User login
Women with recurrent UTIs express fear, frustration
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Fear of antibiotic overuse and frustration with physicians who prescribe them too freely are key sentiments expressed by women with recurrent urinary tract infections (rUTIs), according to findings from a study involving six focus groups.
“Here in our female pelvic medicine reconstructive urology clinic at Cedars-Sinai and at UCLA, we see many women who are referred for evaluation of rUTIs who are very frustrated with their care,” Victoria Scott, MD, Cedars-Sinai Medical Center, Los Angeles, said in an interview.
“So with these focus groups, we saw an opportunity to explore why women are so frustrated and to try and improve the care delivered,” she added.
Findings from the study were published online Sept. 1 in The Journal of Urology.
“There is a need for physicians to modify management strategies ... and to devote more research efforts to improving nonantibiotic options for the prevention and treatment of recurrent urinary tract infections, as well as management strategies that better empower patients,” the authors wrote.
Six focus groups
Four or five participants were included in each of the six focus groups – a total of 29 women. All participants reported a history of symptomatic, culture-proven UTI episodes. They had experienced two or more infections in 6 months or three or more infections within 1 year. Women were predominantly White. Most were employed part- or full-time and held a college degree.
From a qualitative analysis of all focus group transcripts, two main themes emerged:
- The negative impact of taking antibiotics for the prevention and treatment of rUTIs.
- Resentment of the medical profession for the way it managed rUTIs.
The researchers found that participants had a good understanding of the deleterious effects from inappropriate antibiotic use, largely gleaned from media sources and the Internet. “Numerous women stated that they had reached such a level of concern about antibiotics that they would resist taking them for prevention or treatment of infections,” Dr. Scott and colleagues pointed out.
These concerns centered around the risk of developing resistance to antibiotics and the ill effects that antibiotics can have on the gastrointestinal and genitourinary microbiomes. Several women reported that they had developed Clostridium difficile infections after taking antibiotics; one of the patients required hospitalization for the infection.
Women also reported concerns that they had been given an antibiotic needlessly for symptoms that might have been caused by a genitourinary condition other than a UTI. They also reported feeling resentful toward practitioners, particularly if they felt the practitioner was overprescribing antibiotics. Some had resorted to consultations with alternative practitioners, such as herbalists. “A second concern discussed by participants was the feeling of being ignored by physicians,” the authors observed.
In this regard, the women felt that their physicians underestimated the burden that rUTIs had on their lives and the detrimental effect that repeated infections had on their relationships, work, and overall quality of life. “These perceptions led to a prevalent mistrust of physicians,” the investigators wrote. This prompted many women to insist that the medical community devote more effort to the development of nonantibiotic options for the prevention and treatment of UTIs.
Improved management strategies
Asked how physicians might improve their management of rUTIs, Dr. Scott shared a number of suggestions. Cardinal rule No. 1: Have the patient undergo a urinalysis to make sure she does have a UTI. “There is a subset of patients among women with rUTIs who come in with a diagnosis of an rUTI but who really have not had documentation of more than one positive urine culture,” Dr. Scott noted. Such a history suggests that they do not have an rUTI.
It’s imperative that physicians rule out commonly misdiagnosed disorders, such as overactive bladder, as a cause of the patient’s symptoms. Symptoms of overactive bladder and rUTIs often overlap. While waiting for results from the urinalysis to confirm or rule out a UTI, young and healthy women may be prescribed a nonsteroidal anti-inflammatory drug (NSAID), such as naproxen, which can help ameliorate symptoms.
Because UTIs are frequently self-limiting, Dr. Scott and others have found that for young, otherwise healthy women, NSAIDs alone can often resolve symptoms of the UTI without use of an antibiotic. For relatively severe symptoms, a urinary analgesic, such as phenazopyridine (Pyridium), may soothe the lining of the urinary tract and relieve pain. Cystex is an over-the-counter urinary analgesic that women can procure themselves, Dr. Scott added.
If an antibiotic is indicated, those most commonly prescribed for a single episode of acute cystitis are nitrofurantoin and sulfamethoxazole plus trimethoprim (Bactrim). For recurrent UTIs, “patients are a bit more complicated,” Dr. Scott admitted. “I think the best practice is to look back at a woman’s prior urine culture and select an antibiotic that showed good sensitivity in the last positive urine test,” she said.
Prevention starts with behavioral strategies, such as voiding after sexual intercourse and wiping from front to back following urination to avoid introducing fecal bacteria into the urethra. Evidence suggests that premenopausal women who drink at least 1.5 L of water a day have significantly fewer UTI episodes, Dr. Scott noted. There is also “pretty good” evidence that cranberry supplements (not juice) can prevent rUTIs. Use of cranberry supplements is supported by the American Urological Association (conditional recommendation; evidence level of grade C).
For peri- and postmenopausal women, vaginal estrogen may be effective. It’s use for UTI prevention is well supported by the literature. Although not as well supported by evidence, some women find that a supplement such as D-mannose may prevent or treat UTIs by causing bacteria to bind to it rather than to the bladder wall. Probiotics are another possibility, she noted. Empathy can’t hurt, she added.
“A common theme among satisfied women was the sentiment that their physicians understood their problems and had a system in place to allow rapid diagnosis and treatment for UTI episodes,” the authors emphasized.
“[Such attitudes] highlight the need to investigate each patient’s experience and perceptions to allow for shared decision making regarding the management of rUTIs,” they wrote.
Further commentary
Asked to comment on the findings, editorialist Michelle Van Kuiken, MD, assistant professor of urology, University of California, San Francisco, acknowledged that there is not a lot of good evidence to support many of the strategies recommended by the American Urological Association to prevent and treat rUTIs, but she often follows these recommendations anyway. “The one statement in the guidelines that is the most supported by evidence is the use of cranberry supplements, and I do routinely recommended daily use of some form of concentrated cranberry supplements for all of my patients with rUTIs,” she said in an interview.
Dr. Van Kuiken said that vaginal estrogen is a very good option for all postmenopausal women who suffer from rUTIs and that there is growing acceptance of its use for this and other indications. There is some evidence to support D-mannose as well, although it’s not that robust, she acknowledged.
She said the evidence supporting the use of probiotics for this indication is very thin. She does not routinely recommend them for rUTIs, although they are not inherently harmful. “I think for a lot of women who have rUTIs, it can be pretty debilitating and upsetting for them – it can impact travel plans, work, and social events,” Dr. Van Kuiken said.
“Until we develop better diagnostic and therapeutic strategies, validating women’s experiences and concerns with rUTI while limiting unnecessary antibiotics remains our best option,” she wrote.
Dr. Scott and Dr. Van Kuiken have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MRI screening cost effective for women with dense breasts
Alternatively, if a woman worries that the 4-year screening interval is too long, screening mammography may be offered every 2 years, with MRI screening offered for the second 2-year interval, according to the findings. This strategy would still require the patient to undergo MRI breast cancer screening every 4 years.
“MRI is more effective not only for selected patients. It is actually more effective than mammography for all women,” editorialist Christiane Kuhl, MD, PhD, University of Aachen (Germany), said in an interview.
“But the superior diagnostic accuracy of MRI is more often needed for women who are at higher risk for breast cancer, and therefore the cost-effectiveness is easier to achieve in women who are at higher risk,” she added.
The study was published online Sept. 29 in the Journal of the National Cancer Institute.
DENSE trial
The simulation model used for the study was based on results from the Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, which showed that additional MRI screening for women with extremely dense breast tissue led to significantly fewer interval cancers in comparison with mammography alone (P < .001). In the DENSE trial, MRI participants underwent mammography plus MRI at 2-year intervals; the control group underwent mammography alone at 2-year intervals.
In the current study, “screening strategies varied in the number of MRIs and mammograms offered to women aged 50-75 years,” explains Amarens Geuzinge, MSc, University Medical Center, Rotterdam, the Netherlands, and colleagues, “and incremental cost-effectiveness ratios (ICERs) were calculated ... with a willingness-to-pay threshold of 22,000 euros (>$25,000 U.S.),” the investigators add.
Analyses indicated that screening every 2 years with mammography alone cost the least of all strategies that were evaluated, but it also resulted in the lowest number of quality-adjusted life years (QALYs) – in other words, it delivered the least amount of benefit for patients, coauthor Eveline Heijnsdijk, PhD, University Medical Center, Rotterdam, the Netherlands, explained to this news organization.
Offering an additional MRI every 2 years resulted in the highest costs but not the highest number of QALYs and was inferior to the other screening strategies analyzed, she added. Alternating mammography with MRI breast cancer screening, each conducted every 2 years, came close to providing the same benefits to patients as the every-4-year MRI screening strategy, Dr. Heijnsdijk noted.
However, when the authors applied the National Institute for Health and Care Excellence (NICE) threshold, MRI screening every 4 years yielded the highest acceptable incremental cost-effectiveness ratio (ICER), at 15,620 euros per QALYs, whereas screening every 3 years with MRI alone yielded an ICER of 37,181 euros per QALY.
If decision-makers are willing to pay more than 22,000 euros per QALY gained, “MRI every 2 or 3 years can also become cost effective,” the authors add.
Asked how acceptable MRI screening might be if performed only once every 4 years, Dr. Heijnsdijk noted that, in another of their studies, most of the women who had undergone MRI screening for breast cancer said that they would do so again. “MRI is not a pleasant test, but mammography is also not a pleasant test,” she said.
“So many women prefer MRI above mammography, especially because the detection rate with MRI is better than mammography,” she noted. Dr. Heijnsdijk also said that the percentage of women with extremely dense breasts who would be candidates for MRI screening is small – no more than 10% of women.
At a unit cost of slightly under 300 euros for MRI screening – compared with about 100 euros for screening mammography in the Netherlands – the cost of offering 10% of women MRI instead of mammography might increase, but any additional screening costs could be offset by reductions in the need to treat late-stage breast cancer more aggressively.
‘Interval’ cancers
Commenting further on the study, Dr. Kuhl pointed out that from 25% to 45% of cancers that occur in women who have undergone screening mammography are diagnosed as “interval” cancers, even among women who participate in the best mammography programs. “For a long time, people argued that these interval cancers developed only after the last respective mammogram, but that’s not true at all, because we know that with MRI screening, we can reduce the interval cancer rate down to zero,” Dr. Kuhl emphasized.
This is partially explained by the fact that mammography is “particularly blind” when it comes to detecting rapidly growing tumors. “The fact is that mammography has a modality-inherent tendency to preferentially detect slow-growing cancers, whereas rapidly growing tumors are indistinguishable from ubiquitous benign changes like cysts. [This] is why women who undergo screening mammography are frequently not diagnosed with the cancers that we really need to find,” she said.
Although there is ample talk about overdiagnosis when it comes to screening mammography, the overwhelmingly important problem is underdiagnosis. Even in exemplary mammography screening programs, at least 20% of tumors that are diagnosed on mammography have already advanced to a stage that is too late, Dr. Kuhl noted.
This means that at least half of women do not benefit from screening mammography nearly to the extent that they – and their health care practitioners – believe they should, she added. Dr. Kuhl underscored that this does not mean that clinicians should abandon screening mammography.
What it does mean is that physicians need to abandon the one-size-fits-all approach to screening mammography and start stratifying women on the basis of their individual risk of developing breast cancer by taking a family or personal history. Most women do undergo screening mammography at least once, Dr. Kuhl pointed out. From that mammogram, physicians can use information on breast density and breast architecture to better determine individual risk.
“We have good ideas about how to achieve risk stratification, but we’re not using them, because as long as mammography is the answer for everybody, there isn’t much motivation to dig deeper into the issue of how to determine risk,” Dr. Kuhl said.
“But we have to ensure the early diagnosis of aggressive cancers, and it’s exactly MRI that can do this, and we should start with women with very dense breasts because they are doubly underserved by mammography,” she said.
