User login
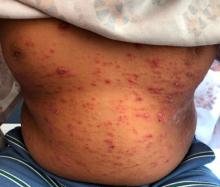
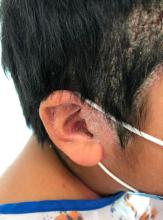
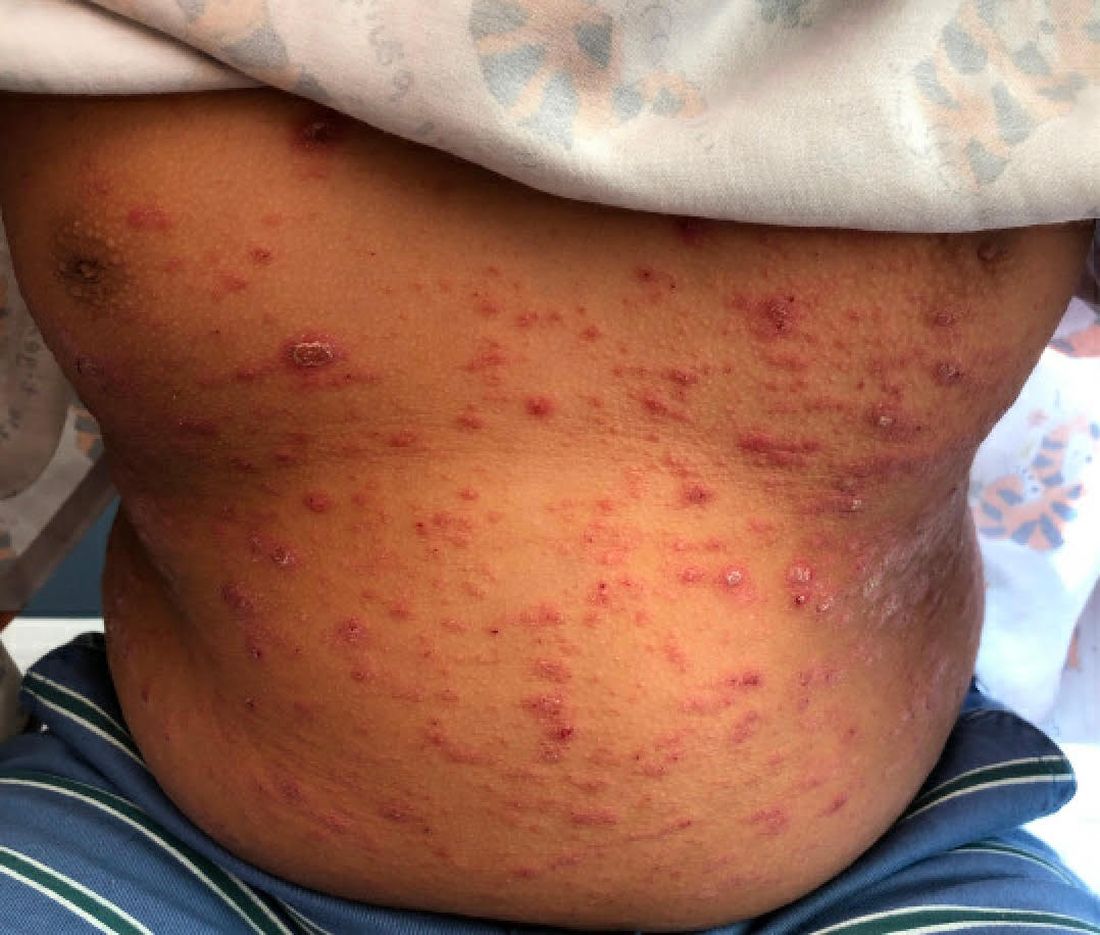
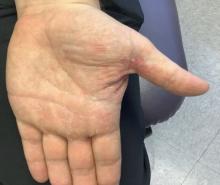
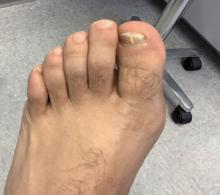
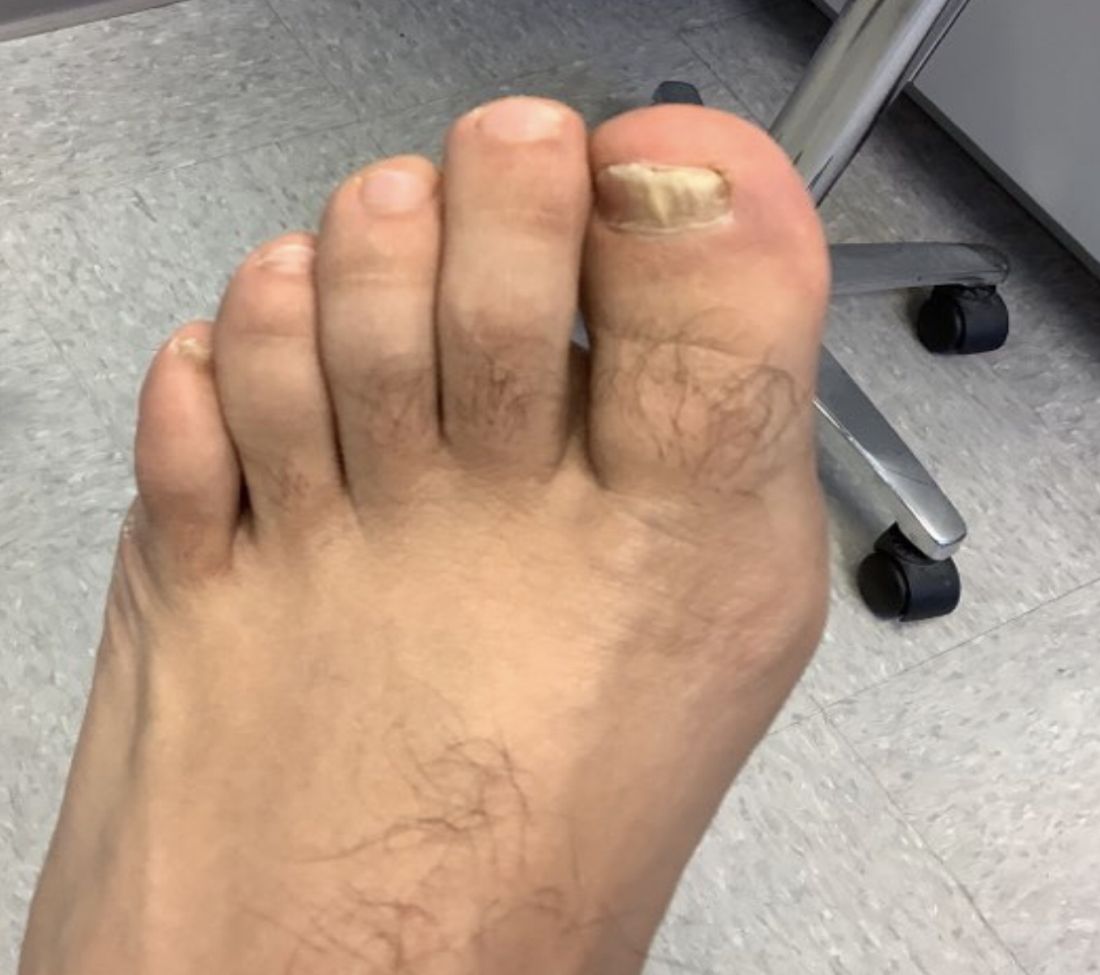
A 9-year-old male presents with multiple thick scaly plaques on scalp, ears, and trunk
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
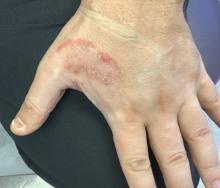
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
Given the characteristic clinical presentation, the most likely diagnosis is psoriasis.
Psoriasis is a chronic immune-mediated disease that is characterized by well-demarcated thick scaly plaques on face, scalp, and intertriginous skin. Psoriasis is more common in adults than children, but the incidence of psoriasis in children has increased over time.1 Clinical presentation of psoriasis includes erythematous hyperkeratotic plaques, usually sharply demarcated. Pediatric patients may have multiple small papules and plaques less than 1 cm in size – “drop-size” – known as guttate lesions. Scalp and facial involvement are common in children. Chronic, inflamed plaques with coarse scale can involve ears, elbows, knees, and umbilicus, and nail changes can include pits, ridges, hyperkeratosis, and onycholysis or “oil spots.” While the diagnosis is clinical, biopsy can sometimes be useful to distinguish psoriasis from other papulosquamous conditions. Psoriasis in children is associated with obesity, higher rates of cardiovascular disease over a lifetime, as well as arthritis and mental health disorders.2
What’s the differential diagnosis?
The differential diagnosis for psoriasis can include papulosquamous diseases such as nummular eczema, pityriasis rosea, and pityriasis rubra pilaris. Tinea corporis may also be considered.
Nummular eczema, also known as “discoid eczema” is characterized by multiple pruritic, coin-shaped, eczematous lesions that may be actively oozing. The term “nummular” is derived from the Latin for “coin,” as lesions are distinct and annular. It is commonly associated with atopic dermatitis, and may be seen with contact dermatitis as well. Oozing, lichenification, hyperpigmentation and limited extent of skin coverage can help distinguish nummular dermatitis from psoriasis.
Pityriasis rosea is a common self-limited disease that is characterized by the appearance of acute, oval, papulosquamous patches on the trunk and proximal areas of the extremities. It usually begins with a characteristic “herald” patch, a single round or oval, sharply demarcated, pink lesion on the chest, neck, or back. Pityriasis rosea and guttate psoriasis may show similar clinical findings but the latter lacks a herald patch and is often preceded by streptococcal throat infection.
Pityriasis rubra pilaris is a rarer inflammatory disease characterized by follicular, hyperkeratotic papules, thick orange waxy palms (palmoplantar keratoderma), and erythroderma. It can also cause hair loss, nail changes, and itching. The rash shows areas with no involvement, “islands of sparing,” which is a signature characteristic of pityriasis rubra pilaris. Skin biopsies are an important diagnostic tool for pityriasis rubra pilaris. In the case of circumscribed pityriasis rubra pilaris, it may look similar to psoriasis, but it can be differentiated in that it is often accompanied by characteristic follicular papules and involvement of the palms, which are more waxy and orange in color.
When evaluating annular scaly patches, it is always important to consider tinea corporis. Tinea corporis will commonly have an annular border of scale with relative clearing in the center of lesions. In addition, when topical corticosteroids are used for prolonged periods, skin fungal infections can develop into “tinea incognito,” with paradoxical worsening since the immune response is suppressed and the fungal infection worsens.
Our patient had been previously treated with topical corticosteroids (medium to high strength) and topical calcineurin inhibitors without significant improvement. Other topical therapies for psoriasis include vitamin analogues, tazarotene, and newer therapies such as topical roflumilast (a phosphodiesterase-4 inhibitor approved for psoriasis in children over 12 years of age).3,4 In addition, as the indications for biological agents have been expanded, there are various options for treating psoriasis in children and adolescents when more active treatment is needed. Systemic therapies for more severe disease include traditional systemic immunosuppressives (for example, methotrexate, cyclosporine) and biologic agents. The four biologic agents currently approved for children are etanercept, ustekinumab, ixekizumab, and secukinumab. Our patient was treated with ustekinumab, which is an injectable biologic agent that blocks interleukin-12/23, with good response to date.
Dr. Al-Nabti is a clinical fellow in the division of pediatric and adolescent dermatology; Dr. Choi is a visiting research physician in the division of pediatric and adolescent dermatology; and Dr. Eichenfield is vice-chair of the department of dermatology and professor of dermatology and pediatrics, all at the University of California, San Diego, and Rady Children’s Hospital, San Diego. They have no relevant disclosures.
References
1. Tollefson MM et al. J Am Acad Dermatol. 2010;62(6):979-87.
2. Menter A et al. J Am Acad Dermatol. 2020;82(1):161-201.
3. Mark G et al. JAMA. 2022;328(11):1073-84.
4. Eichenfield LF et al. Pediatr Dermatol. 2018;35(2):170-81.
A 9-year-old male is seen in the clinic with a 1-year history of multiple thick scaly plaques on scalp, ears, and trunk. He has been treated with hydrocortisone 1% ointment with no change in the lesions. He had upper respiratory tract symptoms 3 weeks prior to the visit.
Examination reveals erythematous, well-demarcated plaques of the anterior scalp with thick overlying micaceous scale with some extension onto the forehead and temples. Additionally, erythematous scaly patches on the ear, axilla, and umbilicus were noted. There was no palmar or plantar involvement. He denied joint swelling, stiffness, or pain in the morning.
Clinician violence: Virtual reality to the rescue?
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
This discussion was recorded on Feb. 21, 2023. This transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome. I’m Dr. Robert Glatter, medical adviser for Medscape Emergency Medicine. Welcome, Dr. Salazar. It’s a pleasure to have you join us today.
Gilberto A. Salazar, MD: The pleasure is all mine, Dr. Glatter. Thank you so much for having me.
Dr. Glatter: This is such an important topic, as you can imagine. Workplace violence is affecting so many providers in hospital emergency departments but also throughout other parts of the hospital.
First, can you describe how the virtual reality (VR) program was designed that you developed and what type of situations it simulates?
Dr. Salazar: We worked in conjunction with the University of Texas at Dallas. They help people like me, subject matter experts in health care, to bring ideas to reality. I worked very closely with a group of engineers from their department in designing a module specifically designed to tackle, as you mentioned, one of our biggest threats in workplace violence.
We decided to bring in a series of competencies and proficiencies that we wanted to bring into the virtual reality space. In leveraging the technology and the expertise from UT Dallas, we were able to make that happen.
Dr. Glatter: I think it’s important to understand, in terms of virtual reality, what type of environment the program creates. Can you describe what a provider who puts the goggles on is experiencing? Do they feel anything? Is there technology that enables this?
Dr. Salazar: Yes, absolutely. We were able to bring to reality a series of scenarios very common from what you and I see in the emergency department on a daily basis. We wanted to immerse a learner into that specific environment. We didn’t feel that a module or something on a computer or a slide set could really bring the reality of what it’s like to interact with a patient who may be escalating or may be aggressive.
We are immersing learners into an actual hospital room to our specifications, very similar to exactly where we practice each and every day, and taking the learners through different situations that we designed with various levels of escalation and aggression, and asking the learner to manage that situation as best as they possibly can using the competencies and proficiencies that we taught them.
Dr. Glatter: Haptic feedback is an important part of the program and also the approach and technique that you’re using. Can you describe what haptic feedback means and what people actually feel?
Dr. Salazar: Absolutely. One of the most unfortunate things in my professional career is physical abuse suffered by people like me and you and our colleagues, nursing personnel, technicians, and others, resulting in injury.
We wanted to provide the most realistic experience that we could design. Haptics engage digital senses other than your auditory and your visuals. They really engage your tactile senses. These haptic vests and gloves and technology allow us to provide a third set of sensory stimuli for the learner.
At one of the modules, we have an actual physical assault that takes place, and the learner is actually able to feel in their body the strikes – of course, not painful – but just bringing in those senses and that stimulus, really leaving the learner with an experience that’s going to be long-lasting.
Dr. Glatter: Feeling that stimulus certainly affects your vital signs. Do you monitor a provider’s vital signs, such as their blood pressure and heart rate, as the situation and the threat escalate? That could potentially trigger some issues in people with prior PTSD or people with other mental health issues. Has that ever been considered in the design of your program?
Dr. Salazar: Yes, 100%. The beautiful thing about haptics is that they can be tailored to our specific parameters. The sensory stimulus that’s provided is actually very mild. It feels more like a tap than an actual strike. It just reminds us that when we’re having or experiencing an actual physical attack, we’re really engaging the senses.
We have an emergency physician or an EMT-paramedic on site at all times during the training so that we can monitor our subjects and make sure that they’re comfortable and healthy.
Dr. Glatter: Do they have actual sensors attached to their bodies that are part of your program or distinct in terms of monitoring their vital signs?
Dr. Salazar: It’s completely different. We have two different systems that we are planning on utilizing. Frankly, in the final version of this virtual reality module, we may not even involve the haptics. We’re going to study it and see how our learners behave and how much information they’re able to acquire and retain.
It may be very possible that just the visuals – the auditory and the immersion taking place within the hospital room – may be enough. It’s very possible that, in the next final version of this, we may find that haptics bring in quite a bit of value, and we may incorporate that. If that is the case, then we will, of course, acquire different technology to monitor the patient’s vital signs.
Dr. Glatter: Clearly, when situations escalate in the department, everyone gets more concerned about the patient, but providers are part of this equation, as you allude to.
In 2022, there was a poll by the American College of Emergency Physicians that stated that 85% of emergency physicians reported an increase in violent activity in their ERs in the past 5 years. Nearly two-thirds of nearly 3,000 emergency physicians surveyed reported being assaulted in the past year. This is an important module that we integrate into training providers in terms of these types of tense situations that can result not only in mental anguish but also in physical injury.
