User login
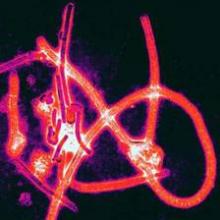
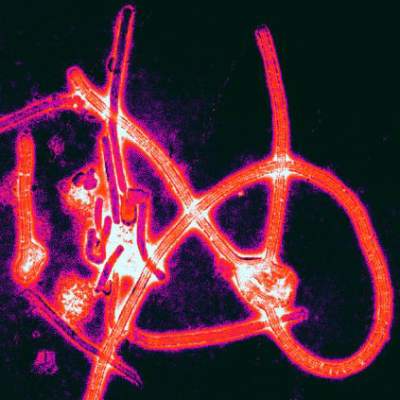
Ebola patients treated in the West showed 81.5% survival
Overall survival was 81.5% for the 27 patients with Ebola virus infection who were treated in the United States or Europe during the recent outbreak, according to a report published online Feb. 18 in the New England Journal of Medicine.
This is markedly higher than the 37%-74% survival reported for the almost 29,000 cases treated in West Africa, where treatment centers were challenged by overwhelming numbers of critically ill patients; limited medical supplies; insufficient numbers of caregivers; limited water, electricity, refrigeration, and other basic resources; and hot, humid working conditions that reduced the time health care personnel could attend to patients while wearing the required protective gear, said Dr. Timothy M. Uyeki of the Centers for Disease Control and Prevention, Atlanta, and his associates.
The investigators performed a retrospective analysis of the medical records of these 27 patients in a descriptive study of their clinical care. The patients were treated from August 2014 through December 2015 at 15 hospitals in 9 countries. Twenty (74%) were medically evacuated from West Africa, three (11%) were Western health care personnel who acquired the disease while caring for patients, and four were “imported” patients who contracted the virus while in West Africa but didn’t become ill until after they traveled to the United States or Europe. Overall, 22 of the patients (81%) were health care personnel, of whom 17 (77%) contracted the virus in West Africa.
Overall mortality was 11.1% after 2 weeks of illness and 18.5% after 4 weeks. The five patients who died were all aged 42 years or older and were significantly older than those who survived. Being 40-45 years old or older has been identified as a risk factor in West Africa as well. Those who died also were hospitalized significantly later after the onset of illness. At least six more patients would have died if they hadn’t received advanced organ support: two required noninvasive ventilation, two required invasive mechanical ventilation, and two required both invasive mechanical ventilation and continuous renal-replacement therapy.
Routine therapies included oral or intravenous fluid replacement, total parenteral nutrition, antiemetics, empiric treatment with multiple antibiotics, nonconvalescent blood products, and respiratory supportive care. Most patients also received investigative therapies such as monoclonal antibody cocktails, antivirals, and treatments aimed at counteracting vascular leakage.
A wide range of possible adverse effects were reported – including systemic inflammatory response syndrome, hypotension, elevated aminotransferase levels, and transfusion-associated acute lung injury – but couldn’t be distinguished from the effects of supportive treatments or even of the virus itself “because of their uncontrolled administration and because most patients received multiple, overlapping investigational therapies,” the researchers said (N Engl J Med. 2016 Feb 18;374:636-46. doi: 10.1056/NEJMoa1504874).
“A key feature” of the lifesaving clinical care was laboratory testing to closely monitor electrolyte levels and hematologic factors. “Our experience suggests that early presentation and receipt of supportive care, IV fluid resuscitation, careful fluid management and electrolyte replacement to correct metabolic abnormalities, nutritional support, and critical care support may reduce mortality among patients with Ebola virus disease,” Dr. Uyeki and his associates said.
They noted that, until now, the infection was not thought to involve a clinically significant respiratory component; however, eight of these patients (30%) presented with cough and nine required ventilatory assistance, and difficulty breathing raised the risk of death.
“The pathophysiological mechanism of pulmonary disease in patients with Ebola virus infection is unknown, but there could be multiple contributing factors, including vascular leak from endothelial infection or cytokine dysregulation or direct damage to Ebola-infected [pulmonary] cells,” they wrote. Aggressive fluid repletion and lung injury from investigational treatments also may have contributed to respiratory involvement.
This study was supported by the working group of the U.S.–European Clinical Network on Clinical Management of Ebola Virus Disease Patients in the U.S. and Europe. Dr. Uyeki reported having no relevant financial disclosures; two of his associates reported ties to Epiguard and Pfizer.
Overall survival was 81.5% for the 27 patients with Ebola virus infection who were treated in the United States or Europe during the recent outbreak, according to a report published online Feb. 18 in the New England Journal of Medicine.
This is markedly higher than the 37%-74% survival reported for the almost 29,000 cases treated in West Africa, where treatment centers were challenged by overwhelming numbers of critically ill patients; limited medical supplies; insufficient numbers of caregivers; limited water, electricity, refrigeration, and other basic resources; and hot, humid working conditions that reduced the time health care personnel could attend to patients while wearing the required protective gear, said Dr. Timothy M. Uyeki of the Centers for Disease Control and Prevention, Atlanta, and his associates.
The investigators performed a retrospective analysis of the medical records of these 27 patients in a descriptive study of their clinical care. The patients were treated from August 2014 through December 2015 at 15 hospitals in 9 countries. Twenty (74%) were medically evacuated from West Africa, three (11%) were Western health care personnel who acquired the disease while caring for patients, and four were “imported” patients who contracted the virus while in West Africa but didn’t become ill until after they traveled to the United States or Europe. Overall, 22 of the patients (81%) were health care personnel, of whom 17 (77%) contracted the virus in West Africa.
Overall mortality was 11.1% after 2 weeks of illness and 18.5% after 4 weeks. The five patients who died were all aged 42 years or older and were significantly older than those who survived. Being 40-45 years old or older has been identified as a risk factor in West Africa as well. Those who died also were hospitalized significantly later after the onset of illness. At least six more patients would have died if they hadn’t received advanced organ support: two required noninvasive ventilation, two required invasive mechanical ventilation, and two required both invasive mechanical ventilation and continuous renal-replacement therapy.
Routine therapies included oral or intravenous fluid replacement, total parenteral nutrition, antiemetics, empiric treatment with multiple antibiotics, nonconvalescent blood products, and respiratory supportive care. Most patients also received investigative therapies such as monoclonal antibody cocktails, antivirals, and treatments aimed at counteracting vascular leakage.
A wide range of possible adverse effects were reported – including systemic inflammatory response syndrome, hypotension, elevated aminotransferase levels, and transfusion-associated acute lung injury – but couldn’t be distinguished from the effects of supportive treatments or even of the virus itself “because of their uncontrolled administration and because most patients received multiple, overlapping investigational therapies,” the researchers said (N Engl J Med. 2016 Feb 18;374:636-46. doi: 10.1056/NEJMoa1504874).
“A key feature” of the lifesaving clinical care was laboratory testing to closely monitor electrolyte levels and hematologic factors. “Our experience suggests that early presentation and receipt of supportive care, IV fluid resuscitation, careful fluid management and electrolyte replacement to correct metabolic abnormalities, nutritional support, and critical care support may reduce mortality among patients with Ebola virus disease,” Dr. Uyeki and his associates said.
They noted that, until now, the infection was not thought to involve a clinically significant respiratory component; however, eight of these patients (30%) presented with cough and nine required ventilatory assistance, and difficulty breathing raised the risk of death.
“The pathophysiological mechanism of pulmonary disease in patients with Ebola virus infection is unknown, but there could be multiple contributing factors, including vascular leak from endothelial infection or cytokine dysregulation or direct damage to Ebola-infected [pulmonary] cells,” they wrote. Aggressive fluid repletion and lung injury from investigational treatments also may have contributed to respiratory involvement.
This study was supported by the working group of the U.S.–European Clinical Network on Clinical Management of Ebola Virus Disease Patients in the U.S. and Europe. Dr. Uyeki reported having no relevant financial disclosures; two of his associates reported ties to Epiguard and Pfizer.
Overall survival was 81.5% for the 27 patients with Ebola virus infection who were treated in the United States or Europe during the recent outbreak, according to a report published online Feb. 18 in the New England Journal of Medicine.
This is markedly higher than the 37%-74% survival reported for the almost 29,000 cases treated in West Africa, where treatment centers were challenged by overwhelming numbers of critically ill patients; limited medical supplies; insufficient numbers of caregivers; limited water, electricity, refrigeration, and other basic resources; and hot, humid working conditions that reduced the time health care personnel could attend to patients while wearing the required protective gear, said Dr. Timothy M. Uyeki of the Centers for Disease Control and Prevention, Atlanta, and his associates.
The investigators performed a retrospective analysis of the medical records of these 27 patients in a descriptive study of their clinical care. The patients were treated from August 2014 through December 2015 at 15 hospitals in 9 countries. Twenty (74%) were medically evacuated from West Africa, three (11%) were Western health care personnel who acquired the disease while caring for patients, and four were “imported” patients who contracted the virus while in West Africa but didn’t become ill until after they traveled to the United States or Europe. Overall, 22 of the patients (81%) were health care personnel, of whom 17 (77%) contracted the virus in West Africa.
Overall mortality was 11.1% after 2 weeks of illness and 18.5% after 4 weeks. The five patients who died were all aged 42 years or older and were significantly older than those who survived. Being 40-45 years old or older has been identified as a risk factor in West Africa as well. Those who died also were hospitalized significantly later after the onset of illness. At least six more patients would have died if they hadn’t received advanced organ support: two required noninvasive ventilation, two required invasive mechanical ventilation, and two required both invasive mechanical ventilation and continuous renal-replacement therapy.
Routine therapies included oral or intravenous fluid replacement, total parenteral nutrition, antiemetics, empiric treatment with multiple antibiotics, nonconvalescent blood products, and respiratory supportive care. Most patients also received investigative therapies such as monoclonal antibody cocktails, antivirals, and treatments aimed at counteracting vascular leakage.
A wide range of possible adverse effects were reported – including systemic inflammatory response syndrome, hypotension, elevated aminotransferase levels, and transfusion-associated acute lung injury – but couldn’t be distinguished from the effects of supportive treatments or even of the virus itself “because of their uncontrolled administration and because most patients received multiple, overlapping investigational therapies,” the researchers said (N Engl J Med. 2016 Feb 18;374:636-46. doi: 10.1056/NEJMoa1504874).
“A key feature” of the lifesaving clinical care was laboratory testing to closely monitor electrolyte levels and hematologic factors. “Our experience suggests that early presentation and receipt of supportive care, IV fluid resuscitation, careful fluid management and electrolyte replacement to correct metabolic abnormalities, nutritional support, and critical care support may reduce mortality among patients with Ebola virus disease,” Dr. Uyeki and his associates said.
They noted that, until now, the infection was not thought to involve a clinically significant respiratory component; however, eight of these patients (30%) presented with cough and nine required ventilatory assistance, and difficulty breathing raised the risk of death.
“The pathophysiological mechanism of pulmonary disease in patients with Ebola virus infection is unknown, but there could be multiple contributing factors, including vascular leak from endothelial infection or cytokine dysregulation or direct damage to Ebola-infected [pulmonary] cells,” they wrote. Aggressive fluid repletion and lung injury from investigational treatments also may have contributed to respiratory involvement.
This study was supported by the working group of the U.S.–European Clinical Network on Clinical Management of Ebola Virus Disease Patients in the U.S. and Europe. Dr. Uyeki reported having no relevant financial disclosures; two of his associates reported ties to Epiguard and Pfizer.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Overall survival was 81.5% for the 27 patients with Ebola virus infection who were treated in the United States or Europe.
Major finding: At least six more patients would have died if they hadn’t received advanced organ support, including invasive mechanical ventilation and continuous renal-replacement therapy.
Data source: A retrospective observational study of the clinical care rendered for all patients treated for Ebola infection in the United States and Europe.
Disclosures: This study was supported by the working group of the U.S.–European Clinical Network on Clinical Management of Ebola Virus Disease Patients in the U.S. and Europe. Dr. Uyeki reported having no relevant financial disclosures; two of his associates reported ties to Epiguard and Pfizer.
Study finds lower-than-expected rate of occult uterine sarcoma
The risk of finding occult uterine sarcoma during hysterectomy for benign indications was lower than expected in a single-center retrospective cohort study, at 0.089%, or 1 in 1,124 hysterectomies, according to a recent analysis.
This is markedly lower than the estimated risks in previous studies, which ranged from 1 in 204 to 1 in 667 procedures for women with presumed myomas. The American College of Obstetricians and Gynecologists estimated the risk to be 1 in 500 hysterectomies, and the Food and Drug Administration pegged it at 1 in 352 based on a pooled analysis of nine studies of women undergoing hysterectomy or myomectomy for presumed myomas. The last estimate in particular has been criticized as inaccurate because of concerns about the quality of data and methodologic flaws of the nine studies, reported Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and her associates (Obstet. Gynecol. 2016;127:468-73.).
