User login
Frail women less likely to initiate hormonal therapy for breast cancer
Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail, according to researchers.
In a prospective cohort study of women aged 65 and older diagnosed with invasive, nonmetastatic estrogen receptor–positive breast cancers (n = 1,062), about a quarter of the women were frail or prefrail (4.9% and 18.7%, respectively) based on a validated frailty score determined at baseline, reported Vanessa Sheppard, Ph.D., of Georgetown University Medical Center, Washington (J. Clin. Oncol. 2014 June 16 [doi:10.1200/JCO.2013.51.7367]).
Overall, 14% of the women in the study did not initiate hormonal therapy. Women considered prefrail or frail were significantly less likely to initiate therapy than were those considered "robust" or nonfrail (odds ratio, 1.63; 95% CI, 1.11-2.40; P =.013). Nonwhite race was also significantly associated with noninitiation of therapy (OR, 1.71; CI, 1.04-2.80; P = 0.33).
Baseline frailty was not predictive of discontinuation of therapy after a median follow-up of 3 years, Dr. Sheppard and colleagues reported.
The findings suggest "women and/or their providers are making informed judgments about the risks and benefits," the researchers wrote. "An alternative explanation is that women with greater frailty may have been concerned about adverse effects based on interactions of hormonal therapy and specific comorbidities, such as cardio- and/or cerebrovascular disease and risk of thromboembolic events," they added.
Limitations of the study include a potential for bias associated with self-reporting (discontinuation of therapy was measured only through self-report) and measurement of frailty only at baseline, the investigators said.
The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.
Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail, according to researchers.
In a prospective cohort study of women aged 65 and older diagnosed with invasive, nonmetastatic estrogen receptor–positive breast cancers (n = 1,062), about a quarter of the women were frail or prefrail (4.9% and 18.7%, respectively) based on a validated frailty score determined at baseline, reported Vanessa Sheppard, Ph.D., of Georgetown University Medical Center, Washington (J. Clin. Oncol. 2014 June 16 [doi:10.1200/JCO.2013.51.7367]).
Overall, 14% of the women in the study did not initiate hormonal therapy. Women considered prefrail or frail were significantly less likely to initiate therapy than were those considered "robust" or nonfrail (odds ratio, 1.63; 95% CI, 1.11-2.40; P =.013). Nonwhite race was also significantly associated with noninitiation of therapy (OR, 1.71; CI, 1.04-2.80; P = 0.33).
Baseline frailty was not predictive of discontinuation of therapy after a median follow-up of 3 years, Dr. Sheppard and colleagues reported.
The findings suggest "women and/or their providers are making informed judgments about the risks and benefits," the researchers wrote. "An alternative explanation is that women with greater frailty may have been concerned about adverse effects based on interactions of hormonal therapy and specific comorbidities, such as cardio- and/or cerebrovascular disease and risk of thromboembolic events," they added.
Limitations of the study include a potential for bias associated with self-reporting (discontinuation of therapy was measured only through self-report) and measurement of frailty only at baseline, the investigators said.
The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.
Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail, according to researchers.
In a prospective cohort study of women aged 65 and older diagnosed with invasive, nonmetastatic estrogen receptor–positive breast cancers (n = 1,062), about a quarter of the women were frail or prefrail (4.9% and 18.7%, respectively) based on a validated frailty score determined at baseline, reported Vanessa Sheppard, Ph.D., of Georgetown University Medical Center, Washington (J. Clin. Oncol. 2014 June 16 [doi:10.1200/JCO.2013.51.7367]).
Overall, 14% of the women in the study did not initiate hormonal therapy. Women considered prefrail or frail were significantly less likely to initiate therapy than were those considered "robust" or nonfrail (odds ratio, 1.63; 95% CI, 1.11-2.40; P =.013). Nonwhite race was also significantly associated with noninitiation of therapy (OR, 1.71; CI, 1.04-2.80; P = 0.33).
Baseline frailty was not predictive of discontinuation of therapy after a median follow-up of 3 years, Dr. Sheppard and colleagues reported.
The findings suggest "women and/or their providers are making informed judgments about the risks and benefits," the researchers wrote. "An alternative explanation is that women with greater frailty may have been concerned about adverse effects based on interactions of hormonal therapy and specific comorbidities, such as cardio- and/or cerebrovascular disease and risk of thromboembolic events," they added.
Limitations of the study include a potential for bias associated with self-reporting (discontinuation of therapy was measured only through self-report) and measurement of frailty only at baseline, the investigators said.
The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Older women with breast cancer are less likely to initiate adjuvant hormonal therapy if they are frail.
Major finding: Odds ratio for noninitiation of hormonal therapy was 1.63 for frail women compared with nonfrail women (95% CI, 1.11 to 2.40; P = .013) after covariate adjustment.
Data source: A prospective cohort of 1,288 older women diagnosed with invasive, nonmetastatic breast cancer recruited from 78 sites from 2004 to 2011, of which 1,062 had estrogen receptor–positive tumors.
Disclosures: The National Institutes of Health, Amgen, and the Cancer and Leukemia Group B (CALGB) Foundation funded the study. Four of Dr. Sheppard’s coauthors, Dr. Gretchen Kimmick, Dr. Eric Winer, Dr. Arti Hurria, and Dr. Claudine Isaacs reported ties with pharmaceutical manufacturers.
Higher risk of death seen with oral steroids in RA interstitial lung disease
The use of prednisone for 3 or more months at a time was associated with a significantly elevated risk of death in patients with rheumatoid arthritis and interstitial lung disease in a retrospective cohort study.
Interstitial lung disease is present in about 5% of patients with rheumatoid arthritis. For years, oral steroids were commonly used in patients with the disease, but today’s rheumatologists "no longer view oral steroids as optimal treatment in RA-ILD [rheumatoid arthritis–associated interstitial lung disease], and our data now confirm that," said Dr. Clive Kelly of Queen Elizabeth Hospital in Gateshead, England, senior investigator of the study. He added that clinicians should avoid long-term treatment with steroids in this patient group whenever possible.
Dr. Kelly led the British Rheumatoid Interstitial Lung (BRILL) Network’s cohort study of 260 patients with RA-ILD diagnosed over a 25-year period. The BRILL study compared patients with RA-ILD and an equal number of RA controls without lung involvement who were matched for age, sex, and time of diagnosis.
At the annual European Congress of Rheumatology, Dr. Kelly reported that steroid-treated RA-ILD patients, who represented nearly 60% of the cohort, had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002). Although the relative risk of respiratory death was significantly increased for RA-ILD patients, regardless of treatment, when compared with RA patients without ILD, the risk was higher in those who had been on steroids (RR, 2.75; 95% CI, 1.6-4.7; P = .0002) than in those who had not received steroids (RR, 2.06; 95% CI, 1.1-3.8; P = .02), the investigators found.
The comparison also revealed other important findings related to RA-ILD. Patients with RA-ILD had significantly higher mortality than did those with RA alone. Over the course of the 25-year study period, however, mortality progressively improved among the RA-ILD patients, with median age at death rising from 63 to 76 years. This steady improvement, Dr. Kelly said, is partly the result of better and earlier diagnosis of lung involvement.
"It’s one of my many missions in life to get rheumatologists to listen to the lungs when they examine the joints in patients with rheumatoid arthritis," he said. "I think we are getting better. We’ve persuaded the British Society for Rheumatology to incorporate lung function testing and clinical examination of the chest into their basic assessment of a rheumatoid patient."
Also likely affecting the improved mortality seen over the cohort’s study period is a change in therapeutic approach. While RA-ILD patients diagnosed in the first half of the study period were likely to have been treated with only prednisone and azathioprine, in the latter half they were more likely to have received cyclophosphamide and methylprednisolone or mycophenolate. Over the last 12 years, more were treated with biologics, and in the final 6 years of the cohort, patients requiring biologics tended to be treated with rituximab, a B-cell inhibitor, rather than anti–tumor necrosis factor (anti-TNF) agents, the BRILL investigators found.
Mortality was lower among RA-ILD patients treated with mycophenolate than in those treated with other immunosuppressive agents. Among biologic agents used in the cohort, rituximab treatment was associated with improved mortality, but anti-TNF inhibitors were seen to be associated with elevated risk of death.
About 95% of RA-ILD patients are anticyclic citrullinated peptide (anti-CCP) antibody positive, compared with 55%-60% of the RA population as a whole, Dr. Kelly said, "so there’s a strong statistical association of seropositivity, and in those who are seropositive, rituximab works well."
The finding that rituximab was associated with improved survival in the cohort not only has implications for RA-ILD, he said, but also, potentially, for people with idiopathic pulmonary fibrosis (IPF) who are anti-CCP antibody positive. "What [rheumatologists] have, and chest physicians traditionally don’t, is access to rituximab and mycophenolate. But these might be worth trying in IPF as well," he said.
Dr. Kelly noted that prospective trials in RA-ILD are beginning to enroll patients with progressive disease to compare azathioprine and mycophenolate, allowing for the use of oral steroids, as well as patients with active RA and ILD to compare anti-TNF inhibitors against rituximab, also allowing oral steroids.
Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
The use of prednisone for 3 or more months at a time was associated with a significantly elevated risk of death in patients with rheumatoid arthritis and interstitial lung disease in a retrospective cohort study.
