User login
-
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]


Pulmonary Vascular & Cardiovascular Network
Pulmonary Vascular Disease Section
Key messages from the 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension
1. Per coverage by the American College of Cardiology, “Pulmonary hypertension (PH) is now defined by a mean pulmonary arterial pressure >20 mm Hg at rest. The definition of pulmonary arterial hypertension (PAH) also implies a pulmonary vascular resistance (PVR) >2 Wood units and pulmonary arterial wedge pressure ≤15 mm Hg.”1 These cut-off values do not translate into new therapeutic recommendations.
2. , involving first suspicion by first-line physicians, then detection by echocardiography, and confirmation with right heart catheterization, preferably in a PH center.
3. Pulmonary vasoreactivity testing is only recommended in patients with idiopathic PAH, heritable PAH, or drug/toxin associated PAH to identify potential candidates for calcium channel blocker therapy. Inhaled nitric oxide or inhaled iloprost are the recommended agents.
4. The role of cardiac MRI in prognostication of patients with PAH has been confirmed such that measures of right ventricular volume, right ventricular ejection fraction, and stroke volume are included as risk assessment variables.
5. The primary limitation of the 2015 ESC/ERS three-strata risk-assessment tool is that 60% to 70% of the patients are classified as intermediate risk (IR). A four-strata risk stratification, dividing the IR group into IR “low” and IR “high” risk, is proposed at follow up.
6. No general recommendation is made for or against the use of anticoagulation in PAH given the absence of robust data and increased risk of bleeding.
7. In patients with PH-ILD, inhaled treprostinil may be considered based on findings from the INCREASE trial, but further long-term outcome data are needed.
8. Improved recognition of the signs of chronic thromboembolic pulmonary hypertension (CTEPH) on CT and echocardiographic imagery at the time of an acute pulmonary embolism (PE) event, along with systematic follow-up of patients with acute PE, is recommended to help mitigate the underdiagnosis of CTEPH.
9. The treatment algorithm for PAH has been simplified, and now includes a focus on cardiopulmonary comorbidities, risk assessment, and treatment goals. Current standards include initial combination therapy and treatment escalation at follow-up, when appropriate.
10. Per coverage by the American College of Cardiology, “The recommendations on sex-related issues in patients with PAH, including pregnancy, have been updated, with information and shared decision making as key points.” Calcium channel blockers, inhaled/IV/subcutaneous prostacyclin analogues, and phosphodiesterase 5 inhibitors all and are considered safe during pregnancy, despite limited data on this use.
11. Per the guideline, “Patients with PAH should be treated with the best standard of pharmacological treatment and be in stable clinical condition before embarking on a supervised rehabilitation program.”2 Additional studies have shown that exercise training has a beneficial impact on 6-minute walk distance, quality of life, World Health Organization function classification, and peak VO2.
12. Immunization of PAH patients against SARS-CoV-2, influenza, and Streptococcus pneumoniae is recommended.
This edition of clinical practice guidelines focuses on early diagnosis of PAH and optimal treatments.
*Mary Jo S. Farmer, MD, PhD
Member-at-Large
Vijay Balasubramanian, MD, MRCP (UK)
Chair
* The authors for this article were listed in the incorrect order in the print edition of CHEST Physician. The order has been corrected here.
References
1. Mukherjee, D. 2022 ESC/ERS guidelines for pulmonary hypertension: key points. American College of Cardiology. August 30, 2022.
2. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618-3731.
Pulmonary Vascular Disease Section
Key messages from the 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension
1. Per coverage by the American College of Cardiology, “Pulmonary hypertension (PH) is now defined by a mean pulmonary arterial pressure >20 mm Hg at rest. The definition of pulmonary arterial hypertension (PAH) also implies a pulmonary vascular resistance (PVR) >2 Wood units and pulmonary arterial wedge pressure ≤15 mm Hg.”1 These cut-off values do not translate into new therapeutic recommendations.
2. , involving first suspicion by first-line physicians, then detection by echocardiography, and confirmation with right heart catheterization, preferably in a PH center.
3. Pulmonary vasoreactivity testing is only recommended in patients with idiopathic PAH, heritable PAH, or drug/toxin associated PAH to identify potential candidates for calcium channel blocker therapy. Inhaled nitric oxide or inhaled iloprost are the recommended agents.
4. The role of cardiac MRI in prognostication of patients with PAH has been confirmed such that measures of right ventricular volume, right ventricular ejection fraction, and stroke volume are included as risk assessment variables.
5. The primary limitation of the 2015 ESC/ERS three-strata risk-assessment tool is that 60% to 70% of the patients are classified as intermediate risk (IR). A four-strata risk stratification, dividing the IR group into IR “low” and IR “high” risk, is proposed at follow up.
6. No general recommendation is made for or against the use of anticoagulation in PAH given the absence of robust data and increased risk of bleeding.
7. In patients with PH-ILD, inhaled treprostinil may be considered based on findings from the INCREASE trial, but further long-term outcome data are needed.
8. Improved recognition of the signs of chronic thromboembolic pulmonary hypertension (CTEPH) on CT and echocardiographic imagery at the time of an acute pulmonary embolism (PE) event, along with systematic follow-up of patients with acute PE, is recommended to help mitigate the underdiagnosis of CTEPH.
9. The treatment algorithm for PAH has been simplified, and now includes a focus on cardiopulmonary comorbidities, risk assessment, and treatment goals. Current standards include initial combination therapy and treatment escalation at follow-up, when appropriate.
10. Per coverage by the American College of Cardiology, “The recommendations on sex-related issues in patients with PAH, including pregnancy, have been updated, with information and shared decision making as key points.” Calcium channel blockers, inhaled/IV/subcutaneous prostacyclin analogues, and phosphodiesterase 5 inhibitors all and are considered safe during pregnancy, despite limited data on this use.
11. Per the guideline, “Patients with PAH should be treated with the best standard of pharmacological treatment and be in stable clinical condition before embarking on a supervised rehabilitation program.”2 Additional studies have shown that exercise training has a beneficial impact on 6-minute walk distance, quality of life, World Health Organization function classification, and peak VO2.
12. Immunization of PAH patients against SARS-CoV-2, influenza, and Streptococcus pneumoniae is recommended.
This edition of clinical practice guidelines focuses on early diagnosis of PAH and optimal treatments.
*Mary Jo S. Farmer, MD, PhD
Member-at-Large
Vijay Balasubramanian, MD, MRCP (UK)
Chair
* The authors for this article were listed in the incorrect order in the print edition of CHEST Physician. The order has been corrected here.
References
1. Mukherjee, D. 2022 ESC/ERS guidelines for pulmonary hypertension: key points. American College of Cardiology. August 30, 2022.
2. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618-3731.
Pulmonary Vascular Disease Section
Key messages from the 2022 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension
1. Per coverage by the American College of Cardiology, “Pulmonary hypertension (PH) is now defined by a mean pulmonary arterial pressure >20 mm Hg at rest. The definition of pulmonary arterial hypertension (PAH) also implies a pulmonary vascular resistance (PVR) >2 Wood units and pulmonary arterial wedge pressure ≤15 mm Hg.”1 These cut-off values do not translate into new therapeutic recommendations.
2. , involving first suspicion by first-line physicians, then detection by echocardiography, and confirmation with right heart catheterization, preferably in a PH center.
3. Pulmonary vasoreactivity testing is only recommended in patients with idiopathic PAH, heritable PAH, or drug/toxin associated PAH to identify potential candidates for calcium channel blocker therapy. Inhaled nitric oxide or inhaled iloprost are the recommended agents.
4. The role of cardiac MRI in prognostication of patients with PAH has been confirmed such that measures of right ventricular volume, right ventricular ejection fraction, and stroke volume are included as risk assessment variables.
5. The primary limitation of the 2015 ESC/ERS three-strata risk-assessment tool is that 60% to 70% of the patients are classified as intermediate risk (IR). A four-strata risk stratification, dividing the IR group into IR “low” and IR “high” risk, is proposed at follow up.
6. No general recommendation is made for or against the use of anticoagulation in PAH given the absence of robust data and increased risk of bleeding.
7. In patients with PH-ILD, inhaled treprostinil may be considered based on findings from the INCREASE trial, but further long-term outcome data are needed.
8. Improved recognition of the signs of chronic thromboembolic pulmonary hypertension (CTEPH) on CT and echocardiographic imagery at the time of an acute pulmonary embolism (PE) event, along with systematic follow-up of patients with acute PE, is recommended to help mitigate the underdiagnosis of CTEPH.
9. The treatment algorithm for PAH has been simplified, and now includes a focus on cardiopulmonary comorbidities, risk assessment, and treatment goals. Current standards include initial combination therapy and treatment escalation at follow-up, when appropriate.
10. Per coverage by the American College of Cardiology, “The recommendations on sex-related issues in patients with PAH, including pregnancy, have been updated, with information and shared decision making as key points.” Calcium channel blockers, inhaled/IV/subcutaneous prostacyclin analogues, and phosphodiesterase 5 inhibitors all and are considered safe during pregnancy, despite limited data on this use.
11. Per the guideline, “Patients with PAH should be treated with the best standard of pharmacological treatment and be in stable clinical condition before embarking on a supervised rehabilitation program.”2 Additional studies have shown that exercise training has a beneficial impact on 6-minute walk distance, quality of life, World Health Organization function classification, and peak VO2.
12. Immunization of PAH patients against SARS-CoV-2, influenza, and Streptococcus pneumoniae is recommended.
This edition of clinical practice guidelines focuses on early diagnosis of PAH and optimal treatments.
*Mary Jo S. Farmer, MD, PhD
Member-at-Large
Vijay Balasubramanian, MD, MRCP (UK)
Chair
* The authors for this article were listed in the incorrect order in the print edition of CHEST Physician. The order has been corrected here.
References
1. Mukherjee, D. 2022 ESC/ERS guidelines for pulmonary hypertension: key points. American College of Cardiology. August 30, 2022.
2. Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J. 2022;43(38):3618-3731.
Diffuse Lung Disease & Transplant Network
Pulmonary Physiology & Rehabilitation Section
Exercise tolerance in untreated sleep apnea
Numerous cardiovascular, respiratory, neuromuscular, and perceptual factors determine exercise tolerance. This makes designing a study to isolate the contribution of one factor difficult.
A recently published study (Elbehairy, et al. Chest. 2022; published online September 29, 2022) explores exercise tolerance in patients with untreated OSA compared with age- and weight-matched controls. The authors found that at an equivalent work rate, patients with OSA had greater minute ventilation, principally due to higher breathing frequency. Dead space volume, dead space ventilation, and dead space to tidal volume ratio (VD/VT) were higher in patients with OSA, likely due to a reduction in pulmonary vessel recruitment relative to ventilation. VD/VT decreased more from rest to peak in controls than in patients with OSA, an adaptation that is expected with exercise. Patients with OSA had greater arterial stiffness measured by pulse wave velocity and higher blood pressures, which may have affected cardiac output augmentation. Patients with OSA also had higher resting mean pulmonary artery pressures and exercise dyspnea scores. Regression models predicting peak oxygen uptake and peak work rate were statistically significant, with predictors being age, pulse wave velocity, and resting mean pulmonary artery pressure. The role of diastolic dysfunction remains to be determined.
Prior studies have shown that some effects of OSA on exercise may be reversed with CPAP treatment (Arias, et al. Eur Heart J. 2006;27[9]:1106-1113; Chalegre, et al. Sleep Breath. 2021;25[3]:1195-1202). Understanding the mechanisms of exercise limitation in OSA will help physicians address symptoms, reinforce CPAP adherence, and design tailored pulmonary rehabilitation programs.
Fatima Zeba, MD
Fellow-in-Training
Pulmonary Physiology & Rehabilitation Section
Exercise tolerance in untreated sleep apnea
Numerous cardiovascular, respiratory, neuromuscular, and perceptual factors determine exercise tolerance. This makes designing a study to isolate the contribution of one factor difficult.
