User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


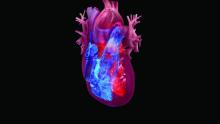
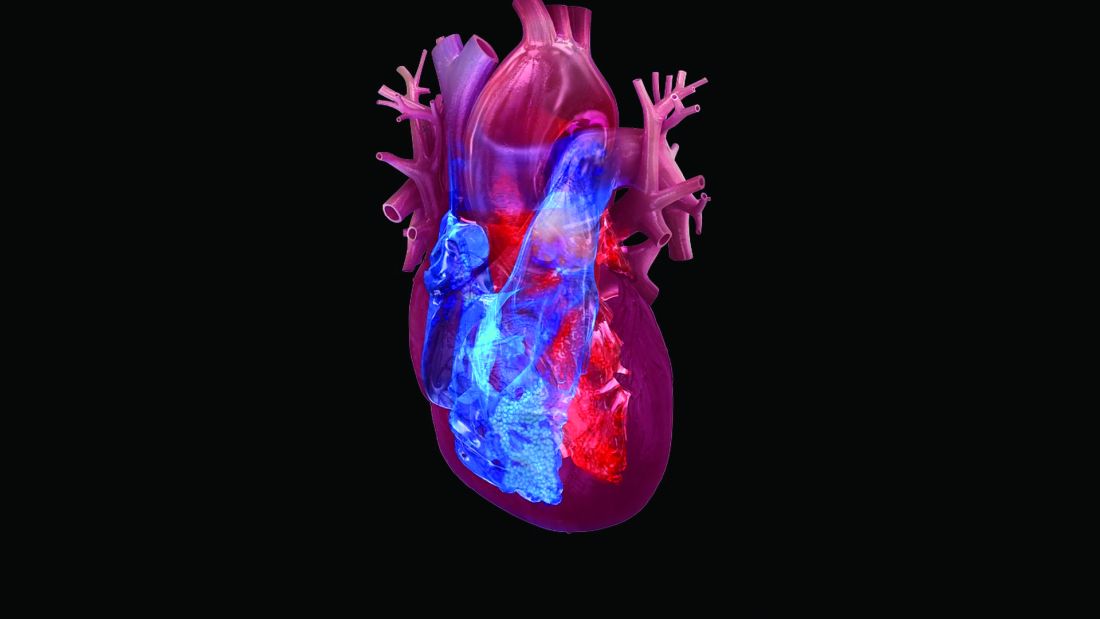
Empagliflozin favorably reshaped left ventricles in HFrEF patients
Treatment with the SGLT2 inhibitor empagliflozin led to significant reductions in both left ventricular end systolic and diastolic volumes in two independent randomized studies of patients with heart failure with reduced ejection fraction.
These results provide important new evidence that one way a drug from this class exerts its beneficial effects on cardiovascular outcomes in these patients is by producing favorable left-ventricular remodeling.
One of the two studies involved only patients with heart failure with reduced ejection fraction (HFrEF) with diabetes and examined treatment impact after 36 weeks. The second study focused exclusively on HFrEF patients without diabetes and followed patients for 6 months. Both studies also generated additional significant evidence of favorable left-ventricular effects.
“The results of these two new trials are incredibly important, as they tell cardiologists one of the mechanisms by which SGLT2 [sodium glucose co-transporter 2] inhibitors reduce heart failure hospitalizations and cardiovascular death,” said Mark C. Petrie, MBChB, professor at the Institute of Cardiovascular & Medical Sciences at the University of Glasgow, and principal investigator for one of the two studies.
“Many cardiologists want to know mechanisms as well as clinical benefit. These remodeling data showing that these drugs reduce the size of abnormally large hearts [and] are also very important for patients,” Dr. Petrie said in an interview. “There have been more than 50 publications on potential mechanisms of benefit of SGLT2 inhibitors in HFrEF, but these are the first randomized, mechanistic data.”
Mechanistic clues follow large cardiovascular outcome trials
Results from a large randomized trial, EMPEROR-Reduced, recently showed that treatment with empagliflozin (Jardiance) on top of standard HFrEF treatment led to significant benefits in patients with or without type 2 diabetes (T2D), compared with placebo, for major cardiovascular and renal endpoints, including the combination of cardiovascular death or hospitalization for heart failure. And results from a second large randomized trial, DAPA-HF, showed similar results with a different drug from the same class, dapagliflozin (Farxiga), in an earlier report.
But while these reports led to quick uptake of these two drugs for the treatment of patients with HFrEF, the means by which these agents exert their HFrEF benefits have been unclear.
“Our study identifies why this drug [empagliflozin] is effective – because it improves heart function, something that has not been understood until now,” Carlos G. Santos-Gallego, MD, lead investigator for the second new report, said in a written statement. “Many doctors are afraid of prescribing a drug they do not understand, and our findings will help clinicians feel more comfortable giving this to patients once approved.”
On the strength of the DAPA-HF results, dapagliflozin received a revised U.S. label in May 2020 that added the indication for treating patients with HFrEF regardless of the whether patients also have T2D, the original indication for prescribing the drug. Many experts anticipate that a similar addition to the label for empagliflozin will soon occur.
EMPA-TROPISM examines patients with no T2D
The single-center study reported by Dr. Santos-Gallego randomized 84 patients with HFrEF and no diabetes to standard treatment with empagliflozin or placebo and measured several parameters in 80 patients who completed the planned 6 months of treatment. The primary endpoints were the changes in both left ventricular end systolic and diastolic volume from baseline in the empagliflozin-treated patients compared with patients on placebo, measured by cardiac MR.
The results showed an average reduction of end systolic volume of 26.6 mL from baseline compared with a small rise in the placebo patients, and an average drop in end diastolic volume of 25.1 mL from baseline compared again with a small increase in the controls. Both differences were statistically significant, reported the senior author of the study, Juan J. Badimon, PhD, in a talk at the virtual scientific sessions of the American Heart Association. Concurrently, the results were published online in the Journal of the American College of Cardiology.
Results from the EMPA-TROPISM study also showed several other significant benefits from empagliflozin treatment, both to left ventricular shape and function as well as to other measures of patient well being. The drug regimen led to an increase in left ventricular ejection fraction, a decrease in left ventricular mass, reduced myocardial fibrosis and aortic stiffness, increased peak oxygen consumption, an increased distance traveled in a 6-minute walk test, and improved quality of life, said Dr. Badimon, professor of medicine and director of the Atherothrombosis Research Unit at the Cardiovascular Institute at the Icahn School of Medicine at Mount Sinai in New York.
SUGAR-DM-HF enrolled only T2D patients
The second study, SUGAR-DM-HF, randomized 105 patients with HFrEF and T2D to treatment with empagliflozin or placebo at any of 15 centers in Scotland, with 92 patients completing the full 36 weeks on treatment. One of the study’s two primary endpoints was the change in left ventricular end systolic volume index, which dropped by an average of 7.9 mL/m2 in patients who received empagliflozin and by 1.5 mL/m2 in the controls, a significant average between-group difference of 6.0 mL/m2, reported Matthew M.Y. Lee, MBChB, at the same meeting.
However, the second primary endpoint, change in left ventricular global longitudinal strain, showed no significant difference in effect on empagliflozin compared with placebo, said Dr. Lee, a cardiologist at the University of Glasgow. Concurrently with his report the results appeared in an article published online in Circulation.
The results also showed a significant drop in left ventricular end diastolic volume index from baseline compared with the control patients, with an average between-group difference in the reduction from baseline of 8.2 mL/m2.
“Reverse cardiac remodeling is a mechanism by which SGLT2 inhibitors reduce heart failure hospitalizations and cardiovascular mortality,” Dr. Lee concluded during his presentation at the meeting.
Although the findings from both studies together provide strong evidence for an effect by empagliflozin on left ventricular shape and function, neither study provides much insight into how this drug exerts these effects. The authors of both studies agreed on several potential explanations, including reductions in cardiac preload and afterload that could reduce left ventricular stretch and volume; a change triggered in myocardial energetics that switches from a metabolism mostly dependent on glucose to one more geared to using fatty acids, ketone bodies, and branched chain amino acids; and a possible drug-induced reduction in oxidative stress and inflammation.
SUGAR-DM-HF was sponsored by a grant from Boehringer Ingelheim, the company that along with Eli Lilly markets empagliflozin (Jardiance). Dr. Lee had no disclosures. Dr. Petrie has been a consultant to Boehringer Ingelheim and Eli Lilly and to several other companies. EMPA-TROPISM was sponsored by a grant from Boehringer Ingelheim. Dr. Badimon and Dr. Santos-Gallego had no disclosures.
Treatment with the SGLT2 inhibitor empagliflozin led to significant reductions in both left ventricular end systolic and diastolic volumes in two independent randomized studies of patients with heart failure with reduced ejection fraction.
These results provide important new evidence that one way a drug from this class exerts its beneficial effects on cardiovascular outcomes in these patients is by producing favorable left-ventricular remodeling.
One of the two studies involved only patients with heart failure with reduced ejection fraction (HFrEF) with diabetes and examined treatment impact after 36 weeks. The second study focused exclusively on HFrEF patients without diabetes and followed patients for 6 months. Both studies also generated additional significant evidence of favorable left-ventricular effects.
“The results of these two new trials are incredibly important, as they tell cardiologists one of the mechanisms by which SGLT2 [sodium glucose co-transporter 2] inhibitors reduce heart failure hospitalizations and cardiovascular death,” said Mark C. Petrie, MBChB, professor at the Institute of Cardiovascular & Medical Sciences at the University of Glasgow, and principal investigator for one of the two studies.
“Many cardiologists want to know mechanisms as well as clinical benefit. These remodeling data showing that these drugs reduce the size of abnormally large hearts [and] are also very important for patients,” Dr. Petrie said in an interview. “There have been more than 50 publications on potential mechanisms of benefit of SGLT2 inhibitors in HFrEF, but these are the first randomized, mechanistic data.”
Mechanistic clues follow large cardiovascular outcome trials
Results from a large randomized trial, EMPEROR-Reduced, recently showed that treatment with empagliflozin (Jardiance) on top of standard HFrEF treatment led to significant benefits in patients with or without type 2 diabetes (T2D), compared with placebo, for major cardiovascular and renal endpoints, including the combination of cardiovascular death or hospitalization for heart failure. And results from a second large randomized trial, DAPA-HF, showed similar results with a different drug from the same class, dapagliflozin (Farxiga), in an earlier report.
But while these reports led to quick uptake of these two drugs for the treatment of patients with HFrEF, the means by which these agents exert their HFrEF benefits have been unclear.
“Our study identifies why this drug [empagliflozin] is effective – because it improves heart function, something that has not been understood until now,” Carlos G. Santos-Gallego, MD, lead investigator for the second new report, said in a written statement. “Many doctors are afraid of prescribing a drug they do not understand, and our findings will help clinicians feel more comfortable giving this to patients once approved.”
