User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
Simple treatments can address bleeding in dermatologic surgery
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
WAIKOLOA, HAWAII – “Nothing can ruin your day more than a lot of bleeding,” said Mohs surgeon Daniel Siegel, MD, clinical professor of dermatology at the State University of New York Downstate Medical Center, New York.

“Part of planning for surgery is to prevent that sort of problem,” and fortunately, and some are very inexpensive. There’s even a “fancy form of potato starch” that can be left in the wound and sewn over, he said in an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation.
During the interview, Dr. Siegel described several options and how to use them. They are good products to have on hand in the clinic, just in case, he said.
SDEF/Global Academy for Medical Education and this news organization and are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Dupilumab conjunctivitis does not always require an ophthalmologist referral
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
WAIKOLOA, HAWAII – Since its approval in 2017, dupilumab (Dupixent) has proven to be a solid addition to the atopic dermatitis (AD) armamentarium.

About 80% to 85% of patients treated with the biologic will achieve a 50% reduction in their Eczema Area and Severity Index score, and some will go on to a 90% reduction, according to Jonathan Silverberg, MD, PhD, of the department of dermatology, Northwestern University, Chicago.
But Dr. Silverberg has seen it in his own practice and said it can be hard to know whether or not to refer to ophthalmology. “We’re often left with this conundrum of ... ‘Is it a side effect of the medication, or is it just because they happen to have hay fever or keratoconjunctivitis or other ophthalmic comorbidities?’ And it’s not always easy to sort out.”
In an interview at the Hawaii Dermatology Seminar, provided by Global Academy for Medical Education/Skin Disease Education Foundation, he offered his advice on managing a patient who develops conjunctivitis during dupilumab treatment, including his treatment tips for when it is safe to handle in the dermatology clinic.
Dr. Silverberg, who was an investigator in the dupilumab phase 3 trials, said that, while dupilumab is the only systemic agent approved by the Food and Drug Administration for treating AD, and more are on the way for AD, there will always still be a role for traditional immunosuppressives. He explained why in the interview and why he favors methotrexate when old school options are in order.
This news organization and SDEF/Global Academy for Medical Education are owned by the same parent company.
EXPERT ANALYSIS FROM THE SDEF HAWAII DERMATOLOGY SEMINAR
Don’t miss early joint involvement in psoriasis
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – advised Alan Menter, MD.
About a third of patients with psoriasis will go on to develop joint involvement, and about half of those will go on to develop permanent joint destruction “if left untreated,” he noted. But early joint involvement has to be caught first, and dermatologists aren’t doing a very good job at early detection, according to Dr. Menter, clinical professor of dermatology at the University of Texas, Dallas.
The consequences, including arthritis mutilans, can be devastating. “It’s vitally important for us to prevent any permanent joint disease by” picking it up early, he said. “Our job as dermatologists is to diagnose it early.”
It’s not hard to do, just a few extra questions and a few extra steps on the physical exam, which takes a minute or two during each visit with psoriasis patients, are needed, he said.
Dr. Menter reviewed questions to ask patients, and explained how to examine patients for joint involvement and alter treatment when it’s found, in an interview at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Onychomycosis that fails terbinafine probably isn’t T. rubrum
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – The work up of a case of onychomycosis doesn’t end with the detection of fungal hyphae.
Trichophyton rubrum remains the most common cause of toenail fungus in the United States, but nondermatophyte molds – Scopulariopsis, Fusarium, and others – are on the rise, so , according to Nathaniel Jellinek, MD, of the department of dermatology, Brown University, Providence, R.I.
Standard in-office potassium hydroxide (KOH) testing can’t distinguish one species of fungus from another, nor can pathology with Gomori methenamine silver (GMS) or Periodic acid-Schiff (PAS) staining. Both culture and polymerase chain reaction (PCR), however, do.
Since few hospitals are equipped to run those tests, Dr. Jellinek uses the Case Western Center for Medical Mycology, in Cleveland, for testing.
In an interview at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation, Dr. Jellinek explained how to speciate, and the importance of doing so.
He also shared his tips on getting good nail clippings and good scrapings for debris for testing, and explained when KOH testing is enough – and when to opt for more advanced diagnostic methods, including PCR, which he said trumps all previous methods.
