User login
Doug Brunk is a San Diego-based award-winning reporter who began covering health care in 1991. Before joining the company, he wrote for the health sciences division of Columbia University and was an associate editor at Contemporary Long Term Care magazine when it won a Jesse H. Neal Award. His work has been syndicated by the Los Angeles Times and he is the author of two books related to the University of Kentucky Wildcats men's basketball program. Doug has a master’s degree in magazine journalism from the S.I. Newhouse School of Public Communications at Syracuse University. Follow him on Twitter @dougbrunk.
How should PRAME be used to evaluate melanocytic lesions?
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
SAN DIEGO – , according to Cora Humberson, MD.
“I’m a fan, but there are issues with it,” Dr. Humberson, dermatopathology coordinator in the department of pathology at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. “It’s all in how you use it.”
PRAME is part of the cancer/testis (CT) antigens, of which more than 40 have now been identified. They are encoded by genes that are normally expressed only in the human germ line, but are also expressed in various tumor types, including melanoma and carcinomas of the bladder, lung, and liver. “The biological function of these antigens is not fully understood, but they may act as a repressor of retinoic acid, potentially inhibiting differentiation, inhibiting proliferation arrest – things that we associate with malignancy,” she said at the meeting, which was hosted by Scripps MD Anderson Cancer Center. “These immunogenic proteins are being pursued as targets for therapeutic cancer vaccines,” she noted.
CT antigens are also being evaluated for their role in oncogenesis, she added. Recapitulation of portions of the germline gene-expression might contribute characteristic features to the neoplastic phenotype, including immortality, invasiveness, immune evasion, and metastatic capacity.
According to Dr. Humberson, PRAME can be used to differentiate comingled nevus and melanoma, to distinguish between nevoid melanoma and nevus, and for melanoma margin assessment in sun-damaged skin. One potential pitfall is that sun-damaged melanocytes may express PRAME. “The older the person and the more sun damage [they have], the more likely you are to see this, but the melanocytes won’t be grouped, they’ll be scattered,” she said.
Another pitfall is that less than 15% of nevi may express PRAME. “PRAME can be expressed in scars, so if you’re looking at a spindle cell lesion, be aware that you might be looking at a scar if you’re seeing PRAME expression,” she added. She also noted that PRAME immunohistochemistry (IHC) expression is not a prognostic biomarker in thin melanomas.
If fewer than 25% of cells in a melanocytic lesion express PRAME, most published assessments of PRAME IHC favor nevi as the diagnosis. “If more than 75% are expressing it, it favors melanoma,” Dr. Humberson said. “There’s a big category in between. It’s not that 30% is more likely benign or that 60% is more likely malignant; you can’t really depend upon [PRAME] if you’re in this range.”
A diagnostic accuracy study found that when more than 75% of cells express PRAME, the marker has a sensitivity of 0.63 and a specificity of 0.97.
Selected PRAME-related published references she recommended include: J Cutan Pathol. 2021;48(9):1115-23; Diagnostics. 2022 Sep 9; 12(9):2197, and J Cutan Pathol. 2022;49(9):829-32.
Dr. Humberson reported having no relevant disclosures.
AT MELANOMA 2023
Long-pulsed 1,064 nm Nd:YAG for nonaggressive BCC ‘effective and easy’
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
SAN DIEGO – After Arisa E. Ortiz, MD, and colleagues published results of a multicenter study reporting that one treatment with the long-pulsed 1,064-nm Nd:YAG laser cleared nonaggressive basal cell carcinoma (BCC) on the trunk and extremities in 90% of patients, she heard from colleagues who were skeptical of the approach.
Maybe it’s just the biopsy alone that’s clearing these tumors, some told her. Others postulated that since the energy was delivered with a 5- to 6-mm spot size at a fluence of 125-140 J/cm2 and a 7- to 10-ms pulse duration, bulk heating likely disrupted the tumors. However, treatments were generally well tolerated, required no anesthesia, and caused no significant adverse events.
“It’s almost scarless,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium. “Sometimes the treatment does leave a mark, but I think the scars are always acceptable. We do have good histologic evidence that we can penetrate 2.15 mm, which is a lot deeper than what the pulsed-dye laser or other superficial wavelengths are able to penetrate.”
Data is well powered to reject the null hypothesis that laser treatment does not have an effect on nodular and superficial BCC lesions, she continued, noting that it is at least comparable if not superior with clearance rates reported for methyl aminolevulinate–PDT (73%), imiquimod cream (83%), and fluorouracil cream (80%). “Maybe we’re not specifically targeting the vasculature [with this approach], but we did some optical coherence tomography imaging and saw that the blood vessels in the tumor were coagulated while the vasculature in the surrounding normal skin were spared,” said Dr. Ortiz, who is also vice president of the American Society for Laser Medicine and Surgery.
In a more recent analysis, she and her colleagues retrospectively analyzed long-term outcomes in 11 patients with BCC who had 16 lesions treated with the 1,064-nm Nd:YAG laser. At a mean of 9 months, 100% of lesions remained clear as determined by clinical observation.
In a subsequent, as yet unpublished study, she and her collaborators followed 34 patients with BCC one year following laser treatment. “Of these, 33 had no recurrence at 1-year follow-up,” Dr. Ortiz said, noting that the one patient with a recurrence was on a biologic agent for Crohn’s disease.
One key advantage of using the long-pulsed 1,064-nm Nd:YAG laser for nonaggressive BCC is the potential for one treatment visit. “They don’t have to come back for suture removal,” she said. “It’s a quick procedure, takes only about 5 minutes. There’s no limitation on activity and there’s minimal wound care, light ointment, and a band-aid; that’s it.”
In addition, she said, there is a lower risk of complications, infections, and bleeding, and there is minimal scarring. It is “also an alternative for treating patients with multiple tumors or those who are poor surgical candidates, such as the elderly and those with Gorlin syndrome.”
Dr. Ortiz avoids treating aggressive subtypes “because we don’t know what margin to treat,” she added. “Avoid the face. I do make some exceptions for patients if they’re elderly or if they’ve had multiple tumors. Monitor for recurrence like you would using any other modality.”
She uses lidocaine without epinephrine to avoid vasoconstriction and treats with the 1,064-nm Nd:YAG laser as follows: a 5-mm spot size, a fluence of 140 J/cm2, and a pulse duration of 8 ms, with no cooling, which are the settings for the Excel V Laser System, she noted. “If you’re using a different Nd:YAG laser, your pulse duration may vary. I do let the device cool in between pulses to avoid bulk heating.”
The immediate endpoint to strive for is slight greying and slight contraction, and the procedure is covered by insurance, billed as malignant destruction/EDC (CPT codes 17260-17266 trunk and 17280-17283 face). “I do biopsy prior to treatment,” she said. “I like the biopsy to be healed when I’m using the laser, so I’ll treat them about a month later.”
As for future directions, Dr. Ortiz and colleagues plan to evaluate the use of gold nanoparticles to more selectively target BCC during treatment with the 1,064-nm Nd:YAG laser. For now, she sees no downside of the procedure for proper candidates. “I do think that patients really like it,” she said. “It’s effective and easy.”
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is also cochair of the MOAS.
AT MOAS 2022
Age-related atopic dermatitis phenotypes evaluated in study
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
, while older adults tend to present with less flexural eczema and the fewest associated signs.
Those are key findings from a study conducted at a single academic medical center, which aimed to identify the age-related clinical phenotypes of AD.
“Previous studies have found differences in the clinical characteristics of AD depending on age of AD onset, ethnic background, and AD severity,” senior author Jonathan I. Silverberg, MD, PhD, MPH, director of clinical research in the department of dermatology at George Washington University, Washington, and his coauthor wrote in the study, which was published online in JAAD International. “However, none have prospectively compared the clinical characteristics and associated signs by age group. Improved understanding of the clinical phenotypes of AD may help guide choice of treatment and improve health outcomes,” they added.
