User login
Saururus chinensis
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
Also known as Asian or Chinese lizard’s tail (or Sam-baekcho in Korea), Saururus chinensis is an East Asian plant used in traditional medicine for various indications including edema, gonorrhea, jaundice, hypertension, leproma, pneumonia, and rheumatoid arthritis.1,2 Specifically, Korean traditional medicine practitioners as well as Native Americans and early colonists in what is now the United States used the botanical to treat cancer, edema, rheumatoid arthritis, and other inflammatory conditions.2-4 Modern research has produced evidence supporting the use of this plant in the dermatologic realm. This column focuses on the relevant bench science and possible applications.
Various beneficial effects
In 2008, Yoo et al. found that the ethanol extract of the dried aerial parts of S. chinensis exhibit anti-inflammatory, antiangiogenic, and antinociceptive properties, which they suggested may partially account for the established therapeutic effects of the plant.2 Also, Lee et al. reported in 2012 on the antiproliferative effects against human cancer cell lines of neolignans found in S. chinensis.5
Antioxidant properties have been associated with S. chinensis. In 2014, Kim et al. reported that S. chinensis extract attenuated the lipopolysaccharide (LPS)-stimulated neuroinflammatory response in BV-2 microglia cells, a result that the authors partly ascribed to the antioxidant constituents (particularly quercetin) of the plant.3
Atopic dermatitis
In 2008, Choi et al. determined that the leaves of S. chinensis impeded the formation of atopic dermatitis–like skin lesions in NC/Nga mice caused by repeated application of picryl chloride, potentially by stimulating the Th1 cell response, thus modulating Th1/Th2 imbalance. They concluded that S. chinensis has potential as an adjunct treatment option for atopic dermatitis.6
Anti-inflammatory activity
In 2010, Bae et al. studied the anti-inflammatory properties of sauchinone, a lignan derived from S. chinensis reputed to exert antioxidant, anti-inflammatory, and hepatoprotective activity,7 using LPS-stimulated RAW264.7 cells. They found that the lignan lowered tumor necrosis factor (TNF)–alpha synthesis by inhibiting the c-Raf-MEK1/2-ERK1/2 phosphorylation pathway, accounting for the anti-inflammatory effects of the S. chinensis constituent.8
More recently, Zhang et al. determined that the ethanol extract of S. chinensis leaves impaired proinflammatory gene expression by blocking the TAK1/AP-1 pathway in LPS-treated RAW264.7 macrophages. They suggested that such suppression is a significant step in the anti-inflammatory function exhibited by the plant.1
Photoprotection
Park et al. investigated in 2013 the beneficial effects of sauchinone. Specifically, they studied potential photoprotective effects of the lignan against UVB in HaCaT human epidermal keratinocytes. They found that sauchinone (5-40 mcm) conferred significant protection as evaluated by cell viability and a toxicity assay. At 20-40 mcm, sauchinone blocked the upregulation of matrix metalloproteinase (MMP)–1 proteins and decrease of type 1 collagen engendered by UVB exposure. The investigators further discovered that sauchinone diminished the synthesis of reactive oxygen species. Overall, they determined that sauchinone imparted protection by suppressing extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 MAPK signaling through the activation of oxidative defense enzymes.7
Potential use as a depigmenting agent
In 2009, Seo et al. isolated the lignans manassantin A and B from S. chinensis and determined that these compounds dose-dependently impeded melanin synthesis in alpha-melanocyte stimulating hormone (alpha-MSH)–activated melanoma B16 cells. They also noted that manassantin A suppressed forskolin- or 3-isobutyl-1-methylxanthine (IBMX)–induced melanin production and diminished cellular levels of IBMX-inducible tyrosinase protein. The lignan had no effect on the catalytic activity of cell-free tyrosinase, an important enzyme in melanin pigment production. The researchers concluded that their results suggest the potential for S. chinensis to be used to treat hyperpigmentation disorders.9
Two years later Lee et al. found that manassantin A, derived from S. chinensis, steadily suppressed the cAMP elevator IBMX- or dibutyryl cAMP-induced melanin synthesis in B16 cells or in melan-a melanocytes by down-regulating the expression of tyrosinase or the TRP1 gene. The lignan also inhibited microphthalmia-associated transcription factor (MITF) induction via the IBMX-activated cAMP-responsive element-binding protein (CREB) pathway, thus preventing the Ser-133 phosphorylation of CREB. The researchers concluded that this molecular disruption of melanin production suggests the potential for the use of manassantin A as a skin depigmenting agent.10
That same year, another S. chinensis lignan gained interest. Yun et al. investigated the effects of the S. chinensis lignan component saucerneol D on melanin synthesis in cAMP-elevated melanocytes. They found that the lignan efficiently impeded melanin product in B16 melanoma cells stimulated with alpha-MSH or other cAMP elevators. Saucerneol D was also credited with down-regulating alpha-MSH–induced gene expression of tyrosinase at the transcription level in B16 cells, suppressing alpha-MSH–induced phosphorylation of CREB in the cells, and inhibiting MITF induction. The investigators concluded that their results point to the potential of the S. chinensis lignan saucerneol D for the treatment of hyperpigmentation disorders.11
In 2012, Chang et al. observed that an extract of S. chinensis and one of its constituent lignans, manassantin B, prevented melanosome transport in normal human melanocytes and Melan-a melanocytes, by interrupting the interaction between melanophilin and myosin Va. The investigators concluded that as a substance that can hinder melanosome transport, manassantin B displays potential for use as depigmenting product.12
The following year, Lee et al. studied the effects of S. chinensis extracts on the melanogenesis signaling pathway activated by alpha-MSH, finding dose-dependent inhibition without provoking cytotoxicity in B16F10 cells. Further, the team found evidence that the depigmenting activity exhibited by S. chinensis extracts may occur as a result of MITF and tyrosinase expression stemming from elevated activity of extracellular signal-regulated kinase (ERK). They concluded that their results support further examination of S. chinensis for its potential to contribute to skin whitening.5
Conclusion
Multiple lignan constituents in this plant-derived ingredient appear to yield anti-inflammatory, antioxidant, photoprotective, and antitumor properties. Its inhibitory effects on melanin production and its antiaging abilities make it worthy of further study and consideration of inclusion in antiaging skin care products.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the division of cosmetic dermatology at the University of Miami in 1997. The third edition of her bestselling textbook, “Cosmetic Dermatology,” was published in 2022. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Johnson & Johnson, and Burt’s Bees. She is the CEO of Skin Type Solutions, a SaaS company used to generate skin care routines in the office and as an e-commerce solution. Write to her at [email protected].
References
1. Zhang J et al. J Ethnopharmacol. 2021 Oct 28;279:114400.
2. Yoo HJ et al. J Ethnopharmacol. 2008 Nov 20;120(2):282-6.
3. Kim BW et al. BMC Complement Altern Med. 2014 Dec 16;14:502.
4. Lee DH et al. Biol Pharm Bull. 2013;36(5):772-9.
5. Lee YJ et al. Biol Pharm Bull. 2012;35(8):1361-6.
6. Choi MS et al. Biol Pharm Bull. 2008 Jan;31(1):51-6.
7. Park G et al. Biol Pharm Bull. 2013;36(7):1134-9.
8. Bae HB et al. Int Immunopharmacol. 2010 Sep;10(9):1022-8.
9. Seo CS et al. Phytother Res. 2009 Nov;23(11):1531-6.
10. Lee HD et al. Exp Dermatol. 2011 Sep;20(9):761-3.
11. Yun JY et al. Arch Pharm Res. 2011 Aug;34(8):1339-45.
12. Chang H et al. Pigment Cell Melanoma Res. 2012 Nov;25(6):765-72.
New Year’s resolutions
I can’t presume to know what issues need addressing in your practice, but I do know the ones I get asked about most often, so I can offer some suggestions that might provide inspiration:
1. Keep your website up to date. Check it now, then make a note to check it regularly. Most people find their physicians online these days, and you don’t want them finding a year-old presentation with outdated photos, personnel, services, and rates. Keep your site current, or hire someone to do it for you.
2. Be an authoritative presence on social media. Like it or not, you should be on Facebook, Twitter (at least for now), Instagram, TikTok – wherever your patients congregate. Medical topics are popular search categories, and they are searching for expert advice. You are the expert. There is a ton of medical misinformation online, and it needs to be countered with accurate, factual data from bona fide experts.
3. Follow colleagues. No need to reinvent the wheel; many physicians have already developed large online followings. Track some of them down, follow them yourself, and use them as inspiration for your own online contributions. Your specialty society probably maintains a presence on Instagram and other sites as well, and they are a good source of topics and tips.
4. Post frequently. We all have a finite amount of time, but a few brief posts per week on various social media platforms will attract more attention, and garner more followers than an occasional long treatise. Add relevant hashtags to get more reach and engagement.
