User login
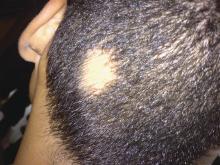
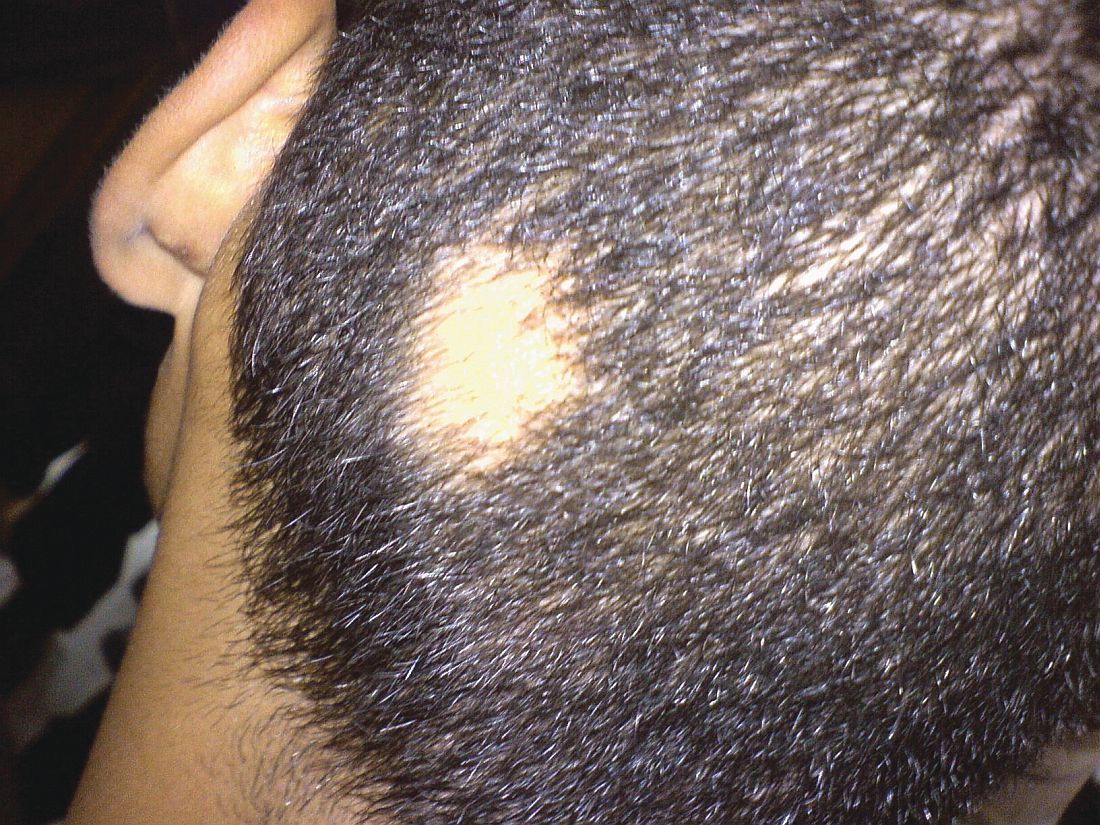
How to get through the tough talks about alopecia areata
CHICAGO – If you can’t set the temptation to hurry aside and take the time to listen, things may not go well, said Neil Prose, MD.
With the caveat that Janus kinase inhibitors show promise, Dr. Prose said that “most children who are destined to lose their hair will probably do so despite all of our best efforts.” Figuring out how to engage children and parents and frame a positive conversation about alopecia can present a real challenge, especially in the context of a busy practice, said Dr. Prose, professor of dermatology and medical director of Patterson Place Pediatric Dermatology at Duke University, Durham, N.C.
“These are very culturally-specific suggestions, but see which ones work for you,” said Dr. Prose, speaking to an international audience at the World Congress of Pediatric Dermatology.
Dr. Prose depicted two opposing images. In one, he said, the patient and you are sitting on opposite sides of the table, with the prospect of hair loss looming between them. By contrast, “imagine what it would take to be on the same side of the table, looking at the problem together,” he said.
There are many barriers that stand in the way of getting you and the patient on the same side of the issue of dealing with severe alopecia areata. The high emotional content of the discussion can be big factor, not just for the patient and family members, but also for you.
“We are often dealing with patient disappointment and, frankly, with our own sense of personal failure” when there isn’t always a good set of options, said Dr. Prose. Other specific aspects of severe pediatric alopecia areata that make the conversation difficult include the high degree of uncertainty that any particular treatment will succeed and a knowledge of how to give patients and family members hope without raising expectations unrealistically.
Coming back to the important first steps of not rushing the visit and being sure to listen, Dr. Prose said that, for him, the process begins before he enters the room, when he takes a moment to clear his mind. “It starts for me just before I open the door to the examining room. As human beings, we are infinitely distractible. It’s very hard for us to simply pay attention.”
Yet, this is vitally important, he said, because families need to be heard. Citing the oft-quoted statistic that, on average, a physician interrupts a patient in the first 17 seconds of the office visit, Dr. Prose said, “Many of us are ‘explainaholics,’ ’’ spending precious visit time talking about what the physician thinks is important.
Still, it’s important to validate parents’ concerns and to alleviate guilt. “Patients’ families sometimes feel guilty because they are so upset and worried – and it’s not cancer,” said Dr. Prose. Potential impacts on quality of life are still huge, and all parents want the best for their children, he pointed out.
One way he likes to begin a follow-up visit is simply to ask, “So, how’s everyone doing?” This opens the door to allow the child and the family to talk about what’s important to them. These may be symptom-related, but social issues also may be what’s looming largest.
In order to decipher how hair loss is affecting a particular child, Dr. Prose said he likes to say, “I need to understand how this is affecting you, so we can decide together where to go from here.” This gives the family control in setting the agenda and begins the process of bringing you to the same side of the table.
Specific prompts that can help you understand how alopecia is affecting a child can include asking about how things are going at school, what the child’s friends know about his or her alopecia, whether there is mocking or bullying occurring, and how the patient, family, and teachers are addressing the global picture.
Parents can be asked whether they are noticing changes in behavior, and it’s a good idea to check in on how parents are coping as well, said Dr. Prose.
To ensure that families feel they’re being heard, and to make sure you are understanding correctly, it’s useful to mirror what’s been said, beginning with a phrase like, “So, what you’re saying is …” Putting a name to the emotions that emerge during the visit also can be useful, using phrases like, “I can imagine that this has been disappointing,” or “It feels like everyone is very worried.”
But, said Dr. Prose, don’t forget about opportunities to praise patients and their families when they’ve come through a tough time well. This validation is important, he said.
When treatment isn’t working, a first place to start is to acknowledge that you, along with the family, wish that things were turning out differently. Then, said Dr. Prose, it can be really important to reappraise treatment goals. After taking the emotional temperature of the room, it may be appropriate to ask, “Is it time to talk about not doing any more treatments?” This question can be put within the framework that hair may or may not regrow spontaneously anyway and that new treatments are emerging that may help in future.
When giving advice or talking about difficult issues, it can be helpful to ask permission, said Dr. Prose. He likes to begin with, “Would it be okay if I ...?” Then, he said, the door can be opened to give advice about school issues, to ask about difficult treatment decisions, or even to share tips learned from other families’ coping methods.
Don’t forget, said Dr. Prose, to refer patients to high-quality information and online support resources, such as the National Alopecia Areata Foundation. The Internet is full of inaccurate and scary information, and patients and families need help with this navigation, he said.
The very last question Dr. Prose asks during a visit is “What other questions do you have?” The question is always framed exactly like this, he said, because it assumes there will be more questions, and it gives families permission to ask more. Although most of the time there aren’t any further questions, Dr. Prose said, “Do not ask the question with your hand on the doorknob!”
Dr. Prose had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
CHICAGO – If you can’t set the temptation to hurry aside and take the time to listen, things may not go well, said Neil Prose, MD.
With the caveat that Janus kinase inhibitors show promise, Dr. Prose said that “most children who are destined to lose their hair will probably do so despite all of our best efforts.” Figuring out how to engage children and parents and frame a positive conversation about alopecia can present a real challenge, especially in the context of a busy practice, said Dr. Prose, professor of dermatology and medical director of Patterson Place Pediatric Dermatology at Duke University, Durham, N.C.
“These are very culturally-specific suggestions, but see which ones work for you,” said Dr. Prose, speaking to an international audience at the World Congress of Pediatric Dermatology.
Dr. Prose depicted two opposing images. In one, he said, the patient and you are sitting on opposite sides of the table, with the prospect of hair loss looming between them. By contrast, “imagine what it would take to be on the same side of the table, looking at the problem together,” he said.
There are many barriers that stand in the way of getting you and the patient on the same side of the issue of dealing with severe alopecia areata. The high emotional content of the discussion can be big factor, not just for the patient and family members, but also for you.
“We are often dealing with patient disappointment and, frankly, with our own sense of personal failure” when there isn’t always a good set of options, said Dr. Prose. Other specific aspects of severe pediatric alopecia areata that make the conversation difficult include the high degree of uncertainty that any particular treatment will succeed and a knowledge of how to give patients and family members hope without raising expectations unrealistically.
Coming back to the important first steps of not rushing the visit and being sure to listen, Dr. Prose said that, for him, the process begins before he enters the room, when he takes a moment to clear his mind. “It starts for me just before I open the door to the examining room. As human beings, we are infinitely distractible. It’s very hard for us to simply pay attention.”
Yet, this is vitally important, he said, because families need to be heard. Citing the oft-quoted statistic that, on average, a physician interrupts a patient in the first 17 seconds of the office visit, Dr. Prose said, “Many of us are ‘explainaholics,’ ’’ spending precious visit time talking about what the physician thinks is important.
Still, it’s important to validate parents’ concerns and to alleviate guilt. “Patients’ families sometimes feel guilty because they are so upset and worried – and it’s not cancer,” said Dr. Prose. Potential impacts on quality of life are still huge, and all parents want the best for their children, he pointed out.
One way he likes to begin a follow-up visit is simply to ask, “So, how’s everyone doing?” This opens the door to allow the child and the family to talk about what’s important to them. These may be symptom-related, but social issues also may be what’s looming largest.
In order to decipher how hair loss is affecting a particular child, Dr. Prose said he likes to say, “I need to understand how this is affecting you, so we can decide together where to go from here.” This gives the family control in setting the agenda and begins the process of bringing you to the same side of the table.
Specific prompts that can help you understand how alopecia is affecting a child can include asking about how things are going at school, what the child’s friends know about his or her alopecia, whether there is mocking or bullying occurring, and how the patient, family, and teachers are addressing the global picture.
Parents can be asked whether they are noticing changes in behavior, and it’s a good idea to check in on how parents are coping as well, said Dr. Prose.
To ensure that families feel they’re being heard, and to make sure you are understanding correctly, it’s useful to mirror what’s been said, beginning with a phrase like, “So, what you’re saying is …” Putting a name to the emotions that emerge during the visit also can be useful, using phrases like, “I can imagine that this has been disappointing,” or “It feels like everyone is very worried.”
But, said Dr. Prose, don’t forget about opportunities to praise patients and their families when they’ve come through a tough time well. This validation is important, he said.
When treatment isn’t working, a first place to start is to acknowledge that you, along with the family, wish that things were turning out differently. Then, said Dr. Prose, it can be really important to reappraise treatment goals. After taking the emotional temperature of the room, it may be appropriate to ask, “Is it time to talk about not doing any more treatments?” This question can be put within the framework that hair may or may not regrow spontaneously anyway and that new treatments are emerging that may help in future.
When giving advice or talking about difficult issues, it can be helpful to ask permission, said Dr. Prose. He likes to begin with, “Would it be okay if I ...?” Then, he said, the door can be opened to give advice about school issues, to ask about difficult treatment decisions, or even to share tips learned from other families’ coping methods.
Don’t forget, said Dr. Prose, to refer patients to high-quality information and online support resources, such as the National Alopecia Areata Foundation. The Internet is full of inaccurate and scary information, and patients and families need help with this navigation, he said.
The very last question Dr. Prose asks during a visit is “What other questions do you have?” The question is always framed exactly like this, he said, because it assumes there will be more questions, and it gives families permission to ask more. Although most of the time there aren’t any further questions, Dr. Prose said, “Do not ask the question with your hand on the doorknob!”
Dr. Prose had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
CHICAGO – If you can’t set the temptation to hurry aside and take the time to listen, things may not go well, said Neil Prose, MD.
With the caveat that Janus kinase inhibitors show promise, Dr. Prose said that “most children who are destined to lose their hair will probably do so despite all of our best efforts.” Figuring out how to engage children and parents and frame a positive conversation about alopecia can present a real challenge, especially in the context of a busy practice, said Dr. Prose, professor of dermatology and medical director of Patterson Place Pediatric Dermatology at Duke University, Durham, N.C.
“These are very culturally-specific suggestions, but see which ones work for you,” said Dr. Prose, speaking to an international audience at the World Congress of Pediatric Dermatology.
Dr. Prose depicted two opposing images. In one, he said, the patient and you are sitting on opposite sides of the table, with the prospect of hair loss looming between them. By contrast, “imagine what it would take to be on the same side of the table, looking at the problem together,” he said.
There are many barriers that stand in the way of getting you and the patient on the same side of the issue of dealing with severe alopecia areata. The high emotional content of the discussion can be big factor, not just for the patient and family members, but also for you.
“We are often dealing with patient disappointment and, frankly, with our own sense of personal failure” when there isn’t always a good set of options, said Dr. Prose. Other specific aspects of severe pediatric alopecia areata that make the conversation difficult include the high degree of uncertainty that any particular treatment will succeed and a knowledge of how to give patients and family members hope without raising expectations unrealistically.
