User login
Negative chest x-ray to rule out pediatric pneumonia
, researchers say.
In a paper published in the September issue of Pediatrics, researchers report the results of a prospective cohort study in 683 children – with a median age of 3.1 years – presenting to emergency departments with suspected pneumonia.
Dr. Susan C. Lipsett, from the division of emergency medicine at Boston Children’s Hospital, and co-authors, wrote that the use of chest radiograph to diagnose pneumonia is thought to have limitations such as its inability to distinguish between bacteria and viral infection, and the possible absence of radiographic presentations early in the disease in patients with dehydration.
In this study, 457 (72.8%) of the children had negative chest radiographs. Of these, 44 were clinically diagnosed with pneumonia, despite the radiograph results, and prescribed antibiotics. These children were more likely to have rales or respiratory distress and less likely to have wheezing compared with the children with negative radiographs who were not initially diagnosed with pneumonia.
Among the remaining 411 children with negative radiographs – who were not prescribed antibiotics – five (1.2%) were subsequently diagnosed with pneumonia within 2 weeks of the radiograph. These five children were all under 3 years of age, but none had been treated with intravenous fluids for dehydration. Only one had radiographic findings of pneumonia on a follow-up visit.
Counting the 44 children diagnosed with pneumonia despite the negative x-ray, chest radiography showed a negative predictive value of 89.2% (95% confidence interval, 85.9%-91.9%). Without those children, the negative predictive value was 98.8% (95% CI, 97%-99.6%).
There were also 113 children (16.5%) with positive chest radiographs, and 72 (10.7%) with equivocal radiographs.
The authors said their results showed that most children with negative chest radiograph would recover fully without needing antibiotics, and argued there was a place for chest radiography in the diagnostic process, to rule out bacterial pneumonia.
“Most clinicians caring for children in the outpatient setting rely on clinical signs and symptoms to determine whether to prescribe an antibiotic for the treatment of pneumonia,” they wrote. “However, given recent literature in which the poor reliability and validity of physical examination findings are cited, reliance on physical examination alone may lead to the overdiagnosis of pneumonia.”
They acknowledged that the lack of a universally accepted gold standard for the diagnosis of pneumonia in children was a significant limitation of the research. In addition, the lack of systematic radiographs meant some children who initially had a negative result and recovered without antibiotics may have shown a positive result on a second scan.
No conflicts of interest were declared.
SOURCE: Lipsett S et al. Pediatrics 2018 142(3):e20180236.
While the results of this study offer reassurance that chest radiograph for suspected pneumonia in children has a high negative predictive value, perhaps a more important question is the accuracy of chest radiography at ruling in bacterial pneumonia – its positive predictive value.
There are reasons to suspect that the positive predictive value of chest radiography may not be as high as the negative predictive value found in this study. This is particularly important given that questions have been raised about the utility of antibiotic therapy in treating Mycoplasma pneumonia infection in children.
Leaving out chest radiography altogether in children with a low clinical suspicion for pneumonia would decreased radi-ation use, cost, and perhaps also unnecessary antibiotic prescriptions.
Matthew D. Garber, MD, is from the department of pediatrics at the University of Florida College of Medicine in Jacksonville and Ricardo A. Quinonez, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital. These comments are taken from an accompanying editorial (Pediatrics 2018, 142(3): e20182025. https://doi.org/10.1542/peds.2018-2025). No conflicts of interest were declared.
While the results of this study offer reassurance that chest radiograph for suspected pneumonia in children has a high negative predictive value, perhaps a more important question is the accuracy of chest radiography at ruling in bacterial pneumonia – its positive predictive value.
There are reasons to suspect that the positive predictive value of chest radiography may not be as high as the negative predictive value found in this study. This is particularly important given that questions have been raised about the utility of antibiotic therapy in treating Mycoplasma pneumonia infection in children.
Leaving out chest radiography altogether in children with a low clinical suspicion for pneumonia would decreased radi-ation use, cost, and perhaps also unnecessary antibiotic prescriptions.
Matthew D. Garber, MD, is from the department of pediatrics at the University of Florida College of Medicine in Jacksonville and Ricardo A. Quinonez, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital. These comments are taken from an accompanying editorial (Pediatrics 2018, 142(3): e20182025. https://doi.org/10.1542/peds.2018-2025). No conflicts of interest were declared.
While the results of this study offer reassurance that chest radiograph for suspected pneumonia in children has a high negative predictive value, perhaps a more important question is the accuracy of chest radiography at ruling in bacterial pneumonia – its positive predictive value.
There are reasons to suspect that the positive predictive value of chest radiography may not be as high as the negative predictive value found in this study. This is particularly important given that questions have been raised about the utility of antibiotic therapy in treating Mycoplasma pneumonia infection in children.
Leaving out chest radiography altogether in children with a low clinical suspicion for pneumonia would decreased radi-ation use, cost, and perhaps also unnecessary antibiotic prescriptions.
Matthew D. Garber, MD, is from the department of pediatrics at the University of Florida College of Medicine in Jacksonville and Ricardo A. Quinonez, MD, is from the department of pediatrics at the Baylor College of Medicine and Texas Children’s Hospital. These comments are taken from an accompanying editorial (Pediatrics 2018, 142(3): e20182025. https://doi.org/10.1542/peds.2018-2025). No conflicts of interest were declared.
, researchers say.
In a paper published in the September issue of Pediatrics, researchers report the results of a prospective cohort study in 683 children – with a median age of 3.1 years – presenting to emergency departments with suspected pneumonia.
Dr. Susan C. Lipsett, from the division of emergency medicine at Boston Children’s Hospital, and co-authors, wrote that the use of chest radiograph to diagnose pneumonia is thought to have limitations such as its inability to distinguish between bacteria and viral infection, and the possible absence of radiographic presentations early in the disease in patients with dehydration.
In this study, 457 (72.8%) of the children had negative chest radiographs. Of these, 44 were clinically diagnosed with pneumonia, despite the radiograph results, and prescribed antibiotics. These children were more likely to have rales or respiratory distress and less likely to have wheezing compared with the children with negative radiographs who were not initially diagnosed with pneumonia.
Among the remaining 411 children with negative radiographs – who were not prescribed antibiotics – five (1.2%) were subsequently diagnosed with pneumonia within 2 weeks of the radiograph. These five children were all under 3 years of age, but none had been treated with intravenous fluids for dehydration. Only one had radiographic findings of pneumonia on a follow-up visit.
Counting the 44 children diagnosed with pneumonia despite the negative x-ray, chest radiography showed a negative predictive value of 89.2% (95% confidence interval, 85.9%-91.9%). Without those children, the negative predictive value was 98.8% (95% CI, 97%-99.6%).
There were also 113 children (16.5%) with positive chest radiographs, and 72 (10.7%) with equivocal radiographs.
The authors said their results showed that most children with negative chest radiograph would recover fully without needing antibiotics, and argued there was a place for chest radiography in the diagnostic process, to rule out bacterial pneumonia.
“Most clinicians caring for children in the outpatient setting rely on clinical signs and symptoms to determine whether to prescribe an antibiotic for the treatment of pneumonia,” they wrote. “However, given recent literature in which the poor reliability and validity of physical examination findings are cited, reliance on physical examination alone may lead to the overdiagnosis of pneumonia.”