5. Participate in trends. When a topic is getting thousands of views, it a trending topic. Post on trending topics, and if you know the trend’s original authors, tag them. That will increase your audience, and the compliment might be reciprocated in the future.
6. Google yourself. You might be surprised by what you find. Being aware of what is being said about you online is a necessary exercise to maintain a healthy online reputation. The good reviews are ego builders, but it’s the bad reviews that you can learn from. They will help you identify your negative personality traits and motivate you to eliminate them.
7. Encrypt your mobile devices. The biggest HIPAA vulnerability in many practices is laptops and tablets carrying confidential patient information; losing one could be a disaster. Encryption software is cheap and readily available, and a lost or stolen mobile device will probably not be treated as a HIPAA breach if it is properly encrypted.
8. Back up your data. Now is an excellent time to verify that the information on your office and personal computers is being backed up – locally and online – on a regular schedule. Don’t wait until something crashes.
9. Keep a closer eye on your office finances. Most physicians delegate the bookkeeping, and that’s fine. But ignoring the financial side completely creates an atmosphere that facilitates embezzlement. Set aside a couple of hours each month to review the books personally. And make sure your employees know you’re doing it.
10. Make sure your long-range financial planning is on track. I’ve said this before, but it can’t be repeated too often. Economic conditions change all the time. Once a year, you should sit down with your accountant and lawyer and make sure your investments are well-diversified and all other aspects of your finances – budgets, credit ratings, insurance coverage, tax situations, college savings, estate plans, retirement accounts – are in the best shape possible.
11. Pay down your debt. Another oldie but goodie. Debt can destroy the best laid retirement plans. If you carry significant debt, set up a plan to pay it off as soon as you can.
12. Take more vacations. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.” If you’ve been working too much, this is the year to start spending more time enjoying your life, your friends and family, and the world. As John Lennon said, “Life is what happens to you while you’re busy making other plans.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I can’t presume to know what issues need addressing in your practice, but I do know the ones I get asked about most often, so I can offer some suggestions that might provide inspiration:
1. Keep your website up to date. Check it now, then make a note to check it regularly. Most people find their physicians online these days, and you don’t want them finding a year-old presentation with outdated photos, personnel, services, and rates. Keep your site current, or hire someone to do it for you.
2. Be an authoritative presence on social media. Like it or not, you should be on Facebook, Twitter (at least for now), Instagram, TikTok – wherever your patients congregate. Medical topics are popular search categories, and they are searching for expert advice. You are the expert. There is a ton of medical misinformation online, and it needs to be countered with accurate, factual data from bona fide experts.
3. Follow colleagues. No need to reinvent the wheel; many physicians have already developed large online followings. Track some of them down, follow them yourself, and use them as inspiration for your own online contributions. Your specialty society probably maintains a presence on Instagram and other sites as well, and they are a good source of topics and tips.
4. Post frequently. We all have a finite amount of time, but a few brief posts per week on various social media platforms will attract more attention, and garner more followers than an occasional long treatise. Add relevant hashtags to get more reach and engagement.
5. Participate in trends. When a topic is getting thousands of views, it a trending topic. Post on trending topics, and if you know the trend’s original authors, tag them. That will increase your audience, and the compliment might be reciprocated in the future.
6. Google yourself. You might be surprised by what you find. Being aware of what is being said about you online is a necessary exercise to maintain a healthy online reputation. The good reviews are ego builders, but it’s the bad reviews that you can learn from. They will help you identify your negative personality traits and motivate you to eliminate them.
7. Encrypt your mobile devices. The biggest HIPAA vulnerability in many practices is laptops and tablets carrying confidential patient information; losing one could be a disaster. Encryption software is cheap and readily available, and a lost or stolen mobile device will probably not be treated as a HIPAA breach if it is properly encrypted.
8. Back up your data. Now is an excellent time to verify that the information on your office and personal computers is being backed up – locally and online – on a regular schedule. Don’t wait until something crashes.
9. Keep a closer eye on your office finances. Most physicians delegate the bookkeeping, and that’s fine. But ignoring the financial side completely creates an atmosphere that facilitates embezzlement. Set aside a couple of hours each month to review the books personally. And make sure your employees know you’re doing it.
10. Make sure your long-range financial planning is on track. I’ve said this before, but it can’t be repeated too often. Economic conditions change all the time. Once a year, you should sit down with your accountant and lawyer and make sure your investments are well-diversified and all other aspects of your finances – budgets, credit ratings, insurance coverage, tax situations, college savings, estate plans, retirement accounts – are in the best shape possible.
11. Pay down your debt. Another oldie but goodie. Debt can destroy the best laid retirement plans. If you carry significant debt, set up a plan to pay it off as soon as you can.
12. Take more vacations. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.” If you’ve been working too much, this is the year to start spending more time enjoying your life, your friends and family, and the world. As John Lennon said, “Life is what happens to you while you’re busy making other plans.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
I can’t presume to know what issues need addressing in your practice, but I do know the ones I get asked about most often, so I can offer some suggestions that might provide inspiration:
1. Keep your website up to date. Check it now, then make a note to check it regularly. Most people find their physicians online these days, and you don’t want them finding a year-old presentation with outdated photos, personnel, services, and rates. Keep your site current, or hire someone to do it for you.
2. Be an authoritative presence on social media. Like it or not, you should be on Facebook, Twitter (at least for now), Instagram, TikTok – wherever your patients congregate. Medical topics are popular search categories, and they are searching for expert advice. You are the expert. There is a ton of medical misinformation online, and it needs to be countered with accurate, factual data from bona fide experts.
3. Follow colleagues. No need to reinvent the wheel; many physicians have already developed large online followings. Track some of them down, follow them yourself, and use them as inspiration for your own online contributions. Your specialty society probably maintains a presence on Instagram and other sites as well, and they are a good source of topics and tips.
4. Post frequently. We all have a finite amount of time, but a few brief posts per week on various social media platforms will attract more attention, and garner more followers than an occasional long treatise. Add relevant hashtags to get more reach and engagement.
5. Participate in trends. When a topic is getting thousands of views, it a trending topic. Post on trending topics, and if you know the trend’s original authors, tag them. That will increase your audience, and the compliment might be reciprocated in the future.
6. Google yourself. You might be surprised by what you find. Being aware of what is being said about you online is a necessary exercise to maintain a healthy online reputation. The good reviews are ego builders, but it’s the bad reviews that you can learn from. They will help you identify your negative personality traits and motivate you to eliminate them.
7. Encrypt your mobile devices. The biggest HIPAA vulnerability in many practices is laptops and tablets carrying confidential patient information; losing one could be a disaster. Encryption software is cheap and readily available, and a lost or stolen mobile device will probably not be treated as a HIPAA breach if it is properly encrypted.
8. Back up your data. Now is an excellent time to verify that the information on your office and personal computers is being backed up – locally and online – on a regular schedule. Don’t wait until something crashes.
9. Keep a closer eye on your office finances. Most physicians delegate the bookkeeping, and that’s fine. But ignoring the financial side completely creates an atmosphere that facilitates embezzlement. Set aside a couple of hours each month to review the books personally. And make sure your employees know you’re doing it.
10. Make sure your long-range financial planning is on track. I’ve said this before, but it can’t be repeated too often. Economic conditions change all the time. Once a year, you should sit down with your accountant and lawyer and make sure your investments are well-diversified and all other aspects of your finances – budgets, credit ratings, insurance coverage, tax situations, college savings, estate plans, retirement accounts – are in the best shape possible.
11. Pay down your debt. Another oldie but goodie. Debt can destroy the best laid retirement plans. If you carry significant debt, set up a plan to pay it off as soon as you can.
12. Take more vacations. Remember Eastern’s First Law: Your last words will NOT be, “I wish I had spent more time in the office.” If you’ve been working too much, this is the year to start spending more time enjoying your life, your friends and family, and the world. As John Lennon said, “Life is what happens to you while you’re busy making other plans.”
