User login
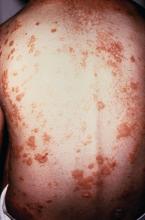
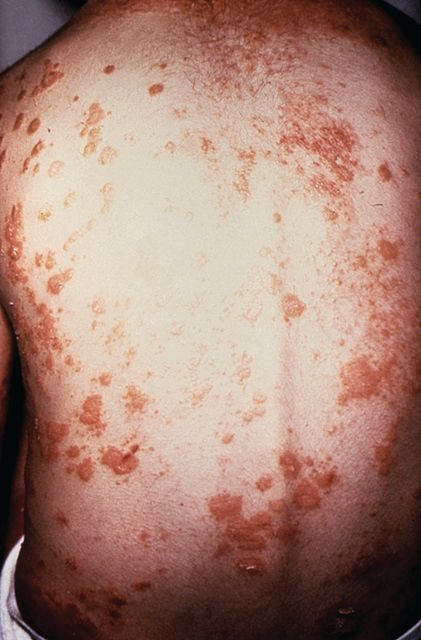
No evidence of subclinical axial involvement seen in skin psoriasis
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
A study of individuals with longstanding skin psoriasis but no clinical arthritis or spondylitis has found no evidence of subclinical involvement of the sacroiliac joint or spine.
The prevalence of sacroiliac lesions on blinded MRI assessment was similar in 20 patients who had skin psoriasis for a median of 23 years and in 22 healthy controls, and no sacroiliac ankylosis was seen in either group. Similarly, there was no significant difference between the two groups in spinal lesions on MRI, nor in any of the five levels of lesion frequency, Vlad A. Bratu, MD, of the department of radiology at University Hospital Basel (Switzerland) and his coauthors reported in Arthritis Care & Research.
On blinded MRI assessment, five (25%) patients with skin psoriasis and two (9.1%) controls were classified as having inflammation of the sacroiliac joint. Three of these patients in the psoriasis group and one in the control group were older than 50, and the three with psoriasis had had the condition for 26-35 years.
Dr. Bratu and his colleagues said that subclinical peripheral joint inflammation on MRI had previously been a common finding in patients who had skin psoriasis but no clinical signs of psoriatic arthritis. But given the limited evidence of concomitant subclinical axial or spinal inflammation in their study, the authors argued there was no support for routine screening for potential subclinical axial inflammation in patients with longstanding skin psoriasis.
They noted that bone marrow edema lesions in at least two sacroiliac joint quadrants were seen in 35% of patients with psoriasis and 23% of healthy controls, a finding that reflected those seen in other studies in healthy individuals.
“If a specificity threshold for a given MRI lesion of at least 0.9 is applied for axial MRI to discriminate between axial SpA [spondyloarthritis] and background variation in healthy controls or in differential diagnostic conditions, no more than 10% of healthy controls in our study should meet this criterion by an individual level data analysis,” they wrote.
The authors also pointed out the impact of age on lesion frequency, which was more evident in spinal lesions.
“This observation supports the hypothesis that some spinal alterations in higher age may reflect degenerative rather than inflammatory changes,” they wrote. “However, there is a gap in knowledge with virtually no evidence about presence and pattern of degenerative versus inflammatory spinal lesions in subjects beyond 50 years of age.”
The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
SOURCE: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: The prevalence of sacroiliac bone marrow lesions was similar between patients with skin psoriasis and healthy controls.
Study details: Case-control study in 20 patients with skin psoriasis and 22 healthy controls.
Disclosures: The study was supported by the Gottfried and Julia Bangerter-Rhyner Foundation. No conflicts of interest were declared.
Source: Bratu V et al. Arthritis Care Res. 2018 Sep 22. doi: 10.1002/acr.23767.
Five-year follow-up confirms safety of antibiotics for uncomplicated appendicitis
Longer-term outcomes of treating uncomplicated acute appendicitis with antibiotics suggest it is a feasible alternative to appendectomy.
Researchers have presented the 5-year follow-up data from the Appendicitis Acuta (APPAC) multicenter randomized clinical trial comparing appendectomy with antibiotic therapy in 530 patients.
They found that 39.1% (100) of the 257 patients randomized to antibiotic therapy – 3 days of intravenous ertapenem followed by 7 days of oral levofloxacin and metronidazole – experienced a recurrence of appendicitis within 5 years and subsequently had surgery.
However the authors noted that seven of these patients were later found not to have appendicitis, so the true success rate for antibiotic treatment was actually 63.7%.
Seventy of the patients who experienced a recurrence underwent surgery in the first year after randomization, 17 in the second year, 3 in the third year, 5 in the fourth year, and the remaining 5 patients in the fifth year, the authors wrote in an article published in JAMA.
However the overall complication rate was similar in patients who were randomized to undergo appendectomy and in those who were initially randomized to the antibiotic group but later experienced a recurrence and underwent surgery.
“No patient initially treated with antibiotics, who ultimately developed recurrent appendicitis, had any complications related to the delay in surgery,” wrote Paulina Salminen, MD, from Turku (Finland) University Hospital and coauthors. “Nearly two-thirds of all patients who initially presented with uncomplicated appendicitis were successfully treated with antibiotics alone, and those who ultimately developed recurrent disease did not experience any adverse outcomes related to the delay in appendectomy.”
Of the 100 patients randomized to antibiotics who underwent appendectomy after a recurrence, 15 were operated on when they were first hospitalized at study admission.
The authors commented that the study design allowed for surgeons to exercise their clinical judgment in choosing when to perform an appendectomy on patients in the antibiotic group, because antibiotics alone was not considered acceptable treatment for appendicitis.
“This led to some patients undergoing appendectomy who did not have appendicitis or who might have been successfully treated with antibiotics or an another course of antibiotics,” they wrote. “Future studies should investigate protocols for further imaging or antibiotic treatment for patients who develop recurrent appendicitis after they were initially treated with antibiotics.”
In the recurrence group, the majority were found to have uncomplicated appendicitis, but complicated appendicitis was seen in two patients between 2 and 5 years after the index admission.
There was a significant 17.9% higher complication rate in the appendectomy group, compared with the antibiotic group – 24.4% versus 6.5% – at 5 years and two patients in the appendectomy group had severe complications requiring reoperation.
They suggested that the higher complication rate with surgery, which was mostly attributable to infections, could be reduced by the use of laparoscopic appendectomy, which is also associated with faster recovery.
The median length of hospital stay was 3 days for both the appendectomy group and the antibiotics-only group, but patients randomized to appendectomy took a median of 22 days of sick leave, compared with 11 days for those randomized to antibiotics (P less than .001).
In the absence of standard protocol on treating appendicitis with antibiotics, the authors noted that they took a conservative approach, using broad-spectrum antibiotics and keeping patients in hospital for 3 days for observation.
“The success of antibiotic treatment for appendicitis calls into question prior beliefs that appendicitis inevitably results in serious intra-abdominal infection if appendectomy is not performed.”
The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
SOURCE: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
“When the earlier results of the APPAC trial were published, showing that 73% of patients with uncomplicated acute appendicitis did not require surgery at 1 year of follow-up, critics raised concerns that many more of these patients would eventually require surgery,” wrote Edward H. Livingston, MD, deputy editor of JAMA in an accompanying editorial (JAMA 2018;320:1245-46).Surgeons have since been waiting until longer-term outcomes were known. These 5-year results are supportive of the antibiotics approach. “They show no increase in major complications in patients who experienced a recurrence and underwent appendectomy after initially being randomized to antibiotic therapy. They challenge the notion that uncomplicated acute appendicitis is a surgical emergency and show that nonsurgical treatment is a reasonable option,” Dr Livingston wrote.
He declared no conflicts of interest.
“When the earlier results of the APPAC trial were published, showing that 73% of patients with uncomplicated acute appendicitis did not require surgery at 1 year of follow-up, critics raised concerns that many more of these patients would eventually require surgery,” wrote Edward H. Livingston, MD, deputy editor of JAMA in an accompanying editorial (JAMA 2018;320:1245-46).Surgeons have since been waiting until longer-term outcomes were known. These 5-year results are supportive of the antibiotics approach. “They show no increase in major complications in patients who experienced a recurrence and underwent appendectomy after initially being randomized to antibiotic therapy. They challenge the notion that uncomplicated acute appendicitis is a surgical emergency and show that nonsurgical treatment is a reasonable option,” Dr Livingston wrote.
He declared no conflicts of interest.
“When the earlier results of the APPAC trial were published, showing that 73% of patients with uncomplicated acute appendicitis did not require surgery at 1 year of follow-up, critics raised concerns that many more of these patients would eventually require surgery,” wrote Edward H. Livingston, MD, deputy editor of JAMA in an accompanying editorial (JAMA 2018;320:1245-46).Surgeons have since been waiting until longer-term outcomes were known. These 5-year results are supportive of the antibiotics approach. “They show no increase in major complications in patients who experienced a recurrence and underwent appendectomy after initially being randomized to antibiotic therapy. They challenge the notion that uncomplicated acute appendicitis is a surgical emergency and show that nonsurgical treatment is a reasonable option,” Dr Livingston wrote.
He declared no conflicts of interest.
Longer-term outcomes of treating uncomplicated acute appendicitis with antibiotics suggest it is a feasible alternative to appendectomy.
Researchers have presented the 5-year follow-up data from the Appendicitis Acuta (APPAC) multicenter randomized clinical trial comparing appendectomy with antibiotic therapy in 530 patients.
They found that 39.1% (100) of the 257 patients randomized to antibiotic therapy – 3 days of intravenous ertapenem followed by 7 days of oral levofloxacin and metronidazole – experienced a recurrence of appendicitis within 5 years and subsequently had surgery.
