User login
Treatment duration for acute otitis media – so many choices
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.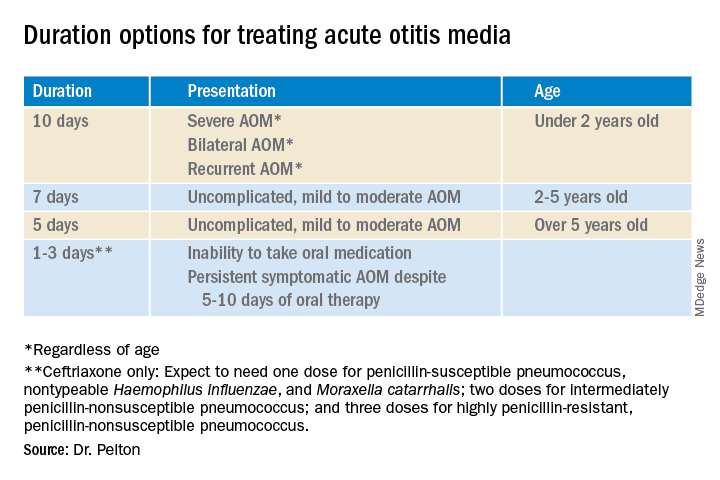
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.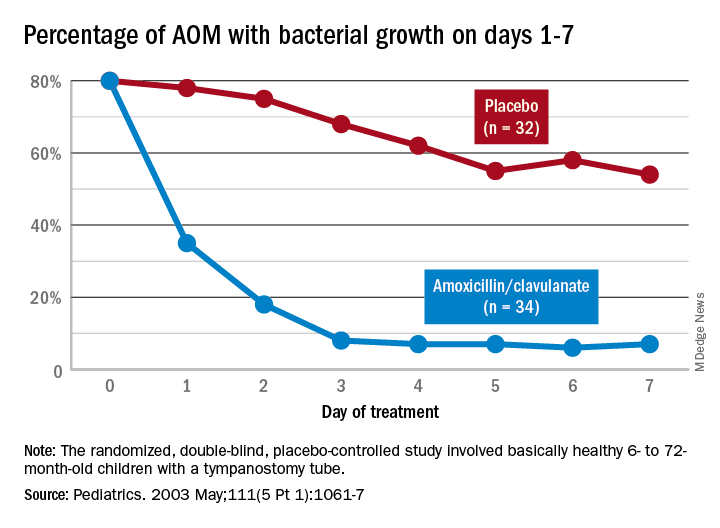
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
Twenty years ago, the dilemma in treating acute otitis media (AOM) was which among 10-plus antibiotics to prescribe. A recent column discussed the evolving pathogen distribution in AOM and its effects on antibiotic choices.1 But here we consider treatment duration. Until the past decade, AOM treatment (except azithromycin) involved 10-day courses. But lately, 10-day antibiotic regimens for uncomplicated infections are disappearing. Shorter-course recommendations are the new norm because of the evolving clinical data showing that an appropriately chosen antibiotic (in partnership with host defenses and source control) resolves infection faster than was previously thought. Shorter courses make sense because of fewer adverse effects, less distortion of normal flora, and less likely induction of pathogen resistance. Table 4.12 in the newest 2021-2024 SOID Redbook lists three antibiotic durations for AOM, and actually there are more than that.
Why so many duration options? Clinical data show that not all AOM is alike and short courses work for subsets of AOM because, besides antibiotics, key elements in AOM resolution are host anatomy and immunity. Bacterial AOM results from a combination of refluxed pathogens in the middle ear being trapped when the eustachian tube malfunctions (infection occurs when middle ear plumbing gets stopped up). If the eustachian tube spontaneously drains and the host immune response slows/stops pathogen growth, no antibiotics are needed. Indeed, a sizable proportion of mild/moderate AOM episodes spontaneously resolve, particularly in children over 2 years old. So a high likelihood of spontaneous remission allows an initial 0-days duration option (watchful waiting) or delayed antibiotics (rescue prescriptions) for older children.
That said, when one chooses to initially prescribe antibiotics for AOM, different durations are recommended. Table 1 has my suggestions.
Data that gave me better microbiological understanding of why oral AOM trials less than 10 days were successful involved purulent AOM drainage from children who had pressure-equalizing (PE) tubes.2 The authors randomized children to either standard-dose amoxicillin-clavulanate or placebo. Of note, 95% of pathogens were susceptible to the antibiotic; 5% were pneumococcus intermediately resistant to penicillin. The authors sampled ear drainage daily for 7 days. Figure 1 shows that cultures remained positive in only around 5% of children by day 3-5 of antibiotics, but viable bacteria persisted through 7 days in over half of placebo recipients. Remember, both groups benefited from a form of source control (drainage of the middle ear via PE tubes). So, if antibiotics can do the job in 3-5 days, why continue antibiotics beyond 5 days?
Anatomy and severity. In children over 5 years old (reasonably mature eustachian tube anatomy) with nonrecurrent (no AOM in past month), nonsevere (no otalgia or high fever) AOM, 5 days is enough. But 2- to 5-year-olds (less mature anatomy) need 7 days and those <2 years old (least mature plumbing) need 10 days. Likewise, severe AOM usually warrants 10 days. Some experts recommend 10 days for bilateral AOM as well.
These age/severity differences make sense because failures are more frequent with:
1. Younger age.3 While not proven, my hypothesis is that “natural” source control (spontaneous internal draining the middle ear into the nasopharynx [NP]) is less frequent in younger children because they have less mature eustachian tube systems. Further, reflux of persisting NP organisms could restart a new AOM episode even if the original pathogen was eliminated by a short 5-day course.
2. Severe AOM. A rationale for longer courses in severe AOM (ear pain, high fever) is that high middle-ear pressures (indicated by degree of tympanic membrane bulging and ear pain) could impede antibiotic penetration, or that high initial bacterial loads (perhaps indicated by systemic fever) require more antibiotic. And finally, return to baseline eustachian tube function may take longer if severe AOM caused enhanced inflammation.
3. Recurrent AOM. (AOM within 1 prior month) – With recurrent AOM, the second “hit” to the eustachian tube may lead to more dysfunction, so a longer antibiotic course may be required to allow more complete source control and more time for more complete functional recovery after a repeated inflammatory injury.
4. Bilateral AOM. Two independent but infected sites mean twice the chance for failure. So, a longer course could allow more time for both sites to undergo “natural” source control.4
More bacteria – more antibiotic? So, is more antibiotic really needed for a higher bacterial load? In vitro this is known as the “inoculum effect,” particularly for beta-lactam drugs, for example, amoxicillin and cephalosporins. Laboratory susceptibility testing is performed with a specifically defined quantity of bacteria (105 bacteria/mL) and the minimum inhibitory concentration (MIC) is the lowest antibiotic concentration that stops bacterial growth. We know that drugs will likely fail if the MIC exceeds the achievable antibiotic concentration at the infection site. But is it as simple as just exceeding the MIC at the infection site? No, pharmacodynamics tell us that overall antibiotic exposure is also important. For example, to be successful, beta-lactam concentrations need to be above the MIC for 40%-50% of the day.
Higher MIC with higher bacterial load. Particularly for beta-lactams, testing with a quantity of bacteria >105/mL produces a higher MIC in vitro. This suggests that clinical failure could occur, even when our in vivo dosing leads to 40%-50% above the “standard” MIC that was obtained from testing the lab standard of 105/mL bacteria, when the infected site’s (middle ear) bacterial load is >105/mL (such higher bacterial loads occur in up to 30% of AOM).5 One way to negate inoculum effect is source control (drain the abscess or debridement), which reduces the bacterial load as well as allowing better antibiotic penetration– both favoring infection resolution. But with suboptimal source control, for example, the middle ear is not drained externally or internally, longer courses (more antibiotic exposure) could aid resolution. Whether the exposure can be administered as higher doses in fewer days or standard doses for more days is debatable but consider that a single parenteral dose of ceftriaxone successfully resolves AOM not attributable to penicillin-nonsusceptible pneumococcus.6Bottom line: Even though the number of potential antibiotics has contracted in the past 20 years, the need to individualize AOM treatment remains important and duration choices are more complex. Indeed, AOM comes in different flavors with patient age, clinical presentation, and episode frequency dictating the choice of duration.
Dr. Christopher J. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. Email him at [email protected].
References
1. Pichichero ME. MDedge. 2022 Jan 11.
2. Ruohola A et al. Pediatrics. 2003;111(5):1061-7.
3. Hoberman A et al. N Engl J Med. 2016;375(25):2446-56.
4. Pichichero ME et al. Otolaryngol Head Neck Surg. 2001;124(4):381-7.
5. Harrison CJ et al. Pediatr Infect Dis. 1985;4(6):641-6.
6. Leibovitz E et al. Pediatr Infect Dis. 2000;19(11):1040-5.
No-shows
I’m not fanatical about dragging stable patients in. If someone is doing fine, having them come in once a year is all I ask. They have better things to do, and I have patients who need my attention more.
Of course, there will always be those who abuse this. They try to drag it out to 18 months, sometimes 2 years. I don’t think having patients drop in for 10-15 minutes once a year to make sure they’re still alive is unreasonable, but maybe that’s just me. Admittedly, during the last 2 years I’ve kind of let it slide a bit, but I think everyone has.
Last week a lady I see for an annual check-in called to make an appointment. She’d been dodging my secretary’s reminders for a few months, so I cut her migraine refill from a 90-day supply to 30 days to encourage her. She called, made an appointment for the following morning, and asked that I send in a refill for 90 days because otherwise her insurance won’t cover it. So, trying to be nice, I did, figuring she was on the schedule now.
Of course, she didn’t show up the next morning. She didn’t cancel, or call in with “I’m sick” or “sorry, I spaced on it” or some other issue. She just no-showed. One of the many banes of outpatient medicine.
Normally I avoid looking at my patients’ online presence, but I got curious. This lady has often suggested I check out her social media account for financial and real estate tips. I never had, until that morning.
Her Twitter account for the last several days was full of reminders to her followers for an in-person seminar on real estate flipping that she was hosting, which, surprisingly, started at the exact time as her appointment with me was supposed to.
I’m pretty sure she ain’t that stupid. She knew exactly what she was doing, and never planned on keeping the appointment. Now she had a 90-day supply of meds and no incentive to follow up with me before then.
Certainly, it’s not the worst thing. The drug involved isn’t controlled, and in 24 years I’ve had patients do far worse.
But it still changes the trust factor in the medical relationship. She isn’t getting another 90-day refill without coming in, and if she has to pay cash for 30 days that’s her problem, not mine. She can avoid that by calling in to schedule before then. Though I doubt she will.