A recently published study (Elbehairy, et al. Chest. 2022; published online September 29, 2022) explores exercise tolerance in patients with untreated OSA compared with age- and weight-matched controls. The authors found that at an equivalent work rate, patients with OSA had greater minute ventilation, principally due to higher breathing frequency. Dead space volume, dead space ventilation, and dead space to tidal volume ratio (VD/VT) were higher in patients with OSA, likely due to a reduction in pulmonary vessel recruitment relative to ventilation. VD/VT decreased more from rest to peak in controls than in patients with OSA, an adaptation that is expected with exercise. Patients with OSA had greater arterial stiffness measured by pulse wave velocity and higher blood pressures, which may have affected cardiac output augmentation. Patients with OSA also had higher resting mean pulmonary artery pressures and exercise dyspnea scores. Regression models predicting peak oxygen uptake and peak work rate were statistically significant, with predictors being age, pulse wave velocity, and resting mean pulmonary artery pressure. The role of diastolic dysfunction remains to be determined.
Prior studies have shown that some effects of OSA on exercise may be reversed with CPAP treatment (Arias, et al. Eur Heart J. 2006;27[9]:1106-1113; Chalegre, et al. Sleep Breath. 2021;25[3]:1195-1202). Understanding the mechanisms of exercise limitation in OSA will help physicians address symptoms, reinforce CPAP adherence, and design tailored pulmonary rehabilitation programs.
Fatima Zeba, MD
Fellow-in-Training
Pulmonary Physiology & Rehabilitation Section
Exercise tolerance in untreated sleep apnea
Numerous cardiovascular, respiratory, neuromuscular, and perceptual factors determine exercise tolerance. This makes designing a study to isolate the contribution of one factor difficult.
A recently published study (Elbehairy, et al. Chest. 2022; published online September 29, 2022) explores exercise tolerance in patients with untreated OSA compared with age- and weight-matched controls. The authors found that at an equivalent work rate, patients with OSA had greater minute ventilation, principally due to higher breathing frequency. Dead space volume, dead space ventilation, and dead space to tidal volume ratio (VD/VT) were higher in patients with OSA, likely due to a reduction in pulmonary vessel recruitment relative to ventilation. VD/VT decreased more from rest to peak in controls than in patients with OSA, an adaptation that is expected with exercise. Patients with OSA had greater arterial stiffness measured by pulse wave velocity and higher blood pressures, which may have affected cardiac output augmentation. Patients with OSA also had higher resting mean pulmonary artery pressures and exercise dyspnea scores. Regression models predicting peak oxygen uptake and peak work rate were statistically significant, with predictors being age, pulse wave velocity, and resting mean pulmonary artery pressure. The role of diastolic dysfunction remains to be determined.
Prior studies have shown that some effects of OSA on exercise may be reversed with CPAP treatment (Arias, et al. Eur Heart J. 2006;27[9]:1106-1113; Chalegre, et al. Sleep Breath. 2021;25[3]:1195-1202). Understanding the mechanisms of exercise limitation in OSA will help physicians address symptoms, reinforce CPAP adherence, and design tailored pulmonary rehabilitation programs.
Fatima Zeba, MD
Fellow-in-Training
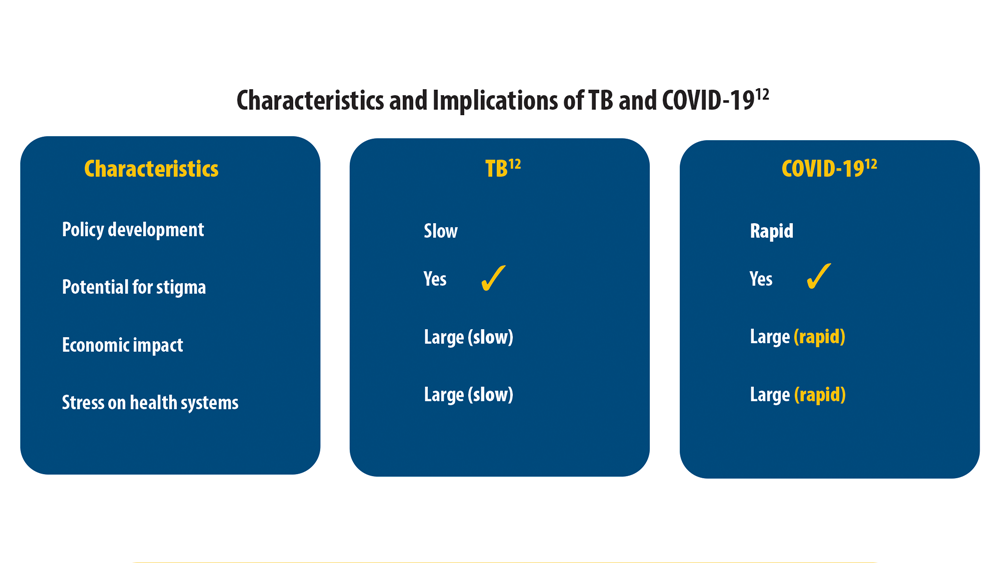
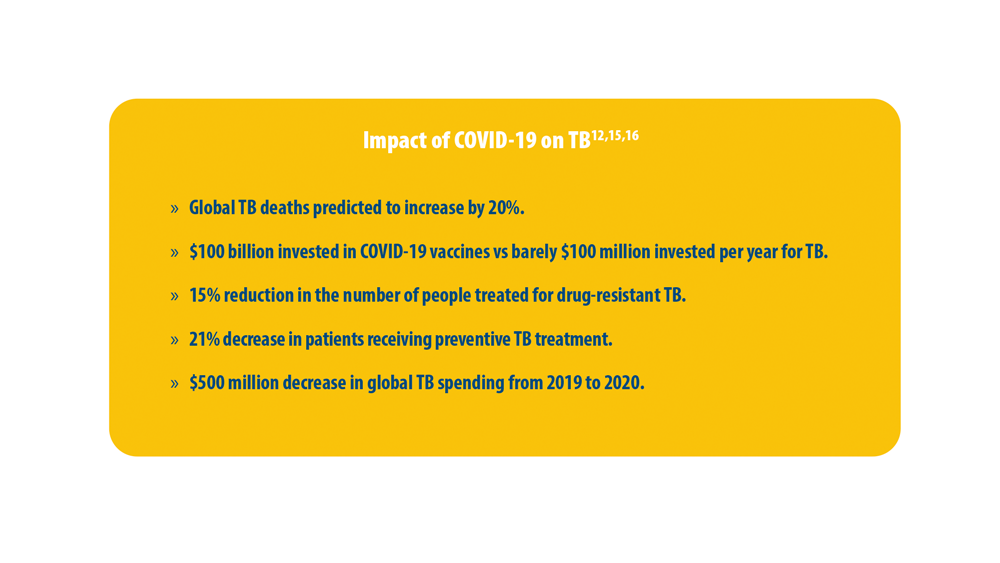
Reducing Tuberculosis Globally and the Impact of COVID-19
1. Tuberculosis fact sheet. World Health Organization. Updated October 14, 2021. Accessed May 24, 2022. https://www.who.int/ news-room/fact-sheets/detail/tuberculosis
2. Tuberculosis deaths rise for the first time in more than a decade due to the COVID-19 pandemic. World Health Organization. Published October 14, 2021. Accessed May 24, 2022. https://www. who.int/news/item/14-10-2021-tuberculosis-deaths-rise-for-thefirst-time-in-more-than-a-decade-due-to-the-covid-19-pandemic
3. Wilson JW, Kissner DG, Escalante P. Cascade of care in the management of latent tuberculosis infection in the United States: a lot to improve and to scale up. Ann Am Thorac Soc. 2021;18(10):1620-1621. doi:10.1513/AnnalsATS.202106-722ED
4. Pedrazzoli D, Wingfield T. Biosocial strategies to address the socioeconomic determinants and consequences of the TB and COVID-19 pandemics. Am J Trop Med Hyg. 2021;104(2):407- 409. doi:10.4269/ajtmh.20-1641
5. Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(9):e1132- e1141. doi:10.1016/S2214-109X(20)30288-6. Erratum in: Lancet Glob Health. 2021;9(1):e23. doi:10.1016/S2214- 109X(20)30433-2
6. Harries AD, Kumar AMV, Satyanarayana S, et al. The growing importance of tuberculosis preventive therapy and how research and innovation can enhance its implementation on the ground. Trop Med Infect Dis. 2020;5(2):61. doi:10.3390/ tropicalmed5020061
7. Ugarte-Gil C, Carrillo-Larco RM, Kirwan DE. Latent tuberculosis infection and non-infectious co-morbidities: diabetes mellitus type 2, chronic kidney disease and rheumatoid arthritis. Int J Infect Dis. 2019;80S:S29-S31. doi:10.1016/j.ijid.2019.02.018
8. Frascella B, Richards AS, Sossen B, et al. Subclinical tuberculosis disease–a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis. 2021;73(3):e830-e841. doi:10.1093/cid/ciaa1402
9. Nathavitharana RR, Garcia-Basteiro AL, Ruhwald M, Cobelens F, Theron G. Reimagining the status quo: how close are we to rapid sputum-free tuberculosis diagnostics for all? EBioMedicine. 2022;78:103939. doi:10.1016/j.ebiom.2022.103939
10. Cattamanchi A, Reza TF, Nalugwa T, et al. Multicomponent strategy with decentralized molecular testing for tuberculosis. N Engl J Med. 2021;385(26):2441-2450. doi:10.1056/NEJMoa2105470
11. Gebreselassie N, Kasaeva T, Zignol M. A global strategy for tuberculosis research and innovation. Eur Respir J. 2020;56(5):2003539. doi:10.1183/13993003.03539-2020
12. Visca D, Ong CWM, Tiberi S, et al. Tuberculosis and COVID19 interaction: a review of biological, clinical and public health effects. Pulmonology. 2021;27(2):151-165. doi:10.1016/j. pulmoe.2020.12.012
13. Saunders MJ, Evans CA. COVID-19, tuberculosis and poverty: preventing a perfect storm. Eur Respir J. 2020;56(1):2001348. doi:10.1183/13993003.01348-2020
14. Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond). 2020;52(12):902-907. doi:10.1080 /23744235.2020.180635
15. Pai M, Kasaeva T, Swaminathan S. Covid-19’s devastating effect on tuberculosis care — a path to recovery. N Engl J Med. 2022;386(16):1490-1493. doi:10.1056/nejmp2118145
16. Dheda K, Perumal T, Moultrie H, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. 2022;10(6):603-622. doi:10.1016/S2213-2600(22)00092-3
1. Tuberculosis fact sheet. World Health Organization. Updated October 14, 2021. Accessed May 24, 2022. https://www.who.int/ news-room/fact-sheets/detail/tuberculosis
2. Tuberculosis deaths rise for the first time in more than a decade due to the COVID-19 pandemic. World Health Organization. Published October 14, 2021. Accessed May 24, 2022. https://www. who.int/news/item/14-10-2021-tuberculosis-deaths-rise-for-thefirst-time-in-more-than-a-decade-due-to-the-covid-19-pandemic
