User login
Joint guidelines offer recommendations for treating peripheral artery disease
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
The report, published in the Journal of the American College of Cardiology, drew on the expertise of a broad panel of experts, including representatives from the American Heart Association, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, and Society for Vascular Medicine.
“Improvements in the diagnosis of peripheral artery disease (PAD) have led to an increasing number of treatment and revascularization methods, especially endovascular interventions,” wrote Steven R. Bailey, MD, who headed the multidisciplinary writing committee. “As new and increasingly sophisticated devices are developed, the medical community needs to understand how best to incorporate these technologies into daily clinical decision making and care, and how to choose between new and more established methods. This project was initiated to respond to this need and to ensure the effective use of peripheral artery revascularization.”
The document is not intended to cover every possible clinical scenario that could employ these interventions, wrote Dr. Bailey, who is the Janey Briscoe Distinguished Chair in Cardiology at the University of Texas, San Antonio, and his coauthors. “Rather, the goal is to provide generalized guidance into the use of these devices and techniques, while understanding that each clinical situation is unique, with physicians using their best judgment and the available evidence base to craft the most beneficial approach for the patient. In all cases, it is assumed that guideline-directed medical therapy should be applied first.”
The panel identified 45 scenarios in key clinical areas in which PAD interventions – either surgical or endovascular procedures – might be employed as first-line therapy. These included renal artery stenosis, lower extremity disease, critical limb ischemia, and asymptomatic artery disease. The report also discussed options for endovascular interventions, and secondary treatment options for lower extremity disease. The panel graded the value of interventions as appropriate, may be appropriate, or rarely appropriate.
“The scenarios in this document are arranged according to the clinical decision points confronting vascular practitioners in everyday clinical practice,” the panel wrote. “These include the presence or absence of symptoms, presence or absence of limb-threatening disease, severity and anatomical location of the culprit lesion, recurrent or de novo disease, the advantage of endovascular or surgical revascularization, and the expected durability of clinical benefit after an intervention.”
Renal artery stenting
Recommendations in this category were largely based on the CORAL (Cardiovascular Outcomes in Renal Atherosclerotic Lesions) study, which recommends best medical therapy as the initial treatment for a newly diagnosed patient. (N Engl J Med 2014;370:13-22).
The optimal medical approach is generally thought to be three antihypertensive medications, one of which should be a diuretic. Primary stenting can be considered for patients with an accelerating decline in renal function and bilateral or solitary significant renal artery stenosis, or moderate stenosis with translesional gradients that exceed threshold measurements. In patients with stable renal function and unilateral significant stenosis, intensifying medical therapy is appropriate. Stenting is rarely appropriate in patients with small, nonviable kidneys.
Lower extremity disease
Recommendations for lower extremity revascularization in patients with claudication are based largely on the 2016 AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease.
For patients with PAD and intermittent claudication, medical therapy and exercise are the first-line treatments. Revascularization should be considered only when this option fails. The appropriateness of intervention depends on the location and length of the lesion.
Intensification of medical therapy or endovascular treatment are appropriate for patients with aortoiliac, superficial femoral artery, and popliteal artery lesions; surgery also may be appropriate here. Medical therapy is appropriate for lesions located below the knee, as well; endovascular approaches also may be appropriate. Surgery for these lesions is rarely appropriate.
Critical limb ischemia
Medical therapy is generally not considered for these patients. But regardless of the lesion location, the panel found either endovascular or surgical treatment appropriate. Indeed, revascularization is the only viable treatment for these patients.
“Revascularization, whether endovascular or surgical, is critical for the reduction of high morbidity and mortality rates associated with limb loss. Mortality rates have been reported to be as high as 20% within 6 months of diagnosis and exceeding 50% after 5 years in patients left untreated. Furthermore, this degree of PAD is commonly associated with excessive cardiovascular events, often surpassing mortality rates associated with even symptomatic coronary artery disease.”
Asymptomatic artery disease
The recommendations in this category address the need to gain arterial access for potentially life-saving cardiovascular procedures. There are no published data in this area, so the recommendations are all based on expert opinion.
To gain access for coronary interventions, endovascular treatment and surgery are both appropriate. For hemodynamic support and large vascular or valvular interventions, endovascular approaches are appropriate, and surgical approaches may be appropriate.
Options for endovascular treatment when deemed appropriate or may be appropriate
Since there is no standardized treatment when an intervention is deemed appropriate, the potential procedures are organized by general lesion location (above or below the inguinal ligament and below the knee), and by lesion length. The recommendations cover the most commonly used endovascular treatment modalities.
“Of note, the use of atherectomy in the iliac artery has been rated Rarely Appropriate in all clinical scenarios,” the team noted. “This rating derives from an absence of data supporting the use of this technology, compared with balloon angioplasty and stenting. Similarly, the use of atherectomy in the superficial femoral and popliteal arteries and below-the-knee vessels also received a lower score, again because of the lack of comparative data relative to technologies with prospectively collected data. The evidence base to judge intervention below the knees is not as developed as other lower-extremity locations, which results in more frequent use of the May Be Appropriate category. The rating panel felt that below-the-knee atherectomy once again lacked comparative evidence to support general use.”
There are some exceptions, “favoring atherectomy include severe calcification and undilatable lesions; however, other technologies had a better evidence base for routine revascularization in most settings.”
Secondary treatment options for lower-extremity disease
This section addresses options for very specific situations, including in-stent restenosis, venous bypass graft failure, and prosthetic bypass graft failure.
“It is recognized that the need for revascularization of a failing conduit, graft, or stent is a marker of adverse outcomes for all of the reparative modalities employed,” the panel wrote. “Literature comparing treatment modalities for in-stent stenosis, venous graft failures, and arterial graft failures is very limited. Therefore, the recommendations primarily reflect consensus based upon current clinical practice.”
The modality choice should probably depend more upon surgeon preference and clinical experience, rather than a blanket recommendation. In general, the panel felt that surgical revascularizations are rarely appropriate for in-stent stenosis, especially if the patient is asymptomatic.
The panel felt that endovascular approaches are generally appropriate for focal stenoses in patients with prior surgical grafts and bioprosthetic material, but in patients with diffused stenosis or thrombosed grafts, both endovascular and surgical approaches were graded as may be appropriate.
“The specific type of therapy [device or surgical procedure] is at the discretion of the clinician, dictated by the clinical scenario plus physician and facility experience.”
Dr. Bailey had no financial disclosures; however, some members of the panel did disclose relationships with device manufacturers and pharmaceutical companies.
SOURCE: Bailey SR et al. J Am Coll Cardiol. 2018 Dec 17.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Bioequivalents lamotrigine, levetiracetam control new-onset focal seizures equally well
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
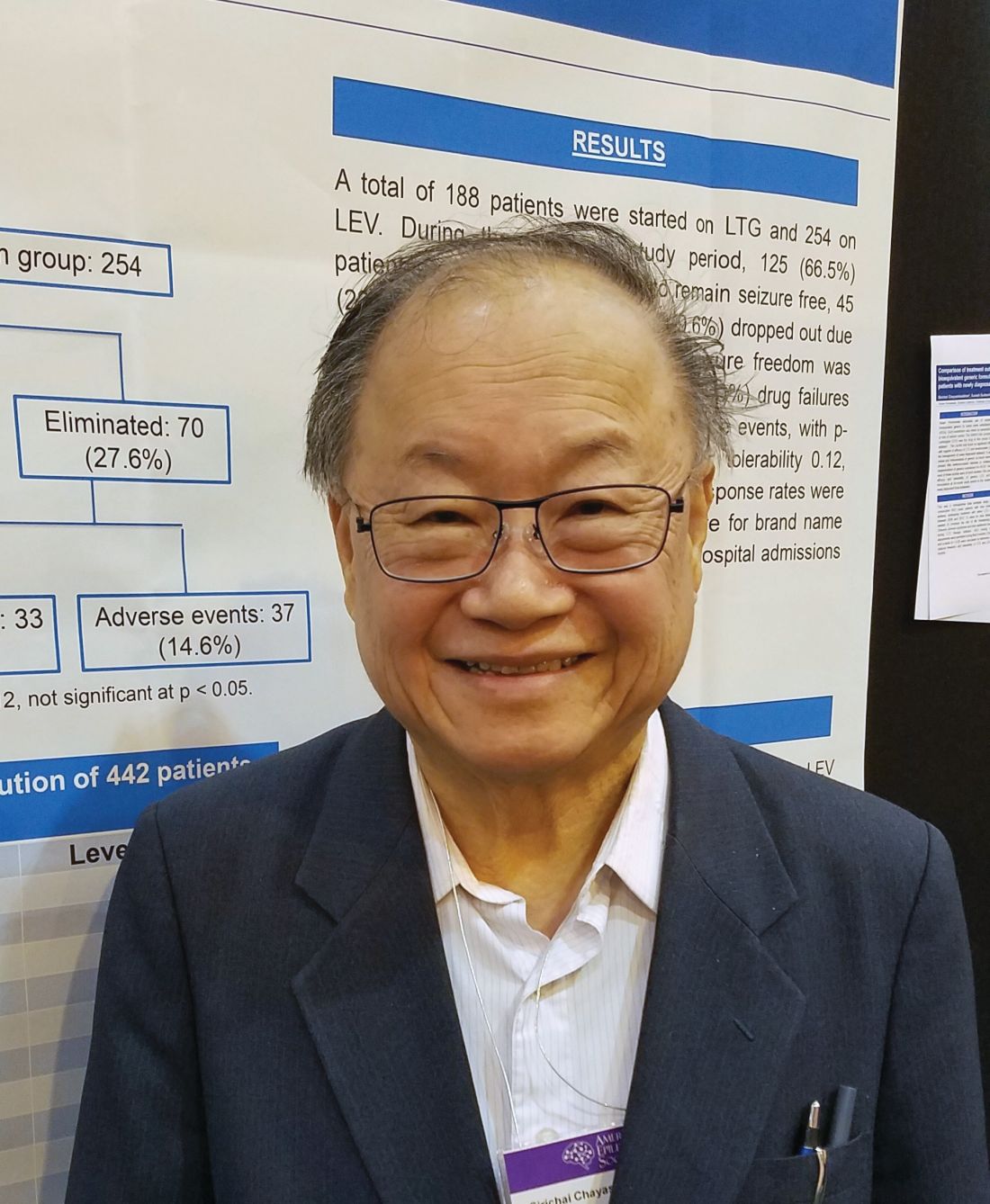
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
NEW ORLEANS – Bioequivalent generic formulations of levetiracetam and lamotrigine reduced seizures by a similar extent over 2 years in a retrospective study of patients with newly diagnosed focal epilepsy.
