User login
Alleviating chemo-related nausea is a huge unmet need
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
This is Mark Kris from chilly New York and Memorial Sloan Kettering. Today I want to talk about a recent article in the Journal of Clinical Oncology that reported a study of a new neurokinin-1 antagonist called fosnetupitant. This was a well-conducted trial that demonstrates the noninferiority of IV fosnetupitant when compared with IV fosaprepitant. By their study criteria, fosnetupitant was not inferior.
But my reason for discussing this is that the paper and the trial miss the point for the field right now. Although the authors talk about the prevention of nausea and vomiting in the introduction, in the paper itself and in the abstract results section, there’s not a single mention about the medication’s ability to control nausea, which is the critical issue for our patients today. You have to go into the supplementary data to find it mentioned, and what you find is that the prevention of nausea is 50% for both the control and this new drug. We control nausea in only half of the patients who receive cisplatin in 2022. That is a huge issue.
When you ask patients what are the effects of cancer treatment that they fear most, that concerns them most, it’s nausea and emesis; indeed, nausea has replaced emesis as the biggest concern. And although this trial used emesis as the main endpoint, and it was useful in defining the drug, it was not useful in coming up with a new treatment that addresses a huge need. Further, the authors talk about an advantage to fosnetupitant based on infusion reactions, but it is a difference of 0.3% vs. 3%. They talk about that sort of thing in the abstract and in the discussion section but don’t include nausea as part of the key endpoint of this trial. Again, you had to dig deeply to find out that, frankly, fosnetupitant was no better than the drugs we already have.
The other concerning point is that we do have another drug that works well. If you go to the American Society of Clinical Oncology or National Comprehensive Cancer Network guidelines for patients receiving high dosages of cisplatin, you find a four-drug regimen, including olanzapine, and that was not used here. Why is olanzapine so critical? It’s an available drug, it’s an inexpensive drug, it’s a safe drug, and it improves nausea by 15%.
So they did this huge trial to show noninferiority, and they neglected to give a drug that could deal with the most serious side effect of cancer therapy – nausea – and improve things by 15%.
A challenge to people in this field: We have to do better. Nausea is a big problem. While noninferiority trials can be helpful for drug development, they’re not really helpful for the field. With a problem of this magnitude, we need better drugs to control nausea. In the meantime, I urge you all to follow the guidelines for high doses of cisplatin. Please use the four-drug regimen that is recommended in the guidelines and widely used in the United States. Going forward, make sure that when we expend huge amounts of energy to develop new agents and report them in our medical journals, that we look for ways to advance care where there are significant gaps in our ability to deliver what we want. Delivering better control of nausea is something we all need to be committed to. It’s a huge unmet need, and I hope future trials will address that need. Our patients will be better for it and we’ll be better in that we’re delivering what patients deserve, what they need, and what they ask for.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for AstraZeneca, Roche/Genentech, and Ariad Pharmaceuticals, and has received research grants from Pfizer, PUMA, and Roche/Genentech.
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
This is Mark Kris from chilly New York and Memorial Sloan Kettering. Today I want to talk about a recent article in the Journal of Clinical Oncology that reported a study of a new neurokinin-1 antagonist called fosnetupitant. This was a well-conducted trial that demonstrates the noninferiority of IV fosnetupitant when compared with IV fosaprepitant. By their study criteria, fosnetupitant was not inferior.
But my reason for discussing this is that the paper and the trial miss the point for the field right now. Although the authors talk about the prevention of nausea and vomiting in the introduction, in the paper itself and in the abstract results section, there’s not a single mention about the medication’s ability to control nausea, which is the critical issue for our patients today. You have to go into the supplementary data to find it mentioned, and what you find is that the prevention of nausea is 50% for both the control and this new drug. We control nausea in only half of the patients who receive cisplatin in 2022. That is a huge issue.
When you ask patients what are the effects of cancer treatment that they fear most, that concerns them most, it’s nausea and emesis; indeed, nausea has replaced emesis as the biggest concern. And although this trial used emesis as the main endpoint, and it was useful in defining the drug, it was not useful in coming up with a new treatment that addresses a huge need. Further, the authors talk about an advantage to fosnetupitant based on infusion reactions, but it is a difference of 0.3% vs. 3%. They talk about that sort of thing in the abstract and in the discussion section but don’t include nausea as part of the key endpoint of this trial. Again, you had to dig deeply to find out that, frankly, fosnetupitant was no better than the drugs we already have.
The other concerning point is that we do have another drug that works well. If you go to the American Society of Clinical Oncology or National Comprehensive Cancer Network guidelines for patients receiving high dosages of cisplatin, you find a four-drug regimen, including olanzapine, and that was not used here. Why is olanzapine so critical? It’s an available drug, it’s an inexpensive drug, it’s a safe drug, and it improves nausea by 15%.
So they did this huge trial to show noninferiority, and they neglected to give a drug that could deal with the most serious side effect of cancer therapy – nausea – and improve things by 15%.
A challenge to people in this field: We have to do better. Nausea is a big problem. While noninferiority trials can be helpful for drug development, they’re not really helpful for the field. With a problem of this magnitude, we need better drugs to control nausea. In the meantime, I urge you all to follow the guidelines for high doses of cisplatin. Please use the four-drug regimen that is recommended in the guidelines and widely used in the United States. Going forward, make sure that when we expend huge amounts of energy to develop new agents and report them in our medical journals, that we look for ways to advance care where there are significant gaps in our ability to deliver what we want. Delivering better control of nausea is something we all need to be committed to. It’s a huge unmet need, and I hope future trials will address that need. Our patients will be better for it and we’ll be better in that we’re delivering what patients deserve, what they need, and what they ask for.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for AstraZeneca, Roche/Genentech, and Ariad Pharmaceuticals, and has received research grants from Pfizer, PUMA, and Roche/Genentech.
This transcript has been edited for clarity. The transcript and an accompanying video first appeared on Medscape.com.
This is Mark Kris from chilly New York and Memorial Sloan Kettering. Today I want to talk about a recent article in the Journal of Clinical Oncology that reported a study of a new neurokinin-1 antagonist called fosnetupitant. This was a well-conducted trial that demonstrates the noninferiority of IV fosnetupitant when compared with IV fosaprepitant. By their study criteria, fosnetupitant was not inferior.
But my reason for discussing this is that the paper and the trial miss the point for the field right now. Although the authors talk about the prevention of nausea and vomiting in the introduction, in the paper itself and in the abstract results section, there’s not a single mention about the medication’s ability to control nausea, which is the critical issue for our patients today. You have to go into the supplementary data to find it mentioned, and what you find is that the prevention of nausea is 50% for both the control and this new drug. We control nausea in only half of the patients who receive cisplatin in 2022. That is a huge issue.
When you ask patients what are the effects of cancer treatment that they fear most, that concerns them most, it’s nausea and emesis; indeed, nausea has replaced emesis as the biggest concern. And although this trial used emesis as the main endpoint, and it was useful in defining the drug, it was not useful in coming up with a new treatment that addresses a huge need. Further, the authors talk about an advantage to fosnetupitant based on infusion reactions, but it is a difference of 0.3% vs. 3%. They talk about that sort of thing in the abstract and in the discussion section but don’t include nausea as part of the key endpoint of this trial. Again, you had to dig deeply to find out that, frankly, fosnetupitant was no better than the drugs we already have.
The other concerning point is that we do have another drug that works well. If you go to the American Society of Clinical Oncology or National Comprehensive Cancer Network guidelines for patients receiving high dosages of cisplatin, you find a four-drug regimen, including olanzapine, and that was not used here. Why is olanzapine so critical? It’s an available drug, it’s an inexpensive drug, it’s a safe drug, and it improves nausea by 15%.
So they did this huge trial to show noninferiority, and they neglected to give a drug that could deal with the most serious side effect of cancer therapy – nausea – and improve things by 15%.
A challenge to people in this field: We have to do better. Nausea is a big problem. While noninferiority trials can be helpful for drug development, they’re not really helpful for the field. With a problem of this magnitude, we need better drugs to control nausea. In the meantime, I urge you all to follow the guidelines for high doses of cisplatin. Please use the four-drug regimen that is recommended in the guidelines and widely used in the United States. Going forward, make sure that when we expend huge amounts of energy to develop new agents and report them in our medical journals, that we look for ways to advance care where there are significant gaps in our ability to deliver what we want. Delivering better control of nausea is something we all need to be committed to. It’s a huge unmet need, and I hope future trials will address that need. Our patients will be better for it and we’ll be better in that we’re delivering what patients deserve, what they need, and what they ask for.
Mark G. Kris, MD, is chief of the thoracic oncology service and the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering Cancer Center in New York City. He reported serving as a director, officer, partner, employee, adviser, consultant, or trustee for AstraZeneca, Roche/Genentech, and Ariad Pharmaceuticals, and has received research grants from Pfizer, PUMA, and Roche/Genentech.
Case report: Male with acute new-onset suicidal ideation tied to SARS-CoV-2
An otherwise healthy 55-year-old male, with no previous psychiatric or medical history, sought care with a family medicine physician for the first time in decades.
Medical symptoms began Oct. 9, 2021, with “some leg weakness and mild sniffles.” Since he was going to be at a public event, he decided to take a PCR test for the SARS-CoV-2 virus on Oct. 13. The patient tested positive.
His symptoms continued to worsen, and he experienced severe body fatigue, sleep disturbance, and lethargy. “A few days after my positive test, the cognitive and physical symptoms dramatically ramped up,” the patient recalled.
Because of those worsening symptoms, on Oct. 20, the patient obtained a new patient appointment with a family medicine physician. After a telemedicine evaluation, the family medicine physician began a multifaceted early outpatient COVID-19 treatment protocol,1 as I (C.M.W.) and colleagues wrote about late last year. However, this treatment began late in the course because of the patient’s initial resistance to seek care.
This early outpatient treatment protocol for COVID-19 included vitamin D3 125 mcg (5,000 ICU), N-acetylcysteine (NAC) 600 mg every day x 30 days; acetylsalicylic acid 325 mg every day x 30 days; azithromycin 250 mg b.i.d. before every meal x 10 days; hydroxychloroquine sulfate 200 mg b.i.d. x 10 days; ivermectin 3 mg, 5 pills daily x 10 days; zinc sulfate 220 mg (50 mg elemental) every day x 30 days; and a prednisone taper (30 mg daily x 3 days, tapering down 5 mg every 3 days). Hydroxyzine 50 mg at bedtime as needed was added for sleep. The patient did not comment to the family physician on any of the psychological or psychiatric symptoms and responded appropriately to questions during the Oct. 20 initial evaluation.
However, he later described that around the time the PCR was positive, For example, he was watching a simple YouTube video for work and “everything was confusing me ... it rattled me, and I couldn’t understand it.” He described his COVID-19 mind as: “The words in my head would come out in a jumbled order, like the message from the words in my brain to my mouth would get crossed. I had trouble spelling and texting. Total cognitive breakdown. I couldn’t do simple mathematics.”
Despite his physical exhaustion, he endured a 3-day period of sleep deprivation. During this time, he recalled looking up at the roof and thinking, “I need to jump off the roof” or thinking, “I might want to throw myself under a bus.” He did not initially reveal his suicidal thoughts to his family medicine physician. After beginning COVID-19 treatment, the patient had two nights of sleep and felt notably improved, and his physical symptoms began to remit. However, the sleeplessness quickly returned “with a vengeance” along with “silly suicidal thoughts.” The thoughts took on a more obsessional quality. For example, he repeatedly thought of jumping out of his second-story bedroom to the living room below and was preoccupied by continually looking at people’s roofs and thinking about jumping. Those thoughts intensified and culminated in his “going missing,” leading his wife to call the police. It was discovered that he had driven to a local bridge and was contemplating jumping off.
After that “going missing” incident, the patient and his wife reached out to their family medicine physician. He reevaluated the patient and, given the new information about the psychiatric symptoms, strongly recommended stat crisis and psychiatric consultation. After discussing the case on the same day, both the family medicine physician and the psychiatrist recommended stat hospital emergency department (ED) assessment on Oct. 29. In the ED, a head CT without contrast at the recommendation of both psychiatrist and family physician, routine electrolytes, CBC with differential, and EKG all were within normal limits. The ED initially discharged him home after crisis evaluation, deciding he was not an imminent risk to himself or others.
The next day, the psychiatrist spoke on the phone with the patient, family medicine physician, and the patient’s wife to arrange an initial assessment. At that time, it remained unclear to all whether the obsessional thoughts had resolved to such a degree that the patient could resist acting upon them. Further, the patient’s sleep architecture had not returned to normal. All agreed another emergency ED assessment was indicated. Ultimately, after voluntary re-evaluation and a difficult hold in the crisis unit, the patient was admitted for psychiatric hospitalization on Oct. 29 and discharged on Nov. 4.
In the psychiatric hospital, venlafaxine XR was started and titrated to 75 mg. The patient was discovered to be hypertensive, and hydrochlorothiazide was started. The discharge diagnosis was major depressive disorder, single episode, severe, without psychotic features.
Posthospitalization course
He was seen for his initial psychiatric outpatient assessment postpsychiatric hospitalization on Nov. 9, as he had not yet been formally evaluated by the psychiatrist because of the emergency situation.
Gabapentin 300 mg by mouth at bedtime was started, and his sleep architecture was restored. The initial plan to titrate venlafaxine XR into dual selective norepinephrine reuptake inhibitor dose range was terminated, and his psychiatrist considered tapering and discontinuing the venlafaxine XR. A clinical examination, additional history, and collateral data no longer necessarily pointed to an active major depressive disorder or even unspecified depressive disorder, though to be sure, the patient was taking 75 mg of venlafaxine XR. While there were seasonal stressors, historically, nothing had risen to the level of MDD.
The obsessions driving his thoughts to jump off buildings and bridges had completely remitted. His cognitive ability returned to baseline with an ability to focus and perform the complicated tasks of his high-intensity work by the Dec. 8 psychiatric examination, where he was accompanied by his wife. He described feeling like, “I snapped back to like I was before this crazy stuff happened.” His mood was reported as, “Very good; like my old self” and this was confirmed by his wife. His affect was calmer and less tense. He was now using gabapentin sparingly for sleep. We continued to entertain discontinuing the venlafaxine XR, considering this recent severe episode likely driven by the COVID-19 virus. The decision was made to continue venlafaxine XR through the winter rather than discontinuing, remaining on the conservative side of treatment. The patient’s diagnosis was changed from “MDD, single episode,” to “mood disorder due to known physiologic condition (COVID-19) (F06.31) with depressive features; resolving.” At the patient’s follow-up examination on Jan. 5, 2022, he was continuing to do well, stating, “The whole series of crazy events happened to someone else.” The hydrochlorothiazide had been discontinued, and the patient’s blood pressure and pulse were normal at 119/81 and 69, respectively. He had made strategic changes at work to lessen stressors during the typically difficult months.
Discussion
Literature has discussed neuropsychiatric sequelae of COVID-19.2 The cited example questions whether psychiatric symptoms are tied directly to the viral infection or to the “host’s immune response.” We believe our case represents a direct neurocognitive/neuropsychiatric insult due to the COVID-19 infection.
This case presents a 55-year-old male with no previous psychiatric or medical history with new onset significant and debilitating cognitive impairment and obsessive thoughts of throwing himself from his bedroom balcony ending up at a bridge struggling with an irrational thought of jumping; ultimately requiring psychiatric hospitalization for acute suicidal thoughts. The patient’s psychiatric symptoms arose prior to any and all medication treatment. The obsessive thoughts correlated both with the onset of SARS-CoV-2 infection and a period of sleep deprivation subsequent to the infection. A course of steroid treatment and taper were started after the onset of neurocognitive-psychiatric symptoms, though there is close timing. We submit that the patient experienced, as part of the initial neurocognitive psychiatric initiating cascade, a COVID-19–induced sleep deprivation that was not etiologic but part of the process; since, even when sleep returned to normal, it was still several weeks before full cognitive function returned to baseline.
An argument could be made for possible MDD or unspecified depressive disorder, as historically there had been work-related stressors for the patient at this time of year because of the chronological nature of his work; though previously nothing presented with obsessional suicidal thinking and nothing with any cognitive impairment – let alone to this incapacitating degree.
The patient describes his seasonal work much like an accountant’s work at the beginning of each year. In the patient’s case, the months of September and October are historically “nonstop, working days,” which then slow down in the winter months for a period of recuperation. In gathering his past history of symptoms, he denied neurovegetative symptoms to meet full diagnostic criteria for MDD or unspecified depressive disorder, absent this episode in the presence of SARS-CoV-2 infection.
We could also consider a contributory negative “organic push” by the viral load and prednisone helping to express an underlying unspecified depression or MDD, but for the profound and unusual presentation. There was little prodrome of depressive symptoms (again, he reported his “typical” extraordinary work burden for this time of year, which is common in his industry).
In this patient, the symptoms have remitted completely. However, the patient is currently taking venlafaxine XR 75 mg. We have considered tapering and discontinuing the venlafaxine – since it is not entirely clear that he needs to be on this medication – so this question remains an open one. We did decide, however, to continue the venlafaxine until after the winter months and to reassess at that time.
Conclusion
The patient presented with new onset psychological and psychiatric symptoms in addition to physiologic symptoms; the former symptoms were not revealed prior to initial family medicine evaluation. As the symptoms worsened, he and his wife sought additional consultation with family physician, psychiatrists, and ED. Steroid treatment may have played a part in exacerbation of symptoms, but the neuropsychiatric cognitive symptoms were present prior to initiation of all pharmacologic and medical treatment. The successful outcome of this case was based upon quick action and collaboration between the family medicine physician, the psychiatrist, and the ED physician. The value of communication, assessment, and action via phone call and text cannot be overstated. Future considerations include further large-scale evaluation of multifaceted early treatment of patients with COVID-19 within the first 72 hours of symptoms to prevent not only hospitalization, morbidity, and mortality, but newly recognized psychological and psychiatric syndromes.3,4
Lastly, fluvoxamine might have been a better choice for adjunctive early treatment of COVID-19.5 As a matter of distinction, if a lingering mood disorder or obsessive-compulsive disorder remain a result of SARS-CoV-2 or if one were to start an antidepressant during the course of illness, it would be reasonable to consider fluvoxamine as a potential first-line agent.
