User login
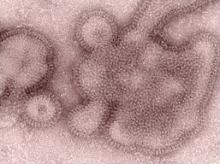
Influenza H3N2v: Efficacy Varies Among Rapid Detection Tests
The ability to detect the recently circulating influenza variant H3N2v was low in some commercially available rapid detection tests, according to an analysis conducted by the Centers for Disease Control and Prevention.
"The ability to detect H3N2v virus varied substantially among the tests. This evaluation emphasizes the fact that a negative [rapid influenza detection test (RIDT)] result should not be considered as conclusive evidence of lack of infection with influenza A (H3N2)v ... Results from RIDTs, both positive and negative, always should be interpreted in the broader context of the circulating influenza strains present in the area, level of clinical suspicion, severity of illness, and risk for complications in a patient with suspected infection," the CDC said Aug. 10 in an early online release from Morbidity and Mortality Weekly Report (2012;61:1-3).
The H3N2v viruses can be definitively identified only at qualified U.S. public health laboratories using a polymerase chain reaction–based influenza diagnostic panel that is not available as a point-of-care test for clinicians. Specimens that test positive for influenza A, H3, and pandemic influenza A markers and negative for H1 and pandemic H1 markers are called "presumptive positive for influenza A(H3N2)v virus," until confirmed as influenza A(H3N2)v, the CDC said.
The CDC analyzed seven Food and Drug Administration–cleared rapid influenza detection tests (RIDTs) for their ability to detect H3N2v viruses, of which 153 infections were reported from four states between July 12 and Aug. 9, 2012. Each of the seven RIDTs – the BinaxNOW, Directigen, FluAlert, QuickVue, Sofia, Xpect, and Veritor – was tested with seven different H3N2 viruses, according to their respective package instructions. Positive and negative controls contained in each RIDT were run before testing the viruses in the study to verify performance of each assay lot, with the exception of FluAlert, which does not provide controls.
Only four of the seven RIDTs (Directigen, Sofia, Veritor, and Xpect) detected all influenza A(H3N2)v viruses. BinaxNOW detected five of seven, and QuickVue detected three of seven. FluAlert detected only one of seven, the CDC said.
More data are needed on the clinical performance of all RIDTs in detecting H3N2v virus in various respiratory specimens, the CDC said.
Clinicians should minimize the occurrence of false RIDT results by strictly following the manufacturer’s instructions, collecting specimens soon after the onset of influenza-like illness – ideally within the first 72 hours – and confirming RIDT results by sending the specimen to a public health laboratory. Additional CDC guidance on interpretation of RIDTs for testing of patients with suspected H3N2v infection is available here.
No disclosure statement was listed.
The ability to detect the recently circulating influenza variant H3N2v was low in some commercially available rapid detection tests, according to an analysis conducted by the Centers for Disease Control and Prevention.
"The ability to detect H3N2v virus varied substantially among the tests. This evaluation emphasizes the fact that a negative [rapid influenza detection test (RIDT)] result should not be considered as conclusive evidence of lack of infection with influenza A (H3N2)v ... Results from RIDTs, both positive and negative, always should be interpreted in the broader context of the circulating influenza strains present in the area, level of clinical suspicion, severity of illness, and risk for complications in a patient with suspected infection," the CDC said Aug. 10 in an early online release from Morbidity and Mortality Weekly Report (2012;61:1-3).
The H3N2v viruses can be definitively identified only at qualified U.S. public health laboratories using a polymerase chain reaction–based influenza diagnostic panel that is not available as a point-of-care test for clinicians. Specimens that test positive for influenza A, H3, and pandemic influenza A markers and negative for H1 and pandemic H1 markers are called "presumptive positive for influenza A(H3N2)v virus," until confirmed as influenza A(H3N2)v, the CDC said.
The CDC analyzed seven Food and Drug Administration–cleared rapid influenza detection tests (RIDTs) for their ability to detect H3N2v viruses, of which 153 infections were reported from four states between July 12 and Aug. 9, 2012. Each of the seven RIDTs – the BinaxNOW, Directigen, FluAlert, QuickVue, Sofia, Xpect, and Veritor – was tested with seven different H3N2 viruses, according to their respective package instructions. Positive and negative controls contained in each RIDT were run before testing the viruses in the study to verify performance of each assay lot, with the exception of FluAlert, which does not provide controls.
Only four of the seven RIDTs (Directigen, Sofia, Veritor, and Xpect) detected all influenza A(H3N2)v viruses. BinaxNOW detected five of seven, and QuickVue detected three of seven. FluAlert detected only one of seven, the CDC said.
More data are needed on the clinical performance of all RIDTs in detecting H3N2v virus in various respiratory specimens, the CDC said.
Clinicians should minimize the occurrence of false RIDT results by strictly following the manufacturer’s instructions, collecting specimens soon after the onset of influenza-like illness – ideally within the first 72 hours – and confirming RIDT results by sending the specimen to a public health laboratory. Additional CDC guidance on interpretation of RIDTs for testing of patients with suspected H3N2v infection is available here.
No disclosure statement was listed.
The ability to detect the recently circulating influenza variant H3N2v was low in some commercially available rapid detection tests, according to an analysis conducted by the Centers for Disease Control and Prevention.
"The ability to detect H3N2v virus varied substantially among the tests. This evaluation emphasizes the fact that a negative [rapid influenza detection test (RIDT)] result should not be considered as conclusive evidence of lack of infection with influenza A (H3N2)v ... Results from RIDTs, both positive and negative, always should be interpreted in the broader context of the circulating influenza strains present in the area, level of clinical suspicion, severity of illness, and risk for complications in a patient with suspected infection," the CDC said Aug. 10 in an early online release from Morbidity and Mortality Weekly Report (2012;61:1-3).
The H3N2v viruses can be definitively identified only at qualified U.S. public health laboratories using a polymerase chain reaction–based influenza diagnostic panel that is not available as a point-of-care test for clinicians. Specimens that test positive for influenza A, H3, and pandemic influenza A markers and negative for H1 and pandemic H1 markers are called "presumptive positive for influenza A(H3N2)v virus," until confirmed as influenza A(H3N2)v, the CDC said.
The CDC analyzed seven Food and Drug Administration–cleared rapid influenza detection tests (RIDTs) for their ability to detect H3N2v viruses, of which 153 infections were reported from four states between July 12 and Aug. 9, 2012. Each of the seven RIDTs – the BinaxNOW, Directigen, FluAlert, QuickVue, Sofia, Xpect, and Veritor – was tested with seven different H3N2 viruses, according to their respective package instructions. Positive and negative controls contained in each RIDT were run before testing the viruses in the study to verify performance of each assay lot, with the exception of FluAlert, which does not provide controls.
Only four of the seven RIDTs (Directigen, Sofia, Veritor, and Xpect) detected all influenza A(H3N2)v viruses. BinaxNOW detected five of seven, and QuickVue detected three of seven. FluAlert detected only one of seven, the CDC said.
More data are needed on the clinical performance of all RIDTs in detecting H3N2v virus in various respiratory specimens, the CDC said.
Clinicians should minimize the occurrence of false RIDT results by strictly following the manufacturer’s instructions, collecting specimens soon after the onset of influenza-like illness – ideally within the first 72 hours – and confirming RIDT results by sending the specimen to a public health laboratory. Additional CDC guidance on interpretation of RIDTs for testing of patients with suspected H3N2v infection is available here.
No disclosure statement was listed.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
CDC Outlines Heterosexual HIV Pre-Exposure Prophylaxis
New federal guidelines outline steps to prevent infection in HIV-negative individuals at high risk of acquiring HIV through heterosexual sex.
The Centers for Disease Control and Prevention issued the interim recommendations pending the debut of a more complete set of HIV prevention guidelines both for heterosexuals and men who have sex with men (MSM).
The guidance comes on the heels of a new drug approval for pre-exposure prophylaxis (PrEP). In July, the Food and Drug Administration approved Truvada, the combination of tenofovir disoproxil fumarate plus emtricitabine (TDF/FTC), for PrEP in HIV-negative men and women who are at high risk for HIV acquisition through sexual intercourse.
Previous CDC guidance in January 2011 addressed PrEP in MSM (MMWR 2011;60:65-8).
The latest CDC document specifically addresses the use of PrEP in individuals who are at risk via heterosexual sex (MMWR 2012;61:586-9). The recommendations include new data about PrEP among heterosexual men and women (N. Engl. J. Med. 2012;367:399-434), as well as new information about pregnancy and other safety issues.
Both interim documents will remain valid until the CDC and other public health agencies complete a comprehensive set of HIV prevention recommendations on PrEP in both MSM and heterosexually active adults at high risk for HIV acquisition, the CDC said.
There are several new recommendations for initiating PrEP in individuals who are at very high risk for acquisition of HIV via penile-vaginal sex with a known HIV-positive partner:
• Prior to initiation: Confirm that the person is HIV-negative immediately before initiating TDF/FTC. Determine if the HIV-infected partner is receiving antiretroviral therapy, and if not, assist with a patient’s transition to care. Screen for hepatitis B and other sexually transmitted infections (STIs) and treat any that are detected. Check creatinine clearance, confirming that the calculated value is 60 mL/min or greater.
Determine if women are planning to become pregnant, are currently pregnant, or are breastfeeding. Women should be advised that PrEP safety during pregnancy has not been fully assessed, but that no harm has been reported. PrEP should not be prescribed for women who are breastfeeding.
• Beginning the PrEP regimen: Prescribe one Truvada tablet daily, with no more than a 90-day supply, renewable only after HIV testing confirms that patient remains HIV uninfected. For women, ensure that a pregnancy test is negative or, if pregnant, that the patient has been informed about PrEP use during pregnancy. Provide risk reduction and PrEP medication-adherence counseling and condoms.
• Follow-up during PrEP: Every 2-3 months, perform an HIV test and a pregnancy test for women, assess risk behaviors and STI symptoms, and test and treat if present. Counsel the individual regarding medication adherence, risk reduction, and condom use. Check serum creatinine and calculate creatinine clearance 3 months after initiation, then every 6 months while on PrEP medication. Test for bacterial STIs every 6 months, regardless of symptoms.
• Discontinuing PrEP: This may be necessary because of patient requests, safety concerns, or acquisition of HIV infection. If HIV status is unknown, a test should be performed. If a patient is HIV-positive, link the patient to HIV care. If active hepatitis B infection is diagnosed, consider continuing medication for that infection. If the individual is pregnant, communicate and coordinate with the prenatal-care provider about the patient’s PrEP use.
New federal guidelines outline steps to prevent infection in HIV-negative individuals at high risk of acquiring HIV through heterosexual sex.
The Centers for Disease Control and Prevention issued the interim recommendations pending the debut of a more complete set of HIV prevention guidelines both for heterosexuals and men who have sex with men (MSM).
The guidance comes on the heels of a new drug approval for pre-exposure prophylaxis (PrEP). In July, the Food and Drug Administration approved Truvada, the combination of tenofovir disoproxil fumarate plus emtricitabine (TDF/FTC), for PrEP in HIV-negative men and women who are at high risk for HIV acquisition through sexual intercourse.
Previous CDC guidance in January 2011 addressed PrEP in MSM (MMWR 2011;60:65-8).
The latest CDC document specifically addresses the use of PrEP in individuals who are at risk via heterosexual sex (MMWR 2012;61:586-9). The recommendations include new data about PrEP among heterosexual men and women (N. Engl. J. Med. 2012;367:399-434), as well as new information about pregnancy and other safety issues.
Both interim documents will remain valid until the CDC and other public health agencies complete a comprehensive set of HIV prevention recommendations on PrEP in both MSM and heterosexually active adults at high risk for HIV acquisition, the CDC said.
