User login
One-quarter of ED sprained ankle diagnoses result in opioid prescription
One-quarter of patients who present at the ED with a , according to the findings of a large study using a national insurance claims database.
M. Kit Delgado, MD, of the Center for Emergency Care Policy and Research at the Perelman School of Medicine, University of Pennsylvania, Philadelphia, and his coauthors analyzed private insurance claims for 30,832 opioid-naive patients who were treated in the emergency department for ankle sprains.
The researchers looked at the initial opioid prescription intensity and duration of subsequent opioid use. The study, published online in Annals of Emergency Medicine, also found a wide variation in prescribing by hospital and geographic region, and an association between higher-potency opioid prescriptions (hydrocodone and oxycodone) and likelihood of extended or prolonged opioid use.
Overall, 25.1% of patients received an opioid prescription, the median tablet quantity was 15 tablets, and median days of opioids supplied was 3 days. Only 5% of patients were prescribed more than 30 tablets, which suggested that the majority of prescriptions written were in agreement with guidelines, the authors wrote.
They noted that nonsteroidal anti-inflammatory drugs are the first-line treatment for ankle sprains, rather than opioids, and are as effective for pain reduction.
Among patients prescribed the equivalent of more than 30 tabs of oxycodone 5 mg, 4.9% of them showed prolonged opioid use – defined as filling four or more subsequent opioid prescriptions in the 30-180 days after the index visit – compared with 1.1% of patients who received fewer than 10 tabs, and 0.5% of those who did not fill an opioid prescription. This represented a nearly fivefold increased probability of transition to prolonged use. For every 26 patients exposed to the higher intensity prescription, 1 would go on to prolonged opioid use.
“We confirmed that the majority of subsequent prescriptions were unlikely to be related to the initial ankle sprain or chronic ankle pain,” the researchers wrote. “This suggests that association between larger prescriptions and increased likelihood of prolonged use could be due to other factors such as patients requesting opioids as default pain control, or the development of dependence or misuse.”
The most commonly prescribed opioid was hydrocodone (64.9%), followed by tramadol (16.2%), oxycodone (14.4%), and codeine (5.5%). The analysis found that patients prescribed higher-potency drugs – namely hydrocodone and oxycodone – were at even greater risk of developing prolonged use.
The researchers noted significant geographic differences in prescribing habits, with 40% of study participants in Arkansas receiving an opioid prescription, compared with 2.8% in North Dakota. Overall, southern states were more likely to overprescribe, and northern states more likely to underprescribe.
Women were at greater risk of transitioning to high-risk prolonged use, as were individuals aged 35-44 years, those with a higher comorbidity burden, or those with a history of drug abuse.
The study was supported by the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the Leonard Davis Institute of Health Economics at the University of Pennsylvania. One author declared an honorarium from the United Health Group.
SOURCE: Delgado MK et al. Ann Emerg Med. 2018. Jul 24. doi: 10.1016/j.annemergmed.2018.06.003.
One-quarter of patients who present at the ED with a , according to the findings of a large study using a national insurance claims database.
M. Kit Delgado, MD, of the Center for Emergency Care Policy and Research at the Perelman School of Medicine, University of Pennsylvania, Philadelphia, and his coauthors analyzed private insurance claims for 30,832 opioid-naive patients who were treated in the emergency department for ankle sprains.
The researchers looked at the initial opioid prescription intensity and duration of subsequent opioid use. The study, published online in Annals of Emergency Medicine, also found a wide variation in prescribing by hospital and geographic region, and an association between higher-potency opioid prescriptions (hydrocodone and oxycodone) and likelihood of extended or prolonged opioid use.
Overall, 25.1% of patients received an opioid prescription, the median tablet quantity was 15 tablets, and median days of opioids supplied was 3 days. Only 5% of patients were prescribed more than 30 tablets, which suggested that the majority of prescriptions written were in agreement with guidelines, the authors wrote.
They noted that nonsteroidal anti-inflammatory drugs are the first-line treatment for ankle sprains, rather than opioids, and are as effective for pain reduction.
Among patients prescribed the equivalent of more than 30 tabs of oxycodone 5 mg, 4.9% of them showed prolonged opioid use – defined as filling four or more subsequent opioid prescriptions in the 30-180 days after the index visit – compared with 1.1% of patients who received fewer than 10 tabs, and 0.5% of those who did not fill an opioid prescription. This represented a nearly fivefold increased probability of transition to prolonged use. For every 26 patients exposed to the higher intensity prescription, 1 would go on to prolonged opioid use.
“We confirmed that the majority of subsequent prescriptions were unlikely to be related to the initial ankle sprain or chronic ankle pain,” the researchers wrote. “This suggests that association between larger prescriptions and increased likelihood of prolonged use could be due to other factors such as patients requesting opioids as default pain control, or the development of dependence or misuse.”
The most commonly prescribed opioid was hydrocodone (64.9%), followed by tramadol (16.2%), oxycodone (14.4%), and codeine (5.5%). The analysis found that patients prescribed higher-potency drugs – namely hydrocodone and oxycodone – were at even greater risk of developing prolonged use.
The researchers noted significant geographic differences in prescribing habits, with 40% of study participants in Arkansas receiving an opioid prescription, compared with 2.8% in North Dakota. Overall, southern states were more likely to overprescribe, and northern states more likely to underprescribe.
Women were at greater risk of transitioning to high-risk prolonged use, as were individuals aged 35-44 years, those with a higher comorbidity burden, or those with a history of drug abuse.
The study was supported by the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the Leonard Davis Institute of Health Economics at the University of Pennsylvania. One author declared an honorarium from the United Health Group.
SOURCE: Delgado MK et al. Ann Emerg Med. 2018. Jul 24. doi: 10.1016/j.annemergmed.2018.06.003.
One-quarter of patients who present at the ED with a , according to the findings of a large study using a national insurance claims database.
M. Kit Delgado, MD, of the Center for Emergency Care Policy and Research at the Perelman School of Medicine, University of Pennsylvania, Philadelphia, and his coauthors analyzed private insurance claims for 30,832 opioid-naive patients who were treated in the emergency department for ankle sprains.
The researchers looked at the initial opioid prescription intensity and duration of subsequent opioid use. The study, published online in Annals of Emergency Medicine, also found a wide variation in prescribing by hospital and geographic region, and an association between higher-potency opioid prescriptions (hydrocodone and oxycodone) and likelihood of extended or prolonged opioid use.
Overall, 25.1% of patients received an opioid prescription, the median tablet quantity was 15 tablets, and median days of opioids supplied was 3 days. Only 5% of patients were prescribed more than 30 tablets, which suggested that the majority of prescriptions written were in agreement with guidelines, the authors wrote.
They noted that nonsteroidal anti-inflammatory drugs are the first-line treatment for ankle sprains, rather than opioids, and are as effective for pain reduction.
Among patients prescribed the equivalent of more than 30 tabs of oxycodone 5 mg, 4.9% of them showed prolonged opioid use – defined as filling four or more subsequent opioid prescriptions in the 30-180 days after the index visit – compared with 1.1% of patients who received fewer than 10 tabs, and 0.5% of those who did not fill an opioid prescription. This represented a nearly fivefold increased probability of transition to prolonged use. For every 26 patients exposed to the higher intensity prescription, 1 would go on to prolonged opioid use.
“We confirmed that the majority of subsequent prescriptions were unlikely to be related to the initial ankle sprain or chronic ankle pain,” the researchers wrote. “This suggests that association between larger prescriptions and increased likelihood of prolonged use could be due to other factors such as patients requesting opioids as default pain control, or the development of dependence or misuse.”
The most commonly prescribed opioid was hydrocodone (64.9%), followed by tramadol (16.2%), oxycodone (14.4%), and codeine (5.5%). The analysis found that patients prescribed higher-potency drugs – namely hydrocodone and oxycodone – were at even greater risk of developing prolonged use.
The researchers noted significant geographic differences in prescribing habits, with 40% of study participants in Arkansas receiving an opioid prescription, compared with 2.8% in North Dakota. Overall, southern states were more likely to overprescribe, and northern states more likely to underprescribe.
Women were at greater risk of transitioning to high-risk prolonged use, as were individuals aged 35-44 years, those with a higher comorbidity burden, or those with a history of drug abuse.
The study was supported by the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the Leonard Davis Institute of Health Economics at the University of Pennsylvania. One author declared an honorarium from the United Health Group.
SOURCE: Delgado MK et al. Ann Emerg Med. 2018. Jul 24. doi: 10.1016/j.annemergmed.2018.06.003.
FROM ANNALS OF EMERGENCY MEDICINE
Key clinical point: Opioids prescribed for sprained ankles increase the risk of prolonged use.
Major finding: High-intensity opioid prescriptions after a sprained ankle were linked to a fivefold higher risk of prolonged use.
Study details: Analysis of health insurance data from 30,832 patients treated in the emergency department for ankle sprains.
Disclosures: The study was supported by the National Institute on Drug Abuse, the National Institute of Child Health and Human Development, and the Leonard Davis Institute of Health Economics at the University of Pennsylvania. One author declared an honorarium from the United Health Group.
Source: Delgado MK et al. Ann Emerg Med. 2018 Jul 24. doi: 10.1016/j.annemergmed.2018.06.003.
Increased COPD mortality with secondhand smoke exposure in childhood
Prolonged exposure to in adulthood, new research has found.
With data from 70,900 never-smoking men and women, most aged 50 years or over, in the Cancer Prevention Study–II Nutrition Cohort, researchers examined associations between childhood and adult secondhand smoke exposure and the risk of death during adulthood from all causes, heart disease, stroke, and COPD. Participants were followed from enrollment in 1992-1993 to 2016-2017.