3. Wilson JW, Kissner DG, Escalante P. Cascade of care in the management of latent tuberculosis infection in the United States: a lot to improve and to scale up. Ann Am Thorac Soc. 2021;18(10):1620-1621. doi:10.1513/AnnalsATS.202106-722ED
4. Pedrazzoli D, Wingfield T. Biosocial strategies to address the socioeconomic determinants and consequences of the TB and COVID-19 pandemics. Am J Trop Med Hyg. 2021;104(2):407- 409. doi:10.4269/ajtmh.20-1641
5. Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(9):e1132- e1141. doi:10.1016/S2214-109X(20)30288-6. Erratum in: Lancet Glob Health. 2021;9(1):e23. doi:10.1016/S2214- 109X(20)30433-2
6. Harries AD, Kumar AMV, Satyanarayana S, et al. The growing importance of tuberculosis preventive therapy and how research and innovation can enhance its implementation on the ground. Trop Med Infect Dis. 2020;5(2):61. doi:10.3390/ tropicalmed5020061
7. Ugarte-Gil C, Carrillo-Larco RM, Kirwan DE. Latent tuberculosis infection and non-infectious co-morbidities: diabetes mellitus type 2, chronic kidney disease and rheumatoid arthritis. Int J Infect Dis. 2019;80S:S29-S31. doi:10.1016/j.ijid.2019.02.018
8. Frascella B, Richards AS, Sossen B, et al. Subclinical tuberculosis disease–a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis. 2021;73(3):e830-e841. doi:10.1093/cid/ciaa1402
9. Nathavitharana RR, Garcia-Basteiro AL, Ruhwald M, Cobelens F, Theron G. Reimagining the status quo: how close are we to rapid sputum-free tuberculosis diagnostics for all? EBioMedicine. 2022;78:103939. doi:10.1016/j.ebiom.2022.103939
10. Cattamanchi A, Reza TF, Nalugwa T, et al. Multicomponent strategy with decentralized molecular testing for tuberculosis. N Engl J Med. 2021;385(26):2441-2450. doi:10.1056/NEJMoa2105470
11. Gebreselassie N, Kasaeva T, Zignol M. A global strategy for tuberculosis research and innovation. Eur Respir J. 2020;56(5):2003539. doi:10.1183/13993003.03539-2020
12. Visca D, Ong CWM, Tiberi S, et al. Tuberculosis and COVID19 interaction: a review of biological, clinical and public health effects. Pulmonology. 2021;27(2):151-165. doi:10.1016/j. pulmoe.2020.12.012
13. Saunders MJ, Evans CA. COVID-19, tuberculosis and poverty: preventing a perfect storm. Eur Respir J. 2020;56(1):2001348. doi:10.1183/13993003.01348-2020
14. Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond). 2020;52(12):902-907. doi:10.1080 /23744235.2020.180635
15. Pai M, Kasaeva T, Swaminathan S. Covid-19’s devastating effect on tuberculosis care — a path to recovery. N Engl J Med. 2022;386(16):1490-1493. doi:10.1056/nejmp2118145
16. Dheda K, Perumal T, Moultrie H, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. 2022;10(6):603-622. doi:10.1016/S2213-2600(22)00092-3
1. Tuberculosis fact sheet. World Health Organization. Updated October 14, 2021. Accessed May 24, 2022. https://www.who.int/ news-room/fact-sheets/detail/tuberculosis
2. Tuberculosis deaths rise for the first time in more than a decade due to the COVID-19 pandemic. World Health Organization. Published October 14, 2021. Accessed May 24, 2022. https://www. who.int/news/item/14-10-2021-tuberculosis-deaths-rise-for-thefirst-time-in-more-than-a-decade-due-to-the-covid-19-pandemic
3. Wilson JW, Kissner DG, Escalante P. Cascade of care in the management of latent tuberculosis infection in the United States: a lot to improve and to scale up. Ann Am Thorac Soc. 2021;18(10):1620-1621. doi:10.1513/AnnalsATS.202106-722ED
4. Pedrazzoli D, Wingfield T. Biosocial strategies to address the socioeconomic determinants and consequences of the TB and COVID-19 pandemics. Am J Trop Med Hyg. 2021;104(2):407- 409. doi:10.4269/ajtmh.20-1641
5. Hogan AB, Jewell BL, Sherrard-Smith E, et al. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(9):e1132- e1141. doi:10.1016/S2214-109X(20)30288-6. Erratum in: Lancet Glob Health. 2021;9(1):e23. doi:10.1016/S2214- 109X(20)30433-2
6. Harries AD, Kumar AMV, Satyanarayana S, et al. The growing importance of tuberculosis preventive therapy and how research and innovation can enhance its implementation on the ground. Trop Med Infect Dis. 2020;5(2):61. doi:10.3390/ tropicalmed5020061
7. Ugarte-Gil C, Carrillo-Larco RM, Kirwan DE. Latent tuberculosis infection and non-infectious co-morbidities: diabetes mellitus type 2, chronic kidney disease and rheumatoid arthritis. Int J Infect Dis. 2019;80S:S29-S31. doi:10.1016/j.ijid.2019.02.018
8. Frascella B, Richards AS, Sossen B, et al. Subclinical tuberculosis disease–a review and analysis of prevalence surveys to inform definitions, burden, associations, and screening methodology. Clin Infect Dis. 2021;73(3):e830-e841. doi:10.1093/cid/ciaa1402
9. Nathavitharana RR, Garcia-Basteiro AL, Ruhwald M, Cobelens F, Theron G. Reimagining the status quo: how close are we to rapid sputum-free tuberculosis diagnostics for all? EBioMedicine. 2022;78:103939. doi:10.1016/j.ebiom.2022.103939
10. Cattamanchi A, Reza TF, Nalugwa T, et al. Multicomponent strategy with decentralized molecular testing for tuberculosis. N Engl J Med. 2021;385(26):2441-2450. doi:10.1056/NEJMoa2105470
11. Gebreselassie N, Kasaeva T, Zignol M. A global strategy for tuberculosis research and innovation. Eur Respir J. 2020;56(5):2003539. doi:10.1183/13993003.03539-2020
12. Visca D, Ong CWM, Tiberi S, et al. Tuberculosis and COVID19 interaction: a review of biological, clinical and public health effects. Pulmonology. 2021;27(2):151-165. doi:10.1016/j. pulmoe.2020.12.012
13. Saunders MJ, Evans CA. COVID-19, tuberculosis and poverty: preventing a perfect storm. Eur Respir J. 2020;56(1):2001348. doi:10.1183/13993003.01348-2020
14. Sy KTL, Haw NJL, Uy J. Previous and active tuberculosis increases risk of death and prolongs recovery in patients with COVID-19. Infect Dis (Lond). 2020;52(12):902-907. doi:10.1080 /23744235.2020.180635
15. Pai M, Kasaeva T, Swaminathan S. Covid-19’s devastating effect on tuberculosis care — a path to recovery. N Engl J Med. 2022;386(16):1490-1493. doi:10.1056/nejmp2118145
16. Dheda K, Perumal T, Moultrie H, et al. The intersecting pandemics of tuberculosis and COVID-19: population-level and patient-level impact, clinical presentation, and corrective interventions. Lancet Respir Med. 2022;10(6):603-622. doi:10.1016/S2213-2600(22)00092-3
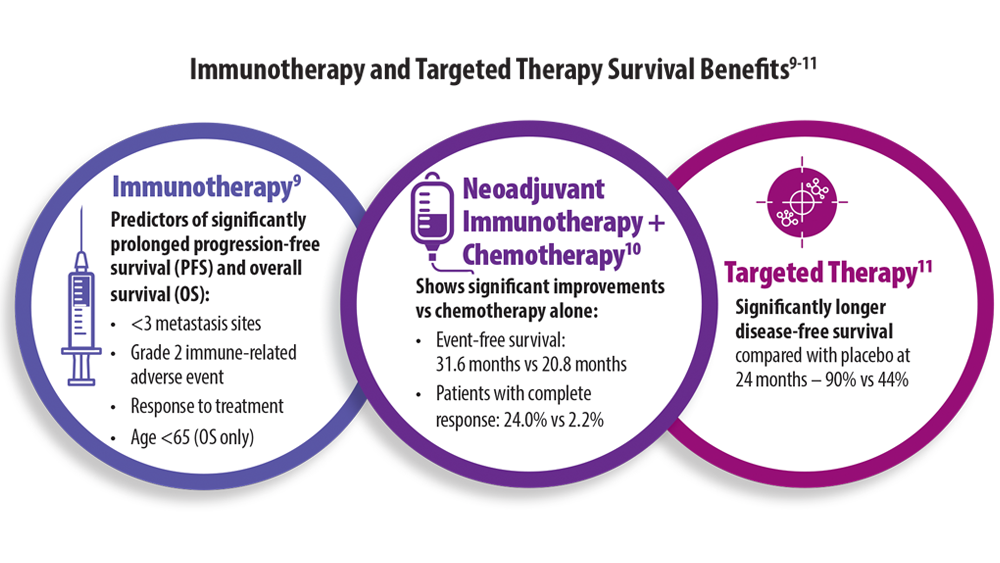
Advances in Lung Cancer Diagnostics and Treatment
1. Cancer facts and figures 2022. American Cancer Society. Accessed June 14, 2022. https://www.cancer.org/content/dam/ cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2022/2022-cancer-facts-and-figures
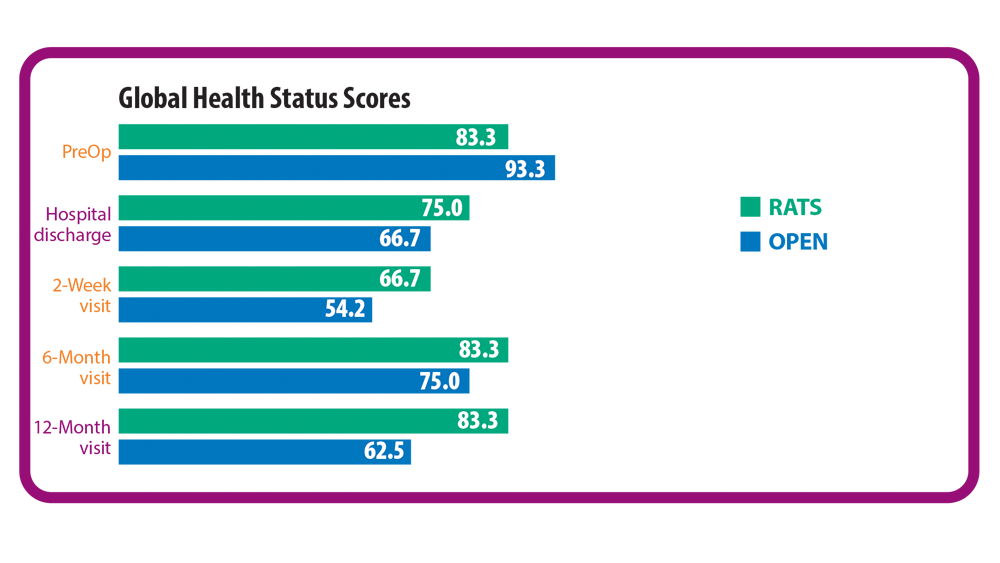
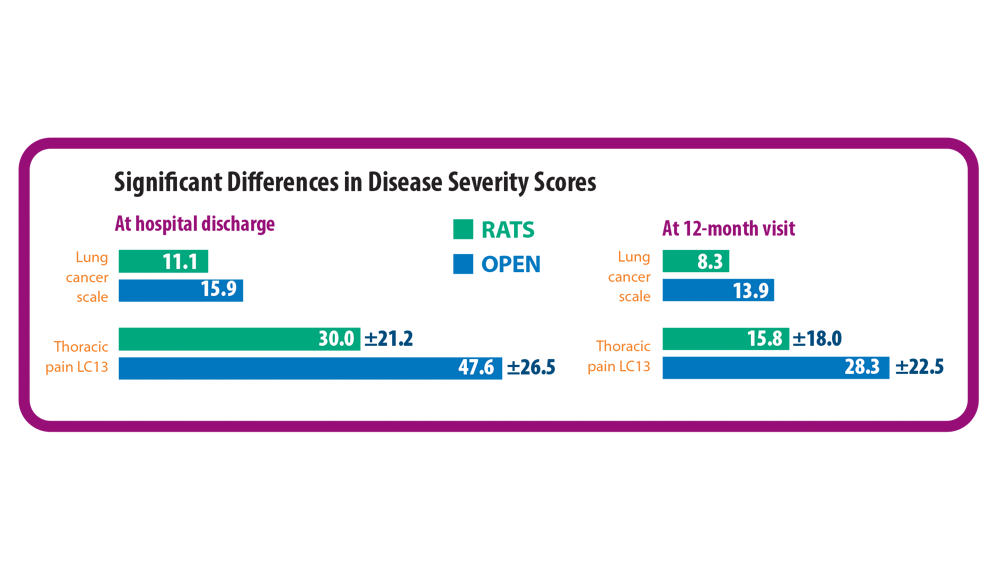
2. Novellis P, Maisonneuve P, Dieci E, et al. Quality of life, postoperative pain, and lymph node dissection in a robotic approach compared to VATS and OPEN for early stage lung cancer. J Clin Med. 2021;10(8):1687. doi:10.3390/jcm10081687
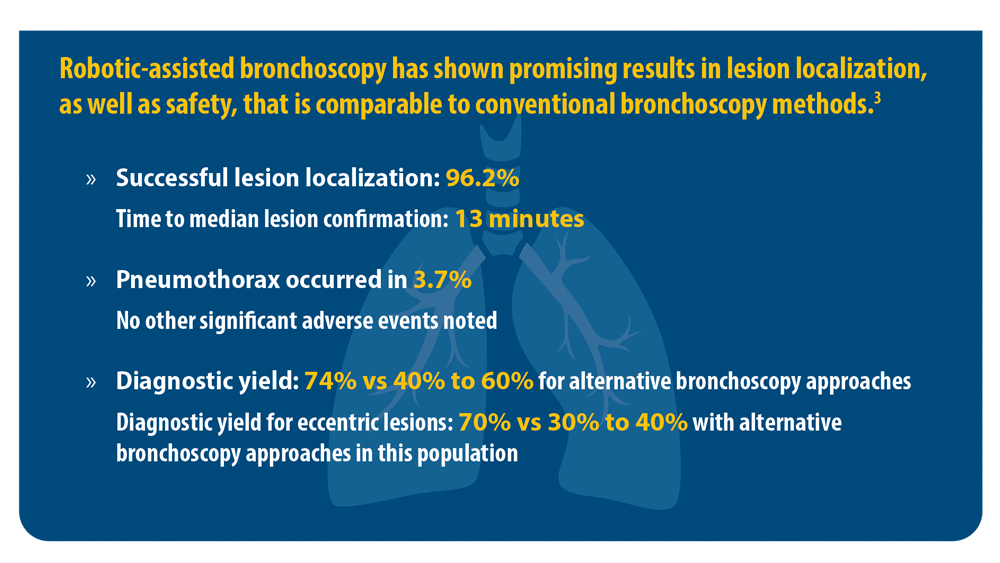
3. Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845-852. doi:10.1016/j. chest.2020.08.2047
4. Current cigarette smoking among adults in the United States. Centers for Disease Control and Prevention. Updated March 17, 2022. Accessed June 15, 2022. https://www.cdc.gov/tobacco/ data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
