User login
Myasthenic Crisis After Recurrent COVID-19 Infection
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
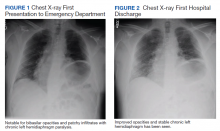
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
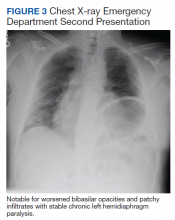
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
A patient with myasthenia gravis who survived 2 COVID-19 infections required plasmapheresis to recover from an acute crisis.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
COVID-19 is still in the early stages of understanding, although it is known to be complicated by individual patient comorbidities. The management and treatment of COVID-19 continues to quickly evolve as more is discovered regarding the virus. Multiple treatments have been preliminarily tested and used under a Food and Drug Administration emergency use authorization (EUA) determination. The long-term success of these therapies, however, is yet to be determined. Additionally, if a patient has a second clinical presentation for COVID-19, it is not known whether this represents latency with subsequent reactivation from the previous infection or a second de novo infection. The uncertainty calls into question the duration of immunity, if any, following a primary infection.
COVID-19 management becomes more complicated when patients have complex medical conditions, such as myasthenia gravis (MG). This autoimmune neuromuscular disorder can present with varying weakness, and many patients are on immunomodulator medications. The weakness can worsen into a myasthenic crisis (MC), resulting in profound weakness of the respiratory muscles. Therefore, patients with MG are at increased risk for COVID-19 and may have a more complicated course when infected.
Our patient with MG presented for severe COVID-19 symptoms twice and later developed MC. He received 2 treatment modalities available under an EUA (remdesivir and convalescent plasma) for COVID-19, resulting in symptom resolution and a negative polymerize chain reaction (PCR) test result for the virus. However, after receiving his typical maintenance therapy of IV immunoglobulin (IVIG) for his MG, he again developed symptoms consistent with COVID-19 and tested positive. After recovering from the second episode of COVID-19, the patient went into MC requiring plasmapheresis.
Case Presentation
A 56-year-old male, US Army veteran presented to Carl R. Darnall Army Medical Center emergency department (ED) 6 days after testing positive for COVID-19, with worsening sputum, cough, congestion, dyspnea, and fever. Due to his MG, the patient had a home oxygen monitor and reported that his oxygenation saturation dropped below 90% with minimal exertion. His medical history was significant for MG, status postthymectomy and radiation treatment, left hemidiaphragm paralysis secondary to phrenic nerve injury, and corticosteroid-induced insulin-dependent diabetes mellitus. His current home medications included pyridostigmine 60 mg 3 times a day, mycophenolate (MMF) 1500 mg twice daily, IV immunoglobulin (IVIG) every 3 weeks, insulin aspart up to 16 U per meal, insulin glargine 30 U twice a day, dulaglutide 0.75 mg every week, and metformin 1000 mg twice daily.
On initial examination, the patient’s heart rate (HR) was 111 beats/min, respiratory rate (RR), 22 breaths/min, blood pressure (BP), 138/88 mm Hg, temperature, 100.9 oF, and his initial pulse oximetry, 91% on room air. On physical examination, the patient was tachypneic, though without other signs of respiratory distress. Lung auscultation revealed no adventitial lung sounds. His cardiac examination was notable only for tachycardia. His neurologic examination demonstrated intact cranial nerves, with 5 out of 5 (scale 1 to 5) strength throughout the upper and lower extremities, sensation was intact to light touch, and he had normal cerebellar function. The rest of the examination was normal.
Initial laboratory investigation was notable for a white blood cell count of 14.15x103 cells/mcL with 84% neutrophils, and 6% lymphocytes. Additional tests revealed a C-reactive protein (CRP) level, 17.97 mg/dL (reference range, 0-0.5 mg/dL), ferritin level, 647 ng/mL (reference range, 22-274 ng/mL), d-dimer, 0.64 mcg/mL (reference range, 0-0.47mcg/mL), and a repeated positive COVID-19 PCR test. A portable chest X-ray showed bibasilar opacities (Figure 1).
The patient was diagnosed with COVID-19 and admitted to the intensive care unit (ICU). In the ICU, the patient received 1 U of convalescent plasma (CP) and started on a course of IV remdesivir 100 mg/d consistent with the EUA. He also received a 5-day course of ceftriaxone and azithromycin for possible community acquired pneumonia (CAP). As part of the patient’s MG maintenance medications, he received IVIG 4 g while in the ICU. Throughout his ICU stay, he required supplemental nasal cannula oxygenation to maintain his oxygen saturation > 93%. After 8 days in the ICU, his oxygen requirements decreased, and the patient was transferred out of the ICU and remdesivir was discontinued. On hospital day 10, a repeat COVID-19 PCR test was negative, inflammatory markers returned to within normal limits, and a repeat chest X-ray showed improvement from admission (Figure 2). Having recovered significantly, he was discharged home.
Three weeks later, the patient again presented to the MTF with 3 days of dyspnea, cough, fever, nausea, and vomiting. One day before symptom onset, he had received his maintenance IVIG infusion. The patient reported that his home oxygen saturation was 82% with minimal exertion. On ED presentation his HR was 107 beats/min, RR, 28 breaths/min, temperature, 98.1 oF, BP 118/71 mm Hg, and oxygen saturation, 92% on 2L nasal cannula. His examination was most notable for tachypnea with accessory muscle use. At this time, his neurologic examination was unchanged from prior admission with grossly intact cranial nerves and symmetric 5 of 5 motor strength in all extremities.
At this second ED visit, laboratory results demonstrated a CRP of 3.44 mg/dL, ferritin 2019 ng/mL, d-dimer, 3.39 mcg/mL, and a positive COVID-19 PCR result. His chest X-ray demonstrated new peripheral opacities compared with the X-ray at discharge (Figure 3). He required ICU admission again for his COVID-19 symptoms.
During his ICU course he continued to require supplemental oxygen by nasal cannula, though never required intubation. This second admission, he was again treated empirically for CAP with levofloxacin 750 mg daily for 5 days. He was discharged after 14 days with symptom resolution and down trending of inflammatory markers, though he was not retested for COVID-19.
Four days after his second discharge, he presented to the ED for a third time with diffuse weakness, dysphagia, and dysarthria of 1 day. His HR was 87/beats/min; RR, 17 breaths/min; temperature, 98.7 oF; BP, 144/81 mm Hg; and oxygen saturation, 98% on room air. His examination was significant for slurred speech, bilateral ptosis, 3 of 5 strength in bilateral finger flexion/abduction, wrist extension, knee and ankle flexion/extension; 4 of 5 strength in bilateral proximal muscle testing of deltoid, and hip; normal sensation, cerebellar function and reflexes. His negative inspiratory force (NIF) maximal effort was −30 cmH2O. He was determined to be in MC without evidence of COIVD-19 symptoms, and laboratory results were within normal limits, including a negative COVID-19 PCR. As he received IVIG as maintenance therapy, plasmapheresis was recommended to treat his MC, which required transfer to an outside civilian facility.
At the outside hospital, the patient underwent 5 rounds of plasmapheresis over 10 days. By the third treatment his strength had returned with resolution of the bulbar symptoms and no supplemental oxygen requirements. The patient was discharged and continued his original dosages of MMF and pyridostigmine. At 3 months, he remained asymptomatic from a COVID-19 standpoint and stable from a MG standpoint.
Discussion
Reinfection with the COVID-19 has been continuously debated with alternative explanations suggested for a positive test after a previous negative PCR test in the setting of symptom resolution.1,2 Proposed causes include dynamic PCR results due to prolonged viral shedding and inaccurate or poorly sensitive tests. The repeat positive cases in these scenarios, however, occurred in asymptomatic patients.1,2 COVID-19 shedding averages 20 to 22 days after symptom onset but has been seen up to 36 days after symptom resolution.2,3 This would suggest that fluctuating results during the immediate postsymptom period may be due to variations in viral shedding load and or sampling error—especially in asymptomatic patients. On the other hand, patients who experience return of symptoms days to weeks after previous convalescence leave clinicians wondering whether this represents clinical latency with reactivation or COVID-19 reinfection. A separate case of initial COVID-19 in a patient that had subsequent clinical recovery with a negative PCR developed recurrent respiratory symptoms and had a positive PCR test only 10 days later, further highlighting the reinfection vs reactivation issue of COVID-19.2 Further understanding of this issue may have implications on the extent of natural immunity following primary infection; potential vaccine dosage schedules; and global public health policies.
Although reactivation may be plausible given his immunomodulatory therapy, our patient’s second COVID-19 symptoms started 40 days after the initial symptoms, and 26 days after the initial course resolution; previous cases of return of severe symptoms occurred between 3 and 6 days.1 Given our patient’s time course between resolution and return of symptoms, if latency is the mechanism at play, this case demonstrates an exceptionally longer latency period than the ones that have been reported. Additionally, if latency is an issue in COVID-19, using remdesivir as a treatment further complicates the understanding of this disease.
Remdesivir, a nucleoside analogue antiviral, was shown to benefit recovery in patients with severe symptoms in the Adaptive COVID-19 Treatment Trial-1 study.4 Our patient had originally been placed on a 10-day course; however, on treatment day 8, his symptoms resolved and the remdesivir was discontinued. This is a similar finding to half the patients in the 10-day arm of the study by McCreary and colleagues.5 Although our patient was asymptomatic 4 weeks after the start of remdesivir, consistent with the majority of patients in the McCreary 10-day study arm, further comparison of the presented patient is limited due to study length and follow-up considerations.5 No previous data exist on reactivation, reinfection, or long-term mortality after being treated with remdesivir for COVID-19 infection.
IVIG is being studied in the treatment of COVID-19 and bears consideration as it relates to our patient. There is no evidence that IVIG used in the treatment of autoimmune diseases increases the risk of infection compared with that of other medications used in the treatment of such diseases. Furthermore, the current guidance from the MG expert panel does not suggest that IVIG increases the risk of contracting COVID-19 aside from the risks of exposure to hospital infrastructure.6 Yet the guidance does not discuss the use of IVIG for MG in patients who are already symptomatic from COVID-19 or for patients recovering from the clinical disease or does it discuss a possible compounding risk of thromboembolic events associated with IVIG and COVID-19.6,7 Our patient received his maintenance IVIG during his first admission without any worsening of symptoms or increased oxygen requirements. The day following our patient’s next scheduled IVIG infusion—while asymptomatic—he again developed respiratory symptoms; this could suggest that IVIG did not contribute to his second clinical course nor protect against.
CP is a treatment modality that has been used and studied in previous infectious outbreaks such as the first severe acute respiratory syndrome, and the H1N1 influenza virus.8 Current data on CP for COVID-19 are limited, but early descriptive studies have shown a benefit in improvement of symptoms 5 days sooner in those requiring supplemental oxygen, but no benefit for those requiring mechanical ventilation.9 Like patients that benefitted in these studies, our patient received CP early, 6 days after first testing positive and onset of symptoms. This patient’s reinfection or return of symptoms draws into question the hindrance or even prevention of long-term immunity from administration of CP.
COVID-19 presents many challenges when managing this patient’s coexisting MG, especially as the patient was already being treated with immunosuppressing therapies. The guidance does recommend continuation of standard MG therapies during hospitalizations, including immunosuppression medications such as MMF.6 Immunosuppression is associated with worsened severity of COVID-19 symptoms, although no relation exists to degree of immunosuppression and severity.7,10 To the best of our knowledge there has been no case report of reinfection or reactivation of COVID-19 associated with immunosuppressive agents used in the treatment of MG.
Our patient also was taking pyridostigmine for the treatment of his MG. There is no evidence this medication increases the risk of infection; but the cholinergic activity can increase bronchial secretions, which could theoretically worsen the COVID-19 respiratory symptoms.6,11 During both ICU admissions, our patient continued pyridostigmine use, observing complete return to baseline after discharge. Given the possible association with worsened respiratory outcomes after the second ICU admission, the balance between managing MG symptoms and COVID-19 symptoms needs further examination.
The patient was in MC during his third presentation to the ED. Although respiratory symptoms may be difficult to differentiate from COVID-19, the additional neurologic symptoms seen in this patient allowed for quick determination of the need for MC treatment. There are many potential etiologies contributing to the development of the MC presented here, and it was likely due to multifactorial precipitants. A common cause of MC is viral upper respiratory infections, further challenging the care of these patients during this pandemic.12 Many medications have been cited as causing a MC, 2 of which our patient received during admission for COVID-19: azithromycin and levoquin.12 Although the patient did not receive hydroxychloroquine, which was still being considered as an appropriate COVID-19 treatment at the time, it also is a drug known for precipitating MC and its use scrutinized in patients with MG.12
A key aspect to diagnosing and guiding therapies in myasthenic crisis in addition to the clinical symptoms of acute weakness is respiratory assessment through the nonaerosolizing NIF test.12 Our patient’s NIF measured < 30 cmH2O when in MC, while the reference range is < 75 cmH2O, and for mechanical ventilation is recommended at 20 cmH2O. Although the patient was maintaining O2 saturation > 95%, his NIF value was concerning, and preparations were made in case of precipitous decline. Compounding the NIF assessment in this patient is his history of left phrenic nerve palsy. Without a documented baseline NIF, results were limited in determining his diaphragm strength.13 Treatment for MC includes IVIG or plasmapheresis, since this patient had failed his maintenance therapy IVIG, plasmapheresis was coordinated for definitive therapy.
Conclusions
Federal facilities have seen an increase in the amount of respiratory complaints over the past months. Although COVID-19 is a concerning diagnosis, it is crucial to consider comorbidities in the diagnostic workup of each, even with a previous recent diagnosis of COVID-19. As treatment recommendations for COVID-19 continue to fluctuate coupled with the limitations and difficulties associated with MG patients, so too treatment and evaluation must be carefully considered at each presentation.
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
1. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: viral relapse, reinfection or inflammatory rebound? J Infect. 2020;81(5):816-846. doi:10.1016/j.jinf.2020.06.073
2. Duggan NM, Ludy SM, Shannon BC, Reisner AT, Wilcox SR. Is novel coronavirus 2019 reinfection possible? Interpreting dynamic SARS-CoV-2 test results. Am J Emerg Med. 2021;39:256.e1-256.e3. doi:10.1016/j.ajem.2020.06.079
3. Li J, Zhang L, Liu B, Song D. Case report: viral shedding for 60 days in a woman with COVID-19. Am J Trop Med Hyg. 2020;102(6):1210-1213. doi:10.4269/ajtmh.20-0275
4. Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of Covid-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994. doi:10.1056/NEJMc2022236
5. McCreary EK, Angus DC. Efficacy of remdesivir in COVID-19. JAMA. 2020;324(11):1041-1042. doi:10.1001/jama.2020.16337
6. International MG/COVID-19 Working Group; Jacob S, Muppidi S, Gordon A, et al. Guidance for the management of myasthenia gravis (MG) and Lambert-Eaton myasthenic syndrome (LEMS) during the COVID-19 pandemic. J Neurol Sci. 2020;412:116803. doi:10.1016/j.jns.2020.116803
7. Anand P, Slama MCC, Kaku M, et al. COVID-19 in patients with myasthenia gravis. Muscle Nerve. 2020;62(2):254-258. doi:10.1002/mus.26918
8. Wooding DJ, Bach H. Treatment of COVID-19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436-1446. doi:10.1016/j.cmi.2020.08.005
9. Salazar E, Perez KK, Ashraf M, et al. Treatment of coronavirus disease 2019 (covid-19) patients with convalescent plasma. Am J Pathol. 2020;190(8):1680-1690. doi:10.1016/j.ajpath.2020.05.014
10. Ryan C, Minc A, Caceres J, et al. Predicting severe outcomes in Covid-19 related illness using only patient demographics, comorbidities and symptoms [published online ahead of print, 2020 Sep 9]. Am J Emerg Med. 2020;S0735-6757(20)30809-3. doi:10.1016/j.ajem.2020.09.017
11. Singh S, Govindarajan R. COVID-19 and generalized myasthenia gravis exacerbation: a case report. Clin Neurol Neurosurg. 2020;196:106045. doi:10.1016/j.clineuro.2020.106045
12. Wendell LC, Levine JM. Myasthenic crisis. Neurohospitalist. 2011;1(1):16-22. doi:10.1177/1941875210382918
13. Dubé BP, Dres M. Diaphragm dysfunction: diagnostic approaches and management strategies. J Clin Med. 2016;5(12):113. Published 2016 Dec 5. doi:10.3390/jcm5120113
Mobile stroke teams treat patients faster and reduce disability
Having a mobile interventional stroke team (MIST) travel to treat stroke patients soon after stroke onset may improve patient outcomes, according to a new study. A retrospective analysis of a pilot program in New York found that
“The use of a Mobile Interventional Stroke Team (MIST) traveling to Thrombectomy Capable Stroke Centers to perform endovascular thrombectomy has been shown to be significantly faster with improved discharge outcomes,” wrote lead author Jacob Morey, a doctoral Candidate at Icahn School of Medicine at Mount Sinai in New York and coauthors in the paper. Prior to this study, “the effect of the MIST model stratified by time of presentation” had yet to be studied.
