User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


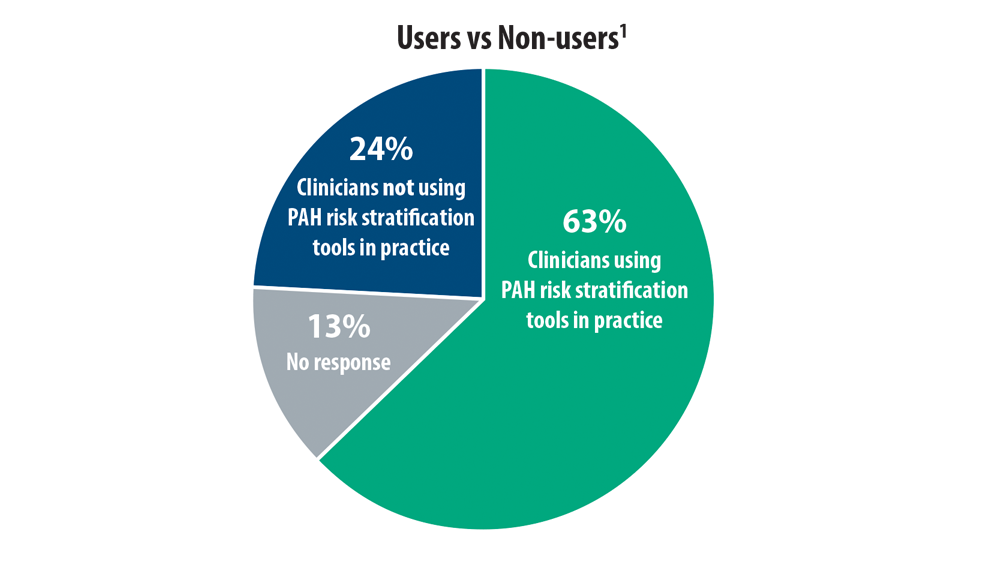
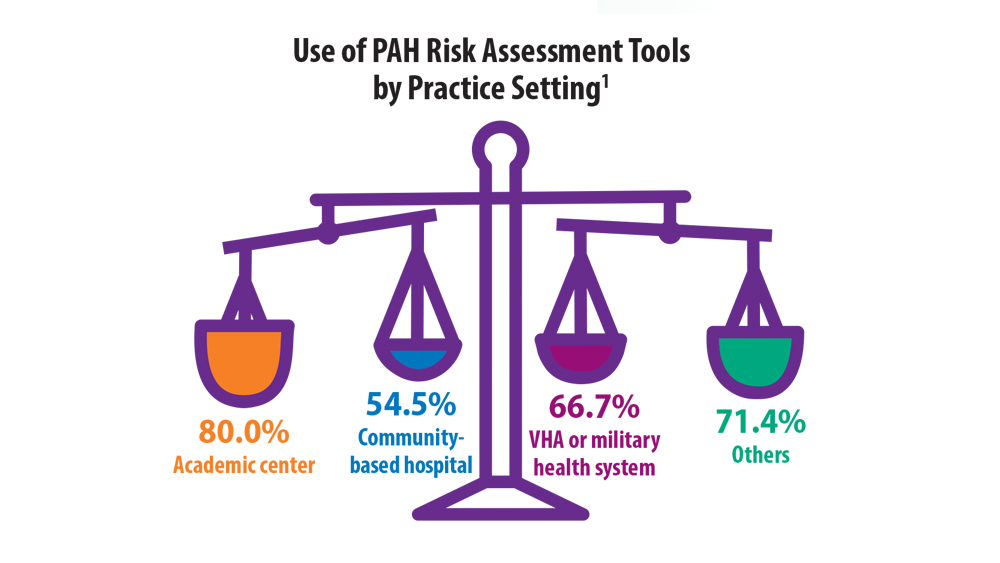
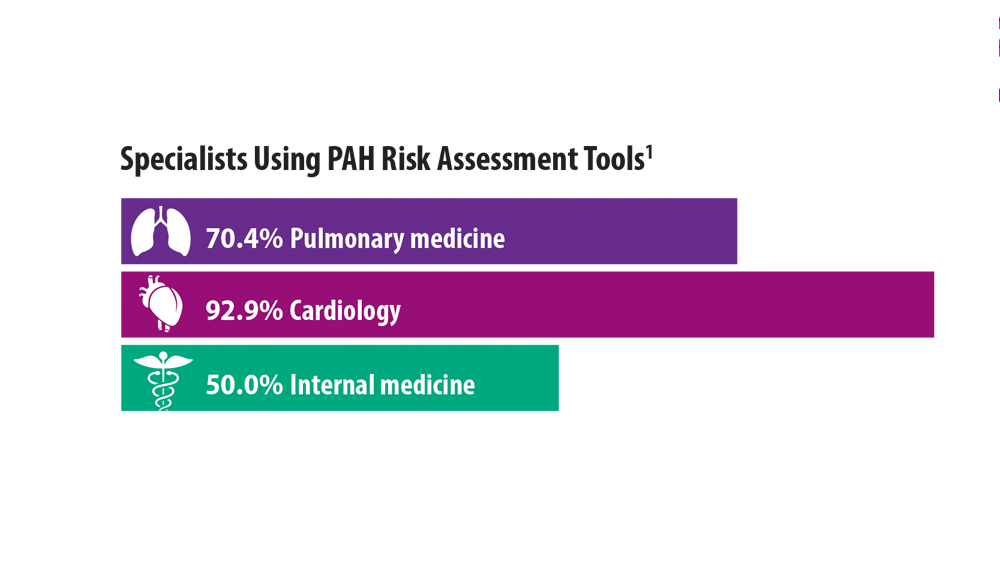
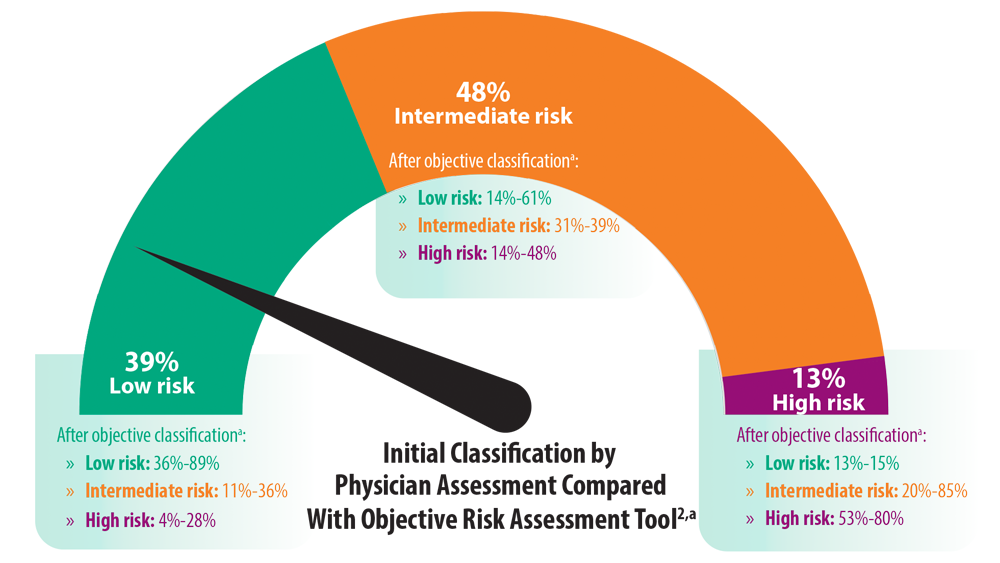
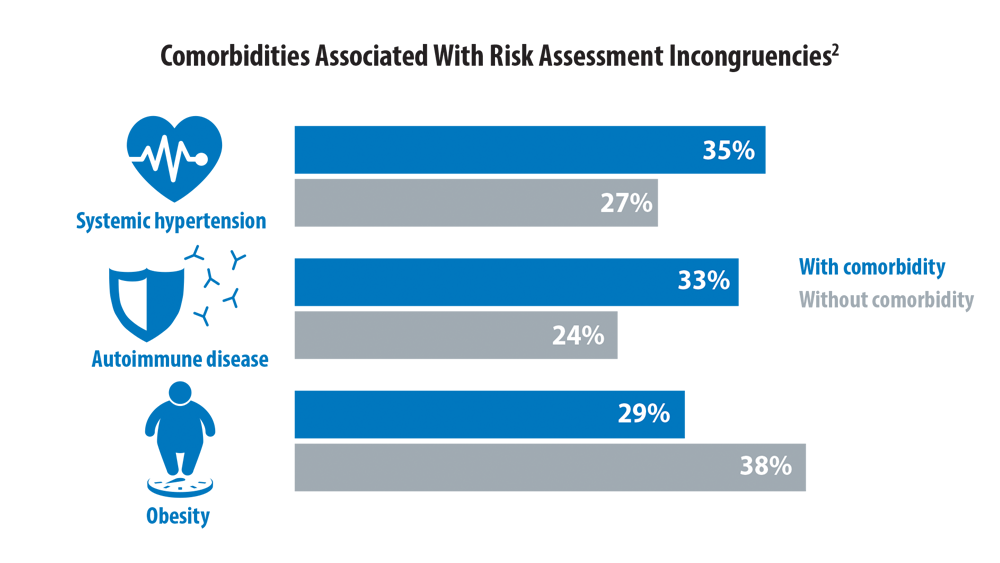
Risk Assessment in Pulmonary Arterial Hypertension
- Sahay S, Balasubramanian V, Memon H, et al. Utilization of risk assessment tools in management of PAH: a PAH provider survey. Pulm Circ. 2022;12(2):e12057. doi:10.1002/pul2.12057
- Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15(11):e0241504. doi:10.1371/journal.pone.0241504
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi:10.1183/13993003.01889-2018
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. doi:10.1183/13993003.00889-2017
- Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. doi:10.1177/2045894020950186
- Sahay S, Balasubramanian V, Memon H, et al. Utilization of risk assessment tools in management of PAH: a PAH provider survey. Pulm Circ. 2022;12(2):e12057. doi:10.1002/pul2.12057
- Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15(11):e0241504. doi:10.1371/journal.pone.0241504
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi:10.1183/13993003.01889-2018
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. doi:10.1183/13993003.00889-2017
- Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. doi:10.1177/2045894020950186
- Sahay S, Balasubramanian V, Memon H, et al. Utilization of risk assessment tools in management of PAH: a PAH provider survey. Pulm Circ. 2022;12(2):e12057. doi:10.1002/pul2.12057
- Sahay S, Tonelli AR, Selej M, Watson Z, Benza RL. Risk assessment in patients with functional class II pulmonary arterial hypertension: comparison of physician gestalt with ESC/ERS and the REVEAL 2.0 risk score. PLoS One. 2020;15(11):e0241504. doi:10.1371/journal.pone.0241504
- Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J. 2019;53(1):1801889. doi:10.1183/13993003.01889-2018
- Boucly A, Weatherald J, Savale L, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J. 2017;50(2):1700889. doi:10.1183/13993003.00889-2017
- Wilson M, Keeley J, Kingman M, Wang J, Rogers F. Current clinical utilization of risk assessment tools in pulmonary arterial hypertension: a descriptive survey of facilitation strategies, patterns, and barriers to use in the United States. Pulm Circ. 2020;10(3):2045894020950186. doi:10.1177/2045894020950186
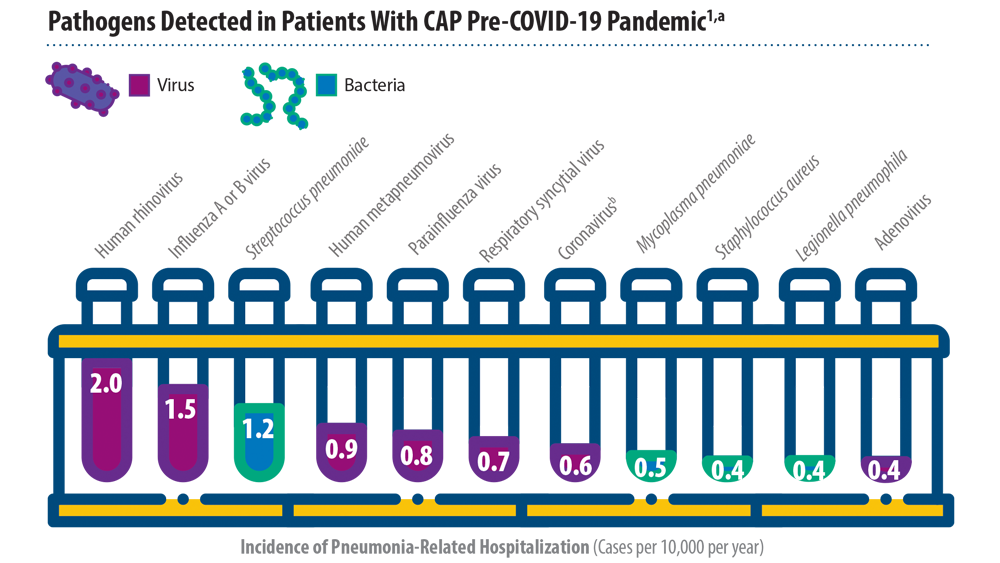
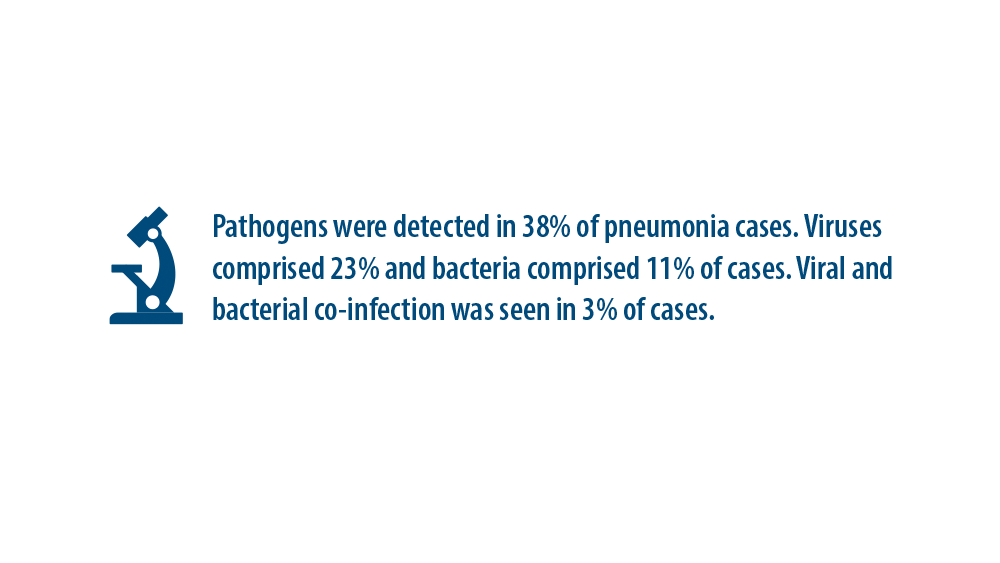
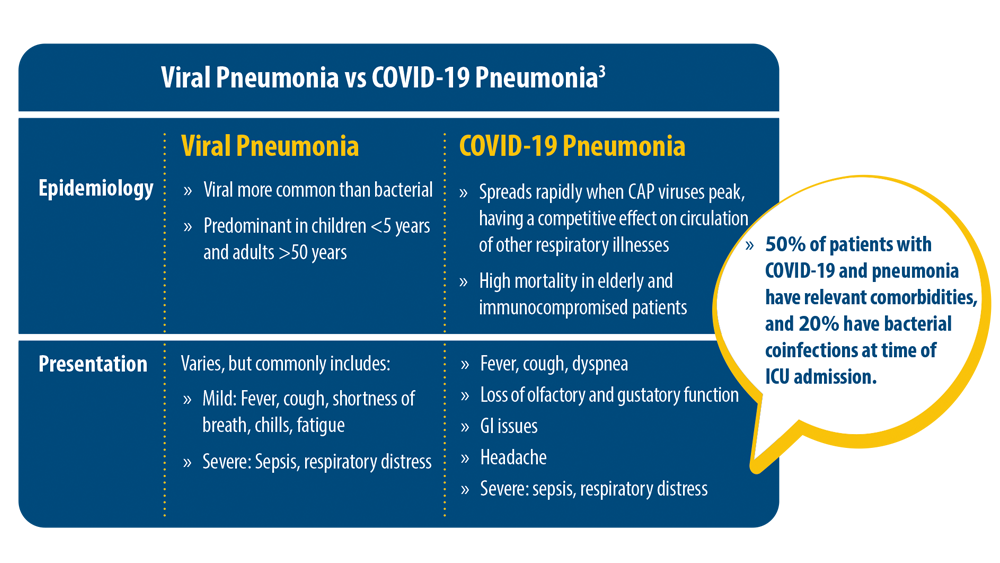
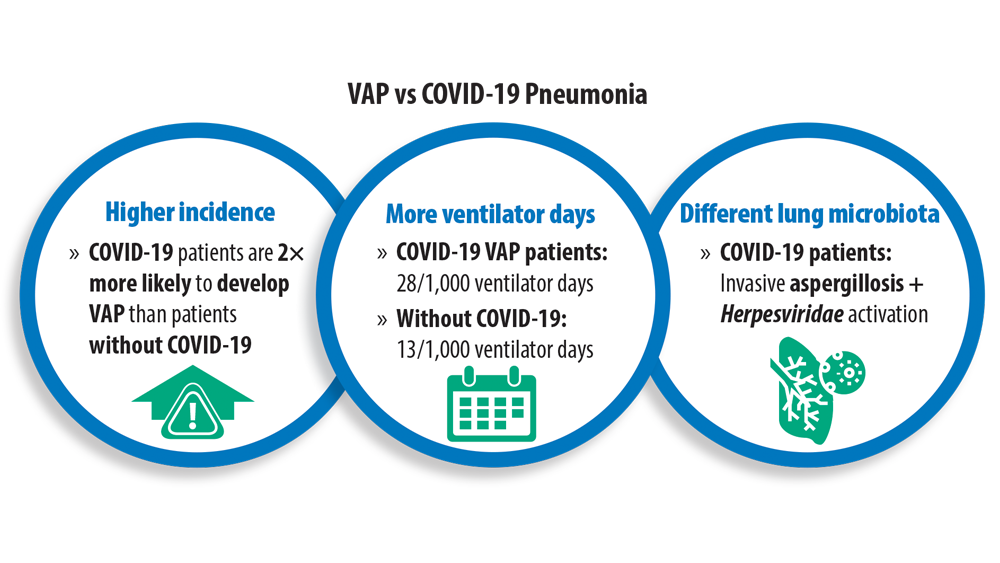
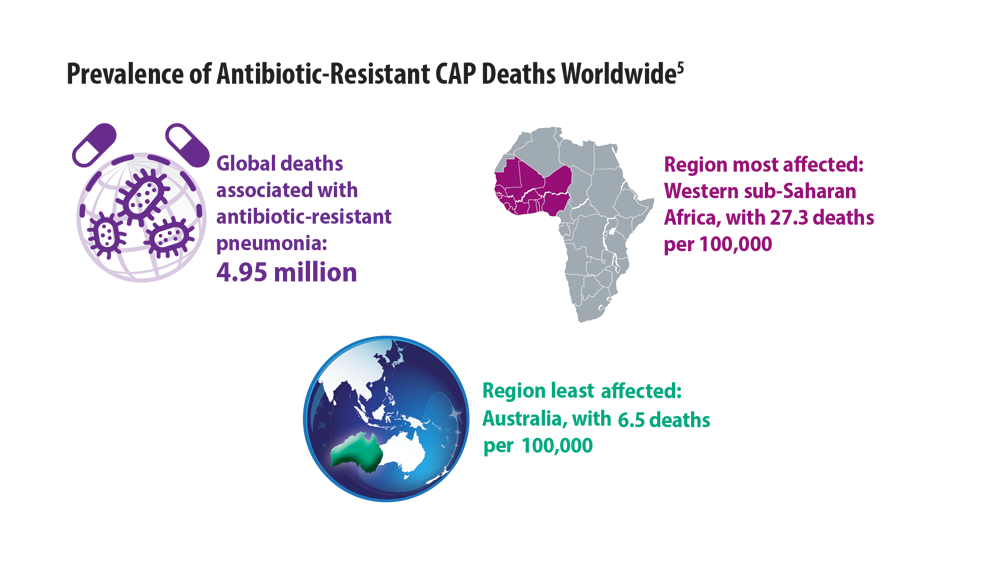
New Pathogens, COVID-19, and Antibiotic Resistance in the Field of Pneumonia
- Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415-427. doi:10.1056/NEJMoa1500245
- Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia. Lancet. 2021;398(10303):906-919. doi:10.1016/S0140-6736(21)00630-9
- Pagliano P, Sellitto C, Conti V, Ascione T, Esposito S. Characteristics of viral pneumonia in the COVID-19 era: an update. Infection. 2021;49(4):607-616. doi:10.1007/s15010-021-01603-y
