User login
Arginine deficiency implicated in novel hemorrhagic fever fatality
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
Deficiency of the amino acid arginine is implicated in the low platelet counts of severe fever with thrombocytopenia syndrome (SFTS), and a measure of global arginine bioavailability had prognostic value for mortality from the causal bunyavirus, according to a metabolomics analysis of serum from SFTS patients.
The new study also reported results from a randomized, controlled trial of intravenous arginine supplementation in SFTS; the 53 patients who received 20 g of arginine once daily had faster viral clearance than the 60 patients who received supportive care only and a placebo infusion (P = .047). Also, SFTS patients who received arginine had quicker resolution of liver transaminase elevations (P = .001).
There was no survival benefit in arginine administration, though the study’s first author, Xiao-Kun Li, MD, and colleagues noted low overall fatality rates in arginine-treated and placebo groups, at 5.7% and 8.3%, respectively.
Severe fever with thrombocytopenia syndrome is caused by a bunyavirus first identified in 2009; SFTS is being seen with increasing frequency in mainland China, Korea, Japan, and the United States. Infection with the virus “is associated with a wide clinical spectrum, with most of the patients having mild disease but more than 10% developing a fatal outcome,” wrote Dr. Li and the other researchers in Science Translational Medicine.
In the case of individuals with SFTS who fare poorly, previous work had implicated a disordered host immune response leading to severe thrombocytopenia with subsequent bleeding and disseminated intravascular coagulation, said Dr. Li and colleagues. The exact pathogenesis of this mechanism had been unknown, however, so the investigators used a metabolomics analysis on serum samples from prospectively observed SFTS patients. “[W]e determined arginine metabolism to be a key pathway that was involved in the interaction between SFTS [virus] and host response,” they wrote.
In a prospective cohort study that used liquid chromatography–tandem mass spectrometry, Dr. Li of the Beijing Institute of Microbiology and Epidemiology and colleagues examined 166 metabolites from 242 clinical samples to perform the metabolomics analysis. Of the SFTS patients in the study, 46 had both acute and convalescent samples that were matched with 46 healthy controls and 46 patients with fever not caused by SFTS. In a separate analysis, a series of samples were drawn from 10 patients who died of SFTS and matched to 10 who survived the infection and 10 healthy controls.
Statistical analyses allowed the investigators to identify metabolomics signatures that were unique for each sample group. Alteration of the arginine metabolism pathway stood out as the most pronounced differentiator in acute SFTS infection and fatality, wrote Dr. Li and coauthors. “By extracting the relative concentrations of arginine-related metabolites along the pathway, we found that arginine RC was significantly reduced in the acute phase of SFTS compared to healthy controls,” they wrote (P less than .001).
Patients who succumbed to SFTS had even lower arginine concentrations than did those who survived; arginine levels climbed during recovery for survivors, but stayed low in serum samples from SFTS fatalities.
There’s a logical mechanism by which arginine could contribute to platelet dysfunction and thrombocytopenia, noted Dr. Li and collaborators: Arginine is a nitric oxide precursor, and this pathway is known to be a potent inhibitor of platelet activation.
Low arginine levels would have the effect of taking the brakes off platelet activation, and the investigators did find increases in platelet-monocyte complexes and platelet apoptosis in SFTS virus infection (P = .007 and P less than .001, respectively), which further suggests “that platelet hyperactivation might contribute to reduced platelet counts in circulation,” they wrote.
Low arginine levels also have the effect of suppressing T-cell activity, and mediators along this pathway were also altered in patients with SFTS, and even more profoundly altered in patients who died of SFTS.
Dr. Li and colleagues probed the metabolomics data to see whether a global arginine bioavailability ratio (GABR), expressed as arginine/(ornithine + citrulline), could be used to prognosticate clinical outcome in SFTS virus infection. After multivariable analysis, they found that decreased GABR was associated with fatality (P = .039). Further, a low GABR early in infection was prognostic of later fatality, with an area under the receiver operating curve (ROC) of 0.713.
In the double-blind, randomized, placebo-controlled trial of arginine supplementation during SFTS, Dr. Li and coinvestigators found that arginine supplementation did not significantly alter most other laboratory values besides liver transaminases. However, blood urea nitrogen concentration was elevated in those who received arginine, and arginine supplementation was also associated with slightly more vomiting. Serum sampling also revealed that platelet activation and T-cell activity were both corrected in patients given arginine, which gives clues to the means by which arginine supplementation might boost host immune response and promote viral clearing and return to homeostasis of clotting pathways.
Limitations of the clinical trial included relatively small sample sizes and the fact that individuals with severe bleeding were excluded from participation in the trial. Also, the study didn’t account for dietary arginine intake, acknowledged Dr. Li and coauthors.
However, the metabolomics and clinical work taken together used state-of-the-art analytic methods and rigorous experimental design to show “the causal relationship between arginine deficiency and platelet deprivation or immunosuppression by SFTSV infection,” wrote Dr. Li and colleagues.
Disturbance in the arginine–nitric oxide pathway is likely “to be a key biochemical pathway that also plays [a] part in other viral hemorrhagic fever,” said the investigators. “The potential of arginine in treating such infectious diseases [with] similar clinical features as SFTS warrants exploration.”
The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
SOURCE: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Low arginine bioavailability was associated with increased risk for severe fever with thrombocytopenia syndrome (SFTS) fatality.
Major finding: Arginine bioavailability had an area under the receiver operating curve of 0.713 for predicting fatality.
Study details: A prospective cohort metabolomics study of 242 serum samples from patients with and without SFTS and a randomized, double-blind, placebo-controlled clinical trial of 113 patients given intravenous arginine supplementation or vehicle alone, in conjunction with supportive care.
Disclosures: The study was partially funded by a Bayer Investigator Award; Dr. Li and coauthors reported no other conflicts of interest.
Source: Li X-K et al. Sci Transl Med. doi: 10.1126/scitranslmed.aat4162.
Opioids don’t treat pain better than ibuprofen after venous ablation surgery
ST. LOUIS – Compared with ibuprofen, opioid pain medication offered little benefit for pain control after venous ablation surgery, in the experience of one surgical center.
Sharing study results at a poster session at the annual meeting of the Midwestern Vascular Surgery Society, Jana Sacco, MD, and her colleagues found that patients who received opioid prescriptions after venous ablations did not have significantly different postsurgical pain than did those who received ibuprofen alone.
The study, conducted against the national backdrop of greater scrutiny of postsurgical opioid prescribing, was the first to look at post–venous ablation pain management strategies, said Dr. Sacco, a resident physician at Henry Ford Hospital, Detroit. Venous ablation surgery can improve quality of life for patients with varicose veins, but best practices for managing postprocedure discomfort had not been clear; some patients receive opioid pain medications, while others are directed to use ibuprofen as needed for pain control.
The retrospective, single-center study assessed pre- and postoperative pain for patients undergoing venous ablation procedures over a 2-year period, said Dr. Sacco.
Patients who were prescribed opioids were compared with patients who were simply asked to take ibuprofen for pain control.
Comparing preoperative to postoperative pain scores, Dr. Sacco and her colleagues defined a change of 2-3 points on a 0-10 Likert scale as “good” improvement; a change of 1 point was defined as “mild” improvement, and no change or worsening was defined as no improvement.
Of the 268 patients for whom postoperative follow-up data were available, 142 received opioid prescriptions, while 126 did not.
Across the entire group of patients studied, those who had moderate to severe preoperative pain had significant improvement in pain after their procedures.
Whether patients received opioid pain medication after their venous ablation was not correlated with the degree of improvement in postprocedure pain scores. Of those who saw no improvement, 30 patients (45%) received opioids and 36 (55%) did not. Of the 89 patients who saw mild postprocedure improvement in pain, 35 (40%) were not discharged on opioids, and of 65 patients who had good improvement in postprocedure pain, 44% were not discharged on opioids (P = .7 for difference across groups).
When Dr. Sacco and her fellow researchers examined such patient characteristics as sex, race, body mass index, smoking status, and CEAP venous severity classification, they did not see any significant differences in pain scores. Similarly, neither the type of procedure (radiofrequency or laser ablation) nor information on whether compression treatment was used was associated with a difference in pain scores.
Dr. Sacco and her coauthors noted that the study was limited by its retrospective nature and the fact that patients were all drawn from a single institution. Additionally, the investigators were only able to ascertain whether opioids had been prescribed, not whether – or how much – medication was actually taken by patients.
“Most patients report an improvement in symptoms after undergoing vein ablation procedures,” reported Dr. Sacco and her colleagues, and most patients also do well with nonopioid pain control regimens. “Overprescribing opioids exposes patients to the risk of narcotic overdose and chronic opioid use and should be used with caution for patients undergoing vein ablation surgery,” they wrote.
Dr. Sacco reported no outside sources of funding and no conflicts of interest.
ST. LOUIS – Compared with ibuprofen, opioid pain medication offered little benefit for pain control after venous ablation surgery, in the experience of one surgical center.
Sharing study results at a poster session at the annual meeting of the Midwestern Vascular Surgery Society, Jana Sacco, MD, and her colleagues found that patients who received opioid prescriptions after venous ablations did not have significantly different postsurgical pain than did those who received ibuprofen alone.
The study, conducted against the national backdrop of greater scrutiny of postsurgical opioid prescribing, was the first to look at post–venous ablation pain management strategies, said Dr. Sacco, a resident physician at Henry Ford Hospital, Detroit. Venous ablation surgery can improve quality of life for patients with varicose veins, but best practices for managing postprocedure discomfort had not been clear; some patients receive opioid pain medications, while others are directed to use ibuprofen as needed for pain control.
The retrospective, single-center study assessed pre- and postoperative pain for patients undergoing venous ablation procedures over a 2-year period, said Dr. Sacco.
Patients who were prescribed opioids were compared with patients who were simply asked to take ibuprofen for pain control.
Comparing preoperative to postoperative pain scores, Dr. Sacco and her colleagues defined a change of 2-3 points on a 0-10 Likert scale as “good” improvement; a change of 1 point was defined as “mild” improvement, and no change or worsening was defined as no improvement.
Of the 268 patients for whom postoperative follow-up data were available, 142 received opioid prescriptions, while 126 did not.
Across the entire group of patients studied, those who had moderate to severe preoperative pain had significant improvement in pain after their procedures.
Whether patients received opioid pain medication after their venous ablation was not correlated with the degree of improvement in postprocedure pain scores. Of those who saw no improvement, 30 patients (45%) received opioids and 36 (55%) did not. Of the 89 patients who saw mild postprocedure improvement in pain, 35 (40%) were not discharged on opioids, and of 65 patients who had good improvement in postprocedure pain, 44% were not discharged on opioids (P = .7 for difference across groups).
When Dr. Sacco and her fellow researchers examined such patient characteristics as sex, race, body mass index, smoking status, and CEAP venous severity classification, they did not see any significant differences in pain scores. Similarly, neither the type of procedure (radiofrequency or laser ablation) nor information on whether compression treatment was used was associated with a difference in pain scores.
Dr. Sacco and her coauthors noted that the study was limited by its retrospective nature and the fact that patients were all drawn from a single institution. Additionally, the investigators were only able to ascertain whether opioids had been prescribed, not whether – or how much – medication was actually taken by patients.
“Most patients report an improvement in symptoms after undergoing vein ablation procedures,” reported Dr. Sacco and her colleagues, and most patients also do well with nonopioid pain control regimens. “Overprescribing opioids exposes patients to the risk of narcotic overdose and chronic opioid use and should be used with caution for patients undergoing vein ablation surgery,” they wrote.
Dr. Sacco reported no outside sources of funding and no conflicts of interest.
ST. LOUIS – Compared with ibuprofen, opioid pain medication offered little benefit for pain control after venous ablation surgery, in the experience of one surgical center.
Sharing study results at a poster session at the annual meeting of the Midwestern Vascular Surgery Society, Jana Sacco, MD, and her colleagues found that patients who received opioid prescriptions after venous ablations did not have significantly different postsurgical pain than did those who received ibuprofen alone.
The study, conducted against the national backdrop of greater scrutiny of postsurgical opioid prescribing, was the first to look at post–venous ablation pain management strategies, said Dr. Sacco, a resident physician at Henry Ford Hospital, Detroit. Venous ablation surgery can improve quality of life for patients with varicose veins, but best practices for managing postprocedure discomfort had not been clear; some patients receive opioid pain medications, while others are directed to use ibuprofen as needed for pain control.
The retrospective, single-center study assessed pre- and postoperative pain for patients undergoing venous ablation procedures over a 2-year period, said Dr. Sacco.
Patients who were prescribed opioids were compared with patients who were simply asked to take ibuprofen for pain control.
Comparing preoperative to postoperative pain scores, Dr. Sacco and her colleagues defined a change of 2-3 points on a 0-10 Likert scale as “good” improvement; a change of 1 point was defined as “mild” improvement, and no change or worsening was defined as no improvement.
Of the 268 patients for whom postoperative follow-up data were available, 142 received opioid prescriptions, while 126 did not.
Across the entire group of patients studied, those who had moderate to severe preoperative pain had significant improvement in pain after their procedures.
Whether patients received opioid pain medication after their venous ablation was not correlated with the degree of improvement in postprocedure pain scores. Of those who saw no improvement, 30 patients (45%) received opioids and 36 (55%) did not. Of the 89 patients who saw mild postprocedure improvement in pain, 35 (40%) were not discharged on opioids, and of 65 patients who had good improvement in postprocedure pain, 44% were not discharged on opioids (P = .7 for difference across groups).
When Dr. Sacco and her fellow researchers examined such patient characteristics as sex, race, body mass index, smoking status, and CEAP venous severity classification, they did not see any significant differences in pain scores. Similarly, neither the type of procedure (radiofrequency or laser ablation) nor information on whether compression treatment was used was associated with a difference in pain scores.
Dr. Sacco and her coauthors noted that the study was limited by its retrospective nature and the fact that patients were all drawn from a single institution. Additionally, the investigators were only able to ascertain whether opioids had been prescribed, not whether – or how much – medication was actually taken by patients.
“Most patients report an improvement in symptoms after undergoing vein ablation procedures,” reported Dr. Sacco and her colleagues, and most patients also do well with nonopioid pain control regimens. “Overprescribing opioids exposes patients to the risk of narcotic overdose and chronic opioid use and should be used with caution for patients undergoing vein ablation surgery,” they wrote.
Dr. Sacco reported no outside sources of funding and no conflicts of interest.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Prescribing opioids after venous ablation surgery didn’t improve pain control over ibuprofen.
Major finding:
Study details: Retrospective, single-institution study of 268 patients undergoing venous ablation surgery.
Disclosures: Dr. Sacco reported no conflicts of interest and no outside sources of funding.
Vascular programs without NIVL curriculum leave trainees feeling unprepared
ST. LOUIS – Many vascular surgery trainees felt unprepared to take the Registered Physician in Vascular Interpretation (RPVI) exam, according to a recent survey. However, trainees in a program without a structured noninvasive vascular laboratory (NIVL) curriculum felt particularly unprepared, said Daisy Chou, MD.
“There is wide variation in NIVL experience amongst vascular surgery training programs,” noted Dr. Chou, a vascular surgery fellow at the Ohio State University, Columbus. She presented survey results at the annual meeting of the Midwestern Vascular Surgical Society. The survey constructed by Dr. Chou and her colleagues went out to trainees in both 0+5 and 5+2 vascular surgery training programs in September, 2017, in 114 unique programs.
Eventually, trainees from just over half of the programs responded (N = 61 programs, 53.5%), said Dr. Chou. Using responses from individual trainees, the authors grouped programs into one of two categories: those whose trainees felt well prepared for the RPVI, and those whose trainees felt unprepared for the RPVI.
In addition to a yes/no question about preparedness, the survey also asked whether training programs had a structured curriculum; respondents were asked to identify specific NIVL-related training activities. The survey asked about individual didactic components, as well as whether the trainee spent individual time with an attending physician and hands-on time with vascular technologists. Respondents were asked about the amount of time, measured in half days per week, spent in the vascular laboratory.
Finally, the survey asked whether trainees took a pre-RPVI exam review course, and whether they passed the RPVI exam on their first attempt.
Overall, 34 of the programs with respondents (55.7%) had structured curricula; the same number included lectures. Twenty programs (32.8%) provided video content, and 29 (47.5%) used textbooks. Just 18 programs (29.5%) assigned articles.
One-on-one time spent with an attending physician and focused on NIVL techniques was reported for 32 programs (52.5%). More programs (n = 37; 60.7%) provided trainees hands-on experience with vascular technologists.
Most programs (n = 32; 52.5%) had trainees spending less than one half day per week in the vascular laboratory, according to survey respondents.
