User login
Monotherapy as good as combo for antibiotic-resistant infections in low-risk patients
VIENNA – A single, well-targeted antibiotic may be enough to effectively combat serous bloodstream infections in patients who have a low baseline mortality risk.
Among these patients, overall mortality was similar among those receiving a single antibiotic and those getting multiple antibiotics (35% vs. 41%). Patients with a high baseline mortality risk, however, did experience a significant 44% survival benefit when treated with a combination regimen, Jesus Rodríguez-Baño, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The finding is important when considering the ever-increasing imperative of antibiotic stewardship, Dr. Rodríguez-Baño said in an interview.
“In areas where these pathogens are common, particularly in intensive care units, where they can become epidemic and infect many patients, the overuse of combination therapy will be fueling the problem,” said Dr. Rodríguez-Baño, head of infectious diseases and clinical microbiology at the University Hospital Virgin Macarena, Seville, Spain. “This is a way to avoid the overuse of some broad-spectrum antibiotics. Selecting the patients who should not receive combination therapy may significantly reduce the total consumption” on a unit.
The retrospective study, dubbed INCREMENT, was conducted at 37 hospitals in 10 countries. It enrolled patients with bloodstream infections caused by extended-spectrum beta-lactamase- or carbapenemase-producing Enterobacteriaceae. Dr. Rodríguez-Baño reported results for 437 patients whose infections were caused by the carbapenemase-producing strain.
It was simultaneously published in Lancet Infectious Diseases (Lancet Inf Dis 2017; DOI: http://dx.doi.org/10.1016/S1473-3099(17)30228-1).
These patients were a mean of 66 years old; most (60%) were male. The primary infective agent was Klebsiella pneumonia (86%); most infections were nosocomial. The origin of infections varied, but most (80%) arose from places other than the urinary or biliary tract. Sources were vascular catheters, pneumonia, intraabdominal, and skin and soft tissue. About half of the patients were in severe sepsis or septic shock when treated.
The group was first divided into those who received appropriate or inappropriate therapy (78% vs. 22%). Appropriate therapy was considered to be the early administration of a drug that could effectively target the infective organism. Next, those who got appropriate therapy were parsed by whether they received mono- or combination therapy (61%, 39%). Finally, these patients were stratified by a specially designed mortality risk score, the INCREMENT Carbapenemase-Producing Enterobacteriaceae (CPE) Mortality Score (Mayo Clinic Proceedings. doi.org/10.1016/j.mayocp.2016.06.024).
- Severe sepsis or shock at presentation (5 points)
- Pitt score of 6 or more (4 points)
- Charlson comorbidity index of 2 or more (3 points)
- Source of bloodstream infection other than urinary or biliary tract (3 points)
- Inappropriate empirical therapy and inappropriate early targeted therapy (2 points)
Patients were considered low risk if they had a score of 0-7, and high of they had a score of 8 or more.
The risk assessment took is quick, easy to figure, and extremely important, Dr. Rodríguez-Baño noted. “This is a very easy-to-use tool that can help us make many patient management decisions. All of the information is already available in the patient’s chart, so it doesn’t require any additional assessments. It’s a very good way to individualize treatment.”
In the initial analysis, all-cause mortality at 30 days was 22% lower among patients who received appropriate early therapy than those who did not (38.5% vs. 60.6%). This translated to a 55% decrease in the risk of death (HR 0.45 in the fully adjusted model).
The investigators next turned their attention toward the group that received appropriate therapy. All-cause 30-day mortality was 41% in those who got monotherapy and 34.8% among those who got combination therapy..
Finally, this group was stratified according to the INCREMENT-CPE mortality risk score.
In the low-risk category, combination therapy did not confer a survival advantage over monotherapy. Death occurred in 20% of those getting monotherapy and 24% receiving combination treatment – not a significant difference (HR 1.21).
Combination therapy did, however, confer a significant survival benefit in the high-risk group. Death occurred in 62% of those receiving monotherapy and 48% of those receiving combination therapy – a 44% risk reduction (HR 0.56).
As long as they were appropriately targeted against the infective organism, all drugs used in the high-mortality risk group were similarly effective at reducing the risk of death. Compared to colistin monotherapy, a combination that included tigecycline reduced the risk of death by 55% (HR 0.45); combination with aminoglycosides by 58% (HR 0.42); and combination with carbapenems by 44% (HR 0.56).
A secondary analysis of this group determined that time was a critical factor in survival. Each day delay after day 2 significantly increased the risk of death, Dr. Rodríguez-Baño said. This 48-hour period gives clinicians a chance to wait for the culture and antibiogram to return, and then choose and initiate the best treatment. Before the results come back, empiric antibiotic therapy is appropriate, but changes should be made immediately after the results come back.
“We tend to think we must give the very best antibiotic at the very first moment that we see a patient with a serious infection,” he said. “But what we found is that it’s not critical to give the perfect antibiotic on the first day. It is critical, however, to give the correct one once you know which bacteria is causing the infection. Since it takes 48 hours for those results to come back, this is perfect timing.”
INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
[email protected]
On Twitter @Alz_gal
VIENNA – A single, well-targeted antibiotic may be enough to effectively combat serous bloodstream infections in patients who have a low baseline mortality risk.
Among these patients, overall mortality was similar among those receiving a single antibiotic and those getting multiple antibiotics (35% vs. 41%). Patients with a high baseline mortality risk, however, did experience a significant 44% survival benefit when treated with a combination regimen, Jesus Rodríguez-Baño, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The finding is important when considering the ever-increasing imperative of antibiotic stewardship, Dr. Rodríguez-Baño said in an interview.
“In areas where these pathogens are common, particularly in intensive care units, where they can become epidemic and infect many patients, the overuse of combination therapy will be fueling the problem,” said Dr. Rodríguez-Baño, head of infectious diseases and clinical microbiology at the University Hospital Virgin Macarena, Seville, Spain. “This is a way to avoid the overuse of some broad-spectrum antibiotics. Selecting the patients who should not receive combination therapy may significantly reduce the total consumption” on a unit.
The retrospective study, dubbed INCREMENT, was conducted at 37 hospitals in 10 countries. It enrolled patients with bloodstream infections caused by extended-spectrum beta-lactamase- or carbapenemase-producing Enterobacteriaceae. Dr. Rodríguez-Baño reported results for 437 patients whose infections were caused by the carbapenemase-producing strain.
It was simultaneously published in Lancet Infectious Diseases (Lancet Inf Dis 2017; DOI: http://dx.doi.org/10.1016/S1473-3099(17)30228-1).
These patients were a mean of 66 years old; most (60%) were male. The primary infective agent was Klebsiella pneumonia (86%); most infections were nosocomial. The origin of infections varied, but most (80%) arose from places other than the urinary or biliary tract. Sources were vascular catheters, pneumonia, intraabdominal, and skin and soft tissue. About half of the patients were in severe sepsis or septic shock when treated.
The group was first divided into those who received appropriate or inappropriate therapy (78% vs. 22%). Appropriate therapy was considered to be the early administration of a drug that could effectively target the infective organism. Next, those who got appropriate therapy were parsed by whether they received mono- or combination therapy (61%, 39%). Finally, these patients were stratified by a specially designed mortality risk score, the INCREMENT Carbapenemase-Producing Enterobacteriaceae (CPE) Mortality Score (Mayo Clinic Proceedings. doi.org/10.1016/j.mayocp.2016.06.024).
- Severe sepsis or shock at presentation (5 points)
- Pitt score of 6 or more (4 points)
- Charlson comorbidity index of 2 or more (3 points)
- Source of bloodstream infection other than urinary or biliary tract (3 points)
- Inappropriate empirical therapy and inappropriate early targeted therapy (2 points)
Patients were considered low risk if they had a score of 0-7, and high of they had a score of 8 or more.
The risk assessment took is quick, easy to figure, and extremely important, Dr. Rodríguez-Baño noted. “This is a very easy-to-use tool that can help us make many patient management decisions. All of the information is already available in the patient’s chart, so it doesn’t require any additional assessments. It’s a very good way to individualize treatment.”
In the initial analysis, all-cause mortality at 30 days was 22% lower among patients who received appropriate early therapy than those who did not (38.5% vs. 60.6%). This translated to a 55% decrease in the risk of death (HR 0.45 in the fully adjusted model).
The investigators next turned their attention toward the group that received appropriate therapy. All-cause 30-day mortality was 41% in those who got monotherapy and 34.8% among those who got combination therapy..
Finally, this group was stratified according to the INCREMENT-CPE mortality risk score.
In the low-risk category, combination therapy did not confer a survival advantage over monotherapy. Death occurred in 20% of those getting monotherapy and 24% receiving combination treatment – not a significant difference (HR 1.21).
Combination therapy did, however, confer a significant survival benefit in the high-risk group. Death occurred in 62% of those receiving monotherapy and 48% of those receiving combination therapy – a 44% risk reduction (HR 0.56).
As long as they were appropriately targeted against the infective organism, all drugs used in the high-mortality risk group were similarly effective at reducing the risk of death. Compared to colistin monotherapy, a combination that included tigecycline reduced the risk of death by 55% (HR 0.45); combination with aminoglycosides by 58% (HR 0.42); and combination with carbapenems by 44% (HR 0.56).
A secondary analysis of this group determined that time was a critical factor in survival. Each day delay after day 2 significantly increased the risk of death, Dr. Rodríguez-Baño said. This 48-hour period gives clinicians a chance to wait for the culture and antibiogram to return, and then choose and initiate the best treatment. Before the results come back, empiric antibiotic therapy is appropriate, but changes should be made immediately after the results come back.
“We tend to think we must give the very best antibiotic at the very first moment that we see a patient with a serious infection,” he said. “But what we found is that it’s not critical to give the perfect antibiotic on the first day. It is critical, however, to give the correct one once you know which bacteria is causing the infection. Since it takes 48 hours for those results to come back, this is perfect timing.”
INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
[email protected]
On Twitter @Alz_gal
VIENNA – A single, well-targeted antibiotic may be enough to effectively combat serous bloodstream infections in patients who have a low baseline mortality risk.
Among these patients, overall mortality was similar among those receiving a single antibiotic and those getting multiple antibiotics (35% vs. 41%). Patients with a high baseline mortality risk, however, did experience a significant 44% survival benefit when treated with a combination regimen, Jesus Rodríguez-Baño, MD, said at the European Society of Clinical Microbiology and Infectious Diseases annual congress.
The finding is important when considering the ever-increasing imperative of antibiotic stewardship, Dr. Rodríguez-Baño said in an interview.
“In areas where these pathogens are common, particularly in intensive care units, where they can become epidemic and infect many patients, the overuse of combination therapy will be fueling the problem,” said Dr. Rodríguez-Baño, head of infectious diseases and clinical microbiology at the University Hospital Virgin Macarena, Seville, Spain. “This is a way to avoid the overuse of some broad-spectrum antibiotics. Selecting the patients who should not receive combination therapy may significantly reduce the total consumption” on a unit.
The retrospective study, dubbed INCREMENT, was conducted at 37 hospitals in 10 countries. It enrolled patients with bloodstream infections caused by extended-spectrum beta-lactamase- or carbapenemase-producing Enterobacteriaceae. Dr. Rodríguez-Baño reported results for 437 patients whose infections were caused by the carbapenemase-producing strain.
It was simultaneously published in Lancet Infectious Diseases (Lancet Inf Dis 2017; DOI: http://dx.doi.org/10.1016/S1473-3099(17)30228-1).