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
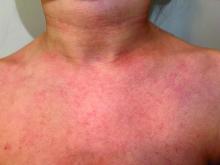
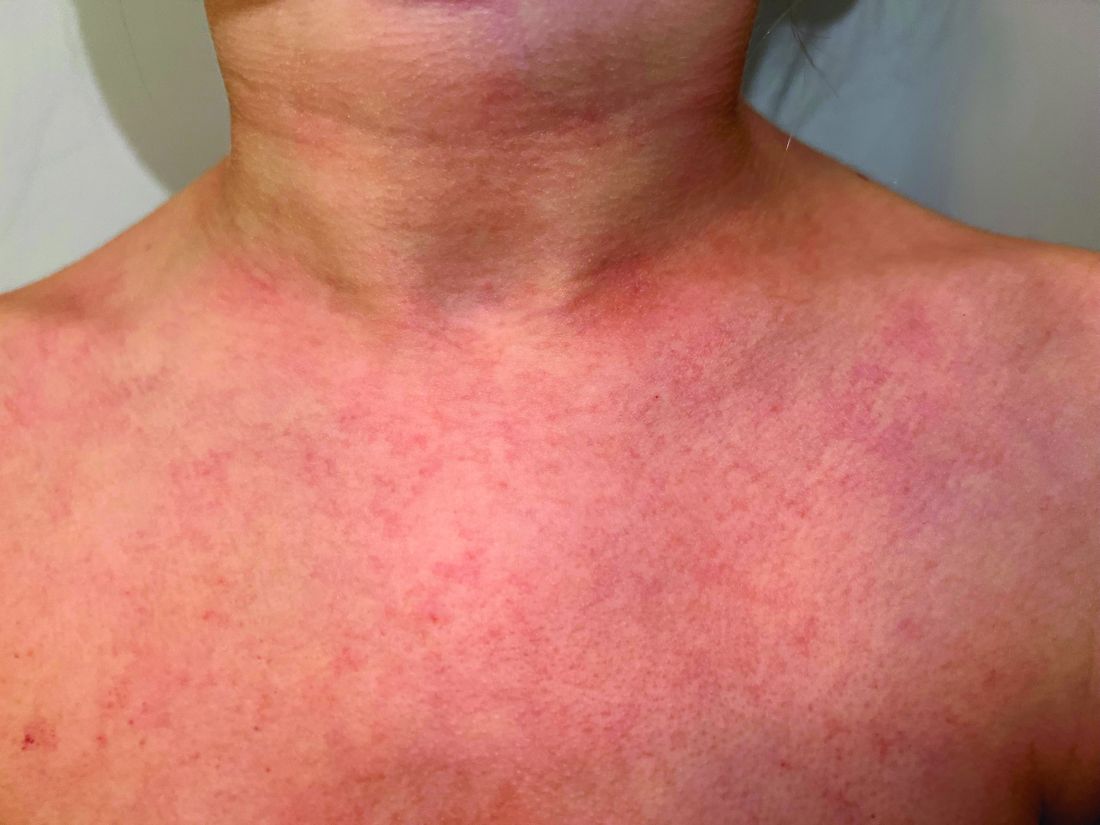
A 9-year old female presented with 1 day of fever, fatigue, and sore throat
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
This condition typically presents in the setting of Streptococcus pyogenes pharyngitis, or strep throat, and is spread via mucosal transfer in close proximity such as classrooms and nurseries. The dermatologic symptoms are a result of the endotoxin produced by S. pyogenes, which is part of the group A Strep bacteria. Clinically, the presentation can be differentiated from an allergic eruption by its relation to acute pharyngitis, insidious onset, and lack of confluence of the lesions. Diagnosis is supported by a throat culture and rapid strep test, although a rapid test lacks reliability in older patients who are less commonly affected and likely to be carriers. First-line treatment is penicillin or amoxicillin, but first-generation cephalosporins, clindamycin, or erythromycin are sufficient if the patient is allergic to penicillins. Prognosis worsens as time between onset and treatment increases, but is overall excellent now with the introduction of antibiotics and improved hygiene.
Scarlet fever is among a list of many common childhood rashes, and it can be difficult to differentiate between these pathologies on clinical presentation. A few notable childhood dermatologic eruptions include erythema infectiosum (fifth disease), roseola (exanthema subitum or sixth disease), and measles. These cases can be distinguished clinically by the age of the patient, distribution, and quality of the symptoms. Laboratory testing may be used to confirm the diagnosis.
Erythema infectiosum is known as fifth disease or slapped-cheek rash because it commonly presents on the cheeks as a pink, maculopapular rash in a reticular pattern. The disease is caused by parvovirus B19 and is accompanied by low fever, malaise, headache, sore throat, and nausea, which precedes the erythematous rash. The facial rash appears first and is followed by patchy eruptions on the extremities. Appearance of the rash typically indicates the patient is no longer contagious, and patients are treated symptomatically with NSAIDs and antihistamines for associated pruritus.
Roseola infantum is commonly caused by human herpesvirus 6 and is usually found in children 3 years and younger. The defining symptom is a high fever, which is paired with a mild cough, runny nose, and diarrhea. A maculopapular rash appears after the fever subsides, starting centrally and spreading outward to the extremities. Although this rash is similar to measles, they can be differentiated by the order of onset. The rash caused by measles begins on the face and mouth (Koplik spots) and moves downward. Additionally, the patient appears generally healthy and the disease is self-limiting in roseola, while patients with measles will appear more ill and require further attention. Measles is caused by the measles virus of the genus Morbillivirus and is highly contagious. It is spread via respiratory route presenting with fever, cough, coryza, and conjunctivitis followed by the rash. Fortunately, the measles vaccine is in widespread use, so cases have declined over the years.
Our patient had a positive strep test. Influenza and coronavirus tests were negative. She was started on daily amoxicillin and the rash resolved within 2 days of taking the antibiotics.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Allmon A et al.. Am Fam Physician. 2015 Aug 1;92(3):211-6.
Moss WJ. Lancet. 2017 Dec 2;390(10111):2490-502.
Mullins TB and Krishnamurthy K. Roseola Infantum, in “StatPearls.” Treasure Islan, Fla.: StatPearls Publishing, 2022.
Pardo S and Perera TB. Scarlet Fever, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2022.
Eliminating the language of blame in lung cancer
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
“Do you smoke?” I asked the patient.
“Yes, and I got what I deserved,” he answered, clearly upset.
I ignored his reaction and continued with the exam, but in retrospect, I should have explained why doctors ask patients this question.
It was not my intention to be rude or blame the patient for his lung cancer diagnosis. Doctors ask patients if they smoke because a smoking history can change the type of treatment and it can be associated with other conditions that may interfere with treatment. It can also determine whether smoking cessation assistance should be offered to the patient. It is crucial that we as doctors know a patient’s medical history, but how we approach sensitive issues may determine if we even get the information we need. In this case, I didn’t explain why I asked the patient if he smoked. Had I taken the time to explain why I needed to know if and how long he smoked and that I was not blaming him for his lung cancer diagnosis, we may have had a more mutually respectful and beneficial relationship.
Almost all of my patients with lung cancer have been asked at one time or another – by a health care provider, friends, or acquaintances – “Do you smoke?” Whether or not they smoked, patients with lung cancer feel the weight of moral judgment being cast upon them by society.
It is common for people who smoke and who go on to develop lung cancer to be weighed down by guilt associated with their diagnosis. Patients with lung cancer face stigma-associated hurdles based on the “I did it to myself” mindset. This societal stigma is not without harm as it can result in emotional responses of guilt and self-blame. This internalized stigma may lead to psychosocial distress and decreased interactions with family, friends, and health care providers. The guilt may drive a patient to forgo lung cancer screening, minimize symptoms, delay seeking treatment, and not advocate for themselves with their physician. Some patients even decide to forgo all treatment.
What about patients who never smoked? They too feel tinged with blame. Many of these patients feel called upon to defend themselves by proclaiming loudly that they have never smoked.
Blame and shame also divides the lung cancer community, resulting in less advocacy. It may also impact research dollars for lung cancer. According to the Lung Cancer Research Foundation, “Despite being the leading cause of cancer mortality, lung cancer receives far less research funding than any other cancer.” By comparison, women with breast cancer are showered with far more resources, supportive services, fundraising events, and certainly more lobbying.
By making unintentional hurtful statements and using judgmental or denigrating language, the lung cancer community may unconsciously be playing a role in perpetuating stigmas associated with lung cancer. That kind of language can come across as blame.
The International Association for the Study of Lung Cancer has developed a language guide to help reduce stigma associated with lung cancer. The aim is to reduce and replace traditional medical language during our patient interactions, presentations, and publications with language that is more empathic and nonjudgmental.
For example, replace the term “cancer patient” with the term “the patient with cancer.” The patient is a person who happens to have been diagnosed with lung cancer, they are not “cancer.” Patients can be very sensitive to language and may misinterpret language that doctors commonly use. Language such as “the patient failed treatment” may be interpreted by patients as a personal failure. In reality, the treatment failed the patient, instead of the other way around. Instead, shift the blame from the patient to the cancer. Adopt terms like “the tumor did not respond to treatment.” Or, “the cancer progressed” instead of “the patient progressed.”
Language around smoking is particularly stigmatizing because it categorizes a person by a behavior. As health care providers, we should consider removing the term “smoker” from our interactions with patients and instead, use “patient who smokes” or ”patient with a smoking history.” Other ways health care providers can reduce stigma triggered by assessing smoking status include using supportive communication skills, providing a rationale for asking smoking related questions, offering help and tobacco cessation and other resources, and displaying empathic behavior, such as maintaining eye contact and a nonjudgmental body position orientated toward the patient.
Many of these common medical phrases were developed to enable efficient communication among health care professionals. Times have changed and patients should not be defined by an illness. They are people first. In addition to improving patient interactions in clinic, using nonjudgmental language whenever possible in presentations and publications is also extremely important, as patients are living longer and getting more involved in research and advocacy.