On the strength of the DAPA-HF results, dapagliflozin received a revised U.S. label in May 2020 that added the indication for treating patients with HFrEF regardless of the whether patients also have T2D, the original indication for prescribing the drug. Many experts anticipate that a similar addition to the label for empagliflozin will soon occur.
EMPA-TROPISM examines patients with no T2D
The single-center study reported by Dr. Santos-Gallego randomized 84 patients with HFrEF and no diabetes to standard treatment with empagliflozin or placebo and measured several parameters in 80 patients who completed the planned 6 months of treatment. The primary endpoints were the changes in both left ventricular end systolic and diastolic volume from baseline in the empagliflozin-treated patients compared with patients on placebo, measured by cardiac MR.
The results showed an average reduction of end systolic volume of 26.6 mL from baseline compared with a small rise in the placebo patients, and an average drop in end diastolic volume of 25.1 mL from baseline compared again with a small increase in the controls. Both differences were statistically significant, reported the senior author of the study, Juan J. Badimon, PhD, in a talk at the virtual scientific sessions of the American Heart Association. Concurrently, the results were published online in the Journal of the American College of Cardiology.
Results from the EMPA-TROPISM study also showed several other significant benefits from empagliflozin treatment, both to left ventricular shape and function as well as to other measures of patient well being. The drug regimen led to an increase in left ventricular ejection fraction, a decrease in left ventricular mass, reduced myocardial fibrosis and aortic stiffness, increased peak oxygen consumption, an increased distance traveled in a 6-minute walk test, and improved quality of life, said Dr. Badimon, professor of medicine and director of the Atherothrombosis Research Unit at the Cardiovascular Institute at the Icahn School of Medicine at Mount Sinai in New York.
SUGAR-DM-HF enrolled only T2D patients
The second study, SUGAR-DM-HF, randomized 105 patients with HFrEF and T2D to treatment with empagliflozin or placebo at any of 15 centers in Scotland, with 92 patients completing the full 36 weeks on treatment. One of the study’s two primary endpoints was the change in left ventricular end systolic volume index, which dropped by an average of 7.9 mL/m2 in patients who received empagliflozin and by 1.5 mL/m2 in the controls, a significant average between-group difference of 6.0 mL/m2, reported Matthew M.Y. Lee, MBChB, at the same meeting.
However, the second primary endpoint, change in left ventricular global longitudinal strain, showed no significant difference in effect on empagliflozin compared with placebo, said Dr. Lee, a cardiologist at the University of Glasgow. Concurrently with his report the results appeared in an article published online in Circulation.
The results also showed a significant drop in left ventricular end diastolic volume index from baseline compared with the control patients, with an average between-group difference in the reduction from baseline of 8.2 mL/m2.
“Reverse cardiac remodeling is a mechanism by which SGLT2 inhibitors reduce heart failure hospitalizations and cardiovascular mortality,” Dr. Lee concluded during his presentation at the meeting.
Although the findings from both studies together provide strong evidence for an effect by empagliflozin on left ventricular shape and function, neither study provides much insight into how this drug exerts these effects. The authors of both studies agreed on several potential explanations, including reductions in cardiac preload and afterload that could reduce left ventricular stretch and volume; a change triggered in myocardial energetics that switches from a metabolism mostly dependent on glucose to one more geared to using fatty acids, ketone bodies, and branched chain amino acids; and a possible drug-induced reduction in oxidative stress and inflammation.
SUGAR-DM-HF was sponsored by a grant from Boehringer Ingelheim, the company that along with Eli Lilly markets empagliflozin (Jardiance). Dr. Lee had no disclosures. Dr. Petrie has been a consultant to Boehringer Ingelheim and Eli Lilly and to several other companies. EMPA-TROPISM was sponsored by a grant from Boehringer Ingelheim. Dr. Badimon and Dr. Santos-Gallego had no disclosures.
Treatment with the SGLT2 inhibitor empagliflozin led to significant reductions in both left ventricular end systolic and diastolic volumes in two independent randomized studies of patients with heart failure with reduced ejection fraction.
These results provide important new evidence that one way a drug from this class exerts its beneficial effects on cardiovascular outcomes in these patients is by producing favorable left-ventricular remodeling.
One of the two studies involved only patients with heart failure with reduced ejection fraction (HFrEF) with diabetes and examined treatment impact after 36 weeks. The second study focused exclusively on HFrEF patients without diabetes and followed patients for 6 months. Both studies also generated additional significant evidence of favorable left-ventricular effects.
“The results of these two new trials are incredibly important, as they tell cardiologists one of the mechanisms by which SGLT2 [sodium glucose co-transporter 2] inhibitors reduce heart failure hospitalizations and cardiovascular death,” said Mark C. Petrie, MBChB, professor at the Institute of Cardiovascular & Medical Sciences at the University of Glasgow, and principal investigator for one of the two studies.
“Many cardiologists want to know mechanisms as well as clinical benefit. These remodeling data showing that these drugs reduce the size of abnormally large hearts [and] are also very important for patients,” Dr. Petrie said in an interview. “There have been more than 50 publications on potential mechanisms of benefit of SGLT2 inhibitors in HFrEF, but these are the first randomized, mechanistic data.”
Mechanistic clues follow large cardiovascular outcome trials
Results from a large randomized trial, EMPEROR-Reduced, recently showed that treatment with empagliflozin (Jardiance) on top of standard HFrEF treatment led to significant benefits in patients with or without type 2 diabetes (T2D), compared with placebo, for major cardiovascular and renal endpoints, including the combination of cardiovascular death or hospitalization for heart failure. And results from a second large randomized trial, DAPA-HF, showed similar results with a different drug from the same class, dapagliflozin (Farxiga), in an earlier report.
But while these reports led to quick uptake of these two drugs for the treatment of patients with HFrEF, the means by which these agents exert their HFrEF benefits have been unclear.
“Our study identifies why this drug [empagliflozin] is effective – because it improves heart function, something that has not been understood until now,” Carlos G. Santos-Gallego, MD, lead investigator for the second new report, said in a written statement. “Many doctors are afraid of prescribing a drug they do not understand, and our findings will help clinicians feel more comfortable giving this to patients once approved.”
On the strength of the DAPA-HF results, dapagliflozin received a revised U.S. label in May 2020 that added the indication for treating patients with HFrEF regardless of the whether patients also have T2D, the original indication for prescribing the drug. Many experts anticipate that a similar addition to the label for empagliflozin will soon occur.
EMPA-TROPISM examines patients with no T2D
The single-center study reported by Dr. Santos-Gallego randomized 84 patients with HFrEF and no diabetes to standard treatment with empagliflozin or placebo and measured several parameters in 80 patients who completed the planned 6 months of treatment. The primary endpoints were the changes in both left ventricular end systolic and diastolic volume from baseline in the empagliflozin-treated patients compared with patients on placebo, measured by cardiac MR.
The results showed an average reduction of end systolic volume of 26.6 mL from baseline compared with a small rise in the placebo patients, and an average drop in end diastolic volume of 25.1 mL from baseline compared again with a small increase in the controls. Both differences were statistically significant, reported the senior author of the study, Juan J. Badimon, PhD, in a talk at the virtual scientific sessions of the American Heart Association. Concurrently, the results were published online in the Journal of the American College of Cardiology.
Results from the EMPA-TROPISM study also showed several other significant benefits from empagliflozin treatment, both to left ventricular shape and function as well as to other measures of patient well being. The drug regimen led to an increase in left ventricular ejection fraction, a decrease in left ventricular mass, reduced myocardial fibrosis and aortic stiffness, increased peak oxygen consumption, an increased distance traveled in a 6-minute walk test, and improved quality of life, said Dr. Badimon, professor of medicine and director of the Atherothrombosis Research Unit at the Cardiovascular Institute at the Icahn School of Medicine at Mount Sinai in New York.
SUGAR-DM-HF enrolled only T2D patients
The second study, SUGAR-DM-HF, randomized 105 patients with HFrEF and T2D to treatment with empagliflozin or placebo at any of 15 centers in Scotland, with 92 patients completing the full 36 weeks on treatment. One of the study’s two primary endpoints was the change in left ventricular end systolic volume index, which dropped by an average of 7.9 mL/m2 in patients who received empagliflozin and by 1.5 mL/m2 in the controls, a significant average between-group difference of 6.0 mL/m2, reported Matthew M.Y. Lee, MBChB, at the same meeting.
However, the second primary endpoint, change in left ventricular global longitudinal strain, showed no significant difference in effect on empagliflozin compared with placebo, said Dr. Lee, a cardiologist at the University of Glasgow. Concurrently with his report the results appeared in an article published online in Circulation.
The results also showed a significant drop in left ventricular end diastolic volume index from baseline compared with the control patients, with an average between-group difference in the reduction from baseline of 8.2 mL/m2.
“Reverse cardiac remodeling is a mechanism by which SGLT2 inhibitors reduce heart failure hospitalizations and cardiovascular mortality,” Dr. Lee concluded during his presentation at the meeting.
Although the findings from both studies together provide strong evidence for an effect by empagliflozin on left ventricular shape and function, neither study provides much insight into how this drug exerts these effects. The authors of both studies agreed on several potential explanations, including reductions in cardiac preload and afterload that could reduce left ventricular stretch and volume; a change triggered in myocardial energetics that switches from a metabolism mostly dependent on glucose to one more geared to using fatty acids, ketone bodies, and branched chain amino acids; and a possible drug-induced reduction in oxidative stress and inflammation.
SUGAR-DM-HF was sponsored by a grant from Boehringer Ingelheim, the company that along with Eli Lilly markets empagliflozin (Jardiance). Dr. Lee had no disclosures. Dr. Petrie has been a consultant to Boehringer Ingelheim and Eli Lilly and to several other companies. EMPA-TROPISM was sponsored by a grant from Boehringer Ingelheim. Dr. Badimon and Dr. Santos-Gallego had no disclosures.
FROM AHA 2020
Escalate HIV adherence strategies amid COVID-19
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
"The writing is on the wall” that virtual care is not meeting the needs of people with HIV who struggled with viral suppression even before the COVID-19 pandemic, said Jason Farley, PhD, ANP-BC, AACRN, associate professor of nursing at Johns Hopkins University, Baltimore. So it’s time for HIV care teams, especially clinics in the Ryan White HIV/AIDS Program, to get creative in bringing wraparound services to patients.
That may mean reallocating the workforce so that one person serves as a community health worker. Or it could mean increasing texts and video calls; helping patients find online support groups to address problems with alcohol or drug use; and conducting an overall assessment of patients’ needs as the pandemic continues.
“The virtual patient-centered medical home may be the new normal after COVID-19, and we have to be thinking about how we use this model with patients for whom it works, but supplement this model in patients that it does not,” Farley said at the virtual Association of Nurses in AIDS Care (ANAC) 2020 Annual Meeting. That work “is essential to our being able to facilitate the best patient outcomes possible.”