Each drug had a specific adverse event profile, with lamotrigine associated with rash and levetiracetam with mood disorders, Sirichai Chayasirisobhon, MD, said at the annual meeting of the American Epilepsy Society. This finding can play into the initial therapeutic decision, said Dr. Chayasirisobhon of Kaiser Permanente Southern California. “If someone comes in with depression or mood disorder, I will start on lamotrigine, not levetiracetam. And we can decrease the chance of rash with a very slow titration, as we did here, starting with just 5 mg/kg and working up over 6 months.”
Although the drugs have a somewhat similar teratogenic profile, Dr. Chayasirisobhon added that he favors lamotrigine for women of childbearing years. “It’s a little bit better choice for them I think.”
His retrospective analysis followed 442 patients from first seizure and medical therapy for 2 years. The generic medications came from Kaiser Permanente’s central pharmacy. They were single-source, with a proven 95% bioequivalence. The main outcome was the percentage of patients who became seizure free and remained so. Any seizure, whether febrile, breakthroughs, or from titration, was considered a failure. These patients were dropped from the study. Any patient who developed a drug-related rash was dropped from the study and started on another medication.
More women than men took lamotrigine (113 vs. 75), whereas more men took levetiracetam (148 vs. 106). Those taking lamotrigine were younger than were those taking levetiracetam (30 vs. 40 years).
At the end of 2 years, there was no statistically significant difference in the primary outcome of being free from seizures (66.5% with lamotrigine vs. 72.4% with levetiracetam). In the lamotrigine group, 33.5% were eliminated from the study, 24% because they had a seizure, and the rest due to an adverse event. In the levetiracetam group, 27.6% were eliminated, 13% because they had a seizure and the rest because of an adverse event.
Adverse events in the lamotrigine group included rash (12), dizziness (3), lethargy (1), and mood changes (2). Among the levetiracetam group, adverse events included dizziness (3), lethargy (7), mood changes (20), slowed thinking (4), depression (2) and headache (1).
“Rash was the main event we saw in this group, and this was even when we did a very slow titration of 5 mg/kg per week,” Dr. Chayasirisobhon said. “Any sign of rash or itching at all, we told them to stop immediately and call us. Fortunately, we had no cases of Steven-Johnson syndrome and all our cases of rash were transient. But in the levetiracetam group, the mood changes are the major thing. Some of the patients became very agitated and aggressive. Whenever we see a patient for the first time, we always ask about mood changes, and we instruct the family to call and report any changes in mood immediately.”
Aside from reproductive age, however, Dr. Chayasirisobhon generally prefers to start new patients on levetiracetam. Its safety profile is remarkable, he said, recounting a case report he published in 2010 (Acta Neurol Taiwan. 2010;19:292-5).
The paper describes a male patient who decided to commit suicide after an argument with his wife. He took his levetiracetam and walked to his father’s grave, swallowing pills the entire time. When he arrived at the grave, he had taken around 65 grams of the medication. “The amazing thing was, he’s still walking, just a little unsteady. Then he decided he’s not ready to die,” Dr. Chayasirisobhon said. “He was able to call 911, so he’s still talking fine. When they checked his level it was so high, but he remained unimpaired except for the unsteady gait and some nystagmus.”
The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
REPORTING FROM AES 2018
Key clinical point:
Major finding: At 2 years, 66.5% of the lamotrigine group and 72.4% of the levetiracetam group were seizure free.
Study details: The retrospective study comprised 442 patients.
Disclosures: The study did not receive outside funding. Dr. Chayasirisobhon had no financial disclosures.
Source: Chayasirisobhon S et al. AES 2018, Abstract 2.147.
Common AEDs confer modestly increased risk of major congenital malformations
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
NEW ORLEANS – The most commonly used antiepileptic drugs modestly increased the risk of major congenital malformations among prenatally exposed infants in the MONEAD study.
Malformations occurred among 5% of pregnancies exposed to the medications – higher than the 2% background rate – but this was still much lower than the 9%-10% rate associated with valproate.
Overall, however, the message of the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic (MONEAD) study is quite reassuring, Kimford J. Meador, MD, said at the annual meeting of the American Epilepsy Society. MONEAD is an ongoing, prospective study to determine both maternal outcomes and long-term childhood neurodevelopmental outcomes associated with the use of antiepileptic drugs (AEDs) during pregnancy.
“The rate of malformations was higher than I thought it would be, and higher than the 2% background rate, but it’s still a modest increase and most babies are born completely normal,” Dr. Meador, professor of neurology and neurosciences at Stanford (Calif.) University, said in an interview. “I think the news here is good, and it’s especially reassuring when you put it in the context that, 60 years ago, there were laws that women with epilepsy couldn’t get married, and some states even had laws to sterilize women. I think that’s absurd when most infants born to these women are without malformations and the risk of miscarriage is very low.”
Another positive finding, he said, is that valproate use among pregnant women is now practically nonexistent. Only 1 of 351 pregnant women with epilepsy and just 2 of a comparator group of 109 nonpregnant women with epilepsy were taking it. That’s great news, said Dr. Meador, who also initiated the NEAD (Neurodevelopmental Effects of Antiepileptic Drugs) study in the early 2000s. NEAD determined the drug’s serious teratogenic potential.
In addition to the cohorts of pregnant and nonpregnant women with epilepsy, 105 healthy pregnant women enrolled in the MONEAD study. Women will be monitored during pregnancy and postpartum to measure maternal outcomes and their children will be monitored from birth through age 6 years to measure their health and developmental outcomes.
The study has six primary outcomes, three for the women and three for their children.
- Determine if women with epilepsy have increased seizures during pregnancy and delineate the contributing factors.
- Determine if C-section rate is increased in women with epilepsy and delineate contributing factors.
- Determine if women with epilepsy have an increased risk for depression during pregnancy and the postpartum period and characterize risk factors.
- Determine the long-term effects of in utero AED exposure on verbal intellectual abilities and other neurobehavioral outcomes.
- Determine if small-for-gestational age and other adverse neonatal outcomes are increased.
- Determine if breastfeeding when taking AEDs impairs the child’s ultimate verbal and other cognitive outcomes.
Rates of miscarriage and neonatal malformations were not primary study outcomes, but the descriptive data were collected and are of high interest, Dr. Meador said.
At baseline, all the women had a mean age of about 30 years. Most (75%) were on monotherapy, 20% were on polytherapy, and the rest were not taking an AED. About 60% had focal epilepsy, 31% had generalized epilepsy, and the remainder had an unclassified seizure disorder. Three subjects had multiple seizure types. The most commonly used AEDs were lamotrigine and levetiracetam (both about 30%); 4% were taking zonisamide, 4% carbamazepine, and 4% oxcarbazepine. Topiramate was being used for 2% of the pregnant woman and 5% of the nonpregnant woman. The combination of lamotrigine and levetiracetam was used for 9.0% of pregnant and 5.5% of nonpregnant women, and other polytherapies in 12.0% of the pregnant and 14.0% of the nonpregnant woman. About 4% of the pregnant and 1% of the nonpregnant women were not taking any AED.
There were 10 (2.8%) spontaneous miscarriages among the pregnant women with epilepsy and none among the healthy pregnant women. Spontaneous miscarriages weren’t associated with acute seizures, and there were no major congenital malformations reported among them. There were also two elective abortions among the pregnant women with epilepsy.
There were 18 major congenital malformations among the pregnant woman with epilepsy (5%). A total of 14 were among pregnancies exposed to monotherapy, 3 were in polytherapy-exposed pregnancies, and 1 was in the group not taking any AEDs.
The malformations were:
- Carbamazepine (one case) – hydronephrosis.
- Gabapentin (one case) – inguinal hernia.
- Lamotrigine (five cases) – aortic coarctation, cryptorchidism, hydronephrosis, pectus excavatum, and morning glory syndrome (a funnel-shaped optic nerve disc associated with impaired visual acuity).
- Levetiracetam (five cases) – atrial septal defect, buried penis syndrome, cryptorchidism, hypoplastic aortic valve, ventricular septal defect.
- Topiramate (one case) – ventricular septal defect.
- Zonisamide (one case) – inguinal hernia, absent pinna.
- Lamotrigine plus clonazepam (one case) – cardiomyopathy.
- Lamotrigine plus levetiracetam (one case) – microcephaly, myelomeningocele, Chiari II malformation.
- Levetiracetam plus phenobarbital (one case) – bilateral inguinal hernia.
MONEAD is funded by the National Institutes of Health; Dr. Meador reported no financial disclosures.
SOURCE: Meador KJ et al. AES 2018, Abstract 3.231.
