User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
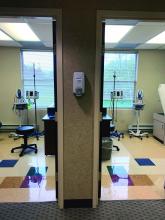
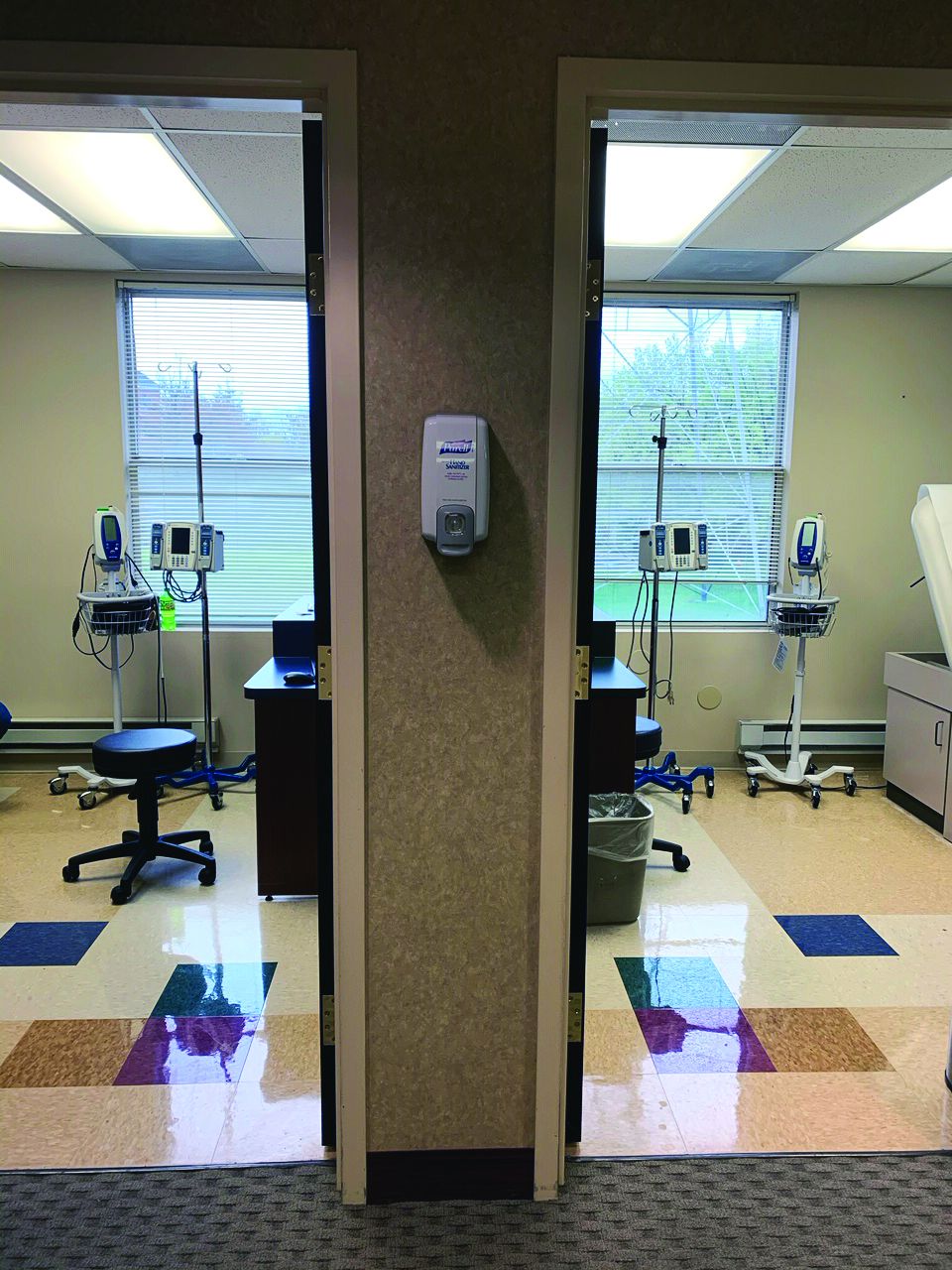
Infusion center directors shuffle treatment services in the era of COVID-19
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
It’s anything but business as usual for clinicians who oversee office-based infusion centers, as they scramble to maintain services for patients considered to be at heightened risk for severe illness should they become infected with COVID-19.
“For many reasons, the guidance for patients right now is that they stay on their medications,” Max I. Hamburger, MD, a managing partner at Rheumatology Associates of Long Island (N.Y.), said in an interview. “Some have decided to stop the drug, and then they call us up to tell us that they’re flaring. The beginning of a flare is tiredness and other things. Now they’re worried: Are they tired because of the disease, or are they tired because they have COVID-19?”
With five office locations located in a region considered to be the epicenter of the COVID-19 pandemic in the United States, Dr. Hamburger and his colleagues are hypervigilant about screening patients for symptoms of the virus before they visit one of the three practice locations that provide infusion services. This starts with an automated phone system that reminds patients of their appointment time. “Part of that robocall now has some questions like, ‘Do you have any symptoms of COVID-19?’ ‘Are you running a fever?’ ‘Do you have any reason to worry about yourself? If so, please call us.’ ” The infusion nurses are also calling the patients in advance of their appointment to check on their status. “When they get to the office location, we ask them again about their general health and check their temperature,” said Dr. Hamburger, who is also founder and executive chairman of United Rheumatology, which is a nationwide rheumatology care management services organization with 650 members in 39 states. “We’re doing everything we can to talk to them about their own state of health and to question them about what I call extended paranoia: like, ‘Who are you living with?’ ‘Who are you hanging out with?’ ‘What are all the six degrees of separation here?’ I want to know what the patient’s husband did last night. I want to know where their kids were over this past week, et cetera. We do everything we can to see if there’s anybody who might have had the slightest [contact with someone who has COVID-19]. Because if I lose my infusion nurse, then I’m up the creek.”
The infusion nurse wears scrubs, a face mask, and latex gloves. She and her staff are using hand sanitizer and cleaning infusion equipment with sanitizing wipes as one might do in a surgical setting. “Every surface is wiped down between patients, and the nurse is changing gloves between patients,” said Dr. Hamburger, who was founding president of the New York State Rheumatology Society before retiring from that post in 2017. “Getting masks has been tough. We’re doing the best we can there. We’re not gloving patients but we’re masking patients.”
As noted in guidance from the American College of Rheumatology and other medical organizations, following the CDC’s recommendation to stay at home during the pandemic has jump-started conversations between physicians and their patients about modifying the time interval between infusions. “If they have been doing well for the last 9 months, we’re having a conversation such as ‘Maybe instead of getting your Orencia every 4 weeks, maybe we’ll push it out to 5 weeks, or maybe we’ll push the Enbrel out to 10 days and the Humira out 3 weeks, et cetera,” Dr. Hamburger said. “One has to be very careful about when you do that, because you don’t want the patient to flare up because it’s hard to get them in, but it is a natural opportunity to look at this. We’re seeing how we can optimize the dose, but I don’t want to send the message that we’re doing this because it changes the patient’s outcome, because there’s zero evidence that it’s a good thing to do in terms of resistance.”
At the infusion centers operated by the Johns Hopkins division of gastroenterology and hepatology, Baltimore, clinicians are not increasing the time interval between infusions for patients at this time. “We’re keeping them as they are, to prevent any flare-ups. Our main goal is to keep patients in remission and out of the hospital,” said Alyssa M. Parian, MD, medical director of the infusion center and associate director of the university’s GI department. “With Remicade specifically, there’s also the risk of developing antibodies if you delay treatment, so we’re basically keeping everyone on track. We’re not recommending a switch from infusions to injectables, and we also are not speeding up infusions, either. Before this pandemic happened, we had already tried to decrease all Remicade infusions from 2 hours to 1 hour for patient satisfaction. The Entyvio is a pretty quick, 30-minute infusion.”
To accommodate patients during this era of physical distancing measures recommended by the Centers for Disease Control and Prevention, Dr. Parian and her infusion nurse manager Elisheva Weiser converted one of their two outpatient GI centers into an infusion-only suite with 12 individual clinic rooms. As soon as patients exit the second-floor elevator, they encounter a workstation prior to entering the office where they are screened for COVID-19 symptoms and their temperature is taken. “If any symptoms or temperature comes back positive, we’re asking them to postpone their treatment and consider COVID testing,” she said.
Instead of one nurse looking after four patients in one room during infusion therapy, now one nurse looks after two patients who are in rooms next to each other. All patients and all staff wear masks while in the center. “We always have physician oversight at our infusion centers,” Dr. Parian said. “We are trying to maintain a ‘COVID-free zone.’ Therefore, no physicians who have served in a hospital ward are allowed in the infusion suite because we don’t want any carriers of COVID-19. Same with the nurses. Additionally, we limit the staff within the suite to only those who are essential and don’t allow anyone to perform telemedicine or urgent clinic visits in this location. Our infusion center staff are on a strict protocol to not come in with any symptoms at all. They are asked to take their temperature before coming in to work.”
She and her colleagues drew from recommendations from the joint GI society message on COVID-19, the Crohn’s and Colitis Foundation, and the International Organization for the Study of Inflammatory Bowel Disease (IOIBD) to inform their approach in serving patients during this unprecedented time. “We went as conservative as possible because these are immunosuppressed patients,” she said. One patient on her panel who receives an infusion every 8 weeks tested positive for COVID-19 between infusions, but was not hospitalized. Dr. Parian said that person will only be treated 14 days after the all symptoms disappear. “That person will wear a mask and will be infused in a separate room,” she said.
In Aventura, Fla., Norman B. Gaylis, MD, and his colleagues at Arthritis & Rheumatic Disease Specialties are looking into shutting down their infusion services during the time period that local public health officials consider to be the peak level of exposure to COVID-19. “We’ve tried to work around that, and bring people in a little early,” said Dr. Gaylis, medical director of rheumatology and infusion services at the practice. “We’ve done our best to mitigate the risk [of exposure] as much as possible.” This includes staggering their caseload by infusing 5 patients at a time, compared with the 15 patients at a time they could treat during prepandemic conditions. “Everyone is at least 20 feet apart,” said Dr. Gaylis, who is a member of the American College of Rheumatology Board of Directors. “While we don’t have the kind of protective garments you might see in an ICU, we still are gowning, gloving, and masking our staff, and trying to practice sterile techniques as much as we can.”
The pandemic has caused him to reflect more broadly on the way he and his colleagues deliver care for patients on infusion therapy. “We see patients who really want their treatment because they feel it’s helpful and beneficial,” he said. “There are also patients who may truly be in remission who could stop [infusion therapy]. We could possibly extend the duration of their therapy, try and push it back.”