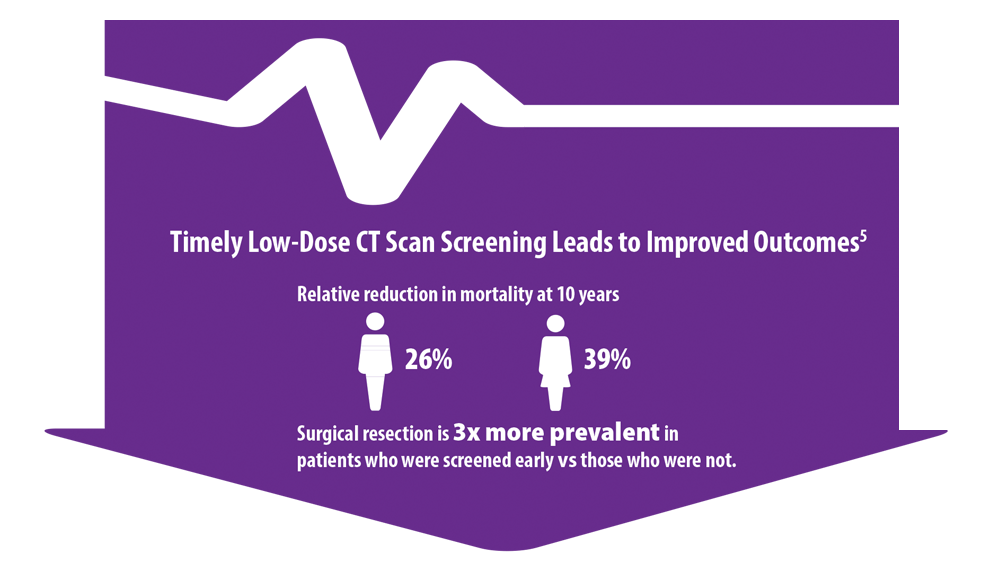
5. Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399-405. doi:10.1513/AnnalsATS.201907- 556CME
6. US Preventive Services Task Force issues final recommendation statement on screening for lung cancer. USPSTF Bulletin. Published March 9, 2021. Accessed June 15, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/lung-cancer-newsbulletin.pdf
7. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
8. Lung cancer screening report. National Cancer Institute Cancer Trends Progress Report. Updated April 2022. Accessed June 15, 2022. https://progressreport.cancer.gov/detection/lung_cancer
9. Huang L, Li L, Zhou Y, et al. Clinical characteristics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer. J Cancer. 2020;11(24):7137-7145. doi:10.7150/ jca.49213
10. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. doi:10.1056/NEJMoa2202170
11. Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
1. Cancer facts and figures 2022. American Cancer Society. Accessed June 14, 2022. https://www.cancer.org/content/dam/ cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2022/2022-cancer-facts-and-figures
2. Novellis P, Maisonneuve P, Dieci E, et al. Quality of life, postoperative pain, and lymph node dissection in a robotic approach compared to VATS and OPEN for early stage lung cancer. J Clin Med. 2021;10(8):1687. doi:10.3390/jcm10081687
3. Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845-852. doi:10.1016/j. chest.2020.08.2047
4. Current cigarette smoking among adults in the United States. Centers for Disease Control and Prevention. Updated March 17, 2022. Accessed June 15, 2022. https://www.cdc.gov/tobacco/ data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
5. Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399-405. doi:10.1513/AnnalsATS.201907- 556CME
6. US Preventive Services Task Force issues final recommendation statement on screening for lung cancer. USPSTF Bulletin. Published March 9, 2021. Accessed June 15, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/lung-cancer-newsbulletin.pdf
7. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
8. Lung cancer screening report. National Cancer Institute Cancer Trends Progress Report. Updated April 2022. Accessed June 15, 2022. https://progressreport.cancer.gov/detection/lung_cancer
9. Huang L, Li L, Zhou Y, et al. Clinical characteristics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer. J Cancer. 2020;11(24):7137-7145. doi:10.7150/ jca.49213
10. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. doi:10.1056/NEJMoa2202170
11. Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
1. Cancer facts and figures 2022. American Cancer Society. Accessed June 14, 2022. https://www.cancer.org/content/dam/ cancer-org/research/cancer-facts-and-statistics/annual-cancerfacts-and-figures/2022/2022-cancer-facts-and-figures
2. Novellis P, Maisonneuve P, Dieci E, et al. Quality of life, postoperative pain, and lymph node dissection in a robotic approach compared to VATS and OPEN for early stage lung cancer. J Clin Med. 2021;10(8):1687. doi:10.3390/jcm10081687
3. Chen AC, Pastis NJ Jr, Mahajan AK, et al. Robotic bronchoscopy for peripheral pulmonary lesions: a multicenter pilot and feasibility study (BENEFIT). Chest. 2021;159(2):845-852. doi:10.1016/j. chest.2020.08.2047
4. Current cigarette smoking among adults in the United States. Centers for Disease Control and Prevention. Updated March 17, 2022. Accessed June 15, 2022. https://www.cdc.gov/tobacco/ data_statistics/fact_sheets/adult_data/cig_smoking/index.htm
5. Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in lung cancer screening: a review. Ann Am Thorac Soc. 2020;17(4):399-405. doi:10.1513/AnnalsATS.201907- 556CME
6. US Preventive Services Task Force issues final recommendation statement on screening for lung cancer. USPSTF Bulletin. Published March 9, 2021. Accessed June 15, 2022. https://www.uspreventiveservicestaskforce.org/uspstf/sites/default/files/file/supporting_documents/lung-cancer-newsbulletin.pdf
7. Mazzone PJ, Silvestri GA, Souter LH, et al. Screening for lung cancer: CHEST guideline and expert panel report. Chest. 2021;160(5):e427-e494. doi:10.1016/j.chest.2021.06.063
8. Lung cancer screening report. National Cancer Institute Cancer Trends Progress Report. Updated April 2022. Accessed June 15, 2022. https://progressreport.cancer.gov/detection/lung_cancer
9. Huang L, Li L, Zhou Y, et al. Clinical characteristics correlate with outcomes of immunotherapy in advanced non-small cell lung cancer. J Cancer. 2020;11(24):7137-7145. doi:10.7150/ jca.49213
10. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985. doi:10.1056/NEJMoa2202170
11. Wu YL, Tsuboi M, He J, et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med. 2020;383(18):1711-1723. doi:10.1056/NEJMoa2027071
Children and COVID: New cases increase for second straight week
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.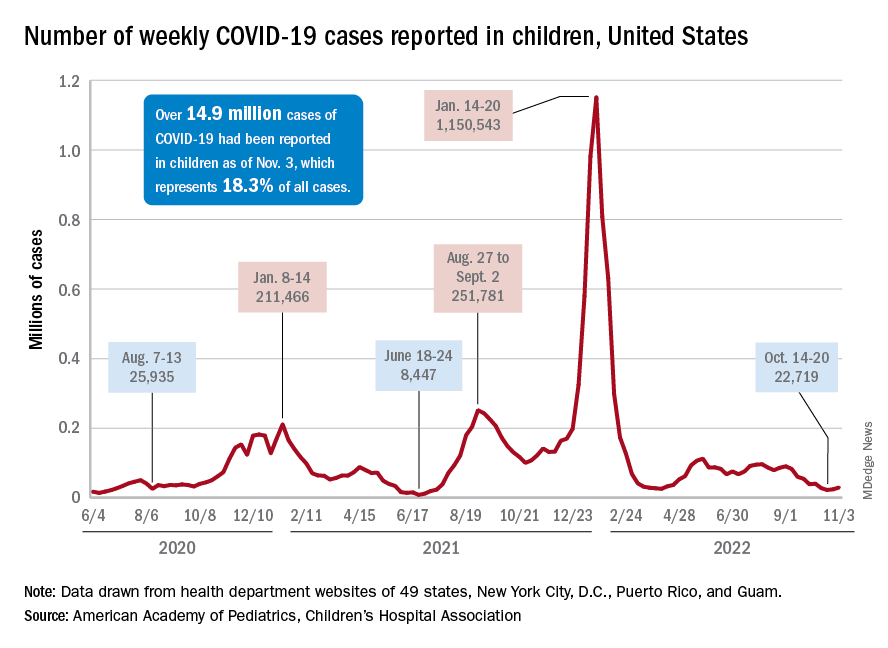
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
New COVID-19 cases rose among U.S. children for the second consecutive week, while hospitals saw signs of renewed activity on the part of SARS-CoV-2.
, when the count fell to its lowest level in more than a year, the American Academy of Pediatrics and the Children’s Hospital Association said in their joint report.
The 7-day average for ED visits with diagnosed COVID was down to just 0.6% of all ED visits for 12- to 15-year-olds as late as Oct. 23 but has moved up to 0.7% since then. Among those aged 16-17 years, the 7-day average was also down to 0.6% for just one day, Oct. 19, but was up to 0.8% as of Nov. 4. So far, though, a similar increase has not yet occurred for ED visits among children aged 0-11 years, the CDC said on its COVID Data Tracker.
The trend is discernible, however, when looking at hospitalizations of children with confirmed COVID. The rate of new admissions of children aged 0-17 years was 0.16 per 100,000 population as late as Oct. 23 but ticked up a notch after that and has been 0.17 per 100,000 since, according to the CDC. As with the ED rate, hospitalizations had been steadily declining since late August.
Vaccine initiation continues to slow
During the week of Oct. 27 to Nov. 2, about 30,000 children under 5 years of age received their initial COVID vaccination. A month earlier (Sept. 29 to Oct. 5), that number was about 40,000. A month before that, about 53,000 children aged 0-5 years received their initial dose, the AAP said in a separate vaccination report based on CDC data.
All of that reduced interest adds up to 7.4% of the age group having received at least one dose and just 3.2% being fully vaccinated as of Nov. 2. Among children aged 5-11 years, the corresponding vaccination rates are 38.9% and 31.8%, while those aged 12-17 years are at 71.3% and 61.1%, the CDC said.
Looking at just the first 20 weeks of the vaccination experience for each age group shows that 1.6 million children under 5 years of age had received at least an initial dose, compared with 8.1 million children aged 5-11 years and 8.1 million children aged 12-15, the AAP said.