There are several new recommendations for initiating PrEP in individuals who are at very high risk for acquisition of HIV via penile-vaginal sex with a known HIV-positive partner:
• Prior to initiation: Confirm that the person is HIV-negative immediately before initiating TDF/FTC. Determine if the HIV-infected partner is receiving antiretroviral therapy, and if not, assist with a patient’s transition to care. Screen for hepatitis B and other sexually transmitted infections (STIs) and treat any that are detected. Check creatinine clearance, confirming that the calculated value is 60 mL/min or greater.
Determine if women are planning to become pregnant, are currently pregnant, or are breastfeeding. Women should be advised that PrEP safety during pregnancy has not been fully assessed, but that no harm has been reported. PrEP should not be prescribed for women who are breastfeeding.
• Beginning the PrEP regimen: Prescribe one Truvada tablet daily, with no more than a 90-day supply, renewable only after HIV testing confirms that patient remains HIV uninfected. For women, ensure that a pregnancy test is negative or, if pregnant, that the patient has been informed about PrEP use during pregnancy. Provide risk reduction and PrEP medication-adherence counseling and condoms.
• Follow-up during PrEP: Every 2-3 months, perform an HIV test and a pregnancy test for women, assess risk behaviors and STI symptoms, and test and treat if present. Counsel the individual regarding medication adherence, risk reduction, and condom use. Check serum creatinine and calculate creatinine clearance 3 months after initiation, then every 6 months while on PrEP medication. Test for bacterial STIs every 6 months, regardless of symptoms.
• Discontinuing PrEP: This may be necessary because of patient requests, safety concerns, or acquisition of HIV infection. If HIV status is unknown, a test should be performed. If a patient is HIV-positive, link the patient to HIV care. If active hepatitis B infection is diagnosed, consider continuing medication for that infection. If the individual is pregnant, communicate and coordinate with the prenatal-care provider about the patient’s PrEP use.
New federal guidelines outline steps to prevent infection in HIV-negative individuals at high risk of acquiring HIV through heterosexual sex.
The Centers for Disease Control and Prevention issued the interim recommendations pending the debut of a more complete set of HIV prevention guidelines both for heterosexuals and men who have sex with men (MSM).
The guidance comes on the heels of a new drug approval for pre-exposure prophylaxis (PrEP). In July, the Food and Drug Administration approved Truvada, the combination of tenofovir disoproxil fumarate plus emtricitabine (TDF/FTC), for PrEP in HIV-negative men and women who are at high risk for HIV acquisition through sexual intercourse.
Previous CDC guidance in January 2011 addressed PrEP in MSM (MMWR 2011;60:65-8).
The latest CDC document specifically addresses the use of PrEP in individuals who are at risk via heterosexual sex (MMWR 2012;61:586-9). The recommendations include new data about PrEP among heterosexual men and women (N. Engl. J. Med. 2012;367:399-434), as well as new information about pregnancy and other safety issues.
Both interim documents will remain valid until the CDC and other public health agencies complete a comprehensive set of HIV prevention recommendations on PrEP in both MSM and heterosexually active adults at high risk for HIV acquisition, the CDC said.
There are several new recommendations for initiating PrEP in individuals who are at very high risk for acquisition of HIV via penile-vaginal sex with a known HIV-positive partner:
• Prior to initiation: Confirm that the person is HIV-negative immediately before initiating TDF/FTC. Determine if the HIV-infected partner is receiving antiretroviral therapy, and if not, assist with a patient’s transition to care. Screen for hepatitis B and other sexually transmitted infections (STIs) and treat any that are detected. Check creatinine clearance, confirming that the calculated value is 60 mL/min or greater.
Determine if women are planning to become pregnant, are currently pregnant, or are breastfeeding. Women should be advised that PrEP safety during pregnancy has not been fully assessed, but that no harm has been reported. PrEP should not be prescribed for women who are breastfeeding.
• Beginning the PrEP regimen: Prescribe one Truvada tablet daily, with no more than a 90-day supply, renewable only after HIV testing confirms that patient remains HIV uninfected. For women, ensure that a pregnancy test is negative or, if pregnant, that the patient has been informed about PrEP use during pregnancy. Provide risk reduction and PrEP medication-adherence counseling and condoms.
• Follow-up during PrEP: Every 2-3 months, perform an HIV test and a pregnancy test for women, assess risk behaviors and STI symptoms, and test and treat if present. Counsel the individual regarding medication adherence, risk reduction, and condom use. Check serum creatinine and calculate creatinine clearance 3 months after initiation, then every 6 months while on PrEP medication. Test for bacterial STIs every 6 months, regardless of symptoms.
• Discontinuing PrEP: This may be necessary because of patient requests, safety concerns, or acquisition of HIV infection. If HIV status is unknown, a test should be performed. If a patient is HIV-positive, link the patient to HIV care. If active hepatitis B infection is diagnosed, consider continuing medication for that infection. If the individual is pregnant, communicate and coordinate with the prenatal-care provider about the patient’s PrEP use.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Glargine, Liraglutide Neck and Neck, Except on Cost
PHILADELPHIA (IMNG) – Daily glargine use among patients with type 2 diabetes produced similar clinical outcomes to those of patients using liraglutide, but with lower associated costs, according to a retrospective analysis of real-world use that was presented at the annual scientific sessions of the American Diabetes Association.
When liraglutide or glargine was added to a combined metformin-plus-sulfonylurea therapy in a previous phase III trial, liraglutide demonstrated a greater reduction in hemoglobin A1c then did glargine (Diabetologia 2009;52:2046-55).
To date, however, no real-world comparative data have been published comparing use of injectable therapy with insulin glargine vs. the glucagonlike peptide–1 agonist liraglutide among patients with type 2 diabetes, said Dr. Philip Levin, director of the diabetes center at Mercy Medical Center, Baltimore.
Administrative claims data were analyzed from a managed care database comprising about 50 U.S. health care plans and 107 million patients. Of those, investigators identified 967 adults who had type 2 diabetes and had initiated injectable pen therapy with either glargine (n = 557) or liraglutide (n = 410) between January and June 2010. All patients who had baseline HbA1c levels less than 7% were excluded, because it’s possible that they were started on liraglutide for weight loss rather than glucose control, Dr. Levin noted.
Because there were significant differences between the two groups at baseline – glargine initiators were older, sicker, less obese, and more likely to be male, and had higher HbA1c levels, among other differences – the 336 eligible patients were propensity matched to make the two groups more comparable. After that was done, the 168 glargine and 168 liraglutide patients did not differ significantly in sex (46% of glargine and 44% of liraglutide patients were women), age (53 years for both groups), mean Charlson comorbidity index (0.29 and 0.36, respectively), or other baseline characteristics.
The group taking glargine stayed on therapy significantly longer than did those on liraglutide (279 vs. 257 day).
Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups, decreasing from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide (reductions of 1.02 and 0.95 percentage points, respectively). The average dose for glargine was 27.4 U/day, and for liraglutide was 1.16 mg/day.
Hypoglycemia was more common with glargine (7.7% vs. 4.7%), but this difference did not reach statistical significance. Hypoglycemia requiring emergency department or inpatient treatment occurred in just 1.1% of both groups.
Health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976. Each of those differences was statistically significant, Dr. Levin said.
These findings will be further explored by the planned INITIATOR study, a large-scale hybrid prospective/retrospective real-world study, he noted.
The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
PHILADELPHIA (IMNG) – Daily glargine use among patients with type 2 diabetes produced similar clinical outcomes to those of patients using liraglutide, but with lower associated costs, according to a retrospective analysis of real-world use that was presented at the annual scientific sessions of the American Diabetes Association.
When liraglutide or glargine was added to a combined metformin-plus-sulfonylurea therapy in a previous phase III trial, liraglutide demonstrated a greater reduction in hemoglobin A1c then did glargine (Diabetologia 2009;52:2046-55).
To date, however, no real-world comparative data have been published comparing use of injectable therapy with insulin glargine vs. the glucagonlike peptide–1 agonist liraglutide among patients with type 2 diabetes, said Dr. Philip Levin, director of the diabetes center at Mercy Medical Center, Baltimore.
Administrative claims data were analyzed from a managed care database comprising about 50 U.S. health care plans and 107 million patients. Of those, investigators identified 967 adults who had type 2 diabetes and had initiated injectable pen therapy with either glargine (n = 557) or liraglutide (n = 410) between January and June 2010. All patients who had baseline HbA1c levels less than 7% were excluded, because it’s possible that they were started on liraglutide for weight loss rather than glucose control, Dr. Levin noted.
Because there were significant differences between the two groups at baseline – glargine initiators were older, sicker, less obese, and more likely to be male, and had higher HbA1c levels, among other differences – the 336 eligible patients were propensity matched to make the two groups more comparable. After that was done, the 168 glargine and 168 liraglutide patients did not differ significantly in sex (46% of glargine and 44% of liraglutide patients were women), age (53 years for both groups), mean Charlson comorbidity index (0.29 and 0.36, respectively), or other baseline characteristics.
The group taking glargine stayed on therapy significantly longer than did those on liraglutide (279 vs. 257 day).
Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups, decreasing from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide (reductions of 1.02 and 0.95 percentage points, respectively). The average dose for glargine was 27.4 U/day, and for liraglutide was 1.16 mg/day.
Hypoglycemia was more common with glargine (7.7% vs. 4.7%), but this difference did not reach statistical significance. Hypoglycemia requiring emergency department or inpatient treatment occurred in just 1.1% of both groups.
Health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976. Each of those differences was statistically significant, Dr. Levin said.
These findings will be further explored by the planned INITIATOR study, a large-scale hybrid prospective/retrospective real-world study, he noted.
The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
PHILADELPHIA (IMNG) – Daily glargine use among patients with type 2 diabetes produced similar clinical outcomes to those of patients using liraglutide, but with lower associated costs, according to a retrospective analysis of real-world use that was presented at the annual scientific sessions of the American Diabetes Association.
When liraglutide or glargine was added to a combined metformin-plus-sulfonylurea therapy in a previous phase III trial, liraglutide demonstrated a greater reduction in hemoglobin A1c then did glargine (Diabetologia 2009;52:2046-55).
To date, however, no real-world comparative data have been published comparing use of injectable therapy with insulin glargine vs. the glucagonlike peptide–1 agonist liraglutide among patients with type 2 diabetes, said Dr. Philip Levin, director of the diabetes center at Mercy Medical Center, Baltimore.
Administrative claims data were analyzed from a managed care database comprising about 50 U.S. health care plans and 107 million patients. Of those, investigators identified 967 adults who had type 2 diabetes and had initiated injectable pen therapy with either glargine (n = 557) or liraglutide (n = 410) between January and June 2010. All patients who had baseline HbA1c levels less than 7% were excluded, because it’s possible that they were started on liraglutide for weight loss rather than glucose control, Dr. Levin noted.
Because there were significant differences between the two groups at baseline – glargine initiators were older, sicker, less obese, and more likely to be male, and had higher HbA1c levels, among other differences – the 336 eligible patients were propensity matched to make the two groups more comparable. After that was done, the 168 glargine and 168 liraglutide patients did not differ significantly in sex (46% of glargine and 44% of liraglutide patients were women), age (53 years for both groups), mean Charlson comorbidity index (0.29 and 0.36, respectively), or other baseline characteristics.
The group taking glargine stayed on therapy significantly longer than did those on liraglutide (279 vs. 257 day).
Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups, decreasing from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide (reductions of 1.02 and 0.95 percentage points, respectively). The average dose for glargine was 27.4 U/day, and for liraglutide was 1.16 mg/day.