Dr. Gaylis emphasized that any discussion about halting infusion therapy requires clinical, serological, and ideally even MRI evidence that the disease is in a dormant state. “You wouldn’t stop treatment in someone who is showing signs in their blood that their disease is still active,” he said. “You’re using all those parameters in that conversation.”
In his clinical opinion, now is not the time to switch patients to self-injectable agents as a perceived matter of convenience. “I don’t really think that’s a good idea because self-injectables are different,” Dr. Gaylis said. “You’re basically switching treatment patterns. The practicality of getting a specialty pharmacy to switch, the insurance companies to cover it, and determine copay for it, is a burden on patients. That’s why I’m against it, because you’re starting a whole new process and problem.”
One patient tested positive for COVID-19 about 3 weeks after an infusion at the facility. “That does lead to a point: Have my staff been tested? We have not had the tests available to us,” Dr. Gaylis said. “One provider had a contact with someone with COVID-19 and stayed home for 2 weeks. That person tested negative. Soon we are going to receive a kit that will allow us to measure IgM and IgG COVID-19 antibodies. Because we’re going to be closed for 2 weeks, measuring us now would be a great way to handle it.”
In rural Western Kentucky, Christopher R. Phillips, MD, and his colleagues at Paducah Rheumatology have arranged for “drive-by” injections for some of their higher-risk patients who require subcutaneous administration of biologic agents. “We have them call us when they’re in the parking lot, and we give them the treatment while they sit in their car,” said Dr. Phillips, who chairs the ACR Insurance Subcommittee and is a member of the ACR COVID-19 Practice and Advocacy Task Force.
For patients who require infusions, they’ve arranged three chairs in the clinic to be at least 6 feet apart, and moved the fourth chair into a separate room. “My infusion nurse knows these patients well; we’re a small community,” he said. “She checks in with them the day before to screen for any symptoms of infection and asks them to call when they get here. A lot of them wait in their car to be brought in. She’ll bring them in, screen for infection symptoms, and check their temperature. She and the receptionist are masked and gloved, and disinfect aggressively between patients. The other thing we are trying to be on top of is making sure that everyone’s insurance coverage is active when they come in, in light of the number of people who have been laid off or had changes in their employment.”
Dr. Phillips has considered increasing the infusion time interval for some patients, but not knowing when current physical distancing guidelines will ease up presents a conundrum. “If I have a patient coming in today, and their next treatment is due in a month, I don’t know how to say that, if we stretch the infusion to 2 months, that things are going to be better,” he said. “For some very well-controlled patients and/or high-risk patients, that is something we’ve done: stretch the interval or skip a treatment. For most patients, our default is to stick with the normal schedule. We feel that, for most patients who have moderate to severe underlying rheumatic disease, the risk of disease flare and subsequent need for steroids may be a larger risk than the treatment itself, though that is an individualized decision.”
To date, Dr. Phillips has not treated a patient who has recovered from COVID-19, but the thought of that scenario gives him pause. “There is some literature suggesting these patients may asymptomatically shed virus for some time after they’ve clinically recovered, but we don’t really know enough about that,” he said. “If I had one of those patients, I’d probably be delaying them for a longer period of time, and I’d be looking for some guidance from the literature on postsymptomatic viral shedding.”
In the meantime, the level of anxiety that many of his patients express during this pandemic is palpable. “They really are between a rock and a hard place,” Dr. Phillips said. “If they come off their effective treatment, they risk flare of a disease that can be life or limb threatening. And yet, because of their disease and their treatment, they’re potentially at increased risk for serious illness if they become infected with COVID-19. We look for ways to try to reassure patients and to comfort them, and work with them to make the best of the situation.”
D.C.-area blacks face increased risk of mortality from SJS/TEN
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
AAP issues guidance on managing infants born to mothers with COVID-19
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
Enhanced team-based CVD care found to benefit diabetes patients
PHOENIX, ARIZ. – Diabetes patients in China who were enrolled in a team-based care intervention with clinical decision support systems significantly reduced their hemoglobin A1c, systolic blood pressure, and LDL cholesterol over 18 months, compared with those who received team-based care alone.
The finding comes from the Diabetes Complication Control in Community Clinics (D4C), a cluster randomized trial conducted in 38 community health centers in Xiamen, China.
“Diabetes has become a major public health challenge worldwide, especially in low- and middle-income countries where populations are large and growing and health care resources are limited,” Jiang He, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. He, chair and professor of epidemiology at Tulane School of Public Health and Tropical Medicine, New Orleans, the prevalence of diabetes has increased rapidly in recent decades in China, from 2.5% in 1994 to 11.6% in 2010. “It was estimated that 114 million Chinese adults had diabetes in 2010,” he said. “Hyperglycemia, high blood pressure, and elevated LDL cholesterol are major risk factors for cardiovascular disease and premature death. The majority of patients with diabetes have multiple uncontrolled CVD risk factors due to suboptimal care. Diabetes and its complications further strain an already overburdened and overwhelmed health care system, especially tertiary care facilities, in China. On the other hand, community health centers are underutilized.”
In D4C, Dr. He and colleagues set out to evaluate changes in CVD risk factors among patients with diabetes after implementing a team-based care model at community health centers in Xiamen, China. They compared the effectiveness of team-based care with clinical decision support systems versus team-based care alone on CVD risk factor control among patients with diabetes at these community health centers.
The study population consisted of 10,942 patients aged 50 years and older with uncontrolled diabetes and at least one of the following three additional CVD risk factors: systolic BP of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg; LDL cholesterol of at least 100 mg/dL, or clinical atherosclerotic cardiovascular disease (ASCVD). At the intervention clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The researchers trained the primary care physicians and nurses, and a clinical decision support system was integrated with guideline-based treatment algorithms for controlling glycemia, blood pressure, and lipids.
At the enhanced care control clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The city health commission trained the primary care physicians and nurses. The intervention lasted for 18 months in both groups.
Dr. He, the D4C study chair, reported findings from 10,942 patients: 5,394 in the intervention group and 5,548 in the enhanced care group. The mean baseline age was similar between the intervention group and the enhanced care group (a mean of 63 years), as was body mass index (a mean of 24.9 kg/m2), hemoglobin A1c (a mean of 8.8 vs. 8.7%, respectively), LDL cholesterol (121.2 vs. 121.1 mg/dL), systolic blood pressure (136.6 vs. 136.9 mm Hg), and diastolic blood pressure (79.7 vs. 79.8 mm Hg).
The researchers found patients in both groups experienced significant reductions in HbA1c, LDL cholesterol, and BP over the 18-month follow-up, but those in the intervention group fared better in all measures. Specifically, the mean change in HbA1c from baseline was –.85% in the intervention group, compared with –.66% in the enhanced care group, while the change in LDL was –19 mg/dL, compared with –12.8 mg/dL, respectively; the change in systolic blood pressure was –8.9 mm Hg vs. –7.7 mm Hg, and the change in 10-year ASCVD risk was .57% vs. .28% (P < .0001 for all associations).
The researchers also observed that the proportions of controlled HbA1c, LDL, and blood pressure at 18 months were higher in the intervention group, compared with the enhanced care group. Specifically, 38% of patients in the intervention group achieved glycemic control, compared with 35% of those in the enhanced care group (P =. 0006), while 48% vs. 39%, respectively, achieved control of LDL cholesterol (P < .0001), and 78% vs. 75% achieved control of blood pressure (P = .0009). In addition, 15% vs. 12% achieved control of all three risk factors at 18 months (P < .0001).
“Implementing team-based care with a clinical decision support system is an effective and sustainable strategy for diabetes control in primary care settings,” Dr. He said at the meeting, which was sponsored by the American Heart Association. “This implementation strategy could be scaled up within primary care settings in China and other low- to middle-income countries to improve CVD risk factor control in patients with diabetes.”
In an interview, session moderator Joshua J. Joseph, MD, of Ohio State University, Columbus, pointed out that since only 12%-15% of study participants achieved control of all three CVD risk factors, “that leaves a great opportunity for [figuring out] how to we get the other 88% or 85% of patients to target levels. That’s going to be important as we think about cardiovascular disease prevention in type 2 diabetes. The more we can use team-based care along with clinical decision support tools, the more we will continue to improve the lives of patients.”
The study was supported by the Xiamen City Health Commission. Dr. He reported having no financial disclosures.
SOURCE: He J et al. EPI/LIFESTYLE 2020, session 7A, abstract 17.
PHOENIX, ARIZ. – Diabetes patients in China who were enrolled in a team-based care intervention with clinical decision support systems significantly reduced their hemoglobin A1c, systolic blood pressure, and LDL cholesterol over 18 months, compared with those who received team-based care alone.
The finding comes from the Diabetes Complication Control in Community Clinics (D4C), a cluster randomized trial conducted in 38 community health centers in Xiamen, China.
“Diabetes has become a major public health challenge worldwide, especially in low- and middle-income countries where populations are large and growing and health care resources are limited,” Jiang He, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. He, chair and professor of epidemiology at Tulane School of Public Health and Tropical Medicine, New Orleans, the prevalence of diabetes has increased rapidly in recent decades in China, from 2.5% in 1994 to 11.6% in 2010. “It was estimated that 114 million Chinese adults had diabetes in 2010,” he said. “Hyperglycemia, high blood pressure, and elevated LDL cholesterol are major risk factors for cardiovascular disease and premature death. The majority of patients with diabetes have multiple uncontrolled CVD risk factors due to suboptimal care. Diabetes and its complications further strain an already overburdened and overwhelmed health care system, especially tertiary care facilities, in China. On the other hand, community health centers are underutilized.”
In D4C, Dr. He and colleagues set out to evaluate changes in CVD risk factors among patients with diabetes after implementing a team-based care model at community health centers in Xiamen, China. They compared the effectiveness of team-based care with clinical decision support systems versus team-based care alone on CVD risk factor control among patients with diabetes at these community health centers.
The study population consisted of 10,942 patients aged 50 years and older with uncontrolled diabetes and at least one of the following three additional CVD risk factors: systolic BP of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg; LDL cholesterol of at least 100 mg/dL, or clinical atherosclerotic cardiovascular disease (ASCVD). At the intervention clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The researchers trained the primary care physicians and nurses, and a clinical decision support system was integrated with guideline-based treatment algorithms for controlling glycemia, blood pressure, and lipids.
At the enhanced care control clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The city health commission trained the primary care physicians and nurses. The intervention lasted for 18 months in both groups.