I try to work with my patients. I really do. Her behavior is rude and inconsiderate, but (at least to me) doesn’t cross the line to firing her from the practice.
But it does make it trickier to be her doctor, since I now know that she isn’t always truthful with me and my staff.
And that sort of thing is important in this field.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not fanatical about dragging stable patients in. If someone is doing fine, having them come in once a year is all I ask. They have better things to do, and I have patients who need my attention more.
Of course, there will always be those who abuse this. They try to drag it out to 18 months, sometimes 2 years. I don’t think having patients drop in for 10-15 minutes once a year to make sure they’re still alive is unreasonable, but maybe that’s just me. Admittedly, during the last 2 years I’ve kind of let it slide a bit, but I think everyone has.
Last week a lady I see for an annual check-in called to make an appointment. She’d been dodging my secretary’s reminders for a few months, so I cut her migraine refill from a 90-day supply to 30 days to encourage her. She called, made an appointment for the following morning, and asked that I send in a refill for 90 days because otherwise her insurance won’t cover it. So, trying to be nice, I did, figuring she was on the schedule now.
Of course, she didn’t show up the next morning. She didn’t cancel, or call in with “I’m sick” or “sorry, I spaced on it” or some other issue. She just no-showed. One of the many banes of outpatient medicine.
Normally I avoid looking at my patients’ online presence, but I got curious. This lady has often suggested I check out her social media account for financial and real estate tips. I never had, until that morning.
Her Twitter account for the last several days was full of reminders to her followers for an in-person seminar on real estate flipping that she was hosting, which, surprisingly, started at the exact time as her appointment with me was supposed to.
I’m pretty sure she ain’t that stupid. She knew exactly what she was doing, and never planned on keeping the appointment. Now she had a 90-day supply of meds and no incentive to follow up with me before then.
Certainly, it’s not the worst thing. The drug involved isn’t controlled, and in 24 years I’ve had patients do far worse.
But it still changes the trust factor in the medical relationship. She isn’t getting another 90-day refill without coming in, and if she has to pay cash for 30 days that’s her problem, not mine. She can avoid that by calling in to schedule before then. Though I doubt she will.
I try to work with my patients. I really do. Her behavior is rude and inconsiderate, but (at least to me) doesn’t cross the line to firing her from the practice.
But it does make it trickier to be her doctor, since I now know that she isn’t always truthful with me and my staff.
And that sort of thing is important in this field.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
I’m not fanatical about dragging stable patients in. If someone is doing fine, having them come in once a year is all I ask. They have better things to do, and I have patients who need my attention more.
Of course, there will always be those who abuse this. They try to drag it out to 18 months, sometimes 2 years. I don’t think having patients drop in for 10-15 minutes once a year to make sure they’re still alive is unreasonable, but maybe that’s just me. Admittedly, during the last 2 years I’ve kind of let it slide a bit, but I think everyone has.
Last week a lady I see for an annual check-in called to make an appointment. She’d been dodging my secretary’s reminders for a few months, so I cut her migraine refill from a 90-day supply to 30 days to encourage her. She called, made an appointment for the following morning, and asked that I send in a refill for 90 days because otherwise her insurance won’t cover it. So, trying to be nice, I did, figuring she was on the schedule now.
Of course, she didn’t show up the next morning. She didn’t cancel, or call in with “I’m sick” or “sorry, I spaced on it” or some other issue. She just no-showed. One of the many banes of outpatient medicine.
Normally I avoid looking at my patients’ online presence, but I got curious. This lady has often suggested I check out her social media account for financial and real estate tips. I never had, until that morning.
Her Twitter account for the last several days was full of reminders to her followers for an in-person seminar on real estate flipping that she was hosting, which, surprisingly, started at the exact time as her appointment with me was supposed to.
I’m pretty sure she ain’t that stupid. She knew exactly what she was doing, and never planned on keeping the appointment. Now she had a 90-day supply of meds and no incentive to follow up with me before then.
Certainly, it’s not the worst thing. The drug involved isn’t controlled, and in 24 years I’ve had patients do far worse.
But it still changes the trust factor in the medical relationship. She isn’t getting another 90-day refill without coming in, and if she has to pay cash for 30 days that’s her problem, not mine. She can avoid that by calling in to schedule before then. Though I doubt she will.
I try to work with my patients. I really do. Her behavior is rude and inconsiderate, but (at least to me) doesn’t cross the line to firing her from the practice.
But it does make it trickier to be her doctor, since I now know that she isn’t always truthful with me and my staff.
And that sort of thing is important in this field.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
ctDNA shows promise for assessing lung cancer treatment response
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
This transcript has been edited for clarity. A version of this article first appeared on Medscape.com.
Hello. This is Mark Kris from Memorial Sloan Kettering, talking today about circulating tumor DNA (ctDNA), an emerging technology for use in perioperative patients. Recently, there have been a number of presentations about the use of ctDNA measurements in patients receiving pre- or postoperative therapies. These are critical therapies because they are given with the intention of improving the chance for cure.
All three of the presentations I’m going to mention have one thing in common: They used the so-called tumor-informed panel. That technology is going to become very important, as shown in these presentations.
I made one of these presentations at the European Society for Medical Oncology Immuno-Oncology virtual meeting in Geneva. In our study, we were able to find genes in the majority of patients who had tumor tissue available. These patients were preoperative surgical candidates. In 72% of these, we were able to find and track ctDNA. When we tracked the DNA in the blood, we saw that the falling levels of DNA were associated with shrinkages of the cancer radiographically – the degree of shrinkage seen in this case in the neoadjuvant examination at the time of surgery and examining the resection specimen after neoadjuvant therapy. Ultimately, the major pathologic responses were associated with clearing or falling DNA as well. Perhaps the most interesting observation is that when you put this DNA information together with the major pathologic response information, all of the patients who had clearance of ctDNA and had a major pathologic response were disease free. I believe that eventually we will use this ctDNA data in conjunction with other measures of benefit to reach a more precise assessment of therapy benefit, and eventually it may be helpful for prognosis as well.
Two other studies also used this technology. One was earlier this year, presented by Patrick Forde at the American Association for Cancer Research meeting. They associated changes in ctDNA using another tumor-informed assay. In that study, using the Archer assay, they were able to show that the ctDNA clearance was associated with a complete pathologic response. So again, combining this information provides a more precise measurement of the benefit of therapy.
Another presentation at ESMO Immuno-Oncology, by Caicun Zhou, looked at the Natera assay, another tumor-informed assay, in a trial of adjuvant atezolizumab. This group showed that patients who had clearance of their ctDNA after surgery had the greatest benefit from subsequent atezolizumab therapy. And even those patients who did not have clearance experienced some benefit of the atezolizumab therapy. In addition, they assessed the degree of benefit associated with whether or not PD-L1 was present. Those patients who had PD-L1 expression experienced the greatest benefit from the atezolizumab. For patients who didn’t have PD-L1 expression, where you wouldn’t expect atezolizumab to have this greater benefit, they didn’t see it.
I believe that ctDNA-informed testing will become more and more useful, both in clinical trials and ultimately in the care of patients with early-stage lung cancers. These tumor-informed assays are going to be standards of care and provide physicians and patients a better estimate of the effectiveness of therapy going forward.
Dr. Kris is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York. He reported serving as a consultant and/or adviser for AstraZeneca, Daiichi Sankyo, and Pfizer, and has received payments for various services from Genentech.
Caring for suicidal youth: An approach for pediatricians
This month’s column is driven by the recent increase of youth in crisis, and COVID-19–related limitations of higher-level services. Suicide is the second leading cause of death among youth1 and populations who face discrimination are at increased risk.2,3
A pediatrician colleague recently asked me about how to support patients who may be at risk. With inpatient units and emergency departments over capacity, properly allocating resources to patients with the most acute needs is crucial. When appropriate, providing preventive suicide care in primary care similarly saves lives.
Case summary
Cassandra is a 16-year-old Black girl who told a friend on Snapchat that she did not want to be alive. The friend told her parents and Cassandra’s parents brought their daughter to an urgent primary care appointment. Cassandra has had a history of difficulty with large transitions like a family move when she was 13. She spent more time in her room for several months before joining the volleyball team and making new friends. She has always done well academically in school but struggled with insomnia and classwork when her high school shifted to remote learning for the 2020-2021 school year because of the pandemic. This year she attends school in person but is unable to play volleyball because of COVID-19 restrictions. Her parents report that she is again spending more time online in her room. She is passing her classes and doing well in math, but overall, her grades have fallen since the pandemic began. She reports recent difficulties with friends and notes feeling hopeless about a changing climate and race relations in the United States.
Discussion
This case example illustrates some factors pediatricians can consider in determining how to proceed in similar circumstances. What are Cassandra’s immediate risk and treatment needs? In cases like Cassandra’s, the American Academy of Pediatrics recommends the ABCD (Assess, Build hope, Connect, Develop a safety plan) approach.4 Preparing practices to deliver this best possible preventive suicide care is essential.
1. Is this patient at imminent risk of harming herself?
Assess: Screen for suicide risk and assess risk level. Several standardized screening tools exist for gauging a patient’s risk. The Ask Suicide Screening Questionnaire (asQ) is a straightforward screening tool (not to be confused with the ASQ Ages and Stages developmental screening). These questionnaires take only a few minutes and next steps are suggested depending on the score (low, moderate, or high risk) and clinical judgment. What matters most is using a standardized screener to directly ask questions about suicide and then follow up appropriately based on risk.
2. What can be done during the visit to promote a good outcome?
Build hope/reasons for living. Validate that people sometimes feel suicidal when things are difficult, but that the feelings come and go and people go on to live meaningful lives. Tell the patients that you care about keeping them safe when the feelings come up. Motivational interviewing can be helpful to reflect back patient-identified reasons for living. Genuinely tell the patients how much you care about their wellbeing.
3. What can be done outside the visit to promote a good outcome?
Connect: Strengthen connections with protective adults. Make a plan to have the patient connect regularly with parents/trusted adults. She could engage in social action, or connect one-on-one. With more structured social opportunities, she will spend less time online. Medical practices can reach out with postcards and phone calls to show that they care about the patient, an intervention called “Caring Contacts” that has been shown to decrease suicide.
4. Once suicide risk is identified, what are specific tools to use during the visit to keep her safe?
Develop a plan for staying safe: Restrict access to lethal means, develop a safety plan and healthy ways of coping. There is a free 2-hour CALM (Counseling on Access to Lethal Means) training to help providers feel competent in restricting access to lethal means prior to increased risk. This resource provides safety plan templates that help identify triggers, specific ways to stay safe, people to talk to, and suicide prevention resources including lifelines (988) and chat options (text 2 letter state to 741741).
