User login
CABG costs more in patients with diabetes
The rate of diabetic coronary artery bypass graft patients has increased more than fivefold in recent decades, and these patients are more likely to have worse outcomes and higher treatment costs, a study showed.
The percentage of patients who had diabetes among all those undergoing coronary artery bypass grafting (CABG) increased from 7% in the 1970s to 37% in the 2000s, according to a database study of 55,501 patients operated on at the Cleveland Clinic.
Patients were identified and preoperative, operative, and postoperative variables were identified, resulting in 45,139 nondiabetic patients assessed and 10,362 diabetic patients (defined as those diabetic patients pharmacologically treated with either insulin or an oral agent) evaluated. The endpoints assessed were in-hospital adverse outcomes as determined by the Society of Thoracic Surgeons National Database, in-hospital direct technical costs, and time-related mortality, according to Dr. Sajjad Raza and his colleagues at the Cleveland Clinic in the August issue of the Journal of Thoracic and Cardiovascular Surgery (150:294-301).
Compared with nondiabetics, diabetic patients undergoing CABG were older and were more likely to be overweight, to be women, and to have a history of heart failure, peripheral arterial disease, carotid disease, hypertension, renal failure, stroke, and advanced coronary artery disease. Over time, the cardiovascular risk profile of the entire population changed, becoming even more pronounced for all patients, but more so for diabetics.
Overall long-term survival at 6 months and at 1, 5 10, 15, and 20 years for diabetic patients was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with 97%, 97%, 90%, 76%, 59%, and 42% for nondiabetic patients, a significant difference at P <.0001.
Propensity matching of similar diabetic and nondiabetic patients showed that deep sternal wound infection and stroke occurred significantly more often in diabetics, although there were no significant differences in cost remaining after matching, even though the length of stay greater than 14 days remained higher for diabetic patients.
Among diabetics, overall survival at 6 months and at 1, 5, 10, 15, and 20 years after CABG was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with overall survival in nondiabetics at 97%, 97%, 90%, 76%, 59%, and 42%, respectively, a significant difference (P <.0001).
“Although long-term survival after CABG is worse in diabetics and high-risk nondiabetics, it is important to note that, in general, high-risk patients reap the greatest survival benefit from CABG. Moreover, using surgical techniques that are associated with better long-term survival after CABG in diabetics could further enhance this survival benefit,” Dr. Raza and his colleagues wrote.
“Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival,” they added. “Diabetic patients and those with a similar high-risk profile set to undergo CABG should be made aware that their risks of postoperative complications are higher than average, and measures should be taken to reduce their postoperative complications,” Dr. Raza and his colleagues concluded.
The authors reported that they had no relevant conflicts of interest.
Patients with diabetes, with or without metabolic syndrome, represent an increasing challenge for cardiac surgery. CABG has been shown to convey a mortality benefit in such patients who also have multivessel disease. This study confirms what most clinicians already know – that the outcomes of patients with diabetes are worse than those in nondiabetic patients, according to Dr. Mani Arsalan and Dr. Michael Mack. “What is particularly important about this study, however, is that it is a single institutional experience with known surgical excellence and a very meticulous and complete outcomes database,” they wrote (J. Thorac. Cardiovasc. Surg. 2015;150:284-5).
Given their findings and the fact that CABG can be expected to remain the mainstay of treatment of multivessel disease in diabetics because of the results of the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, surgeons should pay increased attention to the details of the procedure for these patients. There should be an increased use of bilateral internal mammary arteries, which has been distressingly low, and yet can provide a 23% mortality benefit. “Two arteries are better than one.” Despite the increased risk of deep sternal infection, “the use of skeletonized bilateral internal mammary arteries in young, nonobese diabetic patients with a greater than 10-year life expectancy seems a reasonable risk to take,” Dr. Arsalan and Dr. Mack wrote. In addition, where possible, reaching satisfactory glycemic control before surgery can help decrease early complications. “The weight may be increasingly on our patients, but the real weight is on us as surgeons to help improve their early and long-term survival,” they concluded.
Dr. Arsalan and Dr. Mack are cardiovascular surgeons at Baylor Scott & White Health, Dallas. Their remarks were part of an invited commentary published with the paper.
Patients with diabetes, with or without metabolic syndrome, represent an increasing challenge for cardiac surgery. CABG has been shown to convey a mortality benefit in such patients who also have multivessel disease. This study confirms what most clinicians already know – that the outcomes of patients with diabetes are worse than those in nondiabetic patients, according to Dr. Mani Arsalan and Dr. Michael Mack. “What is particularly important about this study, however, is that it is a single institutional experience with known surgical excellence and a very meticulous and complete outcomes database,” they wrote (J. Thorac. Cardiovasc. Surg. 2015;150:284-5).
Given their findings and the fact that CABG can be expected to remain the mainstay of treatment of multivessel disease in diabetics because of the results of the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, surgeons should pay increased attention to the details of the procedure for these patients. There should be an increased use of bilateral internal mammary arteries, which has been distressingly low, and yet can provide a 23% mortality benefit. “Two arteries are better than one.” Despite the increased risk of deep sternal infection, “the use of skeletonized bilateral internal mammary arteries in young, nonobese diabetic patients with a greater than 10-year life expectancy seems a reasonable risk to take,” Dr. Arsalan and Dr. Mack wrote. In addition, where possible, reaching satisfactory glycemic control before surgery can help decrease early complications. “The weight may be increasingly on our patients, but the real weight is on us as surgeons to help improve their early and long-term survival,” they concluded.
Dr. Arsalan and Dr. Mack are cardiovascular surgeons at Baylor Scott & White Health, Dallas. Their remarks were part of an invited commentary published with the paper.
Patients with diabetes, with or without metabolic syndrome, represent an increasing challenge for cardiac surgery. CABG has been shown to convey a mortality benefit in such patients who also have multivessel disease. This study confirms what most clinicians already know – that the outcomes of patients with diabetes are worse than those in nondiabetic patients, according to Dr. Mani Arsalan and Dr. Michael Mack. “What is particularly important about this study, however, is that it is a single institutional experience with known surgical excellence and a very meticulous and complete outcomes database,” they wrote (J. Thorac. Cardiovasc. Surg. 2015;150:284-5).
Given their findings and the fact that CABG can be expected to remain the mainstay of treatment of multivessel disease in diabetics because of the results of the FREEDOM (Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease) trial, surgeons should pay increased attention to the details of the procedure for these patients. There should be an increased use of bilateral internal mammary arteries, which has been distressingly low, and yet can provide a 23% mortality benefit. “Two arteries are better than one.” Despite the increased risk of deep sternal infection, “the use of skeletonized bilateral internal mammary arteries in young, nonobese diabetic patients with a greater than 10-year life expectancy seems a reasonable risk to take,” Dr. Arsalan and Dr. Mack wrote. In addition, where possible, reaching satisfactory glycemic control before surgery can help decrease early complications. “The weight may be increasingly on our patients, but the real weight is on us as surgeons to help improve their early and long-term survival,” they concluded.
Dr. Arsalan and Dr. Mack are cardiovascular surgeons at Baylor Scott & White Health, Dallas. Their remarks were part of an invited commentary published with the paper.
The rate of diabetic coronary artery bypass graft patients has increased more than fivefold in recent decades, and these patients are more likely to have worse outcomes and higher treatment costs, a study showed.
The percentage of patients who had diabetes among all those undergoing coronary artery bypass grafting (CABG) increased from 7% in the 1970s to 37% in the 2000s, according to a database study of 55,501 patients operated on at the Cleveland Clinic.
Patients were identified and preoperative, operative, and postoperative variables were identified, resulting in 45,139 nondiabetic patients assessed and 10,362 diabetic patients (defined as those diabetic patients pharmacologically treated with either insulin or an oral agent) evaluated. The endpoints assessed were in-hospital adverse outcomes as determined by the Society of Thoracic Surgeons National Database, in-hospital direct technical costs, and time-related mortality, according to Dr. Sajjad Raza and his colleagues at the Cleveland Clinic in the August issue of the Journal of Thoracic and Cardiovascular Surgery (150:294-301).
Compared with nondiabetics, diabetic patients undergoing CABG were older and were more likely to be overweight, to be women, and to have a history of heart failure, peripheral arterial disease, carotid disease, hypertension, renal failure, stroke, and advanced coronary artery disease. Over time, the cardiovascular risk profile of the entire population changed, becoming even more pronounced for all patients, but more so for diabetics.
Overall long-term survival at 6 months and at 1, 5 10, 15, and 20 years for diabetic patients was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with 97%, 97%, 90%, 76%, 59%, and 42% for nondiabetic patients, a significant difference at P <.0001.
Propensity matching of similar diabetic and nondiabetic patients showed that deep sternal wound infection and stroke occurred significantly more often in diabetics, although there were no significant differences in cost remaining after matching, even though the length of stay greater than 14 days remained higher for diabetic patients.
Among diabetics, overall survival at 6 months and at 1, 5, 10, 15, and 20 years after CABG was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with overall survival in nondiabetics at 97%, 97%, 90%, 76%, 59%, and 42%, respectively, a significant difference (P <.0001).
“Although long-term survival after CABG is worse in diabetics and high-risk nondiabetics, it is important to note that, in general, high-risk patients reap the greatest survival benefit from CABG. Moreover, using surgical techniques that are associated with better long-term survival after CABG in diabetics could further enhance this survival benefit,” Dr. Raza and his colleagues wrote.
“Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival,” they added. “Diabetic patients and those with a similar high-risk profile set to undergo CABG should be made aware that their risks of postoperative complications are higher than average, and measures should be taken to reduce their postoperative complications,” Dr. Raza and his colleagues concluded.
The authors reported that they had no relevant conflicts of interest.
The rate of diabetic coronary artery bypass graft patients has increased more than fivefold in recent decades, and these patients are more likely to have worse outcomes and higher treatment costs, a study showed.
The percentage of patients who had diabetes among all those undergoing coronary artery bypass grafting (CABG) increased from 7% in the 1970s to 37% in the 2000s, according to a database study of 55,501 patients operated on at the Cleveland Clinic.
Patients were identified and preoperative, operative, and postoperative variables were identified, resulting in 45,139 nondiabetic patients assessed and 10,362 diabetic patients (defined as those diabetic patients pharmacologically treated with either insulin or an oral agent) evaluated. The endpoints assessed were in-hospital adverse outcomes as determined by the Society of Thoracic Surgeons National Database, in-hospital direct technical costs, and time-related mortality, according to Dr. Sajjad Raza and his colleagues at the Cleveland Clinic in the August issue of the Journal of Thoracic and Cardiovascular Surgery (150:294-301).
Compared with nondiabetics, diabetic patients undergoing CABG were older and were more likely to be overweight, to be women, and to have a history of heart failure, peripheral arterial disease, carotid disease, hypertension, renal failure, stroke, and advanced coronary artery disease. Over time, the cardiovascular risk profile of the entire population changed, becoming even more pronounced for all patients, but more so for diabetics.
Overall long-term survival at 6 months and at 1, 5 10, 15, and 20 years for diabetic patients was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with 97%, 97%, 90%, 76%, 59%, and 42% for nondiabetic patients, a significant difference at P <.0001.
Propensity matching of similar diabetic and nondiabetic patients showed that deep sternal wound infection and stroke occurred significantly more often in diabetics, although there were no significant differences in cost remaining after matching, even though the length of stay greater than 14 days remained higher for diabetic patients.
Among diabetics, overall survival at 6 months and at 1, 5, 10, 15, and 20 years after CABG was 95%, 94%, 80%, 54%, 31%, and 18%, respectively, compared with overall survival in nondiabetics at 97%, 97%, 90%, 76%, 59%, and 42%, respectively, a significant difference (P <.0001).
