User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
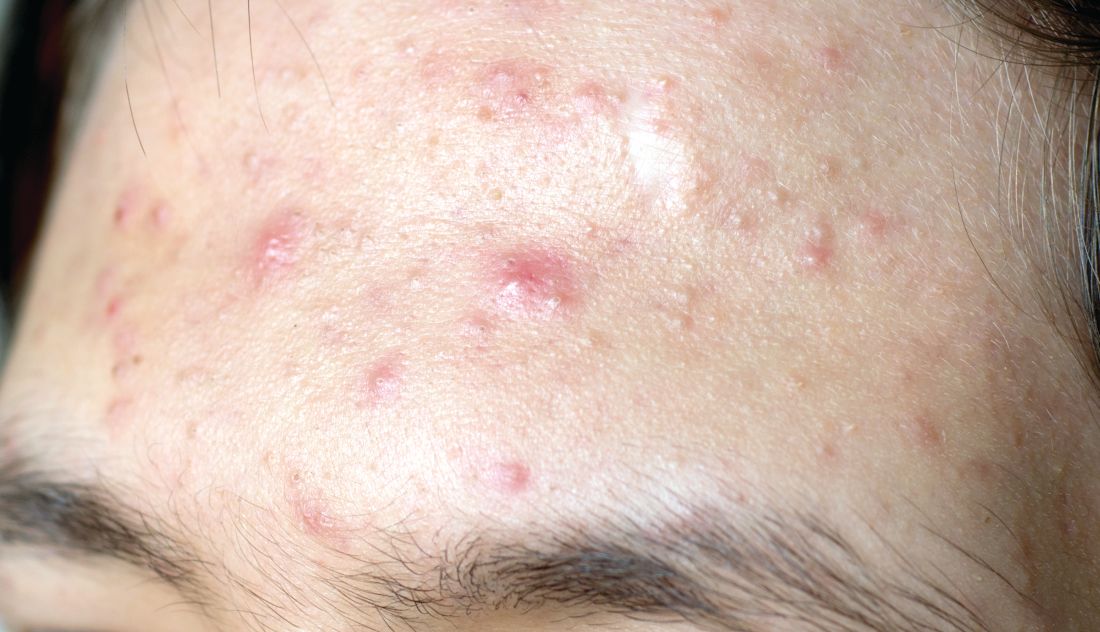
Topical treatment options for acne continue to expand
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
AT MOAS 2022
Cosmetic medicine expert shares male facial aesthetics pearls
SAN DIEGO – .
“Men generally have larger facial muscle mass,” Dr. Green, a dermatologist in Coral Gables, Fla., said at the annual Masters of Aesthetics Symposium. “We need a higher dose to treat them, or they will not be happy. In general, I try to increase the dose by about 50% for my male patients.”
Two early trials of dose adjustments support this practice, he said. In one, 80 men were randomized to receive a total dose of either 20, 40, 60, or 80 U of botulinum toxin type A (Botox) in the glabellar area. The researchers found that the 40, 60, and 80 U doses of botulinum toxin type A were consistently more effective in reducing glabellar lines than the 20 U dose.
In a subsequent study, researchers administered botulinum toxin type A (Dysport) 0.5 to 0.7 mL for men (60, 70, or 80 units), based on procerus/corrugator muscle mass. Efficacy was assessed by a blinded evaluator and patient self-evaluation at several time points up to 150 days post treatment. The median duration of effect was 109 days vs. 0 days for placebo in the blinded evaluator evaluation and 107 days vs. 0 for placebo in the patient self-evaluation.
Most injection algorithms for treating the glabella rely on a 5- or 7-point injection technique, but in 2021, researchers led by Sebastian Cotofana, MD, PhD, of the department of clinical anatomy at Mayo Clinic, Rochester, Minn., reported results from a study of the efficacy and safety of a refined 3-point injection technique targeting horizontal and vertical lines to prevent brow ptosis.
“Prior to this study Sebastian asked me, ‘Why do you guys always inject the body of the muscle?’ ” Dr. Green said. “‘If you inject the origin of the muscle on bone, you could more effectively wipe out the entire muscle’s movement. You’re going to get a better result at a lower dose, so let’s study this.’”
The injection technique involves targeting the midline level of the connecting line between left and right medial canthal ligaments with a 90-degree injection angle with bone contact, as well as the medial and inferior margin of eyebrows with a 45-degree injection angle in relation to midline with frontal bone contact. These three points are located inferior to the traditional (on-label) glabellar frown line injections used to treat the frontalis and the brow depressors.
The researchers used the 5-point glabellar line severity scale to evaluate the time of effect onset and the injection-related outcome 120 days after the treatment in 27 men and 78 women. They found that the onset of the neuromodulator effect occurred in an average of 3.5 days, and no adverse events such as eyebrow ptosis, upper eyelid ptosis, medial eyebrow ptosis, and lateral frontalis hyperactivity occurred during the study period.
“If you inject the origin of these muscles, you can get a brow lift with this technique by avoiding frontalis altogether,” Dr. Green said. “The caveat is, it’s so great at lifting the brows that if you treat the forehead, you may create a midline horizontal ‘shelf’ like I’ve never seen before, where the eyebrows elevate into an immobile superior frontalis.”
To avoid this when treating the forehead as well, he’s learned to split the dose of neuromodulator. “If I was injecting 5 units in the procerus before, I’ll do 2.5 units on nasal bone at the insertion of the muscle and then 2.5 units higher up in the traditional midline procerus injection site,” Dr. Green said.
“Same with the corrugators,” he continued. “Then, remember to inject more superficially in the lateral part, the tail of the corrugators, because the tail of the corrugators is inserting into the undersurface of the dermis. That’s why you see that skin puckering in the lateral brows when people frown. You’re pretty safe to chase that laterally if the brow’s already flat as in men, but I caution you [not] to do that in women, because you may flatten the brow.”
Dr. Green said that he is aware of two cases of lid ptosis from the 3-point technique, one of which happened to him.
“When you’re on the bone with your thumb you can feel that liquid traveling along the bone,” he said. “It can travel all the way to the midline pupil where the levator palpebrae superioris muscle is. I now don’t come in contact with bone with my corrugator origin injections, but rather float the needle a couple of millimeters off bone (in muscle) to hopefully prevent that from happening. Alternatively, some people will compress the brow along frontal bone lateral to that corrugator injection site while they’re injecting to prevent backflow of the neuromodulator.”
Dr. Green reported having received research funding and/or consulting fees from many device and pharmaceutical companies.
SAN DIEGO – .
“Men generally have larger facial muscle mass,” Dr. Green, a dermatologist in Coral Gables, Fla., said at the annual Masters of Aesthetics Symposium. “We need a higher dose to treat them, or they will not be happy. In general, I try to increase the dose by about 50% for my male patients.”
Two early trials of dose adjustments support this practice, he said. In one, 80 men were randomized to receive a total dose of either 20, 40, 60, or 80 U of botulinum toxin type A (Botox) in the glabellar area. The researchers found that the 40, 60, and 80 U doses of botulinum toxin type A were consistently more effective in reducing glabellar lines than the 20 U dose.
In a subsequent study, researchers administered botulinum toxin type A (Dysport) 0.5 to 0.7 mL for men (60, 70, or 80 units), based on procerus/corrugator muscle mass. Efficacy was assessed by a blinded evaluator and patient self-evaluation at several time points up to 150 days post treatment. The median duration of effect was 109 days vs. 0 days for placebo in the blinded evaluator evaluation and 107 days vs. 0 for placebo in the patient self-evaluation.
Most injection algorithms for treating the glabella rely on a 5- or 7-point injection technique, but in 2021, researchers led by Sebastian Cotofana, MD, PhD, of the department of clinical anatomy at Mayo Clinic, Rochester, Minn., reported results from a study of the efficacy and safety of a refined 3-point injection technique targeting horizontal and vertical lines to prevent brow ptosis.
“Prior to this study Sebastian asked me, ‘Why do you guys always inject the body of the muscle?’ ” Dr. Green said. “‘If you inject the origin of the muscle on bone, you could more effectively wipe out the entire muscle’s movement. You’re going to get a better result at a lower dose, so let’s study this.’”
The injection technique involves targeting the midline level of the connecting line between left and right medial canthal ligaments with a 90-degree injection angle with bone contact, as well as the medial and inferior margin of eyebrows with a 45-degree injection angle in relation to midline with frontal bone contact. These three points are located inferior to the traditional (on-label) glabellar frown line injections used to treat the frontalis and the brow depressors.
The researchers used the 5-point glabellar line severity scale to evaluate the time of effect onset and the injection-related outcome 120 days after the treatment in 27 men and 78 women. They found that the onset of the neuromodulator effect occurred in an average of 3.5 days, and no adverse events such as eyebrow ptosis, upper eyelid ptosis, medial eyebrow ptosis, and lateral frontalis hyperactivity occurred during the study period.
“If you inject the origin of these muscles, you can get a brow lift with this technique by avoiding frontalis altogether,” Dr. Green said. “The caveat is, it’s so great at lifting the brows that if you treat the forehead, you may create a midline horizontal ‘shelf’ like I’ve never seen before, where the eyebrows elevate into an immobile superior frontalis.”
To avoid this when treating the forehead as well, he’s learned to split the dose of neuromodulator. “If I was injecting 5 units in the procerus before, I’ll do 2.5 units on nasal bone at the insertion of the muscle and then 2.5 units higher up in the traditional midline procerus injection site,” Dr. Green said.
“Same with the corrugators,” he continued. “Then, remember to inject more superficially in the lateral part, the tail of the corrugators, because the tail of the corrugators is inserting into the undersurface of the dermis. That’s why you see that skin puckering in the lateral brows when people frown. You’re pretty safe to chase that laterally if the brow’s already flat as in men, but I caution you [not] to do that in women, because you may flatten the brow.”
Dr. Green said that he is aware of two cases of lid ptosis from the 3-point technique, one of which happened to him.
“When you’re on the bone with your thumb you can feel that liquid traveling along the bone,” he said. “It can travel all the way to the midline pupil where the levator palpebrae superioris muscle is. I now don’t come in contact with bone with my corrugator origin injections, but rather float the needle a couple of millimeters off bone (in muscle) to hopefully prevent that from happening. Alternatively, some people will compress the brow along frontal bone lateral to that corrugator injection site while they’re injecting to prevent backflow of the neuromodulator.”
Dr. Green reported having received research funding and/or consulting fees from many device and pharmaceutical companies.
SAN DIEGO – .