Terbinafine is still the best option for T. rubrum, but new topicals are better for nondermatophyte molds. There’s also a clever new dosing regimen for terbinafine, one that should put patients at ease about liver toxicity and other concerns. “If you tell them they’re getting 1 month off in the middle, it seems to go over a little easier,” Dr. Jellinek said.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
How to surf the rosacea treatment algorithm
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – according to Linda Stein Gold, MD, director of dermatology research at Henry Ford Hospital in Detroit.

Papules and pustules need an oral or topical anti-inflammatory drug. Background erythema requires an alpha adrenergic agonist. Telangiectasia is best handled by a laser device, and if a patient has a phyma, “you’ve got to use a surgical approach,” she said at the Hawaii Dermatology Seminar provided by Global Academy for Medical Education/Skin Disease Education Foundation. It sounds simple, but there are decisions to be made about what drugs and formulations to use, and when, and when to combine them.
In an interview, Dr. Stein Gold shared her approach to treatment, along with the latest on using ivermectin and brimonidine together, plus her thoughts on new medications under development and the role of the Demodex mite in rosacea.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Don’t fear spironolactone, isotretinoin, OCs for acne
WAIKOLOA, HAWAII – There’s really , of the department of dermatology at the University of Alabama at Birmingham.
There have been concerns with all three in the past, but most of the worries have been recently laid to rest.
The news hasn’t reached everyone, though, so, by and large, they are “tools I think we are not using enough of,” Dr. Harper said in an interview. With isotretinoin, for instance, it really isn’t necessary to do blood work for lipids and liver function every month, a daunting prospect for patients; baseline testing with a repeat at 2 months is sufficient, as long as there’s no dose escalation and results are acceptable, with the exception of a monthly pregnancy test for women, she noted. Meanwhile, there’s no evidence of a link with inflammatory bowel disease, and wound healing isn’t as much of an issue as once thought.
It’s the same story with spironolactone. Hyperkalemia is a long-standing concern, but it turns out that “in healthy young women taking spironolactone for acne, we don’t need to be checking potassium.” As far as breast cancer goes, the potential risk with spironolactone hasn’t panned out in the literature, and there may not be “a link at all,” Dr. Harper said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
There are caveats, of course. Hormonal treatments shouldn’t be used in young women until they’ve established their menstrual cycle. OCs should not be used in smokers, or people who have hypertension or migraines, among other conditions. Also, elevated triglycerides remain a concern with isotretinoin. “The number I would want people to remember is 500 [mg/dL],” the threshold when triglycerides become a problem.
In the interview, Dr. Harper explained the new thinking on these three options, and shared her treatment tips, including what to do if patients’ triglycerides hit the 500 mg/dL mark.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – There’s really , of the department of dermatology at the University of Alabama at Birmingham.
There have been concerns with all three in the past, but most of the worries have been recently laid to rest.
The news hasn’t reached everyone, though, so, by and large, they are “tools I think we are not using enough of,” Dr. Harper said in an interview. With isotretinoin, for instance, it really isn’t necessary to do blood work for lipids and liver function every month, a daunting prospect for patients; baseline testing with a repeat at 2 months is sufficient, as long as there’s no dose escalation and results are acceptable, with the exception of a monthly pregnancy test for women, she noted. Meanwhile, there’s no evidence of a link with inflammatory bowel disease, and wound healing isn’t as much of an issue as once thought.
It’s the same story with spironolactone. Hyperkalemia is a long-standing concern, but it turns out that “in healthy young women taking spironolactone for acne, we don’t need to be checking potassium.” As far as breast cancer goes, the potential risk with spironolactone hasn’t panned out in the literature, and there may not be “a link at all,” Dr. Harper said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
There are caveats, of course. Hormonal treatments shouldn’t be used in young women until they’ve established their menstrual cycle. OCs should not be used in smokers, or people who have hypertension or migraines, among other conditions. Also, elevated triglycerides remain a concern with isotretinoin. “The number I would want people to remember is 500 [mg/dL],” the threshold when triglycerides become a problem.
In the interview, Dr. Harper explained the new thinking on these three options, and shared her treatment tips, including what to do if patients’ triglycerides hit the 500 mg/dL mark.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
WAIKOLOA, HAWAII – There’s really , of the department of dermatology at the University of Alabama at Birmingham.
There have been concerns with all three in the past, but most of the worries have been recently laid to rest.