Early data, tiered interventions
Farley referred to an article published in September in the Journal AIDS that confirmed unpublished data mentioned at the International AIDS Conference 2020. The article reported that viral suppression rates among people with HIV who attended San Francisco’s Ward 86 HIV clinic dropped by 31% from pre-COVID levels.
Of the 1766 people who attended the clinic, about 1 in 5 had detectable HIV viral loads at any point in 2019. But that rate was 31% higher after shelter-in-place orders were issued. And although patients participated in telemedicine visits at more or less the same rate before and after the pandemic (31% vs. 30% no-shows), viral suppression rates dropped. The impact was especially acute for homeless individuals.
“This destabilization occurred despite our population attending telemedicine visits at a higher rate than expected, given the 60% drop in ambulatory care visit volume nationwide,” the authors stated in their article. “Telehealth visits, while offering greater patient convenience, may lead to less access to clinic-based social support services essential to achieving viral suppression among vulnerable groups.”
That’s the challenge HIV clinics now face, Farley said at the ANAC meeting.
He suggested a differentiated care approach in which there are four tiers of care, starting with the standard level of outreach, which may include email, electronic health record blasts, and robo-calls to remind people of their appointments and to refill their medications. Those with sustained viral suppression may only need 90-day automatic refills of their medications. Those who are vulnerable to nonadherence may need to be contacted weekly or more often by the clinic. Such contact could be made by a social worker, a community health worker, or through some form of virtual support.
Patients at tier 4, who have labile viral suppression, need far more than that. These are the 15% of patients with HIV who struggled with viral suppression before the pandemic. They are the patients that Farley’s team focuses on at Baltimore’s John G. Bartlett Specialty Clinic for Infectious Disease.
“We’ve completely deconstructed the patient-centered medical home,” he said of the early move to virtual care. He suggested that clinicians assess their services and ask themselves some questions:
- Has someone on the team reached out to every patient and checked in to see what their biggest needs are, medical or not, during the pandemic? Have they assessed the patient’s ability to receive video calls or text messages?
- How have group-support programs that address stigma or the social determinants of health fared in the transition to virtual medicine?
- Are patients who are in recovery being supported in order that they may engage with recovery programs online?
- How well have counseling services done in engaging people in virtual care? Currently, given the overall increase in mental health challenges during the pandemic, one would expect that the use of mental health counseling is increasing. “If they’re stagnant or going down, someone needs to be reflecting on that issue internally in the clinic,” he said.
- Are patients being contacted regarding the effects that isolation is having on their lives? “The things that would normally allow us to self-mitigate and self-manage these conditions, like going to the gym, meeting with friends, religious services – all of those are being cut,” he said.
- Is there an early alert from an in-person pharmacy to trigger outreach via a community health worker for patients who haven’t picked up their medications in a week or more?
Farley pointed to a 2015 model for an enhanced e-health approach to chronic care management that called for e-support from the community and that was enhanced through virtual communities.
These are some of the approaches Farley has taken at his clinic. He leads a team that focuses specifically on patients who struggled with engagement before the pandemic. Through a grant from the US Department of Health & Human Services’ Health Resources and Services Administration – even before the pandemic – that team has been funding community health workers who have multiple contacts with patients online and virtually and are able to offer what he calls “unapologetically enabling” support for patients so that they are able to focus on their health.
He gave the following example. Before the pandemic, a community health worker on the team had been working with a patient who showed up at every scheduled visit and swore that she was taking her medications, although clearly she was not. A community health worker, who was made available through the grant, was able to recognize that the patient’s biggest challenge in her life was providing childcare for her special-needs child. The community health worker worked with the patient for months to find stable childcare for the child, paid 2 months of rent for the patient so that she would not become homeless, and helped her find transitional housing. When the pandemic hit, the community health worker was already texting and conducting video calls with the patient regularly.
For the past 9 months, that patient has had an undetectable viral load, Farley said.
“Nine months during a pandemic,” Farley reiterated, “and the community health worker keeps working with her, keeps meeting with her.”
Stigma on stigma
The need for this level of support from the clinic may be even more important for people with HIV who acquire COVID-19, said Orlando Harris, PhD, assistant professor of community health systems at the University of California, San Francisco, (UCSF) School of Nursing. HIV-related stigma is a well-known deterrent to care for people living with the virus. During the presentation, Harris asked Farley about the impact of COVID-19 stigma on people with both HIV and COVID-19.
Farley said that patients at his clinic have told him that they have “ostracized” friends who have tested positive for COVID-19. Harris remembered a person with HIV who participated in one of his trials telling the researchers that despite all his precautions – wearing a mask, staying socially distant – he still acquired COVID-19. There was nothing he could have done, Harris said, other than just not go to the grocery store.
The fear of contracting another disease that is associated with stigma, as well as the need to disclose it, can inflame memories of the trauma of being diagnosed with HIV, Harris said. And with patient-centered medical homes struggling to reconstitute their wraparound services via telehealth, he said he wonders whether clinicians should be doing more.
“I worry about people who have survived being diagnosed with HIV in the ‘80s and the ‘90s before antiretroviral therapy showed up on the scene,” he told Medscape Medical News. “I worry that the folks that survived one pandemic [may] be feeling fearful or living in that fear that this new pandemic might take them out. That’s why I’m stressing the need for us to really consider, as clinicians and also as researchers the support systems, the coping mechanisms, the counseling, or what have you to support those living with HIV and vulnerable to COVID-19.”
During telehealth visits, that can be achieved simply by asking people how they are really doing and what their coping mechanisms are.
For their part, the clinicians at San Francisco’s Ward 86 are not trying to provide that support through telehealth on the same level as they were at the beginning of the pandemic, said Matthew Spinelli, MD, assistant professor of medicine, and Monica Gandhi, MD, associate chief of the Division of HIV, Infectious Diseases and Global Medicine, who are both at UCSF and are coauthors of the study.
They still offer telemedicine appointments to patients who request them, said Spinelli. He said about one-third of his patients still prefer to receive their care virtually. The rest have gone back to face-to-face support.
“The analysis led us to promptly open up care as much as possible to our patients, with the idea that telehealth is not cutting it for vulnerable patients with HIV,” Gandhi told Medscape Medical News via email. “We don’t think it’s right for a population who relies on social support from the clinic.”
This article first appeared on Medscape.com.
Lung cancer: Proton beam radiotherapy likely reduces cardiovascular events
The findings were reported at the American Society for Radiation Oncology Annual Meeting 2020.
Patients with lung cancer often have underlying cardiac risk factors, noted lead investigator Timothy Kegelman, MD, PhD, of University of Pennsylvania in Philadelphia.
“The dose to the heart correlates with adverse cardiovascular events following radiation therapy. One strategy to minimize dose to the heart is proton beam radiation,” Dr. Kegelman said.
He and his colleagues retrospectively studied consecutive patients with locally advanced non–small cell lung cancer (NSCLC) treated with chemotherapy plus either proton beam radiotherapy or conventional photon radiotherapy.
The team used electronic health records to ascertain incidence of six cardiovascular events: MI, atrial fibrillation, coronary artery disease, heart failure, stroke, and transient ischemic attack. Patients who had previously experienced an event were not considered as part of the at-risk population for that specific event after radiotherapy.
Analyses were based on 98 patients who received proton beam radiotherapy and 104 patients who received conventional photon radiotherapy.
At baseline, the proton cohort was older, had a heavier smoking history, and had a higher prevalence of previous cardiovascular events (46.9% vs. 31.7%; P = .03).
The total median radiation dose was identical for the proton and photon groups (66.6 Gy), but the former group had significantly lower measures of cardiac radiation dose, including roughly half the mean dose to the heart (6.9 vs. 13.3 Gy).
Outcomes and next steps
At a median follow-up of 29 months, the proton beam radiotherapy group had a significantly lower incidence of transient ischemic attack, compared with the photon radiotherapy group (1.1% vs. 8.2%; P = .04).
The proton group also had numerically lower incidences of MI (2.3% vs. 9.0%; P = .06) and stroke (3.2% vs. 6.1%; P = .50).
The proton and photon groups were similar as far as the incidence of total cardiovascular events (53.1% vs. 47.1%; P = .48) and the 3-year overall survival rate (38.8% vs. 42.1%; P = .99).
“Our future studies aim to examine the potential relationships between grade of cardiac event and type of radiotherapy and dose to cardiac substructures,” Dr. Kegelman commented.
In addition, his institution is participating in RTOG 1308, a phase 3 trial comparing photon and proton beam radiotherapy in patients with inoperable lung cancer that will better assess cardiac-related morbidity and mortality. The trial is expected to be completed by the end of 2025.
Accumulating evidence
“This study adds to a growing body of evidence about the potential importance of heart dose in any radiation modality,” said Daniel Gomez, MD, MBA, of Memorial Sloan Kettering Cancer Center in New York, who was not involved in the study.
The RTOG 0617 trial and the Lung ART trial previously showed correlations between lower radiation dose to the heart and better survival in patients with lung cancer, Dr. Gomez noted.
“It’s been well established that protons can improve heart dose, and therefore it’s been inferred that they may improve outcomes, but the exact mechanisms remain unclear,” Dr. Gomez said.
Proton beam radiotherapy performed well in a single-arm, phase 2 trial among patients with unresectable NSCLC.
“The ongoing phase 3 trial is using a more modern proton technique and has a larger population, with a randomized study design. It will be much more informative,” Dr. Gomez predicted.
The current study did not receive specific funding. Dr. Kegelman disclosed no relevant conflicts of interest. Dr. Gomez disclosed honoraria from Varian.
SOURCE: Kegelman TP et al. ASTRO 2020, Abstract 1046.
The findings were reported at the American Society for Radiation Oncology Annual Meeting 2020.
Patients with lung cancer often have underlying cardiac risk factors, noted lead investigator Timothy Kegelman, MD, PhD, of University of Pennsylvania in Philadelphia.
“The dose to the heart correlates with adverse cardiovascular events following radiation therapy. One strategy to minimize dose to the heart is proton beam radiation,” Dr. Kegelman said.
He and his colleagues retrospectively studied consecutive patients with locally advanced non–small cell lung cancer (NSCLC) treated with chemotherapy plus either proton beam radiotherapy or conventional photon radiotherapy.
The team used electronic health records to ascertain incidence of six cardiovascular events: MI, atrial fibrillation, coronary artery disease, heart failure, stroke, and transient ischemic attack. Patients who had previously experienced an event were not considered as part of the at-risk population for that specific event after radiotherapy.
Analyses were based on 98 patients who received proton beam radiotherapy and 104 patients who received conventional photon radiotherapy.
At baseline, the proton cohort was older, had a heavier smoking history, and had a higher prevalence of previous cardiovascular events (46.9% vs. 31.7%; P = .03).
