User login
Products being developed for AKs therapy may appeal to patients
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
GRAND CAYMAN, CAYMAN ISLANDS – It’s unanimous: Patients with actinic keratoses (AKs) want them to go away quickly, painlessly, and pretty much invisibly. In fact, they’d rather risk developing cancer than deal with weeks of painful, red, oozing crusts.
But unless Ronco comes up with the AK-Away Wand, dermatologists and patients have to face facts, Theodore Rosen, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
“Some AKs are going to just go away, and some are going to just sit there unchanging. Not all AKs are going to turn into squamous cell cancer. But you can’t tell which ones will, and because you can’t predict, they should all be treated. It’s our job to make patients care about this.”
That job starts with the very first conversation, said Dr. Rosen, professor of dermatology, Baylor University, Houston. “The way you frame the information at the very beginning is so important. You have to get the word ‘cancer’ in there.”
Most patients don’t fully grasp the serious threat that a transformed AK can pose, as illustrated by a survey of patients at the Milton S. Hershey Medical Center in Hershey, Pa.. The survey also highlighted the importance of the first discussion with the physician. Almost 550 dermatology clinic patients completed the survey, which presented five AK treatment decision scenarios, asking patients how likely they would be to pursue treatment in each situation (JAMA Dermatol. 2017;153[5]:421-6). Each scenario was factual, but the emphasis on facts varied. The first four questions characterized the lesions as sun damage and stressed the low incidence of malignant transformation (0.5%), and the large percentage that remain unchanged (75%) and spontaneously disappear (25%).
The last question was much simpler and more direct: “Actinic keratoses are precancers. Based on this statement, how likely are you to want treatment?”
“When AK was presented without the word ‘cancer’ in the description, there were lower proportions of individuals who said they would want to receive treatment [about 60%],” Dr. Rosen said. “Presenting AK as a precancer had the highest proportion of patients saying they would prefer treatment – about 92%.”
But current treatments aren’t ideal, at least from the standpoint of patients who prefer fast results with a minimum of erythema, oozing, crusting, and pain. Dr. Rosen looked into his crystal ball and saw a few encouraging treatment options coming down the drug development pike. To make it past regulatory hurdles, though, any new treatment has to hit the sweet spot of approximately 80% lesion clearance, with less than 40% recurrence at 1 year. Whether these investigational protocols can complete that journey remains to be seen.
VDA-1102
VDA-1102, in an ointment formulation, is based on a stress response chemical found in the jasmine plant. It contains a synthetic derivative of methyl jasmonate, a plant stress hormone found in jasmine. According to the patent record for VDA-1102, jasmonates are released in extreme ultraviolet radiation, osmotic shock, heat shock, and pathogen attack to initiate injury response and repair cascades.
The drug stops tumor growth by inhibiting glycolysis; it removes hexokinase 2 (HK2) from mitochondria. HK2 is found only in malignant cells; normal cells have the hexokinase 1 variant. Hexokinase is a key modulator of the transformation of adenosine triphosphate to adenosine diphosphate. As an HK2 modulator, VDA-1102 should, therefore, only induce apoptosis in the malignant cells, Dr. Rosen said.
“In preclinical studies in a hairless mouse model, they were approaching that 80% mark with lesion regression.” But the drug doesn’t induce necrosis or inflammation – a huge plus for patients. “There’s almost nothing in terms of redness, scaling, inflammation, or pain. This could be a really attractive addition to the AK toolkit. Improved aesthetics during treatment translates into improved patient willingness to undergo recurrent treatments. It may also be useful for treating large fields of AK, and in immunosuppressed patients.”
An Israeli company, Vidac Pharma, is conducting a phase 2b study of 150 patients with AK. The big question? Duration of effect – something that can’t be determined in the 21-week study. The company is aiming to launch a phase 3 trial next year.
KX-01
KX-01 (formerly KX2-391), being developed by Athenex, is a dual-action anticancer agent compounded into a 1% ointment. It inhibits both Src kinase and tubulin polymerization. Src regulates several signaling pathways in tumor cells, including proliferation, survival, migration, invasion, and angiogenesis. Tubulin formation is critical for cell replication: Without tubulin polymerization, mitotic spindles can’t form.
The drug passed two phase 3 studies (NCT03285477 and NCT03285490) with flying colors last year, clearing 100% of AK lesions by day 57 when used as field therapy on the head and neck. The studies comprised 702 subjects who applied the active ointment or vehicle once daily for 5 days.
“Local skin reactions were very low and resolved very quickly,” Dr. Rosen said. “But we don’t have any longterm data yet ... we need the 1-year clearance rate to see if it falls in that 40% sweet spot.”
Dr. Rosen disclosed being a consultant for Valeant (Ortho) and Cutanea Life Sciences.
Global Academy and this news organization are owned by the same parent company.
REPORTING FROM CARIBBEAN DERMATOLOGY SYMPOSIUM
Choose your steps for treating chronic spontaneous urticaria
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
GRAND CAYMAN, CAYMAN ISLANDS – in about half of patients.
But for those who don’t respond, treatment guidelines in both the United States and Europe outline a stepwise algorithm that should eventually control symptoms in about 95% of people, without continuous steroid use, Diane Baker, MD, said at the Caribbean Dermatology Symposium, provided by Global Academy for Medical Education.
The guidelines from the American Academy of Allergy, Asthma & Immunology/American College of Allergy, Asthma, and Immunology, and the European Academy of Allergy and Clinical Immunology [EAACI] and the American Academy of Allergy /Global Allergy are markedly similar, said Dr. Baker, a dermatologist in Portland, Ore.
The U.S. document offers a few more choices in its algorithm, while the European document sticks to a more straightforward progression of antihistamine progressing to omalizumab and then to cyclosporine.
“Both guidelines start with monotherapy of a second-generation antihistamine in the licensed dose. This has to be continuous monotherapy though. We still get patients who say, ‘My hives get better with the antihistamine, but they come back when I’m not taking it.’ Yes, patients need to understand that they have to stay on daily doses in order to control symptoms.”
Drug choice is largely physician preference. A 2014 Cochrane review examined 73 studies of H1-histamine blockers in 9,759 participants and found little difference between any of the drugs. “No single H1‐antihistamine stands out as most effective,” the authors concluded. “Cetirizine at 10 mg once daily in the short term and in the intermediate term was found to be effective in completely suppressing urticaria. Evidence is limited for desloratadine given at 5 mg once daily in the intermediate term and at 20 mg in the short term. Levocetirizine at 5 mg in the intermediate but not short term was effective for complete suppression. Levocetirizine 20 mg was effective in the short term, but 10 mg was not,” the study noted (Cochrane Database Syst Rev. 2014 Nov 14;[11]:CD006137).
“In my practice, we use cetirizine,” Dr. Baker said. “But if a patient is on fexofenadine, for example, and doing well, I wouldn’t change that.”
The treatment guidelines agree on the next step for unresponsive patients: Updosing the antihistamine. “You may have to jump up to four times the recommended dose,” she said. “Sometimes we do this gradually, but sometimes I go right ahead to that dose just to get the patient under control. And there’s good evidence that 50%-75% of our patients will be controlled on an updosing regimen. Just keep them on it until they are symptom free, and then you can try reducing it to see how they do.”
But even this can leave up to half of patients still itching. The next treatment step is where the guidelines diverge, Dr. Baker said. The U.S. document suggests trying several other options, including adding another second-generation antihistamine, adding an H2 agonist, a leukotriene receptor antagonist, or a sedating first-generation antihistamine.
“The European recommendation is to go straight to omalizumab,” Dr. Baker said. “They based this recommendation on the finding of insufficient evidence in the literature for any of these other things.”
Instead of recommending omalizumab to antihistamine-resistant patients, the U.S. guidelines suggest a dose-advancement trial of hydroxyzine or doxepin.
But there’s no arguing that omalizumab is highly effective for chronic urticaria, Dr. Baker noted. The 2015 ASTERIA trial perfectly illustrated the drug’s benefit for patients who were still symptomatic on optimal antihistamine treatment (J Invest Dermatol. 2015 Jan;135[1]:67-75).
The 40-week, randomized, double-blind placebo controlled study enrolled 319 patients, who received the injections as a monthly add-on therapy for 24 weeks in doses of 75 mg, 150 mg, or 300 mg or placebo. This was followed by 16 weeks of observation. The primary endpoint was change from baseline in weekly Itch Severity Score (ISS) at week 12.
The omalizumab 300-mg group had the best ISS scores at the end of the study. This group also met nine secondary endpoints, including a decreased time to reach the clinically important response of at least a 5-point ISS decrease.
The drug carries a low risk of adverse events, with just four patients (5%) in the omalizumab 300-mg group developing a serious side effect; none of these were judged to be related to the study drug. There is a very low risk of anaphylaxis associated with omalizumab – about 0.1% in clinical trials and 0.2% in postmarketing observational studies. A 2017 review of three omalizumab studies determined that asthma is the biggest risk factor for such a reaction.