These patients were a mean of 66 years old; most (60%) were male. The primary infective agent was Klebsiella pneumonia (86%); most infections were nosocomial. The origin of infections varied, but most (80%) arose from places other than the urinary or biliary tract. Sources were vascular catheters, pneumonia, intraabdominal, and skin and soft tissue. About half of the patients were in severe sepsis or septic shock when treated.
The group was first divided into those who received appropriate or inappropriate therapy (78% vs. 22%). Appropriate therapy was considered to be the early administration of a drug that could effectively target the infective organism. Next, those who got appropriate therapy were parsed by whether they received mono- or combination therapy (61%, 39%). Finally, these patients were stratified by a specially designed mortality risk score, the INCREMENT Carbapenemase-Producing Enterobacteriaceae (CPE) Mortality Score (Mayo Clinic Proceedings. doi.org/10.1016/j.mayocp.2016.06.024).
- Severe sepsis or shock at presentation (5 points)
- Pitt score of 6 or more (4 points)
- Charlson comorbidity index of 2 or more (3 points)
- Source of bloodstream infection other than urinary or biliary tract (3 points)
- Inappropriate empirical therapy and inappropriate early targeted therapy (2 points)
Patients were considered low risk if they had a score of 0-7, and high of they had a score of 8 or more.
The risk assessment took is quick, easy to figure, and extremely important, Dr. Rodríguez-Baño noted. “This is a very easy-to-use tool that can help us make many patient management decisions. All of the information is already available in the patient’s chart, so it doesn’t require any additional assessments. It’s a very good way to individualize treatment.”
In the initial analysis, all-cause mortality at 30 days was 22% lower among patients who received appropriate early therapy than those who did not (38.5% vs. 60.6%). This translated to a 55% decrease in the risk of death (HR 0.45 in the fully adjusted model).
The investigators next turned their attention toward the group that received appropriate therapy. All-cause 30-day mortality was 41% in those who got monotherapy and 34.8% among those who got combination therapy..
Finally, this group was stratified according to the INCREMENT-CPE mortality risk score.
In the low-risk category, combination therapy did not confer a survival advantage over monotherapy. Death occurred in 20% of those getting monotherapy and 24% receiving combination treatment – not a significant difference (HR 1.21).
Combination therapy did, however, confer a significant survival benefit in the high-risk group. Death occurred in 62% of those receiving monotherapy and 48% of those receiving combination therapy – a 44% risk reduction (HR 0.56).
As long as they were appropriately targeted against the infective organism, all drugs used in the high-mortality risk group were similarly effective at reducing the risk of death. Compared to colistin monotherapy, a combination that included tigecycline reduced the risk of death by 55% (HR 0.45); combination with aminoglycosides by 58% (HR 0.42); and combination with carbapenems by 44% (HR 0.56).
A secondary analysis of this group determined that time was a critical factor in survival. Each day delay after day 2 significantly increased the risk of death, Dr. Rodríguez-Baño said. This 48-hour period gives clinicians a chance to wait for the culture and antibiogram to return, and then choose and initiate the best treatment. Before the results come back, empiric antibiotic therapy is appropriate, but changes should be made immediately after the results come back.
“We tend to think we must give the very best antibiotic at the very first moment that we see a patient with a serious infection,” he said. “But what we found is that it’s not critical to give the perfect antibiotic on the first day. It is critical, however, to give the correct one once you know which bacteria is causing the infection. Since it takes 48 hours for those results to come back, this is perfect timing.”
INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
[email protected]
On Twitter @Alz_gal
Key clinical point:
Major finding: Among these patients, correctly targeted monotherapy decreased the risk of death by 44% – just about the same risk reduction as conferred by combination therapy.
Data source: The INCREMENT retrospective study comprised 437 patients.
Disclosures: INCREMENT was funded in large part by the Spanish Network for Research in Infectious Diseases. Dr. Rodríguez-Baño has been a scientific advisor for Merck, AstraZeneca, and InfectoPharm.
Plazomicin beats gold standard antibiotics in complex, gram-negative bacterial infections
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
VIENNA – An investigational antibiotic effective against several types of gram-negative antibiotic-resistant bacteria has proved its mettle against serious infections of the urinary tract, respiratory tract, and bloodstream.
Plazomicin (Achaogen, San Francisco) posted good results in two phase III studies, handily besting comparator drugs considered gold standard for treating complicated urinary tract infections and pyelonephritis, as well as bloodstream infections and hospital- and ventilator-associated bacterial pneumonia.
Both the EPIC (Evaluating Plazomicin In cUTI) and CARE (Combating Antibiotic Resistant Enterobacteriaceae) trials have provided enough positive data for the company to move forward with a new drug application later this year. The company also plans to seek European Medicines Agency approval in 2018.
Plazomicin is an aminoglycoside that has been modified with several side chains that block aminoglycoside-modifying enzymes, Daniel Cloutier, PhD, principal clinical scientist of Achaogen said at the European Society of Clinical Microbiology and Infectious Disease annual congress.
“Aminoglycoside enzymes tend to travel along with beta-lactamases and carbapenemases as well, so this drug retains potent bactericidal activity against extended spectrum beta-lactamase producing Enterobacteriaceae, carbapenem-resistant Enterobacteriaceae, and aminoglycoside-resistant Enterobacteriaceae,” said Dr. Cloutier, who presented the results of the EPIC trial. The drug is given once-daily as a 30-minute intravenous infusion.
EPIC enrolled 609 adult patients with complicated urinary tract infections or acute pyelonephritis; Dr. Cloutier presented a modified intent-to-treat analysis comprising 388 of these. They were randomized to plazomicin 15 mg/kg every 25 hours or to IV meropenem 1 gram every 24 hours. Treatment proceeded for 4-7 days, after which patients could step down to oral therapy (levofloxacin 500 m/day), for a total of 7-10 days of treatment. About 80% of patients had both IV and oral therapy. At 15-19 days from the first dose, patients had a test of cure; at 24-32 days from first dose, they returned for a safety follow-up.
Patients were a mean of 60 years old. About 60% had a complicated UTI; the rest had acute pyelonephritis. About 35% had a mean creatinine clearance of more than 30-60 mL/min; the rest had a mean clearance of more than 60-90 mL/min.
The primary efficacy endpoint was microbial eradication. Plazomicin performed significantly better than meropenem in this measure (87% vs.72%), a mean difference of about 15%. Patients with pyelonephritis responded marginally better than those with complicated UTI, both groups favoring plazomicin (mean treatment differences of 17.5% and 13.7%, respectively).
The results were similar when the investigators examined groups by whether they needed oral step-down treatment. In the IV-only groups, plazomicin bested meropenem in microbiological eradication by almost 19% (84% vs. 65%). In the IV plus oral therapy group, the mean difference was 14%, also in favor of plazomicin (88% vs. 74%).
Plazomicin was significantly more effective then meropenem in all of the resistant Enterobacteriaceae groups (ESBL-positive, and levofloxacin- and aminoglycoside-resistant). It was also significantly more effective against E. coli (17% treatment difference), Klebsiella pneumonia (9%), and Proteus mirabilis (25%). However, it was 20% less effective than meropenem against E. cloacae.
At late follow-up, 2% of the plazomicin group and 8% of the meropenem group had relapsed – a significant difference.
Plazomicin and meropenem had similar safety profiles. Diarrhea and hypertension were the most common adverse events (about 2% in each group). Headache occurred in 3% of the meropenem patients and 1% of the plazomicin patients. Nausea, vomiting, and anemia occurred in about 1% of each group.
More patients taking plazomicin experienced a serum creatinine clearance increase of at least 0.5 mg/dL during treatment (7% vs. 4%). All but two patients taking plazomicin experienced a full recovery by the last follow-up visit.
The CARE trial was much smaller, comprised of 39 patients who had either bloodstream infections, or hospital-acquired or ventilator-associated bacterial pneumonia caused by carbapenem-resistant Enterobacteriaceae. Lynn Connolly, MD, senior medical director and head of late development at Achaogen, presented the data. Recruitment for such a narrow diagnosis was difficult, and hampered patient accrual, she noted.
CARE’s primary endpoints were a combination of all-cause mortality and significant disease-related complications, and all-cause mortality only, both at 28 days.
Patients were randomized 1:1 to either plazomicin 15 mg/kg every 24 hours, or colistin in a 300-mg loading dose, followed by daily infusions of 5 mg/kg. Everyone, regardless of treatment group, could also receive meropenem or tigecycline if deemed necessary. Treatment lasted 7-14 days. There was a test of cure at 7 days from the last IV dose, a safety assessment at 28 days, and a long-term follow-up at 60 days.
The patients’ mean age was about 65 years. Most (about 80%) had a bloodstream infection; bacterial pneumonias were present in the remainder. Most infections (85%) were monomicrobial, with polymicrobial infections making up the balance. Tigecycline was the favored adjunctive therapy (60%), followed by meropenem.
At day 28, plazomicin was associated with significantly better overall outcomes than colistin. It reduced the combination mortality/significant disease complications endpoint by 26% (23% vs. 50% meropenem). This translated to a 53% relative reduction in the risk of death.
Plazomicin also reduced all-cause mortality only by 28% (12% vs. 40% meropenem). This translated to a relative risk reduction of 70.5%.
The study drug performed well in the subgroup of patients with bloodstream infections, reducing the occurrence of the composite endpoint by 39% (14% vs. 53%), and of the mortality-only endpoint by 33% (7% vs. 40%). This translated to a 63% increased chance of 60-day survival in the plazomicin group.
Almost all patients in each group experienced at least one adverse event; 28% were deemed related to plazomicin and 43% to colistin. Many of these events were related to renal function (33% plazomicin, 52% colistin). Serum creatinine increases of at least 0.5 mg/dL during IV treatment occurred in fewer taking plazomicin (1 vs. 6 taking colistin). Full renal recovery occurred in the patient taking plazomicin, but only in three taking colistin.
“These data suggest that plazomicin could offer an important new treatment option for patients with serious infections due to carbapenem-resistant Enterobacteriaceae,” Dr. Connolly said.
[email protected]
On Twitter @Alz_gal
Reduced-intensity conditioning may not preserve fertility in young girls after bone marrow transplant
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
ORLANDO – Girls who undergo reduced-intensity conditioning for a bone marrow transplant may face fertility problems in the future, even if they experience an outwardly normal puberty.
In the first-ever study to compare high- and low-intensity chemotherapeutic conditioning regimens among young girls, significantly more who underwent the reduced-intensity regimen had normal estradiol, luteinizing hormone, and follicle-stimulating hormone compared with those who had high-intensity conditioning. But anti-Müllerian hormone was low or absent in almost all the girls, no matter which conditioning regimen they had, Jonathan C. Howell, MD, PhD, said at the annual meeting of the Endocrine Society.
While not a perfect predictor of future fertility, anti-Müllerian hormone is a good indicator of ovarian follicular reserve, said Dr. Howell, a pediatric endocrinologist at Cincinnati Children’s Hospital Medical Center.
Dr. Howell and his colleagues, Holly R. Hoefgen, MD, Kasiani C. Myers, MD, and Helen Oquendo-Del Toro, MD, all of Cincinnati Children’s Hospital Medical Center, are following 49 females aged 1-40 years who had preconditioning chemotherapy in advance of hematopoietic stem cell transplantation.