Enacting suicide prevention requires practice readiness and workflow changes. Providers should assess mental health supports in and out of the office, and then rehearse workflow around suicide prevention care. Increasingly, there are embedded case managers or behavioral health providers available. Sometimes local mental health crisis services are the best option. A practice introductory letter to community mental health practitioners can improve later coordination efforts when caring for suicidal youth. Having practice-level support for provider well-being can improve outcomes.
Case follow-up
After interviewing the girl separately, and performing a PHQ-A and an asQ, followed by the Brief Suicide Safety Assessment to screen for acuity, the pediatrician felt confident that Cassandra was suffering from moderate depression and had moderate but not imminent risk of suicide. Options to treat her depression were discussed with Cassandra and her parents, and a referral to therapy was made.
The provider knew that depression care is complementary but not sufficient as standalone suicide prevention. The provider used the asQ pathway to determine next steps. He made a safety plan, and referred her to an outpatient mental health clinician with whom the practice had an established relationship for an urgent mental health evaluation. A follow-up primary care appointment was scheduled within 72 hours to re-check safety and ensure that she had an appointment scheduled to start therapy. A nurse contacted the patient and her family regularly to check on her wellbeing. Her parents made a plan with her volleyball coach to organize outdoor off-season conditioning to help with exercise and socializing. The family removed screens prior to bedtime and sleep improved. At a 3-month follow-up, Cassandra had only mild depressive symptoms and the frequency and intensity of her suicidal ideation had decreased.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont, a Federally Qualified Health Center. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. National Institute of Mental Health: Suicide.
2. Hottes TS et al. Am J Public Health. 2016 May;106(5):e1-12.
3. Bridge JA et al. JAMA Pediatr. 2018;172(7):697-9.
4. Asarnow JR. SAMHSA Center for Adolescent Suicide and Self-Harm..
This month’s column is driven by the recent increase of youth in crisis, and COVID-19–related limitations of higher-level services. Suicide is the second leading cause of death among youth1 and populations who face discrimination are at increased risk.2,3
A pediatrician colleague recently asked me about how to support patients who may be at risk. With inpatient units and emergency departments over capacity, properly allocating resources to patients with the most acute needs is crucial. When appropriate, providing preventive suicide care in primary care similarly saves lives.
Case summary
Cassandra is a 16-year-old Black girl who told a friend on Snapchat that she did not want to be alive. The friend told her parents and Cassandra’s parents brought their daughter to an urgent primary care appointment. Cassandra has had a history of difficulty with large transitions like a family move when she was 13. She spent more time in her room for several months before joining the volleyball team and making new friends. She has always done well academically in school but struggled with insomnia and classwork when her high school shifted to remote learning for the 2020-2021 school year because of the pandemic. This year she attends school in person but is unable to play volleyball because of COVID-19 restrictions. Her parents report that she is again spending more time online in her room. She is passing her classes and doing well in math, but overall, her grades have fallen since the pandemic began. She reports recent difficulties with friends and notes feeling hopeless about a changing climate and race relations in the United States.
Discussion
This case example illustrates some factors pediatricians can consider in determining how to proceed in similar circumstances. What are Cassandra’s immediate risk and treatment needs? In cases like Cassandra’s, the American Academy of Pediatrics recommends the ABCD (Assess, Build hope, Connect, Develop a safety plan) approach.4 Preparing practices to deliver this best possible preventive suicide care is essential.
1. Is this patient at imminent risk of harming herself?
Assess: Screen for suicide risk and assess risk level. Several standardized screening tools exist for gauging a patient’s risk. The Ask Suicide Screening Questionnaire (asQ) is a straightforward screening tool (not to be confused with the ASQ Ages and Stages developmental screening). These questionnaires take only a few minutes and next steps are suggested depending on the score (low, moderate, or high risk) and clinical judgment. What matters most is using a standardized screener to directly ask questions about suicide and then follow up appropriately based on risk.
2. What can be done during the visit to promote a good outcome?
Build hope/reasons for living. Validate that people sometimes feel suicidal when things are difficult, but that the feelings come and go and people go on to live meaningful lives. Tell the patients that you care about keeping them safe when the feelings come up. Motivational interviewing can be helpful to reflect back patient-identified reasons for living. Genuinely tell the patients how much you care about their wellbeing.
3. What can be done outside the visit to promote a good outcome?
Connect: Strengthen connections with protective adults. Make a plan to have the patient connect regularly with parents/trusted adults. She could engage in social action, or connect one-on-one. With more structured social opportunities, she will spend less time online. Medical practices can reach out with postcards and phone calls to show that they care about the patient, an intervention called “Caring Contacts” that has been shown to decrease suicide.
4. Once suicide risk is identified, what are specific tools to use during the visit to keep her safe?
Develop a plan for staying safe: Restrict access to lethal means, develop a safety plan and healthy ways of coping. There is a free 2-hour CALM (Counseling on Access to Lethal Means) training to help providers feel competent in restricting access to lethal means prior to increased risk. This resource provides safety plan templates that help identify triggers, specific ways to stay safe, people to talk to, and suicide prevention resources including lifelines (988) and chat options (text 2 letter state to 741741).
Enacting suicide prevention requires practice readiness and workflow changes. Providers should assess mental health supports in and out of the office, and then rehearse workflow around suicide prevention care. Increasingly, there are embedded case managers or behavioral health providers available. Sometimes local mental health crisis services are the best option. A practice introductory letter to community mental health practitioners can improve later coordination efforts when caring for suicidal youth. Having practice-level support for provider well-being can improve outcomes.
Case follow-up
After interviewing the girl separately, and performing a PHQ-A and an asQ, followed by the Brief Suicide Safety Assessment to screen for acuity, the pediatrician felt confident that Cassandra was suffering from moderate depression and had moderate but not imminent risk of suicide. Options to treat her depression were discussed with Cassandra and her parents, and a referral to therapy was made.
The provider knew that depression care is complementary but not sufficient as standalone suicide prevention. The provider used the asQ pathway to determine next steps. He made a safety plan, and referred her to an outpatient mental health clinician with whom the practice had an established relationship for an urgent mental health evaluation. A follow-up primary care appointment was scheduled within 72 hours to re-check safety and ensure that she had an appointment scheduled to start therapy. A nurse contacted the patient and her family regularly to check on her wellbeing. Her parents made a plan with her volleyball coach to organize outdoor off-season conditioning to help with exercise and socializing. The family removed screens prior to bedtime and sleep improved. At a 3-month follow-up, Cassandra had only mild depressive symptoms and the frequency and intensity of her suicidal ideation had decreased.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont, a Federally Qualified Health Center. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. National Institute of Mental Health: Suicide.
2. Hottes TS et al. Am J Public Health. 2016 May;106(5):e1-12.
3. Bridge JA et al. JAMA Pediatr. 2018;172(7):697-9.
4. Asarnow JR. SAMHSA Center for Adolescent Suicide and Self-Harm..
This month’s column is driven by the recent increase of youth in crisis, and COVID-19–related limitations of higher-level services. Suicide is the second leading cause of death among youth1 and populations who face discrimination are at increased risk.2,3
A pediatrician colleague recently asked me about how to support patients who may be at risk. With inpatient units and emergency departments over capacity, properly allocating resources to patients with the most acute needs is crucial. When appropriate, providing preventive suicide care in primary care similarly saves lives.
Case summary
Cassandra is a 16-year-old Black girl who told a friend on Snapchat that she did not want to be alive. The friend told her parents and Cassandra’s parents brought their daughter to an urgent primary care appointment. Cassandra has had a history of difficulty with large transitions like a family move when she was 13. She spent more time in her room for several months before joining the volleyball team and making new friends. She has always done well academically in school but struggled with insomnia and classwork when her high school shifted to remote learning for the 2020-2021 school year because of the pandemic. This year she attends school in person but is unable to play volleyball because of COVID-19 restrictions. Her parents report that she is again spending more time online in her room. She is passing her classes and doing well in math, but overall, her grades have fallen since the pandemic began. She reports recent difficulties with friends and notes feeling hopeless about a changing climate and race relations in the United States.
Discussion
This case example illustrates some factors pediatricians can consider in determining how to proceed in similar circumstances. What are Cassandra’s immediate risk and treatment needs? In cases like Cassandra’s, the American Academy of Pediatrics recommends the ABCD (Assess, Build hope, Connect, Develop a safety plan) approach.4 Preparing practices to deliver this best possible preventive suicide care is essential.
1. Is this patient at imminent risk of harming herself?
Assess: Screen for suicide risk and assess risk level. Several standardized screening tools exist for gauging a patient’s risk. The Ask Suicide Screening Questionnaire (asQ) is a straightforward screening tool (not to be confused with the ASQ Ages and Stages developmental screening). These questionnaires take only a few minutes and next steps are suggested depending on the score (low, moderate, or high risk) and clinical judgment. What matters most is using a standardized screener to directly ask questions about suicide and then follow up appropriately based on risk.
2. What can be done during the visit to promote a good outcome?
Build hope/reasons for living. Validate that people sometimes feel suicidal when things are difficult, but that the feelings come and go and people go on to live meaningful lives. Tell the patients that you care about keeping them safe when the feelings come up. Motivational interviewing can be helpful to reflect back patient-identified reasons for living. Genuinely tell the patients how much you care about their wellbeing.
3. What can be done outside the visit to promote a good outcome?
Connect: Strengthen connections with protective adults. Make a plan to have the patient connect regularly with parents/trusted adults. She could engage in social action, or connect one-on-one. With more structured social opportunities, she will spend less time online. Medical practices can reach out with postcards and phone calls to show that they care about the patient, an intervention called “Caring Contacts” that has been shown to decrease suicide.
4. Once suicide risk is identified, what are specific tools to use during the visit to keep her safe?
Develop a plan for staying safe: Restrict access to lethal means, develop a safety plan and healthy ways of coping. There is a free 2-hour CALM (Counseling on Access to Lethal Means) training to help providers feel competent in restricting access to lethal means prior to increased risk. This resource provides safety plan templates that help identify triggers, specific ways to stay safe, people to talk to, and suicide prevention resources including lifelines (988) and chat options (text 2 letter state to 741741).
Enacting suicide prevention requires practice readiness and workflow changes. Providers should assess mental health supports in and out of the office, and then rehearse workflow around suicide prevention care. Increasingly, there are embedded case managers or behavioral health providers available. Sometimes local mental health crisis services are the best option. A practice introductory letter to community mental health practitioners can improve later coordination efforts when caring for suicidal youth. Having practice-level support for provider well-being can improve outcomes.
