User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
Rare hematologic malignancy may first present to a dermatologist
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
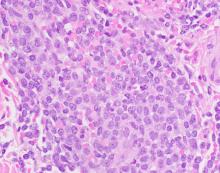
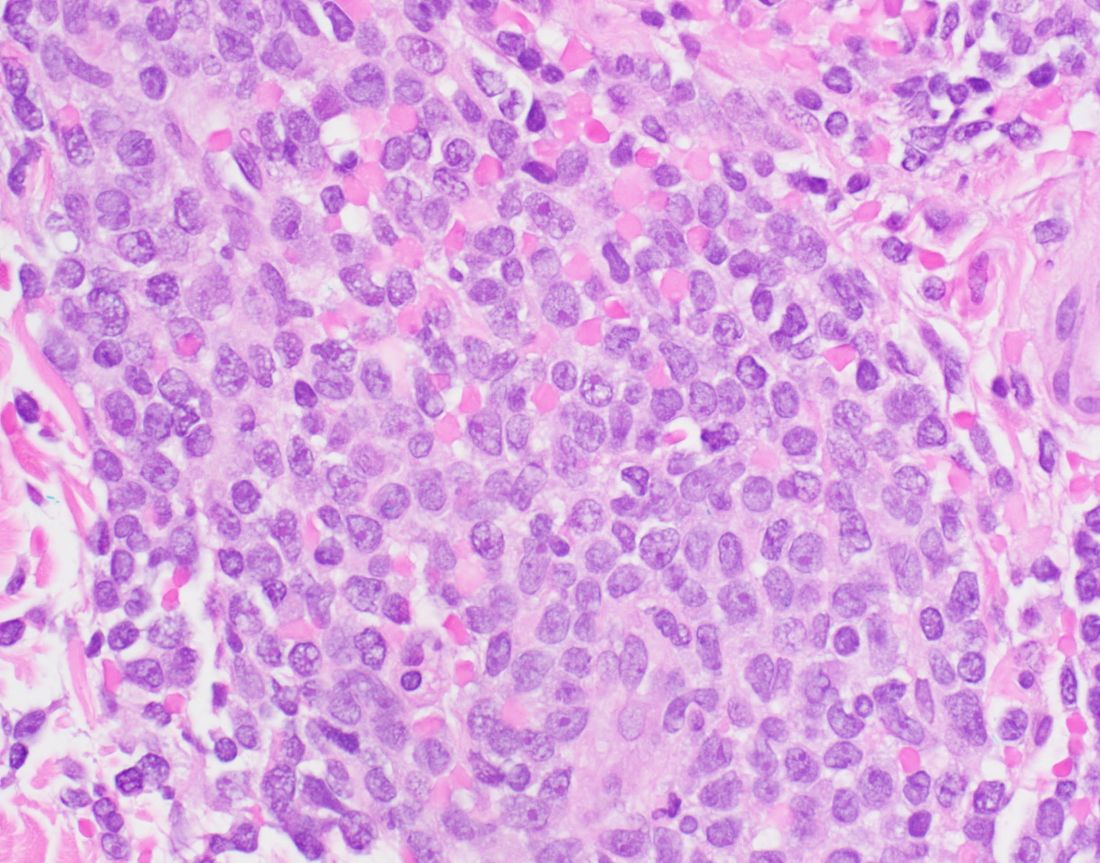
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
in about 80% of cases.
“You won’t see blastic plasmacytoid dendritic cell neoplasm listed on our primary cutaneous lymphoma classifications because it’s not technically a primary cutaneous disease,” Brittney K. DeClerck, MD, said during the annual meeting of the Pacific Dermatologic Association. “It’s a systemic disease that has secondary cutaneous manifestations. That’s a very important distinction to make, in terms of not missing the underlying disease associated with what might be commonly first seen on the skin.”
BPDCN is a malignancy of plasmacytoid dendritic cells, which capture, process, and present antigen, and allow the remainder of the immune system to be activated. “They are mainly derived from the myeloid cell lineage, and possibly from the lymphoid line in a subset of cases,” said Dr. DeClerck, associate professor of clinical pathology and dermatology at the University of Southern California, Los Angeles. “They secrete high levels of type I interferons, which is important for antiviral immunity, but they can also be implicated in severe systemic inflammatory diseases, such as systemic lupus erythematosus and systemic sclerosis.”
BPDCN involves the skin in about 80% of cases, she added, “but invariably at some point it involves the bone marrow and has an acute leukemic presentation, whether or not it happens concurrently with what we see on the skin as dermatologists. We also see variable involvement of the peripheral blood, lymph nodes, and the central nervous system.”
The classification of BPDCN has changed over time based on evolving immunohistochemical markers and technologies. For example, in 1995 it was called agranular CD4+ NK cell leukemia, in 2001 it was called blastic NK-cell lymphoma, in 2005 it was called CD4+/CD56+ hematodermic neoplasm, and in 2008 it was called BPDCN (AML subset). In 2016 it became classified as its own entity: BPDCN.
Because of changing nomenclature, the true incidence of the disease is unknown, but according to the best available literature, 75% of cases occur in men and the median age is between 60 and 70 years, “but all ages can be affected,” Dr. DeClerck said. “Cases seem to come in clusters. Our most recent cluster has been in our pediatric population. At Children’s Hospital Los Angeles, we’ve had three cases in the last couple of years. To me, that was a bit unusual.”
She added that 10%-20% of patients will have either a history of, or will develop another, hematologic malignancy, such as myelodysplastic syndrome (MDS), chronic myelogenous leukemia (CML), or acute myelogenous leukemia (AML).
The general prognosis of BPDCN is poor, and the mean time from onset of lesions to an actual diagnosis is about 6.2 months, which underscores the importance of early diagnosis, Dr. DeClerck said. “There can be some nondescript solitary lesions that patients can present with, so don’t hesitate to biopsy.” The median overall survival is less than 20 months, but patients under 60 years of age have a slightly better prognosis.
Clinical presentation
Clinically, the malignancy presents with variable involvement of the skin, bone marrow, lymph nodes, peripheral blood, and central nervous system. “Patients may have one or all of these,” she said. Because 80% of patients have skin lesions, “dermatologists should be aware of this entity in order to communicate with our pathologists to understand that maybe one biopsy isn’t enough. Several biopsies may be required.”
The most common dermatologic presentation of BPDCN is erythematous to deeply violaceous nodules. Other patients may present with infiltrated ecchymotic plaques or petechial to hyperpigmented macules, patches, and plaques. Biopsy reveals a diffusely infiltrated dermis of markedly atypical large cells, but occasionally can be more subtle. “Early lesions may only be perivascular in nature, so going on high power on anything that looks atypical on low power is important in these cases,” Dr. DeClerck said.
The recommended histochemical stains for suspected BPDCN include CD123, CD4, and CD56. “We need to have other stains to rule out other things, such as negative stains that are going to exclude other T cell and B cell processes, and Merkel cell carcinoma, which can express CD56. We also want to have another confirmatory stain because other things can express CD123, CD4, and CD56. Commonly we use TCL1 or TCF4.”
The differential diagnosis of cutaneous findings includes leukemia cutis, mycosis fungoides, NK/T-cell lymphoma, and cutaneous gamma-delta T-cell lymphoma, while the differential diagnosis of biopsy findings includes AML, acute lymphoblastic leukemia, and NK/T-cell lymphoma.
Treatment of BPDCN
Historically, BPDCN was treated with multiagent high-dose chemotherapy. “Patients would frequently respond early but would relapse quickly, progress, and have a poor outcome,” Dr. DeClerck said. Now, first-line therapy is tagraxofusp-erzs (Elzonris) or multiagent chemotherapy based on where the patient is in the course of disease. Tagraxofusp-erzs is an IL-3 conjugated diphtheria toxic fusion protein which binds to CD123, which was approved by the Food and Drug Administration in 2018 for treating BPDCN. After that initial therapy, it is determined whether the patient has a complete response or failed response, she said. “If they have a complete response, they frequently go on to bone marrow transplantation, which is the only curative therapy at this point for these patients.”