However the authors noted that seven of these patients were later found not to have appendicitis, so the true success rate for antibiotic treatment was actually 63.7%.
Seventy of the patients who experienced a recurrence underwent surgery in the first year after randomization, 17 in the second year, 3 in the third year, 5 in the fourth year, and the remaining 5 patients in the fifth year, the authors wrote in an article published in JAMA.
However the overall complication rate was similar in patients who were randomized to undergo appendectomy and in those who were initially randomized to the antibiotic group but later experienced a recurrence and underwent surgery.
“No patient initially treated with antibiotics, who ultimately developed recurrent appendicitis, had any complications related to the delay in surgery,” wrote Paulina Salminen, MD, from Turku (Finland) University Hospital and coauthors. “Nearly two-thirds of all patients who initially presented with uncomplicated appendicitis were successfully treated with antibiotics alone, and those who ultimately developed recurrent disease did not experience any adverse outcomes related to the delay in appendectomy.”
Of the 100 patients randomized to antibiotics who underwent appendectomy after a recurrence, 15 were operated on when they were first hospitalized at study admission.
The authors commented that the study design allowed for surgeons to exercise their clinical judgment in choosing when to perform an appendectomy on patients in the antibiotic group, because antibiotics alone was not considered acceptable treatment for appendicitis.
“This led to some patients undergoing appendectomy who did not have appendicitis or who might have been successfully treated with antibiotics or an another course of antibiotics,” they wrote. “Future studies should investigate protocols for further imaging or antibiotic treatment for patients who develop recurrent appendicitis after they were initially treated with antibiotics.”
In the recurrence group, the majority were found to have uncomplicated appendicitis, but complicated appendicitis was seen in two patients between 2 and 5 years after the index admission.
There was a significant 17.9% higher complication rate in the appendectomy group, compared with the antibiotic group – 24.4% versus 6.5% – at 5 years and two patients in the appendectomy group had severe complications requiring reoperation.
They suggested that the higher complication rate with surgery, which was mostly attributable to infections, could be reduced by the use of laparoscopic appendectomy, which is also associated with faster recovery.
The median length of hospital stay was 3 days for both the appendectomy group and the antibiotics-only group, but patients randomized to appendectomy took a median of 22 days of sick leave, compared with 11 days for those randomized to antibiotics (P less than .001).
In the absence of standard protocol on treating appendicitis with antibiotics, the authors noted that they took a conservative approach, using broad-spectrum antibiotics and keeping patients in hospital for 3 days for observation.
“The success of antibiotic treatment for appendicitis calls into question prior beliefs that appendicitis inevitably results in serious intra-abdominal infection if appendectomy is not performed.”
The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
SOURCE: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
Longer-term outcomes of treating uncomplicated acute appendicitis with antibiotics suggest it is a feasible alternative to appendectomy.
Researchers have presented the 5-year follow-up data from the Appendicitis Acuta (APPAC) multicenter randomized clinical trial comparing appendectomy with antibiotic therapy in 530 patients.
They found that 39.1% (100) of the 257 patients randomized to antibiotic therapy – 3 days of intravenous ertapenem followed by 7 days of oral levofloxacin and metronidazole – experienced a recurrence of appendicitis within 5 years and subsequently had surgery.
However the authors noted that seven of these patients were later found not to have appendicitis, so the true success rate for antibiotic treatment was actually 63.7%.
Seventy of the patients who experienced a recurrence underwent surgery in the first year after randomization, 17 in the second year, 3 in the third year, 5 in the fourth year, and the remaining 5 patients in the fifth year, the authors wrote in an article published in JAMA.
However the overall complication rate was similar in patients who were randomized to undergo appendectomy and in those who were initially randomized to the antibiotic group but later experienced a recurrence and underwent surgery.
“No patient initially treated with antibiotics, who ultimately developed recurrent appendicitis, had any complications related to the delay in surgery,” wrote Paulina Salminen, MD, from Turku (Finland) University Hospital and coauthors. “Nearly two-thirds of all patients who initially presented with uncomplicated appendicitis were successfully treated with antibiotics alone, and those who ultimately developed recurrent disease did not experience any adverse outcomes related to the delay in appendectomy.”
Of the 100 patients randomized to antibiotics who underwent appendectomy after a recurrence, 15 were operated on when they were first hospitalized at study admission.
The authors commented that the study design allowed for surgeons to exercise their clinical judgment in choosing when to perform an appendectomy on patients in the antibiotic group, because antibiotics alone was not considered acceptable treatment for appendicitis.
“This led to some patients undergoing appendectomy who did not have appendicitis or who might have been successfully treated with antibiotics or an another course of antibiotics,” they wrote. “Future studies should investigate protocols for further imaging or antibiotic treatment for patients who develop recurrent appendicitis after they were initially treated with antibiotics.”
In the recurrence group, the majority were found to have uncomplicated appendicitis, but complicated appendicitis was seen in two patients between 2 and 5 years after the index admission.
There was a significant 17.9% higher complication rate in the appendectomy group, compared with the antibiotic group – 24.4% versus 6.5% – at 5 years and two patients in the appendectomy group had severe complications requiring reoperation.
They suggested that the higher complication rate with surgery, which was mostly attributable to infections, could be reduced by the use of laparoscopic appendectomy, which is also associated with faster recovery.
The median length of hospital stay was 3 days for both the appendectomy group and the antibiotics-only group, but patients randomized to appendectomy took a median of 22 days of sick leave, compared with 11 days for those randomized to antibiotics (P less than .001).
In the absence of standard protocol on treating appendicitis with antibiotics, the authors noted that they took a conservative approach, using broad-spectrum antibiotics and keeping patients in hospital for 3 days for observation.
“The success of antibiotic treatment for appendicitis calls into question prior beliefs that appendicitis inevitably results in serious intra-abdominal infection if appendectomy is not performed.”
The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
SOURCE: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
FROM JAMA
Key clinical point: Antibiotics could be used as an alternative to surgery for uncomplicated acute appendicitis.
Major finding: Around two-thirds of patients with acute appendicitis treated with antibiotics only did not experience a recurrence in 5 years.
Study details: Randomized controlled study in 530 patients with uncomplicated acute appendicitis.
Disclosures: The study was supported by the Mary and Georg C. Ehrnrooth Foundation, the EVO Foundation, and Turku University. One author declared lecture fees from three pharmaceutical companies but no other conflicts of interest were declared.
Source: Salminen P et al. JAMA 2018;320:1259-65. doi: 10.1001/jama.2018.13201.
Obesity plays role in sleep-disordered breathing in pregnancy
The relationship between hypertension during pregnancy and sleep-disordered breathing may be partly mediated by obesity, new research suggests.
An article published in the Journal of Sleep Research details the results of a case-control study in 80 pregnant women – 40 normotensive and 40 with either gestational hypertension or preeclampsia – who were matched on body mass index.
Nearly half of the women in the study (45%) met the criteria for sleep-disordered breathing – defined as a respiratory disturbance index of 5 or above. The incidence was higher among women with hypertension (53%) than among women in the normotensive control group (38%), but the difference was not statistically significant.
There were also no significant differences in median respiratory disturbance index or apnea-hypopnea index between the hypertension and control groups.
However, the incidence of more severe sleep-disordered breathing – a respiratory disturbance index of at least 10 – was significantly greater in the hypertensive group (35% vs. 14%; P = .04). The women with pregnancy-related hypertension also had significantly higher respiratory disturbance index during non–rapid eye movement sleep and when they were sleeping on their back.
The severity of hypertensive disease did not affect the prevalence of sleep-disordered breathing.
Danielle L. Wilson of the Institute for Breathing and Sleep at Austin Health in Melbourne and her coauthors wrote that, while previous research has pointed to a link between hypertension in pregnancy and sleep-disordered breathing, this is the first study to explore the potential confounding role of obesity.
“We found SDB [sleep-disordered breathing] to be more common in our control group than in previous studies, confirming that BMI is an important covariate that requires evaluation in future studies exploring the relationship between SDB and HDP [hypertension during pregnancy],” they reported. “SDB may be a mechanism by which obesity and adverse perinatal outcomes are linked, but given the important contribution of obesity to both SDB and HDP, failing to adjust for this covariate will overestimate the strength of association between SDB and HDP.”
However, they acknowledged there was a significant association between moderate to severe sleep-disordered breathing and hypertension in pregnancy. They suggested this might be a means to increase women’s uptake of clinical review with a sleep physician, which was very low in the study despite it being offered to all women.
“Better engagement may be more likely for women with more severe disease if stronger links with adverse pregnancy outcome are demonstrated,” they wrote.
The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.
The relationship between hypertension during pregnancy and sleep-disordered breathing may be partly mediated by obesity, new research suggests.
An article published in the Journal of Sleep Research details the results of a case-control study in 80 pregnant women – 40 normotensive and 40 with either gestational hypertension or preeclampsia – who were matched on body mass index.
Nearly half of the women in the study (45%) met the criteria for sleep-disordered breathing – defined as a respiratory disturbance index of 5 or above. The incidence was higher among women with hypertension (53%) than among women in the normotensive control group (38%), but the difference was not statistically significant.
There were also no significant differences in median respiratory disturbance index or apnea-hypopnea index between the hypertension and control groups.
However, the incidence of more severe sleep-disordered breathing – a respiratory disturbance index of at least 10 – was significantly greater in the hypertensive group (35% vs. 14%; P = .04). The women with pregnancy-related hypertension also had significantly higher respiratory disturbance index during non–rapid eye movement sleep and when they were sleeping on their back.