The news hasn’t reached everyone, though, so, by and large, they are “tools I think we are not using enough of,” Dr. Harper said in an interview. With isotretinoin, for instance, it really isn’t necessary to do blood work for lipids and liver function every month, a daunting prospect for patients; baseline testing with a repeat at 2 months is sufficient, as long as there’s no dose escalation and results are acceptable, with the exception of a monthly pregnancy test for women, she noted. Meanwhile, there’s no evidence of a link with inflammatory bowel disease, and wound healing isn’t as much of an issue as once thought.
It’s the same story with spironolactone. Hyperkalemia is a long-standing concern, but it turns out that “in healthy young women taking spironolactone for acne, we don’t need to be checking potassium.” As far as breast cancer goes, the potential risk with spironolactone hasn’t panned out in the literature, and there may not be “a link at all,” Dr. Harper said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
There are caveats, of course. Hormonal treatments shouldn’t be used in young women until they’ve established their menstrual cycle. OCs should not be used in smokers, or people who have hypertension or migraines, among other conditions. Also, elevated triglycerides remain a concern with isotretinoin. “The number I would want people to remember is 500 [mg/dL],” the threshold when triglycerides become a problem.
In the interview, Dr. Harper explained the new thinking on these three options, and shared her treatment tips, including what to do if patients’ triglycerides hit the 500 mg/dL mark.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
Exercise type matters for fall prevention among elderly
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
according to a Cochrane Review meta-analysis of 108 randomized controlled trials.
Exercise has been shown to prevent falls in older people, but given the potential consequences, the investigators thought an up-to-date synthesis of the evidence was in order. The analysis focused on people living independently who had not recently been discharged from a hospital. The trials involved 23,407 subjects from 25 countries. The review was exhaustive; the final report is almost 600 pages long (Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2).
The type of exercise matters. The researchers cited “high-certainty evidence” that exercise involving balance and functional training reduces falls. “Tai chi may also prevent falls,” they noted, adding that they were uncertain of the effect of dance, walking, and resistance training by itself. There was no evidence to determine the effects of flexibility or endurance exercises, added the researchers, led by Cathie Sherrington, PhD, of the University of Sydney Institute for Musculoskeletal Health.
Functional exercise mimics everyday movement, with the goal of improving performance. Multidirectional lunges are an example, helping the body prepare for vacuuming, yard work, and other common activities.
“Exercise [programs] carried out in group classes or done at home prescribed by a health professional ... or a trained exercise leader were effective. Exercises were mostly done while standing as this better enhances balance and the ability to do daily activities such as standing up from a low chair or climbing stairs,” according to a Cochrane press release regarding the study.
Overall, exercise reduced the number of falls by 23%, and the number of fallers by 15%, with high-certainty evidence.
Exercise also brought down the number of people facing fall fractures by over 27%, the number of people requiring medical attention for a fall by 39%, and the number ending up in the hospital for a fall by 22%.
Balance and functional exercises reduced the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively. There was low-certainty evidence that tai chi reduces the rate of falls by 19% and the number of people experiencing falls by 20%.
Despite fall prevention, “exercise may make little important difference to health-related quality of life;” when results were converted to EQ-5D and 36-Item Short Form Survey scores, “the respective 95% [confidence intervals] were much smaller than minimally important differences,” the investigators said.
Serious adverse events occurred in participants in one of the 27 trials that reported adverse events. These two serious adverse events were a pelvic stress fracture and an inguinal hernia surgery. Most of the other adverse events reported, all non-serious, were musculoskeletal.
On average, participants were 76 years old, and 77% were women. Disease specific trials – such as exercise for stroke rehabilitation – were excluded.
The work was supported primarily by the Cochrane Bone, Joint and Muscle Trauma Group, based at the University of Manchester, England, and Cochrane’s Acute and Emergency Care Network. There were no industry disclosures.
SOURCE: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
FROM COCHRANE DATABASE OF SYSTEMATIC REVIEWS
Key clinical point: Exercise helps elderly people avoid falls, especially if it focuses on balance and mimics daily activities.
Major finding: Balance and functional exercises reduce the rate of falls by 24%, and the number of fallers by 13%. The effects were even greater when resistance exercises were added to the mix; drops in fall rates and the number of people experiencing falls were 34% and 22%, respectively.
Study details: A meta-analysis of 108 randomized, controlled trials.
Disclosures: The work was supported by Cochrane. There were no industry disclosures.
Source: Sherrington C et al. Cochrane Database Syst Rev. 2019 Jan 31;1:CD012424. doi: 10.1002/14651858.CD012424.pub2.