“Men generally have larger facial muscle mass,” Dr. Green, a dermatologist in Coral Gables, Fla., said at the annual Masters of Aesthetics Symposium. “We need a higher dose to treat them, or they will not be happy. In general, I try to increase the dose by about 50% for my male patients.”
Two early trials of dose adjustments support this practice, he said. In one, 80 men were randomized to receive a total dose of either 20, 40, 60, or 80 U of botulinum toxin type A (Botox) in the glabellar area. The researchers found that the 40, 60, and 80 U doses of botulinum toxin type A were consistently more effective in reducing glabellar lines than the 20 U dose.
In a subsequent study, researchers administered botulinum toxin type A (Dysport) 0.5 to 0.7 mL for men (60, 70, or 80 units), based on procerus/corrugator muscle mass. Efficacy was assessed by a blinded evaluator and patient self-evaluation at several time points up to 150 days post treatment. The median duration of effect was 109 days vs. 0 days for placebo in the blinded evaluator evaluation and 107 days vs. 0 for placebo in the patient self-evaluation.
Most injection algorithms for treating the glabella rely on a 5- or 7-point injection technique, but in 2021, researchers led by Sebastian Cotofana, MD, PhD, of the department of clinical anatomy at Mayo Clinic, Rochester, Minn., reported results from a study of the efficacy and safety of a refined 3-point injection technique targeting horizontal and vertical lines to prevent brow ptosis.
“Prior to this study Sebastian asked me, ‘Why do you guys always inject the body of the muscle?’ ” Dr. Green said. “‘If you inject the origin of the muscle on bone, you could more effectively wipe out the entire muscle’s movement. You’re going to get a better result at a lower dose, so let’s study this.’”
The injection technique involves targeting the midline level of the connecting line between left and right medial canthal ligaments with a 90-degree injection angle with bone contact, as well as the medial and inferior margin of eyebrows with a 45-degree injection angle in relation to midline with frontal bone contact. These three points are located inferior to the traditional (on-label) glabellar frown line injections used to treat the frontalis and the brow depressors.
The researchers used the 5-point glabellar line severity scale to evaluate the time of effect onset and the injection-related outcome 120 days after the treatment in 27 men and 78 women. They found that the onset of the neuromodulator effect occurred in an average of 3.5 days, and no adverse events such as eyebrow ptosis, upper eyelid ptosis, medial eyebrow ptosis, and lateral frontalis hyperactivity occurred during the study period.
“If you inject the origin of these muscles, you can get a brow lift with this technique by avoiding frontalis altogether,” Dr. Green said. “The caveat is, it’s so great at lifting the brows that if you treat the forehead, you may create a midline horizontal ‘shelf’ like I’ve never seen before, where the eyebrows elevate into an immobile superior frontalis.”
To avoid this when treating the forehead as well, he’s learned to split the dose of neuromodulator. “If I was injecting 5 units in the procerus before, I’ll do 2.5 units on nasal bone at the insertion of the muscle and then 2.5 units higher up in the traditional midline procerus injection site,” Dr. Green said.
“Same with the corrugators,” he continued. “Then, remember to inject more superficially in the lateral part, the tail of the corrugators, because the tail of the corrugators is inserting into the undersurface of the dermis. That’s why you see that skin puckering in the lateral brows when people frown. You’re pretty safe to chase that laterally if the brow’s already flat as in men, but I caution you [not] to do that in women, because you may flatten the brow.”
Dr. Green said that he is aware of two cases of lid ptosis from the 3-point technique, one of which happened to him.
“When you’re on the bone with your thumb you can feel that liquid traveling along the bone,” he said. “It can travel all the way to the midline pupil where the levator palpebrae superioris muscle is. I now don’t come in contact with bone with my corrugator origin injections, but rather float the needle a couple of millimeters off bone (in muscle) to hopefully prevent that from happening. Alternatively, some people will compress the brow along frontal bone lateral to that corrugator injection site while they’re injecting to prevent backflow of the neuromodulator.”
Dr. Green reported having received research funding and/or consulting fees from many device and pharmaceutical companies.
AT MOAS 2022
Study evaluates features of alopecia areata in Hispanic/Latinx patients
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
.
Those are among key findings from a retrospective analysis of Hispanic/Latinx patients at the University of California, Irvine (UCI) by Natasha Mesinkovska, MD, PhD, of UCI’s department of dermatology, and her coauthors. The findings were published online in the Journal of the American Academy of Dermatology.
A recent study examined the epidemiology of alopecia areata (AA) in Black patients, wrote Dr. Mesinkovska and coauthors Celine Phong, a UCI medical student, and Amy J. McMichael, MD, professor of dermatology at Wake Forest University, Winston-Salem, N.C. “A similar unmet need exists to describe the characteristics of AA in Hispanic/Latinx (H/L) patients, the prevalent majority in California,” they added.
Drawing from chart reviews, ICD codes, and documented physical exams, they retrospectively identified 197 Hispanic/Latinx patients diagnosed with AA at UCI between 2015 and 2022, including alopecia totalis and alopecia universalis.
Nearly two-thirds of patients with alopecia were female (63%), and their mean age at diagnosis was 33 years. Most patients (79%) presented with patchy pattern AA, 13% had diffuse pattern AA, and only 12% had eyebrow, eyelash, or beard involvement. The most common comorbidity in patients overall was atopy (24%), including allergic rhinitis (12%), asthma (10%), and/or atopic dermatitis (7%).
The authors found that 18% of patients had one or more coexisting autoimmune conditions, most commonly rheumatoid arthritis (9%) and thyroid disease (6%). No patients had celiac disease, myasthenia gravis, or inflammatory bowel disease, but 43% had another dermatologic condition.
In other findings, 22% of patients had vitamin D deficiency, 20% had hyperlipidemia, 18% had obesity, 16% had gastroesophageal reflux disease, and 12% had anemia. At the same time, depression, anxiety, or sleep disorders were identified in 14% of patients.
“Interestingly, the most common autoimmune comorbidity in H/L was rheumatoid arthritis, compared to thyroid disease in Black patients and overall AA patients,” the authors wrote. “This finding may be a reflection of a larger trend, as rheumatoid arthritis in the H/L population has been on the rise.”
The authors acknowledged certain limitations of the study including its small sample size and lack of a control group, and reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Ten recommendations for building and growing a cosmetic dermatology practice
SAN DIEGO – When Omar A. Ibrahimi, MD, PhD, opened his own cosmetic dermatology practice in Stamford, Conn., in 2012, he sensed that he had his work cut out for him.
“I was a fellowship-trained Mohs surgeon who wanted to do aesthetics,” Dr. Ibrahimi, medical director of the Connecticut Skin Institute, recalled during the annual Masters of Aesthetics Symposium. “I was in a geographic area that was new to me. I didn’t know any referring doctors, but I started to network and tried to grow my practice.”
Someone once told him that the “three As” of being a medical specialist are “Available, Affable, and Ability,” so he applied that principle as he began to cultivate relationships with physicians in his geographic area. “I told my referring doctors, ‘If you’re kind enough to send me Mohs cases, I’ll help you out if there’s something you don’t like doing, whether it’s a nail biopsy or treating male genital warts,’” he said. “You want to make it easy for doctors to refer to you, but you also want to make their lives easier.”
Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery, offered . They include:
Know yourself. Do what you love to do, not what you feel like you should do. “Whatever you’re doing in your practice, it should be something that you’re passionate about and excited about,” he said. “I do a mix of Mohs surgery and procedural aesthetic dermatology. Most of my practice is shaped toward energy-based devices and laser procedures. Pick the things that you enjoy doing and try to deliver good results.”
Know your patients. When dermatologists who plan to open their own practice ask Dr. Ibrahimi what kind of laser they should buy, he typically responds by asking them to consider what procedures their patients are asking for. “Depending on where you are geographically and the economic profile of the community in which you practice, it can be a different answer,” Dr. Ibrahimi said. “If you practice in the Northeast and do a lot of medical dermatology, it might mean getting a vascular laser to treat rosacea. If you’re in Southern California, treating pigment might be a bigger concern than treating rosacea.” The annual ASDS Survey on Dermatologic Procedures provides a snapshot of trends and can be useful for decision-making, he said.
Know your practice. “Make sure you are capable of entering the aesthetics field,” he advised. “You cannot have a practice that runs like the DMV, with people waiting 30 to 40 minutes to be seen.” Proper training of staff is also key and representatives from device and injectable companies can provide advice and support. As for marketing, some dermatologists hire a public relations agency, but Dr. Ibrahimi finds that the best source of his referrals is word of mouth. “If I do a good job taking care of patients, they will send their friends and family over to me, but social media is also important,” he said. Taking quality before-and-after photos, and obtaining consent from patients to use them online in educational posts is a good approach, he noted.
Know your market. When Dr. Ibrahimi first opened his practice, offering laser hair removal was not a priority because so many other dermatologists and medical spas in his area were already providing it. With time, though, he added laser hair removal to his menu of treatment offerings because “I knew that if my patients weren’t getting that service from me, they would be getting it from somewhere else,” he said. “Initially it wasn’t important for me, but as my practice matured, I wanted to make sure that I was comprehensive.”
Start cautiously. Think safety first. “I tell people that starting a cosmetic practice is like baseball: don’t try to hit home runs,” Dr. Ibrahimi said. “Just aim for base hits and keep your patients happy. Make sure you deliver safe, good results.” This means knowing everything possible about the devices used in the office, because if the use of a laser is delegated to a staff member and a problem arises, “you have to know everything about how that device works so that you can troubleshoot,” he said. “A lot of problems that arise are from lack of intimacy with your device.”
Seek knowledge. Attend courses in cosmetic dermatology and read literature from journals like Dermatologic Surgery and Lasers in Surgery and Medicine, he advised. “People will see the success, but they won’t know how much hard work it takes to get there,” he said. “You have to develop your reputation to develop the kind of practice that you want.”
Understand the business of aesthetics. Most energy devices carry a steep price tag, and leasing or financing devices come with a monthly payment, he said. “Make sure that what you’re bringing in on that device is going to be sufficient to cover the monthly payment. With something like tissue microcoring, you don’t have to use that five times a day to cover that lease payment. But if you have a vascular laser, you probably need to be treating more than a couple patients per day to make that lease payment. If you can recover the amount the device costs in about a year, that’s going to be a good investment. Many devices come with consumables, so you have to remember that.”
Don’t be afraid to be unique/change directions. Becoming an early adopter of new technologies and procedures can make someone stand out. “Other providers feel more comfortable waiting to allow more data to come out about a new technology before they make a purchase,” he said. “But if you’re established and have a busy practice, that’s an opportunity that can draw people in.”
