User login
Antidepressants may scupper efficacy of MDMA for PTSD
Pooled data from four phase 2 trials reveal that patients with recent SSRI exposure were significantly more likely to continue to meet PTSD diagnostic criteria after methylenedioxymethamphetamine (MDMA)-assisted psychotherapy than their peers who had not recently taken SSRIs.
Although preliminary, the findings have implications for clinical practice if MDMA-assisted psychotherapy is approved by the Food and Drug Administration, Allison Feduccia, PhD, study coauthor and founder of the education platform Psychedelic.Support, said in an interview.
“As psychedelic medicines become available, it’s going to be important that we try to understand what factors impact the response rate and if there are ways that we can improve the treatment outcomes. Allowing for a longer period for tapering completely off SSRIs before initiating MDMA sessions might increase the effectiveness of MDMA,” Dr. Feduccia said.
The study was published online Nov. 20, 2020, in Psychopharmacology (doi: 10.1007/s00213-020-05710-w).
Reduced response
The primary mechanism of action of MDMA involves the same reuptake transporters that are targeted by antidepressant medications commonly prescribed for PTSD. These medications include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), NRIs, and norepinephrine-dopamine reuptake inhibitors (NDRIs).
Prior research shows that, when MDMA is coadministered with a reuptake inhibitor, subjective and psychological effects of the therapy are attenuated.
The researchers sought to determine whether or not recent tapering off of an antidepressant that targets the same primary binding sites as MDMA would affect treatment response. They analyzed data on 50 adults who underwent two sessions of MDMA-assisted psychotherapy in phase 2 clinical trials.
For 16 of these patients, SSRI therapy was tapered off prior to the MDMA sessions. For 34 patients, SSRI therapy was not tapered off, because the patients had not been taking the medication at the time of initial study screening (nontaper group).
The taper protocols specified that medications be tapered gradually over a period of weeks to minimize withdrawal symptoms and for them to be discontinued at least five half-lives of each drug prior to MDMA administration.
Demographics, baseline PTSD, and depression severity were similar between the taper and the nontaper groups. Participants in the studies had chronic PTSD (symptoms lasting >6 months). Severity scores on the Clinician-Administered PTSD Scale for DSM IV (CAPS-IV) were at least 50.
After MDMA-assisted psychotherapy, the nontaper group had significantly lower (better) CAPS-IV total scores, compared with the taper group (mean, 45.7 vs. 70.3; P = .009).
About two-thirds (63.6%) of the nontaper group no longer met PTSD criteria after MDMA-assisted therapy, compared with only 25% of those in the taper group.
The nontaper group also had lower depression symptom severity scores on the Beck Depression Inventory–II, compared with the taper group (mean, 12.7 vs. 22.6; P = .010).
“Another really interesting” observation, said Dr. Feduccia, is that the expected increases in systolic and diastolic blood pressure following MDMA administration were reduced in the taper group, compared with the nontaper group.
“This suggests that MDMA didn’t have the same physiological response in individuals who tapered SSRIs. This should be followed up,” she said.
The investigators offerred several potential mechanisms for the negative effect of recent SSRI use on MDMA-assisted psychotherapy for PTSD.
These include the down-regulation of binding sites (serotonin, dopamine, and/or norepinephrine) related to SSRI use, reduced MDMA treatment-relevant increases in blood pressure in patients with recent SSRI use, and the possibility that withdrawal symptoms from SSRIs may reduce the effectiveness of MDMA psychotherapy.
Important clinical implications
In a comment, Steven R. Thorp, PhD, professor at Alliant International University, San Diego, said the findings are “very interesting” and likely “not well known.”
“There has been great interest in MDMA-assisted psychotherapy in recent years, and if this finding is replicated, it will have important implications for that research,” Dr. Thorp said.
“Although psychotherapy is often preferred by clients with PTSD, compared to medications, and typically shows efficacy that is as strong or stronger (and longer lasting) than medications, many individuals with PTSD are provided with medication only,” Dr. Thorp noted.
“This study suggests that, in addition to the other potential disadvantages of medications (e.g., cost, side effects, potential for addiction), those who take SSRIs, SNRIs, NRIs, and NDRIs for PTSD may also benefit less from MDMA-assisted psychotherapy,” Dr. Thorp added.
The four phase 2 studies used in the analysis were sponsored by the Multidisciplinary Association for Psychedelic Studies, a nonprofit organization. Dr. Feduccia received salary support for full-time employment with MAPS Public Benefit Corporation. Dr. Thorp disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pooled data from four phase 2 trials reveal that patients with recent SSRI exposure were significantly more likely to continue to meet PTSD diagnostic criteria after methylenedioxymethamphetamine (MDMA)-assisted psychotherapy than their peers who had not recently taken SSRIs.
Although preliminary, the findings have implications for clinical practice if MDMA-assisted psychotherapy is approved by the Food and Drug Administration, Allison Feduccia, PhD, study coauthor and founder of the education platform Psychedelic.Support, said in an interview.
“As psychedelic medicines become available, it’s going to be important that we try to understand what factors impact the response rate and if there are ways that we can improve the treatment outcomes. Allowing for a longer period for tapering completely off SSRIs before initiating MDMA sessions might increase the effectiveness of MDMA,” Dr. Feduccia said.
The study was published online Nov. 20, 2020, in Psychopharmacology (doi: 10.1007/s00213-020-05710-w).
Reduced response
The primary mechanism of action of MDMA involves the same reuptake transporters that are targeted by antidepressant medications commonly prescribed for PTSD. These medications include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), NRIs, and norepinephrine-dopamine reuptake inhibitors (NDRIs).
Prior research shows that, when MDMA is coadministered with a reuptake inhibitor, subjective and psychological effects of the therapy are attenuated.
The researchers sought to determine whether or not recent tapering off of an antidepressant that targets the same primary binding sites as MDMA would affect treatment response. They analyzed data on 50 adults who underwent two sessions of MDMA-assisted psychotherapy in phase 2 clinical trials.
For 16 of these patients, SSRI therapy was tapered off prior to the MDMA sessions. For 34 patients, SSRI therapy was not tapered off, because the patients had not been taking the medication at the time of initial study screening (nontaper group).
The taper protocols specified that medications be tapered gradually over a period of weeks to minimize withdrawal symptoms and for them to be discontinued at least five half-lives of each drug prior to MDMA administration.
Demographics, baseline PTSD, and depression severity were similar between the taper and the nontaper groups. Participants in the studies had chronic PTSD (symptoms lasting >6 months). Severity scores on the Clinician-Administered PTSD Scale for DSM IV (CAPS-IV) were at least 50.
After MDMA-assisted psychotherapy, the nontaper group had significantly lower (better) CAPS-IV total scores, compared with the taper group (mean, 45.7 vs. 70.3; P = .009).
About two-thirds (63.6%) of the nontaper group no longer met PTSD criteria after MDMA-assisted therapy, compared with only 25% of those in the taper group.
The nontaper group also had lower depression symptom severity scores on the Beck Depression Inventory–II, compared with the taper group (mean, 12.7 vs. 22.6; P = .010).
“Another really interesting” observation, said Dr. Feduccia, is that the expected increases in systolic and diastolic blood pressure following MDMA administration were reduced in the taper group, compared with the nontaper group.
“This suggests that MDMA didn’t have the same physiological response in individuals who tapered SSRIs. This should be followed up,” she said.
The investigators offerred several potential mechanisms for the negative effect of recent SSRI use on MDMA-assisted psychotherapy for PTSD.
These include the down-regulation of binding sites (serotonin, dopamine, and/or norepinephrine) related to SSRI use, reduced MDMA treatment-relevant increases in blood pressure in patients with recent SSRI use, and the possibility that withdrawal symptoms from SSRIs may reduce the effectiveness of MDMA psychotherapy.
Important clinical implications
In a comment, Steven R. Thorp, PhD, professor at Alliant International University, San Diego, said the findings are “very interesting” and likely “not well known.”
“There has been great interest in MDMA-assisted psychotherapy in recent years, and if this finding is replicated, it will have important implications for that research,” Dr. Thorp said.
“Although psychotherapy is often preferred by clients with PTSD, compared to medications, and typically shows efficacy that is as strong or stronger (and longer lasting) than medications, many individuals with PTSD are provided with medication only,” Dr. Thorp noted.
“This study suggests that, in addition to the other potential disadvantages of medications (e.g., cost, side effects, potential for addiction), those who take SSRIs, SNRIs, NRIs, and NDRIs for PTSD may also benefit less from MDMA-assisted psychotherapy,” Dr. Thorp added.
The four phase 2 studies used in the analysis were sponsored by the Multidisciplinary Association for Psychedelic Studies, a nonprofit organization. Dr. Feduccia received salary support for full-time employment with MAPS Public Benefit Corporation. Dr. Thorp disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pooled data from four phase 2 trials reveal that patients with recent SSRI exposure were significantly more likely to continue to meet PTSD diagnostic criteria after methylenedioxymethamphetamine (MDMA)-assisted psychotherapy than their peers who had not recently taken SSRIs.
Although preliminary, the findings have implications for clinical practice if MDMA-assisted psychotherapy is approved by the Food and Drug Administration, Allison Feduccia, PhD, study coauthor and founder of the education platform Psychedelic.Support, said in an interview.
“As psychedelic medicines become available, it’s going to be important that we try to understand what factors impact the response rate and if there are ways that we can improve the treatment outcomes. Allowing for a longer period for tapering completely off SSRIs before initiating MDMA sessions might increase the effectiveness of MDMA,” Dr. Feduccia said.
The study was published online Nov. 20, 2020, in Psychopharmacology (doi: 10.1007/s00213-020-05710-w).