Airways Disorders Network
Pediatric Chest Medicine Section
CPAP for pediatric OSA: “Off-label” use
Pediatric providers are well aware of the “off-label” uses of medications/devices. While it’s not a stretch to apply “adult” diagnostic and therapeutic criteria to older adolescents, more careful consideration is needed for our younger patients. Typically, adenotonsillectomy is first-line treatment for pediatric OSA, but CPAP can be essential for those for whom surgical intervention is not an option, not an option yet, or has been insufficient (residual OSA). Unfortunately, standard CPAP devices are not approved for use in children, and often have a minimum weight requirement of 30 kg. There are respiratory assist devices and home mechanical ventilators that are approved for use in pediatric patients (minimum weight 13 kg or 5 kg) and designed for more complex ventilatory support, and that also are capable of providing continuous pressure. Alternatively, pediatric providers may proceed with the “off-label” use of simpler CPAP-only medical devices and face obstacles in attaining insurance approval. The recent American Academy of Sleep Medicine position statement (Amos, et al. J Clin Sleep Med. 2022;18[8]:2041-3) acknowledges that CPAP therapy can be safe and effective when management is guided by a pediatric specialist and is typically initiated in a monitored setting (inpatient or polysomnogram). The authors bring up excellent points regarding unique considerations for pediatric CPAP therapy, including the need for desensitization and facial development monitoring, lack of technical/software designed for younger/smaller patients, and limited published data (small and diverse cohorts). Ultimately, evaluation of effectiveness and safety, while distinct, must both be seriously considered in this risk-benefit analysis of care.
Pallavi P. Patwari, MD, FAAP, FAASM
Member-at-Large
Pediatric Chest Medicine Section
CPAP for pediatric OSA: “Off-label” use
Pediatric providers are well aware of the “off-label” uses of medications/devices. While it’s not a stretch to apply “adult” diagnostic and therapeutic criteria to older adolescents, more careful consideration is needed for our younger patients. Typically, adenotonsillectomy is first-line treatment for pediatric OSA, but CPAP can be essential for those for whom surgical intervention is not an option, not an option yet, or has been insufficient (residual OSA). Unfortunately, standard CPAP devices are not approved for use in children, and often have a minimum weight requirement of 30 kg. There are respiratory assist devices and home mechanical ventilators that are approved for use in pediatric patients (minimum weight 13 kg or 5 kg) and designed for more complex ventilatory support, and that also are capable of providing continuous pressure. Alternatively, pediatric providers may proceed with the “off-label” use of simpler CPAP-only medical devices and face obstacles in attaining insurance approval. The recent American Academy of Sleep Medicine position statement (Amos, et al. J Clin Sleep Med. 2022;18[8]:2041-3) acknowledges that CPAP therapy can be safe and effective when management is guided by a pediatric specialist and is typically initiated in a monitored setting (inpatient or polysomnogram). The authors bring up excellent points regarding unique considerations for pediatric CPAP therapy, including the need for desensitization and facial development monitoring, lack of technical/software designed for younger/smaller patients, and limited published data (small and diverse cohorts). Ultimately, evaluation of effectiveness and safety, while distinct, must both be seriously considered in this risk-benefit analysis of care.
Pallavi P. Patwari, MD, FAAP, FAASM
Member-at-Large
Pediatric Chest Medicine Section
CPAP for pediatric OSA: “Off-label” use
Pediatric providers are well aware of the “off-label” uses of medications/devices. While it’s not a stretch to apply “adult” diagnostic and therapeutic criteria to older adolescents, more careful consideration is needed for our younger patients. Typically, adenotonsillectomy is first-line treatment for pediatric OSA, but CPAP can be essential for those for whom surgical intervention is not an option, not an option yet, or has been insufficient (residual OSA). Unfortunately, standard CPAP devices are not approved for use in children, and often have a minimum weight requirement of 30 kg. There are respiratory assist devices and home mechanical ventilators that are approved for use in pediatric patients (minimum weight 13 kg or 5 kg) and designed for more complex ventilatory support, and that also are capable of providing continuous pressure. Alternatively, pediatric providers may proceed with the “off-label” use of simpler CPAP-only medical devices and face obstacles in attaining insurance approval. The recent American Academy of Sleep Medicine position statement (Amos, et al. J Clin Sleep Med. 2022;18[8]:2041-3) acknowledges that CPAP therapy can be safe and effective when management is guided by a pediatric specialist and is typically initiated in a monitored setting (inpatient or polysomnogram). The authors bring up excellent points regarding unique considerations for pediatric CPAP therapy, including the need for desensitization and facial development monitoring, lack of technical/software designed for younger/smaller patients, and limited published data (small and diverse cohorts). Ultimately, evaluation of effectiveness and safety, while distinct, must both be seriously considered in this risk-benefit analysis of care.
Pallavi P. Patwari, MD, FAAP, FAASM
Member-at-Large
EHR-based thromboembolism risk tool boosted prophylaxis
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
CHICAGO – A clinical decision-support tool designed to identify hospitalized patients who need thromboembolism prophylaxis and embedded in a hospital’s electronic health record led to significantly more appropriate prophylaxis, compared with usual care, and significantly cut the 30-day rate of thromboembolism in a randomized, multicenter trial with more than 10,000 patients.
“This is the first time that a clinical decision support tool not only changed [thromboprophylaxis prescribing] behavior but also affected hard outcomes. That’s remarkable,” lead investigator Alex C. Spyropoulos, MD, said in an interview.
Even so, outside experts expressed concerns about certain results and the trial design.
Use of the decision-support risk calculator for thromboembolism in the IMPROVE-DD VTE trial significantly boosted use of appropriate inpatient thromboprophylaxis starting at hospital admission by a relative 52%, and significantly increased outpatient thromboprophylaxis prescribed at discharge by a relative 93% in the study’s two primary endpoints, Dr. Spyropoulos reported at the American Heart Association scientific sessions.
This intervention led to a significant 29% relative reduction in the incidence of total thromboembolic events, both venous and arterial, during hospitalization and through 30 days post discharge.
The absolute thromboembolic event rates were 2.9% among 5,249 patients treated at either of two U.S. hospitals that used the EHR-based risk calculator and 4.0% in 5,450 patients seen at either of two other U.S. hospitals that served as controls and where usual care method identified patients who needed thromboprophylaxis, said Dr. Spyropoulos, professor and director of the anticoagulation and clinical thrombosis services for Northwell Health in New York. This included a 2.7% rate of venous thromboembolism and a 0.25% rate of arterial thromboembolism in the intervention patients, and a 3.3% rate of venous events and a 0.7% rate of arterial events in the controls.
Patients treated at the hospitals that used the EHR-embedded risk calculator also has a numerically lower rate of major bleeding events during hospitalization and 30-day postdischarge follow-up, a 0.15% rate compared with a 0.22% rate in the control patients, a difference that was not significant.
A ‘powerful message’
“It’s a powerful message to see an absolute 1.1% difference in the rate of thromboembolism and a trend to fewer major bleeds. I think this will change practice,” Dr. Spyropoulos added in the interview. “The next step is dissemination.”
But thromboprophylaxis experts cautioned that, while the results looked promising, the findings need more analysis and review, and the intervention may need further testing before it’s ready for widespread use.
For example, one unexpected result was an unexpected 2.1 percentage point increase in all-cause mortality linked with use of the decision-support tool. Total deaths from admission to 30 days after discharge occurred in 9.1% of the patients treated at the two hospitals that used the risk calculator and 7.0% among the control patients, a difference that Dr, Spyropoulos said was likely the result of unbalanced outcomes from COVID-19 infections that had no relevance to the tested intervention. The trial ran during December 2020–January 2022.
But wait – more detail and analysis needed
“I’d like to see more analysis of the data from this trial,” and “there is the issue of increased mortality,” commented Gregory Piazza, MD, director of vascular medicine at Brigham and Women’s Hospital in Boston, and a specialist in thromboembolism prevention and management. He also highlighted the need for greater detail on the arterial thromboembolic events tallied during the study.
With more details and analysis of these findings “we’ll learn more about the true impact” of this intervention, Dr. Piazza said in an interview.
“The increased mortality in the intervention group may have been due to differential treatment and decision-making and confounding and warrants further investigation,” commented Elaine M. Hylek, MD, a professor at Boston University and designated discussant for the report. Selection bias may have contributed to this possible confounding, Dr. Hylek noted.
Other limitations of the study cited by Dr. Hylek included its reliance on individual clinician decision-making to actually prescribe thromboprophylaxis, a lack of information on patient adherence to their thromboprophylaxis prescription, and an overall low rate of appropriate thromboprophylaxis prescribed to patients at discharge. The rates were 7.5% among the controls and 13.6% among patients in the intervention arm. For prescription at the time of hospitalization, the rates were 72.5% among control patients and 80.1% for patients seen at the two hospitals that used the decision-support tool.
The IMPROVE-DD VTE risk assessment tool
The clinical decision-support tool tested is called the IMPROVE-DD VTE risk assessment model, developed over several years by Dr. Spyropoulos and associates; they have also performed multiple validation studies. The model includes eight factors that score 1-3 points if positive that can add up to total scores of 0-14. A score of 0 or 1 is considered low risk, 2 or 3 intermediate risk, and 4 or more high risk. One of the scoring factors is the result of a D-dimer test, which explains the DD part of the name.
The eight factors and point assignments are prior venous thromboembolism: 3 points; known thrombophilia: 2 points; lower limb paralysis: 2 points; current cancer: 2 points; d-dimer level more than twofold the upper limit of normal: 2 points; immobilized for at least 7 days: 1 point; admitted to the ICU or coronary care unit: 1 point; and age greater than 60 years old: 1 point.
Development of the IMPROVE-DD VTE risk calculator received most of its funding from the U.S. Agency for Healthcare Research and Quality, and the risk tool will be available for hospitals and health systems to access at no charge through the agency’s website, Dr. Spyropoulos said. The researchers designed the calculator to operate in any EHR product.
IMPROVE-DD VTE “is a very valid, high-quality tool,” commented Dr. Piazza. “We’ve used some rather blunt tools in the past,” and especially praised inclusion of D-dimer results into the IMPROVE-DD VTE model.
“It’s nice to use a biomarker in addition to clinical factors,” he said. “A biomarker provides a more holistic picture; we can’t do genetic testing on every patient.”
Enrollment focused on higher-risk patients
The study ran at four academic, tertiary-care hospitals in the Northwell Health network in the New York region. It enrolled patients aged more than 60 years who were hospitalized for any of five diagnoses: heart failure; acute respiratory insufficiency, including chronic obstructive lung disease or asthma; acute infectious disease, including COVID-19; acute inflammatory disease, including rheumatic disease; or acute stroke. The study excluded patients with a history of atrial fibrillation, those who used an anticoagulant at home, or those who had received therapeutic anticoagulation within 24 hours of their hospital admission.
The anticoagulant prophylaxis that patients received depended on their calculated risk level – intermediate or high – and whether they were inpatients or being discharged. The anticoagulants that clinicians could prescribe included unfractionated heparin, enoxaparin, fondaparinux, rivaroxaban, and apixaban.
“We’ve been looking for a long time for a tool for medically ill patients that’s like the CHA2DS2-VASc score” for patients with atrial fibrillation. “These powerful data say we now have this, and the EHR provides a vehicle to easily implement it,” Dr. Spyropoulos said.
The IMPROVE-DD VTE study received partial funding from Janssen. Dr. Spyropoulos has been a consultant to Nayer, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen, Pfizer, and Sanofi; adviser to the ATLAS Group; and has received research support from Janssen. Dr. Piazza has received research funding from Bayer, BIG/EKOS, BMS, Janssen, and Portola. Dr. Hylek had been a consultant to Bayer and Ionis, and has received honoraria from Boehringer Ingelheim and Pfizer.