According to Dr. DeClerck, an anti-BCL-2 therapy, venetoclax, can be used for patients with BPDCN as well. National Comprehensive Cancer Network (NCCN) guidelines for the treatment of BPDCN can be found on the NCCN website.
Dr. DeClerck emphasized the importance of reviewing biopsy results with a hematopathologist, “because there are complex leukemias that are beyond what dermatopathologists have been trained in.” Once a patient is diagnosed with BPDCN, she recommends rapid referral to a large center for treatment and possible bone marrow transplantation.
Dr. DeClerck disclosed that she is an adviser for tagraxofusp-erzs manufacturer Stemline Therapeutics.
FROM PDA 2021
FDA approves topical ruxolitinib for atopic dermatitis, first JAK inhibitor for this indication in the U.S.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
The , making it the first topical JAK inhibitor approved for AD – and the first JAK inhibitor approved for this indication – in the United States.
The approval is limited to patients whose AD is not adequately controlled with topical prescription therapies, or when those therapies are not advisable.
“Approval of topical ruxolitinib fills a major gap in the treatment of atopic dermatitis: a safe, effective, and tolerable non-steroidal topical therapy,” Eric L. Simpson, MD, professor of dermatology and director of the Oregon Health & Science University Dermatology Clinical Research Center, Portland, told this news organization. “This approval will allow for long-term treatment without the concern of steroid side effects. From earlier studies, ruxolitinib cream appears to be as effective as a medium-potency topical steroid. These efficacy levels and low incidence of burning will be a welcome addition to our current nonsteroidal therapies.”
The drug’s approval was based on results from two phase 3, randomized studies of identical design involving 1,249 patients aged 12 years and older with AD: TRuE-AD1 and TRuE-AD2. In these studies, ruxolitinib cream demonstrated anti-inflammatory activity, with rapid and sustained antipruritic action, compared with vehicle. In the trials, patients with an Investigator’s Global Assessment (IGA) score of 2 or 3 and 3%-20% of affected body surface area (BSA) were randomized (2:2:1) to twice-daily 0.75% ruxolitinib cream, 1.5% ruxolitinib cream, or vehicle cream for 8 continuous weeks. The 1.5% concentration was approved by the FDA.
A study first published in May of 2021 found that significantly more patients in TRuE-AD1 and TRuE-AD2 achieved IGA treatment success with 0.75% (50% vs. 39%, respectively) and 1.5% ruxolitinib cream (53.8% vs. 51.3%), compared with vehicle (15.1% vs. 7.6%; P < .0001) at week 8. In addition, significant reductions in itch, compared with vehicle, were reported within 12 hours of first applying 1.5% ruxolitinib cream (P < .05).
More key findings from TRuE-AD1 and TRuE-AD2 are scheduled to be presented during the upcoming European Academy of Dermatology and Venereology meeting Sept. 29-Oct. 2, but during the Revolutionizing Atopic Dermatitis Symposium on June 13, Kim Papp, MD, PhD, presented long-term safety data of ruxolitinib cream in patients who were followed for an additional 44 weeks. Dr. Papp, a dermatologist and founder of Probity Medical Research, Waterloo, Ont., reported that 543 patients from TRuE-AD1 and 530 from TRuE-AD2 entered the long-term analysis and that about 78% of these patients completed the study. From weeks 12 to 52, the proportion of patients with an IGA score of 0 or 1 with 0.75% and 1.5% ruxolitinib cream ranged from 62%-77% and 67%-77%, respectively, in TRuE-AD1, to 60%-77% and 72%-80% in TRuE-AD2.
The measured mean total affected BSA was less than 3% throughout the follow-up period in the 1.5% ruxolitinib cream arm in TRuE-AD1 and TRuE-AD2 and was less than 3% in the 0.75% ruxolitinib cream arm during most of the study period.
In a pooled safety analysis, treatment-emergent adverse events (TEAEs) were reported in 60% and 54% of patients who applied 0.75% and 1.5% ruxolitinib cream, respectively, over 44 weeks. The frequency of application-site reactions remained low. Specifically, treatment-related adverse events were reported in 5% of patients who applied 0.75% ruxolitinib cream and in 3% of patients who applied 1.5% ruxolitinib cream; none were serious. TEAEs led to discontinuation in 2% of patients in the 0.75% ruxolitinib cream group, and no patients in the 1.5% ruxolitinib cream group.
Dr. Papp and his colleagues observed that the most common treatment adverse events were upper respiratory tract infections and nasopharyngitis. According to Incyte’s press release, the most common treatment-emergent adverse reactions in patients treated with ruxolitinib during clinical trials were nasopharyngitis, diarrhea, bronchitis, ear infection, eosinophil count increases, urticaria, folliculitis, tonsillitis, and rhinorrhea. The labeling includes boxed warnings for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis, seen with oral JAK inhibitors for inflammatory conditions.
Incyte will market ruxolitinib under the trade name Opzelura.
Dr. Simpson disclosed that he is a consultant to and/or an investigator for several pharmaceutical companies, including Incyte, Regeneron/Sanofi, Eli Lilly and Company, AbbVie, and Pfizer.
Dr. Papp disclosed that he has received honoraria or clinical research grants as a consultant, speaker, scientific officer, advisory board member, and/or steering committee member for several pharmaceutical companies, including Incyte.
Commentary by Robert Sidbury, MD, MPH
Another nonsteroidal topical medication approved for atopic dermatitis (AD)? Thank goodness. Topical ruxolitinib 1.5% cream twice daily for mild to moderate AD demonstrated excellent efficacy vs. placebo in duplicative trials (53.8/51.3% vs. 15.1%/7.6%; P < .001), with a reassuring safety profile. Application site reactions were uncommon, though past experience with other new nonsteroidal agents suggests judgment be reserved on that score. More compelling was the fact that no patients discontinued therapy in the 1.5% arm, and adverse events were mild and self-limited such as nasopharyngitis and diarrhea. This stands in contradistinction to the boxed warning attached to JAK inhibitors (topical and systemic) against a daunting list of destructive possibilities: malignancy, infection, cardiovascular disease, and blood clots. None of these things was seen in these topical ruxolitinib trials.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
Patient risk-benefit thresholds for antibiotic use in dermatologic surgery vary widely
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
(SSI) and had negligible side effects, in a prospective multicenter study.
In addition, a similar proportion of patients preferred to take an antibiotic if there was no SSI reduction and a high risk of adverse events.
Those are two key findings from the study aimed at understanding patient preferences for prophylactic oral antibiotic use following dermatologic surgery, which was published in Dermatologic Surgery.
“Patient risk-benefit thresholds for using antibiotics vary considerably,” the study’s corresponding author, Jeremy R. Etzkorn, MD, MS, of the department of dermatology at the Hospital of the University of Pennsylvania, Philadelphia, told this news organization. “Physicians should appreciate and consider the variation between patients before deciding to send in a prescription after skin surgery.”
To investigate patient preferences about taking antibiotics to prevent SSI relative to antibiotic efficacy and antibiotic-associated adverse drug reactions, Dr. Etzkorn and colleagues at six U.S. medical centers prospectively administered a web-based survey and discrete choice experiment to 388 adults including dermatologic surgery patients and their family members, as well as health care workers (defined as dermatologic surgery patients who work in health care, individuals who work in health care and are accompanying patients to their surgery, or staff in the dermatology clinic.) “A lot has been published about physician preferences and practice patterns with respect to antibiotic prescribing after dermatologic surgery,” Dr. Etzkorn noted. “This is the first study to evaluate patient preferences in a rigorous way.”
