User login
LGBTQ+ teens in homophobic high schools
I am a psychiatrist now but had another life teaching English in public high school for 17 years. My teaching life, in which I was an openly gay teacher, spanned 2001-2018 and was divided between two urban California schools – in Berkeley and San Leandro. I came out by responding honestly to student questions about whether I had a girlfriend, and what I did over the weekend. At Berkeley High my openness wasn’t an issue at all. The school had a vibrant Gay Straight Alliance/GSA for years, there were many openly gay staff and many openly gay students. No students felt the need to come out to me in search of a gay mentor.
Two years later, I began teaching in San Leandro, 20 miles away, and it was a lesson in how even the San Francisco Bay Area, an LGBTQ+ bastion, could harbor homophobia. When I was hired in 2003, San Leandro High had one openly gay teacher, Q. I quickly realized how much braver his coming out was compared with mine in Berkeley.
In San Leandro, gay slurs were heard nonstop in the hallways, no students were out, and by the end of my first year Q had quit, confiding in me that he couldn’t handle the homophobic harassment from students anymore. There was no GSA. A few years ago, two lesbians had held hands during lunch and inspired the wrath of a group of parents who advocated for their expulsion. In response, a teacher tried to introduce gay sensitivity training into his class and the same group of parents tried to get him fired. He was reprimanded by the principal, he countersued in a case that went all the way to the California Supreme Court, and won. Comparing these two local high schools reinforced to me how visibility really matters in creating a childhood experience that is nurturing versus traumatizing.1
Two Chinese girls in love
N and T were two Chinese girls who grew up in San Leandro. They went to the same elementary school and had crushes on each other since then. In their junior year, they joined our first student GSA, becoming president and vice-president. They were out. And, of course, they must’ve known that their families, who would not have been supportive, would become aware. I remember sitting at an outdoor concert when I got a text from N warning me her father had found out and blamed me for having corrupted her. He planned on coming to school to demand I be fired. And such was the unrelenting pressure that N and T faced every time they went home from school and sat at their dinner tables. Eventually, they broke up. They didn’t do so tearfully, but more wearily.
This story illustrates how difficult it is for love between two LGBTQ+ teens to be nurtured. Love in youth can already be volatile because of the lack of emotional regulation and experience. The questioning of identity and the threat of family disintegration at a time when these teens do not have the economic means to protect themselves makes love dangerous. It is no wonder that gay teens are at increased risk for homelessness.2
The family incident that led to the girls’ breakup reveals how culture affects homophobic pressure. N resisted her parents’ disapproval for months, but she capitulated when her father had a heart attack and blamed it on her. “And it’s true,” N confided. “After my parents found out, they were continually stressed. I could see it affect their health. And it breaks my heart to see my dad in the hospital.”
For N, she had not capitulated from fear, but perhaps because of filial piety, or one’s obligation to protect one’s parent. It was a choice between two heartbreaks. Double minorities, like N and T, face a double threat and often can find no safe place. One of my patients who is gay and Black put it best: “It’s like being beaten up at school only to come home to another beating.” This double threat is evidenced by the higher suicide risk of ethnicities who are LGBTQ+ relative to their white counterparts.3
The confusion of a gay athlete
R was a star point guard, a senior who had secured an athletic scholarship, and was recognized as the best athlete in our county. A popular boy, he flaunted his physique and flirted with all the girls. And then when he was enrolled in my class, he began flirting with all the boys, too. There was gossip that R was bisexual. Then one day, not unexpectedly, he came out to me as gay. He admitted he only flirted with girls for his reputation.
By this time many students had come out to me but he flirted with me with his revelation. I corrected him and warned him unequivocally that it was inappropriate but I was worried because I knew he had placed his trust in me. I also knew he came from a homophobic family that was violent – his father had attacked him physically at a school game and our coaches had to pull him off.
Instinctively, I felt I had to have a witness so I confided in another teacher and documented the situation meticulously. Then, one day, just as I feared, he went too far. He stayed after class and said he wanted to show me something on his phone. And that something turned out to be a picture of himself naked. I immediately confiscated the phone and reported it to the administration. This was not how I wanted him to come out: His family notified by the police that he had sexually harassed his teacher, expulsion pending, and scholarship inevitably revoked. Fortunately, we did find a resolution that restored R’s future.
Let’s examine the circumstances that could’ve informed his transgressive behavior. If we consider sexual harassment a form of bullying, R’s history of having a father who publicly bullied him – and may have bullied others in front of him – is a known risk factor.4 It is also common knowledge that organized team sports were and still are a bastion of homophobia and that gay athletes had to accept a culture of explicit homophobia.5
So, it is not hard to understand the constant public pressures that R faced in addition to those from his family. Let’s also consider that appropriate sexual behaviors are not something we are born with, but something that is learned. Of course, inappropriate sexual behavior also happens in the heterosexual world. But heterosexual sexual behavior often has more accepted paths of trial and error. Children experiment with these behaviors and are corrected by adults and older peers as they mature.
However, for homosexual behaviors, there is not usually the fine-tuning about what is appropriate.
Summary
An educational environment where LGBTQ+ persons are highly visible and accepted is a more nurturing environment for LGBTQ teens than one that is not. Specific subcultures within the LGBTQ population involving race, culture, gender, and athletics modulate the experience of coming out and the nature of homophobic oppression.
Dr. Nguyen is a first-year psychiatry resident at the University of San Francisco School of Medicine at Fresno.
References
1. Kosciw JG et al. The effect of negative school climate on academic outcomes for LGBT youth and the role of in-school supports. J Sch Violence. 2013;12(1):45-63.
2. Center for American Progress. Gay and Transgender Youth Homelessness by the Numbers. June 21, 2010).
3. O’Donnell S et al. Increased risk of suicide attempts among Black and Latino lesbians, gay men, and bisexuals. Am J Public Health. 2011;101(6):1055-9.
4. Farrington D and Baldry A. Individual risk factors for school bullying. J Aggress Confl Peace Res. 2010 Jan;2(1):4-16.
5. Anderson E. Openly gay athletes: Contesting hegemonic masculinity in a homophobic environment Gend Soc. 2002 Dec:16(6):860-77.
I am a psychiatrist now but had another life teaching English in public high school for 17 years. My teaching life, in which I was an openly gay teacher, spanned 2001-2018 and was divided between two urban California schools – in Berkeley and San Leandro. I came out by responding honestly to student questions about whether I had a girlfriend, and what I did over the weekend. At Berkeley High my openness wasn’t an issue at all. The school had a vibrant Gay Straight Alliance/GSA for years, there were many openly gay staff and many openly gay students. No students felt the need to come out to me in search of a gay mentor.
Two years later, I began teaching in San Leandro, 20 miles away, and it was a lesson in how even the San Francisco Bay Area, an LGBTQ+ bastion, could harbor homophobia. When I was hired in 2003, San Leandro High had one openly gay teacher, Q. I quickly realized how much braver his coming out was compared with mine in Berkeley.
In San Leandro, gay slurs were heard nonstop in the hallways, no students were out, and by the end of my first year Q had quit, confiding in me that he couldn’t handle the homophobic harassment from students anymore. There was no GSA. A few years ago, two lesbians had held hands during lunch and inspired the wrath of a group of parents who advocated for their expulsion. In response, a teacher tried to introduce gay sensitivity training into his class and the same group of parents tried to get him fired. He was reprimanded by the principal, he countersued in a case that went all the way to the California Supreme Court, and won. Comparing these two local high schools reinforced to me how visibility really matters in creating a childhood experience that is nurturing versus traumatizing.1
Two Chinese girls in love
N and T were two Chinese girls who grew up in San Leandro. They went to the same elementary school and had crushes on each other since then. In their junior year, they joined our first student GSA, becoming president and vice-president. They were out. And, of course, they must’ve known that their families, who would not have been supportive, would become aware. I remember sitting at an outdoor concert when I got a text from N warning me her father had found out and blamed me for having corrupted her. He planned on coming to school to demand I be fired. And such was the unrelenting pressure that N and T faced every time they went home from school and sat at their dinner tables. Eventually, they broke up. They didn’t do so tearfully, but more wearily.
This story illustrates how difficult it is for love between two LGBTQ+ teens to be nurtured. Love in youth can already be volatile because of the lack of emotional regulation and experience. The questioning of identity and the threat of family disintegration at a time when these teens do not have the economic means to protect themselves makes love dangerous. It is no wonder that gay teens are at increased risk for homelessness.2
The family incident that led to the girls’ breakup reveals how culture affects homophobic pressure. N resisted her parents’ disapproval for months, but she capitulated when her father had a heart attack and blamed it on her. “And it’s true,” N confided. “After my parents found out, they were continually stressed. I could see it affect their health. And it breaks my heart to see my dad in the hospital.”
For N, she had not capitulated from fear, but perhaps because of filial piety, or one’s obligation to protect one’s parent. It was a choice between two heartbreaks. Double minorities, like N and T, face a double threat and often can find no safe place. One of my patients who is gay and Black put it best: “It’s like being beaten up at school only to come home to another beating.” This double threat is evidenced by the higher suicide risk of ethnicities who are LGBTQ+ relative to their white counterparts.3
The confusion of a gay athlete
R was a star point guard, a senior who had secured an athletic scholarship, and was recognized as the best athlete in our county. A popular boy, he flaunted his physique and flirted with all the girls. And then when he was enrolled in my class, he began flirting with all the boys, too. There was gossip that R was bisexual. Then one day, not unexpectedly, he came out to me as gay. He admitted he only flirted with girls for his reputation.
By this time many students had come out to me but he flirted with me with his revelation. I corrected him and warned him unequivocally that it was inappropriate but I was worried because I knew he had placed his trust in me. I also knew he came from a homophobic family that was violent – his father had attacked him physically at a school game and our coaches had to pull him off.
Instinctively, I felt I had to have a witness so I confided in another teacher and documented the situation meticulously. Then, one day, just as I feared, he went too far. He stayed after class and said he wanted to show me something on his phone. And that something turned out to be a picture of himself naked. I immediately confiscated the phone and reported it to the administration. This was not how I wanted him to come out: His family notified by the police that he had sexually harassed his teacher, expulsion pending, and scholarship inevitably revoked. Fortunately, we did find a resolution that restored R’s future.
Let’s examine the circumstances that could’ve informed his transgressive behavior. If we consider sexual harassment a form of bullying, R’s history of having a father who publicly bullied him – and may have bullied others in front of him – is a known risk factor.4 It is also common knowledge that organized team sports were and still are a bastion of homophobia and that gay athletes had to accept a culture of explicit homophobia.5
So, it is not hard to understand the constant public pressures that R faced in addition to those from his family. Let’s also consider that appropriate sexual behaviors are not something we are born with, but something that is learned. Of course, inappropriate sexual behavior also happens in the heterosexual world. But heterosexual sexual behavior often has more accepted paths of trial and error. Children experiment with these behaviors and are corrected by adults and older peers as they mature.
However, for homosexual behaviors, there is not usually the fine-tuning about what is appropriate.
Summary
An educational environment where LGBTQ+ persons are highly visible and accepted is a more nurturing environment for LGBTQ teens than one that is not. Specific subcultures within the LGBTQ population involving race, culture, gender, and athletics modulate the experience of coming out and the nature of homophobic oppression.
Dr. Nguyen is a first-year psychiatry resident at the University of San Francisco School of Medicine at Fresno.
References
1. Kosciw JG et al. The effect of negative school climate on academic outcomes for LGBT youth and the role of in-school supports. J Sch Violence. 2013;12(1):45-63.
2. Center for American Progress. Gay and Transgender Youth Homelessness by the Numbers. June 21, 2010).
3. O’Donnell S et al. Increased risk of suicide attempts among Black and Latino lesbians, gay men, and bisexuals. Am J Public Health. 2011;101(6):1055-9.
4. Farrington D and Baldry A. Individual risk factors for school bullying. J Aggress Confl Peace Res. 2010 Jan;2(1):4-16.
5. Anderson E. Openly gay athletes: Contesting hegemonic masculinity in a homophobic environment Gend Soc. 2002 Dec:16(6):860-77.
I am a psychiatrist now but had another life teaching English in public high school for 17 years. My teaching life, in which I was an openly gay teacher, spanned 2001-2018 and was divided between two urban California schools – in Berkeley and San Leandro. I came out by responding honestly to student questions about whether I had a girlfriend, and what I did over the weekend. At Berkeley High my openness wasn’t an issue at all. The school had a vibrant Gay Straight Alliance/GSA for years, there were many openly gay staff and many openly gay students. No students felt the need to come out to me in search of a gay mentor.