The investigators analyzed information in a database for all 10,119 hysterectomies performed for benign indications at their medical center during a 14-year period, and correlated it with data concerning all cases of uterine sarcoma in their center’s tumor registry. A total of 59.4% of these procedures used an abdominal approach, 21.6% were laparoscopic or robot assisted, and 18.9% used a vaginal approach. The most common indications were leiomyomata (37%), abnormal uterine bleeding (28%), and pelvic organ prolapse (11%).
Nine women were found to have an occult uterine sarcoma, including five leiomyosarcomas, two endometrial stromal sarcomas, and two uterine adenocarcinomas.
“All patients had received up-to-date cervical cancer screening and, in the majority of cases, women had received preoperative evaluation with either endometrial sampling or imaging, which did not suggest malignancy. Of the suggested risk factors for sarcoma, it is notable that none of the women we identified were postmenopausal, exposed to pelvic radiation or tamoxifen, nor had a family history of cancer,” the researchers wrote.
Only one patient underwent manual morcellation of a large, bulky uterus before her sarcoma was discovered during total abdominal hysterectomy. The abdominal cavity was then thoroughly explored, and no suspicious lesions were found. This patient later received chemotherapy and had no evidence of disease 3 years later.
The study findings may be helpful for surgical planning and for counseling patients about management options. “It is important to stress that although low, the risk of encountering an occult sarcoma exists. Hence, ongoing efforts to identify potentially safer methods for tissue extraction are essential, as are efforts to improve preoperative identification of malignancies,” the researchers noted.
The study was supported by the University of Texas Southwestern Medical Center. Dr. Kho reported ties to Actamax Surgical Materials and Applied Medical; one of her associates reported ties to AstraZeneca and Genentech.
The risk of finding occult uterine sarcoma during hysterectomy for benign indications was lower than expected in a single-center retrospective cohort study, at 0.089%, or 1 in 1,124 hysterectomies, according to a recent analysis.
This is markedly lower than the estimated risks in previous studies, which ranged from 1 in 204 to 1 in 667 procedures for women with presumed myomas. The American College of Obstetricians and Gynecologists estimated the risk to be 1 in 500 hysterectomies, and the Food and Drug Administration pegged it at 1 in 352 based on a pooled analysis of nine studies of women undergoing hysterectomy or myomectomy for presumed myomas. The last estimate in particular has been criticized as inaccurate because of concerns about the quality of data and methodologic flaws of the nine studies, reported Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and her associates (Obstet. Gynecol. 2016;127:468-73.).
The investigators analyzed information in a database for all 10,119 hysterectomies performed for benign indications at their medical center during a 14-year period, and correlated it with data concerning all cases of uterine sarcoma in their center’s tumor registry. A total of 59.4% of these procedures used an abdominal approach, 21.6% were laparoscopic or robot assisted, and 18.9% used a vaginal approach. The most common indications were leiomyomata (37%), abnormal uterine bleeding (28%), and pelvic organ prolapse (11%).
Nine women were found to have an occult uterine sarcoma, including five leiomyosarcomas, two endometrial stromal sarcomas, and two uterine adenocarcinomas.
“All patients had received up-to-date cervical cancer screening and, in the majority of cases, women had received preoperative evaluation with either endometrial sampling or imaging, which did not suggest malignancy. Of the suggested risk factors for sarcoma, it is notable that none of the women we identified were postmenopausal, exposed to pelvic radiation or tamoxifen, nor had a family history of cancer,” the researchers wrote.
Only one patient underwent manual morcellation of a large, bulky uterus before her sarcoma was discovered during total abdominal hysterectomy. The abdominal cavity was then thoroughly explored, and no suspicious lesions were found. This patient later received chemotherapy and had no evidence of disease 3 years later.
The study findings may be helpful for surgical planning and for counseling patients about management options. “It is important to stress that although low, the risk of encountering an occult sarcoma exists. Hence, ongoing efforts to identify potentially safer methods for tissue extraction are essential, as are efforts to improve preoperative identification of malignancies,” the researchers noted.
The study was supported by the University of Texas Southwestern Medical Center. Dr. Kho reported ties to Actamax Surgical Materials and Applied Medical; one of her associates reported ties to AstraZeneca and Genentech.
The risk of finding occult uterine sarcoma during hysterectomy for benign indications was lower than expected in a single-center retrospective cohort study, at 0.089%, or 1 in 1,124 hysterectomies, according to a recent analysis.
This is markedly lower than the estimated risks in previous studies, which ranged from 1 in 204 to 1 in 667 procedures for women with presumed myomas. The American College of Obstetricians and Gynecologists estimated the risk to be 1 in 500 hysterectomies, and the Food and Drug Administration pegged it at 1 in 352 based on a pooled analysis of nine studies of women undergoing hysterectomy or myomectomy for presumed myomas. The last estimate in particular has been criticized as inaccurate because of concerns about the quality of data and methodologic flaws of the nine studies, reported Dr. Kimberly A. Kho of the University of Texas Southwestern Medical Center, Dallas, and her associates (Obstet. Gynecol. 2016;127:468-73.).
The investigators analyzed information in a database for all 10,119 hysterectomies performed for benign indications at their medical center during a 14-year period, and correlated it with data concerning all cases of uterine sarcoma in their center’s tumor registry. A total of 59.4% of these procedures used an abdominal approach, 21.6% were laparoscopic or robot assisted, and 18.9% used a vaginal approach. The most common indications were leiomyomata (37%), abnormal uterine bleeding (28%), and pelvic organ prolapse (11%).
Nine women were found to have an occult uterine sarcoma, including five leiomyosarcomas, two endometrial stromal sarcomas, and two uterine adenocarcinomas.
“All patients had received up-to-date cervical cancer screening and, in the majority of cases, women had received preoperative evaluation with either endometrial sampling or imaging, which did not suggest malignancy. Of the suggested risk factors for sarcoma, it is notable that none of the women we identified were postmenopausal, exposed to pelvic radiation or tamoxifen, nor had a family history of cancer,” the researchers wrote.
Only one patient underwent manual morcellation of a large, bulky uterus before her sarcoma was discovered during total abdominal hysterectomy. The abdominal cavity was then thoroughly explored, and no suspicious lesions were found. This patient later received chemotherapy and had no evidence of disease 3 years later.
The study findings may be helpful for surgical planning and for counseling patients about management options. “It is important to stress that although low, the risk of encountering an occult sarcoma exists. Hence, ongoing efforts to identify potentially safer methods for tissue extraction are essential, as are efforts to improve preoperative identification of malignancies,” the researchers noted.
The study was supported by the University of Texas Southwestern Medical Center. Dr. Kho reported ties to Actamax Surgical Materials and Applied Medical; one of her associates reported ties to AstraZeneca and Genentech.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: The rate of occult uterine sarcoma in women undergoing hysterectomy for benign indications was lower than expected at 0.089%.
Major finding: A total of 9 out of 10,119 women were found to have an occult uterine sarcoma, including five leiomyosarcomas, two endometrial stromal sarcomas, and two uterine adenocarcinomas.
Data source: A retrospective single-center cohort study involving 10,119 hysterectomies performed during a 14-year period.
Disclosures: The study was supported by the University of Texas Southwestern Medical Center. Dr. Kho reported ties to Actamax Surgical Materials and Applied Medical; one of her associates reported ties to AstraZeneca and Genentech.
Interstitial lung abnormalities linked to higher mortality
In four large, separate research cohorts in which middle-aged and older participants underwent lung CT, interstitial lung abnormalities were associated with a higher-than-average risk of death within 3-9 years, according to a report published online Feb. 16 in JAMA.
These imaging abnormalities were defined as specific patterns of increased lung density affecting more than 5% of any lung zone and included reticular or ground-glass abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, traction bronchiectasis, or pulmonary parenchymal architectural distortion diagnostic of fibrotic lung disease. They were identified in approximately 7% of the 11,691 study participants. The study findings, taken together with those of previous research, “demonstrate that despite often being undiagnosed and asymptomatic, interstitial lung abnormalities may be associated with lower survival rates among older persons,” said Dr. Rachel K. Putman of the pulmonary and critical care division at Brigham and Women’s Hospital and Harvard Medical School, Boston, and her associates.
Previously, interstitial lung abnormalities have been found in the same proportion, 7%, of the general population and have been associated with reduced lung capacity, exercise capacity, and gas exchange. They are more common in families affected by familial interstitial pneumonia and idiopathic pulmonary fibrosis. Given these correlations, “we hypothesized that the presence of interstitial lung abnormalities would be associated with an increased rate of mortality,” the investigators said.
To test this hypothesis, they analyzed data from four large study cohorts that included lung CT: 2,633 participants in the Framingham Heart Study (median follow-up of 4 years after CT), 5,320 in the Age Gene/Environment Susceptibility (AGES)-Reykjavik study (median follow-up, 8.9 years), 2,068 in the Genetic Epidemiology of COPD (COPDGene) study (median follow-up, 6.5 years), and 1,670 participants in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study (median follow-up, 2.9 years).
The absolute rates of all-cause mortality were significantly higher among participants who had interstitial lung abnormalities than among those who did not. Mortality rates were 7% vs. 1% in the FHS, 56% vs. 33% in AGES-Reykjavik, 16% vs. 11% in COPDGene, and 11% vs. 5% in ECLIPSE. After the data were adjusted to account for confounding factors such as age, sex, race, body-mass index, current smoking status, and pack-years of smoking, the lung abnormalities remained strongly associated with a higher risk of death in the FHS (hazard ratio, 2.7), AGES-Reykjavik (HR, 1.3), COPDGene (HR, 1.8), and ECLIPSE (HR, 1.4) studies.
The association remained robust in further analyses restricted only to nonsmoking participants, Dr. Putman and her associates said (JAMA. 2016 Feb 16;315[7]672-81. doi: 10.1001/jama.2016.0518).
The AGES-Reykjavik study was the only one to assess causes of death. In that cohort, participants who had interstitial lung abnormalities were more likely to die of a respiratory cause (13%) than were those who had no such abnormalities (4%) or those who had indeterminate findings on lung CT (6%). After the data were adjusted to account for confounding factors, participants with interstitial lung abnormalities were at much higher risk of dying from a respiratory cause (OR, 2.4) such as respiratory failure or pulmonary fibrosis. In contrast, there was no association between the lung abnormalities and deaths due to cardiovascular disease, cancer, or other causes.
The clinical implications of the association between interstitial lung abnormalities and mortality, particularly respiratory-related mortality, require further investigation, the investigators added.
This study was supported by the National Institutes of Health; the Icelandic Research Fund; the Lanspitali Scientific Fund; the National Cancer Institute; the National Heart, Lung, and Blood Institute; GlaxoSmithKline; the National Institute on Aging; the Icelandic Heart Association; and the Icelandic Parliament. Dr. Putman reported having no relevant financial disclosures; her associates reported numerous ties to industry sources.
In four large, separate research cohorts in which middle-aged and older participants underwent lung CT, interstitial lung abnormalities were associated with a higher-than-average risk of death within 3-9 years, according to a report published online Feb. 16 in JAMA.
These imaging abnormalities were defined as specific patterns of increased lung density affecting more than 5% of any lung zone and included reticular or ground-glass abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, traction bronchiectasis, or pulmonary parenchymal architectural distortion diagnostic of fibrotic lung disease. They were identified in approximately 7% of the 11,691 study participants. The study findings, taken together with those of previous research, “demonstrate that despite often being undiagnosed and asymptomatic, interstitial lung abnormalities may be associated with lower survival rates among older persons,” said Dr. Rachel K. Putman of the pulmonary and critical care division at Brigham and Women’s Hospital and Harvard Medical School, Boston, and her associates.
Previously, interstitial lung abnormalities have been found in the same proportion, 7%, of the general population and have been associated with reduced lung capacity, exercise capacity, and gas exchange. They are more common in families affected by familial interstitial pneumonia and idiopathic pulmonary fibrosis. Given these correlations, “we hypothesized that the presence of interstitial lung abnormalities would be associated with an increased rate of mortality,” the investigators said.
To test this hypothesis, they analyzed data from four large study cohorts that included lung CT: 2,633 participants in the Framingham Heart Study (median follow-up of 4 years after CT), 5,320 in the Age Gene/Environment Susceptibility (AGES)-Reykjavik study (median follow-up, 8.9 years), 2,068 in the Genetic Epidemiology of COPD (COPDGene) study (median follow-up, 6.5 years), and 1,670 participants in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study (median follow-up, 2.9 years).