Have patience and realistic expectations. It’s smart to offer a variety of services, he said, such as medical or surgical dermatology in addition to cosmetic dermatology. “That’s going to help you through any kind of economic downturn,” he said. “Success depends on a lot of factors going right. Make sure you set short- and long-term goals.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
SAN DIEGO – When Omar A. Ibrahimi, MD, PhD, opened his own cosmetic dermatology practice in Stamford, Conn., in 2012, he sensed that he had his work cut out for him.
“I was a fellowship-trained Mohs surgeon who wanted to do aesthetics,” Dr. Ibrahimi, medical director of the Connecticut Skin Institute, recalled during the annual Masters of Aesthetics Symposium. “I was in a geographic area that was new to me. I didn’t know any referring doctors, but I started to network and tried to grow my practice.”
Someone once told him that the “three As” of being a medical specialist are “Available, Affable, and Ability,” so he applied that principle as he began to cultivate relationships with physicians in his geographic area. “I told my referring doctors, ‘If you’re kind enough to send me Mohs cases, I’ll help you out if there’s something you don’t like doing, whether it’s a nail biopsy or treating male genital warts,’” he said. “You want to make it easy for doctors to refer to you, but you also want to make their lives easier.”
Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery, offered . They include:
Know yourself. Do what you love to do, not what you feel like you should do. “Whatever you’re doing in your practice, it should be something that you’re passionate about and excited about,” he said. “I do a mix of Mohs surgery and procedural aesthetic dermatology. Most of my practice is shaped toward energy-based devices and laser procedures. Pick the things that you enjoy doing and try to deliver good results.”
Know your patients. When dermatologists who plan to open their own practice ask Dr. Ibrahimi what kind of laser they should buy, he typically responds by asking them to consider what procedures their patients are asking for. “Depending on where you are geographically and the economic profile of the community in which you practice, it can be a different answer,” Dr. Ibrahimi said. “If you practice in the Northeast and do a lot of medical dermatology, it might mean getting a vascular laser to treat rosacea. If you’re in Southern California, treating pigment might be a bigger concern than treating rosacea.” The annual ASDS Survey on Dermatologic Procedures provides a snapshot of trends and can be useful for decision-making, he said.
Know your practice. “Make sure you are capable of entering the aesthetics field,” he advised. “You cannot have a practice that runs like the DMV, with people waiting 30 to 40 minutes to be seen.” Proper training of staff is also key and representatives from device and injectable companies can provide advice and support. As for marketing, some dermatologists hire a public relations agency, but Dr. Ibrahimi finds that the best source of his referrals is word of mouth. “If I do a good job taking care of patients, they will send their friends and family over to me, but social media is also important,” he said. Taking quality before-and-after photos, and obtaining consent from patients to use them online in educational posts is a good approach, he noted.
Know your market. When Dr. Ibrahimi first opened his practice, offering laser hair removal was not a priority because so many other dermatologists and medical spas in his area were already providing it. With time, though, he added laser hair removal to his menu of treatment offerings because “I knew that if my patients weren’t getting that service from me, they would be getting it from somewhere else,” he said. “Initially it wasn’t important for me, but as my practice matured, I wanted to make sure that I was comprehensive.”
Start cautiously. Think safety first. “I tell people that starting a cosmetic practice is like baseball: don’t try to hit home runs,” Dr. Ibrahimi said. “Just aim for base hits and keep your patients happy. Make sure you deliver safe, good results.” This means knowing everything possible about the devices used in the office, because if the use of a laser is delegated to a staff member and a problem arises, “you have to know everything about how that device works so that you can troubleshoot,” he said. “A lot of problems that arise are from lack of intimacy with your device.”
Seek knowledge. Attend courses in cosmetic dermatology and read literature from journals like Dermatologic Surgery and Lasers in Surgery and Medicine, he advised. “People will see the success, but they won’t know how much hard work it takes to get there,” he said. “You have to develop your reputation to develop the kind of practice that you want.”
Understand the business of aesthetics. Most energy devices carry a steep price tag, and leasing or financing devices come with a monthly payment, he said. “Make sure that what you’re bringing in on that device is going to be sufficient to cover the monthly payment. With something like tissue microcoring, you don’t have to use that five times a day to cover that lease payment. But if you have a vascular laser, you probably need to be treating more than a couple patients per day to make that lease payment. If you can recover the amount the device costs in about a year, that’s going to be a good investment. Many devices come with consumables, so you have to remember that.”
Don’t be afraid to be unique/change directions. Becoming an early adopter of new technologies and procedures can make someone stand out. “Other providers feel more comfortable waiting to allow more data to come out about a new technology before they make a purchase,” he said. “But if you’re established and have a busy practice, that’s an opportunity that can draw people in.”
Have patience and realistic expectations. It’s smart to offer a variety of services, he said, such as medical or surgical dermatology in addition to cosmetic dermatology. “That’s going to help you through any kind of economic downturn,” he said. “Success depends on a lot of factors going right. Make sure you set short- and long-term goals.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
SAN DIEGO – When Omar A. Ibrahimi, MD, PhD, opened his own cosmetic dermatology practice in Stamford, Conn., in 2012, he sensed that he had his work cut out for him.
“I was a fellowship-trained Mohs surgeon who wanted to do aesthetics,” Dr. Ibrahimi, medical director of the Connecticut Skin Institute, recalled during the annual Masters of Aesthetics Symposium. “I was in a geographic area that was new to me. I didn’t know any referring doctors, but I started to network and tried to grow my practice.”
Someone once told him that the “three As” of being a medical specialist are “Available, Affable, and Ability,” so he applied that principle as he began to cultivate relationships with physicians in his geographic area. “I told my referring doctors, ‘If you’re kind enough to send me Mohs cases, I’ll help you out if there’s something you don’t like doing, whether it’s a nail biopsy or treating male genital warts,’” he said. “You want to make it easy for doctors to refer to you, but you also want to make their lives easier.”
Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery, offered . They include:
Know yourself. Do what you love to do, not what you feel like you should do. “Whatever you’re doing in your practice, it should be something that you’re passionate about and excited about,” he said. “I do a mix of Mohs surgery and procedural aesthetic dermatology. Most of my practice is shaped toward energy-based devices and laser procedures. Pick the things that you enjoy doing and try to deliver good results.”
Know your patients. When dermatologists who plan to open their own practice ask Dr. Ibrahimi what kind of laser they should buy, he typically responds by asking them to consider what procedures their patients are asking for. “Depending on where you are geographically and the economic profile of the community in which you practice, it can be a different answer,” Dr. Ibrahimi said. “If you practice in the Northeast and do a lot of medical dermatology, it might mean getting a vascular laser to treat rosacea. If you’re in Southern California, treating pigment might be a bigger concern than treating rosacea.” The annual ASDS Survey on Dermatologic Procedures provides a snapshot of trends and can be useful for decision-making, he said.
Know your practice. “Make sure you are capable of entering the aesthetics field,” he advised. “You cannot have a practice that runs like the DMV, with people waiting 30 to 40 minutes to be seen.” Proper training of staff is also key and representatives from device and injectable companies can provide advice and support. As for marketing, some dermatologists hire a public relations agency, but Dr. Ibrahimi finds that the best source of his referrals is word of mouth. “If I do a good job taking care of patients, they will send their friends and family over to me, but social media is also important,” he said. Taking quality before-and-after photos, and obtaining consent from patients to use them online in educational posts is a good approach, he noted.
Know your market. When Dr. Ibrahimi first opened his practice, offering laser hair removal was not a priority because so many other dermatologists and medical spas in his area were already providing it. With time, though, he added laser hair removal to his menu of treatment offerings because “I knew that if my patients weren’t getting that service from me, they would be getting it from somewhere else,” he said. “Initially it wasn’t important for me, but as my practice matured, I wanted to make sure that I was comprehensive.”
Start cautiously. Think safety first. “I tell people that starting a cosmetic practice is like baseball: don’t try to hit home runs,” Dr. Ibrahimi said. “Just aim for base hits and keep your patients happy. Make sure you deliver safe, good results.” This means knowing everything possible about the devices used in the office, because if the use of a laser is delegated to a staff member and a problem arises, “you have to know everything about how that device works so that you can troubleshoot,” he said. “A lot of problems that arise are from lack of intimacy with your device.”
Seek knowledge. Attend courses in cosmetic dermatology and read literature from journals like Dermatologic Surgery and Lasers in Surgery and Medicine, he advised. “People will see the success, but they won’t know how much hard work it takes to get there,” he said. “You have to develop your reputation to develop the kind of practice that you want.”
Understand the business of aesthetics. Most energy devices carry a steep price tag, and leasing or financing devices come with a monthly payment, he said. “Make sure that what you’re bringing in on that device is going to be sufficient to cover the monthly payment. With something like tissue microcoring, you don’t have to use that five times a day to cover that lease payment. But if you have a vascular laser, you probably need to be treating more than a couple patients per day to make that lease payment. If you can recover the amount the device costs in about a year, that’s going to be a good investment. Many devices come with consumables, so you have to remember that.”
Don’t be afraid to be unique/change directions. Becoming an early adopter of new technologies and procedures can make someone stand out. “Other providers feel more comfortable waiting to allow more data to come out about a new technology before they make a purchase,” he said. “But if you’re established and have a busy practice, that’s an opportunity that can draw people in.”
Have patience and realistic expectations. It’s smart to offer a variety of services, he said, such as medical or surgical dermatology in addition to cosmetic dermatology. “That’s going to help you through any kind of economic downturn,” he said. “Success depends on a lot of factors going right. Make sure you set short- and long-term goals.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
AT MOAS 2022
For optimal results, fractional RF microneedling requires multiple treatments
SAN DIEGO – , according to Catherine M. DiGiorgio, MD.
Most core fractional RF microneedling indications – acne scars, rhytides, skin tightening – require multiple treatments, Dr. DiGiorgio, a laser and cosmetic dermatologist who practices in Boston, said at the annual Masters of Aesthetics Symposium. “That’s an important expectation to set for your patients,” she said. “You also want to select depth and density parameters based on pathophysiology of the condition being treated, and combination treatments always provide the best results. So, whether you’re treating someone for acne scars or rhytides, you want to treat them for their erythema or their dermatoheliosis. The same goes for skin tightening procedures.”
Many nonpolar and bipolar devices are available for use, most of which feature adjustable depths and energies. Tips can be insulated or noninsulated. Generally, the insulated tips are safer for darker skin types because the energy is not delivered to the epidermis. However, the Sylfirm X device from Benev has a noninsulated tip but is safe for all skin types because the energy is delivered from the tip of a conically shaped needle and moves proximally but never reaches the epidermis, said Dr. DiGiorgio. Continuous wave mode is used for tightening and wrinkles while pulsed mode is used for pigment and vascular lesions.