The severity of hypertensive disease did not affect the prevalence of sleep-disordered breathing.
Danielle L. Wilson of the Institute for Breathing and Sleep at Austin Health in Melbourne and her coauthors wrote that, while previous research has pointed to a link between hypertension in pregnancy and sleep-disordered breathing, this is the first study to explore the potential confounding role of obesity.
“We found SDB [sleep-disordered breathing] to be more common in our control group than in previous studies, confirming that BMI is an important covariate that requires evaluation in future studies exploring the relationship between SDB and HDP [hypertension during pregnancy],” they reported. “SDB may be a mechanism by which obesity and adverse perinatal outcomes are linked, but given the important contribution of obesity to both SDB and HDP, failing to adjust for this covariate will overestimate the strength of association between SDB and HDP.”
However, they acknowledged there was a significant association between moderate to severe sleep-disordered breathing and hypertension in pregnancy. They suggested this might be a means to increase women’s uptake of clinical review with a sleep physician, which was very low in the study despite it being offered to all women.
“Better engagement may be more likely for women with more severe disease if stronger links with adverse pregnancy outcome are demonstrated,” they wrote.
The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.
The relationship between hypertension during pregnancy and sleep-disordered breathing may be partly mediated by obesity, new research suggests.
An article published in the Journal of Sleep Research details the results of a case-control study in 80 pregnant women – 40 normotensive and 40 with either gestational hypertension or preeclampsia – who were matched on body mass index.
Nearly half of the women in the study (45%) met the criteria for sleep-disordered breathing – defined as a respiratory disturbance index of 5 or above. The incidence was higher among women with hypertension (53%) than among women in the normotensive control group (38%), but the difference was not statistically significant.
There were also no significant differences in median respiratory disturbance index or apnea-hypopnea index between the hypertension and control groups.
However, the incidence of more severe sleep-disordered breathing – a respiratory disturbance index of at least 10 – was significantly greater in the hypertensive group (35% vs. 14%; P = .04). The women with pregnancy-related hypertension also had significantly higher respiratory disturbance index during non–rapid eye movement sleep and when they were sleeping on their back.
The severity of hypertensive disease did not affect the prevalence of sleep-disordered breathing.
Danielle L. Wilson of the Institute for Breathing and Sleep at Austin Health in Melbourne and her coauthors wrote that, while previous research has pointed to a link between hypertension in pregnancy and sleep-disordered breathing, this is the first study to explore the potential confounding role of obesity.
“We found SDB [sleep-disordered breathing] to be more common in our control group than in previous studies, confirming that BMI is an important covariate that requires evaluation in future studies exploring the relationship between SDB and HDP [hypertension during pregnancy],” they reported. “SDB may be a mechanism by which obesity and adverse perinatal outcomes are linked, but given the important contribution of obesity to both SDB and HDP, failing to adjust for this covariate will overestimate the strength of association between SDB and HDP.”
However, they acknowledged there was a significant association between moderate to severe sleep-disordered breathing and hypertension in pregnancy. They suggested this might be a means to increase women’s uptake of clinical review with a sleep physician, which was very low in the study despite it being offered to all women.
“Better engagement may be more likely for women with more severe disease if stronger links with adverse pregnancy outcome are demonstrated,” they wrote.
The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
SOURCE: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.
FROM JOURNAL OF SLEEP RESEARCH
Key clinical point:
Major finding: The relationship between hypertension in pregnancy and sleep-disordered breathing is only significant for more severe sleep-disordered breathing.
Study details: Prospective case-control study in 80 pregnant women.
Disclosures: The study was supported by the Austin Medical Research Foundation and the Medical Research Foundation for Women and Babies. One author declared a scholarship from a research funding body, and two declared unrelated research support from private industry. No other conflicts of interest were declared.
Source: Wilson D et al. J Sleep Research. 2018 Oct;27(5):e12656.
Cannabinoids may raise pain tolerance, not relieve pain itself
The belief that cannabinoids address chronic pain by relieving it has been challenged by a meta-analysis that finds cannabinoids have effects on pain thresholds that may, instead, make pain more tolerable.
The systematic review and meta-analysis comprised 18 placebo-controlled studies looking at the effect of plant-based or synthetic cannabinoids on experimental pain in 442 healthy participants. The conclusions were published Sept. 19 in JAMA Psychiatry.
“Cannabinoid drugs may prevent the onset of pain by producing small increases in pain thresholds,” wrote Martin J. De Vita and his colleagues, “but may not reduce the intensity of experimental pain already being experienced; instead, cannabinoids may make experimental pain feel less unpleasant and more tolerable, suggesting an influence on affective processes.”
Ten of the studies analyzed measured changes in pain thresholds and showed a small but significant association between cannabinoids and higher pain thresholds (95% confidence interval, 0.054-0.318; P = .006). Five studies examined pain unpleasantness, and showed that cannabinoids were associated with reduced unpleasantness ratings, compared with placebo (95% CI, 0.104-0.472; P = .002).
Among the eight studies that measured pain tolerance, a significant association was found between cannabinoid administration and higher pain tolerance (95% CI, 0.015-0.436; P = .04).
However, the 13 studies looking at pain intensity found no association between cannabinoid use and changes in the intensity of pain (CI, –0.120-0.154), nor were reductions in pain sensitivity to mechanical stimulation found in the three studies that measured mechanical hyperalgesia (95% CI, –0.059-0.244; P = .23).
The analysis did see significant effects according to the type of cannabinoid; plant-based cannabis had significantly stronger associations with reductions in pain unpleasantness compared to dronabinol and other synthetic THC preparations. However, both plant-based cannabis and dronabinol were associated with increases in pain tolerance, whereas other synthetic THC preparations were associated with significant reductions in pain tolerance.
Mr. De Vita, of the department of psychology at Syracuse (N.Y.) University, and his colleagues stressed that the systematic review and meta-analysis focused solely on studies of experimental pain, which “merely approximates features of clinical pain,” and specifically excluded individuals with chronic pain.
The absence of neuropathic pain data is “especially limiting, given that neuropathic pain is the primary condition for which modest empirical evidence exists that supports cannabinoid analgesia,” they wrote.
In particular, they drew attention to the finding that cannabinoids did not appear to have any effect on mechanical hyperalgesia, which they said suggests that cannabinoids might not address central sensitization in people with neuropathic pain.
The authors also raised the question of whether cannabinoids simply relieve pain by making people feel good or “high,” much like other intoxicating substances such as alcohol. They argued that the answer depends on what outcome is desired from treatment.
“If treatment aims to relieve pain without producing intoxication, psychoactive cannabinoids may not suffice,” they wrote. Ultimately, they said, the relief from pain experienced by some patients might be driven largely by an “affective rather than a sensory component.”
Among the limitations cited by the researchers is the focus on studies of experimental pain. “To produce evidence that supports the generalizability of the current findings, pain reactivity research must be conduced in clinical samples,” Mr. De Vita and his colleagues wrote.
The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
SOURCE: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
The belief that cannabinoids address chronic pain by relieving it has been challenged by a meta-analysis that finds cannabinoids have effects on pain thresholds that may, instead, make pain more tolerable.
The systematic review and meta-analysis comprised 18 placebo-controlled studies looking at the effect of plant-based or synthetic cannabinoids on experimental pain in 442 healthy participants. The conclusions were published Sept. 19 in JAMA Psychiatry.
“Cannabinoid drugs may prevent the onset of pain by producing small increases in pain thresholds,” wrote Martin J. De Vita and his colleagues, “but may not reduce the intensity of experimental pain already being experienced; instead, cannabinoids may make experimental pain feel less unpleasant and more tolerable, suggesting an influence on affective processes.”
Ten of the studies analyzed measured changes in pain thresholds and showed a small but significant association between cannabinoids and higher pain thresholds (95% confidence interval, 0.054-0.318; P = .006). Five studies examined pain unpleasantness, and showed that cannabinoids were associated with reduced unpleasantness ratings, compared with placebo (95% CI, 0.104-0.472; P = .002).
Among the eight studies that measured pain tolerance, a significant association was found between cannabinoid administration and higher pain tolerance (95% CI, 0.015-0.436; P = .04).
However, the 13 studies looking at pain intensity found no association between cannabinoid use and changes in the intensity of pain (CI, –0.120-0.154), nor were reductions in pain sensitivity to mechanical stimulation found in the three studies that measured mechanical hyperalgesia (95% CI, –0.059-0.244; P = .23).
The analysis did see significant effects according to the type of cannabinoid; plant-based cannabis had significantly stronger associations with reductions in pain unpleasantness compared to dronabinol and other synthetic THC preparations. However, both plant-based cannabis and dronabinol were associated with increases in pain tolerance, whereas other synthetic THC preparations were associated with significant reductions in pain tolerance.
Mr. De Vita, of the department of psychology at Syracuse (N.Y.) University, and his colleagues stressed that the systematic review and meta-analysis focused solely on studies of experimental pain, which “merely approximates features of clinical pain,” and specifically excluded individuals with chronic pain.
The absence of neuropathic pain data is “especially limiting, given that neuropathic pain is the primary condition for which modest empirical evidence exists that supports cannabinoid analgesia,” they wrote.
In particular, they drew attention to the finding that cannabinoids did not appear to have any effect on mechanical hyperalgesia, which they said suggests that cannabinoids might not address central sensitization in people with neuropathic pain.
The authors also raised the question of whether cannabinoids simply relieve pain by making people feel good or “high,” much like other intoxicating substances such as alcohol. They argued that the answer depends on what outcome is desired from treatment.