They acknowledged that the lack of a universally accepted gold standard for the diagnosis of pneumonia in children was a significant limitation of the research. In addition, the lack of systematic radiographs meant some children who initially had a negative result and recovered without antibiotics may have shown a positive result on a second scan.
No conflicts of interest were declared.
SOURCE: Lipsett S et al. Pediatrics 2018 142(3):e20180236.
, researchers say.
In a paper published in the September issue of Pediatrics, researchers report the results of a prospective cohort study in 683 children – with a median age of 3.1 years – presenting to emergency departments with suspected pneumonia.
Dr. Susan C. Lipsett, from the division of emergency medicine at Boston Children’s Hospital, and co-authors, wrote that the use of chest radiograph to diagnose pneumonia is thought to have limitations such as its inability to distinguish between bacteria and viral infection, and the possible absence of radiographic presentations early in the disease in patients with dehydration.
In this study, 457 (72.8%) of the children had negative chest radiographs. Of these, 44 were clinically diagnosed with pneumonia, despite the radiograph results, and prescribed antibiotics. These children were more likely to have rales or respiratory distress and less likely to have wheezing compared with the children with negative radiographs who were not initially diagnosed with pneumonia.
Among the remaining 411 children with negative radiographs – who were not prescribed antibiotics – five (1.2%) were subsequently diagnosed with pneumonia within 2 weeks of the radiograph. These five children were all under 3 years of age, but none had been treated with intravenous fluids for dehydration. Only one had radiographic findings of pneumonia on a follow-up visit.
Counting the 44 children diagnosed with pneumonia despite the negative x-ray, chest radiography showed a negative predictive value of 89.2% (95% confidence interval, 85.9%-91.9%). Without those children, the negative predictive value was 98.8% (95% CI, 97%-99.6%).
There were also 113 children (16.5%) with positive chest radiographs, and 72 (10.7%) with equivocal radiographs.
The authors said their results showed that most children with negative chest radiograph would recover fully without needing antibiotics, and argued there was a place for chest radiography in the diagnostic process, to rule out bacterial pneumonia.
“Most clinicians caring for children in the outpatient setting rely on clinical signs and symptoms to determine whether to prescribe an antibiotic for the treatment of pneumonia,” they wrote. “However, given recent literature in which the poor reliability and validity of physical examination findings are cited, reliance on physical examination alone may lead to the overdiagnosis of pneumonia.”
They acknowledged that the lack of a universally accepted gold standard for the diagnosis of pneumonia in children was a significant limitation of the research. In addition, the lack of systematic radiographs meant some children who initially had a negative result and recovered without antibiotics may have shown a positive result on a second scan.
No conflicts of interest were declared.
SOURCE: Lipsett S et al. Pediatrics 2018 142(3):e20180236.
FROM PEDIATRICS
Key clinical point: Negative chest radiograph can rule out pneumonia in children.
Major finding: Chest radiograph has a negative predictive value of 89.2% in children with suspected pneumonia.
Study details: Prospective cohort study in 683 children with suspected pneumonia.
Disclosures: No conflicts of interest were declared.
Source: Lipsett S et al. Pediatrics 2018 142(3):e20180236. https://doi.org/10.1542/peds.2018-0236.
Combo produces high response rate in CLL
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Combo treatment yields MRD-negative remissions in CLL
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: The overall response rate for obinutuzumab plus venetoclax in CLL was 95%.
Study details: An ongoing, phase 2, open-label trial in 66 patients with chronic lymphocytic leukemia.
Disclosures: The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Source: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
New MS subtype shows absence of cerebral white matter demyelination
A new subtype of multiple sclerosis called myelocortical multiple sclerosis is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter.
A paper published online Aug. 21 in Lancet Neurology presents the results of a study of the brains and spinal cords of 100 patients who died of multiple sclerosis.
Bruce D. Trapp, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors wrote that while the demyelination of cerebral white matter is a pathologic hallmark of multiple sclerosis, previous research has found only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they wrote.
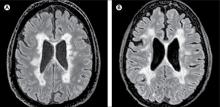
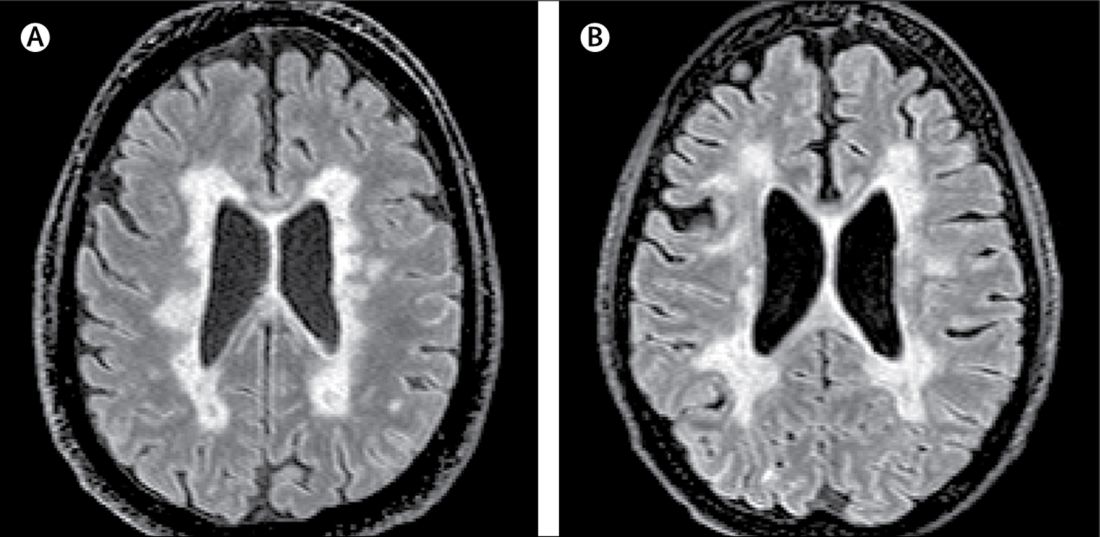
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as ‘myelocortical multiple sclerosis,’ characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals to 12 individuals with typical multiple sclerosis matched by age, sex, MRI protocol, multiple sclerosis disease subtype, disease duration, and Expanded Disability Status Scale.
They found that while individuals with myelocortical multiple sclerosis did not have demyelinated lesions in the cerebral white matter, they had similar areas of demyelinated lesions in the cerebral cortex to individuals with typical multiple sclerosis (median 4.45% vs. 9.74% respectively, P = .5512).
However, the individuals with myelocortical multiple sclerosis had a significantly smaller area of spinal cord demyelination (median 3.81% vs. 13.81%, P = .0083).
Individuals with myelocortical multiple sclerosis also had significantly lower mean cortical neuronal densities, compared with healthy control brains in layer III, layer V, and layer VI. But individuals with typical multiple sclerosis only had a lower cortical neuronal density in layer V when compared with controls.
Researchers also saw that in typical multiple sclerosis, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical multiple sclerosis.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical multiple sclerosis, in T2-weighted, T1-weighted and magnetization transfer ratios (MTR) images.
They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical and with typical multiple sclerosis, although individuals with typical multiple sclerosis had significantly greater MTR lesion volumes.