Interstitial lung disease is present in about 5% of patients with rheumatoid arthritis. For years, oral steroids were commonly used in patients with the disease, but today’s rheumatologists "no longer view oral steroids as optimal treatment in RA-ILD [rheumatoid arthritis–associated interstitial lung disease], and our data now confirm that," said Dr. Clive Kelly of Queen Elizabeth Hospital in Gateshead, England, senior investigator of the study. He added that clinicians should avoid long-term treatment with steroids in this patient group whenever possible.
Dr. Kelly led the British Rheumatoid Interstitial Lung (BRILL) Network’s cohort study of 260 patients with RA-ILD diagnosed over a 25-year period. The BRILL study compared patients with RA-ILD and an equal number of RA controls without lung involvement who were matched for age, sex, and time of diagnosis.
At the annual European Congress of Rheumatology, Dr. Kelly reported that steroid-treated RA-ILD patients, who represented nearly 60% of the cohort, had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002). Although the relative risk of respiratory death was significantly increased for RA-ILD patients, regardless of treatment, when compared with RA patients without ILD, the risk was higher in those who had been on steroids (RR, 2.75; 95% CI, 1.6-4.7; P = .0002) than in those who had not received steroids (RR, 2.06; 95% CI, 1.1-3.8; P = .02), the investigators found.
The comparison also revealed other important findings related to RA-ILD. Patients with RA-ILD had significantly higher mortality than did those with RA alone. Over the course of the 25-year study period, however, mortality progressively improved among the RA-ILD patients, with median age at death rising from 63 to 76 years. This steady improvement, Dr. Kelly said, is partly the result of better and earlier diagnosis of lung involvement.
"It’s one of my many missions in life to get rheumatologists to listen to the lungs when they examine the joints in patients with rheumatoid arthritis," he said. "I think we are getting better. We’ve persuaded the British Society for Rheumatology to incorporate lung function testing and clinical examination of the chest into their basic assessment of a rheumatoid patient."
Also likely affecting the improved mortality seen over the cohort’s study period is a change in therapeutic approach. While RA-ILD patients diagnosed in the first half of the study period were likely to have been treated with only prednisone and azathioprine, in the latter half they were more likely to have received cyclophosphamide and methylprednisolone or mycophenolate. Over the last 12 years, more were treated with biologics, and in the final 6 years of the cohort, patients requiring biologics tended to be treated with rituximab, a B-cell inhibitor, rather than anti–tumor necrosis factor (anti-TNF) agents, the BRILL investigators found.
Mortality was lower among RA-ILD patients treated with mycophenolate than in those treated with other immunosuppressive agents. Among biologic agents used in the cohort, rituximab treatment was associated with improved mortality, but anti-TNF inhibitors were seen to be associated with elevated risk of death.
About 95% of RA-ILD patients are anticyclic citrullinated peptide (anti-CCP) antibody positive, compared with 55%-60% of the RA population as a whole, Dr. Kelly said, "so there’s a strong statistical association of seropositivity, and in those who are seropositive, rituximab works well."
The finding that rituximab was associated with improved survival in the cohort not only has implications for RA-ILD, he said, but also, potentially, for people with idiopathic pulmonary fibrosis (IPF) who are anti-CCP antibody positive. "What [rheumatologists] have, and chest physicians traditionally don’t, is access to rituximab and mycophenolate. But these might be worth trying in IPF as well," he said.
Dr. Kelly noted that prospective trials in RA-ILD are beginning to enroll patients with progressive disease to compare azathioprine and mycophenolate, allowing for the use of oral steroids, as well as patients with active RA and ILD to compare anti-TNF inhibitors against rituximab, also allowing oral steroids.
Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
The use of prednisone for 3 or more months at a time was associated with a significantly elevated risk of death in patients with rheumatoid arthritis and interstitial lung disease in a retrospective cohort study.
Interstitial lung disease is present in about 5% of patients with rheumatoid arthritis. For years, oral steroids were commonly used in patients with the disease, but today’s rheumatologists "no longer view oral steroids as optimal treatment in RA-ILD [rheumatoid arthritis–associated interstitial lung disease], and our data now confirm that," said Dr. Clive Kelly of Queen Elizabeth Hospital in Gateshead, England, senior investigator of the study. He added that clinicians should avoid long-term treatment with steroids in this patient group whenever possible.
Dr. Kelly led the British Rheumatoid Interstitial Lung (BRILL) Network’s cohort study of 260 patients with RA-ILD diagnosed over a 25-year period. The BRILL study compared patients with RA-ILD and an equal number of RA controls without lung involvement who were matched for age, sex, and time of diagnosis.
At the annual European Congress of Rheumatology, Dr. Kelly reported that steroid-treated RA-ILD patients, who represented nearly 60% of the cohort, had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002). Although the relative risk of respiratory death was significantly increased for RA-ILD patients, regardless of treatment, when compared with RA patients without ILD, the risk was higher in those who had been on steroids (RR, 2.75; 95% CI, 1.6-4.7; P = .0002) than in those who had not received steroids (RR, 2.06; 95% CI, 1.1-3.8; P = .02), the investigators found.
The comparison also revealed other important findings related to RA-ILD. Patients with RA-ILD had significantly higher mortality than did those with RA alone. Over the course of the 25-year study period, however, mortality progressively improved among the RA-ILD patients, with median age at death rising from 63 to 76 years. This steady improvement, Dr. Kelly said, is partly the result of better and earlier diagnosis of lung involvement.
"It’s one of my many missions in life to get rheumatologists to listen to the lungs when they examine the joints in patients with rheumatoid arthritis," he said. "I think we are getting better. We’ve persuaded the British Society for Rheumatology to incorporate lung function testing and clinical examination of the chest into their basic assessment of a rheumatoid patient."
Also likely affecting the improved mortality seen over the cohort’s study period is a change in therapeutic approach. While RA-ILD patients diagnosed in the first half of the study period were likely to have been treated with only prednisone and azathioprine, in the latter half they were more likely to have received cyclophosphamide and methylprednisolone or mycophenolate. Over the last 12 years, more were treated with biologics, and in the final 6 years of the cohort, patients requiring biologics tended to be treated with rituximab, a B-cell inhibitor, rather than anti–tumor necrosis factor (anti-TNF) agents, the BRILL investigators found.
Mortality was lower among RA-ILD patients treated with mycophenolate than in those treated with other immunosuppressive agents. Among biologic agents used in the cohort, rituximab treatment was associated with improved mortality, but anti-TNF inhibitors were seen to be associated with elevated risk of death.
About 95% of RA-ILD patients are anticyclic citrullinated peptide (anti-CCP) antibody positive, compared with 55%-60% of the RA population as a whole, Dr. Kelly said, "so there’s a strong statistical association of seropositivity, and in those who are seropositive, rituximab works well."
The finding that rituximab was associated with improved survival in the cohort not only has implications for RA-ILD, he said, but also, potentially, for people with idiopathic pulmonary fibrosis (IPF) who are anti-CCP antibody positive. "What [rheumatologists] have, and chest physicians traditionally don’t, is access to rituximab and mycophenolate. But these might be worth trying in IPF as well," he said.
Dr. Kelly noted that prospective trials in RA-ILD are beginning to enroll patients with progressive disease to compare azathioprine and mycophenolate, allowing for the use of oral steroids, as well as patients with active RA and ILD to compare anti-TNF inhibitors against rituximab, also allowing oral steroids.
Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
FROM THE EULAR CONGRESS 2014
Key clinical point: Rather than corticosteroids, consider using mycophenolate or rituximab in patients with RA-ILD.
Major finding: Steroid-treated RA-ILD patients had an elevated relative risk of all-cause death, compared with those who had never been treated with steroids (RR, 1.65; 95% confidence interval, 1.2-2.3; P = .002).
Data source: A retrospective study of 260 patients with RA-ILD in the British Rheumatoid Interstitial Lung Network.
Disclosures: Dr. Kelly reported that he had no conflicts of interest related to his findings and that none of his fellow BRILL investigators had conflicts.
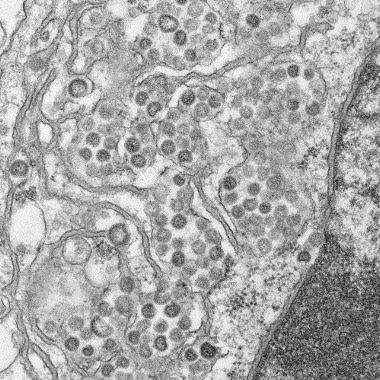
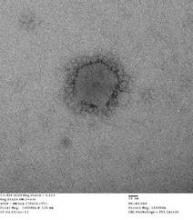
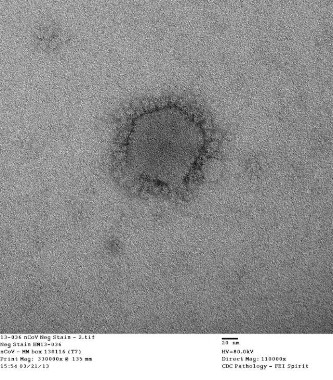
Close contacts of U.S. MERS-CoV cases uninfected
Two U.S. individuals who contracted Middle East Respiratory Syndrome coronavirus (MERS-CoV) did not transmit the virus to members of their households or to health care workers, officials at the Centers for Disease Control and Prevention say.
Both patients with confirmed infections, one in Florida and another in Indiana, had traveled to Saudi Arabia before becoming ill.
Polymerase chain reaction assays and serology revealed no evidence of previous or active infection in any members of their households or healthcare workers who attended them.