Dr. Salazar: One hundred percent. I frankly got tired of seeing my friends and my colleagues suffer both the physical and mental effects of verbal and physical abuse, and I wanted to design a project that was very patient centric while allowing our personnel to really manage these situations a little bit better.
Frankly, we don’t receive great training in this space, and I wanted to rewrite that narrative and make things better for our clinicians out there while remaining patient centric. I wanted to do something about it, and hopefully this dream will become a reality.
Dr. Glatter: Absolutely. There are other data from the Bureau of Labor Statistics stating that health care workers are five times more likely than employees in any other area of work to experience workplace violence. This could, again, range from verbal to physical violence. This is a very important module that you’re developing.
Are there any thoughts to extend this to active-shooter scenarios or any other high-stakes scenarios that you can imagine in the department?
Dr. Salazar: We’re actually working with the same developer that’s helping us with this VR module in developing a mass-casualty incident module so that we can get better training in responding to these very unfortunate high-stakes situations.
Dr. Glatter: In terms of using the module remotely, certainly not requiring resources or having to be in a physical place, can providers in your plan be able to take such a headset home and practice on their own in the sense of being able to deal with a situation? Would this be more reserved for in-department use?
Dr. Salazar: That’s a phenomenal question. I wanted to create the most flexible module that I possibly could. Ideally, a dream scenario is leveraging a simulation center at an academic center and not just do the VR module but also have a brief didactics incorporating a small slide set, some feedback, and some standardized patients. I wanted it to be flexible enough so that folks here in my state, a different state, or even internationally could take advantage of this technology and do it from the comfort of their home.
As you mentioned, this is going to strike some people. It’s going to hit them heavier than others in terms of prior experience as PTSD. For some people, it may be more comfortable to do it in the comfort of their homes. I wanted to create something very flexible and dynamic.
Dr. Glatter: I think that’s ideal. Just one other point. Can you discuss the different levels of competencies involved in this module and how that would be attained?
Dr. Salazar: It’s all evidence based, so we borrowed from literature and the specialties of emergency medicine. We collaborated with psychiatrists within our medical center. We looked at all available literature and methods, proficiencies, competencies, and best practices, and we took all of them together to form something that we think is organized and concise.
We were able to create our own algorithm, but it’s not brand new. We’re just borrowing what we think is the best to create something that the majority of health care personnel are going to be able to relate to and be able to really be proficient at.
This includes things like active listening, bargaining, how to respond, where to put yourself in a situation, and the best possible situation to respond to a scenario, how to prevent things – how to get out of a chokehold, for example. We’re borrowing from several different disciplines and creating something that can be very concise and organized.
Dr. Glatter: Does this program that you’ve developed allow the provider to get feedback in the sense that when they’re in such a danger, their life could be at risk? For example, if they don’t remove themselves in a certain amount of time, this could be lethal.
Dr. Salazar: Yes, 100%. Probably the one thing that differentiates our project from any others is the ability to customize the experience so that a learner who is doing the things that we ask them to do in terms of safety and response is able to get out of a situation successfully within the environment. If they don’t, they get some kind of feedback.
Not to spoil the surprise here, but we’re going to be doing things like looking at decibel meters to see what the volume in the room is doing and how you’re managing the volume and the stimulation within the room. If you are able to maintain the decibel readings at a specific level, you’re going to succeed through the module. If you don’t, we keep the patient escalation going.
Dr. Glatter: There is a debrief built into this type of approach where, in other words, learning points are emphasized – where you could have done better and such.
Dr. Salazar: Yes, absolutely. We are going to be able to get individualized data for each learner so that we can tailor the debrief to their own performance and be able to give them actionable items to work on. It’s a debrief that’s productive and individualized, and folks can walk away with something useful in the end.
Dr. Glatter: Are the data shared or confidential at present?
Dr. Salazar: At this very moment, the data are confidential. We are going to look at how to best use this. We’re hoping to eventually write this up and see how this information can be best used to train personnel.
Eventually, we may see that some of the advice that we’re giving is very common to most folks. Others may require some individualized type of feedback. That said, it remains to be seen, but right now, it’s confidential.
Dr. Glatter: Is this currently being implemented as part of your curriculum for emergency medicine residents?
Dr. Salazar: We’re going to study it first. We’re very excited to include our emergency medicine residents as one of our cohorts that’s going to be undergoing the module, and we’re going to be studying other forms of workplace violence mitigation strategies. We’re really excited about the possibility of this eventually becoming the standard of education for not only our emergency medicine residents, but also health care personnel all over the world.
Dr. Glatter: I’m glad you mentioned that, because obviously nurses, clerks in the department, and anyone who’s working in the department, for that matter, and who interfaces with patients really should undergo such training.
Dr. Salazar: Absolutely. The folks at intake, at check-in, and at kiosks. Do they go through a separate area for screening? You’re absolutely right. There are many folks who interface with patients and all of us are potential victims of workplace violence. We want to give our health care family the best opportunity to succeed in these situations.
Dr. Glatter:: Absolutely. Even EMS providers, being on the front lines and encountering patients in such situations, would benefit, in my opinion.
Dr. Salazar: Yes, absolutely. Behavioral health emergencies and organically induced altered mental status results in injury, both physical and mental, to EMS professionals as well, and there’s good evidence of that. I’ll be very glad to see this type of education make it out to our initial and continuing education efforts for EMS as well.
Dr. Glatter: I want to thank you. This has been very helpful. It’s such an important task that you’ve started to explore, and I look forward to follow-up on this. Again, thank you for your time.
Dr. Salazar: It was my pleasure. Thank you so much for having me.
Dr. Glatter is an attending physician at Lenox Hill Hospital in New York City and assistant professor of emergency medicine at Zucker School of Medicine at Hofstra/Northwell in Hempstead, N.Y. He is an editorial adviser and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Salazar is a board-certified emergency physician and associate professor at UT Southwestern Medicine Center in Dallas. He is involved with the UTSW Emergency Medicine Education Program and serves as the medical director to teach both initial and continuing the emergency medicine education for emergency medical technicians and paramedics, which trains most of the Dallas Fire Rescue personnel and the vast majority for EMS providers in the Dallas County. In addition, he serves as an associate chief of service at Parkland’s emergency department, and liaison to surgical services. A version of this article originally appeared on Medscape.com.
Three surprising studies on exercise restriction and an exercise sweet spot
LIVE HCM: Surprising result No. 1
Rachel Lampert, MD, from Yale University, New Haven, Conn., presented results of the LIVE-HCM observational study of vigorous exercise in more than 1,600 patients with hypertrophic cardiomyopathy (40% female). The investigators aimed to determine whether engagement in vigorous exercise, including competitive sports, is associated with increased risk for life-threatening ventricular arrhythmia and/or mortality in patients with HCM.
Because of the myocardial disease, HCM comes with a risk for ventricular arrhythmia. Prevailing wisdom held that vigorous exercise in these patients would be hazardous. It was all expert opinion; there were no data. Now there are.
Dr. Lampert and colleagues recruited patients from 42 international HCM centers. Patients self-enrolled and the researchers created three groups based on self-reported levels of exercise – vigorous, moderate, and sedentary. The main comparison was between vigorous versus nonvigorous exercisers (including moderate and sedentary). The two groups were mostly matched on baseline characteristics and typical of patients with HCM.
The primary endpoint was a composite of death, resuscitated cardiac arrest, syncope likely caused by an arrhythmia, or an appropriate shock from an ICD.
The event rates were low in all groups and almost identical in vigorous versus nonvigorous exercisers. Sub-group analyses found no increased risk in HCM patients who identified as competitive athletes.
Dr. Lampert said these data “do not support universal restriction of vigorous exercise in patients with HCM.”
Return to play: Surprising result No. 2
Undergraduate student Katherine Martinez from Loyola University, Chicago, presented an observational analysis of 76 elite athletes with genetic heart disease who gained a return-to-play approval from four expert centers in the United States.
The three-step, return-to-play protocol from these specialized centers deserves emphasis. First was the initial evaluation, including two ECGs, 24-hour ECG monitor, echocardiography, and treadmill exercise testing. Second was a discussion between clinicians and patients regarding the athlete’s situation. The third step was to inform coaches and staff of the team and instruct athletes to obtain a personal AED, stay replenished with electrolytes, avoid QT-prolonging drugs, and continue with annual follow-up.
Slightly more than half of these patients had HCM and almost a third had long QT syndrome. Nearly one-third had an ICD implant and 22 were women.
Of the 76 athletes, 73 chose to return to play; however, 4 of these remained disqualified because of their team’s decision. Of the remaining 69, only 3 had one or more breakthrough cardiac events during 200 patient-years of follow-up.
These comprised one male Division I basketball player with HCM who had an ICD shock while moving furniture; another male Division 1 hockey player with long QT syndrome who was taking beta-blockers experienced syncope while coming off the bench and while cooking; and a third male professional hockey player with HCM, on beta-blockers, had syncope without exertion.
The authors concluded that when there was careful evaluation by experts and shared decision-making, a specific plan to return to sport can be put into place for the highest-level athletes.
Masters@Heart: Surprising result No. 3
Ruben De Bosscher MD, PhD, from KU Leuven (Belgium), presented the Masters@Heart study on behalf of a Belgian team of researchers. The question they asked was whether lifelong endurance exercise is associated with more coronary atherosclerosis than standard “normal” exercise levels.
That question brings up the paradox of exercise, which is that numerous observational studies find that exercise strongly associates with lower rates of cardiovascular events, but imaging studies also report high rates of coronary artery calcium in endurance athletes, especially in those who have run multiple marathons.
Masters@Heart investigators sought to explore this paradox by performing detailed coronary imaging in three groups – lifelong athletes, late-onset athletes (after age 30 years), and super-healthy controls. Through advertisements they obtained about 1,100 middle-aged male volunteers (mean age, 55 years). Of these, 605 men were selected at random to participate to reduce the chance of enrolling people who responded to the ads because of health concerns.
Investigators assigned those selected based on self-report of exercise. The control group was notable for their good health: they were free of any risk factors, took (almost) no meds, exercised regularly but not excessively (about 3 hours per week) and had a VO2 max of 122% of predicted.
The groups were well matched on baseline characteristics. Cycling predominated as the exercise of choice (this is a Belgian study after all). All patients had an extensive evaluation including coronary CT imaging.
European Heart Journal published the provocative results.
- Lifelong exercisers had a significantly higher CAC burden than controls, which confirms previous work.
- Lifelong exercisers had a higher percentage of multiple coronary plaques, plaques of at least 50%, and proximal plaques.
- There were no significant differences in the mixture of plaque types in the three groups. About two thirds of the plaques in each group were calcified and the remainder were deemed noncalcified or mixed.
- When looking only at noncalcified plaques, lifelong exercisers tended to have a higher prevalence of multiple plaques, plaques of at least 50%, and proximal plaques.
- So named “vulnerable” plaques were extremely infrequent in all three groups.
The authors concluded that lifelong endurance sport relative to a generic healthy lifestyle was not associated with more favorable coronary plaque composition.
Comments
Each of these three studies provided data where there was none. That is always a good thing.
The major theme from the first two studies is that expert opinion was too cautious. Doctors have long held the idea that patients with genetic heart disease, especially hypertrophic cardiomyopathy, are vulnerable, fragile even, when it comes to vigorous sport.
This new evidence upends this belief, as long as return to sport occurs in the setting of robust patient education and expert evaluation and surveillance.
Paternalism in prohibiting participation in sport because of genetic heart disease has joined the long list of medical reversals.
Masters@Heart provides a slightly different message. It finds that lifelong high-level exercise does not prevent coronary atherosclerosis in men. And, more provocatively, if replicated, might even show that long-term exposure to the biochemical, inflammatory, or hormonal effects of endurance training may actually be atherogenic. Like all good science, these findings raise more questions to explore in the realm of atherogenesis.
Two of the main limitations of the Belgian study was that the control arm was quite healthy; had the comparison arm been typical of sedentary controls in say, the Southeastern United States, the coronary lesions found in longtime exercisers may have looked more favorable. The more significant limitation is the lack of outcomes. Images of coronary arteries remain a surrogate marker. It’s possible that, like statins, higher levels of exercise may stabilize plaque and actually lower the risk for events.
The Belgian authors suggest – as many have – a J-curve of exercise benefits, wherein too little exercise is clearly bad, but too much exercise may also increase risk. In other words, for maximizing health, there may be a Goldilocks amount of exercise.
The problem with this idea comes in its pragmatic translation. The number of lifelong high-level, middle-aged endurance athletes that cite heart health reasons for their affliction is ... almost zero. Nearly everyone I have met in the endurance sport fraternity harbors no notion that racing a bike or running multiple marathons per year is a healthy endeavor.
Paternalism, therefore, would also fall in the realm of limiting lifelong exercise in addicted middle-aged athletes.
Via email, sports cardiologist Michael Emery, MD, reiterated the main immediate message from Masters@Heart: “Exercise does not make you immune from heart disease (which is a message a lot of athletes need to hear honestly).”
I for one cannot give up on endurance exercise. I won’t likely race anymore but I am like the lab rat who needs to run on the wheel. Whether this affects my coronary plaque burden matters not to me.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
LIVE HCM: Surprising result No. 1
Rachel Lampert, MD, from Yale University, New Haven, Conn., presented results of the LIVE-HCM observational study of vigorous exercise in more than 1,600 patients with hypertrophic cardiomyopathy (40% female). The investigators aimed to determine whether engagement in vigorous exercise, including competitive sports, is associated with increased risk for life-threatening ventricular arrhythmia and/or mortality in patients with HCM.
Because of the myocardial disease, HCM comes with a risk for ventricular arrhythmia. Prevailing wisdom held that vigorous exercise in these patients would be hazardous. It was all expert opinion; there were no data. Now there are.
Dr. Lampert and colleagues recruited patients from 42 international HCM centers. Patients self-enrolled and the researchers created three groups based on self-reported levels of exercise – vigorous, moderate, and sedentary. The main comparison was between vigorous versus nonvigorous exercisers (including moderate and sedentary). The two groups were mostly matched on baseline characteristics and typical of patients with HCM.
The primary endpoint was a composite of death, resuscitated cardiac arrest, syncope likely caused by an arrhythmia, or an appropriate shock from an ICD.
The event rates were low in all groups and almost identical in vigorous versus nonvigorous exercisers. Sub-group analyses found no increased risk in HCM patients who identified as competitive athletes.
Dr. Lampert said these data “do not support universal restriction of vigorous exercise in patients with HCM.”
Return to play: Surprising result No. 2
Undergraduate student Katherine Martinez from Loyola University, Chicago, presented an observational analysis of 76 elite athletes with genetic heart disease who gained a return-to-play approval from four expert centers in the United States.
The three-step, return-to-play protocol from these specialized centers deserves emphasis. First was the initial evaluation, including two ECGs, 24-hour ECG monitor, echocardiography, and treadmill exercise testing. Second was a discussion between clinicians and patients regarding the athlete’s situation. The third step was to inform coaches and staff of the team and instruct athletes to obtain a personal AED, stay replenished with electrolytes, avoid QT-prolonging drugs, and continue with annual follow-up.
Slightly more than half of these patients had HCM and almost a third had long QT syndrome. Nearly one-third had an ICD implant and 22 were women.
Of the 76 athletes, 73 chose to return to play; however, 4 of these remained disqualified because of their team’s decision. Of the remaining 69, only 3 had one or more breakthrough cardiac events during 200 patient-years of follow-up.
These comprised one male Division I basketball player with HCM who had an ICD shock while moving furniture; another male Division 1 hockey player with long QT syndrome who was taking beta-blockers experienced syncope while coming off the bench and while cooking; and a third male professional hockey player with HCM, on beta-blockers, had syncope without exertion.
The authors concluded that when there was careful evaluation by experts and shared decision-making, a specific plan to return to sport can be put into place for the highest-level athletes.
Masters@Heart: Surprising result No. 3
Ruben De Bosscher MD, PhD, from KU Leuven (Belgium), presented the Masters@Heart study on behalf of a Belgian team of researchers. The question they asked was whether lifelong endurance exercise is associated with more coronary atherosclerosis than standard “normal” exercise levels.
That question brings up the paradox of exercise, which is that numerous observational studies find that exercise strongly associates with lower rates of cardiovascular events, but imaging studies also report high rates of coronary artery calcium in endurance athletes, especially in those who have run multiple marathons.
Masters@Heart investigators sought to explore this paradox by performing detailed coronary imaging in three groups – lifelong athletes, late-onset athletes (after age 30 years), and super-healthy controls. Through advertisements they obtained about 1,100 middle-aged male volunteers (mean age, 55 years). Of these, 605 men were selected at random to participate to reduce the chance of enrolling people who responded to the ads because of health concerns.
Investigators assigned those selected based on self-report of exercise. The control group was notable for their good health: they were free of any risk factors, took (almost) no meds, exercised regularly but not excessively (about 3 hours per week) and had a VO2 max of 122% of predicted.
The groups were well matched on baseline characteristics. Cycling predominated as the exercise of choice (this is a Belgian study after all). All patients had an extensive evaluation including coronary CT imaging.