“We propose that myelocortical multiple sclerosis is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” the authors wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathological evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical multiple sclerosis.”
The authors noted that their study may have been affected by selection bias, as all the patients in the study had died from complications of advanced multiple sclerosis. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical multiple sclerosis seen in their sample would be similar across the multiple sclerosis population, nor were the findings likely to apply to people with earlier stage disease.
The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, and three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and non-financial support from pharmaceutical companies.
SOURCE: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
A new subtype of multiple sclerosis called myelocortical multiple sclerosis is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter.
A paper published online Aug. 21 in Lancet Neurology presents the results of a study of the brains and spinal cords of 100 patients who died of multiple sclerosis.
Bruce D. Trapp, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors wrote that while the demyelination of cerebral white matter is a pathologic hallmark of multiple sclerosis, previous research has found only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they wrote.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as ‘myelocortical multiple sclerosis,’ characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals to 12 individuals with typical multiple sclerosis matched by age, sex, MRI protocol, multiple sclerosis disease subtype, disease duration, and Expanded Disability Status Scale.
They found that while individuals with myelocortical multiple sclerosis did not have demyelinated lesions in the cerebral white matter, they had similar areas of demyelinated lesions in the cerebral cortex to individuals with typical multiple sclerosis (median 4.45% vs. 9.74% respectively, P = .5512).
However, the individuals with myelocortical multiple sclerosis had a significantly smaller area of spinal cord demyelination (median 3.81% vs. 13.81%, P = .0083).
Individuals with myelocortical multiple sclerosis also had significantly lower mean cortical neuronal densities, compared with healthy control brains in layer III, layer V, and layer VI. But individuals with typical multiple sclerosis only had a lower cortical neuronal density in layer V when compared with controls.
Researchers also saw that in typical multiple sclerosis, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical multiple sclerosis.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical multiple sclerosis, in T2-weighted, T1-weighted and magnetization transfer ratios (MTR) images.
They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical and with typical multiple sclerosis, although individuals with typical multiple sclerosis had significantly greater MTR lesion volumes.
“We propose that myelocortical multiple sclerosis is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” the authors wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathological evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical multiple sclerosis.”
The authors noted that their study may have been affected by selection bias, as all the patients in the study had died from complications of advanced multiple sclerosis. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical multiple sclerosis seen in their sample would be similar across the multiple sclerosis population, nor were the findings likely to apply to people with earlier stage disease.
The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, and three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and non-financial support from pharmaceutical companies.
SOURCE: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
A new subtype of multiple sclerosis called myelocortical multiple sclerosis is characterized by demyelination only in the spinal cord and cerebral cortex and not in the cerebral white matter.
A paper published online Aug. 21 in Lancet Neurology presents the results of a study of the brains and spinal cords of 100 patients who died of multiple sclerosis.
Bruce D. Trapp, PhD, of the Lerner Research Institute at the Cleveland Clinic in Ohio, and his coauthors wrote that while the demyelination of cerebral white matter is a pathologic hallmark of multiple sclerosis, previous research has found only around half of cerebral T2-weighted hyperintense white matter lesions are demyelinated, and these lesions account for less than a third of variance in the rate of brain atrophy.
“In the absence of specific MRI metrics for demyelination, the relationship between cerebral white-matter demyelination and neurodegeneration remains speculative,” they wrote.
In this study, researchers scanned the brains with MRI before autopsy, then took centimeter-thick hemispheric slices to study the white-matter lesions. They identified 12 individuals as having what they describe as ‘myelocortical multiple sclerosis,’ characterized by the absence of areas of cerebral white-matter discoloration indicative of demyelinated lesions.
The authors then compared these individuals to 12 individuals with typical multiple sclerosis matched by age, sex, MRI protocol, multiple sclerosis disease subtype, disease duration, and Expanded Disability Status Scale.
They found that while individuals with myelocortical multiple sclerosis did not have demyelinated lesions in the cerebral white matter, they had similar areas of demyelinated lesions in the cerebral cortex to individuals with typical multiple sclerosis (median 4.45% vs. 9.74% respectively, P = .5512).
However, the individuals with myelocortical multiple sclerosis had a significantly smaller area of spinal cord demyelination (median 3.81% vs. 13.81%, P = .0083).
Individuals with myelocortical multiple sclerosis also had significantly lower mean cortical neuronal densities, compared with healthy control brains in layer III, layer V, and layer VI. But individuals with typical multiple sclerosis only had a lower cortical neuronal density in layer V when compared with controls.
Researchers also saw that in typical multiple sclerosis, neuronal density decreased as the area of brain white-matter demyelination increased. However, this negative linear correlation was not seen in myelocortical multiple sclerosis.
On MRI, researchers were still able to see abnormalities in the cerebral white matter in individuals with myelocortical multiple sclerosis, in T2-weighted, T1-weighted and magnetization transfer ratios (MTR) images.
They also found similar total T2-weighted and T1-weighted lesion volumes in individuals with myelocortical and with typical multiple sclerosis, although individuals with typical multiple sclerosis had significantly greater MTR lesion volumes.
“We propose that myelocortical multiple sclerosis is characterized by spinal cord demyelination, subpial cortical demyelination, and an absence of cerebral white-matter demyelination,” the authors wrote. “Our findings indicate that abnormal cerebral white-matter T2-T1-MTR regions of interest are not always demyelinated, and this pathological evidence suggests that cerebral white-matter demyelination and cortical neuronal degeneration can be independent events in myelocortical multiple sclerosis.”
The authors noted that their study may have been affected by selection bias, as all the patients in the study had died from complications of advanced multiple sclerosis. They suggested that it was therefore not appropriate to conclude that the prevalence of myelocortical multiple sclerosis seen in their sample would be similar across the multiple sclerosis population, nor were the findings likely to apply to people with earlier stage disease.
The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, and three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees, and non-financial support from pharmaceutical companies.
SOURCE: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
FROM LANCET NEUROLOGY
Key clinical point: Researchers have identified a new subtype of multiple sclerosis.
Major finding: Individuals with myelocortical multiple sclerosis show demyelination in the spinal cord and cortex only.
Study details: Post-mortem study of brains and spinal cords of 100 individuals with multiple sclerosis.
Disclosures: The study was funded by the U.S. National Institutes of Health and National Multiple Sclerosis Society. One author was an employee of Renovo Neural, three authors were employees of Biogen. One author declared a pending patent related to automated lesion segmentation from MRI images, and four authors declared funding, fees and non-financial support from pharmaceutical companies.
Source: Trapp B et al. Lancet Neurol. 2018 Aug 21. doi: 10.1016/ S1474-4422(18)30245-X.
No increase in primary ovarian insufficiency with HPV vaccine
The human papillomavirus vaccine does not appear to be associated with an increased risk of ovarian insufficiency, according to researchers.
Allison L. Naleway, PhD, of the Center for Health Research at Kaiser Permanente Northwest, Portland, Ore., and her coauthors wrote that a previous case series had raised concerns about a possible link between the human papillomavirus (HPV) vaccine and primary ovarian insufficiency (POI) in six young women who developed the condition within 12 months of vaccination.
