User login
Diagnosing adolescent ADHD
Pediatricians are increasingly expert in the assessment and treatment of attention-deficit/hyperactivity disorder. But what do you do when adolescents present to your office saying they think they have ADHD? While ADHD is a common and treatable disorder of youth, it is important to take special care when assessing an adolescent. Difficulties with attention and concentration are common symptoms for many different challenges of adolescence, and for ADHD to be the underlying cause, those symptoms must have started prior to adolescence (according to DSM-5, prior to the age of 12). When your adolescent patients or their parents come to your office complaining of inattention, it is important to consider the full range of possible explanations.
Sleep
We have written in this column previously about the challenges that adolescents face in getting adequate sleep consistently. Teenagers, on average, need more than 9 hours of sleep nightly and American teenagers get fewer than 6. This mismatch is because of physiologic shifts that move their natural sleep onset time significantly later, while school still starts early. It’s often compounded by other demands on their time, including homework, extracurricular activities, and the gravitational pull of social connections. Independent teenagers make their own decisions about how to manage their time and may feel sleep is optional, or manage their fatigue with naps and caffeine, both of which will further compromise the quality and efficiency of sleep.
Chronic sleep deprivation will present with difficulties with focus, attention, memory, and cognitive performance. Treatment of this problem with stimulants is likely to make the underlying poor sleep habits even worse. When your patient presents complaining of difficulty concentrating and worsening school performance, be sure to start with a thorough sleep history, and always provide guidance about the body’s need for sleep and healthy sleep habits.
Anxiety
Anxiety disorders are the most common psychiatric illnesses of youth, with estimates of as many as 30% of children and adolescents experiencing one. The true prevalence of ADHD is estimated to be about 4% of the population. Whether social phobia, generalized anxiety disorder, or even posttraumatic stress disorder, anxiety disorders interfere with attention as ruminative worry tends to distract those experiencing it. It can also affect attention and focus indirectly by interfering with restful sleep. Anxiety disorders can be difficult to identify, as the sufferers typically internalize their symptoms. But inquire about specific worries (such as speaking in front of others, meeting new people, or an illness or accident striking themselves or a loved one) and how much time they take up. Explore if worries fill their thoughts during quiet or downtime, and explore more about their worries. You may use a screening instrument such as the Pediatric Symptom Checklist or the SCARED, both of which will indicate a likely problem with anxiety. While it is possible to have comorbid ADHD with an anxiety disorder, the anxiety disorder will likely worsen with stimulants and should be treated first. These are usually curable illnesses and you may find that remission of anxiety symptoms resolves the attentional problems.
Depression
Mood disorders are less common than anxiety disorders in youth, but far more prevalent than ADHD. And depression is usually marked by serious difficulty concentrating across settings (including for things that were previously very interesting). A sullen teenager who is deeply self-critical about school performance would benefit from exploration of associated changes in mood, interests, energy, appetite, sleep, and for feelings of worthlessness, guilt, and suicidal thoughts. The PHQ9A is a simple, free screening instrument that is reasonable to use with every sick visit (and well-check) with your adolescent patients, given the risks of undetected and untreated depression. If your patient presents complaining of poor school performance, always screen for depression. As with anxiety disorders, comorbid ADHD is possible, but it is always recommended to treat the mood disorder first and then to assess for residual ADHD symptoms once the mood disorder is in remission.
Substance abuse
Adolescence is a time of exploration, and drug and alcohol use is common. While attentional impairment will happen with intoxication, occasional or rare use should not lead to consistent impairment in school. But when parents are more worried than their children about a significant change in school performance, it is important to screen for substance abuse. A child with a secret substance use disorder will often present with behavioral changes and deteriorating school performance and might deny any drug or alcohol use to parents. Indeed, stimulants have some street value and some patients may be seeking a stimulant prescription to sell or trade for other drugs. Regular marijuana use may present with only deteriorating school performance and no irritability or other noticeable behavioral changes. Marijuana is seen as safe and even healthy by many teenagers (and even many parents), and some youth may be using it recreationally or to manage difficulties with sleep, anxiety, or mood symptoms.
But there is compelling evidence that marijuana use causes cognitive impairment, including difficulty with sustaining attention, short-term memory, and processing speed, for as long as 24 hours after use. If a teenager is using marijuana daily after school, it is certainly going to interfere, in a dose-dependent manner, with attention and cognitive function. Sustained heavy use can lead to permanent cognitive deficits. It can also trigger or worsen anxiety or mood symptoms (contrary to much popular opinion).
Gathering a thorough substance use history is essential when assessing a teenager for difficulties with focus or attention, especially when these are accompanied by change in behavior and school performance. Remember, it is critical to interview these children without their parents present to invite them to be forthcoming with you.
History
While true ADHD should have been present throughout childhood, it is possible that the symptoms have become noticeable only in adolescence. For patients with very high intelligence and lower levels of impulsivity and hyperactivity, they might easily have “flown under the radar” during their elementary and even middle school years. Their difficulties with attention and focus might become apparent only when the volume and difficulty of schoolwork both are great enough that their intelligence is not enough to get good grades. That is, their problems with executive function, prioritizing, shifting sets, and completing tasks in a timely way make it impossible to keep up good grades when the work gets harder.
Your history should reveal a long history of dreaminess or distractibility, a tendency to lose and forget things, and the other symptoms of inattention. Did they often seem to not be listening when they were younger? Forget to hand in homework? Leave chores unfinished? Leave messes behind everywhere they went? These will not be definitive, but they do reassure that symptoms may have been present for a long time, even if school performance was considered fine until the workload got too large. If such problems were not present before puberty, consider whether a subtle learning disability could be impairing them as they face more challenging academic subjects.