“Words have energy and power with the ability to help, to heal, to hinder, to hurt, to harm, to humiliate, and to humble,” says Yehuda Berg, author and codirector of the Kabbalah Centre International in Los Angeles.
Dr. Schiller is a medical oncologist and founding member of Oncologists United for Climate and Health. She is a former board member of the International Association for the Study of Lung Cancer and a current board member of the Lung Cancer Research Foundation.
Immunity debt and the tripledemic
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Respiratory syncytial virus (RSV) and influenza cases are surging to record numbers this winter in the wake of the COVID-19 pandemic when children were sheltering in the home, receiving virtual education, masking, and hand sanitizing, and when other precautionary health measures were in place.
RSV and flu illness in children now have hospital emergency rooms and pediatric ICUs and wards over capacity. As these respiratory infections increase and variants of SARS-CoV-2 come to dominate, we may expect the full impact of a tripledemic (RSV + flu + SARS-CoV-2).
It has been estimated that RSV causes 33 million lower respiratory infections and 3.6 million hospitalizations annually worldwide in children younger than 5 years old (Lancet. 2022 May 19. doi: 10.1016/S0140-6736(22)00478-0). RSV is typically a seasonal respiratory infection occurring in late fall through early winter, when it gives way to dominance by flu. Thus, we have experienced an out-of-season surge in RSV since it began in early fall 2022, and it persists. A likely explanation for the early and persisting surge in RSV is immunity debt (Infect Dis Now. 2021 Aug. doi: 10.1016/j.idnow.2021.05.004).
Immunity debt is an unintended consequence of prevention of infections that occurred because of public health measures to prevent spread of SARS-CoV-2 infections. The COVID-19 lockdown undoubtedly saved many lives. However, while we were sheltering from SARS-CoV-2 infections, we also were avoiding other infections, especially other respiratory infections such as RSV and flu.
Our group studied this in community-based pediatric practices in Rochester, N.Y. Physician-diagnosed, medically attended infectious disease illness visits were assessed in two child cohorts, age 6-36 months from March 15 to Dec. 31, 2020 (the pandemic period), compared with the same months in 2019 (prepandemic). One hundred forty-four children were included in the pandemic cohort and 215 in the prepandemic cohort. Visits for bronchiolitis were 7.4-fold lower (P = .04), acute otitis media 3.7-fold lower (P < .0001), viral upper respiratory infections (URI) 3.8-fold lower (P < .0001), and croup 27.5-fold lower (P < .0001) in the pandemic than the prepandemic cohort (Front Pediatr. 2021 Sep 13. doi: 10.3389/fped.2021.72248).
The significant reduction in respiratory illness during the COVID-19 epidemic we and others observed resulted in a large pool of children who did not experience RSV or flu infections for an entire year or more. Herd immunity dropped. The susceptible child population increased, including children older than typically seen. We had an immunity debt that had to be repaid, and the repayment is occurring now.
As a consequence of the surge in RSV, interest in prevention has gained more attention. In 1966, tragically, two infant deaths and hospitalization of 80% of the participating infants occurred during a clinical trial of an experimental candidate RSV vaccine, which contained an inactivated version of the entire virus. The severe side effect was later found to be caused by both an antibody and a T-cell problem. The antibody produced in response to the inactivated whole virus didn’t have very good functional activity at blocking or neutralizing the virus. That led to deposition of immune complexes and activation of complement that damaged the airways. The vaccine also triggered a T-cell response with inflammatory cytokine release that added to airway obstruction and lack of clearance of the virus. RSV vaccine development was halted and the bar for further studies was raised very high to ensure safety of any future RSV vaccines. Now, 55 years later, two RSV vaccines and a new preventive monoclonal antibody are nearing licensure.
GlaxoSmithKline (GSK) and Pfizer are in phase 3 clinical trials of a safer RSV vaccine that contains only the RSV surface protein known as protein F. Protein F changes its structure when the virus infects and fuses with human respiratory epithelial cells. The GSK and Pfizer vaccines use a molecular strategy developed at the National Institutes of Health to lock protein F into its original, prefusion configuration. A similar strategy was used by Pfizer/BioNTech and Moderna in their design of mRNA vaccines to the SARS-CoV-2 spike surface protein.
A vaccine with the F protein in its prefusion form takes care of the antibody problem that caused the severe side-effects from the 1966 version of inactivated whole virus vaccine because it induces very high-efficiency, high-potency antibodies that neutralize the RSV. The T-cell response is not as well understood and that is why studies are being done in adults first and then moving to young infants.
The new RSV vaccines are being developed for use in adults over age 60, adults with comorbidities, maternal immunization, and infants. Encouraging results were recently reported by GSK and Pfizer from adult trials. In an interim analysis, Pfizer also recently reported that maternal immunization in the late second or third trimester with their vaccine had an efficacy of 82% within a newborn’s first 90 days of life against severe lower respiratory tract illness. At age 6 months, the efficacy was sustained at 69%. So far, both the GSK and Pfizer RSV vaccines have shown a favorable safety profile.
Another strategy in the RSV prevention field has been a monoclonal antibody. Palivizumab (Synagis, AstraZeneca) is used to prevent severe RSV infections in prematurely born and other infants who are at higher risk of mortality and severe morbidity. Soon there will likely be another monoclonal antibody, called nirsevimab (Beyfortus, AstraZeneca and Sanofi). It is approved in Europe but not yet approved in the United States as I prepare this column. Nirsevimab may be even better than palivizumab – based on phase 3 trial data – and a single injection lasts through an entire normal RSV season while palivizumab requires monthly injections.
Similar to the situation with RSV, the flu season started earlier than usual in fall 2022 and has been picking up steam, likely also because of immunity debt. The WHO estimates that annual epidemics of influenza cause 1 billion infections, 3 million to 5 million severe cases, and 300,000-500,000 deaths. Seasonal flu vaccines provide modest protection. Current flu vaccine formulations consist of the hemagglutinin (H) and neuraminidase (N) proteins but those proteins change sufficiently (called antigenic drift) such that production of the vaccines based on a best guess each year often is not correct for the influenza A or influenza B strains that circulate in a given year (antigenic mismatch).
Public health authorities have long worried about a major change in the composition of the H and N proteins of the influenza virus (called antigenic shift). Preparedness and response to the COVID-19 pandemic was based on preparedness and response to an anticipated influenza pandemic similar to the 1918 flu pandemic. For flu, new “universal” vaccines are in development. Among the candidate vaccines are mRNA vaccines, building on the success of the SARS-CoV-2 mRNA vaccines (Science. 2022 Nov 24. doi: 10.1126/science.abm0271).
Dr. Pichichero is a specialist in pediatric infectious diseases, Center for Infectious Diseases and Immunology, and director of the Research Institute, at Rochester (N.Y.) General Hospital. He has no conflicts of interest to declare.
Diagnosed too late
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
It had only been 3 weeks since I first met this patient. She presented with an advanced case of colon cancer, but instead of treatment,
Within the course of 2 weeks I saw another new patient, but this time with pancreatic cancer that metastasized to the liver. “When can we start treatment?” he asked. Like my female patient with colon cancer, he was diagnosed too late as he was already in an incurable stage. He was shocked to learn that his condition was in stage 4, that achieving remission would be difficult and a cure, not likely. Certainly, standard of care treatments and clinical trials offered him hope, but they were unlikely to change the outcome.
We take a course in this – that is, in giving bad news, but every doctor has his or her own approach. Some are so uncomfortable with the talk, they choose avoidance and adopt the “look like you gotta go approach.” Or, the doctor may schedule another treatment or another test with the intention of avoiding end-of-life discussions. Other doctors opt for straight talk: “I think you should get your affairs in order. You’ve got 3 months to live.” These are extreme behaviors I wouldn’t recommend.
In my practice, I sit with my patients and explain the diagnosis. After discussing all options and the advanced stage and diagnosis, it ultimately comes down to “Win or lose, I will be here to take care of you.” Sometimes there is therapy that may help, but either way, the patient understands that death is a real possibility.
I find that people just want to know if there is hope. A different treatment regimen or a clinical trial may (or may not) extend their life. And while we cannot predict outcomes, we can give them hope. You can’t shut down hope. True for some people the cup is always half empty, but most people want to live and are optimistic no matter how small the chances are.
These conversations are very difficult. I don’t like them, but then I don’t avoid them either. Fortunately, patients don’t usually come to my office for the first visit presenting with advanced disease. In the cases I described above, one patient had been experiencing unexplained weight loss, but didn’t share it with a physician. And, for the patient with pancreatic cancer, other than some discomfort in the last couple of weeks, the disease was not associated with other symptoms. But the absence of symptoms should not in any way rule out a malignant disease. A diagnosis should be based on a complete evaluation of signs and symptoms followed by testing.
