User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Chinese herbal medicine may offer benefits in STEMI: CTS-AMI
CHICAGO – , the CTS-AMI study suggests.
Compared with those assigned to placebo, Chinese patients assigned to tongxinluo had lower rates of 30-day and 1-year major adverse cardiovascular and cerebrovascular events (MACCE), driven by fewer cardiac deaths. Severe STEMI complications were also lower.
Tongxinluo, which contains 10 or more potential active herbs and insects, did not result in severe adverse effects, including major bleeding.
The results were presented at the American Heart Association scientific sessions by Yuejin Yang, MD, PhD, a professor of cardiology at Fuwai Hospital, National Center for CV Disease, Beijing.
He noted that despite reperfusion and optimal medical therapy, patients with STEMI still face high in-hospital mortality, myocardial no-flow, and reperfusion injury, which have no targeted drugs so far worldwide. In addition, “inadequate implementation of timely revascularization for STEMI in China (50-70%) and other developing countries leaves a substantial infarct size in many patients.”
Tongxinluo has been approved for angina and stroke since 1996 in China. Previous preclinical studies and the investigators’ proof-of-concept ENLEAT trial in STEMI suggested tongxinluo could reduce myocardial no-flow and infarction size and protect the cardiomyocytes, Dr. Yang said.
The CTS-AMI trial was conducted at 124 hospitals in mainland China and evenly randomly assigned 3,797 patients with STEMI or new left bundle-branch block within 24 hours of symptom onset to eight capsules of tongxinluo, 2.08 g, or to placebo plus dual antiplatelet therapy before percutaneous coronary intervention (PCI), thrombolysis, or medical management alone, followed by four capsules thrice daily plus guideline-directed therapy for 12 months.
In the modified intention-to-treat cohort of 1,889 tongxinluo- and 1,888 placebo-treated patients, primary PCI was performed in 94.2% and 92.3%, respectively.
The relative risk of 30-day MACCE was reduced 36% in the tongxinluo group, compared with the placebo group (3.39% vs. 5.24%; RR, 0.64; 95% confidence interval, 0.47-0.88).
Among the primary endpoint components, the relative risk of cardiac death was reduced 30% (2.97% vs. 4.24%; RR, 0.70; 95% CI, 0.50-0.99) and MI reinfarction 65% (0 vs. 9 events; RR, 0.35; 95% CI, 0.13-0.99).
Strokes were similar in the tongxinluo and control groups (4 vs. 9; RR, 0.44; 95% CI, 0.14-1.43) and no patient had emergent coronary revascularization at 30 days.
The benefit of the traditional Chinese compound on the primary endpoint was consistent across subgroups, Dr. Yang reported.
At 30 days, severe STEMI complications (11.79% vs. 14.80%; P = .008) and malignant arrhythmias (7.84% vs. 10.20%; P = .011) were lower in the tongxinluo group, whereas mechanical complications (10 vs. 13; P = .526) and cardiogenic shock (2.37% vs. 3.31%; P =.082) were similar.
At 1 year, hazard ratios favored tongxinluo for MACCE (0.64; 95% CI, 0.49-0.82), cardiac death (0.73; 95% CI, 0.55-0.97), MI reinfarction (0.26; 95% CI, 0.10-0.67), and stroke (0.44; 95% CI, 0.21-0.92).
In terms of safety issues, 41 patients receiving tongxinluo and 52 patients receiving placebo had a serious adverse event (2.17% vs. 2.75%; P = .25).
Except for fewer renal injuries with tongxinluo (3.81% vs. 5.30%; P = .029), there were no significant between-group differences in adverse effects including allergic rash, hepatic injury, prolonged activated partial thromboplastin time or prothrombin time, digestive tract hemorrhage, nausea, diarrhea, and headache or dizziness.
“These findings support the use of tongxinluo as an adjunctive therapy in treating STEMI, at least in China and other developing countries,” Dr. Yang concluded.
Invited discussant Kenneth Mahaffey, MD, associate dean, Stanford (Calif.) University, and director of the Stanford Center for Clinical Research, said the results “likely will support use of tongxinluo in China” but that “more studies are needed in other populations and treatment paradigms.”
Asked for further comment by this news organization, Dr. Mahaffey said, “The surprising thing is where are all the MIs? Where are all the revascularization procedures?”
Usually one would expect MIs in about 1% of patients, or about 40 MIs among the 4,000 patients but, he noted, there were zero MIs in the treatment group and 9 among controls.
“We haven’t seen a 30% reduction in cardiovascular death or overall mortality with a therapy in ages with good background therapy,” Dr. Mahaffey said. “We need to see how they ascertained all those events.”
He noted that the results were based on the modified intention-to-treat cohort, which did not include data on 20 patients allocated to treatment, and showed no difference in ST-segment resolution at 2 hours and only a slight difference at 24 hours.
“So even in this trial, for at least some of the data we’ve gotten already that supports the proposed mechanism, it doesn’t show the benefit on that mechanistic substudy. And that’s why we need to see these echoes, the biomarkers, and probably the angios to see: Did it have any effect on the proposed mechanism?” Dr. Mahaffey said.
Finally, information on background therapy is critical for putting the treatment effect into context for other health systems and populations, he said. “Unfortunately, we need to see some additional information to really understand how this will fit in, even in Chinese therapy for STEMI patients, but definitely not outside of China, particularly in the United States, because I don’t know what their background therapy was.”
The study was funded by the National Key Research and Development Program of China. Tongxinluo and placebo were provided by Yiling Pharmacological. The study was designed, conducted, and analyzed independent of the sponsors. Dr. Yang reports no relevant financial conflicts of interest. Dr. Mahaffey reports research funding from the AHA, Apple, Bayer, CIRM, Eidos, Ferring, Gilead, Idorsia, Johnson & Johnson, Luitpold, PAC-12, Precordior, Sanifit, and Verily; consultancy fees from Amgen, Applied Therapeutics, AstraZeneca, CLS Behring, Elsevier, Fibrogen, Inova, Johnson & Johnson, Lexicon, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, Sanofi, and Theravance; and equity in Precordior.
A version of this article first appeared on Medscape.com.
CHICAGO – , the CTS-AMI study suggests.
Compared with those assigned to placebo, Chinese patients assigned to tongxinluo had lower rates of 30-day and 1-year major adverse cardiovascular and cerebrovascular events (MACCE), driven by fewer cardiac deaths. Severe STEMI complications were also lower.
Tongxinluo, which contains 10 or more potential active herbs and insects, did not result in severe adverse effects, including major bleeding.
The results were presented at the American Heart Association scientific sessions by Yuejin Yang, MD, PhD, a professor of cardiology at Fuwai Hospital, National Center for CV Disease, Beijing.
He noted that despite reperfusion and optimal medical therapy, patients with STEMI still face high in-hospital mortality, myocardial no-flow, and reperfusion injury, which have no targeted drugs so far worldwide. In addition, “inadequate implementation of timely revascularization for STEMI in China (50-70%) and other developing countries leaves a substantial infarct size in many patients.”
Tongxinluo has been approved for angina and stroke since 1996 in China. Previous preclinical studies and the investigators’ proof-of-concept ENLEAT trial in STEMI suggested tongxinluo could reduce myocardial no-flow and infarction size and protect the cardiomyocytes, Dr. Yang said.
The CTS-AMI trial was conducted at 124 hospitals in mainland China and evenly randomly assigned 3,797 patients with STEMI or new left bundle-branch block within 24 hours of symptom onset to eight capsules of tongxinluo, 2.08 g, or to placebo plus dual antiplatelet therapy before percutaneous coronary intervention (PCI), thrombolysis, or medical management alone, followed by four capsules thrice daily plus guideline-directed therapy for 12 months.
In the modified intention-to-treat cohort of 1,889 tongxinluo- and 1,888 placebo-treated patients, primary PCI was performed in 94.2% and 92.3%, respectively.
The relative risk of 30-day MACCE was reduced 36% in the tongxinluo group, compared with the placebo group (3.39% vs. 5.24%; RR, 0.64; 95% confidence interval, 0.47-0.88).
Among the primary endpoint components, the relative risk of cardiac death was reduced 30% (2.97% vs. 4.24%; RR, 0.70; 95% CI, 0.50-0.99) and MI reinfarction 65% (0 vs. 9 events; RR, 0.35; 95% CI, 0.13-0.99).
Strokes were similar in the tongxinluo and control groups (4 vs. 9; RR, 0.44; 95% CI, 0.14-1.43) and no patient had emergent coronary revascularization at 30 days.
The benefit of the traditional Chinese compound on the primary endpoint was consistent across subgroups, Dr. Yang reported.
At 30 days, severe STEMI complications (11.79% vs. 14.80%; P = .008) and malignant arrhythmias (7.84% vs. 10.20%; P = .011) were lower in the tongxinluo group, whereas mechanical complications (10 vs. 13; P = .526) and cardiogenic shock (2.37% vs. 3.31%; P =.082) were similar.
At 1 year, hazard ratios favored tongxinluo for MACCE (0.64; 95% CI, 0.49-0.82), cardiac death (0.73; 95% CI, 0.55-0.97), MI reinfarction (0.26; 95% CI, 0.10-0.67), and stroke (0.44; 95% CI, 0.21-0.92).
In terms of safety issues, 41 patients receiving tongxinluo and 52 patients receiving placebo had a serious adverse event (2.17% vs. 2.75%; P = .25).
Except for fewer renal injuries with tongxinluo (3.81% vs. 5.30%; P = .029), there were no significant between-group differences in adverse effects including allergic rash, hepatic injury, prolonged activated partial thromboplastin time or prothrombin time, digestive tract hemorrhage, nausea, diarrhea, and headache or dizziness.
“These findings support the use of tongxinluo as an adjunctive therapy in treating STEMI, at least in China and other developing countries,” Dr. Yang concluded.
Invited discussant Kenneth Mahaffey, MD, associate dean, Stanford (Calif.) University, and director of the Stanford Center for Clinical Research, said the results “likely will support use of tongxinluo in China” but that “more studies are needed in other populations and treatment paradigms.”
Asked for further comment by this news organization, Dr. Mahaffey said, “The surprising thing is where are all the MIs? Where are all the revascularization procedures?”
Usually one would expect MIs in about 1% of patients, or about 40 MIs among the 4,000 patients but, he noted, there were zero MIs in the treatment group and 9 among controls.
“We haven’t seen a 30% reduction in cardiovascular death or overall mortality with a therapy in ages with good background therapy,” Dr. Mahaffey said. “We need to see how they ascertained all those events.”
He noted that the results were based on the modified intention-to-treat cohort, which did not include data on 20 patients allocated to treatment, and showed no difference in ST-segment resolution at 2 hours and only a slight difference at 24 hours.
“So even in this trial, for at least some of the data we’ve gotten already that supports the proposed mechanism, it doesn’t show the benefit on that mechanistic substudy. And that’s why we need to see these echoes, the biomarkers, and probably the angios to see: Did it have any effect on the proposed mechanism?” Dr. Mahaffey said.
Finally, information on background therapy is critical for putting the treatment effect into context for other health systems and populations, he said. “Unfortunately, we need to see some additional information to really understand how this will fit in, even in Chinese therapy for STEMI patients, but definitely not outside of China, particularly in the United States, because I don’t know what their background therapy was.”
