User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
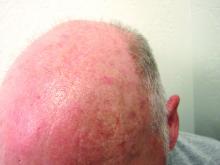
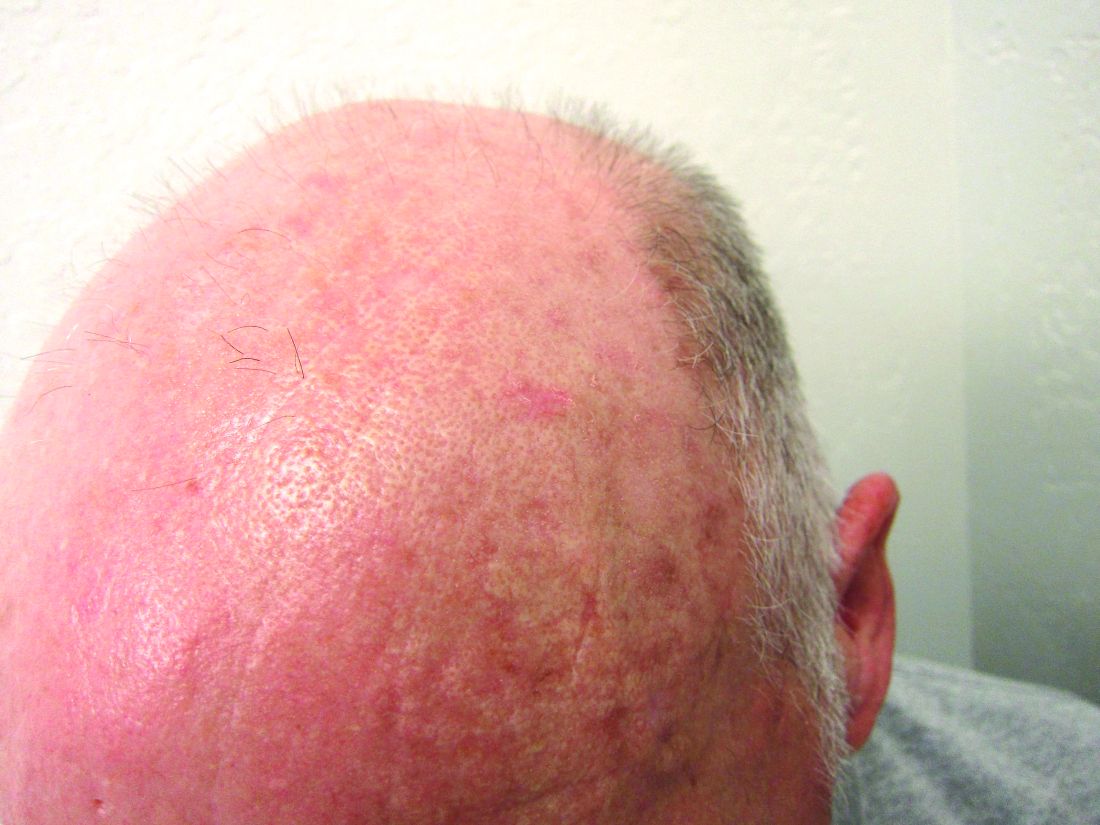
Review eyes nail unit toxicities secondary to targeted cancer therapy
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
while damage to other nail unit anatomic areas can be wide-ranging.
Those are key findings from an evidence-based literature review published on July 21, 2021, in the Journal of the American Academy of Dermatology, as a letter to the editor. “Dermatologic toxicities are often the earliest-presenting and highest-incidence adverse events due to targeted anticancer therapies and immunotherapies,” corresponding author Anisha B. Patel, MD, of the department of dermatology at the University of Texas MD Anderson Cancer Center, Houston, and colleagues wrote. “Nail unit toxicities due to immunotherapy are caused by nonspecific immune activation. Targeted therapies, particularly mitogen-activated protein kinase pathway inhibitors, lead to epidermal thinning of the nail folds and periungual tissue, increasing susceptibility to trauma and penetration by nail plate fragments. Although cutaneous toxicities have been well described, further characterization of nail unit toxicities is needed.”
The researchers searched the PubMed database using the terms nail, nail toxicity, nail dystrophy, paronychia, onycholysis, pyogenic granuloma, onychopathy, targeted therapy, and immunotherapy, and reviewed relevant articles for clinical presentation, diagnosis, incidence, outcomes, and references. They also proposed treatment algorithms for this patient population based on the existing literature and the authors’ collective clinical experience.
Dr. Patel and colleagues found that paronychia and periungual pyogenic granulomas were the most common nail unit toxicities caused by targeted therapy. “Damage to other nail unit anatomic areas includes drug induced or exacerbated lichen planus and psoriasis as well as pigmentary and neoplastic changes,” they wrote. “Onycholysis, onychoschizia, paronychia, psoriasis, lichen planus, and dermatomyositis have been reported with immune checkpoint inhibitors,” with the time of onset during the first week of treatment to several months after treatment has started.
According to National Cancer Institute criteria, nail adverse events associated with medical treatment include nail changes, discoloration, ridging, paronychia, and infection. The severity of nail loss, paronychia, and infection can be graded up to 3 (defined as “severe or medically significant but not life threatening”), while the remainder of nail toxicities may be categorized only as grade 1 (defined as “mild,” with “intervention not indicated”). “High-grade toxicities have been reported, especially with pan-fibroblast growth factor receptor inhibitors,” the authors wrote, referring to a previous study.
