User login
Review shows small benefit, significant safety concerns for flibanserin
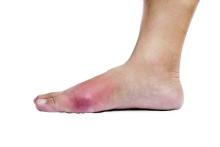
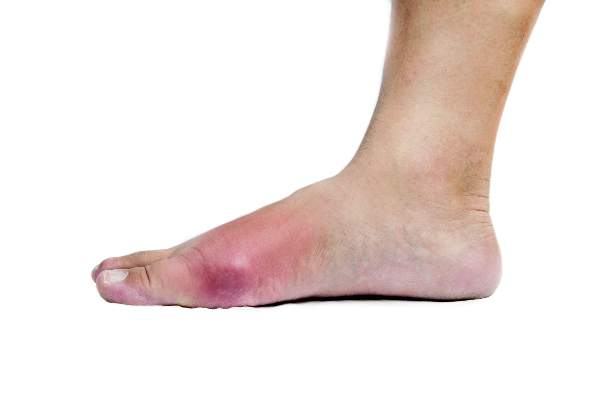
A meta-analysis of eight clinical trials of the newly approved female hypoactive sexual disorder drug flibanserin show the drug yields minimal benefit, while increasing the risk of dizziness, somnolence, nausea, and fatigue to a clinically meaningful degree.
In a study published online Feb. 29 in JAMA Internal Medicine, the researchers wrote that flibanserin has not be sufficiently studied to recommend its use in clinical practice, despite being approved by the Food and Drug Administration in August 2015.
“Before flibanserin can be recommended in guidelines and clinical practice, future studies should include women from diverse populations, particularly women with (a history of) somatic and psychological comorbidities, medication use, and surgical menopause,” wrote Dr. Loes Jaspers of Erasmus University, Rotterdam, the Netherlands, and her associates.
They performed a systematic review of the literature and meta-analysis of five published and three unpublished randomized placebo-controlled trials. Seven of the trials were performed in the U.S. and Canada, while the eighth trial was performed in 13 European countries. About 90% of the 5,914 participants were white, and all were in stable, heterosexual, monogamous relationships for at least 1 year.
At baseline, the mean number of participants’ satisfactory sexual experiences per month was 2.5. The mean score on a scale of sexual desire in a daily electronic diary was 11.5 out of a possible 84 points, and the mean score on the Female Sexual Function Index was 1.8 on a scale of 1.2-6.0.
The primary efficacy outcome – the mean number of satisfactory sexual experiences per month – was 0.49 higher for women receiving 100 mg flibanserin, compared with women receiving placebo. Similarly, the mean improvement in sexual desire score in the daily diary was 1.63 points, and 0.27 points in the Female Sexual Function Index score was 0.27 points. These results remained robust across all subgroup and sensitivity analyses, and indicate that the drug affords “minimal” meaningful improvement, Dr. Jaspers and her associates wrote (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2015.8565).
In contrast, flibanserin clearly influenced safety outcomes, increasing the risk of dizziness, somnolence, nausea, and fatigue to a statistically and clinically significant degree. The risk for dizziness was 4.0 times higher with flibanserin, compared with placebo. Similarly, the risk for somnolence was 3.97 times higher, the risk for nausea was 2.35 times higher, and the risk for fatigue was 1.64 times higher.
The absolute number of series adverse events was small and the risk ratio was no different between flibanserin and placebo, according to the study.
But even though the adverse events tended to be of mild to moderate intensity, it would be premature to conclude that flibanserin is safe, the researchers wrote. “In an open-label extension study including 1,723 women with a median follow-up of 1 year, 723 participants (43%) reported investigator-defined drug-related adverse events, and 143 (8.3%) reported severe adverse events. The continued safety (and efficacy) of flibanserin with long-term use remains to be established.”
Overall, the quality of the evidence in these trials was rated as “very low,” in part because of “limitations in design, indirectness of evidence, and more favorable efficacy outcomes in published compared to unpublished studies,” the researchers noted.
Dr. Jaspers and one of her coauthors work for ErasmusAGE, a center for aging research funded by Nestlé Nutrition and Metagenics. Another coauthor was involved in the European trial as an investigator. No other disclosures were reported.
The flibanserin approval saga is unsatisfying. The Food and Drug Administration approved a marginally effective drug for a non–life-threatening condition in the face of substantial – and unnecessary – uncertainty about its dangers.
Physicians and patients still await answers about how often symptomatic hypotension and syncope occur with alcohol and those results won’t be available for a year to 2.5 years – after the three required postmarketing alcohol studies are reported.
Women with distressing sexual desire problems need good treatments. We all need a drug approval process that delivers good decisions based on adequate evidence.
Dr. Steven Woloshin and Dr. Lisa M. Schwartz are both at the Dartmouth-Hitchcock Medical Center, Lebanon, N.H. They are cofounders of Informulary, a company that provides information about the benefits, harms, and uncertainties of prescription drugs. They reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA Intern Med. 2016 Feb 29. doi:10.1001/jamainternmed.2016.0073).
The flibanserin approval saga is unsatisfying. The Food and Drug Administration approved a marginally effective drug for a non–life-threatening condition in the face of substantial – and unnecessary – uncertainty about its dangers.
Physicians and patients still await answers about how often symptomatic hypotension and syncope occur with alcohol and those results won’t be available for a year to 2.5 years – after the three required postmarketing alcohol studies are reported.
Women with distressing sexual desire problems need good treatments. We all need a drug approval process that delivers good decisions based on adequate evidence.
Dr. Steven Woloshin and Dr. Lisa M. Schwartz are both at the Dartmouth-Hitchcock Medical Center, Lebanon, N.H. They are cofounders of Informulary, a company that provides information about the benefits, harms, and uncertainties of prescription drugs. They reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA Intern Med. 2016 Feb 29. doi:10.1001/jamainternmed.2016.0073).
The flibanserin approval saga is unsatisfying. The Food and Drug Administration approved a marginally effective drug for a non–life-threatening condition in the face of substantial – and unnecessary – uncertainty about its dangers.
Physicians and patients still await answers about how often symptomatic hypotension and syncope occur with alcohol and those results won’t be available for a year to 2.5 years – after the three required postmarketing alcohol studies are reported.
Women with distressing sexual desire problems need good treatments. We all need a drug approval process that delivers good decisions based on adequate evidence.
Dr. Steven Woloshin and Dr. Lisa M. Schwartz are both at the Dartmouth-Hitchcock Medical Center, Lebanon, N.H. They are cofounders of Informulary, a company that provides information about the benefits, harms, and uncertainties of prescription drugs. They reported having no relevant financial disclosures. These remarks are adapted from an accompanying editorial (JAMA Intern Med. 2016 Feb 29. doi:10.1001/jamainternmed.2016.0073).
A meta-analysis of eight clinical trials of the newly approved female hypoactive sexual disorder drug flibanserin show the drug yields minimal benefit, while increasing the risk of dizziness, somnolence, nausea, and fatigue to a clinically meaningful degree.
In a study published online Feb. 29 in JAMA Internal Medicine, the researchers wrote that flibanserin has not be sufficiently studied to recommend its use in clinical practice, despite being approved by the Food and Drug Administration in August 2015.
“Before flibanserin can be recommended in guidelines and clinical practice, future studies should include women from diverse populations, particularly women with (a history of) somatic and psychological comorbidities, medication use, and surgical menopause,” wrote Dr. Loes Jaspers of Erasmus University, Rotterdam, the Netherlands, and her associates.
They performed a systematic review of the literature and meta-analysis of five published and three unpublished randomized placebo-controlled trials. Seven of the trials were performed in the U.S. and Canada, while the eighth trial was performed in 13 European countries. About 90% of the 5,914 participants were white, and all were in stable, heterosexual, monogamous relationships for at least 1 year.
At baseline, the mean number of participants’ satisfactory sexual experiences per month was 2.5. The mean score on a scale of sexual desire in a daily electronic diary was 11.5 out of a possible 84 points, and the mean score on the Female Sexual Function Index was 1.8 on a scale of 1.2-6.0.
The primary efficacy outcome – the mean number of satisfactory sexual experiences per month – was 0.49 higher for women receiving 100 mg flibanserin, compared with women receiving placebo. Similarly, the mean improvement in sexual desire score in the daily diary was 1.63 points, and 0.27 points in the Female Sexual Function Index score was 0.27 points. These results remained robust across all subgroup and sensitivity analyses, and indicate that the drug affords “minimal” meaningful improvement, Dr. Jaspers and her associates wrote (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2015.8565).
In contrast, flibanserin clearly influenced safety outcomes, increasing the risk of dizziness, somnolence, nausea, and fatigue to a statistically and clinically significant degree. The risk for dizziness was 4.0 times higher with flibanserin, compared with placebo. Similarly, the risk for somnolence was 3.97 times higher, the risk for nausea was 2.35 times higher, and the risk for fatigue was 1.64 times higher.
The absolute number of series adverse events was small and the risk ratio was no different between flibanserin and placebo, according to the study.
But even though the adverse events tended to be of mild to moderate intensity, it would be premature to conclude that flibanserin is safe, the researchers wrote. “In an open-label extension study including 1,723 women with a median follow-up of 1 year, 723 participants (43%) reported investigator-defined drug-related adverse events, and 143 (8.3%) reported severe adverse events. The continued safety (and efficacy) of flibanserin with long-term use remains to be established.”
Overall, the quality of the evidence in these trials was rated as “very low,” in part because of “limitations in design, indirectness of evidence, and more favorable efficacy outcomes in published compared to unpublished studies,” the researchers noted.
Dr. Jaspers and one of her coauthors work for ErasmusAGE, a center for aging research funded by Nestlé Nutrition and Metagenics. Another coauthor was involved in the European trial as an investigator. No other disclosures were reported.
A meta-analysis of eight clinical trials of the newly approved female hypoactive sexual disorder drug flibanserin show the drug yields minimal benefit, while increasing the risk of dizziness, somnolence, nausea, and fatigue to a clinically meaningful degree.
In a study published online Feb. 29 in JAMA Internal Medicine, the researchers wrote that flibanserin has not be sufficiently studied to recommend its use in clinical practice, despite being approved by the Food and Drug Administration in August 2015.
“Before flibanserin can be recommended in guidelines and clinical practice, future studies should include women from diverse populations, particularly women with (a history of) somatic and psychological comorbidities, medication use, and surgical menopause,” wrote Dr. Loes Jaspers of Erasmus University, Rotterdam, the Netherlands, and her associates.
They performed a systematic review of the literature and meta-analysis of five published and three unpublished randomized placebo-controlled trials. Seven of the trials were performed in the U.S. and Canada, while the eighth trial was performed in 13 European countries. About 90% of the 5,914 participants were white, and all were in stable, heterosexual, monogamous relationships for at least 1 year.
At baseline, the mean number of participants’ satisfactory sexual experiences per month was 2.5. The mean score on a scale of sexual desire in a daily electronic diary was 11.5 out of a possible 84 points, and the mean score on the Female Sexual Function Index was 1.8 on a scale of 1.2-6.0.
The primary efficacy outcome – the mean number of satisfactory sexual experiences per month – was 0.49 higher for women receiving 100 mg flibanserin, compared with women receiving placebo. Similarly, the mean improvement in sexual desire score in the daily diary was 1.63 points, and 0.27 points in the Female Sexual Function Index score was 0.27 points. These results remained robust across all subgroup and sensitivity analyses, and indicate that the drug affords “minimal” meaningful improvement, Dr. Jaspers and her associates wrote (JAMA Intern Med. 2016 Feb 29. doi: 10.1001/jamainternmed.2015.8565).
In contrast, flibanserin clearly influenced safety outcomes, increasing the risk of dizziness, somnolence, nausea, and fatigue to a statistically and clinically significant degree. The risk for dizziness was 4.0 times higher with flibanserin, compared with placebo. Similarly, the risk for somnolence was 3.97 times higher, the risk for nausea was 2.35 times higher, and the risk for fatigue was 1.64 times higher.
The absolute number of series adverse events was small and the risk ratio was no different between flibanserin and placebo, according to the study.
But even though the adverse events tended to be of mild to moderate intensity, it would be premature to conclude that flibanserin is safe, the researchers wrote. “In an open-label extension study including 1,723 women with a median follow-up of 1 year, 723 participants (43%) reported investigator-defined drug-related adverse events, and 143 (8.3%) reported severe adverse events. The continued safety (and efficacy) of flibanserin with long-term use remains to be established.”
Overall, the quality of the evidence in these trials was rated as “very low,” in part because of “limitations in design, indirectness of evidence, and more favorable efficacy outcomes in published compared to unpublished studies,” the researchers noted.
Dr. Jaspers and one of her coauthors work for ErasmusAGE, a center for aging research funded by Nestlé Nutrition and Metagenics. Another coauthor was involved in the European trial as an investigator. No other disclosures were reported.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Flibanserin has limited benefit and a clinically meaningful increased risk of mild and moderate adverse events.
Major finding: The primary efficacy outcome – the mean number of satisfactory sexual experiences per month – was 0.49 higher for women receiving flibanserin, compared with placebo.
Data source: A systematic review and meta-analysis of five published and three unpublished randomized trials involving nearly 6,000 women.
Disclosures: Dr. Jaspers and one of her coauthors work for ErasmusAGE, a center for aging research funded by Nestlé Nutrition and Metagenics. Another coauthor was involved in the European trial as an investigator. No other disclosures were reported.
