User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


ICU telemedicine turns 40
connected with intensivists at the University Hospitals of Cleveland (Grundy, et al. Crit Care Med. 1982;10[7]:471). After this proof-of-concept report, however, ICU telemedicine gained little traction for nearly 20 years, until Johns Hopkins Hospital established a continuously monitored ICU telemedicine service in a nonintensivist staffed surgical ICU. Their pre/post analysis suggested a 64% decrease in severity-adjusted ICU mortality and greater than 30% decrease in ICU length of stay, ICU complications, and costs (Rosenfeld, et al. Crit Care Med. 2000;28[12]:3925).
Along with better and less costly telemedicine technology, rapid adoption of electronic medical records, and a nationwide intensivist shortage, this and other evidence for the service’s clinical and cost effectiveness has spurred explosive growth in ICU telemedicine in the succeeding 2 decades, with at least 18% of hospitals and 28% of ICU beds supported by ICU telemedicine by 2018 (Ofoma, et al. Crit Care Explor. 2021;4[3]:e0468).
Importantly, what “ICU telemedicine” represents varies substantially across hospitals and even across ICUs within systems. Two-way audiovisual technology is the defining feature, and at a minimum, programs provide intensivists and/or nurses who respond to consultation requests. Commonly, telemedicine clinicians directly connect with patients; monitor labs, hemodynamics, and alarms; and proactively contact on-site clinicians with recommendations or place orders directly into the electronic health record depending on whether the clinician acts as the patients’ primary, co-managing, or consultant provider. A centralized hub and spoke model with telemedicine personnel located at a single, remote center is the most common and best studied ICU telemedicine design. Additional staffing may include respiratory therapists, pharmacists, and advanced practice clinicians in coverage models that range from 24/7 to nocturnal and can also differ in whether patients are monitored continuously or on an as needed basis, triggered by alarms or clinician/nursing concerns.
On-demand services may extend to support for teams responding to medical emergencies inside and sometimes outside the ICU. Another equally important role that ICU telemedicine can provide is helping ensure facilities adhere to ICU quality metrics, such as ventilator bundles, DVT prophylaxis, and daily SAT/SBT.
Unsurprisingly, integrating ICU telemedicine into an existing system is very costly and complex, requiring substantial and thoughtful process redesign to maximize fiscal and clinical return on investment. One vendor of proprietary telemedicine technology, Philips eICU, estimates an implementation cost of $50,000 to $100,000 per bed with annual overhead, software maintenance, and IT staffing of ~20% of implementation costs in addition to clinician staffing of $1-2 million per 100 beds. However, some (but not all) evidence suggests that ICU telemedicine programs pay for themselves over time. An influential report from Sentara Healthcare, an early adopter of ICU telemedicine, described equipment costs of more than $1 million for a total of 103 critical care beds but attributed savings of $460,000 per month to decreased length of stay (Coustasse, et al. The Permanente Journal. 2014;18[4]:76).
Cost savings are great, of course, but ICU telemedicine’s potential to improve clinical outcomes is the real priority. While Sentara’s early report included a 27% decrease in ICU mortality after telemedicine adoption, a 2011 meta-analysis of 13 studies, including 35 ICUs and over 40,000 patients, suggested decreased ICU mortality and LOS with a statistically significant effect on overall hospital mortality and LOS (Young, et al. Arch Intern Med. 2011;171[6]:498). This highlights the Achilles heel of ICU telemedicine evidence: the pretest/posttest studies that dominate this field and likely contribute substantially to the inconsistencies in the evidence base.
In the absence of risk adjustment and control groups, many studies observed postimplementation changes that may reflect trends in patient mix or the effects of unrelated practice changes rather than the causal influence of ICU telemedicine. In fact, in studies using more robust methods, ICU telemedicine’s effect size has been smaller or nonexistent. For example, in 2016, Kahn and colleagues used CMS data to evaluate 132 ICU telemedicine programs using 389 matched controlled hospitals. There was a slight reduction in 90-day mortality (OR=0.96, CI 0.94-0.98) with only 12% showing a statistically significant reduction in mortality. Interestingly, hospitals in urban areas demonstrated greater benefit than rural facilities (Kahn, et al. Medical Care. 2016;54[3]:319).
The heterogeneity of the studied programs (e.g., primary vs consultative role, on-demand vs proactive involvement) and recipient ICUs (e.g., rural vs tertiary care facility, presence of bedside intensivists) further hinders a clear answer to the key question: Would ICU telemedicine benefit my hospital? Fortunately, some recent, well-designed studies have attempted to understand which attributes of ICU telemedicine programs provide results and which ICUs will see the most benefit. In a cohort of 118,990 patients across 56 ICUs, four interventions were associated with lower mortality and reduced LOS: (1) evaluation of patients within 1 hour of ICU admission, (2) frequent leadership review of performance data, (3) ICU best practice compliance, and (4) prompt response to alerts (Lilly, et al. Chest. 2014;145[3]:500). Kahn and colleagues have also investigated this issue, conducting an in-depth ethnographic evaluation of 10 hospitals identified in their 2016 study to have positive, neutral, or negative outcomes after ICU telemedicine implementation (Kahn, et al. Am J Respir Crit Care Med. 2019;199[8]:970). They found that successful programs:
(1) provided consistent services matched to recipient needs;
(2) provided services both proactively and reactively without being obtrusive;
(3) embedded routine engagements unobtrusively into usual routines;
(4) had engaged leadership who set clear expectations and mediated conflicts; and
(5) had bedside clinicians who valued and sought out telemedicine participation in care.
The authors concluded that, “the true value of ICU telemedicine lies not in whether the technology exists but in how it is applied.” However, another recent analysis also suggested that, rather than telemedicine or recipient ICU design, targeting underperforming recipient ICU performance may be the key determinant of whether ICU telemedicine implementation improves outcomes (Fusaro, et al. Crit Care Med. 2019; 47[4]:501). While the finding may reflect regression to the mean, the idea that ICUs with above-expected mortality derive greater benefit from ICU telemedicine support than already well-performing ICUs is certainly logical.
As COVID-19 strained health care systems across the country, we and others found ways to use ICU telemedicine to preserve optimal care delivery for critically ill patients. Our program at Intermountain Healthcare – already supporting 17 ICUs within our 24-hospital health system, as well as 10 external ICUs with experienced critical care physicians, nurses, respiratory therapists, and pharmacists – took on increased responsibility for ICU load balancing and interhospital transfers.
Leveraging telemedicine services also helped community ICUs care for sicker, more complex patients than usual and aided nonintensivist physicians called upon to manage critically ill patients in ad hoc ICUs at referral hospitals. While the pandemic certainly stressed ICU staff, we suspect that telemedicine’s ability to balance caseloads and distribute clinical tasks helped mitigate these stresses. At age 40, ICU telemedicine is both mature and still growing, with continued expansion of bed coverage and the range of services available. Looking ahead, as we confront a national shortage of intensivists, ICU telemedicine likely represents a cost effective and efficient strategy to maintain critical care capacity with the potential to ensure low-cost, high-quality care for all, regardless of location.
Dr. Graham and Dr. Peltan are with the Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah; and Dr. Peltan is also with the Division of Pulmonary & Critical Care Medicine, Department of Medicine, Intermountain Medical Center, Murray, Utah.
connected with intensivists at the University Hospitals of Cleveland (Grundy, et al. Crit Care Med. 1982;10[7]:471). After this proof-of-concept report, however, ICU telemedicine gained little traction for nearly 20 years, until Johns Hopkins Hospital established a continuously monitored ICU telemedicine service in a nonintensivist staffed surgical ICU. Their pre/post analysis suggested a 64% decrease in severity-adjusted ICU mortality and greater than 30% decrease in ICU length of stay, ICU complications, and costs (Rosenfeld, et al. Crit Care Med. 2000;28[12]:3925).
Along with better and less costly telemedicine technology, rapid adoption of electronic medical records, and a nationwide intensivist shortage, this and other evidence for the service’s clinical and cost effectiveness has spurred explosive growth in ICU telemedicine in the succeeding 2 decades, with at least 18% of hospitals and 28% of ICU beds supported by ICU telemedicine by 2018 (Ofoma, et al. Crit Care Explor. 2021;4[3]:e0468).
Importantly, what “ICU telemedicine” represents varies substantially across hospitals and even across ICUs within systems. Two-way audiovisual technology is the defining feature, and at a minimum, programs provide intensivists and/or nurses who respond to consultation requests. Commonly, telemedicine clinicians directly connect with patients; monitor labs, hemodynamics, and alarms; and proactively contact on-site clinicians with recommendations or place orders directly into the electronic health record depending on whether the clinician acts as the patients’ primary, co-managing, or consultant provider. A centralized hub and spoke model with telemedicine personnel located at a single, remote center is the most common and best studied ICU telemedicine design. Additional staffing may include respiratory therapists, pharmacists, and advanced practice clinicians in coverage models that range from 24/7 to nocturnal and can also differ in whether patients are monitored continuously or on an as needed basis, triggered by alarms or clinician/nursing concerns.
On-demand services may extend to support for teams responding to medical emergencies inside and sometimes outside the ICU. Another equally important role that ICU telemedicine can provide is helping ensure facilities adhere to ICU quality metrics, such as ventilator bundles, DVT prophylaxis, and daily SAT/SBT.
Unsurprisingly, integrating ICU telemedicine into an existing system is very costly and complex, requiring substantial and thoughtful process redesign to maximize fiscal and clinical return on investment. One vendor of proprietary telemedicine technology, Philips eICU, estimates an implementation cost of $50,000 to $100,000 per bed with annual overhead, software maintenance, and IT staffing of ~20% of implementation costs in addition to clinician staffing of $1-2 million per 100 beds. However, some (but not all) evidence suggests that ICU telemedicine programs pay for themselves over time. An influential report from Sentara Healthcare, an early adopter of ICU telemedicine, described equipment costs of more than $1 million for a total of 103 critical care beds but attributed savings of $460,000 per month to decreased length of stay (Coustasse, et al. The Permanente Journal. 2014;18[4]:76).
Cost savings are great, of course, but ICU telemedicine’s potential to improve clinical outcomes is the real priority. While Sentara’s early report included a 27% decrease in ICU mortality after telemedicine adoption, a 2011 meta-analysis of 13 studies, including 35 ICUs and over 40,000 patients, suggested decreased ICU mortality and LOS with a statistically significant effect on overall hospital mortality and LOS (Young, et al. Arch Intern Med. 2011;171[6]:498). This highlights the Achilles heel of ICU telemedicine evidence: the pretest/posttest studies that dominate this field and likely contribute substantially to the inconsistencies in the evidence base.
In the absence of risk adjustment and control groups, many studies observed postimplementation changes that may reflect trends in patient mix or the effects of unrelated practice changes rather than the causal influence of ICU telemedicine. In fact, in studies using more robust methods, ICU telemedicine’s effect size has been smaller or nonexistent. For example, in 2016, Kahn and colleagues used CMS data to evaluate 132 ICU telemedicine programs using 389 matched controlled hospitals. There was a slight reduction in 90-day mortality (OR=0.96, CI 0.94-0.98) with only 12% showing a statistically significant reduction in mortality. Interestingly, hospitals in urban areas demonstrated greater benefit than rural facilities (Kahn, et al. Medical Care. 2016;54[3]:319).