Coming back to the important first steps of not rushing the visit and being sure to listen, Dr. Prose said that, for him, the process begins before he enters the room, when he takes a moment to clear his mind. “It starts for me just before I open the door to the examining room. As human beings, we are infinitely distractible. It’s very hard for us to simply pay attention.”
Yet, this is vitally important, he said, because families need to be heard. Citing the oft-quoted statistic that, on average, a physician interrupts a patient in the first 17 seconds of the office visit, Dr. Prose said, “Many of us are ‘explainaholics,’ ’’ spending precious visit time talking about what the physician thinks is important.
Still, it’s important to validate parents’ concerns and to alleviate guilt. “Patients’ families sometimes feel guilty because they are so upset and worried – and it’s not cancer,” said Dr. Prose. Potential impacts on quality of life are still huge, and all parents want the best for their children, he pointed out.
One way he likes to begin a follow-up visit is simply to ask, “So, how’s everyone doing?” This opens the door to allow the child and the family to talk about what’s important to them. These may be symptom-related, but social issues also may be what’s looming largest.
In order to decipher how hair loss is affecting a particular child, Dr. Prose said he likes to say, “I need to understand how this is affecting you, so we can decide together where to go from here.” This gives the family control in setting the agenda and begins the process of bringing you to the same side of the table.
Specific prompts that can help you understand how alopecia is affecting a child can include asking about how things are going at school, what the child’s friends know about his or her alopecia, whether there is mocking or bullying occurring, and how the patient, family, and teachers are addressing the global picture.
Parents can be asked whether they are noticing changes in behavior, and it’s a good idea to check in on how parents are coping as well, said Dr. Prose.
To ensure that families feel they’re being heard, and to make sure you are understanding correctly, it’s useful to mirror what’s been said, beginning with a phrase like, “So, what you’re saying is …” Putting a name to the emotions that emerge during the visit also can be useful, using phrases like, “I can imagine that this has been disappointing,” or “It feels like everyone is very worried.”
But, said Dr. Prose, don’t forget about opportunities to praise patients and their families when they’ve come through a tough time well. This validation is important, he said.
When treatment isn’t working, a first place to start is to acknowledge that you, along with the family, wish that things were turning out differently. Then, said Dr. Prose, it can be really important to reappraise treatment goals. After taking the emotional temperature of the room, it may be appropriate to ask, “Is it time to talk about not doing any more treatments?” This question can be put within the framework that hair may or may not regrow spontaneously anyway and that new treatments are emerging that may help in future.
When giving advice or talking about difficult issues, it can be helpful to ask permission, said Dr. Prose. He likes to begin with, “Would it be okay if I ...?” Then, he said, the door can be opened to give advice about school issues, to ask about difficult treatment decisions, or even to share tips learned from other families’ coping methods.
Don’t forget, said Dr. Prose, to refer patients to high-quality information and online support resources, such as the National Alopecia Areata Foundation. The Internet is full of inaccurate and scary information, and patients and families need help with this navigation, he said.
The very last question Dr. Prose asks during a visit is “What other questions do you have?” The question is always framed exactly like this, he said, because it assumes there will be more questions, and it gives families permission to ask more. Although most of the time there aren’t any further questions, Dr. Prose said, “Do not ask the question with your hand on the doorknob!”
Dr. Prose had no relevant financial disclosures.
[email protected]
On Twitter @karioakes
EXPERT ANALYSIS FROM WCPD 2017
New one-time treatment for head lice found safe for children
CHICAGO – A novel one-time topical treatment for head lice, abametapir, was well tolerated in children as young as 6 months, according to pooled results from 11 clinical trials.
The pooled safety data included results from 11 clinical trials including 1,372 patients. Of these, 700 were aged 6 months to 17 years, and patients were exposed to the novel metalloproteinase inhibitor for 10-20 minutes.
In examining safety data from the pooled trials, Lydie Hazan, MD, of Axis Clinical Trials and her collaborators found that for pediatric patients, most treatment-emergent adverse events (AEs) were skin and subcutaneous tissue-related. The most common AEs were erythema, rash, a burning sensation on the skin, and contact dermatitis.
Data for the three phase II pharmacokinetic trials, the phase II ovicidal efficacy trial, and the two phase III trials were reported separately by Dr. Hazan and her coauthors in a poster presentation at the World Congress of Pediatric Dermatology. The overall incidence of treatment-emergent AEs in the studies ranged from 20% to 29% for patients in the active arms of the trials. For patients who received the vehicle lotion only, the incidence of AEs ranged from 16% to 57%.
Of the 11 trials, 6 involved pediatric patients, with one phase IIB trial, one phase II ovicidal trial, two maximal-use open-label trials, and two phase III randomized, double-blind, vehicle-controlled trials. Of the 920 patients, in most of the trials they received a 10-minute exposure to the study drug (489 received abametapir lotion 0.74%, 431 received vehicle lotion).
Looking just at the phase III trials, 24% of patients in the abametapir arm reported AEs, while 19% of those receiving vehicle reported any AE.
In the two maximal-use pediatric trials, drug exposure ranged from 3.3 g to 200.8 g; AEs in these two trials occurred in 23% of participants.
Safety data collected for all studies also included vital signs, results of physical exams, and laboratory tests; no “clinically meaningful” changes were seen in any of the trials for any of these values, according to Dr. Hazan and her coauthors.
“AEs were mild, not age-related, and primarily in the system organ class of skin and subcutaneous tissue disorders,” said Dr. Hazan and her coauthors.
Abematapir 0.74% lotion had previously been shown to be an effective ovicidal treatment for head lice when used in a single application; the lotion is intended to be applied at home by the patient or caregiver (J Med Entomol. 2017. 54[1]:167-72).*
Dr. Hazan is employed by Axis clinical trials. Other study authors were employed by Hatchtech, which developed abametapir, and by Promius Pharma/Dr. Reddy’s Laboratories, which plans to market abametapir lotion.
*Correction, 8/7/17: An earlier version of this article had an incorrect citation.
[email protected]
On Twitter @karioakes
CHICAGO – A novel one-time topical treatment for head lice, abametapir, was well tolerated in children as young as 6 months, according to pooled results from 11 clinical trials.
The pooled safety data included results from 11 clinical trials including 1,372 patients. Of these, 700 were aged 6 months to 17 years, and patients were exposed to the novel metalloproteinase inhibitor for 10-20 minutes.
In examining safety data from the pooled trials, Lydie Hazan, MD, of Axis Clinical Trials and her collaborators found that for pediatric patients, most treatment-emergent adverse events (AEs) were skin and subcutaneous tissue-related. The most common AEs were erythema, rash, a burning sensation on the skin, and contact dermatitis.
Data for the three phase II pharmacokinetic trials, the phase II ovicidal efficacy trial, and the two phase III trials were reported separately by Dr. Hazan and her coauthors in a poster presentation at the World Congress of Pediatric Dermatology. The overall incidence of treatment-emergent AEs in the studies ranged from 20% to 29% for patients in the active arms of the trials. For patients who received the vehicle lotion only, the incidence of AEs ranged from 16% to 57%.
Of the 11 trials, 6 involved pediatric patients, with one phase IIB trial, one phase II ovicidal trial, two maximal-use open-label trials, and two phase III randomized, double-blind, vehicle-controlled trials. Of the 920 patients, in most of the trials they received a 10-minute exposure to the study drug (489 received abametapir lotion 0.74%, 431 received vehicle lotion).
Looking just at the phase III trials, 24% of patients in the abametapir arm reported AEs, while 19% of those receiving vehicle reported any AE.
In the two maximal-use pediatric trials, drug exposure ranged from 3.3 g to 200.8 g; AEs in these two trials occurred in 23% of participants.
Safety data collected for all studies also included vital signs, results of physical exams, and laboratory tests; no “clinically meaningful” changes were seen in any of the trials for any of these values, according to Dr. Hazan and her coauthors.
“AEs were mild, not age-related, and primarily in the system organ class of skin and subcutaneous tissue disorders,” said Dr. Hazan and her coauthors.
Abematapir 0.74% lotion had previously been shown to be an effective ovicidal treatment for head lice when used in a single application; the lotion is intended to be applied at home by the patient or caregiver (J Med Entomol. 2017. 54[1]:167-72).*
Dr. Hazan is employed by Axis clinical trials. Other study authors were employed by Hatchtech, which developed abametapir, and by Promius Pharma/Dr. Reddy’s Laboratories, which plans to market abametapir lotion.
*Correction, 8/7/17: An earlier version of this article had an incorrect citation.
[email protected]
On Twitter @karioakes
CHICAGO – A novel one-time topical treatment for head lice, abametapir, was well tolerated in children as young as 6 months, according to pooled results from 11 clinical trials.
The pooled safety data included results from 11 clinical trials including 1,372 patients. Of these, 700 were aged 6 months to 17 years, and patients were exposed to the novel metalloproteinase inhibitor for 10-20 minutes.
In examining safety data from the pooled trials, Lydie Hazan, MD, of Axis Clinical Trials and her collaborators found that for pediatric patients, most treatment-emergent adverse events (AEs) were skin and subcutaneous tissue-related. The most common AEs were erythema, rash, a burning sensation on the skin, and contact dermatitis.
Data for the three phase II pharmacokinetic trials, the phase II ovicidal efficacy trial, and the two phase III trials were reported separately by Dr. Hazan and her coauthors in a poster presentation at the World Congress of Pediatric Dermatology. The overall incidence of treatment-emergent AEs in the studies ranged from 20% to 29% for patients in the active arms of the trials. For patients who received the vehicle lotion only, the incidence of AEs ranged from 16% to 57%.
Of the 11 trials, 6 involved pediatric patients, with one phase IIB trial, one phase II ovicidal trial, two maximal-use open-label trials, and two phase III randomized, double-blind, vehicle-controlled trials. Of the 920 patients, in most of the trials they received a 10-minute exposure to the study drug (489 received abametapir lotion 0.74%, 431 received vehicle lotion).
Looking just at the phase III trials, 24% of patients in the abametapir arm reported AEs, while 19% of those receiving vehicle reported any AE.
In the two maximal-use pediatric trials, drug exposure ranged from 3.3 g to 200.8 g; AEs in these two trials occurred in 23% of participants.
Safety data collected for all studies also included vital signs, results of physical exams, and laboratory tests; no “clinically meaningful” changes were seen in any of the trials for any of these values, according to Dr. Hazan and her coauthors.
“AEs were mild, not age-related, and primarily in the system organ class of skin and subcutaneous tissue disorders,” said Dr. Hazan and her coauthors.
Abematapir 0.74% lotion had previously been shown to be an effective ovicidal treatment for head lice when used in a single application; the lotion is intended to be applied at home by the patient or caregiver (J Med Entomol. 2017. 54[1]:167-72).*
Dr. Hazan is employed by Axis clinical trials. Other study authors were employed by Hatchtech, which developed abametapir, and by Promius Pharma/Dr. Reddy’s Laboratories, which plans to market abametapir lotion.
*Correction, 8/7/17: An earlier version of this article had an incorrect citation.
[email protected]
On Twitter @karioakes
AT WCPD 2017
Key clinical point:
Major finding: In pooled clinical trial data, pediatric patients had adverse events at the same rate as adult patients, with overall rates ranging from 20% to 57%.
Data source: Pooled data from 11 clinical trials including 1,372 patients, 700 of whom were aged 6 months to 17 years.
Disclosures: Dr. Hazan is employed by Axis Clinical Trials. Other study authors were employed by Hatchtech, which developed abametapir, and by Promius Pharma/Dr. Reddy’s Laboratories, which plans to market abametapir lotion.
Quality of life preserved with ribociclib + letrozole for advanced breast cancer
CHICAGO – Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better progression-free survival with no drop in quality of life. Health-related quality of life for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
Also, the time to definitive deterioration by 10% or more of the global health status/quality of life scale score was similar between treatment arms (hazard ratio [HR] 0.944; 95% confidence interval [CI] 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK 4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved progression-free survival for postmenopausal patients with hormone receptor-positive, HER2 negative advanced breast cancer, when compared to letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related quality of life and symptoms in the two arms of MONALEESA-2, reporting change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared to end of treatment on the global health status/quality of life subscale of the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire (EORTC QLQ-30).
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr. Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of such key symptoms as fatigue, nausea, and vomiting on quality of life was similar regardless of whether patients received ribociclib or placebo, he said; though symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr. Verma, professor and head of the department of oncology at the University of Calgary, (Alta.), sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the annual meeting of the American Society of Clinical Oncology.
“A clinically meaningful – more than 5-point – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm,” said Dr. Verma. The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core quality of life questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib + letrozole arm and the placebo + letrozole arm. Patients in both arms, said Dr. Verma, were very compliant with questionnaire completion. Over 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression.
It’s important to include these measures, he said, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help to maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” said Dr. Verma.
Dr. Verma reported financial relationships with multiple pharmaceutical companies, including Novartis, which funded the study.
[email protected]
On Twitter @karioakes
CHICAGO – Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better progression-free survival with no drop in quality of life. Health-related quality of life for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
Also, the time to definitive deterioration by 10% or more of the global health status/quality of life scale score was similar between treatment arms (hazard ratio [HR] 0.944; 95% confidence interval [CI] 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK 4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved progression-free survival for postmenopausal patients with hormone receptor-positive, HER2 negative advanced breast cancer, when compared to letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related quality of life and symptoms in the two arms of MONALEESA-2, reporting change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared to end of treatment on the global health status/quality of life subscale of the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire (EORTC QLQ-30).
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr. Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of such key symptoms as fatigue, nausea, and vomiting on quality of life was similar regardless of whether patients received ribociclib or placebo, he said; though symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr. Verma, professor and head of the department of oncology at the University of Calgary, (Alta.), sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the annual meeting of the American Society of Clinical Oncology.