The absolute rates of all-cause mortality were significantly higher among participants who had interstitial lung abnormalities than among those who did not. Mortality rates were 7% vs. 1% in the FHS, 56% vs. 33% in AGES-Reykjavik, 16% vs. 11% in COPDGene, and 11% vs. 5% in ECLIPSE. After the data were adjusted to account for confounding factors such as age, sex, race, body-mass index, current smoking status, and pack-years of smoking, the lung abnormalities remained strongly associated with a higher risk of death in the FHS (hazard ratio, 2.7), AGES-Reykjavik (HR, 1.3), COPDGene (HR, 1.8), and ECLIPSE (HR, 1.4) studies.
The association remained robust in further analyses restricted only to nonsmoking participants, Dr. Putman and her associates said (JAMA. 2016 Feb 16;315[7]672-81. doi: 10.1001/jama.2016.0518).
The AGES-Reykjavik study was the only one to assess causes of death. In that cohort, participants who had interstitial lung abnormalities were more likely to die of a respiratory cause (13%) than were those who had no such abnormalities (4%) or those who had indeterminate findings on lung CT (6%). After the data were adjusted to account for confounding factors, participants with interstitial lung abnormalities were at much higher risk of dying from a respiratory cause (OR, 2.4) such as respiratory failure or pulmonary fibrosis. In contrast, there was no association between the lung abnormalities and deaths due to cardiovascular disease, cancer, or other causes.
The clinical implications of the association between interstitial lung abnormalities and mortality, particularly respiratory-related mortality, require further investigation, the investigators added.
This study was supported by the National Institutes of Health; the Icelandic Research Fund; the Lanspitali Scientific Fund; the National Cancer Institute; the National Heart, Lung, and Blood Institute; GlaxoSmithKline; the National Institute on Aging; the Icelandic Heart Association; and the Icelandic Parliament. Dr. Putman reported having no relevant financial disclosures; her associates reported numerous ties to industry sources.
In four large, separate research cohorts in which middle-aged and older participants underwent lung CT, interstitial lung abnormalities were associated with a higher-than-average risk of death within 3-9 years, according to a report published online Feb. 16 in JAMA.
These imaging abnormalities were defined as specific patterns of increased lung density affecting more than 5% of any lung zone and included reticular or ground-glass abnormalities, diffuse centrilobular nodularity, nonemphysematous cysts, honeycombing, traction bronchiectasis, or pulmonary parenchymal architectural distortion diagnostic of fibrotic lung disease. They were identified in approximately 7% of the 11,691 study participants. The study findings, taken together with those of previous research, “demonstrate that despite often being undiagnosed and asymptomatic, interstitial lung abnormalities may be associated with lower survival rates among older persons,” said Dr. Rachel K. Putman of the pulmonary and critical care division at Brigham and Women’s Hospital and Harvard Medical School, Boston, and her associates.
Previously, interstitial lung abnormalities have been found in the same proportion, 7%, of the general population and have been associated with reduced lung capacity, exercise capacity, and gas exchange. They are more common in families affected by familial interstitial pneumonia and idiopathic pulmonary fibrosis. Given these correlations, “we hypothesized that the presence of interstitial lung abnormalities would be associated with an increased rate of mortality,” the investigators said.
To test this hypothesis, they analyzed data from four large study cohorts that included lung CT: 2,633 participants in the Framingham Heart Study (median follow-up of 4 years after CT), 5,320 in the Age Gene/Environment Susceptibility (AGES)-Reykjavik study (median follow-up, 8.9 years), 2,068 in the Genetic Epidemiology of COPD (COPDGene) study (median follow-up, 6.5 years), and 1,670 participants in the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study (median follow-up, 2.9 years).
The absolute rates of all-cause mortality were significantly higher among participants who had interstitial lung abnormalities than among those who did not. Mortality rates were 7% vs. 1% in the FHS, 56% vs. 33% in AGES-Reykjavik, 16% vs. 11% in COPDGene, and 11% vs. 5% in ECLIPSE. After the data were adjusted to account for confounding factors such as age, sex, race, body-mass index, current smoking status, and pack-years of smoking, the lung abnormalities remained strongly associated with a higher risk of death in the FHS (hazard ratio, 2.7), AGES-Reykjavik (HR, 1.3), COPDGene (HR, 1.8), and ECLIPSE (HR, 1.4) studies.
The association remained robust in further analyses restricted only to nonsmoking participants, Dr. Putman and her associates said (JAMA. 2016 Feb 16;315[7]672-81. doi: 10.1001/jama.2016.0518).
The AGES-Reykjavik study was the only one to assess causes of death. In that cohort, participants who had interstitial lung abnormalities were more likely to die of a respiratory cause (13%) than were those who had no such abnormalities (4%) or those who had indeterminate findings on lung CT (6%). After the data were adjusted to account for confounding factors, participants with interstitial lung abnormalities were at much higher risk of dying from a respiratory cause (OR, 2.4) such as respiratory failure or pulmonary fibrosis. In contrast, there was no association between the lung abnormalities and deaths due to cardiovascular disease, cancer, or other causes.
The clinical implications of the association between interstitial lung abnormalities and mortality, particularly respiratory-related mortality, require further investigation, the investigators added.
This study was supported by the National Institutes of Health; the Icelandic Research Fund; the Lanspitali Scientific Fund; the National Cancer Institute; the National Heart, Lung, and Blood Institute; GlaxoSmithKline; the National Institute on Aging; the Icelandic Heart Association; and the Icelandic Parliament. Dr. Putman reported having no relevant financial disclosures; her associates reported numerous ties to industry sources.
FROM JAMA
Key clinical point: Interstitial lung abnormalities on CT scans appear to signal higher-than-average mortality within 3-9 years.
Major finding: Interstitial lung abnormalities were strongly associated with a higher risk of death in the FHS (hazard ratio, 2.7), AGES-Reykjavik (HR, 1.3), COPDGene (HR, 1.8), and ECLIPSE (HR, 1.4) cohorts.
Data source: An analysis of data in four large prospective cohort studies involving 11,691 adults who underwent lung CT and were followed for a median of 3-9 years.
Disclosures: This study was supported by the National Institutes of Health; the Icelandic Research Fund; the Lanspitali Scientific Fund; the National Cancer Institute; the National Heart, Lung, and Blood Institute; GlaxoSmithKline; the National Institute on Aging; the Icelandic Heart Association; and the Icelandic Parliament. Dr. Putman reported having no relevant financial disclosures; her associates reported numerous ties to industry sources.
Screening for acute HIV infection raises diagnostic yield
Screening a high-prevalence population for acute HIV infection using an antigen/antibody combination assay instead of rapid HIV testing improved the diagnostic yield by 10%, according to a report published online Feb. 16 in JAMA.
Identifying HIV infection during the acute phase is important because that is the most highly infectious stage of the disease. HIV RNA testing using a pooled protocol is effective at this stage but hasn’t been widely adopted “because only 1 RNA assay is U.S. Food and Drug Administration–approved [for this indication], the pooling protocol is logistically complex and time intensive, and it may not be cost-effective,” said Dr. Philip J. Peters of the division of HIV/AIDS prevention, Centers for Disease Control and Prevention, Atlanta, and his associates.
In contrast, combination assays that detect both the p24 antigen and anti-HIV antibodies are faster, are probably cost effective, and are currently recommended by the CDC and the Association of Public Health Laboratories to screen for acute infection. Nevertheless, these assays are not as sensitive as pooled HIV RNA testing, and their accuracy has not been fully established. Dr. Peters and his associates compared the performance of the HIV antigen/antibody combination assay against that of pooled HIV RNA testing (the reference standard) in a high-prevalence population: 86,836 patients treated at 12 centers in San Francisco, New York City, and North Carolina during a 2-year period, including STD clinics and community-based programs.
Just over half of the study population were men who had sex with men, and the median age was 29 years.
The antigen/antibody assay detected 134 of 168 acute infections that had been missed by the rapid HIV test, but it also produced false-positive results for 93 patients. The assay thus had a sensitivity of 79.8%, a specificity of 99.9%, and a positive predictive value of 59%. Relative to rapid HIV testing, the antigen/antibody assay increased the diagnostic yield by 10.4%, Dr. Peters and his associates reported (JAMA. 2016 Feb 16;315[7]:682-690. doi: 10.1001/jama.2016.0286).
As expected, pooled HIV RNA testing performed even better, detecting 164 of the 168 acute infections for a sensitivity of 97.6%, a specificity of 100%, and a positive predictive value of 96.5%. Relative to rapid HIV testing, pooled HIV RNA testing increased the diagnostic yield by 12.4%.
Pooled HIV RNA testing, however, is estimated to cost approximately $160.07 per test, while antigen/antibody combination assays cost only $4.23 each. In addition, antigen/antibody testing requires only 30 minutes (if results are negative) to 60 minutes (if results are positive), while pooled HIV RNA testing requires 6 hours, and the pooling process requires an additional 4-7 days, the investigators noted.
This study was supported by the Centers for Disease Control and Prevention, the San Francisco Department of Public Health, the New York City Department of Health and Mental Hygiene, and the University of North Carolina at Chapel Hill. Dr. Peters and his associates reported having no relevant financial disclosures.
Screening a high-prevalence population for acute HIV infection using an antigen/antibody combination assay instead of rapid HIV testing improved the diagnostic yield by 10%, according to a report published online Feb. 16 in JAMA.
Identifying HIV infection during the acute phase is important because that is the most highly infectious stage of the disease. HIV RNA testing using a pooled protocol is effective at this stage but hasn’t been widely adopted “because only 1 RNA assay is U.S. Food and Drug Administration–approved [for this indication], the pooling protocol is logistically complex and time intensive, and it may not be cost-effective,” said Dr. Philip J. Peters of the division of HIV/AIDS prevention, Centers for Disease Control and Prevention, Atlanta, and his associates.
In contrast, combination assays that detect both the p24 antigen and anti-HIV antibodies are faster, are probably cost effective, and are currently recommended by the CDC and the Association of Public Health Laboratories to screen for acute infection. Nevertheless, these assays are not as sensitive as pooled HIV RNA testing, and their accuracy has not been fully established. Dr. Peters and his associates compared the performance of the HIV antigen/antibody combination assay against that of pooled HIV RNA testing (the reference standard) in a high-prevalence population: 86,836 patients treated at 12 centers in San Francisco, New York City, and North Carolina during a 2-year period, including STD clinics and community-based programs.
Just over half of the study population were men who had sex with men, and the median age was 29 years.
The antigen/antibody assay detected 134 of 168 acute infections that had been missed by the rapid HIV test, but it also produced false-positive results for 93 patients. The assay thus had a sensitivity of 79.8%, a specificity of 99.9%, and a positive predictive value of 59%. Relative to rapid HIV testing, the antigen/antibody assay increased the diagnostic yield by 10.4%, Dr. Peters and his associates reported (JAMA. 2016 Feb 16;315[7]:682-690. doi: 10.1001/jama.2016.0286).
As expected, pooled HIV RNA testing performed even better, detecting 164 of the 168 acute infections for a sensitivity of 97.6%, a specificity of 100%, and a positive predictive value of 96.5%. Relative to rapid HIV testing, pooled HIV RNA testing increased the diagnostic yield by 12.4%.
Pooled HIV RNA testing, however, is estimated to cost approximately $160.07 per test, while antigen/antibody combination assays cost only $4.23 each. In addition, antigen/antibody testing requires only 30 minutes (if results are negative) to 60 minutes (if results are positive), while pooled HIV RNA testing requires 6 hours, and the pooling process requires an additional 4-7 days, the investigators noted.
This study was supported by the Centers for Disease Control and Prevention, the San Francisco Department of Public Health, the New York City Department of Health and Mental Hygiene, and the University of North Carolina at Chapel Hill. Dr. Peters and his associates reported having no relevant financial disclosures.
Screening a high-prevalence population for acute HIV infection using an antigen/antibody combination assay instead of rapid HIV testing improved the diagnostic yield by 10%, according to a report published online Feb. 16 in JAMA.
Identifying HIV infection during the acute phase is important because that is the most highly infectious stage of the disease. HIV RNA testing using a pooled protocol is effective at this stage but hasn’t been widely adopted “because only 1 RNA assay is U.S. Food and Drug Administration–approved [for this indication], the pooling protocol is logistically complex and time intensive, and it may not be cost-effective,” said Dr. Philip J. Peters of the division of HIV/AIDS prevention, Centers for Disease Control and Prevention, Atlanta, and his associates.
In contrast, combination assays that detect both the p24 antigen and anti-HIV antibodies are faster, are probably cost effective, and are currently recommended by the CDC and the Association of Public Health Laboratories to screen for acute infection. Nevertheless, these assays are not as sensitive as pooled HIV RNA testing, and their accuracy has not been fully established. Dr. Peters and his associates compared the performance of the HIV antigen/antibody combination assay against that of pooled HIV RNA testing (the reference standard) in a high-prevalence population: 86,836 patients treated at 12 centers in San Francisco, New York City, and North Carolina during a 2-year period, including STD clinics and community-based programs.