Using EHR data, researchers identified 46 women aged 11-34 years with idiopathic POI – 33 probable cases and 13 possible cases – after excluding cases with known causes. Eighteen of these cases also were excluded because they were diagnosed before the HPV vaccine was available.
They found that only 1 of the remaining 28 patients had been vaccinated against HPV before the symptom onset: a 16-year-old girl who was vaccinated about 23 months before the first clinical evaluation for delayed menarche. Their report was published in Pediatrics.
The adjusted hazard ratio for POI was therefore 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
More than one-half of the 46 confirmed POI cases were diagnosed at age 27 years or older, and only one patient was diagnosed under 15 years of age.
“If POI is triggered by HPV or other adolescent vaccine exposure, we would have expected to see elevated incidence in the younger women who were most likely to be vaccinated, but instead we observed higher incidence in older women (greater than 26 years of age), which is consistent with 1 other population-based study of POI prevalence,” the authors wrote.
They acknowledged that studying POI as a vaccine-related adverse event was challenging because the time from symptom onset to diagnosis was variable. However, they said that 81% of their cohort was followed up for more than 2 years, and a mean of 5.14 years, so the potential for misclassification was “minimal.”
Dr. Naleway and her associates also noted that diagnoses of POI can be difficult to accurately identify, and symptoms may be masked by oral contraceptive use.
“Despite the challenges and limitations discussed above, we believe this study should lessen concern surrounding potential impact on fertility from HPV or other adolescent vaccination,” they wrote.
The Centers for Disease Control and Prevention supported the study. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
SOURCE: Naleway A et al. Pediatrics 2018;42(3):e20180943.
The human papillomavirus vaccine does not appear to be associated with an increased risk of ovarian insufficiency, according to researchers.
Allison L. Naleway, PhD, of the Center for Health Research at Kaiser Permanente Northwest, Portland, Ore., and her coauthors wrote that a previous case series had raised concerns about a possible link between the human papillomavirus (HPV) vaccine and primary ovarian insufficiency (POI) in six young women who developed the condition within 12 months of vaccination.
Using EHR data, researchers identified 46 women aged 11-34 years with idiopathic POI – 33 probable cases and 13 possible cases – after excluding cases with known causes. Eighteen of these cases also were excluded because they were diagnosed before the HPV vaccine was available.
They found that only 1 of the remaining 28 patients had been vaccinated against HPV before the symptom onset: a 16-year-old girl who was vaccinated about 23 months before the first clinical evaluation for delayed menarche. Their report was published in Pediatrics.
The adjusted hazard ratio for POI was therefore 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
More than one-half of the 46 confirmed POI cases were diagnosed at age 27 years or older, and only one patient was diagnosed under 15 years of age.
“If POI is triggered by HPV or other adolescent vaccine exposure, we would have expected to see elevated incidence in the younger women who were most likely to be vaccinated, but instead we observed higher incidence in older women (greater than 26 years of age), which is consistent with 1 other population-based study of POI prevalence,” the authors wrote.
They acknowledged that studying POI as a vaccine-related adverse event was challenging because the time from symptom onset to diagnosis was variable. However, they said that 81% of their cohort was followed up for more than 2 years, and a mean of 5.14 years, so the potential for misclassification was “minimal.”
Dr. Naleway and her associates also noted that diagnoses of POI can be difficult to accurately identify, and symptoms may be masked by oral contraceptive use.
“Despite the challenges and limitations discussed above, we believe this study should lessen concern surrounding potential impact on fertility from HPV or other adolescent vaccination,” they wrote.
The Centers for Disease Control and Prevention supported the study. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
SOURCE: Naleway A et al. Pediatrics 2018;42(3):e20180943.
The human papillomavirus vaccine does not appear to be associated with an increased risk of ovarian insufficiency, according to researchers.
Allison L. Naleway, PhD, of the Center for Health Research at Kaiser Permanente Northwest, Portland, Ore., and her coauthors wrote that a previous case series had raised concerns about a possible link between the human papillomavirus (HPV) vaccine and primary ovarian insufficiency (POI) in six young women who developed the condition within 12 months of vaccination.
Using EHR data, researchers identified 46 women aged 11-34 years with idiopathic POI – 33 probable cases and 13 possible cases – after excluding cases with known causes. Eighteen of these cases also were excluded because they were diagnosed before the HPV vaccine was available.
They found that only 1 of the remaining 28 patients had been vaccinated against HPV before the symptom onset: a 16-year-old girl who was vaccinated about 23 months before the first clinical evaluation for delayed menarche. Their report was published in Pediatrics.
The adjusted hazard ratio for POI was therefore 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
More than one-half of the 46 confirmed POI cases were diagnosed at age 27 years or older, and only one patient was diagnosed under 15 years of age.
“If POI is triggered by HPV or other adolescent vaccine exposure, we would have expected to see elevated incidence in the younger women who were most likely to be vaccinated, but instead we observed higher incidence in older women (greater than 26 years of age), which is consistent with 1 other population-based study of POI prevalence,” the authors wrote.
They acknowledged that studying POI as a vaccine-related adverse event was challenging because the time from symptom onset to diagnosis was variable. However, they said that 81% of their cohort was followed up for more than 2 years, and a mean of 5.14 years, so the potential for misclassification was “minimal.”
Dr. Naleway and her associates also noted that diagnoses of POI can be difficult to accurately identify, and symptoms may be masked by oral contraceptive use.
“Despite the challenges and limitations discussed above, we believe this study should lessen concern surrounding potential impact on fertility from HPV or other adolescent vaccination,” they wrote.
The Centers for Disease Control and Prevention supported the study. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
SOURCE: Naleway A et al. Pediatrics 2018;42(3):e20180943.
FROM PEDIATRICS
Key clinical point:
Major finding: The adjusted hazard ratio for POI was 0.30 after HPV vaccine, compared with 0.88 after Tdap, 1.42 after inactivated influenza vaccine, and 0.94 after meningococcal conjugate vaccine.
Study details: Analysis of medical records data for 46 women with confirmed iatrogenic primary ovarian failure.
Disclosures: The study was supported by the Centers for Disease Control and Prevention. Three authors declared funding from pharmaceutical companies for unrelated studies. No other conflicts of interest were declared.
Source: Naleway A et al. Pediatrics 2018;142(3):e20180943.
Prenatal marijuana use higher in women with severe nausea and vomiting
Pregnant women who experience severe nausea and vomiting have nearly fourfold greater odds of prenatal marijuana use compared with women not experiencing nausea and vomiting, according to data from 220,510 first-trimester screenings.
In a research letter published in JAMA Internal Medicine, researchers reported the results of a health care system data analysis, which found a 3.80-fold greater prevalence of prenatal marijuana use among women with severe nausea and vomiting in pregnancy, compared with those who did not experience nausea and vomiting.
Among women with mild nausea and vomiting in pregnancy, there was still a significant twofold higher prevalence of marijuana use.
“Use of marijuana, an antiemetic, is increasing among pregnant women, and data from two small surveys indicate that women self-report using marijuana to alleviate nausea and vomiting in pregnancy (NVP),” wrote Kelly C. Young-Wolff, PhD, of the division of research at Kaiser Permanente Northern California, Oakland, and her coauthors.
In this study, 2% of the women experienced severe and 15% experienced mild nausea and vomiting during pregnancy.