“Although long-term survival after CABG is worse in diabetics and high-risk nondiabetics, it is important to note that, in general, high-risk patients reap the greatest survival benefit from CABG. Moreover, using surgical techniques that are associated with better long-term survival after CABG in diabetics could further enhance this survival benefit,” Dr. Raza and his colleagues wrote.
“Diabetes is both a marker for high-risk, resource-intensive, and expensive care after CABG and an independent risk factor for reduced long-term survival,” they added. “Diabetic patients and those with a similar high-risk profile set to undergo CABG should be made aware that their risks of postoperative complications are higher than average, and measures should be taken to reduce their postoperative complications,” Dr. Raza and his colleagues concluded.
The authors reported that they had no relevant conflicts of interest.
FROM JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: The percentage of CABG patients with diabetes increased from 7% in the 1970s to 37% in the 2000s. The risk/benefit ratio warrants greater use of bilateral mammary arteries except in obese women with diabetes.
Major finding: Diabetic patients had significantly worse outcomes than nondiabetics with regard to hospital death, deep sternal wound infections, strokes, and renal failure as well as hospital stay and costs.
Data source: A retrospective analysis of a prospective database of patients undergoing first-time CABG at the Cleveland Clinic from 1972 to 2011.
Disclosures: The authors reported that they had no relevant conflicts of interest.
Carrots and sticks lead to smoking cessation
Rewarding smokers for quitting was a more popular option for smoking cessation compared to sanctions-based treatment, but about the same number of participants in either group had quit smoking after 1 year.
Reward-based smoking cessation programs were accepted by 90% of those randomized, compared with 13.7% acceptance of deposit-based programs, and at 6 months, significantly more reward-based participants had ceased smoking, compared with deposit-based participants (15.2% vs. 10.2%). However, as with most such studies, at 1 year, nearly 50% of those who had achieved abstinence at 6 months had returned to smoking in all groups, according to a randomized controlled study of 2,538 CVS Caremark employees and their friends and relatives (ClinicalTrials.gov number, NCT01526265).
Participants were assigned to one of five groups: 468 to usual care only as the control, reported Dr. Scott D. Halpern of the University of Pennsylvania and his colleagues (N. Engl. J. Med. 2015;372:2108-17). They and all participants received information about local smoking cessation resources, cessation guides produced by the American Cancer Society, and, the 41% of the participants receiving health benefits through CVS Caremark had free access to a behavioral-modification program and nicotine-replacement therapy.
In addition, 498 were assigned to individual reward (eligible to receive $200 if they had biochemically confirmed abstinence at each of three times: 14 days, 30 days, and 6 months after quitting, with an additional $200 bonus at 6 months, for a total of $800); 519 to collaborative reward (in addition to the $800, patients were grouped into cohorts of six individuals who would receive an additional $100 per time point if one participant quit smoking to $600 per time point per participant if all six quit); 582 to individual deposit (the $800 dollars included a $150 deposit that would be refunded to participants who quit smoking), and 471 to competitive deposit (included the $150 deposit with the addition of a $450 matching reward per member ($3,600 total), which was redistributed among members who quit at each time point).
In intention-to-treat analyses, all four programs yielded greater rates of sustained cessation through 6 months (range, 9.4%-16.0%) than did usual care (6.0%) (P < .05 for all comparisons).
At 6 months, sustained abstinence was greater with reward-based incentives (15.7%) than with deposit-based (10.2%) (P < .001) and was similar between individual-incentive programs and group-incentive ones (12.1% vs. 13.7%, P = 0.29).
In addition, in instrumental-variable analyses that accounted for differential acceptance, the rate of abstinence at 6 months was 13.2% higher in the deposit-based programs than in the reward-based programs among those who would accept participation in either type of program, the authors stated, indicating that the use of such a program might be of great cost-effectiveness to a health care provider.
Dr. Halpern reported having no relevant conflicts of interest. The National Cancer Institute, the National Institute on Aging, and CVS Caremark supported the study.
Smoking remains the leading cause of preventable deaths in America. It is estimated that employing a smoker costs nearly $6,000 more per year than employing nonsmokers. The article cited, in the New England Journal of Medicine, describes a randomized controlled trial of CVS Caremark employees, their families, and friends who smoke more than five cigarettes per day. The trial compared usual care to four different interventions designed to assess whether a financial incentive would entice greater quitting and abstinence rates.
The researchers looked at whether group participation with potential peer pressure would improve quitting rates. The group-oriented arms were not more effective than individual incentive arms. The psychology of loss aversion versus cash incentive was addressed by asking participants in one group to deposit money and get it back after 6 months of being smoking free. Requiring deposits resulted in decreased participation overall but in those who committed to this arm higher abstinence rates were seen. Overall, incentive rewards over 6 months of participation nearly tripled the rate of smoking cessation compared to usual care.

|
Dr. Michael Liptay |
The severity of cigarette smoking addiction was highlighted by a maximum success rate of around 15% in the best arm as well as a 50% return to smoking rate in those original quitters at 1 year after trial inception. Future innovations in employee benefit design must address the need for shared participation in health maintenance and some form of lifelong risk/reward system that engages people to be responsible for their own health choices.
Dr. Michael Liptay is the medical editor for Thoracic Surgery News.
Smoking remains the leading cause of preventable deaths in America. It is estimated that employing a smoker costs nearly $6,000 more per year than employing nonsmokers. The article cited, in the New England Journal of Medicine, describes a randomized controlled trial of CVS Caremark employees, their families, and friends who smoke more than five cigarettes per day. The trial compared usual care to four different interventions designed to assess whether a financial incentive would entice greater quitting and abstinence rates.
The researchers looked at whether group participation with potential peer pressure would improve quitting rates. The group-oriented arms were not more effective than individual incentive arms. The psychology of loss aversion versus cash incentive was addressed by asking participants in one group to deposit money and get it back after 6 months of being smoking free. Requiring deposits resulted in decreased participation overall but in those who committed to this arm higher abstinence rates were seen. Overall, incentive rewards over 6 months of participation nearly tripled the rate of smoking cessation compared to usual care.

|
Dr. Michael Liptay |
The severity of cigarette smoking addiction was highlighted by a maximum success rate of around 15% in the best arm as well as a 50% return to smoking rate in those original quitters at 1 year after trial inception. Future innovations in employee benefit design must address the need for shared participation in health maintenance and some form of lifelong risk/reward system that engages people to be responsible for their own health choices.
Dr. Michael Liptay is the medical editor for Thoracic Surgery News.
Smoking remains the leading cause of preventable deaths in America. It is estimated that employing a smoker costs nearly $6,000 more per year than employing nonsmokers. The article cited, in the New England Journal of Medicine, describes a randomized controlled trial of CVS Caremark employees, their families, and friends who smoke more than five cigarettes per day. The trial compared usual care to four different interventions designed to assess whether a financial incentive would entice greater quitting and abstinence rates.
The researchers looked at whether group participation with potential peer pressure would improve quitting rates. The group-oriented arms were not more effective than individual incentive arms. The psychology of loss aversion versus cash incentive was addressed by asking participants in one group to deposit money and get it back after 6 months of being smoking free. Requiring deposits resulted in decreased participation overall but in those who committed to this arm higher abstinence rates were seen. Overall, incentive rewards over 6 months of participation nearly tripled the rate of smoking cessation compared to usual care.

|
Dr. Michael Liptay |
The severity of cigarette smoking addiction was highlighted by a maximum success rate of around 15% in the best arm as well as a 50% return to smoking rate in those original quitters at 1 year after trial inception. Future innovations in employee benefit design must address the need for shared participation in health maintenance and some form of lifelong risk/reward system that engages people to be responsible for their own health choices.
Dr. Michael Liptay is the medical editor for Thoracic Surgery News.
Rewarding smokers for quitting was a more popular option for smoking cessation compared to sanctions-based treatment, but about the same number of participants in either group had quit smoking after 1 year.
Reward-based smoking cessation programs were accepted by 90% of those randomized, compared with 13.7% acceptance of deposit-based programs, and at 6 months, significantly more reward-based participants had ceased smoking, compared with deposit-based participants (15.2% vs. 10.2%). However, as with most such studies, at 1 year, nearly 50% of those who had achieved abstinence at 6 months had returned to smoking in all groups, according to a randomized controlled study of 2,538 CVS Caremark employees and their friends and relatives (ClinicalTrials.gov number, NCT01526265).
Participants were assigned to one of five groups: 468 to usual care only as the control, reported Dr. Scott D. Halpern of the University of Pennsylvania and his colleagues (N. Engl. J. Med. 2015;372:2108-17). They and all participants received information about local smoking cessation resources, cessation guides produced by the American Cancer Society, and, the 41% of the participants receiving health benefits through CVS Caremark had free access to a behavioral-modification program and nicotine-replacement therapy.
In addition, 498 were assigned to individual reward (eligible to receive $200 if they had biochemically confirmed abstinence at each of three times: 14 days, 30 days, and 6 months after quitting, with an additional $200 bonus at 6 months, for a total of $800); 519 to collaborative reward (in addition to the $800, patients were grouped into cohorts of six individuals who would receive an additional $100 per time point if one participant quit smoking to $600 per time point per participant if all six quit); 582 to individual deposit (the $800 dollars included a $150 deposit that would be refunded to participants who quit smoking), and 471 to competitive deposit (included the $150 deposit with the addition of a $450 matching reward per member ($3,600 total), which was redistributed among members who quit at each time point).
In intention-to-treat analyses, all four programs yielded greater rates of sustained cessation through 6 months (range, 9.4%-16.0%) than did usual care (6.0%) (P < .05 for all comparisons).
At 6 months, sustained abstinence was greater with reward-based incentives (15.7%) than with deposit-based (10.2%) (P < .001) and was similar between individual-incentive programs and group-incentive ones (12.1% vs. 13.7%, P = 0.29).
In addition, in instrumental-variable analyses that accounted for differential acceptance, the rate of abstinence at 6 months was 13.2% higher in the deposit-based programs than in the reward-based programs among those who would accept participation in either type of program, the authors stated, indicating that the use of such a program might be of great cost-effectiveness to a health care provider.
Dr. Halpern reported having no relevant conflicts of interest. The National Cancer Institute, the National Institute on Aging, and CVS Caremark supported the study.
Rewarding smokers for quitting was a more popular option for smoking cessation compared to sanctions-based treatment, but about the same number of participants in either group had quit smoking after 1 year.
Reward-based smoking cessation programs were accepted by 90% of those randomized, compared with 13.7% acceptance of deposit-based programs, and at 6 months, significantly more reward-based participants had ceased smoking, compared with deposit-based participants (15.2% vs. 10.2%). However, as with most such studies, at 1 year, nearly 50% of those who had achieved abstinence at 6 months had returned to smoking in all groups, according to a randomized controlled study of 2,538 CVS Caremark employees and their friends and relatives (ClinicalTrials.gov number, NCT01526265).
Participants were assigned to one of five groups: 468 to usual care only as the control, reported Dr. Scott D. Halpern of the University of Pennsylvania and his colleagues (N. Engl. J. Med. 2015;372:2108-17). They and all participants received information about local smoking cessation resources, cessation guides produced by the American Cancer Society, and, the 41% of the participants receiving health benefits through CVS Caremark had free access to a behavioral-modification program and nicotine-replacement therapy.