REPORTING FROM AES 2018
Key clinical point:
Major finding: The malformation rate was 5% in exposed pregnancies.
Study details: The MONEAD study comprised 351 pregnant women with epilepsy, 109 nonpregnant women with epilepsy, and 105 healthy pregnant women.
Disclosures: The National Institutes of Health funded the study; Dr. Meador reported no financial disclosures.
Source: Meador KJ et al. AES 2018, Abstract 3.231.
Latest intranasal insulin results for Alzheimer’s muddied by malfunctioning inhaler
BARCELONA –
Instead of doing poorly, patients using the faulty device actually experienced better outcomes than did those who entered the study later and used a more reliable inhaler, Suzanne Craft, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
A secondary analysis of the ViaNase device subgroup “replicated findings in our original studies,” which used the same atomizer, said Dr. Craft, a professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C. “We remain optimistic, but clearly we are at the beginning of understanding optimal insulin doses and delivery techniques for this population.”
The 289-patient, placebo-controlled study was predicated by a prior successful study by Dr. Craft and her colleagues, published in 2012 in JAMA Neurology. That trial randomized 104 patients with amnestic mild cognitive impairment (MCI) or mild-moderate Alzheimer’s to placebo or intranasal insulin 20 or 40 IU. After 4 months, subjects in both insulin groups showed preserved cognition and functional abilities, as well as increased cerebral glucose metabolism.
The ViaNase device was manufactured by Kurve Technology. But the company redesigned it for the new trial, adding an electronic timing component, which Dr. Craft said, was supposed to increase ease of use.
“Unfortunately, there were frequent malfunctions of this mechanism for the first 49 patients – so much so that we had to discontinue using the device and switch to a newer one,” for the other 240 patients in the study. This intranasal drug-delivery system, called the Precisions Olfactory Delivery (POD) device, is made by Impel NeuroPharma. Dr. Craft’s trial is its first investigation in patients with Alzheimer’s disease.
The new study randomized 289 patients with MCI or mild Alzheimer’s to twice-daily sprays with a placebo device, or to intranasal insulin 40 IU for 12 months, followed by a 6-month, open-label period. The primary outcome was the Alzheimer’s Disease Assessment Scale-Cognition measure (ADAS-Cog 12). Secondary outcomes were the Clinical Dementia Rating Scale sum of boxes (CDR-sb) a memory composite measure, activities of daily living, cerebrospinal fluid biomarkers, and MRI of the hippocampus and entorhinal cortex.
Because of the device problems, Dr. Craft conducted separate analyses for the user groups. The primary was an intent-to-treat (ITT), mixed-model, repeat-measures analysis of the 240 using the POD device. The model controlled for age, sex, genetic risk status, and investigation site. An exploratory ITT analysis looked only at the ADAS-Cog 12 in the 49 who used the ViaNase device. Patients were a mean of 71 years old, with a mean Mini Mental State Exam score of 25. About 42% were positive for the high-risk apolipoprotein E epsilon-4 allele.
At 12 months, there was no between-group difference on the ADAS-Cog 12 measure; both groups increased by about 4 points, indicating worsening. Nor were there any changes in any of the Alzheimer’s-related biomarkers: amyloid-beta 40 and 42, total tau, or phosphorylated tau. There was a small but statistically significant difference in the sizes of the hippocampus and entorhinal cortex.
The ViaNase group fared somewhat better in the secondary analysis of the ADAS-Cog12. The measure increased by about 5 points in the placebo group, and about 2.5 points in the insulin group. The significant separation was evident at 3 months and continued to widen over the course of the study.
Compliance was very good in the larger group – around 85%. It was lower in the ViaNase group, probably because of the device’s unreliability. Retention was good in both groups. There were no significant differences in adverse events and no obvious safety issues.
The 6-month, open-label period will close out before the end of the year. In the meantime, Dr. Craft is conducting additional subgroup analyses on the 12-month data.
Dr. Craft has served as a consultant for GlaxoSmithKline and Accera.
SOURCE: Craft S et al. J Prev Alz Dis 2018;5(S1):S9, Abstract OC2.
BARCELONA –
Instead of doing poorly, patients using the faulty device actually experienced better outcomes than did those who entered the study later and used a more reliable inhaler, Suzanne Craft, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
A secondary analysis of the ViaNase device subgroup “replicated findings in our original studies,” which used the same atomizer, said Dr. Craft, a professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C. “We remain optimistic, but clearly we are at the beginning of understanding optimal insulin doses and delivery techniques for this population.”
The 289-patient, placebo-controlled study was predicated by a prior successful study by Dr. Craft and her colleagues, published in 2012 in JAMA Neurology. That trial randomized 104 patients with amnestic mild cognitive impairment (MCI) or mild-moderate Alzheimer’s to placebo or intranasal insulin 20 or 40 IU. After 4 months, subjects in both insulin groups showed preserved cognition and functional abilities, as well as increased cerebral glucose metabolism.
The ViaNase device was manufactured by Kurve Technology. But the company redesigned it for the new trial, adding an electronic timing component, which Dr. Craft said, was supposed to increase ease of use.
“Unfortunately, there were frequent malfunctions of this mechanism for the first 49 patients – so much so that we had to discontinue using the device and switch to a newer one,” for the other 240 patients in the study. This intranasal drug-delivery system, called the Precisions Olfactory Delivery (POD) device, is made by Impel NeuroPharma. Dr. Craft’s trial is its first investigation in patients with Alzheimer’s disease.
The new study randomized 289 patients with MCI or mild Alzheimer’s to twice-daily sprays with a placebo device, or to intranasal insulin 40 IU for 12 months, followed by a 6-month, open-label period. The primary outcome was the Alzheimer’s Disease Assessment Scale-Cognition measure (ADAS-Cog 12). Secondary outcomes were the Clinical Dementia Rating Scale sum of boxes (CDR-sb) a memory composite measure, activities of daily living, cerebrospinal fluid biomarkers, and MRI of the hippocampus and entorhinal cortex.
Because of the device problems, Dr. Craft conducted separate analyses for the user groups. The primary was an intent-to-treat (ITT), mixed-model, repeat-measures analysis of the 240 using the POD device. The model controlled for age, sex, genetic risk status, and investigation site. An exploratory ITT analysis looked only at the ADAS-Cog 12 in the 49 who used the ViaNase device. Patients were a mean of 71 years old, with a mean Mini Mental State Exam score of 25. About 42% were positive for the high-risk apolipoprotein E epsilon-4 allele.
At 12 months, there was no between-group difference on the ADAS-Cog 12 measure; both groups increased by about 4 points, indicating worsening. Nor were there any changes in any of the Alzheimer’s-related biomarkers: amyloid-beta 40 and 42, total tau, or phosphorylated tau. There was a small but statistically significant difference in the sizes of the hippocampus and entorhinal cortex.
The ViaNase group fared somewhat better in the secondary analysis of the ADAS-Cog12. The measure increased by about 5 points in the placebo group, and about 2.5 points in the insulin group. The significant separation was evident at 3 months and continued to widen over the course of the study.
Compliance was very good in the larger group – around 85%. It was lower in the ViaNase group, probably because of the device’s unreliability. Retention was good in both groups. There were no significant differences in adverse events and no obvious safety issues.
The 6-month, open-label period will close out before the end of the year. In the meantime, Dr. Craft is conducting additional subgroup analyses on the 12-month data.
Dr. Craft has served as a consultant for GlaxoSmithKline and Accera.
SOURCE: Craft S et al. J Prev Alz Dis 2018;5(S1):S9, Abstract OC2.
BARCELONA –
Instead of doing poorly, patients using the faulty device actually experienced better outcomes than did those who entered the study later and used a more reliable inhaler, Suzanne Craft, PhD, said at the Clinical Trials on Alzheimer’s Disease conference.
A secondary analysis of the ViaNase device subgroup “replicated findings in our original studies,” which used the same atomizer, said Dr. Craft, a professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C. “We remain optimistic, but clearly we are at the beginning of understanding optimal insulin doses and delivery techniques for this population.”
The 289-patient, placebo-controlled study was predicated by a prior successful study by Dr. Craft and her colleagues, published in 2012 in JAMA Neurology. That trial randomized 104 patients with amnestic mild cognitive impairment (MCI) or mild-moderate Alzheimer’s to placebo or intranasal insulin 20 or 40 IU. After 4 months, subjects in both insulin groups showed preserved cognition and functional abilities, as well as increased cerebral glucose metabolism.
The ViaNase device was manufactured by Kurve Technology. But the company redesigned it for the new trial, adding an electronic timing component, which Dr. Craft said, was supposed to increase ease of use.
“Unfortunately, there were frequent malfunctions of this mechanism for the first 49 patients – so much so that we had to discontinue using the device and switch to a newer one,” for the other 240 patients in the study. This intranasal drug-delivery system, called the Precisions Olfactory Delivery (POD) device, is made by Impel NeuroPharma. Dr. Craft’s trial is its first investigation in patients with Alzheimer’s disease.
The new study randomized 289 patients with MCI or mild Alzheimer’s to twice-daily sprays with a placebo device, or to intranasal insulin 40 IU for 12 months, followed by a 6-month, open-label period. The primary outcome was the Alzheimer’s Disease Assessment Scale-Cognition measure (ADAS-Cog 12). Secondary outcomes were the Clinical Dementia Rating Scale sum of boxes (CDR-sb) a memory composite measure, activities of daily living, cerebrospinal fluid biomarkers, and MRI of the hippocampus and entorhinal cortex.