The total median radiation dose was identical for the proton and photon groups (66.6 Gy), but the former group had significantly lower measures of cardiac radiation dose, including roughly half the mean dose to the heart (6.9 vs. 13.3 Gy).
Outcomes and next steps
At a median follow-up of 29 months, the proton beam radiotherapy group had a significantly lower incidence of transient ischemic attack, compared with the photon radiotherapy group (1.1% vs. 8.2%; P = .04).
The proton group also had numerically lower incidences of MI (2.3% vs. 9.0%; P = .06) and stroke (3.2% vs. 6.1%; P = .50).
The proton and photon groups were similar as far as the incidence of total cardiovascular events (53.1% vs. 47.1%; P = .48) and the 3-year overall survival rate (38.8% vs. 42.1%; P = .99).
“Our future studies aim to examine the potential relationships between grade of cardiac event and type of radiotherapy and dose to cardiac substructures,” Dr. Kegelman commented.
In addition, his institution is participating in RTOG 1308, a phase 3 trial comparing photon and proton beam radiotherapy in patients with inoperable lung cancer that will better assess cardiac-related morbidity and mortality. The trial is expected to be completed by the end of 2025.
Accumulating evidence
“This study adds to a growing body of evidence about the potential importance of heart dose in any radiation modality,” said Daniel Gomez, MD, MBA, of Memorial Sloan Kettering Cancer Center in New York, who was not involved in the study.
The RTOG 0617 trial and the Lung ART trial previously showed correlations between lower radiation dose to the heart and better survival in patients with lung cancer, Dr. Gomez noted.
“It’s been well established that protons can improve heart dose, and therefore it’s been inferred that they may improve outcomes, but the exact mechanisms remain unclear,” Dr. Gomez said.
Proton beam radiotherapy performed well in a single-arm, phase 2 trial among patients with unresectable NSCLC.
“The ongoing phase 3 trial is using a more modern proton technique and has a larger population, with a randomized study design. It will be much more informative,” Dr. Gomez predicted.
The current study did not receive specific funding. Dr. Kegelman disclosed no relevant conflicts of interest. Dr. Gomez disclosed honoraria from Varian.
SOURCE: Kegelman TP et al. ASTRO 2020, Abstract 1046.
The findings were reported at the American Society for Radiation Oncology Annual Meeting 2020.
Patients with lung cancer often have underlying cardiac risk factors, noted lead investigator Timothy Kegelman, MD, PhD, of University of Pennsylvania in Philadelphia.
“The dose to the heart correlates with adverse cardiovascular events following radiation therapy. One strategy to minimize dose to the heart is proton beam radiation,” Dr. Kegelman said.
He and his colleagues retrospectively studied consecutive patients with locally advanced non–small cell lung cancer (NSCLC) treated with chemotherapy plus either proton beam radiotherapy or conventional photon radiotherapy.
The team used electronic health records to ascertain incidence of six cardiovascular events: MI, atrial fibrillation, coronary artery disease, heart failure, stroke, and transient ischemic attack. Patients who had previously experienced an event were not considered as part of the at-risk population for that specific event after radiotherapy.
Analyses were based on 98 patients who received proton beam radiotherapy and 104 patients who received conventional photon radiotherapy.
At baseline, the proton cohort was older, had a heavier smoking history, and had a higher prevalence of previous cardiovascular events (46.9% vs. 31.7%; P = .03).
The total median radiation dose was identical for the proton and photon groups (66.6 Gy), but the former group had significantly lower measures of cardiac radiation dose, including roughly half the mean dose to the heart (6.9 vs. 13.3 Gy).
Outcomes and next steps
At a median follow-up of 29 months, the proton beam radiotherapy group had a significantly lower incidence of transient ischemic attack, compared with the photon radiotherapy group (1.1% vs. 8.2%; P = .04).
The proton group also had numerically lower incidences of MI (2.3% vs. 9.0%; P = .06) and stroke (3.2% vs. 6.1%; P = .50).
The proton and photon groups were similar as far as the incidence of total cardiovascular events (53.1% vs. 47.1%; P = .48) and the 3-year overall survival rate (38.8% vs. 42.1%; P = .99).
“Our future studies aim to examine the potential relationships between grade of cardiac event and type of radiotherapy and dose to cardiac substructures,” Dr. Kegelman commented.
In addition, his institution is participating in RTOG 1308, a phase 3 trial comparing photon and proton beam radiotherapy in patients with inoperable lung cancer that will better assess cardiac-related morbidity and mortality. The trial is expected to be completed by the end of 2025.
Accumulating evidence
“This study adds to a growing body of evidence about the potential importance of heart dose in any radiation modality,” said Daniel Gomez, MD, MBA, of Memorial Sloan Kettering Cancer Center in New York, who was not involved in the study.
The RTOG 0617 trial and the Lung ART trial previously showed correlations between lower radiation dose to the heart and better survival in patients with lung cancer, Dr. Gomez noted.
“It’s been well established that protons can improve heart dose, and therefore it’s been inferred that they may improve outcomes, but the exact mechanisms remain unclear,” Dr. Gomez said.
Proton beam radiotherapy performed well in a single-arm, phase 2 trial among patients with unresectable NSCLC.
“The ongoing phase 3 trial is using a more modern proton technique and has a larger population, with a randomized study design. It will be much more informative,” Dr. Gomez predicted.
The current study did not receive specific funding. Dr. Kegelman disclosed no relevant conflicts of interest. Dr. Gomez disclosed honoraria from Varian.
SOURCE: Kegelman TP et al. ASTRO 2020, Abstract 1046.
FROM ASTRO 2020
Situation ‘dire’ as COVID spike in West, Midwest worsens, experts say
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Coronavirus infections are expected to continue to climb in the upper Midwest and intermountain West of the United States, which will strain an already-maxed-out system as increased hospitalizations and deaths follow, say infectious diseases specialists.
“I think the situation in 2 to 4 weeks is going to be grim,” said Andrew Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah School of Medicine in Salt Lake City, on a call yesterday with reporters, sponsored by the Infectious Diseases Society of America (IDSA).
Cases began rising in Utah in mid-September and have gone up steeply since, increasing from 450 cases per day to 2,650 reported on Nov. 8, according to the Johns Hopkins Coronavirus Resource Center. The New York Times reports that the 7-day rolling average for hospitalizations have gone up 34% and deaths have risen 93%, with 11 deaths this past Tuesday.
Other states in the west – Montana, Idaho, and Wyoming, which reported 1,232 cases on Tuesday and have been averaging 660 cases a day in the last week, according to the Times – are being equally hard hit. The same is true for states in the upper Midwest, including North Dakota, South Dakota, Minnesota, Wisconsin, and Iowa.
Most of the states being hit now have large swaths of rural countryside, which means health resources are limited and spread out, said Pavia.
“The situation really has to be described as dire,” said Pavia, noting that intensive care units in Utah are full, including contingency units that were purpose-built for the pandemic. Physicians and nurses are burned out and in short supply, he said. Instead of a 1:1 or 1:2 nurse-to-ICU patient ratio, the ratio is now 1:4, said Pavia. “Throughout the region, people are facing a crisis in staffing.”
The University of Utah hospital normally takes referrals from Idaho, Wyoming, and northern Arizona, but is prioritizing Utah residents for ICU admission, said Pavia.
Both Pavia and Daniel P. McQuillen, MD, president-elect of IDSA, also noted the shortage of infectious diseases specialists, which began at least a decade ago. McQuillen, senior infectious diseases physician at Beth Israel Lahey Health in Boston, said he and colleagues had done some research earlier this year anticipating the pandemic’s spread, and found that some 80% of counties – including the rural counties in the states now being hit – have one or zero infectious disease specialists.
Those specialists can help improve patient outcomes, explained McQuillen.
Colleges likely driving spike
Pavia said the reasons for sharp increases in the region vary, but there are several areas of commonality. Most of the states didn’t have many cases early in the pandemic, “so perhaps there was less fear of the virus.” There were fewer actions by government officials, driven perhaps by the reluctance to take on individuals who are distrustful of government, he said.
Cases started going up after some events – such as the August motorcycle rally in Sturgis, South Dakota – but the acceleration in September was likely driven by the reopening of colleges across the region, said Pavia.
“Most of the states have kept in-person schooling, and probably more importantly, they’ve kept extracurricular activities in sports,” he said, adding that in many of the areas the weather has turned cooler, driving people indoors.
McQuillen said it has been shown that a significant amount of transmission occurs within homes – and college students may be bringing the virus home and fueling spread, in addition to people not wearing masks while at small family gatherings.
Both he and Pavia said more emphasis needs to be placed on mitigation measures such as mask-wearing as well as on testing. IDSA is starting #MaskUpAmerica, a public service campaign aimed at getting people to wear masks in all community settings, including at work, in churches, at social gatherings, in gyms, and on public transportation.
Pavia said in some places people are refusing to be tested because they don’t want to be quarantined.
Utah Gov. Gary Herbert (R) issued a statewide mask mandate this past weekend and announced some other restrictions, including a 2-week pause on most, but not all, athletic events, according to CBS News. But local pushback could weaken those measures, said Pavia.
Many people are looking to vaccines to usher in a return to normal. But, said Pavia, “vaccines aren’t going to help us out much this winter,” noting that initial doses will be given mostly to first responders and healthcare workers.
“The only way we’re going to get out of this this winter is by doing the things that we’ve been talking about for months – wearing a mask, watching your social distance, and avoiding large gatherings,” he said.
There is an end in sight, said Pavia, but it won’t be in early 2021. “That end is next summer or fall,” he said. “And that’s a hard message to give but it’s really critical.”
McQuillen agreed: “Wearing masks and distancing are exactly all we have probably until middle of next year.”
This article first appeared on Medscape.com.
Nearly one in five develop mental illness following COVID-19
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
One in five COVID-19 patients are diagnosed with a psychiatric disorder such as anxiety or depression within 3 months of testing positive for the virus, new research suggests.
“People have been worried that COVID-19 survivors will be at greater risk of psychiatric disorders, and our findings in a large and detailed study show this to be true,” principal investigator Paul Harrison, BM, DM, professor of psychiatry, University of Oxford, Oxford, United Kingdom, said in a statement.
Health services “need to be ready to provide care, especially since our results are likely to be underestimates of the actual number of cases,” said Harrison.
The study also showed that having a psychiatric disorder independently increases the risk of getting COVID-19 – a finding that’s in line with research published earlier this month.
“Having a psychiatric illness should be added to the list of risk factors for COVID-19,” study coauthor Maxime Taquet, PhD, University of Oxford, said in the release.
The study was published online Nov. 9 in The Lancet Psychiatry.
Double the risk
The investigators took advantage of the TriNetX analytics network, which captured deidentified data from electronic health records of a total of 69.8 million patients from 54 healthcare organizations in the United States.
Of those patients, 62,354 adults were diagnosed with COVID-19 between Jan. 20 and Aug. 1, 2020.