We’ve got to be able to take the time to listen to our patients during these encounters. We may not spend as much time as we should because we’re so busy now and we’re slaves to EMRs. It helps if we take more time to probe symptoms a little longer, especially in the primary care setting.
It is possible for a patient with cancer to be asymptomatic up until the later stages of the disease. A study published in ESMO Open in 2020 found that fewer than half of patients with stage 4 non–small cell lung cancer have only one or two symptoms at diagnosis regardless of whether the patient was a smoker. In this study only 33% of patients reported having a cough and 25% had chest pain.
A study presented in October at the United European Gastroenterology Week found that of 600 pancreatic cancer cases, 46 of these were not detected by CT or MRI conducted 3-18 months prior to diagnosis. Of the 46 cases, 26% were not picked up by the radiologist and the rest were largely as a result of imaging changes over time. Radiology techniques are good, but they cannot pick up lesions that are too small. And some lesions, particularly in pancreatic cancer, can grow and metastasize rather quickly.
When a patient is diagnosed with advanced disease, it is most often simply because of the nature of the disease. But sometimes patients put off scheduling a doctor visit because of fear of the potential for bad news or fear of the doctor belittling their symptoms. Some tell me they were “just hoping the symptoms would disappear.” Waiting too long to see a doctor is never a good idea because timing is crucial. In many cases, there is a small window of opportunity to treat disease if remission is to be achieved.
Dr. Henry is a practicing clinical oncologist with PennMedicine in Philadelphia where he also serves as Vice Chair of the Department of Medicine at Pennsylvania Hospital.
This article was updated 12/7/22.
Medically speaking, 2022 was the best year yet for children
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Headlines from earlier in the fall were grim: Thanks to the COVID-19 pandemic, life expectancy in the United States has fallen for 2 years running. Last year, according to health officials, the average American newborn could hope to reach 76.1 years, down from 79 years in 2019.
So far, so bad. But the headlines don’t tell the full story, which is much less dire. In fact, 2022 is the best year in human history for a child to arrive on Earth.
For a child born this year, in a developed country, into a family with access to good health care, the odds of living into the 22nd century are almost 50%. One in three will live to be 100. Those estimates reflect only incremental progress in medicine and public health, with COVID-19 baked in. They don’t account for biotechnologies beckoning to take control of the cell cycle and aging itself – which could make the outlook much brighter.
For some perspective, consider that a century ago, life expectancy for an American neonate was about 60 years. That 1922 figure was itself nothing short of miraculous, representing a 25% jump since 1901 – a leap that far outstrips the first 2 decades of the current century, during which life expectancy rose by just 2.5 years.
A gain of 2.5 years over 2 decades might not sound impressive, even without COVID-19 causing life expectancy in this country and abroad to sag. But during the pandemic, exciting new technologies that could drive gains in lifespan and healthspan, even bigger than those seen in the early 20th century, have moved closer to clinical reality. Think Star Trek-ish technologies like human hibernation, universal blood, mRNA therapy able to reprogram immune cells to hunt malignancies and fibrotic tissue, even head transplantation.
How long that last one will take to reach a clinic near you is hard to predict, but advances in the needed technology to anastomose cephalic and somatic portions of the spinal cord are mind-boggling. All this means that, from a medical standpoint, the future for babies born in the early 2020s looks dazzlingly bright.
Those sunny rays of optimism likely have failed to pierce the gloom of public discourse. Between “breakthrough infections,” “long COVID,” “Paxlovid rebound,” vaccine-induced myopericarditis, the current respiratory syncytial virus (RSV) outbreak, school shootings, climate change, and the youth mental health crisis, news headlines are undoubtedly frightful.
RSV: What’s old is new again
For the youngest children, the RSV outbreak is currently the scariest story. With social interactions returning toward a pre-COVID state, RSV has rebounded with a vengeance. In many places, pediatric wards are close to, at, or even beyond capacity. With no antiviral treatment for RSV, no licensed vaccine quite yet, and passive immunization (intravenous palivizumab) reserved for children at greatest risk (those under age 6 months and born preterm 35 weeks or earlier), the situation does have the feel of the first year of COVID-19, when treatments were similarly limited.
But let’s keep some perspective. RSV has always been a devastating infection. Prior to COVID-19, in the United States alone RSV killed 100-300 children below age 5 and 6,000-10,000 adults above age 65. The toll has always been worse on the international level. In 2019, 3.6 million people around the world were hospitalized for RSV infections, mostly the very old and the very young. Among causes of death below the age of 5, RSV ranks second only to malaria.
Postvaccine myopericarditis, a favorite concern of the vaccine hesitant, is a real phenomenon in young males. But generally, the condition has a subclinical to mild manifestation and fully resolves within 2 weeks.
Vaccines on the horizon
Monkeypox also was putting a damper on health news in recent months. Yet outreach efforts and selective vaccination and other precautions based on risk stratification appear to have calmed the outbreak. That’s good news, as is the fact that the struggle against malaria may be about to change. After decades of trying, we now have a malaria vaccine with what appears to be 80% efficacy against the infection. The same goes for RSV; finally, not one but two RSV vaccines are showing promise in late-stage clinical trials.
To be sure, for many young people, the times don’t seem so wonderful. The rate of teen suicide is alarming – yet it remains well below that seen in the 1990s. Are social media to blame, or is it something more complex?
If COVID-19 has taught us anything, it’s that development of vaccines and treatments need not take a decade or more. Operation Warp Speed may have seemed like a marketing gimmick and political grandstanding, but you can’t argue with the results.
Keep that perspective in mind to appreciate the moment – which I believe is coming soon – when the same type of intramuscular injection that we now use to trigger immunity against SARS-CoV-2 hits clinics, only this time as a way to cure cancer. Or when you read the stories of young victims of firearm violence who would have died but are rapidly cooled and kept hibernating for hours, so that their wounds can be repaired. And although you may not see that head transplant, one of these new babies might see it, or even might perform the procedure.
Dr. Warmflash is a freelance health and science writer living in Portland, Ore. His recent book, Moon: An Illustrated History: From Ancient Myths to the Colonies of Tomorrow, tells the story of the moon’s role in a plethora of historical events, from the origin of life to early calendar systems, the emergence of science and technology, and the dawn of the Space Age. He reported having no relevant financial disclosures. A version of this article first appeared on Medscape.com.
Visualization can improve sports performance
Over the past 30 years, Dr. Richard W. Cohen has used visualization techniques to help world class tennis players and recreational tennis players become the best they could be.
Visualization should be used in two ways to help players improve. First, to improve technique, after every practice session I have the player think about one shot they did not do well technically, and I have them, in vivo, shadow the shot on the court correctly before they leave the court. That night I tell the player to put themselves in a quiet, relaxed place and, in vitro, visualize themselves hitting the shot the correct way.
Almost always, the next day the players tell me they are hitting that one shot better and are motivated to again think about the one shot that was not technically correct and repeat the in vivo technique with similar great results.
The second way I use visualization for tennis players is to decrease their anxiety before matches. It is important to have some preparatory anxiety to perform optimally but having excessive anxiety will decrease performance. To alleviate excessive anxiety before matches, I have players watch their opponents hit the day before the match for at least 5 minutes to see their strengths and weaknesses. Then, the night before the match, I have them visualize how they will play a big point utilizing their strength into their opponent’s weakness. This rehearsal using imagery the night before a big match will decrease a player’s excessive anxiety and allow them to achieve their best effort in the match.
An example of this is if their opponent has a weak backhand that they can only slice. They visualize hitting wide to their forehand to get into their weak backhand and see themselves going forward and putting away a volley. Visualization used in these two ways helps improve stroke mechanics and match results in players of all levels. These visualization techniques can also be extended to other sports and to help improve life habits.
For example, Dr. Susan A. Cohen has seen that many patients have a decline in their dental health because of fear of going to the dentist to receive the treatment they need. Visualization techniques decrease the patient’s anxiety by rehearsing the possible traumatic events of the dental visit – e.g., the injection of anesthesia before the dental procedure. Visualization of calmness with systematic desensitization has helped decrease anxiety in patients.
In 20 years of clinical experience as a dentist, Dr. Cohen has seen how these techniques have increased compliance in her dental patients. She has also noted that visualizing the results of having a healthy mouth with improved appearance and function leads to an overall willingness to visit the dentist regularly and enjoy the dental experience. These examples demonstrate how visualization can enhance sports performance, quality of life, and overall health.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for over 40 years and is on the editorial advisory board for Clinical Psychiatry News. He has won 18 USTA national tennis championships. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
Over the past 30 years, Dr. Richard W. Cohen has used visualization techniques to help world class tennis players and recreational tennis players become the best they could be.
Visualization should be used in two ways to help players improve. First, to improve technique, after every practice session I have the player think about one shot they did not do well technically, and I have them, in vivo, shadow the shot on the court correctly before they leave the court. That night I tell the player to put themselves in a quiet, relaxed place and, in vitro, visualize themselves hitting the shot the correct way.