The overall prevalence of marijuana use – assessed either by self-report or toxicological test findings – was 5.3%, with 0.7% positive on self-report only, 3.1% positive on toxicologic testing only, and 1.5% positive on both.
The authors said the findings supported the hypothesis that pregnant women were using marijuana to self-medicate for NVP. However, they also noted that clinicians may diagnose NVP more frequently among women who report using marijuana to treat it.
Dr. Young-Wolff and her coauthors said that they would not have been able to distinguish prenatal marijuana use from use before the women knew they were pregnant, “and misclassification is possible given variability in the time that marijuana is detectable in urine.
“The health effects of prenatal marijuana use are unclear, and national guidelines recommend that pregnant women discontinue use,” the authors wrote. “Patients with NVP should be screened for marijuana use and educated about effective and safe NVP treatments.”
The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
Pregnant women who experience severe nausea and vomiting have nearly fourfold greater odds of prenatal marijuana use compared with women not experiencing nausea and vomiting, according to data from 220,510 first-trimester screenings.
In a research letter published in JAMA Internal Medicine, researchers reported the results of a health care system data analysis, which found a 3.80-fold greater prevalence of prenatal marijuana use among women with severe nausea and vomiting in pregnancy, compared with those who did not experience nausea and vomiting.
Among women with mild nausea and vomiting in pregnancy, there was still a significant twofold higher prevalence of marijuana use.
“Use of marijuana, an antiemetic, is increasing among pregnant women, and data from two small surveys indicate that women self-report using marijuana to alleviate nausea and vomiting in pregnancy (NVP),” wrote Kelly C. Young-Wolff, PhD, of the division of research at Kaiser Permanente Northern California, Oakland, and her coauthors.
In this study, 2% of the women experienced severe and 15% experienced mild nausea and vomiting during pregnancy.
The overall prevalence of marijuana use – assessed either by self-report or toxicological test findings – was 5.3%, with 0.7% positive on self-report only, 3.1% positive on toxicologic testing only, and 1.5% positive on both.
The authors said the findings supported the hypothesis that pregnant women were using marijuana to self-medicate for NVP. However, they also noted that clinicians may diagnose NVP more frequently among women who report using marijuana to treat it.
Dr. Young-Wolff and her coauthors said that they would not have been able to distinguish prenatal marijuana use from use before the women knew they were pregnant, “and misclassification is possible given variability in the time that marijuana is detectable in urine.
“The health effects of prenatal marijuana use are unclear, and national guidelines recommend that pregnant women discontinue use,” the authors wrote. “Patients with NVP should be screened for marijuana use and educated about effective and safe NVP treatments.”
The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
Pregnant women who experience severe nausea and vomiting have nearly fourfold greater odds of prenatal marijuana use compared with women not experiencing nausea and vomiting, according to data from 220,510 first-trimester screenings.
In a research letter published in JAMA Internal Medicine, researchers reported the results of a health care system data analysis, which found a 3.80-fold greater prevalence of prenatal marijuana use among women with severe nausea and vomiting in pregnancy, compared with those who did not experience nausea and vomiting.
Among women with mild nausea and vomiting in pregnancy, there was still a significant twofold higher prevalence of marijuana use.
“Use of marijuana, an antiemetic, is increasing among pregnant women, and data from two small surveys indicate that women self-report using marijuana to alleviate nausea and vomiting in pregnancy (NVP),” wrote Kelly C. Young-Wolff, PhD, of the division of research at Kaiser Permanente Northern California, Oakland, and her coauthors.
In this study, 2% of the women experienced severe and 15% experienced mild nausea and vomiting during pregnancy.
The overall prevalence of marijuana use – assessed either by self-report or toxicological test findings – was 5.3%, with 0.7% positive on self-report only, 3.1% positive on toxicologic testing only, and 1.5% positive on both.
The authors said the findings supported the hypothesis that pregnant women were using marijuana to self-medicate for NVP. However, they also noted that clinicians may diagnose NVP more frequently among women who report using marijuana to treat it.
Dr. Young-Wolff and her coauthors said that they would not have been able to distinguish prenatal marijuana use from use before the women knew they were pregnant, “and misclassification is possible given variability in the time that marijuana is detectable in urine.
“The health effects of prenatal marijuana use are unclear, and national guidelines recommend that pregnant women discontinue use,” the authors wrote. “Patients with NVP should be screened for marijuana use and educated about effective and safe NVP treatments.”
The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
SOURCE: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
FROM JAMA INTERNAL MEDICINE
Key clinical point:
Major finding: Women with severe nausea and vomiting in pregnancy have nearly fourfold higher odds of prenatal marijuana use.
Study details: Analysis of health insurance data from 220,510 prenatal screenings.
Disclosures: The study was supported by the National Institute on Drug Abuse and the National Institute of Mental Health. No conflicts of interest were declared.
Source: Young-Wolff K et al. JAMA Intern Med. 2018 Aug 20. doi: 10.1001/jamainternmed.2018.3581.
Lenalidomide may be best maintenance for MM
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma (MM), according to the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, PhD, of the University of Torino in Italy, and her coauthors wrote that, despite the well-recognized importance of novel agent–based maintenance therapy for MM, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published in JAMA Oncology, the researchers reported the results of a systematic review and meta-analysis of 11 prospective, phase 3, randomized, controlled trials of 8 varieties of maintenance therapy in 5073 participants with newly diagnosed MM.
The researchers found that lenalidomide-based regimens showed the best progression-free survival rates, compared with placebo. The hazard ratio (HR) was 0.39 for lenalidomide plus prednisone, and the HR was 0.47 for lenalidomide alone.
In 74% of the network meta-analysis simulations, lenalidomide-based regimens were the most effective options.
Four other maintenance treatment options—thalidomide-interferon (HR, 0.50), thalidomide-bortezomib (HR, 0.58), bortezomib-prednisone (HR, 0.72), and thalidomide alone (HR, 0.73)—also showed progression-free survival gains, but interferon therapy (HR, 0.91) failed to show any benefit.
For overall survival, lenalidomide alone (HR, 0.76) was the best option, followed by thalidomide-bortezomib (HR, 0.82) and bortezomib-prednisone (HR, 0.84). None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplant, they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis—for example, with ISS stage III disease—benefited more from bortezomib-based maintenance.
However, patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points, or the patients’ extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
The authors also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees, and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma (MM), according to the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, PhD, of the University of Torino in Italy, and her coauthors wrote that, despite the well-recognized importance of novel agent–based maintenance therapy for MM, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published in JAMA Oncology, the researchers reported the results of a systematic review and meta-analysis of 11 prospective, phase 3, randomized, controlled trials of 8 varieties of maintenance therapy in 5073 participants with newly diagnosed MM.
The researchers found that lenalidomide-based regimens showed the best progression-free survival rates, compared with placebo. The hazard ratio (HR) was 0.39 for lenalidomide plus prednisone, and the HR was 0.47 for lenalidomide alone.
In 74% of the network meta-analysis simulations, lenalidomide-based regimens were the most effective options.
Four other maintenance treatment options—thalidomide-interferon (HR, 0.50), thalidomide-bortezomib (HR, 0.58), bortezomib-prednisone (HR, 0.72), and thalidomide alone (HR, 0.73)—also showed progression-free survival gains, but interferon therapy (HR, 0.91) failed to show any benefit.