The study was funded by the National Key Research and Development Program of China. Tongxinluo and placebo were provided by Yiling Pharmacological. The study was designed, conducted, and analyzed independent of the sponsors. Dr. Yang reports no relevant financial conflicts of interest. Dr. Mahaffey reports research funding from the AHA, Apple, Bayer, CIRM, Eidos, Ferring, Gilead, Idorsia, Johnson & Johnson, Luitpold, PAC-12, Precordior, Sanifit, and Verily; consultancy fees from Amgen, Applied Therapeutics, AstraZeneca, CLS Behring, Elsevier, Fibrogen, Inova, Johnson & Johnson, Lexicon, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, Sanofi, and Theravance; and equity in Precordior.
A version of this article first appeared on Medscape.com.
CHICAGO – , the CTS-AMI study suggests.
Compared with those assigned to placebo, Chinese patients assigned to tongxinluo had lower rates of 30-day and 1-year major adverse cardiovascular and cerebrovascular events (MACCE), driven by fewer cardiac deaths. Severe STEMI complications were also lower.
Tongxinluo, which contains 10 or more potential active herbs and insects, did not result in severe adverse effects, including major bleeding.
The results were presented at the American Heart Association scientific sessions by Yuejin Yang, MD, PhD, a professor of cardiology at Fuwai Hospital, National Center for CV Disease, Beijing.
He noted that despite reperfusion and optimal medical therapy, patients with STEMI still face high in-hospital mortality, myocardial no-flow, and reperfusion injury, which have no targeted drugs so far worldwide. In addition, “inadequate implementation of timely revascularization for STEMI in China (50-70%) and other developing countries leaves a substantial infarct size in many patients.”
Tongxinluo has been approved for angina and stroke since 1996 in China. Previous preclinical studies and the investigators’ proof-of-concept ENLEAT trial in STEMI suggested tongxinluo could reduce myocardial no-flow and infarction size and protect the cardiomyocytes, Dr. Yang said.
The CTS-AMI trial was conducted at 124 hospitals in mainland China and evenly randomly assigned 3,797 patients with STEMI or new left bundle-branch block within 24 hours of symptom onset to eight capsules of tongxinluo, 2.08 g, or to placebo plus dual antiplatelet therapy before percutaneous coronary intervention (PCI), thrombolysis, or medical management alone, followed by four capsules thrice daily plus guideline-directed therapy for 12 months.
In the modified intention-to-treat cohort of 1,889 tongxinluo- and 1,888 placebo-treated patients, primary PCI was performed in 94.2% and 92.3%, respectively.
The relative risk of 30-day MACCE was reduced 36% in the tongxinluo group, compared with the placebo group (3.39% vs. 5.24%; RR, 0.64; 95% confidence interval, 0.47-0.88).
Among the primary endpoint components, the relative risk of cardiac death was reduced 30% (2.97% vs. 4.24%; RR, 0.70; 95% CI, 0.50-0.99) and MI reinfarction 65% (0 vs. 9 events; RR, 0.35; 95% CI, 0.13-0.99).
Strokes were similar in the tongxinluo and control groups (4 vs. 9; RR, 0.44; 95% CI, 0.14-1.43) and no patient had emergent coronary revascularization at 30 days.
The benefit of the traditional Chinese compound on the primary endpoint was consistent across subgroups, Dr. Yang reported.
At 30 days, severe STEMI complications (11.79% vs. 14.80%; P = .008) and malignant arrhythmias (7.84% vs. 10.20%; P = .011) were lower in the tongxinluo group, whereas mechanical complications (10 vs. 13; P = .526) and cardiogenic shock (2.37% vs. 3.31%; P =.082) were similar.
At 1 year, hazard ratios favored tongxinluo for MACCE (0.64; 95% CI, 0.49-0.82), cardiac death (0.73; 95% CI, 0.55-0.97), MI reinfarction (0.26; 95% CI, 0.10-0.67), and stroke (0.44; 95% CI, 0.21-0.92).
In terms of safety issues, 41 patients receiving tongxinluo and 52 patients receiving placebo had a serious adverse event (2.17% vs. 2.75%; P = .25).
Except for fewer renal injuries with tongxinluo (3.81% vs. 5.30%; P = .029), there were no significant between-group differences in adverse effects including allergic rash, hepatic injury, prolonged activated partial thromboplastin time or prothrombin time, digestive tract hemorrhage, nausea, diarrhea, and headache or dizziness.
“These findings support the use of tongxinluo as an adjunctive therapy in treating STEMI, at least in China and other developing countries,” Dr. Yang concluded.
Invited discussant Kenneth Mahaffey, MD, associate dean, Stanford (Calif.) University, and director of the Stanford Center for Clinical Research, said the results “likely will support use of tongxinluo in China” but that “more studies are needed in other populations and treatment paradigms.”
Asked for further comment by this news organization, Dr. Mahaffey said, “The surprising thing is where are all the MIs? Where are all the revascularization procedures?”
Usually one would expect MIs in about 1% of patients, or about 40 MIs among the 4,000 patients but, he noted, there were zero MIs in the treatment group and 9 among controls.
“We haven’t seen a 30% reduction in cardiovascular death or overall mortality with a therapy in ages with good background therapy,” Dr. Mahaffey said. “We need to see how they ascertained all those events.”
He noted that the results were based on the modified intention-to-treat cohort, which did not include data on 20 patients allocated to treatment, and showed no difference in ST-segment resolution at 2 hours and only a slight difference at 24 hours.
“So even in this trial, for at least some of the data we’ve gotten already that supports the proposed mechanism, it doesn’t show the benefit on that mechanistic substudy. And that’s why we need to see these echoes, the biomarkers, and probably the angios to see: Did it have any effect on the proposed mechanism?” Dr. Mahaffey said.
Finally, information on background therapy is critical for putting the treatment effect into context for other health systems and populations, he said. “Unfortunately, we need to see some additional information to really understand how this will fit in, even in Chinese therapy for STEMI patients, but definitely not outside of China, particularly in the United States, because I don’t know what their background therapy was.”
The study was funded by the National Key Research and Development Program of China. Tongxinluo and placebo were provided by Yiling Pharmacological. The study was designed, conducted, and analyzed independent of the sponsors. Dr. Yang reports no relevant financial conflicts of interest. Dr. Mahaffey reports research funding from the AHA, Apple, Bayer, CIRM, Eidos, Ferring, Gilead, Idorsia, Johnson & Johnson, Luitpold, PAC-12, Precordior, Sanifit, and Verily; consultancy fees from Amgen, Applied Therapeutics, AstraZeneca, CLS Behring, Elsevier, Fibrogen, Inova, Johnson & Johnson, Lexicon, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Quidel, Sanofi, and Theravance; and equity in Precordior.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Key Presentations in Lung Cancer From CHEST 2022
The 2022 CHEST Annual Meeting had several important studies on lung cancer.
Douglas Arenberg, MD, FCCP from the University of Michigan Northville Health Center, reports on content from two papers that focus on the first million persons to have been screened for lung cancer after the initial launch of the American College of Radiology Lung Cancer Screening Registry. The research showed that the medical community is, in fact, doing well in some areas of lung cancer screening but that improvements need to be made in order to reach former tobacco users, who would greatly benefit from these screenings.
He also highlights a series of studies that were discussed regarding smoking cessation at lung evaluations known as the SCALE Collaboration. Effective smoking interventions could enhance the benefits of lung cancer screening by reducing mortality and morbidity resulting from lung cancer.
Finally, Dr Arenberg shares a series of presentations that highlight how the surgical treatment of early-stage lung cancer is creating significant changes in the standard of care.
--
Douglas Arenberg, MD, FCCP Professor of Medicine, Department of Internal Medicine, Division of Pulmonary & Critical Care, University of Michigan; Director of Bronchoscopy and Medical Director for the Lung Cancer Screening and Lung Nodule Clinics, University of Michigan, Ann Arbor, Michigan
Douglas Arenberg, MD, FCCP has disclosed no relevant financial relationships.
The 2022 CHEST Annual Meeting had several important studies on lung cancer.
Douglas Arenberg, MD, FCCP from the University of Michigan Northville Health Center, reports on content from two papers that focus on the first million persons to have been screened for lung cancer after the initial launch of the American College of Radiology Lung Cancer Screening Registry. The research showed that the medical community is, in fact, doing well in some areas of lung cancer screening but that improvements need to be made in order to reach former tobacco users, who would greatly benefit from these screenings.
He also highlights a series of studies that were discussed regarding smoking cessation at lung evaluations known as the SCALE Collaboration. Effective smoking interventions could enhance the benefits of lung cancer screening by reducing mortality and morbidity resulting from lung cancer.
Finally, Dr Arenberg shares a series of presentations that highlight how the surgical treatment of early-stage lung cancer is creating significant changes in the standard of care.
--
Douglas Arenberg, MD, FCCP Professor of Medicine, Department of Internal Medicine, Division of Pulmonary & Critical Care, University of Michigan; Director of Bronchoscopy and Medical Director for the Lung Cancer Screening and Lung Nodule Clinics, University of Michigan, Ann Arbor, Michigan
Douglas Arenberg, MD, FCCP has disclosed no relevant financial relationships.
The 2022 CHEST Annual Meeting had several important studies on lung cancer.
Douglas Arenberg, MD, FCCP from the University of Michigan Northville Health Center, reports on content from two papers that focus on the first million persons to have been screened for lung cancer after the initial launch of the American College of Radiology Lung Cancer Screening Registry. The research showed that the medical community is, in fact, doing well in some areas of lung cancer screening but that improvements need to be made in order to reach former tobacco users, who would greatly benefit from these screenings.
He also highlights a series of studies that were discussed regarding smoking cessation at lung evaluations known as the SCALE Collaboration. Effective smoking interventions could enhance the benefits of lung cancer screening by reducing mortality and morbidity resulting from lung cancer.
Finally, Dr Arenberg shares a series of presentations that highlight how the surgical treatment of early-stage lung cancer is creating significant changes in the standard of care.
--
Douglas Arenberg, MD, FCCP Professor of Medicine, Department of Internal Medicine, Division of Pulmonary & Critical Care, University of Michigan; Director of Bronchoscopy and Medical Director for the Lung Cancer Screening and Lung Nodule Clinics, University of Michigan, Ann Arbor, Michigan
Douglas Arenberg, MD, FCCP has disclosed no relevant financial relationships.

New Medicare physician fee schedule leaves docs fuming over pay cuts
The rule also seeks to ease financial and administrative burdens on accountable care organizations (ACOs).
But physician groups’ initial reactions centered on what the American Medical Association describes as a “damaging across-the-board reduction” of 4.4% in a base calculation, known as a conversion factor.
The reduction is only one of the current threats to physician’s finances, Jack Resneck Jr, MD, AMA’s president, said in a statement. Medicare payment rates also fail to account for inflation in practice costs and COVID-related challenges. Physician’s Medicare payments could be cut by nearly 8.5% in 2023, factoring in other budget cuts, Dr. Resneck said in the statement.
That “would severely impede patient access to care due to the forced closure of physician practices and put further strain on those that remained open during the pandemic,” he said.
A key driver of these cuts is a law that was intended to resolve budget battles between Congress and physicians, while also transitioning Medicare away from fee-for-service payments and pegging reimbursement to judgments about value of care provided. The Centers for Medicare & Medicaid Services thus had little choice about cuts mandated by the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
For AMA and other physician groups, the finalization of the Medicare rule served as a rallying point to build support for pending legislation intended to stave off at least some payment cuts.