At the meeting, Dr. Howell reported data on 23 girls who were in puberty during their treatment (mean age 12 years). The mean follow-up was 4 years, but this varied widely, from 1 to 13 years. Most (16) had high-intensity myeloablation; the remainder had reduced-intensity conditioning. Diagnoses varied between the groups. Among those with high-intensity conditioning, malignancy and bone marrow failure were the most common indications (seven patients each); one patient had an immunodeficiency, and the cause was unknown for another.
Among those who had the reduced-intensity regimen, five had an immunodeficiency and two had bone marrow failure.
The discrepancy in diagnoses between the groups isn’t surprising, Dr. Howell said. “Diagnosis can dictate which treatment patients receive. People with malignancies or a prior history of leukemia or lymphoma often receive the high-intensity conditioning. You want to wipe out every single malignant cell.”
Reduced-intensity conditioning may be an option for patients with other problems such as bone marrow failure, immunodeficiencies, or genetic or metabolic problems. The less-intense regimen does confer some benefits, Dr. Howell noted. “The short-term need for intensive medical therapy while getting the stem cells is less. The medical benefit of these less-intense regimens is certainly there, but the long-term endocrine impact has yet to be defined.”
Most of the girls in the high-intensity regimen group (64%) had high follicle stimulating hormone and luteinizing hormone, suggesting primary ovarian failure; 71% of them also had low estradiol levels. However, all of these hormones were normal in the reduced-intensity group. But regardless of conditioning treatment, anti-Müllerian hormone was abnormally low in nearly all of the patients (87%). Only one girl with myeloablative conditioning and two girls with reduced intensity condition had normal anti-Müllerian levels. “This tells us that fertility potential may not be preserved, despite [their] getting the reduced-intensity conditioning,” Dr. Howell said.
The story here is only beginning to unfold, he said. “Fertility is defined as the ability to conceive a child, and that’s not something we have looked at yet. We would like to know the long-term outcomes of fertility in these patients, and whether they can conceive when they’re ready to start a family. Our goal is to follow these young women into their 20s and 30s, and to see if that’s an opportunity they are able to experience.”
The study is a cooperative project involving the hospital’s divisions of Pediatric and Adolescent Gynecology, Bone Marrow Transplantation and Immunology, and Endocrinology.
Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Fertility preservation talks: The earlier, the better
A talk about fertility preservation can be the first step into a new future for families of children with a cancer diagnosis.
“Talking about your baby having a baby can be the farthest thing from your mind,” when you’re the parent of a child about to undergo cancer treatment, said Dr. Hoefgen. “But we know from survivors that this can be a very important issue in the future. We simply start by telling parents, ‘This will be important to your child at some point, and we want to talk about it now, while there is still something we may be able to do about it.’ ”
Dr. Hoefgen, a staff member at the hospital’s Comprehensive Fertility Care and Preservation Program, said parents “sometimes find it weird” to be talking about unborn grandchildren when they’re consumed with making critical decisions for their own child. But by asking them to consider that child’s long-term future, the discussion offers its own message of hope.
The talks always begin with a basic discussion of how cancer treatments can affect the reproductive organs. The hospital has a series of short animated videos that are very helpful in relaying the information. Another video in that series describes the different methods of fertility preservation: mature oocyte or sperm harvesting, or, for younger patients, removing and freezing ovarian and testicular tissue. Parents and children can watch them together, get grounding in the basics, and be prepared for a productive conversation.
Talks always include the team oncologist, who creates a specialized risk assessment for each patient. The group discusses each preservation method, the risks and benefits, and the cost. But the talks are exploratory, too, helping both clinicians and families understand what’s most important to them, she said.
“Common things that we typically talk about are genetics, religion, and ethics – which may mean different things to different families.”
Dr. Hoefgen and her team reach out to more than 95% of families that face a pediatric cancer diagnosis. After the in-depth discussions, she said, about 20% decide to investigate some form of fertility preservation.
“The most important thing is having the conversation early, while we still have options,” she said.
Dr. Hoefgen had no financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Anti-Müllerian hormone was abnormally low or absent in all treated girls, whether they had reduced-intensity or high-intensity conditioning.
Data source: The prospective study is following 49 females aged 1-40 years.
Disclosures: Neither Dr. Howell nor any of his colleagues had any financial disclosures.
Simple changes shorten door-to-insulin time for diabetic ketoacidosis
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – A few simple adjustments in standing orders shaved 20 minutes off the time one emergency department took to deliver insulin to pediatric patients with diabetic ketoacidosis, according to Mary Ellen Vajravelu, MD.
Door-to-insulin time decreased from a mean of 168 minutes to 146 minutes after the changes went into effect. Most of the time was saved in the period between starting the initial fluid bolus and starting the insulin, she said during an interview at the annual meeting of the Endocrine Society.
A single patient’s experience inspired the quality improvement project.
“A couple of years ago, we saw a patient in the emergency department with diabetic ketoacidosis who had to wait more than 6 hours before insulin was started,” she explained. “Fortunately, there were no adverse clinical outcomes from that,” but the case signaled that the treatment process needed some streamlining.
The International Society for Pediatric and Adolescent Diabetes recommends immediate fluid administration and insulin within 1-2 hours of starting fluid replacement.
The hospital had been following in-house recommendations for fluids, insulin, and labs for patients with diabetic ketoacidosis that were established in 2006 and 2011. When they examined a baseline cohort of patients treated from January to October 2014, they immediately saw that those order sets were not achieving the recommended times.
“We went back and looked at our cases and saw that overall, it was taking more than 2 hours from the time of arrival for these patients to get their insulin,” Dr. Vajravelu said.
She and her colleagues formed a quality improvement team consisting of physicians, nurses, and a pharmacist. They broke down the wait time into three components: arrival to fluid bolus, fluid bolus start to insulin start, and the overall arrival-to-insulin time. They then set specific time goals for each of those periods: giving fluids within 60 minutes of arrival, starting insulin 45-90 minutes from the bolus, and an overall door-to-insulin time of less than 120 minutes.
The team tried to determine key clinical drivers that were affecting these times. One of the first findings was a delay in diagnosing diabetic ketoacidosis (DKA). This was driven by lack of consistency in scoring patient acuity and problems with venous access.
“To address this, we standardized acuity scoring, got an IV team involved, and used point-of-care labs when possible, and educated our providers,” Dr. Vajravelu said.
The team also found that there were delays in ordering the insulin. These were driven by long wait times for intravenous fluids, by waiting for endocrinology consults, and by providers not being familiar with the DKA order sets.
To address these issues, the team changed the order set to use normal saline for all suspected DKA patients without waiting for lab results. The old orders called for delivering the fluid over a 1-hour period; that was changed to running it over 30 minutes, Dr. Vajravelu said. “That shaved off half an hour right there.”
The team examined reasons that insulin infusion was delayed. Pharmacy orders were a big part of this problem, one of which was the way the insulin was sent up from the pharmacy in a bag. “We changed that to being delivered in a syringe and tube system,” she explained.
They also changed the standing order for when insulin was started, from 60 minutes after fluid to 45 minutes.
These changes were instituted in November 2014, and the team trained clinicians on them through January 2015. Since then, the team has reassessed the situation several times, tracking 291 patients in all. They saw improvement in all three time periods, which was significant in two of them.
Arrival to fluid bolus time dropped significantly, from 55 minutes to 53 minutes – an improvement, even though the baseline time was still within the goal. Fluid bolus-to-insulin time fell significantly as well, from 96 minutes to 74 minutes – well within the new goal.
Overall arrival-to-insulin time thus decreased, from 168 minutes to 146 minutes. However, Dr. Vajravelu said, this is still longer than their goal of less than 120 minutes from door to treatment.
“We are now going back to try and see just where the delay still is,” she said. “It may be getting IV access; it may be labs. We need to tease that out.”
Fortunately, the consistent delays in treatment haven’t resulted in any adverse clinical outcomes.
“I think the biggest benefit we will see will be saving time and money in the emergency department,” Dr. Vajravelu predicted. “We hope to get patients out of there faster, getting them admitted into the hospital and treated in the appropriate department. Speeding up care and reducing waste go together in that sense.”
Dr. Vajravelu had no relevant financial disclosures.
[email protected]
On Twitter @Alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Overall door-to-insulin time decreased from 168 minutes to 146 minutes.
Data source: A 2-year quality improvement project involving 291 patients.
Disclosures: Dr. Vajravelu had no relevant financial disclosures.
Dulera inhaler linked to adrenocorticotropic suppression in small case series
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.
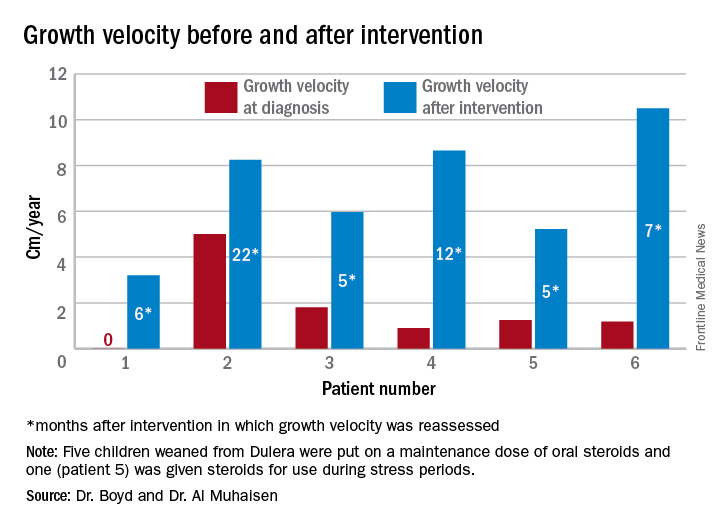
The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.

The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
ORLANDO – A combination corticosteroid asthma inhaler has, for the first time, been associated with growth delay and adrenocorticotropic suppression in children.
The single-center case series is small, but the results highlight the need to regularly monitor growth and adrenal function in children using inhaled mometasone furoate/formoterol fumarate (Dulera; Merck), investigators said at the annual meeting of the Endocrine Society.
“We are hoping to raise awareness of this risk in our pediatric endocrinology colleagues, as well as among allergists, pulmonologists, and pediatricians who treat these children,” said Fadi Al Muhaisen, MD. “These kids should be regularly screened for growth delay and adrenal insufficiency and have their growth plotted at every visit as well.”
Dulera was approved in the United States in 2010 as a maintenance therapy for chronic asthma in adults and children aged 12 years and older. Mometasone furoate is a potent corticosteroid, and formoterol fumarate is a long-acting beta2-adrenergic agonist. The prescribing information says that mometasone furoate exerts less effect on the hypothalamic-pituitary-adrenal axis than other inhaled corticosteroids, and that adrenal suppression is unlikely to occur when used at recommended dosages. These range from a low of 100 mcg/5 mcg, two puffs daily to a maximum dose of 800 mcg/20 mcg daily.
The review involved 18 children, all of whom were seen in the endocrinology clinic for growth failure or short stature and were receiving Dulera for management of their asthma. Of these, eight (44%) had a full adrenal evaluation. Six had biochemical evidence of adrenal suppression and two had normal adrenal function. The remaining 10 patients had not undergone an adrenal evaluation. None of them were on any other inhaled corticosteroid. The six children diagnosed with adrenal insufficiency had a mean age of 9.7 years, but ranged in age from 7 to 12 years. They had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids in the preceding 6 months before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at the time of diagnosis but had been using the higher dose for the preceding 18 months. Three were using concomitant nasal steroids.