In a press statement June 17, Dr. David Swerdlow, who is leading the agency’s MERS-CoV response, called the negative results among contacts that the CDC considered at highest risk for MERS-CoV infection "reassuring."
While the risk MERS-CoV infection in the United States remains low, Dr. Swerdlow added, "it is important that we remain vigilant and quickly identify and respond to any additional importations."
Two U.S. individuals who contracted Middle East Respiratory Syndrome coronavirus (MERS-CoV) did not transmit the virus to members of their households or to health care workers, officials at the Centers for Disease Control and Prevention say.
Both patients with confirmed infections, one in Florida and another in Indiana, had traveled to Saudi Arabia before becoming ill.
Polymerase chain reaction assays and serology revealed no evidence of previous or active infection in any members of their households or healthcare workers who attended them.
In a press statement June 17, Dr. David Swerdlow, who is leading the agency’s MERS-CoV response, called the negative results among contacts that the CDC considered at highest risk for MERS-CoV infection "reassuring."
While the risk MERS-CoV infection in the United States remains low, Dr. Swerdlow added, "it is important that we remain vigilant and quickly identify and respond to any additional importations."
Two U.S. individuals who contracted Middle East Respiratory Syndrome coronavirus (MERS-CoV) did not transmit the virus to members of their households or to health care workers, officials at the Centers for Disease Control and Prevention say.
Both patients with confirmed infections, one in Florida and another in Indiana, had traveled to Saudi Arabia before becoming ill.
Polymerase chain reaction assays and serology revealed no evidence of previous or active infection in any members of their households or healthcare workers who attended them.
In a press statement June 17, Dr. David Swerdlow, who is leading the agency’s MERS-CoV response, called the negative results among contacts that the CDC considered at highest risk for MERS-CoV infection "reassuring."
While the risk MERS-CoV infection in the United States remains low, Dr. Swerdlow added, "it is important that we remain vigilant and quickly identify and respond to any additional importations."
WHO: Basic infection control key to stemming MERS-CoV transmission
The current wave of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections is related to poor infection control in hospitals, officials of the World Health Organization said June 17.
"There is no evidence of sustained person-to-person transmission occurring in communities," Dr. Kenji Fukuda, assistant director general of WHO in Geneva, said during a press conference to disclose findings from the organization’s International Health Regulations Emergency Committee on MERS-CoV. The committee met the day before.
Rather, Dr. Fukuda said, the most recent cases appear to be related to "suboptimal" infection control measures in hospitals. He stressed that the measures required for controlling MERS-CoV transmission in hospital settings were "basic" and could be implemented anywhere, regardless of resources.
"Infection control can really reduce spread in hospitals. This is a critical finding," he said.
WHO officials have reported 701 laboratory confirmed cases of MERS-CoV to date and 249 deaths worldwide. Dr. Fukuda downplayed concerns that Saudi Arabia, where the majority of MERS-CoV cases and deaths have occurred, continues to report inaccurate or out-of-date figures. Significant under-reporting of MERS-CoV cases and deaths was revealed and corrected by Saudi officials early this month, raising the number of cases there by 20%.
"Saudi Arabia is making a very strong effort to catch up," Dr. Fukuda said. "I am confident that the government has really strengthened its efforts to keep on top of the numbers."
He also said that WHO’s infection-prevention recommendations for MERS-CoV have changed to reflect the most recent findings that the virus can be directly transmitted from camels to humans.
"We have a better understanding that camels are a very important source of exposure in the community," he noted. For people working directly with camels, including veterinarians and keepers, Dr. Fukuda said, "recommended practices include leaving clothes in the workplace." Other recommendations include drinking only pasteurized milk and cooking all food thoroughly to prevent another potential source of viral contamination.
Recommendations for pilgrims to Mecca, Saudi Arabia, and for medical delegations accompanying them, "have expanded a lot since last year" to reflect what is known about transmission and exposure. Still, Dr. Fukuda said, WHO officials remain concerned that basic infection control measures be communicated and improved prior to this year’s Hajj in October.
Because so little person-to-person transmission in community settings has been reported so far, he said, "the current situation does not constitute a public health emergency of international concern."
MERS-CoV, however, remains a "serious" public health threat because of its high fatality rate and the fact that much remains unknown about how the virus is transmitted from camels to people, and a lack of understanding about the ultimate source of the virus.
"The gorilla in the room," Dr. Fukuda said, is whether the virus "has the potential to change and become much more transmissible person to person."
The current wave of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections is related to poor infection control in hospitals, officials of the World Health Organization said June 17.
"There is no evidence of sustained person-to-person transmission occurring in communities," Dr. Kenji Fukuda, assistant director general of WHO in Geneva, said during a press conference to disclose findings from the organization’s International Health Regulations Emergency Committee on MERS-CoV. The committee met the day before.
Rather, Dr. Fukuda said, the most recent cases appear to be related to "suboptimal" infection control measures in hospitals. He stressed that the measures required for controlling MERS-CoV transmission in hospital settings were "basic" and could be implemented anywhere, regardless of resources.
"Infection control can really reduce spread in hospitals. This is a critical finding," he said.
WHO officials have reported 701 laboratory confirmed cases of MERS-CoV to date and 249 deaths worldwide. Dr. Fukuda downplayed concerns that Saudi Arabia, where the majority of MERS-CoV cases and deaths have occurred, continues to report inaccurate or out-of-date figures. Significant under-reporting of MERS-CoV cases and deaths was revealed and corrected by Saudi officials early this month, raising the number of cases there by 20%.
"Saudi Arabia is making a very strong effort to catch up," Dr. Fukuda said. "I am confident that the government has really strengthened its efforts to keep on top of the numbers."
He also said that WHO’s infection-prevention recommendations for MERS-CoV have changed to reflect the most recent findings that the virus can be directly transmitted from camels to humans.
"We have a better understanding that camels are a very important source of exposure in the community," he noted. For people working directly with camels, including veterinarians and keepers, Dr. Fukuda said, "recommended practices include leaving clothes in the workplace." Other recommendations include drinking only pasteurized milk and cooking all food thoroughly to prevent another potential source of viral contamination.
Recommendations for pilgrims to Mecca, Saudi Arabia, and for medical delegations accompanying them, "have expanded a lot since last year" to reflect what is known about transmission and exposure. Still, Dr. Fukuda said, WHO officials remain concerned that basic infection control measures be communicated and improved prior to this year’s Hajj in October.
Because so little person-to-person transmission in community settings has been reported so far, he said, "the current situation does not constitute a public health emergency of international concern."
MERS-CoV, however, remains a "serious" public health threat because of its high fatality rate and the fact that much remains unknown about how the virus is transmitted from camels to people, and a lack of understanding about the ultimate source of the virus.
"The gorilla in the room," Dr. Fukuda said, is whether the virus "has the potential to change and become much more transmissible person to person."
The current wave of Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infections is related to poor infection control in hospitals, officials of the World Health Organization said June 17.
"There is no evidence of sustained person-to-person transmission occurring in communities," Dr. Kenji Fukuda, assistant director general of WHO in Geneva, said during a press conference to disclose findings from the organization’s International Health Regulations Emergency Committee on MERS-CoV. The committee met the day before.
Rather, Dr. Fukuda said, the most recent cases appear to be related to "suboptimal" infection control measures in hospitals. He stressed that the measures required for controlling MERS-CoV transmission in hospital settings were "basic" and could be implemented anywhere, regardless of resources.
"Infection control can really reduce spread in hospitals. This is a critical finding," he said.
WHO officials have reported 701 laboratory confirmed cases of MERS-CoV to date and 249 deaths worldwide. Dr. Fukuda downplayed concerns that Saudi Arabia, where the majority of MERS-CoV cases and deaths have occurred, continues to report inaccurate or out-of-date figures. Significant under-reporting of MERS-CoV cases and deaths was revealed and corrected by Saudi officials early this month, raising the number of cases there by 20%.
"Saudi Arabia is making a very strong effort to catch up," Dr. Fukuda said. "I am confident that the government has really strengthened its efforts to keep on top of the numbers."
He also said that WHO’s infection-prevention recommendations for MERS-CoV have changed to reflect the most recent findings that the virus can be directly transmitted from camels to humans.
"We have a better understanding that camels are a very important source of exposure in the community," he noted. For people working directly with camels, including veterinarians and keepers, Dr. Fukuda said, "recommended practices include leaving clothes in the workplace." Other recommendations include drinking only pasteurized milk and cooking all food thoroughly to prevent another potential source of viral contamination.
Recommendations for pilgrims to Mecca, Saudi Arabia, and for medical delegations accompanying them, "have expanded a lot since last year" to reflect what is known about transmission and exposure. Still, Dr. Fukuda said, WHO officials remain concerned that basic infection control measures be communicated and improved prior to this year’s Hajj in October.
Because so little person-to-person transmission in community settings has been reported so far, he said, "the current situation does not constitute a public health emergency of international concern."
MERS-CoV, however, remains a "serious" public health threat because of its high fatality rate and the fact that much remains unknown about how the virus is transmitted from camels to people, and a lack of understanding about the ultimate source of the virus.
"The gorilla in the room," Dr. Fukuda said, is whether the virus "has the potential to change and become much more transmissible person to person."
Risks of two types of polymer stents comparable
Biodegradable biolimus-eluting stents are as safe and effective as durable everolimus-eluting stents at 2 years’ follow-up, with no significant differences seen in rates of target lesion revascularization, mortality, or myocardial infarction.