“If treatment aims to relieve pain without producing intoxication, psychoactive cannabinoids may not suffice,” they wrote. Ultimately, they said, the relief from pain experienced by some patients might be driven largely by an “affective rather than a sensory component.”
Among the limitations cited by the researchers is the focus on studies of experimental pain. “To produce evidence that supports the generalizability of the current findings, pain reactivity research must be conduced in clinical samples,” Mr. De Vita and his colleagues wrote.
The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
SOURCE: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
The belief that cannabinoids address chronic pain by relieving it has been challenged by a meta-analysis that finds cannabinoids have effects on pain thresholds that may, instead, make pain more tolerable.
The systematic review and meta-analysis comprised 18 placebo-controlled studies looking at the effect of plant-based or synthetic cannabinoids on experimental pain in 442 healthy participants. The conclusions were published Sept. 19 in JAMA Psychiatry.
“Cannabinoid drugs may prevent the onset of pain by producing small increases in pain thresholds,” wrote Martin J. De Vita and his colleagues, “but may not reduce the intensity of experimental pain already being experienced; instead, cannabinoids may make experimental pain feel less unpleasant and more tolerable, suggesting an influence on affective processes.”
Ten of the studies analyzed measured changes in pain thresholds and showed a small but significant association between cannabinoids and higher pain thresholds (95% confidence interval, 0.054-0.318; P = .006). Five studies examined pain unpleasantness, and showed that cannabinoids were associated with reduced unpleasantness ratings, compared with placebo (95% CI, 0.104-0.472; P = .002).
Among the eight studies that measured pain tolerance, a significant association was found between cannabinoid administration and higher pain tolerance (95% CI, 0.015-0.436; P = .04).
However, the 13 studies looking at pain intensity found no association between cannabinoid use and changes in the intensity of pain (CI, –0.120-0.154), nor were reductions in pain sensitivity to mechanical stimulation found in the three studies that measured mechanical hyperalgesia (95% CI, –0.059-0.244; P = .23).
The analysis did see significant effects according to the type of cannabinoid; plant-based cannabis had significantly stronger associations with reductions in pain unpleasantness compared to dronabinol and other synthetic THC preparations. However, both plant-based cannabis and dronabinol were associated with increases in pain tolerance, whereas other synthetic THC preparations were associated with significant reductions in pain tolerance.
Mr. De Vita, of the department of psychology at Syracuse (N.Y.) University, and his colleagues stressed that the systematic review and meta-analysis focused solely on studies of experimental pain, which “merely approximates features of clinical pain,” and specifically excluded individuals with chronic pain.
The absence of neuropathic pain data is “especially limiting, given that neuropathic pain is the primary condition for which modest empirical evidence exists that supports cannabinoid analgesia,” they wrote.
In particular, they drew attention to the finding that cannabinoids did not appear to have any effect on mechanical hyperalgesia, which they said suggests that cannabinoids might not address central sensitization in people with neuropathic pain.
The authors also raised the question of whether cannabinoids simply relieve pain by making people feel good or “high,” much like other intoxicating substances such as alcohol. They argued that the answer depends on what outcome is desired from treatment.
“If treatment aims to relieve pain without producing intoxication, psychoactive cannabinoids may not suffice,” they wrote. Ultimately, they said, the relief from pain experienced by some patients might be driven largely by an “affective rather than a sensory component.”
Among the limitations cited by the researchers is the focus on studies of experimental pain. “To produce evidence that supports the generalizability of the current findings, pain reactivity research must be conduced in clinical samples,” Mr. De Vita and his colleagues wrote.
The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
SOURCE: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
FROM JAMA PSYCHIATRY
Key clinical point: Cannabinoids might relieve experimental pain by making people feel good or “high,” much like other intoxicating substances such as alcohol.
Major finding: Cannabinoids improve pain thresholds (95% confidence interval, 0.054-0.3,18; P = .006) and pain unpleasantness (95% CI, 0.104-0.472; P = .002).
Study details: Systematic review and meta-analysis of 18 placebo-controlled studies of experimental pain.
Disclosures: The study was partly supported by the Syracuse University STEM Fellowship, and the National Institute on Alcohol Abuse and Alcoholism. No conflicts of interest were declared.
Source: De Vita MJ et al. JAMA Psychiatry. 2018 Sep 19. doi: 10.1001/jamapsychiatry.2018.2503.
Clearance rates for some antiepileptic drugs rise during pregnancy
Certain antiepileptic drugs show significant increases in clearance rates during pregnancy, which may be associated with increased seizure rates, research suggests.
Paula E. Voinescu, MD, PhD, of Brigham and Women’s Hospital, Boston, and her coauthors conducted a prospective, observational study of 44 pregnancies in 40 women with epilepsy who were treated with antiepileptic drugs. The women were enrolled preconception or in early pregnancy, then kept daily diaries of medication doses, adherence, and seizures, while antiepileptic drug concentrations were measured every 1-3 months.
The investigators found that levetiracetam reached a mean maximal clearance during the first trimester that was 71% higher than baseline clearance (P = .0001), and its clearance level remained high throughout pregnancy.
“This is important information for clinicians who manage women on levetiracetam as they may opt to begin TDM [therapeutic drug monitoring] as early as possible in pregnancy,” the investigators wrote. The report was published online Sept. 5 in Neurology.
The authors noted that there was significant variability between participants in clearance rates of levetiracetam, ranging from 1.42-fold to 2.02-fold baseline clearance, and they suggested that pharmacogenetic differences may play a role.
“Levetiracetam is mainly excreted unchanged by the kidneys, and the changes in clearance are consistent with the time course of increased glomerular filtration rate during pregnancy,” they wrote. “The pathophysiology of the interparticipant variability in clearance fluctuation during pregnancy and of the race influence observed for levetiracetam are difficult to explain with the known pharmacokinetic data.”
Both oxcarbazepine and topiramate also reached significantly higher mean maximal clearances during the second trimester than at baseline (63% and 39% higher, respectively), and their elevated clearance persisted into the third trimester.
However, there were no significant changes in clearance rates of total or free phenytoin or valproic acid during pregnancy.
Among the 15 women for whom researchers had complete seizure history and antiepileptic drug concentrations, 40% experienced a worsening of seizure frequency during at least one trimester.
Overall, lower individualized ratio-to-target concentrations of drug were associated with increased seizure frequency in the first and second trimester, but not in the third trimester.
While the study did not compare seizure outcomes between patients who were managed with TDM and those who were not, the authors suggested that their data supported the use of TDM during pregnancy.
“The finding of lower AED RTC [antiepileptic drug ratio to concentration] at different trimesters suggests that in outpatient clinical practice, possible changes in AED dosing varied by trimester may be clinically important for patient care.”
The study was supported by the National Institutes of Health, the American Brain Foundation, the American Epilepsy Society, the Epilepsy Foundation, and the Karger Fund. Four authors declared support, honoraria, and/or other funding from the pharmaceutical industry, and five also declared support from the study funding bodies.
SOURCE: Voinescu P et al. Neurology. 2018 Sep 5. doi: 10.1212/WNL.0000000000006240.
Certain antiepileptic drugs show significant increases in clearance rates during pregnancy, which may be associated with increased seizure rates, research suggests.
Paula E. Voinescu, MD, PhD, of Brigham and Women’s Hospital, Boston, and her coauthors conducted a prospective, observational study of 44 pregnancies in 40 women with epilepsy who were treated with antiepileptic drugs. The women were enrolled preconception or in early pregnancy, then kept daily diaries of medication doses, adherence, and seizures, while antiepileptic drug concentrations were measured every 1-3 months.
The investigators found that levetiracetam reached a mean maximal clearance during the first trimester that was 71% higher than baseline clearance (P = .0001), and its clearance level remained high throughout pregnancy.
“This is important information for clinicians who manage women on levetiracetam as they may opt to begin TDM [therapeutic drug monitoring] as early as possible in pregnancy,” the investigators wrote. The report was published online Sept. 5 in Neurology.
The authors noted that there was significant variability between participants in clearance rates of levetiracetam, ranging from 1.42-fold to 2.02-fold baseline clearance, and they suggested that pharmacogenetic differences may play a role.
“Levetiracetam is mainly excreted unchanged by the kidneys, and the changes in clearance are consistent with the time course of increased glomerular filtration rate during pregnancy,” they wrote. “The pathophysiology of the interparticipant variability in clearance fluctuation during pregnancy and of the race influence observed for levetiracetam are difficult to explain with the known pharmacokinetic data.”
Both oxcarbazepine and topiramate also reached significantly higher mean maximal clearances during the second trimester than at baseline (63% and 39% higher, respectively), and their elevated clearance persisted into the third trimester.
However, there were no significant changes in clearance rates of total or free phenytoin or valproic acid during pregnancy.
Among the 15 women for whom researchers had complete seizure history and antiepileptic drug concentrations, 40% experienced a worsening of seizure frequency during at least one trimester.
Overall, lower individualized ratio-to-target concentrations of drug were associated with increased seizure frequency in the first and second trimester, but not in the third trimester.
While the study did not compare seizure outcomes between patients who were managed with TDM and those who were not, the authors suggested that their data supported the use of TDM during pregnancy.
“The finding of lower AED RTC [antiepileptic drug ratio to concentration] at different trimesters suggests that in outpatient clinical practice, possible changes in AED dosing varied by trimester may be clinically important for patient care.”
The study was supported by the National Institutes of Health, the American Brain Foundation, the American Epilepsy Society, the Epilepsy Foundation, and the Karger Fund. Four authors declared support, honoraria, and/or other funding from the pharmaceutical industry, and five also declared support from the study funding bodies.