The review found 132 patients with potential anaphylaxis associated with omalizumab. Asthma was the indication for omalizumab therapy in 80%; 43% of patients who provided an anaphylaxis history said that they had experienced a prior non–omalizumab-related reaction.
The U.S. guidelines don’t bring omalizumab into the picture until the final step, which recommends it, cyclosporine, or other unspecified biologics or immunosuppressive agents. At this point, however, the European guidelines move to a cyclosporine recommendation for the very small number of patients who were unresponsive to omalizumab.
Pivotal trials of omalizumab in urticaria used a once-monthly injection schedule, but more recent data suggest that patients who get the drug every 2 weeks may do better, Dr. Baker added. A chart review published in 2016 found a 100% response rate in patients who received twice monthly doses of 300 mg (J Am Acad Dermatol. 2016 Jun;74[6]:1274-6).
Dr. Baker disclosed that she has been a clinical trial investigator for Novartis.
Global Academy and this news organization are owned by the same parent company.
This article was updated 2/1/19.
REPORTING FROM THE CARIBBEAN DERMATOLOGY SYMPOSIUM
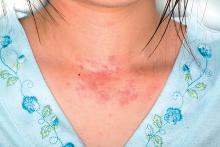
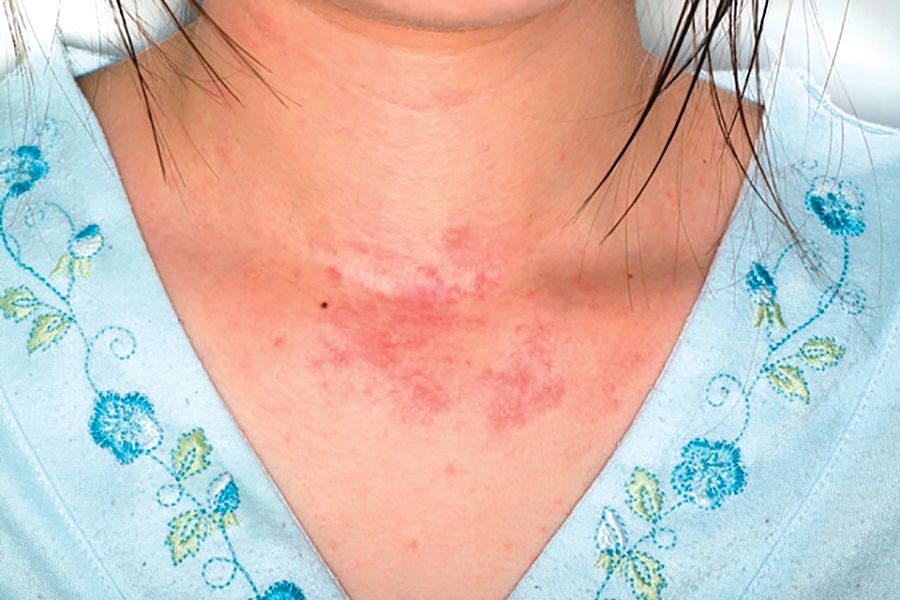
Digoxin-furosemide reduces viral load, diameter of cutaneous warts
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
Topical ionic contraviral therapy decreased the size of cutaneous warts caused by human papillomavirus virus (HPV) by a mean of 3 mm, a significant improvement compared with placebo, in a proof-of-concept study, Dr. Melanie Rijsbergen and her associates reported in the British Journal of Dermatology.
The Dr. Rijsbergen of the Center for Human Drug Research, Leiden, the Netherlands, and her coauthors wrote.
“It has been shown that DNA viruses, such as HPV, rely on potassium influx ... for replication. The cardiac glycoside digoxin and loop diuretic furosemide both inhibit potassium influx by interacting with the cell membrane ion cotransporters,” they said, noting that in 2006, an in vitro study found that “the inhibitory effect on DNA replication was most potent when digoxin and furosemide were combined.”
The placebo-controlled phase 2a trial randomized 80 patients with at least two plantar or common warts to one of four arms: digoxin 0.125% plus furosemide 0.125%; digoxin 0.125%; furosemide 0.125%; or placebo applied once a day for 42 consecutive days. A subset of 20 warts underwent histopathology and immunohistochemistry. In all, 139 warts were treated.
Patients were a mean of 26 years old and had developed warts a mean of 6 years before study onset. They had a mean of three warts each; about half were common and half were plantar.
In an analysis of all treated warts, each active treatment conferred a significant benefit, compared with placebo. The combination treatment was the most effective, with a mean diameter reduction of 3 mm. Warts exposed to digoxin alone or furosemide alone showed a mean reduction of about 2 mm.
At the study’s end, primary wart clearance rates were similar in all treatment groups – around 15%. None of the primary warts in the placebo group cleared. Common warts were more responsive to treatment than were plantar warts (24%-27% vs. 8%-15%). “The increased treatment resistance of plantar warts was previously described and seems to be mainly due to callus formation resulting in a decrease in cutaneous permeability of a drug,” the authors wrote.
The HPV viral load decreased by 94% in warts exposed to the combination therapy – a significant benefit, compared with placebo.
There were no discontinuations because of adverse events, and no serious adverse events related to treatment. There was no topical irritation associated with the treatment.
One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
SOURCE: Rijsbergen M et al. Br J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
FROM THE BRITISH JOURNAL OF DERMATOLOGY
Key clinical point: The combination of digoxin and furosemide in a topical gel reduced the diameter of cutaneous warts caused by HPV.
Major finding: Wart diameter was reduced by a mean of 3 mm among those treated with the combination.
Study details: The randomized, placebo-controlled phase 2a study compared the furosemide-digoxin combination with the two components separately, and placebo separately, in 80 adults.
Disclosures: One author is an employee of Cutanea Life Sciences, which funded the study. Dr. Rijsbergen and the remaining authors declared no financial conflicts.
Source: Rijsbergen M et al. J Dermatol. 2018 Dec 22. doi: 10.1111/bjd.17583.
Warmth and moisture help keep preterm neonates’ skin healthy
The skin of premature infants is very fragile and can take up to 4 weeks to become cornified. Until then, it’s apt to rapidly lose water and heat, putting babies at risk of hypothermia, dehydration, and electrolyte imbalances, Ayan Kusari and his colleagues wrote in Pediatric Dermatology.
The team examined evidence-based skin care in these tiny patients, extracting recommendations from a meta-analysis of 68 studies.
“There are a number of unifying features that distinguish preterm skin from term skin,” wrote Mr. Kusari, a clinical research associate at the Rady Children’s Hospital–San Diego, and his associates. “Preterm skin is thinner, making preterm neonates more susceptible to skin infections and caustic agents. The vernix caseosa is typically thicker in preterm neonates [though thinner in extremely preterm neonates]. Accordingly, there are a number of general principles that can guide skin care for most preterm neonates.”
Bathing
The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
“Colonization by pathogenic bacterial strains, size of the total bacterial population, and incidence of skin infection do not vary between preterm infants bathed every 2 days and preterm infants bathed every 4 days in all studies,” the authors wrote.
These less frequent baths appear to decrease the risk of temperature variability, and tub baths are preferable to sponge baths. “In sponge bathing, wet skin is more exposed to ambient air, which is typically colder than body temperature. Physiological and behavioral parameters in preterm infants are often disrupted during sponge bathing. In contrast, tub bathing results in less variability in body temperature and warmer temperatures after bathing,” Mr. Kusari and his associates found.
However, premoistened baby wipes appeared beneficial, lowering skin pH, which might help “facilitate acid mantle development, infection control, and barrier repair,” they wrote.
Emollients
Seven studies and one meta-analysis examined the use of emollients in preterm infants; there was agreement that emollients do improve skin condition. Plant-based emollients appeared superior to petrolatum-based products.
“In developing countries where oil massage of infants and children is traditional, there appears to be a clear benefit to massage with some oils. In developed countries, research has emphasized petrolatum-based creams and ointments, whose benefits are tempered by the increased risk of serious infections with some products,” Mr. Kusari and his colleagues wrote.
Sunflower seed oil was particularly beneficial in studies carried out in developing countries. A mixture of 70% lanolin and 30% olive oil proved better than olive oil alone. Coconut oil also displayed positive impact on skin condition.
“In contrast, multiple studies show an increased risk of sepsis with the application of petrolatum ointment to preterm neonates,” they noted.
In one study, following the adoption of a new skin care protocol involving regular application of petrolatum‐based ointments for extremely low-birth-weight neonates, researchers in Texas observed a significant, 200% increase in the incidence of systemic candidiasis. A study in Saudi Arabia replicated this finding. The largest study of a petrolatum-based ointment on premature babies was conducted in Vermont and found a statistically significant increase in infection with coagulase-negative staphylococcus (CoNS). “This ... study appears to be the driving force in a Cochrane Database meta-analysis, which concludes that topical emollients are associated with increased CoNS infection in preterm neonates,” the authors wrote.