Because of the device problems, Dr. Craft conducted separate analyses for the user groups. The primary was an intent-to-treat (ITT), mixed-model, repeat-measures analysis of the 240 using the POD device. The model controlled for age, sex, genetic risk status, and investigation site. An exploratory ITT analysis looked only at the ADAS-Cog 12 in the 49 who used the ViaNase device. Patients were a mean of 71 years old, with a mean Mini Mental State Exam score of 25. About 42% were positive for the high-risk apolipoprotein E epsilon-4 allele.
At 12 months, there was no between-group difference on the ADAS-Cog 12 measure; both groups increased by about 4 points, indicating worsening. Nor were there any changes in any of the Alzheimer’s-related biomarkers: amyloid-beta 40 and 42, total tau, or phosphorylated tau. There was a small but statistically significant difference in the sizes of the hippocampus and entorhinal cortex.
The ViaNase group fared somewhat better in the secondary analysis of the ADAS-Cog12. The measure increased by about 5 points in the placebo group, and about 2.5 points in the insulin group. The significant separation was evident at 3 months and continued to widen over the course of the study.
Compliance was very good in the larger group – around 85%. It was lower in the ViaNase group, probably because of the device’s unreliability. Retention was good in both groups. There were no significant differences in adverse events and no obvious safety issues.
The 6-month, open-label period will close out before the end of the year. In the meantime, Dr. Craft is conducting additional subgroup analyses on the 12-month data.
Dr. Craft has served as a consultant for GlaxoSmithKline and Accera.
SOURCE: Craft S et al. J Prev Alz Dis 2018;5(S1):S9, Abstract OC2.
REPORTING FROM CTAD
Resection, neurostimulation combo found successful in eloquent cortical regions
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
NEW ORLEANS – Concurrent surgical resection and implanted strip electrodes eliminated refractory focal seizures in two patients with focal cortical dysplasia and reduced them by 62% in a third patient, according a report presented at the annual meeting of the American Epilepsy Society.
None of the patients had been considered surgical candidates because their seizure foci were in eloquent cortical regions; if fully resected, patients would have experienced marked neurologic deficits. But the combination procedure of flanking the incomplete resected foci with implanted electrodes allowed neurosurgeons to remove less tissue, preserving function while effectively treating previously untreatable seizures, Emily Mirro said at the meeting.
The two-in-one technique makes good surgical sense for these patients, she said in an interview. “If we simply performed the resection and closed without implanting the electrodes, just waiting to see if seizures develop or not, then going back to implant the electrodes, the surgery is riskier and more difficult,” said Ms. Mirro, director of field clinical engineering for NeuroPace, which makes the stimulator system.
At the meeting, she presented three case studies on behalf of primary authors Lawrence Shuer, MD, and Babak Razavi, MD, PhD, both of Stanford (Calif.) University.
The first patient was a 26-year-old with a focal cortical dysplasia in the right parietal region, causing about six seizures each month. At the time of surgery, surgeons flanked the resected region with four cortical strip leads over sensory cortex. The RNS System detected the first postsurgical seizure 1 month afterward. Five months later, the system was enabled at 0.5 milliamps. For the next year, the patient received about 100 stimulations per day, amounting to a total daily stimulation time of about 20 seconds. Electrographic seizures did return, at which point the system increased neurostimulation to about 2,000 per day (a total stimulation time of about 7 minutes per day). At 1.3 years, the patient remains seizure free.
Patient two was a 20-year-old with a left frontal transmantle cortical dysplasia that involved the inferior frontal sulcus. The baseline seizure frequency was about two per day. Surgeons removed the dysplastic area with a 2.0 cm x 0.5 cm resection; the deficit was flanked with two left-front cortical strip leads. In the following 9 days, the patient experienced eight seizures. At 14 days out, the system was enabled at 1 milliamp. This patient became seizure free and remains so at 1.3 years, with about 100 stimulations per day to suppress electrographic abnormalities.
The third patient, also 20 years old, had a left-parietal resection to the margin of the motor cortex. The baseline seizure frequency was up to 150 nocturnal events per month and several seizures during each day as well. The resection was flanked by one strip lead over the motor cortex; one depth lead implanted into it. Immediately after surgery, the patient experienced both electrographic and clinical seizures. The stimulator was enabled a week after surgery at 0.5 milliamps; this was titrated to 3 milliamps over 1.4 years. At last follow-up, the patient had about a 62% reduction in seizure frequency; all are now nocturnal.
None of the patients experienced any peri- or postoperative surgical complications.
Ms. Mirro is an employee of NeuroPace.
SOURCE: Razavi B et al. AES 2018, Abstract 2.315
REPORTING FROM AES 2018
Key clinical point:
Major finding: Two patients became seizure free and one had a 62% reduction in seizures.
Study details: A three-patient case series.
Disclosures: NeuroPace makes the neurostimulator used in the study. The presenter is an employee of NeuroPace.
Source: Razavi B et al. AES 2018, Abstract 2.315.
Transdermal CBD gel decreases recalcitrant focal seizures
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
NEW ORLEANS – A synthetic, transdermal, cannabidiol gel reduced the rate of seizures by half in a group of adults with treatment-resistant focal seizures who were participating in an open-label, long-term extension trial.
A twice-daily, 390-mg dose of the gel, dubbed ZYN002 (Zynerba) for now, was consistently effective in the 24-month STAR 2 extension trial, John Messenheimer, MD, said at the annual meeting of the American Epilepsy Society.
ZYN002 provided continuing coverage for patients who had used the active compound in the randomized phase, and quickly reduced seizures in those who entered on placebo, said Dr. Messenheimer, a consultant neurologist from Moncure, N.C.
The synthetically produced cannabidiol (CBD) transdermal gel ZYN002 is formulated to be applied twice a day to the shoulder. In addition to incompletely controlled focal epilepsies, ZYN002 is also being investigated for fragile X syndrome, developmental and epileptic encephalopathies.
STAR 2 is the extension of STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment of Epilepsy), a 12-week, phase 2a study of the gel. It randomized 181 patients to placebo or to 195 mg or 390 mg CBD gel twice daily.
Patients were a mean of about 40 years old. They had incompletely controlled focal epilepsies, experiencing about 10 seizures per month despite taking a median of three antiepileptic drugs (AEDs). The most commonly used AEDs were levetiracetam (45%), carbamazepine (41%), lamotrigine (33%), lacosamide (28%), and valproate (22%).
By the end of STAR 1, there was an median 18% reduction in seizures from baseline in the 195-mg group, and the 390-mg group experienced a 14% reduction. However, neither of these findings were statistically significant compared with placebo. Dr. Messenheimer said an unusually high 25% placebo response rate contributed to the nonsignificant findings.
Still, patients remained committed to the study, Dr. Messenheimer pointed out: 171 of the 174 STAR trial completers entered the STAR 2 extension. The entire cohort started on the 390-mg dose, and at month 5, they could titrate up to 585 mg or 780 mg daily, or reduce the does to 195 mg twice daily.
At the 18-month point, 76 patients remained in the study. Five discontinued because of an adverse event. Sixty stopped because the gel was ineffective, and the rest exited the study on the decision of an investigator. Dr. Messenheimer presented a responder analysis on 63 of the remaining subjects with full data, as well as an intent-to-treat analysis on the entire STAR 2 cohort.
Among the entire cohort, continued treatment appeared to confer increasing benefit, he said in an interview. By 3 months, the median seizure reduction rate was 25%; it increased to 40% by 6 months and 48% by 9 months. For the next 9 months, the seizure reduction rate stayed steady, hovering at around 55%.
“Among all the patients, we saw an increase in efficacy over 18 months. Half of the patients stayed on 390 mg, and of the half that titrated to higher doses. Most of these went up to 780 mg, but we really didn’t see that the higher doses conferred much benefit over the 390.”
The 63-patient cohort could be viewed as a responder-only analysis, Dr. Messenheimer said, since most of the dropouts occurred in the first few months of the study. Nevertheless, the response rates in the entire 171-person cohort were quite similar, with a 49% reduction by 3 months that increased to a median 55% reduction by 18 months.
The gel was generally well tolerated, although Dr. Messenheimer pointed out three serious adverse events that were probably drug related: two cases of anxiety and one case of increased seizures. Other events that occurred in significantly more of the CBD groups were headaches (12%), upper respiratory infection (11%), lacerations (9%), and fatigue (6%).
There were no liver enzyme abnormalities.
Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
SOURCE: O’Brien TJ et al. AES 2018, Abstract 2.253
REPORTING FROM AES 2018
Key clinical point:
Major finding: The gel reduced uncontrolled focal seizures by a median of about 50%.
Study details: The open-label extension study comprised 171 subjects.
Disclosures: Zynerba sponsored the study; Dr. Messenheimer is a paid consultant for Zynerba.
Source: O’Brien TJ et al. AES 2018, Abstract 2.253
Program decreased seizure frequency for people with epilepsy
NEW ORLEANS – A self-management program that focused on medication adherence, sleep, nutrition, and stress reduction was associated with decreased seizures and improved quality of life for adults with epilepsy.
SMART (Self‐management for people with epilepsy and a history of negative health events) also was associated with improved depression scores and overall quality of life measures in participants, compared with a wait-listed control group, Martha Sajatovic, MD, said at the annual meeting of the American Epilepsy Society.
“I believe what we’re seeing is a result of improved self-management,” said Dr. Sajatovic, the Willard Brown Chair in Neurological Outcomes Research at Case Western Reserve University, Cleveland. “This is multimodal, including better medication adherence, which in turn is related to better communication with the clinician. For example, if patients are not sleeping well or their medicine makes them nauseated or they experience sexual dysfunction, we encourage them to talk to their docs about what they can live with, and what they can’t.”
Presented as a poster during the meeting, the SMART study was also published in Epilepsia.