Dose-dense paclitaxel doesn’t prolong PFS in ovarian cancer patients
Dose-dense paclitaxel failed to prolong progression-free survival beyond that achieved with standard paclitaxel in an international phase III clinical trial involving 692 women with newly diagnosed advanced ovarian cancer, according to a study published online online Feb. 25 in the New England Journal of Medicine.
Dose-dense paclitaxel involves a greater frequency of infusions – weekly vs. every 3 weeks – which is thought to enhance the agent’s antineoplastic effect by facilitating intratumoral perfusion to inhibit angiogenesis. In a recent trial in Japan, this approach was associated with longer overall survival in women with ovarian cancer, compared with conventional paclitaxel dosing, said Dr. John K. Chan of the California Pacific–Palo Alto Medical Foundation, Sutter Cancer Research Institute, San Francisco, and his associates.
They compared the two approaches in an open-label trial involving women with newly diagnosed, untreated, incompletely resected stage III or stage IV epithelial ovarian, fallopian tube, or peritoneal cancer. These patients were treated at 209 clinics in the United States, Canada, and South Korea and followed for a median of 28 months.
Dose-dense paclitaxel didn’t prolong progression-free survival (PFS) compared with conventional paclitaxel (14.7 vs 14.0 months, respectively) in the intention-to-treat analysis or a sensitivity analysis. Moreover, “the effect of the paclitaxel regimen on progression-free survival did not differ significantly between patients who were left with microscopic residual disease and those who were left with macroscopic residual disease, nor did it differ significantly between those who had neoadjuvant chemotherapy followed by interval cytoreduction and those who received primary cytoreduction,” the investigators said.
However, in the subgroup of 112 patients who chose not to receive optional bevacizumab, dose-dense paclitaxel was associated with significantly longer PFS (14.2 months) than was conventional paclitaxel (10.3 months), for an HR of 0.62 (N Engl J Med. 2016 Feb 25. doi: 10.1056/NEJMoa1505067).
Patients who received dose-dense paclitaxel reported poorer quality of life. In particular, severe neuropathy was more frequent among those receiving paclitaxel every week (26%) rather than every 3 weeks (18%). The most common serious adverse events were neutropenia, gastrointestinal disorders (including GI-wall perforation, fistula, or necrosis), thrombocytopenia, infection, and anemia. Severe anemia was more common with weekly paclitaxel, but severe neutropenia was less common in that group.
Dose-dense paclitaxel failed to prolong progression-free survival beyond that achieved with standard paclitaxel in an international phase III clinical trial involving 692 women with newly diagnosed advanced ovarian cancer, according to a study published online online Feb. 25 in the New England Journal of Medicine.
Dose-dense paclitaxel involves a greater frequency of infusions – weekly vs. every 3 weeks – which is thought to enhance the agent’s antineoplastic effect by facilitating intratumoral perfusion to inhibit angiogenesis. In a recent trial in Japan, this approach was associated with longer overall survival in women with ovarian cancer, compared with conventional paclitaxel dosing, said Dr. John K. Chan of the California Pacific–Palo Alto Medical Foundation, Sutter Cancer Research Institute, San Francisco, and his associates.
They compared the two approaches in an open-label trial involving women with newly diagnosed, untreated, incompletely resected stage III or stage IV epithelial ovarian, fallopian tube, or peritoneal cancer. These patients were treated at 209 clinics in the United States, Canada, and South Korea and followed for a median of 28 months.
Dose-dense paclitaxel didn’t prolong progression-free survival (PFS) compared with conventional paclitaxel (14.7 vs 14.0 months, respectively) in the intention-to-treat analysis or a sensitivity analysis. Moreover, “the effect of the paclitaxel regimen on progression-free survival did not differ significantly between patients who were left with microscopic residual disease and those who were left with macroscopic residual disease, nor did it differ significantly between those who had neoadjuvant chemotherapy followed by interval cytoreduction and those who received primary cytoreduction,” the investigators said.
However, in the subgroup of 112 patients who chose not to receive optional bevacizumab, dose-dense paclitaxel was associated with significantly longer PFS (14.2 months) than was conventional paclitaxel (10.3 months), for an HR of 0.62 (N Engl J Med. 2016 Feb 25. doi: 10.1056/NEJMoa1505067).
Patients who received dose-dense paclitaxel reported poorer quality of life. In particular, severe neuropathy was more frequent among those receiving paclitaxel every week (26%) rather than every 3 weeks (18%). The most common serious adverse events were neutropenia, gastrointestinal disorders (including GI-wall perforation, fistula, or necrosis), thrombocytopenia, infection, and anemia. Severe anemia was more common with weekly paclitaxel, but severe neutropenia was less common in that group.
Dose-dense paclitaxel failed to prolong progression-free survival beyond that achieved with standard paclitaxel in an international phase III clinical trial involving 692 women with newly diagnosed advanced ovarian cancer, according to a study published online online Feb. 25 in the New England Journal of Medicine.
Dose-dense paclitaxel involves a greater frequency of infusions – weekly vs. every 3 weeks – which is thought to enhance the agent’s antineoplastic effect by facilitating intratumoral perfusion to inhibit angiogenesis. In a recent trial in Japan, this approach was associated with longer overall survival in women with ovarian cancer, compared with conventional paclitaxel dosing, said Dr. John K. Chan of the California Pacific–Palo Alto Medical Foundation, Sutter Cancer Research Institute, San Francisco, and his associates.
They compared the two approaches in an open-label trial involving women with newly diagnosed, untreated, incompletely resected stage III or stage IV epithelial ovarian, fallopian tube, or peritoneal cancer. These patients were treated at 209 clinics in the United States, Canada, and South Korea and followed for a median of 28 months.
Dose-dense paclitaxel didn’t prolong progression-free survival (PFS) compared with conventional paclitaxel (14.7 vs 14.0 months, respectively) in the intention-to-treat analysis or a sensitivity analysis. Moreover, “the effect of the paclitaxel regimen on progression-free survival did not differ significantly between patients who were left with microscopic residual disease and those who were left with macroscopic residual disease, nor did it differ significantly between those who had neoadjuvant chemotherapy followed by interval cytoreduction and those who received primary cytoreduction,” the investigators said.
However, in the subgroup of 112 patients who chose not to receive optional bevacizumab, dose-dense paclitaxel was associated with significantly longer PFS (14.2 months) than was conventional paclitaxel (10.3 months), for an HR of 0.62 (N Engl J Med. 2016 Feb 25. doi: 10.1056/NEJMoa1505067).
Patients who received dose-dense paclitaxel reported poorer quality of life. In particular, severe neuropathy was more frequent among those receiving paclitaxel every week (26%) rather than every 3 weeks (18%). The most common serious adverse events were neutropenia, gastrointestinal disorders (including GI-wall perforation, fistula, or necrosis), thrombocytopenia, infection, and anemia. Severe anemia was more common with weekly paclitaxel, but severe neutropenia was less common in that group.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Dose-dense paclitaxel failed to prolong progression-free survival in patients with ovarian cancer.
Major finding: Dose-dense paclitaxel didn’t prolong progression-free survival compared with conventional paclitaxel (14.7 vs. 14.0 months).
Data source: An international, open-label, randomized phase III trial involving 692 women followed for a median of 28 months.
Disclosures: The National Cancer Institute and Genentech funded the study. Dr. Chan reported having no relevant disclosures; his associates reported numerous ties to industry sources.
Loss of pulmonary vascular distensibility precedes PH
Loss of distensibility of the pulmonary vasculature may be a marker that allows earlier detection of impending pulmonary hypertension, based on hemodynamic data from the medical records of 90 patients across the spectrum of pulmonary vascular disease.
Normal pulmonary circulation is distensible, allowing distension and recruitment of the pulmonary vasculature during exertion that in turn reduces pulmonary vascular resistance. Loss of this distensibility increases resistance and thus pulmonary arterial pressure, and is a characteristic of mild pulmonary vascular disease. Such disease is a precursor of full-blown pulmonary hypertension (PH), which “is a relatively late hemodynamic event in the evolution of pulmonary vascular disease,” said Dr. Edmund M. T. Lau of Université Paris-Sud, and his associates.
The percentage change in vascular diameter per mm Hg increase in distending pressure has been proposed for estimating the distensibility of resistive pulmonary vessels. This “distensibility value” has been assessed in animal studies and in healthy human subjects, but has not yet been assessed as a possible marker of mild pulmonary vascular disease or PH.
The researchers assessed this distensibility value in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects with no pulmonary vascular disease. The data were obtained from the medical records of these patients, who had undergone right-sided heart catheterization, both at rest and during exercise, over a 6-year period.
The percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg). As expected, the group with PH had the lowest distensibility value, at 0.25%/mm Hg.
Using a cutoff value of 0.76%/mm Hg allowed the researchers to distinguish control subjects from patients with mild disease with a sensitivity of 88% and a specificity of 100%. “To our knowledge, this is the first study to validate the fit of [this] model in subjects with pulmonary vascular disease and to demonstrate that [percentage change in vascular diameter per mm Hg increase in distending pressure] is dramatically reduced in patients who have mild pulmonary vascular disease without manifest PH.
“Taken together, our findings suggest that vascular distensibility is markedly attenuated prior to the development of PH and that [this value] may serve as a useful vascular index in the setting of early disease detection,” Dr. Lau and his associates said (CHEST 2016;149:353-61).
The distensibility value calculated for this study’s control group (1.4%/mm Hg) was slightly lower than that reported in the literature for normal, healthy subjects and in vitro animal vessels (2%/mm Hg). That is likely because the control participants were older than the subjects in previous studies, and vascular distensibility is known to decrease with increasing age, the researchers said.
They added that it might be useful to calculate the distensibility value when patients suspected of having pulmonary vascular disease undergo invasive pulmonary hemodynamic evaluations. “It would be of particular interest to assess [it] in populations at a high risk of developing PH, such as carriers of the BMPR2 mutation and patients with systemic sclerosis.”
Obviously, estimating the distensibility value using noninvasive evaluation would be preferable, the researchers noted. Preliminary studies of healthy control subjects and carriers of the BMPR2 mutation undergoing stress ECG testing have shown that calculating the distensibility value is feasible using Doppler echocardiography data, they added.
This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
These findings have the potential to completely revamp screening for pulmonary vascular disease, but first they must be validated in further research.
It will also be important to determine whether, as the authors suggest, a noninvasive method to determine vascular distensibility can be developed, perhaps using stress echocardiography or stress cardiac magnetic resonance testing. Only then can this measure – the percentage change in vascular diameter per mm Hg increase in distending pressure – translate from the realm of novel research into real-world clinical practice.
Dr. Richa Agarwal is in the department of medicine at Temple University, Philadelphia, and at the Cardiovascular Institute at Allegheny General Hospital, Pittsburgh. Dr. Mardi Gomberg-Maitland is in the department of medicine at the University of Chicago. Dr. Agarwal reported having no relevant financial disclosures; Dr. Gomberg-Maitland reported ties to Actelion, Bayer, GeNo, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, Bellerophon, United Therapeutics, Medscape, and ABComm. Dr. Agarwal and Dr. Gomberg-Maitland made these remarks in an editorial accompanying Dr. Lau’s report (Chest 2016;149:295-7).
These findings have the potential to completely revamp screening for pulmonary vascular disease, but first they must be validated in further research.
It will also be important to determine whether, as the authors suggest, a noninvasive method to determine vascular distensibility can be developed, perhaps using stress echocardiography or stress cardiac magnetic resonance testing. Only then can this measure – the percentage change in vascular diameter per mm Hg increase in distending pressure – translate from the realm of novel research into real-world clinical practice.
Dr. Richa Agarwal is in the department of medicine at Temple University, Philadelphia, and at the Cardiovascular Institute at Allegheny General Hospital, Pittsburgh. Dr. Mardi Gomberg-Maitland is in the department of medicine at the University of Chicago. Dr. Agarwal reported having no relevant financial disclosures; Dr. Gomberg-Maitland reported ties to Actelion, Bayer, GeNo, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, Bellerophon, United Therapeutics, Medscape, and ABComm. Dr. Agarwal and Dr. Gomberg-Maitland made these remarks in an editorial accompanying Dr. Lau’s report (Chest 2016;149:295-7).
These findings have the potential to completely revamp screening for pulmonary vascular disease, but first they must be validated in further research.
It will also be important to determine whether, as the authors suggest, a noninvasive method to determine vascular distensibility can be developed, perhaps using stress echocardiography or stress cardiac magnetic resonance testing. Only then can this measure – the percentage change in vascular diameter per mm Hg increase in distending pressure – translate from the realm of novel research into real-world clinical practice.
Dr. Richa Agarwal is in the department of medicine at Temple University, Philadelphia, and at the Cardiovascular Institute at Allegheny General Hospital, Pittsburgh. Dr. Mardi Gomberg-Maitland is in the department of medicine at the University of Chicago. Dr. Agarwal reported having no relevant financial disclosures; Dr. Gomberg-Maitland reported ties to Actelion, Bayer, GeNo, Gilead, Medtronic, Novartis, Lung Biotechnology, Reata, Bellerophon, United Therapeutics, Medscape, and ABComm. Dr. Agarwal and Dr. Gomberg-Maitland made these remarks in an editorial accompanying Dr. Lau’s report (Chest 2016;149:295-7).
Loss of distensibility of the pulmonary vasculature may be a marker that allows earlier detection of impending pulmonary hypertension, based on hemodynamic data from the medical records of 90 patients across the spectrum of pulmonary vascular disease.