The heterogeneity of the studied programs (e.g., primary vs consultative role, on-demand vs proactive involvement) and recipient ICUs (e.g., rural vs tertiary care facility, presence of bedside intensivists) further hinders a clear answer to the key question: Would ICU telemedicine benefit my hospital? Fortunately, some recent, well-designed studies have attempted to understand which attributes of ICU telemedicine programs provide results and which ICUs will see the most benefit. In a cohort of 118,990 patients across 56 ICUs, four interventions were associated with lower mortality and reduced LOS: (1) evaluation of patients within 1 hour of ICU admission, (2) frequent leadership review of performance data, (3) ICU best practice compliance, and (4) prompt response to alerts (Lilly, et al. Chest. 2014;145[3]:500). Kahn and colleagues have also investigated this issue, conducting an in-depth ethnographic evaluation of 10 hospitals identified in their 2016 study to have positive, neutral, or negative outcomes after ICU telemedicine implementation (Kahn, et al. Am J Respir Crit Care Med. 2019;199[8]:970). They found that successful programs:
(1) provided consistent services matched to recipient needs;
(2) provided services both proactively and reactively without being obtrusive;
(3) embedded routine engagements unobtrusively into usual routines;
(4) had engaged leadership who set clear expectations and mediated conflicts; and
(5) had bedside clinicians who valued and sought out telemedicine participation in care.
The authors concluded that, “the true value of ICU telemedicine lies not in whether the technology exists but in how it is applied.” However, another recent analysis also suggested that, rather than telemedicine or recipient ICU design, targeting underperforming recipient ICU performance may be the key determinant of whether ICU telemedicine implementation improves outcomes (Fusaro, et al. Crit Care Med. 2019; 47[4]:501). While the finding may reflect regression to the mean, the idea that ICUs with above-expected mortality derive greater benefit from ICU telemedicine support than already well-performing ICUs is certainly logical.
As COVID-19 strained health care systems across the country, we and others found ways to use ICU telemedicine to preserve optimal care delivery for critically ill patients. Our program at Intermountain Healthcare – already supporting 17 ICUs within our 24-hospital health system, as well as 10 external ICUs with experienced critical care physicians, nurses, respiratory therapists, and pharmacists – took on increased responsibility for ICU load balancing and interhospital transfers.
Leveraging telemedicine services also helped community ICUs care for sicker, more complex patients than usual and aided nonintensivist physicians called upon to manage critically ill patients in ad hoc ICUs at referral hospitals. While the pandemic certainly stressed ICU staff, we suspect that telemedicine’s ability to balance caseloads and distribute clinical tasks helped mitigate these stresses. At age 40, ICU telemedicine is both mature and still growing, with continued expansion of bed coverage and the range of services available. Looking ahead, as we confront a national shortage of intensivists, ICU telemedicine likely represents a cost effective and efficient strategy to maintain critical care capacity with the potential to ensure low-cost, high-quality care for all, regardless of location.
Dr. Graham and Dr. Peltan are with the Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah; and Dr. Peltan is also with the Division of Pulmonary & Critical Care Medicine, Department of Medicine, Intermountain Medical Center, Murray, Utah.
connected with intensivists at the University Hospitals of Cleveland (Grundy, et al. Crit Care Med. 1982;10[7]:471). After this proof-of-concept report, however, ICU telemedicine gained little traction for nearly 20 years, until Johns Hopkins Hospital established a continuously monitored ICU telemedicine service in a nonintensivist staffed surgical ICU. Their pre/post analysis suggested a 64% decrease in severity-adjusted ICU mortality and greater than 30% decrease in ICU length of stay, ICU complications, and costs (Rosenfeld, et al. Crit Care Med. 2000;28[12]:3925).
Along with better and less costly telemedicine technology, rapid adoption of electronic medical records, and a nationwide intensivist shortage, this and other evidence for the service’s clinical and cost effectiveness has spurred explosive growth in ICU telemedicine in the succeeding 2 decades, with at least 18% of hospitals and 28% of ICU beds supported by ICU telemedicine by 2018 (Ofoma, et al. Crit Care Explor. 2021;4[3]:e0468).
Importantly, what “ICU telemedicine” represents varies substantially across hospitals and even across ICUs within systems. Two-way audiovisual technology is the defining feature, and at a minimum, programs provide intensivists and/or nurses who respond to consultation requests. Commonly, telemedicine clinicians directly connect with patients; monitor labs, hemodynamics, and alarms; and proactively contact on-site clinicians with recommendations or place orders directly into the electronic health record depending on whether the clinician acts as the patients’ primary, co-managing, or consultant provider. A centralized hub and spoke model with telemedicine personnel located at a single, remote center is the most common and best studied ICU telemedicine design. Additional staffing may include respiratory therapists, pharmacists, and advanced practice clinicians in coverage models that range from 24/7 to nocturnal and can also differ in whether patients are monitored continuously or on an as needed basis, triggered by alarms or clinician/nursing concerns.
On-demand services may extend to support for teams responding to medical emergencies inside and sometimes outside the ICU. Another equally important role that ICU telemedicine can provide is helping ensure facilities adhere to ICU quality metrics, such as ventilator bundles, DVT prophylaxis, and daily SAT/SBT.
Unsurprisingly, integrating ICU telemedicine into an existing system is very costly and complex, requiring substantial and thoughtful process redesign to maximize fiscal and clinical return on investment. One vendor of proprietary telemedicine technology, Philips eICU, estimates an implementation cost of $50,000 to $100,000 per bed with annual overhead, software maintenance, and IT staffing of ~20% of implementation costs in addition to clinician staffing of $1-2 million per 100 beds. However, some (but not all) evidence suggests that ICU telemedicine programs pay for themselves over time. An influential report from Sentara Healthcare, an early adopter of ICU telemedicine, described equipment costs of more than $1 million for a total of 103 critical care beds but attributed savings of $460,000 per month to decreased length of stay (Coustasse, et al. The Permanente Journal. 2014;18[4]:76).
Cost savings are great, of course, but ICU telemedicine’s potential to improve clinical outcomes is the real priority. While Sentara’s early report included a 27% decrease in ICU mortality after telemedicine adoption, a 2011 meta-analysis of 13 studies, including 35 ICUs and over 40,000 patients, suggested decreased ICU mortality and LOS with a statistically significant effect on overall hospital mortality and LOS (Young, et al. Arch Intern Med. 2011;171[6]:498). This highlights the Achilles heel of ICU telemedicine evidence: the pretest/posttest studies that dominate this field and likely contribute substantially to the inconsistencies in the evidence base.
In the absence of risk adjustment and control groups, many studies observed postimplementation changes that may reflect trends in patient mix or the effects of unrelated practice changes rather than the causal influence of ICU telemedicine. In fact, in studies using more robust methods, ICU telemedicine’s effect size has been smaller or nonexistent. For example, in 2016, Kahn and colleagues used CMS data to evaluate 132 ICU telemedicine programs using 389 matched controlled hospitals. There was a slight reduction in 90-day mortality (OR=0.96, CI 0.94-0.98) with only 12% showing a statistically significant reduction in mortality. Interestingly, hospitals in urban areas demonstrated greater benefit than rural facilities (Kahn, et al. Medical Care. 2016;54[3]:319).
The heterogeneity of the studied programs (e.g., primary vs consultative role, on-demand vs proactive involvement) and recipient ICUs (e.g., rural vs tertiary care facility, presence of bedside intensivists) further hinders a clear answer to the key question: Would ICU telemedicine benefit my hospital? Fortunately, some recent, well-designed studies have attempted to understand which attributes of ICU telemedicine programs provide results and which ICUs will see the most benefit. In a cohort of 118,990 patients across 56 ICUs, four interventions were associated with lower mortality and reduced LOS: (1) evaluation of patients within 1 hour of ICU admission, (2) frequent leadership review of performance data, (3) ICU best practice compliance, and (4) prompt response to alerts (Lilly, et al. Chest. 2014;145[3]:500). Kahn and colleagues have also investigated this issue, conducting an in-depth ethnographic evaluation of 10 hospitals identified in their 2016 study to have positive, neutral, or negative outcomes after ICU telemedicine implementation (Kahn, et al. Am J Respir Crit Care Med. 2019;199[8]:970). They found that successful programs:
(1) provided consistent services matched to recipient needs;
(2) provided services both proactively and reactively without being obtrusive;
(3) embedded routine engagements unobtrusively into usual routines;
(4) had engaged leadership who set clear expectations and mediated conflicts; and
(5) had bedside clinicians who valued and sought out telemedicine participation in care.
The authors concluded that, “the true value of ICU telemedicine lies not in whether the technology exists but in how it is applied.” However, another recent analysis also suggested that, rather than telemedicine or recipient ICU design, targeting underperforming recipient ICU performance may be the key determinant of whether ICU telemedicine implementation improves outcomes (Fusaro, et al. Crit Care Med. 2019; 47[4]:501). While the finding may reflect regression to the mean, the idea that ICUs with above-expected mortality derive greater benefit from ICU telemedicine support than already well-performing ICUs is certainly logical.
As COVID-19 strained health care systems across the country, we and others found ways to use ICU telemedicine to preserve optimal care delivery for critically ill patients. Our program at Intermountain Healthcare – already supporting 17 ICUs within our 24-hospital health system, as well as 10 external ICUs with experienced critical care physicians, nurses, respiratory therapists, and pharmacists – took on increased responsibility for ICU load balancing and interhospital transfers.
Leveraging telemedicine services also helped community ICUs care for sicker, more complex patients than usual and aided nonintensivist physicians called upon to manage critically ill patients in ad hoc ICUs at referral hospitals. While the pandemic certainly stressed ICU staff, we suspect that telemedicine’s ability to balance caseloads and distribute clinical tasks helped mitigate these stresses. At age 40, ICU telemedicine is both mature and still growing, with continued expansion of bed coverage and the range of services available. Looking ahead, as we confront a national shortage of intensivists, ICU telemedicine likely represents a cost effective and efficient strategy to maintain critical care capacity with the potential to ensure low-cost, high-quality care for all, regardless of location.
Dr. Graham and Dr. Peltan are with the Division of Pulmonary & Critical Care Medicine, Department of Internal Medicine, University of Utah School of Medicine, Salt Lake City, Utah; and Dr. Peltan is also with the Division of Pulmonary & Critical Care Medicine, Department of Medicine, Intermountain Medical Center, Murray, Utah.
Board of Regents meeting, August 16, 2022
The CHEST Board of Regents (BOR) convened a hybrid meeting in Atlanta prior to the pulmonary board review course. Hopefully, many of you had the opportunity to participate in that excellent learning experience. The function of the BOR is to provide direction and oversight for the organization’s strategy and goals, including the development of the many programs that are so expertly crafted by our talented staff, with contributions from our volunteers. Our EVP/CEO, Dr. Robert Musacchio, opened the meeting with a review of the organization’s mid-year progress toward achieving these annual goals. Despite the current economic turmoil and need for flexibility in our COVID landscape, CHEST is on track to meet or exceed the majority of the stated goals. The team continues efforts to achieve our key metrics related to increasing learners, members, and growth in revenue – we anticipate the upcoming annual meeting will only bolster our progress.
Every BOR meeting includes a report from the Finance Committee, which is thoroughly reviewed by the BOR. CHEST investments have fared no better than the rest of the country, but our investment advisors assure us that things will improve.
Similar updates were given by the President of the CHEST Foundation, Dr. Ian Nathanson, who noted that the Foundation will be celebrating its 25th anniversary during CHEST 2022. I would like to personally encourage you to donate and make this year the best year of fundraising. We are eager to bolster our community and patients after the long journey through COVID. Every donation enables more investment in creating access to the profession and in piloting programs across our communities that improve access to care. Thank you to those who have already contributed.