Dr. He, the D4C study chair, reported findings from 10,942 patients: 5,394 in the intervention group and 5,548 in the enhanced care group. The mean baseline age was similar between the intervention group and the enhanced care group (a mean of 63 years), as was body mass index (a mean of 24.9 kg/m2), hemoglobin A1c (a mean of 8.8 vs. 8.7%, respectively), LDL cholesterol (121.2 vs. 121.1 mg/dL), systolic blood pressure (136.6 vs. 136.9 mm Hg), and diastolic blood pressure (79.7 vs. 79.8 mm Hg).
The researchers found patients in both groups experienced significant reductions in HbA1c, LDL cholesterol, and BP over the 18-month follow-up, but those in the intervention group fared better in all measures. Specifically, the mean change in HbA1c from baseline was –.85% in the intervention group, compared with –.66% in the enhanced care group, while the change in LDL was –19 mg/dL, compared with –12.8 mg/dL, respectively; the change in systolic blood pressure was –8.9 mm Hg vs. –7.7 mm Hg, and the change in 10-year ASCVD risk was .57% vs. .28% (P < .0001 for all associations).
The researchers also observed that the proportions of controlled HbA1c, LDL, and blood pressure at 18 months were higher in the intervention group, compared with the enhanced care group. Specifically, 38% of patients in the intervention group achieved glycemic control, compared with 35% of those in the enhanced care group (P =. 0006), while 48% vs. 39%, respectively, achieved control of LDL cholesterol (P < .0001), and 78% vs. 75% achieved control of blood pressure (P = .0009). In addition, 15% vs. 12% achieved control of all three risk factors at 18 months (P < .0001).
“Implementing team-based care with a clinical decision support system is an effective and sustainable strategy for diabetes control in primary care settings,” Dr. He said at the meeting, which was sponsored by the American Heart Association. “This implementation strategy could be scaled up within primary care settings in China and other low- to middle-income countries to improve CVD risk factor control in patients with diabetes.”
In an interview, session moderator Joshua J. Joseph, MD, of Ohio State University, Columbus, pointed out that since only 12%-15% of study participants achieved control of all three CVD risk factors, “that leaves a great opportunity for [figuring out] how to we get the other 88% or 85% of patients to target levels. That’s going to be important as we think about cardiovascular disease prevention in type 2 diabetes. The more we can use team-based care along with clinical decision support tools, the more we will continue to improve the lives of patients.”
The study was supported by the Xiamen City Health Commission. Dr. He reported having no financial disclosures.
SOURCE: He J et al. EPI/LIFESTYLE 2020, session 7A, abstract 17.
PHOENIX, ARIZ. – Diabetes patients in China who were enrolled in a team-based care intervention with clinical decision support systems significantly reduced their hemoglobin A1c, systolic blood pressure, and LDL cholesterol over 18 months, compared with those who received team-based care alone.
The finding comes from the Diabetes Complication Control in Community Clinics (D4C), a cluster randomized trial conducted in 38 community health centers in Xiamen, China.
“Diabetes has become a major public health challenge worldwide, especially in low- and middle-income countries where populations are large and growing and health care resources are limited,” Jiang He, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting.
According to Dr. He, chair and professor of epidemiology at Tulane School of Public Health and Tropical Medicine, New Orleans, the prevalence of diabetes has increased rapidly in recent decades in China, from 2.5% in 1994 to 11.6% in 2010. “It was estimated that 114 million Chinese adults had diabetes in 2010,” he said. “Hyperglycemia, high blood pressure, and elevated LDL cholesterol are major risk factors for cardiovascular disease and premature death. The majority of patients with diabetes have multiple uncontrolled CVD risk factors due to suboptimal care. Diabetes and its complications further strain an already overburdened and overwhelmed health care system, especially tertiary care facilities, in China. On the other hand, community health centers are underutilized.”
In D4C, Dr. He and colleagues set out to evaluate changes in CVD risk factors among patients with diabetes after implementing a team-based care model at community health centers in Xiamen, China. They compared the effectiveness of team-based care with clinical decision support systems versus team-based care alone on CVD risk factor control among patients with diabetes at these community health centers.
The study population consisted of 10,942 patients aged 50 years and older with uncontrolled diabetes and at least one of the following three additional CVD risk factors: systolic BP of at least 140 mm Hg and/or diastolic BP of at least 90 mm Hg; LDL cholesterol of at least 100 mg/dL, or clinical atherosclerotic cardiovascular disease (ASCVD). At the intervention clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The researchers trained the primary care physicians and nurses, and a clinical decision support system was integrated with guideline-based treatment algorithms for controlling glycemia, blood pressure, and lipids.
At the enhanced care control clinics, team-based care was delivered by a team of primary care physicians, nurses, and diabetes specialists. The city health commission trained the primary care physicians and nurses. The intervention lasted for 18 months in both groups.
Dr. He, the D4C study chair, reported findings from 10,942 patients: 5,394 in the intervention group and 5,548 in the enhanced care group. The mean baseline age was similar between the intervention group and the enhanced care group (a mean of 63 years), as was body mass index (a mean of 24.9 kg/m2), hemoglobin A1c (a mean of 8.8 vs. 8.7%, respectively), LDL cholesterol (121.2 vs. 121.1 mg/dL), systolic blood pressure (136.6 vs. 136.9 mm Hg), and diastolic blood pressure (79.7 vs. 79.8 mm Hg).
The researchers found patients in both groups experienced significant reductions in HbA1c, LDL cholesterol, and BP over the 18-month follow-up, but those in the intervention group fared better in all measures. Specifically, the mean change in HbA1c from baseline was –.85% in the intervention group, compared with –.66% in the enhanced care group, while the change in LDL was –19 mg/dL, compared with –12.8 mg/dL, respectively; the change in systolic blood pressure was –8.9 mm Hg vs. –7.7 mm Hg, and the change in 10-year ASCVD risk was .57% vs. .28% (P < .0001 for all associations).
The researchers also observed that the proportions of controlled HbA1c, LDL, and blood pressure at 18 months were higher in the intervention group, compared with the enhanced care group. Specifically, 38% of patients in the intervention group achieved glycemic control, compared with 35% of those in the enhanced care group (P =. 0006), while 48% vs. 39%, respectively, achieved control of LDL cholesterol (P < .0001), and 78% vs. 75% achieved control of blood pressure (P = .0009). In addition, 15% vs. 12% achieved control of all three risk factors at 18 months (P < .0001).
“Implementing team-based care with a clinical decision support system is an effective and sustainable strategy for diabetes control in primary care settings,” Dr. He said at the meeting, which was sponsored by the American Heart Association. “This implementation strategy could be scaled up within primary care settings in China and other low- to middle-income countries to improve CVD risk factor control in patients with diabetes.”
In an interview, session moderator Joshua J. Joseph, MD, of Ohio State University, Columbus, pointed out that since only 12%-15% of study participants achieved control of all three CVD risk factors, “that leaves a great opportunity for [figuring out] how to we get the other 88% or 85% of patients to target levels. That’s going to be important as we think about cardiovascular disease prevention in type 2 diabetes. The more we can use team-based care along with clinical decision support tools, the more we will continue to improve the lives of patients.”
The study was supported by the Xiamen City Health Commission. Dr. He reported having no financial disclosures.
SOURCE: He J et al. EPI/LIFESTYLE 2020, session 7A, abstract 17.
REPORTING FROM EPI/LIFESTYLE 2020
San Diego County CMO vigorously leads COVID-19 response team
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
SAN DIEGO – On the days family physician Nick Yphantides, MD, announces updates on the COVID-19 epidemic to San Diego County residents, he can’t help but think about his late father.
In June of 2009, 75-year-old George Yphantides, a Steinway-trained piano technician who lived in Escondido, Calif., became the third person in the United States to die from complications of the pandemic H1N1 swine flu – just days before a vaccine became available.
“I loved my dad,” Dr. Yphantides, who has been San Diego County’s Chief Medical Officer since the year of his father’s death, said in an interview. “So, when you take a step back and take into consideration my sense of purpose in serving the 3.3 million residents of San Diego County, my passion based on my personal Christian faith, and my activation in terms of what happened to my dad, I have such a storm of internal sense of urgency right now.”
San Diego County and public health officials got experience with COVID-19 in advance of the country’s widely documented cases of community-based transmission. Around 9 pm on Jan. 31, 2020 – the Friday of Super Bowl weekend – Dr. Yphantides answered a phone call from Eric C. McDonald, MD, the county’s medical director of epidemiology. Dr. McDonald informed him that in a few days, a plane full of American citizens traveling from Wuhan, China, would be landing at Marine Corp Air Station Miramar in San Diego for a 2-week quarantine and that the task of providing medical support to any affected individuals fell on county officials.
“I will never forget that phone call,” he said. “We did have two positive cases. What we experienced with those evacuees was amazing surge preparation, and without exaggeration, I have worked 18-20 hours a day since that day.”
Fast forward to March 31, 2020, the county’s confirmed COVID-19 caseload had grown to 734, up 131 from the day before. As of the final day of March, nine people have died, with an age range between 25 and 87 years. Of confirmed cases, 61% are between the ages of 20 and 49, 43% are female, 19% have required hospitalization, 7% have required admission to intensive care, and the mortality rate has been 1.2%. Data currently show optimal proactive hospital capacity.
In the opinion of Dr. Yphantides, the 734 COVID-19 cases represent a tip of the iceberg. “How big is that iceberg? I can’t tell you yet,” he said.
"I see this as the Super Bowl of public health,” he exclaimed.
At least some of Dr. Yphantides’ vigor seems to be fueled by his pride in his team of professionals who have been helping him respond to the surge of COVID-19 cases.
As the county’s CMO, Dr. Yphantides serves as the liaison for the entire Emergency Medical System, the entire local health care delivery system, the entire physician and medical society network, the payor system, and the proportion of the area population using Medi-Cal.
Dr. Yphantides, who attended medical school at the University of California, San Diego, said that, compared with other regions of the country, San Diego County has made “tremendous progress” in overcoming many chronic lifestyle illnesses. For example, cardiovascular disease is no longer the number one cause of death in the county; it’s bookended by cancer and Alzheimer’s disease.
“In the context of the COVID-19 response, [the county’s health care team established] an entire incident command system in our emergency operations center. Our emergency operations center is activated to the top level,” he said.
Dr. Yphantides shares public communication efforts with Dr. McDonald and Wilma J. Wooten, MD, the county’s public health officer. The San Diego County CMO also engages with policymakers, including the board of supervisors, local mayors, state legislators, and national legislators.
“Because of the relational trust capital that I have in this community, I get pulled into unexpected rooms of discussion,” he said. This included meeting with top executives from the San Diego Padres in early March, putting them on notice that the 2020 Major League Baseball season would likely be postponed. (This was officially announced on March 16.)