He and his coinvestigators used a technique from marketing and product research (conjoint analysis/discrete choice experiments) to quantify what patients think about using antibiotics and what trade-offs they are – or are not – willing to make to reduce their risk of infection.
Nearly half of the respondents (47%) were patients, 29% were family members of patients, 19% were health care workers, and the rest were described as patient caregivers or “other.” More than half (59%) were female, the mean age at surgery was 59 years, and 69% had college or postgraduate degrees.
More than half of respondents (55%) would choose to take an antibiotic if it reduced the SSI rate from 5% to 2.5% and if the risk of adverse drug reactions was low (defined as a 1% risk gastrointestinal upset, 0.5% risk itchy skin rash, and 0.01% risk ED visit). Even if an antibiotic could eliminate SSI risk entirely and had a low adverse drug reaction profile, 27% of respondents preferred not to take prophylactic oral antibiotics.
A subgroup analysis revealed that only 21% of health care workers would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 41% of those who do not work in health care. Respondent age also drove treatment choice. For example, only 33% of respondents younger than age 65 would choose a moderate efficacy antibiotic (2.5% SSI risk) with a high adverse effect profile, compared with 45% of those aged 65 years and older.
“We knew patients would likely trade some antibiotic efficacy for some side effects, just as one would trade price for features when shopping for a car,” Dr. Etzkorn said. “We were shocked to see that over a quarter – 27% – of respondents preferred to not take antibiotics even if they were able to prevent all infections and had minimal side effects.”
“It’s interesting that between 27% [and] 55% of patients preferred no operative antibiotic prophylaxis despite a theoretical 100% cure rate for surgical-site infections,” said Lawrence J. Green, MD, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study results.
“I think this mirrors dermatologist’s preferences, as a majority also prefer not to prescribe postoperative antibiotic therapy, unless operating in an area of or a patient with a high risk for infection. It would also be interesting to see if a less educated population would also have similar preferences.”
Dr. Etzkorn acknowledged certain limitations of the study, including that while it evaluated patient reported preferences, it did not include all possible risks and benefits, and “it does not measure actual patient behaviors.”
The researchers reported having no relevant financial disclosures. Dr. Etzkorn disclosed that he serves as a data safety monitoring board member for a clinical trial of Replimmune. Dr. Green disclosed that he is a speaker, consultant, or investigator for numerous pharmaceutical companies.
FROM DERMATOLOGIC SURGERY
Skin ulcers can pose tricky diagnostic challenges
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
In the clinical opinion of Alex G. Ortega-Loayza, MD, MCR, few absolutes drive the initial assessment of patients who present with skin ulcers.
The causes can be neoplastic, infectious, inflammatory, vasculopathic, external, and genetic. “Sometimes they can be of mixed etiology, which make them even more complicated to heal,” Dr. Ortega-Loayza, of the department of dermatology at Oregon Health & Science University, Portland, said during the annual meeting of the Pacific Dermatologic Association.
In a study published in 2019, he and his colleagues at four academic hospitals evaluated characteristics and diagnoses of ulcers in 274 patients with skin ulcers in inpatient dermatology consultation services between July 2015 and July 2018. Most primary teams requesting the consultation (93%) were from nonsurgical specialties. The median age of these patients was 54 years, 45% were male, and 50% had lower-extremity ulcers. Nearly two-thirds of the ulcers (62%) were chronic in nature, while the remaining 38% were acute. The skin ulcer was the chief reason for admission in 49% of cases and 66% were admitted through the ED. In addition, 11% had a superinfected skin ulcer.
The top three etiologies rendered by dermatologists after assessing these patients were pyoderma gangrenosum (17%), infection (13%), and exogenous causes (12%); another 12% remained diagnostically inconclusive after consultation. Diagnostic agreements between the primary team requesting the consultation and the dermatologist were poor to modest.
These data highlights the role of the dermatologists in the workup of skin ulcers of unknown etiology.
“The diagnosis of skin ulcers can be challenging,” Dr. Ortega-Loayza said. “Subjective factors playing a role in the diagnosis of skin ulcers include the type of level of training/experience you’ve had and general awareness and education about skin ulcers.” In addition, there is also a lack of gold-standard diagnostic criteria for atypical/inflammatory ulcers and a lack of specificity of ancillary testing, such as for pyoderma gangrenosum.
Dr. Ortega-Loayza’s basic workup is based on the review of systems and the patient’s comorbidities. Blood work may include CBC, comprehensive metabolic panel, erythrocyte sedimentation rate/C-reactive protein, glucose-6-phosphate dehydrogenase, albumin/prealbumin, autoimmune panels, and hypercoagulable panels. He may order a skin biopsy with H&E staining and microbiological studies, superficial bacterial wound cultures, and vascular studies, such as ankle brachial index (ABI) and chronic venous reflux tests, and Doppler ultrasound, and he might consider an angiogram for certain type of ulcers. Additional imaging studies may include x-ray, CT scan, and/or MRI.
The four key factors to control in patients with skin ulcers, he continued, include effective management of edema (such as compression garments depending on the results of the vascular studies); infection (with topical/oral antibiotics and debridement); the wound microenvironment (with wound dressings), and pain (mainly with nonopioids). “In my practice, we tend to do multilayered compression,” he said. “This can be two- or four-layer. I do light compression if the patient has peripheral arterial disease. I always bring in the patient 2 days later to check on them, or do a telehealth visit, to make sure they are not developing any worsening of the ulcers.”
Infections can be managed with topical antimicrobials such as metronidazole 1% gel and cadexomer iodine. “Iodine can also help dry the wound when you need to do so,” said Dr. Ortega-Loayza, who directs a pyoderma gangrenosum clinic at OHSU. “Debridement can be done with a curette or with commercially available enzymatic products such as Collagenase, PluroGel, and MediHoney.”
When the ulcer is in an active phase (characterized by significant amount of drainage and erythema), he uses one or more of the following products to control the wound microenvironment: zinc oxide, an antimicrobial dressing, a hyperabsorbent dressing, an abdominal pad, and compression.
During the healing phase, with evidence of re-epithelization, he tends to use more foam dressings and continues with compression. His preferred options for managing pain associated with ulcers are medications to control neuropathic pain including initially gabapentin (100 mg-300 mg at bedtime), pregabalin (75 mg twice a day), or duloxetine (extended release, 30 mg once a day). All of these medications can be titrated up based on patients’ needs. Foam dressings with ibuprofen can also provide comfort, he said.
Dr. Ortega-Loayza also provided a few clinical pearls highlighting the role and utility of interleukin-23 inhibitors in the management of patients with pyoderma gangrenosum, oral vitamin K in patients with calciphylaxis, and stanozolol for lipodermatosclerosis. He is also leading the first open-label trial testing a Janus kinase inhibitor – baricitinib – as a treatment for patients with pyoderma gangrenosum.
Dr. Ortega-Loayza disclosed that he is a consultant to Genentech and Guidepoint and is a member of the advisory board for Bristol-Myers Squibb, Boehringer Ingelheim, and Janssen. He also has received research support from Lilly.
FROM PDA 2021
Clinical genetic testing for skin disorders continues to advance
and families of pediatric patients to navigate the landscape.
“Testing options range from targeted variant testing and single-gene testing to exome and genome sequencing,” Gabriele Richard, MD, said at the annual meeting of the Society for Pediatric Dermatology. “It is not always easy to determine which testing is right.”
Increasingly, clinical genomic tests, including exome and genome sequencing, are used for patients with complex phenotypes, and possibly multiple disorders, who might have no diagnosis despite extensive prior testing, said Dr. Richard, medical director at Gaithersburg, Md.–based GeneDx., a molecular diagnostic laboratory that performs comprehensive testing for rare genetic disorders. These tests are also being used more for first-line testing in critically ill patients in the neonatal and pediatric intensive care units, and “have heralded a whole new era of gene and disease discovery,” she added.