Reduced response
The primary mechanism of action of MDMA involves the same reuptake transporters that are targeted by antidepressant medications commonly prescribed for PTSD. These medications include SSRIs, serotonin-norepinephrine reuptake inhibitors (SNRIs), NRIs, and norepinephrine-dopamine reuptake inhibitors (NDRIs).
Prior research shows that, when MDMA is coadministered with a reuptake inhibitor, subjective and psychological effects of the therapy are attenuated.
The researchers sought to determine whether or not recent tapering off of an antidepressant that targets the same primary binding sites as MDMA would affect treatment response. They analyzed data on 50 adults who underwent two sessions of MDMA-assisted psychotherapy in phase 2 clinical trials.
For 16 of these patients, SSRI therapy was tapered off prior to the MDMA sessions. For 34 patients, SSRI therapy was not tapered off, because the patients had not been taking the medication at the time of initial study screening (nontaper group).
The taper protocols specified that medications be tapered gradually over a period of weeks to minimize withdrawal symptoms and for them to be discontinued at least five half-lives of each drug prior to MDMA administration.
Demographics, baseline PTSD, and depression severity were similar between the taper and the nontaper groups. Participants in the studies had chronic PTSD (symptoms lasting >6 months). Severity scores on the Clinician-Administered PTSD Scale for DSM IV (CAPS-IV) were at least 50.
After MDMA-assisted psychotherapy, the nontaper group had significantly lower (better) CAPS-IV total scores, compared with the taper group (mean, 45.7 vs. 70.3; P = .009).
About two-thirds (63.6%) of the nontaper group no longer met PTSD criteria after MDMA-assisted therapy, compared with only 25% of those in the taper group.
The nontaper group also had lower depression symptom severity scores on the Beck Depression Inventory–II, compared with the taper group (mean, 12.7 vs. 22.6; P = .010).
“Another really interesting” observation, said Dr. Feduccia, is that the expected increases in systolic and diastolic blood pressure following MDMA administration were reduced in the taper group, compared with the nontaper group.
“This suggests that MDMA didn’t have the same physiological response in individuals who tapered SSRIs. This should be followed up,” she said.
The investigators offerred several potential mechanisms for the negative effect of recent SSRI use on MDMA-assisted psychotherapy for PTSD.
These include the down-regulation of binding sites (serotonin, dopamine, and/or norepinephrine) related to SSRI use, reduced MDMA treatment-relevant increases in blood pressure in patients with recent SSRI use, and the possibility that withdrawal symptoms from SSRIs may reduce the effectiveness of MDMA psychotherapy.
Important clinical implications
In a comment, Steven R. Thorp, PhD, professor at Alliant International University, San Diego, said the findings are “very interesting” and likely “not well known.”
“There has been great interest in MDMA-assisted psychotherapy in recent years, and if this finding is replicated, it will have important implications for that research,” Dr. Thorp said.
“Although psychotherapy is often preferred by clients with PTSD, compared to medications, and typically shows efficacy that is as strong or stronger (and longer lasting) than medications, many individuals with PTSD are provided with medication only,” Dr. Thorp noted.
“This study suggests that, in addition to the other potential disadvantages of medications (e.g., cost, side effects, potential for addiction), those who take SSRIs, SNRIs, NRIs, and NDRIs for PTSD may also benefit less from MDMA-assisted psychotherapy,” Dr. Thorp added.
The four phase 2 studies used in the analysis were sponsored by the Multidisciplinary Association for Psychedelic Studies, a nonprofit organization. Dr. Feduccia received salary support for full-time employment with MAPS Public Benefit Corporation. Dr. Thorp disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Brain connectivity patterns reliably identify ADHD
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Functional brain connectivity patterns are a stable biomarker of attention-deficit/hyperactivity disorder, new research suggests.
By applying a machine-learning approach to brain-imaging data, investigators were able to identify with 99% accuracy the adult study participants who had been diagnosed with ADHD in childhood.
“Even though the symptoms of ADHD may be less apparent in adulthood, the brain-wiring signature seems to be persistent,” study investigator Christopher McNorgan, PhD, of the department of psychology, State University of New York at Buffalo told this news organization.
The findings were published online Dec. 17, 2020, in Frontiers of Psychology.
Deep-learning neural networks
The researchers analyzed archived functional magnetic resonance imaging (fMRI) and behavioral data for 80 adults (mean age, 24 years; 64 male). Of these participants, 55 were diagnosed with ADHD in childhood and 25 were not.
The fMRI data were obtained during a response inhibition task that tested the individual’s ability to not respond automatically; for example, not saying “Simon Says” after someone else makes the comment.
The behavioral data included scores on the Iowa Gambling Task (IGT), which is used to measure impulsivity and risk taking.
“Usually, but not always, people with ADHD make riskier choices on this task,” Dr. McNorgan noted.
The investigators measured the amount of interconnectedness among different brain regions during the response inhibition task, which was repeated four times.
Patterns of interconnectivity were then fed into a deep-learning neural network that learned which patterns belonged to the ADHD group vs. those without ADHD (control group) and which patterns belonged to the high vs. low scorers on the IGT.
Caveats, cautionary notes
“The trained models are then tested on brain patterns they had never seen before, and we found the models would make the correct ADHD diagnosis and could tell apart the high and low scorers on the IGT 99% of the time,” Dr. McNorgan reported.
“The trained classifiers make predictions by calculating probabilities, and the neural networks learned how each of the brain connections contributes towards the final classification probability. We identified the set of brain connections that had the greatest influence on these probability calculations,” he noted.
Because the network classified both ADHD diagnosis and gambling task performance, the researchers were able to distinguish between connections that predicted ADHD when gambling performance was poor, as is typical for patients with ADHD, and those predicting ADHD when gambling performance was uncharacteristically good.
While more work is needed, the findings have potential clinical relevance, Dr. McNorgan said.
“ADHD can be difficult to diagnose reliably. If expense wasn’t an issue, fMRI may be able to help make diagnosis more reliable and objective,” he added.
However, because individuals with ADHD have different behavioral profiles, such as scoring atypically well on the IGT, additional studies using this approach may help identify brain networks “that are more or less active in those with ADHD that show a particular diagnostic trait,” he said.
“This could help inform what treatments might be more effective for those individuals,” Dr. McNorgan said.
Of course, he added, “clinicians’ diagnostic expertise is still required, as I would not base an ADHD diagnosis solely on the results of a single brain scan.”
No cross-validation
Commenting on the findings for this news organization, Vince Calhoun, PhD, neuroscientist and founding director of the Center for Translational Research in Neuroimaging and Data Science, Atlanta, a joint effort between Georgia State, Georgia Tech, and Emory University, noted some study limitations.
One cautionary note is that the investigators “appear to select relevant regions to include in the model based on activation to the task, then computed the predictions using the subset of regions that showed strong activation. The issue is this was done on the same data, so there was no cross-validation of this ‘feature selection’ step,” said Dr. Calhoun, who was not involved with the research. “This is a type of circularity which can lead to inflated accuracies,” he added.
Dr. Calhoun also noted that “multiple ADHD classification studies” have reported accuracies above 90%. In addition, there were only 80 participants in the current dataset.
“That’s relatively small for making strong claims about high accuracies as has been reported elsewhere,” he said.
Dr. McNorgan and Dr. Calhoun have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FDA curbs use of COVID-19 convalescent plasma, citing new data
The Food and Drug Administration has revised its emergency use authorization for COVID-19 convalescent plasma on the basis of the latest available data.
The revision states that only high-titer COVID-19 convalescent plasma can be used and only in hospitalized patients who are early in the disease course and those with impaired humoral immunity who cannot produce an adequate antibody response.
The revisions stem from new clinical trial data analyzed or reported since the original EUA was issued in August 2020. The original EUA did not have these restrictions.
“This and other changes to the EUA represent important updates to the use of convalescent plasma for the treatment of COVID-19 patients,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing the revisions.
“COVID-19 convalescent plasma used according to the revised EUA may have efficacy, and its known and potential benefits outweigh its known and potential risks,” the FDA said.
The agency said it revoked use of low-titer COVID-19 convalescent plasma on the basis of new data from clinical trials, including randomized, controlled trials, that have failed to demonstrate that low-titer convalescent plasma may be effective in the treatment of hospitalized patients with COVID-19.
The FDA’s updated fact sheet for health care providers on the use of COVID-19 convalescent plasma also notes that transfusion of COVID-19 convalescent plasma late in the disease course, following respiratory failure requiring intubation and mechanical ventilation, hasn’t been found to have clinical benefit.
The revised EUA also includes several additional tests that can be used to manufacture COVID-19 convalescent plasma.
“With this update, nine tests are now included in the EUA for testing plasma donations for anti-SARS-CoV-2 antibodies as a manufacturing step to determine suitability before release,” the FDA said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has revised its emergency use authorization for COVID-19 convalescent plasma on the basis of the latest available data.
The revision states that only high-titer COVID-19 convalescent plasma can be used and only in hospitalized patients who are early in the disease course and those with impaired humoral immunity who cannot produce an adequate antibody response.
The revisions stem from new clinical trial data analyzed or reported since the original EUA was issued in August 2020. The original EUA did not have these restrictions.
“This and other changes to the EUA represent important updates to the use of convalescent plasma for the treatment of COVID-19 patients,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing the revisions.
“COVID-19 convalescent plasma used according to the revised EUA may have efficacy, and its known and potential benefits outweigh its known and potential risks,” the FDA said.
The agency said it revoked use of low-titer COVID-19 convalescent plasma on the basis of new data from clinical trials, including randomized, controlled trials, that have failed to demonstrate that low-titer convalescent plasma may be effective in the treatment of hospitalized patients with COVID-19.
The FDA’s updated fact sheet for health care providers on the use of COVID-19 convalescent plasma also notes that transfusion of COVID-19 convalescent plasma late in the disease course, following respiratory failure requiring intubation and mechanical ventilation, hasn’t been found to have clinical benefit.
The revised EUA also includes several additional tests that can be used to manufacture COVID-19 convalescent plasma.
“With this update, nine tests are now included in the EUA for testing plasma donations for anti-SARS-CoV-2 antibodies as a manufacturing step to determine suitability before release,” the FDA said.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has revised its emergency use authorization for COVID-19 convalescent plasma on the basis of the latest available data.
The revision states that only high-titer COVID-19 convalescent plasma can be used and only in hospitalized patients who are early in the disease course and those with impaired humoral immunity who cannot produce an adequate antibody response.
The revisions stem from new clinical trial data analyzed or reported since the original EUA was issued in August 2020. The original EUA did not have these restrictions.
“This and other changes to the EUA represent important updates to the use of convalescent plasma for the treatment of COVID-19 patients,” Peter Marks, MD, PhD, director, FDA Center for Biologics Evaluation and Research, said in a statement announcing the revisions.
“COVID-19 convalescent plasma used according to the revised EUA may have efficacy, and its known and potential benefits outweigh its known and potential risks,” the FDA said.
The agency said it revoked use of low-titer COVID-19 convalescent plasma on the basis of new data from clinical trials, including randomized, controlled trials, that have failed to demonstrate that low-titer convalescent plasma may be effective in the treatment of hospitalized patients with COVID-19.
The FDA’s updated fact sheet for health care providers on the use of COVID-19 convalescent plasma also notes that transfusion of COVID-19 convalescent plasma late in the disease course, following respiratory failure requiring intubation and mechanical ventilation, hasn’t been found to have clinical benefit.
The revised EUA also includes several additional tests that can be used to manufacture COVID-19 convalescent plasma.
“With this update, nine tests are now included in the EUA for testing plasma donations for anti-SARS-CoV-2 antibodies as a manufacturing step to determine suitability before release,” the FDA said.
A version of this article first appeared on Medscape.com.
FDA extends review period for anticipated Alzheimer’s drug
the drug’s manufacturers have announced. The updated prescription drug user fee act (PDUFA) action date has been pushed forward from March 7 to June 7, 2021.
“As part of the ongoing review, Biogen submitted a response to an information request by the FDA, including additional analyses and clinical data, which the FDA considered a major amendment to the application that will require additional time for review,” Biogen and Eisai said in a statement.
“We are committed to working with the FDA as it completes its review of the aducanumab application. We want to thank the FDA for its continued diligence during the review,” said Biogen CEO Michel Vounatsos.
Biogen submitted the aducanumab application for approval to the FDA in July 2020. The FDA accepted it in August and granted priority review.
Aducanumab is a recombinant human monoclonal antibody targeting beta-amyloid (Abeta). If approved, it would be the first disease-modifying treatment for Alzheimer’s disease.
However, the road to approval has been bumpy. In November, despite high expectations and pleas from patients, caregivers, and advocacy groups, an FDA advisory panel declined to recommend approval of aducanumab.
As previously reported by this news organization, members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee determined that results from Biogen’s one large positive trial did not provide strong enough evidence of efficacy for the treatment of Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
the drug’s manufacturers have announced. The updated prescription drug user fee act (PDUFA) action date has been pushed forward from March 7 to June 7, 2021.
“As part of the ongoing review, Biogen submitted a response to an information request by the FDA, including additional analyses and clinical data, which the FDA considered a major amendment to the application that will require additional time for review,” Biogen and Eisai said in a statement.
“We are committed to working with the FDA as it completes its review of the aducanumab application. We want to thank the FDA for its continued diligence during the review,” said Biogen CEO Michel Vounatsos.
Biogen submitted the aducanumab application for approval to the FDA in July 2020. The FDA accepted it in August and granted priority review.
Aducanumab is a recombinant human monoclonal antibody targeting beta-amyloid (Abeta). If approved, it would be the first disease-modifying treatment for Alzheimer’s disease.
However, the road to approval has been bumpy. In November, despite high expectations and pleas from patients, caregivers, and advocacy groups, an FDA advisory panel declined to recommend approval of aducanumab.
As previously reported by this news organization, members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee determined that results from Biogen’s one large positive trial did not provide strong enough evidence of efficacy for the treatment of Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
the drug’s manufacturers have announced. The updated prescription drug user fee act (PDUFA) action date has been pushed forward from March 7 to June 7, 2021.
“As part of the ongoing review, Biogen submitted a response to an information request by the FDA, including additional analyses and clinical data, which the FDA considered a major amendment to the application that will require additional time for review,” Biogen and Eisai said in a statement.
“We are committed to working with the FDA as it completes its review of the aducanumab application. We want to thank the FDA for its continued diligence during the review,” said Biogen CEO Michel Vounatsos.
Biogen submitted the aducanumab application for approval to the FDA in July 2020. The FDA accepted it in August and granted priority review.
Aducanumab is a recombinant human monoclonal antibody targeting beta-amyloid (Abeta). If approved, it would be the first disease-modifying treatment for Alzheimer’s disease.
However, the road to approval has been bumpy. In November, despite high expectations and pleas from patients, caregivers, and advocacy groups, an FDA advisory panel declined to recommend approval of aducanumab.
As previously reported by this news organization, members of the FDA’s Peripheral and Central Nervous System Drugs Advisory Committee determined that results from Biogen’s one large positive trial did not provide strong enough evidence of efficacy for the treatment of Alzheimer’s disease.
A version of this article first appeared on Medscape.com.
FDA approves intramuscular administration for peginterferon beta-1a in MS
“The new IM administration offers people living with relapsing MS the well-characterized efficacy and safety of Plegridy with the potential for significantly reduced injection site reactions,” Biogen said in a news release announcing the FDA action.
Plegridy is a pegylated version of interferon beta-1a, which prolongs the circulation time of the molecule in the body by increasing its size. The process extends the drug’s half-life, allowing for a less-frequent dosing schedule.
Peginterferon beta-1a administered subcutaneously was first approved by the FDA in 2014 based on data showing it significantly reduces MS relapses, disability progression, and brain lesions.
The FDA approved IM administration for peginterferon beta-1a based on data evaluating bioequivalence and adverse reactions associated with IM administration compared with subcutaneous (SC) administration in healthy volunteers.
Bioequivalence of the IM and SC dosing regimens was confirmed and volunteers receiving the drug through IM administration experienced fewer injection site reactions relative to those receiving SC administration (14.4% vs. 32.1%), the company said.
The overall safety profiles of IM and SC administration were generally similar, with no new safety signals.
The European Commission allowed marketing authorization for IM administration of peginterferon beta-1a in December 2020.
A version of this article first appeared on Medscape.com.
“The new IM administration offers people living with relapsing MS the well-characterized efficacy and safety of Plegridy with the potential for significantly reduced injection site reactions,” Biogen said in a news release announcing the FDA action.
Plegridy is a pegylated version of interferon beta-1a, which prolongs the circulation time of the molecule in the body by increasing its size. The process extends the drug’s half-life, allowing for a less-frequent dosing schedule.
Peginterferon beta-1a administered subcutaneously was first approved by the FDA in 2014 based on data showing it significantly reduces MS relapses, disability progression, and brain lesions.
The FDA approved IM administration for peginterferon beta-1a based on data evaluating bioequivalence and adverse reactions associated with IM administration compared with subcutaneous (SC) administration in healthy volunteers.
Bioequivalence of the IM and SC dosing regimens was confirmed and volunteers receiving the drug through IM administration experienced fewer injection site reactions relative to those receiving SC administration (14.4% vs. 32.1%), the company said.
The overall safety profiles of IM and SC administration were generally similar, with no new safety signals.
The European Commission allowed marketing authorization for IM administration of peginterferon beta-1a in December 2020.
A version of this article first appeared on Medscape.com.
“The new IM administration offers people living with relapsing MS the well-characterized efficacy and safety of Plegridy with the potential for significantly reduced injection site reactions,” Biogen said in a news release announcing the FDA action.
Plegridy is a pegylated version of interferon beta-1a, which prolongs the circulation time of the molecule in the body by increasing its size. The process extends the drug’s half-life, allowing for a less-frequent dosing schedule.
Peginterferon beta-1a administered subcutaneously was first approved by the FDA in 2014 based on data showing it significantly reduces MS relapses, disability progression, and brain lesions.
The FDA approved IM administration for peginterferon beta-1a based on data evaluating bioequivalence and adverse reactions associated with IM administration compared with subcutaneous (SC) administration in healthy volunteers.
Bioequivalence of the IM and SC dosing regimens was confirmed and volunteers receiving the drug through IM administration experienced fewer injection site reactions relative to those receiving SC administration (14.4% vs. 32.1%), the company said.
The overall safety profiles of IM and SC administration were generally similar, with no new safety signals.
The European Commission allowed marketing authorization for IM administration of peginterferon beta-1a in December 2020.
A version of this article first appeared on Medscape.com.
New NIH database will track neurologic effects of COVID-19
“We know COVID-19 can disrupt multiple body systems, but the effects of the virus and the body’s response to COVID-19 infection on the brain, spinal cord, nerves, and muscle can be particularly devastating and contribute to persistence of disability even after the virus is cleared,” said Barbara Karp, MD, program director at the National Institute of Neurological Disorders and Stroke.
“There is an urgent need to understand COVID-19–related neurological problems, which not uncommonly include headaches, fatigue, cognitive difficulties, stroke, pain, and sleep disorders as well as some very rare complications of serious infections,” said Dr. Karp.
The COVID-19 NeuroDatabank/BioBank (NeuroCOVID) is funded by the NINDS. It was created and will be maintained by researchers at NYU Langone Health in New York.
The project is led by Andrea Troxel, ScD, professor of population health, and Eva Petkova, PhD, professor of population health and child and adolescent psychiatry, both at New York University.