European Heart Journal published the provocative results.
- Lifelong exercisers had a significantly higher CAC burden than controls, which confirms previous work.
- Lifelong exercisers had a higher percentage of multiple coronary plaques, plaques of at least 50%, and proximal plaques.
- There were no significant differences in the mixture of plaque types in the three groups. About two thirds of the plaques in each group were calcified and the remainder were deemed noncalcified or mixed.
- When looking only at noncalcified plaques, lifelong exercisers tended to have a higher prevalence of multiple plaques, plaques of at least 50%, and proximal plaques.
- So named “vulnerable” plaques were extremely infrequent in all three groups.
The authors concluded that lifelong endurance sport relative to a generic healthy lifestyle was not associated with more favorable coronary plaque composition.
Comments
Each of these three studies provided data where there was none. That is always a good thing.
The major theme from the first two studies is that expert opinion was too cautious. Doctors have long held the idea that patients with genetic heart disease, especially hypertrophic cardiomyopathy, are vulnerable, fragile even, when it comes to vigorous sport.
This new evidence upends this belief, as long as return to sport occurs in the setting of robust patient education and expert evaluation and surveillance.
Paternalism in prohibiting participation in sport because of genetic heart disease has joined the long list of medical reversals.
Masters@Heart provides a slightly different message. It finds that lifelong high-level exercise does not prevent coronary atherosclerosis in men. And, more provocatively, if replicated, might even show that long-term exposure to the biochemical, inflammatory, or hormonal effects of endurance training may actually be atherogenic. Like all good science, these findings raise more questions to explore in the realm of atherogenesis.
Two of the main limitations of the Belgian study was that the control arm was quite healthy; had the comparison arm been typical of sedentary controls in say, the Southeastern United States, the coronary lesions found in longtime exercisers may have looked more favorable. The more significant limitation is the lack of outcomes. Images of coronary arteries remain a surrogate marker. It’s possible that, like statins, higher levels of exercise may stabilize plaque and actually lower the risk for events.
The Belgian authors suggest – as many have – a J-curve of exercise benefits, wherein too little exercise is clearly bad, but too much exercise may also increase risk. In other words, for maximizing health, there may be a Goldilocks amount of exercise.
The problem with this idea comes in its pragmatic translation. The number of lifelong high-level, middle-aged endurance athletes that cite heart health reasons for their affliction is ... almost zero. Nearly everyone I have met in the endurance sport fraternity harbors no notion that racing a bike or running multiple marathons per year is a healthy endeavor.
Paternalism, therefore, would also fall in the realm of limiting lifelong exercise in addicted middle-aged athletes.
Via email, sports cardiologist Michael Emery, MD, reiterated the main immediate message from Masters@Heart: “Exercise does not make you immune from heart disease (which is a message a lot of athletes need to hear honestly).”
I for one cannot give up on endurance exercise. I won’t likely race anymore but I am like the lab rat who needs to run on the wheel. Whether this affects my coronary plaque burden matters not to me.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
LIVE HCM: Surprising result No. 1
Rachel Lampert, MD, from Yale University, New Haven, Conn., presented results of the LIVE-HCM observational study of vigorous exercise in more than 1,600 patients with hypertrophic cardiomyopathy (40% female). The investigators aimed to determine whether engagement in vigorous exercise, including competitive sports, is associated with increased risk for life-threatening ventricular arrhythmia and/or mortality in patients with HCM.
Because of the myocardial disease, HCM comes with a risk for ventricular arrhythmia. Prevailing wisdom held that vigorous exercise in these patients would be hazardous. It was all expert opinion; there were no data. Now there are.
Dr. Lampert and colleagues recruited patients from 42 international HCM centers. Patients self-enrolled and the researchers created three groups based on self-reported levels of exercise – vigorous, moderate, and sedentary. The main comparison was between vigorous versus nonvigorous exercisers (including moderate and sedentary). The two groups were mostly matched on baseline characteristics and typical of patients with HCM.
The primary endpoint was a composite of death, resuscitated cardiac arrest, syncope likely caused by an arrhythmia, or an appropriate shock from an ICD.
The event rates were low in all groups and almost identical in vigorous versus nonvigorous exercisers. Sub-group analyses found no increased risk in HCM patients who identified as competitive athletes.
Dr. Lampert said these data “do not support universal restriction of vigorous exercise in patients with HCM.”
Return to play: Surprising result No. 2
Undergraduate student Katherine Martinez from Loyola University, Chicago, presented an observational analysis of 76 elite athletes with genetic heart disease who gained a return-to-play approval from four expert centers in the United States.
The three-step, return-to-play protocol from these specialized centers deserves emphasis. First was the initial evaluation, including two ECGs, 24-hour ECG monitor, echocardiography, and treadmill exercise testing. Second was a discussion between clinicians and patients regarding the athlete’s situation. The third step was to inform coaches and staff of the team and instruct athletes to obtain a personal AED, stay replenished with electrolytes, avoid QT-prolonging drugs, and continue with annual follow-up.
Slightly more than half of these patients had HCM and almost a third had long QT syndrome. Nearly one-third had an ICD implant and 22 were women.
Of the 76 athletes, 73 chose to return to play; however, 4 of these remained disqualified because of their team’s decision. Of the remaining 69, only 3 had one or more breakthrough cardiac events during 200 patient-years of follow-up.
These comprised one male Division I basketball player with HCM who had an ICD shock while moving furniture; another male Division 1 hockey player with long QT syndrome who was taking beta-blockers experienced syncope while coming off the bench and while cooking; and a third male professional hockey player with HCM, on beta-blockers, had syncope without exertion.
The authors concluded that when there was careful evaluation by experts and shared decision-making, a specific plan to return to sport can be put into place for the highest-level athletes.
Masters@Heart: Surprising result No. 3
Ruben De Bosscher MD, PhD, from KU Leuven (Belgium), presented the Masters@Heart study on behalf of a Belgian team of researchers. The question they asked was whether lifelong endurance exercise is associated with more coronary atherosclerosis than standard “normal” exercise levels.
That question brings up the paradox of exercise, which is that numerous observational studies find that exercise strongly associates with lower rates of cardiovascular events, but imaging studies also report high rates of coronary artery calcium in endurance athletes, especially in those who have run multiple marathons.
Masters@Heart investigators sought to explore this paradox by performing detailed coronary imaging in three groups – lifelong athletes, late-onset athletes (after age 30 years), and super-healthy controls. Through advertisements they obtained about 1,100 middle-aged male volunteers (mean age, 55 years). Of these, 605 men were selected at random to participate to reduce the chance of enrolling people who responded to the ads because of health concerns.
Investigators assigned those selected based on self-report of exercise. The control group was notable for their good health: they were free of any risk factors, took (almost) no meds, exercised regularly but not excessively (about 3 hours per week) and had a VO2 max of 122% of predicted.
The groups were well matched on baseline characteristics. Cycling predominated as the exercise of choice (this is a Belgian study after all). All patients had an extensive evaluation including coronary CT imaging.
European Heart Journal published the provocative results.
- Lifelong exercisers had a significantly higher CAC burden than controls, which confirms previous work.
- Lifelong exercisers had a higher percentage of multiple coronary plaques, plaques of at least 50%, and proximal plaques.
- There were no significant differences in the mixture of plaque types in the three groups. About two thirds of the plaques in each group were calcified and the remainder were deemed noncalcified or mixed.
- When looking only at noncalcified plaques, lifelong exercisers tended to have a higher prevalence of multiple plaques, plaques of at least 50%, and proximal plaques.
- So named “vulnerable” plaques were extremely infrequent in all three groups.
The authors concluded that lifelong endurance sport relative to a generic healthy lifestyle was not associated with more favorable coronary plaque composition.
Comments
Each of these three studies provided data where there was none. That is always a good thing.
The major theme from the first two studies is that expert opinion was too cautious. Doctors have long held the idea that patients with genetic heart disease, especially hypertrophic cardiomyopathy, are vulnerable, fragile even, when it comes to vigorous sport.
This new evidence upends this belief, as long as return to sport occurs in the setting of robust patient education and expert evaluation and surveillance.
Paternalism in prohibiting participation in sport because of genetic heart disease has joined the long list of medical reversals.
Masters@Heart provides a slightly different message. It finds that lifelong high-level exercise does not prevent coronary atherosclerosis in men. And, more provocatively, if replicated, might even show that long-term exposure to the biochemical, inflammatory, or hormonal effects of endurance training may actually be atherogenic. Like all good science, these findings raise more questions to explore in the realm of atherogenesis.
Two of the main limitations of the Belgian study was that the control arm was quite healthy; had the comparison arm been typical of sedentary controls in say, the Southeastern United States, the coronary lesions found in longtime exercisers may have looked more favorable. The more significant limitation is the lack of outcomes. Images of coronary arteries remain a surrogate marker. It’s possible that, like statins, higher levels of exercise may stabilize plaque and actually lower the risk for events.
The Belgian authors suggest – as many have – a J-curve of exercise benefits, wherein too little exercise is clearly bad, but too much exercise may also increase risk. In other words, for maximizing health, there may be a Goldilocks amount of exercise.
The problem with this idea comes in its pragmatic translation. The number of lifelong high-level, middle-aged endurance athletes that cite heart health reasons for their affliction is ... almost zero. Nearly everyone I have met in the endurance sport fraternity harbors no notion that racing a bike or running multiple marathons per year is a healthy endeavor.
Paternalism, therefore, would also fall in the realm of limiting lifelong exercise in addicted middle-aged athletes.
Via email, sports cardiologist Michael Emery, MD, reiterated the main immediate message from Masters@Heart: “Exercise does not make you immune from heart disease (which is a message a lot of athletes need to hear honestly).”
I for one cannot give up on endurance exercise. I won’t likely race anymore but I am like the lab rat who needs to run on the wheel. Whether this affects my coronary plaque burden matters not to me.
Dr. Mandrola is a clinical electrophysiologist at Baptist Medical Associates, Louisville, Ky. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
White male presents with pruritic, scaly, erythematous patches on his feet and left hand
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
Two feet–one hand syndrome
This condition, also known as ringworm, is a fungal infection caused by a dermatophyte, and presents as a superficial annular or circular rash with a raised, scaly border.
Symptoms include dryness and itchiness, and the lesions may appear red-pink on lighter skin and gray-brown on darker skin types. Although these infections can arise in a variety of combinations, two feet–one hand syndrome occurs in about 60% of cases. Trichophyton rubrum is the most common agent.
Diagnosis is made by patient history, dermoscopic visualization, and staining of skin scraping with KOH or fungal culture. Dermatophytes prefer moist, warm environments, so this disease is prevalent in tropical conditions and associated with moist public areas such as locker rooms and showers. As a result, tinea pedis is also nicknamed “athlete’s foot” for its common presentation in athletes. The fungus spreads easily through contact and can survive on infected surfaces, so patients often self-inoculate by touching/scratching the affected area then touching another body part. Cautions that should be taken to avoid transmission include not sharing personal care products, washing the area and keeping it dry, and avoiding close, humid environments.
The syndrome is highly associated with onychomycosis, which can be more difficult to treat and often requires oral antifungals. Tinea manuum is commonly misdiagnosed as hand dermatitis or eczema and treated with topical steroids, which will exacerbate or flare the tinea.
Two feet–one hand syndrome can typically be treated with over-the-counter topical antifungal medications such as miconazole or clotrimazole. Topical ketoconazole may be prescribed, and oral terbinafine or itraconazole are used in more severe cases when a larger body surface area is affected or in immunocompromised patients.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University, Davie, Fla.; Kiran C. Patel, Tampa Bay Regional Campus; and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Cleveland Clinic. Tinea manuum: Symptoms, causes & treatment. 2022. https://my.clevelandclinic.org/health/diseases/24063-tinea-manuum.
Ugalde-Trejo NX et al. Curr Fungal Infect Rep. 2022 Nov 17. doi: 10.1007/s12281-022-00447-9.
Mizumoto J. Cureus. 2021 Dec 27;13(12):e20758.
What’s it like to take Ozempic? A doctor’s own story
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
With the rising popularity of weight-loss drug injections, I’ve received many questions from patients about the pros, cons, and costs. While Ozempic (semaglutide) is perhaps the best known, it’s technically an agent approved only for type 2 diabetes that has been used off label for obesity. The same substance, semaglutide, is approved for use in obesity, but at a higher dose, under the brand name Wegovy. Alternatives are available, and results will vary depending on the specific agent used and the individual.
Ultimately, I decided to try these new injections for myself. I am not a paid representative for, nor an advocate of, any of these medications; I’m here only to share my personal experience.
In my discussions with patients about weight, I sometimes felt like an imposter. While I was overweight by medical standards, I fortunately had none of the underlying health problems. I wasn’t on medications for blood pressure nor did I have diabetes, but I was counseling people to lose weight and eat better while not always following my own advice.
Since having children and turning 40, my metabolism, like many other women’s, seems to have plummeted. I tried a number of older weight-loss medications, like phentermine and phendimetrazine, under the supervision of medical professionals.
Each time, the efforts worked for a short while, particularly when I followed good portion control and practiced moderate exercise. Once the side effects (that is, tachycardia, palpitations, mood changes, constipation) became intolerable, or I became tired or fearful of being on the medications too long, I’d stop and I would regain some of the weight.
When the newer subcutaneous injectable medications arrived on the scene and I started to talk to my patients about them, I was intrigued by their novel mode of action and seeming benefits.
These medications, glucagonlike peptide–1 (GLP-1) receptor agonists, were first approved for type 2 diabetes, and it soon became apparent that patients were losing significant amounts of weight taking them, so manufacturers conducted further trials in obesity patients without type 2 diabetes.
The first of these, liraglutide, is injected daily and was first approved as Victoza for type 2 diabetes; it later received an additional approval for obesity, in December 2014, as Saxenda.
Semaglutide, another of the new GLP-1 agonists, was first approved for type 2 diabetes as Ozempic but again was found to lead to substantial weight loss, so a subsequent approval of the drug for obesity, as Wegovy, came in June 2021. Semaglutide is injected once a week.
Semaglutide was branded a “game changer” when it was licensed for obesity because the mean weight loss seen in trials was around 15%, more than for any other drug and approaching what could be achieved with bariatric surgery, some doctors said.
These medications work in a different way from the older weight loss drugs, which had focused on the use of amphetamines. The newer medications became very popular because treating obesity helps lower blood glucose, blood pressure, cholesterol, kidney disease risk, and other comorbidities that occur with diabetes. Plus, for most people, there were fewer side effects.
I first tried Saxenda when it arrived on the market, via some samples that our pharmaceutical representative brought, both out of curiosity and to see if it would help me lose the stubborn baby weight. I ended up stopping the daily injections after my second or third week because of nausea and vomiting. I took a break, got a prescription for antinausea medicine, and tried again because it did indeed decrease my appetite. However, when I took my prescription to the pharmacy, my insurance wouldn’t cover it. It happens to doctors, too.
Fast-forward to 2017-2018. The baby weight was still holding on despite lifestyle changes, diet, and exercising. The newer drug classes hit the market, and again we had samples from our reps.
When Ozempic was on backorder, I switched to a low dose of Mounjaro (tirzepatide), a new dual GLP-1 and glucose-dependent insulinotropic polypeptide agonist, approved for type 2 diabetes in May 2022, again using it off label as a weekly injection, as it isn’t currently approved for weight loss. However, it does produce significant weight loss and is awaiting approval for obesity.
With these new medications, I noticed that both my patients and I didn’t complain as much about nausea and vomiting, but I did experience stomach upset, constipation, and acid reflux.
The appetite suppression is effective. It slows down the emptying of the gut so I feel full longer. I’ve lost 30 lb with these weekly injections and would like to lose another 20 lb. I follow a routine of reasonable, portion-controlled eating and moderate exercise (30 minutes of cardiovascular activity at least two to three times a week).
Discontinuing the medications may cause rebound weight gain, especially if I’m no longer following a routine of healthy eating and/or moderate exercise. I deal with minimal constipation by taking stool softeners, and I take antacids for acid reflux.
Here’s what I recommend applying when working with patients who have obesity: First, explain how these medications work. Then conduct a health history to make sure these injections are right for them. Patients with a family history of pancreatic cancer can’t take these medications. You also want to monitor use in patients with a history of hypoglycemia so their blood sugar doesn’t drop too low. It’s also important to make sure your patients are able to afford the medication. My husband takes Ozempic for diabetes, and recently we were told that a refill would cost about $1,500 a month, even with insurance. “Covered” doesn’t necessarily mean affordable.
Take a baseline hemoglobin A1c and repeat it after the patient has been on the medication for 2-3 weeks. Also remind them that they can’t rely solely on the medication but need to practice portion control and healthier eating and to exercise more.
For myself, I want to lose those remaining 20 lb or so by eating healthy and being physically active without having to rely on medication for the rest of my life. Research on these medications is still early so we don’t know the long-term effects yet.
As clinicians, I feel it’s okay to be honest with our patients about our own personal struggles to help them understand that they are not alone and that losing weight is a challenge for everyone.
