User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


FDA grants emergency authorization for Novavax COVID vaccine
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
on July 13.
The vaccine is authorized for adults only. Should the Centers for Disease Control and Prevention follow suit and approve its use, Novavax would join Moderna, Pfizer and Johnson & Johnson on the U.S. market. A CDC panel of advisors is expected to consider the new entry on July 19.
The Novavax vaccine is only for those who have not yet been vaccinated at all.
“Today’s authorization offers adults in the United States who have not yet received a COVID-19 vaccine another option that meets the FDA’s rigorous standards for safety, effectiveness and manufacturing quality needed to support emergency use authorization,” FDA Commissioner Robert Califf, MD, said in a statement. “COVID-19 vaccines remain the best preventive measure against severe disease caused by COVID-19 and I encourage anyone who is eligible for, but has not yet received a COVID-19 vaccine, to consider doing so.”
The Novavax vaccine is protein-based, making it different than mRNA vaccines from Pfizer and Moderna. It contains harmless elements of actual coronavirus spike protein and an ingredient known as a adjuvant that enhances the patient’s immune response.
Clinical trials found the vaccine to be 90.4% effective in preventing mild, moderate or severe COVID-19. Only 17 patients out of 17,200 developed COVID-19 after receiving both doses.
The FDA said, however, that Novavax’s vaccine did show evidence of increased risk of myocarditis – inflammation of the heart – and pericarditis, inflammation of tissue surrounding the heart. In most people both disorders began within 10 days.
A version of this article first appeared on WebMD.com.
Coming soon: More breathable, more comfortable face masks
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Sitting at his desk in Sea Girt, N.J., John Schwind is eager to demonstrate his ReadiMask 365. He holds up what looks like a white sheet of memo paper, peels off a protective liner, and sticks the mask first to his nose. He glides his fingers down his face, over his cheeks, and to his chin, sealing the mask and then demonstrating how easy it is to talk with it in place.
The mask’s medical adhesive sticks directly to the face, without causing breakouts, he said. It doesn’t let air leak and won’t fog his glasses. It’s strapless, so it won’t hurt his ears or make them stick out.
This fall, Mr. Schwind, the CEO of Global Safety First, is hoping to take home $150,000 as one of the two top winners of the federal Mask Innovation Challenge. He has made it to the top 10 but realizes he still has a ton of competition.
After the challenge launched in late 2021, nearly 1,500 submissions were received, says Kumiko Lippold, PhD, a health scientist and manager of the Mask Innovation Challenge. The challenge is run by Dr. Lippold and others at the Division of Research, Innovation, and Ventures (DRIVe), which is part of the Biomedical Advanced Research and Development Authority (BARDA) at the U.S. Department of Health & Human Services.
Like the rest of us, Dr. Lippold knows that masks desperately need a makeover. The aim is not only to get us through this pandemic but also future pandemics and other public health emergencies. “We are focused on building masks for the next pandemic, the next wildfires,” she says.
The project is a partnership among BARDA’s DRIVe, the National Institute for Occupational Safety and Health (NIOSH), and the National Institute of Standards and Technology (NIST).
While NIOSH is a partner in the challenge, giving feedback to mask developers, “the mask challenge is entirely separate from the NIOSH approval process,” Dr. Lippold says. Companies can then pursue NIOSH approval on their own, later, if they wish. The agency certifies only masks and respirators.
Preview of masks to come
“We’ve seen some really amazing things,” Dr. Lippold said of the new designs. She didn’t want to play favorites, so she gave an overview of innovations. Some designs have transparent materials, or partially see-through materials, so facial expressions can be read. “We’ve also seen really unique bio-based materials that are derived from natural products. We’ve seen sensors in some.”
One mask model has origami folds, which increase overall surface and breathing area. Some 3D-printed masks promise a custom fit and take into account whether a person’s nose bridge is low or high.
And the finalists are ...
ReadiMask 365: “I can wear this all day long,” Mr. Schwind said of his new design. It has a nano fiber filter and is flexible. Besides the one in the BARDA challenge, the company has other ReadiMasks on the market. “The most important thing is comfort,” he says. “Second is protection. If they don’t feel they have a good seal, users don’t have confidence in the mask.”
He offers various sizes of ReadiMasks, from small sizes designed for women with smaller faces to extra-large, “for NFL linemen.”
ClearMask: “We are the original clear mask,” says Aaron Hsu, CEO and co-founder of ClearMask in Baltimore. The company began in 2017, and the clear design was inspired by a company co-founder who is deaf. She was scheduled to have surgery, and her sign language interpreter did not show up, leaving her to try to communicate in the operating room with masked health care providers. There were no transparent masks available then, Mr. Hsu says.
“Being able to work with BARDA and getting their wisdom is invaluable,” he says.
The makers of ClearMask think masks are here to stay, at least for some. “I think a certain percentage of the population will continue to wear them, regardless,” said Mr. Hsu. He predicts health care settings will become stricter about wearing masks.
“Even now, when you even walk in to a hospital, you might be required to wear a mask,” he says, even as a visitor. His company’s masks are easy to adjust and are secured around the head, so your ears don’t get sore, he says.
4C Air: The BreSafe transparent mask is semi-transparent and is made of a nanomaterial that provides high levels of filtration and breathability with some transparency.
Air99: Based on origami principles, the Airgami mask is meant to improve fit, breathability, and aesthetics over existing masks. “Airgami fits better, works better and looks better,” says Min Xiao, a company spokesperson. “It won’t fall off the nose or collapse onto the mouth, and eyeglasses fog less, she says. Voices are less muffled.” It’s also reusable, rinseable and can be heat disinfected, she says. It went on the market in November 2020.