The findings were published online on Aug. 5 in Stroke.
MIST model versus drip-and-ship
The researchers analyzed 226 patients who underwent endovascular thrombectomy between January 2017 and February 2020 at four hospitals in the Mount Sinai health system using the NYC MIST Trial and a stroke database. At baseline, all patients were functionally independent as assessed by the modified Rankin Scale (mRS, score of 0-2). 106 patients were treated by a MIST team – staffed by a neurointerventionalist, a fellow or physician assistant, and radiologic technologist – that traveled to the patient’s location. A total of 120 patients were transferred to a comprehensive stroke center (CSC) or a hospital with endovascular thrombectomy expertise. The analysis was stratified based on whether the patient presented in the early time window (≤ 6 hours) or late time window (> 6 hours).
Patients treated in the early time window were significantly more likely to be mobile and able to perform daily tasks (mRS ≤ 2) 90 days after the procedure in the MIST group (54%), compared with the transferred group (28%, P < 0.01). Outcomes did not differ significantly between groups in the late time window (35% vs. 41%, P = 0.77).
Similarly, early-time-window patients in the MIST group were more likely to have higher functionality at discharge, compared with transferred patients, based on the on the National Institutes of Health Stroke Scale (median score of 5.0 vs. 12.0, P < 0.01). There was no significant difference between groups treated in the late time window (median score of 5.0 vs. 11.0, P = 0.11).
“Ischemic strokes often progress rapidly and can cause severe damage because brain tissue dies quickly without oxygen, resulting in serious long-term disabilities or death,“ said Johanna Fifi, MD, of Icahn School of Medicine, said in a statement to the American Heart Association. “Assessing and treating stroke patients in the early window means that a greater number of fast-progressing strokes are identified and treated.”
Time is brain
Endovascular thrombectomy is a time-sensitive surgical procedure to remove large blood clots in acute ischemic stroke that has “historically been limited to comprehensive stroke centers,” the authors wrote in their paper. It is considered the standard of care in ischemic strokes, which make up 90% of all strokes. “Less than 50% of Americans have direct access to endovascular thrombectomy, the others must be transferred to a thrombectomy-capable hospital for treatment, often losing over 2 hours of time to treatment,” said Dr. Fifi. “Every minute is precious in treating stroke, and getting to a center that offers thrombectomy is very important. The MIST model would address this by providing faster access to this potentially life-saving, disability-reducing procedure.”
Access to timely endovascular thrombectomy is gradually improving as “more institutions and cities have implemented the [MIST] model.” Dr. Fifi said.
“This study stresses the importance of ‘time is brain,’ especially for patients in the early time window. Although the study is limited by the observational, retrospective design and was performed at a single integrated center, the findings are provocative,” said Louise McCullough, MD, of the University of Texas Health Science Center at Houston said in a statement to the American Heart Association. “The use of a MIST model highlights the potential benefit of early and urgent treatment for patients with large-vessel stroke. Stroke systems of care need to take advantage of any opportunity to treat patients early, wherever they are.”
The study was partly funded by a Stryker Foundation grant.
Having a mobile interventional stroke team (MIST) travel to treat stroke patients soon after stroke onset may improve patient outcomes, according to a new study. A retrospective analysis of a pilot program in New York found that
“The use of a Mobile Interventional Stroke Team (MIST) traveling to Thrombectomy Capable Stroke Centers to perform endovascular thrombectomy has been shown to be significantly faster with improved discharge outcomes,” wrote lead author Jacob Morey, a doctoral Candidate at Icahn School of Medicine at Mount Sinai in New York and coauthors in the paper. Prior to this study, “the effect of the MIST model stratified by time of presentation” had yet to be studied.
The findings were published online on Aug. 5 in Stroke.
MIST model versus drip-and-ship
The researchers analyzed 226 patients who underwent endovascular thrombectomy between January 2017 and February 2020 at four hospitals in the Mount Sinai health system using the NYC MIST Trial and a stroke database. At baseline, all patients were functionally independent as assessed by the modified Rankin Scale (mRS, score of 0-2). 106 patients were treated by a MIST team – staffed by a neurointerventionalist, a fellow or physician assistant, and radiologic technologist – that traveled to the patient’s location. A total of 120 patients were transferred to a comprehensive stroke center (CSC) or a hospital with endovascular thrombectomy expertise. The analysis was stratified based on whether the patient presented in the early time window (≤ 6 hours) or late time window (> 6 hours).
Patients treated in the early time window were significantly more likely to be mobile and able to perform daily tasks (mRS ≤ 2) 90 days after the procedure in the MIST group (54%), compared with the transferred group (28%, P < 0.01). Outcomes did not differ significantly between groups in the late time window (35% vs. 41%, P = 0.77).
Similarly, early-time-window patients in the MIST group were more likely to have higher functionality at discharge, compared with transferred patients, based on the on the National Institutes of Health Stroke Scale (median score of 5.0 vs. 12.0, P < 0.01). There was no significant difference between groups treated in the late time window (median score of 5.0 vs. 11.0, P = 0.11).
“Ischemic strokes often progress rapidly and can cause severe damage because brain tissue dies quickly without oxygen, resulting in serious long-term disabilities or death,“ said Johanna Fifi, MD, of Icahn School of Medicine, said in a statement to the American Heart Association. “Assessing and treating stroke patients in the early window means that a greater number of fast-progressing strokes are identified and treated.”
Time is brain
Endovascular thrombectomy is a time-sensitive surgical procedure to remove large blood clots in acute ischemic stroke that has “historically been limited to comprehensive stroke centers,” the authors wrote in their paper. It is considered the standard of care in ischemic strokes, which make up 90% of all strokes. “Less than 50% of Americans have direct access to endovascular thrombectomy, the others must be transferred to a thrombectomy-capable hospital for treatment, often losing over 2 hours of time to treatment,” said Dr. Fifi. “Every minute is precious in treating stroke, and getting to a center that offers thrombectomy is very important. The MIST model would address this by providing faster access to this potentially life-saving, disability-reducing procedure.”
Access to timely endovascular thrombectomy is gradually improving as “more institutions and cities have implemented the [MIST] model.” Dr. Fifi said.
“This study stresses the importance of ‘time is brain,’ especially for patients in the early time window. Although the study is limited by the observational, retrospective design and was performed at a single integrated center, the findings are provocative,” said Louise McCullough, MD, of the University of Texas Health Science Center at Houston said in a statement to the American Heart Association. “The use of a MIST model highlights the potential benefit of early and urgent treatment for patients with large-vessel stroke. Stroke systems of care need to take advantage of any opportunity to treat patients early, wherever they are.”
The study was partly funded by a Stryker Foundation grant.
Having a mobile interventional stroke team (MIST) travel to treat stroke patients soon after stroke onset may improve patient outcomes, according to a new study. A retrospective analysis of a pilot program in New York found that
“The use of a Mobile Interventional Stroke Team (MIST) traveling to Thrombectomy Capable Stroke Centers to perform endovascular thrombectomy has been shown to be significantly faster with improved discharge outcomes,” wrote lead author Jacob Morey, a doctoral Candidate at Icahn School of Medicine at Mount Sinai in New York and coauthors in the paper. Prior to this study, “the effect of the MIST model stratified by time of presentation” had yet to be studied.
The findings were published online on Aug. 5 in Stroke.
MIST model versus drip-and-ship
The researchers analyzed 226 patients who underwent endovascular thrombectomy between January 2017 and February 2020 at four hospitals in the Mount Sinai health system using the NYC MIST Trial and a stroke database. At baseline, all patients were functionally independent as assessed by the modified Rankin Scale (mRS, score of 0-2). 106 patients were treated by a MIST team – staffed by a neurointerventionalist, a fellow or physician assistant, and radiologic technologist – that traveled to the patient’s location. A total of 120 patients were transferred to a comprehensive stroke center (CSC) or a hospital with endovascular thrombectomy expertise. The analysis was stratified based on whether the patient presented in the early time window (≤ 6 hours) or late time window (> 6 hours).
Patients treated in the early time window were significantly more likely to be mobile and able to perform daily tasks (mRS ≤ 2) 90 days after the procedure in the MIST group (54%), compared with the transferred group (28%, P < 0.01). Outcomes did not differ significantly between groups in the late time window (35% vs. 41%, P = 0.77).
Similarly, early-time-window patients in the MIST group were more likely to have higher functionality at discharge, compared with transferred patients, based on the on the National Institutes of Health Stroke Scale (median score of 5.0 vs. 12.0, P < 0.01). There was no significant difference between groups treated in the late time window (median score of 5.0 vs. 11.0, P = 0.11).
“Ischemic strokes often progress rapidly and can cause severe damage because brain tissue dies quickly without oxygen, resulting in serious long-term disabilities or death,“ said Johanna Fifi, MD, of Icahn School of Medicine, said in a statement to the American Heart Association. “Assessing and treating stroke patients in the early window means that a greater number of fast-progressing strokes are identified and treated.”
Time is brain
Endovascular thrombectomy is a time-sensitive surgical procedure to remove large blood clots in acute ischemic stroke that has “historically been limited to comprehensive stroke centers,” the authors wrote in their paper. It is considered the standard of care in ischemic strokes, which make up 90% of all strokes. “Less than 50% of Americans have direct access to endovascular thrombectomy, the others must be transferred to a thrombectomy-capable hospital for treatment, often losing over 2 hours of time to treatment,” said Dr. Fifi. “Every minute is precious in treating stroke, and getting to a center that offers thrombectomy is very important. The MIST model would address this by providing faster access to this potentially life-saving, disability-reducing procedure.”
Access to timely endovascular thrombectomy is gradually improving as “more institutions and cities have implemented the [MIST] model.” Dr. Fifi said.
“This study stresses the importance of ‘time is brain,’ especially for patients in the early time window. Although the study is limited by the observational, retrospective design and was performed at a single integrated center, the findings are provocative,” said Louise McCullough, MD, of the University of Texas Health Science Center at Houston said in a statement to the American Heart Association. “The use of a MIST model highlights the potential benefit of early and urgent treatment for patients with large-vessel stroke. Stroke systems of care need to take advantage of any opportunity to treat patients early, wherever they are.”
The study was partly funded by a Stryker Foundation grant.
FROM STROKE
Fulminant Hemorrhagic Bullae of the Upper Extremities Arising in the Setting of IV Placement During Severe COVID-19 Infection: Observations From a Major Consultative Practice
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
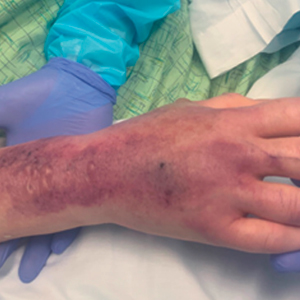
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.
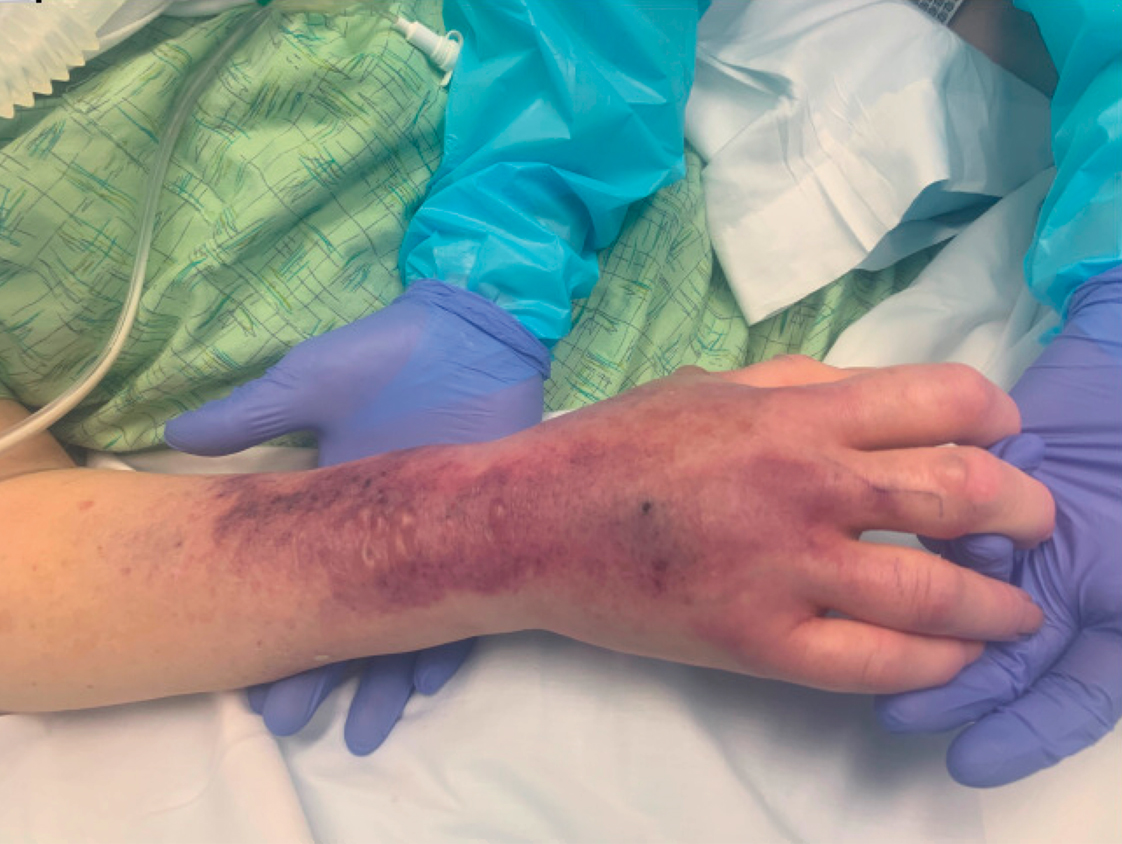
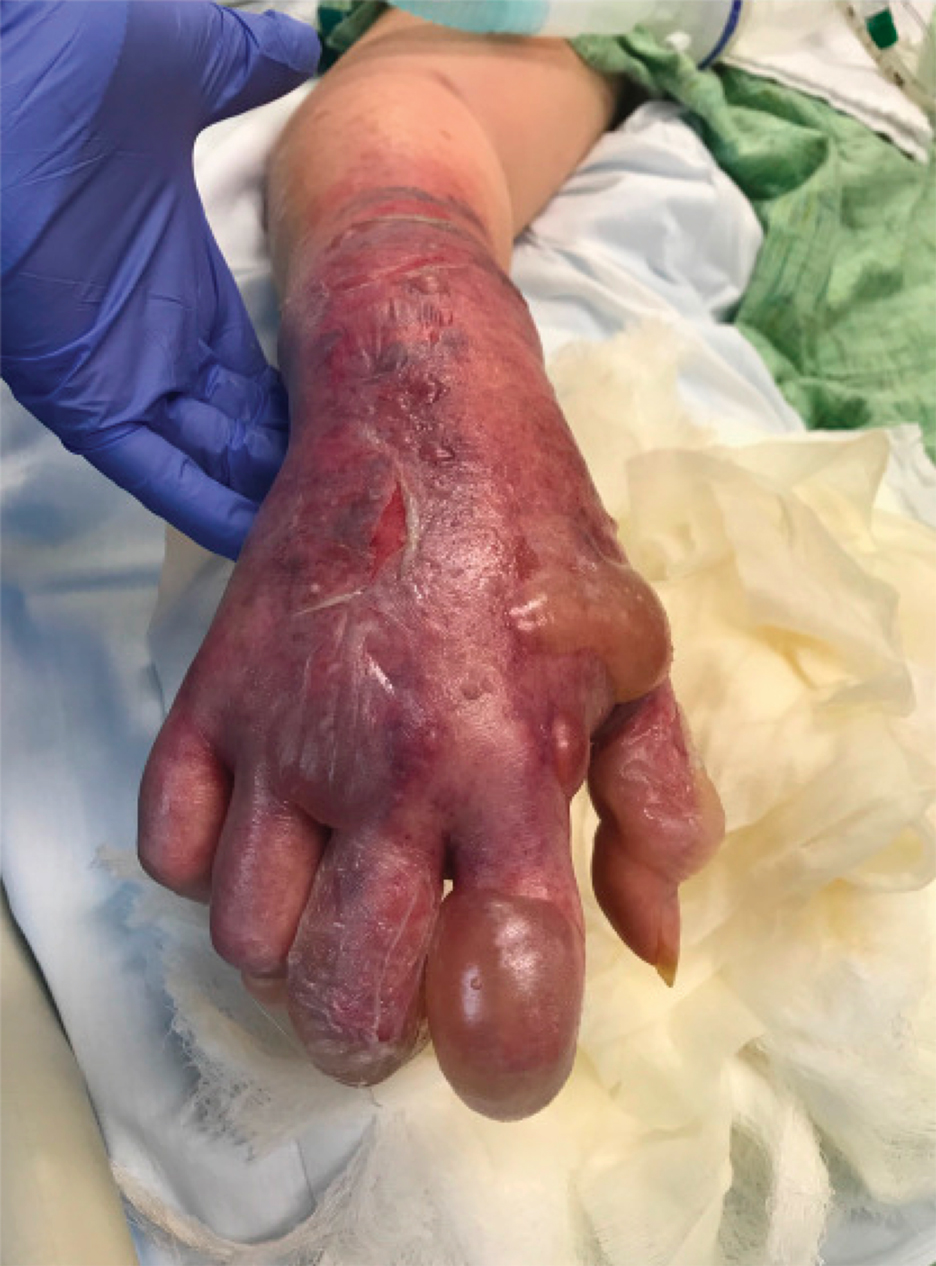
A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.