Case follow-up
After interviewing the girl separately, and performing a PHQ-A and an asQ, followed by the Brief Suicide Safety Assessment to screen for acuity, the pediatrician felt confident that Cassandra was suffering from moderate depression and had moderate but not imminent risk of suicide. Options to treat her depression were discussed with Cassandra and her parents, and a referral to therapy was made.
The provider knew that depression care is complementary but not sufficient as standalone suicide prevention. The provider used the asQ pathway to determine next steps. He made a safety plan, and referred her to an outpatient mental health clinician with whom the practice had an established relationship for an urgent mental health evaluation. A follow-up primary care appointment was scheduled within 72 hours to re-check safety and ensure that she had an appointment scheduled to start therapy. A nurse contacted the patient and her family regularly to check on her wellbeing. Her parents made a plan with her volleyball coach to organize outdoor off-season conditioning to help with exercise and socializing. The family removed screens prior to bedtime and sleep improved. At a 3-month follow-up, Cassandra had only mild depressive symptoms and the frequency and intensity of her suicidal ideation had decreased.
Dr. Spottswood is a child psychiatrist practicing in an integrated care clinic at the Community Health Centers of Burlington, Vermont, a Federally Qualified Health Center. She is the medical director of the Vermont Child Psychiatry Access Program and a clinical assistant professor in the department of psychiatry at the University of Vermont.
References
1. National Institute of Mental Health: Suicide.
2. Hottes TS et al. Am J Public Health. 2016 May;106(5):e1-12.
3. Bridge JA et al. JAMA Pediatr. 2018;172(7):697-9.
4. Asarnow JR. SAMHSA Center for Adolescent Suicide and Self-Harm..
Did you know these things about nicotine? Your patients don’t
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
When asked, young people report that their reasons for starting smoking include rebellion, a new thing to try, and a peer social activity, among others. While you recognize these as developmentally expected drives, it is frustrating and scary that youth don’t realize how their brains are especially sensitive to permanent changes from nicotine.
Smoking even five packs of cigarettes is enough to cause addiction in youth; an influence as powerful as for cocaine or heroin. One pod of a vaping device delivers as much nicotine as one to five packs of cigarettes, depending on the strength and brand. There are no standards for this content and youth often are unaware of any nicotine and chemicals in vapes. Over 90% of adult smokers started before age 18, some as young as 6, mainly because quitting is so difficult. Cigarettes and vaping are not the only sources of nicotine used by youth; others are oral tobacco (chewing tobacco and dip), cigars, pipes, snus (between cheek and gum), hookahs, electronic devices, bidis (tobacco in a tendu leaf), kreteks (tobacco with cloves), and dissolvable tobacco products. Many youth use both cigarettes and noncigarette tobacco.
Given these predispositions, short-term COVID-19 and asthma exacerbation, and the long-lasting detriment of smoking on neurological, cardiac, pulmonary, and emotional health, actually the “leading preventable cause of death,” our job as pediatric providers is to do our best to prevent smoking/vaping or help our patients quit. But adolescent development is notoriously characterized by short-term thinking and feeling immune from long-term health consequences. So what approach has the best results? Focus on aspects of smoking important to the youth now, such as sports performance, bad breath, social stigma, insomnia, cost, lack of benefit for weight loss, and hazardous waste produced. Add to that loss of independence and being manipulated by Big Business by getting them (and targeted minorities) hooked may be salient in our discussion.
Even a brief 3-minute discussion using the AAC (Ask/Assess, Advise, Connect) format has shown effectiveness in getting teens and adults to quit smoking. Our assessment needs to include asking the extent of current use and symptoms of dependence to inform the treatment plan. We need to use their trust in us to advise that quitting is the best thing they can do for their health.
If the youth’s readiness stage is “thinking about stopping” nicotine, our motivational interview–style discussion of pros and cons could include asking “How important is it to you to stop?” and “What are some things that would help you?” If they are open to trying to stop, advise them to set a quit date within 2 weeks and suggest reducing gradually before then (and schedule follow-up). The plan needs to include dealing with the inevitable urges by finding ways to avoid current triggers to smoke (e.g., certain school bathrooms, people drinking or smoking, or stress over homework, conflict at home, etc.). Encourage exercise and meditation to distract and deal with the anxiety; asking family to quit; having a snack handy (such as sugarless gum or sunflower seeds) for when oral cravings develop; and setting rewards for early days of smoke-free success. We need to inform youth that using e-cigs actually reduces rates of success in quitting.
We need to warn youth of the withdrawal symptoms and their usual course when quitting: cravings each lasting 15-20 minutes (starting at 1/2-4 hours); restlessness, sadness, hopelessness (10 hours); irritability, trouble concentrating, insomnia, hunger and weight gain (5-10 pounds over 2 weeks, starting 24 hrs); headaches, dizziness, fatigue (starting 2 days); and anxiety (starting 3 days). There tends to be less brain fog, and less hunger after 2-4 weeks, but depression, anxiety, irritability, cough, constipation, and even suicidal thoughts may last weeks to months. Sounds nasty, right? No wonder quitting is so hard.
Support is crucial to quitting and staying off nicotine. You can provide this but, in addition to friends and family, we should connect youth to free ongoing phone counselors (1-800-QUIT-NOW or 877-44U-QUIT for Spanish), text services (text QUIT to 47848), apps (quit START), or community support.
While behavioral treatments are best for youth with minimal to mild dependence, risk of relapse is minimized with fewer withdrawal symptoms, thus the role for nicotine replacement therapy (NRT) for those with moderate to strong dependence and to help anyone ad lib with cravings. NRT is recommended by the American Academy of Pediatrics (AAP) to supplement counseling, although NRT is not Food and Drug Administration approved and requires a prescription for those under 18.
How can we determine the degree of dependence? Smoking more than 15 cigarettes per day (or vape equivalent) and inhaling even “seldom” counts as “moderate” dependence and more than 26 with difficulty refraining in several situations as “substantial” in the Fagerstrom Tolerance test. Early morning smoking is asked about, important to which NRT to use (gum or lozenge for faster onset). The Hooked on Nicotine Checklist assesses “loss of autonomy” over smoking by any “yes” item and is incorporated in the CRAFFT screen. The recommended dose of NRT and length of weaning is greater in substantial addiction versus moderate. Besides gum, lozenges, patch, inhaler, and nasal spray, you can prescribe bupropion (Wellbutrin or Zyban) or varenicline (Chantix), making note of the black box suicide warning. Combining NRTs is similarly effective compared with varenicline.
Relapse after quitting is more common than not. As for any chronic condition, in relapse we need to query adherence, and consider increasing NRT dose or wean duration, even years. Discussion should have a positive focus on “what was learned” from past attempts in making a new plan that incorporates Relevance, Risks, Rewards, Roadblocks, and Repetition.
Many youth smokers start because their parents smoke. While addressing adults may seem out of scope, we often treat parents when managing scabies, pinworms, meningococcal disease, and even depression for the benefit of the child. The AAP recommends prescribing NRT for parents, when needed.
Nicotine dependence is a chronic relapsing condition with comorbidities of substance use and psychiatric disorders that requires similar monitoring and support as for other chronic conditions we manage and is more likely to shorten lifespan than many.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS. She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Clinical practice policy to protect children from tobacco, nicotine, and tobacco smoke, Pediatrics 2015;136(5):1008-17. doi: 10.1542/peds.2015-31088.
Dietary recommendations for inflammatory rheumatic diseases
This interview is a translation of a video blog that first appeared on Medscape France. It has been edited for clarity.
Weight loss, omega-3 supplements, the Mediterranean diet? What about exclusion diets? Jérémie Sellam, MD, PhD, from Saint-Antoine Hospital in Paris, summarizes the key points of the first set of dietary recommendations of the French Society for Rheumatology.
Transcript
Jérémie Sellam, MD, PhD: Hello, everyone. I’m Professor Jérémie Sellam. I’m a rheumatologist at Saint-Antoine Hospital, which is affiliated with the Sorbonne University in Paris. And I was fortunate enough to coordinate France’s first set of dietary recommendations – in fact, the world’s first set of dietary recommendations – for patients with chronic inflammatory rheumatic diseases. I worked on this project with Claire Daien, MD, PhD, who’s a rheumatologist at Montpellier University Hospital.
The idea of coming up with dietary recommendations for patients with inflammatory rheumatic diseases came, quite simply, from our clinical practice. We see that when patients learn they have polyarthritis or spondyloarthritis, they start to experiment with different diets. Many patients start exclusion diets and experiment in all sorts of ways with the food they eat. And although rheumatologists have been able to find some information here and there in the literature, they’ve been pretty much on their own when trying to come up with advice to give their patients. It was to address this issue that [Dr.] Daien and I set out to form a working group. Because when patients aren’t able to get sound advice and authoritative guidance from their doctors, medical associations, or patient advocacy organizations, they often look for information online, and that information is not always reliable or validated.
This group was made up of rheumatologists, some who work at hospitals and others in private practice. Also involved were physician nutrition specialists and registered dietitians. Operating under the auspices of the French Society for Rheumatology, these multidisciplinary experts conducted out a systematic literature review for the purpose of establishing and drafting recommendations. The result was a declaration of eight general principles and nine recommendations.
General principles
The first of the general principles states that nutritional advice is not a substitute for the pharmacologic treatment of chronic inflammatory rheumatic diseases. As you know, whether it’s methotrexate or biologics, pharmacologic treatments are essential for the proper management of chronic inflammatory rheumatic diseases. We know that these medications have an anti-inflammatory effect, reduce pain, and – particularly in the case of rheumatoid arthritis – have a structural effect. In other words, they prevent joint deterioration and destruction. Now, I can tell you that there’s currently no diet, and no dietary supplement, that has proven to be structurally effective. So, yes, dietary intervention might turn out to be promising for patients with chronic inflammatory rheumatic diseases, but pharmacologic treatment must still be part of the picture.
Another general principle emphasizes that dietary intervention is a way for patients to be actively involved in the overall care of their disease, beyond just taking their medication. We know that patients, when they suffer from chronic diseases, are looking for something more, beyond just taking medications. Encouraging them to take an interest in their diet, asking them about what they eat, giving them advice, and supporting their desire to become involved in this aspect of their treatment plan can give them a sense of empowerment.
Dietary interventions can have articular effects, and I’m going to speak about which interventions you can propose, but also which can be beneficial in terms of cardiovascular health and bone health. All of this is based on the literature. In these recommendations, we’ve taken into account not only laboratory experiments – where this or that diet is given to a mouse with arthritis – but also reviewed randomized controlled trials that compare an intervention group with a control group. This is the benchmark we used to determine whether or not a diet should be recommended.