In addition, 498 were assigned to individual reward (eligible to receive $200 if they had biochemically confirmed abstinence at each of three times: 14 days, 30 days, and 6 months after quitting, with an additional $200 bonus at 6 months, for a total of $800); 519 to collaborative reward (in addition to the $800, patients were grouped into cohorts of six individuals who would receive an additional $100 per time point if one participant quit smoking to $600 per time point per participant if all six quit); 582 to individual deposit (the $800 dollars included a $150 deposit that would be refunded to participants who quit smoking), and 471 to competitive deposit (included the $150 deposit with the addition of a $450 matching reward per member ($3,600 total), which was redistributed among members who quit at each time point).
In intention-to-treat analyses, all four programs yielded greater rates of sustained cessation through 6 months (range, 9.4%-16.0%) than did usual care (6.0%) (P < .05 for all comparisons).
At 6 months, sustained abstinence was greater with reward-based incentives (15.7%) than with deposit-based (10.2%) (P < .001) and was similar between individual-incentive programs and group-incentive ones (12.1% vs. 13.7%, P = 0.29).
In addition, in instrumental-variable analyses that accounted for differential acceptance, the rate of abstinence at 6 months was 13.2% higher in the deposit-based programs than in the reward-based programs among those who would accept participation in either type of program, the authors stated, indicating that the use of such a program might be of great cost-effectiveness to a health care provider.
Dr. Halpern reported having no relevant conflicts of interest. The National Cancer Institute, the National Institute on Aging, and CVS Caremark supported the study.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Reward-based smoking cessation incentive programs were more acceptable and effective than were reward plus penalty-based programs.
Major finding: At 6 months, significantly more reward-based participants had ceased smoking compared with deposit-based participants (15.2% vs. 10.2%).
Data source: A five-group randomized, controlled trial of 2,538 CVS-Caremark employees comparing usual care with four incentive programs aimed at sustained abstinence from smoking.
Disclosures: Dr. Halpern reported having no relevant conflicts of interest. The National Cancer Institute, the National Institute on Aging, and CVS Caremark supported the study.
Perioperative factors influenced open TAAA repair
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
Open thoracoabdominal aortic aneurysm (TAAA) repair produced respectable early outcomes, although preoperative and intraoperative factors were found to influence risk, according to Dr. Joseph S. Coselli, who presented the results of the study he and his colleagues at the Baylor College of Medicine in Houston performed at the annual meeting of the American Association for Thoracic Surgery.
They analyzed data from 3,309 open TAAA repairs performed between October 1986 and December 2014.
“I have been very fortunate to have spent my entire career at Baylor College of Medicine, the epicenter of aortic surgery in the 1950s, ’60s, and ’70s, as well as to have been mentored by Dr. E. Stanley Crawford, who was arguably the finest aortic surgeon of his era. Since transitioning from Dr. Crawford’s surgical practice to my own surgical practice, we have kept his pioneering spirit alive by developing a multimodal strategy for thoracoabdominal aortic aneurysm repair that is based on the Crawford extent of repair and our evolving investigation. We sought to describe our series of over 3,000 TAAA repairs and to identify predictors of early death and other adverse postoperative outcomes,” said Dr. Coselli.
The median patient age was around 67 years, and the repairs involved acute or subacute aortic dissection in about 5% of the cases. Nearly 31% of the case involved chronic dissection, with nearly 22% emergent or urgent repairs and around 5% ruptured aneurysms. Connective tissue disorders were present in roughly 10% of patients. “Operatively, we tend to reserve surgical adjuncts for use in the most-extensive repairs, namely extents I and II TAAA repair; intercostal or lumbar artery reattachment was used in just over half of the repairs, left heart bypass (LHB) was used in around 45% of patients, cold renal perfusion was performed in 58%. and cerebrospinal fluid drainage (CSFD) was used in 45%,” said Dr. Coselli.
There was substantial atherosclerotic disease in older patients, and in nearly 41% of repairs, a visceral vessel procedure was performed.
Unlike many aortic centers that routinely use deep hypothermic circulatory arrest (HCA) for extensive TAAA repair, Dr. Coselli reserved this approach for a small number of highly complex repairs (1.4%) in which the aorta could not be safely clamped.
Of the more than a thousand most extensive (i.e., Crawford extent II) repairs, intercostal/lumbar artery reattachment was used in the vast majority (88%), LHB in 82%, and CSFD in 61%. They used multivariable analysis to identify predictors of operative (30-day or in-hospital) mortality and adverse event, a composite outcome comprising operative death and permanent (present at discharge) spinal cord deficit, renal failure, or stroke, according to Dr. Coselli.
Their results showed an operative mortality rate of 7.5%, a 30-day death rate of 4.8%, with the adverse event outcome occurring in about 14% of repairs. A video of his presentation is available at the AATS website.
The statistically significant predictors of operative death were rupture; renal insufficiency, symptoms, procedures targeting visceral vessels, increasing age, and increasing clamp time, while extent IV repair (the least extensive form of TAAA repair) was inversely associated with death. Their analysis showed that the significant predictors of adverse event were use of HCA, renal insufficiency, rupture, extent II repair, visceral vessel procedures, urgent or emergent repair, increasing age, and increasing clamp time. In addition, they used multivariable analysis to identify predictors of renal failure and paraplegia.
In the 3,060 early survivors, roughly 7% had a life-altering complication at discharge: Nearly 3% of patients had renal failure necessitating dialysis, slightly more than 1% had a unresolved stroke, and about 4% had unresolved paraplegia or paraparesis. Repair failure, primarily pseudoaneurysm, or patch aneurysm, occurred after nearly 3% of repairs, said Dr. Coselli.
Outcomes differed by extent of repair, with the risk being greatest in extent II repair. Actuarial survival was 63.6% at 5 years, 36.8% at 10 years, and 18.3% at 15 years. Freedom from repair failure was nearly 98% at 5 years, around 95% at 10 years, and 94% at 15 years.
“Along with respectable early outcomes, after repair, patients have acceptable long-term survival, and late repair failure was uncommon. Notably, there are several subgroups of patients that do exceedingly well. Paraplegia in young patients with connective tissue disorders, even in the most-extensive repair (extent II), is remarkably rare – these patients do extremely well across the board,” he concluded.
Dr. Cosselli reported that he is a principal investigator and consultant for Medtronic and W.L. Gore & Assoc., as well as being a principal investigator, consultant, and having various financial relationships with Vascutek.
AT THE AATS ANNUAL MEETING
Disruptive medicine: 3-D printing revolution
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
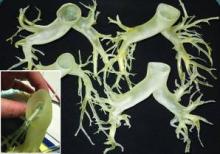
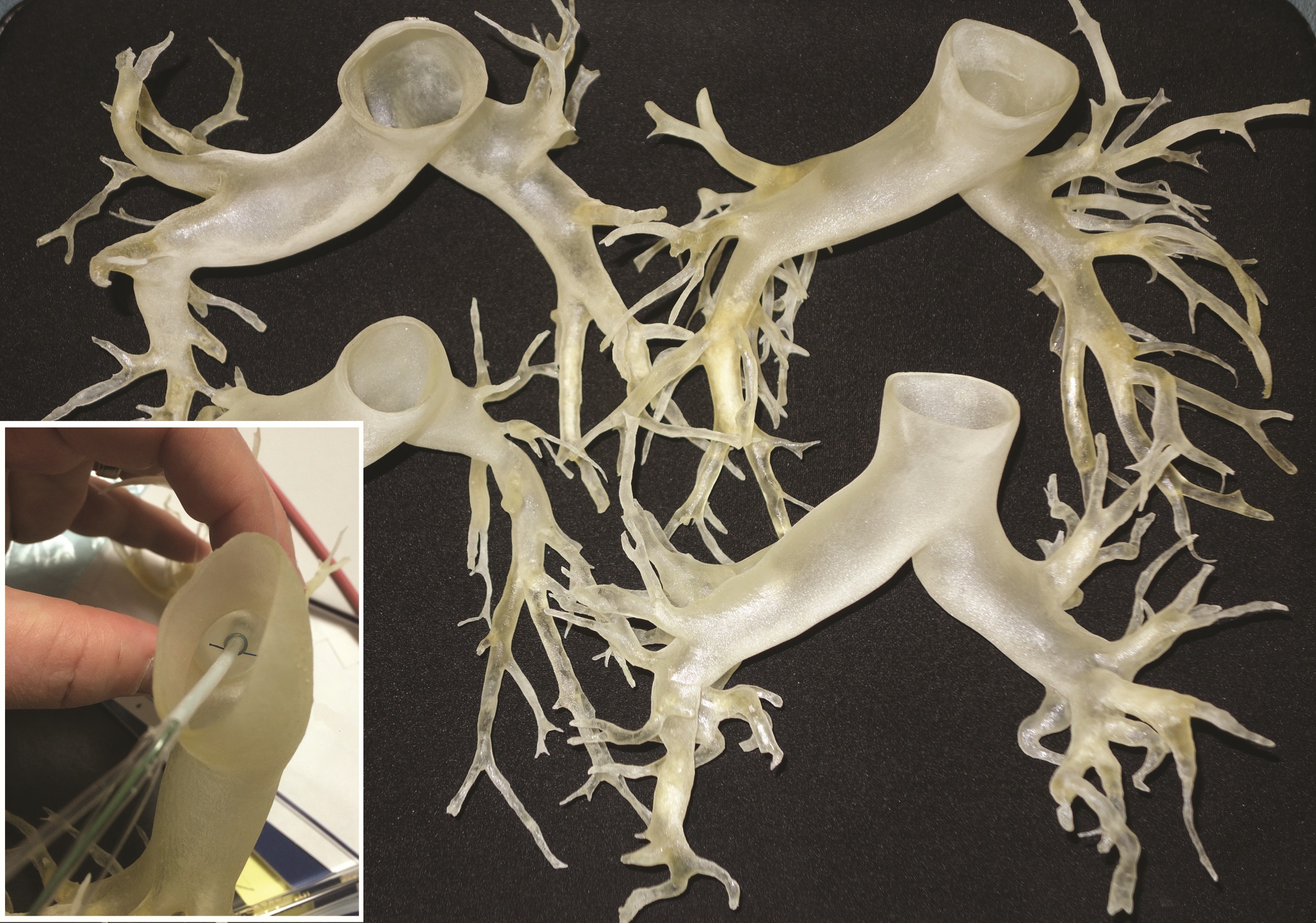
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
Cost comparison favors minimally invasive over conventional AVR
Outcomes were similar, but hospital costs improved with use of mini-aortic valve replacement, compared with conventional AVR, according to the results of a Society of Thoracic Surgeons (STS) database study of 1,341 patients who underwent primary AVR at 17 hospitals.
A propensity match cohort analysis was done to compare patients who had conventional (67%) vs. mini-AVR (33%) performed using either partial sternotomy or right thoracotomy.
Mortality, stroke, renal failure, atrial fibrillation, reoperation for bleeding, and respiratory insufficiency were not statistically significantly different between the two groups. There was also no significant difference in ICU or hospital length of stay between the two groups. However, mini-AVR was associated with both significantly decreased ventilator time (5 vs. 6 hours) and blood product transfusion (25% vs. 32%), according to the report, which was published online and scheduled for the April issue of the Journal of Thoracic and Cardiovascular Surgery (doi:10.1016/j/jtcvs.2015.01.014).
Total hospital cost was significantly lower in the mini-AVR group ($36,348) vs. the conventional repair group ($38,239, P = .02), wrote Dr. Ravi Kiran Ghanta of the University of Virginia, Charlottesville, and his colleagues.
The authors discussed the previously raised issue of longer cross-clamp and bypass times seen in earlier studies of mini-AVR. In their current study, such was not the case, with mini-AVR appearing equivalent with conventional operations. The authors suggested that surgeons have now adopted techniques to reduce bypass and cross-clamp times with mini-AVR.
