User login
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
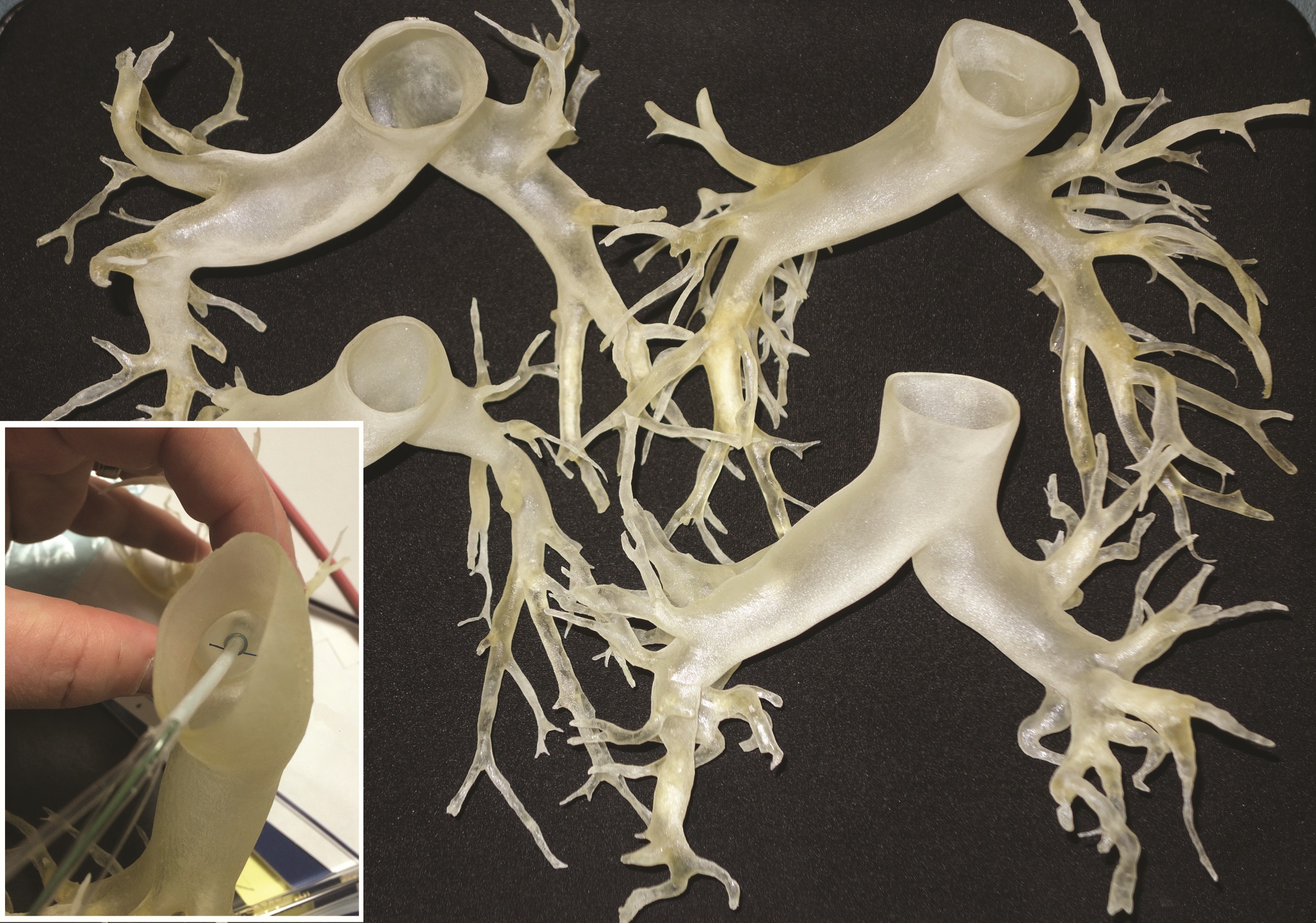
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
“Disruptive medicine: 3-D printing revolution” reports on an important new technology well worth surgeons’ attention. The basic process reported is a simple, but in many ways, revolutionary approach to manufacturing or assembly. 3-D printing is an additive manufacturing process, exactly the opposite from the usual subtractive process. As an example, a block of steel might be milled, drilled and machined into an engine block in a series of processes to remove material from the original piece of steel, a subtractive process.