Temperature regulation
It’s notoriously tough to maintain core temperature in preterm newborns. Six studies in the meta-analysis tackled this issue using impermeable plastic wraps or garments after birth and semipermeable barriers in the weeks after.
“Plastic wraps or bags can help neonates to retain their body heat, and greater skin coverage with plastic devices appears to be associated with a better outcome. In infants less than 28 weeks’ gestational age, the use of polyethylene occlusive wraps prevents heat loss after delivery and results in higher NICU admission temperatures and a lower incidence of hypothermia,” Mr Kusari and his associates wrote.
Semipermeable wraps can be used for an extended period after birth to reduce transepidermal water loss. Seven studies examined this technique, using both adhesive and nonadhesive polyurethane dressings.
“These studies show that semipermeable adhesive membranes decrease water loss, reduce skin breakdown, and decrease erythema while applied, but may strip superficial skin layers when they are removed, leading to a transient post-removal increase in transepidermal water loss. Furthermore, due to their semipermeable design, application of these adhesive membranes does not appear to decrease fluid requirement or affect electrolyte status in preterm neonates; however, skin barrier function is disrupted following removal of plastic tape, with increased transepidermal water loss at sites of tape removal,” the investigators wrote.
Pectin-based dressings and those containing hydrocolloid or acrylate can damage preterm neonatal skin by inflicting medical adhesive-related skin injury, the team wrote; this can involve epidermal stripping, tension injury, shearing, maceration, folliculitis, or contact dermatitis.
Skin sterilization
There’s little consensus when it comes to sterilization choices for preterm neonatal skin about to undergo a venipuncture or other procedure. Popular methods are povidone-iodine and chlorhexidine, with gestational age affecting choice. Iodine-based antiseptics have been associated with thyroid disruption and chlorhexidine with chemical burns.
“Some studies suggest 0.2% chlorhexidine gluconate may be an attractive alternative to povidone-iodine for the very and extremely preterm,” the authors wrote. One study they examined compared chlorhexidine gluconate 0.2% and 0.5% in extremely preterm infants, showing a significant decrease in skin irritation in the lower-concentration group.
But a randomized trial following this finding, which compared 0.2% chlorhexidine gluconate with 10% aqueous povidone-iodine, found no differences in any infection outcome or skin irritation, but there was more thyroid suppression in the povidone-iodine group.
More research is needed, the team concluded.
Cord care
Tincture of time may be the best alternative here.
The investigators examined a meta-analysis of 21 umbilical cord care studies and found that cleaning the cord with antiseptic prolonged the time to cord separation, compared with simple air drying.
“Interestingly, one study does suggest that one-time cleansing with chlorhexidine reduces neonatal mortality when compared to dry cord care; however, most of the existing evidence suggests that antiseptic treatment does not offer a benefit over dry cord care,” they wrote.
“Further studies, particularly in the very preterm and extremely preterm neonates, with an emphasis placed on subclassifying the preterm patient population based on gestational age, are needed to further examine and validate the real‐world utility of these interventions,” Mr. Kusari and his associates concluded. “In the meantime, it may be useful to establish practice guidelines based on the evidence we have presented here.”
The authors reported no relevant financial disclosures.
SOURCE: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
The skin of premature infants is very fragile and can take up to 4 weeks to become cornified. Until then, it’s apt to rapidly lose water and heat, putting babies at risk of hypothermia, dehydration, and electrolyte imbalances, Ayan Kusari and his colleagues wrote in Pediatric Dermatology.
The team examined evidence-based skin care in these tiny patients, extracting recommendations from a meta-analysis of 68 studies.
“There are a number of unifying features that distinguish preterm skin from term skin,” wrote Mr. Kusari, a clinical research associate at the Rady Children’s Hospital–San Diego, and his associates. “Preterm skin is thinner, making preterm neonates more susceptible to skin infections and caustic agents. The vernix caseosa is typically thicker in preterm neonates [though thinner in extremely preterm neonates]. Accordingly, there are a number of general principles that can guide skin care for most preterm neonates.”
Bathing
The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
“Colonization by pathogenic bacterial strains, size of the total bacterial population, and incidence of skin infection do not vary between preterm infants bathed every 2 days and preterm infants bathed every 4 days in all studies,” the authors wrote.
These less frequent baths appear to decrease the risk of temperature variability, and tub baths are preferable to sponge baths. “In sponge bathing, wet skin is more exposed to ambient air, which is typically colder than body temperature. Physiological and behavioral parameters in preterm infants are often disrupted during sponge bathing. In contrast, tub bathing results in less variability in body temperature and warmer temperatures after bathing,” Mr. Kusari and his associates found.
However, premoistened baby wipes appeared beneficial, lowering skin pH, which might help “facilitate acid mantle development, infection control, and barrier repair,” they wrote.
Emollients
Seven studies and one meta-analysis examined the use of emollients in preterm infants; there was agreement that emollients do improve skin condition. Plant-based emollients appeared superior to petrolatum-based products.
“In developing countries where oil massage of infants and children is traditional, there appears to be a clear benefit to massage with some oils. In developed countries, research has emphasized petrolatum-based creams and ointments, whose benefits are tempered by the increased risk of serious infections with some products,” Mr. Kusari and his colleagues wrote.
Sunflower seed oil was particularly beneficial in studies carried out in developing countries. A mixture of 70% lanolin and 30% olive oil proved better than olive oil alone. Coconut oil also displayed positive impact on skin condition.
“In contrast, multiple studies show an increased risk of sepsis with the application of petrolatum ointment to preterm neonates,” they noted.
In one study, following the adoption of a new skin care protocol involving regular application of petrolatum‐based ointments for extremely low-birth-weight neonates, researchers in Texas observed a significant, 200% increase in the incidence of systemic candidiasis. A study in Saudi Arabia replicated this finding. The largest study of a petrolatum-based ointment on premature babies was conducted in Vermont and found a statistically significant increase in infection with coagulase-negative staphylococcus (CoNS). “This ... study appears to be the driving force in a Cochrane Database meta-analysis, which concludes that topical emollients are associated with increased CoNS infection in preterm neonates,” the authors wrote.
Temperature regulation
It’s notoriously tough to maintain core temperature in preterm newborns. Six studies in the meta-analysis tackled this issue using impermeable plastic wraps or garments after birth and semipermeable barriers in the weeks after.
“Plastic wraps or bags can help neonates to retain their body heat, and greater skin coverage with plastic devices appears to be associated with a better outcome. In infants less than 28 weeks’ gestational age, the use of polyethylene occlusive wraps prevents heat loss after delivery and results in higher NICU admission temperatures and a lower incidence of hypothermia,” Mr Kusari and his associates wrote.
Semipermeable wraps can be used for an extended period after birth to reduce transepidermal water loss. Seven studies examined this technique, using both adhesive and nonadhesive polyurethane dressings.
“These studies show that semipermeable adhesive membranes decrease water loss, reduce skin breakdown, and decrease erythema while applied, but may strip superficial skin layers when they are removed, leading to a transient post-removal increase in transepidermal water loss. Furthermore, due to their semipermeable design, application of these adhesive membranes does not appear to decrease fluid requirement or affect electrolyte status in preterm neonates; however, skin barrier function is disrupted following removal of plastic tape, with increased transepidermal water loss at sites of tape removal,” the investigators wrote.
Pectin-based dressings and those containing hydrocolloid or acrylate can damage preterm neonatal skin by inflicting medical adhesive-related skin injury, the team wrote; this can involve epidermal stripping, tension injury, shearing, maceration, folliculitis, or contact dermatitis.
Skin sterilization
There’s little consensus when it comes to sterilization choices for preterm neonatal skin about to undergo a venipuncture or other procedure. Popular methods are povidone-iodine and chlorhexidine, with gestational age affecting choice. Iodine-based antiseptics have been associated with thyroid disruption and chlorhexidine with chemical burns.
“Some studies suggest 0.2% chlorhexidine gluconate may be an attractive alternative to povidone-iodine for the very and extremely preterm,” the authors wrote. One study they examined compared chlorhexidine gluconate 0.2% and 0.5% in extremely preterm infants, showing a significant decrease in skin irritation in the lower-concentration group.
But a randomized trial following this finding, which compared 0.2% chlorhexidine gluconate with 10% aqueous povidone-iodine, found no differences in any infection outcome or skin irritation, but there was more thyroid suppression in the povidone-iodine group.
More research is needed, the team concluded.
Cord care
Tincture of time may be the best alternative here.
The investigators examined a meta-analysis of 21 umbilical cord care studies and found that cleaning the cord with antiseptic prolonged the time to cord separation, compared with simple air drying.