- Maes M, Higginson E, Pereira-Dias J, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19 [published correction appears in Crit Care. 2021 Apr 6;25(1):130]. Crit Care. 2021;25(1):25. doi:10.1186/s13054-021-03460-5
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. doi:10.1016/S0140- 6736(21)02724-0
- Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415-427. doi:10.1056/NEJMoa1500245
- Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia. Lancet. 2021;398(10303):906-919. doi:10.1016/S0140-6736(21)00630-9
- Pagliano P, Sellitto C, Conti V, Ascione T, Esposito S. Characteristics of viral pneumonia in the COVID-19 era: an update. Infection. 2021;49(4):607-616. doi:10.1007/s15010-021-01603-y
- Maes M, Higginson E, Pereira-Dias J, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19 [published correction appears in Crit Care. 2021 Apr 6;25(1):130]. Crit Care. 2021;25(1):25. doi:10.1186/s13054-021-03460-5
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. doi:10.1016/S0140- 6736(21)02724-0
- Jain S, Self WH, Wunderink RG, et al. Community-acquired pneumonia requiring hospitalization among US adults. N Engl J Med. 2015;373(5):415-427. doi:10.1056/NEJMoa1500245
- Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia. Lancet. 2021;398(10303):906-919. doi:10.1016/S0140-6736(21)00630-9
- Pagliano P, Sellitto C, Conti V, Ascione T, Esposito S. Characteristics of viral pneumonia in the COVID-19 era: an update. Infection. 2021;49(4):607-616. doi:10.1007/s15010-021-01603-y
- Maes M, Higginson E, Pereira-Dias J, et al. Ventilator-associated pneumonia in critically ill patients with COVID-19 [published correction appears in Crit Care. 2021 Apr 6;25(1):130]. Crit Care. 2021;25(1):25. doi:10.1186/s13054-021-03460-5
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399(10325):629-655. doi:10.1016/S0140- 6736(21)02724-0
End the year on a wine note at the final Viva La Vino event of 2022
Don’t miss your last chance to join the CHEST Foundation for a celebration of excellent initiatives – and equally excellent wines – at the last Viva La Vino event of 2022, happening on December 1 at 7 pm.
This event will focus on white and red varietals from Piedmont, a region of Northwest Italy. The Piedmont area is known for producing more wines classified as Denominazione di Origine Controllata e Garantita, the highest classification of quality for wines in Italy, than any other region.
Join CHEST CEO Bob Musacchio, PhD, as he guides attendees through a virtual and interactive exploration of the history, varietals, and techniques of Piedmont wines. Plus, hear from other CHEST leaders and friends of the Foundation about the important work currently being done and the evolution of the Foundation’s many initiatives since its inception.
With their ticket, attendees will receive one bottle of white wine and two bottles of red wine – including Paitin Starda Langhe Nebbiolo 2019, Michele Chiarlo Le Madri Roero Arneis 2020, and Massolino Barbera d’Alba 2019 – as well as an Italian-themed snack kit featuring cheese, salami, taralli, and other tasty treats, to complement their imbibes.