The study was supported by the University Medical Center Utrecht, Bayer HealthCare Medical Care, Matakina, and others. Ms. Geuzinge, Dr. Heijnsdijk, and Dr. Kuhl have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alternatively, if a woman worries that the 4-year screening interval is too long, screening mammography may be offered every 2 years, with MRI screening offered for the second 2-year interval, according to the findings. This strategy would still require the patient to undergo MRI breast cancer screening every 4 years.
“MRI is more effective not only for selected patients. It is actually more effective than mammography for all women,” editorialist Christiane Kuhl, MD, PhD, University of Aachen (Germany), said in an interview.
“But the superior diagnostic accuracy of MRI is more often needed for women who are at higher risk for breast cancer, and therefore the cost-effectiveness is easier to achieve in women who are at higher risk,” she added.
The study was published online Sept. 29 in the Journal of the National Cancer Institute.
DENSE trial
The simulation model used for the study was based on results from the Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, which showed that additional MRI screening for women with extremely dense breast tissue led to significantly fewer interval cancers in comparison with mammography alone (P < .001). In the DENSE trial, MRI participants underwent mammography plus MRI at 2-year intervals; the control group underwent mammography alone at 2-year intervals.
In the current study, “screening strategies varied in the number of MRIs and mammograms offered to women aged 50-75 years,” explains Amarens Geuzinge, MSc, University Medical Center, Rotterdam, the Netherlands, and colleagues, “and incremental cost-effectiveness ratios (ICERs) were calculated ... with a willingness-to-pay threshold of 22,000 euros (>$25,000 U.S.),” the investigators add.
Analyses indicated that screening every 2 years with mammography alone cost the least of all strategies that were evaluated, but it also resulted in the lowest number of quality-adjusted life years (QALYs) – in other words, it delivered the least amount of benefit for patients, coauthor Eveline Heijnsdijk, PhD, University Medical Center, Rotterdam, the Netherlands, explained to this news organization.
Offering an additional MRI every 2 years resulted in the highest costs but not the highest number of QALYs and was inferior to the other screening strategies analyzed, she added. Alternating mammography with MRI breast cancer screening, each conducted every 2 years, came close to providing the same benefits to patients as the every-4-year MRI screening strategy, Dr. Heijnsdijk noted.
However, when the authors applied the National Institute for Health and Care Excellence (NICE) threshold, MRI screening every 4 years yielded the highest acceptable incremental cost-effectiveness ratio (ICER), at 15,620 euros per QALYs, whereas screening every 3 years with MRI alone yielded an ICER of 37,181 euros per QALY.
If decision-makers are willing to pay more than 22,000 euros per QALY gained, “MRI every 2 or 3 years can also become cost effective,” the authors add.
Asked how acceptable MRI screening might be if performed only once every 4 years, Dr. Heijnsdijk noted that, in another of their studies, most of the women who had undergone MRI screening for breast cancer said that they would do so again. “MRI is not a pleasant test, but mammography is also not a pleasant test,” she said.
“So many women prefer MRI above mammography, especially because the detection rate with MRI is better than mammography,” she noted. Dr. Heijnsdijk also said that the percentage of women with extremely dense breasts who would be candidates for MRI screening is small – no more than 10% of women.
At a unit cost of slightly under 300 euros for MRI screening – compared with about 100 euros for screening mammography in the Netherlands – the cost of offering 10% of women MRI instead of mammography might increase, but any additional screening costs could be offset by reductions in the need to treat late-stage breast cancer more aggressively.
‘Interval’ cancers
Commenting further on the study, Dr. Kuhl pointed out that from 25% to 45% of cancers that occur in women who have undergone screening mammography are diagnosed as “interval” cancers, even among women who participate in the best mammography programs. “For a long time, people argued that these interval cancers developed only after the last respective mammogram, but that’s not true at all, because we know that with MRI screening, we can reduce the interval cancer rate down to zero,” Dr. Kuhl emphasized.
This is partially explained by the fact that mammography is “particularly blind” when it comes to detecting rapidly growing tumors. “The fact is that mammography has a modality-inherent tendency to preferentially detect slow-growing cancers, whereas rapidly growing tumors are indistinguishable from ubiquitous benign changes like cysts. [This] is why women who undergo screening mammography are frequently not diagnosed with the cancers that we really need to find,” she said.
Although there is ample talk about overdiagnosis when it comes to screening mammography, the overwhelmingly important problem is underdiagnosis. Even in exemplary mammography screening programs, at least 20% of tumors that are diagnosed on mammography have already advanced to a stage that is too late, Dr. Kuhl noted.
This means that at least half of women do not benefit from screening mammography nearly to the extent that they – and their health care practitioners – believe they should, she added. Dr. Kuhl underscored that this does not mean that clinicians should abandon screening mammography.
What it does mean is that physicians need to abandon the one-size-fits-all approach to screening mammography and start stratifying women on the basis of their individual risk of developing breast cancer by taking a family or personal history. Most women do undergo screening mammography at least once, Dr. Kuhl pointed out. From that mammogram, physicians can use information on breast density and breast architecture to better determine individual risk.
“We have good ideas about how to achieve risk stratification, but we’re not using them, because as long as mammography is the answer for everybody, there isn’t much motivation to dig deeper into the issue of how to determine risk,” Dr. Kuhl said.
“But we have to ensure the early diagnosis of aggressive cancers, and it’s exactly MRI that can do this, and we should start with women with very dense breasts because they are doubly underserved by mammography,” she said.
The study was supported by the University Medical Center Utrecht, Bayer HealthCare Medical Care, Matakina, and others. Ms. Geuzinge, Dr. Heijnsdijk, and Dr. Kuhl have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Alternatively, if a woman worries that the 4-year screening interval is too long, screening mammography may be offered every 2 years, with MRI screening offered for the second 2-year interval, according to the findings. This strategy would still require the patient to undergo MRI breast cancer screening every 4 years.
“MRI is more effective not only for selected patients. It is actually more effective than mammography for all women,” editorialist Christiane Kuhl, MD, PhD, University of Aachen (Germany), said in an interview.
“But the superior diagnostic accuracy of MRI is more often needed for women who are at higher risk for breast cancer, and therefore the cost-effectiveness is easier to achieve in women who are at higher risk,” she added.
The study was published online Sept. 29 in the Journal of the National Cancer Institute.
DENSE trial
The simulation model used for the study was based on results from the Dense Tissue and Early Breast Neoplasm Screening (DENSE) trial, which showed that additional MRI screening for women with extremely dense breast tissue led to significantly fewer interval cancers in comparison with mammography alone (P < .001). In the DENSE trial, MRI participants underwent mammography plus MRI at 2-year intervals; the control group underwent mammography alone at 2-year intervals.
In the current study, “screening strategies varied in the number of MRIs and mammograms offered to women aged 50-75 years,” explains Amarens Geuzinge, MSc, University Medical Center, Rotterdam, the Netherlands, and colleagues, “and incremental cost-effectiveness ratios (ICERs) were calculated ... with a willingness-to-pay threshold of 22,000 euros (>$25,000 U.S.),” the investigators add.
Analyses indicated that screening every 2 years with mammography alone cost the least of all strategies that were evaluated, but it also resulted in the lowest number of quality-adjusted life years (QALYs) – in other words, it delivered the least amount of benefit for patients, coauthor Eveline Heijnsdijk, PhD, University Medical Center, Rotterdam, the Netherlands, explained to this news organization.
Offering an additional MRI every 2 years resulted in the highest costs but not the highest number of QALYs and was inferior to the other screening strategies analyzed, she added. Alternating mammography with MRI breast cancer screening, each conducted every 2 years, came close to providing the same benefits to patients as the every-4-year MRI screening strategy, Dr. Heijnsdijk noted.
However, when the authors applied the National Institute for Health and Care Excellence (NICE) threshold, MRI screening every 4 years yielded the highest acceptable incremental cost-effectiveness ratio (ICER), at 15,620 euros per QALYs, whereas screening every 3 years with MRI alone yielded an ICER of 37,181 euros per QALY.
If decision-makers are willing to pay more than 22,000 euros per QALY gained, “MRI every 2 or 3 years can also become cost effective,” the authors add.
Asked how acceptable MRI screening might be if performed only once every 4 years, Dr. Heijnsdijk noted that, in another of their studies, most of the women who had undergone MRI screening for breast cancer said that they would do so again. “MRI is not a pleasant test, but mammography is also not a pleasant test,” she said.
“So many women prefer MRI above mammography, especially because the detection rate with MRI is better than mammography,” she noted. Dr. Heijnsdijk also said that the percentage of women with extremely dense breasts who would be candidates for MRI screening is small – no more than 10% of women.
At a unit cost of slightly under 300 euros for MRI screening – compared with about 100 euros for screening mammography in the Netherlands – the cost of offering 10% of women MRI instead of mammography might increase, but any additional screening costs could be offset by reductions in the need to treat late-stage breast cancer more aggressively.
‘Interval’ cancers
Commenting further on the study, Dr. Kuhl pointed out that from 25% to 45% of cancers that occur in women who have undergone screening mammography are diagnosed as “interval” cancers, even among women who participate in the best mammography programs. “For a long time, people argued that these interval cancers developed only after the last respective mammogram, but that’s not true at all, because we know that with MRI screening, we can reduce the interval cancer rate down to zero,” Dr. Kuhl emphasized.
This is partially explained by the fact that mammography is “particularly blind” when it comes to detecting rapidly growing tumors. “The fact is that mammography has a modality-inherent tendency to preferentially detect slow-growing cancers, whereas rapidly growing tumors are indistinguishable from ubiquitous benign changes like cysts. [This] is why women who undergo screening mammography are frequently not diagnosed with the cancers that we really need to find,” she said.
Although there is ample talk about overdiagnosis when it comes to screening mammography, the overwhelmingly important problem is underdiagnosis. Even in exemplary mammography screening programs, at least 20% of tumors that are diagnosed on mammography have already advanced to a stage that is too late, Dr. Kuhl noted.
This means that at least half of women do not benefit from screening mammography nearly to the extent that they – and their health care practitioners – believe they should, she added. Dr. Kuhl underscored that this does not mean that clinicians should abandon screening mammography.
What it does mean is that physicians need to abandon the one-size-fits-all approach to screening mammography and start stratifying women on the basis of their individual risk of developing breast cancer by taking a family or personal history. Most women do undergo screening mammography at least once, Dr. Kuhl pointed out. From that mammogram, physicians can use information on breast density and breast architecture to better determine individual risk.
“We have good ideas about how to achieve risk stratification, but we’re not using them, because as long as mammography is the answer for everybody, there isn’t much motivation to dig deeper into the issue of how to determine risk,” Dr. Kuhl said.
“But we have to ensure the early diagnosis of aggressive cancers, and it’s exactly MRI that can do this, and we should start with women with very dense breasts because they are doubly underserved by mammography,” she said.
The study was supported by the University Medical Center Utrecht, Bayer HealthCare Medical Care, Matakina, and others. Ms. Geuzinge, Dr. Heijnsdijk, and Dr. Kuhl have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
When children and teens with cancer get COVID-19
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Although most children and adolescents with cancer have mild illness from COVID-19 infection, some do experience severe disease and a small percentage even die, according to a recent analysis.
The findings, published online in Lancet Oncology, represent the first global registry data spanning different income groups to report COVID-19 outcomes in pediatric oncology patients.
“We wanted to create a global pool of evidence to answer the question: Do we see severe [COVID-19] infection [in children with cancer]?” corresponding author Sheena Mukkada, MD, St. Jude Children’s Research Hospital, Memphis, said in an interview.