Dr. Swiner is a family physician in Durham, N.C. She reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Incommunicado no more
A few weeks ago I wrote about my glasses and the discovery that they’d been made incorrectly. The headline for the story was “The Way I See It.”
That’s the opening line from Joni Mitchell’s 1974 song “Free Man in Paris.” But I grew up in a Neil Diamond household (Dad always had Neil Diamond on when he was working at home) so the first time I heard the song was in 1977, when Diamond covered it. In fact, I didn’t even realize it was originally Mitchell’s song until I was in my 50s.
It’s about a world that doesn’t exist anymore.
The song is about music promoter David Geffen and a trip he took to Paris. Back in southern California, he was always working. There were continual phone calls, deals, meetings, and people looking for favors.
But on his trip to Paris in the early 1970s, he became just another person. No one could find him to ask for help or cut a deal. He couldn’t be reached. He felt “unfettered and alive” and could go from “cafe to cabaret,” relax, and enjoy himself.
Medical practice was once that way. You’d check-out patients to your call partners, leave town, and relax for a week or two.
Try doing that today.
We even have a new psychiatric condition – nomophobia – for the fear of not having our mobile phone handy. Every time I leave my house or office I repeat a simple mantra “phone, wallet, keys” as I pat my pockets.
Unless you can part with your gadget – which ain’t easy – no one is a “free man in Paris” (or Tokyo, or Rio, or Beijing) anymore. Even ships have cell service at sea. There are still places on Earth remote enough that you can’t be reached, but they get fewer and smaller every year.
When was the last time you really went somewhere and had no communication with your office at all? Emails, texts, anything? Unless you’re in a shift-work branch of medicine, like ER or hospitalist, I’m going to guess it’s been a while. And even in those branches you probably get emails about administrative matters, scheduling questions, and pointless memos.
Being in solo practice I’ve come to accept this, but it’s a conscious decision on my part. It’s easier than finding a call partner, and if I’m handling my own stuff at least I’m not going to come home to any surprises. So I’ve covered patients from as far west as Hawaii, north as Juneau, south as Panama City, and east as Le Havre.
Granted, this is medicine, and many other jobs don’t require the degree of involvement that it does. But I suspect pretty much any professional - attorney, accountant, executive - still has to deal with work-related stuff while traveling. Back in the 1970s to 1980s my dad, a solo-practice lawyer, had a set time each vacation weekday afternoon where he’d call his secretary to go over stuff. Today it would be by email or texts.
We’ve done this to ourselves. We’ve accepted the trade-off of better connectivity with family and friends for expanding our time at work. The same technology that lets me send in prescription refills from London also lets me send family pictures back from Maui. It’s not easy to draw a solid line between them, and I’m not so sure many of us want to.
Today, 50 years after Ms. Mitchell wrote the song, the idea of being a “free man in Paris” – or anywhere – really doesn’t exist for most of us anymore. You can argue whether that’s good or bad, but it’s where we are.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A few weeks ago I wrote about my glasses and the discovery that they’d been made incorrectly. The headline for the story was “The Way I See It.”
That’s the opening line from Joni Mitchell’s 1974 song “Free Man in Paris.” But I grew up in a Neil Diamond household (Dad always had Neil Diamond on when he was working at home) so the first time I heard the song was in 1977, when Diamond covered it. In fact, I didn’t even realize it was originally Mitchell’s song until I was in my 50s.
It’s about a world that doesn’t exist anymore.
The song is about music promoter David Geffen and a trip he took to Paris. Back in southern California, he was always working. There were continual phone calls, deals, meetings, and people looking for favors.
But on his trip to Paris in the early 1970s, he became just another person. No one could find him to ask for help or cut a deal. He couldn’t be reached. He felt “unfettered and alive” and could go from “cafe to cabaret,” relax, and enjoy himself.
Medical practice was once that way. You’d check-out patients to your call partners, leave town, and relax for a week or two.
Try doing that today.
We even have a new psychiatric condition – nomophobia – for the fear of not having our mobile phone handy. Every time I leave my house or office I repeat a simple mantra “phone, wallet, keys” as I pat my pockets.
Unless you can part with your gadget – which ain’t easy – no one is a “free man in Paris” (or Tokyo, or Rio, or Beijing) anymore. Even ships have cell service at sea. There are still places on Earth remote enough that you can’t be reached, but they get fewer and smaller every year.
When was the last time you really went somewhere and had no communication with your office at all? Emails, texts, anything? Unless you’re in a shift-work branch of medicine, like ER or hospitalist, I’m going to guess it’s been a while. And even in those branches you probably get emails about administrative matters, scheduling questions, and pointless memos.
Being in solo practice I’ve come to accept this, but it’s a conscious decision on my part. It’s easier than finding a call partner, and if I’m handling my own stuff at least I’m not going to come home to any surprises. So I’ve covered patients from as far west as Hawaii, north as Juneau, south as Panama City, and east as Le Havre.
Granted, this is medicine, and many other jobs don’t require the degree of involvement that it does. But I suspect pretty much any professional - attorney, accountant, executive - still has to deal with work-related stuff while traveling. Back in the 1970s to 1980s my dad, a solo-practice lawyer, had a set time each vacation weekday afternoon where he’d call his secretary to go over stuff. Today it would be by email or texts.
We’ve done this to ourselves. We’ve accepted the trade-off of better connectivity with family and friends for expanding our time at work. The same technology that lets me send in prescription refills from London also lets me send family pictures back from Maui. It’s not easy to draw a solid line between them, and I’m not so sure many of us want to.
Today, 50 years after Ms. Mitchell wrote the song, the idea of being a “free man in Paris” – or anywhere – really doesn’t exist for most of us anymore. You can argue whether that’s good or bad, but it’s where we are.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A few weeks ago I wrote about my glasses and the discovery that they’d been made incorrectly. The headline for the story was “The Way I See It.”
That’s the opening line from Joni Mitchell’s 1974 song “Free Man in Paris.” But I grew up in a Neil Diamond household (Dad always had Neil Diamond on when he was working at home) so the first time I heard the song was in 1977, when Diamond covered it. In fact, I didn’t even realize it was originally Mitchell’s song until I was in my 50s.
It’s about a world that doesn’t exist anymore.
The song is about music promoter David Geffen and a trip he took to Paris. Back in southern California, he was always working. There were continual phone calls, deals, meetings, and people looking for favors.
But on his trip to Paris in the early 1970s, he became just another person. No one could find him to ask for help or cut a deal. He couldn’t be reached. He felt “unfettered and alive” and could go from “cafe to cabaret,” relax, and enjoy himself.
Medical practice was once that way. You’d check-out patients to your call partners, leave town, and relax for a week or two.
Try doing that today.
We even have a new psychiatric condition – nomophobia – for the fear of not having our mobile phone handy. Every time I leave my house or office I repeat a simple mantra “phone, wallet, keys” as I pat my pockets.
Unless you can part with your gadget – which ain’t easy – no one is a “free man in Paris” (or Tokyo, or Rio, or Beijing) anymore. Even ships have cell service at sea. There are still places on Earth remote enough that you can’t be reached, but they get fewer and smaller every year.
When was the last time you really went somewhere and had no communication with your office at all? Emails, texts, anything? Unless you’re in a shift-work branch of medicine, like ER or hospitalist, I’m going to guess it’s been a while. And even in those branches you probably get emails about administrative matters, scheduling questions, and pointless memos.
Being in solo practice I’ve come to accept this, but it’s a conscious decision on my part. It’s easier than finding a call partner, and if I’m handling my own stuff at least I’m not going to come home to any surprises. So I’ve covered patients from as far west as Hawaii, north as Juneau, south as Panama City, and east as Le Havre.
Granted, this is medicine, and many other jobs don’t require the degree of involvement that it does. But I suspect pretty much any professional - attorney, accountant, executive - still has to deal with work-related stuff while traveling. Back in the 1970s to 1980s my dad, a solo-practice lawyer, had a set time each vacation weekday afternoon where he’d call his secretary to go over stuff. Today it would be by email or texts.
We’ve done this to ourselves. We’ve accepted the trade-off of better connectivity with family and friends for expanding our time at work. The same technology that lets me send in prescription refills from London also lets me send family pictures back from Maui. It’s not easy to draw a solid line between them, and I’m not so sure many of us want to.
Today, 50 years after Ms. Mitchell wrote the song, the idea of being a “free man in Paris” – or anywhere – really doesn’t exist for most of us anymore. You can argue whether that’s good or bad, but it’s where we are.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
What impact do carbs have on bone health?
I am often asked about the impact of dietary nutrients on bone health, particularly as many patients with low bone density, many with a history of multiple fractures, are referred to me. Many factors affect bone density, an important predictor of fracture risk, including genetics, body weight and muscle mass, bone loading exercise, menstrual status, other hormonal factors, nutritional status, optimal absorption of dietary nutrients, and medication use.
Dietary nutrients include macronutrients (carbohydrates, proteins, fat, and fiber) and micronutrients (such as dietary minerals and vitamins). The importance of micronutrients such as calcium, phosphorus, magnesium, and vitamins C, D, and K in optimizing bone mineralization and bone formation has been well documented.
The impact of protein intake on bone health is slightly more controversial, with some studies suggesting that increased protein intake may be deleterious to bone by increasing acid load, which in turn, increases calcium loss in urine. Overall data analysis from multiple studies support the finding that a higher protein intake is modestly beneficial for bone at certain sites, such as the spine.
Though data regarding the impact of dietary carbohydrates on bone are not as robust, it’s important to understand these effects given the increasing knowledge of the deleterious impact of processed carbohydrates on weight and cardiometabolic outcomes. This leads to the growing recommendations to limit carbohydrates in diet.
Quality and quantity of carbs affect bone health
Available studies suggest that both the quality and quantity of carbohydrates that are in a diet as well as the glycemic index of food may affect bone outcomes. Glycemic index refers to the extent of blood glucose elevation that occurs after the intake of any specific food. Foods with a higher glycemic index cause a rapid increase in blood glucose, whereas those with a low glycemic index result in a slower and more gradual increase. Examples of high–glycemic index food include processed and baked foods (such as breakfast cereals [unless whole grain], pretzels, cookies, doughnuts, pastries, cake, white bread, bagels, croissants, and corn chips), sugar-sweetened beverages, white rice, fast food (such as pizza and burgers), and potatoes. Examples of low glycemic index foods include vegetables, fruits, legumes, dairy and dairy products (without added sugar), whole-grain foods (such as oat porridge), and nuts.
A high–glycemic index diet has been associated with a greater risk for obesity and cardiovascular disease, and with lower bone density, an increased risk for fracture. This has been attributed to acute increases in glucose and insulin levels after consumption of high–glycemic index food, which causes increased oxidative stress and secretion of inflammatory cytokines, such as interleukin 6 and tumor necrosis factor alpha, that activate cells in bone that increase bone loss.
Higher blood glucose concentrations induced by a higher dietary glycemic index can have deleterious effects on osteoblasts, the cells important for bone formation, and increase bone loss through production of advanced glycation end products that affect the cross linking of collagen in bone (important for bone strength), as well as calcium loss in urine. This was recently reported in a study by Garcia-Gavilan and others, in which the authors showed that high dietary glycemic index and dietary glucose load are associated with a higher risk for osteoporosis-related fractures in an older Mediterranean population who are at high risk for cardiovascular events. Similar data were reported by Nouri and coauthors in a study from Iran.
The quantity and quality of dietary carbohydrates may also have an impact on bone. The quality of carbohydrates has been assessed using the carbohydrate quality index (CQI) and the low carbohydrate diet score (LCDS). The CQI takes into account dietary fiber intake, glycemic index, intake of processed vs. whole grain, and solid vs. total carbohydrates in diet. A higher CQI diet is associated with reduced cardiovascular risk. Higher LCDS reflects lower carbohydrate and higher fat and protein intake.
Diets that are rich in refined or processed carbohydrates with added sugar are proinflammatory and increase oxidative stress, which may lead to increased bone loss, low bone density, and increased fracture risk. These foods also have a high glycemic index.
In contrast, diets that are rich in whole grains, legumes, fruits, vegetables, nuts, and olive oil have a lower glycemic index and are beneficial to bone. These diets have a higher CQI and LCDS (as reported by Nouri and coauthors) and provide a rich source of antioxidants, vitamins, minerals, and other nutrients (such as calcium, magnesium, and vitamins B, C, and K), which are all beneficial to bone. Gao and others have reported that implementing a low glycemic index pulse-based diet (lentils, peas, beans) is superior to a regular hospital diet in preventing the increase in bone loss that typically occurs during hospitalization with enforced bed rest.
Most reports of the impact of carbohydrates on bone health are from observational studies. In an interventional study, Dalskov and coauthors randomly assigned children aged 5-18 years who had parents with overweight to one of five diets (high protein/low glycemic index, high protein/high glycemic index, low protein/low glycemic index, low protein/high glycemic index, or regular) for 6 months.
Contrasting with our understanding that protein intake is overall good for bone, this study found that among patients receiving a high–glycemic index diet, those who were on a high-protein diet had greater reductions in a bone formation marker than did those on a low-protein diet, with no major changes observed with the other diets. This suggests the influence of associated dietary nutrients on bone outcomes and that protein intake may modify the effects of dietary carbohydrates on bone formation. Similarly, the fat content of food can alter the glycemic index and thus may modify the impact of dietary carbohydrates on bone.
In summary, available data suggest that the quantity and quality of carbohydrates, including the glycemic index of food, may affect bone health and that it is important to exercise moderation in the consumption of such foods. However, there are only a few studies that have examined these associations, and more studies are necessary to further clarify the impact of dietary carbohydrates on bone as well as any modifications of these effects by other associated food groups. These studies will allow us to refine our recommendations to our patients as we advance our understanding of the impact of the combined effects of various dietary nutrients on bone.
Madhusmita Misra, MD, MPH, is chief of the division of pediatric endocrinology, Mass General for Children, Boston, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
I am often asked about the impact of dietary nutrients on bone health, particularly as many patients with low bone density, many with a history of multiple fractures, are referred to me. Many factors affect bone density, an important predictor of fracture risk, including genetics, body weight and muscle mass, bone loading exercise, menstrual status, other hormonal factors, nutritional status, optimal absorption of dietary nutrients, and medication use.
Dietary nutrients include macronutrients (carbohydrates, proteins, fat, and fiber) and micronutrients (such as dietary minerals and vitamins). The importance of micronutrients such as calcium, phosphorus, magnesium, and vitamins C, D, and K in optimizing bone mineralization and bone formation has been well documented.
The impact of protein intake on bone health is slightly more controversial, with some studies suggesting that increased protein intake may be deleterious to bone by increasing acid load, which in turn, increases calcium loss in urine. Overall data analysis from multiple studies support the finding that a higher protein intake is modestly beneficial for bone at certain sites, such as the spine.
Though data regarding the impact of dietary carbohydrates on bone are not as robust, it’s important to understand these effects given the increasing knowledge of the deleterious impact of processed carbohydrates on weight and cardiometabolic outcomes. This leads to the growing recommendations to limit carbohydrates in diet.
Quality and quantity of carbs affect bone health
Available studies suggest that both the quality and quantity of carbohydrates that are in a diet as well as the glycemic index of food may affect bone outcomes. Glycemic index refers to the extent of blood glucose elevation that occurs after the intake of any specific food. Foods with a higher glycemic index cause a rapid increase in blood glucose, whereas those with a low glycemic index result in a slower and more gradual increase. Examples of high–glycemic index food include processed and baked foods (such as breakfast cereals [unless whole grain], pretzels, cookies, doughnuts, pastries, cake, white bread, bagels, croissants, and corn chips), sugar-sweetened beverages, white rice, fast food (such as pizza and burgers), and potatoes. Examples of low glycemic index foods include vegetables, fruits, legumes, dairy and dairy products (without added sugar), whole-grain foods (such as oat porridge), and nuts.
A high–glycemic index diet has been associated with a greater risk for obesity and cardiovascular disease, and with lower bone density, an increased risk for fracture. This has been attributed to acute increases in glucose and insulin levels after consumption of high–glycemic index food, which causes increased oxidative stress and secretion of inflammatory cytokines, such as interleukin 6 and tumor necrosis factor alpha, that activate cells in bone that increase bone loss.
Higher blood glucose concentrations induced by a higher dietary glycemic index can have deleterious effects on osteoblasts, the cells important for bone formation, and increase bone loss through production of advanced glycation end products that affect the cross linking of collagen in bone (important for bone strength), as well as calcium loss in urine. This was recently reported in a study by Garcia-Gavilan and others, in which the authors showed that high dietary glycemic index and dietary glucose load are associated with a higher risk for osteoporosis-related fractures in an older Mediterranean population who are at high risk for cardiovascular events. Similar data were reported by Nouri and coauthors in a study from Iran.