Dr. Kohanski is a fellowship trained forensic psychiatrist and a diplomate of the American Board of Psychiatry & Neurology. She maintains a private practice in Somerset, N.J., and is a frequent media commentator and medical podcaster. Dr. Kohanski has no conflicts of interest. Dr. Wax is a residency-trained osteopathic family medicine physician in independent private practice in Mullica Hill, N.J. He has authored multiple papers over 2 decades on topics such as SARS-CoV-2 and COVID-19 early treatment. He has been a speaker and media host over 2 decades and served on the National Physicians Council on Healthcare Policy’s congressional subcommittee. Dr. Wax has no conflicts of interest.
References
1. Rev Cardiovasc Med. 2020 Dec 30;21(4):517-30.
2. Brain Behav Immun. 2020 Jul;87:34-9.
3. Trav Med Infect Dis. 2020 May-Jun 35;10738.
4. Kirsch S. “Early treatment for COVID is key to better outcomes.” Times of India. 2021 May 21.
5. Lancet. 2022 Jan 1;10(1):E42-E51.
An otherwise healthy 55-year-old male, with no previous psychiatric or medical history, sought care with a family medicine physician for the first time in decades.
Medical symptoms began Oct. 9, 2021, with “some leg weakness and mild sniffles.” Since he was going to be at a public event, he decided to take a PCR test for the SARS-CoV-2 virus on Oct. 13. The patient tested positive.
His symptoms continued to worsen, and he experienced severe body fatigue, sleep disturbance, and lethargy. “A few days after my positive test, the cognitive and physical symptoms dramatically ramped up,” the patient recalled.
Because of those worsening symptoms, on Oct. 20, the patient obtained a new patient appointment with a family medicine physician. After a telemedicine evaluation, the family medicine physician began a multifaceted early outpatient COVID-19 treatment protocol,1 as I (C.M.W.) and colleagues wrote about late last year. However, this treatment began late in the course because of the patient’s initial resistance to seek care.
This early outpatient treatment protocol for COVID-19 included vitamin D3 125 mcg (5,000 ICU), N-acetylcysteine (NAC) 600 mg every day x 30 days; acetylsalicylic acid 325 mg every day x 30 days; azithromycin 250 mg b.i.d. before every meal x 10 days; hydroxychloroquine sulfate 200 mg b.i.d. x 10 days; ivermectin 3 mg, 5 pills daily x 10 days; zinc sulfate 220 mg (50 mg elemental) every day x 30 days; and a prednisone taper (30 mg daily x 3 days, tapering down 5 mg every 3 days). Hydroxyzine 50 mg at bedtime as needed was added for sleep. The patient did not comment to the family physician on any of the psychological or psychiatric symptoms and responded appropriately to questions during the Oct. 20 initial evaluation.
However, he later described that around the time the PCR was positive, For example, he was watching a simple YouTube video for work and “everything was confusing me ... it rattled me, and I couldn’t understand it.” He described his COVID-19 mind as: “The words in my head would come out in a jumbled order, like the message from the words in my brain to my mouth would get crossed. I had trouble spelling and texting. Total cognitive breakdown. I couldn’t do simple mathematics.”
Despite his physical exhaustion, he endured a 3-day period of sleep deprivation. During this time, he recalled looking up at the roof and thinking, “I need to jump off the roof” or thinking, “I might want to throw myself under a bus.” He did not initially reveal his suicidal thoughts to his family medicine physician. After beginning COVID-19 treatment, the patient had two nights of sleep and felt notably improved, and his physical symptoms began to remit. However, the sleeplessness quickly returned “with a vengeance” along with “silly suicidal thoughts.” The thoughts took on a more obsessional quality. For example, he repeatedly thought of jumping out of his second-story bedroom to the living room below and was preoccupied by continually looking at people’s roofs and thinking about jumping. Those thoughts intensified and culminated in his “going missing,” leading his wife to call the police. It was discovered that he had driven to a local bridge and was contemplating jumping off.
After that “going missing” incident, the patient and his wife reached out to their family medicine physician. He reevaluated the patient and, given the new information about the psychiatric symptoms, strongly recommended stat crisis and psychiatric consultation. After discussing the case on the same day, both the family medicine physician and the psychiatrist recommended stat hospital emergency department (ED) assessment on Oct. 29. In the ED, a head CT without contrast at the recommendation of both psychiatrist and family physician, routine electrolytes, CBC with differential, and EKG all were within normal limits. The ED initially discharged him home after crisis evaluation, deciding he was not an imminent risk to himself or others.
The next day, the psychiatrist spoke on the phone with the patient, family medicine physician, and the patient’s wife to arrange an initial assessment. At that time, it remained unclear to all whether the obsessional thoughts had resolved to such a degree that the patient could resist acting upon them. Further, the patient’s sleep architecture had not returned to normal. All agreed another emergency ED assessment was indicated. Ultimately, after voluntary re-evaluation and a difficult hold in the crisis unit, the patient was admitted for psychiatric hospitalization on Oct. 29 and discharged on Nov. 4.
In the psychiatric hospital, venlafaxine XR was started and titrated to 75 mg. The patient was discovered to be hypertensive, and hydrochlorothiazide was started. The discharge diagnosis was major depressive disorder, single episode, severe, without psychotic features.
Posthospitalization course
He was seen for his initial psychiatric outpatient assessment postpsychiatric hospitalization on Nov. 9, as he had not yet been formally evaluated by the psychiatrist because of the emergency situation.
Gabapentin 300 mg by mouth at bedtime was started, and his sleep architecture was restored. The initial plan to titrate venlafaxine XR into dual selective norepinephrine reuptake inhibitor dose range was terminated, and his psychiatrist considered tapering and discontinuing the venlafaxine XR. A clinical examination, additional history, and collateral data no longer necessarily pointed to an active major depressive disorder or even unspecified depressive disorder, though to be sure, the patient was taking 75 mg of venlafaxine XR. While there were seasonal stressors, historically, nothing had risen to the level of MDD.
The obsessions driving his thoughts to jump off buildings and bridges had completely remitted. His cognitive ability returned to baseline with an ability to focus and perform the complicated tasks of his high-intensity work by the Dec. 8 psychiatric examination, where he was accompanied by his wife. He described feeling like, “I snapped back to like I was before this crazy stuff happened.” His mood was reported as, “Very good; like my old self” and this was confirmed by his wife. His affect was calmer and less tense. He was now using gabapentin sparingly for sleep. We continued to entertain discontinuing the venlafaxine XR, considering this recent severe episode likely driven by the COVID-19 virus. The decision was made to continue venlafaxine XR through the winter rather than discontinuing, remaining on the conservative side of treatment. The patient’s diagnosis was changed from “MDD, single episode,” to “mood disorder due to known physiologic condition (COVID-19) (F06.31) with depressive features; resolving.” At the patient’s follow-up examination on Jan. 5, 2022, he was continuing to do well, stating, “The whole series of crazy events happened to someone else.” The hydrochlorothiazide had been discontinued, and the patient’s blood pressure and pulse were normal at 119/81 and 69, respectively. He had made strategic changes at work to lessen stressors during the typically difficult months.
Discussion
Literature has discussed neuropsychiatric sequelae of COVID-19.2 The cited example questions whether psychiatric symptoms are tied directly to the viral infection or to the “host’s immune response.” We believe our case represents a direct neurocognitive/neuropsychiatric insult due to the COVID-19 infection.
This case presents a 55-year-old male with no previous psychiatric or medical history with new onset significant and debilitating cognitive impairment and obsessive thoughts of throwing himself from his bedroom balcony ending up at a bridge struggling with an irrational thought of jumping; ultimately requiring psychiatric hospitalization for acute suicidal thoughts. The patient’s psychiatric symptoms arose prior to any and all medication treatment. The obsessive thoughts correlated both with the onset of SARS-CoV-2 infection and a period of sleep deprivation subsequent to the infection. A course of steroid treatment and taper were started after the onset of neurocognitive-psychiatric symptoms, though there is close timing. We submit that the patient experienced, as part of the initial neurocognitive psychiatric initiating cascade, a COVID-19–induced sleep deprivation that was not etiologic but part of the process; since, even when sleep returned to normal, it was still several weeks before full cognitive function returned to baseline.
An argument could be made for possible MDD or unspecified depressive disorder, as historically there had been work-related stressors for the patient at this time of year because of the chronological nature of his work; though previously nothing presented with obsessional suicidal thinking and nothing with any cognitive impairment – let alone to this incapacitating degree.
The patient describes his seasonal work much like an accountant’s work at the beginning of each year. In the patient’s case, the months of September and October are historically “nonstop, working days,” which then slow down in the winter months for a period of recuperation. In gathering his past history of symptoms, he denied neurovegetative symptoms to meet full diagnostic criteria for MDD or unspecified depressive disorder, absent this episode in the presence of SARS-CoV-2 infection.
We could also consider a contributory negative “organic push” by the viral load and prednisone helping to express an underlying unspecified depression or MDD, but for the profound and unusual presentation. There was little prodrome of depressive symptoms (again, he reported his “typical” extraordinary work burden for this time of year, which is common in his industry).
In this patient, the symptoms have remitted completely. However, the patient is currently taking venlafaxine XR 75 mg. We have considered tapering and discontinuing the venlafaxine – since it is not entirely clear that he needs to be on this medication – so this question remains an open one. We did decide, however, to continue the venlafaxine until after the winter months and to reassess at that time.
Conclusion
The patient presented with new onset psychological and psychiatric symptoms in addition to physiologic symptoms; the former symptoms were not revealed prior to initial family medicine evaluation. As the symptoms worsened, he and his wife sought additional consultation with family physician, psychiatrists, and ED. Steroid treatment may have played a part in exacerbation of symptoms, but the neuropsychiatric cognitive symptoms were present prior to initiation of all pharmacologic and medical treatment. The successful outcome of this case was based upon quick action and collaboration between the family medicine physician, the psychiatrist, and the ED physician. The value of communication, assessment, and action via phone call and text cannot be overstated. Future considerations include further large-scale evaluation of multifaceted early treatment of patients with COVID-19 within the first 72 hours of symptoms to prevent not only hospitalization, morbidity, and mortality, but newly recognized psychological and psychiatric syndromes.3,4
Lastly, fluvoxamine might have been a better choice for adjunctive early treatment of COVID-19.5 As a matter of distinction, if a lingering mood disorder or obsessive-compulsive disorder remain a result of SARS-CoV-2 or if one were to start an antidepressant during the course of illness, it would be reasonable to consider fluvoxamine as a potential first-line agent.
Dr. Kohanski is a fellowship trained forensic psychiatrist and a diplomate of the American Board of Psychiatry & Neurology. She maintains a private practice in Somerset, N.J., and is a frequent media commentator and medical podcaster. Dr. Kohanski has no conflicts of interest. Dr. Wax is a residency-trained osteopathic family medicine physician in independent private practice in Mullica Hill, N.J. He has authored multiple papers over 2 decades on topics such as SARS-CoV-2 and COVID-19 early treatment. He has been a speaker and media host over 2 decades and served on the National Physicians Council on Healthcare Policy’s congressional subcommittee. Dr. Wax has no conflicts of interest.
References
1. Rev Cardiovasc Med. 2020 Dec 30;21(4):517-30.
2. Brain Behav Immun. 2020 Jul;87:34-9.
3. Trav Med Infect Dis. 2020 May-Jun 35;10738.
4. Kirsch S. “Early treatment for COVID is key to better outcomes.” Times of India. 2021 May 21.
5. Lancet. 2022 Jan 1;10(1):E42-E51.
An otherwise healthy 55-year-old male, with no previous psychiatric or medical history, sought care with a family medicine physician for the first time in decades.
Medical symptoms began Oct. 9, 2021, with “some leg weakness and mild sniffles.” Since he was going to be at a public event, he decided to take a PCR test for the SARS-CoV-2 virus on Oct. 13. The patient tested positive.
His symptoms continued to worsen, and he experienced severe body fatigue, sleep disturbance, and lethargy. “A few days after my positive test, the cognitive and physical symptoms dramatically ramped up,” the patient recalled.
Because of those worsening symptoms, on Oct. 20, the patient obtained a new patient appointment with a family medicine physician. After a telemedicine evaluation, the family medicine physician began a multifaceted early outpatient COVID-19 treatment protocol,1 as I (C.M.W.) and colleagues wrote about late last year. However, this treatment began late in the course because of the patient’s initial resistance to seek care.
This early outpatient treatment protocol for COVID-19 included vitamin D3 125 mcg (5,000 ICU), N-acetylcysteine (NAC) 600 mg every day x 30 days; acetylsalicylic acid 325 mg every day x 30 days; azithromycin 250 mg b.i.d. before every meal x 10 days; hydroxychloroquine sulfate 200 mg b.i.d. x 10 days; ivermectin 3 mg, 5 pills daily x 10 days; zinc sulfate 220 mg (50 mg elemental) every day x 30 days; and a prednisone taper (30 mg daily x 3 days, tapering down 5 mg every 3 days). Hydroxyzine 50 mg at bedtime as needed was added for sleep. The patient did not comment to the family physician on any of the psychological or psychiatric symptoms and responded appropriately to questions during the Oct. 20 initial evaluation.
However, he later described that around the time the PCR was positive, For example, he was watching a simple YouTube video for work and “everything was confusing me ... it rattled me, and I couldn’t understand it.” He described his COVID-19 mind as: “The words in my head would come out in a jumbled order, like the message from the words in my brain to my mouth would get crossed. I had trouble spelling and texting. Total cognitive breakdown. I couldn’t do simple mathematics.”
Despite his physical exhaustion, he endured a 3-day period of sleep deprivation. During this time, he recalled looking up at the roof and thinking, “I need to jump off the roof” or thinking, “I might want to throw myself under a bus.” He did not initially reveal his suicidal thoughts to his family medicine physician. After beginning COVID-19 treatment, the patient had two nights of sleep and felt notably improved, and his physical symptoms began to remit. However, the sleeplessness quickly returned “with a vengeance” along with “silly suicidal thoughts.” The thoughts took on a more obsessional quality. For example, he repeatedly thought of jumping out of his second-story bedroom to the living room below and was preoccupied by continually looking at people’s roofs and thinking about jumping. Those thoughts intensified and culminated in his “going missing,” leading his wife to call the police. It was discovered that he had driven to a local bridge and was contemplating jumping off.
After that “going missing” incident, the patient and his wife reached out to their family medicine physician. He reevaluated the patient and, given the new information about the psychiatric symptoms, strongly recommended stat crisis and psychiatric consultation. After discussing the case on the same day, both the family medicine physician and the psychiatrist recommended stat hospital emergency department (ED) assessment on Oct. 29. In the ED, a head CT without contrast at the recommendation of both psychiatrist and family physician, routine electrolytes, CBC with differential, and EKG all were within normal limits. The ED initially discharged him home after crisis evaluation, deciding he was not an imminent risk to himself or others.
The next day, the psychiatrist spoke on the phone with the patient, family medicine physician, and the patient’s wife to arrange an initial assessment. At that time, it remained unclear to all whether the obsessional thoughts had resolved to such a degree that the patient could resist acting upon them. Further, the patient’s sleep architecture had not returned to normal. All agreed another emergency ED assessment was indicated. Ultimately, after voluntary re-evaluation and a difficult hold in the crisis unit, the patient was admitted for psychiatric hospitalization on Oct. 29 and discharged on Nov. 4.
In the psychiatric hospital, venlafaxine XR was started and titrated to 75 mg. The patient was discovered to be hypertensive, and hydrochlorothiazide was started. The discharge diagnosis was major depressive disorder, single episode, severe, without psychotic features.
Posthospitalization course
He was seen for his initial psychiatric outpatient assessment postpsychiatric hospitalization on Nov. 9, as he had not yet been formally evaluated by the psychiatrist because of the emergency situation.
Gabapentin 300 mg by mouth at bedtime was started, and his sleep architecture was restored. The initial plan to titrate venlafaxine XR into dual selective norepinephrine reuptake inhibitor dose range was terminated, and his psychiatrist considered tapering and discontinuing the venlafaxine XR. A clinical examination, additional history, and collateral data no longer necessarily pointed to an active major depressive disorder or even unspecified depressive disorder, though to be sure, the patient was taking 75 mg of venlafaxine XR. While there were seasonal stressors, historically, nothing had risen to the level of MDD.
The obsessions driving his thoughts to jump off buildings and bridges had completely remitted. His cognitive ability returned to baseline with an ability to focus and perform the complicated tasks of his high-intensity work by the Dec. 8 psychiatric examination, where he was accompanied by his wife. He described feeling like, “I snapped back to like I was before this crazy stuff happened.” His mood was reported as, “Very good; like my old self” and this was confirmed by his wife. His affect was calmer and less tense. He was now using gabapentin sparingly for sleep. We continued to entertain discontinuing the venlafaxine XR, considering this recent severe episode likely driven by the COVID-19 virus. The decision was made to continue venlafaxine XR through the winter rather than discontinuing, remaining on the conservative side of treatment. The patient’s diagnosis was changed from “MDD, single episode,” to “mood disorder due to known physiologic condition (COVID-19) (F06.31) with depressive features; resolving.” At the patient’s follow-up examination on Jan. 5, 2022, he was continuing to do well, stating, “The whole series of crazy events happened to someone else.” The hydrochlorothiazide had been discontinued, and the patient’s blood pressure and pulse were normal at 119/81 and 69, respectively. He had made strategic changes at work to lessen stressors during the typically difficult months.
Discussion
Literature has discussed neuropsychiatric sequelae of COVID-19.2 The cited example questions whether psychiatric symptoms are tied directly to the viral infection or to the “host’s immune response.” We believe our case represents a direct neurocognitive/neuropsychiatric insult due to the COVID-19 infection.
This case presents a 55-year-old male with no previous psychiatric or medical history with new onset significant and debilitating cognitive impairment and obsessive thoughts of throwing himself from his bedroom balcony ending up at a bridge struggling with an irrational thought of jumping; ultimately requiring psychiatric hospitalization for acute suicidal thoughts. The patient’s psychiatric symptoms arose prior to any and all medication treatment. The obsessive thoughts correlated both with the onset of SARS-CoV-2 infection and a period of sleep deprivation subsequent to the infection. A course of steroid treatment and taper were started after the onset of neurocognitive-psychiatric symptoms, though there is close timing. We submit that the patient experienced, as part of the initial neurocognitive psychiatric initiating cascade, a COVID-19–induced sleep deprivation that was not etiologic but part of the process; since, even when sleep returned to normal, it was still several weeks before full cognitive function returned to baseline.