Almost always, the next day the players tell me they are hitting that one shot better and are motivated to again think about the one shot that was not technically correct and repeat the in vivo technique with similar great results.
The second way I use visualization for tennis players is to decrease their anxiety before matches. It is important to have some preparatory anxiety to perform optimally but having excessive anxiety will decrease performance. To alleviate excessive anxiety before matches, I have players watch their opponents hit the day before the match for at least 5 minutes to see their strengths and weaknesses. Then, the night before the match, I have them visualize how they will play a big point utilizing their strength into their opponent’s weakness. This rehearsal using imagery the night before a big match will decrease a player’s excessive anxiety and allow them to achieve their best effort in the match.
An example of this is if their opponent has a weak backhand that they can only slice. They visualize hitting wide to their forehand to get into their weak backhand and see themselves going forward and putting away a volley. Visualization used in these two ways helps improve stroke mechanics and match results in players of all levels. These visualization techniques can also be extended to other sports and to help improve life habits.
For example, Dr. Susan A. Cohen has seen that many patients have a decline in their dental health because of fear of going to the dentist to receive the treatment they need. Visualization techniques decrease the patient’s anxiety by rehearsing the possible traumatic events of the dental visit – e.g., the injection of anesthesia before the dental procedure. Visualization of calmness with systematic desensitization has helped decrease anxiety in patients.
In 20 years of clinical experience as a dentist, Dr. Cohen has seen how these techniques have increased compliance in her dental patients. She has also noted that visualizing the results of having a healthy mouth with improved appearance and function leads to an overall willingness to visit the dentist regularly and enjoy the dental experience. These examples demonstrate how visualization can enhance sports performance, quality of life, and overall health.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for over 40 years and is on the editorial advisory board for Clinical Psychiatry News. He has won 18 USTA national tennis championships. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
Over the past 30 years, Dr. Richard W. Cohen has used visualization techniques to help world class tennis players and recreational tennis players become the best they could be.
Visualization should be used in two ways to help players improve. First, to improve technique, after every practice session I have the player think about one shot they did not do well technically, and I have them, in vivo, shadow the shot on the court correctly before they leave the court. That night I tell the player to put themselves in a quiet, relaxed place and, in vitro, visualize themselves hitting the shot the correct way.
Almost always, the next day the players tell me they are hitting that one shot better and are motivated to again think about the one shot that was not technically correct and repeat the in vivo technique with similar great results.
The second way I use visualization for tennis players is to decrease their anxiety before matches. It is important to have some preparatory anxiety to perform optimally but having excessive anxiety will decrease performance. To alleviate excessive anxiety before matches, I have players watch their opponents hit the day before the match for at least 5 minutes to see their strengths and weaknesses. Then, the night before the match, I have them visualize how they will play a big point utilizing their strength into their opponent’s weakness. This rehearsal using imagery the night before a big match will decrease a player’s excessive anxiety and allow them to achieve their best effort in the match.
An example of this is if their opponent has a weak backhand that they can only slice. They visualize hitting wide to their forehand to get into their weak backhand and see themselves going forward and putting away a volley. Visualization used in these two ways helps improve stroke mechanics and match results in players of all levels. These visualization techniques can also be extended to other sports and to help improve life habits.
For example, Dr. Susan A. Cohen has seen that many patients have a decline in their dental health because of fear of going to the dentist to receive the treatment they need. Visualization techniques decrease the patient’s anxiety by rehearsing the possible traumatic events of the dental visit – e.g., the injection of anesthesia before the dental procedure. Visualization of calmness with systematic desensitization has helped decrease anxiety in patients.
In 20 years of clinical experience as a dentist, Dr. Cohen has seen how these techniques have increased compliance in her dental patients. She has also noted that visualizing the results of having a healthy mouth with improved appearance and function leads to an overall willingness to visit the dentist regularly and enjoy the dental experience. These examples demonstrate how visualization can enhance sports performance, quality of life, and overall health.
Dr. Richard W. Cohen is a psychiatrist who has been in private practice for over 40 years and is on the editorial advisory board for Clinical Psychiatry News. He has won 18 USTA national tennis championships. Dr. Susan A. Cohen has practiced dentistry for over 20 years. The Cohens, who are married, are based in Philadelphia.
New guidelines say pediatricians should screen for anxiety: Now what?
Recently the U.S. Preventive Services Task Force issued a formal recommendation that adolescents and children as young as 8 should be screened for anxiety.1 The advice was based on a review of the research that concluded that anxiety disorders were common in youth (prevalence around 8%), screening was not overly burdensome or dangerous, and treatments were available and effective.
While pediatricians fully appreciate how common clinically significant anxiety is and its impact on the lives of youth, the reception for the recommendations have been mixed. Some are concerned that it could lead to the overprescribing of medications. Arguably, the biggest pushback, however, relates to the question of what to do when a child screens positive in a time when finding an available child and adolescent psychiatrist or other type of pediatric mental health professional can feel next to impossible. The hope of this article is to fill in some of those gaps.
Screening for anxiety disorders
The recommendations suggest using a rating scale as part of the screen but doesn’t dictate which one. A common instrument that has been employed is the Screen for Child Anxiety and Related Disorders, which is a freely available 41-item instrument that has versions for youth self-report and parent-report. A shorter 7-item rating scale, the General Anxiety Disorder–7, and the even shorter GAD-2 (the first two questions of the GAD-7), are also popular but focus, as the name applies, on general anxiety disorder and not related conditions such as social or separation anxiety that can have some different symptoms. These instruments can be given to patients and families in the waiting room or administered with the help of a nurse, physician, or embedded mental health professional. The recommendations do not include specific guidance on how often the screening should be done but repeated screenings are likely important at some interval.
Confirming the diagnosis
Of course, a screening isn’t a formal diagnosis. The American Academy of Pediatrics has expressed the view that the initial diagnosis and treatment for anxiety disorders is well within a pediatrician’s scope of practice, which means further steps are likely required beyond a referral. Fortunately, going from a positive screen to an initial diagnosis does not have to overly laborious and can focus on reviewing the DSM-5 criteria for key anxiety disorders while also ensuring that there isn’t a nonpsychiatric cause driving the symptoms, such as the often cited but rarely seen pheochromocytoma. More common rule-outs include medication-induced anxiety or substance use, excessive caffeine intake, and cardiac arrhythmias. Assessing for current and past trauma or specific causes of the anxiety such as bullying are also important.
It is important to note that it is the rule rather than the exception that youth with clinical levels of anxiety will frequently endorse a number of criteria that span multiple diagnoses including generalized anxiety disorder, social anxiety disorder, and separation anxiety disorder.2 Spending a lot of effort to narrow things down to a single anxiety diagnosis often is unnecessary, as both pharmacologic and nonpharmacologic treatments don’t change all that much between individual diagnoses.
Explaining the diagnosis
In general, I’m a strong proponent of trying to explain any behavioral diagnoses that you make to kids in a way that is accurate but nonstigmatizing. When it comes to anxiety, one parallel I often draw is to our immune system, which most youth understand at least in basic terms. Both our immune system and our anxiety networks are natural and important; as a species, we wouldn’t have lasted long without them. Both are built to assess and respond to threats. Problems can arise, however, if the response is too strong relative to the threat or the response is activated when it doesn’t need to be. Treatment is directed not at ridding ourselves of anxiety but at helping regulate it so it works for us and not against us. Spending a few minutes going through a discussion like this can be very helpful, and perhaps more so than some dry summary of DSM-5 criteria.
Starting treatment
It is important to note that best practice recommendations when it comes to the treatment of anxiety disorder in youth do not suggest medications as the only type of treatment and often urge clinicians to try nonpharmacological interventions first.3 A specific type of psychotherapy called cognitive-behavioral therapy has the strongest scientific support as an effective treatment for anxiety but other modalities, including parenting guidance, can be helpful as well. Consequently, a referral to a good psychotherapist is paramount. For many kids, the key to overcoming anxiety is exposure: which means confronting anxiety slowly, with support, and with specific skills.
If there is a traumatic source of the anxiety, addressing that as much as possible is obviously critical and could involve working with the family or school. For some kids, this may involve frightening things they are seeing online or through other media. Finally, some health promotion activities such as exercise or mindfulness can also be quite useful.
Despite the fact that SSRIs are referred to as antidepressants, there is increasing appreciation that these medications are useful for anxiety, perhaps even more so than for mood. While only one medication, duloxetine, has Food and Drug Administration approval to treat anxiety in children as young as 7, there is good evidence to support the use of many of the most common SSRIs in treating clinical anxiety. Buspirone, beta-blockers, and antihistamine medications like hydroxyzine also can have their place in treatment, while benzodiazepines and antipsychotic medications are generally best avoided for anxious youth, especially in the primary care setting. A short but helpful medication guide with regard to pediatric anxiety has been published by the American Academy of Child and Adolescent Psychiatry.4
Conclusions
Clinical levels of anxiety in children and adolescents are both common and quite treatable, which has prompted new recommendations that primary care clinicians screen for them starting at age 8. While this recommendation may at first seem like yet one more task to fit in, following the guidance can be accomplished with the help of short screening tools and a managed multimodal approach to treatment.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. You can follow him on Twitter and Facebook @PediPsych.