Riboflavin helps visualize urine flow during cystoscopy
By increasing urine color, 400 mg of oral riboflavin the night before gynecologic surgery makes it easier to see and confirm urine flow during intraoperative cystoscopy, according to results of a randomized, blinded, placebo-controlled trial.
The traditional go-to for that purpose, intravenous indigo carmine, has been in short supply, if possible to get at all, so surgeons have been looking for other options. Alternatives include intravenous methylene blue, intravenous fluorescein, and oral phenazopyridine, but each have their own problems, including cost, contraindications, and anaphylaxis.
So the study team turned to riboflavin – vitamin B2 – which, in excess, turns the urine bright, sometimes almost neon yellow. It’s “safe, readily available without prescription, and inexpensive ... and should be considered for routine use,” wrote investigators led by Michael L. Stitely, MD, an ob.gyn. at the University of Otago in Dunedin, New Zealand.
The team randomized 33 women to four 100-mg capsules of riboflavin the night before surgery and 33 to four 1,000-IU capsules of vitamin D3, which served as the placebo. Participants, clinicians, researchers, and study staff all were blinded to group allocation, the investigators noted in Obstetrics & Gynecology.
Surgeons rated urine color a median of 2 (slight yellow) in the riboflavin group, compared with 1 (clear) in the placebo arm, on a 3-point scale (P less than .001). About 13 women on riboflavin got a rating of 3 – strong yellow – versus 1 woman in the placebo arm.
The operating surgeons also said it was easier to visualize urine flow in the riboflavin group, giving a median of 5, compared with 4 in the placebo group, on a 5-point scale (P less than .013). They gave a score of 5 to 19 women in the riboflavin group but only to 8 placebo women, meaning that they “strongly agreed” that it was easy to see urine flow; a score of 4 meant that they simply agreed with the statement.
Overall, surgeons confirmed bilateral urine flow in 30 women (91%) in the riboflavin group, compared with 28 women (85%) in the placebo group (P = .71). When a blinded investigator checked the videos, their assessments of the same parameters correlated with those of the surgeons.
No significant differences were found between the groups in age, height, weight, body mass index, or ethnicity. The most common procedure was a midurethral sling (10 in the riboflavin group; 4 in the placebo arm), followed by cystoscopy with botox (4 in the riboflavin group; 7 in the placebo group). None of the women required intervention for urinary tract injury.
Among the limitations cited was the use of subjective and nonvalidated measures of urine color.
The work was funded by the Healthcare Otago Charitable Trust and the Australasian Gynaecological Endoscopy and Surgery Society. The authors reported no conflicts of interest.
SOURCE: Stitely ML et al. Obstet Gynecol. 2019 Jan 8. doi: 10.1097/AOG.0000000000003063.
By increasing urine color, 400 mg of oral riboflavin the night before gynecologic surgery makes it easier to see and confirm urine flow during intraoperative cystoscopy, according to results of a randomized, blinded, placebo-controlled trial.
The traditional go-to for that purpose, intravenous indigo carmine, has been in short supply, if possible to get at all, so surgeons have been looking for other options. Alternatives include intravenous methylene blue, intravenous fluorescein, and oral phenazopyridine, but each have their own problems, including cost, contraindications, and anaphylaxis.
So the study team turned to riboflavin – vitamin B2 – which, in excess, turns the urine bright, sometimes almost neon yellow. It’s “safe, readily available without prescription, and inexpensive ... and should be considered for routine use,” wrote investigators led by Michael L. Stitely, MD, an ob.gyn. at the University of Otago in Dunedin, New Zealand.
The team randomized 33 women to four 100-mg capsules of riboflavin the night before surgery and 33 to four 1,000-IU capsules of vitamin D3, which served as the placebo. Participants, clinicians, researchers, and study staff all were blinded to group allocation, the investigators noted in Obstetrics & Gynecology.
Surgeons rated urine color a median of 2 (slight yellow) in the riboflavin group, compared with 1 (clear) in the placebo arm, on a 3-point scale (P less than .001). About 13 women on riboflavin got a rating of 3 – strong yellow – versus 1 woman in the placebo arm.
The operating surgeons also said it was easier to visualize urine flow in the riboflavin group, giving a median of 5, compared with 4 in the placebo group, on a 5-point scale (P less than .013). They gave a score of 5 to 19 women in the riboflavin group but only to 8 placebo women, meaning that they “strongly agreed” that it was easy to see urine flow; a score of 4 meant that they simply agreed with the statement.