Federal officials should act soon to block the expected cuts before this season of Congress ends in January, said Anders Gilberg, senior vice president for government affairs at the Medical Group Management Association, in a statement.
“This cannot wait until next Congress – there are claims-processing implications for retroactively applying these policies,” Mr. Gilberg said.
He said MGMA would work with Congress and CMS “to mitigate these cuts and develop sustainable payment policies to allow physician practices to focus on treating patients instead of scrambling to keep their doors open.”
Chronic budget battles
Once seen as a promising resolution to chronic annual budget battles between physicians and Medicare, MACRA has proven a near-universal disappointment. A federal advisory commission in 2018 recommended that Congress scrap MACRA’s Merit-based Incentive Payment System (MIPS) and replace it with a new approach for attempting to tie reimbursement to judgments about the quality of medical care.
MACRA replaced an earlier budgeting approach on Medicare physician pay, known as the sustainable growth rate (SGR). Physician groups successfully lobbied Congress for many years to block threatened Medicare payment cuts. Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that the lawmakers earlier mandated through the SGR.
A similar pattern has emerged as Congress now acts on short-term fixes to stave off MACRA-mandated cuts. A law passed last December postponed cuts in physician pay from MACRA and federal budget laws.
And more than 70 members of the House support a bill (HR 8800) intended to block a slated 4.4% MACRA-related cut in physician pay for 2023. Two physicians, Rep. Ami Bera, MD, (D-CA) and Rep. Larry Bucshon (R-IN) sponsored the bill.
Among the groups backing the bill are the AMA, American Academy of Family Physicians, and American College of Physicians. The lawmakers may try to attach this bill to a large spending measure, known as an omnibus, that Congress will try to clear in December to avoid a partial government shutdown.
In a statement, Tochi Iroku-Malize, MD, MPH, MBA, the president of AAFP, urged Congress to factor in inflation in setting physician reimbursement and to reconsider Medicare’s approach to paying physicians.
“It’s past time to end the untenable physician payment cuts – which have now become an annual threat to the stability of physician practices – caused by Medicare budget neutrality requirements and the ongoing freeze in annual payment updates,” Dr. Iroku-Malize said.
Congress also needs to retool its approach to alternative payment models (APMs) intended to improve the quality of patient care, Dr. Iroku-Malize said.
“Physicians in APMs are better equipped to address unmet social needs and provide other enhanced services that are not supported by fee-for-service payment rates,” Dr. Iroku-Malize said. “However, insufficient Medicare fee-for-service payment rates, inadequate support, and burdensome timelines are undermining the move to value-based care and exacerbating our nation’s underinvestment in primary care.”
Policy changes
But the new rule did have some good news for family physicians, Dr. Iroku-Malize told this news organization in an email.
CMS said it will pay psychologists and social workers to help manage behavioral health needs as part of the primary care team, in addition to their own services. This change will give primary care practices more flexibility to coordinate with behavioral health professionals, Dr. Iroku-Malize noted.
“We know that primary care physicians are the first point of contact for many patients, and behavioral health integration increases critical access to mental health care, decreases stigma for patients, and can prevent more severe medical and behavioral health events,” she wrote.
CMS also eased a supervision requirement for nonphysicians providing behavioral health services.
It intends to allow certain health professionals to provide this care without requiring that a supervising physician or nurse practitioner be physically on site. This shift from direct supervision to what’s called general supervision applies to marriage and family therapists, licensed professional counselors, addiction counselors, certified peer recovery specialists, and behavioral health specialists, CMS said.
Other major policy changes include:
Medicare will pay for telehealth opioid treatment programs allowing patients to initiate treatment with buprenorphine. CMS also clarified that certain programs can bill for opioid use disorder treatment services provided through mobile units, such as vans.
Medicare enrollees may see audiologists for nonacute hearing conditions without an order from a physician or nurse practitioner. The policy is meant to allow audiologists to examine patients to prescribe, fit, or change hearing aids, or to provide hearing tests unrelated to disequilibrium.
CMS created new reimbursement codes for chronic pain management and treatment services to encourage clinicians to see patients with this condition. The codes also are meant to encourage practitioners already treating Medicare patients with chronic pain to spend more time helping them manage their condition “within a trusting, supportive, and ongoing care partnership,” CMS said.
CMS also made changes to the Medicare Shared Savings Program (MSSP) intended to reduce administrative burdens and offer more financial support to practices involved in ACOs. These steps include expanding opportunities for certain low-revenue ACOs to share in savings even if they do not meet a target rate.
A version of this article first appeared on Medscape.com.
The rule also seeks to ease financial and administrative burdens on accountable care organizations (ACOs).
But physician groups’ initial reactions centered on what the American Medical Association describes as a “damaging across-the-board reduction” of 4.4% in a base calculation, known as a conversion factor.
The reduction is only one of the current threats to physician’s finances, Jack Resneck Jr, MD, AMA’s president, said in a statement. Medicare payment rates also fail to account for inflation in practice costs and COVID-related challenges. Physician’s Medicare payments could be cut by nearly 8.5% in 2023, factoring in other budget cuts, Dr. Resneck said in the statement.
That “would severely impede patient access to care due to the forced closure of physician practices and put further strain on those that remained open during the pandemic,” he said.
A key driver of these cuts is a law that was intended to resolve budget battles between Congress and physicians, while also transitioning Medicare away from fee-for-service payments and pegging reimbursement to judgments about value of care provided. The Centers for Medicare & Medicaid Services thus had little choice about cuts mandated by the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
For AMA and other physician groups, the finalization of the Medicare rule served as a rallying point to build support for pending legislation intended to stave off at least some payment cuts.
Federal officials should act soon to block the expected cuts before this season of Congress ends in January, said Anders Gilberg, senior vice president for government affairs at the Medical Group Management Association, in a statement.
“This cannot wait until next Congress – there are claims-processing implications for retroactively applying these policies,” Mr. Gilberg said.
He said MGMA would work with Congress and CMS “to mitigate these cuts and develop sustainable payment policies to allow physician practices to focus on treating patients instead of scrambling to keep their doors open.”
Chronic budget battles
Once seen as a promising resolution to chronic annual budget battles between physicians and Medicare, MACRA has proven a near-universal disappointment. A federal advisory commission in 2018 recommended that Congress scrap MACRA’s Merit-based Incentive Payment System (MIPS) and replace it with a new approach for attempting to tie reimbursement to judgments about the quality of medical care.
MACRA replaced an earlier budgeting approach on Medicare physician pay, known as the sustainable growth rate (SGR). Physician groups successfully lobbied Congress for many years to block threatened Medicare payment cuts. Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that the lawmakers earlier mandated through the SGR.
A similar pattern has emerged as Congress now acts on short-term fixes to stave off MACRA-mandated cuts. A law passed last December postponed cuts in physician pay from MACRA and federal budget laws.
And more than 70 members of the House support a bill (HR 8800) intended to block a slated 4.4% MACRA-related cut in physician pay for 2023. Two physicians, Rep. Ami Bera, MD, (D-CA) and Rep. Larry Bucshon (R-IN) sponsored the bill.
Among the groups backing the bill are the AMA, American Academy of Family Physicians, and American College of Physicians. The lawmakers may try to attach this bill to a large spending measure, known as an omnibus, that Congress will try to clear in December to avoid a partial government shutdown.
In a statement, Tochi Iroku-Malize, MD, MPH, MBA, the president of AAFP, urged Congress to factor in inflation in setting physician reimbursement and to reconsider Medicare’s approach to paying physicians.
“It’s past time to end the untenable physician payment cuts – which have now become an annual threat to the stability of physician practices – caused by Medicare budget neutrality requirements and the ongoing freeze in annual payment updates,” Dr. Iroku-Malize said.
Congress also needs to retool its approach to alternative payment models (APMs) intended to improve the quality of patient care, Dr. Iroku-Malize said.
“Physicians in APMs are better equipped to address unmet social needs and provide other enhanced services that are not supported by fee-for-service payment rates,” Dr. Iroku-Malize said. “However, insufficient Medicare fee-for-service payment rates, inadequate support, and burdensome timelines are undermining the move to value-based care and exacerbating our nation’s underinvestment in primary care.”
Policy changes
But the new rule did have some good news for family physicians, Dr. Iroku-Malize told this news organization in an email.
CMS said it will pay psychologists and social workers to help manage behavioral health needs as part of the primary care team, in addition to their own services. This change will give primary care practices more flexibility to coordinate with behavioral health professionals, Dr. Iroku-Malize noted.
“We know that primary care physicians are the first point of contact for many patients, and behavioral health integration increases critical access to mental health care, decreases stigma for patients, and can prevent more severe medical and behavioral health events,” she wrote.
CMS also eased a supervision requirement for nonphysicians providing behavioral health services.
It intends to allow certain health professionals to provide this care without requiring that a supervising physician or nurse practitioner be physically on site. This shift from direct supervision to what’s called general supervision applies to marriage and family therapists, licensed professional counselors, addiction counselors, certified peer recovery specialists, and behavioral health specialists, CMS said.
Other major policy changes include:
Medicare will pay for telehealth opioid treatment programs allowing patients to initiate treatment with buprenorphine. CMS also clarified that certain programs can bill for opioid use disorder treatment services provided through mobile units, such as vans.
Medicare enrollees may see audiologists for nonacute hearing conditions without an order from a physician or nurse practitioner. The policy is meant to allow audiologists to examine patients to prescribe, fit, or change hearing aids, or to provide hearing tests unrelated to disequilibrium.
CMS created new reimbursement codes for chronic pain management and treatment services to encourage clinicians to see patients with this condition. The codes also are meant to encourage practitioners already treating Medicare patients with chronic pain to spend more time helping them manage their condition “within a trusting, supportive, and ongoing care partnership,” CMS said.
CMS also made changes to the Medicare Shared Savings Program (MSSP) intended to reduce administrative burdens and offer more financial support to practices involved in ACOs. These steps include expanding opportunities for certain low-revenue ACOs to share in savings even if they do not meet a target rate.
A version of this article first appeared on Medscape.com.
The rule also seeks to ease financial and administrative burdens on accountable care organizations (ACOs).
But physician groups’ initial reactions centered on what the American Medical Association describes as a “damaging across-the-board reduction” of 4.4% in a base calculation, known as a conversion factor.
The reduction is only one of the current threats to physician’s finances, Jack Resneck Jr, MD, AMA’s president, said in a statement. Medicare payment rates also fail to account for inflation in practice costs and COVID-related challenges. Physician’s Medicare payments could be cut by nearly 8.5% in 2023, factoring in other budget cuts, Dr. Resneck said in the statement.
That “would severely impede patient access to care due to the forced closure of physician practices and put further strain on those that remained open during the pandemic,” he said.
A key driver of these cuts is a law that was intended to resolve budget battles between Congress and physicians, while also transitioning Medicare away from fee-for-service payments and pegging reimbursement to judgments about value of care provided. The Centers for Medicare & Medicaid Services thus had little choice about cuts mandated by the Medicare Access and CHIP Reauthorization Act (MACRA) of 2015.
For AMA and other physician groups, the finalization of the Medicare rule served as a rallying point to build support for pending legislation intended to stave off at least some payment cuts.