AT AHA 2022
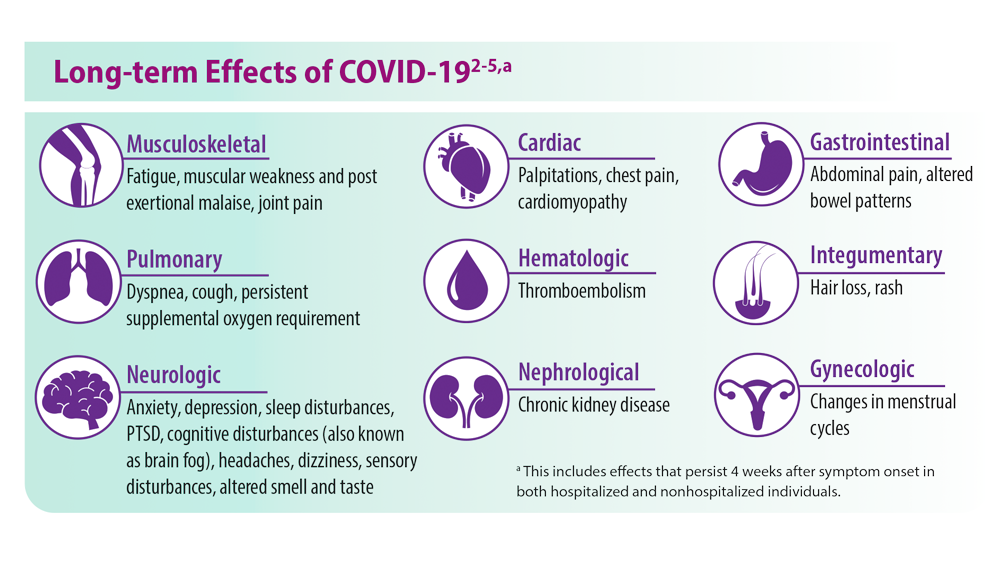
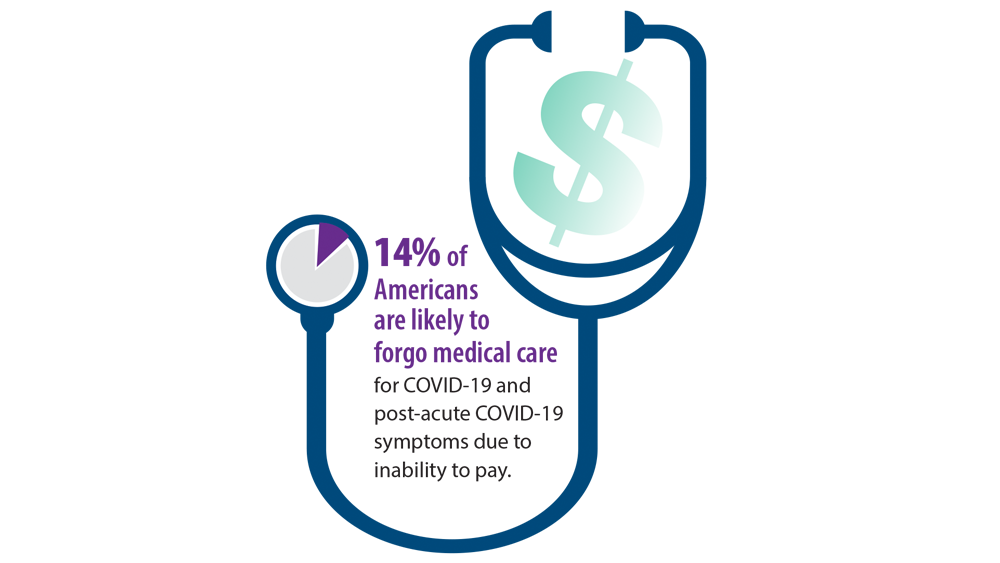
Post-COVID-19 Effects
- Centers for Disease Control and Prevention. COVID data tracker. Updated August 19, 2022. Accessed August 22, 2022. https://covid.cdc.gov/covid-data-tracker
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi:10.1038/s41591-021-01283-z
- Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated May 5, 2022. Accessed June 6, 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-termeffects/index.html
- Ghazanfar H, Kandhi S, Shin D, et al. Impact of COVID-19 on the gastrointestinal tract: a clinical review. Cureus. 2022;14(3):e23333. doi:10.7759/cureus.23333
- Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226(2):270-273. doi:10.1016/j.ajog.2021.09.016
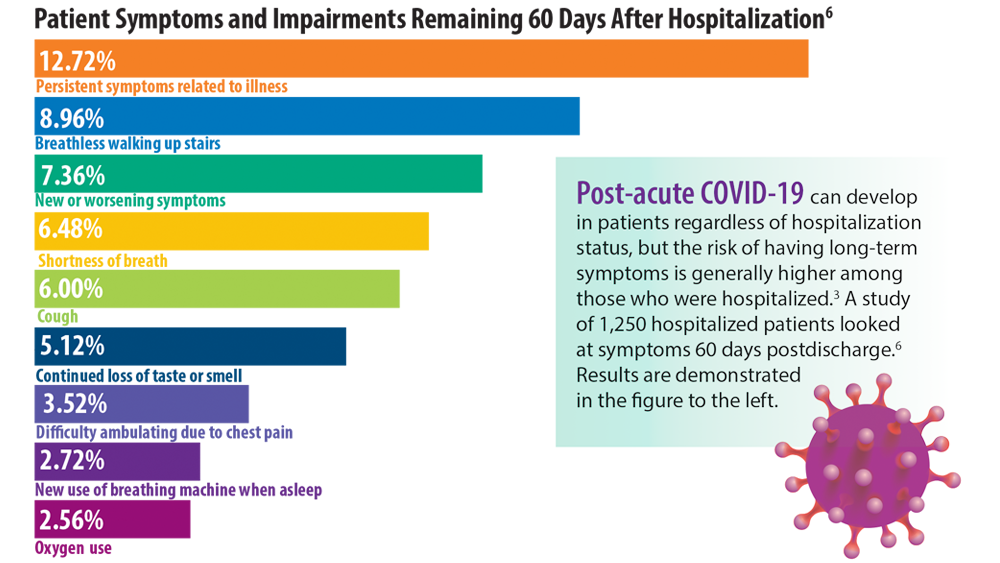
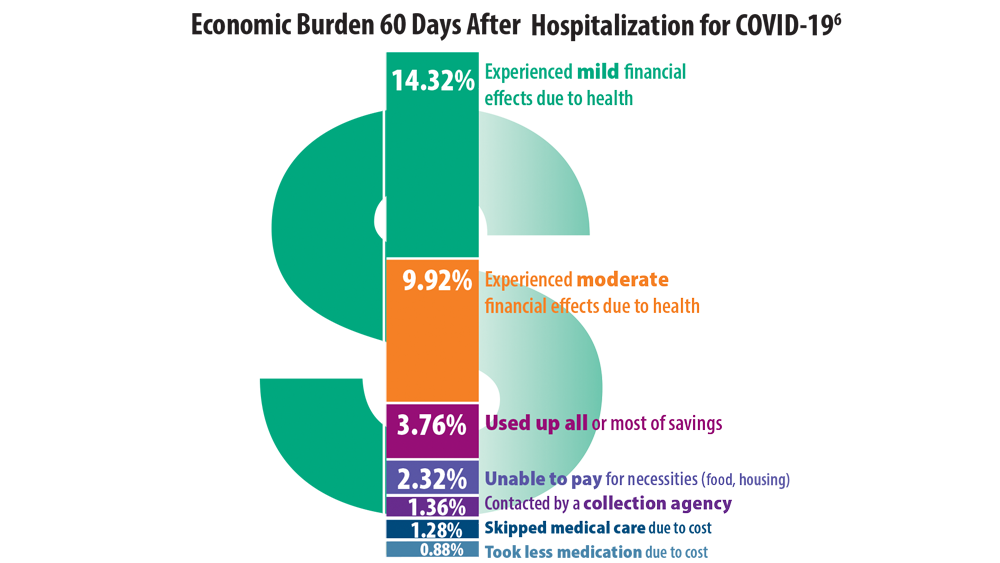
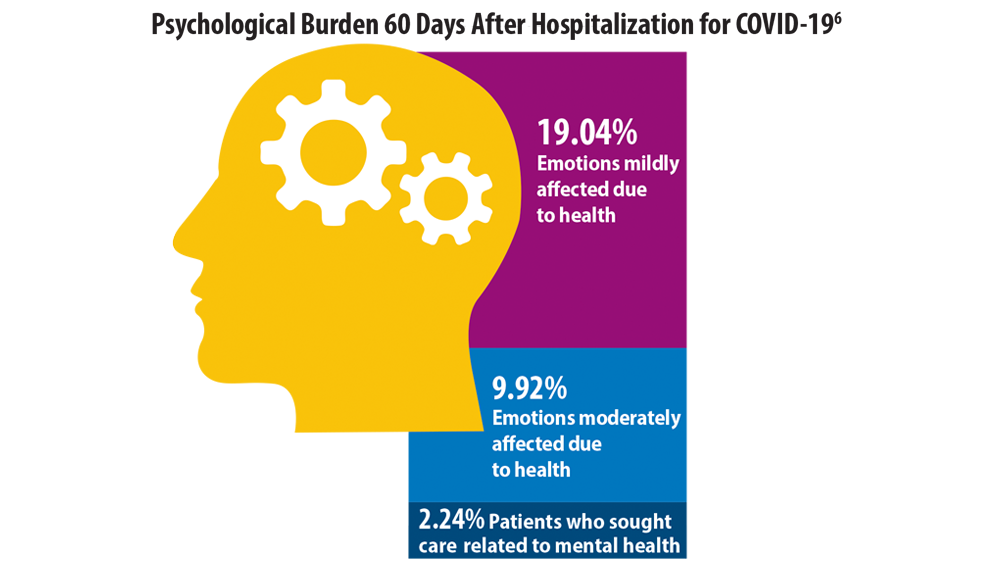
- Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi:10.7326/M20-5661
- Jiang DH, McCoy RG. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35(10):3036-3039. doi:10.1007/s11606-020-06042-3
- Centers for Disease Control and Prevention. COVID data tracker. Updated August 19, 2022. Accessed August 22, 2022. https://covid.cdc.gov/covid-data-tracker
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi:10.1038/s41591-021-01283-z
- Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated May 5, 2022. Accessed June 6, 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-termeffects/index.html
- Ghazanfar H, Kandhi S, Shin D, et al. Impact of COVID-19 on the gastrointestinal tract: a clinical review. Cureus. 2022;14(3):e23333. doi:10.7759/cureus.23333
- Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226(2):270-273. doi:10.1016/j.ajog.2021.09.016
- Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi:10.7326/M20-5661
- Jiang DH, McCoy RG. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35(10):3036-3039. doi:10.1007/s11606-020-06042-3
- Centers for Disease Control and Prevention. COVID data tracker. Updated August 19, 2022. Accessed August 22, 2022. https://covid.cdc.gov/covid-data-tracker
- Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27(4):601-615. doi:10.1038/s41591-021-01283-z
- Centers for Disease Control and Prevention. Long COVID or post-COVID conditions. Updated May 5, 2022. Accessed June 6, 2022. https://www.cdc.gov/coronavirus/2019-ncov/long-termeffects/index.html
- Ghazanfar H, Kandhi S, Shin D, et al. Impact of COVID-19 on the gastrointestinal tract: a clinical review. Cureus. 2022;14(3):e23333. doi:10.7759/cureus.23333
- Khan SM, Shilen A, Heslin KM, et al. SARS-CoV-2 infection and subsequent changes in the menstrual cycle among participants in the Arizona CoVHORT study. Am J Obstet Gynecol. 2022;226(2):270-273. doi:10.1016/j.ajog.2021.09.016
- Chopra V, Flanders SA, O’Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174(4):576-578. doi:10.7326/M20-5661
- Jiang DH, McCoy RG. Planning for the post-COVID syndrome: how payers can mitigate long-term complications of the pandemic. J Gen Intern Med. 2020;35(10):3036-3039. doi:10.1007/s11606-020-06042-3
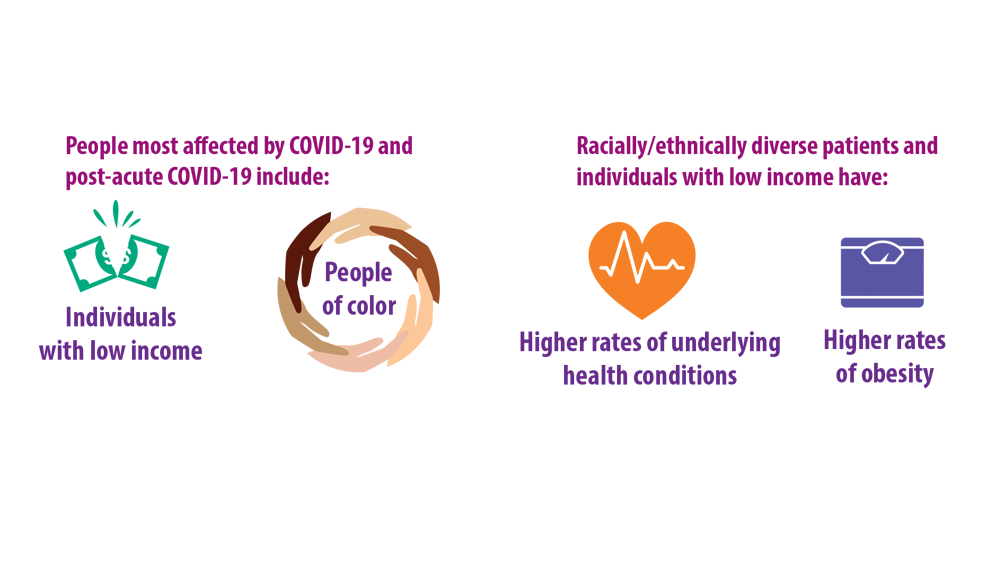
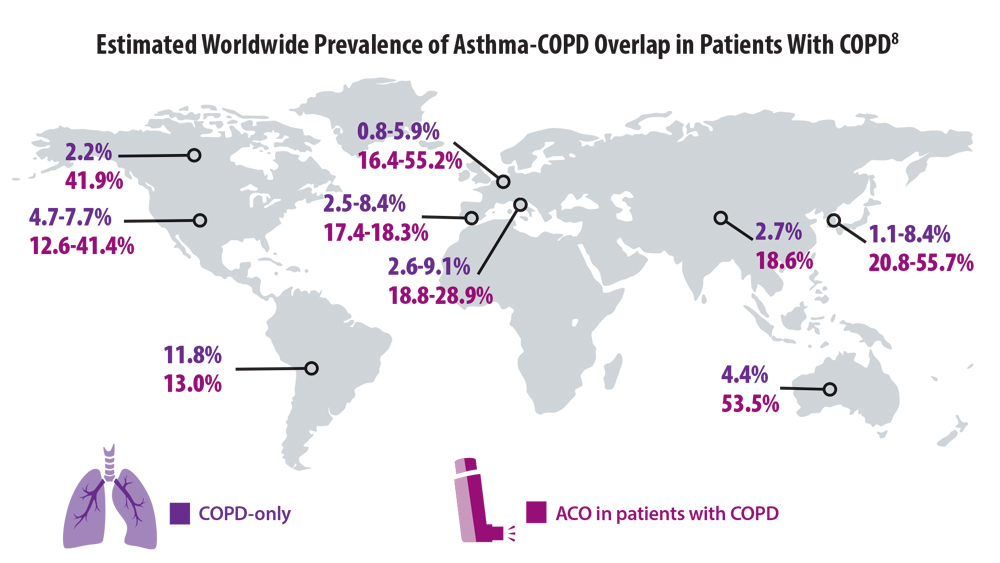
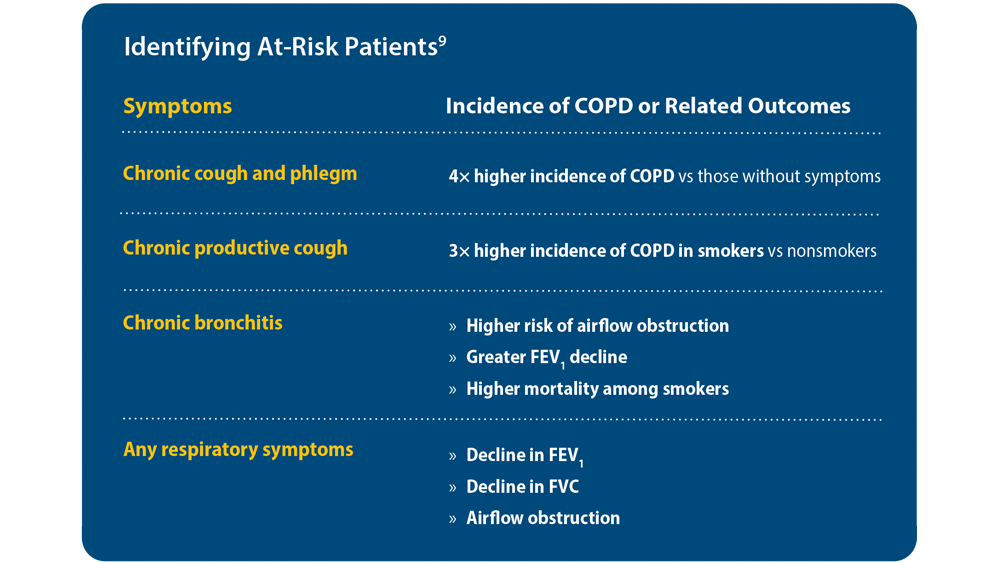
COPD Characteristics and Health Disparities
1. Chronic obstructive pulmonary disease (COPD). Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed May 30, 2022. https://www.cdc.gov/copd/index.html
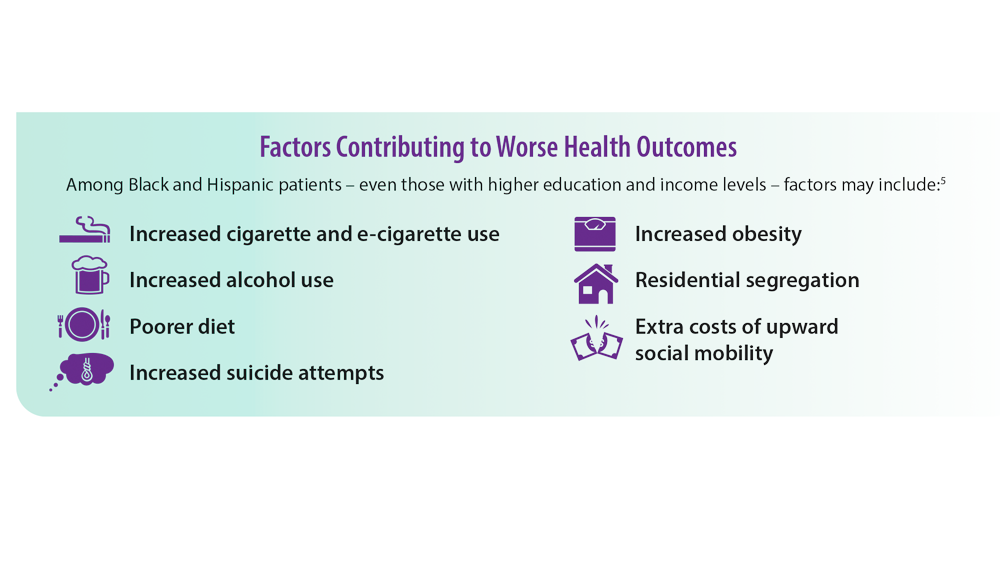
