User login
DLBCL patients at academic centers had significantly better survival
Researchers used the U.S. National Cancer Database to identify patients with a diagnosis of DLBCL from 2004 to 2015. The researchers identified 27,690 patients for the study. The majority of the patients were white (89.3%) and men (53.7%), with an average age of 64 years. A total of 57.6% of the patients had been treated at nonacademic centers and 42.4% at academic centers, and no notable differences were seen in facility choice among the low- to high-risk International Prognostic Index (IPI) risk categories.
The researchers found that overall survival of the DLBCL patients at academic centers was 108.3 months versus 74.5 months at nonacademic centers (P < .001), according to the study published in Clinical Lymphoma, Myeloma and Leukemia.
In addition, the median survival for patients with high-risk disease treated at academic centers was more than twice that of high-risk patients treated at nonacademic centers (33.5 months vs. 14.4 months, respectively; P < .001). Although the median survival for the other risk categories was also improved, the difference was less pronounced in the groups with lower IPI scores, according to the researchers.
Long-term overall survival for all patients with DLBCL at academic centers was significantly improved at both 5 and 10 years (59% and 43% survival, respectively) compared with those patients treated at nonacademic centers (51% and 35% survival, respectively; P < .001).
Speculating on factors that might be involved in this discrepancy in survival, the researchers suggested that academic centers might provide increased access to clinical trials, improved physician expertise, as well as improved treatment facilities and supportive care.
“Our results should prompt further investigation in precisely determining the factors that might support this significant effect on decreased survival among those treated in the community and help ameliorate this discrepancy,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ermann DA et al. Clin Lymphoma Myeloma Leuk. 2020;20(4): e17483.
Researchers used the U.S. National Cancer Database to identify patients with a diagnosis of DLBCL from 2004 to 2015. The researchers identified 27,690 patients for the study. The majority of the patients were white (89.3%) and men (53.7%), with an average age of 64 years. A total of 57.6% of the patients had been treated at nonacademic centers and 42.4% at academic centers, and no notable differences were seen in facility choice among the low- to high-risk International Prognostic Index (IPI) risk categories.
The researchers found that overall survival of the DLBCL patients at academic centers was 108.3 months versus 74.5 months at nonacademic centers (P < .001), according to the study published in Clinical Lymphoma, Myeloma and Leukemia.
In addition, the median survival for patients with high-risk disease treated at academic centers was more than twice that of high-risk patients treated at nonacademic centers (33.5 months vs. 14.4 months, respectively; P < .001). Although the median survival for the other risk categories was also improved, the difference was less pronounced in the groups with lower IPI scores, according to the researchers.
Long-term overall survival for all patients with DLBCL at academic centers was significantly improved at both 5 and 10 years (59% and 43% survival, respectively) compared with those patients treated at nonacademic centers (51% and 35% survival, respectively; P < .001).
Speculating on factors that might be involved in this discrepancy in survival, the researchers suggested that academic centers might provide increased access to clinical trials, improved physician expertise, as well as improved treatment facilities and supportive care.
“Our results should prompt further investigation in precisely determining the factors that might support this significant effect on decreased survival among those treated in the community and help ameliorate this discrepancy,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ermann DA et al. Clin Lymphoma Myeloma Leuk. 2020;20(4): e17483.
Researchers used the U.S. National Cancer Database to identify patients with a diagnosis of DLBCL from 2004 to 2015. The researchers identified 27,690 patients for the study. The majority of the patients were white (89.3%) and men (53.7%), with an average age of 64 years. A total of 57.6% of the patients had been treated at nonacademic centers and 42.4% at academic centers, and no notable differences were seen in facility choice among the low- to high-risk International Prognostic Index (IPI) risk categories.
The researchers found that overall survival of the DLBCL patients at academic centers was 108.3 months versus 74.5 months at nonacademic centers (P < .001), according to the study published in Clinical Lymphoma, Myeloma and Leukemia.
In addition, the median survival for patients with high-risk disease treated at academic centers was more than twice that of high-risk patients treated at nonacademic centers (33.5 months vs. 14.4 months, respectively; P < .001). Although the median survival for the other risk categories was also improved, the difference was less pronounced in the groups with lower IPI scores, according to the researchers.
Long-term overall survival for all patients with DLBCL at academic centers was significantly improved at both 5 and 10 years (59% and 43% survival, respectively) compared with those patients treated at nonacademic centers (51% and 35% survival, respectively; P < .001).
Speculating on factors that might be involved in this discrepancy in survival, the researchers suggested that academic centers might provide increased access to clinical trials, improved physician expertise, as well as improved treatment facilities and supportive care.
“Our results should prompt further investigation in precisely determining the factors that might support this significant effect on decreased survival among those treated in the community and help ameliorate this discrepancy,” the researchers concluded.
The authors reported that they had no conflicts of interest.
SOURCE: Ermann DA et al. Clin Lymphoma Myeloma Leuk. 2020;20(4): e17483.
FROM CLINICAL LYMPHOMA, MYELOMA AND LEUKEMIA
Interleukin-27 increased cytotoxic effects of bone marrow NK cells in CLL
Chronic lymphocytic leukemia is characterized by significant immune perturbation, including significant impairment of natural killer (NK) cells, which leads to disease complications and reduced effectiveness of treatment.
However, the use of , according to an in vitro study conducted by Maral Hemati, a student researcher at the Semnan (Iran) University of Medical Sciences, and colleagues.
Ms. Hemati and her colleagues obtained bone marrow aspirates (BM) and peripheral blood samples (PB) were from 12 untreated CLL patients (9 men and 3 women) with a median age of 61 years. The cells were cultured in vitro, according to their report in International Immunopharmacology.
The researchers found that the use of recombinant human interleukin-27 (IL-27) stimulated NK cells in the cultured BM and PB cells of CLL patients, based upon assessment using cell surface flow cytometry and a cytotoxicity assay.
Treatment with IL-27 also increased CD69 (a marker for NK cell activity) on NK cells both in BM and PB. In addition, , whereas it did not improve NK cell activity of PB, according to the researchers.
The research was supported by Semnan (Iran) University of Medical Sciences. The authors reported that they had no conflicts of interest.
SOURCE: Hemati M et al. Int Immunopharmacol. 2020;82:doi.org/10.1016/j.intimp.2020.106350.
Chronic lymphocytic leukemia is characterized by significant immune perturbation, including significant impairment of natural killer (NK) cells, which leads to disease complications and reduced effectiveness of treatment.
However, the use of , according to an in vitro study conducted by Maral Hemati, a student researcher at the Semnan (Iran) University of Medical Sciences, and colleagues.
Ms. Hemati and her colleagues obtained bone marrow aspirates (BM) and peripheral blood samples (PB) were from 12 untreated CLL patients (9 men and 3 women) with a median age of 61 years. The cells were cultured in vitro, according to their report in International Immunopharmacology.
The researchers found that the use of recombinant human interleukin-27 (IL-27) stimulated NK cells in the cultured BM and PB cells of CLL patients, based upon assessment using cell surface flow cytometry and a cytotoxicity assay.
Treatment with IL-27 also increased CD69 (a marker for NK cell activity) on NK cells both in BM and PB. In addition, , whereas it did not improve NK cell activity of PB, according to the researchers.
The research was supported by Semnan (Iran) University of Medical Sciences. The authors reported that they had no conflicts of interest.
SOURCE: Hemati M et al. Int Immunopharmacol. 2020;82:doi.org/10.1016/j.intimp.2020.106350.
Chronic lymphocytic leukemia is characterized by significant immune perturbation, including significant impairment of natural killer (NK) cells, which leads to disease complications and reduced effectiveness of treatment.
However, the use of , according to an in vitro study conducted by Maral Hemati, a student researcher at the Semnan (Iran) University of Medical Sciences, and colleagues.
Ms. Hemati and her colleagues obtained bone marrow aspirates (BM) and peripheral blood samples (PB) were from 12 untreated CLL patients (9 men and 3 women) with a median age of 61 years. The cells were cultured in vitro, according to their report in International Immunopharmacology.
The researchers found that the use of recombinant human interleukin-27 (IL-27) stimulated NK cells in the cultured BM and PB cells of CLL patients, based upon assessment using cell surface flow cytometry and a cytotoxicity assay.
Treatment with IL-27 also increased CD69 (a marker for NK cell activity) on NK cells both in BM and PB. In addition, , whereas it did not improve NK cell activity of PB, according to the researchers.
The research was supported by Semnan (Iran) University of Medical Sciences. The authors reported that they had no conflicts of interest.
SOURCE: Hemati M et al. Int Immunopharmacol. 2020;82:doi.org/10.1016/j.intimp.2020.106350.
FROM INTERNATIONAL IMMUNOPHARMACOLOGY
Researchers identify a cause of L-DOPA–induced dyskinesia in Parkinson’s disease
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
The conclusion is based on animal studies that were published May 1 in Science Advances. “These studies show that, if we can downregulate RasGRP1 signaling before dopamine replacement, we have an opportunity to greatly improve [patients’] quality of life,” said Srinivasa Subramaniam, PhD, of the department of neuroscience at Scripps Research in Jupiter, Fla., in a press release. Dr. Subramaniam is one of the investigators.
Parkinson’s disease results from the loss of substantia nigral projections neurons, which causes decreased levels of dopamine in the dorsal striatum. Treatment with L-DOPA reduces the disease’s motor symptoms effectively, but ultimately leads to the onset of LID. Previous data suggest that LID results from the abnormal activation of dopamine-1 (D1)–dependent cyclic adenosine 3´,5´-monophosphate (cAMP)/protein kinase A (PKA), extracellular signal–regulated kinase (ERK), and mammalian target of rapamycin kinase complex 1 (mTORC1) signaling in the dorsal striatum.
Animal and biochemical data
Based on earlier animal studies, Dr. Subramaniam and colleagues hypothesized that RasGRP1 might regulate LID. To test this theory, the investigators created lesions in wild-type and RasGRP1 knockout mice to create models of Parkinson’s disease. The investigators saw similar Parkinsonian symptoms in both groups of mice on the drag, rotarod, turning, and open-field tests. After all mice received daily treatment with L-DOPA, RasGRP1 knockout mice had significantly fewer abnormal involuntary movements, compared with the wild-type mice. All aspects of dyskinesia appeared to be equally dampened in the knockout mice.
To analyze whether RasGRP1 deletion affected the efficacy of L-DOPA, the investigators subjected the treated mice to motor tests. Parkinsonian symptoms were decreased among wild-type and knockout mice on the drag and turning tests. “RasGRP1 promoted the adverse effects of L-DOPA but did not interfere with its therapeutic motor effects,” the investigators wrote. Compared with the wild-type mice, the knockout mice had no changes in basal motor behavior or coordination or amphetamine-induced motor activity.
In addition, Dr. Subramaniam and colleagues observed that RasGRP1 levels were increased in the striatum after L-DOPA injection, but not after injection of vehicle control. This and other biochemical findings indicated that striatal RasGRP1 is upregulated in an L-DOPA–dependent manner and is causally linked to the development of LID, according to the investigators.
Other observations indicated that RasGRP1 physiologically activates mTORC1 signaling, which contributes to LID. Using liquid chromatography and mass spectrometry, Dr. Subramaniam and colleagues saw that RasGRP1 acts upstream in response to L-DOPA and regulates a specific and diverse group of proteins to promote LID. When they examined a nonhuman primate model of Parkinson’s disease, they noted similar findings.
New therapeutic targets
“There is an immediate need for new therapeutic targets to stop LID ... in Parkinson’s disease,” said Dr. Subramaniam in a press release. “The treatments now available work poorly and have many additional unwanted side effects. We believe this [study] represents an important step toward better options for people with Parkinson’s disease.”
Future research should attempt to identify the best method of selectively reducing expression of RasGRP1 in the striatum without affecting its expression in other areas of the body, according to Dr. Subramaniam. “The good news is that in mice a total lack of RasGRP1 is not lethal, so we think that blocking RasGRP1 with drugs, or even with gene therapy, may have very few or no major side effects.”
The study was funded by grants from the National Institutes of Health. The investigators reported no conflicts of interest.
SOURCE: Eshraghi M et al. Sci Adv. 2020;6:eaaz7001.
FROM Science Advances
Cardiovascular Effects of Tyrosine Kinase Inhibitors in Patients With Advanced Renal Cell Carcinoma at the VA San Diego Healthcare System (FULL)
Patients who have or are at high risk for developing cardiovascular disease and who are taking tyrosine kinase inhibitors for renal cell carcinoma should receive routine cardiovascular event monitoring during the first 4 months of therapy.
Targeted therapies have transformed the treatment of many malignant diseases by inhibiting molecular pathways involved in tumor growth and oncogenesis. Although these therapies can prevent disease progression, toxicities often result. Renal cell carcinoma (RCC) is one of many cancers that responds well to these therapies.
RCC accounts for 2% to 3% of all malignancies in adults worldwide. About 30% of patients with RCC present with metastatic or advanced disease.1 Cytokine therapy was the standard of care until multitargeted tyrosine kinase inhibitors (TKIs) were developed. Over the past 12 years, the US Food and Drug Administration (FDA) has approved 6 TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib, and sunitinib. Vascular endothelial growth factor receptor (VEGFR) is one of many tyrosine kinase receptors targeted by these medications. This mechanism prevents angiogenesis and consequently increases the risk for hypertension, bleeding, and clot formation.
Given these risks, many patients were excluded from the initial clinical trials of these medications if they had a history of uncontrolled hypertension, advanced heart failure (HF), or a significant cardiovascular (CV) event within 6 months prior to study enrollment. Many of these studies did not report the incidence of CV events (other than hypertension) that occurred during the early trials.2 The recommended monitoring for TKI therapies is focused mainly on blood pressure. For patients on pazopanib and sunitinib therapy, baseline and periodic electrocardiograms (ECGs) are recommended; echocardiograms are recommended only for patients with a history of cardiac disease.3,4 In patients on sorafenib therapy, ECG is recommended for those at risk for corrected QT (QTc) intervalprolongation.5
According to a meta-analysis of the literature published between 1966 and 2013,many studies reported a CV toxicity risk associated with the TKIs used in RCC treatment.6 However, some studies have found modest, not clinically significant changes in cardiac function in patients with advanced disease. In 2013, Hall and colleagues found 73% of patients they studied experienced some type of CV toxicity, whereas only 33% of patients had CV toxicity when hypertension was excluded.7 Interestingly, Rini and colleagues found that RCC patients receiving sunitinib had better response rates and progression-free survival when they developed hypertension compared with those who did not develop hypertension.8
A review of several studies revealed similar numbers in patients on TKI therapy presenting with symptomatic HF, but Hall and colleagues found that 27% of patients developed asymptomatic left ventricular dysfunction.7,9,10 These results suggest routine monitoring may allow for appropriate preventive interventions. In patients receiving TKI therapy, CV events, including QTc prolongation, left ventricular HF, myocardial infarction (MI), hypertension, pulmonary hypertension, and stroke, were commonly reported by investigators.7,9,10 Currently, there are no studies of the incidence of CV events for the 5 TKIs (axitinib, cabozantinib, pazopanib, sorafenib, sunitinib) in this patient population.
TKI therapy may require cardiac monitoring of all patients, as studies have associated TKIs with CV toxicity in varying degrees. Therefore, the authors set out to determine the incidence of CV events as well as time to first CV event in patients with and without a history of CV disease (CVD) who received a TKI for advanced RCC. More frequent monitoring for CV toxicity may present opportunities for clinical interventions for all patients on TKI therapy—especially for those with HF or other diseases in which the goal of therapy is to prevent disease progression. As TKIs have emerged as the standard treatment option for advanced RCC, many patients will continue therapy until disease progression or intolerable toxicity. Identifying and using appropriate monitoring parameters can lead to preventive interventions that allow patients to benefit from TKI therapy longer. At the US Department of Veterans Affairs (VA) San Diego Healthcare System (VASDHS), patients undergo routine cardiac monitoring at the discretion of the provider.
In this retrospective study, the authors wanted to determine the incidence of CV events in patients with and without a history of CVD who were receiving TKIs for advanced RCC. The authors also wanted to evaluate time to CV event from start of therapy in order to determine how often monitoring may be needed. The outcomes of this study may lead to a change in practice and development of monitoring parameters to ensure appropriate and adequate management of TKI therapy in RCC.
Methods
Each year, the VASDHS oncology team diagnose 5 to 10 patients with RCC who begin TKI therapy. When sorafenib was approved by the FDA in 2005, VASDHS estimated that about 100 of its patients had an RCC diagnosis and would be treated with a TKI between December 2005 and July 2017.
The authors identified VASDHS patients with a diagnosis of advanced RCC who received axitinib, cabozantinib, pazopanib, sorafenib, or sunitinib between December 1, 2005 and July 31, 2017. Patients were included if they had been on therapy for at least 30 days. The VASDHS pharmacy informatics team assisted in extracting a list of patients with an ICD-9 or ICD-10 diagnosis of RCC and using prescription fills for any of the 5 TKIs previously noted. Medical records were reviewed for frequency of prescription fills, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, TKI treatment duration, previous history of CVD, ethnicity, and smoking status. If documented, the incidence of CV events was reviewed for each patient at 0, 1, 3, 6, and 12 months. Patients who received medications (Appendix) for their CVD were assessed for adherence based on history of prescription refills from their medical records. Adherence was evaluated for the duration that patients were concurrently taking an oral TKI. The institutional review board at VASDHS approved the study design.
All patients included in this study started TKI therapy since the December 2005 FDA approval of sorafenib, the first oral TKI for treatment of RCC. Each new start was recorded as a separate event, regardless of previous oral TKI therapy. Albiges and colleagues found that the approximate median time from starting TKI therapy to complete response was 12.6 months, and the median duration of TKI therapy after complete response was 10.3 months.11 Based on these results, the follow-up period for patients in this study was 2 years after the start of each TKI therapy. For data analysis, patients were stratified by CVD history (yes or no). In addition, composite outcomes were evaluated to identify a potential cumulative increased risk for CV events for patients who had been on multiple TKI therapies.
For this study, CV toxicities were characterized using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03; severity of adverse events (AEs) was graded 1 to 5. CTCAE commonly has been used to assess AEs in oncology clinical trials. The CV AEs selected for this study included QTc prolongation, hypertension, left ventricular dysfunction, stroke, myocardial infarction (MI), and pulmonary arterial hypertension.
Primary outcomes included incidence of CV events and time to first CV event after initiation of TKI therapy. Secondary outcomes included changes in ECG or echocardiogram results at 0, 1, 3, 6, and 12 months. Secondary outcomes at scheduled time points were not readily available for every patient, but any available time points were gathered to aid in identifying an optimal period for cardiac monitoring. In addition, patients with a history of CVD were evaluated for adherence to common first-line therapies for each disease.
A Fischer exact test was used to compare the incidence of CV events in patients with and without a history of CVD (significance level, α = 0.05). A subgroup analysis was used to compare the incidence of CV events in patients who experienced a CV event (significance level, α = 0.05). A Kaplan-Meier survival curve was used to determine time to first CV event. A log-rank test with significance level set at α = 0.05 also was used.
Results
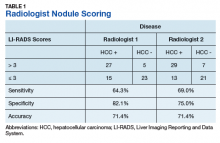
An initial database search identified 134 patients who received TKI therapy at VASDHS between December 1, 2005 and July 31, 2017. According to retrospective chart review, 54 patients met the inclusion criteria for the study (Table 1).