Air Flo Labs: Flo Mask Pro, like the company’s other designs, conducted over 100 3D facial scans across many ethnicities to produce a better fit, says Kevin Ngo, its creator. For the adult masks, two nose bridge sizes are offered. And users can choose a Pro Filter, with 99% filtration, or an Everyday, which is meant to be much more breathable than other masks. “Our silicone gasket is incredibly soft and gentle on the skin,” Mr. Ngo says. “In addition,we offer indents for glasses, which prevent any fogging.” The company began shipping in May; several thousand masks are in use now, Mr. Ngo said.
Georgetown University: This team’s smart mask is made of metallic foams that can be cleaned and reused.
Levi Strauss: The form of the mask can be made by any basic garment factory. It aims to activate the apparel supply chain as another source of low-cost, high-performance masks.
Matregenix: This mask, made of a transparent nanofiber, allows for easier communication while having high filtration.
SEAL Lab: The SINEW mask stands for Smart, Individualized, Near-Face, Extended Wear. The mask used technology to overcome flaws of traditional respirators, with the same degree of protection. It doesn’t make contact with the skin of the wearer’s face.
StaySafeNow: A team from Harvard University developed Crystal Guard, a reusable, cost-effective clear mask. Its developers say it’s meant to be especially useful for essential workers, teachers, and others who have to communicate to do their work.
Bye-bye N95?
“From our perspective, our goal with the mask challenge was not to replace the N95 respirator,” Dr. Lippold says. N95 masks, which NIOSH certifies, are valuable and protect people in high-risk settings. “With the mask challenge, our goal was really to provide the public with a comparable alternative that really meets their specific level of risk.” Working in a health care setting carries a different risk, she says, than going to the grocery store.
“A common complaint with the N95 is that they are very uncomfortable.” It’s a major barrier to compliance, “and we wanted to address that gap. We didn’t directly compare [the entries] to an N95,” she says, although their testing was similar to NIOSH’s. A number of finalists say they will pursue NIOSH approval, she says.
Meanwhile, some of the finalists’ masks are for sale. Air Flo Labs, for instance, has its Flo Mask Pro for sale online, noting that BARDA allowed it to release the test results from NIOSH and NIST.
Getting from 1,500 to 10
In the first phase of the challenge, Dr. Lippold says, “the goal was to engage as wide an audience as possible.” With the second phase, the bar was set a bit higher. Instead of just submitting ideas on paper, companies had to submit prototypes for lab testing. “We got about 80 submissions,” she says.
Those 80 were whittled down to 10 finalists. Teams had sent prototypes, and experts, including those from NIOSH and NIST, rated them, sometimes looking at multiple copies of the masks. Experts looked at how well the masks filtered the air, how breathable they were, and other data. Once the feedback was given to the mask companies, they entered a redesign period. “Scientists can take this data and basically make these prototypes better,” Dr. Lippold says.
The final round of testing will be in September, and the winners will be announced in the fall. The opportunity allowed companies to have their products go through testing they might not otherwise have been able to get, she says.
A version of this article first appeared on WebMD.com.
Hospital-acquired pneumonia is killing patients, yet there is a simple way to stop it
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Four years ago, when Dr. Karen Giuliano went to a Boston hospital for hip replacement surgery, she was given a pale-pink bucket of toiletries issued to patients in many hospitals. Inside were tissues, bar soap, deodorant, toothpaste, and, without a doubt, the worst toothbrush she’d ever seen.
“I couldn’t believe it. I got a toothbrush with no bristles,” she said. “It must have not gone through the bristle machine. It was just a stick.”
To most patients, a useless hospital toothbrush would be a mild inconvenience. But to Dr. Giuliano, a nursing professor at the University of Massachusetts, Amherst, it was a reminder of a pervasive “blind spot” in U.S. hospitals: the stunning consequences of unbrushed teeth.
Hospital patients not getting their teeth brushed, or not brushing their teeth themselves, is believed to be a leading cause of hundreds of thousands of cases of pneumonia a year in patients who have not been put on a ventilator. Pneumonia is among the most common infections that occur in health care facilities, and a majority of cases are nonventilator hospital-acquired pneumonia, or NVHAP, which kills up to 30% of those infected, Dr. Giuliano and other experts said.
But unlike many infections that strike within hospitals, the federal government doesn’t require hospitals to report cases of NVHAP. As a result, few hospitals understand the origin of the illness, track its occurrence, or actively work to prevent it, the experts said.
, according to a growing body of peer-reviewed research papers. Instead, many hospitals often skip teeth brushing to prioritize other tasks and provide only cheap, ineffective toothbrushes, often unaware of the consequences, said Dr. Dian Baker, a Sacramento (Calif.) State nursing professor who has spent more than a decade studying NVHAP.
“I’ll tell you that today the vast majority of the tens of thousands of nurses in hospitals have no idea that pneumonia comes from germs in the mouth,” Dr. Baker said.
Pneumonia occurs when germs trigger an infection in the lungs. Although NVHAP accounts for most of the cases that occur in hospitals, it historically has not received the same attention as pneumonia tied to ventilators, which is easier to identify and study because it occurs among a narrow subset of patients.
NVHAP, a risk for virtually all hospital patients, is often caused by bacteria from the mouth that gathers in the scummy biofilm on unbrushed teeth and is aspirated into the lungs. Patients face a higher risk if they lie flat or remain immobile for long periods, so NVHAP can also be prevented by elevating their heads and getting them out of bed more often.
According to the National Organization for NV-HAP Prevention, which was founded in 2020, this pneumonia infects about 1 in every 100 hospital patients and kills 15%-30% of them. For those who survive, the illness often extends their hospital stay by up to 15 days and makes it much more likely they will be readmitted within a month or transferred to an intensive care unit.