Treatment with most fractional RF microneedling devices is painful so topical anesthesia is required. Dr. DiGiorgio typically uses topical 23% lidocaine and 7% tetracaine. The downtime varies depending on which device is being used. For anesthesia prior to aggressive fractional microneedle RF treatments such as with the Profound RF for skin tightening, Dr. DiGiorgio typically uses a Mesoram needle with a cocktail of 30 ccs of 2% lidocaine with epinephrine, 15 ccs of bicarbonate, and 5 ccs of saline. “More aggressive RF procedures can result in bruising for 7 to 8 days,” she said. “It can be covered with makeup. Wearing masks during the COVID-19 pandemic have also helped patients cover the bruising.”
In her clinical experience, the ideal patient for skin tightening with fractional RF microneedling has mild to moderate skin laxity that does not require surgical intervention. “Nonsurgical treatments provide nonsurgical results,” she said. “If a patient comes in holding their skin back and there is a lot of laxity, this is not going to be the right treatment for that person.”
Dr. DiGiorgio offers fractional RF microneedling in the context of a full-face rejuvenation. She begins by addressing volume loss and dynamic rhytides with injectables prior to skin tightening devices such as fractional RF microneedling or ultrasound-based tightening devices such as Sofwave or Ulthera (also referred to as Ultherapy). “You can add an ablative fractional to target deeper rhytides or pigment-targeting laser to address their dermatoheliosis, which will enhance their results,” she said. “Finally, you can follow up with a thread lift two weeks after the microneedle RF to achieve greater skin tightening. If the thread lift is performed before the microneedle RF, you want to wait about 2 months because the microneedle RF can damage the thread.”
Despite the limited efficacy for tissue tightening with fractional RF microneedling, “it’s a good alternative to lasers, especially for darker skin types,” she said. “Combination treatments will always enhance your results.”
Dr. DiGiorgio disclosed that she is a member of the advisory board for Quthero. She is also a consultant for Revelle and has received equipment from Acclaro.
SAN DIEGO – , according to Catherine M. DiGiorgio, MD.
Most core fractional RF microneedling indications – acne scars, rhytides, skin tightening – require multiple treatments, Dr. DiGiorgio, a laser and cosmetic dermatologist who practices in Boston, said at the annual Masters of Aesthetics Symposium. “That’s an important expectation to set for your patients,” she said. “You also want to select depth and density parameters based on pathophysiology of the condition being treated, and combination treatments always provide the best results. So, whether you’re treating someone for acne scars or rhytides, you want to treat them for their erythema or their dermatoheliosis. The same goes for skin tightening procedures.”
Many nonpolar and bipolar devices are available for use, most of which feature adjustable depths and energies. Tips can be insulated or noninsulated. Generally, the insulated tips are safer for darker skin types because the energy is not delivered to the epidermis. However, the Sylfirm X device from Benev has a noninsulated tip but is safe for all skin types because the energy is delivered from the tip of a conically shaped needle and moves proximally but never reaches the epidermis, said Dr. DiGiorgio. Continuous wave mode is used for tightening and wrinkles while pulsed mode is used for pigment and vascular lesions.
Treatment with most fractional RF microneedling devices is painful so topical anesthesia is required. Dr. DiGiorgio typically uses topical 23% lidocaine and 7% tetracaine. The downtime varies depending on which device is being used. For anesthesia prior to aggressive fractional microneedle RF treatments such as with the Profound RF for skin tightening, Dr. DiGiorgio typically uses a Mesoram needle with a cocktail of 30 ccs of 2% lidocaine with epinephrine, 15 ccs of bicarbonate, and 5 ccs of saline. “More aggressive RF procedures can result in bruising for 7 to 8 days,” she said. “It can be covered with makeup. Wearing masks during the COVID-19 pandemic have also helped patients cover the bruising.”
In her clinical experience, the ideal patient for skin tightening with fractional RF microneedling has mild to moderate skin laxity that does not require surgical intervention. “Nonsurgical treatments provide nonsurgical results,” she said. “If a patient comes in holding their skin back and there is a lot of laxity, this is not going to be the right treatment for that person.”
Dr. DiGiorgio offers fractional RF microneedling in the context of a full-face rejuvenation. She begins by addressing volume loss and dynamic rhytides with injectables prior to skin tightening devices such as fractional RF microneedling or ultrasound-based tightening devices such as Sofwave or Ulthera (also referred to as Ultherapy). “You can add an ablative fractional to target deeper rhytides or pigment-targeting laser to address their dermatoheliosis, which will enhance their results,” she said. “Finally, you can follow up with a thread lift two weeks after the microneedle RF to achieve greater skin tightening. If the thread lift is performed before the microneedle RF, you want to wait about 2 months because the microneedle RF can damage the thread.”
Despite the limited efficacy for tissue tightening with fractional RF microneedling, “it’s a good alternative to lasers, especially for darker skin types,” she said. “Combination treatments will always enhance your results.”
Dr. DiGiorgio disclosed that she is a member of the advisory board for Quthero. She is also a consultant for Revelle and has received equipment from Acclaro.
SAN DIEGO – , according to Catherine M. DiGiorgio, MD.
Most core fractional RF microneedling indications – acne scars, rhytides, skin tightening – require multiple treatments, Dr. DiGiorgio, a laser and cosmetic dermatologist who practices in Boston, said at the annual Masters of Aesthetics Symposium. “That’s an important expectation to set for your patients,” she said. “You also want to select depth and density parameters based on pathophysiology of the condition being treated, and combination treatments always provide the best results. So, whether you’re treating someone for acne scars or rhytides, you want to treat them for their erythema or their dermatoheliosis. The same goes for skin tightening procedures.”
Many nonpolar and bipolar devices are available for use, most of which feature adjustable depths and energies. Tips can be insulated or noninsulated. Generally, the insulated tips are safer for darker skin types because the energy is not delivered to the epidermis. However, the Sylfirm X device from Benev has a noninsulated tip but is safe for all skin types because the energy is delivered from the tip of a conically shaped needle and moves proximally but never reaches the epidermis, said Dr. DiGiorgio. Continuous wave mode is used for tightening and wrinkles while pulsed mode is used for pigment and vascular lesions.
Treatment with most fractional RF microneedling devices is painful so topical anesthesia is required. Dr. DiGiorgio typically uses topical 23% lidocaine and 7% tetracaine. The downtime varies depending on which device is being used. For anesthesia prior to aggressive fractional microneedle RF treatments such as with the Profound RF for skin tightening, Dr. DiGiorgio typically uses a Mesoram needle with a cocktail of 30 ccs of 2% lidocaine with epinephrine, 15 ccs of bicarbonate, and 5 ccs of saline. “More aggressive RF procedures can result in bruising for 7 to 8 days,” she said. “It can be covered with makeup. Wearing masks during the COVID-19 pandemic have also helped patients cover the bruising.”
In her clinical experience, the ideal patient for skin tightening with fractional RF microneedling has mild to moderate skin laxity that does not require surgical intervention. “Nonsurgical treatments provide nonsurgical results,” she said. “If a patient comes in holding their skin back and there is a lot of laxity, this is not going to be the right treatment for that person.”
Dr. DiGiorgio offers fractional RF microneedling in the context of a full-face rejuvenation. She begins by addressing volume loss and dynamic rhytides with injectables prior to skin tightening devices such as fractional RF microneedling or ultrasound-based tightening devices such as Sofwave or Ulthera (also referred to as Ultherapy). “You can add an ablative fractional to target deeper rhytides or pigment-targeting laser to address their dermatoheliosis, which will enhance their results,” she said. “Finally, you can follow up with a thread lift two weeks after the microneedle RF to achieve greater skin tightening. If the thread lift is performed before the microneedle RF, you want to wait about 2 months because the microneedle RF can damage the thread.”
Despite the limited efficacy for tissue tightening with fractional RF microneedling, “it’s a good alternative to lasers, especially for darker skin types,” she said. “Combination treatments will always enhance your results.”
Dr. DiGiorgio disclosed that she is a member of the advisory board for Quthero. She is also a consultant for Revelle and has received equipment from Acclaro.
AT MOAS 2022
‘Dr. Pimple Popper’ offers tips for building a social media presence
SAN DIEGO – In the fall of 2014, Sandra Lee, MD, posted a blackhead extraction video on her Instagram account, a decision that changed her professional life forever.
“I got these crazy comments,” Dr. Lee, a dermatologist who practices in Upland, Calif., recalled at the annual Masters of Aesthetics Symposium. “Either people loved it – they were obsessed – or they thought it was the most disgusting thing they’d ever seen. It created a strong reaction. Either way, they shared it with their friends.”
Soon after she started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious.” “I thought, ‘These videos are so amateur. They’re culling them from the Internet. Or, they’re pinning down their son at the beach and trying to squeeze out a blackhead,’ ” Dr. Lee said. “I thought, ‘I could give them pristine videos,’ ” and that is exactly what she did.
Turning to YouTube as a platform, she began to post videos showing everything from Mohs surgery and Botox injections to keloid removals and ear lobe repair surgeries. With this, . She also grew 16.2 million subscribers on TikTok, 4.5 million followers on Instagram, 2.9 million on Facebook, and 136,700 on Twitter.
About 80% of her followers are women who range between 18 and 40 years of age. “I have over 5 billion views on YouTube, which is mind-blowing,” she said. “That tells you something about the content. It’s not something people watch once. They watch it over and over again.” These include videos compiled as a “bedtime story.”
Dr. Lee offered the following pearls of advice for dermatologists looking to build and maintain a presence on social media:
Use it to showcase what makes you unique. Post what you do on social media, and people will find you. “It’s an opportunity to freely advertise,” Dr. Lee said. “I’m super nitpicky about posting good before-and-after photos. You can also show off how nice and warm and inviting your office is. People come to see me because they know my voice. They know how I interact with patients. That is reason for them enough to travel from far away to see me. It doesn’t mean that I’m the person who is best at treating whatever condition they have.”
Make it interesting. “I say that the special sauce is entertainment and education,” said Dr. Lee, who is in the fifth season of “Dr. Pimple Popper,” her TV show that airs internationally. “The only way you can draw people in is by entertaining them, catching their interest. But I try to trick them into educating them. Five-year-old kids come up to me now and know what a lipoma is. I’m proud of that.”