An argument could be made for possible MDD or unspecified depressive disorder, as historically there had been work-related stressors for the patient at this time of year because of the chronological nature of his work; though previously nothing presented with obsessional suicidal thinking and nothing with any cognitive impairment – let alone to this incapacitating degree.
The patient describes his seasonal work much like an accountant’s work at the beginning of each year. In the patient’s case, the months of September and October are historically “nonstop, working days,” which then slow down in the winter months for a period of recuperation. In gathering his past history of symptoms, he denied neurovegetative symptoms to meet full diagnostic criteria for MDD or unspecified depressive disorder, absent this episode in the presence of SARS-CoV-2 infection.
We could also consider a contributory negative “organic push” by the viral load and prednisone helping to express an underlying unspecified depression or MDD, but for the profound and unusual presentation. There was little prodrome of depressive symptoms (again, he reported his “typical” extraordinary work burden for this time of year, which is common in his industry).
In this patient, the symptoms have remitted completely. However, the patient is currently taking venlafaxine XR 75 mg. We have considered tapering and discontinuing the venlafaxine – since it is not entirely clear that he needs to be on this medication – so this question remains an open one. We did decide, however, to continue the venlafaxine until after the winter months and to reassess at that time.
Conclusion
The patient presented with new onset psychological and psychiatric symptoms in addition to physiologic symptoms; the former symptoms were not revealed prior to initial family medicine evaluation. As the symptoms worsened, he and his wife sought additional consultation with family physician, psychiatrists, and ED. Steroid treatment may have played a part in exacerbation of symptoms, but the neuropsychiatric cognitive symptoms were present prior to initiation of all pharmacologic and medical treatment. The successful outcome of this case was based upon quick action and collaboration between the family medicine physician, the psychiatrist, and the ED physician. The value of communication, assessment, and action via phone call and text cannot be overstated. Future considerations include further large-scale evaluation of multifaceted early treatment of patients with COVID-19 within the first 72 hours of symptoms to prevent not only hospitalization, morbidity, and mortality, but newly recognized psychological and psychiatric syndromes.3,4
Lastly, fluvoxamine might have been a better choice for adjunctive early treatment of COVID-19.5 As a matter of distinction, if a lingering mood disorder or obsessive-compulsive disorder remain a result of SARS-CoV-2 or if one were to start an antidepressant during the course of illness, it would be reasonable to consider fluvoxamine as a potential first-line agent.
Dr. Kohanski is a fellowship trained forensic psychiatrist and a diplomate of the American Board of Psychiatry & Neurology. She maintains a private practice in Somerset, N.J., and is a frequent media commentator and medical podcaster. Dr. Kohanski has no conflicts of interest. Dr. Wax is a residency-trained osteopathic family medicine physician in independent private practice in Mullica Hill, N.J. He has authored multiple papers over 2 decades on topics such as SARS-CoV-2 and COVID-19 early treatment. He has been a speaker and media host over 2 decades and served on the National Physicians Council on Healthcare Policy’s congressional subcommittee. Dr. Wax has no conflicts of interest.
References
1. Rev Cardiovasc Med. 2020 Dec 30;21(4):517-30.
2. Brain Behav Immun. 2020 Jul;87:34-9.
3. Trav Med Infect Dis. 2020 May-Jun 35;10738.
4. Kirsch S. “Early treatment for COVID is key to better outcomes.” Times of India. 2021 May 21.
5. Lancet. 2022 Jan 1;10(1):E42-E51.
“I didn’t want to meet you.” Dispelling myths about palliative care
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
The names of health care professionals and patients cited within the dialogue text have been changed to protect their privacy.
but over the years I have come to realize that she was right – most people, including many within health care, don’t have a good appreciation of what palliative care is or how it can help patients and health care teams.
A recent national survey about cancer-related health information found that of more than 1,000 surveyed Americans, less than 30% professed any knowledge of palliative care. Of those who had some knowledge of palliative care, around 30% believed palliative care was synonymous with hospice.1 Another 15% believed that a patient would have to give up cancer-directed treatments to receive palliative care.1
It’s not giving up
This persistent belief that palliative care is equivalent to hospice, or is tantamount to “giving up,” is one of the most commonly held myths I encounter in everyday practice.
I knock on the exam door and walk in.
A small, trim woman in her late 50s is sitting in a chair, arms folded across her chest, face drawn in.
“Hi,” I start. “I’m Sarah, the palliative care nurse practitioner who works in this clinic. I work closely with Dr. Smith.”
Dr. Smith is the patient’s oncologist.
“I really didn’t want to meet you,” she says in a quiet voice, her eyes large with concern.
I don’t take it personally. Few patients really want to be in the position of needing to meet the palliative care team.
“I looked up palliative care on Google and saw the word hospice.”
“Yeah,” I say. “I hear that a lot. Well, I can reassure you that this isn’t hospice.
In this clinic, our focus is on your cancer symptoms, your treatment side effects, and your quality of life.”
She looks visibly relieved. “Quality of life,” she echoes. “I need more of that.”
“OK,” I say. “So, tell me what you’re struggling with the most right now.”
That’s how many palliative care visits start. I actually prefer if patients haven’t heard of palliative care because it allows me to frame it for them, rather than having to start by addressing a myth or a prior negative experience. Even when patients haven’t had a negative experience with palliative care per se, typically, if they’ve interacted with palliative care in the past, it’s usually because someone they loved died in a hospital setting and it is the memory of that terrible loss that becomes synonymous with their recollection of palliative care.
Many patients I meet have never seen another outpatient palliative care practitioner – and this makes sense – we are still too few and far between. Most established palliative care teams are hospital based and many patients seen in the community do not have easy access to palliative care teams where they receive oncologic care.2 As an embedded practitioner, I see patients in the same exam rooms and infusion centers where they receive their cancer therapies, so I’m effectively woven into the fabric of their oncology experience. Just being there in the cancer center allows me to be in the right place at the right time for the right patients and their care teams.
More than pain management
Another myth I tend to dispel a lot is that palliative care is just a euphemism for “pain management.” I have seen this less lately, but still occasionally in the chart I’ll see documented in a note, “patient is seeing palliative/pain management,” when a patient is seeing me or one of my colleagues. Unfortunately, when providers have limited or outdated views of what palliative care is or the value it brings to patient-centered cancer care, referrals to palliative care tend to be delayed.3
“I really think Ms. Lopez could benefit from seeing palliative care,” an oncology nurse practitioner says to an oncologist.
I’m standing nearby, about to see another patient in one of the exam rooms in our clinic.
“But I don’t think she’s ready. And besides, she doesn’t have any pain,” he says.
He turns to me quizzically. “What do you think?”
“Tell me about the patient,” I ask, taking a few steps in their direction.
“Well, she’s a 64-year-old woman with metastatic cancer.
She has a really poor appetite and is losing some weight.
Seems a bit down, kind of pessimistic about things.
Her scan showed some new growth, so guess I’m not surprised by that.”
“I might be able to help her with the appetite and the mood changes.
I can at least talk with her and see where she’s at,” I offer.
“Alright,” he says. “We’ll put the palliative referral in.”
He hesitates. “But are you sure you want to see her?
She doesn’t have any pain.” He sounds skeptical.
“Yeah, I mean, it sounds like she has symptoms that are bothering her, so I’d be happy to see her. She sounds completely appropriate for palliative care.”
I hear this assumption a lot – that palliative care is somehow equivalent to pain management and that unless a patient’s pain is severe, it’s not worth referring the patient to palliative care. Don’t get me wrong – we do a lot of pain management, but at its heart, palliative care is an interdisciplinary specialty focused on improving or maintaining quality of life for people with serious illness. Because the goal is so broad, care can take many shapes.4
In addition to pain, palliative care clinicians commonly treat nausea, shortness of breath, constipation or diarrhea, poor appetite, fatigue, anxiety, depression, and insomnia.
Palliative care is more than medical or nursing care
A related misconception about palliative care held by many lay people and health care workers alike is that palliative care is primarily medical or nursing care focused mostly on alleviating physical symptoms such as pain or nausea. This couldn’t be further from the truth.
We’ve been talking for a while.
Ms. Lopez tells me about her struggles to maintain her weight while undergoing chemotherapy. She has low-grade nausea that is impacting her ability and desire to eat more and didn’t think that her weight loss was severe enough to warrant taking medication.
We talk about how she may be able to use antinausea medication sparingly to alleviate nausea while also limiting side effects from the medications—which was a big concern for her.
I ask her what else is bothering her.
She tells me that she has always been a strong Catholic and even when life has gotten tough, her faith was never shaken – until now.
She is struggling to understand why she ended up with metastatic cancer at such a relatively young age—why would God do this to her?
She had plans for retirement that have since evaporated in the face of a foreshortened life.
Why did this happen to her of all people? She was completely healthy until her diagnosis.
Her face is wet with tears.
We talk a little about how a diagnosis like this can change so much of a person’s life and identity. I try to validate her experience. She’s clearly suffering from a sense that her life is not what she expected, and she is struggling to integrate how her future looks at this point.
I ask her what conversations with her priest have been like.
At this point you may be wondering where this conversation is going. Why are we talking about Ms. Lopez’s religion? Palliative care is best delivered through high functioning interdisciplinary teams that can include other supportive people in a patient’s life. We work in concert to try to bring comfort to a patient and their family.4 That support network can include nurses, physicians, social workers, and chaplains. In this case, Ms. Lopez had not yet reached out to her priest. She hasn’t had the time or energy to contact her priest given her symptoms.
“Can I contact your priest for you?
Maybe he can visit or call and chat with you?”
She nods and wipes tears away.
“That would be really nice,” she says. “I’d love it if he could pray with me.”
A few hours after the visit, I call Ms. Lopez’s priest.
I ask him to reach out to her and about her request for prayer.
He says he’s been thinking about her and that her presence has been missed at weekly Mass. He thanks me for the call and says he’ll call her tomorrow.
I say my own small prayer for Ms. Lopez and head home, the day’s work completed.
Sarah D'Ambruoso was born and raised in Maine. She completed her undergraduate and graduate nursing education at New York University and UCLA, respectively, and currently works as a palliative care nurse practitioner in an oncology clinic in Los Angeles.
References
1. Cheng BT et al. Patterns of palliative care beliefs among adults in the U.S.: Analysis of a National Cancer Database. J Pain Symptom Manage. 2019 Aug 10. doi: 10.1016/j.jpainsymman.2019.07.030.
2. Finlay E et al. Filling the gap: Creating an outpatient palliative care program in your institution. Am Soc Clin Oncol Educ Book. 2018 May 23. doi: 10.1200/EDBK_200775.
3. Von Roenn JH et al. Barriers and approaches to the successful integration of palliative care and oncology practice. J Natl Compr Canc Netw. 2013 Mar. doi: 10.6004/jnccn.2013.0209.
4. Ferrell BR et al. Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2016 Oct 31. doi: 10.1200/JCO.2016.70.1474.
Withholding anticoagulation for isolated subsegmental pulmonary embolism – Houston, we have a problem
All else being equal, I’d prefer to do nothing. Whether this is nihilism, laziness, or experience is a matter of debate. The American College of Chest Physicians (CHEST) Guidelines on therapy for venous thromboembolism (VTE) opened a door for withholding treatment for isolated subsegmental pulmonary embolism (ISSPE) in 2016 and kept it open in 2021. I was happy to walk through it and withhold therapy if it wasn’t indicated.
ISSPE is truly a conundrum. With advances in technology, the distal vessels in the lung became visible on commercial CT a little more than 10 years ago. The subsegmental branches are located after the fourth bifurcation of the pulmonary arterial system, and the new technology offered resolution adequate to identify clot in these vessels. But the new technology told us nothing about how to manage clot isolated to the subsegmental vasculature.
Autopsy data say clot in these vessels is common, even in patients who were never diagnosed with VTE while they were alive. To some degree then, the pulmonary arterial system is thought to serve as a filter to prevent clot from crossing to the systemic circulation and causing stroke. This led some to speculate that the subsegmental pulmonary arteries are supposed to contain clot and that we simply couldn’t see it before now. If this theory is correct, the practice of providing anticoagulation for ISSPE could increase bleeding without reducing the risk for VTE recurrence.
Management studies generally supported this concept. In 2007, a trial that was published in JAMA randomized patients to two different diagnostic strategies: ventilation-perfusion (VQ) and CT. CT detected more clot than VQ did, so more anticoagulation was given in the CT arm. Yet, the VTE rate during follow-up was not significantly different between arms. The implication? Some of the clots detected by CT were of lesser clinical significance and didn’t need to be treated.
Meta-analytic data from management trials also suggested that some pulmonary emboli (PE) need not be treated. Data also show when compared with patients who have more proximal PE, those with ISSPE have lower pretest probability for VTE, are less symptomatic, and have a lower burden of coexistent lower extremity thrombosis (deep vein thrombosis [DVT]).
In response to this data, the CHEST Guidelines began cautiously providing the option for withholding therapy in patients who were diagnosed with ISSPE in 2016. Their recommendations stated that patients should be stratified for recurrence risk and have lower extremity ultrasonography performed to rule out DVT. A patient with ISSPE, a low recurrence risk, and a negative ultrasound can have anticoagulation withheld. This made perfect sense to me based on what I thought I knew at the time.
Recently published data cast doubt on my nihilism. The first prospective study designed specifically to assess the safety of withholding therapy for ISSPE suggests that this practice could be dangerous. How did this happen? The trial was very well done, and the authors enrolled the right population. All of the patients had ISSPE, low recurrence risk, and negative lower extremity ultrasound. The authors were anticipating a 1% VTE rate at 90 days based on prior data but instead found a rate of 3.1% (1.6%-6.1%). They point out that this rate is not different from those seen in patients with more proximal PE who are treated with anticoagulation. However, they acknowledge that it is higher than what’s considered acceptable and warrants therapeutic anticoagulation.
So what should we do now? We treat ISSPE, that’s what. All the arguments for withholding therapy remain valid, the recurrence rate is reasonably low, and none of the recurrent VTEs in the new study were fatal. There’s still no doubt that some patients with PE won’t benefit from anticoagulation. Unfortunately, we currently lack the tools to identify them. The risk-benefit ratio for recurrence versus bleeding will be tighter with ISSPE, particularly when there’s only one clot. Unless the bleeding risk is elevated though, the ratio still favors treatment.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center.
A version of this article first appeared on Medscape.com.
All else being equal, I’d prefer to do nothing. Whether this is nihilism, laziness, or experience is a matter of debate. The American College of Chest Physicians (CHEST) Guidelines on therapy for venous thromboembolism (VTE) opened a door for withholding treatment for isolated subsegmental pulmonary embolism (ISSPE) in 2016 and kept it open in 2021. I was happy to walk through it and withhold therapy if it wasn’t indicated.
ISSPE is truly a conundrum. With advances in technology, the distal vessels in the lung became visible on commercial CT a little more than 10 years ago. The subsegmental branches are located after the fourth bifurcation of the pulmonary arterial system, and the new technology offered resolution adequate to identify clot in these vessels. But the new technology told us nothing about how to manage clot isolated to the subsegmental vasculature.
Autopsy data say clot in these vessels is common, even in patients who were never diagnosed with VTE while they were alive. To some degree then, the pulmonary arterial system is thought to serve as a filter to prevent clot from crossing to the systemic circulation and causing stroke. This led some to speculate that the subsegmental pulmonary arteries are supposed to contain clot and that we simply couldn’t see it before now. If this theory is correct, the practice of providing anticoagulation for ISSPE could increase bleeding without reducing the risk for VTE recurrence.
Management studies generally supported this concept. In 2007, a trial that was published in JAMA randomized patients to two different diagnostic strategies: ventilation-perfusion (VQ) and CT. CT detected more clot than VQ did, so more anticoagulation was given in the CT arm. Yet, the VTE rate during follow-up was not significantly different between arms. The implication? Some of the clots detected by CT were of lesser clinical significance and didn’t need to be treated.
Meta-analytic data from management trials also suggested that some pulmonary emboli (PE) need not be treated. Data also show when compared with patients who have more proximal PE, those with ISSPE have lower pretest probability for VTE, are less symptomatic, and have a lower burden of coexistent lower extremity thrombosis (deep vein thrombosis [DVT]).
In response to this data, the CHEST Guidelines began cautiously providing the option for withholding therapy in patients who were diagnosed with ISSPE in 2016. Their recommendations stated that patients should be stratified for recurrence risk and have lower extremity ultrasonography performed to rule out DVT. A patient with ISSPE, a low recurrence risk, and a negative ultrasound can have anticoagulation withheld. This made perfect sense to me based on what I thought I knew at the time.
Recently published data cast doubt on my nihilism. The first prospective study designed specifically to assess the safety of withholding therapy for ISSPE suggests that this practice could be dangerous. How did this happen? The trial was very well done, and the authors enrolled the right population. All of the patients had ISSPE, low recurrence risk, and negative lower extremity ultrasound. The authors were anticipating a 1% VTE rate at 90 days based on prior data but instead found a rate of 3.1% (1.6%-6.1%). They point out that this rate is not different from those seen in patients with more proximal PE who are treated with anticoagulation. However, they acknowledge that it is higher than what’s considered acceptable and warrants therapeutic anticoagulation.
So what should we do now? We treat ISSPE, that’s what. All the arguments for withholding therapy remain valid, the recurrence rate is reasonably low, and none of the recurrent VTEs in the new study were fatal. There’s still no doubt that some patients with PE won’t benefit from anticoagulation. Unfortunately, we currently lack the tools to identify them. The risk-benefit ratio for recurrence versus bleeding will be tighter with ISSPE, particularly when there’s only one clot. Unless the bleeding risk is elevated though, the ratio still favors treatment.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center.
A version of this article first appeared on Medscape.com.