Hypoglycemia was more common with glargine (7.7% vs. 4.7%), but this difference did not reach statistical significance. Hypoglycemia requiring emergency department or inpatient treatment occurred in just 1.1% of both groups.
Health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976. Each of those differences was statistically significant, Dr. Levin said.
These findings will be further explored by the planned INITIATOR study, a large-scale hybrid prospective/retrospective real-world study, he noted.
The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups (from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide). However, health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976.
Data Source: The findings come from a retrospective database analysis of real-world use of glargine and liraglutide in 336 patients with type 2 diabetes.
Disclosures: The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
Glargine, Liraglutide Neck and Neck, Except on Cost
PHILADELPHIA – Daily glargine use among patients with type 2 diabetes produced similar clinical outcomes to those of patients using liraglutide, but with lower associated costs, according to a retrospective analysis of real-world use that was presented at the annual scientific sessions of the American Diabetes Association.
When liraglutide or glargine was added to a combined metformin-plus-sulfonylurea therapy in a previous phase III trial, liraglutide demonstrated a greater reduction in hemoglobin A1c than did glargine (Diabetologia 2009;52:2046-55).
To date, however, no real-world comparative data have been published comparing use of injectable therapy with insulin glargine vs. the glucagonlike peptide–1 agonist liraglutide among patients with type 2 diabetes, said Dr. Philip Levin, director of the diabetes center at Mercy Medical Center, Baltimore.
Administrative claims data were analyzed from a managed care database comprising about 50 U.S. health care plans and 107 million patients. Of those, investigators identified 967 adults who had type 2 diabetes and had initiated injectable pen therapy with either glargine (n = 557) or liraglutide (n = 410) between January and June 2010. All patients who had baseline HbA1c levels less than 7% were excluded, because it’s possible that they were started on liraglutide for weight loss rather than glucose control, Dr. Levin noted.
Because there were significant differences between the two groups at baseline – glargine initiators were older, sicker, less obese, and more likely to be male, and had higher HbA1c levels, among other differences – the 336 eligible patients were propensity matched to make the two groups more comparable. After that was done, the 168 glargine and 168 liraglutide patients did not differ significantly in sex (46% of glargine and 44% of liraglutide patients were women), age (53 years for both groups), mean Charlson comorbidity index (0.29 and 0.36, respectively), or other baseline characteristics.
The group taking glargine stayed on therapy significantly longer than did those on liraglutide (279 vs. 257 day).
Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups, decreasing from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide (reductions of 1.02 and 0.95 percentage points, respectively). The average dose for glargine was 27.4 U/day, and for liraglutide was 1.16 mg/day.
Hypoglycemia was more common with glargine (7.7% vs. 4.7%), but this difference did not reach statistical significance. Hypoglycemia requiring emergency department or inpatient treatment occurred in just 1.1% of both groups.
Health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976. Each of those differences was statistically significant, Dr. Levin said.
These findings will be further explored by the planned INITIATOR study, a large-scale hybrid prospective/retrospective real-world study, he noted.
The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
PHILADELPHIA – Daily glargine use among patients with type 2 diabetes produced similar clinical outcomes to those of patients using liraglutide, but with lower associated costs, according to a retrospective analysis of real-world use that was presented at the annual scientific sessions of the American Diabetes Association.
When liraglutide or glargine was added to a combined metformin-plus-sulfonylurea therapy in a previous phase III trial, liraglutide demonstrated a greater reduction in hemoglobin A1c than did glargine (Diabetologia 2009;52:2046-55).
To date, however, no real-world comparative data have been published comparing use of injectable therapy with insulin glargine vs. the glucagonlike peptide–1 agonist liraglutide among patients with type 2 diabetes, said Dr. Philip Levin, director of the diabetes center at Mercy Medical Center, Baltimore.
Administrative claims data were analyzed from a managed care database comprising about 50 U.S. health care plans and 107 million patients. Of those, investigators identified 967 adults who had type 2 diabetes and had initiated injectable pen therapy with either glargine (n = 557) or liraglutide (n = 410) between January and June 2010. All patients who had baseline HbA1c levels less than 7% were excluded, because it’s possible that they were started on liraglutide for weight loss rather than glucose control, Dr. Levin noted.
Because there were significant differences between the two groups at baseline – glargine initiators were older, sicker, less obese, and more likely to be male, and had higher HbA1c levels, among other differences – the 336 eligible patients were propensity matched to make the two groups more comparable. After that was done, the 168 glargine and 168 liraglutide patients did not differ significantly in sex (46% of glargine and 44% of liraglutide patients were women), age (53 years for both groups), mean Charlson comorbidity index (0.29 and 0.36, respectively), or other baseline characteristics.
The group taking glargine stayed on therapy significantly longer than did those on liraglutide (279 vs. 257 day).
Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups, decreasing from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide (reductions of 1.02 and 0.95 percentage points, respectively). The average dose for glargine was 27.4 U/day, and for liraglutide was 1.16 mg/day.
Hypoglycemia was more common with glargine (7.7% vs. 4.7%), but this difference did not reach statistical significance. Hypoglycemia requiring emergency department or inpatient treatment occurred in just 1.1% of both groups.
Health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976. Each of those differences was statistically significant, Dr. Levin said.
These findings will be further explored by the planned INITIATOR study, a large-scale hybrid prospective/retrospective real-world study, he noted.
The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
PHILADELPHIA – Daily glargine use among patients with type 2 diabetes produced similar clinical outcomes to those of patients using liraglutide, but with lower associated costs, according to a retrospective analysis of real-world use that was presented at the annual scientific sessions of the American Diabetes Association.
When liraglutide or glargine was added to a combined metformin-plus-sulfonylurea therapy in a previous phase III trial, liraglutide demonstrated a greater reduction in hemoglobin A1c than did glargine (Diabetologia 2009;52:2046-55).
To date, however, no real-world comparative data have been published comparing use of injectable therapy with insulin glargine vs. the glucagonlike peptide–1 agonist liraglutide among patients with type 2 diabetes, said Dr. Philip Levin, director of the diabetes center at Mercy Medical Center, Baltimore.
Administrative claims data were analyzed from a managed care database comprising about 50 U.S. health care plans and 107 million patients. Of those, investigators identified 967 adults who had type 2 diabetes and had initiated injectable pen therapy with either glargine (n = 557) or liraglutide (n = 410) between January and June 2010. All patients who had baseline HbA1c levels less than 7% were excluded, because it’s possible that they were started on liraglutide for weight loss rather than glucose control, Dr. Levin noted.
Because there were significant differences between the two groups at baseline – glargine initiators were older, sicker, less obese, and more likely to be male, and had higher HbA1c levels, among other differences – the 336 eligible patients were propensity matched to make the two groups more comparable. After that was done, the 168 glargine and 168 liraglutide patients did not differ significantly in sex (46% of glargine and 44% of liraglutide patients were women), age (53 years for both groups), mean Charlson comorbidity index (0.29 and 0.36, respectively), or other baseline characteristics.
The group taking glargine stayed on therapy significantly longer than did those on liraglutide (279 vs. 257 day).
Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups, decreasing from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide (reductions of 1.02 and 0.95 percentage points, respectively). The average dose for glargine was 27.4 U/day, and for liraglutide was 1.16 mg/day.
Hypoglycemia was more common with glargine (7.7% vs. 4.7%), but this difference did not reach statistical significance. Hypoglycemia requiring emergency department or inpatient treatment occurred in just 1.1% of both groups.
Health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976. Each of those differences was statistically significant, Dr. Levin said.
These findings will be further explored by the planned INITIATOR study, a large-scale hybrid prospective/retrospective real-world study, he noted.
The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: Reductions in HbA1c from baseline did not differ significantly between the glargine and liraglutide groups (from 8.96% to 7.94% at 1 year with glargine, and from 8.76% to 7.81% with liraglutide). However, health care costs were significantly lower with glargine. Study drug costs were $1,198 for glargine vs. $2,784 for liraglutide; diabetes-related drug costs were $2,958 vs. $3,988, respectively; and total diabetes-related costs were $5,653 vs. $7,976.
Data Source: The findings come from a retrospective database analysis of real-world use of glargine and liraglutide in 336 patients with type 2 diabetes.
Disclosures: The study was funded by Sanofi-Aventis. Dr. Levin disclosed that he is on the advisory panel for that company, as well as for Novo Nordisk, the maker of liraglutide. He also consults for, receives research support from, or is on the speakers bureau for a long list of other manufacturers of diabetes-related products.
Experimental Peptide Preserves Beta-Cells in Type 1 Diabetes
PHILADELPHIA – An investigational peptide successfully preserved beta-cell function at 2 years in a randomized, double-blind, placebo-controlled phase III trial that enrolled more than 450 patients with newly diagnosed type 1 diabetes.
DiaPep277, a synthetic 24 amino acid peptide derived from human heat shock protein 60, modulates the immune response that leads to autoimmune diabetes by diminishing or blocking the immunological destruction of beta cells. In May 2012, the Food and Drug Administration granted DiaPep277 an Orphan Drug designation for the treatment of type 1 diabetes patients with residual beta cell function, according to statement from Andromeda Biotech, the drug’s developer.
Potential target populations include newly diagnosed adult patients, type 1 diabetic children, people with a high risk of developing type 1 diabetes, and type 1 diabetes patients with slow-progressing disease. Potential benefits include prevention of disease deterioration, improved glycemic control, reduction of daily insulin dose requirements, and delay or reduction of diabetes complications, the company statement said.
Data from the first of two phase III studies – conducted at 40 centers in Europe, Israel, and South Africa – were presented by Dr. Paolo Pozzilli, professor of endocrinology at Università Campus Bio-Medico, Rome. Inclusion criteria for the study were age 16-45 years, no more than 3 months since type 1 diabetes diagnosis, fasting C-peptide greater than 0.2 nmol/L, and positive islet autoantibodies. Subcutaneous injections of DiaPep277 or placebo were given every 3 months. Of the 457 initially randomized, 175 received all nine DiaPep277 injections and 174 received the total of nine placebo injections over the entire 24 month study period.
The primary efficacy end point – change from baseline to end of study in stimulated C-peptide area under curve secretion measured by the glucagon stimulated test – was met. There was significant preservation of C-peptide levels compared to placebo, with a relative change of 23% (P = .037) for all the randomized patients, and an even more significant preservation for the patients who completed 2 years of therapy in full compliance with the study protocol, with a 29% relative change (P = .011).
A secondary end point, the proportion of patients maintaining/achieving a hemoglobin A1c of 7% or less, also was met, with 56% of DiaPep277 patients vs. 44% of those on placebo achieving that goal in the per-protocol evaluation (P = .035). Patients on lower doses of insulin were more likely to achieve an HbA1c of 7% or less, Dr. Pozzilli noted.
Another secondary end point, change from baseline in C-peptide using a mixed-meal tolerance test, was not met, he added.
There were no significant differences between the two groups in those who reported one or more adverse event of any kind (77% for DiaPep277 vs. 71% for placebo), serious adverse events (12% vs. 6%, respectively) or adverse events believed to be drug related (1.3% vs. 0.4%). Hypoglycemia was significantly less frequent in the DiaPep277 group (P = .04).
"We can conclude there is a significant treatment effect without adverse events ... Hopefully we can have something to offer patients with type 1 diabetes at diagnosis," he concluded.
A second large phase III clinical trial of DiaPep277 in newly diagnosed type 1 patients is underway. Results are expected in 2015, he said.
Dr. Pozzilli has consulted for Sanofi, Eli Lilly, Andromeda-Biotech, Roche Diagnostics, Novartis, and Medtronic. Andromeda-Biotech licensed the peptide from Yeda Research & Development Company, the commercial arm of the Weizmann Institute of Science, Rehovot, Israel.