“We have made some decisions that have devastated some people economically. Talk about flipping the switch. We are living and making history every day. It is unbelievable,” he said.
“San Diego is a more aged population compared to many other parts of the country. ... [Part] of the reason why I’m so frantically doing everything I can to prepare, to batten down the hatches, and to optimize our health care delivery system is because we have a population that collectively is more at risk [for more serious complications from COVID-19]. A lot of what drives me is advocacy,” Dr. Yphantides noted.
A colleague’s perspective
Kristi L. Koenig, MD, medical director of emergency medical services for the County of San Diego, characterized Dr. Yphantides’ management style as collaborative. “Under his leadership, we have the perspective of ‘just focus on patient care, get it done, be creative, work together as a team,’ ” said Dr. Koenig, who coedited the textbook, “Koenig and Schultz’s Disaster Medicine: Comprehensive Principles and Practices” (Cambridge University Press, 2016). “He’s decisive and he’s responsive. You don’t have to wait a long time to get a decision, which is very important right now because this is so fast moving.”
Dr. Koenig, who has worked with Dr. Yphantides for 3 years, said that she routinely feeds him information that might help the team navigate its response to COVID-19. “For example, if I see an idea for how to get more [personal protective equipment] and feed it to him, he might have a contact somewhere in a factory that could make the PPE,” she said. “We work together by my reminding him to keep it within the incident command system structure, so that we can coordinate all the resources and not duplicate efforts.”
He uses his personal connections in a way to implement ideas that are beneficial to the overall goal of decreasing morbidity and mortality,” Dr. Koenig added.
Predictions for San Diego County
Dr. Yphantides said he considers San Diego to still be in the calm before the storm and that he is working hard to “board up his community.” The county CMO is also trying to prepare the health care delivery system to optimize its capacity, of doing interventions with hopes of lowering the curve and enhancing the capacity, he said.
When the storm hits, “it’s going be brutal, because we’re going to lose life,” Dr. Yphantides said.
“I am praying that maybe by some of our efforts, instead of a Category 5 storm, it’ll be a Category 3 storm,” he remarked.
The future of health care
Dr. Yphantides views the COVID-19 pandemic as “an absolute game-changer” in terms of what the future of health care delivery will look like in the United States. “Whether the right word is the ‘Amazonification’ of health care, or the ‘Uberization’ of health care, I don’t know, but the essence of how we deliver care is radically being transformed literally before our eyes,” he said. “I would encourage my colleagues to embrace that” and take care of their people by doing whatever it takes under this unprecedented paradigm.
Meanwhile, Dr. Yphantides braces for a potential surge of COVID-19 cases in San Diego County in the coming weeks. He honors the memory of his dad, and he expresses thanks for his mom, who cares for his two teenaged daughters while he helps steward the region’s response to the pandemic.
“Without my mom I could not function in the way that I’m currently functioning,” he said. “So, when you add all of those factors up, and wrap it with a bowtie of sincere love and passion for my community, there’s a fire that’s burning inside of me right now.”
Low-income DC communities have restricted access to iPLEDGE pharmacies
Residents of , results from a survey demonstrated.
Prescription of isotretinoin is regulated by the iPLEDGE program, which strives to ensure that no female patient starts isotretinoin therapy if pregnant and that no female patient on isotretinoin therapy becomes pregnant. “Over the years, many studies have criticized the program by demonstrating that iPLEDGE has promoted health care disparities,” Nidhi Shah said during a virtual meeting held by the George Washington University department of dermatology. “For example, racial minorities and women are more likely to be underprescribed isotretinoin, as well as face more delays in treatment.”
In an effort to evaluate the geographic distribution of iPLEDGE pharmacies in Washington DC, and its correlation with sociodemographic factors, Ms. Shah, a third-year medical student at the George Washington University, Washington, and colleagues obtained a list of active pharmacies in Washington from the local government. They also surveyed each outpatient pharmacy in the District of Columbia to verify their iPLEDGE registration status, for a total of 146 pharmacies.
Ms. Shah reported that 82% of all outpatient pharmacies were enrolled in iPLEDGE. However, enrollment significantly varied by the type of pharmacy. For example, 100% of chain pharmacies were enrolled, compared with 46% of independent pharmacies and 60% of hospital-based pharmacies.
When the researchers evaluated the number and type of iPLEDGE pharmacy by each of the eight wards in Washington, they observed a high density of pharmacies in wards 1 and 2, communities with a generally low proportion of residents who live in poverty, and low density of pharmacies in wards 7 and 8, communities with a higher proportion of residents who live in poverty. In addition, there were more independent than chain pharmacies in wards 7 and 8, and residents in those wards had a greater distance to travel to reach an iPLEDGE pharmacy, compared with residents who live in the other wards.
When Ms. Shah and colleagues examined the correlation between pharmacies per 10,000 residents and specific sociodemographic factors, they observed a strong, positive correlation between iPLEDGE pharmacy density and median household income (P = .0003). On the other hand, there was a strong negative correlation between iPLEDGE pharmacy density and the percentage of individuals with public insurance (P less than .0001), as well as the percentage of nonwhite individuals (P = .0009).
“Our study highlights the lack of isotretinoin-dispensing pharmacies in low-income communities,” Ms. Shah concluded. “Not only are there fewer such pharmacies available in low income communities, but the residents must also travel further to reach them. The spatial heterogeneity of iPLEDGE pharmacies may be an important patient barrier to timely access of isotretinoin, especially for female patients who have a strict 7-day window to collect their medication. We hope that future public health reform works to close this gap.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Ms. Shah reported having no disclosures.
Residents of , results from a survey demonstrated.
Prescription of isotretinoin is regulated by the iPLEDGE program, which strives to ensure that no female patient starts isotretinoin therapy if pregnant and that no female patient on isotretinoin therapy becomes pregnant. “Over the years, many studies have criticized the program by demonstrating that iPLEDGE has promoted health care disparities,” Nidhi Shah said during a virtual meeting held by the George Washington University department of dermatology. “For example, racial minorities and women are more likely to be underprescribed isotretinoin, as well as face more delays in treatment.”
In an effort to evaluate the geographic distribution of iPLEDGE pharmacies in Washington DC, and its correlation with sociodemographic factors, Ms. Shah, a third-year medical student at the George Washington University, Washington, and colleagues obtained a list of active pharmacies in Washington from the local government. They also surveyed each outpatient pharmacy in the District of Columbia to verify their iPLEDGE registration status, for a total of 146 pharmacies.
Ms. Shah reported that 82% of all outpatient pharmacies were enrolled in iPLEDGE. However, enrollment significantly varied by the type of pharmacy. For example, 100% of chain pharmacies were enrolled, compared with 46% of independent pharmacies and 60% of hospital-based pharmacies.
When the researchers evaluated the number and type of iPLEDGE pharmacy by each of the eight wards in Washington, they observed a high density of pharmacies in wards 1 and 2, communities with a generally low proportion of residents who live in poverty, and low density of pharmacies in wards 7 and 8, communities with a higher proportion of residents who live in poverty. In addition, there were more independent than chain pharmacies in wards 7 and 8, and residents in those wards had a greater distance to travel to reach an iPLEDGE pharmacy, compared with residents who live in the other wards.
When Ms. Shah and colleagues examined the correlation between pharmacies per 10,000 residents and specific sociodemographic factors, they observed a strong, positive correlation between iPLEDGE pharmacy density and median household income (P = .0003). On the other hand, there was a strong negative correlation between iPLEDGE pharmacy density and the percentage of individuals with public insurance (P less than .0001), as well as the percentage of nonwhite individuals (P = .0009).
“Our study highlights the lack of isotretinoin-dispensing pharmacies in low-income communities,” Ms. Shah concluded. “Not only are there fewer such pharmacies available in low income communities, but the residents must also travel further to reach them. The spatial heterogeneity of iPLEDGE pharmacies may be an important patient barrier to timely access of isotretinoin, especially for female patients who have a strict 7-day window to collect their medication. We hope that future public health reform works to close this gap.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Ms. Shah reported having no disclosures.
Residents of , results from a survey demonstrated.
Prescription of isotretinoin is regulated by the iPLEDGE program, which strives to ensure that no female patient starts isotretinoin therapy if pregnant and that no female patient on isotretinoin therapy becomes pregnant. “Over the years, many studies have criticized the program by demonstrating that iPLEDGE has promoted health care disparities,” Nidhi Shah said during a virtual meeting held by the George Washington University department of dermatology. “For example, racial minorities and women are more likely to be underprescribed isotretinoin, as well as face more delays in treatment.”
In an effort to evaluate the geographic distribution of iPLEDGE pharmacies in Washington DC, and its correlation with sociodemographic factors, Ms. Shah, a third-year medical student at the George Washington University, Washington, and colleagues obtained a list of active pharmacies in Washington from the local government. They also surveyed each outpatient pharmacy in the District of Columbia to verify their iPLEDGE registration status, for a total of 146 pharmacies.
Ms. Shah reported that 82% of all outpatient pharmacies were enrolled in iPLEDGE. However, enrollment significantly varied by the type of pharmacy. For example, 100% of chain pharmacies were enrolled, compared with 46% of independent pharmacies and 60% of hospital-based pharmacies.
When the researchers evaluated the number and type of iPLEDGE pharmacy by each of the eight wards in Washington, they observed a high density of pharmacies in wards 1 and 2, communities with a generally low proportion of residents who live in poverty, and low density of pharmacies in wards 7 and 8, communities with a higher proportion of residents who live in poverty. In addition, there were more independent than chain pharmacies in wards 7 and 8, and residents in those wards had a greater distance to travel to reach an iPLEDGE pharmacy, compared with residents who live in the other wards.
When Ms. Shah and colleagues examined the correlation between pharmacies per 10,000 residents and specific sociodemographic factors, they observed a strong, positive correlation between iPLEDGE pharmacy density and median household income (P = .0003). On the other hand, there was a strong negative correlation between iPLEDGE pharmacy density and the percentage of individuals with public insurance (P less than .0001), as well as the percentage of nonwhite individuals (P = .0009).
“Our study highlights the lack of isotretinoin-dispensing pharmacies in low-income communities,” Ms. Shah concluded. “Not only are there fewer such pharmacies available in low income communities, but the residents must also travel further to reach them. The spatial heterogeneity of iPLEDGE pharmacies may be an important patient barrier to timely access of isotretinoin, especially for female patients who have a strict 7-day window to collect their medication. We hope that future public health reform works to close this gap.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Ms. Shah reported having no disclosures.