Data were limited to in-hospital costs. Other costs, such as those of rehabilitation and lost productivity, were not included in the analysis. “Including these health-care costs may have increased overall savings with mini-AVR compared to conventional AVR,” the authors noted.
“Mini-AVR is associated with decreased ventilator time, blood product utilization, early discharge, and reduced total hospital cost. In contemporary clinical practice, mini-AVR is safe and cost-effective,” the researchers concluded.
The authors reported that they had no conflicts.
“Measurement of cost and outcome, the determinant of ‘value’ in health care, is assuming increasing importance in the evaluation of all medial interventions, especially those surgical procedures done frequently and at higher cost,” wrote Dr. Verdi J. DiSesa in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.049]).
Although applauding the timeliness and importance of analyzing the costs and outcomes in propensity-matched patients undergoing aortic valve replacement via full sternotomy of mini-AVR using a partial sternotomy or limited thoracotomy incision, he questioned some of the methodology. The cost-to-charge ratio used, which provides an estimate of the cost of services, is not uniform throughout departments in a single hospital, and differs from hospital to hospital, although the latter was accounted for. He suggested that “activity-based costing” would have been a better approach, although more labor intensive. In this approach, every encounter with a caregiver is assigned a unit cost and the cost of a patient encounter is calculated by estimating the number of units of service and multiplying by the unit cost for service.
“This method is demanding of resources but has the potential to become the most accurate and therefore the preferred way to determine the costs of medical services,” he wrote. In addition, the authors included but did not separately analyze patients having AVR via minithoracotomy.
“What might be the conclusions of this research were these patients excluded or analyzed separately?” he asked.
In terms of the propensity model, the matching procedure included only three clinical factors – STS Predicted Rate of Mortality (PROM) score, the operative year, and the surgeon who performed the procedure – resulting in 35% of patients remaining unmatched, which may have biased the results.
This analysis, Dr. DiSesa concluded, is “perhaps an early version of the kind of analyses, which increasingly will be required for the evaluation of the services provided by all health care providers, not only cardiac surgeons.”
Dr. DiSesa is professor of surgery at Temple University, Philadelphia.
“Measurement of cost and outcome, the determinant of ‘value’ in health care, is assuming increasing importance in the evaluation of all medial interventions, especially those surgical procedures done frequently and at higher cost,” wrote Dr. Verdi J. DiSesa in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.049]).
Although applauding the timeliness and importance of analyzing the costs and outcomes in propensity-matched patients undergoing aortic valve replacement via full sternotomy of mini-AVR using a partial sternotomy or limited thoracotomy incision, he questioned some of the methodology. The cost-to-charge ratio used, which provides an estimate of the cost of services, is not uniform throughout departments in a single hospital, and differs from hospital to hospital, although the latter was accounted for. He suggested that “activity-based costing” would have been a better approach, although more labor intensive. In this approach, every encounter with a caregiver is assigned a unit cost and the cost of a patient encounter is calculated by estimating the number of units of service and multiplying by the unit cost for service.
“This method is demanding of resources but has the potential to become the most accurate and therefore the preferred way to determine the costs of medical services,” he wrote. In addition, the authors included but did not separately analyze patients having AVR via minithoracotomy.
“What might be the conclusions of this research were these patients excluded or analyzed separately?” he asked.
In terms of the propensity model, the matching procedure included only three clinical factors – STS Predicted Rate of Mortality (PROM) score, the operative year, and the surgeon who performed the procedure – resulting in 35% of patients remaining unmatched, which may have biased the results.
This analysis, Dr. DiSesa concluded, is “perhaps an early version of the kind of analyses, which increasingly will be required for the evaluation of the services provided by all health care providers, not only cardiac surgeons.”
Dr. DiSesa is professor of surgery at Temple University, Philadelphia.
“Measurement of cost and outcome, the determinant of ‘value’ in health care, is assuming increasing importance in the evaluation of all medial interventions, especially those surgical procedures done frequently and at higher cost,” wrote Dr. Verdi J. DiSesa in his invited commentary (J. Thorac. Cardiovasc. Surg. 2015 [doi:10.1016/j.jtcvs.2015.01.049]).
Although applauding the timeliness and importance of analyzing the costs and outcomes in propensity-matched patients undergoing aortic valve replacement via full sternotomy of mini-AVR using a partial sternotomy or limited thoracotomy incision, he questioned some of the methodology. The cost-to-charge ratio used, which provides an estimate of the cost of services, is not uniform throughout departments in a single hospital, and differs from hospital to hospital, although the latter was accounted for. He suggested that “activity-based costing” would have been a better approach, although more labor intensive. In this approach, every encounter with a caregiver is assigned a unit cost and the cost of a patient encounter is calculated by estimating the number of units of service and multiplying by the unit cost for service.
“This method is demanding of resources but has the potential to become the most accurate and therefore the preferred way to determine the costs of medical services,” he wrote. In addition, the authors included but did not separately analyze patients having AVR via minithoracotomy.
“What might be the conclusions of this research were these patients excluded or analyzed separately?” he asked.
In terms of the propensity model, the matching procedure included only three clinical factors – STS Predicted Rate of Mortality (PROM) score, the operative year, and the surgeon who performed the procedure – resulting in 35% of patients remaining unmatched, which may have biased the results.
This analysis, Dr. DiSesa concluded, is “perhaps an early version of the kind of analyses, which increasingly will be required for the evaluation of the services provided by all health care providers, not only cardiac surgeons.”
Dr. DiSesa is professor of surgery at Temple University, Philadelphia.
Outcomes were similar, but hospital costs improved with use of mini-aortic valve replacement, compared with conventional AVR, according to the results of a Society of Thoracic Surgeons (STS) database study of 1,341 patients who underwent primary AVR at 17 hospitals.
A propensity match cohort analysis was done to compare patients who had conventional (67%) vs. mini-AVR (33%) performed using either partial sternotomy or right thoracotomy.
Mortality, stroke, renal failure, atrial fibrillation, reoperation for bleeding, and respiratory insufficiency were not statistically significantly different between the two groups. There was also no significant difference in ICU or hospital length of stay between the two groups. However, mini-AVR was associated with both significantly decreased ventilator time (5 vs. 6 hours) and blood product transfusion (25% vs. 32%), according to the report, which was published online and scheduled for the April issue of the Journal of Thoracic and Cardiovascular Surgery (doi:10.1016/j/jtcvs.2015.01.014).
Total hospital cost was significantly lower in the mini-AVR group ($36,348) vs. the conventional repair group ($38,239, P = .02), wrote Dr. Ravi Kiran Ghanta of the University of Virginia, Charlottesville, and his colleagues.
The authors discussed the previously raised issue of longer cross-clamp and bypass times seen in earlier studies of mini-AVR. In their current study, such was not the case, with mini-AVR appearing equivalent with conventional operations. The authors suggested that surgeons have now adopted techniques to reduce bypass and cross-clamp times with mini-AVR.
Data were limited to in-hospital costs. Other costs, such as those of rehabilitation and lost productivity, were not included in the analysis. “Including these health-care costs may have increased overall savings with mini-AVR compared to conventional AVR,” the authors noted.
“Mini-AVR is associated with decreased ventilator time, blood product utilization, early discharge, and reduced total hospital cost. In contemporary clinical practice, mini-AVR is safe and cost-effective,” the researchers concluded.
The authors reported that they had no conflicts.
Outcomes were similar, but hospital costs improved with use of mini-aortic valve replacement, compared with conventional AVR, according to the results of a Society of Thoracic Surgeons (STS) database study of 1,341 patients who underwent primary AVR at 17 hospitals.
A propensity match cohort analysis was done to compare patients who had conventional (67%) vs. mini-AVR (33%) performed using either partial sternotomy or right thoracotomy.
Mortality, stroke, renal failure, atrial fibrillation, reoperation for bleeding, and respiratory insufficiency were not statistically significantly different between the two groups. There was also no significant difference in ICU or hospital length of stay between the two groups. However, mini-AVR was associated with both significantly decreased ventilator time (5 vs. 6 hours) and blood product transfusion (25% vs. 32%), according to the report, which was published online and scheduled for the April issue of the Journal of Thoracic and Cardiovascular Surgery (doi:10.1016/j/jtcvs.2015.01.014).
Total hospital cost was significantly lower in the mini-AVR group ($36,348) vs. the conventional repair group ($38,239, P = .02), wrote Dr. Ravi Kiran Ghanta of the University of Virginia, Charlottesville, and his colleagues.
The authors discussed the previously raised issue of longer cross-clamp and bypass times seen in earlier studies of mini-AVR. In their current study, such was not the case, with mini-AVR appearing equivalent with conventional operations. The authors suggested that surgeons have now adopted techniques to reduce bypass and cross-clamp times with mini-AVR.
Data were limited to in-hospital costs. Other costs, such as those of rehabilitation and lost productivity, were not included in the analysis. “Including these health-care costs may have increased overall savings with mini-AVR compared to conventional AVR,” the authors noted.
“Mini-AVR is associated with decreased ventilator time, blood product utilization, early discharge, and reduced total hospital cost. In contemporary clinical practice, mini-AVR is safe and cost-effective,” the researchers concluded.
The authors reported that they had no conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Mortality, stroke, renal failure, and other complications were not significantly different between mini-aortic valve replacement and conventional replacement.
Major finding: Total hospital costs were significantly lower with mini-AVR, compared with conventional replacement ($36,348 vs. $38,239).
Data source: A retrospective data analysis of patient records for isolated AVR repair, extracted from a regional, multi-institutional Society of Thoracic Surgeons database.
Disclosures: The authors reported that they had no relevant conflicts of interest.
Stem cell divisions help dictate cancer risk
The lifetime risk of many different types of cancer are correlated (0.81) with the total number of divisions of their tissue stem cells, a recent study round.
This can allow any of the most common cancer types to be differentiated into replicative (R) or deterministic (D) types, according to the results of a correlative literature review comparing cancer incidence in tissues to their known stem cell behavior. Whether a cancer is R or D has profound implications for prevention and detection, according to a report in Science (2015;347:78-81).
Extreme variation in the lifetime incidence of cancer across various tissues exist, ranging from levels such as 6.9% in the lung down to 0.00072% for laryngeal cartilage, according to Cristian Tomasetti, Ph.D., of the Johns Hopkins Bloomberg School of Public Heath and Dr. Bert Vogelstein of the Johns Hopkins Kimmel Cancer Center, both in Baltimore.
Environmental exposure to known carcinogens seems to be a factor in some, but this cannot explain why cancers of the small intestinal epithelium are three times less common than brain tumors, even though the intestinal cells are exposed to much higher levels of environmental mutagens than are the brain cells, which are protected by the blood-brain barrier. And heredity fails as a complete explanation, with only 5%-10% of cancers having a heritable component.
“If heredity and environment factors cannot fully explain the differences in organ-specific cancer risk, how else can these differences be explained?” the authors asked. They postulated that somatic cell mutation during DNA replication as the result of cell division may be a critical factor, implying that the greater level of cell division, the greater level of mutagenesis, and hence cancer. Stem cells, which both self-renew and are responsible for tissue maintenance were the obvious candidates for such mutations, and recently the technology has developed to detect and quantify them.