John McCleary, 83, of Millinocket, Maine, contracted a likely case of NVHAP in 2008 after he fractured his ankle in a fall and spent 12 days in rehabilitation at a hospital, said his daughter, Kathy Day, a retired nurse and advocate with the Patient Safety Action Network.
Mr. McCleary recovered from the fracture but not from pneumonia. Two days after he returned home, the infection in his lungs caused him to be rushed back to the hospital, where he went into sepsis and spent weeks in treatment before moving to an isolation unit in a nursing home.
He died weeks later, emaciated, largely deaf, unable to eat, and often “too weak to get water through a straw,” his daughter said. After contracting pneumonia, he never walked again.
“It was an astounding assault on his body, from him being here visiting me the week before his fall, to his death just a few months later,” Ms. Day said. “And the whole thing was avoidable.”
While experts describe NVHAP as a largely ignored threat, that appears to be changing.
Last year, a group of researchers – including Dr. Giuliano and Dr. Baker, plus officials from the Centers for Disease Control and Prevention, the Veterans Health Administration, and the Joint Commission – published a “call-to-action” research paper hoping to launch “a national health care conversation about NVHAP prevention.”
The Joint Commission, a nonprofit organization whose accreditation can make or break hospitals, is considering broadening the infection control standards to include more ailments, including NVHAP, said Sylvia Garcia-Houchins, its director of infection prevention and control.
Separately, ECRI, a nonprofit focused on health care safety, this year pinpointed NVHAP as one of its top patient safety concerns.
James Davis, an ECRI infection expert, said the prevalence of NVHAP, while already alarming, is likely “underestimated” and probably worsened as hospitals swelled with patients during the coronavirus pandemic.
“We only know what’s reported,” Mr. Davis said. “Could this be the tip of the iceberg? I would say, in my opinion, probably.”
To better measure the condition, some researchers call for a standardized surveillance definition of NVHAP, which could in time open the door for the federal government to mandate reporting of cases or incentivize prevention. With increasing urgency, researchers are pushing for hospitals not to wait for the federal government to act against NVHAP.
Dr. Baker said she has spoken with hundreds of hospitals about how to prevent NVHAP, but thousands more have yet to take up the cause.
“We are not asking for some big, $300,000 piece of equipment,” Dr. Baker said. “The two things that show the best evidence of preventing this harm are things that should be happening in standard care anyway – brushing teeth and getting patients mobilized.”
That evidence comes from a smattering of studies that show those two strategies can lead to sharp reductions in infection rates.
In California, a study at 21 Kaiser Permanente hospitals used a reprioritization of oral care and getting patients out of bed to reduce rates of hospital-acquired pneumonia by around 70%. At Sutter Medical Center in Sacramento, better oral care reduced NVHAP cases by a yearly average of 35%.
At Orlando Regional Medical Center in Florida, a medical unit and a surgical unit where patients received enhanced oral care reduced NVHAP rates by 85% and 56%, respectively, when compared with similar units that received normal care. A similar study is underway at two hospitals in Illinois.
And the most compelling results come from a veterans’ hospital in Salem, Va., where a 2016 oral care pilot program reduced rates of NVHAP by 92% – saving an estimated 13 lives in just 19 months. The program, the HAPPEN Initiative, has been expanded across the Veterans Health Administration, and experts say it could serve as a model for all U.S. hospitals.
Dr. Michelle Lucatorto, a nursing official who leads HAPPEN, said the program trains nurses to most effectively brush patients’ teeth and educates patients and families on the link between oral care and preventing NVHAP. While teeth brushing may not seem to require training, Dr. Lucatorto made comparisons to how the coronavirus revealed many Americans were doing a lackluster job of another routine hygienic practice: washing their hands.
“Sometimes we are searching for the most complicated intervention,” she said. “We are always looking for that new bypass surgery, or some new technical equipment. And sometimes I think we fail to look at the simple things we can do in our practice to save people’s lives.”
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Pulse oximeters lead to less oxygen supplementation for people of color
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
The new research suggests that skin color–related differences in pulse oximeter readings are in fact impacting clinical decision-making, lead author Eric R. Gottlieb, MD, of Brigham and Women’s Hospital and Massachusetts Institute of Technology, both in Boston, and colleagues wrote. This suggests that technology needs to updated to improve health equity, they continued, in their paper published in JAMA Internal Medicine.
“It has been known for decades that these readings are affected by various surface pigmentations, including nail polish and skin melanin, which may affect light absorption and scattering,” the investigators wrote. “This increases the risk of hidden hypoxemia [among patients with darker skin], in which patients have falsely elevated SpO2 readings, usually defined as 92% or greater, with a blood hemoglobin oxygen saturation less than 88%.”
Although published reports on this phenomenon date back to the 1980s, clinical significance has been largely discounted, they said, citing a 2008 paper on the topic, which stated that “oximetry need not have exact accuracy” to determine if a patient needs oxygen supplementation.
‘We’re not providing equal care’
Questioning the validity of this statement, Dr. Gottlieb and colleagues conducted a retrospective cohort study involving 3,069 patients admitted to intensive care at the Beth Israel Deaconess Medical Center in Boston between 2008 and 2019, thereby excluding patients treated during the COVID-19 pandemic. The population consisted of four races/ethnicities: White (87%), Black (7%), Hispanic (4%), and Asian (3%).
Aligning with previous studies, multivariable linear regression analyses showed that Asian, Black, and Hispanic patients had significantly higher SpO2 readings than White patients in relation to hemoglobin oxygen saturation values, suggesting falsely elevated readings.