The review includes treatment algorithms for paronychia, periungual pyogenic granuloma, nail lichen planus, and psoriasis. “Long-acting and nonselective immunosuppressants are reserved for dose-limiting toxicities, given their unknown effects on already-immunosuppressed patients with cancer and on cancer therapy,” the authors wrote. “A discussion with the oncology department is essential before starting an immunomodulator or immunosuppressant.”
To manage onycholysis, Dr. Patel and colleagues recommended trimming the onycholytic nail plate to its attachment point. “Partial avulsion is used to treat a refractory abscess or painful hemorrhage,” they wrote. “A Pseudomonas superinfection is treated twice daily with a topical antibiotic solution. Brittle nail syndrome is managed with emollients or the application of polyureaurethane, a 16% nail solution, or a hydrosoluble nail lacquer,” they wrote, adding that biotin supplementation is not recommended.
Jonathan Leventhal, MD, who was asked to comment on the study, said that nail toxicity from targeted cancer therapy is one of the most common reasons for consultation in his role as director of the Yale University oncodermatology program at Smilow Cancer Hospital, New Haven, Conn. “When severe, these reactions frequently impact patients’ quality of life,” he said.
“This study is helpful for all dermatologists caring for cancer patients,” with strengths that include “succinctly summarizing the most prevalent conditions and providing a clear and practical algorithm for approaching these nail toxicities,” he said. In addition to targeted agents and immunotherapy, “we commonly see nail toxicities from cytotoxic chemotherapy, which was not reviewed in this paper. Multidisciplinary evaluation and dermatologic involvement is certainly beneficial to make accurate diagnoses and promptly manage these conditions, helping patients stay on their oncologic therapies.”
The researchers reported no financial disclosures. Dr. Leventhal disclosed that he is a member of the advisory board for Regeneron, Sanofi, Bristol-Myers Squibb, and La Roche–Posay. He has also received research funding from Azitra and OnQuality.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Hep B vaccine response varied among youth with inflammatory, autoimmune disorders
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
“Hepatitis B is a common viral infection with 2 billion people worldwide having evidence of prior or current infection, and it can present as an acute or chronic infection,” or with chronic sequelae, including cirrhosis and hepatocellular carcinoma, Alexandra Ritter said during the annual meeting of the Society for Pediatric Dermatology. A three-dose vaccination series is recommended beginning at birth, and in 2016, the Centers for Disease Control and Prevention reported that 90.5% of U.S. children aged 19-35 months had completed the series.
While the vaccine series provides protection in healthy individuals more than 95% of the time, a decreased response has been noted in specific pediatric populations, including those with inflammatory and autoimmune diseases. “This is important to note and investigate further because a decreased vaccine response increases the risk for this high-risk population, and the use of boosters is currently debated,” said Ms. Ritter, who is a fourth-year student at the Medical University of South Carolina, Charleston.
To determine the percent of pediatric patients with inflammatory or autoimmune disease who lack evidence of immunity following the hepatitis B vaccine series, Ms. Ritter and colleagues Abigail Truitt and pediatric dermatologist Lara Wine Lee, MD, PhD, of MUSC, retrospectively reviewed the charts of 160 patients between the ages of 6 months and 21 years, who were diagnosed with an autoimmune or autoinflammatory disease, or inflammatory bowel disease (IBD), and had documented evidence of vaccination and serologic testing prior to the start of immunosuppressive therapy.
Of the 160 patients, 100 (63%) had IBD, 34 (21%) had an autoimmune disease, 26 (16%) had an autoinflammatory disease, 89 (56%) were female, and their mean age was 15 years.
The researchers observed variation in the testing ordered between the three patient groups. Specifically, 88.2% of autoimmune patients had hepatitis B surface antigen (HBsAg) testing, compared with 96.15% of patients with an autoinflammatory disease and 67% of patients with IBD, while 76.47% of patients with an autoimmune disease had hepatitis B core antibody (anti-HBc) testing, compared with 88.46% of patients with an autoinflammatory disease and 31% of patients with IBD.
In addition, 82.35% of patients with an autoimmune disease had HBsAg testing, compared with 100% of patients with an autoinflammatory disease and 94% of patients with IBD.
Of the 148 patients who had HBsAg testing ordered and completed prior to starting an immunosuppressive drug, there was no statistically significant difference in the percent of patients showing evidence of an immune response to the hepatitis B vaccine (32.14% among patients with an autoimmune disease, 34.62% among patients with an autoinflammatory disease, and 31.91% among patients with IBD). Combined, 67.57% of tested negative for the hepatitis B surface antibody.
“Our study showed that the majority of these patients did not show serologic evidence of immunity despite being fully vaccinated,” Ms. Ritter said. “There was also variation in the testing ordered and a more standardized approach is needed in this high-risk population.” She acknowledged certain limitations of the study, including its retrospective design and lack of a control group.
“This brings us to our next question of whether this indicates a failure of the vaccine, or the way immunity is tested,” she continued. “The CDC and the European Consensus Group on Hepatitis B Immunity recommend a cutoff of greater than 10 mIU/mL. Those that achieve immunity are protected for up to 20 years due to immune memory, even if their antibody levels later drop. There have been rare cases of immunocompetent individuals having evidence of transient asymptomatic infections when antibody levels drop. The chronic disease has only been documented in infants born to positive mothers. In hemodialysis patients, however, clinically significant infections have been documented when antibody levels drop.”
The CDC only recommends postvaccination testing to infants born to positive mothers, health care workers at high risk, hemodialysis patients, people with HIV and other immunocompromised people, and needle-sharing partners of chronically infected people. This is completed 1-2 months following the third vaccine dose, and those with antibody levels less than 10 mIU/mL should be revaccinated. “As some groups do not respond to the vaccine series, alternative dosing and the intradermal vaccine have been studied and shown to be effective in certain groups,” she said.