All else being equal, I’d prefer to do nothing. Whether this is nihilism, laziness, or experience is a matter of debate. The American College of Chest Physicians (CHEST) Guidelines on therapy for venous thromboembolism (VTE) opened a door for withholding treatment for isolated subsegmental pulmonary embolism (ISSPE) in 2016 and kept it open in 2021. I was happy to walk through it and withhold therapy if it wasn’t indicated.
ISSPE is truly a conundrum. With advances in technology, the distal vessels in the lung became visible on commercial CT a little more than 10 years ago. The subsegmental branches are located after the fourth bifurcation of the pulmonary arterial system, and the new technology offered resolution adequate to identify clot in these vessels. But the new technology told us nothing about how to manage clot isolated to the subsegmental vasculature.
Autopsy data say clot in these vessels is common, even in patients who were never diagnosed with VTE while they were alive. To some degree then, the pulmonary arterial system is thought to serve as a filter to prevent clot from crossing to the systemic circulation and causing stroke. This led some to speculate that the subsegmental pulmonary arteries are supposed to contain clot and that we simply couldn’t see it before now. If this theory is correct, the practice of providing anticoagulation for ISSPE could increase bleeding without reducing the risk for VTE recurrence.
Management studies generally supported this concept. In 2007, a trial that was published in JAMA randomized patients to two different diagnostic strategies: ventilation-perfusion (VQ) and CT. CT detected more clot than VQ did, so more anticoagulation was given in the CT arm. Yet, the VTE rate during follow-up was not significantly different between arms. The implication? Some of the clots detected by CT were of lesser clinical significance and didn’t need to be treated.
Meta-analytic data from management trials also suggested that some pulmonary emboli (PE) need not be treated. Data also show when compared with patients who have more proximal PE, those with ISSPE have lower pretest probability for VTE, are less symptomatic, and have a lower burden of coexistent lower extremity thrombosis (deep vein thrombosis [DVT]).
In response to this data, the CHEST Guidelines began cautiously providing the option for withholding therapy in patients who were diagnosed with ISSPE in 2016. Their recommendations stated that patients should be stratified for recurrence risk and have lower extremity ultrasonography performed to rule out DVT. A patient with ISSPE, a low recurrence risk, and a negative ultrasound can have anticoagulation withheld. This made perfect sense to me based on what I thought I knew at the time.
Recently published data cast doubt on my nihilism. The first prospective study designed specifically to assess the safety of withholding therapy for ISSPE suggests that this practice could be dangerous. How did this happen? The trial was very well done, and the authors enrolled the right population. All of the patients had ISSPE, low recurrence risk, and negative lower extremity ultrasound. The authors were anticipating a 1% VTE rate at 90 days based on prior data but instead found a rate of 3.1% (1.6%-6.1%). They point out that this rate is not different from those seen in patients with more proximal PE who are treated with anticoagulation. However, they acknowledge that it is higher than what’s considered acceptable and warrants therapeutic anticoagulation.
So what should we do now? We treat ISSPE, that’s what. All the arguments for withholding therapy remain valid, the recurrence rate is reasonably low, and none of the recurrent VTEs in the new study were fatal. There’s still no doubt that some patients with PE won’t benefit from anticoagulation. Unfortunately, we currently lack the tools to identify them. The risk-benefit ratio for recurrence versus bleeding will be tighter with ISSPE, particularly when there’s only one clot. Unless the bleeding risk is elevated though, the ratio still favors treatment.
Aaron B. Holley, MD, is an associate professor of medicine at Uniformed Services University and program director of pulmonary and critical care medicine at Walter Reed National Military Medical Center.
A version of this article first appeared on Medscape.com.
Motherhood and mortality: Navigating miscarriages as a physician
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
One clinic morning in an office visit, I stood next to the door talking, hand on the doorknob ready to exit. My elderly patient was sitting in the chair next to the door, family member in another, as I attempted my exit. Suddenly, as if looking for something, my patient locked her gaze to my abdomen and began to slowly advance herself forward, eyes squinting for a better view. She had found something. Poke, poke, poke. Three pokes in quick succession into my apparently protruding abdomen stoked an internal horror that I dared not release onto my face. How in the hell could she know? My heart sank – the signs were still there.
“There’s something in there,” she said with a seasoned certainty.
“No there’s not,” I said trying hard to hide any emotion.
“Yes, there is,” she said flatly.
“Grannie, no there isn’t,” her family member interrupted, unknowingly saving me. I thanked them again and quickly left the room.
My patient had the ongoings of slowly progressing dementia. Little did she know she was right. Maybe she had known something in another time and space. Either way, I wasn’t prepared to tell the story. She wasn’t prepared to fully understand.
I tried to forge on to see the next patient. Tears began welling in both eyes. I tilted my head back slightly to prevent the water from falling. I wanted to feel offended, but she couldn’t have known the war my body was fighting at the time. I had not yet shared the pregnancy news with this particular patient, and yet her knowing was telling in a sense. I’m learning that the old folks always know.
I was at work, actively having yet another miscarriage. This was the second of two. This most recent time, we found out at 9 weeks that our baby had stopped growing about a week or so earlier. Cue the denial. Cue the rage. Cue the devastation.
Thinking back, with each pregnancy discovery, we did not wait the customary 3 months before telling anyone. Just about everyone knew. We were immediately excited to start sharing with friends, family, coworkers, and even patients early on. We knew the risks in my 40-something age group but were quintessentially optimistic.
I am a family medicine physician with expert-level knowledge and clinical experiences in women’s health counseling, contraception, conception, and pregnancy. In my training, I’ve delivered babies, been elbow-deep searching for wayward tissue from bleeding uteri, and sutured gaping vaginal lacerations. I’ve cried with new mothers at the end of long labors. I’ve been bear-hugged by doting new fathers. I have an abundance of medical knowledge, and yet the pain and struggle of miscarriage over the past 2.5 years has twice reduced me to absolute pieces. There was no course to teach me how to navigate loss within my own body, no textbook to study so that I could test out of the experience. Life hit us dead-on, and I was broken.
I can say that the experience of a miscarriage does not get easier with each subsequent loss. At least for me, the emotions were always raw and tender. Each one was a new gash to my emotional and physical health. My sanity bled out. I was physically exhausted. The struggles of being a health care worker in the midst of a global pandemic I’m sure did not help the situation. My first miscarriage was just before the start of the pandemic. I was in New York visiting family and after dinner at Tavern on the Green, of all places, when I began showing signs. Two days later, I was at the coffee station in our clinic cafeteria adding my cream and sugar when my ob.gyn.’s office called. The hCG levels were probably too low; a miscarriage was likely. I kept my composure, walked out of the cafeteria, got my car keys, went to my car, and proceeded to scream at the top of my lungs for a few minutes. Afterward, I went back to finish up my work and canceled my clinic for the rest of the day.
For my second miscarriage, I was laying in my doctor’s office getting an ultrasound. I had started bleeding the previous day but thought that the subchorionic hemorrhage noted on the last ultrasound might be the culprit. The bleeding was light. That’s the thing about being a pregnant physician: We know too much. The image on the screen looked abnormal, the remnants a ghost of its former self. I knew something was wrong but held out some hope. She searched and turned and pressed the transducer into my belly for a seemingly better view. She apologized for not finding the heartbeat. How is this happening again?
So how does one get through the loss of multiple pregnancies? I know my husband and I worked hard to get through each loss. We did all the right things a good therapist would recommend: Be present in the moment, go with your feelings, allow yourself to feel everything. There were no wrong emotions. Little by little we grieved and healed, grieved and healed. Having a successful pregnancy did help. Miracles are not promised but I believe we were sent one, and her name is Giavonna Barbara. Bookended by miscarriages, she has made me realize just how precious and delicate life really is. She is our absolute world and joy.
I’ve learned twice now that men mourn differently than women. Not any less, just in a different way. There is a pain in the silence that often goes unvocalized, but it is of no less value. My husband and I allowed each other to heal in our own unique ways, and that has made all of the difference. I think I knew I was doing okay when one day I found something funny and I let out the heartiest laugh my belly could muster. A different purpose was renewed. Tears were harder to come by. Hope for the future again sprung eternal. Life went on and so did we.
Looking back, I realize that having a miscarriage and working as a physician in the middle of a global pandemic pushed me to my emotional and physical limits. There is a second-guessing of sorts that occurs. Did the miscarriage happen because I was under so much stress at work? It had happened in the past, was this going to continue to happen?
I can say that I was great at compartmentalizing emotions. I’d try and box them away until I got off of work and then turn them on like a switch once I hit the driver’s seat. It’s easy as a busy physician with so many patients to see, messages to return, notes to write, students and residents to teach, and programs to run to completely tune out the thought of mourning. Temporarily anyway. Work was actually a welcome distraction at times. A purpose. The journey to healing is individualized and can’t be rushed. I like to think that I heal a little bit more every day thinking about the losses and gains that I’ve had. I’m grateful for the experience and growth.
In 2022, I’m looking forward to continuing my healing journey among the twists and turns of the pandemic. I now bring a different level of understanding and empathy to my patients who are undergoing or who have undergone a miscarriage. There will always be a piece of me that viscerally mourns with them. We have a hidden shared experience. I believe I am a better physician because of those lessons learned from my own personal tragedy. Now, I look forward to sharing big belly laughs with my family and friends and savoring the small, quiet moments with my husband and daughter.
A version of this article first appeared on Medscape.com.
The management of inflammatory bowel disease in pregnancy
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).
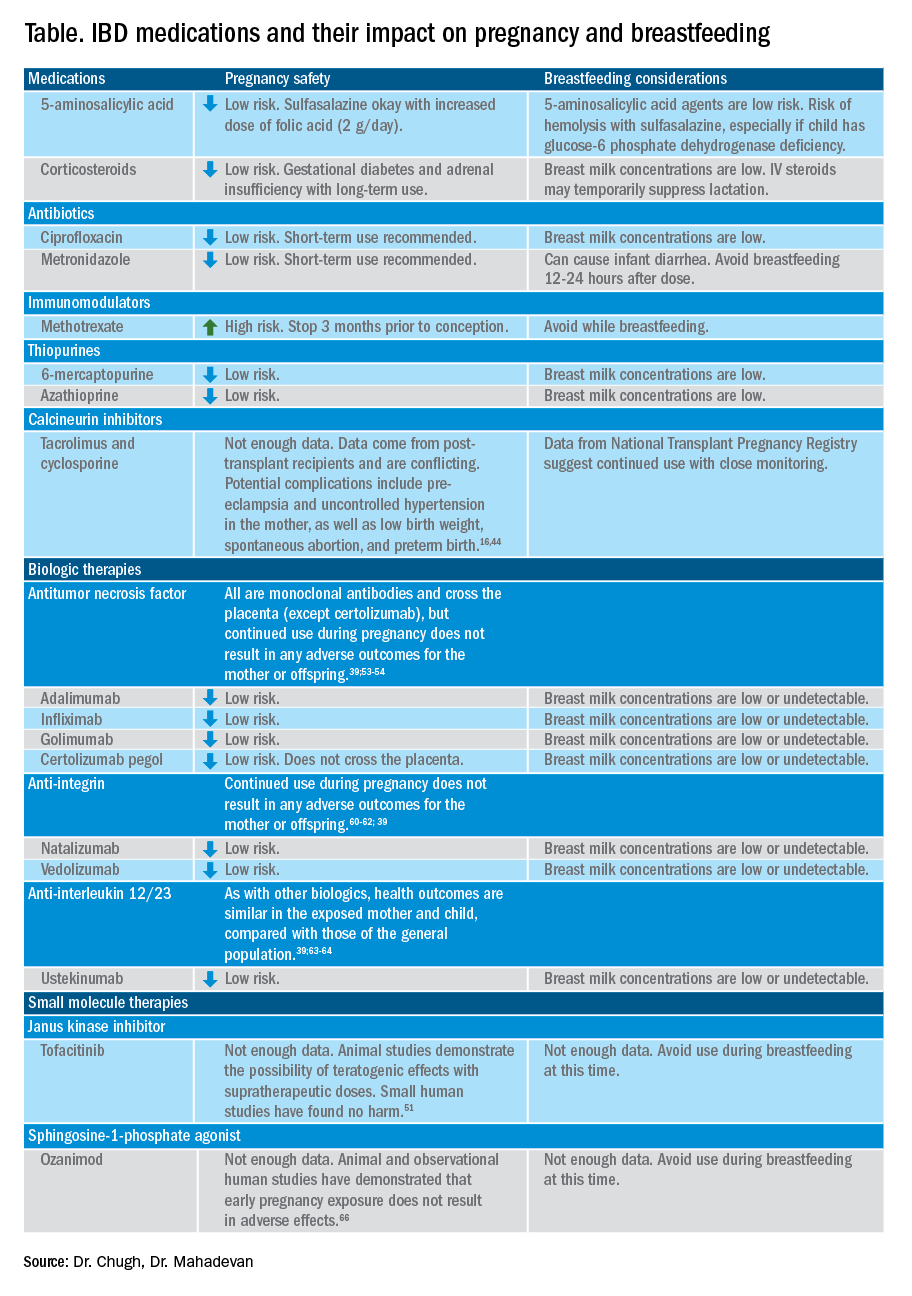
Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34
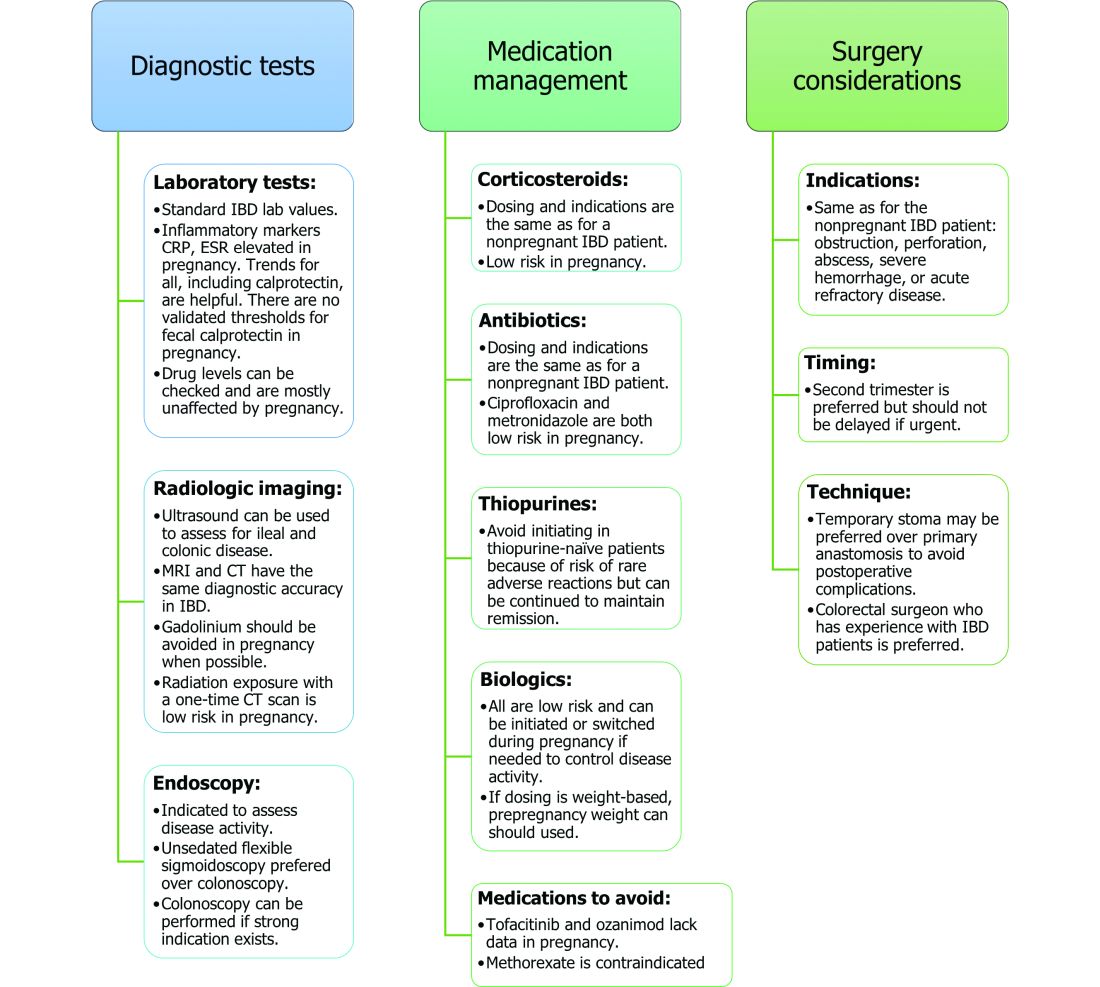
An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
Inflammatory bowel disease (IBD) incidence is rising globally.1-3 In the United States, we have seen a 123% increase in prevalence of IBD among adults and a 133% increase among children from 2007 to 2016, with an annual percentage change of 9.9%.1 The rise of IBD in young people, and the overall higher prevalence in women compared with men, make pregnancy and IBD a topic of increasing importance for gastroenterologists.1 Here, we will discuss management and expectations in women with IBD before conception, during pregnancy, and post partum.
Preconception
Disease activity
Achieving both clinical and endoscopic remission of disease prior to conception is the key to ensuring the best maternal and fetal outcomes. Patients with IBD who conceive while in remission remain in remission 80% of the time.4,5 On the other hand, those who conceive while their disease is active may continue to have active or worsening disease in nearly 70% of cases.4 Active disease has been associated with an increased incidence of preterm birth, low birth weight, and small-for-gestational-age birth.6-8 Active disease can also exacerbate malnutrition and result in poor maternal weight gain, which is associated with intrauterine growth restriction.9,7 Pregnancy outcomes in patients with IBD and quiescent disease are similar to those in the general population.10,11
Health care maintenance
Optimizing maternal health prior to conception is critical. Alcohol, tobacco, recreational drugs, and marijuana should all be avoided. Opioids should be tapered off prior to conception, as continued use may result in neonatal opioid withdrawal syndrome and long-term neurodevelopmental consequences.12,13 In addition, aiming for a healthy body mass index between 18 and 25 months prior to conception allows for better overall pregnancy outcomes.13 Appropriate cancer screening includes colon cancer screening in those with more than 8 years of colitis, regular pap smear for cervical cancer, and annual total body skin cancer examinations for patients on thiopurines and biologic therapies.14
Nutrition
Folic acid supplementation with at least 400 micrograms (mcg) daily is necessary for all women planning pregnancy. Patients with small bowel involvement or history of small bowel resection should have a folate intake of a minimum of 2 grams per day. Adequate vitamin D levels (at least 20 ng/mL) are recommended in all women with IBD. Those with malabsorption should be screened for deficiencies in vitamin B12, folate, and iron.13 These nutritional markers should be evaluated prepregnancy, during the first trimester, and thereafter as needed.15-18
Preconception counseling
Steroid-free remission for at least 3 months prior to conception is recommended and is associated with reduced risk of flare during pregnancy.16,19 IBD medications needed to control disease activity are generally safe preconception and during pregnancy, with some exception (Table).