For overall survival, lenalidomide alone (HR, 0.76) was the best option, followed by thalidomide-bortezomib (HR, 0.82) and bortezomib-prednisone (HR, 0.84). None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplant, they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis—for example, with ISS stage III disease—benefited more from bortezomib-based maintenance.
However, patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points, or the patients’ extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
The authors also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees, and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma (MM), according to the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, PhD, of the University of Torino in Italy, and her coauthors wrote that, despite the well-recognized importance of novel agent–based maintenance therapy for MM, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published in JAMA Oncology, the researchers reported the results of a systematic review and meta-analysis of 11 prospective, phase 3, randomized, controlled trials of 8 varieties of maintenance therapy in 5073 participants with newly diagnosed MM.
The researchers found that lenalidomide-based regimens showed the best progression-free survival rates, compared with placebo. The hazard ratio (HR) was 0.39 for lenalidomide plus prednisone, and the HR was 0.47 for lenalidomide alone.
In 74% of the network meta-analysis simulations, lenalidomide-based regimens were the most effective options.
Four other maintenance treatment options—thalidomide-interferon (HR, 0.50), thalidomide-bortezomib (HR, 0.58), bortezomib-prednisone (HR, 0.72), and thalidomide alone (HR, 0.73)—also showed progression-free survival gains, but interferon therapy (HR, 0.91) failed to show any benefit.
For overall survival, lenalidomide alone (HR, 0.76) was the best option, followed by thalidomide-bortezomib (HR, 0.82) and bortezomib-prednisone (HR, 0.84). None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplant, they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis—for example, with ISS stage III disease—benefited more from bortezomib-based maintenance.
However, patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points, or the patients’ extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
The authors also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees, and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
Lenalidomide best option for myeloma maintenance therapy
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma, say the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, from the division of hematology at the University of Torino (Italy), and her coauthors wrote that despite the well-recognized importance of novel agent–based maintenance therapy for multiple myeloma, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published online in JAMA Oncology, the researchers reported the results of the systematic review and meta-analysis of 11 prospective, phase 3 randomized, controlled trials of eight varieties of maintenance therapy, in 5,073 participants with newly diagnosed multiple myeloma.
Their analysis found that lenalidomide-based regimens showed the best progression-free survival rates (hazard ratio, 0.39 for lenalidomide plus prednisone; HR, 0.47 for lenalidomide alone), compared with placebo, and in 74% of the network meta-analysis simulations, they were the most effective options.
Four other maintenance treatment options - thalidomide-interferon, thalidomide-bortezomib, bortezomib-prednisone, and thalidomide alone – also showed progression-free survival gains – but interferon therapy failed to show any benefit.
However, for overall survival, lenalidomide alone was the best option, followed by thalidomide-bortezomib and bortezomib-prednisone. None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplantation they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis – for example, with ISS stage III disease – benefited more from bortezomib-based maintenance. However patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points or their extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
They also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
SOURCE: Gay F et al. 2018 Aug 9. doi:10.1001/jamaoncol.2018.2961.
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma, say the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, from the division of hematology at the University of Torino (Italy), and her coauthors wrote that despite the well-recognized importance of novel agent–based maintenance therapy for multiple myeloma, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published online in JAMA Oncology, the researchers reported the results of the systematic review and meta-analysis of 11 prospective, phase 3 randomized, controlled trials of eight varieties of maintenance therapy, in 5,073 participants with newly diagnosed multiple myeloma.
Their analysis found that lenalidomide-based regimens showed the best progression-free survival rates (hazard ratio, 0.39 for lenalidomide plus prednisone; HR, 0.47 for lenalidomide alone), compared with placebo, and in 74% of the network meta-analysis simulations, they were the most effective options.
Four other maintenance treatment options - thalidomide-interferon, thalidomide-bortezomib, bortezomib-prednisone, and thalidomide alone – also showed progression-free survival gains – but interferon therapy failed to show any benefit.
However, for overall survival, lenalidomide alone was the best option, followed by thalidomide-bortezomib and bortezomib-prednisone. None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplantation they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis – for example, with ISS stage III disease – benefited more from bortezomib-based maintenance. However patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points or their extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
They also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
SOURCE: Gay F et al. 2018 Aug 9. doi:10.1001/jamaoncol.2018.2961.
Lenalidomide may be the best maintenance treatment option for patients with newly diagnosed multiple myeloma, say the authors of a systematic review and meta-analysis.
Francesca M. Gay, MD, from the division of hematology at the University of Torino (Italy), and her coauthors wrote that despite the well-recognized importance of novel agent–based maintenance therapy for multiple myeloma, there is a lack of direct or indirect comparisons between the available regimens.
In a paper published online in JAMA Oncology, the researchers reported the results of the systematic review and meta-analysis of 11 prospective, phase 3 randomized, controlled trials of eight varieties of maintenance therapy, in 5,073 participants with newly diagnosed multiple myeloma.
Their analysis found that lenalidomide-based regimens showed the best progression-free survival rates (hazard ratio, 0.39 for lenalidomide plus prednisone; HR, 0.47 for lenalidomide alone), compared with placebo, and in 74% of the network meta-analysis simulations, they were the most effective options.
Four other maintenance treatment options - thalidomide-interferon, thalidomide-bortezomib, bortezomib-prednisone, and thalidomide alone – also showed progression-free survival gains – but interferon therapy failed to show any benefit.
However, for overall survival, lenalidomide alone was the best option, followed by thalidomide-bortezomib and bortezomib-prednisone. None of the other regimens considered showed benefits for overall survival.
“Long-term use of lenalidomide undoubtedly has advantages, owing to the lack of neuropathy, which is the main factor limiting the long-term use of both thalidomide and bortezomib,” the authors wrote.
When the authors restricted their analysis to trials conducted in the setting of autologous stem cell transplantation they found similar results, with lenalidomide-based regimens having the best progression-free and overall survival.
Patients with a good prognosis and standard-risk chromosomal abnormalities also did best with lenalidomide-based maintenance, while those with a poor prognosis – for example, with ISS stage III disease – benefited more from bortezomib-based maintenance. However patients with high-risk chromosomal abnormalities gained no advantage from any regimen, which the authors suggested may relate to small sample size, different cut-off points or their extremely poor prognosis.
The authors noted that their analysis did not take into account adverse events, drug discontinuations, or quality of life but focused solely on progression-free survival and overall survival.
“An increase in second primary malignant disease with prolonged lenalidomide therapy has been reported, but the survival benefit overcame the risk in all the trials,” they wrote.
They also commented that better treatment options are needed for patients with aggressive disease, and there are ongoing trials looking at second-generation proteasome inhibitors, immunomodulatory agents, and monoclonal antibodies for maintenance therapy.
Most authors declared research funding, advisory board positions, fees and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
SOURCE: Gay F et al. 2018 Aug 9. doi:10.1001/jamaoncol.2018.2961.
FROM JAMA ONCOLOGY
Key clinical point: Lenalidomide is the best option for maintenance therapy in multiple myeloma.