“Interestingly, one study does suggest that one-time cleansing with chlorhexidine reduces neonatal mortality when compared to dry cord care; however, most of the existing evidence suggests that antiseptic treatment does not offer a benefit over dry cord care,” they wrote.
“Further studies, particularly in the very preterm and extremely preterm neonates, with an emphasis placed on subclassifying the preterm patient population based on gestational age, are needed to further examine and validate the real‐world utility of these interventions,” Mr. Kusari and his associates concluded. “In the meantime, it may be useful to establish practice guidelines based on the evidence we have presented here.”
The authors reported no relevant financial disclosures.
SOURCE: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
The skin of premature infants is very fragile and can take up to 4 weeks to become cornified. Until then, it’s apt to rapidly lose water and heat, putting babies at risk of hypothermia, dehydration, and electrolyte imbalances, Ayan Kusari and his colleagues wrote in Pediatric Dermatology.
The team examined evidence-based skin care in these tiny patients, extracting recommendations from a meta-analysis of 68 studies.
“There are a number of unifying features that distinguish preterm skin from term skin,” wrote Mr. Kusari, a clinical research associate at the Rady Children’s Hospital–San Diego, and his associates. “Preterm skin is thinner, making preterm neonates more susceptible to skin infections and caustic agents. The vernix caseosa is typically thicker in preterm neonates [though thinner in extremely preterm neonates]. Accordingly, there are a number of general principles that can guide skin care for most preterm neonates.”
Bathing
The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
“Colonization by pathogenic bacterial strains, size of the total bacterial population, and incidence of skin infection do not vary between preterm infants bathed every 2 days and preterm infants bathed every 4 days in all studies,” the authors wrote.
These less frequent baths appear to decrease the risk of temperature variability, and tub baths are preferable to sponge baths. “In sponge bathing, wet skin is more exposed to ambient air, which is typically colder than body temperature. Physiological and behavioral parameters in preterm infants are often disrupted during sponge bathing. In contrast, tub bathing results in less variability in body temperature and warmer temperatures after bathing,” Mr. Kusari and his associates found.
However, premoistened baby wipes appeared beneficial, lowering skin pH, which might help “facilitate acid mantle development, infection control, and barrier repair,” they wrote.
Emollients
Seven studies and one meta-analysis examined the use of emollients in preterm infants; there was agreement that emollients do improve skin condition. Plant-based emollients appeared superior to petrolatum-based products.
“In developing countries where oil massage of infants and children is traditional, there appears to be a clear benefit to massage with some oils. In developed countries, research has emphasized petrolatum-based creams and ointments, whose benefits are tempered by the increased risk of serious infections with some products,” Mr. Kusari and his colleagues wrote.
Sunflower seed oil was particularly beneficial in studies carried out in developing countries. A mixture of 70% lanolin and 30% olive oil proved better than olive oil alone. Coconut oil also displayed positive impact on skin condition.
“In contrast, multiple studies show an increased risk of sepsis with the application of petrolatum ointment to preterm neonates,” they noted.
In one study, following the adoption of a new skin care protocol involving regular application of petrolatum‐based ointments for extremely low-birth-weight neonates, researchers in Texas observed a significant, 200% increase in the incidence of systemic candidiasis. A study in Saudi Arabia replicated this finding. The largest study of a petrolatum-based ointment on premature babies was conducted in Vermont and found a statistically significant increase in infection with coagulase-negative staphylococcus (CoNS). “This ... study appears to be the driving force in a Cochrane Database meta-analysis, which concludes that topical emollients are associated with increased CoNS infection in preterm neonates,” the authors wrote.
Temperature regulation
It’s notoriously tough to maintain core temperature in preterm newborns. Six studies in the meta-analysis tackled this issue using impermeable plastic wraps or garments after birth and semipermeable barriers in the weeks after.
“Plastic wraps or bags can help neonates to retain their body heat, and greater skin coverage with plastic devices appears to be associated with a better outcome. In infants less than 28 weeks’ gestational age, the use of polyethylene occlusive wraps prevents heat loss after delivery and results in higher NICU admission temperatures and a lower incidence of hypothermia,” Mr Kusari and his associates wrote.
Semipermeable wraps can be used for an extended period after birth to reduce transepidermal water loss. Seven studies examined this technique, using both adhesive and nonadhesive polyurethane dressings.
“These studies show that semipermeable adhesive membranes decrease water loss, reduce skin breakdown, and decrease erythema while applied, but may strip superficial skin layers when they are removed, leading to a transient post-removal increase in transepidermal water loss. Furthermore, due to their semipermeable design, application of these adhesive membranes does not appear to decrease fluid requirement or affect electrolyte status in preterm neonates; however, skin barrier function is disrupted following removal of plastic tape, with increased transepidermal water loss at sites of tape removal,” the investigators wrote.
Pectin-based dressings and those containing hydrocolloid or acrylate can damage preterm neonatal skin by inflicting medical adhesive-related skin injury, the team wrote; this can involve epidermal stripping, tension injury, shearing, maceration, folliculitis, or contact dermatitis.
Skin sterilization
There’s little consensus when it comes to sterilization choices for preterm neonatal skin about to undergo a venipuncture or other procedure. Popular methods are povidone-iodine and chlorhexidine, with gestational age affecting choice. Iodine-based antiseptics have been associated with thyroid disruption and chlorhexidine with chemical burns.
“Some studies suggest 0.2% chlorhexidine gluconate may be an attractive alternative to povidone-iodine for the very and extremely preterm,” the authors wrote. One study they examined compared chlorhexidine gluconate 0.2% and 0.5% in extremely preterm infants, showing a significant decrease in skin irritation in the lower-concentration group.
But a randomized trial following this finding, which compared 0.2% chlorhexidine gluconate with 10% aqueous povidone-iodine, found no differences in any infection outcome or skin irritation, but there was more thyroid suppression in the povidone-iodine group.
More research is needed, the team concluded.
Cord care
Tincture of time may be the best alternative here.
The investigators examined a meta-analysis of 21 umbilical cord care studies and found that cleaning the cord with antiseptic prolonged the time to cord separation, compared with simple air drying.
“Interestingly, one study does suggest that one-time cleansing with chlorhexidine reduces neonatal mortality when compared to dry cord care; however, most of the existing evidence suggests that antiseptic treatment does not offer a benefit over dry cord care,” they wrote.
“Further studies, particularly in the very preterm and extremely preterm neonates, with an emphasis placed on subclassifying the preterm patient population based on gestational age, are needed to further examine and validate the real‐world utility of these interventions,” Mr. Kusari and his associates concluded. “In the meantime, it may be useful to establish practice guidelines based on the evidence we have presented here.”
The authors reported no relevant financial disclosures.
SOURCE: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: Limiting baths, using plant-based emollients, and using plastic wraps benefit preterm neonates’ skin early in life.
Major finding: The team identified eight studies of bathing preterm neonates and concluded that a daily bath isn’t necessary.
Study details: A meta-analysis of 68 studies.
Disclosures: The authors reported no relevant financial disclosures.
Source: Kusari A et al. Pediatr Dermatol. 2018 Dec 12. doi: 10.1111/pde.13725.
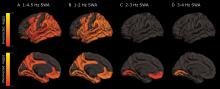
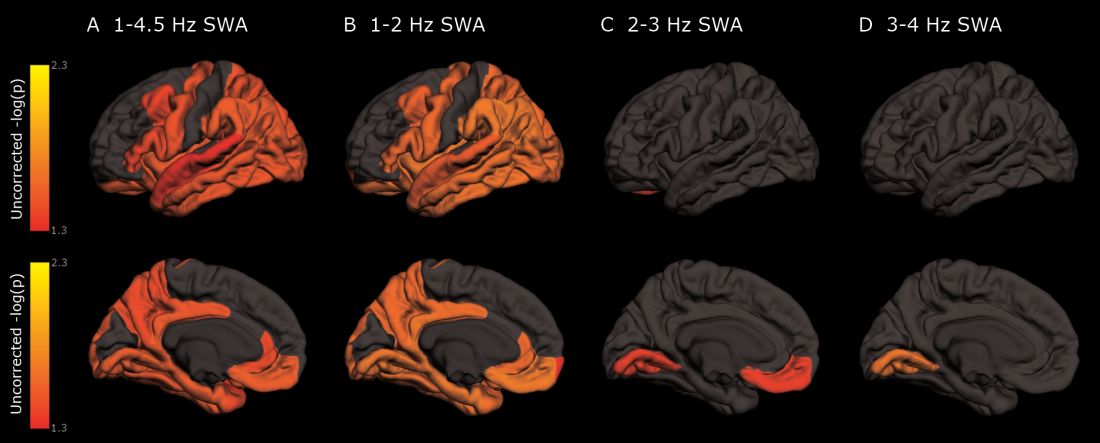
As deep sleep decreases, Alzheimer’s pathology – particularly tau – increases
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
The protein was evident in areas associated with memory consolidation, typically affected in Alzheimer’s disease: the entorhinal, parahippocampal, inferior parietal, insula, isthmus cingulate, lingual, supramarginal, and orbitofrontal regions.
Because the findings were observed in a population of cognitively normal and minimally impaired subjects, they suggest a role for sleep studies in assessing the risk for cognitive decline and Alzheimer’s disease, and in monitoring patients with the disease, reported Brendan P. Lucey, MD, and his colleagues. The report is in Science and Translational Medicine (Sci Transl Med. 2019 Jan 9;11:eaau6550).
“With the rising incidence of Alzheimer’s disease in an aging population, our findings have potential application in both clinical trials and patient screening for Alzheimer’s disease to noninvasively monitor for progression of Alzheimer’s disease pathology,” wrote Dr. Lucey, director of the Sleep Medicine Center and assistant professor of neurology at Washington University in St. Louis. “For instance, periodically measuring non-REM slow wave activity, in conjunction with other biomarkers, may have utility for monitoring Alzheimer’s disease risk or response to an Alzheimer’s disease treatment.”
Dr. Lucey and his colleagues examined sleep architecture and tau and amyloid deposition in 119 subjects enrolled in longitudinal aging studies. For 6 nights, subjects slept with a single-channel EEG monitor on. They also underwent cognitive testing and genotyping for Alzheimer’s disease risk factors.
Subjects were a mean of 74 years old. Almost 80% had normal cognition as measured by the Clinical Dementia Rating Scale (CDR); the remainder had very mild cognitive impairment (CDR 0.5)
Among those with positive biomarker findings, sleep architecture was altered in several ways: lower REM latency, lower wake after sleep onset, prolonged sleep-onset latency, and longer self-reported total sleep time. The differences were evident in those with normal cognition, but even more pronounced in those with mild cognitive impairment. Despite the longer sleep times, however, sleep efficiency was decreased.
Decreased non-REM slow wave activity was associated with increased tau deposition. The protein was largely concentrated in areas of typical Alzheimer’s disease pathology (entorhinal, parahippocampal, orbital frontal, precuneus, inferior parietal, and inferior temporal regions). There were no significant associations between non-REM slow wave activity and amyloid deposits.
Other sleep parameters, however, were associated with amyloid, including REM latency and sleep latency, “suggesting that as amyloid-beta deposition increased, the time to fall asleep and enter REM sleep decreased,” the investigators said.
Those with tau pathology also slept longer, reporting more daytime naps. “This suggests that participants with greater tau pathology experienced daytime sleepiness despite increased total sleep time.”
“These results, coupled with the non-REM slow wave activity findings, suggest that the quality of sleep decreases with increasing tau despite increased sleep time.” Questions about napping should probably be included in dementia screening discussions, they said.
The study was largely funded by the National Institutes of Health. Dr. Lucey had no financial conflicts.
SOURCE: Lucey BP et al. Sci Transl Med 2019 Jan 9;11:eaau6550.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Cognitively normal subjects with tau deposition experience altered sleep patterns.
Major finding: Decreased time in non-REM deep sleep was associated with increased tau pathology in Alzheimer’s-affected brain regions and in cerebrospinal fluid.
Study details: The prospective longitudinal study comprised 119 subjects.
Disclosures: The authors reported no relevant financial disclosures.
Source: Lucey BP et al. Sci Transl Med. 2019 Jan 9;11:eaau6550.
Treating OSA with positive airway pressure decreased amyloid levels in CSF
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
Soluble amyloid-beta in cerebrospinal fluid (CSF) decreased when subjects with obstructive sleep apnea used a positive airway pressure device with good adherence, suggesting that improving sleep could reduce the risk of Alzheimer’s disease in this population.
The small decrease in cerebrospinal amyloid-beta 40 (Ab40) and Ab42 hints at decreased neuronal release of the neurotoxic protein, wrote Yo-El S. Ju, MD, and her colleagues. The report was published online in Annals of Neurology.
Alzheimer’s disease (AD) biomarker studies typically find decreased CSF levels associated with increased Ab brain plaques. But before plaques form, increased soluble Ab in CSF is a risk factor for aggregation. Thus, higher soluble Ab levels in mid-life may suggest a risk of later Ab pathology, wrote Dr. Ju of Washington University, St. Louis.
“We tested individuals without any AD pathology as assessed by Ab42 [in CSF], a highly sensitive biomarker of amyloid plaques,” Dr. Ju and her coauthors wrote. “This means our study findings can be extrapolated to the large population of people with OSA [obstructive sleep apnea], many of whom are middle-aged or younger, and have many years to accrue benefit from AD risk reduction ... The effect of OSA on SWA [slow wave activity], Ab, and possibly tau, is a probable proximal step in a cascade whereby OSA increases the risk of AD.”
The researchers recruited 35 subjects with mild to severe OSA and without abnormal Ab levels in CSF. Subjects used auto-titrating positive airway pressure (PAP) for 1-4 months; 18 were sufficiently compliant to be included in the analysis (more than 4 hours on more than 70% of 30 preceding nights as recorded by the machine). CSF was obtained after a baseline polysomnogram and after the treatment period lasting 1-4 months.
Of the 18 analyzed patients, 7 had mild OSA and 11 had moderate to severe OSA. They were an average of nearly 57 years old with a mean body mass index of 30.4 kg/m2; 7 patients had hypertension.
PAP treatment was effective, indicated by a normalized apnea-hypopnea index and decreased time in hypoxemia. Total sleep time and sleep efficiency were unchanged, but slow-wave activity did increase. As expected, hourly arousals and time in hypoxemia decreased, and hypoxic nadir shifted from an oxygen saturation of 82.5% to 91%.
“As a group, there was no significant change in Ab with treatment,” the researchers wrote. But a correlational analysis found that “greater improvement in OSA was associated with greater decrease in Ab40 and Ab42. Additionally, we found that change in tau negatively correlated with OSA improvement.”
The team suggested a two-factor model to explain the relationship between OSA and Ab levels. “Due to decreased SWA, there would be relatively increased release of Ab into the [interstitial fluid]. However, as OSA severity worsens, pressure effects of obstructive respiratory events impede the clearance of Ab and tau out of the interstitial space, resulting in lower levels in the CSF and an inverse U-shaped curve. In this model, a small improvement in OSA may result in an increase in Ab or tau, whereas a larger improvement in OSA – that ameliorates both SWA and clearance mechanisms – will result in a decrease in Ab and tau.”
The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
SOURCE: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
FROM ANNALS OF NEUROLOGY
Key clinical point:
Major finding: After treatment, a correlational analysis found decreases in amyloid-beta 40 and 42.
Study details: The prospective, interventional study comprised 18 subjects.
Disclosures: The project was funded in part by Philips-Respironics, which provided the devices, and by the National Institutes of Health. Philips-Respironics had no input or role in any other part of the study. The authors had no financial disclosures.
Source: Ju YS et al. Ann Neurol. 2018 Dec 31. doi: 10.1002/ana.25408.
FDA expands Essure’s postmarketing surveillance study
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
The study, ordered in 2016, will now run 5 years instead of 3, and the cohort will be enlarged to add any women who elect implantation while the device is still on the market, FDA Commissioner Scott Gottlieb, MD, announced in a press statement. The agency also added a key biological measure: All patients with Essure will undergo regular blood work to evaluate proinflammatory markers that could be device related.
“We’re requiring additional blood testing of patients enrolled in follow-up visits during the study to learn more about patients’ levels of certain inflammatory markers that can be indicators of increased inflammation,” Dr. Gottlieb said. “This could help us better evaluate potential immune reactions to the device and whether these findings are associated with symptoms that patients have reported related to Essure.”
The device has been associated with severe problems in some patients, he noted.
“I personally had the opportunity to meet with women who have been adversely affected by Essure to listen and learn about their concerns. Some of the women I spoke with developed significant medical problems that they ascribe to their use of the product. We remain committed to these women and to improving how we monitor the safety of medical devices, including those related to women’s health.”
The study expansion comes as Bayer is facing more than 16,000 lawsuits over adverse events associated with Essure implantation.
Since its approval, Essure is estimated to have been used by more than 750,000 patients worldwide. Bayer claims the device is 99% effective in preventing pregnancy, but it’s also been associated with some serious risks, including persistent pain, perforation of the uterus and fallopian tubes, and migration of the coils into the pelvis or abdomen. In view of these – and more than 15,000 adverse events reported to the FDA – the agency announced new restrictions on Essure earlier this year. Those restrictions, plus a prior boxed warning on the label, contributed to about a 70% decline in U.S. sales, which Bayer says prompted the discontinuation.
The open-label prospective observational study will compare women who have the Essure device to a matched cohort that underwent laparoscopic tubal ligation. The main safety endpoints are chronic pelvic pain and abnormal uterine bleeding, as well as the new measure of inflammatory markers. As of Dec. 3, 791 patients have been enrolled (293 in the Essure arm and 498 in the laparoscopic tubal ligation arm).
Women who have the implant now and remain free of any adverse events should probably keep the device, Dr. Gottlieb advised.
“We believe women who’ve been using Essure successfully to prevent pregnancy can and should continue to do so. Women who suspect the device may be related to symptoms they are experiencing, such as persistent pain, should talk to their doctor on what steps may be appropriate. Device removal has its own risks. Patients should discuss the benefits and risks of any procedure with their health care providers before deciding on the best option for them.”
Atopic dermatitis associated with increased suicidality
Patients with atopic dermatitis might face up to a 44% increased risk of suicidal ideation and are 36% more likely to attempt suicide than those without the disorder, a large meta-analysis has determined.
The analysis, which included data from studies published as far back as 1945, also found some correlation of increased suicide risk and increasing disease severity, although the numbers were small, Jeena K. Sandhu and her colleagues reported in JAMA Dermatology.
Both physical and psychological factors could be involved in the link, wrote Ms. Sandhu, a medical student at the University of Missouri–Kansas City, and her coauthors.
“Atopic dermatitis is associated with multiple physical comorbidities, such as asthma, allergic rhinitis, metabolic syndrome, and sleep disturbances, which all contribute to the overall physical burden of the disease. Many patients also have a profound psychosocial burden. Because of the visibility of the disease, patients may experience shame, embarrassment, and stigmatization,” they wrote.
But the disease also is associated with high levels of proinflammatory cytokines, and those proteins have been isolated in the cerebrospinal fluid of patients who have attempted suicide, the investigators noted. “Treatments targeting cytokines, such as interleukin-4 and interleukin-13, have been shown to decrease symptoms of depression and anxiety in patients with atopic dermatitis.”
The investigators plumbed several databases of medical literature, searching for studies that mentioned both atopic dermatitis (AD) and suicide, suicidal ideation, or suicidal behavior. They found 15 studies, published from 1945 to May 2018. Most (13) were cross sectional; the remainder were cohort studies. Together, they comprised a total of 4.7 million subjects, 310,681 of whom had AD. The analysis looked at risks in three areas: suicidal ideation, suicide attempts, and completed suicides.
Of the studies, 11 investigated suicidal ideation. Pooled data determined that patients with AD were a significant 44% more likely to experience suicidal ideation than those without the disease.
Three studies mentioned suicide attempts and had complete data for pooling. Taken together, they showed a significant 36% increased risk of attempted suicide among patients with AD, compared with those without the disorder.
Two studies investigated the prevalence of completed suicides among patients. One did report a significantly increased risk of 40%, compared with the control group, but it failed to report the number of suicides in the control group. The other study found no increased risk of completed suicides in patients with either mild or moderate to severe disease, compared with controls.
Two studies involved only pediatric patients. One, conducted in Korea, found a significant 23% increased risk of suicidal ideation and a 31% increased risk of attempted suicide. The other failed to find any increased risks in the overall analysis, but did find small increases in the risks of ideation and attempt in girls with AD, compared with healthy controls.
the team concluded. “Dermatology providers may use several tools to screen patients for suicidality. Asking patients about suicidal ideation with a question may be integrated into a patient visit. If a patient screens positive for suicidality, the dermatology provider should send a referral to the patient’s primary care or mental health provider for follow-up care. If the patient reports an orchestrated plan to commit suicide, this patient should be urgently referred to the emergency department for further assessment.”
Ms. Sandhu reported no financial disclosures.
SOURCE: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
Patients with atopic dermatitis might face up to a 44% increased risk of suicidal ideation and are 36% more likely to attempt suicide than those without the disorder, a large meta-analysis has determined.
The analysis, which included data from studies published as far back as 1945, also found some correlation of increased suicide risk and increasing disease severity, although the numbers were small, Jeena K. Sandhu and her colleagues reported in JAMA Dermatology.
Both physical and psychological factors could be involved in the link, wrote Ms. Sandhu, a medical student at the University of Missouri–Kansas City, and her coauthors.
“Atopic dermatitis is associated with multiple physical comorbidities, such as asthma, allergic rhinitis, metabolic syndrome, and sleep disturbances, which all contribute to the overall physical burden of the disease. Many patients also have a profound psychosocial burden. Because of the visibility of the disease, patients may experience shame, embarrassment, and stigmatization,” they wrote.
But the disease also is associated with high levels of proinflammatory cytokines, and those proteins have been isolated in the cerebrospinal fluid of patients who have attempted suicide, the investigators noted. “Treatments targeting cytokines, such as interleukin-4 and interleukin-13, have been shown to decrease symptoms of depression and anxiety in patients with atopic dermatitis.”
The investigators plumbed several databases of medical literature, searching for studies that mentioned both atopic dermatitis (AD) and suicide, suicidal ideation, or suicidal behavior. They found 15 studies, published from 1945 to May 2018. Most (13) were cross sectional; the remainder were cohort studies. Together, they comprised a total of 4.7 million subjects, 310,681 of whom had AD. The analysis looked at risks in three areas: suicidal ideation, suicide attempts, and completed suicides.
Of the studies, 11 investigated suicidal ideation. Pooled data determined that patients with AD were a significant 44% more likely to experience suicidal ideation than those without the disease.
Three studies mentioned suicide attempts and had complete data for pooling. Taken together, they showed a significant 36% increased risk of attempted suicide among patients with AD, compared with those without the disorder.
Two studies investigated the prevalence of completed suicides among patients. One did report a significantly increased risk of 40%, compared with the control group, but it failed to report the number of suicides in the control group. The other study found no increased risk of completed suicides in patients with either mild or moderate to severe disease, compared with controls.
Two studies involved only pediatric patients. One, conducted in Korea, found a significant 23% increased risk of suicidal ideation and a 31% increased risk of attempted suicide. The other failed to find any increased risks in the overall analysis, but did find small increases in the risks of ideation and attempt in girls with AD, compared with healthy controls.
the team concluded. “Dermatology providers may use several tools to screen patients for suicidality. Asking patients about suicidal ideation with a question may be integrated into a patient visit. If a patient screens positive for suicidality, the dermatology provider should send a referral to the patient’s primary care or mental health provider for follow-up care. If the patient reports an orchestrated plan to commit suicide, this patient should be urgently referred to the emergency department for further assessment.”
Ms. Sandhu reported no financial disclosures.
SOURCE: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
Patients with atopic dermatitis might face up to a 44% increased risk of suicidal ideation and are 36% more likely to attempt suicide than those without the disorder, a large meta-analysis has determined.
The analysis, which included data from studies published as far back as 1945, also found some correlation of increased suicide risk and increasing disease severity, although the numbers were small, Jeena K. Sandhu and her colleagues reported in JAMA Dermatology.
Both physical and psychological factors could be involved in the link, wrote Ms. Sandhu, a medical student at the University of Missouri–Kansas City, and her coauthors.
“Atopic dermatitis is associated with multiple physical comorbidities, such as asthma, allergic rhinitis, metabolic syndrome, and sleep disturbances, which all contribute to the overall physical burden of the disease. Many patients also have a profound psychosocial burden. Because of the visibility of the disease, patients may experience shame, embarrassment, and stigmatization,” they wrote.
But the disease also is associated with high levels of proinflammatory cytokines, and those proteins have been isolated in the cerebrospinal fluid of patients who have attempted suicide, the investigators noted. “Treatments targeting cytokines, such as interleukin-4 and interleukin-13, have been shown to decrease symptoms of depression and anxiety in patients with atopic dermatitis.”
The investigators plumbed several databases of medical literature, searching for studies that mentioned both atopic dermatitis (AD) and suicide, suicidal ideation, or suicidal behavior. They found 15 studies, published from 1945 to May 2018. Most (13) were cross sectional; the remainder were cohort studies. Together, they comprised a total of 4.7 million subjects, 310,681 of whom had AD. The analysis looked at risks in three areas: suicidal ideation, suicide attempts, and completed suicides.
Of the studies, 11 investigated suicidal ideation. Pooled data determined that patients with AD were a significant 44% more likely to experience suicidal ideation than those without the disease.
Three studies mentioned suicide attempts and had complete data for pooling. Taken together, they showed a significant 36% increased risk of attempted suicide among patients with AD, compared with those without the disorder.
Two studies investigated the prevalence of completed suicides among patients. One did report a significantly increased risk of 40%, compared with the control group, but it failed to report the number of suicides in the control group. The other study found no increased risk of completed suicides in patients with either mild or moderate to severe disease, compared with controls.
Two studies involved only pediatric patients. One, conducted in Korea, found a significant 23% increased risk of suicidal ideation and a 31% increased risk of attempted suicide. The other failed to find any increased risks in the overall analysis, but did find small increases in the risks of ideation and attempt in girls with AD, compared with healthy controls.
the team concluded. “Dermatology providers may use several tools to screen patients for suicidality. Asking patients about suicidal ideation with a question may be integrated into a patient visit. If a patient screens positive for suicidality, the dermatology provider should send a referral to the patient’s primary care or mental health provider for follow-up care. If the patient reports an orchestrated plan to commit suicide, this patient should be urgently referred to the emergency department for further assessment.”
Ms. Sandhu reported no financial disclosures.
SOURCE: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
FROM JAMA DERMATOLOGY
Key clinical point: Suicidal ideation and suicide attempts seem to be more common among people with atopic dermatitis than those without the disease.
Major finding: Patients were 44% more likely to have suicidal ideation and 36% more likely to attempt suicide.
Study details: The meta-analysis comprised 15 studies with a total of 4.7 million participants, 310,681 of whom had the disease.
Disclosures: Ms. Sandhu reported no financial disclosures.
Source: Sandhu JK et al. JAMA Dermatol. 2018 Dec 12. doi: 10.1001/jamadermatol.2018.4566.
Hippocampal abnormalities seen in epilepsy subtypes may be congenital
NEW ORLEANS – , although to a lesser extent, based on findings from two studies presented at the annual meeting of the American Epilepsy Society.
While the studies suggest an imaging endophenotype associated with these disorders, it’s unclear if a larger degree of abnormality causes disease manifestation, or whether there are other predisposing actors at work.
“What our study tells us is that hippocampal abnormalities can occur in the absence of seizure,” Marian Galovic, MD, said in an interview. “It may be that, in some cases, hippocampal abnormalities could be the cause, rather than the consequence, of seizures.”
Dr. Galovic of University College London was on hand to discuss the work of his colleague, Lili Long, MD, PhD, of the Xiangya Hospital of Central South University, Changsha, China. Visa issues prevented her from attending the meeting.
The study included 18 sibling pairs in which the affected siblings had sporadic, nonlesional temporal lobe epilepsy (TLE), involving the right lobe in 12 and the left in 6. The patients, siblings, and 18 healthy, age-matched controls underwent clinical, electrophysiologic, and high-resolution structural neuroimaging.
The researchers compared overall hippocampal volumes between groups and determined the subregional extent of hippocampal abnormalities using shape analysis. They also looked at whole-brain differences in cortical thickness and folding complexity.
As expected, median hippocampal volumes were largest in the healthy controls (left = 2.82 mL, right = 2.94 mL), and smallest in patients. Patients with left TLE had a median left hippocampal volume of 2.23 mL, while those with right TLE had a median right hippocampal volume of 1.92 mL.
However, volume in the unaffected siblings was a surprise. Like the patients, these subjects also had significant reductions in hippocampal volume when compared with controls (left = 2.47 mL, right = 2.65 mL). “The atrophy was relatively similar in siblings and patients, although not as pronounced in siblings,” Dr. Galovic said. “It was mostly unilateral in the siblings and bilateral in the patients, but it was still more pronounced on the side where the epilepsy of the affected sibling was coming from.”
Patients and siblings also shared morphologic variations of the hippocampus, with atrophy more pronounced on the right than the left. The right lateral body and anterior head of the hippocampus were most affected, Dr. Galovic said, with reductions in the right cornu ammonis 1 subfield and subiculum.
Widespread cortical thinning was present in patients, including the pericentral, frontal, and temporal areas. Unaffected siblings also showed cortical thinning, but this was mostly restricted to the right postcentral gyrus. Patients and siblings also demonstrated increased cortical folding complexity, but in different areas: predominantly frontal in patients, but predominantly parieto-occipital in siblings. Both were significantly different than healthy control subjects.
The study didn’t examine any association with memory, which is often impaired in patients with TLE. However, Dr. Galovic said, “We have just submitted for publication a study in which we did find an association between focal hippocampal atrophy and memory performance.”
A different study by a team at University College London looked at hippocampal structure and function in patients with juvenile myoclonic epilepsy (JME) and their unaffected siblings. The imaging study, lead by Lorenzo Caciagli, MD, of the university comprised 37 patients with JME, 16 unaffected siblings, and 20 healthy controls. It employed multimodal MRI and neuropsychological measures to examine the form and function of the mesiotemporal lobe.
The subjects were matched for age, sex, handedness, and hemispheric dominance, which was assessed with language lateralization indices. This measures the number of active voxels on functional MRI, showing which hemisphere is dominant for language.
Both patients and their siblings showed reductions in left hippocampal volume on the order of 5%-8%, significantly smaller than the volumes seen in healthy controls. About half of patients and half of siblings also showed either unilateral or bilateral hippocampal malrotation. This was present in just 15% of controls, another significant difference. The structural differences weren’t associated with seizure control or age at disease onset, or with any impairments in verbal or visual memory. But when the investigators performed functional mapping, they found unusual patterns of hippocampal activation in both patients and siblings, pointing to a dysfunction of verbal encoding. In patients, there appeared to be distinct patterns of underactivation along the hippocampal long axis, regardless of whether malrotation was present. But among patients who had malrotation, the left posterior hippocampus showed more activation during visual memory.
The team concluded that the hippocampal abnormalities in volume, shape, and positioning in patients with JME and their siblings are related to functional reorganization. The abnormalities probably occur during prenatal neurodevelopment, they noted.
“Cosegregation of imaging patterns in patients and their siblings is suggestive of genetic imaging phenotypes, and independent of disease activity,” Dr. Caciagli and his coinvestigators wrote in their abstract.
Funding for the TLE study came from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and Xiangya Hospital. Funding for the JME study came from a variety of U.K. charities and government agencies.
SOURCES: Long L et al. AES 2018, Abstract 2.183; Caciagli L et al. AES 2018, Abstract 2.166.
NEW ORLEANS – , although to a lesser extent, based on findings from two studies presented at the annual meeting of the American Epilepsy Society.
While the studies suggest an imaging endophenotype associated with these disorders, it’s unclear if a larger degree of abnormality causes disease manifestation, or whether there are other predisposing actors at work.
“What our study tells us is that hippocampal abnormalities can occur in the absence of seizure,” Marian Galovic, MD, said in an interview. “It may be that, in some cases, hippocampal abnormalities could be the cause, rather than the consequence, of seizures.”
Dr. Galovic of University College London was on hand to discuss the work of his colleague, Lili Long, MD, PhD, of the Xiangya Hospital of Central South University, Changsha, China. Visa issues prevented her from attending the meeting.
The study included 18 sibling pairs in which the affected siblings had sporadic, nonlesional temporal lobe epilepsy (TLE), involving the right lobe in 12 and the left in 6. The patients, siblings, and 18 healthy, age-matched controls underwent clinical, electrophysiologic, and high-resolution structural neuroimaging.
The researchers compared overall hippocampal volumes between groups and determined the subregional extent of hippocampal abnormalities using shape analysis. They also looked at whole-brain differences in cortical thickness and folding complexity.
As expected, median hippocampal volumes were largest in the healthy controls (left = 2.82 mL, right = 2.94 mL), and smallest in patients. Patients with left TLE had a median left hippocampal volume of 2.23 mL, while those with right TLE had a median right hippocampal volume of 1.92 mL.
However, volume in the unaffected siblings was a surprise. Like the patients, these subjects also had significant reductions in hippocampal volume when compared with controls (left = 2.47 mL, right = 2.65 mL). “The atrophy was relatively similar in siblings and patients, although not as pronounced in siblings,” Dr. Galovic said. “It was mostly unilateral in the siblings and bilateral in the patients, but it was still more pronounced on the side where the epilepsy of the affected sibling was coming from.”

Patients and siblings also shared morphologic variations of the hippocampus, with atrophy more pronounced on the right than the left. The right lateral body and anterior head of the hippocampus were most affected, Dr. Galovic said, with reductions in the right cornu ammonis 1 subfield and subiculum.
Widespread cortical thinning was present in patients, including the pericentral, frontal, and temporal areas. Unaffected siblings also showed cortical thinning, but this was mostly restricted to the right postcentral gyrus. Patients and siblings also demonstrated increased cortical folding complexity, but in different areas: predominantly frontal in patients, but predominantly parieto-occipital in siblings. Both were significantly different than healthy control subjects.

The study didn’t examine any association with memory, which is often impaired in patients with TLE. However, Dr. Galovic said, “We have just submitted for publication a study in which we did find an association between focal hippocampal atrophy and memory performance.”
A different study by a team at University College London looked at hippocampal structure and function in patients with juvenile myoclonic epilepsy (JME) and their unaffected siblings. The imaging study, lead by Lorenzo Caciagli, MD, of the university comprised 37 patients with JME, 16 unaffected siblings, and 20 healthy controls. It employed multimodal MRI and neuropsychological measures to examine the form and function of the mesiotemporal lobe.
The subjects were matched for age, sex, handedness, and hemispheric dominance, which was assessed with language lateralization indices. This measures the number of active voxels on functional MRI, showing which hemisphere is dominant for language.
Both patients and their siblings showed reductions in left hippocampal volume on the order of 5%-8%, significantly smaller than the volumes seen in healthy controls. About half of patients and half of siblings also showed either unilateral or bilateral hippocampal malrotation. This was present in just 15% of controls, another significant difference. The structural differences weren’t associated with seizure control or age at disease onset, or with any impairments in verbal or visual memory. But when the investigators performed functional mapping, they found unusual patterns of hippocampal activation in both patients and siblings, pointing to a dysfunction of verbal encoding. In patients, there appeared to be distinct patterns of underactivation along the hippocampal long axis, regardless of whether malrotation was present. But among patients who had malrotation, the left posterior hippocampus showed more activation during visual memory.
The team concluded that the hippocampal abnormalities in volume, shape, and positioning in patients with JME and their siblings are related to functional reorganization. The abnormalities probably occur during prenatal neurodevelopment, they noted.
“Cosegregation of imaging patterns in patients and their siblings is suggestive of genetic imaging phenotypes, and independent of disease activity,” Dr. Caciagli and his coinvestigators wrote in their abstract.
Funding for the TLE study came from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and Xiangya Hospital. Funding for the JME study came from a variety of U.K. charities and government agencies.
SOURCES: Long L et al. AES 2018, Abstract 2.183; Caciagli L et al. AES 2018, Abstract 2.166.
NEW ORLEANS – , although to a lesser extent, based on findings from two studies presented at the annual meeting of the American Epilepsy Society.
While the studies suggest an imaging endophenotype associated with these disorders, it’s unclear if a larger degree of abnormality causes disease manifestation, or whether there are other predisposing actors at work.
“What our study tells us is that hippocampal abnormalities can occur in the absence of seizure,” Marian Galovic, MD, said in an interview. “It may be that, in some cases, hippocampal abnormalities could be the cause, rather than the consequence, of seizures.”
Dr. Galovic of University College London was on hand to discuss the work of his colleague, Lili Long, MD, PhD, of the Xiangya Hospital of Central South University, Changsha, China. Visa issues prevented her from attending the meeting.
The study included 18 sibling pairs in which the affected siblings had sporadic, nonlesional temporal lobe epilepsy (TLE), involving the right lobe in 12 and the left in 6. The patients, siblings, and 18 healthy, age-matched controls underwent clinical, electrophysiologic, and high-resolution structural neuroimaging.
The researchers compared overall hippocampal volumes between groups and determined the subregional extent of hippocampal abnormalities using shape analysis. They also looked at whole-brain differences in cortical thickness and folding complexity.
As expected, median hippocampal volumes were largest in the healthy controls (left = 2.82 mL, right = 2.94 mL), and smallest in patients. Patients with left TLE had a median left hippocampal volume of 2.23 mL, while those with right TLE had a median right hippocampal volume of 1.92 mL.
However, volume in the unaffected siblings was a surprise. Like the patients, these subjects also had significant reductions in hippocampal volume when compared with controls (left = 2.47 mL, right = 2.65 mL). “The atrophy was relatively similar in siblings and patients, although not as pronounced in siblings,” Dr. Galovic said. “It was mostly unilateral in the siblings and bilateral in the patients, but it was still more pronounced on the side where the epilepsy of the affected sibling was coming from.”

Patients and siblings also shared morphologic variations of the hippocampus, with atrophy more pronounced on the right than the left. The right lateral body and anterior head of the hippocampus were most affected, Dr. Galovic said, with reductions in the right cornu ammonis 1 subfield and subiculum.
Widespread cortical thinning was present in patients, including the pericentral, frontal, and temporal areas. Unaffected siblings also showed cortical thinning, but this was mostly restricted to the right postcentral gyrus. Patients and siblings also demonstrated increased cortical folding complexity, but in different areas: predominantly frontal in patients, but predominantly parieto-occipital in siblings. Both were significantly different than healthy control subjects.

The study didn’t examine any association with memory, which is often impaired in patients with TLE. However, Dr. Galovic said, “We have just submitted for publication a study in which we did find an association between focal hippocampal atrophy and memory performance.”
A different study by a team at University College London looked at hippocampal structure and function in patients with juvenile myoclonic epilepsy (JME) and their unaffected siblings. The imaging study, lead by Lorenzo Caciagli, MD, of the university comprised 37 patients with JME, 16 unaffected siblings, and 20 healthy controls. It employed multimodal MRI and neuropsychological measures to examine the form and function of the mesiotemporal lobe.
The subjects were matched for age, sex, handedness, and hemispheric dominance, which was assessed with language lateralization indices. This measures the number of active voxels on functional MRI, showing which hemisphere is dominant for language.
Both patients and their siblings showed reductions in left hippocampal volume on the order of 5%-8%, significantly smaller than the volumes seen in healthy controls. About half of patients and half of siblings also showed either unilateral or bilateral hippocampal malrotation. This was present in just 15% of controls, another significant difference. The structural differences weren’t associated with seizure control or age at disease onset, or with any impairments in verbal or visual memory. But when the investigators performed functional mapping, they found unusual patterns of hippocampal activation in both patients and siblings, pointing to a dysfunction of verbal encoding. In patients, there appeared to be distinct patterns of underactivation along the hippocampal long axis, regardless of whether malrotation was present. But among patients who had malrotation, the left posterior hippocampus showed more activation during visual memory.
The team concluded that the hippocampal abnormalities in volume, shape, and positioning in patients with JME and their siblings are related to functional reorganization. The abnormalities probably occur during prenatal neurodevelopment, they noted.
“Cosegregation of imaging patterns in patients and their siblings is suggestive of genetic imaging phenotypes, and independent of disease activity,” Dr. Caciagli and his coinvestigators wrote in their abstract.
Funding for the TLE study came from the National Natural Science Foundation of China, the Ministry of Science and Technology of China, and Xiangya Hospital. Funding for the JME study came from a variety of U.K. charities and government agencies.
SOURCES: Long L et al. AES 2018, Abstract 2.183; Caciagli L et al. AES 2018, Abstract 2.166.
REPORTING FROM AES 2018
FDA aims to boost safety of platelets for transfusion
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.
The Food and Drug Administration is asking for comments on its
The draft document, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” will be open for public comment through Feb. 4, 2019.
It is the first update to the policy document since 2016.
In the draft guidance, the FDA recommended three strategies for platelets stored for 5 days from collection. For apheresis platelets and prestorage pools, the FDA suggested an initial primary culture followed by a secondary culture on day 3 or day 4 or an initial primary culture followed by secondary testing with a rapid test. The third strategy – for apheresis platelets – is pathogen reduction alone.
The FDA also outlined three strategies for testing platelets stored for 7 days, all of which apply to apheresis platelets. The methods include an initial primary culture followed by a secondary culture no earlier than day 4, using a device labeled as a safety measure; an initial primary culture followed by a secondary rapid test, labeled as a safety measure; or large volume delayed sampling.
The supply of blood and blood components in the United States is among the safest in the world, FDA Commissioner Scott Gottlieb, MD, said in a statement. The FDA’s continuously updated protocols are intended to keep it that way.
“Blood and blood components are some of the most critical medical products American patients depend upon,” Dr. Gottlieb wrote. “But there remains risk, albeit uncommon, of contamination with infectious diseases, particularly with blood products that are stored at room temperature. While we’ve made great strides in reducing the risk of blood contamination through donor screening and laboratory testing, we continue to support innovations and blood product alternatives that can better keep pace with emerging pathogens and reduce some of the logistical challenges and costs associated with ensuring the safety of blood products.”
Since the 2016 guidance document was issued, new strategies for bacterial detection have become available that could potentially reduce the risk of contamination of platelets and permit extension of platelet dating up to 7 days, including bacterial testing strategies using culture-based devices, rapid bacterial detection devices, and the implementation of pathogen reduction technology.
The recommendations in the draft guidance incorporate ideas put forth during a July 2018 meeting of the agency’s Blood Products Advisory Committee. Committee members were asked to discuss the advantages and disadvantages of various strategies to control the risk of bacterial contamination in platelets, including the scientific evidence and the operational considerations involved. Their comments have been incorporated into the new draft guidance document.
In late November 2018, the FDA held a public workshop to encourage a scientific discussion on a range of pathogen reduction topics, including the development of novel technologies. “The ideal pathogen reduction technology would: be relatively inexpensive, be simple to implement on whole blood, allow treated blood to subsequently be separated into components or alternatively could be performed on apheresis products, inactivate a broad range of pathogens, and would have no adverse effect on product safety or product yield,” the FDA noted in a statement.