Overall, surgeons confirmed bilateral urine flow in 30 women (91%) in the riboflavin group, compared with 28 women (85%) in the placebo group (P = .71). When a blinded investigator checked the videos, their assessments of the same parameters correlated with those of the surgeons.
No significant differences were found between the groups in age, height, weight, body mass index, or ethnicity. The most common procedure was a midurethral sling (10 in the riboflavin group; 4 in the placebo arm), followed by cystoscopy with botox (4 in the riboflavin group; 7 in the placebo group). None of the women required intervention for urinary tract injury.
Among the limitations cited was the use of subjective and nonvalidated measures of urine color.
The work was funded by the Healthcare Otago Charitable Trust and the Australasian Gynaecological Endoscopy and Surgery Society. The authors reported no conflicts of interest.
SOURCE: Stitely ML et al. Obstet Gynecol. 2019 Jan 8. doi: 10.1097/AOG.0000000000003063.
By increasing urine color, 400 mg of oral riboflavin the night before gynecologic surgery makes it easier to see and confirm urine flow during intraoperative cystoscopy, according to results of a randomized, blinded, placebo-controlled trial.
The traditional go-to for that purpose, intravenous indigo carmine, has been in short supply, if possible to get at all, so surgeons have been looking for other options. Alternatives include intravenous methylene blue, intravenous fluorescein, and oral phenazopyridine, but each have their own problems, including cost, contraindications, and anaphylaxis.
So the study team turned to riboflavin – vitamin B2 – which, in excess, turns the urine bright, sometimes almost neon yellow. It’s “safe, readily available without prescription, and inexpensive ... and should be considered for routine use,” wrote investigators led by Michael L. Stitely, MD, an ob.gyn. at the University of Otago in Dunedin, New Zealand.
The team randomized 33 women to four 100-mg capsules of riboflavin the night before surgery and 33 to four 1,000-IU capsules of vitamin D3, which served as the placebo. Participants, clinicians, researchers, and study staff all were blinded to group allocation, the investigators noted in Obstetrics & Gynecology.
Surgeons rated urine color a median of 2 (slight yellow) in the riboflavin group, compared with 1 (clear) in the placebo arm, on a 3-point scale (P less than .001). About 13 women on riboflavin got a rating of 3 – strong yellow – versus 1 woman in the placebo arm.
The operating surgeons also said it was easier to visualize urine flow in the riboflavin group, giving a median of 5, compared with 4 in the placebo group, on a 5-point scale (P less than .013). They gave a score of 5 to 19 women in the riboflavin group but only to 8 placebo women, meaning that they “strongly agreed” that it was easy to see urine flow; a score of 4 meant that they simply agreed with the statement.
Overall, surgeons confirmed bilateral urine flow in 30 women (91%) in the riboflavin group, compared with 28 women (85%) in the placebo group (P = .71). When a blinded investigator checked the videos, their assessments of the same parameters correlated with those of the surgeons.
No significant differences were found between the groups in age, height, weight, body mass index, or ethnicity. The most common procedure was a midurethral sling (10 in the riboflavin group; 4 in the placebo arm), followed by cystoscopy with botox (4 in the riboflavin group; 7 in the placebo group). None of the women required intervention for urinary tract injury.
Among the limitations cited was the use of subjective and nonvalidated measures of urine color.
The work was funded by the Healthcare Otago Charitable Trust and the Australasian Gynaecological Endoscopy and Surgery Society. The authors reported no conflicts of interest.
SOURCE: Stitely ML et al. Obstet Gynecol. 2019 Jan 8. doi: 10.1097/AOG.0000000000003063.
FROM OBSTETRICS & GYNECOLOGY
Key clinical point: Oral riboflavin the night before gynecologic surgery is a sound alternative to indigo carmine for cystoscopy visualization.
Major finding: Surgeons rated urine color a median of 2 (slight yellow) in the riboflavin group, compared with 1 (clear) in the placebo arm on a 3-point scale (P less than .001). About 13 women on riboflavin got a rating of 3 – strong yellow – versus 1 woman in the placebo arm.
Study details: A randomized trial with 66 women.
Disclosures: The work was funded by the Healthcare Otago Charitable Trust and the Australasian Gynaecological Endoscopy and Surgery Society. The authors reported no conflicts of interest.