The morning session was completed with excellent presentations by the Chief Learning Officer/Education SVP, Richard Schuch and Publisher/Communications SVP, Nicki Augustyn. Rich provided an update on the education strategy and how it will change to keep up with the ever-changing needs of learners. He also made the observation that CHEST cannot do this alone, and partnering with companies to assist in new methods of content delivery will be important for the future of the organization. Nicki presented data regarding the current membership structure, as well as the effect of the pandemic on membership over the last 2 years.
In the afternoon session, the BOR and staff spent over 2 hours on the topic of advocacy. CHEST has become more active in the area of advocacy for both patients and the medical profession, specifically in the areas of pulmonary, critical care, and sleep medicine. The Health Policy and Advocacy Committee (HPAC) currently has workgroups working in five different areas, including: oxygen, pulmonary rehabilitation, coding and billing, noninvasive ventilation, and tobacco and vaping. However, CHEST is often asked to sign on to or support the advocacy efforts of other organizations, including other medical societies, patient groups, and industry groups. At times, the decision to support or not support is easy. While there is a process to make that decision, this session helped better define the process and started to create some norms around when CHEST itself should lead its own statement on a particular issue.
The BOR will meet a total of six times this year, either remotely or in person, to make certain that CHEST continues to fulfill its mission “to champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.”
The CHEST Board of Regents (BOR) convened a hybrid meeting in Atlanta prior to the pulmonary board review course. Hopefully, many of you had the opportunity to participate in that excellent learning experience. The function of the BOR is to provide direction and oversight for the organization’s strategy and goals, including the development of the many programs that are so expertly crafted by our talented staff, with contributions from our volunteers. Our EVP/CEO, Dr. Robert Musacchio, opened the meeting with a review of the organization’s mid-year progress toward achieving these annual goals. Despite the current economic turmoil and need for flexibility in our COVID landscape, CHEST is on track to meet or exceed the majority of the stated goals. The team continues efforts to achieve our key metrics related to increasing learners, members, and growth in revenue – we anticipate the upcoming annual meeting will only bolster our progress.
Every BOR meeting includes a report from the Finance Committee, which is thoroughly reviewed by the BOR. CHEST investments have fared no better than the rest of the country, but our investment advisors assure us that things will improve.
Similar updates were given by the President of the CHEST Foundation, Dr. Ian Nathanson, who noted that the Foundation will be celebrating its 25th anniversary during CHEST 2022. I would like to personally encourage you to donate and make this year the best year of fundraising. We are eager to bolster our community and patients after the long journey through COVID. Every donation enables more investment in creating access to the profession and in piloting programs across our communities that improve access to care. Thank you to those who have already contributed.
The morning session was completed with excellent presentations by the Chief Learning Officer/Education SVP, Richard Schuch and Publisher/Communications SVP, Nicki Augustyn. Rich provided an update on the education strategy and how it will change to keep up with the ever-changing needs of learners. He also made the observation that CHEST cannot do this alone, and partnering with companies to assist in new methods of content delivery will be important for the future of the organization. Nicki presented data regarding the current membership structure, as well as the effect of the pandemic on membership over the last 2 years.
In the afternoon session, the BOR and staff spent over 2 hours on the topic of advocacy. CHEST has become more active in the area of advocacy for both patients and the medical profession, specifically in the areas of pulmonary, critical care, and sleep medicine. The Health Policy and Advocacy Committee (HPAC) currently has workgroups working in five different areas, including: oxygen, pulmonary rehabilitation, coding and billing, noninvasive ventilation, and tobacco and vaping. However, CHEST is often asked to sign on to or support the advocacy efforts of other organizations, including other medical societies, patient groups, and industry groups. At times, the decision to support or not support is easy. While there is a process to make that decision, this session helped better define the process and started to create some norms around when CHEST itself should lead its own statement on a particular issue.
The BOR will meet a total of six times this year, either remotely or in person, to make certain that CHEST continues to fulfill its mission “to champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.”
The CHEST Board of Regents (BOR) convened a hybrid meeting in Atlanta prior to the pulmonary board review course. Hopefully, many of you had the opportunity to participate in that excellent learning experience. The function of the BOR is to provide direction and oversight for the organization’s strategy and goals, including the development of the many programs that are so expertly crafted by our talented staff, with contributions from our volunteers. Our EVP/CEO, Dr. Robert Musacchio, opened the meeting with a review of the organization’s mid-year progress toward achieving these annual goals. Despite the current economic turmoil and need for flexibility in our COVID landscape, CHEST is on track to meet or exceed the majority of the stated goals. The team continues efforts to achieve our key metrics related to increasing learners, members, and growth in revenue – we anticipate the upcoming annual meeting will only bolster our progress.
Every BOR meeting includes a report from the Finance Committee, which is thoroughly reviewed by the BOR. CHEST investments have fared no better than the rest of the country, but our investment advisors assure us that things will improve.
Similar updates were given by the President of the CHEST Foundation, Dr. Ian Nathanson, who noted that the Foundation will be celebrating its 25th anniversary during CHEST 2022. I would like to personally encourage you to donate and make this year the best year of fundraising. We are eager to bolster our community and patients after the long journey through COVID. Every donation enables more investment in creating access to the profession and in piloting programs across our communities that improve access to care. Thank you to those who have already contributed.
The morning session was completed with excellent presentations by the Chief Learning Officer/Education SVP, Richard Schuch and Publisher/Communications SVP, Nicki Augustyn. Rich provided an update on the education strategy and how it will change to keep up with the ever-changing needs of learners. He also made the observation that CHEST cannot do this alone, and partnering with companies to assist in new methods of content delivery will be important for the future of the organization. Nicki presented data regarding the current membership structure, as well as the effect of the pandemic on membership over the last 2 years.
In the afternoon session, the BOR and staff spent over 2 hours on the topic of advocacy. CHEST has become more active in the area of advocacy for both patients and the medical profession, specifically in the areas of pulmonary, critical care, and sleep medicine. The Health Policy and Advocacy Committee (HPAC) currently has workgroups working in five different areas, including: oxygen, pulmonary rehabilitation, coding and billing, noninvasive ventilation, and tobacco and vaping. However, CHEST is often asked to sign on to or support the advocacy efforts of other organizations, including other medical societies, patient groups, and industry groups. At times, the decision to support or not support is easy. While there is a process to make that decision, this session helped better define the process and started to create some norms around when CHEST itself should lead its own statement on a particular issue.
The BOR will meet a total of six times this year, either remotely or in person, to make certain that CHEST continues to fulfill its mission “to champion the prevention, diagnosis, and treatment of chest diseases through education, communication, and research.”
Pulmonary Vascular Disease & Cardiovascular Disease Network
Cardiovascular Medicine & Surgery Section
Emerging role of cardiopulmonary obstetric critical care
, with 23.8 women dying per 100,000 live births (Hoyert DL, Miniño AM. Maternal mortality in the United States. National Vital Statistics Reports; vol 69 no 2. Hyattsville, MD: National Center for Health Statistics. 2020). The care of this vulnerable population testifies to the quality of care provided across the country. Some of these poor outcomes are directly attributed to in-hospital deaths due to preexisting or newly discovered heart or lung diseases, such as valvular heart diseases, cardiomyopathies, pulmonary arterial hypertension, eclampsia, or other etiologies. With the development of advanced heart and lung programs across the nation capable of providing mechanical circulatory support and extracorporeal life support, we believe that incorporating a heart-lung-OB team approach to high-risk cases can identify knowledge gaps early and predict and prevent maternal complications.
In this proposed model, patients funnel to the hub facility to be cared for by a team of intensive care physicians, advanced heart failure physicians, cardiovascular and obstetric anesthesiologists, and maternal/fetal medicine physicians, with the potential addition of an adult ECMO team member.
A team huddle, using a virtual platform, would be organized by a designated OB coordinator at the patient’s admission with follow-up huddles every 2 to 3 days, to ensure the team stays engaged through delivery into the postpartum period. Value could be added with subsequent cardiac or pulmonary rehabilitation. With an emphasis on shared decision making, we can make it a national priority to save every woman during the birthing process.
Bindu Akkanti, MD, FCCP, Member-at-Large
Mark Warner, MD, FCCP, Member-at-Large
Cardiovascular Medicine & Surgery Section
Emerging role of cardiopulmonary obstetric critical care
, with 23.8 women dying per 100,000 live births (Hoyert DL, Miniño AM. Maternal mortality in the United States. National Vital Statistics Reports; vol 69 no 2. Hyattsville, MD: National Center for Health Statistics. 2020). The care of this vulnerable population testifies to the quality of care provided across the country. Some of these poor outcomes are directly attributed to in-hospital deaths due to preexisting or newly discovered heart or lung diseases, such as valvular heart diseases, cardiomyopathies, pulmonary arterial hypertension, eclampsia, or other etiologies. With the development of advanced heart and lung programs across the nation capable of providing mechanical circulatory support and extracorporeal life support, we believe that incorporating a heart-lung-OB team approach to high-risk cases can identify knowledge gaps early and predict and prevent maternal complications.
In this proposed model, patients funnel to the hub facility to be cared for by a team of intensive care physicians, advanced heart failure physicians, cardiovascular and obstetric anesthesiologists, and maternal/fetal medicine physicians, with the potential addition of an adult ECMO team member.
A team huddle, using a virtual platform, would be organized by a designated OB coordinator at the patient’s admission with follow-up huddles every 2 to 3 days, to ensure the team stays engaged through delivery into the postpartum period. Value could be added with subsequent cardiac or pulmonary rehabilitation. With an emphasis on shared decision making, we can make it a national priority to save every woman during the birthing process.
Bindu Akkanti, MD, FCCP, Member-at-Large
Mark Warner, MD, FCCP, Member-at-Large
Cardiovascular Medicine & Surgery Section
Emerging role of cardiopulmonary obstetric critical care
, with 23.8 women dying per 100,000 live births (Hoyert DL, Miniño AM. Maternal mortality in the United States. National Vital Statistics Reports; vol 69 no 2. Hyattsville, MD: National Center for Health Statistics. 2020). The care of this vulnerable population testifies to the quality of care provided across the country. Some of these poor outcomes are directly attributed to in-hospital deaths due to preexisting or newly discovered heart or lung diseases, such as valvular heart diseases, cardiomyopathies, pulmonary arterial hypertension, eclampsia, or other etiologies. With the development of advanced heart and lung programs across the nation capable of providing mechanical circulatory support and extracorporeal life support, we believe that incorporating a heart-lung-OB team approach to high-risk cases can identify knowledge gaps early and predict and prevent maternal complications.
In this proposed model, patients funnel to the hub facility to be cared for by a team of intensive care physicians, advanced heart failure physicians, cardiovascular and obstetric anesthesiologists, and maternal/fetal medicine physicians, with the potential addition of an adult ECMO team member.
A team huddle, using a virtual platform, would be organized by a designated OB coordinator at the patient’s admission with follow-up huddles every 2 to 3 days, to ensure the team stays engaged through delivery into the postpartum period. Value could be added with subsequent cardiac or pulmonary rehabilitation. With an emphasis on shared decision making, we can make it a national priority to save every woman during the birthing process.