FDA okays emergency use of convalescent plasma for seriously ill COVID-19 patients
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
As the proportion of patients infected with COVID-19 continues to rise in the United States, the Food and Drug Administration is facilitating access to COVID-19 convalescent plasma for use in patients with serious or immediately life-threatening COVID-19 infections.
While clinical trials are underway to evaluate the safety and efficacy of administering convalescent plasma to patients with COVID-19, the FDA is granting clinicians permission for use of investigational convalescent plasma under single-patient emergency Investigational New Drug Applications (INDs), since no known cure exists and a vaccine is more than 1 year away from becoming available.
This allows the use of an investigational drug for the treatment of an individual patient by a licensed physician upon FDA authorization. This does not include the use of COVID-19 convalescent plasma for the prevention of infection, according to a statement issued by the agency on March 24.
“It is possible that convalescent plasma that contains antibodies to SARS-CoV-2 (the virus that causes COVID-19) might be effective against the infection,” the FDA statement reads. “Use of convalescent plasma has been studied in outbreaks of other respiratory infections, including the 2009-2010 H1N1 influenza virus pandemic, 2003 SARS-CoV-1 epidemic, and the 2012 MERS-CoV epidemic. Although promising, convalescent plasma has not been shown to be effective in every disease studied.”
“I think the FDA got caught initially a little flat-footed when it came to the development of COVID-19 tests, but they’re quickly catching up,” Peter J. Pitts, who was the FDA’s associate commissioner from 2002 to 2004, said in an interview. “I think that the attitude now is, ‘If it’s safe, let’s create a pathway to see how these things work in the real world.’ I think that’s going to be as true for treatments to lessen the symptoms and shorten the duration of the disease, as well as convalescent plasma as a potential alternative to a yet-to-be-developed vaccine.”
At the University of Washington School of Medicine, Seattle, Terry B. Gernsheimer, MD, and her colleagues are recruiting recovered COVID-19 patients to donate plasma for seriously ill patients affected with the virus. “The thought of using convalescent plasma makes total sense, because it’s immediately available, and it’s something that we can try to give people,” said Dr. Gernsheimer, a hematologist who is professor of medicine at the medical school. “It’s been used in China, and reports should be coming out shortly about their experience with this.”
In a case series that appeared in JAMA on March 27 (doi: 10.1001/jama.2020.4783), Chinese researchers led by Chenguang Shen, PhD, reported findings from five critically ill COVID-19 patients with acute respiratory distress syndrome who received a transfusion with convalescent plasma at Shenzhen Third People’s Hospital 10 and 22 days after hospital admission. The patients ranged in age from 36 to 73 years, three were men, and all were receiving mechanical ventilation at the time of treatment.
Dr. Shen and colleagues reported that viral loads decreased and became negative within 12 days following the transfusion. Three of the patients were discharged from the hospital after a length of stay that ranged from 51 to 55 days, and two remain in stable condition at 37 days after the transfusion. The researchers pointed out that all patients received antiviral agents, including interferon and lopinavir/ritonavir, during and following convalescent plasma treatment, “which also may have contributed to the viral clearance observed.”
Under the FDA policy on emergency IND use, COVID-19 convalescent plasma must only be collected from recovered individuals if they are eligible to donate blood, required testing must be performed, and the donation must be found suitable.
Potential donors “are going to be screened the way all blood donors are screened,” Dr. Gernsheimer said. “It’s not going to be any less safe than any unit of plasma that’s on the shelf that comes from our volunteer donors. There are always transfusion reactions that we have to worry about, [and] there are potentially unknown pathogens that we don’t yet know about that we are not yet testing for. It’s the regular risk we see with any unit of plasma.”
She added that COVID-19 survivors appear to start increasing their titer of the antibody around day 28. “We’ll be looking for recovered individuals who have had a documented infection, and whose symptoms started about 28 days before we collect,” she said.
The FDA advises clinicians to address several considerations for donor eligibility, including prior diagnosis of COVID-19 documented by a laboratory test; complete resolution of symptoms at least 14 days prior to donation; female donors negative for HLA antibodies or male donors, and negative results for COVID-19 either from one or more nasopharyngeal swab specimens or by a molecular diagnostic test from blood. [A partial list of available tests can be accessed on the FDA website.] The agency also advises that donors have defined SARS-CoV-2–neutralizing antibody titers, if testing can be conducted (optimally greater than 1:320).
Patients eligible to receive COVID-19 convalescent plasma must have a severe or immediately life-threatening infection with laboratory-confirmed COVID-19. The agency defines severe disease as dyspnea, respiratory frequency of 30 per minute or greater, blood oxygen saturation of 93% or less, partial pressure of arterial oxygen to fraction of inspired oxygen ratio of less than 300, and/or lung infiltrates of greater than 50% within 24-48 hours. Life-threatening disease is defined as respiratory failure, septic shock, and/or multiple organ dysfunction or failure. Patients must provide informed consent.
The potential risks of receiving COVID-19 convalescent plasma remain unknown, according to Dr. Gernsheimer. “What some people have thought about is, could there be such an inflammatory response with the virus that we would initially see these patients get worse?” she said. “My understanding is that has not occurred in China yet, but we don’t have all those data. But we always worry if we have something that’s going to cause inflammation around an infection, for example, that could initially make it more difficult to breathe if it’s a lung infection. So far, my understanding is that has not been seen.”
For COVID-19 convalescent plasma authorization requests that require a response within 4-8 hours, requesting clinicians may complete form 3296 and submit it by email to [email protected].
For COVID-19 convalescent plasma authorization requests that require a response in less than 4 hours, or if the clinician is unable to complete and submit form 3926 because of extenuating circumstances, verbal authorization can be sought by calling the FDA’s Office of Emergency Operations at 1-866-300-4374.
The FDA is working with the National Institutes of Health, the Centers for Disease Control and Prevention, and other government partners to develop protocols for use by multiple investigators in order to coordinate the collection and use of COVID-19 convalescent plasma.
“It’s crucial that data be captured for every patient so that we really understand what safety and effectiveness looks like on as close to a real-world level as we can, as quickly as we can,” said Mr. Pitts, who is president and cofounder of the Center for Medicine in the Public Interest, and who also does consulting work for the FDA. “I understand that health care professionals are overworked and overburdened right now. I applaud them for their heroic work. But that doesn’t mean that we can shirk off collecting the data. When I was at the FDA, I helped address the SARS epidemic. The agency attitude at that point was, ‘Let’s get things that just might work through the process, as long as the cure isn’t going to be worse than the disease.’ I think that’s the attitude that’s leading the charge today.”
Survey explores the role of social media in choosing a dermatologist
Fewer than one-quarter of consumers rely heavily on social media for choosing a dermatologist, results from an online survey suggest.
In a video presentation during a virtual meeting held by the George Washington University department of dermatology, Kamaria Nelson, MD, said that, as of 2019, 79% of Americans have a social media account, with the majority using platforms such as Facebook, Instagram, Twitter, and YouTube. “There’s also a high predominance of social media use in the dermatology field, with many dermatologists assuming that it will improve their personal brands,” said Dr. Nelson, a research fellow in the department of dermatology at George Washington University, Washington. “Some even hire social media managers to monitor online reviews and mitigate any damage. So, although social media is commonly used, it’s unknown if it impacts patient knowledge, access to health care, or provider choice.”
To evaluate how social media influences patients when choosing a dermatologist, she and her colleagues used Survey Monkey to create a 10-item questionnaire that they distributed to 1,481 individuals in the general U.S. population in May 2019. Individuals qualified for the study if they used social media and if they had ever been to a dermatologist. Dr. Nelson reported that 726 individuals (58%) qualified for the survey and 715 completed it, for a response rate of 98%. The researchers used Chi-square tests to compare frequency and importance of social media by visit type, age, gender, and educational level.
When the respondents were stratified by visit type, 43% who saw a dermatologist for cosmetic reasons were more likely to view social media as “extremely important” or “very important,” compared with 15% of patients who saw a dermatologist for medical reasons (P less than .0001).
When stratified by age, about 12% of respondents between the ages of 18 and 44 years considered social media as extremely important when choosing a dermatologist, compared with only 9% of those aged 45-60 years and about 2% of those older than age 60 (P less than .0001).
When stratified by educational level, 30% of respondents with a high school degree or less were more likely to view social media as extremely important or very important when choosing a dermatologist, while 62% of those with more than a high school degree were more likely to view social media as “not at all important” or only “slightly important” (P = .0006).
One of the survey questions was, “When choosing a dermatologist to see, how important is his or her social media site?” Only 9% of respondents said extremely important, 13% said very important, 21% said “moderately important,” 24% said slightly important, and 33% said not at all important. “This left about 22% of respondents who viewed the social media site as extremely important or very important when choosing a dermatologist,” Dr. Nelson said.
Factors deemed important on a dermatologist’s social media profile were patient reviews (68%), years of experience (61%), and the amount of medical information written by the dermatologist (59%).
“There seems to be a low reliance on social media when selecting a dermatologist,” Dr. Nelson concluded. “We also found that cosmetic patients, patients with lower levels of education, and younger patients were more likely to value social media. Therefore, social media may only be useful for targeting specific patient populations. When doing so, medical information written by a provider is most often desired.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Nelson reported having no disclosures.
Fewer than one-quarter of consumers rely heavily on social media for choosing a dermatologist, results from an online survey suggest.
In a video presentation during a virtual meeting held by the George Washington University department of dermatology, Kamaria Nelson, MD, said that, as of 2019, 79% of Americans have a social media account, with the majority using platforms such as Facebook, Instagram, Twitter, and YouTube. “There’s also a high predominance of social media use in the dermatology field, with many dermatologists assuming that it will improve their personal brands,” said Dr. Nelson, a research fellow in the department of dermatology at George Washington University, Washington. “Some even hire social media managers to monitor online reviews and mitigate any damage. So, although social media is commonly used, it’s unknown if it impacts patient knowledge, access to health care, or provider choice.”
To evaluate how social media influences patients when choosing a dermatologist, she and her colleagues used Survey Monkey to create a 10-item questionnaire that they distributed to 1,481 individuals in the general U.S. population in May 2019. Individuals qualified for the study if they used social media and if they had ever been to a dermatologist. Dr. Nelson reported that 726 individuals (58%) qualified for the survey and 715 completed it, for a response rate of 98%. The researchers used Chi-square tests to compare frequency and importance of social media by visit type, age, gender, and educational level.
When the respondents were stratified by visit type, 43% who saw a dermatologist for cosmetic reasons were more likely to view social media as “extremely important” or “very important,” compared with 15% of patients who saw a dermatologist for medical reasons (P less than .0001).
