User login
Mean platelet volume higher in DVT patients with PE than without
Mean platelet volume (MPV) has been associated with deep vein thrombosis (DVT), but it also appears to be a potential predictor of the presence of pulmonary embolism (PE), according to a retrospective analysis of patients reported by Dr. Atilla Icli of Ahi Evran University, Kirsehir, Turkey, and his colleagues.
The researchers assessed three groups of patients among those admitted to the emergency department or outpatient clinic of two university hospitals during 2005-2014. The patient study groups consisted of 98 patients with DVT and PE (48 men; mean age, 56.8 years); 97 patients with DVT without PE (48 men; mean age, 54.2 years); and 98 patients admitted with suspicion of DVT, but confirmed to be free of DVT and PE (45 men; mean age, 55.0 years) (Heart Lung Circulation. 2015 Nov;24[11]:1081-6).
There were no statistically significant differences between the groups with regard to sex, age, body mass index, or comorbidities such as hypertension or diabetes. Blood samples were drawn from all patients and assessed for MPV.
MPV was significantly higher in all DVT patients than controls (9.3 femtoliter vs. 7.9 fL; P less than .001) and in DVT patients with PE as compared to DVT patients without PE (9.9 fL vs. 8.7 fL, respectively; P less than .001). A cut-off point was seen where the rate of PE was significantly higher in patients with MPV greater than 9.15 fL, as compared with those with MPV equal to or below 9.15 fL. In addition, the presence of PE in patients with DVT was independently associated with MPV (odds ratio, 22.2; P less than .001).
“Our findings suggest that MPVs are elevated in patients with DVT, and higher MPVs may be associated with this group of patients,” Dr. Icli and his colleagues concluded.
The authors indicated that they had no relevant conflicts.
Read the full study online in Heart, Lung and Circulation.
Mean platelet volume (MPV) has been associated with deep vein thrombosis (DVT), but it also appears to be a potential predictor of the presence of pulmonary embolism (PE), according to a retrospective analysis of patients reported by Dr. Atilla Icli of Ahi Evran University, Kirsehir, Turkey, and his colleagues.
The researchers assessed three groups of patients among those admitted to the emergency department or outpatient clinic of two university hospitals during 2005-2014. The patient study groups consisted of 98 patients with DVT and PE (48 men; mean age, 56.8 years); 97 patients with DVT without PE (48 men; mean age, 54.2 years); and 98 patients admitted with suspicion of DVT, but confirmed to be free of DVT and PE (45 men; mean age, 55.0 years) (Heart Lung Circulation. 2015 Nov;24[11]:1081-6).
There were no statistically significant differences between the groups with regard to sex, age, body mass index, or comorbidities such as hypertension or diabetes. Blood samples were drawn from all patients and assessed for MPV.
MPV was significantly higher in all DVT patients than controls (9.3 femtoliter vs. 7.9 fL; P less than .001) and in DVT patients with PE as compared to DVT patients without PE (9.9 fL vs. 8.7 fL, respectively; P less than .001). A cut-off point was seen where the rate of PE was significantly higher in patients with MPV greater than 9.15 fL, as compared with those with MPV equal to or below 9.15 fL. In addition, the presence of PE in patients with DVT was independently associated with MPV (odds ratio, 22.2; P less than .001).
“Our findings suggest that MPVs are elevated in patients with DVT, and higher MPVs may be associated with this group of patients,” Dr. Icli and his colleagues concluded.
The authors indicated that they had no relevant conflicts.
Read the full study online in Heart, Lung and Circulation.
Mean platelet volume (MPV) has been associated with deep vein thrombosis (DVT), but it also appears to be a potential predictor of the presence of pulmonary embolism (PE), according to a retrospective analysis of patients reported by Dr. Atilla Icli of Ahi Evran University, Kirsehir, Turkey, and his colleagues.
The researchers assessed three groups of patients among those admitted to the emergency department or outpatient clinic of two university hospitals during 2005-2014. The patient study groups consisted of 98 patients with DVT and PE (48 men; mean age, 56.8 years); 97 patients with DVT without PE (48 men; mean age, 54.2 years); and 98 patients admitted with suspicion of DVT, but confirmed to be free of DVT and PE (45 men; mean age, 55.0 years) (Heart Lung Circulation. 2015 Nov;24[11]:1081-6).
There were no statistically significant differences between the groups with regard to sex, age, body mass index, or comorbidities such as hypertension or diabetes. Blood samples were drawn from all patients and assessed for MPV.
MPV was significantly higher in all DVT patients than controls (9.3 femtoliter vs. 7.9 fL; P less than .001) and in DVT patients with PE as compared to DVT patients without PE (9.9 fL vs. 8.7 fL, respectively; P less than .001). A cut-off point was seen where the rate of PE was significantly higher in patients with MPV greater than 9.15 fL, as compared with those with MPV equal to or below 9.15 fL. In addition, the presence of PE in patients with DVT was independently associated with MPV (odds ratio, 22.2; P less than .001).
“Our findings suggest that MPVs are elevated in patients with DVT, and higher MPVs may be associated with this group of patients,” Dr. Icli and his colleagues concluded.
The authors indicated that they had no relevant conflicts.
Read the full study online in Heart, Lung and Circulation.
FROM HEART, LUNG AND CIRCULATION
Endovascular treatment of acute IVC thrombosis found effective
Endovascular treatment of acute thrombosis of the inferior vena cava is safe and effective with excellent short-term results, according to the results of a 10-year retrospective review reported by Dr. Khanjan H. Nagarsheth and his colleagues at the Staten Island University (N.Y.) Hospital.
Dr. Nagarsheth and his colleagues assessed 25 patients (44% men) with a mean age of 50.3 years, who underwent catheter-directed treatment in either the operating room or the angiography suite for acute (existing for less than 2 weeks) symptomatic IVC thrombosis (Annals Vasc Surg. 2015; 29:1373-9).
All patients had a prior history of deep vein thrombosis; 21 patients had an IVC filter at presentation.
Endovascular treatment was successful in all 25 patients. The greater majority of patients (22, 88%) received both catheter-directed thrombolysis (CDT) and pharmacomechanical thrombectomy; the remaining 3 patients received CDT alone.
Significant (greater than 50% luminal gain) angiographic resolution of the venous thromboembolism was achieved in all patients, with 23 (92%) reporting moderate to complete symptomatic improvement immediately after the procedure. At the median follow-up of 54.3 weeks, symptomatic improvement was noted in all patients, and complete symptom resolution was seen in 16 (89%). None of the IVC filters were removed and 67% of patients had a patent IVC at last follow-up.
“An aggressive endovascular approach to treatment of [acute thrombosis of the] IVC is warranted even in the presence of a thrombosed caval filter,” the authors concluded.
The authors did not indicate the existence of any conflicts.
Read the full study online in the Annals of Vascular Surgery.
Endovascular treatment of acute thrombosis of the inferior vena cava is safe and effective with excellent short-term results, according to the results of a 10-year retrospective review reported by Dr. Khanjan H. Nagarsheth and his colleagues at the Staten Island University (N.Y.) Hospital.
Dr. Nagarsheth and his colleagues assessed 25 patients (44% men) with a mean age of 50.3 years, who underwent catheter-directed treatment in either the operating room or the angiography suite for acute (existing for less than 2 weeks) symptomatic IVC thrombosis (Annals Vasc Surg. 2015; 29:1373-9).
All patients had a prior history of deep vein thrombosis; 21 patients had an IVC filter at presentation.
Endovascular treatment was successful in all 25 patients. The greater majority of patients (22, 88%) received both catheter-directed thrombolysis (CDT) and pharmacomechanical thrombectomy; the remaining 3 patients received CDT alone.
Significant (greater than 50% luminal gain) angiographic resolution of the venous thromboembolism was achieved in all patients, with 23 (92%) reporting moderate to complete symptomatic improvement immediately after the procedure. At the median follow-up of 54.3 weeks, symptomatic improvement was noted in all patients, and complete symptom resolution was seen in 16 (89%). None of the IVC filters were removed and 67% of patients had a patent IVC at last follow-up.
“An aggressive endovascular approach to treatment of [acute thrombosis of the] IVC is warranted even in the presence of a thrombosed caval filter,” the authors concluded.
The authors did not indicate the existence of any conflicts.
Read the full study online in the Annals of Vascular Surgery.
Endovascular treatment of acute thrombosis of the inferior vena cava is safe and effective with excellent short-term results, according to the results of a 10-year retrospective review reported by Dr. Khanjan H. Nagarsheth and his colleagues at the Staten Island University (N.Y.) Hospital.
Dr. Nagarsheth and his colleagues assessed 25 patients (44% men) with a mean age of 50.3 years, who underwent catheter-directed treatment in either the operating room or the angiography suite for acute (existing for less than 2 weeks) symptomatic IVC thrombosis (Annals Vasc Surg. 2015; 29:1373-9).
All patients had a prior history of deep vein thrombosis; 21 patients had an IVC filter at presentation.
Endovascular treatment was successful in all 25 patients. The greater majority of patients (22, 88%) received both catheter-directed thrombolysis (CDT) and pharmacomechanical thrombectomy; the remaining 3 patients received CDT alone.
Significant (greater than 50% luminal gain) angiographic resolution of the venous thromboembolism was achieved in all patients, with 23 (92%) reporting moderate to complete symptomatic improvement immediately after the procedure. At the median follow-up of 54.3 weeks, symptomatic improvement was noted in all patients, and complete symptom resolution was seen in 16 (89%). None of the IVC filters were removed and 67% of patients had a patent IVC at last follow-up.
“An aggressive endovascular approach to treatment of [acute thrombosis of the] IVC is warranted even in the presence of a thrombosed caval filter,” the authors concluded.
The authors did not indicate the existence of any conflicts.
Read the full study online in the Annals of Vascular Surgery.
FROM THE ANNALS OF VASCULAR SURGERY
ESC model predicts recurrent VTE risk
Recurrent venous thromboembolism was significantly associated with a higher European Society of Cardiology (ESC) pulmonary embolism score. This association was independent of other factors such as age, sex, and body mass index, according to a long-term, prospective follow-up study of 627 patients with a first episode of pulmonary embolism.