In a paper published in the September issue of the American Journal of Preventive Medicine, they reported that individuals who had lived with a smoker throughout childhood (16-18 years) had a 31% higher risk of dying from COPD, compared with individuals not exposed to secondhand smoke during childhood (95% confidence interval 1.05-1.65, P = .06). Any exposure to secondhand smoke in childhood – defined as one or more hours of exposure per week – was associated with a 21% higher risk of COPD mortality.
“It is established that SHS [secondhand smoke] exposure in childhood can result in asthma, chronic wheezing, respiratory infections, and decreased lung function and growth in children,” wrote W. Ryan Diver, a cancer epidemiologist, and his colleagues from the Epidemiology Research Program at the American Cancer Society. “These respiratory illnesses in early life are associated with worse lung function in adolescence and adulthood, as indicated by a lower forced expiratory volume in a 1 second plateau, and ultimately diagnosis of COPD.”
The researchers did not see a significant association between childhood secondhand smoke exposure and the risk of death from ischemic heart disease or stroke in adulthood. But the authors said that there was compelling evidence that secondhand smoke exposure during childhood contributed to increased arterial stiffness, autonomic dysfunction, and other vascular effects, which had led to the hypothesis that such exposure might influence the risk of heart disease and stroke mortality in adulthood. They suggested that this effect might have been more apparent in generations born in the 1950s and 1960s, where both parents were likely to smoke at home, as opposed to just one parent, which would have meant higher levels of exposure to secondhand smoke.
Adult exposure to secondhand smoke showed a significant dose-response relationship with overall mortality. Those exposed for 10 or more hours a week showed a 9% higher risk of death from all causes (95% CI 1.04-1.14, P less than .0001), as well as a significant 27% higher risk of death from ischemic heart disease.
Any exposure to secondhand smoke in adulthood was associated with a 14% higher risk of stroke, with a trend of increasing risk with increasing exposure.
The researchers also saw a significant association between secondhand smoke exposure and COPD mortality, but only in adults who reported being exposed both outside and inside the home.
The authors noted that the most recent Surgeon General’s report found that the evidence on secondhand smoke and increased risk of death from COPD was “suggestive but not sufficient,” and further research was needed.
“The associations observed with both childhood exposure to SHS and adult exposure to SHS add to the mounting data relating SHS to COPD,” they wrote.
One limitation of the study was that it relied on self-report, which in the case of childhood exposure would have required some participants to recall at least 3 decades back. It also did not capture whether it was the mother or father who smoked, which meant the authors could not account for possible effects of smoking during pregnancy.
The investigators noted that “more than 50 years after the publication of the first Surgeon General report on smoking and health, these findings suggest that researchers and scientists still do not fully understand the long-term health consequences of smoking, particularly, the potential delayed effects of childhood SHS exposure in later adulthood.” Despite this, the authors said the findings “provide further support for implementation of smoke-free air laws, smoke-free home policies, and clinical interventions to reduce SHS exposure.”
No conflicts of interest were declared.
SOURCE: Diver WR et al. Am J Prev Med 2018;55[3]:345-52. doi: 10.1016/j.amepre.2018.05.005.
Prolonged exposure to in adulthood, new research has found.
With data from 70,900 never-smoking men and women, most aged 50 years or over, in the Cancer Prevention Study–II Nutrition Cohort, researchers examined associations between childhood and adult secondhand smoke exposure and the risk of death during adulthood from all causes, heart disease, stroke, and COPD. Participants were followed from enrollment in 1992-1993 to 2016-2017.
In a paper published in the September issue of the American Journal of Preventive Medicine, they reported that individuals who had lived with a smoker throughout childhood (16-18 years) had a 31% higher risk of dying from COPD, compared with individuals not exposed to secondhand smoke during childhood (95% confidence interval 1.05-1.65, P = .06). Any exposure to secondhand smoke in childhood – defined as one or more hours of exposure per week – was associated with a 21% higher risk of COPD mortality.
“It is established that SHS [secondhand smoke] exposure in childhood can result in asthma, chronic wheezing, respiratory infections, and decreased lung function and growth in children,” wrote W. Ryan Diver, a cancer epidemiologist, and his colleagues from the Epidemiology Research Program at the American Cancer Society. “These respiratory illnesses in early life are associated with worse lung function in adolescence and adulthood, as indicated by a lower forced expiratory volume in a 1 second plateau, and ultimately diagnosis of COPD.”
The researchers did not see a significant association between childhood secondhand smoke exposure and the risk of death from ischemic heart disease or stroke in adulthood. But the authors said that there was compelling evidence that secondhand smoke exposure during childhood contributed to increased arterial stiffness, autonomic dysfunction, and other vascular effects, which had led to the hypothesis that such exposure might influence the risk of heart disease and stroke mortality in adulthood. They suggested that this effect might have been more apparent in generations born in the 1950s and 1960s, where both parents were likely to smoke at home, as opposed to just one parent, which would have meant higher levels of exposure to secondhand smoke.
Adult exposure to secondhand smoke showed a significant dose-response relationship with overall mortality. Those exposed for 10 or more hours a week showed a 9% higher risk of death from all causes (95% CI 1.04-1.14, P less than .0001), as well as a significant 27% higher risk of death from ischemic heart disease.
Any exposure to secondhand smoke in adulthood was associated with a 14% higher risk of stroke, with a trend of increasing risk with increasing exposure.
The researchers also saw a significant association between secondhand smoke exposure and COPD mortality, but only in adults who reported being exposed both outside and inside the home.
The authors noted that the most recent Surgeon General’s report found that the evidence on secondhand smoke and increased risk of death from COPD was “suggestive but not sufficient,” and further research was needed.
“The associations observed with both childhood exposure to SHS and adult exposure to SHS add to the mounting data relating SHS to COPD,” they wrote.
One limitation of the study was that it relied on self-report, which in the case of childhood exposure would have required some participants to recall at least 3 decades back. It also did not capture whether it was the mother or father who smoked, which meant the authors could not account for possible effects of smoking during pregnancy.
The investigators noted that “more than 50 years after the publication of the first Surgeon General report on smoking and health, these findings suggest that researchers and scientists still do not fully understand the long-term health consequences of smoking, particularly, the potential delayed effects of childhood SHS exposure in later adulthood.” Despite this, the authors said the findings “provide further support for implementation of smoke-free air laws, smoke-free home policies, and clinical interventions to reduce SHS exposure.”
No conflicts of interest were declared.
SOURCE: Diver WR et al. Am J Prev Med 2018;55[3]:345-52. doi: 10.1016/j.amepre.2018.05.005.
Prolonged exposure to in adulthood, new research has found.
With data from 70,900 never-smoking men and women, most aged 50 years or over, in the Cancer Prevention Study–II Nutrition Cohort, researchers examined associations between childhood and adult secondhand smoke exposure and the risk of death during adulthood from all causes, heart disease, stroke, and COPD. Participants were followed from enrollment in 1992-1993 to 2016-2017.
In a paper published in the September issue of the American Journal of Preventive Medicine, they reported that individuals who had lived with a smoker throughout childhood (16-18 years) had a 31% higher risk of dying from COPD, compared with individuals not exposed to secondhand smoke during childhood (95% confidence interval 1.05-1.65, P = .06). Any exposure to secondhand smoke in childhood – defined as one or more hours of exposure per week – was associated with a 21% higher risk of COPD mortality.
“It is established that SHS [secondhand smoke] exposure in childhood can result in asthma, chronic wheezing, respiratory infections, and decreased lung function and growth in children,” wrote W. Ryan Diver, a cancer epidemiologist, and his colleagues from the Epidemiology Research Program at the American Cancer Society. “These respiratory illnesses in early life are associated with worse lung function in adolescence and adulthood, as indicated by a lower forced expiratory volume in a 1 second plateau, and ultimately diagnosis of COPD.”
The researchers did not see a significant association between childhood secondhand smoke exposure and the risk of death from ischemic heart disease or stroke in adulthood. But the authors said that there was compelling evidence that secondhand smoke exposure during childhood contributed to increased arterial stiffness, autonomic dysfunction, and other vascular effects, which had led to the hypothesis that such exposure might influence the risk of heart disease and stroke mortality in adulthood. They suggested that this effect might have been more apparent in generations born in the 1950s and 1960s, where both parents were likely to smoke at home, as opposed to just one parent, which would have meant higher levels of exposure to secondhand smoke.
Adult exposure to secondhand smoke showed a significant dose-response relationship with overall mortality. Those exposed for 10 or more hours a week showed a 9% higher risk of death from all causes (95% CI 1.04-1.14, P less than .0001), as well as a significant 27% higher risk of death from ischemic heart disease.
Any exposure to secondhand smoke in adulthood was associated with a 14% higher risk of stroke, with a trend of increasing risk with increasing exposure.
The researchers also saw a significant association between secondhand smoke exposure and COPD mortality, but only in adults who reported being exposed both outside and inside the home.
The authors noted that the most recent Surgeon General’s report found that the evidence on secondhand smoke and increased risk of death from COPD was “suggestive but not sufficient,” and further research was needed.
“The associations observed with both childhood exposure to SHS and adult exposure to SHS add to the mounting data relating SHS to COPD,” they wrote.
One limitation of the study was that it relied on self-report, which in the case of childhood exposure would have required some participants to recall at least 3 decades back. It also did not capture whether it was the mother or father who smoked, which meant the authors could not account for possible effects of smoking during pregnancy.
The investigators noted that “more than 50 years after the publication of the first Surgeon General report on smoking and health, these findings suggest that researchers and scientists still do not fully understand the long-term health consequences of smoking, particularly, the potential delayed effects of childhood SHS exposure in later adulthood.” Despite this, the authors said the findings “provide further support for implementation of smoke-free air laws, smoke-free home policies, and clinical interventions to reduce SHS exposure.”
No conflicts of interest were declared.