The quantity and quality of dietary carbohydrates may also have an impact on bone. The quality of carbohydrates has been assessed using the carbohydrate quality index (CQI) and the low carbohydrate diet score (LCDS). The CQI takes into account dietary fiber intake, glycemic index, intake of processed vs. whole grain, and solid vs. total carbohydrates in diet. A higher CQI diet is associated with reduced cardiovascular risk. Higher LCDS reflects lower carbohydrate and higher fat and protein intake.
Diets that are rich in refined or processed carbohydrates with added sugar are proinflammatory and increase oxidative stress, which may lead to increased bone loss, low bone density, and increased fracture risk. These foods also have a high glycemic index.
In contrast, diets that are rich in whole grains, legumes, fruits, vegetables, nuts, and olive oil have a lower glycemic index and are beneficial to bone. These diets have a higher CQI and LCDS (as reported by Nouri and coauthors) and provide a rich source of antioxidants, vitamins, minerals, and other nutrients (such as calcium, magnesium, and vitamins B, C, and K), which are all beneficial to bone. Gao and others have reported that implementing a low glycemic index pulse-based diet (lentils, peas, beans) is superior to a regular hospital diet in preventing the increase in bone loss that typically occurs during hospitalization with enforced bed rest.
Most reports of the impact of carbohydrates on bone health are from observational studies. In an interventional study, Dalskov and coauthors randomly assigned children aged 5-18 years who had parents with overweight to one of five diets (high protein/low glycemic index, high protein/high glycemic index, low protein/low glycemic index, low protein/high glycemic index, or regular) for 6 months.
Contrasting with our understanding that protein intake is overall good for bone, this study found that among patients receiving a high–glycemic index diet, those who were on a high-protein diet had greater reductions in a bone formation marker than did those on a low-protein diet, with no major changes observed with the other diets. This suggests the influence of associated dietary nutrients on bone outcomes and that protein intake may modify the effects of dietary carbohydrates on bone formation. Similarly, the fat content of food can alter the glycemic index and thus may modify the impact of dietary carbohydrates on bone.
In summary, available data suggest that the quantity and quality of carbohydrates, including the glycemic index of food, may affect bone health and that it is important to exercise moderation in the consumption of such foods. However, there are only a few studies that have examined these associations, and more studies are necessary to further clarify the impact of dietary carbohydrates on bone as well as any modifications of these effects by other associated food groups. These studies will allow us to refine our recommendations to our patients as we advance our understanding of the impact of the combined effects of various dietary nutrients on bone.
Madhusmita Misra, MD, MPH, is chief of the division of pediatric endocrinology, Mass General for Children, Boston, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
I am often asked about the impact of dietary nutrients on bone health, particularly as many patients with low bone density, many with a history of multiple fractures, are referred to me. Many factors affect bone density, an important predictor of fracture risk, including genetics, body weight and muscle mass, bone loading exercise, menstrual status, other hormonal factors, nutritional status, optimal absorption of dietary nutrients, and medication use.
Dietary nutrients include macronutrients (carbohydrates, proteins, fat, and fiber) and micronutrients (such as dietary minerals and vitamins). The importance of micronutrients such as calcium, phosphorus, magnesium, and vitamins C, D, and K in optimizing bone mineralization and bone formation has been well documented.
The impact of protein intake on bone health is slightly more controversial, with some studies suggesting that increased protein intake may be deleterious to bone by increasing acid load, which in turn, increases calcium loss in urine. Overall data analysis from multiple studies support the finding that a higher protein intake is modestly beneficial for bone at certain sites, such as the spine.
Though data regarding the impact of dietary carbohydrates on bone are not as robust, it’s important to understand these effects given the increasing knowledge of the deleterious impact of processed carbohydrates on weight and cardiometabolic outcomes. This leads to the growing recommendations to limit carbohydrates in diet.
Quality and quantity of carbs affect bone health
Available studies suggest that both the quality and quantity of carbohydrates that are in a diet as well as the glycemic index of food may affect bone outcomes. Glycemic index refers to the extent of blood glucose elevation that occurs after the intake of any specific food. Foods with a higher glycemic index cause a rapid increase in blood glucose, whereas those with a low glycemic index result in a slower and more gradual increase. Examples of high–glycemic index food include processed and baked foods (such as breakfast cereals [unless whole grain], pretzels, cookies, doughnuts, pastries, cake, white bread, bagels, croissants, and corn chips), sugar-sweetened beverages, white rice, fast food (such as pizza and burgers), and potatoes. Examples of low glycemic index foods include vegetables, fruits, legumes, dairy and dairy products (without added sugar), whole-grain foods (such as oat porridge), and nuts.
A high–glycemic index diet has been associated with a greater risk for obesity and cardiovascular disease, and with lower bone density, an increased risk for fracture. This has been attributed to acute increases in glucose and insulin levels after consumption of high–glycemic index food, which causes increased oxidative stress and secretion of inflammatory cytokines, such as interleukin 6 and tumor necrosis factor alpha, that activate cells in bone that increase bone loss.
Higher blood glucose concentrations induced by a higher dietary glycemic index can have deleterious effects on osteoblasts, the cells important for bone formation, and increase bone loss through production of advanced glycation end products that affect the cross linking of collagen in bone (important for bone strength), as well as calcium loss in urine. This was recently reported in a study by Garcia-Gavilan and others, in which the authors showed that high dietary glycemic index and dietary glucose load are associated with a higher risk for osteoporosis-related fractures in an older Mediterranean population who are at high risk for cardiovascular events. Similar data were reported by Nouri and coauthors in a study from Iran.
The quantity and quality of dietary carbohydrates may also have an impact on bone. The quality of carbohydrates has been assessed using the carbohydrate quality index (CQI) and the low carbohydrate diet score (LCDS). The CQI takes into account dietary fiber intake, glycemic index, intake of processed vs. whole grain, and solid vs. total carbohydrates in diet. A higher CQI diet is associated with reduced cardiovascular risk. Higher LCDS reflects lower carbohydrate and higher fat and protein intake.
Diets that are rich in refined or processed carbohydrates with added sugar are proinflammatory and increase oxidative stress, which may lead to increased bone loss, low bone density, and increased fracture risk. These foods also have a high glycemic index.
In contrast, diets that are rich in whole grains, legumes, fruits, vegetables, nuts, and olive oil have a lower glycemic index and are beneficial to bone. These diets have a higher CQI and LCDS (as reported by Nouri and coauthors) and provide a rich source of antioxidants, vitamins, minerals, and other nutrients (such as calcium, magnesium, and vitamins B, C, and K), which are all beneficial to bone. Gao and others have reported that implementing a low glycemic index pulse-based diet (lentils, peas, beans) is superior to a regular hospital diet in preventing the increase in bone loss that typically occurs during hospitalization with enforced bed rest.
Most reports of the impact of carbohydrates on bone health are from observational studies. In an interventional study, Dalskov and coauthors randomly assigned children aged 5-18 years who had parents with overweight to one of five diets (high protein/low glycemic index, high protein/high glycemic index, low protein/low glycemic index, low protein/high glycemic index, or regular) for 6 months.
Contrasting with our understanding that protein intake is overall good for bone, this study found that among patients receiving a high–glycemic index diet, those who were on a high-protein diet had greater reductions in a bone formation marker than did those on a low-protein diet, with no major changes observed with the other diets. This suggests the influence of associated dietary nutrients on bone outcomes and that protein intake may modify the effects of dietary carbohydrates on bone formation. Similarly, the fat content of food can alter the glycemic index and thus may modify the impact of dietary carbohydrates on bone.
In summary, available data suggest that the quantity and quality of carbohydrates, including the glycemic index of food, may affect bone health and that it is important to exercise moderation in the consumption of such foods. However, there are only a few studies that have examined these associations, and more studies are necessary to further clarify the impact of dietary carbohydrates on bone as well as any modifications of these effects by other associated food groups. These studies will allow us to refine our recommendations to our patients as we advance our understanding of the impact of the combined effects of various dietary nutrients on bone.
Madhusmita Misra, MD, MPH, is chief of the division of pediatric endocrinology, Mass General for Children, Boston, and serves or has served as a director, officer, partner, employee, advisor, consultant, or trustee for AbbVie, Sanofi, and Ipsen.
A version of this article first appeared on Medscape.com.
Robotic peritoneal vaginoplasty
When the Food and Drug Administration first approved the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for adult use in 2000, it altered the face of minimally invasive surgery across a multitude of specialties. Improved three-dimensional visualization and enhanced instrument articulation facilitates complex dissections and intracorporeal suturing. While the standard of care for gender-affirming vaginoplasty remains the single-stage penile inversion vaginoplasty, robotic procedures are quickly emerging as alternative options for both primary and revisional surgeries.
The single-stage penile inversion vaginoplasty requires an adequate amount of penoscrotal tissue not only to line a neovaginal canal that measures 12-15 cm, but also to create external vulvar structures. While this is often sufficient in most candidates, there is an increasing number of patients who are receiving puberty blockers, resulting in penoscrotal hypoplasia.
Alternatively, there are patients who experience loss of vaginal depth and vaginal stenosis who seek revisional surgeries. Additional donor sites for skin grafting are available and include the lower abdomen and thighs, although patients may not want these donor site scars. With these donor sites, there is also concern about graft contracture, which could lead to recurrent vaginal stenosis.1 Robotic peritoneal vaginoplasty and robotic enteric vaginoplasty can serve as additional options for patients seeking revisional surgery or who have insufficient genital skin. One benefit of using peritoneal flaps is that they are hairless and are well vascularized with minimal donor site morbidity.1 Currently, there are two predominant techniques that utilize peritoneal flaps: the modified Davydov procedure and the tubularized urachus-peritoneal hinge flap.
The modified Davydov technique, which originated in the treatment of congenital vaginal agenesis in cisgender women, involves the creation of anterior and posterior peritoneal flaps. This type of peritoneal vaginoplasty is more commonly utilized for primary cases.
Ideally, there is a robotic surgeon (typically a urologist) working in tandem with the perineal surgeon. The robotic surgeon makes a horizontal incision along the peritoneal ridge at the rectovesical junction and continues the dissection within Denonvilliers fascia, between the prostate and rectum, to the pelvic floor. This dissection is like that performed in a robot-assisted laparoscopic prostatectomy.
Simultaneously, the perineal surgeon will break through the pelvic floor with assistance of the robotic view. Peritoneal flaps are raised from the anterior rectum and posterior bladder.2,3 In primary cases, the penoscrotal flap is introduced into the abdomen from the perineum and sutured to the anterior and posterior peritoneum to create a circumferential canal. At the apex of the neovagina, these anterior and posterior flaps are then sutured together.2,3
The tubularized urachus-peritoneal hinge flap technique is predominantly used for revision cases in patients who experienced neovaginal shortening and desire increased neovaginal depth. As peritoneal reach is limited, candidates for this procedure must have both adequate width and neovaginal canal depth.4 Once intra-abdominal access is achieved, an anterior peritoneal flap is mobilized to the level of the bladder and rotated 180 degrees inferiorly.4 The superior aspect of the flap is flipped is mobilized and is sutured to the peritoneum at the apex of the neovaginal canal.
The main benefit of these procedures, compared with traditional techniques, is increased neovaginal depth. The average vaginal length in patients undergoing peritoneal vaginoplasties is 14.2 cm, compared with 11.6 cm achieved in those using skin grafts.1,3 However, many surgeons report achieving 14-15 cm of depth with the traditional vaginoplasty. There are insufficient short- and long-term data for the peritoneal technique to recommend this as a first-line procedure.
Complications for peritoneal vaginoplasty procedures are similar to those of single-stage penile inversion vaginoplasty cases but with additional operative risks associated with laparoscopic/robotic surgery. These risks include injury to viscera and major vessels during initial intra-abdominal access, intra-abdominal adhesions, port site hernias, need to convert to an open procedure, and equipment malfunction.2 Additional postoperative risks include pelvic abscess formation, dehiscence of the peritoneal-vaginal incision, and peritoneal perforation during dilation.2,3 Surgeons and institutions must also weigh the cost of using the robot versus the cost of additional revisional surgical procedures. While initial studies evaluating robotic peritoneal vaginoplasty procedures have yielded promising preliminary results, additional studies are warranted.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Salibian AA et al. Plast Reconstr Surg. 2021;147(4):634e-43e.
2. Dy GW et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:237-48.
3. Jacoby A et al. J Urol. 2019;201(6):1171-5.
4. Smith SM et al. J Sex Med. 2022;10(6):100572.
When the Food and Drug Administration first approved the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for adult use in 2000, it altered the face of minimally invasive surgery across a multitude of specialties. Improved three-dimensional visualization and enhanced instrument articulation facilitates complex dissections and intracorporeal suturing. While the standard of care for gender-affirming vaginoplasty remains the single-stage penile inversion vaginoplasty, robotic procedures are quickly emerging as alternative options for both primary and revisional surgeries.
The single-stage penile inversion vaginoplasty requires an adequate amount of penoscrotal tissue not only to line a neovaginal canal that measures 12-15 cm, but also to create external vulvar structures. While this is often sufficient in most candidates, there is an increasing number of patients who are receiving puberty blockers, resulting in penoscrotal hypoplasia.
Alternatively, there are patients who experience loss of vaginal depth and vaginal stenosis who seek revisional surgeries. Additional donor sites for skin grafting are available and include the lower abdomen and thighs, although patients may not want these donor site scars. With these donor sites, there is also concern about graft contracture, which could lead to recurrent vaginal stenosis.1 Robotic peritoneal vaginoplasty and robotic enteric vaginoplasty can serve as additional options for patients seeking revisional surgery or who have insufficient genital skin. One benefit of using peritoneal flaps is that they are hairless and are well vascularized with minimal donor site morbidity.1 Currently, there are two predominant techniques that utilize peritoneal flaps: the modified Davydov procedure and the tubularized urachus-peritoneal hinge flap.
The modified Davydov technique, which originated in the treatment of congenital vaginal agenesis in cisgender women, involves the creation of anterior and posterior peritoneal flaps. This type of peritoneal vaginoplasty is more commonly utilized for primary cases.
Ideally, there is a robotic surgeon (typically a urologist) working in tandem with the perineal surgeon. The robotic surgeon makes a horizontal incision along the peritoneal ridge at the rectovesical junction and continues the dissection within Denonvilliers fascia, between the prostate and rectum, to the pelvic floor. This dissection is like that performed in a robot-assisted laparoscopic prostatectomy.
Simultaneously, the perineal surgeon will break through the pelvic floor with assistance of the robotic view. Peritoneal flaps are raised from the anterior rectum and posterior bladder.2,3 In primary cases, the penoscrotal flap is introduced into the abdomen from the perineum and sutured to the anterior and posterior peritoneum to create a circumferential canal. At the apex of the neovagina, these anterior and posterior flaps are then sutured together.2,3
The tubularized urachus-peritoneal hinge flap technique is predominantly used for revision cases in patients who experienced neovaginal shortening and desire increased neovaginal depth. As peritoneal reach is limited, candidates for this procedure must have both adequate width and neovaginal canal depth.4 Once intra-abdominal access is achieved, an anterior peritoneal flap is mobilized to the level of the bladder and rotated 180 degrees inferiorly.4 The superior aspect of the flap is flipped is mobilized and is sutured to the peritoneum at the apex of the neovaginal canal.
The main benefit of these procedures, compared with traditional techniques, is increased neovaginal depth. The average vaginal length in patients undergoing peritoneal vaginoplasties is 14.2 cm, compared with 11.6 cm achieved in those using skin grafts.1,3 However, many surgeons report achieving 14-15 cm of depth with the traditional vaginoplasty. There are insufficient short- and long-term data for the peritoneal technique to recommend this as a first-line procedure.
Complications for peritoneal vaginoplasty procedures are similar to those of single-stage penile inversion vaginoplasty cases but with additional operative risks associated with laparoscopic/robotic surgery. These risks include injury to viscera and major vessels during initial intra-abdominal access, intra-abdominal adhesions, port site hernias, need to convert to an open procedure, and equipment malfunction.2 Additional postoperative risks include pelvic abscess formation, dehiscence of the peritoneal-vaginal incision, and peritoneal perforation during dilation.2,3 Surgeons and institutions must also weigh the cost of using the robot versus the cost of additional revisional surgical procedures. While initial studies evaluating robotic peritoneal vaginoplasty procedures have yielded promising preliminary results, additional studies are warranted.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Salibian AA et al. Plast Reconstr Surg. 2021;147(4):634e-43e.
2. Dy GW et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:237-48.
3. Jacoby A et al. J Urol. 2019;201(6):1171-5.
4. Smith SM et al. J Sex Med. 2022;10(6):100572.
When the Food and Drug Administration first approved the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, Calif.) for adult use in 2000, it altered the face of minimally invasive surgery across a multitude of specialties. Improved three-dimensional visualization and enhanced instrument articulation facilitates complex dissections and intracorporeal suturing. While the standard of care for gender-affirming vaginoplasty remains the single-stage penile inversion vaginoplasty, robotic procedures are quickly emerging as alternative options for both primary and revisional surgeries.
The single-stage penile inversion vaginoplasty requires an adequate amount of penoscrotal tissue not only to line a neovaginal canal that measures 12-15 cm, but also to create external vulvar structures. While this is often sufficient in most candidates, there is an increasing number of patients who are receiving puberty blockers, resulting in penoscrotal hypoplasia.