Along with coauthor Sheena Chatrath, a dermatology research fellow in the department, Dr. Silverberg prospectively reviewed self-reported questionnaires that were completed by 380 patients prior to their visit at GWU’s eczema clinic between 2013 and 2019. Questions included age of AD onset, sociodemographics, Visual Analog Scale (VAS) itch and sleep for Scoring AD, and Numeric Rating Scale (NRS) for skin pain and itch. The researchers used the Eczema Area Severity Index to assess AD severity and a dermatologist conducted full body skin exams, noting the distribution of AD involvement as well as associated signs.
Of the 380 patients, 6.1% were younger than aged 18 years, 46.3% were young adults aged 18-39 years, and 47.6% were older adults 40 years of age and older.
Compared with pediatric patients, both young and older adults were less likely to experience AD on the ankles (adjusted odds ratio [aOR], 0.41 and 0.43, respectively), moderate to severe AD lesions on flexures (aOR, 0.47 and 0.30), pityriasis alba (aOR, 0.24 and 0.07), oozing lesions (aOR, 0.44 and 0.35), and moderate to severe excoriations (aOR, 0.49 and 0.44).
In children, severe itch was more common, reported in 47.1%, compared with 43.4% of the young adults and 38.6% of the older adults, and itch was less severe among the young and older adults. “Interestingly, despite increased itch in pediatric patients, we found no difference in the severity of skin pain across all age groups,” the researchers wrote. “Moreover, pediatric patients reported skin pain less often than adult patients. This may be due to age-related differences of pain perception.”
In other findings, compared with pediatric patients, young adults were more likely to experience AD around the eyes (aOR, 2.92), while older adults were less likely to experience AD on elbows (aOR, 0.34), nipples (aOR, 0.40), knees (aOR, 0.27), and less likely to have keratosis pilaris (aOR, 0.38), and lichenification (aOR, 0.47).
Dr. Silverberg and Ms. Chatrath used latent class analysis to identify four classes for distribution of AD lesions. In this model, class 1 had low probabilities of AD involvement at all sites examined and class 2 had low probabilities of scalp, face, and foot involvement, and intermediate probability of all other AD sites. Class 3 had low probabilities of hand and foot involvement, high probability of facial erythema, and intermediate probability of all other AD signs, while class 4 had intermediate probability of postauricular and foot involvement, and high probability of all other AD sites examined.
“Pediatric patients were most commonly in class 4 (33.3%), followed by class 1 and 2 (26.7%), and least commonly in class 3 (13.3%),” the authors wrote. “In young adults, class 4 and 1 were most common (32.4% and 29.4%), followed by class 2 (27.9%), and least commonly class 3 (10.3%). In older adults, class 1 was most common (40.3%), followed by class 4 (23.6%), and least commonly classes 2 and 3 (18.1%).”
The researchers also used latent class analysis to identify four classes for the signs and symptoms of AD. In this model, class 1 had zero-low probability of all AD signs and class 2 had low probability of all AD signs. Class 3 had high probability of oozing lesions and low probability of all other signs, while class 4 had high probability of xerosis, intermediate probability of ichthyosis and palmar hyperlinearity, and low probability of all other AD signs.
In all three groups, the most common class was class 1 (85.6% of older adults, 81.8% of younger adults, and 82.6% of pediatric patients). Among the pediatric patients, they wrote, “class 3 was the second most common (8.7%), followed by class 2 and 4 (4.4%).” Among the young adults, 9.7% were in class 2, 5.7% were in class 4, and 2.8% were in class 3; and among the older adults, 8.3% were in class 4, 4.4% were in class 2, and 1.67% were in class 3.
Zelma Chiesa Fuxench, MD, of the department of dermatology at the University of Pennsylvania, Philadelphia, who was asked to comment on the study, said that while AD is traditionally largely thought of as a disease of children primarily involving the flexural areas, “this study provides additional evidence to support that AD is more than just a disease of childhood with a fixed clinical presentation, but is a heterogeneous disease whose clinical presentation varies across different population groups.”
While the study provides insight into the clinical differences that may be observed across AD groups, “care must be taken when interpreting these results as the study was done in a single center with observations collected during one single visit,” she added. “AD is not a ‘static’ disease; its presentation can stay the same in one patient but can vary even in another patient throughout their lifetime. Therefore, studies of a more prospective nature that evaluate the change in clinical presentation using multiple measures throughout time in these individuals would be a step forward to better understand if these phenotypic differences truly exist and, as such, what implications could they have for treatment selection.”
This study was supported by grants from the Agency for Healthcare Research and Quality and the Dermatology Foundation. The researchers reported having no disclosures. Dr. Chiesa Fuxench disclosed serving as a consultant for the Asthma and Allergy Foundation of America, National Eczema Association, Pfizer, AbbVie, and Incyte, for which she has received honoraria for AD-related work. She is the recipient of research grants through Regeneron, Sanofi, Tioga, Vanda, Menlo Therapeutics, Leo Pharma, and Eli Lilly for work related to AD as well as honoraria for continuing medical education work related to AD sponsored through educational grants from Regeneron/Sanofi and Pfizer.
FROM JAAD INTERNATIONAL
AAD unveils updated guidelines for topical AD treatment in adults
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
, and topical phosphodiesterase-4 (PDE-4) and Janus kinase (JAK) inhibitors. The guidelines also conditionally recommend the use of bathing and wet wrap therapy but recommend against the use of topical antimicrobials, antiseptics, and antihistamines.
The development updates the AAD’s 2014 recommendations for managing AD with topical therapies, published almost 9 years ago. “At that time, the only U.S. FDA–approved systemic medication for atopic dermatitis was prednisone – universally felt amongst dermatologists to be the least appropriate systemic medication for this condition, at least chronically,” Robert Sidbury, MD, MPH, who cochaired a 14-member multidisciplinary work group that assembled the updated guidelines, told this news organization in an interview.
“Since 2017, there have been two different biologic medications approved for moderate to severe AD (dupilumab and tralokinumab) with certainly a third or more right around the corner. There have been two new oral agents approved for moderate to severe AD – upadacitinib and abrocitinib – with others on the way,” he noted. While these are not topical therapies, the purview of the newly released guidelines, he said, “there have also been new topical medications approved since that time (crisaborole and ruxolitinib). It was high time for an update.”
For the new guidelines, which were published online in the Journal of the American Academy of Dermatology, Dr. Sidbury, chief of the division of dermatology at Seattle Children’s Hospital, guidelines cochair Dawn M. R. Davis, MD, a dermatologist at Mayo Clinic, Rochester, Minn., and colleagues conducted a systematic review of evidence regarding the use of nonprescription topical agents such as moisturizers, bathing practices, and wet wraps, as well as topical pharmacologic modalities such as corticosteroids, calcineurin inhibitors, JAK inhibitors, PDE-4 inhibitors, antimicrobials, and antihistamines.
Next, the work group applied the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) approach for assessing the certainty of the evidence and formulating and grading clinical recommendations based on relevant randomized trials in the medical literature.
12 recommendations
Of the 12 recommendations made for adults with AD, the work group ranked 7 as “strong” based on the evidence reviewed, and the rest as “conditional.” The “strong” recommendations include the use of moisturizers; the use of tacrolimus 0.03% or 0.1%; the use of pimecrolimus 1% cream for mild to moderate AD; use of topical steroids; intermittent use of medium-potency topical corticosteroids as maintenance therapy to reduce flares and relapse; the use of the topical PDE-4 inhibitor crisaborole, and the use of the topical JAK inhibitor ruxolitinib.
Regarding ruxolitinib cream 1.5%, the work group advised that the treatment area “should not exceed 20% body surface area, and a maximum of 60 grams should be applied per week; these stipulations are aimed at reducing systemic absorption, as black box warnings include serious infections, mortality, malignancies (for example, lymphoma), major adverse cardiovascular events, and thrombosis.”
Conditional recommendations in the guidelines include those for bathing for treatment and maintenance and the use of wet dressings, and those against the use of topical antimicrobials, topical antihistamines, and topical antiseptics.
According to Dr. Sidbury, the topic of bathing generated robust discussion among the work group members. “Though [each group member] has strong opinions and individual practice styles, they were also able to recognize that the evidence is all that matters in a project like this, which led to a ‘conditional’ recommendation regarding bathing frequency backed by ‘low’ evidence,” he said. “While this may seem like ‘guidance’ that doesn’t ‘guide,’ I would argue it informs the guideline consumer exactly where we are in terms of this question and allows them to use their best judgment and experience as their true north here.”
In the realm of topical steroids, Dr. Sidbury said that topical steroid addiction (TSA) and topical steroid withdrawal (TSW) have been a “controversial but persistent concern” from some patients and providers. “Two systematic reviews of this topic were mentioned, and it was made clear that the evidence base [for the concepts] is weak,” he said. “With that important caveat ,the guideline committee delineated both a definition of TSW/TSA and potential risk factors.”
Dr. Sidbury marveled at the potential impact of newer medicines such as crisaborole and ruxolitinib on younger AD patients as well. Crisaborole is now Food and Drug Administration approved down to 3 months of age for mild to moderate AD. “This is extraordinary and expands treatment options for all providers at an age when parents and providers are most conservative in their practice,” he said. “Ruxolitinib, also nonsteroidal, is FDA approved for mild to moderate AD down to 12 years of age. Having spent a good percentage of my practice years either being able to offer only topical steroids, or later topical steroids and topical calcineurin inhibitors like tacrolimus or pimecrolimus, having additional options is wonderful.”
In the guidelines, the work group noted that “significant gaps remain” in current understanding of various topical AD therapies. “Studies are needed which examine quality of life and other patient-important outcomes, changes to the cutaneous microbiome, as well as long term follow-up, and use in special and diverse populations (e.g., pregnancy, lactation, immunosuppression, multiple comorbidities, skin of color, pediatric),” they wrote. “Furthermore, increased use of new systemic AD treatment options (dupilumab, tralokinumab, abrocitinib, upadacitinib) in patients with moderate to severe disease may result in a selection bias toward milder disease in current and future AD topical therapy studies.”
Use of topical therapies to manage AD in pediatric patients will be covered in a forthcoming AAD guideline. The first updated AD guideline, on comorbidities associated with AD in adults, was released in January 2022.
Dr. Sidbury reported that he serves as an advisory board member for Pfizer, a principal investigator for Regeneron, an investigator for Brickell Biotech and Galderma USA, and a consultant for Galderma Global and Microes. Other work group members reported having financial disclosures with many pharmaceutical companies.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Picosecond lasers for tattoo removal could benefit from enhancements, expert says
SAN DIEGO – When picosecond lasers hit the market about 10 years ago, they became a game-changer for tattoo removal, boasting the delivery of energy that is about threefold faster than with nanosecond lasers.
However, picosecond lasers are far from perfect even in the hands of the most experienced clinicians, according to Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford. “They have been very difficult to build from an engineering perspective,” he said at the annual Masters of Aesthetics Symposium. It took a long time for these lasers to come to the market, and they are still fairly expensive and require a lot of maintenance, he noted. In addition, “they are also not quite as ‘picosecond’ as they need to be. I think there is definitely room to improve, but this is the gold standard.”
Today, . Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be less than 10 nanoseconds, with shorter pulses better at capturing the ink particles that are smaller than average.
Black is the most common tattoo color dermatologists treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “Other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
Dr. Ibrahimi underscored the importance of setting realistic expectations during consults with patients seeking options for tattoo removal. Even with picosecond laser technology, many treatments are typically required and “a good patient consultation is key to setting proper expectations,” he said. “If you promise someone results in 4 to 5 treatments like many of the device companies will say you can achieve, you’re going to have a large group of patients who are disappointed.”
The clinical endpoint to strive for during tattoo removal is whitening of the ink, which typically fades about 20 minutes after treatment. That whitening corresponds to cavitation, or the production of gas vacuoles in the cells that were holding the ink. This discovery led to a technique intended to enhance tattoo removal. In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the “R20” method, was more effective than a single-pass treatment (P < .01).
“Subsequent to this, there has been conflicting data on whether this is truly effective or not,” said Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery. “Most of us agree that one additional pass would be helpful, but when you’re doing this in the private practice setting, it’s often challenging because patients aren’t necessarily willing to pay more for more than just one pass for their tattoo removal.”
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin (PFD) for use during tattoo removal, which has been shown to reduce epidermal whitening. The patch contains a fluorocarbon “that is very good at dissolving gas, and it is already widely used in medicine,” he said. When applied, “it almost instantaneously takes the whitening away; you don’t have to wait the 20 minutes to do your second pass.”
A different technology designed to speed up tattoo removal is the Resonic Rapid Acoustic Pulse device (marketed as Resonic, from Allergan Aesthetics), which is cleared by the FDA for use as an accessory to the 1064 nm Q-switched laser for black tattoo removal in patients with skin types I-III. “This uses acoustic pulses of sound waves; they’re rapid and powerful,” Dr. Ibrahimi said. “They can clear those cavitation bubbles much like the PFD patches do. It’s also thought that they further disperse the tattoo ink particles by supplementing the laser energy as well. It is also purported to alter the body’s healing response, or immune response, which is important in tattoo clearing.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie (which owns Allergan), Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
SAN DIEGO – When picosecond lasers hit the market about 10 years ago, they became a game-changer for tattoo removal, boasting the delivery of energy that is about threefold faster than with nanosecond lasers.
However, picosecond lasers are far from perfect even in the hands of the most experienced clinicians, according to Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford. “They have been very difficult to build from an engineering perspective,” he said at the annual Masters of Aesthetics Symposium. It took a long time for these lasers to come to the market, and they are still fairly expensive and require a lot of maintenance, he noted. In addition, “they are also not quite as ‘picosecond’ as they need to be. I think there is definitely room to improve, but this is the gold standard.”
Today, . Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be less than 10 nanoseconds, with shorter pulses better at capturing the ink particles that are smaller than average.
Black is the most common tattoo color dermatologists treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “Other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
Dr. Ibrahimi underscored the importance of setting realistic expectations during consults with patients seeking options for tattoo removal. Even with picosecond laser technology, many treatments are typically required and “a good patient consultation is key to setting proper expectations,” he said. “If you promise someone results in 4 to 5 treatments like many of the device companies will say you can achieve, you’re going to have a large group of patients who are disappointed.”
The clinical endpoint to strive for during tattoo removal is whitening of the ink, which typically fades about 20 minutes after treatment. That whitening corresponds to cavitation, or the production of gas vacuoles in the cells that were holding the ink. This discovery led to a technique intended to enhance tattoo removal. In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the “R20” method, was more effective than a single-pass treatment (P < .01).
“Subsequent to this, there has been conflicting data on whether this is truly effective or not,” said Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery. “Most of us agree that one additional pass would be helpful, but when you’re doing this in the private practice setting, it’s often challenging because patients aren’t necessarily willing to pay more for more than just one pass for their tattoo removal.”
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin (PFD) for use during tattoo removal, which has been shown to reduce epidermal whitening. The patch contains a fluorocarbon “that is very good at dissolving gas, and it is already widely used in medicine,” he said. When applied, “it almost instantaneously takes the whitening away; you don’t have to wait the 20 minutes to do your second pass.”
A different technology designed to speed up tattoo removal is the Resonic Rapid Acoustic Pulse device (marketed as Resonic, from Allergan Aesthetics), which is cleared by the FDA for use as an accessory to the 1064 nm Q-switched laser for black tattoo removal in patients with skin types I-III. “This uses acoustic pulses of sound waves; they’re rapid and powerful,” Dr. Ibrahimi said. “They can clear those cavitation bubbles much like the PFD patches do. It’s also thought that they further disperse the tattoo ink particles by supplementing the laser energy as well. It is also purported to alter the body’s healing response, or immune response, which is important in tattoo clearing.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie (which owns Allergan), Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
SAN DIEGO – When picosecond lasers hit the market about 10 years ago, they became a game-changer for tattoo removal, boasting the delivery of energy that is about threefold faster than with nanosecond lasers.
However, picosecond lasers are far from perfect even in the hands of the most experienced clinicians, according to Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford. “They have been very difficult to build from an engineering perspective,” he said at the annual Masters of Aesthetics Symposium. It took a long time for these lasers to come to the market, and they are still fairly expensive and require a lot of maintenance, he noted. In addition, “they are also not quite as ‘picosecond’ as they need to be. I think there is definitely room to improve, but this is the gold standard.”
Today, . Tattoo ink particles average about 0.1 mcm in size, and the thermal relaxation size works out to be less than 10 nanoseconds, with shorter pulses better at capturing the ink particles that are smaller than average.
Black is the most common tattoo color dermatologists treat. “For that, you can typically use a 1064, which has the highest absorption, but you can also use many of the other wavelengths,” he said. “Other colors are less common, followed by red, for which you would use a 532-nm wavelength.”
Dr. Ibrahimi underscored the importance of setting realistic expectations during consults with patients seeking options for tattoo removal. Even with picosecond laser technology, many treatments are typically required and “a good patient consultation is key to setting proper expectations,” he said. “If you promise someone results in 4 to 5 treatments like many of the device companies will say you can achieve, you’re going to have a large group of patients who are disappointed.”
The clinical endpoint to strive for during tattoo removal is whitening of the ink, which typically fades about 20 minutes after treatment. That whitening corresponds to cavitation, or the production of gas vacuoles in the cells that were holding the ink. This discovery led to a technique intended to enhance tattoo removal. In 2012, R. Rox Anderson, MD, director of the Wellman Center for Photomedicine at Massachusetts General Hospital, and colleagues published results of a study that compared a single Q-switched laser treatment pass with four treatment passes separated by 20 minutes. After treating 18 tattoos in 12 adults, they found that the technique, known as the “R20” method, was more effective than a single-pass treatment (P < .01).
“Subsequent to this, there has been conflicting data on whether this is truly effective or not,” said Dr. Ibrahimi, who is also on the board of directors for the American Society for Dermatologic Surgery and the American Society for Laser Medicine and Surgery. “Most of us agree that one additional pass would be helpful, but when you’re doing this in the private practice setting, it’s often challenging because patients aren’t necessarily willing to pay more for more than just one pass for their tattoo removal.”
Another recent advance is use of a topical square silicone patch infused with perfluorodecalin (PFD) for use during tattoo removal, which has been shown to reduce epidermal whitening. The patch contains a fluorocarbon “that is very good at dissolving gas, and it is already widely used in medicine,” he said. When applied, “it almost instantaneously takes the whitening away; you don’t have to wait the 20 minutes to do your second pass.”
A different technology designed to speed up tattoo removal is the Resonic Rapid Acoustic Pulse device (marketed as Resonic, from Allergan Aesthetics), which is cleared by the FDA for use as an accessory to the 1064 nm Q-switched laser for black tattoo removal in patients with skin types I-III. “This uses acoustic pulses of sound waves; they’re rapid and powerful,” Dr. Ibrahimi said. “They can clear those cavitation bubbles much like the PFD patches do. It’s also thought that they further disperse the tattoo ink particles by supplementing the laser energy as well. It is also purported to alter the body’s healing response, or immune response, which is important in tattoo clearing.”
Dr. Ibrahimi disclosed that he is a member of the Advisory Board for Accure Acne, AbbVie (which owns Allergan), Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero. He also holds stock in many device and pharmaceutical companies.
AT MOAS 2022
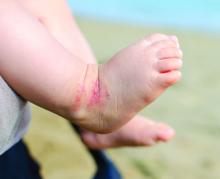
Study spotlights clinicopathologic features, survival outcomes of pediatric melanoma
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
.
“Cutaneous melanomas are rare in children and much less common in adolescents than in later life,” researchers led by Mary-Ann El Sharouni, PhD, wrote in the study, which was published online in the Journal of the American Academy of Dermatology. “Management of these young patients currently follows guidelines developed for adults. Better understanding of melanoma occurring in the first 2 decades of life is, therefore, warranted.”
Drawing from two datasets – one from the Netherlands and the other from Melanoma Institute Australia (MIA) at the University of Sydney – Dr. El Sharouni of the MIA and of the department of dermatology at University Medical Center Utrecht in the Netherlands, and colleagues, evaluated all patients younger than 20 years of age who were diagnosed with invasive melanoma between January 2000 and December 2014. The pooled cohort included 397 Dutch and 117 Australian individuals. Of these, 62 were children and 452 were adolescents. To determine melanoma subtypes, the researchers reevaluated pathology reports and used multivariate Cox models to calculate recurrence-free survival (RFS) and overall survival (OS).
The median Breslow thickness was 2.7 mm in children and 1.0 mm in adolescents. Most patients (83%) had conventional melanoma, which consisted of superficial spreading, nodular, desmoplastic, and acral lentiginous forms, while 78 had spitzoid melanoma and 8 had melanoma associated with a congenital nevus. The 10-year RFS was 91.5% in children and 86.4% in adolescents (P =.32), while the 10-year OS was 100% in children and 92.7% in adolescents (P = .09).
On multivariable analysis, which was possible only for the adolescent cohort because of the small number of children, ulceration status and anatomic site were associated with RFS and OS, whereas age, sex, mitotic index, sentinel node status, and melanoma subtype were not. Breslow thickness > 4 mm was associated with worse RFS. As for affected anatomic site, those with melanomas located on the upper and lower limbs had a better overall RFS and OS compared with those who had head or neck melanomas.
The authors acknowledged certain limitation of the analysis, including its retrospective design and the small number of children. “Our data suggest that adolescent melanomas are often similar to adult-type melanomas, whilst those which occur in young children frequently occur via different molecular mechanisms,” they concluded. “In the future it is likely that further understanding of these molecular mechanisms and ability to classify melanomas based on their molecular characteristics will assist in further refining prognostic estimates and possible guiding treatment for young patients with melanoma.”
Rebecca M. Thiede, MD, assistant program director of the division of dermatology at the University of Arizona, Tucson, who was asked to comment on the study, said that the analysis “greatly contributes to dermatology, as we are still learning the differences between melanoma in children and adolescents versus adults.
This study found that adolescents with melanoma had worse survival if mitosis were present and/or located on head/neck, which could aid in aggressiveness of treatment.”
A key strength of analysis, she continued, is the large sample size of 514 patients, “given that melanoma in this population is very rare. A limitation which [the researchers] brought up is the discrepancy of diagnosis via histopathology of melanoma in children versus adults. The study relied on the pathology report given the retrospective nature of this [analysis, and it] was based on Australian and Dutch populations, which may limit its scope in other countries.”
Dr. El Sharouni was supported by a research fellowship grant from the European Academy of Dermatology and Venereology (EADV), while two of her coauthors, Richard A. Scolyer, MD, and John F. Thompson, MD, were recipients of an Australian National Health and Medical Research Council Program Grant. The study was also supported by a research program grant from Cancer Institute New South Wales. Dr. Thiede reported having no financial disclosures.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Infantile hemangioma: Analysis underscores importance of early propranolol treatment
, results from a post-hoc analysis of phase 2 and 3 clinical trial data showed.
“It is widely accepted that oral propranolol should be started early to improve the success rate, but proposed thresholds have lacked supportive data,” researchers led by Christine Léauté-Labrèze, MD, of the department of dermatology at Pellegrin Children’s Hospital, Bordeaux, France, wrote in the study, which was published online in Pediatric Dermatology. In the pivotal phase 2/3 trial of propranolol of 460 infants, published in 2015, the mean initiation of treatment was 104 days, they added, but “in real-life studies, most infants are referred later than this.”
In addition, a European expert consensus panel set the ideal age for a patient to be seen by a specialist at between 3 and 5 weeks of age, while an American Academy of Pediatrics Clinical Practice Guideline set the ideal age at 1 month.
To determine factors associated with a higher success rate with oral propranolol treatment, such as age at treatment initiation, the researchers analyzed data from the pivotal phase 2-3 clinical trial of oral propranolol in IH. They used Generalized Additive Model (GAM) charts with Generalized Linear Models (GLM), then a rule discovery algorithm, to identify subgroups presenting a high probability of occurrence of the predefined outcome: success at 6 months of treatment (defined as complete or nearly complete resolution of the target hemangioma). Study coauthors were Ilona J. Frieden, MD, of the department of dermatology at the University of California, San Francisco, and director of the UCSF Birthmarks & Vascular Anomalies Center; and Alain Delarue, MD, of medical affairs at Pierre Fabre Dermatologie, Lavaur, France, which markets the pediatric formulation of propranolol approved by the Food and Drug Administration in 2014 for treating IH.
They found that patients who started oral propranolol 3 mg/kg/day before the age of 10 weeks had a success rate of 86%, while those who started treatment after 10 weeks of age had a success rate of 60%. “Our clinical experience suggested that starting early propranolol gave better results on infantile hemangiomas; however, we were surprised” by the significance of the difference, the three study authors stated in an e-mail reply to this news organization.
“It therefore seemed essential to communicate the importance of early treatment to maximize the possibilities of recovery for children. Our findings support early treatment of at-risk infantile hemangiomas, without waiting for complications such as ulceration and/or functional consequences,” they added.
In their e-mail reply, the authors stated that treatment of high-risk IH should be initiated whenever possible before 10 weeks of age. Ideally, infants should be examined by a practitioner between 2 and 5 weeks of age and referred to a specialized center if they have features of an at-risk IH. Tools such as the Infantile Hemangioma Referral Score (IHReS) and consensus guidelines such as the AAP Clinical Practice Guideline “can help guide clinicians seeing newborns and young infants to recognize which IH may need early intervention,” they stated.
For rural-based providers whose patients and their families may not live close to an expert center, the study authors especially recommend using the IHReS scoring tool, which is readily available online and “will be very helpful in assessing whether patients need referral.” For those who do, they added, “triage using photographs is an excellent way to reach out to a referral center for advice and possible urgent referral.” In addition, a recent study emphasized that telemedicine using either live interactive portals or store-and-forward can be helpful in evaluation and management of patients with IH.
Dr. Léauté-Labrèze and colleagues acknowledged certain limitations of the analysis, including the fact that it was performed post-hoc on an existing study and the challenge of translating its findings into clinical practice.
The three study authors were also authors of the 2015 NEJM study; Dr. Léauté-Labrèze was the lead author.
Dr. Léauté-Labrèze disclosed that she has served as a speaker and consultant for Pierre Fabre. Dr. Delarue is an employee of the company. Dr. Frieden reported having no disclosures relevant to the analysis.
, results from a post-hoc analysis of phase 2 and 3 clinical trial data showed.
“It is widely accepted that oral propranolol should be started early to improve the success rate, but proposed thresholds have lacked supportive data,” researchers led by Christine Léauté-Labrèze, MD, of the department of dermatology at Pellegrin Children’s Hospital, Bordeaux, France, wrote in the study, which was published online in Pediatric Dermatology. In the pivotal phase 2/3 trial of propranolol of 460 infants, published in 2015, the mean initiation of treatment was 104 days, they added, but “in real-life studies, most infants are referred later than this.”
In addition, a European expert consensus panel set the ideal age for a patient to be seen by a specialist at between 3 and 5 weeks of age, while an American Academy of Pediatrics Clinical Practice Guideline set the ideal age at 1 month.
To determine factors associated with a higher success rate with oral propranolol treatment, such as age at treatment initiation, the researchers analyzed data from the pivotal phase 2-3 clinical trial of oral propranolol in IH. They used Generalized Additive Model (GAM) charts with Generalized Linear Models (GLM), then a rule discovery algorithm, to identify subgroups presenting a high probability of occurrence of the predefined outcome: success at 6 months of treatment (defined as complete or nearly complete resolution of the target hemangioma). Study coauthors were Ilona J. Frieden, MD, of the department of dermatology at the University of California, San Francisco, and director of the UCSF Birthmarks & Vascular Anomalies Center; and Alain Delarue, MD, of medical affairs at Pierre Fabre Dermatologie, Lavaur, France, which markets the pediatric formulation of propranolol approved by the Food and Drug Administration in 2014 for treating IH.
They found that patients who started oral propranolol 3 mg/kg/day before the age of 10 weeks had a success rate of 86%, while those who started treatment after 10 weeks of age had a success rate of 60%. “Our clinical experience suggested that starting early propranolol gave better results on infantile hemangiomas; however, we were surprised” by the significance of the difference, the three study authors stated in an e-mail reply to this news organization.
“It therefore seemed essential to communicate the importance of early treatment to maximize the possibilities of recovery for children. Our findings support early treatment of at-risk infantile hemangiomas, without waiting for complications such as ulceration and/or functional consequences,” they added.
In their e-mail reply, the authors stated that treatment of high-risk IH should be initiated whenever possible before 10 weeks of age. Ideally, infants should be examined by a practitioner between 2 and 5 weeks of age and referred to a specialized center if they have features of an at-risk IH. Tools such as the Infantile Hemangioma Referral Score (IHReS) and consensus guidelines such as the AAP Clinical Practice Guideline “can help guide clinicians seeing newborns and young infants to recognize which IH may need early intervention,” they stated.
For rural-based providers whose patients and their families may not live close to an expert center, the study authors especially recommend using the IHReS scoring tool, which is readily available online and “will be very helpful in assessing whether patients need referral.” For those who do, they added, “triage using photographs is an excellent way to reach out to a referral center for advice and possible urgent referral.” In addition, a recent study emphasized that telemedicine using either live interactive portals or store-and-forward can be helpful in evaluation and management of patients with IH.
Dr. Léauté-Labrèze and colleagues acknowledged certain limitations of the analysis, including the fact that it was performed post-hoc on an existing study and the challenge of translating its findings into clinical practice.
The three study authors were also authors of the 2015 NEJM study; Dr. Léauté-Labrèze was the lead author.
Dr. Léauté-Labrèze disclosed that she has served as a speaker and consultant for Pierre Fabre. Dr. Delarue is an employee of the company. Dr. Frieden reported having no disclosures relevant to the analysis.
, results from a post-hoc analysis of phase 2 and 3 clinical trial data showed.
“It is widely accepted that oral propranolol should be started early to improve the success rate, but proposed thresholds have lacked supportive data,” researchers led by Christine Léauté-Labrèze, MD, of the department of dermatology at Pellegrin Children’s Hospital, Bordeaux, France, wrote in the study, which was published online in Pediatric Dermatology. In the pivotal phase 2/3 trial of propranolol of 460 infants, published in 2015, the mean initiation of treatment was 104 days, they added, but “in real-life studies, most infants are referred later than this.”
In addition, a European expert consensus panel set the ideal age for a patient to be seen by a specialist at between 3 and 5 weeks of age, while an American Academy of Pediatrics Clinical Practice Guideline set the ideal age at 1 month.
To determine factors associated with a higher success rate with oral propranolol treatment, such as age at treatment initiation, the researchers analyzed data from the pivotal phase 2-3 clinical trial of oral propranolol in IH. They used Generalized Additive Model (GAM) charts with Generalized Linear Models (GLM), then a rule discovery algorithm, to identify subgroups presenting a high probability of occurrence of the predefined outcome: success at 6 months of treatment (defined as complete or nearly complete resolution of the target hemangioma). Study coauthors were Ilona J. Frieden, MD, of the department of dermatology at the University of California, San Francisco, and director of the UCSF Birthmarks & Vascular Anomalies Center; and Alain Delarue, MD, of medical affairs at Pierre Fabre Dermatologie, Lavaur, France, which markets the pediatric formulation of propranolol approved by the Food and Drug Administration in 2014 for treating IH.
They found that patients who started oral propranolol 3 mg/kg/day before the age of 10 weeks had a success rate of 86%, while those who started treatment after 10 weeks of age had a success rate of 60%. “Our clinical experience suggested that starting early propranolol gave better results on infantile hemangiomas; however, we were surprised” by the significance of the difference, the three study authors stated in an e-mail reply to this news organization.
“It therefore seemed essential to communicate the importance of early treatment to maximize the possibilities of recovery for children. Our findings support early treatment of at-risk infantile hemangiomas, without waiting for complications such as ulceration and/or functional consequences,” they added.
In their e-mail reply, the authors stated that treatment of high-risk IH should be initiated whenever possible before 10 weeks of age. Ideally, infants should be examined by a practitioner between 2 and 5 weeks of age and referred to a specialized center if they have features of an at-risk IH. Tools such as the Infantile Hemangioma Referral Score (IHReS) and consensus guidelines such as the AAP Clinical Practice Guideline “can help guide clinicians seeing newborns and young infants to recognize which IH may need early intervention,” they stated.
For rural-based providers whose patients and their families may not live close to an expert center, the study authors especially recommend using the IHReS scoring tool, which is readily available online and “will be very helpful in assessing whether patients need referral.” For those who do, they added, “triage using photographs is an excellent way to reach out to a referral center for advice and possible urgent referral.” In addition, a recent study emphasized that telemedicine using either live interactive portals or store-and-forward can be helpful in evaluation and management of patients with IH.
Dr. Léauté-Labrèze and colleagues acknowledged certain limitations of the analysis, including the fact that it was performed post-hoc on an existing study and the challenge of translating its findings into clinical practice.
The three study authors were also authors of the 2015 NEJM study; Dr. Léauté-Labrèze was the lead author.
Dr. Léauté-Labrèze disclosed that she has served as a speaker and consultant for Pierre Fabre. Dr. Delarue is an employee of the company. Dr. Frieden reported having no disclosures relevant to the analysis.
FROM PEDIATRIC DERMATOLOGY
Do collagen supplements benefit the skin?
SAN DIEGO – When patients ask if collagen supplements can benefit their skin, what should you tell them?
According to Ava Shamban, MD, a dermatologist who practices in Santa Monica, Calif., And in her opinion, more research is needed to establish knowledge of the effects and physiologic mechanism of collagen supplementation.
“Collagen is the most abundant protein in the skin; it is found only in animal flesh like meat and fish that contain connective tissue,” she said at the annual Masters of Aesthetics Symposium. “We produce less collagen as we age. External factors can slow down our collagen production, including smoking, sun exposure, lack of sleep/exercise, and alcohol consumption.”
Though human studies are lacking, some trials have found that collagen supplements may improve skin hydration and elasticity. “Maybe there’s some benefit, but the digestive process breaks collagen down into amino acids, so I don’t buy it,” she said.
At the meeting, Dr. Shamban discussed other topics related to the effect of supplements and nutrition on the skin:
Can Nutrafol reverse permanent hair loss? “It definitely doesn’t do that,” she said. “Can it help regrow hair? Perhaps.” Nutrafol is an over-the-counter supplement that aims to relieve moderate hair thinning or strengthen hair to prevent breakage, and is physician-formulated with medical-grade ingredients that target root causes of thinning such as stress, lifestyle, hormones, and nutrition.
As for biotin, “we now know that high levels of biotin can actually cause hair loss,” she said. “If you have advanced hair loss, supplements may not work for you. There is no hair regrowth supplement that can bring back a dead hair follicle. Can it help a miniaturized hair follicle? Maybe. Platelet-rich plasma injections have been shown to stimulate hair growth, but only if the follicle is miniaturized, not if it’s totally gone.”
How does the human microbiome affect skin? In a review of sequencing surveys of healthy adults, “the composition of microbial communities was found to be primarily dependent on the physiology of the skin site, with changes in the relative abundance of bacterial taxa associated with moist, dry, and sebaceous environments,” the authors reported . “The microbiome is the genetic material of all the microbes that live inside the body, including bacteria, fungi, protozoa, and viruses,” Dr. Shamban said. “The more diverse the microbiota is, the healthier it’s considered. That diversity is enriched through a diet full of various vegetables and fruits.”
Nearly all adults are colonized with Cutibacterium acnes (formerly Propionibacterium acnes), but only a minority have acne, which highlights the importance of studying diseases in the broader context of host genetics, immune or barrier defects, the microbiome, and the environment, she added. For example, the decreased diversity of the skin microbiome in people with atopic dermatitis has been linked to a reduction in environmental biodiversity in the areas surrounding their homes.
Do adaptogens have a role in skin care? Adaptogens such as ashwagandha, elderberry, ginseng, licorice root, neem, moringa, and reishi mushrooms have been used in Chinese and Ayurvedic medicine for centuries and are purported to promote adaptability, resilience, and survival of living organisms in stress. They appear to affect the neuroendocrine immune system and at low doses may function as mild stress mimetics.
“The idea is that combining adaptogens into skin care can reinforce and support the skin’s resistance against stressors that can accelerate visible signs of aging,” said Dr. Shamban. “They share some similarities with antioxidants in that their main purpose is to protect the body from external stressors such as UV rays, oxidation, and pollution.” More studies should be conducted to verify effectiveness, she said, “but Eastern practices that have incorporated it for centuries shouldn’t be fully dismissed. Most doctors believe adaptogens are safe, but how they interact with the mechanics of the body’s stress response system remains a mystery.”
Embrace the consumption of micronutrients. Inspired by work from dermatologist Zoe Diana Draelos, MD, Dr. Shamban advises patients to eat a “rainbow of different colored foods” every day, especially those rich in vitamins A, C, and E. Green foods are generally rich in vitamin E, brown foods are rich in trace minerals, and blue/purple foods are rich in antioxidants. “It’s always best to get nutrients from a rich, healthy diet, but sometimes our skin requires extra help,” she said.
A randomized, placebo-controlled, double-blind study by French researchers, which showed that skin is prone to seasonal changes during the winter, particularly in exposed areas, also looked at whether a daily micronutrient supplement with ingredients that included green tea extract, blackcurrant seed oil, and magnesium, had an impact on the negative effects of winter weather on the skin. “The data indicate that oral micronutrient supplementation can be a safe treatment, with no serious side effects, and may prevent or even eliminate the negative effects of winter on the skin,” she said.
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – When patients ask if collagen supplements can benefit their skin, what should you tell them?
According to Ava Shamban, MD, a dermatologist who practices in Santa Monica, Calif., And in her opinion, more research is needed to establish knowledge of the effects and physiologic mechanism of collagen supplementation.
“Collagen is the most abundant protein in the skin; it is found only in animal flesh like meat and fish that contain connective tissue,” she said at the annual Masters of Aesthetics Symposium. “We produce less collagen as we age. External factors can slow down our collagen production, including smoking, sun exposure, lack of sleep/exercise, and alcohol consumption.”
Though human studies are lacking, some trials have found that collagen supplements may improve skin hydration and elasticity. “Maybe there’s some benefit, but the digestive process breaks collagen down into amino acids, so I don’t buy it,” she said.
At the meeting, Dr. Shamban discussed other topics related to the effect of supplements and nutrition on the skin:
Can Nutrafol reverse permanent hair loss? “It definitely doesn’t do that,” she said. “Can it help regrow hair? Perhaps.” Nutrafol is an over-the-counter supplement that aims to relieve moderate hair thinning or strengthen hair to prevent breakage, and is physician-formulated with medical-grade ingredients that target root causes of thinning such as stress, lifestyle, hormones, and nutrition.
As for biotin, “we now know that high levels of biotin can actually cause hair loss,” she said. “If you have advanced hair loss, supplements may not work for you. There is no hair regrowth supplement that can bring back a dead hair follicle. Can it help a miniaturized hair follicle? Maybe. Platelet-rich plasma injections have been shown to stimulate hair growth, but only if the follicle is miniaturized, not if it’s totally gone.”
How does the human microbiome affect skin? In a review of sequencing surveys of healthy adults, “the composition of microbial communities was found to be primarily dependent on the physiology of the skin site, with changes in the relative abundance of bacterial taxa associated with moist, dry, and sebaceous environments,” the authors reported . “The microbiome is the genetic material of all the microbes that live inside the body, including bacteria, fungi, protozoa, and viruses,” Dr. Shamban said. “The more diverse the microbiota is, the healthier it’s considered. That diversity is enriched through a diet full of various vegetables and fruits.”
Nearly all adults are colonized with Cutibacterium acnes (formerly Propionibacterium acnes), but only a minority have acne, which highlights the importance of studying diseases in the broader context of host genetics, immune or barrier defects, the microbiome, and the environment, she added. For example, the decreased diversity of the skin microbiome in people with atopic dermatitis has been linked to a reduction in environmental biodiversity in the areas surrounding their homes.
Do adaptogens have a role in skin care? Adaptogens such as ashwagandha, elderberry, ginseng, licorice root, neem, moringa, and reishi mushrooms have been used in Chinese and Ayurvedic medicine for centuries and are purported to promote adaptability, resilience, and survival of living organisms in stress. They appear to affect the neuroendocrine immune system and at low doses may function as mild stress mimetics.
“The idea is that combining adaptogens into skin care can reinforce and support the skin’s resistance against stressors that can accelerate visible signs of aging,” said Dr. Shamban. “They share some similarities with antioxidants in that their main purpose is to protect the body from external stressors such as UV rays, oxidation, and pollution.” More studies should be conducted to verify effectiveness, she said, “but Eastern practices that have incorporated it for centuries shouldn’t be fully dismissed. Most doctors believe adaptogens are safe, but how they interact with the mechanics of the body’s stress response system remains a mystery.”
Embrace the consumption of micronutrients. Inspired by work from dermatologist Zoe Diana Draelos, MD, Dr. Shamban advises patients to eat a “rainbow of different colored foods” every day, especially those rich in vitamins A, C, and E. Green foods are generally rich in vitamin E, brown foods are rich in trace minerals, and blue/purple foods are rich in antioxidants. “It’s always best to get nutrients from a rich, healthy diet, but sometimes our skin requires extra help,” she said.
A randomized, placebo-controlled, double-blind study by French researchers, which showed that skin is prone to seasonal changes during the winter, particularly in exposed areas, also looked at whether a daily micronutrient supplement with ingredients that included green tea extract, blackcurrant seed oil, and magnesium, had an impact on the negative effects of winter weather on the skin. “The data indicate that oral micronutrient supplementation can be a safe treatment, with no serious side effects, and may prevent or even eliminate the negative effects of winter on the skin,” she said.
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – When patients ask if collagen supplements can benefit their skin, what should you tell them?
According to Ava Shamban, MD, a dermatologist who practices in Santa Monica, Calif., And in her opinion, more research is needed to establish knowledge of the effects and physiologic mechanism of collagen supplementation.
“Collagen is the most abundant protein in the skin; it is found only in animal flesh like meat and fish that contain connective tissue,” she said at the annual Masters of Aesthetics Symposium. “We produce less collagen as we age. External factors can slow down our collagen production, including smoking, sun exposure, lack of sleep/exercise, and alcohol consumption.”
Though human studies are lacking, some trials have found that collagen supplements may improve skin hydration and elasticity. “Maybe there’s some benefit, but the digestive process breaks collagen down into amino acids, so I don’t buy it,” she said.
At the meeting, Dr. Shamban discussed other topics related to the effect of supplements and nutrition on the skin:
Can Nutrafol reverse permanent hair loss? “It definitely doesn’t do that,” she said. “Can it help regrow hair? Perhaps.” Nutrafol is an over-the-counter supplement that aims to relieve moderate hair thinning or strengthen hair to prevent breakage, and is physician-formulated with medical-grade ingredients that target root causes of thinning such as stress, lifestyle, hormones, and nutrition.
As for biotin, “we now know that high levels of biotin can actually cause hair loss,” she said. “If you have advanced hair loss, supplements may not work for you. There is no hair regrowth supplement that can bring back a dead hair follicle. Can it help a miniaturized hair follicle? Maybe. Platelet-rich plasma injections have been shown to stimulate hair growth, but only if the follicle is miniaturized, not if it’s totally gone.”
How does the human microbiome affect skin? In a review of sequencing surveys of healthy adults, “the composition of microbial communities was found to be primarily dependent on the physiology of the skin site, with changes in the relative abundance of bacterial taxa associated with moist, dry, and sebaceous environments,” the authors reported . “The microbiome is the genetic material of all the microbes that live inside the body, including bacteria, fungi, protozoa, and viruses,” Dr. Shamban said. “The more diverse the microbiota is, the healthier it’s considered. That diversity is enriched through a diet full of various vegetables and fruits.”
Nearly all adults are colonized with Cutibacterium acnes (formerly Propionibacterium acnes), but only a minority have acne, which highlights the importance of studying diseases in the broader context of host genetics, immune or barrier defects, the microbiome, and the environment, she added. For example, the decreased diversity of the skin microbiome in people with atopic dermatitis has been linked to a reduction in environmental biodiversity in the areas surrounding their homes.
Do adaptogens have a role in skin care? Adaptogens such as ashwagandha, elderberry, ginseng, licorice root, neem, moringa, and reishi mushrooms have been used in Chinese and Ayurvedic medicine for centuries and are purported to promote adaptability, resilience, and survival of living organisms in stress. They appear to affect the neuroendocrine immune system and at low doses may function as mild stress mimetics.
“The idea is that combining adaptogens into skin care can reinforce and support the skin’s resistance against stressors that can accelerate visible signs of aging,” said Dr. Shamban. “They share some similarities with antioxidants in that their main purpose is to protect the body from external stressors such as UV rays, oxidation, and pollution.” More studies should be conducted to verify effectiveness, she said, “but Eastern practices that have incorporated it for centuries shouldn’t be fully dismissed. Most doctors believe adaptogens are safe, but how they interact with the mechanics of the body’s stress response system remains a mystery.”
Embrace the consumption of micronutrients. Inspired by work from dermatologist Zoe Diana Draelos, MD, Dr. Shamban advises patients to eat a “rainbow of different colored foods” every day, especially those rich in vitamins A, C, and E. Green foods are generally rich in vitamin E, brown foods are rich in trace minerals, and blue/purple foods are rich in antioxidants. “It’s always best to get nutrients from a rich, healthy diet, but sometimes our skin requires extra help,” she said.
A randomized, placebo-controlled, double-blind study by French researchers, which showed that skin is prone to seasonal changes during the winter, particularly in exposed areas, also looked at whether a daily micronutrient supplement with ingredients that included green tea extract, blackcurrant seed oil, and magnesium, had an impact on the negative effects of winter weather on the skin. “The data indicate that oral micronutrient supplementation can be a safe treatment, with no serious side effects, and may prevent or even eliminate the negative effects of winter on the skin,” she said.
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
AT MOAS 2022
Long-term maintenance required in melasma patients
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
SAN DIEGO –
“They need to understand that melasma is going to require long-term maintenance,” Dr. Ortiz, director of laser and cosmetic dermatology at the University of California, San Diego, said at the annual Masters of Aesthetics Symposium.
Hydroquinone is a mainstay of melasma therapy, but instead of the commonly used 4% formulation, she prefers to use 12% hydroquinone with 6% kojic acid in VersaBase cream. “It’s a high concentration but the VersaBase makes it more tolerable,” she said. “I have patients take a pea-sized amount and mix it in a regular moisturizer. It’s too strong to spot treat, so it goes on the whole face.”
Mindful that chronic hydroquinone use can cause ochronosis (permanent darkening), she has patients alternate with a nonhydroquinone bleaching agent such as lignin peroxidase, oligopeptide, Lytera, Melaplex, 4-n-butylresorcinol, Cysteamine cream, tranexamic acid, or oral antioxidants. In a study sponsored by SkinMedica, investigators conducted a randomized, double-blind, half-face study in females with moderate to severe facial hyperpigmentation to assess the efficacy and tolerability of three new skin brightener formulations containing SMA-432, a prostaglandin E2 inhibitor, compared with 4% hydroquinone. They found that the nonhydroquinone skin formulations were better tolerated and were just as effective as 4% hydroquinone.
Chemical peels and laser treatments
Chemical peels are another treatment option for melasma, but Dr. Ortiz prefers glycolic peels over salicylic and other peels, “because there is no downtime,” she said.
As for laser-based approaches, melasma patients respond best to low energy devices such as the 1,927-nm fractional diode laser at a 3.75% density. “This also can increase the skin permeability of topicals, so when you’re combining it with hydroquinone it can be more effective,” she said.
In an observational study of 27 women with refractory melasma, with phototypes II-V, New York City–based dermatologist Arielle Kauvar, MD, combined microdermabrasion with the Q-switched Nd:YAG laser. “The settings she used were very low fluence, so there was no clinical endpoint or no whitening,” said Dr. Ortiz, vice president of the American Society for Laser Medicine and Surgery (ASLMS). Specifically, Dr. Kauvar used the laser at 1.6-2 J/cm2 with a 5- or 6-mm spot size immediately following microdermabrasion every 4 weeks; Patients received an average of 2.6 treatments, and were assessed 3-12 months after the last treatment. Study participants were on a standard skin care regimen of a broad spectrum sunscreen, hydroquinone, and tretinoin or vitamin C.
Most of the patients showed at least 50% clearance of melasma 1 month after the first treatment, and 81% showed more than 75% clearance of melasma; remission lasted at least 6 months.
“I personally prefer to use picosecond over Q-switched lasers, because they deliver the energy faster, and you can use a 1,064-nm picosecond laser that is safe in all skin types,” Dr. Ortiz said. “There is minimal downtime, and it doesn’t require anesthesia. You have to consider these things when you’re treating melasma, because this usually requires monthly treatments. If you do something that requires a week of downtime every month, it’s not practical for patients.”
In a study published in 2021, Dr. Ortiz and Tanya Greywal, MD, used three passes of the 1,064-nm Nd:YAG laser to treat melasma in 10 patients with skin types II-V. The device had a 650-microsecond pulse duration, a 6-mm spot size, and an energy mode of 11-14 J/cm2. The researchers observed a mean melasma improvement of 26%-50% as early as 3 weeks. “There was no downtime, and no anesthesia was required,” Dr. Ortiz said.
Researchers have discovered a vascular component to melasma, which may have treatment implications. Houston-based dermatologist Paul M. Friedman, MD, and his colleagues used spectrocolorimetry to detect an underlying prominent vascular component in a retrospective review of 11 patients with melasma, with skin types II-IV. They determined that melasma lesions exhibiting subtle or subclinical telangiectatic erythema may be improved by combining vascular-targeted laser therapy with fractional low-powered diode laser therapy.
“So, combining a vascular laser with a 1,927-nm fractional diode laser showed more improvement than with just the diode laser alone,” said Dr. Ortiz, who was not involved with the analysis.
To optimize results following the laser treatment of melasma, she uses one application of clobetasol immediately after the procedure. “This can help reduce swelling and inflammation to decrease the risk of postinflammatory hyperpigmentation,” she said. “You can also use a skin cooling system like Cryomodulation for controlled cooling.”
Tranexamic acid and PLE
Another strategy for melasma patients involves oral treatment with extract of Polypodium leucotomos (PLE), a fern from the Polypodiaceae family with antioxidant properties that has been shown to be photoprotective against UVA and UVB radiation. “I explain to my patients that it’s like an internal sunscreen,” Dr. Ortiz said. “It does not replace your external sunscreen, but it adds extra protection.”
In a pilot placebo-controlled study of patients with melasma on their normal regimen of hydroquinone and sunscreen, 40 Asian patients with melasma were randomized to receive either oral PLE supplementation or placebo for 12 weeks. The authors found that PLE significantly improved and accelerated the outcome reached with hydroquinone and sunscreen from about the first month of treatment, compared with placebo.
Dr. Ortiz discussed the role of oral tranexamic acid, an antifibrinolytic, procoagulant agent that is approved by the Food and Drug Administration for the treatment of menorrhagia and to prevent hemorrhage in patients with hemophilia undergoing tooth extractions. “This is a game changer for melasma treatment,” she said, but its use has been limited by the risk for thromboembolism.
In a study of 561 patients with melasma, 90% improved after a median treatment duration of 4 months, and only 7% had side effects, most commonly abdominal bloating and pain. Treatment was discontinued in one patient who developed a deep vein thrombosis, and was diagnosed with familial protein S deficiency.
The daily dosing of tranexamic acid for menorrhagia is 3,900 mg daily, while the dose for treating melasma has ranged from 500 mg to 1,500 mg per day, Dr. Ortiz said. It’s available as a 650-mg tablet in the United States. “I prescribe 325 mg twice a day, but studies have shown that 650 mg once a day is just as effective,” she said.
Prior to prescribing tranexamic acid, Dr. Ortiz does not order labs, but she performs an extensive history of current illness and does not prescribe it in patients with an increased risk of clotting, including people who smoke and those who take oral contraceptives or are on hormone supplementation. Use is also contraindicated in people with a current malignancy, those with a history of stroke or DVT, and those who have any clotting disorder.
Dr. Ortiz disclosed having financial relationships with several pharmaceutical and device companies. She is cochair of the Masters of Aesthetics Symposium.
AT MOAS 2022
Why it’s important to offer cosmeceuticals in a cosmetic practice
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
SAN DIEGO – , advised Ava Shamban, MD.
It’s important to provide patients with high-quality products to take home with them and cosmeceuticals contain biologically active ingredients that enhance skin care efficacy, Dr. Shamban, a dermatologist who practices in Santa Monica, Calif., said at the annual Masters of Aesthetics Symposium. “You can do all the lasers, injectables, and peels that you want, but if you’re not giving your patients high-quality products to take home with them, you’re not doing your job,” she commented.
“Look for brands that are formulated and tested for effectiveness,” she added. “In my office, we like to have products that are designed for specific issues to accompany prescription products, everything from rosacea, acne, melasma, and eczema to psoriasis.”
Dr. Shamban, author of the 2011 book, “Heal Your Skin: The Breakthrough Plan for Renewal,” recommends that dermatologists devise a questionnaire for patients asking them to list their skin-related concerns and use the responses to create a list of products for them to use at home. Provide clear instructions on use, including proper layering of products, how often to use them, and the correct amount to apply. “If you’re not going to do this, someone else will,” she said. Next, instruct them that cosmeceuticals must be used routinely to achieve optimal benefit. “Nothing happens overnight, and be wary of anyone that promises you otherwise,” Dr. Shamban said. “Offering cosmeceuticals helps bridge the gap between at-home routines and in-office treatments. If in-office procedures are a marathon, view the consistent use of the right products at home as your training.”
During her presentation, she showed a photo of the “beauty bar,” the dedicated space with a counter and shelves for displaying skin care products in her Santa Monica office. “It’s good to set something up like this in your office, even if it’s just a little corner, because it gives it authority,” Dr. Shamban said. “Encourage clients to explore the beauty bar after their appointment with you.” She emphasized the importance of offering a wide range of products to accommodate different lifestyles, budgets, skin types, ages, and specific skin concerns, and training staff about the products. “There is never a one-size-fits-all approach to skincare; it’s all about the individual,” she said. “It’s never about pushing product; it’s always about educating patients.”
Dr. Shamban disclosed that she conducts clinical trials for many pharmaceutical and device companies.
AT MOAS 2022