In addition, unprovoked PE and varicose veins of the lower limbs also increased recurrence risk, while longer anticoagulation treatment reduced it (Intnl J Cardiol. 2016;202:275-81).
Dr. Shuai Zhang of the Beijing Institute of Respiratory Medicine and coauthors categorized their patients into three groups according to the ESC risk stratification model (Eur Heart J. 2008;29:2276-315). These were low-, intermediate-, and high-risk groups. Of the 627 patients, 84 had suspected VTE recurrence, 68 of whom had this confirmed by imaging diagnosis and were designated as the recurrent group. The 1-, 2-, and 5-year cumulative incidences of recurrent VTE were 4.5%. 7.3%, and 13.9%, respectively.
The researchers compared the 68 recurrent to the 559 nonrecurrent patients; all patients had had a first episode of PE.
Compared with nonrecurrent VTE patients, the recurrent group had significantly more high-risk and intermediate-risk patients, and the high-risk and intermediate-risk patients overall had significantly more cumulative recurrent events than the low-risk group. Multivariate analysis confirmed the association between higher risk in ESC stratification and VTE recurrence independent of age, sex, and body mass index.
Based on these results, the authors stated that, “The severity of disease should be considered in determining the initial treatment and duration of follow-up anticoagulation.”
“These finding support the notion that optimized therapy based on risk stratification is valuable while treating PE patients,” they concluded.
The authors reported having no conflicts of interest.
Read the full study online in the International Journal of Cardiology.
Recurrent venous thromboembolism was significantly associated with a higher European Society of Cardiology (ESC) pulmonary embolism score. This association was independent of other factors such as age, sex, and body mass index, according to a long-term, prospective follow-up study of 627 patients with a first episode of pulmonary embolism.
In addition, unprovoked PE and varicose veins of the lower limbs also increased recurrence risk, while longer anticoagulation treatment reduced it (Intnl J Cardiol. 2016;202:275-81).
Dr. Shuai Zhang of the Beijing Institute of Respiratory Medicine and coauthors categorized their patients into three groups according to the ESC risk stratification model (Eur Heart J. 2008;29:2276-315). These were low-, intermediate-, and high-risk groups. Of the 627 patients, 84 had suspected VTE recurrence, 68 of whom had this confirmed by imaging diagnosis and were designated as the recurrent group. The 1-, 2-, and 5-year cumulative incidences of recurrent VTE were 4.5%. 7.3%, and 13.9%, respectively.
The researchers compared the 68 recurrent to the 559 nonrecurrent patients; all patients had had a first episode of PE.
Compared with nonrecurrent VTE patients, the recurrent group had significantly more high-risk and intermediate-risk patients, and the high-risk and intermediate-risk patients overall had significantly more cumulative recurrent events than the low-risk group. Multivariate analysis confirmed the association between higher risk in ESC stratification and VTE recurrence independent of age, sex, and body mass index.
Based on these results, the authors stated that, “The severity of disease should be considered in determining the initial treatment and duration of follow-up anticoagulation.”
“These finding support the notion that optimized therapy based on risk stratification is valuable while treating PE patients,” they concluded.
The authors reported having no conflicts of interest.
Read the full study online in the International Journal of Cardiology.
Recurrent venous thromboembolism was significantly associated with a higher European Society of Cardiology (ESC) pulmonary embolism score. This association was independent of other factors such as age, sex, and body mass index, according to a long-term, prospective follow-up study of 627 patients with a first episode of pulmonary embolism.
In addition, unprovoked PE and varicose veins of the lower limbs also increased recurrence risk, while longer anticoagulation treatment reduced it (Intnl J Cardiol. 2016;202:275-81).
Dr. Shuai Zhang of the Beijing Institute of Respiratory Medicine and coauthors categorized their patients into three groups according to the ESC risk stratification model (Eur Heart J. 2008;29:2276-315). These were low-, intermediate-, and high-risk groups. Of the 627 patients, 84 had suspected VTE recurrence, 68 of whom had this confirmed by imaging diagnosis and were designated as the recurrent group. The 1-, 2-, and 5-year cumulative incidences of recurrent VTE were 4.5%. 7.3%, and 13.9%, respectively.
The researchers compared the 68 recurrent to the 559 nonrecurrent patients; all patients had had a first episode of PE.
Compared with nonrecurrent VTE patients, the recurrent group had significantly more high-risk and intermediate-risk patients, and the high-risk and intermediate-risk patients overall had significantly more cumulative recurrent events than the low-risk group. Multivariate analysis confirmed the association between higher risk in ESC stratification and VTE recurrence independent of age, sex, and body mass index.
Based on these results, the authors stated that, “The severity of disease should be considered in determining the initial treatment and duration of follow-up anticoagulation.”
“These finding support the notion that optimized therapy based on risk stratification is valuable while treating PE patients,” they concluded.
The authors reported having no conflicts of interest.
Read the full study online in the International Journal of Cardiology.
FROM THE INTERNATIONAL JOURNAL OF CARDIOLOGY
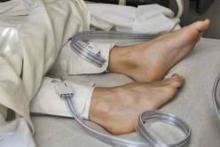
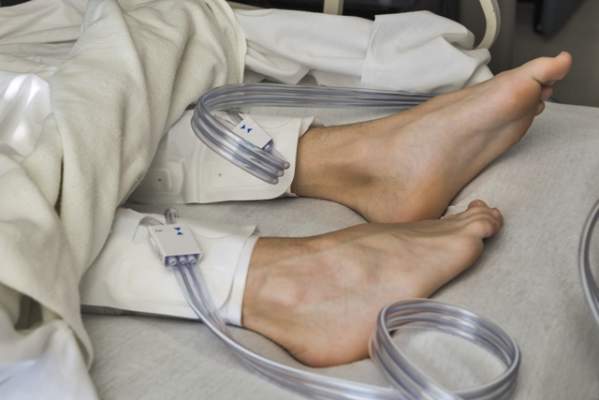
IPC maintains first-line status in preventing DVT in most surgical patients
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
Intermittent pneumatic compression remains the consensus choice as the sole prophylactic agent for deep vein thrombosis in low- or moderate-risk surgical patients, according to a literature analysis published online ahead of print in September in the Journal of Vascular Surgery: Venous and Lymphatic Disorders.
Dr. Nirvana Sadaghianloo of the University of Nice (France) Sophia Antipolis and Dr. Alan Dardik of Yale University in New Haven, Conn., used the MEDLINE and Cochrane libraries to find individual studies and meta-analyses published in English since 2011 assessing the efficacy of intermittent pneumatic compression (IPC) in preventing deep vein thrombosis (DVT), which included the American College of Chest Physicians ninth edition guidelines (2012). They stated that, although the overall quality of studies regarding the use of IPC was low, IPC showed efficacy in prevention of DVT for more than 30 years (J Vasc Surg Venous Lymphat Disord. 2015 doi: 10.1016/j.jvsv.2015.07.006).
“IPC represents a good alternative to pharmacologic agents when the risk of thrombosis is moderate or low or when the risk of bleeding is high or may have serious consequences for the patient,” they stated.
However, they also found that most recommendations suggested that, in high-risk patients, IPC plays a role primarily as an additional modality to provide additional benefit in preventing DVT when is used in combination with pharmacologic therapy. They highlighted how the choice of any thromboprophylactic agent required a systematic risk assessment as a critical prerequisite.
Overall, risk stratification was most frequently assessed by the Caprini or Rogers score for most general, abdominal-pelvic, bariatric, vascular, plastic, and gynecologic surgery patients and by the Padua Prediction Score for hospitalized medical patients. In addition, major orthopedic surgery patients and stroke patients with restricted mobility were usually considered high risk, Dr. Sadaghianloo and Dr. Dardik said.
From their assessment of the literature, they determined that “further studies are needed to assess practical clinical questions that remain unanswered, including optimal cuff length and location, sequence and duration of pressure, and whether use of IPC in an outpatient setting can be effective and achieve good compliance.”
The authors reported that they had no conflicts of interest.
FROM JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISORDERS
Key clinical point: IPC is efficacious as the sole prophylactic agent in low- or moderate-risk surgical patients and in patients with high risk of bleeding with drug therapy.
Major finding: In high-risk patients, IPC is an added modality for preventing DVT in combination with pharmacologic prophylaxis.
Data source: Researchers performed an assessment of the literature in English since 2011 in MEDLINE and the Cochrane libraries.
Disclosures: The authors reported that they had no conflicts of interest.
Low incidence of DVT seen in routine MRI of damaged knees
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
The incidence of deep vein thrombosis (DVT) in knees undergoing routine assessment of damage was found to be low but suggests the need to interrogate the popliteal vein for evidence of thrombosis, according to Dr. Ryan M. Shulman of the joint department of medical imaging of University Health Network and Mount Sinai Hospital, Toronto, and his colleagues.
They documented the appearance and determined the prevalence of findings suspicious for popliteal vein thrombosis on magnetic resonance imaging (MRI) assessment of the knee joint by retrospectively reviewing 2,888 MRI examinations.
MRI images were classified as showing either normal appearing popliteal vein or findings suspicious for popliteal vein thrombosis. They found 2,879 of the MRI studies as having a normal appearing popliteal vein, with 9 showing findings suspicious for popliteal vein thrombosis.
“Although the prevalence of MR findings is low (0.3%), our findings reiterate the need to interrogate the popliteal vein for evidence of thrombosis,” Dr. Shulman and his colleagues concluded.
Find the full study online the Journal of Clinical Imaging 2015 (doi: 10.1016/j.clinimag.2015.09.008).
FROM JOURNAL OF CLINICAL IMAGING
3-D–printed devices mitigate pediatric tracheobronchomalacia
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
As illustrated in this article, the investigators demonstrate the potential for the future of 3-D–printing in medicine. While there have been numerous reports of utilizing 3-D–printing for a generation of personalized prostheses, these have been used in static circumstances in which the prosthesis is not required to change over time. In pediatric applications, as the child grows, the prosthesis also needs to adapt to this growth, thereby necessitating “4-D” printing.

|
Dr. Sai Yendamuri |
In three patients, the authors have applied this 4-D–printing paradigm to devise external bronchial splints to alleviate life-threatening tracheobronchomalacia using a novel design and a bioresorbable material, resulting in superb medium-term outcomes for patients with an otherwise dire prognosis.