SOURCE: Diver WR et al. Am J Prev Med 2018;55[3]:345-52. doi: 10.1016/j.amepre.2018.05.005.
FROM THE AMERICAN JOURNAL OF PREVENTIVE MEDICINE
Key clinical point: Childhood secondhand smoke exposure is linked to increased COPD mortality in adulthood.
Major finding: Adults who were exposed to secondhand smoke throughout childhood have a 31% higher risk of COPD mortality than do those not exposed.
Study details: Retrospective cohort study of 70,900 never-smoking men and women enrolled in the Cancer Prevention Study–II Nutrition Cohort.
Disclosures: No conflicts of interest were declared.
Source: Diver W et al. Am J Prev Med. 2018;55[3]:345-52. doi: 10.1016/j.amepre.2018.05.005.
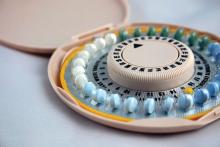
Hormonal contraceptive use linked to leukemia risk in offspring
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
Estrogenic compounds could have a number of effects on the genomic machinery, that could in turn lead to an increased risk of leukemia in offspring. It may be that oral contraceptives cause epigenetic changes to fetal hematopoietic stem cells that lead to gene rearrangements and oxidative damage, which could then influence the risk of developing childhood leukemia.
This study opens a new avenue of investigation for a risk factor that might increase a child’s susceptibility to leukemia and is important in shedding more light on dose-response associations of exposures.
Dr. Maria S. Pombo-de-Oliveira is from the pediatric hematology-oncology research program at the Instituto Nacional de Câncer in Rio de Janeiro. These comments are adapted from an accompanying editorial (Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045[18]30509-6). Dr. Pombo-de-Oliveira reported having no conflicts of interest.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
A nationwide cohort study found an association between a woman’s use of hormonal contraceptives and a small increased risk of nonlymphoid leukemia in her offspring.
Maternal use of hormonal contraception either during pregnancy or in the 3 months beforehand was associated with a 46% higher risk of any leukemia in the children (P = .011), compared with no use, Marie Hargreave, PhD, of the Danish Cancer Society Research Center and her coauthors reported in Lancet Oncology.
The study of 1,185,157 children born between 1996 and 2014 included data from the Danish Cancer Registry and Danish National Prescription Registry and followed children for a median of 9.3 years.
Use during pregnancy was associated with a 78% higher risk of any leukemia in the offspring (P = .070), and contraception use that stopped more than 3 months before pregnancy was associated with a 25% higher risk of any leukemia (P = .039).
The researchers estimated that maternal use of hormonal contraceptives up to and including during pregnancy would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia in Denmark from contraceptive use from 1996 to 2014.
The increased risk appeared to be limited to nonlymphoid leukemia only. The risk with recent use was more than twofold higher (HR, 2.17), compared with nonuse, and use during pregnancy was associated with a nearly fourfold increase in the risk of leukemia (HR, 3.87).
“Sex hormones are considered to be potent carcinogens, and the causal association between in-utero exposure to the oestrogen analogue diethylstilbestrol and subsequent risk for adenocarcinoma of the vagina is firmly established,” Dr. Hargreave and her colleagues wrote. “The mechanism by which maternal use of hormones increases cancer risk in children is, however, still not clear.”
Recent use of combined oral contraceptive products was associated with a more than twofold increased risk of nonlymphoid leukemia in offspring, compared with no use. However progestin-only oral contraceptives and emergency contraception did not appear to increase in the risk of lymphoid or nonlymphoid leukemia.
The association was strongest in children aged 6-10 years, which the authors suggested was likely because the incidence of nonlymphoid leukemia increases after the age of 6 years.
While acknowledging that the small increase in leukemia risk was not a major safety concern for hormonal contraceptives, the authors commented that the results suggested the intrauterine hormonal environment could be a direction for research into the causes of leukemia.
The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
SOURCE: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: Recent maternal hormonal contraceptive use was linked to one additional case of leukemia per 47,170 children.
Study details: Danish nationwide cohort study in 1,185,157 children.
Disclosures: The study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
Source: Hargreave M et al. Lancet Oncol. 2018 Sep 6. doi: 10.1016/S1470-2045(18)30479-0.
Adults with autism, comorbid depression less likely to get talk therapy
Adults with autism spectrum disorders are less likely to receive individual talk therapy, but when they do, they make use of it more than adults without autism.
Using Medicaid data, Brenna B. Maddox, PhD, of the University of Pennsylvania, Philadelphia, and her colleagues presented the results of a study looking at treatment use by 268 adults with autism spectrum disorder (ASD) and 1,072 without, all of whom had depression or anxiety. They published their results in Research in Autism Spectrum Disorders.
Participants with ASD were less likely than those without ASD to receive either individual talk therapy (57.1% vs. 64.3%, P less than .05) or group talk therapy (0.7% vs. 2.9%, P less than .05). However, among those who did receive talk therapy, people with ASD had a significantly higher monthly mean number of visits than those without ASD (0.64 vs. 0.44, P less than .001).
“As predicted, adults with ASD and anxiety/depression were less likely to receive individual talk therapy, consistent with reports that they often experience difficulty accessing mental health services,” wrote Dr. Maddox and her colleagues.
The authors suggested the increased number of talk therapy visits by adults with ASD could reflect a need for more session time for those patients, compared with those without ASD. For example, cognitive-behavioral therapy programs are often adapted to ASD patients by increasing the number of sessions.
“An alternative explanation is that talk therapy in the community is less effective with adults with ASD, who therefore stay in therapy for a longer period in pursuit of greater symptom relief,” the authors wrote.
However, they also commented on the fact that both groups received less than one session a month and that it was unlikely that anxiety and depression symptoms would be well addressed with so few visits. “This finding highlights that the problem of limited access to mental health care is not exclusive to adults with ASD.”
Almost twice as many people with ASD used antipsychotics (39.6% vs. 21.5%, P less than .001) and stimulants (11.9% vs. 6.1%, P less than .001), compared with people without ASD. There were similar numbers of benzodiazepine and antidepressant users in both groups, but people with ASD were prescribed all medication types for a significantly higher number of days per month.
Those with ASD also were more likely to be prescribed different classes of medications at the same time. However, they also were less likely to show substance use.
Dr. Maddox and her colleagues cited several limitations. For example, the diagnosis of psychiatric and medical conditions was made using ICD-9 codes, and those conditions were not validated using clinical evaluation.
The study was supported by the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Maddox BB et al. Res Autism Spectr Disord. 2018 Apr 11. doi: 10.1016/j.rasd.2018.03.009.
Adults with autism spectrum disorders are less likely to receive individual talk therapy, but when they do, they make use of it more than adults without autism.
Using Medicaid data, Brenna B. Maddox, PhD, of the University of Pennsylvania, Philadelphia, and her colleagues presented the results of a study looking at treatment use by 268 adults with autism spectrum disorder (ASD) and 1,072 without, all of whom had depression or anxiety. They published their results in Research in Autism Spectrum Disorders.
Participants with ASD were less likely than those without ASD to receive either individual talk therapy (57.1% vs. 64.3%, P less than .05) or group talk therapy (0.7% vs. 2.9%, P less than .05). However, among those who did receive talk therapy, people with ASD had a significantly higher monthly mean number of visits than those without ASD (0.64 vs. 0.44, P less than .001).
“As predicted, adults with ASD and anxiety/depression were less likely to receive individual talk therapy, consistent with reports that they often experience difficulty accessing mental health services,” wrote Dr. Maddox and her colleagues.
The authors suggested the increased number of talk therapy visits by adults with ASD could reflect a need for more session time for those patients, compared with those without ASD. For example, cognitive-behavioral therapy programs are often adapted to ASD patients by increasing the number of sessions.
“An alternative explanation is that talk therapy in the community is less effective with adults with ASD, who therefore stay in therapy for a longer period in pursuit of greater symptom relief,” the authors wrote.
However, they also commented on the fact that both groups received less than one session a month and that it was unlikely that anxiety and depression symptoms would be well addressed with so few visits. “This finding highlights that the problem of limited access to mental health care is not exclusive to adults with ASD.”
Almost twice as many people with ASD used antipsychotics (39.6% vs. 21.5%, P less than .001) and stimulants (11.9% vs. 6.1%, P less than .001), compared with people without ASD. There were similar numbers of benzodiazepine and antidepressant users in both groups, but people with ASD were prescribed all medication types for a significantly higher number of days per month.
Those with ASD also were more likely to be prescribed different classes of medications at the same time. However, they also were less likely to show substance use.
Dr. Maddox and her colleagues cited several limitations. For example, the diagnosis of psychiatric and medical conditions was made using ICD-9 codes, and those conditions were not validated using clinical evaluation.
The study was supported by the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Maddox BB et al. Res Autism Spectr Disord. 2018 Apr 11. doi: 10.1016/j.rasd.2018.03.009.
Adults with autism spectrum disorders are less likely to receive individual talk therapy, but when they do, they make use of it more than adults without autism.
Using Medicaid data, Brenna B. Maddox, PhD, of the University of Pennsylvania, Philadelphia, and her colleagues presented the results of a study looking at treatment use by 268 adults with autism spectrum disorder (ASD) and 1,072 without, all of whom had depression or anxiety. They published their results in Research in Autism Spectrum Disorders.
Participants with ASD were less likely than those without ASD to receive either individual talk therapy (57.1% vs. 64.3%, P less than .05) or group talk therapy (0.7% vs. 2.9%, P less than .05). However, among those who did receive talk therapy, people with ASD had a significantly higher monthly mean number of visits than those without ASD (0.64 vs. 0.44, P less than .001).
“As predicted, adults with ASD and anxiety/depression were less likely to receive individual talk therapy, consistent with reports that they often experience difficulty accessing mental health services,” wrote Dr. Maddox and her colleagues.