If you have ruled out anxiety, mood, and substance use concerns, and helped them to address a sleep deficit, then you can proceed. It is worthwhile to get Vanderbilt Assessments as you would for a younger child. If they meet criteria, discuss the risks and benefits of medication, executive skills coaching, and environmental adjustments (extra time for tests, a less stimulating environment) that can help them explore academic challenges without the discouragement that ADHD can bring.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Pediatricians are increasingly expert in the assessment and treatment of attention-deficit/hyperactivity disorder. But what do you do when adolescents present to your office saying they think they have ADHD? While ADHD is a common and treatable disorder of youth, it is important to take special care when assessing an adolescent. Difficulties with attention and concentration are common symptoms for many different challenges of adolescence, and for ADHD to be the underlying cause, those symptoms must have started prior to adolescence (according to DSM-5, prior to the age of 12). When your adolescent patients or their parents come to your office complaining of inattention, it is important to consider the full range of possible explanations.
Sleep
We have written in this column previously about the challenges that adolescents face in getting adequate sleep consistently. Teenagers, on average, need more than 9 hours of sleep nightly and American teenagers get fewer than 6. This mismatch is because of physiologic shifts that move their natural sleep onset time significantly later, while school still starts early. It’s often compounded by other demands on their time, including homework, extracurricular activities, and the gravitational pull of social connections. Independent teenagers make their own decisions about how to manage their time and may feel sleep is optional, or manage their fatigue with naps and caffeine, both of which will further compromise the quality and efficiency of sleep.
Chronic sleep deprivation will present with difficulties with focus, attention, memory, and cognitive performance. Treatment of this problem with stimulants is likely to make the underlying poor sleep habits even worse. When your patient presents complaining of difficulty concentrating and worsening school performance, be sure to start with a thorough sleep history, and always provide guidance about the body’s need for sleep and healthy sleep habits.
Anxiety
Anxiety disorders are the most common psychiatric illnesses of youth, with estimates of as many as 30% of children and adolescents experiencing one. The true prevalence of ADHD is estimated to be about 4% of the population. Whether social phobia, generalized anxiety disorder, or even posttraumatic stress disorder, anxiety disorders interfere with attention as ruminative worry tends to distract those experiencing it. It can also affect attention and focus indirectly by interfering with restful sleep. Anxiety disorders can be difficult to identify, as the sufferers typically internalize their symptoms. But inquire about specific worries (such as speaking in front of others, meeting new people, or an illness or accident striking themselves or a loved one) and how much time they take up. Explore if worries fill their thoughts during quiet or downtime, and explore more about their worries. You may use a screening instrument such as the Pediatric Symptom Checklist or the SCARED, both of which will indicate a likely problem with anxiety. While it is possible to have comorbid ADHD with an anxiety disorder, the anxiety disorder will likely worsen with stimulants and should be treated first. These are usually curable illnesses and you may find that remission of anxiety symptoms resolves the attentional problems.
Depression
Mood disorders are less common than anxiety disorders in youth, but far more prevalent than ADHD. And depression is usually marked by serious difficulty concentrating across settings (including for things that were previously very interesting). A sullen teenager who is deeply self-critical about school performance would benefit from exploration of associated changes in mood, interests, energy, appetite, sleep, and for feelings of worthlessness, guilt, and suicidal thoughts. The PHQ9A is a simple, free screening instrument that is reasonable to use with every sick visit (and well-check) with your adolescent patients, given the risks of undetected and untreated depression. If your patient presents complaining of poor school performance, always screen for depression. As with anxiety disorders, comorbid ADHD is possible, but it is always recommended to treat the mood disorder first and then to assess for residual ADHD symptoms once the mood disorder is in remission.
Substance abuse
Adolescence is a time of exploration, and drug and alcohol use is common. While attentional impairment will happen with intoxication, occasional or rare use should not lead to consistent impairment in school. But when parents are more worried than their children about a significant change in school performance, it is important to screen for substance abuse. A child with a secret substance use disorder will often present with behavioral changes and deteriorating school performance and might deny any drug or alcohol use to parents. Indeed, stimulants have some street value and some patients may be seeking a stimulant prescription to sell or trade for other drugs. Regular marijuana use may present with only deteriorating school performance and no irritability or other noticeable behavioral changes. Marijuana is seen as safe and even healthy by many teenagers (and even many parents), and some youth may be using it recreationally or to manage difficulties with sleep, anxiety, or mood symptoms.
But there is compelling evidence that marijuana use causes cognitive impairment, including difficulty with sustaining attention, short-term memory, and processing speed, for as long as 24 hours after use. If a teenager is using marijuana daily after school, it is certainly going to interfere, in a dose-dependent manner, with attention and cognitive function. Sustained heavy use can lead to permanent cognitive deficits. It can also trigger or worsen anxiety or mood symptoms (contrary to much popular opinion).
Gathering a thorough substance use history is essential when assessing a teenager for difficulties with focus or attention, especially when these are accompanied by change in behavior and school performance. Remember, it is critical to interview these children without their parents present to invite them to be forthcoming with you.
History
While true ADHD should have been present throughout childhood, it is possible that the symptoms have become noticeable only in adolescence. For patients with very high intelligence and lower levels of impulsivity and hyperactivity, they might easily have “flown under the radar” during their elementary and even middle school years. Their difficulties with attention and focus might become apparent only when the volume and difficulty of schoolwork both are great enough that their intelligence is not enough to get good grades. That is, their problems with executive function, prioritizing, shifting sets, and completing tasks in a timely way make it impossible to keep up good grades when the work gets harder.