The findings come from NEXT (NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial), a 3-year randomized trial led by Dr. Masahiro Natsuaki of Saiseikai Fukuoka (Japan) General Hospital. They were presented at the annual meeting of the American College of Cardiology and published in JAMA (doi:10.1001/jama.2014.3584).
NEXT compared the biodegradable polymer biolimus-eluting stent (BP-BES) to the durable polymer everolimus-eluting stent (DP-EES), measuring target lesion revascularization and whether BP-BES carries a risk for excess mortality or MI, compared with DP-EES, as shorter studies and meta-analyses have suggested.
Dr. Natsuaki and colleagues randomized 3,235 patients from nearly 100 treatment centers to BP-BES (n = 1,617) or DP-EES (n = 1,618), with 98% of all patients completing follow-up. Mortality and MI were comparable for both stents (7.8% for BP-BES vs. 7.7% for DP-EES; noninferiority, P = .003), and the need for target lesion revascularization was also comparable for both stents (6.2% vs. 6%; noninferiority, P less than .001). The researchers noted that "2 years is not long enough to confirm the long-term safety of BP-BES, and the study was underpowered for the interim analysis. Follow-up at 3 years will be important."
NEXT was sponsored by Terumo Japan, maker of the biodegradable stents. Two investigators disclosed they are advisers for Terumo Japan and Abbott Vascular Japan, maker of the durable polymer stents used.
Biodegradable biolimus-eluting stents are as safe and effective as durable everolimus-eluting stents at 2 years’ follow-up, with no significant differences seen in rates of target lesion revascularization, mortality, or myocardial infarction.
The findings come from NEXT (NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial), a 3-year randomized trial led by Dr. Masahiro Natsuaki of Saiseikai Fukuoka (Japan) General Hospital. They were presented at the annual meeting of the American College of Cardiology and published in JAMA (doi:10.1001/jama.2014.3584).
NEXT compared the biodegradable polymer biolimus-eluting stent (BP-BES) to the durable polymer everolimus-eluting stent (DP-EES), measuring target lesion revascularization and whether BP-BES carries a risk for excess mortality or MI, compared with DP-EES, as shorter studies and meta-analyses have suggested.
Dr. Natsuaki and colleagues randomized 3,235 patients from nearly 100 treatment centers to BP-BES (n = 1,617) or DP-EES (n = 1,618), with 98% of all patients completing follow-up. Mortality and MI were comparable for both stents (7.8% for BP-BES vs. 7.7% for DP-EES; noninferiority, P = .003), and the need for target lesion revascularization was also comparable for both stents (6.2% vs. 6%; noninferiority, P less than .001). The researchers noted that "2 years is not long enough to confirm the long-term safety of BP-BES, and the study was underpowered for the interim analysis. Follow-up at 3 years will be important."
NEXT was sponsored by Terumo Japan, maker of the biodegradable stents. Two investigators disclosed they are advisers for Terumo Japan and Abbott Vascular Japan, maker of the durable polymer stents used.
Biodegradable biolimus-eluting stents are as safe and effective as durable everolimus-eluting stents at 2 years’ follow-up, with no significant differences seen in rates of target lesion revascularization, mortality, or myocardial infarction.
The findings come from NEXT (NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial), a 3-year randomized trial led by Dr. Masahiro Natsuaki of Saiseikai Fukuoka (Japan) General Hospital. They were presented at the annual meeting of the American College of Cardiology and published in JAMA (doi:10.1001/jama.2014.3584).
NEXT compared the biodegradable polymer biolimus-eluting stent (BP-BES) to the durable polymer everolimus-eluting stent (DP-EES), measuring target lesion revascularization and whether BP-BES carries a risk for excess mortality or MI, compared with DP-EES, as shorter studies and meta-analyses have suggested.
Dr. Natsuaki and colleagues randomized 3,235 patients from nearly 100 treatment centers to BP-BES (n = 1,617) or DP-EES (n = 1,618), with 98% of all patients completing follow-up. Mortality and MI were comparable for both stents (7.8% for BP-BES vs. 7.7% for DP-EES; noninferiority, P = .003), and the need for target lesion revascularization was also comparable for both stents (6.2% vs. 6%; noninferiority, P less than .001). The researchers noted that "2 years is not long enough to confirm the long-term safety of BP-BES, and the study was underpowered for the interim analysis. Follow-up at 3 years will be important."
NEXT was sponsored by Terumo Japan, maker of the biodegradable stents. Two investigators disclosed they are advisers for Terumo Japan and Abbott Vascular Japan, maker of the durable polymer stents used.
Major finding: Mortality and myocardial infarction were comparable for BP-BES (7.8%) vs. DP-EES (7.7%); noninferiority, P = .003), as was the need for target lesion revascularization (6.2% vs. 6%; noninferiority, P less than .001).
Data source: The NOBORI Biolimus-Eluting vs. XIENCE/PROMUS Everolimus-Eluting Stent Trial, a randomized trial of 3,235 patients.
Disclosures: NEXT was sponsored by Terumo Japan, the maker of BP-BES. Two investigators disclosed that they serve as advisers for Terumo Japan and Abbott Vascular Japan, maker of DP-EES.
Treat to Target Shows Durable Improvements in Psoriatic Arthritis
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
Thanks to anti–tumor necrosis factor inhibitors and other highly effective biologic therapies, rheumatologists are increasingly embracing treat to target, a strategy in which patients are closely monitored and medications adjusted until a patient has the least disease activity possible.
Ample evidence from randomized, controlled trials has shown treat to target – sometimes referred to as tight control – to result in better outcomes than standard therapy in rheumatoid arthritis patients.
But in psoriatic arthritis (PsA), a more heterogeneous disorder with skin and nail manifestations as well as joint and connective tissue involvement, remission has historically been less well defined. Only in recent years have endpoints been developed and validated for minimal disease activity in PsA, and evidence in support of a treat-to-target approach is now slowly trickling in.
At the annual European Congress of Rheumatology, Dr. Arthur Kavanaugh of the University of California, San Diego, presented results from a 5-year extension of a randomized, controlled trial of golimumab in patients with PsA that showed better long-term outcomes in those able to achieve minimal disease activity (MDA) through a treat-to-target strategy.
"I think [the study] does provide some evidence suggesting that treat to target could well be a valuable goal for PsA, Dr. Kavanaugh said. "Right now, RA has the advantage of more studies showing this."
Dr. Kavanaugh and his colleagues’ study used data from an open-label extension of a clinical trial in which about 400 patients were randomized to receive golimumab at 50 mg or 100 mg, or placebo; all placebo patients were crossed over to golimumab treatment at 24 weeks and during the long-term, open-label extension of the trial, were followed for as long as 252 weeks. Patients were assessed at 14, 24, and 52 weeks, then yearly until week 252. The investigators used a validated composite endpoint that included measures of skin, joint, and enthesis involvement.
About half of the patients (44.2% of those randomized to placebo and 51.7% of those randomized to active treatment) achieved persistent MDA over three or more consecutive time points during follow-up, and investigators saw significantly better clinically meaningful Health Assessment Questionnaire improvement and less radiographic progression in patients who had achieved MDA, regardless of treatment allocation.
Patients who achieved MDA after crossing over had improvements that were similar to those who started on golimumab at baseline, suggesting that delayed treatment initiation does not result in a worsening of physical function or radiographic progression.
"When it comes to patients, we cannot always control when we will see them in the course of disease," Dr. Kavanaugh said. "These data suggest that even though a patient may show up with months of uncontrolled disease, it is still a viable goal to treat them to try to get MDA."
Dr. Kavanaugh said that further studies in PsA patients were needed to confirm that treat-to-target strategies resulted in better long-term disease outcomes. "As always in medicine, it is important to have verification from multiple studies. It would be helpful to have data from studies using medications with other mechanisms of action to support treat to target," he added.
Dr. Kavanaugh disclosed research and grant support from Abbott, Amgen, Janssen, and UCB. His coauthors on the study disclosed financial support from additional manufacturers, and three were employees of Janssen.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
Thanks to anti–tumor necrosis factor inhibitors and other highly effective biologic therapies, rheumatologists are increasingly embracing treat to target, a strategy in which patients are closely monitored and medications adjusted until a patient has the least disease activity possible.
Ample evidence from randomized, controlled trials has shown treat to target – sometimes referred to as tight control – to result in better outcomes than standard therapy in rheumatoid arthritis patients.
But in psoriatic arthritis (PsA), a more heterogeneous disorder with skin and nail manifestations as well as joint and connective tissue involvement, remission has historically been less well defined. Only in recent years have endpoints been developed and validated for minimal disease activity in PsA, and evidence in support of a treat-to-target approach is now slowly trickling in.
At the annual European Congress of Rheumatology, Dr. Arthur Kavanaugh of the University of California, San Diego, presented results from a 5-year extension of a randomized, controlled trial of golimumab in patients with PsA that showed better long-term outcomes in those able to achieve minimal disease activity (MDA) through a treat-to-target strategy.
"I think [the study] does provide some evidence suggesting that treat to target could well be a valuable goal for PsA, Dr. Kavanaugh said. "Right now, RA has the advantage of more studies showing this."
Dr. Kavanaugh and his colleagues’ study used data from an open-label extension of a clinical trial in which about 400 patients were randomized to receive golimumab at 50 mg or 100 mg, or placebo; all placebo patients were crossed over to golimumab treatment at 24 weeks and during the long-term, open-label extension of the trial, were followed for as long as 252 weeks. Patients were assessed at 14, 24, and 52 weeks, then yearly until week 252. The investigators used a validated composite endpoint that included measures of skin, joint, and enthesis involvement.
About half of the patients (44.2% of those randomized to placebo and 51.7% of those randomized to active treatment) achieved persistent MDA over three or more consecutive time points during follow-up, and investigators saw significantly better clinically meaningful Health Assessment Questionnaire improvement and less radiographic progression in patients who had achieved MDA, regardless of treatment allocation.
Patients who achieved MDA after crossing over had improvements that were similar to those who started on golimumab at baseline, suggesting that delayed treatment initiation does not result in a worsening of physical function or radiographic progression.
"When it comes to patients, we cannot always control when we will see them in the course of disease," Dr. Kavanaugh said. "These data suggest that even though a patient may show up with months of uncontrolled disease, it is still a viable goal to treat them to try to get MDA."
Dr. Kavanaugh said that further studies in PsA patients were needed to confirm that treat-to-target strategies resulted in better long-term disease outcomes. "As always in medicine, it is important to have verification from multiple studies. It would be helpful to have data from studies using medications with other mechanisms of action to support treat to target," he added.
Dr. Kavanaugh disclosed research and grant support from Abbott, Amgen, Janssen, and UCB. His coauthors on the study disclosed financial support from additional manufacturers, and three were employees of Janssen.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
Earn 0.25 hours AMA PRA Category 1 credit: Read this article, and click the link at the end to take the posttest.
Thanks to anti–tumor necrosis factor inhibitors and other highly effective biologic therapies, rheumatologists are increasingly embracing treat to target, a strategy in which patients are closely monitored and medications adjusted until a patient has the least disease activity possible.
Ample evidence from randomized, controlled trials has shown treat to target – sometimes referred to as tight control – to result in better outcomes than standard therapy in rheumatoid arthritis patients.
But in psoriatic arthritis (PsA), a more heterogeneous disorder with skin and nail manifestations as well as joint and connective tissue involvement, remission has historically been less well defined. Only in recent years have endpoints been developed and validated for minimal disease activity in PsA, and evidence in support of a treat-to-target approach is now slowly trickling in.
At the annual European Congress of Rheumatology, Dr. Arthur Kavanaugh of the University of California, San Diego, presented results from a 5-year extension of a randomized, controlled trial of golimumab in patients with PsA that showed better long-term outcomes in those able to achieve minimal disease activity (MDA) through a treat-to-target strategy.
"I think [the study] does provide some evidence suggesting that treat to target could well be a valuable goal for PsA, Dr. Kavanaugh said. "Right now, RA has the advantage of more studies showing this."
Dr. Kavanaugh and his colleagues’ study used data from an open-label extension of a clinical trial in which about 400 patients were randomized to receive golimumab at 50 mg or 100 mg, or placebo; all placebo patients were crossed over to golimumab treatment at 24 weeks and during the long-term, open-label extension of the trial, were followed for as long as 252 weeks. Patients were assessed at 14, 24, and 52 weeks, then yearly until week 252. The investigators used a validated composite endpoint that included measures of skin, joint, and enthesis involvement.
About half of the patients (44.2% of those randomized to placebo and 51.7% of those randomized to active treatment) achieved persistent MDA over three or more consecutive time points during follow-up, and investigators saw significantly better clinically meaningful Health Assessment Questionnaire improvement and less radiographic progression in patients who had achieved MDA, regardless of treatment allocation.
Patients who achieved MDA after crossing over had improvements that were similar to those who started on golimumab at baseline, suggesting that delayed treatment initiation does not result in a worsening of physical function or radiographic progression.
"When it comes to patients, we cannot always control when we will see them in the course of disease," Dr. Kavanaugh said. "These data suggest that even though a patient may show up with months of uncontrolled disease, it is still a viable goal to treat them to try to get MDA."
Dr. Kavanaugh said that further studies in PsA patients were needed to confirm that treat-to-target strategies resulted in better long-term disease outcomes. "As always in medicine, it is important to have verification from multiple studies. It would be helpful to have data from studies using medications with other mechanisms of action to support treat to target," he added.
Dr. Kavanaugh disclosed research and grant support from Abbott, Amgen, Janssen, and UCB. His coauthors on the study disclosed financial support from additional manufacturers, and three were employees of Janssen.
To earn 0.25 hours AMA PRA Category 1 credit after reading this article, take the post-test here.
FROM THE EULAR CONGRESS 2014
Treat to target shows durable improvements in psoriatic arthritis
Thanks to anti–tumor necrosis factor inhibitors and other highly effective biologic therapies, rheumatologists are increasingly embracing treat to target, a strategy in which patients are closely monitored and medications adjusted until a patient has the least disease activity possible.
Ample evidence from randomized, controlled trials has shown treat to target – sometimes referred to as tight control – to result in better outcomes than standard therapy in rheumatoid arthritis patients.
But in psoriatic arthritis (PsA), a more heterogeneous disorder with skin and nail manifestations as well as joint and connective tissue involvement, remission has historically been less well defined. Only in recent years have endpoints been developed and validated for minimal disease activity in PsA, and evidence in support of a treat-to-target approach is now slowly trickling in.
At the annual European Congress of Rheumatology, Dr. Arthur Kavanaugh of the University of California, San Diego, presented results from a 5-year extension of a randomized, controlled trial of golimumab in patients with PsA that showed better long-term outcomes in those able to achieve minimal disease activity (MDA) through a treat-to-target strategy.
"I think [the study] does provide some evidence suggesting that treat to target could well be a valuable goal for PsA, Dr. Kavanaugh said. "Right now, RA has the advantage of more studies showing this."
Dr. Kavanaugh and his colleagues’ study used data from an open-label extension of a clinical trial in which about 400 patients were randomized to receive golimumab at 50 mg or 100 mg, or placebo; all placebo patients were crossed over to golimumab treatment at 24 weeks and during the long-term, open-label extension of the trial, were followed for as long as 252 weeks. Patients were assessed at 14, 24, and 52 weeks, then yearly until week 252. The investigators used a validated composite endpoint that included measures of skin, joint, and enthesis involvement.
About half of the patients (44.2% of those randomized to placebo and 51.7% of those randomized to active treatment) achieved persistent MDA over three or more consecutive time points during follow-up, and investigators saw significantly better clinically meaningful Health Assessment Questionnaire improvement and less radiographic progression in patients who had achieved MDA, regardless of treatment allocation.
Patients who achieved MDA after crossing over had improvements that were similar to those who started on golimumab at baseline, suggesting that delayed treatment initiation does not result in a worsening of physical function or radiographic progression.
"When it comes to patients, we cannot always control when we will see them in the course of disease," Dr. Kavanaugh said. "These data suggest that even though a patient may show up with months of uncontrolled disease, it is still a viable goal to treat them to try to get MDA."
Dr. Kavanaugh said that further studies in PsA patients were needed to confirm that treat-to-target strategies resulted in better long-term disease outcomes. "As always in medicine, it is important to have verification from multiple studies. It would be helpful to have data from studies using medications with other mechanisms of action to support treat to target," he added.
Dr. Kavanaugh disclosed research and grant support from Abbott, Amgen, Janssen, and UCB. His coauthors on the study disclosed financial support from additional manufacturers, and three were employees of Janssen.
Thanks to anti–tumor necrosis factor inhibitors and other highly effective biologic therapies, rheumatologists are increasingly embracing treat to target, a strategy in which patients are closely monitored and medications adjusted until a patient has the least disease activity possible.
Ample evidence from randomized, controlled trials has shown treat to target – sometimes referred to as tight control – to result in better outcomes than standard therapy in rheumatoid arthritis patients.
But in psoriatic arthritis (PsA), a more heterogeneous disorder with skin and nail manifestations as well as joint and connective tissue involvement, remission has historically been less well defined. Only in recent years have endpoints been developed and validated for minimal disease activity in PsA, and evidence in support of a treat-to-target approach is now slowly trickling in.
At the annual European Congress of Rheumatology, Dr. Arthur Kavanaugh of the University of California, San Diego, presented results from a 5-year extension of a randomized, controlled trial of golimumab in patients with PsA that showed better long-term outcomes in those able to achieve minimal disease activity (MDA) through a treat-to-target strategy.
"I think [the study] does provide some evidence suggesting that treat to target could well be a valuable goal for PsA, Dr. Kavanaugh said. "Right now, RA has the advantage of more studies showing this."
Dr. Kavanaugh and his colleagues’ study used data from an open-label extension of a clinical trial in which about 400 patients were randomized to receive golimumab at 50 mg or 100 mg, or placebo; all placebo patients were crossed over to golimumab treatment at 24 weeks and during the long-term, open-label extension of the trial, were followed for as long as 252 weeks. Patients were assessed at 14, 24, and 52 weeks, then yearly until week 252. The investigators used a validated composite endpoint that included measures of skin, joint, and enthesis involvement.
About half of the patients (44.2% of those randomized to placebo and 51.7% of those randomized to active treatment) achieved persistent MDA over three or more consecutive time points during follow-up, and investigators saw significantly better clinically meaningful Health Assessment Questionnaire improvement and less radiographic progression in patients who had achieved MDA, regardless of treatment allocation.
Patients who achieved MDA after crossing over had improvements that were similar to those who started on golimumab at baseline, suggesting that delayed treatment initiation does not result in a worsening of physical function or radiographic progression.
"When it comes to patients, we cannot always control when we will see them in the course of disease," Dr. Kavanaugh said. "These data suggest that even though a patient may show up with months of uncontrolled disease, it is still a viable goal to treat them to try to get MDA."
Dr. Kavanaugh said that further studies in PsA patients were needed to confirm that treat-to-target strategies resulted in better long-term disease outcomes. "As always in medicine, it is important to have verification from multiple studies. It would be helpful to have data from studies using medications with other mechanisms of action to support treat to target," he added.
Dr. Kavanaugh disclosed research and grant support from Abbott, Amgen, Janssen, and UCB. His coauthors on the study disclosed financial support from additional manufacturers, and three were employees of Janssen.
Thanks to anti–tumor necrosis factor inhibitors and other highly effective biologic therapies, rheumatologists are increasingly embracing treat to target, a strategy in which patients are closely monitored and medications adjusted until a patient has the least disease activity possible.
Ample evidence from randomized, controlled trials has shown treat to target – sometimes referred to as tight control – to result in better outcomes than standard therapy in rheumatoid arthritis patients.
But in psoriatic arthritis (PsA), a more heterogeneous disorder with skin and nail manifestations as well as joint and connective tissue involvement, remission has historically been less well defined. Only in recent years have endpoints been developed and validated for minimal disease activity in PsA, and evidence in support of a treat-to-target approach is now slowly trickling in.
At the annual European Congress of Rheumatology, Dr. Arthur Kavanaugh of the University of California, San Diego, presented results from a 5-year extension of a randomized, controlled trial of golimumab in patients with PsA that showed better long-term outcomes in those able to achieve minimal disease activity (MDA) through a treat-to-target strategy.
"I think [the study] does provide some evidence suggesting that treat to target could well be a valuable goal for PsA, Dr. Kavanaugh said. "Right now, RA has the advantage of more studies showing this."
Dr. Kavanaugh and his colleagues’ study used data from an open-label extension of a clinical trial in which about 400 patients were randomized to receive golimumab at 50 mg or 100 mg, or placebo; all placebo patients were crossed over to golimumab treatment at 24 weeks and during the long-term, open-label extension of the trial, were followed for as long as 252 weeks. Patients were assessed at 14, 24, and 52 weeks, then yearly until week 252. The investigators used a validated composite endpoint that included measures of skin, joint, and enthesis involvement.
About half of the patients (44.2% of those randomized to placebo and 51.7% of those randomized to active treatment) achieved persistent MDA over three or more consecutive time points during follow-up, and investigators saw significantly better clinically meaningful Health Assessment Questionnaire improvement and less radiographic progression in patients who had achieved MDA, regardless of treatment allocation.
Patients who achieved MDA after crossing over had improvements that were similar to those who started on golimumab at baseline, suggesting that delayed treatment initiation does not result in a worsening of physical function or radiographic progression.
"When it comes to patients, we cannot always control when we will see them in the course of disease," Dr. Kavanaugh said. "These data suggest that even though a patient may show up with months of uncontrolled disease, it is still a viable goal to treat them to try to get MDA."
Dr. Kavanaugh said that further studies in PsA patients were needed to confirm that treat-to-target strategies resulted in better long-term disease outcomes. "As always in medicine, it is important to have verification from multiple studies. It would be helpful to have data from studies using medications with other mechanisms of action to support treat to target," he added.
Dr. Kavanaugh disclosed research and grant support from Abbott, Amgen, Janssen, and UCB. His coauthors on the study disclosed financial support from additional manufacturers, and three were employees of Janssen.
FROM THE EULAR CONGRESS 2014
MERS virus can be transmitted from camels to humans, case report shows
A case report on a camel-to-human transmission of Middle East respiratory syndrome coronavirus (MERS-CoV) offers the first conclusive evidence that the virus can be transmitted directly from camels to humans.
The report, published online June 4 in the New England Journal of Medicine (doi:10.1056/NEJMoa1401505) by Esam I. Azhar, Ph.D. and his colleagues at King Abdulaziz University Hospital, in Jeddah, Saudi Arabia, describes a 44-year old Saudi man who, in late October 2013, had applied medicine to the nasal passage of a camel with rhinorrhea.
After becoming ill with respiratory symptoms a week later, he was hospitalized, and investigators visited his stables to collect nasal fluid and blood samples from his animals. Nasal swabs revealed active MERS-CoV infection in one of the nine camels the man owned, and serologic samples from other camels in his herd showed evidence of recent prior infection, at a time when serum screening by immunofluorescence assay had yet to reveal MERS-CoV antibodies in the patient. This meant, the researchers noted, that the camels had been infected before the patient.
Using nasal swab samples, Dr. Azhar and his colleagues carried out reverse-transcriptase–polymerase-chain-reaction detection, isolation, and sequencing of MERS-CoV from the camel with active infection and the patient. Viral isolates from both camel and human were shown to be identical on genetic sequencing. Previously, antibodies cross-reactive to MERS-CoV had been identified in camels, but without isolation and comparison of virus from both animal and human, the camels’ role as reservoirs or intermediate hosts for transmitting the virus to humans could not be confirmed.
In this case, Dr. Azhar and his colleagues wrote in their analysis, "direct cross-species transmission had probably occurred between the two without any intermediate host." The patient died after 2 weeks’ hospitalization, but all of his animals appeared to have cleared the virus following acute infection, suggesting that camels act only as transient hosts of the virus. "The exact reservoir that maintains the virus in its ecologic niche has yet to be identified," the investigators wrote.
None of the study’s authors reported conflicts of interest.
A case report on a camel-to-human transmission of Middle East respiratory syndrome coronavirus (MERS-CoV) offers the first conclusive evidence that the virus can be transmitted directly from camels to humans.
The report, published online June 4 in the New England Journal of Medicine (doi:10.1056/NEJMoa1401505) by Esam I. Azhar, Ph.D. and his colleagues at King Abdulaziz University Hospital, in Jeddah, Saudi Arabia, describes a 44-year old Saudi man who, in late October 2013, had applied medicine to the nasal passage of a camel with rhinorrhea.
After becoming ill with respiratory symptoms a week later, he was hospitalized, and investigators visited his stables to collect nasal fluid and blood samples from his animals. Nasal swabs revealed active MERS-CoV infection in one of the nine camels the man owned, and serologic samples from other camels in his herd showed evidence of recent prior infection, at a time when serum screening by immunofluorescence assay had yet to reveal MERS-CoV antibodies in the patient. This meant, the researchers noted, that the camels had been infected before the patient.
Using nasal swab samples, Dr. Azhar and his colleagues carried out reverse-transcriptase–polymerase-chain-reaction detection, isolation, and sequencing of MERS-CoV from the camel with active infection and the patient. Viral isolates from both camel and human were shown to be identical on genetic sequencing. Previously, antibodies cross-reactive to MERS-CoV had been identified in camels, but without isolation and comparison of virus from both animal and human, the camels’ role as reservoirs or intermediate hosts for transmitting the virus to humans could not be confirmed.
In this case, Dr. Azhar and his colleagues wrote in their analysis, "direct cross-species transmission had probably occurred between the two without any intermediate host." The patient died after 2 weeks’ hospitalization, but all of his animals appeared to have cleared the virus following acute infection, suggesting that camels act only as transient hosts of the virus. "The exact reservoir that maintains the virus in its ecologic niche has yet to be identified," the investigators wrote.
None of the study’s authors reported conflicts of interest.
A case report on a camel-to-human transmission of Middle East respiratory syndrome coronavirus (MERS-CoV) offers the first conclusive evidence that the virus can be transmitted directly from camels to humans.
The report, published online June 4 in the New England Journal of Medicine (doi:10.1056/NEJMoa1401505) by Esam I. Azhar, Ph.D. and his colleagues at King Abdulaziz University Hospital, in Jeddah, Saudi Arabia, describes a 44-year old Saudi man who, in late October 2013, had applied medicine to the nasal passage of a camel with rhinorrhea.
After becoming ill with respiratory symptoms a week later, he was hospitalized, and investigators visited his stables to collect nasal fluid and blood samples from his animals. Nasal swabs revealed active MERS-CoV infection in one of the nine camels the man owned, and serologic samples from other camels in his herd showed evidence of recent prior infection, at a time when serum screening by immunofluorescence assay had yet to reveal MERS-CoV antibodies in the patient. This meant, the researchers noted, that the camels had been infected before the patient.
Using nasal swab samples, Dr. Azhar and his colleagues carried out reverse-transcriptase–polymerase-chain-reaction detection, isolation, and sequencing of MERS-CoV from the camel with active infection and the patient. Viral isolates from both camel and human were shown to be identical on genetic sequencing. Previously, antibodies cross-reactive to MERS-CoV had been identified in camels, but without isolation and comparison of virus from both animal and human, the camels’ role as reservoirs or intermediate hosts for transmitting the virus to humans could not be confirmed.
In this case, Dr. Azhar and his colleagues wrote in their analysis, "direct cross-species transmission had probably occurred between the two without any intermediate host." The patient died after 2 weeks’ hospitalization, but all of his animals appeared to have cleared the virus following acute infection, suggesting that camels act only as transient hosts of the virus. "The exact reservoir that maintains the virus in its ecologic niche has yet to be identified," the investigators wrote.
None of the study’s authors reported conflicts of interest.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
CDC: Illinois man cleared of MERS-CoV; not infected by Indiana patient
An Indiana man infected with Middle East Respiratory Syndrome Coronavirus had not transmitted the virus to a colleague in Illinois, as was previously suspected, Centers for Disease Control and Prevention officials said May 28.