Federal officials should act soon to block the expected cuts before this season of Congress ends in January, said Anders Gilberg, senior vice president for government affairs at the Medical Group Management Association, in a statement.
“This cannot wait until next Congress – there are claims-processing implications for retroactively applying these policies,” Mr. Gilberg said.
He said MGMA would work with Congress and CMS “to mitigate these cuts and develop sustainable payment policies to allow physician practices to focus on treating patients instead of scrambling to keep their doors open.”
Chronic budget battles
Once seen as a promising resolution to chronic annual budget battles between physicians and Medicare, MACRA has proven a near-universal disappointment. A federal advisory commission in 2018 recommended that Congress scrap MACRA’s Merit-based Incentive Payment System (MIPS) and replace it with a new approach for attempting to tie reimbursement to judgments about the quality of medical care.
MACRA replaced an earlier budgeting approach on Medicare physician pay, known as the sustainable growth rate (SGR). Physician groups successfully lobbied Congress for many years to block threatened Medicare payment cuts. Between 2003 and April 2014, Congress passed 17 laws overriding the cuts to physician pay that the lawmakers earlier mandated through the SGR.
A similar pattern has emerged as Congress now acts on short-term fixes to stave off MACRA-mandated cuts. A law passed last December postponed cuts in physician pay from MACRA and federal budget laws.
And more than 70 members of the House support a bill (HR 8800) intended to block a slated 4.4% MACRA-related cut in physician pay for 2023. Two physicians, Rep. Ami Bera, MD, (D-CA) and Rep. Larry Bucshon (R-IN) sponsored the bill.
Among the groups backing the bill are the AMA, American Academy of Family Physicians, and American College of Physicians. The lawmakers may try to attach this bill to a large spending measure, known as an omnibus, that Congress will try to clear in December to avoid a partial government shutdown.
In a statement, Tochi Iroku-Malize, MD, MPH, MBA, the president of AAFP, urged Congress to factor in inflation in setting physician reimbursement and to reconsider Medicare’s approach to paying physicians.
“It’s past time to end the untenable physician payment cuts – which have now become an annual threat to the stability of physician practices – caused by Medicare budget neutrality requirements and the ongoing freeze in annual payment updates,” Dr. Iroku-Malize said.
Congress also needs to retool its approach to alternative payment models (APMs) intended to improve the quality of patient care, Dr. Iroku-Malize said.
“Physicians in APMs are better equipped to address unmet social needs and provide other enhanced services that are not supported by fee-for-service payment rates,” Dr. Iroku-Malize said. “However, insufficient Medicare fee-for-service payment rates, inadequate support, and burdensome timelines are undermining the move to value-based care and exacerbating our nation’s underinvestment in primary care.”
Policy changes
But the new rule did have some good news for family physicians, Dr. Iroku-Malize told this news organization in an email.
CMS said it will pay psychologists and social workers to help manage behavioral health needs as part of the primary care team, in addition to their own services. This change will give primary care practices more flexibility to coordinate with behavioral health professionals, Dr. Iroku-Malize noted.
“We know that primary care physicians are the first point of contact for many patients, and behavioral health integration increases critical access to mental health care, decreases stigma for patients, and can prevent more severe medical and behavioral health events,” she wrote.
CMS also eased a supervision requirement for nonphysicians providing behavioral health services.
It intends to allow certain health professionals to provide this care without requiring that a supervising physician or nurse practitioner be physically on site. This shift from direct supervision to what’s called general supervision applies to marriage and family therapists, licensed professional counselors, addiction counselors, certified peer recovery specialists, and behavioral health specialists, CMS said.
Other major policy changes include:
Medicare will pay for telehealth opioid treatment programs allowing patients to initiate treatment with buprenorphine. CMS also clarified that certain programs can bill for opioid use disorder treatment services provided through mobile units, such as vans.
Medicare enrollees may see audiologists for nonacute hearing conditions without an order from a physician or nurse practitioner. The policy is meant to allow audiologists to examine patients to prescribe, fit, or change hearing aids, or to provide hearing tests unrelated to disequilibrium.
CMS created new reimbursement codes for chronic pain management and treatment services to encourage clinicians to see patients with this condition. The codes also are meant to encourage practitioners already treating Medicare patients with chronic pain to spend more time helping them manage their condition “within a trusting, supportive, and ongoing care partnership,” CMS said.
CMS also made changes to the Medicare Shared Savings Program (MSSP) intended to reduce administrative burdens and offer more financial support to practices involved in ACOs. These steps include expanding opportunities for certain low-revenue ACOs to share in savings even if they do not meet a target rate.
A version of this article first appeared on Medscape.com.
Man with COVID finally tests negative after 411 days
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
according to experts in the United Kingdom.
The man was treated with a mixture of neutralizing monoclonal antibodies, King’s College London said in a news release.
The man, 59, tested positive in December 2020 and tested negative in January 2022. He had a weakened immune system because of a previous kidney transplant. He received three doses of vaccine and his symptoms lessened, but he kept testing positive for COVID.
To find out if the man had a persistent infection or had been infected several times, doctors did a genetic analysis of the virus.
“This revealed that the patient’s infection was a persistent infection with an early COVID variant – a variation of the original Wuhan variant that was dominant in the United Kingdom in the later months of 2020. Analysis found the patient’s virus had multiple mutations since he was first infected,” King’s College said.
The doctors treated him with a Regeneron treatment that is no longer widely used because it’s not effective against newer COVID variants.
“Some new variants of the virus are resistant to all the antibody treatments available in the United Kingdom and Europe. Some people with weakened immune systems are still at risk of severe illness and becoming persistently infected. We are still working to understand the best way to protect and treat them,” Luke Snell, MD, from the King’s College School of Immunology & Microbial Sciences, said in the news release.
This is one of the longest known cases of COVID infection. Another man in England was infected with COVID for 505 days before his death, which King’s College said was the longest known COVID infection.
A version of this article first appeared on WebMD.com.
COVID bivalent booster better vs. recent Omicron subvariants: Pfizer
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
the company reported on Nov. 4, supporting calls by public health officials for eligible people to get this booster before a potential COVID-19 surge this winter.
The company’s ongoing phase 2/3 study of their Omicron BA.4 and BA.5 bivalent – which targets both the virus’ original strain and the two subvariants – shows that the vaccine offered the strongest protection in people older than 55 years.
One month after receiving a 30-mcg booster with the bivalent vaccine, those older than 55 had four times more neutralizing antibodies against these Omicron subvariants, compared with people who received the original monovalent vaccine as a booster in the study.
Researchers compared the geometric mean titer (GMT) levels of these antibodies in three groups before and 1 month after boosting. The 36 people older than 55 years in the released study findings had an GMT level of 896 with the bivalent booster, a level 13 times higher than before this immunization.
For the 38 adults ages 18-55 in the study, the GMT level increased to 606 at 1 month after the bivalent booster, an increase of almost 10-fold, compared with baseline. In a comparator group of 40 people receiving the original vaccine as a fourth dose, the GMT level was 236, or threefold higher than before their booster shot.
The newly released data is “very encouraging and consistent now with three studies all showing a substantial 3-4 fold increased level of neutralizing antibodies versus BA.5 as compared with the original booster,” said Eric Topol, MD, director of the Scripps Research Translational Institute in La Jolla, Calif., and editor-in-chief of Medscape Medical News.
Pfizer and BioNTech announced the updated findings in a Nov. 4 press release.
A booster dose of the BA.4/BA.5-adapted bivalent vaccine is authorized for emergency use by the Food and Drug Administration for ages 5 years and older. The safety and tolerability profile of the Pfizer/BioNTech bivalent booster remains favorable and similar to the original COVID-19 vaccine, the company reported.
Until recently, the BA.5 Omicron variant was the dominant strain in the United States, but is now getting elbowed out by the subvariants BQ.1.1, BQ.1, and BA.4.6, which together make up almost 45% of the circulating virus.
Some skepticism
“It is important to note that these data are press-release level, which does not allow a view of the data totality,” Hana El Sahly, MD, professor of molecular virology and microbiology, Baylor College of Medicine, Houston, said in an interview.
“For example, there may be significant differences between the groups, and the release mentions at least one difference that is of importance: the interval since the last vaccination which often affects the response to subsequent boosting,” she said.
Dr. El Sahly added that the findings are not surprising. “In the short term, a variant-specific vaccine produces a higher level of antibody against the variant in the vaccine than the vaccines based on the ancestral strains.”
More researcher results are warranted. “These data do not indicate that these differences between the two vaccines translate into a meaningful clinical benefit at a population level,” Dr. El Sahly said.
An uncertain winter ahead
“As we head into the holiday season, we hope these updated data will encourage people to seek out a COVID-19 bivalent booster as soon as they are eligible in order to maintain high levels of protection against the widely circulating Omicron BA.4 and BA.5 sublineages,” Albert Bourla, Pfizer chairman and CEO, stated in the release.
The updated data from the Pfizer/BioNTech study are “all the more reason to get a booster, with added protection also versus BQ.1.1, which will soon become dominant in the U.S.,” Dr. Topol predicted.
It is unclear when the next surge will happen, as COVID-19 does not always follow a seasonal pattern, at least not yet, Dr. El Sahly said. “Regardless, it is reasonable to recommend additional vaccine doses to immunocompromised and frail or older persons. More importantly, influenza vaccination and being up to date on pneumococcal vaccines are highly recommended as soon as feasible, given the early and intense flu season.”
A version of this article first appeared on Medscape.com.
Avoid routine early ECMO in severe cardiogenic shock: ECMO-CS
CHICAGO – Routine early, expeditious use of extracorporeal membrane oxygenation (ECMO) is a common strategy in patients with severe cardiogenic shock, but a less aggressive initial approach may be just as effective, a randomized trial suggests.
In the study that assigned patients with “rapidly deteriorating or severe” cardiogenic shock to one or the other approach, clinical outcomes were no better for those who received immediate ECMO than for those initially managed with inotropes and vasopressors, researchers said.
The conservative strategy, importantly, allowed for downstream ECMO in the event of hemodynamic deterioration, which occurred in a substantial 39% of cases, observed Petr Ostadal, MD, PhD, when presenting the results at the American Heart Association scientific sessions.
Dr. Ostadal of Na Homolce Hospital, Prague, is also first author on the published report of the study, called Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS), which was published the same day in Circulation.
The trial makes a firm case for preferring the conservative initial approach over routine early ECMO in the kind of patients it entered, Larry A. Allen, MD, MHS, University of Coloradoat Denver, Aurora, told this news organization.
More than 60% of the trial’s 117 patients had shock secondary to an acute coronary syndrome; another 23% were in heart failure decompensation.
A preference for the conservative initial approach would be welcome, he said. The early aggressive ECMO approach is resource intensive and carries some important risks, such as stroke or coagulopathy, said Dr. Allen, who is not connected with ECMO-CS. Yet it is increasingly the go-to approach in such patients, based primarily on observational data.
Although early ECMO apparently didn’t benefit patients in this study in their specific stage of cardiogenic shock, Dr. Allen observed, it would presumably help some, but identifying them in practice presents challenges. “Defining where people are in the spectrum of early versus middle versus late cardiogenic shock is actually very tricky.”
It will therefore be important, he said, to identify ways to predict which conservatively managed patients do well with the strategy, and which are most at risk for hemodynamic deterioration and for whom ECMO should be readily available.