To assess the psychiatric sequelae of COVID-19, the investigators created propensity score–matched cohorts of patients who had received a diagnosis of other conditions that represented a range of common acute presentations.
In 14 to 90 days after being diagnosed with COVID-19, 5.8% of patients received a first recorded diagnosis of psychiatric illness. Among patients with health problems other than COVID, 2.5% to 3.4% of patients received a psychiatric diagnosis, the authors report. The risk was greatest for anxiety disorders, depression, and insomnia.
Older COVID-19 patients had a two- to threefold increased risk for a first dementia diagnosis, a finding that supports an earlier UK study.
Some of this excess risk could reflect misdiagnosed cases of delirium or transient cognitive impairment due to reversible cerebral events, the authors noted.
The study also revealed a bidirectional relationship between mental illness and COVID-19. Individuals with a psychiatric diagnosis were about 65% more likely to be diagnosed with COVID-19 in comparison with their counterparts who did not have mental illness, independently of known physical health risk factors for COVID-19.
“We did not anticipate that psychiatric history would be an independent risk factor for COVID-19. This finding appears robust, being observed in all age strata and in both sexes, and was substantial,” the authors write.
At present, “we don’t understand what the explanation is for the associations between COVID and mental illness. We are looking into this in more detail to try and understand better what subgroups are particularly vulnerable in this regard,” Harrison told Medscape Medical News.
“Ambitious” research
Commenting on the findings for Medscape Medical News, Roy H. Perlis, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, said this is “an ambitious effort to understand the short-term consequences of COVID in terms of brain diseases.”
Perlis said he’s not particularly surprised by the increase in psychiatric diagnoses among COVID-19 patients.
“After COVID infection, people are more likely to get close medical follow-up than usual. They’re more likely to be accessing the healthcare system; after all, they’ve already had COVID, so they’re probably less fearful of seeing their doctor. But, that probably also means they’re more likely to get a new diagnosis of something like depression,” he said.
Dementia may be the clearest illustration of this, Perlis said. “It seems less likely that dementia develops a month after COVID; more likely, something that happens during the illness leads someone to be more likely to diagnose dementia later on,” he noted.
Perlis cautioned against being “unnecessarily alarmed” by the findings in this study.
“We know that rates of depression in the UK and the US, as in much of the world, are substantially elevated right now. Much of this is likely a consequence of the stress and disruption that accompanies the pandemic,” said Perlis.
The study was funded by the National Institute for Health Research. Harrison has disclosed no relevant financial relationships. One author is an employee of TriNetX. Perlis has received consulting fees for service on scientific advisory boards of Belle Artificial Intelligence, Burrage Capital, Genomind, Psy Therapeutics, Outermost Therapeutics, RID Ventures, and Takeda. He holds equity in Psy Therapeutics and Outermost Therapeutics.
This article first appeared on Medscape.com.
New reports guide return to play in athletes with COVID-19
Increasingly, clinicians are being called upon to advise athletes who have recovered from COVID-19 on when it is safe for them to return to play.
Now, they have two reports that offer more insights into the cardiotoxic effects of COVID-19 on the athletic heart.
In the first report, researchers report a high prevalence of pericardial involvement in college-student athletes who have recovered from COVID-19 and give their practical advice on how to let these athletes return to play safely.
In the second report, an expert panel of sports cardiologists provides a comprehensive guide to the appropriate imaging of athletes who may have cardiovascular complications from COVID-19.
Both are published in JACC: Cardiovascular Imaging.
“We were asked by the editors of JACC to submit this paper, and the impetus for it was the fact that there are so many athletes returning after being infected with COVID-19, we need to try and give guidance to cardiologists as to how best to evaluate these athletes,” Dermot Phelan, MD, PhD, Sanger Heart and Vascular Institute, Atrium Health, Charlotte, N.C., and lead author of the consensus statement, said in an interview.
The consensus statement acknowledges that information about the cardiovascular complications of COVID-19 continues to evolve. Meanwhile, pathologies such as myocarditis, pericarditis, and right ventricular dysfunction, in the absence of significant clinical symptoms, in athletes who have been affected by COVID-19 remain of considerable concern.
It also emphasizes the unique challenges the average cardiologist faces in distinguishing between what is normal for an athlete’s heart and what is true pathology after COVID-19 infection; details how different imaging modalities can help in screening, evaluating, and monitoring athletes with suspected cardiovascular complications of COVID-19 infection; and discusses the strengths and limitations of these modalities.
Finally, the consensus statement provides some well-needed guidance on return-to-play decision-making, for both the athlete and the clinician.
Athletic remodeling or covid-19 damage?
Athletes can develop certain cardiovascular characteristics because of their athletic activity, and sometimes, this can cloud the diagnostic picture.
“Is this change due to the effects of COVID-19, or is it just because this is an athlete’s heart? This was an international expert consensus, made up of sports cardiologists from all over the world who have a lot of experience in dealing with athletes,” Dr. Phelan said. “We were trying to relay the important information to the cardiologist who is not used to dealing with athletes on a day-to-day basis, as to what they might expect to find in that athlete, and what is not an expected finding and should be tested further.”
Phelan, a sports cardiologist, is familiar with what is normal for an athlete’s heart and what is pathology.
“We know that athletes, particularly long-term endurance athletes, develop changes in the heart that can affect not only the electrics but the structure of the heart, and sometimes, that overlaps with abnormalities with pathology. This can be a challenge for the nonsports cardiologist to differentiate,” he said.
Phelan and his group have written two other consensus documents on the management of cardiovascular problems that develop in some athletes who have been infected with COVID-19.
The first was published in May in JAMA Cardiology, and the second, which revised some of the original recommendations made in the first document, was published online Oct. 26 in JAMA Cardiology.
The first set of recommendations called for imaging studies to be done in all athletes, but the second set states that athletes who recover and are asymptomatic do not need extensive (and expensive) imaging tests.
“These two papers work hand in hand,” Dr. Phelan said. “In May, we had very little experience with COVID, and there was a lot of concern about hospitalized patients having a very high incidence of heart disease. We published those recommendations, but we recognized at the time that we had very little data and that we would reconsider once we had more experience with data.
“This current set of recommendations that we have put forth here are for those athletes who do need to get further testing, so it’s a step beyond,” Dr. Phelan added. “So the second iteration states that young athletes who had mild or no symptoms didn’t need to go through all of that cardiac testing, but others do need it.”
To do widespread cardiovascular imaging for many individuals would be very costly. Realistically, there are not that many centers in the United States that have all the sophisticated equipment required to do such testing, Dr. Phelan noted.
“One of our major points is difficulty obtaining the test, but also the cost; these are very expensive tests. There are limitations. They are useful when used in the correct context,” he said.
To play or not to play, that is the question
Partho P. Sengupta, MD, DM, had to answer that question for more than 50 young athletes who were returning to college at West Virginia University, anxious to be back with their teams and on the playing field. They had been infected with COVID-19 and needed to know when they could return to play.
Dr. Sengupta, who is also an author for the Phelan et al consensus statement on imaging, said there was a lot of pressure – from all the various stakeholders, and from anxious parents, worried college athletes, their teammates, and the university – to determine if the youngsters could return to play.
The fear was that COVID-19 infection left the young athlete’s heart vulnerable to myocarditis and, thus, sudden death on the playing field after strenuous activity.
“At the time we were doing this imaging, there was a lot of concern in the media, and papers were coming out reporting a lot of cardiac involvement or myocarditis associated with COVID-19. Nobody really knew what to do,” he explained.
“There were all kinds of questions, concerns. The parents were putting pressure on us, the athletes wanted to know, the teams, the university. So we put together a team and completed all of the examinations, including testing of blood markers, within a 2-week period. These young athletes, they’re scared, they’re worried and anxious, they don’t know what’s going to happen with their scholarship, so there was some urgency to this work,” Dr. Sengupta said.
“We had to screen all comers within a very short period. We had 54 consecutive patients, gave them full screening, full battery of tests, blood tests, all in a 2-week period,” he said.
Speed was of the essence, and Dr. Sengupta and his team rolled up their sleeves and got to work “We had to know who was safe to clear to return to play and who might need extra follow-up.”
Screening echocardiograms
They performed screening echocardiograms on 54 consecutive college athletes who had tested positive for COVID-19 on reverse transcription polymerase chain reaction nasal swab testing or who showed that they had IgG antibodies against COVID-19. The screening echocardiograms were done after the athletes had quarantined for at least 14 days and were no longer infectious.
Most (85%) were male, and the mean age was 19 years. A total of 16 (30%) athletes were asymptomatic, 36 (66%) reported mild COVID-19 related symptoms, and two (4%) reported moderate symptoms.
Of the 54 athletes who were initially screened with echocardiography, 48 (11 asymptomatic, 37 symptomatic), went on to have cardiac magnetic resonance imaging.
Results showed that more than half the athletes (27; 56.3%), showed some cardiac abnormality. The most common was pericardial late enhancement with associated pericardial effusion, affecting 19 (39.5%) athletes.
Of these, six (12.5%) had reduced global longitudinal strain (GLS) or an increased native T1.
One patient showed myocardial enhancement.
Additionally, seven athletes (14.6%) had reduced left ventricular ejection fraction or reduced GLS with or without increased native T1. Native T2 levels were normal in all subjects and no specific imaging features of myocardial inflammation were identified.
Participants were brought back to receive the results of their tests and to get an individualized plan about their safe return to play 3 to 5 weeks after they had ceased to be infectious with COVID-19.
“We saw pericardial inflammation that was resolving. We did not see any blood biomarkers to suggest that there was active inflammation going on,” he said. “We also did not see any muscle inflammation, but we did see pockets of fluid in over a third of our athletes.”
Fortunately, most were deemed able to get back to playing safely, despite having evidence of pericardial inflammation.
This was on strict condition that they be monitored very closely for any adverse events that might occur as they began to exercise again.
“Once they go back to the field to start exercising and practicing, it is under great supervision. We instructed all of our sports physicians and other team managers that these people need to be observed very carefully. So as long as they were asymptomatic, even though the signs of pericardial inflammation were there, if there were no signs of inflammation in the blood, we let them go back to play, closely monitored,” Dr. Sengupta said.
A small number remained very symptomatic at the end of the 5 weeks and were referred to cardiac rehabilitation, Dr. Sengupta said. “They were tired, fatigued, short of breath, even 5 weeks after they got over COVID, so we sent them for cardiac rehab to help them get conditioned again.”
The researchers plan to reevaluate and reimage all of the athletes in another 3 months to monitor their cardiac health.
Dr. Sengupta acknowledged the limitations of this single-center, nonrandomized, controlled report, but insists reports such as this add a bit more to what we are learning about COVID-19 every day.
“These kids were coming to us and asking questions. You have to use the best science you have available to you at that point in time. Some people ask why we did not have a control group, but how do you design a control population in the midst of a pandemic? The science may or may not be perfect, I agree, but the information we obtained is important,” he said.