Two years later, I began teaching in San Leandro, 20 miles away, and it was a lesson in how even the San Francisco Bay Area, an LGBTQ+ bastion, could harbor homophobia. When I was hired in 2003, San Leandro High had one openly gay teacher, Q. I quickly realized how much braver his coming out was compared with mine in Berkeley.
In San Leandro, gay slurs were heard nonstop in the hallways, no students were out, and by the end of my first year Q had quit, confiding in me that he couldn’t handle the homophobic harassment from students anymore. There was no GSA. A few years ago, two lesbians had held hands during lunch and inspired the wrath of a group of parents who advocated for their expulsion. In response, a teacher tried to introduce gay sensitivity training into his class and the same group of parents tried to get him fired. He was reprimanded by the principal, he countersued in a case that went all the way to the California Supreme Court, and won. Comparing these two local high schools reinforced to me how visibility really matters in creating a childhood experience that is nurturing versus traumatizing.1
Two Chinese girls in love
N and T were two Chinese girls who grew up in San Leandro. They went to the same elementary school and had crushes on each other since then. In their junior year, they joined our first student GSA, becoming president and vice-president. They were out. And, of course, they must’ve known that their families, who would not have been supportive, would become aware. I remember sitting at an outdoor concert when I got a text from N warning me her father had found out and blamed me for having corrupted her. He planned on coming to school to demand I be fired. And such was the unrelenting pressure that N and T faced every time they went home from school and sat at their dinner tables. Eventually, they broke up. They didn’t do so tearfully, but more wearily.
This story illustrates how difficult it is for love between two LGBTQ+ teens to be nurtured. Love in youth can already be volatile because of the lack of emotional regulation and experience. The questioning of identity and the threat of family disintegration at a time when these teens do not have the economic means to protect themselves makes love dangerous. It is no wonder that gay teens are at increased risk for homelessness.2
The family incident that led to the girls’ breakup reveals how culture affects homophobic pressure. N resisted her parents’ disapproval for months, but she capitulated when her father had a heart attack and blamed it on her. “And it’s true,” N confided. “After my parents found out, they were continually stressed. I could see it affect their health. And it breaks my heart to see my dad in the hospital.”
For N, she had not capitulated from fear, but perhaps because of filial piety, or one’s obligation to protect one’s parent. It was a choice between two heartbreaks. Double minorities, like N and T, face a double threat and often can find no safe place. One of my patients who is gay and Black put it best: “It’s like being beaten up at school only to come home to another beating.” This double threat is evidenced by the higher suicide risk of ethnicities who are LGBTQ+ relative to their white counterparts.3
The confusion of a gay athlete
R was a star point guard, a senior who had secured an athletic scholarship, and was recognized as the best athlete in our county. A popular boy, he flaunted his physique and flirted with all the girls. And then when he was enrolled in my class, he began flirting with all the boys, too. There was gossip that R was bisexual. Then one day, not unexpectedly, he came out to me as gay. He admitted he only flirted with girls for his reputation.
By this time many students had come out to me but he flirted with me with his revelation. I corrected him and warned him unequivocally that it was inappropriate but I was worried because I knew he had placed his trust in me. I also knew he came from a homophobic family that was violent – his father had attacked him physically at a school game and our coaches had to pull him off.
Instinctively, I felt I had to have a witness so I confided in another teacher and documented the situation meticulously. Then, one day, just as I feared, he went too far. He stayed after class and said he wanted to show me something on his phone. And that something turned out to be a picture of himself naked. I immediately confiscated the phone and reported it to the administration. This was not how I wanted him to come out: His family notified by the police that he had sexually harassed his teacher, expulsion pending, and scholarship inevitably revoked. Fortunately, we did find a resolution that restored R’s future.
Let’s examine the circumstances that could’ve informed his transgressive behavior. If we consider sexual harassment a form of bullying, R’s history of having a father who publicly bullied him – and may have bullied others in front of him – is a known risk factor.4 It is also common knowledge that organized team sports were and still are a bastion of homophobia and that gay athletes had to accept a culture of explicit homophobia.5
So, it is not hard to understand the constant public pressures that R faced in addition to those from his family. Let’s also consider that appropriate sexual behaviors are not something we are born with, but something that is learned. Of course, inappropriate sexual behavior also happens in the heterosexual world. But heterosexual sexual behavior often has more accepted paths of trial and error. Children experiment with these behaviors and are corrected by adults and older peers as they mature.
However, for homosexual behaviors, there is not usually the fine-tuning about what is appropriate.
Summary
An educational environment where LGBTQ+ persons are highly visible and accepted is a more nurturing environment for LGBTQ teens than one that is not. Specific subcultures within the LGBTQ population involving race, culture, gender, and athletics modulate the experience of coming out and the nature of homophobic oppression.
Dr. Nguyen is a first-year psychiatry resident at the University of San Francisco School of Medicine at Fresno.
References
1. Kosciw JG et al. The effect of negative school climate on academic outcomes for LGBT youth and the role of in-school supports. J Sch Violence. 2013;12(1):45-63.
2. Center for American Progress. Gay and Transgender Youth Homelessness by the Numbers. June 21, 2010).
3. O’Donnell S et al. Increased risk of suicide attempts among Black and Latino lesbians, gay men, and bisexuals. Am J Public Health. 2011;101(6):1055-9.
4. Farrington D and Baldry A. Individual risk factors for school bullying. J Aggress Confl Peace Res. 2010 Jan;2(1):4-16.
5. Anderson E. Openly gay athletes: Contesting hegemonic masculinity in a homophobic environment Gend Soc. 2002 Dec:16(6):860-77.
Cultivating strength: Psychological well-being after nonfatal suicide attempts
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
A study of three separate nationally representative samples of nearly 9,000 U.S. military veterans found psychological well-being – defined in terms of having a high sense of purpose, social connectedness, and happiness – to be significantly diminished among veteran suicide attempt survivors relative to nonattempters, even decades after their last attempt.1
Despite the trend toward diminished well-being, many veterans who survived a suicide attempt reported average to optimal levels of well-being. Specifically, 52%-60% of veterans reporting a prior suicide attempt also reported experiencing as much purpose, social connection, and happiness as veterans without a suicide attempt history. Remarkably, a small subset (2-7%) of veteran attempt survivors even reported higher levels of well-being than veterans without a suicide attempt history.
Thus,
These data are notable because, in 2021, approximately 1.4 million U.S. adults made a nonfatal suicide attempt. Historically, suicide research has understandably emphasized the study of risk factors that increase the likelihood that someone dies by suicide. Given that a prior suicide attempt is among the top risk factors for suicide, virtually all research on suicide attempt survivors has focused on their elevated risk for future suicidality. Yet, 9 out of 10 people who have made a nonfatal suicide attempt do not go on to die by suicide. It is thus critical to investigate the quality of life of the millions of suicide attempt survivors.
To date, we know little about a question keenly important to suicide attempt survivors and their loved ones: What is the possibility of rebuilding a meaningful, high-quality life after a suicide attempt?
In addition to reporting on the prevalence of high levels of psychological well-being after a nonfatal suicide attempt, it is pivotal to investigate factors that may help facilitate this outcome. To that end, we identified personal characteristics associated with high levels of well-being. Notably, it was malleable psychological strengths such as optimism and a curious mindset, more than the mere absence of symptoms, that were linked to higher levels of well-being among veteran suicide attempt survivors.
Current suicide prevention interventions and treatments, which often focus on mitigating immediate suicide risk by treating symptoms, may be overlooking the importance of cultivating and building psychological strengths that may help promote greater well-being and enriched lives. Moreover, treatments that emphasize such strengths might be particularly fruitful in mitigating suicide risk in veterans, as veterans may be more receptive to prevention and treatment initiatives that embrace the cultivation and bolstering of strengths that are inherent in military culture and values, such as resilience and perseverance in the face of life challenges.2
One notable caveat to this study is that the data were cross-sectional, meaning they were collected at a single time point. As such, the authors cannot conclude that factors such as curiosity necessarily caused higher levels of well-being in veterans, as opposed to well-being causing higher levels of curiosity.
Similarly, while one can infer that psychological well-being was near-absent at the time of a suicide attempt, well-being of attempt survivors was not assessed before their attempt. Longitudinal studies that follow attempt survivors over time are needed to understand how well-being changes over time for suicide attempt survivors and the causal chain in what predicts that change.
Nevertheless, the results of this large, multicohort study serve as an important first step toward a more comprehensive view of prognosis after a suicide attempt. Just as the process that leads to a suicide attempt is complex, so too is the process of recovery after an attempt. While this study provides sound estimates of well-being outcomes and some possible candidates that might facilitate these outcomes, a critical next step for future research is to replicate and extend these findings. To do so, it is pivotal to extend the assessment scope beyond symptom-based measures and include measures of well-being.
Additionally, the investment in resources into longer-term examinations following suicide attempts is essential to understand different pathways toward achieving greater well-being. Providing hope is vital and potentially lifesaving, as one of the most common experiences reported before a suicide attempt is an unremitting sense of hopelessness. Continued research on well-being has the potential to impart a more balanced, nuanced prognosis after a suicide attempt that challenges perceptions of an invariably bleak prospect of recovery after suicidality.
Collectively, these results highlight the importance of broadening the scope of how the mental health field views and treats psychiatric difficulties to include a greater focus on recovery-based outcomes and personal strengths that help facilitate recovery from adverse life experiences such as suicide attempts.
People desire lives that they enjoy and find meaningful, and having a history of suicide attempts does not preclude the prospect of such a life. It is time that suicide research reflects the vast landscape of potential outcomes after a suicide attempt that goes beyond the prediction of future suicide risk.
Mr. Brown is a doctoral student of clinical psychology at the University of South Florida, Tampa. Dr. Rottenberg is director of the Mood and Emotion Lab and area director of the department of clinical psychology, University of South Florida.
References
1. Brown BA et al. Psychological well-being in US veterans with non-fatal suicide attempts: A multi-cohort population-based study. J Affect Disord. 2022 Oct 1;314:34-43. doi: 10.1016/j.jad.2022.07.003.
2. Bryan CJ et al. Understanding and preventing military suicide. Arch Suicide Res. 2012;16(2):95-110. doi: 10.1080/13811118.2012.667321.
Notes on direct admission of pediatric patients
Scenario: Yesterday you saw a 6-month-old infant with what appeared to be viral gastroenteritis and mild dehydration. When you called his parents today to check on his condition he was not improving despite your recommendations about his diet and oral rehydration. Should you have him brought to your office for a reevaluation, have his parents take him to the local emergency department for evaluation and probable hospital admission, or ask his parents to take him to the hospital telling them that you will call and arrange for a direct admission.
Obviously, I haven’t given you enough background information to allow you to give me an answer you are comfortable with. What time of day is it? Is it a holiday weekend? What’s the weather like? How far is it from the patient’s home to your office? To the emergency department? How is the local ED staffed? Are there hospitalists? What is their training?
Whether or not you choose to see the patient first in the office, is direct admission to the hospital an option that you are likely to choose? What steps do you take to see that it happens smoothly?
At least one-quarter of the unscheduled pediatric hospitalizations begin with a direct admission, meaning that the patients are not first evaluated in that hospital’s ED. In a recent policy statement, the American Academy of Pediatrics Committee on Hospital Care explored the pluses and minuses of direct admission and issued a list of seven recommendations. Among the concerns raised by the authors are “potential delays in initial evaluation and treatment, inconsistent admission processes, and difficulties in determining the appropriateness of patients for direct admission.” The committee makes it clear that they understand each community has it own strengths and challenges and the unique needs of each patient make it difficult to define a set of recommendations that fits all.
However, as I read through the committee’s seven recommendations, one leapt off the screen as a unifying concept that should apply in every situation. Recommendation No. 2 reads, “[There should be] clear systems of communication between members of the health care team and with families of children requiring admission.”
First, who is on this “health care team”? Are you a team member with the hospital folks – the ED nurses and doctors, the hospitalists, the floor nurses? Do you share an employer? Are you in the same town? Have your ever met them face to face? Do you do so regularly?