When stratified by age, about 12% of respondents between the ages of 18 and 44 years considered social media as extremely important when choosing a dermatologist, compared with only 9% of those aged 45-60 years and about 2% of those older than age 60 (P less than .0001).
When stratified by educational level, 30% of respondents with a high school degree or less were more likely to view social media as extremely important or very important when choosing a dermatologist, while 62% of those with more than a high school degree were more likely to view social media as “not at all important” or only “slightly important” (P = .0006).
One of the survey questions was, “When choosing a dermatologist to see, how important is his or her social media site?” Only 9% of respondents said extremely important, 13% said very important, 21% said “moderately important,” 24% said slightly important, and 33% said not at all important. “This left about 22% of respondents who viewed the social media site as extremely important or very important when choosing a dermatologist,” Dr. Nelson said.
Factors deemed important on a dermatologist’s social media profile were patient reviews (68%), years of experience (61%), and the amount of medical information written by the dermatologist (59%).
“There seems to be a low reliance on social media when selecting a dermatologist,” Dr. Nelson concluded. “We also found that cosmetic patients, patients with lower levels of education, and younger patients were more likely to value social media. Therefore, social media may only be useful for targeting specific patient populations. When doing so, medical information written by a provider is most often desired.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Nelson reported having no disclosures.
Fewer than one-quarter of consumers rely heavily on social media for choosing a dermatologist, results from an online survey suggest.
In a video presentation during a virtual meeting held by the George Washington University department of dermatology, Kamaria Nelson, MD, said that, as of 2019, 79% of Americans have a social media account, with the majority using platforms such as Facebook, Instagram, Twitter, and YouTube. “There’s also a high predominance of social media use in the dermatology field, with many dermatologists assuming that it will improve their personal brands,” said Dr. Nelson, a research fellow in the department of dermatology at George Washington University, Washington. “Some even hire social media managers to monitor online reviews and mitigate any damage. So, although social media is commonly used, it’s unknown if it impacts patient knowledge, access to health care, or provider choice.”
To evaluate how social media influences patients when choosing a dermatologist, she and her colleagues used Survey Monkey to create a 10-item questionnaire that they distributed to 1,481 individuals in the general U.S. population in May 2019. Individuals qualified for the study if they used social media and if they had ever been to a dermatologist. Dr. Nelson reported that 726 individuals (58%) qualified for the survey and 715 completed it, for a response rate of 98%. The researchers used Chi-square tests to compare frequency and importance of social media by visit type, age, gender, and educational level.
When the respondents were stratified by visit type, 43% who saw a dermatologist for cosmetic reasons were more likely to view social media as “extremely important” or “very important,” compared with 15% of patients who saw a dermatologist for medical reasons (P less than .0001).
When stratified by age, about 12% of respondents between the ages of 18 and 44 years considered social media as extremely important when choosing a dermatologist, compared with only 9% of those aged 45-60 years and about 2% of those older than age 60 (P less than .0001).
When stratified by educational level, 30% of respondents with a high school degree or less were more likely to view social media as extremely important or very important when choosing a dermatologist, while 62% of those with more than a high school degree were more likely to view social media as “not at all important” or only “slightly important” (P = .0006).
One of the survey questions was, “When choosing a dermatologist to see, how important is his or her social media site?” Only 9% of respondents said extremely important, 13% said very important, 21% said “moderately important,” 24% said slightly important, and 33% said not at all important. “This left about 22% of respondents who viewed the social media site as extremely important or very important when choosing a dermatologist,” Dr. Nelson said.
Factors deemed important on a dermatologist’s social media profile were patient reviews (68%), years of experience (61%), and the amount of medical information written by the dermatologist (59%).
“There seems to be a low reliance on social media when selecting a dermatologist,” Dr. Nelson concluded. “We also found that cosmetic patients, patients with lower levels of education, and younger patients were more likely to value social media. Therefore, social media may only be useful for targeting specific patient populations. When doing so, medical information written by a provider is most often desired.”
The virtual meeting included presentations that had been slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic. Dr. Nelson reported having no disclosures.
Low fitness level linked to higher risk of heart failure in diabetes
PHOENIX – Lower baseline fitness and greater decline in fitness over time are independently associated with a higher risk of heart failure in patients with diabetes, results from a large analysis showed.
“Diabetes is an important risk factor for the development of heart failure, and the diagnosis of diabetes in newly diagnosed cases of heart failure has been increasing,” Ambarish Pandey, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “Type 2 diabetes is associated with increased burden of traditional risk factors such as hypertension, kidney dysfunction, and dyslipidemia – each of which in turn increase the risk of both atherothrombotic disease as well as heart failure.”
Recent data from the Swedish National Diabetes Register have shown that optimal management of these risk factors in patients with type 2 diabetes can actually mitigate the risk of atherosclerotic events such as acute MI, but the risk of heart failure does not significantly lower with optimal management of these traditional cardiovascular risk factors (N Engl J Med. 2018;379:633-44). “These findings highlight that novel approaches that go beyond just managing traditional cardiovascular risk factors are needed for prevention of heart failure in patients with type 2 diabetes,” said Dr. Pandey, of the division of cardiology at the University of Texas Southwestern Medical Center, Dallas. “Our group has demonstrated that physical inactivity and low levels of fitness are associated with a higher risk of heart failure. We have also shown that the protective effect of physical activity against heart failure risk is stronger against heart failure with preserved ejection fraction, which is a subtype of heart failure that is increasing in prevalence and has no effective therapies.”
Dr. Pandey and his colleagues set out to test the research hypothesis that fitness decline and increases in body mass index over time are significantly associated with a higher risk of heart failure. To do this, they drew from the LookAHEAD Trial, a multicenter analysis of 5,145 overweight or obese patients with type 2 diabetes who were randomized to an intensive lifestyle intervention or to usual care. The intervention consisted of a caloric intake goal of 1,200 to 1,800 kcal per day and engaging in at least 175 minutes per week of physical activity. Participants were stratified into one of three fitness group levels: low, moderate, and high, from 5 metabolic equivalents (METs) in the lowest fitness tertile to 9 METs in the highest fitness tertile. The primary outcome of the trial was adverse cardiovascular events. The intervention was implemented for almost 10 years, and patients were followed for up to 12 years from baseline.
The heart failure outcomes were not systematically adjudicated in the primary LookAHEAD trial, so Dr. Pandey and colleagues conducted an ancillary study of all incident hospitalizations in the study and followed them for 2 additional years. Overall, the researchers identified 257 incident heart failure events. The cumulative incidence of heart failure for the usual care versus the intensive lifestyle intervention arm was not statistically different (an event rate of 4.53 vs. 4.32 per 1,000 person-years, respectively; hazard ratio, 0.96). “This demonstrated that the intensive lifestyle intervention in the LookAHEAD trial did not significantly modify the risk of heart failure,” Dr. Pandey said.
However, an adjusted analysis revealed that the risk of heart failure was 39% lower in the moderately fit group and 62% lower in the high fit group, compared with the low-fitness group. Among heart failure subtypes, the risk of heart failure with preserved ejection fraction (HFpEF) was 40% lower in the moderately fit group and 77% lower in the high-fitness group. On the other hand, baseline level of fitness level was not associated with risk of heart failure reduced ejection fraction (HFrEF) after the researchers adjusted for cardiovascular risk factors.
Next, Dr. Pandey and his colleagues used Cox modeling to examine the association of baseline and longitudinal changes in fitness and BMI with risk of heart failure. For change in fitness and BMI analysis, they used the 4-year follow-up data in 3,092 participants who underwent repeat fitness testing and had available data on BMI. They excluded patients who developed heart failure within the first 4 years of the study.
The mean age of the ancillary study population was about 60 years, and there was a lower proportion of women in the high fitness tertile (41%). The researchers observed a graded, inverse association between higher fitness levels and lower risk of heart failure such that increasing fitness from baseline was associated with a substantial decrease in the risk of heart failure. Specifically, a 10% decline in fitness over the 4 years of follow-up was associated with a 11% increase in the overall risk of heart failure (HR, 1.11). “This was largely consistent with the two heart failure subtypes,” he said. Similarly, a 10% increase in BMI over the 4 years of follow-up was associated with a 25% increase in the overall risk of heart failure (HR 1.25). On the other hand, a 10% decrease BMI was associated with a 20% decrease in the risk of heart failure (HR .80). This was also largely consistent for both heart failure subtypes. According to co-lead investigator Kershaw Patel, MD, “these findings suggest that therapies targeting large and sustained improvements in fitness and weight loss may modify the risk of heart failure among patients with diabetes.”
“Lower fitness at baseline was more strongly associated with the risk of HFpEF vs. HFrEF, and greater weight loss over follow-up is associated with a lower risk of heart failure independent of changes in other risk factors,” Dr. Pandey concluded at the meeting, which was sponsored by the American Heart Association.
In an interview, session moderator Joshua J. Joseph, MD, said that it remains unclear what type of setting is ideal for carrying out cardiorespiratory fitness in this patient population. “What is the supervision needed for that to occur?” asked Dr. Joseph, of The Ohio State University, Columbus. “Can patients do this on their own, or do they need guidance? What is the best approach? That’s the question we all have to answer individually in our own communities.”
Dr. Pandey reported having no disclosures.
SOURCE: Pandey A. Epi/Lifestyle 2020, Abstract 16.
PHOENIX – Lower baseline fitness and greater decline in fitness over time are independently associated with a higher risk of heart failure in patients with diabetes, results from a large analysis showed.
“Diabetes is an important risk factor for the development of heart failure, and the diagnosis of diabetes in newly diagnosed cases of heart failure has been increasing,” Ambarish Pandey, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “Type 2 diabetes is associated with increased burden of traditional risk factors such as hypertension, kidney dysfunction, and dyslipidemia – each of which in turn increase the risk of both atherothrombotic disease as well as heart failure.”
Recent data from the Swedish National Diabetes Register have shown that optimal management of these risk factors in patients with type 2 diabetes can actually mitigate the risk of atherosclerotic events such as acute MI, but the risk of heart failure does not significantly lower with optimal management of these traditional cardiovascular risk factors (N Engl J Med. 2018;379:633-44). “These findings highlight that novel approaches that go beyond just managing traditional cardiovascular risk factors are needed for prevention of heart failure in patients with type 2 diabetes,” said Dr. Pandey, of the division of cardiology at the University of Texas Southwestern Medical Center, Dallas. “Our group has demonstrated that physical inactivity and low levels of fitness are associated with a higher risk of heart failure. We have also shown that the protective effect of physical activity against heart failure risk is stronger against heart failure with preserved ejection fraction, which is a subtype of heart failure that is increasing in prevalence and has no effective therapies.”