SOURCE: Voinescu P et al. Neurology. 2018 Sep 5. doi: 10.1212/WNL.0000000000006240.
Certain antiepileptic drugs show significant increases in clearance rates during pregnancy, which may be associated with increased seizure rates, research suggests.
Paula E. Voinescu, MD, PhD, of Brigham and Women’s Hospital, Boston, and her coauthors conducted a prospective, observational study of 44 pregnancies in 40 women with epilepsy who were treated with antiepileptic drugs. The women were enrolled preconception or in early pregnancy, then kept daily diaries of medication doses, adherence, and seizures, while antiepileptic drug concentrations were measured every 1-3 months.
The investigators found that levetiracetam reached a mean maximal clearance during the first trimester that was 71% higher than baseline clearance (P = .0001), and its clearance level remained high throughout pregnancy.
“This is important information for clinicians who manage women on levetiracetam as they may opt to begin TDM [therapeutic drug monitoring] as early as possible in pregnancy,” the investigators wrote. The report was published online Sept. 5 in Neurology.
The authors noted that there was significant variability between participants in clearance rates of levetiracetam, ranging from 1.42-fold to 2.02-fold baseline clearance, and they suggested that pharmacogenetic differences may play a role.
“Levetiracetam is mainly excreted unchanged by the kidneys, and the changes in clearance are consistent with the time course of increased glomerular filtration rate during pregnancy,” they wrote. “The pathophysiology of the interparticipant variability in clearance fluctuation during pregnancy and of the race influence observed for levetiracetam are difficult to explain with the known pharmacokinetic data.”
Both oxcarbazepine and topiramate also reached significantly higher mean maximal clearances during the second trimester than at baseline (63% and 39% higher, respectively), and their elevated clearance persisted into the third trimester.
However, there were no significant changes in clearance rates of total or free phenytoin or valproic acid during pregnancy.
Among the 15 women for whom researchers had complete seizure history and antiepileptic drug concentrations, 40% experienced a worsening of seizure frequency during at least one trimester.
Overall, lower individualized ratio-to-target concentrations of drug were associated with increased seizure frequency in the first and second trimester, but not in the third trimester.
While the study did not compare seizure outcomes between patients who were managed with TDM and those who were not, the authors suggested that their data supported the use of TDM during pregnancy.
“The finding of lower AED RTC [antiepileptic drug ratio to concentration] at different trimesters suggests that in outpatient clinical practice, possible changes in AED dosing varied by trimester may be clinically important for patient care.”
The study was supported by the National Institutes of Health, the American Brain Foundation, the American Epilepsy Society, the Epilepsy Foundation, and the Karger Fund. Four authors declared support, honoraria, and/or other funding from the pharmaceutical industry, and five also declared support from the study funding bodies.
SOURCE: Voinescu P et al. Neurology. 2018 Sep 5. doi: 10.1212/WNL.0000000000006240.
FROM NEUROLOGY
Key clinical point: Some antiepileptic drugs show higher clearance rates during pregnancy, which could increase seizure risk.
Major finding: The mean maximal clearance rate of levetiracetam in the first trimester is 71% higher than baseline.
Study details: Prospective observational study in 40 women with 44 pregnancies.
Disclosures: The study was supported by the National Institutes of Health, the American Brain Foundation, the American Epilepsy Society, the Epilepsy Foundation, and the Karger Fund. Four authors declared support, honoraria, and/or other funding from the pharmaceutical industry, and five also declared support from the study funding bodies.
Source: Voinescu P et al. Neurology. 2018 Sep 5. doi: 10.1212/WNL.0000000000006240
What’s in that e-cigarette? It may be cannabis
Nearly 1 in 11 U.S. middle and high school students have used a cannabis product in an e-cigarette, according to a school-based survey of 20,675 students.
The survey found that 8.9% of students in grades 6-12 said they had used an e-cigarette with marijuana, tetrahydrocannabinol or hash oil, or tetrahydrocannabinol wax. Among the students who reported ever using e-cigarettes, 30.6% had used a cannabis product in the device. The findings were published in JAMA Pediatrics.
This translated to around 1.7 million high school students and 425,000 middle school students who had ever used cannabis in e-cigarettes; figures the authors said were consistent with or higher than previous reports among U.S. and Canadian students.
Katrina F. Trivers, PhD, and her colleagues from the Centers for Disease Control and Prevention, noted that the U.S. Surgeon General has found e-cigarette aerosol can contain potentially harmful ingredients. Additionally, the National Academies of Sciences has said youth cannabis use can harm learning and memory.
“Strategies to reduce cannabis use in e-cigarettes are critical for protecting young people from these potential health risks,” the researchers wrote.
Male students and high school students were significantly more likely to report using cannabis products in an e-cigarette (10.6% and 12.4%, respectively), compared with female or middle school students.
Among current users of e-cigarettes, 39.5% reported using cannabis in the e-cigarette, while among those who used other tobacco products, 38.5% used cannabis in e-cigarettes. Higher e-cigarette use was also associated with use of cannabis products in e-cigarettes.
Living with someone who used tobacco products was associated with a higher incidence of cannabis in e-cigarette use (13%). Researchers also saw a higher use of cannabis in e-cigarettes among students of Hispanic ethnicity, compared with other ethnicities.
In 2015, around one-third of U.S. middle and high school students said they had used nonnicotine substances in e-cigarettes, but the use of cannabis in e-cigarettes could increase as several states consider legalizing cannabis sales for adults. “Given the high concurrent use of tobacco and other substances, it is important to monitor the substances youth use in e-cigarettes,” they wrote.
The researchers reported having no financial disclosures.
SOURCE: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
Nearly 1 in 11 U.S. middle and high school students have used a cannabis product in an e-cigarette, according to a school-based survey of 20,675 students.
The survey found that 8.9% of students in grades 6-12 said they had used an e-cigarette with marijuana, tetrahydrocannabinol or hash oil, or tetrahydrocannabinol wax. Among the students who reported ever using e-cigarettes, 30.6% had used a cannabis product in the device. The findings were published in JAMA Pediatrics.
This translated to around 1.7 million high school students and 425,000 middle school students who had ever used cannabis in e-cigarettes; figures the authors said were consistent with or higher than previous reports among U.S. and Canadian students.
Katrina F. Trivers, PhD, and her colleagues from the Centers for Disease Control and Prevention, noted that the U.S. Surgeon General has found e-cigarette aerosol can contain potentially harmful ingredients. Additionally, the National Academies of Sciences has said youth cannabis use can harm learning and memory.
“Strategies to reduce cannabis use in e-cigarettes are critical for protecting young people from these potential health risks,” the researchers wrote.
Male students and high school students were significantly more likely to report using cannabis products in an e-cigarette (10.6% and 12.4%, respectively), compared with female or middle school students.
Among current users of e-cigarettes, 39.5% reported using cannabis in the e-cigarette, while among those who used other tobacco products, 38.5% used cannabis in e-cigarettes. Higher e-cigarette use was also associated with use of cannabis products in e-cigarettes.
Living with someone who used tobacco products was associated with a higher incidence of cannabis in e-cigarette use (13%). Researchers also saw a higher use of cannabis in e-cigarettes among students of Hispanic ethnicity, compared with other ethnicities.
In 2015, around one-third of U.S. middle and high school students said they had used nonnicotine substances in e-cigarettes, but the use of cannabis in e-cigarettes could increase as several states consider legalizing cannabis sales for adults. “Given the high concurrent use of tobacco and other substances, it is important to monitor the substances youth use in e-cigarettes,” they wrote.
The researchers reported having no financial disclosures.
SOURCE: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
Nearly 1 in 11 U.S. middle and high school students have used a cannabis product in an e-cigarette, according to a school-based survey of 20,675 students.
The survey found that 8.9% of students in grades 6-12 said they had used an e-cigarette with marijuana, tetrahydrocannabinol or hash oil, or tetrahydrocannabinol wax. Among the students who reported ever using e-cigarettes, 30.6% had used a cannabis product in the device. The findings were published in JAMA Pediatrics.
This translated to around 1.7 million high school students and 425,000 middle school students who had ever used cannabis in e-cigarettes; figures the authors said were consistent with or higher than previous reports among U.S. and Canadian students.
Katrina F. Trivers, PhD, and her colleagues from the Centers for Disease Control and Prevention, noted that the U.S. Surgeon General has found e-cigarette aerosol can contain potentially harmful ingredients. Additionally, the National Academies of Sciences has said youth cannabis use can harm learning and memory.
“Strategies to reduce cannabis use in e-cigarettes are critical for protecting young people from these potential health risks,” the researchers wrote.
Male students and high school students were significantly more likely to report using cannabis products in an e-cigarette (10.6% and 12.4%, respectively), compared with female or middle school students.
Among current users of e-cigarettes, 39.5% reported using cannabis in the e-cigarette, while among those who used other tobacco products, 38.5% used cannabis in e-cigarettes. Higher e-cigarette use was also associated with use of cannabis products in e-cigarettes.
Living with someone who used tobacco products was associated with a higher incidence of cannabis in e-cigarette use (13%). Researchers also saw a higher use of cannabis in e-cigarettes among students of Hispanic ethnicity, compared with other ethnicities.
In 2015, around one-third of U.S. middle and high school students said they had used nonnicotine substances in e-cigarettes, but the use of cannabis in e-cigarettes could increase as several states consider legalizing cannabis sales for adults. “Given the high concurrent use of tobacco and other substances, it is important to monitor the substances youth use in e-cigarettes,” they wrote.
The researchers reported having no financial disclosures.