Be authentic. You may be using social media to promote your dermatology practice, but it’s important for followers to get a glimpse of your nonwork personality as well. Maybe that means posting a photo of yourself at a concert, baseball game, or dinner with family and friends. “Show that you have a sense of humor, because you want them to like you,” Dr. Lee added. “That’s why someone follows you, because they want to be your friend. They enjoy spending time with you on the Internet. It’s like gambling. In order to win, you have to play. So, you have to post.”
Avoid hot-button topics. “I don’t post about my kids, and I try to choose sponsorships wisely,” she said. “I do very few branding deals. Be careful about your brand and how you present yourself. Present yourself in an authentic way, but not in a way that hurts yourself or the dermatology profession.”
Be mindful of the time investment. “It’s like running a whole other business,” Dr. Lee said. “There are also trolls out there, so you have to have thick skin.”
Don’t sweat it if you don’t want to engage. “Not everybody wants to do it, and not everybody will be good at it, but that’s okay,” she said.
Dr. Lee reported having no relevant disclosures.
SAN DIEGO – In the fall of 2014, Sandra Lee, MD, posted a blackhead extraction video on her Instagram account, a decision that changed her professional life forever.
“I got these crazy comments,” Dr. Lee, a dermatologist who practices in Upland, Calif., recalled at the annual Masters of Aesthetics Symposium. “Either people loved it – they were obsessed – or they thought it was the most disgusting thing they’d ever seen. It created a strong reaction. Either way, they shared it with their friends.”
Soon after she started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious.” “I thought, ‘These videos are so amateur. They’re culling them from the Internet. Or, they’re pinning down their son at the beach and trying to squeeze out a blackhead,’ ” Dr. Lee said. “I thought, ‘I could give them pristine videos,’ ” and that is exactly what she did.
Turning to YouTube as a platform, she began to post videos showing everything from Mohs surgery and Botox injections to keloid removals and ear lobe repair surgeries. With this, . She also grew 16.2 million subscribers on TikTok, 4.5 million followers on Instagram, 2.9 million on Facebook, and 136,700 on Twitter.
About 80% of her followers are women who range between 18 and 40 years of age. “I have over 5 billion views on YouTube, which is mind-blowing,” she said. “That tells you something about the content. It’s not something people watch once. They watch it over and over again.” These include videos compiled as a “bedtime story.”
Dr. Lee offered the following pearls of advice for dermatologists looking to build and maintain a presence on social media:
Use it to showcase what makes you unique. Post what you do on social media, and people will find you. “It’s an opportunity to freely advertise,” Dr. Lee said. “I’m super nitpicky about posting good before-and-after photos. You can also show off how nice and warm and inviting your office is. People come to see me because they know my voice. They know how I interact with patients. That is reason for them enough to travel from far away to see me. It doesn’t mean that I’m the person who is best at treating whatever condition they have.”
Make it interesting. “I say that the special sauce is entertainment and education,” said Dr. Lee, who is in the fifth season of “Dr. Pimple Popper,” her TV show that airs internationally. “The only way you can draw people in is by entertaining them, catching their interest. But I try to trick them into educating them. Five-year-old kids come up to me now and know what a lipoma is. I’m proud of that.”
Be authentic. You may be using social media to promote your dermatology practice, but it’s important for followers to get a glimpse of your nonwork personality as well. Maybe that means posting a photo of yourself at a concert, baseball game, or dinner with family and friends. “Show that you have a sense of humor, because you want them to like you,” Dr. Lee added. “That’s why someone follows you, because they want to be your friend. They enjoy spending time with you on the Internet. It’s like gambling. In order to win, you have to play. So, you have to post.”
Avoid hot-button topics. “I don’t post about my kids, and I try to choose sponsorships wisely,” she said. “I do very few branding deals. Be careful about your brand and how you present yourself. Present yourself in an authentic way, but not in a way that hurts yourself or the dermatology profession.”
Be mindful of the time investment. “It’s like running a whole other business,” Dr. Lee said. “There are also trolls out there, so you have to have thick skin.”
Don’t sweat it if you don’t want to engage. “Not everybody wants to do it, and not everybody will be good at it, but that’s okay,” she said.
Dr. Lee reported having no relevant disclosures.
SAN DIEGO – In the fall of 2014, Sandra Lee, MD, posted a blackhead extraction video on her Instagram account, a decision that changed her professional life forever.
“I got these crazy comments,” Dr. Lee, a dermatologist who practices in Upland, Calif., recalled at the annual Masters of Aesthetics Symposium. “Either people loved it – they were obsessed – or they thought it was the most disgusting thing they’d ever seen. It created a strong reaction. Either way, they shared it with their friends.”
Soon after she started posting videos, she discovered Reddit, which has a subreddit for “popping addicts” and the “pop-curious.” “I thought, ‘These videos are so amateur. They’re culling them from the Internet. Or, they’re pinning down their son at the beach and trying to squeeze out a blackhead,’ ” Dr. Lee said. “I thought, ‘I could give them pristine videos,’ ” and that is exactly what she did.
Turning to YouTube as a platform, she began to post videos showing everything from Mohs surgery and Botox injections to keloid removals and ear lobe repair surgeries. With this, . She also grew 16.2 million subscribers on TikTok, 4.5 million followers on Instagram, 2.9 million on Facebook, and 136,700 on Twitter.
About 80% of her followers are women who range between 18 and 40 years of age. “I have over 5 billion views on YouTube, which is mind-blowing,” she said. “That tells you something about the content. It’s not something people watch once. They watch it over and over again.” These include videos compiled as a “bedtime story.”
Dr. Lee offered the following pearls of advice for dermatologists looking to build and maintain a presence on social media:
Use it to showcase what makes you unique. Post what you do on social media, and people will find you. “It’s an opportunity to freely advertise,” Dr. Lee said. “I’m super nitpicky about posting good before-and-after photos. You can also show off how nice and warm and inviting your office is. People come to see me because they know my voice. They know how I interact with patients. That is reason for them enough to travel from far away to see me. It doesn’t mean that I’m the person who is best at treating whatever condition they have.”
Make it interesting. “I say that the special sauce is entertainment and education,” said Dr. Lee, who is in the fifth season of “Dr. Pimple Popper,” her TV show that airs internationally. “The only way you can draw people in is by entertaining them, catching their interest. But I try to trick them into educating them. Five-year-old kids come up to me now and know what a lipoma is. I’m proud of that.”
Be authentic. You may be using social media to promote your dermatology practice, but it’s important for followers to get a glimpse of your nonwork personality as well. Maybe that means posting a photo of yourself at a concert, baseball game, or dinner with family and friends. “Show that you have a sense of humor, because you want them to like you,” Dr. Lee added. “That’s why someone follows you, because they want to be your friend. They enjoy spending time with you on the Internet. It’s like gambling. In order to win, you have to play. So, you have to post.”
Avoid hot-button topics. “I don’t post about my kids, and I try to choose sponsorships wisely,” she said. “I do very few branding deals. Be careful about your brand and how you present yourself. Present yourself in an authentic way, but not in a way that hurts yourself or the dermatology profession.”
Be mindful of the time investment. “It’s like running a whole other business,” Dr. Lee said. “There are also trolls out there, so you have to have thick skin.”
Don’t sweat it if you don’t want to engage. “Not everybody wants to do it, and not everybody will be good at it, but that’s okay,” she said.
Dr. Lee reported having no relevant disclosures.
AT MOAS 2022
Experts dispel incorrect dogmas in aesthetic medicine
At least once a week,
Those images may help Dr. Stankiewicz understand patient preferences in terms of lip size and proportion, but she points out that shape is unique to each person. “I tell them: ‘All we can do is enhance that lip shape with filler. We can’t give you somebody else’s lip shape with an injection of filler.’ ”
During a virtual course on laser and aesthetic skin therapy, she and Omar A. Ibrahimi, MD, PhD, dispelled this and other false dogmas that they hear from some clinicians who practice aesthetic medicine and the patients who see them.
Wait 1 year before treating traumatic and surgical scars with vascular and fractional CO2 lasers. “I don’t think this is controversial anymore, because there is a boatload of data, which has shown that early treatment can prevent hypertrophic scarring and promote scar maturation,” said Dr. Stankiewicz, who practices dermatology in Park City, Utah. “Histology has also shown more organized dermal collagen from early treatment. Of course, there will be situations where you may want to hold off, like doing an ablative fractional [laser treatment] over the scar of a joint replacement ... where you may risk infection.” In her clinic, she routinely treats scars on the same day as suture removal, “as long as the healing looks appropriate.”
Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, Stamford, also jumps on treating scars early. For a patient with postacne erythema, for example, he will use a pulsed-dye laser, which he believes will prevent scars from becoming atrophic.
Used equipment is a better investment than new equipment. While purchasing used laser and light devices can save money, especially when starting out, be wary of potential pitfalls, including the fact that many devices have disposable tips. “If your laser isn’t certified or you’re not the authorized owner of the device, you won’t be able to buy the disposables,” Dr. Stankiewicz noted. “So, before you buy a used device, ensure that you can buy them.”
Also, consider the cost of service if the device breaks down, she advised. Some lasers are complicated to service and others have codes set by the manufacturer so that only contracted engineers can work on them. “Otherwise, third-party engineers and service providers have to figure out how to crack the code to get into the machine,” she said. “If you’re in the situation where you have to ask the manufacturer to service your device, you have to pay a lot of money to recertify your device. Then you’ve lost all the savings you thought you made by buying a used machine.” She prefers to negotiate a good deal on a new device. “Often, a very good deal on a new device can rival the offer of a used one.”
Dr. Ibrahimi recalled buying a used fractional laser that came with a 30-day guarantee, but it stopped working around day 45. “I didn’t have much recourse there,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “You can’t go back to the company [for repair] unless you pay a recertification fee.”
Avoid exercise after Botox treatment. Although inverted yoga poses and lying down should be avoided for several hours after receiving Botox, there are no other limits to other forms of exercise post treatment, Dr. Stankiewicz said. If she suspects that a patient will develop bruising on one or more injection sites, she treats the areas with a laser. “Doing this on the same day as Botox treatment doesn’t always stop or treat bruising, many times it does.”
Another myth she hears is that it is not safe to fly in an airplane after Botox treatment. “That recommendation comes from the fact that the atmospheric pressure is lower in an airplane, so we worry about the risk of Botox spread,” Dr. Stankiewicz said. “But I practice at 7,000 feet above sea level, which is the same atmospheric pressure as that in an airplane,” she added, noting Botox is administered throughout the day in her practice and she does not see increased complications or worry about spread.
Clinician self-treatment is okay. In the opinion of Dr. Stankiewicz, aesthetic clinicians who treat themselves “have a fool for a patient.” She added: “Although no one is going to blame you and may not even know if you give yourself a little Botox touch-up at home, glorifying self-treatment on social media must stop. It’s dangerous and it can be ineffective.”