Funds raised from Viva La Vino benefit the Harold Amos Medical Faculty Development Program (AMFDP) and CHEST initiatives to improve patient care. The AMFDP offers 4-year postdoctoral research awards to physicians, dentists, and nurses from historically marginalized backgrounds. Learn more about the recipient of this year’s grant, George Alba, MD, in the September issue of CHEST Physician.
Don’t miss your last chance to join the CHEST Foundation for a celebration of excellent initiatives – and equally excellent wines – at the last Viva La Vino event of 2022, happening on December 1 at 7 pm.
This event will focus on white and red varietals from Piedmont, a region of Northwest Italy. The Piedmont area is known for producing more wines classified as Denominazione di Origine Controllata e Garantita, the highest classification of quality for wines in Italy, than any other region.
Join CHEST CEO Bob Musacchio, PhD, as he guides attendees through a virtual and interactive exploration of the history, varietals, and techniques of Piedmont wines. Plus, hear from other CHEST leaders and friends of the Foundation about the important work currently being done and the evolution of the Foundation’s many initiatives since its inception.
With their ticket, attendees will receive one bottle of white wine and two bottles of red wine – including Paitin Starda Langhe Nebbiolo 2019, Michele Chiarlo Le Madri Roero Arneis 2020, and Massolino Barbera d’Alba 2019 – as well as an Italian-themed snack kit featuring cheese, salami, taralli, and other tasty treats, to complement their imbibes.
Funds raised from Viva La Vino benefit the Harold Amos Medical Faculty Development Program (AMFDP) and CHEST initiatives to improve patient care. The AMFDP offers 4-year postdoctoral research awards to physicians, dentists, and nurses from historically marginalized backgrounds. Learn more about the recipient of this year’s grant, George Alba, MD, in the September issue of CHEST Physician.
Don’t miss your last chance to join the CHEST Foundation for a celebration of excellent initiatives – and equally excellent wines – at the last Viva La Vino event of 2022, happening on December 1 at 7 pm.
This event will focus on white and red varietals from Piedmont, a region of Northwest Italy. The Piedmont area is known for producing more wines classified as Denominazione di Origine Controllata e Garantita, the highest classification of quality for wines in Italy, than any other region.
Join CHEST CEO Bob Musacchio, PhD, as he guides attendees through a virtual and interactive exploration of the history, varietals, and techniques of Piedmont wines. Plus, hear from other CHEST leaders and friends of the Foundation about the important work currently being done and the evolution of the Foundation’s many initiatives since its inception.
With their ticket, attendees will receive one bottle of white wine and two bottles of red wine – including Paitin Starda Langhe Nebbiolo 2019, Michele Chiarlo Le Madri Roero Arneis 2020, and Massolino Barbera d’Alba 2019 – as well as an Italian-themed snack kit featuring cheese, salami, taralli, and other tasty treats, to complement their imbibes.
Funds raised from Viva La Vino benefit the Harold Amos Medical Faculty Development Program (AMFDP) and CHEST initiatives to improve patient care. The AMFDP offers 4-year postdoctoral research awards to physicians, dentists, and nurses from historically marginalized backgrounds. Learn more about the recipient of this year’s grant, George Alba, MD, in the September issue of CHEST Physician.
Chest Infections & Disaster Response Network
Disaster Response & Global Health Section
Responding to firearm violence in America
We think of disasters as sudden, calamitous events, but it does not take much imagination to recognize the loss of lives in America from firearm violence as a type of disaster. In 2020, 45,222 people died from gun-related injuries, an increase of 5,155 (14%) since 2019 (Kegler, et al. MMWR Morb Mortal Wkly Rep. 2022;71[19]:656). This is the highest death rate since 1994, and includes increases in both homicides and suicides. Mass shootings constitute a fraction of this total, but there have already been 530 deaths from mass shooting incidents in 2022.
Opinions about the appropriate degree of firearm regulations remain divided, but the need to improve our response as clinicians is clear. The National Center for Disaster Medicine and Public Health recently published consensus recommendations for healthcare response in mass shootings (Goolsby, et al. J Am Coll Surg. 2022; published online July 18, 2022). These recommendations address readiness training, triage, communications, public education, patient tracking, family reunification, and mental health services.
Stop the Bleed is a program originally based on the military’s Tactical Combat Casualty Care standards. It offers training on hemorrhage control for both the public and clinicians, similar to basic life support programs. It encourages bystanders to become trained and empowered to help in a bleeding emergency before professional help arrives. Opportunities for training are a frequent offering at the CHEST Annual Meeting, and additional information can be found at https://www.stopthebleed.org.
Stella Ogake, MD
Disaster Response & Global Health Section
Responding to firearm violence in America
We think of disasters as sudden, calamitous events, but it does not take much imagination to recognize the loss of lives in America from firearm violence as a type of disaster. In 2020, 45,222 people died from gun-related injuries, an increase of 5,155 (14%) since 2019 (Kegler, et al. MMWR Morb Mortal Wkly Rep. 2022;71[19]:656). This is the highest death rate since 1994, and includes increases in both homicides and suicides. Mass shootings constitute a fraction of this total, but there have already been 530 deaths from mass shooting incidents in 2022.
Opinions about the appropriate degree of firearm regulations remain divided, but the need to improve our response as clinicians is clear. The National Center for Disaster Medicine and Public Health recently published consensus recommendations for healthcare response in mass shootings (Goolsby, et al. J Am Coll Surg. 2022; published online July 18, 2022). These recommendations address readiness training, triage, communications, public education, patient tracking, family reunification, and mental health services.
Stop the Bleed is a program originally based on the military’s Tactical Combat Casualty Care standards. It offers training on hemorrhage control for both the public and clinicians, similar to basic life support programs. It encourages bystanders to become trained and empowered to help in a bleeding emergency before professional help arrives. Opportunities for training are a frequent offering at the CHEST Annual Meeting, and additional information can be found at https://www.stopthebleed.org.
Stella Ogake, MD
Disaster Response & Global Health Section
Responding to firearm violence in America
We think of disasters as sudden, calamitous events, but it does not take much imagination to recognize the loss of lives in America from firearm violence as a type of disaster. In 2020, 45,222 people died from gun-related injuries, an increase of 5,155 (14%) since 2019 (Kegler, et al. MMWR Morb Mortal Wkly Rep. 2022;71[19]:656). This is the highest death rate since 1994, and includes increases in both homicides and suicides. Mass shootings constitute a fraction of this total, but there have already been 530 deaths from mass shooting incidents in 2022.
Opinions about the appropriate degree of firearm regulations remain divided, but the need to improve our response as clinicians is clear. The National Center for Disaster Medicine and Public Health recently published consensus recommendations for healthcare response in mass shootings (Goolsby, et al. J Am Coll Surg. 2022; published online July 18, 2022). These recommendations address readiness training, triage, communications, public education, patient tracking, family reunification, and mental health services.
Stop the Bleed is a program originally based on the military’s Tactical Combat Casualty Care standards. It offers training on hemorrhage control for both the public and clinicians, similar to basic life support programs. It encourages bystanders to become trained and empowered to help in a bleeding emergency before professional help arrives. Opportunities for training are a frequent offering at the CHEST Annual Meeting, and additional information can be found at https://www.stopthebleed.org.
Stella Ogake, MD
Time travel and thoughts on leadership
This is an odd column for me to write. First, because of the nature of print publication, this writing for the November issue is being crafted just before the annual meeting is to be held in Nashville. Therefore, while I have a pretty good sense of what is in store for CHEST 2022, I have yet to see the final product, or the audience’s reaction to it. However, I will make some bold predictions as to what occurred therein:
- Even in the context of 3 years of separation, thousands CHEST members gathered in droves to rekindle friendships and to experience the best education in pulmonary, critical care, and sleep medicine that the world has to offer, leading to our second-biggest meeting ever.
- Neil Pasricha’s presentation helped attendees rekindle the “Art of Happiness.”
- Hundreds of attendees participated in, and successfully solved, our newest escape room, “Starship Relics.”
- Our valued CHEST members were able to successfully thwart Dr. Didactic and save the future of educational innovation.
- “CHEST After Hours” trended on social media and will become a normal and highly-anticipated part of the CHEST meeting moving forward.
- The most uncomfortable moment of the meeting centered on mayonnaise; for those of you who know what I am referencing, I am a little sorry…but only a little.
- Despite my best efforts, we were not able to recruit Neil Patrick Harris to participate.
Predicting the future of medical meetings is something we’ve spent a lot of time trying to do over the last year as we developed plans for CHEST 2022. But given the talented individuals involved in that planning, foreseeing the meeting’s success did not require any time travel; it was hardly a difficult task at all. Program Chair Subani Chandra and Vice-Chair Aneesa Das were exactly the people we needed at the helm for this all-important return to in-person meetings, and I cannot thank them enough for their creativity, effort, and leadership in bringing CHEST 2022 to fruition. And while I expect to have been seven-for-seven in my predictions above, I do hope I got that NPH one wrong.
The other reason that this column was a challenge to craft is because it represents my final formal presidential missive in these esteemed pages. And as I planned this final walk of the path, I gave careful consideration to the message with which I wanted to conclude my year. And as I put together my predictions for the future, my mind also turned to the past, considering things I wish I had known (or spent more time considering) as I started this journey. Some of this information may prove useful to the next generation of CHEST leaders, and some may be already well engrained for those of you with leadership experience. Here, in no particular order, are some thoughts for those of you in the audience who are considering future leadership opportunities at CHEST (or elsewhere in life; I suspect some of this advice is applicable to other venues). That said, the recommendations also come from yours truly, so take them with an appropriately large grain of salt, as your mileage may vary, and reasonable people could take issue here or there.
- The most important conversations should happen in person. The past 3 years have shown us the amazing things that modern technology can accomplish, but when it comes to providing important information, asking for input on a crucial issue, or providing feedback on a sensitive matter, there is no adequate substitute for a discussion in which all parties are in the same room.
- You are going to get things wrong sometimes; sometimes, this is because there wasn’t a way to get a right answer, and sometimes it will be because you tried something that didn’t work. You will learn far more from one of these experiences than from a dozen things that went as well as (or better than) expected.
- It is profoundly difficult to change someone’s mind if you aren’t interacting with them. I believe there is no gap so large that warrants breakdown of communication. Going that extra mile to talk to people who have a drastically different opinion than your own is the only way that you might be able to change someone’s mind and is a great way to ensure that your own opinion withstands pushback. With the growth of social media over the last decade, we’ve gotten very good at blocking people on social media; while this can sometimes be good (or even necessary) for emotional well-being, there can be value to interacting with such folks in a real-world environment.
- You do not have to bring everything to the table. The best leaders surround themselves with other really smart folks who, in aggregate, will provide support in areas in which you are deficient. That said, you need to know where these gaps in your knowledge and experience are, and when it is the right time to listen to those trusted advisors.
- When it comes time to identify folks for your “cabinet,” make sure to choose people who think differently than you and who may disagree with you on some fundamental things. Surrounding yourself with friends and close colleagues can lead to groupthink and poor decision making. The best results often stem from challenging and difficult decision-making processes.
- As a corollary to the above, every leader will bring their own sensibility and personality to the role. Make sure to bring yours, even if it involves silly jokes about holding a medical meeting in a former President’s basement or getting another former President to eat a big spoonful of the aforementioned condiment.
- Fun is important. Fun builds relationships, and teams, and trust. Make sure you are having it, as much as you possibly can, throughout your leadership tenure.
On that note, I will sign off for good, at least in these pages. I’ll still be bumbling around, proposing new educational experiences, hosting Pardon the Interruption, and serving as a sounding board for anyone who wants to chat. But I cannot wait to see what the next 3 years bring for our organization, under the leadership of Drs. Addrizzo-Harris, Buckley, and Howington. And for those of you who are just taking your first steps in leadership, and who will be following in their footsteps years down the road, I hope that you get just as much enjoyment from and fulfilment in the role of President as I have. #SchulmanOut
This is an odd column for me to write. First, because of the nature of print publication, this writing for the November issue is being crafted just before the annual meeting is to be held in Nashville. Therefore, while I have a pretty good sense of what is in store for CHEST 2022, I have yet to see the final product, or the audience’s reaction to it. However, I will make some bold predictions as to what occurred therein:
- Even in the context of 3 years of separation, thousands CHEST members gathered in droves to rekindle friendships and to experience the best education in pulmonary, critical care, and sleep medicine that the world has to offer, leading to our second-biggest meeting ever.