Misconceptions regarding heritability of IBD have sometimes discouraged men and women from having children. While genetics may increase susceptibility, environmental and other factors are involved as well. The concordance rates for monozygotic twins range from 33.3%-58.3% for Crohn’s disease and 13.4%-27.9% for ulcerative colitis (UC).20 The risk of a child developing IBD is higher in those who have multiple relatives with IBD and whose parents had IBD at the time of conception.21 While genetic testing for IBD loci is available, it is not commonly performed at this time as many genes are involved.22
Pregnancy
Coordinated care
A complete team of specialists with coordinated care among all providers is needed for optimal maternal and fetal outcomes.23,24 A gastroenterologist, ideally an IBD specialist, should follow the patient throughout pregnancy, seeing the patient at least once during the first or second trimester and as needed during pregnancy.16 A high-risk obstetrician or maternal-fetal medicine specialist should be involved early in pregnancy, as well. Open communication among all disciplines ensures that a common message is conveyed to the patient.16,24 A nutritionist, mental health provider, and lactation specialist knowledgeable about IBD drugs may be of assistance, as well.16
Disease activity
While women with IBD are at increased risk of spontaneous abortion, preterm birth, and labor complications, this risk is mitigated by controlling disease activity.25 The risk of preterm birth, small-for-gestational-age birth, and delivery via C-section is much higher in women with moderate-to-high disease activity, compared with those with low disease activity.26 The presence of active perianal disease mandates C-section over vaginal delivery. Fourth-degree lacerations following vaginal delivery are most common among those patients with perianal disease.26,27 Stillbirths were shown to be increased only in those with active IBD when compared with non-IBD comparators and inactive IBD.28-31;11
Noninvasive methods for disease monitoring are preferred in pregnancy, but serum markers such as erythrocyte sedimentation rate and C-reactive protein may not be reliable in the pregnant patient (Figure).32 Fecal calprotectin does rise in correlation with disease activity, but exact thresholds have not been validated in pregnancy.33,34

An unsedated, unprepped flexible sigmoidoscopy can be safely performed throughout pregnancy.35 When there is a strong indication, a complete colonoscopy can be performed in the pregnant patient as well.36 Current American Society for Gastrointestinal Endoscopy (ASGE) guidelines suggest placing the patient in the left lateral tilt position to avoid decreased maternal and placental perfusion via compression of the aorta or inferior vena cava and performing endoscopy during the second trimester, although trimester-specific timing is not always feasible by indication.37
Medication use and safety
IBD medications are a priority topic of concern among pregnant patients or those considering conception.38 Comprehensive data from the PIANO (Pregnancy in Inflammatory Bowel Disease and Neonatal Outcomes) registry has shown that most IBD drugs do not result in adverse pregnancy outcomes and should be continued.39 The use of biologics and thiopurines, either in combination or alone, is not related to an increased risk of congenital malformations, spontaneous abortion, preterm birth, low birth weight, or infections during the child’s first year of life.7,39 Developmental milestones also remain unaffected.39 Here, we will discuss safety considerations during pregnancy (see Table).
5-aminosalycylic acid. 5-aminosalicylic acid (5-ASA) agents are generally low risk during pregnancy and should be continued.40-41 Sulfasalazine does interfere with folate metabolism, but by increasing folic acid supplementation to 2 grams per day, sulfasalazine can be continued throughout pregnancy, as well.42
Corticosteroids. Intrapartum corticosteroid use is associated with an increased risk of gestational diabetes and adrenal insufficiency when used long term.43-45 Short-term use may, however, be necessary to control an acute flare. The lowest dose for the shortest duration possible is recommended. Because of its high first-pass metabolism, budesonide is considered low risk in pregnancy.
Methotrexate. Methotrexate needs to be stopped at least 3 months prior to conception and should be avoided throughout pregnancy. Use during pregnancy can result in spontaneous abortions, as well as embryotoxicity.46
Thiopurines (6-mercaptopurine and azathioprine). Patients who are taking thiopurines prior to conception to maintain remission can continue to do so. Data on thiopurines from the PIANO registry has shown no increase in spontaneous abortions, congenital malformations, low birth weight, preterm birth, rates of infection in the child, or developmental delays.47-51
Calcineurin inhibitors (cyclosporine and tacrolimus). Calcineurin inhibitors are reserved for the management of acute severe UC. Safety data on calcineurin inhibitors is conflicting, and there is not enough information at this time to identify risk during pregnancy. Cyclosporine can be used for salvage therapy if absolutely needed, and there are case reports of its successful using during pregnancy.16,52
Biologic therapies. With the exception of certolizumab, all of the currently used biologics are actively transported across the placenta.39,53,54 Intrapartum use of biologic therapies does not worsen pregnancy or neonatal outcomes, including the risk for intensive care unit admission, infections, and developmental milestones.39,47
While drug concentrations may vary slightly during pregnancy, these changes are not substantial enough to warrant more frequent monitoring or dose adjustments, and prepregnancy weight should be used for dosing.55,56
Antitumor necrosis factor agents used in IBD include infliximab, adalimumab, certolizumab, and golimumab.57 All are low risk for pregnant patients and their offspring. Dosage timings can be adjusted, but not stopped, to minimize exposure to the child; however, it cannot be adjusted for certolizumab pegol because of its lack of placental transfer.58-59
Natalizumab and vedolizumab are integrin receptor antagonists and are also low risk in pregnancy.57;60-62;39
Ustekinumab, an interleukin-12/23 antagonist, can be found in infant serum and cord blood, as well. Health outcomes are similar in the exposed mother and child, however, compared with those of the general population.39;63-64
Small molecule drugs. Unlike monoclonal antibodies, which do not cross the placenta in large amounts until early in the second trimester, small molecules can cross in the first trimester during the critical period of organogenesis.
The two small molecule agents currently approved for use in UC are tofacitinib, a janus kinase inhibitor, and ozanimod, a sphingosine-1-phosphate receptor agonist.65-66 Further data are still needed to make recommendations on the use of tofacitinib and ozanimod in pregnancy. At this time, we recommend weighing the risks (unknown risk to human pregnancy) vs. benefits (controlled disease activity with clear risk of harm to mother and baby from flare) in the individual patient before counseling on use in pregnancy.
Delivery
Mode of delivery
The mode of delivery should be determined by the obstetrician. C-section is recommended for patients with active perianal disease or, in some cases, a history of ileal pouch anal anastomosis (IPAA).67-68 Vaginal delivery in the setting of perianal disease has been shown to increase the risk of fourth-degree laceration and anal sphincter dysfunction in the future.26-27 Anorectal motility may be impacted by IPAA construction and vaginal delivery independently of each other. It is therefore suggested that vaginal delivery be avoided in patients with a history of IPAA to avoid compounding the risk. Some studies do not show clear harm from vaginal delivery in the setting of IPAA, however, and informed decision making among all stakeholders should be had.27;69-70
Anticoagulation
The incidence of venous thromboembolism (VTE) is elevated in patients with IBD during pregnancy, and up to 12 weeks postpartum, compared with pregnant patients without IBD.71-72 VTE for prophylaxis is indicated in the pregnant patient while hospitalized and potentially thereafter depending on the patient’s risk factors, which may include obesity, prior personal history of VTE, heart failure, and prolonged immobility. Unfractionated heparin, low molecular weight heparin, and warfarin are safe for breastfeeding women.16,73
Postpartum care of mother
There is a risk of postpartum flare, occurring in about one third of patients in the first 6 months postpartum.74-75 De-escalating therapy during delivery or immediately postpartum is a predictor of a postpartum flare.75 If no infection is present and the timing interval is appropriate, biologic therapies should be continued and can be resumed 24 hours after a vaginal delivery and 48 hours after a C-section.16,76
NSAIDs and opioids can be used for pain relief but should be avoided in the long-term to prevent flares (NSAIDs) and infant sedation (associated with opioids) when used while breastfeeding.77 The LactMed database is an excellent resource for clarification on risk of medication use while breastfeeding.78
In particular, contraception should be addressed postpartum. Exogenous estrogen use increases the risk of VTE, which is already increased in IBD; nonestrogen containing, long-acting reversible contraception is preferred.79-80 Progestin-only implants or intrauterine devices may be used first line. The efficacy of oral contraceptives is theoretically reduced in those with rapid bowel transit, active small bowel inflammation, and prior small bowel resection, so adding another form of contraception is recommended.16,81
Source: American Gastroenterological Association
Postdelivery care of baby
Breastfeeding
Guidelines regarding medication use during breastfeeding are similar to those in pregnancy (see Table). Breastfeeding on biologics and thiopurines can continue without interruption in the child. Thiopurine concentrations in breast milk are low or undetectable.82,78 TNF receptor antagonists, anti-integrin therapies, and ustekinumab are found in low to undetectable levels in breast milk, as well.78
On the other hand, the active metabolite of methotrexate is detectable in breast milk and most sources recommend not breastfeeding on methotrexate. At doses used in IBD (15-25 milligrams per week), some experts have suggested avoiding breastfeeding for 24 hours following a dose.57,78 It is the practice of this author to recommend not breastfeeding at all on methotrexate.
5-ASA therapies are low risk for breastfeeding, but alternatives to sulfasalazine are preferred. The sulfapyridine metabolite transfers to breast milk and may cause hemolysis in infants born with a glucose-6-phosphate dehydrogenase deficiency.78
With regards to calcineurin inhibitors, tacrolimus appears in breast milk in low quantities, while cyclosporine levels are variable. Data from the National Transplantation Pregnancy Registry suggest that these medications can be used at the time of breastfeeding with close monitoring.78
There is not enough data on small molecule therapies at this time to support breastfeeding safety, and it is our practice to not recommend breastfeeding in this scenario.
The transfer of steroids to the child via breast milk does occur but at subtherapeutic levels.16 Budesonide has high first pass metabolism and is low risk during breastfeeding.83-84 As far as is known, IBD maintenance medications do not suppress lactation. The use of intravenous corticosteroids can, however, temporarily decrease milk production.16,85
Vaccines
Vaccination of infants can proceed as indicated by the Center for Disease Control and Prevention guidelines, with one exception. If the child’s mother was exposed to any biologic agents (not including certolizumab) during the third trimester, any live vaccines should be withheld in the first 6 months of life. In the United States, this restriction currently only applies to the rotavirus vaccine, which is administered starting at the age of 2 months.16,86 Notably, inadvertent administration of the rotavirus vaccine in the biologic-exposed child does not appear to result in any adverse effects.87 Immunity is achieved even if the child is exposed to IBD therapies through breast milk.88
Developmental milestones
Infant exposure to biologics and thiopurines has not been shown to result in any developmental delays. The PIANO study measured developmental milestones at 48 months from birth and found no differences when compared with validated population norms.39 A separate study observing childhood development up to 7 years of age in patients born to mothers with IBD found similar cognitive scores and motor development when compared with those born to mothers without IBD.89
Conclusion
Women considering conception should be optimized prior to pregnancy and maintained on appropriate medications throughout pregnancy and lactation to achieve a healthy pregnancy for both mother and baby. To date, biologics and thiopurines are not associated with adverse pregnancy outcomes. More data are needed for small molecules.
Dr. Chugh is an advanced inflammatory bowel disease fellow in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan is professor of medicine and codirector at the Center for Colitis and Crohn’s Disease in the division of gastroenterology at the University of California San Francisco. Dr. Mahadevan has potential conflicts related to AbbVie, Janssen, BMS, Takeda, Pfizer, Lilly, Gilead, Arena, and Prometheus Biosciences.
References
1. Ye Y et al. Inflamm Bowel Dis. 2020;26:619-25.
2. Sykora J et al. World J Gastroenterol. 2018;24:2741-63.
3. Murakami Y et al. J Gastroenterol 2019;54:1070-7.
4. Hashash JG and Kane S. Gastroenterol Hepatol. (N Y) 2015;11:96-102.
5. Miller JP. J R Soc Med. 1986;79:221-5.
6. Cornish J et al. Gut. 2007;56:830-7.
7. Leung KK et al. Inflamm Bowel Dis. 2021;27:550-62.
8. O’Toole A et al. Dig Dis Sci. 2015;60:2750-61.
9. Nguyen GC et al. Inflamm Bowel Dis. 2008;14:1105-11.
10. Lee HH et al. Aliment Pharmacol Ther. 2020;51:861-9.
11. Kim MA et al. J Crohns Colitis. 2021;15:719-32.
12. Conradt E et al. Pediatrics. 2019;144.
13. ACOG Committee Opinion No. 762: Prepregnancy Counseling. Obstet Gynecol. 2019;133:e78-e89.
14. Farraye FA et al. Am J Gastroenterol. 2017;112:241-58.
15. Lee S et al. J Crohns Colitis. 2018;12:702-9.
16. Mahadevan U et al. Inflamm Bowel Dis. 2019;25:627-41.
17. Ward MG et al. Inflamm. Bowel Dis 2015;21:2839-47.
18. Battat R et al. Inflamm Bowel Dis. 2014;20:1120-8.
19. Pedersen N et al. Aliment Pharmacol Ther. 2013;38:501-12.
20. Annese V. Pharmacol Res. 2020;159:104892.
21. Bennett RA et al. Gastroenterology. 1991;100:1638-43.
22. Turpin W et al. Inflamm Bowel Dis. 2018;24:1133-48.
23. de Lima A et al. Clin Gastroenterol Hepatol. 2016;14:1285-92 e1.
24. Selinger C et al. Frontline Gastroenterol. 2021;12:182-7.
25. Mahadevan U et al. Gastroenterology. 2007;133:1106-12.
26. Hatch Q et al. Dis Colon Rectum. 2014;57:174-8.
27. Foulon A et al. Inflamm Bowel Dis. 2017;23:712-20.
28. Norgard B et al. Am J Gastroenterol. 2007;102:1947-54.
29. Broms G et al. Scand J Gastroenterol 2016;51:1462-9.
30. Meyer A et al. Aliment Pharmacol Ther. 2020;52:1480-90.
31. Kammerlander H et al. Inflamm Bowel Dis. 2017;23:1011-8.
32. Tandon P et al. J Clin Gastroenterol. 2019;53:574-81.
33. Kammerlander H et al. Inflamm Bowel Dis. 2018;24:839-48.
34. Julsgaard M et al. Inflamm Bowel Dis. 2017;23:1240-6.
35. Ko MS et al. Dig Dis Sci. 2020;65:2979-85.
36. Cappell MS et al. J Reprod Med. 2010;55:115-23.
37. Committee ASoP et al. Gastrointest Endosc. 2012;76:18-24.
38. Aboubakr A et al. Dig Dis Sci. 2021;66:1829-35.
39. Mahadevan U et al. Gastroenterology. 2021;160:1131-9.
40. Diav-Citrin O et al. Gastroenterology. 1998;114:23-8.
41. Rahimi R et al. Reprod Toxicol. 2008;25:271-5.
42. Norgard B et al. Aliment Pharmacol Ther. 2001;15:483-6.
43. Leung YP et al. J Crohns Colitis. 2015;9:223-30.
44. Schulze H et al. Aliment Pharmacol Ther. 2014;40:991-1008.
45. Szymanska E et al. J Gynecol Obstet Hum Reprod. 2021;50:101777.
46. Weber-Schoendorfer C et al. Arthritis Rheumatol. 2014;66:1101-10.
47. Nielsen OH et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):74-87.e3.
48. Coelho J et al. Gut. 2011;60:198-203.
49. Sheikh M et al. J Crohns Colitis. 2015;9:680-4.
50. Kanis SL et al. Clin Gastroenterol Hepatol. 2017;15:1232-41 e1.
51. Mahadevan U et al. Inflamm Bowel Dis. 2018;24:2494-500.
52. Rosen MH et al. Inflamm Bowel Dis. 2020;26:971-3.
53. Porter C et al. J Reprod Immunol. 2016;116:7-12.
54. Mahadevan U et al. Clin Gastroenterol Hepatol. 2013;11:286-92; quiz e24.
55. Picardo S and Seow CH. Best Pract Res Clin Gastroenterol. 2020;44-5:101670.
56. Flanagan E et al. Aliment Pharmacol Ther. 2020;52:1551-62.
57. Singh S et al. Gastroenterology. 2021;160:2512-56 e9.
58. de Lima A et al. Gut. 2016;65:1261-8.
59. Julsgaard M et al. Inflamm Bowel Dis. 2020;26:93-102.
60. Wils P et al. Aliment Pharmacol Ther. 2021;53:460-70.
61. Mahadevan U et al. Aliment Pharmacol Ther. 2017;45:941-50.
62. Bar-Gil Shitrit A et al. Am J Gastroenterol. 2019;114:1172-5.
63. Klenske E et al. J Crohns Colitis. 2019;13:267-9.
64. Matro R et al. Gastroenterology. 2018;155:696-704.
65. Feuerstein JD et al. Gastroenterology. 2020;158:1450-61.
66. Sandborn WJ et al. J Crohns Colitis. 2021 Jul 5;15(7):1120-1129.
67. Lamb CA et al. Gut. 2019;68:s1-s106.
68. Nguyen GC et al. Gastroenterology. 2016;150:734-57 e1.
69. Ravid A et al. Dis Colon Rectum. 2002;45:1283-8.
70. Seligman NS et al. J Matern Fetal Neonatal Med. 2011;24:525-30.
71. Kim YH et al. Medicine (Baltimore). 2019;98:e17309.
72. Hansen AT et al. J Thromb Haemost. 2017;15:702-8.
73. Bates SM et al. J Thromb Thrombolysis. 2016;41:92-128.
74. Bennett A et al. Inflamm Bowel Dis. 2021 May 17;izab104.
75. Yu A et al. Inflamm Bowel Dis. 2020;26:1926-32.
76. Mahadevan U et al. Gastroenterology. 2017;152:451-62 e2.
77. Long MD et al. J Clin Gastroenterol. 2016;50:152-6.
78. Drugs and Lactation Database (LactMed). 2006 ed. Bethesda, MD: National Library of Medicine (US), 2006-2021.
79. Khalili H et al. Gut. 2013;62:1153-9.
80. Long MD and Hutfless S. Gastroenterology. 2016;150:1518-20.
81. Centers for Disease Control and Prevention. U S. Medical Eligibility Criteria for Contraceptive Use, 2010. MMWR Recomm Rep. 2010;59:1-86.