I assume you call the ED or the pediatric floor to arrange a direct admit? Maybe you don’t. I can recall working in situations where several infamous “local docs” would just send the patients in with a scribbled note (or not) and no phone call. Will you be speaking to folks who are even vaguely familiar with you or even your name? Do you get to speak with people who will be hands on with the patient?
Obviously, where I’m going with this is that, if you and the hospital staff are truly on the same health care team, communication should flow freely among the members and having some familiarity allows this to happen more smoothly. It can start on our end as the referring physician by making the call personally. Likewise, the receiving hospital must make frontline people available so you can speak with staff who will be working with the patient. Do you have enough information to tell the family what to expect?
Of course legible and complete records are a must. But nothing beats personal contact and a name. If you can tell a parent “I spoke to Martha, the nurse who will meet you on the floor,” that can be a giant first step forward in the healing process.
Most of us trained at hospitals that accepted direct admit patients and can remember the challenges. And most of us recall EDs that weren’t pediatric friendly. Whether our local situation favors direct admission or ED preadmission evaluation, it is our job to make the communication flow with the patient’s safety and the family’s comfort in mind.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Scenario: Yesterday you saw a 6-month-old infant with what appeared to be viral gastroenteritis and mild dehydration. When you called his parents today to check on his condition he was not improving despite your recommendations about his diet and oral rehydration. Should you have him brought to your office for a reevaluation, have his parents take him to the local emergency department for evaluation and probable hospital admission, or ask his parents to take him to the hospital telling them that you will call and arrange for a direct admission.
Obviously, I haven’t given you enough background information to allow you to give me an answer you are comfortable with. What time of day is it? Is it a holiday weekend? What’s the weather like? How far is it from the patient’s home to your office? To the emergency department? How is the local ED staffed? Are there hospitalists? What is their training?
Whether or not you choose to see the patient first in the office, is direct admission to the hospital an option that you are likely to choose? What steps do you take to see that it happens smoothly?
At least one-quarter of the unscheduled pediatric hospitalizations begin with a direct admission, meaning that the patients are not first evaluated in that hospital’s ED. In a recent policy statement, the American Academy of Pediatrics Committee on Hospital Care explored the pluses and minuses of direct admission and issued a list of seven recommendations. Among the concerns raised by the authors are “potential delays in initial evaluation and treatment, inconsistent admission processes, and difficulties in determining the appropriateness of patients for direct admission.” The committee makes it clear that they understand each community has it own strengths and challenges and the unique needs of each patient make it difficult to define a set of recommendations that fits all.
However, as I read through the committee’s seven recommendations, one leapt off the screen as a unifying concept that should apply in every situation. Recommendation No. 2 reads, “[There should be] clear systems of communication between members of the health care team and with families of children requiring admission.”
First, who is on this “health care team”? Are you a team member with the hospital folks – the ED nurses and doctors, the hospitalists, the floor nurses? Do you share an employer? Are you in the same town? Have your ever met them face to face? Do you do so regularly?
I assume you call the ED or the pediatric floor to arrange a direct admit? Maybe you don’t. I can recall working in situations where several infamous “local docs” would just send the patients in with a scribbled note (or not) and no phone call. Will you be speaking to folks who are even vaguely familiar with you or even your name? Do you get to speak with people who will be hands on with the patient?
Obviously, where I’m going with this is that, if you and the hospital staff are truly on the same health care team, communication should flow freely among the members and having some familiarity allows this to happen more smoothly. It can start on our end as the referring physician by making the call personally. Likewise, the receiving hospital must make frontline people available so you can speak with staff who will be working with the patient. Do you have enough information to tell the family what to expect?
Of course legible and complete records are a must. But nothing beats personal contact and a name. If you can tell a parent “I spoke to Martha, the nurse who will meet you on the floor,” that can be a giant first step forward in the healing process.
Most of us trained at hospitals that accepted direct admit patients and can remember the challenges. And most of us recall EDs that weren’t pediatric friendly. Whether our local situation favors direct admission or ED preadmission evaluation, it is our job to make the communication flow with the patient’s safety and the family’s comfort in mind.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Scenario: Yesterday you saw a 6-month-old infant with what appeared to be viral gastroenteritis and mild dehydration. When you called his parents today to check on his condition he was not improving despite your recommendations about his diet and oral rehydration. Should you have him brought to your office for a reevaluation, have his parents take him to the local emergency department for evaluation and probable hospital admission, or ask his parents to take him to the hospital telling them that you will call and arrange for a direct admission.
Obviously, I haven’t given you enough background information to allow you to give me an answer you are comfortable with. What time of day is it? Is it a holiday weekend? What’s the weather like? How far is it from the patient’s home to your office? To the emergency department? How is the local ED staffed? Are there hospitalists? What is their training?
Whether or not you choose to see the patient first in the office, is direct admission to the hospital an option that you are likely to choose? What steps do you take to see that it happens smoothly?
At least one-quarter of the unscheduled pediatric hospitalizations begin with a direct admission, meaning that the patients are not first evaluated in that hospital’s ED. In a recent policy statement, the American Academy of Pediatrics Committee on Hospital Care explored the pluses and minuses of direct admission and issued a list of seven recommendations. Among the concerns raised by the authors are “potential delays in initial evaluation and treatment, inconsistent admission processes, and difficulties in determining the appropriateness of patients for direct admission.” The committee makes it clear that they understand each community has it own strengths and challenges and the unique needs of each patient make it difficult to define a set of recommendations that fits all.
However, as I read through the committee’s seven recommendations, one leapt off the screen as a unifying concept that should apply in every situation. Recommendation No. 2 reads, “[There should be] clear systems of communication between members of the health care team and with families of children requiring admission.”
First, who is on this “health care team”? Are you a team member with the hospital folks – the ED nurses and doctors, the hospitalists, the floor nurses? Do you share an employer? Are you in the same town? Have your ever met them face to face? Do you do so regularly?
I assume you call the ED or the pediatric floor to arrange a direct admit? Maybe you don’t. I can recall working in situations where several infamous “local docs” would just send the patients in with a scribbled note (or not) and no phone call. Will you be speaking to folks who are even vaguely familiar with you or even your name? Do you get to speak with people who will be hands on with the patient?
Obviously, where I’m going with this is that, if you and the hospital staff are truly on the same health care team, communication should flow freely among the members and having some familiarity allows this to happen more smoothly. It can start on our end as the referring physician by making the call personally. Likewise, the receiving hospital must make frontline people available so you can speak with staff who will be working with the patient. Do you have enough information to tell the family what to expect?
Of course legible and complete records are a must. But nothing beats personal contact and a name. If you can tell a parent “I spoke to Martha, the nurse who will meet you on the floor,” that can be a giant first step forward in the healing process.
Most of us trained at hospitals that accepted direct admit patients and can remember the challenges. And most of us recall EDs that weren’t pediatric friendly. Whether our local situation favors direct admission or ED preadmission evaluation, it is our job to make the communication flow with the patient’s safety and the family’s comfort in mind.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Navigating your childcare options in a post-COVID world
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.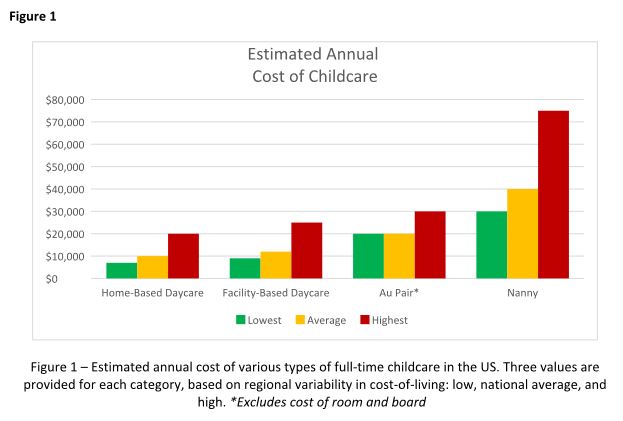
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
When we found out we were expecting our first child, we were ecstatic. Our excitement soon gave way to panic, however, as we realized that we needed a plan for childcare. As full-time physicians early in our careers, neither of us was prepared to drop to part-time or become a stay-at-home caregiver. Not knowing where to start, we turned to our friends and colleagues, and of course, the Internet, for advice on our options.
In our research, we discovered three things. First, with COVID-19, the cost of childcare has skyrocketed, and availability has decreased. Second, there are several options for childcare, each with its own benefits and drawbacks. Third, there is no one-size-fits-all solution.
Family
Using family members to provide childcare is often cost-effective and provides a familiar, supportive environment for children. Proximity does not guarantee a willingness or ability to provide long-term care, however, and it can cause strain on family relationships, lead to intrusions and boundary issues, and create feelings of obligation and guilt. It is important to have very honest, up-front discussions with family members about hopes and expectations if this is your childcare plan.
Daycare, facility-based
Daycare centers are commercial facilities that offer care to multiple children of varying ages, starting from as young as 6 weeks. They have trained professionals and provide structured activities and educational programs for children. Many daycares also provide snacks and lunch, which is included in their tuition. They are a popular choice for families seeking full-time childcare and the social and educational benefits that come with a structured setting.
Daycares also have some downsides. They usually operate during normal workday hours, from 7:00 a.m. to 6:00 p.m., which may not be convenient for physicians who work outside of these hours. Even with feasible hours, getting children dressed, ready, and dropped off each morning could add significant time and stress to your morning routine. Additionally, most daycares have policies that prohibit attendance if a child is sick or febrile, which is a common occurrence, particularly for daycare kids. In case of an illness outbreak, the daycare may even close for several days. Both scenarios require at least one parent to take a day off or have an alternative childcare plan available on short notice.
Availability of daycare can be limited, particularly since the COVID pandemic, creating waitlists that can be several months long. Early registration, even during pregnancy, is recommended to secure a spot. It can be helpful to find out if your employer has an agreement with a specific daycare that has “physician-friendly” hours and gives waitlist priority to trainees or even attending physicians. The cost of daycare for one child is typically affordable, around $12,000 per year on average, but can be as high as $25,000 in cities with high cost of living. A sibling discount may be offered, but the cost of daycare for multiple children could still exceed in-home childcare options.1
Daycare, home-based (also known as family care centers)
Family care centers offer a home-like alternative to daycares, with smaller staff-to-child ratios and often more personalized care. They are favored by families seeking a more intimate setting. They might offer more flexible scheduling and are typically less expensive than facility-based daycares, at up to 25% lower cost.1 They may lack the same structure and educational opportunities as facility-based daycares, however, and are not subject to the same health and safety regulations.
Nannies
Nannies are professional caregivers who provide in-home childcare services. Their responsibilities may include feeding, changing, dressing, bathing, and playing with children. In some cases, they may also be expected to do light housekeeping tasks like meal preparation, laundry, and cleaning. It is common for nannies in high-demand markets to refuse to perform these additional tasks, however. Nannies are preferred by families with hectic schedules due to their flexibility. They can work early, late, or even overnight shifts, and provide care in the comfort of your home, avoiding the hassle of drop-off and pick-up times. Nannies also can provide personalized care to meet each child’s specific needs, and they can care for children who are sick or febrile.
When hiring a nanny, it is important to have a written contract outlining their expected hours, wages, benefits, and duties to prevent misunderstandings in the future. Finding a trustworthy and reliable nanny can be a challenge, and families have several options for finding one. They can post jobs on free websites and browse nanny CVs or use a fee-based nanny agency. The cost of using an agency can range from a few hundred to several thousand dollars, so it is important to ask friends and colleagues for recommendations before paying for an agency’s services.
The cost of hiring a nanny is one of its main drawbacks. Nannies typically earn $15 to $30 per hour, and if they work in the family’s home, they are typically considered “household employees” by the IRS. Household employees are entitled to overtime pay for work beyond 40 hours per week, and the employer (you!) is responsible for payroll taxes, withholding, and providing an annual W-2 tax statement.2 There are affordable online nanny payroll services that handle payroll and tax-filing to simplify the process, however. The average annual cost of a full-time nanny is around $40,000 and can be as high as $75,000 in some markets.1 A nanny-share with other families can lower costs, but it may also result in less control over the caregiver and schedule.
It is important to consult a tax professional or the IRS for guidance on nanny wages, taxes, and payroll, as a nanny might rarely be considered an “independent contractor” if they meet certain criteria.