“A clinically meaningful – more than 5-point – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm,” said Dr. Verma. The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core quality of life questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib + letrozole arm and the placebo + letrozole arm. Patients in both arms, said Dr. Verma, were very compliant with questionnaire completion. Over 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression.
It’s important to include these measures, he said, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help to maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” said Dr. Verma.
Dr. Verma reported financial relationships with multiple pharmaceutical companies, including Novartis, which funded the study.
[email protected]
On Twitter @karioakes
CHICAGO – Patients with advanced breast cancer whose aromatase inhibitor therapy was supplemented with a cycline-dependent kinase inhibitor had better progression-free survival with no drop in quality of life. Health-related quality of life for patients on the combination therapy was equivalent to that of patients on monotherapy in most aspects, but patients receiving both therapies had a sustained and clinically meaningful decrease in pain.
Also, the time to definitive deterioration by 10% or more of the global health status/quality of life scale score was similar between treatment arms (hazard ratio [HR] 0.944; 95% confidence interval [CI] 0.720-1.237).
The MONALEESA-2 trial had previously shown that the CDK 4/6 inhibitor ribociclib, when added to the aromatase inhibitor letrozole, significantly improved progression-free survival for postmenopausal patients with hormone receptor-positive, HER2 negative advanced breast cancer, when compared to letrozole in combination with placebo.
Sunil Verma, MD, reported on health-related quality of life and symptoms in the two arms of MONALEESA-2, reporting change from baseline, time to a definitive 10% deterioration, and the mean scores for on-treatment time compared to end of treatment on the global health status/quality of life subscale of the European Organization for Research and Treatment of Cancer 30-item core quality of life questionnaire (EORTC QLQ-30).
“During treatment, overall health-related quality of life was maintained from baseline and was similar in both arms,” said Dr. Verma, the study’s first author. Changes during treatment were not statistically significant, and did not reach the predetermined threshold for a clinically meaningful difference. The effect of such key symptoms as fatigue, nausea, and vomiting on quality of life was similar regardless of whether patients received ribociclib or placebo, he said; though symptom scores were slightly higher for patients in the active arm of the study, the results were not clinically significant.
Reporting validated, cancer-specific patient-reported outcomes from the trial, Dr. Verma, professor and head of the department of oncology at the University of Calgary, (Alta.), sought to “highlight the patient experience with a focus on health-related quality of life and symptoms,” he said during his presentation at the annual meeting of the American Society of Clinical Oncology.
“A clinically meaningful – more than 5-point – improvement from baseline in pain score was maintained up to and including cycle 15 in the ribociclib plus letrozole arm,” said Dr. Verma. The placebo arm had a mild, clinically insignificant improvement at most assessment points. For both treatment arms, pain scores increased a bit above baseline levels at the time of disease progression or end of therapy, he said.
Patients completed the EORTC 30-item core quality of life questionnaire at their screening visit, and then every 8 weeks for the first 18 months. Then, they completed the questionnaires every 12 weeks until they experienced disease progression, died, were lost to follow-up or withdrew from the study, or stopped treatment.
Statistical analysis of the questionnaire results took into account the patients’ baseline scores, treatments received, and how they were stratified. Investigators assessed both statistical significance and the clinical meaningfulness of changes, defined as a change of 5-10 points.
In MONALEESA-2, 334 patients each were allocated to the ribociclib + letrozole arm and the placebo + letrozole arm. Patients in both arms, said Dr. Verma, were very compliant with questionnaire completion. Over 90% of patients who were eligible completed questionnaire through cycle 19 of ribociclib or placebo. He explained that the overall numbers completing the questionnaire declined with time, as more patients had disease progression.
It’s important to include these measures, he said, because patients and their families care about “the quality of the time gained,” so patient-reported outcomes should be a part of risk-benefit assessments for new cancer therapies. “While delaying disease progression may help to maintain patient quality of life, the addition of novel treatments to existing therapies can add toxicities, which may diminish quality of life,” said Dr. Verma.
Dr. Verma reported financial relationships with multiple pharmaceutical companies, including Novartis, which funded the study.
[email protected]
On Twitter @karioakes
AT ASCO 2017
Key clinical point:
Major finding: Quality of life was sustained and pain scores decreased when ribociclib was added to letrozole for patients with advanced breast cancer.
Data source: Double-blind, placebo-controlled phase III trial of letrozole plus ribociclib compared to letrozole plus placebo in 668 patients with advanced hormone receptor-positive, HER-2 negative breast cancer.
Disclosures: Dr. Verma reported financial relationships with multiple companies, including Novartis, which markets ribociclib.
Dacomitinib boosts PFS in advanced NSCLC
CHICAGO – The clear advantage goes to the second-generation tyrosine kinase inhibitor in a new trial comparing dacomitinib to gefitinib for advanced non–small cell lung cancer.
In a randomized, open-label phase III trial designed as a head-to-head comparison of the two drugs for the first-line treatment of advanced non–small cell lung cancer (NSCLC), “the blinded, independent review showed that we have a median progression-free survival (PFS) of 14.7 months versus 9.2 months,” said first author Tony Mok, MD, professor and chair of the department of clinical oncology at the Chinese University of Hong Kong. This PFS rate, he said, “is among the highest of randomized phase III trials in the first-line setting.”
Two years into the study, those taking dacomitinib had triple the PFS rate of those on gefitinib (30.6% versus 9.6%). The overall hazard ratio (HR) for PFS with dacomitinib compared to gefitinib was 0.59 (95% confidence interval [CI], 0.47-0.74, P less than .0001).
A previous single-arm phase II trial of the drug, ARCHER 2017, showed a response rate of 75.6% and a median PFS of 18.2 months for patients with NSCLC and an EGFR-activating mutation.
“Based on these data, we thought it was likely that we could have a hypothesis for dacomitinib to be superior to gefitinib, a first-generation TKI [tyrosine kinase inhibitor], in terms of progression-free survival,” Dr. Mok said in a press conference at the annual meeting of the American Society of Clinical Oncology. Dacomitinib is a second-generation TKI.
Patients in the new study, ARCHER 1050, had advanced NSCLC with EGFR-activating mutations and no prior systemic treatment for their advanced disease. In addition, patients had good performance status, could not have had prior TKI exposure, and could not have CNS metastases. This last exclusion, explained Dr. Mok, was because investigators were uncertain about dacomitinib’s CNS penetration at the time of study design, and because gefitinib may also not be the best therapeutic choice for CNS metastases.
Patients were randomized 1:1 to receive either dacomitinib 45 mg orally daily (n = 227), or gefitinib 250 mg orally daily (n = 225). Patients were stratified by whether or not they were ethnically Asian, and by whether they had EGFR mutation of exon 19 or exon 21. Patients were balanced in terms of age, gender, ethnicity, smoking, and performance status between arms. About 75% of the patients were Asian, and 65% were nonsmokers.
The international study enrolled patients from 71 centers in Asia and Europe. At the time of the data cutoff, investigators saw PFS events totaling 59.9% in the dacomitinib arm and 79.6% in the gefitinib arm. Patients were followed for PFS for a median of 22.1 months. “We have relatively mature data,” said Dr. Mok, except for overall survival, with only 36.9% of events occurring at the time of the data cutoff.
The primary endpoint in the open-label trial was PFS in the intention-to-treat population, as assessed by an independent, blinded reviewer. Dr. Mok said that the study was powered to see at least 256 PFS events, and to see an improvement in PFS for dacomitinib that equated to an HR of no more than 0.667. This would translate to median PFS for dacomitinib of 14.3 months versus 9.5 months for gefitinib, values Dr. Mok said were “reasonable.” And, he pointed out, the study results fell almost exactly in line with these predictions, though the actual HR was a bit lower than predicted.
An analysis of PFS by subgroup, also conducted by independent review, found that dacomitinib was favored for all subgroups except for non-Asian patients, for whom the HR was 0.89 but did not reach statistical significance. Since these patients made up about one-fourth of the study population, said Dr. Mok, small sample size was a potential issue. “But we have to ask ourselves the question, do they really perform worse than the Asians, if they have a response?”
To attempt to answer this question, the investigators performed an exploratory analysis of the 72 non-Asian patients who had responded to therapy. Among this group, they saw data similar to that of the overall group, with an HR of 0.547 (95% CI, 0.321-0.933, P less than .0123).
Secondary endpoints included investigator-assessed PFS, overall survival, objective response rate, duration of response, quality of life, and safety assessments.
Objective response rates were similar between arms, at 74.9% for dacomitinib and 71.6% for gefitinib (P = .3883). However, the median duration of response was significantly longer for those on dacomitinib (14.8 versus 8.3 months, P less than .0001).
“This may be best explained by looking into the depth of the response,” said Dr. Mok. Patient-level data showed that dacomitinib patients had a larger reduction in target lesion size; “this may reflect a more potent inhibition of EGFR,” he said.
With the more potent inhibition, however, came more frequent grade 3 adverse events involving diarrhea, dermatitis, stomatitis, and paronychia for those on dacomitinib; however, noted Dr. Mok, serious transaminase elevations were more common in the gefitinib group. “There is no new signal” for concerning toxicity, he said. Dose reductions were more common in dacomitinib than in gefitinib (66.1% versus 18%), but there are two tiers of dose reductions permissible with dacomitinib, giving some flexibility.
Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which sponsored the study.
[email protected]
On Twitter @karioakes
CHICAGO – The clear advantage goes to the second-generation tyrosine kinase inhibitor in a new trial comparing dacomitinib to gefitinib for advanced non–small cell lung cancer.
In a randomized, open-label phase III trial designed as a head-to-head comparison of the two drugs for the first-line treatment of advanced non–small cell lung cancer (NSCLC), “the blinded, independent review showed that we have a median progression-free survival (PFS) of 14.7 months versus 9.2 months,” said first author Tony Mok, MD, professor and chair of the department of clinical oncology at the Chinese University of Hong Kong. This PFS rate, he said, “is among the highest of randomized phase III trials in the first-line setting.”
Two years into the study, those taking dacomitinib had triple the PFS rate of those on gefitinib (30.6% versus 9.6%). The overall hazard ratio (HR) for PFS with dacomitinib compared to gefitinib was 0.59 (95% confidence interval [CI], 0.47-0.74, P less than .0001).
A previous single-arm phase II trial of the drug, ARCHER 2017, showed a response rate of 75.6% and a median PFS of 18.2 months for patients with NSCLC and an EGFR-activating mutation.
“Based on these data, we thought it was likely that we could have a hypothesis for dacomitinib to be superior to gefitinib, a first-generation TKI [tyrosine kinase inhibitor], in terms of progression-free survival,” Dr. Mok said in a press conference at the annual meeting of the American Society of Clinical Oncology. Dacomitinib is a second-generation TKI.
Patients in the new study, ARCHER 1050, had advanced NSCLC with EGFR-activating mutations and no prior systemic treatment for their advanced disease. In addition, patients had good performance status, could not have had prior TKI exposure, and could not have CNS metastases. This last exclusion, explained Dr. Mok, was because investigators were uncertain about dacomitinib’s CNS penetration at the time of study design, and because gefitinib may also not be the best therapeutic choice for CNS metastases.
Patients were randomized 1:1 to receive either dacomitinib 45 mg orally daily (n = 227), or gefitinib 250 mg orally daily (n = 225). Patients were stratified by whether or not they were ethnically Asian, and by whether they had EGFR mutation of exon 19 or exon 21. Patients were balanced in terms of age, gender, ethnicity, smoking, and performance status between arms. About 75% of the patients were Asian, and 65% were nonsmokers.
The international study enrolled patients from 71 centers in Asia and Europe. At the time of the data cutoff, investigators saw PFS events totaling 59.9% in the dacomitinib arm and 79.6% in the gefitinib arm. Patients were followed for PFS for a median of 22.1 months. “We have relatively mature data,” said Dr. Mok, except for overall survival, with only 36.9% of events occurring at the time of the data cutoff.
The primary endpoint in the open-label trial was PFS in the intention-to-treat population, as assessed by an independent, blinded reviewer. Dr. Mok said that the study was powered to see at least 256 PFS events, and to see an improvement in PFS for dacomitinib that equated to an HR of no more than 0.667. This would translate to median PFS for dacomitinib of 14.3 months versus 9.5 months for gefitinib, values Dr. Mok said were “reasonable.” And, he pointed out, the study results fell almost exactly in line with these predictions, though the actual HR was a bit lower than predicted.
An analysis of PFS by subgroup, also conducted by independent review, found that dacomitinib was favored for all subgroups except for non-Asian patients, for whom the HR was 0.89 but did not reach statistical significance. Since these patients made up about one-fourth of the study population, said Dr. Mok, small sample size was a potential issue. “But we have to ask ourselves the question, do they really perform worse than the Asians, if they have a response?”
To attempt to answer this question, the investigators performed an exploratory analysis of the 72 non-Asian patients who had responded to therapy. Among this group, they saw data similar to that of the overall group, with an HR of 0.547 (95% CI, 0.321-0.933, P less than .0123).
Secondary endpoints included investigator-assessed PFS, overall survival, objective response rate, duration of response, quality of life, and safety assessments.
Objective response rates were similar between arms, at 74.9% for dacomitinib and 71.6% for gefitinib (P = .3883). However, the median duration of response was significantly longer for those on dacomitinib (14.8 versus 8.3 months, P less than .0001).
“This may be best explained by looking into the depth of the response,” said Dr. Mok. Patient-level data showed that dacomitinib patients had a larger reduction in target lesion size; “this may reflect a more potent inhibition of EGFR,” he said.
With the more potent inhibition, however, came more frequent grade 3 adverse events involving diarrhea, dermatitis, stomatitis, and paronychia for those on dacomitinib; however, noted Dr. Mok, serious transaminase elevations were more common in the gefitinib group. “There is no new signal” for concerning toxicity, he said. Dose reductions were more common in dacomitinib than in gefitinib (66.1% versus 18%), but there are two tiers of dose reductions permissible with dacomitinib, giving some flexibility.
Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which sponsored the study.
[email protected]
On Twitter @karioakes
CHICAGO – The clear advantage goes to the second-generation tyrosine kinase inhibitor in a new trial comparing dacomitinib to gefitinib for advanced non–small cell lung cancer.
In a randomized, open-label phase III trial designed as a head-to-head comparison of the two drugs for the first-line treatment of advanced non–small cell lung cancer (NSCLC), “the blinded, independent review showed that we have a median progression-free survival (PFS) of 14.7 months versus 9.2 months,” said first author Tony Mok, MD, professor and chair of the department of clinical oncology at the Chinese University of Hong Kong. This PFS rate, he said, “is among the highest of randomized phase III trials in the first-line setting.”
Two years into the study, those taking dacomitinib had triple the PFS rate of those on gefitinib (30.6% versus 9.6%). The overall hazard ratio (HR) for PFS with dacomitinib compared to gefitinib was 0.59 (95% confidence interval [CI], 0.47-0.74, P less than .0001).
A previous single-arm phase II trial of the drug, ARCHER 2017, showed a response rate of 75.6% and a median PFS of 18.2 months for patients with NSCLC and an EGFR-activating mutation.
“Based on these data, we thought it was likely that we could have a hypothesis for dacomitinib to be superior to gefitinib, a first-generation TKI [tyrosine kinase inhibitor], in terms of progression-free survival,” Dr. Mok said in a press conference at the annual meeting of the American Society of Clinical Oncology. Dacomitinib is a second-generation TKI.
Patients in the new study, ARCHER 1050, had advanced NSCLC with EGFR-activating mutations and no prior systemic treatment for their advanced disease. In addition, patients had good performance status, could not have had prior TKI exposure, and could not have CNS metastases. This last exclusion, explained Dr. Mok, was because investigators were uncertain about dacomitinib’s CNS penetration at the time of study design, and because gefitinib may also not be the best therapeutic choice for CNS metastases.
Patients were randomized 1:1 to receive either dacomitinib 45 mg orally daily (n = 227), or gefitinib 250 mg orally daily (n = 225). Patients were stratified by whether or not they were ethnically Asian, and by whether they had EGFR mutation of exon 19 or exon 21. Patients were balanced in terms of age, gender, ethnicity, smoking, and performance status between arms. About 75% of the patients were Asian, and 65% were nonsmokers.
The international study enrolled patients from 71 centers in Asia and Europe. At the time of the data cutoff, investigators saw PFS events totaling 59.9% in the dacomitinib arm and 79.6% in the gefitinib arm. Patients were followed for PFS for a median of 22.1 months. “We have relatively mature data,” said Dr. Mok, except for overall survival, with only 36.9% of events occurring at the time of the data cutoff.
The primary endpoint in the open-label trial was PFS in the intention-to-treat population, as assessed by an independent, blinded reviewer. Dr. Mok said that the study was powered to see at least 256 PFS events, and to see an improvement in PFS for dacomitinib that equated to an HR of no more than 0.667. This would translate to median PFS for dacomitinib of 14.3 months versus 9.5 months for gefitinib, values Dr. Mok said were “reasonable.” And, he pointed out, the study results fell almost exactly in line with these predictions, though the actual HR was a bit lower than predicted.
An analysis of PFS by subgroup, also conducted by independent review, found that dacomitinib was favored for all subgroups except for non-Asian patients, for whom the HR was 0.89 but did not reach statistical significance. Since these patients made up about one-fourth of the study population, said Dr. Mok, small sample size was a potential issue. “But we have to ask ourselves the question, do they really perform worse than the Asians, if they have a response?”
To attempt to answer this question, the investigators performed an exploratory analysis of the 72 non-Asian patients who had responded to therapy. Among this group, they saw data similar to that of the overall group, with an HR of 0.547 (95% CI, 0.321-0.933, P less than .0123).
Secondary endpoints included investigator-assessed PFS, overall survival, objective response rate, duration of response, quality of life, and safety assessments.
Objective response rates were similar between arms, at 74.9% for dacomitinib and 71.6% for gefitinib (P = .3883). However, the median duration of response was significantly longer for those on dacomitinib (14.8 versus 8.3 months, P less than .0001).
“This may be best explained by looking into the depth of the response,” said Dr. Mok. Patient-level data showed that dacomitinib patients had a larger reduction in target lesion size; “this may reflect a more potent inhibition of EGFR,” he said.
With the more potent inhibition, however, came more frequent grade 3 adverse events involving diarrhea, dermatitis, stomatitis, and paronychia for those on dacomitinib; however, noted Dr. Mok, serious transaminase elevations were more common in the gefitinib group. “There is no new signal” for concerning toxicity, he said. Dose reductions were more common in dacomitinib than in gefitinib (66.1% versus 18%), but there are two tiers of dose reductions permissible with dacomitinib, giving some flexibility.
Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which sponsored the study.
[email protected]
On Twitter @karioakes
AT ASCO 2017
Key clinical point:
Major finding: At 2 years, the dacomitinib arm had triple the PFS rate of the gefitinib arm (30.6% versus 9.6%).
Data source: Randomized, open-label phase III clinical trial of 452 patients who received dacomitinib or gefitinib for first-line therapy for advanced non–small cell lung cancer.
Disclosures: Dr. Mok reported financial relationships with multiple pharmaceutical companies, including Pfizer and SFJ Pharmaceuticals, which funded the study.
Is female genital cosmetic surgery going mainstream?
Experts describe the field of female genital cosmetic surgery as the “Wild West,” but the lack of regulation and consensus has not kept it from exploding in recent years.
More than 10,000 labiaplasties were performed in 2016, a 23% jump over the previous year, and the procedures are offered by more than 35% of all plastic surgeons, according to data from the American Society for Aesthetic Plastic Surgery. As another indicator of increasing attention to the appearance of female genitalia, a 2013 survey of U.S. women revealed that more than 80% performed some sort of pubic hair grooming (JAMA Dermatol. 2016;152[10]:1106-13).
Dr. Iglesia recounted being contacted by a National Gallery of Art staff member, who, when confronted with Gustave Courbet’s L’Origine du Monde, an 1866 below-the-waist portrait of a nude woman, asked, “Is this normal? Do women have this much hair?” Dr. Iglesia said she reassured the staff member that the woman in the portrait did indeed have a normal female Tanner stage IV or V escutcheon. However, she said, social media and other images in the popular press have essentially erased female pubic hair from the public eye, even in explicit imagery that involves female nudity.
“This is an ideal that men and women are seeing in social media, in pornography, and even in the lay press.” And now, she said, “We’re in a new era of sex surgeries, with these ‘nips and tucks’ below the belt.”
Labiaplasty
The combination of a newly-hairless genital region, together with portrayals of adult women with a “Barbie doll” appearance, may contribute to women feeling self-conscious about labia minora protruding beyond the labia majora. Dr. Iglesia, who is section director for female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, Washington, D.C., said this is true even though the normal length of labia minora can range from 7 mm to 5 cm.
That’s where labiaplasty comes in. The procedure, which can be performed with conventional surgical techniques or with a laser, is sometimes done for functional reasons.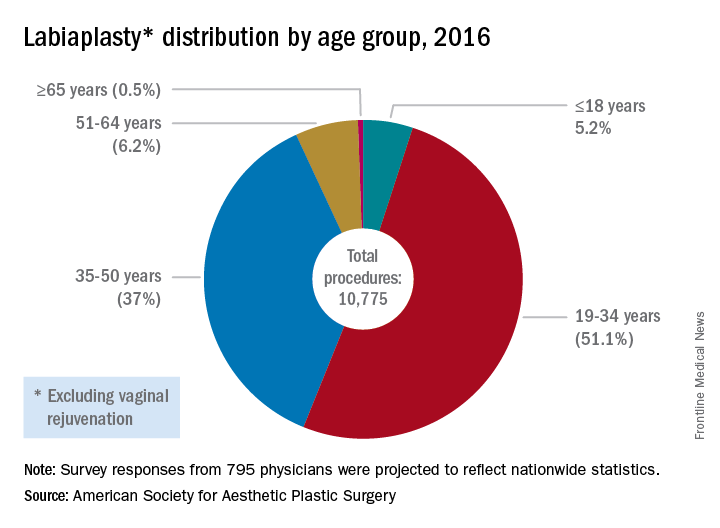
The waters are murkier when labiaplasty is performed for cosmetic reasons, to get that “Barbie doll” look, with some offices advertising the procedure as “designer lips,” Dr. Iglesia said.
In 2007, ACOG issued a committee opinion expressing concern about the lack of data and sometimes deceptive marketing practices surrounding a number of cosmetic vaginal surgeries (Obstet Gynecol 2007;110:737–8). The policy was reaffirmed in 2017.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/is-it-appropriate-to-perform-gynecologic-procedures-such-as-labiaplasty-for-cosmetic-reasons?iframe=1"}]Similarly, the Society of Obstetricians and Gynaecologists of Canada issued a 2013 statement about labiaplasty and other female genital cosmetic surgeries saying that “there is little evidence to support any of the female genital cosmetic surgeries in terms of improvement to sexual satisfaction or self-images. Physicians choosing to proceed with these cosmetic procedures should not promote these surgeries for the enhancement of sexual function and advertising of female genital cosmetic surgical procedures should be avoided.”
However, Mickey Karram, MD, who is director of the urogynecology program at Christ Hospital, Cincinnatti, said that informed consent is the key to dealing appropriately with these procedures.
“If a patient is physically bothered from a cosmetic standpoint that her labia are larger than she thinks they should be, and they are bothering her, is it appropriate or inappropriate to potentially discuss with her a labiaplasty?” Dr. Karram said at the ACOG meeting. For the patient who understands the risk and is also clear that the procedure is not medically necessary, he said he “feels strongly” that labiaplasty should be an option.
Fractional laser
The introduction of the fractional laser to gynecology is also adding to the debate about the appropriate integration of gynecologic procedures that may have nonmedical uses, such as vaginal “tightening.” Used primarily intravaginally, these devices have shallow penetration and are meant to stimulate collagen, proteoglycan, and hyaluronic acid synthesis with minimal tissue damage and downtime. One such device, the MonaLisa Touch, is marketed in the United States by Cynosure.
These energy sources hold great promise for the genitourinary syndrome of menopause (GSM) and other conditions, Dr. Karram said. “Many of these energy sources are being promoted for actual disease states, like vulvovaginal atrophy and lichen sclerosus,” he said.
Dr. Iglesia is not so sure: “This is not the fountain of youth.” She pointed out that the vasculature and innervation of the vagina and vulva are complex, with the outer one-eighth of the vagina being much more highly innervated. Laser treatment with a shallow penetration depth may not get at all of the issues that contribute to GSM.
“Is marketing ahead of the science? I would say yes,” she said. “There’s too much hype about this curing vaginal dryness and making your sex life better.”
Dr. Zahn also urged caution with the use of this technology. “The data are very limited, but, despite this, it’s become a very popular and highly-advertised approach. We need larger studies and more longitudinal data. This is especially true since one of the proposed ways this device works is by stimulating fibrosis. In every other body system, fibrosis stimulation may result in scarring. We have no idea if this is the case with this device. If it is, its application could result in worsening of bodily function, especially in regard to dyspareunia,” he said. “We clearly need more data.”
In 2016, ACOG issued a position statement about the fractional carbon dioxide and yttrium-aluminum-garnet laser systems that had received clearance from the Food and Drug Administration. The statement advised both ob.gyns. and patients that “this technology is, in fact, neither approved nor cleared by the FDA for the specific indication of treating vulvovaginal atrophy.”
Both Dr. Karram and Dr. Iglesia are investigators in an ongoing randomized, placebo- and sham-controlled trial comparing vaginal estrogen and laser therapy used both in conjunction and singly.
‘No-go’ procedures
Though Dr. Karram and Dr. Iglesia disagree on whether cosmetic labiaplasty is appropriate, they were in agreement that certain procedures are so untested, or have such potential risk with no proven benefit, that they should not be performed at all. The procedures on both physicians’ “no-go” lists included clitoral unhooding, G-spot amplification, “revirginification” in any form, vulval recontouring with autologous fat, and the so-called “O-shot,” injections of platelet-rich plasma that are touted as augmenting the sexual experience.
What’s to be done?
There is also agreement that a lack of common terminology is a significant problem. Step one, Dr. Karram said, is doing away with the term vaginal rejuvenation. “This is a terrible term. … There’s no real definition for this term.” He called for a multidisciplinary working group that would bring together gynecologists, plastic surgeons, and dermatologists to begin the work of terminology standardization.
From there, he proposed that the group develop a classification system that clarifies whether procedures are being done for cosmetic reasons, to enhance the sexual experience, or to address a specific disease state. Finally, he said, the group should recommend standardized outcome metrics that can be used to study the various interventions.
Dr. Zahn applauded this notion. “It’s a great point. I agree that multiple disciplines should be involved in examining outcomes, statistics, and criteria for evaluating procedures.”
And gynecologists should be leading this effort, Dr. Karram suggested. “Who knows this anatomy the best? We do.” He added, “If it’s going to be addressed, it should be addressed by us.”
But, Dr. Iglesia said she worries about vulnerable populations, such as adolescents and cancer survivors, who may undergo surgeries, for which the benefits may not outweigh the potential risks. For labiaplasty and laser resurfacing techniques, there have been a small number of studies on outcomes and patient satisfaction that have generally been conducted at single centers with no comparison arms and limited follow-up, she said.
“I also am concerned about pain, scarring, altered sensation, painful sex that could develop, wound complications, and what happens over time,” especially when these procedures may be performed on adolescents or women in their 20s or 30s who may later go on to have children, Dr. Iglesia said.