Major finding: Lenalidomide-based maintenance regimens show the best progression-free and overall survival in multiple myeloma.
Study details: Systematic review and network meta-analysis of 11 studies in 5073 participants with newly diagnosed multiple myeloma.
Disclosures: Most authors declared research funding, advisory board positions, fees and honoraria from the pharmaceutical industry, including lenalidomide manufacturer Celgene.
Source: Gay F et al. 2018 Aug 9. doi: 10.1001/jamaoncol.2018.2961.
Shared decision making falls short for lung cancer screening
A small study of discussions between clinicians and patients about has highlighted a lack of shared decision making and information about potential harms.
“Our findings are consistent with increasingly robust evidence that patients, members of the public, and clinicians tend to overestimate the benefits and underestimate the harms of medical interventions, including treatments, tests, or screening tests,” wrote Alison T. Brenner, PhD, and her colleagues at the University of North Carolina at Chapel Hill, in a presentation of the findings in JAMA Internal Medicine.
The researchers transcribed conversations between 14 patients – who were eligible for lung cancer screening because of their age – and their primary care or pulmonary care physicians. They found that not one physician adequately explained false positives or their consequences, such as the possibility of additional imaging and invasive diagnostic procedures, nor did any discuss the potential for diagnosis and treatment of cancer that would not have affected the individual during his or her lifetime (overdiagnosis).
Researchers used a 12-item scoring system for physician behaviors, with 0-4 points allocated to each item. The items included telling patients there was more than one way to deal with the identified problem, explaining the pros and cons of the available options, exploring patients’ fears and concerns, and offering the patient clear opportunities to ask questions.
Mean scores for each item ranged from 0 to 0.79. Two conversations met the baseline skill criteria – a score of two points – for one item each, two other conversations met the baseline skill criteria for two items. But for 8 of the 12 items, not one conversation achieved even a baseline skill score. The mean total visit length was 13:07 minutes, and the mean time spent discussing LCS was 0:59 minute (range, 0:16-2:19 minutes).
“Although experts disagree on how well the existing evidence suggests an overall net benefit of LCS [lung cancer screening], consensus has emerged on the importance of shared decision making,” wrote the investigators. Current U.S. Preventive Services Task Force recommendations stress that lung cancer screening should not occur without a shared decision-making process, including a thorough discussion of benefits and harms.
The authors said that, while their study was small, it did raise concerns that shared decision making in practice is a long way from what is recommended by the guidelines.
“The fact that the main drivers of harms from LCS (false positives and their sequelae, as well as overdiagnosis) were not adequately explained by physicians is troubling,” they wrote. “However, these findings are consistent with other evidence that discussions between patients and physicians regarding preference-sensitive cancer screening decisions are imbalanced with respect to explaining the pros and cons.”
Based on these findings, the authors called for urgent discussions between clinical leaders, policy makers, and researchers about how to involve patients more meaningfully in discussions about lung cancer screening.
“Until more is known, we believe that guideline and policy makers should not assume that recommending SDM [shared decision-making] for cancer-screening decisions with a ‘tenuous balance of benefits and harms,’ like LCS, will protect patients who would value avoiding screening harms.”
The study was supported by the North Carolina Translational and Clinical Sciences Institute and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Brenner A et al. JAMA Intern Med. 2018; Aug 13. doi: 10.1001/jamainternmed.2018.3054.
The results of this first real-world study of the U.S. Preventive Services Task Force recommendations on lung cancer screening – which comes 4 years after the recommendations were made – are disappointing. Even the highest-scoring conversations made no mention of possible harms, such as a 98% false-positive rate, additional testing, and the small increased cancer risk from radiation.
Rita F. Redberg, MD, is from the department of medicine in the division of cardiology at the University of California, San Francisco, and the editor of JAMA Internal Medicine. These comments are taken from an accompanying editorial (JAMA Int Med. 2018 Aug 13. doi: 10.1001/jamainternmed.2018.3527). Dr. Redberg chaired the April 2014 Medicare Evidence Development & Coverage Advisory Committee meeting on lung cancer screening.
The results of this first real-world study of the U.S. Preventive Services Task Force recommendations on lung cancer screening – which comes 4 years after the recommendations were made – are disappointing. Even the highest-scoring conversations made no mention of possible harms, such as a 98% false-positive rate, additional testing, and the small increased cancer risk from radiation.
Rita F. Redberg, MD, is from the department of medicine in the division of cardiology at the University of California, San Francisco, and the editor of JAMA Internal Medicine. These comments are taken from an accompanying editorial (JAMA Int Med. 2018 Aug 13. doi: 10.1001/jamainternmed.2018.3527). Dr. Redberg chaired the April 2014 Medicare Evidence Development & Coverage Advisory Committee meeting on lung cancer screening.
The results of this first real-world study of the U.S. Preventive Services Task Force recommendations on lung cancer screening – which comes 4 years after the recommendations were made – are disappointing. Even the highest-scoring conversations made no mention of possible harms, such as a 98% false-positive rate, additional testing, and the small increased cancer risk from radiation.
Rita F. Redberg, MD, is from the department of medicine in the division of cardiology at the University of California, San Francisco, and the editor of JAMA Internal Medicine. These comments are taken from an accompanying editorial (JAMA Int Med. 2018 Aug 13. doi: 10.1001/jamainternmed.2018.3527). Dr. Redberg chaired the April 2014 Medicare Evidence Development & Coverage Advisory Committee meeting on lung cancer screening.
A small study of discussions between clinicians and patients about has highlighted a lack of shared decision making and information about potential harms.
“Our findings are consistent with increasingly robust evidence that patients, members of the public, and clinicians tend to overestimate the benefits and underestimate the harms of medical interventions, including treatments, tests, or screening tests,” wrote Alison T. Brenner, PhD, and her colleagues at the University of North Carolina at Chapel Hill, in a presentation of the findings in JAMA Internal Medicine.
The researchers transcribed conversations between 14 patients – who were eligible for lung cancer screening because of their age – and their primary care or pulmonary care physicians. They found that not one physician adequately explained false positives or their consequences, such as the possibility of additional imaging and invasive diagnostic procedures, nor did any discuss the potential for diagnosis and treatment of cancer that would not have affected the individual during his or her lifetime (overdiagnosis).
Researchers used a 12-item scoring system for physician behaviors, with 0-4 points allocated to each item. The items included telling patients there was more than one way to deal with the identified problem, explaining the pros and cons of the available options, exploring patients’ fears and concerns, and offering the patient clear opportunities to ask questions.
Mean scores for each item ranged from 0 to 0.79. Two conversations met the baseline skill criteria – a score of two points – for one item each, two other conversations met the baseline skill criteria for two items. But for 8 of the 12 items, not one conversation achieved even a baseline skill score. The mean total visit length was 13:07 minutes, and the mean time spent discussing LCS was 0:59 minute (range, 0:16-2:19 minutes).
“Although experts disagree on how well the existing evidence suggests an overall net benefit of LCS [lung cancer screening], consensus has emerged on the importance of shared decision making,” wrote the investigators. Current U.S. Preventive Services Task Force recommendations stress that lung cancer screening should not occur without a shared decision-making process, including a thorough discussion of benefits and harms.
The authors said that, while their study was small, it did raise concerns that shared decision making in practice is a long way from what is recommended by the guidelines.