SMART is an 8-week online educational program delivered by a nurse educator and a “peer educator,” a person with epilepsy who has had at least three negative health events. The first session is an in-person visit during which the team gets acquainted and discusses goals. The remaining sessions are self-paced and delivered on computer tablets provided by the investigators.
SMART didn’t just focus on the physical issues of living with epilepsy, Dr. Sajatovic said in an interview. Sessions also discussed the stigma still associated with the disorder, and myths that unnecessarily inflate perceptions. Discussions include goal setting, epilepsy complications and how to manage them, the importance of good sleep hygiene, problem-solving skills, nutrition and substance abuse, exercise, and how to deal with medication side effects.
“One thing we really stressed was sharing information in a way that was accessible to all patients and fostered self-motivation,” she said. “Most of our participants had never been in a program like this before. It was very empowering for many.”
The researchers chose participants who were socioeconomically challenged for this project; 88% made less than $25,000 per year and 74% were unemployed. The mean age of participants was 41 years, 70% were black, and most had been living with epilepsy at least half of their life. About 70% lived alone, and 70% had experienced at least one seizure within the month before enrolling. Mental health comorbidities were common; 69% had depression, 32% had anxiety, and 13% had PTSD.
The study enrolled 120 people, who were evenly divided between the intervention group and the wait-list group. The primary outcome was the change in total negative health events from baseline to the study’s end. Negative health events were seizures and ED or hospital admissions for any other causes including attempts at self-harm, falls, and accidents.
Secondary outcomes included changes in depression scores as measured by the Montgomery-Åsberg Depression Rating Scale and the 9-item Patient Health Questionnaire. Quality of life was measured using the 10-item Quality of Life in Epilepsy; functional status was measured using the 36-Item Short-Form Health Survey.
At baseline, the total mean 6-month negative health events count was 15, with 13 events being seizures. The other events were hospital or ED visits for other reasons.
At the end of the study, the intervention group experienced a significant mean decrease of 10 fewer negative health events, compared with a decrease of 2 in the wait-listed group. This was largely driven by a mean of 7.8 fewer seizures in the active group, compared with a decrease of about 1.0 in the wait-listed group. The 6-month ER and hospitalization counts did not significantly change.
Among the secondary outcomes, depression, overall health, and quality of life all improved significantly in the intervention group, compared with the wait-listed group. The intervention group also had significant decreases in depression measures and improvements in daily function measures, Dr. Sajatovic said.
“It was so gratifying to see this. Most of our participants had never been in a program like this before. It was a chance for them to take control of their epilepsy, instead of simply having it control them,” she said.
This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic had no financial disclosures related to this presentation.
SOURCE: Sajatovic M et al.
NEW ORLEANS – A self-management program that focused on medication adherence, sleep, nutrition, and stress reduction was associated with decreased seizures and improved quality of life for adults with epilepsy.
SMART (Self‐management for people with epilepsy and a history of negative health events) also was associated with improved depression scores and overall quality of life measures in participants, compared with a wait-listed control group, Martha Sajatovic, MD, said at the annual meeting of the American Epilepsy Society.
“I believe what we’re seeing is a result of improved self-management,” said Dr. Sajatovic, the Willard Brown Chair in Neurological Outcomes Research at Case Western Reserve University, Cleveland. “This is multimodal, including better medication adherence, which in turn is related to better communication with the clinician. For example, if patients are not sleeping well or their medicine makes them nauseated or they experience sexual dysfunction, we encourage them to talk to their docs about what they can live with, and what they can’t.”
Presented as a poster during the meeting, the SMART study was also published in Epilepsia.
SMART is an 8-week online educational program delivered by a nurse educator and a “peer educator,” a person with epilepsy who has had at least three negative health events. The first session is an in-person visit during which the team gets acquainted and discusses goals. The remaining sessions are self-paced and delivered on computer tablets provided by the investigators.
SMART didn’t just focus on the physical issues of living with epilepsy, Dr. Sajatovic said in an interview. Sessions also discussed the stigma still associated with the disorder, and myths that unnecessarily inflate perceptions. Discussions include goal setting, epilepsy complications and how to manage them, the importance of good sleep hygiene, problem-solving skills, nutrition and substance abuse, exercise, and how to deal with medication side effects.
“One thing we really stressed was sharing information in a way that was accessible to all patients and fostered self-motivation,” she said. “Most of our participants had never been in a program like this before. It was very empowering for many.”
The researchers chose participants who were socioeconomically challenged for this project; 88% made less than $25,000 per year and 74% were unemployed. The mean age of participants was 41 years, 70% were black, and most had been living with epilepsy at least half of their life. About 70% lived alone, and 70% had experienced at least one seizure within the month before enrolling. Mental health comorbidities were common; 69% had depression, 32% had anxiety, and 13% had PTSD.
The study enrolled 120 people, who were evenly divided between the intervention group and the wait-list group. The primary outcome was the change in total negative health events from baseline to the study’s end. Negative health events were seizures and ED or hospital admissions for any other causes including attempts at self-harm, falls, and accidents.
Secondary outcomes included changes in depression scores as measured by the Montgomery-Åsberg Depression Rating Scale and the 9-item Patient Health Questionnaire. Quality of life was measured using the 10-item Quality of Life in Epilepsy; functional status was measured using the 36-Item Short-Form Health Survey.
At baseline, the total mean 6-month negative health events count was 15, with 13 events being seizures. The other events were hospital or ED visits for other reasons.
At the end of the study, the intervention group experienced a significant mean decrease of 10 fewer negative health events, compared with a decrease of 2 in the wait-listed group. This was largely driven by a mean of 7.8 fewer seizures in the active group, compared with a decrease of about 1.0 in the wait-listed group. The 6-month ER and hospitalization counts did not significantly change.
Among the secondary outcomes, depression, overall health, and quality of life all improved significantly in the intervention group, compared with the wait-listed group. The intervention group also had significant decreases in depression measures and improvements in daily function measures, Dr. Sajatovic said.
“It was so gratifying to see this. Most of our participants had never been in a program like this before. It was a chance for them to take control of their epilepsy, instead of simply having it control them,” she said.
This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic had no financial disclosures related to this presentation.
SOURCE: Sajatovic M et al.
NEW ORLEANS – A self-management program that focused on medication adherence, sleep, nutrition, and stress reduction was associated with decreased seizures and improved quality of life for adults with epilepsy.
SMART (Self‐management for people with epilepsy and a history of negative health events) also was associated with improved depression scores and overall quality of life measures in participants, compared with a wait-listed control group, Martha Sajatovic, MD, said at the annual meeting of the American Epilepsy Society.
“I believe what we’re seeing is a result of improved self-management,” said Dr. Sajatovic, the Willard Brown Chair in Neurological Outcomes Research at Case Western Reserve University, Cleveland. “This is multimodal, including better medication adherence, which in turn is related to better communication with the clinician. For example, if patients are not sleeping well or their medicine makes them nauseated or they experience sexual dysfunction, we encourage them to talk to their docs about what they can live with, and what they can’t.”
Presented as a poster during the meeting, the SMART study was also published in Epilepsia.
SMART is an 8-week online educational program delivered by a nurse educator and a “peer educator,” a person with epilepsy who has had at least three negative health events. The first session is an in-person visit during which the team gets acquainted and discusses goals. The remaining sessions are self-paced and delivered on computer tablets provided by the investigators.
SMART didn’t just focus on the physical issues of living with epilepsy, Dr. Sajatovic said in an interview. Sessions also discussed the stigma still associated with the disorder, and myths that unnecessarily inflate perceptions. Discussions include goal setting, epilepsy complications and how to manage them, the importance of good sleep hygiene, problem-solving skills, nutrition and substance abuse, exercise, and how to deal with medication side effects.
“One thing we really stressed was sharing information in a way that was accessible to all patients and fostered self-motivation,” she said. “Most of our participants had never been in a program like this before. It was very empowering for many.”
The researchers chose participants who were socioeconomically challenged for this project; 88% made less than $25,000 per year and 74% were unemployed. The mean age of participants was 41 years, 70% were black, and most had been living with epilepsy at least half of their life. About 70% lived alone, and 70% had experienced at least one seizure within the month before enrolling. Mental health comorbidities were common; 69% had depression, 32% had anxiety, and 13% had PTSD.
The study enrolled 120 people, who were evenly divided between the intervention group and the wait-list group. The primary outcome was the change in total negative health events from baseline to the study’s end. Negative health events were seizures and ED or hospital admissions for any other causes including attempts at self-harm, falls, and accidents.
Secondary outcomes included changes in depression scores as measured by the Montgomery-Åsberg Depression Rating Scale and the 9-item Patient Health Questionnaire. Quality of life was measured using the 10-item Quality of Life in Epilepsy; functional status was measured using the 36-Item Short-Form Health Survey.
At baseline, the total mean 6-month negative health events count was 15, with 13 events being seizures. The other events were hospital or ED visits for other reasons.
At the end of the study, the intervention group experienced a significant mean decrease of 10 fewer negative health events, compared with a decrease of 2 in the wait-listed group. This was largely driven by a mean of 7.8 fewer seizures in the active group, compared with a decrease of about 1.0 in the wait-listed group. The 6-month ER and hospitalization counts did not significantly change.
Among the secondary outcomes, depression, overall health, and quality of life all improved significantly in the intervention group, compared with the wait-listed group. The intervention group also had significant decreases in depression measures and improvements in daily function measures, Dr. Sajatovic said.
“It was so gratifying to see this. Most of our participants had never been in a program like this before. It was a chance for them to take control of their epilepsy, instead of simply having it control them,” she said.
This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic had no financial disclosures related to this presentation.