Bindu Akkanti, MD, FCCP, Member-at-Large
Mark Warner, MD, FCCP, Member-at-Large
Diffuse Lung Disease & Transplant Network
Lung Transplant Section
Strengthening lung transplant education
The number of lung transplants (LT) performed reached an all-time high in 2019 with a 52.3% increase over the previous decade. Transplants are being performed in older and sicker patients with 35% of recipients being over 65 years of age and 25% with lung allocation scores (LAS) over 60. (Valapour, et al. Am J Transplant. 2021;21[Suppl 2]:441). This growth has led to an increased demand for transplant pulmonologists. There are about 15 dedicated LT fellowship programs located at 68 transplant centers with widely variable curricula. The vast majority of the 160 general pulmonary and critical care medicine (PCCM) fellowship programs do not have access to hands-on clinical transplant training and are guided by vague ACGME guidelines. A U.S. national survey (Town JA, et al. Ann Am Thorac Soc. 2016;13[4]:568) of PCCM programs found that about 41% of centers did not have a transplant curriculum, and training was very variable. Another report found that a structured educational LT curriculum at a transplant center was associated with improved performance of PCCM fellows (Hayes, et al. Teach Learn Med. 2013;25[1]:59). The lack of a structured curriculum and wide variability coupled with lack of information about the training pathways impedes effective training.
Recognizing these issues, the lung transplant steering committee developed two webinars for the online CHEST learning portal (tinyurl.com/53pnne2k). These provide resources and information for fellows and junior faculty interested in a transplant pulmonology career as well as discuss needs and opportunities to develop a program for specialized training in LT. There is need for a multipronged approach addressing:
–Increase access to specialized transplant education for PCCM fellows.
–Develop a uniform structured curriculum for lung transplant education engaging the PCCM and transplant fellowship program directors as stakeholders.
–Increase collaboration between the transplant fellowship programs to address gaps in training.
Hakim Azhfar Ali, MBBS, FCCP
Member-at-Large
Lung Transplant Section
Strengthening lung transplant education
The number of lung transplants (LT) performed reached an all-time high in 2019 with a 52.3% increase over the previous decade. Transplants are being performed in older and sicker patients with 35% of recipients being over 65 years of age and 25% with lung allocation scores (LAS) over 60. (Valapour, et al. Am J Transplant. 2021;21[Suppl 2]:441). This growth has led to an increased demand for transplant pulmonologists. There are about 15 dedicated LT fellowship programs located at 68 transplant centers with widely variable curricula. The vast majority of the 160 general pulmonary and critical care medicine (PCCM) fellowship programs do not have access to hands-on clinical transplant training and are guided by vague ACGME guidelines. A U.S. national survey (Town JA, et al. Ann Am Thorac Soc. 2016;13[4]:568) of PCCM programs found that about 41% of centers did not have a transplant curriculum, and training was very variable. Another report found that a structured educational LT curriculum at a transplant center was associated with improved performance of PCCM fellows (Hayes, et al. Teach Learn Med. 2013;25[1]:59). The lack of a structured curriculum and wide variability coupled with lack of information about the training pathways impedes effective training.
Recognizing these issues, the lung transplant steering committee developed two webinars for the online CHEST learning portal (tinyurl.com/53pnne2k). These provide resources and information for fellows and junior faculty interested in a transplant pulmonology career as well as discuss needs and opportunities to develop a program for specialized training in LT. There is need for a multipronged approach addressing:
–Increase access to specialized transplant education for PCCM fellows.
–Develop a uniform structured curriculum for lung transplant education engaging the PCCM and transplant fellowship program directors as stakeholders.
–Increase collaboration between the transplant fellowship programs to address gaps in training.
Hakim Azhfar Ali, MBBS, FCCP
Member-at-Large
Lung Transplant Section
Strengthening lung transplant education
The number of lung transplants (LT) performed reached an all-time high in 2019 with a 52.3% increase over the previous decade. Transplants are being performed in older and sicker patients with 35% of recipients being over 65 years of age and 25% with lung allocation scores (LAS) over 60. (Valapour, et al. Am J Transplant. 2021;21[Suppl 2]:441). This growth has led to an increased demand for transplant pulmonologists. There are about 15 dedicated LT fellowship programs located at 68 transplant centers with widely variable curricula. The vast majority of the 160 general pulmonary and critical care medicine (PCCM) fellowship programs do not have access to hands-on clinical transplant training and are guided by vague ACGME guidelines. A U.S. national survey (Town JA, et al. Ann Am Thorac Soc. 2016;13[4]:568) of PCCM programs found that about 41% of centers did not have a transplant curriculum, and training was very variable. Another report found that a structured educational LT curriculum at a transplant center was associated with improved performance of PCCM fellows (Hayes, et al. Teach Learn Med. 2013;25[1]:59). The lack of a structured curriculum and wide variability coupled with lack of information about the training pathways impedes effective training.
Recognizing these issues, the lung transplant steering committee developed two webinars for the online CHEST learning portal (tinyurl.com/53pnne2k). These provide resources and information for fellows and junior faculty interested in a transplant pulmonology career as well as discuss needs and opportunities to develop a program for specialized training in LT. There is need for a multipronged approach addressing:
–Increase access to specialized transplant education for PCCM fellows.
–Develop a uniform structured curriculum for lung transplant education engaging the PCCM and transplant fellowship program directors as stakeholders.
–Increase collaboration between the transplant fellowship programs to address gaps in training.
Hakim Azhfar Ali, MBBS, FCCP
Member-at-Large
Diffuse Lung Disease & Transplant Network
Occupational & Environmental Health Section
Quaternary ammonium compounds: exposure and lung disease
Quaternary ammonium compounds (QACS) are a common ingredient in many major commercial disinfectant products. During the COVID pandemic, the use of QACS increased due to their efficacy in inactivating enveloped viruses such as SARS-COV-2 (Hora, et al. Environ Sci & Technol Letters. 2020;7[9]:622).
Increasing data suggest a link between QAC exposure and occupational lung disease (Migueres, et al. J Allergy Clin Immunol Pract. 2021;9[9]:3387). Historically, exposure to QACs has been highest in health care workers. This is reflected in the increased risk of obstructive lung disease seen among nursing and operating room staff (Xie, et al. JAMA Netw Open. 2021;4[9] :e2125749). In the setting of enhanced COVID-19 cleaning protocols, QACS are increasingly utilized outside of the health care setting. Custodians and janitorial staff may face increased and potentially underrecognized exposure to these compounds. In addition to the direct harms of COVID-19, we may see an increase in occupational obstructive lung disease as a result of cleaning product exposure. Early diagnosis and exposure removal is crucial to prevent a new epidemic of occupational asthma.
Maeve MacMurdo, MBChB
Member-at-Large
Occupational & Environmental Health Section
Quaternary ammonium compounds: exposure and lung disease
Quaternary ammonium compounds (QACS) are a common ingredient in many major commercial disinfectant products. During the COVID pandemic, the use of QACS increased due to their efficacy in inactivating enveloped viruses such as SARS-COV-2 (Hora, et al. Environ Sci & Technol Letters. 2020;7[9]:622).
Increasing data suggest a link between QAC exposure and occupational lung disease (Migueres, et al. J Allergy Clin Immunol Pract. 2021;9[9]:3387). Historically, exposure to QACs has been highest in health care workers. This is reflected in the increased risk of obstructive lung disease seen among nursing and operating room staff (Xie, et al. JAMA Netw Open. 2021;4[9] :e2125749). In the setting of enhanced COVID-19 cleaning protocols, QACS are increasingly utilized outside of the health care setting. Custodians and janitorial staff may face increased and potentially underrecognized exposure to these compounds. In addition to the direct harms of COVID-19, we may see an increase in occupational obstructive lung disease as a result of cleaning product exposure. Early diagnosis and exposure removal is crucial to prevent a new epidemic of occupational asthma.
Maeve MacMurdo, MBChB
Member-at-Large
Occupational & Environmental Health Section
Quaternary ammonium compounds: exposure and lung disease
Quaternary ammonium compounds (QACS) are a common ingredient in many major commercial disinfectant products. During the COVID pandemic, the use of QACS increased due to their efficacy in inactivating enveloped viruses such as SARS-COV-2 (Hora, et al. Environ Sci & Technol Letters. 2020;7[9]:622).
Increasing data suggest a link between QAC exposure and occupational lung disease (Migueres, et al. J Allergy Clin Immunol Pract. 2021;9[9]:3387). Historically, exposure to QACs has been highest in health care workers. This is reflected in the increased risk of obstructive lung disease seen among nursing and operating room staff (Xie, et al. JAMA Netw Open. 2021;4[9] :e2125749). In the setting of enhanced COVID-19 cleaning protocols, QACS are increasingly utilized outside of the health care setting. Custodians and janitorial staff may face increased and potentially underrecognized exposure to these compounds. In addition to the direct harms of COVID-19, we may see an increase in occupational obstructive lung disease as a result of cleaning product exposure. Early diagnosis and exposure removal is crucial to prevent a new epidemic of occupational asthma.
Maeve MacMurdo, MBChB
Member-at-Large
Critical Care Network
Palliative and End-of-Life Care Section
Time-limited trials of critical care
Many patients die in the ICU, often after long courses of aggressive interventions, with potentially nonbeneficial treatments. Surrogate decision makers are tasked with decisions to initiate or forgo treatments based on recommendations from clinicians in the face of prognostic uncertainty and emotional duress. A strategy that has been adopted by ICU clinicians to address this has been proposing a “time-limited trial” (TLT) of ICU-specific interventions. A TLT involves clinicians partnering with patients and their surrogate decision makers in a shared decision-making model, proposing initiation of treatments for a set time, evaluating for specific measures of what is considered beneficial, and deciding to continue treatment or stop if without benefit. with palliative care teams (Vink EE, et al. Intensive Care Med. 2018;44:1369). Recent research about TLT in the ICU has found that when executed well, TLTs can improve quality of care and provide patients with the care they desire and can benefit from (Vink, et al). Additionally, the use of an education intervention for ICU clinicians regarding protocolled TLT interventions was associated with improved quality of family meetings, and, importantly, a reduced intensity and duration of ICU treatments (Chang DW, et al. JAMA Intern Med. 2021;181[6]:786).
Bradley Hayward, MD
Member-at-Large
Palliative and End-of-Life Care Section
Time-limited trials of critical care
Many patients die in the ICU, often after long courses of aggressive interventions, with potentially nonbeneficial treatments. Surrogate decision makers are tasked with decisions to initiate or forgo treatments based on recommendations from clinicians in the face of prognostic uncertainty and emotional duress. A strategy that has been adopted by ICU clinicians to address this has been proposing a “time-limited trial” (TLT) of ICU-specific interventions. A TLT involves clinicians partnering with patients and their surrogate decision makers in a shared decision-making model, proposing initiation of treatments for a set time, evaluating for specific measures of what is considered beneficial, and deciding to continue treatment or stop if without benefit. with palliative care teams (Vink EE, et al. Intensive Care Med. 2018;44:1369). Recent research about TLT in the ICU has found that when executed well, TLTs can improve quality of care and provide patients with the care they desire and can benefit from (Vink, et al). Additionally, the use of an education intervention for ICU clinicians regarding protocolled TLT interventions was associated with improved quality of family meetings, and, importantly, a reduced intensity and duration of ICU treatments (Chang DW, et al. JAMA Intern Med. 2021;181[6]:786).