Your history should reveal a long history of dreaminess or distractibility, a tendency to lose and forget things, and the other symptoms of inattention. Did they often seem to not be listening when they were younger? Forget to hand in homework? Leave chores unfinished? Leave messes behind everywhere they went? These will not be definitive, but they do reassure that symptoms may have been present for a long time, even if school performance was considered fine until the workload got too large. If such problems were not present before puberty, consider whether a subtle learning disability could be impairing them as they face more challenging academic subjects.
If you have ruled out anxiety, mood, and substance use concerns, and helped them to address a sleep deficit, then you can proceed. It is worthwhile to get Vanderbilt Assessments as you would for a younger child. If they meet criteria, discuss the risks and benefits of medication, executive skills coaching, and environmental adjustments (extra time for tests, a less stimulating environment) that can help them explore academic challenges without the discouragement that ADHD can bring.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Pediatricians are increasingly expert in the assessment and treatment of attention-deficit/hyperactivity disorder. But what do you do when adolescents present to your office saying they think they have ADHD? While ADHD is a common and treatable disorder of youth, it is important to take special care when assessing an adolescent. Difficulties with attention and concentration are common symptoms for many different challenges of adolescence, and for ADHD to be the underlying cause, those symptoms must have started prior to adolescence (according to DSM-5, prior to the age of 12). When your adolescent patients or their parents come to your office complaining of inattention, it is important to consider the full range of possible explanations.
Sleep
We have written in this column previously about the challenges that adolescents face in getting adequate sleep consistently. Teenagers, on average, need more than 9 hours of sleep nightly and American teenagers get fewer than 6. This mismatch is because of physiologic shifts that move their natural sleep onset time significantly later, while school still starts early. It’s often compounded by other demands on their time, including homework, extracurricular activities, and the gravitational pull of social connections. Independent teenagers make their own decisions about how to manage their time and may feel sleep is optional, or manage their fatigue with naps and caffeine, both of which will further compromise the quality and efficiency of sleep.
Chronic sleep deprivation will present with difficulties with focus, attention, memory, and cognitive performance. Treatment of this problem with stimulants is likely to make the underlying poor sleep habits even worse. When your patient presents complaining of difficulty concentrating and worsening school performance, be sure to start with a thorough sleep history, and always provide guidance about the body’s need for sleep and healthy sleep habits.
Anxiety
Anxiety disorders are the most common psychiatric illnesses of youth, with estimates of as many as 30% of children and adolescents experiencing one. The true prevalence of ADHD is estimated to be about 4% of the population. Whether social phobia, generalized anxiety disorder, or even posttraumatic stress disorder, anxiety disorders interfere with attention as ruminative worry tends to distract those experiencing it. It can also affect attention and focus indirectly by interfering with restful sleep. Anxiety disorders can be difficult to identify, as the sufferers typically internalize their symptoms. But inquire about specific worries (such as speaking in front of others, meeting new people, or an illness or accident striking themselves or a loved one) and how much time they take up. Explore if worries fill their thoughts during quiet or downtime, and explore more about their worries. You may use a screening instrument such as the Pediatric Symptom Checklist or the SCARED, both of which will indicate a likely problem with anxiety. While it is possible to have comorbid ADHD with an anxiety disorder, the anxiety disorder will likely worsen with stimulants and should be treated first. These are usually curable illnesses and you may find that remission of anxiety symptoms resolves the attentional problems.
Depression
Mood disorders are less common than anxiety disorders in youth, but far more prevalent than ADHD. And depression is usually marked by serious difficulty concentrating across settings (including for things that were previously very interesting). A sullen teenager who is deeply self-critical about school performance would benefit from exploration of associated changes in mood, interests, energy, appetite, sleep, and for feelings of worthlessness, guilt, and suicidal thoughts. The PHQ9A is a simple, free screening instrument that is reasonable to use with every sick visit (and well-check) with your adolescent patients, given the risks of undetected and untreated depression. If your patient presents complaining of poor school performance, always screen for depression. As with anxiety disorders, comorbid ADHD is possible, but it is always recommended to treat the mood disorder first and then to assess for residual ADHD symptoms once the mood disorder is in remission.
Substance abuse
Adolescence is a time of exploration, and drug and alcohol use is common. While attentional impairment will happen with intoxication, occasional or rare use should not lead to consistent impairment in school. But when parents are more worried than their children about a significant change in school performance, it is important to screen for substance abuse. A child with a secret substance use disorder will often present with behavioral changes and deteriorating school performance and might deny any drug or alcohol use to parents. Indeed, stimulants have some street value and some patients may be seeking a stimulant prescription to sell or trade for other drugs. Regular marijuana use may present with only deteriorating school performance and no irritability or other noticeable behavioral changes. Marijuana is seen as safe and even healthy by many teenagers (and even many parents), and some youth may be using it recreationally or to manage difficulties with sleep, anxiety, or mood symptoms.
But there is compelling evidence that marijuana use causes cognitive impairment, including difficulty with sustaining attention, short-term memory, and processing speed, for as long as 24 hours after use. If a teenager is using marijuana daily after school, it is certainly going to interfere, in a dose-dependent manner, with attention and cognitive function. Sustained heavy use can lead to permanent cognitive deficits. It can also trigger or worsen anxiety or mood symptoms (contrary to much popular opinion).
Gathering a thorough substance use history is essential when assessing a teenager for difficulties with focus or attention, especially when these are accompanied by change in behavior and school performance. Remember, it is critical to interview these children without their parents present to invite them to be forthcoming with you.
History
While true ADHD should have been present throughout childhood, it is possible that the symptoms have become noticeable only in adolescence. For patients with very high intelligence and lower levels of impulsivity and hyperactivity, they might easily have “flown under the radar” during their elementary and even middle school years. Their difficulties with attention and focus might become apparent only when the volume and difficulty of schoolwork both are great enough that their intelligence is not enough to get good grades. That is, their problems with executive function, prioritizing, shifting sets, and completing tasks in a timely way make it impossible to keep up good grades when the work gets harder.