“I think part of what ECMO-CS tells us is that, if a patient is stable on intravenous inotropic and vasopressor support, you can defer ECMO while you’re thinking about the patient – about their larger context and the right medical decision-making for them.”
The trial randomly assigned 122 patients with rapidly deteriorating or severe cardiogenic shock to the immediate-ECMO or the conservative strategy at four centers in the Czech Republic. The 117 patients for whom informed consent could be obtained were included in the analysis, 58 and 59 patients, respectively. Their mean age was about 65 years and three-fourths were male.
The primary endpoint, the only endpoint for which the study was powered, consisted of death from any cause, resuscitated circulatory arrest, or use of a different form of mechanical circulatory support (MCS) by 30 days.
It occurred in 63.8% of patients assigned to immediate ECMO and 71.2% of those in the conservative strategy group, for a hazard ratio of 0.72 (95% confidence interval, 0.46-1.12; P = .21).
As individual endpoints, rates of death from any cause and resuscitated arrest did not significantly differ between the groups, but conservatively managed patients more often used another form of MCS. The HRs were 1.11 (95% CI, 0.66-1.87) for death from any cause, 0.79 (95% CI, 0.27-2.28) for resuscitated cardiac arrest, and 0.38 (95% CI, 0.18-0.79) for use of another MCS device.
The rates for serious adverse events – including bleeding, ischemia, stroke, pneumonia, or sepsis – were similar at 60.3% in the early-ECMO group and 61% in group with conservative initial management, Dr. Ostadal reported.
Other than the 23 patients in the conservative initial strategy group who went on to receive ECMO (1.9 days after randomization, on average), 1 went on to undergo implantation with a HeartMate (Abbott) ventricular assist device and 3 received an Impella pump (Abiomed).
Six patients in the early-ECMO group were already receiving intra-aortic balloon pump (IABP) support at randomization, two underwent temporary implantation with a Centrimag device (Abbott), and three went on to receive a HeartMate device, the published report notes.
ECMO is the optimal first choice for MCS in such patients with cardiogenic shock who need a circulatory support device, especially because it also oxygenates the blood, Dr. Ostadal told this news organization.
But ECMO doesn’t help with ventricular unloading. Indeed, it can sometimes reduce ventricular preload, especially if right-heart pressures are low. So MCS devices that unload the ventricle, typically an IABP, can complement ECMO.
Dr. Ostadal speculates, however, that there may be a better pairing option. “Impella plus ECMO, I think, is the combination which has a future,” he said, for patients in cardiogenic shock who need a short-term percutaneous hemodynamic support device. Impella “supports the whole circulation” and unloads the left ventricle.
“A balloon pump in combination with ECMO is still not a bad choice. It’s very cheap in comparison with Impella.” But in his opinion, Dr. Ostadal said, “The combination of Impella plus ECMO is more efficient from a hemodynamic point of view.”
As the published report notes, ongoing randomized trials looking at ECMO plus other MCS devices in cardiogenic shock include ECLS-SHOCK, with a projected enrollment of 420 patients, and EURO-SHOCK, aiming for a similar number of patients; both compare routine ECMO to conservative management.
In addition, ANCHOR, in which ECMO is combined with IABP, and DanShock, which looks at early use of Impella rather than ECMO, are enrolling patients with shock secondary to acute coronary syndromes.
Dr. Ostadal disclosed consulting for Getinge, Edwards, Medtronic, Biomedica, and Xenios/Fresenius, and receiving research support from Xenios/Fresenius. Dr. Allen disclosed modest or significant relationships with ACI Clinical, Novartis, UpToDate, Boston Scientific, and Cytokinetics.
A version of this article first appeared on Medscape.com.
CHICAGO – Routine early, expeditious use of extracorporeal membrane oxygenation (ECMO) is a common strategy in patients with severe cardiogenic shock, but a less aggressive initial approach may be just as effective, a randomized trial suggests.
In the study that assigned patients with “rapidly deteriorating or severe” cardiogenic shock to one or the other approach, clinical outcomes were no better for those who received immediate ECMO than for those initially managed with inotropes and vasopressors, researchers said.
The conservative strategy, importantly, allowed for downstream ECMO in the event of hemodynamic deterioration, which occurred in a substantial 39% of cases, observed Petr Ostadal, MD, PhD, when presenting the results at the American Heart Association scientific sessions.
Dr. Ostadal of Na Homolce Hospital, Prague, is also first author on the published report of the study, called Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS), which was published the same day in Circulation.
The trial makes a firm case for preferring the conservative initial approach over routine early ECMO in the kind of patients it entered, Larry A. Allen, MD, MHS, University of Coloradoat Denver, Aurora, told this news organization.
More than 60% of the trial’s 117 patients had shock secondary to an acute coronary syndrome; another 23% were in heart failure decompensation.
A preference for the conservative initial approach would be welcome, he said. The early aggressive ECMO approach is resource intensive and carries some important risks, such as stroke or coagulopathy, said Dr. Allen, who is not connected with ECMO-CS. Yet it is increasingly the go-to approach in such patients, based primarily on observational data.
Although early ECMO apparently didn’t benefit patients in this study in their specific stage of cardiogenic shock, Dr. Allen observed, it would presumably help some, but identifying them in practice presents challenges. “Defining where people are in the spectrum of early versus middle versus late cardiogenic shock is actually very tricky.”
It will therefore be important, he said, to identify ways to predict which conservatively managed patients do well with the strategy, and which are most at risk for hemodynamic deterioration and for whom ECMO should be readily available.
“I think part of what ECMO-CS tells us is that, if a patient is stable on intravenous inotropic and vasopressor support, you can defer ECMO while you’re thinking about the patient – about their larger context and the right medical decision-making for them.”
The trial randomly assigned 122 patients with rapidly deteriorating or severe cardiogenic shock to the immediate-ECMO or the conservative strategy at four centers in the Czech Republic. The 117 patients for whom informed consent could be obtained were included in the analysis, 58 and 59 patients, respectively. Their mean age was about 65 years and three-fourths were male.
The primary endpoint, the only endpoint for which the study was powered, consisted of death from any cause, resuscitated circulatory arrest, or use of a different form of mechanical circulatory support (MCS) by 30 days.
It occurred in 63.8% of patients assigned to immediate ECMO and 71.2% of those in the conservative strategy group, for a hazard ratio of 0.72 (95% confidence interval, 0.46-1.12; P = .21).
As individual endpoints, rates of death from any cause and resuscitated arrest did not significantly differ between the groups, but conservatively managed patients more often used another form of MCS. The HRs were 1.11 (95% CI, 0.66-1.87) for death from any cause, 0.79 (95% CI, 0.27-2.28) for resuscitated cardiac arrest, and 0.38 (95% CI, 0.18-0.79) for use of another MCS device.
The rates for serious adverse events – including bleeding, ischemia, stroke, pneumonia, or sepsis – were similar at 60.3% in the early-ECMO group and 61% in group with conservative initial management, Dr. Ostadal reported.
Other than the 23 patients in the conservative initial strategy group who went on to receive ECMO (1.9 days after randomization, on average), 1 went on to undergo implantation with a HeartMate (Abbott) ventricular assist device and 3 received an Impella pump (Abiomed).
Six patients in the early-ECMO group were already receiving intra-aortic balloon pump (IABP) support at randomization, two underwent temporary implantation with a Centrimag device (Abbott), and three went on to receive a HeartMate device, the published report notes.
ECMO is the optimal first choice for MCS in such patients with cardiogenic shock who need a circulatory support device, especially because it also oxygenates the blood, Dr. Ostadal told this news organization.
But ECMO doesn’t help with ventricular unloading. Indeed, it can sometimes reduce ventricular preload, especially if right-heart pressures are low. So MCS devices that unload the ventricle, typically an IABP, can complement ECMO.
Dr. Ostadal speculates, however, that there may be a better pairing option. “Impella plus ECMO, I think, is the combination which has a future,” he said, for patients in cardiogenic shock who need a short-term percutaneous hemodynamic support device. Impella “supports the whole circulation” and unloads the left ventricle.
“A balloon pump in combination with ECMO is still not a bad choice. It’s very cheap in comparison with Impella.” But in his opinion, Dr. Ostadal said, “The combination of Impella plus ECMO is more efficient from a hemodynamic point of view.”
As the published report notes, ongoing randomized trials looking at ECMO plus other MCS devices in cardiogenic shock include ECLS-SHOCK, with a projected enrollment of 420 patients, and EURO-SHOCK, aiming for a similar number of patients; both compare routine ECMO to conservative management.
In addition, ANCHOR, in which ECMO is combined with IABP, and DanShock, which looks at early use of Impella rather than ECMO, are enrolling patients with shock secondary to acute coronary syndromes.
Dr. Ostadal disclosed consulting for Getinge, Edwards, Medtronic, Biomedica, and Xenios/Fresenius, and receiving research support from Xenios/Fresenius. Dr. Allen disclosed modest or significant relationships with ACI Clinical, Novartis, UpToDate, Boston Scientific, and Cytokinetics.
A version of this article first appeared on Medscape.com.
CHICAGO – Routine early, expeditious use of extracorporeal membrane oxygenation (ECMO) is a common strategy in patients with severe cardiogenic shock, but a less aggressive initial approach may be just as effective, a randomized trial suggests.
In the study that assigned patients with “rapidly deteriorating or severe” cardiogenic shock to one or the other approach, clinical outcomes were no better for those who received immediate ECMO than for those initially managed with inotropes and vasopressors, researchers said.
The conservative strategy, importantly, allowed for downstream ECMO in the event of hemodynamic deterioration, which occurred in a substantial 39% of cases, observed Petr Ostadal, MD, PhD, when presenting the results at the American Heart Association scientific sessions.
Dr. Ostadal of Na Homolce Hospital, Prague, is also first author on the published report of the study, called Extracorporeal Membrane Oxygenation in the Therapy of Cardiogenic Shock (ECMO-CS), which was published the same day in Circulation.
The trial makes a firm case for preferring the conservative initial approach over routine early ECMO in the kind of patients it entered, Larry A. Allen, MD, MHS, University of Coloradoat Denver, Aurora, told this news organization.
More than 60% of the trial’s 117 patients had shock secondary to an acute coronary syndrome; another 23% were in heart failure decompensation.
A preference for the conservative initial approach would be welcome, he said. The early aggressive ECMO approach is resource intensive and carries some important risks, such as stroke or coagulopathy, said Dr. Allen, who is not connected with ECMO-CS. Yet it is increasingly the go-to approach in such patients, based primarily on observational data.
Although early ECMO apparently didn’t benefit patients in this study in their specific stage of cardiogenic shock, Dr. Allen observed, it would presumably help some, but identifying them in practice presents challenges. “Defining where people are in the spectrum of early versus middle versus late cardiogenic shock is actually very tricky.”
It will therefore be important, he said, to identify ways to predict which conservatively managed patients do well with the strategy, and which are most at risk for hemodynamic deterioration and for whom ECMO should be readily available.
“I think part of what ECMO-CS tells us is that, if a patient is stable on intravenous inotropic and vasopressor support, you can defer ECMO while you’re thinking about the patient – about their larger context and the right medical decision-making for them.”
The trial randomly assigned 122 patients with rapidly deteriorating or severe cardiogenic shock to the immediate-ECMO or the conservative strategy at four centers in the Czech Republic. The 117 patients for whom informed consent could be obtained were included in the analysis, 58 and 59 patients, respectively. Their mean age was about 65 years and three-fourths were male.