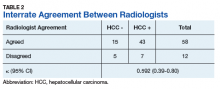
Patients without a history of CVD (17%) did not experience any CV events while on TKI therapy. Of the patients with a history of CVD, 9 (20%) experienced ≥ 1 CV event. Fifty-five percent of the events experienced were hypertension. One patient experienced QTc prolongation, and 2 patients experienced MI. As already noted, each new start of TKI was recorded as a separate event, regardless of previous TKI therapy. Among patients with a history of CVD, 2 experienced 2 CV events. Overall, 11 CV events occurred among patients who received ≥ 1 TKI, corresponding to an overall incidence of 24% (Table 2). 
Of the 13 patients who were exposed to ≥ 2 TKI therapies, 2 experienced a CV event. Both patients were started on sunitinib and were switched to sorafenib. One of these used sunitinib for 7 months, experienced a partial response and was switched to sorafenib (with a 3-month break between therapies). The second patient was on sunitinib for 24 months, with multiple doses held because of low blood counts and diarrhea. While on sunitinib, this patient experienced a HF exacerbation, determined to be caused by the underlying disease. This event occurred 17 months after sunitinib was started, and therapy was continued for another 7 months. The patient was switched to sorafenib because of poor tolerability and disease progression. While on sorafenib, this patient experienced grade 1 QTc prolongation.
Discussion
Of the available oral TKI therapies for RCC, sunitinib and sorafenib have the most data associated with nonhypertensive CV toxicity.2,7-10,12 Instudies, the percentage of patients who experienced CV toxicity while on sunitinib or sorafenib has ranged widely, from 2.7% to 33.8%; the variance may be attributable to differences in how institutions report CV toxicities.7-9
According to the prescribing information for TKIs, hypertension is frequently reported as an AE for all 5 TKIs, and BP monitoring is recommended.3,4 However, the development of hypertension with these TKIs has been associated with response to therapy.7 With pazopanib, sorafenib, and sunitinib, there is a higher incidence of other AEs: edema, HF, MI, and QTc prolongation. Baseline ECG is recommended for all patients started on pazopanib and sunitinib and for patients with a history of CVD who are started on sorafenib. An ECG is recommended for patients with a history of CVD who are started on pazopanib and sunitinib.
Even with the medication prescribing information recommendations, it is unclear how frequently patients should be monitored. At VASDHS, CV monitoring for any patient started on a TKI remains at the discretion of the oncologist. There are concerns that ordering cardiac monitoring tests, which might be unnecessary, will change or guide therapy. In this study, data evaluation revealed 1 patient who experienced a CV event had a CVD history that was not documented in the patient’s medical history. It is important that providers obtain a detailed clinical assessment of patients CV history during each visit to determine whether CV monitoring should be considered. Patients also may benefit from additional counseling to emphasize the importance of adherence to CV medication therapy to reduce the incidence of these events.
Data from this study indicate that routine CV monitoring should be considered in patients with CVD, in keeping with current medication prescribing information recommendations. Of the patients who had a CV event, 54% experienced hypertension, 18% MI, and 28% stroke, QTc prolongation, or congestive HF.
Limitations
This retrospective study had several limitations. Many patients did not have a baseline cardiac monitoring test or any monitoring during therapy. Often, a cardiac test was performed only when the patient was symptomatic or experiencing a CV event. In addition, because of intolerance or nonadherence to therapy, many patients discontinued treatment early, before completing 30 days. That axitinib and cabozantinib are newer therapies and not first-line at VASDHS during the data collection period accounts for the small number of patients on these therapies. Therapy was shorter for patients started on pazopanib, axitinib, and cabozantinib than it was for patients on sunitinib and sorafenib. Duration of therapy may affect treatment-related events, but the majority of patients in this study experienced an event within 4 months of therapy. About half of the patients who experienced an event were nonadherent to their CV medication regimen. Another potential limitation is that this study was conducted at VASDHS, where most patients are male (RCC incidence is 2:1 male:female).
Conclusion
In this study, CV events occurred in 24% of patients with a history of CVD; 11% of these events were nonhypertensive. Baseline cardiac monitoring was not performed for most patients started on TKI therapy, but tests were performed once patients became symptomatic. The study results suggest that high-risk patients should undergo routine cardiac monitoring during the first 4 months of TKI therapy, in keeping with medication package insert monitoring recommendations. Cardiac monitoring of high-risk patients will allow for earlier identification of cardiac decline and offer opportunities for interventions, such as pharmacist-driven protocols to start CV medications. Implementation of this study’s recommendations should be evaluated to determine whether outcomes improve with routine cardiac monitoring in these high-risk patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, FrontlineMedical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
1. Rini, BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
2. Tolcher AW, Appleman LJ, Shapiro GI, et al. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67(4):751-764.
3. Votrient [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
4. Sutent [package insert]. New York, NY: Pfizer Labs; 2018.
5. Nexavar [package insert]. Wayne, NJ; Bayer HealthCare Pharmaceuticals Inc; 2018.
6. Ghatalia P, Morgan CJ, Je Y, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228–237.
7. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72-78.
8. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763-773.
9. Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450-3456.
10. Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204-5212.
11. Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482-487.
12. Jang S, Zheng C, Tsai HT, et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016;122(1):124-130
13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
14. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. JACC. 2017;70(6):776-803.
15. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
16. O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC. 2013;61(4):e78-e140.
17. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228.
18. Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
Patients who have or are at high risk for developing cardiovascular disease and who are taking tyrosine kinase inhibitors for renal cell carcinoma should receive routine cardiovascular event monitoring during the first 4 months of therapy.
Patients who have or are at high risk for developing cardiovascular disease and who are taking tyrosine kinase inhibitors for renal cell carcinoma should receive routine cardiovascular event monitoring during the first 4 months of therapy.
Targeted therapies have transformed the treatment of many malignant diseases by inhibiting molecular pathways involved in tumor growth and oncogenesis. Although these therapies can prevent disease progression, toxicities often result. Renal cell carcinoma (RCC) is one of many cancers that responds well to these therapies.
RCC accounts for 2% to 3% of all malignancies in adults worldwide. About 30% of patients with RCC present with metastatic or advanced disease.1 Cytokine therapy was the standard of care until multitargeted tyrosine kinase inhibitors (TKIs) were developed. Over the past 12 years, the US Food and Drug Administration (FDA) has approved 6 TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib, and sunitinib. Vascular endothelial growth factor receptor (VEGFR) is one of many tyrosine kinase receptors targeted by these medications. This mechanism prevents angiogenesis and consequently increases the risk for hypertension, bleeding, and clot formation.
Given these risks, many patients were excluded from the initial clinical trials of these medications if they had a history of uncontrolled hypertension, advanced heart failure (HF), or a significant cardiovascular (CV) event within 6 months prior to study enrollment. Many of these studies did not report the incidence of CV events (other than hypertension) that occurred during the early trials.2 The recommended monitoring for TKI therapies is focused mainly on blood pressure. For patients on pazopanib and sunitinib therapy, baseline and periodic electrocardiograms (ECGs) are recommended; echocardiograms are recommended only for patients with a history of cardiac disease.3,4 In patients on sorafenib therapy, ECG is recommended for those at risk for corrected QT (QTc) intervalprolongation.5
According to a meta-analysis of the literature published between 1966 and 2013,many studies reported a CV toxicity risk associated with the TKIs used in RCC treatment.6 However, some studies have found modest, not clinically significant changes in cardiac function in patients with advanced disease. In 2013, Hall and colleagues found 73% of patients they studied experienced some type of CV toxicity, whereas only 33% of patients had CV toxicity when hypertension was excluded.7 Interestingly, Rini and colleagues found that RCC patients receiving sunitinib had better response rates and progression-free survival when they developed hypertension compared with those who did not develop hypertension.8
A review of several studies revealed similar numbers in patients on TKI therapy presenting with symptomatic HF, but Hall and colleagues found that 27% of patients developed asymptomatic left ventricular dysfunction.7,9,10 These results suggest routine monitoring may allow for appropriate preventive interventions. In patients receiving TKI therapy, CV events, including QTc prolongation, left ventricular HF, myocardial infarction (MI), hypertension, pulmonary hypertension, and stroke, were commonly reported by investigators.7,9,10 Currently, there are no studies of the incidence of CV events for the 5 TKIs (axitinib, cabozantinib, pazopanib, sorafenib, sunitinib) in this patient population.
TKI therapy may require cardiac monitoring of all patients, as studies have associated TKIs with CV toxicity in varying degrees. Therefore, the authors set out to determine the incidence of CV events as well as time to first CV event in patients with and without a history of CV disease (CVD) who received a TKI for advanced RCC. More frequent monitoring for CV toxicity may present opportunities for clinical interventions for all patients on TKI therapy—especially for those with HF or other diseases in which the goal of therapy is to prevent disease progression. As TKIs have emerged as the standard treatment option for advanced RCC, many patients will continue therapy until disease progression or intolerable toxicity. Identifying and using appropriate monitoring parameters can lead to preventive interventions that allow patients to benefit from TKI therapy longer. At the US Department of Veterans Affairs (VA) San Diego Healthcare System (VASDHS), patients undergo routine cardiac monitoring at the discretion of the provider.
In this retrospective study, the authors wanted to determine the incidence of CV events in patients with and without a history of CVD who were receiving TKIs for advanced RCC. The authors also wanted to evaluate time to CV event from start of therapy in order to determine how often monitoring may be needed. The outcomes of this study may lead to a change in practice and development of monitoring parameters to ensure appropriate and adequate management of TKI therapy in RCC.
Methods
Each year, the VASDHS oncology team diagnose 5 to 10 patients with RCC who begin TKI therapy. When sorafenib was approved by the FDA in 2005, VASDHS estimated that about 100 of its patients had an RCC diagnosis and would be treated with a TKI between December 2005 and July 2017.
The authors identified VASDHS patients with a diagnosis of advanced RCC who received axitinib, cabozantinib, pazopanib, sorafenib, or sunitinib between December 1, 2005 and July 31, 2017. Patients were included if they had been on therapy for at least 30 days. The VASDHS pharmacy informatics team assisted in extracting a list of patients with an ICD-9 or ICD-10 diagnosis of RCC and using prescription fills for any of the 5 TKIs previously noted. Medical records were reviewed for frequency of prescription fills, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, TKI treatment duration, previous history of CVD, ethnicity, and smoking status. If documented, the incidence of CV events was reviewed for each patient at 0, 1, 3, 6, and 12 months. Patients who received medications (Appendix) for their CVD were assessed for adherence based on history of prescription refills from their medical records. Adherence was evaluated for the duration that patients were concurrently taking an oral TKI. The institutional review board at VASDHS approved the study design.
All patients included in this study started TKI therapy since the December 2005 FDA approval of sorafenib, the first oral TKI for treatment of RCC. Each new start was recorded as a separate event, regardless of previous oral TKI therapy. Albiges and colleagues found that the approximate median time from starting TKI therapy to complete response was 12.6 months, and the median duration of TKI therapy after complete response was 10.3 months.11 Based on these results, the follow-up period for patients in this study was 2 years after the start of each TKI therapy. For data analysis, patients were stratified by CVD history (yes or no). In addition, composite outcomes were evaluated to identify a potential cumulative increased risk for CV events for patients who had been on multiple TKI therapies.
For this study, CV toxicities were characterized using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03; severity of adverse events (AEs) was graded 1 to 5. CTCAE commonly has been used to assess AEs in oncology clinical trials. The CV AEs selected for this study included QTc prolongation, hypertension, left ventricular dysfunction, stroke, myocardial infarction (MI), and pulmonary arterial hypertension.
Primary outcomes included incidence of CV events and time to first CV event after initiation of TKI therapy. Secondary outcomes included changes in ECG or echocardiogram results at 0, 1, 3, 6, and 12 months. Secondary outcomes at scheduled time points were not readily available for every patient, but any available time points were gathered to aid in identifying an optimal period for cardiac monitoring. In addition, patients with a history of CVD were evaluated for adherence to common first-line therapies for each disease.
A Fischer exact test was used to compare the incidence of CV events in patients with and without a history of CVD (significance level, α = 0.05). A subgroup analysis was used to compare the incidence of CV events in patients who experienced a CV event (significance level, α = 0.05). A Kaplan-Meier survival curve was used to determine time to first CV event. A log-rank test with significance level set at α = 0.05 also was used.
Results
An initial database search identified 134 patients who received TKI therapy at VASDHS between December 1, 2005 and July 31, 2017. According to retrospective chart review, 54 patients met the inclusion criteria for the study (Table 1).
Patients without a history of CVD (17%) did not experience any CV events while on TKI therapy. Of the patients with a history of CVD, 9 (20%) experienced ≥ 1 CV event. Fifty-five percent of the events experienced were hypertension. One patient experienced QTc prolongation, and 2 patients experienced MI. As already noted, each new start of TKI was recorded as a separate event, regardless of previous TKI therapy. Among patients with a history of CVD, 2 experienced 2 CV events. Overall, 11 CV events occurred among patients who received ≥ 1 TKI, corresponding to an overall incidence of 24% (Table 2). 
Of the 13 patients who were exposed to ≥ 2 TKI therapies, 2 experienced a CV event. Both patients were started on sunitinib and were switched to sorafenib. One of these used sunitinib for 7 months, experienced a partial response and was switched to sorafenib (with a 3-month break between therapies). The second patient was on sunitinib for 24 months, with multiple doses held because of low blood counts and diarrhea. While on sunitinib, this patient experienced a HF exacerbation, determined to be caused by the underlying disease. This event occurred 17 months after sunitinib was started, and therapy was continued for another 7 months. The patient was switched to sorafenib because of poor tolerability and disease progression. While on sorafenib, this patient experienced grade 1 QTc prolongation.
Discussion
Of the available oral TKI therapies for RCC, sunitinib and sorafenib have the most data associated with nonhypertensive CV toxicity.2,7-10,12 Instudies, the percentage of patients who experienced CV toxicity while on sunitinib or sorafenib has ranged widely, from 2.7% to 33.8%; the variance may be attributable to differences in how institutions report CV toxicities.7-9
According to the prescribing information for TKIs, hypertension is frequently reported as an AE for all 5 TKIs, and BP monitoring is recommended.3,4 However, the development of hypertension with these TKIs has been associated with response to therapy.7 With pazopanib, sorafenib, and sunitinib, there is a higher incidence of other AEs: edema, HF, MI, and QTc prolongation. Baseline ECG is recommended for all patients started on pazopanib and sunitinib and for patients with a history of CVD who are started on sorafenib. An ECG is recommended for patients with a history of CVD who are started on pazopanib and sunitinib.
Even with the medication prescribing information recommendations, it is unclear how frequently patients should be monitored. At VASDHS, CV monitoring for any patient started on a TKI remains at the discretion of the oncologist. There are concerns that ordering cardiac monitoring tests, which might be unnecessary, will change or guide therapy. In this study, data evaluation revealed 1 patient who experienced a CV event had a CVD history that was not documented in the patient’s medical history. It is important that providers obtain a detailed clinical assessment of patients CV history during each visit to determine whether CV monitoring should be considered. Patients also may benefit from additional counseling to emphasize the importance of adherence to CV medication therapy to reduce the incidence of these events.
Data from this study indicate that routine CV monitoring should be considered in patients with CVD, in keeping with current medication prescribing information recommendations. Of the patients who had a CV event, 54% experienced hypertension, 18% MI, and 28% stroke, QTc prolongation, or congestive HF.
Limitations
This retrospective study had several limitations. Many patients did not have a baseline cardiac monitoring test or any monitoring during therapy. Often, a cardiac test was performed only when the patient was symptomatic or experiencing a CV event. In addition, because of intolerance or nonadherence to therapy, many patients discontinued treatment early, before completing 30 days. That axitinib and cabozantinib are newer therapies and not first-line at VASDHS during the data collection period accounts for the small number of patients on these therapies. Therapy was shorter for patients started on pazopanib, axitinib, and cabozantinib than it was for patients on sunitinib and sorafenib. Duration of therapy may affect treatment-related events, but the majority of patients in this study experienced an event within 4 months of therapy. About half of the patients who experienced an event were nonadherent to their CV medication regimen. Another potential limitation is that this study was conducted at VASDHS, where most patients are male (RCC incidence is 2:1 male:female).
Conclusion
In this study, CV events occurred in 24% of patients with a history of CVD; 11% of these events were nonhypertensive. Baseline cardiac monitoring was not performed for most patients started on TKI therapy, but tests were performed once patients became symptomatic. The study results suggest that high-risk patients should undergo routine cardiac monitoring during the first 4 months of TKI therapy, in keeping with medication package insert monitoring recommendations. Cardiac monitoring of high-risk patients will allow for earlier identification of cardiac decline and offer opportunities for interventions, such as pharmacist-driven protocols to start CV medications. Implementation of this study’s recommendations should be evaluated to determine whether outcomes improve with routine cardiac monitoring in these high-risk patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, FrontlineMedical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
Targeted therapies have transformed the treatment of many malignant diseases by inhibiting molecular pathways involved in tumor growth and oncogenesis. Although these therapies can prevent disease progression, toxicities often result. Renal cell carcinoma (RCC) is one of many cancers that responds well to these therapies.
RCC accounts for 2% to 3% of all malignancies in adults worldwide. About 30% of patients with RCC present with metastatic or advanced disease.1 Cytokine therapy was the standard of care until multitargeted tyrosine kinase inhibitors (TKIs) were developed. Over the past 12 years, the US Food and Drug Administration (FDA) has approved 6 TKIs for the treatment of RCC: axitinib, cabozantinib, lenvatinib, pazopanib, sorafenib, and sunitinib. Vascular endothelial growth factor receptor (VEGFR) is one of many tyrosine kinase receptors targeted by these medications. This mechanism prevents angiogenesis and consequently increases the risk for hypertension, bleeding, and clot formation.
Given these risks, many patients were excluded from the initial clinical trials of these medications if they had a history of uncontrolled hypertension, advanced heart failure (HF), or a significant cardiovascular (CV) event within 6 months prior to study enrollment. Many of these studies did not report the incidence of CV events (other than hypertension) that occurred during the early trials.2 The recommended monitoring for TKI therapies is focused mainly on blood pressure. For patients on pazopanib and sunitinib therapy, baseline and periodic electrocardiograms (ECGs) are recommended; echocardiograms are recommended only for patients with a history of cardiac disease.3,4 In patients on sorafenib therapy, ECG is recommended for those at risk for corrected QT (QTc) intervalprolongation.5
According to a meta-analysis of the literature published between 1966 and 2013,many studies reported a CV toxicity risk associated with the TKIs used in RCC treatment.6 However, some studies have found modest, not clinically significant changes in cardiac function in patients with advanced disease. In 2013, Hall and colleagues found 73% of patients they studied experienced some type of CV toxicity, whereas only 33% of patients had CV toxicity when hypertension was excluded.7 Interestingly, Rini and colleagues found that RCC patients receiving sunitinib had better response rates and progression-free survival when they developed hypertension compared with those who did not develop hypertension.8
A review of several studies revealed similar numbers in patients on TKI therapy presenting with symptomatic HF, but Hall and colleagues found that 27% of patients developed asymptomatic left ventricular dysfunction.7,9,10 These results suggest routine monitoring may allow for appropriate preventive interventions. In patients receiving TKI therapy, CV events, including QTc prolongation, left ventricular HF, myocardial infarction (MI), hypertension, pulmonary hypertension, and stroke, were commonly reported by investigators.7,9,10 Currently, there are no studies of the incidence of CV events for the 5 TKIs (axitinib, cabozantinib, pazopanib, sorafenib, sunitinib) in this patient population.