Via a literature search, the authors identified 31 tissue types in which stem cells had been quantitatively assessed, then plotted the total number of stem cell divisions during an average human lifetime for each of these tissues on the X axis, and the lifetime risk in the United States for the associated cancer types from sources such as the Surveillance, Epidemiology, and End Results (SEER) database. Not only was there a strikingly high positive correlation (0.81), which indicated that 65% of the differences of cancer risk among different tissues can be explained by the total number of stem cell divisions in these tissues, the correlation extended across five orders of magnitude, “thereby applying to cancers with enormous differences in incidence,” according to Dr. Tomasetti and Dr. Vogelstein.
They then proceeded to attempt to distinguish the effect of this cell-replicative component from environmental and hereditary factors that contribute to the incidence of cancer. They defined an extra risk score (ERS) as the log product of the lifetime risk of cancer and the total number of stem cell divisions. They then used unsupervised machine learning methods to classify tumors based only on this score into two groups. The result was 9 tumors with high scores and 22 tumors with low ERS scores. If the ERS was high, it meant that there were added factors, such as heredity and environment, contributing to increase the cancer incidence. These they referred to as D-tumors (deterministic). If the ERS was low, that meant that stochastic factors during cell division were the main contributors to incidence, which they called R-tumors (replicative). Upon inspection, the D-tumors were indeed those that had been previously found to have a high hereditary or environmental component. A notable D-tumor, for example, was lung cancer in smokers, while lung cancer in nonsmokers was designated an R-tumor.
“These results have could have important public health implications,” the researchers indicated.
“The maximum fraction of tumors that are preventable through primary prevention (such as vaccines against infectious agents or altered lifestyle) may be evaluated from their ERS. For nonhereditary D-tumors, this fraction is high and primary prevention may make a major impact. ... For R-tumors, primary prevention measures are not likely to be effective, and secondary prevention should be the major focus,” Dr. Tomasetti and Dr. Vogelstein concluded.
The authors reported no relevant disclosures.
This paper published in Science addresses the question of why different tissues in the body are more prone to carcinogenesis than others. The authors hypothesize that environmental factors or inherited predispositions do not explain these differences. An example given is melanocytes and basal epidermal cells in the skin. Each are exposed to the same carcinogen (UV light) at an identical dose, yet melanomas are much less common than are basal cell carcinomas. The explanation arrived at by the authors focuses on stem cells in each tissue.
Stem cells are the only cells that can self-renew and serve to maintain a tissue’s architecture and development. The authors performed a literature search and plotted the total number of stem cell divisions during the average lifetime of a human vs. the lifetime risk for cancer in that tissue type. A linear correlation was performed and found that 65% of the differences in cancer risk among different tissues were explained by total number of stem cell divisions in those tissues. In other words, the more stem cells divide in a tissue, the greater chance of mutations occurring leading to malignancy.
The simple yet elegant concept helps explain the melanoma and basal cell carcinoma differences as well as why those with familial adenomatous polyposis (APC) are 30 times more likely to develop colon carcinoma than duodenal cancer. Human colons have 150-fold more stem cell divisions than the small intestine. Amazingly, mice have more stem cell divisions in their small intestines and in the presence of APC mutation for adenomatous polyps, small intestine tumors are more common than ones in the colon. The authors’ findings suggest in cases where these tumors develop independent of environmental and hereditary factors, secondary prevention such as early detection need to be the driving focus to improve clinical outcomes.
Dr. Michael J. Liptay is the Mary and John Bent Professor and chairman of cardiovascular and thoracic surgery, director of cardiothoracic surgery, and chief of thoracic surgery at Rush University Medical Center, Chicago, and the medical editor of Thoracic Surgery News.
This paper published in Science addresses the question of why different tissues in the body are more prone to carcinogenesis than others. The authors hypothesize that environmental factors or inherited predispositions do not explain these differences. An example given is melanocytes and basal epidermal cells in the skin. Each are exposed to the same carcinogen (UV light) at an identical dose, yet melanomas are much less common than are basal cell carcinomas. The explanation arrived at by the authors focuses on stem cells in each tissue.
Stem cells are the only cells that can self-renew and serve to maintain a tissue’s architecture and development. The authors performed a literature search and plotted the total number of stem cell divisions during the average lifetime of a human vs. the lifetime risk for cancer in that tissue type. A linear correlation was performed and found that 65% of the differences in cancer risk among different tissues were explained by total number of stem cell divisions in those tissues. In other words, the more stem cells divide in a tissue, the greater chance of mutations occurring leading to malignancy.
The simple yet elegant concept helps explain the melanoma and basal cell carcinoma differences as well as why those with familial adenomatous polyposis (APC) are 30 times more likely to develop colon carcinoma than duodenal cancer. Human colons have 150-fold more stem cell divisions than the small intestine. Amazingly, mice have more stem cell divisions in their small intestines and in the presence of APC mutation for adenomatous polyps, small intestine tumors are more common than ones in the colon. The authors’ findings suggest in cases where these tumors develop independent of environmental and hereditary factors, secondary prevention such as early detection need to be the driving focus to improve clinical outcomes.
Dr. Michael J. Liptay is the Mary and John Bent Professor and chairman of cardiovascular and thoracic surgery, director of cardiothoracic surgery, and chief of thoracic surgery at Rush University Medical Center, Chicago, and the medical editor of Thoracic Surgery News.
This paper published in Science addresses the question of why different tissues in the body are more prone to carcinogenesis than others. The authors hypothesize that environmental factors or inherited predispositions do not explain these differences. An example given is melanocytes and basal epidermal cells in the skin. Each are exposed to the same carcinogen (UV light) at an identical dose, yet melanomas are much less common than are basal cell carcinomas. The explanation arrived at by the authors focuses on stem cells in each tissue.
Stem cells are the only cells that can self-renew and serve to maintain a tissue’s architecture and development. The authors performed a literature search and plotted the total number of stem cell divisions during the average lifetime of a human vs. the lifetime risk for cancer in that tissue type. A linear correlation was performed and found that 65% of the differences in cancer risk among different tissues were explained by total number of stem cell divisions in those tissues. In other words, the more stem cells divide in a tissue, the greater chance of mutations occurring leading to malignancy.
The simple yet elegant concept helps explain the melanoma and basal cell carcinoma differences as well as why those with familial adenomatous polyposis (APC) are 30 times more likely to develop colon carcinoma than duodenal cancer. Human colons have 150-fold more stem cell divisions than the small intestine. Amazingly, mice have more stem cell divisions in their small intestines and in the presence of APC mutation for adenomatous polyps, small intestine tumors are more common than ones in the colon. The authors’ findings suggest in cases where these tumors develop independent of environmental and hereditary factors, secondary prevention such as early detection need to be the driving focus to improve clinical outcomes.
Dr. Michael J. Liptay is the Mary and John Bent Professor and chairman of cardiovascular and thoracic surgery, director of cardiothoracic surgery, and chief of thoracic surgery at Rush University Medical Center, Chicago, and the medical editor of Thoracic Surgery News.
The lifetime risk of many different types of cancer are correlated (0.81) with the total number of divisions of their tissue stem cells, a recent study round.
This can allow any of the most common cancer types to be differentiated into replicative (R) or deterministic (D) types, according to the results of a correlative literature review comparing cancer incidence in tissues to their known stem cell behavior. Whether a cancer is R or D has profound implications for prevention and detection, according to a report in Science (2015;347:78-81).
Extreme variation in the lifetime incidence of cancer across various tissues exist, ranging from levels such as 6.9% in the lung down to 0.00072% for laryngeal cartilage, according to Cristian Tomasetti, Ph.D., of the Johns Hopkins Bloomberg School of Public Heath and Dr. Bert Vogelstein of the Johns Hopkins Kimmel Cancer Center, both in Baltimore.
Environmental exposure to known carcinogens seems to be a factor in some, but this cannot explain why cancers of the small intestinal epithelium are three times less common than brain tumors, even though the intestinal cells are exposed to much higher levels of environmental mutagens than are the brain cells, which are protected by the blood-brain barrier. And heredity fails as a complete explanation, with only 5%-10% of cancers having a heritable component.
“If heredity and environment factors cannot fully explain the differences in organ-specific cancer risk, how else can these differences be explained?” the authors asked. They postulated that somatic cell mutation during DNA replication as the result of cell division may be a critical factor, implying that the greater level of cell division, the greater level of mutagenesis, and hence cancer. Stem cells, which both self-renew and are responsible for tissue maintenance were the obvious candidates for such mutations, and recently the technology has developed to detect and quantify them.
Via a literature search, the authors identified 31 tissue types in which stem cells had been quantitatively assessed, then plotted the total number of stem cell divisions during an average human lifetime for each of these tissues on the X axis, and the lifetime risk in the United States for the associated cancer types from sources such as the Surveillance, Epidemiology, and End Results (SEER) database. Not only was there a strikingly high positive correlation (0.81), which indicated that 65% of the differences of cancer risk among different tissues can be explained by the total number of stem cell divisions in these tissues, the correlation extended across five orders of magnitude, “thereby applying to cancers with enormous differences in incidence,” according to Dr. Tomasetti and Dr. Vogelstein.
They then proceeded to attempt to distinguish the effect of this cell-replicative component from environmental and hereditary factors that contribute to the incidence of cancer. They defined an extra risk score (ERS) as the log product of the lifetime risk of cancer and the total number of stem cell divisions. They then used unsupervised machine learning methods to classify tumors based only on this score into two groups. The result was 9 tumors with high scores and 22 tumors with low ERS scores. If the ERS was high, it meant that there were added factors, such as heredity and environment, contributing to increase the cancer incidence. These they referred to as D-tumors (deterministic). If the ERS was low, that meant that stochastic factors during cell division were the main contributors to incidence, which they called R-tumors (replicative). Upon inspection, the D-tumors were indeed those that had been previously found to have a high hereditary or environmental component. A notable D-tumor, for example, was lung cancer in smokers, while lung cancer in nonsmokers was designated an R-tumor.
“These results have could have important public health implications,” the researchers indicated.
“The maximum fraction of tumors that are preventable through primary prevention (such as vaccines against infectious agents or altered lifestyle) may be evaluated from their ERS. For nonhereditary D-tumors, this fraction is high and primary prevention may make a major impact. ... For R-tumors, primary prevention measures are not likely to be effective, and secondary prevention should be the major focus,” Dr. Tomasetti and Dr. Vogelstein concluded.
The authors reported no relevant disclosures.
The lifetime risk of many different types of cancer are correlated (0.81) with the total number of divisions of their tissue stem cells, a recent study round.
This can allow any of the most common cancer types to be differentiated into replicative (R) or deterministic (D) types, according to the results of a correlative literature review comparing cancer incidence in tissues to their known stem cell behavior. Whether a cancer is R or D has profound implications for prevention and detection, according to a report in Science (2015;347:78-81).
Extreme variation in the lifetime incidence of cancer across various tissues exist, ranging from levels such as 6.9% in the lung down to 0.00072% for laryngeal cartilage, according to Cristian Tomasetti, Ph.D., of the Johns Hopkins Bloomberg School of Public Heath and Dr. Bert Vogelstein of the Johns Hopkins Kimmel Cancer Center, both in Baltimore.
Environmental exposure to known carcinogens seems to be a factor in some, but this cannot explain why cancers of the small intestinal epithelium are three times less common than brain tumors, even though the intestinal cells are exposed to much higher levels of environmental mutagens than are the brain cells, which are protected by the blood-brain barrier. And heredity fails as a complete explanation, with only 5%-10% of cancers having a heritable component.
“If heredity and environment factors cannot fully explain the differences in organ-specific cancer risk, how else can these differences be explained?” the authors asked. They postulated that somatic cell mutation during DNA replication as the result of cell division may be a critical factor, implying that the greater level of cell division, the greater level of mutagenesis, and hence cancer. Stem cells, which both self-renew and are responsible for tissue maintenance were the obvious candidates for such mutations, and recently the technology has developed to detect and quantify them.