2. Stellefson M, Wang MQ, Kinder C. Racial disparities in health risk indicators reported by Alabamians diagnosed with COPD. Int J Environ Res Public Health. 2021;18(18):9662. doi:10.3390/ ijerph18189662
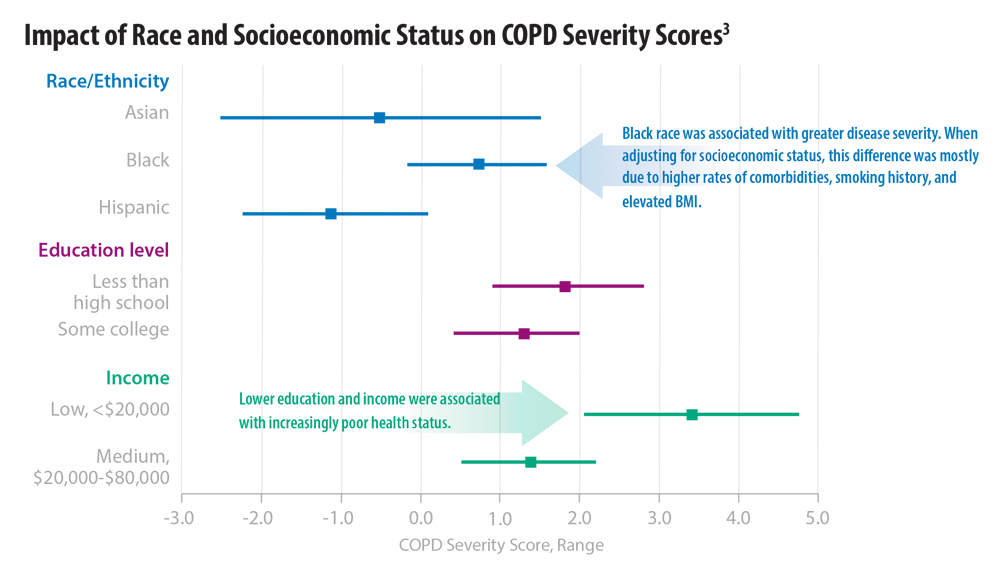
3. Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65(1):26-34. doi:10.1136/jech.2009.089722
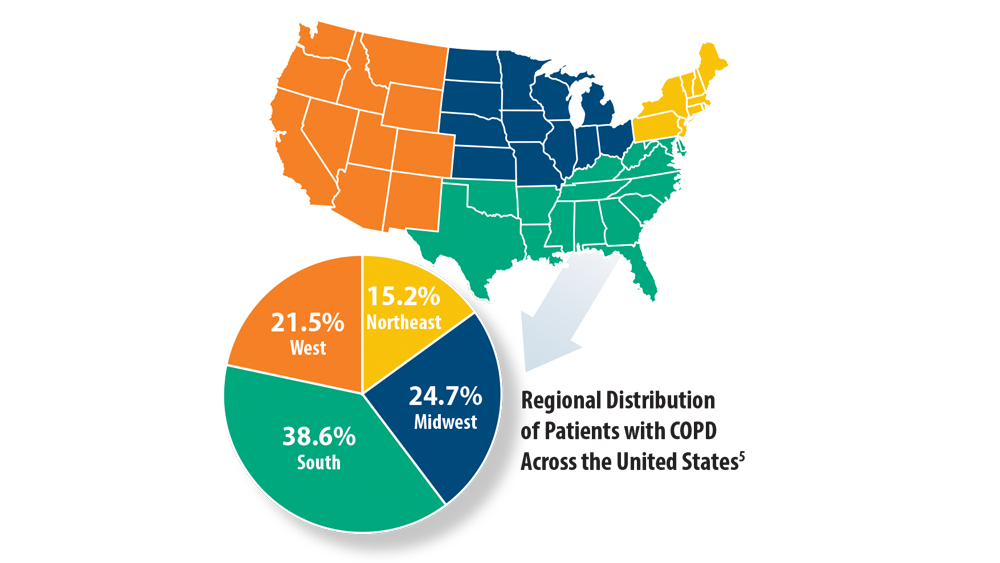
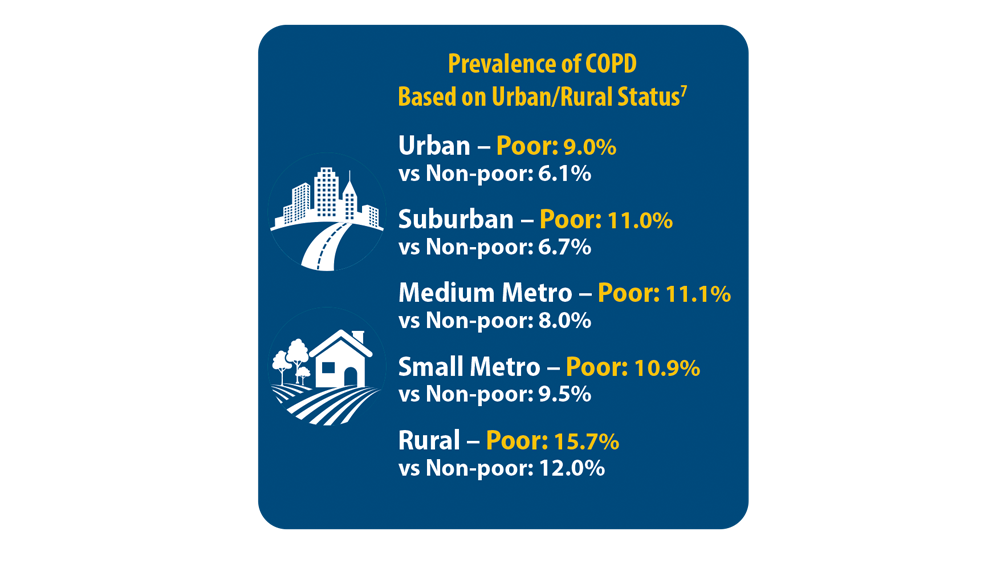
4. Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease – United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(7):205- 211. doi:10.15585/mmwr.mm6707a1
5. Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
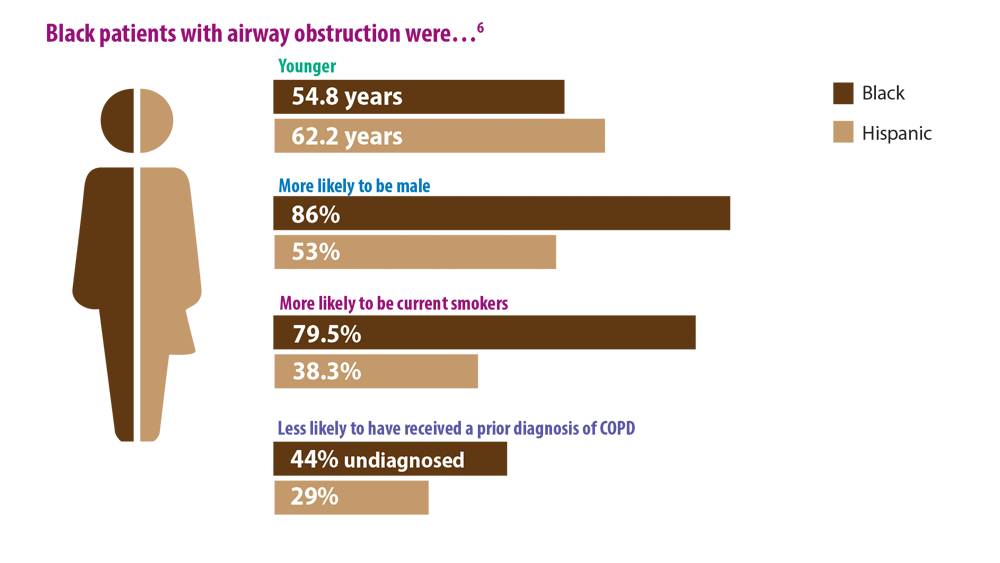
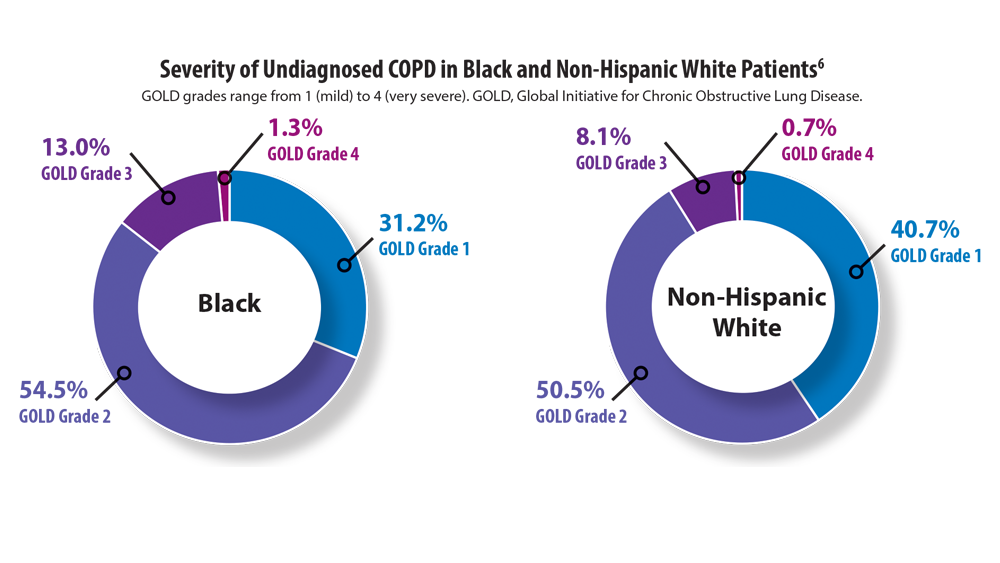
6. Mamary AJ, Stewart JI, Kinney GL, et al. Race and gender disparities are evident in COPD underdiagnoses across all severities of measured airflow obstruction. Chronic Obstr Pulm Dis. 2018;5(3):177-184. doi:10.15326/jcopdf.5.3.2017.0145
7. Woo H, Brigham EP, Allbright K, et al. Racial segregation and respiratory outcomes among urban black residents with and at risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;204(5):536-545. doi:10.1164/rccm.202009- 3721OC
8. Hosseini M, Almasi-Hashiani A, Sepidarkish M, Maroufizadeh S. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and meta-analysis. Respir Res. 2019;20(1):229. doi:10.1186/s12931-019-1198-4
9. Han MK, Agusti A, Celli BR, et al. From GOLD 0 to pre-COPD. Am J Respir Crit Care Med. 2021;203(4):414-423. doi:10.1164/ rccm.202008-3328PP
1. Chronic obstructive pulmonary disease (COPD). Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed May 30, 2022. https://www.cdc.gov/copd/index.html
2. Stellefson M, Wang MQ, Kinder C. Racial disparities in health risk indicators reported by Alabamians diagnosed with COPD. Int J Environ Res Public Health. 2021;18(18):9662. doi:10.3390/ ijerph18189662
3. Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65(1):26-34. doi:10.1136/jech.2009.089722
4. Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease – United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(7):205- 211. doi:10.15585/mmwr.mm6707a1
5. Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
6. Mamary AJ, Stewart JI, Kinney GL, et al. Race and gender disparities are evident in COPD underdiagnoses across all severities of measured airflow obstruction. Chronic Obstr Pulm Dis. 2018;5(3):177-184. doi:10.15326/jcopdf.5.3.2017.0145
7. Woo H, Brigham EP, Allbright K, et al. Racial segregation and respiratory outcomes among urban black residents with and at risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;204(5):536-545. doi:10.1164/rccm.202009- 3721OC
8. Hosseini M, Almasi-Hashiani A, Sepidarkish M, Maroufizadeh S. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and meta-analysis. Respir Res. 2019;20(1):229. doi:10.1186/s12931-019-1198-4
9. Han MK, Agusti A, Celli BR, et al. From GOLD 0 to pre-COPD. Am J Respir Crit Care Med. 2021;203(4):414-423. doi:10.1164/ rccm.202008-3328PP
1. Chronic obstructive pulmonary disease (COPD). Centers for Disease Control and Prevention. Updated February 22, 2021. Accessed May 30, 2022. https://www.cdc.gov/copd/index.html
2. Stellefson M, Wang MQ, Kinder C. Racial disparities in health risk indicators reported by Alabamians diagnosed with COPD. Int J Environ Res Public Health. 2021;18(18):9662. doi:10.3390/ ijerph18189662
3. Eisner MD, Blanc PD, Omachi TA, et al. Socioeconomic status, race and COPD health outcomes. J Epidemiol Community Health. 2011;65(1):26-34. doi:10.1136/jech.2009.089722