Further modeling showed that these same patient groups also received lower oxygen delivery rates, which were not explained directly by race/ethnicity, but instead were mediated by the discrepancy between SpO2 and hemoglobin oxygen saturation values. In other words, physicians were responding consistently to pulse oximetry readings, rather than exhibiting a direct racial/ethnic bias in their clinical decision-making.
“We’re not providing equal care,” Dr. Gottlieb said in an interview. “It’s not that the patients are sicker, or have other socioeconomic explanations for why this happens to them. It’s us. It’s our technology. And that’s something that really has to be fixed.”
The investigators offered a cautionary view of corrective algorithms, as these “have exacerbated disparities and are subject to ethical concerns;” for example, with glomerular filtration rate estimations in Black patients.
Dr. Gottlieb also cautioned against action by individual physicians, who may now be inclined to change how they interpret pulse oximeter readings based on a patient’s race or ethnicity.
“I don’t think that we can expect physicians, every time they see a patient, to be second guessing whether the number basically reflects the truth,” he said.
Instead, Dr. Gottlieb suggested that the burden of change rests upon the shoulders of institutions, including hospitals and device manufacturers, both of which “really need to take the responsibility” for making sure that pulse oximeters are “equitable and have similar performance across races.”
While Dr. Gottlieb said that skin color likely plays the greatest role in measurement discrepancies, he encouraged stakeholders “to think broadly about this, and not just assume that it’s entirely skin color,” noting a small amount of evidence indicating that blood chemistry may also play a role. Still, he predicted that colorimetry – the direct measurement of skin color – will probably be incorporated into pulse oximeters of the future.
Black patients 3X more likely to have hidden hypoxia than White patients
Michael Sjoding, MD, of the University of Michigan, Ann Arbor, was one of the first to raise awareness of skin color–related issues with pulse oximeters during the throes of the COVID-19 pandemic. His study, which involved more than 10,000 patients, showed that Black patients were threefold more likely to have hidden hypoxia than White patients.
The present study shows that such discrepancies are indeed clinically significant, Dr. Sjoding said in an interview. And these data are needed, he added, to bring about change.
“What is being asked is potentially a big deal,” Dr. Sjoding said. “Pulse oximeters are everywhere, and it would be a big undertaking to redesign pulse oximeters and purchase new pulse oximeters. You need a compelling body of evidence to do that. I think it’s there now, clearly. So I’m hopeful that we’re going to finally move forward, towards having devices that we are confident work accurately in everyone.”
Why it has taken so long to gather this evidence, however, is a thornier topic, considering race-related discrepancies in pulse oximeter readings were first documented more than 3 decades ago.
“We sort of rediscovered something that had been known and had been described in the past,” Dr. Sjoding said. He explained how he and many of his colleagues had completed pulmonary fellowships, yet none of them knew of these potential issues with pulse oximeters until they began to observe differences in their own patients during the pandemic.
“I’ll give previous generations of researchers the benefit of the doubt,” Dr. Sjoding said, pointing out that techniques in data gathering and analysis have advanced considerably over the years. “The types of studies that were done before were very different than what we did.”
Yet Dr. Sjoding entertained the possibility that other factors may have been at play.
“I think definitely there’s a social commentary on prioritization of research,” he said.
The study was supported by grants from the National Institutes of Health. The investigators and Dr. Sjoding reported no conflicts of interest.
FROM JAMA INTERNAL MEDICINE
New KRAS inhibitor shows promise in NSCLC
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
In a phase 2 cohort study, who had previously been treated with platinum-based chemotherapy and immune therapy.
Adagrasib targets KRAS (G12C), which had long been thought undruggable until research published in 2013 revealed a new binding pocket that did not compete directly against the protein’s natural binding partner. The new trial further validates the approach. “It supports that clinically effective targeted therapies can be developed for patients with KRAS (G12C)–mutant NSCLC,” said Pasi Jänne, MD, PhD, who is the lead author of the study describing the new results published online in the New England Journal of Medicine.
KRAS is the most frequently mutated oncogene in human cancers. A mutated form is found in about 25% of NSCLCs. KRAS plays a key role in cell signaling governing growth, maturation, and cell death. The mutated form is linked to cancer growth and spread. Patients with mutated KRAS have few effective treatment options.
Adagrasib is currently under study and not yet approved by the Food and Drug Administration. However, sotorasib (Lumakras, Amgen), which also inhibits KRAS (G12C), was approved in May 2021 by the FDA for KRAS (G12C)–mutated NSCLC. There are some key differences between the drugs. Adagrasib has a half-life of 23 hours versus 5 hours for sotorasib, and the newer drug has the potential to penetrate the central nervous system. That could be an important consideration in NSCLC since it often metastasizes to the brain. “Having pharmacological approaches to treat brain metastases is a wonderful new therapeutic option for lung cancer patients,” said Dr. Jänne, who is director of the Lowe Center for Thoracic Oncology at Dana Farber Cancer Institute, Boston.
Adagrasib is being investigated as part of the KRYSTAL-1 study, alone and as part of combinations in various solid tumors. Previously treated NSCLC KRAS (G12C) patients are also being enrolled in a phase 3 study of adagrasib combined with docetaxel, as well as another phase 2 study of adagrasib combined with pembrolizumab as first-line therapy for NSCLC KRAS (G12C).
Adagrasib is likely to remain a second-line therapy following chemotherapy and immunotherapy. “The activity by itself at the moment is not sufficient to be a first-line treatment. That may change in the future in combination with a standard of care agent or in a subset of patients with KRAS (G12C)–mutant NSCLC, although no subset with higher efficacy has been identified to date. Identification of predictive biomarkers for patients likely to benefit from single agent or an adagrasib combination treatment remains a high priority,” Dr. Jänne said.
The study included 116 patients who had previously been treated with platinum-based chemotherapy and anti–programmed death 1 or programmed death–ligand 1 therapy. They received 600 mg oral adagrasib twice per day over a median follow-up period of 12.9 months. About 42.9% (95% confidence interval, 33.5%-52.6%) experienced a confirmed objective response with a median duration of 8.5 months (95% CI, 6.2-13.8 months). The median progression-free survival was 6.5 months (95% CI, 4.7-8.4 months). After a median follow-up of 15.6 months, the median overall survival was 12.6 months (95% CI, 9.2-19.2 months). The estimated overall survival at 1 year was 50.8% (95% CI, 40.9%-60.0%).
33 patients had stable central nervous system metastases that had been previously treated. About 33.3% had an intracranial confirmed objective response (95% CI, 18.0-51.8%) with a median duration of response of 11.2 months (95% CI, 2.99 months to not available).
Adverse events are similar to what is seen with other targeted therapies, according to Dr. Jänne. 97.4% of patient reported a treatment-related adverse event; 52.6% had grade 1-2 adverse events, and 44.8% had grade 3 adverse events. 6.9% discontinued the drug as a result.
Dr. Jänne has consulted for Mirati Therapeutics and is a member of its scientific advisory board. The study was funded by Mirati Therapeutics.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Children and COVID: Vaccination a harder sell in the summer
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.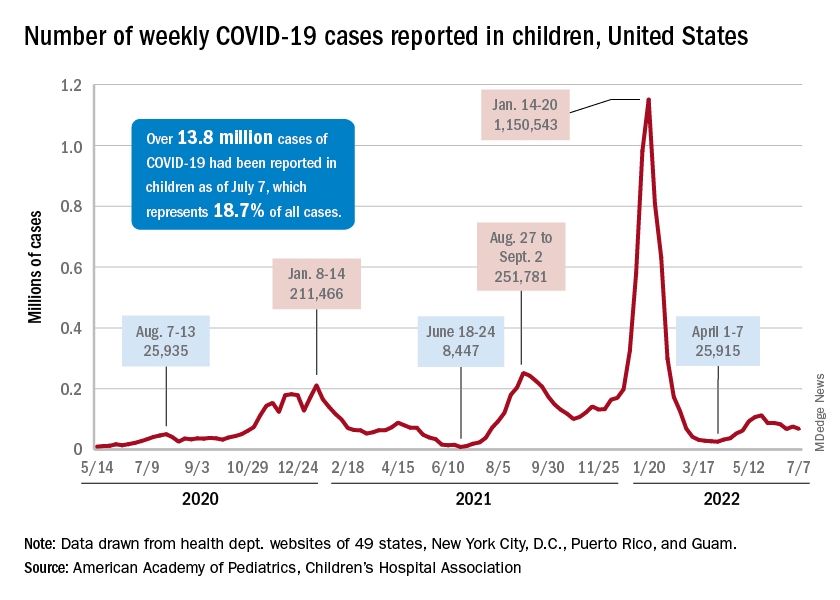
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.
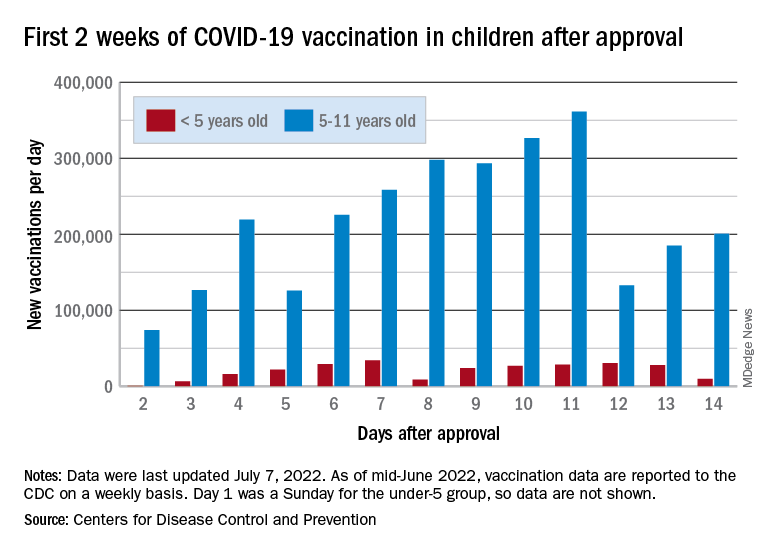
Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
The COVID-19 vaccination effort in the youngest children has begun much more slowly than the most recent rollout for older children, according to the Centers for Disease Control and Prevention.
in early November of 2021, based on CDC data last updated on July 7.
That approval, of course, came between the Delta and Omicron surges, when awareness was higher. The low initial uptake among those under age 5, however, was not unexpected by the Biden administration. “That number in and of itself is very much in line with our expectation, and we’re eager to continue working closely with partners to build on this start,” a senior administration official told ABC News.
With approval of the vaccine occurring after the school year was over, parents’ thoughts have been focused more on vacations and less on vaccinations. “Even before these vaccines officially became available, this was going to be a different rollout; it was going to take more time,” the official explained.
Incidence measures continue on different paths
New COVID-19 cases dropped during the latest reporting week (July 1-7), returning to the downward trend that began in late May and then stopped for 1 week (June 24-30), when cases were up by 12.4%, according to the American Academy of Pediatrics and the Children’s Hospital Association.

Children also represent a smaller share of cases, probably because of underreporting. “There has been a notable decline in the portion of reported weekly COVID-19 cases that are children,” the two groups said in their weekly COVID report. Although “cases are likely increasingly underreported for all age groups, this decline indicates that children are disproportionately undercounted in reported COVID-19 cases.”