The question, she said, is not just whether gynecologists are better equipped than plastic surgeons or dermatologists to be performing female genital cosmetic surgery, “but should we be doing this at all?”
Dr. Zahn emphasized the need for evidence to guide decision making. “There has to be data that there is benefit and that the benefit outweighs the potential harm. There is no data on most cosmetic gynecologic procedures. If there are no data, they shouldn’t be done because we would not have the information necessary to appropriately counsel patients,” he said.
Dr. Karram has a financial relationship with Cynosure, which markets the MonaLisa Touch system in the United States. Dr. Iglesia reported that she had no relevant financial disclosures. Dr. Zahn is employed by ACOG.
[email protected]
On Twitter @karioakes
Experts describe the field of female genital cosmetic surgery as the “Wild West,” but the lack of regulation and consensus has not kept it from exploding in recent years.
More than 10,000 labiaplasties were performed in 2016, a 23% jump over the previous year, and the procedures are offered by more than 35% of all plastic surgeons, according to data from the American Society for Aesthetic Plastic Surgery. As another indicator of increasing attention to the appearance of female genitalia, a 2013 survey of U.S. women revealed that more than 80% performed some sort of pubic hair grooming (JAMA Dermatol. 2016;152[10]:1106-13).
Dr. Iglesia recounted being contacted by a National Gallery of Art staff member, who, when confronted with Gustave Courbet’s L’Origine du Monde, an 1866 below-the-waist portrait of a nude woman, asked, “Is this normal? Do women have this much hair?” Dr. Iglesia said she reassured the staff member that the woman in the portrait did indeed have a normal female Tanner stage IV or V escutcheon. However, she said, social media and other images in the popular press have essentially erased female pubic hair from the public eye, even in explicit imagery that involves female nudity.
“This is an ideal that men and women are seeing in social media, in pornography, and even in the lay press.” And now, she said, “We’re in a new era of sex surgeries, with these ‘nips and tucks’ below the belt.”
Labiaplasty
The combination of a newly-hairless genital region, together with portrayals of adult women with a “Barbie doll” appearance, may contribute to women feeling self-conscious about labia minora protruding beyond the labia majora. Dr. Iglesia, who is section director for female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, Washington, D.C., said this is true even though the normal length of labia minora can range from 7 mm to 5 cm.
That’s where labiaplasty comes in. The procedure, which can be performed with conventional surgical techniques or with a laser, is sometimes done for functional reasons.
The waters are murkier when labiaplasty is performed for cosmetic reasons, to get that “Barbie doll” look, with some offices advertising the procedure as “designer lips,” Dr. Iglesia said.
In 2007, ACOG issued a committee opinion expressing concern about the lack of data and sometimes deceptive marketing practices surrounding a number of cosmetic vaginal surgeries (Obstet Gynecol 2007;110:737–8). The policy was reaffirmed in 2017.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/is-it-appropriate-to-perform-gynecologic-procedures-such-as-labiaplasty-for-cosmetic-reasons?iframe=1"}]Similarly, the Society of Obstetricians and Gynaecologists of Canada issued a 2013 statement about labiaplasty and other female genital cosmetic surgeries saying that “there is little evidence to support any of the female genital cosmetic surgeries in terms of improvement to sexual satisfaction or self-images. Physicians choosing to proceed with these cosmetic procedures should not promote these surgeries for the enhancement of sexual function and advertising of female genital cosmetic surgical procedures should be avoided.”
However, Mickey Karram, MD, who is director of the urogynecology program at Christ Hospital, Cincinnatti, said that informed consent is the key to dealing appropriately with these procedures.
“If a patient is physically bothered from a cosmetic standpoint that her labia are larger than she thinks they should be, and they are bothering her, is it appropriate or inappropriate to potentially discuss with her a labiaplasty?” Dr. Karram said at the ACOG meeting. For the patient who understands the risk and is also clear that the procedure is not medically necessary, he said he “feels strongly” that labiaplasty should be an option.
Fractional laser
The introduction of the fractional laser to gynecology is also adding to the debate about the appropriate integration of gynecologic procedures that may have nonmedical uses, such as vaginal “tightening.” Used primarily intravaginally, these devices have shallow penetration and are meant to stimulate collagen, proteoglycan, and hyaluronic acid synthesis with minimal tissue damage and downtime. One such device, the MonaLisa Touch, is marketed in the United States by Cynosure.
These energy sources hold great promise for the genitourinary syndrome of menopause (GSM) and other conditions, Dr. Karram said. “Many of these energy sources are being promoted for actual disease states, like vulvovaginal atrophy and lichen sclerosus,” he said.
Dr. Iglesia is not so sure: “This is not the fountain of youth.” She pointed out that the vasculature and innervation of the vagina and vulva are complex, with the outer one-eighth of the vagina being much more highly innervated. Laser treatment with a shallow penetration depth may not get at all of the issues that contribute to GSM.
“Is marketing ahead of the science? I would say yes,” she said. “There’s too much hype about this curing vaginal dryness and making your sex life better.”
Dr. Zahn also urged caution with the use of this technology. “The data are very limited, but, despite this, it’s become a very popular and highly-advertised approach. We need larger studies and more longitudinal data. This is especially true since one of the proposed ways this device works is by stimulating fibrosis. In every other body system, fibrosis stimulation may result in scarring. We have no idea if this is the case with this device. If it is, its application could result in worsening of bodily function, especially in regard to dyspareunia,” he said. “We clearly need more data.”
In 2016, ACOG issued a position statement about the fractional carbon dioxide and yttrium-aluminum-garnet laser systems that had received clearance from the Food and Drug Administration. The statement advised both ob.gyns. and patients that “this technology is, in fact, neither approved nor cleared by the FDA for the specific indication of treating vulvovaginal atrophy.”
Both Dr. Karram and Dr. Iglesia are investigators in an ongoing randomized, placebo- and sham-controlled trial comparing vaginal estrogen and laser therapy used both in conjunction and singly.
‘No-go’ procedures
Though Dr. Karram and Dr. Iglesia disagree on whether cosmetic labiaplasty is appropriate, they were in agreement that certain procedures are so untested, or have such potential risk with no proven benefit, that they should not be performed at all. The procedures on both physicians’ “no-go” lists included clitoral unhooding, G-spot amplification, “revirginification” in any form, vulval recontouring with autologous fat, and the so-called “O-shot,” injections of platelet-rich plasma that are touted as augmenting the sexual experience.
What’s to be done?
There is also agreement that a lack of common terminology is a significant problem. Step one, Dr. Karram said, is doing away with the term vaginal rejuvenation. “This is a terrible term. … There’s no real definition for this term.” He called for a multidisciplinary working group that would bring together gynecologists, plastic surgeons, and dermatologists to begin the work of terminology standardization.
From there, he proposed that the group develop a classification system that clarifies whether procedures are being done for cosmetic reasons, to enhance the sexual experience, or to address a specific disease state. Finally, he said, the group should recommend standardized outcome metrics that can be used to study the various interventions.
Dr. Zahn applauded this notion. “It’s a great point. I agree that multiple disciplines should be involved in examining outcomes, statistics, and criteria for evaluating procedures.”
And gynecologists should be leading this effort, Dr. Karram suggested. “Who knows this anatomy the best? We do.” He added, “If it’s going to be addressed, it should be addressed by us.”
But, Dr. Iglesia said she worries about vulnerable populations, such as adolescents and cancer survivors, who may undergo surgeries, for which the benefits may not outweigh the potential risks. For labiaplasty and laser resurfacing techniques, there have been a small number of studies on outcomes and patient satisfaction that have generally been conducted at single centers with no comparison arms and limited follow-up, she said.
“I also am concerned about pain, scarring, altered sensation, painful sex that could develop, wound complications, and what happens over time,” especially when these procedures may be performed on adolescents or women in their 20s or 30s who may later go on to have children, Dr. Iglesia said.
The question, she said, is not just whether gynecologists are better equipped than plastic surgeons or dermatologists to be performing female genital cosmetic surgery, “but should we be doing this at all?”
Dr. Zahn emphasized the need for evidence to guide decision making. “There has to be data that there is benefit and that the benefit outweighs the potential harm. There is no data on most cosmetic gynecologic procedures. If there are no data, they shouldn’t be done because we would not have the information necessary to appropriately counsel patients,” he said.
Dr. Karram has a financial relationship with Cynosure, which markets the MonaLisa Touch system in the United States. Dr. Iglesia reported that she had no relevant financial disclosures. Dr. Zahn is employed by ACOG.
[email protected]
On Twitter @karioakes
Experts describe the field of female genital cosmetic surgery as the “Wild West,” but the lack of regulation and consensus has not kept it from exploding in recent years.
More than 10,000 labiaplasties were performed in 2016, a 23% jump over the previous year, and the procedures are offered by more than 35% of all plastic surgeons, according to data from the American Society for Aesthetic Plastic Surgery. As another indicator of increasing attention to the appearance of female genitalia, a 2013 survey of U.S. women revealed that more than 80% performed some sort of pubic hair grooming (JAMA Dermatol. 2016;152[10]:1106-13).
Dr. Iglesia recounted being contacted by a National Gallery of Art staff member, who, when confronted with Gustave Courbet’s L’Origine du Monde, an 1866 below-the-waist portrait of a nude woman, asked, “Is this normal? Do women have this much hair?” Dr. Iglesia said she reassured the staff member that the woman in the portrait did indeed have a normal female Tanner stage IV or V escutcheon. However, she said, social media and other images in the popular press have essentially erased female pubic hair from the public eye, even in explicit imagery that involves female nudity.
“This is an ideal that men and women are seeing in social media, in pornography, and even in the lay press.” And now, she said, “We’re in a new era of sex surgeries, with these ‘nips and tucks’ below the belt.”
Labiaplasty
The combination of a newly-hairless genital region, together with portrayals of adult women with a “Barbie doll” appearance, may contribute to women feeling self-conscious about labia minora protruding beyond the labia majora. Dr. Iglesia, who is section director for female pelvic medicine and reconstructive surgery at MedStar Washington Hospital Center, Washington, D.C., said this is true even though the normal length of labia minora can range from 7 mm to 5 cm.
That’s where labiaplasty comes in. The procedure, which can be performed with conventional surgical techniques or with a laser, is sometimes done for functional reasons.
The waters are murkier when labiaplasty is performed for cosmetic reasons, to get that “Barbie doll” look, with some offices advertising the procedure as “designer lips,” Dr. Iglesia said.
In 2007, ACOG issued a committee opinion expressing concern about the lack of data and sometimes deceptive marketing practices surrounding a number of cosmetic vaginal surgeries (Obstet Gynecol 2007;110:737–8). The policy was reaffirmed in 2017.
[polldaddy:{"method":"iframe","type":"survey","src":"//newspolls2017.polldaddy.com/s/is-it-appropriate-to-perform-gynecologic-procedures-such-as-labiaplasty-for-cosmetic-reasons?iframe=1"}]Similarly, the Society of Obstetricians and Gynaecologists of Canada issued a 2013 statement about labiaplasty and other female genital cosmetic surgeries saying that “there is little evidence to support any of the female genital cosmetic surgeries in terms of improvement to sexual satisfaction or self-images. Physicians choosing to proceed with these cosmetic procedures should not promote these surgeries for the enhancement of sexual function and advertising of female genital cosmetic surgical procedures should be avoided.”
However, Mickey Karram, MD, who is director of the urogynecology program at Christ Hospital, Cincinnatti, said that informed consent is the key to dealing appropriately with these procedures.
“If a patient is physically bothered from a cosmetic standpoint that her labia are larger than she thinks they should be, and they are bothering her, is it appropriate or inappropriate to potentially discuss with her a labiaplasty?” Dr. Karram said at the ACOG meeting. For the patient who understands the risk and is also clear that the procedure is not medically necessary, he said he “feels strongly” that labiaplasty should be an option.
Fractional laser
The introduction of the fractional laser to gynecology is also adding to the debate about the appropriate integration of gynecologic procedures that may have nonmedical uses, such as vaginal “tightening.” Used primarily intravaginally, these devices have shallow penetration and are meant to stimulate collagen, proteoglycan, and hyaluronic acid synthesis with minimal tissue damage and downtime. One such device, the MonaLisa Touch, is marketed in the United States by Cynosure.
These energy sources hold great promise for the genitourinary syndrome of menopause (GSM) and other conditions, Dr. Karram said. “Many of these energy sources are being promoted for actual disease states, like vulvovaginal atrophy and lichen sclerosus,” he said.
Dr. Iglesia is not so sure: “This is not the fountain of youth.” She pointed out that the vasculature and innervation of the vagina and vulva are complex, with the outer one-eighth of the vagina being much more highly innervated. Laser treatment with a shallow penetration depth may not get at all of the issues that contribute to GSM.
“Is marketing ahead of the science? I would say yes,” she said. “There’s too much hype about this curing vaginal dryness and making your sex life better.”
Dr. Zahn also urged caution with the use of this technology. “The data are very limited, but, despite this, it’s become a very popular and highly-advertised approach. We need larger studies and more longitudinal data. This is especially true since one of the proposed ways this device works is by stimulating fibrosis. In every other body system, fibrosis stimulation may result in scarring. We have no idea if this is the case with this device. If it is, its application could result in worsening of bodily function, especially in regard to dyspareunia,” he said. “We clearly need more data.”
In 2016, ACOG issued a position statement about the fractional carbon dioxide and yttrium-aluminum-garnet laser systems that had received clearance from the Food and Drug Administration. The statement advised both ob.gyns. and patients that “this technology is, in fact, neither approved nor cleared by the FDA for the specific indication of treating vulvovaginal atrophy.”
Both Dr. Karram and Dr. Iglesia are investigators in an ongoing randomized, placebo- and sham-controlled trial comparing vaginal estrogen and laser therapy used both in conjunction and singly.