“Right now, I don’t think we have enough science, and we are still learning. It is very difficult to predict who will develop the heart muscle disease or the pericardial disease,” Dr. Sengupta said. “We had to do our work quickly to give answers to the young athletes, their parents, their teammates, their university, as soon as possible, and we were doing this under pandemic conditions.”
The work was supported by the National Science Foundation National Institute of General Medical Sciences of the National Institutes of Health. Dr. Phelan reported no relevant financial relationships. Dr. Sengupta reported that he is a consultant for HeartSciences, Kencor Health, and Ultromics.
This article first appeared on Medscape.com.
Increasingly, clinicians are being called upon to advise athletes who have recovered from COVID-19 on when it is safe for them to return to play.
Now, they have two reports that offer more insights into the cardiotoxic effects of COVID-19 on the athletic heart.
In the first report, researchers report a high prevalence of pericardial involvement in college-student athletes who have recovered from COVID-19 and give their practical advice on how to let these athletes return to play safely.
In the second report, an expert panel of sports cardiologists provides a comprehensive guide to the appropriate imaging of athletes who may have cardiovascular complications from COVID-19.
Both are published in JACC: Cardiovascular Imaging.
“We were asked by the editors of JACC to submit this paper, and the impetus for it was the fact that there are so many athletes returning after being infected with COVID-19, we need to try and give guidance to cardiologists as to how best to evaluate these athletes,” Dermot Phelan, MD, PhD, Sanger Heart and Vascular Institute, Atrium Health, Charlotte, N.C., and lead author of the consensus statement, said in an interview.
The consensus statement acknowledges that information about the cardiovascular complications of COVID-19 continues to evolve. Meanwhile, pathologies such as myocarditis, pericarditis, and right ventricular dysfunction, in the absence of significant clinical symptoms, in athletes who have been affected by COVID-19 remain of considerable concern.
It also emphasizes the unique challenges the average cardiologist faces in distinguishing between what is normal for an athlete’s heart and what is true pathology after COVID-19 infection; details how different imaging modalities can help in screening, evaluating, and monitoring athletes with suspected cardiovascular complications of COVID-19 infection; and discusses the strengths and limitations of these modalities.
Finally, the consensus statement provides some well-needed guidance on return-to-play decision-making, for both the athlete and the clinician.
Athletic remodeling or covid-19 damage?
Athletes can develop certain cardiovascular characteristics because of their athletic activity, and sometimes, this can cloud the diagnostic picture.
“Is this change due to the effects of COVID-19, or is it just because this is an athlete’s heart? This was an international expert consensus, made up of sports cardiologists from all over the world who have a lot of experience in dealing with athletes,” Dr. Phelan said. “We were trying to relay the important information to the cardiologist who is not used to dealing with athletes on a day-to-day basis, as to what they might expect to find in that athlete, and what is not an expected finding and should be tested further.”
Phelan, a sports cardiologist, is familiar with what is normal for an athlete’s heart and what is pathology.
“We know that athletes, particularly long-term endurance athletes, develop changes in the heart that can affect not only the electrics but the structure of the heart, and sometimes, that overlaps with abnormalities with pathology. This can be a challenge for the nonsports cardiologist to differentiate,” he said.
Phelan and his group have written two other consensus documents on the management of cardiovascular problems that develop in some athletes who have been infected with COVID-19.
The first was published in May in JAMA Cardiology, and the second, which revised some of the original recommendations made in the first document, was published online Oct. 26 in JAMA Cardiology.
The first set of recommendations called for imaging studies to be done in all athletes, but the second set states that athletes who recover and are asymptomatic do not need extensive (and expensive) imaging tests.
“These two papers work hand in hand,” Dr. Phelan said. “In May, we had very little experience with COVID, and there was a lot of concern about hospitalized patients having a very high incidence of heart disease. We published those recommendations, but we recognized at the time that we had very little data and that we would reconsider once we had more experience with data.
“This current set of recommendations that we have put forth here are for those athletes who do need to get further testing, so it’s a step beyond,” Dr. Phelan added. “So the second iteration states that young athletes who had mild or no symptoms didn’t need to go through all of that cardiac testing, but others do need it.”
To do widespread cardiovascular imaging for many individuals would be very costly. Realistically, there are not that many centers in the United States that have all the sophisticated equipment required to do such testing, Dr. Phelan noted.
“One of our major points is difficulty obtaining the test, but also the cost; these are very expensive tests. There are limitations. They are useful when used in the correct context,” he said.
To play or not to play, that is the question
Partho P. Sengupta, MD, DM, had to answer that question for more than 50 young athletes who were returning to college at West Virginia University, anxious to be back with their teams and on the playing field. They had been infected with COVID-19 and needed to know when they could return to play.
Dr. Sengupta, who is also an author for the Phelan et al consensus statement on imaging, said there was a lot of pressure – from all the various stakeholders, and from anxious parents, worried college athletes, their teammates, and the university – to determine if the youngsters could return to play.
The fear was that COVID-19 infection left the young athlete’s heart vulnerable to myocarditis and, thus, sudden death on the playing field after strenuous activity.
“At the time we were doing this imaging, there was a lot of concern in the media, and papers were coming out reporting a lot of cardiac involvement or myocarditis associated with COVID-19. Nobody really knew what to do,” he explained.
“There were all kinds of questions, concerns. The parents were putting pressure on us, the athletes wanted to know, the teams, the university. So we put together a team and completed all of the examinations, including testing of blood markers, within a 2-week period. These young athletes, they’re scared, they’re worried and anxious, they don’t know what’s going to happen with their scholarship, so there was some urgency to this work,” Dr. Sengupta said.
“We had to screen all comers within a very short period. We had 54 consecutive patients, gave them full screening, full battery of tests, blood tests, all in a 2-week period,” he said.
Speed was of the essence, and Dr. Sengupta and his team rolled up their sleeves and got to work “We had to know who was safe to clear to return to play and who might need extra follow-up.”
Screening echocardiograms
They performed screening echocardiograms on 54 consecutive college athletes who had tested positive for COVID-19 on reverse transcription polymerase chain reaction nasal swab testing or who showed that they had IgG antibodies against COVID-19. The screening echocardiograms were done after the athletes had quarantined for at least 14 days and were no longer infectious.
Most (85%) were male, and the mean age was 19 years. A total of 16 (30%) athletes were asymptomatic, 36 (66%) reported mild COVID-19 related symptoms, and two (4%) reported moderate symptoms.
Of the 54 athletes who were initially screened with echocardiography, 48 (11 asymptomatic, 37 symptomatic), went on to have cardiac magnetic resonance imaging.
Results showed that more than half the athletes (27; 56.3%), showed some cardiac abnormality. The most common was pericardial late enhancement with associated pericardial effusion, affecting 19 (39.5%) athletes.
Of these, six (12.5%) had reduced global longitudinal strain (GLS) or an increased native T1.
One patient showed myocardial enhancement.
Additionally, seven athletes (14.6%) had reduced left ventricular ejection fraction or reduced GLS with or without increased native T1. Native T2 levels were normal in all subjects and no specific imaging features of myocardial inflammation were identified.
Participants were brought back to receive the results of their tests and to get an individualized plan about their safe return to play 3 to 5 weeks after they had ceased to be infectious with COVID-19.
“We saw pericardial inflammation that was resolving. We did not see any blood biomarkers to suggest that there was active inflammation going on,” he said. “We also did not see any muscle inflammation, but we did see pockets of fluid in over a third of our athletes.”
Fortunately, most were deemed able to get back to playing safely, despite having evidence of pericardial inflammation.
This was on strict condition that they be monitored very closely for any adverse events that might occur as they began to exercise again.
“Once they go back to the field to start exercising and practicing, it is under great supervision. We instructed all of our sports physicians and other team managers that these people need to be observed very carefully. So as long as they were asymptomatic, even though the signs of pericardial inflammation were there, if there were no signs of inflammation in the blood, we let them go back to play, closely monitored,” Dr. Sengupta said.
A small number remained very symptomatic at the end of the 5 weeks and were referred to cardiac rehabilitation, Dr. Sengupta said. “They were tired, fatigued, short of breath, even 5 weeks after they got over COVID, so we sent them for cardiac rehab to help them get conditioned again.”
The researchers plan to reevaluate and reimage all of the athletes in another 3 months to monitor their cardiac health.
Dr. Sengupta acknowledged the limitations of this single-center, nonrandomized, controlled report, but insists reports such as this add a bit more to what we are learning about COVID-19 every day.
“These kids were coming to us and asking questions. You have to use the best science you have available to you at that point in time. Some people ask why we did not have a control group, but how do you design a control population in the midst of a pandemic? The science may or may not be perfect, I agree, but the information we obtained is important,” he said.
“Right now, I don’t think we have enough science, and we are still learning. It is very difficult to predict who will develop the heart muscle disease or the pericardial disease,” Dr. Sengupta said. “We had to do our work quickly to give answers to the young athletes, their parents, their teammates, their university, as soon as possible, and we were doing this under pandemic conditions.”
The work was supported by the National Science Foundation National Institute of General Medical Sciences of the National Institutes of Health. Dr. Phelan reported no relevant financial relationships. Dr. Sengupta reported that he is a consultant for HeartSciences, Kencor Health, and Ultromics.
This article first appeared on Medscape.com.
Increasingly, clinicians are being called upon to advise athletes who have recovered from COVID-19 on when it is safe for them to return to play.
Now, they have two reports that offer more insights into the cardiotoxic effects of COVID-19 on the athletic heart.
In the first report, researchers report a high prevalence of pericardial involvement in college-student athletes who have recovered from COVID-19 and give their practical advice on how to let these athletes return to play safely.
In the second report, an expert panel of sports cardiologists provides a comprehensive guide to the appropriate imaging of athletes who may have cardiovascular complications from COVID-19.
Both are published in JACC: Cardiovascular Imaging.
“We were asked by the editors of JACC to submit this paper, and the impetus for it was the fact that there are so many athletes returning after being infected with COVID-19, we need to try and give guidance to cardiologists as to how best to evaluate these athletes,” Dermot Phelan, MD, PhD, Sanger Heart and Vascular Institute, Atrium Health, Charlotte, N.C., and lead author of the consensus statement, said in an interview.
The consensus statement acknowledges that information about the cardiovascular complications of COVID-19 continues to evolve. Meanwhile, pathologies such as myocarditis, pericarditis, and right ventricular dysfunction, in the absence of significant clinical symptoms, in athletes who have been affected by COVID-19 remain of considerable concern.
It also emphasizes the unique challenges the average cardiologist faces in distinguishing between what is normal for an athlete’s heart and what is true pathology after COVID-19 infection; details how different imaging modalities can help in screening, evaluating, and monitoring athletes with suspected cardiovascular complications of COVID-19 infection; and discusses the strengths and limitations of these modalities.