Via a literature search, the authors identified 31 tissue types in which stem cells had been quantitatively assessed, then plotted the total number of stem cell divisions during an average human lifetime for each of these tissues on the X axis, and the lifetime risk in the United States for the associated cancer types from sources such as the Surveillance, Epidemiology, and End Results (SEER) database. Not only was there a strikingly high positive correlation (0.81), which indicated that 65% of the differences of cancer risk among different tissues can be explained by the total number of stem cell divisions in these tissues, the correlation extended across five orders of magnitude, “thereby applying to cancers with enormous differences in incidence,” according to Dr. Tomasetti and Dr. Vogelstein.
They then proceeded to attempt to distinguish the effect of this cell-replicative component from environmental and hereditary factors that contribute to the incidence of cancer. They defined an extra risk score (ERS) as the log product of the lifetime risk of cancer and the total number of stem cell divisions. They then used unsupervised machine learning methods to classify tumors based only on this score into two groups. The result was 9 tumors with high scores and 22 tumors with low ERS scores. If the ERS was high, it meant that there were added factors, such as heredity and environment, contributing to increase the cancer incidence. These they referred to as D-tumors (deterministic). If the ERS was low, that meant that stochastic factors during cell division were the main contributors to incidence, which they called R-tumors (replicative). Upon inspection, the D-tumors were indeed those that had been previously found to have a high hereditary or environmental component. A notable D-tumor, for example, was lung cancer in smokers, while lung cancer in nonsmokers was designated an R-tumor.
“These results have could have important public health implications,” the researchers indicated.
“The maximum fraction of tumors that are preventable through primary prevention (such as vaccines against infectious agents or altered lifestyle) may be evaluated from their ERS. For nonhereditary D-tumors, this fraction is high and primary prevention may make a major impact. ... For R-tumors, primary prevention measures are not likely to be effective, and secondary prevention should be the major focus,” Dr. Tomasetti and Dr. Vogelstein concluded.
The authors reported no relevant disclosures.
FROM SCIENCE
Key clinical point: Only a third of the variation in cancer risk among tissues is because of the environment or inheritance, and this has implications with regard to prevention and detection.
Major finding: The lifetime risk of many different types of cancer are correlated (0.81) with the total number of divisions of their tissue stem cells.
Data source: Researchers performed a literature review to correlate cancer incidence in a variety of tissues with the nature, number, and hierarchical division patterns of the tissue’s stem cells.
Disclosures: The researchers reported no relevant disclosures.
Greater surgeon experience linked to better long-term survival in NSCLC
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
How do you measure surgeon experience? “What is more important, the surgeon maturity, the volume of surgeries, or a reference oncologic hospital? I believe that experience in surgery is not mathematics, but is an art that any one can be trained to do,” Dr. Daniele Cristina Cataneo wrote in her invited editorial commentary [doi: 10.1016/j.jtcvs.2014.12.073].
This is borne out by this study, she added. The authors have answered the question – surgeon experience has no impact on perioperative outcomes, but can affect long-term survival based upon N2 resection. This means that the pertinent component of experience can be trained, i.e., sampling more nodes. She concluded that with appropriate training, a surgeon could be rendered “experienced” to operate in early stage lung cancer – not only without complications, but also, by increasing N2 resection, with improved survival.
Dr. Cataneo is an associate professor of thoracic surgery at the Botucatu School of Medicine, Sao Paulo State University, Brazil.
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
Surgeon experience may be a factor in long-term survival of patients after lung resection for non–small cell lung cancer (NSCLC) but the correlation between the two is not straightforward.
Postfellowship surgeon experience did not influence perioperative outcomes in pathologic stage I NSCLC. However, a moderate level of experience was associated with greater utilization of video-assisted thoracic surgery, higher mediastinal lymph noted yield, and improved 5-year survival, according to the results of a single center, retrospective review of a lung cancer database.
Between January 2000 and December 2012, 800 patients underwent resection for pathologic stage I NSCLC by eight surgeons – comprising 638 lobectomies (79.8%) and 162 sublobar resections (20.2%).
Experience was based on the number of years at the time of surgery beyond the individual’s completion of a cardiothoracic surgery fellowship. The low-experience (LE) group was defined as operations conducted within the first 5 years of practice after specialty training. The moderate-experience (ME) group comprised surgeons with experience of 5-15 years. The high-experience (HE) group comprised surgeons with more than 15 years post fellowship, according to Paul J. Scheel III and colleagues in the division of cardiothoracic surgery, Washington University, St. Louis.
Over the complete time period, operations were performed by six different surgeons in the LE group, five surgeons in the ME group, and two surgeons in the HE group. By multiple criteria in previous publications, “all the operators involved in our study are specialty trained in thoracic surgery, and are high-volume surgeons,” which eliminates some potential confounders, according to the report, which was published online and in the April issue of The Journal of Thoracic and Cardiovascular Surgery. [doi:10.1016/j.jtcvs.2014.12.032].
The number of mediastinal (N2) lymph node stations sampled per operation was highest for the ME group and lowest for the HE group: LE = 2.8, ME = 3.5, and HE = 2.3, all of which were significantly different across all groups.
The risk of perioperative morbidity defined by STS criteria was not significantly different: with LE = 30.3%, ME = 22.8%, and HE = 28.9%, all similar (P = .163). There were no differences seen in length of hospital stay or perioperative mortality between the groups.
Unadjusted 5-year survival, however, was significantly higher in the ME group (76.9%) compared to the LE group (67.5%, P < .001) and the HE group (71.4%, P = .006). In addition, the ME group surgeons were significantly more likely to have used video-assisted thoracic surgery (VATS) than were the other two groups.
In their discussion, the researchers pointed out a possible reason for the difference seen in mortality: “We noted that the ME group tended to have a higher yield of lymph nodes and this also correlated with survival. It is plausible that surgeons who are in the early stage of their career may be completely focused on ‘getting the specimen out’ with less attention being paid to nodal sampling with its added operative time and perceived additional morbidity.” HE surgeons may have lower yields because “the importance of nodal sampling has been predominately realized over the last 2 decades,” they pointed out.
“Patients operated on by moderately experienced surgeons may have better long-term survival after resection for pathologic stage I lung cancer. Expanding this study to a larger patient and surgeon population would be needed to validate the results and identify the underlying causes for these differences in order to provide the best patient care,” the researchers concluded.
The authors reported having no conflicts of interest.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point:Insufficient lymph-node sampling by less-experienced surgeons may be a reason why patients with stage I NSCLC had better long-term survival if operated on by moderate- and high-experience surgeons.
Major finding: Short-term outcomes in pathologic stage I NSCLC were not affected by surgeon experience, but 5-year survival was significantly lower for the low-experience compared to the moderate-experience surgeons (76.9% vs. 67.5%).
Data source: An institutional database analysis was conducted of 800 operations on stage I NSCLC patients performed from 2000 to 2012.
Disclosures: The authors reported having no conflicts of interest.
Privacy issues transformed in the genomic era
Researchers are attempting to develop new models to deal with patient privacy issues, including profound levels of patient participation in the dissemination of personal data, in the new era of genomic medicine, according to an article in the January issue of Science written by Jennifer Couzin-Frankel, based on interviews with participants and investigators in the RUDY trial and other projects.
The RUDY rheumatology registry in London is an example of such a new approach. RUDY, a registry used for research into rare bone diseases and vasculitis, has an emphasis on patients’ self-reported outcomes. In order to alleviate patients’ fears of loss of privacy because of data sharing, the study offers patients various controls over dissemination, according to one of the study’s principal investigators, Dr. Kassim Javaid, a University of Oxford (England) rheumatologist, wrote Ms. Couzin-Frankel (Science 2015;347:501-3).
Patients in the study can determine whether their blood, scans, and medical histories can be shared with other researchers. In addition, they will have access to a clinical trial Web page where they can find out whether their tissues samples are being used by other research groups.
This model is thought to not only increase the sense of patient participation and authority in the study, but also to provide transparency and accountability as to how their information is being used. Dr. Javaid refers to this strategy as “dynamic consent,” because patients can choose which portions of the study to participate in and whether to restrict their data and samples to RUDY investigators or to allow others to study them.
The problem of maintaining privacy is profound, because DNA sequencing provides such a potent means of individual identification. Ms. Couzin-Frankel cites in particular a case published in Science (2013;339:321-4), in which a group working with Dr. Yaniv Erlich showed that a man could be uniquely identified based on a partial DNA sequence of his Y chromosome, age, and U.S. state of residence, which is the type of information commonly posted in DNA databases widely accessible to the research community. By combining this information with data he found for others in the same family on popular genealogy databases, where more than 100,000 people already have posted DNA markers, Dr. Erlich could not only identify the donors of the DNA but also could identify their family members as far as second cousins once removed.
Although such data can be encrypted, reported Ms. Couzin-Frankel, Dr. Erlich pointed out that this can render the information largely useless for research and doesn’t even always protect the donor.
In contrast, however, many participants in trials and databases, such as the database/Web group PatientsLikeMe, see patient altruism as key. PatientsLikeMe has recruited more 300,000 people with more than 2,300 different diseases, who are sharing their health data, how they’re faring in clinical trials, and acting as a support group to one another in order to help medical scientists and drug companies further research their conditions. Patients voluntarily agree to this free flow of information, even though it decreases their privacy.
In another example in which patients cite altruistic reasons for giving up their privacy, the Personal Genome Project at Harvard Medical School in Boston, founded by geneticist George M. Church, Ph.D., has nearly 4,000 participants sharing their DNA sequences and health histories online for anyone to view. The Personal Genome Project even has a “real name” option, which allows participants to post their identity, Ms. Couzin-Frankel reports.
The Science article author, Ms. Couzin-Frankel, is a science journalist with no relevant financial conflicts. The RUDY study is funded by the University of Oxford and the U.K. National Institute for Health Research.
The article in Science raises important ethical questions, re: the deidentification of data and obtaining [of] informed consent when data or tissue or DNA alone can actually be used to identify the patient.

|
Dr. Jennifer S. Lawton |
When we obtain informed consent for participation in clinical trials, we ask our patients to take a “leap of faith” and trust us to maintain their privacy. Our patients personally gain little or nothing for participation, yet they altruistically allow their data or tissue to be used to improve the lives of others. Just as we do not want our Social Security number, bank account information, and credit card numbers to be public knowledge, we also do not want our genetic information – with potential risks of developing or predispositions for life-threatening diseases – public knowledge (or accessible to employers and insurance companies). These questions will be the topic of many ethical discussions in the future, and will provide an opportunity for innovative and advanced technology and methods to protect personal information.
Dr. Jennifer S. Lawton is the Acquired Cardiovascular Disease section editor for Thoracic Surgery News.
The article in Science raises important ethical questions, re: the deidentification of data and obtaining [of] informed consent when data or tissue or DNA alone can actually be used to identify the patient.

|
Dr. Jennifer S. Lawton |
When we obtain informed consent for participation in clinical trials, we ask our patients to take a “leap of faith” and trust us to maintain their privacy. Our patients personally gain little or nothing for participation, yet they altruistically allow their data or tissue to be used to improve the lives of others. Just as we do not want our Social Security number, bank account information, and credit card numbers to be public knowledge, we also do not want our genetic information – with potential risks of developing or predispositions for life-threatening diseases – public knowledge (or accessible to employers and insurance companies). These questions will be the topic of many ethical discussions in the future, and will provide an opportunity for innovative and advanced technology and methods to protect personal information.