The authors suggested the increased number of talk therapy visits by adults with ASD could reflect a need for more session time for those patients, compared with those without ASD. For example, cognitive-behavioral therapy programs are often adapted to ASD patients by increasing the number of sessions.
“An alternative explanation is that talk therapy in the community is less effective with adults with ASD, who therefore stay in therapy for a longer period in pursuit of greater symptom relief,” the authors wrote.
However, they also commented on the fact that both groups received less than one session a month and that it was unlikely that anxiety and depression symptoms would be well addressed with so few visits. “This finding highlights that the problem of limited access to mental health care is not exclusive to adults with ASD.”
Almost twice as many people with ASD used antipsychotics (39.6% vs. 21.5%, P less than .001) and stimulants (11.9% vs. 6.1%, P less than .001), compared with people without ASD. There were similar numbers of benzodiazepine and antidepressant users in both groups, but people with ASD were prescribed all medication types for a significantly higher number of days per month.
Those with ASD also were more likely to be prescribed different classes of medications at the same time. However, they also were less likely to show substance use.
Dr. Maddox and her colleagues cited several limitations. For example, the diagnosis of psychiatric and medical conditions was made using ICD-9 codes, and those conditions were not validated using clinical evaluation.
The study was supported by the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Maddox BB et al. Res Autism Spectr Disord. 2018 Apr 11. doi: 10.1016/j.rasd.2018.03.009.
FROM RESEARCH IN AUTISM SPECTRUM DISORDERS
Key clinical point: Therapists may need more session time for adults with autism spectrum disorder.
Major finding: Among adults with autism who experience anxiety and depression, 57.1% received talk therapy, compared with 64.3% of those without autism.
Study details: A study of Medicaid claims data for 268 adults with autism and 1,072 without.
Disclosures: The study was supported by the National Institute of Mental Health. No conflicts of interest were declared.
Source: Maddox BB et al. Res Autism Spectr Disord. 2018 Apr 11. doi: 10.1016/j.rasd.2018.03.009.
Single-dose influenza drug baloxavir similar to oseltamivir in efficacy
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
These two studies of baloxavir show that the drug has a clinical benefit similar to that of oseltamivir in individuals with uncomplicated influenza infection. As a single-dose treatment, baloxavir has the advantage in reducing concerns about adherence compared to the treatment regimen for oseltamivir, which requires 5 days of twice-daily dosing.
However, these studies should be viewed as the first step. While baloxavir showed significantly greater reductions in viral load at 24 hours and a shorter duration of infectious virus detection than did oseltamivir or placebo, it also induced the emergence of viral escape mutants with reduced susceptibility.
It’s not yet known whether these influenza viruses with reduced susceptibility are transmissible, and whether surveillance for I38T and other markers will be needed. We also need trials to identify which patients are most likely to benefit from baloxavir, and the timing for treatment.
Timothy M. Uyeki, MD, is with the Influenza Division at the National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention. These comments are taken from an editorial (N Engl J Med. 2018;397:975-7. doi: 10.1056/NEJMe1810815. No conflicts of interest were declared.
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
A new single-dose influenza antiviral drug appears significantly better than placebo at relieving the symptoms of infection, and reduces viral load faster than does oseltamivir, new research suggests.
Baloxavir marboxil – a selective inhibitor of influenza cap-dependent endonuclease – was tested in two randomized, double-blind, controlled trials. The first was a double-blind, placebo-controlled, dose-ranging, phase 2 randomized trial of 389 Japanese adults aged 20-64 years with acute uncomplicated influenza from December 2015 through March 2016. The second was a phase 3 randomized controlled trial of 1,366 patients comparing baloxavir with placebo and oseltamivir.
The phase 2 study showed patients treated with 10 mg, 20 mg or 40 mg oral dose of baloxavir experienced a significantly shorter median time to symptom alleviation compared with placebo (54.2, 51, 49.5, and 77.7 hours, respectively), according to a paper published in the Sept. 6 edition of the New England Journal of Medicine.
In addition, all three doses showed significantly greater reductions in influenza virus titers on days 2 and 3, compared with placebo.
The phase 3 trial CAPSTONE-1 (NCT02954354) was a double-blind, placebo- and oseltamivir-controlled, randomized trial that enrolled outpatients aged 12-64 years with influenza-like illness in the United States and Japan from December 2016 through March 2017. Patients aged 20-64 years received a single, weight-based oral dose of baloxavir (40 mg for patients weighing more than 80 kg, 80 mg for those weighing 80 kg or less) on day 1 only or oseltamivir at a dose of 75 mg twice daily or matching placebos on a 5-day regimen.
Patients aged 12-19 years were randomly assigned to receive either baloxavir or placebo on day 1 only, according to the researchers.
The median time to alleviation of symptoms was similar in the baloxavir (53.5 hours) and oseltamivir group (53.8 hours). However, patients taking baloxavir had significantly faster declines in infectious viral load compared with those taking oseltamivir, which was taken as a 75-mg dose twice daily for 5 days. In addition, patients who were treated with baloxavir within 24 hours of symptom onset showed significantly shorter time to alleviation of symptoms compared with placebo than did those who started treatment more than 24 hours after symptoms began.
Adverse events related to the study drug were more common among patients taking oseltamivir (8.4%) compared with those taking baloxavir (4.4%) or placebo (3.9%). In the phase 2 study, the adverse event rate was lower in the three baloxavir dosage groups compared with the placebo group. The study also showed a similar low frequency of complications requiring antibiotic treatment in both the baloxavir, oseltamivir, and placebo arms.
Some patients did show evidence of decreased susceptibility to baloxavir; for example, PA I38T/M amino acid substitutions were seen in 9.7% of the patients taking baloxavir but none of randomly selected patients in the placebo group of the phase 3 trial.
“These trials showed that single doses of the cap-dependent endonuclease inhibitor baloxavir were superior to placebo in alleviating influenza symptoms in patients with uncomplicated influenza, without clinically significant side effects,” wrote Dr. Frederick G. Hayden of the University of Virginia, Charlottesville, and his coauthors.
“The antiviral effects that were observed with baloxavir in patients with uncomplicated influenza provide encouragement with respect to its potential value in treating complicated or severe influenza infections,” they noted.
Because the treatment was inhibitory for influenza virus strains that were resistant to neuraminidase inhibitors or M2 ion-channel inhibitors, it could be a treatment option for patients infected with those viruses, the researchers added.
CAPSTONE-2, a randomized, controlled trial involving patients at high risk for influenza complications (NCT02949011) is in progress.
The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
SOURCE: Hayden F et al. N Engl J Med. 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Single-dose influenza antiviral baloxavir shows efficacy similar to that of oseltamivir.
Major finding: Baloxavir shows similar time to alleviation of influenza symptoms compared with oseltamivir, but greater reductions in viral load.
Study details: Phase 2 and phase 3 randomized controlled trials in 389 and 1,366 otherwise healthy patients with influenza.
Disclosures: The study was supported by Shionogi, which developed baloxavir. Seven authors declared fees from the pharmaceutical industry, including Shionogi. Six authors were employees of Shionogi, one also holding stock. No other conflicts of interest were declared.
Source: Hayden F et al. N Engl J Med 2018;379:913-23. doi: 10.1056/NEJMoa1716197.
Syphilis surge drives USPSTF reaffirmation of early screening for all pregnant women
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
I strongly concur with the U.S. Preventive Services Task Force recommendation on early screening for syphilis infection in all pregnant women. There is benefit to screening all women for syphilis in early pregnancy given the risks of miscarriage, congenital syphilis, and maternal illness – if untreated. Additionally, in women who live in high prevalence areas or with high-risk behaviors for acquiring syphilis, testing should be performed again in the third trimester and at delivery. Also, all women with a fetal death after 20 weeks should be tested or retested if testing was done earlier in pregnancy.
Martina Badell, MD , is a maternal-fetal medicine specialist at Emory University and director of the Emory University Hospital Midtown Perinatal Center, both in Atlanta. Dr. Badell reported no relevant financial conflicts. She was asked to comment on the USPSTF recommendation.
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
Against the backdrop of a near doubling in the incidence of congenital syphilis in the United States, the U.S. Preventive Services Task Force has reaffirmed its 2009 recommendation to screen all pregnant women for syphilis as early as possible in pregnancy.
The advice was the task force’s primary recommendation, based on a systematic review of seven studies and backed by the highest grade of evidence, in a statement published in JAMA. Untreated syphilis can be transmitted to the fetus at any time during pregnancy or birth, and congenital syphilis is associated with significant neonatal morbidity – including bone deformities and neurologic impairment – as well as stillbirth and neonatal death.
The prevalence of congenital syphilis was in decline from 2008 to 2012, but then increased by 87% from 2012 to 2016 – from 8.4 cases per 100,000 live births in 2012 to 15.7 cases in 2016. The increase coincided with rising national rates of syphilis among women of reproductive age – from 0.9 cases of primary and secondary syphilis infection per 100,000 women in 2012 to 1.9 cases in 2016.
Additionally, the task force recommended that pregnant women who had not received prenatal care be screened at delivery.
“Although nearly 70% of infants with congenital syphilis are born to mothers who received prenatal care, detection, and treatment of maternal syphilis often occurs too late to treat the fetus and prevent congenital syphilis,” wrote Susan J. Curry, PhD, from the University of Iowa, Iowa City, and her coauthors. “Recent data suggest that while screening rates for syphilis infection are generally high, the proportion of women screened earlier in pregnancy remains low (for example, 20% of women are screened only at the time of delivery).”
The review pointed to an observational study of the impact of the introduction of syphilis screening during pregnancy in China. That study of more than 2 million women showed that screening for syphilis in pregnancy increased from 89.8% of women in 2002 to 97.2% of women in 2012 and was associated with a decrease in the incidence of congenital syphilis from 109.3 cases to 9.4 cases per 100,000 live births.
The group found convincing evidence that screening reduced both the incidence of congenital syphilis and the risk of adverse outcomes related to maternal infection and that the potential harms of screening – such as false positives – were small.
The paper also referenced guidelines from the Centers for Disease Control and Prevention, the American Academy of Pediatrics, and the American College of Obstetricians and Gynecologists that high-risk women – such as those living in areas or communities with a higher prevalence of syphilis, women with HIV, or with a history of incarceration or sex work – should also be rescreened early in the third trimester and again at delivery. Similarly, women who are exposed to an infected partner also should be rescreened.
Further, the task force recommended screening for nonpregnant adolescents and adults at increased risk of syphilis infection.
In terms of treatment, the CDC currently recommends parenteral penicillin G benzathine as the treatment of choice for syphilis in pregnant women. However, the task force recommended clinicians consult the CDC website for updates.
The authors noted that no studies that met the inclusion criteria examined whether penicillin use during pregnancy was associated with any harm or looked at serious adverse events in women with a history of penicillin allergy.
“Because the review was primarily focused on screening, it did not address the efficacy of alternative antibiotic treatments [e.g., ceftriaxone] in pregnant women [with or without penicillin allergies],” the authors wrote.
The research was funded by the U.S. Department of Health and Human Services. No conflicts of interest were reported.
SOURCE: Curry S et al. JAMA. 2018;320:911-7.
FROM JAMA
Self-management intervention for epilepsy achieves health improvements
Self-management of epilepsy using a group-format, remote intervention improved mood, quality of life, and health functioning in high-risk individuals in a randomized, controlled trial.
In the 6-month trial, 120 individuals with epilepsy who had experienced at least one epilepsy-related health event in the previous 6 months were randomized either to a wait-list control group or a novel self‐management intervention.
The eight-session intervention, known as SMART, focused on modifiable factors that can be addressed with self-management, such as stress, substance abuse, routine, nutrition, and social support. It was delivered remotely over 8-10 weeks, either by telephone or online, after an initial in-person session.
“SMART combines the portability and low cost of a Web‐based intervention with the personally salient components of behavior modeling obtained by interacting with individuals who have ‘walked the walk’ in living with epilepsy,” Martha Sajatovic, MD, of Case Western Reserve University, Cleveland, and her coauthors wrote about their study published in Epilepsia.
Over the 6-month follow-up period, researchers found that individuals randomized to the intervention had a mean of 10.16 fewer negative health events, compared with a mean of 1.93 fewer events in the control group (P = .04).
When the authors looked at subcategories of negative health event counts – such as past 3-day seizure count or past 6‐month ED and hospitalization count – the differences were not significant. There was also no difference in seizure severity.
However, the study also showed significant improvements in participants’ self-rated depressive symptom severity, observer-rated depressive symptom severity, quality of life, and health functioning – both physical and mental – compared with controls. Intervention participants also reported significant improvements on the Epilepsy Self-Efficacy and Epilepsy Self-Management scales.
The majority of participants (94.2%) said the intervention was useful and that it covered the most important issues for them. A total of 92.3% believed the benefits of the SMART intervention were worth the hassle of taking part.
“SMART’s strengths are its foundation based on participatory research methods and an evidence‐based intervention, its use of peer educators facilitating empowerment and training, multimode delivery using traditional group format and telehealth approaches to eliminate barriers to care, and efficacy even in people who have long‐standing epilepsy,” the authors wrote.
“It is possible that SMART, which uses people with epilepsy as guides to help others learn to cope with the challenges of living with this common chronic neurologic condition, may help to alleviate some of the factors that prevent people with epilepsy from optimizing their quality of life.”
The study was supported by the Centers for Disease Control and Prevention. Three authors declared research grants, consultancies, royalties, or speaking positions with the private sector.
SOURCE: Sajatovic M et al. Epilepsia. 2018 Aug 10. doi: 10.1111/epi.14527.
Self-management of epilepsy using a group-format, remote intervention improved mood, quality of life, and health functioning in high-risk individuals in a randomized, controlled trial.
In the 6-month trial, 120 individuals with epilepsy who had experienced at least one epilepsy-related health event in the previous 6 months were randomized either to a wait-list control group or a novel self‐management intervention.
The eight-session intervention, known as SMART, focused on modifiable factors that can be addressed with self-management, such as stress, substance abuse, routine, nutrition, and social support. It was delivered remotely over 8-10 weeks, either by telephone or online, after an initial in-person session.
“SMART combines the portability and low cost of a Web‐based intervention with the personally salient components of behavior modeling obtained by interacting with individuals who have ‘walked the walk’ in living with epilepsy,” Martha Sajatovic, MD, of Case Western Reserve University, Cleveland, and her coauthors wrote about their study published in Epilepsia.
Over the 6-month follow-up period, researchers found that individuals randomized to the intervention had a mean of 10.16 fewer negative health events, compared with a mean of 1.93 fewer events in the control group (P = .04).
When the authors looked at subcategories of negative health event counts – such as past 3-day seizure count or past 6‐month ED and hospitalization count – the differences were not significant. There was also no difference in seizure severity.
However, the study also showed significant improvements in participants’ self-rated depressive symptom severity, observer-rated depressive symptom severity, quality of life, and health functioning – both physical and mental – compared with controls. Intervention participants also reported significant improvements on the Epilepsy Self-Efficacy and Epilepsy Self-Management scales.
The majority of participants (94.2%) said the intervention was useful and that it covered the most important issues for them. A total of 92.3% believed the benefits of the SMART intervention were worth the hassle of taking part.
“SMART’s strengths are its foundation based on participatory research methods and an evidence‐based intervention, its use of peer educators facilitating empowerment and training, multimode delivery using traditional group format and telehealth approaches to eliminate barriers to care, and efficacy even in people who have long‐standing epilepsy,” the authors wrote.
“It is possible that SMART, which uses people with epilepsy as guides to help others learn to cope with the challenges of living with this common chronic neurologic condition, may help to alleviate some of the factors that prevent people with epilepsy from optimizing their quality of life.”
The study was supported by the Centers for Disease Control and Prevention. Three authors declared research grants, consultancies, royalties, or speaking positions with the private sector.
SOURCE: Sajatovic M et al. Epilepsia. 2018 Aug 10. doi: 10.1111/epi.14527.
Self-management of epilepsy using a group-format, remote intervention improved mood, quality of life, and health functioning in high-risk individuals in a randomized, controlled trial.
In the 6-month trial, 120 individuals with epilepsy who had experienced at least one epilepsy-related health event in the previous 6 months were randomized either to a wait-list control group or a novel self‐management intervention.
The eight-session intervention, known as SMART, focused on modifiable factors that can be addressed with self-management, such as stress, substance abuse, routine, nutrition, and social support. It was delivered remotely over 8-10 weeks, either by telephone or online, after an initial in-person session.
“SMART combines the portability and low cost of a Web‐based intervention with the personally salient components of behavior modeling obtained by interacting with individuals who have ‘walked the walk’ in living with epilepsy,” Martha Sajatovic, MD, of Case Western Reserve University, Cleveland, and her coauthors wrote about their study published in Epilepsia.
Over the 6-month follow-up period, researchers found that individuals randomized to the intervention had a mean of 10.16 fewer negative health events, compared with a mean of 1.93 fewer events in the control group (P = .04).
When the authors looked at subcategories of negative health event counts – such as past 3-day seizure count or past 6‐month ED and hospitalization count – the differences were not significant. There was also no difference in seizure severity.
However, the study also showed significant improvements in participants’ self-rated depressive symptom severity, observer-rated depressive symptom severity, quality of life, and health functioning – both physical and mental – compared with controls. Intervention participants also reported significant improvements on the Epilepsy Self-Efficacy and Epilepsy Self-Management scales.
The majority of participants (94.2%) said the intervention was useful and that it covered the most important issues for them. A total of 92.3% believed the benefits of the SMART intervention were worth the hassle of taking part.
“SMART’s strengths are its foundation based on participatory research methods and an evidence‐based intervention, its use of peer educators facilitating empowerment and training, multimode delivery using traditional group format and telehealth approaches to eliminate barriers to care, and efficacy even in people who have long‐standing epilepsy,” the authors wrote.
“It is possible that SMART, which uses people with epilepsy as guides to help others learn to cope with the challenges of living with this common chronic neurologic condition, may help to alleviate some of the factors that prevent people with epilepsy from optimizing their quality of life.”
The study was supported by the Centers for Disease Control and Prevention. Three authors declared research grants, consultancies, royalties, or speaking positions with the private sector.
SOURCE: Sajatovic M et al. Epilepsia. 2018 Aug 10. doi: 10.1111/epi.14527.
FROM EPILEPSIA
Key clinical point:
Major finding: A remote, self-management intervention for epilepsy was associated with significantly fewer negative health events, compared with being in a wait-list control group.
Study details: A randomized, controlled trial in 120 individuals with epilepsy.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. Three authors declared research grants, consultancies, royalties, or speaking positions with the private sector.
Source: Sajatovic M et al. Epilepsia. 2018 Aug 10. doi: 10.1111/epi.14527.
Sustained minimal disease activity in PsA reduced CV risk factors
Achieving sustained minimal disease activity in psoriatic arthritis could slow or prevent the progression of carotid atherosclerosis and arterial stiffness, new research suggests.
A prospective cohort study, published online Aug. 25 in Arthritis & Rheumatology, involved 90 patients with psoriatic arthritis (PsA) who were followed for at least 24 months. High-resolution carotid ultrasound and arterial stiffness markers were assessed annually.
Overall, 57 patients (63%) achieved minimal disease activity (MDA) – defined as meeting five or more cutoffs for seven domains of disease activity – at 1 year of follow-up, 69% achieved it by 2 years, and 46% sustained MDA from the 1-year follow-up to the 2-year follow-up.
The 41 patients who sustained MDA over the follow-up points showed significantly lower odds of progression of atherosclerotic carotid plaques (odds ratio = 0.273; P = .024). They were also significantly less likely to show increases in total carotid plaque area, increased intima-media thickness, or increased measures of arterial stiffness. The researchers also noted a trend toward improvements in pulse wave velocity in this group.
Patients who achieved sustained MDA had lower disease activity at baseline, and a higher proportion of them were already in a state of MDA at baseline, compared with the group of patients who did not achieved sustained MDA.
Sustained MDA appeared to be key to reducing atherosclerosis, as researchers did not see any significant differences in measures of arterial stiffness between those who achieved MDA – sustained or otherwise – at 2 years and those who did not, after adjusting for baseline differences and use of disease-modifying antirheumatic drugs (DMARDs).
“The most important finding in this study is that long-term control of inflammation is important in preventing progression of subclinical atherosclerosis and arterial stiffness, independent of traditional CV risk factors,” wrote Isaac T. Cheng of Prince of Wales Hospital at The Chinese University of Hong Kong, and his coauthors. “These data suggest that low stable disease activity over a prolonged period of time may have a significant protective effect against CVD in PsA compared with those patients with intermittent flare-ups.”
The study also looked at treatment effect. More patients treated with biologic DMARDs achieved MDA, but the researchers saw no association between the use of conventional synthetic DMARDs and the likelihood of achieving MDA.
They noted that the protective effects of achieving sustained MDA seemed to be independent of biologic DMARDs, “suggesting that controlling disease activity using various combinations of conventional and biological DMARD may be useful in improving CV risk in these patients.”
However, the investigators noted that the study was conducted in Hong Kong, where biologics are not reimbursed by the government. Patients in the study had to pay for these medications themselves, and some could not afford them.
They raised the possibility that early initiation with biologic DMARDs might further improve cardiovascular outcomes, but said this needed to be addressed in future studies.
“These data support the EULAR recommendation that disease activity should be controlled optimally in order to lower CVD risk in patients with PsA,” the authors wrote. “Data from the current study confirm that using a treat-to-target strategy, MDA is indeed an achievable target even in a health care system with resource constrain.”
The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
SOURCE: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
Achieving sustained minimal disease activity in psoriatic arthritis could slow or prevent the progression of carotid atherosclerosis and arterial stiffness, new research suggests.
A prospective cohort study, published online Aug. 25 in Arthritis & Rheumatology, involved 90 patients with psoriatic arthritis (PsA) who were followed for at least 24 months. High-resolution carotid ultrasound and arterial stiffness markers were assessed annually.
Overall, 57 patients (63%) achieved minimal disease activity (MDA) – defined as meeting five or more cutoffs for seven domains of disease activity – at 1 year of follow-up, 69% achieved it by 2 years, and 46% sustained MDA from the 1-year follow-up to the 2-year follow-up.
The 41 patients who sustained MDA over the follow-up points showed significantly lower odds of progression of atherosclerotic carotid plaques (odds ratio = 0.273; P = .024). They were also significantly less likely to show increases in total carotid plaque area, increased intima-media thickness, or increased measures of arterial stiffness. The researchers also noted a trend toward improvements in pulse wave velocity in this group.
Patients who achieved sustained MDA had lower disease activity at baseline, and a higher proportion of them were already in a state of MDA at baseline, compared with the group of patients who did not achieved sustained MDA.
Sustained MDA appeared to be key to reducing atherosclerosis, as researchers did not see any significant differences in measures of arterial stiffness between those who achieved MDA – sustained or otherwise – at 2 years and those who did not, after adjusting for baseline differences and use of disease-modifying antirheumatic drugs (DMARDs).
“The most important finding in this study is that long-term control of inflammation is important in preventing progression of subclinical atherosclerosis and arterial stiffness, independent of traditional CV risk factors,” wrote Isaac T. Cheng of Prince of Wales Hospital at The Chinese University of Hong Kong, and his coauthors. “These data suggest that low stable disease activity over a prolonged period of time may have a significant protective effect against CVD in PsA compared with those patients with intermittent flare-ups.”
The study also looked at treatment effect. More patients treated with biologic DMARDs achieved MDA, but the researchers saw no association between the use of conventional synthetic DMARDs and the likelihood of achieving MDA.
They noted that the protective effects of achieving sustained MDA seemed to be independent of biologic DMARDs, “suggesting that controlling disease activity using various combinations of conventional and biological DMARD may be useful in improving CV risk in these patients.”
However, the investigators noted that the study was conducted in Hong Kong, where biologics are not reimbursed by the government. Patients in the study had to pay for these medications themselves, and some could not afford them.
They raised the possibility that early initiation with biologic DMARDs might further improve cardiovascular outcomes, but said this needed to be addressed in future studies.
“These data support the EULAR recommendation that disease activity should be controlled optimally in order to lower CVD risk in patients with PsA,” the authors wrote. “Data from the current study confirm that using a treat-to-target strategy, MDA is indeed an achievable target even in a health care system with resource constrain.”
The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
SOURCE: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
Achieving sustained minimal disease activity in psoriatic arthritis could slow or prevent the progression of carotid atherosclerosis and arterial stiffness, new research suggests.
A prospective cohort study, published online Aug. 25 in Arthritis & Rheumatology, involved 90 patients with psoriatic arthritis (PsA) who were followed for at least 24 months. High-resolution carotid ultrasound and arterial stiffness markers were assessed annually.
Overall, 57 patients (63%) achieved minimal disease activity (MDA) – defined as meeting five or more cutoffs for seven domains of disease activity – at 1 year of follow-up, 69% achieved it by 2 years, and 46% sustained MDA from the 1-year follow-up to the 2-year follow-up.
The 41 patients who sustained MDA over the follow-up points showed significantly lower odds of progression of atherosclerotic carotid plaques (odds ratio = 0.273; P = .024). They were also significantly less likely to show increases in total carotid plaque area, increased intima-media thickness, or increased measures of arterial stiffness. The researchers also noted a trend toward improvements in pulse wave velocity in this group.
Patients who achieved sustained MDA had lower disease activity at baseline, and a higher proportion of them were already in a state of MDA at baseline, compared with the group of patients who did not achieved sustained MDA.
Sustained MDA appeared to be key to reducing atherosclerosis, as researchers did not see any significant differences in measures of arterial stiffness between those who achieved MDA – sustained or otherwise – at 2 years and those who did not, after adjusting for baseline differences and use of disease-modifying antirheumatic drugs (DMARDs).
“The most important finding in this study is that long-term control of inflammation is important in preventing progression of subclinical atherosclerosis and arterial stiffness, independent of traditional CV risk factors,” wrote Isaac T. Cheng of Prince of Wales Hospital at The Chinese University of Hong Kong, and his coauthors. “These data suggest that low stable disease activity over a prolonged period of time may have a significant protective effect against CVD in PsA compared with those patients with intermittent flare-ups.”
The study also looked at treatment effect. More patients treated with biologic DMARDs achieved MDA, but the researchers saw no association between the use of conventional synthetic DMARDs and the likelihood of achieving MDA.
They noted that the protective effects of achieving sustained MDA seemed to be independent of biologic DMARDs, “suggesting that controlling disease activity using various combinations of conventional and biological DMARD may be useful in improving CV risk in these patients.”
However, the investigators noted that the study was conducted in Hong Kong, where biologics are not reimbursed by the government. Patients in the study had to pay for these medications themselves, and some could not afford them.
They raised the possibility that early initiation with biologic DMARDs might further improve cardiovascular outcomes, but said this needed to be addressed in future studies.
“These data support the EULAR recommendation that disease activity should be controlled optimally in order to lower CVD risk in patients with PsA,” the authors wrote. “Data from the current study confirm that using a treat-to-target strategy, MDA is indeed an achievable target even in a health care system with resource constrain.”
The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
SOURCE: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point:
Major finding: Patients who achieved sustained minimal disease activity had lower odds of progression of atherosclerotic carotid plaques.
Study details: Prospective cohort study of 90 patients with psoriatic arthritis.
Disclosures: The Health and Medical Research Fund supported the study. No conflicts of interest were declared.
Source: Cheng I et al. Arthritis Rheumatol. 2018 Aug 25. doi: 10.1002/art.40695.
Spinraza shows motor improvements in older children with SMA type 1
Treatment with nusinersen (Spinraza) produced significant improvements in motor function in children with spinal muscular atrophy type 1 even if treatment was initiated at a later age, new research has suggested.
In a paper published online Aug. 29 in Neurology, researchers presented the results of a prospective cohort study in 33 children who ranged in age from 8.3 months to 9.4 years and had spinal muscular atrophy type 1. In this study, the children were treated with the antisense oligonucleotide nusinersen, which increases production of functional survival motor neuron (SMN) protein. The patients participated in the trial as a part of an Expanded Access Program for nusinersen that’s operated by its manufacturer, Biogen.
All previous trials of nusinersen have enrolled patients younger than 7 months, wrote Karolina Aragon-Gawinska, MD, of the Institute I-motion at Armand Trousseau Hospital, Paris, and her coauthors. The benefits of the drug in older patients were unknown.
The disease has a median survival of 8-13.5 months of age, yet all patients in the study were alive at 6 months after starting the treatment. Researchers saw a 1.5-point median improvement in motor milestones – measured using the Hammersmith Infant Neurologic Examination (HINE) Part 2 – and five patients were able to sit up without support for more than 30 seconds.
On the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders scale, which assesses the motor skills of infants with spinal muscular atrophy, there was a median improvement of four points at 6 months.
There were no significant changes in the need for nutritional support. However, eight patients showed worsening respiratory condition at 6 months, compared with baseline. Three patients – all of whom had a more severe form of the disease by virtue of only having two copies of the nearly identical SMN2 gene – showed no significant motor progress and were placed on full-time ventilation.
Overall, the number of copies of SMN2 gene did not appear to affect the need for ventilator or nutritional support.
“The response to treatment was highly variable, but new motor acquisitions were attained even in 8-year-old patients,” the authors wrote. “In some patients, respiratory worsening was observed despite motor improvement, suggesting a slower action of nusinersen on the respiratory symptoms and the possible intercurrent infections that might destabilize these weak patients.”
They noted that while previous studies had included patients with only two copies of the SMN2 gene – and hence more severe disease – around half the patients in this study had three copies, which may explain why even older patients showed significant responses to treatment.
“Patients with three SMN2 copies were older and had a longer disease duration than patients with two SMN2 copies, which may partially explain the absence of copy number effect.”
The study was funded by the Institute of Myology and AFM-Telethon. Eight authors reported involvement with pharmaceutical-sponsored trials, consultancies, or other funding from the pharmaceutical industry, including Biogen. No other conflicts of interest were declared.
SOURCE: Aragon-Gawinska K et al. Neurology. 2018 Aug 29. doi: 10.1212/WNL.0000000000006281.
Treatment with nusinersen (Spinraza) produced significant improvements in motor function in children with spinal muscular atrophy type 1 even if treatment was initiated at a later age, new research has suggested.
In a paper published online Aug. 29 in Neurology, researchers presented the results of a prospective cohort study in 33 children who ranged in age from 8.3 months to 9.4 years and had spinal muscular atrophy type 1. In this study, the children were treated with the antisense oligonucleotide nusinersen, which increases production of functional survival motor neuron (SMN) protein. The patients participated in the trial as a part of an Expanded Access Program for nusinersen that’s operated by its manufacturer, Biogen.
All previous trials of nusinersen have enrolled patients younger than 7 months, wrote Karolina Aragon-Gawinska, MD, of the Institute I-motion at Armand Trousseau Hospital, Paris, and her coauthors. The benefits of the drug in older patients were unknown.
The disease has a median survival of 8-13.5 months of age, yet all patients in the study were alive at 6 months after starting the treatment. Researchers saw a 1.5-point median improvement in motor milestones – measured using the Hammersmith Infant Neurologic Examination (HINE) Part 2 – and five patients were able to sit up without support for more than 30 seconds.
On the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders scale, which assesses the motor skills of infants with spinal muscular atrophy, there was a median improvement of four points at 6 months.
There were no significant changes in the need for nutritional support. However, eight patients showed worsening respiratory condition at 6 months, compared with baseline. Three patients – all of whom had a more severe form of the disease by virtue of only having two copies of the nearly identical SMN2 gene – showed no significant motor progress and were placed on full-time ventilation.
Overall, the number of copies of SMN2 gene did not appear to affect the need for ventilator or nutritional support.
“The response to treatment was highly variable, but new motor acquisitions were attained even in 8-year-old patients,” the authors wrote. “In some patients, respiratory worsening was observed despite motor improvement, suggesting a slower action of nusinersen on the respiratory symptoms and the possible intercurrent infections that might destabilize these weak patients.”
They noted that while previous studies had included patients with only two copies of the SMN2 gene – and hence more severe disease – around half the patients in this study had three copies, which may explain why even older patients showed significant responses to treatment.
“Patients with three SMN2 copies were older and had a longer disease duration than patients with two SMN2 copies, which may partially explain the absence of copy number effect.”
The study was funded by the Institute of Myology and AFM-Telethon. Eight authors reported involvement with pharmaceutical-sponsored trials, consultancies, or other funding from the pharmaceutical industry, including Biogen. No other conflicts of interest were declared.
SOURCE: Aragon-Gawinska K et al. Neurology. 2018 Aug 29. doi: 10.1212/WNL.0000000000006281.
Treatment with nusinersen (Spinraza) produced significant improvements in motor function in children with spinal muscular atrophy type 1 even if treatment was initiated at a later age, new research has suggested.
In a paper published online Aug. 29 in Neurology, researchers presented the results of a prospective cohort study in 33 children who ranged in age from 8.3 months to 9.4 years and had spinal muscular atrophy type 1. In this study, the children were treated with the antisense oligonucleotide nusinersen, which increases production of functional survival motor neuron (SMN) protein. The patients participated in the trial as a part of an Expanded Access Program for nusinersen that’s operated by its manufacturer, Biogen.
All previous trials of nusinersen have enrolled patients younger than 7 months, wrote Karolina Aragon-Gawinska, MD, of the Institute I-motion at Armand Trousseau Hospital, Paris, and her coauthors. The benefits of the drug in older patients were unknown.
The disease has a median survival of 8-13.5 months of age, yet all patients in the study were alive at 6 months after starting the treatment. Researchers saw a 1.5-point median improvement in motor milestones – measured using the Hammersmith Infant Neurologic Examination (HINE) Part 2 – and five patients were able to sit up without support for more than 30 seconds.
On the Children’s Hospital of Philadelphia Infant Test of Neuromuscular Disorders scale, which assesses the motor skills of infants with spinal muscular atrophy, there was a median improvement of four points at 6 months.
There were no significant changes in the need for nutritional support. However, eight patients showed worsening respiratory condition at 6 months, compared with baseline. Three patients – all of whom had a more severe form of the disease by virtue of only having two copies of the nearly identical SMN2 gene – showed no significant motor progress and were placed on full-time ventilation.
Overall, the number of copies of SMN2 gene did not appear to affect the need for ventilator or nutritional support.
“The response to treatment was highly variable, but new motor acquisitions were attained even in 8-year-old patients,” the authors wrote. “In some patients, respiratory worsening was observed despite motor improvement, suggesting a slower action of nusinersen on the respiratory symptoms and the possible intercurrent infections that might destabilize these weak patients.”
They noted that while previous studies had included patients with only two copies of the SMN2 gene – and hence more severe disease – around half the patients in this study had three copies, which may explain why even older patients showed significant responses to treatment.
“Patients with three SMN2 copies were older and had a longer disease duration than patients with two SMN2 copies, which may partially explain the absence of copy number effect.”
The study was funded by the Institute of Myology and AFM-Telethon. Eight authors reported involvement with pharmaceutical-sponsored trials, consultancies, or other funding from the pharmaceutical industry, including Biogen. No other conflicts of interest were declared.
SOURCE: Aragon-Gawinska K et al. Neurology. 2018 Aug 29. doi: 10.1212/WNL.0000000000006281.
FROM NEUROLOGY
Key clinical point: Nusinersen can produce motor improvements in older children with spinal muscular atrophy type 1.
Major finding: Nusinersen treatment showed a median 1.5-point improvement in HINE-2 score after 6 months.
Study details: Prospective cohort study in 33 children with spinal muscular atrophy type 1.
Disclosures: The study was funded by the Institute of Myology and AFM-Telethon. Eight authors reported involvement with pharmaceutical-sponsored trials, consultancies, or other funding from the pharmaceutical industry, including from nusinersen manufacturer Biogen. No other conflicts of interest were declared.
Source: Aragon-Gawinska K et al. Neurology. 2018 Aug 29. doi: 10.1212/WNL.0000000000006281.
36% of soldiers who attempt suicide have no mental health diagnosis
About one-third of enlisted soldiers who attempt suicide do not have a history of mental illness, say the authors of a retrospective longitudinal study among U.S. Army personnel.
However, health care use, exposure to violent crime, and first year of service all were significantly associated with suicide attempts.
The study, published online Aug. 29 in JAMA Psychiatry, analyzed administrative data from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) for 9,650 enlisted soldiers (74.8% male) who had a documented suicide attempt.
Overall, 36.3% of the soldiers studied who attempted suicide did not have a previous diagnosis of a mental health disorder. Having a history of mental illness was associated with sixfold higher odds of suicide attempt after adjusting for sociodemographic and service-related variables.
Among those without a history of mental health disorders, the first year of service was associated with sixfold higher odds of a suicide attempt (95% confidence interval, 4.7-7.7), and nearly 60% of those without a previous diagnosis who attempted suicide did so in their first year of service.
“This factor is noteworthy, because, as in the general population, most transitions from ideation to attempt among soldiers occur within 1 year of ideation onset,” wrote Robert J. Ursano, MD, professor of psychiatry and neurosciences at the Center for the Study of Traumatic Stress at the Uniformed Services University of the Health Sciences, Bethesda, Md., and his coauthors.
Among individuals without a history of mental health disorders, women had 2.6-fold higher odds (95% CI, 2.4-2.8) of a suicide attempt. The authors suggested that these
“Given the large proportion of first-year soldiers in the group without previous [mental health diagnosis], it is possible that women face additional stressors and challenges in the initial months of service,” they wrote.
Previous deployment was associated with 2.4-fold higher odds of suicide attempt; a 2-month delay in promotion was associated with 2.1-fold higher odds. In addition, a demotion in the past year was associated with 60% higher odds of a suicide attempt.
Health care use also showed associations with suicide attempts. Individuals who had visited outpatient services eight or more times in the past 2 months had more than threefold higher odds of suicide attempt. Those with an injury-related visit to outpatient services in the previous month had threefold higher odds, and those who had an injury-related visit to inpatient services had a 3.8-fold higher odds.
Exposure to minor violent crime and perpetration of major violent crime or family violence also were associated with higher odds of suicide attempt.
The authors cited several limitations. One is that the study did not capture unreported suicide attempts, mental disorders, or crimes.