Targeted variant testing is used for known familial variants, to test family members for carrier status and segregation, and to make a prenatal diagnosis, she said. Single-gene testing is available for most genes and has its place for conditions that can be clinically well-recognized, such as ichthyosis vulgaris, Darier disease, or Papillon-Lefèvre syndrome.
Specific tests for identifying gene deletions or duplications are exon-level microarrays, multiplex ligation-dependent probe amplification (MLPA), and chromosomal microarray analysis. “The latter has been successful in identifying diseases causing chromosomal abnormalities in over 10% of cases overall,” Dr. Richard said. An example of a skin disorder is X-linked ichthyosis caused by a deletion of the steroid sulfatase locus in more than 95% of affected males, she said.
“However, the current staple of molecular diagnostic testing is multigene next-generation sequencing (NGS) panels, which allow you to interrogate two to hundreds of genes concurrently, including sequencing and deletion duplication testing.” These tests are the most cost effective, she said, and are available for almost any genodermatosis or group of disorders with overlapping phenotypes, such as albinism or ichthyosis, epidermolysis bullosa and skin fragility, ectodermal dysplasia, or porphyria. According to Dr. Richard, the diagnostic outcomes of NGS panels mainly depend on test indication, panel size and gene curation, age of onset, and prevailing inheritance pattern of disorders.
Her recommended criteria for distinguishing the myriad of available NGS panels include checking gene content, technical sensitivity of sequencing and deletion/duplication analysis, quality of variant interpretation and reporting, turn-around time, and available familial follow-up testing. “If a family might consider future prenatal diagnosis, choose the lab that performs prenatal and diagnostic testing,” Dr. Richard said. “Equally important are client services such as ease of ordering, insurance coverage, and the ability to determine out-of-pocket cost to patients.”
Resources that enable consumers to compare panel content, methodology, turnaround time, and other parameters include the Genetic Testing Registry (GTR) operated by the National Center for Biotechnology Information, and Concert Genetics, a genetic testing company. The National Society of Genetic Counselors also offers a searchable database for finding a genetic counselor.
Exome sequencing includes the coding sequences of about 20,000 genes and has an average depth of 50 to about 150 reads. “It is a phenotype-driven test where only select variants are being reported fitting the phenotype,” Dr. Richard said. “The outcome of exome and genome sequencing much depends on optimization of bioinformatic pipelines and tools.” Besides small sequence variants, exome sequencing is able to identify a variety of different types of disease-causing variants, such as gene copy number variants seen in about 6% of positive cases, mosaicism, regions of homozygosity, uniparental disomy, and other unusual events and is cost effective.
Whole-genome sequencing, meanwhile, includes the entire genome, particularly noncoding regions, and has an average depth of more than 30 reads. “It’s based on single-molecule sequencing, has longer reads and more uniform coverage, compared to exome sequencing,” she said. “Higher cost, variant interpretation, and lack of coverage by payers are still presenting challenges for genome sequencing.” Genome sequencing can be done in a day or less.
According to diagnostic outcomes based on 280,000 individuals including 125,000 probands from GeneDx data, a definitive diagnosis was made in 26% of probands, of which 2.8% had more than one diagnostic finding and 1.8% had actionable secondary findings. In addition, 7% of the variants were found in candidate genes; 31% of probands had variants of uncertain significance, while 36% tested negative. “Nevertheless, the diagnostic yield of exome sequencing depends on the phenotype and cohort studied,” Dr. Richard continued.
At her company, she said, the highest positive rate is for multiple congenital anomalies (34%), skeletal system abnormalities (30%), and nervous system abnormalities (29%). Trio testing – the concurrent analysis of both biological parents and proband for all genes – “is a critical factor for success,” she added. “It not only improves the variant calling because we have three times the data and increases test sensitivity, it also provides more certain results, determines inheritance and allows for detection of parental mosaicism.”
According to Dr. Richard, trio testing has a one-third higher diagnostic rate than sequencing of the proband alone. Citing a published prospective study that compiled data from eight different exome- and genome-sequencing studies in critically ill neonates and children, trio testing made it possible to make a genetic diagnosis in up to 58% of children.
Whole-genome sequencing is estimated to have a 5%-10% higher diagnostic rate than exome sequencing. “However, we are still a ways away from using it as a routine diagnostic test for all test indications,” Dr. Richard said. “Automation, special bioinformatics algorithms and databases, and combination of genome sequencing with mRNA sequencing are being explored and built to further improve the diagnostic yield.”
Dr. Richard had no disclosures other than being an employee of GeneDx.
and families of pediatric patients to navigate the landscape.
“Testing options range from targeted variant testing and single-gene testing to exome and genome sequencing,” Gabriele Richard, MD, said at the annual meeting of the Society for Pediatric Dermatology. “It is not always easy to determine which testing is right.”
Increasingly, clinical genomic tests, including exome and genome sequencing, are used for patients with complex phenotypes, and possibly multiple disorders, who might have no diagnosis despite extensive prior testing, said Dr. Richard, medical director at Gaithersburg, Md.–based GeneDx., a molecular diagnostic laboratory that performs comprehensive testing for rare genetic disorders. These tests are also being used more for first-line testing in critically ill patients in the neonatal and pediatric intensive care units, and “have heralded a whole new era of gene and disease discovery,” she added.
Targeted variant testing is used for known familial variants, to test family members for carrier status and segregation, and to make a prenatal diagnosis, she said. Single-gene testing is available for most genes and has its place for conditions that can be clinically well-recognized, such as ichthyosis vulgaris, Darier disease, or Papillon-Lefèvre syndrome.
Specific tests for identifying gene deletions or duplications are exon-level microarrays, multiplex ligation-dependent probe amplification (MLPA), and chromosomal microarray analysis. “The latter has been successful in identifying diseases causing chromosomal abnormalities in over 10% of cases overall,” Dr. Richard said. An example of a skin disorder is X-linked ichthyosis caused by a deletion of the steroid sulfatase locus in more than 95% of affected males, she said.
“However, the current staple of molecular diagnostic testing is multigene next-generation sequencing (NGS) panels, which allow you to interrogate two to hundreds of genes concurrently, including sequencing and deletion duplication testing.” These tests are the most cost effective, she said, and are available for almost any genodermatosis or group of disorders with overlapping phenotypes, such as albinism or ichthyosis, epidermolysis bullosa and skin fragility, ectodermal dysplasia, or porphyria. According to Dr. Richard, the diagnostic outcomes of NGS panels mainly depend on test indication, panel size and gene curation, age of onset, and prevailing inheritance pattern of disorders.
Her recommended criteria for distinguishing the myriad of available NGS panels include checking gene content, technical sensitivity of sequencing and deletion/duplication analysis, quality of variant interpretation and reporting, turn-around time, and available familial follow-up testing. “If a family might consider future prenatal diagnosis, choose the lab that performs prenatal and diagnostic testing,” Dr. Richard said. “Equally important are client services such as ease of ordering, insurance coverage, and the ability to determine out-of-pocket cost to patients.”
Resources that enable consumers to compare panel content, methodology, turnaround time, and other parameters include the Genetic Testing Registry (GTR) operated by the National Center for Biotechnology Information, and Concert Genetics, a genetic testing company. The National Society of Genetic Counselors also offers a searchable database for finding a genetic counselor.
Exome sequencing includes the coding sequences of about 20,000 genes and has an average depth of 50 to about 150 reads. “It is a phenotype-driven test where only select variants are being reported fitting the phenotype,” Dr. Richard said. “The outcome of exome and genome sequencing much depends on optimization of bioinformatic pipelines and tools.” Besides small sequence variants, exome sequencing is able to identify a variety of different types of disease-causing variants, such as gene copy number variants seen in about 6% of positive cases, mosaicism, regions of homozygosity, uniparental disomy, and other unusual events and is cost effective.
Whole-genome sequencing, meanwhile, includes the entire genome, particularly noncoding regions, and has an average depth of more than 30 reads. “It’s based on single-molecule sequencing, has longer reads and more uniform coverage, compared to exome sequencing,” she said. “Higher cost, variant interpretation, and lack of coverage by payers are still presenting challenges for genome sequencing.” Genome sequencing can be done in a day or less.
According to diagnostic outcomes based on 280,000 individuals including 125,000 probands from GeneDx data, a definitive diagnosis was made in 26% of probands, of which 2.8% had more than one diagnostic finding and 1.8% had actionable secondary findings. In addition, 7% of the variants were found in candidate genes; 31% of probands had variants of uncertain significance, while 36% tested negative. “Nevertheless, the diagnostic yield of exome sequencing depends on the phenotype and cohort studied,” Dr. Richard continued.
At her company, she said, the highest positive rate is for multiple congenital anomalies (34%), skeletal system abnormalities (30%), and nervous system abnormalities (29%). Trio testing – the concurrent analysis of both biological parents and proband for all genes – “is a critical factor for success,” she added. “It not only improves the variant calling because we have three times the data and increases test sensitivity, it also provides more certain results, determines inheritance and allows for detection of parental mosaicism.”
According to Dr. Richard, trio testing has a one-third higher diagnostic rate than sequencing of the proband alone. Citing a published prospective study that compiled data from eight different exome- and genome-sequencing studies in critically ill neonates and children, trio testing made it possible to make a genetic diagnosis in up to 58% of children.
Whole-genome sequencing is estimated to have a 5%-10% higher diagnostic rate than exome sequencing. “However, we are still a ways away from using it as a routine diagnostic test for all test indications,” Dr. Richard said. “Automation, special bioinformatics algorithms and databases, and combination of genome sequencing with mRNA sequencing are being explored and built to further improve the diagnostic yield.”
Dr. Richard had no disclosures other than being an employee of GeneDx.
and families of pediatric patients to navigate the landscape.
“Testing options range from targeted variant testing and single-gene testing to exome and genome sequencing,” Gabriele Richard, MD, said at the annual meeting of the Society for Pediatric Dermatology. “It is not always easy to determine which testing is right.”
Increasingly, clinical genomic tests, including exome and genome sequencing, are used for patients with complex phenotypes, and possibly multiple disorders, who might have no diagnosis despite extensive prior testing, said Dr. Richard, medical director at Gaithersburg, Md.–based GeneDx., a molecular diagnostic laboratory that performs comprehensive testing for rare genetic disorders. These tests are also being used more for first-line testing in critically ill patients in the neonatal and pediatric intensive care units, and “have heralded a whole new era of gene and disease discovery,” she added.
Targeted variant testing is used for known familial variants, to test family members for carrier status and segregation, and to make a prenatal diagnosis, she said. Single-gene testing is available for most genes and has its place for conditions that can be clinically well-recognized, such as ichthyosis vulgaris, Darier disease, or Papillon-Lefèvre syndrome.
Specific tests for identifying gene deletions or duplications are exon-level microarrays, multiplex ligation-dependent probe amplification (MLPA), and chromosomal microarray analysis. “The latter has been successful in identifying diseases causing chromosomal abnormalities in over 10% of cases overall,” Dr. Richard said. An example of a skin disorder is X-linked ichthyosis caused by a deletion of the steroid sulfatase locus in more than 95% of affected males, she said.
“However, the current staple of molecular diagnostic testing is multigene next-generation sequencing (NGS) panels, which allow you to interrogate two to hundreds of genes concurrently, including sequencing and deletion duplication testing.” These tests are the most cost effective, she said, and are available for almost any genodermatosis or group of disorders with overlapping phenotypes, such as albinism or ichthyosis, epidermolysis bullosa and skin fragility, ectodermal dysplasia, or porphyria. According to Dr. Richard, the diagnostic outcomes of NGS panels mainly depend on test indication, panel size and gene curation, age of onset, and prevailing inheritance pattern of disorders.
Her recommended criteria for distinguishing the myriad of available NGS panels include checking gene content, technical sensitivity of sequencing and deletion/duplication analysis, quality of variant interpretation and reporting, turn-around time, and available familial follow-up testing. “If a family might consider future prenatal diagnosis, choose the lab that performs prenatal and diagnostic testing,” Dr. Richard said. “Equally important are client services such as ease of ordering, insurance coverage, and the ability to determine out-of-pocket cost to patients.”
Resources that enable consumers to compare panel content, methodology, turnaround time, and other parameters include the Genetic Testing Registry (GTR) operated by the National Center for Biotechnology Information, and Concert Genetics, a genetic testing company. The National Society of Genetic Counselors also offers a searchable database for finding a genetic counselor.
Exome sequencing includes the coding sequences of about 20,000 genes and has an average depth of 50 to about 150 reads. “It is a phenotype-driven test where only select variants are being reported fitting the phenotype,” Dr. Richard said. “The outcome of exome and genome sequencing much depends on optimization of bioinformatic pipelines and tools.” Besides small sequence variants, exome sequencing is able to identify a variety of different types of disease-causing variants, such as gene copy number variants seen in about 6% of positive cases, mosaicism, regions of homozygosity, uniparental disomy, and other unusual events and is cost effective.
Whole-genome sequencing, meanwhile, includes the entire genome, particularly noncoding regions, and has an average depth of more than 30 reads. “It’s based on single-molecule sequencing, has longer reads and more uniform coverage, compared to exome sequencing,” she said. “Higher cost, variant interpretation, and lack of coverage by payers are still presenting challenges for genome sequencing.” Genome sequencing can be done in a day or less.
According to diagnostic outcomes based on 280,000 individuals including 125,000 probands from GeneDx data, a definitive diagnosis was made in 26% of probands, of which 2.8% had more than one diagnostic finding and 1.8% had actionable secondary findings. In addition, 7% of the variants were found in candidate genes; 31% of probands had variants of uncertain significance, while 36% tested negative. “Nevertheless, the diagnostic yield of exome sequencing depends on the phenotype and cohort studied,” Dr. Richard continued.
At her company, she said, the highest positive rate is for multiple congenital anomalies (34%), skeletal system abnormalities (30%), and nervous system abnormalities (29%). Trio testing – the concurrent analysis of both biological parents and proband for all genes – “is a critical factor for success,” she added. “It not only improves the variant calling because we have three times the data and increases test sensitivity, it also provides more certain results, determines inheritance and allows for detection of parental mosaicism.”
According to Dr. Richard, trio testing has a one-third higher diagnostic rate than sequencing of the proband alone. Citing a published prospective study that compiled data from eight different exome- and genome-sequencing studies in critically ill neonates and children, trio testing made it possible to make a genetic diagnosis in up to 58% of children.
Whole-genome sequencing is estimated to have a 5%-10% higher diagnostic rate than exome sequencing. “However, we are still a ways away from using it as a routine diagnostic test for all test indications,” Dr. Richard said. “Automation, special bioinformatics algorithms and databases, and combination of genome sequencing with mRNA sequencing are being explored and built to further improve the diagnostic yield.”
Dr. Richard had no disclosures other than being an employee of GeneDx.
FROM SPD 2021
Six shifts driving the future of medicine, strategist says
Contact lenses that detect glucose in tears. Capsules embedded in clothes that can be used to counteract the risk of sensitive skin conditions.
These are just .
At the annual meeting of the Society for Pediatric Dermatology, Zayna Khayat, PhD, said that the future of medicine is driven by six shifts pulling society from a past oriented around the health care system – the buildings, clinicians, and payers – to a patient-oriented perspective. “That doesn’t just happen on its own,” said Dr. Khayat, a future strategist at Toronto-based SE Health. “There are big forces that are pulling us to the future whether we want it to or not. One is that patients have woken up. They have grown to have power in many other complex decisions in their life, and they’re expecting no less from our health care system.”
During her presentation, she discussed the six shifts:
1. The timing of service placement. The traditional model of medicine is “an intermittent and interventional science that waits for the symptoms and goes in and either fixes or manages them,” she said. “So, it’s not really health care; it’s sick care. That’s been fine in the industrial era when we needed to get medicine to stop catastrophic events. Not only is it shifting to be proactive and preventative but it’s shifting to a new science of medicine called predictive medicine.”
As for proactive and preventative care, she continued, each patient’s choice of behaviors related to diet, exercise, and stress “mingles with DNA to produce health, yet we spend about 90% of our resources on sick care. Now, health systems are moving their resources to things like education, housing, transportation, food security, equity, and racial divides. ... This is trickling down to how we train health care professionals. We know that patients live very little of their time in formal care settings, so all of their health is created – or destroyed – well outside of the clinical setting. We train our health professionals mostly in a clinical setting. Health systems are now starting to reimagine how training happens so we can train people to understand the fully loaded context of their patients’ lives.”
2. A shift in precision. For all its advances and science breakthroughs, medicine “is still quite crude,” said Dr. Khayat, who is also an adjunct professor in the Rotman School of Management at the University of Toronto. “It’s very analog, based on a one-size-fits-all approach. In the business world, we call this a segment of one: the idea that in some clinical trial, a result was produced that was based on the average of everybody, and therefore we just give everybody what worked for the average. ... We don’t need to have that trade-off anymore, because it won’t be a trade-off of higher cost to tailor down to an N of 1. It will be highly personalized, intelligent medicine, very precise.”
3. A shift from institution-centered to person-centered care. “The artifacts that health care was built on are very analog and are going to get decentralized out of buildings, dephysicalized, disintermediated,” she predicted. “We’ll have a seamless digital physical experience, expanded channels through which patients can access their services. Pick a channel that makes sense for the patient and don’t let care follow the place but rather let care follow the person.”
4. A shift in care duration, from episodic and intermittent care to more continuous care. “With very little input you should know what’s going on at any point in time instead of time-sharing access to diagnostics and to clinicians,” Dr. Khayat said. Wrist-worn devices that gather personal omics “are now really democratized, with every aspect of a diagnostic clinic available within or connected to a smartphone. This allows for data to be gathered and shared with clinicians, including tools under the skin that can get some of the biochemical data in real time instead of poking and prodding and waiting for a diagnostic lab.” These devices, she said, will become easier to use, cheaper, and will work faster, and provide much better data “at almost zero cost.”
Technologies being developed include tattoos that can read biomarkers, innovations in clothing that can detect biochemical reactions in the skin, underwear that can read vital signs, and contact lenses that can measure glucose levels. “The skin will become a major noninvasive way to obtain information,” she said.
5. A shift in power from providers to patients. “It’s estimated that about 80% of health care decisions could be self-managed by people in their communities,” Dr. Khayat said.
6. A shift from volume-based to value-based care. “Because we’ve been obsessed with the system, we’ve paid for stuff like visits, pills, MRI scans, et cetera,” she said. “We don’t need to do that anymore. Health systems don’t want to keep paying for stuff if they don’t see the results. Because of all the other shifts, we can pay for results. Some call this value-based care. I call it fee-for-health.”
She noted that the future of medicine is underpinned by innovations in AI/predictalytics, voice recognition, virtual reality, blockchain, IoT sensors, 3D printing, omics, robotics, autonomous transport, neurotechnology, nanobiology, and cellular therapy. “They’re moving at a very fast pace because they don’t need the kind of cost, capital, and expertise that the previous tools did,” she said. “This is the promise that technology can bring.”
Dr. Khayat disclosed that she has been a workshop participant for Roche Canada.
Contact lenses that detect glucose in tears. Capsules embedded in clothes that can be used to counteract the risk of sensitive skin conditions.
These are just .
At the annual meeting of the Society for Pediatric Dermatology, Zayna Khayat, PhD, said that the future of medicine is driven by six shifts pulling society from a past oriented around the health care system – the buildings, clinicians, and payers – to a patient-oriented perspective. “That doesn’t just happen on its own,” said Dr. Khayat, a future strategist at Toronto-based SE Health. “There are big forces that are pulling us to the future whether we want it to or not. One is that patients have woken up. They have grown to have power in many other complex decisions in their life, and they’re expecting no less from our health care system.”
During her presentation, she discussed the six shifts:
1. The timing of service placement. The traditional model of medicine is “an intermittent and interventional science that waits for the symptoms and goes in and either fixes or manages them,” she said. “So, it’s not really health care; it’s sick care. That’s been fine in the industrial era when we needed to get medicine to stop catastrophic events. Not only is it shifting to be proactive and preventative but it’s shifting to a new science of medicine called predictive medicine.”
As for proactive and preventative care, she continued, each patient’s choice of behaviors related to diet, exercise, and stress “mingles with DNA to produce health, yet we spend about 90% of our resources on sick care. Now, health systems are moving their resources to things like education, housing, transportation, food security, equity, and racial divides. ... This is trickling down to how we train health care professionals. We know that patients live very little of their time in formal care settings, so all of their health is created – or destroyed – well outside of the clinical setting. We train our health professionals mostly in a clinical setting. Health systems are now starting to reimagine how training happens so we can train people to understand the fully loaded context of their patients’ lives.”
2. A shift in precision. For all its advances and science breakthroughs, medicine “is still quite crude,” said Dr. Khayat, who is also an adjunct professor in the Rotman School of Management at the University of Toronto. “It’s very analog, based on a one-size-fits-all approach. In the business world, we call this a segment of one: the idea that in some clinical trial, a result was produced that was based on the average of everybody, and therefore we just give everybody what worked for the average. ... We don’t need to have that trade-off anymore, because it won’t be a trade-off of higher cost to tailor down to an N of 1. It will be highly personalized, intelligent medicine, very precise.”
3. A shift from institution-centered to person-centered care. “The artifacts that health care was built on are very analog and are going to get decentralized out of buildings, dephysicalized, disintermediated,” she predicted. “We’ll have a seamless digital physical experience, expanded channels through which patients can access their services. Pick a channel that makes sense for the patient and don’t let care follow the place but rather let care follow the person.”
4. A shift in care duration, from episodic and intermittent care to more continuous care. “With very little input you should know what’s going on at any point in time instead of time-sharing access to diagnostics and to clinicians,” Dr. Khayat said. Wrist-worn devices that gather personal omics “are now really democratized, with every aspect of a diagnostic clinic available within or connected to a smartphone. This allows for data to be gathered and shared with clinicians, including tools under the skin that can get some of the biochemical data in real time instead of poking and prodding and waiting for a diagnostic lab.” These devices, she said, will become easier to use, cheaper, and will work faster, and provide much better data “at almost zero cost.”
Technologies being developed include tattoos that can read biomarkers, innovations in clothing that can detect biochemical reactions in the skin, underwear that can read vital signs, and contact lenses that can measure glucose levels. “The skin will become a major noninvasive way to obtain information,” she said.
5. A shift in power from providers to patients. “It’s estimated that about 80% of health care decisions could be self-managed by people in their communities,” Dr. Khayat said.
6. A shift from volume-based to value-based care. “Because we’ve been obsessed with the system, we’ve paid for stuff like visits, pills, MRI scans, et cetera,” she said. “We don’t need to do that anymore. Health systems don’t want to keep paying for stuff if they don’t see the results. Because of all the other shifts, we can pay for results. Some call this value-based care. I call it fee-for-health.”
She noted that the future of medicine is underpinned by innovations in AI/predictalytics, voice recognition, virtual reality, blockchain, IoT sensors, 3D printing, omics, robotics, autonomous transport, neurotechnology, nanobiology, and cellular therapy. “They’re moving at a very fast pace because they don’t need the kind of cost, capital, and expertise that the previous tools did,” she said. “This is the promise that technology can bring.”
Dr. Khayat disclosed that she has been a workshop participant for Roche Canada.