Dr. Jennifer S. Lawton is the Acquired Cardiovascular Disease section editor for Thoracic Surgery News.
The article in Science raises important ethical questions, re: the deidentification of data and obtaining [of] informed consent when data or tissue or DNA alone can actually be used to identify the patient.

|
Dr. Jennifer S. Lawton |
When we obtain informed consent for participation in clinical trials, we ask our patients to take a “leap of faith” and trust us to maintain their privacy. Our patients personally gain little or nothing for participation, yet they altruistically allow their data or tissue to be used to improve the lives of others. Just as we do not want our Social Security number, bank account information, and credit card numbers to be public knowledge, we also do not want our genetic information – with potential risks of developing or predispositions for life-threatening diseases – public knowledge (or accessible to employers and insurance companies). These questions will be the topic of many ethical discussions in the future, and will provide an opportunity for innovative and advanced technology and methods to protect personal information.
Dr. Jennifer S. Lawton is the Acquired Cardiovascular Disease section editor for Thoracic Surgery News.
Researchers are attempting to develop new models to deal with patient privacy issues, including profound levels of patient participation in the dissemination of personal data, in the new era of genomic medicine, according to an article in the January issue of Science written by Jennifer Couzin-Frankel, based on interviews with participants and investigators in the RUDY trial and other projects.
The RUDY rheumatology registry in London is an example of such a new approach. RUDY, a registry used for research into rare bone diseases and vasculitis, has an emphasis on patients’ self-reported outcomes. In order to alleviate patients’ fears of loss of privacy because of data sharing, the study offers patients various controls over dissemination, according to one of the study’s principal investigators, Dr. Kassim Javaid, a University of Oxford (England) rheumatologist, wrote Ms. Couzin-Frankel (Science 2015;347:501-3).
Patients in the study can determine whether their blood, scans, and medical histories can be shared with other researchers. In addition, they will have access to a clinical trial Web page where they can find out whether their tissues samples are being used by other research groups.
This model is thought to not only increase the sense of patient participation and authority in the study, but also to provide transparency and accountability as to how their information is being used. Dr. Javaid refers to this strategy as “dynamic consent,” because patients can choose which portions of the study to participate in and whether to restrict their data and samples to RUDY investigators or to allow others to study them.
The problem of maintaining privacy is profound, because DNA sequencing provides such a potent means of individual identification. Ms. Couzin-Frankel cites in particular a case published in Science (2013;339:321-4), in which a group working with Dr. Yaniv Erlich showed that a man could be uniquely identified based on a partial DNA sequence of his Y chromosome, age, and U.S. state of residence, which is the type of information commonly posted in DNA databases widely accessible to the research community. By combining this information with data he found for others in the same family on popular genealogy databases, where more than 100,000 people already have posted DNA markers, Dr. Erlich could not only identify the donors of the DNA but also could identify their family members as far as second cousins once removed.
Although such data can be encrypted, reported Ms. Couzin-Frankel, Dr. Erlich pointed out that this can render the information largely useless for research and doesn’t even always protect the donor.
In contrast, however, many participants in trials and databases, such as the database/Web group PatientsLikeMe, see patient altruism as key. PatientsLikeMe has recruited more 300,000 people with more than 2,300 different diseases, who are sharing their health data, how they’re faring in clinical trials, and acting as a support group to one another in order to help medical scientists and drug companies further research their conditions. Patients voluntarily agree to this free flow of information, even though it decreases their privacy.
In another example in which patients cite altruistic reasons for giving up their privacy, the Personal Genome Project at Harvard Medical School in Boston, founded by geneticist George M. Church, Ph.D., has nearly 4,000 participants sharing their DNA sequences and health histories online for anyone to view. The Personal Genome Project even has a “real name” option, which allows participants to post their identity, Ms. Couzin-Frankel reports.
The Science article author, Ms. Couzin-Frankel, is a science journalist with no relevant financial conflicts. The RUDY study is funded by the University of Oxford and the U.K. National Institute for Health Research.
Researchers are attempting to develop new models to deal with patient privacy issues, including profound levels of patient participation in the dissemination of personal data, in the new era of genomic medicine, according to an article in the January issue of Science written by Jennifer Couzin-Frankel, based on interviews with participants and investigators in the RUDY trial and other projects.
The RUDY rheumatology registry in London is an example of such a new approach. RUDY, a registry used for research into rare bone diseases and vasculitis, has an emphasis on patients’ self-reported outcomes. In order to alleviate patients’ fears of loss of privacy because of data sharing, the study offers patients various controls over dissemination, according to one of the study’s principal investigators, Dr. Kassim Javaid, a University of Oxford (England) rheumatologist, wrote Ms. Couzin-Frankel (Science 2015;347:501-3).
Patients in the study can determine whether their blood, scans, and medical histories can be shared with other researchers. In addition, they will have access to a clinical trial Web page where they can find out whether their tissues samples are being used by other research groups.
This model is thought to not only increase the sense of patient participation and authority in the study, but also to provide transparency and accountability as to how their information is being used. Dr. Javaid refers to this strategy as “dynamic consent,” because patients can choose which portions of the study to participate in and whether to restrict their data and samples to RUDY investigators or to allow others to study them.
The problem of maintaining privacy is profound, because DNA sequencing provides such a potent means of individual identification. Ms. Couzin-Frankel cites in particular a case published in Science (2013;339:321-4), in which a group working with Dr. Yaniv Erlich showed that a man could be uniquely identified based on a partial DNA sequence of his Y chromosome, age, and U.S. state of residence, which is the type of information commonly posted in DNA databases widely accessible to the research community. By combining this information with data he found for others in the same family on popular genealogy databases, where more than 100,000 people already have posted DNA markers, Dr. Erlich could not only identify the donors of the DNA but also could identify their family members as far as second cousins once removed.
Although such data can be encrypted, reported Ms. Couzin-Frankel, Dr. Erlich pointed out that this can render the information largely useless for research and doesn’t even always protect the donor.
In contrast, however, many participants in trials and databases, such as the database/Web group PatientsLikeMe, see patient altruism as key. PatientsLikeMe has recruited more 300,000 people with more than 2,300 different diseases, who are sharing their health data, how they’re faring in clinical trials, and acting as a support group to one another in order to help medical scientists and drug companies further research their conditions. Patients voluntarily agree to this free flow of information, even though it decreases their privacy.
In another example in which patients cite altruistic reasons for giving up their privacy, the Personal Genome Project at Harvard Medical School in Boston, founded by geneticist George M. Church, Ph.D., has nearly 4,000 participants sharing their DNA sequences and health histories online for anyone to view. The Personal Genome Project even has a “real name” option, which allows participants to post their identity, Ms. Couzin-Frankel reports.
The Science article author, Ms. Couzin-Frankel, is a science journalist with no relevant financial conflicts. The RUDY study is funded by the University of Oxford and the U.K. National Institute for Health Research.
FROM SCIENCE
Key clinical point: The RUDY rheumatology registry represents a new data-sharing model based on patient-researcher trust.
Major finding: Openness, full documentation, and reviews are the key to creating a community of trust that obviates the need for cryptographic protection of DNA sequences.
Data source: A review and interview-based summary of new modes of data-sharing in medical research, focusing on the RUDY trial.
Disclosures: Jennifer Couzin-Frankel, a science journalist, has no relevant financial conflicts. The RUDY study is funded by the University of Oxford (England) and the U.K. National Institute for Health Research.
Baseline QOL measures not associated with outcomes in high-risk operable lung cancer patients
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).

|
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).

|
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
This research is a noteworthy contribution because there source of prospectively acquired QOL data for such a high-risk group facing lung surgery, Dr. Michael T. Jaklitsch said in his invited editorial commentary (J. Thorac. Cardiovasc. Surg. 2015 Dec. 2 [doi:10.1016/j.jtcvs.2014.11.068]).

|
Dr. Michael T. Jaklitsch |
Although there was no evidence of predictive ability of QOL data for this population, “the predictive value of self-assessment may be more powerful in a broader population, however, than in a selected high-risk population such as the Alliance Z4032 trial,” he said. “The amount of pulmonary impairment required to enter this trial was likely the prime determinant of morbidity.” Thus QOL tools may be predictive with certain populations, but not in others. Overall, however, one benefit of self-assessment tools is that they allow patients to be seen more as people than as disease cases by the surgeons.
“Self-assessment tools allow our patients to tell us more completely about themselves and frequently become a springboard to discuss fears of the near future after surgery and what that might look like,” he added. “They allow us to ask our patients more completely, ‘How are you doing?’ ”
Dr. Jaklitsch is a thoracic surgeon at Brigham and Woman’s Hospital, Harvard Medical School, Boston.
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
Poor baseline quality-of-life scores were not predictive of worse overall or recurrence-free survival, or of higher risk for adverse events following sublobar resection in high-risk surgical patients with lung cancer.
In addition, quality of life (QOL) and dyspnea scores did not deteriorate significantly overall, based upon the results of a prospective, multicenter study. Low dyspnea scores at baseline, however, did predict subsequent poor overall survival, according to Dr. Hiran C. Fernando of the Boston Medical Center and his colleagues.
The researchers assessed QOL using the 36-item Short-Form Health Survey (SF36) and the dyspnea score from the University of California, San Diego, Shortness of Breath Questionnaire (SOBQ). Both were measured at baseline, 3, 12, and 24 months. The SF36 scores were further broken down into the physical component summary (PCS) and the mental component summary (MCS), according to their report published online and in the March issue of the Journal of Thoracic and Cardiovascular Surgery (2014 Nov. 13 [doi:10.1016/j.jtcvs.2014.11.003]) .
A total of 212 eligible patients in the American College of Surgeons Oncology Group Z4032 trial were randomized to sublobar resection (108 patients) or sublobar resection with brachytherapy (104). The mean age was about 70.5 years, and 56% were women. There were no significant differences in baseline QOL scores between arms. Baseline PCS and MCS scores were at least 1 standard deviation below those of the U.S. general population in 65% and 46.5% of the patients, respectively.
Overall, there were no significant differences in grade 3+ adverse events, overall survival, or recurrence-free survival seen in patients with baseline scores greater than or equal to median QOL scores or less than median scores. There was, however, significantly worse overall survival for patients with baseline SOBQ scores less than or equal to median. In addition, a 10-point drop in SOBQ score at 12 months also predicted poor overall survival, according to Dr. Fernando and his associates.
In terms of results for operative procedures and tumor types, there was a significantly higher percentage of patients with a decline of 10 points or more in SOBQ scores with segmentectomy, compared with wedge resection (40.5% vs. 21.9%) at 12 months, with thoracotomy vs. video-assisted thoracic surgery (VATS) (38.8% vs. 20.4%, P = .03) at 12 months, and for T1b vs. T1a tumors (46.9% vs. 23.5%) at 24 months. In addition, there was a significantly greater than or equal to 10-point improvement in PCS scores at 3 months with VATS vs. thoracotomy (16.5% vs. 3.6%).
The researchers pointed out that, although QOL measurements can be useful to help decide the optimal surgery procedure, it has even more relevance when considering surgical versus nonsurgical therapies, such as using stereotactic body radiation therapy, for high-risk patients with early-stage lung cancer.
“Some advantages relating to minimizing postoperative dyspnea, as measured by the SOBQ, were gained by using VATS (rather than thoracotomy) or wedge resection (rather than segmentectomy). In addition, VATS, as opposed to thoracotomy, patients had improved PCS scores at 3 months, lending support to the preferential use of VATS when SR is performed,” the researchers concluded.
The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Baseline quality-of-life measures were not predictive of outcomes after lung cancer surgery in high-risk operable patients.