In a cohort of 1,319 pediatric patients followed for 30 days, Dr. Mukkada and colleagues reported that 80% of these patients had asymptomatic to moderate disease from COVID-19, while 1 in 5 experienced severe or critical illness and almost 4% died – four times the mortality rate observed in published cohorts of general pediatric patients.
The results highlight that “children and adolescents with cancer generally recover without incident from COVID-19, but can have a severe course of infection,” the authors concluded.
And knowing that some children can get very sick, investigators wanted “to identify who these patients are so that we can prioritize and protect that group,” she added.
Echoing that sentiment, Kathy Pritchard-Jones, MD, president of the International Society of Paediatric Oncology and coauthor on the study, noted in a press release that, “by working together to create this global registry, we have enabled hospitals around the world to rapidly share and learn how COVID-19 is affecting children with cancer.”
Dr. Pritchard-Jones commented that overall these results provide reassurance that “many children can continue their cancer treatment safely, but they also highlight important clinical features that may predict a more severe clinical course and the need for greater vigilance for some patients.”
Inside the Global Registry data
The Global Registry of COVID-19 in Childhood Cancer, created jointly by St. Jude Children’s Research Hospital and SIOP, included data from 131 institutions in 45 countries. Children recruited into the registry between April 2020 and February 2021 ranged in age from infancy to 18 years old.
Most patients remained asymptomatic (35%) or experienced mild to moderate illness (45%), though 20% did develop severe or critical illness.
The investigators highlighted several factors associated with a greater risk of developing more severe illness from COVID-19, which included cancer type, intensity of therapy, age, absolute lymphocyte count, and presence of comorbidities or COVID-19 symptoms.
Notably, more than 80% of either severe or critical infections occurred in patients with hematologic malignancies – with 56% of cases in patients with acute lymphoblastic lymphoma or acute lymphoblastic leukemia – followed by extracranial solid tumors (15.8%), and central nervous system tumors (2.7%).
In patients with acute lymphoblastic leukemia or acute lymphoblastic lymphoma, severe or critical disease was most common in those receiving induction therapy (30%), relapse or refractory therapy (30%), and those in the maintenance or continuation phase of therapy (19%).
Older age was associated with a higher likelihood of having severe disease – with the lowest risk in infants (9.7%) and the highest in the 15- to 18-year-old cohort (27.3%).
Patients with lymphopenia who had an absolute lymphocyte count of 300 cells per mm3 or less and an absolute neutrophil count of 500 cells per mm3 or more also had an elevated risk of severe illness from COVID-19.
Regarding whether the presence of lymphopenia or neutropenia should change the treatment approach, Dr. Mukkada noted that, when possible, these patients should receive antiviral treatment, such as remdesivir, if the center has antivirals, or be prioritized for hospital admission.
Modifying cancer treatment might be recommended if patients are highly lymphopenic or have very low neutrophil counts, but a more effective strategy is simply to ensure that age-eligible children and adolescents with cancer or who have had a hematopoietic stem-cell transplantation have been fully vaccinated against COVID-19. For children who are not yet age-eligible, everyone around them should be vaccinated.
Pediatric patients in low- and middle-income countries were also more likely to have severe or critical outcomes from COVID-19 (41.7%), compared with patients in other income groups (23.9%).
The impact of COVID-19 “has been felt in every corner of the world, but particularly in low- and middle-income countries, compared to high-income countries,” senior author Carlos Rodriguez-Galindo, MD, global director at St. Jude, said in a statement.
In terms of the intersection of cancer treatment and COVID diagnosis, almost 83% of pediatric patients were receiving treatment for their cancer. Chemotherapy was withheld in about 45% of these patients and some modification to the treatment regimen occurred in almost 56% of participants on active therapy.
“Treatment modifications were least common in patients from upper-middle–income countries, compared with other income groups,” the authors wrote.
Although an interesting observation, Dr. Mukkada noted that the registry data could not explain why treatment modifications occurred less frequently in upper-middle income countries as opposed to high-income and lower-income countries.
U.K. Monitoring Project
Not all studies, however, have found that COVID-19 infection is significantly more severe in children with cancer. In a 2020 report from the U.K. Paediatric Coronavirus Cancer Monitoring Project, researchers evaluated all children in the United Kingdom under the age of 16 diagnosed with COVID and cancer.
“[Given that] we had complete coverage of every center in the U.K. that cares for children with cancer, we are confident that we picked up at least all the severe or critical cases,” lead author Gerard Millen, MD, honorary clinical research fellow, University of Birmingham (England), said in an interview.
Between March 2020 and July 2020, Dr. Millen and colleagues identified 54 positive cases of COVID-19, 15 (28%) of which were asymptomatic, 34 (63%) mild, and 4 (7.4%) severe or critical – more in line with the incidence of severe illness reported in the general pediatric population.
“Thankfully, we had no children with cancer in the U.K. who died from COVID-19,” Dr. Millen noted. “Overall, in the U.K., we have taken the approach that the majority of children with cancer in this country are at very low risk from COVID-19 and that we do not have good evidence to modify their treatment.”
Dr. Millen pointed out that the data in the U.K. study were “remarkably similar” to those from the high-income countries in the global St. Jude/SIOP cohort, where 7.4% of patients in that cohort had severe or critical disease, compared with 7.4% of patients from their own U.K. cohort.
“I think many of the key differences between the two cohorts reflect the fact that access to treatment in many low- to middle-income countries is more challenging with many factors contributing to overall poorer outcomes for both cancer and noncancer metrics,” Dr. Millen said.
Both the U.K. and registry studies were performed prior to vaccinations becoming available to older children, and before the emergence of certain variants, including the Delta variant, which is responsible for the most recent surge of COVID-19 infections around the world.
Data on COVID-19 vaccination in children with cancer are limited but promising so far.
As for whether the Delta variant might affect outcomes for children with cancer and COVID-19, Dr. Mukkada could only speculate, but she noted that “what we are hearing anecdotally about the [Delta] disease being more severe, even in patients who don’t have cancer, is leading us to say that we can’t close the registry yet. We are still actively enrolling children.”
The study was funded by the American Lebanese Syrian Associated Charities and the National Cancer Institute. The study authors and Dr. Millen disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Immunotherapy for cancer patients with poor PS needs a rethink
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The findings have prompted an expert to argue against the use of immunotherapy for such patients, who may have little time left and very little chance of benefiting.
“It is quite clear from clinical practice that most patients with limited PS do very poorly and do not benefit from immune check point inhibitors (ICI),” Jason Luke, MD, UPMC Hillman Cancer Center and the University of Pittsburgh, said in an email.
“So, my strong opinion is that patients should not be getting an immunotherapy just because it might not cause as many side effects as chemotherapy,” he added.
“Instead of giving an immunotherapy with little chance of success, patients and families deserve to have a direct conversation about what realistic expectations [might be] and how we as the oncology community can support them to achieve whatever their personal goals are in the time that they have left,” he emphasized.
Dr. Luke was the lead author of an editorial in which he commented on the study. Both the study and the editorial were published online in JCO Oncology Practice.
Variety of cancers
The study was conducted by Mridula Krishnan, MD, Nebraska Medicine Fred and Pamela Buffett Cancer Center, Omaha, Nebraska, and colleagues.
The team reviewed 257 patients who had been treated with either a programmed cell death protein–1 inhibitor or programmed cell death–ligand-1 inhibitor for a variety of advanced cancers. The drugs included pembrolizumab (Keytruda), nivolumab (Opdivo), atezolizumab (Tecentique), durvalumab (Imfinzi), and avelumab (Bavencio).
Most of the patients (71%) had good PS, with an Eastern Cooperative Oncology Group (ECOG) PS of 0-1 on initiation of immunotherapy; 29% of patients had poor PS, with an ECOG PS of greater than or equal to 2.
“The primary outcome was OS stratified by ECOG PS 0-1 versus ≥2,” note the authors. Across all tumor types, OS was superior for patients in the ECOG 0-1 PS group, the investigators note. The median OS was 12.6 months, compared with only 3.1 months for patients in the ECOG greater than or equal to 2 group (P < .001).
Moreover, overall response rates for patients with a poor PS were low. Only 8%, or 6 of 75 patients with an ECOG PS of greater than or equal to 2, achieved an objective response by RECIST criteria.
This compared to an overall response rate of 23% for patients with an ECOG PS of 0-1, the investigators note (P = .005).
Interestingly, the hospice referral rate for patients with a poor PS (67%) was similar to that of patients with a PS of 1-2 (61.9%), Dr. Krishnan and colleagues observe.
Those with a poor PS were more like to die in-hospital (28.6%) than were patients with a good PS (15.1%; P = .035). The authors point out that it is well known that outcomes with chemotherapy are worse among patients who experience a decline in functional reserve, owing to increased susceptibility to toxicity and complications.
“Regardless of age, patients with ECOG PS >2 usually have poor tolerability to chemotherapy, and this correlates with worse survival outcome,” they emphasize. There is as yet no clear guidance regarding the impact of PS on ICI treatment response, although “there should be,” Dr. Luke believes.
“In a patient with declining performance status, especially ECOG PS 3-4 but potentially 2 as well, there is little likelihood that the functional and immune reserve of the patient will be adequate to mount a robust antitumor response,” he elaborated.
“It’s not impossible, but trying for it should not come at the expense of engaging about end-of-life care and maximizing the palliative opportunities that many only have a short window of time in which to pursue,” he added.
Again, Dr. Luke strongly believes that just giving an ICI without engaging in a frank conversation with the patient and their families – which happens all too often, he feels – is absolutely not the way to go when treating patients with a poor PS and little time left.
“Patients and families might be better served by having a more direct and frank conversation about what the likelihood [is] that ICI therapy will actually do,” Dr. Luke stressed.
In their editorial, Dr. Luke and colleagues write: “Overall, we as an oncology community need to improve our communication with patients regarding goals of care and end-of-life considerations as opposed to reflexive treatment initiation,” he writes.
“Our duty, first and foremost, should focus on the person sitting in front of us – taking a step back may be the best way to move forward with compassionate care,” they add.
The authors and editorialists have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pandemic derails small success in lowering diabetes-related amputations
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.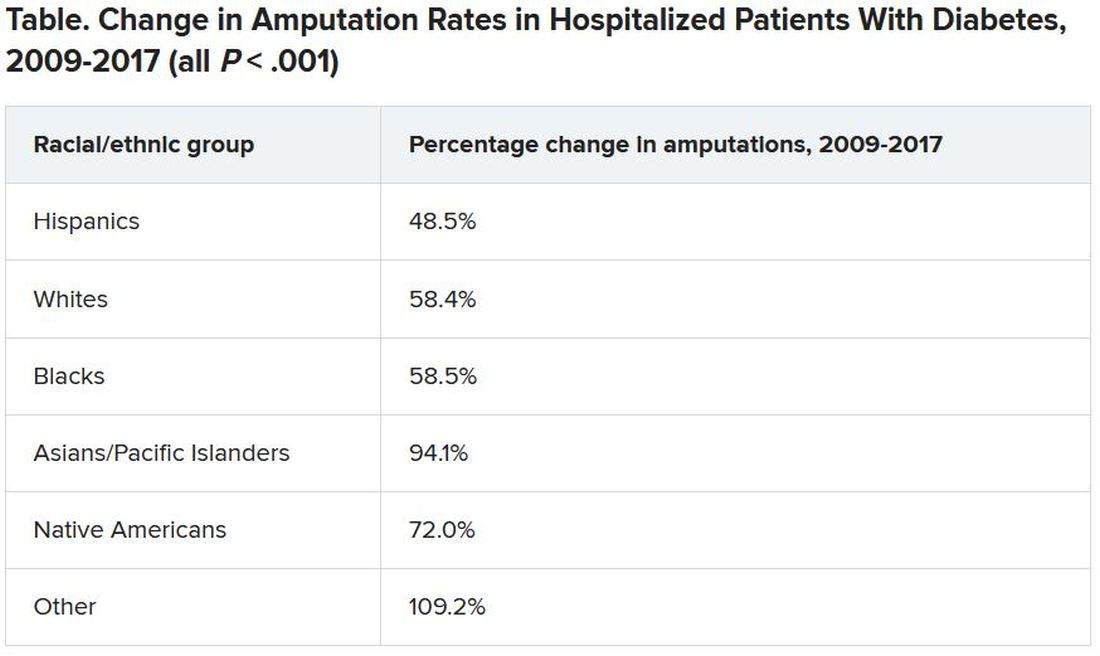
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Rates of minor diabetes-related lower extremity amputations (LEAs) in hospitalized patients increased between 2009 and 2017 in all racial and ethnic groups, in both rural and urban areas, and in all geographic regions across the United States, a new retrospective, observational study indicates.