Au pair
An au pair is a live-in childcare provider who travels to a host family’s home from a foreign country on a special J-1 visa. The goal is to provide care for children and participate in cultural exchange activities. Au pairs bring many benefits, such as cost savings compared to traditional childcare options and greater flexibility and customization. They can work up to 10 hours per day and 45 hours a week, performing tasks such as light housekeeping, meal preparation, and transportation for the children. Host families must provide a safe and comfortable living environment, including a private room, meals, and some travel and education expenses.1
The process of hiring an au pair involves working with a designated agency that matches families with applicants and sponsors the J-1 visa. The entire process can take several months, and average program fees cost around $10,000 per placement. Au pairs are hired on a 12-month J-1 visa, which can be extended for up to an additional 12 months, allowing families up to 2 years with the same au pair before needing to find a new placement.
Au pairs earn a minimum weekly stipend of $195.75, set forth by the U.S. State Department.3 Currently, au pairs are not subject to local and state wage requirements, but legal proceedings in various states have recently questioned whether au pairs should be protected under local regulations. Massachusetts has been the most progressive, explicitly protecting au pairs as domestic workers under state labor laws, raising their weekly stipend to roughly $600 to comply with state minimum wage requirements.4 The federal government is expected to provide clarity on this issue, but for the time being, au pairs remain an affordable alternative to a nanny in most states.
Conclusion
Choosing childcare is a complicated process with multiple factors to consider. Figure 1 breaks down the estimated annual cost of each of the options outlined above for a single child in low, average, and high cost-of-living areas. But your decision likely hinges on much more than just cost, and may include family dynamics, scheduling needs, and personal preferences. Gather as much advice and information as possible, but remember to trust your instincts and make the decision that works best for your family. At the end of the day, what matters most is the happiness and well-being of your child.
Dr. Hathorn and Dr. Creighton are married, and both work full-time with a 1-year-old child. Dr. Hathorn is a bariatric and advanced therapeutic endoscopist at the University of North Carolina at Chapel Hill. Dr. Creighton is an anesthesiologist at UNC Chapel Hill. Neither reported any conflicts of interest.
References
1. Care.com. This is how much childcare costs in 2022. 2022 Jun 15.
2. Internal Revenue Service. Publication 926 - Household Employer’s Tax Guide 2023.
3. U.S. Department of State. Au Pair.
4. Commonwealth of Massachusetts. Domestic workers.
Disclaimer
The financial and tax information presented in this article are believed to be true and accurate at the time of writing. However, it’s important to note that tax laws and regulations are subject to change. The authors are not certified financial advisers or tax specialists. It is recommended to seek verification from a local tax expert or the Internal Revenue Service to discuss your specific situation.
From private practice to academic medicine: My journey and lessons learned
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Loyalty.
This is a quality that I value in relationships. Loyalty was a significant factor contributing to my postfellowship commitment to private practice. In 2001, I graduated from physician assistant school and accepted a job with a private practice GI group in Omaha. I was fortunate to work with supportive gastroenterologists who encouraged me to pursue further training after I expressed an interest in medical school. My goal was to become a gastroenterologist but like every medical student, I would keep an open mind. My decision did not waver, and the support from my first mentors continued. As I graduated from fellowship in 2014, I gravitated toward the same private practice largely based on loyalty and my experience as a PA.

COURTESY AMERICAN GASTROENTEROLOGICAL ASSOCIATION
My experience in private practice was positive. My focus at that time and currently is clinical medicine with a focus on inflammatory bowel disease (IBD) patients. My colleagues were supportive, and I worked with a great team of nurses and APPs. I cared for many patients in both the inpatient and outpatient setting and had an opportunity to complete a high volume and variety of procedures. Overall, the various aspects of my job were rewarding. However, something was missing, and I made personal and professional adjustments. My schedule pulled me from valued family time with my kids (mostly) in the early mornings, therefore, I altered my work schedule. My clinical interest in IBD was diluted by the emphasis to see mostly general GI patients, as is the case for many in private practice. I missed the academic environment, especially working with medical students, residents, and fellows, so I occasionally had residents shadow me. Unfortunately, adjustments did not “fix” that missing component – to me, this was a job that did not feel like a career. I was not professionally fulfilled and on several occasions during the 6 years in private practice, I connected with mentors from my medical training to explore career options while trying to define what was missing.
During the latter part of years 5 and 6, it became apparent to me that loyalty pulled me toward working with a great group of supportive gastroenterologists, but it became increasingly more apparent that this job was not in line with my career goals. I had identified that I wanted to actively participate in medical education while practicing as a gastroenterologist in an academic setting. Additionally, time with my family was a critical part to the work-life integration.
My approach to the next step in my journey was different than my initial job. My goal was to define what was important, as in what were my absolute requirements for career satisfaction and where was I willing to be flexible. Each of us has different absolute and relative requirements based on our values, and I neglected to clearly identify these components with my first job. Admittedly, I have (at times) struggled to acknowledge my values, because I might somehow appear less committed to a career. Owning these values has provided clarity in my path from private practice to academic medicine. During the 3 years I have been in my current position, I have stepped into a leadership role in the University of Nebraska Medicine GI fellowship program while providing clinical medicine at the Fred Paustian IBD Center at Nebraska Medicine. In addition, I continue to have an active endoscopy schedule and derive great satisfaction teaching the fellows how to be effective endoscopists. Personally, the difference in compensation between academic medicine and private practice was not an important factor, although this is a factor for some people (and that’s okay).
When I graduated from fellowship, my path seemed clear, and I did not anticipate the road ahead. However, with each hurdle, I was gifted with lessons that would prove to be valuable as I moved ahead. Thank you for giving me the space to share my story.
Lessons that have helped in my journey from private practice to academics
- Mentorship: Find mentors, not just one mentor. Over the years, I have had several mentors, but what I recognize is that, early in my career, I did not have a mentor. Although a mentor cannot make decisions for you, he/she can provide guidance from a place of experience (both career and life experience).
- Define a mission statement: My mentor pushed me to first define my values and then my mission statement. This serves as the foundation that I reference when making decisions that will impact my family and my career. For example, if I am invited to participate on a committee, I look at how this will impact my family and whether it aligns with my mission. This helps to clarify what I am willing to say yes to and what to pass along to another colleague. Remember that last part ... if you are saying no to something, suggest another colleague for the opportunity.
- Advocate for yourself: Only you know what is best for you. Sometimes the path to discovering what that is can be tortuous and require guidance. Throughout my journey, I worked with colleagues who were supportive of my journey back to medical school and supportive of my job in private practice, but only I could define what a career meant to me.
- Assume positive intent: In medicine, we frequently work in a high-stakes, stressful environment. Assume positive intent in your interactions with others, especially colleagues. This will serve you well.
- Life happens: Each of us will experience an unexpected event in our personal life or career path or both. This will be okay. The path forward may look different and require a pivot. This unexpected event might be that you find your job leaves you wanting something more or something different. Your journey will be right for you.
Dr. Hutchins is an assistant professor in the division of gastroenterology and hepatology at the University of Nebraska Medical Center, Omaha. She reported no conflicts of interest.
Will new guidelines widen the gap in treating childhood obesity?
In the United States, the Centers for Disease Control and Prevention estimates that nearly one in five children have obesity. Since the 1980s, the number of children with obesity has been increasing, with each generation reaching higher rates and greater weights at earlier ages. Even with extensive efforts from parents, clinicians, educators, and policymakers to limit the excessive weight gain among children, the number of obesity and severe obesity diagnoses keeps rising.
In response to this critical public health challenge, the American Academy of Pediatrics (AAP) introduced new clinical practice guidelines for the evaluation and management of obesity in children and adolescents. Developed by an expert panel, the new AAP guidelines present a departure in the conceptualization of obesity, recognizing the role that social determinants of health play in contributing to excessive weight gain.
As a community health researcher who investigates disparities in childhood obesity, I applaud the paradigm shift from the AAP. I specifically endorse the recognition that obesity is a very serious metabolic disease that won’t go away unless we introduce systemic changes and effective treatments.
However, I, like so many of my colleagues and anyone aware of the access barriers to the recommended treatments, worry about the consequences that the new guidelines will have in the context of current and future health disparities.
A recent study, published in Pediatrics, showed that childhood obesity disparities are widening. Younger generations of children are reaching higher weights at younger ages. These alarming trends are greater among Black children and children growing up with the greatest socioeconomic disadvantages. The new AAP guidelines – even while driven by good intentions – can exacerbate these differences and set children who are able to live healthy lives further apart from those with disproportionate obesity risks, who lack access to the treatments recommended by the AAP.
Rather than “watchful waiting,” to see if children outgrow obesity, the new guidelines call for “aggressive treatment,” as reported by this news organization. At least 26 hours of in-person intensive health behavior and lifestyle counseling and treatment are recommended for children aged 2 years old or older who meet the obesity criteria. For children aged 12 years or older, the AAP recommends complementing lifestyle counseling with pharmacotherapy. This breakthrough welcomes the use of promising antiobesity medications (for example, orlistat, Wegovy [semaglutide], Saxenda [liraglutide], Qsymia (phentermine and topiramate]) approved by the Food and Drug Administration for long-term use in children aged 12 and up. For children 13 years or older with severe obesity, bariatric surgery should be considered.
Will cost barriers continue to increase disparity?
The very promising semaglutide (Wegovy) is a GLP-1–based medication currently offered for about $1,000 per month. As with other chronic diseases, children should be prepared to take obesity medications for prolonged periods of time. A study conducted in adults found that when the medication is suspended, any weight loss can be regained. The costs of bariatric surgery total over $20,000.
In the U.S. health care system, at current prices, very few of the children in need of the medications or surgical treatments have access to them. Most private health insurance companies and Medicaid reject coverage for childhood obesity treatments. Barriers to treatment access are greater for Black and Hispanic children, children growing up in poverty, and children living in the U.S. South region, all of whom are more likely to develop obesity earlier in life than their White and wealthier counterparts.
The AAP recognized that a substantial time and financial commitment is required to follow the new treatment recommendations. Members of the AAP Expert Committee that developed the guidelines stated that they are “aware of the multitude of barriers to treatment that patients and their families face.”
Nevertheless, the recognition of the role of social determinants of health in the development of childhood obesity didn’t motivate the introduction of treatment options that aren’t unattainable for most U.S. families.
It’s important to step away from the conclusion that because of the price tag, at the population level, the new AAP guidelines will be inconsequential. This conclusion fails to recognize the potential harm that the guidelines may introduce. In the context of childhood obesity disparities, the new treatment recommendations probably will widen the childhood obesity prevalence gap between the haves – who will benefit from the options available to reduce childhood obesity – and the have-nots, whose obesity rates will continue with their growth.
We live in a world of the haves and have-nots. This applies to financial resources as well as obesity rates. In the long term, the optimists hope that the GLP-1 medications will become ubiquitous, generics will be developed, and insurance companies will expand coverage and grant access to most children in need of effective obesity treatment options. Until this happens, unless intentional policies are promptly introduced, childhood obesity disparities will continue to widen.
To avoid the increasing disparities, brave and intentional actions are required. A lack of attention dealt to this known problem will result in a lost opportunity for the AAP, legislators, and others in a position to help U.S. children.
Liliana Aguayo, PhD, MPH, is assistant professor, Clinical Research Track, Hubert Department of Global Health, Emory University, Atlanta. A version of this article first appeared on Medscape.com.
In the United States, the Centers for Disease Control and Prevention estimates that nearly one in five children have obesity. Since the 1980s, the number of children with obesity has been increasing, with each generation reaching higher rates and greater weights at earlier ages. Even with extensive efforts from parents, clinicians, educators, and policymakers to limit the excessive weight gain among children, the number of obesity and severe obesity diagnoses keeps rising.
In response to this critical public health challenge, the American Academy of Pediatrics (AAP) introduced new clinical practice guidelines for the evaluation and management of obesity in children and adolescents. Developed by an expert panel, the new AAP guidelines present a departure in the conceptualization of obesity, recognizing the role that social determinants of health play in contributing to excessive weight gain.
As a community health researcher who investigates disparities in childhood obesity, I applaud the paradigm shift from the AAP. I specifically endorse the recognition that obesity is a very serious metabolic disease that won’t go away unless we introduce systemic changes and effective treatments.
However, I, like so many of my colleagues and anyone aware of the access barriers to the recommended treatments, worry about the consequences that the new guidelines will have in the context of current and future health disparities.