When it comes to monitoring immunocompromised individuals and giving booster shots, however, there are conflicting recommendations. The CDC recommends yearly testing and booster shots when levels drop below 10 mIU/mL only in hemodialysis patients, while the European Consensus Group recommends testing every 6-12 months for immunocompromised individuals and boosters when their levels drop below 10 mIU/mL.
“The CDC has not yet determined if other immunocompromised individuals should receive a booster, with more research required, but studies have shown it to be effective,” Ms. Ritter said. In a similar study looking at evidence of immunity in children with connective tissue disease who were on immunosuppressive treatment, 50% had no evidence of protective antibodies, compared with 96% in the control group. “In that study, a booster shot was given, and protective antibody concentrations were found at follow-up,” she said.
The researchers reported having no financial disclosures.
[email protected]
FROM SPD 2021
Age, distance from dermatology clinic <p>predict number of melanomas diagnosed
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
Among patients from a single dermatology practice who were diagnosed with two or more melanomas over an 8-year period, 45% lived more than 20 miles away from the practice, and almost 60% were 70 years of age and older, results from single-center study showed.
“Dermatologists have known that many people are underdiagnosed for melanoma, but now our research supports that the problem is especially concentrated among older patients living in remote areas,” corresponding author Rose Parisi, MBA, said in an interview. “With this information, dermatologists should consider identifying and reaching out to their patients in this at-risk subpopulation, increasing the frequency of full-body skin exams, and collaborating with primary care physicians to educate them about melanoma’s dangers.”
In a study published online Aug. 3 in the Journal of the American Academy of Dermatology, Ms. Parisi of Albany Medical College, New York, and colleagues drew from the electronic medical records of a single-specialty private dermatology practice that serves urban, suburban, and rural patient populations to identify 346 melanoma pathology reports from patients cared for between 2012 and 2020. They limited their investigation to those diagnosed with biopsy-confirmed melanoma and analyzed the number of melanomas, Breslow depth, follow-up full-body skin exams, family history of melanoma, gender, insurance, and age (categorized as younger than 70 years and 70 years or older). To determine patient travel distance, they calculated the miles between the ZIP codes of the patient’s residence and the dermatology practice.
Regression analysis revealed that the . Specifically, among patients diagnosed with two or more melanomas, 45.0% lived more than 20 miles away and 21.3% lived less than 15 miles away; 59.6% were age 70 and older, while 40.4% were younger than age 70 (P less than .01).
No statistically significant association was observed between travel distance and Breslow depth or follow-up full-body skin exams within 1 year following diagnosis.
In other findings, among patients who lived more than 20 miles from the practice, those aged 70 and older were diagnosed with 0.56 more melanomas than patients between the ages of 58 and 70 (P = .00003), and 0.31 more melanomas than patients who lived 15-20 miles away (P = .014). No statistically significant differences in the number of melanomas diagnosed were observed between patients in either age group who lived fewer than 15 miles from the office.
“We were surprised that the combination of age and patient distance to diagnosing dermatology provider was such a powerful predictor of the number of diagnosed melanomas,” Ms. Parisi said. “It’s probably due to less mobility among older patients living in more remote areas, and it puts them at higher risk of multiple melanomas. This was something we haven’t seen in the dermatology literature.”
She and her coauthors acknowledged that the limited sampling of patients from a single practice “may not generalize across all urban and rural settings, and results must be considered preliminary,” they wrote. However, “our findings reveal an important vulnerability among older patients in nonurban areas, and efforts to improve access to melanoma diagnosis should be concentrated on this geodemographic segment.”
Nikolai Klebanov, MD, of the department of dermatology at Massachusetts General Hospital, Boston, who was asked to comment on the study, described what was addressed in the study as a “timely and an important topic.”
In an interview, he said, “there is less access to dermatologists and other medical specialists outside of large metropolitan and suburban areas,” and there are other health disparities affecting people living in rural or more underserved areas, which, he added, “also became exacerbated by the COVID-19 pandemic.”
For future studies on this topic, Dr. Klebanov said that he would be interested to see diagnoses measured per person-year rather than the total number of melanomas diagnosed. “More elderly patients may also be those who have ‘stuck with the practice’ for longer, and had a longer follow-up that gives more time to catch more melanomas,” he said.
“Adjusting for median income using ZIP codes could also help adjust for socioeconomic status, which would help with external validity of the study. Income relationships to geography are not the same in all cities; some have wealthy suburbs within 20 miles, while some have more underserved and rural areas at that distance.”
Neither the researchers nor Dr. Klebanov reported having financial disclosures.
FROM JAMA DERMATOLOGY
Which AK treatment has the best long-term efficacy? A study reviews the data
The four results from a systemic review and meta-analysis suggest.
To date, many studies have reported that “most interventions are superior to placebo in terms of lesion clearance and improving the cosmetic image,” corresponding author Markus V. Heppt, MD, MSc, and colleagues wrote in a study published online Aug. 4, 2021, in JAMA Dermatology.
“However, most randomized clinical trials (RCTs) and meta-analyses focused on short-term outcomes that are evaluated within 3-6 months after treatment, although AK is increasingly being considered a chronic condition and reducing the incidence of cSCC [cutaneous squamous cell carcinoma] should be the ultimate goal of treatment,” they said. In addition, most treatments have been compared with placebo “and head-to-head comparisons are widely lacking, limiting the possibility to cross compare distinct active treatments. To this end, no evidence-based recommendation regarding the long-term efficacy of interventions for AK exists.”