Self-treatment can also impair judgment and the objectivity of cosmetic therapies. “Also, when you’re pointing a laser at your own face and posting it on social media, it gives viewers the impression that this is not a serious medical treatment when it really is,” she emphasized. In addition, “when you treat yourself, you lose the ability to see the proper clinical endpoint. You also lose the ability to see the angle and the appropriate position for injection to avoid intervascular occlusion.”
Neither Dr. Stankiewicz nor Dr. Ibrahimi reported having relevant financial disclosures.
At least once a week,
Those images may help Dr. Stankiewicz understand patient preferences in terms of lip size and proportion, but she points out that shape is unique to each person. “I tell them: ‘All we can do is enhance that lip shape with filler. We can’t give you somebody else’s lip shape with an injection of filler.’ ”
During a virtual course on laser and aesthetic skin therapy, she and Omar A. Ibrahimi, MD, PhD, dispelled this and other false dogmas that they hear from some clinicians who practice aesthetic medicine and the patients who see them.
Wait 1 year before treating traumatic and surgical scars with vascular and fractional CO2 lasers. “I don’t think this is controversial anymore, because there is a boatload of data, which has shown that early treatment can prevent hypertrophic scarring and promote scar maturation,” said Dr. Stankiewicz, who practices dermatology in Park City, Utah. “Histology has also shown more organized dermal collagen from early treatment. Of course, there will be situations where you may want to hold off, like doing an ablative fractional [laser treatment] over the scar of a joint replacement ... where you may risk infection.” In her clinic, she routinely treats scars on the same day as suture removal, “as long as the healing looks appropriate.”
Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, Stamford, also jumps on treating scars early. For a patient with postacne erythema, for example, he will use a pulsed-dye laser, which he believes will prevent scars from becoming atrophic.
Used equipment is a better investment than new equipment. While purchasing used laser and light devices can save money, especially when starting out, be wary of potential pitfalls, including the fact that many devices have disposable tips. “If your laser isn’t certified or you’re not the authorized owner of the device, you won’t be able to buy the disposables,” Dr. Stankiewicz noted. “So, before you buy a used device, ensure that you can buy them.”
Also, consider the cost of service if the device breaks down, she advised. Some lasers are complicated to service and others have codes set by the manufacturer so that only contracted engineers can work on them. “Otherwise, third-party engineers and service providers have to figure out how to crack the code to get into the machine,” she said. “If you’re in the situation where you have to ask the manufacturer to service your device, you have to pay a lot of money to recertify your device. Then you’ve lost all the savings you thought you made by buying a used machine.” She prefers to negotiate a good deal on a new device. “Often, a very good deal on a new device can rival the offer of a used one.”
Dr. Ibrahimi recalled buying a used fractional laser that came with a 30-day guarantee, but it stopped working around day 45. “I didn’t have much recourse there,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “You can’t go back to the company [for repair] unless you pay a recertification fee.”
Avoid exercise after Botox treatment. Although inverted yoga poses and lying down should be avoided for several hours after receiving Botox, there are no other limits to other forms of exercise post treatment, Dr. Stankiewicz said. If she suspects that a patient will develop bruising on one or more injection sites, she treats the areas with a laser. “Doing this on the same day as Botox treatment doesn’t always stop or treat bruising, many times it does.”
Another myth she hears is that it is not safe to fly in an airplane after Botox treatment. “That recommendation comes from the fact that the atmospheric pressure is lower in an airplane, so we worry about the risk of Botox spread,” Dr. Stankiewicz said. “But I practice at 7,000 feet above sea level, which is the same atmospheric pressure as that in an airplane,” she added, noting Botox is administered throughout the day in her practice and she does not see increased complications or worry about spread.
Clinician self-treatment is okay. In the opinion of Dr. Stankiewicz, aesthetic clinicians who treat themselves “have a fool for a patient.” She added: “Although no one is going to blame you and may not even know if you give yourself a little Botox touch-up at home, glorifying self-treatment on social media must stop. It’s dangerous and it can be ineffective.”
Self-treatment can also impair judgment and the objectivity of cosmetic therapies. “Also, when you’re pointing a laser at your own face and posting it on social media, it gives viewers the impression that this is not a serious medical treatment when it really is,” she emphasized. In addition, “when you treat yourself, you lose the ability to see the proper clinical endpoint. You also lose the ability to see the angle and the appropriate position for injection to avoid intervascular occlusion.”
Neither Dr. Stankiewicz nor Dr. Ibrahimi reported having relevant financial disclosures.
At least once a week,
Those images may help Dr. Stankiewicz understand patient preferences in terms of lip size and proportion, but she points out that shape is unique to each person. “I tell them: ‘All we can do is enhance that lip shape with filler. We can’t give you somebody else’s lip shape with an injection of filler.’ ”
During a virtual course on laser and aesthetic skin therapy, she and Omar A. Ibrahimi, MD, PhD, dispelled this and other false dogmas that they hear from some clinicians who practice aesthetic medicine and the patients who see them.
Wait 1 year before treating traumatic and surgical scars with vascular and fractional CO2 lasers. “I don’t think this is controversial anymore, because there is a boatload of data, which has shown that early treatment can prevent hypertrophic scarring and promote scar maturation,” said Dr. Stankiewicz, who practices dermatology in Park City, Utah. “Histology has also shown more organized dermal collagen from early treatment. Of course, there will be situations where you may want to hold off, like doing an ablative fractional [laser treatment] over the scar of a joint replacement ... where you may risk infection.” In her clinic, she routinely treats scars on the same day as suture removal, “as long as the healing looks appropriate.”
Dr. Ibrahimi, a dermatologist and medical director of the Connecticut Skin Institute, Stamford, also jumps on treating scars early. For a patient with postacne erythema, for example, he will use a pulsed-dye laser, which he believes will prevent scars from becoming atrophic.
Used equipment is a better investment than new equipment. While purchasing used laser and light devices can save money, especially when starting out, be wary of potential pitfalls, including the fact that many devices have disposable tips. “If your laser isn’t certified or you’re not the authorized owner of the device, you won’t be able to buy the disposables,” Dr. Stankiewicz noted. “So, before you buy a used device, ensure that you can buy them.”
Also, consider the cost of service if the device breaks down, she advised. Some lasers are complicated to service and others have codes set by the manufacturer so that only contracted engineers can work on them. “Otherwise, third-party engineers and service providers have to figure out how to crack the code to get into the machine,” she said. “If you’re in the situation where you have to ask the manufacturer to service your device, you have to pay a lot of money to recertify your device. Then you’ve lost all the savings you thought you made by buying a used machine.” She prefers to negotiate a good deal on a new device. “Often, a very good deal on a new device can rival the offer of a used one.”
Dr. Ibrahimi recalled buying a used fractional laser that came with a 30-day guarantee, but it stopped working around day 45. “I didn’t have much recourse there,” he said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “You can’t go back to the company [for repair] unless you pay a recertification fee.”
Avoid exercise after Botox treatment. Although inverted yoga poses and lying down should be avoided for several hours after receiving Botox, there are no other limits to other forms of exercise post treatment, Dr. Stankiewicz said. If she suspects that a patient will develop bruising on one or more injection sites, she treats the areas with a laser. “Doing this on the same day as Botox treatment doesn’t always stop or treat bruising, many times it does.”
Another myth she hears is that it is not safe to fly in an airplane after Botox treatment. “That recommendation comes from the fact that the atmospheric pressure is lower in an airplane, so we worry about the risk of Botox spread,” Dr. Stankiewicz said. “But I practice at 7,000 feet above sea level, which is the same atmospheric pressure as that in an airplane,” she added, noting Botox is administered throughout the day in her practice and she does not see increased complications or worry about spread.
Clinician self-treatment is okay. In the opinion of Dr. Stankiewicz, aesthetic clinicians who treat themselves “have a fool for a patient.” She added: “Although no one is going to blame you and may not even know if you give yourself a little Botox touch-up at home, glorifying self-treatment on social media must stop. It’s dangerous and it can be ineffective.”
Self-treatment can also impair judgment and the objectivity of cosmetic therapies. “Also, when you’re pointing a laser at your own face and posting it on social media, it gives viewers the impression that this is not a serious medical treatment when it really is,” she emphasized. In addition, “when you treat yourself, you lose the ability to see the proper clinical endpoint. You also lose the ability to see the angle and the appropriate position for injection to avoid intervascular occlusion.”
Neither Dr. Stankiewicz nor Dr. Ibrahimi reported having relevant financial disclosures.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Researchers use AI to diagnose infantile hemangioma
a proof-of-concept study reported.
Early diagnosis of infantile hemangiomas “is essential, as there is a narrow window of opportunity to treat high-risk lesions,” April J. Zhang, MD, and coauthors noted in the study. “AI algorithms optimized for image classification through use of convolutional neural networks have been widely utilized to classify lesions in which images are readily standardized, such as skin cancers and onychomycosis.”
The results were published in Pediatric Dermatology.
Dr. Zhang, of the department of dermatology at the Medical College of Wisconsin, Milwaukee, and colleagues trained a convoluted neural network to diagnose infantile hemangiomas based on clinical images from pediatric dermatology patients treated at Children’s Wisconsin between 2002 and 2019.
They used Microsoft’s ResNet-50, a publicly available network architecture, to train a binary infantile hemangioma classifier to group images as infantile hemangiomas or non–infantile hemangiomas. The team randomly split data from the model into training, validation, and test groups.
The preliminary data set contained 14,811 images, about half of which were facial lesions. The training group of images achieved an accuracy of 61.5%. Next, Dr. Zhang and colleagues limited the data set to facial-only lesions and removed poor-quality images, which left 5,834 images in the final data set: 4,110 infantile hemangiomas and 1,724 non–infantile hemangiomas. This model achieved an overall accuracy of 91.7%, with a sensitivity of 93% and a specificity of 90.5%.
“Our study is the first to demonstrate the applicability of AI in the pediatric dermatology population,” the authors wrote. “With current nationwide shortages in pediatric dermatologists, AI has the potential to improve patient access and outcomes through enhanced rapid diagnostic capabilities.”
They acknowledged certain limitations of the study, including a data set with greater numbers of infantile hemangiomas, compared with non–infantile hemangiomas.
“Random oversampling of the non–infantile hemangioma data set was used to combat this but may lead to model overfitting, where a model performs well on its training data but is unable to generalize to new data,” they wrote. “As infantile hemangiomas are rarely biopsied, expert clinical diagnoses were used as the gold standard without pathologic confirmation.”