“We’ve built a pretty comprehensive database that will accept deidentified patient information about new neurological issues that coincide with their COVID disease or worsening of preexisting neurological problems,” said Dr. Troxel. “In addition, we have a bio repository that will accept almost any kind of biological sample, such as blood, plasma, cerebrospinal fluid, and tissue,” she said.
“Neuroimages are very difficult to store because the files are so enormous, but we’ve had some questions about that, and we’re looking into whether we can accommodate neuroimages,” Dr. Troxel noted.
Dr. Troxel said a “blast of information and invitations” has gone out in an effort to acquire data and biospecimens. “We’ve been really pleased with the amount of interest already, interest not only from large academic medical centers, as you might expect, but also from some smaller stand-alone clinics and even some individuals who have either experienced some of these neurological problems of COVID or know those who have and are really eager to try to provide information,” she added.
Researchers interested in using data and biosamples from the database may submit requests to the NeuroCOVID Steering Committee. More information is available online on the NeuroCOVID website.
A version of this article first appeared on Medscape.com.
“We know COVID-19 can disrupt multiple body systems, but the effects of the virus and the body’s response to COVID-19 infection on the brain, spinal cord, nerves, and muscle can be particularly devastating and contribute to persistence of disability even after the virus is cleared,” said Barbara Karp, MD, program director at the National Institute of Neurological Disorders and Stroke.
“There is an urgent need to understand COVID-19–related neurological problems, which not uncommonly include headaches, fatigue, cognitive difficulties, stroke, pain, and sleep disorders as well as some very rare complications of serious infections,” said Dr. Karp.
The COVID-19 NeuroDatabank/BioBank (NeuroCOVID) is funded by the NINDS. It was created and will be maintained by researchers at NYU Langone Health in New York.
The project is led by Andrea Troxel, ScD, professor of population health, and Eva Petkova, PhD, professor of population health and child and adolescent psychiatry, both at New York University.
“We’ve built a pretty comprehensive database that will accept deidentified patient information about new neurological issues that coincide with their COVID disease or worsening of preexisting neurological problems,” said Dr. Troxel. “In addition, we have a bio repository that will accept almost any kind of biological sample, such as blood, plasma, cerebrospinal fluid, and tissue,” she said.
“Neuroimages are very difficult to store because the files are so enormous, but we’ve had some questions about that, and we’re looking into whether we can accommodate neuroimages,” Dr. Troxel noted.
Dr. Troxel said a “blast of information and invitations” has gone out in an effort to acquire data and biospecimens. “We’ve been really pleased with the amount of interest already, interest not only from large academic medical centers, as you might expect, but also from some smaller stand-alone clinics and even some individuals who have either experienced some of these neurological problems of COVID or know those who have and are really eager to try to provide information,” she added.
Researchers interested in using data and biosamples from the database may submit requests to the NeuroCOVID Steering Committee. More information is available online on the NeuroCOVID website.
A version of this article first appeared on Medscape.com.
“We know COVID-19 can disrupt multiple body systems, but the effects of the virus and the body’s response to COVID-19 infection on the brain, spinal cord, nerves, and muscle can be particularly devastating and contribute to persistence of disability even after the virus is cleared,” said Barbara Karp, MD, program director at the National Institute of Neurological Disorders and Stroke.
“There is an urgent need to understand COVID-19–related neurological problems, which not uncommonly include headaches, fatigue, cognitive difficulties, stroke, pain, and sleep disorders as well as some very rare complications of serious infections,” said Dr. Karp.
The COVID-19 NeuroDatabank/BioBank (NeuroCOVID) is funded by the NINDS. It was created and will be maintained by researchers at NYU Langone Health in New York.
The project is led by Andrea Troxel, ScD, professor of population health, and Eva Petkova, PhD, professor of population health and child and adolescent psychiatry, both at New York University.
“We’ve built a pretty comprehensive database that will accept deidentified patient information about new neurological issues that coincide with their COVID disease or worsening of preexisting neurological problems,” said Dr. Troxel. “In addition, we have a bio repository that will accept almost any kind of biological sample, such as blood, plasma, cerebrospinal fluid, and tissue,” she said.
“Neuroimages are very difficult to store because the files are so enormous, but we’ve had some questions about that, and we’re looking into whether we can accommodate neuroimages,” Dr. Troxel noted.
Dr. Troxel said a “blast of information and invitations” has gone out in an effort to acquire data and biospecimens. “We’ve been really pleased with the amount of interest already, interest not only from large academic medical centers, as you might expect, but also from some smaller stand-alone clinics and even some individuals who have either experienced some of these neurological problems of COVID or know those who have and are really eager to try to provide information,” she added.
Researchers interested in using data and biosamples from the database may submit requests to the NeuroCOVID Steering Committee. More information is available online on the NeuroCOVID website.
A version of this article first appeared on Medscape.com.
Plant-based or keto diet? Novel study yields surprising results
For appetite control, a low-fat, plant-based diet has advantages over a low-carbohydrate, animal-based ketogenic diet, although the keto diet wins when it comes to keeping post-meal glucose and insulin levels in check, new research suggests.
In a highly controlled crossover study conducted at the National Institutes of Health, people consumed fewer daily calories when on a low-fat, plant-based diet, but their insulin and blood glucose levels were higher than when they followed a low-carbohydrate, animal-based diet.
“There is this somewhat-outdated idea now that higher-fat diets, because they have more calories per gram, tend to make people overeat – something called the passive overconsumption model,” senior investigator Kevin Hall, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, said in an interview.
The other more popular model these days, he explained, is the carbohydrate-insulin model, which holds that following a diet high in carbohydrates and sugar that causes insulin levels to spike will increase hunger and cause a person to overeat.
In this study, Dr. Hall and colleagues tested these two hypotheses head to head.
“The short answer is that we got exactly the opposite predictions from the carbohydrate-insulin model of obesity. In other words, instead of making people eat more and gaining weight and body fat, they actually ended up eating less on that diet and losing body fat compared to the higher-fat diet,” Dr. Hall said.
“Yet, the passive overconsumption model also failed, because despite them eating a very energy-dense diet and high fat, they didn’t gain weight and gain body fat. And so both of these models of why people overeat and gain weight seem to be inadequate in our study,” he said. “This suggests that things are a little bit more complicated.”
The study was published online Jan. 21, 2021 in Nature Medicine.
Pros and cons to both diets
For the study, the researchers housed 20 healthy adults who did not have diabetes for 4 continuous weeks at the NIH Clinical Center. The mean age of the participants was 29.9 years, and the mean body mass index was 27.8 kg/m2.
The participants were randomly allocated to consume ad libitum either a plant-based, low-fat diet (10.3% fat, 75.2% carbohydrate) with low-energy density (about 1 kcal/g−1), or an animal-based, ketogenic, low-carbohydrate diet (75.8% fat, 10.0% carbohydrate) with high energy density (about 2 kcal/g−1) for 2 weeks. They then crossed over to the alternate diet for 2 weeks.
Both diets contained about 14% protein and were matched for total calories, although the low-carb diet had twice as many calories per gram of food than the low-fat diet. Participants could eat what and however much they chose of the meals they were given.
One participant withdrew, owing to hypoglycemia during the low-carbohydrate diet phase. For the primary outcome, the researchers compared mean daily ad libitum energy intake between each 2-week diet period.
They found that energy intake from the low-fat diet was reduced by approximately 550-700 kcal/d−1, compared with the low-carbohydrate keto diet. Yet, despite the large differences in calorie intake, participants reported no differences in hunger, enjoyment of meals, or fullness between the two diets.
Participants lost weight on both diets (about 1-2 kg on average), but only the low-fat diet led to a significant loss of body fat.
“Interestingly, our findings suggest benefits to both diets, at least in the short term,” Dr. Hall said in a news release.
“While the low-fat, plant-based diet helps curb appetite, the animal-based, low-carb diet resulted in lower and more steady insulin and glucose levels. We don’t yet know if these differences would be sustained over the long term,” he said.
Dr. Hall added that it’s important to note that the study was not designed to make diet recommendations for weight loss, and the results might have been different had the participants been actively trying to lose weight.
“In fact, they didn’t even know what the study was about; we just said we want you to eat the two diets, and we’re going to see what happens in your body either as you eat as much or as little as you want,” he said.
“It’s a bit of a mixed bag in terms of which diet might be better for an individual. I think you can interpret this study as that there are positives and negatives for both diets,” Dr. Hall said.
Diet ‘tribes’
In a comment, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said it’s important to note that “a ‘low-carb diet’ has yet to be defined, and many definitions exist.
“We really need a standard definition of what constitutes ‘low-carb’ so that studies can be designed and evaluated in a consistent manner. It’s problematic because, without a standard definition, the ‘diet tribe’ researchers (keto versus plant-based) always seem to find the answer that is in their own favor,” Dr. Wallace said. “This study does seem to use less than 20 grams of carbs per day, which in my mind is pretty low carb.”
Perhaps the most important caveat, he added, is that, in the real world, “most people don’t adhere to these very strict diets – not even for 2 weeks.”
The study was supported by the NIDDK Intramural Research Program, with additional NIH support from a National Institute of Nursing Research grant. One author has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, serves on the scientific advisory council for Kerry Taste and Nutrition, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. Dr. Hall and the other authors disclosed no relevant financial relationships. Dr. Wallace is principal and CEO of the Think Healthy Group, editor of the Journal of Dietary Supplements, and deputy editor of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
For appetite control, a low-fat, plant-based diet has advantages over a low-carbohydrate, animal-based ketogenic diet, although the keto diet wins when it comes to keeping post-meal glucose and insulin levels in check, new research suggests.
In a highly controlled crossover study conducted at the National Institutes of Health, people consumed fewer daily calories when on a low-fat, plant-based diet, but their insulin and blood glucose levels were higher than when they followed a low-carbohydrate, animal-based diet.