The six children evaluated had been using the medication for a mean of 1.3 years, although that varied widely, from just a few months to about 2 years. Only one had been on oral steroids during the 2 years before coming to the endocrinology clinic. Five were using the 200 mcg/5 mcg dose, two puffs daily; one child was taking one puff daily of 100 mcg/5 mcg at time of diagnosis, but had been using the higher dose for 18 months before that. Three were using concomitant nasal steroids.
All presented with growth failure, with bone age 1-3 years behind chronological age. One child was referred to the clinic after an emergency department visit for headache, nausea, diarrhea, and fatigue – symptoms of adrenal failure. That child had an adrenocroticotropin (ACTH) level of 10 pg/mL. Both his random peak cortisol measures after ACTH stimulation were less than 1 mcg/mL.
ACTH levels in four of the children were less than 5-6 pg/ml, with random and peak stimulated cortisols of around 1 mcg/mL. One patient had an ACTH level of 68 pg/mL, a random cortisol of less than 1 mcg/mL, and a peak stimulated cortisol of 8.7 mcg/mL.

The results were all normal in the four subjects who had repeat adrenal function evaluation after intervention. Adrenal recovery took a mean of 20 months (5-30 months).
Growth accelerated rapidly after intervention, which was either initiation of maintenance oral steroids and discontinuation of Dulera or, in one patient, after Dulera was weaned. At time of adrenal insufficiency diagnosis, four patients had grown 1-2 cm in the prior year; one had not grown at all, and one had grown about 4.5 cm. After discontinuing or weaning the medication, all experienced growth spurts: 3 cm/year in 6 months; 8 cm/year in 22 months; 6 cm/year in 5 months; 8 cm/year in 12 months; 5 cm/year in 5 months; and 10 cm/year in 7 months.
There were no exacerbations in asthma, despite discontinuing the inhaled medication, Dr. Al Muhaisen said. Changing the asthma treatment required some open discussion between the investigators and the treating pulmonologists, he noted.
“We had some back-and-forth discussions, being very frank that we thought the adrenal insufficiency was directly related to this medication and that we needed to wean it and stop it as soon as possible.”
Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
AT ENDO 2017
Key clinical point:
Major finding: Of eight children who had an adrenal workup at an endocrinology clinic, six had adrenal suppression.
Data source: The case series comprised 18 children taking Dulera who presented with growth failure.
Disclosures: Neither Dr. Al Muhaisen nor Dr. Boyd had any financial disclosures.
Most children with growth disorders benefit from growth hormone
ORLANDO – About 80% of children with growth disorders will attain their expected normal adult height if adequately treated with growth hormone, a large observational study has determined.
Somatropin recombinant human growth hormone seemed most effective for patients with growth hormone deficiency, idiopathic short stature, and for those who were born small for gestational age. Patients who had Turner syndrome or a homeobox-deficiency gene didn’t do quite as well, although most did still improve their final height.
The Genetics and Neuroendocrinology of Short Stature International Study (GENESIS) followed more than 22,000 children who were treated in 30 countries from 1999 to 2015.
All of the patients were treated with somatropin recombinant human growth hormone (Humatrope). Eli Lilly, which makes the medication, sponsored the study and presented the results during a poster session at the annual meeting of the Endocrine Society. Representatives at the poster declined to discuss results with the media during the open session.
“The results speak for themselves,” said Cheri Deal, MD, PhD, of the University of Montreal, Quebec, one of the presenting authors. “It’s an incredible story.”
The GENESIS cohort comprised more than 22,000 children who were treated for a variety of growth disorders at 827 international sites.
Subanalyses have been published, but this is the first release of the data on 5,076 patients with full follow-up. Most of the patients (61%) received the medication for growth hormone deficiency. Other indications were Turner syndrome (14%), idiopathic short stature (11%), short-stature homeobox-containing gene deficiency (3%), small for gestational age (5%), and chronic renal insufficiency (0.3%). Other diagnoses, plus unknown causes, made up the remainder of the diagnoses.
The investigators, led by Christopher J. Child, PhD, an Eli Lilly scientist, split the cohort into diagnostic groups and treatment subgroups: those who attained normal adult height, those who attained normal adult height with at least 4 years of treatment, and those who gained normal adult height who started treatment at younger than 10 years of age.
At baseline, those with growth hormone deficiency were a mean of 11 years old; their height was a mean of 2.4 standard deviations (SDS) below normal. They received a mean of 6 years of treatment; 86% achieved a normal adult height, with an overall gain of 1.39 SDS. Those who started somatropin before age 10 years made bigger gains. The final height gain was 1.78 SDS after a mean of 9.5 years of treatment.
At baseline, those with idiopathic short stature were a mean of 12 years old; their mean height was 2.3 SDS below normal. They received a mean of 4.7 years of treatment; 82% achieved normal height, with a gain of 1.1 SDS. Starting somatropin at a younger age (mean 8 years) improved outcomes slightly, with a final height gain of 1.6 SDS after 8 years of treatment.
Patients with Turner syndrome were a mean of 10 years when they started treatment; their mean height was 2.65 SDS below normal. They received a mean of 6.4 years of treatment; 66% achieved normal adult height, with a mean height gain of 0.95 SDS. Early treatment didn’t alter outcomes much in this group. After a mean of 8.5 years of treatment, 65% achieved normal height, with a height gain of 0.84 SDS.
Patients with a short-stature homeobox-containing gene deficiency were a mean of 11 years when they started treatment. At that time, their height was a mean of 2.36 SDS below normal. They were treated for a mean of 4.7 years; 76% achieved a normal adult height, with a mean gain of 0.86 SDS. Early treatment did improve outcomes a bit. After a mean treatment time of 7 years, 78% gained normal adult height, with a height gain of 0.84 SDS.
Children who were small for gestational age started treatment when they were a mean of 10.5 years. At that time, their mean height was 2.57 SDS below normal. They were treated for a mean of 5.4 years; 78% attained a normal adult height, with a mean gain of 1.1 SDS. Early treatment improved outcomes. After a mean treatment time of 7.8 years, 81% achieved normal adult height, with a mean gain of 1.41 SDS.
The less-robust gains seen in the children with genetically influenced growth disorders “wasn’t unexpected” and is in line with what has been observed in a number of clinical trials, the investigators said.
Eli Lilly manufactures the somatropin product used in the study, and it sponsored the study. Dr. Child is an employee of the company. Dr. Deal did not have any financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – About 80% of children with growth disorders will attain their expected normal adult height if adequately treated with growth hormone, a large observational study has determined.
Somatropin recombinant human growth hormone seemed most effective for patients with growth hormone deficiency, idiopathic short stature, and for those who were born small for gestational age. Patients who had Turner syndrome or a homeobox-deficiency gene didn’t do quite as well, although most did still improve their final height.
The Genetics and Neuroendocrinology of Short Stature International Study (GENESIS) followed more than 22,000 children who were treated in 30 countries from 1999 to 2015.
All of the patients were treated with somatropin recombinant human growth hormone (Humatrope). Eli Lilly, which makes the medication, sponsored the study and presented the results during a poster session at the annual meeting of the Endocrine Society. Representatives at the poster declined to discuss results with the media during the open session.
“The results speak for themselves,” said Cheri Deal, MD, PhD, of the University of Montreal, Quebec, one of the presenting authors. “It’s an incredible story.”
The GENESIS cohort comprised more than 22,000 children who were treated for a variety of growth disorders at 827 international sites.
Subanalyses have been published, but this is the first release of the data on 5,076 patients with full follow-up. Most of the patients (61%) received the medication for growth hormone deficiency. Other indications were Turner syndrome (14%), idiopathic short stature (11%), short-stature homeobox-containing gene deficiency (3%), small for gestational age (5%), and chronic renal insufficiency (0.3%). Other diagnoses, plus unknown causes, made up the remainder of the diagnoses.
The investigators, led by Christopher J. Child, PhD, an Eli Lilly scientist, split the cohort into diagnostic groups and treatment subgroups: those who attained normal adult height, those who attained normal adult height with at least 4 years of treatment, and those who gained normal adult height who started treatment at younger than 10 years of age.
At baseline, those with growth hormone deficiency were a mean of 11 years old; their height was a mean of 2.4 standard deviations (SDS) below normal. They received a mean of 6 years of treatment; 86% achieved a normal adult height, with an overall gain of 1.39 SDS. Those who started somatropin before age 10 years made bigger gains. The final height gain was 1.78 SDS after a mean of 9.5 years of treatment.
At baseline, those with idiopathic short stature were a mean of 12 years old; their mean height was 2.3 SDS below normal. They received a mean of 4.7 years of treatment; 82% achieved normal height, with a gain of 1.1 SDS. Starting somatropin at a younger age (mean 8 years) improved outcomes slightly, with a final height gain of 1.6 SDS after 8 years of treatment.
Patients with Turner syndrome were a mean of 10 years when they started treatment; their mean height was 2.65 SDS below normal. They received a mean of 6.4 years of treatment; 66% achieved normal adult height, with a mean height gain of 0.95 SDS. Early treatment didn’t alter outcomes much in this group. After a mean of 8.5 years of treatment, 65% achieved normal height, with a height gain of 0.84 SDS.
Patients with a short-stature homeobox-containing gene deficiency were a mean of 11 years when they started treatment. At that time, their height was a mean of 2.36 SDS below normal. They were treated for a mean of 4.7 years; 76% achieved a normal adult height, with a mean gain of 0.86 SDS. Early treatment did improve outcomes a bit. After a mean treatment time of 7 years, 78% gained normal adult height, with a height gain of 0.84 SDS.
Children who were small for gestational age started treatment when they were a mean of 10.5 years. At that time, their mean height was 2.57 SDS below normal. They were treated for a mean of 5.4 years; 78% attained a normal adult height, with a mean gain of 1.1 SDS. Early treatment improved outcomes. After a mean treatment time of 7.8 years, 81% achieved normal adult height, with a mean gain of 1.41 SDS.
The less-robust gains seen in the children with genetically influenced growth disorders “wasn’t unexpected” and is in line with what has been observed in a number of clinical trials, the investigators said.
Eli Lilly manufactures the somatropin product used in the study, and it sponsored the study. Dr. Child is an employee of the company. Dr. Deal did not have any financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – About 80% of children with growth disorders will attain their expected normal adult height if adequately treated with growth hormone, a large observational study has determined.
Somatropin recombinant human growth hormone seemed most effective for patients with growth hormone deficiency, idiopathic short stature, and for those who were born small for gestational age. Patients who had Turner syndrome or a homeobox-deficiency gene didn’t do quite as well, although most did still improve their final height.
The Genetics and Neuroendocrinology of Short Stature International Study (GENESIS) followed more than 22,000 children who were treated in 30 countries from 1999 to 2015.
All of the patients were treated with somatropin recombinant human growth hormone (Humatrope). Eli Lilly, which makes the medication, sponsored the study and presented the results during a poster session at the annual meeting of the Endocrine Society. Representatives at the poster declined to discuss results with the media during the open session.
“The results speak for themselves,” said Cheri Deal, MD, PhD, of the University of Montreal, Quebec, one of the presenting authors. “It’s an incredible story.”
The GENESIS cohort comprised more than 22,000 children who were treated for a variety of growth disorders at 827 international sites.