- Neil Pasricha’s presentation helped attendees rekindle the “Art of Happiness.”
- Hundreds of attendees participated in, and successfully solved, our newest escape room, “Starship Relics.”
- Our valued CHEST members were able to successfully thwart Dr. Didactic and save the future of educational innovation.
- “CHEST After Hours” trended on social media and will become a normal and highly-anticipated part of the CHEST meeting moving forward.
- The most uncomfortable moment of the meeting centered on mayonnaise; for those of you who know what I am referencing, I am a little sorry…but only a little.
- Despite my best efforts, we were not able to recruit Neil Patrick Harris to participate.
Predicting the future of medical meetings is something we’ve spent a lot of time trying to do over the last year as we developed plans for CHEST 2022. But given the talented individuals involved in that planning, foreseeing the meeting’s success did not require any time travel; it was hardly a difficult task at all. Program Chair Subani Chandra and Vice-Chair Aneesa Das were exactly the people we needed at the helm for this all-important return to in-person meetings, and I cannot thank them enough for their creativity, effort, and leadership in bringing CHEST 2022 to fruition. And while I expect to have been seven-for-seven in my predictions above, I do hope I got that NPH one wrong.
The other reason that this column was a challenge to craft is because it represents my final formal presidential missive in these esteemed pages. And as I planned this final walk of the path, I gave careful consideration to the message with which I wanted to conclude my year. And as I put together my predictions for the future, my mind also turned to the past, considering things I wish I had known (or spent more time considering) as I started this journey. Some of this information may prove useful to the next generation of CHEST leaders, and some may be already well engrained for those of you with leadership experience. Here, in no particular order, are some thoughts for those of you in the audience who are considering future leadership opportunities at CHEST (or elsewhere in life; I suspect some of this advice is applicable to other venues). That said, the recommendations also come from yours truly, so take them with an appropriately large grain of salt, as your mileage may vary, and reasonable people could take issue here or there.
- The most important conversations should happen in person. The past 3 years have shown us the amazing things that modern technology can accomplish, but when it comes to providing important information, asking for input on a crucial issue, or providing feedback on a sensitive matter, there is no adequate substitute for a discussion in which all parties are in the same room.
- You are going to get things wrong sometimes; sometimes, this is because there wasn’t a way to get a right answer, and sometimes it will be because you tried something that didn’t work. You will learn far more from one of these experiences than from a dozen things that went as well as (or better than) expected.
- It is profoundly difficult to change someone’s mind if you aren’t interacting with them. I believe there is no gap so large that warrants breakdown of communication. Going that extra mile to talk to people who have a drastically different opinion than your own is the only way that you might be able to change someone’s mind and is a great way to ensure that your own opinion withstands pushback. With the growth of social media over the last decade, we’ve gotten very good at blocking people on social media; while this can sometimes be good (or even necessary) for emotional well-being, there can be value to interacting with such folks in a real-world environment.
- You do not have to bring everything to the table. The best leaders surround themselves with other really smart folks who, in aggregate, will provide support in areas in which you are deficient. That said, you need to know where these gaps in your knowledge and experience are, and when it is the right time to listen to those trusted advisors.
- When it comes time to identify folks for your “cabinet,” make sure to choose people who think differently than you and who may disagree with you on some fundamental things. Surrounding yourself with friends and close colleagues can lead to groupthink and poor decision making. The best results often stem from challenging and difficult decision-making processes.
- As a corollary to the above, every leader will bring their own sensibility and personality to the role. Make sure to bring yours, even if it involves silly jokes about holding a medical meeting in a former President’s basement or getting another former President to eat a big spoonful of the aforementioned condiment.
- Fun is important. Fun builds relationships, and teams, and trust. Make sure you are having it, as much as you possibly can, throughout your leadership tenure.
On that note, I will sign off for good, at least in these pages. I’ll still be bumbling around, proposing new educational experiences, hosting Pardon the Interruption, and serving as a sounding board for anyone who wants to chat. But I cannot wait to see what the next 3 years bring for our organization, under the leadership of Drs. Addrizzo-Harris, Buckley, and Howington. And for those of you who are just taking your first steps in leadership, and who will be following in their footsteps years down the road, I hope that you get just as much enjoyment from and fulfilment in the role of President as I have. #SchulmanOut
This is an odd column for me to write. First, because of the nature of print publication, this writing for the November issue is being crafted just before the annual meeting is to be held in Nashville. Therefore, while I have a pretty good sense of what is in store for CHEST 2022, I have yet to see the final product, or the audience’s reaction to it. However, I will make some bold predictions as to what occurred therein:
- Even in the context of 3 years of separation, thousands CHEST members gathered in droves to rekindle friendships and to experience the best education in pulmonary, critical care, and sleep medicine that the world has to offer, leading to our second-biggest meeting ever.
- Neil Pasricha’s presentation helped attendees rekindle the “Art of Happiness.”
- Hundreds of attendees participated in, and successfully solved, our newest escape room, “Starship Relics.”
- Our valued CHEST members were able to successfully thwart Dr. Didactic and save the future of educational innovation.
- “CHEST After Hours” trended on social media and will become a normal and highly-anticipated part of the CHEST meeting moving forward.
- The most uncomfortable moment of the meeting centered on mayonnaise; for those of you who know what I am referencing, I am a little sorry…but only a little.
- Despite my best efforts, we were not able to recruit Neil Patrick Harris to participate.
Predicting the future of medical meetings is something we’ve spent a lot of time trying to do over the last year as we developed plans for CHEST 2022. But given the talented individuals involved in that planning, foreseeing the meeting’s success did not require any time travel; it was hardly a difficult task at all. Program Chair Subani Chandra and Vice-Chair Aneesa Das were exactly the people we needed at the helm for this all-important return to in-person meetings, and I cannot thank them enough for their creativity, effort, and leadership in bringing CHEST 2022 to fruition. And while I expect to have been seven-for-seven in my predictions above, I do hope I got that NPH one wrong.
The other reason that this column was a challenge to craft is because it represents my final formal presidential missive in these esteemed pages. And as I planned this final walk of the path, I gave careful consideration to the message with which I wanted to conclude my year. And as I put together my predictions for the future, my mind also turned to the past, considering things I wish I had known (or spent more time considering) as I started this journey. Some of this information may prove useful to the next generation of CHEST leaders, and some may be already well engrained for those of you with leadership experience. Here, in no particular order, are some thoughts for those of you in the audience who are considering future leadership opportunities at CHEST (or elsewhere in life; I suspect some of this advice is applicable to other venues). That said, the recommendations also come from yours truly, so take them with an appropriately large grain of salt, as your mileage may vary, and reasonable people could take issue here or there.
- The most important conversations should happen in person. The past 3 years have shown us the amazing things that modern technology can accomplish, but when it comes to providing important information, asking for input on a crucial issue, or providing feedback on a sensitive matter, there is no adequate substitute for a discussion in which all parties are in the same room.
- You are going to get things wrong sometimes; sometimes, this is because there wasn’t a way to get a right answer, and sometimes it will be because you tried something that didn’t work. You will learn far more from one of these experiences than from a dozen things that went as well as (or better than) expected.
- It is profoundly difficult to change someone’s mind if you aren’t interacting with them. I believe there is no gap so large that warrants breakdown of communication. Going that extra mile to talk to people who have a drastically different opinion than your own is the only way that you might be able to change someone’s mind and is a great way to ensure that your own opinion withstands pushback. With the growth of social media over the last decade, we’ve gotten very good at blocking people on social media; while this can sometimes be good (or even necessary) for emotional well-being, there can be value to interacting with such folks in a real-world environment.
- You do not have to bring everything to the table. The best leaders surround themselves with other really smart folks who, in aggregate, will provide support in areas in which you are deficient. That said, you need to know where these gaps in your knowledge and experience are, and when it is the right time to listen to those trusted advisors.
- When it comes time to identify folks for your “cabinet,” make sure to choose people who think differently than you and who may disagree with you on some fundamental things. Surrounding yourself with friends and close colleagues can lead to groupthink and poor decision making. The best results often stem from challenging and difficult decision-making processes.
- As a corollary to the above, every leader will bring their own sensibility and personality to the role. Make sure to bring yours, even if it involves silly jokes about holding a medical meeting in a former President’s basement or getting another former President to eat a big spoonful of the aforementioned condiment.
- Fun is important. Fun builds relationships, and teams, and trust. Make sure you are having it, as much as you possibly can, throughout your leadership tenure.