In terms of preparedness, respondents for over half of the programs did not respond to the question asking whether they felt prepared for the RPVI, presumably because they had not yet taken the exam. This, acknowledged Dr. Chou, was a significant limitation of the survey. There was a timing problem: Trainees were surveyed at the start of the 2017-2018 academic year, but the RPVI exam isn’t usually taken until the end of the final year of training, with review courses taken not long before that.
Of the 32 programs with trainees who reported taking the RPVI exam, 18 had trainees who felt unprepared, and 14 program had trainees who felt well prepared. About a quarter of programs (N = 15; 24.6%) had trainees who took a review course prior to taking the exam.
Dr. Chou and her colleagues then examined the survey responses another way, seeing what differentiated the programs whose trainees felt well prepared from those with trainees who felt unprepared.
Statistically, the clear standout was whether the program had a structured curriculum: The 14 programs with a structured curriculum all had students who reported feeling well prepared. Just one-third of the 18 programs with unprepared students had a structured curriculum, which was a significant difference (P = .0001).
Also, programs that assigned articles and those that gave formal lectures were more likely to have students who felt prepared to sit for the RPVI exam (P = .002 and .004, respectively). A higher number of programs that gave trainees hands-on time with vascular technologists had trainees who felt prepared, but the difference wasn’t quite statistically significant (P = .05).
Having taken a review course prior to the exam was associated with feeling well prepared (P = .03).
Dr. Chou and her colleagues performed a logistic regression analysis to arrive at the educational components associated with the highest odds for trainees feeling well prepared. Lectures and articles came out on top in this analysis (odds ratios for feeling well prepared, 15.88 and 15.97, respectively). Hands-on time with vascular technologists had an odds ratio of 5.12 for feeling prepared.
Taking a review course boosted preparedness as well, with an odds ratio of 11.85 for feeling well prepared for the RPVI exam. This created a bit of a conundrum for the investigators, said Dr. Chou: “All well prepared programs had a structured NIVL curriculum, but most of their trainees still took an RPVI review course, so it’s unclear if the structured curriculum or the review course is responsible for trainees feeling well prepared for the RPVI exam,” she said.
An important caveat to the analysis of survey results, said Dr. Chou, is that “It’s unknown how these results will translate into pass rates.
“Vascular surgery leadership should not leave NIVL education to review courses,” said Dr. Chou. The ultimate goal, she said, should be to achieve expertise in the service of providing better patient care. To this end, Dr. Chou and her coauthors recommend that a structured NIVL curriculum be incorporated into vascular surgery training, and that the program include time spent with vascular technologists, a formal lecture-based component, and structured reading, as is provided by a journal club.
Dr. Chou reported no conflicts of interest, and no external sources of funding.
ST. LOUIS – Many vascular surgery trainees felt unprepared to take the Registered Physician in Vascular Interpretation (RPVI) exam, according to a recent survey. However, trainees in a program without a structured noninvasive vascular laboratory (NIVL) curriculum felt particularly unprepared, said Daisy Chou, MD.
“There is wide variation in NIVL experience amongst vascular surgery training programs,” noted Dr. Chou, a vascular surgery fellow at the Ohio State University, Columbus. She presented survey results at the annual meeting of the Midwestern Vascular Surgical Society. The survey constructed by Dr. Chou and her colleagues went out to trainees in both 0+5 and 5+2 vascular surgery training programs in September, 2017, in 114 unique programs.
Eventually, trainees from just over half of the programs responded (N = 61 programs, 53.5%), said Dr. Chou. Using responses from individual trainees, the authors grouped programs into one of two categories: those whose trainees felt well prepared for the RPVI, and those whose trainees felt unprepared for the RPVI.
In addition to a yes/no question about preparedness, the survey also asked whether training programs had a structured curriculum; respondents were asked to identify specific NIVL-related training activities. The survey asked about individual didactic components, as well as whether the trainee spent individual time with an attending physician and hands-on time with vascular technologists. Respondents were asked about the amount of time, measured in half days per week, spent in the vascular laboratory.
Finally, the survey asked whether trainees took a pre-RPVI exam review course, and whether they passed the RPVI exam on their first attempt.
Overall, 34 of the programs with respondents (55.7%) had structured curricula; the same number included lectures. Twenty programs (32.8%) provided video content, and 29 (47.5%) used textbooks. Just 18 programs (29.5%) assigned articles.
One-on-one time spent with an attending physician and focused on NIVL techniques was reported for 32 programs (52.5%). More programs (n = 37; 60.7%) provided trainees hands-on experience with vascular technologists.
Most programs (n = 32; 52.5%) had trainees spending less than one half day per week in the vascular laboratory, according to survey respondents.
In terms of preparedness, respondents for over half of the programs did not respond to the question asking whether they felt prepared for the RPVI, presumably because they had not yet taken the exam. This, acknowledged Dr. Chou, was a significant limitation of the survey. There was a timing problem: Trainees were surveyed at the start of the 2017-2018 academic year, but the RPVI exam isn’t usually taken until the end of the final year of training, with review courses taken not long before that.
Of the 32 programs with trainees who reported taking the RPVI exam, 18 had trainees who felt unprepared, and 14 program had trainees who felt well prepared. About a quarter of programs (N = 15; 24.6%) had trainees who took a review course prior to taking the exam.
Dr. Chou and her colleagues then examined the survey responses another way, seeing what differentiated the programs whose trainees felt well prepared from those with trainees who felt unprepared.
Statistically, the clear standout was whether the program had a structured curriculum: The 14 programs with a structured curriculum all had students who reported feeling well prepared. Just one-third of the 18 programs with unprepared students had a structured curriculum, which was a significant difference (P = .0001).
Also, programs that assigned articles and those that gave formal lectures were more likely to have students who felt prepared to sit for the RPVI exam (P = .002 and .004, respectively). A higher number of programs that gave trainees hands-on time with vascular technologists had trainees who felt prepared, but the difference wasn’t quite statistically significant (P = .05).
Having taken a review course prior to the exam was associated with feeling well prepared (P = .03).
Dr. Chou and her colleagues performed a logistic regression analysis to arrive at the educational components associated with the highest odds for trainees feeling well prepared. Lectures and articles came out on top in this analysis (odds ratios for feeling well prepared, 15.88 and 15.97, respectively). Hands-on time with vascular technologists had an odds ratio of 5.12 for feeling prepared.
Taking a review course boosted preparedness as well, with an odds ratio of 11.85 for feeling well prepared for the RPVI exam. This created a bit of a conundrum for the investigators, said Dr. Chou: “All well prepared programs had a structured NIVL curriculum, but most of their trainees still took an RPVI review course, so it’s unclear if the structured curriculum or the review course is responsible for trainees feeling well prepared for the RPVI exam,” she said.
An important caveat to the analysis of survey results, said Dr. Chou, is that “It’s unknown how these results will translate into pass rates.
“Vascular surgery leadership should not leave NIVL education to review courses,” said Dr. Chou. The ultimate goal, she said, should be to achieve expertise in the service of providing better patient care. To this end, Dr. Chou and her coauthors recommend that a structured NIVL curriculum be incorporated into vascular surgery training, and that the program include time spent with vascular technologists, a formal lecture-based component, and structured reading, as is provided by a journal club.
Dr. Chou reported no conflicts of interest, and no external sources of funding.
ST. LOUIS – Many vascular surgery trainees felt unprepared to take the Registered Physician in Vascular Interpretation (RPVI) exam, according to a recent survey. However, trainees in a program without a structured noninvasive vascular laboratory (NIVL) curriculum felt particularly unprepared, said Daisy Chou, MD.
“There is wide variation in NIVL experience amongst vascular surgery training programs,” noted Dr. Chou, a vascular surgery fellow at the Ohio State University, Columbus. She presented survey results at the annual meeting of the Midwestern Vascular Surgical Society. The survey constructed by Dr. Chou and her colleagues went out to trainees in both 0+5 and 5+2 vascular surgery training programs in September, 2017, in 114 unique programs.
Eventually, trainees from just over half of the programs responded (N = 61 programs, 53.5%), said Dr. Chou. Using responses from individual trainees, the authors grouped programs into one of two categories: those whose trainees felt well prepared for the RPVI, and those whose trainees felt unprepared for the RPVI.
In addition to a yes/no question about preparedness, the survey also asked whether training programs had a structured curriculum; respondents were asked to identify specific NIVL-related training activities. The survey asked about individual didactic components, as well as whether the trainee spent individual time with an attending physician and hands-on time with vascular technologists. Respondents were asked about the amount of time, measured in half days per week, spent in the vascular laboratory.
Finally, the survey asked whether trainees took a pre-RPVI exam review course, and whether they passed the RPVI exam on their first attempt.
Overall, 34 of the programs with respondents (55.7%) had structured curricula; the same number included lectures. Twenty programs (32.8%) provided video content, and 29 (47.5%) used textbooks. Just 18 programs (29.5%) assigned articles.
One-on-one time spent with an attending physician and focused on NIVL techniques was reported for 32 programs (52.5%). More programs (n = 37; 60.7%) provided trainees hands-on experience with vascular technologists.
Most programs (n = 32; 52.5%) had trainees spending less than one half day per week in the vascular laboratory, according to survey respondents.
In terms of preparedness, respondents for over half of the programs did not respond to the question asking whether they felt prepared for the RPVI, presumably because they had not yet taken the exam. This, acknowledged Dr. Chou, was a significant limitation of the survey. There was a timing problem: Trainees were surveyed at the start of the 2017-2018 academic year, but the RPVI exam isn’t usually taken until the end of the final year of training, with review courses taken not long before that.
Of the 32 programs with trainees who reported taking the RPVI exam, 18 had trainees who felt unprepared, and 14 program had trainees who felt well prepared. About a quarter of programs (N = 15; 24.6%) had trainees who took a review course prior to taking the exam.
Dr. Chou and her colleagues then examined the survey responses another way, seeing what differentiated the programs whose trainees felt well prepared from those with trainees who felt unprepared.
Statistically, the clear standout was whether the program had a structured curriculum: The 14 programs with a structured curriculum all had students who reported feeling well prepared. Just one-third of the 18 programs with unprepared students had a structured curriculum, which was a significant difference (P = .0001).
Also, programs that assigned articles and those that gave formal lectures were more likely to have students who felt prepared to sit for the RPVI exam (P = .002 and .004, respectively). A higher number of programs that gave trainees hands-on time with vascular technologists had trainees who felt prepared, but the difference wasn’t quite statistically significant (P = .05).
Having taken a review course prior to the exam was associated with feeling well prepared (P = .03).
Dr. Chou and her colleagues performed a logistic regression analysis to arrive at the educational components associated with the highest odds for trainees feeling well prepared. Lectures and articles came out on top in this analysis (odds ratios for feeling well prepared, 15.88 and 15.97, respectively). Hands-on time with vascular technologists had an odds ratio of 5.12 for feeling prepared.
Taking a review course boosted preparedness as well, with an odds ratio of 11.85 for feeling well prepared for the RPVI exam. This created a bit of a conundrum for the investigators, said Dr. Chou: “All well prepared programs had a structured NIVL curriculum, but most of their trainees still took an RPVI review course, so it’s unclear if the structured curriculum or the review course is responsible for trainees feeling well prepared for the RPVI exam,” she said.
An important caveat to the analysis of survey results, said Dr. Chou, is that “It’s unknown how these results will translate into pass rates.
“Vascular surgery leadership should not leave NIVL education to review courses,” said Dr. Chou. The ultimate goal, she said, should be to achieve expertise in the service of providing better patient care. To this end, Dr. Chou and her coauthors recommend that a structured NIVL curriculum be incorporated into vascular surgery training, and that the program include time spent with vascular technologists, a formal lecture-based component, and structured reading, as is provided by a journal club.
Dr. Chou reported no conflicts of interest, and no external sources of funding.
REPORTING FROM MIDWESTERN VASCULAR 2018
Key clinical point: Many vascular surgery trainees do not feel prepared to take the RPVI exam.
Major finding: Lectures and textbook reading were highly associated with feeling prepared (P = .002 and .004, respectively).
Study details: Survey of trainees in 114 vascular surgery training programs.
Disclosures: The author reported no outside sources of funding, and no conflicts of interest.
NHLBI commits to a sickle cell cure
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
“We have new exigency and intensity of effort to enable curative strategies for sickle cell disease to move forward,” said W. Keith Hoots, MD, the director of the division of blood diseases at NHLBI.
The key word in the cure effort is partnership – whether it’s among federal agencies, with public and private organizations, or with patients and families.
“Developmental strategies are built on partnerships to enhance care and accelerate cure for sickle cell disease in the U.S. and worldwide,” Dr. Hoots said at the 12th annual symposium of the Foundation for Sickle Cell Disease Research in Washington.
The reach also extends internationally. Supporting research in sub-Saharan Africa has promised to accelerate the clinical trial process by bringing advanced research capabilities to a region with a very high per capita rate of SCD. While in the United States, infrastructure is being built for a future research network, with the goal of developing a secure database of shared elements that harmonize and unite existing data.
Future cohort studies, enhanced newborn screening, and higher uptake of hydroxyurea will all be supported as part of this effort, Dr. Hoots said.
In the United States, patients can participate in a meaningful way as citizen-scientists, as new technology makes it possible to crowdsource high-quality data collection securely.
And including both community organizations and primary care providers in the “circle of partners” means not only that advances are brought out to patients expeditiously but also that the voices of patients and families have a clear channel back to researchers and policy makers through formal patient engagement and lay participation at all levels, Dr. Hoots said.
“The number of presently interested partners may surprise you,” Dr. Hoots said.
This multifaceted approach allows for “multiple shots on goal, with the acceptance that there could potentially be some failures,” Dr. Hoots said. Keeping all players better connected, though, should allow efforts to be redirected when needed, with a particular focus on accelerating work toward genetic therapies for SCD.
Perhaps the flagship effort is the Cure Sickle Cell Disease Initiative, a new partnership focused on accelerating cure-focused SCD research by filling in gaps left in the network of other funding strategies.
NHLBI named Edward J. Benz Jr., MD, the president and CEO emeritus of Boston’s Dana-Farber Cancer Institute, as the executive director and the Emmes Corporation, a contract research organization with expertise in clinical trials, as the coordinating center.
Traveling the last mile
New strategies also need to focus on how to boost uptake of such currently available best practices in SCD treatment as hydroxyurea use. To that end, Dr. Hoots said, NHLBI is drawing on implementation science, a discipline that, in a medical setting, can help solve such “last-mile” problems as bringing best practices in SCD treatment to patients.
In clinical practice, this might look like solving transportation issues for family members so that appointments aren’t missed and hydroxyurea prescriptions are filled. For researchers, implementation science can help with thorny details of participant recruitment and retention.
Established in 2016, the Sickle Cell Disease Implementation Consortium comprises nine U.S. research centers and NHLBI, which are each seeking to recruit at least 300 participants with SCD, aged 15-45 years, to study effective identification of barriers to care, and the best means to overcome them.
However, Dr. Hoots said, NHLBI also will continue funding SCD research through the traditional investigator-initiated application process, in conjunction with “a suite of specialized programs that can support translational and clinical research in SCD.”
Some of the features rolling out within the Cure SCD Initiative are included in direct response to stakeholder feedback about pressing needs and top priorities. For example, an economic case needed to be made in order for insurance companies, public and private alike, to reimburse for genetic SCD treatments. This requires an understanding of the lifetime cost burden of SCD, as well as determining what the long-term follow-up of costs of gene therapy will be.
Patients, family members and those providing primary care for SCD patients all agreed that clinical trials should have endpoints that reflect meaningful outcomes for patients and should be designed with the input of both patients and providers.
When queried, sickle cell disease researchers expressed a need to identify common data elements in SCD research, and wished for a secure yet accessible national data warehouse for data from gene and cell therapy trials.
At present, there are three clinical trials of curative stem cell approaches for SCD registered with the Blood and Marrow Transplant Clinical Trials Network and several more early phase clinical trials underway, Dr. Hoots said. A primary focus is the use of autologous cells for genomic editing, gene therapy, and erythroid-specific vectors.
Genetic research
As an example of the new collaboration, research centers and biotechnology companies sent their cell and genetic therapy experts to an NIH-sponsored gathering in March 2017. By pooling expertise in this way, the group was able to “identify some unprecedented opportunities, as well as some necessary barriers to overcome,” he said. These players continue to collaborate in the ongoing clinical trials of novel – and potentially curative – SCD therapies.
The TOPMed (Trans-Omics for Precision Medicine) program is a key mechanism to support SCD-related genetic research. For example, Dr. Hoots said, TOPMed is being used in support of whole-genome sequencing in a longitudinal cohort of patients with SCD who receive transfusion care at four large centers in Brazil.