Bradley Hayward, MD
Member-at-Large
Palliative and End-of-Life Care Section
Time-limited trials of critical care
Many patients die in the ICU, often after long courses of aggressive interventions, with potentially nonbeneficial treatments. Surrogate decision makers are tasked with decisions to initiate or forgo treatments based on recommendations from clinicians in the face of prognostic uncertainty and emotional duress. A strategy that has been adopted by ICU clinicians to address this has been proposing a “time-limited trial” (TLT) of ICU-specific interventions. A TLT involves clinicians partnering with patients and their surrogate decision makers in a shared decision-making model, proposing initiation of treatments for a set time, evaluating for specific measures of what is considered beneficial, and deciding to continue treatment or stop if without benefit. with palliative care teams (Vink EE, et al. Intensive Care Med. 2018;44:1369). Recent research about TLT in the ICU has found that when executed well, TLTs can improve quality of care and provide patients with the care they desire and can benefit from (Vink, et al). Additionally, the use of an education intervention for ICU clinicians regarding protocolled TLT interventions was associated with improved quality of family meetings, and, importantly, a reduced intensity and duration of ICU treatments (Chang DW, et al. JAMA Intern Med. 2021;181[6]:786).
Bradley Hayward, MD
Member-at-Large
PCCM diversity grant recipient looks to inhibit platelet endothelial interactions via NEDD9 to improve acute lung injury
In February, The American College of Chest Physicians (CHEST), the American Thoracic Society, and the American Lung Association announced a partnership with the prestigious Harold Amos Medical Faculty Development Program (AMFDP), a Robert Wood Johnson Foundation initiative, to sponsor a scholar in pulmonary and critical care medicine.
George Alba, MD, is a pulmonary and critical care physician investigator at Massachusetts General Hospital. Dr. Alba studied English Literature and Biology as an undergraduate at Washington University in St. Louis, where he worked in a developmental biology laboratory; earned his MD at the Mount Sinai School of Medicine, where he graduated AOA with Distinction in Medical Education; and then completed both Internal Medicine and Pulmonary and Critical Care Medicine training at Massachusetts General Hospital.
During his fellowship, Dr. Alba specialized in pulmonary and critical care medicine because he appreciated the variety that comes with working in the intensive care unit.
“I love the medical complexity, the physiology, and the decision-making,” said Dr. Alba. “I’ve always enjoyed all aspects of clinical medicine, so it was hard to choose a path, but the benefit of the ICU is that it allows me to take care of a spectrum of medical illness across all subspecialties.”
He continued, “What I loved about pulmonary, specifically, was that I could see patients in the hospital and in the ICU, perform procedures, and still have a longitudinal relationship with patients in the clinic, which gave me a very flexible, wide grasp of medicine.”
Growing up in a close-knit Cuban family and community, Dr. Alba was raised speaking Spanish at home and learned English primarily in school. Being bilingual helped him in medicine greatly: in clinic, in the hospital, and in the ICU, he is able to communicate directly with Spanish-speaking patients and their families. This became critically important during the COVID-19 pandemic when Chelsea, a primarily Hispanic community in Boston, was disproportionately impacted. The patients greatly benefited from Spanish-speaking clinicians to communicate with their family members who were unable to visit due to the infection control policies in place.
As an instructor of medicine at Harvard Medical School and pulmonary and critical care physician at Massachusetts General, Dr. Alba is actively engaged in clinical care, teaching, and research focusing primarily on mechanisms of pulmonary vascular dysfunction in lung disease.
Dr. Alba’s AMFDP award project is titled “Pulmonary Endothelial NEDD9 and Acute Lung Injury,” and through the proposed scientific aims, he looks to advance NEDD9 antagonism as a potential therapeutic target in acute respiratory distress syndrome (ARDS.) He is being co-mentored by Bradley Maron, MD, a pulmonary vascular disease researcher at Brigham and Women’s Hospital, and Eric Schmidt, MD, an endothelial biologist and expert in animal models of acute lung injury at Massachusetts General Hospital.
This is especially relevant research during the COVID-19 pandemic, as patients with severe lung injury frequently develop clotting in the lung blood vessels. Dr. Alba’s prior work demonstrated that NEDD9 is a pulmonary endothelial protein that is upregulated by hypoxia, that it binds to activated platelets to promote platelet adhesion and clotting, and that inhibition of NEDD9-platelet interactions with a custom antibody can decrease clotting in the lungs of animals. He recently showed that pulmonary endothelial NEDD9 is increased in patients with ARDS who demonstrate blood vessel clotting.
Now, Dr. Alba seeks to use a custom-made anti-NEDD9 antibody to block platelet adhesion in animal models of ARDS to decrease the extent of lung injury. While aspirin and anticoagulants have been unhelpful in treating ARDS in prior trials, Dr. Alba believes that circulating pulmonary endothelial protein NEDD9 can serve as a biomarker to identify subgroups of ARDS who may benefit from earlier targeted antithrombotic therapy.
Dr. Alba hopes that one day the anti-NEDD9 antibody may become one such therapeutic option for patients. The AMFDP will help support his ongoing work.
“Growing up, I saw through my father’s example how education unlocks opportunities. Our community came together to help him on this path. Now a retired doctor of osteopathy in neonatology, he inspired me to pursue a career in medicine,” said Dr. Alba. “This award comes at a critical time in my junior faculty career: It allows me to continue pursuing my research in a meaningful way while also gaining new skills that will be critical for my ongoing career development.”
Dr. Alba continued, “Programs like the Robert Wood Johnson Foundation initiative that specifically try to increase the number of individuals traditionally underrepresented in academia are key and would not be possible without the support of groups like CHEST, the American Lung Association, and the American Thoracic Society.
These programs help folks who may have other external barriers to being in academia, including socioeconomic pressures, lack of resources
Dr. Alba is also committed to paying it forward: “I want to ensure that the type of invested mentorship I experienced to help get me this far is not a matter of serendipity for the fortunate few, but rather a standard for all students and trainees, especially those from underrepresented backgrounds.”
In February, The American College of Chest Physicians (CHEST), the American Thoracic Society, and the American Lung Association announced a partnership with the prestigious Harold Amos Medical Faculty Development Program (AMFDP), a Robert Wood Johnson Foundation initiative, to sponsor a scholar in pulmonary and critical care medicine.
George Alba, MD, is a pulmonary and critical care physician investigator at Massachusetts General Hospital. Dr. Alba studied English Literature and Biology as an undergraduate at Washington University in St. Louis, where he worked in a developmental biology laboratory; earned his MD at the Mount Sinai School of Medicine, where he graduated AOA with Distinction in Medical Education; and then completed both Internal Medicine and Pulmonary and Critical Care Medicine training at Massachusetts General Hospital.
During his fellowship, Dr. Alba specialized in pulmonary and critical care medicine because he appreciated the variety that comes with working in the intensive care unit.
“I love the medical complexity, the physiology, and the decision-making,” said Dr. Alba. “I’ve always enjoyed all aspects of clinical medicine, so it was hard to choose a path, but the benefit of the ICU is that it allows me to take care of a spectrum of medical illness across all subspecialties.”
He continued, “What I loved about pulmonary, specifically, was that I could see patients in the hospital and in the ICU, perform procedures, and still have a longitudinal relationship with patients in the clinic, which gave me a very flexible, wide grasp of medicine.”
Growing up in a close-knit Cuban family and community, Dr. Alba was raised speaking Spanish at home and learned English primarily in school. Being bilingual helped him in medicine greatly: in clinic, in the hospital, and in the ICU, he is able to communicate directly with Spanish-speaking patients and their families. This became critically important during the COVID-19 pandemic when Chelsea, a primarily Hispanic community in Boston, was disproportionately impacted. The patients greatly benefited from Spanish-speaking clinicians to communicate with their family members who were unable to visit due to the infection control policies in place.
As an instructor of medicine at Harvard Medical School and pulmonary and critical care physician at Massachusetts General, Dr. Alba is actively engaged in clinical care, teaching, and research focusing primarily on mechanisms of pulmonary vascular dysfunction in lung disease.
Dr. Alba’s AMFDP award project is titled “Pulmonary Endothelial NEDD9 and Acute Lung Injury,” and through the proposed scientific aims, he looks to advance NEDD9 antagonism as a potential therapeutic target in acute respiratory distress syndrome (ARDS.) He is being co-mentored by Bradley Maron, MD, a pulmonary vascular disease researcher at Brigham and Women’s Hospital, and Eric Schmidt, MD, an endothelial biologist and expert in animal models of acute lung injury at Massachusetts General Hospital.
This is especially relevant research during the COVID-19 pandemic, as patients with severe lung injury frequently develop clotting in the lung blood vessels. Dr. Alba’s prior work demonstrated that NEDD9 is a pulmonary endothelial protein that is upregulated by hypoxia, that it binds to activated platelets to promote platelet adhesion and clotting, and that inhibition of NEDD9-platelet interactions with a custom antibody can decrease clotting in the lungs of animals. He recently showed that pulmonary endothelial NEDD9 is increased in patients with ARDS who demonstrate blood vessel clotting.
Now, Dr. Alba seeks to use a custom-made anti-NEDD9 antibody to block platelet adhesion in animal models of ARDS to decrease the extent of lung injury. While aspirin and anticoagulants have been unhelpful in treating ARDS in prior trials, Dr. Alba believes that circulating pulmonary endothelial protein NEDD9 can serve as a biomarker to identify subgroups of ARDS who may benefit from earlier targeted antithrombotic therapy.
Dr. Alba hopes that one day the anti-NEDD9 antibody may become one such therapeutic option for patients. The AMFDP will help support his ongoing work.
“Growing up, I saw through my father’s example how education unlocks opportunities. Our community came together to help him on this path. Now a retired doctor of osteopathy in neonatology, he inspired me to pursue a career in medicine,” said Dr. Alba. “This award comes at a critical time in my junior faculty career: It allows me to continue pursuing my research in a meaningful way while also gaining new skills that will be critical for my ongoing career development.”
Dr. Alba continued, “Programs like the Robert Wood Johnson Foundation initiative that specifically try to increase the number of individuals traditionally underrepresented in academia are key and would not be possible without the support of groups like CHEST, the American Lung Association, and the American Thoracic Society.
These programs help folks who may have other external barriers to being in academia, including socioeconomic pressures, lack of resources
Dr. Alba is also committed to paying it forward: “I want to ensure that the type of invested mentorship I experienced to help get me this far is not a matter of serendipity for the fortunate few, but rather a standard for all students and trainees, especially those from underrepresented backgrounds.”
In February, The American College of Chest Physicians (CHEST), the American Thoracic Society, and the American Lung Association announced a partnership with the prestigious Harold Amos Medical Faculty Development Program (AMFDP), a Robert Wood Johnson Foundation initiative, to sponsor a scholar in pulmonary and critical care medicine.
George Alba, MD, is a pulmonary and critical care physician investigator at Massachusetts General Hospital. Dr. Alba studied English Literature and Biology as an undergraduate at Washington University in St. Louis, where he worked in a developmental biology laboratory; earned his MD at the Mount Sinai School of Medicine, where he graduated AOA with Distinction in Medical Education; and then completed both Internal Medicine and Pulmonary and Critical Care Medicine training at Massachusetts General Hospital.
During his fellowship, Dr. Alba specialized in pulmonary and critical care medicine because he appreciated the variety that comes with working in the intensive care unit.
“I love the medical complexity, the physiology, and the decision-making,” said Dr. Alba. “I’ve always enjoyed all aspects of clinical medicine, so it was hard to choose a path, but the benefit of the ICU is that it allows me to take care of a spectrum of medical illness across all subspecialties.”
He continued, “What I loved about pulmonary, specifically, was that I could see patients in the hospital and in the ICU, perform procedures, and still have a longitudinal relationship with patients in the clinic, which gave me a very flexible, wide grasp of medicine.”