Dr. Sai Yendamuri is an attending surgeon at the department of thoracic surgery, and director, Thoracic Surgery Research Laboratory, and an associate professor of oncology at Roswell Park Cancer Institute, Buffalo, N.Y. He is also an associate medical editor for Thoracic Surgery News.
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
Immediate and continued life-sustaining improvement was seen in three pediatric patients implanted with 3-D–printed tracheobronchial splints as a treatment for terminal tracheobronchomalacia (TBM), a condition of excessive collapse of the airways during respiration leading to cardiopulmonary arrest.
The particular value of such 3-D–printable biomaterials to pediatric surgery is their ability to adopt a 4-D modality – to exhibit specifically engineered shape changes in response to surrounding tissue growth over a defined time period. In addition to their malleability, these devices also are designed to biodegrade. These features have proven especially useful as seen in this medical device emergency use exemption study performed at the University of Michigan, according to a report published in Science Translational Medicine (2015 Apr 29. [doi: 10.1126/scitranslmed.3010825]).
“Our multidisciplinary team designed an archetype device to allow radial expansion of the affected airway over the critical growth period while resisting external compression and intrinsic collapse,” wrote Dr. Robert J. Morrison of the University of Michigan, Ann Arbor, and his colleagues.
The study population involved three infant boys, aged 3 months, 5 months, and 16 months at time of treatment. In each patient, a sternotomy exposed their affected airways. The 3-D–printed splint, consisting of conjoined rib-like C-shaped arches was placed around the affected airway and secured with polypropylene sutures. The splint counters external pressure on the airway and holds it open. Because the splint is malleable, with an expandable opening placed opposite to the main collapsing pressure, it is capable of expanding as the airway grows.
Examination of the airway immediately after placement demonstrated patency, which was confirmed 1 month later. Results showed the benefit of the splints for all three patients, although total results were complicated by additional comorbidities:
• Patient 1: Blood gases returned to normal immediately after implantation and remained normal at 3 months’ follow-up. A week after implantation, weaning from mechanical ventilation was initiated and, 3 weeks after the procedure, the child was discharged to home. Repeat imaging at 1, 3, 6, 12, and 39 months postoperatively demonstrated continued resolution of the TBM, with evidence of fragmentation and degradation of the splint at 39 months.
• Patient 2: Immediately after implantation of the device, blood gases improved greatly and the left lung perfused. The patient had opioid and benzodiazepine dependence from long-term ventilator support, requiring a longer controlled wean from the ventilator. Four weeks after surgery, the patient was transitioned to a portable ventilator system, completely weaned at 15 weeks, and discharged from the hospital to home for the first time in his life.
• Patient 3: After implantation, the patient ceased experiencing life-threatening desaturation episodes and showed sustained improvement in blood gases. Imaging showed continued patency of the left main bronchus with resolution of left-lung air trapping. However, at 14 months post implantation, he remained on permanent ventilator support, “presumably because of distal left segmental bronchomalacia beyond what the splint was designed to address,” according to Dr. Morrison and his colleagues.
“We report successful implantation of patient-specific bioresorbable airway splints for the treatment of severe TBM. The personalized splints conformed to the patients’ individual geometries and expanded with airway growth (in the ‘fourth dimension’),” the researchers summarized.
“The three pediatric patients implanted with these 3-D–printed airway splints had a terminal form of TBM. The clinical improvement in each case was immediate and sustained, suggesting that improvement is not attributable to the natural history of the disease alone,” they concluded.
The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: The use of 3-D–printed airway implants mitigated life-threatening tracheobronchomalacia (TBM) in three infants.
Major finding: Three infants with a terminal form of TBM ceased exhibiting life-threatening airway disease and showed continued growth of pulmonary airways after 3-D tracheal implants.
Data source: A study performed at the University of Michigan, Ann Arbor, of three infants with terminal TBM who received a medical device emergency use exemption for a 3-D tracheal implant.
Disclosures: The study was funded by the National Institutes of Health. Two of the study authors were coinventors of the device for which they have filed a patent. There were no other disclosures.
CTS Innovation: The promise of tissue engineering for heart valve replacement and repair
Bioengineered tissues are showing promise in the treatment of valvular heart disease, according to the results of several early clinical and preclinical studies demonstrating the benefits of using heterologous substrates as scaffolding capable of promoting in vivo cell infiltration and remodeling.
Ultimately, cardiac tissue engineering seeks to develop the ideal material for replacement and repair of a variety of heart components – a material able to integrate, function, and, when necessary, grow and develop in a manner undifferentiated from normal cellular structures.
One of the most important areas of focus for such efforts is the development of heart valves and valve patches suitable for use in replacement or repair. The need is obvious: “The fact that literature continues to be published debating the best type of valve prosthesis is proof that, to date, the ideal valve substitute has not been found,” according to Cristian Rosu, Ph.D. and Dr. Edward G. Soltesz of the Cleveland Clinic (Semin Thorac Cardiovasc Surg. 2015 June 30 [doi: 10.1053/j.semtcvs.2015.06.007]).
Dr. Roşu and Dr. Soltesz go on to say that this lack of an ideal prosthetic heart valve leaves surgeons and their patients with a difficult choice at the time of valve surgery. The focus of their concern relates to how current bioprostheses are being used in younger and younger patients, and how these devices have a finite lifespan – requiring eventual reoperation and replacement, perhaps multiple times. In addition, calcification is a prominent and well-known risk of bioprosthetic valves, especially in children (Circulation. 2014 Jul 1;130[1]:51-60).
As for mechanical valves, although durable, they require a lifetime of anticoagulation therapy to prevent thrombosis. And the lack of growth potential found in both mechanical and current bioprosthetic devices is obviously a bane to pediatric valve therapy.
The latest efforts in tissue engineering therefore seek to develop valves and valve components that may be more permanent solutions by better mimicking natural valves.
But better mimicking of a natural valve is not an easy task, when such valves exist in “a near-perfect correlation of structure and function, enabling the valve to avoid excess stress on the cusps while simultaneously withstanding the wear and tear of 40-million repetitive deformations per year, equivalent to some 3 billion over a 75-year lifetime.” (“Principles of Tissue Engineering,” 4th ed. [London: Academic Press 2014, p. 813]).
The “holy grail” of tissue engineering, therefore, is to provide a completely in vitro–developed, autologous, fully cellularized, functional scaffolding for implantation that can live up to these requirements. In order to do this, extensive research into the search for the best cell sources and growth matrices and methods is underway, as illustrated by several recent reviews (Front Cell Dev Biol. 30 June 2015 [doi.org/10.3389/fcell.2015.00039] and Mater Sci Eng C. 2015 March 1;48:556-65).
Candidate cell types include a variety of embryonic stem cells, as well as adult cell types that have proven amenable to rejuvenation and redifferentiation (Adv Drug Deliv Rev. 2014;69-70:254-69).
In one example of the quest for completely in vitro human-tissue designed valves, Dr. Jean Dubé of Laval University, Quebec, and his colleagues reported research on a human tissue–engineered trileaflet heart valve assembled in vitro using human fibroblasts. These cells self-assembled into living tissue sheets when cultured in the presence of sodium ascorbate. These sheets could be layered together to create a thick construct, with the ultimate goal of replacing the use of bovine pericardium tissue implants with ones made of autologous cells from the patient (Acta Biomater. 2014 Aug;10[8]:3563-70).
Currently, however, many of the preclinical studies of fully tissue engineered heart valves have shown retraction of the heart valve leaflets as a major mechanism of functional failure. This retraction is caused by both passive and active cell stress and passive matrix stress, according to a review by Inge A.E.W. van Loosdregt, Ph.D., and her colleagues at the Eindhoven (the Netherlands) University of Technology (J Biomech. 2014 Jun 27;47[9]:2064-9).
While all of these developmental issues regarding the use of fully tissue-engineered valves are being worked out, early clinical applications are already being found for a new generation of valve prostheses that take an intermediate approach, one that uses an implanted scaffolding material that allows autologous cell infiltration and replacement in vivo.
Two of the most prominent examples of these scaffoldings currently in investigation and in early clinical use for heart valve repair or replacement are the bovine pericardium-derived CardioCel (Admedus, Brisbane, Australia) and the porcine intestinal submucosa-derived CorMatrix ECM(CorMatrix Cardiovascular, Roswell, Ga.). CorMatrix ECM was approved by the Food and Drug Administration in 2005 for pericardial repair and reconstruction, and in 2007 for cardiac tissue repair. The FDA approved CardioCel in 2014 for use in the United States in pericardial closure and for the repair of cardiac and vascular defects in both adults and children.
Both technologies rely on the concept of matrix infiltration by autologous cells after implantation in order to create a living mimic of the patient’s own tissues.
CardioCel is a highly treated, bovine pericardium-derived, decellularized collagen matrix. It showed significant resistance to calcification in mitral and pulmonary implants in a juvenile sheep model as reported by Dr. Christian P. Brizard at Royal Children’s Hospital, Melbourne, and his colleagues (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3194-201). These investigators replaced the posterior leaflet of the mitral valve and one of the pulmonary valve cusps with patches in 10-month-old ewes. They compared the use of CardioCel in six ewes to a control group of four ewes repaired with autologous pericardium that was treated intraoperatively with glutaraldehyde, which is the standard default used at their institution for more than 2 decades as the best material for valve repair in their pediatric patients.
The primary end points of the study were thickening and calcium content. They found that all animals survived with normal valve echocardiography until sacrifice at 7 months. They reported that the bovine pericardium patches allowed accurate valve repair at both systemic and pulmonary pressure with preserved mechanical properties and more-controlled healing and without calcification as compared with the controls. (Calcification is a known major risk factor for the eventual failure of bioprosthetic valves.) Additionally, the bovine pericardium patched valves showed the in vivo development of dense but thin cellularized outer layers of mature collagen I, compared with the controls, which had outer layers that were much less dense and showed the presence of immature collagen III.