On that note, I will sign off for good, at least in these pages. I’ll still be bumbling around, proposing new educational experiences, hosting Pardon the Interruption, and serving as a sounding board for anyone who wants to chat. But I cannot wait to see what the next 3 years bring for our organization, under the leadership of Drs. Addrizzo-Harris, Buckley, and Howington. And for those of you who are just taking your first steps in leadership, and who will be following in their footsteps years down the road, I hope that you get just as much enjoyment from and fulfilment in the role of President as I have. #SchulmanOut
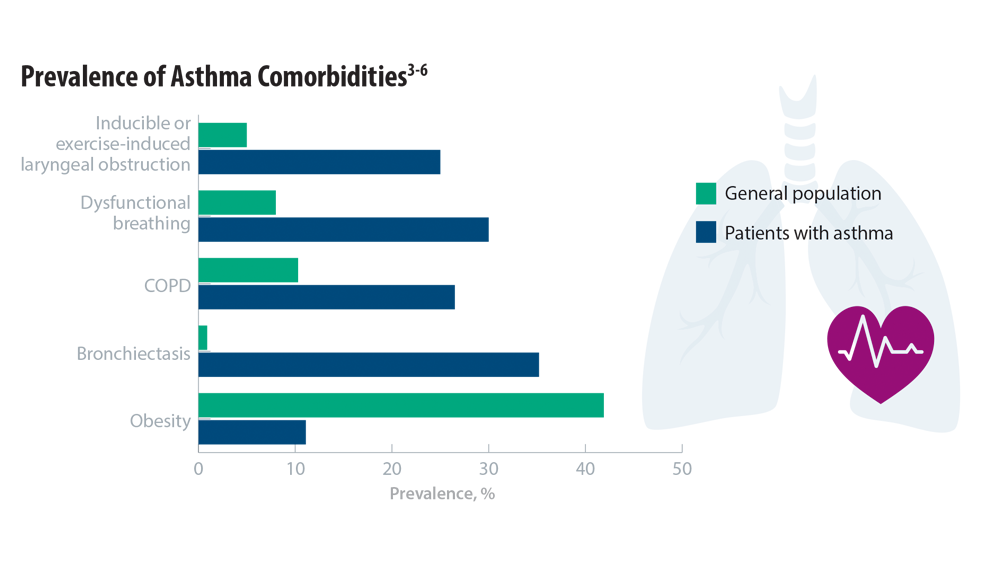
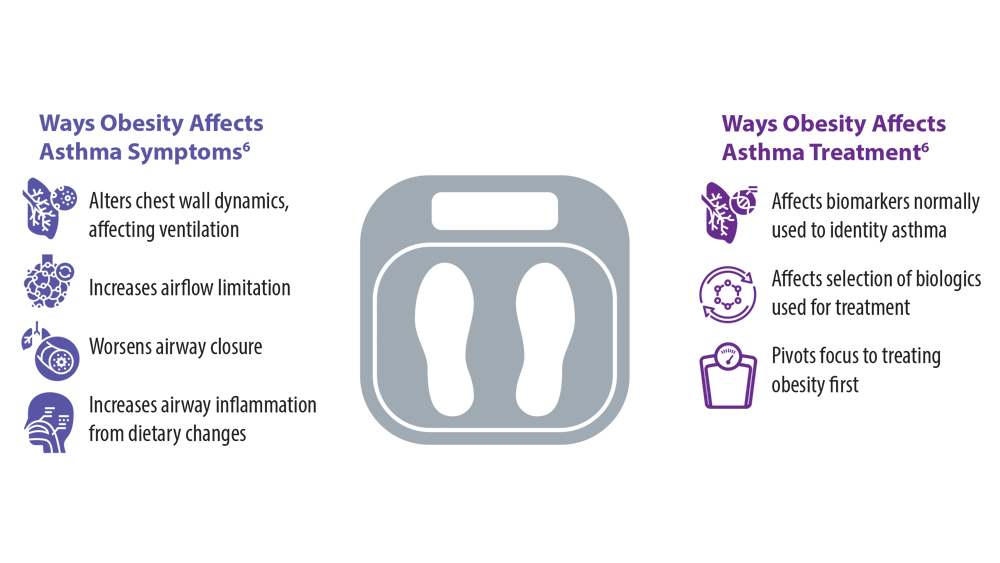
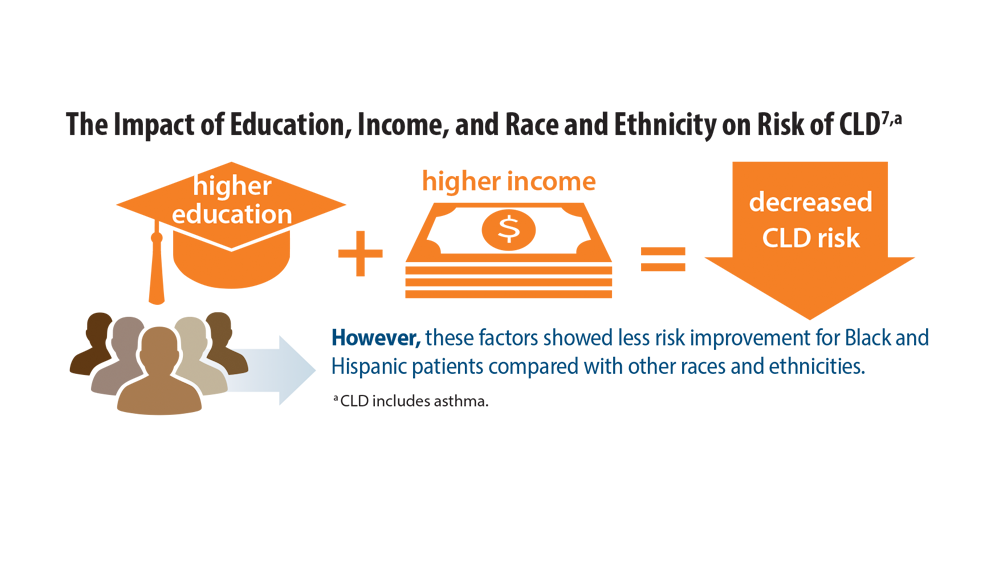
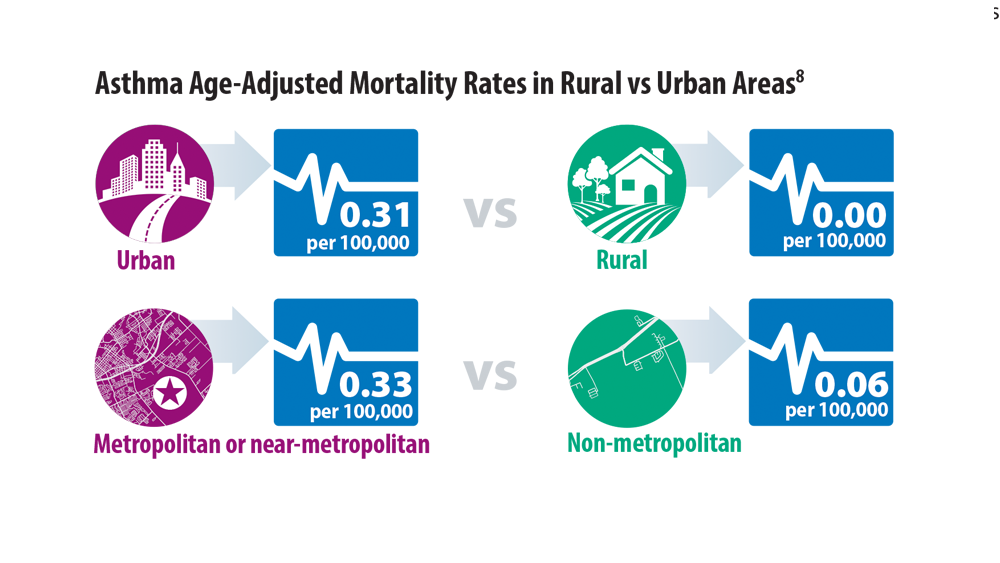
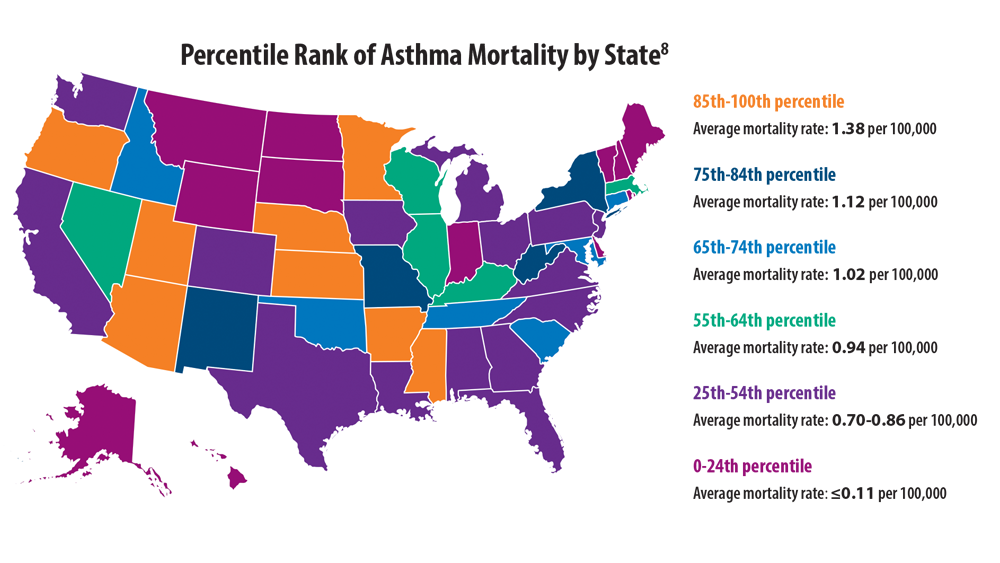
Comorbidities, Racial Disparities, and Geographic Differences in Asthma
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
- Wenzel M. Gasping for a diagnosis: pediatric vocal cord dysfunction. J Pediatr Health Care. 2019;33(1):5-13. doi:10.1016/j.pedhc.2018.03.002
- Mogensen I, James A, Malinovschi A. Systemic and breath biomarkers for asthma: an update. Curr Opin Allergy Clin Immunol. 2020;20(1):71-79. doi:10.1097/ACI.0000000000000599
- Gibson PG, McDonald VM, Granchelli A, Olin JT. Asthma and comorbid conditions—pulmonary comorbidity. J Allergy Clin Immunol Pract. 2021;9(11):3868-3875. doi:10.1016/j. jaip.2021.08.028
- Peters U, Dixon AE, Forno E. Obesity and asthma. J Allergy Clin Immunol. 2018;141(4):1169-1179. doi:10.1016/j.jaci.2018.02.004
- Adult obesity facts. Centers for Disease Control and Prevention. Published May 17, 2022. Accessed June 7, 2022. https://www.cdc.gov/obesity/data/adult.html
- Sharma V, Cowan DC. Obesity, inflammation, and severe asthma: an update. Curr Allergy Asthma Rep. 2021;21(12):46. doi:10.1007/s11882-021-01024-9
- Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
- Bleecker ER, Gandhi H, Gilbert I, Murphy KR, Chupp GL. Mapping geographic variability of severe uncontrolled asthma in the United States: management implications. Ann Allergy Asthma Immunol. 2022;128(1):78-88. doi:10.1016/j.anai.2021.09.025
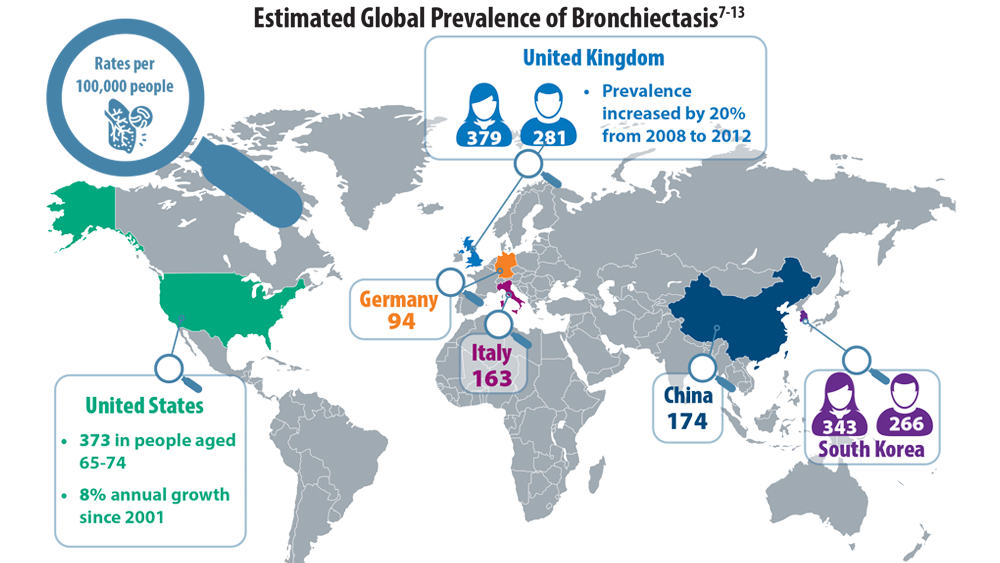
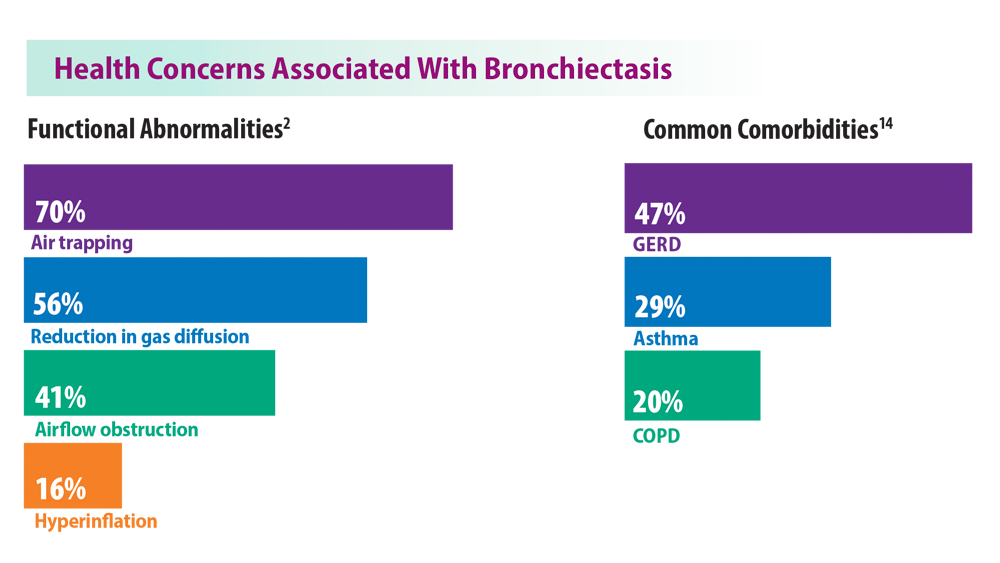
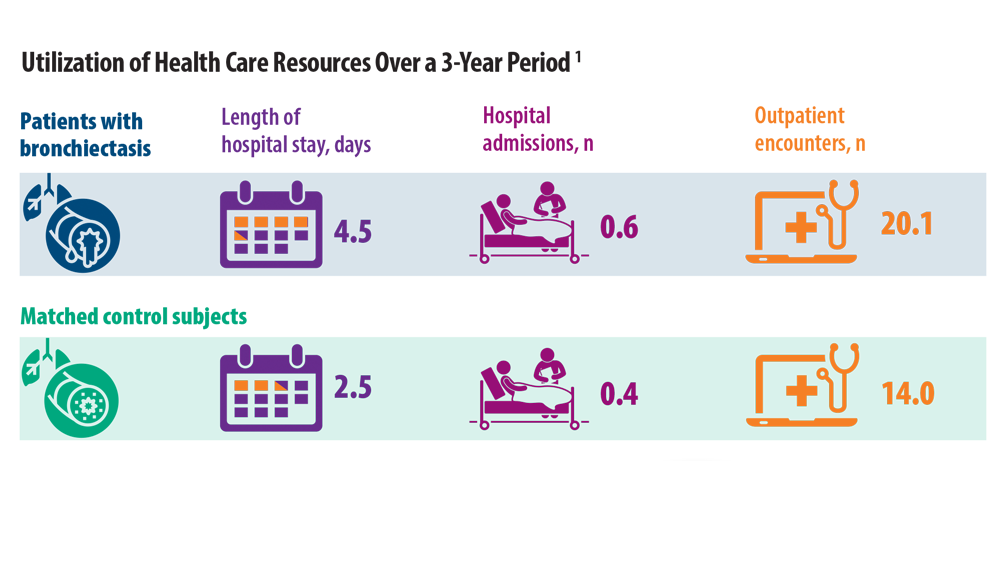
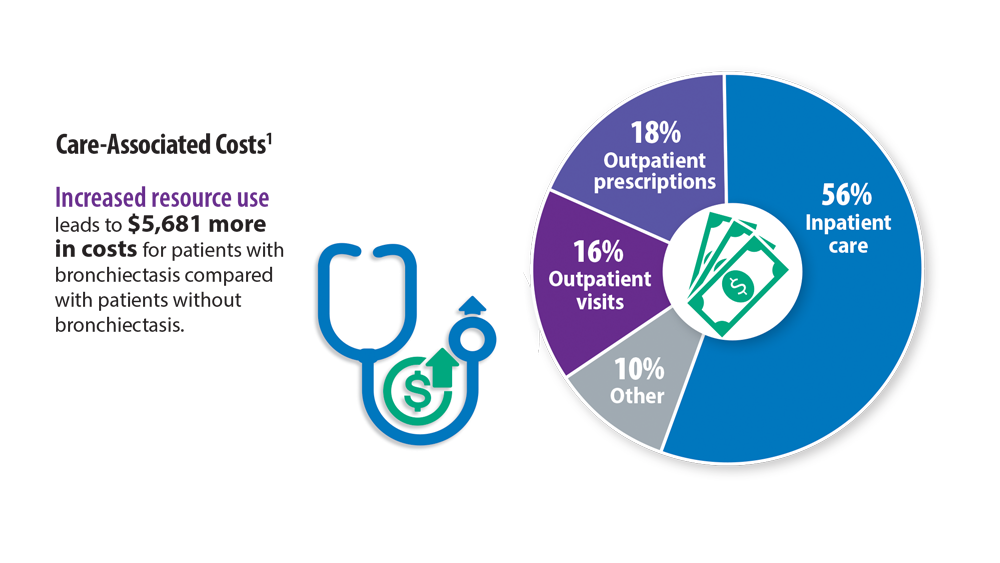
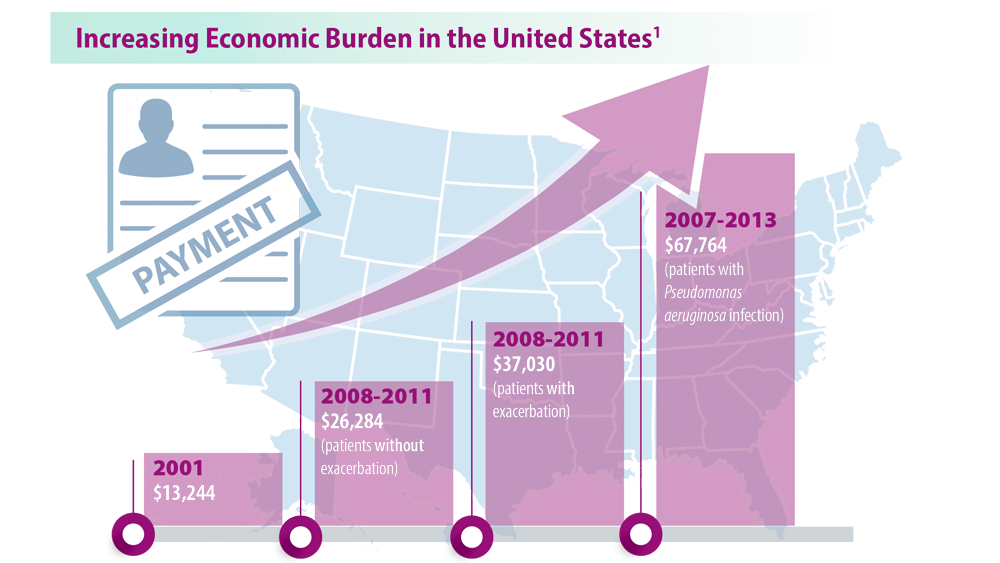
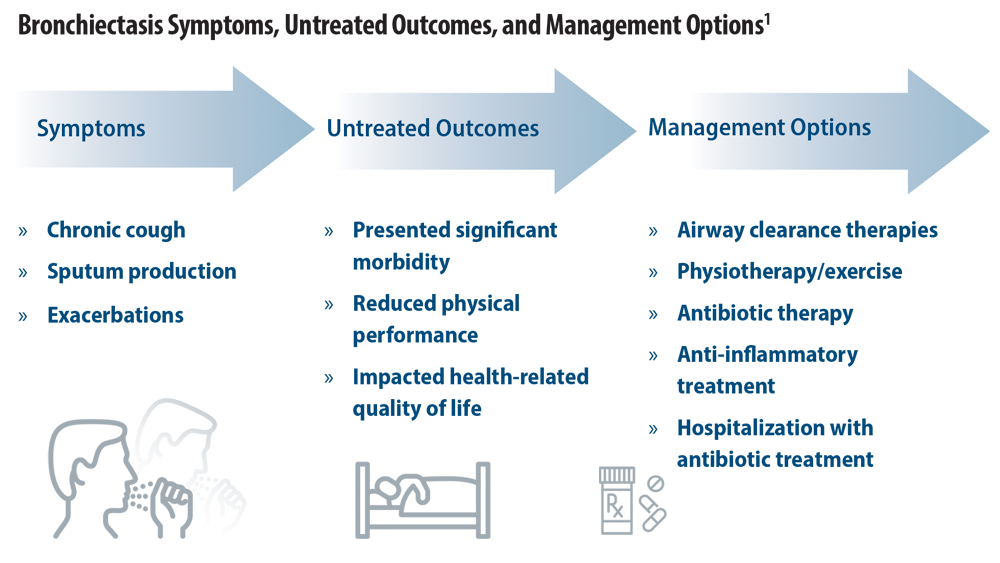
Rising Incidence of Bronchiectasis and Associated Burdens
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis – known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. doi:10.1186/s12890-019-0818-6
- Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7-22. doi:10.1016/j.ccm.2021.11.001
- Emmons EE. Bronchiectasis. Medscape. Updated September 15, 2020. Accessed June 24, 2022. https://emedicine.medscape.com/article/296961-overview
- World Populating Ageing 2019: highlights (ST/ESA/SER.A/430). United Nations Department of Economic and Social Affairs, Population Division. Published 2019. Accessed July 28, 2022. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
- O’Donnell AE. Bronchiectasis update. Curr Opin Infect Dis. 2018;31(2):194-198. doi:10.1097/QCO.0000000000000445
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
- Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi:10.1177/1479972317709649
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432-439. doi:10.1378/chest.11-2209
- Bronchiectasis statistics. British Lung Foundation. Accessed June 24, 2022. https://statistics.blf.org.uk/bronchiectasis
- Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6):1900499. doi:10.1183/13993003.00499-2019
- Aliberti S, Sotigiu G, Lapi F, Gramegna A, Cricelli C, Blasi F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med. 2020;20(1):15. doi:10.1186/s12890-020-1050-0
- Park DI, Kang S, Choi S. Evaluating the prevalence and incidence of bronchiectasis and nontuberculous mycobacteria in South Korea using the nationwide population data. Int J Environ Res Public Health. 2021;18(17):9029. doi:10.3390/ijerph18179029
- Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013-2017: a nationwide population-based cohort study. Respir Res. 2022;23:111. doi:10.1186/s12931-022-02023-8