‘No-go’ procedures
Though Dr. Karram and Dr. Iglesia disagree on whether cosmetic labiaplasty is appropriate, they were in agreement that certain procedures are so untested, or have such potential risk with no proven benefit, that they should not be performed at all. The procedures on both physicians’ “no-go” lists included clitoral unhooding, G-spot amplification, “revirginification” in any form, vulval recontouring with autologous fat, and the so-called “O-shot,” injections of platelet-rich plasma that are touted as augmenting the sexual experience.
What’s to be done?
There is also agreement that a lack of common terminology is a significant problem. Step one, Dr. Karram said, is doing away with the term vaginal rejuvenation. “This is a terrible term. … There’s no real definition for this term.” He called for a multidisciplinary working group that would bring together gynecologists, plastic surgeons, and dermatologists to begin the work of terminology standardization.
From there, he proposed that the group develop a classification system that clarifies whether procedures are being done for cosmetic reasons, to enhance the sexual experience, or to address a specific disease state. Finally, he said, the group should recommend standardized outcome metrics that can be used to study the various interventions.
Dr. Zahn applauded this notion. “It’s a great point. I agree that multiple disciplines should be involved in examining outcomes, statistics, and criteria for evaluating procedures.”
And gynecologists should be leading this effort, Dr. Karram suggested. “Who knows this anatomy the best? We do.” He added, “If it’s going to be addressed, it should be addressed by us.”
But, Dr. Iglesia said she worries about vulnerable populations, such as adolescents and cancer survivors, who may undergo surgeries, for which the benefits may not outweigh the potential risks. For labiaplasty and laser resurfacing techniques, there have been a small number of studies on outcomes and patient satisfaction that have generally been conducted at single centers with no comparison arms and limited follow-up, she said.
“I also am concerned about pain, scarring, altered sensation, painful sex that could develop, wound complications, and what happens over time,” especially when these procedures may be performed on adolescents or women in their 20s or 30s who may later go on to have children, Dr. Iglesia said.
The question, she said, is not just whether gynecologists are better equipped than plastic surgeons or dermatologists to be performing female genital cosmetic surgery, “but should we be doing this at all?”
Dr. Zahn emphasized the need for evidence to guide decision making. “There has to be data that there is benefit and that the benefit outweighs the potential harm. There is no data on most cosmetic gynecologic procedures. If there are no data, they shouldn’t be done because we would not have the information necessary to appropriately counsel patients,” he said.
Dr. Karram has a financial relationship with Cynosure, which markets the MonaLisa Touch system in the United States. Dr. Iglesia reported that she had no relevant financial disclosures. Dr. Zahn is employed by ACOG.
[email protected]
On Twitter @karioakes
Female genital mutilation is seen by most ob.gyns.
SAN DIEGO – Nearly 60% of ob.gyns. have seen patients who have experienced female genital mutilation (FGM), according to the results of a survey of 288 fellows of the American College of Obstetricians and Gynecologists.
Additionally, the survey found that there are few guidelines for care of these patients. Among fellows who had seen patients with FGM, 80.1% said their institutions had no policies or guidelines for management of these patients. Additionally, just 56.7% of fellows who had treated these women were aware that federal laws prohibit FGM.
The findings came as a surprise to the study’s senior author, Alireza Shamshirsaz, MD, a maternal-fetal medicine specialist at Baylor College of Medicine, Houston. “I really see the gap of knowledge of [ob.gyns.] in the States, and the emergent need for guidelines and regulation.”
Ob.gyns. who cared for women who had experienced FGM reported that they used a combination of approaches in treating these patients. Nearly half of physicians (45.8%) said they generally treated FGM patients the same as their other patients, but nearly three-quarters said they also discuss potential complications of FGM procedures. About 28% of physicians reported providing information about surgical repair options, while less than 5% provided mental health referrals.
For minors who had experienced FGM, low numbers of survey respondents made law enforcement referrals (7.7%) or social service referrals (7.7%). There were a few respondents (5.4%) who said they didn’t feel comfortable managing patients who had experienced FGM.
In another surprise to the researchers, genital mutilation procedures were not all performed abroad. Three ob.gyns. reported caring for patients who had previously undergone FGM in the United States.
The findings highlight the need for structured education in ob.gyn. residency training programs regarding how to approach at-risk patients and those who have experienced FGM, Dr. Shamshirsaz said in an interview. He also called for specific guidelines from ACOG regarding virginity testing and hymenoplasty and for clearer statutes to guide physicians when patients request FGM or virginity testing procedures.
“All [ob.gyns.] should be aware that their at-risk patients may have a history of honor-related practices and should obtain a full history in a culturally sensitive and professionally responsible manner so that they may respond to and address the unique needs of these patients,” the researchers wrote.
The researchers reported having no relevant financial disclosures. The study was supported by a grant from ACOG.
[email protected]
On Twitter @karioakes
SAN DIEGO – Nearly 60% of ob.gyns. have seen patients who have experienced female genital mutilation (FGM), according to the results of a survey of 288 fellows of the American College of Obstetricians and Gynecologists.
Additionally, the survey found that there are few guidelines for care of these patients. Among fellows who had seen patients with FGM, 80.1% said their institutions had no policies or guidelines for management of these patients. Additionally, just 56.7% of fellows who had treated these women were aware that federal laws prohibit FGM.
The findings came as a surprise to the study’s senior author, Alireza Shamshirsaz, MD, a maternal-fetal medicine specialist at Baylor College of Medicine, Houston. “I really see the gap of knowledge of [ob.gyns.] in the States, and the emergent need for guidelines and regulation.”
Ob.gyns. who cared for women who had experienced FGM reported that they used a combination of approaches in treating these patients. Nearly half of physicians (45.8%) said they generally treated FGM patients the same as their other patients, but nearly three-quarters said they also discuss potential complications of FGM procedures. About 28% of physicians reported providing information about surgical repair options, while less than 5% provided mental health referrals.
For minors who had experienced FGM, low numbers of survey respondents made law enforcement referrals (7.7%) or social service referrals (7.7%). There were a few respondents (5.4%) who said they didn’t feel comfortable managing patients who had experienced FGM.
In another surprise to the researchers, genital mutilation procedures were not all performed abroad. Three ob.gyns. reported caring for patients who had previously undergone FGM in the United States.
The findings highlight the need for structured education in ob.gyn. residency training programs regarding how to approach at-risk patients and those who have experienced FGM, Dr. Shamshirsaz said in an interview. He also called for specific guidelines from ACOG regarding virginity testing and hymenoplasty and for clearer statutes to guide physicians when patients request FGM or virginity testing procedures.
“All [ob.gyns.] should be aware that their at-risk patients may have a history of honor-related practices and should obtain a full history in a culturally sensitive and professionally responsible manner so that they may respond to and address the unique needs of these patients,” the researchers wrote.
The researchers reported having no relevant financial disclosures. The study was supported by a grant from ACOG.
[email protected]
On Twitter @karioakes
SAN DIEGO – Nearly 60% of ob.gyns. have seen patients who have experienced female genital mutilation (FGM), according to the results of a survey of 288 fellows of the American College of Obstetricians and Gynecologists.
Additionally, the survey found that there are few guidelines for care of these patients. Among fellows who had seen patients with FGM, 80.1% said their institutions had no policies or guidelines for management of these patients. Additionally, just 56.7% of fellows who had treated these women were aware that federal laws prohibit FGM.
The findings came as a surprise to the study’s senior author, Alireza Shamshirsaz, MD, a maternal-fetal medicine specialist at Baylor College of Medicine, Houston. “I really see the gap of knowledge of [ob.gyns.] in the States, and the emergent need for guidelines and regulation.”
Ob.gyns. who cared for women who had experienced FGM reported that they used a combination of approaches in treating these patients. Nearly half of physicians (45.8%) said they generally treated FGM patients the same as their other patients, but nearly three-quarters said they also discuss potential complications of FGM procedures. About 28% of physicians reported providing information about surgical repair options, while less than 5% provided mental health referrals.
For minors who had experienced FGM, low numbers of survey respondents made law enforcement referrals (7.7%) or social service referrals (7.7%). There were a few respondents (5.4%) who said they didn’t feel comfortable managing patients who had experienced FGM.
In another surprise to the researchers, genital mutilation procedures were not all performed abroad. Three ob.gyns. reported caring for patients who had previously undergone FGM in the United States.
The findings highlight the need for structured education in ob.gyn. residency training programs regarding how to approach at-risk patients and those who have experienced FGM, Dr. Shamshirsaz said in an interview. He also called for specific guidelines from ACOG regarding virginity testing and hymenoplasty and for clearer statutes to guide physicians when patients request FGM or virginity testing procedures.
“All [ob.gyns.] should be aware that their at-risk patients may have a history of honor-related practices and should obtain a full history in a culturally sensitive and professionally responsible manner so that they may respond to and address the unique needs of these patients,” the researchers wrote.
The researchers reported having no relevant financial disclosures. The study was supported by a grant from ACOG.
[email protected]
On Twitter @karioakes
AT ACOG 2017
Key clinical point:
Major finding: Of the 288 ACOG fellows surveyed, 58.6% have seen patients who have experienced female genital mutilation.
Data source: An anonymous online survey of 288 randomly-selected and stratified ACOG fellows.
Disclosures: The study was supported by a grant from ACOG. The researchers reported having no relevant financial disclosures.
VIDEO: Immune therapy effective, durable in treatment-naive melanoma brain metastases
CHICAGO – Immune therapy shows promise for use in the treatment of melanoma brain metastases, especially for treatment-naive patients, judging from the findings of a new phase II randomized study.
For patients with asymptomatic brain metastases from melanoma who had not had prior treatment, nivolumab combined with ipilimumab produced a 50% intracranial response rate after at least 12 weeks of therapy. When nivolumab alone was given to untreated patients, the intracranial response rate was 21%, Georgina Long MD, PhD, co–medical director of the Melanoma Institute Australia , said during a video interview at the annual meeting of the American Society of Clinical Oncology.
“If you look at progression-free survival by response, none of our complete responders have progressed,” said Dr. Long. “And this is with a median follow-up of 16.4 months.” The partial responders have also done well, with little progression, she said. “Remember, these patients usually survive only a few weeks.”
The Anti-PD1 Brain Collaboration study, a phase II clinical trial, enrolled patients with melanoma brain metastases at least 5 mm but less than 40 mm in diameter who had not received previous anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death protein 1 (anti-PD-1), or anti-programmed death-ligand 1 (anti-PD-L1) therapies. Patients were permitted to have had previous BRAF and MEK inhibitor therapies. Asymptomatic patients who had no previous local brain therapy (i.e., radiation treatment or surgery) were randomized 1:1 to receive nivolumab alone, or nivolumab plus ipilimumab.
The nivolumab arm received 3 mg/kg by intravenous infusion every 2 weeks. The combination arm began with nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four doses. After this, they also received nivolumab 3 mg/kg monotherapy every 2 weeks.
The third cohort – a small group of 15 patients who received nivolumab alone – either had symptomatic brain metastases or leptomeningeal disease and could have had previous brain surgery or radiotherapy. Unlike the first two cohorts, they were also permitted to be on up to 10 mg/day of prednisone; these patients received nivolumab alone at 3 mg/kg every 2 weeks.
For all patients, immune therapy was given until the disease progressed, consent was withdrawn, or patients experienced unacceptable toxicity or they died.
“We were most interested in the randomized cohorts,” said Dr. Long. Interestingly, she said, ipilimumab became available in Australia when 27 patients were enrolled in the nivolumab arm and 26 to the combination arm. “So we stopped the monotherapy arm, and the rest of the 60 patients to be recruited all went into the combination arm,” she said. A total of 76 patients were recruited, 33 into the combination arm, 27 to the asymptomatic nivolumab monotherapy arm, and 16 to the symptomatic and/or previously treated arm.
Data analysis from the point of the data cut included 67 patients who were followed for a period ranging from 5 to 34 months. Intracranial disease was evaluated by gadolinium-enhanced MRI and modified Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
“The results of the trial were very interesting,” said Dr. Long. The nivolumab plus ipilimumab combo resulted in an overall 42% intracranial response rate, while nivolumab alone produced an overall intracranial response rate of 21%. However, patients in either arm who had prior BRAF or MEK inhibitor exposure “didn’t do too well on immunotherapy,” said Dr. Long, noting that the response rate was just 16% for these patients. These were, she said, “small numbers, but still, an interesting signal there.”
When comparing the secondary endpoint of extracranial response to intracranial response on a per-patient basis, Dr. Long and her collaborators could see that “the intracranial and extracranial results were mostly concordant.”
Analysis of the additional secondary endpoints of progression-free survival (PFS) and overall survival (OS) also showed an interesting pattern, said Dr. Long. After an initial drop-off period of about 5 months, the curves for patients in all arms have stabilized, so that patients who were responders are maintaining that response. The overall 6-month PFS rate for the combination cohort was 47%, with a durable response: “If you look at the curve, it’s flattened out since that stage, and we haven’t had any progression since that time,” said Dr. Long. The PFS rate was 29% for the cohort receiving nivolumab alone. “Activity is highest when nivolumab and ipilimumab are given upfront,” said Dr. Long.
For asymptomatic patients pretreated with BRAF or MEK inhibitors, “activity is low,” said Dr. Long, with an intracranial response rate of 16% in both cohorts.
Symptomatic patients who were more heavily pretreated fared even worse: “The activity of nivolumab monotherapy is low after multiple modality therapy or in leptomeningeal melanoma,” said Dr. Long. The intracranial response rate in the third cohort was just 6%.
The combination therapy cohort had the most treatment-related adverse events, with 96% of patients experiencing some adverse event. About half (12/26, 46%) had grade 3 or 4 events, and the same number had a serious adverse event. Seven patients (27%) discontinued therapy because of treatment-related adverse events in the combination study arm. However, said Dr. Long, this side effect profile is in keeping with what has been seen in other studies of combination therapy with nivolumab and ipilimumab. “There were not unexpected adverse events,” she said.
Dr. Long reported relationships with Bristol-Myers Squibb, Merck, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Immune therapy shows promise for use in the treatment of melanoma brain metastases, especially for treatment-naive patients, judging from the findings of a new phase II randomized study.
For patients with asymptomatic brain metastases from melanoma who had not had prior treatment, nivolumab combined with ipilimumab produced a 50% intracranial response rate after at least 12 weeks of therapy. When nivolumab alone was given to untreated patients, the intracranial response rate was 21%, Georgina Long MD, PhD, co–medical director of the Melanoma Institute Australia , said during a video interview at the annual meeting of the American Society of Clinical Oncology.
“If you look at progression-free survival by response, none of our complete responders have progressed,” said Dr. Long. “And this is with a median follow-up of 16.4 months.” The partial responders have also done well, with little progression, she said. “Remember, these patients usually survive only a few weeks.”
The Anti-PD1 Brain Collaboration study, a phase II clinical trial, enrolled patients with melanoma brain metastases at least 5 mm but less than 40 mm in diameter who had not received previous anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death protein 1 (anti-PD-1), or anti-programmed death-ligand 1 (anti-PD-L1) therapies. Patients were permitted to have had previous BRAF and MEK inhibitor therapies. Asymptomatic patients who had no previous local brain therapy (i.e., radiation treatment or surgery) were randomized 1:1 to receive nivolumab alone, or nivolumab plus ipilimumab.
The nivolumab arm received 3 mg/kg by intravenous infusion every 2 weeks. The combination arm began with nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four doses. After this, they also received nivolumab 3 mg/kg monotherapy every 2 weeks.
The third cohort – a small group of 15 patients who received nivolumab alone – either had symptomatic brain metastases or leptomeningeal disease and could have had previous brain surgery or radiotherapy. Unlike the first two cohorts, they were also permitted to be on up to 10 mg/day of prednisone; these patients received nivolumab alone at 3 mg/kg every 2 weeks.
For all patients, immune therapy was given until the disease progressed, consent was withdrawn, or patients experienced unacceptable toxicity or they died.
“We were most interested in the randomized cohorts,” said Dr. Long. Interestingly, she said, ipilimumab became available in Australia when 27 patients were enrolled in the nivolumab arm and 26 to the combination arm. “So we stopped the monotherapy arm, and the rest of the 60 patients to be recruited all went into the combination arm,” she said. A total of 76 patients were recruited, 33 into the combination arm, 27 to the asymptomatic nivolumab monotherapy arm, and 16 to the symptomatic and/or previously treated arm.
Data analysis from the point of the data cut included 67 patients who were followed for a period ranging from 5 to 34 months. Intracranial disease was evaluated by gadolinium-enhanced MRI and modified Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
“The results of the trial were very interesting,” said Dr. Long. The nivolumab plus ipilimumab combo resulted in an overall 42% intracranial response rate, while nivolumab alone produced an overall intracranial response rate of 21%. However, patients in either arm who had prior BRAF or MEK inhibitor exposure “didn’t do too well on immunotherapy,” said Dr. Long, noting that the response rate was just 16% for these patients. These were, she said, “small numbers, but still, an interesting signal there.”
When comparing the secondary endpoint of extracranial response to intracranial response on a per-patient basis, Dr. Long and her collaborators could see that “the intracranial and extracranial results were mostly concordant.”
Analysis of the additional secondary endpoints of progression-free survival (PFS) and overall survival (OS) also showed an interesting pattern, said Dr. Long. After an initial drop-off period of about 5 months, the curves for patients in all arms have stabilized, so that patients who were responders are maintaining that response. The overall 6-month PFS rate for the combination cohort was 47%, with a durable response: “If you look at the curve, it’s flattened out since that stage, and we haven’t had any progression since that time,” said Dr. Long. The PFS rate was 29% for the cohort receiving nivolumab alone. “Activity is highest when nivolumab and ipilimumab are given upfront,” said Dr. Long.
For asymptomatic patients pretreated with BRAF or MEK inhibitors, “activity is low,” said Dr. Long, with an intracranial response rate of 16% in both cohorts.
Symptomatic patients who were more heavily pretreated fared even worse: “The activity of nivolumab monotherapy is low after multiple modality therapy or in leptomeningeal melanoma,” said Dr. Long. The intracranial response rate in the third cohort was just 6%.
The combination therapy cohort had the most treatment-related adverse events, with 96% of patients experiencing some adverse event. About half (12/26, 46%) had grade 3 or 4 events, and the same number had a serious adverse event. Seven patients (27%) discontinued therapy because of treatment-related adverse events in the combination study arm. However, said Dr. Long, this side effect profile is in keeping with what has been seen in other studies of combination therapy with nivolumab and ipilimumab. “There were not unexpected adverse events,” she said.
Dr. Long reported relationships with Bristol-Myers Squibb, Merck, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Immune therapy shows promise for use in the treatment of melanoma brain metastases, especially for treatment-naive patients, judging from the findings of a new phase II randomized study.
For patients with asymptomatic brain metastases from melanoma who had not had prior treatment, nivolumab combined with ipilimumab produced a 50% intracranial response rate after at least 12 weeks of therapy. When nivolumab alone was given to untreated patients, the intracranial response rate was 21%, Georgina Long MD, PhD, co–medical director of the Melanoma Institute Australia , said during a video interview at the annual meeting of the American Society of Clinical Oncology.
“If you look at progression-free survival by response, none of our complete responders have progressed,” said Dr. Long. “And this is with a median follow-up of 16.4 months.” The partial responders have also done well, with little progression, she said. “Remember, these patients usually survive only a few weeks.”
The Anti-PD1 Brain Collaboration study, a phase II clinical trial, enrolled patients with melanoma brain metastases at least 5 mm but less than 40 mm in diameter who had not received previous anti-cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4), anti-programmed cell death protein 1 (anti-PD-1), or anti-programmed death-ligand 1 (anti-PD-L1) therapies. Patients were permitted to have had previous BRAF and MEK inhibitor therapies. Asymptomatic patients who had no previous local brain therapy (i.e., radiation treatment or surgery) were randomized 1:1 to receive nivolumab alone, or nivolumab plus ipilimumab.
The nivolumab arm received 3 mg/kg by intravenous infusion every 2 weeks. The combination arm began with nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks for four doses. After this, they also received nivolumab 3 mg/kg monotherapy every 2 weeks.
The third cohort – a small group of 15 patients who received nivolumab alone – either had symptomatic brain metastases or leptomeningeal disease and could have had previous brain surgery or radiotherapy. Unlike the first two cohorts, they were also permitted to be on up to 10 mg/day of prednisone; these patients received nivolumab alone at 3 mg/kg every 2 weeks.
For all patients, immune therapy was given until the disease progressed, consent was withdrawn, or patients experienced unacceptable toxicity or they died.
“We were most interested in the randomized cohorts,” said Dr. Long. Interestingly, she said, ipilimumab became available in Australia when 27 patients were enrolled in the nivolumab arm and 26 to the combination arm. “So we stopped the monotherapy arm, and the rest of the 60 patients to be recruited all went into the combination arm,” she said. A total of 76 patients were recruited, 33 into the combination arm, 27 to the asymptomatic nivolumab monotherapy arm, and 16 to the symptomatic and/or previously treated arm.
Data analysis from the point of the data cut included 67 patients who were followed for a period ranging from 5 to 34 months. Intracranial disease was evaluated by gadolinium-enhanced MRI and modified Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria.
“The results of the trial were very interesting,” said Dr. Long. The nivolumab plus ipilimumab combo resulted in an overall 42% intracranial response rate, while nivolumab alone produced an overall intracranial response rate of 21%. However, patients in either arm who had prior BRAF or MEK inhibitor exposure “didn’t do too well on immunotherapy,” said Dr. Long, noting that the response rate was just 16% for these patients. These were, she said, “small numbers, but still, an interesting signal there.”
When comparing the secondary endpoint of extracranial response to intracranial response on a per-patient basis, Dr. Long and her collaborators could see that “the intracranial and extracranial results were mostly concordant.”
Analysis of the additional secondary endpoints of progression-free survival (PFS) and overall survival (OS) also showed an interesting pattern, said Dr. Long. After an initial drop-off period of about 5 months, the curves for patients in all arms have stabilized, so that patients who were responders are maintaining that response. The overall 6-month PFS rate for the combination cohort was 47%, with a durable response: “If you look at the curve, it’s flattened out since that stage, and we haven’t had any progression since that time,” said Dr. Long. The PFS rate was 29% for the cohort receiving nivolumab alone. “Activity is highest when nivolumab and ipilimumab are given upfront,” said Dr. Long.
For asymptomatic patients pretreated with BRAF or MEK inhibitors, “activity is low,” said Dr. Long, with an intracranial response rate of 16% in both cohorts.
Symptomatic patients who were more heavily pretreated fared even worse: “The activity of nivolumab monotherapy is low after multiple modality therapy or in leptomeningeal melanoma,” said Dr. Long. The intracranial response rate in the third cohort was just 6%.
The combination therapy cohort had the most treatment-related adverse events, with 96% of patients experiencing some adverse event. About half (12/26, 46%) had grade 3 or 4 events, and the same number had a serious adverse event. Seven patients (27%) discontinued therapy because of treatment-related adverse events in the combination study arm. However, said Dr. Long, this side effect profile is in keeping with what has been seen in other studies of combination therapy with nivolumab and ipilimumab. “There were not unexpected adverse events,” she said.
Dr. Long reported relationships with Bristol-Myers Squibb, Merck, and Roche.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT ASCO 2017
EMEUNET tailors EULAR experience for young rheumatologists
Young rheumatologists and researchers will find plenty of relevant content at this year’s EULAR Congress in Madrid, June 14-17, thanks to a dedicated presentation track. Other tailored opportunities include networking events, mentorship for first-time attendees to help them make the most of their EULAR experience, and a unique opportunity for small group discussion and networking with key opinion leaders in rheumatology in the so-called mentor-mentee meetings.
The Young Rheumatologists track provides three sessions with a special focus on researchers and clinicians who are early in their careers, Sofia Ramiro, MD, PhD, explained in an interview. Dr. Ramiro chairs the steering committee of the Emerging Eular Network (EMEUNET), a network of young clinicians and researchers in the field of rheumatology in Europe.
Another session will zero in on osteoarthritis, vasculitis, spondyloarthritis, and rheumatoid arthritis, with a shorter lecture format and more time left for a question-and-answer session and discussion. With a group of younger rheumatologists in attendance, “the sessions are somewhat more informal,” promoting a comfortable and interactive environment for discussion and learning, Dr. Ramiro said.
The third session in the Young Rheumatologists track will consist of case discussions focused on how to counsel and take care of women who have rheumatoid arthritis and would like to become pregnant. Two patient cases will be presented and discussed by leaders in the field. “Again, the idea is to make these presentations as real-world as possible,” Dr. Ramiro said.
The EMEUNET booth will be in the EULAR Village, and, for the first time, the booth will be incorporated in the EULAR booth, as a “pillar” under the bigger EULAR umbrella. Dr. Ramiro said to be sure to stay tuned for a surprise associated with EULAR’s 70th anniversary. On the evening of Thursday, June 15, EMEUNET will host a networking event.
On the morning of Friday, June 16, mentor-mentee meetings organized by EMEUNET link five to six young attendees with mentors, according to area of interest. Sign up is available online, allowing small group discussion with leaders in academic rheumatology. This year, meetings will be led by Iain McInnes, PhD (Glasgow, Scotland), Josef Smolen, MD (Vienna), and William Dixon, MBBS, PhD (Manchester, England). Mentorship topics can include the incorporation of research into a clinical career, general career advice, and insight into international collaboration, Dr. Ramiro said.
“These are usually very well-attended meetings and very popular,” she said. “People who have participated in them always give us very good feedback and are very enthusiastic about how easily accessible these very famous key opinion leaders are and what good advice they give to them.”
Finally, the Ambassador program helps first-time attendees get the most out of EULAR. “I think that we all know that the first time we attend such a huge conference the experience can be daunting,” Dr. Ramiro said. Now in its third year, the ambassador program pairs an EMEUNET member with up to six first-timers. The ambassador helps the newcomers decide which sessions to attend and remains available through mobile phone, social media, and the meeting app throughout the meeting.
All of EMEUNET’s activities during EULAR support the organization’s aim of “widening collaboration and fostering collaboration among young researchers and clinicians,” Dr. Ramiro said. “The ultimate aim is to improve and promote education in the area of our diseases and to foster research collaborations,” she said of the 1,500-member strong organization.
Young rheumatologists and researchers will find plenty of relevant content at this year’s EULAR Congress in Madrid, June 14-17, thanks to a dedicated presentation track. Other tailored opportunities include networking events, mentorship for first-time attendees to help them make the most of their EULAR experience, and a unique opportunity for small group discussion and networking with key opinion leaders in rheumatology in the so-called mentor-mentee meetings.
The Young Rheumatologists track provides three sessions with a special focus on researchers and clinicians who are early in their careers, Sofia Ramiro, MD, PhD, explained in an interview. Dr. Ramiro chairs the steering committee of the Emerging Eular Network (EMEUNET), a network of young clinicians and researchers in the field of rheumatology in Europe.
Another session will zero in on osteoarthritis, vasculitis, spondyloarthritis, and rheumatoid arthritis, with a shorter lecture format and more time left for a question-and-answer session and discussion. With a group of younger rheumatologists in attendance, “the sessions are somewhat more informal,” promoting a comfortable and interactive environment for discussion and learning, Dr. Ramiro said.