TKI therapy may require cardiac monitoring of all patients, as studies have associated TKIs with CV toxicity in varying degrees. Therefore, the authors set out to determine the incidence of CV events as well as time to first CV event in patients with and without a history of CV disease (CVD) who received a TKI for advanced RCC. More frequent monitoring for CV toxicity may present opportunities for clinical interventions for all patients on TKI therapy—especially for those with HF or other diseases in which the goal of therapy is to prevent disease progression. As TKIs have emerged as the standard treatment option for advanced RCC, many patients will continue therapy until disease progression or intolerable toxicity. Identifying and using appropriate monitoring parameters can lead to preventive interventions that allow patients to benefit from TKI therapy longer. At the US Department of Veterans Affairs (VA) San Diego Healthcare System (VASDHS), patients undergo routine cardiac monitoring at the discretion of the provider.
In this retrospective study, the authors wanted to determine the incidence of CV events in patients with and without a history of CVD who were receiving TKIs for advanced RCC. The authors also wanted to evaluate time to CV event from start of therapy in order to determine how often monitoring may be needed. The outcomes of this study may lead to a change in practice and development of monitoring parameters to ensure appropriate and adequate management of TKI therapy in RCC.
Methods
Each year, the VASDHS oncology team diagnose 5 to 10 patients with RCC who begin TKI therapy. When sorafenib was approved by the FDA in 2005, VASDHS estimated that about 100 of its patients had an RCC diagnosis and would be treated with a TKI between December 2005 and July 2017.
The authors identified VASDHS patients with a diagnosis of advanced RCC who received axitinib, cabozantinib, pazopanib, sorafenib, or sunitinib between December 1, 2005 and July 31, 2017. Patients were included if they had been on therapy for at least 30 days. The VASDHS pharmacy informatics team assisted in extracting a list of patients with an ICD-9 or ICD-10 diagnosis of RCC and using prescription fills for any of the 5 TKIs previously noted. Medical records were reviewed for frequency of prescription fills, age, sex, Eastern Cooperative Oncology Group (ECOG) performance status, TKI treatment duration, previous history of CVD, ethnicity, and smoking status. If documented, the incidence of CV events was reviewed for each patient at 0, 1, 3, 6, and 12 months. Patients who received medications (Appendix) for their CVD were assessed for adherence based on history of prescription refills from their medical records. Adherence was evaluated for the duration that patients were concurrently taking an oral TKI. The institutional review board at VASDHS approved the study design.
All patients included in this study started TKI therapy since the December 2005 FDA approval of sorafenib, the first oral TKI for treatment of RCC. Each new start was recorded as a separate event, regardless of previous oral TKI therapy. Albiges and colleagues found that the approximate median time from starting TKI therapy to complete response was 12.6 months, and the median duration of TKI therapy after complete response was 10.3 months.11 Based on these results, the follow-up period for patients in this study was 2 years after the start of each TKI therapy. For data analysis, patients were stratified by CVD history (yes or no). In addition, composite outcomes were evaluated to identify a potential cumulative increased risk for CV events for patients who had been on multiple TKI therapies.
For this study, CV toxicities were characterized using Common Terminology Criteria for Adverse Events (CTCAE) version 4.03; severity of adverse events (AEs) was graded 1 to 5. CTCAE commonly has been used to assess AEs in oncology clinical trials. The CV AEs selected for this study included QTc prolongation, hypertension, left ventricular dysfunction, stroke, myocardial infarction (MI), and pulmonary arterial hypertension.
Primary outcomes included incidence of CV events and time to first CV event after initiation of TKI therapy. Secondary outcomes included changes in ECG or echocardiogram results at 0, 1, 3, 6, and 12 months. Secondary outcomes at scheduled time points were not readily available for every patient, but any available time points were gathered to aid in identifying an optimal period for cardiac monitoring. In addition, patients with a history of CVD were evaluated for adherence to common first-line therapies for each disease.
A Fischer exact test was used to compare the incidence of CV events in patients with and without a history of CVD (significance level, α = 0.05). A subgroup analysis was used to compare the incidence of CV events in patients who experienced a CV event (significance level, α = 0.05). A Kaplan-Meier survival curve was used to determine time to first CV event. A log-rank test with significance level set at α = 0.05 also was used.
Results
An initial database search identified 134 patients who received TKI therapy at VASDHS between December 1, 2005 and July 31, 2017. According to retrospective chart review, 54 patients met the inclusion criteria for the study (Table 1).
Patients without a history of CVD (17%) did not experience any CV events while on TKI therapy. Of the patients with a history of CVD, 9 (20%) experienced ≥ 1 CV event. Fifty-five percent of the events experienced were hypertension. One patient experienced QTc prolongation, and 2 patients experienced MI. As already noted, each new start of TKI was recorded as a separate event, regardless of previous TKI therapy. Among patients with a history of CVD, 2 experienced 2 CV events. Overall, 11 CV events occurred among patients who received ≥ 1 TKI, corresponding to an overall incidence of 24% (Table 2). 
Of the 13 patients who were exposed to ≥ 2 TKI therapies, 2 experienced a CV event. Both patients were started on sunitinib and were switched to sorafenib. One of these used sunitinib for 7 months, experienced a partial response and was switched to sorafenib (with a 3-month break between therapies). The second patient was on sunitinib for 24 months, with multiple doses held because of low blood counts and diarrhea. While on sunitinib, this patient experienced a HF exacerbation, determined to be caused by the underlying disease. This event occurred 17 months after sunitinib was started, and therapy was continued for another 7 months. The patient was switched to sorafenib because of poor tolerability and disease progression. While on sorafenib, this patient experienced grade 1 QTc prolongation.
Discussion
Of the available oral TKI therapies for RCC, sunitinib and sorafenib have the most data associated with nonhypertensive CV toxicity.2,7-10,12 Instudies, the percentage of patients who experienced CV toxicity while on sunitinib or sorafenib has ranged widely, from 2.7% to 33.8%; the variance may be attributable to differences in how institutions report CV toxicities.7-9
According to the prescribing information for TKIs, hypertension is frequently reported as an AE for all 5 TKIs, and BP monitoring is recommended.3,4 However, the development of hypertension with these TKIs has been associated with response to therapy.7 With pazopanib, sorafenib, and sunitinib, there is a higher incidence of other AEs: edema, HF, MI, and QTc prolongation. Baseline ECG is recommended for all patients started on pazopanib and sunitinib and for patients with a history of CVD who are started on sorafenib. An ECG is recommended for patients with a history of CVD who are started on pazopanib and sunitinib.
Even with the medication prescribing information recommendations, it is unclear how frequently patients should be monitored. At VASDHS, CV monitoring for any patient started on a TKI remains at the discretion of the oncologist. There are concerns that ordering cardiac monitoring tests, which might be unnecessary, will change or guide therapy. In this study, data evaluation revealed 1 patient who experienced a CV event had a CVD history that was not documented in the patient’s medical history. It is important that providers obtain a detailed clinical assessment of patients CV history during each visit to determine whether CV monitoring should be considered. Patients also may benefit from additional counseling to emphasize the importance of adherence to CV medication therapy to reduce the incidence of these events.
Data from this study indicate that routine CV monitoring should be considered in patients with CVD, in keeping with current medication prescribing information recommendations. Of the patients who had a CV event, 54% experienced hypertension, 18% MI, and 28% stroke, QTc prolongation, or congestive HF.
Limitations
This retrospective study had several limitations. Many patients did not have a baseline cardiac monitoring test or any monitoring during therapy. Often, a cardiac test was performed only when the patient was symptomatic or experiencing a CV event. In addition, because of intolerance or nonadherence to therapy, many patients discontinued treatment early, before completing 30 days. That axitinib and cabozantinib are newer therapies and not first-line at VASDHS during the data collection period accounts for the small number of patients on these therapies. Therapy was shorter for patients started on pazopanib, axitinib, and cabozantinib than it was for patients on sunitinib and sorafenib. Duration of therapy may affect treatment-related events, but the majority of patients in this study experienced an event within 4 months of therapy. About half of the patients who experienced an event were nonadherent to their CV medication regimen. Another potential limitation is that this study was conducted at VASDHS, where most patients are male (RCC incidence is 2:1 male:female).
Conclusion
In this study, CV events occurred in 24% of patients with a history of CVD; 11% of these events were nonhypertensive. Baseline cardiac monitoring was not performed for most patients started on TKI therapy, but tests were performed once patients became symptomatic. The study results suggest that high-risk patients should undergo routine cardiac monitoring during the first 4 months of TKI therapy, in keeping with medication package insert monitoring recommendations. Cardiac monitoring of high-risk patients will allow for earlier identification of cardiac decline and offer opportunities for interventions, such as pharmacist-driven protocols to start CV medications. Implementation of this study’s recommendations should be evaluated to determine whether outcomes improve with routine cardiac monitoring in these high-risk patients.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, FrontlineMedical Communications Inc., the US Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects— before administering pharmacologic therapy to patients.
1. Rini, BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
2. Tolcher AW, Appleman LJ, Shapiro GI, et al. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67(4):751-764.
3. Votrient [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
4. Sutent [package insert]. New York, NY: Pfizer Labs; 2018.
5. Nexavar [package insert]. Wayne, NJ; Bayer HealthCare Pharmaceuticals Inc; 2018.
6. Ghatalia P, Morgan CJ, Je Y, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228–237.
7. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72-78.
8. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763-773.
9. Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450-3456.
10. Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204-5212.
11. Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482-487.
12. Jang S, Zheng C, Tsai HT, et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016;122(1):124-130
13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
14. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. JACC. 2017;70(6):776-803.
15. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
16. O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC. 2013;61(4):e78-e140.
17. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228.
18. Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
1. Rini, BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931-1939.
2. Tolcher AW, Appleman LJ, Shapiro GI, et al. A phase I open-label study evaluating the cardiovascular safety of sorafenib in patients with advanced cancer. Cancer Chemother Pharmacol. 2011;67(4):751-764.
3. Votrient [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017.
4. Sutent [package insert]. New York, NY: Pfizer Labs; 2018.
5. Nexavar [package insert]. Wayne, NJ; Bayer HealthCare Pharmaceuticals Inc; 2018.
6. Ghatalia P, Morgan CJ, Je Y, et al. Congestive heart failure with vascular endothelial growth factor receptor tyrosine kinase inhibitors. Crit Rev Oncol Hematol 2015;94:228–237.
7. Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72-78.
8. Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763-773.
9. Richards CJ, Je Y, Schutz FA, et al. Incidence and risk of congestive heart failure in patients with renal and nonrenal cell carcinoma treated with sunitinib. J Clin Oncol. 2011;29(25):3450-3456.
10. Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2008;26(32):5204-5212.
11. Albiges L, Oudard S, Negrier S, et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol. 2012;30(5):482-487.
12. Jang S, Zheng C, Tsai HT, et al. Cardiovascular toxicity after antiangiogenic therapy in persons older than 65 years with advanced renal cell carcinoma. Cancer. 2016;122(1):124-130
13. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520.
14. Yancy CW, Jessup M, Bozkurt B, et al. ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. JACC. 2017;70(6):776-803.
15. Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236.
16. O’Gara PT, Kushner FG, Ascheim DD, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. JACC. 2013;61(4):e78-e140.
17. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64(24):e139-e228.
18. Galiè N, Humbert M, Vachiery JL, et al; ESC Scientific Document Group. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
Sharing Cancer Care Information Across VA Health Care Systems (FULL)
A telementoring program based on the Specialty Care Access Network Extension for Community Healthcare Outcomes model shared information about cancer care across VA health Care systems.
In 2016, the Cancer Care Coordinator at the US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACT) in West Haven partnered with the VA New England Healthcare System to use its telementoring program. The VA Specialty Care Access Network Extension for Community Healthcare Outcomes (VA ECHO) was used to present a series of educational conferences on cancer care. This article describes our experience implementing the program and reviews participant feedback gathered from voluntary surveys.
Background
In 2011, the Veterans Health Administration (VHA) Office of Healthcare Transformation launched VA ECHO, a telementoring program for primary care providers (PCPs) and patient-aligned care team staff. VACT was selected as 1 of 7 hub sites across the US. The VA ECHO system uses video and online technology to provide PCPs with case-based specialist consultation and didactic education. The system enables providers at any VA location to participate in online and telephone conferences in real time. The presentations are recorded and made available online to VA providers through a secure site.
VA ECHO is based on the highly successful Project ECHO model established by Sanjeev Arora and the University of New Mexico in 2007.1 The rationale for Project ECHO was that patient care could be improved by increasing the competence of PCPs in the management of complex diseases by providing access to disease specialists through a case-based learning approach that used technology, which it termed knowledge networks, to connect the PCPs to specialists.
The original model addressed management of hepatitis C in a medically underserved area where half of the population was widely geographically dispersed, making the provision of specialty care challenging. Developers identified 6 characteristics that make a disease appropriate for treatment using the Project ECHO knowledge network model:
- The disease is common;
- Management of the disease is complex;
- Treatment for the disease is evolving;
- The disease has a high societal impact;
- There are serious outcomes if the disease is not treated; and
- Disease management improves outcomes.1
VA ECHO conferences are available to all VA personnel. Staff can subscribe to an e-mail group list to be alerted to conference times and topics. Participants can connect directly to the conference using Microsoft Outlook Lync or Skype (Redmond, WA) and see the slides in real time on their computer as they listen to the presentation. The presentations are recorded, and the slides with audio can be accessed easily on the VA ECHO SharePoint site for download, enabling VA staff to listen to conferences at their convenience (Figure).
VA Cancer ECHO
The impetus to create a series of talks related to cancer care using VA ECHO was the frequent and often time-consuming requests we received from colleagues at other VA sites for information about areas of cancer care, such as survivorship and cancer care coordination. It was felt that presenting cancer care information as a VA ECHO series would make this information available to a large group of providers at one time, making the method more time effective than sharing the information via one-on-one conversations.
The cancer care coordinator originally conceived this as a 3-part, 1-time series to present work done at VACT in the areas of survivorship, psychosocial distress monitoring, and coordination of cancer care using the VA Cancer Care Tracking System, an online tracking tool. Information about the series was disseminated via VA group e-mail lists for oncology providers and via the existing VA ECHO subscriber invitation process. The 3-presentation series garnered positive feedback and had attendance that ranged from 49 to 75 participants (mean, 60). Participants expressed enthusiasm for the format via e-mail and phone feedback directly to the West Haven staff.
Expansion
The success of this original 3-part series led to a trial of an ongoing Cancer Care Conference series (Conference) using VA ECHO. This was a novel use of VA ECHO and was outside its traditional format, which is geared to discussion of individual cases and clinical knowledge. Nevertheless, this new style of communication has been embraced by a wide range of VA cancer care professionals.
One reason we considered expanding the program was that oncology fit the framework of the original Project ECHO knowledge network model. Cancer is common at the VA, which cares for 175,000 patients with cancer annually.2 The management of cancer is complex involving many disciplines working together, and treatments are constantly changing. In addition, cancer has a high societal impact; there are serious outcomes both in terms of patient survival and patient symptom burden. And lastly, outcomes are improved with proactive disease management that is informed by the most current, evidence-based medicine.
The Conference was conceived as a forum for providers across disciplines to share best practices and discuss common challenges in caring for veterans with cancer. We invited participants to submit proposals for presentations related to cancer care initiatives at their VA sites. Potential speakers across all areas of care for veterans with cancer were invited to submit possible topics for the conference. The submissions were reviewed by the moderators in an effort to create a series of talks on a variety of topics across all aspects of care for oncology patients in the VA. This process of effectively crowd-sourcing educational content inspires providers to think more creatively about their practice and quality improvement projects and has sparked an ongoing dialogue about quality initiatives among VA oncology providers across disciplines and geographic locations. As a result, this approach also has enabled participants to learn from colleagues who work at a wide range of rural and urban VA locations throughout the country and to network with colleagues who are working on similar quality initiatives and challenges related to caring for veterans with cancer.
Program
The first Conference talk was in October 2016. It encompassed ten 1-hour talks during the 2016 to 2017 academic year. Speakers were recruited from the VACT West Haven campus and from several other VA sites nationwide. Topics included survivorship, psychosocial distress, palliative care, cancer navigation, and establishing a clinical trials program.
In its first year, the Conference series had 260 unique attendees representing such disciplines as medicine, nursing, social work, pharmacy, psychology, and clinic administration and representing all 21 Veterans Integrated Services Networks (VISNs). Speakers including oncologists, hepatologists, cancer care coordinators, health psychologists, and a research coordinator gave presentations on psychosocial distress screening and issues, cognitive behavioral therapy for cancer pain, cancer navigation, cancer case tracking, VISN-based liver cancer tumor tracker and liver tumor board, starting a VA-based clinical trial, palliative care, and survivorship.
The Conference accounted for 508 continuing medical education (CME) hours, which accounted for one-third of the total CME hours generated by the VACT West Haven VA ECHO program. Highlights of the talks were presented at the 2017 Association of VA Hematology/Oncology annual meeting in Denver, Colorado.
During the second year of the Conference, speakers were recruited to address new American College of Surgeons Commission on Cancer (CoC) requirements regarding survivorship treatment summaries for a subset of cancer survivors.3 The focus on survivorship was driven by ongoing feedback from participants who were working on initiatives to implement this process at their VA sites and wanted to learn from peers involved in this process throughout the VA system. Several speakers gave talks on implementing survivorship care at their VA and specifically on the use of computerized patient record system templates to create survivorship treatment summaries for veterans in accordance with CoC standards.
Since the first Conference in 2016, the number of unique attendees grew by 20% to 327 in 2018. During its first 2 years, participants have earned a total of 1,095 CME credits through Yale University CME. Conferences are usually broadcast at noon eastern time so that providers can take advantage of sessions during lunch breaks.
Participant Surveys
Attendees were invited to participate in voluntary, anonymous surveys to obtain feedback on and to receive input on topics of interest for future talks. Participants also were asked to comment on resources that they utilized to be updated on practice changes (Table 1).
The Conference has led to increased awareness of other continuing education opportunities available through VA ECHO-Connecticut. Of survey participants, 20% reported that they had attended other VA ECHO conferences.
The survey samples are self-selecting and may not necessarily be representative of the Conference participants or of the VA oncology interdisciplinary team as a whole; however, the relatively large number of survey participants provides some confidence that these survey results can help inform future planning for this and other continuing education opportunities for VA oncology providers.
An additional online survey was designed to elucidate whether participants were incorporating knowledge gained from the Conference in their cancer care practice. Half of the 32 participants strongly agreed with the following statement: “Participation in the VA Cancer Care Conference has added to my knowledge of information relevant to my practice,” and 13 more agreed with the statement for a total of 90.6% of those surveyed responding affirmatively. Only 3 participants neither agreed nor disagreed, and none disagreed with the statement. More than half of the participants reported that they made changes to their practice or plan to make changes as a result of the Conference.
Conculsion
The VA ECHO program established at the VACT West Haven campus in 2012 now offers regular monthly or bimonthly conferences in 9 specialties: pain, liver/hepatitis C, neurology, nephrology, cardiology, diabetes/endocrinology, mental health and addiction, nursing grand rounds, and cancer care. The VACT ECHO program is led by a medical director, and each specialty has a clinical director who conducts sessions and recruits other specialists from their department.