Subanalyses have been published, but this is the first release of the data on 5,076 patients with full follow-up. Most of the patients (61%) received the medication for growth hormone deficiency. Other indications were Turner syndrome (14%), idiopathic short stature (11%), short-stature homeobox-containing gene deficiency (3%), small for gestational age (5%), and chronic renal insufficiency (0.3%). Other diagnoses, plus unknown causes, made up the remainder of the diagnoses.
The investigators, led by Christopher J. Child, PhD, an Eli Lilly scientist, split the cohort into diagnostic groups and treatment subgroups: those who attained normal adult height, those who attained normal adult height with at least 4 years of treatment, and those who gained normal adult height who started treatment at younger than 10 years of age.
At baseline, those with growth hormone deficiency were a mean of 11 years old; their height was a mean of 2.4 standard deviations (SDS) below normal. They received a mean of 6 years of treatment; 86% achieved a normal adult height, with an overall gain of 1.39 SDS. Those who started somatropin before age 10 years made bigger gains. The final height gain was 1.78 SDS after a mean of 9.5 years of treatment.
At baseline, those with idiopathic short stature were a mean of 12 years old; their mean height was 2.3 SDS below normal. They received a mean of 4.7 years of treatment; 82% achieved normal height, with a gain of 1.1 SDS. Starting somatropin at a younger age (mean 8 years) improved outcomes slightly, with a final height gain of 1.6 SDS after 8 years of treatment.
Patients with Turner syndrome were a mean of 10 years when they started treatment; their mean height was 2.65 SDS below normal. They received a mean of 6.4 years of treatment; 66% achieved normal adult height, with a mean height gain of 0.95 SDS. Early treatment didn’t alter outcomes much in this group. After a mean of 8.5 years of treatment, 65% achieved normal height, with a height gain of 0.84 SDS.
Patients with a short-stature homeobox-containing gene deficiency were a mean of 11 years when they started treatment. At that time, their height was a mean of 2.36 SDS below normal. They were treated for a mean of 4.7 years; 76% achieved a normal adult height, with a mean gain of 0.86 SDS. Early treatment did improve outcomes a bit. After a mean treatment time of 7 years, 78% gained normal adult height, with a height gain of 0.84 SDS.
Children who were small for gestational age started treatment when they were a mean of 10.5 years. At that time, their mean height was 2.57 SDS below normal. They were treated for a mean of 5.4 years; 78% attained a normal adult height, with a mean gain of 1.1 SDS. Early treatment improved outcomes. After a mean treatment time of 7.8 years, 81% achieved normal adult height, with a mean gain of 1.41 SDS.
The less-robust gains seen in the children with genetically influenced growth disorders “wasn’t unexpected” and is in line with what has been observed in a number of clinical trials, the investigators said.
Eli Lilly manufactures the somatropin product used in the study, and it sponsored the study. Dr. Child is an employee of the company. Dr. Deal did not have any financial disclosures.
[email protected]
On Twitter @Alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Overall, 80% of the treated children reached normal adult height.
Data source: The prospective observational cohort study comprised 5,076 children.
Disclosures: Eli Lilly manufactures the somatropin product used in the study, and it sponsored the study. Dr. Child is an employee of the company. Dr. Deal did not have any financial disclosures.
Osilodrostat maintained cortisol control in Cushing’s syndrome
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
[email protected]
On Twitter @alz_gal
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
[email protected]
On Twitter @alz_gal
ORLANDO – Osilodrostat, a drug that normalized cortisol in 89% of patients with Cushing’s syndrome who took it during a phase II study, continued to exert a sustained benefit during a 31-month extension phase.
In an intent-to-treat analysis, all of the 16 patients who entered the LINC-2 extension study responded well to the medication, with no lapse in cortisol control, Rosario Pivonello, MD, said at the annual meeting of the Endocrine Society.
Osilodrostat, made by Novartis, is an oral inhibitor of 11 beta–hydroxylase. The enzyme catalyzes the last step of cortisol synthesis in the adrenal cortex. The drug was granted orphan status in 2014 by the European Medicines Agency.
In the LINC-2 study, 19 patients took osilodrostat at an initial dose of either 4 mg/day or 10 mg/day, if baseline urinary-free cortisol exceeded three times the upper normal limit. The dose was escalated every 2 weeks to up to 60 mg/day, until cortisol levels were at or below the upper limit of normal. In this study, the main efficacy endpoint was normalization of cortisol, or at least a 50% decrease from baseline at weeks 10 and 22.
Overall response was 89%. Osilodrostat treatment reduced urinary-free cortisol in all patients, and 79% had normal cortisol levels at week 22. The most common adverse events were nausea, diarrhea, asthenia, and adrenal insufficiency. New or worsening hirsutism and/or acne were reported among four female patients, all of whom had increased testosterone levels.
The LINC-2 extension study enrolled 16 patients from the phase II cohort, all of whom had responded to the medication. They were allowed to continue on their existing effective dose through the 31-month period.
Dr. Pivonello presented response curves that tracked cortisol levels from treatment initiation in the LINC-2 study. The median baseline cortisol level was about 1,500 nmol per 24 hours. By the fourth week of treatment, this had normalized in all of the patients who entered the extension phase. The response curve showed continued, stable cortisol suppression throughout the entire 31-month period.
Four patients dropped out during the course of the study. Dr. Pivonello didn’t discuss the reasons for these dropouts. He did break down the results by response, imputing the missing data from these four patients. In this analysis, the majority (87.5%) were fully controlled, with urinary-free cortisol in the normal range. The remainder were partially controlled, experiencing at least a 50% decrease in cortisol from their baseline levels. These responses were stable, with no patient experiencing loss of control over the follow-up period.
The 12 remaining patients are still taking the medication, and they experienced other clinical improvements as well. Systolic blood pressure decreased by a mean of 2.2% (from 130 mm Hg to 127 mm Hg). Diastolic blood pressure also improved, by 6% (from 85 mm Hg to 80 mm Hg).
Fasting plasma glucose dropped from a mean of 89 mg/dL to 82 mg/dL. Weight decreased from a mean of 84 kg to 74 kg, with a corresponding decrease in body mass index, from 29.6 kg/m2 to 26.2 kg/m2.
Serum aldosterone decreased along with cortisol, dropping from a mean of 168 pmol/L to just 19 pmol/L. Adrenocorticotropic hormone increased, as did 11-deoxycortisol, 11-deoxycorticosterone, and testosterone.
Pituitary tumor size was measured in six patients. It increased in three and decreased in three. Dr. Pivonello didn’t discuss why this might have occurred.
The most common adverse events were asthenia, adrenal insufficiency, diarrhea, fatigue, headache, nausea, and acne. These moderated over time in both number and severity.
However, there were eight serious adverse events among three patients, including prolonged Q-T interval on electrocardiogram, food poisoning, gastroenteritis, headache, noncardiac chest pain, symptoms related to pituitary tumor (two patients), and uncontrolled Cushing’s syndrome.
Two patients experienced hypokalemia. Six experienced mild events related to hypocortisolism.
Novartis is pursuing the drug with two placebo-controlled phase III studies (LINC-3 and LINC-4), Dr. Pivonello said. An additional phase II study is being conducted in Japan.
Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
[email protected]
On Twitter @alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Every patient in the study responded either fully (87.5%) or partially, with a decrease of at least 50% in cortisol from baseline (12.5%), with no loss of control.
Data source: The 31-month extension study comprised 16 patients who had responded well to the drug in a phase II trial.
Disclosures: Dr. Pivonello has received consulting fees and honoraria from Novartis, which sponsored the study.
Long-acting growth hormone moves forward based on positive phase II data
ORLANDO – An extended-release human growth hormone formulation proved safe and effective in both children and adults, offering the prospect of a less-rigorous dosing schedule and potentially better patient compliance with treatment.
The two phase II studies examined somavaratan, which is being developed by Versartis of Menlo Park, Calif. Kevin Yuen, MD, an endocrinologist at the Swedish Medical Center, Seattle, and Wayne V. Moore, MD, a pediatric endocrinologist at Children’s Mercy Hospital, Kansas City, Mo., presented the data at the annual meeting of the Endocrine Society.
“Despite the fact that human growth hormone is a proven treatment for growth hormone deficiency, daily use of our current formulations can be a factor that affects compliance,” said Dr. Yuen. He cited a 2008 study of 158 men taking growth hormone, which found that only one-third were highly compliant (Endocr Pract. 2008 Mar;14[2]:143-54). “And even among this group, there were 21 doses missed over just a 3-month period.”
The group of 55 international experts described several strategies for creating long-acting growth hormone formulations, including depot formulations, pegylation, prodrugs, noncovalent albumin binding growth hormone compounds, and growth hormone fusion proteins. These preparations are currently in various stages of development, with some already approved in Europe and Asia.
Somavaratan (VRS-317) is a fusion protein produced in Escherichia coli. The active portion is recombinant human growth hormone, which is bound to long chains of hydrophilic amino acids. This reduces renal filtration, Dr. Yuen said. The growth hormone loses some potency in this construct, but its delayed clearance, with a half-life 30-60 times longer than recombinant human growth hormone (rhGH) allows it to exert a prolonged effect in target tissue.
Of the two phase II studies of the molecule, one was conducted in 64 prepubertal children who were naive to any growth hormone treatment, and one in 36 adults with adult-onset growth hormone deficiency. The company also has made these presentations available online.
The pediatric study reported 3-year data on the cohort, which began treatment of children at a mean age of 7 years. At baseline, the children were about 2.6 standard deviations (SDs) below their expected height, and their mean IGF-1 levels, about 1.7 SDs below. They showed a mean maximum stimulated growth hormone level of 5.4 ng/mL. Although they were a mean of 7.8 years chronologically at the study’s outset, their mean bone age was 6.4 years.
The first 12 months of the study consisted of dose-ranging trials, with initial doses of 5 mg/kg each month, then 2.5 mg/kg twice a month, and then 1.15 mg/kg weekly. During the last 2 years of the study, all children were taking 3.5 mg/kg, once a month.
Within the first year, all children taking the 3.5-mg/kg dose had achieved normal IGF-1 levels, which were consistent with levels achieved in the ANSWER registry dataset of somatropin (rDNA origin) injection (Norditropin) recombinant human growth hormone (Clin Epidemiol. 2013;5:119-27).
Height velocity improved, as did height standard deviation. By year 3, patients were a mean of 1.25 SDs below expected height – a significant improvement over baseline. These findings were almost superimposable with those in the ANSWER registry. Bone age and chronological age came into alignment within the first year and that association was maintained throughout the study – again, in almost superimposable curves with the registry data.
Somavaratan exerted no untoward metabolic effects. There were no adverse changes in body mass index. At baseline, the mean hemoglobin A1c was 5.2%; this was unchanged at 3 years. No patient developed diabetes. The most commonly reported adverse event was injection site pain (48%). Injection site erythema was reported in 5% of patients, but no injection site nodules occurred.
Other adverse events were headache, extremity pain, arthralgia, and musculoskeletal pain. Although the numbers were small overall, reports did increase after all the children were switched to the 3.5-mg/kg dose. However, they occurred in 5% or less of the patient group. There were no withdrawals due to adverse events.
The second trial was a dose-ranging study conducted in 49 adults aged 23-70 years. They all had been diagnosed with adult-onset growth hormone deficiency, but had stable pituitary function. If they were on any growth hormone therapy, they underwent a 14-day washout period.
The subjects were divided and dosed by age and gender. All subjects received one injection per month for 5 months.