SOURCE: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
FROM JAMA PEDIATRICS
Key clinical point:
Major finding: A survey of U.S. students showed that 8.9% have used cannabis in an e-cigarette. Among e-cigarette users, cannabis use in e-cigarettes was reported by 30.6% of students.
Study details: A school-based survey of 20,675 students.
Disclosures: The researchers reported having no financial disclosures.
Source: Trivers KF et al. JAMA Pediatr. 2018 Sep 17. doi: 10.1001/jamapediatrics.2018.1920.
TKIs feasible and effective in Brazilian population with clear cell RCC
The use of tyrosine kinase inhibitors (TKIs) sunitinib and pazopanib to treat metastatic clear cell renal cell carcinoma (RCC) is feasible and effective in Brazil, even though limitations of the public health system mean not all patients have access to these or other therapies, researchers wrote.
In a retrospective analysis of 222 patients with advanced clear cell RCC who were treated with either sunitinib (n = 109) or pazopanib (n = 113), overall survival was 15.2 months with sunitinib and 14.2 months with pazopanib, investigators reported in the Journal of Global Oncology.
Overall survival rates, based on Memorial Sloan Kettering Cancer Center risk categories, were 32.9 months for patients with low risk disease, 15.9 months for intermediate risk patients, and 8.1 months for patient with poor risk – which constituted 29% of the patient population.
Pedro I. Velho, MD, of Johns Hopkins University, Baltimore, and his coauthors noted that these survival rates were lower than those seen in phase 3 trials in the same population.
They suggested this could be caused by the fact that the patients in this real-world study had worse baseline prognoses than those seen in previous clinical trials.
However, they also pointed out that, in the Brazilian public health system, there was no standard systemic treatment option outside clinical trials for patients who have progressed on a TKI as a first-line treatment. Only around half the patients in this study (101) would have been eligible for participation in a clinical trial.
“In our opinion, it is crucial to extend the access to these TKIs for the Brazilian population and also patients in other countries with limited access to targeted therapies,” the authors wrote.
However, the 14 patients who were eligible for treatment with nivolumab under a compassionate use program showed a significantly better overall survival rate, compared with patients who did not have access to a second-line therapy (36.7 months vs. 13.6 months; P less than .001).
“Also, it is imperative to provide systemic sequential treatment options for this population as an attempt to improve survival and offer the best outcomes for patients with metastatic RCC.”
The median duration of treatment with sunitinib was 6.4 months, and with pazopanib, it was 6.7 months. Adverse events were responsible for discontinuation of treatment in 28.4% of patients on sunitinib and 22.1% of patients on pazopanib, with fatigue, diarrhea, nausea and vomiting as the most common toxicities in both groups.
Six authors declared honoraria, funding, expenses or consultancies with the pharmaceutical industry. Two authors had no conflicts of interest to declare.
SOURCE: Velho PI et al. J Glob Oncol. 2018 Sep 10. doi: 10.1200/JGO.18.00073.
The use of tyrosine kinase inhibitors (TKIs) sunitinib and pazopanib to treat metastatic clear cell renal cell carcinoma (RCC) is feasible and effective in Brazil, even though limitations of the public health system mean not all patients have access to these or other therapies, researchers wrote.
In a retrospective analysis of 222 patients with advanced clear cell RCC who were treated with either sunitinib (n = 109) or pazopanib (n = 113), overall survival was 15.2 months with sunitinib and 14.2 months with pazopanib, investigators reported in the Journal of Global Oncology.
Overall survival rates, based on Memorial Sloan Kettering Cancer Center risk categories, were 32.9 months for patients with low risk disease, 15.9 months for intermediate risk patients, and 8.1 months for patient with poor risk – which constituted 29% of the patient population.
Pedro I. Velho, MD, of Johns Hopkins University, Baltimore, and his coauthors noted that these survival rates were lower than those seen in phase 3 trials in the same population.
They suggested this could be caused by the fact that the patients in this real-world study had worse baseline prognoses than those seen in previous clinical trials.
However, they also pointed out that, in the Brazilian public health system, there was no standard systemic treatment option outside clinical trials for patients who have progressed on a TKI as a first-line treatment. Only around half the patients in this study (101) would have been eligible for participation in a clinical trial.
“In our opinion, it is crucial to extend the access to these TKIs for the Brazilian population and also patients in other countries with limited access to targeted therapies,” the authors wrote.
However, the 14 patients who were eligible for treatment with nivolumab under a compassionate use program showed a significantly better overall survival rate, compared with patients who did not have access to a second-line therapy (36.7 months vs. 13.6 months; P less than .001).
“Also, it is imperative to provide systemic sequential treatment options for this population as an attempt to improve survival and offer the best outcomes for patients with metastatic RCC.”
The median duration of treatment with sunitinib was 6.4 months, and with pazopanib, it was 6.7 months. Adverse events were responsible for discontinuation of treatment in 28.4% of patients on sunitinib and 22.1% of patients on pazopanib, with fatigue, diarrhea, nausea and vomiting as the most common toxicities in both groups.
Six authors declared honoraria, funding, expenses or consultancies with the pharmaceutical industry. Two authors had no conflicts of interest to declare.
SOURCE: Velho PI et al. J Glob Oncol. 2018 Sep 10. doi: 10.1200/JGO.18.00073.
The use of tyrosine kinase inhibitors (TKIs) sunitinib and pazopanib to treat metastatic clear cell renal cell carcinoma (RCC) is feasible and effective in Brazil, even though limitations of the public health system mean not all patients have access to these or other therapies, researchers wrote.
In a retrospective analysis of 222 patients with advanced clear cell RCC who were treated with either sunitinib (n = 109) or pazopanib (n = 113), overall survival was 15.2 months with sunitinib and 14.2 months with pazopanib, investigators reported in the Journal of Global Oncology.
Overall survival rates, based on Memorial Sloan Kettering Cancer Center risk categories, were 32.9 months for patients with low risk disease, 15.9 months for intermediate risk patients, and 8.1 months for patient with poor risk – which constituted 29% of the patient population.
Pedro I. Velho, MD, of Johns Hopkins University, Baltimore, and his coauthors noted that these survival rates were lower than those seen in phase 3 trials in the same population.
They suggested this could be caused by the fact that the patients in this real-world study had worse baseline prognoses than those seen in previous clinical trials.
However, they also pointed out that, in the Brazilian public health system, there was no standard systemic treatment option outside clinical trials for patients who have progressed on a TKI as a first-line treatment. Only around half the patients in this study (101) would have been eligible for participation in a clinical trial.
“In our opinion, it is crucial to extend the access to these TKIs for the Brazilian population and also patients in other countries with limited access to targeted therapies,” the authors wrote.
However, the 14 patients who were eligible for treatment with nivolumab under a compassionate use program showed a significantly better overall survival rate, compared with patients who did not have access to a second-line therapy (36.7 months vs. 13.6 months; P less than .001).
“Also, it is imperative to provide systemic sequential treatment options for this population as an attempt to improve survival and offer the best outcomes for patients with metastatic RCC.”
The median duration of treatment with sunitinib was 6.4 months, and with pazopanib, it was 6.7 months. Adverse events were responsible for discontinuation of treatment in 28.4% of patients on sunitinib and 22.1% of patients on pazopanib, with fatigue, diarrhea, nausea and vomiting as the most common toxicities in both groups.
Six authors declared honoraria, funding, expenses or consultancies with the pharmaceutical industry. Two authors had no conflicts of interest to declare.
SOURCE: Velho PI et al. J Glob Oncol. 2018 Sep 10. doi: 10.1200/JGO.18.00073.
FROM THE JOURNAL OF GLOBAL ONCOLOGY
Key clinical point: Tyrosine kinase inhibitors are feasible for treating clear cell renal cell carcinoma in a Brazilian population.
Major finding: Overall survival in Brazilian patients with clear cell RCC was 15.2 months with sunitinib and 14.2 months with pazopanib.
Study details: Retrospective cohort study in 222 patients with advanced clear cell RCC.
Disclosures: Six authors declared honoraria, funding, expenses, or consultancies with the pharmaceutical industry. Two authors had no conflicts of interest to declare.
Source: Velho PI et al. J Glob Onc. 2018 Sep 10. doi: 10.1200/JGO.18.00073.
Women, older patients at greater risk of more aggressive PBC
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
A large, real-world study of primary biliary cholangitis has revealed that patients who are female, older, or have other autoimmune diseases are likely to have a more progressed and aggressive disease profile.
In the Journal of Clinical Gastroenterology, researchers reported the findings of a medical records database study involving 15,875 patients with primary biliary cholangitis (PBC) – previously known as primary biliary cirrhosis – a chronic, autoimmune form of liver disease.
Overall, more than one-third of patients (38.3%) had high levels of alkaline phosphatase – a marker for treatment nonresponse, defined as at least 1.5 times the upper limit of the normal range, which is also an indicator of adverse outcomes and of progression to high-risk liver disease.
These patients were more likely to be female, less likely to be insured by Medicaid, and more likely to have been diagnosed more than 1 year ago than patients whose alkaline phosphatase levels were not high. They were also more likely to be older, from the Midwest or Southern regions of the United States, have cirrhosis, or have other autoimmune diseases such as Sjögren’s syndrome and RA.
Patients with high alkaline phosphatase also showed higher aminotransferase and bilirubin, more cirrhosis, pruritus, and jaundice, but lower albumin.
Conversely, male patients had a higher incidence of cirrhosis, the study found. Other factors independently associated with cirrhosis included older age, having Medicaid insurance, having high alkaline phosphatase, and certain autoimmune conditions including type 1 diabetes, autoimmune hepatitis, and ulcerative colitis.
In patients with cirrhosis, the authors saw higher serum levels of AST and bilirubin, but lower albumin and platelets.