The authors reported having no financial disclosures.
a proof-of-concept study reported.
Early diagnosis of infantile hemangiomas “is essential, as there is a narrow window of opportunity to treat high-risk lesions,” April J. Zhang, MD, and coauthors noted in the study. “AI algorithms optimized for image classification through use of convolutional neural networks have been widely utilized to classify lesions in which images are readily standardized, such as skin cancers and onychomycosis.”
The results were published in Pediatric Dermatology.
Dr. Zhang, of the department of dermatology at the Medical College of Wisconsin, Milwaukee, and colleagues trained a convoluted neural network to diagnose infantile hemangiomas based on clinical images from pediatric dermatology patients treated at Children’s Wisconsin between 2002 and 2019.
They used Microsoft’s ResNet-50, a publicly available network architecture, to train a binary infantile hemangioma classifier to group images as infantile hemangiomas or non–infantile hemangiomas. The team randomly split data from the model into training, validation, and test groups.
The preliminary data set contained 14,811 images, about half of which were facial lesions. The training group of images achieved an accuracy of 61.5%. Next, Dr. Zhang and colleagues limited the data set to facial-only lesions and removed poor-quality images, which left 5,834 images in the final data set: 4,110 infantile hemangiomas and 1,724 non–infantile hemangiomas. This model achieved an overall accuracy of 91.7%, with a sensitivity of 93% and a specificity of 90.5%.
“Our study is the first to demonstrate the applicability of AI in the pediatric dermatology population,” the authors wrote. “With current nationwide shortages in pediatric dermatologists, AI has the potential to improve patient access and outcomes through enhanced rapid diagnostic capabilities.”
They acknowledged certain limitations of the study, including a data set with greater numbers of infantile hemangiomas, compared with non–infantile hemangiomas.
“Random oversampling of the non–infantile hemangioma data set was used to combat this but may lead to model overfitting, where a model performs well on its training data but is unable to generalize to new data,” they wrote. “As infantile hemangiomas are rarely biopsied, expert clinical diagnoses were used as the gold standard without pathologic confirmation.”
The authors reported having no financial disclosures.
a proof-of-concept study reported.
Early diagnosis of infantile hemangiomas “is essential, as there is a narrow window of opportunity to treat high-risk lesions,” April J. Zhang, MD, and coauthors noted in the study. “AI algorithms optimized for image classification through use of convolutional neural networks have been widely utilized to classify lesions in which images are readily standardized, such as skin cancers and onychomycosis.”
The results were published in Pediatric Dermatology.
Dr. Zhang, of the department of dermatology at the Medical College of Wisconsin, Milwaukee, and colleagues trained a convoluted neural network to diagnose infantile hemangiomas based on clinical images from pediatric dermatology patients treated at Children’s Wisconsin between 2002 and 2019.
They used Microsoft’s ResNet-50, a publicly available network architecture, to train a binary infantile hemangioma classifier to group images as infantile hemangiomas or non–infantile hemangiomas. The team randomly split data from the model into training, validation, and test groups.
The preliminary data set contained 14,811 images, about half of which were facial lesions. The training group of images achieved an accuracy of 61.5%. Next, Dr. Zhang and colleagues limited the data set to facial-only lesions and removed poor-quality images, which left 5,834 images in the final data set: 4,110 infantile hemangiomas and 1,724 non–infantile hemangiomas. This model achieved an overall accuracy of 91.7%, with a sensitivity of 93% and a specificity of 90.5%.
“Our study is the first to demonstrate the applicability of AI in the pediatric dermatology population,” the authors wrote. “With current nationwide shortages in pediatric dermatologists, AI has the potential to improve patient access and outcomes through enhanced rapid diagnostic capabilities.”
They acknowledged certain limitations of the study, including a data set with greater numbers of infantile hemangiomas, compared with non–infantile hemangiomas.
“Random oversampling of the non–infantile hemangioma data set was used to combat this but may lead to model overfitting, where a model performs well on its training data but is unable to generalize to new data,” they wrote. “As infantile hemangiomas are rarely biopsied, expert clinical diagnoses were used as the gold standard without pathologic confirmation.”
The authors reported having no financial disclosures.
FROM PEDIATRIC DERMATOLOGY
Parental atopic dermatitis, asthma linked to risk of AD in offspring
of life, an analysis of a large birth cohort found.
“The prevalence of AD in children has increased dramatically in recent years, and most studies reporting the impact of parental atopic history on AD are based on older data,” wrote the study authors, led by Cathal O’Connor, MD. “Given the recent interest in early intervention to prevent AD and other allergic diseases, enhanced early identification of infants at risk of AD is increasingly important.”
The detailed analysis of AD risk associated with parental atopy in early life “may help to risk stratify infants to optimize early interventions for prevention or early treatment of AD,” they wrote.
The study was published in Pediatric Dermatology.
For the analysis, Dr. O’Connor of the department of pediatrics and child health at University College Cork (Ireland) and colleagues conducted a secondary analysis of the Cork Babies After Scope: Evaluating the Longitudinal Impact Using Neurological and Nutritional Endpoints (BASELINE) Birth Cohort Study.
The study recruited 2,183 healthy first-born babies between August 2009 and October 2011 to examine the effects of environmental factors during pregnancy and infancy on childhood health and development. Skin barrier assessments were performed at birth, 2 months, 6 months, 12 months, and 24 months using a validated open chamber system to measure transepidermal water loss.
Parental atopy was self-reported at 2 months. Parents were asked at 2 months if the infant had an “itchy rash on the face or in the folds of the arms or legs,” as a screening question for AD. Experienced health care personnel used UK Working Party criteria to diagnose AD at 6, 12, and 24 months.
Complete data on AD status was available for 1,505 children in the cohort. Dr. O’Connor and colleagues calculated an overall AD prevalence of 18.6% at 6 months, 15.2% at 12 months, and 16.5% at 24 months.
Overall prevalence of AD was highest at 6 months. The study showed a similar or slightly higher impact of paternal atopy on offspring AD development, compared to maternal atopy.
Multivariable logistic regression analysis revealed that the odds of AD were 1.57 at 6 months and 1.66 at 12 months for maternal AD; 1.90 at 6 months and 1.85 at 24 months for paternal AD; 1.76 at 6 months and 1.75 at 12 months for maternal asthma; and 1.70 at 6 months, 1.86 at 12 months, and 1.99 at 24 months for paternal asthma.
“Parental allergic rhinitis was not associated with AD in offspring in the first 2 years, except for maternal rhinitis at 24 months [an adjusted odds ratio of 1.79],” the authors wrote. “The genetic predisposition to allergic rhinitis, given the key role of aeroallergen sensitization in its pathogenesis, may not be associated with early onset AD, but may have a greater impact in later onset or persistent AD.”
The authors acknowledged certain limitations of the study, including the fact that it was a secondary data analysis, and that parental AD, asthma, and rhinitis were self-reported, “which may reduce reliability and may contribute to the differences seen between the impact of maternal and paternal reported atopy on offspring,” they wrote. “Data on siblings were not captured, as participants in the study were first-born children. Filaggrin mutational analysis was not performed, which would have provided richer detail.”
Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, who was asked to comment on the work, said that the study confirms the well-known association between parental atopy and the risk of atopy in offspring, which has been shown in several studies dating back decades.
“The authors try to parse risk based on maternal or paternal or biparental history of AD and/or asthma and/or rhinitis, but this type of nuanced analysis when diagnosis is based solely on parental report may be an over-reach,” she said.
“Given that this data supports the association between parental atopy and risk of AD in infants at various time points, the clinically relevant immediate next question is how can we leverage this knowledge to prevent onset of AD in infants at risk?” she said. “To date, interventions such as early introduction of emollients have been evaluated with mixed results.”
A recent Cochrane analysis concluded that, based on available data, skin care interventions such as emollient use during the first year of life in otherwise healthy infants is probably not effective for preventing eczema and may increase risk of skin infection.
“Effects of skin care interventions on risk of asthma are also uncertain,” said Dr. Cordoro, who is also chief of the division of pediatric dermatology at UCSF.
“In sum, this study offers additional data in support of the link between atopy in parents and offspring,” she said. “Understanding how to mitigate risk and prevent atopy requires unraveling of the complex interplay between genetic, environmental, immunologic, microbial and other factors. For now, dermatologists are unable to make broad evidence-based recommendations for otherwise healthy (i.e., with normal skin) but at-risk infants in terms of approaches to skin care that might prevent eczema and asthma.”
of life, an analysis of a large birth cohort found.
“The prevalence of AD in children has increased dramatically in recent years, and most studies reporting the impact of parental atopic history on AD are based on older data,” wrote the study authors, led by Cathal O’Connor, MD. “Given the recent interest in early intervention to prevent AD and other allergic diseases, enhanced early identification of infants at risk of AD is increasingly important.”
The detailed analysis of AD risk associated with parental atopy in early life “may help to risk stratify infants to optimize early interventions for prevention or early treatment of AD,” they wrote.
The study was published in Pediatric Dermatology.
For the analysis, Dr. O’Connor of the department of pediatrics and child health at University College Cork (Ireland) and colleagues conducted a secondary analysis of the Cork Babies After Scope: Evaluating the Longitudinal Impact Using Neurological and Nutritional Endpoints (BASELINE) Birth Cohort Study.
The study recruited 2,183 healthy first-born babies between August 2009 and October 2011 to examine the effects of environmental factors during pregnancy and infancy on childhood health and development. Skin barrier assessments were performed at birth, 2 months, 6 months, 12 months, and 24 months using a validated open chamber system to measure transepidermal water loss.
Parental atopy was self-reported at 2 months. Parents were asked at 2 months if the infant had an “itchy rash on the face or in the folds of the arms or legs,” as a screening question for AD. Experienced health care personnel used UK Working Party criteria to diagnose AD at 6, 12, and 24 months.
Complete data on AD status was available for 1,505 children in the cohort. Dr. O’Connor and colleagues calculated an overall AD prevalence of 18.6% at 6 months, 15.2% at 12 months, and 16.5% at 24 months.
Overall prevalence of AD was highest at 6 months. The study showed a similar or slightly higher impact of paternal atopy on offspring AD development, compared to maternal atopy.
Multivariable logistic regression analysis revealed that the odds of AD were 1.57 at 6 months and 1.66 at 12 months for maternal AD; 1.90 at 6 months and 1.85 at 24 months for paternal AD; 1.76 at 6 months and 1.75 at 12 months for maternal asthma; and 1.70 at 6 months, 1.86 at 12 months, and 1.99 at 24 months for paternal asthma.