On May 17, the CDC disclosed that the Illinois resident, who had attended an extended business meeting with the confirmed Indiana MERS-CoV patient prior to that patient’s hospitalization, had tested positive for MERS-CoV antibodies on serological assays. The case was particularly worrisome because it suggested transmission through a more casual level of contact than has been seen in most MERS cases to date.
However, CDC officials now say that additional testing with a neutralizing antibody assay, a more definitive blood test that takes 5 or more days to produce results, revealed that the Illinois man had not been infected with MERS-CoV.
Thus far only two U.S. residents have been confirmed with MERS-CoV, the Indiana patient and a Florida patient. Both became ill after travel to Saudi Arabia. None of their stateside contacts has yet tested positive, though voluntary testing of family members, colleagues, fellow passengers on flights, and attending health care workers continues.
In a news conference May 28, Mark Pallansch, Ph.D., director of CDC’s Division of Viral Diseases at the National Center for Immunization and Respiratory Diseases, acknowledged that the two serological assays used in preliminary testing – the enzyme-linked immunosorbent assay (ELISA) and the immunofluorescent assay (IFA), have high specificity in excluding infection, but can result in false positives.
In the case of the Illinois resident, "there are several potential explanations for our preliminary results – that is still something not completely understood," Dr. Pallansch said. The most common and likely explanation, he said, is some cross-reactivity with another type of coronavirus.
Dr. Pallansch said that CDC would continue its protocol of using ELISA and IFA, with any positive results followed by the neutralizing antibody assay.
The neutralizing antibody assay, he said, uses live MERS-CoV and must be conducted in a high-containment lab, making it difficult to test hundreds of samples; thus initial screening with the other assays would continue.
Various serology assays are being developed around the world, including by the CDC, that may supplant the assays currently being used in the initial serological detection of MERS-CoV, Dr. Pallansch reported.
Dr. David Swerdlow, who is leading CDC’s MERS-CoV response, said during the press conference that the CDC would continue to maintain its high level of vigilance, which includes disclosing to the public any initial positive results pending confirmation, and asking individuals who test positive on first assays to take significant measures to avoid contacts that could result in transmission.
Before definitive serological results were returned, the Illinois man was asked to wear a mask, to avoid close contact with other people, and to not come in contact with crowds. He was fully compliant with those requests, Dr. Swerdlow said.
"We can’t have all the tests back in order to take public health action," Dr. Swerdlow said, noting that MERS-CoV has been seen associated with a 30% fatality rate.
"It’s our priority to make sure the public is protected," he added. While there have been only two imported cases to date in the United States, and no infections yet found to have occurred as a result of local transmission, MERS-CoV "can and likely will enter our country again."
An Indiana man infected with Middle East Respiratory Syndrome Coronavirus had not transmitted the virus to a colleague in Illinois, as was previously suspected, Centers for Disease Control and Prevention officials said May 28.
On May 17, the CDC disclosed that the Illinois resident, who had attended an extended business meeting with the confirmed Indiana MERS-CoV patient prior to that patient’s hospitalization, had tested positive for MERS-CoV antibodies on serological assays. The case was particularly worrisome because it suggested transmission through a more casual level of contact than has been seen in most MERS cases to date.
However, CDC officials now say that additional testing with a neutralizing antibody assay, a more definitive blood test that takes 5 or more days to produce results, revealed that the Illinois man had not been infected with MERS-CoV.
Thus far only two U.S. residents have been confirmed with MERS-CoV, the Indiana patient and a Florida patient. Both became ill after travel to Saudi Arabia. None of their stateside contacts has yet tested positive, though voluntary testing of family members, colleagues, fellow passengers on flights, and attending health care workers continues.
In a news conference May 28, Mark Pallansch, Ph.D., director of CDC’s Division of Viral Diseases at the National Center for Immunization and Respiratory Diseases, acknowledged that the two serological assays used in preliminary testing – the enzyme-linked immunosorbent assay (ELISA) and the immunofluorescent assay (IFA), have high specificity in excluding infection, but can result in false positives.
In the case of the Illinois resident, "there are several potential explanations for our preliminary results – that is still something not completely understood," Dr. Pallansch said. The most common and likely explanation, he said, is some cross-reactivity with another type of coronavirus.
Dr. Pallansch said that CDC would continue its protocol of using ELISA and IFA, with any positive results followed by the neutralizing antibody assay.
The neutralizing antibody assay, he said, uses live MERS-CoV and must be conducted in a high-containment lab, making it difficult to test hundreds of samples; thus initial screening with the other assays would continue.
Various serology assays are being developed around the world, including by the CDC, that may supplant the assays currently being used in the initial serological detection of MERS-CoV, Dr. Pallansch reported.
Dr. David Swerdlow, who is leading CDC’s MERS-CoV response, said during the press conference that the CDC would continue to maintain its high level of vigilance, which includes disclosing to the public any initial positive results pending confirmation, and asking individuals who test positive on first assays to take significant measures to avoid contacts that could result in transmission.
Before definitive serological results were returned, the Illinois man was asked to wear a mask, to avoid close contact with other people, and to not come in contact with crowds. He was fully compliant with those requests, Dr. Swerdlow said.
"We can’t have all the tests back in order to take public health action," Dr. Swerdlow said, noting that MERS-CoV has been seen associated with a 30% fatality rate.
"It’s our priority to make sure the public is protected," he added. While there have been only two imported cases to date in the United States, and no infections yet found to have occurred as a result of local transmission, MERS-CoV "can and likely will enter our country again."
An Indiana man infected with Middle East Respiratory Syndrome Coronavirus had not transmitted the virus to a colleague in Illinois, as was previously suspected, Centers for Disease Control and Prevention officials said May 28.
On May 17, the CDC disclosed that the Illinois resident, who had attended an extended business meeting with the confirmed Indiana MERS-CoV patient prior to that patient’s hospitalization, had tested positive for MERS-CoV antibodies on serological assays. The case was particularly worrisome because it suggested transmission through a more casual level of contact than has been seen in most MERS cases to date.
However, CDC officials now say that additional testing with a neutralizing antibody assay, a more definitive blood test that takes 5 or more days to produce results, revealed that the Illinois man had not been infected with MERS-CoV.
Thus far only two U.S. residents have been confirmed with MERS-CoV, the Indiana patient and a Florida patient. Both became ill after travel to Saudi Arabia. None of their stateside contacts has yet tested positive, though voluntary testing of family members, colleagues, fellow passengers on flights, and attending health care workers continues.
In a news conference May 28, Mark Pallansch, Ph.D., director of CDC’s Division of Viral Diseases at the National Center for Immunization and Respiratory Diseases, acknowledged that the two serological assays used in preliminary testing – the enzyme-linked immunosorbent assay (ELISA) and the immunofluorescent assay (IFA), have high specificity in excluding infection, but can result in false positives.
In the case of the Illinois resident, "there are several potential explanations for our preliminary results – that is still something not completely understood," Dr. Pallansch said. The most common and likely explanation, he said, is some cross-reactivity with another type of coronavirus.
Dr. Pallansch said that CDC would continue its protocol of using ELISA and IFA, with any positive results followed by the neutralizing antibody assay.
The neutralizing antibody assay, he said, uses live MERS-CoV and must be conducted in a high-containment lab, making it difficult to test hundreds of samples; thus initial screening with the other assays would continue.
Various serology assays are being developed around the world, including by the CDC, that may supplant the assays currently being used in the initial serological detection of MERS-CoV, Dr. Pallansch reported.
Dr. David Swerdlow, who is leading CDC’s MERS-CoV response, said during the press conference that the CDC would continue to maintain its high level of vigilance, which includes disclosing to the public any initial positive results pending confirmation, and asking individuals who test positive on first assays to take significant measures to avoid contacts that could result in transmission.
Before definitive serological results were returned, the Illinois man was asked to wear a mask, to avoid close contact with other people, and to not come in contact with crowds. He was fully compliant with those requests, Dr. Swerdlow said.
"We can’t have all the tests back in order to take public health action," Dr. Swerdlow said, noting that MERS-CoV has been seen associated with a 30% fatality rate.
"It’s our priority to make sure the public is protected," he added. While there have been only two imported cases to date in the United States, and no infections yet found to have occurred as a result of local transmission, MERS-CoV "can and likely will enter our country again."
Yearly monitoring does not predict fractures after bisphosphonate cessation
Fracture risk in women who discontinue therapy with bisphosphonates can be assessed by measuring bone mineral density at the time of discontinuation, but subsequent frequent monitoring appears to have little predictive value, according to researchers.
The findings were published online May 5 in JAMA Internal Medicine (doi:10.1001/jamainternmed.2014.1232).
Dr. Douglas Bauer of the University of California, San Francisco, and his colleagues looked at data from a trial of about 1,000 postmenopausal women aged 61-86 who had been treated for 4-5 years with alendronate and were randomized to an additional 5 years of alendronate treatment or placebo.
Among the 437 women assigned to placebo, 22% (n = 94) experienced one or more fractures during follow-up. Hip and neck BMD were assessed via dual-energy x-ray absorptiometry (DXA) at baseline and at 1 and 3 years, and bone turnover markers (BTMs) were also analyzed.
Neither 1-year changes in hip DXA nor 1- or 3-year changes in BTM levels were associated with fracture risk. Only age and lower hip BMD at the time of treatment discontinuation were significantly predictive of fracture, according to Dr. Bauer and his associates.