Just over half of the study population were men who had sex with men, and the median age was 29 years.
The antigen/antibody assay detected 134 of 168 acute infections that had been missed by the rapid HIV test, but it also produced false-positive results for 93 patients. The assay thus had a sensitivity of 79.8%, a specificity of 99.9%, and a positive predictive value of 59%. Relative to rapid HIV testing, the antigen/antibody assay increased the diagnostic yield by 10.4%, Dr. Peters and his associates reported (JAMA. 2016 Feb 16;315[7]:682-690. doi: 10.1001/jama.2016.0286).
As expected, pooled HIV RNA testing performed even better, detecting 164 of the 168 acute infections for a sensitivity of 97.6%, a specificity of 100%, and a positive predictive value of 96.5%. Relative to rapid HIV testing, pooled HIV RNA testing increased the diagnostic yield by 12.4%.
Pooled HIV RNA testing, however, is estimated to cost approximately $160.07 per test, while antigen/antibody combination assays cost only $4.23 each. In addition, antigen/antibody testing requires only 30 minutes (if results are negative) to 60 minutes (if results are positive), while pooled HIV RNA testing requires 6 hours, and the pooling process requires an additional 4-7 days, the investigators noted.
This study was supported by the Centers for Disease Control and Prevention, the San Francisco Department of Public Health, the New York City Department of Health and Mental Hygiene, and the University of North Carolina at Chapel Hill. Dr. Peters and his associates reported having no relevant financial disclosures.
FROM JAMA
Key clinical point: Screening a high-prevalence population for acute HIV infection using an antigen/antibody combination assay instead of rapid HIV testing improved the diagnostic yield by 10%.
Major finding: The antigen/antibody assay had a sensitivity of 79.8%, a specificity of 99.9%, and a positive predictive value of 59%.
Data source: A 2-year prospective multicenter study involving 86,836 participants seeking HIV testing.
Disclosures: This study was supported by the Centers for Disease Control and Prevention, the San Francisco Department of Public Health, the New York City Department of Health and Mental Hygiene, and the University of North Carolina at Chapel Hill. Dr. Peters and his associates reported having no relevant financial disclosures.
Dexamethasone harmful for patients with HIV-associated cryptococcal meningitis
Adjunctive dexamethasone not only failed to decrease mortality, it actually induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis, according to a report published online Feb. 11 in the New England Journal of Medicine.
Glucocorticoids are inexpensive, readily available, and relatively safe for patients with central nervous system infections, and they are widely used for HIV-associated cryptococcal meningitis in regions where the burden of the infection is highest. Glucocorticoids are even recommended for this indication in some international guidelines, as they are thought to reduce intracranial pressure and inflammatory complications. But evidence of their usefulness from randomized controlled trials is sparse, said Dr. Jeremy Day of Oxford University Clinical Research Unit, Wellcome Trust Major Overseas Programme Vietnam, Ho Chi Minh City, and his associates.
They performed a double-blind, randomized trial to compare the effectiveness and safety of adjunctive dexamethasone against placebo, which involved 451 patients treated for 6 weeks at 13 hospitals in Indonesia, Laos, Thailand, Vietnam, Malawi, and Uganda. The study participants also received standard antifungal therapy including amphotericin B and fluconazole, as well as antiretroviral therapy and Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole.
The trial was stopped prematurely when the safety committee found “dexamethasone was causing harm across key outcomes, including fungal clearance, adverse events, and disability outcomes.” Consequently, the study didn’t have the statistical power to show an effect of dexamethasone on the primary outcome measure – mortality at 10 weeks after randomization (4 weeks after study treatment ended).
Dexamethasone did reduce intracranial pressure more rapidly than placebo, but this effect didn’t translate into improved survival. Mortality at 10 weeks was 47% for the 224 patients assigned to dexamethasone and 41% for the 226 patients assigned to placebo, a nonsignificant difference. However, the drug’s effects changed over time: Hazard ratios for death were 0.77 at days 1-22 but rose to 1.94 at days 23-43 and to 2.50 at days 44-71. By 6-month follow-up, mortality risk showed a trend toward harm with dexamethasone, and was 9% higher in the intention-to-treat population and 11% higher in the per-protocol population analyses, Dr. Day and his associates said (N. Engl J Med. 2016 Feb 11;374[6]: doi:10.1056/NEJMoa1509024). The rate of disability or death was significantly higher with dexamethasone than with placebo at both 10 weeks and 6 months, with odds ratios for a good outcome of only 0.42 and 0.49, respectively. Infections or infestations developed in 48 patients (21%) taking dexamethasone but only 25 (11%) of those taking placebo. Gastrointestinal disorders (13% vs. 7%), renal or urinary disorders (10% vs. 3%), and cardiac disorders (4% vs. 0%) also were significantly more frequent with dexamethasone, as were episodes of hyperglycemia, hypercreatinemia, hyperkalemia, and hyponatremia.
This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
Adjunctive dexamethasone not only failed to decrease mortality, it actually induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis, according to a report published online Feb. 11 in the New England Journal of Medicine.
Glucocorticoids are inexpensive, readily available, and relatively safe for patients with central nervous system infections, and they are widely used for HIV-associated cryptococcal meningitis in regions where the burden of the infection is highest. Glucocorticoids are even recommended for this indication in some international guidelines, as they are thought to reduce intracranial pressure and inflammatory complications. But evidence of their usefulness from randomized controlled trials is sparse, said Dr. Jeremy Day of Oxford University Clinical Research Unit, Wellcome Trust Major Overseas Programme Vietnam, Ho Chi Minh City, and his associates.
They performed a double-blind, randomized trial to compare the effectiveness and safety of adjunctive dexamethasone against placebo, which involved 451 patients treated for 6 weeks at 13 hospitals in Indonesia, Laos, Thailand, Vietnam, Malawi, and Uganda. The study participants also received standard antifungal therapy including amphotericin B and fluconazole, as well as antiretroviral therapy and Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole.
The trial was stopped prematurely when the safety committee found “dexamethasone was causing harm across key outcomes, including fungal clearance, adverse events, and disability outcomes.” Consequently, the study didn’t have the statistical power to show an effect of dexamethasone on the primary outcome measure – mortality at 10 weeks after randomization (4 weeks after study treatment ended).
Dexamethasone did reduce intracranial pressure more rapidly than placebo, but this effect didn’t translate into improved survival. Mortality at 10 weeks was 47% for the 224 patients assigned to dexamethasone and 41% for the 226 patients assigned to placebo, a nonsignificant difference. However, the drug’s effects changed over time: Hazard ratios for death were 0.77 at days 1-22 but rose to 1.94 at days 23-43 and to 2.50 at days 44-71. By 6-month follow-up, mortality risk showed a trend toward harm with dexamethasone, and was 9% higher in the intention-to-treat population and 11% higher in the per-protocol population analyses, Dr. Day and his associates said (N. Engl J Med. 2016 Feb 11;374[6]: doi:10.1056/NEJMoa1509024). The rate of disability or death was significantly higher with dexamethasone than with placebo at both 10 weeks and 6 months, with odds ratios for a good outcome of only 0.42 and 0.49, respectively. Infections or infestations developed in 48 patients (21%) taking dexamethasone but only 25 (11%) of those taking placebo. Gastrointestinal disorders (13% vs. 7%), renal or urinary disorders (10% vs. 3%), and cardiac disorders (4% vs. 0%) also were significantly more frequent with dexamethasone, as were episodes of hyperglycemia, hypercreatinemia, hyperkalemia, and hyponatremia.
This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
Adjunctive dexamethasone not only failed to decrease mortality, it actually induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis, according to a report published online Feb. 11 in the New England Journal of Medicine.
Glucocorticoids are inexpensive, readily available, and relatively safe for patients with central nervous system infections, and they are widely used for HIV-associated cryptococcal meningitis in regions where the burden of the infection is highest. Glucocorticoids are even recommended for this indication in some international guidelines, as they are thought to reduce intracranial pressure and inflammatory complications. But evidence of their usefulness from randomized controlled trials is sparse, said Dr. Jeremy Day of Oxford University Clinical Research Unit, Wellcome Trust Major Overseas Programme Vietnam, Ho Chi Minh City, and his associates.
They performed a double-blind, randomized trial to compare the effectiveness and safety of adjunctive dexamethasone against placebo, which involved 451 patients treated for 6 weeks at 13 hospitals in Indonesia, Laos, Thailand, Vietnam, Malawi, and Uganda. The study participants also received standard antifungal therapy including amphotericin B and fluconazole, as well as antiretroviral therapy and Pneumocystis prophylaxis with trimethoprim-sulfamethoxazole.
The trial was stopped prematurely when the safety committee found “dexamethasone was causing harm across key outcomes, including fungal clearance, adverse events, and disability outcomes.” Consequently, the study didn’t have the statistical power to show an effect of dexamethasone on the primary outcome measure – mortality at 10 weeks after randomization (4 weeks after study treatment ended).
Dexamethasone did reduce intracranial pressure more rapidly than placebo, but this effect didn’t translate into improved survival. Mortality at 10 weeks was 47% for the 224 patients assigned to dexamethasone and 41% for the 226 patients assigned to placebo, a nonsignificant difference. However, the drug’s effects changed over time: Hazard ratios for death were 0.77 at days 1-22 but rose to 1.94 at days 23-43 and to 2.50 at days 44-71. By 6-month follow-up, mortality risk showed a trend toward harm with dexamethasone, and was 9% higher in the intention-to-treat population and 11% higher in the per-protocol population analyses, Dr. Day and his associates said (N. Engl J Med. 2016 Feb 11;374[6]: doi:10.1056/NEJMoa1509024). The rate of disability or death was significantly higher with dexamethasone than with placebo at both 10 weeks and 6 months, with odds ratios for a good outcome of only 0.42 and 0.49, respectively. Infections or infestations developed in 48 patients (21%) taking dexamethasone but only 25 (11%) of those taking placebo. Gastrointestinal disorders (13% vs. 7%), renal or urinary disorders (10% vs. 3%), and cardiac disorders (4% vs. 0%) also were significantly more frequent with dexamethasone, as were episodes of hyperglycemia, hypercreatinemia, hyperkalemia, and hyponatremia.
This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Dexamethasone failed to decrease mortality and induced more disability and adverse events than placebo in patients with HIV-associated cryptococcal meningitis.
Major finding: The hazard ratio for death in the dexamethasone group relative to the placebo group was 0.77 at days 1-22 but rose to 1.94 at days 23-43, and to 2.50 at days 44-71.
Data source: An international, randomized, double-blind, placebo-controlled trial involving 451 patients treated for 6 weeks and followed for a further 4 weeks.
Disclosures: This study was supported by the United Kingdom Department for International Development, the Wellcome Trust, and the U.K. Medical Research Council through the Joint Global Health Trials Program. Dr. Day and his associates reported having no relevant financial disclosures.
Caplacizumab induces rapid resolution of acute TTP
Caplacizumab induces faster resolution of acute episodes of acquired thrombotic thrombocytopenic purpura than does conventional therapy by blocking further platelet aggregation mediated by von Willebrand factor, according to a report published online Feb. 11 in the New England Journal of Medicine.
Faster normalization of the platelet count “prevents further consumption of platelets into microthrombi, and the consequent progression of tissue ischemia.” This in turn should prevent further ischemic injury to the brain, heart, and kidneys in both the short and the long term, said Dr. Flora Peyvandi of the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Ospedale Maggiore Policlinico, Milan, and her associates.
The investigators assessed caplacizumab, an anti–von Willebrand factor immunoglobulin, as a potential treatment for acquired TTP in a manufacturer-sponsored phase II trial involving 75 patients treated at 56 medical centers worldwide. All the study participants received standard treatment – daily plasma exchange and immunosuppressive therapy. In addition, they were randomly assigned to receive daily caplacizumab (36 patients) or placebo injections (39 patients) after each plasma exchange procedure and for 30 days following the final procedure, for a maximum of 90 days.
“Caplacizumab rapidly neutralized its target as indicated by suppression of von Willebrand factor–ristocetin cofactor activity to a mean of less than 20% by day 1 and throughout the treatment period,” Dr. Peyvandi and her associates said. These values returned to baseline levels within 1 week of treatment cessation.