The recommendations
As for the recommendations themselves, we wanted to start off by emphasizing weight loss and what can be called weight-loss support. There’s a link between obesity and the risk of developing rheumatoid arthritis, and also psoriatic arthropathy. And the more overweight a patient is, the more active their disease. In other words, patients with obesity are going to experience more pain, more instances of wakefulness, and more morning stiffness than their normal-weight peers. They’re also going to show symptoms that suggest that disease activity is not controlled well.
Several randomized controlled studies have shown that weight loss will improve systemic joint symptoms. In one particular study, patients with psoriatic arthropathy were started on [tumor necrosis factor] inhibitor therapy and one group followed a prescribed diet and the other had no restrictions on eating. More patients in the diet group than in the no-diet group achieved minimal disease activity. Of course, in some cases – for example, patients with complicated morbid obesity – it might be necessary to have a discussion about bariatric surgery.
But practically speaking, how does one proceed? First of all, patients should be weighed at each visit and, if they’re overweight or obese, the subject should be broached. But even after that conversation, the reality remains that it’s not easy to lose weight. So in the recommendations, we focused on the fact that it shouldn’t be left to the rheumatologist or treating physician alone to handle this challenging aspect of treatment. They should incorporate dietary and nutritional care by reaching out to a dietician or, in the case of complicated obesity – especially when the BMI is higher than 35 kg/m2 – they can refer patients to a nutrition expert who can manage the patient’s obesity, come up with a weight-loss plan, and handle any complications that might arise.
We don’t speak about a low-calorie diet in the recommendations because a diet has a beginning and an end and, quite often, patients regain weight after stopping a diet. Instead, we speak about weight-loss support to point out that weight loss maintained through dietary changes brings about long-term control of disease activity.
In addition, we make two positive recommendations, which overlap, that can help patients control their disease: a Mediterranean diet and omega-3 supplements. One study showed that after participants with rheumatoid arthritis followed the Mediterranean diet for 1 year, those who also took omega-3 fish oil supplements were twice as likely to achieve remission (40% vs. 20%). This explains the interest in having omega-3 as part of the diet. Other studies have shown a broad benefit of the Mediterranean diet.
We know this diet: Fish, especially fatty fish; meat, but not every day, and white meat is best; and fruits and vegetables. In addition, exercise and stay hydrated. All of this can help patients who want to use diet as a means to control their disease. And, as I said earlier, studies have shown that omega-3 supplements have beneficial effects. These are essential polyunsaturated fatty acids, which can help control the disease and joint symptoms.
We also provide some exclusionary recommendations. Not all studies are done well, but it’s clear that there are no major benefits – in fact, no benefit at all – from vegan diets, gluten-free diets, or dairy-free diets. And with these diets, patients run the risk of developing deficiencies, so it’s important that patients are aware of this. We also have to keep in mind that exclusion diets can increase social isolation. Patients need to take part in meals; such gatherings are times for sharing and having social interactions. And I would say that they must be told that there are no data in the literature in support of these diets. But if they ever insist on this kind of intervention, I think that it’s better to advise them to do it under the supervision of a dietician and nutritionist, especially to prevent the development of deficiencies. We’re talking about deficiencies in things like calcium, vitamin B12, and selenium.
Conclusion
As you can see, we have positive recommendations when the patient wants to do something beyond pharmacologic treatment: the Mediterranean diet and omega-3 supplements. And we have negative recommendations, marked by a warning about the risk of developing deficiencies. But I think we all understand the importance of paying close attention to how our patients are experimenting with food. Their diets and eating habits can give us ideas for research and reviews that could allow us to deepen our understanding of the effect of diet on disease, because currently, the quality of the data on some of the diets and types of dietary interventions out there is rather tenuous.
Thank you for listening. I’d also like to thank Claire Daien, MD, PhD, for conducting this project with me so that we could come up with all of these recommendations. I’m also grateful to the following nutrition societies and associations who were our partners: the French Society of Nutrition, the French-Speaking Society of Clinical Nutrition and Metabolism, the French Association for the Study of Obesity, and the French Association of Dieticians and Nutritionists. And patient associations, too, must be recognized, as some of their members participated: the French National Association Against Rheumatoid Arthritis, the French Spondyloarthritis Association, and the French Association for Polyarthritis and Chronic Inflammatory Rheumatic Diseases.
This interview is a translation of a video blog that first appeared on Medscape France. It has been edited for clarity.
Weight loss, omega-3 supplements, the Mediterranean diet? What about exclusion diets? Jérémie Sellam, MD, PhD, from Saint-Antoine Hospital in Paris, summarizes the key points of the first set of dietary recommendations of the French Society for Rheumatology.
Transcript
Jérémie Sellam, MD, PhD: Hello, everyone. I’m Professor Jérémie Sellam. I’m a rheumatologist at Saint-Antoine Hospital, which is affiliated with the Sorbonne University in Paris. And I was fortunate enough to coordinate France’s first set of dietary recommendations – in fact, the world’s first set of dietary recommendations – for patients with chronic inflammatory rheumatic diseases. I worked on this project with Claire Daien, MD, PhD, who’s a rheumatologist at Montpellier University Hospital.
The idea of coming up with dietary recommendations for patients with inflammatory rheumatic diseases came, quite simply, from our clinical practice. We see that when patients learn they have polyarthritis or spondyloarthritis, they start to experiment with different diets. Many patients start exclusion diets and experiment in all sorts of ways with the food they eat. And although rheumatologists have been able to find some information here and there in the literature, they’ve been pretty much on their own when trying to come up with advice to give their patients. It was to address this issue that [Dr.] Daien and I set out to form a working group. Because when patients aren’t able to get sound advice and authoritative guidance from their doctors, medical associations, or patient advocacy organizations, they often look for information online, and that information is not always reliable or validated.
This group was made up of rheumatologists, some who work at hospitals and others in private practice. Also involved were physician nutrition specialists and registered dietitians. Operating under the auspices of the French Society for Rheumatology, these multidisciplinary experts conducted out a systematic literature review for the purpose of establishing and drafting recommendations. The result was a declaration of eight general principles and nine recommendations.
General principles
The first of the general principles states that nutritional advice is not a substitute for the pharmacologic treatment of chronic inflammatory rheumatic diseases. As you know, whether it’s methotrexate or biologics, pharmacologic treatments are essential for the proper management of chronic inflammatory rheumatic diseases. We know that these medications have an anti-inflammatory effect, reduce pain, and – particularly in the case of rheumatoid arthritis – have a structural effect. In other words, they prevent joint deterioration and destruction. Now, I can tell you that there’s currently no diet, and no dietary supplement, that has proven to be structurally effective. So, yes, dietary intervention might turn out to be promising for patients with chronic inflammatory rheumatic diseases, but pharmacologic treatment must still be part of the picture.
Another general principle emphasizes that dietary intervention is a way for patients to be actively involved in the overall care of their disease, beyond just taking their medication. We know that patients, when they suffer from chronic diseases, are looking for something more, beyond just taking medications. Encouraging them to take an interest in their diet, asking them about what they eat, giving them advice, and supporting their desire to become involved in this aspect of their treatment plan can give them a sense of empowerment.
Dietary interventions can have articular effects, and I’m going to speak about which interventions you can propose, but also which can be beneficial in terms of cardiovascular health and bone health. All of this is based on the literature. In these recommendations, we’ve taken into account not only laboratory experiments – where this or that diet is given to a mouse with arthritis – but also reviewed randomized controlled trials that compare an intervention group with a control group. This is the benchmark we used to determine whether or not a diet should be recommended.
The recommendations
As for the recommendations themselves, we wanted to start off by emphasizing weight loss and what can be called weight-loss support. There’s a link between obesity and the risk of developing rheumatoid arthritis, and also psoriatic arthropathy. And the more overweight a patient is, the more active their disease. In other words, patients with obesity are going to experience more pain, more instances of wakefulness, and more morning stiffness than their normal-weight peers. They’re also going to show symptoms that suggest that disease activity is not controlled well.
Several randomized controlled studies have shown that weight loss will improve systemic joint symptoms. In one particular study, patients with psoriatic arthropathy were started on [tumor necrosis factor] inhibitor therapy and one group followed a prescribed diet and the other had no restrictions on eating. More patients in the diet group than in the no-diet group achieved minimal disease activity. Of course, in some cases – for example, patients with complicated morbid obesity – it might be necessary to have a discussion about bariatric surgery.
But practically speaking, how does one proceed? First of all, patients should be weighed at each visit and, if they’re overweight or obese, the subject should be broached. But even after that conversation, the reality remains that it’s not easy to lose weight. So in the recommendations, we focused on the fact that it shouldn’t be left to the rheumatologist or treating physician alone to handle this challenging aspect of treatment. They should incorporate dietary and nutritional care by reaching out to a dietician or, in the case of complicated obesity – especially when the BMI is higher than 35 kg/m2 – they can refer patients to a nutrition expert who can manage the patient’s obesity, come up with a weight-loss plan, and handle any complications that might arise.
We don’t speak about a low-calorie diet in the recommendations because a diet has a beginning and an end and, quite often, patients regain weight after stopping a diet. Instead, we speak about weight-loss support to point out that weight loss maintained through dietary changes brings about long-term control of disease activity.
In addition, we make two positive recommendations, which overlap, that can help patients control their disease: a Mediterranean diet and omega-3 supplements. One study showed that after participants with rheumatoid arthritis followed the Mediterranean diet for 1 year, those who also took omega-3 fish oil supplements were twice as likely to achieve remission (40% vs. 20%). This explains the interest in having omega-3 as part of the diet. Other studies have shown a broad benefit of the Mediterranean diet.
We know this diet: Fish, especially fatty fish; meat, but not every day, and white meat is best; and fruits and vegetables. In addition, exercise and stay hydrated. All of this can help patients who want to use diet as a means to control their disease. And, as I said earlier, studies have shown that omega-3 supplements have beneficial effects. These are essential polyunsaturated fatty acids, which can help control the disease and joint symptoms.
We also provide some exclusionary recommendations. Not all studies are done well, but it’s clear that there are no major benefits – in fact, no benefit at all – from vegan diets, gluten-free diets, or dairy-free diets. And with these diets, patients run the risk of developing deficiencies, so it’s important that patients are aware of this. We also have to keep in mind that exclusion diets can increase social isolation. Patients need to take part in meals; such gatherings are times for sharing and having social interactions. And I would say that they must be told that there are no data in the literature in support of these diets. But if they ever insist on this kind of intervention, I think that it’s better to advise them to do it under the supervision of a dietician and nutritionist, especially to prevent the development of deficiencies. We’re talking about deficiencies in things like calcium, vitamin B12, and selenium.