4. Croft JB, Wheaton AG, Liu Y, et al. Urban-rural county and state differences in chronic obstructive pulmonary disease – United States, 2015. MMWR Morb Mortal Wkly Rep. 2018;67(7):205- 211. doi:10.15585/mmwr.mm6707a1
5. Assari S, Chalian H, Bazargan M. Race, ethnicity, socioeconomic status, and chronic lung disease in the U.S. Res Health Sci. 2020;5(1):48-63. doi:10.22158/rhs.v5n1p48
6. Mamary AJ, Stewart JI, Kinney GL, et al. Race and gender disparities are evident in COPD underdiagnoses across all severities of measured airflow obstruction. Chronic Obstr Pulm Dis. 2018;5(3):177-184. doi:10.15326/jcopdf.5.3.2017.0145
7. Woo H, Brigham EP, Allbright K, et al. Racial segregation and respiratory outcomes among urban black residents with and at risk of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2021;204(5):536-545. doi:10.1164/rccm.202009- 3721OC
8. Hosseini M, Almasi-Hashiani A, Sepidarkish M, Maroufizadeh S. Global prevalence of asthma-COPD overlap (ACO) in the general population: a systematic review and meta-analysis. Respir Res. 2019;20(1):229. doi:10.1186/s12931-019-1198-4
9. Han MK, Agusti A, Celli BR, et al. From GOLD 0 to pre-COPD. Am J Respir Crit Care Med. 2021;203(4):414-423. doi:10.1164/ rccm.202008-3328PP
New Treatment Pathways for Cystic Fibrosis
- Cystic Fibrosis Foundation. What is cystic fibrosis? https://www. cff.org/intro-cf/about-cystic-fibrosis. Accessed June 17, 2022.
- Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809-1819. doi:10.1056/NEJMoa1908639
- McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol. 2021;56(6):1496-1503. doi:10.1002/ppul.25285
- O’Connor KE, Goodwin DL, NeSmith A, et al. Elexacaftor/ tezacaftor/ivacaftor resolves subfertility in females with CF: a two center case series. J Cyst Fibros. 2021;20(3):399-401. doi:10.1016/j.jcf.2020.12.011
- Shteinberg M, Taylor-Cousar JL, Durieu I, Cohen-Cymberknoh M. Fertility and Pregnancy in Cystic Fibrosis. Chest. 2021;160(6):2051-2060. doi:10.1016/j.chest.2021.07.024
- Cystic Fibrosis Foundation. What is cystic fibrosis? https://www. cff.org/intro-cf/about-cystic-fibrosis. Accessed June 17, 2022.
- Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809-1819. doi:10.1056/NEJMoa1908639
- McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol. 2021;56(6):1496-1503. doi:10.1002/ppul.25285
- O’Connor KE, Goodwin DL, NeSmith A, et al. Elexacaftor/ tezacaftor/ivacaftor resolves subfertility in females with CF: a two center case series. J Cyst Fibros. 2021;20(3):399-401. doi:10.1016/j.jcf.2020.12.011
- Shteinberg M, Taylor-Cousar JL, Durieu I, Cohen-Cymberknoh M. Fertility and Pregnancy in Cystic Fibrosis. Chest. 2021;160(6):2051-2060. doi:10.1016/j.chest.2021.07.024
- Cystic Fibrosis Foundation. What is cystic fibrosis? https://www. cff.org/intro-cf/about-cystic-fibrosis. Accessed June 17, 2022.
- Middleton PG, Mall MA, Dřevínek P, et al. Elexacaftor-tezacaftor-ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 2019;381(19):1809-1819. doi:10.1056/NEJMoa1908639
- McGarry ME, McColley SA. Cystic fibrosis patients of minority race and ethnicity less likely eligible for CFTR modulators based on CFTR genotype. Pediatr Pulmonol. 2021;56(6):1496-1503. doi:10.1002/ppul.25285
- O’Connor KE, Goodwin DL, NeSmith A, et al. Elexacaftor/ tezacaftor/ivacaftor resolves subfertility in females with CF: a two center case series. J Cyst Fibros. 2021;20(3):399-401. doi:10.1016/j.jcf.2020.12.011
- Shteinberg M, Taylor-Cousar JL, Durieu I, Cohen-Cymberknoh M. Fertility and Pregnancy in Cystic Fibrosis. Chest. 2021;160(6):2051-2060. doi:10.1016/j.chest.2021.07.024