The third session in the Young Rheumatologists track will consist of case discussions focused on how to counsel and take care of women who have rheumatoid arthritis and would like to become pregnant. Two patient cases will be presented and discussed by leaders in the field. “Again, the idea is to make these presentations as real-world as possible,” Dr. Ramiro said.
The EMEUNET booth will be in the EULAR Village, and, for the first time, the booth will be incorporated in the EULAR booth, as a “pillar” under the bigger EULAR umbrella. Dr. Ramiro said to be sure to stay tuned for a surprise associated with EULAR’s 70th anniversary. On the evening of Thursday, June 15, EMEUNET will host a networking event.
On the morning of Friday, June 16, mentor-mentee meetings organized by EMEUNET link five to six young attendees with mentors, according to area of interest. Sign up is available online, allowing small group discussion with leaders in academic rheumatology. This year, meetings will be led by Iain McInnes, PhD (Glasgow, Scotland), Josef Smolen, MD (Vienna), and William Dixon, MBBS, PhD (Manchester, England). Mentorship topics can include the incorporation of research into a clinical career, general career advice, and insight into international collaboration, Dr. Ramiro said.
“These are usually very well-attended meetings and very popular,” she said. “People who have participated in them always give us very good feedback and are very enthusiastic about how easily accessible these very famous key opinion leaders are and what good advice they give to them.”
Finally, the Ambassador program helps first-time attendees get the most out of EULAR. “I think that we all know that the first time we attend such a huge conference the experience can be daunting,” Dr. Ramiro said. Now in its third year, the ambassador program pairs an EMEUNET member with up to six first-timers. The ambassador helps the newcomers decide which sessions to attend and remains available through mobile phone, social media, and the meeting app throughout the meeting.
All of EMEUNET’s activities during EULAR support the organization’s aim of “widening collaboration and fostering collaboration among young researchers and clinicians,” Dr. Ramiro said. “The ultimate aim is to improve and promote education in the area of our diseases and to foster research collaborations,” she said of the 1,500-member strong organization.
Young rheumatologists and researchers will find plenty of relevant content at this year’s EULAR Congress in Madrid, June 14-17, thanks to a dedicated presentation track. Other tailored opportunities include networking events, mentorship for first-time attendees to help them make the most of their EULAR experience, and a unique opportunity for small group discussion and networking with key opinion leaders in rheumatology in the so-called mentor-mentee meetings.
The Young Rheumatologists track provides three sessions with a special focus on researchers and clinicians who are early in their careers, Sofia Ramiro, MD, PhD, explained in an interview. Dr. Ramiro chairs the steering committee of the Emerging Eular Network (EMEUNET), a network of young clinicians and researchers in the field of rheumatology in Europe.
Another session will zero in on osteoarthritis, vasculitis, spondyloarthritis, and rheumatoid arthritis, with a shorter lecture format and more time left for a question-and-answer session and discussion. With a group of younger rheumatologists in attendance, “the sessions are somewhat more informal,” promoting a comfortable and interactive environment for discussion and learning, Dr. Ramiro said.
The third session in the Young Rheumatologists track will consist of case discussions focused on how to counsel and take care of women who have rheumatoid arthritis and would like to become pregnant. Two patient cases will be presented and discussed by leaders in the field. “Again, the idea is to make these presentations as real-world as possible,” Dr. Ramiro said.
The EMEUNET booth will be in the EULAR Village, and, for the first time, the booth will be incorporated in the EULAR booth, as a “pillar” under the bigger EULAR umbrella. Dr. Ramiro said to be sure to stay tuned for a surprise associated with EULAR’s 70th anniversary. On the evening of Thursday, June 15, EMEUNET will host a networking event.
On the morning of Friday, June 16, mentor-mentee meetings organized by EMEUNET link five to six young attendees with mentors, according to area of interest. Sign up is available online, allowing small group discussion with leaders in academic rheumatology. This year, meetings will be led by Iain McInnes, PhD (Glasgow, Scotland), Josef Smolen, MD (Vienna), and William Dixon, MBBS, PhD (Manchester, England). Mentorship topics can include the incorporation of research into a clinical career, general career advice, and insight into international collaboration, Dr. Ramiro said.
“These are usually very well-attended meetings and very popular,” she said. “People who have participated in them always give us very good feedback and are very enthusiastic about how easily accessible these very famous key opinion leaders are and what good advice they give to them.”
Finally, the Ambassador program helps first-time attendees get the most out of EULAR. “I think that we all know that the first time we attend such a huge conference the experience can be daunting,” Dr. Ramiro said. Now in its third year, the ambassador program pairs an EMEUNET member with up to six first-timers. The ambassador helps the newcomers decide which sessions to attend and remains available through mobile phone, social media, and the meeting app throughout the meeting.
All of EMEUNET’s activities during EULAR support the organization’s aim of “widening collaboration and fostering collaboration among young researchers and clinicians,” Dr. Ramiro said. “The ultimate aim is to improve and promote education in the area of our diseases and to foster research collaborations,” she said of the 1,500-member strong organization.
VIDEO: Immunotherapy ups disease control rate in relapsed mesothelioma
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
CHICAGO – Early data from a phase II trial of immune checkpoint inhibitors to treat relapsed mesothelioma give hope that immunotherapy may be an effective therapeutic option for the rapidly progressive, currently incurable cancer.
Reporting on 12 weeks of data from the randomized multicenter trial, Arnaud Scherpereel, MD, the study’s first author, said in a video interview, “We were very pleased to see that we were able to increase ... the disease control rate to 44% with nivolumab, and 50% with nivolumab plus ipilimumab. This was translated into a overall survival gain for these patients.” The best previous disease control rate seen with other therapies was less than 30%, said Dr. Scherpereel at the annual meeting of the American Society of Clinical Oncology.
Discussing the early results in a video interview, Dr. Scherpereel, head of the pulmonary and thoracic oncology department at the University Hospital of Lille, France noted that the median overall survival for the nivolumab patients was 10.4 months, and has not yet been reached for the nivolumab plus ipilimumab patients. Further, he said in a press briefing, “Tumors shrunk in 18% of patients treated with nivolumab and 26% of those treated with nivolumab plus ipilimumab.”
The French MAPS-2 study has enrolled 125 adult patients with malignant pleural mesothelioma who had measurable disease progression after one or two prior lines of chemotherapy, including pemetrexed/platinum doublet. Patients were randomized 1:1 to receive either nivolumab or nivolumab plus ipilimumab, until disease control or unacceptable toxicity was reached, for a maximum of 2 years. Patients were mostly (80%) male, with a median age of 71.8 years, and most had the epithelioid malignant pleural mesothelioma subtype.
In commentary at the press briefing announcing the findings, ASCO expert Michael Sabel, MD, said, “I need to emphasize that this is amazing, in that we are seeing [the use of] checkpoint inhibitors expanding beyond melanoma, to other cancers that we thought were not amenable to immunotherapy approaches.”
“This is a great example of how basic cancer research in one field can expand across others,” said Dr. Sabel of the departments of surgery and surgical oncology at the University of Michigan, Ann Arbor.
Most side effects were not severe, but there were three potentially drug-related deaths in the nivolumab-ipilimumab combo arm: one patient died of fulminant hepatitis, one from metabolic encephalitis, and one from acute renal failure. “There is no identified factor that is predictive” in terms of which patients will have the more significant known adverse effects of checkpoint inhibitors, said Dr. Scherpereel. Patients, caregivers, and health care professionals all need to be alert to the possibility of adverse events and act promptly if concerning symptoms crop up, he said.
Dr. Scherpereel said that though his study group has not yet reported the quality of life findings from MAPS-2, he sees that his patients who are study participants are doing better. “In my patients, they have a very good tolerance to this treatment compared to chemotherapy. They have less dyspnea, less chest pain. Clearly, we hope to get these drugs into the routine very quickly for them.”
Bristol-Myers Squibb manufactures both nivolumab and ipilimumab and provided the study drugs. Dr. Sabel disclosed a financial relationship with Merck. Dr. Scherpereel has no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
[email protected]
On Twitter @karioakes
AT ASCO 2017
VIDEO: Routine genomic testing identifies actionable alterations in 52% of tumors
CHICAGO –
Molecular profiling, including genetic sequencing and copy number variation analysis, was performed in 1944 tumors from patients with advanced tumors enrolled in the profiLER study. Of the tumors screened, mutations deemed actionable were identified in 1,004 (52%), with 394 patients having two or more actionable targets, and the remainder having one identified targeted treatment. A molecular targeted treatment was recommended for 676 patients (35% of those tested).
“We showed that the patients who did receive the molecular targeted agents were doing better in terms of overall survival,” said Olivier Tredan, MD, PhD, the study’s lead investigator. Noting that these are trends as the trial was not randomized, he reported that the overall survival (OS) for those receiving targeted treatments was 53.7% at 3 years, compared with 46.1% for those who did not receive targeted treatment. The trend continued out to 5 years, with the OS for the targeted treatment group at 34.8%, compared with 28.1% OS for those who did not receive targeted treatment, he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many patients either were too sick to receive the recommended treatment or died before they could be treated, Dr. Tredan said in a video interview.
Of the patients who did receive targeted treatment, over 60% received mTOR inhibitors. The next most common therapies were multitarget tyrosine kinase receptor (TKR)–inhibiting/antiangiogenic therapies, received by about one-third of patients. Fewer than one in five patients received any other therapies. Tumor types were colorectal, gynecological, breast, head and neck carcinomas, sarcomas, and brain tumors.
A new randomized clinical study, profiLER 2, is planned. The new study will pit a 315-gene commercial test against the 69-gene test used in profiLER 1, to see whether casting a wider net yields more targets for therapy.
Still, knowing that a treatment might help is useful only if the patient can actually receive the drug, said Dr. Tredan. “What we want is more molecular targeted agent initiation, so we need to have larger screening programs, but we need also to have access to novel targeted agents.”
Dr. Tredan reported financial relationships with Bayer, GlaxoSmithKline, and Novartis.
[email protected]
On Twitter @karioakes
CHICAGO –
Molecular profiling, including genetic sequencing and copy number variation analysis, was performed in 1944 tumors from patients with advanced tumors enrolled in the profiLER study. Of the tumors screened, mutations deemed actionable were identified in 1,004 (52%), with 394 patients having two or more actionable targets, and the remainder having one identified targeted treatment. A molecular targeted treatment was recommended for 676 patients (35% of those tested).
“We showed that the patients who did receive the molecular targeted agents were doing better in terms of overall survival,” said Olivier Tredan, MD, PhD, the study’s lead investigator. Noting that these are trends as the trial was not randomized, he reported that the overall survival (OS) for those receiving targeted treatments was 53.7% at 3 years, compared with 46.1% for those who did not receive targeted treatment. The trend continued out to 5 years, with the OS for the targeted treatment group at 34.8%, compared with 28.1% OS for those who did not receive targeted treatment, he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many patients either were too sick to receive the recommended treatment or died before they could be treated, Dr. Tredan said in a video interview.
Of the patients who did receive targeted treatment, over 60% received mTOR inhibitors. The next most common therapies were multitarget tyrosine kinase receptor (TKR)–inhibiting/antiangiogenic therapies, received by about one-third of patients. Fewer than one in five patients received any other therapies. Tumor types were colorectal, gynecological, breast, head and neck carcinomas, sarcomas, and brain tumors.
A new randomized clinical study, profiLER 2, is planned. The new study will pit a 315-gene commercial test against the 69-gene test used in profiLER 1, to see whether casting a wider net yields more targets for therapy.
Still, knowing that a treatment might help is useful only if the patient can actually receive the drug, said Dr. Tredan. “What we want is more molecular targeted agent initiation, so we need to have larger screening programs, but we need also to have access to novel targeted agents.”
Dr. Tredan reported financial relationships with Bayer, GlaxoSmithKline, and Novartis.
[email protected]
On Twitter @karioakes
CHICAGO –
Molecular profiling, including genetic sequencing and copy number variation analysis, was performed in 1944 tumors from patients with advanced tumors enrolled in the profiLER study. Of the tumors screened, mutations deemed actionable were identified in 1,004 (52%), with 394 patients having two or more actionable targets, and the remainder having one identified targeted treatment. A molecular targeted treatment was recommended for 676 patients (35% of those tested).
“We showed that the patients who did receive the molecular targeted agents were doing better in terms of overall survival,” said Olivier Tredan, MD, PhD, the study’s lead investigator. Noting that these are trends as the trial was not randomized, he reported that the overall survival (OS) for those receiving targeted treatments was 53.7% at 3 years, compared with 46.1% for those who did not receive targeted treatment. The trend continued out to 5 years, with the OS for the targeted treatment group at 34.8%, compared with 28.1% OS for those who did not receive targeted treatment, he said at the annual meeting of the American Society of Clinical Oncology.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Many patients either were too sick to receive the recommended treatment or died before they could be treated, Dr. Tredan said in a video interview.
Of the patients who did receive targeted treatment, over 60% received mTOR inhibitors. The next most common therapies were multitarget tyrosine kinase receptor (TKR)–inhibiting/antiangiogenic therapies, received by about one-third of patients. Fewer than one in five patients received any other therapies. Tumor types were colorectal, gynecological, breast, head and neck carcinomas, sarcomas, and brain tumors.
A new randomized clinical study, profiLER 2, is planned. The new study will pit a 315-gene commercial test against the 69-gene test used in profiLER 1, to see whether casting a wider net yields more targets for therapy.
Still, knowing that a treatment might help is useful only if the patient can actually receive the drug, said Dr. Tredan. “What we want is more molecular targeted agent initiation, so we need to have larger screening programs, but we need also to have access to novel targeted agents.”
Dr. Tredan reported financial relationships with Bayer, GlaxoSmithKline, and Novartis.
[email protected]
On Twitter @karioakes
AT ASCO 2017