Major finding: A significantly greater improvement in the physical component of quality of life at 3 months and in dyspnea at 1 year was seen from using VATS, compared with thoracotomy.
Data source: Researchers reviewed self-assessment QOL data from 212 eligible high-risk operable patients from the ACSOG Z4032 trial who had sublobar resections with or without brachytherapy using VATS or thoracotomy.
Disclosures: The study was supported by the National Cancer Institute. Dr. Fernando reported receiving consulting fees from Galil and CSA Medical.
A percutaneous modification of RVAD placement shows promise
The use of a modified percutaneous placement technique for right ventricular assist device (RVAD) implantation showed the potential to lessen complications and improve outcomes, according to the results of a retrospective review of 21 patients with right ventricular failure (RVF) implanted with RVADs using this technique.
“This study shows the feasibility and safety of the proposed RVAD implantation technique for various forms of perioperative RVF. A satisfactory outcome can be achieved with a minimal rate of complications,” according to Dr. Diyar Saeed and colleagues at the Clinic for Cardiovascular Surgery, Heinrich-Heine University of Dusseldorf (Germany). Their report appears in the March issue of The Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j/jtcvs.2014.10.104].
Although this technique has been previously described anecdotally in a few case reports, this study is the largest series reported to date, according to the authors.
A total of 63 left ventricular assist devices (LVADs) were placed during the study period (January 2010 to February 2014); 17 (27%) of these patients subsequently received RVADs. An additional four patients were included who received RVADs after developing postcardiotomy RVF. The mean patient age of the RVAD patients 58 years, and 71% were men, The primary diagnosis was ischemic cardiomyopathy in 57% of the patients, dilative cardiomyopathy in 24%, and acute myocardial infarction in 19% .
The initial operation for RVAD implantation required a median sternotomy and was performed via cardiopulmonary bypass in 14 patients, LVAD support in 3 patients, ECMO support in 3 patients, and off-pump in 1 patient. A dacron graft was attached to the pulmonary artery and passed through a subxiphoid exit, where the RVAD outflow cannula was inserted. The inflow cannula was percutaneously cannulated in the femoral vein, and the sternum was primarily closed.
The median duration of RVAD support was 9 days (range 2-88 days). Explantation of the RVAD was performed by pulling and ligating the outflow graft followed by closure of the insertion site. The RVAD inflow cannula was removed and direct pressure applied.
The overall outcomes were that 52% of patients were successfully weaned from the RVAD; 38% of patients died; and 10% of patients received cardiac transplants, giving a survival rate to discharge or heart transplantation of 62%. The overall 1-year survival rate was 52%, but this comprised an improved survival rate of the 4 postcardiotomy patients (75%) compared with the 17 LVAD patients who received an RVAD (47%). Because of the small population size, these latter differences were not significant.
The main drawback of the technique is the limited mobility of the patients owing to the presence of the inflow cannula in the groin, according to the authors, with the majority of the patients remaining bed or chair bound.
“Early extubation, extended support duration, and reduction of resternotomy risks may be the main advantage of this technique. The RVAD removal can be reproducibly performed under minimal anesthesia and without the need for resternotomy. [However], the survival rate remains limited in patients requiring RVAD support after LVAD implantation,” the researchers concluded.
The authors reported having no financial disclosures.
What makes this series unique is the fact that Dr. Saeed and his colleagues placed the RVAD inflow and outflow cannulae percutaneously to avoid the need for resternotomy for removal, according to the invited editorial commentary by Dr. Robert L. Kormos [doi:10.1016/j/jtcvs.2014.11.031].
“Although this is an expanded observational study, it should make us consider further trials to define the real benefits of this approach,” he stated. “The lingering question for all of us, however, is: How does the clinical community define the need for these systems? The balance, of course, will be between utilizing a lower clinical threshold for implantation of LVADs, thus reducing the inherent RV dysfunction, versus ease of insertion and management of percutaneous RVADs, which could lead to earlier RV support decisions, obviating the syndrome of multiorgan failure that accompanies RV failure, and promoting the potential for RV recovery.”
Dr. Kormos is is a professor of surgery at the Presbyterian University Hospital, University of Pittsburgh.
What makes this series unique is the fact that Dr. Saeed and his colleagues placed the RVAD inflow and outflow cannulae percutaneously to avoid the need for resternotomy for removal, according to the invited editorial commentary by Dr. Robert L. Kormos [doi:10.1016/j/jtcvs.2014.11.031].
“Although this is an expanded observational study, it should make us consider further trials to define the real benefits of this approach,” he stated. “The lingering question for all of us, however, is: How does the clinical community define the need for these systems? The balance, of course, will be between utilizing a lower clinical threshold for implantation of LVADs, thus reducing the inherent RV dysfunction, versus ease of insertion and management of percutaneous RVADs, which could lead to earlier RV support decisions, obviating the syndrome of multiorgan failure that accompanies RV failure, and promoting the potential for RV recovery.”
Dr. Kormos is is a professor of surgery at the Presbyterian University Hospital, University of Pittsburgh.
What makes this series unique is the fact that Dr. Saeed and his colleagues placed the RVAD inflow and outflow cannulae percutaneously to avoid the need for resternotomy for removal, according to the invited editorial commentary by Dr. Robert L. Kormos [doi:10.1016/j/jtcvs.2014.11.031].
“Although this is an expanded observational study, it should make us consider further trials to define the real benefits of this approach,” he stated. “The lingering question for all of us, however, is: How does the clinical community define the need for these systems? The balance, of course, will be between utilizing a lower clinical threshold for implantation of LVADs, thus reducing the inherent RV dysfunction, versus ease of insertion and management of percutaneous RVADs, which could lead to earlier RV support decisions, obviating the syndrome of multiorgan failure that accompanies RV failure, and promoting the potential for RV recovery.”
Dr. Kormos is is a professor of surgery at the Presbyterian University Hospital, University of Pittsburgh.
The use of a modified percutaneous placement technique for right ventricular assist device (RVAD) implantation showed the potential to lessen complications and improve outcomes, according to the results of a retrospective review of 21 patients with right ventricular failure (RVF) implanted with RVADs using this technique.
“This study shows the feasibility and safety of the proposed RVAD implantation technique for various forms of perioperative RVF. A satisfactory outcome can be achieved with a minimal rate of complications,” according to Dr. Diyar Saeed and colleagues at the Clinic for Cardiovascular Surgery, Heinrich-Heine University of Dusseldorf (Germany). Their report appears in the March issue of The Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j/jtcvs.2014.10.104].
Although this technique has been previously described anecdotally in a few case reports, this study is the largest series reported to date, according to the authors.
A total of 63 left ventricular assist devices (LVADs) were placed during the study period (January 2010 to February 2014); 17 (27%) of these patients subsequently received RVADs. An additional four patients were included who received RVADs after developing postcardiotomy RVF. The mean patient age of the RVAD patients 58 years, and 71% were men, The primary diagnosis was ischemic cardiomyopathy in 57% of the patients, dilative cardiomyopathy in 24%, and acute myocardial infarction in 19% .
The initial operation for RVAD implantation required a median sternotomy and was performed via cardiopulmonary bypass in 14 patients, LVAD support in 3 patients, ECMO support in 3 patients, and off-pump in 1 patient. A dacron graft was attached to the pulmonary artery and passed through a subxiphoid exit, where the RVAD outflow cannula was inserted. The inflow cannula was percutaneously cannulated in the femoral vein, and the sternum was primarily closed.
The median duration of RVAD support was 9 days (range 2-88 days). Explantation of the RVAD was performed by pulling and ligating the outflow graft followed by closure of the insertion site. The RVAD inflow cannula was removed and direct pressure applied.
The overall outcomes were that 52% of patients were successfully weaned from the RVAD; 38% of patients died; and 10% of patients received cardiac transplants, giving a survival rate to discharge or heart transplantation of 62%. The overall 1-year survival rate was 52%, but this comprised an improved survival rate of the 4 postcardiotomy patients (75%) compared with the 17 LVAD patients who received an RVAD (47%). Because of the small population size, these latter differences were not significant.
The main drawback of the technique is the limited mobility of the patients owing to the presence of the inflow cannula in the groin, according to the authors, with the majority of the patients remaining bed or chair bound.
“Early extubation, extended support duration, and reduction of resternotomy risks may be the main advantage of this technique. The RVAD removal can be reproducibly performed under minimal anesthesia and without the need for resternotomy. [However], the survival rate remains limited in patients requiring RVAD support after LVAD implantation,” the researchers concluded.
The authors reported having no financial disclosures.
The use of a modified percutaneous placement technique for right ventricular assist device (RVAD) implantation showed the potential to lessen complications and improve outcomes, according to the results of a retrospective review of 21 patients with right ventricular failure (RVF) implanted with RVADs using this technique.
“This study shows the feasibility and safety of the proposed RVAD implantation technique for various forms of perioperative RVF. A satisfactory outcome can be achieved with a minimal rate of complications,” according to Dr. Diyar Saeed and colleagues at the Clinic for Cardiovascular Surgery, Heinrich-Heine University of Dusseldorf (Germany). Their report appears in the March issue of The Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j/jtcvs.2014.10.104].
Although this technique has been previously described anecdotally in a few case reports, this study is the largest series reported to date, according to the authors.
A total of 63 left ventricular assist devices (LVADs) were placed during the study period (January 2010 to February 2014); 17 (27%) of these patients subsequently received RVADs. An additional four patients were included who received RVADs after developing postcardiotomy RVF. The mean patient age of the RVAD patients 58 years, and 71% were men, The primary diagnosis was ischemic cardiomyopathy in 57% of the patients, dilative cardiomyopathy in 24%, and acute myocardial infarction in 19% .
The initial operation for RVAD implantation required a median sternotomy and was performed via cardiopulmonary bypass in 14 patients, LVAD support in 3 patients, ECMO support in 3 patients, and off-pump in 1 patient. A dacron graft was attached to the pulmonary artery and passed through a subxiphoid exit, where the RVAD outflow cannula was inserted. The inflow cannula was percutaneously cannulated in the femoral vein, and the sternum was primarily closed.
The median duration of RVAD support was 9 days (range 2-88 days). Explantation of the RVAD was performed by pulling and ligating the outflow graft followed by closure of the insertion site. The RVAD inflow cannula was removed and direct pressure applied.
The overall outcomes were that 52% of patients were successfully weaned from the RVAD; 38% of patients died; and 10% of patients received cardiac transplants, giving a survival rate to discharge or heart transplantation of 62%. The overall 1-year survival rate was 52%, but this comprised an improved survival rate of the 4 postcardiotomy patients (75%) compared with the 17 LVAD patients who received an RVAD (47%). Because of the small population size, these latter differences were not significant.
The main drawback of the technique is the limited mobility of the patients owing to the presence of the inflow cannula in the groin, according to the authors, with the majority of the patients remaining bed or chair bound.
“Early extubation, extended support duration, and reduction of resternotomy risks may be the main advantage of this technique. The RVAD removal can be reproducibly performed under minimal anesthesia and without the need for resternotomy. [However], the survival rate remains limited in patients requiring RVAD support after LVAD implantation,” the researchers concluded.
The authors reported having no financial disclosures.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: Use of a percutaneous modification of right ventricular assist device (RVAD) implantation appears to provide extended support duration and reduces the resternotomy risks, compared with conventional RVAD implantation.
Major finding: The survival rates to discharge or heart transplantation, and to 1 year, were 62% and 52%, respectively.
Data source: A retrospective review of 21 patients with RVF implanted with RVADs via the modified percutaneous placement technique.
Disclosures: The authors reported having no financial disclosures.