These renewed efforts, set against the backdrop of paradigm-shifting genetic therapies, represent new promise for a generation of individuals with SCD, Dr. Hoots said. “It takes all of us to address the SCD challenge.”
ASH initiatives
NHLBI isn’t alone in making SCD a priority. The American Society of Hematology also is putting a spotlight on the condition.
The ASH multifaceted sickle cell disease (SCD) initiative addresses the disease burden both within the United States and globally, said LaTasha Lee, PhD, senior manager of sickle cell disease policy and programs for ASH.
Speaking at the 12th annual symposium of the Foundation for Sickle Cell Disease Research, Dr. Lee said that four prongs make up the initiative: disease research, attention to global issues, a renewed focus on access to care in the United States, and work to develop ASH’s new SCD guidelines.
New guidelines on the management of acute and chronic complications of SCD are in the works, with an anticipated 2019 date for publication of five separate guidelines. Topics covered in the guidelines will include pain, cerebrovascular disease, cardiopulmonary and kidney disease, transfusion support, and stem cell transplantation.
UN aims to eradicate TB by 2030
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.
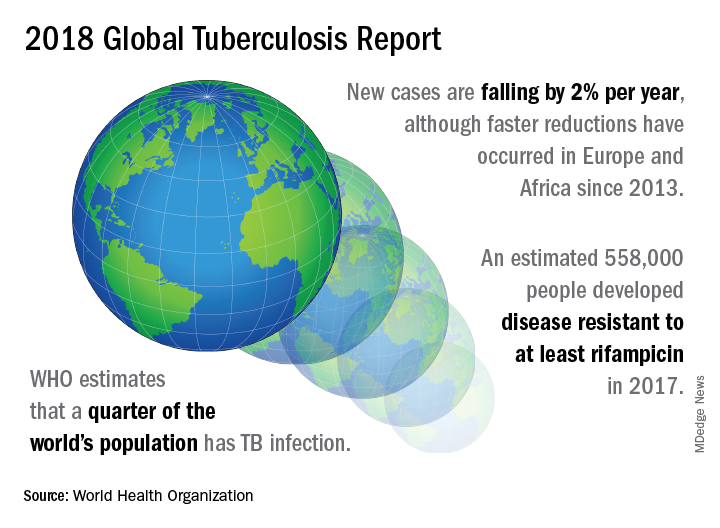
On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
A concerted a lethal disease affecting one-quarter of the world’s population by the year 2030.

On September 26 the United Nations General Assembly will convene a high-level meeting of global stakeholders to solidify the eradication plan, addressing the global crisis of tuberculosis (TB), the world’s most deadly infectious disease.
“We must seize the moment,” said Tereza Kasaeva, MD, director of the World Health Organization’s global TB program, speaking at a telebriefing and press conference accompanying the release of the World Health Organization’s annual global tuberculosis report. “It’s unacceptable in the 21st century that millions lose their lives to this preventable and curable disease.”
TB caused 1.6 million deaths globally in 2017, and the World Health Organization (WHO) estimates that of the 10 million new cases of TB last year, 558,000 are multi-drug resistant (MDR) infections.
Though death rates and new cases are falling globally each year, significantly more resources are needed to boost access to preventive treatment for latent TB infection; “Most people needing it are not yet accessing care,” according to the press briefing accompanying the report.
A review and commentary on TB incubation and latency published in BMJ (2018;362:k2738 doi: 10.1136/bmj.k2738; e-pub 23 Aug 2018) has called into question the focus preventive treatment of latent cases at the expense of reaching those most likely to die from TB (e.g., HIV patients, children of individuals living with active TB). The authors state that “latent” TB is identified by indirect evidence of present or past infection with Mycobacterium tuberculosis as inferred by a detectable adaptive immune response to M tuberculosis antigens. Active TB infection is overwhelmingly the result of a primary infection and almost always occurs within two years.
In order to meet the ambitious goal of TB eradication by the year 2030, treatment coverage must rise to 90% globally from the current 64%, according to the report.
Progress in southern Africa and in the Russian Federation, where efforts have led to a 30% reduction in TB mortality and a decrease in incidence of 5% per year, show that steep reductions in TB are possible when resources are brought to bear on the problem, said Dr. Kasaeva. “We should acknowledge that actions in some countries and regions show that progress can accelerate,” she said. Still, she noted, “Four thousand lives per day are lost to TB. Tuberculosis is the leading killer of people living with HIV, and the major cause of deaths related to antimicrobial resistance” at a global level.
Two thirds of all TB cases occur in eight countries, with India, China, and Indonesia leading this group. About half of the cases of MDR TB occur in India, China, and Russia, said Dr. Kasaeva, and globally only one in four individuals with MDR TB who need access to treatment have received it. “We need to urgently tackle the multidrug resistant TB public health crisis,” she said.
Major impediments to successful public health efforts against TB are underdiagnosis and underreporting: It is estimated that 3.6 million of 2017’s 10 million new cases were not officially recorded or reported. Countries where these problems are most serious include India, Indonesia, and Nigeria. Fewer than half of the children with TB are reported globally, according to the report.
People living with HIV/AIDS who are also infected with TB number nearly 1,000,000, but only about half of these were officially reported in 2017.
In terms of prevention priorities, WHO has recommended targeting treatment of latent TB in two groups: People living with HIV/AIDS, and children under the age of 5 years who live in households with TB-infected individuals.
“To enable these actions,” said Dr. Kasaeva, “we need strengthened commitments not just for TB care, but for overall health services. So the aim for universal coverage is real.” Underreporting is particularly prevalent in lower income countries with large unregulated private sectors, she said, though India and Indonesia have taken corrective steps to increase reporting.
A meaningful global initiative will not come cheap: The current annual shortfall in funding for TB prevention, diagnosis, and treatment is about $3.5 billion. By the year 2022, the gap between funding and what’s needed to stay on track for the 2030 target will be over $6 billion, said Dr. Kasaeva.
The best use of increased resources for TB eradication will be in locally focused efforts, said Irene Koek, MD, the United States Agency for International Development’s deputy administrator for global health. “It is likely that each region requires a tailored response.” Further, “to improve quality of care we need to ensure that services are patient centered,” she said at the press conference.
To that end, Dr. Koek expects that at the upcoming high-level meeting, the United Nations member states will be called on to develop an open framework, with clear accountability for monitoring and reviewing progress. The road forward should “celebrate accomplishments and acknowledge shortcomings,” she said. Some recent studies have shown that treatment success rates above 80% for patients with MDR TB can be achieved.
“Lessons learned from these experiences should be documented and shared in order to replicate success globally,” said Dr. Koek.
The United States, said Dr. Koek, is the leading global investor in TB research and treatment. “We welcome increased partnerships, especially with countries with the highest burden, to end global suffering from this disease.”
Eric Goosby, MD, the United Nations special envoy on TB, used his speaking time to lend some perspective to the social framework around TB’s longtime lethality.
There are aspects of TB infection that differentiate it from HIV/AIDS, said Dr. Goosby, who has spent most of his clinical and public health career on HIV/AIDS treatment and prevention. In contrast to an infection that at present requires a lifetime of treatment, TB can ordinarily be treated in 6 months, making it an unpleasant episode that an individual may be eager to move past. Additionally, the fact that TB has had a “hold on the world since the time of the ancient Egyptians” may paradoxically have served to lessen urgency in research and treatment efforts, he noted.
Dr. Goosby also spoke of the stigma surrounding TB, whose sufferers are likely to be facing dire poverty, malnutrition, and other infectious disease burdens. Civil society concerned with TB, he said, has spoken up “for those without a voice, for those who have difficulty advocating for themselves.”
Dr. Kasaeva agreed, noting that TB “affects the poorest of the poor, which makes it extraordinarily difficult for activism to come from that population.”
However, others have spoken for those affected, said Dr. Goosby. “The TB civil society has put its heart and soul this last year into gathering political will from leaders around the world…. It’s not a passive effort; it involves a lot of work.” During the past year of concerted effort, he said, “All of us have known the difficulty of pushing a political leader up that learning curve.”
As the upcoming high-level meeting approaches, those who have been working on the effort can feel the momentum, said Dr. Goosby. Still, he noted, “While there’s a significant step forward, this is not the time for a victory dance. This is really the time for a reflection...Do we understand the burden in our respective countries, and has the response been adequate?”
The goal for the meeting is to have leaders “step up to commit, not for one day, or for one meeting, but for the duration of the effort,” said Dr. Goosby. “We must make sure that the words that we hear next week from our leaders translate into action...Next week the world will say, ‘No more. No longer. No one is immune to TB. Tuberculosis is preventable; tuberculosis is treatable; tuberculosis is curable.’”
The BMJ commentary, by Marcel A. Behr, MD, of McGill International TB Centre, Infectious Diseases and Immunity in Global Health Program, McGill University Health Centre Research Institute, and his colleagues, recommend caution when building a prevention strategy around treating many millions of individuals with “latent” TB. They wrote, “Immunoreactivity to TB does not necessarily indicate the presence of live bacteria, as reactivity can persist after infection has been cleared. Classifying two billion people with evidence of immunoreactivity as having latent TB infection may divert fundamental research and public health interventions away from transmissible active TB disease and newly infected people at highest risk of progression to disease.”
This story was updated on 09/24/2018
FROM A WORLD HEALTH ORGANIZATION PRESS CONFERENCE
Severe maternal morbidity climbs; racial disparities persist
The rate of severe maternal morbidity in the United States has climbed steadily since 2006, increasing 45% overall in the decade ending in 2015, according to a new report from the Agency for Healthcare Research and Quality that also found large ethnic and racial, geographic, and socioeconomic variation in rates of severe maternal morbidity.
The longstanding increased risk for severe maternal morbidity for black women, compared with white women, continued essentially unchanged, with black women 112%-115% more likely to experience any of 21 conditions and procedures that defined severe maternal morbidity in the report. Disparities also existed between white women and those of Hispanic or Asian/Pacific Islander origin, but those gaps are narrowing, according to the report.
“Black women, Hispanic women, and women of other races/ethnicities were overrepresented among deliveries involving severe maternal morbidity, as compared with white women,” wrote Kathryn Fingar, PhD, and her coauthors. “White women constituted a lower percentage of deliveries with any severe maternal morbidity than they did other deliveries” – 23% lower.
The 21 indicators, developed by the Centers for Disease Control and Prevention, range from conditions such as renal failure and sepsis to in-hospital procedures such as blood transfusion and hysterectomy. Women were considered to have severe maternal morbidity if any of the indicators were present, regardless of whether in-hospital death occurred.

Dr. Fingar of IBM Watson Health, Cambridge, Mass., and her collaborators summarized data from AHRQ’s Healthcare Cost and Utilization Project (H-CUP) in the statistical brief. The two most common procedures that indicate severe maternal morbidity are blood transfusion and hysterectomy, and these indicators were tracked in deliveries where women had a condition that served as one of the severe maternal morbidity indicators.
Dr. Fingar and her colleagues noted that they excluded data for the final quarter of 2015, because that is when the transition from the 9th to the 10th edition of the International Classifications of Diseases was made.
In addition to the overall increase from 101.3 to 146.6 women per 10,000 experiencing any severe maternal morbidity, the incidence of blood transfusion during a delivery hospitalization, either alone or in conjunction with other indicators, rose from 78.9 to 121.1 women per 10,000, an increase of 54%.
Showing that increased blood transfusions were a major driver of the jump in severe maternal morbidity, the composite increase in the rates of all other indicators went from 34 to 42 per delivery hospitalization, an increase of just 24%, or less than half the increase in blood transfusions.
There was significant variation in trends over time for the rates of the other indicators: acute renal failure, shock, the need for mechanical ventilation, and sepsis all increased by at least 100% (range, 104%-134%), and the rates of aneurysm increased by 99%. Rates of other indicators fell; pulmonary edema, embolism, eclampsia, myocardial infarction, cerebrovascular disorders, serious anesthesia complications, and intraoperative heart failure and arrest all declined by 25%-53% during the data-gathering period.
Looking at the data another way, 78% of cases of severe maternal morbidity in 2006 and 83% in 2015 involved a blood transfusion, making it the most common indicator. Far fewer delivery hospitalizations involved disseminated intravascular coagulation (DIC) and hysterectomy, the next most common indicators, which were seen in just 8% of cases.
Blood transfusions were most likely in women with shock (72%), amniotic fluid embolism (63%), sickle cell disease with crisis (54%), and DIC (51%).
One in three women who experienced shock during delivery had a hysterectomy, as did more than 20% of women who experienced adult respiratory distress syndrome or cardiac arrest (or ventricular fibrillation).
Women delivering at the youngest and oldest ends of the age spectrum were more likely to experience severe maternal morbidity. For women younger than 20 years of age, the rate was 206 per 10,000. In the group of women aged 40 years and over, the rate of any severe maternal morbidity was 248 per 10,000 delivery hospitalizations. Women in their 20s and 30s had rates of 136 and 143 per 10,000, respectively.
Besides race/ethnicity and age, a variety of other patient characteristics were associated with increased rates of severe maternal morbidity and mortality, with higher rates seen in women from the poorest quartile than the wealthiest (177 versus 122 per 10,000, respectively). Women with Medicaid were more likely than those with private insurance to experience severe maternal morbidity during a delivery hospitalization (175 versus 121 per 10,000, respectively).
Similarly, severe maternal morbidity was more common in safety net hospitals and in minority-serving hospitals (182 versus 128 and 176 versus 123 per 10,000, respectively) than other hospitals.
Regionally, severe maternal morbidity was most common in the Northeast and the South, at 165 and 164 per 10,000, respectively, compared with rates of 132 and 116 in the West and Midwest, respectively.
Hospital deaths per 100,000 delivery hospitalizations were 248% higher for black than white women in 2006. By the end of 2015, that figure declined modestly to 193%, with absolute rates of 19 versus 5.5 deaths per 100,000 delivery hospitalizations for black versus white women in 2006. In 2015, the absolute rates were 11 versus 4 per 100,000.
The study was conducted by the Agency for Healthcare Research and Quality. There were no reported conflicts of interest.
SOURCE: Fingar K et al. Agency for Healthcare Research and Quality Statistical Brief #243.
The data here confirm, again, that we have a crisis in maternal health care in the United States. Not only is severe maternal morbidity increasing, but disparities in outcomes between white and black women persist, such that severe maternal morbidity is twice as common among black women as it is among white women.
It’s tempting to attribute the gap to social determinants of health that are outside the control of the obstetrician-gynecologist. But education doesn’t protect black women: the New York City Department of Health and Mental Hygiene found that non-Hispanic black women with a college degree have higher rates of severe maternal morbidity than women of other race/ethnicities who never graduated high school. It’s a stark finding that’s illustrated by the heartbreaking story of Shalon Irving, a PhD epidemiologist at the Centers for Disease Control and Prevention, whose postpartum death was reported by ProPublica last winter.
One contributing factor is likely to be implicit biases that affect how well we see and hear patients who are different from us. I was mortified to see my own implicit biases when I took the Project Implicit Social Attitudes test (https://implicit.harvard.edu/implicit/). These aren’t the beliefs that we choose – they are the patterns that we absorb from our day-to-day lives. If you search Google images for “pregnancy,” “motherhood,” or “breastfeeding,” the overwhelming majority of images feature white women, whereas the phrase “welfare queen” is used to denigrate the reproduction of women of color. That’s the world we live in, and these patterns reinforce mental short cuts that are more likely to drive our behavior when we’re pressed for time, as we are every day in clinical practice. In one study in a pediatric emergency department, the busier the shift, the higher the providers’ implicit bias at the end of the shift.
We might start by looking at the images on our web sites and hanging in our offices and hospital corridors – do they look like the women we care for? Do they celebrate pregnancy and parenthood for diverse families? And how can we make sure that we pause, before we walk into a patient’s room, so that we can be fully present to her individual strengths and vulnerabilities, and tailor our care to her as an individual?
Alison Stuebe, MD, of the University of North Carolina, Chapel Hill, is medical director of lactation services and on the steering committee for Moms Rising North Carolina, the Breastfeeding Expert Work Group for the American College of Obstetricians and Gynecologists, and a board member of the Society for Maternal-Fetal Medicine. She was asked to comment on the AHRQ report by this newspaper.
The data here confirm, again, that we have a crisis in maternal health care in the United States. Not only is severe maternal morbidity increasing, but disparities in outcomes between white and black women persist, such that severe maternal morbidity is twice as common among black women as it is among white women.