Contact lenses that detect glucose in tears. Capsules embedded in clothes that can be used to counteract the risk of sensitive skin conditions.
These are just .
At the annual meeting of the Society for Pediatric Dermatology, Zayna Khayat, PhD, said that the future of medicine is driven by six shifts pulling society from a past oriented around the health care system – the buildings, clinicians, and payers – to a patient-oriented perspective. “That doesn’t just happen on its own,” said Dr. Khayat, a future strategist at Toronto-based SE Health. “There are big forces that are pulling us to the future whether we want it to or not. One is that patients have woken up. They have grown to have power in many other complex decisions in their life, and they’re expecting no less from our health care system.”
During her presentation, she discussed the six shifts:
1. The timing of service placement. The traditional model of medicine is “an intermittent and interventional science that waits for the symptoms and goes in and either fixes or manages them,” she said. “So, it’s not really health care; it’s sick care. That’s been fine in the industrial era when we needed to get medicine to stop catastrophic events. Not only is it shifting to be proactive and preventative but it’s shifting to a new science of medicine called predictive medicine.”
As for proactive and preventative care, she continued, each patient’s choice of behaviors related to diet, exercise, and stress “mingles with DNA to produce health, yet we spend about 90% of our resources on sick care. Now, health systems are moving their resources to things like education, housing, transportation, food security, equity, and racial divides. ... This is trickling down to how we train health care professionals. We know that patients live very little of their time in formal care settings, so all of their health is created – or destroyed – well outside of the clinical setting. We train our health professionals mostly in a clinical setting. Health systems are now starting to reimagine how training happens so we can train people to understand the fully loaded context of their patients’ lives.”
2. A shift in precision. For all its advances and science breakthroughs, medicine “is still quite crude,” said Dr. Khayat, who is also an adjunct professor in the Rotman School of Management at the University of Toronto. “It’s very analog, based on a one-size-fits-all approach. In the business world, we call this a segment of one: the idea that in some clinical trial, a result was produced that was based on the average of everybody, and therefore we just give everybody what worked for the average. ... We don’t need to have that trade-off anymore, because it won’t be a trade-off of higher cost to tailor down to an N of 1. It will be highly personalized, intelligent medicine, very precise.”
3. A shift from institution-centered to person-centered care. “The artifacts that health care was built on are very analog and are going to get decentralized out of buildings, dephysicalized, disintermediated,” she predicted. “We’ll have a seamless digital physical experience, expanded channels through which patients can access their services. Pick a channel that makes sense for the patient and don’t let care follow the place but rather let care follow the person.”
4. A shift in care duration, from episodic and intermittent care to more continuous care. “With very little input you should know what’s going on at any point in time instead of time-sharing access to diagnostics and to clinicians,” Dr. Khayat said. Wrist-worn devices that gather personal omics “are now really democratized, with every aspect of a diagnostic clinic available within or connected to a smartphone. This allows for data to be gathered and shared with clinicians, including tools under the skin that can get some of the biochemical data in real time instead of poking and prodding and waiting for a diagnostic lab.” These devices, she said, will become easier to use, cheaper, and will work faster, and provide much better data “at almost zero cost.”
Technologies being developed include tattoos that can read biomarkers, innovations in clothing that can detect biochemical reactions in the skin, underwear that can read vital signs, and contact lenses that can measure glucose levels. “The skin will become a major noninvasive way to obtain information,” she said.
5. A shift in power from providers to patients. “It’s estimated that about 80% of health care decisions could be self-managed by people in their communities,” Dr. Khayat said.
6. A shift from volume-based to value-based care. “Because we’ve been obsessed with the system, we’ve paid for stuff like visits, pills, MRI scans, et cetera,” she said. “We don’t need to do that anymore. Health systems don’t want to keep paying for stuff if they don’t see the results. Because of all the other shifts, we can pay for results. Some call this value-based care. I call it fee-for-health.”
She noted that the future of medicine is underpinned by innovations in AI/predictalytics, voice recognition, virtual reality, blockchain, IoT sensors, 3D printing, omics, robotics, autonomous transport, neurotechnology, nanobiology, and cellular therapy. “They’re moving at a very fast pace because they don’t need the kind of cost, capital, and expertise that the previous tools did,” she said. “This is the promise that technology can bring.”
Dr. Khayat disclosed that she has been a workshop participant for Roche Canada.
FROM SPD 2021
Targeted therapies for vascular anomalies continue to be refined
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
FROM SPD 2021
Expert shares vulvovaginal candidiasis treatment pearls
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
that was approved in June 2021, Aruna Venkatesan, MD, recommends.
“Ibrexafungerp, an inhibitor of beta (1-3)–glucan synthase, is important for many reasons,” Dr. Venkatesan, chief of dermatology and director of the genital dermatology clinic at Santa Clara Valley Medical Center, San Jose, Calif., said during the annual meeting of the Pacific Dermatologic Association. “It’s one of the few drugs that can be used to treat Candida glabrata when C. glabrata is resistant to azoles and echinocandins. As the second-most common Candida species after C. albicans, C. glabrata is more common in immunosuppressed patients and it can cause mucosal and invasive disease, so ibrexafungerp is a welcome addition to our treatment armamentarium,” said Dr. Venkatesan, clinical professor of dermatology (affiliated) at Stanford (Calif.) Hospital and Clinics, adding that that vulvovaginal candidiasis can be tricky to diagnose. “In medical school, we learned that yeast infection in a woman presents as white, curd-like discharge, but that’s actually a minority of patients.”
For a patient who is being treated with topical steroids or estrogen for a genital condition, but is experiencing worsening itch, redness, or thick white discharge, she recommends performing a KOH exam.
“Instead of using a 15-blade scalpel, as we are used to performing on the skin for tinea, take a sterile [cotton swab], and swab the affected area. You can then apply it to a slide and perform a KOH exam as you normally would. Then look for yeast elements under the microscope. I also find it helpful to send for fungal culture to get speciation, especially in someone who’s not responding to therapy. This is because non-albicans yeast can be more resistant to azoles and require a different treatment plan.”
Often, patients with vulvovaginal candidiasis who present to her clinic are referred from an ob.gyn. and other general practitioners because they have failed a topical or oral azole. “I tend to avoid the topicals,” said Dr. Venkatesan, who is also president-elect of the North American chapter of the International Society for the Study of Vulvovaginal Disease. “If the culture shows C. albicans, I usually treat with oral fluconazole, 150 mg or 200 mg once, and consider repeat weekly dosing. Many patients come to me because they have recurrent refractory disease, so giving it once weekly for 6-8 weeks while they work on their potential risk factors such as diabetic blood sugar control is sensible.”
Non-albicans yeast can be resistant to azoles. If the fungal culture shows C. glabrata in such patients, “consider a course of intravaginal boric acid suppositories,” she advised. “These used to be difficult to give patients, because you would either have to send the prescription to a compounding pharmacy, or have the patients buy the capsules and boric acid crystals separately and make them themselves. That always made me nervous because of the chance of errors. The safety and the concern of taking it by mouth is an issue.” But now, intravaginal boric acid suppositories are available on Amazon and other web sites, and are relatively affordable, she said, adding, “just make sure the patient doesn’t take it by mouth as this is very toxic.”
Dr. Venkatesan reported having no financial disclosures.
FROM PDA 2021
Ask about itch and joint pain in pediatric psoriasis patients, expert advises
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
During the annual meeting of the Society for Pediatric Dermatology, Amy S. Paller, MD, MS, marveled on the remarkable advances in the treatment of inflammatory skin disorders during the past 2 decades.
“We’ve come a long way, from disease features being red, thick, and scaly and being treated with nonspecific therapy like topical steroids, keratolytics, and tar, to understanding disease pathogenesis and finding new targeted therapies for inflammatory skin disorders in children,” said Dr. Paller, professor and chair of the department of dermatology at Northwestern University, Chicago. “There are now studies moving forward with gene correction, gene replacement, the gene product replaced, or pathway inhibition to prevent the effects of genetic change.”