Other measures, however, have been rising slowly but steadily since the spring. New admissions of patients aged 0-17 years with confirmed COVID, which were down to 0.13 per 100,000 population in early April, had climbed to 0.39 per 100,000 by July 7, the CDC said on its COVID Data Tracker.
Emergency department visits continue to show the same upward trend, despite a small decline in early June. A COVID diagnosis was involved in just 0.5% of ED visits in children aged 0-11 years on March 26, but by July 6 the rate was 4.7%. Increases were not as high among older children: From 0.3% on March 26 to 2.5% on July 6 for those aged 12-15 and from 0.3% to 2.4% for 16- and 17-year-olds, according to the CDC.
Transplanted pig hearts functioned normally in deceased persons on ventilator support
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.

Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.

Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
A team of surgeons successfully transplanted genetically engineered pig hearts into two recently deceased people whose bodies were being maintained on ventilatory support – not in the hope of restoring life, but as a proof-of-concept experiment in xenotransplantation that could eventually help to ease the critical shortage of donor organs.
The surgeries were performed on June 16 and July 6, 2022, using porcine hearts from animals genetically engineered to prevent organ rejection and promote adaptive immunity by human recipients
without utilizing unapproved devices or techniques or medications,” said Nader Moazami, MD, surgical director of heart transplantation and chief of the division of heart and lung transplantation and mechanical circulatory support at NYU Langone Health, New York.

Through 72 hours of postoperative monitoring “we evaluated the heart for functionality and the heart function was completely normal with excellent contractility,” he said at a press briefing announcing early results of the experimental program.
He acknowledged that for the first of the two procedures some surgical modification of the pig heart was required, primarily because of size differences between the donor and recipient.
“Nevertheless, we learned a tremendous amount from the first operation, and when that experience was translated into the second operation it even performed better,” he said.
Alex Reyentovich, MD, medical director of heart transplantation and director of the NYU Langone advanced heart failure program noted that “there are 6 million individuals with heart failure in the United States. About 100,000 of those individuals have end-stage heart failure, and we only do about 3,500 heart transplants a year in the United States, so we have a tremendous deficiency in organs, and there are many people dying waiting for a heart.”
Infection protocols
To date there has been only one xenotransplant of a genetically modified pig heart into a living human recipient, David Bennett Sr., age 57. The surgery, performed at the University of Maryland in January 2022, was initially successful, with the patient able to sit up in bed a few days after the procedure, and the heart performing like a “rock star” according to transplant surgeon Bartley Griffith, MD.
However, Mr. Bennett died 2 months after the procedure from compromise of the organ by an as yet undetermined cause, of which one may have been the heart's infection by porcine cytomegalovirus (CMV).

The NYU team, mindful of this potential setback, used more sensitive assays to screen the donor organs for porcine CMV, and implemented protocols to prevent and to monitor for potential zoonotic transmission of porcine endogenous retrovirus.
The procedure used a dedicated operating room and equipment that will not be used for clinical procedures, the team emphasized.
An organ transplant specialist who was not involved in the study commented that there can be unwelcome surprises even with the most rigorous infection prophylaxis protocols.
“I think these are important steps, but they don’t resolve the question of infectious risk. Sometimes viruses or latent infections are only manifested later,” said Jay A. Fishman, MD, associate director of the Massachusetts General Hospital Transplant Center and director of the transplant infectious diseases and compromised host program at the hospital, which is in Boston.
“I think these are important steps, but as you may recall from the Maryland heart transplant experience, when porcine cytomegalovirus was activated, it was a long way into that patient’s course, and so we just don’t know whether something would have been reactivated later,” he said in an interview.
Dr. Fishman noted that experience with xenotransplantation at the University of Maryland and other centers has suggested that immunosuppressive regimens used for human-to-human transplants may not be suited for animal-to-human grafts.
The hearts were taken from pigs genetically modified with knockouts of four porcine genes to prevent rejection – including a gene for a growth hormone that would otherwise cause the heart to continue to expand in the recipient’s chest – and with the addition of six human transgenes encoding for expression of proteins regulating biologic pathways that might be disrupted by incompatibilities across species.
Vietnam veteran
The organ recipients were recently deceased patients who had expressed the clear wish to be organ donors but whose organs were for clinical reasons unsuitable for transplant.
The first recipient was Lawrence Kelly, a Vietnam War veteran and welder who died from heart failure at the age of 72.
“He was an organ donor, and would be so happy to know how much his contribution to this research will help people like him with this heart disease. He was a hero his whole life, and he went out a hero,” said Alice Michael, Mr. Kelly’s partner of 33 years, who also spoke at the briefing.
“It was, I think, one of the most incredible things to see a pig heart pounding away and beating inside the chest of a human being,” said Robert A. Montgomery, MD, DPhil, director of the NYU Transplant Institute, and himself a heart transplant recipient.
Dr. Fishman said he had no relevant conflicts of interest.
This article was updated on 7/12/22 and 7/14/22.
Drugging the undruggable
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
including 68% of pancreatic tumors and 20% of all non–small cell lung cancers (NSCLC).
We now have a treatment – sotorasib – for patients with locally advanced or metastatic NSCLC that is driven by a KRAS mutation (G12C). And, now, there is a second treatment – adagrasib – under study, which, according to a presentation recently made at the annual meeting of the American Society of Clinical Oncology, looks promising.
Ras is a membrane-bound regulatory protein (G protein) belonging to the family of guanosine triphosphatases (GTPases). Ras functions as a guanosine diphosphate/triphosphate binary switch by cycling between the active GTP-bound and the inactive GDP-bound states in response to extracellular stimuli. The KRAS (G12C) mutation affects the active form of KRAS and results in abnormally high concentrations of GTP-bound KRAS leading to hyperactivation of downstream oncogenic pathways and uncontrolled cell growth, specifically of ERK and MEK signaling pathways.