Your history should reveal a long history of dreaminess or distractibility, a tendency to lose and forget things, and the other symptoms of inattention. Did they often seem to not be listening when they were younger? Forget to hand in homework? Leave chores unfinished? Leave messes behind everywhere they went? These will not be definitive, but they do reassure that symptoms may have been present for a long time, even if school performance was considered fine until the workload got too large. If such problems were not present before puberty, consider whether a subtle learning disability could be impairing them as they face more challenging academic subjects.
If you have ruled out anxiety, mood, and substance use concerns, and helped them to address a sleep deficit, then you can proceed. It is worthwhile to get Vanderbilt Assessments as you would for a younger child. If they meet criteria, discuss the risks and benefits of medication, executive skills coaching, and environmental adjustments (extra time for tests, a less stimulating environment) that can help them explore academic challenges without the discouragement that ADHD can bring.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Ways to lessen toxic effects of chemo in older adults
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
Age-related changes that potentiate adverse drug reactions include alterations in absorption, distribution, metabolism, and excretion. As such, older patients often require adjustments in medications to optimize safety and use. Medication adjustment is especially important for older patients on complex medication regimens for multiple conditions, such as those undergoing cancer treatment. Three recent high-quality randomized trials evaluated the use of geriatric assessment (GA) in older adults with cancer.1-3
Interdisciplinary GA can identify aging-related conditions associated with poor outcomes in older patients with cancer (e.g., toxic effects of chemotherapy) and provide recommendations aimed at improving health outcomes. The results of these trials suggest that interdisciplinary GA can improve care outcomes and oncologists’ communication for older adults with cancer, and should be considered an emerging standard of care.
Geriatric assessment and chemotherapy-related toxic effects
A cluster randomized trial1 at City of Hope National Medical Center conducted between August 2015 and February 2019 enrolled 613 participants and randomly assigned them to receive a GA-guided intervention or usual standard of care in a 2-to-1 ratio. Participants were eligible for the study if they were aged ≥65 years; had a diagnosis of solid malignant neoplasm of any stage; were starting a new chemotherapy regimen; and were fluent in English, Spanish, or Chinese.
The intervention included a GA at baseline followed by assessments focused on six common areas: sleep problems, problems with eating and feeding, incontinence, confusion, evidence of falls, and skin breakdown. An interdisciplinary team (oncologist, nurse practitioner, pharmacist, physical therapist, occupational therapist, social worker, and nutritionist) performed the assessment and developed a plan of care. Interventions were multifactorial and could include referral to specialists; recommendations for medication changes; symptom management; nutritional intervention with diet recommendations and supplementation; and interventions targeting social, spiritual, and functional well-being. Follow-up by a nurse practitioner continued until completion of chemotherapy or 6 months after starting chemotherapy, whichever was earlier.
The primary outcome was grade 3 or higher chemotherapy-related toxic effects using National Cancer Institute criteria, and secondary outcomes were advance directive completion, emergency room visits and unplanned hospitalizations, and survival up to 12 months. Results showed a 10% absolute reduction in the incidence of grade 3 or higher toxic effects (P = .02), with a number needed to treat of 10. Advance directive completion also increased by 15%, but no differences were observed for other outcomes. This study offers high-quality evidence that a GA-based intervention can reduce toxic effects of chemotherapy regimens for older adults with cancer.
Geriatric assessment in community oncology practices
A recent study by Supriya G. Mohile, MD, and colleagues2 is the first nationwide multicenter clinical trial to demonstrate the effects of GA and GA-guided management. This study was conducted in 40 oncology practices from the University of Rochester National Cancer Institute Community Oncology Research Program network. Centers were randomly assigned to intervention or usual care (362 patients treated by 68 oncologists in the intervention group and 371 patients treated by 91 oncologists in the usual-care group). Eligibility criteria were age ≥70 years; impairment in at least one GA domain other than polypharmacy; incurable advanced solid tumor or lymphoma with a plan to start new cancer treatment with a high risk for toxic effects within 4 weeks; and English language fluency. Both study groups underwent a baseline GA that assessed patients’ physical performance, functional status, comorbidity, cognition, nutrition, social support, polypharmacy, and psychological status. For the intervention group, a summary and management recommendations were provided to the treating oncologists.
The primary outcome was grade 3 or higher toxic effects within 3 months of starting a new regimen; secondary outcomes included treatment intensity and survival and GA outcomes within 3 months. A smaller proportion of patients in the intervention group experienced toxicity (51% vs. 71%), with an absolute risk reduction of 20%. Patients in the intervention group also had fewer falls and a greater reduction in medications used; there were no other differences in secondary outcomes. This study offers very strong and generalizable evidence that incorporating GA in the care of older adults with cancer at risk for toxicity can reduce toxicity as well as improve other outcomes, such as falls and polypharmacy.
Geriatric assessment and oncologist-patient communication
A secondary analysis3 of data from Dr. Mohile and colleagues2 evaluated the effect of GA-guided recommendations on oncologist-patient communication regarding comorbidities. Patients (n = 541) included in this analysis were 76.6 years of age on average and had 3.2 (standard deviation, 1.9) comorbid conditions. All patients underwent GA, but only oncologists in the intervention arm received GA-based recommendations. Clinical encounters between oncologist and patient immediately following the GA were audio recorded and analyzed to examine communication between oncologists and participants as it relates to chronic comorbid conditions.