Dr. Pandey and his colleagues set out to test the research hypothesis that fitness decline and increases in body mass index over time are significantly associated with a higher risk of heart failure. To do this, they drew from the LookAHEAD Trial, a multicenter analysis of 5,145 overweight or obese patients with type 2 diabetes who were randomized to an intensive lifestyle intervention or to usual care. The intervention consisted of a caloric intake goal of 1,200 to 1,800 kcal per day and engaging in at least 175 minutes per week of physical activity. Participants were stratified into one of three fitness group levels: low, moderate, and high, from 5 metabolic equivalents (METs) in the lowest fitness tertile to 9 METs in the highest fitness tertile. The primary outcome of the trial was adverse cardiovascular events. The intervention was implemented for almost 10 years, and patients were followed for up to 12 years from baseline.
The heart failure outcomes were not systematically adjudicated in the primary LookAHEAD trial, so Dr. Pandey and colleagues conducted an ancillary study of all incident hospitalizations in the study and followed them for 2 additional years. Overall, the researchers identified 257 incident heart failure events. The cumulative incidence of heart failure for the usual care versus the intensive lifestyle intervention arm was not statistically different (an event rate of 4.53 vs. 4.32 per 1,000 person-years, respectively; hazard ratio, 0.96). “This demonstrated that the intensive lifestyle intervention in the LookAHEAD trial did not significantly modify the risk of heart failure,” Dr. Pandey said.
However, an adjusted analysis revealed that the risk of heart failure was 39% lower in the moderately fit group and 62% lower in the high fit group, compared with the low-fitness group. Among heart failure subtypes, the risk of heart failure with preserved ejection fraction (HFpEF) was 40% lower in the moderately fit group and 77% lower in the high-fitness group. On the other hand, baseline level of fitness level was not associated with risk of heart failure reduced ejection fraction (HFrEF) after the researchers adjusted for cardiovascular risk factors.
Next, Dr. Pandey and his colleagues used Cox modeling to examine the association of baseline and longitudinal changes in fitness and BMI with risk of heart failure. For change in fitness and BMI analysis, they used the 4-year follow-up data in 3,092 participants who underwent repeat fitness testing and had available data on BMI. They excluded patients who developed heart failure within the first 4 years of the study.
The mean age of the ancillary study population was about 60 years, and there was a lower proportion of women in the high fitness tertile (41%). The researchers observed a graded, inverse association between higher fitness levels and lower risk of heart failure such that increasing fitness from baseline was associated with a substantial decrease in the risk of heart failure. Specifically, a 10% decline in fitness over the 4 years of follow-up was associated with a 11% increase in the overall risk of heart failure (HR, 1.11). “This was largely consistent with the two heart failure subtypes,” he said. Similarly, a 10% increase in BMI over the 4 years of follow-up was associated with a 25% increase in the overall risk of heart failure (HR 1.25). On the other hand, a 10% decrease BMI was associated with a 20% decrease in the risk of heart failure (HR .80). This was also largely consistent for both heart failure subtypes. According to co-lead investigator Kershaw Patel, MD, “these findings suggest that therapies targeting large and sustained improvements in fitness and weight loss may modify the risk of heart failure among patients with diabetes.”
“Lower fitness at baseline was more strongly associated with the risk of HFpEF vs. HFrEF, and greater weight loss over follow-up is associated with a lower risk of heart failure independent of changes in other risk factors,” Dr. Pandey concluded at the meeting, which was sponsored by the American Heart Association.
In an interview, session moderator Joshua J. Joseph, MD, said that it remains unclear what type of setting is ideal for carrying out cardiorespiratory fitness in this patient population. “What is the supervision needed for that to occur?” asked Dr. Joseph, of The Ohio State University, Columbus. “Can patients do this on their own, or do they need guidance? What is the best approach? That’s the question we all have to answer individually in our own communities.”
Dr. Pandey reported having no disclosures.
SOURCE: Pandey A. Epi/Lifestyle 2020, Abstract 16.
PHOENIX – Lower baseline fitness and greater decline in fitness over time are independently associated with a higher risk of heart failure in patients with diabetes, results from a large analysis showed.
“Diabetes is an important risk factor for the development of heart failure, and the diagnosis of diabetes in newly diagnosed cases of heart failure has been increasing,” Ambarish Pandey, MD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “Type 2 diabetes is associated with increased burden of traditional risk factors such as hypertension, kidney dysfunction, and dyslipidemia – each of which in turn increase the risk of both atherothrombotic disease as well as heart failure.”
Recent data from the Swedish National Diabetes Register have shown that optimal management of these risk factors in patients with type 2 diabetes can actually mitigate the risk of atherosclerotic events such as acute MI, but the risk of heart failure does not significantly lower with optimal management of these traditional cardiovascular risk factors (N Engl J Med. 2018;379:633-44). “These findings highlight that novel approaches that go beyond just managing traditional cardiovascular risk factors are needed for prevention of heart failure in patients with type 2 diabetes,” said Dr. Pandey, of the division of cardiology at the University of Texas Southwestern Medical Center, Dallas. “Our group has demonstrated that physical inactivity and low levels of fitness are associated with a higher risk of heart failure. We have also shown that the protective effect of physical activity against heart failure risk is stronger against heart failure with preserved ejection fraction, which is a subtype of heart failure that is increasing in prevalence and has no effective therapies.”
Dr. Pandey and his colleagues set out to test the research hypothesis that fitness decline and increases in body mass index over time are significantly associated with a higher risk of heart failure. To do this, they drew from the LookAHEAD Trial, a multicenter analysis of 5,145 overweight or obese patients with type 2 diabetes who were randomized to an intensive lifestyle intervention or to usual care. The intervention consisted of a caloric intake goal of 1,200 to 1,800 kcal per day and engaging in at least 175 minutes per week of physical activity. Participants were stratified into one of three fitness group levels: low, moderate, and high, from 5 metabolic equivalents (METs) in the lowest fitness tertile to 9 METs in the highest fitness tertile. The primary outcome of the trial was adverse cardiovascular events. The intervention was implemented for almost 10 years, and patients were followed for up to 12 years from baseline.
The heart failure outcomes were not systematically adjudicated in the primary LookAHEAD trial, so Dr. Pandey and colleagues conducted an ancillary study of all incident hospitalizations in the study and followed them for 2 additional years. Overall, the researchers identified 257 incident heart failure events. The cumulative incidence of heart failure for the usual care versus the intensive lifestyle intervention arm was not statistically different (an event rate of 4.53 vs. 4.32 per 1,000 person-years, respectively; hazard ratio, 0.96). “This demonstrated that the intensive lifestyle intervention in the LookAHEAD trial did not significantly modify the risk of heart failure,” Dr. Pandey said.
However, an adjusted analysis revealed that the risk of heart failure was 39% lower in the moderately fit group and 62% lower in the high fit group, compared with the low-fitness group. Among heart failure subtypes, the risk of heart failure with preserved ejection fraction (HFpEF) was 40% lower in the moderately fit group and 77% lower in the high-fitness group. On the other hand, baseline level of fitness level was not associated with risk of heart failure reduced ejection fraction (HFrEF) after the researchers adjusted for cardiovascular risk factors.
Next, Dr. Pandey and his colleagues used Cox modeling to examine the association of baseline and longitudinal changes in fitness and BMI with risk of heart failure. For change in fitness and BMI analysis, they used the 4-year follow-up data in 3,092 participants who underwent repeat fitness testing and had available data on BMI. They excluded patients who developed heart failure within the first 4 years of the study.
The mean age of the ancillary study population was about 60 years, and there was a lower proportion of women in the high fitness tertile (41%). The researchers observed a graded, inverse association between higher fitness levels and lower risk of heart failure such that increasing fitness from baseline was associated with a substantial decrease in the risk of heart failure. Specifically, a 10% decline in fitness over the 4 years of follow-up was associated with a 11% increase in the overall risk of heart failure (HR, 1.11). “This was largely consistent with the two heart failure subtypes,” he said. Similarly, a 10% increase in BMI over the 4 years of follow-up was associated with a 25% increase in the overall risk of heart failure (HR 1.25). On the other hand, a 10% decrease BMI was associated with a 20% decrease in the risk of heart failure (HR .80). This was also largely consistent for both heart failure subtypes. According to co-lead investigator Kershaw Patel, MD, “these findings suggest that therapies targeting large and sustained improvements in fitness and weight loss may modify the risk of heart failure among patients with diabetes.”
“Lower fitness at baseline was more strongly associated with the risk of HFpEF vs. HFrEF, and greater weight loss over follow-up is associated with a lower risk of heart failure independent of changes in other risk factors,” Dr. Pandey concluded at the meeting, which was sponsored by the American Heart Association.
In an interview, session moderator Joshua J. Joseph, MD, said that it remains unclear what type of setting is ideal for carrying out cardiorespiratory fitness in this patient population. “What is the supervision needed for that to occur?” asked Dr. Joseph, of The Ohio State University, Columbus. “Can patients do this on their own, or do they need guidance? What is the best approach? That’s the question we all have to answer individually in our own communities.”
Dr. Pandey reported having no disclosures.
SOURCE: Pandey A. Epi/Lifestyle 2020, Abstract 16.
REPORTING FROM EPI/LIFESTYLE 2020
AFib-related cardiovascular deaths on the rise
PHOENIX, ARIZ. – Cardiovascular deaths and death rates related to atrial fibrillation have risen since 1999, with significant acceleration following 2009, results from a cross-sectional analysis of national data show.
“AFib is the most common arrhythmia disorder in the United States and it is estimated that it will effect more than 12 million Americans by 2030,” Yoshihiro Tanaka, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “The predicted lifetime risk ranges from 25% to 35%, and AFib is associated with an increased risk for heart failure, stroke, and death.”
A recent review reported that declines in total heart disease mortality rates in the United States have plateaued since 2011 (JAMA 2019;322[8]:780-2). However, it is not well understood what factors such as AFib contribute to this rate of plateau. In an effort to quantify U.S. trends in AFib-related CVD death rates, Dr. Tanaka and colleagues conducted a serial cross-sectional analysis of death certificate data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER) database during 1999-2017.
Outcomes included age-adjusted mortality per 100,000 based on the 2000 U.S. standard population. The researchers also used joinpoint regression to calculate the average annual percentage change over time and conducted subgroup analyses by race and sex and across two age groups: 35-64 years and 65-84 years.