Growing up in a close-knit Cuban family and community, Dr. Alba was raised speaking Spanish at home and learned English primarily in school. Being bilingual helped him in medicine greatly: in clinic, in the hospital, and in the ICU, he is able to communicate directly with Spanish-speaking patients and their families. This became critically important during the COVID-19 pandemic when Chelsea, a primarily Hispanic community in Boston, was disproportionately impacted. The patients greatly benefited from Spanish-speaking clinicians to communicate with their family members who were unable to visit due to the infection control policies in place.
As an instructor of medicine at Harvard Medical School and pulmonary and critical care physician at Massachusetts General, Dr. Alba is actively engaged in clinical care, teaching, and research focusing primarily on mechanisms of pulmonary vascular dysfunction in lung disease.
Dr. Alba’s AMFDP award project is titled “Pulmonary Endothelial NEDD9 and Acute Lung Injury,” and through the proposed scientific aims, he looks to advance NEDD9 antagonism as a potential therapeutic target in acute respiratory distress syndrome (ARDS.) He is being co-mentored by Bradley Maron, MD, a pulmonary vascular disease researcher at Brigham and Women’s Hospital, and Eric Schmidt, MD, an endothelial biologist and expert in animal models of acute lung injury at Massachusetts General Hospital.
This is especially relevant research during the COVID-19 pandemic, as patients with severe lung injury frequently develop clotting in the lung blood vessels. Dr. Alba’s prior work demonstrated that NEDD9 is a pulmonary endothelial protein that is upregulated by hypoxia, that it binds to activated platelets to promote platelet adhesion and clotting, and that inhibition of NEDD9-platelet interactions with a custom antibody can decrease clotting in the lungs of animals. He recently showed that pulmonary endothelial NEDD9 is increased in patients with ARDS who demonstrate blood vessel clotting.
Now, Dr. Alba seeks to use a custom-made anti-NEDD9 antibody to block platelet adhesion in animal models of ARDS to decrease the extent of lung injury. While aspirin and anticoagulants have been unhelpful in treating ARDS in prior trials, Dr. Alba believes that circulating pulmonary endothelial protein NEDD9 can serve as a biomarker to identify subgroups of ARDS who may benefit from earlier targeted antithrombotic therapy.
Dr. Alba hopes that one day the anti-NEDD9 antibody may become one such therapeutic option for patients. The AMFDP will help support his ongoing work.
“Growing up, I saw through my father’s example how education unlocks opportunities. Our community came together to help him on this path. Now a retired doctor of osteopathy in neonatology, he inspired me to pursue a career in medicine,” said Dr. Alba. “This award comes at a critical time in my junior faculty career: It allows me to continue pursuing my research in a meaningful way while also gaining new skills that will be critical for my ongoing career development.”
Dr. Alba continued, “Programs like the Robert Wood Johnson Foundation initiative that specifically try to increase the number of individuals traditionally underrepresented in academia are key and would not be possible without the support of groups like CHEST, the American Lung Association, and the American Thoracic Society.
These programs help folks who may have other external barriers to being in academia, including socioeconomic pressures, lack of resources
Dr. Alba is also committed to paying it forward: “I want to ensure that the type of invested mentorship I experienced to help get me this far is not a matter of serendipity for the fortunate few, but rather a standard for all students and trainees, especially those from underrepresented backgrounds.”
Is another COVID-19 booster really needed?
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Many countries around the globe are starting to roll out another booster of the COVID-19 vaccine but, with public interest waning and a sense of normalcy firmly installed in our minds, this may prove an ill-fated effort, unless authorities can provide a coherent answer to the question “Is another jab really needed?” (The short answer is a firm “yes,” of course.)
In what we could call the “chronic” phase of the pandemic, most countries have now settled for a certain number of daily cases and a (relatively low) number of complications and deaths. It’s the vaccines that have afforded us this peace of mind, lest we forget. But they are different to other vaccines that we are more familiar with, such as the MMR that we get as kids and then forget about for the rest of our lives. As good as the different COVID-19 vaccines are, they never came with the promise of generating lifelong antibodies. We knew early on that the immunity they provide slowly wanes with time. That doesn’t mean that those who have their vaccination records up to date (which included a booster probably earlier in 2022) are suddenly exposed. Data suggest that although people several months past their last booster would now be more prone to getting reinfected, the protection against severe disease still hangs around 85%. In other words, their chances of ending up in the hospital are low.
Why worry, then, about further boosting the immune system? The same studies show that an additional jab would increase this percentage up to 99%. Is this roughly 10% improvement really worth another worldwide vaccination campaign? Well, this is a numbers game, after all. The current form of the virus is extremely infectious, and the Northern Hemisphere is heading toward the cold months of the year, which we have seen in past years increases COVID-19 contagions, as you would expect from any airborne virus. Thus, it’s easy to expect a new peak in the number of cases, especially considering that we are not going to apply any of the usual restrictions to prevent this. In these conditions, extending the safety net to a further 10% of the population would substantially reduce the total number of victims. It seems like a good investment of resources.
We can be more surgical about it and direct this new vaccination campaign to the population most likely to end up in the hospital. People with concomitant pathologies are at the top of the list, but it’s also an age issue. On the basis of different studies of the most common ages of admission, the cutoff point for the booster varies from country to country, with the lowest being 50 and in other cases hovering around 65 years of age. Given the safety of these vaccines, if we can afford it, the wider we cast the net, the better, but at least we should make every effort to fully vaccinate the higher age brackets.
The final question is which vaccine to give. There are confounding studies about the importance of switching to Omicron-specific jabs, which are finally available. Although this seems like a good idea, since Omicron infections elicit a more effective range of antibodies and new variants seem to better escape our defenses, recent studies suggest that there actually may not be so much difference with the old formula.
The conclusion? This regimen of yearly boosters for some may be the scenario for the upcoming years, similar to what we already do for the flu, so we should get used to it.
Dr. Macip is associate professor, department of molecular and cellular biology, University of Leicester (England). He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Mutation burden predicts ICI response in lung cancer
FROM JAMA ONCOLOGY
after treatment with PD-1 and PD-L1 inhibitors. The findings could supplement other biomarkers, and suggest that chemotherapy could be avoided in some patients.
“We found a TMB value … of 19 mutations per megabase was a strong discriminator of response and nonresponse, and that corresponds to approximately the 90th percentile for TMB in our dataset. That is a higher threshold than has been previously proposed to be used for a TMB cutoff across different datasets or in lung cancer, but it did seem to be a strong discriminator of response, and that also translated into an improvement in progression free survival and overall survival in patients treated with immunotherapy,” said study coauthor Mark Awad, MD, PhD, in a podcast hosted by JAMA. He is a cancer researcher at Harvard Medical School, Boston. The research was published online in JAMA Oncology.
The value was reinforced when the team looked at deciles of TMB, from the lowest 10%, 20%, up to 90%. “It did seem like there was an inflection point, but only at the really higher levels of TMB – above the 80th, or especially the 90th percentile for TMB. That’s where it seemed to make a big difference in terms of improvements in response rate, progression-free, and overall survival,” Dr. Awad said.
The values of TMB and levels of PD-L1 expression also interacted in a useful way. “If you’re looking at PD-L1 on one axis and TMB on the other, it does seem that higher PD-L1 and the higher TMB can really identify patients with strong and great outcomes to immune checkpoint inhibitors. By contrast, low PD-L1 and low TMB really identifies patients that are not likely to benefit from immunotherapy alone and obviously might need to escalate it or more intensified therapy,” he said.
The results could help inform clinical decisions, though Dr. Awad included a caveat that the study was retrospective. In particular, patients with high TMB levels who might not tolerate chemotherapy well could be candidates for immunotherapy alone, “if you feel like there would be time to try immunotherapy alone rather than chemoimmunotherapy, and hopefully spare or avoid some of the chemotherapy toxicities, with the understanding that you wouldn’t want a patient’s disease to rapidly progress. You have to choose these cases carefully,” he said.
Dr. Awad suggested that TMB can be used alongside other factors such as PD-L1 mutations, KRAS mutation status, STK-11, and KEAP1 mutations. “I think all of these features will start to tip the scales one way or the other in terms of using immunotherapy alone or immunotherapy in combination with chemotherapy, and hopefully as new trials are developed, TMB and other predictive biomarkers can be used to stratify populations within a trial to hopefully ensure balance between treatment arms, and also to identify cancers that are less likely to respond to immune checkpoint inhibitors, such that we can really develop more tailored regimens for patients that will or won’t be as likely to respond to immunotherapy.”
The study included 1,552 patients with advanced NSCLC, with a median age of 66; 53.5% were women. The median TMB was 9.82 mutations per megabase. The researchers categorized patients as low TMB (fewer than 19 mutations per megabase) or high TMB (19 or more mutations). The high TMB group associated with better outcomes after treatment with PD-1/PD-L1 inhibitors, including overall response rate, progression-free survival, and overall survival. The associations occurred in the discovery cohort as well as two other independent cohorts. The same relationship occurred in all PD-L1 tumor proportion score subgroups.
Patients with NSCLCs with high TMB as well as PD-L1 expression of 50% or higher had an overall response rate of 57% and had the longest PFS and OS with ICI treatment (18.1 months and 47.7 months, respectively). On the other hand, patients with low TMB and PD-L1–negative NSCLC had the lowest ORR at 8.7% and the shortest PFS and OS (2.1 months and 10.4 months, respectively).
Dr. Awad has consulted for Achilles, AbbVie, Neon, Maverick, Nektar, and Hegrui. He has received grants and personal fees from Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, and Lilly. He has received personal fees from Maverick, Blueprint Medicine, Syndax, Ariad, Nektar, Gritstone, ArcherDx, Mirati, NextCure, Novartis, EMD Serono, and NovaRx.
FROM JAMA ONCOLOGY
after treatment with PD-1 and PD-L1 inhibitors. The findings could supplement other biomarkers, and suggest that chemotherapy could be avoided in some patients.
“We found a TMB value … of 19 mutations per megabase was a strong discriminator of response and nonresponse, and that corresponds to approximately the 90th percentile for TMB in our dataset. That is a higher threshold than has been previously proposed to be used for a TMB cutoff across different datasets or in lung cancer, but it did seem to be a strong discriminator of response, and that also translated into an improvement in progression free survival and overall survival in patients treated with immunotherapy,” said study coauthor Mark Awad, MD, PhD, in a podcast hosted by JAMA. He is a cancer researcher at Harvard Medical School, Boston. The research was published online in JAMA Oncology.
The value was reinforced when the team looked at deciles of TMB, from the lowest 10%, 20%, up to 90%. “It did seem like there was an inflection point, but only at the really higher levels of TMB – above the 80th, or especially the 90th percentile for TMB. That’s where it seemed to make a big difference in terms of improvements in response rate, progression-free, and overall survival,” Dr. Awad said.
The values of TMB and levels of PD-L1 expression also interacted in a useful way. “If you’re looking at PD-L1 on one axis and TMB on the other, it does seem that higher PD-L1 and the higher TMB can really identify patients with strong and great outcomes to immune checkpoint inhibitors. By contrast, low PD-L1 and low TMB really identifies patients that are not likely to benefit from immunotherapy alone and obviously might need to escalate it or more intensified therapy,” he said.
The results could help inform clinical decisions, though Dr. Awad included a caveat that the study was retrospective. In particular, patients with high TMB levels who might not tolerate chemotherapy well could be candidates for immunotherapy alone, “if you feel like there would be time to try immunotherapy alone rather than chemoimmunotherapy, and hopefully spare or avoid some of the chemotherapy toxicities, with the understanding that you wouldn’t want a patient’s disease to rapidly progress. You have to choose these cases carefully,” he said.