Technology is leading the way in generating new therapeutic advances, she continued, beyond traditional “omics” to lipidomics, metabolomics, glycomics, and kinomics. “This has enabled us to find new genetic disorders and their causes, to look at changes in gene expression patterns, and to look at changes in protein expression patterns that give us clues as to how to move forward with better therapy,” she said. “When we’re talking about new insights into pathogenesis-based therapy, we’re talking largely about understanding the pathways that lead to either inflammation or promoting cell proliferation and abnormal differentiation.”
Treating pediatric psoriasis
. “First of all, ask about itch and pain with these patients,” she advised. “Interviews have shown that 61% of children experience some itch, 39% have pain or stinging, and in the ixekizumab trials, 72% had what’s considered meaningful itch, with at least 4 out of 10 (mean intensity 5.3) on the itch numeric rating scale. Little is known about the itch associated with psoriasis and its underlying cause – unrelated to the IL-4/IL-13 pathway activation of atopic dermatitis – but it’s worth asking about. I find that itch of the scalp is especially a problem in psoriasis.”
Physicians should also ask pediatric psoriasis patients about joint pain, because about 1% of them have psoriatic arthritis, which is much less common than in adults, “but important to find and manage,” she added. Dr. Paller recommends the new R-JET rapid joint exam technique, which is accompanied by a three-question survey and body diagram that facilitates identification of true arthritis, “so you can know how quickly to refer”.
Several studies have described an increased risk of metabolic syndrome in adolescents with pediatric psoriasis and now in prepubertal children with the disease. In a recent study of 60 consecutive prepubertal children with psoriasis, 70% of whom had mild disease, 40% were overweight or obese, 53% had central obesity, 27% had high levels of the HOMA-IR (homeostasis model assessment of insulin resistance) despite generally normal levels of fasting glucose, and 30% met criteria for metabolic syndrome.
“This really struck me because our AAD [American Academy of Dermatology] guidelines did not recommend screening for type 2 diabetes in prepubertal children, even if overweight, because the risk is so small,” Dr. Paller said. “This report suggests that we may need to reconsider this recommendation in prepubertal children with psoriasis.”
Meanwhile, the number of medications approved by the Food and Drug Administration and the European Medicines Agency for children with psoriasis who are 6 years of age and above continues to expand, including tumor necrosis factor (TNF) inhibitors, interleukin (IL)-23 inhibitors, and IL-17 inhibitors. Most children can now achieve a PASI 90 within 12 weeks with the IL-23 inhibitor ustekinumab and the IL-17 inhibitors ixekizumab and secukinumab, Dr. Paller said.
In the ixekizumab trial, there are head-to-head comparison data in a European arm that involved the use of etanercept, she said. “What’s most noticeable is the significant difference in those who were able to achieve PASI 90 or above with this IL-17 inhibitor, versus etanercept,” which she added, raises the question of whether aiming for a PASI 75 is adequate, "or should we strive for PASI 90?” A pediatric psoriasis study published in 2020 found that the greatest improvement in quality of life was associated with a PASI 90 and use of systemic treatments.
Looking forward, phase 3 clinical trials are underway in pediatric patients with moderate to severe psoriasis for guselkumab, tildrakizumab, risankizumab, certolizumab, bimekizumab, and brodalumab. “The cost of all of these biologics is high, however. I remind everyone that we still have methotrexate,” she said. “The risk of side effects with our low-dose methotrexate treatment for psoriasis remains low, but methotrexate doesn’t hit these [high] PASI numbers and it’s much slower in its onset than biologics.”
Dr. Paller disclosed that she is a consultant to and/or an investigator for AbbVie, Arena, Bausch, Bristol Myers Squibb, Dermavant, Eli Lilly, Incyte, Forte, LEO Pharma, LifeMax, Pfizer, RAPT Therapeutics, Regeneron, and Sanofi.
Commentary by Robert Sidbury, MD, MPH
Dr. Paller reminds us of some essential features of pediatric psoriasis:
• It can hurt. Ask your patients if it does.
• It can itch. Look for excoriations, especially in the scalp.
• It is often associated with metabolic syndrome, so check relevant biometrics and labs, and consider coincident insulin resistance.
• Our traditional clinical trial target of PASI75, or a 75% reduction in body surface area involvement, is just not good enough. Studies have shown that the most meaningful quality-of-life gains come at PASI90 or above.
• With our newer biologics, such as IL-12/23 blockers (for instance, ustekinumab) and IL-17 blockers (for example, ixekizumab and secukinumab), PASI90 and better is a reasonable expectation, not a pipe dream.
Dr. Sidbury is chief of dermatology at Seattle Children's Hospital and professor, department of pediatrics, University of Washington, Seattle. He is a site principal investigator for dupilumab trials, for which the hospital has a contract with Regeneron.
This article was updated 6/16/22.
FROM SPD 2021
Study evaluates OTC treatments for molluscum contagiosum
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
“It’s important for clinicians who see children with molluscum to be aware of the many products marketed to patients and to be able to provide objective information about them,” senior author Elaine Siegfried, MD, said in an interview following the annual meeting of the Society for Pediatric Dermatology, where the abstract was presented during a poster session.
In the text of their abstract, Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and coauthors Isaac Hoft, of Open Mind Holistics in Ft. Collins, Colo., and Samantha K. Ong, BA, a student at SLU, noted that MC primarily infects children, with an annual incidence of 8%. “Although the disease is self-limited, associated symptoms, contagion and an average 1-year duration prompt concern and frequent medical visits,” they wrote.
The optimal treatment for MC has not been defined and there is currently no approved medication approved for the condition, although three products are in development: VP-102 (cantharidin) by Verrica Pharmaceuticals; SB206, a topical antiviral by Novan; and 10%-15% KOH formulation by the Gurina Foundation.
But many OTC products have been marketed to treat the condition. To identify the OTC products and to assess accompanying information related to safety, efficacy, and cost, the researchers performed an internet search using the terms “molluscum” plus “treatment,” “treatment at home,” “relief,” and “medication.” Eight products were identified for analysis: Conzerol (Elroselabs), Molleave (Innovative Med), Mollenol (Jeva Laboratories), MolluscumBLAST (Revitalize Life Organics), Molluscum Away Patches (Molluscum Away), Naturasil (Nature’s Innovation), Terrasil (Advanced Skincare % Topical Solutions), and Zymaderm (Naturopathix). Package sizes ranged from 0.78 to 1.5 ounces, and prices ranged from about $19 to almost $55.
Dr. Siegfried and colleagues found that all products provided instructions on application and use but most package labels did not include sufficient information about their plant-based ingredients or appropriate dosing. Six of the eight products contained Thuja occidentalis (Arbor vitae), a coniferous cedar whose essential oil has been used in homeopathic products for its anti-inflammatory and antiviral properties. Lemon extract, tea tree oil, and other botanicals were present in no more than three products each. Only two of the products provided information about the number of lesions that could be treated per package.
“The lack of national oversight as well as robust methods for high-level data analysis make safety and efficacy unclear for a Thuja extract marketed to treat MC,” the researchers wrote. “Numerous adverse drug events and positive intradermal skin tests related to Thuja have been reported.”
Dr. Siegfried added that many OTC products offer a money-back guarantee, “so when seeing a patient who failed to respond to one of these products, encourage them, at least, to request a refund, but to also submit a comment about lack of efficacy, in order to provide more balanced Internet information.”
Dr. Siegfried disclosed that she has served as an investigator and consultant for Verrica Pharmaceuticals, and as a consultant and Data Safety Monitoring board member for Novan, two of the companies currently developing drugs to treat molluscum. Her coauthors had no conflicts of interest to disclose.
FROM SPD 2021