In all, 522,104 AFib-related CVD deaths were identified during 1999-2017. Dr. Tanaka reported that age-adjusted mortality increased from 16.0 per 100,000 persons in 1999 to 22.2 per 100,000 person in 2017, with an acceleration following an inflection point in 2009. Specifically, the average annual percentage change in AFib-related CVD deaths rose from 0.4% in 2009 to 3.5% in 2017 (P < .001). “These increases were consistent across all race-sex subgroups,” said Dr. Tanaka, of the department of preventive medicine at Northwestern University, Chicago. “Relative increases were also greater in younger compared with older adults, although the absolute number of deaths in younger adults was less.”
The researchers observed that age-adjusted mortality increased across blacks and whites in both age groups, with a more pronounced increase among black and white men. Black men had the highest age-adjusted mortality among persons aged 35-64 (6.5 per 100,000 persons, compared with 4.2 among white men, 2.8 in black women, and 1.6 in white women 1.6 per 100,000). At the same time, white men had the highest age-adjusted mortality rate among those aged 65-84 years (112.5 per 100,000 persons, compared with 87.7 in black men, 77.4 in white women, and 61.3 in black women).
In an interview, one of the session’s moderators, Alvaro Alonso, MD, PhD, said that the study’s reliance on mortality data is a limitation. “You have to be careful with that, because it’s not the whole picture,” said Dr. Alonso, professor of epidemiology at the Rollins School of Public Health at Emory University, Atlanta. “It could be an underestimation of what is going on. The increase in recent years is probably due to a higher awareness of AFib as a risk factor for stroke; it’s more on the radar. Also, around 2009-2010, we started having new anticoagulants for AFib. It’s getting diagnosed more. When you look at coronary heart disease and stroke, there has been a decrease over time. In mortality and incidence of AFib, we don’t have that. That’s probably because we don’t know very much about what the risk factors for AFib are and how to prevent it.”
Dr. Tanaka said that the cause of increase in AFib-related CVD mortality can be classified into two major categories: a balance between case fatality of AFib and the prevalence of AFib. “The case fatality rate should have decreased over the last years,” he said at the meeting, which was sponsored by the American Heart Association. “In contrast, in the context of the aging of the population, the prevalence of AFib increased over the past years. Contributing factors include increasing awareness of AFib, a change in coding between ICD-9 and ICD-10, and a change in coding practices by physicians.”
Strengths of the study, he said, include its large sample size and the fact that the researchers were able to capture data from all death certificates filed in the United States. Limitations include the fact that the data “do not identify if changes in age-adjusted mortality rates are due to changing incidence or to case fatality rates,” he said. “CDC WONDER does not allow us to explore causes of these descriptive findings, but this would be an important next step.”
Dr. Tanaka reported having no financial disclosures.
SOURCE: Tanaka Y. EPI/Lifestyle 2020, Session 5, Abstract 15.
PHOENIX, ARIZ. – Cardiovascular deaths and death rates related to atrial fibrillation have risen since 1999, with significant acceleration following 2009, results from a cross-sectional analysis of national data show.
“AFib is the most common arrhythmia disorder in the United States and it is estimated that it will effect more than 12 million Americans by 2030,” Yoshihiro Tanaka, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “The predicted lifetime risk ranges from 25% to 35%, and AFib is associated with an increased risk for heart failure, stroke, and death.”
A recent review reported that declines in total heart disease mortality rates in the United States have plateaued since 2011 (JAMA 2019;322[8]:780-2). However, it is not well understood what factors such as AFib contribute to this rate of plateau. In an effort to quantify U.S. trends in AFib-related CVD death rates, Dr. Tanaka and colleagues conducted a serial cross-sectional analysis of death certificate data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER) database during 1999-2017.
Outcomes included age-adjusted mortality per 100,000 based on the 2000 U.S. standard population. The researchers also used joinpoint regression to calculate the average annual percentage change over time and conducted subgroup analyses by race and sex and across two age groups: 35-64 years and 65-84 years.
In all, 522,104 AFib-related CVD deaths were identified during 1999-2017. Dr. Tanaka reported that age-adjusted mortality increased from 16.0 per 100,000 persons in 1999 to 22.2 per 100,000 person in 2017, with an acceleration following an inflection point in 2009. Specifically, the average annual percentage change in AFib-related CVD deaths rose from 0.4% in 2009 to 3.5% in 2017 (P < .001). “These increases were consistent across all race-sex subgroups,” said Dr. Tanaka, of the department of preventive medicine at Northwestern University, Chicago. “Relative increases were also greater in younger compared with older adults, although the absolute number of deaths in younger adults was less.”
The researchers observed that age-adjusted mortality increased across blacks and whites in both age groups, with a more pronounced increase among black and white men. Black men had the highest age-adjusted mortality among persons aged 35-64 (6.5 per 100,000 persons, compared with 4.2 among white men, 2.8 in black women, and 1.6 in white women 1.6 per 100,000). At the same time, white men had the highest age-adjusted mortality rate among those aged 65-84 years (112.5 per 100,000 persons, compared with 87.7 in black men, 77.4 in white women, and 61.3 in black women).
In an interview, one of the session’s moderators, Alvaro Alonso, MD, PhD, said that the study’s reliance on mortality data is a limitation. “You have to be careful with that, because it’s not the whole picture,” said Dr. Alonso, professor of epidemiology at the Rollins School of Public Health at Emory University, Atlanta. “It could be an underestimation of what is going on. The increase in recent years is probably due to a higher awareness of AFib as a risk factor for stroke; it’s more on the radar. Also, around 2009-2010, we started having new anticoagulants for AFib. It’s getting diagnosed more. When you look at coronary heart disease and stroke, there has been a decrease over time. In mortality and incidence of AFib, we don’t have that. That’s probably because we don’t know very much about what the risk factors for AFib are and how to prevent it.”
Dr. Tanaka said that the cause of increase in AFib-related CVD mortality can be classified into two major categories: a balance between case fatality of AFib and the prevalence of AFib. “The case fatality rate should have decreased over the last years,” he said at the meeting, which was sponsored by the American Heart Association. “In contrast, in the context of the aging of the population, the prevalence of AFib increased over the past years. Contributing factors include increasing awareness of AFib, a change in coding between ICD-9 and ICD-10, and a change in coding practices by physicians.”
Strengths of the study, he said, include its large sample size and the fact that the researchers were able to capture data from all death certificates filed in the United States. Limitations include the fact that the data “do not identify if changes in age-adjusted mortality rates are due to changing incidence or to case fatality rates,” he said. “CDC WONDER does not allow us to explore causes of these descriptive findings, but this would be an important next step.”
Dr. Tanaka reported having no financial disclosures.
SOURCE: Tanaka Y. EPI/Lifestyle 2020, Session 5, Abstract 15.
PHOENIX, ARIZ. – Cardiovascular deaths and death rates related to atrial fibrillation have risen since 1999, with significant acceleration following 2009, results from a cross-sectional analysis of national data show.
“AFib is the most common arrhythmia disorder in the United States and it is estimated that it will effect more than 12 million Americans by 2030,” Yoshihiro Tanaka, MD, PhD, said at the Epidemiology and Prevention/Lifestyle and Cardiometabolic Health meeting. “The predicted lifetime risk ranges from 25% to 35%, and AFib is associated with an increased risk for heart failure, stroke, and death.”
A recent review reported that declines in total heart disease mortality rates in the United States have plateaued since 2011 (JAMA 2019;322[8]:780-2). However, it is not well understood what factors such as AFib contribute to this rate of plateau. In an effort to quantify U.S. trends in AFib-related CVD death rates, Dr. Tanaka and colleagues conducted a serial cross-sectional analysis of death certificate data from the Centers for Disease Control and Prevention’s Wide-Ranging Online Data for Epidemiologic Research (WONDER) database during 1999-2017.
Outcomes included age-adjusted mortality per 100,000 based on the 2000 U.S. standard population. The researchers also used joinpoint regression to calculate the average annual percentage change over time and conducted subgroup analyses by race and sex and across two age groups: 35-64 years and 65-84 years.
In all, 522,104 AFib-related CVD deaths were identified during 1999-2017. Dr. Tanaka reported that age-adjusted mortality increased from 16.0 per 100,000 persons in 1999 to 22.2 per 100,000 person in 2017, with an acceleration following an inflection point in 2009. Specifically, the average annual percentage change in AFib-related CVD deaths rose from 0.4% in 2009 to 3.5% in 2017 (P < .001). “These increases were consistent across all race-sex subgroups,” said Dr. Tanaka, of the department of preventive medicine at Northwestern University, Chicago. “Relative increases were also greater in younger compared with older adults, although the absolute number of deaths in younger adults was less.”
The researchers observed that age-adjusted mortality increased across blacks and whites in both age groups, with a more pronounced increase among black and white men. Black men had the highest age-adjusted mortality among persons aged 35-64 (6.5 per 100,000 persons, compared with 4.2 among white men, 2.8 in black women, and 1.6 in white women 1.6 per 100,000). At the same time, white men had the highest age-adjusted mortality rate among those aged 65-84 years (112.5 per 100,000 persons, compared with 87.7 in black men, 77.4 in white women, and 61.3 in black women).
In an interview, one of the session’s moderators, Alvaro Alonso, MD, PhD, said that the study’s reliance on mortality data is a limitation. “You have to be careful with that, because it’s not the whole picture,” said Dr. Alonso, professor of epidemiology at the Rollins School of Public Health at Emory University, Atlanta. “It could be an underestimation of what is going on. The increase in recent years is probably due to a higher awareness of AFib as a risk factor for stroke; it’s more on the radar. Also, around 2009-2010, we started having new anticoagulants for AFib. It’s getting diagnosed more. When you look at coronary heart disease and stroke, there has been a decrease over time. In mortality and incidence of AFib, we don’t have that. That’s probably because we don’t know very much about what the risk factors for AFib are and how to prevent it.”
Dr. Tanaka said that the cause of increase in AFib-related CVD mortality can be classified into two major categories: a balance between case fatality of AFib and the prevalence of AFib. “The case fatality rate should have decreased over the last years,” he said at the meeting, which was sponsored by the American Heart Association. “In contrast, in the context of the aging of the population, the prevalence of AFib increased over the past years. Contributing factors include increasing awareness of AFib, a change in coding between ICD-9 and ICD-10, and a change in coding practices by physicians.”
Strengths of the study, he said, include its large sample size and the fact that the researchers were able to capture data from all death certificates filed in the United States. Limitations include the fact that the data “do not identify if changes in age-adjusted mortality rates are due to changing incidence or to case fatality rates,” he said. “CDC WONDER does not allow us to explore causes of these descriptive findings, but this would be an important next step.”
Dr. Tanaka reported having no financial disclosures.
SOURCE: Tanaka Y. EPI/Lifestyle 2020, Session 5, Abstract 15.
REPORTING FROM EPI/LIFESTYLE 2020