A recent study, published in Pediatrics, showed that childhood obesity disparities are widening. Younger generations of children are reaching higher weights at younger ages. These alarming trends are greater among Black children and children growing up with the greatest socioeconomic disadvantages. The new AAP guidelines – even while driven by good intentions – can exacerbate these differences and set children who are able to live healthy lives further apart from those with disproportionate obesity risks, who lack access to the treatments recommended by the AAP.
Rather than “watchful waiting,” to see if children outgrow obesity, the new guidelines call for “aggressive treatment,” as reported by this news organization. At least 26 hours of in-person intensive health behavior and lifestyle counseling and treatment are recommended for children aged 2 years old or older who meet the obesity criteria. For children aged 12 years or older, the AAP recommends complementing lifestyle counseling with pharmacotherapy. This breakthrough welcomes the use of promising antiobesity medications (for example, orlistat, Wegovy [semaglutide], Saxenda [liraglutide], Qsymia (phentermine and topiramate]) approved by the Food and Drug Administration for long-term use in children aged 12 and up. For children 13 years or older with severe obesity, bariatric surgery should be considered.
Will cost barriers continue to increase disparity?
The very promising semaglutide (Wegovy) is a GLP-1–based medication currently offered for about $1,000 per month. As with other chronic diseases, children should be prepared to take obesity medications for prolonged periods of time. A study conducted in adults found that when the medication is suspended, any weight loss can be regained. The costs of bariatric surgery total over $20,000.
In the U.S. health care system, at current prices, very few of the children in need of the medications or surgical treatments have access to them. Most private health insurance companies and Medicaid reject coverage for childhood obesity treatments. Barriers to treatment access are greater for Black and Hispanic children, children growing up in poverty, and children living in the U.S. South region, all of whom are more likely to develop obesity earlier in life than their White and wealthier counterparts.
The AAP recognized that a substantial time and financial commitment is required to follow the new treatment recommendations. Members of the AAP Expert Committee that developed the guidelines stated that they are “aware of the multitude of barriers to treatment that patients and their families face.”
Nevertheless, the recognition of the role of social determinants of health in the development of childhood obesity didn’t motivate the introduction of treatment options that aren’t unattainable for most U.S. families.
It’s important to step away from the conclusion that because of the price tag, at the population level, the new AAP guidelines will be inconsequential. This conclusion fails to recognize the potential harm that the guidelines may introduce. In the context of childhood obesity disparities, the new treatment recommendations probably will widen the childhood obesity prevalence gap between the haves – who will benefit from the options available to reduce childhood obesity – and the have-nots, whose obesity rates will continue with their growth.
We live in a world of the haves and have-nots. This applies to financial resources as well as obesity rates. In the long term, the optimists hope that the GLP-1 medications will become ubiquitous, generics will be developed, and insurance companies will expand coverage and grant access to most children in need of effective obesity treatment options. Until this happens, unless intentional policies are promptly introduced, childhood obesity disparities will continue to widen.
To avoid the increasing disparities, brave and intentional actions are required. A lack of attention dealt to this known problem will result in a lost opportunity for the AAP, legislators, and others in a position to help U.S. children.
Liliana Aguayo, PhD, MPH, is assistant professor, Clinical Research Track, Hubert Department of Global Health, Emory University, Atlanta. A version of this article first appeared on Medscape.com.
In the United States, the Centers for Disease Control and Prevention estimates that nearly one in five children have obesity. Since the 1980s, the number of children with obesity has been increasing, with each generation reaching higher rates and greater weights at earlier ages. Even with extensive efforts from parents, clinicians, educators, and policymakers to limit the excessive weight gain among children, the number of obesity and severe obesity diagnoses keeps rising.
In response to this critical public health challenge, the American Academy of Pediatrics (AAP) introduced new clinical practice guidelines for the evaluation and management of obesity in children and adolescents. Developed by an expert panel, the new AAP guidelines present a departure in the conceptualization of obesity, recognizing the role that social determinants of health play in contributing to excessive weight gain.
As a community health researcher who investigates disparities in childhood obesity, I applaud the paradigm shift from the AAP. I specifically endorse the recognition that obesity is a very serious metabolic disease that won’t go away unless we introduce systemic changes and effective treatments.
However, I, like so many of my colleagues and anyone aware of the access barriers to the recommended treatments, worry about the consequences that the new guidelines will have in the context of current and future health disparities.
A recent study, published in Pediatrics, showed that childhood obesity disparities are widening. Younger generations of children are reaching higher weights at younger ages. These alarming trends are greater among Black children and children growing up with the greatest socioeconomic disadvantages. The new AAP guidelines – even while driven by good intentions – can exacerbate these differences and set children who are able to live healthy lives further apart from those with disproportionate obesity risks, who lack access to the treatments recommended by the AAP.
Rather than “watchful waiting,” to see if children outgrow obesity, the new guidelines call for “aggressive treatment,” as reported by this news organization. At least 26 hours of in-person intensive health behavior and lifestyle counseling and treatment are recommended for children aged 2 years old or older who meet the obesity criteria. For children aged 12 years or older, the AAP recommends complementing lifestyle counseling with pharmacotherapy. This breakthrough welcomes the use of promising antiobesity medications (for example, orlistat, Wegovy [semaglutide], Saxenda [liraglutide], Qsymia (phentermine and topiramate]) approved by the Food and Drug Administration for long-term use in children aged 12 and up. For children 13 years or older with severe obesity, bariatric surgery should be considered.
Will cost barriers continue to increase disparity?
The very promising semaglutide (Wegovy) is a GLP-1–based medication currently offered for about $1,000 per month. As with other chronic diseases, children should be prepared to take obesity medications for prolonged periods of time. A study conducted in adults found that when the medication is suspended, any weight loss can be regained. The costs of bariatric surgery total over $20,000.
In the U.S. health care system, at current prices, very few of the children in need of the medications or surgical treatments have access to them. Most private health insurance companies and Medicaid reject coverage for childhood obesity treatments. Barriers to treatment access are greater for Black and Hispanic children, children growing up in poverty, and children living in the U.S. South region, all of whom are more likely to develop obesity earlier in life than their White and wealthier counterparts.
The AAP recognized that a substantial time and financial commitment is required to follow the new treatment recommendations. Members of the AAP Expert Committee that developed the guidelines stated that they are “aware of the multitude of barriers to treatment that patients and their families face.”
Nevertheless, the recognition of the role of social determinants of health in the development of childhood obesity didn’t motivate the introduction of treatment options that aren’t unattainable for most U.S. families.
It’s important to step away from the conclusion that because of the price tag, at the population level, the new AAP guidelines will be inconsequential. This conclusion fails to recognize the potential harm that the guidelines may introduce. In the context of childhood obesity disparities, the new treatment recommendations probably will widen the childhood obesity prevalence gap between the haves – who will benefit from the options available to reduce childhood obesity – and the have-nots, whose obesity rates will continue with their growth.
We live in a world of the haves and have-nots. This applies to financial resources as well as obesity rates. In the long term, the optimists hope that the GLP-1 medications will become ubiquitous, generics will be developed, and insurance companies will expand coverage and grant access to most children in need of effective obesity treatment options. Until this happens, unless intentional policies are promptly introduced, childhood obesity disparities will continue to widen.
To avoid the increasing disparities, brave and intentional actions are required. A lack of attention dealt to this known problem will result in a lost opportunity for the AAP, legislators, and others in a position to help U.S. children.
Liliana Aguayo, PhD, MPH, is assistant professor, Clinical Research Track, Hubert Department of Global Health, Emory University, Atlanta. A version of this article first appeared on Medscape.com.
Firing patients
One might assume that, just as patients are free to accept or reject their doctors, physicians have an equal right to reject their patients; to a certain extent, that is true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, gender, sexual orientation, and so on. However, the evolution of ever-larger practice environments has raised new questions.
While verbal abuse, inappropriate treatment demands (particularly for controlled substances), refusal to adhere to mutually agreed treatment plans, and failure to keep appointments or pay bills remain the most common reasons for dismissal, evolving practice environments may require us to modify our responses.
What happens, for example, when a patient is banned from a large clinic that employs most of that community’s physicians, or is the only practice in town with the specialists required by that patient? The medical profession does have an obligation to not exclude such patients from care.
In a large cross-specialty system or consolidated specialist practice, firing a patient has a very different level of consequences than in a small office. There must be a balance between separating patients and doctors who don’t get along and seeing that the patient in question receives competent treatment. The physician, as the professional, has a higher standard to live up to with respect to handling this kind of situation.
If the problem is a personality conflict, the solution may be as simple as transferring the patient to another caregiver within the practice. While it does not make sense for a patient to continue seeing a doctor who does not want to see them, it also does not make sense to ban a patient from a large system where there could well be one or more other doctors who would be a good match. If a patient is unable to pay outstanding bills, a large clinic might prohibit them from making new appointments until they have worked out a payment plan rather than firing them outright.
If you are part of a large practice, take the time to research your group’s official policies for dealing with such situations. If there is no written policy, you might want to start that discussion with your colleagues.
The point is that in any practice, large or small, firing a patient should be a last resort. Try to make every effort to resolve the problem amicably. Communicate with the patient in question, explain your concerns, and discuss options for resolution. Take time to listen to the patient, as they may have an explanation (rational or not) for their objectionable behavior.
You can also send a letter, repeating your concerns and proposed solutions, as further documentation of your efforts to achieve an amicable resolution. All verbal and written warnings must, of course, be documented. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating them could lead to intervention by your state licensing board. There is also the risk of civil litigation, which is typically not covered by malpractice policies, and may not be covered by your general liability policy either.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
One might assume that, just as patients are free to accept or reject their doctors, physicians have an equal right to reject their patients; to a certain extent, that is true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, gender, sexual orientation, and so on. However, the evolution of ever-larger practice environments has raised new questions.
While verbal abuse, inappropriate treatment demands (particularly for controlled substances), refusal to adhere to mutually agreed treatment plans, and failure to keep appointments or pay bills remain the most common reasons for dismissal, evolving practice environments may require us to modify our responses.
What happens, for example, when a patient is banned from a large clinic that employs most of that community’s physicians, or is the only practice in town with the specialists required by that patient? The medical profession does have an obligation to not exclude such patients from care.
In a large cross-specialty system or consolidated specialist practice, firing a patient has a very different level of consequences than in a small office. There must be a balance between separating patients and doctors who don’t get along and seeing that the patient in question receives competent treatment. The physician, as the professional, has a higher standard to live up to with respect to handling this kind of situation.
If the problem is a personality conflict, the solution may be as simple as transferring the patient to another caregiver within the practice. While it does not make sense for a patient to continue seeing a doctor who does not want to see them, it also does not make sense to ban a patient from a large system where there could well be one or more other doctors who would be a good match. If a patient is unable to pay outstanding bills, a large clinic might prohibit them from making new appointments until they have worked out a payment plan rather than firing them outright.
If you are part of a large practice, take the time to research your group’s official policies for dealing with such situations. If there is no written policy, you might want to start that discussion with your colleagues.
The point is that in any practice, large or small, firing a patient should be a last resort. Try to make every effort to resolve the problem amicably. Communicate with the patient in question, explain your concerns, and discuss options for resolution. Take time to listen to the patient, as they may have an explanation (rational or not) for their objectionable behavior.
You can also send a letter, repeating your concerns and proposed solutions, as further documentation of your efforts to achieve an amicable resolution. All verbal and written warnings must, of course, be documented. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating them could lead to intervention by your state licensing board. There is also the risk of civil litigation, which is typically not covered by malpractice policies, and may not be covered by your general liability policy either.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
One might assume that, just as patients are free to accept or reject their doctors, physicians have an equal right to reject their patients; to a certain extent, that is true. There are no specific laws prohibiting a provider from terminating a patient relationship for any reason, other than a discriminatory one – race, nationality, religion, age, gender, sexual orientation, and so on. However, the evolution of ever-larger practice environments has raised new questions.
While verbal abuse, inappropriate treatment demands (particularly for controlled substances), refusal to adhere to mutually agreed treatment plans, and failure to keep appointments or pay bills remain the most common reasons for dismissal, evolving practice environments may require us to modify our responses.
What happens, for example, when a patient is banned from a large clinic that employs most of that community’s physicians, or is the only practice in town with the specialists required by that patient? The medical profession does have an obligation to not exclude such patients from care.