82. Angelberger S et al. J Crohns Colitis. 2011;5:95-100.
83. Vestergaard T et al. Scand J Gastroenterol. 2018;53:1459-62.
84. Beaulieu DB et al. Inflamm Bowel Dis. 2009;15:25-8.
85. Anderson PO. Breastfeed Med. 2017;12:199-201.
86. Wodi AP et al. MMWR Morb Mortal Wkly Rep. 2021;70:189-92.
87. Chiarella-Redfern H et al. Inflamm Bowel Dis. 2022 Jan 5;28(1):79-86.
88. Beaulieu DB et al. Clin Gastroenterol Hepatol. 2018;16:99-105.
89. Friedman S et al. J Crohns Colitis. 2020 Dec 2;14(12):1709-1716.
The power of physician advocacy
February is National Cancer Prevention Month. With approximately 4.8 million new cases and 3.4 million deaths worldwide annually, GI cancers represent roughly a quarter of the global cancer incidence and over a third of all cancer-related deaths, according to one study.
which remains a central focus of our clinical and endoscopic practice as gastroenterologists. This includes important studies that demonstrate the value of upper endoscopy in reducing GI cancer mortality, illustrate the potential promise of artificial intelligence in improving early detection of gastric cancer, and link adenoma detection rate to long-term survival in patients who undergo CRC screening with flexible sigmoidoscopy. We also report on a focused update from the U.S. Multi-Society Task Force on colorectal cancer, which thoughtfully reviews the data supporting a shift in the age of initiation of average-risk CRC screening from 50 to 45 years.
On the policy front, AGA and its partners have worked tirelessly for many years to eliminate financial barriers to colorectal cancer (CRC) screening through national advocacy efforts. These efforts resulted in closure of the so-called Medicare “colonoscopy loophole” through legislation included in the COVID-19 relief bill – as a result, out-of-pocket costs for patients undergoing a screening colonoscopy that results in polypectomy are disallowed as of January 2022. The Biden Administration recently issued guidance in January in response to multisociety advocacy efforts: Private insurers must provide coverage without cost sharing for a follow-up colonoscopy after a positive stool-based CRC screening test for plan or policy years starting on or after May 31, 2022. Removing these financial barriers to care is particularly critical to efforts to improve CRC screening rates among medically underserved communities.
These achievements highlight the power of physician advocacy in inspiring policy changes that directly improve the health and well-being of our patients. I encourage you to visit the AGA website to learn how you can contribute to ongoing advocacy efforts.
Megan A. Adams, MD, JD, MSc
Editor in Chief
February is National Cancer Prevention Month. With approximately 4.8 million new cases and 3.4 million deaths worldwide annually, GI cancers represent roughly a quarter of the global cancer incidence and over a third of all cancer-related deaths, according to one study.
which remains a central focus of our clinical and endoscopic practice as gastroenterologists. This includes important studies that demonstrate the value of upper endoscopy in reducing GI cancer mortality, illustrate the potential promise of artificial intelligence in improving early detection of gastric cancer, and link adenoma detection rate to long-term survival in patients who undergo CRC screening with flexible sigmoidoscopy. We also report on a focused update from the U.S. Multi-Society Task Force on colorectal cancer, which thoughtfully reviews the data supporting a shift in the age of initiation of average-risk CRC screening from 50 to 45 years.
On the policy front, AGA and its partners have worked tirelessly for many years to eliminate financial barriers to colorectal cancer (CRC) screening through national advocacy efforts. These efforts resulted in closure of the so-called Medicare “colonoscopy loophole” through legislation included in the COVID-19 relief bill – as a result, out-of-pocket costs for patients undergoing a screening colonoscopy that results in polypectomy are disallowed as of January 2022. The Biden Administration recently issued guidance in January in response to multisociety advocacy efforts: Private insurers must provide coverage without cost sharing for a follow-up colonoscopy after a positive stool-based CRC screening test for plan or policy years starting on or after May 31, 2022. Removing these financial barriers to care is particularly critical to efforts to improve CRC screening rates among medically underserved communities.
These achievements highlight the power of physician advocacy in inspiring policy changes that directly improve the health and well-being of our patients. I encourage you to visit the AGA website to learn how you can contribute to ongoing advocacy efforts.
Megan A. Adams, MD, JD, MSc
Editor in Chief
February is National Cancer Prevention Month. With approximately 4.8 million new cases and 3.4 million deaths worldwide annually, GI cancers represent roughly a quarter of the global cancer incidence and over a third of all cancer-related deaths, according to one study.
which remains a central focus of our clinical and endoscopic practice as gastroenterologists. This includes important studies that demonstrate the value of upper endoscopy in reducing GI cancer mortality, illustrate the potential promise of artificial intelligence in improving early detection of gastric cancer, and link adenoma detection rate to long-term survival in patients who undergo CRC screening with flexible sigmoidoscopy. We also report on a focused update from the U.S. Multi-Society Task Force on colorectal cancer, which thoughtfully reviews the data supporting a shift in the age of initiation of average-risk CRC screening from 50 to 45 years.
On the policy front, AGA and its partners have worked tirelessly for many years to eliminate financial barriers to colorectal cancer (CRC) screening through national advocacy efforts. These efforts resulted in closure of the so-called Medicare “colonoscopy loophole” through legislation included in the COVID-19 relief bill – as a result, out-of-pocket costs for patients undergoing a screening colonoscopy that results in polypectomy are disallowed as of January 2022. The Biden Administration recently issued guidance in January in response to multisociety advocacy efforts: Private insurers must provide coverage without cost sharing for a follow-up colonoscopy after a positive stool-based CRC screening test for plan or policy years starting on or after May 31, 2022. Removing these financial barriers to care is particularly critical to efforts to improve CRC screening rates among medically underserved communities.
These achievements highlight the power of physician advocacy in inspiring policy changes that directly improve the health and well-being of our patients. I encourage you to visit the AGA website to learn how you can contribute to ongoing advocacy efforts.
Megan A. Adams, MD, JD, MSc
Editor in Chief
When the wrong history repeats itself
Fifteen years ago, Mrs. Smith was hospitalized for a dural sinus thrombosis.
This is a scary enough diagnosis as it is, but with the miracle of modern medicine she did great. She still checks in with me every year or so, but hasn’t had any recurrence.
Three years ago she tripped over her dog (amazing how often that seems to happen) and broke her arm. She landed in the hospital and needed orthopedic surgery, so they consulted me about the safety of getting her off the antiplatelet agent she was taking since stopping Coumadin.
When I arrived someone had already written an admitting note, which included a past history of “subdural hematoma, maintained on daily aspirin.”
Where this error came from, I don’t know. When I asked Mrs. Smith, she was quite clear on her correct diagnosis, and said she’d given it to the person who admitted her. So I dictated a consult, and typed it into my progress note each day. My notes made it clear that she’d had a dural sinus thrombosis and not a subdural hematoma.
This isn’t just nitpicking, obviously. They’re entirely different disorders. While the point may not be critical to her needing wrist surgery, these are medical records, and for this, and future hospital stays and for physicians to be aware of.
I signed off after a few days and didn’t think much of it until I was faxed a copy of her discharge summary. Which listed “subdural hematoma, maintained on daily aspirin.”
Apparently no one read my notes. Not that I’m really surprised.
We’re now 3 years later. As do many patients of her age, Mrs. Smith has landed in the hospital a few times since then. COVID, syncope, another fall. In each one of them the “subdural hematoma, maintained on daily aspirin” shows up.
At the most recent incident, the hospital’s neurologist called and asked me why I was treating a subdural hematoma with aspirin, then said Mrs. Smith had told him it was a dural sinus thrombosis. I said she was right, and he said “that makes more sense” and that he’d put it in his note.
He did, but it didn’t change anything. The discharge summary still listed “subdural hematoma, maintained on daily aspirin.”
At some point resistance is futile.
The stupidity of the whole thing is frustrating, as is knowing that it’s not just Mrs. Smith. The same scenario of incorrect history and medications is propagated from visit to visit. Taking a history is too time consuming for some. It’s easier to just read off, or cut and paste, a previous note. In cases where the patient can’t give a history I understand this. But when they can it’s just being too rushed – or lazy – to care.
It’s easy to blame EMRs as the culprits. Bashing them is fashionable. But in this case I can’t. They make it easier, but it’s nothing new. I remember a night almost 30 years ago when I was doing an admission at the Phoenix VA. When I picked up the most recent volume of the patient’s old chart to look at labs, the previous H&P said “see old chart.”
The problem is human nature. Not the computer.
But in this field the fallout can be serious – the wrong precautions taken, or medication given, based on a nonexistent contraindication. In medicine the stakes are high. Our decisions are only as good as the information we base them on, and if that information is wrong ...
Shortcuts have consequences.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Fifteen years ago, Mrs. Smith was hospitalized for a dural sinus thrombosis.
This is a scary enough diagnosis as it is, but with the miracle of modern medicine she did great. She still checks in with me every year or so, but hasn’t had any recurrence.
Three years ago she tripped over her dog (amazing how often that seems to happen) and broke her arm. She landed in the hospital and needed orthopedic surgery, so they consulted me about the safety of getting her off the antiplatelet agent she was taking since stopping Coumadin.
When I arrived someone had already written an admitting note, which included a past history of “subdural hematoma, maintained on daily aspirin.”
Where this error came from, I don’t know. When I asked Mrs. Smith, she was quite clear on her correct diagnosis, and said she’d given it to the person who admitted her. So I dictated a consult, and typed it into my progress note each day. My notes made it clear that she’d had a dural sinus thrombosis and not a subdural hematoma.
This isn’t just nitpicking, obviously. They’re entirely different disorders. While the point may not be critical to her needing wrist surgery, these are medical records, and for this, and future hospital stays and for physicians to be aware of.
I signed off after a few days and didn’t think much of it until I was faxed a copy of her discharge summary. Which listed “subdural hematoma, maintained on daily aspirin.”
Apparently no one read my notes. Not that I’m really surprised.
We’re now 3 years later. As do many patients of her age, Mrs. Smith has landed in the hospital a few times since then. COVID, syncope, another fall. In each one of them the “subdural hematoma, maintained on daily aspirin” shows up.
At the most recent incident, the hospital’s neurologist called and asked me why I was treating a subdural hematoma with aspirin, then said Mrs. Smith had told him it was a dural sinus thrombosis. I said she was right, and he said “that makes more sense” and that he’d put it in his note.
He did, but it didn’t change anything. The discharge summary still listed “subdural hematoma, maintained on daily aspirin.”
At some point resistance is futile.
The stupidity of the whole thing is frustrating, as is knowing that it’s not just Mrs. Smith. The same scenario of incorrect history and medications is propagated from visit to visit. Taking a history is too time consuming for some. It’s easier to just read off, or cut and paste, a previous note. In cases where the patient can’t give a history I understand this. But when they can it’s just being too rushed – or lazy – to care.
It’s easy to blame EMRs as the culprits. Bashing them is fashionable. But in this case I can’t. They make it easier, but it’s nothing new. I remember a night almost 30 years ago when I was doing an admission at the Phoenix VA. When I picked up the most recent volume of the patient’s old chart to look at labs, the previous H&P said “see old chart.”
The problem is human nature. Not the computer.
But in this field the fallout can be serious – the wrong precautions taken, or medication given, based on a nonexistent contraindication. In medicine the stakes are high. Our decisions are only as good as the information we base them on, and if that information is wrong ...
Shortcuts have consequences.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Fifteen years ago, Mrs. Smith was hospitalized for a dural sinus thrombosis.
This is a scary enough diagnosis as it is, but with the miracle of modern medicine she did great. She still checks in with me every year or so, but hasn’t had any recurrence.
Three years ago she tripped over her dog (amazing how often that seems to happen) and broke her arm. She landed in the hospital and needed orthopedic surgery, so they consulted me about the safety of getting her off the antiplatelet agent she was taking since stopping Coumadin.
When I arrived someone had already written an admitting note, which included a past history of “subdural hematoma, maintained on daily aspirin.”
Where this error came from, I don’t know. When I asked Mrs. Smith, she was quite clear on her correct diagnosis, and said she’d given it to the person who admitted her. So I dictated a consult, and typed it into my progress note each day. My notes made it clear that she’d had a dural sinus thrombosis and not a subdural hematoma.
This isn’t just nitpicking, obviously. They’re entirely different disorders. While the point may not be critical to her needing wrist surgery, these are medical records, and for this, and future hospital stays and for physicians to be aware of.
I signed off after a few days and didn’t think much of it until I was faxed a copy of her discharge summary. Which listed “subdural hematoma, maintained on daily aspirin.”
Apparently no one read my notes. Not that I’m really surprised.
We’re now 3 years later. As do many patients of her age, Mrs. Smith has landed in the hospital a few times since then. COVID, syncope, another fall. In each one of them the “subdural hematoma, maintained on daily aspirin” shows up.
At the most recent incident, the hospital’s neurologist called and asked me why I was treating a subdural hematoma with aspirin, then said Mrs. Smith had told him it was a dural sinus thrombosis. I said she was right, and he said “that makes more sense” and that he’d put it in his note.
He did, but it didn’t change anything. The discharge summary still listed “subdural hematoma, maintained on daily aspirin.”
At some point resistance is futile.
The stupidity of the whole thing is frustrating, as is knowing that it’s not just Mrs. Smith. The same scenario of incorrect history and medications is propagated from visit to visit. Taking a history is too time consuming for some. It’s easier to just read off, or cut and paste, a previous note. In cases where the patient can’t give a history I understand this. But when they can it’s just being too rushed – or lazy – to care.
It’s easy to blame EMRs as the culprits. Bashing them is fashionable. But in this case I can’t. They make it easier, but it’s nothing new. I remember a night almost 30 years ago when I was doing an admission at the Phoenix VA. When I picked up the most recent volume of the patient’s old chart to look at labs, the previous H&P said “see old chart.”
The problem is human nature. Not the computer.
But in this field the fallout can be serious – the wrong precautions taken, or medication given, based on a nonexistent contraindication. In medicine the stakes are high. Our decisions are only as good as the information we base them on, and if that information is wrong ...
Shortcuts have consequences.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Will I really feel better if I eat fermented foods?
I’m in a crowded commercial kitchen, and everywhere I look I see bottles of colorful drinks and jars holding faded vegetables suspended in brine. The smell of fermented cabbage permeates the room. I open a mason jar, which lets out a loud hiss. I’d spent months researching the gut-brain axis during my PhD, hoping to understand the role that fermented food may play in our mental health. So I enrolled in a class on how to make fermented foods.
The teacher is praising these ancient foods as a magical cure for every ailment you can imagine. I’m uncomfortable – not because of the smell, but because I’ve never found a scientific article that definitively supported this idea. I’m subconsciously applying a fact filter and wondering what the other unsuspecting students must think. I let this slide, since I’m here to learn the art of fermentation. I bravely take a spoonful of sauerkraut. The salty brine overwhelms my senses. Gulp!
If you’ve ever eaten sauerkraut, kimchi, tempeh, kombucha, or kefir, then you’ve had a fermented food (or drink). The first time I gave them a proper go (with a mind open to enjoying them), I noticed the sour, vinegar-like taste and the noticeable absence of sugar. It didn’t take me long to get used to the taste. After a while of drinking my bubbly kombucha, I noticed that my palate had adapted and sweet flavors felt overpowering.
Fermentation is a natural process of curdling or culturing that has been used for thousands of years to preserve foods. Fermented foods and drinks are made through “desired microbial growth and enzymatic conversions of food components” (as opposed to undesirable microbial growth, which happens when your food spoils). Fermented foods are made either by the bacteria and yeast already present in the environment/food material or by introducing bacteria or yeast to help start the fermentation process.
For example, when I made sauerkraut, I shredded the cabbage, added salt, then pummeled and squeezed the cabbage until it released its own juices, which also allowed the “probiotic” lactic acid bacteria in the cabbage to kickstart the fermentation process. Probiotic bacteria like Lactobacillus and Bifidobacterium are considered probiotic good bugs, and are also present in many yogurts and cheeses.
We can’t necessarily call our sauerkraut a “probiotic food” because we don’t know the exact probiotic strains that are in our sauerkraut and whether they are present in the correct “probiotic” dose. It’s also worth noting that foods and drinks that are produced by fermentation don’t necessarily need to have live bacteria in them when you eat them to still be considered a fermented food. For example, sourdough is born from a bubbly live starter culture that contains yeast and bacteria, but once cooked it might no longer have any live bacteria in it.
So, what about the health claims?
Microbial fermentation may interact with health through multiple different biological pathways. It can enhance the nutritional composition of the final food, create bioactive compounds, and change the composition of the gut microbiota (potentially outcompeting harmful pathogens). The lactic acid bacteria in fermented food might also help to influence your immune system and strengthen your intestinal barrier. Some fermented foods, like tempeh, also contain prebiotics; these are fibers that escape your digestion and are broken down by your gut bacteria, including your lactic acid bacteria, which feed off prebiotic fiber to help grow their colonies. In a recent diet experiment, a high-fiber diet was compared with a diet high in fermented foods (eg, yogurt, fermented vegetables, kefir, fermented cheese); those who ate higher fermented food had lower markers of inflammation and an increased diversity of gut microbiota (which is thought to be a good thing in adults). So, in theory, fermented foods sound good.