Teleconferencing can provide opportunities for colleagues living in distant locations to connect; share best practices, common goals, and challenges; and initiate ongoing and lasting relationships. The Conference draws the most diverse audience by discipline of all the VA ECHO conferences hosted at VACT (Table 2).
Traditionally, the national VA ECHO program has been a forum for specialists to discuss clinical case presentations for the benefit of primary care providers and to deliver didactics about chronic clinical conditions. Our Cancer Care Management VA ECHO has explored new ground by discussing material that has helped sites set up and enhance cancer care clinics and disseminate best practices for cancer survivorship and other aspects of cancer care. As a result, this conference has attracted and provided a forum for the most diverse audience of staff among VA ECHO clinics, with participation from clinic administrators to social workers to primary care providers to tumor registrars.
Through the creation of the Conference, > 300 individuals who care for veterans with cancer have been provided with a regular forum at which to connect with colleagues, receive updates on new treatment options for their patients, and learn about and share best practices specific to VA oncology patients. The VA ECHO technology creates a resource that can be accessed by all VA staff from their desktop computer. The VA ECHO SharePoint saves the slides of the Conference presentations both with and without audio to enable staff who can’t participate in real time to access the information at their convenience.
The Conference has facilitated networking among VA oncology providers who have common interests. Conference participants also have participated in other VA ECHO conferences in disciplines beyond oncology. Participants in the Conference also are encouraged to participate as speakers by presenting quality improvement initiatives at their VA site. This novel approach to generating content for this educational series has led to a dynamic interchange of ideas and increased networking among VA providers related to their practice and quality improvement initiatives at their VA sites. The Conference provides a regular forum for VA staff across a wide range of disciplines to share personal experiences, successes, and frustrations and to get feedback from colleagues.
The Conference combines a structured approach to presenting VA-specific educational content related to cancer care and multiple mechanisms that encourage staff to participate in an ongoing dialogue related to quality initiatives both on the phone during the Conference, online using Outlook LYNC or Skype to ask questions during the Conference, and during conversations on group e-mail. The Conference promotes staff engagement at little or no extra cost to the VA. For more information about the VA ECHO Cancer Care Conference or to submit a presentation for consideration for a future session, please contact [email protected] or [email protected].
1. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
2. Hematology and oncology federal health care data trends. Fed Pract. 2017;33(suppl 5):S12-S15.
3. American College of Surgeons Commission on Cancer. Cancer Program Standards: Ensuring Patient Centered Care, 2016 Edition. https://www.facs.org/quality-programs/cancer/coc/standards. Accessed March 14, 2018.
A telementoring program based on the Specialty Care Access Network Extension for Community Healthcare Outcomes model shared information about cancer care across VA health Care systems.
A telementoring program based on the Specialty Care Access Network Extension for Community Healthcare Outcomes model shared information about cancer care across VA health Care systems.
In 2016, the Cancer Care Coordinator at the US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACT) in West Haven partnered with the VA New England Healthcare System to use its telementoring program. The VA Specialty Care Access Network Extension for Community Healthcare Outcomes (VA ECHO) was used to present a series of educational conferences on cancer care. This article describes our experience implementing the program and reviews participant feedback gathered from voluntary surveys.
Background
In 2011, the Veterans Health Administration (VHA) Office of Healthcare Transformation launched VA ECHO, a telementoring program for primary care providers (PCPs) and patient-aligned care team staff. VACT was selected as 1 of 7 hub sites across the US. The VA ECHO system uses video and online technology to provide PCPs with case-based specialist consultation and didactic education. The system enables providers at any VA location to participate in online and telephone conferences in real time. The presentations are recorded and made available online to VA providers through a secure site.
VA ECHO is based on the highly successful Project ECHO model established by Sanjeev Arora and the University of New Mexico in 2007.1 The rationale for Project ECHO was that patient care could be improved by increasing the competence of PCPs in the management of complex diseases by providing access to disease specialists through a case-based learning approach that used technology, which it termed knowledge networks, to connect the PCPs to specialists.
The original model addressed management of hepatitis C in a medically underserved area where half of the population was widely geographically dispersed, making the provision of specialty care challenging. Developers identified 6 characteristics that make a disease appropriate for treatment using the Project ECHO knowledge network model:
- The disease is common;
- Management of the disease is complex;
- Treatment for the disease is evolving;
- The disease has a high societal impact;
- There are serious outcomes if the disease is not treated; and
- Disease management improves outcomes.1
VA ECHO conferences are available to all VA personnel. Staff can subscribe to an e-mail group list to be alerted to conference times and topics. Participants can connect directly to the conference using Microsoft Outlook Lync or Skype (Redmond, WA) and see the slides in real time on their computer as they listen to the presentation. The presentations are recorded, and the slides with audio can be accessed easily on the VA ECHO SharePoint site for download, enabling VA staff to listen to conferences at their convenience (Figure).
VA Cancer ECHO
The impetus to create a series of talks related to cancer care using VA ECHO was the frequent and often time-consuming requests we received from colleagues at other VA sites for information about areas of cancer care, such as survivorship and cancer care coordination. It was felt that presenting cancer care information as a VA ECHO series would make this information available to a large group of providers at one time, making the method more time effective than sharing the information via one-on-one conversations.
The cancer care coordinator originally conceived this as a 3-part, 1-time series to present work done at VACT in the areas of survivorship, psychosocial distress monitoring, and coordination of cancer care using the VA Cancer Care Tracking System, an online tracking tool. Information about the series was disseminated via VA group e-mail lists for oncology providers and via the existing VA ECHO subscriber invitation process. The 3-presentation series garnered positive feedback and had attendance that ranged from 49 to 75 participants (mean, 60). Participants expressed enthusiasm for the format via e-mail and phone feedback directly to the West Haven staff.
Expansion
The success of this original 3-part series led to a trial of an ongoing Cancer Care Conference series (Conference) using VA ECHO. This was a novel use of VA ECHO and was outside its traditional format, which is geared to discussion of individual cases and clinical knowledge. Nevertheless, this new style of communication has been embraced by a wide range of VA cancer care professionals.
One reason we considered expanding the program was that oncology fit the framework of the original Project ECHO knowledge network model. Cancer is common at the VA, which cares for 175,000 patients with cancer annually.2 The management of cancer is complex involving many disciplines working together, and treatments are constantly changing. In addition, cancer has a high societal impact; there are serious outcomes both in terms of patient survival and patient symptom burden. And lastly, outcomes are improved with proactive disease management that is informed by the most current, evidence-based medicine.
The Conference was conceived as a forum for providers across disciplines to share best practices and discuss common challenges in caring for veterans with cancer. We invited participants to submit proposals for presentations related to cancer care initiatives at their VA sites. Potential speakers across all areas of care for veterans with cancer were invited to submit possible topics for the conference. The submissions were reviewed by the moderators in an effort to create a series of talks on a variety of topics across all aspects of care for oncology patients in the VA. This process of effectively crowd-sourcing educational content inspires providers to think more creatively about their practice and quality improvement projects and has sparked an ongoing dialogue about quality initiatives among VA oncology providers across disciplines and geographic locations. As a result, this approach also has enabled participants to learn from colleagues who work at a wide range of rural and urban VA locations throughout the country and to network with colleagues who are working on similar quality initiatives and challenges related to caring for veterans with cancer.
Program
The first Conference talk was in October 2016. It encompassed ten 1-hour talks during the 2016 to 2017 academic year. Speakers were recruited from the VACT West Haven campus and from several other VA sites nationwide. Topics included survivorship, psychosocial distress, palliative care, cancer navigation, and establishing a clinical trials program.
In its first year, the Conference series had 260 unique attendees representing such disciplines as medicine, nursing, social work, pharmacy, psychology, and clinic administration and representing all 21 Veterans Integrated Services Networks (VISNs). Speakers including oncologists, hepatologists, cancer care coordinators, health psychologists, and a research coordinator gave presentations on psychosocial distress screening and issues, cognitive behavioral therapy for cancer pain, cancer navigation, cancer case tracking, VISN-based liver cancer tumor tracker and liver tumor board, starting a VA-based clinical trial, palliative care, and survivorship.
The Conference accounted for 508 continuing medical education (CME) hours, which accounted for one-third of the total CME hours generated by the VACT West Haven VA ECHO program. Highlights of the talks were presented at the 2017 Association of VA Hematology/Oncology annual meeting in Denver, Colorado.
During the second year of the Conference, speakers were recruited to address new American College of Surgeons Commission on Cancer (CoC) requirements regarding survivorship treatment summaries for a subset of cancer survivors.3 The focus on survivorship was driven by ongoing feedback from participants who were working on initiatives to implement this process at their VA sites and wanted to learn from peers involved in this process throughout the VA system. Several speakers gave talks on implementing survivorship care at their VA and specifically on the use of computerized patient record system templates to create survivorship treatment summaries for veterans in accordance with CoC standards.
Since the first Conference in 2016, the number of unique attendees grew by 20% to 327 in 2018. During its first 2 years, participants have earned a total of 1,095 CME credits through Yale University CME. Conferences are usually broadcast at noon eastern time so that providers can take advantage of sessions during lunch breaks.
Participant Surveys
Attendees were invited to participate in voluntary, anonymous surveys to obtain feedback on and to receive input on topics of interest for future talks. Participants also were asked to comment on resources that they utilized to be updated on practice changes (Table 1).
The Conference has led to increased awareness of other continuing education opportunities available through VA ECHO-Connecticut. Of survey participants, 20% reported that they had attended other VA ECHO conferences.
The survey samples are self-selecting and may not necessarily be representative of the Conference participants or of the VA oncology interdisciplinary team as a whole; however, the relatively large number of survey participants provides some confidence that these survey results can help inform future planning for this and other continuing education opportunities for VA oncology providers.
An additional online survey was designed to elucidate whether participants were incorporating knowledge gained from the Conference in their cancer care practice. Half of the 32 participants strongly agreed with the following statement: “Participation in the VA Cancer Care Conference has added to my knowledge of information relevant to my practice,” and 13 more agreed with the statement for a total of 90.6% of those surveyed responding affirmatively. Only 3 participants neither agreed nor disagreed, and none disagreed with the statement. More than half of the participants reported that they made changes to their practice or plan to make changes as a result of the Conference.
Conculsion
The VA ECHO program established at the VACT West Haven campus in 2012 now offers regular monthly or bimonthly conferences in 9 specialties: pain, liver/hepatitis C, neurology, nephrology, cardiology, diabetes/endocrinology, mental health and addiction, nursing grand rounds, and cancer care. The VACT ECHO program is led by a medical director, and each specialty has a clinical director who conducts sessions and recruits other specialists from their department.
Teleconferencing can provide opportunities for colleagues living in distant locations to connect; share best practices, common goals, and challenges; and initiate ongoing and lasting relationships. The Conference draws the most diverse audience by discipline of all the VA ECHO conferences hosted at VACT (Table 2).
Traditionally, the national VA ECHO program has been a forum for specialists to discuss clinical case presentations for the benefit of primary care providers and to deliver didactics about chronic clinical conditions. Our Cancer Care Management VA ECHO has explored new ground by discussing material that has helped sites set up and enhance cancer care clinics and disseminate best practices for cancer survivorship and other aspects of cancer care. As a result, this conference has attracted and provided a forum for the most diverse audience of staff among VA ECHO clinics, with participation from clinic administrators to social workers to primary care providers to tumor registrars.
Through the creation of the Conference, > 300 individuals who care for veterans with cancer have been provided with a regular forum at which to connect with colleagues, receive updates on new treatment options for their patients, and learn about and share best practices specific to VA oncology patients. The VA ECHO technology creates a resource that can be accessed by all VA staff from their desktop computer. The VA ECHO SharePoint saves the slides of the Conference presentations both with and without audio to enable staff who can’t participate in real time to access the information at their convenience.
The Conference has facilitated networking among VA oncology providers who have common interests. Conference participants also have participated in other VA ECHO conferences in disciplines beyond oncology. Participants in the Conference also are encouraged to participate as speakers by presenting quality improvement initiatives at their VA site. This novel approach to generating content for this educational series has led to a dynamic interchange of ideas and increased networking among VA providers related to their practice and quality improvement initiatives at their VA sites. The Conference provides a regular forum for VA staff across a wide range of disciplines to share personal experiences, successes, and frustrations and to get feedback from colleagues.
The Conference combines a structured approach to presenting VA-specific educational content related to cancer care and multiple mechanisms that encourage staff to participate in an ongoing dialogue related to quality initiatives both on the phone during the Conference, online using Outlook LYNC or Skype to ask questions during the Conference, and during conversations on group e-mail. The Conference promotes staff engagement at little or no extra cost to the VA. For more information about the VA ECHO Cancer Care Conference or to submit a presentation for consideration for a future session, please contact [email protected] or [email protected].
In 2016, the Cancer Care Coordinator at the US Department of Veterans Affairs (VA) Connecticut Healthcare System (VACT) in West Haven partnered with the VA New England Healthcare System to use its telementoring program. The VA Specialty Care Access Network Extension for Community Healthcare Outcomes (VA ECHO) was used to present a series of educational conferences on cancer care. This article describes our experience implementing the program and reviews participant feedback gathered from voluntary surveys.
Background
In 2011, the Veterans Health Administration (VHA) Office of Healthcare Transformation launched VA ECHO, a telementoring program for primary care providers (PCPs) and patient-aligned care team staff. VACT was selected as 1 of 7 hub sites across the US. The VA ECHO system uses video and online technology to provide PCPs with case-based specialist consultation and didactic education. The system enables providers at any VA location to participate in online and telephone conferences in real time. The presentations are recorded and made available online to VA providers through a secure site.
VA ECHO is based on the highly successful Project ECHO model established by Sanjeev Arora and the University of New Mexico in 2007.1 The rationale for Project ECHO was that patient care could be improved by increasing the competence of PCPs in the management of complex diseases by providing access to disease specialists through a case-based learning approach that used technology, which it termed knowledge networks, to connect the PCPs to specialists.
The original model addressed management of hepatitis C in a medically underserved area where half of the population was widely geographically dispersed, making the provision of specialty care challenging. Developers identified 6 characteristics that make a disease appropriate for treatment using the Project ECHO knowledge network model:
- The disease is common;
- Management of the disease is complex;
- Treatment for the disease is evolving;
- The disease has a high societal impact;
- There are serious outcomes if the disease is not treated; and
- Disease management improves outcomes.1
VA ECHO conferences are available to all VA personnel. Staff can subscribe to an e-mail group list to be alerted to conference times and topics. Participants can connect directly to the conference using Microsoft Outlook Lync or Skype (Redmond, WA) and see the slides in real time on their computer as they listen to the presentation. The presentations are recorded, and the slides with audio can be accessed easily on the VA ECHO SharePoint site for download, enabling VA staff to listen to conferences at their convenience (Figure).
VA Cancer ECHO
The impetus to create a series of talks related to cancer care using VA ECHO was the frequent and often time-consuming requests we received from colleagues at other VA sites for information about areas of cancer care, such as survivorship and cancer care coordination. It was felt that presenting cancer care information as a VA ECHO series would make this information available to a large group of providers at one time, making the method more time effective than sharing the information via one-on-one conversations.
The cancer care coordinator originally conceived this as a 3-part, 1-time series to present work done at VACT in the areas of survivorship, psychosocial distress monitoring, and coordination of cancer care using the VA Cancer Care Tracking System, an online tracking tool. Information about the series was disseminated via VA group e-mail lists for oncology providers and via the existing VA ECHO subscriber invitation process. The 3-presentation series garnered positive feedback and had attendance that ranged from 49 to 75 participants (mean, 60). Participants expressed enthusiasm for the format via e-mail and phone feedback directly to the West Haven staff.
Expansion
The success of this original 3-part series led to a trial of an ongoing Cancer Care Conference series (Conference) using VA ECHO. This was a novel use of VA ECHO and was outside its traditional format, which is geared to discussion of individual cases and clinical knowledge. Nevertheless, this new style of communication has been embraced by a wide range of VA cancer care professionals.
One reason we considered expanding the program was that oncology fit the framework of the original Project ECHO knowledge network model. Cancer is common at the VA, which cares for 175,000 patients with cancer annually.2 The management of cancer is complex involving many disciplines working together, and treatments are constantly changing. In addition, cancer has a high societal impact; there are serious outcomes both in terms of patient survival and patient symptom burden. And lastly, outcomes are improved with proactive disease management that is informed by the most current, evidence-based medicine.
The Conference was conceived as a forum for providers across disciplines to share best practices and discuss common challenges in caring for veterans with cancer. We invited participants to submit proposals for presentations related to cancer care initiatives at their VA sites. Potential speakers across all areas of care for veterans with cancer were invited to submit possible topics for the conference. The submissions were reviewed by the moderators in an effort to create a series of talks on a variety of topics across all aspects of care for oncology patients in the VA. This process of effectively crowd-sourcing educational content inspires providers to think more creatively about their practice and quality improvement projects and has sparked an ongoing dialogue about quality initiatives among VA oncology providers across disciplines and geographic locations. As a result, this approach also has enabled participants to learn from colleagues who work at a wide range of rural and urban VA locations throughout the country and to network with colleagues who are working on similar quality initiatives and challenges related to caring for veterans with cancer.
Program
The first Conference talk was in October 2016. It encompassed ten 1-hour talks during the 2016 to 2017 academic year. Speakers were recruited from the VACT West Haven campus and from several other VA sites nationwide. Topics included survivorship, psychosocial distress, palliative care, cancer navigation, and establishing a clinical trials program.
In its first year, the Conference series had 260 unique attendees representing such disciplines as medicine, nursing, social work, pharmacy, psychology, and clinic administration and representing all 21 Veterans Integrated Services Networks (VISNs). Speakers including oncologists, hepatologists, cancer care coordinators, health psychologists, and a research coordinator gave presentations on psychosocial distress screening and issues, cognitive behavioral therapy for cancer pain, cancer navigation, cancer case tracking, VISN-based liver cancer tumor tracker and liver tumor board, starting a VA-based clinical trial, palliative care, and survivorship.
The Conference accounted for 508 continuing medical education (CME) hours, which accounted for one-third of the total CME hours generated by the VACT West Haven VA ECHO program. Highlights of the talks were presented at the 2017 Association of VA Hematology/Oncology annual meeting in Denver, Colorado.
During the second year of the Conference, speakers were recruited to address new American College of Surgeons Commission on Cancer (CoC) requirements regarding survivorship treatment summaries for a subset of cancer survivors.3 The focus on survivorship was driven by ongoing feedback from participants who were working on initiatives to implement this process at their VA sites and wanted to learn from peers involved in this process throughout the VA system. Several speakers gave talks on implementing survivorship care at their VA and specifically on the use of computerized patient record system templates to create survivorship treatment summaries for veterans in accordance with CoC standards.
Since the first Conference in 2016, the number of unique attendees grew by 20% to 327 in 2018. During its first 2 years, participants have earned a total of 1,095 CME credits through Yale University CME. Conferences are usually broadcast at noon eastern time so that providers can take advantage of sessions during lunch breaks.
Participant Surveys
Attendees were invited to participate in voluntary, anonymous surveys to obtain feedback on and to receive input on topics of interest for future talks. Participants also were asked to comment on resources that they utilized to be updated on practice changes (Table 1).
The Conference has led to increased awareness of other continuing education opportunities available through VA ECHO-Connecticut. Of survey participants, 20% reported that they had attended other VA ECHO conferences.
The survey samples are self-selecting and may not necessarily be representative of the Conference participants or of the VA oncology interdisciplinary team as a whole; however, the relatively large number of survey participants provides some confidence that these survey results can help inform future planning for this and other continuing education opportunities for VA oncology providers.
An additional online survey was designed to elucidate whether participants were incorporating knowledge gained from the Conference in their cancer care practice. Half of the 32 participants strongly agreed with the following statement: “Participation in the VA Cancer Care Conference has added to my knowledge of information relevant to my practice,” and 13 more agreed with the statement for a total of 90.6% of those surveyed responding affirmatively. Only 3 participants neither agreed nor disagreed, and none disagreed with the statement. More than half of the participants reported that they made changes to their practice or plan to make changes as a result of the Conference.
Conculsion
The VA ECHO program established at the VACT West Haven campus in 2012 now offers regular monthly or bimonthly conferences in 9 specialties: pain, liver/hepatitis C, neurology, nephrology, cardiology, diabetes/endocrinology, mental health and addiction, nursing grand rounds, and cancer care. The VACT ECHO program is led by a medical director, and each specialty has a clinical director who conducts sessions and recruits other specialists from their department.
Teleconferencing can provide opportunities for colleagues living in distant locations to connect; share best practices, common goals, and challenges; and initiate ongoing and lasting relationships. The Conference draws the most diverse audience by discipline of all the VA ECHO conferences hosted at VACT (Table 2).
Traditionally, the national VA ECHO program has been a forum for specialists to discuss clinical case presentations for the benefit of primary care providers and to deliver didactics about chronic clinical conditions. Our Cancer Care Management VA ECHO has explored new ground by discussing material that has helped sites set up and enhance cancer care clinics and disseminate best practices for cancer survivorship and other aspects of cancer care. As a result, this conference has attracted and provided a forum for the most diverse audience of staff among VA ECHO clinics, with participation from clinic administrators to social workers to primary care providers to tumor registrars.
Through the creation of the Conference, > 300 individuals who care for veterans with cancer have been provided with a regular forum at which to connect with colleagues, receive updates on new treatment options for their patients, and learn about and share best practices specific to VA oncology patients. The VA ECHO technology creates a resource that can be accessed by all VA staff from their desktop computer. The VA ECHO SharePoint saves the slides of the Conference presentations both with and without audio to enable staff who can’t participate in real time to access the information at their convenience.
The Conference has facilitated networking among VA oncology providers who have common interests. Conference participants also have participated in other VA ECHO conferences in disciplines beyond oncology. Participants in the Conference also are encouraged to participate as speakers by presenting quality improvement initiatives at their VA site. This novel approach to generating content for this educational series has led to a dynamic interchange of ideas and increased networking among VA providers related to their practice and quality improvement initiatives at their VA sites. The Conference provides a regular forum for VA staff across a wide range of disciplines to share personal experiences, successes, and frustrations and to get feedback from colleagues.
The Conference combines a structured approach to presenting VA-specific educational content related to cancer care and multiple mechanisms that encourage staff to participate in an ongoing dialogue related to quality initiatives both on the phone during the Conference, online using Outlook LYNC or Skype to ask questions during the Conference, and during conversations on group e-mail. The Conference promotes staff engagement at little or no extra cost to the VA. For more information about the VA ECHO Cancer Care Conference or to submit a presentation for consideration for a future session, please contact [email protected] or [email protected].
1. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
2. Hematology and oncology federal health care data trends. Fed Pract. 2017;33(suppl 5):S12-S15.
3. American College of Surgeons Commission on Cancer. Cancer Program Standards: Ensuring Patient Centered Care, 2016 Edition. https://www.facs.org/quality-programs/cancer/coc/standards. Accessed March 14, 2018.
1. Arora S, Geppert CM, Kalishman S, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82(2):154-160.
2. Hematology and oncology federal health care data trends. Fed Pract. 2017;33(suppl 5):S12-S15.
3. American College of Surgeons Commission on Cancer. Cancer Program Standards: Ensuring Patient Centered Care, 2016 Edition. https://www.facs.org/quality-programs/cancer/coc/standards. Accessed March 14, 2018.
Liver Imaging Reporting and Data System in Patients at High Risk for Hepatocellular Carcinoma in the Memphis Veterans Affairs Population (FULL)
Although hepatocellular carcinoma can be difficult to detect, use of the LI-RADS algorithm could lead to earlier identification in at-risk patients.
Hepatocellular carcinoma (HCC) is the third most common cause of death from cancer worldwide.1 Liver cancer is the fifth most common cancer in men and the seventh in women.2 The highest incidence rates are in sub-Saharan Africa and Southeast Asia where hepatitis B virus is endemic. The incidence of HCC in western countries is increasing, particularly due to the rise of hepatitis C virus (HCV) as well as alcoholic liver disease and nonalcoholic fatty liver disease. The incidence of HCC has tripled in the US in the past 2 decades.1-3
HCC can be diagnosed by radiographic images without the need for biopsy if the typical imaging features are present.3 The European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend screening abdominal ultrasonography at 6-month intervals for high-risk patients.3,4 High-risk patients include patients with cirrhosis, especially those with hepatitis B or C.3
If screening ultrasonography detects a nodule, size determines whether a follow-up ultrasound is needed vs obtaining a contrast-enhanced dynamic computed tomography (CT) scan or a magnetic resonance image (MRI).3 If ultrasonography detects a nodule > 1 cm in diameter, then a dynamic CT or MRI is performed. Characteristic hyperenhancement during later arterial phase and washout during the venous or delayed phase is associated with a nearly 100% specificity for HCC diagnosis.5 Arterial-enhancing contrast is required when using CT and MRI because HCC is a hypervascular lesion.6 The portal venous blood dilutes the majority of the liver’s arterial blood; therefore, the liver does not enhance during the arterial phase, while HCC will show maximum enhancement.7 Furthermore, HCC should demonstrate a “washout” of contrast during the venous phase on CT and MRI.4 Standard imaging protocol dictates that 4 phases are needed to properly diagnose HCC including unenhanced, arterial, venous, and delayed.4
Regular surveillance increases the likelihood of detecting HCC before the presentation of clinical symptoms and facilitates receipt of curative therapy.8-10 Patients with viral hepatitis and cirrhosis with HCC found on screening are more likely to have earlier-stage disease and survive longer from the time of diagnosis.11 Furthermore, it has been observed that HCC detected by surveillance is significantly more likely to undergo curative therapy compared with incidental or symptomatic detection of HCC.9
Technical improvements in imaging techniques include advancement in contrast agents, multidetector row helical CT, and the flexibility/range of pulse sequences available in MRI.7 Even with technical improvements in all modalities used in HCC imaging, detecting HCC remains difficult, especially when detecting the small (< 2 cm) lesions in a cirrhotic liver.7 Interpretation of imaging also remains a challenge as HCC does not always fit strict criteria: lack of “washout” in a hypervascular lesion, determining small HCC lesions from benign nodules, and hypovascular/isovascular HCC.5 Radiologic differentials in the diagnosis of HCC include transient hepatic intensity difference (THID)/transient hepatic attenuation difference (THAD), arterio-portal shunt, and regenerative nodules.12 In the common clinical setting, patients undergo multiple imaging studies that are interpreted by multiple radiologists, which can add to the difficulty in the diagnosis of HCC.13
The radiology community recognized the inconsistencies and complexities of HCC imaging. Therefore, the American College of Radiology endorsed the Liver Imaging Reporting and Data System (LI-RADS), which had the goal of reducing variability in lesion interpretation through standardization and improving communication with clinicians.14 LI-RADS uses a diagnostic algorithm for CT and MRI that categorizes observed liver findings in high-risk individuals based on the probability or relative risk of HCC without assigning a formal diagnosis.14 LI-RADS takes into account arterial phase enhancement, tumor size, washout appearance, the presence and nature of a capsule, and threshold growth.15 LI-RADS categorizes an observed liver finding on a scale of 1 to 5, with 1 corresponding to a definitely benign finding and 5 with definitive HCC.14 Furthermore, LI-RADS sought to limit the technical variabilities among institutions.
LI-RADS was launched in 2011 and has been utilized by many clinical practices while continuing to be expanded and updated.16 Recent studies examined the specificity of LI-RADS as well as interreader variability.17,18 For nodules viewed on MRI, both LI-RADS categories 4 and 5 had high specificity for HCC.17 When looking at interreader repeatability, LI-RADS showed moderate agreement among experts using the diagnostic algorithm.19 Further studies have compared LI-RADS with the AASLD guidelines and the Organ Procurement and Transplantation Network (OPTN) guidelines.16 When compared with other guidelines, LI-RADS expands the definition of indeterminate findings into probably benign, intermediate probability of HCC, and probably HCC, which corresponds to LI-RADS categories 2, 3, and 4.16
We looked retrospectively at a group of patients previously diagnosed with HCC to see whether utilizing the LI-RADS scoring system within our screening system might have allowed an earlier prediction of HCC and a timelier intervention. Prior to this investigation the LI-RADS system was not used for HCC screening at our US Department of Veterans Affairs (VA) facility. We examined screened patients at the Memphis VA Medical Center (MVAMC) in Tennessee who were subsequently diagnosed with HCC to see which LI-RADS category the last surveillance CT prior to diagnosis would fall into, 6 months to a year prior to the diagnosis of HCC. Our control population was a group of patients screened with CT for their liver nodules who were found not to have HCC.
Methods
Patients at MVAMC with cirrhosis and patients with chronic hepatitis B are routinely screened with ultrasound, CT, or MRI in accordance with the AASLD, EASL, and VA guidelines. Of 303 patients with HCV and cirrhosis under care in 2015, 242 (81%) received imaging to screen for HCC according to the VA National Hepatitis C Registry 2015 (Personal Communication, Population Health Service, Office of Patient Care Services).The LI-RADS scoring system was not applied as a standard screening methodology.
Under an institutional review board-approved protocol, we reviewed the charts of all patients diagnosed with HCC at MVAMC from 2009 to 2014, utilizing ICD-9 code of 155.0 for HCC. We identified within these charts patients who had a surveillance CT image performed within a 6- to 13-month period prior to the CTs that diagnosed HCC (prediagnostic HCC CT). Furthermore, we reviewed the charts of all patients diagnosed with benign liver nodules at MVAMC from 2009 to 2014, utilizing the ICD-9 code of 573.8 for other specified disorders of the liver.
Within these charts, we found patients who had a surveillance CT image performed and who were followed after that image with additional imaging for ≥ 2 years or who had a liver biopsy negative for HCC (benign surveillance CT). We compared these 2 sets of CTs utilizing LI-RADS criteria. Once these patients were identified, a list of the CTs to be examined were given to 2 MVAMC radiologists who specialize in CT.
No identifying information of the patients was included, and a 13-digit number unique to each CT exam identified the CTs to be reviewed. Radiologist 1 and 2 examined the CTs on the MVAMC Picture Archiving and Communication System (PACS). Both radiologists were asked to give each nodule a score according to LI-RADS v2014 diagnostic algorithm (Figure).
We hypothesized that the prediagnostic CT images of patients eventually determined to have HCC would have a LI-RADS score of 4 (LR4) or LR5. Furthermore, we hypothesized that the CT images of the benign liver nodule patients would have a score ≤ LR3. If there was a disagreement between the radiologists in terms of a malignant score (LR4 or LR5) vs a benign score (≤ LR3), then a third radiologist (radiologist 3) provided a score for these nodules. The third, tiebreaker radiologist was given the scores of both prior radiologists and asked to choose which score was correct.
Statistical analysis was then applied to the data to determine the sensitivity, specificity, and diagnostic accuracy in diagnosing eventual HCC, as well as the false-negative and false-positive rates of radiologists 1 and 2. Raw data also were used to determine the agreement between raters by calculating the κ statistic with a 95% CI.
Results
A total of 70 nodules were examined by radiologists 1 and 2 with 42 of the nodules in the prediagnostic HCC CTs and 28 of the nodules in the benign surveillance CTs.
Radiologist 1 identified 11 patients with LR4 and 21 patients with LR5. His scores showed a sensitivity of 64.3% and specificity of 82.1% with accuracy of 71.4% for LI-RADS in identifying eventual HCC. The false-negative rate of the LI-RADS diagnostic algorithm for radiologist 1 was 35.7% and the false-positive rate was 17.9%. Radiologist 2 identified 17 patients LR4 and 19 patients with LR5. Radiologist 2’s scores showed a sensitivity of 69.0% and specificity of 75.0% with accuracy of 71.4% for LI-RADS in identifying eventual HCC.The false-negative rate of the LI-RADS diagnostic algorithm for radiologist 2 was 31.0% and false-positive rate of 25.0%. The κ statistic was calculated to determine the interrater agreement. The radiologists agreed on 58 of 70 samples; 15 without HCC and 43 with HCC. The κ statistic was 0.592, which indicates moderate agreement (Table 2).
Discussion
If HCC is diagnosed late in the disease process based on symptomatology and not on surveillance imaging, the likelihood of receiving early and potential curative therapy greatly declines as was shown in a systemic literature review.9 Surveillance imaging and lesion interpretation by various radiologists has been difficult to standardize as new technologic advances continue to occur in the imaging of HCC.14 LI-RADS was initiated to help standardize CT and MRI interpretation and reporting of hepatic nodules. As a dynamic algorithm, it continues to adjust with new advances in imaging techniques with the most recent updates being made to the algorithm in 2014.14,19 LI-RADS applies to patients at high risk for HCC most often who are already enrolled in a surveillance program.19 The MVAMC has a high incidence of patients with cirrhosis who are at risk for HCC, which is why we chose it as our study population.
LI-RADS can be applied to both MRI and CT imaging. Much of the recent literature have looked at LI-RADS in terms of MRI. A group in China looked at 100 pathologically confirmed patients and assigned a LI-RADS score to the MRI at the time of diagnosis and showed that MRI LI-RADS scoring was highly sensitive and specific in the diagnosis of HCC.20 This study did note a numeric difference in the specificity of LI-RADS algorithm depending on how LR3 scores were viewed. If a LR3 score was considered negative rather than positive for HCC, then the specificity increased by almost 20%.20
Another study looked at patients with liver nodules ≤ 20 mm found on ultrasound and obtained MRIs and biopsies on these patients, assigning the MRI a LI-RADs score.17 Darnell and colleagues found that MRI LR4 and LR5 have a high specificity for HCC. However, 29 of the 42 LR3 lesions examined were found to be HCC.17 Furthermore, Choi and colleagues retrospectively looked at patients in a HCC surveillance program who had undergone MRI as part of the program and assigned LI-RADS scores to these MRIs.21 Their study showed that LR5 criteria on gadoxetate disodium-enhanced MRI has excellent positive predictive value (PPV) for diagnosing HCC, and LR4 showed good PPV.21
In our study, we chose to look at LI-RADS in terms of surveillance CT scans 6 to 13 months prior to the diagnosis of HCC to see whether this method would allow us to intervene earlier with more aggressive diagnostics or therapy in those suspected of having HCC. Although Choi and colleagues looked retrospectively at MRI surveillance imaging, most of the prior studies have looked at LI-RADS scoring in imaging at the time of diagnosis.17,20,21 By looking at surveillance CT scans, we sought to determine LI-RADS sensitivity, specificity, and diagnostic accuracy as a screening tool compared with CT evaluations without LI-RADS scoring.
We also chose to look at CT scans since most of the prior studies have looked at the more detailed and often more expensive MRIs. For both radiologists 1 and 2, the sensitivity was > 60% and specificity was > 70% with a diagnostic accuracy of 71.4% in predicting a diagnosis of HCC in future scans. Although there was high false negative of > 30% for both radiologists, we did consider LR3 as negative for HCC. As Darnell and colleagues’ study of MRI LI-RADS shows, LR3 may need to be revised in the future as its ambiguity can lead to false-negatives.17 Our results also showed moderate interreader agreement, which has been seen in previous studies with LI-RADS.18
Some studies have compared MRI with CT imaging in terms of LI-RADs classification of hepatic nodules to find out whether concordance was seen.22,23 Both studies found that there was substantial discordance between MRI and CT with CT often underscoring hepatic nodules.22,23 In Zhang and colleagues, interclass agreement between CT and MRI varied the most in terms of arterial enhancement with CT producing false-negative findings.22 CT also underestimated LI-RADS score by 16.9% for LR3, 37.3% for LR4, and 8.5% for LR5 in this study.22 Furthermore, Corwin and colleagues found a significant upgrade in terms of LI-RADS categorization with MRI for 42.5% of observations.23 In this study, upgraded LI-RADS scores on MRI included 2 upgraded to LR5V (Figure), 15 upgraded to LR5, and 12 upgraded to LR4.23
Our study shows that the LI-RADS algorithm has a good sensitivity, specificity, and diagnostic accuracy as a screening tool, predicting HCC in scans earlier than standard CT evaluation. In our study, the patients with HCC were shown to have higher LI-RADS scores on prediagnostic imaging, while the benign liver nodule patients were shown to have lower LI-RADS scores. This data would suggest that a LI-RADS score given to surveillance CT of LR4 or higher should recommend either a biopsy or follow-up imaging after a short interval. If LI-RADS is applied to surveillance CTs in patients at risk for HCC, a diagnosis of HCC may be arrived at earlier as compared with not using the LI-RADS algorithm. Earlier detection may lead to earlier intervention and improved treatment outcomes.
Limitations
Limitations to our study occurred because radiologist 3 did not review all of the images nor score them. Radiologist 3 was limited to 12 images where there was disagreement and was limited to 2 scores to choose from for each image. Further limitations include that this study was performed at a single center. Our study focused on one imaging modality and did not include ultrasounds or MRIs. We did not compare the demographics of our patients with those of other VA hospitals. The radiologists interpreted the images individually, and their subjectivity was another limitation.
Conclusion
In the MVAMC population, LI-RADS showed a good sensitivity, specificity, and diagnostic accuracy for CT surveillance scans in patient at high risk for HCC at an earlier time point than did standard evaluation by very experienced CT radiologists. Higher LI-RADS scores on surveillance CTs had good diagnostic accuracy for the probable future diagnosis of HCC, whereas lower LI-RADS scores had a good diagnostic accuracy for probable benign nodules. Utilizing the LI-RADS algorithm on all surveillance CTs in patients at high risk for HCC may lead to obtaining MRIs or follow-up CTs sooner for suspicious nodules, leading to an earlier diagnosis of HCC and possible earlier and more effective intervention.
1. El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
2. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
3. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
4. Selvapatt N, House H, Brown A. Hepatocellular carcinoma surveillance: are we utilizing it? J Clin Gastroenterol. 2016;50(1):e8-e12.
5. Lee JM, Yoon JH, Joo I, Woo HS. Recent advances in CT and MR imaging for evaluation of hepatocellular carcinoma. Liver Cancer. 2012;1(1):22-40.
6. Chou R, Cuevas C, Fu R, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systemic review and meta-analysis. Ann Intern Med. 2015;162(10):697-711.
7. Ariff B, Lloyd CR, Khan S, et al. Imaging of liver cancer. World J Gastroenterol. 2009;15(11):1289-1300.
8. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31(2):330-335.
9. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624.
10. Nusbaum, JD, Smirniotopoulos J, Wright HC, et al. The effect of hepatocellular carcinoma surveillance in an urban population with liver cirrhosis. J Clin Gastroenterol. 2015;49(10):e91-e95.
11. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systemic review. Ann Intern Med. 2014;161(4):261-269.
12. Shah S, Shukla A, Paunipagar B. Radiological features of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(suppl 3):S63-S66.
13. You MW, Kim SY, Kim KW, et al. Recent advances in the imaging of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21(1):95-103.
14. American College of Radiology. Liver reporting and data system (LI-RADS). https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS. Accessed April 10, 2018.
15. Anis M. Imaging of hepatocellular carcinoma: new approaches to diagnosis. Clin Liver Dis. 2015;19(2):325-340.
16. Mitchell D, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61(3):1056-1065.
17. Darnell A, Forner A, Rimola J, et al. Liver imaging reporting and data system with MR imaging: evaluation in nodules 20 mm or smaller detected in cirrhosis at screening US. Radiology. 2015; 275(3):698-707.
18. Davenport MS, Khalatbari S, Liu PS, et al. Repeatability of diagnostic features and scoring systems for hepatocellular carcinoma by using MR imaging. Radiology. 2014;272(1):132-142.
19. An C, Rakhmonova G, Choi JY, Kim MJ. Liver imaging reporting and data system (LI-RADS) version 2014: understanding and application of the diagnostic algorithm. Clin Mol Hepatol. 2016;22(2):296-307.
20. Zhao W, Li W, Yi X, et al. [Diagnostic value of liver imaging reporting and data system on primary hepatocellular carcinoma] [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41(4):380-387.
21. Choi SH, Byun JH, Kim SY, et al. Liver imaging reporting and data system v2014 with gadoxetate disodium-enhanced magnetic resonance imaging: validation of LIRADS category 4 and 5 criteria. Invest Radiol. 2016;51(8):483-490.
22. Zhang YD, Zhu FP, Xu X, et al. Liver imaging reporting and data system: substantial discordance between CT and MR for imaging classification of hepatic nodules. Acad Radiol. 2016;23(3):344-352.
23. Corwin MT, Fananapazir G, Jin M, Lamba R, Bashir MR. Difference in liver imaging and reporting data system categorization between MRI and CT. Am J Roentgenol. 2016;206(2):307-312.
Although hepatocellular carcinoma can be difficult to detect, use of the LI-RADS algorithm could lead to earlier identification in at-risk patients.
Although hepatocellular carcinoma can be difficult to detect, use of the LI-RADS algorithm could lead to earlier identification in at-risk patients.
Hepatocellular carcinoma (HCC) is the third most common cause of death from cancer worldwide.1 Liver cancer is the fifth most common cancer in men and the seventh in women.2 The highest incidence rates are in sub-Saharan Africa and Southeast Asia where hepatitis B virus is endemic. The incidence of HCC in western countries is increasing, particularly due to the rise of hepatitis C virus (HCV) as well as alcoholic liver disease and nonalcoholic fatty liver disease. The incidence of HCC has tripled in the US in the past 2 decades.1-3
HCC can be diagnosed by radiographic images without the need for biopsy if the typical imaging features are present.3 The European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend screening abdominal ultrasonography at 6-month intervals for high-risk patients.3,4 High-risk patients include patients with cirrhosis, especially those with hepatitis B or C.3
If screening ultrasonography detects a nodule, size determines whether a follow-up ultrasound is needed vs obtaining a contrast-enhanced dynamic computed tomography (CT) scan or a magnetic resonance image (MRI).3 If ultrasonography detects a nodule > 1 cm in diameter, then a dynamic CT or MRI is performed. Characteristic hyperenhancement during later arterial phase and washout during the venous or delayed phase is associated with a nearly 100% specificity for HCC diagnosis.5 Arterial-enhancing contrast is required when using CT and MRI because HCC is a hypervascular lesion.6 The portal venous blood dilutes the majority of the liver’s arterial blood; therefore, the liver does not enhance during the arterial phase, while HCC will show maximum enhancement.7 Furthermore, HCC should demonstrate a “washout” of contrast during the venous phase on CT and MRI.4 Standard imaging protocol dictates that 4 phases are needed to properly diagnose HCC including unenhanced, arterial, venous, and delayed.4
Regular surveillance increases the likelihood of detecting HCC before the presentation of clinical symptoms and facilitates receipt of curative therapy.8-10 Patients with viral hepatitis and cirrhosis with HCC found on screening are more likely to have earlier-stage disease and survive longer from the time of diagnosis.11 Furthermore, it has been observed that HCC detected by surveillance is significantly more likely to undergo curative therapy compared with incidental or symptomatic detection of HCC.9
Technical improvements in imaging techniques include advancement in contrast agents, multidetector row helical CT, and the flexibility/range of pulse sequences available in MRI.7 Even with technical improvements in all modalities used in HCC imaging, detecting HCC remains difficult, especially when detecting the small (< 2 cm) lesions in a cirrhotic liver.7 Interpretation of imaging also remains a challenge as HCC does not always fit strict criteria: lack of “washout” in a hypervascular lesion, determining small HCC lesions from benign nodules, and hypovascular/isovascular HCC.5 Radiologic differentials in the diagnosis of HCC include transient hepatic intensity difference (THID)/transient hepatic attenuation difference (THAD), arterio-portal shunt, and regenerative nodules.12 In the common clinical setting, patients undergo multiple imaging studies that are interpreted by multiple radiologists, which can add to the difficulty in the diagnosis of HCC.13
The radiology community recognized the inconsistencies and complexities of HCC imaging. Therefore, the American College of Radiology endorsed the Liver Imaging Reporting and Data System (LI-RADS), which had the goal of reducing variability in lesion interpretation through standardization and improving communication with clinicians.14 LI-RADS uses a diagnostic algorithm for CT and MRI that categorizes observed liver findings in high-risk individuals based on the probability or relative risk of HCC without assigning a formal diagnosis.14 LI-RADS takes into account arterial phase enhancement, tumor size, washout appearance, the presence and nature of a capsule, and threshold growth.15 LI-RADS categorizes an observed liver finding on a scale of 1 to 5, with 1 corresponding to a definitely benign finding and 5 with definitive HCC.14 Furthermore, LI-RADS sought to limit the technical variabilities among institutions.
LI-RADS was launched in 2011 and has been utilized by many clinical practices while continuing to be expanded and updated.16 Recent studies examined the specificity of LI-RADS as well as interreader variability.17,18 For nodules viewed on MRI, both LI-RADS categories 4 and 5 had high specificity for HCC.17 When looking at interreader repeatability, LI-RADS showed moderate agreement among experts using the diagnostic algorithm.19 Further studies have compared LI-RADS with the AASLD guidelines and the Organ Procurement and Transplantation Network (OPTN) guidelines.16 When compared with other guidelines, LI-RADS expands the definition of indeterminate findings into probably benign, intermediate probability of HCC, and probably HCC, which corresponds to LI-RADS categories 2, 3, and 4.16
We looked retrospectively at a group of patients previously diagnosed with HCC to see whether utilizing the LI-RADS scoring system within our screening system might have allowed an earlier prediction of HCC and a timelier intervention. Prior to this investigation the LI-RADS system was not used for HCC screening at our US Department of Veterans Affairs (VA) facility. We examined screened patients at the Memphis VA Medical Center (MVAMC) in Tennessee who were subsequently diagnosed with HCC to see which LI-RADS category the last surveillance CT prior to diagnosis would fall into, 6 months to a year prior to the diagnosis of HCC. Our control population was a group of patients screened with CT for their liver nodules who were found not to have HCC.
Methods
Patients at MVAMC with cirrhosis and patients with chronic hepatitis B are routinely screened with ultrasound, CT, or MRI in accordance with the AASLD, EASL, and VA guidelines. Of 303 patients with HCV and cirrhosis under care in 2015, 242 (81%) received imaging to screen for HCC according to the VA National Hepatitis C Registry 2015 (Personal Communication, Population Health Service, Office of Patient Care Services).The LI-RADS scoring system was not applied as a standard screening methodology.
Under an institutional review board-approved protocol, we reviewed the charts of all patients diagnosed with HCC at MVAMC from 2009 to 2014, utilizing ICD-9 code of 155.0 for HCC. We identified within these charts patients who had a surveillance CT image performed within a 6- to 13-month period prior to the CTs that diagnosed HCC (prediagnostic HCC CT). Furthermore, we reviewed the charts of all patients diagnosed with benign liver nodules at MVAMC from 2009 to 2014, utilizing the ICD-9 code of 573.8 for other specified disorders of the liver.
Within these charts, we found patients who had a surveillance CT image performed and who were followed after that image with additional imaging for ≥ 2 years or who had a liver biopsy negative for HCC (benign surveillance CT). We compared these 2 sets of CTs utilizing LI-RADS criteria. Once these patients were identified, a list of the CTs to be examined were given to 2 MVAMC radiologists who specialize in CT.
No identifying information of the patients was included, and a 13-digit number unique to each CT exam identified the CTs to be reviewed. Radiologist 1 and 2 examined the CTs on the MVAMC Picture Archiving and Communication System (PACS). Both radiologists were asked to give each nodule a score according to LI-RADS v2014 diagnostic algorithm (Figure).
We hypothesized that the prediagnostic CT images of patients eventually determined to have HCC would have a LI-RADS score of 4 (LR4) or LR5. Furthermore, we hypothesized that the CT images of the benign liver nodule patients would have a score ≤ LR3. If there was a disagreement between the radiologists in terms of a malignant score (LR4 or LR5) vs a benign score (≤ LR3), then a third radiologist (radiologist 3) provided a score for these nodules. The third, tiebreaker radiologist was given the scores of both prior radiologists and asked to choose which score was correct.
Statistical analysis was then applied to the data to determine the sensitivity, specificity, and diagnostic accuracy in diagnosing eventual HCC, as well as the false-negative and false-positive rates of radiologists 1 and 2. Raw data also were used to determine the agreement between raters by calculating the κ statistic with a 95% CI.
Results
A total of 70 nodules were examined by radiologists 1 and 2 with 42 of the nodules in the prediagnostic HCC CTs and 28 of the nodules in the benign surveillance CTs.
Radiologist 1 identified 11 patients with LR4 and 21 patients with LR5. His scores showed a sensitivity of 64.3% and specificity of 82.1% with accuracy of 71.4% for LI-RADS in identifying eventual HCC. The false-negative rate of the LI-RADS diagnostic algorithm for radiologist 1 was 35.7% and the false-positive rate was 17.9%. Radiologist 2 identified 17 patients LR4 and 19 patients with LR5. Radiologist 2’s scores showed a sensitivity of 69.0% and specificity of 75.0% with accuracy of 71.4% for LI-RADS in identifying eventual HCC.The false-negative rate of the LI-RADS diagnostic algorithm for radiologist 2 was 31.0% and false-positive rate of 25.0%. The κ statistic was calculated to determine the interrater agreement. The radiologists agreed on 58 of 70 samples; 15 without HCC and 43 with HCC. The κ statistic was 0.592, which indicates moderate agreement (Table 2).
Discussion
If HCC is diagnosed late in the disease process based on symptomatology and not on surveillance imaging, the likelihood of receiving early and potential curative therapy greatly declines as was shown in a systemic literature review.9 Surveillance imaging and lesion interpretation by various radiologists has been difficult to standardize as new technologic advances continue to occur in the imaging of HCC.14 LI-RADS was initiated to help standardize CT and MRI interpretation and reporting of hepatic nodules. As a dynamic algorithm, it continues to adjust with new advances in imaging techniques with the most recent updates being made to the algorithm in 2014.14,19 LI-RADS applies to patients at high risk for HCC most often who are already enrolled in a surveillance program.19 The MVAMC has a high incidence of patients with cirrhosis who are at risk for HCC, which is why we chose it as our study population.
LI-RADS can be applied to both MRI and CT imaging. Much of the recent literature have looked at LI-RADS in terms of MRI. A group in China looked at 100 pathologically confirmed patients and assigned a LI-RADS score to the MRI at the time of diagnosis and showed that MRI LI-RADS scoring was highly sensitive and specific in the diagnosis of HCC.20 This study did note a numeric difference in the specificity of LI-RADS algorithm depending on how LR3 scores were viewed. If a LR3 score was considered negative rather than positive for HCC, then the specificity increased by almost 20%.20
Another study looked at patients with liver nodules ≤ 20 mm found on ultrasound and obtained MRIs and biopsies on these patients, assigning the MRI a LI-RADs score.17 Darnell and colleagues found that MRI LR4 and LR5 have a high specificity for HCC. However, 29 of the 42 LR3 lesions examined were found to be HCC.17 Furthermore, Choi and colleagues retrospectively looked at patients in a HCC surveillance program who had undergone MRI as part of the program and assigned LI-RADS scores to these MRIs.21 Their study showed that LR5 criteria on gadoxetate disodium-enhanced MRI has excellent positive predictive value (PPV) for diagnosing HCC, and LR4 showed good PPV.21
In our study, we chose to look at LI-RADS in terms of surveillance CT scans 6 to 13 months prior to the diagnosis of HCC to see whether this method would allow us to intervene earlier with more aggressive diagnostics or therapy in those suspected of having HCC. Although Choi and colleagues looked retrospectively at MRI surveillance imaging, most of the prior studies have looked at LI-RADS scoring in imaging at the time of diagnosis.17,20,21 By looking at surveillance CT scans, we sought to determine LI-RADS sensitivity, specificity, and diagnostic accuracy as a screening tool compared with CT evaluations without LI-RADS scoring.
We also chose to look at CT scans since most of the prior studies have looked at the more detailed and often more expensive MRIs. For both radiologists 1 and 2, the sensitivity was > 60% and specificity was > 70% with a diagnostic accuracy of 71.4% in predicting a diagnosis of HCC in future scans. Although there was high false negative of > 30% for both radiologists, we did consider LR3 as negative for HCC. As Darnell and colleagues’ study of MRI LI-RADS shows, LR3 may need to be revised in the future as its ambiguity can lead to false-negatives.17 Our results also showed moderate interreader agreement, which has been seen in previous studies with LI-RADS.18
Some studies have compared MRI with CT imaging in terms of LI-RADs classification of hepatic nodules to find out whether concordance was seen.22,23 Both studies found that there was substantial discordance between MRI and CT with CT often underscoring hepatic nodules.22,23 In Zhang and colleagues, interclass agreement between CT and MRI varied the most in terms of arterial enhancement with CT producing false-negative findings.22 CT also underestimated LI-RADS score by 16.9% for LR3, 37.3% for LR4, and 8.5% for LR5 in this study.22 Furthermore, Corwin and colleagues found a significant upgrade in terms of LI-RADS categorization with MRI for 42.5% of observations.23 In this study, upgraded LI-RADS scores on MRI included 2 upgraded to LR5V (Figure), 15 upgraded to LR5, and 12 upgraded to LR4.23
Our study shows that the LI-RADS algorithm has a good sensitivity, specificity, and diagnostic accuracy as a screening tool, predicting HCC in scans earlier than standard CT evaluation. In our study, the patients with HCC were shown to have higher LI-RADS scores on prediagnostic imaging, while the benign liver nodule patients were shown to have lower LI-RADS scores. This data would suggest that a LI-RADS score given to surveillance CT of LR4 or higher should recommend either a biopsy or follow-up imaging after a short interval. If LI-RADS is applied to surveillance CTs in patients at risk for HCC, a diagnosis of HCC may be arrived at earlier as compared with not using the LI-RADS algorithm. Earlier detection may lead to earlier intervention and improved treatment outcomes.
Limitations
Limitations to our study occurred because radiologist 3 did not review all of the images nor score them. Radiologist 3 was limited to 12 images where there was disagreement and was limited to 2 scores to choose from for each image. Further limitations include that this study was performed at a single center. Our study focused on one imaging modality and did not include ultrasounds or MRIs. We did not compare the demographics of our patients with those of other VA hospitals. The radiologists interpreted the images individually, and their subjectivity was another limitation.
Conclusion
In the MVAMC population, LI-RADS showed a good sensitivity, specificity, and diagnostic accuracy for CT surveillance scans in patient at high risk for HCC at an earlier time point than did standard evaluation by very experienced CT radiologists. Higher LI-RADS scores on surveillance CTs had good diagnostic accuracy for the probable future diagnosis of HCC, whereas lower LI-RADS scores had a good diagnostic accuracy for probable benign nodules. Utilizing the LI-RADS algorithm on all surveillance CTs in patients at high risk for HCC may lead to obtaining MRIs or follow-up CTs sooner for suspicious nodules, leading to an earlier diagnosis of HCC and possible earlier and more effective intervention.
Hepatocellular carcinoma (HCC) is the third most common cause of death from cancer worldwide.1 Liver cancer is the fifth most common cancer in men and the seventh in women.2 The highest incidence rates are in sub-Saharan Africa and Southeast Asia where hepatitis B virus is endemic. The incidence of HCC in western countries is increasing, particularly due to the rise of hepatitis C virus (HCV) as well as alcoholic liver disease and nonalcoholic fatty liver disease. The incidence of HCC has tripled in the US in the past 2 decades.1-3
HCC can be diagnosed by radiographic images without the need for biopsy if the typical imaging features are present.3 The European Association for the Study of Liver Disease (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend screening abdominal ultrasonography at 6-month intervals for high-risk patients.3,4 High-risk patients include patients with cirrhosis, especially those with hepatitis B or C.3
If screening ultrasonography detects a nodule, size determines whether a follow-up ultrasound is needed vs obtaining a contrast-enhanced dynamic computed tomography (CT) scan or a magnetic resonance image (MRI).3 If ultrasonography detects a nodule > 1 cm in diameter, then a dynamic CT or MRI is performed. Characteristic hyperenhancement during later arterial phase and washout during the venous or delayed phase is associated with a nearly 100% specificity for HCC diagnosis.5 Arterial-enhancing contrast is required when using CT and MRI because HCC is a hypervascular lesion.6 The portal venous blood dilutes the majority of the liver’s arterial blood; therefore, the liver does not enhance during the arterial phase, while HCC will show maximum enhancement.7 Furthermore, HCC should demonstrate a “washout” of contrast during the venous phase on CT and MRI.4 Standard imaging protocol dictates that 4 phases are needed to properly diagnose HCC including unenhanced, arterial, venous, and delayed.4
Regular surveillance increases the likelihood of detecting HCC before the presentation of clinical symptoms and facilitates receipt of curative therapy.8-10 Patients with viral hepatitis and cirrhosis with HCC found on screening are more likely to have earlier-stage disease and survive longer from the time of diagnosis.11 Furthermore, it has been observed that HCC detected by surveillance is significantly more likely to undergo curative therapy compared with incidental or symptomatic detection of HCC.9
Technical improvements in imaging techniques include advancement in contrast agents, multidetector row helical CT, and the flexibility/range of pulse sequences available in MRI.7 Even with technical improvements in all modalities used in HCC imaging, detecting HCC remains difficult, especially when detecting the small (< 2 cm) lesions in a cirrhotic liver.7 Interpretation of imaging also remains a challenge as HCC does not always fit strict criteria: lack of “washout” in a hypervascular lesion, determining small HCC lesions from benign nodules, and hypovascular/isovascular HCC.5 Radiologic differentials in the diagnosis of HCC include transient hepatic intensity difference (THID)/transient hepatic attenuation difference (THAD), arterio-portal shunt, and regenerative nodules.12 In the common clinical setting, patients undergo multiple imaging studies that are interpreted by multiple radiologists, which can add to the difficulty in the diagnosis of HCC.13
The radiology community recognized the inconsistencies and complexities of HCC imaging. Therefore, the American College of Radiology endorsed the Liver Imaging Reporting and Data System (LI-RADS), which had the goal of reducing variability in lesion interpretation through standardization and improving communication with clinicians.14 LI-RADS uses a diagnostic algorithm for CT and MRI that categorizes observed liver findings in high-risk individuals based on the probability or relative risk of HCC without assigning a formal diagnosis.14 LI-RADS takes into account arterial phase enhancement, tumor size, washout appearance, the presence and nature of a capsule, and threshold growth.15 LI-RADS categorizes an observed liver finding on a scale of 1 to 5, with 1 corresponding to a definitely benign finding and 5 with definitive HCC.14 Furthermore, LI-RADS sought to limit the technical variabilities among institutions.