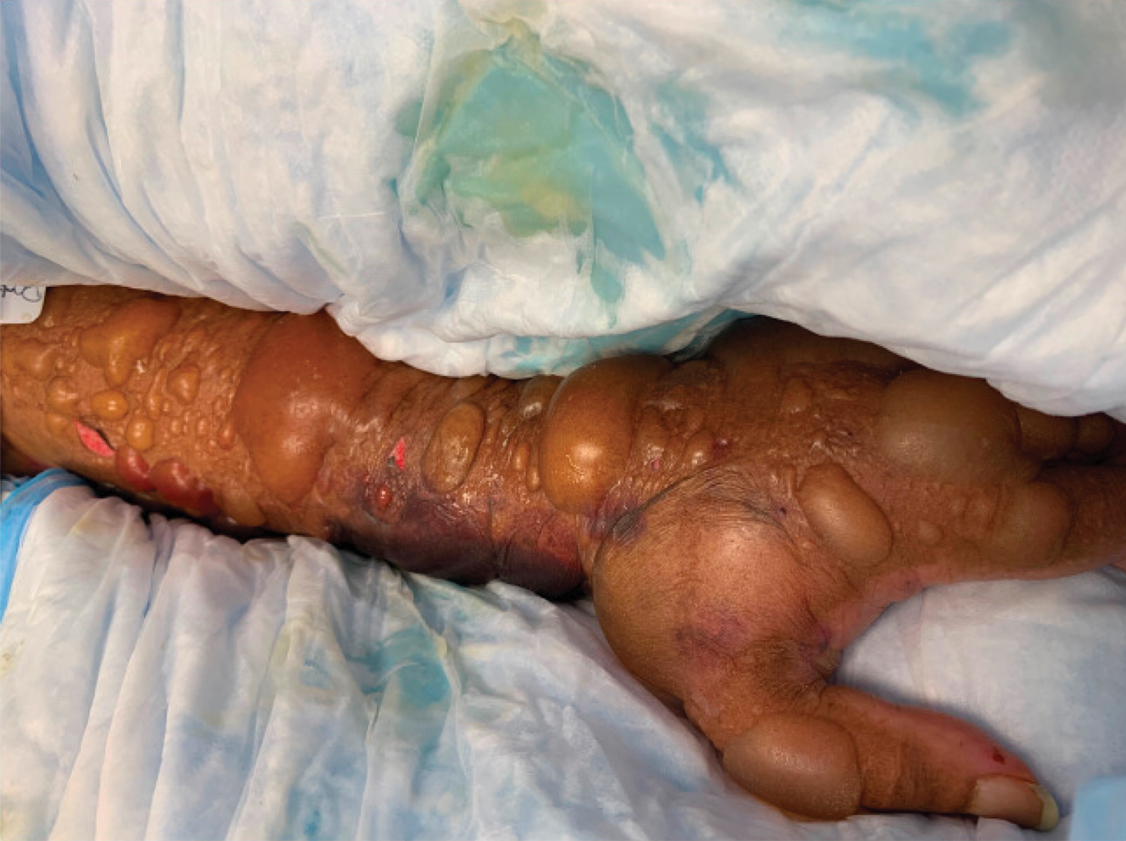
Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
To the Editor:
A range of dermatologic manifestations of COVID-19 have been reported, including nonspecific maculopapular exanthems, urticaria, and varicellalike eruptions.1 Additionally, there have been sporadic accounts of cutaneous vasculopathic signs such as perniolike lesions, acro-ischemia, livedo reticularis, and retiform purpura.2 We describe exuberant hemorrhagic bullae occurring on the extremities of 2 critically ill patients with COVID-19. We hypothesized that the bullae were vasculopathic in nature and possibly exacerbated by peripheral intravenous (IV)–related injury.
A 62-year-old woman with a history of diabetes mellitus and chronic obstructive pulmonary disease was admitted to the intensive care unit for acute hypoxemic respiratory failure secondary to COVID-19 infection. Dermatology was consulted for evaluation of blisters on the right arm. A new peripheral IV line was inserted into the patient’s right forearm for treatment of secondary methicillin-resistant Staphylococcus aureus pneumonia. The peripheral IV was inserted into the right proximal forearm for 2 days prior to development of ecchymosis and blisters. Intravenous medications included vancomycin, cefepime, methylprednisolone, and famotidine, as well as maintenance fluids (normal saline). Physical examination revealed extensive confluent ecchymoses with overlying tense bullae (Figure 1). Notable laboratory findings included an elevated D-dimer (peak of 8.67 μg/mL fibrinogen-equivalent units [FEUs], reference range <0.5 μg/mL FEU) and fibrinogen (789 mg/dL, reference range 200–400 mg/dL) levels. Three days later she developed worsening edema of the right arm, accompanied by more extensive bullae formation (Figure 2). Computed tomography of the right arm showed extensive subcutaneous stranding and subcutaneous edema. An orthopedic consultation determined that there was no compartment syndrome, and surgical intervention was not recommended. The patient’s course was complicated by multiorgan failure, and she died 18 days after admission.


A 67-year-old man with coronary artery disease, diabetes mellitus, and hemiparesis secondary to stroke was admitted to the intensive care unit due to hypoxemia secondary to COVID-19 pneumonia. Dermatology was consulted for the evaluation of blisters on both arms. The right forearm peripheral IV line was used for 4 days prior to the development of cutaneous symptoms. Intravenous medications included cefepime, famotidine, and methylprednisolone. The left forearm peripheral IV line was in place for 1 day prior to the development of blisters and was used for the infusion of maintenance fluids (lactated Ringer’s solution). On the first day of the eruption, small bullae were noted at sites of prior peripheral IV lines (Figure 3). On day 3 of admission, the eruption progressed to larger and more confluent tense bullae with ecchymosis (Figure 4). Additionally, laboratory test results were notable for an elevated D-dimer (peak of >20.00 ug/mL FEU) and fibrinogen (748 mg/dL) levels. Computed tomography of the arms showed extensive subcutaneous stranding and fluid along the fascial planes of the arms, with no gas or abscess formation. Surgical intervention was not recommended following an orthopedic consultation. The patient’s course was complicated by acute kidney injury and rhabdomyolysis; he was later discharged to a skilled nursing facility in stable condition.


Reports from China indicate that approximately 50% of COVID-19 patients have elevated D-dimer levels and are at risk for thrombosis.3 We hypothesize that the exuberant hemorrhagic bullous eruptions in our 2 cases may be mediated in part by a hypercoagulable state secondary to COVID-19 infection combined with IV-related trauma or extravasation injury. However, a direct cytotoxic effect of the virus cannot be entirely excluded as a potential inciting factor. Other entities considered in the differential for localized bullae included trauma-induced bullous pemphigoid as well as bullous cellulitis. Both patients were treated with high-dose steroids as well as broad-spectrum antibiotics, which were expected to lead to improvement in symptoms of bullous pemphigoid and cellulitis, respectively; however, they did not lead to symptom improvement.
Extravasation injury results from unintentional administration of potentially vesicant substances into tissues surrounding the intended vascular channel.4 The mechanism of action of these injuries is postulated to arise from direct tissue injury from cytotoxic substances, elevated osmotic pressure, and reduced blood supply if vasoconstrictive substances are infused.5 In our patients, these injuries also may have promoted vascular occlusion leading to the brisk reaction observed. Although ecchymoses typically are associated with hypocoagulable states, both of our patients were noted to have normal platelet levels throughout hospitalization. Additionally, findings of elevated D-dimer and fibrinogen levels point to a hypercoagulable state. However, there is a possibility of platelet dysfunction leading to the observed cutaneous findings of ecchymoses. Thrombocytopenia is a common finding in patients with COVID-19 and is found to be associated with increased in-hospital mortality.6 Additional study of these reactions is needed given the propensity for multiorgan failure and death in patients with COVID-19 from suspected diffuse microvascular damage.3
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387
- Zhang Y, Cao W, Xiao M, et al. Clinical and coagulation characteristics of 7 patients with critical COVID-19 pneumonia and acro-ischemia [in Chinese][published online March 28, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E006.
- Mei H, Hu Y. Characteristics, causes, diagnosis and treatment of coagulation dysfunction in patients with COVID-19 [in Chinese][published online March 14, 2020]. Zhonghua Xue Ye Xue Za Zhi. 2020;41:E002.
- Sauerland C, Engelking C, Wickham R, et al. Vesicant extravasation part I: mechanisms, pathogenesis, and nursing care to reduce risk. Oncol Nurs Forum. 2006;33:1134-1141.
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632.
- Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469‐1472.
Practice Points
- Hemorrhagic bullae are an uncommon cutaneous manifestation of COVID-19 infection in hospitalized individuals.
- Although there is no reported treatment for COVID-19–associated hemorrhagic bullae, we recommend supportive care and management of underlying etiology.
Becoming vaccine ambassadors: A new role for psychiatrists
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15
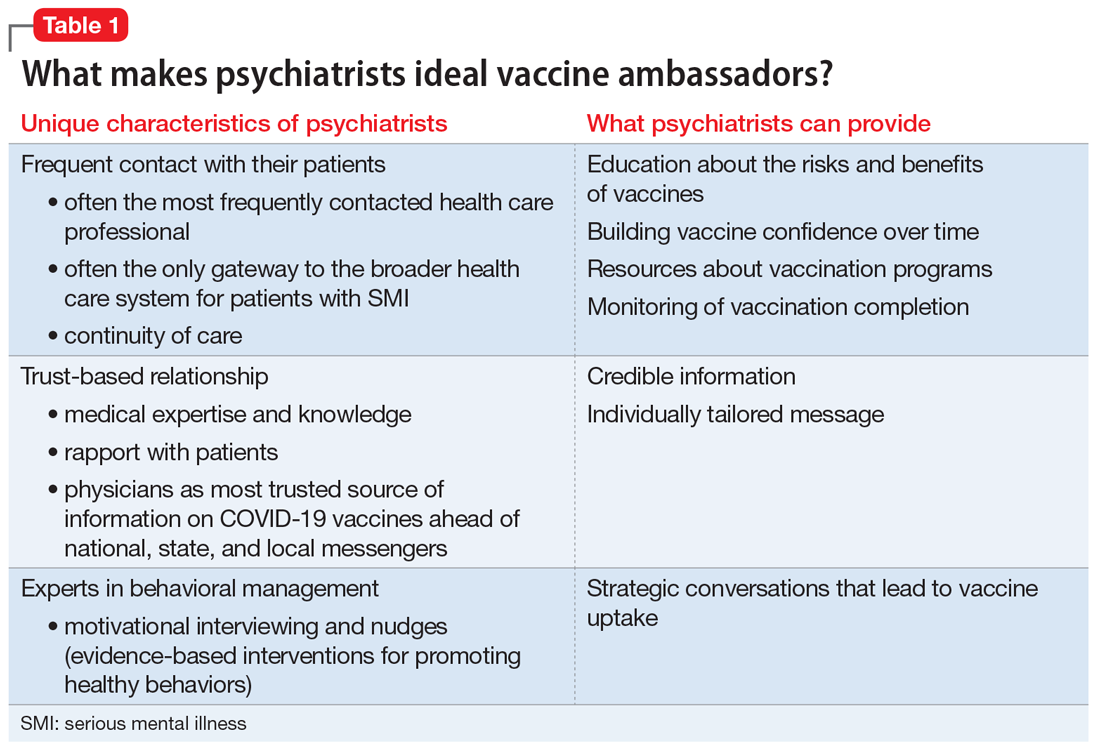
Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41
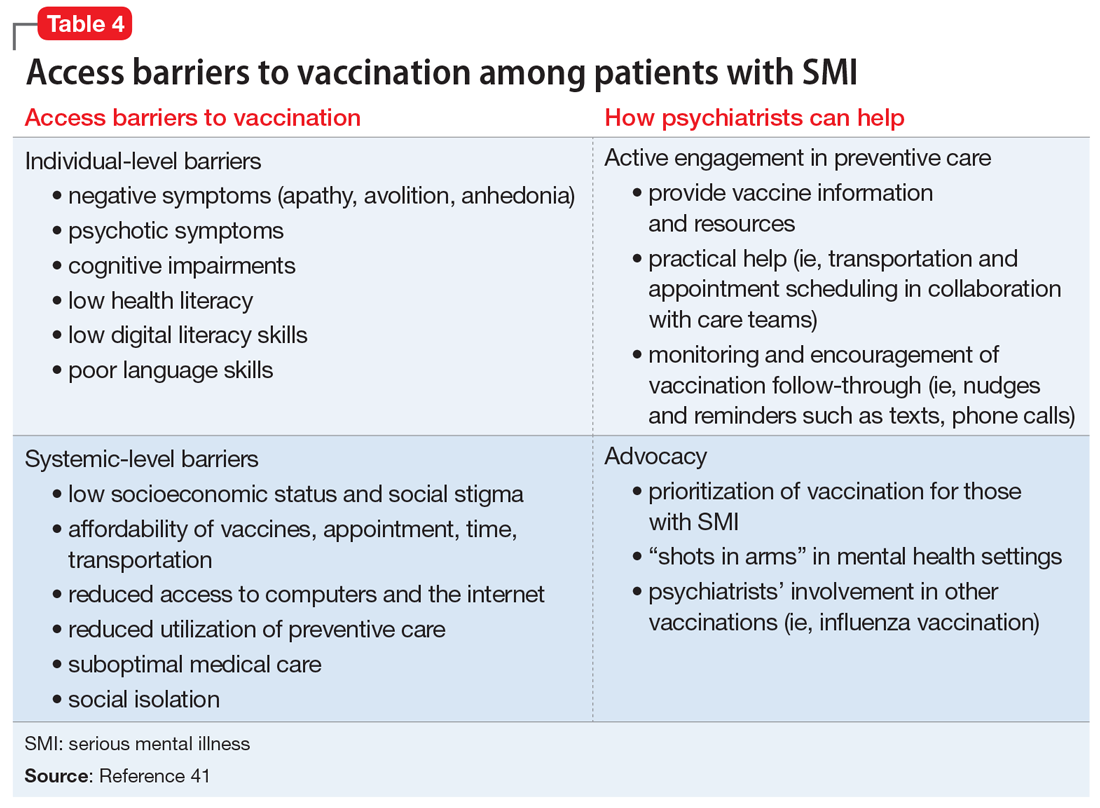
Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.
Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15

Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41

Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.

Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
After more than 600,000 deaths in the United States from the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), several safe and effective vaccines against the virus have become available. Vaccines are the most effective preventive measure against COVID-19 and the most promising way to achieve herd immunity to end the current pandemic. However, obstacles to reaching this goal include vaccine skepticism, structural barriers, or simple inertia to get vaccinated. These challenges provide opportunities for psychiatrists to use their medical knowledge and expertise, applying behavior management techniques such as motivational interviewing and nudging to encourage their patients to get vaccinated. In particular, marginalized patients with serious mental illness (SMI), who are subject to disproportionately high rates of COVID-19 infection and more severe outcomes,1 have much to gain if psychiatrists become involved in the COVID-19 vaccination campaign.
In this article, we define vaccine hesitancy and highlight what makes psychiatrists ideal vaccine ambassadors, given their unique skill set and longitudinal, trust-based connection with their patients. We expand on the particular vulnerabilities of patients with SMI, including structural barriers to vaccination that lead to health disparities and inequity. Finally, building on “The ABCs of successful vaccinations” framework published in
What is vaccine hesitancy?
The World Health Organization (WHO) defines vaccine hesitancy as a “delay in acceptance or refusal of vaccines despite availability of vaccine services.”3,4 Vaccine hesitancy occurs on a continuum ranging from uncertainty about accepting a vaccine to absolute refusal.4,5 It involves a complex decision-making process driven by contextual, individual, and social influences, and vaccine-specific issues.4 In the “3C” model developed by the WHO Strategic Advisory Group of Experts (SAGE) Working Group, vaccine hesitancy is influenced by confidence (trust in vaccines, in the health care system, and in policy makers), complacency (lower perceived risk), and convenience (availability, affordability, accessibility, language and health literacy, appeal of vaccination program).4
In 2019, the WHO named vaccine hesitancy as one of the top 10 global health threats.3 Hesitancy to receive COVID-19 vaccines may be particularly high because of their rapid development. In addition, the tumultuous political environment that often featured inconsistent messaging about the virus, its dangers, and its transmission since the early days of the pandemic created widespread public confusion and doubt as scientific understandings evolved. “Anti-vaxxer” movements that completely rejected vaccine efficacy disseminated misinformation online. Followers of these movements may have such extreme overvalued ideas that any effort to persuade them otherwise with scientific evidence will accomplish very little.6,7 Therefore, focusing on individuals who are “sitting on the fence” about getting vaccinated can be more productive because they represent a much larger group than those who adamantly refuse vaccines, and they may be more amenable to changing beliefs and behaviors.8
The US Census Bureau’s Household Pulse Survey asked, “How likely are you to accept the vaccine?”9 As of late June 2021, 11.4% of US adults reported they would “definitely not get a vaccine” or “probably not get a vaccine,” and that number increases to 16.9% when including those who are “unsure,” although there is wide geographical variability.10
A recent study in Denmark showed that willingness to receive the COVID-19 vaccine was slightly lower among patients with mental illness (84.8%) compared with the general population (89.5%).11 Given the small difference, vaccine hesitancy was not considered to be a major barrier for vaccination among patients with mental illness in Denmark. This is similar to the findings of a pre-pandemic study at a community mental health clinic in the United States involving other vaccinations, which suggested that 84% of patients with SMI perceived vaccinations as safe, effective, and important.12 In this clinic, identified barriers to vaccinations in general among patients with SMI included lack of awareness and knowledge (42.2%), accessibility (16.3%), personal cost (13.3%), fears about immunization (10.4%), and lack of recommendations by primary care providers (PCPs) (1.5%).12
It is critical to distinguish attitude-driven vaccine hesitancy from a lack of education and opportunity to receive a vaccine. Particularly disadvantaged communities may be mislabeled as “vaccine hesitant” when in fact they may not have the ability to be as proactive as other population groups (eg, difficulty scheduling appointments over the Internet).
Continue to: What makes psychiatrists ideal vaccine ambassadors?
What makes psychiatrists ideal vaccine ambassadors?
There are several reasons psychiatrists can be well-positioned to contribute to the success of vaccination campaigns (Table 1). These include their frequent contact with patients and their care teams, the high trust those patients have in them, and their medical expertise and skills in applied behavioral and social science techniques, including motivational interviewing and nudging. Vaccination efforts and outreach are more effective when led by the clinician with whom the patient has the most contact because resolving vaccine hesitancy is not a one-time discussion but requires ongoing communication, persistence, and consistency.13 Patients may contact their psychiatrists more frequently than their other clinicians, including PCPs. For this reason, psychiatrists can serve as the gateway to health care, particularly for patients with SMI.14 In addition, interruptions in nonemergency services caused by the COVID-19 pandemic may affect vaccine delivery because patients may have been unable to see their PCPs regularly during the pandemic.15

Psychiatrists’ medical expertise and their ability to develop rapport with their patients promote trust-building. Receiving credible information from a trusted source such as a patient’s psychiatrist can be impactful. A recent poll suggested that individual health care clinicians have been consistently identified as the most trusted sources for vaccine information, including for the COVID-19 vaccines.16 There is also higher trust when there is greater continuity of care both in terms of length of time the patient has known the clinician and the number of consultations,17 an inherent part of psychiatric practice. In addition, research has shown that patients trust their psychiatrists as much as they trust their general practitioners.18
Psychiatrists are experts in behavior change, promoting healthy behaviors through motivational interviewing and nudging. They also have experience with managing patients who hold overvalued ideas as well as dealing with uncertainty, given their scientific and medical training.
Motivational interviewing is a patient-centered, collaborative approach widely used by psychiatrists to treat unhealthy behaviors such as substance use. Clinicians elicit and strengthen the patient’s desire and motivation for change while respecting their autonomy. Instead of presenting persuasive facts, the clinician creates a welcoming, nonthreatening, safe environment by engaging patients in open dialogue, reflecting back the patients’ concerns with empathy, helping them realize contradictions in behavior, and supporting self-sufficiency.19 In a nonpsychiatric setting, studies have shown the effectiveness of motivational interviewing in increasing uptake of human papillomavirus vaccines and of pediatric vaccines.20
Nudging, which comes from behavioral economics and psychology, underscores the importance of structuring a choice architecture in changing the way people make their everyday decisions.21 Nudging still gives people a choice and respects autonomy, but it leads patients to more efficient and productive decision-making. Many nudges are based around giving good “default options” because people often do not make efforts to deviate from default options. In addition, social nudges are powerful, giving people a social reference point and normalizing certain behaviors.21 Psychiatrists have become skilled in nudging from working with patients with varying levels of insight and cognitive capabilities. That is, they give simple choices, prompts, and frequent feedback to reinforce “good” decisions and to discourage “bad” decisions.
Continue to: Managing overvalued ideas
Managing overvalued ideas. Psychiatrists are also well-versed in having discussions with patients who hold irrational beliefs (psychosis) or overvalued ideas. For example, psychiatrists frequently manage anorexia nervosa and hypochondria, which are rooted in overvalued ideas.7 While psychiatrists may not be able to directly confront the overvalued ideas, they can work around such ideas while waiting for more flexible moments. Similarly, managing patients with intense emotional commitment7 to commonly held anti-vaccination ideas may not be much different. Psychiatrists can work around resistance until patients may be less strongly attached to those overvalued ideas in instances when other techniques, such as motivational interviewing and nudging, may be more effective.
Managing uncertainty. Psychiatrists are experts in managing “not knowing” and uncertainty. Due to their medical scientific training, they are familiar with the process of science, and how understanding changes through trial and error. In contrast, most patients usually only see the end product (ie, a drug comes to market). Discussions with patients that acknowledge uncertainty and emphasize that changes in what is known are expected and appropriate as scientific knowledge evolves could help preempt skepticism when messages are updated.
Why do patients with SMI need more help?
SMI as a high-risk group. Patients with SMI are part of a “tragic” epidemiologic triad of agent-host-environment15 that places them at remarkably elevated risk for COVID-19 infection and more serious complications and death when infected.1 After age, a diagnosis of a schizophrenia spectrum disorder is the second largest predictor of mortality from COVID-19, with a 2.7-fold increase in mortality.22 This is how the elements of the triad come together: SARS-Cov-2 is a highly infectious agent affecting individuals who are vulnerable hosts because of their high frequency of medical comorbidities, including cardiovascular disease, type 2 diabetes, and respiratory tract diseases, which are all risk factors for worse outcomes due to COVID-19.23 In addition, SMI is associated with socioeconomic risk factors for SARS-Cov-2 infection, including poverty, homelessness, and crowded settings such as jails, group homes, hospitals, and shelters, which constitute ideal environments for high transmission of the virus.
Structural barriers to vaccination. Studies have suggested lower rates of vaccination among people with SMI for various other infectious diseases compared with the general population.12 For example, in 1 outpatient mental health setting, influenza vaccination rates were 24% to 28%, which was lower than the national vaccination rate of 40.9% for the same influenza season (2010 to 2011).24 More recently, a study in Israel examining the COVID-19 vaccination rate among >25,000 patients with schizophrenia suggested under-vaccination of this cohort. The results showed that the odds of getting the COVID-19 vaccination were significantly lower in the schizophrenia group compared with the general population (odds ratio = 0.80, 95% CI: 0.77 to 0.83).25
Patients with SMI encounter considerable system-level barriers to vaccinations in general, such as reduced access to health care due to cost and a lack of transportation,12 the digital divide given their reduced access to the internet and computers for information and scheduling,26 and lack of vaccination recommendations from their PCPs.12 Studies have also shown that patients with SMI often receive suboptimal medical care because of stigmatization and discrimination.27 They also have lower rates of preventive care utilization, seeking medical services only in times of crisis and seeking mental health services more often than physical health care.28-30
Continue to: Patients with SMI face...
Patients with SMI face additional individual challenges that impede vaccine uptake, such as lack of knowledge and awareness about the virus and vaccinations, general cognitive impairment, low digital literacy skills,31 low language literacy and educational attainment, baseline delusions, and negative symptoms such as apathy, avolition, and anhedonia.1 Thus, even if they overcome the external barriers and obtain vaccine-related information, these patients may experience difficulty in understanding the content and applying this information to their personal circumstances as a result of low health literacy.
How psychiatrists can help
The concept of using mental health care sites and trained clinicians to increase medical disease prevention is not new. The rigorously tested intervention model STIRR (Screen, Test, Immunize, Reduce risk, and Refer) uses co-located nurse practitioners in community mental health centers to provide risk assessment, counseling, and blood testing for hepatitis and HIV, as well as on-site vaccinations for hepatitis to patients dually diagnosed with SMI and substance use disorders.32
Prioritization of patients with SMI for vaccine eligibility does not directly lead to vaccine uptake. Patients with SMI need extra support from their primary point of health care contact, namely their psychiatrists. Psychiatrists may bring a set of specialized skills uniquely suited to this moment to address vaccine hesitancy and overall lack of vaccine resources and awareness. Freudenreich et al2 recently proposed “The ABCs of Successful Vaccinations” framework that psychiatrists can use in their interactions with patients to encourage vaccination by focusing on:
- attitudes towards vaccination
- barriers to vaccination
- completed vaccination series.
Understand attitudes toward vaccination. Decision-making may be an emotional and psychological experience that is informed by thoughts and feelings,34 and psychiatrists are uniquely positioned to tailor messages to individual patients by using motivational interviewing and applying nudging techniques.8 Given the large role of the pandemic in everyday life, it would be natural to address vaccine-related concerns in the course of routine rapport-building. Table 219,34-38 shows example phrases of COVID-19 vaccine messages that are based on communication strategies that have demonstrated success in health behavior domains (including vaccinations).39

Continue to: First, a strong recommendation...
First, a strong recommendation should be made using the presumptive approach.40 If vaccine hesitancy is detected, psychiatrists should next attempt to understand patients’ reasoning with open-ended questions to probe vaccine-related concerns. Motivational interviewing can then be used to target the fence sitters (rather than anti-vaxxers).6 Psychiatrists can also communicate with therapists about the need for further follow up on patients’ hesitancies.
When assuring patients of vaccine safety and efficacy, it is helpful to explain the vaccine development process, including FDA approval, extensive clinical trials, monitoring, and the distribution process. Providing clear, transparent, accurate information about the risks and benefits of the vaccines is important, as well as monitoring misinformation and developing convincing counter messages that elicit positive emotions toward the vaccines.41 Examples of messages to counter common vaccine-related concerns and misinformation are shown in Table 3.42-44

Know the barriers to vaccination. The role of the psychiatrist is to help patients, particularly those with SMIs, overcome logistical barriers and address hesitancy, which are both essential for vaccine uptake. Psychiatrists can help identify actual barriers (eg, transportation, digital access for information and scheduling) and perceived barriers, improve information access, and help patients obtain self-efficacy to take the actions needed to get vaccinated, particularly by collaborating with and communicating these concerns to other social services (Table 4).41

Monitor for vaccination series completion. Especially for vaccines that require more than a single dose over time, patients need more reminders, nudges, practical support, and encouragement to complete vaccination. A surprising degree of confusion regarding the timing of protection and benefit from the second COVID-19 injection (for the 2-injection vaccines) was uncovered in a recent survey of >1,000 US adults who had received their vaccinations in February 2021.45 Attentive monitoring of vaccination series completion by psychiatrists can thus increase the likelihood that a patient will follow through (Table 4).41 This can be as simple as asking about completion of the series during appointments, but further aided by communicating to the larger care team (social workers, care managers, care coordinators) when identifying that the patient may need further assistance.
The Figure2,6,7,19,40 summarizes the steps that psychiatrists can take to help patients get vaccinated by assessing attitudes towards vaccination (vaccine hesitancy), helping to remove barriers to vaccination, and ensuring via patient follow-up that a vaccine series is completed.

Continue to: Active involvement is key
Active involvement is key
The active involvement of psychiatrists in COVID-19 vaccination efforts can protect patients from the virus, reduce health disparities among patients with SMI, and promote herd immunity, helping to end the pandemic. Psychiatry practices can serve as ideal platforms to deliver evidence-based COVID-19 vaccine information and encourage vaccine uptake, particularly for marginalized populations.
Vaccination programs in mental health practices can even be conceptualized as a moral mandate in the spirit of addressing distributive injustice. The population management challenges of individual-level barriers and follow-through could be dramatically reduced—if not nearly eliminated—through policy-level changes that allow vaccinations to be administered in places where patients with SMI are already engaged: that is, “shots in arms” in mental health settings. As noted, some studies have shown that mental health settings can play a key role in other preventive care campaigns, such as the annual influenza and hepatitis vaccinations, and thus the incorporation of preventive care need not be limited to just COVID-19 vaccination efforts.
The COVID-19 pandemic is an opportunity to rethink the role of psychiatrists and psychiatric offices and clinics in preventive health care. The health risks and disparities of patients with SMI require the proactive involvement of psychiatrists at both the level of their individual patients and at the federal and state levels to advocate for policy changes that can benefit these populations. Overall, psychiatrists occupy a special role within the medical establishment that enables them to uniquely advocate for patients with SMI and ensure they are not forgotten during the COVID-19 pandemic.
Bottom Line
Psychiatrists could apply behavior management techniques such as motivational interviewing and nudging to address vaccine hesitancy in their patients and move them to accepting the COVID-19 vaccination. This could be particularly valuable for patients with serious mental illness, who face increased risks from COVID-19 and additional barriers to getting vaccinated.
Related Resources
- American Psychiatric Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-Coronavirus
- Baddeley M. Behavioural economics: a very short introduction. Oxford University Press; 2017.
- Centers for Disease Control and Prevention. Vaccines for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/index.html
- Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
- Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
1. Mazereel V, Van Assche K, Detraux J, et al. COVID-19 vaccination for people with severe mental illness: why, what, and how? Lancet Psychiatry. 2021;8(5):444-450.
2. Freudenreich O, Van Alphen MU, Lim C. The ABCs of successful vaccinations: a role for psychiatry. Current Psychiatry. 2021;20(3):48-50.
3. World Health Organization (WHO). Ten threats to global health in 2019. Accessed July 2, 2021. https://www.who.int/news-room/spotlight/ten-threats-to-global-health-in-2019
4. MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161-4164.
5. McClure CC, Cataldi JR, O’Leary ST. Vaccine hesitancy: where we are and where we are going. Clin Ther. 2017;39(8):1550-1562.
6. Betsch C, Korn L, Holtmann C. Don’t try to convert the antivaccinators, instead target the fence-sitters. Proc Natl Acad Sci. 2015;112(49):E6725-E6726.
7. Rahman T, Hartz SM, Xiong W, et al. Extreme overvalued beliefs. J Am Acad Psychiatry Law. 2020;48(3):319-326.
8. Leask J. Target the fence-sitters. Nature. 2011;473(7348):443-445.
9. United States Census Bureau. Household Pulse Survey COVID-19 Vaccination Tracker. Updated June 30, 2021. Accessed July 2, 2021. https://www.census.gov/library/visualizations/interactive/household-pulse-survey-covid-19-vaccination-tracker.html
10. United States Census Bureau. Measuring household experiences during the coronavirus pandemic. Updated May 5, 2021. Accessed July 2, 2021. https://www.census.gov/data/experimental-data-products/household-pulse-survey.html
11. Jefsen OH, Kølbæk P, Gil Y, et al. COVID-19 vaccine willingness among patients with mental illness compared with the general population. Acta Neuropsychiatrica. 2021:1-24. doi:10.1017/neu.2021.15
12. Miles LW, Williams N, Luthy KE, et al. Adult vaccination rates in the mentally ill population: an outpatient improvement project. J Am Psychiatr Nurses Assoc. 2020;26(2):172-180.
13. Lewandowsky S, Ecker UK, Seifert CM, et al. Misinformation and its correction: continued influence and successful debiasing. Psychol Sci Public Interest. 2012;13(3):106-131.
14. Druss BG, Rosenheck RA. Locus of mental health treatment in an integrated service system. Psychiatr Serv. 2000;51(7):890-892.
15. Freudenreich O, Kontos N, Querques J. COVID-19 and patients with serious mental illness. Current Psychiatry. 2020;19(9):24-35.
16. Hamel L, Kirzinger A, Muñana C, et al. KFF COVID-19 vaccine monitor: December 2020. Accessed July 2, 2021. https://www.kff.org/coronavirus-covid-19/report/kff-covid-19-vaccine-monitor-december-2020/
17. Kai J, Crosland A. Perspectives of people with enduring mental ill health from a community-based qualitative study. Br J Gen Pract. 2001;51(470):730-736.
18. Mather G, Baker D, Laugharne R. Patient trust in psychiatrists. Psychosis. 2012;4(2):161-167.
19. Miller WR, Rollnick S. Motivational interviewing: helping people change. Guilford Press; 2012.
20. Reno JE, O’Leary S, Garrett K, et al. Improving provider communication about HPV vaccines for vaccine-hesitant parents through the use of motivational interviewing. J Health Commun. 2018;23(4):313-320.
21. Baddeley M. Behavioural economics: a very short introduction. Volume 505. Oxford University Press; 2017.
22. Nemani K, Li C, Olfson M, et al. Association of psychiatric disorders with mortality among patients with COVID-19. JAMA Psychiatry. 2021;78(4):380-386.
23. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52.
24. Lorenz RA, Norris MM, Norton LC, et al. Factors associated with influenza vaccination decisions among patients with mental illness. Int J Psychiatry Med. 2013;46(1):1-13.
25. Bitan DT. Patients with schizophrenia are under‐vaccinated for COVID‐19: a report from Israel. World Psychiatry. 2021;20(2):300.
26. Robotham D, Satkunanathan S, Doughty L, et al. Do we still have a digital divide in mental health? A five-year survey follow-up. J Med Internet Res. 2016;18(11):e309.
27. De Hert M, Cohen D, Bobes J, et al. Physical illness in patients with severe mental disorders. II. Barriers to care, monitoring and treatment guidelines, plus recommendations at the system and individual level. World Psychiatry. 2011;10(2):138.
28. Carrà G, Bartoli F, Carretta D, et al. The prevalence of metabolic syndrome in people with severe mental illness: a mediation analysis. Soc Psychiatry Psychiatr Epidemiol. 2014;49(11):1739-1746.
29. Lin MT, Burgess JF, Carey K. The association between serious psychological distress and emergency department utilization among young adults in the USA. Soc Psychiatry Psychiatr Epidemiol. 2012;47(6):939-947.
30. DeCoux M. Acute versus primary care: the health care decision making process for individuals with severe mental illness. Issues Ment Health Nurs. 2005;26(9):935-951.
31. Hoffman L, Wisniewski H, Hays R, et al. Digital opportunities for outcomes in recovery services (DOORS): a pragmatic hands-on group approach toward increasing digital health and smartphone competencies, autonomy, relatedness, and alliance for those with serious mental illness. J Psychiatr Pract. 2020;26(2):80-88.
32. Rosenberg SD, Goldberg RW, Dixon LB, et al. Assessing the STIRR model of best practices for blood-borne infections of clients with severe mental illness. Psychiatr Serv. 2010;61(9):885-891.
33. Slade EP, Rosenberg S, Dixon LB, et al. Costs of a public health model to increase receipt of hepatitis-related services for persons with mental illness. Psychiatr Serv. 2013;64(2):127-133.
34. Brewer NT, Chapman GB, Rothman AJ, et al. Increasing vaccination: putting psychological science into action. Psychol Sci Public Interest. 2017;18(3):149-207.
35. Nabet B, Gable J, Eder J, et al. PolicyLab evidence to action brief: addressing vaccine hesitancy to protect children & communities against preventable diseases. Children’s Hospital of Philadelphia. Published Spring 2017. Accessed July 2, 2021. https://policylab.chop.edu/sites/default/files/pdf/publications/Addressing_Vaccine_Hesitancy.pdf
36. Opel DJ, Heritage J, Taylor JA, et al. The architecture of provider-parent vaccine discussions at health supervision visits. Pediatrics. 2013;132(6):1037-1046.
37. Betsch C, Böhm R, Korn L, et al. On the benefits of explaining herd immunity in vaccine advocacy. Nat Hum Behav. 2017;1(3):1-6.
38. Shen F, Sheer VC, Li R. Impact of narratives on persuasion in health communication: a meta-analysis. J Advert. 2015;44(2):105-113.
39. Parkerson N, Leader A. Vaccine hesitancy in the era of COVID. Population Health Leadership Series: PopTalk webinars. Paper 26. Published February 10, 2021. https://jdc.jefferson.edu/phlspoptalk/26/
40. Dempsey AF, O’Leary ST. Human papillomavirus vaccination: narrative review of studies on how providers’ vaccine communication affects attitudes and uptake. Acad Pediatr. 2018;18(2):S23-S27.
41. Chou W, Burgdorf C, Gaysynsky A, et al. COVID-19 vaccination communication: applying behavioral and social science to address vaccine hesitancy and foster vaccine confidence. National Institutes of Health. Published 2020. https://obssr.od.nih.gov/sites/obssr/files/inline-files/OBSSR_VaccineWhitePaper_FINAL_508.pdf
42. International Society for Vaccines and the MJH Life Sciences COVID-19 coalition. Building confidence in COVID-19 vaccination: a toolbox of talks from leaders in the field. March 9, 2021. https://globalmeet.webcasts.com/starthere.jsp?ei=1435659&tp_key=59ed660099
43. Centers for Disease Control and Prevention. Frequently asked questions about COVID-19 vaccination. Accessed July 2, 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/faq.html
44. Singh BR, Gandharava S, Gandharva R. Covid-19 vaccines and community immunity. Infectious Diseases Research. 2021;2(1):5.
45. Goldfarb JL, Kreps S, Brownstein JS, et al. Beyond the first dose - Covid-19 vaccine follow-through and continued protective measures. N Engl J Med. 2021;85(2):101-103.
CDC to show vaccinated people infected with Delta remain contagious
and infect others, the New York Times reported on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the New York Times said. The bottom line is that the CDC data shows people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on Jul 29 that the CDC’s updated mask guidance followed an outbreak on Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
A version of this article first appeared on Medscape.com.
and infect others, the New York Times reported on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the New York Times said. The bottom line is that the CDC data shows people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on Jul 29 that the CDC’s updated mask guidance followed an outbreak on Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
A version of this article first appeared on Medscape.com.
and infect others, the New York Times reported on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the New York Times said. The bottom line is that the CDC data shows people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on Jul 29 that the CDC’s updated mask guidance followed an outbreak on Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
A version of this article first appeared on Medscape.com.
Short sleep is linked to future dementia
, according to a new analysis of data from the Whitehall II cohort study.
Previous work had identified links between short sleep duration and dementia risk, but few studies examined sleep habits long before onset of dementia. Those that did produced inconsistent results, according to Séverine Sabia, PhD, who is a research associate at Inserm (France) and the University College London.
“One potential reason for these inconstancies is the large range of ages of the study populations, and the small number of participants within each sleep duration group. The novelty of our study is to examine this association among almost 8,000 participants with a follow-up of 30 years, using repeated measures of sleep duration starting in midlife to consider sleep duration at specific ages,” Dr. Sabia said in an interview. She presented the research at the 2021 Alzheimer’s Association International Conference.
Those previous studies found a U-shaped association between sleep duration and dementia risk, with lowest risk associated with 7-8 hours of sleep, but greater risk for shorter and longer durations. However, because the studies had follow-up periods shorter than 10 years, they are at greater risk of reverse causation bias. Longer follow-up studies tended to have small sample sizes or to focus on older adults.
The longer follow-up in the current study makes for a more compelling case, said Claire Sexton, DPhil, director of Scientific Programs & Outreach for the Alzheimer’s Association. Observations of short or long sleep closer to the onset of symptoms could just be a warning sign of dementia. “But looking at age 50, age 60 ... if you’re seeing those relationships, then it’s less likely that it is just purely prodromal,” said Dr. Sexton. But it still doesn’t necessarily confirm causation. “It could also be a risk factor,” Dr. Sexton added.
Multifactorial risk
Dr. Sabia also noted that the magnitude of risk was similar to that seen with smoking or obesity, and many factors play a role in dementia risk. “Even if the risk of dementia was 30% higher in those with persistent short sleep duration, in absolute terms, the percentage of those with persistent short duration who developed dementia was 8%, and 6% in those with persistent sleep duration of 7 hours. Dementia is a multifactorial disease, which means that several factors are likely to influence its onset. Sleep duration is one of them, but if a person has poor sleep and does not manage to increase it, there are other important prevention measures. It is important to keep a healthy lifestyle and cardiometabolic measures in the normal range. All together it is likely to be beneficial for brain health in later life,” she said.
Dr. Sexton agreed. “With sleep we’re still trying to tease apart what aspect of sleep is important. Is it the sleep duration? Is it the quality of sleep? Is it certain sleep stages?” she said.
Regardless of sleep’s potential influence on dementia risk, both Dr. Sexton and Dr. Sabia noted the importance of sleep for general health. “These types of problems are very prevalent, so it’s good for people to be aware of them. And then if they notice any problems with their sleep, or any changes, to go and see their health care provider, and to be discussing them, and then to be investigating the cause, and to see whether changes in sleep hygiene and treatments for insomnia could address these sleep problems,” said Dr. Sexton.
Decades of data
During the Whitehall II study, researchers assessed average sleep duration (“How many hours of sleep do you have on an average weeknight?”) six times over 30 years of follow-up. Dr. Sabia’s group extracted self-reported sleep duration data at ages 50, 60, and 70. Short sleep duration was defined as fewer than 5 hours, or 6 hours. Normal sleep duration was defined as 7 hours. Long duration was defined as 8 hours or more.
A questioner during the Q&A period noted that this grouping is a little unusual. Many studies define 7-8 hours as normal. Dr. Sabia answered that they were unable to examine periods of 9 hours or more due to the nature of the data, and the lowest associated risk was found at 7 hours.
The researchers analyzed data from 7,959 participants (33.0% women). At age 50, compared with 7 hours of sleep, 6 or few hours of sleep was associated with a higher risk of dementia over the ensuing 25 years of follow-up (hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.01-1.48). The same was true at age 60 (15 years of follow-up HR, 1.37; 95% CI, 1.10-1.72). There was a trend at age 70 (8 years follow-up; HR, 1.24; 95% CI, 0.98-1.57). For 8 or more hours of sleep, there were trends toward increased risk at age 50 (HR, 1.25; 95% CI, 0.98-1.60). Long sleep at age 60 and 70 was associated with heightened risk, but the confidence intervals were well outside statistical significance.
Twenty percent of participants had persistent short sleep over the course of follow-up, 37% had persistent normal sleep, and 7% had persistent long sleep. Seven percent of participants experienced a change from normal sleep to short sleep, 16% had a change from short sleep to normal sleep, and 13% had a change from normal sleep to long sleep.
Persistent short sleep between age 50 and 70 was associated with a 30% increased risk of dementia (HR, 1.30; 95% CI, 1.00-1.69). There were no statistically significant associations between dementia risk and any of the changing sleep pattern groups.
Dr. Sabia and Dr. Sexton have no relevant financial disclosures.
, according to a new analysis of data from the Whitehall II cohort study.
Previous work had identified links between short sleep duration and dementia risk, but few studies examined sleep habits long before onset of dementia. Those that did produced inconsistent results, according to Séverine Sabia, PhD, who is a research associate at Inserm (France) and the University College London.
“One potential reason for these inconstancies is the large range of ages of the study populations, and the small number of participants within each sleep duration group. The novelty of our study is to examine this association among almost 8,000 participants with a follow-up of 30 years, using repeated measures of sleep duration starting in midlife to consider sleep duration at specific ages,” Dr. Sabia said in an interview. She presented the research at the 2021 Alzheimer’s Association International Conference.
Those previous studies found a U-shaped association between sleep duration and dementia risk, with lowest risk associated with 7-8 hours of sleep, but greater risk for shorter and longer durations. However, because the studies had follow-up periods shorter than 10 years, they are at greater risk of reverse causation bias. Longer follow-up studies tended to have small sample sizes or to focus on older adults.
The longer follow-up in the current study makes for a more compelling case, said Claire Sexton, DPhil, director of Scientific Programs & Outreach for the Alzheimer’s Association. Observations of short or long sleep closer to the onset of symptoms could just be a warning sign of dementia. “But looking at age 50, age 60 ... if you’re seeing those relationships, then it’s less likely that it is just purely prodromal,” said Dr. Sexton. But it still doesn’t necessarily confirm causation. “It could also be a risk factor,” Dr. Sexton added.
Multifactorial risk
Dr. Sabia also noted that the magnitude of risk was similar to that seen with smoking or obesity, and many factors play a role in dementia risk. “Even if the risk of dementia was 30% higher in those with persistent short sleep duration, in absolute terms, the percentage of those with persistent short duration who developed dementia was 8%, and 6% in those with persistent sleep duration of 7 hours. Dementia is a multifactorial disease, which means that several factors are likely to influence its onset. Sleep duration is one of them, but if a person has poor sleep and does not manage to increase it, there are other important prevention measures. It is important to keep a healthy lifestyle and cardiometabolic measures in the normal range. All together it is likely to be beneficial for brain health in later life,” she said.
Dr. Sexton agreed. “With sleep we’re still trying to tease apart what aspect of sleep is important. Is it the sleep duration? Is it the quality of sleep? Is it certain sleep stages?” she said.
Regardless of sleep’s potential influence on dementia risk, both Dr. Sexton and Dr. Sabia noted the importance of sleep for general health. “These types of problems are very prevalent, so it’s good for people to be aware of them. And then if they notice any problems with their sleep, or any changes, to go and see their health care provider, and to be discussing them, and then to be investigating the cause, and to see whether changes in sleep hygiene and treatments for insomnia could address these sleep problems,” said Dr. Sexton.
Decades of data
During the Whitehall II study, researchers assessed average sleep duration (“How many hours of sleep do you have on an average weeknight?”) six times over 30 years of follow-up. Dr. Sabia’s group extracted self-reported sleep duration data at ages 50, 60, and 70. Short sleep duration was defined as fewer than 5 hours, or 6 hours. Normal sleep duration was defined as 7 hours. Long duration was defined as 8 hours or more.
A questioner during the Q&A period noted that this grouping is a little unusual. Many studies define 7-8 hours as normal. Dr. Sabia answered that they were unable to examine periods of 9 hours or more due to the nature of the data, and the lowest associated risk was found at 7 hours.
The researchers analyzed data from 7,959 participants (33.0% women). At age 50, compared with 7 hours of sleep, 6 or few hours of sleep was associated with a higher risk of dementia over the ensuing 25 years of follow-up (hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.01-1.48). The same was true at age 60 (15 years of follow-up HR, 1.37; 95% CI, 1.10-1.72). There was a trend at age 70 (8 years follow-up; HR, 1.24; 95% CI, 0.98-1.57). For 8 or more hours of sleep, there were trends toward increased risk at age 50 (HR, 1.25; 95% CI, 0.98-1.60). Long sleep at age 60 and 70 was associated with heightened risk, but the confidence intervals were well outside statistical significance.
Twenty percent of participants had persistent short sleep over the course of follow-up, 37% had persistent normal sleep, and 7% had persistent long sleep. Seven percent of participants experienced a change from normal sleep to short sleep, 16% had a change from short sleep to normal sleep, and 13% had a change from normal sleep to long sleep.
Persistent short sleep between age 50 and 70 was associated with a 30% increased risk of dementia (HR, 1.30; 95% CI, 1.00-1.69). There were no statistically significant associations between dementia risk and any of the changing sleep pattern groups.
Dr. Sabia and Dr. Sexton have no relevant financial disclosures.
, according to a new analysis of data from the Whitehall II cohort study.
Previous work had identified links between short sleep duration and dementia risk, but few studies examined sleep habits long before onset of dementia. Those that did produced inconsistent results, according to Séverine Sabia, PhD, who is a research associate at Inserm (France) and the University College London.
“One potential reason for these inconstancies is the large range of ages of the study populations, and the small number of participants within each sleep duration group. The novelty of our study is to examine this association among almost 8,000 participants with a follow-up of 30 years, using repeated measures of sleep duration starting in midlife to consider sleep duration at specific ages,” Dr. Sabia said in an interview. She presented the research at the 2021 Alzheimer’s Association International Conference.
Those previous studies found a U-shaped association between sleep duration and dementia risk, with lowest risk associated with 7-8 hours of sleep, but greater risk for shorter and longer durations. However, because the studies had follow-up periods shorter than 10 years, they are at greater risk of reverse causation bias. Longer follow-up studies tended to have small sample sizes or to focus on older adults.
The longer follow-up in the current study makes for a more compelling case, said Claire Sexton, DPhil, director of Scientific Programs & Outreach for the Alzheimer’s Association. Observations of short or long sleep closer to the onset of symptoms could just be a warning sign of dementia. “But looking at age 50, age 60 ... if you’re seeing those relationships, then it’s less likely that it is just purely prodromal,” said Dr. Sexton. But it still doesn’t necessarily confirm causation. “It could also be a risk factor,” Dr. Sexton added.
Multifactorial risk
Dr. Sabia also noted that the magnitude of risk was similar to that seen with smoking or obesity, and many factors play a role in dementia risk. “Even if the risk of dementia was 30% higher in those with persistent short sleep duration, in absolute terms, the percentage of those with persistent short duration who developed dementia was 8%, and 6% in those with persistent sleep duration of 7 hours. Dementia is a multifactorial disease, which means that several factors are likely to influence its onset. Sleep duration is one of them, but if a person has poor sleep and does not manage to increase it, there are other important prevention measures. It is important to keep a healthy lifestyle and cardiometabolic measures in the normal range. All together it is likely to be beneficial for brain health in later life,” she said.
Dr. Sexton agreed. “With sleep we’re still trying to tease apart what aspect of sleep is important. Is it the sleep duration? Is it the quality of sleep? Is it certain sleep stages?” she said.
Regardless of sleep’s potential influence on dementia risk, both Dr. Sexton and Dr. Sabia noted the importance of sleep for general health. “These types of problems are very prevalent, so it’s good for people to be aware of them. And then if they notice any problems with their sleep, or any changes, to go and see their health care provider, and to be discussing them, and then to be investigating the cause, and to see whether changes in sleep hygiene and treatments for insomnia could address these sleep problems,” said Dr. Sexton.
Decades of data
During the Whitehall II study, researchers assessed average sleep duration (“How many hours of sleep do you have on an average weeknight?”) six times over 30 years of follow-up. Dr. Sabia’s group extracted self-reported sleep duration data at ages 50, 60, and 70. Short sleep duration was defined as fewer than 5 hours, or 6 hours. Normal sleep duration was defined as 7 hours. Long duration was defined as 8 hours or more.
A questioner during the Q&A period noted that this grouping is a little unusual. Many studies define 7-8 hours as normal. Dr. Sabia answered that they were unable to examine periods of 9 hours or more due to the nature of the data, and the lowest associated risk was found at 7 hours.
The researchers analyzed data from 7,959 participants (33.0% women). At age 50, compared with 7 hours of sleep, 6 or few hours of sleep was associated with a higher risk of dementia over the ensuing 25 years of follow-up (hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.01-1.48). The same was true at age 60 (15 years of follow-up HR, 1.37; 95% CI, 1.10-1.72). There was a trend at age 70 (8 years follow-up; HR, 1.24; 95% CI, 0.98-1.57). For 8 or more hours of sleep, there were trends toward increased risk at age 50 (HR, 1.25; 95% CI, 0.98-1.60). Long sleep at age 60 and 70 was associated with heightened risk, but the confidence intervals were well outside statistical significance.
Twenty percent of participants had persistent short sleep over the course of follow-up, 37% had persistent normal sleep, and 7% had persistent long sleep. Seven percent of participants experienced a change from normal sleep to short sleep, 16% had a change from short sleep to normal sleep, and 13% had a change from normal sleep to long sleep.
Persistent short sleep between age 50 and 70 was associated with a 30% increased risk of dementia (HR, 1.30; 95% CI, 1.00-1.69). There were no statistically significant associations between dementia risk and any of the changing sleep pattern groups.
Dr. Sabia and Dr. Sexton have no relevant financial disclosures.
FROM AAIC 2021
Surgeon marks ‘right’ instead of ‘left’ testicle, then operates
Wrong-site surgery
Florida regulators have imposed a fine and other measures on a Tampa doctor who made a crucial error prior to his patient’s testicular surgery, as a story in the Miami Herald, among other news sites, reports.
On Sept. 10, 2019, a patient referred to in state documents as “C.F.” showed up for a procedure – a varicocelectomy – that would remove the enlarged veins in his left testicle. His doctor that day was Raul Fernandez-Crespo, MD, a urologist who had been licensed to practice in Florida since April of the same year. Dr. Fernandez-Crespo completed his urology residency at the University of Puerto Rico in 2019.
Following a conversation with C.F., Dr. Fernandez-Crespo designated what he believed was the proper surgical site – his patient’s right testicle.
He then proceeded to operate, but at some point during the procedure – news accounts don’t make clear when or how he became aware of his error – he realized C.F. had actually consented to a left-testicle varicocelectomy. With his patient still sedated, Dr. Fernandez-Crespo also completed the second procedure.
His mistake came to the attention of the Department of Health, which filed an administrative complaint against the surgeon. On June 17, 2021, the department’s medical licensing body, the Florida Board of Medicine, handed down its final order about the case.
In addition to imposing a $2,500 fine on Dr. Fernandez-Crespo and issuing “a letter of concern” – a public document that can be used as evidence in any relevant future disciplinary action against him – regulators said the surgeon must reimburse $2,045.56 to the department for its case-related administrative costs; take a 5-hour CME course in risk management or attend 8 hours of board disciplinary hearings; and, finally, give a 1-hour lecture on wrong-site surgeries at a board-approved medical facility.
Before this, Dr. Fernandez-Crespo had no previous disciplinary history with the Florida Board of Medicine.
Huge judgment after fertility procedure goes wrong
A Connecticut couple whose fertility and prenatal care at a state university health center proved disastrous will receive millions of dollars in damages, according to a report in the Hartford Courant.
In 2014, Jean-Marie Monroe-Lynch and her husband, Aaron Lynch, went to UConn Health, in Farmington, for treatment of Jean-Marie’s infertility. Her care was overseen by the Center for Advanced Reproductive Services (CARS), a private company then under contract with UConn Health. (The contract, which ended in 2014, obligated UConn to provide CARS providers with medical malpractice coverage.)
There, Jean-Marie was inseminated with sperm from a donor who turned out to be a carrier for cytomegalovirus (CMV), the herpes virus that can cause severe birth defects, or fetal death, when contracted by a pregnant woman. The insemination resulted in a twin pregnancy, a boy and a girl. The girl, Shay, died in utero after several of her organs became infected with CMV; the boy, Joshua, was born with severe mental and physical disabilities.
In their suit, Ms. Monroe-Lynch and her husband alleged that they were never cautioned about the risks associated with using a sperm donor whose blood had tested positive for CMV antibodies. Their suit further alleged that, at the 20-week ultrasound, UConn’s prenatal team failed to detect evidence of congenital CMV infection and again failed, at the 22-week ultrasound, to properly recognize and respond to abnormal findings.
“They totally dropped the ball,” said the couple’s attorney. “If you’re a pregnant woman and contract the virus for the first time, the results can be devastating.”
CARS disputes this conclusion, arguing that the plaintiffs failed to prove as a “matter of scientific fact” that Ms. Monroe-Lynch was infected with CMV as the result of her intrauterine insemination.
But Superior Court Judge Mark H. Taylor disagreed. In his 107-page ruling, he said that the court “agrees with the vast majority of superior courts, concluding that a physician providing obstetric care owes a direct duty to a mother to prevent harm to her child during gestation and delivery.”
Jean-Marie Monroe-Lynch and Aaron Lynch received a $37.6 million award, consisting of $24.1 million in economic damages and $13.5 million in noneconomic damages.
Their surviving child, Joshua, will reportedly require a lifetime of medical and other care. In the meantime, UConn Health vows to appeal the Superior Court’s decision.
COVID patient’s relative demands justice for fatal outcome
An Indiana man whose grandfather recently died after suffering a stroke is calling on state lawmakers to rethink legislation passed earlier this year to protect health care providers during the COVID-19 pandemic, according to a story reported by CBS4Indy.
Late last year, Daniel Enlow’s 83-year-old grandfather, Edward Rigney, was checked into Eskenazi Hospital, in Indianapolis. Mr. Rigney suffered from COPD and had also been diagnosed with COVID-19.
At some point during his hospitalization, medical staff attempted to place what seems to have been an arterial line in order to monitor his condition. During the procedure, or at some point shortly thereafter, an “iatrogenic air embolus” was released into his veins and caused a stroke, according to medical records and Mr. Rigney’s death certificate.
“I started asking for medical records because I wanted to know what was happening leading up to it in black and white in front of me,” said Mr. Enlow, who wished to present his evidence to a medical review panel, as required by Indiana law. The first step in this process would have been to consult with a medical malpractice attorney, but several declined to take his case.
Why? Because a pair of bills passed by Indiana legislators in early 2021 make COVID-19–related suits – even tangentially related ones – potentially difficult to take to court.
The bills raised the bar to file a medical malpractice claim in COVID-19 cases and to allow only those that involve “gross negligence or willful or wanton misconduct.”
“In the vast majority of cases, it’s impossible to prove that,” said Fred Schultz, immediate past president of the Indiana Trial Lawyers Association, who lobbied against the legislation.
The bills were never designed to offer “blanket freedom,” said GOP State Senator Aaron Freeman, sponsor of one of the bills. “If something is being used in a way that it is a complete bar to certain claims, then maybe we need to go back and look at it and open that up a little bit and make it less restrictive. I’m certainly open to having those conversations.”
Meanwhile, Mr. Enlow has vowed to keep pushing in the name of his late grandfather. The hospital’s parent company, Eskenazi Health, has declined to comment.
A version of this article first appeared on Medscape.com.
Wrong-site surgery
Florida regulators have imposed a fine and other measures on a Tampa doctor who made a crucial error prior to his patient’s testicular surgery, as a story in the Miami Herald, among other news sites, reports.
On Sept. 10, 2019, a patient referred to in state documents as “C.F.” showed up for a procedure – a varicocelectomy – that would remove the enlarged veins in his left testicle. His doctor that day was Raul Fernandez-Crespo, MD, a urologist who had been licensed to practice in Florida since April of the same year. Dr. Fernandez-Crespo completed his urology residency at the University of Puerto Rico in 2019.
Following a conversation with C.F., Dr. Fernandez-Crespo designated what he believed was the proper surgical site – his patient’s right testicle.
He then proceeded to operate, but at some point during the procedure – news accounts don’t make clear when or how he became aware of his error – he realized C.F. had actually consented to a left-testicle varicocelectomy. With his patient still sedated, Dr. Fernandez-Crespo also completed the second procedure.
His mistake came to the attention of the Department of Health, which filed an administrative complaint against the surgeon. On June 17, 2021, the department’s medical licensing body, the Florida Board of Medicine, handed down its final order about the case.
In addition to imposing a $2,500 fine on Dr. Fernandez-Crespo and issuing “a letter of concern” – a public document that can be used as evidence in any relevant future disciplinary action against him – regulators said the surgeon must reimburse $2,045.56 to the department for its case-related administrative costs; take a 5-hour CME course in risk management or attend 8 hours of board disciplinary hearings; and, finally, give a 1-hour lecture on wrong-site surgeries at a board-approved medical facility.
Before this, Dr. Fernandez-Crespo had no previous disciplinary history with the Florida Board of Medicine.
Huge judgment after fertility procedure goes wrong
A Connecticut couple whose fertility and prenatal care at a state university health center proved disastrous will receive millions of dollars in damages, according to a report in the Hartford Courant.
In 2014, Jean-Marie Monroe-Lynch and her husband, Aaron Lynch, went to UConn Health, in Farmington, for treatment of Jean-Marie’s infertility. Her care was overseen by the Center for Advanced Reproductive Services (CARS), a private company then under contract with UConn Health. (The contract, which ended in 2014, obligated UConn to provide CARS providers with medical malpractice coverage.)
There, Jean-Marie was inseminated with sperm from a donor who turned out to be a carrier for cytomegalovirus (CMV), the herpes virus that can cause severe birth defects, or fetal death, when contracted by a pregnant woman. The insemination resulted in a twin pregnancy, a boy and a girl. The girl, Shay, died in utero after several of her organs became infected with CMV; the boy, Joshua, was born with severe mental and physical disabilities.
In their suit, Ms. Monroe-Lynch and her husband alleged that they were never cautioned about the risks associated with using a sperm donor whose blood had tested positive for CMV antibodies. Their suit further alleged that, at the 20-week ultrasound, UConn’s prenatal team failed to detect evidence of congenital CMV infection and again failed, at the 22-week ultrasound, to properly recognize and respond to abnormal findings.
“They totally dropped the ball,” said the couple’s attorney. “If you’re a pregnant woman and contract the virus for the first time, the results can be devastating.”
CARS disputes this conclusion, arguing that the plaintiffs failed to prove as a “matter of scientific fact” that Ms. Monroe-Lynch was infected with CMV as the result of her intrauterine insemination.
But Superior Court Judge Mark H. Taylor disagreed. In his 107-page ruling, he said that the court “agrees with the vast majority of superior courts, concluding that a physician providing obstetric care owes a direct duty to a mother to prevent harm to her child during gestation and delivery.”
Jean-Marie Monroe-Lynch and Aaron Lynch received a $37.6 million award, consisting of $24.1 million in economic damages and $13.5 million in noneconomic damages.
Their surviving child, Joshua, will reportedly require a lifetime of medical and other care. In the meantime, UConn Health vows to appeal the Superior Court’s decision.
COVID patient’s relative demands justice for fatal outcome
An Indiana man whose grandfather recently died after suffering a stroke is calling on state lawmakers to rethink legislation passed earlier this year to protect health care providers during the COVID-19 pandemic, according to a story reported by CBS4Indy.
Late last year, Daniel Enlow’s 83-year-old grandfather, Edward Rigney, was checked into Eskenazi Hospital, in Indianapolis. Mr. Rigney suffered from COPD and had also been diagnosed with COVID-19.
At some point during his hospitalization, medical staff attempted to place what seems to have been an arterial line in order to monitor his condition. During the procedure, or at some point shortly thereafter, an “iatrogenic air embolus” was released into his veins and caused a stroke, according to medical records and Mr. Rigney’s death certificate.
“I started asking for medical records because I wanted to know what was happening leading up to it in black and white in front of me,” said Mr. Enlow, who wished to present his evidence to a medical review panel, as required by Indiana law. The first step in this process would have been to consult with a medical malpractice attorney, but several declined to take his case.
Why? Because a pair of bills passed by Indiana legislators in early 2021 make COVID-19–related suits – even tangentially related ones – potentially difficult to take to court.
The bills raised the bar to file a medical malpractice claim in COVID-19 cases and to allow only those that involve “gross negligence or willful or wanton misconduct.”
“In the vast majority of cases, it’s impossible to prove that,” said Fred Schultz, immediate past president of the Indiana Trial Lawyers Association, who lobbied against the legislation.
The bills were never designed to offer “blanket freedom,” said GOP State Senator Aaron Freeman, sponsor of one of the bills. “If something is being used in a way that it is a complete bar to certain claims, then maybe we need to go back and look at it and open that up a little bit and make it less restrictive. I’m certainly open to having those conversations.”
Meanwhile, Mr. Enlow has vowed to keep pushing in the name of his late grandfather. The hospital’s parent company, Eskenazi Health, has declined to comment.
A version of this article first appeared on Medscape.com.
Wrong-site surgery
Florida regulators have imposed a fine and other measures on a Tampa doctor who made a crucial error prior to his patient’s testicular surgery, as a story in the Miami Herald, among other news sites, reports.
On Sept. 10, 2019, a patient referred to in state documents as “C.F.” showed up for a procedure – a varicocelectomy – that would remove the enlarged veins in his left testicle. His doctor that day was Raul Fernandez-Crespo, MD, a urologist who had been licensed to practice in Florida since April of the same year. Dr. Fernandez-Crespo completed his urology residency at the University of Puerto Rico in 2019.
Following a conversation with C.F., Dr. Fernandez-Crespo designated what he believed was the proper surgical site – his patient’s right testicle.
He then proceeded to operate, but at some point during the procedure – news accounts don’t make clear when or how he became aware of his error – he realized C.F. had actually consented to a left-testicle varicocelectomy. With his patient still sedated, Dr. Fernandez-Crespo also completed the second procedure.
His mistake came to the attention of the Department of Health, which filed an administrative complaint against the surgeon. On June 17, 2021, the department’s medical licensing body, the Florida Board of Medicine, handed down its final order about the case.
In addition to imposing a $2,500 fine on Dr. Fernandez-Crespo and issuing “a letter of concern” – a public document that can be used as evidence in any relevant future disciplinary action against him – regulators said the surgeon must reimburse $2,045.56 to the department for its case-related administrative costs; take a 5-hour CME course in risk management or attend 8 hours of board disciplinary hearings; and, finally, give a 1-hour lecture on wrong-site surgeries at a board-approved medical facility.
Before this, Dr. Fernandez-Crespo had no previous disciplinary history with the Florida Board of Medicine.
Huge judgment after fertility procedure goes wrong
A Connecticut couple whose fertility and prenatal care at a state university health center proved disastrous will receive millions of dollars in damages, according to a report in the Hartford Courant.
In 2014, Jean-Marie Monroe-Lynch and her husband, Aaron Lynch, went to UConn Health, in Farmington, for treatment of Jean-Marie’s infertility. Her care was overseen by the Center for Advanced Reproductive Services (CARS), a private company then under contract with UConn Health. (The contract, which ended in 2014, obligated UConn to provide CARS providers with medical malpractice coverage.)
There, Jean-Marie was inseminated with sperm from a donor who turned out to be a carrier for cytomegalovirus (CMV), the herpes virus that can cause severe birth defects, or fetal death, when contracted by a pregnant woman. The insemination resulted in a twin pregnancy, a boy and a girl. The girl, Shay, died in utero after several of her organs became infected with CMV; the boy, Joshua, was born with severe mental and physical disabilities.
In their suit, Ms. Monroe-Lynch and her husband alleged that they were never cautioned about the risks associated with using a sperm donor whose blood had tested positive for CMV antibodies. Their suit further alleged that, at the 20-week ultrasound, UConn’s prenatal team failed to detect evidence of congenital CMV infection and again failed, at the 22-week ultrasound, to properly recognize and respond to abnormal findings.
“They totally dropped the ball,” said the couple’s attorney. “If you’re a pregnant woman and contract the virus for the first time, the results can be devastating.”
CARS disputes this conclusion, arguing that the plaintiffs failed to prove as a “matter of scientific fact” that Ms. Monroe-Lynch was infected with CMV as the result of her intrauterine insemination.
But Superior Court Judge Mark H. Taylor disagreed. In his 107-page ruling, he said that the court “agrees with the vast majority of superior courts, concluding that a physician providing obstetric care owes a direct duty to a mother to prevent harm to her child during gestation and delivery.”