The primary endpoint of the study, median time to normalization of the platelet count, was significantly reduced by 39% with caplacizumab compared with placebo. Among the 69 patients who had not undergone an initial plasma-exchange session before study enrollment, the median time to response was 3.0 days with caplacizumab and 4.9 days with placebo. And among the six patients who had undergone an initial plasma-exchange session before enrollment, the median time to response was 2.4 days with caplacizumab and 4.3 days with placebo, Dr. Peyvandi and her associates said (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMoa1505533).
At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group. Three patients in the caplacizumab group had TTP exacerbations, compared with 11 in the placebo group. Post hoc analyses showed that the mean number of plasma-exchange days (5.9 vs. 7.9) and the mean volume of plasma administered (19.9 liters vs. 28.3 liters) were lower with caplacizumab than with placebo. And post hoc analyses of markers of end-organ damage, such as lactate dehydrogenase, troponin T, troponin 1, and creatinine levels, showed more rapid normalization with caplacizumab.
As expected, the number of patients who had bleeding-related adverse events was higher with the active treatment (19 patients) than with placebo (14 patients), but these events “were generally mild” and didn’t require treatment. Serious bleeding events occurred in 2 patients in each study group and included subarachnoid hemorrhage, retinal hemorrhage, and metrorrhagia in the caplacizumab group and cerebral hemorrhage and hematuria in the placebo group.
The study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
These study findings prompt speculation that effective treatment of TTP will require a combination of interventions that target different aspects of the disorder’s pathophysiology.
First, using caplacizumab or similar agents to interfere with the binding of von Willebrand factor to platelets will prevent formation of new microthrombi. Second, replacing the specific cleaving metalloprotease ADAMTS13 using normal plasma will restore von Willebrand factor multimers to the appropriate size. And third, inducing disaggregation of platelet-rich thrombi will be the final step, but we haven’t yet found the means to accomplish that.
Dr. Agnes Veyradier is at the Institute of Hematology at the French Reference Center for Thrombotic Microangiopathies, Paris, and University Paris Diderot. Dr. Veyradier reported having no relevant financial disclosures and made these remarks in an editorial accompanying Dr. Peyvandi’s report (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMe151876).
These study findings prompt speculation that effective treatment of TTP will require a combination of interventions that target different aspects of the disorder’s pathophysiology.
First, using caplacizumab or similar agents to interfere with the binding of von Willebrand factor to platelets will prevent formation of new microthrombi. Second, replacing the specific cleaving metalloprotease ADAMTS13 using normal plasma will restore von Willebrand factor multimers to the appropriate size. And third, inducing disaggregation of platelet-rich thrombi will be the final step, but we haven’t yet found the means to accomplish that.
Dr. Agnes Veyradier is at the Institute of Hematology at the French Reference Center for Thrombotic Microangiopathies, Paris, and University Paris Diderot. Dr. Veyradier reported having no relevant financial disclosures and made these remarks in an editorial accompanying Dr. Peyvandi’s report (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMe151876).
These study findings prompt speculation that effective treatment of TTP will require a combination of interventions that target different aspects of the disorder’s pathophysiology.
First, using caplacizumab or similar agents to interfere with the binding of von Willebrand factor to platelets will prevent formation of new microthrombi. Second, replacing the specific cleaving metalloprotease ADAMTS13 using normal plasma will restore von Willebrand factor multimers to the appropriate size. And third, inducing disaggregation of platelet-rich thrombi will be the final step, but we haven’t yet found the means to accomplish that.
Dr. Agnes Veyradier is at the Institute of Hematology at the French Reference Center for Thrombotic Microangiopathies, Paris, and University Paris Diderot. Dr. Veyradier reported having no relevant financial disclosures and made these remarks in an editorial accompanying Dr. Peyvandi’s report (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMe151876).
Caplacizumab induces faster resolution of acute episodes of acquired thrombotic thrombocytopenic purpura than does conventional therapy by blocking further platelet aggregation mediated by von Willebrand factor, according to a report published online Feb. 11 in the New England Journal of Medicine.
Faster normalization of the platelet count “prevents further consumption of platelets into microthrombi, and the consequent progression of tissue ischemia.” This in turn should prevent further ischemic injury to the brain, heart, and kidneys in both the short and the long term, said Dr. Flora Peyvandi of the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Ospedale Maggiore Policlinico, Milan, and her associates.
The investigators assessed caplacizumab, an anti–von Willebrand factor immunoglobulin, as a potential treatment for acquired TTP in a manufacturer-sponsored phase II trial involving 75 patients treated at 56 medical centers worldwide. All the study participants received standard treatment – daily plasma exchange and immunosuppressive therapy. In addition, they were randomly assigned to receive daily caplacizumab (36 patients) or placebo injections (39 patients) after each plasma exchange procedure and for 30 days following the final procedure, for a maximum of 90 days.
“Caplacizumab rapidly neutralized its target as indicated by suppression of von Willebrand factor–ristocetin cofactor activity to a mean of less than 20% by day 1 and throughout the treatment period,” Dr. Peyvandi and her associates said. These values returned to baseline levels within 1 week of treatment cessation.
The primary endpoint of the study, median time to normalization of the platelet count, was significantly reduced by 39% with caplacizumab compared with placebo. Among the 69 patients who had not undergone an initial plasma-exchange session before study enrollment, the median time to response was 3.0 days with caplacizumab and 4.9 days with placebo. And among the six patients who had undergone an initial plasma-exchange session before enrollment, the median time to response was 2.4 days with caplacizumab and 4.3 days with placebo, Dr. Peyvandi and her associates said (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMoa1505533).
At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group. Three patients in the caplacizumab group had TTP exacerbations, compared with 11 in the placebo group. Post hoc analyses showed that the mean number of plasma-exchange days (5.9 vs. 7.9) and the mean volume of plasma administered (19.9 liters vs. 28.3 liters) were lower with caplacizumab than with placebo. And post hoc analyses of markers of end-organ damage, such as lactate dehydrogenase, troponin T, troponin 1, and creatinine levels, showed more rapid normalization with caplacizumab.
As expected, the number of patients who had bleeding-related adverse events was higher with the active treatment (19 patients) than with placebo (14 patients), but these events “were generally mild” and didn’t require treatment. Serious bleeding events occurred in 2 patients in each study group and included subarachnoid hemorrhage, retinal hemorrhage, and metrorrhagia in the caplacizumab group and cerebral hemorrhage and hematuria in the placebo group.
The study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
Caplacizumab induces faster resolution of acute episodes of acquired thrombotic thrombocytopenic purpura than does conventional therapy by blocking further platelet aggregation mediated by von Willebrand factor, according to a report published online Feb. 11 in the New England Journal of Medicine.
Faster normalization of the platelet count “prevents further consumption of platelets into microthrombi, and the consequent progression of tissue ischemia.” This in turn should prevent further ischemic injury to the brain, heart, and kidneys in both the short and the long term, said Dr. Flora Peyvandi of the Angelo Bianchi Bonomi Hemophilia and Thrombosis Center, Ospedale Maggiore Policlinico, Milan, and her associates.
The investigators assessed caplacizumab, an anti–von Willebrand factor immunoglobulin, as a potential treatment for acquired TTP in a manufacturer-sponsored phase II trial involving 75 patients treated at 56 medical centers worldwide. All the study participants received standard treatment – daily plasma exchange and immunosuppressive therapy. In addition, they were randomly assigned to receive daily caplacizumab (36 patients) or placebo injections (39 patients) after each plasma exchange procedure and for 30 days following the final procedure, for a maximum of 90 days.
“Caplacizumab rapidly neutralized its target as indicated by suppression of von Willebrand factor–ristocetin cofactor activity to a mean of less than 20% by day 1 and throughout the treatment period,” Dr. Peyvandi and her associates said. These values returned to baseline levels within 1 week of treatment cessation.
The primary endpoint of the study, median time to normalization of the platelet count, was significantly reduced by 39% with caplacizumab compared with placebo. Among the 69 patients who had not undergone an initial plasma-exchange session before study enrollment, the median time to response was 3.0 days with caplacizumab and 4.9 days with placebo. And among the six patients who had undergone an initial plasma-exchange session before enrollment, the median time to response was 2.4 days with caplacizumab and 4.3 days with placebo, Dr. Peyvandi and her associates said (N Engl J Med. 2016 Feb 11;374[6]. doi:10.1056/NEJMoa1505533).
At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group. Three patients in the caplacizumab group had TTP exacerbations, compared with 11 in the placebo group. Post hoc analyses showed that the mean number of plasma-exchange days (5.9 vs. 7.9) and the mean volume of plasma administered (19.9 liters vs. 28.3 liters) were lower with caplacizumab than with placebo. And post hoc analyses of markers of end-organ damage, such as lactate dehydrogenase, troponin T, troponin 1, and creatinine levels, showed more rapid normalization with caplacizumab.
As expected, the number of patients who had bleeding-related adverse events was higher with the active treatment (19 patients) than with placebo (14 patients), but these events “were generally mild” and didn’t require treatment. Serious bleeding events occurred in 2 patients in each study group and included subarachnoid hemorrhage, retinal hemorrhage, and metrorrhagia in the caplacizumab group and cerebral hemorrhage and hematuria in the placebo group.
The study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Caplacizumab induces more rapid resolution of an acute TTP episode than does conventional therapy.
Major finding: At 1-month follow-up, 81% of the caplacizumab group showed complete remission, compared with 46% of the placebo group.
Data source: An international single-blind placebo-controlled phase II trial involving 75 patients treated over a 3.5-year period.
Disclosures: This study was supported by Ablynx, maker of caplacizumab, which also designed and conducted the study, analyzed the data, and prepared the manuscript. Dr. Peyvandi reported ties to Ablynx, Alexion, Biotest, Kedrion Biopharma, Novo Nordisk, Baxter, Bayer, CSL Behring, Grifols, LFB, Roche, and Sobi; her associates’ financial disclosures are available at NEJM.org.
Behavioral interventions cut inappropriate antibiotic prescribing
Two behavioral interventions for primary care clinicians cut the rate of inappropriate antibiotic prescribing for acute respiratory tract infections significantly, according to a report published online Feb. 9 in JAMA.
Compared with a control condition that included clinician education, the two interventions reduced inappropriate prescribing by 5.2% and 7.0%, respectively.
“We believe these effect sizes are clinically significant, especially when measured against control clinicians who were motivated to join a trial, knew they were being monitored, and who had relatively low antibiotic prescribing rates at baseline,” said Daniella Meeker, Ph.D., of the Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, and her associates.
In a cluster-randomized trial involving 248 clinicians at 47 primary care practices in Boston and Los Angeles, the investigators designed three interventions and tested various combinations of them against a control condition during an 18-month period. The number of inappropriate antibiotic prescriptions given during this intervention period was then compared with that during a baseline period, the 18 months preceding the intervention.
The analysis included 14,753 patient visits for acute respiratory tract infections during the baseline period and 16,959 visits during the intervention period.
The first behavioral intervention, termed “accountable justification,” used an alert each time a clinician prescribed an antibiotic in a patient’s electronic health record – a prompt asking for an explicit justification for doing so. That approach was based on the hope that to preserve their reputations, clinicians would tailor their behavior to fall in line with norms followed by their peers and recommended in clinical guidelines.
The second intervention, “peer comparison,” used monthly e-mails to inform clinicians whether or not they were “top performers” (within the lowest decile) for inappropriate prescribing in their geographical region. The emails included the number and proportion of antibiotic prescriptions they wrote inappropriately for acute upper respiratory tract infections, compared with the proportion written by top performers.
The mean rate of antibiotic prescribing decreased during the intervention in all the study groups, including the control group, which showed an absolute decrease of 11% (from 24.1% to 13.1%). The absolute decrease was significantly greater, at 18.1%, in the accountable justification group (from 23.2% to 5.2%) and at 16.3% in the peer comparison group (from 19.9% to 3.7%), Dr. Meeker and her associates said (JAMA. 2016 Feb 9;315[6]:562-70). The rate of return visits for possible bacterial infections within 30 days of the index visit was used as a measure of safety for withholding antibiotic prescriptions. This rate was 0.4% in the control group. The only intervention group that showed a “modestly higher” rate of return visits was the one that used both the accountable justification and the peer comparison interventions together, for which the rate of return visits was 1.4%.
The study was supported by the American Recovery and Reinvestment Act of 2009, the National Institutes of Health, the National Institute on Aging, the Agency for Healthcare Research and Quality, the University of Southern California’s Medical Information Network for Experimental Research, and the Patient-Centered Outcomes Research Institute. Dr. Meeker and her associates reported having no relevant financial disclosures.
Even though the reductions in inappropriate prescribing in this study might be considered modest, they were real, important, and potentially sustainable.
Baseline levels of inappropriate prescribing were already low to start with among the study participants, which suggests that they already were judicious prescribers in relation to national averages. In addition, the control group participants knew their antibiotic prescribing was being monitored and may have decreased it, consciously or unconsciously. Both of these factors may have blunted the potential effectiveness of the interventions.