Cohort A comprised 21 men and women aged 35 years or older, who took 0.6 mg/kg per month. Cohort B comprised six men and women younger than 35 years, who took 0.8 mg/kg per month. Cohort C comprised eight women taking oral estrogen contraceptives. These women received 1 mg/kg per month.
The cohorts were similar in body mass index and weight, but they did differ significantly in baseline IGF-1 levels. In cohort A, the level was 0.52 SDs below normal. In cohort B, it was 2.89 SDs below normal, and in cohort C, it was 2.29 SDs below.
Overall, somavaratan induced a rapid and dramatic increase in IGF-1 that tailed off over 30 days. By day 8 after injection, IGF-1 had risen from a mean baseline of -1 SDs to more than 2 SDs above. By day 22, it had returned to baseline levels. The response to the fifth injection was identical to that of the first, Dr. Yuen said.
Response varied somewhat by cohort, with the younger, mixed-gender group responding the most dramatically, with a mean increase of about 4 SDs from baseline. This put the group above the maximum response target of 1.5 SDs.
The older, mixed-gender cohort experienced about a 3 SDs increase – also above the target level. The women taking estrogen experienced the flattest response, gaining about 2 SDs. However, the response curve was nearly identical, with a rapid, sharp increase in IGF-1 within the first week, followed by a gradual decline to baseline by 22 days.
The adverse event profile was not quite as benign as it was in the pediatric study. Virtually all patients experienced at least one adverse event. A third were mild and 58% moderate. The rest were serious, with one severe and one life-threatening event. Dr. Yuen did not discuss adverse events; these were, however, included in supplementary slides available on the Versartis Inc. slide set.
The finding of a predictable, 3-week tailing-off of efficacy, combined with the fact that patients responded so dramatically, exceeding the maximum target of a 1.5 SDs increase in IGF-1, has prompted a new dosing protocol for the somavaratan open-label extension study, which includes all the phase II completers, plus an additional 40 adult patients.
Doses will be titrated to each subject’s individual IGF-1 responses, based on the IGF-I level 7 days post dose until a maintenance dose is reached. Subjects receiving somavaratan in a previous somavaratan study will have their dose decreased by half (minimum dose of 20 mg, or 40 mg for women on estrogen).
Dr. Yuen is a member of the Versartis advisory board. Dr. Moore has received research support from the company.
[email protected]
On Twitter @Alz_Gal
ORLANDO – An extended-release human growth hormone formulation proved safe and effective in both children and adults, offering the prospect of a less-rigorous dosing schedule and potentially better patient compliance with treatment.
The two phase II studies examined somavaratan, which is being developed by Versartis of Menlo Park, Calif. Kevin Yuen, MD, an endocrinologist at the Swedish Medical Center, Seattle, and Wayne V. Moore, MD, a pediatric endocrinologist at Children’s Mercy Hospital, Kansas City, Mo., presented the data at the annual meeting of the Endocrine Society.
“Despite the fact that human growth hormone is a proven treatment for growth hormone deficiency, daily use of our current formulations can be a factor that affects compliance,” said Dr. Yuen. He cited a 2008 study of 158 men taking growth hormone, which found that only one-third were highly compliant (Endocr Pract. 2008 Mar;14[2]:143-54). “And even among this group, there were 21 doses missed over just a 3-month period.”
The group of 55 international experts described several strategies for creating long-acting growth hormone formulations, including depot formulations, pegylation, prodrugs, noncovalent albumin binding growth hormone compounds, and growth hormone fusion proteins. These preparations are currently in various stages of development, with some already approved in Europe and Asia.
Somavaratan (VRS-317) is a fusion protein produced in Escherichia coli. The active portion is recombinant human growth hormone, which is bound to long chains of hydrophilic amino acids. This reduces renal filtration, Dr. Yuen said. The growth hormone loses some potency in this construct, but its delayed clearance, with a half-life 30-60 times longer than recombinant human growth hormone (rhGH) allows it to exert a prolonged effect in target tissue.
Of the two phase II studies of the molecule, one was conducted in 64 prepubertal children who were naive to any growth hormone treatment, and one in 36 adults with adult-onset growth hormone deficiency. The company also has made these presentations available online.
The pediatric study reported 3-year data on the cohort, which began treatment of children at a mean age of 7 years. At baseline, the children were about 2.6 standard deviations (SDs) below their expected height, and their mean IGF-1 levels, about 1.7 SDs below. They showed a mean maximum stimulated growth hormone level of 5.4 ng/mL. Although they were a mean of 7.8 years chronologically at the study’s outset, their mean bone age was 6.4 years.
The first 12 months of the study consisted of dose-ranging trials, with initial doses of 5 mg/kg each month, then 2.5 mg/kg twice a month, and then 1.15 mg/kg weekly. During the last 2 years of the study, all children were taking 3.5 mg/kg, once a month.
Within the first year, all children taking the 3.5-mg/kg dose had achieved normal IGF-1 levels, which were consistent with levels achieved in the ANSWER registry dataset of somatropin (rDNA origin) injection (Norditropin) recombinant human growth hormone (Clin Epidemiol. 2013;5:119-27).
Height velocity improved, as did height standard deviation. By year 3, patients were a mean of 1.25 SDs below expected height – a significant improvement over baseline. These findings were almost superimposable with those in the ANSWER registry. Bone age and chronological age came into alignment within the first year and that association was maintained throughout the study – again, in almost superimposable curves with the registry data.
Somavaratan exerted no untoward metabolic effects. There were no adverse changes in body mass index. At baseline, the mean hemoglobin A1c was 5.2%; this was unchanged at 3 years. No patient developed diabetes. The most commonly reported adverse event was injection site pain (48%). Injection site erythema was reported in 5% of patients, but no injection site nodules occurred.
Other adverse events were headache, extremity pain, arthralgia, and musculoskeletal pain. Although the numbers were small overall, reports did increase after all the children were switched to the 3.5-mg/kg dose. However, they occurred in 5% or less of the patient group. There were no withdrawals due to adverse events.
The second trial was a dose-ranging study conducted in 49 adults aged 23-70 years. They all had been diagnosed with adult-onset growth hormone deficiency, but had stable pituitary function. If they were on any growth hormone therapy, they underwent a 14-day washout period.
The subjects were divided and dosed by age and gender. All subjects received one injection per month for 5 months.
Cohort A comprised 21 men and women aged 35 years or older, who took 0.6 mg/kg per month. Cohort B comprised six men and women younger than 35 years, who took 0.8 mg/kg per month. Cohort C comprised eight women taking oral estrogen contraceptives. These women received 1 mg/kg per month.
The cohorts were similar in body mass index and weight, but they did differ significantly in baseline IGF-1 levels. In cohort A, the level was 0.52 SDs below normal. In cohort B, it was 2.89 SDs below normal, and in cohort C, it was 2.29 SDs below.
Overall, somavaratan induced a rapid and dramatic increase in IGF-1 that tailed off over 30 days. By day 8 after injection, IGF-1 had risen from a mean baseline of -1 SDs to more than 2 SDs above. By day 22, it had returned to baseline levels. The response to the fifth injection was identical to that of the first, Dr. Yuen said.
Response varied somewhat by cohort, with the younger, mixed-gender group responding the most dramatically, with a mean increase of about 4 SDs from baseline. This put the group above the maximum response target of 1.5 SDs.
The older, mixed-gender cohort experienced about a 3 SDs increase – also above the target level. The women taking estrogen experienced the flattest response, gaining about 2 SDs. However, the response curve was nearly identical, with a rapid, sharp increase in IGF-1 within the first week, followed by a gradual decline to baseline by 22 days.
The adverse event profile was not quite as benign as it was in the pediatric study. Virtually all patients experienced at least one adverse event. A third were mild and 58% moderate. The rest were serious, with one severe and one life-threatening event. Dr. Yuen did not discuss adverse events; these were, however, included in supplementary slides available on the Versartis Inc. slide set.
The finding of a predictable, 3-week tailing-off of efficacy, combined with the fact that patients responded so dramatically, exceeding the maximum target of a 1.5 SDs increase in IGF-1, has prompted a new dosing protocol for the somavaratan open-label extension study, which includes all the phase II completers, plus an additional 40 adult patients.
Doses will be titrated to each subject’s individual IGF-1 responses, based on the IGF-I level 7 days post dose until a maintenance dose is reached. Subjects receiving somavaratan in a previous somavaratan study will have their dose decreased by half (minimum dose of 20 mg, or 40 mg for women on estrogen).
Dr. Yuen is a member of the Versartis advisory board. Dr. Moore has received research support from the company.
[email protected]
On Twitter @Alz_Gal
ORLANDO – An extended-release human growth hormone formulation proved safe and effective in both children and adults, offering the prospect of a less-rigorous dosing schedule and potentially better patient compliance with treatment.
The two phase II studies examined somavaratan, which is being developed by Versartis of Menlo Park, Calif. Kevin Yuen, MD, an endocrinologist at the Swedish Medical Center, Seattle, and Wayne V. Moore, MD, a pediatric endocrinologist at Children’s Mercy Hospital, Kansas City, Mo., presented the data at the annual meeting of the Endocrine Society.
“Despite the fact that human growth hormone is a proven treatment for growth hormone deficiency, daily use of our current formulations can be a factor that affects compliance,” said Dr. Yuen. He cited a 2008 study of 158 men taking growth hormone, which found that only one-third were highly compliant (Endocr Pract. 2008 Mar;14[2]:143-54). “And even among this group, there were 21 doses missed over just a 3-month period.”
The group of 55 international experts described several strategies for creating long-acting growth hormone formulations, including depot formulations, pegylation, prodrugs, noncovalent albumin binding growth hormone compounds, and growth hormone fusion proteins. These preparations are currently in various stages of development, with some already approved in Europe and Asia.
Somavaratan (VRS-317) is a fusion protein produced in Escherichia coli. The active portion is recombinant human growth hormone, which is bound to long chains of hydrophilic amino acids. This reduces renal filtration, Dr. Yuen said. The growth hormone loses some potency in this construct, but its delayed clearance, with a half-life 30-60 times longer than recombinant human growth hormone (rhGH) allows it to exert a prolonged effect in target tissue.
Of the two phase II studies of the molecule, one was conducted in 64 prepubertal children who were naive to any growth hormone treatment, and one in 36 adults with adult-onset growth hormone deficiency. The company also has made these presentations available online.
The pediatric study reported 3-year data on the cohort, which began treatment of children at a mean age of 7 years. At baseline, the children were about 2.6 standard deviations (SDs) below their expected height, and their mean IGF-1 levels, about 1.7 SDs below. They showed a mean maximum stimulated growth hormone level of 5.4 ng/mL. Although they were a mean of 7.8 years chronologically at the study’s outset, their mean bone age was 6.4 years.
The first 12 months of the study consisted of dose-ranging trials, with initial doses of 5 mg/kg each month, then 2.5 mg/kg twice a month, and then 1.15 mg/kg weekly. During the last 2 years of the study, all children were taking 3.5 mg/kg, once a month.
Within the first year, all children taking the 3.5-mg/kg dose had achieved normal IGF-1 levels, which were consistent with levels achieved in the ANSWER registry dataset of somatropin (rDNA origin) injection (Norditropin) recombinant human growth hormone (Clin Epidemiol. 2013;5:119-27).
Height velocity improved, as did height standard deviation. By year 3, patients were a mean of 1.25 SDs below expected height – a significant improvement over baseline. These findings were almost superimposable with those in the ANSWER registry. Bone age and chronological age came into alignment within the first year and that association was maintained throughout the study – again, in almost superimposable curves with the registry data.