Source: Stitely ML et al. Obstet Gynecol. 2019 Jan 8. doi: 10.1097/AOG.0000000000003063.
Adolescence does not rule out bullous pemphigoid
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Although there are only 14 cases in the literature, it should still be kept in mind, wrote investigators led by Aikaterini Patsatsi, MD, PhD, of Aristotle University, Thessaloniki, Greece, and senior author Victoria Werth, MD, of the University of Pennsylvania, Philadelphia.
The good news is that the course of adolescent bullous pemphigoid “seems favorable, with long remission after disease control,” the investigators reported in Pediatric Dermatology.
Bullous pemphigoid (BP) is the most common autoimmune blistering disease in the elderly, but is rare in children, with the majority of pediatric cases occurring in early childhood. Even so, BP is still possible in adolescents, and should be worked up with “salt‐split skin [testing] in all cases, and the detection of circulating anti-BP180 and anti‐BP230 autoantibodies by ELISA [enzyme-linked immunosorbent assay] tests, not routinely done for this diagnosis,” the investigators wrote.
BP hasn’t been well characterized in teenagers, so Dr. Patsatsi and her associates searched Medline for “bullous pemphigoid in childhood and adolescence,” “childhood bullous pemphigoid,” “juvenile bullous pemphigoid,” and “autoimmune blistering and autoimmune bullous diseases in childhood.”
It turned out that “all authors agree that the management plan should be the least aggressive possible” with “the addition of immunomodulating agents such as dapsone, azathioprine, mycophenolate mofetil, or doxycycline/niacinamide,” although systemic steroids were used in 13 of the 14 cases, the investigators wrote.
They found nine cases in children aged 10‐13 years (six in girls, two in boys, and one case with no sex identified), with the first case reported in 1970. Five had mucosal involvement. One case was diagnosed as localized BP of the perineum. The children were treated with systemic prednisone (eight of nine), in combination with dapsone (two of nine), azathioprine (two of nine), and erythromycin/nicotinamide (one of nine). Three relapsed; there was no report of what was done for them or how they fared.
“The clinical features of BP in this age range include a pruritic generalized bullous eruption, similar to ... adult BP, with frequent involvement of the oral mucosa,” Dr. Patsatsi and her associates wrote.
The team also found five cases in children aged 14‐17 years (three girls, two boys), with the first reported in 1994. None had mucosal involvement. Treatment included systemic prednisone (five of five), in combination with dapsone (three of five), azathioprine (two of five), doxycycline/nicotinamide (one of five), and mycophenolate mofetil (one of five). Two cases relapsed; subsequent treatment and outcomes weren’t reported.
The clinical features again were similar to those seen in adults, “with disseminated tense blisters and erosions,” the investigators noted.
Only one case was reported in adolescents aged 18-21 years, though it was excluded from the review because it overlapped with pemphigus vulgaris.
No funding and no relevant financial disclosures were reported for the work.
SOURCE: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: The course of adolescent bullous pemphigoid appears favorable, with long remission after the disease is controlled.
Major finding: The investigators found nine cases in children aged 10‐13 years, and five cases in children aged 14‐17 years.
Study details: A search in Medline detected 14 adolescents with a diagnosis of bullous pemphigoid.
Disclosures: No funding and no relevant financial disclosures were reported for the work.
Source: Patsatsi A et al. Pediatr Dermatol. 2018 Dec 19. doi: 10.1111/pde.13717.