Alternatively, there are patients who experience loss of vaginal depth and vaginal stenosis who seek revisional surgeries. Additional donor sites for skin grafting are available and include the lower abdomen and thighs, although patients may not want these donor site scars. With these donor sites, there is also concern about graft contracture, which could lead to recurrent vaginal stenosis.1 Robotic peritoneal vaginoplasty and robotic enteric vaginoplasty can serve as additional options for patients seeking revisional surgery or who have insufficient genital skin. One benefit of using peritoneal flaps is that they are hairless and are well vascularized with minimal donor site morbidity.1 Currently, there are two predominant techniques that utilize peritoneal flaps: the modified Davydov procedure and the tubularized urachus-peritoneal hinge flap.
The modified Davydov technique, which originated in the treatment of congenital vaginal agenesis in cisgender women, involves the creation of anterior and posterior peritoneal flaps. This type of peritoneal vaginoplasty is more commonly utilized for primary cases.
Ideally, there is a robotic surgeon (typically a urologist) working in tandem with the perineal surgeon. The robotic surgeon makes a horizontal incision along the peritoneal ridge at the rectovesical junction and continues the dissection within Denonvilliers fascia, between the prostate and rectum, to the pelvic floor. This dissection is like that performed in a robot-assisted laparoscopic prostatectomy.
Simultaneously, the perineal surgeon will break through the pelvic floor with assistance of the robotic view. Peritoneal flaps are raised from the anterior rectum and posterior bladder.2,3 In primary cases, the penoscrotal flap is introduced into the abdomen from the perineum and sutured to the anterior and posterior peritoneum to create a circumferential canal. At the apex of the neovagina, these anterior and posterior flaps are then sutured together.2,3
The tubularized urachus-peritoneal hinge flap technique is predominantly used for revision cases in patients who experienced neovaginal shortening and desire increased neovaginal depth. As peritoneal reach is limited, candidates for this procedure must have both adequate width and neovaginal canal depth.4 Once intra-abdominal access is achieved, an anterior peritoneal flap is mobilized to the level of the bladder and rotated 180 degrees inferiorly.4 The superior aspect of the flap is flipped is mobilized and is sutured to the peritoneum at the apex of the neovaginal canal.
The main benefit of these procedures, compared with traditional techniques, is increased neovaginal depth. The average vaginal length in patients undergoing peritoneal vaginoplasties is 14.2 cm, compared with 11.6 cm achieved in those using skin grafts.1,3 However, many surgeons report achieving 14-15 cm of depth with the traditional vaginoplasty. There are insufficient short- and long-term data for the peritoneal technique to recommend this as a first-line procedure.
Complications for peritoneal vaginoplasty procedures are similar to those of single-stage penile inversion vaginoplasty cases but with additional operative risks associated with laparoscopic/robotic surgery. These risks include injury to viscera and major vessels during initial intra-abdominal access, intra-abdominal adhesions, port site hernias, need to convert to an open procedure, and equipment malfunction.2 Additional postoperative risks include pelvic abscess formation, dehiscence of the peritoneal-vaginal incision, and peritoneal perforation during dilation.2,3 Surgeons and institutions must also weigh the cost of using the robot versus the cost of additional revisional surgical procedures. While initial studies evaluating robotic peritoneal vaginoplasty procedures have yielded promising preliminary results, additional studies are warranted.
Dr. Brandt is an ob.gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pa.
References
1. Salibian AA et al. Plast Reconstr Surg. 2021;147(4):634e-43e.
2. Dy GW et al. In: Nikolavsky D and Blakely SA, eds. Urological care for the transgender patient: A comprehensive guide. Switzerland: Springer, 2021:237-48.
3. Jacoby A et al. J Urol. 2019;201(6):1171-5.
4. Smith SM et al. J Sex Med. 2022;10(6):100572.
U.S. vs. French guidelines for osteoporosis treatment
Lille, France – Bernard Cortet, MD, PhD, chairperson of the Osteoporosis Research and Information Group and head of the rheumatology department at Lille (France) University Hospital, has agreed to compare the new U.S. guidelines to the 2018 French recommendations written under the aegis of the French Society for Rheumatology and GRIO. Dr. Cortet participated in drafting the French recommendations.
Question: The ACP “strongly” recommends initial pharmacologic treatment with bisphosphonate antiresorptive drugs (alendronate, ibandronate, risedronate, zoledronate) in postmenopausal females diagnosed with primary osteoporosis. Isn’t this what the SFR–GRIO have been recommending for many years?
Answer: The ACP reinforces its stance by arguing that in postmenopausal females with primary osteoporosis, bisphosphonates have the most favorable balance between benefits, harms, patient values and preferences, and cost among the drug classes that were evaluated. In addition to net clinical benefits, bisphosphonates are much cheaper than other pharmacologic treatments and are available in generic oral and injectable formulations.
Our French recommendations specify the choice of drug based on the type of fracture in women and on their bone mineral density (BMD). However, bisphosphonates are definitely given pride of place. When treatment for osteoporosis needs to be started, most of the time, a bisphosphonate is the treatment of choice.
Nevertheless, as also highlighted by the ACP, a more “aggressive” approach must be considered for more severe cases.
In the case of a severe fracture, the French recommendations indicate that all treatments can be prescribed. However, zoledronic acid should be favored as first-line treatment for a hip fracture. In other cases – with or without a nonsevere fracture – the therapeutic indication depends on the BMD values, and in difficult cases, on tools such as FRAX [the Fracture Risk Assessment Tool].
Our guidance strongly recommends opting for an injection in other contexts, such as significant decrease in bone density, presence of comorbidities, poor treatment compliance, brain function disorders, and polymedication.
Q. But it’s not really as simple as prescribing a bisphosphonate, is it?
A. You’re right, many people find the idea of taking bisphosphonates worrying because of associated jaw problems – osteonecrosis of the jaw – or atypical femoral fractures, based on what they’ve read on the Internet, where these serious adverse events are on display front and center with no mention of how often they actually happen and, often, failing to mention how effective bisphosphonates truly are.
These complications are real, but fortunately rare, especially during the first 5 years of treatment. To put this into context, for bisphosphonates, there’s one case of osteonecrosis of the jaw for every 10,000. And for denosumab, there are five cases for every 10,000. For atypical fractures, there’s one case for every 30,000 to 50,000.
Q. The U.S. guidelines also recommend that clinicians use a RANK ligand inhibitor – denosumab, also an antiresorptive drug – as second-line medical treatment. This is to reduce the risk of fractures in postmenopausal women diagnosed with primary osteoporosis and presenting with contraindications or side effects of bisphosphonates. Do you support the use of denosumab as second-line treatment?
A. French legislation classifies it as a second-line treatment, after bisphosphonates. However, there are arguments in favor of prescribing it as first-line treatment in some contexts. If denosumab is to be prescribed – via a twice-yearly subcutaneous injection – full compliance must be observed. If a patient is to stop taking denosumab, an opinion from a medical professional is required before treatment can be discontinued, and then treatment with bisphosphonates must be prescribed.
Q. The ACP recommends that clinicians use either a sclerostin inhibitor – romosozumab – or recombinant human parathyroid hormone – teriparatide – two anabolic agents, followed by a bisphosphonate, with the aim of reducing the risk of fractures. This is only used in women with primary osteoporosis who are at a very high risk of fracture. As romosozumab is not available in France, it’s not really worth discussing its use. Does this strategy seem advisable to you, though?
A. The main issue is what is understood by “women at a very high risk of fracture.” There’s no consensus on the definition of what constitutes a woman at a very high risk of fracture, but we can assume that it involves the combination of low BMD and at least one severe fracture.
The role of anabolic bone treatment, as [the ACP] has defined it, seems logical to me, because in cases of severe osteoporosis with fracture, the risk of recurrence is very high in the next 2-3 years. In a study comparing risedronate and teriparatide in cases of severe osteoporosis, teriparatide was more effective in reducing the recurrence of vertebral fractures.
The favorable opinion of the French National Authority for Health in relation to medical coverage for romosozumab in the treatment of severe postmenopausal osteoporosis in women under the age of 75 years with a history of severe fractures, a T-score less than –2.5, and no previous history of coronary artery disease dates to 2021. This is because medical coverage for this specific group was not listed in the marketing authorization (MA) description for this drug.
But the review by the Economic Committee for Health Products failed to reach a consensus regarding the price. Today, in theory, romosozumab can be dispensed in France by hospital pharmacies, because it is approved for use in public hospitals. Romosozumab is a very interesting drug for relatively young women, especially those with multiple vertebral fractures. This injectable treatment is more effective than teriparatide in increasing BMD values and more effective than alendronate in preventing the recurrence of fractures.
Regarding medical coverage, as it stands, in cases where patients have a T-score less than or equal to –3, the 2018 SFR–GRIO recommends starting treatment even if the patient has no fractures. In cases with severe fractures combined with very low BMD (T-score ≤ –3), injectable treatments may be used to reach a bone density target (T-score > –2.5 to –2 for the hip) at the end of the treatment plan. [These treatments include] zoledronic acid, denosumab (in case of bisphosphonate failure or intolerance), or a treatment plan with teriparatide (covered by medical insurance if the patient has at least two vertebral fractures) followed by an antiresorptive drug (bisphosphonate or denosumab).
Romosozumab is a humanized monoclonal antibody (IgG2) that binds to sclerostin and acts as an inhibitor. This increases bone formation because of the activation of [bone lining cells], the production of bone matrix by osteoblasts, and the recruitment of osteochondroprogenitor cells. Moreover, romosozumab causes changes in the expression of osteoclast mediators, which decreases bone resorption. Together, these two effects that increase bone formation and decrease bone resorption lead to the rapid increase of trabecular and cortical bone mass, as well as improvements in bone structure and strength.
Women treated with a bone anabolic agent must take an antiresorptive agent at the end of their treatment so that the benefits from the treatment remain in the long term. The French and U.S. guidelines line up on this point.
In patients with two prevalent vertebral fractures, the U.S. guidelines state that teriparatide can be prescribed as first-line treatment at diagnosis in the absence of any contraindications. We agree on this point as well.
Moreover, in women under the age of 70 years with osteoporosis requiring treatment, French experts recommend prescribing raloxifene, a selective estrogen-receptor modulator. This is if the risk of nonvertebral fracture is low, as defined by the absence of the following criteria: low hip T-score, risk of falling, and history of nonvertebral fracture. Opportunities for its use are limited, and it doesn’t even figure among the U.S. recommendations.
Q. The ACP recommends that clinicians adopt an individualized approach regarding whether to start medical treatment with a bisphosphonate in women over age 65 years with low bone mass (osteopenia) to reduce the risk of fractures. If treatment is started, they›re of the opinion that a bisphosphonate must be used. What are the recommendations in France?
A. It should be noted that this recommendation by the ACP is conditional because of the low-certainty evidence.
Here’s a brief reminder of important things to note: a T-score between –2.5 and –1 indicates osteopenia; a T-score less than or equal to –2.5 indicates osteoporosis; a T-score less than or equal to –2.5 with one or several fractures indicates severe osteoporosis. The French recommendations state that treatment is not justified if a patient’s T-score is higher than –2 and there’s no presence of fractures, even with risk factors (and/or multiple falls). For T-scores less than or equal to –2 and higher than –3, the decision to prescribe depends on the specialist.
Q. The ACP recommends that clinicians use bisphosphonates for the initial medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis.
A. The ACP recommends that clinicians use a RANK ligand inhibitor – denosumab – as second-line medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis who present with contraindications or who are experiencing side effects of bisphosphonates. This treatment is not covered by health insurance for men in France.
Between 20% and 25% of clinical osteoporotic fractures occur in men. After age 50 years, men are roughly 20% more likely to experience an osteoporotic fracture in their lifetime. The French recommendations regarding the management and treatment of osteoporosis in men were published in 2021.
In the case of severe fractures (vertebrae, pelvis, upper end of the femur, distal femur, proximal humerus) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –1.
In the case of nonsevere fractures (particularly wrist and ankle) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –2. If there are no fractures, osteoporosis treatment is recommended in men at risk of bone fragility or of falling and if one of the T-scores is less than or equal to –3. In patients who had a fracture of the upper end of the femur attributable to bone fragility, zoledronic acid is recommended as first-line treatment.
For men with a severe nonvertebral fracture, single vertebral fracture, or nonsevere fracture, two treatments are indicated and covered by health insurance in France: zoledronic acid and risedronate. In men with at least two vertebral fractures, the following treatments are indicated and covered by health insurance in France: teriparatide and risedronate. In this case, teriparatide is prescribed for a period of 18 months. It must be followed by a prescription of oral or intravenous bisphosphonates.
Q. What is your take on the HAS update to the proper use of osteoporosis medication that’s just been published?
A. Like in the 2018 SFR–GRIO guidelines, no update has been made to the section on postmenopausal osteoporosis, except for the HAS introduction to the proper use of romosozumab, even though it’s not covered by health insurance in France.
In accordance with the MA, it doesn’t make sense to include this drug on the list of treatment options available for women with and without fractures, as it’s not included in the HAS-selected list of drugs covered by health insurance in France.
But I’m glad that the HAS has adopted the GRIO and SFR recommendations regarding corticosteroid-induced osteoporosis. Preventive treatment for corticosteroid-induced osteoporosis must be considered as soon as the daily dose of corticosteroids reaches or exceeds the equivalent of 7.5 mg of prednisone and when the estimated duration of corticosteroid therapy exceeds 3 months.
In summary, in women and men over the age of 50 years, the intake of the equivalent of 7.5 mg/day or more of prednisone or a history of a low-trauma fracture or being age 70 years or older, even with a T-score less than or equal to –2.5 for one of the two sites, indicates prescribing a bisphosphonate. Teriparatide is indicated if the patient has two vertebral fractures.
This article was translated from Medscape’s French edition.
A version of this article first appeared on Medscape.com.
Lille, France – Bernard Cortet, MD, PhD, chairperson of the Osteoporosis Research and Information Group and head of the rheumatology department at Lille (France) University Hospital, has agreed to compare the new U.S. guidelines to the 2018 French recommendations written under the aegis of the French Society for Rheumatology and GRIO. Dr. Cortet participated in drafting the French recommendations.
Question: The ACP “strongly” recommends initial pharmacologic treatment with bisphosphonate antiresorptive drugs (alendronate, ibandronate, risedronate, zoledronate) in postmenopausal females diagnosed with primary osteoporosis. Isn’t this what the SFR–GRIO have been recommending for many years?
Answer: The ACP reinforces its stance by arguing that in postmenopausal females with primary osteoporosis, bisphosphonates have the most favorable balance between benefits, harms, patient values and preferences, and cost among the drug classes that were evaluated. In addition to net clinical benefits, bisphosphonates are much cheaper than other pharmacologic treatments and are available in generic oral and injectable formulations.
Our French recommendations specify the choice of drug based on the type of fracture in women and on their bone mineral density (BMD). However, bisphosphonates are definitely given pride of place. When treatment for osteoporosis needs to be started, most of the time, a bisphosphonate is the treatment of choice.
Nevertheless, as also highlighted by the ACP, a more “aggressive” approach must be considered for more severe cases.
In the case of a severe fracture, the French recommendations indicate that all treatments can be prescribed. However, zoledronic acid should be favored as first-line treatment for a hip fracture. In other cases – with or without a nonsevere fracture – the therapeutic indication depends on the BMD values, and in difficult cases, on tools such as FRAX [the Fracture Risk Assessment Tool].
Our guidance strongly recommends opting for an injection in other contexts, such as significant decrease in bone density, presence of comorbidities, poor treatment compliance, brain function disorders, and polymedication.
Q. But it’s not really as simple as prescribing a bisphosphonate, is it?
A. You’re right, many people find the idea of taking bisphosphonates worrying because of associated jaw problems – osteonecrosis of the jaw – or atypical femoral fractures, based on what they’ve read on the Internet, where these serious adverse events are on display front and center with no mention of how often they actually happen and, often, failing to mention how effective bisphosphonates truly are.
These complications are real, but fortunately rare, especially during the first 5 years of treatment. To put this into context, for bisphosphonates, there’s one case of osteonecrosis of the jaw for every 10,000. And for denosumab, there are five cases for every 10,000. For atypical fractures, there’s one case for every 30,000 to 50,000.
Q. The U.S. guidelines also recommend that clinicians use a RANK ligand inhibitor – denosumab, also an antiresorptive drug – as second-line medical treatment. This is to reduce the risk of fractures in postmenopausal women diagnosed with primary osteoporosis and presenting with contraindications or side effects of bisphosphonates. Do you support the use of denosumab as second-line treatment?
A. French legislation classifies it as a second-line treatment, after bisphosphonates. However, there are arguments in favor of prescribing it as first-line treatment in some contexts. If denosumab is to be prescribed – via a twice-yearly subcutaneous injection – full compliance must be observed. If a patient is to stop taking denosumab, an opinion from a medical professional is required before treatment can be discontinued, and then treatment with bisphosphonates must be prescribed.