In another case of valvular use of the CardioCel material, at the recent American Association for Thoracic Surgery Mitral Conclave 2015, M. Bonnie Ghosh-Dastidar, Ph.D., and her colleagues from the Royal Brompton Hospital, London, reported on a severely ill patient with significant mitral regurgitation and an infected mitral valve with large vegetations on the anterior leaflets. The infected tissue was resected and a large patch of CardioCel bovine pericardium was used to reconstruct the leaflets. Postoperative assessment showed a competent mitral valve with good area of leaflet coaptation, according to Dr. Ghosh-Dastidar.
In his invited commentary on the animal-model research by Dr. Brizard, Dr. Niv Ad, director of cardiac surgery research at Inova Heart and Vascular Institute, Falls Church, Va., said that despite the promise of the CardioCel patches, there were a number of alternative approaches being investigated. “Other engineered materials currently being study include processes such as lyophilization, which has shown promising results in reducing inflammation. Another proposed approach is the use of decellularized vessels and patches with the promise of normal remodeling and growth, such as extracellular matrix and its potential for tissue regeneration.”
Dr. Ad also stated that, “the key bioengineering challenge is to determine how biologic, structural, and mechanical factors interact and function in vivo. The understanding of these factors will prove critical to the development of a clinically viable tissue-engineered heart valve,” (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3202-3).
An example of the use of extracellular matrix material referred to by Dr. Ad is the CorMatrix ECM, which is an extracellular matrix material derived from porcine small-intestinal submucosa, processed and decellularized.
Dr. Marc W. Gerdisch and his colleagues from the Franciscan St. Francis Heart Center, Indianapolis, reported on the results of treating 19 patients with mitral valve disease using the CorMatrix patch material for partial or subtotal leaflet repair or extension. There were three deaths unrelated to the repair and no instances of perioperative or late stroke. Two patients with a history of cancer and cancer therapy experienced failure of the initial repair, requiring reintervention. However, the other mitral valve repair patients continued to show good valvular function and no calcification on echocardiographic follow-up of 4 days to 48 months (Thorac Cardiovasc Surg. 2014 Oct;148[4]:1370-8).
And in 2015, CorMatrix Cardiovascular announced FDA approval of an investigational device exemption for an early feasibility study of their CorMatrix ECM Tricuspid Heart Valve in up to 15 patients at 5 U.S. centers (NCT02397668). Indications are for the surgical management of tricuspid valve disease not amenable to annuloplasty or repair, including tricuspid valve disease secondary to congenital heart disease in pediatric patients and adult endocarditis patients. The CorMatrix ECM Tricuspid Valve is a flexible, unstented valve acting as a 3‐D scaffold designed to function immediately as a prosthetic, but one constructed to permit native cellular infiltration and remodeling.
Ultimately, both the CardioCel and the CorMatrix materials are just the tip of an iceberg of research into tissue engineering as a clinical tool. And success and the wider adoption of any current or developing technologies will require the results of far-more-extensive studies and long-term clinical results.
At some future date, engineered total organ substitutes or the routine injection of genetically engineered stem cells may provide a practical alternative for treating a diseased or damaged heart. But for now, the most-likely scenario is the continued exploration of tissue engineering to develop the most durable, growth-, stress-, and biologically compatible patches, valves, and vasculatures possible – defined tools that can be adapted to current surgical techniques, designed to obtain the best repair of congenital or acquired heart disease conditions.
As with any transformative technology in medicine, the path from laboratory success and optimistic short-term clinical results to durable, long-term postoperative benefits may be a rocky one. But given the current enthusiasm, it certainly looks as if it will be a well-traveled road.
Dr. Brizard reported consulting and lecturing fees from Admedus. His research was supported with the assistance of a grant from Admedus. Dr. Gerdisch reported consulting fees and equity ownership in CorMatrix Cardiovascular.
Bioengineered tissues are showing promise in the treatment of valvular heart disease, according to the results of several early clinical and preclinical studies demonstrating the benefits of using heterologous substrates as scaffolding capable of promoting in vivo cell infiltration and remodeling.
Ultimately, cardiac tissue engineering seeks to develop the ideal material for replacement and repair of a variety of heart components – a material able to integrate, function, and, when necessary, grow and develop in a manner undifferentiated from normal cellular structures.
One of the most important areas of focus for such efforts is the development of heart valves and valve patches suitable for use in replacement or repair. The need is obvious: “The fact that literature continues to be published debating the best type of valve prosthesis is proof that, to date, the ideal valve substitute has not been found,” according to Cristian Rosu, Ph.D. and Dr. Edward G. Soltesz of the Cleveland Clinic (Semin Thorac Cardiovasc Surg. 2015 June 30 [doi: 10.1053/j.semtcvs.2015.06.007]).
Dr. Roşu and Dr. Soltesz go on to say that this lack of an ideal prosthetic heart valve leaves surgeons and their patients with a difficult choice at the time of valve surgery. The focus of their concern relates to how current bioprostheses are being used in younger and younger patients, and how these devices have a finite lifespan – requiring eventual reoperation and replacement, perhaps multiple times. In addition, calcification is a prominent and well-known risk of bioprosthetic valves, especially in children (Circulation. 2014 Jul 1;130[1]:51-60).
As for mechanical valves, although durable, they require a lifetime of anticoagulation therapy to prevent thrombosis. And the lack of growth potential found in both mechanical and current bioprosthetic devices is obviously a bane to pediatric valve therapy.
The latest efforts in tissue engineering therefore seek to develop valves and valve components that may be more permanent solutions by better mimicking natural valves.
But better mimicking of a natural valve is not an easy task, when such valves exist in “a near-perfect correlation of structure and function, enabling the valve to avoid excess stress on the cusps while simultaneously withstanding the wear and tear of 40-million repetitive deformations per year, equivalent to some 3 billion over a 75-year lifetime.” (“Principles of Tissue Engineering,” 4th ed. [London: Academic Press 2014, p. 813]).
The “holy grail” of tissue engineering, therefore, is to provide a completely in vitro–developed, autologous, fully cellularized, functional scaffolding for implantation that can live up to these requirements. In order to do this, extensive research into the search for the best cell sources and growth matrices and methods is underway, as illustrated by several recent reviews (Front Cell Dev Biol. 30 June 2015 [doi.org/10.3389/fcell.2015.00039] and Mater Sci Eng C. 2015 March 1;48:556-65).
Candidate cell types include a variety of embryonic stem cells, as well as adult cell types that have proven amenable to rejuvenation and redifferentiation (Adv Drug Deliv Rev. 2014;69-70:254-69).
In one example of the quest for completely in vitro human-tissue designed valves, Dr. Jean Dubé of Laval University, Quebec, and his colleagues reported research on a human tissue–engineered trileaflet heart valve assembled in vitro using human fibroblasts. These cells self-assembled into living tissue sheets when cultured in the presence of sodium ascorbate. These sheets could be layered together to create a thick construct, with the ultimate goal of replacing the use of bovine pericardium tissue implants with ones made of autologous cells from the patient (Acta Biomater. 2014 Aug;10[8]:3563-70).
Currently, however, many of the preclinical studies of fully tissue engineered heart valves have shown retraction of the heart valve leaflets as a major mechanism of functional failure. This retraction is caused by both passive and active cell stress and passive matrix stress, according to a review by Inge A.E.W. van Loosdregt, Ph.D., and her colleagues at the Eindhoven (the Netherlands) University of Technology (J Biomech. 2014 Jun 27;47[9]:2064-9).
While all of these developmental issues regarding the use of fully tissue-engineered valves are being worked out, early clinical applications are already being found for a new generation of valve prostheses that take an intermediate approach, one that uses an implanted scaffolding material that allows autologous cell infiltration and replacement in vivo.
Two of the most prominent examples of these scaffoldings currently in investigation and in early clinical use for heart valve repair or replacement are the bovine pericardium-derived CardioCel (Admedus, Brisbane, Australia) and the porcine intestinal submucosa-derived CorMatrix ECM(CorMatrix Cardiovascular, Roswell, Ga.). CorMatrix ECM was approved by the Food and Drug Administration in 2005 for pericardial repair and reconstruction, and in 2007 for cardiac tissue repair. The FDA approved CardioCel in 2014 for use in the United States in pericardial closure and for the repair of cardiac and vascular defects in both adults and children.
Both technologies rely on the concept of matrix infiltration by autologous cells after implantation in order to create a living mimic of the patient’s own tissues.
CardioCel is a highly treated, bovine pericardium-derived, decellularized collagen matrix. It showed significant resistance to calcification in mitral and pulmonary implants in a juvenile sheep model as reported by Dr. Christian P. Brizard at Royal Children’s Hospital, Melbourne, and his colleagues (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3194-201). These investigators replaced the posterior leaflet of the mitral valve and one of the pulmonary valve cusps with patches in 10-month-old ewes. They compared the use of CardioCel in six ewes to a control group of four ewes repaired with autologous pericardium that was treated intraoperatively with glutaraldehyde, which is the standard default used at their institution for more than 2 decades as the best material for valve repair in their pediatric patients.
The primary end points of the study were thickening and calcium content. They found that all animals survived with normal valve echocardiography until sacrifice at 7 months. They reported that the bovine pericardium patches allowed accurate valve repair at both systemic and pulmonary pressure with preserved mechanical properties and more-controlled healing and without calcification as compared with the controls. (Calcification is a known major risk factor for the eventual failure of bioprosthetic valves.) Additionally, the bovine pericardium patched valves showed the in vivo development of dense but thin cellularized outer layers of mature collagen I, compared with the controls, which had outer layers that were much less dense and showed the presence of immature collagen III.
In another case of valvular use of the CardioCel material, at the recent American Association for Thoracic Surgery Mitral Conclave 2015, M. Bonnie Ghosh-Dastidar, Ph.D., and her colleagues from the Royal Brompton Hospital, London, reported on a severely ill patient with significant mitral regurgitation and an infected mitral valve with large vegetations on the anterior leaflets. The infected tissue was resected and a large patch of CardioCel bovine pericardium was used to reconstruct the leaflets. Postoperative assessment showed a competent mitral valve with good area of leaflet coaptation, according to Dr. Ghosh-Dastidar.
In his invited commentary on the animal-model research by Dr. Brizard, Dr. Niv Ad, director of cardiac surgery research at Inova Heart and Vascular Institute, Falls Church, Va., said that despite the promise of the CardioCel patches, there were a number of alternative approaches being investigated. “Other engineered materials currently being study include processes such as lyophilization, which has shown promising results in reducing inflammation. Another proposed approach is the use of decellularized vessels and patches with the promise of normal remodeling and growth, such as extracellular matrix and its potential for tissue regeneration.”