The relative hazard ratio was 1.87 (95% confidence interval, 1.20-2.92) for fracture risk in lowest tertile of baseline hip BMD, and 1.54 (95% CI, 1.26-1.85) per 5-year increase in age.
Yearly monitoring – recommended by many experts after discontinuation of bisphosphonates – should not be considered predictive of fractures, the researchers noted. It was "somewhat surprising that short-term changes in these individual measurements are not associated with fracture risk after discontinuation," they wrote.
The researchers cautioned clinicians and patients contemplating a drug holiday after 5 years of treatment to "be aware that short-term monitoring to detect individuals at higher risk who might resume bisphosphonate therapy, or initiate another therapy, may not add to risk prediction over and above age and BMD measured at the time of discontinuation."
The study’s limitations include its relatively small number of fractures observed, reducing its statistical power, and the use of a single bisphosphonate medication, Dr. Bauer acknowledged.
The researchers received no outside funding, though their findings were derived from an analysis of the placebo group of the FLEX trial, a manufacturer-sponsored study comparing extended alendronate sodium treatment with placebo in a large cohort of women treated 5 years on alendronate (JAMA 2006;296:2927-38).
Dr. Bauer reported no conflicts of interest related to the study; other study authors disclosed financial relationships with Amgen, GlaxoSmithKline, Merck, Novartis, Nycomed, and Eli Lilly.
In an era when we know much more about how to start than how to stop alendronate therapy, the results of Bauer and colleagues suggest that identification of patients at high risk of fracture after treatment discontinuation is best accomplished by BMD measurement at the time of discontinuation rather than frequent short-term monitoring with BMD or bone turnover marker measurements after treatment discontinuation.
The study is convincing because of its reliance on a clinical fracture outcome rather than surrogate measures such as rates of BMD loss or changes in bone turnover marker levels. Future studies should be longer in duration to accumulate more evidence regarding predictors of long-term fracture incidence after bisphosphonate withdrawal and to identify an outcomes-based bisphosphonate washout period for trials of sequential therapy.
Dr. Margaret L. Gourlay is in the department of family medicine at the University of North Carolina, Chapel Hill, and Dr. Kristine E. Ensrud is in the school of public health at the University of Minnesota, Minneapolis. Dr. Ensrud disclosed a consulting relationship with Merck Sharp & Dohme. Their comments were taken from an editorial (JAMA Intern. Med. 2014 May 5 [doi:10.1001/jamainternmed.2014.162]).
In an era when we know much more about how to start than how to stop alendronate therapy, the results of Bauer and colleagues suggest that identification of patients at high risk of fracture after treatment discontinuation is best accomplished by BMD measurement at the time of discontinuation rather than frequent short-term monitoring with BMD or bone turnover marker measurements after treatment discontinuation.
The study is convincing because of its reliance on a clinical fracture outcome rather than surrogate measures such as rates of BMD loss or changes in bone turnover marker levels. Future studies should be longer in duration to accumulate more evidence regarding predictors of long-term fracture incidence after bisphosphonate withdrawal and to identify an outcomes-based bisphosphonate washout period for trials of sequential therapy.
Dr. Margaret L. Gourlay is in the department of family medicine at the University of North Carolina, Chapel Hill, and Dr. Kristine E. Ensrud is in the school of public health at the University of Minnesota, Minneapolis. Dr. Ensrud disclosed a consulting relationship with Merck Sharp & Dohme. Their comments were taken from an editorial (JAMA Intern. Med. 2014 May 5 [doi:10.1001/jamainternmed.2014.162]).
In an era when we know much more about how to start than how to stop alendronate therapy, the results of Bauer and colleagues suggest that identification of patients at high risk of fracture after treatment discontinuation is best accomplished by BMD measurement at the time of discontinuation rather than frequent short-term monitoring with BMD or bone turnover marker measurements after treatment discontinuation.
The study is convincing because of its reliance on a clinical fracture outcome rather than surrogate measures such as rates of BMD loss or changes in bone turnover marker levels. Future studies should be longer in duration to accumulate more evidence regarding predictors of long-term fracture incidence after bisphosphonate withdrawal and to identify an outcomes-based bisphosphonate washout period for trials of sequential therapy.
Dr. Margaret L. Gourlay is in the department of family medicine at the University of North Carolina, Chapel Hill, and Dr. Kristine E. Ensrud is in the school of public health at the University of Minnesota, Minneapolis. Dr. Ensrud disclosed a consulting relationship with Merck Sharp & Dohme. Their comments were taken from an editorial (JAMA Intern. Med. 2014 May 5 [doi:10.1001/jamainternmed.2014.162]).
Fracture risk in women who discontinue therapy with bisphosphonates can be assessed by measuring bone mineral density at the time of discontinuation, but subsequent frequent monitoring appears to have little predictive value, according to researchers.
The findings were published online May 5 in JAMA Internal Medicine (doi:10.1001/jamainternmed.2014.1232).
Dr. Douglas Bauer of the University of California, San Francisco, and his colleagues looked at data from a trial of about 1,000 postmenopausal women aged 61-86 who had been treated for 4-5 years with alendronate and were randomized to an additional 5 years of alendronate treatment or placebo.
Among the 437 women assigned to placebo, 22% (n = 94) experienced one or more fractures during follow-up. Hip and neck BMD were assessed via dual-energy x-ray absorptiometry (DXA) at baseline and at 1 and 3 years, and bone turnover markers (BTMs) were also analyzed.
Neither 1-year changes in hip DXA nor 1- or 3-year changes in BTM levels were associated with fracture risk. Only age and lower hip BMD at the time of treatment discontinuation were significantly predictive of fracture, according to Dr. Bauer and his associates.
The relative hazard ratio was 1.87 (95% confidence interval, 1.20-2.92) for fracture risk in lowest tertile of baseline hip BMD, and 1.54 (95% CI, 1.26-1.85) per 5-year increase in age.
Yearly monitoring – recommended by many experts after discontinuation of bisphosphonates – should not be considered predictive of fractures, the researchers noted. It was "somewhat surprising that short-term changes in these individual measurements are not associated with fracture risk after discontinuation," they wrote.
The researchers cautioned clinicians and patients contemplating a drug holiday after 5 years of treatment to "be aware that short-term monitoring to detect individuals at higher risk who might resume bisphosphonate therapy, or initiate another therapy, may not add to risk prediction over and above age and BMD measured at the time of discontinuation."
The study’s limitations include its relatively small number of fractures observed, reducing its statistical power, and the use of a single bisphosphonate medication, Dr. Bauer acknowledged.
The researchers received no outside funding, though their findings were derived from an analysis of the placebo group of the FLEX trial, a manufacturer-sponsored study comparing extended alendronate sodium treatment with placebo in a large cohort of women treated 5 years on alendronate (JAMA 2006;296:2927-38).
Dr. Bauer reported no conflicts of interest related to the study; other study authors disclosed financial relationships with Amgen, GlaxoSmithKline, Merck, Novartis, Nycomed, and Eli Lilly.
Fracture risk in women who discontinue therapy with bisphosphonates can be assessed by measuring bone mineral density at the time of discontinuation, but subsequent frequent monitoring appears to have little predictive value, according to researchers.
The findings were published online May 5 in JAMA Internal Medicine (doi:10.1001/jamainternmed.2014.1232).
Dr. Douglas Bauer of the University of California, San Francisco, and his colleagues looked at data from a trial of about 1,000 postmenopausal women aged 61-86 who had been treated for 4-5 years with alendronate and were randomized to an additional 5 years of alendronate treatment or placebo.
Among the 437 women assigned to placebo, 22% (n = 94) experienced one or more fractures during follow-up. Hip and neck BMD were assessed via dual-energy x-ray absorptiometry (DXA) at baseline and at 1 and 3 years, and bone turnover markers (BTMs) were also analyzed.
Neither 1-year changes in hip DXA nor 1- or 3-year changes in BTM levels were associated with fracture risk. Only age and lower hip BMD at the time of treatment discontinuation were significantly predictive of fracture, according to Dr. Bauer and his associates.
The relative hazard ratio was 1.87 (95% confidence interval, 1.20-2.92) for fracture risk in lowest tertile of baseline hip BMD, and 1.54 (95% CI, 1.26-1.85) per 5-year increase in age.
Yearly monitoring – recommended by many experts after discontinuation of bisphosphonates – should not be considered predictive of fractures, the researchers noted. It was "somewhat surprising that short-term changes in these individual measurements are not associated with fracture risk after discontinuation," they wrote.
The researchers cautioned clinicians and patients contemplating a drug holiday after 5 years of treatment to "be aware that short-term monitoring to detect individuals at higher risk who might resume bisphosphonate therapy, or initiate another therapy, may not add to risk prediction over and above age and BMD measured at the time of discontinuation."
The study’s limitations include its relatively small number of fractures observed, reducing its statistical power, and the use of a single bisphosphonate medication, Dr. Bauer acknowledged.
The researchers received no outside funding, though their findings were derived from an analysis of the placebo group of the FLEX trial, a manufacturer-sponsored study comparing extended alendronate sodium treatment with placebo in a large cohort of women treated 5 years on alendronate (JAMA 2006;296:2927-38).
Dr. Bauer reported no conflicts of interest related to the study; other study authors disclosed financial relationships with Amgen, GlaxoSmithKline, Merck, Novartis, Nycomed, and Eli Lilly.