References
1. U.S. Preventive Services Task Force. JAMA. 2022;328(14):1438-44.
2. Strawn JR. Curr Psychiatry. 2012;11(9):16-21.
3. Walter HJ et al. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1107-24.
4. Anxiety Disorders: Parents’ Medication Guide Workgroup. “Anxiety disorders: Parents’ medication guide.” Washington D.C.: American Academy of Child & Adolescent Psychiatry, 2020.
Recently the U.S. Preventive Services Task Force issued a formal recommendation that adolescents and children as young as 8 should be screened for anxiety.1 The advice was based on a review of the research that concluded that anxiety disorders were common in youth (prevalence around 8%), screening was not overly burdensome or dangerous, and treatments were available and effective.
While pediatricians fully appreciate how common clinically significant anxiety is and its impact on the lives of youth, the reception for the recommendations have been mixed. Some are concerned that it could lead to the overprescribing of medications. Arguably, the biggest pushback, however, relates to the question of what to do when a child screens positive in a time when finding an available child and adolescent psychiatrist or other type of pediatric mental health professional can feel next to impossible. The hope of this article is to fill in some of those gaps.
Screening for anxiety disorders
The recommendations suggest using a rating scale as part of the screen but doesn’t dictate which one. A common instrument that has been employed is the Screen for Child Anxiety and Related Disorders, which is a freely available 41-item instrument that has versions for youth self-report and parent-report. A shorter 7-item rating scale, the General Anxiety Disorder–7, and the even shorter GAD-2 (the first two questions of the GAD-7), are also popular but focus, as the name applies, on general anxiety disorder and not related conditions such as social or separation anxiety that can have some different symptoms. These instruments can be given to patients and families in the waiting room or administered with the help of a nurse, physician, or embedded mental health professional. The recommendations do not include specific guidance on how often the screening should be done but repeated screenings are likely important at some interval.
Confirming the diagnosis
Of course, a screening isn’t a formal diagnosis. The American Academy of Pediatrics has expressed the view that the initial diagnosis and treatment for anxiety disorders is well within a pediatrician’s scope of practice, which means further steps are likely required beyond a referral. Fortunately, going from a positive screen to an initial diagnosis does not have to overly laborious and can focus on reviewing the DSM-5 criteria for key anxiety disorders while also ensuring that there isn’t a nonpsychiatric cause driving the symptoms, such as the often cited but rarely seen pheochromocytoma. More common rule-outs include medication-induced anxiety or substance use, excessive caffeine intake, and cardiac arrhythmias. Assessing for current and past trauma or specific causes of the anxiety such as bullying are also important.
It is important to note that it is the rule rather than the exception that youth with clinical levels of anxiety will frequently endorse a number of criteria that span multiple diagnoses including generalized anxiety disorder, social anxiety disorder, and separation anxiety disorder.2 Spending a lot of effort to narrow things down to a single anxiety diagnosis often is unnecessary, as both pharmacologic and nonpharmacologic treatments don’t change all that much between individual diagnoses.
Explaining the diagnosis
In general, I’m a strong proponent of trying to explain any behavioral diagnoses that you make to kids in a way that is accurate but nonstigmatizing. When it comes to anxiety, one parallel I often draw is to our immune system, which most youth understand at least in basic terms. Both our immune system and our anxiety networks are natural and important; as a species, we wouldn’t have lasted long without them. Both are built to assess and respond to threats. Problems can arise, however, if the response is too strong relative to the threat or the response is activated when it doesn’t need to be. Treatment is directed not at ridding ourselves of anxiety but at helping regulate it so it works for us and not against us. Spending a few minutes going through a discussion like this can be very helpful, and perhaps more so than some dry summary of DSM-5 criteria.
Starting treatment
It is important to note that best practice recommendations when it comes to the treatment of anxiety disorder in youth do not suggest medications as the only type of treatment and often urge clinicians to try nonpharmacological interventions first.3 A specific type of psychotherapy called cognitive-behavioral therapy has the strongest scientific support as an effective treatment for anxiety but other modalities, including parenting guidance, can be helpful as well. Consequently, a referral to a good psychotherapist is paramount. For many kids, the key to overcoming anxiety is exposure: which means confronting anxiety slowly, with support, and with specific skills.
If there is a traumatic source of the anxiety, addressing that as much as possible is obviously critical and could involve working with the family or school. For some kids, this may involve frightening things they are seeing online or through other media. Finally, some health promotion activities such as exercise or mindfulness can also be quite useful.
Despite the fact that SSRIs are referred to as antidepressants, there is increasing appreciation that these medications are useful for anxiety, perhaps even more so than for mood. While only one medication, duloxetine, has Food and Drug Administration approval to treat anxiety in children as young as 7, there is good evidence to support the use of many of the most common SSRIs in treating clinical anxiety. Buspirone, beta-blockers, and antihistamine medications like hydroxyzine also can have their place in treatment, while benzodiazepines and antipsychotic medications are generally best avoided for anxious youth, especially in the primary care setting. A short but helpful medication guide with regard to pediatric anxiety has been published by the American Academy of Child and Adolescent Psychiatry.4
Conclusions
Clinical levels of anxiety in children and adolescents are both common and quite treatable, which has prompted new recommendations that primary care clinicians screen for them starting at age 8. While this recommendation may at first seem like yet one more task to fit in, following the guidance can be accomplished with the help of short screening tools and a managed multimodal approach to treatment.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. You can follow him on Twitter and Facebook @PediPsych.
References
1. U.S. Preventive Services Task Force. JAMA. 2022;328(14):1438-44.
2. Strawn JR. Curr Psychiatry. 2012;11(9):16-21.
3. Walter HJ et al. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1107-24.
4. Anxiety Disorders: Parents’ Medication Guide Workgroup. “Anxiety disorders: Parents’ medication guide.” Washington D.C.: American Academy of Child & Adolescent Psychiatry, 2020.
Recently the U.S. Preventive Services Task Force issued a formal recommendation that adolescents and children as young as 8 should be screened for anxiety.1 The advice was based on a review of the research that concluded that anxiety disorders were common in youth (prevalence around 8%), screening was not overly burdensome or dangerous, and treatments were available and effective.
While pediatricians fully appreciate how common clinically significant anxiety is and its impact on the lives of youth, the reception for the recommendations have been mixed. Some are concerned that it could lead to the overprescribing of medications. Arguably, the biggest pushback, however, relates to the question of what to do when a child screens positive in a time when finding an available child and adolescent psychiatrist or other type of pediatric mental health professional can feel next to impossible. The hope of this article is to fill in some of those gaps.
Screening for anxiety disorders
The recommendations suggest using a rating scale as part of the screen but doesn’t dictate which one. A common instrument that has been employed is the Screen for Child Anxiety and Related Disorders, which is a freely available 41-item instrument that has versions for youth self-report and parent-report. A shorter 7-item rating scale, the General Anxiety Disorder–7, and the even shorter GAD-2 (the first two questions of the GAD-7), are also popular but focus, as the name applies, on general anxiety disorder and not related conditions such as social or separation anxiety that can have some different symptoms. These instruments can be given to patients and families in the waiting room or administered with the help of a nurse, physician, or embedded mental health professional. The recommendations do not include specific guidance on how often the screening should be done but repeated screenings are likely important at some interval.
Confirming the diagnosis
Of course, a screening isn’t a formal diagnosis. The American Academy of Pediatrics has expressed the view that the initial diagnosis and treatment for anxiety disorders is well within a pediatrician’s scope of practice, which means further steps are likely required beyond a referral. Fortunately, going from a positive screen to an initial diagnosis does not have to overly laborious and can focus on reviewing the DSM-5 criteria for key anxiety disorders while also ensuring that there isn’t a nonpsychiatric cause driving the symptoms, such as the often cited but rarely seen pheochromocytoma. More common rule-outs include medication-induced anxiety or substance use, excessive caffeine intake, and cardiac arrhythmias. Assessing for current and past trauma or specific causes of the anxiety such as bullying are also important.
It is important to note that it is the rule rather than the exception that youth with clinical levels of anxiety will frequently endorse a number of criteria that span multiple diagnoses including generalized anxiety disorder, social anxiety disorder, and separation anxiety disorder.2 Spending a lot of effort to narrow things down to a single anxiety diagnosis often is unnecessary, as both pharmacologic and nonpharmacologic treatments don’t change all that much between individual diagnoses.