Still wanting to understand more, and dispel a few myths, a team of researchers and I investigated what’s known about the link between fermented foods and mental health. We looked at the pathways by which fermented foods might affect mental health, such as by reducing inflammation and strengthening the intestinal barrier. These pathways are relevant because they might reduce your brain’s exposure to certain inflammatory molecules that can impact brain function and mental health.
Fermented foods also contain neurotransmitters that are important to mental health. Research about fermented food and mental health is still in its early infancy. Animal studies provide experimental evidence that fermented foods can help with symptoms of depression and anxiety – but that’s in animals. The problem is in knowing how the animal findings relate to our human experience.
We found eight studies in humans that experimented with fermented foods (for example, fermented milk products) to measure their impact on depression, anxiety, and stress in adults, but the studies were all so different that we were unable to make firm conclusions. It is still difficult to know what the active ingredient in fermented foods is. Is it the microbes? Is it the byproducts? Is it the nutrition? And how much of each is needed, and what are safe levels of each? We really need more studies, with detailed descriptions of exactly what is in each food being tested. At this stage, there is not enough human evidence to make firm clinical recommendations for eating fermented food to improve mental health symptoms.
I’ve since moved on from sauerkraut to making sourdough bread as a COVID lockdown project (as this involves a fermented starter culture). When my delicious fresh bread comes out of the oven, my world is paused for a few minutes, and my family mill around to enjoy the warm, fresh bread. While it may be too soon to tell whether fermented foods help our mental health, my sourdough itself has sure helped us.
Dr. Dawson is a nutritionist and bioinformatician research fellow at the Food & Mood Centre at Deakin University, Geelong, Australia. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I’m in a crowded commercial kitchen, and everywhere I look I see bottles of colorful drinks and jars holding faded vegetables suspended in brine. The smell of fermented cabbage permeates the room. I open a mason jar, which lets out a loud hiss. I’d spent months researching the gut-brain axis during my PhD, hoping to understand the role that fermented food may play in our mental health. So I enrolled in a class on how to make fermented foods.
The teacher is praising these ancient foods as a magical cure for every ailment you can imagine. I’m uncomfortable – not because of the smell, but because I’ve never found a scientific article that definitively supported this idea. I’m subconsciously applying a fact filter and wondering what the other unsuspecting students must think. I let this slide, since I’m here to learn the art of fermentation. I bravely take a spoonful of sauerkraut. The salty brine overwhelms my senses. Gulp!
If you’ve ever eaten sauerkraut, kimchi, tempeh, kombucha, or kefir, then you’ve had a fermented food (or drink). The first time I gave them a proper go (with a mind open to enjoying them), I noticed the sour, vinegar-like taste and the noticeable absence of sugar. It didn’t take me long to get used to the taste. After a while of drinking my bubbly kombucha, I noticed that my palate had adapted and sweet flavors felt overpowering.
Fermentation is a natural process of curdling or culturing that has been used for thousands of years to preserve foods. Fermented foods and drinks are made through “desired microbial growth and enzymatic conversions of food components” (as opposed to undesirable microbial growth, which happens when your food spoils). Fermented foods are made either by the bacteria and yeast already present in the environment/food material or by introducing bacteria or yeast to help start the fermentation process.
For example, when I made sauerkraut, I shredded the cabbage, added salt, then pummeled and squeezed the cabbage until it released its own juices, which also allowed the “probiotic” lactic acid bacteria in the cabbage to kickstart the fermentation process. Probiotic bacteria like Lactobacillus and Bifidobacterium are considered probiotic good bugs, and are also present in many yogurts and cheeses.
We can’t necessarily call our sauerkraut a “probiotic food” because we don’t know the exact probiotic strains that are in our sauerkraut and whether they are present in the correct “probiotic” dose. It’s also worth noting that foods and drinks that are produced by fermentation don’t necessarily need to have live bacteria in them when you eat them to still be considered a fermented food. For example, sourdough is born from a bubbly live starter culture that contains yeast and bacteria, but once cooked it might no longer have any live bacteria in it.
So, what about the health claims?
Microbial fermentation may interact with health through multiple different biological pathways. It can enhance the nutritional composition of the final food, create bioactive compounds, and change the composition of the gut microbiota (potentially outcompeting harmful pathogens). The lactic acid bacteria in fermented food might also help to influence your immune system and strengthen your intestinal barrier. Some fermented foods, like tempeh, also contain prebiotics; these are fibers that escape your digestion and are broken down by your gut bacteria, including your lactic acid bacteria, which feed off prebiotic fiber to help grow their colonies. In a recent diet experiment, a high-fiber diet was compared with a diet high in fermented foods (eg, yogurt, fermented vegetables, kefir, fermented cheese); those who ate higher fermented food had lower markers of inflammation and an increased diversity of gut microbiota (which is thought to be a good thing in adults). So, in theory, fermented foods sound good.
Still wanting to understand more, and dispel a few myths, a team of researchers and I investigated what’s known about the link between fermented foods and mental health. We looked at the pathways by which fermented foods might affect mental health, such as by reducing inflammation and strengthening the intestinal barrier. These pathways are relevant because they might reduce your brain’s exposure to certain inflammatory molecules that can impact brain function and mental health.
Fermented foods also contain neurotransmitters that are important to mental health. Research about fermented food and mental health is still in its early infancy. Animal studies provide experimental evidence that fermented foods can help with symptoms of depression and anxiety – but that’s in animals. The problem is in knowing how the animal findings relate to our human experience.
We found eight studies in humans that experimented with fermented foods (for example, fermented milk products) to measure their impact on depression, anxiety, and stress in adults, but the studies were all so different that we were unable to make firm conclusions. It is still difficult to know what the active ingredient in fermented foods is. Is it the microbes? Is it the byproducts? Is it the nutrition? And how much of each is needed, and what are safe levels of each? We really need more studies, with detailed descriptions of exactly what is in each food being tested. At this stage, there is not enough human evidence to make firm clinical recommendations for eating fermented food to improve mental health symptoms.
I’ve since moved on from sauerkraut to making sourdough bread as a COVID lockdown project (as this involves a fermented starter culture). When my delicious fresh bread comes out of the oven, my world is paused for a few minutes, and my family mill around to enjoy the warm, fresh bread. While it may be too soon to tell whether fermented foods help our mental health, my sourdough itself has sure helped us.
Dr. Dawson is a nutritionist and bioinformatician research fellow at the Food & Mood Centre at Deakin University, Geelong, Australia. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
I’m in a crowded commercial kitchen, and everywhere I look I see bottles of colorful drinks and jars holding faded vegetables suspended in brine. The smell of fermented cabbage permeates the room. I open a mason jar, which lets out a loud hiss. I’d spent months researching the gut-brain axis during my PhD, hoping to understand the role that fermented food may play in our mental health. So I enrolled in a class on how to make fermented foods.
The teacher is praising these ancient foods as a magical cure for every ailment you can imagine. I’m uncomfortable – not because of the smell, but because I’ve never found a scientific article that definitively supported this idea. I’m subconsciously applying a fact filter and wondering what the other unsuspecting students must think. I let this slide, since I’m here to learn the art of fermentation. I bravely take a spoonful of sauerkraut. The salty brine overwhelms my senses. Gulp!
If you’ve ever eaten sauerkraut, kimchi, tempeh, kombucha, or kefir, then you’ve had a fermented food (or drink). The first time I gave them a proper go (with a mind open to enjoying them), I noticed the sour, vinegar-like taste and the noticeable absence of sugar. It didn’t take me long to get used to the taste. After a while of drinking my bubbly kombucha, I noticed that my palate had adapted and sweet flavors felt overpowering.
Fermentation is a natural process of curdling or culturing that has been used for thousands of years to preserve foods. Fermented foods and drinks are made through “desired microbial growth and enzymatic conversions of food components” (as opposed to undesirable microbial growth, which happens when your food spoils). Fermented foods are made either by the bacteria and yeast already present in the environment/food material or by introducing bacteria or yeast to help start the fermentation process.
For example, when I made sauerkraut, I shredded the cabbage, added salt, then pummeled and squeezed the cabbage until it released its own juices, which also allowed the “probiotic” lactic acid bacteria in the cabbage to kickstart the fermentation process. Probiotic bacteria like Lactobacillus and Bifidobacterium are considered probiotic good bugs, and are also present in many yogurts and cheeses.
We can’t necessarily call our sauerkraut a “probiotic food” because we don’t know the exact probiotic strains that are in our sauerkraut and whether they are present in the correct “probiotic” dose. It’s also worth noting that foods and drinks that are produced by fermentation don’t necessarily need to have live bacteria in them when you eat them to still be considered a fermented food. For example, sourdough is born from a bubbly live starter culture that contains yeast and bacteria, but once cooked it might no longer have any live bacteria in it.
So, what about the health claims?
Microbial fermentation may interact with health through multiple different biological pathways. It can enhance the nutritional composition of the final food, create bioactive compounds, and change the composition of the gut microbiota (potentially outcompeting harmful pathogens). The lactic acid bacteria in fermented food might also help to influence your immune system and strengthen your intestinal barrier. Some fermented foods, like tempeh, also contain prebiotics; these are fibers that escape your digestion and are broken down by your gut bacteria, including your lactic acid bacteria, which feed off prebiotic fiber to help grow their colonies. In a recent diet experiment, a high-fiber diet was compared with a diet high in fermented foods (eg, yogurt, fermented vegetables, kefir, fermented cheese); those who ate higher fermented food had lower markers of inflammation and an increased diversity of gut microbiota (which is thought to be a good thing in adults). So, in theory, fermented foods sound good.
Still wanting to understand more, and dispel a few myths, a team of researchers and I investigated what’s known about the link between fermented foods and mental health. We looked at the pathways by which fermented foods might affect mental health, such as by reducing inflammation and strengthening the intestinal barrier. These pathways are relevant because they might reduce your brain’s exposure to certain inflammatory molecules that can impact brain function and mental health.
Fermented foods also contain neurotransmitters that are important to mental health. Research about fermented food and mental health is still in its early infancy. Animal studies provide experimental evidence that fermented foods can help with symptoms of depression and anxiety – but that’s in animals. The problem is in knowing how the animal findings relate to our human experience.
We found eight studies in humans that experimented with fermented foods (for example, fermented milk products) to measure their impact on depression, anxiety, and stress in adults, but the studies were all so different that we were unable to make firm conclusions. It is still difficult to know what the active ingredient in fermented foods is. Is it the microbes? Is it the byproducts? Is it the nutrition? And how much of each is needed, and what are safe levels of each? We really need more studies, with detailed descriptions of exactly what is in each food being tested. At this stage, there is not enough human evidence to make firm clinical recommendations for eating fermented food to improve mental health symptoms.
I’ve since moved on from sauerkraut to making sourdough bread as a COVID lockdown project (as this involves a fermented starter culture). When my delicious fresh bread comes out of the oven, my world is paused for a few minutes, and my family mill around to enjoy the warm, fresh bread. While it may be too soon to tell whether fermented foods help our mental health, my sourdough itself has sure helped us.
Dr. Dawson is a nutritionist and bioinformatician research fellow at the Food & Mood Centre at Deakin University, Geelong, Australia. She disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The must-read acute care medicine articles from 2021
When 2021 began, there appeared to be light at the end of the long and dark COVID-19 pandemic. A vaccine was introduced, the “curve” had been flattened, and by spring, businesses were slowly starting to open. Whereas the medical literature of 2020 seemed to be almost entirely focused on COVID-19, medical writers, researchers, and educators seemed to slowly start turning more attention back to non–COVID-related topics in 2021.
Unfortunately, as I write this, the Omicron variant of the coronavirus is in full swing, and much of our attention has once again turned back to COVID-19. However, we are able to look back on 2021 and acknowledge a wealth of fantastic original research articles and guidelines which have improved patient care in many ways.
Specifically, I have chosen articles that did not appear to gain widespread notoriety in emergency medicine but are, nevertheless, worthy of your time and attention. Note that this write-up serves as a summary only, and I encourage interested readers to peruse the full manuscripts for further details. I am limiting my recap to two articles.
Recommendations on difficult airway management
Emergency physicians are well trained in airway management, and a major part of that training includes the preintubation anatomic assessment of the airway. However, there are few recommendations on the physiological considerations for airway management.
A set of recommendations from the Society for Airway Management was written primarily with anesthesiologists in mind, but many of the recommendations listed below are very relevant to emergency physicians as well. The authors make recommendations for patients who are hypoxic or hypotensive prior to induction, for patients with right ventricular dysfunction, for patients with severe metabolic acidosis, and for neurologically injured patients. Some of the key pearls follow.
Patients with hypoxemia
- The importance of preoxygenation before intubation is once again emphasized, and this can be performed using high-flow oxygen for at least 3 minutes, or (in a cooperative patient) with eight vital capacity breaths.
- Maintenance of oxygenation during the apneic period should be continued. Apneic oxygenation can be provided with a nasal cannula at 15 liters per minute or with a high-flow nasal oxygen system at 40-70 LPM.
- For patients with significant shunt physiology or reduced functional residual capacity (for example, late pregnancy, obesity, or acute respiratory distress syndrome), preoxygenation should be performed with positive end expiratory pressure (PEEP) using noninvasive positive pressure ventilation or bag-valve mask ventilation with a PEEP valve. When higher levels of PEEP are required, an extraglottic device should be considered during preoxygenation.
- For patients with refractory hypoxemia, awake intubation to maintain spontaneous respirations should be considered.
- Patients should be preoxygenated in the upright position when possible.
- Ramped-up position (head elevated so as to bring the external auditory canal in the same horizontal line as the sternal notch) should be performed when possible in order to improve the grade of view, improve oxygenation, and reduce aspiration.
Patients with hypotension
- Patients should be screened for high risk for hemodynamic collapse prior to administration of induction medications and intubation by assessing the stroke index. A stroke index greater than 0.7 predicts a high risk. These patients should receive hemodynamic optimization (for example, intravenous fluids, administration of vasopressors) whenever possible, prior to administration of induction medications and intubation.
- Vasopressor infusions are preferable to bolus-dosed vasopressors. However, if vasopressor infusions are not possible, bolus-dosed vasopressors should be available and used to maintain systemic pressure during and after the intubation until an infusion can be started. When bolus-dosed vasopressors are used, diluted epinephrine should be considered as the vasopressor of choice in patients with depressed myocardial function.
Patients with right ventricular (RV) dysfunction
- Patients should be screened for significant RV dysfunction prior to intubation because of their high risk for hemodynamic decompensation with positive pressure ventilation.
- RV dysfunction may sometimes worsen with fluid administration. Fluid-intolerant patients may instead need RV afterload reduction with inhaled or intravenous pulmonary vasodilators.
- Patients with RV failure–induced shock should be considered for preintubation extracorporeal membrane oxygenation if available.
- Patients with RV volume overload should receive diuresis prior to intubation.
- Ventilator settings should aim to avoid hypercapnia, maintain low airway pressures, and use a higher PEEP to avoid atelectasis.
Patients with severe metabolic acidosis
- Patients with severe metabolic acidosis are at high risk for decompensation after intubation because of volume depletion and inadequate alveolar ventilation, resulting in profound acidosis.
- Patients with high minute ventilation prior to intubation should be considered for awake intubation to maintain spontaneous respirations. Otherwise, consider a spontaneous breathing mode after intubation with a high minute ventilation (that is, use a higher-than-normal respiratory rate on the ventilator in order to reproduce the preintubation minute ventilation). Apnea time should be minimized in order to minimize worsening acidosis.
- Preintubation bicarbonate boluses to prevent worsening acidosis are controversial and lack data showing any benefit.
Neurologically injured patients
- Eucapnia and normoxia should be maintained before, during, and after intubation to maintain stable cerebral blood flow.
- Hemodynamically neutral induction agents should be used.
- Patients should be positioned with the head of bed elevated to 30° upright when possible.
- Limit PEEP post intubation in order to promote venous drainage.
Evidence update for the treatment of anaphylaxis
The treatment of anaphylaxis is considered bread and butter in emergency and acute care medicine, but a great deal of what we have learned over the years is not well supported by the literature. In an article published in Resuscitation, the Anaphylaxis Working Group of the Resuscitation Council of the United Kingdom performed an evidence review regarding the emergency treatment of anaphylaxis.
A summary of key points includes:
- Anaphylaxis is defined as a systemic hypersensitivity reaction, usually rapid in onset, with potentially life-threatening compromise in airway, breathing, and/or circulation.
- The most important treatment is epinephrine (EPI), with an initial recommended dose in adults of 0.5 mg administered via the intramuscular (IM) route. Up to 10% of patients have a suboptimal response to one dose, but 98% will respond by the third dose; therefore, these authors recommend repeating the IM EPI every 5 minutes, if needed, up to three doses. There is no evidence to support any alternative or additional vasopressors, and so they should only be used if EPI is ineffective. Intravenous EPI is not recommended initially except in the perioperative setting where close monitoring can be performed. If intravenous EPI is used, the authors recommend an intravenous infusion rather than bolus dosing.
- Intravenous fluid bolus dosing is recommended in the majority of cases of anaphylaxis, regardless of presence or absence of hemodynamic compromise, because of the profound reduction in venous tone and third-spacing that typically occurs.
- Antihistamines are not recommended in early treatment. They are only effective for reversing skin manifestations of anaphylaxis (which EPI treats as well), and the sedation they produce can confound the proper ongoing evaluation of the patient. Furthermore, the use of antihistamines early in the treatment of anaphylaxis has been found to produce delays in proper use of EPI.
- Steroids are not recommended in early treatment. They help only with the late phase of inflammatory response, but despite that, there is no good evidence that they decrease the biphasic response of anaphylaxis. There is some emerging evidence that the use of steroids may actually be associated with increased morbidity even after correcting for anaphylaxis severity. The authors recommended the use of steroids in anaphylaxis only for patients with poorly controlled asthma and possibly for patients with refractory anaphylaxis. Inhaled beta-agonists are recommended in anaphylaxis only for patients with lower respiratory tract symptoms caused by anaphylaxis, but warned that the inhaled beta-agonists should not delay proper use of EPI.