Somavaratan exerted no untoward metabolic effects. There were no adverse changes in body mass index. At baseline, the mean hemoglobin A1c was 5.2%; this was unchanged at 3 years. No patient developed diabetes. The most commonly reported adverse event was injection site pain (48%). Injection site erythema was reported in 5% of patients, but no injection site nodules occurred.
Other adverse events were headache, extremity pain, arthralgia, and musculoskeletal pain. Although the numbers were small overall, reports did increase after all the children were switched to the 3.5-mg/kg dose. However, they occurred in 5% or less of the patient group. There were no withdrawals due to adverse events.
The second trial was a dose-ranging study conducted in 49 adults aged 23-70 years. They all had been diagnosed with adult-onset growth hormone deficiency, but had stable pituitary function. If they were on any growth hormone therapy, they underwent a 14-day washout period.
The subjects were divided and dosed by age and gender. All subjects received one injection per month for 5 months.
Cohort A comprised 21 men and women aged 35 years or older, who took 0.6 mg/kg per month. Cohort B comprised six men and women younger than 35 years, who took 0.8 mg/kg per month. Cohort C comprised eight women taking oral estrogen contraceptives. These women received 1 mg/kg per month.
The cohorts were similar in body mass index and weight, but they did differ significantly in baseline IGF-1 levels. In cohort A, the level was 0.52 SDs below normal. In cohort B, it was 2.89 SDs below normal, and in cohort C, it was 2.29 SDs below.
Overall, somavaratan induced a rapid and dramatic increase in IGF-1 that tailed off over 30 days. By day 8 after injection, IGF-1 had risen from a mean baseline of -1 SDs to more than 2 SDs above. By day 22, it had returned to baseline levels. The response to the fifth injection was identical to that of the first, Dr. Yuen said.
Response varied somewhat by cohort, with the younger, mixed-gender group responding the most dramatically, with a mean increase of about 4 SDs from baseline. This put the group above the maximum response target of 1.5 SDs.
The older, mixed-gender cohort experienced about a 3 SDs increase – also above the target level. The women taking estrogen experienced the flattest response, gaining about 2 SDs. However, the response curve was nearly identical, with a rapid, sharp increase in IGF-1 within the first week, followed by a gradual decline to baseline by 22 days.
The adverse event profile was not quite as benign as it was in the pediatric study. Virtually all patients experienced at least one adverse event. A third were mild and 58% moderate. The rest were serious, with one severe and one life-threatening event. Dr. Yuen did not discuss adverse events; these were, however, included in supplementary slides available on the Versartis Inc. slide set.
The finding of a predictable, 3-week tailing-off of efficacy, combined with the fact that patients responded so dramatically, exceeding the maximum target of a 1.5 SDs increase in IGF-1, has prompted a new dosing protocol for the somavaratan open-label extension study, which includes all the phase II completers, plus an additional 40 adult patients.
Doses will be titrated to each subject’s individual IGF-1 responses, based on the IGF-I level 7 days post dose until a maintenance dose is reached. Subjects receiving somavaratan in a previous somavaratan study will have their dose decreased by half (minimum dose of 20 mg, or 40 mg for women on estrogen).
Dr. Yuen is a member of the Versartis advisory board. Dr. Moore has received research support from the company.
[email protected]
On Twitter @Alz_Gal
AT ENDO 2017
Key clinical point:
Major finding: In the 64-patient pediatric study, the molecule raised growth velocity and IGF-1 levels by several standard deviations. In the 48-patient adult study, it raised IGF-1 levels by up to 4 standard deviations.
Data source: A 64-patient pediatric dose-ranging study and a 48-patient adult study.
Disclosures: Versartis sponsored the studies. Dr. Yuen is on the company’s advisory board. Dr. Moore has received research support from the company.
Strontium, ketamine target troublesome itch
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
[email protected]
On Twitter @alz_gal
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
[email protected]
On Twitter @alz_gal
ORLANDO – Two drugs that target ion channels in nerves are being used to quiet neurogenic itch.
The powerful anesthetic ketamine and the element strontium have both been formulated into topical compounds that do very well in quelling itches that have been stubbornly resistant to other therapies, Gil Yosipovitch, MD, said at the annual meeting of the American Academy of Dermatology.
Both studies employed a 4% strontium hydrogel that is available over the counter (TriCalm). The product is designed to alleviate skin irritation (itching, burning, or stinging sensations), according to the manufacturer’s website.
The first study, published in 2013, comprised 32 healthy subjects in whom itch was induced with cowhage before and after skin treatment with the strontium gel, a control vehicle, topical 1% hydrocortisone, and topical 2% diphenhydramine (Acta Derm Venereol. 2013 Sep 4;93[5]:520-6).
Strontium significantly reduced the peak intensity and duration of itch relative to all three of the comparators.
A confirmatory study was published in 2015. The vehicle-controlled, randomized, crossover study recorded cowhage-induced itch intensity and duration in 48 healthy subjects before and after skin treatment with TriCalm, 2% diphenhydramine, 1% hydrocortisone, and hydrogel vehicle (Clin Cosmet Investig Dermatol. 2015 Apr 24;8:223-9). The results were similar, Dr. Yosipovitch said.
TriCalm effectively reduced peak itch intensity by about 3 points on a visual analog scale – a 41% reduction. Itch duration was reduced by 40%. These results were both clinically and statistically significantly better than those achieved by the other active comparators and the vehicle control.
Dr. Yosipovitch said the gel is most effective on nonhistaminergic itches, including those with a neurogenic component, nummular eczema, and facial itch.
“The most powerful antipruritic we have seen in the last 3 years, however, is topical ketamine,” Dr. Yosipovitch said. Typically formulated in 2%-10% creams, the anesthetic is usually combined with amitriptyline and lidocaine. “I see this as the most effective topical antipruritic and antinociceptive we have been using.”
Ketamine is an antagonist of the n-methyl-D-aspartate (NMDA) glutamate receptor and an ion channel protein. Amitriptyline serves primarily as a voltage-gated sodium channel antagonist, and lidocaine as a local anesthetic.
Mark Davis, MD, professor of dermatology at the Mayo Clinic, Rochester, Minn., and associates initially investigated 0.5% ketamine in a topical combination with amitriptyline 1% in a cream. The compound was remarkably effective for a 41-year-old man with a recalcitrant case of brachioradial pruritus – a neuropathic condition characterized by upper-extremity itching (JAMA Dermatol. 2013;149[2]:148-50). The patient had already failed treatment with halobetasol propionate, pimecrolimus, capsaicin, doxepin hydrochloride creams, and oral hydroxyzine hydrochloride and desloratadine. However, he had complete resolution of the itch soon after using the combination cream two to three times daily. At last follow-up, 4 years later, he was still using it at least once daily and continued to obtain complete relief.
Dr. Yosipovitch said this case was followed by a retrospective study of 16 patients who had used the 0.5% ketamine cream with either 1% or 2% amitriptyline for recalcitrant pruritus. The etiologies included neurodermatitis, pruritus caused by postherpetic neuralgia, nostalgia paresthetica, anesthesia dolorosa, nasal pruritus, and diabetic neuropathy (J Am Acad Dermatol. 2013 Aug;69[2]:320-1).
They used the medication one to five times per day for a mean duration of 10 months. Of the 16 patients, two had complete relief; two had substantial relief; six had some relief; five had no relief; and one reported increased itching.
Most recently, Dr. Yosipovitch and associates reported the results of a retrospective case review of 96 patients with a variety of pruritic conditions. The most frequent indications were neuropathic conditions (29%) and prurigo nodularis (19%). Most patients got a compounded cream of 10% ketamine, 5% amitriptyline, and 2% lidocaine;16 patients got a compound with 5% ketamine. The medication worked quickly, providing itch relief within a median of about 4 minutes, with an average of about a 50% decrease in itch rating (J Am Acad Dermatol. 2017 Apr;76[4]:760-1).
Forty patients participated in a pharmacy-administered telephone survey that assessed medication tolerability and efficacy. Of these, 23 patients (58%) had relief “to a great extent” and 14 (35%) “to a moderate extent.”
There were mild side effects (burning and redness at the application site) in 16 patients. “We attributed this mainly to the lidocaine component,” Dr. Yosipovitch said. “Itch reduction lasted from 30 minutes to 7 hours, so we think this is quite a powerful tool. I now often use this topical for patients with severe intractable itch.”
He added that a case report of encephalopathy associated with the cream has recently surfaced. The patient was an elderly man with Parkinson’s disease who had been using 10% ketamine compounded with amitriptyline and lidocaine for 4 days. He gradually increased the use until he was applying it onto almost all of his upper body. The day after this extensive application, the patient presented to an emergency department with slurred speech, ataxia, and altered mental status (JAMA Dermatol. 2016;152[12]:1390-1).
“So a word of warning here: I don’t recommend using it all over the body,” Dr. Yosipovitch said.
Dr. Yosipovitch has financial relationships with numerous companies that are investigating antipruritic compounds, including strontium.
[email protected]
On Twitter @alz_gal
EXPERT ANALYSIS FROM AAD 17
Temozolomide may help half of patients with aggressive pituitary tumors
ORLANDO – Temozolomide, an alkylating agent approved for glioblastoma, improved long-term survival in about half of patients who took it for aggressive pituitary tumors, a retrospective study has determined.
The study, conducted by members of the French Society of Endocrinology, comprised 43 patients. Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond, Gérald Raverot, MD, said at the annual meeting of the Endocrine Society.
“Despite the very good response we saw in some patients, we also saw a high risk of recurrence, with a median of about 30 months,” for relapse, he noted. “And a second course of temozolomide always failed.”
When used for aggressive pituitary tumors, temozolomide is usually given in a conventional scheme of up to 12 cycles. It’s typically reserved for tumors that have responded poorly to other treatment regimens, Dr. Raverot said.
The drug has not been widely studied in patients with aggressive pituitary tumors, although there have been a number of case reports suggesting that can be beneficial. Data on about 90 patients have been published. The largest series to date appeared in 2015 and comprised 24 patients. It found about a 50% response rate to the drug. Two patients had a complete regression and seven patients had a partial regression of tumor mass. Tumor mass shrunk to less than 30% in three patients, less than 50% in three, and less than 75% in one.
Because of both the promise temozolomide shows in these very tough cases, and the paucity of descriptive and clinical data, Dr. Raverot and his colleagues conducted a multi-center study that spanned 21 facilities in France and comprised 43 patients who were treated from 2006-2016. The intent was to evaluate efficacy at the end of treatment, or at last follow-up in the case of those who were still being treated. Tumor response was defined as a decrease of more than 30% in the largest tumor diameter; hormonal response was more than a 50% decrease in baseline hormone levels. The endpoint was overall survival and relapse-free survival.
Of the 43 patients, 29 were men. The group’s mean age at diagnosis was 43 years, and the mean age at temozolomide treatment, 53 years. Fourteen of the tumors were carcinomas and 12 were silent or initially silent.
About half of the tumors (23) were adrenocorticotropic hormone-producing. Other tumor types were prolactin-secreting (13) and growth hormone-secreting (3); an additional three tumors secreted both prolactin and growth hormone.
Most patients (36) underwent a typical temozolomide protocol. This consisted of at least one 5-day cycle of 150 mg/m2/day every 28 days, followed by 250 mg/m2/day thereafter. The median number of cycles was 6.5, but this ranged from 1-24 cycles.