“Parental allergic rhinitis was not associated with AD in offspring in the first 2 years, except for maternal rhinitis at 24 months [an adjusted odds ratio of 1.79],” the authors wrote. “The genetic predisposition to allergic rhinitis, given the key role of aeroallergen sensitization in its pathogenesis, may not be associated with early onset AD, but may have a greater impact in later onset or persistent AD.”
The authors acknowledged certain limitations of the study, including the fact that it was a secondary data analysis, and that parental AD, asthma, and rhinitis were self-reported, “which may reduce reliability and may contribute to the differences seen between the impact of maternal and paternal reported atopy on offspring,” they wrote. “Data on siblings were not captured, as participants in the study were first-born children. Filaggrin mutational analysis was not performed, which would have provided richer detail.”
Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, who was asked to comment on the work, said that the study confirms the well-known association between parental atopy and the risk of atopy in offspring, which has been shown in several studies dating back decades.
“The authors try to parse risk based on maternal or paternal or biparental history of AD and/or asthma and/or rhinitis, but this type of nuanced analysis when diagnosis is based solely on parental report may be an over-reach,” she said.
“Given that this data supports the association between parental atopy and risk of AD in infants at various time points, the clinically relevant immediate next question is how can we leverage this knowledge to prevent onset of AD in infants at risk?” she said. “To date, interventions such as early introduction of emollients have been evaluated with mixed results.”
A recent Cochrane analysis concluded that, based on available data, skin care interventions such as emollient use during the first year of life in otherwise healthy infants is probably not effective for preventing eczema and may increase risk of skin infection.
“Effects of skin care interventions on risk of asthma are also uncertain,” said Dr. Cordoro, who is also chief of the division of pediatric dermatology at UCSF.
“In sum, this study offers additional data in support of the link between atopy in parents and offspring,” she said. “Understanding how to mitigate risk and prevent atopy requires unraveling of the complex interplay between genetic, environmental, immunologic, microbial and other factors. For now, dermatologists are unable to make broad evidence-based recommendations for otherwise healthy (i.e., with normal skin) but at-risk infants in terms of approaches to skin care that might prevent eczema and asthma.”
of life, an analysis of a large birth cohort found.
“The prevalence of AD in children has increased dramatically in recent years, and most studies reporting the impact of parental atopic history on AD are based on older data,” wrote the study authors, led by Cathal O’Connor, MD. “Given the recent interest in early intervention to prevent AD and other allergic diseases, enhanced early identification of infants at risk of AD is increasingly important.”
The detailed analysis of AD risk associated with parental atopy in early life “may help to risk stratify infants to optimize early interventions for prevention or early treatment of AD,” they wrote.
The study was published in Pediatric Dermatology.
For the analysis, Dr. O’Connor of the department of pediatrics and child health at University College Cork (Ireland) and colleagues conducted a secondary analysis of the Cork Babies After Scope: Evaluating the Longitudinal Impact Using Neurological and Nutritional Endpoints (BASELINE) Birth Cohort Study.
The study recruited 2,183 healthy first-born babies between August 2009 and October 2011 to examine the effects of environmental factors during pregnancy and infancy on childhood health and development. Skin barrier assessments were performed at birth, 2 months, 6 months, 12 months, and 24 months using a validated open chamber system to measure transepidermal water loss.
Parental atopy was self-reported at 2 months. Parents were asked at 2 months if the infant had an “itchy rash on the face or in the folds of the arms or legs,” as a screening question for AD. Experienced health care personnel used UK Working Party criteria to diagnose AD at 6, 12, and 24 months.
Complete data on AD status was available for 1,505 children in the cohort. Dr. O’Connor and colleagues calculated an overall AD prevalence of 18.6% at 6 months, 15.2% at 12 months, and 16.5% at 24 months.
Overall prevalence of AD was highest at 6 months. The study showed a similar or slightly higher impact of paternal atopy on offspring AD development, compared to maternal atopy.
Multivariable logistic regression analysis revealed that the odds of AD were 1.57 at 6 months and 1.66 at 12 months for maternal AD; 1.90 at 6 months and 1.85 at 24 months for paternal AD; 1.76 at 6 months and 1.75 at 12 months for maternal asthma; and 1.70 at 6 months, 1.86 at 12 months, and 1.99 at 24 months for paternal asthma.
“Parental allergic rhinitis was not associated with AD in offspring in the first 2 years, except for maternal rhinitis at 24 months [an adjusted odds ratio of 1.79],” the authors wrote. “The genetic predisposition to allergic rhinitis, given the key role of aeroallergen sensitization in its pathogenesis, may not be associated with early onset AD, but may have a greater impact in later onset or persistent AD.”
The authors acknowledged certain limitations of the study, including the fact that it was a secondary data analysis, and that parental AD, asthma, and rhinitis were self-reported, “which may reduce reliability and may contribute to the differences seen between the impact of maternal and paternal reported atopy on offspring,” they wrote. “Data on siblings were not captured, as participants in the study were first-born children. Filaggrin mutational analysis was not performed, which would have provided richer detail.”
Kelly M. Cordoro, MD, professor of dermatology and pediatrics at the University of California, San Francisco, who was asked to comment on the work, said that the study confirms the well-known association between parental atopy and the risk of atopy in offspring, which has been shown in several studies dating back decades.
“The authors try to parse risk based on maternal or paternal or biparental history of AD and/or asthma and/or rhinitis, but this type of nuanced analysis when diagnosis is based solely on parental report may be an over-reach,” she said.
“Given that this data supports the association between parental atopy and risk of AD in infants at various time points, the clinically relevant immediate next question is how can we leverage this knowledge to prevent onset of AD in infants at risk?” she said. “To date, interventions such as early introduction of emollients have been evaluated with mixed results.”
A recent Cochrane analysis concluded that, based on available data, skin care interventions such as emollient use during the first year of life in otherwise healthy infants is probably not effective for preventing eczema and may increase risk of skin infection.
“Effects of skin care interventions on risk of asthma are also uncertain,” said Dr. Cordoro, who is also chief of the division of pediatric dermatology at UCSF.
“In sum, this study offers additional data in support of the link between atopy in parents and offspring,” she said. “Understanding how to mitigate risk and prevent atopy requires unraveling of the complex interplay between genetic, environmental, immunologic, microbial and other factors. For now, dermatologists are unable to make broad evidence-based recommendations for otherwise healthy (i.e., with normal skin) but at-risk infants in terms of approaches to skin care that might prevent eczema and asthma.”
FROM PEDIATRIC DERMATOLOGY
Study eyes sunscreens marketed to individuals with skin of color
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
, and more than 40% contain a UV blocker that may create a white cast.
Those are among the findings from a study by Michelle Xiong, a medical student at Brown University, Providence, R.I., and Erin M. Warshaw, MD, of the department of dermatology at Park Nicollet/Health Partners Health Services, Minneapolis, which was published online in the Journal of the American Academy of Dermatology.
“There is increasing awareness of the negative effects of ultraviolet (UV) light in individuals with skin of color (SOC), especially in regards to pigmentation disorders induced and/or exacerbated by UV exposure,” the authors wrote. “As a result, there has been a surge in sunscreens marketed to this population. We aimed to characterize cost, marketing claims, and potential allergenic ingredients in sunscreens marketed to individuals with SOC.”
Between December 2021 and October 2022, the researchers used the following search terms on Google: “sunscreen” plus “skin of 36 color,” “dark skin,” “brown skin,” “LatinX skin,” and/or “Black skin.” They extracted price, marketing claims, and ingredients from manufacturers’ websites and used 90 allergens contained in the American Contact Dermatitis Society 2020 Core series to identify potential allergens. Next, they combined cross-reactors/synonyms into allergen categories based on ACDS Contact Allergen Management Plan (CAMP) cross-reactor classification. If multiple ingredients in a sunscreen were represented by a single allergen category, it was counted only once. A similar approach was utilized for marketing categories.
A total of 12 sunscreens were included in the analysis: Absolute Joi, Black Girl Sunscreen, Black Girl Sunscreen Make It Matte, Bolden SPF Brightening Moisturizer, Eleven on the Defense Unrivaled Sun Serum, Kinlo Golden Rays Sunscreen, Live Tinted Hueguard 3-in-1 Mineral Sunscreen, Mele Dew The Most Sheer Moisturizer SPF30 Broad Spectrum Sunscreen, Mele No Shade Sunscreen Oil, Specific Beauty Active Radiance Day Moi, Unsun Mineral Sunscreen, and Urban Skin Rx Complexion Protection. Their average cost was $19.30 per ounce (range, $6.33-$50.00) and common marketing claims for these products were “no white cast” (91.7%), being free of an ingredient (83.3%), and “moisturizing” (75%).
Of the 12 sunscreens, 7 (58.3%) contained a chemical sunscreen agent, 5 (41.7%) contained a physical UV blocker, and all contained at least one allergen. The average number of allergens per product was 4.7, most commonly fragrance/botanicals (83.3%), tocopherol (83.3%), sodium benzoates/derivatives (58.3%), and sorbitan sesquiolate/derivatives (58.3%).
“Average cost of sunscreens marketed to individuals with SOC was $19.30/oz, much higher than the median price of $3.32/oz reported in a separate study of 65 popular sunscreens,” the study authors wrote. “As many of the sunscreens in our study were sold by smaller businesses, higher prices may be due to higher production costs or a perceived smaller market.”
The authors expressed surprise that five sunscreens marketed to individuals with SOC contained a physical UV blocker which may create a white cast. They contacted the manufacturers of these five sunscreens and confirmed that three used micronized formulations. “While ingested/inhaled nanoparticles of titanium dioxide may cause tissue effects, most studies of topical products show excellent safety,” they wrote.
They also noted that the average of 4.7 allergens per product observed in the analysis was similar to the average of 4.9 seen in a separate study of 52 popular sunscreens. “However, that study only included 34 allergens while this study evaluated 90 allergens,” the authors wrote. “Consumers and providers should be aware sunscreens marketed to individuals with SOC may cause allergic contact dermatitis,” they commented.
“It is interesting to see how costly these products are now compared to store bought and general commercially available sunscreens several years ago,” said Lawrence J. Green, clinical professor of dermatology at George Washington University, Washington, who was asked to comment on the study. “However, to me that is not surprising as products marketed and targeted to specific populations are often priced at a premium. It wasn’t clear to me how many of these specialized online SOC sunscreens are tinted. I wish the authors had compared the cost of tinted sunscreens in general to nontinted sunscreens because tinted ones are more useful for SOC, because when rubbed in, they can readily match SOC and can also offer protection in the visible light spectrum.”
The authors reported having no financial disclosures; the study had no funding source. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY