User login
Added rituximab was effective in children and adolescents with high-risk B-cell NHL
The addition of rituximab to standard chemotherapy was a more effective therapy in children and adolescents with high-risk, high-grade,mature B-cell non-Hodgkin lymphoma than the use of chemotherapy alone, according to a study published in the New England Journal of Medicine. The addition of rituximab resulted in long-term complete remission in the vast majority of patients, reported Veronique Minard-Colin, MD, of the Gustave Roussy Institute, Villejuif Cedex, France, and her colleagues on behalf of the European Intergroup for Childhood Non-Hodgkin Lymphoma and the Children’s Oncology Group.
The researchers performed an open-label, randomized, phase 3 trial of 328 patients younger than 18 years of age with high-risk, mature B-cell non-Hodgkin’s lymphoma (stage III with an elevated lactate dehydrogenase level or stage IV) or acute leukemia to compare the addition of six doses of rituximab to standard lymphomes malins B (LMB) chemotherapy with standard LMB chemotherapy alone. There were 164 patients assigned to each group. The primary end point of the study was event-free survival; overall survival and toxic effects were also followed.
The majority of patients had Burkitt’s lymphoma: 139 (84.8%) in the rituximab-chemotherapy group and 142 (86.6%) in the chemotherapy-alone group, with diffuse large B-cell lymphoma being the second most common cancer: 19 (11.6%) vs. 12 (7.3%), respectively.
Event-free survival at 3 years was 93.9% (95% confidence interval, 89.1-96.7) in the rituximab-chemotherapy group and 82.3% (95% CI, 75.7-87.5) in the chemotherapy group.
Higher 3-year overall survival was also observed (95.1% in the rituximab-chemotherapy group vs. 87.3% in the chemotherapy group; hazard ratio for death, 0.36; 95% CI, 0.16 -0.82).
Eight patients in the rituximab-chemotherapy group died (4 deaths were disease related, 3 were treatment related, and 1 was from a second cancer), as did 20 in the chemotherapy group (17 deaths disease related, and 3 treatment related); HR, 0.36; 95% CI, 0.16-0.82.
The incidence of acute adverse events of grade 4 or higher after prephase treatment was 33.3% in the rituximab-chemotherapy group and 24.2% in the chemotherapy group, a nonsignificant difference (P = .07). However, around twice as many patients in the rituximab-chemotherapy group had a low IgG level at 1 year after trial inclusion, compared with the chemotherapy-alone group, which could indicate the potential for more frequent infections in the long term, the researchers stated.
“An assessment of the long-term effects of combining rituximab with this chemotherapy regimen in children with non-Hodgkin lymphoma, including data on immune status, will be useful,” they added.
The study was funded by the French Ministry of Health, Cancer Research UK, the National Institute for Health Research Clinical Research Network, the Children’s Cancer Foundation Hong Kong, the U.S. National Cancer Institute, and F. Hoffmann–La Roche–Genentech. Several of the authors reported consulting for and institutional and grant funding from F. Hoffmann-LaRoche, which markets rituximab, as well as relationships with other pharmaceutical companies.
SOURCE: Minard-Colin V et al. N Engl J Med. 2020;382:2207-19.
The addition of rituximab to standard chemotherapy was a more effective therapy in children and adolescents with high-risk, high-grade,mature B-cell non-Hodgkin lymphoma than the use of chemotherapy alone, according to a study published in the New England Journal of Medicine. The addition of rituximab resulted in long-term complete remission in the vast majority of patients, reported Veronique Minard-Colin, MD, of the Gustave Roussy Institute, Villejuif Cedex, France, and her colleagues on behalf of the European Intergroup for Childhood Non-Hodgkin Lymphoma and the Children’s Oncology Group.
The researchers performed an open-label, randomized, phase 3 trial of 328 patients younger than 18 years of age with high-risk, mature B-cell non-Hodgkin’s lymphoma (stage III with an elevated lactate dehydrogenase level or stage IV) or acute leukemia to compare the addition of six doses of rituximab to standard lymphomes malins B (LMB) chemotherapy with standard LMB chemotherapy alone. There were 164 patients assigned to each group. The primary end point of the study was event-free survival; overall survival and toxic effects were also followed.
The majority of patients had Burkitt’s lymphoma: 139 (84.8%) in the rituximab-chemotherapy group and 142 (86.6%) in the chemotherapy-alone group, with diffuse large B-cell lymphoma being the second most common cancer: 19 (11.6%) vs. 12 (7.3%), respectively.
Event-free survival at 3 years was 93.9% (95% confidence interval, 89.1-96.7) in the rituximab-chemotherapy group and 82.3% (95% CI, 75.7-87.5) in the chemotherapy group.
Higher 3-year overall survival was also observed (95.1% in the rituximab-chemotherapy group vs. 87.3% in the chemotherapy group; hazard ratio for death, 0.36; 95% CI, 0.16 -0.82).
Eight patients in the rituximab-chemotherapy group died (4 deaths were disease related, 3 were treatment related, and 1 was from a second cancer), as did 20 in the chemotherapy group (17 deaths disease related, and 3 treatment related); HR, 0.36; 95% CI, 0.16-0.82.
The incidence of acute adverse events of grade 4 or higher after prephase treatment was 33.3% in the rituximab-chemotherapy group and 24.2% in the chemotherapy group, a nonsignificant difference (P = .07). However, around twice as many patients in the rituximab-chemotherapy group had a low IgG level at 1 year after trial inclusion, compared with the chemotherapy-alone group, which could indicate the potential for more frequent infections in the long term, the researchers stated.
“An assessment of the long-term effects of combining rituximab with this chemotherapy regimen in children with non-Hodgkin lymphoma, including data on immune status, will be useful,” they added.
The study was funded by the French Ministry of Health, Cancer Research UK, the National Institute for Health Research Clinical Research Network, the Children’s Cancer Foundation Hong Kong, the U.S. National Cancer Institute, and F. Hoffmann–La Roche–Genentech. Several of the authors reported consulting for and institutional and grant funding from F. Hoffmann-LaRoche, which markets rituximab, as well as relationships with other pharmaceutical companies.
SOURCE: Minard-Colin V et al. N Engl J Med. 2020;382:2207-19.
The addition of rituximab to standard chemotherapy was a more effective therapy in children and adolescents with high-risk, high-grade,mature B-cell non-Hodgkin lymphoma than the use of chemotherapy alone, according to a study published in the New England Journal of Medicine. The addition of rituximab resulted in long-term complete remission in the vast majority of patients, reported Veronique Minard-Colin, MD, of the Gustave Roussy Institute, Villejuif Cedex, France, and her colleagues on behalf of the European Intergroup for Childhood Non-Hodgkin Lymphoma and the Children’s Oncology Group.
The researchers performed an open-label, randomized, phase 3 trial of 328 patients younger than 18 years of age with high-risk, mature B-cell non-Hodgkin’s lymphoma (stage III with an elevated lactate dehydrogenase level or stage IV) or acute leukemia to compare the addition of six doses of rituximab to standard lymphomes malins B (LMB) chemotherapy with standard LMB chemotherapy alone. There were 164 patients assigned to each group. The primary end point of the study was event-free survival; overall survival and toxic effects were also followed.
The majority of patients had Burkitt’s lymphoma: 139 (84.8%) in the rituximab-chemotherapy group and 142 (86.6%) in the chemotherapy-alone group, with diffuse large B-cell lymphoma being the second most common cancer: 19 (11.6%) vs. 12 (7.3%), respectively.
Event-free survival at 3 years was 93.9% (95% confidence interval, 89.1-96.7) in the rituximab-chemotherapy group and 82.3% (95% CI, 75.7-87.5) in the chemotherapy group.
Higher 3-year overall survival was also observed (95.1% in the rituximab-chemotherapy group vs. 87.3% in the chemotherapy group; hazard ratio for death, 0.36; 95% CI, 0.16 -0.82).
Eight patients in the rituximab-chemotherapy group died (4 deaths were disease related, 3 were treatment related, and 1 was from a second cancer), as did 20 in the chemotherapy group (17 deaths disease related, and 3 treatment related); HR, 0.36; 95% CI, 0.16-0.82.
The incidence of acute adverse events of grade 4 or higher after prephase treatment was 33.3% in the rituximab-chemotherapy group and 24.2% in the chemotherapy group, a nonsignificant difference (P = .07). However, around twice as many patients in the rituximab-chemotherapy group had a low IgG level at 1 year after trial inclusion, compared with the chemotherapy-alone group, which could indicate the potential for more frequent infections in the long term, the researchers stated.
“An assessment of the long-term effects of combining rituximab with this chemotherapy regimen in children with non-Hodgkin lymphoma, including data on immune status, will be useful,” they added.
The study was funded by the French Ministry of Health, Cancer Research UK, the National Institute for Health Research Clinical Research Network, the Children’s Cancer Foundation Hong Kong, the U.S. National Cancer Institute, and F. Hoffmann–La Roche–Genentech. Several of the authors reported consulting for and institutional and grant funding from F. Hoffmann-LaRoche, which markets rituximab, as well as relationships with other pharmaceutical companies.
SOURCE: Minard-Colin V et al. N Engl J Med. 2020;382:2207-19.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding rituximab to chemotherapy was effective in children and adolescents with high-risk, high-grade, mature B-cell non-Hodgkin lymphoma.
Major finding: Higher 3-year overall survival was observed (95.1% in the rituximab-chemotherapy group vs. 87.3% in the chemotherapy group).
Study details: Analysis of 328 patients who underwent randomization to standard chemotherapy vs. chemo plus rituximab (164 patients per group).
Disclosures: The study was funded by the French Ministry of Health, Cancer Research UK, the National Institute for Health Research Clinical Research Network, the Children’s Cancer Foundation Hong Kong, the U.S. National Cancer Institute, and F. Hoffmann–La Roche–Genentech. Several of the authors reported consulting for and institutional and grant funding from F. Hoffmann-LaRoche, which markets rituximab, as well as relationships with other pharmaceutical companies.
Source: Minard-Colin V et al. N Engl J Med. 2020; 382:2207-19.
MCC response varies based on immunosuppression type, especially CLL
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
Patients with Merkel cell carcinoma and chronic immunosuppression may fare better or worse on immunotherapy based on the reason for immunosuppression, according to recent research at the annual meeting of the Society for Investigative Dermatology, held virtually.
About 10% of patients with Merkel cell carcinoma (MCC) are immunosuppressed at diagnosis, and these patients tend to have a more aggressive disease course and worse disease-specific survival compared with immunocompetent patients, Lauren Zawacki, a research assistant in the Nghiem Lab at the University of Washington, Seattle, said in her presentation. Although patients are receiving immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 as treatments, the efficacy and side effects on immunosuppressed patients have not been well studied because many of these patients are not eligible for clinical trials.
Ms. Zawacki and colleagues analyzed data from a prospective Seattle registry of 1,442 patients with MCC, identifying 179 patients with MCC who had chronic immunosuppression due to chronic lymphocytic leukemia (CLL), solid organ transplants, autoimmune disorders, other hematological malignancies, and HIV and AIDS. Non-Hodgkin lymphoma comprised 7 of 8 patients in the group with other hematological malignancies, and Crohn’s disease made up 5 of 6 patients in the autoimmune disorder group. Of the 179 patients with MCC and immunosuppression, 31 patients were treated with either anti-PD-1 or anti-PD-L1 therapy.
There was an objective response rate of 52%, with 14 patients having a complete response, 2 patients having a partial response, and 15 patients experiencing disease progression. Of the patients with disease progression, 11 died of MCC. The response rate in immunocompromised patients is similar to results seen by her group in immunocompetent patients (Nghiem P et al. N Engl J Med 2016; 374:2542-52), said Ms. Zawacki. “While the overall objective response rate is comparable between immunocompetent and immunosuppressed patients, the response rates vary greatly between the different types of immunosuppression,” she said.
When grouping response rates by immunosuppression type, they found 2 of 11 patients with CLL (18%) and 2 of 6 patients with autoimmune disease (33%) had an objective response, while 2 of 3 patients with HIV/AIDS (66%) and 7 of 7 patients with other hematologic malignancies (100%) had an objective response.
“While the numbers of the cohort are small, there still seems to be a considerable difference in the response rate between the different types of immune suppression, which is critical when we’re treating patients who typically have a more aggressive disease course,” said Ms. Zawacki.
In particular, the finding of no patients with MCC and CLL achieving a complete response interested Ms. Zawacki and her colleagues, since about one-fourth of patients in the Seattle registry have this combination of disease. “Not only did none of the CLL patients have a complete response, but 7 out of the 11 patients with CLL died from MCC,” she explained. When examining further, the researchers found 45% of patients in this group discontinued because of side effects of immunotherapy and had a median time to recurrence of 1.5 months. “This finding suggests that CLL in particular plays a large role in impairing the function of the immune system, leading to not only a more aggressive disease course, but a poorer response to immunotherapy,” she said.
“There is a significant need for improved interventions for patients with CLL and autoimmune disorders,” she added. “Research for immunosuppressed patients is critical given the associated aggressive disease course and their lack of inclusion in clinical trials.”
Ms. Zawacki acknowledged the small number of patients in the study as a limitation, and patients who received follow-up at outside facilities may have received slightly different care, which could impact adverse event reporting or reasons for study discontinuation.
“A multi-institutional study would be beneficial to expand the number of patients in that cohort and to help confirm the trend observed in this study. In addition, future studies should assess the role of combination systemic therapy, such as neutron radiation and immunotherapy together in order to see if the objective response can be approved among immunosuppressed patients,” she said.
This study was supported by funding from the MCC Patient Gift Fund, the National Cancer Institute, and a grant from NIH. Ms. Zawacki reports no relevant conflicts of interest.
SOURCE: Zawacki L. SID 2020, Abstract 497.
FROM SID 2020
Neurologists’ pay gets a boost, most happy with career choice
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.
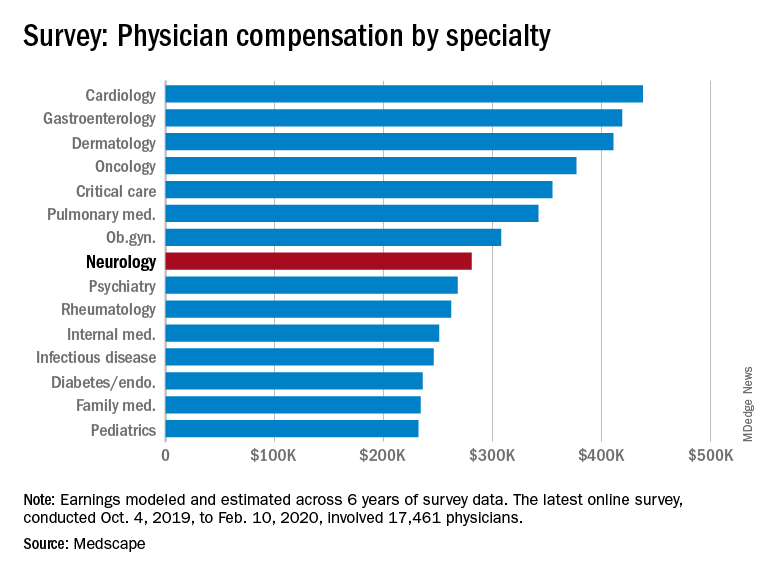
Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
findings from the newly released Medscape Neurologist Compensation Report 2020 show.
Neurologists’ average annual income this year rose to $280,000, up from $267,000 last year. More than half of neurologists (53%) feel fairly compensated, similar to last year’s percentage.

Neurologists are below the middle earners of all physician specialties. At $280,000 in annual compensation for patient care, neurologists rank ninth from the bottom, just below allergists/immunologists ($301,000) but ahead of psychiatrists ($268,000), rheumatologists ($262,000), and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in over 30 specialties.
COVID-19 impact
An important caveat is that the data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, data show that since the start of the crisis, physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they had closed their practices, at least temporarily.
There continues to be a gender pay gap in neurology, with male neurologists earning about 26% more than their female peers ($299,000 vs. $237,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
More than half of all physicians (56%) say they receive an incentive bonus. Neurologists report that they are eligible for an annual incentive bonus of $35,000. Average annual incentive bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one third of physicians overall who receive incentive bonuses say the prospect of receiving the bonus has encouraged them to work longer hours. A higher percentage of neurologists (41%) say their potential bonus influenced them to increase their work hours.
Fifty-eight percent of neurologists achieve more than three quarters of their potential annual incentive bonus. On average, neurologists achieve about two thirds of their potential bonus, the same proportion as for physicians overall.
However, COVID-19 may change that. Experts who were interviewed recently by Medscape noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male neurologists spend 37.7 hours per week seeing patients, somewhat more hours per week than female neurologists (36.1 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, neurologists spend 16.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5), infectious disease physicians (18.5), and psychiatrists (18.3). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a neurologist? Being good at what they do/finding answers, diagnoses tops the list (33%), followed by making the world a better place/helping others (26%), relationships with and gratitude from patients (18%), and making good money at a job they like (11%). A few cited teaching (5%) and pride in their profession (4%).
The most challenging part of practicing neurology is having to follow so many rules and regulations (26%). Other challenges include having to work long hours (18%), dealing with difficult patients (17%), trouble getting fair reimbursement (13%), and working with electronic health records (10%).
Despite the challenges, if they had to do it all over again, 73% of neurologists would still choose medicine as a career, and 86% would again choose neurology.
Other key findings in the latest report regarding neurologists include the following:
- At 18%, neurologists rank near the middle among physicians with regard to losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- 29% of neurologists say they use physician assistants (PAs) to treat patients in their practices, and 53% use nurse practitioners (NPs); 38% use neither for patient care. Of neurologists who work with PAs and NPs in their offices, 49% say these employees have helped boost profitability.
- Two-thirds of neurologists say they will continue taking new and current Medicare/Medicaid patients; none say they will not take new Medicare patients; and 26% are undecided.
- Neurologists participate in various payment methods; 78% are reimbursed via insurance, 35% have fee-for-service arrangements, and 28% are in accountable care organizations.
- Nearly 40% of neurologists expect to participate in the merit-based incentive payment system option, and 10% expect to participate in alternative payment models.
This article first appeared on Medscape.com.
Framingham risk score may also predict cognitive decline
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
“In the absence of effective treatments for dementia, we need to monitor and control cardiovascular risk burden as a way to maintain patient’s cognitive health as they age,” said Weili Xu, PhD, Department of Epidemiology and Biostatistics, School of Public Health, Tianjin Medical University, Tianjin, China, in a press release.
“Given the progressive increase in the number of dementia cases worldwide, our findings have both clinical and public health relevance.”
Dr. Xu and first author Ruixue Song, MSc, also from Tianjin Medical University, published their findings online ahead of print May 18 in the Journal of the American College of Cardiology.
The World Health Organization projects that up to 82 million people will have dementia by 2050. Given the lack of effective treatments for dementia, identifying modifiable risk factors for cognitive decline and aggressively managing them is an increasingly appealing strategy.
Assessing cardiovascular risk and cognition
The researchers followed 1,588 dementia-free participants from the Rush Memory and Aging Project for 21 years (median, 5.8 years). FGCRS was assessed at baseline and categorized into tertiles (lowest, middle, and highest). Mean age of the studied population was 79.5 years, 75.8% of participants were female, and mean Framingham score was 15.6 (range, 4 to 28).
Annual evaluations included assessment of episodic memory (memory of everyday events), semantic memory (long-term memory), working memory (short-term memory), visuospatial ability (capacity to identify visual and spatial relationships among objects), and perceptual speed (ability to accurately and completely compare letters, numbers, objects, pictures, or patterns) using 19 tests to derive a composite score.
A subsample (n = 378) of participants underwent MRI, and structural total and regional brain volumes were estimated.
Linear regression was used to estimate beta-coefficients for the relationship between cardiovascular risk burden at baseline and longitudinally. If the beta-coefficient is negative, the interpretation is that for every 1-unit increase in the predictor variable (FGCRS), the outcome variable (cognitive function) will decrease by the beta-coefficient value.
At baseline, higher FGCRS was related to small but consistent (although not usually statistically significant) decreases in hippocampal volume, gray matter, and total brain volume.
Considered longitudinally, participants in the highest-risk tertile of FGCRS experienced faster decline in global cognition (beta = −0.019), episodic memory (beta = −0.023), working memory (beta = −0.021), and perceptual speed (beta = −0.027) during follow-up (P < .05 for all) than those in the lowest-risk tertile.
The declines in semantic memory (beta = –0.012) and visuospatial ability (beta = –0.010) did not reach statistical significance.
Bringing dementia prevention into the exam room early
Commenting on the research, Costantino Iadecola, MD, director of the Feil Family Brain and Mind Research Institute at Weill Cornell Medicine in New York City, said the study has immediate clinical usefulness.
“The link between the cardiovascular risk factors and dementia is well known, but in your doctor’s office, that link is not seen. If your GP or cardiologist sees you with high blood pressure, he’s not immediately going to think about the risk of dementia 20 years later,” said Dr. Iadecola.
“What this study does is it directly links a simple score that’s commonly used to assess cardiovascular risk to dementia risk, which can be used to counsel patients and, hopefully, reduce the risk of both cardiovascular disease and cognitive disorders.”
Dr. Iadecola wrote an editorial together with Neal S. Parikh, MD, MS, also from Weill Cornell Medicine, that accompanied the findings of the trial.
Even neurologists sometimes fail to make the connection between vascular risk and dementia, he said. “They think that by making a stroke patient move their hand better, they’re treating them, but 30% of stroke patients get dementia 6 or 8 months later and they’re missing this link between cerebrovascular pathology and dementia.
Dr. Iadecola is one of 26 experts who authored the recent Berlin Manifesto, an effort led by Vladimir Hachinski, MD, professor of neurology and epidemiology at Western University in Ontario, Canada, to raise awareness of the link between cardiovascular and brain health.
Dr. Hachinski coined the term “brain attack” and devised the Hachinski Ischemic Score that remains the standard for identifying a vascular component of cognitive impairment.
The current study has some strengths and limitations, noted Dr. Iadecola. The average age of participants was 80 years, which is appropriate given the high risk for cognitive decline at this age, but the generalizability of the study may be limited given that most participants were white women.
Going forward, he said, rigorous studies are needed to confirm these findings and to determine how to best prevent dementia through treatment of individual cardiovascular risk factors.
Dr. Xu has received grants from nonindustry entities, including the Swedish Research Council and the National Natural Science Foundation of China. The study was funded by the European Union’s Horizon 320230 research and innovation program. Dr. Iadecola is a member of the scientific advisory board for Broadview Ventures.
This article appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
AAN publishes ethical guidance on patient care during the pandemic
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
The document, which was published online May 15 in Neurology, reviews adaptations to the inpatient and outpatient settings and addresses the need to develop protocols for the allocation of scarce medical resources. The guidance is the product of a joint committee of the AAN, the American Neurological Association, the Child Neurology Society, and the Neurocritical Care Society Ethics Committee.
“Now is one of the most challenging times of our careers as neurologists,” said James C. Stevens, MD, president of the AAN, in a press release. “Clinics and hospitals are adapting to caring for the most ill, managing scarce resources, and trying to protect people without the disease. As neurologists, we must continue to adapt our daily practice, continue to care for our most ill neurology patients, and help contribute to the care of those afflicted with COVID-19.”
The role of telehealth
The authors recommended that ordinary appointments be held using telehealth, which, they say, already has become part of patient care. Telehealth enables neurologists to continue providing care while reducing the risk of exposure to and spread of SARS-CoV-2. The disadvantages of telehealth are that it limits physical examinations and behavioral health examinations, the authors acknowledged. “Each clinician should decide, in concert with his or her patient, if an in-person evaluation warrants the risk of an encounter,” according to the guidance.
Neurologists also should advise their patients that their neurologic condition could affect their relative risk of hospitalization and death resulting from COVID-19. Patients with multiple sclerosis or myasthenia gravis, for example, may be receiving corticosteroids or immunomodulatory therapies that make them more vulnerable to COVID-19 infection. “Even if desired services are available, neurologists and their patients ought to consider whether their care plans can safely be delayed in order to mitigate risk,” wrote the authors. Neurologists must try to maintain the customary standard of care, however, for patients with neurologic disease severe enough to warrant hospitalization, such as stroke or epilepsy.
The potential need for triage
Resources such as ventilators and ICU beds are limited, and health care facilities have had to triage them during the pandemic. Patients with a neurologic disease that decreases their likelihood of survival from a respiratory illness may not be offered these resources. Neurologists should discuss with patients and decision makers the ways in which reduced resources might affect patient care. Neurologists must “be aware of the burden of disease in their local community and how healthcare leaders plan on coping with a surge,” according to the guidance.
Advance directives, which should be a standard part of clinical care, take on increased importance during the pandemic. Patients who have not completed advance care planning documents should be encouraged to do so, according to the authors. These documents include patients’ preferences for “do not attempt resuscitation” status. Nevertheless, “we must assure patients with chronic illness that diminished resources in this healthcare crisis will not restrict their access to comfort and palliative care,” the document states.
Scarce resource allocation protocols
In the event that a surge in patients overwhelms a hospital’s contingencies and forces it to operate in crisis mode, it should have a scarce resource allocation protocol in place.
“This will surely be the most challenging aspect of patient care during this pandemic public health emergency,” wrote the authors. To ensure transparency and to mitigate the emotional effect of these decisions on patients and clinicians, scarce resource allocation protocols should be developed by teams that include intensivists, clinical ethicists, and nursing representatives who are not directly involved in the care of the critically ill patients. The goal of these protocols is to maximize the number of lives saved. They generally include an initial patient assessment followed by regular reevaluations to determine whether patients using scarce resources are benefiting less than other patients who need the same resources. The protocols should consider not only patients with COVID-19 infection, but also patients with stroke, traumatic injury, influenza, and heart failure who may need the same resources. Race, gender, ethnicity, socioeconomics, and perceived social worth should not influence care decisions, according to the guidance. Validated mortality prediction scales, such as the Glasgow Outcome Scale, can contribute to care decisions. Obtaining community input into these protocols will ensure trust in the health care system.
“If the situation necessitates hard decisions, we need to be fair, objective, transparent, and adamantly preserve our professional integrity,” wrote the authors. “Through it all, we owe it to our patients and families, as well as ourselves, to maintain our own health and wellness.”
The guidance was developed without funding, and the authors reported no relevant disclosures.
SOURCE: Rubin MA et al. Neurology. 2020 May 15. doi: 10.1212/WNL.0000000000009744.
FROM NEUROLOGY
Race and location appear to play a role in the incidence of CLL and DLBCL
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
Exposure to carcinogens has been implicated in the development of non-Hodgkin lymphoma (NHL), suggesting that an examination of the environment on a population-based level might provide some insights. On that basis, researchers performed a study that found that living in an urban vs. rural area was associated with an increased risk of developing non-Hodgkin lymphoma (NHL) among diverse, urban populations.
The study, published online in Clinical Lymphoma, Myeloma & Leukemia, found an increased incidence of diffuse large B-cell lymphoma (DLBCL) in urban vs. rural Hispanics, and a similar increased incidence of chronic lymphocytic leukemia (CLL) in non-metropolitan urban non-Hispanic blacks.
A total of 482,096 adults aged 20 years and older with incident NHL were reported to 21 Surveillance, Epidemiology,and End Results (SEER) population-based registries for the period 2000 to 2016. Deanna Blansky of the Albert Einstein College of Medicine, Bronx, N.Y., and her colleagues compared patients by NHL subtype and urban-rural status, using rural-urban continuum codes from the U.S. Department of Agriculture.
The researchers found 136,197 DLBCL, 70,882 follicular lymphoma (FL), and 120,319 CLL cases of patients aged ≥ 20 years. The DLBCL patients comprised 73.6% non-Hispanic white, 11.8% Hispanic, and 7.3% non-Hispanic black, with a similar distribution observed for FL and CLL. Patients were adjusted for age, sex, and family poverty.
The study showed that, overall, there was a higher DLBCL incidence rate in metropolitan urban areas, compared with rural areas overall (incidence rate ratio [IRR] = 1.20, 95% confidence interval [CI] 1.11-1.30). Most pronounced was an increased DLBCL incidence among Hispanics in urban areas, compared with rural areas (rural IRR = 1.00; non-metropolitan urban IRR = 1.32, 95% CI 1.16-1.51; metropolitan urban = 1.55, 95% CI 1.36-1.76).
In contrast, metropolitan urban areas had a lower overall incidence of CLL than rural areas (8.4 vs. 9.7 per 100,000; IRR = .87; 95% CI .86-.89).
However, increased CLL incidence rates were found to be associated with non-metropolitan urban areas, compared with rural areas (IRR = 1.19; 95% CI 1.10-1.28), particularly among non-Hispanic Blacks (IRR = 1.49, 95% CI 1.27-1.72).
Unlike DLBCL and CLL, there were no differences observed in FL incidence rates by urban-rural status after adjusting for age, sex, and family poverty rates, the researchers reported.
“Overall, our findings suggest that factors related to urban status may be associated with DLBCL and CLL pathogenesis. Our results may help provide epidemiological clues to understanding the racial disparities seen among hematological malignancies, particularly regarding the risk of DLBCL in Hispanics and CLL in non-Hispanic Blacks,” the researchers concluded.
The study was sponsored by the U.S. National Institutes of Health. The researchers did not report conflict information.
SOURCE: Blansky D et al. Clin Lymphoma Myeloma Leuk. 2020 May 15; doi.org/10.1016/j.clml.2020.05.010.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Blood pressure lowering lessens risk of dementia, cognitive decline
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
“Although observational studies report hypertension to be an important risk factor for dementia, the benefit of blood pressure lowering on dementia or cognitive impairment in clinical trials is modest and lower than the risk reduction for stroke,” wrote Diarmaid Hughes, MB, of the NUI Galway and Saolta University Hospital Group in Galway, Ireland, and coauthors. They added, however, that “these findings have the potential to inform public health strategies to reduce the burden of dementia globally.” The study was published online ahead of print May 19 in JAMA.
A rich data set
To assess the relationship between lowering blood pressure and cognitive issues, the researchers performed a systemic search of randomized, clinical trials that compared blood pressure lowering via antihypertensive agents with a control, had at least 1 year of follow-up, included more than 1,000 participants, and reported on either dementia, cognitive impairment, cognitive decline, or a change in cognitive test scores as outcomes. Of the 14 studies deemed eligible, 12 reported either the incidence of dementia (n = 9) or a composite of dementia or cognitive impairment (n = 3) at follow-up and thus were included in the primary meta-analysis. The other two studies were used for secondary outcomes only.
The studies included 96,158 participants in total – 42.2% were women – and their mean age was 69 years. At baseline, participants’ mean systolic blood pressure was 154 mm Hg and their mean diastolic blood pressure was 83.3 mm Hg. The mean duration of follow-up was 49.24 months.
In the 12 trials that reported dementia or cognitive impairment, blood pressure lowering via antihypertensive agents, compared with control, was significantly associated with a reduction in those two outcomes (7.0% vs. 7.5% over a mean trial follow-up of 4.1 years; odds ratio, 0.93; 95% confidence interval, 0.88-0.98; absolute risk reduction, 0.39%; 95% CI, 0.09%-0.68%). Blood pressure lowering, compared with control, was also significantly associated with a reduction in cognitive decline (20.2% vs. 21.1% over a mean trial follow-up of 4.1 years; OR, 0.93; 95% CI, 0.88-0.99; ARR, 0.71%; 95% CI, 0.19%-1.2%) in the eight trials that reported it as an outcome. An analysis of the eight trials that reported a change in cognitive scores did not find a significant association between that outcome and blood pressure lowering.
Subpopulations should be examined
“This is a very broad brush stroke study, albeit a definitive one,” Richard J. Caselli, MD, of the Mayo Clinic in Phoenix said in an interview. “With all the thousands of people in this meta-analysis, there are going to be subpopulations of patients with certain characteristics or common conditions in which blood pressure lowering might have a bigger or a lesser impact on their risk factor. Is there a difference between certain racial groups? Does it matter what antihypertensive strategies are used? You can look at the interactions between blood pressure lowering and other conditions: diabetes, head injuries, air pollution, certain genetic risk factors. There are a number of additional findings that could come from a very rich data set like this.”
The authors acknowledged their study’s limitations, including the challenges of performing a meta-analysis of studies that drew from different populations and had potentially different definitions of dementia, cognitive impairment, and cognitive decline outcomes. In addition, the low incidence of dementia across clinical trials limited the researchers, and its underdetection in trials and the potential of survivor bias for healthier participants with blood pressure reductions were noted as “unmeasured sources of potential error.”
Three authors reported receiving grants or personal fees from the Wellcome Trust and the Health Research Board, the Chief Scientist Office, and Bayer AG, respectively.
SOURCE: Hughes D et al. JAMA. 2020 May 19. doi: 10.1001/jama.2020.4249.
FROM JAMA
Yoga is a good adjunct to migraine therapy
in Neurology.
The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
in Neurology.

The structured yoga program resulted in “remarkably improved” outcomes at 3 months of follow-up in CONTAIN, with both headache frequency and use of medications cut in half, compared with baseline, according to the investigators.
Compared with the control group on standard antimigraine medications alone, the yoga group demonstrated significantly greater reductions in pain intensity, headache frequency, pill counts, and validated measures of disability and headache impact on daily life (see graphic).
“The good news is that practicing something as simple and accessible as yoga may help much more than medications alone. And all you need is a mat,” observed Dr. Bhatia, professor of neurology at the All India Institute of Medical Sciences in New Delhi.
The single-center, open-label, blinded-assessment CONTAIN trial included 160 adult episodic migraine patients ages 18-50 years experiencing 4-14 headaches per month. They were randomized to prophylactic and acute rescue medications alone or in combination with yoga instruction by a qualified yoga therapist in a class that met at the medical center 3 days per week for 1 month. This was followed by practice of the hour-long yoga program at home 5 days per week for the next 2 months, with twice-monthly telephone calls from the yoga center to encourage adherence and encouragement to call if questions arose. Both groups received counseling about the importance of lifestyle changes that may help with migraine, including diet, physical activity, adequate sleep, and stress reduction. Outcomes were assessed in an intent-to-treat analysis.
The yoga program included specific relaxation exercises, breathing techniques, meditation, and yoga postures, or asanas. The migraine-tailored program was vetted by yoga experts at five renowned Indian yoga centers.
No safety issues arose with the yoga program.
The investigators noted that the 47% reduction in migraine medication pill count and 49% decrease in headache frequency over the course of 3 months in the adjunctive yoga group have important implications, not only in a limited-resource country such as India, but also in the United States, where Americans spend an estimated $3.2 billion annually on prescription and over the counter headache medications, and the indirect cost associated with lost productivity due to migraine has been put at $13 billion per year.
Dr. Bhatia and colleagues speculated that the observed benefits of add-on yoga in migraineurs may involve previously described improved vagal tone and parasympathetic drive coupled with decreased sympathetic tone, increased nitric oxide levels, and loosening of stiff muscles, which can trigger headaches.
Real-life goals
Commenting on the research, neurologist Holly Yancy, DO, a headache specialist at the Banner Health - University Medicine Neuroscience Institute in Phoenix, said she was impressed by the high quality of this well-designed, adequately powered study of a complementary and alternative therapy.
“The primary and secondary endpoints were real-life goals of migraine treatment that we strive to achieve in clinical practice – and they were met in the study,” she observed. “To start with a month of in-house yoga classes to instill a baseline competence in yoga prior to transitioning to home practice and to provide resources for ongoing assistance for questions were nice touches.”
She noted that the control group also experienced reductions in migraine frequency, severity, and disability scores, albeit of significantly lesser magnitude than in the yoga group. This underscores how important it is in clinical practice to spend time counseling migraine patients on lifestyle choices.
“A trial such as this provides neurologists and other health care providers with an accessible, evidence-based treatment for migraines that can be used with other preventive treatments to decrease the frequency and the amount of medication their patients are taking. In addition, it is a behavioral therapy that can decrease triggers and potentially help patients cope with pain,” Dr. Yancy said.
“I suspect I’ll not hesitate to recommend yoga as an adjunctive treatment for patients in my clinic that are physically capable. I think it would be logical to try to extrapolate the concept to a chronic migraine population as well, though it would be ideal to base that recommendation on another study conducted with a chronic migraine population.”
Dr. Bhatia and his coinvestigators reported having no financial conflicts regarding their study, funded by the Government of India and the All India Institute of Medical Sciences.
SOURCE: Kumar A et al. Neurology. 2020 May 6. doi: 10.1212/WNL.0000000000009473.
FROM NEUROLOGY
Satralizumab monotherapy reduces NMOSD relapse rate
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
according to trial results published in the Lancet Neurology.
The findings help confirm the role of interleukin-6 in the pathobiology of aquaporin-4 autoantibody (AQP4-IgG)–seropositive disease. For patients who are AQP4-IgG seronegative, however, “there is insufficient evidence to indicate a risk reduction” with this drug, the investigators wrote. In addition, satralizumab did not significantly affect pain or fatigue.
“The limitations of the study include the relatively small group sizes and low number of relapses. Despite these limitations, a significant treatment benefit was observed with satralizumab, compared with placebo in the study population, with efficacy and safety comparable to satralizumab used in combination with immunosuppressants,” reported lead author Anthony Traboulsee, MD, professor of neurology at the University of British Columbia, Vancouver, and colleagues.
Satralizumab is a humanized monoclonal antibody targeting the IL-6 receptor. A prior phase 3 study, SakuraSky, found that the drug reduces the risk of NMOSD relapse when added to immunosuppressant therapy. To assess the safety and efficacy of satralizumab monotherapy, Dr. Traboulsee and colleagues conducted SakuraStar, a randomized, double-blind, placebo-controlled trial.
Evaluating drug as monotherapy
The phase 3 trial enrolled 95 patients aged 18-74 years at 44 sites in 13 countries. The investigators included patients with AQP4-IgG–seropositive or –seronegative neuromyelitis optica using the 2006 Wingerchuk criteria or with AQP4-IgG–seropositive NMOSD with at least one event of longitudinally extensive myelitis or optic neuritis. The researchers limited the number of AQP4-IgG–seronegative patients to about 30% of the study population. Eligible participants had at least one NMOSD attack or relapse in the past 12 months and a score of 6.5 or less on the Expanded Disability Status Scale (EDSS). The investigators excluded patients with a clinical relapse in the 30 days before study baseline. Participants were randomly assigned 2:1 to receive satralizumab 120 mg or placebo subcutaneously at weeks 0, 2, 4, and every 4 weeks thereafter. Concomitant immunosuppressant use was prohibited, although corticosteroids and intravenous immunoglobulin were permitted as rescue therapy for the treatment of relapse.
The primary endpoint was time to first relapse, and the trial was designed to continue until 44 relapses occurred or for 1.5 years after the last patient entered the trial, whichever occurred first.
“Because even one NMOSD attack can have serious neurological consequences, this design allowed patients who had an attack on placebo to enter the open-label phase and receive the active drug,” the researchers wrote. “The trial design used unequal randomization to minimize exposure to placebo; because patients were not permitted to receive concomitant immunosuppressant treatment in this trial, the design limited the number of patients not receiving any treatment for the disorder. Placebo was selected with the consideration that no drugs were approved for the treatment of NMOSD when the trial was designed.” Recent trials of eculizumab, inebilizumab, and satralizumab have found that the agents are effective treatments for NMOSD. In 2019, the Food and Drug Administration approved eculizumab, a complement inhibitor, for the treatment of AQP4-IgG–seropositive NMOSD.
For the primary endpoint of SakuraStar, the researchers defined relapses as new or worsening objective neurologic symptoms with at least one of the following:
- Increase of 1 or more EDSS points from a baseline EDSS score of more than 0, or increase of 2 or more EDSS points from a baseline EDSS score of 0
- Increase of 2 or more points on at least one appropriate symptom-specific functional system score for pyramidal, cerebellar, brain stem, sensory, bowel or bladder, or a single eye
- Increase of 1 or more points on more than one symptom-specific functional system score with a baseline of at least 1
- Increase of 1 or more points on a single-eye symptom-specific functional system score with a baseline score of at least 1
In addition, symptoms had to be attributable to NMOSD; persist for more than 24 hours; and not be attributable to confounding clinical factors such as fever, infection, injury, change in mood, or adverse reactions to medications. Researchers assessed EDSS and functional system scores within 7 days of a patient reporting symptoms.
The double-blind treatment period ended 1.5 years after the last enrolled patient was assigned to satralizumab or placebo. More than 80% of the participants were women, including 73% of the satralizumab group and 97% of the placebo group. In all, 95 participants were assigned to a treatment between 2014 and 2017 – 63 to satralizumab and 32 to placebo. Relapses occurred in 19 patients receiving satralizumab (30%) and 16 receiving placebo (50%). The hazard ratio was 0.45.
“Patients treated with placebo showed a shorter time to relapse and a higher withdrawal rate than did patients treated with satralizumab,” wrote Dr. Traboulsee and colleagues. The Kaplan-Meier method suggested that 76% of patients on satralizumab had not relapsed at 48 weeks, compared with 62% of patients on placebo. And at 96 weeks, 72% of patients on satralizumab had not relapsed, compared with 51% of patients on placebo.
Among patients who were AQP4-IgG seropositive, the proportion with protocol-defined relapse was 22% in the satralizumab group versus 57% in the placebo group. Among patients who were AQP4-IgG seronegative, the proportion with a protocol-defined relapse was 46% in the satralizumab group versus 33% in the placebo group.
The most common adverse events were urinary tract infection and upper respiratory tract infection, and most adverse events were mild to moderate. A higher rate of severe adverse events was reported in the satralizumab group than in the placebo group (32.1 vs. 9.9 events per 100 patient-years). The investigators considered most of the severe adverse events unrelated to the study treatment. “None of the severe adverse events led to discontinuation of the study drug except one severe event of pneumonia in the satralizumab group,” the researchers wrote.
Data confirm efficacy of IL-6 blockade
“Satralizumab was well tolerated and no meaningful adverse effects from the drug were reported and no deaths occurred,” said Michael Levy, MD, PhD, director of the NMO clinic and research laboratory at Massachusetts General Hospital, Boston, in an accompanying editorial. “This trial of satralizumab was done shortly after the completion of a parallel trial of satralizumab in patients with NMOSD in which the same dose of satralizumab reduced the risk of relapse by 62%, compared with placebo. The main difference between these two trials is that, in the first published study, participants were permitted to use background immunotherapy; otherwise, the trial designs were nearly identical, and the enrolled participants are comparable. Together, the findings from these studies suggest that background therapy seems to provide only a small additional benefit to satralizumab alone.”
Dr. Levy also discussed findings from a phase 2 study of tocilizumab for the prevention of relapse in patients with NMOSD published in the same issue of the Lancet Neurology. The satralizumab and tocilizumab data have “confirmed that IL-6 blockade reduces the risk of relapse in patients with NMOSD,” Dr. Levy said. “IL-6 is a crucial component of the immune system, but when IL-6 production is altered during autoimmune attacks and sepsis, there can be severe consequences.”
The phase 2 trial of tocilizumab, which was described at the 2019 annual congress of the European Committee for Treatment and Research in Multiple Sclerosis, included 118 patients, 87% of whom were AQP4-IgG seropositive. Patients received intravenous tocilizumab or oral azathioprine for up to 60 weeks. Overall, 14% of patients in the tocilizumab group relapsed, compared with 47% of patients in the azathioprine group.
“The main differences between this trial of tocilizumab and the two satralizumab trials are that the tocilizumab was administered intravenously, rather than subcutaneously, the study duration was approximately 1 year, and the investigators were not masked to the treatment allocation,” Dr. Levy said. “Similar to satralizumab, adverse effects with tocilizumab were mild, including asymptomatic elevations in liver enzymes and an increased incidence of respiratory and urinary infections, with no significant differences identified between the tocilizumab and azathioprine groups.”
Various immunopathologic mechanisms may influence outcomes in NMOSD. While satralizumab and tocilizumab target IL-6, eculizumab is a C5 complement inhibitor and inebilizumab is a CD19 B-cell depleting monoclonal antibody, Dr. Levy said. “The safety concerns regarding these approaches are all substantially outweighed by the benefit of preventing NMOSD relapses.”
A need to understand AQP4-IgG–seronegative disease
SakuraStar “provides convincing data for the efficacy of satralizumab monotherapy in NMOSD with subgroup analysis showing that the benefit was seen in AQP4-IgG seropositive patients,” commented Dean M. Wingerchuk, MD, director of the division of multiple sclerosis and autoimmune neurology at the Mayo Clinic in Phoenix. “The results help confirm the key role of IL-6 in the pathobiology of AQP4-IgG–seropositive disease.”
Questions about AQP4-IgG seronegative disease remain. “The results are quite similar to the SakuraSky trial, in which satralizumab was used in conjunction with other background immunosuppressive therapies, suggesting that the primary benefit may be derived primarily from satralizumab. Both trials also showed that satralizumab did not benefit the NMOSD without AQP4-IgG subgroup though the subject numbers are rather small. We need to know more about the clinical and laboratory characteristics of the seronegative patients as they likely comprise a heterogeneous group. For example, did any of them have other autoantibodies such as MOG-IgG? Depending on the results, those details may help us understand the relative role of IL-6 in AQP4-IgG–seronegative subgroups, an important area of further study.”
SakuraStar was funded by Chugai Pharmaceutical, a member of the Roche group. Dr. Traboulsee reported grants, personal fees, and nonfinancial support from Chugai Pharmaceutical during the study, and several coauthors were employees of Chugai Pharmaceutical. Additional coauthors reported personal fees from Chugai, Roche, and other companies. Dr. Levy has received consulting fees from Alexion, Viela Bio, Chugai Pharmaceutical, Quest Diagnostics, and UCB Pharmaceuticals.
SOURCE: Traboulsee A et al. Lancet Neurol. 2020;19(5):402-12.
FROM THE LANCET NEUROLOGY
Updated AAN advisory outlines when PFO closure may be option for patients with stroke
Patients with an embolic-appearing infarct who are younger than 60 years, have undergone a thorough evaluation to rule out other stroke mechanisms, and have discussed with doctors the potential risks and benefits may be candidates for the procedure.
“For patients with cryptogenic stroke and PFO, percutaneous PFO closure probably reduces the risk of stroke recurrence with [a hazard ratio] of 0.41 and an absolute risk reduction of 3.4% at 5 years; probably is associated with a periprocedural complication rate of 3.9%; and probably is associated with the development of serious nonperiprocedural atrial fibrillation, with a relative risk of 2.72,” according to the advisory authors’ meta-analysis.
Most procedural complications and instances of atrial fibrillation were “self-limited and are of uncertain long-term clinical consequence, given the lower rate of stroke in patients whose PFOs were closed,” the authors said. “Subgroup analysis suggests that the overall benefit seen across trials may not extend to those patients with small shunts and small, deep infarcts.” The authors estimated that the number of patients who need to be treated to prevent one stroke at 5 years is 29.
The advisory updates 2016 guidance that said clinicians should not routinely offer PFO closure outside of a research setting. Since then, three trials published in 2017 the New England Journal of Medicine (RESPECT, CLOSE, and REDUCE) and one trial published in 2018 in the Journal of the American College of Cardiology (DEFENSE-PFO) found that PFO closure reduces the risk of recurrent stroke in patients with a PFO who have had a cryptogenic stroke, compared with medical therapy alone. In addition, the Food and Drug Administration approved the Amplatzer PFO Occluder and Gore Cardioform Septal Occluder. These developments necessitated the practice advisory, the authors said. The advisory was published online April 29 in Neurology. It is endorsed by the American Heart Association/American Stroke Association, the Society for Cardiovascular Angiography and Interventions, and the European Academy of Neurology.
Systematic review
For the update, Steven R. Messé, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and a panel of neurologists, internists, and cardiologists with expertise in stroke and PFO systematically reviewed relevant randomized studies published through August 2019 and conducted meta-analyses to make their recommendations. The literature search identified eight articles that met inclusion criteria, including one article that provided follow-up from a trial that had been included in the previous practice advisory.
“The risk of a second stroke in people with PFO and no other possible causes of stroke is very low, approximately 1% per year while being treated with just medication alone,” Dr. Messé said in a news release. “Also, it is difficult to determine with absolute certainty that the PFO is the cause of a person’s stroke. So it is important that people with PFO are educated about the benefits and risks of PFO closure.” For patients who opt to take medication only, doctors may consider prescribing antiplatelet or anticoagulant drugs, according to the advisory. “All patients with previous stroke should be treated with an antithrombotic medication indefinitely if there is no bleeding contraindication, regardless of whether a PFO is present or if it is closed,” Dr. Messé and colleagues wrote. “However, specific antithrombotic management for patients with stroke thought to be caused by PFO remains uncertain.”
Calls for thorough work-up
“If an alternative plausible higher-risk mechanism of stroke is identified, it is likely that the PFO was an innocent bystander,” the authors said. “Secondary stroke prevention is optimized by targeting the most likely etiology of the preceding event. ... The randomized PFO closure trials all mandated thorough evaluations for participants before enrollment ... to rule out other stroke mechanisms; moreover, all studies required TEE [transesophageal echocardiography] to characterize the PFO and ensure that it was the most likely etiology for the initial event.”
In patients being considered for PFO closure, clinicians should obtain brain imaging to confirm stroke size and distribution (level B); obtain vascular imaging of the cervical and intracranial vessels to look for dissection, vasculopathy, and atherosclerosis (level B); and perform hypercoagulable studies (level B), according to the advisory. Clinicians must perform a baseline ECG to look for atrial fibrillation (level A), and patients thought to be at risk of atrial fibrillation should receive prolonged cardiac monitoring for at least 28 days (level B).
Before PFO closure, a clinician with expertise in stroke should assess the patient to ensure that the PFO is the most plausible mechanism of stroke (level B). “If a higher-risk alternative mechanism of stroke is identified, clinicians should not routinely recommend PFO closure (level B),” the authors said. Patients also should be assessed by a clinician with expertise in assessing the anatomic features of a PFO and performing PFO closure (level B).
The randomized trials focused on patients whose PFOs were closed within 6 months of a stroke, and registry studies are needed to assess long-term outcomes, noted Dr. Messé and colleagues. “It remains unclear whether closure provides a similar benefit in these patients who otherwise still fit the studies’ inclusion criteria,” the authors said. “Long-term and large-scale safety registries for patients who have received PFO closure are needed to assess the risk of device erosion, fracture, embolization, and thrombotic and endocarditis risks, and the effect of residual shunts and incidence of atrial fibrillation.”
About 25% of the general adult population has a PFO. “It’s important to note that having a PFO is common, and that most people with PFO will never know they have it because it usually does not cause any problems,” Dr. Messé said. “However, while there is generally a very low risk of stroke in patients with PFO, in younger people who have had a stroke without any other possible causes identified, closing the PFO may reduce the risk of having another stroke better than medication alone.”
The practice advisory was developed with financial support from the AAN. Dr. Messé and most of the authors had no relevant conflicts of interest. Several authors disclosed ties to medical device and pharmaceutical companies.
SOURCE: Messé SR et al. Neurology. 2020 Apr 29. doi: 10.1212/WNL.0000000000009443.
Patients with an embolic-appearing infarct who are younger than 60 years, have undergone a thorough evaluation to rule out other stroke mechanisms, and have discussed with doctors the potential risks and benefits may be candidates for the procedure.
“For patients with cryptogenic stroke and PFO, percutaneous PFO closure probably reduces the risk of stroke recurrence with [a hazard ratio] of 0.41 and an absolute risk reduction of 3.4% at 5 years; probably is associated with a periprocedural complication rate of 3.9%; and probably is associated with the development of serious nonperiprocedural atrial fibrillation, with a relative risk of 2.72,” according to the advisory authors’ meta-analysis.
Most procedural complications and instances of atrial fibrillation were “self-limited and are of uncertain long-term clinical consequence, given the lower rate of stroke in patients whose PFOs were closed,” the authors said. “Subgroup analysis suggests that the overall benefit seen across trials may not extend to those patients with small shunts and small, deep infarcts.” The authors estimated that the number of patients who need to be treated to prevent one stroke at 5 years is 29.
The advisory updates 2016 guidance that said clinicians should not routinely offer PFO closure outside of a research setting. Since then, three trials published in 2017 the New England Journal of Medicine (RESPECT, CLOSE, and REDUCE) and one trial published in 2018 in the Journal of the American College of Cardiology (DEFENSE-PFO) found that PFO closure reduces the risk of recurrent stroke in patients with a PFO who have had a cryptogenic stroke, compared with medical therapy alone. In addition, the Food and Drug Administration approved the Amplatzer PFO Occluder and Gore Cardioform Septal Occluder. These developments necessitated the practice advisory, the authors said. The advisory was published online April 29 in Neurology. It is endorsed by the American Heart Association/American Stroke Association, the Society for Cardiovascular Angiography and Interventions, and the European Academy of Neurology.
Systematic review
For the update, Steven R. Messé, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and a panel of neurologists, internists, and cardiologists with expertise in stroke and PFO systematically reviewed relevant randomized studies published through August 2019 and conducted meta-analyses to make their recommendations. The literature search identified eight articles that met inclusion criteria, including one article that provided follow-up from a trial that had been included in the previous practice advisory.
“The risk of a second stroke in people with PFO and no other possible causes of stroke is very low, approximately 1% per year while being treated with just medication alone,” Dr. Messé said in a news release. “Also, it is difficult to determine with absolute certainty that the PFO is the cause of a person’s stroke. So it is important that people with PFO are educated about the benefits and risks of PFO closure.” For patients who opt to take medication only, doctors may consider prescribing antiplatelet or anticoagulant drugs, according to the advisory. “All patients with previous stroke should be treated with an antithrombotic medication indefinitely if there is no bleeding contraindication, regardless of whether a PFO is present or if it is closed,” Dr. Messé and colleagues wrote. “However, specific antithrombotic management for patients with stroke thought to be caused by PFO remains uncertain.”
Calls for thorough work-up
“If an alternative plausible higher-risk mechanism of stroke is identified, it is likely that the PFO was an innocent bystander,” the authors said. “Secondary stroke prevention is optimized by targeting the most likely etiology of the preceding event. ... The randomized PFO closure trials all mandated thorough evaluations for participants before enrollment ... to rule out other stroke mechanisms; moreover, all studies required TEE [transesophageal echocardiography] to characterize the PFO and ensure that it was the most likely etiology for the initial event.”
In patients being considered for PFO closure, clinicians should obtain brain imaging to confirm stroke size and distribution (level B); obtain vascular imaging of the cervical and intracranial vessels to look for dissection, vasculopathy, and atherosclerosis (level B); and perform hypercoagulable studies (level B), according to the advisory. Clinicians must perform a baseline ECG to look for atrial fibrillation (level A), and patients thought to be at risk of atrial fibrillation should receive prolonged cardiac monitoring for at least 28 days (level B).
Before PFO closure, a clinician with expertise in stroke should assess the patient to ensure that the PFO is the most plausible mechanism of stroke (level B). “If a higher-risk alternative mechanism of stroke is identified, clinicians should not routinely recommend PFO closure (level B),” the authors said. Patients also should be assessed by a clinician with expertise in assessing the anatomic features of a PFO and performing PFO closure (level B).
The randomized trials focused on patients whose PFOs were closed within 6 months of a stroke, and registry studies are needed to assess long-term outcomes, noted Dr. Messé and colleagues. “It remains unclear whether closure provides a similar benefit in these patients who otherwise still fit the studies’ inclusion criteria,” the authors said. “Long-term and large-scale safety registries for patients who have received PFO closure are needed to assess the risk of device erosion, fracture, embolization, and thrombotic and endocarditis risks, and the effect of residual shunts and incidence of atrial fibrillation.”
About 25% of the general adult population has a PFO. “It’s important to note that having a PFO is common, and that most people with PFO will never know they have it because it usually does not cause any problems,” Dr. Messé said. “However, while there is generally a very low risk of stroke in patients with PFO, in younger people who have had a stroke without any other possible causes identified, closing the PFO may reduce the risk of having another stroke better than medication alone.”
The practice advisory was developed with financial support from the AAN. Dr. Messé and most of the authors had no relevant conflicts of interest. Several authors disclosed ties to medical device and pharmaceutical companies.
SOURCE: Messé SR et al. Neurology. 2020 Apr 29. doi: 10.1212/WNL.0000000000009443.
Patients with an embolic-appearing infarct who are younger than 60 years, have undergone a thorough evaluation to rule out other stroke mechanisms, and have discussed with doctors the potential risks and benefits may be candidates for the procedure.
“For patients with cryptogenic stroke and PFO, percutaneous PFO closure probably reduces the risk of stroke recurrence with [a hazard ratio] of 0.41 and an absolute risk reduction of 3.4% at 5 years; probably is associated with a periprocedural complication rate of 3.9%; and probably is associated with the development of serious nonperiprocedural atrial fibrillation, with a relative risk of 2.72,” according to the advisory authors’ meta-analysis.
Most procedural complications and instances of atrial fibrillation were “self-limited and are of uncertain long-term clinical consequence, given the lower rate of stroke in patients whose PFOs were closed,” the authors said. “Subgroup analysis suggests that the overall benefit seen across trials may not extend to those patients with small shunts and small, deep infarcts.” The authors estimated that the number of patients who need to be treated to prevent one stroke at 5 years is 29.
The advisory updates 2016 guidance that said clinicians should not routinely offer PFO closure outside of a research setting. Since then, three trials published in 2017 the New England Journal of Medicine (RESPECT, CLOSE, and REDUCE) and one trial published in 2018 in the Journal of the American College of Cardiology (DEFENSE-PFO) found that PFO closure reduces the risk of recurrent stroke in patients with a PFO who have had a cryptogenic stroke, compared with medical therapy alone. In addition, the Food and Drug Administration approved the Amplatzer PFO Occluder and Gore Cardioform Septal Occluder. These developments necessitated the practice advisory, the authors said. The advisory was published online April 29 in Neurology. It is endorsed by the American Heart Association/American Stroke Association, the Society for Cardiovascular Angiography and Interventions, and the European Academy of Neurology.
Systematic review
For the update, Steven R. Messé, MD, of the Hospital of the University of Pennsylvania in Philadelphia, and a panel of neurologists, internists, and cardiologists with expertise in stroke and PFO systematically reviewed relevant randomized studies published through August 2019 and conducted meta-analyses to make their recommendations. The literature search identified eight articles that met inclusion criteria, including one article that provided follow-up from a trial that had been included in the previous practice advisory.
“The risk of a second stroke in people with PFO and no other possible causes of stroke is very low, approximately 1% per year while being treated with just medication alone,” Dr. Messé said in a news release. “Also, it is difficult to determine with absolute certainty that the PFO is the cause of a person’s stroke. So it is important that people with PFO are educated about the benefits and risks of PFO closure.” For patients who opt to take medication only, doctors may consider prescribing antiplatelet or anticoagulant drugs, according to the advisory. “All patients with previous stroke should be treated with an antithrombotic medication indefinitely if there is no bleeding contraindication, regardless of whether a PFO is present or if it is closed,” Dr. Messé and colleagues wrote. “However, specific antithrombotic management for patients with stroke thought to be caused by PFO remains uncertain.”
Calls for thorough work-up
“If an alternative plausible higher-risk mechanism of stroke is identified, it is likely that the PFO was an innocent bystander,” the authors said. “Secondary stroke prevention is optimized by targeting the most likely etiology of the preceding event. ... The randomized PFO closure trials all mandated thorough evaluations for participants before enrollment ... to rule out other stroke mechanisms; moreover, all studies required TEE [transesophageal echocardiography] to characterize the PFO and ensure that it was the most likely etiology for the initial event.”
In patients being considered for PFO closure, clinicians should obtain brain imaging to confirm stroke size and distribution (level B); obtain vascular imaging of the cervical and intracranial vessels to look for dissection, vasculopathy, and atherosclerosis (level B); and perform hypercoagulable studies (level B), according to the advisory. Clinicians must perform a baseline ECG to look for atrial fibrillation (level A), and patients thought to be at risk of atrial fibrillation should receive prolonged cardiac monitoring for at least 28 days (level B).
Before PFO closure, a clinician with expertise in stroke should assess the patient to ensure that the PFO is the most plausible mechanism of stroke (level B). “If a higher-risk alternative mechanism of stroke is identified, clinicians should not routinely recommend PFO closure (level B),” the authors said. Patients also should be assessed by a clinician with expertise in assessing the anatomic features of a PFO and performing PFO closure (level B).
The randomized trials focused on patients whose PFOs were closed within 6 months of a stroke, and registry studies are needed to assess long-term outcomes, noted Dr. Messé and colleagues. “It remains unclear whether closure provides a similar benefit in these patients who otherwise still fit the studies’ inclusion criteria,” the authors said. “Long-term and large-scale safety registries for patients who have received PFO closure are needed to assess the risk of device erosion, fracture, embolization, and thrombotic and endocarditis risks, and the effect of residual shunts and incidence of atrial fibrillation.”
About 25% of the general adult population has a PFO. “It’s important to note that having a PFO is common, and that most people with PFO will never know they have it because it usually does not cause any problems,” Dr. Messé said. “However, while there is generally a very low risk of stroke in patients with PFO, in younger people who have had a stroke without any other possible causes identified, closing the PFO may reduce the risk of having another stroke better than medication alone.”
The practice advisory was developed with financial support from the AAN. Dr. Messé and most of the authors had no relevant conflicts of interest. Several authors disclosed ties to medical device and pharmaceutical companies.
SOURCE: Messé SR et al. Neurology. 2020 Apr 29. doi: 10.1212/WNL.0000000000009443.
FROM NEUROLOGY