Dr. Awad suggested that TMB can be used alongside other factors such as PD-L1 mutations, KRAS mutation status, STK-11, and KEAP1 mutations. “I think all of these features will start to tip the scales one way or the other in terms of using immunotherapy alone or immunotherapy in combination with chemotherapy, and hopefully as new trials are developed, TMB and other predictive biomarkers can be used to stratify populations within a trial to hopefully ensure balance between treatment arms, and also to identify cancers that are less likely to respond to immune checkpoint inhibitors, such that we can really develop more tailored regimens for patients that will or won’t be as likely to respond to immunotherapy.”
The study included 1,552 patients with advanced NSCLC, with a median age of 66; 53.5% were women. The median TMB was 9.82 mutations per megabase. The researchers categorized patients as low TMB (fewer than 19 mutations per megabase) or high TMB (19 or more mutations). The high TMB group associated with better outcomes after treatment with PD-1/PD-L1 inhibitors, including overall response rate, progression-free survival, and overall survival. The associations occurred in the discovery cohort as well as two other independent cohorts. The same relationship occurred in all PD-L1 tumor proportion score subgroups.
Patients with NSCLCs with high TMB as well as PD-L1 expression of 50% or higher had an overall response rate of 57% and had the longest PFS and OS with ICI treatment (18.1 months and 47.7 months, respectively). On the other hand, patients with low TMB and PD-L1–negative NSCLC had the lowest ORR at 8.7% and the shortest PFS and OS (2.1 months and 10.4 months, respectively).
Dr. Awad has consulted for Achilles, AbbVie, Neon, Maverick, Nektar, and Hegrui. He has received grants and personal fees from Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, and Lilly. He has received personal fees from Maverick, Blueprint Medicine, Syndax, Ariad, Nektar, Gritstone, ArcherDx, Mirati, NextCure, Novartis, EMD Serono, and NovaRx.
FROM JAMA ONCOLOGY
after treatment with PD-1 and PD-L1 inhibitors. The findings could supplement other biomarkers, and suggest that chemotherapy could be avoided in some patients.
“We found a TMB value … of 19 mutations per megabase was a strong discriminator of response and nonresponse, and that corresponds to approximately the 90th percentile for TMB in our dataset. That is a higher threshold than has been previously proposed to be used for a TMB cutoff across different datasets or in lung cancer, but it did seem to be a strong discriminator of response, and that also translated into an improvement in progression free survival and overall survival in patients treated with immunotherapy,” said study coauthor Mark Awad, MD, PhD, in a podcast hosted by JAMA. He is a cancer researcher at Harvard Medical School, Boston. The research was published online in JAMA Oncology.
The value was reinforced when the team looked at deciles of TMB, from the lowest 10%, 20%, up to 90%. “It did seem like there was an inflection point, but only at the really higher levels of TMB – above the 80th, or especially the 90th percentile for TMB. That’s where it seemed to make a big difference in terms of improvements in response rate, progression-free, and overall survival,” Dr. Awad said.
The values of TMB and levels of PD-L1 expression also interacted in a useful way. “If you’re looking at PD-L1 on one axis and TMB on the other, it does seem that higher PD-L1 and the higher TMB can really identify patients with strong and great outcomes to immune checkpoint inhibitors. By contrast, low PD-L1 and low TMB really identifies patients that are not likely to benefit from immunotherapy alone and obviously might need to escalate it or more intensified therapy,” he said.
The results could help inform clinical decisions, though Dr. Awad included a caveat that the study was retrospective. In particular, patients with high TMB levels who might not tolerate chemotherapy well could be candidates for immunotherapy alone, “if you feel like there would be time to try immunotherapy alone rather than chemoimmunotherapy, and hopefully spare or avoid some of the chemotherapy toxicities, with the understanding that you wouldn’t want a patient’s disease to rapidly progress. You have to choose these cases carefully,” he said.
Dr. Awad suggested that TMB can be used alongside other factors such as PD-L1 mutations, KRAS mutation status, STK-11, and KEAP1 mutations. “I think all of these features will start to tip the scales one way or the other in terms of using immunotherapy alone or immunotherapy in combination with chemotherapy, and hopefully as new trials are developed, TMB and other predictive biomarkers can be used to stratify populations within a trial to hopefully ensure balance between treatment arms, and also to identify cancers that are less likely to respond to immune checkpoint inhibitors, such that we can really develop more tailored regimens for patients that will or won’t be as likely to respond to immunotherapy.”
The study included 1,552 patients with advanced NSCLC, with a median age of 66; 53.5% were women. The median TMB was 9.82 mutations per megabase. The researchers categorized patients as low TMB (fewer than 19 mutations per megabase) or high TMB (19 or more mutations). The high TMB group associated with better outcomes after treatment with PD-1/PD-L1 inhibitors, including overall response rate, progression-free survival, and overall survival. The associations occurred in the discovery cohort as well as two other independent cohorts. The same relationship occurred in all PD-L1 tumor proportion score subgroups.
Patients with NSCLCs with high TMB as well as PD-L1 expression of 50% or higher had an overall response rate of 57% and had the longest PFS and OS with ICI treatment (18.1 months and 47.7 months, respectively). On the other hand, patients with low TMB and PD-L1–negative NSCLC had the lowest ORR at 8.7% and the shortest PFS and OS (2.1 months and 10.4 months, respectively).
Dr. Awad has consulted for Achilles, AbbVie, Neon, Maverick, Nektar, and Hegrui. He has received grants and personal fees from Genentech, Bristol-Myers Squibb, Merck, AstraZeneca, and Lilly. He has received personal fees from Maverick, Blueprint Medicine, Syndax, Ariad, Nektar, Gritstone, ArcherDx, Mirati, NextCure, Novartis, EMD Serono, and NovaRx.
Salt pills for patients with acute decompensated heart failure?
Restriction of dietary salt to alleviate or prevent volume overload in patients with acute decompensated heart failure (ADHF) is common hospital practice, but without a solid evidence base. A trial testing whether taking salt pills might have benefits for patients with ADHF undergoing intensive diuresis, therefore, may seem a bit counterintuitive.
In just such a randomized, placebo-controlled trial, the approach made no difference to weight loss on diuresis, a proxy for volume reduction, or to serum creatinine levels in ADHF patients receiving high-dose intravenous diuretic therapy.
The patients consumed the extra salt during their intravenous therapy in the form of tablets providing 6 g sodium chloride daily on top of their hospital-provided, low-sodium meals.
During that time, serum sodium levels remained stable for the 34 patients assigned to the salt tablets but dropped significantly in the 31 given placebo pills.
They lost about the same weight, averages of 4 kg and 4.6 kg (8.8-10 lb), respectively, and their urine output was also similar. Patients who took the salt tablets showed less of an increase in blood urea nitrogen (BUN) at both 96 hours and at discharge.
The findings “challenge the routine practice of sodium chloride restriction in acute heart failure, something done thousands of times a day, millions of times a year,” Robert A. Montgomery, MD, Cleveland Clinic, said when presenting the study at the annual scientific meeting of the Heart Failure Society of America.
The trial, called OSPREY-AHF (Oral Sodium to Preserve Renal Efficiency in Acute Heart Failure), also may encourage a shift in ADHF management from a preoccupation with salt restriction to focus more on fighting fluid retention.
OSPREY-HF took on “an established practice that doesn’t have much high-quality evidentiary support,” one guided primarily by consensus and observational data, Montgomery said in an interview.
There are also potential downsides to dietary sodium restriction, including some that may complicate or block ADHF therapies.
“Low-sodium diets can be associated with decreased caloric intake and nutritional quality,” Dr. Montgomery observed. And observational studies suggest that “patients who are on a low sodium diet can develop increased neurohormonal activation. The kidney is not sensing salt, and so starts ramping up the hormones,” which promotes diuretic resistance.
But emerging evidence also suggests “that giving sodium chloride in the form of hypertonic saline can help patients who are diuretic resistant.” The intervention, which appears to attenuate the neurohormonal activation associated with high-dose intravenous diuretics, Dr. Montgomery noted, helped inspire the design of OSPREY-AHF.
Edema consists of “a gallon of water and a pinch of salt, so we really should stop being so salt-centric and think much more about water as the problem in decompensated heart failure,” said John G.F. Cleland, MD, PhD, during the question-and-answer period after Montgomery’s presentation. Dr. Cleland, of the University of Glasgow Institute of Health and Wellbeing, is not connected to OSPREY-AHF.
“I think that maybe we overinterpret how important salt is” as a focus of volume management in ADHF, offered David Lanfear, MD, Henry Ford Health System, Detroit, who is also not part of the study.
OSPREY-AHF was well conducted but applies to a “very specific” clinical setting, Dr. Lanfear said in an interview. “These people are getting aggressive diuresis, a big dose and continuous infusion. It’s not everybody that has heart failure.”
Although the study was small, “I think it will fuel interest in this area and, probably, further investigation,” he said. The trial on its own won’t change practice, “but it will raise some eyebrows.”
The trial included patients with ADHF who have been “admitted to a cardiovascular medicine floor, not the intensive care unit” and were receiving at least 10 mg per hour of furosemide. It excluded any who were “hypernatremic or severely hyponatremic,” said Dr. Montgomery when presenting the study. They were required to have an initial estimated glomerular filtration rate (eGFR) of at least 15 mL/min per 1.73 m2.
The patients were randomly assigned double blind at a single center to receive tablets providing 2 g sodium chloride or placebo pills – 34 and 31 patients, respectively – three times daily during intravenous diuresis.
At 96 hours, the two groups showed no difference in change in creatinine levels or change in weight, both primary endpoints. Nor did they differ in urine output or change in eGFR. But serum sodium levels fell further, and BUN levels went up more in those given placebo.
The two groups showed no differences in hospital length of stay, use of renal replacement therapy at 90 days, ICU time during the index hospitalization, 30-day readmission, or 90-day mortality – although the trial wasn’t powered for clinical outcomes, Dr. Montgomery reported.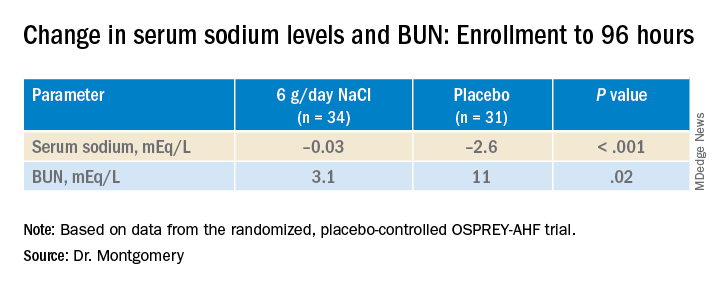
"We have patients who complain about their sodium-restricted diet, we have patients that have cachexia, who have a lot of complaints about provider-ordered meals and recommendations,” Dr. Montgomery explained in an interview.
Clinicians provide education and invest a lot of effort into getting patients with heart failure to start and maintain a low-sodium diet, he said. “But a low-sodium diet, in prior studies – and our study adds to this – is not a lever that actually seems to positively or adversely affect patients.”
Dr. Montgomery pointed to the recently published SODIUM-HF trial comparing low-sodium and unrestricted-sodium diets in outpatients with heart failure. It saw no clinical benefit from the low-sodium intervention.
Until studies show, potentially, that sodium restriction in hospitalized patients with heart failure makes a clinical difference, Dr. Montgomery said, “I’d say we should invest our time in things that we know are the most helpful, like getting them on guideline-directed medical therapy, when instead we spend an enormous amount of time counseling on and enforcing dietary restriction.”