“There is this somewhat-outdated idea now that higher-fat diets, because they have more calories per gram, tend to make people overeat – something called the passive overconsumption model,” senior investigator Kevin Hall, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, said in an interview.
The other more popular model these days, he explained, is the carbohydrate-insulin model, which holds that following a diet high in carbohydrates and sugar that causes insulin levels to spike will increase hunger and cause a person to overeat.
In this study, Dr. Hall and colleagues tested these two hypotheses head to head.
“The short answer is that we got exactly the opposite predictions from the carbohydrate-insulin model of obesity. In other words, instead of making people eat more and gaining weight and body fat, they actually ended up eating less on that diet and losing body fat compared to the higher-fat diet,” Dr. Hall said.
“Yet, the passive overconsumption model also failed, because despite them eating a very energy-dense diet and high fat, they didn’t gain weight and gain body fat. And so both of these models of why people overeat and gain weight seem to be inadequate in our study,” he said. “This suggests that things are a little bit more complicated.”
The study was published online Jan. 21, 2021 in Nature Medicine.
Pros and cons to both diets
For the study, the researchers housed 20 healthy adults who did not have diabetes for 4 continuous weeks at the NIH Clinical Center. The mean age of the participants was 29.9 years, and the mean body mass index was 27.8 kg/m2.
The participants were randomly allocated to consume ad libitum either a plant-based, low-fat diet (10.3% fat, 75.2% carbohydrate) with low-energy density (about 1 kcal/g−1), or an animal-based, ketogenic, low-carbohydrate diet (75.8% fat, 10.0% carbohydrate) with high energy density (about 2 kcal/g−1) for 2 weeks. They then crossed over to the alternate diet for 2 weeks.
Both diets contained about 14% protein and were matched for total calories, although the low-carb diet had twice as many calories per gram of food than the low-fat diet. Participants could eat what and however much they chose of the meals they were given.
One participant withdrew, owing to hypoglycemia during the low-carbohydrate diet phase. For the primary outcome, the researchers compared mean daily ad libitum energy intake between each 2-week diet period.
They found that energy intake from the low-fat diet was reduced by approximately 550-700 kcal/d−1, compared with the low-carbohydrate keto diet. Yet, despite the large differences in calorie intake, participants reported no differences in hunger, enjoyment of meals, or fullness between the two diets.
Participants lost weight on both diets (about 1-2 kg on average), but only the low-fat diet led to a significant loss of body fat.
“Interestingly, our findings suggest benefits to both diets, at least in the short term,” Dr. Hall said in a news release.
“While the low-fat, plant-based diet helps curb appetite, the animal-based, low-carb diet resulted in lower and more steady insulin and glucose levels. We don’t yet know if these differences would be sustained over the long term,” he said.
Dr. Hall added that it’s important to note that the study was not designed to make diet recommendations for weight loss, and the results might have been different had the participants been actively trying to lose weight.
“In fact, they didn’t even know what the study was about; we just said we want you to eat the two diets, and we’re going to see what happens in your body either as you eat as much or as little as you want,” he said.
“It’s a bit of a mixed bag in terms of which diet might be better for an individual. I think you can interpret this study as that there are positives and negatives for both diets,” Dr. Hall said.
Diet ‘tribes’
In a comment, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said it’s important to note that “a ‘low-carb diet’ has yet to be defined, and many definitions exist.
“We really need a standard definition of what constitutes ‘low-carb’ so that studies can be designed and evaluated in a consistent manner. It’s problematic because, without a standard definition, the ‘diet tribe’ researchers (keto versus plant-based) always seem to find the answer that is in their own favor,” Dr. Wallace said. “This study does seem to use less than 20 grams of carbs per day, which in my mind is pretty low carb.”
Perhaps the most important caveat, he added, is that, in the real world, “most people don’t adhere to these very strict diets – not even for 2 weeks.”
The study was supported by the NIDDK Intramural Research Program, with additional NIH support from a National Institute of Nursing Research grant. One author has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, serves on the scientific advisory council for Kerry Taste and Nutrition, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. Dr. Hall and the other authors disclosed no relevant financial relationships. Dr. Wallace is principal and CEO of the Think Healthy Group, editor of the Journal of Dietary Supplements, and deputy editor of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
For appetite control, a low-fat, plant-based diet has advantages over a low-carbohydrate, animal-based ketogenic diet, although the keto diet wins when it comes to keeping post-meal glucose and insulin levels in check, new research suggests.
In a highly controlled crossover study conducted at the National Institutes of Health, people consumed fewer daily calories when on a low-fat, plant-based diet, but their insulin and blood glucose levels were higher than when they followed a low-carbohydrate, animal-based diet.
“There is this somewhat-outdated idea now that higher-fat diets, because they have more calories per gram, tend to make people overeat – something called the passive overconsumption model,” senior investigator Kevin Hall, PhD, National Institute of Diabetes and Digestive and Kidney Diseases, said in an interview.
The other more popular model these days, he explained, is the carbohydrate-insulin model, which holds that following a diet high in carbohydrates and sugar that causes insulin levels to spike will increase hunger and cause a person to overeat.
In this study, Dr. Hall and colleagues tested these two hypotheses head to head.
“The short answer is that we got exactly the opposite predictions from the carbohydrate-insulin model of obesity. In other words, instead of making people eat more and gaining weight and body fat, they actually ended up eating less on that diet and losing body fat compared to the higher-fat diet,” Dr. Hall said.
“Yet, the passive overconsumption model also failed, because despite them eating a very energy-dense diet and high fat, they didn’t gain weight and gain body fat. And so both of these models of why people overeat and gain weight seem to be inadequate in our study,” he said. “This suggests that things are a little bit more complicated.”
The study was published online Jan. 21, 2021 in Nature Medicine.
Pros and cons to both diets
For the study, the researchers housed 20 healthy adults who did not have diabetes for 4 continuous weeks at the NIH Clinical Center. The mean age of the participants was 29.9 years, and the mean body mass index was 27.8 kg/m2.
The participants were randomly allocated to consume ad libitum either a plant-based, low-fat diet (10.3% fat, 75.2% carbohydrate) with low-energy density (about 1 kcal/g−1), or an animal-based, ketogenic, low-carbohydrate diet (75.8% fat, 10.0% carbohydrate) with high energy density (about 2 kcal/g−1) for 2 weeks. They then crossed over to the alternate diet for 2 weeks.
Both diets contained about 14% protein and were matched for total calories, although the low-carb diet had twice as many calories per gram of food than the low-fat diet. Participants could eat what and however much they chose of the meals they were given.
One participant withdrew, owing to hypoglycemia during the low-carbohydrate diet phase. For the primary outcome, the researchers compared mean daily ad libitum energy intake between each 2-week diet period.
They found that energy intake from the low-fat diet was reduced by approximately 550-700 kcal/d−1, compared with the low-carbohydrate keto diet. Yet, despite the large differences in calorie intake, participants reported no differences in hunger, enjoyment of meals, or fullness between the two diets.
Participants lost weight on both diets (about 1-2 kg on average), but only the low-fat diet led to a significant loss of body fat.
“Interestingly, our findings suggest benefits to both diets, at least in the short term,” Dr. Hall said in a news release.
“While the low-fat, plant-based diet helps curb appetite, the animal-based, low-carb diet resulted in lower and more steady insulin and glucose levels. We don’t yet know if these differences would be sustained over the long term,” he said.
Dr. Hall added that it’s important to note that the study was not designed to make diet recommendations for weight loss, and the results might have been different had the participants been actively trying to lose weight.
“In fact, they didn’t even know what the study was about; we just said we want you to eat the two diets, and we’re going to see what happens in your body either as you eat as much or as little as you want,” he said.
“It’s a bit of a mixed bag in terms of which diet might be better for an individual. I think you can interpret this study as that there are positives and negatives for both diets,” Dr. Hall said.
Diet ‘tribes’
In a comment, Taylor Wallace, PhD, adjunct professor, department of nutrition and food studies, George Mason University, Fairfax, Va., said it’s important to note that “a ‘low-carb diet’ has yet to be defined, and many definitions exist.
“We really need a standard definition of what constitutes ‘low-carb’ so that studies can be designed and evaluated in a consistent manner. It’s problematic because, without a standard definition, the ‘diet tribe’ researchers (keto versus plant-based) always seem to find the answer that is in their own favor,” Dr. Wallace said. “This study does seem to use less than 20 grams of carbs per day, which in my mind is pretty low carb.”
Perhaps the most important caveat, he added, is that, in the real world, “most people don’t adhere to these very strict diets – not even for 2 weeks.”
The study was supported by the NIDDK Intramural Research Program, with additional NIH support from a National Institute of Nursing Research grant. One author has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, serves on the scientific advisory council for Kerry Taste and Nutrition, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. Dr. Hall and the other authors disclosed no relevant financial relationships. Dr. Wallace is principal and CEO of the Think Healthy Group, editor of the Journal of Dietary Supplements, and deputy editor of the Journal of the American College of Nutrition.
A version of this article first appeared on Medscape.com.
‘Alarming finding’ in schizophrenia patients with COVID-19
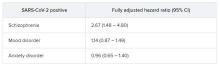
Schizophrenia spectrum disorder is associated with a significantly increased risk of dying from COVID-19, new research shows.
After adjusting for demographic and medical risk factors, the investigators found that patients who had been diagnosed with schizophrenia were two to three times more likely to die of COVID-19 if they contracted the disease.
“ and efforts should be taken to reduce risk of infection [social distancing, masks, etc.], particularly in people with schizophrenia who live in congregate living situations [hospitals and group residences],” Donald Goff, MD, department of psychiatry, New York University Langone Medical Center, said in an interview.
The study was published online Jan. 27 in JAMA Psychiatry.