In contrast, major lower extremity amputation rates held steady during the study period with a few exceptions.
There was also a decline in major-to-minor amputation ratios, especially among Native Americans – a sign that diabetes was being better managed and foot ulcers were being caught earlier, preventing the need for a major amputation above the foot or below or above the knee.
Minor LEAs include the loss of a toe, toes, or a foot.
“While I know an amputation is devastating either way, having a minor amputation is better than having a major amputation, and trends [at least to 2017] show that comprehensive foot examinations are paying off,” lead author Marvellous Akinlotan, PhD, MPH, a research associate at the Southwest Rural Health Research Center in Bryan, Texas, said in an interview.
Asked to comment, Marcia Ory, PhD, MPH, director of the Center for Population Health & Aging, Texas A&M School of Public Health, College Station, who was not involved in the study, said: “It points to some successes, but it also points to the need for continued education and preventive care to reduce all types of amputations.”
The study was published online in Diabetes Care.
Amputations increased during COVID-19
However, the study was conducted prior to the COVID-19 pandemic, and amputation rates appear to have significantly worsened during the past 18 months.
In a summary of recent evidence collated by the Amputee Coalition, the authors point out that not only does COVID-19 itself put patients at higher risk for limb loss because severe infection increases the risk of blood clots, but patients with diabetes appear to have been far more likely to undergo any level of amputation during the pandemic than before it began.
In a study of patients with diabetes attending a foot and ankle surgery service in Ohio, the risk of having any level of amputation was 10.8 times higher during compared with before the pandemic. And of patients undergoing any amputation, the odds for receiving a major amputation was 3.1 times higher than before the pandemic.
Telehealth and web-based options for diabetes care and education could help improve health outcomes, particularly during lockdowns.
“Having a diabetes-related amputation is life-changing – it brings disability and functional limitations to the individual – and within the health care system, it reflects the failure of secondary prevention efforts, which ideally should slow the progression of diabetic complications,” noted Dr. Akinlotan.
Race and geography affect risk of amputation
In their study, Dr. Akinlotan and colleagues used data from the National Inpatient Sample to identify trends in LEAs among patients primarily hospitalized for diabetes in the United States between 2009 and 2017.
“The primary outcome variable was documentation of either minor or major LEA during a diabetes-related admission,” they explain.
Minor LEAs increased significantly across all ethnic groups.
Although major amputation rates remained steady, “we did find that some groups remained at risk for having a major amputation,” Dr. Akinlotan noted.
White populations, people in the Midwest, and rural areas saw notable increases in major LEAs, as did “... Blacks, Hispanics, [and] those living in the South,” she said.
Patients need to be encouraged to monitor and control their blood glucose, to offset modifiable risk factors, and to seek regular medical attention to prevent an insidious diabetic complication from developing further, she said.
“It’s important for patients to know that continuing care is necessary,” Dr. Akinlotan stressed. “Diabetes is chronic and complex, but it can be managed, so that’s the good news.”
Dr. Ory agrees: “Effective management will require an all-in approach, with doctors and patients working together.
“Given the limited time in doctor-patient encounters, physicians can benefit patients by referring them to evidence-based, self-management education programs, which are proliferating around in the county,” she added.
The authors and Dr. Ory have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Real-world COVID-19 vaccine protection high in transplant patients
Real-world protection from COVID-19 vaccination is better than expected in transplant recipients, reducing the risk of symptomatic infection by almost 80% in those who have had both doses compared with unvaccinated controls, a new transplant registry analysis shows.
“Persons who have received an organ transplant are considered to be at increased risk for COVID-19 and for a severe outcome because their immune systems are necessarily suppressed to ensure their transplants are successful and lasting,” lead author Saima Aslam, MD, professor of medicine, University of California, San Diego, said in a statement.
Because numerous studies have demonstrated reduced antibody responses to SARS-CoV-2 in solid organ transplant recipients and variable effect on T-cell responses, there has been a need to study clinical effectiveness and breakthrough infections in those who are vaccinated, they explained.
“These findings offer strong evidence that getting vaccinated provides significant protection,” Dr. Aslam noted.
The investigators say that recent data from France, as well as other studies, show an increased rate of detectable antibodies following a third dose of the Pfizer-BioNTech COVID-19 vaccine in organ transplant recipients, “but based on our data it is unclear if a third dose is clinically warranted.”
The researchers stressed that almost half of the solid organ transplant recipients analyzed in the study had not been vaccinated at all, even by the beginning of 2021 when the United States was well into a third wave of COVID-19 infections.
So there is still a significant need, the authors said, to continue to improve outreach efforts to those in the transplant community and promote the benefits of being fully vaccinated.
The study was published online recently in Transplant Infectious Disease.
Transplant registry
The researchers analyzed clinical data from the UC San Diego transplant registry from Jan. 1 through June 2 of this year, with 2,151 solid organ transplant recipients identified. The patients had received a variety of solid organ transplants including kidney, liver, lung, and heart; the largest percentage received a donor kidney.
Among all patients, 912 were fully vaccinated and 1,239 were not (1,151 of those 1,239 received no vaccine at all and 88 had been partially vaccinated; these 1,239 served as the control group).
Fully vaccinated patients had received two shots of either the Pfizer-BioNTech COVID-19 or the Moderna vaccine, or a single dose of the Johnson & Johnson vaccine. The majority, at nearly 70%, had received the Moderna vaccine. The mean age of the cohort was 57 years and the median time since patients had undergone transplantation was almost 5 years (57.5 months).
During the 6-month study interval, 65 cases of COVID-19 were documented in the group overall. Only 4 cases occurred among fully vaccinated individuals whereas 61 cases occurred among the unvaccinated, including in 2 patients who had been partially vaccinated.
Among the four cases that occurred among the fully vaccinated, two were considered mild and were treated on an outpatient basis, and the other two were moderate, requiring hospitalization and treatment with remdesivir.
There were no COVID-19–related deaths among the 4 patients who experienced breakthrough infections, whereas 2 (3.3%) of 61 of control patients died of COVID-19–related causes.
The authors noted that the incidence rate for COVID-19 was 0.065 per 1,000-person days among the fully vaccinated compared with an incidence rate of 0.34 per 1,000-person days in the control group.
Booster doses for especially vulnerable transplant recipients?
“These findings are encouraging for a couple of reasons,” said coauthor Kristin Mekeel, MD, chief of transplant and hepatobiliary surgery at UCSD.
“First, they demonstrate real-world clinical effectiveness of COVID-19 vaccination in a vulnerable population,” she noted.
“Second, the effectiveness is better than expected,” she added, “given that studies have found that only about half of solid organ transplant recipients develop detectable antibodies after vaccination.”
Although calls for patients who are immunosuppressed to receive a third booster dose of a COVID-19 vaccine may not be necessary, “prioritizing at-risk subsets of transplant recipients based on immunological profiles and clinical characteristics for a third vaccine dose could be considered,” they said, adding it’s still vitally important for transplant patients to continue to mask and practice social distancing.
And it is especially important for transplant recipients to encourage household members to get vaccinated, too, especially given the current COVID-19 surge in San Diego.
The study was funded by the Cystic Fibrosis Foundation.
Dr. Aslam reports receiving grants from the Cystic Fibrosis Foundation as well as honoraria from Gilead and Merck. Study author Susan J. Little, MD, received grant funding from Gilead Sciences. Dr. Mekeel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Real-world protection from COVID-19 vaccination is better than expected in transplant recipients, reducing the risk of symptomatic infection by almost 80% in those who have had both doses compared with unvaccinated controls, a new transplant registry analysis shows.
“Persons who have received an organ transplant are considered to be at increased risk for COVID-19 and for a severe outcome because their immune systems are necessarily suppressed to ensure their transplants are successful and lasting,” lead author Saima Aslam, MD, professor of medicine, University of California, San Diego, said in a statement.
Because numerous studies have demonstrated reduced antibody responses to SARS-CoV-2 in solid organ transplant recipients and variable effect on T-cell responses, there has been a need to study clinical effectiveness and breakthrough infections in those who are vaccinated, they explained.
“These findings offer strong evidence that getting vaccinated provides significant protection,” Dr. Aslam noted.
The investigators say that recent data from France, as well as other studies, show an increased rate of detectable antibodies following a third dose of the Pfizer-BioNTech COVID-19 vaccine in organ transplant recipients, “but based on our data it is unclear if a third dose is clinically warranted.”
The researchers stressed that almost half of the solid organ transplant recipients analyzed in the study had not been vaccinated at all, even by the beginning of 2021 when the United States was well into a third wave of COVID-19 infections.
So there is still a significant need, the authors said, to continue to improve outreach efforts to those in the transplant community and promote the benefits of being fully vaccinated.
The study was published online recently in Transplant Infectious Disease.
Transplant registry
The researchers analyzed clinical data from the UC San Diego transplant registry from Jan. 1 through June 2 of this year, with 2,151 solid organ transplant recipients identified. The patients had received a variety of solid organ transplants including kidney, liver, lung, and heart; the largest percentage received a donor kidney.
Among all patients, 912 were fully vaccinated and 1,239 were not (1,151 of those 1,239 received no vaccine at all and 88 had been partially vaccinated; these 1,239 served as the control group).
Fully vaccinated patients had received two shots of either the Pfizer-BioNTech COVID-19 or the Moderna vaccine, or a single dose of the Johnson & Johnson vaccine. The majority, at nearly 70%, had received the Moderna vaccine. The mean age of the cohort was 57 years and the median time since patients had undergone transplantation was almost 5 years (57.5 months).
During the 6-month study interval, 65 cases of COVID-19 were documented in the group overall. Only 4 cases occurred among fully vaccinated individuals whereas 61 cases occurred among the unvaccinated, including in 2 patients who had been partially vaccinated.
Among the four cases that occurred among the fully vaccinated, two were considered mild and were treated on an outpatient basis, and the other two were moderate, requiring hospitalization and treatment with remdesivir.
There were no COVID-19–related deaths among the 4 patients who experienced breakthrough infections, whereas 2 (3.3%) of 61 of control patients died of COVID-19–related causes.
The authors noted that the incidence rate for COVID-19 was 0.065 per 1,000-person days among the fully vaccinated compared with an incidence rate of 0.34 per 1,000-person days in the control group.
Booster doses for especially vulnerable transplant recipients?
“These findings are encouraging for a couple of reasons,” said coauthor Kristin Mekeel, MD, chief of transplant and hepatobiliary surgery at UCSD.
“First, they demonstrate real-world clinical effectiveness of COVID-19 vaccination in a vulnerable population,” she noted.
“Second, the effectiveness is better than expected,” she added, “given that studies have found that only about half of solid organ transplant recipients develop detectable antibodies after vaccination.”
Although calls for patients who are immunosuppressed to receive a third booster dose of a COVID-19 vaccine may not be necessary, “prioritizing at-risk subsets of transplant recipients based on immunological profiles and clinical characteristics for a third vaccine dose could be considered,” they said, adding it’s still vitally important for transplant patients to continue to mask and practice social distancing.
And it is especially important for transplant recipients to encourage household members to get vaccinated, too, especially given the current COVID-19 surge in San Diego.
The study was funded by the Cystic Fibrosis Foundation.
Dr. Aslam reports receiving grants from the Cystic Fibrosis Foundation as well as honoraria from Gilead and Merck. Study author Susan J. Little, MD, received grant funding from Gilead Sciences. Dr. Mekeel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Real-world protection from COVID-19 vaccination is better than expected in transplant recipients, reducing the risk of symptomatic infection by almost 80% in those who have had both doses compared with unvaccinated controls, a new transplant registry analysis shows.
“Persons who have received an organ transplant are considered to be at increased risk for COVID-19 and for a severe outcome because their immune systems are necessarily suppressed to ensure their transplants are successful and lasting,” lead author Saima Aslam, MD, professor of medicine, University of California, San Diego, said in a statement.
Because numerous studies have demonstrated reduced antibody responses to SARS-CoV-2 in solid organ transplant recipients and variable effect on T-cell responses, there has been a need to study clinical effectiveness and breakthrough infections in those who are vaccinated, they explained.
“These findings offer strong evidence that getting vaccinated provides significant protection,” Dr. Aslam noted.
The investigators say that recent data from France, as well as other studies, show an increased rate of detectable antibodies following a third dose of the Pfizer-BioNTech COVID-19 vaccine in organ transplant recipients, “but based on our data it is unclear if a third dose is clinically warranted.”
The researchers stressed that almost half of the solid organ transplant recipients analyzed in the study had not been vaccinated at all, even by the beginning of 2021 when the United States was well into a third wave of COVID-19 infections.
So there is still a significant need, the authors said, to continue to improve outreach efforts to those in the transplant community and promote the benefits of being fully vaccinated.
The study was published online recently in Transplant Infectious Disease.
Transplant registry
The researchers analyzed clinical data from the UC San Diego transplant registry from Jan. 1 through June 2 of this year, with 2,151 solid organ transplant recipients identified. The patients had received a variety of solid organ transplants including kidney, liver, lung, and heart; the largest percentage received a donor kidney.
Among all patients, 912 were fully vaccinated and 1,239 were not (1,151 of those 1,239 received no vaccine at all and 88 had been partially vaccinated; these 1,239 served as the control group).
Fully vaccinated patients had received two shots of either the Pfizer-BioNTech COVID-19 or the Moderna vaccine, or a single dose of the Johnson & Johnson vaccine. The majority, at nearly 70%, had received the Moderna vaccine. The mean age of the cohort was 57 years and the median time since patients had undergone transplantation was almost 5 years (57.5 months).
During the 6-month study interval, 65 cases of COVID-19 were documented in the group overall. Only 4 cases occurred among fully vaccinated individuals whereas 61 cases occurred among the unvaccinated, including in 2 patients who had been partially vaccinated.
Among the four cases that occurred among the fully vaccinated, two were considered mild and were treated on an outpatient basis, and the other two were moderate, requiring hospitalization and treatment with remdesivir.
There were no COVID-19–related deaths among the 4 patients who experienced breakthrough infections, whereas 2 (3.3%) of 61 of control patients died of COVID-19–related causes.
The authors noted that the incidence rate for COVID-19 was 0.065 per 1,000-person days among the fully vaccinated compared with an incidence rate of 0.34 per 1,000-person days in the control group.
Booster doses for especially vulnerable transplant recipients?
“These findings are encouraging for a couple of reasons,” said coauthor Kristin Mekeel, MD, chief of transplant and hepatobiliary surgery at UCSD.
“First, they demonstrate real-world clinical effectiveness of COVID-19 vaccination in a vulnerable population,” she noted.
“Second, the effectiveness is better than expected,” she added, “given that studies have found that only about half of solid organ transplant recipients develop detectable antibodies after vaccination.”
Although calls for patients who are immunosuppressed to receive a third booster dose of a COVID-19 vaccine may not be necessary, “prioritizing at-risk subsets of transplant recipients based on immunological profiles and clinical characteristics for a third vaccine dose could be considered,” they said, adding it’s still vitally important for transplant patients to continue to mask and practice social distancing.
And it is especially important for transplant recipients to encourage household members to get vaccinated, too, especially given the current COVID-19 surge in San Diego.
The study was funded by the Cystic Fibrosis Foundation.
Dr. Aslam reports receiving grants from the Cystic Fibrosis Foundation as well as honoraria from Gilead and Merck. Study author Susan J. Little, MD, received grant funding from Gilead Sciences. Dr. Mekeel has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
More children with high-risk brain cancer now surviving
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
A practice-changing study that used molecular testing to distinguish between subtypes of medulloblastoma has shown a significant improvement in survival for children with high-risk disease who underwent treatment intensification with carboplatin.
“Each of the four subgroups of medulloblastoma has a different prognosis, but for this particular subgroup, 20 fewer children out of every 100 would have survived prior to this study,” James Olson, MD, professor of medicine, French Hutchinson Cancer Research Center, University of Washington, Seattle, said in an interview.
“This is the reason for celebration – for now and forevermore, we can expect 20 more children with high-risk, group 3 medulloblastoma to survive,” he said.
“We recommend that all children with high-risk, group 3 medulloblastoma receive carboplatin and all children in the other subgroups do not, because we don’t want them to experience the toxicity without benefit,” Dr. Olson said.
The study was published online July 22, 2021, in JAMA Oncology.
Hematologic toxicity was more pronounced in the carboplatin arm in the induction phase of the protocol, and toxicity persisted into the first cycles of maintenance therapy. On the other hand, “there weren’t enough additional side effects to recommend children not get carboplatin if they would benefit from it,” Dr. Olson noted.
At least 75% of children with newly diagnosed medulloblastoma survive, although those with high-risk, group 3 disease have a substantially poorer prognosis than those with other molecular subtypes.
However, if a child with medulloblastoma experiences relapse, “the likelihood of survival is near zero, so it’s important to get it right the first time,” Dr. Olson said.
One of the patients who took part in this trial, Sammy Loch of Seattle, is now 27 years old and has been cancer free for 11 years.
She was diagnosed with medulloblastoma when in high school. At the time of her diagnosis, she was asked by her pediatric oncologist at Seattle Children’s Hospital about taking part in the study. After careful consideration, she agreed.
“Participating in research was my way to give back and pay it forward,” Ms. Loch said in a statement. “It’s really exciting to know more people will survive because of the research I was involved in,” she added. She continues to pay her debt forward, serving as a therapist for people with chronic health conditions and raising funds for pediatric cancer research.
Patients had high-risk features
The study involved 261 evaluable patients (median age, 8.6 years). All patients had high-risk features, including metastatic disease (72.4% of the group), diffuse anaplastic histologic characteristics (22.2%), and incomplete surgical resection (5.4%), defined as residual tumor greater than 1.5 cm2.
“All patients received 36 Gy craniospinal radiotherapy with boost to the posterior fossa of 55.8 Gy cumulative dose with conventional fractionation of 1.8 Gy/d,” Dr. Olson and colleagues explain. Patients also received six doses of vincristine 1.5 mg/m2 weekly during radiotherapy and were randomly assigned to receive carboplatin 35 mg/m2 for a total of 30 doses given daily prior to radiotherapy or placebo.
This regimen was followed by maintenance therapy, which consisted of six 28-day cycles of the combination of cisplatin 75 mg/m2 on day 1; cyclophosphamide 1,000 mg/m2 on days 2 and 3; and vincristine 1.5 mg/m2 on days 1 and 8.
Patients were originally assigned to receive an additional 12 cycles of isotretinoin or placebo, to be given during and after maintenance therapy. However, randomization to isotretinoin was discontinued early because of futility.
The study was initially powered to evaluate medulloblastoma as a single disease. However, as a result of biologic insights gained after the study was conceived, it was amended to include a molecular subgroup analysis to better distinguish patients who might truly benefit from intensified therapy, the authors explained.
Study results
The World Health Organization categorizes tumors of the central nervous system into four groups. The authors followed this system of categorization for their patients with medulloblastoma. The four groups are WNT, in which WNT signaling pathway is activated; SHH, in which the SHH signaling pathway is activated; with or without TP53 mutation (provisionally designated group 3); and non-WNT/non-SHH (provisionally designed group 4)
The primary endpoint of the trial was event-free survival (EFS). In the patient population overall, there was no significant difference regarding this endpoint among those who received carboplatin and those who did not (EFS at 5 years, 66.4% vs. 59.2%).
However, there was a significant improvement among the patients in subgroup 3. Among those patients, EFS at 5 years was 73.2% with carboplatin versus 53.7% without (P = .047).
Similarly, in the overall group, there was no significant improvement in overall survival (OS) at 5 years from the addition of carboplatin (77.6% vs. 68.8% without carboplatin). However, the OS at 5 years varied widely between the different subtypes. There was again a significant improvement in OS at 5 years among the patients in subgroup 3 (82% with carboplation vs 63.7% without).
The beneficial effects from the addition of carboplatin on both endpoints were seen exclusively in patients in group 3, the authors emphasized.
“The WNT group does really well with less therapy, so if we treated all children the same, we would likely be overtreating WNT children and undertreating group 3 children,” Dr. Olson observed. “Genetic analysis is essential.”
In an earlier study, Dr. Olson and colleagues found that 70% of children with primitive neuroectodermal tumor of the CNS and pineoblastoma had been misdiagnosed even by outstanding children’s oncology centers because clinicians were relying on microscopic diagnosis.
“With molecular diagnosis, we were able to learn that many of these children had completely different diseases that require complexly different treatments, so doing diagnosis by molecular classification is absolutely essential,” he reemphasized.
“Glimmers of hope”
This study provides clinicians with “glimmers of hope” that children with high-risk, group 3 medulloblastoma will experience improvements in survival, wrote Allison Martin, MD, Albert Einstein College of Medicine, New York, and Sadhana Jackson, MD, National Institutes of Health, Bethesda, M.d., in an accompanying editorial.
The editorialists hope that “the treatment paradigm for all patients with high-risk disease can be improved through incorporation of detailed molecular analyses.”
However, they pointed out that DNA methylation and other advanced testing methods used to distinguish subgroups 3 and 4 in this study are not widely available, even at most Children’s Oncology Group member institutions. (Dr. Olson countered that, even if these sophisticated tests are not available at all pediatric oncology centers, tests will be performed if clinicians send tissue to the few sites that are equipped to conduct them.)
The editorialists also noted that therapy intensification with carboplatin is associated with an increased risk for adverse effects – “underscoring the importance of correctly identifying patients who could benefit from this intervention and avoid unnecessary toxic effects.”
The study was funded by the National Cancer Institute. Dr. Olson has disclosed no relevant financial relationships. Dr. Martin reported that she previously owed shares in Celgene, which she has subsequently sold.
A version of this article first appeared on Medscape.com.
Semaglutide boosts weight loss following endoscopic gastroplasty
Combining minimally invasive endoscopic sleeve gastroplasty with a weekly injection of the glucagonlike peptide–1 agonist semaglutide (Ozempic, Novo Nordisk) leads to significantly greater weight loss than ESG alone in patients with diabetes and excess weight who are not candidates for bariatric surgery, new research shows.
During minimally invasive ESG, a flexible endoscope equipped with an endoscopic suturing device is inserted down the esophagus and into the stomach. The endoscopist then applies the sutures to the upper portion of the stomach, minimizing its size to restrict the amount of food a patient can ingest.
“Our stomachs can stretch back a bit, but we can use the suturing device again,” explained the lead investigator of the research Anna Carolina Hoff, MD, founder and clinical director of Angioskope Brazil in São José dos Campos.
“It’s important that patients with diabetes lose as much weight as possible because, if they lose about 10% of their total body weight, they have a great improvement in their glycemic levels, and some patients can even stop taking their [antidiabetic] medications,” Dr. Hoff said in an interview.
“And we found that by adding the GLP-1 agonist [semaglutide], we could increase weight loss from, on average, about 16%-18% of total body weight with ESG alone to up to 27%, so it’s a great metabolic combination,” she noted.
Dr. Hoff presented the findings at the annual Digestive Disease Week® (DDW).
Asked to comment, Scott Kahan, MD, MPH, director, National Center for Weight and Wellness, George Washington University, Washington, cautioned that it’s still early days for minimally invasive ESG.
“It is reasonable to assume that the long-term outcomes [with ESG] won’t be as good or durable over time as with bariatric surgery, but ... we will have to see.”
However, “we know that, typically, combinations of therapeutic options work better than a one-off option, so I think the real benefit of this study – outside the specific procedure and this specific medication – is that it is a very valuable proof-of-principle study showing that combinations do work better,” Dr. Kahan said in an interview.
Minimally invasive endoscopic sleeve gastroplasty
ESG is a surrogate for laparoscopic sleeve gastrectomy that can offer the benefits of such a procedure to those who don’t qualify for, or don’t wish to pursue, bariatric surgery. It can be performed at an earlier stage of disease, in those with a body mass index of 30 mg/kg2, whereas generally people are not offered bariatric procedures unless they have a BMI of at least 35 with comorbidities or a BMI of at least 40 if they do not have comorbidities.
Subcutaneous semaglutide is already approved for the treatment of type 2 diabetes in adults at doses of up to 1 mg/week; higher doses are needed for weight loss. Novo Nordisk has been investigating higher doses for weight loss in the STEP trial program, which is now complete, and the company has submitted the data to the Food and Drug Administration and European Medicines Agency for an additional indication of adults with obesity (BMI ≥30) or who are overweight (BMI ≥27) and who have at least one weight-related comorbidity, as an adjunct to a reduced-calorie diet and increased physical activity, with a decision expected soon.
Novo Nordisk has also developed an oral form of semaglutide, which has been approved as a once-daily agent for type 2 diabetes (Rybelsus) in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
Patients lost fat mass as well as excess weight
The Brazilian study involved 58 patients with obesity or overweight who also had diabetes and were undergoing minimally invasive ESG; they were further randomized to receive semaglutide or placebo.
The GLP-1 agonist (or sham placebo) was initiated 1 month after participants had undergone the procedure and patients were monitored each month for weight loss and type of fat loss achieved with the combination versus ESG alone. The initial dose of semaglutide used was 0.25 mg subcutaneous a week but could be titrated up to a maximum dose of 1.5 mg.
At the end of 11 months of active treatment versus placebo (12 months after ESG), patients who received additional semaglutide lost 86.3% of their excess body weight – the amount of weight patients needed to lose to reach normal BMI – compared with only 60.4% for ESG controls.
Specifically, the mean percentage total body weight loss at the end of 12 months was 25.2% for those in the combination group, compared with 18.6% for those treated with ESG alone (P < .001).
More importantly, patients in the combination group lost 12.6% of their body fat mass, compared with 9% for ESG controls, while mean A1c levels fell more in those treated with additional semaglutide compared with controls (P = .0394).
Indeed, five patients in the combination group reverted to a nondiabetic state and were able to discontinue antidiabetic medications altogether, Dr. Hoff noted.
“Our main goal is not just to lose weight but to lose body mass fat, which is very different from just losing weight,” she explained.
If patients lose weight but still maintain a high percentage of body fat mass, they have what she refers to as “sarcopenic obesity” because in this state patients have lost a lot of muscle mass but still have high levels of metabolically active visceral fat. Among many other inflammatory complexes, metabolically active visceral fat contains a large number of inflammasomes, and it is the latter that have been associated with obesity-related cancers.
“Obesity is a progressive disease, so what we are trying to do here is buy time for patients so they do not progress to [bariatric] surgery, and this approach gives patients a chance to act earlier before obesity takes over and more metabolic consequences occur,” Dr. Hoff emphasized.
So, when combined with semaglutide, “we now have a minimally invasive procedure that can be just as successful [as surgery] and which can be made available to even more people looking to lose a significant amount of weight,” she concluded.
Dr. Hoff and Dr. Kahan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Combining minimally invasive endoscopic sleeve gastroplasty with a weekly injection of the glucagonlike peptide–1 agonist semaglutide (Ozempic, Novo Nordisk) leads to significantly greater weight loss than ESG alone in patients with diabetes and excess weight who are not candidates for bariatric surgery, new research shows.
During minimally invasive ESG, a flexible endoscope equipped with an endoscopic suturing device is inserted down the esophagus and into the stomach. The endoscopist then applies the sutures to the upper portion of the stomach, minimizing its size to restrict the amount of food a patient can ingest.
“Our stomachs can stretch back a bit, but we can use the suturing device again,” explained the lead investigator of the research Anna Carolina Hoff, MD, founder and clinical director of Angioskope Brazil in São José dos Campos.
“It’s important that patients with diabetes lose as much weight as possible because, if they lose about 10% of their total body weight, they have a great improvement in their glycemic levels, and some patients can even stop taking their [antidiabetic] medications,” Dr. Hoff said in an interview.
“And we found that by adding the GLP-1 agonist [semaglutide], we could increase weight loss from, on average, about 16%-18% of total body weight with ESG alone to up to 27%, so it’s a great metabolic combination,” she noted.
Dr. Hoff presented the findings at the annual Digestive Disease Week® (DDW).
Asked to comment, Scott Kahan, MD, MPH, director, National Center for Weight and Wellness, George Washington University, Washington, cautioned that it’s still early days for minimally invasive ESG.
“It is reasonable to assume that the long-term outcomes [with ESG] won’t be as good or durable over time as with bariatric surgery, but ... we will have to see.”
However, “we know that, typically, combinations of therapeutic options work better than a one-off option, so I think the real benefit of this study – outside the specific procedure and this specific medication – is that it is a very valuable proof-of-principle study showing that combinations do work better,” Dr. Kahan said in an interview.
Minimally invasive endoscopic sleeve gastroplasty
ESG is a surrogate for laparoscopic sleeve gastrectomy that can offer the benefits of such a procedure to those who don’t qualify for, or don’t wish to pursue, bariatric surgery. It can be performed at an earlier stage of disease, in those with a body mass index of 30 mg/kg2, whereas generally people are not offered bariatric procedures unless they have a BMI of at least 35 with comorbidities or a BMI of at least 40 if they do not have comorbidities.
Subcutaneous semaglutide is already approved for the treatment of type 2 diabetes in adults at doses of up to 1 mg/week; higher doses are needed for weight loss. Novo Nordisk has been investigating higher doses for weight loss in the STEP trial program, which is now complete, and the company has submitted the data to the Food and Drug Administration and European Medicines Agency for an additional indication of adults with obesity (BMI ≥30) or who are overweight (BMI ≥27) and who have at least one weight-related comorbidity, as an adjunct to a reduced-calorie diet and increased physical activity, with a decision expected soon.
Novo Nordisk has also developed an oral form of semaglutide, which has been approved as a once-daily agent for type 2 diabetes (Rybelsus) in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
Patients lost fat mass as well as excess weight
The Brazilian study involved 58 patients with obesity or overweight who also had diabetes and were undergoing minimally invasive ESG; they were further randomized to receive semaglutide or placebo.
The GLP-1 agonist (or sham placebo) was initiated 1 month after participants had undergone the procedure and patients were monitored each month for weight loss and type of fat loss achieved with the combination versus ESG alone. The initial dose of semaglutide used was 0.25 mg subcutaneous a week but could be titrated up to a maximum dose of 1.5 mg.
At the end of 11 months of active treatment versus placebo (12 months after ESG), patients who received additional semaglutide lost 86.3% of their excess body weight – the amount of weight patients needed to lose to reach normal BMI – compared with only 60.4% for ESG controls.
Specifically, the mean percentage total body weight loss at the end of 12 months was 25.2% for those in the combination group, compared with 18.6% for those treated with ESG alone (P < .001).
More importantly, patients in the combination group lost 12.6% of their body fat mass, compared with 9% for ESG controls, while mean A1c levels fell more in those treated with additional semaglutide compared with controls (P = .0394).
Indeed, five patients in the combination group reverted to a nondiabetic state and were able to discontinue antidiabetic medications altogether, Dr. Hoff noted.
“Our main goal is not just to lose weight but to lose body mass fat, which is very different from just losing weight,” she explained.
If patients lose weight but still maintain a high percentage of body fat mass, they have what she refers to as “sarcopenic obesity” because in this state patients have lost a lot of muscle mass but still have high levels of metabolically active visceral fat. Among many other inflammatory complexes, metabolically active visceral fat contains a large number of inflammasomes, and it is the latter that have been associated with obesity-related cancers.
“Obesity is a progressive disease, so what we are trying to do here is buy time for patients so they do not progress to [bariatric] surgery, and this approach gives patients a chance to act earlier before obesity takes over and more metabolic consequences occur,” Dr. Hoff emphasized.
So, when combined with semaglutide, “we now have a minimally invasive procedure that can be just as successful [as surgery] and which can be made available to even more people looking to lose a significant amount of weight,” she concluded.
Dr. Hoff and Dr. Kahan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Combining minimally invasive endoscopic sleeve gastroplasty with a weekly injection of the glucagonlike peptide–1 agonist semaglutide (Ozempic, Novo Nordisk) leads to significantly greater weight loss than ESG alone in patients with diabetes and excess weight who are not candidates for bariatric surgery, new research shows.
During minimally invasive ESG, a flexible endoscope equipped with an endoscopic suturing device is inserted down the esophagus and into the stomach. The endoscopist then applies the sutures to the upper portion of the stomach, minimizing its size to restrict the amount of food a patient can ingest.
“Our stomachs can stretch back a bit, but we can use the suturing device again,” explained the lead investigator of the research Anna Carolina Hoff, MD, founder and clinical director of Angioskope Brazil in São José dos Campos.
“It’s important that patients with diabetes lose as much weight as possible because, if they lose about 10% of their total body weight, they have a great improvement in their glycemic levels, and some patients can even stop taking their [antidiabetic] medications,” Dr. Hoff said in an interview.
“And we found that by adding the GLP-1 agonist [semaglutide], we could increase weight loss from, on average, about 16%-18% of total body weight with ESG alone to up to 27%, so it’s a great metabolic combination,” she noted.
Dr. Hoff presented the findings at the annual Digestive Disease Week® (DDW).
Asked to comment, Scott Kahan, MD, MPH, director, National Center for Weight and Wellness, George Washington University, Washington, cautioned that it’s still early days for minimally invasive ESG.
“It is reasonable to assume that the long-term outcomes [with ESG] won’t be as good or durable over time as with bariatric surgery, but ... we will have to see.”
However, “we know that, typically, combinations of therapeutic options work better than a one-off option, so I think the real benefit of this study – outside the specific procedure and this specific medication – is that it is a very valuable proof-of-principle study showing that combinations do work better,” Dr. Kahan said in an interview.
Minimally invasive endoscopic sleeve gastroplasty
ESG is a surrogate for laparoscopic sleeve gastrectomy that can offer the benefits of such a procedure to those who don’t qualify for, or don’t wish to pursue, bariatric surgery. It can be performed at an earlier stage of disease, in those with a body mass index of 30 mg/kg2, whereas generally people are not offered bariatric procedures unless they have a BMI of at least 35 with comorbidities or a BMI of at least 40 if they do not have comorbidities.
Subcutaneous semaglutide is already approved for the treatment of type 2 diabetes in adults at doses of up to 1 mg/week; higher doses are needed for weight loss. Novo Nordisk has been investigating higher doses for weight loss in the STEP trial program, which is now complete, and the company has submitted the data to the Food and Drug Administration and European Medicines Agency for an additional indication of adults with obesity (BMI ≥30) or who are overweight (BMI ≥27) and who have at least one weight-related comorbidity, as an adjunct to a reduced-calorie diet and increased physical activity, with a decision expected soon.
Novo Nordisk has also developed an oral form of semaglutide, which has been approved as a once-daily agent for type 2 diabetes (Rybelsus) in doses of 7 mg and 14 mg to improve glycemic control along with diet and exercise. It is the first GLP-1 agonist available in tablet form.
Patients lost fat mass as well as excess weight
The Brazilian study involved 58 patients with obesity or overweight who also had diabetes and were undergoing minimally invasive ESG; they were further randomized to receive semaglutide or placebo.
The GLP-1 agonist (or sham placebo) was initiated 1 month after participants had undergone the procedure and patients were monitored each month for weight loss and type of fat loss achieved with the combination versus ESG alone. The initial dose of semaglutide used was 0.25 mg subcutaneous a week but could be titrated up to a maximum dose of 1.5 mg.
At the end of 11 months of active treatment versus placebo (12 months after ESG), patients who received additional semaglutide lost 86.3% of their excess body weight – the amount of weight patients needed to lose to reach normal BMI – compared with only 60.4% for ESG controls.
Specifically, the mean percentage total body weight loss at the end of 12 months was 25.2% for those in the combination group, compared with 18.6% for those treated with ESG alone (P < .001).
More importantly, patients in the combination group lost 12.6% of their body fat mass, compared with 9% for ESG controls, while mean A1c levels fell more in those treated with additional semaglutide compared with controls (P = .0394).
Indeed, five patients in the combination group reverted to a nondiabetic state and were able to discontinue antidiabetic medications altogether, Dr. Hoff noted.
“Our main goal is not just to lose weight but to lose body mass fat, which is very different from just losing weight,” she explained.
If patients lose weight but still maintain a high percentage of body fat mass, they have what she refers to as “sarcopenic obesity” because in this state patients have lost a lot of muscle mass but still have high levels of metabolically active visceral fat. Among many other inflammatory complexes, metabolically active visceral fat contains a large number of inflammasomes, and it is the latter that have been associated with obesity-related cancers.
“Obesity is a progressive disease, so what we are trying to do here is buy time for patients so they do not progress to [bariatric] surgery, and this approach gives patients a chance to act earlier before obesity takes over and more metabolic consequences occur,” Dr. Hoff emphasized.
So, when combined with semaglutide, “we now have a minimally invasive procedure that can be just as successful [as surgery] and which can be made available to even more people looking to lose a significant amount of weight,” she concluded.
Dr. Hoff and Dr. Kahan have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NSCLC survival on immunotherapy much lower in ‘real world’
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
Real-world use of the immune checkpoint inhibitors for first-line treatment of advanced non–small cell lung cancer (NSCLC) provides nowhere near the same survival advantage as seen in clinical trials, according to a retrospective cohort study of nearly 20,000 Medicare patients.
For example, the median overall survival (OS) in the “real world” was 11.4 months for patients treated with pembrolizumab (Keytruda, Merck) monotherapy – approximately 15 months shorter than the median OS among pembrolizumab-treated participants in the KEYNOTE-024 trial.
Indeed, OS was shorter for Medicare patients treated with an immune checkpoint inhibitor alone than it was for patients treated with a chemoimmunotherapy regimen of platinum plus pemetrexed plus pembrolizumab, at a median of 12.9 months – which in itself was approximately 10 months shorter than survival outcomes with this triplet therapy in the KEYNOTE-189 trial.
“These results, based on the nationwide experience for patients on Medicare, may inform discussions between physicians and patients with respect to expectations for outcomes among older patients with NSCLC,” lead author Kenneth Kehl, MD, assistant professor of medicine, Harvard Medical School, Boston, said in a statement.
Deborah Schrag, MD, chief, division of population sciences, Dana-Farber Cancer Institute, Boston, and Harvard Medical School, agreed, adding in the same statement that “this information empowers patients and clinicians with realistic expectations and equips them to make informed decisions.”
The study was published online May 21 in JAMA Network Open and was done in conjunction with the Health Data Analytics Institute, an analytics firm that applies artificial intelligence for measuring health risks.
Systemic therapy
For the study, the team analyzed Medicare data for 19,529 patients (median age, 73.8 years) who had all initiated first palliative-intent systemic therapy for lung cancer between January 2016 and December 2018. Some 3,079 patients received pembrolizumab monotherapy, 5,159 patients received a platinum-based regimen plus pemetrexed, 9,866 received a platinum plus a taxane, and 1,425 received platinum, pemetrexed, and pembrolizumab.
The authors noted that uptake of pembrolizumab-containing regimens in the Medicare population was rapid.
In the second quarter of 2016, pembrolizumab was used in only 0.7% of first-line treatments for advanced NSCLC, but increased to 42.4% of first-line treatments 2 years later, in the third quarter of 2018.
“The primary outcome was OS, which was measured using the restricted mean survival time (RMST),” Dr. Kehl and colleagues noted.
After propensity-score stratification, patients who received pembrolizumab had an adjusted RMST of 11 months compared with an adjusted RMST of 11.1 months for those who received the combination of platinum plus pemetrexed.
Survival was statistically worse for patients who received pembrolizumab than it was for those treated with a platinum/taxane combination, although the magnitude of difference between the two groups was small, at 0.7 months (P < .001). Patients who received the platinum/pemetrexed/pembrolizumab triplet had an adjusted RMST of 11.7 months, which was significantly better than the adjusted RMST of 11.2 months for patients who received the platinum/pemetrexed doublet, but the magnitude of the difference between these two groups was small, at 0.5 months (P = .02), the investigators added.
Different patient groups
Patients who received immunotherapy alone may have been more ill than those who received chemotherapy, the authors suggested. Patients who were 70 years of age or older, who were female, and who had a higher baseline mortality risk were more likely to receive single-agent pembrolizumab than chemotherapy, they noted. “Indeed, immunotherapy may be construed as a potential first-line treatment for patients who would otherwise have been deemed too frail for treatment at all, including patients older than 80 years,” they observed.
It is also possible that the Medicare patients included in the current analysis may differ substantively from advanced NSCLC participants enrolled in clinical trials, they wrote. For example, the median age of the Medicare cohort was approximately 10 years older than the median age of participants in both KEYNOTE-024 and KEYNOTE-189, the authors pointed out.
“If clinicians recommend immunotherapy disproportionately to Medicare patients with poor performance status or greater comorbidity – perhaps even if PD-L1 (programmed cell death-ligand-1) expression levels are below thresholds associated with the most substantial immunotherapy benefit – it may not be surprising that large survival improvements associated with immunotherapy were not observed in this analysis,” Dr. Kehl and colleagues suggested.
It is possible that durable benefit from immunotherapy, at least among some subgroups of patients included in the Medicare analysis, might have become more evident with additional follow-up beyond 18 months, they noted. However, they added, in “both KEYNOTE-024 and KEYNOTE-189, pembrolizumab was associated with substantial improvements in overall survival by that point.
“These results may inform prognostic considerations in practice and reinforce the importance of understanding patient selection dynamics in assessing the value and clinical utility of transformative treatment strategies,” they cautioned.
Dr. Kehl has reported receiving personal fees from Aetion, Roche, and IBM. Dr. Schrag has reported receiving personal fees from JAMA for editorial services and travel reimbursement/speaker fees from Pfizer.
A version of this article first appeared on Medscape.com.
USPSTF final recommendation on CRC screening: 45 is the new 50
Screening for colorectal cancer (CRC) should now begin at the age of 45 and not 50 for average-risk individuals in the United States, notes the final recommendation from the U.S. Preventive Services Task Force.
The recommendation finalizes draft guidelines issued in October 2020 and mandates insurance coverage to ensure equal access to CRC screening regardless of a patient’s insurance status.
The USPSTF’s final recommendations also now align with those of the American Cancer Society, which lowered the age for initiation of CRC screening to 45 years in 2018.
“New statistics project an alarming rise in the incidence of young-onset colorectal cancer, projected to be the leading cause of cancer death in patients aged 20-49 by 2040,” commented Kimmie Ng, MD, MPH, director, Young-Onset Colorectal Cancer Center, Dana-Farber Cancer Institute, Boston, and lead author of a JAMA editorial about the new guideline.
“We must take bold steps to translate the lowered age of beginning screening into meaningful decreases in CRC incidence and mortality,” she emphasized.
The USPSTF recommendations and substantial evidence supporting them were published online May 18, 2021, in JAMA.
Risk factors for CRC
As the USPSTF authors noted, age is one of the most important risk factors for CRC, with nearly 94% of all new cases of CRC occurring in adults 45 years of age and older. Justification for the lower age of CRC screening initiation was based on simulation models showing that initiation of screening at the age of 45 was associated with an estimated additional 22-27 life-years gained, compared with starting at the age of 50.
The USPSTF continues to recommend screening for CRC in all adults aged between 50 and 75 years, lowering the age for screening to 45 years in recognition of the fact that, in 2020, 11% of colon cancers and 15% of rectal cancers occurred in patients under the age of 50.
The USPSTF also continues to conclude that there is a “small net benefit” of screening for CRC in adults aged between 76 and 85 years who have been previously screened.
However, the decision to screen patients in this age group should be based on individual risk factors for CRC, a patient’s overall health status, and personal preference. Perhaps self-evidently, adults in this age group who have never been screened for CRC are more likely to benefit from CRC screening than those who have been previously screened.
Similar to the previous guidelines released in 2016, the updated USPSTF recommendations continue to offer a menu of screening strategies, although the frequency of screening for each of the screening strategies varies. Recommended screening strategies include:
- High-sensitivity guaiac fecal occult blood test or fecal immunochemical test (FIT) every year
- Stool DNA-FIT every 1-3 years
- CT colonography every 5 years
- every 5 years
- Flexible sigmoidoscopy every 10 years plus annual FIT
- screening every 10 years
“Based on the evidence, there are many tests available that can effectively screen for colorectal cancer and the right test is the one that gets done,” USPSTF member Martha Kubik, PhD, RN, said in a statement.
“To encourage screening and help patients select the best test for them, we urge primary care clinicians to talk about the pros and cons of the various recommended options with their patients,” she added.
An accompanying review of the effectiveness, accuracy, and potential harms of CRC screening methods underscores how different screening tests have different levels of evidence demonstrating their ability to detect cancer, precursor lesions, or both, as well as their ability to reduce mortality from cancer.
Eligible patients
Currently, fewer than 70% of eligible patients in the United States undergo CRC screening, Dr. Ng pointed out in the editorial. In addition, CRC disproportionately affects African American patients, who are about 20% more likely to get CRC and about 40% more likely to die from it, compared with other patient groups. Modeling studies published along with the USPSTF recommendations showed equal benefit for screening regardless of race and gender, underscoring the importance of screening adherence, especially in patient populations disproportionately affected by CRC.
“Far too many people in the U.S. are not receiving this lifesaving preventive service,” USPSTF vice chair Michael Barry, MD, said in a statement.
“We hope that this new recommendation to screen people ages 45-49, coupled with our long-standing recommendation to screen people 50-75, will prevent more people from dying from colorectal cancer,” he added.
Dr. Ng echoed this sentiment in her editorial: “The USPSTF recommendation for beginning colorectal cancer screening for average-risk adults at age 45 years has moved the field one step forward and indicates that ‘45 is the new 50,’ ” she observed.
“Lowering the recommended age to initiate screening will make colorectal cancer screening available to millions more people in the United States and, hopefully, many more lives will be saved by catching colorectal cancer earlier as well as by preventing colorectal cancer,” Dr. Ng affirmed.
“AGA fully supports the decision of the U.S. Preventive Services Task Force to reduce the age at which to initiate screening among individuals at average risk for development of colorectal cancer to 45 years,” said John Inadomi, MD, AGAF, AGA Institute President. “This decision harmonizes the recommendations between the major U.S. screening guidelines including the American Cancer Society and American College of Physicians. Furthermore, we expect the U.S. Multi-Society Task force, of which the AGA is a member, to also reduce the age at which we recommend initiation screening to 45 years. We expect this important change to save lives and improve the health of the U.S. population.” Read more at https://gastro.org/news/aga-gi-societies-support-lowering-crc-screening-age/.
All members of the USPSTF received travel reimbursement and an honorarium for participating in USPSTF meetings.
Dr. Ng reported receiving nonfinancial support from Pharmavite as well as grants from the Evergrande Group, Janssen, Revolution Medicines, Genentech, and Gilead Sciences. She has also reported receiving personal fees from Seattle Genetics, Array Biopharma, BiomX, and X-Biotix Therapeutics.
A version of this article first appeared on Medscape.com.
Screening for colorectal cancer (CRC) should now begin at the age of 45 and not 50 for average-risk individuals in the United States, notes the final recommendation from the U.S. Preventive Services Task Force.
The recommendation finalizes draft guidelines issued in October 2020 and mandates insurance coverage to ensure equal access to CRC screening regardless of a patient’s insurance status.
The USPSTF’s final recommendations also now align with those of the American Cancer Society, which lowered the age for initiation of CRC screening to 45 years in 2018.
“New statistics project an alarming rise in the incidence of young-onset colorectal cancer, projected to be the leading cause of cancer death in patients aged 20-49 by 2040,” commented Kimmie Ng, MD, MPH, director, Young-Onset Colorectal Cancer Center, Dana-Farber Cancer Institute, Boston, and lead author of a JAMA editorial about the new guideline.
“We must take bold steps to translate the lowered age of beginning screening into meaningful decreases in CRC incidence and mortality,” she emphasized.
The USPSTF recommendations and substantial evidence supporting them were published online May 18, 2021, in JAMA.
Risk factors for CRC
As the USPSTF authors noted, age is one of the most important risk factors for CRC, with nearly 94% of all new cases of CRC occurring in adults 45 years of age and older. Justification for the lower age of CRC screening initiation was based on simulation models showing that initiation of screening at the age of 45 was associated with an estimated additional 22-27 life-years gained, compared with starting at the age of 50.
The USPSTF continues to recommend screening for CRC in all adults aged between 50 and 75 years, lowering the age for screening to 45 years in recognition of the fact that, in 2020, 11% of colon cancers and 15% of rectal cancers occurred in patients under the age of 50.
The USPSTF also continues to conclude that there is a “small net benefit” of screening for CRC in adults aged between 76 and 85 years who have been previously screened.
However, the decision to screen patients in this age group should be based on individual risk factors for CRC, a patient’s overall health status, and personal preference. Perhaps self-evidently, adults in this age group who have never been screened for CRC are more likely to benefit from CRC screening than those who have been previously screened.
Similar to the previous guidelines released in 2016, the updated USPSTF recommendations continue to offer a menu of screening strategies, although the frequency of screening for each of the screening strategies varies. Recommended screening strategies include:
- High-sensitivity guaiac fecal occult blood test or fecal immunochemical test (FIT) every year
- Stool DNA-FIT every 1-3 years
- CT colonography every 5 years
- every 5 years
- Flexible sigmoidoscopy every 10 years plus annual FIT
- screening every 10 years
“Based on the evidence, there are many tests available that can effectively screen for colorectal cancer and the right test is the one that gets done,” USPSTF member Martha Kubik, PhD, RN, said in a statement.
“To encourage screening and help patients select the best test for them, we urge primary care clinicians to talk about the pros and cons of the various recommended options with their patients,” she added.
An accompanying review of the effectiveness, accuracy, and potential harms of CRC screening methods underscores how different screening tests have different levels of evidence demonstrating their ability to detect cancer, precursor lesions, or both, as well as their ability to reduce mortality from cancer.
Eligible patients
Currently, fewer than 70% of eligible patients in the United States undergo CRC screening, Dr. Ng pointed out in the editorial. In addition, CRC disproportionately affects African American patients, who are about 20% more likely to get CRC and about 40% more likely to die from it, compared with other patient groups. Modeling studies published along with the USPSTF recommendations showed equal benefit for screening regardless of race and gender, underscoring the importance of screening adherence, especially in patient populations disproportionately affected by CRC.
“Far too many people in the U.S. are not receiving this lifesaving preventive service,” USPSTF vice chair Michael Barry, MD, said in a statement.
“We hope that this new recommendation to screen people ages 45-49, coupled with our long-standing recommendation to screen people 50-75, will prevent more people from dying from colorectal cancer,” he added.
Dr. Ng echoed this sentiment in her editorial: “The USPSTF recommendation for beginning colorectal cancer screening for average-risk adults at age 45 years has moved the field one step forward and indicates that ‘45 is the new 50,’ ” she observed.
“Lowering the recommended age to initiate screening will make colorectal cancer screening available to millions more people in the United States and, hopefully, many more lives will be saved by catching colorectal cancer earlier as well as by preventing colorectal cancer,” Dr. Ng affirmed.
“AGA fully supports the decision of the U.S. Preventive Services Task Force to reduce the age at which to initiate screening among individuals at average risk for development of colorectal cancer to 45 years,” said John Inadomi, MD, AGAF, AGA Institute President. “This decision harmonizes the recommendations between the major U.S. screening guidelines including the American Cancer Society and American College of Physicians. Furthermore, we expect the U.S. Multi-Society Task force, of which the AGA is a member, to also reduce the age at which we recommend initiation screening to 45 years. We expect this important change to save lives and improve the health of the U.S. population.” Read more at https://gastro.org/news/aga-gi-societies-support-lowering-crc-screening-age/.
All members of the USPSTF received travel reimbursement and an honorarium for participating in USPSTF meetings.
Dr. Ng reported receiving nonfinancial support from Pharmavite as well as grants from the Evergrande Group, Janssen, Revolution Medicines, Genentech, and Gilead Sciences. She has also reported receiving personal fees from Seattle Genetics, Array Biopharma, BiomX, and X-Biotix Therapeutics.
A version of this article first appeared on Medscape.com.
Screening for colorectal cancer (CRC) should now begin at the age of 45 and not 50 for average-risk individuals in the United States, notes the final recommendation from the U.S. Preventive Services Task Force.
The recommendation finalizes draft guidelines issued in October 2020 and mandates insurance coverage to ensure equal access to CRC screening regardless of a patient’s insurance status.
The USPSTF’s final recommendations also now align with those of the American Cancer Society, which lowered the age for initiation of CRC screening to 45 years in 2018.
“New statistics project an alarming rise in the incidence of young-onset colorectal cancer, projected to be the leading cause of cancer death in patients aged 20-49 by 2040,” commented Kimmie Ng, MD, MPH, director, Young-Onset Colorectal Cancer Center, Dana-Farber Cancer Institute, Boston, and lead author of a JAMA editorial about the new guideline.
“We must take bold steps to translate the lowered age of beginning screening into meaningful decreases in CRC incidence and mortality,” she emphasized.
The USPSTF recommendations and substantial evidence supporting them were published online May 18, 2021, in JAMA.
Risk factors for CRC
As the USPSTF authors noted, age is one of the most important risk factors for CRC, with nearly 94% of all new cases of CRC occurring in adults 45 years of age and older. Justification for the lower age of CRC screening initiation was based on simulation models showing that initiation of screening at the age of 45 was associated with an estimated additional 22-27 life-years gained, compared with starting at the age of 50.
The USPSTF continues to recommend screening for CRC in all adults aged between 50 and 75 years, lowering the age for screening to 45 years in recognition of the fact that, in 2020, 11% of colon cancers and 15% of rectal cancers occurred in patients under the age of 50.
The USPSTF also continues to conclude that there is a “small net benefit” of screening for CRC in adults aged between 76 and 85 years who have been previously screened.
However, the decision to screen patients in this age group should be based on individual risk factors for CRC, a patient’s overall health status, and personal preference. Perhaps self-evidently, adults in this age group who have never been screened for CRC are more likely to benefit from CRC screening than those who have been previously screened.
Similar to the previous guidelines released in 2016, the updated USPSTF recommendations continue to offer a menu of screening strategies, although the frequency of screening for each of the screening strategies varies. Recommended screening strategies include:
- High-sensitivity guaiac fecal occult blood test or fecal immunochemical test (FIT) every year
- Stool DNA-FIT every 1-3 years
- CT colonography every 5 years
- every 5 years
- Flexible sigmoidoscopy every 10 years plus annual FIT
- screening every 10 years
“Based on the evidence, there are many tests available that can effectively screen for colorectal cancer and the right test is the one that gets done,” USPSTF member Martha Kubik, PhD, RN, said in a statement.
“To encourage screening and help patients select the best test for them, we urge primary care clinicians to talk about the pros and cons of the various recommended options with their patients,” she added.
An accompanying review of the effectiveness, accuracy, and potential harms of CRC screening methods underscores how different screening tests have different levels of evidence demonstrating their ability to detect cancer, precursor lesions, or both, as well as their ability to reduce mortality from cancer.
Eligible patients
Currently, fewer than 70% of eligible patients in the United States undergo CRC screening, Dr. Ng pointed out in the editorial. In addition, CRC disproportionately affects African American patients, who are about 20% more likely to get CRC and about 40% more likely to die from it, compared with other patient groups. Modeling studies published along with the USPSTF recommendations showed equal benefit for screening regardless of race and gender, underscoring the importance of screening adherence, especially in patient populations disproportionately affected by CRC.
“Far too many people in the U.S. are not receiving this lifesaving preventive service,” USPSTF vice chair Michael Barry, MD, said in a statement.
“We hope that this new recommendation to screen people ages 45-49, coupled with our long-standing recommendation to screen people 50-75, will prevent more people from dying from colorectal cancer,” he added.
Dr. Ng echoed this sentiment in her editorial: “The USPSTF recommendation for beginning colorectal cancer screening for average-risk adults at age 45 years has moved the field one step forward and indicates that ‘45 is the new 50,’ ” she observed.
“Lowering the recommended age to initiate screening will make colorectal cancer screening available to millions more people in the United States and, hopefully, many more lives will be saved by catching colorectal cancer earlier as well as by preventing colorectal cancer,” Dr. Ng affirmed.
“AGA fully supports the decision of the U.S. Preventive Services Task Force to reduce the age at which to initiate screening among individuals at average risk for development of colorectal cancer to 45 years,” said John Inadomi, MD, AGAF, AGA Institute President. “This decision harmonizes the recommendations between the major U.S. screening guidelines including the American Cancer Society and American College of Physicians. Furthermore, we expect the U.S. Multi-Society Task force, of which the AGA is a member, to also reduce the age at which we recommend initiation screening to 45 years. We expect this important change to save lives and improve the health of the U.S. population.” Read more at https://gastro.org/news/aga-gi-societies-support-lowering-crc-screening-age/.
All members of the USPSTF received travel reimbursement and an honorarium for participating in USPSTF meetings.
Dr. Ng reported receiving nonfinancial support from Pharmavite as well as grants from the Evergrande Group, Janssen, Revolution Medicines, Genentech, and Gilead Sciences. She has also reported receiving personal fees from Seattle Genetics, Array Biopharma, BiomX, and X-Biotix Therapeutics.
A version of this article first appeared on Medscape.com.