At the ASCO annual meeting in June, Spira and colleagues reported the results of cohort A of the KRYSTAL-1 study evaluating adagrasib as second-line therapy patients with advanced solid tumors harboring a KRAS (G12C) mutation. Like sotorasib, adagrasib is a KRAS (G12C) inhibitor that irreversibly and selectively binds KRAS (G12C), locking it in its inactive state. In this study, patients had to have failed first-line chemotherapy and immunotherapy with 43% of lung cancer patients responding. The 12-month overall survival (OS) was 51%, median overall survival was 12.6 and median progression-free survival (PFS) was 6.5 months. Twenty-five patients with KRAS (G12C)–mutant NSCLC and active, untreated central nervous system metastases received adagrasib in a phase 1b cohort. The intracranial overall response rate was 31.6% and median intracranial PFS was 4.2 months. Systemic ORR was 35.0% (7/20), the disease control rate was 80.0% (16/20) and median duration of response was 9.6 months. Based on these data, a phase 3 trial evaluating adagrasib monotherapy versus docetaxel in previously treated patients with KRAS (G12C) mutant NSCLC is ongoing.
The Food and Drug Administration approval of sotorasib in 2021 was, in part, based on the results of a single-arm, phase 2, second-line study of patients who had previously received platinum-based chemotherapy and/or immunotherapy. An ORR rate of 37.1% was reported with a median PFS of 6.8 months and median OS of 12.5 months leading to the FDA approval. Responses were observed across the range of baseline PD-L1 expression levels: 48% of PD-L1 negative, 39% with PD-L1 between 1%-49%, and 22% of patients with a PD-L1 of greater than 50% having a response.
The major toxicities observed in these studies were gastrointestinal (diarrhea, nausea, vomiting) and hepatic (elevated liver enzymes). About 97% of patients on adagrasib experienced any treatment-related adverse events, and 43% experienced a grade 3 or 4 treatment-related adverse event leading to dose reduction in 52% of patients, a dose interruption in 61% of patients, and a 7% discontinuation rate. About 70% of patients treated with sotorasib had a treatment-related adverse event of any grade, and 21% reported grade 3 or 4 treatment-related adverse events.
A subgroup in the KRYSTAL-1 trial reported an intracranial ORR of 32% in patients with active, untreated CNS metastases. Median overall survival has not yet reached concordance between systemic and intracranial disease control was 88%. In addition, preliminary data from two patients with untreated CNS metastases from a phase 1b cohort found cerebrospinal fluid concentrations of adagrasib with a mean ratio of unbound brain-to-plasma concentration of 0.47, which is comparable or exceeds values for known CNS-penetrant tyrosine kinase inhibitors.
Unfortunately, KRAS (G12C) is not the only KRAS mutation out there. There are a myriad of others, such as G12V and G12D. Hopefully, we will be seeing more drugs aimed at this set of important mutations. Another question, of course, is when and if these drugs will move to the first-line setting.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Inflation and health care: The prognosis for doctors
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Rampant inflation doesn’t just mean a spike in everyday expenses like gas and groceries. It’s also bound to have a significant impact on the cost of health care – and on your practice. A recent report from McKinsey & Company predicts that the current inflationary spiral will force health care providers to charge higher reimbursement rates, and those costs inevitably will be passed along to both employers and consumers. Bottom line: Your patients will likely have to pay more out of pocket.
How, precisely, will inflation affect your practice, and what’s the best way to minimize the damage?
Step 1: Maintain operational standards
“Based on the conversations we’ve had with our physician clients that own practices, we see the potential for cost inflation to outrun revenue inflation over the next year,” said Michael Ashley Schulman, CFA, partner and chief investment officer at Running Point Capital, El Segundo, Calif. “Staff wages, as well as office equipment and medical supply costs, are increasing faster than insurance and Medicare/Medicaid reimbursement amounts.” Even so, topflight employees are essential to keep your practice running smoothly. Prioritize excellent nursing. Instead of adding a new hire, compensate your best nurse as well as possible. The same goes for an efficient office manager: On that front, too, you should go the extra mile, even if it means trimming expenses elsewhere.
Step 2: Plan ahead for insurance challenges
Many insurers, including Medicare, set health care costs a year in advance, based on projected growth. This means insurance payouts will stay largely the same for the time being. “Almost all physicians employed by large groups won’t see costs due to inflation rise until next year,” said Mark V. Pauly, PhD, Bendheim Professor in the department of health care management at the University of Pennsylvania, Philadelphia. “For self-employed physicians, there will also be a cushion.”
“The big issue with inflation is that more patients will likely be underinsured,” said Tiffany Johnson, MBA, CFP, co-CEO and financial advisor at Piece of Wealth Planning in Atlanta. “With more out-of-pocket costs ... these patients may not seek out medical treatment or go to see a specialist if they do not believe it is necessary.” A new study from Johns Hopkins found that patients under financial pressure often delay or forgo medical treatment because of food insecurity. Compassionate care is the solution: Direct these patients to financial aid and other resources they may qualify for. That way, they can continue to receive the care they need from you, and your need to pass on costs may be lower.
Step 3: Rely on your affiliated health care organization
These are tough times when it comes to expansion. “Since we are in an environment where inflation and interest rates are both high, it will be much harder for physicians to have the capital to invest in new technology to grow or advance their practice,” Ms. Johnson said. With that in mind, keep the lines of communication between you and your affiliated hospital/health care organization more open than ever. Combining practices with another doctor is one way to increase revenue; you might ask if any affiliated doctors are seeking to team up. It’s also vital to attend meetings and pay close attention to budget cuts your organization may be making. And don’t be shy about asking your administrator for profit-boosting recommendations.