It’s tempting to attribute the gap to social determinants of health that are outside the control of the obstetrician-gynecologist. But education doesn’t protect black women: the New York City Department of Health and Mental Hygiene found that non-Hispanic black women with a college degree have higher rates of severe maternal morbidity than women of other race/ethnicities who never graduated high school. It’s a stark finding that’s illustrated by the heartbreaking story of Shalon Irving, a PhD epidemiologist at the Centers for Disease Control and Prevention, whose postpartum death was reported by ProPublica last winter.
One contributing factor is likely to be implicit biases that affect how well we see and hear patients who are different from us. I was mortified to see my own implicit biases when I took the Project Implicit Social Attitudes test (https://implicit.harvard.edu/implicit/). These aren’t the beliefs that we choose – they are the patterns that we absorb from our day-to-day lives. If you search Google images for “pregnancy,” “motherhood,” or “breastfeeding,” the overwhelming majority of images feature white women, whereas the phrase “welfare queen” is used to denigrate the reproduction of women of color. That’s the world we live in, and these patterns reinforce mental short cuts that are more likely to drive our behavior when we’re pressed for time, as we are every day in clinical practice. In one study in a pediatric emergency department, the busier the shift, the higher the providers’ implicit bias at the end of the shift.
We might start by looking at the images on our web sites and hanging in our offices and hospital corridors – do they look like the women we care for? Do they celebrate pregnancy and parenthood for diverse families? And how can we make sure that we pause, before we walk into a patient’s room, so that we can be fully present to her individual strengths and vulnerabilities, and tailor our care to her as an individual?
Alison Stuebe, MD, of the University of North Carolina, Chapel Hill, is medical director of lactation services and on the steering committee for Moms Rising North Carolina, the Breastfeeding Expert Work Group for the American College of Obstetricians and Gynecologists, and a board member of the Society for Maternal-Fetal Medicine. She was asked to comment on the AHRQ report by this newspaper.
The data here confirm, again, that we have a crisis in maternal health care in the United States. Not only is severe maternal morbidity increasing, but disparities in outcomes between white and black women persist, such that severe maternal morbidity is twice as common among black women as it is among white women.
It’s tempting to attribute the gap to social determinants of health that are outside the control of the obstetrician-gynecologist. But education doesn’t protect black women: the New York City Department of Health and Mental Hygiene found that non-Hispanic black women with a college degree have higher rates of severe maternal morbidity than women of other race/ethnicities who never graduated high school. It’s a stark finding that’s illustrated by the heartbreaking story of Shalon Irving, a PhD epidemiologist at the Centers for Disease Control and Prevention, whose postpartum death was reported by ProPublica last winter.
One contributing factor is likely to be implicit biases that affect how well we see and hear patients who are different from us. I was mortified to see my own implicit biases when I took the Project Implicit Social Attitudes test (https://implicit.harvard.edu/implicit/). These aren’t the beliefs that we choose – they are the patterns that we absorb from our day-to-day lives. If you search Google images for “pregnancy,” “motherhood,” or “breastfeeding,” the overwhelming majority of images feature white women, whereas the phrase “welfare queen” is used to denigrate the reproduction of women of color. That’s the world we live in, and these patterns reinforce mental short cuts that are more likely to drive our behavior when we’re pressed for time, as we are every day in clinical practice. In one study in a pediatric emergency department, the busier the shift, the higher the providers’ implicit bias at the end of the shift.
We might start by looking at the images on our web sites and hanging in our offices and hospital corridors – do they look like the women we care for? Do they celebrate pregnancy and parenthood for diverse families? And how can we make sure that we pause, before we walk into a patient’s room, so that we can be fully present to her individual strengths and vulnerabilities, and tailor our care to her as an individual?
Alison Stuebe, MD, of the University of North Carolina, Chapel Hill, is medical director of lactation services and on the steering committee for Moms Rising North Carolina, the Breastfeeding Expert Work Group for the American College of Obstetricians and Gynecologists, and a board member of the Society for Maternal-Fetal Medicine. She was asked to comment on the AHRQ report by this newspaper.
The rate of severe maternal morbidity in the United States has climbed steadily since 2006, increasing 45% overall in the decade ending in 2015, according to a new report from the Agency for Healthcare Research and Quality that also found large ethnic and racial, geographic, and socioeconomic variation in rates of severe maternal morbidity.
The longstanding increased risk for severe maternal morbidity for black women, compared with white women, continued essentially unchanged, with black women 112%-115% more likely to experience any of 21 conditions and procedures that defined severe maternal morbidity in the report. Disparities also existed between white women and those of Hispanic or Asian/Pacific Islander origin, but those gaps are narrowing, according to the report.
“Black women, Hispanic women, and women of other races/ethnicities were overrepresented among deliveries involving severe maternal morbidity, as compared with white women,” wrote Kathryn Fingar, PhD, and her coauthors. “White women constituted a lower percentage of deliveries with any severe maternal morbidity than they did other deliveries” – 23% lower.
The 21 indicators, developed by the Centers for Disease Control and Prevention, range from conditions such as renal failure and sepsis to in-hospital procedures such as blood transfusion and hysterectomy. Women were considered to have severe maternal morbidity if any of the indicators were present, regardless of whether in-hospital death occurred.

Dr. Fingar of IBM Watson Health, Cambridge, Mass., and her collaborators summarized data from AHRQ’s Healthcare Cost and Utilization Project (H-CUP) in the statistical brief. The two most common procedures that indicate severe maternal morbidity are blood transfusion and hysterectomy, and these indicators were tracked in deliveries where women had a condition that served as one of the severe maternal morbidity indicators.
Dr. Fingar and her colleagues noted that they excluded data for the final quarter of 2015, because that is when the transition from the 9th to the 10th edition of the International Classifications of Diseases was made.
In addition to the overall increase from 101.3 to 146.6 women per 10,000 experiencing any severe maternal morbidity, the incidence of blood transfusion during a delivery hospitalization, either alone or in conjunction with other indicators, rose from 78.9 to 121.1 women per 10,000, an increase of 54%.
Showing that increased blood transfusions were a major driver of the jump in severe maternal morbidity, the composite increase in the rates of all other indicators went from 34 to 42 per delivery hospitalization, an increase of just 24%, or less than half the increase in blood transfusions.
There was significant variation in trends over time for the rates of the other indicators: acute renal failure, shock, the need for mechanical ventilation, and sepsis all increased by at least 100% (range, 104%-134%), and the rates of aneurysm increased by 99%. Rates of other indicators fell; pulmonary edema, embolism, eclampsia, myocardial infarction, cerebrovascular disorders, serious anesthesia complications, and intraoperative heart failure and arrest all declined by 25%-53% during the data-gathering period.
Looking at the data another way, 78% of cases of severe maternal morbidity in 2006 and 83% in 2015 involved a blood transfusion, making it the most common indicator. Far fewer delivery hospitalizations involved disseminated intravascular coagulation (DIC) and hysterectomy, the next most common indicators, which were seen in just 8% of cases.
Blood transfusions were most likely in women with shock (72%), amniotic fluid embolism (63%), sickle cell disease with crisis (54%), and DIC (51%).
One in three women who experienced shock during delivery had a hysterectomy, as did more than 20% of women who experienced adult respiratory distress syndrome or cardiac arrest (or ventricular fibrillation).
Women delivering at the youngest and oldest ends of the age spectrum were more likely to experience severe maternal morbidity. For women younger than 20 years of age, the rate was 206 per 10,000. In the group of women aged 40 years and over, the rate of any severe maternal morbidity was 248 per 10,000 delivery hospitalizations. Women in their 20s and 30s had rates of 136 and 143 per 10,000, respectively.
Besides race/ethnicity and age, a variety of other patient characteristics were associated with increased rates of severe maternal morbidity and mortality, with higher rates seen in women from the poorest quartile than the wealthiest (177 versus 122 per 10,000, respectively). Women with Medicaid were more likely than those with private insurance to experience severe maternal morbidity during a delivery hospitalization (175 versus 121 per 10,000, respectively).
Similarly, severe maternal morbidity was more common in safety net hospitals and in minority-serving hospitals (182 versus 128 and 176 versus 123 per 10,000, respectively) than other hospitals.
Regionally, severe maternal morbidity was most common in the Northeast and the South, at 165 and 164 per 10,000, respectively, compared with rates of 132 and 116 in the West and Midwest, respectively.
Hospital deaths per 100,000 delivery hospitalizations were 248% higher for black than white women in 2006. By the end of 2015, that figure declined modestly to 193%, with absolute rates of 19 versus 5.5 deaths per 100,000 delivery hospitalizations for black versus white women in 2006. In 2015, the absolute rates were 11 versus 4 per 100,000.
The study was conducted by the Agency for Healthcare Research and Quality. There were no reported conflicts of interest.
SOURCE: Fingar K et al. Agency for Healthcare Research and Quality Statistical Brief #243.
The rate of severe maternal morbidity in the United States has climbed steadily since 2006, increasing 45% overall in the decade ending in 2015, according to a new report from the Agency for Healthcare Research and Quality that also found large ethnic and racial, geographic, and socioeconomic variation in rates of severe maternal morbidity.
The longstanding increased risk for severe maternal morbidity for black women, compared with white women, continued essentially unchanged, with black women 112%-115% more likely to experience any of 21 conditions and procedures that defined severe maternal morbidity in the report. Disparities also existed between white women and those of Hispanic or Asian/Pacific Islander origin, but those gaps are narrowing, according to the report.
“Black women, Hispanic women, and women of other races/ethnicities were overrepresented among deliveries involving severe maternal morbidity, as compared with white women,” wrote Kathryn Fingar, PhD, and her coauthors. “White women constituted a lower percentage of deliveries with any severe maternal morbidity than they did other deliveries” – 23% lower.
The 21 indicators, developed by the Centers for Disease Control and Prevention, range from conditions such as renal failure and sepsis to in-hospital procedures such as blood transfusion and hysterectomy. Women were considered to have severe maternal morbidity if any of the indicators were present, regardless of whether in-hospital death occurred.

Dr. Fingar of IBM Watson Health, Cambridge, Mass., and her collaborators summarized data from AHRQ’s Healthcare Cost and Utilization Project (H-CUP) in the statistical brief. The two most common procedures that indicate severe maternal morbidity are blood transfusion and hysterectomy, and these indicators were tracked in deliveries where women had a condition that served as one of the severe maternal morbidity indicators.
Dr. Fingar and her colleagues noted that they excluded data for the final quarter of 2015, because that is when the transition from the 9th to the 10th edition of the International Classifications of Diseases was made.
In addition to the overall increase from 101.3 to 146.6 women per 10,000 experiencing any severe maternal morbidity, the incidence of blood transfusion during a delivery hospitalization, either alone or in conjunction with other indicators, rose from 78.9 to 121.1 women per 10,000, an increase of 54%.
Showing that increased blood transfusions were a major driver of the jump in severe maternal morbidity, the composite increase in the rates of all other indicators went from 34 to 42 per delivery hospitalization, an increase of just 24%, or less than half the increase in blood transfusions.
There was significant variation in trends over time for the rates of the other indicators: acute renal failure, shock, the need for mechanical ventilation, and sepsis all increased by at least 100% (range, 104%-134%), and the rates of aneurysm increased by 99%. Rates of other indicators fell; pulmonary edema, embolism, eclampsia, myocardial infarction, cerebrovascular disorders, serious anesthesia complications, and intraoperative heart failure and arrest all declined by 25%-53% during the data-gathering period.
Looking at the data another way, 78% of cases of severe maternal morbidity in 2006 and 83% in 2015 involved a blood transfusion, making it the most common indicator. Far fewer delivery hospitalizations involved disseminated intravascular coagulation (DIC) and hysterectomy, the next most common indicators, which were seen in just 8% of cases.
Blood transfusions were most likely in women with shock (72%), amniotic fluid embolism (63%), sickle cell disease with crisis (54%), and DIC (51%).
One in three women who experienced shock during delivery had a hysterectomy, as did more than 20% of women who experienced adult respiratory distress syndrome or cardiac arrest (or ventricular fibrillation).
Women delivering at the youngest and oldest ends of the age spectrum were more likely to experience severe maternal morbidity. For women younger than 20 years of age, the rate was 206 per 10,000. In the group of women aged 40 years and over, the rate of any severe maternal morbidity was 248 per 10,000 delivery hospitalizations. Women in their 20s and 30s had rates of 136 and 143 per 10,000, respectively.
Besides race/ethnicity and age, a variety of other patient characteristics were associated with increased rates of severe maternal morbidity and mortality, with higher rates seen in women from the poorest quartile than the wealthiest (177 versus 122 per 10,000, respectively). Women with Medicaid were more likely than those with private insurance to experience severe maternal morbidity during a delivery hospitalization (175 versus 121 per 10,000, respectively).
Similarly, severe maternal morbidity was more common in safety net hospitals and in minority-serving hospitals (182 versus 128 and 176 versus 123 per 10,000, respectively) than other hospitals.
Regionally, severe maternal morbidity was most common in the Northeast and the South, at 165 and 164 per 10,000, respectively, compared with rates of 132 and 116 in the West and Midwest, respectively.
Hospital deaths per 100,000 delivery hospitalizations were 248% higher for black than white women in 2006. By the end of 2015, that figure declined modestly to 193%, with absolute rates of 19 versus 5.5 deaths per 100,000 delivery hospitalizations for black versus white women in 2006. In 2015, the absolute rates were 11 versus 4 per 100,000.
The study was conducted by the Agency for Healthcare Research and Quality. There were no reported conflicts of interest.
SOURCE: Fingar K et al. Agency for Healthcare Research and Quality Statistical Brief #243.
FROM AN AHRQ STATISTICAL BRIEF
Key clinical point:
Major finding: Black women were over 100% more likely to experience severe maternal morbidity, compared with white women.
Study details: Statistical analysis of Healthcare Cost and Utilization Project (H-CUP) data.
Disclosures: The study was funded by the Agency for Healthcare Research and Quality. The authors reported no conflicts of interest.
Source: Fingar K et al. Agency for Healthcare Research and Quality Statistical Brief #243.
So it’s pediatric onychomycosis. Now what?
CHICAGO – Though research shows that nail fungus occurs in just 0.3% of pediatric patients in the United States, that’s not what Sheila Friedlander, MD, is seeing in her southern California practice, where it’s not uncommon to see children whose nails, toe nails in particular, have fungal involvement.
said Dr. Friedlander during a nail-focused session at the annual summer meeting of the American Academy of Dermatology. Dr. Friedlander, professor of dermatology and pediatrics at the University of California San Diego and Rady Children’s Hospital, said that she suspects that more participation in organized sports at a young age may be contributing to the increase, with occlusive sports footwear replacing bare feet or sandals for more hours of the day, presenting more opportunities for toenail trauma in sports such as soccer.
When making the clinical call about a nail problem, bear in mind that the younger the child, the less likely a nail problem is fungal, Dr. Friedlander noted. “Little children are much less likely than older children to have nail fungus. Pediatric nails are thinner, and they are faster growing, with better blood supply to the matrix.”
And if frank onychomadesis is observed, think about the time of year, and ask about recent fevers and rashes, because coxsackievirus may be the culprit. “Be not afraid, and look everywhere if the nail is confusing to you,” she said. In all ages, the diagnosis is primarily clinical, “but I culture them, I ‘PAS’ [periodic acid-Schiff stain] them, too. If you do both, you’ll increase your yield,” Dr. Friedlander said, adding, “the beauty of PAS is you can use it to give your families an answer very soon.”
Once you’ve established that fungus is to blame for a nail problem, there’s a conundrum: There are no Food and Drug Administration-approved therapies, either topical or systemic, for pediatric onychomycosis, Dr. Friedlander said. She, along with coauthors and first author Aditya Gupta, MD, of Mediprobe Research, London, Ontario, Canada, recently published an article reviewing the safety and efficacy of antifungal agents in this age group (Pediatr Dermatol. 2018 Jun 26. doi: 10.1111/pde.13561).
Reviewing information available in the United States and Canada, Dr. Friedlander and her coauthors came up with three topical and four oral options for children, along with recommendations for dosage and duration.
In response to an audience question about the use of topical antifungal treatment for nail involvement, Dr. Friedlander responded, “I think topicals would be great for kids, but it’s for kids where there is no nail matrix involvement. Also, cost is a problem. Nobody will cover it. But some families are willing to do this to avoid systemic therapy,” and if the family budget can accommodate a topical choice, it’s a logical option, she said, noting that partial reimbursement via a coupon system is available from some pharmaceutical companies.