Jean-Marie Monroe-Lynch and Aaron Lynch received a $37.6 million award, consisting of $24.1 million in economic damages and $13.5 million in noneconomic damages.
Their surviving child, Joshua, will reportedly require a lifetime of medical and other care. In the meantime, UConn Health vows to appeal the Superior Court’s decision.
COVID patient’s relative demands justice for fatal outcome
An Indiana man whose grandfather recently died after suffering a stroke is calling on state lawmakers to rethink legislation passed earlier this year to protect health care providers during the COVID-19 pandemic, according to a story reported by CBS4Indy.
Late last year, Daniel Enlow’s 83-year-old grandfather, Edward Rigney, was checked into Eskenazi Hospital, in Indianapolis. Mr. Rigney suffered from COPD and had also been diagnosed with COVID-19.
At some point during his hospitalization, medical staff attempted to place what seems to have been an arterial line in order to monitor his condition. During the procedure, or at some point shortly thereafter, an “iatrogenic air embolus” was released into his veins and caused a stroke, according to medical records and Mr. Rigney’s death certificate.
“I started asking for medical records because I wanted to know what was happening leading up to it in black and white in front of me,” said Mr. Enlow, who wished to present his evidence to a medical review panel, as required by Indiana law. The first step in this process would have been to consult with a medical malpractice attorney, but several declined to take his case.
Why? Because a pair of bills passed by Indiana legislators in early 2021 make COVID-19–related suits – even tangentially related ones – potentially difficult to take to court.
The bills raised the bar to file a medical malpractice claim in COVID-19 cases and to allow only those that involve “gross negligence or willful or wanton misconduct.”
“In the vast majority of cases, it’s impossible to prove that,” said Fred Schultz, immediate past president of the Indiana Trial Lawyers Association, who lobbied against the legislation.
The bills were never designed to offer “blanket freedom,” said GOP State Senator Aaron Freeman, sponsor of one of the bills. “If something is being used in a way that it is a complete bar to certain claims, then maybe we need to go back and look at it and open that up a little bit and make it less restrictive. I’m certainly open to having those conversations.”
Meanwhile, Mr. Enlow has vowed to keep pushing in the name of his late grandfather. The hospital’s parent company, Eskenazi Health, has declined to comment.
A version of this article first appeared on Medscape.com.
New investigational helmet device shrinks glioblastoma
This is the first time that the wearable Oncomagnetic device was tried with a patient.
The patient had end-stage recurrent glioblastoma and had undergone all standard therapy options. He wore the device for 5 weeks but died from an unrelated injury, so the treatment period was cut short.
A brain scan showed a 31% reduction of contrast-enhanced tumor volume, and an autopsy of his brain confirmed the rapid response to the treatment.
The case study was published online on July 22, 2021, in Frontiers in Oncology.
“I believe that there is a great potential with this device,” said study author David S. Baskin, MD, director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the department of neurosurgery at Houston Methodist Hospital. “This is a very exciting time.”
The team is now treating several patients with glioblastoma under compassionate use.
In an independent comment, Adilia Hormigo, MD, PhD, director of the neuro-oncology program at the Tisch Cancer Institute, Mount Sinai Health System, New York, noted that a clinical trial is needed to evaluate the device. “But this is an interesting idea, and we have to be open-minded in treating this fatal disease.”
Oscillating magnetic fields
The Oncomagnetic device consists of three oncoscillators that are attached to the outside of a helmet and are connected to a microprocessor-based electronic controller powered by a rechargeable battery.
It consists of a series of rotating magnets that produce oscillating magnetic fields that cover the entire brain, including the upper part of the brain stem. The device induces rapid apoptosis of glioblastoma cells, Dr. Baskin explained. Its mechanism of action involves disruption of the electron transport in the mitochondrial respiratory chain, causing an elevation of reactive oxygen species and caspase-dependent cancer cell death.
Dr. Baskin emphasized that the new Oncomagnetic device is very different from the Optune device (Novocare), which is already approved by the Food and Drug Administration and has been shown to increase survival among patients with glioblastoma. Optune uses tumor-treating fields (TTFs), which are electromagnetic waves that are delivered via an electric field generator through four transducer arrays that are placed on a shaved scalp. Preclinical studies indicated that the TTFs disrupt cell division by disrupting several steps in the mitotic process that are crucial for cell division.
Both of these devices “are using a type of external maneuver” rather than invasive intracranial approaches, said Dr. Hormingo. The experimental Oncomagnetic device may have an advantage in that it needs to be worn by the patient for fewer hours, she commented. A better understanding of the physics and underlying mechanism is needed, however. Clinical trials are an essential next step.
Most common brain cancer in adults
Glioblastoma is the most common malignant tumor of the brain in adults. Outcomes continue to be dismal. In more than 40 years, median survival has only modestly improved.
“We haven’t gotten very far with glioblastoma despite millions of dollars in research,” Dr. Baskin said. “With treatment, survival is about 15 months, and those are not very good months.”
Out of the box
Standard treatments for glioblastoma include surgery, radiotherapy, and chemotherapy, and many patients cannot tolerate some of these, Dr. Baskin noted. Hence, there is a great need for a different therapeutic approach that yields better outcomes with lower toxicity.
“We didn’t want to develop another chemotherapeutic agent that would help you live another 2 months,” he said in an interview. “We were trying to think out of the box.
“If you want to do something that will really make a difference in an aggressive tumor like glioblastoma, you have to attack something so basic that the tumor can’t evade it,” he said. “For example, with temozolomide, if it is unmethylated, the tumor can repair the DNA damage from the chemotherapy. Even if you’re sensitive to begin with, over time, the tumor will eventually become resistant.”
The new device stems from work by Dr. Baskin and colleagues on mitochondria, which he describes as the powerhouse of the cell. “Mitochondrial DNA can’t repair itself, so if you damage the mitochondria, you will damage the cell, and theoretically, it cannot repair itself,” he said.
In preclinical models, the oscillating magnetic fields generated by the new device were shown to kill patient-derived glioblastoma cells in cell culture without having cytotoxic effects on cortical neurons and normal human astrocytes. Animal studies also showed that it was effective and nontoxic, explained Dr. Baskin.
However, getting the device to human clinical trials has been slow going. “We wanted to start an early-phase trial for an investigational device, but the FDA is overwhelmed with COVID-related applications,” he said. “That has taken priority, and we understand that. So we were able to evaluate it on a patient through compassionate use via the [Food and Drug Administration]–approved Expanded Access Program.”
Exciting possibilities
The patient was a 53-year-old man who had undergone radiotherapy and chemotherapy, and the tumor was progressing. Imaging revealed the presence of leptomeningeal disease, which is associated with a poor outcome and a median survival of 3.5-3.9 months.
The patient was fitted with the helmet device and wore it under supervision for the first 3 days of treatment, during which time the strength of the oscillating magnetic fields was escalated. After this initial supervised phase, the treatment continued at home without supervision, using the same regimen as on the third day.
Treatment was first administered for 2 hours while under supervision and was then gradually increased to a maximum of 6 hours per day. The patient was evaluated clinically on days 7, 16, 30, and 44 after initiation of treatment. No serious adverse events were reported during treatment. The patient’s wife reported subjective improvement in speech and cognitive function.
Dr. Baskin noted that the patient had been experiencing falls for the past year and a half before treatment was initiated. “And then he tripped and fell and sustained a head injury that he subsequently died from,” he said.
Autopsy results confirmed the rapid response to treatment, and tumor shrinkage appeared to correlate with the treatment dose.
“Our results in the laboratory and with this patient open a new world of noninvasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future,” Dr. Baskin commented.
He said his team has experimented with this approach with other tumor types in the laboratory, including triple-negative breast cancer and lung cancer. “We’ve only tried it in a culture so far, but it seems to melt the cancer cells,” he said.
The work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute and several foundations. Dr. Baskin and two coauthors are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
A version of this article first appeared on Medscape.com.
This is the first time that the wearable Oncomagnetic device was tried with a patient.
The patient had end-stage recurrent glioblastoma and had undergone all standard therapy options. He wore the device for 5 weeks but died from an unrelated injury, so the treatment period was cut short.
A brain scan showed a 31% reduction of contrast-enhanced tumor volume, and an autopsy of his brain confirmed the rapid response to the treatment.
The case study was published online on July 22, 2021, in Frontiers in Oncology.
“I believe that there is a great potential with this device,” said study author David S. Baskin, MD, director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the department of neurosurgery at Houston Methodist Hospital. “This is a very exciting time.”
The team is now treating several patients with glioblastoma under compassionate use.
In an independent comment, Adilia Hormigo, MD, PhD, director of the neuro-oncology program at the Tisch Cancer Institute, Mount Sinai Health System, New York, noted that a clinical trial is needed to evaluate the device. “But this is an interesting idea, and we have to be open-minded in treating this fatal disease.”
Oscillating magnetic fields
The Oncomagnetic device consists of three oncoscillators that are attached to the outside of a helmet and are connected to a microprocessor-based electronic controller powered by a rechargeable battery.
It consists of a series of rotating magnets that produce oscillating magnetic fields that cover the entire brain, including the upper part of the brain stem. The device induces rapid apoptosis of glioblastoma cells, Dr. Baskin explained. Its mechanism of action involves disruption of the electron transport in the mitochondrial respiratory chain, causing an elevation of reactive oxygen species and caspase-dependent cancer cell death.
Dr. Baskin emphasized that the new Oncomagnetic device is very different from the Optune device (Novocare), which is already approved by the Food and Drug Administration and has been shown to increase survival among patients with glioblastoma. Optune uses tumor-treating fields (TTFs), which are electromagnetic waves that are delivered via an electric field generator through four transducer arrays that are placed on a shaved scalp. Preclinical studies indicated that the TTFs disrupt cell division by disrupting several steps in the mitotic process that are crucial for cell division.
Both of these devices “are using a type of external maneuver” rather than invasive intracranial approaches, said Dr. Hormingo. The experimental Oncomagnetic device may have an advantage in that it needs to be worn by the patient for fewer hours, she commented. A better understanding of the physics and underlying mechanism is needed, however. Clinical trials are an essential next step.
Most common brain cancer in adults
Glioblastoma is the most common malignant tumor of the brain in adults. Outcomes continue to be dismal. In more than 40 years, median survival has only modestly improved.
“We haven’t gotten very far with glioblastoma despite millions of dollars in research,” Dr. Baskin said. “With treatment, survival is about 15 months, and those are not very good months.”
Out of the box
Standard treatments for glioblastoma include surgery, radiotherapy, and chemotherapy, and many patients cannot tolerate some of these, Dr. Baskin noted. Hence, there is a great need for a different therapeutic approach that yields better outcomes with lower toxicity.
“We didn’t want to develop another chemotherapeutic agent that would help you live another 2 months,” he said in an interview. “We were trying to think out of the box.
“If you want to do something that will really make a difference in an aggressive tumor like glioblastoma, you have to attack something so basic that the tumor can’t evade it,” he said. “For example, with temozolomide, if it is unmethylated, the tumor can repair the DNA damage from the chemotherapy. Even if you’re sensitive to begin with, over time, the tumor will eventually become resistant.”
The new device stems from work by Dr. Baskin and colleagues on mitochondria, which he describes as the powerhouse of the cell. “Mitochondrial DNA can’t repair itself, so if you damage the mitochondria, you will damage the cell, and theoretically, it cannot repair itself,” he said.
In preclinical models, the oscillating magnetic fields generated by the new device were shown to kill patient-derived glioblastoma cells in cell culture without having cytotoxic effects on cortical neurons and normal human astrocytes. Animal studies also showed that it was effective and nontoxic, explained Dr. Baskin.
However, getting the device to human clinical trials has been slow going. “We wanted to start an early-phase trial for an investigational device, but the FDA is overwhelmed with COVID-related applications,” he said. “That has taken priority, and we understand that. So we were able to evaluate it on a patient through compassionate use via the [Food and Drug Administration]–approved Expanded Access Program.”
Exciting possibilities
The patient was a 53-year-old man who had undergone radiotherapy and chemotherapy, and the tumor was progressing. Imaging revealed the presence of leptomeningeal disease, which is associated with a poor outcome and a median survival of 3.5-3.9 months.
The patient was fitted with the helmet device and wore it under supervision for the first 3 days of treatment, during which time the strength of the oscillating magnetic fields was escalated. After this initial supervised phase, the treatment continued at home without supervision, using the same regimen as on the third day.
Treatment was first administered for 2 hours while under supervision and was then gradually increased to a maximum of 6 hours per day. The patient was evaluated clinically on days 7, 16, 30, and 44 after initiation of treatment. No serious adverse events were reported during treatment. The patient’s wife reported subjective improvement in speech and cognitive function.
Dr. Baskin noted that the patient had been experiencing falls for the past year and a half before treatment was initiated. “And then he tripped and fell and sustained a head injury that he subsequently died from,” he said.
Autopsy results confirmed the rapid response to treatment, and tumor shrinkage appeared to correlate with the treatment dose.
“Our results in the laboratory and with this patient open a new world of noninvasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future,” Dr. Baskin commented.
He said his team has experimented with this approach with other tumor types in the laboratory, including triple-negative breast cancer and lung cancer. “We’ve only tried it in a culture so far, but it seems to melt the cancer cells,” he said.
The work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute and several foundations. Dr. Baskin and two coauthors are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
A version of this article first appeared on Medscape.com.
This is the first time that the wearable Oncomagnetic device was tried with a patient.
The patient had end-stage recurrent glioblastoma and had undergone all standard therapy options. He wore the device for 5 weeks but died from an unrelated injury, so the treatment period was cut short.
A brain scan showed a 31% reduction of contrast-enhanced tumor volume, and an autopsy of his brain confirmed the rapid response to the treatment.
The case study was published online on July 22, 2021, in Frontiers in Oncology.
“I believe that there is a great potential with this device,” said study author David S. Baskin, MD, director of the Kenneth R. Peak Center for Brain and Pituitary Tumor Treatment in the department of neurosurgery at Houston Methodist Hospital. “This is a very exciting time.”
The team is now treating several patients with glioblastoma under compassionate use.
In an independent comment, Adilia Hormigo, MD, PhD, director of the neuro-oncology program at the Tisch Cancer Institute, Mount Sinai Health System, New York, noted that a clinical trial is needed to evaluate the device. “But this is an interesting idea, and we have to be open-minded in treating this fatal disease.”
Oscillating magnetic fields
The Oncomagnetic device consists of three oncoscillators that are attached to the outside of a helmet and are connected to a microprocessor-based electronic controller powered by a rechargeable battery.
It consists of a series of rotating magnets that produce oscillating magnetic fields that cover the entire brain, including the upper part of the brain stem. The device induces rapid apoptosis of glioblastoma cells, Dr. Baskin explained. Its mechanism of action involves disruption of the electron transport in the mitochondrial respiratory chain, causing an elevation of reactive oxygen species and caspase-dependent cancer cell death.
Dr. Baskin emphasized that the new Oncomagnetic device is very different from the Optune device (Novocare), which is already approved by the Food and Drug Administration and has been shown to increase survival among patients with glioblastoma. Optune uses tumor-treating fields (TTFs), which are electromagnetic waves that are delivered via an electric field generator through four transducer arrays that are placed on a shaved scalp. Preclinical studies indicated that the TTFs disrupt cell division by disrupting several steps in the mitotic process that are crucial for cell division.
Both of these devices “are using a type of external maneuver” rather than invasive intracranial approaches, said Dr. Hormingo. The experimental Oncomagnetic device may have an advantage in that it needs to be worn by the patient for fewer hours, she commented. A better understanding of the physics and underlying mechanism is needed, however. Clinical trials are an essential next step.
Most common brain cancer in adults
Glioblastoma is the most common malignant tumor of the brain in adults. Outcomes continue to be dismal. In more than 40 years, median survival has only modestly improved.
“We haven’t gotten very far with glioblastoma despite millions of dollars in research,” Dr. Baskin said. “With treatment, survival is about 15 months, and those are not very good months.”
Out of the box
Standard treatments for glioblastoma include surgery, radiotherapy, and chemotherapy, and many patients cannot tolerate some of these, Dr. Baskin noted. Hence, there is a great need for a different therapeutic approach that yields better outcomes with lower toxicity.
“We didn’t want to develop another chemotherapeutic agent that would help you live another 2 months,” he said in an interview. “We were trying to think out of the box.
“If you want to do something that will really make a difference in an aggressive tumor like glioblastoma, you have to attack something so basic that the tumor can’t evade it,” he said. “For example, with temozolomide, if it is unmethylated, the tumor can repair the DNA damage from the chemotherapy. Even if you’re sensitive to begin with, over time, the tumor will eventually become resistant.”
The new device stems from work by Dr. Baskin and colleagues on mitochondria, which he describes as the powerhouse of the cell. “Mitochondrial DNA can’t repair itself, so if you damage the mitochondria, you will damage the cell, and theoretically, it cannot repair itself,” he said.