Step 4: Revisit vendor relationships
Find out if your vendors will continue to supply you with the goods you need at reasonable rates, and switch now if they won’t. Be proactive. “Test new medical suppliers,” Mr. Schulman advised. “Reread equipment leasing contracts to check if the interest rates have increased. See if buyout, prepay, or refinancing options are more economical. Also, investigate [bringing down] your rental expense by reducing square footage or moving to a lower-cost location.” In light of ongoing supply chain issues, it’s wise to consider alternative products. But stay focused on quality – you don’t want to be stuck with cheap, possibly defective equipment. Spend where it’s essential and cut the fat somewhere else.
Step 5: Don’t waste your assets
Analyze your budget in minute detail. “Now is the time to review your current inventory and overhead costs,” Ms. Johnson said. “Many physicians let their office staff handle the restocking of inventory and office supplies. While this can be efficient for their practice, it also leaves room for unnecessary business expenses.” Take a cold, hard look at your supply closet – what’s in there that you can live without? Don’t reorder it. Then seek out any revenue stream you may be overlooking. “It’s important to review billing to make sure all the services are reimbursable,” Ms. Johnson added. Small mistakes can yield dividends if you find them.
Step 6: Be poised to pivot
Get creative. “To minimize a profit decline, use video consulting – it’s more efficient and less equipment intensive,” Mr. Schulman said. “Look at how remote work and flexible hours can maximize the work your practice accomplishes while cutting office costs.”
Ms. Johnson suggests adding concierge services, noting that “concierge doctors offer personalized care and direct access for an up-front fee.” With this approach, you may see fewer patients, but your payout paperwork will decrease, and that up-front fee can be profitable. Another outside-the-box idea: Start making house calls. A Scripps study found that home health visits requested via app can result in patient care delivered by a doctor and medical assistant in less than 2 hours. House calls can be an effective and profitable solution when it comes to providing nonemergency care and preventive treatment to patients who aren’t mobile, not to mention patients who just appreciate the convenience.
Step 7: Maintain transparency
Any economic changes your practice will implement must be communicated to your staff and patients clearly and directly. Keep everyone in the loop and be ready to answer questions immediately. Show those you work with and care for that, regardless of the economy, it’s they who matter to you most. That simple reassurance will prove invaluable.
A version of this article first appeared on Medscape.com.
Biologics reduce exacerbations in severe asthma
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
, based on data from more than 2,000 individuals.
The development of biologics to target specific inflammatory pathways “has transformed the management of uncontrolled SA,” but data on the real-world use of biologics in severe asthma patients treated by subspecialists are limited, wrote Reynold A. Panettieri, Jr., MD, of Rutgers, State University of New Jersey, New Brunswick, and colleagues.
In a study published in the Annals of Allergy, Asthma & Immunology, the researchers reviewed data from CHRONICLE, an ongoing, prospective, real-world noninterventional study of adults aged 18 years and older with severe asthma in the United States.
The study population included 2,847 patients enrolled in the CHRONICLE study between February 2018 and February 2021; 68.8% were women, 74.6% were White. The patients ranged in age from 18 to 89 years, with a mean age of 54.2 years.
Biologic use was defined as patients who started or had ongoing use of biologics between 12 months before enrollment and the patient’s most recent data collection. Switches were defined as stopping one biologic and starting another within 6 months; stops were defined as discontinuing a biologic without switching to another within 6 months. A total of 66% of the patients were using biologics at the time of study enrollment. The most common biologic was omalizumab (47%), followed by benralizumab (27%), mepolizumab (26%), and dupilumab (18%).
Overall, 89% of the patients had ongoing biologic use, 16% had biologic switches, and 13% had stops.
Patients who started biologics or switched biologics had significant reductions in asthma exacerbations at 6 months, compared with nonbiologic users of 58% (1.80 vs. 0.76 per patient-year) and 49% (1.47 vs. 0.75 per patient-year), respectively (P < .001 for both). Asthma exacerbations declined by 70% among biologics users for whom data were available for 12 months before and 12 months after starting biologics.
Exacerbations decreased at 6 months after biologic initiation across all subgroups of patients, notably patients with pre-biologic FEV1 < 80% and patients with FEV1 ≥ 80% (66% and 53%, respectively); never smokers and current/former smokers (63% and 50%, respectively); and patients with COPD and without COPD (58% and 52%, respectively).
The researchers also found a greater reduction in exacerbations among patients who switched from anti-IgE therapy to anti–IL-5/IL-5R/IL-4R therapy, compared with those who switched among anti–IL-5/IL-5R/IL-4R therapies (58% vs. 46%).
Patients who stopped or switched biologics appeared to have more severe or treatment-refractory disease than those with ongoing biologic use, the researchers noted. The most common reason for stopping or switching was worsening symptoms.
The study findings were limited by several factors, including the focus only on adults in the United States with subspecialist-treated SA, which may limit generalizability to children or other populations, the researchers noted. Other limitations included the variation in clinical decisions and insurance coverage and the inability to conduct longitudinal assessments, they said.
The results demonstrate that starting or switching biologics was consistently associated with fewer exacerbations in severe asthma. However, more research is needed to determine why some patients were not receiving biologics because they were not considered clinically eligible by their subspecialist health care providers, the researchers concluded.
The current study and the CHRONICLE study were supported by AstraZeneca. Lead author Dr. Panettieri disclosed serving on the advisory boards for and receiving grant support from AstraZeneca, Sanofi, Genentech, Regeneron, and Novartis.
FROM THE ANNALS OF ALLERGY, ASTHMA & IMMUNOLOGY