Where appropriate, ciclopirox 8%, efinaconazole 10%, and tavaborole 5% can each be considered. Dr. Friedlander cited one study she coauthored, which reported that 70% of pediatric participants with nonmatrix onychomycosis saw effective treatment, with a 71% mycological cure rate (P = .03), after 32 weeks of treatment with ciclopirox lacquer versus vehicle (Pediatr Dermatol. 2013 May-Jun;30[3]:316-22).
Systemic therapies – which, when studied, have been given at tinea capitis doses – could include griseofulvin, terbinafine, itraconazole, and fluconazole.
In terms of oral options, Dr. Friedlander said, griseofulvin has some practical limitations. While prolonged treatment is required in any case, terbinafine may produce results in about 3 months, whereas griseofulvin may require up to 9 months of therapy. “I always try to use terbinafine … griseofulvin takes a year and a day,” she said.
She also shared some tips to improve pediatric adherence with oral antifungals: “You can tell parents to crush terbinafine tablets and mix in peanut butter or applesauce to improve adherence. Griseofulvin can be flavored by the pharmacy, but volumes are big with griseofulvin, so it’s a challenge to get kids to take it all,” she said.
Dr. Friedlander reported that she had no relevant financial disclosures.
[email protected]
CHICAGO – Though research shows that nail fungus occurs in just 0.3% of pediatric patients in the United States, that’s not what Sheila Friedlander, MD, is seeing in her southern California practice, where it’s not uncommon to see children whose nails, toe nails in particular, have fungal involvement.
said Dr. Friedlander during a nail-focused session at the annual summer meeting of the American Academy of Dermatology. Dr. Friedlander, professor of dermatology and pediatrics at the University of California San Diego and Rady Children’s Hospital, said that she suspects that more participation in organized sports at a young age may be contributing to the increase, with occlusive sports footwear replacing bare feet or sandals for more hours of the day, presenting more opportunities for toenail trauma in sports such as soccer.
When making the clinical call about a nail problem, bear in mind that the younger the child, the less likely a nail problem is fungal, Dr. Friedlander noted. “Little children are much less likely than older children to have nail fungus. Pediatric nails are thinner, and they are faster growing, with better blood supply to the matrix.”
And if frank onychomadesis is observed, think about the time of year, and ask about recent fevers and rashes, because coxsackievirus may be the culprit. “Be not afraid, and look everywhere if the nail is confusing to you,” she said. In all ages, the diagnosis is primarily clinical, “but I culture them, I ‘PAS’ [periodic acid-Schiff stain] them, too. If you do both, you’ll increase your yield,” Dr. Friedlander said, adding, “the beauty of PAS is you can use it to give your families an answer very soon.”
Once you’ve established that fungus is to blame for a nail problem, there’s a conundrum: There are no Food and Drug Administration-approved therapies, either topical or systemic, for pediatric onychomycosis, Dr. Friedlander said. She, along with coauthors and first author Aditya Gupta, MD, of Mediprobe Research, London, Ontario, Canada, recently published an article reviewing the safety and efficacy of antifungal agents in this age group (Pediatr Dermatol. 2018 Jun 26. doi: 10.1111/pde.13561).
Reviewing information available in the United States and Canada, Dr. Friedlander and her coauthors came up with three topical and four oral options for children, along with recommendations for dosage and duration.
In response to an audience question about the use of topical antifungal treatment for nail involvement, Dr. Friedlander responded, “I think topicals would be great for kids, but it’s for kids where there is no nail matrix involvement. Also, cost is a problem. Nobody will cover it. But some families are willing to do this to avoid systemic therapy,” and if the family budget can accommodate a topical choice, it’s a logical option, she said, noting that partial reimbursement via a coupon system is available from some pharmaceutical companies.
Where appropriate, ciclopirox 8%, efinaconazole 10%, and tavaborole 5% can each be considered. Dr. Friedlander cited one study she coauthored, which reported that 70% of pediatric participants with nonmatrix onychomycosis saw effective treatment, with a 71% mycological cure rate (P = .03), after 32 weeks of treatment with ciclopirox lacquer versus vehicle (Pediatr Dermatol. 2013 May-Jun;30[3]:316-22).
Systemic therapies – which, when studied, have been given at tinea capitis doses – could include griseofulvin, terbinafine, itraconazole, and fluconazole.
In terms of oral options, Dr. Friedlander said, griseofulvin has some practical limitations. While prolonged treatment is required in any case, terbinafine may produce results in about 3 months, whereas griseofulvin may require up to 9 months of therapy. “I always try to use terbinafine … griseofulvin takes a year and a day,” she said.
She also shared some tips to improve pediatric adherence with oral antifungals: “You can tell parents to crush terbinafine tablets and mix in peanut butter or applesauce to improve adherence. Griseofulvin can be flavored by the pharmacy, but volumes are big with griseofulvin, so it’s a challenge to get kids to take it all,” she said.
Dr. Friedlander reported that she had no relevant financial disclosures.
[email protected]
CHICAGO – Though research shows that nail fungus occurs in just 0.3% of pediatric patients in the United States, that’s not what Sheila Friedlander, MD, is seeing in her southern California practice, where it’s not uncommon to see children whose nails, toe nails in particular, have fungal involvement.
said Dr. Friedlander during a nail-focused session at the annual summer meeting of the American Academy of Dermatology. Dr. Friedlander, professor of dermatology and pediatrics at the University of California San Diego and Rady Children’s Hospital, said that she suspects that more participation in organized sports at a young age may be contributing to the increase, with occlusive sports footwear replacing bare feet or sandals for more hours of the day, presenting more opportunities for toenail trauma in sports such as soccer.
When making the clinical call about a nail problem, bear in mind that the younger the child, the less likely a nail problem is fungal, Dr. Friedlander noted. “Little children are much less likely than older children to have nail fungus. Pediatric nails are thinner, and they are faster growing, with better blood supply to the matrix.”
And if frank onychomadesis is observed, think about the time of year, and ask about recent fevers and rashes, because coxsackievirus may be the culprit. “Be not afraid, and look everywhere if the nail is confusing to you,” she said. In all ages, the diagnosis is primarily clinical, “but I culture them, I ‘PAS’ [periodic acid-Schiff stain] them, too. If you do both, you’ll increase your yield,” Dr. Friedlander said, adding, “the beauty of PAS is you can use it to give your families an answer very soon.”
Once you’ve established that fungus is to blame for a nail problem, there’s a conundrum: There are no Food and Drug Administration-approved therapies, either topical or systemic, for pediatric onychomycosis, Dr. Friedlander said. She, along with coauthors and first author Aditya Gupta, MD, of Mediprobe Research, London, Ontario, Canada, recently published an article reviewing the safety and efficacy of antifungal agents in this age group (Pediatr Dermatol. 2018 Jun 26. doi: 10.1111/pde.13561).
Reviewing information available in the United States and Canada, Dr. Friedlander and her coauthors came up with three topical and four oral options for children, along with recommendations for dosage and duration.
In response to an audience question about the use of topical antifungal treatment for nail involvement, Dr. Friedlander responded, “I think topicals would be great for kids, but it’s for kids where there is no nail matrix involvement. Also, cost is a problem. Nobody will cover it. But some families are willing to do this to avoid systemic therapy,” and if the family budget can accommodate a topical choice, it’s a logical option, she said, noting that partial reimbursement via a coupon system is available from some pharmaceutical companies.
Where appropriate, ciclopirox 8%, efinaconazole 10%, and tavaborole 5% can each be considered. Dr. Friedlander cited one study she coauthored, which reported that 70% of pediatric participants with nonmatrix onychomycosis saw effective treatment, with a 71% mycological cure rate (P = .03), after 32 weeks of treatment with ciclopirox lacquer versus vehicle (Pediatr Dermatol. 2013 May-Jun;30[3]:316-22).
Systemic therapies – which, when studied, have been given at tinea capitis doses – could include griseofulvin, terbinafine, itraconazole, and fluconazole.
In terms of oral options, Dr. Friedlander said, griseofulvin has some practical limitations. While prolonged treatment is required in any case, terbinafine may produce results in about 3 months, whereas griseofulvin may require up to 9 months of therapy. “I always try to use terbinafine … griseofulvin takes a year and a day,” she said.
She also shared some tips to improve pediatric adherence with oral antifungals: “You can tell parents to crush terbinafine tablets and mix in peanut butter or applesauce to improve adherence. Griseofulvin can be flavored by the pharmacy, but volumes are big with griseofulvin, so it’s a challenge to get kids to take it all,” she said.
Dr. Friedlander reported that she had no relevant financial disclosures.
[email protected]
EXPERT ANALYSIS FROM SUMMER AAD 2018
CDC releases guidelines for pediatric mTBI
and should base management and prognostication on clinical decision-making tools and symptom rating scales, according to new practice guidelines issued by a working group of the Centers for Disease Control and Prevention (JAMA Pediatrics. 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2853.
The guidelines were released simultaneously with a systematic review, conducted by the same authors, of the existing literature regarding pediatric mTBI (JAMA Pediatrics 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2847). As the evaluators sorted through the literature to find high-quality studies for this population, the funnel rapidly narrowed: From an initial pool of over 15,000 studies conducted between 1990 and 2015, findings from just 75 studies were eventually included in the systematic review.
The review’s findings formed the basis for the guidelines and allowed Angela Lumba-Brown, MD, a pediatric emergency medicine physician at Stanford (Calif.) University, and her coauthors to ascribe a level of confidence in the inference from study data for a given recommendation. Recommendations also are categorized by strength and accordingly indicate that clinicians “should” or “may” follow them. Exceptions are carved out for practices, such as the use of hypertonic 3% saline solution for acute headache in the ED, that should not be used outside research settings.
In the end, the guidelines cover 19 main topics, sorted into guidance regarding the diagnosis, prognosis, and management and treatment of mTBI in children.
Diagnosis
The recommendations regarding mTBI diagnosis center around determining which children are at risk for significant intracranial injury (ICI). The guidelines recommend, with moderate confidence, that clinicians usually should not obtain a head CT for children with mTBI. Validated clinical decision rules should be used for risk stratification to determine which children can safely avoid imaging and which children should be considered for head CT, wrote Dr. Lumba-Brown and her coauthors. Magnetic resonance imaging is not recommended for initial evaluation of mTBI, nor should skull radiographs be ordered in the absence of clinical suspicion for skull fracture.
From the systematic review, Dr. Lumba-Brown and her colleagues found that several risk factors taken together may mean that significant ICI is more likely. These include patient age younger than 2 years; any vomiting, loss of consciousness, or amnesia; a severe mechanism of injury, severe or worsening headache, or nonfrontal scalp hematoma; a Glasgow Coma Scale (GCS) score of less than 15; and clinical suspicion for skull fracture. Clinicians should give consideration to the risks of ionizing radiation to the head, and balance this against their assessment of risk for severe – and perhaps actionable – injury.
A validated symptom rating scale, used in an age-appropriate way, should be used as part of the evaluation of children with mTBI. For children aged 6 and older, the Graded Symptom Checklist is an appropriate tool within 2 days after injury, while the Post Concussion Symptom Scale as part of computerized neurocognitive testing can differentiate which high school athletes have mTBI when used within 4 days of injury, according to the guidelines, which also identify other validated symptom rating scales.
The guidelines authors recommend, with high confidence, that serum biomarkers should not be used outside of research settings in the diagnosis of mTBI in children at present.
Prognosis
Families should be counseled that symptoms mostly resolve within 1-3 months for up to 80% of children with mTBI, but families also should know that “each child’s recovery from mTBI is unique and will follow its own trajectory,” wrote Dr. Lumba-Brown and her coauthors, in a moderate-strength recommendation.
Some factors have been associated with slower recovery from mTBI, and either upon evaluation for mTBI or in routine sports examinations, families should be told about this potential if risk factors are present, said the guidelines, although the evidence supporting the associations is of “varying strength,” wrote Dr. Lumba-Brown and her coauthors. Children with previous mTBIs and those with a history of premorbid neurologic and psychiatric problems, learning problems, or family and social stress all may have delayed recovery. For children with ICI, lower cognitive ability also is associated with delayed recovery.
Demographic factors such as lower socioeconomic status and being of Hispanic ethnicity also may increase the risk for delayed mTBI recovery. Older children and adolescents may recover more slowly. Those with more severe initial presentation and more symptoms in the immediate post-mTBI phase also may have a slower recovery course, said Dr. Lumba-Brown and her coauthors.
A validated prediction rule can be used in the ED to gather information about these discrete risk factors to guide family counseling, according to the guidelines, which note that research has found that “an empirically derived set of risk factors predicted the risk of persistent post-concussion symptoms at 28 days” for children seen in the ED with mTBI.
During the recovery phase, a combination of tools should be used to track recovery from mTBI; these can include validated symptom scales, validated cognitive testing, reaction time measures, and, in adolescent athletes, balance testing. Using a combination of tools is a valuable strategy, the researchers wrote. “No single assessment tool is strongly predictive of outcome in children with mTBI,” they noted.
When prognosis is poor, or recovery is not proceeding as expected, clinicians should have a low threshold for initiating other interventions and referrals.
Management and treatment
Although the guideline authors acknowledged significant knowledge gaps in all areas of pediatric mTBI diagnosis and management, evidence is especially scant for best practices for treatment, rest, and return to play and school after a child sustains mTBI, said Dr. Lumba-Brown and her coauthors.
However, families should be given information about warning signs for serious head injury and how to monitor symptoms, as well as information about mTBI and the expected recovery course. Other forward-looking instructions should cover the importance of preventing new head injuries, managing the gradual return to normal cognitive and physical activities, and clear instructions regarding return to school and recreational activities. The guideline authors made a strong recommendation to provide this information, with high confidence in the data.
However, little strong evidence points the way to a clear set of criteria for when children are ready for school, play, and athletic participation. These decisions must be customized to the individual child, and decision making, particularly about return to school and academic activities, should be a collaborative affair, with schools, clinicians, and families all communicating to make sure the pace of return to normal life is keeping pace with the child’s recovery. “Because postconcussive symptoms resolve at different rates in different children after mTBI, individualization of return-to-school programming is necessary,” wrote Dr. Lumba-Brown and her coauthors.
The guideline authors cite evidence that “suggests that early rest (within the first 3 days) may be beneficial but that inactivity beyond this period for most children may worsen their self-reported symptoms.”
Psychosocial support may be beneficial for certain children, wrote the researchers, drawing on evidence showing that such support is beneficial in frank TBI, and is probably beneficial in mTBI.
Active rehabilitation as tolerated is recommended after an initial period of rest, with exertion kept to a level that does not exacerbate symptoms. Children should not participate in contact activities until symptoms are fully resolved.
A posttraumatic headache that is severe or worsens in the ED should prompt consideration of emergent neuroimaging, according to the guidelines. In the postacute phase, however, children can have nonopioid analgesia, although parents should know about such risks as rebound headache. When chronic headache follows a mTBI, the guidelines recommend that clinicians refer patients for a multidisciplinary evaluation that can assess the many factors – including analgesic overuse – that can be contributors.
Drawing on the larger body of adult TBI research, the authors recommend that insufficient or disordered sleep be addressed, because “the maintenance of appropriate sleep and the management of disrupted sleep may be a critical target of treatment for the child with mTBI.”
Children who suffer a mTBI may experience cognitive dysfunction as a direct result of injury to the brain or secondary to the effects of other symptoms such as sleep disruptions, headache pain, fatigue, or low tolerance of frustration. Clinicians may want to perform or refer their patients for a neuropsychological evaluation to determine what is causing the cognitive dysfunction, the authors said.
Dr. Lumba-Brown and her coauthors, who formed the CDC’s Pediatric Mild Traumatic Brain Injury Guideline Workgroup, also recommended that clinicians use the term “mild traumatic brain injury” to describe head injuries that cause confusion or disorientation, without loss of consciousness, or loss of consciousness of up to 30 minutes or less, or posttraumatic amnesia of less than 24 hours duration, and that are associated with a GCS of 13-15 by 30 minutes after injury or at the time of initial medical assessment. This practice, they said, may reduce the risk of misinterpretation by medical professionals and the public that can occur when the terms “mTBI,” “concussion,” and “minor head injury” all may refer to the same injury.
The CDC has developed a suite of materials to assist both health care providers and the public in guideline implementation. The agency also is using its HEADS UP campaign to publicize the guidelines and related materials, and plans ongoing evaluation of the guidelines and implementation materials.
Many study authors, including Dr. Lumba-Brown, had relationships with medical device or pharmaceutical companies. The systematic review and guideline development were funded by the CDC.
A growing realization that mTBI can have persistent and significant deleterious effects has informed medical and public attitudes toward concussion in children, which now results in almost 1 million annual ED visits.