Q. The ACP recommends that clinicians use either a sclerostin inhibitor – romosozumab – or recombinant human parathyroid hormone – teriparatide – two anabolic agents, followed by a bisphosphonate, with the aim of reducing the risk of fractures. This is only used in women with primary osteoporosis who are at a very high risk of fracture. As romosozumab is not available in France, it’s not really worth discussing its use. Does this strategy seem advisable to you, though?
A. The main issue is what is understood by “women at a very high risk of fracture.” There’s no consensus on the definition of what constitutes a woman at a very high risk of fracture, but we can assume that it involves the combination of low BMD and at least one severe fracture.
The role of anabolic bone treatment, as [the ACP] has defined it, seems logical to me, because in cases of severe osteoporosis with fracture, the risk of recurrence is very high in the next 2-3 years. In a study comparing risedronate and teriparatide in cases of severe osteoporosis, teriparatide was more effective in reducing the recurrence of vertebral fractures.
The favorable opinion of the French National Authority for Health in relation to medical coverage for romosozumab in the treatment of severe postmenopausal osteoporosis in women under the age of 75 years with a history of severe fractures, a T-score less than –2.5, and no previous history of coronary artery disease dates to 2021. This is because medical coverage for this specific group was not listed in the marketing authorization (MA) description for this drug.
But the review by the Economic Committee for Health Products failed to reach a consensus regarding the price. Today, in theory, romosozumab can be dispensed in France by hospital pharmacies, because it is approved for use in public hospitals. Romosozumab is a very interesting drug for relatively young women, especially those with multiple vertebral fractures. This injectable treatment is more effective than teriparatide in increasing BMD values and more effective than alendronate in preventing the recurrence of fractures.
Regarding medical coverage, as it stands, in cases where patients have a T-score less than or equal to –3, the 2018 SFR–GRIO recommends starting treatment even if the patient has no fractures. In cases with severe fractures combined with very low BMD (T-score ≤ –3), injectable treatments may be used to reach a bone density target (T-score > –2.5 to –2 for the hip) at the end of the treatment plan. [These treatments include] zoledronic acid, denosumab (in case of bisphosphonate failure or intolerance), or a treatment plan with teriparatide (covered by medical insurance if the patient has at least two vertebral fractures) followed by an antiresorptive drug (bisphosphonate or denosumab).
Romosozumab is a humanized monoclonal antibody (IgG2) that binds to sclerostin and acts as an inhibitor. This increases bone formation because of the activation of [bone lining cells], the production of bone matrix by osteoblasts, and the recruitment of osteochondroprogenitor cells. Moreover, romosozumab causes changes in the expression of osteoclast mediators, which decreases bone resorption. Together, these two effects that increase bone formation and decrease bone resorption lead to the rapid increase of trabecular and cortical bone mass, as well as improvements in bone structure and strength.
Women treated with a bone anabolic agent must take an antiresorptive agent at the end of their treatment so that the benefits from the treatment remain in the long term. The French and U.S. guidelines line up on this point.
In patients with two prevalent vertebral fractures, the U.S. guidelines state that teriparatide can be prescribed as first-line treatment at diagnosis in the absence of any contraindications. We agree on this point as well.
Moreover, in women under the age of 70 years with osteoporosis requiring treatment, French experts recommend prescribing raloxifene, a selective estrogen-receptor modulator. This is if the risk of nonvertebral fracture is low, as defined by the absence of the following criteria: low hip T-score, risk of falling, and history of nonvertebral fracture. Opportunities for its use are limited, and it doesn’t even figure among the U.S. recommendations.
Q. The ACP recommends that clinicians adopt an individualized approach regarding whether to start medical treatment with a bisphosphonate in women over age 65 years with low bone mass (osteopenia) to reduce the risk of fractures. If treatment is started, they›re of the opinion that a bisphosphonate must be used. What are the recommendations in France?
A. It should be noted that this recommendation by the ACP is conditional because of the low-certainty evidence.
Here’s a brief reminder of important things to note: a T-score between –2.5 and –1 indicates osteopenia; a T-score less than or equal to –2.5 indicates osteoporosis; a T-score less than or equal to –2.5 with one or several fractures indicates severe osteoporosis. The French recommendations state that treatment is not justified if a patient’s T-score is higher than –2 and there’s no presence of fractures, even with risk factors (and/or multiple falls). For T-scores less than or equal to –2 and higher than –3, the decision to prescribe depends on the specialist.
Q. The ACP recommends that clinicians use bisphosphonates for the initial medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis.
A. The ACP recommends that clinicians use a RANK ligand inhibitor – denosumab – as second-line medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis who present with contraindications or who are experiencing side effects of bisphosphonates. This treatment is not covered by health insurance for men in France.
Between 20% and 25% of clinical osteoporotic fractures occur in men. After age 50 years, men are roughly 20% more likely to experience an osteoporotic fracture in their lifetime. The French recommendations regarding the management and treatment of osteoporosis in men were published in 2021.
In the case of severe fractures (vertebrae, pelvis, upper end of the femur, distal femur, proximal humerus) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –1.
In the case of nonsevere fractures (particularly wrist and ankle) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –2. If there are no fractures, osteoporosis treatment is recommended in men at risk of bone fragility or of falling and if one of the T-scores is less than or equal to –3. In patients who had a fracture of the upper end of the femur attributable to bone fragility, zoledronic acid is recommended as first-line treatment.
For men with a severe nonvertebral fracture, single vertebral fracture, or nonsevere fracture, two treatments are indicated and covered by health insurance in France: zoledronic acid and risedronate. In men with at least two vertebral fractures, the following treatments are indicated and covered by health insurance in France: teriparatide and risedronate. In this case, teriparatide is prescribed for a period of 18 months. It must be followed by a prescription of oral or intravenous bisphosphonates.
Q. What is your take on the HAS update to the proper use of osteoporosis medication that’s just been published?
A. Like in the 2018 SFR–GRIO guidelines, no update has been made to the section on postmenopausal osteoporosis, except for the HAS introduction to the proper use of romosozumab, even though it’s not covered by health insurance in France.
In accordance with the MA, it doesn’t make sense to include this drug on the list of treatment options available for women with and without fractures, as it’s not included in the HAS-selected list of drugs covered by health insurance in France.
But I’m glad that the HAS has adopted the GRIO and SFR recommendations regarding corticosteroid-induced osteoporosis. Preventive treatment for corticosteroid-induced osteoporosis must be considered as soon as the daily dose of corticosteroids reaches or exceeds the equivalent of 7.5 mg of prednisone and when the estimated duration of corticosteroid therapy exceeds 3 months.
In summary, in women and men over the age of 50 years, the intake of the equivalent of 7.5 mg/day or more of prednisone or a history of a low-trauma fracture or being age 70 years or older, even with a T-score less than or equal to –2.5 for one of the two sites, indicates prescribing a bisphosphonate. Teriparatide is indicated if the patient has two vertebral fractures.
This article was translated from Medscape’s French edition.
A version of this article first appeared on Medscape.com.
Lille, France – Bernard Cortet, MD, PhD, chairperson of the Osteoporosis Research and Information Group and head of the rheumatology department at Lille (France) University Hospital, has agreed to compare the new U.S. guidelines to the 2018 French recommendations written under the aegis of the French Society for Rheumatology and GRIO. Dr. Cortet participated in drafting the French recommendations.
Question: The ACP “strongly” recommends initial pharmacologic treatment with bisphosphonate antiresorptive drugs (alendronate, ibandronate, risedronate, zoledronate) in postmenopausal females diagnosed with primary osteoporosis. Isn’t this what the SFR–GRIO have been recommending for many years?
Answer: The ACP reinforces its stance by arguing that in postmenopausal females with primary osteoporosis, bisphosphonates have the most favorable balance between benefits, harms, patient values and preferences, and cost among the drug classes that were evaluated. In addition to net clinical benefits, bisphosphonates are much cheaper than other pharmacologic treatments and are available in generic oral and injectable formulations.
Our French recommendations specify the choice of drug based on the type of fracture in women and on their bone mineral density (BMD). However, bisphosphonates are definitely given pride of place. When treatment for osteoporosis needs to be started, most of the time, a bisphosphonate is the treatment of choice.
Nevertheless, as also highlighted by the ACP, a more “aggressive” approach must be considered for more severe cases.
In the case of a severe fracture, the French recommendations indicate that all treatments can be prescribed. However, zoledronic acid should be favored as first-line treatment for a hip fracture. In other cases – with or without a nonsevere fracture – the therapeutic indication depends on the BMD values, and in difficult cases, on tools such as FRAX [the Fracture Risk Assessment Tool].
Our guidance strongly recommends opting for an injection in other contexts, such as significant decrease in bone density, presence of comorbidities, poor treatment compliance, brain function disorders, and polymedication.
Q. But it’s not really as simple as prescribing a bisphosphonate, is it?
A. You’re right, many people find the idea of taking bisphosphonates worrying because of associated jaw problems – osteonecrosis of the jaw – or atypical femoral fractures, based on what they’ve read on the Internet, where these serious adverse events are on display front and center with no mention of how often they actually happen and, often, failing to mention how effective bisphosphonates truly are.
These complications are real, but fortunately rare, especially during the first 5 years of treatment. To put this into context, for bisphosphonates, there’s one case of osteonecrosis of the jaw for every 10,000. And for denosumab, there are five cases for every 10,000. For atypical fractures, there’s one case for every 30,000 to 50,000.
Q. The U.S. guidelines also recommend that clinicians use a RANK ligand inhibitor – denosumab, also an antiresorptive drug – as second-line medical treatment. This is to reduce the risk of fractures in postmenopausal women diagnosed with primary osteoporosis and presenting with contraindications or side effects of bisphosphonates. Do you support the use of denosumab as second-line treatment?
A. French legislation classifies it as a second-line treatment, after bisphosphonates. However, there are arguments in favor of prescribing it as first-line treatment in some contexts. If denosumab is to be prescribed – via a twice-yearly subcutaneous injection – full compliance must be observed. If a patient is to stop taking denosumab, an opinion from a medical professional is required before treatment can be discontinued, and then treatment with bisphosphonates must be prescribed.
Q. The ACP recommends that clinicians use either a sclerostin inhibitor – romosozumab – or recombinant human parathyroid hormone – teriparatide – two anabolic agents, followed by a bisphosphonate, with the aim of reducing the risk of fractures. This is only used in women with primary osteoporosis who are at a very high risk of fracture. As romosozumab is not available in France, it’s not really worth discussing its use. Does this strategy seem advisable to you, though?
A. The main issue is what is understood by “women at a very high risk of fracture.” There’s no consensus on the definition of what constitutes a woman at a very high risk of fracture, but we can assume that it involves the combination of low BMD and at least one severe fracture.
The role of anabolic bone treatment, as [the ACP] has defined it, seems logical to me, because in cases of severe osteoporosis with fracture, the risk of recurrence is very high in the next 2-3 years. In a study comparing risedronate and teriparatide in cases of severe osteoporosis, teriparatide was more effective in reducing the recurrence of vertebral fractures.
The favorable opinion of the French National Authority for Health in relation to medical coverage for romosozumab in the treatment of severe postmenopausal osteoporosis in women under the age of 75 years with a history of severe fractures, a T-score less than –2.5, and no previous history of coronary artery disease dates to 2021. This is because medical coverage for this specific group was not listed in the marketing authorization (MA) description for this drug.
But the review by the Economic Committee for Health Products failed to reach a consensus regarding the price. Today, in theory, romosozumab can be dispensed in France by hospital pharmacies, because it is approved for use in public hospitals. Romosozumab is a very interesting drug for relatively young women, especially those with multiple vertebral fractures. This injectable treatment is more effective than teriparatide in increasing BMD values and more effective than alendronate in preventing the recurrence of fractures.
Regarding medical coverage, as it stands, in cases where patients have a T-score less than or equal to –3, the 2018 SFR–GRIO recommends starting treatment even if the patient has no fractures. In cases with severe fractures combined with very low BMD (T-score ≤ –3), injectable treatments may be used to reach a bone density target (T-score > –2.5 to –2 for the hip) at the end of the treatment plan. [These treatments include] zoledronic acid, denosumab (in case of bisphosphonate failure or intolerance), or a treatment plan with teriparatide (covered by medical insurance if the patient has at least two vertebral fractures) followed by an antiresorptive drug (bisphosphonate or denosumab).
Romosozumab is a humanized monoclonal antibody (IgG2) that binds to sclerostin and acts as an inhibitor. This increases bone formation because of the activation of [bone lining cells], the production of bone matrix by osteoblasts, and the recruitment of osteochondroprogenitor cells. Moreover, romosozumab causes changes in the expression of osteoclast mediators, which decreases bone resorption. Together, these two effects that increase bone formation and decrease bone resorption lead to the rapid increase of trabecular and cortical bone mass, as well as improvements in bone structure and strength.
Women treated with a bone anabolic agent must take an antiresorptive agent at the end of their treatment so that the benefits from the treatment remain in the long term. The French and U.S. guidelines line up on this point.
In patients with two prevalent vertebral fractures, the U.S. guidelines state that teriparatide can be prescribed as first-line treatment at diagnosis in the absence of any contraindications. We agree on this point as well.
Moreover, in women under the age of 70 years with osteoporosis requiring treatment, French experts recommend prescribing raloxifene, a selective estrogen-receptor modulator. This is if the risk of nonvertebral fracture is low, as defined by the absence of the following criteria: low hip T-score, risk of falling, and history of nonvertebral fracture. Opportunities for its use are limited, and it doesn’t even figure among the U.S. recommendations.
Q. The ACP recommends that clinicians adopt an individualized approach regarding whether to start medical treatment with a bisphosphonate in women over age 65 years with low bone mass (osteopenia) to reduce the risk of fractures. If treatment is started, they›re of the opinion that a bisphosphonate must be used. What are the recommendations in France?
A. It should be noted that this recommendation by the ACP is conditional because of the low-certainty evidence.
Here’s a brief reminder of important things to note: a T-score between –2.5 and –1 indicates osteopenia; a T-score less than or equal to –2.5 indicates osteoporosis; a T-score less than or equal to –2.5 with one or several fractures indicates severe osteoporosis. The French recommendations state that treatment is not justified if a patient’s T-score is higher than –2 and there’s no presence of fractures, even with risk factors (and/or multiple falls). For T-scores less than or equal to –2 and higher than –3, the decision to prescribe depends on the specialist.
Q. The ACP recommends that clinicians use bisphosphonates for the initial medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis.
A. The ACP recommends that clinicians use a RANK ligand inhibitor – denosumab – as second-line medical treatment to reduce the risk of fractures in men diagnosed with primary osteoporosis who present with contraindications or who are experiencing side effects of bisphosphonates. This treatment is not covered by health insurance for men in France.
Between 20% and 25% of clinical osteoporotic fractures occur in men. After age 50 years, men are roughly 20% more likely to experience an osteoporotic fracture in their lifetime. The French recommendations regarding the management and treatment of osteoporosis in men were published in 2021.
In the case of severe fractures (vertebrae, pelvis, upper end of the femur, distal femur, proximal humerus) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –1.
In the case of nonsevere fractures (particularly wrist and ankle) attributable to bone fragility, osteoporosis treatment is recommended if one of the T-scores is less than or equal to –2. If there are no fractures, osteoporosis treatment is recommended in men at risk of bone fragility or of falling and if one of the T-scores is less than or equal to –3. In patients who had a fracture of the upper end of the femur attributable to bone fragility, zoledronic acid is recommended as first-line treatment.
For men with a severe nonvertebral fracture, single vertebral fracture, or nonsevere fracture, two treatments are indicated and covered by health insurance in France: zoledronic acid and risedronate. In men with at least two vertebral fractures, the following treatments are indicated and covered by health insurance in France: teriparatide and risedronate. In this case, teriparatide is prescribed for a period of 18 months. It must be followed by a prescription of oral or intravenous bisphosphonates.
Q. What is your take on the HAS update to the proper use of osteoporosis medication that’s just been published?
A. Like in the 2018 SFR–GRIO guidelines, no update has been made to the section on postmenopausal osteoporosis, except for the HAS introduction to the proper use of romosozumab, even though it’s not covered by health insurance in France.
In accordance with the MA, it doesn’t make sense to include this drug on the list of treatment options available for women with and without fractures, as it’s not included in the HAS-selected list of drugs covered by health insurance in France.
But I’m glad that the HAS has adopted the GRIO and SFR recommendations regarding corticosteroid-induced osteoporosis. Preventive treatment for corticosteroid-induced osteoporosis must be considered as soon as the daily dose of corticosteroids reaches or exceeds the equivalent of 7.5 mg of prednisone and when the estimated duration of corticosteroid therapy exceeds 3 months.
In summary, in women and men over the age of 50 years, the intake of the equivalent of 7.5 mg/day or more of prednisone or a history of a low-trauma fracture or being age 70 years or older, even with a T-score less than or equal to –2.5 for one of the two sites, indicates prescribing a bisphosphonate. Teriparatide is indicated if the patient has two vertebral fractures.