- The optimal observation period before discharge for stable patients is unknown. The authors noted the recommendations of the Joint Task Force on Practice Parameters of the American Academy of Allergy, Asthma, & Immunology and the American College of Allergy, Asthma, and Immunology: Biphasic reactions were more common in patients with severe initial symptoms – for example, those requiring more than one dose of EPI; therefore, these patients are recommended to have “extended observation.” Lower-risk patients with resolved symptoms of anaphylaxis can be observed for 1 hour, which would capture 95% of biphasic reactions in this group of patients.
Summary and other honorable mentions
There you have it. My two favorite practice-changing (non–COVID-19) articles of 2021. Not surprisingly, both articles deal largely with airway and hemodynamic concerns – the ABC’s of emergency medicine. Although these bulleted pearls provide key points from these two articles, the full discussions of those key points in the articles would provide a great deal more education than I can provide in this brief write-up, and so I strongly encourage everyone to read the full articles.
I also encourage readers to peruse the following “honorable mention” articles: Stiell and colleagues published a “Best Practices Checklist” on behalf of the Canadian Association of Emergency Physicians pertaining to the management of acute atrial fibrillation and atrial flutter; and on behalf of the American Heart Association (in collaboration with several other major organizations), Gulati and colleagues published the 2021 Guideline for the Evaluation and Diagnosis of Chest Pain. Both publications show us how we should strive to manage atrial fibrillation and chest pain, respectively, in the emergency department for years to come.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore.
A version of this article first appeared on Medscape.com.
When 2021 began, there appeared to be light at the end of the long and dark COVID-19 pandemic. A vaccine was introduced, the “curve” had been flattened, and by spring, businesses were slowly starting to open. Whereas the medical literature of 2020 seemed to be almost entirely focused on COVID-19, medical writers, researchers, and educators seemed to slowly start turning more attention back to non–COVID-related topics in 2021.
Unfortunately, as I write this, the Omicron variant of the coronavirus is in full swing, and much of our attention has once again turned back to COVID-19. However, we are able to look back on 2021 and acknowledge a wealth of fantastic original research articles and guidelines which have improved patient care in many ways.
Specifically, I have chosen articles that did not appear to gain widespread notoriety in emergency medicine but are, nevertheless, worthy of your time and attention. Note that this write-up serves as a summary only, and I encourage interested readers to peruse the full manuscripts for further details. I am limiting my recap to two articles.
Recommendations on difficult airway management
Emergency physicians are well trained in airway management, and a major part of that training includes the preintubation anatomic assessment of the airway. However, there are few recommendations on the physiological considerations for airway management.
A set of recommendations from the Society for Airway Management was written primarily with anesthesiologists in mind, but many of the recommendations listed below are very relevant to emergency physicians as well. The authors make recommendations for patients who are hypoxic or hypotensive prior to induction, for patients with right ventricular dysfunction, for patients with severe metabolic acidosis, and for neurologically injured patients. Some of the key pearls follow.
Patients with hypoxemia
- The importance of preoxygenation before intubation is once again emphasized, and this can be performed using high-flow oxygen for at least 3 minutes, or (in a cooperative patient) with eight vital capacity breaths.
- Maintenance of oxygenation during the apneic period should be continued. Apneic oxygenation can be provided with a nasal cannula at 15 liters per minute or with a high-flow nasal oxygen system at 40-70 LPM.
- For patients with significant shunt physiology or reduced functional residual capacity (for example, late pregnancy, obesity, or acute respiratory distress syndrome), preoxygenation should be performed with positive end expiratory pressure (PEEP) using noninvasive positive pressure ventilation or bag-valve mask ventilation with a PEEP valve. When higher levels of PEEP are required, an extraglottic device should be considered during preoxygenation.
- For patients with refractory hypoxemia, awake intubation to maintain spontaneous respirations should be considered.
- Patients should be preoxygenated in the upright position when possible.
- Ramped-up position (head elevated so as to bring the external auditory canal in the same horizontal line as the sternal notch) should be performed when possible in order to improve the grade of view, improve oxygenation, and reduce aspiration.
Patients with hypotension
- Patients should be screened for high risk for hemodynamic collapse prior to administration of induction medications and intubation by assessing the stroke index. A stroke index greater than 0.7 predicts a high risk. These patients should receive hemodynamic optimization (for example, intravenous fluids, administration of vasopressors) whenever possible, prior to administration of induction medications and intubation.
- Vasopressor infusions are preferable to bolus-dosed vasopressors. However, if vasopressor infusions are not possible, bolus-dosed vasopressors should be available and used to maintain systemic pressure during and after the intubation until an infusion can be started. When bolus-dosed vasopressors are used, diluted epinephrine should be considered as the vasopressor of choice in patients with depressed myocardial function.
Patients with right ventricular (RV) dysfunction
- Patients should be screened for significant RV dysfunction prior to intubation because of their high risk for hemodynamic decompensation with positive pressure ventilation.
- RV dysfunction may sometimes worsen with fluid administration. Fluid-intolerant patients may instead need RV afterload reduction with inhaled or intravenous pulmonary vasodilators.
- Patients with RV failure–induced shock should be considered for preintubation extracorporeal membrane oxygenation if available.
- Patients with RV volume overload should receive diuresis prior to intubation.
- Ventilator settings should aim to avoid hypercapnia, maintain low airway pressures, and use a higher PEEP to avoid atelectasis.
Patients with severe metabolic acidosis
- Patients with severe metabolic acidosis are at high risk for decompensation after intubation because of volume depletion and inadequate alveolar ventilation, resulting in profound acidosis.
- Patients with high minute ventilation prior to intubation should be considered for awake intubation to maintain spontaneous respirations. Otherwise, consider a spontaneous breathing mode after intubation with a high minute ventilation (that is, use a higher-than-normal respiratory rate on the ventilator in order to reproduce the preintubation minute ventilation). Apnea time should be minimized in order to minimize worsening acidosis.
- Preintubation bicarbonate boluses to prevent worsening acidosis are controversial and lack data showing any benefit.
Neurologically injured patients
- Eucapnia and normoxia should be maintained before, during, and after intubation to maintain stable cerebral blood flow.
- Hemodynamically neutral induction agents should be used.
- Patients should be positioned with the head of bed elevated to 30° upright when possible.
- Limit PEEP post intubation in order to promote venous drainage.
Evidence update for the treatment of anaphylaxis
The treatment of anaphylaxis is considered bread and butter in emergency and acute care medicine, but a great deal of what we have learned over the years is not well supported by the literature. In an article published in Resuscitation, the Anaphylaxis Working Group of the Resuscitation Council of the United Kingdom performed an evidence review regarding the emergency treatment of anaphylaxis.
A summary of key points includes:
- Anaphylaxis is defined as a systemic hypersensitivity reaction, usually rapid in onset, with potentially life-threatening compromise in airway, breathing, and/or circulation.
- The most important treatment is epinephrine (EPI), with an initial recommended dose in adults of 0.5 mg administered via the intramuscular (IM) route. Up to 10% of patients have a suboptimal response to one dose, but 98% will respond by the third dose; therefore, these authors recommend repeating the IM EPI every 5 minutes, if needed, up to three doses. There is no evidence to support any alternative or additional vasopressors, and so they should only be used if EPI is ineffective. Intravenous EPI is not recommended initially except in the perioperative setting where close monitoring can be performed. If intravenous EPI is used, the authors recommend an intravenous infusion rather than bolus dosing.
- Intravenous fluid bolus dosing is recommended in the majority of cases of anaphylaxis, regardless of presence or absence of hemodynamic compromise, because of the profound reduction in venous tone and third-spacing that typically occurs.
- Antihistamines are not recommended in early treatment. They are only effective for reversing skin manifestations of anaphylaxis (which EPI treats as well), and the sedation they produce can confound the proper ongoing evaluation of the patient. Furthermore, the use of antihistamines early in the treatment of anaphylaxis has been found to produce delays in proper use of EPI.
- Steroids are not recommended in early treatment. They help only with the late phase of inflammatory response, but despite that, there is no good evidence that they decrease the biphasic response of anaphylaxis. There is some emerging evidence that the use of steroids may actually be associated with increased morbidity even after correcting for anaphylaxis severity. The authors recommended the use of steroids in anaphylaxis only for patients with poorly controlled asthma and possibly for patients with refractory anaphylaxis. Inhaled beta-agonists are recommended in anaphylaxis only for patients with lower respiratory tract symptoms caused by anaphylaxis, but warned that the inhaled beta-agonists should not delay proper use of EPI.
- The optimal observation period before discharge for stable patients is unknown. The authors noted the recommendations of the Joint Task Force on Practice Parameters of the American Academy of Allergy, Asthma, & Immunology and the American College of Allergy, Asthma, and Immunology: Biphasic reactions were more common in patients with severe initial symptoms – for example, those requiring more than one dose of EPI; therefore, these patients are recommended to have “extended observation.” Lower-risk patients with resolved symptoms of anaphylaxis can be observed for 1 hour, which would capture 95% of biphasic reactions in this group of patients.
Summary and other honorable mentions
There you have it. My two favorite practice-changing (non–COVID-19) articles of 2021. Not surprisingly, both articles deal largely with airway and hemodynamic concerns – the ABC’s of emergency medicine. Although these bulleted pearls provide key points from these two articles, the full discussions of those key points in the articles would provide a great deal more education than I can provide in this brief write-up, and so I strongly encourage everyone to read the full articles.
I also encourage readers to peruse the following “honorable mention” articles: Stiell and colleagues published a “Best Practices Checklist” on behalf of the Canadian Association of Emergency Physicians pertaining to the management of acute atrial fibrillation and atrial flutter; and on behalf of the American Heart Association (in collaboration with several other major organizations), Gulati and colleagues published the 2021 Guideline for the Evaluation and Diagnosis of Chest Pain. Both publications show us how we should strive to manage atrial fibrillation and chest pain, respectively, in the emergency department for years to come.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore.
A version of this article first appeared on Medscape.com.
When 2021 began, there appeared to be light at the end of the long and dark COVID-19 pandemic. A vaccine was introduced, the “curve” had been flattened, and by spring, businesses were slowly starting to open. Whereas the medical literature of 2020 seemed to be almost entirely focused on COVID-19, medical writers, researchers, and educators seemed to slowly start turning more attention back to non–COVID-related topics in 2021.
Unfortunately, as I write this, the Omicron variant of the coronavirus is in full swing, and much of our attention has once again turned back to COVID-19. However, we are able to look back on 2021 and acknowledge a wealth of fantastic original research articles and guidelines which have improved patient care in many ways.
Specifically, I have chosen articles that did not appear to gain widespread notoriety in emergency medicine but are, nevertheless, worthy of your time and attention. Note that this write-up serves as a summary only, and I encourage interested readers to peruse the full manuscripts for further details. I am limiting my recap to two articles.
Recommendations on difficult airway management
Emergency physicians are well trained in airway management, and a major part of that training includes the preintubation anatomic assessment of the airway. However, there are few recommendations on the physiological considerations for airway management.
A set of recommendations from the Society for Airway Management was written primarily with anesthesiologists in mind, but many of the recommendations listed below are very relevant to emergency physicians as well. The authors make recommendations for patients who are hypoxic or hypotensive prior to induction, for patients with right ventricular dysfunction, for patients with severe metabolic acidosis, and for neurologically injured patients. Some of the key pearls follow.
Patients with hypoxemia
- The importance of preoxygenation before intubation is once again emphasized, and this can be performed using high-flow oxygen for at least 3 minutes, or (in a cooperative patient) with eight vital capacity breaths.
- Maintenance of oxygenation during the apneic period should be continued. Apneic oxygenation can be provided with a nasal cannula at 15 liters per minute or with a high-flow nasal oxygen system at 40-70 LPM.
- For patients with significant shunt physiology or reduced functional residual capacity (for example, late pregnancy, obesity, or acute respiratory distress syndrome), preoxygenation should be performed with positive end expiratory pressure (PEEP) using noninvasive positive pressure ventilation or bag-valve mask ventilation with a PEEP valve. When higher levels of PEEP are required, an extraglottic device should be considered during preoxygenation.
- For patients with refractory hypoxemia, awake intubation to maintain spontaneous respirations should be considered.
- Patients should be preoxygenated in the upright position when possible.
- Ramped-up position (head elevated so as to bring the external auditory canal in the same horizontal line as the sternal notch) should be performed when possible in order to improve the grade of view, improve oxygenation, and reduce aspiration.
Patients with hypotension
- Patients should be screened for high risk for hemodynamic collapse prior to administration of induction medications and intubation by assessing the stroke index. A stroke index greater than 0.7 predicts a high risk. These patients should receive hemodynamic optimization (for example, intravenous fluids, administration of vasopressors) whenever possible, prior to administration of induction medications and intubation.
- Vasopressor infusions are preferable to bolus-dosed vasopressors. However, if vasopressor infusions are not possible, bolus-dosed vasopressors should be available and used to maintain systemic pressure during and after the intubation until an infusion can be started. When bolus-dosed vasopressors are used, diluted epinephrine should be considered as the vasopressor of choice in patients with depressed myocardial function.
Patients with right ventricular (RV) dysfunction
- Patients should be screened for significant RV dysfunction prior to intubation because of their high risk for hemodynamic decompensation with positive pressure ventilation.
- RV dysfunction may sometimes worsen with fluid administration. Fluid-intolerant patients may instead need RV afterload reduction with inhaled or intravenous pulmonary vasodilators.
- Patients with RV failure–induced shock should be considered for preintubation extracorporeal membrane oxygenation if available.
- Patients with RV volume overload should receive diuresis prior to intubation.
- Ventilator settings should aim to avoid hypercapnia, maintain low airway pressures, and use a higher PEEP to avoid atelectasis.
Patients with severe metabolic acidosis
- Patients with severe metabolic acidosis are at high risk for decompensation after intubation because of volume depletion and inadequate alveolar ventilation, resulting in profound acidosis.
- Patients with high minute ventilation prior to intubation should be considered for awake intubation to maintain spontaneous respirations. Otherwise, consider a spontaneous breathing mode after intubation with a high minute ventilation (that is, use a higher-than-normal respiratory rate on the ventilator in order to reproduce the preintubation minute ventilation). Apnea time should be minimized in order to minimize worsening acidosis.
- Preintubation bicarbonate boluses to prevent worsening acidosis are controversial and lack data showing any benefit.
Neurologically injured patients
- Eucapnia and normoxia should be maintained before, during, and after intubation to maintain stable cerebral blood flow.
- Hemodynamically neutral induction agents should be used.
- Patients should be positioned with the head of bed elevated to 30° upright when possible.
- Limit PEEP post intubation in order to promote venous drainage.
Evidence update for the treatment of anaphylaxis
The treatment of anaphylaxis is considered bread and butter in emergency and acute care medicine, but a great deal of what we have learned over the years is not well supported by the literature. In an article published in Resuscitation, the Anaphylaxis Working Group of the Resuscitation Council of the United Kingdom performed an evidence review regarding the emergency treatment of anaphylaxis.
A summary of key points includes:
- Anaphylaxis is defined as a systemic hypersensitivity reaction, usually rapid in onset, with potentially life-threatening compromise in airway, breathing, and/or circulation.
- The most important treatment is epinephrine (EPI), with an initial recommended dose in adults of 0.5 mg administered via the intramuscular (IM) route. Up to 10% of patients have a suboptimal response to one dose, but 98% will respond by the third dose; therefore, these authors recommend repeating the IM EPI every 5 minutes, if needed, up to three doses. There is no evidence to support any alternative or additional vasopressors, and so they should only be used if EPI is ineffective. Intravenous EPI is not recommended initially except in the perioperative setting where close monitoring can be performed. If intravenous EPI is used, the authors recommend an intravenous infusion rather than bolus dosing.
- Intravenous fluid bolus dosing is recommended in the majority of cases of anaphylaxis, regardless of presence or absence of hemodynamic compromise, because of the profound reduction in venous tone and third-spacing that typically occurs.
- Antihistamines are not recommended in early treatment. They are only effective for reversing skin manifestations of anaphylaxis (which EPI treats as well), and the sedation they produce can confound the proper ongoing evaluation of the patient. Furthermore, the use of antihistamines early in the treatment of anaphylaxis has been found to produce delays in proper use of EPI.
- Steroids are not recommended in early treatment. They help only with the late phase of inflammatory response, but despite that, there is no good evidence that they decrease the biphasic response of anaphylaxis. There is some emerging evidence that the use of steroids may actually be associated with increased morbidity even after correcting for anaphylaxis severity. The authors recommended the use of steroids in anaphylaxis only for patients with poorly controlled asthma and possibly for patients with refractory anaphylaxis. Inhaled beta-agonists are recommended in anaphylaxis only for patients with lower respiratory tract symptoms caused by anaphylaxis, but warned that the inhaled beta-agonists should not delay proper use of EPI.
- The optimal observation period before discharge for stable patients is unknown. The authors noted the recommendations of the Joint Task Force on Practice Parameters of the American Academy of Allergy, Asthma, & Immunology and the American College of Allergy, Asthma, and Immunology: Biphasic reactions were more common in patients with severe initial symptoms – for example, those requiring more than one dose of EPI; therefore, these patients are recommended to have “extended observation.” Lower-risk patients with resolved symptoms of anaphylaxis can be observed for 1 hour, which would capture 95% of biphasic reactions in this group of patients.
Summary and other honorable mentions
There you have it. My two favorite practice-changing (non–COVID-19) articles of 2021. Not surprisingly, both articles deal largely with airway and hemodynamic concerns – the ABC’s of emergency medicine. Although these bulleted pearls provide key points from these two articles, the full discussions of those key points in the articles would provide a great deal more education than I can provide in this brief write-up, and so I strongly encourage everyone to read the full articles.
I also encourage readers to peruse the following “honorable mention” articles: Stiell and colleagues published a “Best Practices Checklist” on behalf of the Canadian Association of Emergency Physicians pertaining to the management of acute atrial fibrillation and atrial flutter; and on behalf of the American Heart Association (in collaboration with several other major organizations), Gulati and colleagues published the 2021 Guideline for the Evaluation and Diagnosis of Chest Pain. Both publications show us how we should strive to manage atrial fibrillation and chest pain, respectively, in the emergency department for years to come.
Amal Mattu, MD, is a professor, vice chair of education, and codirector of the emergency cardiology fellowship in the department of emergency medicine at the University of Maryland, Baltimore.
A version of this article first appeared on Medscape.com.











