User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Dr. Fauci: Extraordinary challenges, scientific triumphs with COVID-19
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
“Vaccines have been the bright light of this extraordinary challenge that we’ve gone through,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases.
In an address for the opening ceremony of the American Thoracic Society’s virtual international conference, Dr. Fauci emphasized the role of basic and clinical research and government support for science in helping turn the tide of the COVID-19 pandemic.
“A few weeks ago, I wrote an editorial in Science, because there was some misunderstanding about how and why we were able to go from a realization of a new pathogen in January of 2020, to getting doses of vaccines in the arms of individuals – a highly efficacious vaccine – 11 months later. Truly, an unprecedented accomplishment,” he said.
“But as I said in the editorial, the speed and efficiency with which these highly efficacious vaccines were developed, and their potential for saving millions of lives, are due to an extraordinary multidisciplinary effort, involving basic, preclinical, and clinical science that had been underway – out of the spotlight – for decades and decades before the unfolding of the COVID-19 pandemic, a fact that very few people really appreciate: namely, the importance of investment in biomedical research.”
The general addresses the troops
Perhaps no other audience is so well suited to receive Dr. Fauci’s speech as those who are currently attending (virtually) the ATS conference, including researchers who scrutinize the virus from every angle to describe its workings and identify its vulnerabilities, epidemiologists who study viral transmission and look for ways to thwart it, public health workers who fan out to communities across the country to push vaccine acceptance, and clinicians who specialize in critical care and pulmonary medicine, many of whom staff the respiratory floors and intensive care units where the most severely ill patients are treated.
Speaking about the lessons learned and challenges remaining from the COVID-19 pandemic, Dr. Fauci briefly reviewed the epidemiology, virology and transmission, diagnostics, and clinical course of SARS-CoV-2 infections and the therapeutics and vaccines for COVID-19.
Epidemiology
The pandemic began in December 2019 with recognition of a novel type of pneumonia in the Wuhan District of Central China, Dr. Fauci noted.
“Very quickly thereafter, in the first week of January 2020, the Chinese identified a new strain of coronavirus as [the] source of the outbreak. Fast forward to where we are right now: We have experienced and are experiencing the most devastating pandemic of a respiratory illness in the last 102 years, with already approximately 160 million individuals having been infected – and this is clearly a gross undercounting – and also 3.3 million deaths, again, very likely an undercounting,” he said.
According to the Centers for Disease Control and Prevention, as of May 9, 2021, there were approximately 32.5 million cases of COVID-19 and 578,520 deaths in the United States. Those cases and deaths occurred largely in three surges in the United States, in early spring, early summer, and late fall of 2020.
Virology and transmission
SARS-CoV-2 is a beta-coronavirus in the same subgenus as SARS-CoV-1 and some bat coronaviruses, Dr. Fauci explained. The viral genome is large, about 30,000 kilobases, and it has four structural proteins, most importantly the S or “spike” protein that allows the virus to attach to and fuse with cell membranes by binding to the ACE2 receptor on tissues in the upper and lower respiratory tract, gastrointestinal tract, cardiovascular system, and other organ systems.
The virus is transmitted mainly through exposure to respiratory droplets within 6 feet of an infected person, or sometimes through droplets or particles that remain in the air over time and various distances.
Contact with contaminated surfaces, once feared as a means of transmission, is now understood to be less common.
The virus has been detected in stool, blood, semen, and ocular secretions, although the role of transmission through these sources is still unknown.
“Some very interesting characteristics of this virus, really quite unique compared to other viruses, certainly other respiratory viruses, is [that] about a third to 40% of people who are infected never develop any symptoms,” Dr. Fauci said. “Importantly, and very problematic to what we do to contain it – particularly with regard to identification, isolation, and contract tracing – between 50% and 60% of the transmissions occur either from someone who will never develop symptoms, or someone in the presymptomatic phase of disease.”
The fundamentals of preventing acquisition and transmission are as familiar to most Americans now as the Pledge of Allegiance: universal mask wearing, physical distancing, avoiding crowds and congregate settings, preference for outdoor over indoor settings, and frequent hand washing, he noted.
Diagnostics
Tests for SARS-CoV-2 infection fall into three basic categories: molecular tests such as polymerase chain reaction (PCR) that are highly specific and highly sensitive for actual infections, antigen tests that detect the viral protein rather than the nucleic acids, and antibody tests to detect serum proteins made in response to viral infection.
Antigen testing is used largely for broader surveillance of groups of individuals to detect viral penetrance within that group, Dr. Fauci noted.
Clinical course
The clinical course of COVID-19 has some interesting characteristics but is not substantially different from a flu-like syndrome, Dr. Fauci said.
Symptoms and signs common to both types of infections include fever, cough, fatigue, anorexia, dyspnea, and myalgias, but the loss of smell and/or taste preceding the onset of respiratory symptoms is a unique feature of COVID-19.
Dr. Fauci cited data on more than 44,000 individuals with confirmed COVID-19 in China that showed that a large majority (81%) of cases were mild or moderate in nature, but 14% of patients experienced severe disease, and 5% were critically ill. The case-fatality rate in this study was 2.3%.
People at increased risk for severe disease include older adults and those of any age with certain comorbidities.
Manifestations of severe COVID-19 infections in adults can include neurological disorders, hyperinflammation, acute respiratory distress syndrome, cardiac dysfunction, hypercoagulability, and acute kidney injury.
In children, COVID-19 has been associated with a multisystem inflammatory syndrome (MIS-C) similar to Kawasaki disease.
In a substantial number of cases, the effects of COVID-19 can linger for 6 months or longer, Dr. Fauci said, pointing to a study from the University of Washington in Seattle.
Investigators there found that approximately 30% of patients enrolled at their center reported persistent symptoms for as long as 9 months after the initial illness, with fatigue as the most commonly reported symptom. One-third of outpatients with mild disease also reported persistent symptoms.
Therapeutics
Therapeutics that are either approved by the Food and Drug Administration, have emergency use authorization, or are in clinical trials for early or moderate disease include remdesivir (Veklury, Gilead Sciences), monoclonal antibodies, convalescent plasma, antiviral agents, hyperimmune globulin, anticoagulants, and immunomodulators.
Options for moderate to severe to advanced disease include dexamethasone, baricitinib (Olumiant, Eli Lilly and Company) plus remdesivir, and immunomodulators such as infliximab (Remicade, Janssen Biotech), and biosimilars.
Vaccines
Finally, Dr. Fauci reviewed the current state of vaccines, including the three with emergency use authorization from the FDA as of this writing: two nucleic acid, messenger RNA-based (mRNA) vaccines from Moderna and Pfizer/BioNTech, and an adenoviral vector-based vaccine from Johnson & Johnson.
Other vaccines in development or in use elsewhere in the world include recombinant protein and adjuvant approaches by GlaxoSmithKline and Sanofi (in a phase 2 clinical trial launched in February 2021) and by Novavax.
The three vaccines in use in the United States were highly efficacious in both clinical trials, with efficacy of about 95% for the mRNA vaccines and 67% for the Johnson & Johnson vaccine.
The real-world performance of these vaccines has been even more impressive, however.
For example, the Johnson & Johnson vaccine had 72% efficacy at preventing moderate to severe COVID 19 in the United States, 68% in Brazil, and 64% in South Africa, and 85% efficacy against severe disease across all regions studied, Dr. Fauci said.
He cited a study of 22,234 employees of the University of Texas Southwestern Medical Center in Dallas who were vaccinated under a program started on Dec. 15, 2020. The COVID-19 infection rate among these vaccinated employees was 0.05%.
Dr. Fauci recounted the experience in Israel, where the highly transmissible B.1.1.7 strain of SARS-CoV-2 is predominant. A chart of the progress shows clearly that as the vaccine doses delivered steadily increased, the number of COVID-19 cases began a precipitous decline.
Horse race
Fittingly for a speech presented on the day that the Preakness Stakes – the second leg in thoroughbred racing’s Triple Crown – was run, Dr. Fauci closed with a cartoon showing two racehorses, labeled “SARS-CoV-2” and “Vaccines,” nearly neck-and-neck, but with vaccines having a slight lead.
“We are in a race against the virus. The vaccines, and the virus: If we vaccinate the overwhelming proportion of our population, we will without a doubt be able to crush the outbreak in the same way as we have done with other viral-borne diseases like measles, smallpox, and polio.
“So, the message is: Get vaccinated,” he concluded.
Long-term use of prescription sleep meds unsupported by new research
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
a new study shows.
“While there are good data from [randomized, controlled trials] that these medications improve sleep disturbances in the short term,” few studies have examined whether they provide long-term benefits, stated the authors of the paper, which was published in BMJ Open.
“The current observational study does not support use of sleep medications over the long term, as there were no self-reported differences at 1 or 2 years of follow-up comparing sleep medication users with nonusers,” author Daniel H. Solomon, MD, MPH, from Brigham and Women’s Hospital, Boston, and colleagues wrote.
Women included in the analysis were drawn from the Study of Women’s Health Across the Nation (SWAN), an ongoing multicenter, longitudinal study examining women during the menopausal transition. The average age of the women included in the cohort was 49.5 years and approximately half were White. All women reported a sleep disturbance on at least 3 nights per week during a 2-week interval. At follow up, women were asked to use a Likert scale to rate three aspects of sleep: difficulty initiating sleep, frequent awakening, and waking up early. On the scale, 1 represented having no difficulties on any nights, 3 represented having difficulties 1-2 nights per week, and 5 represented having difficulty 5-7 nights per week.
Women already using prescription sleep medication at their baseline visit were excluded from the study. Medications used included benzodiazepines, selective BZD receptor agonists, and other hypnotics.
Over the 21 years of follow-up in the SWAN study (1995-2016), Dr. Solomon and colleagues identified 238 women using sleep medication and these were compared with a cohort of 447 propensity score–matched non–sleep medication uses. Overall, the 685 women included were similar in characteristics to each other as well as to the other potentially eligible women not included in the analysis.
Sleep disturbance patterns compared
At baseline, sleep disturbance patterns were similar between the two groups. Among medication users, the mean score for difficulty initiating sleep was 2.7 (95% confidence interval, 2.5-2.9), waking frequently 3.8 (95% CI, 3.6-3.9), and waking early 2.9 (95% CI, 2.7-3.1). Among the nonusers, the baseline scores were 2.6 (95% CI, 2.5-2.7), 3.7 (95% CI, 3.6-3.8), and 2.7 (95% CI, 2.5-2.8), respectively. After 1 year, there was no statistically significant difference in scores between the two groups. The average ratings for medication users were 2.6 (95% CI, 2.3-2.8) for difficulty initiating sleep, 3.8 (95% CI, 3.6-4.0) for waking frequently, and 2.8 (95% CI, 2.6-3.0) for waking early.
Average ratings among nonusers were 2.3 (95% CI, 2.2-2.4), 3.5 (95% CI, 3.3-3.6), and 2.5 (95% CI, 2.3-2.6), respectively.
After 2 years, there were still no statistically significant reductions in sleep disturbances among those taking prescription sleep medications, compared with those not taking medication.
The researchers noted that approximately half of the women in this cohort were current or past tobacco users and that 20% were moderate to heavy alcohol users.
More work-up, not more medication, needed
The study authors acknowledged the limitations of an observational study and noted that, since participants only reported medication use and sleep disturbances at annual visits, they did not know whether patients’ medication use was intermittent or of any interim outcomes. Additionally, the authors pointed out that those classified as “nonusers” may have been using over-the-counter medication.
“Investigations should look at detailed-use patterns, on a daily or weekly basis, with frequent outcomes data,” Dr. Solomon said in an interview. “While our data shed new light on chronic use, we only had data collected on an annual basis; daily or weekly data would provide more granular information.”
Regarding clinician prescribing practices, Dr. Solomon said, “short-term, intermittent use can be helpful, but use these agents sparingly” and “educate patients that chronic regular use of medications for sleep is not associated with improvement in sleep disturbances.”
Commenting on the study, Andrea Matsumura, MD, a sleep specialist at the Oregon Clinic in Portland, echoed this sentiment: “When someone says they are having trouble sleeping this is the tip of the iceberg and it warrants an evaluation to determine if someone has a breathing disorder, a circadian disorder, a life situation, or a type of insomnia that is driving the sleeplessness.”
“I think this study supports what we all should know,” Dr. Matsumura concluded. “Sleep aids are not meant to be used long term” and should not be used for longer than 2 weeks without further work-up.
Funding for this study was provided through a grant from the National Institutes of Health. Dr. Solomon has received salary support from research grants to Brigham and Women’s Hospital for unrelated work from AbbVie, Amgen, Corrona, Genentech and Pfizer. The other authors and Dr. Matsumura have reported no relevant financial relationships.
FROM BMJ OPEN
E-cigarettes linked to wheeze, shortness of breath
The use of e-cigarettes is linked to a higher frequency of self-reported wheezing and shortness of breath in adolescents and young adults, according to an online survey. The association was present even after controlling for cigarette and cannabis use.
Previous studies of adolescents and young adults have shown associations between e-cigarette use and wheeze, shortness of breath, and asthma. The Youth Risk Behavior Surveillance (YRBS) survey by the Centers for Disease Control and Prevention and other health agencies, conducted from 2015 to 2017, found that 63.5% of youth who used e-cigarettes also used some combination of cigarettes and cannabis. Combined use was associated with a 55%-65% increased odds of self-reported asthma.
The Population Assessment of Tobacco and Health (PATH) study, which was published in October 2020, had similar findings, though it did not find an association between e-cigarette use alone and wheezing.
“The findings from the current study highlight that we need to keep asking young people about respiratory symptoms, couse of other tobacco products, as well as cannabis use. As more products, including cannabis and various e-cigarette devices, enter the market, assessing respiratory health will be important both where adolescents and young adults receive their health care and in research,” Alayna Tackett, PhD, said in an interview. Dr. Tackett presented the study at the American Thoracic Society’s virtual international conference. She is an assistant professor of preventive medicine at the University of Southern California, Los Angeles.
“I found [the study] very interesting because it seems to be identifying a physiologic response to these e-cigarettes,” said Christopher Pascoe, MD, who was asked to comment. “And they were so young [age 14-21 years]. The fact that these symptoms of wheezing and shortness of breath are coming from people who are this young suggests that there may be chronic problems showing up later with continued use of these devices.”
Dr. Pascoe is an assistant professor of physiology and pathophysiology at the University of Manitoba, Winnipeg, where he also works with the Children’s Hospital Research Institute of Manitoba. His own research examines lung tissue harvested from pneumothorax surgeries in smokers and e-cigarette users to identify markers of inflammation.
He called the research a “good start” at unraveling the impacts of e-cigarettes and smoking, since some people use both products. “The fact that there was still a twofold increase in odds for wheezing, shortness of breath among people who use these e-cigarettes, but weren’t using cannabis and weren’t using cigarettes. I think it’s novel, and it suggests that there is an effect [of e-cigarettes alone].”
The study is based on a self-reported data, which is a significant limitation, especially considering that asthma is often overreported. “Self-report can be fraught with things, but I think it’s an interesting starting point for trying to recruit people who are just e-cigarette users and following them up further,” said Dr. Pascoe.
The researchers surveyed 2,931 individuals aged 14-21 years between Aug. 6 and Aug.30, 2020, with an average age of 18.9 years. Of the respondents, 80% were women and girls, and 75% were White. The high percentage of women and girls was unusual. Dr. Tackett provided no explanation for the atypical demographic but noted that the current study used convenience sampling.
The survey asked about use of e-cigarettes, cigarettes, and cannabis in the past 30 days, as well as asthma diagnosis and respiratory symptoms over the same period. The methodology employed survey management company Lucid, which recruited, collected data from, and provided compensation to participants.
A total of 24% of participants reported asthma, 13% reported wheeze, and 20% reported shortness of breath. Among 1,414 respondents who reported e-cigarette use in the past 30 days, 15% also said they had used cigarettes, and 37% said they had used cannabis.
After controlling for age, birth sex, and race/ethnicity, compared with self-reported never e-cigarette users, there was an association between past 30-day e-cigarette use and self-reported asthma (odds ratio, 1.4; 95% CI, 1.1-1.7), wheeze (OR, 3.1; 95% CI, 2.3-4.2), and shortness of breath (OR, 2.9; 95% CI, 2.3-3.6). After the researchers controlled for past 30-day cigarette cannabis use, the association with asthma was no longer statistically significant (OR, 1.11; 95% CI, 0.87-1.41), but the association with wheeze (OR, 2.3; 95% CI, 1.6-3.0) and shortness of breath (OR, 2.1; 95% CI, 1.6-2.8) remained.
Dr. Tackett noted that wheeze and shortness of breath are only two indicators of respiratory health, and more research needs to be done. Her team is conducting follow-up studies using objective measurement tools such as home-based spirometry in adolescents and young adults who exclusively use e-cigarettes and who have never used e-cigarettes.
“We need to better understand the complex relationships between use of these products and whether multiple product use is associated with worse respiratory outcomes,” said Dr. Tackett.
Dr. Pascoe and Dr. Tackett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of e-cigarettes is linked to a higher frequency of self-reported wheezing and shortness of breath in adolescents and young adults, according to an online survey. The association was present even after controlling for cigarette and cannabis use.
Previous studies of adolescents and young adults have shown associations between e-cigarette use and wheeze, shortness of breath, and asthma. The Youth Risk Behavior Surveillance (YRBS) survey by the Centers for Disease Control and Prevention and other health agencies, conducted from 2015 to 2017, found that 63.5% of youth who used e-cigarettes also used some combination of cigarettes and cannabis. Combined use was associated with a 55%-65% increased odds of self-reported asthma.
The Population Assessment of Tobacco and Health (PATH) study, which was published in October 2020, had similar findings, though it did not find an association between e-cigarette use alone and wheezing.
“The findings from the current study highlight that we need to keep asking young people about respiratory symptoms, couse of other tobacco products, as well as cannabis use. As more products, including cannabis and various e-cigarette devices, enter the market, assessing respiratory health will be important both where adolescents and young adults receive their health care and in research,” Alayna Tackett, PhD, said in an interview. Dr. Tackett presented the study at the American Thoracic Society’s virtual international conference. She is an assistant professor of preventive medicine at the University of Southern California, Los Angeles.
“I found [the study] very interesting because it seems to be identifying a physiologic response to these e-cigarettes,” said Christopher Pascoe, MD, who was asked to comment. “And they were so young [age 14-21 years]. The fact that these symptoms of wheezing and shortness of breath are coming from people who are this young suggests that there may be chronic problems showing up later with continued use of these devices.”
Dr. Pascoe is an assistant professor of physiology and pathophysiology at the University of Manitoba, Winnipeg, where he also works with the Children’s Hospital Research Institute of Manitoba. His own research examines lung tissue harvested from pneumothorax surgeries in smokers and e-cigarette users to identify markers of inflammation.
He called the research a “good start” at unraveling the impacts of e-cigarettes and smoking, since some people use both products. “The fact that there was still a twofold increase in odds for wheezing, shortness of breath among people who use these e-cigarettes, but weren’t using cannabis and weren’t using cigarettes. I think it’s novel, and it suggests that there is an effect [of e-cigarettes alone].”
The study is based on a self-reported data, which is a significant limitation, especially considering that asthma is often overreported. “Self-report can be fraught with things, but I think it’s an interesting starting point for trying to recruit people who are just e-cigarette users and following them up further,” said Dr. Pascoe.
The researchers surveyed 2,931 individuals aged 14-21 years between Aug. 6 and Aug.30, 2020, with an average age of 18.9 years. Of the respondents, 80% were women and girls, and 75% were White. The high percentage of women and girls was unusual. Dr. Tackett provided no explanation for the atypical demographic but noted that the current study used convenience sampling.
The survey asked about use of e-cigarettes, cigarettes, and cannabis in the past 30 days, as well as asthma diagnosis and respiratory symptoms over the same period. The methodology employed survey management company Lucid, which recruited, collected data from, and provided compensation to participants.
A total of 24% of participants reported asthma, 13% reported wheeze, and 20% reported shortness of breath. Among 1,414 respondents who reported e-cigarette use in the past 30 days, 15% also said they had used cigarettes, and 37% said they had used cannabis.
After controlling for age, birth sex, and race/ethnicity, compared with self-reported never e-cigarette users, there was an association between past 30-day e-cigarette use and self-reported asthma (odds ratio, 1.4; 95% CI, 1.1-1.7), wheeze (OR, 3.1; 95% CI, 2.3-4.2), and shortness of breath (OR, 2.9; 95% CI, 2.3-3.6). After the researchers controlled for past 30-day cigarette cannabis use, the association with asthma was no longer statistically significant (OR, 1.11; 95% CI, 0.87-1.41), but the association with wheeze (OR, 2.3; 95% CI, 1.6-3.0) and shortness of breath (OR, 2.1; 95% CI, 1.6-2.8) remained.
Dr. Tackett noted that wheeze and shortness of breath are only two indicators of respiratory health, and more research needs to be done. Her team is conducting follow-up studies using objective measurement tools such as home-based spirometry in adolescents and young adults who exclusively use e-cigarettes and who have never used e-cigarettes.
“We need to better understand the complex relationships between use of these products and whether multiple product use is associated with worse respiratory outcomes,” said Dr. Tackett.
Dr. Pascoe and Dr. Tackett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of e-cigarettes is linked to a higher frequency of self-reported wheezing and shortness of breath in adolescents and young adults, according to an online survey. The association was present even after controlling for cigarette and cannabis use.
Previous studies of adolescents and young adults have shown associations between e-cigarette use and wheeze, shortness of breath, and asthma. The Youth Risk Behavior Surveillance (YRBS) survey by the Centers for Disease Control and Prevention and other health agencies, conducted from 2015 to 2017, found that 63.5% of youth who used e-cigarettes also used some combination of cigarettes and cannabis. Combined use was associated with a 55%-65% increased odds of self-reported asthma.
The Population Assessment of Tobacco and Health (PATH) study, which was published in October 2020, had similar findings, though it did not find an association between e-cigarette use alone and wheezing.
“The findings from the current study highlight that we need to keep asking young people about respiratory symptoms, couse of other tobacco products, as well as cannabis use. As more products, including cannabis and various e-cigarette devices, enter the market, assessing respiratory health will be important both where adolescents and young adults receive their health care and in research,” Alayna Tackett, PhD, said in an interview. Dr. Tackett presented the study at the American Thoracic Society’s virtual international conference. She is an assistant professor of preventive medicine at the University of Southern California, Los Angeles.
“I found [the study] very interesting because it seems to be identifying a physiologic response to these e-cigarettes,” said Christopher Pascoe, MD, who was asked to comment. “And they were so young [age 14-21 years]. The fact that these symptoms of wheezing and shortness of breath are coming from people who are this young suggests that there may be chronic problems showing up later with continued use of these devices.”
Dr. Pascoe is an assistant professor of physiology and pathophysiology at the University of Manitoba, Winnipeg, where he also works with the Children’s Hospital Research Institute of Manitoba. His own research examines lung tissue harvested from pneumothorax surgeries in smokers and e-cigarette users to identify markers of inflammation.
He called the research a “good start” at unraveling the impacts of e-cigarettes and smoking, since some people use both products. “The fact that there was still a twofold increase in odds for wheezing, shortness of breath among people who use these e-cigarettes, but weren’t using cannabis and weren’t using cigarettes. I think it’s novel, and it suggests that there is an effect [of e-cigarettes alone].”
The study is based on a self-reported data, which is a significant limitation, especially considering that asthma is often overreported. “Self-report can be fraught with things, but I think it’s an interesting starting point for trying to recruit people who are just e-cigarette users and following them up further,” said Dr. Pascoe.
The researchers surveyed 2,931 individuals aged 14-21 years between Aug. 6 and Aug.30, 2020, with an average age of 18.9 years. Of the respondents, 80% were women and girls, and 75% were White. The high percentage of women and girls was unusual. Dr. Tackett provided no explanation for the atypical demographic but noted that the current study used convenience sampling.
The survey asked about use of e-cigarettes, cigarettes, and cannabis in the past 30 days, as well as asthma diagnosis and respiratory symptoms over the same period. The methodology employed survey management company Lucid, which recruited, collected data from, and provided compensation to participants.
A total of 24% of participants reported asthma, 13% reported wheeze, and 20% reported shortness of breath. Among 1,414 respondents who reported e-cigarette use in the past 30 days, 15% also said they had used cigarettes, and 37% said they had used cannabis.
After controlling for age, birth sex, and race/ethnicity, compared with self-reported never e-cigarette users, there was an association between past 30-day e-cigarette use and self-reported asthma (odds ratio, 1.4; 95% CI, 1.1-1.7), wheeze (OR, 3.1; 95% CI, 2.3-4.2), and shortness of breath (OR, 2.9; 95% CI, 2.3-3.6). After the researchers controlled for past 30-day cigarette cannabis use, the association with asthma was no longer statistically significant (OR, 1.11; 95% CI, 0.87-1.41), but the association with wheeze (OR, 2.3; 95% CI, 1.6-3.0) and shortness of breath (OR, 2.1; 95% CI, 1.6-2.8) remained.
Dr. Tackett noted that wheeze and shortness of breath are only two indicators of respiratory health, and more research needs to be done. Her team is conducting follow-up studies using objective measurement tools such as home-based spirometry in adolescents and young adults who exclusively use e-cigarettes and who have never used e-cigarettes.
“We need to better understand the complex relationships between use of these products and whether multiple product use is associated with worse respiratory outcomes,” said Dr. Tackett.
Dr. Pascoe and Dr. Tackett disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Dapagliflozin misses as treatment for COVID-19 but leaves intriguing signal for benefit
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
FROM ACC 2021
Novel rehab program fights frailty, boosts capacity in advanced HF
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.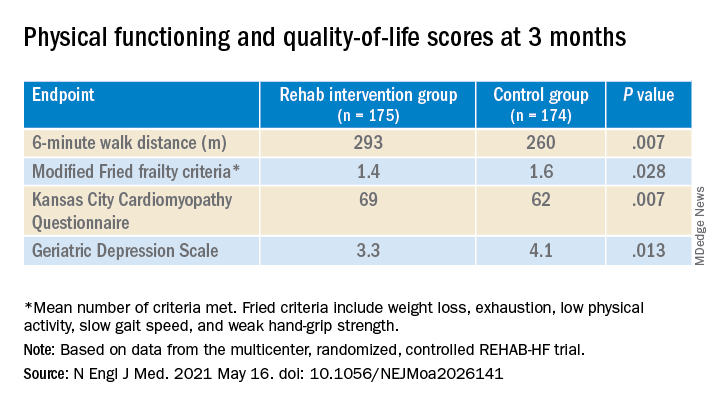
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
A novel physical rehabilitation program for patients with advanced heart failure that aimed to improve their ability to exercise before focusing on endurance was successful in a randomized trial in ways that seem to have eluded some earlier exercise-training studies in the setting of HF.
The often-frail patients following the training regimen, initiated before discharge from hospitalization for acute decompensation, worked on capabilities such as mobility, balance, and strength deemed necessary if exercises meant to build exercise capacity were to succeed.
A huge percentage stayed with the 12-week program, which featured personalized, one-on-one training from a physical therapist. The patients benefited, with improvements in balance, walking ability, and strength, which were followed by significant gains in 6-minute walk distance (6MWD) and measures of physical functioning, frailty, and quality of life. The patients then continued elements of the program at home out to 6 months.
At that time, death and rehospitalizations did not differ between those assigned to the regimen and similar patients who had not participated in the program, although the trial wasn’t powered for clinical events.
The rehab strategy seemed to work across a wide range of patient subgroups. In particular, there was evidence that the benefits were more pronounced in patients with HF and preserved ejection fraction (HFpEF) than in those with HF and reduced ejection fraction (HFrEF), observed Dalane W. Kitzman, MD, Wake Forest University, Winston-Salem, N.C.
Dr. Kitzman presented results from the REHAB-HF (Rehabilitation Therapy in Older Acute Heart Failure Patients) trial at the annual scientific sessions of the American College of Cardiology and is lead author on its same-day publication in the New England Journal of Medicine.
An earlier pilot program unexpectedly showed that such patients recently hospitalized with HF “have significant impairments in mobility and balance,” he explained. If so, “it would be hazardous to subject them to traditional endurance training, such as walking-based treadmill or even bicycle.”
The unusual program, said Dr. Kitzman, looks to those issues before engaging the patients in endurance exercise by addressing mobility, balance, and basic strength – enough to repeatedly stand up from a sitting position, for example. “If you’re not able to stand with confidence, then you’re not able to walk on a treadmill.”
This model of exercise rehab “is used in geriatrics research, and enables them to safely increase endurance. It’s well known from geriatric studies that if you go directly to endurance in these, frail, older patients, you have little improvement and often have injuries and falls,” he added.
Guidance from telemedicine?
The functional outcomes examined in REHAB-HF “are the ones that matter to patients the most,” observed Eileen M. Handberg, PhD, of Shands Hospital at the University of Florida, Gainesville, at a presentation on the trial for the media.
“This is about being able to get out of a chair without assistance, not falling, walking farther, and feeling better as opposed to the more traditional outcome measure that has been used in cardiac rehab trials, which has been the exercise treadmill test – which most patients don’t have the capacity to do very well anyway,” said Dr. Handberg, who is not a part of REHAB-HF.
“This opens up rehab, potentially, to the more sick, who also need a better quality of life,” she said.
However, many patients invited to participate in the trial could not because they lived too far from the program, Dr. Handberg observed. “It would be nice to see if the lessons from COVID-19 might apply to this population” by making participation possible remotely, “perhaps using family members as rehab assistance,” she said.
“I was really very impressed that you had 83% adherence to a home exercise 6 months down the road, which far eclipses what we had in HF-ACTION,” said Vera Bittner, MD, University of Alabama at Birmingham, as the invited discussant following Dr. Kitzman’s formal presentation of the trial. “And it certainly eclipses what we see in the typical cardiac rehab program.”
Both Dr. Bittner and Dr. Kitzman participated in HF-ACTION, a randomized exercise-training trial for patients with chronic, stable HFrEF who were all-around less sick than those in REHAB-HF.
Four functional domains
Historically, HF exercise or rehab trials have excluded patients hospitalized with acute decompensation, and third-party reimbursement often has not covered such programs because of a lack of supporting evidence and a supposed potential for harm, Dr. Kitzman said.
Entry to REHAB-HF required the patients to be fit enough to walk 4 meters, with or without a walker or other assistant device, and to have been in the hospital for at least 24 hours with a primary diagnosis of acute decompensated HF.
The intervention relied on exercises aimed at improving the four functional domains of strength, balance, mobility, and – when those three were sufficiently developed – endurance, Dr. Kitzman and associates wrote in their published report.
“The intervention was initiated in the hospital when feasible and was subsequently transitioned to an outpatient facility as soon as possible after discharge,” they wrote. Afterward, “a key goal of the intervention during the first 3 months [the outpatient phase] was to prepare the patient to transition to the independent maintenance phase (months 4-6).”
The study’s control patients “received frequent calls from study staff to try to approximate the increased attention received by the intervention group,” Dr. Kitzman said in an interview. “They were allowed to receive all usual care as ordered by their treating physicians. This included, if ordered, standard physical therapy or cardiac rehabilitation” in 43% of the control cohort. Of the trial’s 349 patients, those assigned to the intervention scored significantly higher on the three-component Short Physical Performance Battery (SPPB) at 12 weeks than those assigned to a usual care approach that included, for some, more conventional cardiac rehabilitation (8.3 vs. 6.9; P < .001).
The SPPB, validated in trials as a proxy for clinical outcomes includes tests of balance while standing, gait speed during a 4-minute walk, and strength. The latter is the test that measures time needed to rise from a chair five times.
They also showed consistent gains in other measures of physical functioning and quality of life by 12 weeks months.
The observed SPPB treatment effect is “impressive” and “compares very favorably with previously reported estimates,” observed an accompanying editorial from Stefan D. Anker, MD, PhD, of the German Center for Cardiovascular Research and Charité Universitätsmedizin, Berlin, and Andrew J.S. Coats, DM, of the University of Warwick, Coventry, England.
“Similarly, the between-group differences seen in 6-minute walk distance (34 m) and gait speed (0.12 m/s) are clinically meaningful and sizable.”
They propose that some of the substantial quality-of-life benefit in the intervention group “may be due to better physical performance, and that part may be due to improvements in psychosocial factors and mood. It appears that exercise also resulted in patients becoming happier, or at least less depressed, as evidenced by the positive results on the Geriatric Depression Scale.”
Similar results across most subgroups
In subgroup analyses, the intervention was successful against the standard-care approach in both men and women at all ages and regardless of ejection fraction; symptom status; and whether the patient had diabetes, ischemic heart disease, or atrial fibrillation, or was obese.
Clinical outcomes were not significantly different at 6 months. The rate of death from any cause was 13% for the intervention group and 10% for the control group. There were 194 and 213 hospitalizations from any cause, respectively.
Not included in the trial’s current publication but soon to be published, Dr. Kitzman said when interviewed, is a comparison of outcomes in patients with HFpEF and HFrEF. “We found at baseline that those with HFpEF had worse impairment in physical function, quality of life, and frailty. After the intervention, there appeared to be consistently larger improvements in all outcomes, including SPPB, 6-minute walk, qualify of life, and frailty, in HFpEF versus HFrEF.”
The signals of potential benefit in HFpEF extended to clinical endpoints, he said. In contrast to similar rates of all-cause rehospitalization in HFrEF, “in patients with HFpEF, rehospitalizations were 17% lower in the intervention group, compared to the control group.” Still, he noted, the interaction P value wasn’t significant.
However, Dr. Kitzman added, mortality in the intervention group, compared with the control group, was reduced by 35% among patients with HFpEF, “but was 250% higher in HFrEF,” with a significant interaction P value.
He was careful to note that, as a phase 2 trial, REHAB-HF was underpowered for clinical events, “and even the results in the HFpEF group should not be seen as adequate evidence to change clinical care.” They were from an exploratory analysis that included relatively few events.
“Because definitive demonstration of improvement in clinical events is critical for altering clinical care guidelines and for third-party payer reimbursement decisions, we believe that a subsequent phase 3 trial is needed and are currently planning toward that,” Dr. Kitzman said.
The study was supported by research grants from the National Institutes of Health, the Kermit Glenn Phillips II Chair in Cardiovascular Medicine, and the Oristano Family Fund at Wake Forest. Dr. Kitzman disclosed receiving consulting fees or honoraria from AbbVie, AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, CinRx, Corviamedical, GlaxoSmithKline, and Merck; and having an unspecified relationship with Gilead. Dr. Handberg disclosed receiving grants from Aastom Biosciences, Abbott Laboratories, Amgen, Amorcyte, AstraZeneca, Biocardia, Boehringer Ingelheim, Capricor, Cytori Therapeutics, Department of Defense, Direct Flow Medical, Everyfit, Gilead, Ionis, Medtronic, Merck, Mesoblast, Relypsa, and Sanofi-Aventis. Dr. Bittner discloses receiving consulting fees or honoraria from Pfizer and Sanofi; receiving research grants from Amgen and The Medicines Company; and having unspecified relationships with AstraZeneca, DalCor, Esperion, and Sanofi-Aventis. Dr. Anker reported receiving grants and personal fees from Abbott Vascular and Vifor; personal fees from Bayer, Boehringer Ingelheim, Novartis, Servier, Cardiac Dimensions, Thermo Fisher Scientific, AstraZeneca, Occlutech, Actimed, and Respicardia. Dr. Coats disclosed receiving personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, WL Gore, Impulse Dynamics, and Respicardia.
A version of this article first appeared on Medscape.com.
Omics analysis links blood type to COVID-19
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Checkpoint inhibitors earn spot in new ESMO SCLC guidelines
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
In new small cell lung cancer guidelines, the European Society of Medical Oncology calls for upfront atezolizumab or durvalumab in combination with four to six cycles of etoposide and a platinum for stage 4 disease.
The strong recommendation is based on two phase 3 trials that showed improved overall survival when the checkpoint inhibitors were added to standard chemotherapy. “With very similar results, and in the context of a severe unmet need, both trials justify the need for immunotherapy in the frontline setting” and established “new standards of care” for stage 4 disease, the group said. “Atezolizumab or durvalumab in combination with a platinum plus etoposide should be offered to all eligible chemotherapy-naive patients” with a performance status of 0-1, said the group led by Anne-Marie Dingemans, MD, PhD, a pulmonology professor at Maastricht (the Netherlands) University Medical Center.
Alessio Cortellini, MD, a consulting oncologist and visiting researcher at Imperial College London, said he strongly endorses the recommendation when asked for comment.
“The addition of a PD-L1 inhibitor to a platinum/etoposide backbone is the first strategy that has led to a significant benefit in terms of overall survival. After decades of disappointing results, the bar has” been raised, said Dr. Cortellini.
New inhibitor
EMSO also incorporated the new RNA polymerase II inhibitor lurbinectedin into their guidelines as an option for patients progressing on or after first-line platinum-based chemotherapy.
The agent was approved by the U.S. Food and Drug Administration in June 2020 for metastatic small cell lung cancer (SCLC) with disease progression on or after platinum-based chemotherapy.
The recommendations – more than 50 in all – are based on a literature review and expert opinion, and cover SCLC diagnosis, staging, treatment, and follow-up, with flowcharts outlining treatment pathways.
Atezolizumab earned the endorsement following the IMpower133 trial, which showed a median overall survival of 12.3 months for atezolizumab in combination with carboplatin and etoposide, versus 10.3 months on chemotherapy alone; 34% of the atezolizumab group was alive at 18 months versus 21% in the placebo arm.
The durvalumab recommendation is based on the CASPIAN trial, in which the addition of durvalumab to platinum plus etoposide improved median overall survival from 10.5 to 12.9 months; 32% of durvalumab patients were alive at 18 months versus 24.8% in the chemotherapy-alone arm.
ESMO said “it is important to stress that, in both trials,” patients were in good clinical condition with a median age in the early 60s, so relatively young for SCLC. Also, the modest benefits “clearly emphasize the need for” biomarkers that predict response in order to better select patients.
Immunotherapy has improved cancer treatment across many malignancies and continues to be actively investigated in SCLC, but so far only atezolizumab and durvalumab have phase 3 evidence of benefit.
Makers of the blockbuster checkpoint inhibitors nivolumab and pembrolizumab recently withdrew their FDA approval for stage 4 SCLC that’s progressed after platinum-based chemotherapy and at least one other line of therapy; phase 3 trials failed to confirm the modest survival benefit found in early studies.
Lurbinectedin earned its place in the guidelines based on a single-arm study with 105 relapsed patients that showed an overall response rate of 22.2% in platinum-resistant and 45% in platinum-sensitive patients, with a median overall survival of 9.3 months.
The jury is still out, however. A phase 3 trial of lurbinectedin plus doxorubicin versus topotecan or CAV [cyclophosphamide, adriamycin, and vincristine] for advanced recurrent disease failed to meet its endpoint of superior overall survival, according to a recent press release from the its maker.
“It might be a bit early to discuss” routine use of lurbinectedin, although having it available is good “since literally nothing works in the second line setting,” Dr. Cortellini said.
There was no external funding for the work. The authors had numerous ties to pharmaceutical companies, including Dr. Dingemans who reported adviser and speakers fees and/or research funding from Roche, Lilly, Bristol-Myers Squib, and others. Dr. Costellini reported speakers fees from Novartis, Astrazeneca, and Astellas and consultant payments from Bristol-Myers Squibb, Roche, MSD, and AstraZeneca.
FROM ANNALS OF ONCOLOGY
New STRENGTH analysis reignites debate on omega-3 CV benefits
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
Questions over the cardiovascular benefits shown in the REDUCE-IT trial with icosapent ethyl, a high-dose eicosapentaenoic acid (EPA) product, have been reignited with a new analysis from the STRENGTH trial showing no benefit of a high-dose combined omega-3 fatty acid product in patients who achieved the highest EPA levels and no harm in those with the highest levels of docosahexaenoic acid (DHA).
STRENGTH investigator Steven Nissen, MD, said these new results add to concerns about the positive result in the previously reported REDUCE-IT trial and suggest that “there is no strong evidence of a benefit of fish oil in preventing major cardiovascular events.”
But Dr. Nissen, who is chair of the department of cardiovascular medicine at the Cleveland Clinic in Ohio, pointed out evidence of harm, with both REDUCE-IT and STRENGTH showing an increase in atrial fibrillation with the high-dose omega-3 fatty acid products.
“Fish oils increase the risk of atrial fibrillation substantially, and there is no solid evidence that they help the heart in any way,” he stated.
The new STRENGTH analysis was presented at the annual scientific sessions of the American College of Cardiology. and was simultaneously published in JAMA Cardiology.
The REDUCE-IT trial showed a large 25% relative-risk reduction in cardiovascular events in patients taking icosapent ethyl (Vascepa, Amarin), a high-dose purified formulation of EPA, compared with patients taking a mineral oil placebo. But a similar trial, STRENGTH, showed no effect of a similar high dose of the mixed EPA/DHA product (Epanova, AstraZeneca), compared with a corn oil placebo.
The different results from these two studies have led to many questions about how the benefits seen in REDUCE-IT were brought about, and why they weren’t replicated in the STRENGTH study.
Dr. Nissen noted that several hypotheses have been proposed. These include a potential adverse effect of the mineral oil placebo in the REDUCE-IT trial, which may have elevated risk in the placebo treatment group and led to a false-positive result for icosapent ethyl. Another possibility is that the moderately higher plasma levels of EPA achieved in REDUCE-IT were responsible for the observed benefits or that the coadministration of DHA in STRENGTH may have counteracted the potential beneficial effects of EPA.
The current post hoc analysis of STRENGTH was conducted to address these latter two possibilities. It aimed to assess the association between cardiovascular outcomes and achieved levels of EPA, DHA, or changes in levels of these fatty acids.
“In our new analysis, among patients treated with fish oil, we found no evidence that EPA is beneficial or that DHA is harmful,” Dr. Nissen said.
Results of the new analysis showed an absence of a benefit from achieving high levels of EPA or harm from achieving high levels of DHA which, the authors say, “strengthens the concerns that the choice of comparator may have influenced the divergent results observed in the two trials.”
“Unlike corn oil, which is inert, mineral oil has major adverse effects, increasing LDL by 10.9% and CRP [C-reactive protein] by 32% in the REDUCE-IT trial,” Dr. Nissen said. “If you give a toxic placebo, then the active drug may falsely look really good.”
The STRENGTH trial randomly assigned 13,078 individuals at high risk for major cardiovascular events to receive 4 g daily of the EPA/DHA combined product (omega-3 carboxylic acid) or corn oil as the placebo. Main results, reported previously, showed no difference between the two groups in terms of the primary outcome – a composite of cardiovascular death, myocardial infarction, stroke, coronary revascularization, or unstable angina requiring hospitalization.
The current analysis, in 10,382 patients with available omega-3 fatty acid levels, looked at event rates according to tertiles of achieved EPA and DHA levels. The median plasma EPA level for patients taking the omega-3 product was 89 mcg/mL, with the top tertile achieving levels of 151 mcg/mL (a 443% increase). Dr. Nissen pointed out that this was higher than the median level of EPA reported in the REDUCE-IT trial (144 mcg/mL).
The median level of DHA was 91 mcg/mL, rising to 118 mcg/mL (a 68% increase) in the top tertile in the STRENGTH analysis.
Results showed no difference in the occurrence of the prespecified primary outcome among patients treated with omega-3 carboxylic acid who were in the top tertile of achieved EPA levels at 1 year (event rate, 11.3%), compared with patients treated with corn oil (11.0%), a nonsignificant difference (hazard ratio, 0.98; P = .81).
For DHA, patients in the top tertile of achieved DHA levels had an event rate of 11.4% vs. 11.0% in the corn oil group, also a nonsignificant difference (HR, 1.02; P = .85)
Sensitivity analyses based on the highest tertile of change in EPA or DHA levels showed similarly neutral results.
Because plasma levels may not reflect tissue levels of EPA or DHA, additional analyses assessed red blood cell EPA and DHA levels, neither of which showed any evidence of benefit or harm.
“These findings suggest that supplementation of omega-3 fatty acids in high-risk cardiovascular patients is neutral even at the highest achieved levels,” Dr. Nissen said. “And, in the context of increased risk of atrial fibrillation in omega-3 trials, they cast uncertainty over whether there is net benefit or harm with any omega-3 preparation,” he concluded.
He suggested that the choice of placebo comparator may play an important role in determining outcome for trials of omega-3 products, adding that further research is needed with trials specifically designed to compare corn oil with mineral oil and compare purified EPA with other formulations of omega-3 fatty acids.
At an press conference, Dr. Nissen said he could not recommend use of omega-3 fatty acid products for cardiovascular risk reduction given the uncertainty over the benefit in REDUCE-IT.
“We need replication, and the problem is STRENGTH did not replicate REDUCE-IT,” he stated.
REDUCE-IT investigator responds
The discussant of the STRENGTH analysis at the ACC presentation, Deepak L. Bhatt, MD, who was lead investigator of the REDUCE-IT trial, suggested that one conclusion could be that “an absence of a relationship in a negative trial doesn’t tell us that much other than that specific drug doesn’t work.”
Dr. Bhatt, who is executive director of interventional cardiovascular programs at Brigham and Women’s Hospital Heart & Vascular Center, Boston, said in an interview that comparisons should not be made between different trials using different products.
“I commend the STRENGTH investigators on a well-conducted trial that provided a definitive answer about the specific drug they studied, finding no benefit. But in a completely negative trial, I wouldn’t necessarily expect to see a relationship between any biomarker and outcome,” he said.
“With respect to icosapent ethyl (pure EPA), every cardiovascular trial to date has been positive: REDUCE-IT (randomized, placebo-controlled), JELIS (randomized, no placebo), EVAPORATE (randomized, placebo-controlled), CHERRY (randomized, no placebo), and some smaller ones,” Dr. Bhatt added. “Both REDUCE-IT and JELIS found associations between higher levels of EPA and lower rates of cardiovascular events, suggesting that higher EPA levels attained specifically with icosapent ethyl are beneficial.”
Pointing out that all the glucagonlike peptide–1 agonists lower glucose, for example, but not all reduce cardiovascular events, Dr. Bhatt said it was best to focus on clinical trial results and not overly focus on biomarker changes.
“Yes, the drug in STRENGTH raised EPA (and raised DHA, as well as lowering triglycerides), but the drug in REDUCE-IT and JELIS raised EPA much more, without raising DHA – and more importantly, the increase in EPA was via a totally different drug, with many different properties,” he added.
In his discussion of the study at the ACC presentation, Dr. Bhatt pointed out that in the STRENGTH trial overall there was no reduction in major adverse cardiovascular events despite a 19% reduction in triglycerides, which he said was a “very interesting disconnect.” He asked Dr. Nissen what he thought the reason was for the observation in this analysis of no relationship between EPA or DHA level and triglyceride reduction.
Dr. Nissen said that was an interesting point. “When we look at the two trials, they both reduced triglyceride levels by an almost identical amount, 19%, but we don’t see a relationship with that and EPA levels achieved.” He suggested this may be because of different threshold levels.
Dr. Bhatt also noted that high-intensity statin use was lower in the patients with higher EPA levels in the STRENGTH analysis, but Dr. Nissen countered: “I don’t think that was enough of a difference to explain the lack of an effect.”
Invited commentator on the new analysis at an ACC press conference, Eileen Handberg, PhD, said it was important to try to understand the reasons behind the different results of the STRENGTH and REDUCE-IT trials. “These new findings are important because they explain potentially why these outcomes are different,” she stated.
Dr. Handberg, who is professor of medicine at the University of Florida, Gainesville, said she hoped the additional research called for by Dr. Nissen would go ahead as a head-to-head study of the two omega-3 products or of the two different placebo oils.
The STRENGTH trial was sponsored by Astra Zeneca. Dr. Nissen reports research grants from AbbVie, Amgen, Astra Zeneca, Eli Lilly, Esperion Therapeutics, MEDTRONIC, MyoKardia, Novartis, Novo Nordisk, Pfizer, and Silence Therapeutics. Dr. Bhatt reports constant fees/honoraria from CellProthera, Elsevier Practice Update Cardiology, K2P, Level Ex, Medtelligence, MJH Life Sciences, and WebMD; data safety monitoring board activities with Contego; other roles with TobeSoft, Belvoir Publications, Cardax, Cereno Scientific, Clinical Cardiology, Elsevier, HMP Global, Janssen Pharmaceuticals, Journal of Invasive Cardiology, Medscape Cardiology, Merck, MyoKardia, Novo Nordisk, PhaseBio, PLx Pharma, Regado Biosciences, and Slack Publications/Cardiology Research Foundation; and research grants from Abbott, Afimmune, Amarin, Amgen, Astra Zeneca, Bayer Healthcare Pharmaceuticals, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, Cardax, Chiesi, Eisai, Eli Lilly, Ethicon, FlowCo, Forest Laboratories, Fractyl, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Lexicon, MEDTRONIC, MyoKardia, Owkin, Pfizer, PhaseBio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, Takeda, and The Medicines Company.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
LAAOS III: Surgical LAA closure cuts AFib stroke risk by one third
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
Left atrial appendage occlusion performed at the time of other heart surgery reduces the risk for stroke by about one-third in high-risk patients with atrial fibrillation (AFib), according to results of the Left Atrial Appendage Occlusion Study III (LAAOS III).
At 3.8 years’ follow-up, the primary endpoint of ischemic stroke or systemic embolism occurred in 4.8% of patients randomly assigned to left atrial appendage occlusion (LAAO) and 7.0% of those with no occlusion. This translated into a 33% relative risk reduction (hazard ratio, 0.67; 95% confidence interval, 0.53-0.85; P = .001).
In a landmark analysis, the effect was present early on but was more pronounced after the first 30 days, reducing the relative risk by 42% (HR, 0.58; 95% CI, 0.42-0.80), the researchers report.
The reduction in ongoing stroke risk was on top of oral anticoagulation (OAC) and consistent across all subgroups, Richard Whitlock, MD, PhD, professor of surgery, McMaster University, Hamilton, Ont., reported in a late-breaking trial session at the annual scientific sessions of the American College of Cardiology.
The procedure was safe and added, on average, just 6 minutes to cardiopulmonary bypass time, according to the results, simultaneously published in the New England Journal of Medicine.
“Any patient who comes to the operating room who fits the profile of a LAAOS III patient – so has atrial fibrillation and an elevated stroke risk based on their CHA2DS2-VASc score – the appendage should come off,” he said in an interview.
Commenting during the formal discussion, panelist Michael J. Mack, MD, of Baylor Health Care System in Houston, said, “This is potentially a game-changing, practice-changing study” but asked if there are any patients who shouldn’t undergo LAAO, such as those with heart failure (HF).
Dr. Whitlock said about 10%-15% of patients coming for heart surgery have a history of AFib and “as surgeons, you do need to individualize therapy. If you have a very frail patient, have concerns about tissue quality, you really need to think about how you would occlude the left atrial appendage or if you would occlude.”
Reassuringly, he noted, the data show no increase in HF hospitalizations and a beneficial effect on stroke among patients with HF and those with low ejection fractions, below 50%.
Observational data on surgical occlusion have been inconsistent, and current guidelines offer a weak recommendation in patients with AFib who have a contraindication to long-term anticoagulation. This is the first study to definitively prove that ischemic stroke is reduced by managing the left atrial appendage, he said in an interview.
“The previous percutaneous trials failed to demonstrate that; they demonstrated noninferiority but it was driven primarily by the avoidance of hemorrhagic events or strokes through taking patients off oral anticoagulation,” he said.
The results should translate into a class I guideline recommendation, he added. “This opens up a new paradigm of treatment for atrial fibrillation and stroke prevention in that it is really the first study that has looked at the additive effects of managing the left atrial appendage in addition to oral anticoagulation, and it’s protective on top of oral anticoagulation. That is a paradigm shift.”
In an accompanying editorial, Richard L. Page, MD, University of Vermont in Burlington, said the trial provides no insight on the possible benefit of surgical occlusion in patients unable to receive anticoagulation or with a lower CHA2DS2-VASc score, but he agreed a class I recommendation is likely for the population studied.
“I hope and anticipate that the results of this paper will strengthen the guideline indications for surgical left atrial appendage occlusion and will increase the number of cardiac surgeons who routinely perform this add-on procedure,” he said. “While many already perform this procedure, cardiac surgeons should now feel more comfortable that surgical left atrial appendage occlusion is indicated and supported by high-quality randomized data.”
Unfortunately, LAAOS III does not answer the question of whether patients can come off anticoagulation, but it does show surgical occlusion provides added protection from strokes, which can be huge with atrial fibrillation, Dr. Whitlock said.
“I spoke with a patient today who is an active 66-year-old individual on a [direct oral anticoagulant], and his stroke risk has been further reduced by 30%-40%, so he was ecstatic to hear the results,” Dr. Whitlock said. “I think it’s peace of mind.”
Global, nonindustry effort
LAAOS III investigators at 105 centers in 27 countries enrolled 4,811 patients undergoing cardiac surgery (mean age, 71 years; 68% male) who had a CHA2DS2-VASc score of at least 2.
In all, 4,770 were randomly assigned to no LAAO or occlusion via the preferred technique of amputation with suture closure of the stump as well as stapler occlusion, or epicardial device closure with the AtriClip (AtriCure) or TigerPaw (Maquet Medical). The treating team, researchers, and patients were blinded to assignment.
Patients were followed every 6 months with a validated stroke questionnaire. The trial was stopped early by the data safety monitoring board after the second interim analysis.
The mean CHA2DS2-VASc score was 4.2, one-third of patients had permanent AFib, 9% had a history of stroke, and more than two-thirds underwent a valve procedure, which makes LAAOS III unique, as many previous trials excluded valvular AFib, Dr. Whitlock pointed out.
Operative outcomes in the LAAO and no-LAAO groups were as follows:
- Bypass time: mean, 119 minutes vs. 113 minutes.
- Cross-clamp time: mean, 86 minutes vs. 82 minutes.
- Chest tube output: median, 520 mL vs. 500 mL.
- Reoperation for bleeding: both, 4.0%.
- Prolonged hospitalization due to HF: 5 vs. 14 events.
- 30-day mortality: 3.7% vs 4.0%.
The primary safety outcome of HF hospitalization at 3.8 years occurred in 7.7% of patients with LAAO and 6.8% without occlusion (HR, 1.13; 95% CI, 0.92-1.40), despite concerns that taking off the appendage could worsen HF risk by impairing renal clearance of salt and water.
“There’s observational data on either side of the fence, so it was an important endpoint that people were concerned about,” Dr. Whitlock told this news organization. “We had a data collection firm dedicated to admission for heart failure to really tease that out and, in the end, we saw no adverse effect.”
Although rates of ischemic stroke at 3.8 years were lower with LAAO than without (4.2% vs. 6.6%; HR, 0.62; 95% CI, 0.48-0.80), there was no difference in systemic embolism (0.3% for both) or death (22.6% vs. 22.5%).
In LAAOS III, fewer than 2% of the deaths were attributed to stroke, which is consistent with large stroke registries, Dr. Whitlock said. “Stroke is not what causes people with atrial fibrillation to die; it’s actually the progression on to heart failure.”
The positive effect on stroke was consistent across all subgroups, including sex, age, rheumatic heart disease, type of OAC at baseline, CHA2DS2-VASc score (≤4 vs. >4), type of surgery, history of heart failure or hypertension, and prior stroke/transient ischemic attack/systemic embolism.
Panelist Anne B. Curtis, MD, State University of New York at Buffalo, expressed surprise that about half of patients at baseline were not receiving anticoagulation and questioned whether event rates varied among those who did and didn’t stay on OAC.
Dr. Whitlock noted that OAC is often underused in AFib and that analyses showed the effects were consistent whether patients were on or off anticoagulants.
The study was sponsored by the Population Health Research Institute, McMaster University. Dr. Whitlock reported no relevant disclosures. Dr. Curtis reported consultant fees/honoraria from Abbott, Janssen, Medtronic, Milestone Pharmaceuticals, and Sanofi Aventis, and data safety monitoring board participation for Medtronic.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
ADAPTABLE: Low-dose aspirin as good as high-dose in CHD?
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
No significant difference in cardiovascular events or major bleeding was shown between patients with established coronary heart disease assigned to a daily aspirin dose of 81 mg and those receiving a dose of 325 mg in the 15,000-patient ADAPTABLE trial.
Although substantial dose switching occurred in the trial, particularly from the higher to the lower dose, lead investigator W. Schuyler Jones, MD, believes the results support the use of the 81-mg dose in most patients.
“While we would have liked to see higher adherence to the assigned doses, we think the results of the trial are reliable,” Dr. Jones said in an interview.
The real-world, open-label, pragmatic trial also involved an innovative low-cost design allowing researchers to identify and communicate with eligible patients directly, opening up a new cost-effective method to conduct clinical research going forward.