SOURCE: Sajatovic M et al.
REPORTING FROM AES 2018
Key clinical point: Patients with epilepsy had fewer seizures and improved quality of life after 6 months of participation in a program that teaches self-management techniques.
Major finding: The intervention group had a mean of 7.8 fewer seizures, compared with their baseline count during the 6-month study.
Study details: The prospective study randomized 120 people to either the intervention group or a wait-list group.
Disclosures: This study was supported by a grant from the Centers for Disease Control and Prevention. Dr. Sajatovic reported no financial disclosures related to this presentation.
Source: Sajatovic M et al. AES
PCOS linked to increased cancer risk in premenopausal women
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
based on an analysis of nearly 3.5 million women in a large Swedish database.
Women with PCOS had a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and more than a doubling in the risk of ovarian and pancreatic cancers. Once women reached menopausal status, however, their cancer risk was comparable to that of women without a history of PCOS.
“Several carcinogenic processes are associated with PCOS, including dyslipidemia, hyperinsulinemia, and chronic inflammation,” wrote Weimin Ye, MD, PhD, of the Karolinska Institutet, Stockholm, and his colleagues. “Our study indicates that cancer may need to be added to the spectrum of long-term health consequences of PCOS and warrants increased surveillance among those patients.”
The research letter was published online in JAMA Oncology.
The team examined the relationship between PCOS and primary cancers in about 3.5 million women over a span of up to 24 years (1985-2009), although the mean follow-up time was not mentioned. To examine the potential impact of menopause, they conducted separate multivariate logistic regression analyses for those younger than 51 years, and those aged 51 years or older. The analyses controlled for use of some medications (metformin, oral contraceptives, and hormone therapy); as well as educational level (a proxy for socioeconomic status); smoking; parity (a proxy for fertility); parental cancers; and diabetes.
Overall, 14,764 women had been diagnosed with PCOS; they were a mean of 28 years at baseline and 182 developed a primary cancer 1 year or more after PCOS diagnosis.
These women had a 15% overall increased risk of cancer, compared with women without PCOS.
The risks for specific cancers also were increased, compared with women without PCOS, including endometrial (hazard ratio, 2.62), ovarian (HR, 2.16), endocrine (HR, 1.92), pancreatic (HR, 3.4), kidney (HR, 3.0), and skeletal and hematopoietic (HR, 1.69) cancers.
The risks were associated with younger age, however. In the group under age 51 years, the overall risk was 22% higher. The increased risk of specific cancers were endometrial (HR, 6.45), ovarian (HR, 2.55), pancreatic (HR, 6.68), kidney (HR, 4.57), and endocrine (not thyroid) gland (HR, 2.9) cancers.
The authors had no relevant financial disclosures.
SOURCE: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
FROM JAMA ONCOLOGY
Key clinical point: Polycystic ovarian syndrome may be associated with increased cancer risks among younger women.
Major finding: Among premenopausal women, there was a sixfold increased risk of endometrial cancer, a tripling of endocrine gland cancers, and a more than doubling in the risk of ovarian and pancreatic cancers
Study details: The study examined risks in 3.5 million women with up to 24 years of follow-up.
Disclosures: The study authors had no financial disclosures.
Source: Yin W et al. JAMA Oncol. 2018 Nov 29. doi:10.1001/jamaoncol.2018.5188.
Three commonly used quick cognitive assessments often yield flawed results
a retrospective analysis has concluded.
The likelihood of a false-positive or false-negative result declined sharply when all three tests were given, however; only about 2% of patients were misclassified in all three, David Llewellyn, PhD, and his colleagues reported in Neurology: Clinical Practice.
The Mini Mental State Examination (MMSE), Memory Impairment Screen (MIS), and animal naming (AN) were susceptible to different measurement biases, wrote Dr. Llewellyn of the University of Exeter (U.K.).
Just one variable – an informant’s perception of the patient’s memory as unimpaired – consistently predicted inaccuracy in all three tests. Most of the patients in this category carried the diagnosis of cognitively impaired but not demented (CIND), a finding that has important clinical implications.
“These participants may be in the very early stages of conversion to dementia. ... Therefore, of those with low or borderline cognitive assessment results, reassessment to detect further decline may be appropriate.”
The study comprised 824 patients included in the Aging, Demographics and Memory Study, which is a subsample of the Health and Retirement Study. They completed the tests from 2001-2004, during which time they were a mean of 82 years old. A panel of experts adjudicated diagnoses, which they then parsed into all-cause dementia, CIND, or cognitively normal. The testing included a self and informant assessment of memory decline. The investigators also looked at 22 predictors of cognition, including patient characteristics, apolipoprotein E carriage (ApoE e4), and sociodemographic factors.
The prevalence of dementia was 35.3%; of the nondemented patients, 43% met the criteria for CIND. The team found that 35.7% of cases were misclassified by at least one test, 13.4% by two, and 1.7% by all three.
The MMSE was the least accurate, with a 21% misclassification rate, reflected in an 18.6% false-positive rate for those without dementia and a 2.4% rate of false-negative for those with dementia.
The MIS had a 16% misclassification rate, with a 9.5% rate of false-positive for those with no dementia and a 6.3% rate of false-negative for those without.
The AN had a 14% misclassification rate, with a 6.8% false-positive rate for those without dementia and a 7.7% false-negative rate for those with dementia.
For the MMSE, MIS, and AN, the number of participants with false-positives that met the criteria for CIND were 74.5%, 82.1%, and 82.1%, respectively.
In the final multivariate model, seven variables predicted misclassification, including black ethnicity for the MMSE; age, visual impairment, ApoeE4 noncarrier, and depression for the MIS; and no hyperlipidemia and normal informant memory assessment for the AN. Lower years of education and heart problems predicted misclassification on both the MMSE and AN.
An absence of informant-related poor memory predicted misclassification on all three tests.
“Failing to detect dementia can delay access to treatment and support, whereas false alarms lead to unnecessary investigations, causing pressure on health care systems,” Dr. Llewellyn said in a press statement. “Identifying people with dementia in a timely fashion is important, particularly as new methods of treatment come onstream. Our findings show that we desperately need more accurate and less biased ways of detecting dementia swiftly in clinic.”
The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.
SOURCE: Llewellyn D et al. Neuro Clin Pract. 2019;1:1-9.
a retrospective analysis has concluded.
The likelihood of a false-positive or false-negative result declined sharply when all three tests were given, however; only about 2% of patients were misclassified in all three, David Llewellyn, PhD, and his colleagues reported in Neurology: Clinical Practice.
The Mini Mental State Examination (MMSE), Memory Impairment Screen (MIS), and animal naming (AN) were susceptible to different measurement biases, wrote Dr. Llewellyn of the University of Exeter (U.K.).
Just one variable – an informant’s perception of the patient’s memory as unimpaired – consistently predicted inaccuracy in all three tests. Most of the patients in this category carried the diagnosis of cognitively impaired but not demented (CIND), a finding that has important clinical implications.
“These participants may be in the very early stages of conversion to dementia. ... Therefore, of those with low or borderline cognitive assessment results, reassessment to detect further decline may be appropriate.”
The study comprised 824 patients included in the Aging, Demographics and Memory Study, which is a subsample of the Health and Retirement Study. They completed the tests from 2001-2004, during which time they were a mean of 82 years old. A panel of experts adjudicated diagnoses, which they then parsed into all-cause dementia, CIND, or cognitively normal. The testing included a self and informant assessment of memory decline. The investigators also looked at 22 predictors of cognition, including patient characteristics, apolipoprotein E carriage (ApoE e4), and sociodemographic factors.
The prevalence of dementia was 35.3%; of the nondemented patients, 43% met the criteria for CIND. The team found that 35.7% of cases were misclassified by at least one test, 13.4% by two, and 1.7% by all three.
The MMSE was the least accurate, with a 21% misclassification rate, reflected in an 18.6% false-positive rate for those without dementia and a 2.4% rate of false-negative for those with dementia.
The MIS had a 16% misclassification rate, with a 9.5% rate of false-positive for those with no dementia and a 6.3% rate of false-negative for those without.
The AN had a 14% misclassification rate, with a 6.8% false-positive rate for those without dementia and a 7.7% false-negative rate for those with dementia.
For the MMSE, MIS, and AN, the number of participants with false-positives that met the criteria for CIND were 74.5%, 82.1%, and 82.1%, respectively.
In the final multivariate model, seven variables predicted misclassification, including black ethnicity for the MMSE; age, visual impairment, ApoeE4 noncarrier, and depression for the MIS; and no hyperlipidemia and normal informant memory assessment for the AN. Lower years of education and heart problems predicted misclassification on both the MMSE and AN.
An absence of informant-related poor memory predicted misclassification on all three tests.
“Failing to detect dementia can delay access to treatment and support, whereas false alarms lead to unnecessary investigations, causing pressure on health care systems,” Dr. Llewellyn said in a press statement. “Identifying people with dementia in a timely fashion is important, particularly as new methods of treatment come onstream. Our findings show that we desperately need more accurate and less biased ways of detecting dementia swiftly in clinic.”
The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.
SOURCE: Llewellyn D et al. Neuro Clin Pract. 2019;1:1-9.
a retrospective analysis has concluded.
The likelihood of a false-positive or false-negative result declined sharply when all three tests were given, however; only about 2% of patients were misclassified in all three, David Llewellyn, PhD, and his colleagues reported in Neurology: Clinical Practice.
The Mini Mental State Examination (MMSE), Memory Impairment Screen (MIS), and animal naming (AN) were susceptible to different measurement biases, wrote Dr. Llewellyn of the University of Exeter (U.K.).