Progress at the laboratory bench has elucidated much of the neurometabolic cascade that occurs with the insult of mTBI, and has allowed researchers to document the path of brain healing after injury. Neuroimaging now can go beyond static images to trace neural networks and detect previously unseen and subtle functional deficits engendered by mTBI.
In particular, 21st century magnetic resonance imaging (MRI) has shown increased sensitivity over CT alone. In the TRACK-TBI study, over one in four patients whose CTs were read as normal had MRI findings consistent with trauma-induced pathology. Both multimodal MRI and serum biomarkers show promise, although more research regarding their utility is needed, particularly in the case of proteomic biomarkers.
Still, high-quality studies of pediatric mTBI are scant, and translation of burgeoning research into clinical practice is severely impeded by the numerous knowledge gaps that exist in the field.
Dr. Lumba-Brown and her colleagues have synthesized research that supports a neurobiopsychosocial model of mTBI in children that comes into play most prominently in the postacute phase, when non–injury-related factors such as demographics, socioeconomic status, and premorbid psychological conditions are strong mediators of the recovery trajectory.
With children as with adults, scant research guides the path forward for treatment and recovery from mTBI. For children, clinicians are still grappling with issues surrounding return to full participation in the academic and recreational activities of the school environment.
Data from two currently active studies should help light the way forward, however. The TRACK-TBI study, funded by the National Institutes of Health, will include almost 200 children among its 2,700 enrollees who have sustained all levels of TBI.
The Concussion Assessment, Research, and Education (CARE) Consortium is funded jointly by the National College Athletic Association and the Department of Defense. Between student athletes and military cadets, over 40,000 individuals are now part of the study.
The two studies’ testing modalities and methodologies align, offering the opportunity for a powerful pooled analysis that includes civilians, athletes, and those in the military.
Until then, these guidelines provide a way forward to an individualized approach to the best care for a child with mTBI.
Michael McCrea, PhD, is professor of neurology and neurosurgery, and director of brain injury research at the Medical College of Wisconsin, Milwaukee. Geoff Manley, MD, PhD, is professor of neurologic surgery at the University of California, San Francisco. Neither author reported conflicts of interest. These remarks were drawn from an editorial accompanying the guidelines and systematic review (JAMA Pediatrics. 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2846).
A growing realization that mTBI can have persistent and significant deleterious effects has informed medical and public attitudes toward concussion in children, which now results in almost 1 million annual ED visits.
Progress at the laboratory bench has elucidated much of the neurometabolic cascade that occurs with the insult of mTBI, and has allowed researchers to document the path of brain healing after injury. Neuroimaging now can go beyond static images to trace neural networks and detect previously unseen and subtle functional deficits engendered by mTBI.
In particular, 21st century magnetic resonance imaging (MRI) has shown increased sensitivity over CT alone. In the TRACK-TBI study, over one in four patients whose CTs were read as normal had MRI findings consistent with trauma-induced pathology. Both multimodal MRI and serum biomarkers show promise, although more research regarding their utility is needed, particularly in the case of proteomic biomarkers.
Still, high-quality studies of pediatric mTBI are scant, and translation of burgeoning research into clinical practice is severely impeded by the numerous knowledge gaps that exist in the field.
Dr. Lumba-Brown and her colleagues have synthesized research that supports a neurobiopsychosocial model of mTBI in children that comes into play most prominently in the postacute phase, when non–injury-related factors such as demographics, socioeconomic status, and premorbid psychological conditions are strong mediators of the recovery trajectory.
With children as with adults, scant research guides the path forward for treatment and recovery from mTBI. For children, clinicians are still grappling with issues surrounding return to full participation in the academic and recreational activities of the school environment.
Data from two currently active studies should help light the way forward, however. The TRACK-TBI study, funded by the National Institutes of Health, will include almost 200 children among its 2,700 enrollees who have sustained all levels of TBI.
The Concussion Assessment, Research, and Education (CARE) Consortium is funded jointly by the National College Athletic Association and the Department of Defense. Between student athletes and military cadets, over 40,000 individuals are now part of the study.
The two studies’ testing modalities and methodologies align, offering the opportunity for a powerful pooled analysis that includes civilians, athletes, and those in the military.
Until then, these guidelines provide a way forward to an individualized approach to the best care for a child with mTBI.
Michael McCrea, PhD, is professor of neurology and neurosurgery, and director of brain injury research at the Medical College of Wisconsin, Milwaukee. Geoff Manley, MD, PhD, is professor of neurologic surgery at the University of California, San Francisco. Neither author reported conflicts of interest. These remarks were drawn from an editorial accompanying the guidelines and systematic review (JAMA Pediatrics. 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2846).
A growing realization that mTBI can have persistent and significant deleterious effects has informed medical and public attitudes toward concussion in children, which now results in almost 1 million annual ED visits.
Progress at the laboratory bench has elucidated much of the neurometabolic cascade that occurs with the insult of mTBI, and has allowed researchers to document the path of brain healing after injury. Neuroimaging now can go beyond static images to trace neural networks and detect previously unseen and subtle functional deficits engendered by mTBI.
In particular, 21st century magnetic resonance imaging (MRI) has shown increased sensitivity over CT alone. In the TRACK-TBI study, over one in four patients whose CTs were read as normal had MRI findings consistent with trauma-induced pathology. Both multimodal MRI and serum biomarkers show promise, although more research regarding their utility is needed, particularly in the case of proteomic biomarkers.
Still, high-quality studies of pediatric mTBI are scant, and translation of burgeoning research into clinical practice is severely impeded by the numerous knowledge gaps that exist in the field.
Dr. Lumba-Brown and her colleagues have synthesized research that supports a neurobiopsychosocial model of mTBI in children that comes into play most prominently in the postacute phase, when non–injury-related factors such as demographics, socioeconomic status, and premorbid psychological conditions are strong mediators of the recovery trajectory.
With children as with adults, scant research guides the path forward for treatment and recovery from mTBI. For children, clinicians are still grappling with issues surrounding return to full participation in the academic and recreational activities of the school environment.
Data from two currently active studies should help light the way forward, however. The TRACK-TBI study, funded by the National Institutes of Health, will include almost 200 children among its 2,700 enrollees who have sustained all levels of TBI.
The Concussion Assessment, Research, and Education (CARE) Consortium is funded jointly by the National College Athletic Association and the Department of Defense. Between student athletes and military cadets, over 40,000 individuals are now part of the study.
The two studies’ testing modalities and methodologies align, offering the opportunity for a powerful pooled analysis that includes civilians, athletes, and those in the military.
Until then, these guidelines provide a way forward to an individualized approach to the best care for a child with mTBI.
Michael McCrea, PhD, is professor of neurology and neurosurgery, and director of brain injury research at the Medical College of Wisconsin, Milwaukee. Geoff Manley, MD, PhD, is professor of neurologic surgery at the University of California, San Francisco. Neither author reported conflicts of interest. These remarks were drawn from an editorial accompanying the guidelines and systematic review (JAMA Pediatrics. 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2846).
and should base management and prognostication on clinical decision-making tools and symptom rating scales, according to new practice guidelines issued by a working group of the Centers for Disease Control and Prevention (JAMA Pediatrics. 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2853.
The guidelines were released simultaneously with a systematic review, conducted by the same authors, of the existing literature regarding pediatric mTBI (JAMA Pediatrics 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2847). As the evaluators sorted through the literature to find high-quality studies for this population, the funnel rapidly narrowed: From an initial pool of over 15,000 studies conducted between 1990 and 2015, findings from just 75 studies were eventually included in the systematic review.
The review’s findings formed the basis for the guidelines and allowed Angela Lumba-Brown, MD, a pediatric emergency medicine physician at Stanford (Calif.) University, and her coauthors to ascribe a level of confidence in the inference from study data for a given recommendation. Recommendations also are categorized by strength and accordingly indicate that clinicians “should” or “may” follow them. Exceptions are carved out for practices, such as the use of hypertonic 3% saline solution for acute headache in the ED, that should not be used outside research settings.
In the end, the guidelines cover 19 main topics, sorted into guidance regarding the diagnosis, prognosis, and management and treatment of mTBI in children.
Diagnosis
The recommendations regarding mTBI diagnosis center around determining which children are at risk for significant intracranial injury (ICI). The guidelines recommend, with moderate confidence, that clinicians usually should not obtain a head CT for children with mTBI. Validated clinical decision rules should be used for risk stratification to determine which children can safely avoid imaging and which children should be considered for head CT, wrote Dr. Lumba-Brown and her coauthors. Magnetic resonance imaging is not recommended for initial evaluation of mTBI, nor should skull radiographs be ordered in the absence of clinical suspicion for skull fracture.
From the systematic review, Dr. Lumba-Brown and her colleagues found that several risk factors taken together may mean that significant ICI is more likely. These include patient age younger than 2 years; any vomiting, loss of consciousness, or amnesia; a severe mechanism of injury, severe or worsening headache, or nonfrontal scalp hematoma; a Glasgow Coma Scale (GCS) score of less than 15; and clinical suspicion for skull fracture. Clinicians should give consideration to the risks of ionizing radiation to the head, and balance this against their assessment of risk for severe – and perhaps actionable – injury.
A validated symptom rating scale, used in an age-appropriate way, should be used as part of the evaluation of children with mTBI. For children aged 6 and older, the Graded Symptom Checklist is an appropriate tool within 2 days after injury, while the Post Concussion Symptom Scale as part of computerized neurocognitive testing can differentiate which high school athletes have mTBI when used within 4 days of injury, according to the guidelines, which also identify other validated symptom rating scales.
The guidelines authors recommend, with high confidence, that serum biomarkers should not be used outside of research settings in the diagnosis of mTBI in children at present.
Prognosis
Families should be counseled that symptoms mostly resolve within 1-3 months for up to 80% of children with mTBI, but families also should know that “each child’s recovery from mTBI is unique and will follow its own trajectory,” wrote Dr. Lumba-Brown and her coauthors, in a moderate-strength recommendation.
Some factors have been associated with slower recovery from mTBI, and either upon evaluation for mTBI or in routine sports examinations, families should be told about this potential if risk factors are present, said the guidelines, although the evidence supporting the associations is of “varying strength,” wrote Dr. Lumba-Brown and her coauthors. Children with previous mTBIs and those with a history of premorbid neurologic and psychiatric problems, learning problems, or family and social stress all may have delayed recovery. For children with ICI, lower cognitive ability also is associated with delayed recovery.
Demographic factors such as lower socioeconomic status and being of Hispanic ethnicity also may increase the risk for delayed mTBI recovery. Older children and adolescents may recover more slowly. Those with more severe initial presentation and more symptoms in the immediate post-mTBI phase also may have a slower recovery course, said Dr. Lumba-Brown and her coauthors.
A validated prediction rule can be used in the ED to gather information about these discrete risk factors to guide family counseling, according to the guidelines, which note that research has found that “an empirically derived set of risk factors predicted the risk of persistent post-concussion symptoms at 28 days” for children seen in the ED with mTBI.
During the recovery phase, a combination of tools should be used to track recovery from mTBI; these can include validated symptom scales, validated cognitive testing, reaction time measures, and, in adolescent athletes, balance testing. Using a combination of tools is a valuable strategy, the researchers wrote. “No single assessment tool is strongly predictive of outcome in children with mTBI,” they noted.
When prognosis is poor, or recovery is not proceeding as expected, clinicians should have a low threshold for initiating other interventions and referrals.
Management and treatment
Although the guideline authors acknowledged significant knowledge gaps in all areas of pediatric mTBI diagnosis and management, evidence is especially scant for best practices for treatment, rest, and return to play and school after a child sustains mTBI, said Dr. Lumba-Brown and her coauthors.
However, families should be given information about warning signs for serious head injury and how to monitor symptoms, as well as information about mTBI and the expected recovery course. Other forward-looking instructions should cover the importance of preventing new head injuries, managing the gradual return to normal cognitive and physical activities, and clear instructions regarding return to school and recreational activities. The guideline authors made a strong recommendation to provide this information, with high confidence in the data.
However, little strong evidence points the way to a clear set of criteria for when children are ready for school, play, and athletic participation. These decisions must be customized to the individual child, and decision making, particularly about return to school and academic activities, should be a collaborative affair, with schools, clinicians, and families all communicating to make sure the pace of return to normal life is keeping pace with the child’s recovery. “Because postconcussive symptoms resolve at different rates in different children after mTBI, individualization of return-to-school programming is necessary,” wrote Dr. Lumba-Brown and her coauthors.
The guideline authors cite evidence that “suggests that early rest (within the first 3 days) may be beneficial but that inactivity beyond this period for most children may worsen their self-reported symptoms.”
Psychosocial support may be beneficial for certain children, wrote the researchers, drawing on evidence showing that such support is beneficial in frank TBI, and is probably beneficial in mTBI.
Active rehabilitation as tolerated is recommended after an initial period of rest, with exertion kept to a level that does not exacerbate symptoms. Children should not participate in contact activities until symptoms are fully resolved.
A posttraumatic headache that is severe or worsens in the ED should prompt consideration of emergent neuroimaging, according to the guidelines. In the postacute phase, however, children can have nonopioid analgesia, although parents should know about such risks as rebound headache. When chronic headache follows a mTBI, the guidelines recommend that clinicians refer patients for a multidisciplinary evaluation that can assess the many factors – including analgesic overuse – that can be contributors.
Drawing on the larger body of adult TBI research, the authors recommend that insufficient or disordered sleep be addressed, because “the maintenance of appropriate sleep and the management of disrupted sleep may be a critical target of treatment for the child with mTBI.”
Children who suffer a mTBI may experience cognitive dysfunction as a direct result of injury to the brain or secondary to the effects of other symptoms such as sleep disruptions, headache pain, fatigue, or low tolerance of frustration. Clinicians may want to perform or refer their patients for a neuropsychological evaluation to determine what is causing the cognitive dysfunction, the authors said.
Dr. Lumba-Brown and her coauthors, who formed the CDC’s Pediatric Mild Traumatic Brain Injury Guideline Workgroup, also recommended that clinicians use the term “mild traumatic brain injury” to describe head injuries that cause confusion or disorientation, without loss of consciousness, or loss of consciousness of up to 30 minutes or less, or posttraumatic amnesia of less than 24 hours duration, and that are associated with a GCS of 13-15 by 30 minutes after injury or at the time of initial medical assessment. This practice, they said, may reduce the risk of misinterpretation by medical professionals and the public that can occur when the terms “mTBI,” “concussion,” and “minor head injury” all may refer to the same injury.
The CDC has developed a suite of materials to assist both health care providers and the public in guideline implementation. The agency also is using its HEADS UP campaign to publicize the guidelines and related materials, and plans ongoing evaluation of the guidelines and implementation materials.
Many study authors, including Dr. Lumba-Brown, had relationships with medical device or pharmaceutical companies. The systematic review and guideline development were funded by the CDC.
and should base management and prognostication on clinical decision-making tools and symptom rating scales, according to new practice guidelines issued by a working group of the Centers for Disease Control and Prevention (JAMA Pediatrics. 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2853.
The guidelines were released simultaneously with a systematic review, conducted by the same authors, of the existing literature regarding pediatric mTBI (JAMA Pediatrics 2018 Sep 4. doi: 10.1001/jamapediatrics.2018.2847). As the evaluators sorted through the literature to find high-quality studies for this population, the funnel rapidly narrowed: From an initial pool of over 15,000 studies conducted between 1990 and 2015, findings from just 75 studies were eventually included in the systematic review.
The review’s findings formed the basis for the guidelines and allowed Angela Lumba-Brown, MD, a pediatric emergency medicine physician at Stanford (Calif.) University, and her coauthors to ascribe a level of confidence in the inference from study data for a given recommendation. Recommendations also are categorized by strength and accordingly indicate that clinicians “should” or “may” follow them. Exceptions are carved out for practices, such as the use of hypertonic 3% saline solution for acute headache in the ED, that should not be used outside research settings.
In the end, the guidelines cover 19 main topics, sorted into guidance regarding the diagnosis, prognosis, and management and treatment of mTBI in children.
Diagnosis
The recommendations regarding mTBI diagnosis center around determining which children are at risk for significant intracranial injury (ICI). The guidelines recommend, with moderate confidence, that clinicians usually should not obtain a head CT for children with mTBI. Validated clinical decision rules should be used for risk stratification to determine which children can safely avoid imaging and which children should be considered for head CT, wrote Dr. Lumba-Brown and her coauthors. Magnetic resonance imaging is not recommended for initial evaluation of mTBI, nor should skull radiographs be ordered in the absence of clinical suspicion for skull fracture.