Guardian angel or watchdog? Pills of capecitabine contain sensors
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
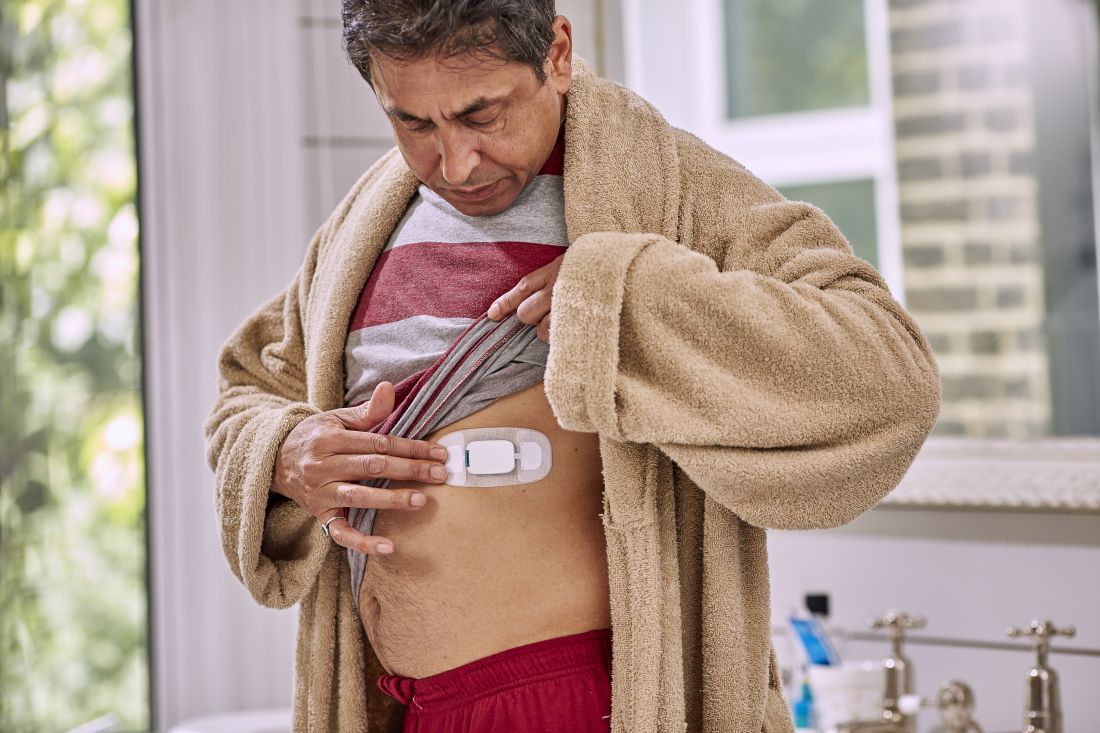
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.
Recently, a woman with advanced colorectal cancer at the University of Minnesota, Minneapolis, was taking capecitabine (Xeloda) in the morning but skipping her evening dose.
Her hands hurt, and she couldn’t open the childproof cap. Her daughter had been doing it for her in the morning but wasn’t around to help out at night.
It was the kind of problem that might have gone on for days or weeks until the next clinic visit, and, even then, be addressed only if the woman remembered to mention it.
But that’s not what happened. The care team realized pretty much right away that she was skipping the p.m. dose because the woman was taking her capecitabine in a gel cap with an adherence sensor.
The sensor, a sandwich of copper, silicon, and magnesium in a millimeter square, sent out an electric ping when she took her dose, activated by stomach acid; the ping was picked up by an adhesive bandage patch the woman wore, which relayed the signal to an app on her smartphone; the phone passed it on to a server cloud that the woman had given her providers permission to access.
They monitored her adherence on a Web portal, along with heart rate and activity data, also captured by the patch. The system is called Proteus Discover, from Proteus Digital Health.
Instead of taking days or weeks, the care team quickly realized that she wasn’t taking her evening dose of capecitabine. They contacted her, replaced the childproof cap, and twice-daily dosing resumed.
Expanded use in oncology?
Seven other advanced colorectal cancer patients have participated in the University of Minnesota (UM) pilot project, the first use of the device in oncology. “It’s gone so well that it’s annoying to me to not have this for all my patients. I’m already feeling frustrated that I can’t just put this in all the drugs that I give orally,” said Edward Greeno, MD, an oncologist/hematologist at the university, and the Proteus point man.
“You would assume that cancer patients would be hypercompliant, but it turns out they have the same compliance problems” as other patients, plus additional hurdles, he said, including complex regimens and drug toxicity.
“Every patient we have approached so far has been enthusiastic. They might be a little bit annoyed that I know they haven’t been taking their pills, but they are also glad that I am there to hold them responsible and help them. My intention is to roll this out much more broadly,” he said. That just might happen. Dr. Greeno is working with Proteus to roll the sensor out at UM and oncology programs elsewhere.
A slow, careful rollout
The device was cleared by the Food and Drug Administration in 2012. After more than 180,000 ingestions, there have been no safety issues, besides occasional skin irritation from the patch, which is waterproof and meant to be worn for a week, then replaced. It pings if it’s taken off. The sensor is passes through the body like food.
Patients can communicate with providers over the phone app, which also sends reminders when it’s time to take the next pill.