Six patients were treated according to the Stupp protocol for temozolomide in glioblastoma. This consists of daily temozolomide 75 mg/m2 with concomitant radiotherapy for 6 weeks, followed by a standard temozolomide protocol. Four patients underwent 6 cycles; one patient 12 cycles, and one patient, 17 cycles.
An additional four patients had concomitant radiotherapy within 4 months of their temozolomide treatment.
The overall response rate was 51% (22 patients). Dr. Raverot attempted to identify clinical characteristics predictive of response. There was no association with gender, age at diagnosis or age at temozolomide treatment, tumor type, whether or not the tumor was a carcinoma, or what type of hormone it secreted. Nor was there a response associated with hypermethylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene.
Dr. Raverot found only one positive association with response. Tumors that were silent or initially silent (12) were much less likely to respond than secreting tumors. Of the 21 nonresponsive tumors, 10 were silent (45%). Of the 22 responsive tumors, only 2 were silent (9%).
Dr. Raverot also analyzed response by protocol and found intriguing results. Of the 10 patients who had concomitant radiotherapy, seven responded and three did not. Patients who underwent the Stupp protocol also tended to do better, he said. “Of the six who had this, five responded, so this is interesting.”
However, he cautioned, both of these positive associations are based on such small numbers that it’s impossible to draw firm conclusions.
Dr. Raverot had survival data on 38 patients with a median follow-up of 16 months after the end of treatment. Of these, 20 were responders and 18 were non-responders. Death had occurred in 13 of the nonresponders and five responders.
Of the 20 responders, 10 were still controlled at the time of last follow-up, and 10 had relapsed at a median of 5 months after treatment cessation. Five of these patients had a second course of temozolomide, but none of them responded to it, Dr. Raverot said. Three of these patients have died and two are still living.
“We looked at other salvage treatments for them, but none of these therapies could control the disease. Unfortunately, we just don’t have good treatment options for these patients. And even among those with good treatment response, there is a risk of early recurrence, with a median time of 30 months to relapse. The second course of temozolomide always fails. So we have now some questions about who we should maintain on treatment. We don’t have this answered yet, and we need to.”
Dr. Raverot had no financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – Temozolomide, an alkylating agent approved for glioblastoma, improved long-term survival in about half of patients who took it for aggressive pituitary tumors, a retrospective study has determined.
The study, conducted by members of the French Society of Endocrinology, comprised 43 patients. Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond, Gérald Raverot, MD, said at the annual meeting of the Endocrine Society.
“Despite the very good response we saw in some patients, we also saw a high risk of recurrence, with a median of about 30 months,” for relapse, he noted. “And a second course of temozolomide always failed.”
When used for aggressive pituitary tumors, temozolomide is usually given in a conventional scheme of up to 12 cycles. It’s typically reserved for tumors that have responded poorly to other treatment regimens, Dr. Raverot said.
The drug has not been widely studied in patients with aggressive pituitary tumors, although there have been a number of case reports suggesting that can be beneficial. Data on about 90 patients have been published. The largest series to date appeared in 2015 and comprised 24 patients. It found about a 50% response rate to the drug. Two patients had a complete regression and seven patients had a partial regression of tumor mass. Tumor mass shrunk to less than 30% in three patients, less than 50% in three, and less than 75% in one.
Because of both the promise temozolomide shows in these very tough cases, and the paucity of descriptive and clinical data, Dr. Raverot and his colleagues conducted a multi-center study that spanned 21 facilities in France and comprised 43 patients who were treated from 2006-2016. The intent was to evaluate efficacy at the end of treatment, or at last follow-up in the case of those who were still being treated. Tumor response was defined as a decrease of more than 30% in the largest tumor diameter; hormonal response was more than a 50% decrease in baseline hormone levels. The endpoint was overall survival and relapse-free survival.
Of the 43 patients, 29 were men. The group’s mean age at diagnosis was 43 years, and the mean age at temozolomide treatment, 53 years. Fourteen of the tumors were carcinomas and 12 were silent or initially silent.
About half of the tumors (23) were adrenocorticotropic hormone-producing. Other tumor types were prolactin-secreting (13) and growth hormone-secreting (3); an additional three tumors secreted both prolactin and growth hormone.
Most patients (36) underwent a typical temozolomide protocol. This consisted of at least one 5-day cycle of 150 mg/m2/day every 28 days, followed by 250 mg/m2/day thereafter. The median number of cycles was 6.5, but this ranged from 1-24 cycles.
Six patients were treated according to the Stupp protocol for temozolomide in glioblastoma. This consists of daily temozolomide 75 mg/m2 with concomitant radiotherapy for 6 weeks, followed by a standard temozolomide protocol. Four patients underwent 6 cycles; one patient 12 cycles, and one patient, 17 cycles.
An additional four patients had concomitant radiotherapy within 4 months of their temozolomide treatment.
The overall response rate was 51% (22 patients). Dr. Raverot attempted to identify clinical characteristics predictive of response. There was no association with gender, age at diagnosis or age at temozolomide treatment, tumor type, whether or not the tumor was a carcinoma, or what type of hormone it secreted. Nor was there a response associated with hypermethylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene.
Dr. Raverot found only one positive association with response. Tumors that were silent or initially silent (12) were much less likely to respond than secreting tumors. Of the 21 nonresponsive tumors, 10 were silent (45%). Of the 22 responsive tumors, only 2 were silent (9%).
Dr. Raverot also analyzed response by protocol and found intriguing results. Of the 10 patients who had concomitant radiotherapy, seven responded and three did not. Patients who underwent the Stupp protocol also tended to do better, he said. “Of the six who had this, five responded, so this is interesting.”
However, he cautioned, both of these positive associations are based on such small numbers that it’s impossible to draw firm conclusions.
Dr. Raverot had survival data on 38 patients with a median follow-up of 16 months after the end of treatment. Of these, 20 were responders and 18 were non-responders. Death had occurred in 13 of the nonresponders and five responders.
Of the 20 responders, 10 were still controlled at the time of last follow-up, and 10 had relapsed at a median of 5 months after treatment cessation. Five of these patients had a second course of temozolomide, but none of them responded to it, Dr. Raverot said. Three of these patients have died and two are still living.
“We looked at other salvage treatments for them, but none of these therapies could control the disease. Unfortunately, we just don’t have good treatment options for these patients. And even among those with good treatment response, there is a risk of early recurrence, with a median time of 30 months to relapse. The second course of temozolomide always fails. So we have now some questions about who we should maintain on treatment. We don’t have this answered yet, and we need to.”
Dr. Raverot had no financial disclosures.
[email protected]
On Twitter @Alz_gal
ORLANDO – Temozolomide, an alkylating agent approved for glioblastoma, improved long-term survival in about half of patients who took it for aggressive pituitary tumors, a retrospective study has determined.
The study, conducted by members of the French Society of Endocrinology, comprised 43 patients. Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond, Gérald Raverot, MD, said at the annual meeting of the Endocrine Society.
“Despite the very good response we saw in some patients, we also saw a high risk of recurrence, with a median of about 30 months,” for relapse, he noted. “And a second course of temozolomide always failed.”
When used for aggressive pituitary tumors, temozolomide is usually given in a conventional scheme of up to 12 cycles. It’s typically reserved for tumors that have responded poorly to other treatment regimens, Dr. Raverot said.
The drug has not been widely studied in patients with aggressive pituitary tumors, although there have been a number of case reports suggesting that can be beneficial. Data on about 90 patients have been published. The largest series to date appeared in 2015 and comprised 24 patients. It found about a 50% response rate to the drug. Two patients had a complete regression and seven patients had a partial regression of tumor mass. Tumor mass shrunk to less than 30% in three patients, less than 50% in three, and less than 75% in one.
Because of both the promise temozolomide shows in these very tough cases, and the paucity of descriptive and clinical data, Dr. Raverot and his colleagues conducted a multi-center study that spanned 21 facilities in France and comprised 43 patients who were treated from 2006-2016. The intent was to evaluate efficacy at the end of treatment, or at last follow-up in the case of those who were still being treated. Tumor response was defined as a decrease of more than 30% in the largest tumor diameter; hormonal response was more than a 50% decrease in baseline hormone levels. The endpoint was overall survival and relapse-free survival.
Of the 43 patients, 29 were men. The group’s mean age at diagnosis was 43 years, and the mean age at temozolomide treatment, 53 years. Fourteen of the tumors were carcinomas and 12 were silent or initially silent.
About half of the tumors (23) were adrenocorticotropic hormone-producing. Other tumor types were prolactin-secreting (13) and growth hormone-secreting (3); an additional three tumors secreted both prolactin and growth hormone.
Most patients (36) underwent a typical temozolomide protocol. This consisted of at least one 5-day cycle of 150 mg/m2/day every 28 days, followed by 250 mg/m2/day thereafter. The median number of cycles was 6.5, but this ranged from 1-24 cycles.
Six patients were treated according to the Stupp protocol for temozolomide in glioblastoma. This consists of daily temozolomide 75 mg/m2 with concomitant radiotherapy for 6 weeks, followed by a standard temozolomide protocol. Four patients underwent 6 cycles; one patient 12 cycles, and one patient, 17 cycles.
An additional four patients had concomitant radiotherapy within 4 months of their temozolomide treatment.
The overall response rate was 51% (22 patients). Dr. Raverot attempted to identify clinical characteristics predictive of response. There was no association with gender, age at diagnosis or age at temozolomide treatment, tumor type, whether or not the tumor was a carcinoma, or what type of hormone it secreted. Nor was there a response associated with hypermethylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene.
Dr. Raverot found only one positive association with response. Tumors that were silent or initially silent (12) were much less likely to respond than secreting tumors. Of the 21 nonresponsive tumors, 10 were silent (45%). Of the 22 responsive tumors, only 2 were silent (9%).
Dr. Raverot also analyzed response by protocol and found intriguing results. Of the 10 patients who had concomitant radiotherapy, seven responded and three did not. Patients who underwent the Stupp protocol also tended to do better, he said. “Of the six who had this, five responded, so this is interesting.”
However, he cautioned, both of these positive associations are based on such small numbers that it’s impossible to draw firm conclusions.
Dr. Raverot had survival data on 38 patients with a median follow-up of 16 months after the end of treatment. Of these, 20 were responders and 18 were non-responders. Death had occurred in 13 of the nonresponders and five responders.
Of the 20 responders, 10 were still controlled at the time of last follow-up, and 10 had relapsed at a median of 5 months after treatment cessation. Five of these patients had a second course of temozolomide, but none of them responded to it, Dr. Raverot said. Three of these patients have died and two are still living.
“We looked at other salvage treatments for them, but none of these therapies could control the disease. Unfortunately, we just don’t have good treatment options for these patients. And even among those with good treatment response, there is a risk of early recurrence, with a median time of 30 months to relapse. The second course of temozolomide always fails. So we have now some questions about who we should maintain on treatment. We don’t have this answered yet, and we need to.”
Dr. Raverot had no financial disclosures.
[email protected]
On Twitter @Alz_gal
AT ENDO 2017
Key clinical point:
Major finding: Of the 51% who responded to the treatment, the median overall survival time was 44 months, compared to just 16 months for patients who didn’t respond.
Data source: The retrospective study comprised 43 patients treated in France.
Disclosures: Dr. Raverot had no financial disclosures.