Conclusion
As you can see, we have positive recommendations when the patient wants to do something beyond pharmacologic treatment: the Mediterranean diet and omega-3 supplements. And we have negative recommendations, marked by a warning about the risk of developing deficiencies. But I think we all understand the importance of paying close attention to how our patients are experimenting with food. Their diets and eating habits can give us ideas for research and reviews that could allow us to deepen our understanding of the effect of diet on disease, because currently, the quality of the data on some of the diets and types of dietary interventions out there is rather tenuous.
Thank you for listening. I’d also like to thank Claire Daien, MD, PhD, for conducting this project with me so that we could come up with all of these recommendations. I’m also grateful to the following nutrition societies and associations who were our partners: the French Society of Nutrition, the French-Speaking Society of Clinical Nutrition and Metabolism, the French Association for the Study of Obesity, and the French Association of Dieticians and Nutritionists. And patient associations, too, must be recognized, as some of their members participated: the French National Association Against Rheumatoid Arthritis, the French Spondyloarthritis Association, and the French Association for Polyarthritis and Chronic Inflammatory Rheumatic Diseases.
This interview is a translation of a video blog that first appeared on Medscape France. It has been edited for clarity.
Weight loss, omega-3 supplements, the Mediterranean diet? What about exclusion diets? Jérémie Sellam, MD, PhD, from Saint-Antoine Hospital in Paris, summarizes the key points of the first set of dietary recommendations of the French Society for Rheumatology.
Transcript
Jérémie Sellam, MD, PhD: Hello, everyone. I’m Professor Jérémie Sellam. I’m a rheumatologist at Saint-Antoine Hospital, which is affiliated with the Sorbonne University in Paris. And I was fortunate enough to coordinate France’s first set of dietary recommendations – in fact, the world’s first set of dietary recommendations – for patients with chronic inflammatory rheumatic diseases. I worked on this project with Claire Daien, MD, PhD, who’s a rheumatologist at Montpellier University Hospital.
The idea of coming up with dietary recommendations for patients with inflammatory rheumatic diseases came, quite simply, from our clinical practice. We see that when patients learn they have polyarthritis or spondyloarthritis, they start to experiment with different diets. Many patients start exclusion diets and experiment in all sorts of ways with the food they eat. And although rheumatologists have been able to find some information here and there in the literature, they’ve been pretty much on their own when trying to come up with advice to give their patients. It was to address this issue that [Dr.] Daien and I set out to form a working group. Because when patients aren’t able to get sound advice and authoritative guidance from their doctors, medical associations, or patient advocacy organizations, they often look for information online, and that information is not always reliable or validated.
This group was made up of rheumatologists, some who work at hospitals and others in private practice. Also involved were physician nutrition specialists and registered dietitians. Operating under the auspices of the French Society for Rheumatology, these multidisciplinary experts conducted out a systematic literature review for the purpose of establishing and drafting recommendations. The result was a declaration of eight general principles and nine recommendations.
General principles
The first of the general principles states that nutritional advice is not a substitute for the pharmacologic treatment of chronic inflammatory rheumatic diseases. As you know, whether it’s methotrexate or biologics, pharmacologic treatments are essential for the proper management of chronic inflammatory rheumatic diseases. We know that these medications have an anti-inflammatory effect, reduce pain, and – particularly in the case of rheumatoid arthritis – have a structural effect. In other words, they prevent joint deterioration and destruction. Now, I can tell you that there’s currently no diet, and no dietary supplement, that has proven to be structurally effective. So, yes, dietary intervention might turn out to be promising for patients with chronic inflammatory rheumatic diseases, but pharmacologic treatment must still be part of the picture.
Another general principle emphasizes that dietary intervention is a way for patients to be actively involved in the overall care of their disease, beyond just taking their medication. We know that patients, when they suffer from chronic diseases, are looking for something more, beyond just taking medications. Encouraging them to take an interest in their diet, asking them about what they eat, giving them advice, and supporting their desire to become involved in this aspect of their treatment plan can give them a sense of empowerment.
Dietary interventions can have articular effects, and I’m going to speak about which interventions you can propose, but also which can be beneficial in terms of cardiovascular health and bone health. All of this is based on the literature. In these recommendations, we’ve taken into account not only laboratory experiments – where this or that diet is given to a mouse with arthritis – but also reviewed randomized controlled trials that compare an intervention group with a control group. This is the benchmark we used to determine whether or not a diet should be recommended.
The recommendations
As for the recommendations themselves, we wanted to start off by emphasizing weight loss and what can be called weight-loss support. There’s a link between obesity and the risk of developing rheumatoid arthritis, and also psoriatic arthropathy. And the more overweight a patient is, the more active their disease. In other words, patients with obesity are going to experience more pain, more instances of wakefulness, and more morning stiffness than their normal-weight peers. They’re also going to show symptoms that suggest that disease activity is not controlled well.
Several randomized controlled studies have shown that weight loss will improve systemic joint symptoms. In one particular study, patients with psoriatic arthropathy were started on [tumor necrosis factor] inhibitor therapy and one group followed a prescribed diet and the other had no restrictions on eating. More patients in the diet group than in the no-diet group achieved minimal disease activity. Of course, in some cases – for example, patients with complicated morbid obesity – it might be necessary to have a discussion about bariatric surgery.
But practically speaking, how does one proceed? First of all, patients should be weighed at each visit and, if they’re overweight or obese, the subject should be broached. But even after that conversation, the reality remains that it’s not easy to lose weight. So in the recommendations, we focused on the fact that it shouldn’t be left to the rheumatologist or treating physician alone to handle this challenging aspect of treatment. They should incorporate dietary and nutritional care by reaching out to a dietician or, in the case of complicated obesity – especially when the BMI is higher than 35 kg/m2 – they can refer patients to a nutrition expert who can manage the patient’s obesity, come up with a weight-loss plan, and handle any complications that might arise.
We don’t speak about a low-calorie diet in the recommendations because a diet has a beginning and an end and, quite often, patients regain weight after stopping a diet. Instead, we speak about weight-loss support to point out that weight loss maintained through dietary changes brings about long-term control of disease activity.
In addition, we make two positive recommendations, which overlap, that can help patients control their disease: a Mediterranean diet and omega-3 supplements. One study showed that after participants with rheumatoid arthritis followed the Mediterranean diet for 1 year, those who also took omega-3 fish oil supplements were twice as likely to achieve remission (40% vs. 20%). This explains the interest in having omega-3 as part of the diet. Other studies have shown a broad benefit of the Mediterranean diet.
We know this diet: Fish, especially fatty fish; meat, but not every day, and white meat is best; and fruits and vegetables. In addition, exercise and stay hydrated. All of this can help patients who want to use diet as a means to control their disease. And, as I said earlier, studies have shown that omega-3 supplements have beneficial effects. These are essential polyunsaturated fatty acids, which can help control the disease and joint symptoms.
We also provide some exclusionary recommendations. Not all studies are done well, but it’s clear that there are no major benefits – in fact, no benefit at all – from vegan diets, gluten-free diets, or dairy-free diets. And with these diets, patients run the risk of developing deficiencies, so it’s important that patients are aware of this. We also have to keep in mind that exclusion diets can increase social isolation. Patients need to take part in meals; such gatherings are times for sharing and having social interactions. And I would say that they must be told that there are no data in the literature in support of these diets. But if they ever insist on this kind of intervention, I think that it’s better to advise them to do it under the supervision of a dietician and nutritionist, especially to prevent the development of deficiencies. We’re talking about deficiencies in things like calcium, vitamin B12, and selenium.
Conclusion
As you can see, we have positive recommendations when the patient wants to do something beyond pharmacologic treatment: the Mediterranean diet and omega-3 supplements. And we have negative recommendations, marked by a warning about the risk of developing deficiencies. But I think we all understand the importance of paying close attention to how our patients are experimenting with food. Their diets and eating habits can give us ideas for research and reviews that could allow us to deepen our understanding of the effect of diet on disease, because currently, the quality of the data on some of the diets and types of dietary interventions out there is rather tenuous.
Thank you for listening. I’d also like to thank Claire Daien, MD, PhD, for conducting this project with me so that we could come up with all of these recommendations. I’m also grateful to the following nutrition societies and associations who were our partners: the French Society of Nutrition, the French-Speaking Society of Clinical Nutrition and Metabolism, the French Association for the Study of Obesity, and the French Association of Dieticians and Nutritionists. And patient associations, too, must be recognized, as some of their members participated: the French National Association Against Rheumatoid Arthritis, the French Spondyloarthritis Association, and the French Association for Polyarthritis and Chronic Inflammatory Rheumatic Diseases.
Docs react: NyQuil chicken and endless eye mucus
It’s the season of love. In that spirit, Lean in and get a whiff of the latest good, bad, and ugly videos making the rounds on the internet’s most perplexing platform. But don’t get too close; these videos are especially ripe.
The bad: NyQuil chicken
You know something bad has happened when your TikTok search ends with a warning from the app that says “Learn how to recognize harmful trends and hoaxes.” That’s what shows up now when you try to find out what the “NyQuil chicken” or “sleepy chicken” trend is (or was) all about.
TikTok videos, including this one from TikTok user @janelleandkate, show users trying out a trend meant to cook up a meal that will also cure your cold symptoms. The trend involves cooking chicken in a pan full of the cold and flu medicine NyQuil. The NyQuil chicken idea stems from a Twitter meme from 2017, so it is possible that some of the recent videos are fake (blue food coloring is easy to get, people).
However, in the instance that people believe the videos to be real and want to try the trend out, it is important to warn that this shouldn’t be attempted.
Aaron Hartman, MD, assistant clinical professor of family medicine at Virginia Commonwealth University, told the website Mic about the trend’s dangers: “When you cook cough medicine like NyQuil, however, you boil off the water and alcohol in it, leaving the chicken saturated with a super concentrated amount of drugs in the meat. If you ate one of those cutlets completely cooked, it’d be as if you’re actually consuming a quarter to half a bottle of NyQuil.”
And that’s not good for anyone. What ever happened to an old fashioned herb marinade?
The good: Can you fart yourself blind? Doc explains
It’s something we’ve all wondered about, right?
TikTok and YouTube’s mainstay plastic surgeon Anthony Youn, MD, took it upon himself to reply to a comment saying “I once farted so hard I went blind for 3 minutes.” This phenomenon, according to Dr. Youn, is very rare, but not impossible, though we wouldn’t exactly want to try it for ourselves.
In the humorous (but very informative!) video, Dr. Youn explains that particularly pungent flatulence can contain large amounts of hydrogen sulfide, a gas that is known for smelling like rotten eggs. According to the Occupational Safety and Health Administration, hydrogen sulfide is produced in a number of industries, like oil and gas refining, mining, and paper processing. Exposure to higher concentrations of hydrogen sulfide can be dangerous, with prolonged exposure at a 2-5 parts per million (ppm) concentration causing nausea, headaches, and airway problems in some asthma patients. At very high concentrations, it can be fatal.