Dr. Jones, a cardiologist and associate professor of medicine at Duke University Medical Center, Durham, N.C., presented the ADAPTABLE results at the annual scientific sessions of the American College of Cardiology. They were simultaneously published online in the New England Journal of Medicine.
He noted there were mixed signals in the results. “For example, the main intent-to-treat analysis showed a trend to a lower rate of all-cause death in the 81-mg group, but the subgroup of patients who stayed on the 325-mg dose throughout the study had a lower event rate. But overall, there was no difference.”
Dr. Jones said the investigators had the following take-home messages to patients: “If a patient is already taking 81 mg, staying on this dose is probably right given the similar study results for the primary endpoint and that we didn’t find conclusive evidence that 325 mg is better. But for patients who have tolerated 325 mg long term, then they may want to stay on this dose as it may be associated with moderate benefit.”
Dr. Jones pointed out that, overall, patients who switched doses tended to do worse, but he suggested this may have been more to do with underlying reasons for switching rather than the different dose itself. “For example, switching often happens after bleeding or bruising, which can also often preempt an ischemic event, and other illnesses, such as cancer or atrial fibrillation, can also lead patients to change doses.”
“With the caveat that this trial did not include new patients (the vast majority of patients had been taking aspirin previously) the results support the approach of starting new patients on 81 mg, which is what we have been seeing in evolving clinical practice in recent years,” he added.
Dr. Jones explained that the trial set out to answer the simple but important question about the best dose of aspirin in patients with heart disease.
“Aspirin has been established as an appropriate long-term medication for patients with ischemic heart disease since the 1980s, but we really don’t have any good information on the correct dose.
He noted that the U.S. guidelines suggest any dose in the range of 81 mg to 325 mg daily can be used, whereas the European guidelines recommend 81 mg daily, although this is mainly based on observational data and expert opinion; there is little hard, randomized-trial evidence.
The ADAPTABLE trial randomly assigned 15,076 patients with established heart disease to receive 81 mg or 325 mg of aspirin. Before randomization, 96% of those with available information were already taking aspirin, 85% of whom were taking 81 mg.
After a mean follow-up of 26 months, the primary efficacy endpoint – a composite of all-cause death, myocardial infarction, or stroke – had occurred in 7.28% of the 81-mg group and 7.51% of the 325-mg group (hazard ratio, 1.02; 95% confidence interval, 0.91-1.14).
The main safety endpoint, hospitalization for major bleeding with an associated blood transfusion, occurred in 0.63% of the 81-mg group and 0.60% of the 325-mg group (HR, 1.18; 95% CI, 0.79-1.77).
“The bleeding safety endpoint looked similar, which may be counterintuitive to what may have been expected,” Dr. Jones commented. “However, the safety endpoint was very stringent. We still haven’t analyzed all the less serious ADR [adverse drug event]/bleeding data, but overall, it does appear to be balanced.”
He added: “Most cardiologists probably may not have expected to see much difference in efficacy between these two doses but would maybe have anticipated a lower bleeding rate with the low dose. I was a little surprised to see such a low bleeding rate in the 325-mg group.”
Patients assigned to 325 mg had a higher incidence of dose switching (41.6%) than those assigned to 81 mg (7.1%) and were more likely to discontinue treatment (11.1% vs. 7.0%). This resulted in fewer median days of exposure to the assigned dose in the 325-mg group (434 vs. 650 days).
“This was an open-label study, and such studies always suffer from a degree of infidelity to the assigned treatment group,” Dr. Jones said. “In ADAPTABLE, this was unbalanced in that a much greater number of patients switched from 325 mg to 81 mg than the other way round.”
“But our results do reflect what happens in normal life,” he added. “People behaved in the study like they do in the real world. They sometimes changed their dose and sometimes stopped taking aspirin altogether. So, I think the results are an accurate representation of the real world.”
A sensitivity analysis based on which dose the patient actually reported taking showed a higher risk for death, MI, or stroke in patients who took 81 mg than those who took 325 mg (HR, 1.25; 95% CI, 1.10-1.43). But as with any postrandomization analysis, this approach has many inherent biases, Dr. Jones cautioned.
Innovative study design
The ADAPTABLE study used an innovative low-cost design, which involved direct communication with the patients themselves.
Using the National Patient-Centered Clinical Research Network (PCORnet), a group of 40 U.S. centers committed to compiling data in a common format, invitations to enroll in the study were sent to eligible patients identified from medical records. Consent and randomization took place on the patient web portal.
Participants then purchased aspirin at the assigned dose themselves, and all follow-up was done virtually or on the phone, with outcomes ascertained remotely (from patient reports, electronic medical records, and insurance claims) without adjudication.
“This is a pretty neat way to do clinical research, enabling us to conduct a 15,000-patient trial on a very tight budget,” Dr. Jones commented.
He estimated that the trial cost around $18 to $19 million. “No industry funder would have sponsored such a study of aspirin, and a typical trial with this many patients conducted in the traditional way would have cost at least 5 or 10 times more,” he said.
“This is the first time this type of study has been done in the U.S. on such a large scale, and it opens up this method for future research.”
He explained that this design, communicating directly with patients, somewhat limits the questions that can be addressed. “As aspirin is purchased over the counter by patients themselves, this is a question that lent itself to be answered in this way.”
Another innovative design feature was the inclusion of “patient partners,” with one patient nominated by each center to be part of the organization of the trial. “This helped keep the research relevant to what patients care about.
They also helped with the recruitment strategy and communication with participants. I think this is something we need to continue and prioritize in clinical research going forward,” Dr. Jones noted.
‘Pioneering’ trial
Discussants of the study at the ACC presentation congratulated the investigators on conducting such an innovative trial.
Donald Lloyd-Jones, MD, chair of preventive medicine at Northwestern University, Chicago, said, “This is really a pioneering large pragmatic trial, and we’re going to need to see more of these over the next few years. The most important legacy from this trial for me is that you did it, and that you showed us many of the promises and some of the pitfalls of these large pragmatic designs.”
Akshay Desai, MD, associate professor of medicine, Harvard Medical School, Boston, added: “This was an innovative approach to answering an important question for daily clinical practice.”
On the results of the study, Dr. Lloyd-Jones said, “Maybe the outcomes were not too surprising, and I certainly endorse your cautious status quo statement about patients staying on the dose that they are on.”
But he suggested that the bleeding safety outcomes were perhaps a little unexpected, being a little lower in the lower-dose group, and he asked whether there was a sensitivity analysis looking at bleeding on a per protocol basis. Dr. Jones answered that this was planned.
Dr. Desai also raised questions about the “unusual bleeding endpoint,” noting that the rates of bleeding were far lower than would be expected, compared with other clinical trials.
Dr. Jones replied that the bleeding endpoint with blood product transfusion was chosen to allow the researchers to accurately identify these events in claims codes. He said the endpoint probably mirrored the GUSTO (Global Use of Strategies to Open Occluded Coronary Arteries) severe bleeding classification.
In an editorial accompanying the publication of ADAPTABLE, Colin Baigent, FMedSci, says the study provides proof of principle that large pragmatic randomized trials can be conducted in the United States.
But Dr. Baigent, who is professor of epidemiology and director of the Medical Research Council Population Health Research Unit at the University of Oxford (England), says that the high degree of switching between dosages that occurred during the trial gives rise to some uncertainty about the results.
“Because switching was not likely to have been at random, bias arising from this degree of crossover could have obscured a true difference in efficacy or safety (or both), and moreover it is also not possible to conclude that the lack of any significant difference between the two dose groups implies equivalence of the effects of the doses,” he writes.
He suggests that a pilot study may have identified a preference for the 81-mg dose and allowed methods to facilitate equipoise, such as a run-in period with both doses, and only patients adhering being considered for randomization.
But Dr. Baigent concludes that the ADAPTABLE trial is a “major achievement” in that it paves the way for low-cost randomized trials in the United States, which should allow many more clinical questions to be answered.
The trial was supported by an award from the Patient-Centred Outcomes Research Institute. Dr. Schuyler Jones reports consultant fees/honoraria from Bayer Healthcare and Janssen and research grants from Boehringer Ingelheim, Bristol Myers Squibb, and the Patient-Centered Outcomes Research Institute. Dr. Baigent reports grants from Boehringer Ingelheim, Medical Research Council, British Heart Foundation, and National Institute of Health Research, outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM ACC 2021
