The Army STARRS was supported by the Department of the Army, the U.S. Department of Health & Human Services, the National Institutes of Health, the National Institute of Mental Health and the Department of Defense. Two authors were employed by the NIMH, and two were Army liaisons or consultants.
SOURCE: Ursano RJ et al. JAMA Psychiatry. 2018 Aug 29. doi: 10.1001/jamapsychiatry.2018.2069.
An increase in suicide rates in the Army during the 2000s led to an increased focus on suicide prevention. However, at the time these study data were being collected, treatment for suicide ideation outside deployment largely occurred in the context of mental health clinics, wrote Mark A. Reger, PhD, Derek J. Smolenski, PhD, and Sarah P. Carter.
Therefore, individuals without mental health symptoms might have been less likely to be identified as being at risk, and therefore, less likely to receive evidence-based treatment. This suggests that greater focus is needed on suicide prevention in other settings, and particularly on combating the stigma associated with a mental health diagnosis – which is often around concerns of career effects from such a diagnosis.
To the Army’s credit, it has shifted toward integrating mental health services into new locations and services – including embedded behavioral health teams – with the aim of increasing access, decreasing stigma, and improving consultation with command.
Dr. Reger and Ms. Carter are affiliated with the Veterans Affairs Puget Sound Health Care System in Seattle, and Dr. Smolenski is with the Psychological Health Center of Excellence at the Defense Health Agency in Tacoma, Wash. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Aug 29. doi: 10.1001/jamapsychiatry.2018.2042). No conflicts of interest were declared.
An increase in suicide rates in the Army during the 2000s led to an increased focus on suicide prevention. However, at the time these study data were being collected, treatment for suicide ideation outside deployment largely occurred in the context of mental health clinics, wrote Mark A. Reger, PhD, Derek J. Smolenski, PhD, and Sarah P. Carter.
Therefore, individuals without mental health symptoms might have been less likely to be identified as being at risk, and therefore, less likely to receive evidence-based treatment. This suggests that greater focus is needed on suicide prevention in other settings, and particularly on combating the stigma associated with a mental health diagnosis – which is often around concerns of career effects from such a diagnosis.
To the Army’s credit, it has shifted toward integrating mental health services into new locations and services – including embedded behavioral health teams – with the aim of increasing access, decreasing stigma, and improving consultation with command.
Dr. Reger and Ms. Carter are affiliated with the Veterans Affairs Puget Sound Health Care System in Seattle, and Dr. Smolenski is with the Psychological Health Center of Excellence at the Defense Health Agency in Tacoma, Wash. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Aug 29. doi: 10.1001/jamapsychiatry.2018.2042). No conflicts of interest were declared.
An increase in suicide rates in the Army during the 2000s led to an increased focus on suicide prevention. However, at the time these study data were being collected, treatment for suicide ideation outside deployment largely occurred in the context of mental health clinics, wrote Mark A. Reger, PhD, Derek J. Smolenski, PhD, and Sarah P. Carter.
Therefore, individuals without mental health symptoms might have been less likely to be identified as being at risk, and therefore, less likely to receive evidence-based treatment. This suggests that greater focus is needed on suicide prevention in other settings, and particularly on combating the stigma associated with a mental health diagnosis – which is often around concerns of career effects from such a diagnosis.
To the Army’s credit, it has shifted toward integrating mental health services into new locations and services – including embedded behavioral health teams – with the aim of increasing access, decreasing stigma, and improving consultation with command.
Dr. Reger and Ms. Carter are affiliated with the Veterans Affairs Puget Sound Health Care System in Seattle, and Dr. Smolenski is with the Psychological Health Center of Excellence at the Defense Health Agency in Tacoma, Wash. These comments are taken from an accompanying editorial (JAMA Psychiatry. 2018 Aug 29. doi: 10.1001/jamapsychiatry.2018.2042). No conflicts of interest were declared.
About one-third of enlisted soldiers who attempt suicide do not have a history of mental illness, say the authors of a retrospective longitudinal study among U.S. Army personnel.
However, health care use, exposure to violent crime, and first year of service all were significantly associated with suicide attempts.
The study, published online Aug. 29 in JAMA Psychiatry, analyzed administrative data from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) for 9,650 enlisted soldiers (74.8% male) who had a documented suicide attempt.
Overall, 36.3% of the soldiers studied who attempted suicide did not have a previous diagnosis of a mental health disorder. Having a history of mental illness was associated with sixfold higher odds of suicide attempt after adjusting for sociodemographic and service-related variables.
Among those without a history of mental health disorders, the first year of service was associated with sixfold higher odds of a suicide attempt (95% confidence interval, 4.7-7.7), and nearly 60% of those without a previous diagnosis who attempted suicide did so in their first year of service.
“This factor is noteworthy, because, as in the general population, most transitions from ideation to attempt among soldiers occur within 1 year of ideation onset,” wrote Robert J. Ursano, MD, professor of psychiatry and neurosciences at the Center for the Study of Traumatic Stress at the Uniformed Services University of the Health Sciences, Bethesda, Md., and his coauthors.
Among individuals without a history of mental health disorders, women had 2.6-fold higher odds (95% CI, 2.4-2.8) of a suicide attempt. The authors suggested that these
“Given the large proportion of first-year soldiers in the group without previous [mental health diagnosis], it is possible that women face additional stressors and challenges in the initial months of service,” they wrote.
Previous deployment was associated with 2.4-fold higher odds of suicide attempt; a 2-month delay in promotion was associated with 2.1-fold higher odds. In addition, a demotion in the past year was associated with 60% higher odds of a suicide attempt.
Health care use also showed associations with suicide attempts. Individuals who had visited outpatient services eight or more times in the past 2 months had more than threefold higher odds of suicide attempt. Those with an injury-related visit to outpatient services in the previous month had threefold higher odds, and those who had an injury-related visit to inpatient services had a 3.8-fold higher odds.
Exposure to minor violent crime and perpetration of major violent crime or family violence also were associated with higher odds of suicide attempt.
The authors cited several limitations. One is that the study did not capture unreported suicide attempts, mental disorders, or crimes.
The Army STARRS was supported by the Department of the Army, the U.S. Department of Health & Human Services, the National Institutes of Health, the National Institute of Mental Health and the Department of Defense. Two authors were employed by the NIMH, and two were Army liaisons or consultants.
SOURCE: Ursano RJ et al. JAMA Psychiatry. 2018 Aug 29. doi: 10.1001/jamapsychiatry.2018.2069.
About one-third of enlisted soldiers who attempt suicide do not have a history of mental illness, say the authors of a retrospective longitudinal study among U.S. Army personnel.
However, health care use, exposure to violent crime, and first year of service all were significantly associated with suicide attempts.
The study, published online Aug. 29 in JAMA Psychiatry, analyzed administrative data from the Army Study to Assess Risk and Resilience in Servicemembers (Army STARRS) for 9,650 enlisted soldiers (74.8% male) who had a documented suicide attempt.
Overall, 36.3% of the soldiers studied who attempted suicide did not have a previous diagnosis of a mental health disorder. Having a history of mental illness was associated with sixfold higher odds of suicide attempt after adjusting for sociodemographic and service-related variables.
Among those without a history of mental health disorders, the first year of service was associated with sixfold higher odds of a suicide attempt (95% confidence interval, 4.7-7.7), and nearly 60% of those without a previous diagnosis who attempted suicide did so in their first year of service.
“This factor is noteworthy, because, as in the general population, most transitions from ideation to attempt among soldiers occur within 1 year of ideation onset,” wrote Robert J. Ursano, MD, professor of psychiatry and neurosciences at the Center for the Study of Traumatic Stress at the Uniformed Services University of the Health Sciences, Bethesda, Md., and his coauthors.
Among individuals without a history of mental health disorders, women had 2.6-fold higher odds (95% CI, 2.4-2.8) of a suicide attempt. The authors suggested that these
“Given the large proportion of first-year soldiers in the group without previous [mental health diagnosis], it is possible that women face additional stressors and challenges in the initial months of service,” they wrote.
Previous deployment was associated with 2.4-fold higher odds of suicide attempt; a 2-month delay in promotion was associated with 2.1-fold higher odds. In addition, a demotion in the past year was associated with 60% higher odds of a suicide attempt.
Health care use also showed associations with suicide attempts. Individuals who had visited outpatient services eight or more times in the past 2 months had more than threefold higher odds of suicide attempt. Those with an injury-related visit to outpatient services in the previous month had threefold higher odds, and those who had an injury-related visit to inpatient services had a 3.8-fold higher odds.
Exposure to minor violent crime and perpetration of major violent crime or family violence also were associated with higher odds of suicide attempt.
The authors cited several limitations. One is that the study did not capture unreported suicide attempts, mental disorders, or crimes.
The Army STARRS was supported by the Department of the Army, the U.S. Department of Health & Human Services, the National Institutes of Health, the National Institute of Mental Health and the Department of Defense. Two authors were employed by the NIMH, and two were Army liaisons or consultants.
SOURCE: Ursano RJ et al. JAMA Psychiatry. 2018 Aug 29. doi: 10.1001/jamapsychiatry.2018.2069.
FROM JAMA PSYCHIATRY
Key clinical point: Many U.S. Army soldiers who attempt suicide and have no mental health diagnosis probably have undetected mental health disorders.
Major finding: More than one-third of soldiers who have attempted suicide do not have a history of mental health diagnosis.
Study details: Retrospective cohort study in 9,650 enlisted soldiers who had a documented suicide attempt.
Disclosures: The Army STARRS was supported by the Department of the Army, the U.S. Department of Health & Human Services, the National Institutes of Health, the National Institute of Mental Health, and the Department of Defense. Two authors were employed by the NIMH, and two were Army liaisons or consultants.
Source: Ursano RJ et al. JAMA Psychiatry. 2018 Aug 29. doi: 10.1001/jamapsychiatry.2018.2069.