Normal pulmonary circulation is distensible, allowing distension and recruitment of the pulmonary vasculature during exertion that in turn reduces pulmonary vascular resistance. Loss of this distensibility increases resistance and thus pulmonary arterial pressure, and is a characteristic of mild pulmonary vascular disease. Such disease is a precursor of full-blown pulmonary hypertension (PH), which “is a relatively late hemodynamic event in the evolution of pulmonary vascular disease,” said Dr. Edmund M. T. Lau of Université Paris-Sud, and his associates.
The percentage change in vascular diameter per mm Hg increase in distending pressure has been proposed for estimating the distensibility of resistive pulmonary vessels. This “distensibility value” has been assessed in animal studies and in healthy human subjects, but has not yet been assessed as a possible marker of mild pulmonary vascular disease or PH.
The researchers assessed this distensibility value in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects with no pulmonary vascular disease. The data were obtained from the medical records of these patients, who had undergone right-sided heart catheterization, both at rest and during exercise, over a 6-year period.
The percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg). As expected, the group with PH had the lowest distensibility value, at 0.25%/mm Hg.
Using a cutoff value of 0.76%/mm Hg allowed the researchers to distinguish control subjects from patients with mild disease with a sensitivity of 88% and a specificity of 100%. “To our knowledge, this is the first study to validate the fit of [this] model in subjects with pulmonary vascular disease and to demonstrate that [percentage change in vascular diameter per mm Hg increase in distending pressure] is dramatically reduced in patients who have mild pulmonary vascular disease without manifest PH.
“Taken together, our findings suggest that vascular distensibility is markedly attenuated prior to the development of PH and that [this value] may serve as a useful vascular index in the setting of early disease detection,” Dr. Lau and his associates said (CHEST 2016;149:353-61).
The distensibility value calculated for this study’s control group (1.4%/mm Hg) was slightly lower than that reported in the literature for normal, healthy subjects and in vitro animal vessels (2%/mm Hg). That is likely because the control participants were older than the subjects in previous studies, and vascular distensibility is known to decrease with increasing age, the researchers said.
They added that it might be useful to calculate the distensibility value when patients suspected of having pulmonary vascular disease undergo invasive pulmonary hemodynamic evaluations. “It would be of particular interest to assess [it] in populations at a high risk of developing PH, such as carriers of the BMPR2 mutation and patients with systemic sclerosis.”
Obviously, estimating the distensibility value using noninvasive evaluation would be preferable, the researchers noted. Preliminary studies of healthy control subjects and carriers of the BMPR2 mutation undergoing stress ECG testing have shown that calculating the distensibility value is feasible using Doppler echocardiography data, they added.
This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
Loss of distensibility of the pulmonary vasculature may be a marker that allows earlier detection of impending pulmonary hypertension, based on hemodynamic data from the medical records of 90 patients across the spectrum of pulmonary vascular disease.
Normal pulmonary circulation is distensible, allowing distension and recruitment of the pulmonary vasculature during exertion that in turn reduces pulmonary vascular resistance. Loss of this distensibility increases resistance and thus pulmonary arterial pressure, and is a characteristic of mild pulmonary vascular disease. Such disease is a precursor of full-blown pulmonary hypertension (PH), which “is a relatively late hemodynamic event in the evolution of pulmonary vascular disease,” said Dr. Edmund M. T. Lau of Université Paris-Sud, and his associates.
The percentage change in vascular diameter per mm Hg increase in distending pressure has been proposed for estimating the distensibility of resistive pulmonary vessels. This “distensibility value” has been assessed in animal studies and in healthy human subjects, but has not yet been assessed as a possible marker of mild pulmonary vascular disease or PH.
The researchers assessed this distensibility value in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects with no pulmonary vascular disease. The data were obtained from the medical records of these patients, who had undergone right-sided heart catheterization, both at rest and during exercise, over a 6-year period.
The percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg). As expected, the group with PH had the lowest distensibility value, at 0.25%/mm Hg.
Using a cutoff value of 0.76%/mm Hg allowed the researchers to distinguish control subjects from patients with mild disease with a sensitivity of 88% and a specificity of 100%. “To our knowledge, this is the first study to validate the fit of [this] model in subjects with pulmonary vascular disease and to demonstrate that [percentage change in vascular diameter per mm Hg increase in distending pressure] is dramatically reduced in patients who have mild pulmonary vascular disease without manifest PH.
“Taken together, our findings suggest that vascular distensibility is markedly attenuated prior to the development of PH and that [this value] may serve as a useful vascular index in the setting of early disease detection,” Dr. Lau and his associates said (CHEST 2016;149:353-61).
The distensibility value calculated for this study’s control group (1.4%/mm Hg) was slightly lower than that reported in the literature for normal, healthy subjects and in vitro animal vessels (2%/mm Hg). That is likely because the control participants were older than the subjects in previous studies, and vascular distensibility is known to decrease with increasing age, the researchers said.
They added that it might be useful to calculate the distensibility value when patients suspected of having pulmonary vascular disease undergo invasive pulmonary hemodynamic evaluations. “It would be of particular interest to assess [it] in populations at a high risk of developing PH, such as carriers of the BMPR2 mutation and patients with systemic sclerosis.”
Obviously, estimating the distensibility value using noninvasive evaluation would be preferable, the researchers noted. Preliminary studies of healthy control subjects and carriers of the BMPR2 mutation undergoing stress ECG testing have shown that calculating the distensibility value is feasible using Doppler echocardiography data, they added.
This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
FROM CHEST
Key clinical point: Loss of distensibility of the pulmonary vasculature precedes the development of pulmonary hypertension and may serve as a marker allowing earlier detection of the disorder.
Major finding: During exercise, the percentage change in vascular diameter per mm Hg increase in distending pressure was “strikingly reduced” (0.45%/mm Hg) in the mild pulmonary vascular disease group compared with the control group (1.4%/mm Hg).
Data source: A retrospective single-center comparison of pulmonary vascular distensibility in 31 patients with PH, 33 with mild pulmonary vascular disease but no PH as yet, and 26 control subjects.
Disclosures: This study was supported by Fonds de Dotation Recherche en Santé Respiratoire, Fondation du Souffle, and the INSERM–University of Sydney Exchange Grant. Dr. Lau reported having no relevant financial disclosures; his associates reported ties to Actelion, Aires, Bayer, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and United Therapeutics Corporation.
Vaccine coverage remains low in U.S. adults
Vaccine coverage of adults in the United States continues to be low for the seven vaccines currently recommended by the Centers for Disease Control and Prevention, according to a study published in MMWR.
Illness attributable to vaccine-preventable diseases is more prevalent among adults than among children, and is especially high among the oldest adults.
For example, an estimated 50%-70% of influenza-related hospitalizations, 80%-90% of influenza-related deaths, and 50% of invasive pneumococcal disease each year occur in people aged 65 years and older, noted Dr. Walter W. Williams of the National Center for Immunization and Respiratory Diseases, CDC, Atlanta, and his associates.
The influenza vaccine is recommended for all adults every year; the Td (pneumococcal, tetanus, diphtheria), Tdap (tetanus, diphtheria, acellular pertussis), hepatitis A, hepatitis B, herpes zoster, and HPV (human papillomavirus) vaccines are routinely recommended for many adults based on patient age, underlying medical conditions, lifestyle factors, occupation, travel, and other considerations.
However, adult vaccination coverage is low because of limited public awareness about the need for these vaccines, lack of vaccine requirements for adults like the immunization requirements in place for children, failure to incorporate routine vaccine assessments and recommendations in adult health care visits, the costs of stocking vaccines and providing vaccine services, inadequate or inconsistent payment for vaccines and for vaccine administration, and the tendency to prioritize acute over preventive medical care, they noted.
The investigators assessed nationwide vaccine coverage of adults using data from the 2013 and 2014 National Health Interview Survey, which annually collects health information from a large, nationally representative sample of households via in-person interviews. The 2014 sample included 36,324 adults who self-reported their vaccination status (Morb Mortal Wkly Rep Surveill Summ. 2016;65:1-36).
Coverage for Tdap slightly increased 2.9 percentage points to 20.1% of the eligible population in 2014, the most recent year for which data are available, and coverage for herpes zoster among high-risk patients aged 60 and older slightly increased 3.6 percentage points to 27.9% of the population. Coverage for the other five vaccines remained steady and low: for the influenza vaccine, 43.2% of the population; for the pneumococcal vaccine, 20.3% of high-risk younger adults and 61.3% of very high-risk older adults; for the Td vaccine, 62.2%; for hepatitis A, 9%; for hepatitis B, 24.5%; and for HPV, coverage was 8.2% for men and 40.2% for women aged 19-26 years.
As expected, vaccine coverage was lower for people who didn’t have health insurance, didn’t have a usual site for receiving health care, and didn’t have any physician contacts during the preceding year. However, even among adults who had health insurance and had 10 or more physician contacts within the past year, up to 88% reported that they hadn’t received vaccinations that were recommended for everyone or for specific populations.
Racial and ethnic gaps in vaccine coverage persisted for five of these vaccines and actually worsened for the Tdap and herpes zoster vaccines.
Advice from health care providers is strongly associated with delivery of adult vaccinations. To address these gaps in vaccine coverage, clinicians should consider routine assessment of all adult patients’ vaccine needs, use of a reminder/recall system, use of standing-order programs for vaccination, and assessment of their practices’ vaccination rates. “Efforts are also needed to identify adults who do not have a regular provider or insurance and who report fewer health care visits,” Dr. Williams and his associates said.
The CDC updates vaccine recommendations annually and publishes them in the U.S. Adult Immunization Schedule, which is “a ready resource for persons who provide healthcare services in various settings,” they added.
The CDC supported the study. The authors’ financial disclosures were not provided.
It is unfortunate that vaccination rates for preventable conditions continue to be stagnant at low levels. As stated in the article, there are a multitude of reasons proposed for this (and likely others - such as a very vocal anti-vaccination movement), and until prevention efforts are given the time and monetary attention similar to that of disease management, this trend will continue. A follow-up to this study would be informative to assess the effect of the Affordable Care Act's mandate to cover these preventive services.
Dr. Eric Gartman, FCCP, is a member of the editorial advisory board for CHEST Physician.
It is unfortunate that vaccination rates for preventable conditions continue to be stagnant at low levels. As stated in the article, there are a multitude of reasons proposed for this (and likely others - such as a very vocal anti-vaccination movement), and until prevention efforts are given the time and monetary attention similar to that of disease management, this trend will continue. A follow-up to this study would be informative to assess the effect of the Affordable Care Act's mandate to cover these preventive services.
Dr. Eric Gartman, FCCP, is a member of the editorial advisory board for CHEST Physician.
It is unfortunate that vaccination rates for preventable conditions continue to be stagnant at low levels. As stated in the article, there are a multitude of reasons proposed for this (and likely others - such as a very vocal anti-vaccination movement), and until prevention efforts are given the time and monetary attention similar to that of disease management, this trend will continue. A follow-up to this study would be informative to assess the effect of the Affordable Care Act's mandate to cover these preventive services.
Dr. Eric Gartman, FCCP, is a member of the editorial advisory board for CHEST Physician.
Vaccine coverage of adults in the United States continues to be low for the seven vaccines currently recommended by the Centers for Disease Control and Prevention, according to a study published in MMWR.
Illness attributable to vaccine-preventable diseases is more prevalent among adults than among children, and is especially high among the oldest adults.
For example, an estimated 50%-70% of influenza-related hospitalizations, 80%-90% of influenza-related deaths, and 50% of invasive pneumococcal disease each year occur in people aged 65 years and older, noted Dr. Walter W. Williams of the National Center for Immunization and Respiratory Diseases, CDC, Atlanta, and his associates.
The influenza vaccine is recommended for all adults every year; the Td (pneumococcal, tetanus, diphtheria), Tdap (tetanus, diphtheria, acellular pertussis), hepatitis A, hepatitis B, herpes zoster, and HPV (human papillomavirus) vaccines are routinely recommended for many adults based on patient age, underlying medical conditions, lifestyle factors, occupation, travel, and other considerations.
However, adult vaccination coverage is low because of limited public awareness about the need for these vaccines, lack of vaccine requirements for adults like the immunization requirements in place for children, failure to incorporate routine vaccine assessments and recommendations in adult health care visits, the costs of stocking vaccines and providing vaccine services, inadequate or inconsistent payment for vaccines and for vaccine administration, and the tendency to prioritize acute over preventive medical care, they noted.
The investigators assessed nationwide vaccine coverage of adults using data from the 2013 and 2014 National Health Interview Survey, which annually collects health information from a large, nationally representative sample of households via in-person interviews. The 2014 sample included 36,324 adults who self-reported their vaccination status (Morb Mortal Wkly Rep Surveill Summ. 2016;65:1-36).
Coverage for Tdap slightly increased 2.9 percentage points to 20.1% of the eligible population in 2014, the most recent year for which data are available, and coverage for herpes zoster among high-risk patients aged 60 and older slightly increased 3.6 percentage points to 27.9% of the population. Coverage for the other five vaccines remained steady and low: for the influenza vaccine, 43.2% of the population; for the pneumococcal vaccine, 20.3% of high-risk younger adults and 61.3% of very high-risk older adults; for the Td vaccine, 62.2%; for hepatitis A, 9%; for hepatitis B, 24.5%; and for HPV, coverage was 8.2% for men and 40.2% for women aged 19-26 years.