In the intervention arm, more discussions regarding comorbidities took place, and more participants’ concerns about comorbidities were acknowledged. More importantly, participants in the intervention group were 2.4 times more likely to have their concerns about comorbidities addressed through referral or education, compared with the usual-care group (P = .004). Moreover, 41% of oncologists in the intervention arm modified dosage or cancer treatment schedule because of concern about tolerability or comorbidities. This study demonstrates beneficial effects of GA in increasing communication and perhaps consideration of comorbidities of older adults when planning cancer treatment.
Dr. Hung is professor of geriatrics and palliative care at Mount Sinai Hospital, New York. He disclosed no relevant conflicts of interest.
References
1. Li D et al. JAMA Oncol. 2021;7:e214158.
2. Mohile SG et al. Lancet. 2021;398:1894-1904.
3. Kleckner AS et al. JCO Oncol Pract. 2022;18:e9-19.
A version of this article first appeared on Medscape.com.
The context of our lives
Neuroscience expands our knowledge of relational and social worlds
Psychiatry may be emerging from the era of psychopharmacology and entering the era of the brain, but these reductionist, jingoistic labels do little justice to the need to acknowledge and incorporate the context of our lives into our theories and treatments. Yet psychiatrists who embrace context have much to celebrate in evolving neuroscience research.
One aptly named article – ’Families that fire together smile together’ – illustrates the fundamental connection between parent and child.1 In the functional MRIs (fMRIs) taken of these parent-child dyads (n = 76), the dyads with similar resting state connectomes also have similar day-to-day emotional states, as reflected in their diary entries. Their empathic states were identified in the multivoxel patterns in the fusiform face area of the brain.2 Another study of fMRIs and parent-child dyads (n = 93) found that the parental functional connectomes (fbc) predicted their children’s externalizing and internalizing problems. The maternal fbcs were correlated with the daughter-mother relationship, and to the daughter’s internalizing problems, suggesting a potential future focus on gendered relationships.3
The implications for psychotherapy are clear: These studies show that empathic connection between parent and child results in a better outcome for the child. Patient and psychotherapist can choose from a range of psychotherapeutic interventions that promote empathy, from providing behavioral tasks that support connection between parent and child to more in-depth family interventions. Family interventions that promote empathy include increasing the family’s understanding of the importance of empathic connection and providing a safe space to help establish empathic connection.
Studying prosocial behavior, Lukas Lengersdorff and colleagues found that fMRIs of male participants (n = 96) reflected stronger activity when they were acting on behalf of the other, rather than when acting for themselves.4 During this prosocial learning fMRI study, there was stronger engagement of the ventromedial prefrontal cortex (PFC) and higher connectivity between the ventromedial PFC and the right temporoparietal junction (rTPJ). Protecting others from harm appears to be associated with neural mechanisms that support self-relevant learning, but with the added recruitment of structures associated with the social brain. This study shows what we already know – that our brains are wired for social context. This research supports psychotherapeutic interventions aimed at creating interpersonal connection, not just at an intimate level, but also at the prosocial level, such as caring and helping others.
When social interactions are coded, the default mode network (DMN) shows increased activity. Participants (n = 11) in another study had heightened medial PFC–rTPJ connectivity, not only during rest that followed the experimental social encoding, but also during rest that followed a subsequent, nonsocial task.5 Engaging portions of the DMN during live social interactions when actively decoding the social environment, and later engaging these regions when relaxing after the social interaction, appears to facilitate social functioning. Our brains are wired to respond to context. This research underscores the positive impact of interventions such as group therapy and support groups, two underutilized modalities.
Neuroscience evaluation of our relationships provides depth to studies that fall under the medical paradigm of the gene/environment interaction. One of the most elegant in psychiatry is the Finnish study of a sample of offspring of mothers with schizophrenia who gave their children up for adoption.6 This sample of index offspring (n = 155) was compared blindly with matched controls (n = 186) of adopted/away offspring of parents without schizophrenia. The genetic effect manifested only as a psychiatric disorder in the presence of a disturbed family environment. We can now extrapolate certain possible mechanisms from the studies mentioned above: That the deficits lie in the activity or lack of activity in the DMN and associated areas, and in the generation of connectomes responsible for empathic connections.
Neuroscience expands our knowledge of our relational and social worlds, but can psychiatry make the case for inclusion of context in our conceptualization of psychiatric distress? From time to time, inroads are made, for example, the Global Assessment of Relational Functioning was incorporated into the DSM-IV-R and the Cultural Formulation Interview is in the DSM-5. However, without a sustained paradigm shift that places the gene/environment paradigm at the core of psychiatry, these efforts will rise and fall as the pioneers in these fields rise and fall.
A major barrier to moving the gene/environment paradigm more centrally in psychiatry is the prominence of individualism as an American ideal. As the neuroscience of context develops, we will be able to argue more robustly for a contextual approach to patient care.
A second barrier is the difficulty of teaching and learning about complexity. It is easy to learn how to use the DSM to make a diagnosis, to understand when and how to prescribe medications, but it is much more difficult to understand how to incorporate the complexity of life and the context within which we live, into our lexicon of psychiatric theories and treatments. As Tanya Luhrmann, PhD, points out in her study of the process of psychiatric training, residents are intimidated by the need to learn the many psychological theories and their practice; learning about medications is much simpler and takes much less time and effort.7
Nevertheless, context is embraced by several psychiatric subspecialties. Family psychiatrists recognize the power of relational dynamics in the family, and their role in shaping the individual. From understanding family communication patterns, to understanding how roles in the family get allocated, family psychiatry has well established tools for assessment and many evidence-based treatments that focus on changing relational dynamics. Social and community psychiatrists emphasize the role of race, poverty, and access, and support the assessment and treatment of the underprivileged. Cultural psychiatrists recognize that each culture has its own way of constructing identities and shaping our experiences, its own conceptualization of illness and specific idioms of distress. Cultural psychiatrists focus on sensitizing the general psychiatrist to these nuances. Child psychiatrists involve parents, and geriatric psychiatrists involve guardians. General psychiatrists understand context when, for example, understanding the role of trauma in the development of an individual, recognizing that its impact is contingent on the context within which the trauma occurs.