“The fact that the main drivers of harms from LCS (false positives and their sequelae, as well as overdiagnosis) were not adequately explained by physicians is troubling,” they wrote. “However, these findings are consistent with other evidence that discussions between patients and physicians regarding preference-sensitive cancer screening decisions are imbalanced with respect to explaining the pros and cons.”
Based on these findings, the authors called for urgent discussions between clinical leaders, policy makers, and researchers about how to involve patients more meaningfully in discussions about lung cancer screening.
“Until more is known, we believe that guideline and policy makers should not assume that recommending SDM [shared decision-making] for cancer-screening decisions with a ‘tenuous balance of benefits and harms,’ like LCS, will protect patients who would value avoiding screening harms.”
The study was supported by the North Carolina Translational and Clinical Sciences Institute and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Brenner A et al. JAMA Intern Med. 2018; Aug 13. doi: 10.1001/jamainternmed.2018.3054.
A small study of discussions between clinicians and patients about has highlighted a lack of shared decision making and information about potential harms.
“Our findings are consistent with increasingly robust evidence that patients, members of the public, and clinicians tend to overestimate the benefits and underestimate the harms of medical interventions, including treatments, tests, or screening tests,” wrote Alison T. Brenner, PhD, and her colleagues at the University of North Carolina at Chapel Hill, in a presentation of the findings in JAMA Internal Medicine.
The researchers transcribed conversations between 14 patients – who were eligible for lung cancer screening because of their age – and their primary care or pulmonary care physicians. They found that not one physician adequately explained false positives or their consequences, such as the possibility of additional imaging and invasive diagnostic procedures, nor did any discuss the potential for diagnosis and treatment of cancer that would not have affected the individual during his or her lifetime (overdiagnosis).
Researchers used a 12-item scoring system for physician behaviors, with 0-4 points allocated to each item. The items included telling patients there was more than one way to deal with the identified problem, explaining the pros and cons of the available options, exploring patients’ fears and concerns, and offering the patient clear opportunities to ask questions.
Mean scores for each item ranged from 0 to 0.79. Two conversations met the baseline skill criteria – a score of two points – for one item each, two other conversations met the baseline skill criteria for two items. But for 8 of the 12 items, not one conversation achieved even a baseline skill score. The mean total visit length was 13:07 minutes, and the mean time spent discussing LCS was 0:59 minute (range, 0:16-2:19 minutes).
“Although experts disagree on how well the existing evidence suggests an overall net benefit of LCS [lung cancer screening], consensus has emerged on the importance of shared decision making,” wrote the investigators. Current U.S. Preventive Services Task Force recommendations stress that lung cancer screening should not occur without a shared decision-making process, including a thorough discussion of benefits and harms.
The authors said that, while their study was small, it did raise concerns that shared decision making in practice is a long way from what is recommended by the guidelines.
“The fact that the main drivers of harms from LCS (false positives and their sequelae, as well as overdiagnosis) were not adequately explained by physicians is troubling,” they wrote. “However, these findings are consistent with other evidence that discussions between patients and physicians regarding preference-sensitive cancer screening decisions are imbalanced with respect to explaining the pros and cons.”
Based on these findings, the authors called for urgent discussions between clinical leaders, policy makers, and researchers about how to involve patients more meaningfully in discussions about lung cancer screening.
“Until more is known, we believe that guideline and policy makers should not assume that recommending SDM [shared decision-making] for cancer-screening decisions with a ‘tenuous balance of benefits and harms,’ like LCS, will protect patients who would value avoiding screening harms.”
The study was supported by the North Carolina Translational and Clinical Sciences Institute and the National Cancer Institute. No conflicts of interest were declared.
SOURCE: Brenner A et al. JAMA Intern Med. 2018; Aug 13. doi: 10.1001/jamainternmed.2018.3054.
FROM JAMA INTERNAL MEDICINE
Key clinical point: Few clinicians are following recommendations about shared decision-making with patients regarding lung cancer screening.
Major finding: No physicians in the study explained false positives and their risks in discussions with patients about lung cancer screening.
Study details: Content analysis of 14 recorded consultations about lung cancer screening.
Disclosures: The study was supported by the North Carolina Translational and Clinical Sciences Institute and the National Cancer Institute. No conflicts of interest were declared.
Source: Brenner A et al. JAMA Intern Med. 2018; Aug 13. doi: 10.1001/jamainternmed.2018.3054.
No increase in autism risk with prenatal Tdap
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
A retrospective cohort study in more than 80,000 children has found no evidence of an increased risk of autism spectrum disorder associated with prenatal tetanus, diphtheria, and acellular pertussis (Tdap) immunization.
Of 81,993 children born between 2011 and 2014, 1,341 children (1.6%) were diagnosed with autism spectrum disorder. The incidence of autism spectrum disorder was 3.78 per 1,000 person-years in the Tdap-vaccinated group, and 4.05 per 1,000 person years in the unvaccinated group, representing an unadjusted hazard ratio of 0.98 and an adjusted hazard ratio of 0.85. This was consistent across all the birth cohorts.
Prenatal immunization rates with the prenatal Tdap vaccine ranged from 26% of the 2012 birth cohort to 79% of the 2014 birth cohort, and mean gestational age at vaccination was 28 weeks.
Tracy A. Becerra-Culqui, PhD, MPH, and colleagues of the department of research and evaluation at Kaiser Permanente Southern California, Pasadena, said this was the first study to look at the risk of autism spectrum disorder after maternal exposure to the Tdap vaccine, to their knowledge. “Our results potentially indicate that the maternal Tdap vaccine affects immune trajectories protecting infants against infections that would otherwise lead to neurodevelopmental alterations.”
They highlighted several strengths of their study. One was that maternal Tdap vaccination and information on autism spectrum disorder both were derived from EHRs and therefore not subject to recall bias. The study, published online in Pediatrics, also included children diagnosed with autism spectrum disorder from age 1 year onwards, reflecting the latest evidence on screening and diagnosis of autism.
“Our weighting procedures enabled us to balance the Tdap-exposed and -unexposed groups to compare two populations that were comparable in important measured confounding factors,” Dr. Becerra-Culqui and associates noted.
The investigators found that women who received the Tdap vaccine during pregnancy were more likely to be Asian American or Pacific Islander, to have a bachelor’s degree or higher, be nulliparous, to have also been vaccinated prenatally against influenza, and to deliver at term, compared with unvaccinated women.
However the authors did note that their follow-up was limited to 6.5 years for the earliest birth cohort, and 3.5 years for the latest cohort, so they may not have picked up children who received a later diagnosis of autism spectrum disorder.
The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
SOURCE: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.
FROM PEDIATRICS
Key clinical point:
Major finding: The adjusted hazard ratio for autism spectrum disorder in children exposed to the prenatal Tdap vaccine is 0.98, compared with unvaccinated children.
Study details: A retrospective cohort study in 81,993 children exposed to the prenatal Tdap vaccine.
Disclosures: The study was supported by Kaiser Permanente Southern California. Five authors declared funding from GlaxoSmithKline, Bayer AG, or the Centers for Disease Control and Prevention for unrelated or separate studies.
Source: Becerra-Culqui T et al. Pediatrics. 2018;142(3):e20180120.