Just one variable – an informant’s perception of the patient’s memory as unimpaired – consistently predicted inaccuracy in all three tests. Most of the patients in this category carried the diagnosis of cognitively impaired but not demented (CIND), a finding that has important clinical implications.
“These participants may be in the very early stages of conversion to dementia. ... Therefore, of those with low or borderline cognitive assessment results, reassessment to detect further decline may be appropriate.”
The study comprised 824 patients included in the Aging, Demographics and Memory Study, which is a subsample of the Health and Retirement Study. They completed the tests from 2001-2004, during which time they were a mean of 82 years old. A panel of experts adjudicated diagnoses, which they then parsed into all-cause dementia, CIND, or cognitively normal. The testing included a self and informant assessment of memory decline. The investigators also looked at 22 predictors of cognition, including patient characteristics, apolipoprotein E carriage (ApoE e4), and sociodemographic factors.
The prevalence of dementia was 35.3%; of the nondemented patients, 43% met the criteria for CIND. The team found that 35.7% of cases were misclassified by at least one test, 13.4% by two, and 1.7% by all three.
The MMSE was the least accurate, with a 21% misclassification rate, reflected in an 18.6% false-positive rate for those without dementia and a 2.4% rate of false-negative for those with dementia.
The MIS had a 16% misclassification rate, with a 9.5% rate of false-positive for those with no dementia and a 6.3% rate of false-negative for those without.
The AN had a 14% misclassification rate, with a 6.8% false-positive rate for those without dementia and a 7.7% false-negative rate for those with dementia.
For the MMSE, MIS, and AN, the number of participants with false-positives that met the criteria for CIND were 74.5%, 82.1%, and 82.1%, respectively.
In the final multivariate model, seven variables predicted misclassification, including black ethnicity for the MMSE; age, visual impairment, ApoeE4 noncarrier, and depression for the MIS; and no hyperlipidemia and normal informant memory assessment for the AN. Lower years of education and heart problems predicted misclassification on both the MMSE and AN.
An absence of informant-related poor memory predicted misclassification on all three tests.
“Failing to detect dementia can delay access to treatment and support, whereas false alarms lead to unnecessary investigations, causing pressure on health care systems,” Dr. Llewellyn said in a press statement. “Identifying people with dementia in a timely fashion is important, particularly as new methods of treatment come onstream. Our findings show that we desperately need more accurate and less biased ways of detecting dementia swiftly in clinic.”
The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.
SOURCE: Llewellyn D et al. Neuro Clin Pract. 2019;1:1-9.
FROM NEUROLOGY: CLINICAL PRACTICE
Key clinical point: Used alone, the MMSE, Memory Impairment Screen, and animal naming tests may not correctly flag patients with memory problems.
Major finding: More than a third of patients received an inaccurate diagnosis from at least one of the tests.
Study details: The retrospective study comprised 824 patients.
Disclosures: The study was supported by the Halpin Trust, the Mary Kinross Charitable Trust, the Engineering and Physical Sciences Research Council, and the U.K. National Institute for Health Research. None of the authors reported any financial conflicts relevant to the work.Source: Llewellyn D et al. Neuro Clin Pract. 2019;9(1):1-9.
CDC: No medical therapy can yet be recommended for acute flaccid myelitis
The updated guidance on managing acute flaccid myelitis is unlikely to relieve the frustrations of physicians struggling to treat the condition.
After reviewing the extant data on the baffling disorder, the Centers for Disease Control and Prevention found no evidence that corticosteroids, interferon, antivirals, or any other immunologic or biologic therapy is an effective treatment.
All of the treatments mentioned in the guidance have been used anecdotally, and often for cases proven to be associated with enterovirus-related cases. However, there are no well validated studies confirming benefit for any of these approaches, the agency said in its clinical management document.
Acute flaccid myelitis (AFM) has stricken 90 patients in the United States this year and another 252 cases are being investigated, according to new data from the CDC. The number of confirmed cases is triple that seen in 2017. Whether the disease is an infectious or autoimmune process, or something else entirely, remains unknown.
In response to the outbreak – the largest since 2014 – an expert panel of 4 CDC staff physicians reviewed the literature to find what, if any, treatments were effective; another 14 external experts provided input on the recommendations. At this point, nothing can be officially recommended, the agency said.
Corticosteroids
Corticosteroids should not be administered to most patients with AFM. In addition to “a theoretical concern” about the potential adverse effects of these drugs in acute infections, there is some hard evidence that they are associated with worse outcomes in enteroviral neuroinvasive diseases, particularly those caused by EV-71.
This observation, following a 2012 outbreak in Cambodia, led a World Health Organization commission to conclude that corticosteroids were contraindicated in the management of EV-71–associated neuroinvasive disease. This year, there has been an uptick in EV-A71-associated neurologic disease.
The CDC did hedge its advice on corticosteroids a bit in the setting of AFM, however. “There may be theoretical benefit for steroids in the setting of severe cord swelling or long tract signs suggesting white matter involvement, where steroids may salvage tissue that may be harmed due to an ongoing immune/inflammatory response. While AFM is clinically and radiographically defined by the predominance of gray matter damage in the spinal cord, some patients may have some white matter involvement. It is not clear if these different patterns are important relative to therapeutic considerations.”
Nevertheless, the agency does not recommend corticosteroid use for these patients. “The possible benefits of the use of corticosteroids to manage spinal cord edema or white matter involvement in AFM should be balanced with the possible harm due to immunosuppression in the setting of possible viral infection.”
IVIG
While IVIG holds some theoretical benefit for AFM, there are no high-level human data, the guidelines state. The treatment is generally safe and well tolerated, but the few reports of its use in AFM did not show clear benefit. These include two case series. One suggested an acute improvement of neurologic status, but no long-term resolution of deficits. The other indicated neither significant improvement nor deterioration.
However, current practice at Children’s Hospital of Philadelphia is to initiate IVIG therapy at AFM diagnosis in hopes of boosting humoral immunity.
Nevertheless, the CDC said, “For IVIG to modify disease in an active viral infectious process, early administration is likely required, and possibly prior to exposure,” and the treatment cannot be recommended.
Plasma exchange
Plasma exchange in combination with IVIG and corticosteroids was ineffective in a case series of four Argentinian children, although a single case published last year found that the combination was associated with significant improvement. However, there are not enough data to recommend this approach.
Fluoxetine
Fluoxetine’s antiviral potential turned up in a high-throughput screening project to identify novel compounds with antiviral efficacy against enteroviruses. In 2012, researchers from the University of California, Los Angeles, tested more than 1,000 compounds and found that the SSRI is a potent inhibitor of coxsackievirus. A later project at the National Institutes of Health replicated this finding, and determined that fluoxetine inhibited several enteroviruses, including the AFM suspect, EV-D68.
Fluoxetine concentrates more highly in the central nervous system than it does in plasma, but its antiviral properties have nothing to do with neurotransmitter activity. Rather, it appears to inhibit protein 2C, a highly conserved nonstructural protein that’s crucial to the assembly of RNA into virion particles.
In early November, a retrospective study examined fluoxetine’s use in 30 AFM patients, compared with 26 who did not receive it. The primary outcome was change in summative limb strength score. The study did little to clarify any benefit, however. The authors concluded that fluoxetine was preferentially given to patients with EDV-68 infections. They had more severe impairment at nadir, and at the last follow-up of about 1 year, they had worse outcomes.
“There is no clear human evidence for efficacy of fluoxetine in the treatment of AFM based on a single retrospective evaluation conducted in patients with AFM, and data from a mouse model also did not support efficacy,” the CDC said.
Antiviral medications
The CDC is quite clear on its recommendation that these drugs are not indicated in AFM, since it is not yet proven to be an infectious process.
“Any guidance regarding antiviral medications should be interpreted with great caution, given the unknowns about the pathogenesis of this illness at present ... Testing has been conducted at CDC for antiviral activity of compounds pleconaril, pocapavir, and vapendavir and none have significant activity against currently circulating strains of EV-D68 at clinically relevant concentrations.”
Interferon
There is some anecdotal evidence that interferon alpha-2b was beneficial in treating a polio-like syndrome associated with West Nile virus and Saint Louis encephalitis. “Although there are limited in vitro, animal, and anecdotal human data suggesting activity of some interferons against viral infections, sufficient data are lacking in the setting of AFM,” the agency said. “There is no indication that interferon should be used for the treatment of AFM, and there is concern about the potential for harm from the use of interferon given the immunomodulatory effects in the setting of possible ongoing viral replication.”
SOURCE: CDC Acute Flaccid Myelitis: Interim Considerations for Clinical Management
The updated guidance on managing acute flaccid myelitis is unlikely to relieve the frustrations of physicians struggling to treat the condition.
After reviewing the extant data on the baffling disorder, the Centers for Disease Control and Prevention found no evidence that corticosteroids, interferon, antivirals, or any other immunologic or biologic therapy is an effective treatment.
All of the treatments mentioned in the guidance have been used anecdotally, and often for cases proven to be associated with enterovirus-related cases. However, there are no well validated studies confirming benefit for any of these approaches, the agency said in its clinical management document.
Acute flaccid myelitis (AFM) has stricken 90 patients in the United States this year and another 252 cases are being investigated, according to new data from the CDC. The number of confirmed cases is triple that seen in 2017. Whether the disease is an infectious or autoimmune process, or something else entirely, remains unknown.
In response to the outbreak – the largest since 2014 – an expert panel of 4 CDC staff physicians reviewed the literature to find what, if any, treatments were effective; another 14 external experts provided input on the recommendations. At this point, nothing can be officially recommended, the agency said.