3-D printing is an additive manufacturing process whereby materials (metal, plastic, other) are layered together to make a complex three-dimensional solid object. Working from a CAD file, material is laid down in successive layers until the entire object is created. Each layer deposited can be imagined as a thinly sliced horizontal cross-section of the eventual object. As such, 3-D printing is really a stack of 2-D prints. The technique was invented by Charles Hull in 1986 and is revolutionizing prototyping and, in some cases, manufacturing. Applications have included prototyping, metal casting, architectural design and building, and in 3-D design and visualization: this application is well-demonstrated in the medical application of pulmonary artery reconstruction outlined in this article. In this case, complex anatomy can be easily visualized on a solid full-scale model of the pulmonary vasculature, and treatment plans more easily formulated and even modeled.
An extensive, thoughtful discussion of medical 3-D printing can be found in the November 24, 2014 The New Yorker article entitled “Print Thyself: How 3-D printing is revolutionizing medicine.” Other medical applications described include 3-D reconstruction in complex craniofacial repairs, modeling of abnormalities in the tracheobronchial tree to design surgical strategies to manage airway stenosis, and in reconstructive modeling for complex traumatic injuries in bone and soft tissue.
Beyond such applications, concepts have evolved to areas of 3-D printing using mixtures of cells and matrix as an approach to the engineering and additive assembly of complex tissues, and even organs. Thus, functional organs might someday be produced; a massive step beyond simple prototyping.
As in many areas of science and technology, the field moves quickly. The March 20, 2015 issue of Science published a report on “Continuous liquid interface production (CLIP) of 3D objects.” This true 3-D printing process is up to 100 times faster than current technologies and is compatible with producing objects from soft elastic materials, ceramics and biologics! More to come…
Thomas M. Krummel, MD, FACS, is the Emile Holman Professor and Chair, Department of Surgery at Stanford University and the Co-Director, Biodesign Innovation Program at Stanford. He is also a member of the Board of Directors of the Fogarty Institute for Innovation.
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.
The emergence of 3-D printing is beginning to look like a case of ‘disruptive medicine.’ Exploratory research in this area is ongoing in cardiology and orthopaedic and plastic surgery, and the experimental applications multiply daily.
Currently, 3-D printed models are being used for simulation training, preprocedural planning, development of personalized surgical equipment, and in a few cases, temporary structures for insertion in patients. As 3-D printers become cheaper, costs for their use in medicine are expected to decline
This rapidly developing technology is being applied in cardiothoracic surgery. The 3-D printing technology was used to construct flexible 3-D models of 10 human patient pulmonary arteries as part of a project to develop a new delivery catheter for regional lung chemotherapy.
Computed tomography and CT angiography in combination with software-driven segmentation techniques were used for generation and adjustment of 3-D polygon mesh to form reconstructed models of the pulmonary arteries. The reconstructed models were exported as stereolithographic data sets and further processed, according to Sergei N. Kurenov of the department of thoracic surgery, Roswell Park Cancer Institute, Buffalo, N.Y., and his colleagues.
How the process works
In this process, producing the anatomical pulmonary artery models required a series of steps: data acquisition from the patient CT digital data, 3-D visualization and segmentation, surface rendering and creating a 3-D polygon mesh, geometrical surface preparation – simplification, refinement, and geometry fixing, and the hollowing of an existing volume to “thicken” the walls.
Three contrast CT data sets with a 0.625-mm, 1-mm, and 2-mm slice thickness were gathered for each patient.