Dr. Ad also stated that, “the key bioengineering challenge is to determine how biologic, structural, and mechanical factors interact and function in vivo. The understanding of these factors will prove critical to the development of a clinically viable tissue-engineered heart valve,” (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3202-3).
An example of the use of extracellular matrix material referred to by Dr. Ad is the CorMatrix ECM, which is an extracellular matrix material derived from porcine small-intestinal submucosa, processed and decellularized.
Dr. Marc W. Gerdisch and his colleagues from the Franciscan St. Francis Heart Center, Indianapolis, reported on the results of treating 19 patients with mitral valve disease using the CorMatrix patch material for partial or subtotal leaflet repair or extension. There were three deaths unrelated to the repair and no instances of perioperative or late stroke. Two patients with a history of cancer and cancer therapy experienced failure of the initial repair, requiring reintervention. However, the other mitral valve repair patients continued to show good valvular function and no calcification on echocardiographic follow-up of 4 days to 48 months (Thorac Cardiovasc Surg. 2014 Oct;148[4]:1370-8).
And in 2015, CorMatrix Cardiovascular announced FDA approval of an investigational device exemption for an early feasibility study of their CorMatrix ECM Tricuspid Heart Valve in up to 15 patients at 5 U.S. centers (NCT02397668). Indications are for the surgical management of tricuspid valve disease not amenable to annuloplasty or repair, including tricuspid valve disease secondary to congenital heart disease in pediatric patients and adult endocarditis patients. The CorMatrix ECM Tricuspid Valve is a flexible, unstented valve acting as a 3‐D scaffold designed to function immediately as a prosthetic, but one constructed to permit native cellular infiltration and remodeling.
Ultimately, both the CardioCel and the CorMatrix materials are just the tip of an iceberg of research into tissue engineering as a clinical tool. And success and the wider adoption of any current or developing technologies will require the results of far-more-extensive studies and long-term clinical results.
At some future date, engineered total organ substitutes or the routine injection of genetically engineered stem cells may provide a practical alternative for treating a diseased or damaged heart. But for now, the most-likely scenario is the continued exploration of tissue engineering to develop the most durable, growth-, stress-, and biologically compatible patches, valves, and vasculatures possible – defined tools that can be adapted to current surgical techniques, designed to obtain the best repair of congenital or acquired heart disease conditions.
As with any transformative technology in medicine, the path from laboratory success and optimistic short-term clinical results to durable, long-term postoperative benefits may be a rocky one. But given the current enthusiasm, it certainly looks as if it will be a well-traveled road.
Dr. Brizard reported consulting and lecturing fees from Admedus. His research was supported with the assistance of a grant from Admedus. Dr. Gerdisch reported consulting fees and equity ownership in CorMatrix Cardiovascular.
Bioengineered tissues are showing promise in the treatment of valvular heart disease, according to the results of several early clinical and preclinical studies demonstrating the benefits of using heterologous substrates as scaffolding capable of promoting in vivo cell infiltration and remodeling.
Ultimately, cardiac tissue engineering seeks to develop the ideal material for replacement and repair of a variety of heart components – a material able to integrate, function, and, when necessary, grow and develop in a manner undifferentiated from normal cellular structures.
One of the most important areas of focus for such efforts is the development of heart valves and valve patches suitable for use in replacement or repair. The need is obvious: “The fact that literature continues to be published debating the best type of valve prosthesis is proof that, to date, the ideal valve substitute has not been found,” according to Cristian Rosu, Ph.D. and Dr. Edward G. Soltesz of the Cleveland Clinic (Semin Thorac Cardiovasc Surg. 2015 June 30 [doi: 10.1053/j.semtcvs.2015.06.007]).
Dr. Roşu and Dr. Soltesz go on to say that this lack of an ideal prosthetic heart valve leaves surgeons and their patients with a difficult choice at the time of valve surgery. The focus of their concern relates to how current bioprostheses are being used in younger and younger patients, and how these devices have a finite lifespan – requiring eventual reoperation and replacement, perhaps multiple times. In addition, calcification is a prominent and well-known risk of bioprosthetic valves, especially in children (Circulation. 2014 Jul 1;130[1]:51-60).
As for mechanical valves, although durable, they require a lifetime of anticoagulation therapy to prevent thrombosis. And the lack of growth potential found in both mechanical and current bioprosthetic devices is obviously a bane to pediatric valve therapy.
The latest efforts in tissue engineering therefore seek to develop valves and valve components that may be more permanent solutions by better mimicking natural valves.
But better mimicking of a natural valve is not an easy task, when such valves exist in “a near-perfect correlation of structure and function, enabling the valve to avoid excess stress on the cusps while simultaneously withstanding the wear and tear of 40-million repetitive deformations per year, equivalent to some 3 billion over a 75-year lifetime.” (“Principles of Tissue Engineering,” 4th ed. [London: Academic Press 2014, p. 813]).
The “holy grail” of tissue engineering, therefore, is to provide a completely in vitro–developed, autologous, fully cellularized, functional scaffolding for implantation that can live up to these requirements. In order to do this, extensive research into the search for the best cell sources and growth matrices and methods is underway, as illustrated by several recent reviews (Front Cell Dev Biol. 30 June 2015 [doi.org/10.3389/fcell.2015.00039] and Mater Sci Eng C. 2015 March 1;48:556-65).
Candidate cell types include a variety of embryonic stem cells, as well as adult cell types that have proven amenable to rejuvenation and redifferentiation (Adv Drug Deliv Rev. 2014;69-70:254-69).
In one example of the quest for completely in vitro human-tissue designed valves, Dr. Jean Dubé of Laval University, Quebec, and his colleagues reported research on a human tissue–engineered trileaflet heart valve assembled in vitro using human fibroblasts. These cells self-assembled into living tissue sheets when cultured in the presence of sodium ascorbate. These sheets could be layered together to create a thick construct, with the ultimate goal of replacing the use of bovine pericardium tissue implants with ones made of autologous cells from the patient (Acta Biomater. 2014 Aug;10[8]:3563-70).
Currently, however, many of the preclinical studies of fully tissue engineered heart valves have shown retraction of the heart valve leaflets as a major mechanism of functional failure. This retraction is caused by both passive and active cell stress and passive matrix stress, according to a review by Inge A.E.W. van Loosdregt, Ph.D., and her colleagues at the Eindhoven (the Netherlands) University of Technology (J Biomech. 2014 Jun 27;47[9]:2064-9).
While all of these developmental issues regarding the use of fully tissue-engineered valves are being worked out, early clinical applications are already being found for a new generation of valve prostheses that take an intermediate approach, one that uses an implanted scaffolding material that allows autologous cell infiltration and replacement in vivo.
Two of the most prominent examples of these scaffoldings currently in investigation and in early clinical use for heart valve repair or replacement are the bovine pericardium-derived CardioCel (Admedus, Brisbane, Australia) and the porcine intestinal submucosa-derived CorMatrix ECM(CorMatrix Cardiovascular, Roswell, Ga.). CorMatrix ECM was approved by the Food and Drug Administration in 2005 for pericardial repair and reconstruction, and in 2007 for cardiac tissue repair. The FDA approved CardioCel in 2014 for use in the United States in pericardial closure and for the repair of cardiac and vascular defects in both adults and children.
Both technologies rely on the concept of matrix infiltration by autologous cells after implantation in order to create a living mimic of the patient’s own tissues.
CardioCel is a highly treated, bovine pericardium-derived, decellularized collagen matrix. It showed significant resistance to calcification in mitral and pulmonary implants in a juvenile sheep model as reported by Dr. Christian P. Brizard at Royal Children’s Hospital, Melbourne, and his colleagues (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3194-201). These investigators replaced the posterior leaflet of the mitral valve and one of the pulmonary valve cusps with patches in 10-month-old ewes. They compared the use of CardioCel in six ewes to a control group of four ewes repaired with autologous pericardium that was treated intraoperatively with glutaraldehyde, which is the standard default used at their institution for more than 2 decades as the best material for valve repair in their pediatric patients.
The primary end points of the study were thickening and calcium content. They found that all animals survived with normal valve echocardiography until sacrifice at 7 months. They reported that the bovine pericardium patches allowed accurate valve repair at both systemic and pulmonary pressure with preserved mechanical properties and more-controlled healing and without calcification as compared with the controls. (Calcification is a known major risk factor for the eventual failure of bioprosthetic valves.) Additionally, the bovine pericardium patched valves showed the in vivo development of dense but thin cellularized outer layers of mature collagen I, compared with the controls, which had outer layers that were much less dense and showed the presence of immature collagen III.
In another case of valvular use of the CardioCel material, at the recent American Association for Thoracic Surgery Mitral Conclave 2015, M. Bonnie Ghosh-Dastidar, Ph.D., and her colleagues from the Royal Brompton Hospital, London, reported on a severely ill patient with significant mitral regurgitation and an infected mitral valve with large vegetations on the anterior leaflets. The infected tissue was resected and a large patch of CardioCel bovine pericardium was used to reconstruct the leaflets. Postoperative assessment showed a competent mitral valve with good area of leaflet coaptation, according to Dr. Ghosh-Dastidar.
In his invited commentary on the animal-model research by Dr. Brizard, Dr. Niv Ad, director of cardiac surgery research at Inova Heart and Vascular Institute, Falls Church, Va., said that despite the promise of the CardioCel patches, there were a number of alternative approaches being investigated. “Other engineered materials currently being study include processes such as lyophilization, which has shown promising results in reducing inflammation. Another proposed approach is the use of decellularized vessels and patches with the promise of normal remodeling and growth, such as extracellular matrix and its potential for tissue regeneration.”
Dr. Ad also stated that, “the key bioengineering challenge is to determine how biologic, structural, and mechanical factors interact and function in vivo. The understanding of these factors will prove critical to the development of a clinically viable tissue-engineered heart valve,” (J Thorac Cardiovasc Surg. 2014 Dec;148[6]:3202-3).
An example of the use of extracellular matrix material referred to by Dr. Ad is the CorMatrix ECM, which is an extracellular matrix material derived from porcine small-intestinal submucosa, processed and decellularized.
Dr. Marc W. Gerdisch and his colleagues from the Franciscan St. Francis Heart Center, Indianapolis, reported on the results of treating 19 patients with mitral valve disease using the CorMatrix patch material for partial or subtotal leaflet repair or extension. There were three deaths unrelated to the repair and no instances of perioperative or late stroke. Two patients with a history of cancer and cancer therapy experienced failure of the initial repair, requiring reintervention. However, the other mitral valve repair patients continued to show good valvular function and no calcification on echocardiographic follow-up of 4 days to 48 months (Thorac Cardiovasc Surg. 2014 Oct;148[4]:1370-8).
And in 2015, CorMatrix Cardiovascular announced FDA approval of an investigational device exemption for an early feasibility study of their CorMatrix ECM Tricuspid Heart Valve in up to 15 patients at 5 U.S. centers (NCT02397668). Indications are for the surgical management of tricuspid valve disease not amenable to annuloplasty or repair, including tricuspid valve disease secondary to congenital heart disease in pediatric patients and adult endocarditis patients. The CorMatrix ECM Tricuspid Valve is a flexible, unstented valve acting as a 3‐D scaffold designed to function immediately as a prosthetic, but one constructed to permit native cellular infiltration and remodeling.
Ultimately, both the CardioCel and the CorMatrix materials are just the tip of an iceberg of research into tissue engineering as a clinical tool. And success and the wider adoption of any current or developing technologies will require the results of far-more-extensive studies and long-term clinical results.
At some future date, engineered total organ substitutes or the routine injection of genetically engineered stem cells may provide a practical alternative for treating a diseased or damaged heart. But for now, the most-likely scenario is the continued exploration of tissue engineering to develop the most durable, growth-, stress-, and biologically compatible patches, valves, and vasculatures possible – defined tools that can be adapted to current surgical techniques, designed to obtain the best repair of congenital or acquired heart disease conditions.
As with any transformative technology in medicine, the path from laboratory success and optimistic short-term clinical results to durable, long-term postoperative benefits may be a rocky one. But given the current enthusiasm, it certainly looks as if it will be a well-traveled road.
Dr. Brizard reported consulting and lecturing fees from Admedus. His research was supported with the assistance of a grant from Admedus. Dr. Gerdisch reported consulting fees and equity ownership in CorMatrix Cardiovascular.
Warfarin therapy highly unpredictable in real-world practice
Only a minority of patients with atrial fibrillation or venous thromboembolism achieved good anticoagulation control while on warfarin, according to a population-based data analysis by Ana Filipa Macedo and her colleagues at Boehringer Ingelheim in the United Kingdom. In fact, only 44% of 140,078 AF patients and 36% of 70,371 VTE patients assessed had an optimal International Normalized Ratio (INR) time in therapeutic range (TTR) more than 70% of the time while on warfarin.
Patient characteristics associated with a significant increase in time spent above or below the recommended INR range of 2.0-3.0 were current smoking, the use of NSAIDs, age less than 45 years, and a body mass index less than 18.5 kg/m2 in both AF and VTE patients, whereas patients with VTE alone had predictors of poor control that included chronic obstructive pulmonary disease or asthma, heart failure, and active cancer.
“The study provides evidence that in the first 12 months of warfarin use, there is a high amount of unpredictable variability in an individual’s TTR,” the researchers summarized. “These findings confirm the difficulty of achieving high-quality anticoagulation with warfarin in real-world clinical practice and can be used to identify patients who require closer monitoring or innovative management strategies,” the authors added.
More of the study can be read here (Thromb Res. 2015 Aug; 136(2):250-260.).
The study received funding from Boehringer Ingelheim, and the authors disclosed that they were all employees of the company, which makes a variety of cardiovascular therapies, including anticoagulants.
Only a minority of patients with atrial fibrillation or venous thromboembolism achieved good anticoagulation control while on warfarin, according to a population-based data analysis by Ana Filipa Macedo and her colleagues at Boehringer Ingelheim in the United Kingdom. In fact, only 44% of 140,078 AF patients and 36% of 70,371 VTE patients assessed had an optimal International Normalized Ratio (INR) time in therapeutic range (TTR) more than 70% of the time while on warfarin.
Patient characteristics associated with a significant increase in time spent above or below the recommended INR range of 2.0-3.0 were current smoking, the use of NSAIDs, age less than 45 years, and a body mass index less than 18.5 kg/m2 in both AF and VTE patients, whereas patients with VTE alone had predictors of poor control that included chronic obstructive pulmonary disease or asthma, heart failure, and active cancer.
“The study provides evidence that in the first 12 months of warfarin use, there is a high amount of unpredictable variability in an individual’s TTR,” the researchers summarized. “These findings confirm the difficulty of achieving high-quality anticoagulation with warfarin in real-world clinical practice and can be used to identify patients who require closer monitoring or innovative management strategies,” the authors added.
More of the study can be read here (Thromb Res. 2015 Aug; 136(2):250-260.).
The study received funding from Boehringer Ingelheim, and the authors disclosed that they were all employees of the company, which makes a variety of cardiovascular therapies, including anticoagulants.
Only a minority of patients with atrial fibrillation or venous thromboembolism achieved good anticoagulation control while on warfarin, according to a population-based data analysis by Ana Filipa Macedo and her colleagues at Boehringer Ingelheim in the United Kingdom. In fact, only 44% of 140,078 AF patients and 36% of 70,371 VTE patients assessed had an optimal International Normalized Ratio (INR) time in therapeutic range (TTR) more than 70% of the time while on warfarin.
Patient characteristics associated with a significant increase in time spent above or below the recommended INR range of 2.0-3.0 were current smoking, the use of NSAIDs, age less than 45 years, and a body mass index less than 18.5 kg/m2 in both AF and VTE patients, whereas patients with VTE alone had predictors of poor control that included chronic obstructive pulmonary disease or asthma, heart failure, and active cancer.
“The study provides evidence that in the first 12 months of warfarin use, there is a high amount of unpredictable variability in an individual’s TTR,” the researchers summarized. “These findings confirm the difficulty of achieving high-quality anticoagulation with warfarin in real-world clinical practice and can be used to identify patients who require closer monitoring or innovative management strategies,” the authors added.
More of the study can be read here (Thromb Res. 2015 Aug; 136(2):250-260.).
The study received funding from Boehringer Ingelheim, and the authors disclosed that they were all employees of the company, which makes a variety of cardiovascular therapies, including anticoagulants.
FROM THROMBOSIS RESEARCH
Dickkopf-3 overexpression linked to tumor traits in esophageal adenocarcinoma
Esophageal adenocarcinoma is fatal to 90% of patients, indicating a profound need for new therapeutic agents, according to Zhuewn Wang and her colleagues.
The search for genes involved in oncogenesis provides one avenue for finding potential targets. Ms. Wang and her colleagues performed a laboratory study at the University of Michigan, Ann Arbor, to examine the results of the overexpression of DKK3 (the gene for the Dickkopf-3 protein [DKK3]) in DKK3-transfected tissue-cultured esophageal adenocarcinoma (EAC) cell lines. They found that DKK3 overexpression correlated with significantly increased proliferation and Matrigel invasion ability – both known to be important oncogenic traits (J. Thorac. Cardiovasc. Surg. 2015;150:377-85).
DKK3 was overexpressed (greater than twofold) in 76% (72/95) of esophageal adenocarcinomas tested. In addition, the DKK3 protein was present at moderate to high levels in 47% (29/62) of esophageal adenocarcinomas as shown by tissue microarray. Nodal metastases were also significantly increased in patients with esophageal adenocarcinomas highly overexpressing DKK3 (28/32) vs. non–highly expressing EAC tumors (42/63).
In vitro studies showed that stable transfection of DKK3 in an EAC cell line significantly increased proliferation and Matrigel invasion. The researchers also found that the levels of SMAD4, a key mediator of the transforming growth factor–beta pathway, increased after activin treatment of the transfected cell line, and siSMAD4 significantly decreased Matrigel invasion, suggesting that DKK3 acts through the transforming growth factor–beta pathway, according to the researchers.
Additionally, the transfected cells showed increased endothelial tube formation, and they were significantly more resistant to 5-fluorouracil and cisplatin. This finding correlates with the fact that DKK3 expression was found to be significantly higher in chemoresistant esophageal adenocarcinomas, the investigators reported.
In their animal model system (NOD/SCIDg mice), injection of the transfected cells resulted in tumors at all sites (8/8), whereas vector-only cells grew in only one of eight sites.
“The results of the current study suggest that DKK3 may play an important role in tumor growth and invasion in EAC. DKK3 is overexpressed in a significant number of esophageal adenocarcinomas and targeting DKK3 and its downstream mediators may be beneficial in the prevention and treatment of micrometastatic disease and potentially decreasing disease recurrence,” the researchers concluded.
The authors reported that they had no relevant financial conflicts.
The number of EAC cell lines currently available for study is limited. Despite the fact that the majority of EAC human tumors overexpress Dickkopf-3 in vivo, none of the cell lines used in this study had significant expression. Therefore the authors had to use a forced overexpression transfection strategy to evaluate EAC tumor biology, according to Dr. David R. Jones (J. Thorac. Cardovasc. Surg. 2015;150:288).
This is less than optimal because of an inability to silence Dickkopf-3 to determine if it is sufficient or merely required to drive tumorigenesis or aggressiveness. A more glaring and overarching problem is the lack of an adequate animal model, Dr. Jones added. The xenograft flank model may not recapitulate EAC tumorigenesis or metastases. “Currently there are no well-characterized EAC genetically engineered mouse models that faithfully reproduce EAC,” he pointed out. “The lack of EAC animal models is a major barrier to the preclinical evaluation of novel therapies, target validation, and pathway discovery and confirmation.”
Dr. Jones is a cardiothoracic surgeon at Memorial Sloan Kettering Cancer Center, New York. He made these remarks in his invited commentary.
The number of EAC cell lines currently available for study is limited. Despite the fact that the majority of EAC human tumors overexpress Dickkopf-3 in vivo, none of the cell lines used in this study had significant expression. Therefore the authors had to use a forced overexpression transfection strategy to evaluate EAC tumor biology, according to Dr. David R. Jones (J. Thorac. Cardovasc. Surg. 2015;150:288).
This is less than optimal because of an inability to silence Dickkopf-3 to determine if it is sufficient or merely required to drive tumorigenesis or aggressiveness. A more glaring and overarching problem is the lack of an adequate animal model, Dr. Jones added. The xenograft flank model may not recapitulate EAC tumorigenesis or metastases. “Currently there are no well-characterized EAC genetically engineered mouse models that faithfully reproduce EAC,” he pointed out. “The lack of EAC animal models is a major barrier to the preclinical evaluation of novel therapies, target validation, and pathway discovery and confirmation.”
Dr. Jones is a cardiothoracic surgeon at Memorial Sloan Kettering Cancer Center, New York. He made these remarks in his invited commentary.
The number of EAC cell lines currently available for study is limited. Despite the fact that the majority of EAC human tumors overexpress Dickkopf-3 in vivo, none of the cell lines used in this study had significant expression. Therefore the authors had to use a forced overexpression transfection strategy to evaluate EAC tumor biology, according to Dr. David R. Jones (J. Thorac. Cardovasc. Surg. 2015;150:288).
This is less than optimal because of an inability to silence Dickkopf-3 to determine if it is sufficient or merely required to drive tumorigenesis or aggressiveness. A more glaring and overarching problem is the lack of an adequate animal model, Dr. Jones added. The xenograft flank model may not recapitulate EAC tumorigenesis or metastases. “Currently there are no well-characterized EAC genetically engineered mouse models that faithfully reproduce EAC,” he pointed out. “The lack of EAC animal models is a major barrier to the preclinical evaluation of novel therapies, target validation, and pathway discovery and confirmation.”
Dr. Jones is a cardiothoracic surgeon at Memorial Sloan Kettering Cancer Center, New York. He made these remarks in his invited commentary.
Esophageal adenocarcinoma is fatal to 90% of patients, indicating a profound need for new therapeutic agents, according to Zhuewn Wang and her colleagues.
The search for genes involved in oncogenesis provides one avenue for finding potential targets. Ms. Wang and her colleagues performed a laboratory study at the University of Michigan, Ann Arbor, to examine the results of the overexpression of DKK3 (the gene for the Dickkopf-3 protein [DKK3]) in DKK3-transfected tissue-cultured esophageal adenocarcinoma (EAC) cell lines. They found that DKK3 overexpression correlated with significantly increased proliferation and Matrigel invasion ability – both known to be important oncogenic traits (J. Thorac. Cardiovasc. Surg. 2015;150:377-85).
DKK3 was overexpressed (greater than twofold) in 76% (72/95) of esophageal adenocarcinomas tested. In addition, the DKK3 protein was present at moderate to high levels in 47% (29/62) of esophageal adenocarcinomas as shown by tissue microarray. Nodal metastases were also significantly increased in patients with esophageal adenocarcinomas highly overexpressing DKK3 (28/32) vs. non–highly expressing EAC tumors (42/63).
In vitro studies showed that stable transfection of DKK3 in an EAC cell line significantly increased proliferation and Matrigel invasion. The researchers also found that the levels of SMAD4, a key mediator of the transforming growth factor–beta pathway, increased after activin treatment of the transfected cell line, and siSMAD4 significantly decreased Matrigel invasion, suggesting that DKK3 acts through the transforming growth factor–beta pathway, according to the researchers.
Additionally, the transfected cells showed increased endothelial tube formation, and they were significantly more resistant to 5-fluorouracil and cisplatin. This finding correlates with the fact that DKK3 expression was found to be significantly higher in chemoresistant esophageal adenocarcinomas, the investigators reported.
In their animal model system (NOD/SCIDg mice), injection of the transfected cells resulted in tumors at all sites (8/8), whereas vector-only cells grew in only one of eight sites.
“The results of the current study suggest that DKK3 may play an important role in tumor growth and invasion in EAC. DKK3 is overexpressed in a significant number of esophageal adenocarcinomas and targeting DKK3 and its downstream mediators may be beneficial in the prevention and treatment of micrometastatic disease and potentially decreasing disease recurrence,” the researchers concluded.
The authors reported that they had no relevant financial conflicts.
Esophageal adenocarcinoma is fatal to 90% of patients, indicating a profound need for new therapeutic agents, according to Zhuewn Wang and her colleagues.
The search for genes involved in oncogenesis provides one avenue for finding potential targets. Ms. Wang and her colleagues performed a laboratory study at the University of Michigan, Ann Arbor, to examine the results of the overexpression of DKK3 (the gene for the Dickkopf-3 protein [DKK3]) in DKK3-transfected tissue-cultured esophageal adenocarcinoma (EAC) cell lines. They found that DKK3 overexpression correlated with significantly increased proliferation and Matrigel invasion ability – both known to be important oncogenic traits (J. Thorac. Cardiovasc. Surg. 2015;150:377-85).
DKK3 was overexpressed (greater than twofold) in 76% (72/95) of esophageal adenocarcinomas tested. In addition, the DKK3 protein was present at moderate to high levels in 47% (29/62) of esophageal adenocarcinomas as shown by tissue microarray. Nodal metastases were also significantly increased in patients with esophageal adenocarcinomas highly overexpressing DKK3 (28/32) vs. non–highly expressing EAC tumors (42/63).
In vitro studies showed that stable transfection of DKK3 in an EAC cell line significantly increased proliferation and Matrigel invasion. The researchers also found that the levels of SMAD4, a key mediator of the transforming growth factor–beta pathway, increased after activin treatment of the transfected cell line, and siSMAD4 significantly decreased Matrigel invasion, suggesting that DKK3 acts through the transforming growth factor–beta pathway, according to the researchers.
Additionally, the transfected cells showed increased endothelial tube formation, and they were significantly more resistant to 5-fluorouracil and cisplatin. This finding correlates with the fact that DKK3 expression was found to be significantly higher in chemoresistant esophageal adenocarcinomas, the investigators reported.
In their animal model system (NOD/SCIDg mice), injection of the transfected cells resulted in tumors at all sites (8/8), whereas vector-only cells grew in only one of eight sites.
“The results of the current study suggest that DKK3 may play an important role in tumor growth and invasion in EAC. DKK3 is overexpressed in a significant number of esophageal adenocarcinomas and targeting DKK3 and its downstream mediators may be beneficial in the prevention and treatment of micrometastatic disease and potentially decreasing disease recurrence,” the researchers concluded.
The authors reported that they had no relevant financial conflicts.
FROM THE JOURNAL OF THORACIC AND CARDIOVASCULAR SURGERY
Key clinical point: DKK3 may be important in mediating invasion in esophageal adenocarcinoma and could be a novel target for treating and preventing metastasis.
Major finding: Dickkopf-3 overexpression correlated with tumorigenesis and aggressiveness traits in an esophageal adenocarcinoma cell line, and the gene was overexpressed in primary EAC tumors relative to normal esophageal tissue.
Data source: Laboratory studies using cell culture and a mouse model.
Disclosures: The authors reported that they had no relevant financial conflicts.
Talent: Too much or too little depends on the kind of team
Talent facilitates team performance, but only up to a point, depending on the type of team. For highly interdependent teams, there is a threshold where the benefits of more talent decrease and eventually become detrimental rather than beneficial, according to an analysis of five studies performed by Roderick I. Swaab and his colleagues.
Surveys across industries and countries show that organizations consider talent attraction is their top priority, presumably based on the belief that more talent is better and the relationship between talent and team performance is linear and monotonic, they stated in Psychological Science (2014;25:1581-91)
The researchers analyzed the results of five studies they performed to investigate this relationship, comparing the impact of talent on team performance in the highly interdependent sports soccer (World Cup performance) and basketball (NBA), compared with the less interdependent sport of baseball (MLB).
In the case of soccer, team performance data were based on the average Fédération Internationale de Football Association (FIFA) rankings of national teams during the 2010 and 2014 World Cup qualification periods. Top talent was assessed by dividing the team’s numbers of players in each national team active in one of the world’s elite clubs divided by the total number of players on the national team.
In the case of the NBA, top talent was determined using the individual players’ estimated wins added (EWA) as determined over a 10-year period (2002-2012), with an index determining whether a player was in the top one-third (1) or not (0). Team performance was measured using each team’s end-of-year win percentage.
For baseball, top talent was determined using a player’s wins above replacement statistic (WAR), which is the number of wins a player contributes relative to a freely available minor-league player. Team performance was measured using each team’s win percentage.
In their results, basketball and soccer, the two most interdependent of the three sports, both showed a significant quadratic effect in which top talent benefited performance only up to a point, after which the marginal benefit of talent decreased and turned negative.
These results were in contrast to those seen with baseball. “As we predicted, the effect of top talent never turned negative in baseball, a sport in which task interdependence is relatively low. Thus there was no too-much-talent effect in baseball unlike in football [soccer] and basketball” according to the researchers.
“We predict that the too-much-talent effect will be found in other organizational contexts as well,” they added.
“Just as a colony of high-performing chickens competing for dominance suffers decrements in overall egg production and increases in bird mortality, teams with too much talent appear to divert attention away from coordination as team members peck at each other in their attempts to establish intergroup standing. In many cases, too much talent can be the seed of failure,” Mr. Swaab and his colleagues concluded.
The authors all declared that they had no conflicts of interest relative to the paper.
Mr. Swaab and his colleagues hypothesized the “too-much-talent effect” and found that talent often facilitates team performance, but only up to a point. They report that the relationship between talent and performance is not linear and monotonic. In contrast, they found that the relationship in football and basketball eventually turns negative (Psychol. Science 2014;25;1581-91). We have all heard the phrase, “There is no ‘I’ in team,” and it is clear that in certain sports, a team is necessary. No single cyclist can win the Tour de France without a strong team of support, and a soccer striker who is arrogant and feels he or she can score alone will fail to pass to the open teammate time and time again. We have all seen this strategy fail to result in a win. In contrast, one role of the midfielder in soccer is to provide selfless service to the striker – for the benefit of the team.