So far, Proteus has worked with ten health systems in the United States, and more are in the works. Commercialization efforts have focused mostly on blood pressure, cholesterol, and type 2 diabetes drugs, the bad boys of drug adherence, but the system has also been piloted for hepatitis C treatment, and trials are underway for HIV preexposure prophylaxis.
Among the company’s many favorable studies, the system has already been demonstrated to be a viable alternative to directly observed therapy in tuberculosis, the current gold-standard, but hugely labor and resource intensive (PLoS One. 2013;8[1]:e53373. doi: 10.1371/journal.pone.0053373).
Proteus can’t be picked up at the local Walgreens. The company works closely with clients and is being careful in its rollout. For one thing, each sensor has to be programed for the specific drug it’s being used with, but also, and as with any new technology, business and payment models are still being worked out.
UM’s partner in the oncology project, Fairview Health Services, pays Proteus when patients hit an adherence rate of 80%, but how much they pay is a proprietary secret. “Most of the cost issue is still not in the public domain,” said Scooter Plowman, MD, the company’s medical director.
In 2017, FDA approved a version of the antipsychotic aripiprazole embedded with the Proteus sensor. The rollout of “Abilify MyCite” by Otsuka Pharmaceuticals has been similarly cautious, under contract with health systems.
“Otsuka has been very smart in the approach they are taking,” Dr. Plowman said.
He wouldn’t give details, but Proteus is in talks with other pharmaceutical makers to bring pills with sensors to the market.
A new fix for an old problem
Proteus isn’t alone in the ingestible event marker (IEM) market. The FDA is reviewing a rival sensor from etectRx, in Gainesville, Fla.
The technology is a little different; the etectRx sensor is a microchip made out of magnesium and silver chloride that’s embedded on the inside of a gel cap. Instead of an electric blip, it sends out a radio wave when activated by stomach acid. It’s larger than the Proteus offering, but still has room to spare in a gel cap.
The signal is stronger, so patients wear a neck pendant instead of a patch to pick it up. The pendant does not capture heart rate or activity data. It’s not meant to be worn continuously and can come off after it pings the system.
The two systems are otherwise similar; etectRx also uses a phone app to relay adherence data to a server cloud clinicians can access, with patient permission. As with Proteus, everything works as long as the phone is on. President and CEO Harry Travis anticipates clearance in 2019.
Adherence is a huge and well-known problem in medicine; only about half of patients take medications as they are prescribed. People end up in the ED or the hospital with problems that might have been avoided. Providers and payers want solutions.
Industry is bringing technology to bear on the problem. The payoff will be huge for the winners; analysts project multiple billion dollar growth in the adherence technology sector.
Most companies, however, are pinning their hopes on indirect approaches, bottle caps that ping when opened, for instance, or coaching apps for smart phones. IEMs seem to be ahead of the curve.
Guardian angel or watchdog?
Whether that’s a good thing or bad thing depends on who you talk to, but patients do seem more likely to take their medications if a sensor is on board.
Dr. Plowman said he thinks IEMs improve adherence because, with their own health at stake, patients want to do better, and IEM systems provide the extra help they need, complete with positive feedback.
But patients also know they are being watched. The technology is barely off the ground, but concerns have already been raised about surveillance. It’s not hard to imagine insurance companies demanding proof of adherence before paying for expensive drugs. There are privacy concerns as well; everything is encrypted with IEMs, but hackers are clever.
Dr. Plowman and Mr. Travis acknowledged the concerns, and also that there’s no way to know how IEMs – if they take off – will play out in coming decades; it’s a lot like the Internet in 1992.
The intent is for the systems to remain voluntary, as they are now, perhaps with inducements for patients to use them, maybe lower insurance premiums.
“There is something inherently personal about swallowable data,” Dr. Plowman said. “It’s something we take tremendous efforts to protect.” As for compulsory use, “we take enormous strides to prevent that. It’s a major priority.”
“Always, there will be an opt-out” option, said Mr. Travis.
It’s important to consider the potential for IEMs to move medicine forward. When patients with acute bone fractures in one study, for instance, were sent home with the usual handful of oxycodone tablets, it turned out that they only took a median of six. Researchers knew that because the subjects took their oxycodone in an etectRx capsule. It’s was an important insight in the midst of an opioid epidemic (Anesth Analg. 2017 Dec;125[6]:2105-12).
Dr. Greeno is an adviser for Proteus; the company covers his travel costs.