Dr. Jeffrey S. Gerber is in the division of infectious diseases at the Children’s Hospital of Philadelphia and in the department of pediatrics at the University of Pennsylvania, Philadelphia. He reported having no conflicts of interest. Dr. Gerber made these remarks in an editorial accompanying Dr. Meeker’s report (JAMA. 2016 Feb 9;315[6]:558-9).
Even though the reductions in inappropriate prescribing in this study might be considered modest, they were real, important, and potentially sustainable.
Baseline levels of inappropriate prescribing were already low to start with among the study participants, which suggests that they already were judicious prescribers in relation to national averages. In addition, the control group participants knew their antibiotic prescribing was being monitored and may have decreased it, consciously or unconsciously. Both of these factors may have blunted the potential effectiveness of the interventions.
Dr. Jeffrey S. Gerber is in the division of infectious diseases at the Children’s Hospital of Philadelphia and in the department of pediatrics at the University of Pennsylvania, Philadelphia. He reported having no conflicts of interest. Dr. Gerber made these remarks in an editorial accompanying Dr. Meeker’s report (JAMA. 2016 Feb 9;315[6]:558-9).
Even though the reductions in inappropriate prescribing in this study might be considered modest, they were real, important, and potentially sustainable.
Baseline levels of inappropriate prescribing were already low to start with among the study participants, which suggests that they already were judicious prescribers in relation to national averages. In addition, the control group participants knew their antibiotic prescribing was being monitored and may have decreased it, consciously or unconsciously. Both of these factors may have blunted the potential effectiveness of the interventions.
Dr. Jeffrey S. Gerber is in the division of infectious diseases at the Children’s Hospital of Philadelphia and in the department of pediatrics at the University of Pennsylvania, Philadelphia. He reported having no conflicts of interest. Dr. Gerber made these remarks in an editorial accompanying Dr. Meeker’s report (JAMA. 2016 Feb 9;315[6]:558-9).
Two behavioral interventions for primary care clinicians cut the rate of inappropriate antibiotic prescribing for acute respiratory tract infections significantly, according to a report published online Feb. 9 in JAMA.
Compared with a control condition that included clinician education, the two interventions reduced inappropriate prescribing by 5.2% and 7.0%, respectively.
“We believe these effect sizes are clinically significant, especially when measured against control clinicians who were motivated to join a trial, knew they were being monitored, and who had relatively low antibiotic prescribing rates at baseline,” said Daniella Meeker, Ph.D., of the Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, and her associates.
In a cluster-randomized trial involving 248 clinicians at 47 primary care practices in Boston and Los Angeles, the investigators designed three interventions and tested various combinations of them against a control condition during an 18-month period. The number of inappropriate antibiotic prescriptions given during this intervention period was then compared with that during a baseline period, the 18 months preceding the intervention.
The analysis included 14,753 patient visits for acute respiratory tract infections during the baseline period and 16,959 visits during the intervention period.
The first behavioral intervention, termed “accountable justification,” used an alert each time a clinician prescribed an antibiotic in a patient’s electronic health record – a prompt asking for an explicit justification for doing so. That approach was based on the hope that to preserve their reputations, clinicians would tailor their behavior to fall in line with norms followed by their peers and recommended in clinical guidelines.
The second intervention, “peer comparison,” used monthly e-mails to inform clinicians whether or not they were “top performers” (within the lowest decile) for inappropriate prescribing in their geographical region. The emails included the number and proportion of antibiotic prescriptions they wrote inappropriately for acute upper respiratory tract infections, compared with the proportion written by top performers.
The mean rate of antibiotic prescribing decreased during the intervention in all the study groups, including the control group, which showed an absolute decrease of 11% (from 24.1% to 13.1%). The absolute decrease was significantly greater, at 18.1%, in the accountable justification group (from 23.2% to 5.2%) and at 16.3% in the peer comparison group (from 19.9% to 3.7%), Dr. Meeker and her associates said (JAMA. 2016 Feb 9;315[6]:562-70). The rate of return visits for possible bacterial infections within 30 days of the index visit was used as a measure of safety for withholding antibiotic prescriptions. This rate was 0.4% in the control group. The only intervention group that showed a “modestly higher” rate of return visits was the one that used both the accountable justification and the peer comparison interventions together, for which the rate of return visits was 1.4%.
The study was supported by the American Recovery and Reinvestment Act of 2009, the National Institutes of Health, the National Institute on Aging, the Agency for Healthcare Research and Quality, the University of Southern California’s Medical Information Network for Experimental Research, and the Patient-Centered Outcomes Research Institute. Dr. Meeker and her associates reported having no relevant financial disclosures.
Two behavioral interventions for primary care clinicians cut the rate of inappropriate antibiotic prescribing for acute respiratory tract infections significantly, according to a report published online Feb. 9 in JAMA.
Compared with a control condition that included clinician education, the two interventions reduced inappropriate prescribing by 5.2% and 7.0%, respectively.
“We believe these effect sizes are clinically significant, especially when measured against control clinicians who were motivated to join a trial, knew they were being monitored, and who had relatively low antibiotic prescribing rates at baseline,” said Daniella Meeker, Ph.D., of the Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, and her associates.
In a cluster-randomized trial involving 248 clinicians at 47 primary care practices in Boston and Los Angeles, the investigators designed three interventions and tested various combinations of them against a control condition during an 18-month period. The number of inappropriate antibiotic prescriptions given during this intervention period was then compared with that during a baseline period, the 18 months preceding the intervention.
The analysis included 14,753 patient visits for acute respiratory tract infections during the baseline period and 16,959 visits during the intervention period.
The first behavioral intervention, termed “accountable justification,” used an alert each time a clinician prescribed an antibiotic in a patient’s electronic health record – a prompt asking for an explicit justification for doing so. That approach was based on the hope that to preserve their reputations, clinicians would tailor their behavior to fall in line with norms followed by their peers and recommended in clinical guidelines.
The second intervention, “peer comparison,” used monthly e-mails to inform clinicians whether or not they were “top performers” (within the lowest decile) for inappropriate prescribing in their geographical region. The emails included the number and proportion of antibiotic prescriptions they wrote inappropriately for acute upper respiratory tract infections, compared with the proportion written by top performers.
The mean rate of antibiotic prescribing decreased during the intervention in all the study groups, including the control group, which showed an absolute decrease of 11% (from 24.1% to 13.1%). The absolute decrease was significantly greater, at 18.1%, in the accountable justification group (from 23.2% to 5.2%) and at 16.3% in the peer comparison group (from 19.9% to 3.7%), Dr. Meeker and her associates said (JAMA. 2016 Feb 9;315[6]:562-70). The rate of return visits for possible bacterial infections within 30 days of the index visit was used as a measure of safety for withholding antibiotic prescriptions. This rate was 0.4% in the control group. The only intervention group that showed a “modestly higher” rate of return visits was the one that used both the accountable justification and the peer comparison interventions together, for which the rate of return visits was 1.4%.
The study was supported by the American Recovery and Reinvestment Act of 2009, the National Institutes of Health, the National Institute on Aging, the Agency for Healthcare Research and Quality, the University of Southern California’s Medical Information Network for Experimental Research, and the Patient-Centered Outcomes Research Institute. Dr. Meeker and her associates reported having no relevant financial disclosures.
FROM JAMA
Key clinical point: Two behavioral interventions for primary care clinicians cut the rate of inappropriate antibiotic prescribing for acute respiratory tract infections.
Major finding: The absolute decrease in inappropriate antibiotics prescribing was significantly greater – at 18.1% in the “accountable justification” group and at 16.3% in the “peer comparison” group – than the control group (11%).
Data source: A cluster-randomized clinical trial involving 248 clinicians at 47 primary care practices in Boston and Los Angeles.
Disclosures: The study was supported by the American Recovery and Reinvestment Act of 2009, the National Institutes of Health, the National Institute on Aging, the Agency for Healthcare Research and Quality, the University of Southern California’s Medical Information Network for Experimental Research, and the Patient-Centered Outcomes Research Institute. Dr. Meeker and her associates reported having no relevant financial disclosures.
ACP guideline: CBT, antidepressants similarly effective for major depression
Cognitive behavioral therapy (CBT) and second-generation antidepressants have similar efficacy and are both viable choices for adult patients who have major depressive disorder, according to a new clinical practice guideline published online Feb. 8 in the Annals of Internal Medicine.
“Although second-generation antidepressants are often initially prescribed for patients with depression, CBT is a reasonable approach for initial treatment and should be strongly considered as an alternative,” said Amir Qaseem, M.D., Ph.D., of the American College of Physicians, Philadelphia, and his associates on the clinical guidelines committee (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M15-2570).
Until now, the relative benefits and harms of antidepressant medications vs. other therapies were unclear. The guideline was intended “to summarize and grade the evidence on the comparative effectiveness and safety of nonpharmacologic treatments and [antidepressants], alone or in combination.”
It is based on a systematic review and meta-analysis of 45 English-, German-, and Italian-language randomized, controlled trials of at least 6 weeks’ duration published from 1990 to September 2015, as well as relevant unpublished research. The Evidence-Based Practice Center of the federal Agency for Healthcare Research and Quality and the University of North Carolina at Chapel Hill conducted the review.
The nonpharmacologic treatments that were assessed included:
• Cognitive therapy, which aims to correct negative thoughts and false self-beliefs.
• CBT, which includes a behavioral component such as activity scheduling and homework.
• Acceptance and commitment therapy, which uses mindfulness techniques to help patients accept problems and counter negative thoughts;
• Interpersonal therapy, which focuses on relationships.
• Psychodynamic therapy, which targets past experiences and conscious and unconscious feelings.
• Third-wave CBT, which focuses on thought processes to help patients achieve awareness and acceptance.
The complementary and alternative treatments that were examined included acupuncture, meditation, yoga, and the use of omega-3 fatty acid supplements, SAMe, or St. John’s wort. Exercise as practiced individually, in informal groups, or in classes also was assessed.
The second-generation antidepressants that were assessed included selective serotonin reuptake inhibitors (such as fluoxetine, sertraline, and paroxetine), serotonin norepinephrine reuptake inhibitors (such as venlafaxine and duloxetine), selective serotonin norepinephrine reuptake inhibitors, and bupropion, mirtazapine, nefazodone, and trazodone.
The outcomes of interest with antidepressants were response rates, remission rates, speed of response, speed of remission, relapse rates, adverse events, quality of life, functional capacity, reduction of suicidality, and reduction of hospitalization.
Moderate-quality evidence showed no significant difference between antidepressants and CBT in either treatment response or discontinuation of treatment because of adverse effects.
Therefore, the guideline strongly recommends that “clinicians select between either CBT or second-generation antidepressants to treat patients with major depressive disorder after discussing treatment effects, adverse effect profiles, cost, accessibility, and preferences with the patient,” Dr. Qaseem and his associates said.
The guideline also states that most patients do not achieve remission after initial treatment with antidepressants, so “switching therapies or augmenting with additional interventions may be warranted.”
Regarding other treatments and other outcomes, the evidence was considered too sparse and of such low quality that it would not support any other recommendations at this time. Most of the reviewed studies were flawed by high dropout rates, dosing inequalities, small sample sizes, and poor evaluation of adverse events, according to Dr. Gerald Gartlehner and his associates at the Evidence-Based Practice Center, Chapel Hill, N.C. (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M15-1813).
The guideline and the review/meta-analysis of the literature are available at www.annals.org. The guideline work was supported exclusively by the American College of Physicians. Dr. Qaseem disclosed no conflicts of interest. Complete disclosures for the members of the clinical guideline committee are available at www.acponline.org.
Generalist physicians should seize the day and implement the new ACP guidelines for their adult patients who have depression.
Integrating care for depression into primary health care can be especially effective if clinicians provide support for patient self-management, conduct follow-up that includes careful assessment of treatment response and adherence, coordinate with mental health specialists to increase patient access to the full range of psychological treatments, and pursue more intensive treatment for refractory depression.
To screen patients for depression, the Patient Health Questionnaire-2 is a validated tool that can easily be administered verbally. But all practice staff must understand the importance of screening, must know how to introduce screening to patients, must understand patients’ concerns about confidentiality, and must administer the screen verbatim.
Dr. John W. Williams Jr. is at Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gary R. Maslow is at Duke University, Durham, N.C. Their financial disclosures are available at www.acponline.org. Dr. Williams and Dr. Maslow made these remarks in an editorial accompanying the ACP’s clinical practice guideline (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M16-0104).
Generalist physicians should seize the day and implement the new ACP guidelines for their adult patients who have depression.
Integrating care for depression into primary health care can be especially effective if clinicians provide support for patient self-management, conduct follow-up that includes careful assessment of treatment response and adherence, coordinate with mental health specialists to increase patient access to the full range of psychological treatments, and pursue more intensive treatment for refractory depression.
To screen patients for depression, the Patient Health Questionnaire-2 is a validated tool that can easily be administered verbally. But all practice staff must understand the importance of screening, must know how to introduce screening to patients, must understand patients’ concerns about confidentiality, and must administer the screen verbatim.
Dr. John W. Williams Jr. is at Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gary R. Maslow is at Duke University, Durham, N.C. Their financial disclosures are available at www.acponline.org. Dr. Williams and Dr. Maslow made these remarks in an editorial accompanying the ACP’s clinical practice guideline (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M16-0104).
Generalist physicians should seize the day and implement the new ACP guidelines for their adult patients who have depression.
Integrating care for depression into primary health care can be especially effective if clinicians provide support for patient self-management, conduct follow-up that includes careful assessment of treatment response and adherence, coordinate with mental health specialists to increase patient access to the full range of psychological treatments, and pursue more intensive treatment for refractory depression.
To screen patients for depression, the Patient Health Questionnaire-2 is a validated tool that can easily be administered verbally. But all practice staff must understand the importance of screening, must know how to introduce screening to patients, must understand patients’ concerns about confidentiality, and must administer the screen verbatim.
Dr. John W. Williams Jr. is at Durham Veterans Affairs Medical Center, Durham, N.C. Dr. Gary R. Maslow is at Duke University, Durham, N.C. Their financial disclosures are available at www.acponline.org. Dr. Williams and Dr. Maslow made these remarks in an editorial accompanying the ACP’s clinical practice guideline (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M16-0104).
Cognitive behavioral therapy (CBT) and second-generation antidepressants have similar efficacy and are both viable choices for adult patients who have major depressive disorder, according to a new clinical practice guideline published online Feb. 8 in the Annals of Internal Medicine.
“Although second-generation antidepressants are often initially prescribed for patients with depression, CBT is a reasonable approach for initial treatment and should be strongly considered as an alternative,” said Amir Qaseem, M.D., Ph.D., of the American College of Physicians, Philadelphia, and his associates on the clinical guidelines committee (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M15-2570).
Until now, the relative benefits and harms of antidepressant medications vs. other therapies were unclear. The guideline was intended “to summarize and grade the evidence on the comparative effectiveness and safety of nonpharmacologic treatments and [antidepressants], alone or in combination.”
It is based on a systematic review and meta-analysis of 45 English-, German-, and Italian-language randomized, controlled trials of at least 6 weeks’ duration published from 1990 to September 2015, as well as relevant unpublished research. The Evidence-Based Practice Center of the federal Agency for Healthcare Research and Quality and the University of North Carolina at Chapel Hill conducted the review.
The nonpharmacologic treatments that were assessed included:
• Cognitive therapy, which aims to correct negative thoughts and false self-beliefs.
• CBT, which includes a behavioral component such as activity scheduling and homework.
• Acceptance and commitment therapy, which uses mindfulness techniques to help patients accept problems and counter negative thoughts;
• Interpersonal therapy, which focuses on relationships.
• Psychodynamic therapy, which targets past experiences and conscious and unconscious feelings.
• Third-wave CBT, which focuses on thought processes to help patients achieve awareness and acceptance.
The complementary and alternative treatments that were examined included acupuncture, meditation, yoga, and the use of omega-3 fatty acid supplements, SAMe, or St. John’s wort. Exercise as practiced individually, in informal groups, or in classes also was assessed.
The second-generation antidepressants that were assessed included selective serotonin reuptake inhibitors (such as fluoxetine, sertraline, and paroxetine), serotonin norepinephrine reuptake inhibitors (such as venlafaxine and duloxetine), selective serotonin norepinephrine reuptake inhibitors, and bupropion, mirtazapine, nefazodone, and trazodone.
The outcomes of interest with antidepressants were response rates, remission rates, speed of response, speed of remission, relapse rates, adverse events, quality of life, functional capacity, reduction of suicidality, and reduction of hospitalization.
Moderate-quality evidence showed no significant difference between antidepressants and CBT in either treatment response or discontinuation of treatment because of adverse effects.
Therefore, the guideline strongly recommends that “clinicians select between either CBT or second-generation antidepressants to treat patients with major depressive disorder after discussing treatment effects, adverse effect profiles, cost, accessibility, and preferences with the patient,” Dr. Qaseem and his associates said.
The guideline also states that most patients do not achieve remission after initial treatment with antidepressants, so “switching therapies or augmenting with additional interventions may be warranted.”
Regarding other treatments and other outcomes, the evidence was considered too sparse and of such low quality that it would not support any other recommendations at this time. Most of the reviewed studies were flawed by high dropout rates, dosing inequalities, small sample sizes, and poor evaluation of adverse events, according to Dr. Gerald Gartlehner and his associates at the Evidence-Based Practice Center, Chapel Hill, N.C. (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M15-1813).
The guideline and the review/meta-analysis of the literature are available at www.annals.org. The guideline work was supported exclusively by the American College of Physicians. Dr. Qaseem disclosed no conflicts of interest. Complete disclosures for the members of the clinical guideline committee are available at www.acponline.org.
Cognitive behavioral therapy (CBT) and second-generation antidepressants have similar efficacy and are both viable choices for adult patients who have major depressive disorder, according to a new clinical practice guideline published online Feb. 8 in the Annals of Internal Medicine.
“Although second-generation antidepressants are often initially prescribed for patients with depression, CBT is a reasonable approach for initial treatment and should be strongly considered as an alternative,” said Amir Qaseem, M.D., Ph.D., of the American College of Physicians, Philadelphia, and his associates on the clinical guidelines committee (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M15-2570).
Until now, the relative benefits and harms of antidepressant medications vs. other therapies were unclear. The guideline was intended “to summarize and grade the evidence on the comparative effectiveness and safety of nonpharmacologic treatments and [antidepressants], alone or in combination.”
It is based on a systematic review and meta-analysis of 45 English-, German-, and Italian-language randomized, controlled trials of at least 6 weeks’ duration published from 1990 to September 2015, as well as relevant unpublished research. The Evidence-Based Practice Center of the federal Agency for Healthcare Research and Quality and the University of North Carolina at Chapel Hill conducted the review.
The nonpharmacologic treatments that were assessed included:
• Cognitive therapy, which aims to correct negative thoughts and false self-beliefs.
• CBT, which includes a behavioral component such as activity scheduling and homework.
• Acceptance and commitment therapy, which uses mindfulness techniques to help patients accept problems and counter negative thoughts;
• Interpersonal therapy, which focuses on relationships.
• Psychodynamic therapy, which targets past experiences and conscious and unconscious feelings.
• Third-wave CBT, which focuses on thought processes to help patients achieve awareness and acceptance.
The complementary and alternative treatments that were examined included acupuncture, meditation, yoga, and the use of omega-3 fatty acid supplements, SAMe, or St. John’s wort. Exercise as practiced individually, in informal groups, or in classes also was assessed.
The second-generation antidepressants that were assessed included selective serotonin reuptake inhibitors (such as fluoxetine, sertraline, and paroxetine), serotonin norepinephrine reuptake inhibitors (such as venlafaxine and duloxetine), selective serotonin norepinephrine reuptake inhibitors, and bupropion, mirtazapine, nefazodone, and trazodone.
The outcomes of interest with antidepressants were response rates, remission rates, speed of response, speed of remission, relapse rates, adverse events, quality of life, functional capacity, reduction of suicidality, and reduction of hospitalization.
Moderate-quality evidence showed no significant difference between antidepressants and CBT in either treatment response or discontinuation of treatment because of adverse effects.
Therefore, the guideline strongly recommends that “clinicians select between either CBT or second-generation antidepressants to treat patients with major depressive disorder after discussing treatment effects, adverse effect profiles, cost, accessibility, and preferences with the patient,” Dr. Qaseem and his associates said.
The guideline also states that most patients do not achieve remission after initial treatment with antidepressants, so “switching therapies or augmenting with additional interventions may be warranted.”
Regarding other treatments and other outcomes, the evidence was considered too sparse and of such low quality that it would not support any other recommendations at this time. Most of the reviewed studies were flawed by high dropout rates, dosing inequalities, small sample sizes, and poor evaluation of adverse events, according to Dr. Gerald Gartlehner and his associates at the Evidence-Based Practice Center, Chapel Hill, N.C. (Ann Intern Med. 2016 Feb 9; doi: 10.7326/M15-1813).
The guideline and the review/meta-analysis of the literature are available at www.annals.org. The guideline work was supported exclusively by the American College of Physicians. Dr. Qaseem disclosed no conflicts of interest. Complete disclosures for the members of the clinical guideline committee are available at www.acponline.org.
FROM ANNALS OF INTERNAL MEDICINE
Key clinical point: Cognitive behavioral therapy and antidepressant medications are similarly effective for adults with major depressive disorder.
Major finding: Moderate-quality evidence from 45 clinical trials showed no significant difference between antidepressants and cognitive behavioral therapy in either treatment response or discontinuation of treatment due to adverse effects.
Data source: A comprehensive review of the literature and compilation of a new clinical practice guideline by the American College of Physicians for primary care clinicians.
Disclosures: The guideline work was supported exclusively by the American College of Physicians. Dr. Qaseem disclosed no conflicts of interest. Complete disclosures for Dr. Williams, Dr. Maslow, and the members of the clinical guideline committee are available at www.acponline.org.
Survivors of childhood ALL show significant attention problems
Survivors of childhood acute lymphoblastic leukemia (ALL) who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later, according to a report published online Feb. 8 in the Journal of Clinical Oncology.
The attention problems were isolated from intelligence and academic performance, but they still “significantly and negatively impact real-world functioning,” said Lisa M. Jacola, Ph.D. of the department of psychology and her associates at St. Jude Children’s Research Hospital, Memphis (J Clin Oncol. 2016 Feb 8. [doi: 10.1200/JCO.2015.64.3205]).
Intrathecal chemotherapy has largely replaced cranial radiation therapy to protect the CNS in childhood ALL, but most research concerning neurocognitive outcomes after this treatment has been retrospective and has involved small sample sizes. So investigators performed a prospective study using data from an ongoing trial involving a representative cohort of more than 400 patients aged 1-18 years who were treated at a single center during a 7-year period.
These patients were classified as low- or high-risk based on comprehensive biologic and clinical factors, such as blast cell immunophenotype and genotype, presenting clinical features, and early treatment response. They then received intrathecal methotrexate, cytarabine, and hydrocortisone in doses appropriate to their risk status, as well as standardized leucovorin followed by mercaptopurine and vincristine plus dexamethasone.
The researchers focused on the 211 patients who underwent comprehensive neurocognitive assessment 2 years after diagnosis and treatment. “The overall group did not significantly differ from normative expectations on measures of global intelligence (estimated IQ), academic skills (reading, math, or spelling), and learning and memory.” However, half of them were rated as below average on several measures of attention. In addition, caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems in the children.
This adverse effect was strongest among children who were younger than age 5 at diagnosis and among those at high risk who received more aggressive treatment.
“Our findings ... emphasize the importance of routine neurocognitive monitoring of all survivors treated with contemporary therapy.” But early detection will only be helpful if effective interventions are developed to remediate these attention and behavior problems. Research has shown that pharmacologic therapies “have reduced acceptability among survivors of childhood cancer,” so nonpharmacologic approaches would be especially useful, the researchers added.
Survivors of childhood acute lymphoblastic leukemia (ALL) who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later, according to a report published online Feb. 8 in the Journal of Clinical Oncology.
The attention problems were isolated from intelligence and academic performance, but they still “significantly and negatively impact real-world functioning,” said Lisa M. Jacola, Ph.D. of the department of psychology and her associates at St. Jude Children’s Research Hospital, Memphis (J Clin Oncol. 2016 Feb 8. [doi: 10.1200/JCO.2015.64.3205]).
Intrathecal chemotherapy has largely replaced cranial radiation therapy to protect the CNS in childhood ALL, but most research concerning neurocognitive outcomes after this treatment has been retrospective and has involved small sample sizes. So investigators performed a prospective study using data from an ongoing trial involving a representative cohort of more than 400 patients aged 1-18 years who were treated at a single center during a 7-year period.
These patients were classified as low- or high-risk based on comprehensive biologic and clinical factors, such as blast cell immunophenotype and genotype, presenting clinical features, and early treatment response. They then received intrathecal methotrexate, cytarabine, and hydrocortisone in doses appropriate to their risk status, as well as standardized leucovorin followed by mercaptopurine and vincristine plus dexamethasone.
The researchers focused on the 211 patients who underwent comprehensive neurocognitive assessment 2 years after diagnosis and treatment. “The overall group did not significantly differ from normative expectations on measures of global intelligence (estimated IQ), academic skills (reading, math, or spelling), and learning and memory.” However, half of them were rated as below average on several measures of attention. In addition, caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems in the children.
This adverse effect was strongest among children who were younger than age 5 at diagnosis and among those at high risk who received more aggressive treatment.
“Our findings ... emphasize the importance of routine neurocognitive monitoring of all survivors treated with contemporary therapy.” But early detection will only be helpful if effective interventions are developed to remediate these attention and behavior problems. Research has shown that pharmacologic therapies “have reduced acceptability among survivors of childhood cancer,” so nonpharmacologic approaches would be especially useful, the researchers added.
Survivors of childhood acute lymphoblastic leukemia (ALL) who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later, according to a report published online Feb. 8 in the Journal of Clinical Oncology.
The attention problems were isolated from intelligence and academic performance, but they still “significantly and negatively impact real-world functioning,” said Lisa M. Jacola, Ph.D. of the department of psychology and her associates at St. Jude Children’s Research Hospital, Memphis (J Clin Oncol. 2016 Feb 8. [doi: 10.1200/JCO.2015.64.3205]).
Intrathecal chemotherapy has largely replaced cranial radiation therapy to protect the CNS in childhood ALL, but most research concerning neurocognitive outcomes after this treatment has been retrospective and has involved small sample sizes. So investigators performed a prospective study using data from an ongoing trial involving a representative cohort of more than 400 patients aged 1-18 years who were treated at a single center during a 7-year period.
These patients were classified as low- or high-risk based on comprehensive biologic and clinical factors, such as blast cell immunophenotype and genotype, presenting clinical features, and early treatment response. They then received intrathecal methotrexate, cytarabine, and hydrocortisone in doses appropriate to their risk status, as well as standardized leucovorin followed by mercaptopurine and vincristine plus dexamethasone.
The researchers focused on the 211 patients who underwent comprehensive neurocognitive assessment 2 years after diagnosis and treatment. “The overall group did not significantly differ from normative expectations on measures of global intelligence (estimated IQ), academic skills (reading, math, or spelling), and learning and memory.” However, half of them were rated as below average on several measures of attention. In addition, caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems in the children.
This adverse effect was strongest among children who were younger than age 5 at diagnosis and among those at high risk who received more aggressive treatment.
“Our findings ... emphasize the importance of routine neurocognitive monitoring of all survivors treated with contemporary therapy.” But early detection will only be helpful if effective interventions are developed to remediate these attention and behavior problems. Research has shown that pharmacologic therapies “have reduced acceptability among survivors of childhood cancer,” so nonpharmacologic approaches would be especially useful, the researchers added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of childhood ALL who underwent CNS-directed chemotherapy show significant attention problems that impair their functioning 2 years later.
Major finding: 50% of the study participants were rated as below average on several measures of attention, and caregivers reported a significantly greater than expected frequency of hyperactivity, impulsivity, and learning problems.
Data source: A prospective longitudinal analysis of neurocognitive function in 211 ALL survivors assessed 2 years after diagnosis and treatment.
Disclosures: This study was supported by the National Cancer Institute and American Lebanese Syrian Associated Charities. Dr. Jacola reported having no relevant financial disclosures; one of her associates reported receiving research funding from Sigma Tau Pharmaceuticals.
5% prevalence of expiratory central airway collapse in COPDGene study
Among current and former smokers with or without chronic obstructive pulmonary disease (COPD), expiratory central airway collapse develops in approximately 5% and is associated with a worse respiratory-related quality of life, greater dyspnea, and an increased rate of total and severe exacerbations of pulmonary problems, according to a report published online Feb. 2 in JAMA.
Until recently, expiratory central airway collapse (ECAC) could only be studied using bronchoscopy, so it was not very well characterized. For example, the estimated prevalence among patients with known respiratory problems ranged from 1% to 53%. With the increasing use of noninvasive imaging techniques, the condition is being recognized more often, especially in association with smoking and COPD, but it still remains poorly understood, said Dr. Surya P. Bhatt of the division of pulmonary, allergy, and critical care medicine, University of Alabama at Birmingham, and his associates.
To assess the prevalence and clinical significance of ECAC, the investigators analyzed paired CT images of inspiratory and expiratory scans collected in the multicenter COPDGene study, focusing on scans for 8,820 current and former smokers aged 45-80 years (mean age, 59.7 years) who enrolled from local communities across the United States at 21 participating medical centers. Approximately 57% of the study participants were men, 66% were white and 34% were African American, 52% were active smokers, and 44% had COPD.
A total of 443 cases of ECAC were identified, for a prevalence of 5%.
ECAC was more common in participants with COPD (5.9%) than in those without COPD (4.3%), and the prevalence increased with increasing severity of COPD. Study subjects with ECAC were older than those without the condition, with a mean age of 65 years, compared with 59 years. ECAC also was more frequent among women than men (7.2% vs 3.1%), and among whites than blacks (6.2% vs 2.5%). Participants with ECAC had a higher body-mass index, a higher prevalence of chronic bronchitis, and more pack-years of smoking than those without ECAC.
In the primary data analysis, adults with ECAC had a worse respiratory-related quality of life than those without the condition, as measured using the St. George’s Respiratory Questionnaire. This association remained robust, and was independent of the degree of airflow obstruction and the severity of COPD, after the data were adjusted to account for patient demographics, structural lung disease, and forced expiratory volume in 1 second. “We speculate that ECAC might explain some cases of dyspnea disproportionate to apparent obstructive airways disease measured by CT, spirometry, or both,” Dr. Bhatt and his associates said.
Participants with ECAC also had more severe dyspnea as measured by the modified Medical Research Council score but did not have a shorter walking distance on the 6-minute walk test (JAMA 2016 Feb 2. doi: 10.1001/jama.2015.19431).A subset of 7,456 study participants were assessed at 3- to 6-month intervals for a median of 4.3 years. Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years). Mortality, however, was not significantly different between participants who had ECAC (9.9%) and those who did not (9.6%).
“Whether some of these [exacerbations] represent decompensated ECAC or whether ECAC is a marker for future respiratory events needs to be investigated. Our results suggest that ECAC might contribute to symptoms independent of underlying disease and also may serve as a CT-based biomarker of poor respiratory outcomes,” the investigators said.
This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Among current and former smokers with or without chronic obstructive pulmonary disease (COPD), expiratory central airway collapse develops in approximately 5% and is associated with a worse respiratory-related quality of life, greater dyspnea, and an increased rate of total and severe exacerbations of pulmonary problems, according to a report published online Feb. 2 in JAMA.
Until recently, expiratory central airway collapse (ECAC) could only be studied using bronchoscopy, so it was not very well characterized. For example, the estimated prevalence among patients with known respiratory problems ranged from 1% to 53%. With the increasing use of noninvasive imaging techniques, the condition is being recognized more often, especially in association with smoking and COPD, but it still remains poorly understood, said Dr. Surya P. Bhatt of the division of pulmonary, allergy, and critical care medicine, University of Alabama at Birmingham, and his associates.
To assess the prevalence and clinical significance of ECAC, the investigators analyzed paired CT images of inspiratory and expiratory scans collected in the multicenter COPDGene study, focusing on scans for 8,820 current and former smokers aged 45-80 years (mean age, 59.7 years) who enrolled from local communities across the United States at 21 participating medical centers. Approximately 57% of the study participants were men, 66% were white and 34% were African American, 52% were active smokers, and 44% had COPD.
A total of 443 cases of ECAC were identified, for a prevalence of 5%.
ECAC was more common in participants with COPD (5.9%) than in those without COPD (4.3%), and the prevalence increased with increasing severity of COPD. Study subjects with ECAC were older than those without the condition, with a mean age of 65 years, compared with 59 years. ECAC also was more frequent among women than men (7.2% vs 3.1%), and among whites than blacks (6.2% vs 2.5%). Participants with ECAC had a higher body-mass index, a higher prevalence of chronic bronchitis, and more pack-years of smoking than those without ECAC.
In the primary data analysis, adults with ECAC had a worse respiratory-related quality of life than those without the condition, as measured using the St. George’s Respiratory Questionnaire. This association remained robust, and was independent of the degree of airflow obstruction and the severity of COPD, after the data were adjusted to account for patient demographics, structural lung disease, and forced expiratory volume in 1 second. “We speculate that ECAC might explain some cases of dyspnea disproportionate to apparent obstructive airways disease measured by CT, spirometry, or both,” Dr. Bhatt and his associates said.
Participants with ECAC also had more severe dyspnea as measured by the modified Medical Research Council score but did not have a shorter walking distance on the 6-minute walk test (JAMA 2016 Feb 2. doi: 10.1001/jama.2015.19431).A subset of 7,456 study participants were assessed at 3- to 6-month intervals for a median of 4.3 years. Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years). Mortality, however, was not significantly different between participants who had ECAC (9.9%) and those who did not (9.6%).
“Whether some of these [exacerbations] represent decompensated ECAC or whether ECAC is a marker for future respiratory events needs to be investigated. Our results suggest that ECAC might contribute to symptoms independent of underlying disease and also may serve as a CT-based biomarker of poor respiratory outcomes,” the investigators said.
This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
Among current and former smokers with or without chronic obstructive pulmonary disease (COPD), expiratory central airway collapse develops in approximately 5% and is associated with a worse respiratory-related quality of life, greater dyspnea, and an increased rate of total and severe exacerbations of pulmonary problems, according to a report published online Feb. 2 in JAMA.
Until recently, expiratory central airway collapse (ECAC) could only be studied using bronchoscopy, so it was not very well characterized. For example, the estimated prevalence among patients with known respiratory problems ranged from 1% to 53%. With the increasing use of noninvasive imaging techniques, the condition is being recognized more often, especially in association with smoking and COPD, but it still remains poorly understood, said Dr. Surya P. Bhatt of the division of pulmonary, allergy, and critical care medicine, University of Alabama at Birmingham, and his associates.
To assess the prevalence and clinical significance of ECAC, the investigators analyzed paired CT images of inspiratory and expiratory scans collected in the multicenter COPDGene study, focusing on scans for 8,820 current and former smokers aged 45-80 years (mean age, 59.7 years) who enrolled from local communities across the United States at 21 participating medical centers. Approximately 57% of the study participants were men, 66% were white and 34% were African American, 52% were active smokers, and 44% had COPD.
A total of 443 cases of ECAC were identified, for a prevalence of 5%.
ECAC was more common in participants with COPD (5.9%) than in those without COPD (4.3%), and the prevalence increased with increasing severity of COPD. Study subjects with ECAC were older than those without the condition, with a mean age of 65 years, compared with 59 years. ECAC also was more frequent among women than men (7.2% vs 3.1%), and among whites than blacks (6.2% vs 2.5%). Participants with ECAC had a higher body-mass index, a higher prevalence of chronic bronchitis, and more pack-years of smoking than those without ECAC.
In the primary data analysis, adults with ECAC had a worse respiratory-related quality of life than those without the condition, as measured using the St. George’s Respiratory Questionnaire. This association remained robust, and was independent of the degree of airflow obstruction and the severity of COPD, after the data were adjusted to account for patient demographics, structural lung disease, and forced expiratory volume in 1 second. “We speculate that ECAC might explain some cases of dyspnea disproportionate to apparent obstructive airways disease measured by CT, spirometry, or both,” Dr. Bhatt and his associates said.
Participants with ECAC also had more severe dyspnea as measured by the modified Medical Research Council score but did not have a shorter walking distance on the 6-minute walk test (JAMA 2016 Feb 2. doi: 10.1001/jama.2015.19431).A subset of 7,456 study participants were assessed at 3- to 6-month intervals for a median of 4.3 years. Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years). Mortality, however, was not significantly different between participants who had ECAC (9.9%) and those who did not (9.6%).
“Whether some of these [exacerbations] represent decompensated ECAC or whether ECAC is a marker for future respiratory events needs to be investigated. Our results suggest that ECAC might contribute to symptoms independent of underlying disease and also may serve as a CT-based biomarker of poor respiratory outcomes,” the investigators said.
This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.
FROM JAMA
Key clinical point: Expiratory central airway collapse is associated with a worse respiratory-related quality of life among current and former smokers.
Major finding: Compared with participants who did not have ECAC, those who did developed more total exacerbations of pulmonary problems (35 vs. 58 events per 100 person-years) and more severe exacerbations requiring hospitalization (10 vs. 17 events per 100 person-years).
Data source: A cross-sectional cohort study comparing inspiratory and expiratory CT scans for 8,820 adults participating in a COPD study.
Disclosures: This study was supported by the National Heart, Lung, and Blood Institute. Dr. Bhatt reported having no relevant financial disclosures; his associates reported ties to numerous industry sources.