Explaining the diagnosis
In general, I’m a strong proponent of trying to explain any behavioral diagnoses that you make to kids in a way that is accurate but nonstigmatizing. When it comes to anxiety, one parallel I often draw is to our immune system, which most youth understand at least in basic terms. Both our immune system and our anxiety networks are natural and important; as a species, we wouldn’t have lasted long without them. Both are built to assess and respond to threats. Problems can arise, however, if the response is too strong relative to the threat or the response is activated when it doesn’t need to be. Treatment is directed not at ridding ourselves of anxiety but at helping regulate it so it works for us and not against us. Spending a few minutes going through a discussion like this can be very helpful, and perhaps more so than some dry summary of DSM-5 criteria.
Starting treatment
It is important to note that best practice recommendations when it comes to the treatment of anxiety disorder in youth do not suggest medications as the only type of treatment and often urge clinicians to try nonpharmacological interventions first.3 A specific type of psychotherapy called cognitive-behavioral therapy has the strongest scientific support as an effective treatment for anxiety but other modalities, including parenting guidance, can be helpful as well. Consequently, a referral to a good psychotherapist is paramount. For many kids, the key to overcoming anxiety is exposure: which means confronting anxiety slowly, with support, and with specific skills.
If there is a traumatic source of the anxiety, addressing that as much as possible is obviously critical and could involve working with the family or school. For some kids, this may involve frightening things they are seeing online or through other media. Finally, some health promotion activities such as exercise or mindfulness can also be quite useful.
Despite the fact that SSRIs are referred to as antidepressants, there is increasing appreciation that these medications are useful for anxiety, perhaps even more so than for mood. While only one medication, duloxetine, has Food and Drug Administration approval to treat anxiety in children as young as 7, there is good evidence to support the use of many of the most common SSRIs in treating clinical anxiety. Buspirone, beta-blockers, and antihistamine medications like hydroxyzine also can have their place in treatment, while benzodiazepines and antipsychotic medications are generally best avoided for anxious youth, especially in the primary care setting. A short but helpful medication guide with regard to pediatric anxiety has been published by the American Academy of Child and Adolescent Psychiatry.4
Conclusions
Clinical levels of anxiety in children and adolescents are both common and quite treatable, which has prompted new recommendations that primary care clinicians screen for them starting at age 8. While this recommendation may at first seem like yet one more task to fit in, following the guidance can be accomplished with the help of short screening tools and a managed multimodal approach to treatment.
Dr. Rettew is a child and adolescent psychiatrist with Lane County Behavioral Health in Eugene, Ore., and Oregon Health & Science University, Portland. You can follow him on Twitter and Facebook @PediPsych.
References
1. U.S. Preventive Services Task Force. JAMA. 2022;328(14):1438-44.
2. Strawn JR. Curr Psychiatry. 2012;11(9):16-21.
3. Walter HJ et al. J Am Acad Child Adolesc Psychiatry. 2020;59(10):1107-24.
4. Anxiety Disorders: Parents’ Medication Guide Workgroup. “Anxiety disorders: Parents’ medication guide.” Washington D.C.: American Academy of Child & Adolescent Psychiatry, 2020.
Dialing down the negativity
I don’t do email. Or texting. You want to talk to me and my staff? Pick up a phone.
Some people say I’m old fashioned, or not patient-friendly, or whatever.
I don’t care.
To me there are too many issues with things that can get missed in emails, too many security concerns, too many ways to alter them so it looks like something different was said.
Now, a recent study of an EHR system found that 3% of emails from patients had negative, if not downright nasty, sentiments expressed to their physicians.
Here’s some examples:
“I hope and expect that you will spend eternity in hell. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
The study also noted that the most common expletive used by patients is the F-bomb, and that words with violent connotations, such as “shoot,” “fight,” and “kill” were often used in such emails. The last are definitely concerning in an era of increased violence directed at doctors and other health care workers who are just trying to do their jobs.
Now, I know doctors are a microcosm of society. Like patients, most are decent people trying their best, but a few are ... not particularly nice.
But still, I don’t think we, or anyone for that matter, need to be getting emails of this nature. It certainly doesn’t put anyone in a good position, or allow for objective, unbiased, care. Even if they’re only 3% of emails, that can still be quite a few.
Who needs that?
One of the issues with email is that it’s easy to type something nasty and hit “send,” then later have it occur to you that maybe you should have calmed down first. Granted, that sort of thing can (and does) happen when talking to another person (by phone or in person), but it’s harder.
Direct personal contact, especially face-to-face, appears to lessen impulsive reactions for most. The other person isn’t an invisible email address, they’re someone you’re talking to. You can read tone-of-voice and facial expressions. Again, I’m aware people still can lose their cool in person, but it’s harder.
and running into the next exam room. Plus, it ensures that all noncritical patient interactions occur during business hours, when we’re in doctor mode, rather than at 2:45 a.m. when we look at the iPhone while waiting for the dog to come back in. That’s a terrible time to receive and send medical (or any) emails for both doctor and patient.
A lot rides on every one of my patient interactions, and that’s why I still want them done directly. If that makes me old-fashioned, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t do email. Or texting. You want to talk to me and my staff? Pick up a phone.
Some people say I’m old fashioned, or not patient-friendly, or whatever.
I don’t care.
To me there are too many issues with things that can get missed in emails, too many security concerns, too many ways to alter them so it looks like something different was said.
Now, a recent study of an EHR system found that 3% of emails from patients had negative, if not downright nasty, sentiments expressed to their physicians.
Here’s some examples:
“I hope and expect that you will spend eternity in hell. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
The study also noted that the most common expletive used by patients is the F-bomb, and that words with violent connotations, such as “shoot,” “fight,” and “kill” were often used in such emails. The last are definitely concerning in an era of increased violence directed at doctors and other health care workers who are just trying to do their jobs.
Now, I know doctors are a microcosm of society. Like patients, most are decent people trying their best, but a few are ... not particularly nice.
But still, I don’t think we, or anyone for that matter, need to be getting emails of this nature. It certainly doesn’t put anyone in a good position, or allow for objective, unbiased, care. Even if they’re only 3% of emails, that can still be quite a few.
Who needs that?
One of the issues with email is that it’s easy to type something nasty and hit “send,” then later have it occur to you that maybe you should have calmed down first. Granted, that sort of thing can (and does) happen when talking to another person (by phone or in person), but it’s harder.
Direct personal contact, especially face-to-face, appears to lessen impulsive reactions for most. The other person isn’t an invisible email address, they’re someone you’re talking to. You can read tone-of-voice and facial expressions. Again, I’m aware people still can lose their cool in person, but it’s harder.
and running into the next exam room. Plus, it ensures that all noncritical patient interactions occur during business hours, when we’re in doctor mode, rather than at 2:45 a.m. when we look at the iPhone while waiting for the dog to come back in. That’s a terrible time to receive and send medical (or any) emails for both doctor and patient.
A lot rides on every one of my patient interactions, and that’s why I still want them done directly. If that makes me old-fashioned, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I don’t do email. Or texting. You want to talk to me and my staff? Pick up a phone.
Some people say I’m old fashioned, or not patient-friendly, or whatever.
I don’t care.
To me there are too many issues with things that can get missed in emails, too many security concerns, too many ways to alter them so it looks like something different was said.
Now, a recent study of an EHR system found that 3% of emails from patients had negative, if not downright nasty, sentiments expressed to their physicians.
Here’s some examples:
“I hope and expect that you will spend eternity in hell. You are an abusive, nasty, cheap person.”
“Your office is full of liars, hypocrites and I will do everything in my power to prevent anyone from going to your bullsh** office again.”
The study also noted that the most common expletive used by patients is the F-bomb, and that words with violent connotations, such as “shoot,” “fight,” and “kill” were often used in such emails. The last are definitely concerning in an era of increased violence directed at doctors and other health care workers who are just trying to do their jobs.
Now, I know doctors are a microcosm of society. Like patients, most are decent people trying their best, but a few are ... not particularly nice.
But still, I don’t think we, or anyone for that matter, need to be getting emails of this nature. It certainly doesn’t put anyone in a good position, or allow for objective, unbiased, care. Even if they’re only 3% of emails, that can still be quite a few.
Who needs that?
One of the issues with email is that it’s easy to type something nasty and hit “send,” then later have it occur to you that maybe you should have calmed down first. Granted, that sort of thing can (and does) happen when talking to another person (by phone or in person), but it’s harder.
Direct personal contact, especially face-to-face, appears to lessen impulsive reactions for most. The other person isn’t an invisible email address, they’re someone you’re talking to. You can read tone-of-voice and facial expressions. Again, I’m aware people still can lose their cool in person, but it’s harder.
and running into the next exam room. Plus, it ensures that all noncritical patient interactions occur during business hours, when we’re in doctor mode, rather than at 2:45 a.m. when we look at the iPhone while waiting for the dog to come back in. That’s a terrible time to receive and send medical (or any) emails for both doctor and patient.
A lot rides on every one of my patient interactions, and that’s why I still want them done directly. If that makes me old-fashioned, so be it.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.