In preclinical models, the oscillating magnetic fields generated by the new device were shown to kill patient-derived glioblastoma cells in cell culture without having cytotoxic effects on cortical neurons and normal human astrocytes. Animal studies also showed that it was effective and nontoxic, explained Dr. Baskin.
However, getting the device to human clinical trials has been slow going. “We wanted to start an early-phase trial for an investigational device, but the FDA is overwhelmed with COVID-related applications,” he said. “That has taken priority, and we understand that. So we were able to evaluate it on a patient through compassionate use via the [Food and Drug Administration]–approved Expanded Access Program.”
Exciting possibilities
The patient was a 53-year-old man who had undergone radiotherapy and chemotherapy, and the tumor was progressing. Imaging revealed the presence of leptomeningeal disease, which is associated with a poor outcome and a median survival of 3.5-3.9 months.
The patient was fitted with the helmet device and wore it under supervision for the first 3 days of treatment, during which time the strength of the oscillating magnetic fields was escalated. After this initial supervised phase, the treatment continued at home without supervision, using the same regimen as on the third day.
Treatment was first administered for 2 hours while under supervision and was then gradually increased to a maximum of 6 hours per day. The patient was evaluated clinically on days 7, 16, 30, and 44 after initiation of treatment. No serious adverse events were reported during treatment. The patient’s wife reported subjective improvement in speech and cognitive function.
Dr. Baskin noted that the patient had been experiencing falls for the past year and a half before treatment was initiated. “And then he tripped and fell and sustained a head injury that he subsequently died from,” he said.
Autopsy results confirmed the rapid response to treatment, and tumor shrinkage appeared to correlate with the treatment dose.
“Our results in the laboratory and with this patient open a new world of noninvasive and nontoxic therapy for brain cancer, with many exciting possibilities for the future,” Dr. Baskin commented.
He said his team has experimented with this approach with other tumor types in the laboratory, including triple-negative breast cancer and lung cancer. “We’ve only tried it in a culture so far, but it seems to melt the cancer cells,” he said.
The work was supported by a grant from the Translational Research Initiative of the Houston Methodist Research Institute and several foundations. Dr. Baskin and two coauthors are listed as inventors on a U.S. patent application filed by Houston Methodist Hospital for the device used in this report.
A version of this article first appeared on Medscape.com.
Prevalence of dementia before age 65 much higher than expected
Results of a large meta-analysis show that currently 3.9 million individuals are living with young-onset dementia. Among these patients, symptoms of the disease start before age 65.
Recent global young-onset dementia estimates have ranged from 42.3 to 54.0 per 100,000 population, the researchers noted. However, the new study, which included 74 global studies with 2.7 million participants, shows that the global age-standardized prevalence of young-onset dementia is 119.00 per 100,000 among individuals aged 30-64 years; there was little difference in prevalence between men and women. On the basis of the latest population estimates, these new prevalence data imply that there are approximately 175,000 persons with young-onset dementia in the United States.
Although the new global estimate of young-onset dementia is higher than previously thought, “it is still probably an underestimation owing to lack of high-quality data. This should raise awareness for policy makers and health care professionals to organize more and better care for this subgroup of individuals with dementia,” wrote the investigators, with first author Stevie Hendriks, MSc, Maastricht (the Netherlands) University, and the Young-Onset Dementia Epidemiology Study Group.
The study was published online July 19, 2021, in JAMA Neurology.
‘Essential’ data
Young-onset dementia is exceedingly rare in those aged 30-63 years (1.1 per 100,000) but is more prevalent at age 60-64 years (77.4 per 100,000). “Our findings fit the general observation that prevalence of dementia increases exponentially from 60 years of age onward,” they wrote.
The prevalence of young-onset dementia was similar in men and women, lower in the United States than in Europe, highest in upper- to middle-income countries, and highest for Alzheimer’s disease, followed by vascular dementia and frontotemporal dementia.
Monitoring the prevalence of young-onset dementia is “essential” to adequately plan and organize health services, the investigators noted.
To ensure more accurate prevalence estimates in the future, “efforts should be made to conduct more cohort studies and to standardize procedures and reporting of prevalence studies. In addition, more data are needed from low-income countries as well as studies that include younger age ranges,” they said.
New insights
In an accompanying editorial, David S. Knopman, MD, department of neurology, Mayo Clinic, Rochester, Minn., noted that the study provides new insights into an “underappreciated problem.”.
Young-onset dementia is a “particularly disheartening diagnosis because it affects individuals in their prime years, in the midst of their careers, and while raising families,” Dr. Knopman wrote.
“Most dementia care is geared for older patients, and as a consequence, services are rarely available to address the needs of someone diagnosed with dementia in their 50s who has dependent children at home and a spouse who must continue working. Understanding the prevalence and incidence of young-onset dementia is a first step in addressing this challenge,” Dr. Knopman wrote.
He noted that the authors of this analysis have “done a service to the dementia community by collecting and analyzing the dozens of individual studies of young-onset dementia.
“The product, a rationally derived estimate of dementia prevalence across the population aged 30-64 years, provides a basis for initiating more efforts to improve methods for timely diagnosis and to address the unique needs of patients with young-onset dementia,” Dr. Knopman concluded.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis show that currently 3.9 million individuals are living with young-onset dementia. Among these patients, symptoms of the disease start before age 65.
Recent global young-onset dementia estimates have ranged from 42.3 to 54.0 per 100,000 population, the researchers noted. However, the new study, which included 74 global studies with 2.7 million participants, shows that the global age-standardized prevalence of young-onset dementia is 119.00 per 100,000 among individuals aged 30-64 years; there was little difference in prevalence between men and women. On the basis of the latest population estimates, these new prevalence data imply that there are approximately 175,000 persons with young-onset dementia in the United States.
Although the new global estimate of young-onset dementia is higher than previously thought, “it is still probably an underestimation owing to lack of high-quality data. This should raise awareness for policy makers and health care professionals to organize more and better care for this subgroup of individuals with dementia,” wrote the investigators, with first author Stevie Hendriks, MSc, Maastricht (the Netherlands) University, and the Young-Onset Dementia Epidemiology Study Group.
The study was published online July 19, 2021, in JAMA Neurology.
‘Essential’ data
Young-onset dementia is exceedingly rare in those aged 30-63 years (1.1 per 100,000) but is more prevalent at age 60-64 years (77.4 per 100,000). “Our findings fit the general observation that prevalence of dementia increases exponentially from 60 years of age onward,” they wrote.
The prevalence of young-onset dementia was similar in men and women, lower in the United States than in Europe, highest in upper- to middle-income countries, and highest for Alzheimer’s disease, followed by vascular dementia and frontotemporal dementia.
Monitoring the prevalence of young-onset dementia is “essential” to adequately plan and organize health services, the investigators noted.
To ensure more accurate prevalence estimates in the future, “efforts should be made to conduct more cohort studies and to standardize procedures and reporting of prevalence studies. In addition, more data are needed from low-income countries as well as studies that include younger age ranges,” they said.
New insights
In an accompanying editorial, David S. Knopman, MD, department of neurology, Mayo Clinic, Rochester, Minn., noted that the study provides new insights into an “underappreciated problem.”.
Young-onset dementia is a “particularly disheartening diagnosis because it affects individuals in their prime years, in the midst of their careers, and while raising families,” Dr. Knopman wrote.
“Most dementia care is geared for older patients, and as a consequence, services are rarely available to address the needs of someone diagnosed with dementia in their 50s who has dependent children at home and a spouse who must continue working. Understanding the prevalence and incidence of young-onset dementia is a first step in addressing this challenge,” Dr. Knopman wrote.
He noted that the authors of this analysis have “done a service to the dementia community by collecting and analyzing the dozens of individual studies of young-onset dementia.
“The product, a rationally derived estimate of dementia prevalence across the population aged 30-64 years, provides a basis for initiating more efforts to improve methods for timely diagnosis and to address the unique needs of patients with young-onset dementia,” Dr. Knopman concluded.
A version of this article first appeared on Medscape.com.
Results of a large meta-analysis show that currently 3.9 million individuals are living with young-onset dementia. Among these patients, symptoms of the disease start before age 65.
Recent global young-onset dementia estimates have ranged from 42.3 to 54.0 per 100,000 population, the researchers noted. However, the new study, which included 74 global studies with 2.7 million participants, shows that the global age-standardized prevalence of young-onset dementia is 119.00 per 100,000 among individuals aged 30-64 years; there was little difference in prevalence between men and women. On the basis of the latest population estimates, these new prevalence data imply that there are approximately 175,000 persons with young-onset dementia in the United States.
Although the new global estimate of young-onset dementia is higher than previously thought, “it is still probably an underestimation owing to lack of high-quality data. This should raise awareness for policy makers and health care professionals to organize more and better care for this subgroup of individuals with dementia,” wrote the investigators, with first author Stevie Hendriks, MSc, Maastricht (the Netherlands) University, and the Young-Onset Dementia Epidemiology Study Group.
The study was published online July 19, 2021, in JAMA Neurology.
‘Essential’ data
Young-onset dementia is exceedingly rare in those aged 30-63 years (1.1 per 100,000) but is more prevalent at age 60-64 years (77.4 per 100,000). “Our findings fit the general observation that prevalence of dementia increases exponentially from 60 years of age onward,” they wrote.
The prevalence of young-onset dementia was similar in men and women, lower in the United States than in Europe, highest in upper- to middle-income countries, and highest for Alzheimer’s disease, followed by vascular dementia and frontotemporal dementia.
Monitoring the prevalence of young-onset dementia is “essential” to adequately plan and organize health services, the investigators noted.
To ensure more accurate prevalence estimates in the future, “efforts should be made to conduct more cohort studies and to standardize procedures and reporting of prevalence studies. In addition, more data are needed from low-income countries as well as studies that include younger age ranges,” they said.
New insights
In an accompanying editorial, David S. Knopman, MD, department of neurology, Mayo Clinic, Rochester, Minn., noted that the study provides new insights into an “underappreciated problem.”.
Young-onset dementia is a “particularly disheartening diagnosis because it affects individuals in their prime years, in the midst of their careers, and while raising families,” Dr. Knopman wrote.
“Most dementia care is geared for older patients, and as a consequence, services are rarely available to address the needs of someone diagnosed with dementia in their 50s who has dependent children at home and a spouse who must continue working. Understanding the prevalence and incidence of young-onset dementia is a first step in addressing this challenge,” Dr. Knopman wrote.
He noted that the authors of this analysis have “done a service to the dementia community by collecting and analyzing the dozens of individual studies of young-onset dementia.
“The product, a rationally derived estimate of dementia prevalence across the population aged 30-64 years, provides a basis for initiating more efforts to improve methods for timely diagnosis and to address the unique needs of patients with young-onset dementia,” Dr. Knopman concluded.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Can a supplement that mimics the keto diet reduce seizures?
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
early research suggests. However, at least one expert has concerns.
In an open-label feasibility study, researchers assessed a liquid supplement known as K.Vita (Vitaflo International), which contains both decanoic acid and octanoic acid.
Although the study was small, the findings are promising, said coinvestigator Matthew Walker, MD, PhD, University College London Institute of Neurology, department of clinical and experimental epilepsy.
“The dietary supplement was reasonably well tolerated and while we weren’t specifically looking for efficacy here, we did see some patients had quite dramatic results in terms of reduced seizures,” Dr. Walker said.
Unlike the ketogenic diet, this dietary supplement is “very easy” to follow, involves only minor dietary modifications, and doesn’t require the intervention of a dietitian, he added.
The findings were published online July 23, 2021, in Brain Communications.
Key ingredients
In the ketogenic diet, the body uses body fat as its primary fuel source. The switch from carbohydrates to fat for body fuel results in built-up ketones.
Previous research shows the ketogenic diet is effective in reducing seizures in some patients with epilepsy. However, many patients find it difficult to tolerate, especially for extended periods. Dr. Walker also noted that ketones may have other long-term side effects, including osteoporosis.
He added that his team was keen to learn what elements of the ketogenic diet affect seizures. “Interestingly, we found that one of the fats used in the ketogenic diet, decanoic acid, has quite marked antiseizure effects,” Dr. Walker said.
Previous research has shown that decanoic acid, a medium-chain triglyceride–derived fatty acid, can cross the blood-brain barrier and decrease excitatory neurotransmission and network excitability in vitro.
Dr. Walker noted that ketones are necessary in order to reduce seizures.
“Rather than have a very high-fat, low-carbohydrate diet that causes ketones, we thought ‘why don’t we use a diet in which we just use mainly this fat, this decanoic acid, and avoid ketosis,’ ” he said.
The researchers then went to work developing the K.Vita dietary supplement, which mainly contains decanoic acid but also another fat, octanoic acid.
Assessing feasibility
The feasibility study included 61 patients (59% female) who began taking the supplement. Of these, 35 were children (aged 3-18 years) and 26 were adults. The children had Dravet syndrome or another genetically driven form of epilepsy, while most of the adults had a focal epilepsy.
All participants had failed multiple antiseizure medications – a median of 3 for children and 10 for adults who completed the trial. Of the 61 original participants, 20 (19 children and 1 adult) had tried the ketogenic diet but had stopped it for various reasons, including noncompliance and lack of efficacy.
The liquid supplement was introduced gradually. The amount administered was based on weight in the children and was a standard amount in adults, with the target being 240 mL.
Participants consumed the supplement in equal servings taken at regular intervals as part of a meal or snack. They could take it alone or mix it with yogurt or another food.
Patients with feeding tubes took the supplement immediately before or after or mixed into an enteral feed, with a water flush afterward.
Researchers provided patients and caregivers with guidance on excluding highly refined sugary foods and beverages. Starchy foods such as bread, pasta, rice, and potatoes were not restricted.
The study consisted of three visits: baseline, 5 weeks, and 12 weeks, in addition to regular phone and email contact. Participants were also asked to keep a seizure diary.
Highly acceptable to patients
Overall, the study withdrawal rate was 33%. After a protocol change involving a slower introduction of the supplement, there were fewer withdrawals, Dr. Walker reported. He noted that the proportion of participants who completed the study (41 of 61) is “much better than with most studies of adults following the ketogenic diet.”
The most frequently reported gastrointestinal symptoms with the supplement were bloating and constipation, but these were predominantly mild and tended to decrease over time. This, said Dr. Walker, contrasts to the ketogenic diet where side effects tend to persist.
There was no significant change in body weight or body mass index. “We did not see weight gain as a problem at all,” Dr. Walker said.
Of 15 caregivers and 19 adults who returned an acceptability questionnaire, 84% agreed or strongly agreed the supplement had a good flavor (strawberry); 88% liked the appearance and color; 77% liked the texture and consistency; and 88% agreed or strongly agreed it was easy to take.
About one-third of adults and two-thirds of caregivers said they believed the supplement reduced seizures.
50% seizure reduction
Only three children and one adult became ketotic. This is typically classified as a beta-hydroxybutyrate (BHB) greater than 1 mmol/L (10.4 mg/dL). The BHB levels detected were markedly lower than those observed in individuals following a ketogenic diet, the investigators note.
Of the 41 participants, 19 completed the diaries. There were also data from physician recordings, so researchers were able to retrieve seizure frequencies for 32 of the 41 (78%). Of these 32 patients, 14 (44%) had a 50% or greater reduction in seizures. Overall, children and adults “responded similarly,” Dr. Walker said.
He acknowledged the study numbers are small and emphasized that larger studies are needed to determine efficacy. He also hopes for a future randomized controlled trial comparing K.Vita with another supplement that contains different types of fats.
Interestingly, the product has already “passed” the regulatory approval process in the United Kingdom, so it can be labeled as a medicinal food and should be available for use at the beginning of 2022, Dr. Walker said.
Study concerns
Asked to comment on the findings, Daniel Goldenholz, MD, PhD, instructor in the department of neurology, Beth Israel Deaconess Medical Center, Boston, said the supplement may be helpful, but he has concerns about the study.
Many patients with epilepsy are “desperate” for therapies that will help treat their seizures, said Dr. Goldenholz, who was not involved with the research. “If there’s a dietary therapy that has the potential for being helpful, I’m loving that. I need that. My patients are begging for something that works.” It is “really exciting” that researchers are working on that goal, Dr. Goldenholz added.
However, he noted that it is too soon to start talking to patients about this new product. He also pointed out that a significant fraction of the study participants dropped out, many because they couldn’t tolerate the supplement. In addition, others didn’t produce a seizure diary.
Dr. Goldenholz and colleagues have published several studies showing that patients with no intervention at all can sometimes show a reduction in seizures compared with their baseline results.
“We found sizable 50% reductions attributable entirely to the natural fluctuations in seizure rates, rather than any therapy at all, he said.
Dr. Goldenholz added that he hopes to see future studies on this topic, and on similar therapies “with sufficient data and more reliable metrics for efficacy.”
The study was funded by Vitaflo International. Dr. Walker reports having received grants from Vitaflo International and personal fees from UCB Pharma, Eisai, and Sage. In addition, along with colleagues, he has a patent (Nutritional product) pending.
A version of this article first appeared on Medscape.com.
FROM BRAIN COMMUNICATION