Neuroscience clarifies the neural pathways involved in the development of empathic and social behaviors. Our psychological theories and practice must reflect this advancement. We can teach the relevant neuroscience along with basic concepts such as child-parent relationships. We must assess an individual’s degree of fit within their family and community. Apart from asking relational questions, such as who in your world is important to you, we can use well recognized tools to help us bring context to the forefront. An easy tool is the three generational genogram, or an ecomap, which allows each individual to see where they sit in the context of their world.8 Cultural influences, societal, religious, and family influences can be drawn on the genogram, highlighting both formal and hidden family narratives. In addition, we can share how the brain works with our patients; the science of empathy and social behaviors shows us that our need for interpersonal connection is hardwired.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Lee TH et al. Families that fire together smile together: Resting state connectome similarity and daily emotional synchrony in parent-child dyads. Neuroimage. 2017 May 15;152:31-37. doi: 10.1016/j.neuroimage.2017.02.078.
2. Lee TH et al. Love flows downstream: Mothers’ and children’s neural representation similarity in perceiving distress of self and family. Soc Cogn Affect Neurosci. 2017 Dec 1;12(12):1916-27. doi: 10.1093/scan/nsx125.
3. Itahashi T et al. Functional connectomes linking child-parent relationships with psychological problems in adolescence. Neuroimage. 2020 Oct 1;219:117013. doi: 10.1016/j.neuroimage.2020.117013.
4. Lengersdorff LL et al. When implicit prosociality trumps selfishness: The neural valuation system underpins more optimal choices when learning to avoid harm to others than to oneself. J Neurosci. 2020 Sep 16;40(38):7286-99. doi: 10.1523/JNEUROSCI.0842-20.2020.
5. Meyer ML et al. Evidence that default network connectivity during rest consolidates social information. Cereb Cortex. 2019 May 1;29(5):1910-20. doi: 10.1093/cercor/bhy071.
6. Tienari P et al. The Finnish adoptive family study of schizophrenia. Implications for family research. Br J Psychiatry Suppl. 1994 Apr;(23):20-6.
7. Luhrmann, TM. Of two minds: The growing disorder in American psychiatry. New York, NY: Alfred A. Knopf, 2000.
8. Libbon R et al. Family skills for the resident toolbox: The 10-min. Genogram, Ecomap, and Prescribing Homework. Acad Psychiatry. 2019 Aug;43(4):435-439. doi: 10.1007/s40596-019-01054-6.
Neuroscience expands our knowledge of relational and social worlds
Neuroscience expands our knowledge of relational and social worlds
Psychiatry may be emerging from the era of psychopharmacology and entering the era of the brain, but these reductionist, jingoistic labels do little justice to the need to acknowledge and incorporate the context of our lives into our theories and treatments. Yet psychiatrists who embrace context have much to celebrate in evolving neuroscience research.
One aptly named article – ’Families that fire together smile together’ – illustrates the fundamental connection between parent and child.1 In the functional MRIs (fMRIs) taken of these parent-child dyads (n = 76), the dyads with similar resting state connectomes also have similar day-to-day emotional states, as reflected in their diary entries. Their empathic states were identified in the multivoxel patterns in the fusiform face area of the brain.2 Another study of fMRIs and parent-child dyads (n = 93) found that the parental functional connectomes (fbc) predicted their children’s externalizing and internalizing problems. The maternal fbcs were correlated with the daughter-mother relationship, and to the daughter’s internalizing problems, suggesting a potential future focus on gendered relationships.3
The implications for psychotherapy are clear: These studies show that empathic connection between parent and child results in a better outcome for the child. Patient and psychotherapist can choose from a range of psychotherapeutic interventions that promote empathy, from providing behavioral tasks that support connection between parent and child to more in-depth family interventions. Family interventions that promote empathy include increasing the family’s understanding of the importance of empathic connection and providing a safe space to help establish empathic connection.
Studying prosocial behavior, Lukas Lengersdorff and colleagues found that fMRIs of male participants (n = 96) reflected stronger activity when they were acting on behalf of the other, rather than when acting for themselves.4 During this prosocial learning fMRI study, there was stronger engagement of the ventromedial prefrontal cortex (PFC) and higher connectivity between the ventromedial PFC and the right temporoparietal junction (rTPJ). Protecting others from harm appears to be associated with neural mechanisms that support self-relevant learning, but with the added recruitment of structures associated with the social brain. This study shows what we already know – that our brains are wired for social context. This research supports psychotherapeutic interventions aimed at creating interpersonal connection, not just at an intimate level, but also at the prosocial level, such as caring and helping others.
When social interactions are coded, the default mode network (DMN) shows increased activity. Participants (n = 11) in another study had heightened medial PFC–rTPJ connectivity, not only during rest that followed the experimental social encoding, but also during rest that followed a subsequent, nonsocial task.5 Engaging portions of the DMN during live social interactions when actively decoding the social environment, and later engaging these regions when relaxing after the social interaction, appears to facilitate social functioning. Our brains are wired to respond to context. This research underscores the positive impact of interventions such as group therapy and support groups, two underutilized modalities.
Neuroscience evaluation of our relationships provides depth to studies that fall under the medical paradigm of the gene/environment interaction. One of the most elegant in psychiatry is the Finnish study of a sample of offspring of mothers with schizophrenia who gave their children up for adoption.6 This sample of index offspring (n = 155) was compared blindly with matched controls (n = 186) of adopted/away offspring of parents without schizophrenia. The genetic effect manifested only as a psychiatric disorder in the presence of a disturbed family environment. We can now extrapolate certain possible mechanisms from the studies mentioned above: That the deficits lie in the activity or lack of activity in the DMN and associated areas, and in the generation of connectomes responsible for empathic connections.