Thankfully, a person’s gas is not at all that dangerous. When it comes to the commentor’s claim, Dr. Youn says that something else hydrogen sulfide can do is reduce blood pressure.
“If it reduces blood pressure to the central retinal artery,” Dr. Youn says, “your silent but deadly toot could theoretically make you go blind.”
Thank goodness we can lay that question to rest.
The ugly: Eye boogers from hell
Get a look at this!
This video from @mikaylaadiorr has amassed over 8 million likes and over 89,000 comments, and shows someone, who we can assume is Mikayla, pulling some sort of long string-like material out of the corner of her eye. It’s like a clown’s never-ending handkerchief, only goopy.
These mucus eye strings are caused by untreated eye conditions, like dry eye or pink eye (conjunctivitis), but pulling the mucus out is actually a symptom of what is called mucus fishing syndrome. As you know, our eyes are covered in layers of mucus and tears, which keeps our eyeballs lubricated and also protects us from bacteria and viruses. It’s possible to dry out the eyes by pulling some mucus off, but our eyes aren’t big fans of that, so they’ll create more mucus to keep from drying out.
A person who might get a bit addicted to pulling the strings out has likely developed mucus fishing syndrome, which is considered a body-focused repetitive behavior (BFRB); other BFRBs include skin-picking (dermatillomania) or picking hairs out (trichotillomania).
Popular TikToker and Oregon ophthalmologist Will Flanary, MD, aka Dr. Glaucomflecken, responded to the videos, which have been encouraging others to try it.
“This is called mucus fishing syndrome,” the ophthalmologist explained via text captions in his video. “The trauma from pulling mucus out of your eye causes more mucus to form. You get caught in a never-ending cycle that gets worse over time. So…stop it.”
Fingers off the mucus, people.
A version of this article first appeared on Medscape.com.
It’s the season of love. In that spirit, Lean in and get a whiff of the latest good, bad, and ugly videos making the rounds on the internet’s most perplexing platform. But don’t get too close; these videos are especially ripe.
The bad: NyQuil chicken
You know something bad has happened when your TikTok search ends with a warning from the app that says “Learn how to recognize harmful trends and hoaxes.” That’s what shows up now when you try to find out what the “NyQuil chicken” or “sleepy chicken” trend is (or was) all about.
TikTok videos, including this one from TikTok user @janelleandkate, show users trying out a trend meant to cook up a meal that will also cure your cold symptoms. The trend involves cooking chicken in a pan full of the cold and flu medicine NyQuil. The NyQuil chicken idea stems from a Twitter meme from 2017, so it is possible that some of the recent videos are fake (blue food coloring is easy to get, people).
However, in the instance that people believe the videos to be real and want to try the trend out, it is important to warn that this shouldn’t be attempted.
Aaron Hartman, MD, assistant clinical professor of family medicine at Virginia Commonwealth University, told the website Mic about the trend’s dangers: “When you cook cough medicine like NyQuil, however, you boil off the water and alcohol in it, leaving the chicken saturated with a super concentrated amount of drugs in the meat. If you ate one of those cutlets completely cooked, it’d be as if you’re actually consuming a quarter to half a bottle of NyQuil.”
And that’s not good for anyone. What ever happened to an old fashioned herb marinade?
The good: Can you fart yourself blind? Doc explains
It’s something we’ve all wondered about, right?
TikTok and YouTube’s mainstay plastic surgeon Anthony Youn, MD, took it upon himself to reply to a comment saying “I once farted so hard I went blind for 3 minutes.” This phenomenon, according to Dr. Youn, is very rare, but not impossible, though we wouldn’t exactly want to try it for ourselves.
In the humorous (but very informative!) video, Dr. Youn explains that particularly pungent flatulence can contain large amounts of hydrogen sulfide, a gas that is known for smelling like rotten eggs. According to the Occupational Safety and Health Administration, hydrogen sulfide is produced in a number of industries, like oil and gas refining, mining, and paper processing. Exposure to higher concentrations of hydrogen sulfide can be dangerous, with prolonged exposure at a 2-5 parts per million (ppm) concentration causing nausea, headaches, and airway problems in some asthma patients. At very high concentrations, it can be fatal.
Thankfully, a person’s gas is not at all that dangerous. When it comes to the commentor’s claim, Dr. Youn says that something else hydrogen sulfide can do is reduce blood pressure.
“If it reduces blood pressure to the central retinal artery,” Dr. Youn says, “your silent but deadly toot could theoretically make you go blind.”
Thank goodness we can lay that question to rest.
The ugly: Eye boogers from hell
Get a look at this!
This video from @mikaylaadiorr has amassed over 8 million likes and over 89,000 comments, and shows someone, who we can assume is Mikayla, pulling some sort of long string-like material out of the corner of her eye. It’s like a clown’s never-ending handkerchief, only goopy.
These mucus eye strings are caused by untreated eye conditions, like dry eye or pink eye (conjunctivitis), but pulling the mucus out is actually a symptom of what is called mucus fishing syndrome. As you know, our eyes are covered in layers of mucus and tears, which keeps our eyeballs lubricated and also protects us from bacteria and viruses. It’s possible to dry out the eyes by pulling some mucus off, but our eyes aren’t big fans of that, so they’ll create more mucus to keep from drying out.
A person who might get a bit addicted to pulling the strings out has likely developed mucus fishing syndrome, which is considered a body-focused repetitive behavior (BFRB); other BFRBs include skin-picking (dermatillomania) or picking hairs out (trichotillomania).
Popular TikToker and Oregon ophthalmologist Will Flanary, MD, aka Dr. Glaucomflecken, responded to the videos, which have been encouraging others to try it.
“This is called mucus fishing syndrome,” the ophthalmologist explained via text captions in his video. “The trauma from pulling mucus out of your eye causes more mucus to form. You get caught in a never-ending cycle that gets worse over time. So…stop it.”
Fingers off the mucus, people.
A version of this article first appeared on Medscape.com.
It’s the season of love. In that spirit, Lean in and get a whiff of the latest good, bad, and ugly videos making the rounds on the internet’s most perplexing platform. But don’t get too close; these videos are especially ripe.
The bad: NyQuil chicken
You know something bad has happened when your TikTok search ends with a warning from the app that says “Learn how to recognize harmful trends and hoaxes.” That’s what shows up now when you try to find out what the “NyQuil chicken” or “sleepy chicken” trend is (or was) all about.
TikTok videos, including this one from TikTok user @janelleandkate, show users trying out a trend meant to cook up a meal that will also cure your cold symptoms. The trend involves cooking chicken in a pan full of the cold and flu medicine NyQuil. The NyQuil chicken idea stems from a Twitter meme from 2017, so it is possible that some of the recent videos are fake (blue food coloring is easy to get, people).
However, in the instance that people believe the videos to be real and want to try the trend out, it is important to warn that this shouldn’t be attempted.
Aaron Hartman, MD, assistant clinical professor of family medicine at Virginia Commonwealth University, told the website Mic about the trend’s dangers: “When you cook cough medicine like NyQuil, however, you boil off the water and alcohol in it, leaving the chicken saturated with a super concentrated amount of drugs in the meat. If you ate one of those cutlets completely cooked, it’d be as if you’re actually consuming a quarter to half a bottle of NyQuil.”
And that’s not good for anyone. What ever happened to an old fashioned herb marinade?
The good: Can you fart yourself blind? Doc explains
It’s something we’ve all wondered about, right?
TikTok and YouTube’s mainstay plastic surgeon Anthony Youn, MD, took it upon himself to reply to a comment saying “I once farted so hard I went blind for 3 minutes.” This phenomenon, according to Dr. Youn, is very rare, but not impossible, though we wouldn’t exactly want to try it for ourselves.
In the humorous (but very informative!) video, Dr. Youn explains that particularly pungent flatulence can contain large amounts of hydrogen sulfide, a gas that is known for smelling like rotten eggs. According to the Occupational Safety and Health Administration, hydrogen sulfide is produced in a number of industries, like oil and gas refining, mining, and paper processing. Exposure to higher concentrations of hydrogen sulfide can be dangerous, with prolonged exposure at a 2-5 parts per million (ppm) concentration causing nausea, headaches, and airway problems in some asthma patients. At very high concentrations, it can be fatal.
Thankfully, a person’s gas is not at all that dangerous. When it comes to the commentor’s claim, Dr. Youn says that something else hydrogen sulfide can do is reduce blood pressure.
“If it reduces blood pressure to the central retinal artery,” Dr. Youn says, “your silent but deadly toot could theoretically make you go blind.”
Thank goodness we can lay that question to rest.
The ugly: Eye boogers from hell
Get a look at this!
This video from @mikaylaadiorr has amassed over 8 million likes and over 89,000 comments, and shows someone, who we can assume is Mikayla, pulling some sort of long string-like material out of the corner of her eye. It’s like a clown’s never-ending handkerchief, only goopy.
These mucus eye strings are caused by untreated eye conditions, like dry eye or pink eye (conjunctivitis), but pulling the mucus out is actually a symptom of what is called mucus fishing syndrome. As you know, our eyes are covered in layers of mucus and tears, which keeps our eyeballs lubricated and also protects us from bacteria and viruses. It’s possible to dry out the eyes by pulling some mucus off, but our eyes aren’t big fans of that, so they’ll create more mucus to keep from drying out.
A person who might get a bit addicted to pulling the strings out has likely developed mucus fishing syndrome, which is considered a body-focused repetitive behavior (BFRB); other BFRBs include skin-picking (dermatillomania) or picking hairs out (trichotillomania).
Popular TikToker and Oregon ophthalmologist Will Flanary, MD, aka Dr. Glaucomflecken, responded to the videos, which have been encouraging others to try it.
“This is called mucus fishing syndrome,” the ophthalmologist explained via text captions in his video. “The trauma from pulling mucus out of your eye causes more mucus to form. You get caught in a never-ending cycle that gets worse over time. So…stop it.”
Fingers off the mucus, people.
A version of this article first appeared on Medscape.com.
Enough is enough: the pandemic and loss of female oncologists
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Imagine this: As a young girl, you decide you want to become a doctor when you grow up. You spend countless hours studying, researching, and volunteering to eventually make it into medical school. Four years later, you graduate top of your class and match into your first-choice residency program. You are so proud of yourself!
During your last year of residency, a pandemic takes the entire world by storm. You persevere through your last 14 months of residency that included additional time in the ICU, not seeing your colleagues, and interviewing for your new job all from your own living room. After all of this, you finally get to start doing what you have been waiting to do for the past decade: train with the brilliant minds in hematology and oncology.