Support for this study was provided by Cleveland Clinic Heart Vascular and Thoracic Institute’s Wilson Grant and Kaufman Center for Heart Failure Treatment and Recovery Grant. Dr. Lanfear disclosed research support from SomaLogic and Lilly; consulting for Abbott Laboratories, AstraZeneca, Janssen, Martin Pharmaceuticals, and Amgen; and serving on advisory panels for Illumina and Cytokinetics. Dr. Montgomery and Dr. Cleland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Restriction of dietary salt to alleviate or prevent volume overload in patients with acute decompensated heart failure (ADHF) is common hospital practice, but without a solid evidence base. A trial testing whether taking salt pills might have benefits for patients with ADHF undergoing intensive diuresis, therefore, may seem a bit counterintuitive.
In just such a randomized, placebo-controlled trial, the approach made no difference to weight loss on diuresis, a proxy for volume reduction, or to serum creatinine levels in ADHF patients receiving high-dose intravenous diuretic therapy.
The patients consumed the extra salt during their intravenous therapy in the form of tablets providing 6 g sodium chloride daily on top of their hospital-provided, low-sodium meals.
During that time, serum sodium levels remained stable for the 34 patients assigned to the salt tablets but dropped significantly in the 31 given placebo pills.
They lost about the same weight, averages of 4 kg and 4.6 kg (8.8-10 lb), respectively, and their urine output was also similar. Patients who took the salt tablets showed less of an increase in blood urea nitrogen (BUN) at both 96 hours and at discharge.
The findings “challenge the routine practice of sodium chloride restriction in acute heart failure, something done thousands of times a day, millions of times a year,” Robert A. Montgomery, MD, Cleveland Clinic, said when presenting the study at the annual scientific meeting of the Heart Failure Society of America.
The trial, called OSPREY-AHF (Oral Sodium to Preserve Renal Efficiency in Acute Heart Failure), also may encourage a shift in ADHF management from a preoccupation with salt restriction to focus more on fighting fluid retention.
OSPREY-HF took on “an established practice that doesn’t have much high-quality evidentiary support,” one guided primarily by consensus and observational data, Montgomery said in an interview.
There are also potential downsides to dietary sodium restriction, including some that may complicate or block ADHF therapies.
“Low-sodium diets can be associated with decreased caloric intake and nutritional quality,” Dr. Montgomery observed. And observational studies suggest that “patients who are on a low sodium diet can develop increased neurohormonal activation. The kidney is not sensing salt, and so starts ramping up the hormones,” which promotes diuretic resistance.
But emerging evidence also suggests “that giving sodium chloride in the form of hypertonic saline can help patients who are diuretic resistant.” The intervention, which appears to attenuate the neurohormonal activation associated with high-dose intravenous diuretics, Dr. Montgomery noted, helped inspire the design of OSPREY-AHF.
Edema consists of “a gallon of water and a pinch of salt, so we really should stop being so salt-centric and think much more about water as the problem in decompensated heart failure,” said John G.F. Cleland, MD, PhD, during the question-and-answer period after Montgomery’s presentation. Dr. Cleland, of the University of Glasgow Institute of Health and Wellbeing, is not connected to OSPREY-AHF.
“I think that maybe we overinterpret how important salt is” as a focus of volume management in ADHF, offered David Lanfear, MD, Henry Ford Health System, Detroit, who is also not part of the study.
OSPREY-AHF was well conducted but applies to a “very specific” clinical setting, Dr. Lanfear said in an interview. “These people are getting aggressive diuresis, a big dose and continuous infusion. It’s not everybody that has heart failure.”
Although the study was small, “I think it will fuel interest in this area and, probably, further investigation,” he said. The trial on its own won’t change practice, “but it will raise some eyebrows.”
The trial included patients with ADHF who have been “admitted to a cardiovascular medicine floor, not the intensive care unit” and were receiving at least 10 mg per hour of furosemide. It excluded any who were “hypernatremic or severely hyponatremic,” said Dr. Montgomery when presenting the study. They were required to have an initial estimated glomerular filtration rate (eGFR) of at least 15 mL/min per 1.73 m2.
The patients were randomly assigned double blind at a single center to receive tablets providing 2 g sodium chloride or placebo pills – 34 and 31 patients, respectively – three times daily during intravenous diuresis.
At 96 hours, the two groups showed no difference in change in creatinine levels or change in weight, both primary endpoints. Nor did they differ in urine output or change in eGFR. But serum sodium levels fell further, and BUN levels went up more in those given placebo.
The two groups showed no differences in hospital length of stay, use of renal replacement therapy at 90 days, ICU time during the index hospitalization, 30-day readmission, or 90-day mortality – although the trial wasn’t powered for clinical outcomes, Dr. Montgomery reported.
"We have patients who complain about their sodium-restricted diet, we have patients that have cachexia, who have a lot of complaints about provider-ordered meals and recommendations,” Dr. Montgomery explained in an interview.
Clinicians provide education and invest a lot of effort into getting patients with heart failure to start and maintain a low-sodium diet, he said. “But a low-sodium diet, in prior studies – and our study adds to this – is not a lever that actually seems to positively or adversely affect patients.”
Dr. Montgomery pointed to the recently published SODIUM-HF trial comparing low-sodium and unrestricted-sodium diets in outpatients with heart failure. It saw no clinical benefit from the low-sodium intervention.
Until studies show, potentially, that sodium restriction in hospitalized patients with heart failure makes a clinical difference, Dr. Montgomery said, “I’d say we should invest our time in things that we know are the most helpful, like getting them on guideline-directed medical therapy, when instead we spend an enormous amount of time counseling on and enforcing dietary restriction.”
Support for this study was provided by Cleveland Clinic Heart Vascular and Thoracic Institute’s Wilson Grant and Kaufman Center for Heart Failure Treatment and Recovery Grant. Dr. Lanfear disclosed research support from SomaLogic and Lilly; consulting for Abbott Laboratories, AstraZeneca, Janssen, Martin Pharmaceuticals, and Amgen; and serving on advisory panels for Illumina and Cytokinetics. Dr. Montgomery and Dr. Cleland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Restriction of dietary salt to alleviate or prevent volume overload in patients with acute decompensated heart failure (ADHF) is common hospital practice, but without a solid evidence base. A trial testing whether taking salt pills might have benefits for patients with ADHF undergoing intensive diuresis, therefore, may seem a bit counterintuitive.
In just such a randomized, placebo-controlled trial, the approach made no difference to weight loss on diuresis, a proxy for volume reduction, or to serum creatinine levels in ADHF patients receiving high-dose intravenous diuretic therapy.
The patients consumed the extra salt during their intravenous therapy in the form of tablets providing 6 g sodium chloride daily on top of their hospital-provided, low-sodium meals.
During that time, serum sodium levels remained stable for the 34 patients assigned to the salt tablets but dropped significantly in the 31 given placebo pills.
They lost about the same weight, averages of 4 kg and 4.6 kg (8.8-10 lb), respectively, and their urine output was also similar. Patients who took the salt tablets showed less of an increase in blood urea nitrogen (BUN) at both 96 hours and at discharge.
The findings “challenge the routine practice of sodium chloride restriction in acute heart failure, something done thousands of times a day, millions of times a year,” Robert A. Montgomery, MD, Cleveland Clinic, said when presenting the study at the annual scientific meeting of the Heart Failure Society of America.
The trial, called OSPREY-AHF (Oral Sodium to Preserve Renal Efficiency in Acute Heart Failure), also may encourage a shift in ADHF management from a preoccupation with salt restriction to focus more on fighting fluid retention.
OSPREY-HF took on “an established practice that doesn’t have much high-quality evidentiary support,” one guided primarily by consensus and observational data, Montgomery said in an interview.
There are also potential downsides to dietary sodium restriction, including some that may complicate or block ADHF therapies.
“Low-sodium diets can be associated with decreased caloric intake and nutritional quality,” Dr. Montgomery observed. And observational studies suggest that “patients who are on a low sodium diet can develop increased neurohormonal activation. The kidney is not sensing salt, and so starts ramping up the hormones,” which promotes diuretic resistance.
But emerging evidence also suggests “that giving sodium chloride in the form of hypertonic saline can help patients who are diuretic resistant.” The intervention, which appears to attenuate the neurohormonal activation associated with high-dose intravenous diuretics, Dr. Montgomery noted, helped inspire the design of OSPREY-AHF.
Edema consists of “a gallon of water and a pinch of salt, so we really should stop being so salt-centric and think much more about water as the problem in decompensated heart failure,” said John G.F. Cleland, MD, PhD, during the question-and-answer period after Montgomery’s presentation. Dr. Cleland, of the University of Glasgow Institute of Health and Wellbeing, is not connected to OSPREY-AHF.
“I think that maybe we overinterpret how important salt is” as a focus of volume management in ADHF, offered David Lanfear, MD, Henry Ford Health System, Detroit, who is also not part of the study.
OSPREY-AHF was well conducted but applies to a “very specific” clinical setting, Dr. Lanfear said in an interview. “These people are getting aggressive diuresis, a big dose and continuous infusion. It’s not everybody that has heart failure.”
Although the study was small, “I think it will fuel interest in this area and, probably, further investigation,” he said. The trial on its own won’t change practice, “but it will raise some eyebrows.”
The trial included patients with ADHF who have been “admitted to a cardiovascular medicine floor, not the intensive care unit” and were receiving at least 10 mg per hour of furosemide. It excluded any who were “hypernatremic or severely hyponatremic,” said Dr. Montgomery when presenting the study. They were required to have an initial estimated glomerular filtration rate (eGFR) of at least 15 mL/min per 1.73 m2.
The patients were randomly assigned double blind at a single center to receive tablets providing 2 g sodium chloride or placebo pills – 34 and 31 patients, respectively – three times daily during intravenous diuresis.
At 96 hours, the two groups showed no difference in change in creatinine levels or change in weight, both primary endpoints. Nor did they differ in urine output or change in eGFR. But serum sodium levels fell further, and BUN levels went up more in those given placebo.
The two groups showed no differences in hospital length of stay, use of renal replacement therapy at 90 days, ICU time during the index hospitalization, 30-day readmission, or 90-day mortality – although the trial wasn’t powered for clinical outcomes, Dr. Montgomery reported.
"We have patients who complain about their sodium-restricted diet, we have patients that have cachexia, who have a lot of complaints about provider-ordered meals and recommendations,” Dr. Montgomery explained in an interview.
Clinicians provide education and invest a lot of effort into getting patients with heart failure to start and maintain a low-sodium diet, he said. “But a low-sodium diet, in prior studies – and our study adds to this – is not a lever that actually seems to positively or adversely affect patients.”
Dr. Montgomery pointed to the recently published SODIUM-HF trial comparing low-sodium and unrestricted-sodium diets in outpatients with heart failure. It saw no clinical benefit from the low-sodium intervention.
Until studies show, potentially, that sodium restriction in hospitalized patients with heart failure makes a clinical difference, Dr. Montgomery said, “I’d say we should invest our time in things that we know are the most helpful, like getting them on guideline-directed medical therapy, when instead we spend an enormous amount of time counseling on and enforcing dietary restriction.”
Support for this study was provided by Cleveland Clinic Heart Vascular and Thoracic Institute’s Wilson Grant and Kaufman Center for Heart Failure Treatment and Recovery Grant. Dr. Lanfear disclosed research support from SomaLogic and Lilly; consulting for Abbott Laboratories, AstraZeneca, Janssen, Martin Pharmaceuticals, and Amgen; and serving on advisory panels for Illumina and Cytokinetics. Dr. Montgomery and Dr. Cleland disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HFSA 2022