LI-RADS was launched in 2011 and has been utilized by many clinical practices while continuing to be expanded and updated.16 Recent studies examined the specificity of LI-RADS as well as interreader variability.17,18 For nodules viewed on MRI, both LI-RADS categories 4 and 5 had high specificity for HCC.17 When looking at interreader repeatability, LI-RADS showed moderate agreement among experts using the diagnostic algorithm.19 Further studies have compared LI-RADS with the AASLD guidelines and the Organ Procurement and Transplantation Network (OPTN) guidelines.16 When compared with other guidelines, LI-RADS expands the definition of indeterminate findings into probably benign, intermediate probability of HCC, and probably HCC, which corresponds to LI-RADS categories 2, 3, and 4.16
We looked retrospectively at a group of patients previously diagnosed with HCC to see whether utilizing the LI-RADS scoring system within our screening system might have allowed an earlier prediction of HCC and a timelier intervention. Prior to this investigation the LI-RADS system was not used for HCC screening at our US Department of Veterans Affairs (VA) facility. We examined screened patients at the Memphis VA Medical Center (MVAMC) in Tennessee who were subsequently diagnosed with HCC to see which LI-RADS category the last surveillance CT prior to diagnosis would fall into, 6 months to a year prior to the diagnosis of HCC. Our control population was a group of patients screened with CT for their liver nodules who were found not to have HCC.
Methods
Patients at MVAMC with cirrhosis and patients with chronic hepatitis B are routinely screened with ultrasound, CT, or MRI in accordance with the AASLD, EASL, and VA guidelines. Of 303 patients with HCV and cirrhosis under care in 2015, 242 (81%) received imaging to screen for HCC according to the VA National Hepatitis C Registry 2015 (Personal Communication, Population Health Service, Office of Patient Care Services).The LI-RADS scoring system was not applied as a standard screening methodology.
Under an institutional review board-approved protocol, we reviewed the charts of all patients diagnosed with HCC at MVAMC from 2009 to 2014, utilizing ICD-9 code of 155.0 for HCC. We identified within these charts patients who had a surveillance CT image performed within a 6- to 13-month period prior to the CTs that diagnosed HCC (prediagnostic HCC CT). Furthermore, we reviewed the charts of all patients diagnosed with benign liver nodules at MVAMC from 2009 to 2014, utilizing the ICD-9 code of 573.8 for other specified disorders of the liver.
Within these charts, we found patients who had a surveillance CT image performed and who were followed after that image with additional imaging for ≥ 2 years or who had a liver biopsy negative for HCC (benign surveillance CT). We compared these 2 sets of CTs utilizing LI-RADS criteria. Once these patients were identified, a list of the CTs to be examined were given to 2 MVAMC radiologists who specialize in CT.
No identifying information of the patients was included, and a 13-digit number unique to each CT exam identified the CTs to be reviewed. Radiologist 1 and 2 examined the CTs on the MVAMC Picture Archiving and Communication System (PACS). Both radiologists were asked to give each nodule a score according to LI-RADS v2014 diagnostic algorithm (Figure).
We hypothesized that the prediagnostic CT images of patients eventually determined to have HCC would have a LI-RADS score of 4 (LR4) or LR5. Furthermore, we hypothesized that the CT images of the benign liver nodule patients would have a score ≤ LR3. If there was a disagreement between the radiologists in terms of a malignant score (LR4 or LR5) vs a benign score (≤ LR3), then a third radiologist (radiologist 3) provided a score for these nodules. The third, tiebreaker radiologist was given the scores of both prior radiologists and asked to choose which score was correct.
Statistical analysis was then applied to the data to determine the sensitivity, specificity, and diagnostic accuracy in diagnosing eventual HCC, as well as the false-negative and false-positive rates of radiologists 1 and 2. Raw data also were used to determine the agreement between raters by calculating the κ statistic with a 95% CI.
Results
A total of 70 nodules were examined by radiologists 1 and 2 with 42 of the nodules in the prediagnostic HCC CTs and 28 of the nodules in the benign surveillance CTs.
Radiologist 1 identified 11 patients with LR4 and 21 patients with LR5. His scores showed a sensitivity of 64.3% and specificity of 82.1% with accuracy of 71.4% for LI-RADS in identifying eventual HCC. The false-negative rate of the LI-RADS diagnostic algorithm for radiologist 1 was 35.7% and the false-positive rate was 17.9%. Radiologist 2 identified 17 patients LR4 and 19 patients with LR5. Radiologist 2’s scores showed a sensitivity of 69.0% and specificity of 75.0% with accuracy of 71.4% for LI-RADS in identifying eventual HCC.The false-negative rate of the LI-RADS diagnostic algorithm for radiologist 2 was 31.0% and false-positive rate of 25.0%. The κ statistic was calculated to determine the interrater agreement. The radiologists agreed on 58 of 70 samples; 15 without HCC and 43 with HCC. The κ statistic was 0.592, which indicates moderate agreement (Table 2).
Discussion
If HCC is diagnosed late in the disease process based on symptomatology and not on surveillance imaging, the likelihood of receiving early and potential curative therapy greatly declines as was shown in a systemic literature review.9 Surveillance imaging and lesion interpretation by various radiologists has been difficult to standardize as new technologic advances continue to occur in the imaging of HCC.14 LI-RADS was initiated to help standardize CT and MRI interpretation and reporting of hepatic nodules. As a dynamic algorithm, it continues to adjust with new advances in imaging techniques with the most recent updates being made to the algorithm in 2014.14,19 LI-RADS applies to patients at high risk for HCC most often who are already enrolled in a surveillance program.19 The MVAMC has a high incidence of patients with cirrhosis who are at risk for HCC, which is why we chose it as our study population.
LI-RADS can be applied to both MRI and CT imaging. Much of the recent literature have looked at LI-RADS in terms of MRI. A group in China looked at 100 pathologically confirmed patients and assigned a LI-RADS score to the MRI at the time of diagnosis and showed that MRI LI-RADS scoring was highly sensitive and specific in the diagnosis of HCC.20 This study did note a numeric difference in the specificity of LI-RADS algorithm depending on how LR3 scores were viewed. If a LR3 score was considered negative rather than positive for HCC, then the specificity increased by almost 20%.20
Another study looked at patients with liver nodules ≤ 20 mm found on ultrasound and obtained MRIs and biopsies on these patients, assigning the MRI a LI-RADs score.17 Darnell and colleagues found that MRI LR4 and LR5 have a high specificity for HCC. However, 29 of the 42 LR3 lesions examined were found to be HCC.17 Furthermore, Choi and colleagues retrospectively looked at patients in a HCC surveillance program who had undergone MRI as part of the program and assigned LI-RADS scores to these MRIs.21 Their study showed that LR5 criteria on gadoxetate disodium-enhanced MRI has excellent positive predictive value (PPV) for diagnosing HCC, and LR4 showed good PPV.21
In our study, we chose to look at LI-RADS in terms of surveillance CT scans 6 to 13 months prior to the diagnosis of HCC to see whether this method would allow us to intervene earlier with more aggressive diagnostics or therapy in those suspected of having HCC. Although Choi and colleagues looked retrospectively at MRI surveillance imaging, most of the prior studies have looked at LI-RADS scoring in imaging at the time of diagnosis.17,20,21 By looking at surveillance CT scans, we sought to determine LI-RADS sensitivity, specificity, and diagnostic accuracy as a screening tool compared with CT evaluations without LI-RADS scoring.
We also chose to look at CT scans since most of the prior studies have looked at the more detailed and often more expensive MRIs. For both radiologists 1 and 2, the sensitivity was > 60% and specificity was > 70% with a diagnostic accuracy of 71.4% in predicting a diagnosis of HCC in future scans. Although there was high false negative of > 30% for both radiologists, we did consider LR3 as negative for HCC. As Darnell and colleagues’ study of MRI LI-RADS shows, LR3 may need to be revised in the future as its ambiguity can lead to false-negatives.17 Our results also showed moderate interreader agreement, which has been seen in previous studies with LI-RADS.18
Some studies have compared MRI with CT imaging in terms of LI-RADs classification of hepatic nodules to find out whether concordance was seen.22,23 Both studies found that there was substantial discordance between MRI and CT with CT often underscoring hepatic nodules.22,23 In Zhang and colleagues, interclass agreement between CT and MRI varied the most in terms of arterial enhancement with CT producing false-negative findings.22 CT also underestimated LI-RADS score by 16.9% for LR3, 37.3% for LR4, and 8.5% for LR5 in this study.22 Furthermore, Corwin and colleagues found a significant upgrade in terms of LI-RADS categorization with MRI for 42.5% of observations.23 In this study, upgraded LI-RADS scores on MRI included 2 upgraded to LR5V (Figure), 15 upgraded to LR5, and 12 upgraded to LR4.23
Our study shows that the LI-RADS algorithm has a good sensitivity, specificity, and diagnostic accuracy as a screening tool, predicting HCC in scans earlier than standard CT evaluation. In our study, the patients with HCC were shown to have higher LI-RADS scores on prediagnostic imaging, while the benign liver nodule patients were shown to have lower LI-RADS scores. This data would suggest that a LI-RADS score given to surveillance CT of LR4 or higher should recommend either a biopsy or follow-up imaging after a short interval. If LI-RADS is applied to surveillance CTs in patients at risk for HCC, a diagnosis of HCC may be arrived at earlier as compared with not using the LI-RADS algorithm. Earlier detection may lead to earlier intervention and improved treatment outcomes.
Limitations
Limitations to our study occurred because radiologist 3 did not review all of the images nor score them. Radiologist 3 was limited to 12 images where there was disagreement and was limited to 2 scores to choose from for each image. Further limitations include that this study was performed at a single center. Our study focused on one imaging modality and did not include ultrasounds or MRIs. We did not compare the demographics of our patients with those of other VA hospitals. The radiologists interpreted the images individually, and their subjectivity was another limitation.
Conclusion
In the MVAMC population, LI-RADS showed a good sensitivity, specificity, and diagnostic accuracy for CT surveillance scans in patient at high risk for HCC at an earlier time point than did standard evaluation by very experienced CT radiologists. Higher LI-RADS scores on surveillance CTs had good diagnostic accuracy for the probable future diagnosis of HCC, whereas lower LI-RADS scores had a good diagnostic accuracy for probable benign nodules. Utilizing the LI-RADS algorithm on all surveillance CTs in patients at high risk for HCC may lead to obtaining MRIs or follow-up CTs sooner for suspicious nodules, leading to an earlier diagnosis of HCC and possible earlier and more effective intervention.
1. El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
2. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
3. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
4. Selvapatt N, House H, Brown A. Hepatocellular carcinoma surveillance: are we utilizing it? J Clin Gastroenterol. 2016;50(1):e8-e12.
5. Lee JM, Yoon JH, Joo I, Woo HS. Recent advances in CT and MR imaging for evaluation of hepatocellular carcinoma. Liver Cancer. 2012;1(1):22-40.
6. Chou R, Cuevas C, Fu R, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systemic review and meta-analysis. Ann Intern Med. 2015;162(10):697-711.
7. Ariff B, Lloyd CR, Khan S, et al. Imaging of liver cancer. World J Gastroenterol. 2009;15(11):1289-1300.
8. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31(2):330-335.
9. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624.
10. Nusbaum, JD, Smirniotopoulos J, Wright HC, et al. The effect of hepatocellular carcinoma surveillance in an urban population with liver cirrhosis. J Clin Gastroenterol. 2015;49(10):e91-e95.
11. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systemic review. Ann Intern Med. 2014;161(4):261-269.
12. Shah S, Shukla A, Paunipagar B. Radiological features of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(suppl 3):S63-S66.
13. You MW, Kim SY, Kim KW, et al. Recent advances in the imaging of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21(1):95-103.
14. American College of Radiology. Liver reporting and data system (LI-RADS). https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS. Accessed April 10, 2018.
15. Anis M. Imaging of hepatocellular carcinoma: new approaches to diagnosis. Clin Liver Dis. 2015;19(2):325-340.
16. Mitchell D, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61(3):1056-1065.
17. Darnell A, Forner A, Rimola J, et al. Liver imaging reporting and data system with MR imaging: evaluation in nodules 20 mm or smaller detected in cirrhosis at screening US. Radiology. 2015; 275(3):698-707.
18. Davenport MS, Khalatbari S, Liu PS, et al. Repeatability of diagnostic features and scoring systems for hepatocellular carcinoma by using MR imaging. Radiology. 2014;272(1):132-142.
19. An C, Rakhmonova G, Choi JY, Kim MJ. Liver imaging reporting and data system (LI-RADS) version 2014: understanding and application of the diagnostic algorithm. Clin Mol Hepatol. 2016;22(2):296-307.
20. Zhao W, Li W, Yi X, et al. [Diagnostic value of liver imaging reporting and data system on primary hepatocellular carcinoma] [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41(4):380-387.
21. Choi SH, Byun JH, Kim SY, et al. Liver imaging reporting and data system v2014 with gadoxetate disodium-enhanced magnetic resonance imaging: validation of LIRADS category 4 and 5 criteria. Invest Radiol. 2016;51(8):483-490.
22. Zhang YD, Zhu FP, Xu X, et al. Liver imaging reporting and data system: substantial discordance between CT and MR for imaging classification of hepatic nodules. Acad Radiol. 2016;23(3):344-352.
23. Corwin MT, Fananapazir G, Jin M, Lamba R, Bashir MR. Difference in liver imaging and reporting data system categorization between MRI and CT. Am J Roentgenol. 2016;206(2):307-312.
1. El–Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
2. El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
3. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
4. Selvapatt N, House H, Brown A. Hepatocellular carcinoma surveillance: are we utilizing it? J Clin Gastroenterol. 2016;50(1):e8-e12.
5. Lee JM, Yoon JH, Joo I, Woo HS. Recent advances in CT and MR imaging for evaluation of hepatocellular carcinoma. Liver Cancer. 2012;1(1):22-40.
6. Chou R, Cuevas C, Fu R, et al. Imaging techniques for the diagnosis of hepatocellular carcinoma: a systemic review and meta-analysis. Ann Intern Med. 2015;162(10):697-711.
7. Ariff B, Lloyd CR, Khan S, et al. Imaging of liver cancer. World J Gastroenterol. 2009;15(11):1289-1300.
8. Yuen MF, Cheng CC, Lauder IJ, Lam SK, Ooi CG, Lai CL. Early detection of hepatocellular carcinoma increases the chance of treatment: Hong Kong experience. Hepatology. 2000;31(2):330-335.
9. Singal AG, Pillai A, Tiro J. Early detection, curative treatment, and survival rates for hepatocellular carcinoma surveillance in patients with cirrhosis: a meta-analysis. PLoS Med. 2014;11(4):e1001624.
10. Nusbaum, JD, Smirniotopoulos J, Wright HC, et al. The effect of hepatocellular carcinoma surveillance in an urban population with liver cirrhosis. J Clin Gastroenterol. 2015;49(10):e91-e95.
11. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systemic review. Ann Intern Med. 2014;161(4):261-269.
12. Shah S, Shukla A, Paunipagar B. Radiological features of hepatocellular carcinoma. J Clin Exp Hepatol. 2014;4(suppl 3):S63-S66.
13. You MW, Kim SY, Kim KW, et al. Recent advances in the imaging of hepatocellular carcinoma. Clin Mol Hepatol. 2015;21(1):95-103.
14. American College of Radiology. Liver reporting and data system (LI-RADS). https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS. Accessed April 10, 2018.
15. Anis M. Imaging of hepatocellular carcinoma: new approaches to diagnosis. Clin Liver Dis. 2015;19(2):325-340.
16. Mitchell D, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61(3):1056-1065.
17. Darnell A, Forner A, Rimola J, et al. Liver imaging reporting and data system with MR imaging: evaluation in nodules 20 mm or smaller detected in cirrhosis at screening US. Radiology. 2015; 275(3):698-707.
18. Davenport MS, Khalatbari S, Liu PS, et al. Repeatability of diagnostic features and scoring systems for hepatocellular carcinoma by using MR imaging. Radiology. 2014;272(1):132-142.
19. An C, Rakhmonova G, Choi JY, Kim MJ. Liver imaging reporting and data system (LI-RADS) version 2014: understanding and application of the diagnostic algorithm. Clin Mol Hepatol. 2016;22(2):296-307.
20. Zhao W, Li W, Yi X, et al. [Diagnostic value of liver imaging reporting and data system on primary hepatocellular carcinoma] [in Chinese]. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2016;41(4):380-387.
21. Choi SH, Byun JH, Kim SY, et al. Liver imaging reporting and data system v2014 with gadoxetate disodium-enhanced magnetic resonance imaging: validation of LIRADS category 4 and 5 criteria. Invest Radiol. 2016;51(8):483-490.
22. Zhang YD, Zhu FP, Xu X, et al. Liver imaging reporting and data system: substantial discordance between CT and MR for imaging classification of hepatic nodules. Acad Radiol. 2016;23(3):344-352.
23. Corwin MT, Fananapazir G, Jin M, Lamba R, Bashir MR. Difference in liver imaging and reporting data system categorization between MRI and CT. Am J Roentgenol. 2016;206(2):307-312.
Are patients with epilepsy at increased risk of COVID-19 infection?
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
Chronic conditions such as lung disease, diabetes, and heart disease frequently receive attention for increasing the risk of complications for people who contract the coronavirus. Meanwhile, many members of the epilepsy community continue to wonder how the virus affects them. To address these concerns, the Epilepsy Foundation has released information that answers many common questions that people with epilepsy have about how COVID-19 can impact their health.
Perhaps the most pressing of these questions is: Does epilepsy increase the risk or severity of the coronavirus?
“The most common thing we’re hearing from patients in my practice is their proactive concern for being at increased risk for getting the coronavirus,” confirmed Selim Benbadis, MD, division director, epilepsy, EEG, and sleep medicine at the University of South Florida in Tampa. “Epilepsy patients are not at increased risk for complications from the coronavirus because epilepsy does not affect the immune system.”
In other words, people who have epilepsy face the same health challenges as people who do not have the condition and are otherwise healthy. For this reason, people who have epilepsy should exercise the same habits and preventative measures that healthy people would typically take, such as social distancing; avoiding contact with sick people; washing hands regularly; disinfecting surfaces regularly; and avoiding touching hands, eyes, nose and mouth.
However, as Dr. Benbadis explained, the high fever associated with coronavirus can trigger seizures. The increased risk is another reason people who have epilepsy should do their best to avoid getting sick.
Seizure medications do not increase COVID-19 risk but other conditions can
Similarly, epilepsy medications do not increase the risk of contracting the disease.