The scans were processed using commercial software packages. Because of the high variability of curvature and embedding in complex anatomical scenes with other vessels interference, the pulmary artery segmentation using the software tools required a clear understanding of the patient’s anatomy, which took 4-8 hours for the experienced operator, according to the researchers.
After further computer processing of the virtual reconstructed pulmonary model, it was sent to the 3-D printer, which used a rubberlike material that is elastic and semitransparent, behaving similarly to polyurethane.
The 10 unique models were successfully created with no print failures, although the original plan of using a 1-mm mural thickness proved too fragile, so the entire group was printed with a 1.5-mm wall. The design process took 8 hours from CT image to stereolithographic model, and printing required an overall total of 97 hours, according to the report published online in the Journal of Thoracic and Cardiovascular Surgery [doi:10.1016/j.jtcvs.2014.12.059].
Accurate models of individual patients’ anatomy
The physical measurements of the model were accurate for clinical purposes, with the 95% confidence levels for the 10 models demonstrating equivalence. Anatomic measurements using this process could be useful for general pulmonary artery catheter design, according to the authors. These measurements showed sufficient similarity for a design to be created that would be effective for most patients, although this finding would have to be validated with a larger sample of patients.
“While many of the measurements could have been made with software analysis of the 3-D files, some measurements were greatly facilitated by bending the model and aligning the physical catheter. These measurements represent distance beyond which a catheter might cause damage,” they added.
Biological 3-D printing of organs
Gut is a perfect beginning project for 3-D printing, Dr. John Geibel said at the 2015 American Gastroenterological Association Tech Summit, which was sponsored by the AGA Center for GI Innovation and Technology. It has a very simple shape – just a long hollow tube. Epithelial cells grow and turn over very quickly, suggesting that a length of artificial intestine could be grown relatively quickly. And although intestine is composed of a number of distinct layers, a 3-D bioprinter would have no trouble laying down concentric circles of each one to recreate their natural morphology.
“It will take time. It will take planning. But this is going to happen,” said Dr. Geibel of Yale University, New Haven, Conn.
To create a length of intestine, the print heads of a bioprinter would be loaded with cells from all of the gut layers – the serosa, the different muscle strata, the mucosa. Each would be laid down in its respective anatomic ring, supported all around by a hydrogel. The print sequence would be repeated over and over until the required length of intestine was created. From then, Dr. Geibel said, it would be only a matter of days before the cells knit themselves together so well that the gel could be dissolved and the new tissue ready for transplant.
Liver would likely be the next organ up for printing, with the ultimate goal of creating fully transplantable organs. The need is enormous, and can’t be overstated. Patients who need a new liver wait an average of 4 years before they receive one.
The liver is much more complicated than a length of gut. It is cellularly complex and highly vascularized. But liver-printing is already a reality. Bioprinted “3-D liver-in-a-dish,” created by San Diego–based Organovo, has function, if not form. The cells work together; they grow, divide, and secrete bile acids. However, they exist as a formless, nonvascular blob.
As it stands (or rather, lies) now, bioprinted liver is a perfect preclinical model – perfectly replicating how the liver would respond to drugs without any of the messy adverse events that hurt patients. But it needs some backbone, or more accurately, some matrix, in order to morph again and grow into a complete organ. A liver-shaped collagen matrix could provide the necessary frame for cells to grow in and around; tunnels through it would form pathways for a similarly engineered vasculature.
The project to create 3-D models of pulmonary arteries is one of many ongoing efforts in this field. “Going forward, this technology competes with virtual educational media for health care professionals, trainees, and patients. Complex anatomy can be visualized easily on a scale model at the operating table (rather than by manipulating a nonsterile pointing device on a computer). The [pulmonary arteries] we printed could be used in a relatively low-cost lifelike [video-assisted thoracoscopic] lobectomy trainer,” the authors stated, while acknowledging the current issue of cost and time.
Printing services were funded by an unrestricted grant from Incodema3D, which employs Dan Sammons, one of the authors of the study. The other authors had no relevant financial disclosures.