The study included 7,348 adults with laboratory-confirmed SARS-CoV-2 infection from the NYU Langone Health System; 75 (1.0%) had a history of schizophrenia spectrum disorder, 564 (7.7%) had a history of a mood disorder, and 360 (4.9%) had a history of an anxiety disorder.
Overall, 864 patients (11.8%) died or were discharged to hospice within 45 days of a positive SARS-CoV-2 test.
In the fully adjusted model, a premorbid diagnosis of schizophrenia spectrum disorder, but not mood or anxiety disorder, was significantly associated with an increased risk of dying from COVID-19 within 45 days.
”A higher risk with schizophrenia spectrum diagnoses was expected based on previous studies of all-cause mortality, but the magnitude of the increase after adjusting for comorbid medical risk factors was unexpected,” the researchers wrote in the study, first authored by Katlyn Nemani, MD, research assistant professor of psychiatry at NYU Langone.
‘Alarming finding’
In an interview, Luming Li, MD, Yale New Haven (Conn.) Psychiatric Hospital, noted that, although the number patients with schizophrenia spectrum disorders in the sample is “fairly low,” she was not surprised by the increased risk for death from COVID-19.
“Schizophrenia falls into the serious mental illness category, and these patients are more often predisposed to homelessness, comorbid medical and substance use, living in congregate settings, lower socioeconomic status, etc,” Dr. Li noted.
Dr. Li’s advice for clinicians who treat patients who have schizophrenia during the COVID-19 pandemic is to minimize their risk in various care settings through the use of personal protective equipment and other infection prevention techniques.
“If a patient does contract COVID-19, make sure patient’s care is escalated appropriately, given the higher risk for mortality in patients with schizophrenia spectrum disorders,” she said.
Tom Pollak, PhD, MRCPsych, King’s College London, said that it has been known for some time that patients with serious mental illness have poorer physical health outcomes. More recently, it has been shown that those who have been diagnosed with psychiatric disorders appear to be at greater risk for poor COVID-19 outcomes.
“This study is the first to specifically highlight schizophrenia spectrum disorders as being particularly at risk. This is an alarming finding. These patients are already amongst the most vulnerable members of society and are probably underserved by most health care systems worldwide,” Dr. Pollak said in a statement.
“Although these findings need urgent replication in larger samples, there are clear reasons for policymakers to take notice now, including giving immediate consideration for prioritization of patients with serious mental illness in nationwide COVID-19 vaccination programs,” he added.
Matthew Hotopf, PhD, FRCPsych, FMedSci, also with King’s College London, said that the New York group has identified people with severe mental disorders as “a high-risk group, and this has immediate public health implications regarding vaccination – that’s the important message of the paper.
“Schizophrenia and other severe psychiatric disorders are risk factors for mortality in the general population before COVID. This is a group with a 10- to 20-year reduction in life expectancy – more than for many diseases we associated with early death,” said Dr. Hotopf.
“The reasons for this are multifactorial, including social deprivation, lifestyle factors (people with schizophrenia smoke more and have high rates of obesity), harms associated with some medications used to treat psychosis, and differential access to health care,” he noted.
“In COVID, we know that deprivation is associated with a much higher mortality, so we would therefore expect that people with severe mental illness will be particularly disadvantaged,” he said.
The study had no specific funding. Dr. Goff has received research support and travel reimbursement from Avanir Pharmaceuticals and Takeda. Dr. Nemani, Dr. Li, Dr. Pollak, and Dr. Hotopf disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schizophrenia spectrum disorder is associated with a significantly increased risk of dying from COVID-19, new research shows.
After adjusting for demographic and medical risk factors, the investigators found that patients who had been diagnosed with schizophrenia were two to three times more likely to die of COVID-19 if they contracted the disease.
“ and efforts should be taken to reduce risk of infection [social distancing, masks, etc.], particularly in people with schizophrenia who live in congregate living situations [hospitals and group residences],” Donald Goff, MD, department of psychiatry, New York University Langone Medical Center, said in an interview.
The study was published online Jan. 27 in JAMA Psychiatry.
The study included 7,348 adults with laboratory-confirmed SARS-CoV-2 infection from the NYU Langone Health System; 75 (1.0%) had a history of schizophrenia spectrum disorder, 564 (7.7%) had a history of a mood disorder, and 360 (4.9%) had a history of an anxiety disorder.
Overall, 864 patients (11.8%) died or were discharged to hospice within 45 days of a positive SARS-CoV-2 test.
In the fully adjusted model, a premorbid diagnosis of schizophrenia spectrum disorder, but not mood or anxiety disorder, was significantly associated with an increased risk of dying from COVID-19 within 45 days.
”A higher risk with schizophrenia spectrum diagnoses was expected based on previous studies of all-cause mortality, but the magnitude of the increase after adjusting for comorbid medical risk factors was unexpected,” the researchers wrote in the study, first authored by Katlyn Nemani, MD, research assistant professor of psychiatry at NYU Langone.
‘Alarming finding’
In an interview, Luming Li, MD, Yale New Haven (Conn.) Psychiatric Hospital, noted that, although the number patients with schizophrenia spectrum disorders in the sample is “fairly low,” she was not surprised by the increased risk for death from COVID-19.
“Schizophrenia falls into the serious mental illness category, and these patients are more often predisposed to homelessness, comorbid medical and substance use, living in congregate settings, lower socioeconomic status, etc,” Dr. Li noted.
Dr. Li’s advice for clinicians who treat patients who have schizophrenia during the COVID-19 pandemic is to minimize their risk in various care settings through the use of personal protective equipment and other infection prevention techniques.
“If a patient does contract COVID-19, make sure patient’s care is escalated appropriately, given the higher risk for mortality in patients with schizophrenia spectrum disorders,” she said.
Tom Pollak, PhD, MRCPsych, King’s College London, said that it has been known for some time that patients with serious mental illness have poorer physical health outcomes. More recently, it has been shown that those who have been diagnosed with psychiatric disorders appear to be at greater risk for poor COVID-19 outcomes.
“This study is the first to specifically highlight schizophrenia spectrum disorders as being particularly at risk. This is an alarming finding. These patients are already amongst the most vulnerable members of society and are probably underserved by most health care systems worldwide,” Dr. Pollak said in a statement.
“Although these findings need urgent replication in larger samples, there are clear reasons for policymakers to take notice now, including giving immediate consideration for prioritization of patients with serious mental illness in nationwide COVID-19 vaccination programs,” he added.
Matthew Hotopf, PhD, FRCPsych, FMedSci, also with King’s College London, said that the New York group has identified people with severe mental disorders as “a high-risk group, and this has immediate public health implications regarding vaccination – that’s the important message of the paper.
“Schizophrenia and other severe psychiatric disorders are risk factors for mortality in the general population before COVID. This is a group with a 10- to 20-year reduction in life expectancy – more than for many diseases we associated with early death,” said Dr. Hotopf.
“The reasons for this are multifactorial, including social deprivation, lifestyle factors (people with schizophrenia smoke more and have high rates of obesity), harms associated with some medications used to treat psychosis, and differential access to health care,” he noted.
“In COVID, we know that deprivation is associated with a much higher mortality, so we would therefore expect that people with severe mental illness will be particularly disadvantaged,” he said.
The study had no specific funding. Dr. Goff has received research support and travel reimbursement from Avanir Pharmaceuticals and Takeda. Dr. Nemani, Dr. Li, Dr. Pollak, and Dr. Hotopf disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Schizophrenia spectrum disorder is associated with a significantly increased risk of dying from COVID-19, new research shows.
After adjusting for demographic and medical risk factors, the investigators found that patients who had been diagnosed with schizophrenia were two to three times more likely to die of COVID-19 if they contracted the disease.
“ and efforts should be taken to reduce risk of infection [social distancing, masks, etc.], particularly in people with schizophrenia who live in congregate living situations [hospitals and group residences],” Donald Goff, MD, department of psychiatry, New York University Langone Medical Center, said in an interview.
The study was published online Jan. 27 in JAMA Psychiatry.
The study included 7,348 adults with laboratory-confirmed SARS-CoV-2 infection from the NYU Langone Health System; 75 (1.0%) had a history of schizophrenia spectrum disorder, 564 (7.7%) had a history of a mood disorder, and 360 (4.9%) had a history of an anxiety disorder.
Overall, 864 patients (11.8%) died or were discharged to hospice within 45 days of a positive SARS-CoV-2 test.
In the fully adjusted model, a premorbid diagnosis of schizophrenia spectrum disorder, but not mood or anxiety disorder, was significantly associated with an increased risk of dying from COVID-19 within 45 days.
”A higher risk with schizophrenia spectrum diagnoses was expected based on previous studies of all-cause mortality, but the magnitude of the increase after adjusting for comorbid medical risk factors was unexpected,” the researchers wrote in the study, first authored by Katlyn Nemani, MD, research assistant professor of psychiatry at NYU Langone.
‘Alarming finding’
In an interview, Luming Li, MD, Yale New Haven (Conn.) Psychiatric Hospital, noted that, although the number patients with schizophrenia spectrum disorders in the sample is “fairly low,” she was not surprised by the increased risk for death from COVID-19.
“Schizophrenia falls into the serious mental illness category, and these patients are more often predisposed to homelessness, comorbid medical and substance use, living in congregate settings, lower socioeconomic status, etc,” Dr. Li noted.
Dr. Li’s advice for clinicians who treat patients who have schizophrenia during the COVID-19 pandemic is to minimize their risk in various care settings through the use of personal protective equipment and other infection prevention techniques.
“If a patient does contract COVID-19, make sure patient’s care is escalated appropriately, given the higher risk for mortality in patients with schizophrenia spectrum disorders,” she said.
Tom Pollak, PhD, MRCPsych, King’s College London, said that it has been known for some time that patients with serious mental illness have poorer physical health outcomes. More recently, it has been shown that those who have been diagnosed with psychiatric disorders appear to be at greater risk for poor COVID-19 outcomes.
“This study is the first to specifically highlight schizophrenia spectrum disorders as being particularly at risk. This is an alarming finding. These patients are already amongst the most vulnerable members of society and are probably underserved by most health care systems worldwide,” Dr. Pollak said in a statement.
“Although these findings need urgent replication in larger samples, there are clear reasons for policymakers to take notice now, including giving immediate consideration for prioritization of patients with serious mental illness in nationwide COVID-19 vaccination programs,” he added.
Matthew Hotopf, PhD, FRCPsych, FMedSci, also with King’s College London, said that the New York group has identified people with severe mental disorders as “a high-risk group, and this has immediate public health implications regarding vaccination – that’s the important message of the paper.
“Schizophrenia and other severe psychiatric disorders are risk factors for mortality in the general population before COVID. This is a group with a 10- to 20-year reduction in life expectancy – more than for many diseases we associated with early death,” said Dr. Hotopf.
“The reasons for this are multifactorial, including social deprivation, lifestyle factors (people with schizophrenia smoke more and have high rates of obesity), harms associated with some medications used to treat psychosis, and differential access to health care,” he noted.
“In COVID, we know that deprivation is associated with a much higher mortality, so we would therefore expect that people with severe mental illness will be particularly disadvantaged,” he said.
The study had no specific funding. Dr. Goff has received research support and travel reimbursement from Avanir Pharmaceuticals and Takeda. Dr. Nemani, Dr. Li, Dr. Pollak, and Dr. Hotopf disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
APA apologizes for past support of racism in psychiatry
The American Psychiatric Association has issued a formal apology for its past support of structural racism in psychiatry.
The apology, issued Jan. 18, coincided with the federal holiday honoring the life and work of civil rights activist Dr. Martin Luther King Jr.
“We apologize for our role in perpetrating structural racism in this country, and we hope to begin to make amends for APA’s and psychiatry’s history of actions, intentional and not, that hurt Black, indigenous, and people of color,” APA President Jeffrey Geller, MD, MPH, said in a statement.
The apology was written and issued by the APA Board of Trustees. It acknowledges practices and events in psychiatry that contributed to racial inequality, and expresses the organization’s commitment to developing antiracist policies that promote equity in mental health for all.
“This apology is one important step we needed to take to move forward to a more equitable future. The board is issuing this document on Martin Luther King Jr. Day, because we hope that it honors his life’s work of reconciliation and equality. We do not take that legacy or his call to action lightly and will continue our important work,” said Dr. Geller.
One involved the Eastern State Hospital in Williamsburg, Va., the nation’s first psychiatric care facility, founded in 1773.
Eastern State, which for a time in the 1800s was called the Eastern Lunatic Asylum, was not segregated when founded. However, 70 years later, when the 13 founders of what is now the APA met to discuss improvements in mental health care delivery, the treatment system they created and the organization they founded aligned with that era’s racist social and political policies. In this system, Black patients received psychiatric care separately from White patients, the APA said.
The APA also acknowledged failing to act in Black Americans’ best interest at critical points in the United States’ sociopolitical evolution throughout the 19th and 20th centuries.
“This inactivity was notably evident while white supremacists lynched Black people during the Reconstruction Era as well as when Jim Crow segregation was in effect, which led to ‘separate but equal’ standards of care starting in 1896,” the APA said.
Later, the APA failed to declare support for Brown v. Board of Education of Topeka in 1954, along with further major civil rights legislation designed to improve social and psychological conditions for Black people, the organization admitted.
Throughout the decades that followed, psychiatric misdiagnosis among Black, indigenous, and people of color populations were also common, the APA acknowledged.
For example, late 20th century psychiatrists commonly attributed their minority patients’ frustrations to schizophrenia, while categorizing similar behaviors as “neuroticism” in White patients.
The APA pointed to one study which found that APA members diagnosed more Black than White patients with schizophrenia, even when both had otherwise identical clinical presentations.
“This reveals the basis for embedded discrimination within psychiatry that has contributed to reduced quality of care” for Black, indigenous, and people of color, and “perpetuation of dangerous stereotypes,” the APA said.
Saul Levin, MD, the APA’s medical director and CEO, said the Board of Trustees has taken “an important step in issuing this apology. The APA administration is committed to working toward inclusion, health equity, and fairness that everyone deserves.”
The APA Board of Trustees began drafting the apology late last year after it concluded that events and persistent inequities in health care and psychiatry had highlighted an organizational need for action.
The APA’s Presidential Task Force on Structural Racism is continuing with efforts to educate and engage members on the issue and implement changes within the organization.
A version of this article first appeared on Medscape.com.
The American Psychiatric Association has issued a formal apology for its past support of structural racism in psychiatry.
The apology, issued Jan. 18, coincided with the federal holiday honoring the life and work of civil rights activist Dr. Martin Luther King Jr.
“We apologize for our role in perpetrating structural racism in this country, and we hope to begin to make amends for APA’s and psychiatry’s history of actions, intentional and not, that hurt Black, indigenous, and people of color,” APA President Jeffrey Geller, MD, MPH, said in a statement.
The apology was written and issued by the APA Board of Trustees. It acknowledges practices and events in psychiatry that contributed to racial inequality, and expresses the organization’s commitment to developing antiracist policies that promote equity in mental health for all.
“This apology is one important step we needed to take to move forward to a more equitable future. The board is issuing this document on Martin Luther King Jr. Day, because we hope that it honors his life’s work of reconciliation and equality. We do not take that legacy or his call to action lightly and will continue our important work,” said Dr. Geller.
One involved the Eastern State Hospital in Williamsburg, Va., the nation’s first psychiatric care facility, founded in 1773.
Eastern State, which for a time in the 1800s was called the Eastern Lunatic Asylum, was not segregated when founded. However, 70 years later, when the 13 founders of what is now the APA met to discuss improvements in mental health care delivery, the treatment system they created and the organization they founded aligned with that era’s racist social and political policies. In this system, Black patients received psychiatric care separately from White patients, the APA said.
The APA also acknowledged failing to act in Black Americans’ best interest at critical points in the United States’ sociopolitical evolution throughout the 19th and 20th centuries.
“This inactivity was notably evident while white supremacists lynched Black people during the Reconstruction Era as well as when Jim Crow segregation was in effect, which led to ‘separate but equal’ standards of care starting in 1896,” the APA said.
Later, the APA failed to declare support for Brown v. Board of Education of Topeka in 1954, along with further major civil rights legislation designed to improve social and psychological conditions for Black people, the organization admitted.
Throughout the decades that followed, psychiatric misdiagnosis among Black, indigenous, and people of color populations were also common, the APA acknowledged.
For example, late 20th century psychiatrists commonly attributed their minority patients’ frustrations to schizophrenia, while categorizing similar behaviors as “neuroticism” in White patients.
The APA pointed to one study which found that APA members diagnosed more Black than White patients with schizophrenia, even when both had otherwise identical clinical presentations.
“This reveals the basis for embedded discrimination within psychiatry that has contributed to reduced quality of care” for Black, indigenous, and people of color, and “perpetuation of dangerous stereotypes,” the APA said.
Saul Levin, MD, the APA’s medical director and CEO, said the Board of Trustees has taken “an important step in issuing this apology. The APA administration is committed to working toward inclusion, health equity, and fairness that everyone deserves.”
The APA Board of Trustees began drafting the apology late last year after it concluded that events and persistent inequities in health care and psychiatry had highlighted an organizational need for action.
The APA’s Presidential Task Force on Structural Racism is continuing with efforts to educate and engage members on the issue and implement changes within the organization.
A version of this article first appeared on Medscape.com.
The American Psychiatric Association has issued a formal apology for its past support of structural racism in psychiatry.
The apology, issued Jan. 18, coincided with the federal holiday honoring the life and work of civil rights activist Dr. Martin Luther King Jr.
“We apologize for our role in perpetrating structural racism in this country, and we hope to begin to make amends for APA’s and psychiatry’s history of actions, intentional and not, that hurt Black, indigenous, and people of color,” APA President Jeffrey Geller, MD, MPH, said in a statement.
The apology was written and issued by the APA Board of Trustees. It acknowledges practices and events in psychiatry that contributed to racial inequality, and expresses the organization’s commitment to developing antiracist policies that promote equity in mental health for all.
“This apology is one important step we needed to take to move forward to a more equitable future. The board is issuing this document on Martin Luther King Jr. Day, because we hope that it honors his life’s work of reconciliation and equality. We do not take that legacy or his call to action lightly and will continue our important work,” said Dr. Geller.
One involved the Eastern State Hospital in Williamsburg, Va., the nation’s first psychiatric care facility, founded in 1773.
Eastern State, which for a time in the 1800s was called the Eastern Lunatic Asylum, was not segregated when founded. However, 70 years later, when the 13 founders of what is now the APA met to discuss improvements in mental health care delivery, the treatment system they created and the organization they founded aligned with that era’s racist social and political policies. In this system, Black patients received psychiatric care separately from White patients, the APA said.
The APA also acknowledged failing to act in Black Americans’ best interest at critical points in the United States’ sociopolitical evolution throughout the 19th and 20th centuries.
“This inactivity was notably evident while white supremacists lynched Black people during the Reconstruction Era as well as when Jim Crow segregation was in effect, which led to ‘separate but equal’ standards of care starting in 1896,” the APA said.
Later, the APA failed to declare support for Brown v. Board of Education of Topeka in 1954, along with further major civil rights legislation designed to improve social and psychological conditions for Black people, the organization admitted.
Throughout the decades that followed, psychiatric misdiagnosis among Black, indigenous, and people of color populations were also common, the APA acknowledged.
For example, late 20th century psychiatrists commonly attributed their minority patients’ frustrations to schizophrenia, while categorizing similar behaviors as “neuroticism” in White patients.
The APA pointed to one study which found that APA members diagnosed more Black than White patients with schizophrenia, even when both had otherwise identical clinical presentations.
“This reveals the basis for embedded discrimination within psychiatry that has contributed to reduced quality of care” for Black, indigenous, and people of color, and “perpetuation of dangerous stereotypes,” the APA said.
Saul Levin, MD, the APA’s medical director and CEO, said the Board of Trustees has taken “an important step in issuing this apology. The APA administration is committed to working toward inclusion, health equity, and fairness that everyone deserves.”
The APA Board of Trustees began drafting the apology late last year after it concluded that events and persistent inequities in health care and psychiatry had highlighted an organizational need for action.
The APA’s Presidential Task Force on Structural Racism is continuing with efforts to educate and engage members on the issue and implement changes within the organization.
A version of this article first appeared on Medscape.com.
FDA clears device to remove dead pancreatic tissue
The Food and Drug Administration has approved the EndoRotor System (Interscope, Inc.) for removal of necrotic tissue in patients with walled-off pancreatic necrosis (WOPN).
“This device has shown its potential to provide a minimally invasive way to remove harmful necrotic pancreatic tissue in patients with walled-off pancreatic necrosis,” Charles Viviano, MD, PhD, acting director, Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office, FDA Center for Devices and Radiological Health, said in a statement.
“Currently, in order to remove dead tissue from a patient’s necrotic pancreatic cavity, health care providers need to perform an invasive surgery or use other endoscopic tools not specifically indicated to treat this condition. With [this] marketing authorization, patients with walled-off pancreatic necrosis now have a new treatment option,” said Dr. Viviano.
WOPN is a potentially deadly condition that occurs in about 15% of patients with severe pancreatitis. Often, the dead tissue must be removed.
The EndoRotor System is made up of a power console, foot control, specimen trap, and single-use catheter.
The device is used to perform endoscopic necrosectomy. In this procedure, a stent is used to create a portal between the stomach and the necrotic cavity in the pancreas to accommodate a standard endoscope through which the EndoRotor cuts and removes necrotized tissue.
The FDA approved the EndoRotor System on the basis of a clinical trial involving 30 patients with WOPN who underwent a total of 63 direct endoscopic necrosectomies with the EndoRotor System (average, 2.1 procedures per patient).
The effectiveness of the EndoRotor System was determined by how well it cleared pancreatic necrotic tissue measured during CT with contrast before and after the procedure, endoscopy, or MRI 14 to 28 days after the last procedure.
Results showed an average 85% reduction in the amount of necrotic tissue, with half of the patients having 98.5% clearance of necrotic tissue, the FDA said.
Three patients suffered procedure-related serious adverse events (10% complication rate). Two patients experienced gastrointestinal bleeding. One patient had a pneumoperitoneum and later died after suffering from sepsis and multiorgan system failure caused by massive collections of infected pancreatic necrotic tissue.
Other serious adverse events, which were thought to be due to the patient’s underlying condition and not related to the device or procedure, included hematemesis, deep vein thrombosis, and pancreatitis.
The EndoRotor System should not be used for patients with known or suspected pancreatic cancer, and the device will carry a boxed warning stating this.
The FDA said it knows of one patient who died from pancreatic cancer 3 months after having necrotic pancreatic tissue removed with the EndoRotor System.
“This patient did not have a diagnosis of pancreatic cancer prior to treatment, although the patient’s outcome is believed to be unrelated to the device or procedure,” the FDA said.
The EndoRotor System should be used only after patients have undergone other procedures to drain the WOPN.
It is also not appropriate for patients with walled-off necrosis who have a documented pseudoaneurysm greater than 1 cm within the cavity or with intervening gastric varices or unavoidable blood vessels within the access tract.
The EndoRotor System was approved under the de novo premarket review pathway for new low- to moderate-risk devices.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the EndoRotor System (Interscope, Inc.) for removal of necrotic tissue in patients with walled-off pancreatic necrosis (WOPN).
“This device has shown its potential to provide a minimally invasive way to remove harmful necrotic pancreatic tissue in patients with walled-off pancreatic necrosis,” Charles Viviano, MD, PhD, acting director, Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office, FDA Center for Devices and Radiological Health, said in a statement.
“Currently, in order to remove dead tissue from a patient’s necrotic pancreatic cavity, health care providers need to perform an invasive surgery or use other endoscopic tools not specifically indicated to treat this condition. With [this] marketing authorization, patients with walled-off pancreatic necrosis now have a new treatment option,” said Dr. Viviano.
WOPN is a potentially deadly condition that occurs in about 15% of patients with severe pancreatitis. Often, the dead tissue must be removed.
The EndoRotor System is made up of a power console, foot control, specimen trap, and single-use catheter.
The device is used to perform endoscopic necrosectomy. In this procedure, a stent is used to create a portal between the stomach and the necrotic cavity in the pancreas to accommodate a standard endoscope through which the EndoRotor cuts and removes necrotized tissue.
The FDA approved the EndoRotor System on the basis of a clinical trial involving 30 patients with WOPN who underwent a total of 63 direct endoscopic necrosectomies with the EndoRotor System (average, 2.1 procedures per patient).
The effectiveness of the EndoRotor System was determined by how well it cleared pancreatic necrotic tissue measured during CT with contrast before and after the procedure, endoscopy, or MRI 14 to 28 days after the last procedure.
Results showed an average 85% reduction in the amount of necrotic tissue, with half of the patients having 98.5% clearance of necrotic tissue, the FDA said.
Three patients suffered procedure-related serious adverse events (10% complication rate). Two patients experienced gastrointestinal bleeding. One patient had a pneumoperitoneum and later died after suffering from sepsis and multiorgan system failure caused by massive collections of infected pancreatic necrotic tissue.
Other serious adverse events, which were thought to be due to the patient’s underlying condition and not related to the device or procedure, included hematemesis, deep vein thrombosis, and pancreatitis.
The EndoRotor System should not be used for patients with known or suspected pancreatic cancer, and the device will carry a boxed warning stating this.
The FDA said it knows of one patient who died from pancreatic cancer 3 months after having necrotic pancreatic tissue removed with the EndoRotor System.
“This patient did not have a diagnosis of pancreatic cancer prior to treatment, although the patient’s outcome is believed to be unrelated to the device or procedure,” the FDA said.
The EndoRotor System should be used only after patients have undergone other procedures to drain the WOPN.
It is also not appropriate for patients with walled-off necrosis who have a documented pseudoaneurysm greater than 1 cm within the cavity or with intervening gastric varices or unavoidable blood vessels within the access tract.
The EndoRotor System was approved under the de novo premarket review pathway for new low- to moderate-risk devices.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved the EndoRotor System (Interscope, Inc.) for removal of necrotic tissue in patients with walled-off pancreatic necrosis (WOPN).
“This device has shown its potential to provide a minimally invasive way to remove harmful necrotic pancreatic tissue in patients with walled-off pancreatic necrosis,” Charles Viviano, MD, PhD, acting director, Reproductive, Gastro-Renal, Urological, General Hospital Device and Human Factors Office, FDA Center for Devices and Radiological Health, said in a statement.
“Currently, in order to remove dead tissue from a patient’s necrotic pancreatic cavity, health care providers need to perform an invasive surgery or use other endoscopic tools not specifically indicated to treat this condition. With [this] marketing authorization, patients with walled-off pancreatic necrosis now have a new treatment option,” said Dr. Viviano.
WOPN is a potentially deadly condition that occurs in about 15% of patients with severe pancreatitis. Often, the dead tissue must be removed.
The EndoRotor System is made up of a power console, foot control, specimen trap, and single-use catheter.
The device is used to perform endoscopic necrosectomy. In this procedure, a stent is used to create a portal between the stomach and the necrotic cavity in the pancreas to accommodate a standard endoscope through which the EndoRotor cuts and removes necrotized tissue.
The FDA approved the EndoRotor System on the basis of a clinical trial involving 30 patients with WOPN who underwent a total of 63 direct endoscopic necrosectomies with the EndoRotor System (average, 2.1 procedures per patient).
The effectiveness of the EndoRotor System was determined by how well it cleared pancreatic necrotic tissue measured during CT with contrast before and after the procedure, endoscopy, or MRI 14 to 28 days after the last procedure.
Results showed an average 85% reduction in the amount of necrotic tissue, with half of the patients having 98.5% clearance of necrotic tissue, the FDA said.
Three patients suffered procedure-related serious adverse events (10% complication rate). Two patients experienced gastrointestinal bleeding. One patient had a pneumoperitoneum and later died after suffering from sepsis and multiorgan system failure caused by massive collections of infected pancreatic necrotic tissue.
Other serious adverse events, which were thought to be due to the patient’s underlying condition and not related to the device or procedure, included hematemesis, deep vein thrombosis, and pancreatitis.
The EndoRotor System should not be used for patients with known or suspected pancreatic cancer, and the device will carry a boxed warning stating this.
The FDA said it knows of one patient who died from pancreatic cancer 3 months after having necrotic pancreatic tissue removed with the EndoRotor System.
“This patient did not have a diagnosis of pancreatic cancer prior to treatment, although the patient’s outcome is believed to be unrelated to the device or procedure,” the FDA said.
The EndoRotor System should be used only after patients have undergone other procedures to drain the WOPN.
It is also not appropriate for patients with walled-off necrosis who have a documented pseudoaneurysm greater than 1 cm within the cavity or with intervening gastric varices or unavoidable blood vessels within the access tract.
The EndoRotor System was approved under the de novo premarket review pathway for new low- to moderate-risk devices.
A version of this article first appeared on Medscape.com.