Finally, the consensus statement provides some well-needed guidance on return-to-play decision-making, for both the athlete and the clinician.
Athletic remodeling or covid-19 damage?
Athletes can develop certain cardiovascular characteristics because of their athletic activity, and sometimes, this can cloud the diagnostic picture.
“Is this change due to the effects of COVID-19, or is it just because this is an athlete’s heart? This was an international expert consensus, made up of sports cardiologists from all over the world who have a lot of experience in dealing with athletes,” Dr. Phelan said. “We were trying to relay the important information to the cardiologist who is not used to dealing with athletes on a day-to-day basis, as to what they might expect to find in that athlete, and what is not an expected finding and should be tested further.”
Phelan, a sports cardiologist, is familiar with what is normal for an athlete’s heart and what is pathology.
“We know that athletes, particularly long-term endurance athletes, develop changes in the heart that can affect not only the electrics but the structure of the heart, and sometimes, that overlaps with abnormalities with pathology. This can be a challenge for the nonsports cardiologist to differentiate,” he said.
Phelan and his group have written two other consensus documents on the management of cardiovascular problems that develop in some athletes who have been infected with COVID-19.
The first was published in May in JAMA Cardiology, and the second, which revised some of the original recommendations made in the first document, was published online Oct. 26 in JAMA Cardiology.
The first set of recommendations called for imaging studies to be done in all athletes, but the second set states that athletes who recover and are asymptomatic do not need extensive (and expensive) imaging tests.
“These two papers work hand in hand,” Dr. Phelan said. “In May, we had very little experience with COVID, and there was a lot of concern about hospitalized patients having a very high incidence of heart disease. We published those recommendations, but we recognized at the time that we had very little data and that we would reconsider once we had more experience with data.
“This current set of recommendations that we have put forth here are for those athletes who do need to get further testing, so it’s a step beyond,” Dr. Phelan added. “So the second iteration states that young athletes who had mild or no symptoms didn’t need to go through all of that cardiac testing, but others do need it.”
To do widespread cardiovascular imaging for many individuals would be very costly. Realistically, there are not that many centers in the United States that have all the sophisticated equipment required to do such testing, Dr. Phelan noted.
“One of our major points is difficulty obtaining the test, but also the cost; these are very expensive tests. There are limitations. They are useful when used in the correct context,” he said.
To play or not to play, that is the question
Partho P. Sengupta, MD, DM, had to answer that question for more than 50 young athletes who were returning to college at West Virginia University, anxious to be back with their teams and on the playing field. They had been infected with COVID-19 and needed to know when they could return to play.
Dr. Sengupta, who is also an author for the Phelan et al consensus statement on imaging, said there was a lot of pressure – from all the various stakeholders, and from anxious parents, worried college athletes, their teammates, and the university – to determine if the youngsters could return to play.
The fear was that COVID-19 infection left the young athlete’s heart vulnerable to myocarditis and, thus, sudden death on the playing field after strenuous activity.
“At the time we were doing this imaging, there was a lot of concern in the media, and papers were coming out reporting a lot of cardiac involvement or myocarditis associated with COVID-19. Nobody really knew what to do,” he explained.
“There were all kinds of questions, concerns. The parents were putting pressure on us, the athletes wanted to know, the teams, the university. So we put together a team and completed all of the examinations, including testing of blood markers, within a 2-week period. These young athletes, they’re scared, they’re worried and anxious, they don’t know what’s going to happen with their scholarship, so there was some urgency to this work,” Dr. Sengupta said.
“We had to screen all comers within a very short period. We had 54 consecutive patients, gave them full screening, full battery of tests, blood tests, all in a 2-week period,” he said.
Speed was of the essence, and Dr. Sengupta and his team rolled up their sleeves and got to work “We had to know who was safe to clear to return to play and who might need extra follow-up.”
Screening echocardiograms
They performed screening echocardiograms on 54 consecutive college athletes who had tested positive for COVID-19 on reverse transcription polymerase chain reaction nasal swab testing or who showed that they had IgG antibodies against COVID-19. The screening echocardiograms were done after the athletes had quarantined for at least 14 days and were no longer infectious.
Most (85%) were male, and the mean age was 19 years. A total of 16 (30%) athletes were asymptomatic, 36 (66%) reported mild COVID-19 related symptoms, and two (4%) reported moderate symptoms.
Of the 54 athletes who were initially screened with echocardiography, 48 (11 asymptomatic, 37 symptomatic), went on to have cardiac magnetic resonance imaging.
Results showed that more than half the athletes (27; 56.3%), showed some cardiac abnormality. The most common was pericardial late enhancement with associated pericardial effusion, affecting 19 (39.5%) athletes.
Of these, six (12.5%) had reduced global longitudinal strain (GLS) or an increased native T1.
One patient showed myocardial enhancement.
Additionally, seven athletes (14.6%) had reduced left ventricular ejection fraction or reduced GLS with or without increased native T1. Native T2 levels were normal in all subjects and no specific imaging features of myocardial inflammation were identified.
Participants were brought back to receive the results of their tests and to get an individualized plan about their safe return to play 3 to 5 weeks after they had ceased to be infectious with COVID-19.
“We saw pericardial inflammation that was resolving. We did not see any blood biomarkers to suggest that there was active inflammation going on,” he said. “We also did not see any muscle inflammation, but we did see pockets of fluid in over a third of our athletes.”
Fortunately, most were deemed able to get back to playing safely, despite having evidence of pericardial inflammation.
This was on strict condition that they be monitored very closely for any adverse events that might occur as they began to exercise again.
“Once they go back to the field to start exercising and practicing, it is under great supervision. We instructed all of our sports physicians and other team managers that these people need to be observed very carefully. So as long as they were asymptomatic, even though the signs of pericardial inflammation were there, if there were no signs of inflammation in the blood, we let them go back to play, closely monitored,” Dr. Sengupta said.
A small number remained very symptomatic at the end of the 5 weeks and were referred to cardiac rehabilitation, Dr. Sengupta said. “They were tired, fatigued, short of breath, even 5 weeks after they got over COVID, so we sent them for cardiac rehab to help them get conditioned again.”
The researchers plan to reevaluate and reimage all of the athletes in another 3 months to monitor their cardiac health.
Dr. Sengupta acknowledged the limitations of this single-center, nonrandomized, controlled report, but insists reports such as this add a bit more to what we are learning about COVID-19 every day.
“These kids were coming to us and asking questions. You have to use the best science you have available to you at that point in time. Some people ask why we did not have a control group, but how do you design a control population in the midst of a pandemic? The science may or may not be perfect, I agree, but the information we obtained is important,” he said.
“Right now, I don’t think we have enough science, and we are still learning. It is very difficult to predict who will develop the heart muscle disease or the pericardial disease,” Dr. Sengupta said. “We had to do our work quickly to give answers to the young athletes, their parents, their teammates, their university, as soon as possible, and we were doing this under pandemic conditions.”
The work was supported by the National Science Foundation National Institute of General Medical Sciences of the National Institutes of Health. Dr. Phelan reported no relevant financial relationships. Dr. Sengupta reported that he is a consultant for HeartSciences, Kencor Health, and Ultromics.
This article first appeared on Medscape.com.
AMA creates COVID-19 CPT codes for Pfizer, Moderna vaccines
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
The largest U.S. physician organization on Tuesday took a step to prepare for future payments for administration of two leading COVID-19 vaccine candidates, publishing new billing codes tailored to track each use of these medications.
The The new codes apply to the experimental vaccine being developed by Pfizer, in collaboration with a smaller German firm BioNTech, and to the similar product expected from Moderna, according to an AMA press release.
Positive news has emerged this week about both of these vaccines, which were developed using a newer – and as yet unproven – approach. They seek to use messenger RNA to instruct cells to produce a target protein for SARS-CoV-2.
New York–based Pfizer on Monday announced interim phase 3 data that was widely viewed as promising. Pfizer said the vaccine appeared to be 90% effective in preventing COVID-19 in trial volunteers who were without evidence of prior infection of the virus.
In a press release, Pfizer said it plans to ask the Food and Drug Administration to consider a special clearance, known as an emergency-use authorization, “soon after” a safety milestone is achieved in its vaccine trial. That milestone could be reached this month.
Moderna said it was on track to report early data from a late-stage trial of its experimental coronavirus vaccine later this month, and could file with the FDA for an emergency-use authorization in early December, according to a Reuters report.
The severity of the global pandemic has put the FDA under pressure to move quickly on approval of COVID-19 vaccines, based on limited data, while also working to make sure these products are safe. The creation of CPT codes for each of two coronavirus vaccines, as well as accompanying administration codes, will set up a way to keep tabs on each dose of each of these shots, the AMA said.
“Correlating each coronavirus vaccine with its own unique CPT code provides analytical advantages to help track, allocate and optimize resources as an immunization program ramps up in the United States,” AMA President Susan R. Bailey, MD, said in the release.
AMA plans to introduce more vaccine-specific CPT codes as more vaccine candidates approach FDA review. These vaccine-specific CPT codes can go into effect only after the FDA grants a clearance.
The newly created Category I CPT codes and long descriptors for the vaccine products are:
- 91300; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3mL dosage, diluent reconstituted, for intramuscular use (Pfizer/BioNTech)
- 91301; severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5mL dosage, for intramuscular use (Moderna)
These two administrative codes would apply to the Pfizer-BioNTech shot:
- 0001A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; first dose.
- 0002A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 30 mcg/0.3 mL dosage, diluent reconstituted; second dose.
And these two administrative codes would apply to the Moderna shot:
- 0011A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; first dose.
- 0012A; Immunization administration by intramuscular injection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease [COVID-19]) vaccine, mRNA-LNP, spike protein, preservative free, 100 mcg/0.5 mL dosage; second dose.
A version of this article originally appeared on Medscape.com.
Launching a virtual Listening Tour
How do we discuss race and lung health issues that impact our most deserving, underserved communities? Continuously and uncomfortably. As the Executive Director of the CHEST Foundation and as a young Black man, I am hopeful that we, as CHEST, can lead these uncomfortable conversations to better our communities. Our ability to listen and deliver support to our most-deserving communities is critical in how we fulfill our mission. CHEST continues to be a leader in lung health because we choose to give a voice and a platform in support of better lung health – especially to those who are disproportionately affected by lung disease, specifically addressing the quality of care they receive and bringing to light the fact that too often these patients are forgotten by the rest of society.
As cases of COVID-19 and civil unrest continue to swell across our nation, we, the CHEST Foundation, have launched a virtual Listening Tour. We are taking this pragmatic, and more importantly, passionate approach to addressing health disparities by identifying and addressing barriers and issues affecting our most deserving and disproportionately underserved communities. By bringing together these communities’ patients and caregivers, local leaders, involved businesses, and our CHEST members in a virtual community gathering, we intend to clearly define the needs of each community, elevate those needs to a national level, and work to collaborate with and support these local communities and leaders to address their most-pressing issues.