All of a sudden, You start to question: If these incredible women have decided that the sacrifice this career requires is too much, then (1) How will I survive? and (2) Did I make a huge mistake in my career decision? Spoiler alert: This girl is me.
The World Health Organization defines burnout as a “syndrome conceptualized as resulting from chronic workplace stress that has not been successfully managed. It is characterized by energy depletion or exhaustion, increased mental distance from one’s job, and reduced professional efficacy.”
We know that 33% of oncologists are feeling burned out right now, according to the Medscape National Physician Burnout & Suicide Report 2021. Of the 51% of female physicians that are burned out, work-life balance has been identified as the biggest workplace concern to them. Research has shown that hours per week devoted to direct patient care is the dominant predictor of burnout for practicing oncologists. But in academic oncology, that is followed by grant deadlines, manuscript rejections, and the constant reminders that you are a new face in oncology, a specialty that was previously male-dominated.
In less than a year, we have had several key female oncologists leave our cancer center. While some made the decision to retire early, two of them chose to pivot their careers and leave clinical medicine to assist with drug development and clinical trials. Although this is extremely important work for cancer care, I was shocked to hear that these amazing and successful clinicians were choosing to remove all direct patient care from their practice, when for many of them, patient care was what motivated them to pursue medicine in the first place. They were loved by their patients, respected as researchers, and well known as educators within the division.
One shared that she no longer felt like she could be a good mother, wife, or daughter with what was currently being demanded of her to have a successful academic career. In hearing this news, I was saddened to have to say goodbye to a mentor of mine and immediately started second-guessing my career choice. I felt that my goal of having an impactful career and prosperous home life was not only unattainable but potentially unrealistic.
While we know that female physicians already experience a greater degree of burnout, the pandemic has only added fuel to the fire. This is especially true in cancer care. It has been estimated that new cancer diagnosis have decreased by as much as 23% since the beginning of the pandemic. This delay in diagnosis will lead to patients presenting with more advanced disease, busier clinic schedules, and worsened clinical outcomes for years to come. With no end in sight, I worry what this will mean for women currently in oncology, in addition to those in training or deciding if they should pursue this as a career.
Extrapolating evidence from prior epidemics, physicians are at increased risk for burnout due to immediate and long-term effects from this pandemic. We need to act now to not only continue addressing previously existing individual and organizational causes of burnout but also develop strategies to provide support for the COVID-19–specific impacts on oncologists’ well-being. An editorial published by the American Society of Clinical Oncology provides helpful suggestions on how to do this.
A recent cross-sectional survey found that 22% of academic female oncologists were likely or very likely to pursue a career outside of academia in the next 5 years. Losing these women would be detrimental to the field. This would mean a significant number of patients losing their long-term oncologists with whom they have years of care, trainees losing their professional and research mentors to guide and help mold them into successful independent practitioners and researchers, and arguably most important, little girls losing role models to show them that regardless of their gender, they can become an oncologist.Dr. Poterala is a current hematology and oncology fellow at the University of Wisconsin Carbone Cancer Center, Madison. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Enjoy the ride
She was a 20-year-old barista when we first met, working her way through college.
I was a newly minted attending physician. I’d stopped at the place she worked for coffee on the way to my office. When I got up to the front she was wearing sunglasses and apologized for them. She said she was having bad headaches, and couldn’t get into a doctor she’d been referred to. Feeling bad for her, and needing patients, I handed her my card.
She showed up a few days later, a little nervous as she’d never met a “regular” outside the coffee place before, and had brought her sister along for support.
She was back last week. Now she’s head of human resources for the same chain of local coffee shops. She’s married, with kids, a mortgage, and a minivan.
We were talking about our chance meeting and reminiscing. Her migraines had taken a few medication trials to control, but after a year or 2 we’d found the right one for her and she’s been on it since.
Like many of my longtime patients, she moved past calling me “doctor” long ago. Our one to two visits a year are now more social than medical, chatting about our kids, dogs, and lives.
The same passage of time that brings us from grade school, to medical school, to medical practice takes others along with it. We may not see the changes of days, but when they drop by only once a year it’s obvious. Just like the way we don’t see daily changes in family and friends, but when we look at old pictures we’re shocked by how different they (not to mention ourselves) look.
We all follow the same course around the sun, usually facing the same milestones and similar memories on the trip. Our long-term patients, like distant relatives, may only come by infrequently, so the changes are greater. I’m sure they say the same things about me. “I saw Dr. Block today; boy, he’s really gone gray.”
I don’t mind that (too much) anymore. My thinning, graying, hair (I hope) makes me look a little more distinguished, although my complete lack of fashion sense more than goes the other way.
The river only goes in one direction, carrying us, our patients, and our families, all along with it. We often lose track of time’s effects on us until we see the changes it has brought to another.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
She was a 20-year-old barista when we first met, working her way through college.
I was a newly minted attending physician. I’d stopped at the place she worked for coffee on the way to my office. When I got up to the front she was wearing sunglasses and apologized for them. She said she was having bad headaches, and couldn’t get into a doctor she’d been referred to. Feeling bad for her, and needing patients, I handed her my card.
She showed up a few days later, a little nervous as she’d never met a “regular” outside the coffee place before, and had brought her sister along for support.
She was back last week. Now she’s head of human resources for the same chain of local coffee shops. She’s married, with kids, a mortgage, and a minivan.
We were talking about our chance meeting and reminiscing. Her migraines had taken a few medication trials to control, but after a year or 2 we’d found the right one for her and she’s been on it since.
Like many of my longtime patients, she moved past calling me “doctor” long ago. Our one to two visits a year are now more social than medical, chatting about our kids, dogs, and lives.
The same passage of time that brings us from grade school, to medical school, to medical practice takes others along with it. We may not see the changes of days, but when they drop by only once a year it’s obvious. Just like the way we don’t see daily changes in family and friends, but when we look at old pictures we’re shocked by how different they (not to mention ourselves) look.
We all follow the same course around the sun, usually facing the same milestones and similar memories on the trip. Our long-term patients, like distant relatives, may only come by infrequently, so the changes are greater. I’m sure they say the same things about me. “I saw Dr. Block today; boy, he’s really gone gray.”
I don’t mind that (too much) anymore. My thinning, graying, hair (I hope) makes me look a little more distinguished, although my complete lack of fashion sense more than goes the other way.
The river only goes in one direction, carrying us, our patients, and our families, all along with it. We often lose track of time’s effects on us until we see the changes it has brought to another.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
She was a 20-year-old barista when we first met, working her way through college.
I was a newly minted attending physician. I’d stopped at the place she worked for coffee on the way to my office. When I got up to the front she was wearing sunglasses and apologized for them. She said she was having bad headaches, and couldn’t get into a doctor she’d been referred to. Feeling bad for her, and needing patients, I handed her my card.
She showed up a few days later, a little nervous as she’d never met a “regular” outside the coffee place before, and had brought her sister along for support.
She was back last week. Now she’s head of human resources for the same chain of local coffee shops. She’s married, with kids, a mortgage, and a minivan.
We were talking about our chance meeting and reminiscing. Her migraines had taken a few medication trials to control, but after a year or 2 we’d found the right one for her and she’s been on it since.
Like many of my longtime patients, she moved past calling me “doctor” long ago. Our one to two visits a year are now more social than medical, chatting about our kids, dogs, and lives.
The same passage of time that brings us from grade school, to medical school, to medical practice takes others along with it. We may not see the changes of days, but when they drop by only once a year it’s obvious. Just like the way we don’t see daily changes in family and friends, but when we look at old pictures we’re shocked by how different they (not to mention ourselves) look.
We all follow the same course around the sun, usually facing the same milestones and similar memories on the trip. Our long-term patients, like distant relatives, may only come by infrequently, so the changes are greater. I’m sure they say the same things about me. “I saw Dr. Block today; boy, he’s really gone gray.”
I don’t mind that (too much) anymore. My thinning, graying, hair (I hope) makes me look a little more distinguished, although my complete lack of fashion sense more than goes the other way.
The river only goes in one direction, carrying us, our patients, and our families, all along with it. We often lose track of time’s effects on us until we see the changes it has brought to another.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
A test for cannabis-caused impairment
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
You have a 16-year-old patient who has been doing poorly in school. He has withdrawn from his social group and quit the sports in which he excelled. He admits to using marijuana “maybe once or twice a week.” But you and his parents suspect that it is much more often and contributing to the change in his behavior and school performance.
They would prefer he not use marijuana at all but could maybe be comfortable with some arrangement in which their son could demonstrate that his usage was indeed limited to once or twice on the weekends. They ask for your help with crafting a contract that might include “some urine or blood test” that would allow them to be sure their son was adhering to the contract.
You explain to them that there are hazards associated with setting up contracts such as the one they are proposing. One revolving around the issue of trust. Another being that he may be addicted to the point that a compromise that includes scaling back his usage is unlikely to succeed. And, finally, you tell them that because of marijuana’s pharmacokinetics, their son’s urine tests will always be positive and not reflective of the how much he is using or whether he is intoxicated.
Scenarios similar to this are increasingly common for those of us living in states that have legalized recreational cannabis use. The absence of a laboratory test that can determine when a person is impaired by marijuana has made life difficult for law enforcement officers accustomed to relying on breath and blood tests for alcohol to confirm their suspicion that a driver is under the influence.
In addition, because marijuana is still detectable days after it is used, many well-paying jobs go unfilled when potential applicants are hesitant to submit to a required drug test. The quirky pharmacokinetics of cannabis are well-known to the recreational users and they see no reason to risk failing a urine test regardless of how good the job may be.
This lack of a reliable indicator of cannabis intoxication has not gone unnoticed, and in a recent study published in the journal Neuropharmacology, researchers at Massachusetts General Hospital in Boston report some hopeful results using fNIRS brain scanning. The investigators observed an increase in the level of oxygenated hemoglobin concentration (HbO), which is a type of neural activity signature, in the prefrontal cortex region of the volunteers who reported being impaired.
While a brain scan may sound like an unwieldy tool to use on roadside sobriety stops, the researchers report that portable scanners – some using skull cap sensors – could be easily adapted for use by law enforcement in the field. This technology also could be used by employers on the job site to test truck drivers and heavy machine operators at the beginning of each shift, thereby allaying the fears of responsible cannabis users.
This technology might be helpful to you in advising the parents of the 16-year-old you suspect of heavy usage. It would certainly help in confirming the suspicion that he is using more often than he claims. However, the contract the parents propose still may not work. If this young man demonstrates on multiple attempts that his word can’t be trusted, technology isn’t going to be the answer.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].