“The medications patients take to treat their epilepsy do not affect their immune system,” said Andrew Wilner, MD, associate professor of neurology at the University of Tennessee Health Science Center, Memphis. There are a few exceptions – such as adrenocorticotropic hormone and everolimus – but doctors rarely use these drugs to treat epilepsy.
However, there are some situations and conditions that may pose a risk for people who contact the coronavirus. For instance, people who have problems swallowing their food and tend to suck food down their windpipes are more likely to develop pneumonia. Also, much like the general population, having diabetes, heart disease, or lung problems increase the chances of developing complications from the virus.
The best ways to avoid additional risks in epilepsy
Because of the pandemic, people who have epilepsy may have found that many of their doctors’ appointments have been canceled. Many clinics and medical practices have done this in order minimize exposing people who have acute illnesses to the virus. By focusing more on patients with acute conditions, doctors and nurses can better tend to patients with acute problems. As a result, practices have shifted to providing patient care using telemedicine as much as possible.
“Telemedicine services have surged, and I’ve been saying for years that telemedicine was going to grow,” Dr. Benbadis said. “It’s more convenient, and I believe that we’re going to see increased use of telemedicine long after the coronavirus pandemic is over.”
Aside from communicating with their doctors, the Epilepsy Foundation and Dr. Wilner stress that the best way for people who have epilepsy to stay healthy is by taking their medications on a regular basis exactly as prescribed.
“Taking mediation correctly and regularly is the best strategy for epilepsy patients to avoid unnecessary hospitalizations,” Dr. Wilner said. “If they have breakthrough seizures and get sent to the emergency room, then they risk being exposed to the virus in the ER.”
Also, because ERs are more crowded than usual, the Epilepsy Foundation encourages people who suspect they have the coronavirus to call their doctor’s office first. The goal is to try to make sure that people who have severe or life-threatening symptoms have access to treatment in the ER.
As with the general population, the first thing that epilepsy patients who suspect they have the coronavirus should do is call his or her doctor’s office. The health care professional taking the call will ask the patient a series of questions to determine whether the patient has COVID-19 or another condition or needs to seek emergency medical attention.
Fever, cough, and trouble breathing fall among the most commonly reported symptoms of the coronavirus. In many cases, health care providers recommend that people with mild versions of these symptoms stay at home.
Helpful tips
The Epilepsy Foundation offers tips on signs to look for when trying to figure out when a seizure requires an ER visit. These are:
- Seizures in which awareness is lost for more than 5 minutes and no reversal medications are available.
- Seizures with an unusual pattern or duration.
- Seizures that cannot be treated safely at home or are not responding to rescue medication even after the medication has had enough time to work.
- Seizures that occur after a severe blow to the head.
Additionally, while COVID-19 can cause death and sudden death in patients, the virus does not cause sudden unexpected death in epilepsy (SUDEP). Because SUDEP is extremely rare, Dr. Benbadis said that there is no information to suggest that contracting the coronavirus will increase the risk,
Finally, no shortages of seizures medications have been reported as a result of COVID-19. However, there were shortages of generic levetiracetam immediate-release and levetiracetam extended-release medications prior to and during COVID-19. Experts expect the shortage to continue.
Overall, people who have epilepsy should be able to stay healthy – provided they exercise healthy and preventative habits.
“The majority of epilepsy patients should be reassured that if they continue their usual care, take their meds as directed, get adequate sleep, nutritious diet, they’re not at any increased risk compared to the general population,” said Dr. Wilner.
Dr. Benbadis reported the following disclosures: consultant for Bioserenity (DigiTrace), Brain Sentinel, Cavion, Ceribell, Eisai, Greenwich, LivaNova, Neuropace, SK biopharmaceuticals, Sunovion; speakers bureau for Eisai, Greenwich, LivaNova, Sunovion; Florida Medical Director of Stratus/Alliance; Member: Epilepsy Study Consortium; grant support from Cavion, LivaNova, Greenwich, SK biopharmaceuticals, Sunovion, Takeda, UCB, Xenon; royalties as an author or editor for Emedicine-Medscape-WebMD, UpToDate; editorial board for the Epilepsy.com (Epilepsy Foundation) controversy section, Emedicine-Medscape-WebMD, Epileptic Disorders, Epilepsy and Behavior, and Expert Review of Neurotherapeutics. Dr. Wilner reports Medical Advisory Board of Accordant Health Services, Greensboro, S.C., and book royalties: “The Locum Life: A Physician’s Guide to Locum Tenens,” Lulu Press.
COVID-19: Experts hasten to head off mental health crisis
The COVID-19 pandemic is already affecting mental health at a population level, with increased anxiety, feelings of isolation, and concerns about access to mental health care.
Two U.K. surveys were conducted to inform research priorities for mental health research and in an effort to head off a mental health crisis. The U.K. charity MQ conducted a “stakeholder” survey of 2,198 individuals who had a lived experience of mental illness, while Ipsos MORI conducted a poll of 1,099 members of the public.
The online surveys were conducted in late March, the same week the U.K.’s nationwide lockdown measures were announced. Respondents were asked about their biggest mental health and well-being concerns and coping strategies as they relate to the COVID-19 pandemic.
Results showed that across the two surveys, respondents’ primary concern was anxiety, which was cited in 750 responses.
In addition, respondents were worried about being social isolated, becoming mentally unwell, and having a lack of access to mental health services, as well as the impact of the pandemic on personal relationships.
The findings were used by a panel of experts to inform a position paper published in the Lancet Psychiatry. The paper outlines a proposed government response to curb the long-term “profound” and “pervasive” impact of the pandemic on mental health.
‘Unprecedented response’ needed
“Governments must find evidence-based ways to boost the resilience of our societies and ... to treat those with mental ill health remotely to come out of this pandemic in good mental health,” coauthor of the paper Emily A. Holmes, PhD, of the department of psychology at Uppsala (Sweden) University, said in a press release.
“Frontline medical staff and vulnerable groups such as the elderly and those with serious mental health conditions must be prioritized for rapid mental health support,” she added.
The position paper authors call for “moment-to-moment” monitoring of anxiety, depression, self-harm, and suicide, as well as using digital technology and rapid deployment of evidence-based programs and treatments.
Patients will need to be accessible via computer, cell phone, and other remote technologies in order to receive treatment during physical isolation. However, they noted that there is no “one-size-fits-all” approach, and novel approaches custom tailored to particular populations, including frontline health care workers, are necessary.
“To make a real difference we will need to harness the tools of our digital age, finding smart new ways to measure the mental health of individuals remotely, finding creative ways to boost resilience, and finding ways to treat people in their homes. This effort must be considered central to our global response to the pandemic,” coauthor Ed Bullmore, PhD, of the department of psychiatry at the University of Cambridge (England), said in a statement.
Dr. Bullmore added that it will take “unprecedented research response if we are to limit the negative consequences of this pandemic on the mental health of our society now and in the future.”
Most vulnerable will bear the brunt
During a webinar held to discuss the paper, Matthew Hotopf, PhD, of the Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, cautioned that society’s most vulnerable citizens will bear the brunt of the pandemic’s mental health consequences.
“These individuals often have unstable housing, unstable work, and are disadvantaged in terms of their physical health and their mental health,” with a “very significant gap” in life expectancy versus the rest of the population, he said. The COVID-19 pandemic will widen the gap between “the haves and the have nots.”
“People with established and significant mental disorders are one version of the ‘have nots’ but actually it applies to a lot of people,” said Dr. Hotopf, noting that his experience of lockdown is “very different” from that of someone “living in overcrowded, unstable accommodation, with kids running around and maybe a partner who has problems with anger control.”
The authors of the position paper noted that the COVID-19 pandemic highlights several important research priorities that need to be addressed in the coming weeks and months. These include:
- Understanding the effect of COVID-19 on risk of anxiety, depression, and other outcomes, such as self-harm and suicide
- Understanding how to create physical and social supports to ensure mental health in a climate of physical distancing
- Determining the mental health consequences of social isolation for vulnerable groups, and how can these be mitigated under pandemic conditions
- Understanding the mental health impact of media reporting of COVID-19 in traditional and social media
- Determining the best methods for promoting successful adherence to behavioral advice about COVID-19 while enabling mental well-being and minimizing distress
Another area highlighted by the experts is the potential for neuropsychiatric sequelae in individuals infected with COVID-19. They called for “experimental medicine studies to validate clinical biomarkers and repurpose new treatments for the potentially neurotoxic effects of the virus.”
The authors/investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic is already affecting mental health at a population level, with increased anxiety, feelings of isolation, and concerns about access to mental health care.
Two U.K. surveys were conducted to inform research priorities for mental health research and in an effort to head off a mental health crisis. The U.K. charity MQ conducted a “stakeholder” survey of 2,198 individuals who had a lived experience of mental illness, while Ipsos MORI conducted a poll of 1,099 members of the public.
The online surveys were conducted in late March, the same week the U.K.’s nationwide lockdown measures were announced. Respondents were asked about their biggest mental health and well-being concerns and coping strategies as they relate to the COVID-19 pandemic.
Results showed that across the two surveys, respondents’ primary concern was anxiety, which was cited in 750 responses.
In addition, respondents were worried about being social isolated, becoming mentally unwell, and having a lack of access to mental health services, as well as the impact of the pandemic on personal relationships.
The findings were used by a panel of experts to inform a position paper published in the Lancet Psychiatry. The paper outlines a proposed government response to curb the long-term “profound” and “pervasive” impact of the pandemic on mental health.
‘Unprecedented response’ needed
“Governments must find evidence-based ways to boost the resilience of our societies and ... to treat those with mental ill health remotely to come out of this pandemic in good mental health,” coauthor of the paper Emily A. Holmes, PhD, of the department of psychology at Uppsala (Sweden) University, said in a press release.
“Frontline medical staff and vulnerable groups such as the elderly and those with serious mental health conditions must be prioritized for rapid mental health support,” she added.
The position paper authors call for “moment-to-moment” monitoring of anxiety, depression, self-harm, and suicide, as well as using digital technology and rapid deployment of evidence-based programs and treatments.
Patients will need to be accessible via computer, cell phone, and other remote technologies in order to receive treatment during physical isolation. However, they noted that there is no “one-size-fits-all” approach, and novel approaches custom tailored to particular populations, including frontline health care workers, are necessary.
“To make a real difference we will need to harness the tools of our digital age, finding smart new ways to measure the mental health of individuals remotely, finding creative ways to boost resilience, and finding ways to treat people in their homes. This effort must be considered central to our global response to the pandemic,” coauthor Ed Bullmore, PhD, of the department of psychiatry at the University of Cambridge (England), said in a statement.
Dr. Bullmore added that it will take “unprecedented research response if we are to limit the negative consequences of this pandemic on the mental health of our society now and in the future.”
Most vulnerable will bear the brunt
During a webinar held to discuss the paper, Matthew Hotopf, PhD, of the Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, cautioned that society’s most vulnerable citizens will bear the brunt of the pandemic’s mental health consequences.
“These individuals often have unstable housing, unstable work, and are disadvantaged in terms of their physical health and their mental health,” with a “very significant gap” in life expectancy versus the rest of the population, he said. The COVID-19 pandemic will widen the gap between “the haves and the have nots.”
“People with established and significant mental disorders are one version of the ‘have nots’ but actually it applies to a lot of people,” said Dr. Hotopf, noting that his experience of lockdown is “very different” from that of someone “living in overcrowded, unstable accommodation, with kids running around and maybe a partner who has problems with anger control.”
The authors of the position paper noted that the COVID-19 pandemic highlights several important research priorities that need to be addressed in the coming weeks and months. These include:
- Understanding the effect of COVID-19 on risk of anxiety, depression, and other outcomes, such as self-harm and suicide
- Understanding how to create physical and social supports to ensure mental health in a climate of physical distancing
- Determining the mental health consequences of social isolation for vulnerable groups, and how can these be mitigated under pandemic conditions
- Understanding the mental health impact of media reporting of COVID-19 in traditional and social media
- Determining the best methods for promoting successful adherence to behavioral advice about COVID-19 while enabling mental well-being and minimizing distress
Another area highlighted by the experts is the potential for neuropsychiatric sequelae in individuals infected with COVID-19. They called for “experimental medicine studies to validate clinical biomarkers and repurpose new treatments for the potentially neurotoxic effects of the virus.”
The authors/investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic is already affecting mental health at a population level, with increased anxiety, feelings of isolation, and concerns about access to mental health care.
Two U.K. surveys were conducted to inform research priorities for mental health research and in an effort to head off a mental health crisis. The U.K. charity MQ conducted a “stakeholder” survey of 2,198 individuals who had a lived experience of mental illness, while Ipsos MORI conducted a poll of 1,099 members of the public.
The online surveys were conducted in late March, the same week the U.K.’s nationwide lockdown measures were announced. Respondents were asked about their biggest mental health and well-being concerns and coping strategies as they relate to the COVID-19 pandemic.
Results showed that across the two surveys, respondents’ primary concern was anxiety, which was cited in 750 responses.
In addition, respondents were worried about being social isolated, becoming mentally unwell, and having a lack of access to mental health services, as well as the impact of the pandemic on personal relationships.
The findings were used by a panel of experts to inform a position paper published in the Lancet Psychiatry. The paper outlines a proposed government response to curb the long-term “profound” and “pervasive” impact of the pandemic on mental health.
‘Unprecedented response’ needed
“Governments must find evidence-based ways to boost the resilience of our societies and ... to treat those with mental ill health remotely to come out of this pandemic in good mental health,” coauthor of the paper Emily A. Holmes, PhD, of the department of psychology at Uppsala (Sweden) University, said in a press release.
“Frontline medical staff and vulnerable groups such as the elderly and those with serious mental health conditions must be prioritized for rapid mental health support,” she added.
The position paper authors call for “moment-to-moment” monitoring of anxiety, depression, self-harm, and suicide, as well as using digital technology and rapid deployment of evidence-based programs and treatments.
Patients will need to be accessible via computer, cell phone, and other remote technologies in order to receive treatment during physical isolation. However, they noted that there is no “one-size-fits-all” approach, and novel approaches custom tailored to particular populations, including frontline health care workers, are necessary.
“To make a real difference we will need to harness the tools of our digital age, finding smart new ways to measure the mental health of individuals remotely, finding creative ways to boost resilience, and finding ways to treat people in their homes. This effort must be considered central to our global response to the pandemic,” coauthor Ed Bullmore, PhD, of the department of psychiatry at the University of Cambridge (England), said in a statement.
Dr. Bullmore added that it will take “unprecedented research response if we are to limit the negative consequences of this pandemic on the mental health of our society now and in the future.”
Most vulnerable will bear the brunt
During a webinar held to discuss the paper, Matthew Hotopf, PhD, of the Institute of Psychiatry, Psychology, and Neuroscience at King’s College London, cautioned that society’s most vulnerable citizens will bear the brunt of the pandemic’s mental health consequences.
“These individuals often have unstable housing, unstable work, and are disadvantaged in terms of their physical health and their mental health,” with a “very significant gap” in life expectancy versus the rest of the population, he said. The COVID-19 pandemic will widen the gap between “the haves and the have nots.”
“People with established and significant mental disorders are one version of the ‘have nots’ but actually it applies to a lot of people,” said Dr. Hotopf, noting that his experience of lockdown is “very different” from that of someone “living in overcrowded, unstable accommodation, with kids running around and maybe a partner who has problems with anger control.”
The authors of the position paper noted that the COVID-19 pandemic highlights several important research priorities that need to be addressed in the coming weeks and months. These include:
- Understanding the effect of COVID-19 on risk of anxiety, depression, and other outcomes, such as self-harm and suicide
- Understanding how to create physical and social supports to ensure mental health in a climate of physical distancing
- Determining the mental health consequences of social isolation for vulnerable groups, and how can these be mitigated under pandemic conditions
- Understanding the mental health impact of media reporting of COVID-19 in traditional and social media
- Determining the best methods for promoting successful adherence to behavioral advice about COVID-19 while enabling mental well-being and minimizing distress
Another area highlighted by the experts is the potential for neuropsychiatric sequelae in individuals infected with COVID-19. They called for “experimental medicine studies to validate clinical biomarkers and repurpose new treatments for the potentially neurotoxic effects of the virus.”
The authors/investigators disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA approves ibrutinib-rituximab combo for newly diagnosed CLL, SLL in adults
The Food and Drug Administration has expanded the indication for ibrutinib (Imbruvica) to allow its combination with rituximab for frontline treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adults.
The approval, announced April 21, was based on findings from the randomized, controlled, open-label, phase 3 E1912 trial of 529 patients, which demonstrated significantly improved progression-free survival (PFS) among those who received ibrutinib plus rituximab, compared with those who received fludarabine, cyclophosphamide, and rituximab (FCR) (87% vs. 75%; hazard ratio, 0.34). Median PFS was not reached in either arm after a median follow-up of 37 months.
E1912 was the first study to show superiority of a chemotherapy-free regimen over FCR chemoimmunotherapy, considered the gold standard for newly diagnosed CLL and SLL for the past 2 decades.
The recommended dosage for the newly approved combination is a once-daily 420-mg dose of ibrutinib taken with a glass of water, with rituximab initiation in the second cycle at doses of 50 mg/m2 on day 1, 325 mg/m2 on day 2, and 500 mg/m2 on days 1-5 of subsequent cycles for a total of six cycles.
The Food and Drug Administration has expanded the indication for ibrutinib (Imbruvica) to allow its combination with rituximab for frontline treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adults.
The approval, announced April 21, was based on findings from the randomized, controlled, open-label, phase 3 E1912 trial of 529 patients, which demonstrated significantly improved progression-free survival (PFS) among those who received ibrutinib plus rituximab, compared with those who received fludarabine, cyclophosphamide, and rituximab (FCR) (87% vs. 75%; hazard ratio, 0.34). Median PFS was not reached in either arm after a median follow-up of 37 months.
E1912 was the first study to show superiority of a chemotherapy-free regimen over FCR chemoimmunotherapy, considered the gold standard for newly diagnosed CLL and SLL for the past 2 decades.
The recommended dosage for the newly approved combination is a once-daily 420-mg dose of ibrutinib taken with a glass of water, with rituximab initiation in the second cycle at doses of 50 mg/m2 on day 1, 325 mg/m2 on day 2, and 500 mg/m2 on days 1-5 of subsequent cycles for a total of six cycles.
The Food and Drug Administration has expanded the indication for ibrutinib (Imbruvica) to allow its combination with rituximab for frontline treatment of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) in adults.
The approval, announced April 21, was based on findings from the randomized, controlled, open-label, phase 3 E1912 trial of 529 patients, which demonstrated significantly improved progression-free survival (PFS) among those who received ibrutinib plus rituximab, compared with those who received fludarabine, cyclophosphamide, and rituximab (FCR) (87% vs. 75%; hazard ratio, 0.34). Median PFS was not reached in either arm after a median follow-up of 37 months.
E1912 was the first study to show superiority of a chemotherapy-free regimen over FCR chemoimmunotherapy, considered the gold standard for newly diagnosed CLL and SLL for the past 2 decades.
The recommended dosage for the newly approved combination is a once-daily 420-mg dose of ibrutinib taken with a glass of water, with rituximab initiation in the second cycle at doses of 50 mg/m2 on day 1, 325 mg/m2 on day 2, and 500 mg/m2 on days 1-5 of subsequent cycles for a total of six cycles.
Expert outlines strategies for managing migraine in women
Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.