Stories are what connect us and move us forward. We are confident that this virtual Listening Tour will be an opportunity for constituents to tell their own stories and learn from each other, while allowing the CHEST organization, through the CHEST Foundation, to act as the arbiter for pulmonary health and provide a path forward to create equity for those suffering from chronic lung disease.
We need your support to challenge these longstanding disparities in chest medicine. Help us advance these critical conversations and move the needle toward equality by contributing today at chestfoundation.org/donate.
How do we discuss race and lung health issues that impact our most deserving, underserved communities? Continuously and uncomfortably. As the Executive Director of the CHEST Foundation and as a young Black man, I am hopeful that we, as CHEST, can lead these uncomfortable conversations to better our communities. Our ability to listen and deliver support to our most-deserving communities is critical in how we fulfill our mission. CHEST continues to be a leader in lung health because we choose to give a voice and a platform in support of better lung health – especially to those who are disproportionately affected by lung disease, specifically addressing the quality of care they receive and bringing to light the fact that too often these patients are forgotten by the rest of society.
As cases of COVID-19 and civil unrest continue to swell across our nation, we, the CHEST Foundation, have launched a virtual Listening Tour. We are taking this pragmatic, and more importantly, passionate approach to addressing health disparities by identifying and addressing barriers and issues affecting our most deserving and disproportionately underserved communities. By bringing together these communities’ patients and caregivers, local leaders, involved businesses, and our CHEST members in a virtual community gathering, we intend to clearly define the needs of each community, elevate those needs to a national level, and work to collaborate with and support these local communities and leaders to address their most-pressing issues.
Stories are what connect us and move us forward. We are confident that this virtual Listening Tour will be an opportunity for constituents to tell their own stories and learn from each other, while allowing the CHEST organization, through the CHEST Foundation, to act as the arbiter for pulmonary health and provide a path forward to create equity for those suffering from chronic lung disease.
We need your support to challenge these longstanding disparities in chest medicine. Help us advance these critical conversations and move the needle toward equality by contributing today at chestfoundation.org/donate.
How do we discuss race and lung health issues that impact our most deserving, underserved communities? Continuously and uncomfortably. As the Executive Director of the CHEST Foundation and as a young Black man, I am hopeful that we, as CHEST, can lead these uncomfortable conversations to better our communities. Our ability to listen and deliver support to our most-deserving communities is critical in how we fulfill our mission. CHEST continues to be a leader in lung health because we choose to give a voice and a platform in support of better lung health – especially to those who are disproportionately affected by lung disease, specifically addressing the quality of care they receive and bringing to light the fact that too often these patients are forgotten by the rest of society.
As cases of COVID-19 and civil unrest continue to swell across our nation, we, the CHEST Foundation, have launched a virtual Listening Tour. We are taking this pragmatic, and more importantly, passionate approach to addressing health disparities by identifying and addressing barriers and issues affecting our most deserving and disproportionately underserved communities. By bringing together these communities’ patients and caregivers, local leaders, involved businesses, and our CHEST members in a virtual community gathering, we intend to clearly define the needs of each community, elevate those needs to a national level, and work to collaborate with and support these local communities and leaders to address their most-pressing issues.
Stories are what connect us and move us forward. We are confident that this virtual Listening Tour will be an opportunity for constituents to tell their own stories and learn from each other, while allowing the CHEST organization, through the CHEST Foundation, to act as the arbiter for pulmonary health and provide a path forward to create equity for those suffering from chronic lung disease.
We need your support to challenge these longstanding disparities in chest medicine. Help us advance these critical conversations and move the needle toward equality by contributing today at chestfoundation.org/donate.
This month in the journal CHEST®
Editor’s picks
International perspective on the new 2019 IDSA/ATS CAP guideline: A critical appraisal by a global expert panel. By Dr. Mathias Pletz, et al.
Development of an accurate bedside swallowing evaluation decision tree algorithm for detecting aspiration in acute respiratory failure survivors. By Dr. Marc Moss, et al.
How I Do It: Managing fatigue in patients with interstitial lung disease. By Dr. Marlies Wijsenbeek, et al.
Life-threatening and non-life-threatening complications associated with coughing: A scoping review. By Dr. Richard S. Irwin, MD, Master FCCP, et al.
Obstructive Sleep Apnea in Professional Transport Operations: Safety, Regulatory, and Economic Impact. By Dr. Indira Gurubhagavatula, et al.
Editor’s picks
Editor’s picks
International perspective on the new 2019 IDSA/ATS CAP guideline: A critical appraisal by a global expert panel. By Dr. Mathias Pletz, et al.
Development of an accurate bedside swallowing evaluation decision tree algorithm for detecting aspiration in acute respiratory failure survivors. By Dr. Marc Moss, et al.
How I Do It: Managing fatigue in patients with interstitial lung disease. By Dr. Marlies Wijsenbeek, et al.
Life-threatening and non-life-threatening complications associated with coughing: A scoping review. By Dr. Richard S. Irwin, MD, Master FCCP, et al.
Obstructive Sleep Apnea in Professional Transport Operations: Safety, Regulatory, and Economic Impact. By Dr. Indira Gurubhagavatula, et al.
International perspective on the new 2019 IDSA/ATS CAP guideline: A critical appraisal by a global expert panel. By Dr. Mathias Pletz, et al.
Development of an accurate bedside swallowing evaluation decision tree algorithm for detecting aspiration in acute respiratory failure survivors. By Dr. Marc Moss, et al.
How I Do It: Managing fatigue in patients with interstitial lung disease. By Dr. Marlies Wijsenbeek, et al.
Life-threatening and non-life-threatening complications associated with coughing: A scoping review. By Dr. Richard S. Irwin, MD, Master FCCP, et al.
Obstructive Sleep Apnea in Professional Transport Operations: Safety, Regulatory, and Economic Impact. By Dr. Indira Gurubhagavatula, et al.
CHEST and ATS respond to proposed fee schedule
CHEST and the American Thoracic Society (ATS) submitted joint comments regarding the proposed Medicare Physician Fee Schedule for 2021 to CMS Administrator Seema Verma on topics of direct interest to members. The letter focuses on:
Medicare payment for critical care services: Further to the joint letter from CHEST, ATS, and the Society of Critical Care Medicine to Department of Health and Human Services Secretary Azar (see article in September 2020 Washington Watchline), the concerns related to the proposed 8% reduction in reimbursement for critical care services are explained, particularly relating to the role of critical care providers during the pandemic. They call for waiving budget neutrality or utilizing the public health emergency declaration to ensure appropriate patient care.
E/M payment changes: ATS and CHEST voice support for the proposed changes to evaluation and management (E/M) office visits and the increased reimbursement for the cognitive component of E/M medicine. They urge CMS to use its authority to waive the budget neutrality requirements while implementing the E/M changes.
Adoption of RUC-recommended values for pulmonary services: They urge CMS to finalize values for specific pulmonary services while acknowledging thanks for the adoption of the Relative Value Scale Update Committee (RUC)-recommended physician work values for a range of Current Procedural Terminology codes.
Telehealth services: While commending CMS for actions related to telehealth to provide care during the pandemic, they suggest it is now appropriate to sunset the telehealth listing for critical care services as providers have acquired additional experience in treating COVID-19.
GPC1X descriptors and utilization projections: They urge CMS to clarify the descriptors and seek additional comments on primary and ongoing health-care services.
Watch for reports of ongoing efforts from CHEST as the fee schedule process continues. Details of other activities in support of CHEST members appear in the November issue of Washington Watchline.
Reprinted from the November 2020 issue of Washington Watchline.
CHEST and the American Thoracic Society (ATS) submitted joint comments regarding the proposed Medicare Physician Fee Schedule for 2021 to CMS Administrator Seema Verma on topics of direct interest to members. The letter focuses on:
Medicare payment for critical care services: Further to the joint letter from CHEST, ATS, and the Society of Critical Care Medicine to Department of Health and Human Services Secretary Azar (see article in September 2020 Washington Watchline), the concerns related to the proposed 8% reduction in reimbursement for critical care services are explained, particularly relating to the role of critical care providers during the pandemic. They call for waiving budget neutrality or utilizing the public health emergency declaration to ensure appropriate patient care.
E/M payment changes: ATS and CHEST voice support for the proposed changes to evaluation and management (E/M) office visits and the increased reimbursement for the cognitive component of E/M medicine. They urge CMS to use its authority to waive the budget neutrality requirements while implementing the E/M changes.
Adoption of RUC-recommended values for pulmonary services: They urge CMS to finalize values for specific pulmonary services while acknowledging thanks for the adoption of the Relative Value Scale Update Committee (RUC)-recommended physician work values for a range of Current Procedural Terminology codes.
Telehealth services: While commending CMS for actions related to telehealth to provide care during the pandemic, they suggest it is now appropriate to sunset the telehealth listing for critical care services as providers have acquired additional experience in treating COVID-19.
GPC1X descriptors and utilization projections: They urge CMS to clarify the descriptors and seek additional comments on primary and ongoing health-care services.
Watch for reports of ongoing efforts from CHEST as the fee schedule process continues. Details of other activities in support of CHEST members appear in the November issue of Washington Watchline.
Reprinted from the November 2020 issue of Washington Watchline.
CHEST and the American Thoracic Society (ATS) submitted joint comments regarding the proposed Medicare Physician Fee Schedule for 2021 to CMS Administrator Seema Verma on topics of direct interest to members. The letter focuses on:
Medicare payment for critical care services: Further to the joint letter from CHEST, ATS, and the Society of Critical Care Medicine to Department of Health and Human Services Secretary Azar (see article in September 2020 Washington Watchline), the concerns related to the proposed 8% reduction in reimbursement for critical care services are explained, particularly relating to the role of critical care providers during the pandemic. They call for waiving budget neutrality or utilizing the public health emergency declaration to ensure appropriate patient care.
E/M payment changes: ATS and CHEST voice support for the proposed changes to evaluation and management (E/M) office visits and the increased reimbursement for the cognitive component of E/M medicine. They urge CMS to use its authority to waive the budget neutrality requirements while implementing the E/M changes.
Adoption of RUC-recommended values for pulmonary services: They urge CMS to finalize values for specific pulmonary services while acknowledging thanks for the adoption of the Relative Value Scale Update Committee (RUC)-recommended physician work values for a range of Current Procedural Terminology codes.
Telehealth services: While commending CMS for actions related to telehealth to provide care during the pandemic, they suggest it is now appropriate to sunset the telehealth listing for critical care services as providers have acquired additional experience in treating COVID-19.
GPC1X descriptors and utilization projections: They urge CMS to clarify the descriptors and seek additional comments on primary and ongoing health-care services.
Watch for reports of ongoing efforts from CHEST as the fee schedule process continues. Details of other activities in support of CHEST members appear in the November issue of Washington Watchline.
Reprinted from the November 2020 issue of Washington Watchline.