Corticosteroids
Corticosteroids should not be administered to most patients with AFM. In addition to “a theoretical concern” about the potential adverse effects of these drugs in acute infections, there is some hard evidence that they are associated with worse outcomes in enteroviral neuroinvasive diseases, particularly those caused by EV-71.
This observation, following a 2012 outbreak in Cambodia, led a World Health Organization commission to conclude that corticosteroids were contraindicated in the management of EV-71–associated neuroinvasive disease. This year, there has been an uptick in EV-A71-associated neurologic disease.
The CDC did hedge its advice on corticosteroids a bit in the setting of AFM, however. “There may be theoretical benefit for steroids in the setting of severe cord swelling or long tract signs suggesting white matter involvement, where steroids may salvage tissue that may be harmed due to an ongoing immune/inflammatory response. While AFM is clinically and radiographically defined by the predominance of gray matter damage in the spinal cord, some patients may have some white matter involvement. It is not clear if these different patterns are important relative to therapeutic considerations.”
Nevertheless, the agency does not recommend corticosteroid use for these patients. “The possible benefits of the use of corticosteroids to manage spinal cord edema or white matter involvement in AFM should be balanced with the possible harm due to immunosuppression in the setting of possible viral infection.”
IVIG
While IVIG holds some theoretical benefit for AFM, there are no high-level human data, the guidelines state. The treatment is generally safe and well tolerated, but the few reports of its use in AFM did not show clear benefit. These include two case series. One suggested an acute improvement of neurologic status, but no long-term resolution of deficits. The other indicated neither significant improvement nor deterioration.
However, current practice at Children’s Hospital of Philadelphia is to initiate IVIG therapy at AFM diagnosis in hopes of boosting humoral immunity.
Nevertheless, the CDC said, “For IVIG to modify disease in an active viral infectious process, early administration is likely required, and possibly prior to exposure,” and the treatment cannot be recommended.
Plasma exchange
Plasma exchange in combination with IVIG and corticosteroids was ineffective in a case series of four Argentinian children, although a single case published last year found that the combination was associated with significant improvement. However, there are not enough data to recommend this approach.
Fluoxetine
Fluoxetine’s antiviral potential turned up in a high-throughput screening project to identify novel compounds with antiviral efficacy against enteroviruses. In 2012, researchers from the University of California, Los Angeles, tested more than 1,000 compounds and found that the SSRI is a potent inhibitor of coxsackievirus. A later project at the National Institutes of Health replicated this finding, and determined that fluoxetine inhibited several enteroviruses, including the AFM suspect, EV-D68.
Fluoxetine concentrates more highly in the central nervous system than it does in plasma, but its antiviral properties have nothing to do with neurotransmitter activity. Rather, it appears to inhibit protein 2C, a highly conserved nonstructural protein that’s crucial to the assembly of RNA into virion particles.
In early November, a retrospective study examined fluoxetine’s use in 30 AFM patients, compared with 26 who did not receive it. The primary outcome was change in summative limb strength score. The study did little to clarify any benefit, however. The authors concluded that fluoxetine was preferentially given to patients with EDV-68 infections. They had more severe impairment at nadir, and at the last follow-up of about 1 year, they had worse outcomes.
“There is no clear human evidence for efficacy of fluoxetine in the treatment of AFM based on a single retrospective evaluation conducted in patients with AFM, and data from a mouse model also did not support efficacy,” the CDC said.
Antiviral medications
The CDC is quite clear on its recommendation that these drugs are not indicated in AFM, since it is not yet proven to be an infectious process.
“Any guidance regarding antiviral medications should be interpreted with great caution, given the unknowns about the pathogenesis of this illness at present ... Testing has been conducted at CDC for antiviral activity of compounds pleconaril, pocapavir, and vapendavir and none have significant activity against currently circulating strains of EV-D68 at clinically relevant concentrations.”
Interferon
There is some anecdotal evidence that interferon alpha-2b was beneficial in treating a polio-like syndrome associated with West Nile virus and Saint Louis encephalitis. “Although there are limited in vitro, animal, and anecdotal human data suggesting activity of some interferons against viral infections, sufficient data are lacking in the setting of AFM,” the agency said. “There is no indication that interferon should be used for the treatment of AFM, and there is concern about the potential for harm from the use of interferon given the immunomodulatory effects in the setting of possible ongoing viral replication.”
SOURCE: CDC Acute Flaccid Myelitis: Interim Considerations for Clinical Management
The updated guidance on managing acute flaccid myelitis is unlikely to relieve the frustrations of physicians struggling to treat the condition.
After reviewing the extant data on the baffling disorder, the Centers for Disease Control and Prevention found no evidence that corticosteroids, interferon, antivirals, or any other immunologic or biologic therapy is an effective treatment.
All of the treatments mentioned in the guidance have been used anecdotally, and often for cases proven to be associated with enterovirus-related cases. However, there are no well validated studies confirming benefit for any of these approaches, the agency said in its clinical management document.
Acute flaccid myelitis (AFM) has stricken 90 patients in the United States this year and another 252 cases are being investigated, according to new data from the CDC. The number of confirmed cases is triple that seen in 2017. Whether the disease is an infectious or autoimmune process, or something else entirely, remains unknown.
In response to the outbreak – the largest since 2014 – an expert panel of 4 CDC staff physicians reviewed the literature to find what, if any, treatments were effective; another 14 external experts provided input on the recommendations. At this point, nothing can be officially recommended, the agency said.
Corticosteroids
Corticosteroids should not be administered to most patients with AFM. In addition to “a theoretical concern” about the potential adverse effects of these drugs in acute infections, there is some hard evidence that they are associated with worse outcomes in enteroviral neuroinvasive diseases, particularly those caused by EV-71.
This observation, following a 2012 outbreak in Cambodia, led a World Health Organization commission to conclude that corticosteroids were contraindicated in the management of EV-71–associated neuroinvasive disease. This year, there has been an uptick in EV-A71-associated neurologic disease.
The CDC did hedge its advice on corticosteroids a bit in the setting of AFM, however. “There may be theoretical benefit for steroids in the setting of severe cord swelling or long tract signs suggesting white matter involvement, where steroids may salvage tissue that may be harmed due to an ongoing immune/inflammatory response. While AFM is clinically and radiographically defined by the predominance of gray matter damage in the spinal cord, some patients may have some white matter involvement. It is not clear if these different patterns are important relative to therapeutic considerations.”
Nevertheless, the agency does not recommend corticosteroid use for these patients. “The possible benefits of the use of corticosteroids to manage spinal cord edema or white matter involvement in AFM should be balanced with the possible harm due to immunosuppression in the setting of possible viral infection.”
IVIG
While IVIG holds some theoretical benefit for AFM, there are no high-level human data, the guidelines state. The treatment is generally safe and well tolerated, but the few reports of its use in AFM did not show clear benefit. These include two case series. One suggested an acute improvement of neurologic status, but no long-term resolution of deficits. The other indicated neither significant improvement nor deterioration.
However, current practice at Children’s Hospital of Philadelphia is to initiate IVIG therapy at AFM diagnosis in hopes of boosting humoral immunity.
Nevertheless, the CDC said, “For IVIG to modify disease in an active viral infectious process, early administration is likely required, and possibly prior to exposure,” and the treatment cannot be recommended.
Plasma exchange
Plasma exchange in combination with IVIG and corticosteroids was ineffective in a case series of four Argentinian children, although a single case published last year found that the combination was associated with significant improvement. However, there are not enough data to recommend this approach.
Fluoxetine
Fluoxetine’s antiviral potential turned up in a high-throughput screening project to identify novel compounds with antiviral efficacy against enteroviruses. In 2012, researchers from the University of California, Los Angeles, tested more than 1,000 compounds and found that the SSRI is a potent inhibitor of coxsackievirus. A later project at the National Institutes of Health replicated this finding, and determined that fluoxetine inhibited several enteroviruses, including the AFM suspect, EV-D68.
Fluoxetine concentrates more highly in the central nervous system than it does in plasma, but its antiviral properties have nothing to do with neurotransmitter activity. Rather, it appears to inhibit protein 2C, a highly conserved nonstructural protein that’s crucial to the assembly of RNA into virion particles.
In early November, a retrospective study examined fluoxetine’s use in 30 AFM patients, compared with 26 who did not receive it. The primary outcome was change in summative limb strength score. The study did little to clarify any benefit, however. The authors concluded that fluoxetine was preferentially given to patients with EDV-68 infections. They had more severe impairment at nadir, and at the last follow-up of about 1 year, they had worse outcomes.
“There is no clear human evidence for efficacy of fluoxetine in the treatment of AFM based on a single retrospective evaluation conducted in patients with AFM, and data from a mouse model also did not support efficacy,” the CDC said.
Antiviral medications
The CDC is quite clear on its recommendation that these drugs are not indicated in AFM, since it is not yet proven to be an infectious process.
“Any guidance regarding antiviral medications should be interpreted with great caution, given the unknowns about the pathogenesis of this illness at present ... Testing has been conducted at CDC for antiviral activity of compounds pleconaril, pocapavir, and vapendavir and none have significant activity against currently circulating strains of EV-D68 at clinically relevant concentrations.”
Interferon
There is some anecdotal evidence that interferon alpha-2b was beneficial in treating a polio-like syndrome associated with West Nile virus and Saint Louis encephalitis. “Although there are limited in vitro, animal, and anecdotal human data suggesting activity of some interferons against viral infections, sufficient data are lacking in the setting of AFM,” the agency said. “There is no indication that interferon should be used for the treatment of AFM, and there is concern about the potential for harm from the use of interferon given the immunomodulatory effects in the setting of possible ongoing viral replication.”
SOURCE: CDC Acute Flaccid Myelitis: Interim Considerations for Clinical Management