As expected, vaccine coverage was lower for people who didn’t have health insurance, didn’t have a usual site for receiving health care, and didn’t have any physician contacts during the preceding year. However, even among adults who had health insurance and had 10 or more physician contacts within the past year, up to 88% reported that they hadn’t received vaccinations that were recommended for everyone or for specific populations.
Racial and ethnic gaps in vaccine coverage persisted for five of these vaccines and actually worsened for the Tdap and herpes zoster vaccines.
Advice from health care providers is strongly associated with delivery of adult vaccinations. To address these gaps in vaccine coverage, clinicians should consider routine assessment of all adult patients’ vaccine needs, use of a reminder/recall system, use of standing-order programs for vaccination, and assessment of their practices’ vaccination rates. “Efforts are also needed to identify adults who do not have a regular provider or insurance and who report fewer health care visits,” Dr. Williams and his associates said.
The CDC updates vaccine recommendations annually and publishes them in the U.S. Adult Immunization Schedule, which is “a ready resource for persons who provide healthcare services in various settings,” they added.
The CDC supported the study. The authors’ financial disclosures were not provided.
Vaccine coverage of adults in the United States continues to be low for the seven vaccines currently recommended by the Centers for Disease Control and Prevention, according to a study published in MMWR.
Illness attributable to vaccine-preventable diseases is more prevalent among adults than among children, and is especially high among the oldest adults.
For example, an estimated 50%-70% of influenza-related hospitalizations, 80%-90% of influenza-related deaths, and 50% of invasive pneumococcal disease each year occur in people aged 65 years and older, noted Dr. Walter W. Williams of the National Center for Immunization and Respiratory Diseases, CDC, Atlanta, and his associates.
The influenza vaccine is recommended for all adults every year; the Td (pneumococcal, tetanus, diphtheria), Tdap (tetanus, diphtheria, acellular pertussis), hepatitis A, hepatitis B, herpes zoster, and HPV (human papillomavirus) vaccines are routinely recommended for many adults based on patient age, underlying medical conditions, lifestyle factors, occupation, travel, and other considerations.
However, adult vaccination coverage is low because of limited public awareness about the need for these vaccines, lack of vaccine requirements for adults like the immunization requirements in place for children, failure to incorporate routine vaccine assessments and recommendations in adult health care visits, the costs of stocking vaccines and providing vaccine services, inadequate or inconsistent payment for vaccines and for vaccine administration, and the tendency to prioritize acute over preventive medical care, they noted.
The investigators assessed nationwide vaccine coverage of adults using data from the 2013 and 2014 National Health Interview Survey, which annually collects health information from a large, nationally representative sample of households via in-person interviews. The 2014 sample included 36,324 adults who self-reported their vaccination status (Morb Mortal Wkly Rep Surveill Summ. 2016;65:1-36).
Coverage for Tdap slightly increased 2.9 percentage points to 20.1% of the eligible population in 2014, the most recent year for which data are available, and coverage for herpes zoster among high-risk patients aged 60 and older slightly increased 3.6 percentage points to 27.9% of the population. Coverage for the other five vaccines remained steady and low: for the influenza vaccine, 43.2% of the population; for the pneumococcal vaccine, 20.3% of high-risk younger adults and 61.3% of very high-risk older adults; for the Td vaccine, 62.2%; for hepatitis A, 9%; for hepatitis B, 24.5%; and for HPV, coverage was 8.2% for men and 40.2% for women aged 19-26 years.
As expected, vaccine coverage was lower for people who didn’t have health insurance, didn’t have a usual site for receiving health care, and didn’t have any physician contacts during the preceding year. However, even among adults who had health insurance and had 10 or more physician contacts within the past year, up to 88% reported that they hadn’t received vaccinations that were recommended for everyone or for specific populations.
Racial and ethnic gaps in vaccine coverage persisted for five of these vaccines and actually worsened for the Tdap and herpes zoster vaccines.
Advice from health care providers is strongly associated with delivery of adult vaccinations. To address these gaps in vaccine coverage, clinicians should consider routine assessment of all adult patients’ vaccine needs, use of a reminder/recall system, use of standing-order programs for vaccination, and assessment of their practices’ vaccination rates. “Efforts are also needed to identify adults who do not have a regular provider or insurance and who report fewer health care visits,” Dr. Williams and his associates said.
The CDC updates vaccine recommendations annually and publishes them in the U.S. Adult Immunization Schedule, which is “a ready resource for persons who provide healthcare services in various settings,” they added.
The CDC supported the study. The authors’ financial disclosures were not provided.
from Morbidity and Mortality Weekly Report
Key clinical point: Vaccine coverage of adults in the United States remains low for the seven vaccines currently recommended by the Centers for Disease Control and Prevention.
Major finding: Vaccine coverage was 20.1% for Tdap, 27.9% for herpes zoster, 43.2% for influenza, 20.3%-61.3% for pneumococcal disease, 62.2% for Td, 9.0% for hepatitis A, 24.5% for hepatitis B, and 8.2%-40.2% for HPV.
Data source: An analysis of self-reported vaccine status in a nationally representative sample of 36,324 adults participating in the cross-sectional National Health Interview Survey.
Disclosures: The CDC supported the study. The authors’ financial disclosures were not provided.
New postop pain guideline: Multiple approaches to target different pain mechanisms
Clinicians should use multiple approaches to target different pain mechanisms when treating postsurgical pain, according to a Clinical Practice Guideline for this aspect of patient care published by the American Pain Society in the February issue of the Journal of Pain.
Postoperative pain management reportedly is inadequate for more than half of the adults and children who undergo surgery each year. In this setting, inadequate pain relief can contribute to impaired recovery, decreased physical function, postoperative complications, and poor quality of life, and it may even set the stage for the development of chronic pain, said Dr. Roger Chou, lead author of the guideline and professor of medicine and of medical informatics and clinical epidemiology, Oregon Health and Science University, Portland, and his associates.
The guideline includes 32 evidence-based recommendations for all clinicians who treat postoperative pain. It was compiled by a 23-member expert panel based on their analysis of 858 primary studies and 107 systematic reviews of the literature. Despite the panel’s efforts to obtain top-level evidence, of 32 recommendations, the panel rated only 4 as supported by high-quality evidence, and 11 recommendations were on the basis of low-quality evidence. The panel members had expertise in anesthesia, pain medicine, surgery, obstetrics and gynecology, primary care, pediatrics, hospital medicine, nursing, physical therapy, and psychology, said Dr. Chou, who is also director of the Pacific Northwest Evidence-Based Practice Center, Portland, and his associates.
The first strong recommendation, based on high-quality evidence, is to use a variety of medications and techniques that act on both the central and peripheral nervous systems and have been associated with both superior pain relief and decreased opioid consumption, compared with single agents or methods. For example, continuous nonopioid analgesia such as acetaminophen or NSAIDs can be combined with opioids that act on different receptors and with nonpharmacologic therapies. The guideline addresses several specific situations, and summarizes in a table the multimodal options for common surgeries such as laparotomy, total hip or knee replacement, coronary artery bypass graft, and cesarean section.
The guideline also strongly recommends that management of postsurgical pain should begin preoperatively, with assessment of the patient’s medical and psychiatric comorbidities; concomitant medications; and history of any chronic pain, substance abuse, and previous postoperative treatments and responses. Clinicians should use a validated pain assessment tool to track patients’ responses to postsurgical pain treatment and adjust treatment plans accordingly.
The guideline suggests that clinicians consider nonpharmacologic techniques including transcutaneous electrical nerve stimulation and cognitive-behavioral modalities such as guided imagery, relaxation methods, hypnosis, and intraoperative suggestion. At the very least, these are noninvasive and don’t appear to be associated with any harm. However, the guideline cautions that the evidence is insufficient to support cognitive-behavioral modalities in children. It also cannot recommend for or against several alternative treatments for which there is not yet sufficient evidence of efficacy, including acupuncture, massage, and cold therapy, Dr. Chou and his associates said (J Pain. 2016;17:131-57).
For adults who have no contraindications, a preoperative dose of celecoxib is strongly recommended because it improves postoperative pain and also reduces the need for opioids. One contraindication to celecoxib is CABG surgery, because of its attendant risk of adverse cardiovascular events. Preoperative doses of gabapentin or pregabalin also are strongly recommended as part of multimodal postsurgical analgesia, particularly for operations associated with substantial pain.
The guideline also strongly recommends epidural or spinal analgesia for major thoracic or abdominal procedures, especially for patients at risk for cardiac complications, pulmonary complications, or prolonged ileus. Compared with systemic analgesia, these forms appear to decrease the risk of death, venous thromboembolism, myocardial infarction, pneumonia, and respiratory depression. However, patients who receive neuraxial analgesia should still be carefully monitored, as they may develop respiratory depression, hypotension, or motor weakness, or the intervention might mask symptoms of compartment syndrome.
According to the guideline, all facilities that offer surgery should also provide clinicians with access to a pain specialist. The specialist should be available to consult for patients with inadequately controlled postoperative pain and to assist with diagnosis, interventions, or management of comorbid conditions. They may be especially useful for advising clinicians regarding patients who have opioid tolerance, especially if there is a history of substance abuse or addiction. Pain treatment should not be withheld from such patients “because of fears of worsening addiction or precipitation of relapse. In addition to the ethical requirement to address postoperative pain, poorly treated pain can be a trigger for relapse,” the guideline states.
The guideline also addresses the transition to outpatient care and identifies some research gaps. “Research is urgently needed on optimal methods for managing patients who receive opioids before surgery, effectiveness of opioid-sparing multimodal regimens, optimal methods of pain assessment and monitoring, and a number of areas related to management of perioperative pain in infants and children,” the panel concluded.
Funding for this guideline was provided by the American Pain Society. Dr. Chou’s and his associates’ financial disclosures are available at www.jpain.org.
Clinicians should use multiple approaches to target different pain mechanisms when treating postsurgical pain, according to a Clinical Practice Guideline for this aspect of patient care published by the American Pain Society in the February issue of the Journal of Pain.
Postoperative pain management reportedly is inadequate for more than half of the adults and children who undergo surgery each year. In this setting, inadequate pain relief can contribute to impaired recovery, decreased physical function, postoperative complications, and poor quality of life, and it may even set the stage for the development of chronic pain, said Dr. Roger Chou, lead author of the guideline and professor of medicine and of medical informatics and clinical epidemiology, Oregon Health and Science University, Portland, and his associates.
The guideline includes 32 evidence-based recommendations for all clinicians who treat postoperative pain. It was compiled by a 23-member expert panel based on their analysis of 858 primary studies and 107 systematic reviews of the literature. Despite the panel’s efforts to obtain top-level evidence, of 32 recommendations, the panel rated only 4 as supported by high-quality evidence, and 11 recommendations were on the basis of low-quality evidence. The panel members had expertise in anesthesia, pain medicine, surgery, obstetrics and gynecology, primary care, pediatrics, hospital medicine, nursing, physical therapy, and psychology, said Dr. Chou, who is also director of the Pacific Northwest Evidence-Based Practice Center, Portland, and his associates.
The first strong recommendation, based on high-quality evidence, is to use a variety of medications and techniques that act on both the central and peripheral nervous systems and have been associated with both superior pain relief and decreased opioid consumption, compared with single agents or methods. For example, continuous nonopioid analgesia such as acetaminophen or NSAIDs can be combined with opioids that act on different receptors and with nonpharmacologic therapies. The guideline addresses several specific situations, and summarizes in a table the multimodal options for common surgeries such as laparotomy, total hip or knee replacement, coronary artery bypass graft, and cesarean section.
The guideline also strongly recommends that management of postsurgical pain should begin preoperatively, with assessment of the patient’s medical and psychiatric comorbidities; concomitant medications; and history of any chronic pain, substance abuse, and previous postoperative treatments and responses. Clinicians should use a validated pain assessment tool to track patients’ responses to postsurgical pain treatment and adjust treatment plans accordingly.
The guideline suggests that clinicians consider nonpharmacologic techniques including transcutaneous electrical nerve stimulation and cognitive-behavioral modalities such as guided imagery, relaxation methods, hypnosis, and intraoperative suggestion. At the very least, these are noninvasive and don’t appear to be associated with any harm. However, the guideline cautions that the evidence is insufficient to support cognitive-behavioral modalities in children. It also cannot recommend for or against several alternative treatments for which there is not yet sufficient evidence of efficacy, including acupuncture, massage, and cold therapy, Dr. Chou and his associates said (J Pain. 2016;17:131-57).
For adults who have no contraindications, a preoperative dose of celecoxib is strongly recommended because it improves postoperative pain and also reduces the need for opioids. One contraindication to celecoxib is CABG surgery, because of its attendant risk of adverse cardiovascular events. Preoperative doses of gabapentin or pregabalin also are strongly recommended as part of multimodal postsurgical analgesia, particularly for operations associated with substantial pain.
The guideline also strongly recommends epidural or spinal analgesia for major thoracic or abdominal procedures, especially for patients at risk for cardiac complications, pulmonary complications, or prolonged ileus. Compared with systemic analgesia, these forms appear to decrease the risk of death, venous thromboembolism, myocardial infarction, pneumonia, and respiratory depression. However, patients who receive neuraxial analgesia should still be carefully monitored, as they may develop respiratory depression, hypotension, or motor weakness, or the intervention might mask symptoms of compartment syndrome.
According to the guideline, all facilities that offer surgery should also provide clinicians with access to a pain specialist. The specialist should be available to consult for patients with inadequately controlled postoperative pain and to assist with diagnosis, interventions, or management of comorbid conditions. They may be especially useful for advising clinicians regarding patients who have opioid tolerance, especially if there is a history of substance abuse or addiction. Pain treatment should not be withheld from such patients “because of fears of worsening addiction or precipitation of relapse. In addition to the ethical requirement to address postoperative pain, poorly treated pain can be a trigger for relapse,” the guideline states.
The guideline also addresses the transition to outpatient care and identifies some research gaps. “Research is urgently needed on optimal methods for managing patients who receive opioids before surgery, effectiveness of opioid-sparing multimodal regimens, optimal methods of pain assessment and monitoring, and a number of areas related to management of perioperative pain in infants and children,” the panel concluded.
Funding for this guideline was provided by the American Pain Society. Dr. Chou’s and his associates’ financial disclosures are available at www.jpain.org.
Clinicians should use multiple approaches to target different pain mechanisms when treating postsurgical pain, according to a Clinical Practice Guideline for this aspect of patient care published by the American Pain Society in the February issue of the Journal of Pain.
Postoperative pain management reportedly is inadequate for more than half of the adults and children who undergo surgery each year. In this setting, inadequate pain relief can contribute to impaired recovery, decreased physical function, postoperative complications, and poor quality of life, and it may even set the stage for the development of chronic pain, said Dr. Roger Chou, lead author of the guideline and professor of medicine and of medical informatics and clinical epidemiology, Oregon Health and Science University, Portland, and his associates.
The guideline includes 32 evidence-based recommendations for all clinicians who treat postoperative pain. It was compiled by a 23-member expert panel based on their analysis of 858 primary studies and 107 systematic reviews of the literature. Despite the panel’s efforts to obtain top-level evidence, of 32 recommendations, the panel rated only 4 as supported by high-quality evidence, and 11 recommendations were on the basis of low-quality evidence. The panel members had expertise in anesthesia, pain medicine, surgery, obstetrics and gynecology, primary care, pediatrics, hospital medicine, nursing, physical therapy, and psychology, said Dr. Chou, who is also director of the Pacific Northwest Evidence-Based Practice Center, Portland, and his associates.
The first strong recommendation, based on high-quality evidence, is to use a variety of medications and techniques that act on both the central and peripheral nervous systems and have been associated with both superior pain relief and decreased opioid consumption, compared with single agents or methods. For example, continuous nonopioid analgesia such as acetaminophen or NSAIDs can be combined with opioids that act on different receptors and with nonpharmacologic therapies. The guideline addresses several specific situations, and summarizes in a table the multimodal options for common surgeries such as laparotomy, total hip or knee replacement, coronary artery bypass graft, and cesarean section.
The guideline also strongly recommends that management of postsurgical pain should begin preoperatively, with assessment of the patient’s medical and psychiatric comorbidities; concomitant medications; and history of any chronic pain, substance abuse, and previous postoperative treatments and responses. Clinicians should use a validated pain assessment tool to track patients’ responses to postsurgical pain treatment and adjust treatment plans accordingly.
The guideline suggests that clinicians consider nonpharmacologic techniques including transcutaneous electrical nerve stimulation and cognitive-behavioral modalities such as guided imagery, relaxation methods, hypnosis, and intraoperative suggestion. At the very least, these are noninvasive and don’t appear to be associated with any harm. However, the guideline cautions that the evidence is insufficient to support cognitive-behavioral modalities in children. It also cannot recommend for or against several alternative treatments for which there is not yet sufficient evidence of efficacy, including acupuncture, massage, and cold therapy, Dr. Chou and his associates said (J Pain. 2016;17:131-57).
For adults who have no contraindications, a preoperative dose of celecoxib is strongly recommended because it improves postoperative pain and also reduces the need for opioids. One contraindication to celecoxib is CABG surgery, because of its attendant risk of adverse cardiovascular events. Preoperative doses of gabapentin or pregabalin also are strongly recommended as part of multimodal postsurgical analgesia, particularly for operations associated with substantial pain.
The guideline also strongly recommends epidural or spinal analgesia for major thoracic or abdominal procedures, especially for patients at risk for cardiac complications, pulmonary complications, or prolonged ileus. Compared with systemic analgesia, these forms appear to decrease the risk of death, venous thromboembolism, myocardial infarction, pneumonia, and respiratory depression. However, patients who receive neuraxial analgesia should still be carefully monitored, as they may develop respiratory depression, hypotension, or motor weakness, or the intervention might mask symptoms of compartment syndrome.
According to the guideline, all facilities that offer surgery should also provide clinicians with access to a pain specialist. The specialist should be available to consult for patients with inadequately controlled postoperative pain and to assist with diagnosis, interventions, or management of comorbid conditions. They may be especially useful for advising clinicians regarding patients who have opioid tolerance, especially if there is a history of substance abuse or addiction. Pain treatment should not be withheld from such patients “because of fears of worsening addiction or precipitation of relapse. In addition to the ethical requirement to address postoperative pain, poorly treated pain can be a trigger for relapse,” the guideline states.
The guideline also addresses the transition to outpatient care and identifies some research gaps. “Research is urgently needed on optimal methods for managing patients who receive opioids before surgery, effectiveness of opioid-sparing multimodal regimens, optimal methods of pain assessment and monitoring, and a number of areas related to management of perioperative pain in infants and children,” the panel concluded.
Funding for this guideline was provided by the American Pain Society. Dr. Chou’s and his associates’ financial disclosures are available at www.jpain.org.
FROM THE JOURNAL OF PAIN
Post-TAVR mortality lower in women
Women undergoing transcatheter aortic valve replacement (TAVR) for severe aortic stenosis have lower 1-year mortality than men, even though their 30-day rates of vascular complications and major bleeding are worse, according to a report published online Feb. 23 in Annals of Internal Medicine.
“These findings for TAVR directly contrast with the abundant literature on aortic valve surgery, in which female sex has been shown to be an established risk factor for adverse prognosis,” said Dr. Susheel Kodali of Columbia University, New York and New York–Presbyterian Hospital, and his associates.
Previous studies assessing sex-specific differences in outcomes after TAVR, which have been small in size and limited to the experience at only one or two medical centers, have yielded conflicting results. Dr. Kodali and his associates examined sex-specific outcomes in what they described as the largest such study to date – a secondary analysis of data from a large clinical trial and a patient registry, involving 2,559 patients treated at 25 sites in the United States, Canada, and Germany. Their analysis involved nearly equal numbers of women (1,220) and men (1,339).
One-year unadjusted all-cause mortality was significantly lower for women (19.0%) than men (25.9%), for an HR of 0.72. In a further analysis that adjusted for numerous potential confounding factors, female sex remained independently associated with lower 1-year mortality, also with an HR of 0.72. Women also had a lower rate of rehospitalization at 1 year (15.8% vs. 18.9%; HR, 0.82). In contrast, stroke incidence did not differ significantly between women and men (5.2% vs. 4.5%; HR, 1.16).
Mortality was lower for women regardless of access route: 17.4% (vs. 24.0% in men) for the transfemoral approach and 20.8% (vs. 28.8% in men) for the transapical approach, the investigators said (Ann Intern Med. 2016 Feb 23. doi: 10.7326/M15-0121).
This mortality benefit occurred despite the fact that women had higher rates of vascular complications (17.3% vs. 10.0%) and of major bleeding (10.5% vs. 7.7%) at 30 days.
Although this study was not designed to elucidate why women have lower mortality than men following TAVR, there are several plausible reasons. First, men in this study had a greater burden of comorbid conditions than women.
Second, women had smaller annulus sizes and greater ejection fractions than men at baseline, and they also had less aortic regurgitation after undergoing the procedure. “These echocardiographic differences may [represent] better preoperative preservation of contractility and superior hemodynamics in women after the procedure,” Dr. Kodali and his associates said.
Third, previous studies have reported that after undergoing surgical aortic valve replacement, women with aortic stenosis have less cardiac fibrosis and more rapid left-ventricular remodeling than men do. “These salutary benefits may extend to women having TAVR” as well, the investigators added.
Women undergoing transcatheter aortic valve replacement (TAVR) for severe aortic stenosis have lower 1-year mortality than men, even though their 30-day rates of vascular complications and major bleeding are worse, according to a report published online Feb. 23 in Annals of Internal Medicine.
“These findings for TAVR directly contrast with the abundant literature on aortic valve surgery, in which female sex has been shown to be an established risk factor for adverse prognosis,” said Dr. Susheel Kodali of Columbia University, New York and New York–Presbyterian Hospital, and his associates.
Previous studies assessing sex-specific differences in outcomes after TAVR, which have been small in size and limited to the experience at only one or two medical centers, have yielded conflicting results. Dr. Kodali and his associates examined sex-specific outcomes in what they described as the largest such study to date – a secondary analysis of data from a large clinical trial and a patient registry, involving 2,559 patients treated at 25 sites in the United States, Canada, and Germany. Their analysis involved nearly equal numbers of women (1,220) and men (1,339).
One-year unadjusted all-cause mortality was significantly lower for women (19.0%) than men (25.9%), for an HR of 0.72. In a further analysis that adjusted for numerous potential confounding factors, female sex remained independently associated with lower 1-year mortality, also with an HR of 0.72. Women also had a lower rate of rehospitalization at 1 year (15.8% vs. 18.9%; HR, 0.82). In contrast, stroke incidence did not differ significantly between women and men (5.2% vs. 4.5%; HR, 1.16).
Mortality was lower for women regardless of access route: 17.4% (vs. 24.0% in men) for the transfemoral approach and 20.8% (vs. 28.8% in men) for the transapical approach, the investigators said (Ann Intern Med. 2016 Feb 23. doi: 10.7326/M15-0121).
This mortality benefit occurred despite the fact that women had higher rates of vascular complications (17.3% vs. 10.0%) and of major bleeding (10.5% vs. 7.7%) at 30 days.
Although this study was not designed to elucidate why women have lower mortality than men following TAVR, there are several plausible reasons. First, men in this study had a greater burden of comorbid conditions than women.
Second, women had smaller annulus sizes and greater ejection fractions than men at baseline, and they also had less aortic regurgitation after undergoing the procedure. “These echocardiographic differences may [represent] better preoperative preservation of contractility and superior hemodynamics in women after the procedure,” Dr. Kodali and his associates said.
Third, previous studies have reported that after undergoing surgical aortic valve replacement, women with aortic stenosis have less cardiac fibrosis and more rapid left-ventricular remodeling than men do. “These salutary benefits may extend to women having TAVR” as well, the investigators added.
Women undergoing transcatheter aortic valve replacement (TAVR) for severe aortic stenosis have lower 1-year mortality than men, even though their 30-day rates of vascular complications and major bleeding are worse, according to a report published online Feb. 23 in Annals of Internal Medicine.
“These findings for TAVR directly contrast with the abundant literature on aortic valve surgery, in which female sex has been shown to be an established risk factor for adverse prognosis,” said Dr. Susheel Kodali of Columbia University, New York and New York–Presbyterian Hospital, and his associates.
Previous studies assessing sex-specific differences in outcomes after TAVR, which have been small in size and limited to the experience at only one or two medical centers, have yielded conflicting results. Dr. Kodali and his associates examined sex-specific outcomes in what they described as the largest such study to date – a secondary analysis of data from a large clinical trial and a patient registry, involving 2,559 patients treated at 25 sites in the United States, Canada, and Germany. Their analysis involved nearly equal numbers of women (1,220) and men (1,339).
One-year unadjusted all-cause mortality was significantly lower for women (19.0%) than men (25.9%), for an HR of 0.72. In a further analysis that adjusted for numerous potential confounding factors, female sex remained independently associated with lower 1-year mortality, also with an HR of 0.72. Women also had a lower rate of rehospitalization at 1 year (15.8% vs. 18.9%; HR, 0.82). In contrast, stroke incidence did not differ significantly between women and men (5.2% vs. 4.5%; HR, 1.16).
Mortality was lower for women regardless of access route: 17.4% (vs. 24.0% in men) for the transfemoral approach and 20.8% (vs. 28.8% in men) for the transapical approach, the investigators said (Ann Intern Med. 2016 Feb 23. doi: 10.7326/M15-0121).
This mortality benefit occurred despite the fact that women had higher rates of vascular complications (17.3% vs. 10.0%) and of major bleeding (10.5% vs. 7.7%) at 30 days.
Although this study was not designed to elucidate why women have lower mortality than men following TAVR, there are several plausible reasons. First, men in this study had a greater burden of comorbid conditions than women.
Second, women had smaller annulus sizes and greater ejection fractions than men at baseline, and they also had less aortic regurgitation after undergoing the procedure. “These echocardiographic differences may [represent] better preoperative preservation of contractility and superior hemodynamics in women after the procedure,” Dr. Kodali and his associates said.
Third, previous studies have reported that after undergoing surgical aortic valve replacement, women with aortic stenosis have less cardiac fibrosis and more rapid left-ventricular remodeling than men do. “These salutary benefits may extend to women having TAVR” as well, the investigators added.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: One-year mortality after transcatheter aortic valve replacement is lower for women than men.
Major finding: One-year unadjusted all-cause mortality was significantly lower for women (19.0%) than men (25.9%), for an HR of 0.72.
Data source: A secondary analysis of data from a large international trial and patient registry, involving 1,220 women and 1,339 men undergoing TAVR.
Disclosures: This study was supported by Edwards Lifesciences, maker of the cardiac valves used in this study. Dr. Kodali reported ties to Edwards Lifesciences, Medtronic, and Thubrikar Aortic Valve; his associates reported ties to numerous industry sources.
Prednisolone, indomethacin similarly effective for acute gout
Oral prednisolone is as effective as indomethacin for relieving pain in acute gout and should be considered a first-line treatment option, according to a report published online Feb. 22 in Annals of Internal Medicine.
Colchicine and nonsteroidal anti-inflammatory drugs have been considered the first-line treatment for acute gout for many years. “However, their use is limited in elderly adults and in patients with comorbid conditions (such as renal insufficiency or gastrointestinal disease) because of their potential adverse effects and drug interactions,” said Dr. Timothy Hudson Rainer of the emergency medicine academic unit, Cardiff (Wales) University, and his associates.
They performed a double-blind, randomized trial comparing oral indomethacin against oral prednisolone in 416 patients who presented during a 2-year period to the emergency departments of four Hong Kong hospitals, where acute gout typically is treated in the ED. Those who were randomized to indomethacin initially received 50 mg (two 25-mg tablets) of the drug three times a day and six tablets of oral placebo prednisolone once a day for 2 days, followed by 25 mg of indomethacin three times a day and six tablets of placebo prednisolone once a day for 3 days. Prednisolone-treated patients initially received 30 mg (three 10-mg tablets) of the drug once a day and two tablets of placebo indomethacin three times a day for 2 days, followed by 30 mg (three 10-mg tablets) of prednisolone once a day and one tablet of placebo indomethacin three times a day for 3 days. All the patients received 1 g oral paracetamol to be taken every 6 hours as needed. The mean patient age was 65 years, and most (74%) of the study participants had a history of recurrent gout. The patients were followed for 2 weeks.
Scores on several measures of joint pain, redness, and tenderness were equivalent between the two treatment groups throughout the study period. During a 2-hour period at the emergency department, 100-mm visual analog scale (VAS) pain scores at rest declined by 6.54 mm/hour with indomethacin and by 5.05 mm/hour with prednisolone, and with activity, the declines were 11.69 mm/hour and 11.38 mm/hour, respectively. VAS scores declined during days 1-14 of treatment by similar mean amounts both at rest (1.80 mm/day for indomethacin and 1.68 mm/day for prednisolone) and with activity (2.96 mm/day vs. 3.19 mm/day, respectively). All VAS pain score improvements except for the one at rest in the ED exceeded 13 mm, meeting the definition for clinically meaningful improvement. The number of patients who showed clinically meaningful declines in pain scores also was equivalent between the two groups in both the intention-to-treat and the per-protocol analyses, the investigators said (Ann Intern Med. 2016 Feb 23. doi: 10.7326/M14-2070).
Both groups also showed similar responses in secondary endpoints of improvement in redness and tenderness of the affected joints, need for additional paracetamol, and patient satisfaction with analgesia.
There were no serious adverse events, but seven patients in the indomethacin group and one in the prednisolone group discontinued treatment because of adverse signs or symptoms. This included abdominal pain, dizziness, and lethargy among patients taking indomethacin and mild hyperkalemia in the patient taking prednisolone. The rate of minor adverse events was significantly higher with indomethacin (19%) than with prednisolone (6%).
“Our study provides robust evidence that oral corticosteroids are as effective at treating pain and as acceptable to patients as NSAIDs,” Dr. Rainer and his associates noted.
This trial was supported by the Hong Kong government’s Health and Health Services Research Grant Committee. Dr. Rainer and his associates reported having no relevant financial disclosures.
Oral prednisolone is as effective as indomethacin for relieving pain in acute gout and should be considered a first-line treatment option, according to a report published online Feb. 22 in Annals of Internal Medicine.
Colchicine and nonsteroidal anti-inflammatory drugs have been considered the first-line treatment for acute gout for many years. “However, their use is limited in elderly adults and in patients with comorbid conditions (such as renal insufficiency or gastrointestinal disease) because of their potential adverse effects and drug interactions,” said Dr. Timothy Hudson Rainer of the emergency medicine academic unit, Cardiff (Wales) University, and his associates.
They performed a double-blind, randomized trial comparing oral indomethacin against oral prednisolone in 416 patients who presented during a 2-year period to the emergency departments of four Hong Kong hospitals, where acute gout typically is treated in the ED. Those who were randomized to indomethacin initially received 50 mg (two 25-mg tablets) of the drug three times a day and six tablets of oral placebo prednisolone once a day for 2 days, followed by 25 mg of indomethacin three times a day and six tablets of placebo prednisolone once a day for 3 days. Prednisolone-treated patients initially received 30 mg (three 10-mg tablets) of the drug once a day and two tablets of placebo indomethacin three times a day for 2 days, followed by 30 mg (three 10-mg tablets) of prednisolone once a day and one tablet of placebo indomethacin three times a day for 3 days. All the patients received 1 g oral paracetamol to be taken every 6 hours as needed. The mean patient age was 65 years, and most (74%) of the study participants had a history of recurrent gout. The patients were followed for 2 weeks.
Scores on several measures of joint pain, redness, and tenderness were equivalent between the two treatment groups throughout the study period. During a 2-hour period at the emergency department, 100-mm visual analog scale (VAS) pain scores at rest declined by 6.54 mm/hour with indomethacin and by 5.05 mm/hour with prednisolone, and with activity, the declines were 11.69 mm/hour and 11.38 mm/hour, respectively. VAS scores declined during days 1-14 of treatment by similar mean amounts both at rest (1.80 mm/day for indomethacin and 1.68 mm/day for prednisolone) and with activity (2.96 mm/day vs. 3.19 mm/day, respectively). All VAS pain score improvements except for the one at rest in the ED exceeded 13 mm, meeting the definition for clinically meaningful improvement. The number of patients who showed clinically meaningful declines in pain scores also was equivalent between the two groups in both the intention-to-treat and the per-protocol analyses, the investigators said (Ann Intern Med. 2016 Feb 23. doi: 10.7326/M14-2070).
Both groups also showed similar responses in secondary endpoints of improvement in redness and tenderness of the affected joints, need for additional paracetamol, and patient satisfaction with analgesia.
There were no serious adverse events, but seven patients in the indomethacin group and one in the prednisolone group discontinued treatment because of adverse signs or symptoms. This included abdominal pain, dizziness, and lethargy among patients taking indomethacin and mild hyperkalemia in the patient taking prednisolone. The rate of minor adverse events was significantly higher with indomethacin (19%) than with prednisolone (6%).
“Our study provides robust evidence that oral corticosteroids are as effective at treating pain and as acceptable to patients as NSAIDs,” Dr. Rainer and his associates noted.
This trial was supported by the Hong Kong government’s Health and Health Services Research Grant Committee. Dr. Rainer and his associates reported having no relevant financial disclosures.
Oral prednisolone is as effective as indomethacin for relieving pain in acute gout and should be considered a first-line treatment option, according to a report published online Feb. 22 in Annals of Internal Medicine.
Colchicine and nonsteroidal anti-inflammatory drugs have been considered the first-line treatment for acute gout for many years. “However, their use is limited in elderly adults and in patients with comorbid conditions (such as renal insufficiency or gastrointestinal disease) because of their potential adverse effects and drug interactions,” said Dr. Timothy Hudson Rainer of the emergency medicine academic unit, Cardiff (Wales) University, and his associates.
They performed a double-blind, randomized trial comparing oral indomethacin against oral prednisolone in 416 patients who presented during a 2-year period to the emergency departments of four Hong Kong hospitals, where acute gout typically is treated in the ED. Those who were randomized to indomethacin initially received 50 mg (two 25-mg tablets) of the drug three times a day and six tablets of oral placebo prednisolone once a day for 2 days, followed by 25 mg of indomethacin three times a day and six tablets of placebo prednisolone once a day for 3 days. Prednisolone-treated patients initially received 30 mg (three 10-mg tablets) of the drug once a day and two tablets of placebo indomethacin three times a day for 2 days, followed by 30 mg (three 10-mg tablets) of prednisolone once a day and one tablet of placebo indomethacin three times a day for 3 days. All the patients received 1 g oral paracetamol to be taken every 6 hours as needed. The mean patient age was 65 years, and most (74%) of the study participants had a history of recurrent gout. The patients were followed for 2 weeks.
Scores on several measures of joint pain, redness, and tenderness were equivalent between the two treatment groups throughout the study period. During a 2-hour period at the emergency department, 100-mm visual analog scale (VAS) pain scores at rest declined by 6.54 mm/hour with indomethacin and by 5.05 mm/hour with prednisolone, and with activity, the declines were 11.69 mm/hour and 11.38 mm/hour, respectively. VAS scores declined during days 1-14 of treatment by similar mean amounts both at rest (1.80 mm/day for indomethacin and 1.68 mm/day for prednisolone) and with activity (2.96 mm/day vs. 3.19 mm/day, respectively). All VAS pain score improvements except for the one at rest in the ED exceeded 13 mm, meeting the definition for clinically meaningful improvement. The number of patients who showed clinically meaningful declines in pain scores also was equivalent between the two groups in both the intention-to-treat and the per-protocol analyses, the investigators said (Ann Intern Med. 2016 Feb 23. doi: 10.7326/M14-2070).
Both groups also showed similar responses in secondary endpoints of improvement in redness and tenderness of the affected joints, need for additional paracetamol, and patient satisfaction with analgesia.
There were no serious adverse events, but seven patients in the indomethacin group and one in the prednisolone group discontinued treatment because of adverse signs or symptoms. This included abdominal pain, dizziness, and lethargy among patients taking indomethacin and mild hyperkalemia in the patient taking prednisolone. The rate of minor adverse events was significantly higher with indomethacin (19%) than with prednisolone (6%).
“Our study provides robust evidence that oral corticosteroids are as effective at treating pain and as acceptable to patients as NSAIDs,” Dr. Rainer and his associates noted.
This trial was supported by the Hong Kong government’s Health and Health Services Research Grant Committee. Dr. Rainer and his associates reported having no relevant financial disclosures.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Oral prednisolone is as effective as indomethacin for relieving pain in acute gout and should be considered a first-line treatment option.
Major finding: VAS scores declined during days 1-14 of treatment by similar mean amounts both at rest (1.80 mm/day for indomethacin and 1.68 mm/day for prednisolone) and with activity (2.96 mm/day vs. 3.19 mm/day, respectively).
Data source: A multicenter, double-blind, randomized clinical trial involving 416 patients presenting to an ED for acute gout.
Disclosures: This trial was supported by the Hong Kong government’s Health and Health Services Research Grant Committee. Dr. Rainer and his associates reported having no relevant financial disclosures.
Cardiovascular Abnormalities Persist After Preeclamptic Pregnancy
For 12-18 months following a preeclamptic pregnancy, cardiovascular abnormalities persist in some women and appear to raise their risk for recurrent preeclampsia in a subsequent pregnancy, according to a case-control study published online Feb. 22 in Hypertension.
If these findings are confirmed in further studies, it might become possible to identify women at high risk for recurrent preeclampsia by performing a cardiovascular assessment before they attempt a second pregnancy, said Dr. Herbert Valensise of the department of obstetrics and gynecology, Tor Vergata University, Rome, and his associates.
An estimated 3%-8% of pregnancies are complicated by preeclampsia, and women who have preeclampsia once are seven times more likely to have a recurrence in subsequent pregnancies than are women who never had the disorder. At present, it is difficult to predict which women will have a recurrence. The investigators assessed cardiac function in women who had completed a first pregnancy and had not yet attempted a second pregnancy; 75 of these women had early preeclampsia during their initial pregnancies and were compared with 147 control subjects who did not. All these study participants were then followed through their second pregnancies to determine whether preeclampsia recurred.
The 22 women who had recurrent preeclampsia in their second pregnancies showed several cardiovascular abnormalities during the nonpregnant state, compared with the control subjects and the 53 women who did not develop preeclampsia in their second pregnancies. The affected women had lower stroke volume (63 mL vs. 73 mL and 70 mL, respectively), elevated total vascular resistance (1,638 vs. 1,341 and 1,383 dyne·s−1·cm−5), lower cardiac output (4.6 vs. 5.3 and 5.2 L), higher diastolic blood pressure (77 vs. 68 and 69 mm Hg), greater thickness of the left-ventricular wall (35 vs. 28 and 33), and “a tendency to a concentric geometry of the left ventricle,” Dr. Valensise and his associates said. All differences were statistically significant at P less than .05 (Hypertension. 2016 Feb 22. doi: 10.1161/HYPERTENSIONAHA.115.06674).
These findings “suggest that cardiac dysfunction and cardiovascular maladaptation precede the appearance of a recurrent preeclampsia months before a second pregnancy,” they said.
The preeclampsia during the first pregnancy was no more severe among women who had a recurrence than it was among the women who did not, so the severity of the first episode was not a useful guide for predicting recurrence, the investigators added.
No funding source was identified for this study. Dr. Valensise and his associates reported having no relevant financial disclosures.
For 12-18 months following a preeclamptic pregnancy, cardiovascular abnormalities persist in some women and appear to raise their risk for recurrent preeclampsia in a subsequent pregnancy, according to a case-control study published online Feb. 22 in Hypertension.
If these findings are confirmed in further studies, it might become possible to identify women at high risk for recurrent preeclampsia by performing a cardiovascular assessment before they attempt a second pregnancy, said Dr. Herbert Valensise of the department of obstetrics and gynecology, Tor Vergata University, Rome, and his associates.