To determine the long-term clearance rates of treatments used in adults with AK, a precursor of cSCC, Dr. Heppt, of the department of dermatology at University Hospital Erlangen (Germany), and colleagues drew from 15 randomized clinical trials that reported sustained clearance rates after at least 12 months of treatment and were published up to April 6, 2020. They conducted the review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline and its extension for network meta-analyses (PRIMSA-NMA) and using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process to determine the certainty of the evidence for network meta-analyses.
The study population included 4,252 patients. Among 10 studies included in a network meta-analysis for the outcome of participant complete clearance, ALA-PDT showed the most favorable risk ratio profile, compared with placebo (RR, 8.06; moderate-quality evidence on GRADE), followed by imiquimod, 5% (RR, 5.98; very-low-quality evidence on GRADE); MAL-PDT (RR, 5.95; low-quality evidence on GRADE); and cryosurgery (RR, 4.76; very-low-quality evidence on GRADE).
ALA-PDT had the highest RR in the network meta-analyses for lesion-specific clearance (RR, 5.08; moderate-quality evidence on GRADE).
“Although ALA-PDT showed the most favorable RR and was ranked best among all interventions, the relative efficacy values and treatment rankings must be interpreted with caution,” because of the low certainty of evidence and few direct, head-to-head comparisons, the authors emphasized. “In particular, it remains elusive how to translate the distinct RR values into clinical relevance. We are hesitant to derive hierarchical or algorithmic treatment recommendations from our results.”
“The current meta-analysis notes that there are conflicting results in different studies,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn. who was asked to comment on the study. “Sustained participant complete clearance of actinic keratoses at 12 months is used as an outcome measure, although the authors comment that prevention/reduction of squamous cell carcinoma might be the more valid outcome measure.”
In her clinical experience, Dr. Ko said that patients often have good, sustained clearance of AKs with field treatment using a topical medication like 5-fluorouracil. “Patients can also have a good result with photodynamic therapy,” she said. “The paper’s results therefore do reflect what I have seen in my own practice. I also agree with the authors that, while it is difficult to measure, a meaningful outcome for patients is reduction/prevention of squamous cell carcinoma. It would be useful to have data on which treatment of actinic keratosis is best to reduce/prevent squamous cell carcinoma.”
The authors acknowledged limitations of the study, including the fact that field-directed treatments such as imiquimod, PDT, and fluorouracil were compared with lesion-directed approaches such as cryosurgery, “which may limit the generalizability of our results.” They concluded that their analysis “provides data that might contribute to an evidence-based framework to guide the selection of interventions for AK with proven long-term efficacy and sustained AK clearance.”
The analysis did not include data on tirbanibulin, a first-in-class dual Src kinase and tubulin polymerization inhibitor that was approved by the FDA for the topical treatment of AKs on the face or scalp in December 2020.
Dr. Heppt disclosed that he has been a member of the advisory boards of Almirall Hermal and Sanofi-Aventis and has received speaker’s honoraria from Galderma and Biofrontera. Many of his coauthors also reported having relevant financial disclosures. Dr. Ko reported having no relevant disclosures.
The four results from a systemic review and meta-analysis suggest.
To date, many studies have reported that “most interventions are superior to placebo in terms of lesion clearance and improving the cosmetic image,” corresponding author Markus V. Heppt, MD, MSc, and colleagues wrote in a study published online Aug. 4, 2021, in JAMA Dermatology.
“However, most randomized clinical trials (RCTs) and meta-analyses focused on short-term outcomes that are evaluated within 3-6 months after treatment, although AK is increasingly being considered a chronic condition and reducing the incidence of cSCC [cutaneous squamous cell carcinoma] should be the ultimate goal of treatment,” they said. In addition, most treatments have been compared with placebo “and head-to-head comparisons are widely lacking, limiting the possibility to cross compare distinct active treatments. To this end, no evidence-based recommendation regarding the long-term efficacy of interventions for AK exists.”
To determine the long-term clearance rates of treatments used in adults with AK, a precursor of cSCC, Dr. Heppt, of the department of dermatology at University Hospital Erlangen (Germany), and colleagues drew from 15 randomized clinical trials that reported sustained clearance rates after at least 12 months of treatment and were published up to April 6, 2020. They conducted the review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline and its extension for network meta-analyses (PRIMSA-NMA) and using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process to determine the certainty of the evidence for network meta-analyses.
The study population included 4,252 patients. Among 10 studies included in a network meta-analysis for the outcome of participant complete clearance, ALA-PDT showed the most favorable risk ratio profile, compared with placebo (RR, 8.06; moderate-quality evidence on GRADE), followed by imiquimod, 5% (RR, 5.98; very-low-quality evidence on GRADE); MAL-PDT (RR, 5.95; low-quality evidence on GRADE); and cryosurgery (RR, 4.76; very-low-quality evidence on GRADE).
ALA-PDT had the highest RR in the network meta-analyses for lesion-specific clearance (RR, 5.08; moderate-quality evidence on GRADE).
“Although ALA-PDT showed the most favorable RR and was ranked best among all interventions, the relative efficacy values and treatment rankings must be interpreted with caution,” because of the low certainty of evidence and few direct, head-to-head comparisons, the authors emphasized. “In particular, it remains elusive how to translate the distinct RR values into clinical relevance. We are hesitant to derive hierarchical or algorithmic treatment recommendations from our results.”
“The current meta-analysis notes that there are conflicting results in different studies,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn. who was asked to comment on the study. “Sustained participant complete clearance of actinic keratoses at 12 months is used as an outcome measure, although the authors comment that prevention/reduction of squamous cell carcinoma might be the more valid outcome measure.”