This article was translated from Medscape’s French edition.
A version of this article first appeared on Medscape.com.
Emergency birth on a plane: Two doctors earn their wings
Emergencies happen anywhere, anytime, and sometimes medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
In December 2017, I was a second-year urology resident at Cleveland Clinic. I’d gone to New Delhi to attend my best friend’s wedding. My flight back was New Delhi to Paris to JFK via Air France. I didn’t sleep on the first flight. So, on the second, I wanted to get some rest, because I had to go back to work the next day. I put on a movie and tried to snooze. As the saying goes in residency, you sleep when you can.
About 3 hours later, a flight attendant made an announcement in French, but I didn’t really hear it. Then they announced in English that they needed a physician. I noticed some flight attendants walking frantically around the economy cabin asking, “Is there a doctor on the plane?” Turns out there were two – the woman sitting next to me happened to be a pediatrician with Doctors Without Borders. I volunteered.
The flight attendant told me a woman was having abdominal pain. I thought it would be something straightforward. Usually, medical emergencies on planes involve chest pain or a panic attack or a vasovagal syncopal episode. Well, I was in for a ride that day.
She said she was 37 or 38 weeks in. I said, “Okay, if you’re having this significant abdominal pain, then I need to examine you.” So we decided to move her to the first-class cabin, which was empty (I never did ask why – but it was good we had room to work).
Next step, I went back to my seat and asked the pediatrician if she could assist. My plan was to simply get the passenger through the flight, and as soon as we landed, she would go to the hospital.
There was room to lie down in first class. The pediatrician and I examined her, and she appeared fine. She was traveling with her 4-year-old daughter, and the flight attendants were taking care of her. Everything was okay.
The pilot came back and asked if we would need an emergency landing. I asked him how far it was to JFK – 4 hours. He said the closest place to land would be the Azores Islands, which is Portuguese territory, 2 hours away.
The problem: Even if we made it to the Azores, the hospital there was a very basic facility with no obstetric care available. And by the time the ambulance picked her up and got her there, it would still be 2 or 3 hours total. I said, “No, let’s just observe and continue our course.” Inside my head, I was hoping and praying to God that was the right decision.
Within an hour, everything changed.
The woman’s pain got worse, and she started having contractions. Then her water broke.
Things progressed quickly from there. The contractions progressively got worse and worse. The interval between them got smaller and smaller. The next time we examined her, we could see the baby’s head beginning to crown.
At that point, we had to decide – are we going to deliver? We were in the middle of the North Atlantic Ocean. There was nothing around us. We were 35,000 feet in the air, surrounded by blue.
The crew wanted us to sign a Good Samaritan agreement. So, we did that. And then I said, “Okay, let’s just go for it.”
We got the plane’s medical kit. They had IV fluids, so I started an IV. I was able to monitor the woman’s blood pressure. They had the usual drugs for doing ACLS [advanced cardiac life support], running the code, and things like that. But they didn’t have a suturing kit or a laceration kit. They didn’t have a scalpel. There was nothing else.
Honestly, there was a lot of panic going through my head. I started thinking about what could go wrong. I’d done an ob.gyn. rotation in medical school and delivered seven babies before it was over. But a plane – even the first-class cabin – is in no way, shape, or form like a delivery room. I was really scared she would hemorrhage out or something.
So, internally, I was having a meltdown. Sij, you have to keep it together right now, because there’s no one else that’s going to do this. Just give it your best shot. And that’s what I did.
I asked the pilot to go to an altitude that would minimize any turbulence, and we were very lucky that the notorious North Atlantic air wasn’t choppy.
More luck: This was the passenger’s second baby, and I was counting on second deliveries being easier. The pediatrician, the flight attendants, and I came together as a team. Two flight attendants had given birth before, so they held the patient’s hand and guided her to push. I was “downstairs” waiting to catch.
She was in some pain. At this point, usually people get an epidural. I kept thinking about what drugs were safe in pregnancy, but I wasn’t sure. I don’t know if they even had morphine or anything on the plane. We gave her some Tylenol.
It didn’t take long. After about 30 minutes, the baby’s head emerged. I was able to navigate it out, avoiding any shoulder dystocia. There’s a certain technique that you learn in medical school, which thankfully came back to me. I caught it – it was a boy born right there in a first-class seat.
I gave him to the pediatrician, and she did the Apgar score, calculating his breathing and appearance. Then my job was to make sure there were no postpartum complications.
I ended up using a piece of string in the kit to tie around the umbilical cord, and then I cut it with scissors. After that, the woman was able to deliver the placenta. She did have some vaginal bleeding, but that resolved by just holding pressure.
The baby was fine. Mom was doing great. No complications. It was a miracle. I was the right person at the right place at the right time. I just think it was something from God.
The pilot made an announcement, “We’re en route to JFK, and there’s an additional passenger on this plane now.”
When we landed, I had very little time because I had to catch my flight to Cleveland. I didn’t even process what had happened.
A few days later, I got this package from Air France with a very expensive bottle of champagne along with a travel voucher. I heard from the mom by email – she and baby were doing fine.
Eventually, the media relations people at Cleveland Clinic heard about the incident, and it became a story that went viral. That was very weird, because I’m usually someone who’s private. All through my residency, people would introduce me with, “Remember that guy who delivered a baby on a plane? That’s him.”
I’m so thankful for everyone who was on that team. It was very beautiful because it was people from different cultures, backgrounds, and faiths who came together to achieve something so miraculous. The patient was Nigerian. The flight attendants were French. The pediatrician and I were American.
That just shows you the power of teamwork and how humanity can come together. Medicine, surgery – everything, in fact – is a team sport.
Sij Hemal, MD, graduated from urology residency at the Cleveland Clinic and is currently a robotic urologic oncology and minimally invasive surgery fellow at the University of Southern California, Los Angeles.
A version of this article originally appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
In December 2017, I was a second-year urology resident at Cleveland Clinic. I’d gone to New Delhi to attend my best friend’s wedding. My flight back was New Delhi to Paris to JFK via Air France. I didn’t sleep on the first flight. So, on the second, I wanted to get some rest, because I had to go back to work the next day. I put on a movie and tried to snooze. As the saying goes in residency, you sleep when you can.
About 3 hours later, a flight attendant made an announcement in French, but I didn’t really hear it. Then they announced in English that they needed a physician. I noticed some flight attendants walking frantically around the economy cabin asking, “Is there a doctor on the plane?” Turns out there were two – the woman sitting next to me happened to be a pediatrician with Doctors Without Borders. I volunteered.
The flight attendant told me a woman was having abdominal pain. I thought it would be something straightforward. Usually, medical emergencies on planes involve chest pain or a panic attack or a vasovagal syncopal episode. Well, I was in for a ride that day.
She said she was 37 or 38 weeks in. I said, “Okay, if you’re having this significant abdominal pain, then I need to examine you.” So we decided to move her to the first-class cabin, which was empty (I never did ask why – but it was good we had room to work).
Next step, I went back to my seat and asked the pediatrician if she could assist. My plan was to simply get the passenger through the flight, and as soon as we landed, she would go to the hospital.
There was room to lie down in first class. The pediatrician and I examined her, and she appeared fine. She was traveling with her 4-year-old daughter, and the flight attendants were taking care of her. Everything was okay.
The pilot came back and asked if we would need an emergency landing. I asked him how far it was to JFK – 4 hours. He said the closest place to land would be the Azores Islands, which is Portuguese territory, 2 hours away.
The problem: Even if we made it to the Azores, the hospital there was a very basic facility with no obstetric care available. And by the time the ambulance picked her up and got her there, it would still be 2 or 3 hours total. I said, “No, let’s just observe and continue our course.” Inside my head, I was hoping and praying to God that was the right decision.
Within an hour, everything changed.
The woman’s pain got worse, and she started having contractions. Then her water broke.
Things progressed quickly from there. The contractions progressively got worse and worse. The interval between them got smaller and smaller. The next time we examined her, we could see the baby’s head beginning to crown.
At that point, we had to decide – are we going to deliver? We were in the middle of the North Atlantic Ocean. There was nothing around us. We were 35,000 feet in the air, surrounded by blue.
The crew wanted us to sign a Good Samaritan agreement. So, we did that. And then I said, “Okay, let’s just go for it.”
We got the plane’s medical kit. They had IV fluids, so I started an IV. I was able to monitor the woman’s blood pressure. They had the usual drugs for doing ACLS [advanced cardiac life support], running the code, and things like that. But they didn’t have a suturing kit or a laceration kit. They didn’t have a scalpel. There was nothing else.
Honestly, there was a lot of panic going through my head. I started thinking about what could go wrong. I’d done an ob.gyn. rotation in medical school and delivered seven babies before it was over. But a plane – even the first-class cabin – is in no way, shape, or form like a delivery room. I was really scared she would hemorrhage out or something.
So, internally, I was having a meltdown. Sij, you have to keep it together right now, because there’s no one else that’s going to do this. Just give it your best shot. And that’s what I did.
I asked the pilot to go to an altitude that would minimize any turbulence, and we were very lucky that the notorious North Atlantic air wasn’t choppy.
More luck: This was the passenger’s second baby, and I was counting on second deliveries being easier. The pediatrician, the flight attendants, and I came together as a team. Two flight attendants had given birth before, so they held the patient’s hand and guided her to push. I was “downstairs” waiting to catch.
She was in some pain. At this point, usually people get an epidural. I kept thinking about what drugs were safe in pregnancy, but I wasn’t sure. I don’t know if they even had morphine or anything on the plane. We gave her some Tylenol.
It didn’t take long. After about 30 minutes, the baby’s head emerged. I was able to navigate it out, avoiding any shoulder dystocia. There’s a certain technique that you learn in medical school, which thankfully came back to me. I caught it – it was a boy born right there in a first-class seat.
I gave him to the pediatrician, and she did the Apgar score, calculating his breathing and appearance. Then my job was to make sure there were no postpartum complications.
I ended up using a piece of string in the kit to tie around the umbilical cord, and then I cut it with scissors. After that, the woman was able to deliver the placenta. She did have some vaginal bleeding, but that resolved by just holding pressure.
The baby was fine. Mom was doing great. No complications. It was a miracle. I was the right person at the right place at the right time. I just think it was something from God.
The pilot made an announcement, “We’re en route to JFK, and there’s an additional passenger on this plane now.”
When we landed, I had very little time because I had to catch my flight to Cleveland. I didn’t even process what had happened.
A few days later, I got this package from Air France with a very expensive bottle of champagne along with a travel voucher. I heard from the mom by email – she and baby were doing fine.
Eventually, the media relations people at Cleveland Clinic heard about the incident, and it became a story that went viral. That was very weird, because I’m usually someone who’s private. All through my residency, people would introduce me with, “Remember that guy who delivered a baby on a plane? That’s him.”
I’m so thankful for everyone who was on that team. It was very beautiful because it was people from different cultures, backgrounds, and faiths who came together to achieve something so miraculous. The patient was Nigerian. The flight attendants were French. The pediatrician and I were American.
That just shows you the power of teamwork and how humanity can come together. Medicine, surgery – everything, in fact – is a team sport.
Sij Hemal, MD, graduated from urology residency at the Cleveland Clinic and is currently a robotic urologic oncology and minimally invasive surgery fellow at the University of Southern California, Los Angeles.
A version of this article originally appeared on Medscape.com.
Emergencies happen anywhere, anytime, and sometimes medical professionals find themselves in situations where they are the only ones who can help. Is There a Doctor in the House? is a series telling these stories.
In December 2017, I was a second-year urology resident at Cleveland Clinic. I’d gone to New Delhi to attend my best friend’s wedding. My flight back was New Delhi to Paris to JFK via Air France. I didn’t sleep on the first flight. So, on the second, I wanted to get some rest, because I had to go back to work the next day. I put on a movie and tried to snooze. As the saying goes in residency, you sleep when you can.
About 3 hours later, a flight attendant made an announcement in French, but I didn’t really hear it. Then they announced in English that they needed a physician. I noticed some flight attendants walking frantically around the economy cabin asking, “Is there a doctor on the plane?” Turns out there were two – the woman sitting next to me happened to be a pediatrician with Doctors Without Borders. I volunteered.
The flight attendant told me a woman was having abdominal pain. I thought it would be something straightforward. Usually, medical emergencies on planes involve chest pain or a panic attack or a vasovagal syncopal episode. Well, I was in for a ride that day.
She said she was 37 or 38 weeks in. I said, “Okay, if you’re having this significant abdominal pain, then I need to examine you.” So we decided to move her to the first-class cabin, which was empty (I never did ask why – but it was good we had room to work).
Next step, I went back to my seat and asked the pediatrician if she could assist. My plan was to simply get the passenger through the flight, and as soon as we landed, she would go to the hospital.
There was room to lie down in first class. The pediatrician and I examined her, and she appeared fine. She was traveling with her 4-year-old daughter, and the flight attendants were taking care of her. Everything was okay.
The pilot came back and asked if we would need an emergency landing. I asked him how far it was to JFK – 4 hours. He said the closest place to land would be the Azores Islands, which is Portuguese territory, 2 hours away.
The problem: Even if we made it to the Azores, the hospital there was a very basic facility with no obstetric care available. And by the time the ambulance picked her up and got her there, it would still be 2 or 3 hours total. I said, “No, let’s just observe and continue our course.” Inside my head, I was hoping and praying to God that was the right decision.
Within an hour, everything changed.
The woman’s pain got worse, and she started having contractions. Then her water broke.
Things progressed quickly from there. The contractions progressively got worse and worse. The interval between them got smaller and smaller. The next time we examined her, we could see the baby’s head beginning to crown.
At that point, we had to decide – are we going to deliver? We were in the middle of the North Atlantic Ocean. There was nothing around us. We were 35,000 feet in the air, surrounded by blue.
The crew wanted us to sign a Good Samaritan agreement. So, we did that. And then I said, “Okay, let’s just go for it.”
We got the plane’s medical kit. They had IV fluids, so I started an IV. I was able to monitor the woman’s blood pressure. They had the usual drugs for doing ACLS [advanced cardiac life support], running the code, and things like that. But they didn’t have a suturing kit or a laceration kit. They didn’t have a scalpel. There was nothing else.
Honestly, there was a lot of panic going through my head. I started thinking about what could go wrong. I’d done an ob.gyn. rotation in medical school and delivered seven babies before it was over. But a plane – even the first-class cabin – is in no way, shape, or form like a delivery room. I was really scared she would hemorrhage out or something.
So, internally, I was having a meltdown. Sij, you have to keep it together right now, because there’s no one else that’s going to do this. Just give it your best shot. And that’s what I did.
I asked the pilot to go to an altitude that would minimize any turbulence, and we were very lucky that the notorious North Atlantic air wasn’t choppy.
More luck: This was the passenger’s second baby, and I was counting on second deliveries being easier. The pediatrician, the flight attendants, and I came together as a team. Two flight attendants had given birth before, so they held the patient’s hand and guided her to push. I was “downstairs” waiting to catch.
She was in some pain. At this point, usually people get an epidural. I kept thinking about what drugs were safe in pregnancy, but I wasn’t sure. I don’t know if they even had morphine or anything on the plane. We gave her some Tylenol.
It didn’t take long. After about 30 minutes, the baby’s head emerged. I was able to navigate it out, avoiding any shoulder dystocia. There’s a certain technique that you learn in medical school, which thankfully came back to me. I caught it – it was a boy born right there in a first-class seat.
I gave him to the pediatrician, and she did the Apgar score, calculating his breathing and appearance. Then my job was to make sure there were no postpartum complications.
I ended up using a piece of string in the kit to tie around the umbilical cord, and then I cut it with scissors. After that, the woman was able to deliver the placenta. She did have some vaginal bleeding, but that resolved by just holding pressure.
The baby was fine. Mom was doing great. No complications. It was a miracle. I was the right person at the right place at the right time. I just think it was something from God.
The pilot made an announcement, “We’re en route to JFK, and there’s an additional passenger on this plane now.”
When we landed, I had very little time because I had to catch my flight to Cleveland. I didn’t even process what had happened.
A few days later, I got this package from Air France with a very expensive bottle of champagne along with a travel voucher. I heard from the mom by email – she and baby were doing fine.
Eventually, the media relations people at Cleveland Clinic heard about the incident, and it became a story that went viral. That was very weird, because I’m usually someone who’s private. All through my residency, people would introduce me with, “Remember that guy who delivered a baby on a plane? That’s him.”
I’m so thankful for everyone who was on that team. It was very beautiful because it was people from different cultures, backgrounds, and faiths who came together to achieve something so miraculous. The patient was Nigerian. The flight attendants were French. The pediatrician and I were American.
That just shows you the power of teamwork and how humanity can come together. Medicine, surgery – everything, in fact – is a team sport.
Sij Hemal, MD, graduated from urology residency at the Cleveland Clinic and is currently a robotic urologic oncology and minimally invasive surgery fellow at the University of Southern California, Los Angeles.
A version of this article originally appeared on Medscape.com.