- Hayoung Choi, H, Yang, B, N. Hyewon et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. Aug 2019, 54 (2) 1900194; doi:10.1183/13993003.00194-2019.
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis – known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. doi:10.1186/s12890-019-0818-6
- Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7-22. doi:10.1016/j.ccm.2021.11.001
- Emmons EE. Bronchiectasis. Medscape. Updated September 15, 2020. Accessed June 24, 2022. https://emedicine.medscape.com/article/296961-overview
- World Populating Ageing 2019: highlights (ST/ESA/SER.A/430). United Nations Department of Economic and Social Affairs, Population Division. Published 2019. Accessed July 28, 2022. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
- O’Donnell AE. Bronchiectasis update. Curr Opin Infect Dis. 2018;31(2):194-198. doi:10.1097/QCO.0000000000000445
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
- Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi:10.1177/1479972317709649
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432-439. doi:10.1378/chest.11-2209
- Bronchiectasis statistics. British Lung Foundation. Accessed June 24, 2022. https://statistics.blf.org.uk/bronchiectasis
- Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6):1900499. doi:10.1183/13993003.00499-2019
- Aliberti S, Sotigiu G, Lapi F, Gramegna A, Cricelli C, Blasi F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med. 2020;20(1):15. doi:10.1186/s12890-020-1050-0
- Park DI, Kang S, Choi S. Evaluating the prevalence and incidence of bronchiectasis and nontuberculous mycobacteria in South Korea using the nationwide population data. Int J Environ Res Public Health. 2021;18(17):9029. doi:10.3390/ijerph18179029
- Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013-2017: a nationwide population-based cohort study. Respir Res. 2022;23:111. doi:10.1186/s12931-022-02023-8
- Hayoung Choi, H, Yang, B, N. Hyewon et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. Aug 2019, 54 (2) 1900194; doi:10.1183/13993003.00194-2019.
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis – known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54. doi:10.1186/s12890-019-0818-6
- Cohen R, Shteinberg M. Diagnosis and evaluation of bronchiectasis. Clin Chest Med. 2022;43(1):7-22. doi:10.1016/j.ccm.2021.11.001
- Emmons EE. Bronchiectasis. Medscape. Updated September 15, 2020. Accessed June 24, 2022. https://emedicine.medscape.com/article/296961-overview
- World Populating Ageing 2019: highlights (ST/ESA/SER.A/430). United Nations Department of Economic and Social Affairs, Population Division. Published 2019. Accessed July 28, 2022. https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
- O’Donnell AE. Bronchiectasis update. Curr Opin Infect Dis. 2018;31(2):194-198. doi:10.1097/QCO.0000000000000445
- Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi:10.1183/13993003.00629-2017
- Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi:10.1177/1479972317709649
- Seitz AE, Olivier KN, Adjemian J, Holland SM, Prevots DR. Trends in bronchiectasis among Medicare beneficiaries in the United States, 2000 to 2007. Chest. 2012;142(2):432-439. doi:10.1378/chest.11-2209
- Bronchiectasis statistics. British Lung Foundation. Accessed June 24, 2022. https://statistics.blf.org.uk/bronchiectasis
- Ringshausen FC, Rademacher J, Pink I, et al. Increasing bronchiectasis prevalence in Germany, 2009-2017: a population-based cohort study. Eur Respir J. 2019;54(6):1900499. doi:10.1183/13993003.00499-2019
- Aliberti S, Sotigiu G, Lapi F, Gramegna A, Cricelli C, Blasi F. Prevalence and incidence of bronchiectasis in Italy. BMC Pulm Med. 2020;20(1):15. doi:10.1186/s12890-020-1050-0
- Park DI, Kang S, Choi S. Evaluating the prevalence and incidence of bronchiectasis and nontuberculous mycobacteria in South Korea using the nationwide population data. Int J Environ Res Public Health. 2021;18(17):9029. doi:10.3390/ijerph18179029
- Feng J, Sun L, Sun X, et al. Increasing prevalence and burden of bronchiectasis in urban Chinese adults, 2013-2017: a nationwide population-based cohort study. Respir Res. 2022;23:111. doi:10.1186/s12931-022-02023-8
- Hayoung Choi, H, Yang, B, N. Hyewon et al. Population-based prevalence of bronchiectasis and associated comorbidities in South Korea. Eur Respir J. Aug 2019, 54 (2) 1900194; doi:10.1183/13993003.00194-2019.