In a large cross-specialty system or consolidated specialist practice, firing a patient has a very different level of consequences than in a small office. There must be a balance between separating patients and doctors who don’t get along and seeing that the patient in question receives competent treatment. The physician, as the professional, has a higher standard to live up to with respect to handling this kind of situation.
If the problem is a personality conflict, the solution may be as simple as transferring the patient to another caregiver within the practice. While it does not make sense for a patient to continue seeing a doctor who does not want to see them, it also does not make sense to ban a patient from a large system where there could well be one or more other doctors who would be a good match. If a patient is unable to pay outstanding bills, a large clinic might prohibit them from making new appointments until they have worked out a payment plan rather than firing them outright.
If you are part of a large practice, take the time to research your group’s official policies for dealing with such situations. If there is no written policy, you might want to start that discussion with your colleagues.
The point is that in any practice, large or small, firing a patient should be a last resort. Try to make every effort to resolve the problem amicably. Communicate with the patient in question, explain your concerns, and discuss options for resolution. Take time to listen to the patient, as they may have an explanation (rational or not) for their objectionable behavior.
You can also send a letter, repeating your concerns and proposed solutions, as further documentation of your efforts to achieve an amicable resolution. All verbal and written warnings must, of course, be documented. If the patient has a managed care policy, we review the managed care contract, which sometimes includes specific requirements for dismissal of its patients.
When such efforts fail, we send the patient two letters – one certified with return receipt, the other by conventional first class, in case the patient refuses the certified copy – explaining the reason for dismissal, and that care will be discontinued in 30 days from the letter’s date. (Most attorneys and medical associations agree that 30 days is sufficient reasonable notice.) We offer to provide care during the interim period, include a list of names and contact information for potential alternate providers, and offer to transfer records after receiving written permission.
Following these precautions will usually protect you from charges of “patient abandonment,” which is generally defined as the unilateral severance by the physician of the physician-patient relationship without giving the patient sufficient advance notice to obtain the services of another practitioner, and at a time when the patient still requires medical attention.
Some states have their own unique definitions of patient abandonment. You should check with your state’s health department, and your attorney, for any unusual requirements in your state, because violating them could lead to intervention by your state licensing board. There is also the risk of civil litigation, which is typically not covered by malpractice policies, and may not be covered by your general liability policy either.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Can particles in dairy and beef cause cancer and MS?
In Western diets, dairy and beef are ubiquitous: Milk goes with coffee, melted cheese with pizza, and chili with rice. But what if dairy products and beef contained a new kind of pathogen that could infect you as a child and trigger cancer or multiple sclerosis (MS) 40-70 years later?
However, in two joint statements, the German Federal Institute for Risk Assessment (BfR) and the Max Rubner Institute (MRI) have rejected such theories.
In 2008, Harald zur Hausen, MD, DSc, received the Nobel Prize in Medicine for his discovery that human papillomaviruses cause cervical cancer. His starting point was the observation that sexually abstinent women, such as nuns, rarely develop this cancer. So it was possible to draw the conclusion that pathogens are transmitted during sexual intercourse, explain Dr. zur Hausen and his wife Ethel-Michele de Villiers, PhD, both of DKFZ Heidelberg.
Papillomaviruses, as well as human herpes and Epstein-Barr viruses (EBV), polyomaviruses, and retroviruses, cause cancer in a direct way: by inserting their genes into the DNA of human cells. With a latency of a few years to a few decades, the proteins formed through expression stimulate malignant growth by altering the regulating host gene.
Acid radicals
However, viruses – just like bacteria and parasites – can also indirectly trigger cancer. One mechanism for this triggering is the disruption of immune defenses, as shown by the sometimes drastically increased tumor incidence with AIDS or with immunosuppressants after transplants. Chronic inflammation is a second mechanism that generates acid radicals and thereby causes random mutations in replicating cells. Examples include stomach cancer caused by Helicobacter pylori and liver cancer caused by Schistosoma, liver fluke, and hepatitis B and C viruses.
According to Dr. de Villiers and Dr. zur Hausen, there are good reasons to believe that other pathogens could cause chronic inflammation and thereby lead to cancer. Epidemiologic data suggest that dairy and meat products from European cows (Bos taurus) are a potential source. This is because colon cancer and breast cancer commonly occur in places where these foods are heavily consumed (that is, in North America, Argentina, Europe, and Australia). In contrast, the rate is low in India, where cows are revered as holy animals. Also noteworthy is that women with a lactose intolerance rarely develop breast cancer.
Viral progeny
In fact, the researchers found single-stranded DNA rings that originated in viruses, which they named bovine meat and milk factors (BMMF), in the intestines of patients with colon cancer. They reported, “This new class of pathogen deserves, in our opinion at least, to become the focus of cancer development and further chronic diseases.” They also detected elevated levels of acid radicals in these areas (that is, oxidative stress), which is typical for chronic inflammation.
The researchers assume that infants, whose immune system is not yet fully matured, ingest the BMMF as soon as they have dairy. Therefore, there is no need for adults to avoid dairy or beef because everyone is infected anyway, said Dr. zur Hausen.
‘Breast milk is healthy’
Dr. De Villiers and Dr. zur Hausen outlined more evidence of cancer-triggering pathogens. Mothers who have breastfed are less likely, especially after multiple pregnancies, to develop tumors in various organs or to have MS and type 2 diabetes. The authors attribute the protective effect to oligosaccharides in breast milk, which begin to be formed midway through the pregnancy. They bind to lectin receptors and, in so doing, mask the terminal molecule onto which the viruses need to dock. As a result, their port of entry into the cells is blocked.
The oligosaccharides also protect the baby against life-threatening infections by blocking access by rotaviruses and noroviruses. In this way, especially if breastfeeding lasts a long time – around 1 year – the period of incomplete immunocompetence is bridged.
Colon cancer
To date, it has been assumed that around 20% of all cancerous diseases globally are caused by infections, said the researchers. But if the suspected BMMF cases are included, this figure rises to 50%, even to around 80%, for colon cancer. If the suspicion is confirmed, the consequences for prevention and therapy would be significant.
The voice of a Nobel prize winner undoubtedly carries weight, but at the time, Dr. zur Hausen still had to convince a host of skeptics with his discovery that a viral infection is a major cause of cervical cancer. Nonetheless, some indicators suggest that he and his wife have found a dead end this time.
Institutional skepticism
When his working group made the results public in February 2019, the DKFZ felt the need to give an all-clear signal in response to alarmed press reports. There is no reason to see dairy and meat consumption as something negative. Similarly, in their first joint statement, the BfR and the MRI judged the data to be insufficient and called for further studies. Multiple research teams began to focus on BMMF as a result. In what foods can they be found? Are they more common in patients with cancer than in healthy people? Are they infectious? Do they cause inflammation and cancer?
The findings presented in a second statement by the BfR and MRI at the end of November 2022 contradicted the claims made by the DKFZ scientists across the board. In no way do BMMF represent new pathogens. They are variants of already known DNA sequences. In addition, they are present in numerous animal-based and plant-based foods, including pork, fish, fruit, vegetables, and nuts.
BMMF do not possess the ability to infect human cells, the institutes said. The proof that they are damaging to one’s health was also absent. It is true that the incidence of intestinal tumors correlates positively with the consumption of red and processed meat – which in no way signifies causality – but dairy products are linked to a reduced risk. On the other hand, breast cancer cannot be associated with the consumption of beef or dairy.
Therefore, both institutes recommend continuing to use these products as supplementary diet for infants because of their micronutrients. They further stated that the products are safe for people of all ages.
Association with MS?
Unperturbed, Dr. de Villiers and Dr. zur Hausen went one step further in their current article. They posited that MS is also associated with the consumption of dairy products and beef. Here too geographic distribution prompted the idea to look for BMMF in the brain lesions of patients with MS. The researchers isolated ring-shaped DNA molecules that proved to be closely related to BMMF from dairy and cattle blood. “The result was electrifying for us.”
However, there are several other factors to consider, such as vitamin D3 deficiency. This is because the incidence of MS decreases the further you travel from the poles toward the equator (that is, as solar radiation increases). Also, EBV clearly plays a role because patients with MS display increased titers of EBV antibodies. One study also showed that people in Antarctica excreted reactivated EBV in their saliva during winter and that vitamin D3 stopped the viral secretion.
Under these conditions, the researchers hypothesized that MS is caused by a double infection of brain cells by EBV and BMMF. EBV is reactivated by a lack of vitamin D3, and the BMMF multiply and are eventually converted into proteins. A focal immunoreaction causes the Schwann cells and oligodendrocytes to malfunction, which leads to the destruction of the myelin sheaths around the nerve fibers.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In Western diets, dairy and beef are ubiquitous: Milk goes with coffee, melted cheese with pizza, and chili with rice. But what if dairy products and beef contained a new kind of pathogen that could infect you as a child and trigger cancer or multiple sclerosis (MS) 40-70 years later?
However, in two joint statements, the German Federal Institute for Risk Assessment (BfR) and the Max Rubner Institute (MRI) have rejected such theories.
In 2008, Harald zur Hausen, MD, DSc, received the Nobel Prize in Medicine for his discovery that human papillomaviruses cause cervical cancer. His starting point was the observation that sexually abstinent women, such as nuns, rarely develop this cancer. So it was possible to draw the conclusion that pathogens are transmitted during sexual intercourse, explain Dr. zur Hausen and his wife Ethel-Michele de Villiers, PhD, both of DKFZ Heidelberg.
Papillomaviruses, as well as human herpes and Epstein-Barr viruses (EBV), polyomaviruses, and retroviruses, cause cancer in a direct way: by inserting their genes into the DNA of human cells. With a latency of a few years to a few decades, the proteins formed through expression stimulate malignant growth by altering the regulating host gene.
Acid radicals
However, viruses – just like bacteria and parasites – can also indirectly trigger cancer. One mechanism for this triggering is the disruption of immune defenses, as shown by the sometimes drastically increased tumor incidence with AIDS or with immunosuppressants after transplants. Chronic inflammation is a second mechanism that generates acid radicals and thereby causes random mutations in replicating cells. Examples include stomach cancer caused by Helicobacter pylori and liver cancer caused by Schistosoma, liver fluke, and hepatitis B and C viruses.
According to Dr. de Villiers and Dr. zur Hausen, there are good reasons to believe that other pathogens could cause chronic inflammation and thereby lead to cancer. Epidemiologic data suggest that dairy and meat products from European cows (Bos taurus) are a potential source. This is because colon cancer and breast cancer commonly occur in places where these foods are heavily consumed (that is, in North America, Argentina, Europe, and Australia). In contrast, the rate is low in India, where cows are revered as holy animals. Also noteworthy is that women with a lactose intolerance rarely develop breast cancer.
Viral progeny
In fact, the researchers found single-stranded DNA rings that originated in viruses, which they named bovine meat and milk factors (BMMF), in the intestines of patients with colon cancer. They reported, “This new class of pathogen deserves, in our opinion at least, to become the focus of cancer development and further chronic diseases.” They also detected elevated levels of acid radicals in these areas (that is, oxidative stress), which is typical for chronic inflammation.
The researchers assume that infants, whose immune system is not yet fully matured, ingest the BMMF as soon as they have dairy. Therefore, there is no need for adults to avoid dairy or beef because everyone is infected anyway, said Dr. zur Hausen.
‘Breast milk is healthy’
Dr. De Villiers and Dr. zur Hausen outlined more evidence of cancer-triggering pathogens. Mothers who have breastfed are less likely, especially after multiple pregnancies, to develop tumors in various organs or to have MS and type 2 diabetes. The authors attribute the protective effect to oligosaccharides in breast milk, which begin to be formed midway through the pregnancy. They bind to lectin receptors and, in so doing, mask the terminal molecule onto which the viruses need to dock. As a result, their port of entry into the cells is blocked.
The oligosaccharides also protect the baby against life-threatening infections by blocking access by rotaviruses and noroviruses. In this way, especially if breastfeeding lasts a long time – around 1 year – the period of incomplete immunocompetence is bridged.
Colon cancer
To date, it has been assumed that around 20% of all cancerous diseases globally are caused by infections, said the researchers. But if the suspected BMMF cases are included, this figure rises to 50%, even to around 80%, for colon cancer. If the suspicion is confirmed, the consequences for prevention and therapy would be significant.
The voice of a Nobel prize winner undoubtedly carries weight, but at the time, Dr. zur Hausen still had to convince a host of skeptics with his discovery that a viral infection is a major cause of cervical cancer. Nonetheless, some indicators suggest that he and his wife have found a dead end this time.
Institutional skepticism
When his working group made the results public in February 2019, the DKFZ felt the need to give an all-clear signal in response to alarmed press reports. There is no reason to see dairy and meat consumption as something negative. Similarly, in their first joint statement, the BfR and the MRI judged the data to be insufficient and called for further studies. Multiple research teams began to focus on BMMF as a result. In what foods can they be found? Are they more common in patients with cancer than in healthy people? Are they infectious? Do they cause inflammation and cancer?
The findings presented in a second statement by the BfR and MRI at the end of November 2022 contradicted the claims made by the DKFZ scientists across the board. In no way do BMMF represent new pathogens. They are variants of already known DNA sequences. In addition, they are present in numerous animal-based and plant-based foods, including pork, fish, fruit, vegetables, and nuts.
BMMF do not possess the ability to infect human cells, the institutes said. The proof that they are damaging to one’s health was also absent. It is true that the incidence of intestinal tumors correlates positively with the consumption of red and processed meat – which in no way signifies causality – but dairy products are linked to a reduced risk. On the other hand, breast cancer cannot be associated with the consumption of beef or dairy.
Therefore, both institutes recommend continuing to use these products as supplementary diet for infants because of their micronutrients. They further stated that the products are safe for people of all ages.
Association with MS?
Unperturbed, Dr. de Villiers and Dr. zur Hausen went one step further in their current article. They posited that MS is also associated with the consumption of dairy products and beef. Here too geographic distribution prompted the idea to look for BMMF in the brain lesions of patients with MS. The researchers isolated ring-shaped DNA molecules that proved to be closely related to BMMF from dairy and cattle blood. “The result was electrifying for us.”
However, there are several other factors to consider, such as vitamin D3 deficiency. This is because the incidence of MS decreases the further you travel from the poles toward the equator (that is, as solar radiation increases). Also, EBV clearly plays a role because patients with MS display increased titers of EBV antibodies. One study also showed that people in Antarctica excreted reactivated EBV in their saliva during winter and that vitamin D3 stopped the viral secretion.
Under these conditions, the researchers hypothesized that MS is caused by a double infection of brain cells by EBV and BMMF. EBV is reactivated by a lack of vitamin D3, and the BMMF multiply and are eventually converted into proteins. A focal immunoreaction causes the Schwann cells and oligodendrocytes to malfunction, which leads to the destruction of the myelin sheaths around the nerve fibers.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
In Western diets, dairy and beef are ubiquitous: Milk goes with coffee, melted cheese with pizza, and chili with rice. But what if dairy products and beef contained a new kind of pathogen that could infect you as a child and trigger cancer or multiple sclerosis (MS) 40-70 years later?
However, in two joint statements, the German Federal Institute for Risk Assessment (BfR) and the Max Rubner Institute (MRI) have rejected such theories.
In 2008, Harald zur Hausen, MD, DSc, received the Nobel Prize in Medicine for his discovery that human papillomaviruses cause cervical cancer. His starting point was the observation that sexually abstinent women, such as nuns, rarely develop this cancer. So it was possible to draw the conclusion that pathogens are transmitted during sexual intercourse, explain Dr. zur Hausen and his wife Ethel-Michele de Villiers, PhD, both of DKFZ Heidelberg.
Papillomaviruses, as well as human herpes and Epstein-Barr viruses (EBV), polyomaviruses, and retroviruses, cause cancer in a direct way: by inserting their genes into the DNA of human cells. With a latency of a few years to a few decades, the proteins formed through expression stimulate malignant growth by altering the regulating host gene.
Acid radicals
However, viruses – just like bacteria and parasites – can also indirectly trigger cancer. One mechanism for this triggering is the disruption of immune defenses, as shown by the sometimes drastically increased tumor incidence with AIDS or with immunosuppressants after transplants. Chronic inflammation is a second mechanism that generates acid radicals and thereby causes random mutations in replicating cells. Examples include stomach cancer caused by Helicobacter pylori and liver cancer caused by Schistosoma, liver fluke, and hepatitis B and C viruses.
According to Dr. de Villiers and Dr. zur Hausen, there are good reasons to believe that other pathogens could cause chronic inflammation and thereby lead to cancer. Epidemiologic data suggest that dairy and meat products from European cows (Bos taurus) are a potential source. This is because colon cancer and breast cancer commonly occur in places where these foods are heavily consumed (that is, in North America, Argentina, Europe, and Australia). In contrast, the rate is low in India, where cows are revered as holy animals. Also noteworthy is that women with a lactose intolerance rarely develop breast cancer.
Viral progeny
In fact, the researchers found single-stranded DNA rings that originated in viruses, which they named bovine meat and milk factors (BMMF), in the intestines of patients with colon cancer. They reported, “This new class of pathogen deserves, in our opinion at least, to become the focus of cancer development and further chronic diseases.” They also detected elevated levels of acid radicals in these areas (that is, oxidative stress), which is typical for chronic inflammation.
The researchers assume that infants, whose immune system is not yet fully matured, ingest the BMMF as soon as they have dairy. Therefore, there is no need for adults to avoid dairy or beef because everyone is infected anyway, said Dr. zur Hausen.
‘Breast milk is healthy’
Dr. De Villiers and Dr. zur Hausen outlined more evidence of cancer-triggering pathogens. Mothers who have breastfed are less likely, especially after multiple pregnancies, to develop tumors in various organs or to have MS and type 2 diabetes. The authors attribute the protective effect to oligosaccharides in breast milk, which begin to be formed midway through the pregnancy. They bind to lectin receptors and, in so doing, mask the terminal molecule onto which the viruses need to dock. As a result, their port of entry into the cells is blocked.
The oligosaccharides also protect the baby against life-threatening infections by blocking access by rotaviruses and noroviruses. In this way, especially if breastfeeding lasts a long time – around 1 year – the period of incomplete immunocompetence is bridged.
Colon cancer
To date, it has been assumed that around 20% of all cancerous diseases globally are caused by infections, said the researchers. But if the suspected BMMF cases are included, this figure rises to 50%, even to around 80%, for colon cancer. If the suspicion is confirmed, the consequences for prevention and therapy would be significant.
The voice of a Nobel prize winner undoubtedly carries weight, but at the time, Dr. zur Hausen still had to convince a host of skeptics with his discovery that a viral infection is a major cause of cervical cancer. Nonetheless, some indicators suggest that he and his wife have found a dead end this time.
Institutional skepticism
When his working group made the results public in February 2019, the DKFZ felt the need to give an all-clear signal in response to alarmed press reports. There is no reason to see dairy and meat consumption as something negative. Similarly, in their first joint statement, the BfR and the MRI judged the data to be insufficient and called for further studies. Multiple research teams began to focus on BMMF as a result. In what foods can they be found? Are they more common in patients with cancer than in healthy people? Are they infectious? Do they cause inflammation and cancer?
The findings presented in a second statement by the BfR and MRI at the end of November 2022 contradicted the claims made by the DKFZ scientists across the board. In no way do BMMF represent new pathogens. They are variants of already known DNA sequences. In addition, they are present in numerous animal-based and plant-based foods, including pork, fish, fruit, vegetables, and nuts.
BMMF do not possess the ability to infect human cells, the institutes said. The proof that they are damaging to one’s health was also absent. It is true that the incidence of intestinal tumors correlates positively with the consumption of red and processed meat – which in no way signifies causality – but dairy products are linked to a reduced risk. On the other hand, breast cancer cannot be associated with the consumption of beef or dairy.
Therefore, both institutes recommend continuing to use these products as supplementary diet for infants because of their micronutrients. They further stated that the products are safe for people of all ages.
Association with MS?
Unperturbed, Dr. de Villiers and Dr. zur Hausen went one step further in their current article. They posited that MS is also associated with the consumption of dairy products and beef. Here too geographic distribution prompted the idea to look for BMMF in the brain lesions of patients with MS. The researchers isolated ring-shaped DNA molecules that proved to be closely related to BMMF from dairy and cattle blood. “The result was electrifying for us.”
However, there are several other factors to consider, such as vitamin D3 deficiency. This is because the incidence of MS decreases the further you travel from the poles toward the equator (that is, as solar radiation increases). Also, EBV clearly plays a role because patients with MS display increased titers of EBV antibodies. One study also showed that people in Antarctica excreted reactivated EBV in their saliva during winter and that vitamin D3 stopped the viral secretion.
Under these conditions, the researchers hypothesized that MS is caused by a double infection of brain cells by EBV and BMMF. EBV is reactivated by a lack of vitamin D3, and the BMMF multiply and are eventually converted into proteins. A focal immunoreaction causes the Schwann cells and oligodendrocytes to malfunction, which leads to the destruction of the myelin sheaths around the nerve fibers.
This article was translated from the Medscape German Edition. A version appeared on Medscape.com.
Dodging PE
I was (probably) the bane of my elementary school nurse.
I hated PE (I know, who didn’t?). But I also had childhood asthma. So it was an easy out to go to the school nurse, Mrs. Reed, because I was having an asthma attack, or at least claiming to have one.
She’d put me in a chair to “keep an eye” on me, occasionally have me take the prescription drug my pediatrician had ordered (Marax – anyone else remember that?), and knew to send me back to class about 5 minutes before PE was over.
Maybe Mrs. Reed liked me. Maybe it was just the path of least resistance to let me dodge PE. Maybe she’d hated PE, too, and was sympathetic. Who knows?
So twice a week through years of elementary school she and I went through the same routine of my showing up in her office. No matter how busy she was, she always told me to take a seat and do a therapeutic application of her stethoscope. She often told others who noticed my frequent visits that I was “a sickly child” even though I knew she saw through me and said it with a sense of sarcasm and humor.
Of course, life goes on, and one day 20 years ago Mrs. Reed showed up on my hospital census as a new consult after she’d had a minor stroke.
She remembered me very well. Her first comment, said with the same tone I recalled, was that she was amazed I’d lived to adulthood after having been such “a sickly child.” We both laughed.
Now, in her late 80s, she still comes to see me for this and that. Sometimes we reminisce about the intertwined journey our lives have taken us on. Sometimes she asks if I’ve been to PE recently.
Like any patient, she occasionally shows up on the wrong day, or at the wrong time. I always do my best to see her, though.
After all, I owe her big time for letting me dodge PE.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I was (probably) the bane of my elementary school nurse.
I hated PE (I know, who didn’t?). But I also had childhood asthma. So it was an easy out to go to the school nurse, Mrs. Reed, because I was having an asthma attack, or at least claiming to have one.
She’d put me in a chair to “keep an eye” on me, occasionally have me take the prescription drug my pediatrician had ordered (Marax – anyone else remember that?), and knew to send me back to class about 5 minutes before PE was over.
Maybe Mrs. Reed liked me. Maybe it was just the path of least resistance to let me dodge PE. Maybe she’d hated PE, too, and was sympathetic. Who knows?
So twice a week through years of elementary school she and I went through the same routine of my showing up in her office. No matter how busy she was, she always told me to take a seat and do a therapeutic application of her stethoscope. She often told others who noticed my frequent visits that I was “a sickly child” even though I knew she saw through me and said it with a sense of sarcasm and humor.
Of course, life goes on, and one day 20 years ago Mrs. Reed showed up on my hospital census as a new consult after she’d had a minor stroke.
She remembered me very well. Her first comment, said with the same tone I recalled, was that she was amazed I’d lived to adulthood after having been such “a sickly child.” We both laughed.
Now, in her late 80s, she still comes to see me for this and that. Sometimes we reminisce about the intertwined journey our lives have taken us on. Sometimes she asks if I’ve been to PE recently.
Like any patient, she occasionally shows up on the wrong day, or at the wrong time. I always do my best to see her, though.
After all, I owe her big time for letting me dodge PE.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I was (probably) the bane of my elementary school nurse.
I hated PE (I know, who didn’t?). But I also had childhood asthma. So it was an easy out to go to the school nurse, Mrs. Reed, because I was having an asthma attack, or at least claiming to have one.
She’d put me in a chair to “keep an eye” on me, occasionally have me take the prescription drug my pediatrician had ordered (Marax – anyone else remember that?), and knew to send me back to class about 5 minutes before PE was over.
Maybe Mrs. Reed liked me. Maybe it was just the path of least resistance to let me dodge PE. Maybe she’d hated PE, too, and was sympathetic. Who knows?