PHILADELPHIA – An investigational peptide successfully preserved beta-cell function at 2 years in a randomized, double-blind, placebo-controlled phase III trial that enrolled more than 450 patients with newly diagnosed type 1 diabetes.
DiaPep277, a synthetic 24 amino acid peptide derived from human heat shock protein 60, modulates the immune response that leads to autoimmune diabetes by diminishing or blocking the immunological destruction of beta cells. In May 2012, the Food and Drug Administration granted DiaPep277 an Orphan Drug designation for the treatment of type 1 diabetes patients with residual beta cell function, according to statement from Andromeda Biotech, the drug’s developer.
Potential target populations include newly diagnosed adult patients, type 1 diabetic children, people with a high risk of developing type 1 diabetes, and type 1 diabetes patients with slow-progressing disease. Potential benefits include prevention of disease deterioration, improved glycemic control, reduction of daily insulin dose requirements, and delay or reduction of diabetes complications, the company statement said.
Data from the first of two phase III studies – conducted at 40 centers in Europe, Israel, and South Africa – were presented by Dr. Paolo Pozzilli, professor of endocrinology at Università Campus Bio-Medico, Rome. Inclusion criteria for the study were age 16-45 years, no more than 3 months since type 1 diabetes diagnosis, fasting C-peptide greater than 0.2 nmol/L, and positive islet autoantibodies. Subcutaneous injections of DiaPep277 or placebo were given every 3 months. Of the 457 initially randomized, 175 received all nine DiaPep277 injections and 174 received the total of nine placebo injections over the entire 24 month study period.
The primary efficacy end point – change from baseline to end of study in stimulated C-peptide area under curve secretion measured by the glucagon stimulated test – was met. There was significant preservation of C-peptide levels compared to placebo, with a relative change of 23% (P = .037) for all the randomized patients, and an even more significant preservation for the patients who completed 2 years of therapy in full compliance with the study protocol, with a 29% relative change (P = .011).
A secondary end point, the proportion of patients maintaining/achieving a hemoglobin A1c of 7% or less, also was met, with 56% of DiaPep277 patients vs. 44% of those on placebo achieving that goal in the per-protocol evaluation (P = .035). Patients on lower doses of insulin were more likely to achieve an HbA1c of 7% or less, Dr. Pozzilli noted.
Another secondary end point, change from baseline in C-peptide using a mixed-meal tolerance test, was not met, he added.
There were no significant differences between the two groups in those who reported one or more adverse event of any kind (77% for DiaPep277 vs. 71% for placebo), serious adverse events (12% vs. 6%, respectively) or adverse events believed to be drug related (1.3% vs. 0.4%). Hypoglycemia was significantly less frequent in the DiaPep277 group (P = .04).
"We can conclude there is a significant treatment effect without adverse events ... Hopefully we can have something to offer patients with type 1 diabetes at diagnosis," he concluded.
A second large phase III clinical trial of DiaPep277 in newly diagnosed type 1 patients is underway. Results are expected in 2015, he said.
Dr. Pozzilli has consulted for Sanofi, Eli Lilly, Andromeda-Biotech, Roche Diagnostics, Novartis, and Medtronic. Andromeda-Biotech licensed the peptide from Yeda Research & Development Company, the commercial arm of the Weizmann Institute of Science, Rehovot, Israel.
PHILADELPHIA – An investigational peptide successfully preserved beta-cell function at 2 years in a randomized, double-blind, placebo-controlled phase III trial that enrolled more than 450 patients with newly diagnosed type 1 diabetes.
DiaPep277, a synthetic 24 amino acid peptide derived from human heat shock protein 60, modulates the immune response that leads to autoimmune diabetes by diminishing or blocking the immunological destruction of beta cells. In May 2012, the Food and Drug Administration granted DiaPep277 an Orphan Drug designation for the treatment of type 1 diabetes patients with residual beta cell function, according to statement from Andromeda Biotech, the drug’s developer.
Potential target populations include newly diagnosed adult patients, type 1 diabetic children, people with a high risk of developing type 1 diabetes, and type 1 diabetes patients with slow-progressing disease. Potential benefits include prevention of disease deterioration, improved glycemic control, reduction of daily insulin dose requirements, and delay or reduction of diabetes complications, the company statement said.
Data from the first of two phase III studies – conducted at 40 centers in Europe, Israel, and South Africa – were presented by Dr. Paolo Pozzilli, professor of endocrinology at Università Campus Bio-Medico, Rome. Inclusion criteria for the study were age 16-45 years, no more than 3 months since type 1 diabetes diagnosis, fasting C-peptide greater than 0.2 nmol/L, and positive islet autoantibodies. Subcutaneous injections of DiaPep277 or placebo were given every 3 months. Of the 457 initially randomized, 175 received all nine DiaPep277 injections and 174 received the total of nine placebo injections over the entire 24 month study period.
The primary efficacy end point – change from baseline to end of study in stimulated C-peptide area under curve secretion measured by the glucagon stimulated test – was met. There was significant preservation of C-peptide levels compared to placebo, with a relative change of 23% (P = .037) for all the randomized patients, and an even more significant preservation for the patients who completed 2 years of therapy in full compliance with the study protocol, with a 29% relative change (P = .011).
A secondary end point, the proportion of patients maintaining/achieving a hemoglobin A1c of 7% or less, also was met, with 56% of DiaPep277 patients vs. 44% of those on placebo achieving that goal in the per-protocol evaluation (P = .035). Patients on lower doses of insulin were more likely to achieve an HbA1c of 7% or less, Dr. Pozzilli noted.
Another secondary end point, change from baseline in C-peptide using a mixed-meal tolerance test, was not met, he added.
There were no significant differences between the two groups in those who reported one or more adverse event of any kind (77% for DiaPep277 vs. 71% for placebo), serious adverse events (12% vs. 6%, respectively) or adverse events believed to be drug related (1.3% vs. 0.4%). Hypoglycemia was significantly less frequent in the DiaPep277 group (P = .04).
"We can conclude there is a significant treatment effect without adverse events ... Hopefully we can have something to offer patients with type 1 diabetes at diagnosis," he concluded.
A second large phase III clinical trial of DiaPep277 in newly diagnosed type 1 patients is underway. Results are expected in 2015, he said.
Dr. Pozzilli has consulted for Sanofi, Eli Lilly, Andromeda-Biotech, Roche Diagnostics, Novartis, and Medtronic. Andromeda-Biotech licensed the peptide from Yeda Research & Development Company, the commercial arm of the Weizmann Institute of Science, Rehovot, Israel.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: There was significant preservation of C-peptide levels, compared with placebo, with a relative change of 23% (P = .037) for all the randomized patients and an even more significant preservation for the patients who completed 2 years of therapy in full compliance with the study protocol, with a 29% relative change (P = .011).
Data Source: The data come from a randomized, controlled, double-blind phase III trial that enrolled 457 patients with newly diagnosed type 1 diabetes.
Disclosures: The study was funded by Andromeda Biotech. Dr. Pozzilli has consulted for that company, as well as for Sanofi, Eli Lilly, Roche Diagnostics, Novartis, and Medtronic.
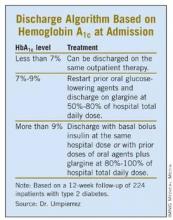
Admission HbA1c Aids Discharge Planning
PHILADELPHIA – An algorithm based on hemoglobin A1c levels at admission was safe and effective in guiding diabetes therapy after discharge, based on a prospective, randomized pilot study of 224 inpatients with type 2 diabetes.
Average HbA1c dropped from 8.4% on admission to 7.9% at 4 weeks and to 7.3% at 12 weeks after discharge, said Dr. Guillermo E. Umpierrez, professor of medicine at Emory University, and chief of diabetes and endocrinology at Grady Memorial Hospital, Atlanta.
"If we can do this at Grady Hospital, a county hospital in downtown Atlanta, I think most of you can do it in your facilities. We see these patients within 2 weeks of discharge. I think frequent followup and education are key to [the success of] this type of program," Dr. Umpierrez said.
The patients were placed into one of three post-discharge treatment groups based on their HbA1c levels at admission. Patients with admission HbA1c values of less than 7% resumed their previous outpatient treatments at discharge. Those with HbA1c values of 7%-9% resumed their outpatient oral agents at discharge along with once-daily glargine at 50%-80% of the hospital dose. Patients admitted with a HbA1c level above 9% were discharged on their oral agents plus glargine at 80%-100% of the hospital dose or with the same basal and bolus insulin doses as they’d been given in the hospital.
The treatment goal after discharge was a blood glucose range of 70-130 mg/dL (fasting and premeal) and a HbA1c value below 7%, Dr. Umpierrez said.
For the group discharged on oral agents alone, average HbA1c fell from 6.9% at admission to 6.6% at 12 week follow-up. For those discharged on oral agents plus glargine, average HbA1c fell from 9.2% at admission to 7.5% at 12 weeks. For those discharged on glargine plus glulisine, average HbA1c fell from 11.1% to 8.0% at 12 weeks, Dr. Umpierrez reported.
Hypoglycemia with blood glucose levels below 70 mg/dL occurred post-discharge in 22% of those discharged on oral agents, 30% on oral agents plus glargine, and 44% on glargine plus glulisine. However, hypoglycemia with blood glucose values less than 40 mg/dl occurred in 3% of all patients and in 6% of the group on glargine plus glulisine.
The 224 study patients were a subset of the 375 participants in Sanofi-Aventis’ multicenter "Basal Plus" trial. That study of medical and surgical inpatients with previously diagnosed type 2 diabetes compared the efficacy and safety of a daily dose of glargine plus corrective doses of glulisine ("basal plus") to basal-bolus insulin and sliding scale regular insulin regimens.
At admission, the mean blood glucose level was 204 mg/dL (range 140-400 mg/dL), and the mean admission HbA1c was 8.4%. A total of 150 patients were randomized to the basal-bolus regimen, 148 to "basal plus" regimen, and 77 to the sliding scale insulin regimen.
During hospitalization, patients in the basal-bolus group were started at 0.5 U/kg, given half as glargine once daily and half as glulisine before meals. The "basal plus" group received 0.25 U/kg of glargine once daily plus correction doses of glulisine before meals for blood glucose values above 140 mg/dL. Sliding scale insulin was given four times a day for blood glucose values above 140 mg/dL.
Results from the in-hospital patients in the basal plus trial were reported by Dr. Umpierrez and his fellow researchers in a poster. The basal plus and basal bolus regimens resulted in similar glycemic control, with worse outcomes for those on sliding scale insulin. Mean daily blood glucose values after day 1 were 172 mg/dL for sliding scale insulin, 156 mg/dL for basal bolus, and 163 mg/dL for basal plus. Treatment failures, defined as more than two consecutive blood glucose values or a mean daily glucose value greater than 240 mg/dL, occurred in 19% on sliding scale insulin and in 2% on the basal plus regimen (P less than .001). Glucose values of less than 70 mg/dL occurred in 16% of inpatients and in 1.7% of blood glucose readings overall. Glucose levels of less than 40 mg/dL occurred in 1% of the basal bolus and the basal plus groups, and in none of the inpatients on sliding scale insulin.
There were no differences in length of stay or complications including wound infections, pneumonia, respiratory or renal failure and bacteremia between groups, the investigators reported.
This study was funded by Sanofi-Aventis. Dr. Umpierrez disclosed that he has also received research support from Merck.
PHILADELPHIA – An algorithm based on hemoglobin A1c levels at admission was safe and effective in guiding diabetes therapy after discharge, based on a prospective, randomized pilot study of 224 inpatients with type 2 diabetes.
Average HbA1c dropped from 8.4% on admission to 7.9% at 4 weeks and to 7.3% at 12 weeks after discharge, said Dr. Guillermo E. Umpierrez, professor of medicine at Emory University, and chief of diabetes and endocrinology at Grady Memorial Hospital, Atlanta.
"If we can do this at Grady Hospital, a county hospital in downtown Atlanta, I think most of you can do it in your facilities. We see these patients within 2 weeks of discharge. I think frequent followup and education are key to [the success of] this type of program," Dr. Umpierrez said.
The patients were placed into one of three post-discharge treatment groups based on their HbA1c levels at admission. Patients with admission HbA1c values of less than 7% resumed their previous outpatient treatments at discharge. Those with HbA1c values of 7%-9% resumed their outpatient oral agents at discharge along with once-daily glargine at 50%-80% of the hospital dose. Patients admitted with a HbA1c level above 9% were discharged on their oral agents plus glargine at 80%-100% of the hospital dose or with the same basal and bolus insulin doses as they’d been given in the hospital.
The treatment goal after discharge was a blood glucose range of 70-130 mg/dL (fasting and premeal) and a HbA1c value below 7%, Dr. Umpierrez said.
For the group discharged on oral agents alone, average HbA1c fell from 6.9% at admission to 6.6% at 12 week follow-up. For those discharged on oral agents plus glargine, average HbA1c fell from 9.2% at admission to 7.5% at 12 weeks. For those discharged on glargine plus glulisine, average HbA1c fell from 11.1% to 8.0% at 12 weeks, Dr. Umpierrez reported.
Hypoglycemia with blood glucose levels below 70 mg/dL occurred post-discharge in 22% of those discharged on oral agents, 30% on oral agents plus glargine, and 44% on glargine plus glulisine. However, hypoglycemia with blood glucose values less than 40 mg/dl occurred in 3% of all patients and in 6% of the group on glargine plus glulisine.
The 224 study patients were a subset of the 375 participants in Sanofi-Aventis’ multicenter "Basal Plus" trial. That study of medical and surgical inpatients with previously diagnosed type 2 diabetes compared the efficacy and safety of a daily dose of glargine plus corrective doses of glulisine ("basal plus") to basal-bolus insulin and sliding scale regular insulin regimens.
At admission, the mean blood glucose level was 204 mg/dL (range 140-400 mg/dL), and the mean admission HbA1c was 8.4%. A total of 150 patients were randomized to the basal-bolus regimen, 148 to "basal plus" regimen, and 77 to the sliding scale insulin regimen.
During hospitalization, patients in the basal-bolus group were started at 0.5 U/kg, given half as glargine once daily and half as glulisine before meals. The "basal plus" group received 0.25 U/kg of glargine once daily plus correction doses of glulisine before meals for blood glucose values above 140 mg/dL. Sliding scale insulin was given four times a day for blood glucose values above 140 mg/dL.
Results from the in-hospital patients in the basal plus trial were reported by Dr. Umpierrez and his fellow researchers in a poster. The basal plus and basal bolus regimens resulted in similar glycemic control, with worse outcomes for those on sliding scale insulin. Mean daily blood glucose values after day 1 were 172 mg/dL for sliding scale insulin, 156 mg/dL for basal bolus, and 163 mg/dL for basal plus. Treatment failures, defined as more than two consecutive blood glucose values or a mean daily glucose value greater than 240 mg/dL, occurred in 19% on sliding scale insulin and in 2% on the basal plus regimen (P less than .001). Glucose values of less than 70 mg/dL occurred in 16% of inpatients and in 1.7% of blood glucose readings overall. Glucose levels of less than 40 mg/dL occurred in 1% of the basal bolus and the basal plus groups, and in none of the inpatients on sliding scale insulin.
There were no differences in length of stay or complications including wound infections, pneumonia, respiratory or renal failure and bacteremia between groups, the investigators reported.
This study was funded by Sanofi-Aventis. Dr. Umpierrez disclosed that he has also received research support from Merck.
PHILADELPHIA – An algorithm based on hemoglobin A1c levels at admission was safe and effective in guiding diabetes therapy after discharge, based on a prospective, randomized pilot study of 224 inpatients with type 2 diabetes.
Average HbA1c dropped from 8.4% on admission to 7.9% at 4 weeks and to 7.3% at 12 weeks after discharge, said Dr. Guillermo E. Umpierrez, professor of medicine at Emory University, and chief of diabetes and endocrinology at Grady Memorial Hospital, Atlanta.
"If we can do this at Grady Hospital, a county hospital in downtown Atlanta, I think most of you can do it in your facilities. We see these patients within 2 weeks of discharge. I think frequent followup and education are key to [the success of] this type of program," Dr. Umpierrez said.
The patients were placed into one of three post-discharge treatment groups based on their HbA1c levels at admission. Patients with admission HbA1c values of less than 7% resumed their previous outpatient treatments at discharge. Those with HbA1c values of 7%-9% resumed their outpatient oral agents at discharge along with once-daily glargine at 50%-80% of the hospital dose. Patients admitted with a HbA1c level above 9% were discharged on their oral agents plus glargine at 80%-100% of the hospital dose or with the same basal and bolus insulin doses as they’d been given in the hospital.
The treatment goal after discharge was a blood glucose range of 70-130 mg/dL (fasting and premeal) and a HbA1c value below 7%, Dr. Umpierrez said.
For the group discharged on oral agents alone, average HbA1c fell from 6.9% at admission to 6.6% at 12 week follow-up. For those discharged on oral agents plus glargine, average HbA1c fell from 9.2% at admission to 7.5% at 12 weeks. For those discharged on glargine plus glulisine, average HbA1c fell from 11.1% to 8.0% at 12 weeks, Dr. Umpierrez reported.
Hypoglycemia with blood glucose levels below 70 mg/dL occurred post-discharge in 22% of those discharged on oral agents, 30% on oral agents plus glargine, and 44% on glargine plus glulisine. However, hypoglycemia with blood glucose values less than 40 mg/dl occurred in 3% of all patients and in 6% of the group on glargine plus glulisine.
The 224 study patients were a subset of the 375 participants in Sanofi-Aventis’ multicenter "Basal Plus" trial. That study of medical and surgical inpatients with previously diagnosed type 2 diabetes compared the efficacy and safety of a daily dose of glargine plus corrective doses of glulisine ("basal plus") to basal-bolus insulin and sliding scale regular insulin regimens.
At admission, the mean blood glucose level was 204 mg/dL (range 140-400 mg/dL), and the mean admission HbA1c was 8.4%. A total of 150 patients were randomized to the basal-bolus regimen, 148 to "basal plus" regimen, and 77 to the sliding scale insulin regimen.
During hospitalization, patients in the basal-bolus group were started at 0.5 U/kg, given half as glargine once daily and half as glulisine before meals. The "basal plus" group received 0.25 U/kg of glargine once daily plus correction doses of glulisine before meals for blood glucose values above 140 mg/dL. Sliding scale insulin was given four times a day for blood glucose values above 140 mg/dL.
Results from the in-hospital patients in the basal plus trial were reported by Dr. Umpierrez and his fellow researchers in a poster. The basal plus and basal bolus regimens resulted in similar glycemic control, with worse outcomes for those on sliding scale insulin. Mean daily blood glucose values after day 1 were 172 mg/dL for sliding scale insulin, 156 mg/dL for basal bolus, and 163 mg/dL for basal plus. Treatment failures, defined as more than two consecutive blood glucose values or a mean daily glucose value greater than 240 mg/dL, occurred in 19% on sliding scale insulin and in 2% on the basal plus regimen (P less than .001). Glucose values of less than 70 mg/dL occurred in 16% of inpatients and in 1.7% of blood glucose readings overall. Glucose levels of less than 40 mg/dL occurred in 1% of the basal bolus and the basal plus groups, and in none of the inpatients on sliding scale insulin.
There were no differences in length of stay or complications including wound infections, pneumonia, respiratory or renal failure and bacteremia between groups, the investigators reported.
This study was funded by Sanofi-Aventis. Dr. Umpierrez disclosed that he has also received research support from Merck.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: In the postdischarge cohort, hemoglobin A1c dropped from 8.7% on admission to 7.3% at 12 weeks after discharge.
Data Source: The 224 post-discharge study patients were a subset of 375 inpatients in a trial that compared the efficacy and safety of a daily dose of glargine plus corrective doses of glulisine to basal-bolus insulin and sliding scale regular insulin regimens.
Disclosures: This study was funded by Sanofi-Aventis. Dr. Umpierrez disclosed that he has also received research support from Merck.
HIV Incidence Higher in Young Black MSM Community
WASHINGTON – The rate of new HIV infections among black men who have sex with men was 2.8% per year, nearly 50% higher than the rate seen in white men who have sex with men in the United States, a longitudinal cohort study conducted in six U.S. cities has found.
"Culturally tailored interventions that encourage repeated HIV/[sexually transmitted infection] testing [and] engagement with treatment/prevention, and that address social factors such as poverty, are urgently needed" for black men who have sex with men (MSM), said Beryl Koblin, Ph.D., an epidemiologist at the school of public health at Columbia University, New York.
The findings come from a study conducted within the HIV Prevention Trials Network (HPTN). The study, HPTN 061, is the largest cohort of prospectively followed black MSM in the United States. Funded by the National Institutes of Health, HPTN 061 is designed to determine the feasibility and acceptability of a multicomponent intervention for black MSM, including peer health programs. These incidence data are among the early findings from the study, Dr. Koblin said.
The study was conducted in Atlanta, Boston, Los Angeles, New York, San Francisco, and Washington between July 2009 and October 2010. Black MSM were recruited from the community or referred by sexual partners. Eligibility criteria were men who identified as men or were male at birth; were identified as black, African American, Caribbean, African, or multiethnic black; were at least 18 years old; and had had at least one episode of unprotected anal intercourse with a man in the past 6 months.
The men completed questionnaires regarding their social and sexual networks and were tested for gonorrhea, chlamydia, and syphilis. They received risk-reduction counseling and offers of services from a peer community navigator to link to clinical and social services. Those who tested positive for any infection were guided to medical care services. Participants were offered incentives to refer up to five black sexual partners for participation in the study.
A total of 1,553 black MSM enrolled, of whom 1,379 (89%) reported no prior HIV diagnosis. Of the 96% who agreed to HIV testing, 12% (165) were newly diagnosed as HIV positive, including 3 men with acute HIV infection. Among the 1,168 men who were uninfected at baseline, a little over a third were aged 30 years or younger; fewer than half had a college education or more. Fewer than a third worked full or part time, and two-thirds had an annual income of less than $20,000. About 14% had a sexually transmitted infection (STI) at the time of enrollment, Dr. Koblin said at a press briefing.
After 1 year of follow-up, among the 1,009 who had at least one follow-up test at 6 months or 1 year, the overall incidence of new HIV infection was 2.8%. Men aged 18-30 years and younger had a significantly higher HIV incidence than did those who were older (5.9% vs. 1.0%).
Among men aged 30 and younger, the incidence of new HIV infections among black MSM was three times the rate seen in white MSM of the same age, Dr. Koblin said.
Men who reported having unprotected anal intercourse had an annual HIV incidence rate of 5%, and the annual incidence rate among those with an STI at baseline was 6%. For those who reported having male partners only, the annual incidence rate was 4%. For men identifying as exclusively gay/homosexual, the incidence was 5%, compared with just 1.5% for those who identified as bisexual.
Compared with the 1,168 HIV-negative men, the 174 who reported a prior HIV diagnosis and the 165 who were newly diagnosed were more likely to be unemployed (82% for both HIV-positive groups vs. 65% for those who were HIV negative), to have household incomes less than $50,000 per year (94% vs. 87%), to have a homosexual or gay identity (69% of those previously diagnosed and 58% of the newly diagnosed vs. 47% of those who were HIV negative), and to have had unprotected receptive anal intercourse (55%, 57%, and 43%, respectively.
The HIV-negative men were more likely to have had a female partner in the past 6 months (49% vs. 25% of those previously diagnosed and 35% of the newly diagnosed). Other STIs were twice as common among both HIV-positive groups (25% and 27%, respectively), compared with 13% among the HIV-negative subjects.
"These data are important in terms of identifying [at risk] subpopulations," she said, adding that particular focus should be paid to young men, those reporting receptive anal sex at baseline, those reporting exclusively male partners and who are gay identified, and those who had an STI diagnosed at baseline.
Dr. Koblin stated that she had no financial disclosures.
WASHINGTON – The rate of new HIV infections among black men who have sex with men was 2.8% per year, nearly 50% higher than the rate seen in white men who have sex with men in the United States, a longitudinal cohort study conducted in six U.S. cities has found.
"Culturally tailored interventions that encourage repeated HIV/[sexually transmitted infection] testing [and] engagement with treatment/prevention, and that address social factors such as poverty, are urgently needed" for black men who have sex with men (MSM), said Beryl Koblin, Ph.D., an epidemiologist at the school of public health at Columbia University, New York.
The findings come from a study conducted within the HIV Prevention Trials Network (HPTN). The study, HPTN 061, is the largest cohort of prospectively followed black MSM in the United States. Funded by the National Institutes of Health, HPTN 061 is designed to determine the feasibility and acceptability of a multicomponent intervention for black MSM, including peer health programs. These incidence data are among the early findings from the study, Dr. Koblin said.
The study was conducted in Atlanta, Boston, Los Angeles, New York, San Francisco, and Washington between July 2009 and October 2010. Black MSM were recruited from the community or referred by sexual partners. Eligibility criteria were men who identified as men or were male at birth; were identified as black, African American, Caribbean, African, or multiethnic black; were at least 18 years old; and had had at least one episode of unprotected anal intercourse with a man in the past 6 months.
The men completed questionnaires regarding their social and sexual networks and were tested for gonorrhea, chlamydia, and syphilis. They received risk-reduction counseling and offers of services from a peer community navigator to link to clinical and social services. Those who tested positive for any infection were guided to medical care services. Participants were offered incentives to refer up to five black sexual partners for participation in the study.
A total of 1,553 black MSM enrolled, of whom 1,379 (89%) reported no prior HIV diagnosis. Of the 96% who agreed to HIV testing, 12% (165) were newly diagnosed as HIV positive, including 3 men with acute HIV infection. Among the 1,168 men who were uninfected at baseline, a little over a third were aged 30 years or younger; fewer than half had a college education or more. Fewer than a third worked full or part time, and two-thirds had an annual income of less than $20,000. About 14% had a sexually transmitted infection (STI) at the time of enrollment, Dr. Koblin said at a press briefing.
After 1 year of follow-up, among the 1,009 who had at least one follow-up test at 6 months or 1 year, the overall incidence of new HIV infection was 2.8%. Men aged 18-30 years and younger had a significantly higher HIV incidence than did those who were older (5.9% vs. 1.0%).
Among men aged 30 and younger, the incidence of new HIV infections among black MSM was three times the rate seen in white MSM of the same age, Dr. Koblin said.
Men who reported having unprotected anal intercourse had an annual HIV incidence rate of 5%, and the annual incidence rate among those with an STI at baseline was 6%. For those who reported having male partners only, the annual incidence rate was 4%. For men identifying as exclusively gay/homosexual, the incidence was 5%, compared with just 1.5% for those who identified as bisexual.
Compared with the 1,168 HIV-negative men, the 174 who reported a prior HIV diagnosis and the 165 who were newly diagnosed were more likely to be unemployed (82% for both HIV-positive groups vs. 65% for those who were HIV negative), to have household incomes less than $50,000 per year (94% vs. 87%), to have a homosexual or gay identity (69% of those previously diagnosed and 58% of the newly diagnosed vs. 47% of those who were HIV negative), and to have had unprotected receptive anal intercourse (55%, 57%, and 43%, respectively.
The HIV-negative men were more likely to have had a female partner in the past 6 months (49% vs. 25% of those previously diagnosed and 35% of the newly diagnosed). Other STIs were twice as common among both HIV-positive groups (25% and 27%, respectively), compared with 13% among the HIV-negative subjects.
"These data are important in terms of identifying [at risk] subpopulations," she said, adding that particular focus should be paid to young men, those reporting receptive anal sex at baseline, those reporting exclusively male partners and who are gay identified, and those who had an STI diagnosed at baseline.
Dr. Koblin stated that she had no financial disclosures.
WASHINGTON – The rate of new HIV infections among black men who have sex with men was 2.8% per year, nearly 50% higher than the rate seen in white men who have sex with men in the United States, a longitudinal cohort study conducted in six U.S. cities has found.
"Culturally tailored interventions that encourage repeated HIV/[sexually transmitted infection] testing [and] engagement with treatment/prevention, and that address social factors such as poverty, are urgently needed" for black men who have sex with men (MSM), said Beryl Koblin, Ph.D., an epidemiologist at the school of public health at Columbia University, New York.
The findings come from a study conducted within the HIV Prevention Trials Network (HPTN). The study, HPTN 061, is the largest cohort of prospectively followed black MSM in the United States. Funded by the National Institutes of Health, HPTN 061 is designed to determine the feasibility and acceptability of a multicomponent intervention for black MSM, including peer health programs. These incidence data are among the early findings from the study, Dr. Koblin said.
The study was conducted in Atlanta, Boston, Los Angeles, New York, San Francisco, and Washington between July 2009 and October 2010. Black MSM were recruited from the community or referred by sexual partners. Eligibility criteria were men who identified as men or were male at birth; were identified as black, African American, Caribbean, African, or multiethnic black; were at least 18 years old; and had had at least one episode of unprotected anal intercourse with a man in the past 6 months.
The men completed questionnaires regarding their social and sexual networks and were tested for gonorrhea, chlamydia, and syphilis. They received risk-reduction counseling and offers of services from a peer community navigator to link to clinical and social services. Those who tested positive for any infection were guided to medical care services. Participants were offered incentives to refer up to five black sexual partners for participation in the study.
A total of 1,553 black MSM enrolled, of whom 1,379 (89%) reported no prior HIV diagnosis. Of the 96% who agreed to HIV testing, 12% (165) were newly diagnosed as HIV positive, including 3 men with acute HIV infection. Among the 1,168 men who were uninfected at baseline, a little over a third were aged 30 years or younger; fewer than half had a college education or more. Fewer than a third worked full or part time, and two-thirds had an annual income of less than $20,000. About 14% had a sexually transmitted infection (STI) at the time of enrollment, Dr. Koblin said at a press briefing.
After 1 year of follow-up, among the 1,009 who had at least one follow-up test at 6 months or 1 year, the overall incidence of new HIV infection was 2.8%. Men aged 18-30 years and younger had a significantly higher HIV incidence than did those who were older (5.9% vs. 1.0%).
Among men aged 30 and younger, the incidence of new HIV infections among black MSM was three times the rate seen in white MSM of the same age, Dr. Koblin said.
Men who reported having unprotected anal intercourse had an annual HIV incidence rate of 5%, and the annual incidence rate among those with an STI at baseline was 6%. For those who reported having male partners only, the annual incidence rate was 4%. For men identifying as exclusively gay/homosexual, the incidence was 5%, compared with just 1.5% for those who identified as bisexual.
Compared with the 1,168 HIV-negative men, the 174 who reported a prior HIV diagnosis and the 165 who were newly diagnosed were more likely to be unemployed (82% for both HIV-positive groups vs. 65% for those who were HIV negative), to have household incomes less than $50,000 per year (94% vs. 87%), to have a homosexual or gay identity (69% of those previously diagnosed and 58% of the newly diagnosed vs. 47% of those who were HIV negative), and to have had unprotected receptive anal intercourse (55%, 57%, and 43%, respectively.
The HIV-negative men were more likely to have had a female partner in the past 6 months (49% vs. 25% of those previously diagnosed and 35% of the newly diagnosed). Other STIs were twice as common among both HIV-positive groups (25% and 27%, respectively), compared with 13% among the HIV-negative subjects.
"These data are important in terms of identifying [at risk] subpopulations," she said, adding that particular focus should be paid to young men, those reporting receptive anal sex at baseline, those reporting exclusively male partners and who are gay identified, and those who had an STI diagnosed at baseline.
Dr. Koblin stated that she had no financial disclosures.
AT THE 19TH INTERNATIONAL AIDS CONFERENCE
Major Finding: After 1 year of follow-up, among the 1,009 black MSM who had at least one follow-up test at 6 months or 1 year, the overall incidence of new HIV infection was 2.8%. Men aged 18-30 years and younger had a significantly higher HIV incidence than did those who were older (5.9% vs. 1.0%).
Data Source: Findings are based on a longitudinal cohort study conducted in six U.S. cities
Disclosures: Dr. Koblin stated that she had no financial disclosures.
Adult Immunization Program Needs Shot in the Arm
ATLANTA – Despite federal efforts to make adult immunization a higher priority, recent increases in use have been modest at best, according to Dr. Walter W. Williams an epidemiologist with the Centers for Disease Control and Prevention.
In a comparison of 2009 and 2010 data from the NHIS (National Health Interview Survey), an annual in-home survey that includes 7,624 noninstitutionalized adults aged 19-64 years, Dr. Williams found that the use of tetanus-diphtheria-acellular pertussis (Tdap) vaccination, for example, increased by almost 2%, rising to 8%. Herpes zoster vaccination rates among those aged 60 years and older increased by 4%, rising to 14%. And human papillomavirus (HPV) vaccination rates among women aged 19-26 years increased by nearly 4%, rising to 21%.
Racial disparities were seen for nearly all immunization rates, with non-Hispanic whites generally having higher rates than did black, Hispanic, or Asian adults. Pneumococcal vaccine coverage among adults aged 65 years and older was 64% for whites, compared with 46% for blacks and 39% for Hispanics/Latinos. Receipt of zoster vaccine among those at least aged 60 years was 17% for whites, compared with 5% for blacks and 4% for Hispanics/Latinos.
"These data highlight the problems that we have with our adult program or lack thereof," said Kristen R. Ehresmann, R.N., the director of the infectious disease epidemiology, prevention and control division of the Minnesota Department of Health, St. Paul.
Sara Rosenbaum, an attorney and a member of the CDC’s Advisory Committee on Immunization Practices, concurred. "I think these data are testament to the fact that we don’t really have an adult immunization program," she said, noting the lack of funding.
Ms. Rosenbaum, who teaches at George Washington University in Washington, also noted that "the zoster numbers tell us that there’s something terribly wrong right now with the way Medicare immunization coverage works for zoster," referring to the current system whereby Medicare covers Zostavax under Part D (prescription drug coverage), which physicians can’t bill directly.
But Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases countered that although the Medicare issue has been a "headache," the primary concern about Zostavax has been a supply problem, which is now resolved. Moreover, the vaccine is recommended for adults beginning at age 60, and most who are aged 60-65 are insured privately. "We actually have an opportunity now that the supply is good for every stakeholder to try to take advantage of a good vaccine for a very common bad disease, at least in the population that couldn’t access it because of supply, and now they can," she said.
Sandra A. Fryhofer, the ACIP liaison from the American College of Physicians (ACP), said that she found these latest data "very sobering. ... The excitement that we in this room share for adult vaccination has to go outside this room and out to our physicians and other providers."
Part of the problem for physicians in the trenches may be the failure of their reminder systems, said ACIP member Dr. Jonathan Temte, a family physician. "When I see children in my practice, it’s largely a well-care [visit] and vaccines are a big part [of those]. When I see my typical older adult for well care, I’m seeing six or seven or eight comorbid conditions at the same time. ... For adult immunizations, having reminder systems that are flawlessly built into your [electronic medical record] systems are so very important."
However, "most of us ... unfortunately have even learned how to ignore the warnings that pop up on our EMR screens because of all the chaos. We need to do a better job with those reminder systems," said Dr. Temte, professor of family medicine at the University of Wisconsin, Madison.
Signs that adult vaccination is moving up on the priority list include the fact it has gained increasing amounts of airtime at the thrice-yearly meetings held by ACIP. Back in the 1990s, the agendas for those meetings may have included one or two adult-focused topics amidst a much longer list of pediatric vaccine issues.
Today, some ACIP agendas are almost entirely geared toward adults. At the recent June meeting, topics included the use of the 13-valent conjugate pneumococcal vaccine for immunocompromised adults, hepatitis B vaccine for health care personnel in whom protection is uncertain, and postexposure prophylaxis with anthrax vaccine, as well as an entire session specifically focused on the woefully low rates of vaccine coverage for prevention of noninfluenza conditions among adults.
Dr. Carolyn B. Bridges, associate director for Adult Immunizations at the CDC’s Immunization Services Division, reported on the first-ever National Adult Immunization Summit, held May 15-17 in Atlanta. Sponsored by the American Medical Association, the National Vaccine Program Office, and the CDC, the meeting was patterned after the National Influenza Vaccine Summit (www.preventinfluenza.org).
Key action items were identified by five working groups that were formed to address patient, provider, and decision-maker education; access and collaboration; and quality/performance measures. In addition, an Interagency Adult Immunization Task Force was initiated within the U.S. Department of Health and Human Services.
Among the needs identified were improved documentation/communication via immunization information systems and EMRs; decreased policy and legal barriers for vaccine providers; increased education and incentivization of providers, such as via performance or quality measures; and decreased complexity of the adult vaccination schedule. Over the next several weeks, the AMA, the CDC, and the NVPO will develop an initial list of key action items and will prepare proceedings of the summit for submission to a peer-reviewed journal. Another Adult Immunization Summit is anticipated for 2013, Dr. Bridges said.
In the meantime, here’s a helpful resource list developed from the summit.
None of the sources in this story reported having conflicts of interest.
ATLANTA – Despite federal efforts to make adult immunization a higher priority, recent increases in use have been modest at best, according to Dr. Walter W. Williams an epidemiologist with the Centers for Disease Control and Prevention.
In a comparison of 2009 and 2010 data from the NHIS (National Health Interview Survey), an annual in-home survey that includes 7,624 noninstitutionalized adults aged 19-64 years, Dr. Williams found that the use of tetanus-diphtheria-acellular pertussis (Tdap) vaccination, for example, increased by almost 2%, rising to 8%. Herpes zoster vaccination rates among those aged 60 years and older increased by 4%, rising to 14%. And human papillomavirus (HPV) vaccination rates among women aged 19-26 years increased by nearly 4%, rising to 21%.
Racial disparities were seen for nearly all immunization rates, with non-Hispanic whites generally having higher rates than did black, Hispanic, or Asian adults. Pneumococcal vaccine coverage among adults aged 65 years and older was 64% for whites, compared with 46% for blacks and 39% for Hispanics/Latinos. Receipt of zoster vaccine among those at least aged 60 years was 17% for whites, compared with 5% for blacks and 4% for Hispanics/Latinos.
"These data highlight the problems that we have with our adult program or lack thereof," said Kristen R. Ehresmann, R.N., the director of the infectious disease epidemiology, prevention and control division of the Minnesota Department of Health, St. Paul.
Sara Rosenbaum, an attorney and a member of the CDC’s Advisory Committee on Immunization Practices, concurred. "I think these data are testament to the fact that we don’t really have an adult immunization program," she said, noting the lack of funding.
Ms. Rosenbaum, who teaches at George Washington University in Washington, also noted that "the zoster numbers tell us that there’s something terribly wrong right now with the way Medicare immunization coverage works for zoster," referring to the current system whereby Medicare covers Zostavax under Part D (prescription drug coverage), which physicians can’t bill directly.
But Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases countered that although the Medicare issue has been a "headache," the primary concern about Zostavax has been a supply problem, which is now resolved. Moreover, the vaccine is recommended for adults beginning at age 60, and most who are aged 60-65 are insured privately. "We actually have an opportunity now that the supply is good for every stakeholder to try to take advantage of a good vaccine for a very common bad disease, at least in the population that couldn’t access it because of supply, and now they can," she said.
Sandra A. Fryhofer, the ACIP liaison from the American College of Physicians (ACP), said that she found these latest data "very sobering. ... The excitement that we in this room share for adult vaccination has to go outside this room and out to our physicians and other providers."
Part of the problem for physicians in the trenches may be the failure of their reminder systems, said ACIP member Dr. Jonathan Temte, a family physician. "When I see children in my practice, it’s largely a well-care [visit] and vaccines are a big part [of those]. When I see my typical older adult for well care, I’m seeing six or seven or eight comorbid conditions at the same time. ... For adult immunizations, having reminder systems that are flawlessly built into your [electronic medical record] systems are so very important."
However, "most of us ... unfortunately have even learned how to ignore the warnings that pop up on our EMR screens because of all the chaos. We need to do a better job with those reminder systems," said Dr. Temte, professor of family medicine at the University of Wisconsin, Madison.
Signs that adult vaccination is moving up on the priority list include the fact it has gained increasing amounts of airtime at the thrice-yearly meetings held by ACIP. Back in the 1990s, the agendas for those meetings may have included one or two adult-focused topics amidst a much longer list of pediatric vaccine issues.
Today, some ACIP agendas are almost entirely geared toward adults. At the recent June meeting, topics included the use of the 13-valent conjugate pneumococcal vaccine for immunocompromised adults, hepatitis B vaccine for health care personnel in whom protection is uncertain, and postexposure prophylaxis with anthrax vaccine, as well as an entire session specifically focused on the woefully low rates of vaccine coverage for prevention of noninfluenza conditions among adults.
Dr. Carolyn B. Bridges, associate director for Adult Immunizations at the CDC’s Immunization Services Division, reported on the first-ever National Adult Immunization Summit, held May 15-17 in Atlanta. Sponsored by the American Medical Association, the National Vaccine Program Office, and the CDC, the meeting was patterned after the National Influenza Vaccine Summit (www.preventinfluenza.org).
Key action items were identified by five working groups that were formed to address patient, provider, and decision-maker education; access and collaboration; and quality/performance measures. In addition, an Interagency Adult Immunization Task Force was initiated within the U.S. Department of Health and Human Services.
Among the needs identified were improved documentation/communication via immunization information systems and EMRs; decreased policy and legal barriers for vaccine providers; increased education and incentivization of providers, such as via performance or quality measures; and decreased complexity of the adult vaccination schedule. Over the next several weeks, the AMA, the CDC, and the NVPO will develop an initial list of key action items and will prepare proceedings of the summit for submission to a peer-reviewed journal. Another Adult Immunization Summit is anticipated for 2013, Dr. Bridges said.
In the meantime, here’s a helpful resource list developed from the summit.
None of the sources in this story reported having conflicts of interest.
ATLANTA – Despite federal efforts to make adult immunization a higher priority, recent increases in use have been modest at best, according to Dr. Walter W. Williams an epidemiologist with the Centers for Disease Control and Prevention.
In a comparison of 2009 and 2010 data from the NHIS (National Health Interview Survey), an annual in-home survey that includes 7,624 noninstitutionalized adults aged 19-64 years, Dr. Williams found that the use of tetanus-diphtheria-acellular pertussis (Tdap) vaccination, for example, increased by almost 2%, rising to 8%. Herpes zoster vaccination rates among those aged 60 years and older increased by 4%, rising to 14%. And human papillomavirus (HPV) vaccination rates among women aged 19-26 years increased by nearly 4%, rising to 21%.
Racial disparities were seen for nearly all immunization rates, with non-Hispanic whites generally having higher rates than did black, Hispanic, or Asian adults. Pneumococcal vaccine coverage among adults aged 65 years and older was 64% for whites, compared with 46% for blacks and 39% for Hispanics/Latinos. Receipt of zoster vaccine among those at least aged 60 years was 17% for whites, compared with 5% for blacks and 4% for Hispanics/Latinos.
"These data highlight the problems that we have with our adult program or lack thereof," said Kristen R. Ehresmann, R.N., the director of the infectious disease epidemiology, prevention and control division of the Minnesota Department of Health, St. Paul.
Sara Rosenbaum, an attorney and a member of the CDC’s Advisory Committee on Immunization Practices, concurred. "I think these data are testament to the fact that we don’t really have an adult immunization program," she said, noting the lack of funding.
Ms. Rosenbaum, who teaches at George Washington University in Washington, also noted that "the zoster numbers tell us that there’s something terribly wrong right now with the way Medicare immunization coverage works for zoster," referring to the current system whereby Medicare covers Zostavax under Part D (prescription drug coverage), which physicians can’t bill directly.
But Dr. Anne Schuchat, director of the CDC’s National Center for Immunization and Respiratory Diseases countered that although the Medicare issue has been a "headache," the primary concern about Zostavax has been a supply problem, which is now resolved. Moreover, the vaccine is recommended for adults beginning at age 60, and most who are aged 60-65 are insured privately. "We actually have an opportunity now that the supply is good for every stakeholder to try to take advantage of a good vaccine for a very common bad disease, at least in the population that couldn’t access it because of supply, and now they can," she said.
Sandra A. Fryhofer, the ACIP liaison from the American College of Physicians (ACP), said that she found these latest data "very sobering. ... The excitement that we in this room share for adult vaccination has to go outside this room and out to our physicians and other providers."
Part of the problem for physicians in the trenches may be the failure of their reminder systems, said ACIP member Dr. Jonathan Temte, a family physician. "When I see children in my practice, it’s largely a well-care [visit] and vaccines are a big part [of those]. When I see my typical older adult for well care, I’m seeing six or seven or eight comorbid conditions at the same time. ... For adult immunizations, having reminder systems that are flawlessly built into your [electronic medical record] systems are so very important."
However, "most of us ... unfortunately have even learned how to ignore the warnings that pop up on our EMR screens because of all the chaos. We need to do a better job with those reminder systems," said Dr. Temte, professor of family medicine at the University of Wisconsin, Madison.
Signs that adult vaccination is moving up on the priority list include the fact it has gained increasing amounts of airtime at the thrice-yearly meetings held by ACIP. Back in the 1990s, the agendas for those meetings may have included one or two adult-focused topics amidst a much longer list of pediatric vaccine issues.
Today, some ACIP agendas are almost entirely geared toward adults. At the recent June meeting, topics included the use of the 13-valent conjugate pneumococcal vaccine for immunocompromised adults, hepatitis B vaccine for health care personnel in whom protection is uncertain, and postexposure prophylaxis with anthrax vaccine, as well as an entire session specifically focused on the woefully low rates of vaccine coverage for prevention of noninfluenza conditions among adults.
Dr. Carolyn B. Bridges, associate director for Adult Immunizations at the CDC’s Immunization Services Division, reported on the first-ever National Adult Immunization Summit, held May 15-17 in Atlanta. Sponsored by the American Medical Association, the National Vaccine Program Office, and the CDC, the meeting was patterned after the National Influenza Vaccine Summit (www.preventinfluenza.org).
Key action items were identified by five working groups that were formed to address patient, provider, and decision-maker education; access and collaboration; and quality/performance measures. In addition, an Interagency Adult Immunization Task Force was initiated within the U.S. Department of Health and Human Services.
Among the needs identified were improved documentation/communication via immunization information systems and EMRs; decreased policy and legal barriers for vaccine providers; increased education and incentivization of providers, such as via performance or quality measures; and decreased complexity of the adult vaccination schedule. Over the next several weeks, the AMA, the CDC, and the NVPO will develop an initial list of key action items and will prepare proceedings of the summit for submission to a peer-reviewed journal. Another Adult Immunization Summit is anticipated for 2013, Dr. Bridges said.
In the meantime, here’s a helpful resource list developed from the summit.
None of the sources in this story reported having conflicts of interest.
AT A MEETING OF THE CENTERS FOR DISEASE CONTROL AND PREVENTION'S ADVISORY COMMITTEE ON IMMUNIZATION PRACTICE
Major Finding: Compared with 2009 NHIS estimates, only modest increases in adult use were seen in 2010 for the Tdap vaccination (1.6% rise, to 8.2%), herpes zoster vaccination (4.4% rise, to 14.4%), and HPV vaccination among women aged 19-26 years (3.6% rise, to 20.7%).
Data Source: The findings come from 7,624 noninstitutionalized adults aged 19-64 years who were interviewed for the 2010 NHIS, an annual in-home survey conducted by the CDC.
Disclosures: The study was funded by the CDC. None of the speakers reported disclosures.
In Depth: HIV/AIDS Researchers Focus on a Cure
With the International AIDS Society officially shifting its focus from controlling to curing HIV infection, Dr. Steven Deeks talks about how the approach to HIV/AIDS has progressed to the point that a cure is conceivable. And he outlines the roles that clinicians can play as research efforts shift toward finding a cure. See the related story here.
With the International AIDS Society officially shifting its focus from controlling to curing HIV infection, Dr. Steven Deeks talks about how the approach to HIV/AIDS has progressed to the point that a cure is conceivable. And he outlines the roles that clinicians can play as research efforts shift toward finding a cure. See the related story here.
With the International AIDS Society officially shifting its focus from controlling to curing HIV infection, Dr. Steven Deeks talks about how the approach to HIV/AIDS has progressed to the point that a cure is conceivable. And he outlines the roles that clinicians can play as research efforts shift toward finding a cure. See the related story here.
Genetic Screening Targets Maturity-Onset Diabetes of Youth
PHILADELPHIA – In people aged 20-40 years who have been diagnosed with diabetes, routine genetic screening for maturity-onset diabetes of youth could result in more targeted treatment. But the costs of genetic testing need to come down before routine screening becomes cost effective.
Using a simulation model of type 2 diabetes complications that accounted for the natural history of disease by genetic subtypes, investigators found that genetic screening added 0.01 quality-adjusted life-years (19.22 vs. 19.21 with no screening) at an increased total cost of $1,727 ($41,033 vs. $39,306). The incremental cost-effectiveness ratio (or cost per quality-adjusted life-years) was $143,409.
Genetic testing becomes more cost effective as the cost of the test decreases. If the prevalence of maturity-onset diabetes of youth (MODY) in the screened population is 5% or greater, and the cost of the test drops to less than $1,000, then the incremental cost-effectiveness ratio would go to $50,000, which is considered to be the conventional cost-effectiveness threshold. If the test cost falls to $200, then the screening policy would be cost saving, presuming that the MODY prevalence in the screened population was 20% or higher, said Dr. Rochelle N. Naylor, a pediatric endocrinology fellow at the University of Chicago’s National Center for Monogenic Diabetes.
MODY is caused by mutations in 11 different genes; just 3 of them account for 80% of cases of MODY. Screening can aid treatment decisions because mutations in either HNF1A or HNF4A lead to a progressive insulin secretory defect for which sulfonylureas are the established first-line therapy. Mutations in GCK, on the other hand, result in permanent but mild blood glucose elevations and don’t require treatment.
But MODY accounts for 2% of diabetes and is rarely diagnosed because genetic testing isn’t routinely obtained. Patients are typically misdiagnosed as having either type 1 or type 2 diabetes, and are treated with insulin or medications other than sulfonylureas, Dr. Naylor said.
The study simulated diabetes costs and complications in incident cases of diabetes that were diagnosed in a hypothetical population of patients aged 20-40 years. Included in the model were variables based on published data on the three MODY subtypes as well as type 2 diabetes complications data from the U.K. Prospective Diabetes Study. The model assumed a MODY prevalence of 2% in the population, with 35% of cases having GCK mutations and 65% having HNF1A or HNF4A mutations, and with a one-time testing cost of $2,000. For the simulated no-testing policy, the model assumed that treatment decisions would be based on the assumed diagnosis of type 2 diabetes.
The model assumed that 75% of MODY patients with HNF1A or HNF4A mutations would be treated with sulfonylureas and that all patients with GCK MODY would discontinue therapy.
A calculator to predict the likelihood of MODY is available at www.diabetesgenes.org. Targeted populations could be expanded as genetic testing costs fall, Dr. Naylor said.
Dr. Naylor said she has no personal disclosures, but that the University of Chicago’s National Center for Monogenic Diabetes receives royalties from Athena Diagnostics for genetic testing for diabetes genes.
PHILADELPHIA – In people aged 20-40 years who have been diagnosed with diabetes, routine genetic screening for maturity-onset diabetes of youth could result in more targeted treatment. But the costs of genetic testing need to come down before routine screening becomes cost effective.
Using a simulation model of type 2 diabetes complications that accounted for the natural history of disease by genetic subtypes, investigators found that genetic screening added 0.01 quality-adjusted life-years (19.22 vs. 19.21 with no screening) at an increased total cost of $1,727 ($41,033 vs. $39,306). The incremental cost-effectiveness ratio (or cost per quality-adjusted life-years) was $143,409.
Genetic testing becomes more cost effective as the cost of the test decreases. If the prevalence of maturity-onset diabetes of youth (MODY) in the screened population is 5% or greater, and the cost of the test drops to less than $1,000, then the incremental cost-effectiveness ratio would go to $50,000, which is considered to be the conventional cost-effectiveness threshold. If the test cost falls to $200, then the screening policy would be cost saving, presuming that the MODY prevalence in the screened population was 20% or higher, said Dr. Rochelle N. Naylor, a pediatric endocrinology fellow at the University of Chicago’s National Center for Monogenic Diabetes.
MODY is caused by mutations in 11 different genes; just 3 of them account for 80% of cases of MODY. Screening can aid treatment decisions because mutations in either HNF1A or HNF4A lead to a progressive insulin secretory defect for which sulfonylureas are the established first-line therapy. Mutations in GCK, on the other hand, result in permanent but mild blood glucose elevations and don’t require treatment.
But MODY accounts for 2% of diabetes and is rarely diagnosed because genetic testing isn’t routinely obtained. Patients are typically misdiagnosed as having either type 1 or type 2 diabetes, and are treated with insulin or medications other than sulfonylureas, Dr. Naylor said.
The study simulated diabetes costs and complications in incident cases of diabetes that were diagnosed in a hypothetical population of patients aged 20-40 years. Included in the model were variables based on published data on the three MODY subtypes as well as type 2 diabetes complications data from the U.K. Prospective Diabetes Study. The model assumed a MODY prevalence of 2% in the population, with 35% of cases having GCK mutations and 65% having HNF1A or HNF4A mutations, and with a one-time testing cost of $2,000. For the simulated no-testing policy, the model assumed that treatment decisions would be based on the assumed diagnosis of type 2 diabetes.
The model assumed that 75% of MODY patients with HNF1A or HNF4A mutations would be treated with sulfonylureas and that all patients with GCK MODY would discontinue therapy.
A calculator to predict the likelihood of MODY is available at www.diabetesgenes.org. Targeted populations could be expanded as genetic testing costs fall, Dr. Naylor said.
Dr. Naylor said she has no personal disclosures, but that the University of Chicago’s National Center for Monogenic Diabetes receives royalties from Athena Diagnostics for genetic testing for diabetes genes.
PHILADELPHIA – In people aged 20-40 years who have been diagnosed with diabetes, routine genetic screening for maturity-onset diabetes of youth could result in more targeted treatment. But the costs of genetic testing need to come down before routine screening becomes cost effective.
Using a simulation model of type 2 diabetes complications that accounted for the natural history of disease by genetic subtypes, investigators found that genetic screening added 0.01 quality-adjusted life-years (19.22 vs. 19.21 with no screening) at an increased total cost of $1,727 ($41,033 vs. $39,306). The incremental cost-effectiveness ratio (or cost per quality-adjusted life-years) was $143,409.
Genetic testing becomes more cost effective as the cost of the test decreases. If the prevalence of maturity-onset diabetes of youth (MODY) in the screened population is 5% or greater, and the cost of the test drops to less than $1,000, then the incremental cost-effectiveness ratio would go to $50,000, which is considered to be the conventional cost-effectiveness threshold. If the test cost falls to $200, then the screening policy would be cost saving, presuming that the MODY prevalence in the screened population was 20% or higher, said Dr. Rochelle N. Naylor, a pediatric endocrinology fellow at the University of Chicago’s National Center for Monogenic Diabetes.
MODY is caused by mutations in 11 different genes; just 3 of them account for 80% of cases of MODY. Screening can aid treatment decisions because mutations in either HNF1A or HNF4A lead to a progressive insulin secretory defect for which sulfonylureas are the established first-line therapy. Mutations in GCK, on the other hand, result in permanent but mild blood glucose elevations and don’t require treatment.
But MODY accounts for 2% of diabetes and is rarely diagnosed because genetic testing isn’t routinely obtained. Patients are typically misdiagnosed as having either type 1 or type 2 diabetes, and are treated with insulin or medications other than sulfonylureas, Dr. Naylor said.
The study simulated diabetes costs and complications in incident cases of diabetes that were diagnosed in a hypothetical population of patients aged 20-40 years. Included in the model were variables based on published data on the three MODY subtypes as well as type 2 diabetes complications data from the U.K. Prospective Diabetes Study. The model assumed a MODY prevalence of 2% in the population, with 35% of cases having GCK mutations and 65% having HNF1A or HNF4A mutations, and with a one-time testing cost of $2,000. For the simulated no-testing policy, the model assumed that treatment decisions would be based on the assumed diagnosis of type 2 diabetes.
The model assumed that 75% of MODY patients with HNF1A or HNF4A mutations would be treated with sulfonylureas and that all patients with GCK MODY would discontinue therapy.
A calculator to predict the likelihood of MODY is available at www.diabetesgenes.org. Targeted populations could be expanded as genetic testing costs fall, Dr. Naylor said.
Dr. Naylor said she has no personal disclosures, but that the University of Chicago’s National Center for Monogenic Diabetes receives royalties from Athena Diagnostics for genetic testing for diabetes genes.
AT THE ANNUAL SCIENTIFIC SESSIONS OF THE AMERICAN DIABETES ASSOCIATION
Major Finding: The screening policy added 0.01 quality-adjusted life years (19.22 vs. 19.21 with no screening).
Data Source: The study simulated the costs and complications in incident cases of diabetes diagnosed in a hypothetical population of patients aged 20-40 years.
Disclosures: Dr. Naylor reported no personal disclosures. The National Center for Monogenic Diabetes receives royalties from Athena Diagnostics for genetic testing for diabetes genes.