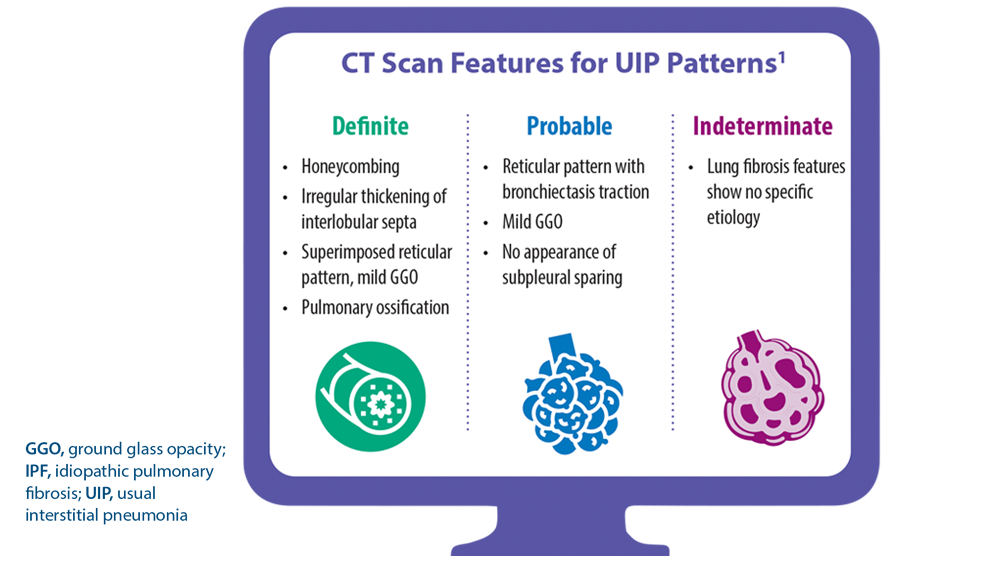
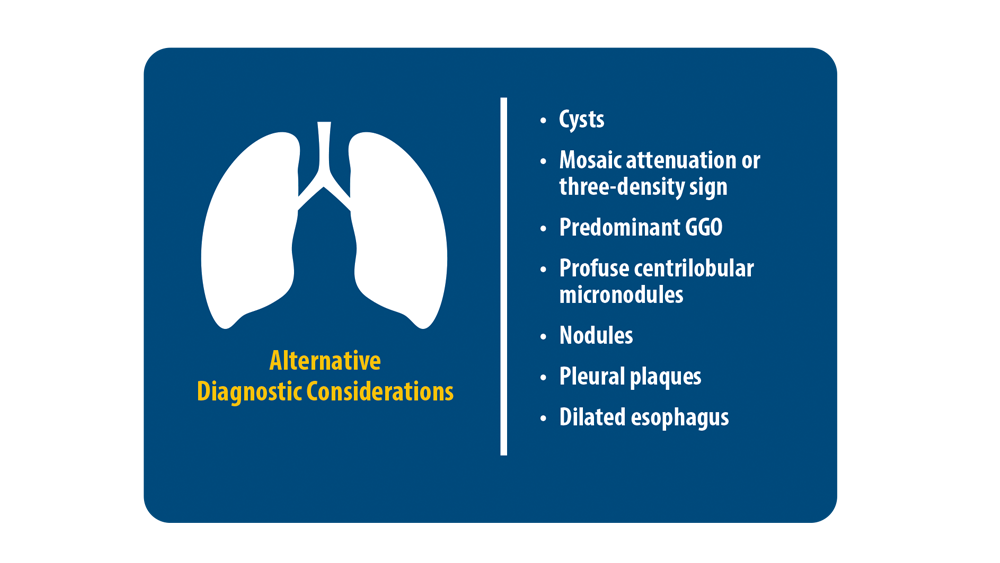
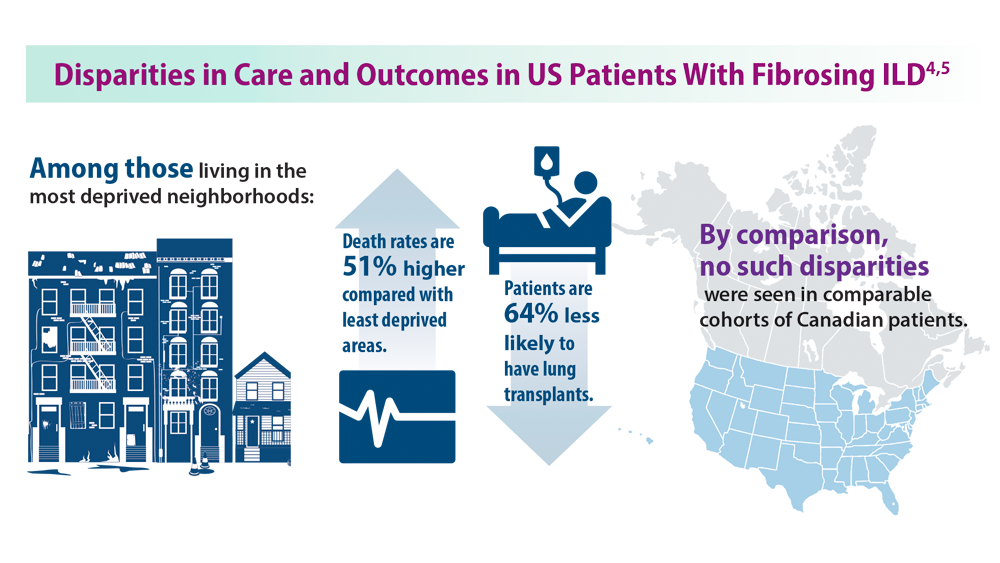
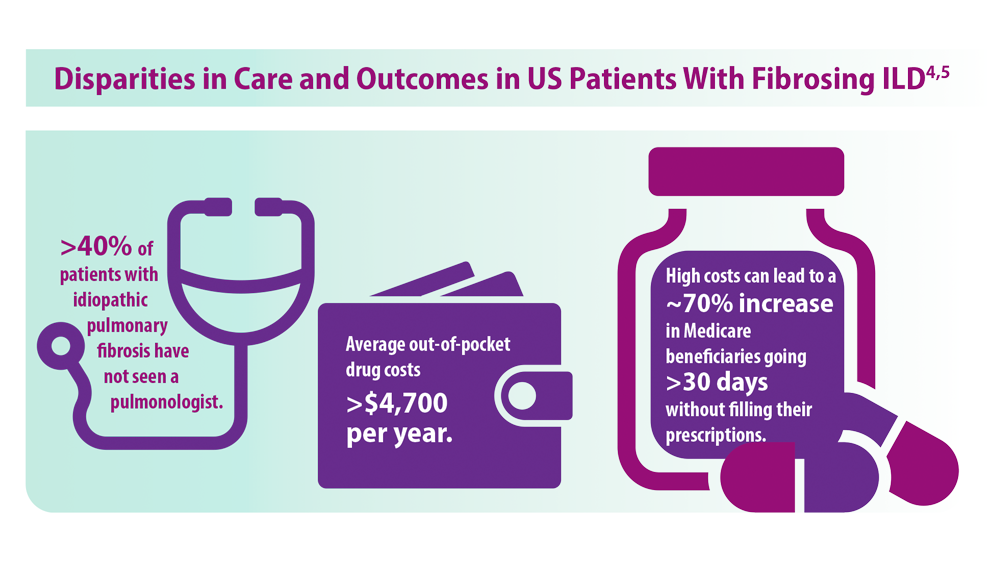
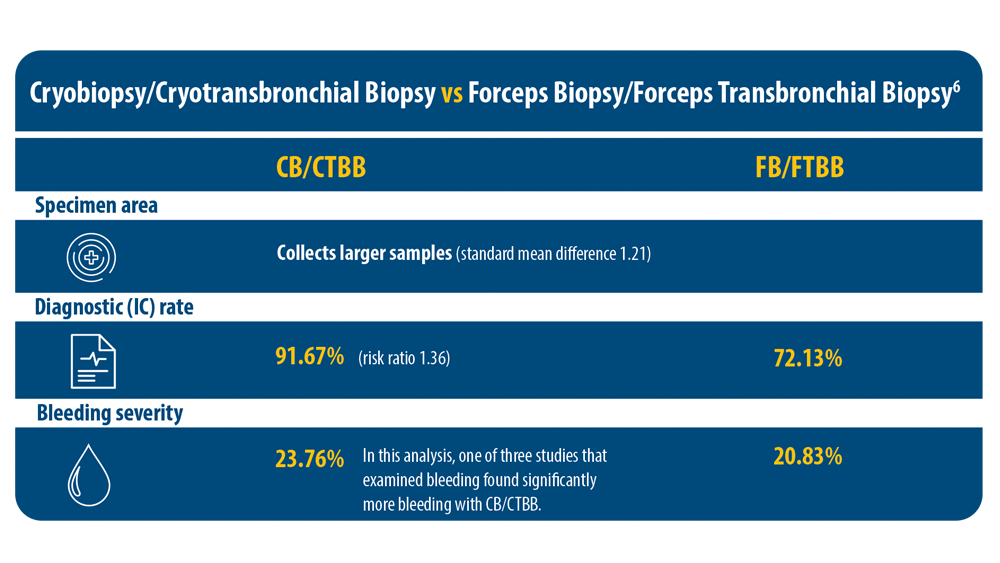
ILD: Diagnostic Considerations and Socioeconomic Barriers
1. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/ rccm.202202-0399ST
2. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report (podcast). Chest. 2021;160(2). Published August 5, 2021. Accessed July 11, 2022. https://www.podbean.com/ew/pb-jgzb7-10980b0
3. Kheir F, Uribe Becerra JP, Bissell B, et al. Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Ann Am Thorac Soc. 2022;19(7):1193-1202. doi:10.1513/ AnnalsATS.202102-198OC
4. Goobie GC, Ryerson CJ, Johannson KA, et al. Neighborhoodlevel disadvantage impacts on patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2022;205(4):459-467. doi:10.1164/rccm.202109-2065OC
5. Gaffney AW, Podolanczuk AJ. Inequity and the interstitium: pushing back on disparities in fibrosing lung disease in the United States and Canada. Am J Respir Crit Care Med. 2022;205(4):385-387. doi:10.1164/rccm.202111-2652ED
6. Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21(5):834-841. doi:10.1111/resp.12770
1. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/ rccm.202202-0399ST
2. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report (podcast). Chest. 2021;160(2). Published August 5, 2021. Accessed July 11, 2022. https://www.podbean.com/ew/pb-jgzb7-10980b0
3. Kheir F, Uribe Becerra JP, Bissell B, et al. Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Ann Am Thorac Soc. 2022;19(7):1193-1202. doi:10.1513/ AnnalsATS.202102-198OC
4. Goobie GC, Ryerson CJ, Johannson KA, et al. Neighborhoodlevel disadvantage impacts on patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2022;205(4):459-467. doi:10.1164/rccm.202109-2065OC
5. Gaffney AW, Podolanczuk AJ. Inequity and the interstitium: pushing back on disparities in fibrosing lung disease in the United States and Canada. Am J Respir Crit Care Med. 2022;205(4):385-387. doi:10.1164/rccm.202111-2652ED
6. Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21(5):834-841. doi:10.1111/resp.12770
1. Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2022;205(9):e18-e47. doi:10.1164/ rccm.202202-0399ST
2. Diagnosis and evaluation of hypersensitivity pneumonitis: CHEST guideline and expert panel report (podcast). Chest. 2021;160(2). Published August 5, 2021. Accessed July 11, 2022. https://www.podbean.com/ew/pb-jgzb7-10980b0
3. Kheir F, Uribe Becerra JP, Bissell B, et al. Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Ann Am Thorac Soc. 2022;19(7):1193-1202. doi:10.1513/ AnnalsATS.202102-198OC
4. Goobie GC, Ryerson CJ, Johannson KA, et al. Neighborhoodlevel disadvantage impacts on patients with fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2022;205(4):459-467. doi:10.1164/rccm.202109-2065OC
5. Gaffney AW, Podolanczuk AJ. Inequity and the interstitium: pushing back on disparities in fibrosing lung disease in the United States and Canada. Am J Respir Crit Care Med. 2022;205(4):385-387. doi:10.1164/rccm.202111-2652ED
6. Ganganah O, Guo SL, Chiniah M, Li YS. Efficacy and safety of cryobiopsy versus forceps biopsy for interstitial lung diseases and lung tumours: a systematic review and meta-analysis. Respirology. 2016;21(5):834-841. doi:10.1111/resp.12770
CDC warns of early uptick in respiratory disease
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention is warning of an early surge in respiratory disease caused by multiple viruses. As influenza viruses, respiratory syncytial virus (RSV), SARS-CoV-2, and rhinovirus/enterovirus simultaneously circulate, the agency cautioned that this confluence of viral activity could strain the health care system, according to a CDC Health Network Alert advisory issued Nov. 4.