The primary endpoint, the only endpoint for which the study was powered, consisted of death from any cause, resuscitated circulatory arrest, or use of a different form of mechanical circulatory support (MCS) by 30 days.
It occurred in 63.8% of patients assigned to immediate ECMO and 71.2% of those in the conservative strategy group, for a hazard ratio of 0.72 (95% confidence interval, 0.46-1.12; P = .21).
As individual endpoints, rates of death from any cause and resuscitated arrest did not significantly differ between the groups, but conservatively managed patients more often used another form of MCS. The HRs were 1.11 (95% CI, 0.66-1.87) for death from any cause, 0.79 (95% CI, 0.27-2.28) for resuscitated cardiac arrest, and 0.38 (95% CI, 0.18-0.79) for use of another MCS device.
The rates for serious adverse events – including bleeding, ischemia, stroke, pneumonia, or sepsis – were similar at 60.3% in the early-ECMO group and 61% in group with conservative initial management, Dr. Ostadal reported.
Other than the 23 patients in the conservative initial strategy group who went on to receive ECMO (1.9 days after randomization, on average), 1 went on to undergo implantation with a HeartMate (Abbott) ventricular assist device and 3 received an Impella pump (Abiomed).
Six patients in the early-ECMO group were already receiving intra-aortic balloon pump (IABP) support at randomization, two underwent temporary implantation with a Centrimag device (Abbott), and three went on to receive a HeartMate device, the published report notes.
ECMO is the optimal first choice for MCS in such patients with cardiogenic shock who need a circulatory support device, especially because it also oxygenates the blood, Dr. Ostadal told this news organization.
But ECMO doesn’t help with ventricular unloading. Indeed, it can sometimes reduce ventricular preload, especially if right-heart pressures are low. So MCS devices that unload the ventricle, typically an IABP, can complement ECMO.
Dr. Ostadal speculates, however, that there may be a better pairing option. “Impella plus ECMO, I think, is the combination which has a future,” he said, for patients in cardiogenic shock who need a short-term percutaneous hemodynamic support device. Impella “supports the whole circulation” and unloads the left ventricle.
“A balloon pump in combination with ECMO is still not a bad choice. It’s very cheap in comparison with Impella.” But in his opinion, Dr. Ostadal said, “The combination of Impella plus ECMO is more efficient from a hemodynamic point of view.”
As the published report notes, ongoing randomized trials looking at ECMO plus other MCS devices in cardiogenic shock include ECLS-SHOCK, with a projected enrollment of 420 patients, and EURO-SHOCK, aiming for a similar number of patients; both compare routine ECMO to conservative management.
In addition, ANCHOR, in which ECMO is combined with IABP, and DanShock, which looks at early use of Impella rather than ECMO, are enrolling patients with shock secondary to acute coronary syndromes.
Dr. Ostadal disclosed consulting for Getinge, Edwards, Medtronic, Biomedica, and Xenios/Fresenius, and receiving research support from Xenios/Fresenius. Dr. Allen disclosed modest or significant relationships with ACI Clinical, Novartis, UpToDate, Boston Scientific, and Cytokinetics.
A version of this article first appeared on Medscape.com.
AT AHA 2022
Inpatient sleep medicine: An invaluable service for hospital medicine
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.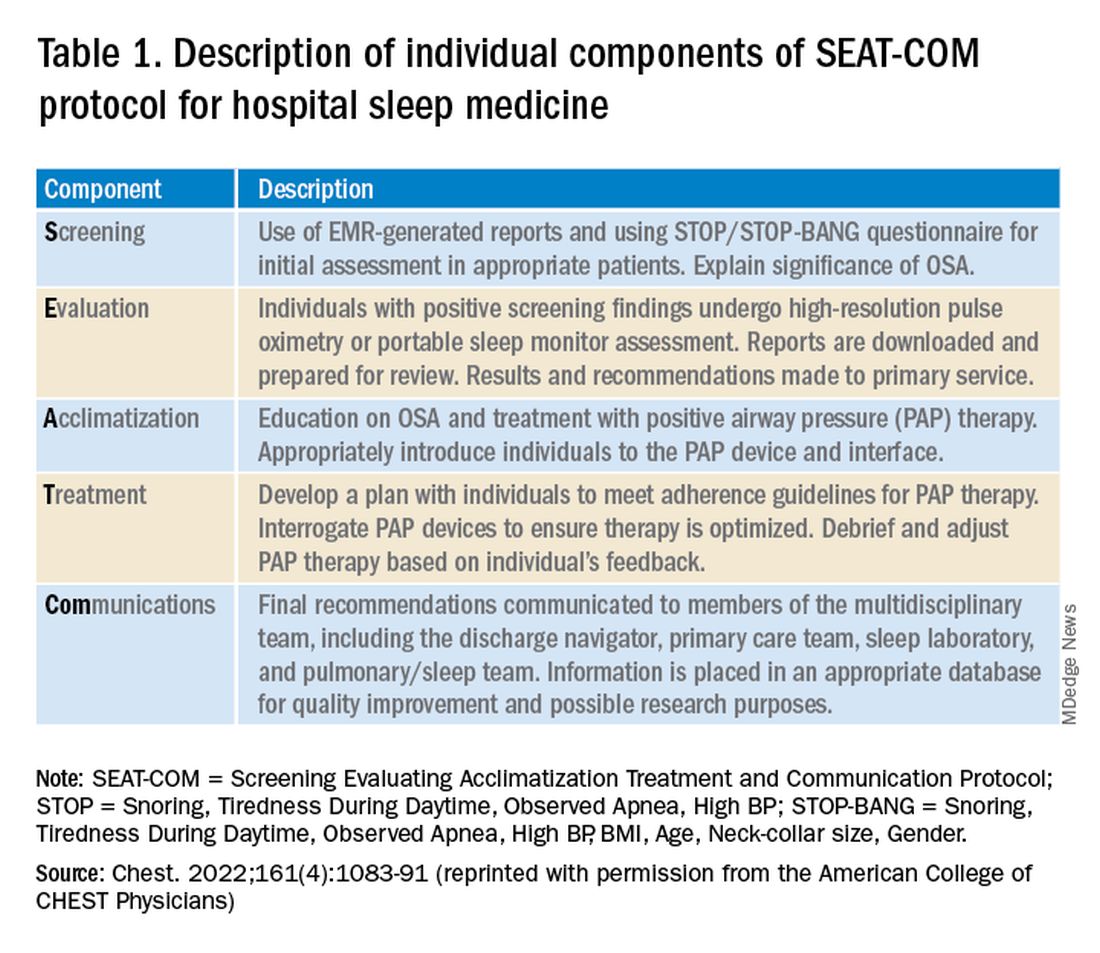
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Estimates suggest that nearly 1 billion adults worldwide could have sleep apnea (Benjafield AV, et al. Lancet Respir Med. 2019;7[8]:687-698). Even with the current widespread use of portable sleep testing, cheap and innovative models of OSA care will need to be developed to address this growing epidemic. This fact is particularly true for communities with significant health disparities, as the evidence suggests diagnostic rates for OSA are extremely poor in these areas (Stansbury R, et al. J Clin Sleep Med. 2022;18[3]:817-824). Current models of care for OSA are predominantly outpatient based. Hospital sleep medicine offers a potential mechanism to capture patients with OSA who would otherwise go undiagnosed and potentially suffer adverse health outcomes from untreated disease.
What is hospital sleep medicine?
Hospital sleep medicine includes the evaluation and management of sleep disorders, including, but not limited to, insomnia, restless legs syndrome, and circadian rhythm disorders, in hospitalized patients. Our program centers around proactive screening and early recognition of sleep-disordered breathing (SDB). Patients at high risk for SDB are identified upon entry to the hospital. These individuals are educated about the disease process and how it impacts comorbid health conditions. Recommendations are provided to the primary team regarding the appropriate screening test for SDB; positive airway pressure trials; mask fitting and acclimation; and coordination with care management in the discharge process, including scheduling follow-up care and diagnostic sleep studies. This program has become an integral part of our comprehensive sleep program, which includes inpatient, outpatient, and sleep center care and utilizes a multidisciplinary team approach including sleep specialists, sleep technologists, respiratory therapists, nurses, information technology professionals, and discharge planners, as well as ambulatory sleep clinics and sleep laboratories.
Evidence for hospital sleep medicine
While there has been interest in hospital-based sleep medicine since 2000, the most well-validated clinical pathway was first described by Sharma and colleagues in 2015 (Sharma, et al. J Clin Sleep Med. 2015;11[7]:717-723). This initial application of a formal sleep program demonstrated a high prevalence of SDB in hospitalized adult patients and led to a substantial increase in SDB diagnoses in the system. Subsequent studies have demonstrated improved outcomes, particularly in patients with cardiopulmonary disease. For example, there are data to suggest that hospitalized patients with congestive heart failure or COPD have increased rates of readmission, and early diagnosis and intervention are associated with decreased rates of subsequent readmission and ED visits (Konikkara J, et al. Hosp Pract. 2016;44[1]:41-47; Sharma S, et al. Am J Cardiol. 2016;117[6]:940-945). Long-term data also suggest survival benefit (Sharma S, et al. Am J Med. 2017;130[10]:1184-1191). Adherence to inpatient PAP trials has also been shown to predict outpatient follow-up and adherence to PAP therapy (Sharma S, et al. Sleep Breath. 2022; published online June 18, 2022).
Establishing a team
Establishing a hospital sleep medicine program requires upfront investment and training and begins with educating key stakeholders. Support from executive administration and various departments including respiratory, sleep medicine, information technology, nursing, physicians, mid-level providers, and discharge planning is essential. Data are available, as outlined here, showing significant improvement in patient outcomes with a hospital sleep medicine program. This information can garner significant enthusiasm from leadership to support the initiation of a program. A more detailed account of key program elements, inpatient protocols, and technologies utilized is available in our recent review (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Table 1 from this article is highlighted here and outlines the essential components (SEAT-COM) of our hospital sleep medicine model. While each component of this model is important, we stress the importance of care coordination, timely diagnostic testing, and treatment, as significant delays can lead to inadequate time for acclimatization and optimization of therapy. It is important to note that the practice of hospital sleep medicine does not supplant clinic-based approaches, but rather serves to facilitate and enhance outpatient diagnosis and treatment.
Current questions
Data to date suggest a hospital sleep medicine program positively influences important clinical endpoints in hospitalized patients identified to be at risk for SDB. However, much of the published research is based on retrospective and prospective analysis of established clinical programs. Further, most studies have been completed at large, urban-based academic medical centers. Our group has recently completed a validation study in our local rural population, but larger multicenter trials involving more diverse communities and health systems are needed to better understand outcomes and further refine the optimal timing of screening and intervention for SDB in hospitalized patients (Stansbury, et al. Sleep Breath. 2022; published online January 20, 2022).
A common question that arises is the program’s impact regarding payment for rendered service in the context of Medicare’s prospective payment system. Given that the program focuses on screening for SDB and does not utilize formal testing for diagnosis, there is no additional cost for diagnostic tests or procedural codes. Thus, the diagnosis-related group is not impacted (Sharma S, Stansbury R. Chest. 2022;161[4]:1083-1091). Importantly, hospital sleep medicine has the potential for cost savings given the reduction in hospital readmissions and decreased adverse events during a patient’s hospital stay. The economics of the initial investment in a hospital sleep program versus potential savings from improved patient outcomes warrants evaluation.