The cardiothoracic surgery world that we all work in can benefit from the findings reported by Swaab and colleagues. The individual with the “talent” in the cardiothoracic surgery realm – the skilled surgeon – may not be the best at building teams that work well together, and putting together several talented surgeons does not make an outstanding surgical team. Surgeons are not trained to be leaders, team builders, or to model collaborative behavior. A good leader requires emotional intelligence to be aware of others around her and of the successes and failures of the team. Lynda Gratton and Tamara J. Erickson found that the most productive, innovative teams were led by people who were both task- and relationship-oriented and that these leaders changed their style during the project (Eight Ways to Build Collaborative Teams, Harvard Bushiness Review, November 2007). Their findings were similar to those reported by Mr. Swaab and his colleagues in that they also found that the greater the proportion of highly educated specialists on a team, the more likely the team is to disintegrate into unproductive conflicts.
If we wish to establish world-renowned service lines or surgical programs, we would be well served by understanding that a great team requires collaboration, cohesion, and diversity. Diversity not only in talent but also in background and experiences. In addition, surgeon leaders cannot score all of the goals alone; they need an integrated, cohesive, diverse, and collaborative team to provide excellent patient care.
Dr. Jennifer S. Lawton is a professor of surgery at the division of cardiothoracic surgery, Washington University, St. Louis. She is also an associate medical editor for Vascular Specialist.
Mr. Swaab and his colleagues hypothesized the “too-much-talent effect” and found that talent often facilitates team performance, but only up to a point. They report that the relationship between talent and performance is not linear and monotonic. In contrast, they found that the relationship in football and basketball eventually turns negative (Psychol. Science 2014;25;1581-91). We have all heard the phrase, “There is no ‘I’ in team,” and it is clear that in certain sports, a team is necessary. No single cyclist can win the Tour de France without a strong team of support, and a soccer striker who is arrogant and feels he or she can score alone will fail to pass to the open teammate time and time again. We have all seen this strategy fail to result in a win. In contrast, one role of the midfielder in soccer is to provide selfless service to the striker – for the benefit of the team.
The cardiothoracic surgery world that we all work in can benefit from the findings reported by Swaab and colleagues. The individual with the “talent” in the cardiothoracic surgery realm – the skilled surgeon – may not be the best at building teams that work well together, and putting together several talented surgeons does not make an outstanding surgical team. Surgeons are not trained to be leaders, team builders, or to model collaborative behavior. A good leader requires emotional intelligence to be aware of others around her and of the successes and failures of the team. Lynda Gratton and Tamara J. Erickson found that the most productive, innovative teams were led by people who were both task- and relationship-oriented and that these leaders changed their style during the project (Eight Ways to Build Collaborative Teams, Harvard Bushiness Review, November 2007). Their findings were similar to those reported by Mr. Swaab and his colleagues in that they also found that the greater the proportion of highly educated specialists on a team, the more likely the team is to disintegrate into unproductive conflicts.
If we wish to establish world-renowned service lines or surgical programs, we would be well served by understanding that a great team requires collaboration, cohesion, and diversity. Diversity not only in talent but also in background and experiences. In addition, surgeon leaders cannot score all of the goals alone; they need an integrated, cohesive, diverse, and collaborative team to provide excellent patient care.
Dr. Jennifer S. Lawton is a professor of surgery at the division of cardiothoracic surgery, Washington University, St. Louis. She is also an associate medical editor for Vascular Specialist.
Mr. Swaab and his colleagues hypothesized the “too-much-talent effect” and found that talent often facilitates team performance, but only up to a point. They report that the relationship between talent and performance is not linear and monotonic. In contrast, they found that the relationship in football and basketball eventually turns negative (Psychol. Science 2014;25;1581-91). We have all heard the phrase, “There is no ‘I’ in team,” and it is clear that in certain sports, a team is necessary. No single cyclist can win the Tour de France without a strong team of support, and a soccer striker who is arrogant and feels he or she can score alone will fail to pass to the open teammate time and time again. We have all seen this strategy fail to result in a win. In contrast, one role of the midfielder in soccer is to provide selfless service to the striker – for the benefit of the team.
The cardiothoracic surgery world that we all work in can benefit from the findings reported by Swaab and colleagues. The individual with the “talent” in the cardiothoracic surgery realm – the skilled surgeon – may not be the best at building teams that work well together, and putting together several talented surgeons does not make an outstanding surgical team. Surgeons are not trained to be leaders, team builders, or to model collaborative behavior. A good leader requires emotional intelligence to be aware of others around her and of the successes and failures of the team. Lynda Gratton and Tamara J. Erickson found that the most productive, innovative teams were led by people who were both task- and relationship-oriented and that these leaders changed their style during the project (Eight Ways to Build Collaborative Teams, Harvard Bushiness Review, November 2007). Their findings were similar to those reported by Mr. Swaab and his colleagues in that they also found that the greater the proportion of highly educated specialists on a team, the more likely the team is to disintegrate into unproductive conflicts.
If we wish to establish world-renowned service lines or surgical programs, we would be well served by understanding that a great team requires collaboration, cohesion, and diversity. Diversity not only in talent but also in background and experiences. In addition, surgeon leaders cannot score all of the goals alone; they need an integrated, cohesive, diverse, and collaborative team to provide excellent patient care.
Dr. Jennifer S. Lawton is a professor of surgery at the division of cardiothoracic surgery, Washington University, St. Louis. She is also an associate medical editor for Vascular Specialist.
Talent facilitates team performance, but only up to a point, depending on the type of team. For highly interdependent teams, there is a threshold where the benefits of more talent decrease and eventually become detrimental rather than beneficial, according to an analysis of five studies performed by Roderick I. Swaab and his colleagues.
Surveys across industries and countries show that organizations consider talent attraction is their top priority, presumably based on the belief that more talent is better and the relationship between talent and team performance is linear and monotonic, they stated in Psychological Science (2014;25:1581-91)
The researchers analyzed the results of five studies they performed to investigate this relationship, comparing the impact of talent on team performance in the highly interdependent sports soccer (World Cup performance) and basketball (NBA), compared with the less interdependent sport of baseball (MLB).
In the case of soccer, team performance data were based on the average Fédération Internationale de Football Association (FIFA) rankings of national teams during the 2010 and 2014 World Cup qualification periods. Top talent was assessed by dividing the team’s numbers of players in each national team active in one of the world’s elite clubs divided by the total number of players on the national team.
In the case of the NBA, top talent was determined using the individual players’ estimated wins added (EWA) as determined over a 10-year period (2002-2012), with an index determining whether a player was in the top one-third (1) or not (0). Team performance was measured using each team’s end-of-year win percentage.
For baseball, top talent was determined using a player’s wins above replacement statistic (WAR), which is the number of wins a player contributes relative to a freely available minor-league player. Team performance was measured using each team’s win percentage.
In their results, basketball and soccer, the two most interdependent of the three sports, both showed a significant quadratic effect in which top talent benefited performance only up to a point, after which the marginal benefit of talent decreased and turned negative.
These results were in contrast to those seen with baseball. “As we predicted, the effect of top talent never turned negative in baseball, a sport in which task interdependence is relatively low. Thus there was no too-much-talent effect in baseball unlike in football [soccer] and basketball” according to the researchers.
“We predict that the too-much-talent effect will be found in other organizational contexts as well,” they added.
“Just as a colony of high-performing chickens competing for dominance suffers decrements in overall egg production and increases in bird mortality, teams with too much talent appear to divert attention away from coordination as team members peck at each other in their attempts to establish intergroup standing. In many cases, too much talent can be the seed of failure,” Mr. Swaab and his colleagues concluded.
The authors all declared that they had no conflicts of interest relative to the paper.
Talent facilitates team performance, but only up to a point, depending on the type of team. For highly interdependent teams, there is a threshold where the benefits of more talent decrease and eventually become detrimental rather than beneficial, according to an analysis of five studies performed by Roderick I. Swaab and his colleagues.
Surveys across industries and countries show that organizations consider talent attraction is their top priority, presumably based on the belief that more talent is better and the relationship between talent and team performance is linear and monotonic, they stated in Psychological Science (2014;25:1581-91)
The researchers analyzed the results of five studies they performed to investigate this relationship, comparing the impact of talent on team performance in the highly interdependent sports soccer (World Cup performance) and basketball (NBA), compared with the less interdependent sport of baseball (MLB).
In the case of soccer, team performance data were based on the average Fédération Internationale de Football Association (FIFA) rankings of national teams during the 2010 and 2014 World Cup qualification periods. Top talent was assessed by dividing the team’s numbers of players in each national team active in one of the world’s elite clubs divided by the total number of players on the national team.
In the case of the NBA, top talent was determined using the individual players’ estimated wins added (EWA) as determined over a 10-year period (2002-2012), with an index determining whether a player was in the top one-third (1) or not (0). Team performance was measured using each team’s end-of-year win percentage.
For baseball, top talent was determined using a player’s wins above replacement statistic (WAR), which is the number of wins a player contributes relative to a freely available minor-league player. Team performance was measured using each team’s win percentage.
In their results, basketball and soccer, the two most interdependent of the three sports, both showed a significant quadratic effect in which top talent benefited performance only up to a point, after which the marginal benefit of talent decreased and turned negative.
These results were in contrast to those seen with baseball. “As we predicted, the effect of top talent never turned negative in baseball, a sport in which task interdependence is relatively low. Thus there was no too-much-talent effect in baseball unlike in football [soccer] and basketball” according to the researchers.
“We predict that the too-much-talent effect will be found in other organizational contexts as well,” they added.
“Just as a colony of high-performing chickens competing for dominance suffers decrements in overall egg production and increases in bird mortality, teams with too much talent appear to divert attention away from coordination as team members peck at each other in their attempts to establish intergroup standing. In many cases, too much talent can be the seed of failure,” Mr. Swaab and his colleagues concluded.
The authors all declared that they had no conflicts of interest relative to the paper.
FROM PSYCHOLOGICAL SCIENCE
Key clinical point: Talent facilitates team performance, but it’s only up to a point, depending on the type of team.
Major finding: For highly interdependent teams, there is a threshold where the benefits of more talent decrease and eventually become detrimental rather than beneficial.
Data source: Five studies compared the impact of talent on team performance in the highly interdependent sports soccer (World Cup performance) and basketball (NBA), compared with the less interdependent sport of baseball (MLB)
Disclosures: The authors of the study reported no relevant disclosures.