So twice a week through years of elementary school she and I went through the same routine of my showing up in her office. No matter how busy she was, she always told me to take a seat and do a therapeutic application of her stethoscope. She often told others who noticed my frequent visits that I was “a sickly child” even though I knew she saw through me and said it with a sense of sarcasm and humor.
Of course, life goes on, and one day 20 years ago Mrs. Reed showed up on my hospital census as a new consult after she’d had a minor stroke.
She remembered me very well. Her first comment, said with the same tone I recalled, was that she was amazed I’d lived to adulthood after having been such “a sickly child.” We both laughed.
Now, in her late 80s, she still comes to see me for this and that. Sometimes we reminisce about the intertwined journey our lives have taken us on. Sometimes she asks if I’ve been to PE recently.
Like any patient, she occasionally shows up on the wrong day, or at the wrong time. I always do my best to see her, though.
After all, I owe her big time for letting me dodge PE.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The 2023 ‘Meddy’ awards
Without further ado (or comedy skits or musical numbers or extended tributes or commercials), the Meddys go to ...
Best depiction of emergency medicine’s rollercoaster
M*A*S*H (1970)
The original film, not the TV show, jumps from Frank Burns being hauled away in a straitjacket to a soldier’s spurting neck wound. Hawkeye Pierce calmly steps in and we see the entire sequence of him applying pressure, then stepping back to gown-and-glove (“it’s going to spurt a bit”), then jumping back in with arterial sutures, quipping, “Baby, we’re gonna see some stitchin’ like you never saw before.” After that, cocktail hour. Yes, medicine in Hollywood can be overdramatized and even inaccurate, but Robert Altman’s take on the novel by former U.S. Army surgeon Richard Hooker still stands tall for just how crazy emergency medicine can be.
Best ‘is there a doctor in the house?’ moment
Field of Dreams (1989)
When Ray Kinsella’s daughter gets knocked off the back of the bleachers, everything stops. No one knows what to do … except Doc “Moonlight” Graham, who gives up his life’s (and afterlife’s) dream to step off the field and save the girl from choking to death. Burt Lancaster, in his final movie role, embodies everything people wish a doctor to be: Calm, kind, and able to offer a quick, effective solution to a crisis. “Hey rookie! You were good.” Yes, he sure was.
Most unethical doctor
Elvis (2022)
No doctor wants to be remembered as the guy who killed Elvis. But that legacy clings to Dr. George Nichopoulos, Elvis’s personal physician in the 1970s. In Elvis, Dr. Nichopoulos, played by Tony Nixon, hovers in the background, enabling the King’s worsening addictions. Taking late-night calls for narcotics and injecting the unconscious star with stimulants, “unethical” is an understatement for the fictional “Dr. Nick.” The real Dr. Nichopoulos was acquitted of wrongdoing in Elvis’ death, although there is little doubt that the thousands of medication doses he prescribed played a role. When his license was finally revoked for overprescribing in the 1990s, the obliging doc reportedly claimed, “I cared too much.”
Best self-use of a defibrillator
Casino Royale (2006)
We expect backlash in the post-award press conference since James Bond technically only attempted to self-defibrillate in the passenger seat of his car. He never attached the device to the leads. Vesper Lynd had to pick up his slack and save the day. Also, supporters of fellow self-defibrillating nominee Jason Statham in Crank will no doubt raise a stink on Twitter. But we stand by our choice because it was such an, ahem, heart-stopper of a scene.
Best worst patient lying about an injury
Tár (2022)
Love it or hate it, few recent movies have been as polarizing as Tár. Cate Blanchett’s portrayal of a musical genius might be toweringly brilliant or outrageously offensive (or both) depending on whom you ask. But clearly the character has a loose relationship with facts. More than a few doctors might have raised an eyebrow had Lydia Tár appeared with injuries to her face, claiming to have been attacked in a mugging. In reality, Lydia tripped and fell while pursuing an attractive young cellist into a hazardous basement. Did she lie to protect her image, preserve her marriage, or – like many patients – avoid a lecture on unhealthy behavior? We pick D, all of the above.
Best therapy for a speech disorder
The King’s Speech (2010)
Public speaking might cause anxiety for many of us, but how about doing it in front of a global radio audience while wrestling with a speech disorder? Based on a true story, The King’s Speech revealed that terrifying experience for England’s King George VI. Enter Lionel Logue, played by Geoffrey Rush. Irreverent, unconventional, and untrained, the Australian pioneer in speech and language therapy uses a range of strategies – some of which are still used today – to help the royal find his voice. But when singing, shouting swear words, and provoking rage don’t do the trick, Mr. Logue turns to psychotherapy to unearth the childhood traumas at the root of the king’s disability. Experience, as Mr. Logue tells his patient, matters just as much as “letters after your name.”
A version of this article first appeared on Medscape.com.
Without further ado (or comedy skits or musical numbers or extended tributes or commercials), the Meddys go to ...
Best depiction of emergency medicine’s rollercoaster
M*A*S*H (1970)
The original film, not the TV show, jumps from Frank Burns being hauled away in a straitjacket to a soldier’s spurting neck wound. Hawkeye Pierce calmly steps in and we see the entire sequence of him applying pressure, then stepping back to gown-and-glove (“it’s going to spurt a bit”), then jumping back in with arterial sutures, quipping, “Baby, we’re gonna see some stitchin’ like you never saw before.” After that, cocktail hour. Yes, medicine in Hollywood can be overdramatized and even inaccurate, but Robert Altman’s take on the novel by former U.S. Army surgeon Richard Hooker still stands tall for just how crazy emergency medicine can be.
Best ‘is there a doctor in the house?’ moment
Field of Dreams (1989)
When Ray Kinsella’s daughter gets knocked off the back of the bleachers, everything stops. No one knows what to do … except Doc “Moonlight” Graham, who gives up his life’s (and afterlife’s) dream to step off the field and save the girl from choking to death. Burt Lancaster, in his final movie role, embodies everything people wish a doctor to be: Calm, kind, and able to offer a quick, effective solution to a crisis. “Hey rookie! You were good.” Yes, he sure was.
Most unethical doctor
Elvis (2022)
No doctor wants to be remembered as the guy who killed Elvis. But that legacy clings to Dr. George Nichopoulos, Elvis’s personal physician in the 1970s. In Elvis, Dr. Nichopoulos, played by Tony Nixon, hovers in the background, enabling the King’s worsening addictions. Taking late-night calls for narcotics and injecting the unconscious star with stimulants, “unethical” is an understatement for the fictional “Dr. Nick.” The real Dr. Nichopoulos was acquitted of wrongdoing in Elvis’ death, although there is little doubt that the thousands of medication doses he prescribed played a role. When his license was finally revoked for overprescribing in the 1990s, the obliging doc reportedly claimed, “I cared too much.”
Best self-use of a defibrillator
Casino Royale (2006)
We expect backlash in the post-award press conference since James Bond technically only attempted to self-defibrillate in the passenger seat of his car. He never attached the device to the leads. Vesper Lynd had to pick up his slack and save the day. Also, supporters of fellow self-defibrillating nominee Jason Statham in Crank will no doubt raise a stink on Twitter. But we stand by our choice because it was such an, ahem, heart-stopper of a scene.
Best worst patient lying about an injury
Tár (2022)
Love it or hate it, few recent movies have been as polarizing as Tár. Cate Blanchett’s portrayal of a musical genius might be toweringly brilliant or outrageously offensive (or both) depending on whom you ask. But clearly the character has a loose relationship with facts. More than a few doctors might have raised an eyebrow had Lydia Tár appeared with injuries to her face, claiming to have been attacked in a mugging. In reality, Lydia tripped and fell while pursuing an attractive young cellist into a hazardous basement. Did she lie to protect her image, preserve her marriage, or – like many patients – avoid a lecture on unhealthy behavior? We pick D, all of the above.
Best therapy for a speech disorder
The King’s Speech (2010)
Public speaking might cause anxiety for many of us, but how about doing it in front of a global radio audience while wrestling with a speech disorder? Based on a true story, The King’s Speech revealed that terrifying experience for England’s King George VI. Enter Lionel Logue, played by Geoffrey Rush. Irreverent, unconventional, and untrained, the Australian pioneer in speech and language therapy uses a range of strategies – some of which are still used today – to help the royal find his voice. But when singing, shouting swear words, and provoking rage don’t do the trick, Mr. Logue turns to psychotherapy to unearth the childhood traumas at the root of the king’s disability. Experience, as Mr. Logue tells his patient, matters just as much as “letters after your name.”
A version of this article first appeared on Medscape.com.
Without further ado (or comedy skits or musical numbers or extended tributes or commercials), the Meddys go to ...
Best depiction of emergency medicine’s rollercoaster
M*A*S*H (1970)
The original film, not the TV show, jumps from Frank Burns being hauled away in a straitjacket to a soldier’s spurting neck wound. Hawkeye Pierce calmly steps in and we see the entire sequence of him applying pressure, then stepping back to gown-and-glove (“it’s going to spurt a bit”), then jumping back in with arterial sutures, quipping, “Baby, we’re gonna see some stitchin’ like you never saw before.” After that, cocktail hour. Yes, medicine in Hollywood can be overdramatized and even inaccurate, but Robert Altman’s take on the novel by former U.S. Army surgeon Richard Hooker still stands tall for just how crazy emergency medicine can be.
Best ‘is there a doctor in the house?’ moment
Field of Dreams (1989)
When Ray Kinsella’s daughter gets knocked off the back of the bleachers, everything stops. No one knows what to do … except Doc “Moonlight” Graham, who gives up his life’s (and afterlife’s) dream to step off the field and save the girl from choking to death. Burt Lancaster, in his final movie role, embodies everything people wish a doctor to be: Calm, kind, and able to offer a quick, effective solution to a crisis. “Hey rookie! You were good.” Yes, he sure was.
Most unethical doctor
Elvis (2022)
No doctor wants to be remembered as the guy who killed Elvis. But that legacy clings to Dr. George Nichopoulos, Elvis’s personal physician in the 1970s. In Elvis, Dr. Nichopoulos, played by Tony Nixon, hovers in the background, enabling the King’s worsening addictions. Taking late-night calls for narcotics and injecting the unconscious star with stimulants, “unethical” is an understatement for the fictional “Dr. Nick.” The real Dr. Nichopoulos was acquitted of wrongdoing in Elvis’ death, although there is little doubt that the thousands of medication doses he prescribed played a role. When his license was finally revoked for overprescribing in the 1990s, the obliging doc reportedly claimed, “I cared too much.”
Best self-use of a defibrillator
Casino Royale (2006)
We expect backlash in the post-award press conference since James Bond technically only attempted to self-defibrillate in the passenger seat of his car. He never attached the device to the leads. Vesper Lynd had to pick up his slack and save the day. Also, supporters of fellow self-defibrillating nominee Jason Statham in Crank will no doubt raise a stink on Twitter. But we stand by our choice because it was such an, ahem, heart-stopper of a scene.
Best worst patient lying about an injury
Tár (2022)
Love it or hate it, few recent movies have been as polarizing as Tár. Cate Blanchett’s portrayal of a musical genius might be toweringly brilliant or outrageously offensive (or both) depending on whom you ask. But clearly the character has a loose relationship with facts. More than a few doctors might have raised an eyebrow had Lydia Tár appeared with injuries to her face, claiming to have been attacked in a mugging. In reality, Lydia tripped and fell while pursuing an attractive young cellist into a hazardous basement. Did she lie to protect her image, preserve her marriage, or – like many patients – avoid a lecture on unhealthy behavior? We pick D, all of the above.
Best therapy for a speech disorder
The King’s Speech (2010)
Public speaking might cause anxiety for many of us, but how about doing it in front of a global radio audience while wrestling with a speech disorder? Based on a true story, The King’s Speech revealed that terrifying experience for England’s King George VI. Enter Lionel Logue, played by Geoffrey Rush. Irreverent, unconventional, and untrained, the Australian pioneer in speech and language therapy uses a range of strategies – some of which are still used today – to help the royal find his voice. But when singing, shouting swear words, and provoking rage don’t do the trick, Mr. Logue turns to psychotherapy to unearth the childhood traumas at the root of the king’s disability. Experience, as Mr. Logue tells his patient, matters just as much as “letters after your name.”
A version of this article first appeared on Medscape.com.