“This early increase in disease incidence highlights the importance of optimizing respiratory virus prevention and treatment measures, including prompt vaccination and antiviral treatment,” the alert stated.
The CDC reports that RSV activity is increasing nationally, but in some areas – such as the South and Mountain West – cases appear to be trending downward.
Influenza cases continue to climb, with the virus activity being the highest in the South, Mid-Atlantic, and the south-central West Coast, according to CDC data. “In fact, we’re seeing the highest influenza hospitalization rates going back a decade,” said José Romero, MD, director of the CDC’s National Center for Immunization and Respiratory Diseases, during a press briefing. The agency estimates that there have been 1.6 million illnesses, 13,000 hospitalizations, and 730 deaths from the flu so far this season. As of Nov. 4, there have been two pediatric deaths.
COVID-19 cases appear to have plateaued in the past three weeks, Dr. Romero said; however, the CDC expects that there will be “high-level circulation of SARS-CoV-2 this fall and winter,” the health alert stated.
The CDC advised that all eligible individuals aged 6-months or older should be vaccinated against COVID-19 and influenza. To protect against RSV-hospitalization, high-risk children should receive the monoclonal antibody drug palivizumab (Synagis). High-risk children include infants born before 29 weeks, children younger than age 2 with chronic lung disease or hemodynamically significant congenital heart disease, and children with suppressed immune systems or neuromuscular disorders.
Any patient with confirmed or suspected flu who is hospitalized, at higher risk for influenza complications, or who has a severe or progressive illness should be treated as early as possible with antivirals, such as oral oseltamivir (Tamiflu).
Patients with confirmed SARS-CoV-2 infection with increased risk of complications should also be treated with antivirals, such as nirmatrelvir and ritonavir (Paxlovid) or remdesivir (Veklury).
Patients should also be reminded to wash their hands frequently, cover coughs and sneezes, stay home when sick, and avoid close contact with people who are sick, the CDC advised.
“There’s no doubt that we will face some challenges this winter,” said Dawn O’Connell, HHS Assistant Secretary for Preparedness and Response, “but it’s important to remember that RSV and flu are not new, and we have safe and effective vaccines for COVID-19 and the flu.”
A version of this article first appeared on Medscape.com.
New trial suggests CV benefit with EPA: RESPECT-EPA
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
The open-label randomized RESPECT-EPA study showed a reduction of borderline statistical significance in its primary endpoint of a composite of cardiovascular death, nonfatal myocardial infarction, nonfatal ischemic stroke, unstable angina, and coronary revascularization in patients allocated to the EPA product at a dosage of 1,800 mg/day.
The results were presented at the American Heart Association scientific sessions by Hiroyuki Daida, MD, Juntendo University Graduate School of Medicine, Japan.
However, the trial has several limitations, including a high number of patient withdrawals or protocol deviations, and as such, its conclusions are uncertain.
Regardless, it has inevitably added to the debate on the cardiovascular benefits of EPA, which were shown in the REDUCE-IT trial. However, that trial has been dogged with controversy because of concerns that the mineral oil placebo used may have had an adverse effect.
Commenting on the new RESPECT-EPA trial for this article, lead investigator of the REDUCE-IT trial, Deepak Bhatt, MD, said the results were consistent with REDUCE-IT and another previous Japanese trial, the Japan EPA Lipid Intervention Study (JELIS), and added to the evidence supporting cardiovascular benefits of EPA.
“In isolation, this study may not be viewed as showing conclusive benefits, but looking at the totality of the data from this trial and from the field more widely, this together shows a convincing cardiovascular benefit with EPA,” Dr. Bhatt said. “We now have 3 randomized controlled trials all showing benefits of highly purified EPA in reducing cardiovascular events.”
However, long-time critic of the REDUCE-IT trial, Steve Nissen, MD, Cleveland Clinic, was not at all impressed with the RESPECT-EPA trial and does not believe it should be used to support the EPA data from REDUCE-IT.
“The many limitations of the RESPECT-EPA trial make it uninterpretable. It just doesn’t meet contemporary standards for clinical trials,” Dr. Nissen said in an interview. “I don’t think it sheds any light at all on the debate over the efficacy of EPA in cardiovascular disease.”
Dr. Nissen was the lead investigator of another largescale trial, STRENGTH, which showed no benefit of a different high dose omega-3 fatty acid product including a combination of EPA and docosahexaenoic acid (DHA).
In his AHA presentation on the RESPECT-EPA study, Dr. Daida explained as background that in 2005, JELIS first demonstrated a beneficial effect of highly purified EPA on cardiovascular outcomes in patients with and without coronary artery disease.
Recently, optimal medical therapy, particularly with high-intensity statins, has become the gold standard of care for patients with coronary artery disease, but they are still at substantially high residual risk, he noted.
Despite of the evidence provided by JELIS, the conflicting results in recent omega-3 fatty acid trials (REDUCE-IT and STRENGTH) have led to an intense controversy regarding the relevance of EPA intervention on top of the latest optimal medical therapy, Dr. Daida said.
The current study – Randomized trial for Evaluating the Secondary Prevention Efficacy of Combination Therapy Statin and EPA (RESPECT-EPA) – was conducted to determine the effect of highly purified EPA on cardiovascular events in Japanese patients with chronic coronary artery disease and a low EPA/arachidonic acid (AA) ratio (< 0.4), who were already receiving statins.
They were randomly assigned to highly purified EPA (icosapent ethyl, 1,800 mg/day) plus statin therapy or to statin therapy alone.
The enrollment period started in 2013 and continued for 4 years. Patients were followed for a further 4 years from the end of the enrollment period.
The trial included 2,506 patients, 1,249 assigned to the EPA group and 1,257 to the control group. In both groups there were a high number of early withdrawals or protocol deviations (647 in the EPA group and 350 in the control group).
The analysis was conducted on 1,225 patients in the EPA group and 1,235 patients in the control group, although at 6 years’ follow-up there were fewer than 400 patients in each arm.
Baseline characteristics showed median low-density lipoprotein (LDL) cholesterol levels of 80 mg/dL, EPA levels of 45 mcg/mL, and triglyceride levels of 120 mg/dL.
The primary endpoint, a composite of cardiovascular death, nonfatal MI, nonfatal ischemic stroke, unstable angina, and coronary revascularization showed a borderline significant reduction in the EPA group at 6 years since the start of randomization (10.9% vs. 14.9%; hazard ratio, 0.785; P = .0547).
The secondary endpoint, a composite of sudden cardiac death, MI, unstable angina, and coronary revascularization, showed a significant reduction in the EPA group (8.0% vs. 11.3%; HR, 0.734; P = .0306).
In terms of adverse events, there was an increase in gastrointestinal disorders (3.4% vs. 1.2%) and new-onset atrial fibrillation (3.1% vs. 1.6%) in the EPA group.
In a post hoc analysis, which excluded patients with an increase of more than 30 mcg/mL in the control group (182 patients) and those with an increase of less than 30 mcg/mL in the EPA group (259 patients), the primary endpoint showed a significant reduction the EPA group (HR, 0.725; P = .0202).
Dr. Daida noted that limitations of the study included a lower than expected event rate (suggesting that the study may be underpowered), an open-label design, and the fact that baseline levels of EPA in this Japanese population would be higher than those in Western countries.
‘Massive loss’ of patients
Critiquing the study, Dr. Nissen highlighted the large dropout and protocol violation rate.
“There was a massive loss of patients over the 6- to 8-year follow-up, and the Kaplan-Meier curves didn’t start to diverge until after 4 years, by which time many patients had dropped out. It would have been a very selective population that lasted 6 years in the study. Patients that drop out are different to those that stay in, so they are cherry-picking the patients that persist in the trial. There is enormous bias here,” he commented.
“Another weakness is the open-label design. Everyone knew who is getting what. Blinding is important in a study. And there was no control treatment in this trial,” he noted.
The researchers also selected patients with low EPA levels at baseline, Dr. Nissen added. “That is completely different hypothesis to what was tested in the REDUCE-IT and STRENGTH trials. And even with all these problems, the results are still statistically insignificant.”
On the post hoc subgroup analysis showing a significant benefit, Dr. Nissen said, “they compared a subgroup in the active treatment arm who had large increases in EPA to a subgroup of control patients who had the smallest increase in EPA. That would be like comparing patients who had the largest reductions in LDL in a statin trial to those in the control arm who had no reductions or increases in LDL. That’s scientifically totally inappropriate.”
Supportive data
But Dr. Bhatt argues that the RESPECT-EPA trial supports the two previous trials showing benefits of EPA.
“Some may quibble with the P value, but to me this study has shown clear results, with obvious separation of the Kaplan-Meier curves,” he said.
“It is an investigator-initiated study, which is good in principle but has some of the usual caveats of such a study in that – probably as a consequence of budget constraints – it has an open-label design and is underpowered. But as they did not use a placebo and still showed a benefit of EPA, that helps resolve the issue of the placebo used in REDUCE-IT for those who were concerned about it,” Dr. Bhatt noted.
He pointed out that the 1,800-mg dose of EPA is the same dose used in the JELIS trial and is the dose used in Japan. The REDUCE-IT trial used a higher dose (4 g), but in general, Japanese people have higher levels of EPA than Western populations, he explained.
“While this trial included patients with lower levels of EPA, what is considered low in Japan is much higher than average American levels,” he added.
Magnitude of benefit uncertain?
Discussant of the study at the Late Breaking Clinical Trials session, Pam R. Taub, MD, professor of medicine at the University of California, San Diego School of Medicine, said, “Despite being underpowered with a sample size of 2,460, RESPECT-EPA shows benefit in decreasing composite coronary events.”
“There is benefit with EPA, but the magnitude of benefit is uncertain,” she stated.
Dr. Taub pointed out that there is a signal across studies for new-onset atrial fibrillation, but the absolute increase is “rather small.”
She noted that more mechanistic and clinical data are needed to hone in on which patients will derive the most benefit, such as those with elevated high-sensitivity C-reactive protein or highest change in EPA levels. But she concluded that in clinical practice, physicians could consider addition of EPA for reduction of residual risk in secondary prevention patients.
The RESPECT-EPA study was supported by the Japan Heart Foundation. Dr. Daida reports peakers’ bureau/honorarium fees from Novartis Pharma, Bayer Yakuhin, Sanofi, Kowa Company, Taisho Pharmaceutical, Abbott Medical Japan, Otsuka Pharmaceutical, Amgen, MSD, Daiichi Sankyo, Pfizer Japan, FUKUDA DENSHI, Tsumura, and TOA EIYO and research funding from Philips Japan, FUJIFILM Holdings, Asahi Kasei, Inter Reha, TOHO HOLDINGS, GLORY, BMS, Abbott Japan, and Boehringer Ingelheim Japan.
A version of this article first appeared on Medscape.com.
FROM AHA 2022