In her clinical experience, Dr. Ko said that patients often have good, sustained clearance of AKs with field treatment using a topical medication like 5-fluorouracil. “Patients can also have a good result with photodynamic therapy,” she said. “The paper’s results therefore do reflect what I have seen in my own practice. I also agree with the authors that, while it is difficult to measure, a meaningful outcome for patients is reduction/prevention of squamous cell carcinoma. It would be useful to have data on which treatment of actinic keratosis is best to reduce/prevent squamous cell carcinoma.”
The authors acknowledged limitations of the study, including the fact that field-directed treatments such as imiquimod, PDT, and fluorouracil were compared with lesion-directed approaches such as cryosurgery, “which may limit the generalizability of our results.” They concluded that their analysis “provides data that might contribute to an evidence-based framework to guide the selection of interventions for AK with proven long-term efficacy and sustained AK clearance.”
The analysis did not include data on tirbanibulin, a first-in-class dual Src kinase and tubulin polymerization inhibitor that was approved by the FDA for the topical treatment of AKs on the face or scalp in December 2020.
Dr. Heppt disclosed that he has been a member of the advisory boards of Almirall Hermal and Sanofi-Aventis and has received speaker’s honoraria from Galderma and Biofrontera. Many of his coauthors also reported having relevant financial disclosures. Dr. Ko reported having no relevant disclosures.
The four results from a systemic review and meta-analysis suggest.
To date, many studies have reported that “most interventions are superior to placebo in terms of lesion clearance and improving the cosmetic image,” corresponding author Markus V. Heppt, MD, MSc, and colleagues wrote in a study published online Aug. 4, 2021, in JAMA Dermatology.
“However, most randomized clinical trials (RCTs) and meta-analyses focused on short-term outcomes that are evaluated within 3-6 months after treatment, although AK is increasingly being considered a chronic condition and reducing the incidence of cSCC [cutaneous squamous cell carcinoma] should be the ultimate goal of treatment,” they said. In addition, most treatments have been compared with placebo “and head-to-head comparisons are widely lacking, limiting the possibility to cross compare distinct active treatments. To this end, no evidence-based recommendation regarding the long-term efficacy of interventions for AK exists.”
To determine the long-term clearance rates of treatments used in adults with AK, a precursor of cSCC, Dr. Heppt, of the department of dermatology at University Hospital Erlangen (Germany), and colleagues drew from 15 randomized clinical trials that reported sustained clearance rates after at least 12 months of treatment and were published up to April 6, 2020. They conducted the review by following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline and its extension for network meta-analyses (PRIMSA-NMA) and using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) process to determine the certainty of the evidence for network meta-analyses.
The study population included 4,252 patients. Among 10 studies included in a network meta-analysis for the outcome of participant complete clearance, ALA-PDT showed the most favorable risk ratio profile, compared with placebo (RR, 8.06; moderate-quality evidence on GRADE), followed by imiquimod, 5% (RR, 5.98; very-low-quality evidence on GRADE); MAL-PDT (RR, 5.95; low-quality evidence on GRADE); and cryosurgery (RR, 4.76; very-low-quality evidence on GRADE).
ALA-PDT had the highest RR in the network meta-analyses for lesion-specific clearance (RR, 5.08; moderate-quality evidence on GRADE).
“Although ALA-PDT showed the most favorable RR and was ranked best among all interventions, the relative efficacy values and treatment rankings must be interpreted with caution,” because of the low certainty of evidence and few direct, head-to-head comparisons, the authors emphasized. “In particular, it remains elusive how to translate the distinct RR values into clinical relevance. We are hesitant to derive hierarchical or algorithmic treatment recommendations from our results.”
“The current meta-analysis notes that there are conflicting results in different studies,” said Christine Ko, MD, professor of dermatology and pathology at Yale University, New Haven, Conn. who was asked to comment on the study. “Sustained participant complete clearance of actinic keratoses at 12 months is used as an outcome measure, although the authors comment that prevention/reduction of squamous cell carcinoma might be the more valid outcome measure.”
In her clinical experience, Dr. Ko said that patients often have good, sustained clearance of AKs with field treatment using a topical medication like 5-fluorouracil. “Patients can also have a good result with photodynamic therapy,” she said. “The paper’s results therefore do reflect what I have seen in my own practice. I also agree with the authors that, while it is difficult to measure, a meaningful outcome for patients is reduction/prevention of squamous cell carcinoma. It would be useful to have data on which treatment of actinic keratosis is best to reduce/prevent squamous cell carcinoma.”
The authors acknowledged limitations of the study, including the fact that field-directed treatments such as imiquimod, PDT, and fluorouracil were compared with lesion-directed approaches such as cryosurgery, “which may limit the generalizability of our results.” They concluded that their analysis “provides data that might contribute to an evidence-based framework to guide the selection of interventions for AK with proven long-term efficacy and sustained AK clearance.”
The analysis did not include data on tirbanibulin, a first-in-class dual Src kinase and tubulin polymerization inhibitor that was approved by the FDA for the topical treatment of AKs on the face or scalp in December 2020.
Dr. Heppt disclosed that he has been a member of the advisory boards of Almirall Hermal and Sanofi-Aventis and has received speaker’s honoraria from Galderma and Biofrontera. Many of his coauthors also reported having relevant financial disclosures. Dr. Ko reported having no relevant disclosures.
FROM JAMA DERMATOLOGY
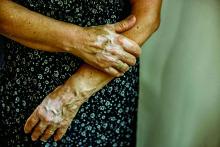
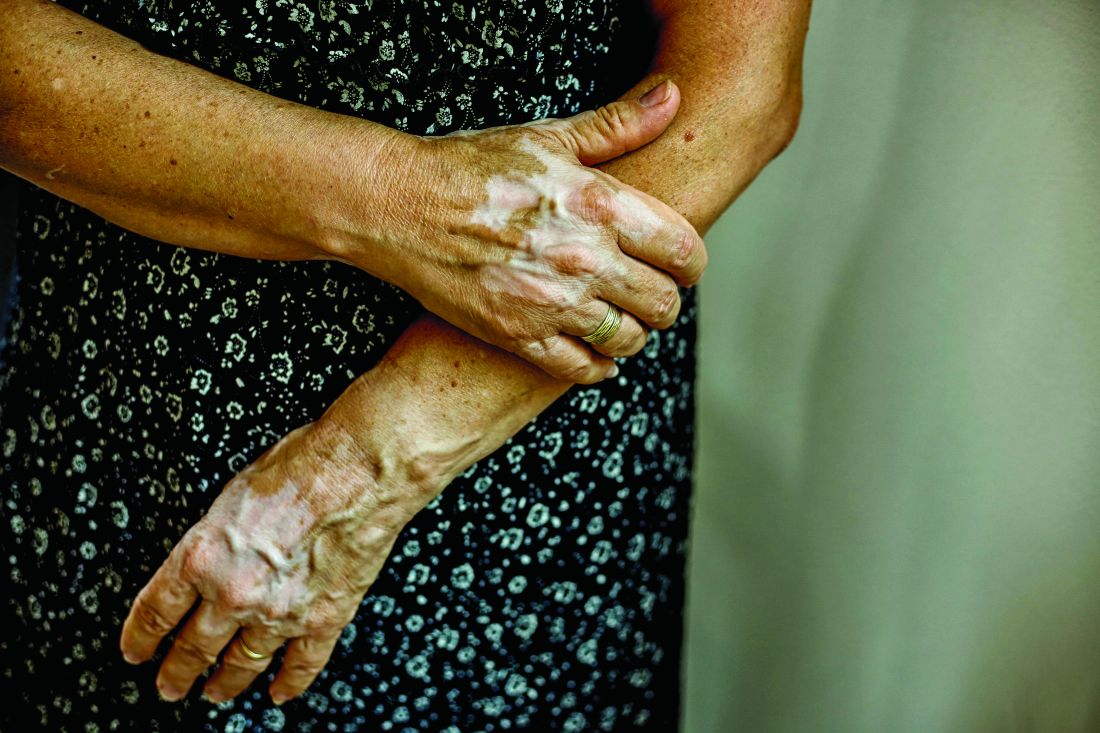
Insurance coverage for vitiligo varies widely in the U.S., analysis finds
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
, which may disproportionately affect patients of color.
Those are the conclusions from an analysis of vitiligo treatment coverage policies across major health insurers in the United States.
“Vitiligo can be less noticeable in patients with lighter skin types, becoming apparent only when affected patches fail to tan,” first authors Andrew Blundell, MD, MSc, and Moniyka Sachar, MD, wrote in a study published online on July 16 in Pediatric Dermatology. However, they pointed out that, in patients with darker skin types, “vitiligo can be far more evident due to the stark contrast of involved versus uninvolved skin, and as such can lead to a significant impact on quality of life, as well as heightened stigmatization.”
Nevertheless, they noted many health care insurers consider vitiligo as a cosmetic condition, and do not cover treatments, and for the 1%-2% of the general population with vitiligo, “this lack of recognition from health care insurers makes treatments both less accessible and affordable, and only further marginalizes patients with this condition.”
Dr. Blundell, of San Juan Bautista School of Medicine, Caguas, P.R., and Dr. Sachar, of the department of dermatology at Brown University, Providence, R.I., and colleagues surveyed 15 commercial health care insurers, 50 BlueCross BlueShield plans, Medicare, Medicaid, and Veterans Affairs to determine the level of treatment coverage for vitiligo. They looked at office visits, medications (the topical calcineurin inhibitors [TCIs] pimecrolimus, and tacrolimus), excimer laser therapy, and phototherapy (psoralen with UVA [PUVA] and narrow-band UVB [nbUVB]). They collected information from medical policies available online or by direct contact with the plans in 2018.
The researchers reported data from 17 organizations with regional or national coverage policies for vitiligo treatment and two others – BlueCross BlueShield and Medicaid – which had policies that differed by state and plan. Of the 17 organizations, only 12% did not cover TCIs, 56% did not cover nbUVB phototherapy, 53% did not cover PUVA phototherapy, and 41% did not cover laser therapy.
As for BlueCross BlueShield, the health plan did not cover pimecrolimus and tacrolimus in 39% and 35% of states, respectively. At the same time, NbUVB and PUVA therapy were not covered in 20% and 10% of states, respectively, while excimer laser therapy was not covered in 82% of states.
Of accessible Medicaid information from 32 states, 11 did not cover topicals, 5 did not cover nbUVB, 4 did not cover PUVA, and 7 did not cover laser therapy. “The two most commonly cited reasons for denial of coverage were (a) vitiligo is considered a cosmetic condition and (b) certain therapies are not FDA-approved for vitiligo, though they may be approved for other skin conditions,” the study authors wrote.
While the analysis revealed that topical TCI therapy is more widely covered by insurance companies, compared with phototherapy, “multiple studies have shown that a combination of both topical and phototherapy is more effective in treating vitiligo than either alone,” they noted. “Vitiligo treatments can delay the progression of the disease and result in better outcomes when started early, furthering the need for insurance coverage of these treatments. If all proven and accepted vitiligo treatments were covered by their health insurers, patients would have better access, as well as timely and affordable ways by which to limit depigmentation and to repigment affected areas.”
In addition, lack of access to treatments “may increase health disparities among already-marginalized groups, such as children and adults of darker skin phototypes,” they wrote.
Seemal R. Desai, MD, who was asked to comment on the study, said that the findings resonate with him based on his clinical experience as a dermatologist at the University of Texas Southwestern Medical Center in Dallas and in clinical practice. “Vitiligo has a high psychological impact, continues to increase in its prevalence, and has been shown to be an autoimmune, chronic, inflammatory skin disease, yet we’re still having challenges with treatment,” said Dr. Desai, who is also a member of the board of directors for the American Academy of Dermatology and the Global Vitiligo Foundation (GVF).
He said that he is working with the AAD, the GVF, and other stakeholders to improve treatment coverage. For example, in Massachusetts, the Tufts Health Plan had stopped covering treatment for vitiligo. “Through a series of advocacy efforts, that was reversed a couple of years ago,” said Dr. Desai, who is also a past president of the Skin of Color Society. “We also have seen isolated reports of Medicaid and Medicare coverage where local contractors aren’t following national Centers for Medicare and Medicaid Service directive guidance. The challenge becomes, how do you get consistency in treatment coverage, and how do you make sure patients continue to get access to treatment?”
Turning the tide will require “a concerted effort” by dermatologists to engage with the payers, he added. “I’ve had to get on the phone with countless insurance companies on behalf of my patients and make them understand the comorbidities associated with vitiligo, sending them copies of studies that show it’s an autoimmune disease linked to thyroid issues,” Dr. Desai continued. “We talk a lot about the psychological burden and quality of life. There’s still a lot of work to be done in this sphere, but I think we’re making progress.”
With hopes that Janus kinase (JAK) inhibitors and other new products being investigated will soon be approved as a treatment option for vitiligo, Dr. Desai said that now is the time to standardize coverage for patients. “It’s important that we start talking about insurance coverage and denial issues now and get ahead of it, so that when we get those JAK inhibitors available, we don’t fight coverage decisions then.”
The researchers acknowledged certain limitations of the study, including the fact that it was based on insurance coverage from 2017 to 2018 and the lack of easily available state Medicaid policies.
The study coauthors were Colleen K. Gabel, MD, of the University of Massachusetts, Worcester, and Lionel G. Bercovitch, MD, of Brown University. None of the study authors reported financial disclosures.
Dr. Desai disclosed that he has conducted vitiligo research trials and has done consulting work for several pharmaceutical companies.
FROM PEDIATRIC DERMATOLOGY
Study highlights impact of acne in adult women on quality of life, mental health
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
FROM JAMA DERMATOLOGY
When is MRI useful in the management of congenital melanocytic nevi?
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
When used for appropriate patients, results from a small multi-institutional study showed.
“The majority of congenital nevi are considered low risk for cutaneous and/or systemic complications,” Holly Neale said at the annual meeting of the Society for Pediatric Dermatology. “However, a subset of children born with higher-risk congenital nevi require close monitoring, as some features of congenital nevi have been associated with cutaneous melanoma, central nervous system melanoma, melanin in the brain or spine, and structural irregularities in the brain or spine. It’s important to understand which congenital nevi are considered higher risk in order to guide management and counseling decisions.”
One major management decision is to do a screening magnetic resonance image of the CNS to evaluate for neurologic involvement, said Ms. Neale, a fourth-year medical student at the University of Massachusetts, Worcester. Prior studies have shown that congenital nevi that are bigger than 20 cm, posterior axial location, and having more than one congenital nevus may predict CNS abnormalities, while recent guidelines from experts in the field suggest that any child with more than one congenital nevus at birth undergo screening MRI.
“However, guidelines are evolving, and more data is required to better understand the CNS abnormalities and patient outcomes for children with congenital nevi,” said Ms. Neale, who spent the past year as a pediatric dermatology research fellow at Massachusetts General Hospital, Boston.
To address this knowledge gap, she and colleagues at the University of Massachusetts, Massachusetts General Hospital, and Boston Children’s Hospital performed a retrospective chart review between Jan. 1, 2009, and Dec. 31, 2019, of individuals ages 18 and younger who had an MRI of the brain or spine with at least one dermatologist-diagnosed nevus as identified via key words in the medical record. Of the 909 patients screened, 46 met inclusion criteria, evenly split between males and females.
The most common location of the largest nevus was the trunk (in 41% of patients), followed by lesions that spanned multiple regions. More than one-third of patients had giant nevi (greater than 40 cm).
“The majority of images were considered nonconcerning, which includes normal, benign, or other findings such as trauma related, infectious, or orthopedic, which we did not classify as abnormal as it did not guide our study question,” Ms. Neale said. Specifically, 8% of spine images and 27% of brain images were considered “concerning,” defined as any finding that prompted further workup or monitoring, which includes findings concerning for melanin.
The most common brain finding was melanin (in eight children), and one child with brain melanin also had findings suggestive of melanin in the thoracic spine. The most common finding in spine MRIs was fatty filum (in four children), requiring intervention for tethering in only one individual. No cases of cutaneous melanoma developed during the study period, and only one patient with abnormal imaging had CNS melanoma, which was fatal.
All patients with findings suggestive of CNS melanin had more than four nevi present at birth, which is in line with current imaging screening guidelines. In addition, children with concerning imaging had higher rates of death, neurodevelopmental problems, seizures, and neurosurgery, compared with their counterparts with unremarkable imaging findings. Describing preliminary analyses, Ms. Neale said that a chi square analysis was performed to test statistical significance of these differences, “and neurosurgery was the only variable that children with concerning imaging were significantly more likely to experience, although sample size limits detection for the other variables.”
The authors concluded that MRI is a helpful tool when used in the appropriate clinical context for the management of congenital nevi. “As more children undergo imaging, we may discover more nonmelanin abnormalities,” she said.
Joseph M. Lam, MD, who was asked to comment on the study, said that the increased risk of CNS melanin in patients with larger lesions and in those with multiple lesions confirms previous reports.
“It is interesting to note that some patients with nonconcerning imaging results still had neurodevelopmental problems and seizures, albeit at a lower rate than those with concerning imaging results,” said Dr. Lam, a pediatric dermatologist at British Columbia Children’s Hospital, Vancouver. “The lack of a control group for comparison of rates of neurological sequelae, such as NDP, seizures and nonmelanin structural anomalies, limits the generalizability of the findings. However, this is a nice study that helps us understand better the CNS anomalies in CMN.”
Ms. Neale acknowledged certain limitations of the study, including the lack of a control group without CMN, the small number of patients, the potential for referral bias, and its retrospective design. Also, the proximity of the study period does not allow for chronic follow-up and detection of the development of melanoma or other problems in the future.
Ms. Neale and associates reported having no relevant financial disclosures. Dr. Lam disclosed that he has received speaker fees from Pierre Fabre.
FROM SPD 2021
Common outcome measures for AD lack adequate reporting of race, skin tone
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2021
Genetic testing for neurofibromatosis 1: An imperfect science
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
FROM SPD 2021
The first signs of elusive dysautonomia may appear on the skin
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
During the annual meeting of the Society for Pediatric Dermatology, Adelaide A. Hebert, MD, defined dysautonomia as an umbrella term describing conditions that result in a malfunction of the autonomic nervous system. “This encompasses both the sympathetic and the parasympathetic components of the nervous system,” said Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. “Clinical findings may be neurometabolic, developmental, and/or degenerative,” representing a “whole constellation of issues” that physicians may encounter in practice, she noted. Of particular interest is postural orthostatic tachycardia syndrome (POTS), which affects between 1 million and 3 million people in the United States. Typical symptoms include lightheadedness, fainting, and a rapid increase in heartbeat after standing up from a seated position. Other conditions associated with dysautonomia include neurocardiogenic syncope and multiple system atrophy.
Dysautonomia can impact the brain, heart, mouth, blood vessels, eyes, immune cells, and bladder, as well as the skin. Patient presentations vary with symptoms that can range from mild to debilitating. The average time from symptom onset to diagnosis of dysautonomia is 7 years. “It is very difficult to put together these mysterious symptoms that patients have unless one really thinks about dysautonomia as a possible diagnosis,” Dr. Hebert said.
One of the common symptoms that she has seen in her clinical practice is joint hypermobility. “There is a known association between dysautonomia and hypermobile-type Ehlers-Danlos syndrome (EDS), and these patients often have hyperhidrosis,” she said. “So, keep in mind that you could see hypermobility, especially in those with EDS, with associated hyperhidrosis and dysautonomia.” Two key references that she recommends to clinicians when evaluating patients with possible dysautonomia are a study on postural tachycardia in hypermobile EDS, and an article on cardiovascular autonomic dysfunction in hypermobile EDS.
The Beighton Scoring System, which measures joint mobility on a 9-point scale, involves assessment of the joint mobility of the knuckle of both pinky fingers, the base of both thumbs, the elbows, knees, and spine. An instructional video on how to perform a joint hypermobility assessment is available on the Ehler-Danlos Society website.
Literature review
In March 2021, Dr. Hebert and colleagues from other medical specialties published a summary of the literature on cutaneous manifestations in dysautonomia, with an emphasis on syndromes of orthostatic intolerance. “We had neurology, cardiology, along with dermatology involved in contributing the findings they had seen in the UTHealth McGovern Dysautonomia Center of Excellence as there was a dearth of literature that taught us about the cutaneous manifestations of orthostatic intolerance syndromes,” Dr. Hebert said.
One study included in the review showed that 23 out of 26 patients with POTS had at least one of the following cutaneous manifestations: flushing, Raynaud’s phenomenon, evanescent hyperemia, livedo reticularis, erythromelalgia, and hypo- or hyperhidrosis. “If you see a patient with any of these findings, you want to think about the possibility of dysautonomia,” she said, adding that urticaria can also be a finding.
To screen for dysautonomia, she advised, “ask patients if they have difficulty sitting or standing upright, if they have indigestion or other gastric symptoms, abnormal blood vessel functioning such as low or high blood pressure, increased or decreased sweating, changes in urinary frequency or urinary incontinence, or challenges with vision.”
If the patient answers yes to two or more of these questions, she said, consider a referral to neurology and/or cardiology or a center of excellence for further evaluation with tilt-table testing and other screening tools. She also recommended a review published in 2015 that describes the dermatological manifestations of postural tachycardia syndrome and includes illustrated cases.
One of Dr. Hebert’s future dermatology residents assembled a composite of data from the Dysautonomia Center of Excellence, and in the study, found that, compared with males, females with dysautonomia suffer more from excessive sweating, paleness of the face, pale extremities, swelling, cyanosis, cold intolerance, flushing, and hot flashes.
Dr. Hebert disclosed that she has been a consultant to and an adviser for several pharmaceutical companies.
FROM SPD 2021