Neuroscience expands our knowledge of our relational and social worlds, but can psychiatry make the case for inclusion of context in our conceptualization of psychiatric distress? From time to time, inroads are made, for example, the Global Assessment of Relational Functioning was incorporated into the DSM-IV-R and the Cultural Formulation Interview is in the DSM-5. However, without a sustained paradigm shift that places the gene/environment paradigm at the core of psychiatry, these efforts will rise and fall as the pioneers in these fields rise and fall.
A major barrier to moving the gene/environment paradigm more centrally in psychiatry is the prominence of individualism as an American ideal. As the neuroscience of context develops, we will be able to argue more robustly for a contextual approach to patient care.
A second barrier is the difficulty of teaching and learning about complexity. It is easy to learn how to use the DSM to make a diagnosis, to understand when and how to prescribe medications, but it is much more difficult to understand how to incorporate the complexity of life and the context within which we live, into our lexicon of psychiatric theories and treatments. As Tanya Luhrmann, PhD, points out in her study of the process of psychiatric training, residents are intimidated by the need to learn the many psychological theories and their practice; learning about medications is much simpler and takes much less time and effort.7
Nevertheless, context is embraced by several psychiatric subspecialties. Family psychiatrists recognize the power of relational dynamics in the family, and their role in shaping the individual. From understanding family communication patterns, to understanding how roles in the family get allocated, family psychiatry has well established tools for assessment and many evidence-based treatments that focus on changing relational dynamics. Social and community psychiatrists emphasize the role of race, poverty, and access, and support the assessment and treatment of the underprivileged. Cultural psychiatrists recognize that each culture has its own way of constructing identities and shaping our experiences, its own conceptualization of illness and specific idioms of distress. Cultural psychiatrists focus on sensitizing the general psychiatrist to these nuances. Child psychiatrists involve parents, and geriatric psychiatrists involve guardians. General psychiatrists understand context when, for example, understanding the role of trauma in the development of an individual, recognizing that its impact is contingent on the context within which the trauma occurs.
Neuroscience clarifies the neural pathways involved in the development of empathic and social behaviors. Our psychological theories and practice must reflect this advancement. We can teach the relevant neuroscience along with basic concepts such as child-parent relationships. We must assess an individual’s degree of fit within their family and community. Apart from asking relational questions, such as who in your world is important to you, we can use well recognized tools to help us bring context to the forefront. An easy tool is the three generational genogram, or an ecomap, which allows each individual to see where they sit in the context of their world.8 Cultural influences, societal, religious, and family influences can be drawn on the genogram, highlighting both formal and hidden family narratives. In addition, we can share how the brain works with our patients; the science of empathy and social behaviors shows us that our need for interpersonal connection is hardwired.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Lee TH et al. Families that fire together smile together: Resting state connectome similarity and daily emotional synchrony in parent-child dyads. Neuroimage. 2017 May 15;152:31-37. doi: 10.1016/j.neuroimage.2017.02.078.
2. Lee TH et al. Love flows downstream: Mothers’ and children’s neural representation similarity in perceiving distress of self and family. Soc Cogn Affect Neurosci. 2017 Dec 1;12(12):1916-27. doi: 10.1093/scan/nsx125.
3. Itahashi T et al. Functional connectomes linking child-parent relationships with psychological problems in adolescence. Neuroimage. 2020 Oct 1;219:117013. doi: 10.1016/j.neuroimage.2020.117013.
4. Lengersdorff LL et al. When implicit prosociality trumps selfishness: The neural valuation system underpins more optimal choices when learning to avoid harm to others than to oneself. J Neurosci. 2020 Sep 16;40(38):7286-99. doi: 10.1523/JNEUROSCI.0842-20.2020.
5. Meyer ML et al. Evidence that default network connectivity during rest consolidates social information. Cereb Cortex. 2019 May 1;29(5):1910-20. doi: 10.1093/cercor/bhy071.
6. Tienari P et al. The Finnish adoptive family study of schizophrenia. Implications for family research. Br J Psychiatry Suppl. 1994 Apr;(23):20-6.
7. Luhrmann, TM. Of two minds: The growing disorder in American psychiatry. New York, NY: Alfred A. Knopf, 2000.
8. Libbon R et al. Family skills for the resident toolbox: The 10-min. Genogram, Ecomap, and Prescribing Homework. Acad Psychiatry. 2019 Aug;43(4):435-439. doi: 10.1007/s40596-019-01054-6.
Psychiatry may be emerging from the era of psychopharmacology and entering the era of the brain, but these reductionist, jingoistic labels do little justice to the need to acknowledge and incorporate the context of our lives into our theories and treatments. Yet psychiatrists who embrace context have much to celebrate in evolving neuroscience research.
One aptly named article – ’Families that fire together smile together’ – illustrates the fundamental connection between parent and child.1 In the functional MRIs (fMRIs) taken of these parent-child dyads (n = 76), the dyads with similar resting state connectomes also have similar day-to-day emotional states, as reflected in their diary entries. Their empathic states were identified in the multivoxel patterns in the fusiform face area of the brain.2 Another study of fMRIs and parent-child dyads (n = 93) found that the parental functional connectomes (fbc) predicted their children’s externalizing and internalizing problems. The maternal fbcs were correlated with the daughter-mother relationship, and to the daughter’s internalizing problems, suggesting a potential future focus on gendered relationships.3
The implications for psychotherapy are clear: These studies show that empathic connection between parent and child results in a better outcome for the child. Patient and psychotherapist can choose from a range of psychotherapeutic interventions that promote empathy, from providing behavioral tasks that support connection between parent and child to more in-depth family interventions. Family interventions that promote empathy include increasing the family’s understanding of the importance of empathic connection and providing a safe space to help establish empathic connection.