From the systematic review, Dr. Lumba-Brown and her colleagues found that several risk factors taken together may mean that significant ICI is more likely. These include patient age younger than 2 years; any vomiting, loss of consciousness, or amnesia; a severe mechanism of injury, severe or worsening headache, or nonfrontal scalp hematoma; a Glasgow Coma Scale (GCS) score of less than 15; and clinical suspicion for skull fracture. Clinicians should give consideration to the risks of ionizing radiation to the head, and balance this against their assessment of risk for severe – and perhaps actionable – injury.
A validated symptom rating scale, used in an age-appropriate way, should be used as part of the evaluation of children with mTBI. For children aged 6 and older, the Graded Symptom Checklist is an appropriate tool within 2 days after injury, while the Post Concussion Symptom Scale as part of computerized neurocognitive testing can differentiate which high school athletes have mTBI when used within 4 days of injury, according to the guidelines, which also identify other validated symptom rating scales.
The guidelines authors recommend, with high confidence, that serum biomarkers should not be used outside of research settings in the diagnosis of mTBI in children at present.
Prognosis
Families should be counseled that symptoms mostly resolve within 1-3 months for up to 80% of children with mTBI, but families also should know that “each child’s recovery from mTBI is unique and will follow its own trajectory,” wrote Dr. Lumba-Brown and her coauthors, in a moderate-strength recommendation.
Some factors have been associated with slower recovery from mTBI, and either upon evaluation for mTBI or in routine sports examinations, families should be told about this potential if risk factors are present, said the guidelines, although the evidence supporting the associations is of “varying strength,” wrote Dr. Lumba-Brown and her coauthors. Children with previous mTBIs and those with a history of premorbid neurologic and psychiatric problems, learning problems, or family and social stress all may have delayed recovery. For children with ICI, lower cognitive ability also is associated with delayed recovery.
Demographic factors such as lower socioeconomic status and being of Hispanic ethnicity also may increase the risk for delayed mTBI recovery. Older children and adolescents may recover more slowly. Those with more severe initial presentation and more symptoms in the immediate post-mTBI phase also may have a slower recovery course, said Dr. Lumba-Brown and her coauthors.
A validated prediction rule can be used in the ED to gather information about these discrete risk factors to guide family counseling, according to the guidelines, which note that research has found that “an empirically derived set of risk factors predicted the risk of persistent post-concussion symptoms at 28 days” for children seen in the ED with mTBI.
During the recovery phase, a combination of tools should be used to track recovery from mTBI; these can include validated symptom scales, validated cognitive testing, reaction time measures, and, in adolescent athletes, balance testing. Using a combination of tools is a valuable strategy, the researchers wrote. “No single assessment tool is strongly predictive of outcome in children with mTBI,” they noted.
When prognosis is poor, or recovery is not proceeding as expected, clinicians should have a low threshold for initiating other interventions and referrals.
Management and treatment
Although the guideline authors acknowledged significant knowledge gaps in all areas of pediatric mTBI diagnosis and management, evidence is especially scant for best practices for treatment, rest, and return to play and school after a child sustains mTBI, said Dr. Lumba-Brown and her coauthors.
However, families should be given information about warning signs for serious head injury and how to monitor symptoms, as well as information about mTBI and the expected recovery course. Other forward-looking instructions should cover the importance of preventing new head injuries, managing the gradual return to normal cognitive and physical activities, and clear instructions regarding return to school and recreational activities. The guideline authors made a strong recommendation to provide this information, with high confidence in the data.
However, little strong evidence points the way to a clear set of criteria for when children are ready for school, play, and athletic participation. These decisions must be customized to the individual child, and decision making, particularly about return to school and academic activities, should be a collaborative affair, with schools, clinicians, and families all communicating to make sure the pace of return to normal life is keeping pace with the child’s recovery. “Because postconcussive symptoms resolve at different rates in different children after mTBI, individualization of return-to-school programming is necessary,” wrote Dr. Lumba-Brown and her coauthors.
The guideline authors cite evidence that “suggests that early rest (within the first 3 days) may be beneficial but that inactivity beyond this period for most children may worsen their self-reported symptoms.”
Psychosocial support may be beneficial for certain children, wrote the researchers, drawing on evidence showing that such support is beneficial in frank TBI, and is probably beneficial in mTBI.
Active rehabilitation as tolerated is recommended after an initial period of rest, with exertion kept to a level that does not exacerbate symptoms. Children should not participate in contact activities until symptoms are fully resolved.
A posttraumatic headache that is severe or worsens in the ED should prompt consideration of emergent neuroimaging, according to the guidelines. In the postacute phase, however, children can have nonopioid analgesia, although parents should know about such risks as rebound headache. When chronic headache follows a mTBI, the guidelines recommend that clinicians refer patients for a multidisciplinary evaluation that can assess the many factors – including analgesic overuse – that can be contributors.
Drawing on the larger body of adult TBI research, the authors recommend that insufficient or disordered sleep be addressed, because “the maintenance of appropriate sleep and the management of disrupted sleep may be a critical target of treatment for the child with mTBI.”
Children who suffer a mTBI may experience cognitive dysfunction as a direct result of injury to the brain or secondary to the effects of other symptoms such as sleep disruptions, headache pain, fatigue, or low tolerance of frustration. Clinicians may want to perform or refer their patients for a neuropsychological evaluation to determine what is causing the cognitive dysfunction, the authors said.
Dr. Lumba-Brown and her coauthors, who formed the CDC’s Pediatric Mild Traumatic Brain Injury Guideline Workgroup, also recommended that clinicians use the term “mild traumatic brain injury” to describe head injuries that cause confusion or disorientation, without loss of consciousness, or loss of consciousness of up to 30 minutes or less, or posttraumatic amnesia of less than 24 hours duration, and that are associated with a GCS of 13-15 by 30 minutes after injury or at the time of initial medical assessment. This practice, they said, may reduce the risk of misinterpretation by medical professionals and the public that can occur when the terms “mTBI,” “concussion,” and “minor head injury” all may refer to the same injury.
The CDC has developed a suite of materials to assist both health care providers and the public in guideline implementation. The agency also is using its HEADS UP campaign to publicize the guidelines and related materials, and plans ongoing evaluation of the guidelines and implementation materials.
Many study authors, including Dr. Lumba-Brown, had relationships with medical device or pharmaceutical companies. The systematic review and guideline development were funded by the CDC.
FROM JAMA PEDIATRICS
Pancreatic abnormalities found in one-quarter of patients with high-risk germline mutations
according to a recent study. However, screening individuals under the age of 50 years, even those with risk factors, is a low-yield strategy.
In a retrospective analysis of 86 asymptomatic adult patients with high-risk germline mutations followed at a single center, screening by a variety of imaging modalities showed a pancreatic abnormality in about one-quarter of patients (23 of 86, 26.7%). No cancers were detected on initial screening or during a median 29.8-month follow-up period, though the investigators saw more abnormalities in patients over the age of 60 years (P = .043).
The mutations were detected through genetic screening at the University of Texas MD Anderson Cancer Center, Houston, where the study was conducted.
“At our institution, based on oncology and/or medical genetics assessments, patients with a personal history of breast cancer and/or family history of [pancreatic cancer] are screened for BRCAs and BRCA1 mutations. Patients with other types of cancers in the family are screened for P53, STK11, MSH2, ATM, and APC mutations,” explained first author R. Tomás DaVee, MD, of the division of gastroenterology, hepatology, and nutrition at the University of Texas, Houston, and his collaborators.
Patients were considered to have a family history of pancreatic cancer (PC) if any first-, second-, or third-degree relative had PC; overall, this amounted to 37 patients (43%). For the study, familial PC was defined as having either two first-degree or three or more relatives of any degree diagnosed with PC.
Patients, who were a median age of 48.5 years, were included in the study if they had any of the germline mutations, which are associated with an increase in relative risk of PC ranging from 2.26 for BRCA1 to 132 for STK11/LKB1, the mutation that causes Peutz-Jeghers syndrome. Patients who had a family history alone, without high-risk germline mutations, were excluded from the study. Most participants (79.8%) were women, and most of the participants were BRCA2 or BRCA1 positive (58.1% and 16.3%, respectively).
Of the abnormalities found, almost half (47.8%) were cysts, and almost as many (43.5%) were hyperechoic strands and foci. Two patients (8.7% of abnormalities) had mild pancreatic duct dilation. Patients who had only fatty infiltration or pancreas divisum were classified as having normal variations rather than abnormalities.
The pancreatic abnormalities had been found through endoscopic ultrasound (EUS), CT, or MRI, though the study’s primary aim was to look at outcomes and diagnostic yield for high-risk germline mutation patients receiving EUS. A secondary aim of the study, said the authors, was to compare EUS with both MRI and CT as methods to screen for early PC.
In this regard, Dr. DaVee and his coauthors wrote that “EUS-based screening conferred a higher yield in detecting abnormal pancreatic findings, compared with MRI [P = .007].” For MRI in comparison with EUS as the criterion standard, sensitivity and specificity were 55.6% and 93.8% for detecting pancreatic abnormalities. CT fared a little worse, with sensitivity of 50% and specificity of 89.5% for detecting pancreatic abnormalities.
Overall, the investigators noted that “the frequency of [pancreatic abnormalities] in our study is lower than reported in some studies using alternate criteria.” The relatively young age of the study cohort may be partly responsible for this finding, they said, adding that “our data further support evidence that abnormal pancreatic findings increase with age ... in asymptomatic [high-risk individuals], including the subtle finding of hyperechoic strands and foci.”
To improve survival, “premalignant and early PC identification is key,” wrote Dr. DaVee and his coauthors, pointing out that, of the 10% of patients diagnosed with PC when it is still localized, the 5-year survival rate is about 31.5%, compared with the overall 8.2% of individuals with PC who are still alive 5 years after their diagnosis. The latter figure, they noted, is a small improvement over the 3% 5-year survival figure from 1975.
Since population-wide screening for PC would not have a favorable cost-benefit profile, the challenge is identifying which individuals might benefit from targeted screening for PC. Dr. DaVee and his colleagues suggested that, as a general rule, high-risk individuals aged younger than 50 years need not be screened, and that EUS be used for the index screening, with MRI used to follow individuals with unremarkable initial screenings.
None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
SOURCE: DaVee RT et al. Gastrointest Endosc. 2018 Jun; 87(6):1443-50.
according to a recent study. However, screening individuals under the age of 50 years, even those with risk factors, is a low-yield strategy.
In a retrospective analysis of 86 asymptomatic adult patients with high-risk germline mutations followed at a single center, screening by a variety of imaging modalities showed a pancreatic abnormality in about one-quarter of patients (23 of 86, 26.7%). No cancers were detected on initial screening or during a median 29.8-month follow-up period, though the investigators saw more abnormalities in patients over the age of 60 years (P = .043).
The mutations were detected through genetic screening at the University of Texas MD Anderson Cancer Center, Houston, where the study was conducted.
“At our institution, based on oncology and/or medical genetics assessments, patients with a personal history of breast cancer and/or family history of [pancreatic cancer] are screened for BRCAs and BRCA1 mutations. Patients with other types of cancers in the family are screened for P53, STK11, MSH2, ATM, and APC mutations,” explained first author R. Tomás DaVee, MD, of the division of gastroenterology, hepatology, and nutrition at the University of Texas, Houston, and his collaborators.
Patients were considered to have a family history of pancreatic cancer (PC) if any first-, second-, or third-degree relative had PC; overall, this amounted to 37 patients (43%). For the study, familial PC was defined as having either two first-degree or three or more relatives of any degree diagnosed with PC.
Patients, who were a median age of 48.5 years, were included in the study if they had any of the germline mutations, which are associated with an increase in relative risk of PC ranging from 2.26 for BRCA1 to 132 for STK11/LKB1, the mutation that causes Peutz-Jeghers syndrome. Patients who had a family history alone, without high-risk germline mutations, were excluded from the study. Most participants (79.8%) were women, and most of the participants were BRCA2 or BRCA1 positive (58.1% and 16.3%, respectively).
Of the abnormalities found, almost half (47.8%) were cysts, and almost as many (43.5%) were hyperechoic strands and foci. Two patients (8.7% of abnormalities) had mild pancreatic duct dilation. Patients who had only fatty infiltration or pancreas divisum were classified as having normal variations rather than abnormalities.
The pancreatic abnormalities had been found through endoscopic ultrasound (EUS), CT, or MRI, though the study’s primary aim was to look at outcomes and diagnostic yield for high-risk germline mutation patients receiving EUS. A secondary aim of the study, said the authors, was to compare EUS with both MRI and CT as methods to screen for early PC.
In this regard, Dr. DaVee and his coauthors wrote that “EUS-based screening conferred a higher yield in detecting abnormal pancreatic findings, compared with MRI [P = .007].” For MRI in comparison with EUS as the criterion standard, sensitivity and specificity were 55.6% and 93.8% for detecting pancreatic abnormalities. CT fared a little worse, with sensitivity of 50% and specificity of 89.5% for detecting pancreatic abnormalities.
Overall, the investigators noted that “the frequency of [pancreatic abnormalities] in our study is lower than reported in some studies using alternate criteria.” The relatively young age of the study cohort may be partly responsible for this finding, they said, adding that “our data further support evidence that abnormal pancreatic findings increase with age ... in asymptomatic [high-risk individuals], including the subtle finding of hyperechoic strands and foci.”
To improve survival, “premalignant and early PC identification is key,” wrote Dr. DaVee and his coauthors, pointing out that, of the 10% of patients diagnosed with PC when it is still localized, the 5-year survival rate is about 31.5%, compared with the overall 8.2% of individuals with PC who are still alive 5 years after their diagnosis. The latter figure, they noted, is a small improvement over the 3% 5-year survival figure from 1975.
Since population-wide screening for PC would not have a favorable cost-benefit profile, the challenge is identifying which individuals might benefit from targeted screening for PC. Dr. DaVee and his colleagues suggested that, as a general rule, high-risk individuals aged younger than 50 years need not be screened, and that EUS be used for the index screening, with MRI used to follow individuals with unremarkable initial screenings.
None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
SOURCE: DaVee RT et al. Gastrointest Endosc. 2018 Jun; 87(6):1443-50.
according to a recent study. However, screening individuals under the age of 50 years, even those with risk factors, is a low-yield strategy.
In a retrospective analysis of 86 asymptomatic adult patients with high-risk germline mutations followed at a single center, screening by a variety of imaging modalities showed a pancreatic abnormality in about one-quarter of patients (23 of 86, 26.7%). No cancers were detected on initial screening or during a median 29.8-month follow-up period, though the investigators saw more abnormalities in patients over the age of 60 years (P = .043).
The mutations were detected through genetic screening at the University of Texas MD Anderson Cancer Center, Houston, where the study was conducted.
“At our institution, based on oncology and/or medical genetics assessments, patients with a personal history of breast cancer and/or family history of [pancreatic cancer] are screened for BRCAs and BRCA1 mutations. Patients with other types of cancers in the family are screened for P53, STK11, MSH2, ATM, and APC mutations,” explained first author R. Tomás DaVee, MD, of the division of gastroenterology, hepatology, and nutrition at the University of Texas, Houston, and his collaborators.
Patients were considered to have a family history of pancreatic cancer (PC) if any first-, second-, or third-degree relative had PC; overall, this amounted to 37 patients (43%). For the study, familial PC was defined as having either two first-degree or three or more relatives of any degree diagnosed with PC.
Patients, who were a median age of 48.5 years, were included in the study if they had any of the germline mutations, which are associated with an increase in relative risk of PC ranging from 2.26 for BRCA1 to 132 for STK11/LKB1, the mutation that causes Peutz-Jeghers syndrome. Patients who had a family history alone, without high-risk germline mutations, were excluded from the study. Most participants (79.8%) were women, and most of the participants were BRCA2 or BRCA1 positive (58.1% and 16.3%, respectively).
Of the abnormalities found, almost half (47.8%) were cysts, and almost as many (43.5%) were hyperechoic strands and foci. Two patients (8.7% of abnormalities) had mild pancreatic duct dilation. Patients who had only fatty infiltration or pancreas divisum were classified as having normal variations rather than abnormalities.
The pancreatic abnormalities had been found through endoscopic ultrasound (EUS), CT, or MRI, though the study’s primary aim was to look at outcomes and diagnostic yield for high-risk germline mutation patients receiving EUS. A secondary aim of the study, said the authors, was to compare EUS with both MRI and CT as methods to screen for early PC.
In this regard, Dr. DaVee and his coauthors wrote that “EUS-based screening conferred a higher yield in detecting abnormal pancreatic findings, compared with MRI [P = .007].” For MRI in comparison with EUS as the criterion standard, sensitivity and specificity were 55.6% and 93.8% for detecting pancreatic abnormalities. CT fared a little worse, with sensitivity of 50% and specificity of 89.5% for detecting pancreatic abnormalities.