An estimated 3%-8% of pregnancies are complicated by preeclampsia, and women who have preeclampsia once are seven times more likely to have a recurrence in subsequent pregnancies than are women who never had the disorder. At present, it is difficult to predict which women will have a recurrence. The investigators assessed cardiac function in women who had completed a first pregnancy and had not yet attempted a second pregnancy; 75 of these women had early preeclampsia during their initial pregnancies and were compared with 147 control subjects who did not. All these study participants were then followed through their second pregnancies to determine whether preeclampsia recurred.
The 22 women who had recurrent preeclampsia in their second pregnancies showed several cardiovascular abnormalities during the nonpregnant state, compared with the control subjects and the 53 women who did not develop preeclampsia in their second pregnancies. The affected women had lower stroke volume (63 mL vs. 73 mL and 70 mL, respectively), elevated total vascular resistance (1,638 vs. 1,341 and 1,383 dyne·s−1·cm−5), lower cardiac output (4.6 vs. 5.3 and 5.2 L), higher diastolic blood pressure (77 vs. 68 and 69 mm Hg), greater thickness of the left-ventricular wall (35 vs. 28 and 33), and “a tendency to a concentric geometry of the left ventricle,” Dr. Valensise and his associates said. All differences were statistically significant at P less than .05 (Hypertension. 2016 Feb 22. doi: 10.1161/HYPERTENSIONAHA.115.06674).
These findings “suggest that cardiac dysfunction and cardiovascular maladaptation precede the appearance of a recurrent preeclampsia months before a second pregnancy,” they said.
The preeclampsia during the first pregnancy was no more severe among women who had a recurrence than it was among the women who did not, so the severity of the first episode was not a useful guide for predicting recurrence, the investigators added.
No funding source was identified for this study. Dr. Valensise and his associates reported having no relevant financial disclosures.
For 12-18 months following a preeclamptic pregnancy, cardiovascular abnormalities persist in some women and appear to raise their risk for recurrent preeclampsia in a subsequent pregnancy, according to a case-control study published online Feb. 22 in Hypertension.
If these findings are confirmed in further studies, it might become possible to identify women at high risk for recurrent preeclampsia by performing a cardiovascular assessment before they attempt a second pregnancy, said Dr. Herbert Valensise of the department of obstetrics and gynecology, Tor Vergata University, Rome, and his associates.
An estimated 3%-8% of pregnancies are complicated by preeclampsia, and women who have preeclampsia once are seven times more likely to have a recurrence in subsequent pregnancies than are women who never had the disorder. At present, it is difficult to predict which women will have a recurrence. The investigators assessed cardiac function in women who had completed a first pregnancy and had not yet attempted a second pregnancy; 75 of these women had early preeclampsia during their initial pregnancies and were compared with 147 control subjects who did not. All these study participants were then followed through their second pregnancies to determine whether preeclampsia recurred.
The 22 women who had recurrent preeclampsia in their second pregnancies showed several cardiovascular abnormalities during the nonpregnant state, compared with the control subjects and the 53 women who did not develop preeclampsia in their second pregnancies. The affected women had lower stroke volume (63 mL vs. 73 mL and 70 mL, respectively), elevated total vascular resistance (1,638 vs. 1,341 and 1,383 dyne·s−1·cm−5), lower cardiac output (4.6 vs. 5.3 and 5.2 L), higher diastolic blood pressure (77 vs. 68 and 69 mm Hg), greater thickness of the left-ventricular wall (35 vs. 28 and 33), and “a tendency to a concentric geometry of the left ventricle,” Dr. Valensise and his associates said. All differences were statistically significant at P less than .05 (Hypertension. 2016 Feb 22. doi: 10.1161/HYPERTENSIONAHA.115.06674).
These findings “suggest that cardiac dysfunction and cardiovascular maladaptation precede the appearance of a recurrent preeclampsia months before a second pregnancy,” they said.
The preeclampsia during the first pregnancy was no more severe among women who had a recurrence than it was among the women who did not, so the severity of the first episode was not a useful guide for predicting recurrence, the investigators added.
No funding source was identified for this study. Dr. Valensise and his associates reported having no relevant financial disclosures.
FROM HYPERTENSION
Cardiovascular abnormalities persist after preeclamptic pregnancy
For 12-18 months following a preeclamptic pregnancy, cardiovascular abnormalities persist in some women and appear to raise their risk for recurrent preeclampsia in a subsequent pregnancy, according to a case-control study published online Feb. 22 in Hypertension.
If these findings are confirmed in further studies, it might become possible to identify women at high risk for recurrent preeclampsia by performing a cardiovascular assessment before they attempt a second pregnancy, said Dr. Herbert Valensise of the department of obstetrics and gynecology, Tor Vergata University, Rome, and his associates.
An estimated 3%-8% of pregnancies are complicated by preeclampsia, and women who have preeclampsia once are seven times more likely to have a recurrence in subsequent pregnancies than are women who never had the disorder. At present, it is difficult to predict which women will have a recurrence. The investigators assessed cardiac function in women who had completed a first pregnancy and had not yet attempted a second pregnancy; 75 of these women had early preeclampsia during their initial pregnancies and were compared with 147 control subjects who did not. All these study participants were then followed through their second pregnancies to determine whether preeclampsia recurred.
The 22 women who had recurrent preeclampsia in their second pregnancies showed several cardiovascular abnormalities during the nonpregnant state, compared with the control subjects and the 53 women who did not develop preeclampsia in their second pregnancies. The affected women had lower stroke volume (63 mL vs. 73 mL and 70 mL, respectively), elevated total vascular resistance (1,638 vs. 1,341 and 1,383 dyne·s−1·cm−5), lower cardiac output (4.6 vs. 5.3 and 5.2 L), higher diastolic blood pressure (77 vs. 68 and 69 mm Hg), greater thickness of the left-ventricular wall (35 vs. 28 and 33), and “a tendency to a concentric geometry of the left ventricle,” Dr. Valensise and his associates said. All differences were statistically significant at P less than .05 (Hypertension. 2016 Feb 22. doi: 10.1161/HYPERTENSIONAHA.115.06674).
These findings “suggest that cardiac dysfunction and cardiovascular maladaptation precede the appearance of a recurrent preeclampsia months before a second pregnancy,” they said.
The preeclampsia during the first pregnancy was no more severe among women who had a recurrence than it was among the women who did not, so the severity of the first episode was not a useful guide for predicting recurrence, the investigators added.
No funding source was identified for this study. Dr. Valensise and his associates reported having no relevant financial disclosures.
For 12-18 months following a preeclamptic pregnancy, cardiovascular abnormalities persist in some women and appear to raise their risk for recurrent preeclampsia in a subsequent pregnancy, according to a case-control study published online Feb. 22 in Hypertension.
If these findings are confirmed in further studies, it might become possible to identify women at high risk for recurrent preeclampsia by performing a cardiovascular assessment before they attempt a second pregnancy, said Dr. Herbert Valensise of the department of obstetrics and gynecology, Tor Vergata University, Rome, and his associates.
An estimated 3%-8% of pregnancies are complicated by preeclampsia, and women who have preeclampsia once are seven times more likely to have a recurrence in subsequent pregnancies than are women who never had the disorder. At present, it is difficult to predict which women will have a recurrence. The investigators assessed cardiac function in women who had completed a first pregnancy and had not yet attempted a second pregnancy; 75 of these women had early preeclampsia during their initial pregnancies and were compared with 147 control subjects who did not. All these study participants were then followed through their second pregnancies to determine whether preeclampsia recurred.
The 22 women who had recurrent preeclampsia in their second pregnancies showed several cardiovascular abnormalities during the nonpregnant state, compared with the control subjects and the 53 women who did not develop preeclampsia in their second pregnancies. The affected women had lower stroke volume (63 mL vs. 73 mL and 70 mL, respectively), elevated total vascular resistance (1,638 vs. 1,341 and 1,383 dyne·s−1·cm−5), lower cardiac output (4.6 vs. 5.3 and 5.2 L), higher diastolic blood pressure (77 vs. 68 and 69 mm Hg), greater thickness of the left-ventricular wall (35 vs. 28 and 33), and “a tendency to a concentric geometry of the left ventricle,” Dr. Valensise and his associates said. All differences were statistically significant at P less than .05 (Hypertension. 2016 Feb 22. doi: 10.1161/HYPERTENSIONAHA.115.06674).
These findings “suggest that cardiac dysfunction and cardiovascular maladaptation precede the appearance of a recurrent preeclampsia months before a second pregnancy,” they said.
The preeclampsia during the first pregnancy was no more severe among women who had a recurrence than it was among the women who did not, so the severity of the first episode was not a useful guide for predicting recurrence, the investigators added.
No funding source was identified for this study. Dr. Valensise and his associates reported having no relevant financial disclosures.
For 12-18 months following a preeclamptic pregnancy, cardiovascular abnormalities persist in some women and appear to raise their risk for recurrent preeclampsia in a subsequent pregnancy, according to a case-control study published online Feb. 22 in Hypertension.
If these findings are confirmed in further studies, it might become possible to identify women at high risk for recurrent preeclampsia by performing a cardiovascular assessment before they attempt a second pregnancy, said Dr. Herbert Valensise of the department of obstetrics and gynecology, Tor Vergata University, Rome, and his associates.
An estimated 3%-8% of pregnancies are complicated by preeclampsia, and women who have preeclampsia once are seven times more likely to have a recurrence in subsequent pregnancies than are women who never had the disorder. At present, it is difficult to predict which women will have a recurrence. The investigators assessed cardiac function in women who had completed a first pregnancy and had not yet attempted a second pregnancy; 75 of these women had early preeclampsia during their initial pregnancies and were compared with 147 control subjects who did not. All these study participants were then followed through their second pregnancies to determine whether preeclampsia recurred.
The 22 women who had recurrent preeclampsia in their second pregnancies showed several cardiovascular abnormalities during the nonpregnant state, compared with the control subjects and the 53 women who did not develop preeclampsia in their second pregnancies. The affected women had lower stroke volume (63 mL vs. 73 mL and 70 mL, respectively), elevated total vascular resistance (1,638 vs. 1,341 and 1,383 dyne·s−1·cm−5), lower cardiac output (4.6 vs. 5.3 and 5.2 L), higher diastolic blood pressure (77 vs. 68 and 69 mm Hg), greater thickness of the left-ventricular wall (35 vs. 28 and 33), and “a tendency to a concentric geometry of the left ventricle,” Dr. Valensise and his associates said. All differences were statistically significant at P less than .05 (Hypertension. 2016 Feb 22. doi: 10.1161/HYPERTENSIONAHA.115.06674).
These findings “suggest that cardiac dysfunction and cardiovascular maladaptation precede the appearance of a recurrent preeclampsia months before a second pregnancy,” they said.
The preeclampsia during the first pregnancy was no more severe among women who had a recurrence than it was among the women who did not, so the severity of the first episode was not a useful guide for predicting recurrence, the investigators added.
No funding source was identified for this study. Dr. Valensise and his associates reported having no relevant financial disclosures.
FROM HYPERTENSION
Key clinical point: For months following a preeclamptic pregnancy, cardiovascular abnormalities persist in some women and appear to raise their risk for recurrent preeclampsia in a subsequent pregnancy.
Major finding: In the nonpregnant state, women who went on to develop recurrent preeclampsia showed elevated total vascular resistance, lower cardiac output, higher diastolic blood pressure, greater thickness of the left-ventricular wall, and concentric geometry of the left ventricle.
Data source: A case-control study comparing CV abnormalities between 75 nonpregnant women who had preeclampsia during a previous pregnancy and 147 control subjects who did not.
Disclosures: No funding source was identified for this study. Dr. Valensise and his associates reported having no relevant financial disclosures.
Testosterone: Moderate sexual, mood benefits; no vitality benefit
Testosterone therapy provides small to moderate benefits in sexual function and mood, but none in walking distance or vitality among symptomatic older men with low serum levels of the hormone, according to a report published online February 18 in the New England Journal of Medicine.
These are among the long-awaited findings of the first three of the seven testosterone trials, which all are parallel randomized double-blind placebo-controlled studies involving 790 men at 12 sites and which were called for by the Institute of Medicine because existing evidence supporting the use of testosterone treatment was inadequate and equivocal. All seven studies have been completed, but only the results of the sexual function trial, the physical function trial, and the vitality trial are reported here.
The findings apply only to men similar to the study participants: those aged 65 years or older who have average serum testosterone levels of less than 275 ng/dL and report corresponding sexual, physical, or mood symptoms. The testosterone trials were funded chiefly by the National Institutes of Health; AbbVie also provided financial support and donated the testosterone and placebo gels used in the studies, but did not participate in the design or conduct of the trials or in the collection, analysis, or reporting of the data, said Dr. Peter J. Snyder of the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and his associates.
The investigators noted that relatively few (14.7%) of the more than 51,000 men who were screened to participate in the testosterone trials had sufficiently low testosterone to qualify, and only 790 men (1.5%) ultimately enrolled. The average age was 72 years, and approximately 90% of the participants were white. Coexisting conditions were common, including obesity (63%), hypertension (72%), a history of MI (15%), diabetes (33%), and sleep apnea (20%).
Compared with placebo, 1 year of testosterone treatment raised serum levels to the mid-normal range for younger men (those aged 19-40 years).
The primary outcome of the sexual function trial – change from baseline in the score for sexual activity on the psychosexual daily questionnaire – increased more in men who received active treatment than in those who received placebo. Secondary outcomes of increased sexual desire and increased erectile function also favored the testosterone group, and participants in that group were more likely to report that their sexual desire had improved with treatment. The findings were similar across participants in all three of the testosterone trials, the investigators said (New Engl J Med. 2016 Feb 18 doi: 10.1056/NEJMoa1506119). The primary outcome of the physical function trial, the percentage of men who increased their distance on the 6-minute walk test by at least 50 meters, was not significantly different between the testosterone (20.3%) and placebo (12.1%) groups, nor was the change from baseline in 6-minute walk distance or the percentage of men whose score on the physical-function domain of the short-form health survey increased by 8 points or more. However, when participants in all three testosterone trials were considered as a whole, the active-treatment group showed significantly greater improvement in all these domains. And men in the testosterone group were more likely to report that their walking ability had improved with treatment.
The primary outcome of the vitality trial, an increase of at least 4 points in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale, also did not differ between the men who received testosterone and those who received placebo. But again, when participants in all three testosterone trials were considered as a whole, men in the testosterone group showed a greater improvement since baseline in SF-36 vitality scores, as well as greater increases in Positive and Negative Affect Schedule (PANAS) positive-affect scores and greater declines in PANAS negative-affect scores. The men given testosterone also were more likely to report that their energy was better after treatment.
These findings suggest that even though testosterone therapy had only small to moderate effects on sexual function and on some measures of physical function and mood, those effects might be clinically relevant, Dr. Snyder and his associates said.
Regarding adverse effects, 3 cases of prostate cancer developed in the active-treatment group and 1 in the placebo group, and there was no difference between the two groups in urinary symptoms. However, the sample size was too small to reliably assess the treatment’s effect on risk of these conditions. Similarly, no pattern of increased cardiovascular risk was identified, but the sample size was too small to detect anything but a large increase in this risk, they added.
This study was supported by the National Institute on Aging, the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, AbbVie, and several other groups. Dr. Snyder reported receiving consulting fees from Watson Laboratories; his associates reported ties to numerous industry sources.
Data from these three trials show that testosterone supplementation yields some benefits, but they were modest, tended to wane toward the end of the year of treatment, and were not as robust as those reported with use of phosphodiesterase type 5 inhibitors. These results might be insufficient to support a decision to initiate testosterone therapy in symptomatic older men.
Although no major toxicities were apparent and effects on hemoglobin and prostate-specific antigen levels appeared to be minor, larger and more extended trials are needed to establish the adverse effects of testosterone supplementation, especially regarding prostate and cardiovascular health.
In addition, the fact that only 1.5% of the more than 51,000 men who were screened were actually enrolled in these studies clearly limits the generalizability of their conclusions. Most testosterone prescriptions are written for middle-aged men, but we cannot assume that the benefits, lack of benefits, and adverse-event profiles of these study participants apply to younger men, those with higher testosterone levels, or those with other demographic and clinical characteristics.
Dr. Eric S. Orwoll is in the department of medicine at Oregon Health and Science University, Portland. He reported receiving grants and personal fees from Eli Lilly, Merck, and Amgen. Dr. Orwoll made these remarks in an editorial accompanying Dr. Snyder’s report (New Engl J Med. 2016 Feb 18. doi: 10.1056/NEJMe1600196).
Data from these three trials show that testosterone supplementation yields some benefits, but they were modest, tended to wane toward the end of the year of treatment, and were not as robust as those reported with use of phosphodiesterase type 5 inhibitors. These results might be insufficient to support a decision to initiate testosterone therapy in symptomatic older men.
Although no major toxicities were apparent and effects on hemoglobin and prostate-specific antigen levels appeared to be minor, larger and more extended trials are needed to establish the adverse effects of testosterone supplementation, especially regarding prostate and cardiovascular health.
In addition, the fact that only 1.5% of the more than 51,000 men who were screened were actually enrolled in these studies clearly limits the generalizability of their conclusions. Most testosterone prescriptions are written for middle-aged men, but we cannot assume that the benefits, lack of benefits, and adverse-event profiles of these study participants apply to younger men, those with higher testosterone levels, or those with other demographic and clinical characteristics.
Dr. Eric S. Orwoll is in the department of medicine at Oregon Health and Science University, Portland. He reported receiving grants and personal fees from Eli Lilly, Merck, and Amgen. Dr. Orwoll made these remarks in an editorial accompanying Dr. Snyder’s report (New Engl J Med. 2016 Feb 18. doi: 10.1056/NEJMe1600196).
Data from these three trials show that testosterone supplementation yields some benefits, but they were modest, tended to wane toward the end of the year of treatment, and were not as robust as those reported with use of phosphodiesterase type 5 inhibitors. These results might be insufficient to support a decision to initiate testosterone therapy in symptomatic older men.
Although no major toxicities were apparent and effects on hemoglobin and prostate-specific antigen levels appeared to be minor, larger and more extended trials are needed to establish the adverse effects of testosterone supplementation, especially regarding prostate and cardiovascular health.
In addition, the fact that only 1.5% of the more than 51,000 men who were screened were actually enrolled in these studies clearly limits the generalizability of their conclusions. Most testosterone prescriptions are written for middle-aged men, but we cannot assume that the benefits, lack of benefits, and adverse-event profiles of these study participants apply to younger men, those with higher testosterone levels, or those with other demographic and clinical characteristics.
Dr. Eric S. Orwoll is in the department of medicine at Oregon Health and Science University, Portland. He reported receiving grants and personal fees from Eli Lilly, Merck, and Amgen. Dr. Orwoll made these remarks in an editorial accompanying Dr. Snyder’s report (New Engl J Med. 2016 Feb 18. doi: 10.1056/NEJMe1600196).
Testosterone therapy provides small to moderate benefits in sexual function and mood, but none in walking distance or vitality among symptomatic older men with low serum levels of the hormone, according to a report published online February 18 in the New England Journal of Medicine.
These are among the long-awaited findings of the first three of the seven testosterone trials, which all are parallel randomized double-blind placebo-controlled studies involving 790 men at 12 sites and which were called for by the Institute of Medicine because existing evidence supporting the use of testosterone treatment was inadequate and equivocal. All seven studies have been completed, but only the results of the sexual function trial, the physical function trial, and the vitality trial are reported here.
The findings apply only to men similar to the study participants: those aged 65 years or older who have average serum testosterone levels of less than 275 ng/dL and report corresponding sexual, physical, or mood symptoms. The testosterone trials were funded chiefly by the National Institutes of Health; AbbVie also provided financial support and donated the testosterone and placebo gels used in the studies, but did not participate in the design or conduct of the trials or in the collection, analysis, or reporting of the data, said Dr. Peter J. Snyder of the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and his associates.
The investigators noted that relatively few (14.7%) of the more than 51,000 men who were screened to participate in the testosterone trials had sufficiently low testosterone to qualify, and only 790 men (1.5%) ultimately enrolled. The average age was 72 years, and approximately 90% of the participants were white. Coexisting conditions were common, including obesity (63%), hypertension (72%), a history of MI (15%), diabetes (33%), and sleep apnea (20%).
Compared with placebo, 1 year of testosterone treatment raised serum levels to the mid-normal range for younger men (those aged 19-40 years).
The primary outcome of the sexual function trial – change from baseline in the score for sexual activity on the psychosexual daily questionnaire – increased more in men who received active treatment than in those who received placebo. Secondary outcomes of increased sexual desire and increased erectile function also favored the testosterone group, and participants in that group were more likely to report that their sexual desire had improved with treatment. The findings were similar across participants in all three of the testosterone trials, the investigators said (New Engl J Med. 2016 Feb 18 doi: 10.1056/NEJMoa1506119). The primary outcome of the physical function trial, the percentage of men who increased their distance on the 6-minute walk test by at least 50 meters, was not significantly different between the testosterone (20.3%) and placebo (12.1%) groups, nor was the change from baseline in 6-minute walk distance or the percentage of men whose score on the physical-function domain of the short-form health survey increased by 8 points or more. However, when participants in all three testosterone trials were considered as a whole, the active-treatment group showed significantly greater improvement in all these domains. And men in the testosterone group were more likely to report that their walking ability had improved with treatment.
The primary outcome of the vitality trial, an increase of at least 4 points in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale, also did not differ between the men who received testosterone and those who received placebo. But again, when participants in all three testosterone trials were considered as a whole, men in the testosterone group showed a greater improvement since baseline in SF-36 vitality scores, as well as greater increases in Positive and Negative Affect Schedule (PANAS) positive-affect scores and greater declines in PANAS negative-affect scores. The men given testosterone also were more likely to report that their energy was better after treatment.
These findings suggest that even though testosterone therapy had only small to moderate effects on sexual function and on some measures of physical function and mood, those effects might be clinically relevant, Dr. Snyder and his associates said.
Regarding adverse effects, 3 cases of prostate cancer developed in the active-treatment group and 1 in the placebo group, and there was no difference between the two groups in urinary symptoms. However, the sample size was too small to reliably assess the treatment’s effect on risk of these conditions. Similarly, no pattern of increased cardiovascular risk was identified, but the sample size was too small to detect anything but a large increase in this risk, they added.
This study was supported by the National Institute on Aging, the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, AbbVie, and several other groups. Dr. Snyder reported receiving consulting fees from Watson Laboratories; his associates reported ties to numerous industry sources.
Testosterone therapy provides small to moderate benefits in sexual function and mood, but none in walking distance or vitality among symptomatic older men with low serum levels of the hormone, according to a report published online February 18 in the New England Journal of Medicine.
These are among the long-awaited findings of the first three of the seven testosterone trials, which all are parallel randomized double-blind placebo-controlled studies involving 790 men at 12 sites and which were called for by the Institute of Medicine because existing evidence supporting the use of testosterone treatment was inadequate and equivocal. All seven studies have been completed, but only the results of the sexual function trial, the physical function trial, and the vitality trial are reported here.
The findings apply only to men similar to the study participants: those aged 65 years or older who have average serum testosterone levels of less than 275 ng/dL and report corresponding sexual, physical, or mood symptoms. The testosterone trials were funded chiefly by the National Institutes of Health; AbbVie also provided financial support and donated the testosterone and placebo gels used in the studies, but did not participate in the design or conduct of the trials or in the collection, analysis, or reporting of the data, said Dr. Peter J. Snyder of the division of endocrinology, diabetes, and metabolism at the University of Pennsylvania, Philadelphia, and his associates.
The investigators noted that relatively few (14.7%) of the more than 51,000 men who were screened to participate in the testosterone trials had sufficiently low testosterone to qualify, and only 790 men (1.5%) ultimately enrolled. The average age was 72 years, and approximately 90% of the participants were white. Coexisting conditions were common, including obesity (63%), hypertension (72%), a history of MI (15%), diabetes (33%), and sleep apnea (20%).
Compared with placebo, 1 year of testosterone treatment raised serum levels to the mid-normal range for younger men (those aged 19-40 years).
The primary outcome of the sexual function trial – change from baseline in the score for sexual activity on the psychosexual daily questionnaire – increased more in men who received active treatment than in those who received placebo. Secondary outcomes of increased sexual desire and increased erectile function also favored the testosterone group, and participants in that group were more likely to report that their sexual desire had improved with treatment. The findings were similar across participants in all three of the testosterone trials, the investigators said (New Engl J Med. 2016 Feb 18 doi: 10.1056/NEJMoa1506119). The primary outcome of the physical function trial, the percentage of men who increased their distance on the 6-minute walk test by at least 50 meters, was not significantly different between the testosterone (20.3%) and placebo (12.1%) groups, nor was the change from baseline in 6-minute walk distance or the percentage of men whose score on the physical-function domain of the short-form health survey increased by 8 points or more. However, when participants in all three testosterone trials were considered as a whole, the active-treatment group showed significantly greater improvement in all these domains. And men in the testosterone group were more likely to report that their walking ability had improved with treatment.
The primary outcome of the vitality trial, an increase of at least 4 points in Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) scale, also did not differ between the men who received testosterone and those who received placebo. But again, when participants in all three testosterone trials were considered as a whole, men in the testosterone group showed a greater improvement since baseline in SF-36 vitality scores, as well as greater increases in Positive and Negative Affect Schedule (PANAS) positive-affect scores and greater declines in PANAS negative-affect scores. The men given testosterone also were more likely to report that their energy was better after treatment.
These findings suggest that even though testosterone therapy had only small to moderate effects on sexual function and on some measures of physical function and mood, those effects might be clinically relevant, Dr. Snyder and his associates said.
Regarding adverse effects, 3 cases of prostate cancer developed in the active-treatment group and 1 in the placebo group, and there was no difference between the two groups in urinary symptoms. However, the sample size was too small to reliably assess the treatment’s effect on risk of these conditions. Similarly, no pattern of increased cardiovascular risk was identified, but the sample size was too small to detect anything but a large increase in this risk, they added.
This study was supported by the National Institute on Aging, the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, AbbVie, and several other groups. Dr. Snyder reported receiving consulting fees from Watson Laboratories; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Testosterone therapy provides small to moderate benefits in sexual function and mood but none in walking distance or vitality among older men with low serum levels of the hormone.
Major finding: The percentage of men who increased their distance on the 6-minute walk test by at least 50 meters was not significantly different between the testosterone and placebo groups (20.3% vs. 12.1%), for an OR of 1.42.
Data source: Three multicenter independent but parallel double-blind placebo-controlled randomized clinical trials involving 790 men treated for 1 year.
Disclosures: This study was supported by the National Institute on Aging, the National Heart, Lung, and Blood Institute, the National Institute of Neurological Disorders and Stroke, AbbVie, and several other groups. Dr. Snyder reported receiving consulting fees from Watson Laboratories; his associates reported ties to numerous industry sources.