Conclusion
SDB is a prevalent disorder with potential deleterious impacts on a patient’s health. Despite this, it is underrecognized and, thus, undertreated. Hospital sleep medicine is a growing model of care that may expand our capability for early diagnosis and intervention. Studies have demonstrated benefits to patients, particularly those with cardiopulmonary disease. However, additional studies are required to further validate hospital-based sleep medicine in more diverse populations and environments.
Dr. Del Prado Rico and Dr. Stansbury are with the Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Health Science Center North, West Virginia University. Dr. Stansbury is also with the Division of Pulmonary, Allergy, and Critical Care Medicine, Department of Medicine, University of Pittsburgh.
Numbers of adolescents who vape within 5 minutes of waking jumps
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
Vaping has become the dominant form of tobacco use by adolescents in the United States immediately after waking up, according to an analysis of a survey on teen tobacco use published in JAMA Network Open.
By 2019, Stanton Glantz, PhD, and associates found, “more e-cigarette users were using their first tobacco product within 5 minutes of waking than users of cigarettes and all other tobacco products combined.” Use upon waking is an indicator of addiction.
That number changed drastically from 2014 when less than 1% of sole-e-cigarette users were using e-cigarettes first thing in the morning to 10.3% by 2021. The numbers did not change for sole cigarette smokers or sole smokeless tobacco users, but did increase by half (odds ratio per year, 1.49) for sole cigar users.
In addition, among adolescents who currently use any tobacco product, the proportion whose first tobacco product was e-cigarettes increased from 27.2% in 2014 to 78.3% in 2019 and remained close to that at 77% in 2021.
Meanwhile, the number of young people using e-cigarettes peaked in 2019 and has been declining.
By 2019, the Centers for Disease Control and Prevention estimated that 5.3 million middle and high school students were using e-cigarettes. That number dropped to 3.6 million in 2020 and to 2.1 million in 2021 during the COVID-19 pandemic.
Researchers suspect more addictive nicotine
This increasing intensity of use may reflect the higher nicotine delivery and addiction liability of modern e-cigarettes that use protonated nicotine, which makes nicotine easier to inhale than older versions of e-cigarettes, which used freebase nicotine, Dr. Glantz and associates wrote.
The change in nicotine came in 2015 with the introduction of Juul products, they said, “which added benzoic acid to the nicotine e-liquid to lower the pH level and form protonated nicotine.”
The researchers advised: “Clinicians should question all their patients about nicotine and tobacco product use, including e-cigarettes and other new nicotine products.”
Raghu Appasani, MD, a psychiatrist who specializes in adolescent addiction and a clinical fellow at University of California, San Francisco, said in an interview that users often misunderstand the potential health effects of e-cigarettes and mistakenly think of them as a safe alternative to cigarettes.
All medical providers have a responsibility to ask patients about nicotine and tobacco products, Dr. Appasani said.
‘Be curious, not judgmental’
Dr. Appasani advised: “Be curious with your approach. This may uncover that maybe they use [e-cigarettes] to fit into a social scene or have stressors at home or in school. Most likely there is an underlying issue that has led to their use. Perhaps there is untreated anxiety and/or depression. Be curious, not judgmental.”
It is also important to ask about social and psychological factors that may be contributing to use and help the user think through how the use is affecting life in their home, school, and social settings, Dr. Appasani said.
He said he was not surprised by the findings as e-cigarettes allow easy access to smoking and it’s easier to hide the habit. The flavoring often get kids hooked originally.
The authors wrote: “These findings suggest that clinicians need to be ready to address youth addiction to these new highly addictive nicotine products during many clinical encounters, and stronger regulation is needed, including comprehensive bans on the sale of flavored tobacco products.”
Just more than half of the survey respondents (51.1%) were male and average age was 14. Researchers analyzed data from the National Youth Tobacco Survey, a nationally representative survey of middle and high school students.
They used the Youth Behavioral Risk Factor Surveillance System from 2015 to 2019 as a confirmatory analysis.
This study was supported in part by grants from the National Cancer Institute. Dr. Glantz received personal fees from the World Health Organization outside the submitted work. One coauthor reported serving as a paid expert witness against the tobacco industry outside the submitted work. No other disclosures were reported. Dr. Appasani declared no relevant financial relationships.
FROM JAMA NETWORK OPEN
Pulmonologist consult at COPD admission reduces risk of return
according to a retrospective cohort review presented at the annual meeting of the American College of Chest Physicians (CHEST).
“When stratified by severity of COPD at the time of admission, the difference in the readmission rate was even greater,” reported Nakisa Hekmat-Joo, MD, a third-year resident at Staten Island University Hospital, New York.
Just as protocols have been developed for prompt initiation of antibiotics in patients with septicemia or prompt revascularization in patients with ST-segment elevated myocardial infarction (STEMI), Dr. Hekmat-Joo said the data from this study warrant a larger trial to evaluate whether an AECOPD admission protocol is warranted to improve outcomes and lower costs.
In this study, all AECOPD admissions were included from a recent 2-year period at two Staten Island hospitals. Of these, 198 patients received a pulmonologist consult within 24 hours. The remaining 92 patients were not evaluated by pulmonologists but were admitted and then managed by residents, internists, or others.
The primary outcome was length of stay (LOS). Although the slightly lower LOS in pulmonologist-treated group did not approach significance (4.16 vs. 4.21 days; P = .88), the readmission rate at 90 days, which was a secondary outcome, was reduced by almost half (30.1% vs. 57.6%; P < .0001).
At admission, there was no significant difference between those receiving a pulmonologist consult and those who did not. The average O2 saturation was lower in the group seen by a pulmonologist (93% vs. 95.4%; P < .0001), but the most striking difference was the low relative readmission rate, which remained significant after controlling for severity and pulmonary function.
“When we stratified patients for baseline severity, the advantage of a pulmonologist consult was even greater for those with the most severe disease,” Dr. Hekmat-Joo said. Among those with the greatest severity, the 90-day readmission rate was nearly three times greater in the absence of a pulmonologist consult (72% vs. 28%).
Although the comparison of outcomes for those receiving a pulmonologist consult vs. those who did not was adjusted for COPD severity, the potential for pulmonologist consults to be ordered for those patients who looked the sickest would have likely worked against the study result.
“We speculate that pulmonologists were more likely than internists to treat beyond standard guidelines, particularly in the event of greater severity,” Dr. Hekmat-Joo explained. These steps might include earlier use of noninvasive positive pressure ventilation or earlier initiation of rehabilitation strategies.
There were several signals that a pulmonologist consult led to more rigorous care.
“The average time to follow-up after hospitalization was 23 days for the pulmonologist group and 66 days for the nonpulmonologist group,” said Dr. Hekmat-Joo, noting this difference was highly significant (P = .0052).
Based on these results, Dr. Hekmat-Joo and her co-investigators are now working on a protocol for COPD admissions that involves a pulmonologist consult within 24 hours of admission. She hopes to test this protocol in a prospective trial.
“COPD remains a major cause of death and consumes enormous health care resources. About 30% of the cost of COPD care is due to readmissions,” she said, noting that readmissions adversely impact quality of life.
Asked if there was sufficient staff at her institution to allow for a pulmonologist consult with every COPD admission, Dr. Hekmat-Joo acknowledged that this has to be demonstrated, but compelling evidence of a benefit might prompt a redistribution of resources.
“If we can show that readmissions are substantially reduced, adding staff to perform these consults would be a good investment,” said Dr. Hekmat-Joo, indicating that improved outcomes could also attract the attention of third-party payers and those tracking quality-of-care metrics.
There is a strong rationale for a randomized prospective trial to confirm the value of a pulmonologist consultation following admission for an acute exacerbation of COPD, according to Nicola A. Hanania, MD, director, Airways Clinical Research Center, Baylor College of Medicine, Houston.
The potential for benefit as seen in this retrospective study is a rational expectation and might be related to more appropriate therapy upon discharge as well as to earlier and more rigorous follow-up, according to Dr. Hanania. Although he cautioned that there is a meaningful risk of selection bias in a retrospective study, he thinks this study “is certainly probing an important issue.”
“Mortality from a hospitalized COPD exacerbation exceeds that of a myocardial infarction,” Dr. Hanania pointed out. Noting that all patients with an MI are evaluated by a cardiologist, he sees the logic of a pulmonologist consult – although he acknowledged that evidence is needed.
“I strongly believe that a prospective study is feasible and will answer the question in an unbiased manner if done properly,” he said in an interview. If a multicenter, well-controlled study was positive, it could change practice.
In the event of a study showing major clinical benefits, particularly a reduction in mortality, “I believe it is feasible to have a pulmonary consult to see every COPD exacerbation patient admitted to the hospital,” Dr. Hanania said.
Dr. Hekmat-Joo reports no relevant financial relationships. Dr. Hanania has financial relationships with AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mylan, Novartis, Regeneron, Sanofi, and Sunovion.
A version of this article first appeared on Medscape.com.
according to a retrospective cohort review presented at the annual meeting of the American College of Chest Physicians (CHEST).
“When stratified by severity of COPD at the time of admission, the difference in the readmission rate was even greater,” reported Nakisa Hekmat-Joo, MD, a third-year resident at Staten Island University Hospital, New York.
Just as protocols have been developed for prompt initiation of antibiotics in patients with septicemia or prompt revascularization in patients with ST-segment elevated myocardial infarction (STEMI), Dr. Hekmat-Joo said the data from this study warrant a larger trial to evaluate whether an AECOPD admission protocol is warranted to improve outcomes and lower costs.
In this study, all AECOPD admissions were included from a recent 2-year period at two Staten Island hospitals. Of these, 198 patients received a pulmonologist consult within 24 hours. The remaining 92 patients were not evaluated by pulmonologists but were admitted and then managed by residents, internists, or others.
The primary outcome was length of stay (LOS). Although the slightly lower LOS in pulmonologist-treated group did not approach significance (4.16 vs. 4.21 days; P = .88), the readmission rate at 90 days, which was a secondary outcome, was reduced by almost half (30.1% vs. 57.6%; P < .0001).
At admission, there was no significant difference between those receiving a pulmonologist consult and those who did not. The average O2 saturation was lower in the group seen by a pulmonologist (93% vs. 95.4%; P < .0001), but the most striking difference was the low relative readmission rate, which remained significant after controlling for severity and pulmonary function.
“When we stratified patients for baseline severity, the advantage of a pulmonologist consult was even greater for those with the most severe disease,” Dr. Hekmat-Joo said. Among those with the greatest severity, the 90-day readmission rate was nearly three times greater in the absence of a pulmonologist consult (72% vs. 28%).
Although the comparison of outcomes for those receiving a pulmonologist consult vs. those who did not was adjusted for COPD severity, the potential for pulmonologist consults to be ordered for those patients who looked the sickest would have likely worked against the study result.
“We speculate that pulmonologists were more likely than internists to treat beyond standard guidelines, particularly in the event of greater severity,” Dr. Hekmat-Joo explained. These steps might include earlier use of noninvasive positive pressure ventilation or earlier initiation of rehabilitation strategies.
There were several signals that a pulmonologist consult led to more rigorous care.