Overall, the investigators noted that “the frequency of [pancreatic abnormalities] in our study is lower than reported in some studies using alternate criteria.” The relatively young age of the study cohort may be partly responsible for this finding, they said, adding that “our data further support evidence that abnormal pancreatic findings increase with age ... in asymptomatic [high-risk individuals], including the subtle finding of hyperechoic strands and foci.”
To improve survival, “premalignant and early PC identification is key,” wrote Dr. DaVee and his coauthors, pointing out that, of the 10% of patients diagnosed with PC when it is still localized, the 5-year survival rate is about 31.5%, compared with the overall 8.2% of individuals with PC who are still alive 5 years after their diagnosis. The latter figure, they noted, is a small improvement over the 3% 5-year survival figure from 1975.
Since population-wide screening for PC would not have a favorable cost-benefit profile, the challenge is identifying which individuals might benefit from targeted screening for PC. Dr. DaVee and his colleagues suggested that, as a general rule, high-risk individuals aged younger than 50 years need not be screened, and that EUS be used for the index screening, with MRI used to follow individuals with unremarkable initial screenings.
None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
SOURCE: DaVee RT et al. Gastrointest Endosc. 2018 Jun; 87(6):1443-50.
FROM GASTROINTESTINAL ENDOSCOPY
Key clinical point: Pancreatic abnormalities increased with age in high-risk individuals.
Major finding: Imaging found pancreatic abnormalities in 23 of 86 high-risk individuals (26.7%).
Study details: A retrospective, single-center study of 86 patients with germline mutations placing them at high risk for pancreatic cancer.
Disclosures: None of the authors reported relevant conflicts of interest, and no outside source of funding was reported.
Source: DaVee RT et al. Gastrointest Endosc. 2018 Jun;87(6):1443-540.
Diclofenac’s cardiovascular risk confirmed in novel Nordic study
Those beginning diclofenac had a 50% increased 30-day risk for a composite outcome of major adverse cardiovascular events (MACE) compared with individuals who didn’t initiate an NSAID or acetaminophen (95% confidence interval for incidence rate ratio, 1.4-1.7).
The risk was still significantly elevated when the study’s first author, Morten Schmidt, MD, and his colleagues compared diclofenac initiation with beginning other NSAIDs or acetaminophen. Compared with those starting ibuprofen or acetaminophen, the MACE risk was elevated 20% in diclofenac initiators (95% CI, 1.1-1.3 for both). Initiating diclofenac was associated with 30% greater risk for MACE compared with initiating naproxen (95% CI, 1.1-1.5).
“Diclofenac is the most frequently used NSAID in low-, middle-, and high-income countries and is available over the counter in most countries; therefore, its cardiovascular risk profile is of major clinical and public health importance,” wrote Dr. Schmidt and his coauthors.
In all, the study included 1,370,832 individuals who initiated diclofenac, 3,878,454 ibuprofen initiators, 291,490 naproxen initiators, and 764,781 acetaminophen initiators. Those starting diclofenac were compared with those starting other medications, and with 1,303,209 individuals who sought health care but did not start one of the medications.
The researchers used the longstanding and complete Danish health registry system to their advantage in designing a cohort trial that was modeled to resemble a clinical trial. For each month, beginning in 1996 and continuing through 2016, Dr. Schmidt and his collaborators assembled propensity-matched cohorts of individuals to compare each study group. The study design achieved many of the aims of a clinical trial while working within the ethical constraints of studying medications now known to elevate cardiovascular risk.
For each 30-day period, the investigators were then able to track and compare cardiovascular outcomes for each group. Each month, data for a new cohort were collected, beginning a new “clinical trial.” Individuals could be included in more than one month’s worth of “trial” data as long as they continued to meet inclusion criteria.
The completeness of Danish health data meant that the researchers were confident in data about comorbidities, other prescription medications, and outcomes.
Dr. Schmidt and his colleagues performed subgroup and sensitivity analyses to look at the extent to which preexisting risks for cardiovascular disease mediated MACE risk on diclofenac initiation. They found that diclofenac initiators in the highest risk group had up to 40 excess cardiovascular events per year – about half of them fatal – that were attributable to starting the medication. Although that group had the highest absolute risk, however, “the relative risks were highest in those with the lowest baseline risk,” wrote the investigators.
In addition to looking at rates of MACE, secondary outcomes for the study included evaluating the association between medication use or non-use and each individual component of the composite primary outcome. These included first-time occurrences of the nonfatal endpoints of atrial fibrillation or flutter, ischemic (but not hemorrhagic) stroke, heart failure, and myocardial infarction. Cardiac death was death from any cardiac cause.
“Supporting use of a combined endpoint, event rates consistently increased for all individual outcomes” for diclofenac initiators compared with those who did not start an NSAID, wrote Dr. Schmidt and his colleagues.
Individuals were excluded if they had known cardiovascular, kidney, liver, or ulcer disease, and if they had malignancy or serious mental health diagnoses such as dementia or schizophrenia. Participants, aged a mean 48-56 years, had to be at least 18 years of age and could not have filled a prescription for an NSAID within the previous 12 months. Men made up 36.6%-46.3% of the cohorts.
Dr. Schmidt, of Aarhus (Denmark) University, and his collaborators said that in comparison with other NSAIDs, the short half-life of diclofenac means that a supratherapeutic plasma concentration of diclofenac soon after initiation achieves not just cyclooxygenase-2 (COX-2), but also COX-1 inhibition. However, after those high levels fall, patients taking diclofenac spend a substantial period of time with unopposed COX-2 inhibition, a state that is known to be prothrombotic, and also associated with blood pressure elevation, atherogenesis, and worsening of heart failure.
Diclofenac and ibuprofen had similar gastrointestinal bleeding risks, and both medications were associated with a higher risk of bleeding than were ibuprofen, acetaminophen, or no medication.
“Comparing diclofenac initiation with no NSAID initiation, the consistency between our results and those of previous meta-analyses of both trial and observational data provides strong evidence to guide clinical decision making,” said Dr. Schmidt and his coauthors.
“Considering its cardiovascular and gastrointestinal risks, however, there is little justification to initiate diclofenac treatment before other traditional NSAIDs,” noted the investigators. “It is time to acknowledge the potential health risk of diclofenac and to reduce its use.”
The study was funded by the Department of Clinical Epidemiology Research Foundation, University of Aarhus, and by the Program for Clinical Research Infrastructure, funded by the Lundbeck Foundation, Novo Nordisk Foundation, and the Danish Research Council. The authors reported that they had no relevant conflicts of interest.
SOURCE: Schmidt M et al. BMJ 2018;362:k3426
Those beginning diclofenac had a 50% increased 30-day risk for a composite outcome of major adverse cardiovascular events (MACE) compared with individuals who didn’t initiate an NSAID or acetaminophen (95% confidence interval for incidence rate ratio, 1.4-1.7).
The risk was still significantly elevated when the study’s first author, Morten Schmidt, MD, and his colleagues compared diclofenac initiation with beginning other NSAIDs or acetaminophen. Compared with those starting ibuprofen or acetaminophen, the MACE risk was elevated 20% in diclofenac initiators (95% CI, 1.1-1.3 for both). Initiating diclofenac was associated with 30% greater risk for MACE compared with initiating naproxen (95% CI, 1.1-1.5).
“Diclofenac is the most frequently used NSAID in low-, middle-, and high-income countries and is available over the counter in most countries; therefore, its cardiovascular risk profile is of major clinical and public health importance,” wrote Dr. Schmidt and his coauthors.
In all, the study included 1,370,832 individuals who initiated diclofenac, 3,878,454 ibuprofen initiators, 291,490 naproxen initiators, and 764,781 acetaminophen initiators. Those starting diclofenac were compared with those starting other medications, and with 1,303,209 individuals who sought health care but did not start one of the medications.
The researchers used the longstanding and complete Danish health registry system to their advantage in designing a cohort trial that was modeled to resemble a clinical trial. For each month, beginning in 1996 and continuing through 2016, Dr. Schmidt and his collaborators assembled propensity-matched cohorts of individuals to compare each study group. The study design achieved many of the aims of a clinical trial while working within the ethical constraints of studying medications now known to elevate cardiovascular risk.
For each 30-day period, the investigators were then able to track and compare cardiovascular outcomes for each group. Each month, data for a new cohort were collected, beginning a new “clinical trial.” Individuals could be included in more than one month’s worth of “trial” data as long as they continued to meet inclusion criteria.
The completeness of Danish health data meant that the researchers were confident in data about comorbidities, other prescription medications, and outcomes.
Dr. Schmidt and his colleagues performed subgroup and sensitivity analyses to look at the extent to which preexisting risks for cardiovascular disease mediated MACE risk on diclofenac initiation. They found that diclofenac initiators in the highest risk group had up to 40 excess cardiovascular events per year – about half of them fatal – that were attributable to starting the medication. Although that group had the highest absolute risk, however, “the relative risks were highest in those with the lowest baseline risk,” wrote the investigators.
In addition to looking at rates of MACE, secondary outcomes for the study included evaluating the association between medication use or non-use and each individual component of the composite primary outcome. These included first-time occurrences of the nonfatal endpoints of atrial fibrillation or flutter, ischemic (but not hemorrhagic) stroke, heart failure, and myocardial infarction. Cardiac death was death from any cardiac cause.
“Supporting use of a combined endpoint, event rates consistently increased for all individual outcomes” for diclofenac initiators compared with those who did not start an NSAID, wrote Dr. Schmidt and his colleagues.
Individuals were excluded if they had known cardiovascular, kidney, liver, or ulcer disease, and if they had malignancy or serious mental health diagnoses such as dementia or schizophrenia. Participants, aged a mean 48-56 years, had to be at least 18 years of age and could not have filled a prescription for an NSAID within the previous 12 months. Men made up 36.6%-46.3% of the cohorts.
Dr. Schmidt, of Aarhus (Denmark) University, and his collaborators said that in comparison with other NSAIDs, the short half-life of diclofenac means that a supratherapeutic plasma concentration of diclofenac soon after initiation achieves not just cyclooxygenase-2 (COX-2), but also COX-1 inhibition. However, after those high levels fall, patients taking diclofenac spend a substantial period of time with unopposed COX-2 inhibition, a state that is known to be prothrombotic, and also associated with blood pressure elevation, atherogenesis, and worsening of heart failure.
Diclofenac and ibuprofen had similar gastrointestinal bleeding risks, and both medications were associated with a higher risk of bleeding than were ibuprofen, acetaminophen, or no medication.
“Comparing diclofenac initiation with no NSAID initiation, the consistency between our results and those of previous meta-analyses of both trial and observational data provides strong evidence to guide clinical decision making,” said Dr. Schmidt and his coauthors.
“Considering its cardiovascular and gastrointestinal risks, however, there is little justification to initiate diclofenac treatment before other traditional NSAIDs,” noted the investigators. “It is time to acknowledge the potential health risk of diclofenac and to reduce its use.”
The study was funded by the Department of Clinical Epidemiology Research Foundation, University of Aarhus, and by the Program for Clinical Research Infrastructure, funded by the Lundbeck Foundation, Novo Nordisk Foundation, and the Danish Research Council. The authors reported that they had no relevant conflicts of interest.
SOURCE: Schmidt M et al. BMJ 2018;362:k3426
Those beginning diclofenac had a 50% increased 30-day risk for a composite outcome of major adverse cardiovascular events (MACE) compared with individuals who didn’t initiate an NSAID or acetaminophen (95% confidence interval for incidence rate ratio, 1.4-1.7).
The risk was still significantly elevated when the study’s first author, Morten Schmidt, MD, and his colleagues compared diclofenac initiation with beginning other NSAIDs or acetaminophen. Compared with those starting ibuprofen or acetaminophen, the MACE risk was elevated 20% in diclofenac initiators (95% CI, 1.1-1.3 for both). Initiating diclofenac was associated with 30% greater risk for MACE compared with initiating naproxen (95% CI, 1.1-1.5).
“Diclofenac is the most frequently used NSAID in low-, middle-, and high-income countries and is available over the counter in most countries; therefore, its cardiovascular risk profile is of major clinical and public health importance,” wrote Dr. Schmidt and his coauthors.
In all, the study included 1,370,832 individuals who initiated diclofenac, 3,878,454 ibuprofen initiators, 291,490 naproxen initiators, and 764,781 acetaminophen initiators. Those starting diclofenac were compared with those starting other medications, and with 1,303,209 individuals who sought health care but did not start one of the medications.
The researchers used the longstanding and complete Danish health registry system to their advantage in designing a cohort trial that was modeled to resemble a clinical trial. For each month, beginning in 1996 and continuing through 2016, Dr. Schmidt and his collaborators assembled propensity-matched cohorts of individuals to compare each study group. The study design achieved many of the aims of a clinical trial while working within the ethical constraints of studying medications now known to elevate cardiovascular risk.
For each 30-day period, the investigators were then able to track and compare cardiovascular outcomes for each group. Each month, data for a new cohort were collected, beginning a new “clinical trial.” Individuals could be included in more than one month’s worth of “trial” data as long as they continued to meet inclusion criteria.
The completeness of Danish health data meant that the researchers were confident in data about comorbidities, other prescription medications, and outcomes.
Dr. Schmidt and his colleagues performed subgroup and sensitivity analyses to look at the extent to which preexisting risks for cardiovascular disease mediated MACE risk on diclofenac initiation. They found that diclofenac initiators in the highest risk group had up to 40 excess cardiovascular events per year – about half of them fatal – that were attributable to starting the medication. Although that group had the highest absolute risk, however, “the relative risks were highest in those with the lowest baseline risk,” wrote the investigators.
In addition to looking at rates of MACE, secondary outcomes for the study included evaluating the association between medication use or non-use and each individual component of the composite primary outcome. These included first-time occurrences of the nonfatal endpoints of atrial fibrillation or flutter, ischemic (but not hemorrhagic) stroke, heart failure, and myocardial infarction. Cardiac death was death from any cardiac cause.
“Supporting use of a combined endpoint, event rates consistently increased for all individual outcomes” for diclofenac initiators compared with those who did not start an NSAID, wrote Dr. Schmidt and his colleagues.
Individuals were excluded if they had known cardiovascular, kidney, liver, or ulcer disease, and if they had malignancy or serious mental health diagnoses such as dementia or schizophrenia. Participants, aged a mean 48-56 years, had to be at least 18 years of age and could not have filled a prescription for an NSAID within the previous 12 months. Men made up 36.6%-46.3% of the cohorts.
Dr. Schmidt, of Aarhus (Denmark) University, and his collaborators said that in comparison with other NSAIDs, the short half-life of diclofenac means that a supratherapeutic plasma concentration of diclofenac soon after initiation achieves not just cyclooxygenase-2 (COX-2), but also COX-1 inhibition. However, after those high levels fall, patients taking diclofenac spend a substantial period of time with unopposed COX-2 inhibition, a state that is known to be prothrombotic, and also associated with blood pressure elevation, atherogenesis, and worsening of heart failure.
Diclofenac and ibuprofen had similar gastrointestinal bleeding risks, and both medications were associated with a higher risk of bleeding than were ibuprofen, acetaminophen, or no medication.
“Comparing diclofenac initiation with no NSAID initiation, the consistency between our results and those of previous meta-analyses of both trial and observational data provides strong evidence to guide clinical decision making,” said Dr. Schmidt and his coauthors.
“Considering its cardiovascular and gastrointestinal risks, however, there is little justification to initiate diclofenac treatment before other traditional NSAIDs,” noted the investigators. “It is time to acknowledge the potential health risk of diclofenac and to reduce its use.”
The study was funded by the Department of Clinical Epidemiology Research Foundation, University of Aarhus, and by the Program for Clinical Research Infrastructure, funded by the Lundbeck Foundation, Novo Nordisk Foundation, and the Danish Research Council. The authors reported that they had no relevant conflicts of interest.
SOURCE: Schmidt M et al. BMJ 2018;362:k3426
FROM BMJ
Key clinical point: Those starting diclofenac had increased risk for cardiovascular events or cardiac death.
Major finding: Risk for major adverse cardiovascular events was increased by 50% compared with noninitiators.
Study details: Retrospective propensity-matched cohort study using national databases and registries.
Disclosures: The study was supported by the Department of Clinical Epidemiology Research Foundation of the University of Aarhus, Denmark, and by the Program for Clinical Research Infrastructure, funded by the Lundbeck Foundation, Novo Nordisk Foundation, and the Danish Research Council. The authors reported that they had no relevant conflicts of interest.
Source: Schmidt M et al. BMJ 2018;362:k3426.