Zobair M. Younossi, MD, from the Center for Liver Diseases at Inova Fairfax Hospital, Falls Church, Virginia, and his coauthors said the results suggest many patients with PBC have progressed further in their condition than previously thought.
“This implies that a heightened focus on these patients with a goal toward treating more optimally should be considered to reduce their probability of disease progression,” they wrote. “Once cirrhosis develops, adverse patient outcomes such as increased mortality and adverse health care system outcomes such as excessive resource utilization increases substantially.”
The authors noted that most patients were female and white – consistent with previous reports of PBC – but the mean age of 60 years was older than expected.
“Our data suggest that PBC patients may be getting older and this could have major implications for Medicare,” they wrote. The study also examined how patients used health care resources, and found those with alkaline phosphatase levels more than 1.5 times the upper range of normal had significantly higher use. For example, they had significantly more all-cause and disease-related visits to the doctor and more use of outpatient resources for all causes.
They also had significantly more cumulative days of treatment with ursodeoxycholic acid – the standard treatment for PBC – at 528.4 days, compared with 41.6 days in individuals without high alkaline phosphatase levels. However they were no more likely to undergo imaging procedures.
Patients with cirrhosis were also more likely to have higher levels of health care utilization, compared with patients without cirrhosis, particularly use of outpatient services, inpatient stays, and ED visits. The authors also noted that patients with Medicaid but not Medicare had a higher rate of abdominal procedures.
Given that more advanced disease and presence of cirrhosis were both major drivers of increased health care use, the authors called for better identification and treatment of these patients. “This should not only potentially improve patients’ long-term outcomes but also aid in the reduction or delay of conceivably costly health resource utilization,” they wrote.
Two authors declared research funding or consulting fees from the pharmaceutical industry, and one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
SOURCE: Younossi ZM et al. J Clin Gastroenterol. 2018 Aug 24. doi: 10.1097/MCG.0000000000001120.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY
Key clinical point: Women, older individuals, and patients with other autoimmune diseases are more likely to have worse primary biliary cholangitis (PBC).
Major finding: More than one-third of patients with PBC have high levels of alkaline phosphatase.
Study details: An analysis of medical records for 15,875 patients with PBC.
Disclosures: Two authors declared research funding or consulting fees from the pharmaceutical industry; one author was an employee of Intercept Pharmaceuticals. No other conflicts of interest were declared.
Source: Younossi ZM et al. J Clin Gastroenterol. 2018 August 24. doi: 10.1097/MCG.0000000000001120.
Huntington’s progression tracks with levels of mutant huntingtin, neurofilament light
Concentrations of mutant huntingtin protein and neurofilament light proteins in cerebrospinal fluid and blood may be the first signs of progression in Huntington’s disease, according to a paper published online Sept. 12 in Science Translational Medicine.
In a cohort of 40 Huntington’s mutation carriers with manifest disease, 20 carriers without clinical symptoms, and 20 healthy controls, researchers examined levels of mutant huntingtin (mHTT) and neurofilament light (NfL) protein in biofluids, in parallel with clinical evaluations and MRI imaging.
They found that concentrations of mHTT in the cerebrospinal fluid (CSF) and concentrations of NfL proteins in the CSF and plasma were significantly higher in participants with manifest Huntington’s disease (HD) than in those without manifest disease or in controls.
Researchers also saw that CSF concentrations of mHTT showed the earliest detectable change in progression of the disease, followed by plasma and CSF levels of NfL. After that came changes in caudate and global brain volume, motor score, word reading, and other clinical measures.
“These results suggest that as our understanding grows further, analysis of mHTT and NfL might be useful for developing HD therapeutics and for clinical management,” wrote Lauren M. Byrne of the Huntington’s Disease Centre at the University College London Institute of Neurology and her coauthors.
Plasma concentrations of NfL showed the strongest association with clinical severity, even after adjusting for the number of CAG (or cytosine, adenine, and guanine) repeats – a measure of disease severity – and age.
“Our previous work suggests that NfL is a dynamic marker of ongoing neuronal damage in HD that predicts subsequent progression,” the authors wrote. “This perhaps reflects that NfL, as a marker of axonal damage, has a more direct relationship with the development of clinical manifestations and brain atrophy.”
NfL concentrations in CSF more closely predicted brain volume than did plasma NfL or CSF concentrations of mHTT.
In participants who carried the Huntington’s mutation, CSF concentrations of mHTT and NfL were strongly correlated. Researchers also noted that mutation carriers had a significantly higher CSF-to-plasma ratio of NfL than did controls.
The study also showed that mHTT in the CSF and NfL in the cerebrospinal fluid and plasma, were very stable within individuals over 4-8 weeks.
“The very high intraclass correlation values of the three markers revealed them to be highly stable, suggesting that intraindividual variation in these analytes is likely to be a minimal source of noise in natural history and therapeutic studies,” the authors wrote.
This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
SOURCE: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
Concentrations of mutant huntingtin protein and neurofilament light proteins in cerebrospinal fluid and blood may be the first signs of progression in Huntington’s disease, according to a paper published online Sept. 12 in Science Translational Medicine.
In a cohort of 40 Huntington’s mutation carriers with manifest disease, 20 carriers without clinical symptoms, and 20 healthy controls, researchers examined levels of mutant huntingtin (mHTT) and neurofilament light (NfL) protein in biofluids, in parallel with clinical evaluations and MRI imaging.
They found that concentrations of mHTT in the cerebrospinal fluid (CSF) and concentrations of NfL proteins in the CSF and plasma were significantly higher in participants with manifest Huntington’s disease (HD) than in those without manifest disease or in controls.
Researchers also saw that CSF concentrations of mHTT showed the earliest detectable change in progression of the disease, followed by plasma and CSF levels of NfL. After that came changes in caudate and global brain volume, motor score, word reading, and other clinical measures.
“These results suggest that as our understanding grows further, analysis of mHTT and NfL might be useful for developing HD therapeutics and for clinical management,” wrote Lauren M. Byrne of the Huntington’s Disease Centre at the University College London Institute of Neurology and her coauthors.
Plasma concentrations of NfL showed the strongest association with clinical severity, even after adjusting for the number of CAG (or cytosine, adenine, and guanine) repeats – a measure of disease severity – and age.
“Our previous work suggests that NfL is a dynamic marker of ongoing neuronal damage in HD that predicts subsequent progression,” the authors wrote. “This perhaps reflects that NfL, as a marker of axonal damage, has a more direct relationship with the development of clinical manifestations and brain atrophy.”
NfL concentrations in CSF more closely predicted brain volume than did plasma NfL or CSF concentrations of mHTT.
In participants who carried the Huntington’s mutation, CSF concentrations of mHTT and NfL were strongly correlated. Researchers also noted that mutation carriers had a significantly higher CSF-to-plasma ratio of NfL than did controls.
The study also showed that mHTT in the CSF and NfL in the cerebrospinal fluid and plasma, were very stable within individuals over 4-8 weeks.
“The very high intraclass correlation values of the three markers revealed them to be highly stable, suggesting that intraindividual variation in these analytes is likely to be a minimal source of noise in natural history and therapeutic studies,” the authors wrote.
This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
SOURCE: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
Concentrations of mutant huntingtin protein and neurofilament light proteins in cerebrospinal fluid and blood may be the first signs of progression in Huntington’s disease, according to a paper published online Sept. 12 in Science Translational Medicine.
In a cohort of 40 Huntington’s mutation carriers with manifest disease, 20 carriers without clinical symptoms, and 20 healthy controls, researchers examined levels of mutant huntingtin (mHTT) and neurofilament light (NfL) protein in biofluids, in parallel with clinical evaluations and MRI imaging.
They found that concentrations of mHTT in the cerebrospinal fluid (CSF) and concentrations of NfL proteins in the CSF and plasma were significantly higher in participants with manifest Huntington’s disease (HD) than in those without manifest disease or in controls.
Researchers also saw that CSF concentrations of mHTT showed the earliest detectable change in progression of the disease, followed by plasma and CSF levels of NfL. After that came changes in caudate and global brain volume, motor score, word reading, and other clinical measures.
“These results suggest that as our understanding grows further, analysis of mHTT and NfL might be useful for developing HD therapeutics and for clinical management,” wrote Lauren M. Byrne of the Huntington’s Disease Centre at the University College London Institute of Neurology and her coauthors.
Plasma concentrations of NfL showed the strongest association with clinical severity, even after adjusting for the number of CAG (or cytosine, adenine, and guanine) repeats – a measure of disease severity – and age.
“Our previous work suggests that NfL is a dynamic marker of ongoing neuronal damage in HD that predicts subsequent progression,” the authors wrote. “This perhaps reflects that NfL, as a marker of axonal damage, has a more direct relationship with the development of clinical manifestations and brain atrophy.”
NfL concentrations in CSF more closely predicted brain volume than did plasma NfL or CSF concentrations of mHTT.
In participants who carried the Huntington’s mutation, CSF concentrations of mHTT and NfL were strongly correlated. Researchers also noted that mutation carriers had a significantly higher CSF-to-plasma ratio of NfL than did controls.
The study also showed that mHTT in the CSF and NfL in the cerebrospinal fluid and plasma, were very stable within individuals over 4-8 weeks.
“The very high intraclass correlation values of the three markers revealed them to be highly stable, suggesting that intraindividual variation in these analytes is likely to be a minimal source of noise in natural history and therapeutic studies,” the authors wrote.
This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
SOURCE: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: Cerebrospinal levels of mutant huntingtin could be earliest sign of Huntington’s disease progression.