“The average time to follow-up after hospitalization was 23 days for the pulmonologist group and 66 days for the nonpulmonologist group,” said Dr. Hekmat-Joo, noting this difference was highly significant (P = .0052).
Based on these results, Dr. Hekmat-Joo and her co-investigators are now working on a protocol for COPD admissions that involves a pulmonologist consult within 24 hours of admission. She hopes to test this protocol in a prospective trial.
“COPD remains a major cause of death and consumes enormous health care resources. About 30% of the cost of COPD care is due to readmissions,” she said, noting that readmissions adversely impact quality of life.
Asked if there was sufficient staff at her institution to allow for a pulmonologist consult with every COPD admission, Dr. Hekmat-Joo acknowledged that this has to be demonstrated, but compelling evidence of a benefit might prompt a redistribution of resources.
“If we can show that readmissions are substantially reduced, adding staff to perform these consults would be a good investment,” said Dr. Hekmat-Joo, indicating that improved outcomes could also attract the attention of third-party payers and those tracking quality-of-care metrics.
There is a strong rationale for a randomized prospective trial to confirm the value of a pulmonologist consultation following admission for an acute exacerbation of COPD, according to Nicola A. Hanania, MD, director, Airways Clinical Research Center, Baylor College of Medicine, Houston.
The potential for benefit as seen in this retrospective study is a rational expectation and might be related to more appropriate therapy upon discharge as well as to earlier and more rigorous follow-up, according to Dr. Hanania. Although he cautioned that there is a meaningful risk of selection bias in a retrospective study, he thinks this study “is certainly probing an important issue.”
“Mortality from a hospitalized COPD exacerbation exceeds that of a myocardial infarction,” Dr. Hanania pointed out. Noting that all patients with an MI are evaluated by a cardiologist, he sees the logic of a pulmonologist consult – although he acknowledged that evidence is needed.
“I strongly believe that a prospective study is feasible and will answer the question in an unbiased manner if done properly,” he said in an interview. If a multicenter, well-controlled study was positive, it could change practice.
In the event of a study showing major clinical benefits, particularly a reduction in mortality, “I believe it is feasible to have a pulmonary consult to see every COPD exacerbation patient admitted to the hospital,” Dr. Hanania said.
Dr. Hekmat-Joo reports no relevant financial relationships. Dr. Hanania has financial relationships with AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mylan, Novartis, Regeneron, Sanofi, and Sunovion.
A version of this article first appeared on Medscape.com.
according to a retrospective cohort review presented at the annual meeting of the American College of Chest Physicians (CHEST).
“When stratified by severity of COPD at the time of admission, the difference in the readmission rate was even greater,” reported Nakisa Hekmat-Joo, MD, a third-year resident at Staten Island University Hospital, New York.
Just as protocols have been developed for prompt initiation of antibiotics in patients with septicemia or prompt revascularization in patients with ST-segment elevated myocardial infarction (STEMI), Dr. Hekmat-Joo said the data from this study warrant a larger trial to evaluate whether an AECOPD admission protocol is warranted to improve outcomes and lower costs.
In this study, all AECOPD admissions were included from a recent 2-year period at two Staten Island hospitals. Of these, 198 patients received a pulmonologist consult within 24 hours. The remaining 92 patients were not evaluated by pulmonologists but were admitted and then managed by residents, internists, or others.
The primary outcome was length of stay (LOS). Although the slightly lower LOS in pulmonologist-treated group did not approach significance (4.16 vs. 4.21 days; P = .88), the readmission rate at 90 days, which was a secondary outcome, was reduced by almost half (30.1% vs. 57.6%; P < .0001).
At admission, there was no significant difference between those receiving a pulmonologist consult and those who did not. The average O2 saturation was lower in the group seen by a pulmonologist (93% vs. 95.4%; P < .0001), but the most striking difference was the low relative readmission rate, which remained significant after controlling for severity and pulmonary function.
“When we stratified patients for baseline severity, the advantage of a pulmonologist consult was even greater for those with the most severe disease,” Dr. Hekmat-Joo said. Among those with the greatest severity, the 90-day readmission rate was nearly three times greater in the absence of a pulmonologist consult (72% vs. 28%).
Although the comparison of outcomes for those receiving a pulmonologist consult vs. those who did not was adjusted for COPD severity, the potential for pulmonologist consults to be ordered for those patients who looked the sickest would have likely worked against the study result.
“We speculate that pulmonologists were more likely than internists to treat beyond standard guidelines, particularly in the event of greater severity,” Dr. Hekmat-Joo explained. These steps might include earlier use of noninvasive positive pressure ventilation or earlier initiation of rehabilitation strategies.
There were several signals that a pulmonologist consult led to more rigorous care.
“The average time to follow-up after hospitalization was 23 days for the pulmonologist group and 66 days for the nonpulmonologist group,” said Dr. Hekmat-Joo, noting this difference was highly significant (P = .0052).
Based on these results, Dr. Hekmat-Joo and her co-investigators are now working on a protocol for COPD admissions that involves a pulmonologist consult within 24 hours of admission. She hopes to test this protocol in a prospective trial.
“COPD remains a major cause of death and consumes enormous health care resources. About 30% of the cost of COPD care is due to readmissions,” she said, noting that readmissions adversely impact quality of life.
Asked if there was sufficient staff at her institution to allow for a pulmonologist consult with every COPD admission, Dr. Hekmat-Joo acknowledged that this has to be demonstrated, but compelling evidence of a benefit might prompt a redistribution of resources.
“If we can show that readmissions are substantially reduced, adding staff to perform these consults would be a good investment,” said Dr. Hekmat-Joo, indicating that improved outcomes could also attract the attention of third-party payers and those tracking quality-of-care metrics.
There is a strong rationale for a randomized prospective trial to confirm the value of a pulmonologist consultation following admission for an acute exacerbation of COPD, according to Nicola A. Hanania, MD, director, Airways Clinical Research Center, Baylor College of Medicine, Houston.
The potential for benefit as seen in this retrospective study is a rational expectation and might be related to more appropriate therapy upon discharge as well as to earlier and more rigorous follow-up, according to Dr. Hanania. Although he cautioned that there is a meaningful risk of selection bias in a retrospective study, he thinks this study “is certainly probing an important issue.”
“Mortality from a hospitalized COPD exacerbation exceeds that of a myocardial infarction,” Dr. Hanania pointed out. Noting that all patients with an MI are evaluated by a cardiologist, he sees the logic of a pulmonologist consult – although he acknowledged that evidence is needed.
“I strongly believe that a prospective study is feasible and will answer the question in an unbiased manner if done properly,” he said in an interview. If a multicenter, well-controlled study was positive, it could change practice.
In the event of a study showing major clinical benefits, particularly a reduction in mortality, “I believe it is feasible to have a pulmonary consult to see every COPD exacerbation patient admitted to the hospital,” Dr. Hanania said.
Dr. Hekmat-Joo reports no relevant financial relationships. Dr. Hanania has financial relationships with AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Mylan, Novartis, Regeneron, Sanofi, and Sunovion.
A version of this article first appeared on Medscape.com.
FROM CHEST 2022
Working while sick: Why doctors don’t stay home when ill
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.
The reasons are likely as varied as, “you weren’t feeling bad enough to miss work,” “you couldn’t afford to miss pay,” “you had too many patients to see,” or “too much work to do.”
In Medscape’s Employed Physicians Report: Loving the Focus, Hating the Bureaucracy, 61% of physicians reported that they sometimes or often come to work sick. Only 2% of respondents said they never come to work unwell.
Medscape wanted to know more about how often you call in sick, how often you come to work feeling unwell, what symptoms you have, and the dogma of your workplace culture regarding sick days. Not to mention the brutal ethos that starts in medical school, in which calling in sick shows weakness or is unacceptable.
So, we polled 2,347 physicians in the United States and abroad and asked them about their sniffling, sneezing, cold, flu, and fever symptoms, and, of course, COVID. Results were split about 50-50 among male and female physicians. The poll ran from Sept. 28 through Oct. 11.
Coming to work sick
It’s no surprise that the majority of physicians who were polled (85%) have come to work sick in 2022. In the last prepandemic year (2019), about 70% came to work feeling sick one to five times, and 13% worked while sick six to ten times.
When asked about the symptoms that they’ve previously come to work with, 48% of U.S. physicians said multiple symptoms. They gave high marks for runny nose, cough, congestion, and sore throat. Only 27% have worked with a fever, 22% have worked with other symptoms, and 7% have worked with both strep throat and COVID.
“My workplace, especially in the COVID years, accommodates persons who honestly do not feel well enough to report. Sooner or later, everyone covers for someone else who has to be out,” says Kenneth Abbott, MD, an oncologist in Maryland.
The culture of working while sick
Why doctors come to work when they’re sick is complicated. The overwhelming majority of U.S. respondents cited professional obligations; 73% noted that they feel a professional obligation to their patients, and 72% feel a professional obligation to their co-workers. Half of the polled U.S. physicians said they didn’t feel bad enough to stay home, while 48% said they had too much work to do to stay home.
Some 45% said the expectation at their workplace is to come to work unless seriously ill; 43% had too many patients to see; and 18% didn’t think they were contagious when they headed to work sick. Unfortunately, 15% chose to work while sick because otherwise they would lose pay.
In light of these responses, it’s not surprising that 93% reported they’d seen other medical professionals working when sick.
“My schedule is almost always booked weeks in advance. If someone misses or has to cancel their appointment, they typically have 2-4 weeks to wait to get back in. If I was sick and a full day of patients (or God forbid more than a day) had to be canceled because I called in, it’s so much more work when I return,” says Caitlin Briggs, MD, a psychiatrist in Lexington, Ky.
Doctors’ workplace sick day policy
Most employees’ benefits allow at least a few sick days, but doctors who treat society’s ill patients don’t seem to stay home from work when they’re suffering. So, we asked physicians, official policy aside, whether they thought going to work sick was expected in their workplace. The majority (76%) said yes, while 24% said no.
“Unless I’m dying or extremely contagious, I usually work. At least now, I have the telehealth option. Not saying any of this is right, but it’s the reality we deal with and the choice we must make,” says Dr. Briggs.
Additionally, 58% of polled physicians said their workplace did not have a clearly defined policy against coming to work sick, while 20% said theirs did, and 22% weren’t sure.
“The first thing I heard on the subject as a medical student was that sick people come to the hospital, so if you’re sick, then you come to the hospital too ... to work. If you can’t work, then you will be admitted. Another aphorism was from Churchill, that ‘most of the world’s work is done by people who don’t feel very well,’ ” says Paul Andreason, MD, a psychiatrist in Bethesda, Md.
Working in the time of COVID
Working while ill during ordinary times is one thing, but what about working in the time of COVID? Has the pandemic changed the culture of coming to work sick because medical facilities, such as doctor’s offices and hospitals, don’t want their staff coming in when they have COVID?
Surprisingly, when we asked physicians whether the pandemic has made it more or less acceptable to come to work sick, only 61% thought COVID has made it less acceptable to work while sick, while 16% thought it made it more acceptable, and 23% said there’s no change.
“I draw the line at fevers/chills, feeling like you’ve just been run over, or significant enteritis,” says Dr. Abbott. “Also, if I have to take palliative meds that interfere with alertness, I’m not doing my patients any favors.”
While a minority of physicians may call in sick, most still suffer through their sneezing, coughing, chills, and fever while seeing patients as usual.
A version of this article first appeared on Medscape.com.