Major finding: Changing levels of mutant huntingtin in the cerebrospinal fluid are the first sign of disease progression.
Study details: Cohort study in 60 Huntington’s disease mutation carriers and 20 controls.
Disclosures: This work was supported by the Medical Research Council U.K., the CHDI Foundation, the Wellcome Trust, the U.K. Department of Health’s National Institute for Health Research Biomedical Research Centres funding scheme, the U.K. Dementia Research Institute, F. Hoffmann-La Roche, the Horizon 2020 Framework Programme, and the Engineering and Physical Sciences Research Council. A number of authors disclosed consulting or serving on advisory boards for F. Hoffmann-La Roche and/or other companies. Three authors are full-time employees of F. Hoffmann-La Roche.
Source: Byrne L et al. Sci Transl Med. 2018;10:eaat7108. doi: 10.1126/scitranslmed.aat7108.
Hormonal contraceptives tied to leukemia in progeny
A nationwide cohort study suggests an association between a woman’s use of hormonal contraceptives and leukemia in her offspring.
Children of mothers who used hormonal contraception, either during pregnancy or in the 3 months beforehand, had a 1.5-fold greater risk of leukemia, when compared to children of mothers who had never used hormonal contraception.
This increased risk translated to one additional case of leukemia per about 50,000 children exposed to hormonal contraceptives.
The increased risk appeared limited to non-lymphoid leukemia.
Marie Hargreave, PhD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, and her colleagues reported these findings in The Lancet Oncology.
The study included 1,185,157 children born between 1996 and 2014 and followed for a median of 9.3 years. Data on these children were collected from the Danish Medical Birth Registry and the Danish Cancer Registry.
The researchers looked at redeemed prescriptions from the Danish National Prescription Registry to determine the mothers’ contraceptive use and divided the women into three categories:
- Mothers who had never used hormonal contraceptives
- Those with previous hormonal contraceptive use, defined as greater than 3 months before the start of pregnancy
- Mothers with recent contraceptive use, defined as during or within 3 months of pregnancy.
Results
There were 606 children diagnosed with leukemia in the study cohort—465 with lymphoid leukemia and 141 with non-lymphoid leukemia.
Overall, children born to mothers with previous or recent use of hormonal contraceptives had a significantly increased risk of developing any leukemia. The hazard ratios (HRs) were as follows:
- Previous use of hormonal contraceptives—HR=1.25 (P=0.039)
- Recent use—HR=1.46 (P=0.011)
- Use within 3 months of pregnancy—HR=1.42 (P=0.025)
- Use during pregnancy—HR=1.78 (P=0.070).
The risk of lymphoid leukemia did not increase significantly with maternal use of hormonal contraceptives. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.23 (P=0.089)
- Recent use—HR=1.27 (P=0.167)
- Use within 3 months of pregnancy—HR=1.28 (P=0.173)
- Use during pregnancy—HR=1.22 (P=0.635).
However, the risk of non-lymphoid leukemia was significantly increased in children born to mothers with recent hormonal contraceptive use. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.33 (P=0.232)
- Recent use—HR=2.17 (P=0.008)
- Use within 3 months of pregnancy—HR=1.95 (P=0.033)
- Use during pregnancy—HR=3.87 (P=0.006).
The association between recent contraceptive use and any leukemia was strongest in children ages 6 to 10 years. The researchers said this was not surprising because the incidence of non-lymphoid leukemia increases after the age of 6.
The researchers estimated that a mother’s recent use of hormonal contraceptives would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia over the study period.
This low risk of leukemia “is not a major concern with regard to the safety of hormonal contraceptives,” the researchers said.
However, the findings do suggest the intrauterine hormonal environment affects leukemia development in children, and this should be explored in future research.
This study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations, and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
A nationwide cohort study suggests an association between a woman’s use of hormonal contraceptives and leukemia in her offspring.
Children of mothers who used hormonal contraception, either during pregnancy or in the 3 months beforehand, had a 1.5-fold greater risk of leukemia, when compared to children of mothers who had never used hormonal contraception.
This increased risk translated to one additional case of leukemia per about 50,000 children exposed to hormonal contraceptives.
The increased risk appeared limited to non-lymphoid leukemia.
Marie Hargreave, PhD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, and her colleagues reported these findings in The Lancet Oncology.
The study included 1,185,157 children born between 1996 and 2014 and followed for a median of 9.3 years. Data on these children were collected from the Danish Medical Birth Registry and the Danish Cancer Registry.
The researchers looked at redeemed prescriptions from the Danish National Prescription Registry to determine the mothers’ contraceptive use and divided the women into three categories:
- Mothers who had never used hormonal contraceptives
- Those with previous hormonal contraceptive use, defined as greater than 3 months before the start of pregnancy
- Mothers with recent contraceptive use, defined as during or within 3 months of pregnancy.
Results
There were 606 children diagnosed with leukemia in the study cohort—465 with lymphoid leukemia and 141 with non-lymphoid leukemia.
Overall, children born to mothers with previous or recent use of hormonal contraceptives had a significantly increased risk of developing any leukemia. The hazard ratios (HRs) were as follows:
- Previous use of hormonal contraceptives—HR=1.25 (P=0.039)
- Recent use—HR=1.46 (P=0.011)
- Use within 3 months of pregnancy—HR=1.42 (P=0.025)
- Use during pregnancy—HR=1.78 (P=0.070).
The risk of lymphoid leukemia did not increase significantly with maternal use of hormonal contraceptives. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.23 (P=0.089)
- Recent use—HR=1.27 (P=0.167)
- Use within 3 months of pregnancy—HR=1.28 (P=0.173)
- Use during pregnancy—HR=1.22 (P=0.635).
However, the risk of non-lymphoid leukemia was significantly increased in children born to mothers with recent hormonal contraceptive use. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.33 (P=0.232)
- Recent use—HR=2.17 (P=0.008)
- Use within 3 months of pregnancy—HR=1.95 (P=0.033)
- Use during pregnancy—HR=3.87 (P=0.006).
The association between recent contraceptive use and any leukemia was strongest in children ages 6 to 10 years. The researchers said this was not surprising because the incidence of non-lymphoid leukemia increases after the age of 6.
The researchers estimated that a mother’s recent use of hormonal contraceptives would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia over the study period.
This low risk of leukemia “is not a major concern with regard to the safety of hormonal contraceptives,” the researchers said.
However, the findings do suggest the intrauterine hormonal environment affects leukemia development in children, and this should be explored in future research.
This study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations, and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.
A nationwide cohort study suggests an association between a woman’s use of hormonal contraceptives and leukemia in her offspring.
Children of mothers who used hormonal contraception, either during pregnancy or in the 3 months beforehand, had a 1.5-fold greater risk of leukemia, when compared to children of mothers who had never used hormonal contraception.
This increased risk translated to one additional case of leukemia per about 50,000 children exposed to hormonal contraceptives.
The increased risk appeared limited to non-lymphoid leukemia.
Marie Hargreave, PhD, of the Danish Cancer Society Research Center in Copenhagen, Denmark, and her colleagues reported these findings in The Lancet Oncology.
The study included 1,185,157 children born between 1996 and 2014 and followed for a median of 9.3 years. Data on these children were collected from the Danish Medical Birth Registry and the Danish Cancer Registry.
The researchers looked at redeemed prescriptions from the Danish National Prescription Registry to determine the mothers’ contraceptive use and divided the women into three categories:
- Mothers who had never used hormonal contraceptives
- Those with previous hormonal contraceptive use, defined as greater than 3 months before the start of pregnancy
- Mothers with recent contraceptive use, defined as during or within 3 months of pregnancy.
Results
There were 606 children diagnosed with leukemia in the study cohort—465 with lymphoid leukemia and 141 with non-lymphoid leukemia.
Overall, children born to mothers with previous or recent use of hormonal contraceptives had a significantly increased risk of developing any leukemia. The hazard ratios (HRs) were as follows:
- Previous use of hormonal contraceptives—HR=1.25 (P=0.039)
- Recent use—HR=1.46 (P=0.011)
- Use within 3 months of pregnancy—HR=1.42 (P=0.025)
- Use during pregnancy—HR=1.78 (P=0.070).
The risk of lymphoid leukemia did not increase significantly with maternal use of hormonal contraceptives. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.23 (P=0.089)
- Recent use—HR=1.27 (P=0.167)
- Use within 3 months of pregnancy—HR=1.28 (P=0.173)
- Use during pregnancy—HR=1.22 (P=0.635).
However, the risk of non-lymphoid leukemia was significantly increased in children born to mothers with recent hormonal contraceptive use. The HRs were as follows:
- Previous use of hormonal contraceptives—HR=1.33 (P=0.232)
- Recent use—HR=2.17 (P=0.008)
- Use within 3 months of pregnancy—HR=1.95 (P=0.033)
- Use during pregnancy—HR=3.87 (P=0.006).
The association between recent contraceptive use and any leukemia was strongest in children ages 6 to 10 years. The researchers said this was not surprising because the incidence of non-lymphoid leukemia increases after the age of 6.
The researchers estimated that a mother’s recent use of hormonal contraceptives would have resulted in about one additional case of leukemia per 47,170 children; in other words, 25 additional cases of leukemia over the study period.
This low risk of leukemia “is not a major concern with regard to the safety of hormonal contraceptives,” the researchers said.
However, the findings do suggest the intrauterine hormonal environment affects leukemia development in children, and this should be explored in future research.
This study was supported by the Danish Cancer Research Foundation and other foundations. One author reported grants from the sponsoring foundations, and another author reported speaking fees from Jazz Pharmaceuticals and Shire Pharmaceuticals.