Studying prosocial behavior, Lukas Lengersdorff and colleagues found that fMRIs of male participants (n = 96) reflected stronger activity when they were acting on behalf of the other, rather than when acting for themselves.4 During this prosocial learning fMRI study, there was stronger engagement of the ventromedial prefrontal cortex (PFC) and higher connectivity between the ventromedial PFC and the right temporoparietal junction (rTPJ). Protecting others from harm appears to be associated with neural mechanisms that support self-relevant learning, but with the added recruitment of structures associated with the social brain. This study shows what we already know – that our brains are wired for social context. This research supports psychotherapeutic interventions aimed at creating interpersonal connection, not just at an intimate level, but also at the prosocial level, such as caring and helping others.
When social interactions are coded, the default mode network (DMN) shows increased activity. Participants (n = 11) in another study had heightened medial PFC–rTPJ connectivity, not only during rest that followed the experimental social encoding, but also during rest that followed a subsequent, nonsocial task.5 Engaging portions of the DMN during live social interactions when actively decoding the social environment, and later engaging these regions when relaxing after the social interaction, appears to facilitate social functioning. Our brains are wired to respond to context. This research underscores the positive impact of interventions such as group therapy and support groups, two underutilized modalities.
Neuroscience evaluation of our relationships provides depth to studies that fall under the medical paradigm of the gene/environment interaction. One of the most elegant in psychiatry is the Finnish study of a sample of offspring of mothers with schizophrenia who gave their children up for adoption.6 This sample of index offspring (n = 155) was compared blindly with matched controls (n = 186) of adopted/away offspring of parents without schizophrenia. The genetic effect manifested only as a psychiatric disorder in the presence of a disturbed family environment. We can now extrapolate certain possible mechanisms from the studies mentioned above: That the deficits lie in the activity or lack of activity in the DMN and associated areas, and in the generation of connectomes responsible for empathic connections.
Neuroscience expands our knowledge of our relational and social worlds, but can psychiatry make the case for inclusion of context in our conceptualization of psychiatric distress? From time to time, inroads are made, for example, the Global Assessment of Relational Functioning was incorporated into the DSM-IV-R and the Cultural Formulation Interview is in the DSM-5. However, without a sustained paradigm shift that places the gene/environment paradigm at the core of psychiatry, these efforts will rise and fall as the pioneers in these fields rise and fall.
A major barrier to moving the gene/environment paradigm more centrally in psychiatry is the prominence of individualism as an American ideal. As the neuroscience of context develops, we will be able to argue more robustly for a contextual approach to patient care.
A second barrier is the difficulty of teaching and learning about complexity. It is easy to learn how to use the DSM to make a diagnosis, to understand when and how to prescribe medications, but it is much more difficult to understand how to incorporate the complexity of life and the context within which we live, into our lexicon of psychiatric theories and treatments. As Tanya Luhrmann, PhD, points out in her study of the process of psychiatric training, residents are intimidated by the need to learn the many psychological theories and their practice; learning about medications is much simpler and takes much less time and effort.7
Nevertheless, context is embraced by several psychiatric subspecialties. Family psychiatrists recognize the power of relational dynamics in the family, and their role in shaping the individual. From understanding family communication patterns, to understanding how roles in the family get allocated, family psychiatry has well established tools for assessment and many evidence-based treatments that focus on changing relational dynamics. Social and community psychiatrists emphasize the role of race, poverty, and access, and support the assessment and treatment of the underprivileged. Cultural psychiatrists recognize that each culture has its own way of constructing identities and shaping our experiences, its own conceptualization of illness and specific idioms of distress. Cultural psychiatrists focus on sensitizing the general psychiatrist to these nuances. Child psychiatrists involve parents, and geriatric psychiatrists involve guardians. General psychiatrists understand context when, for example, understanding the role of trauma in the development of an individual, recognizing that its impact is contingent on the context within which the trauma occurs.
Neuroscience clarifies the neural pathways involved in the development of empathic and social behaviors. Our psychological theories and practice must reflect this advancement. We can teach the relevant neuroscience along with basic concepts such as child-parent relationships. We must assess an individual’s degree of fit within their family and community. Apart from asking relational questions, such as who in your world is important to you, we can use well recognized tools to help us bring context to the forefront. An easy tool is the three generational genogram, or an ecomap, which allows each individual to see where they sit in the context of their world.8 Cultural influences, societal, religious, and family influences can be drawn on the genogram, highlighting both formal and hidden family narratives. In addition, we can share how the brain works with our patients; the science of empathy and social behaviors shows us that our need for interpersonal connection is hardwired.
Dr. Heru is professor of psychiatry at the University of Colorado Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest to disclose. Contact Dr. Heru at [email protected].
References
1. Lee TH et al. Families that fire together smile together: Resting state connectome similarity and daily emotional synchrony in parent-child dyads. Neuroimage. 2017 May 15;152:31-37. doi: 10.1016/j.neuroimage.2017.02.078.
2. Lee TH et al. Love flows downstream: Mothers’ and children’s neural representation similarity in perceiving distress of self and family. Soc Cogn Affect Neurosci. 2017 Dec 1;12(12):1916-27. doi: 10.1093/scan/nsx125.
3. Itahashi T et al. Functional connectomes linking child-parent relationships with psychological problems in adolescence. Neuroimage. 2020 Oct 1;219:117013. doi: 10.1016/j.neuroimage.2020.117013.
4. Lengersdorff LL et al. When implicit prosociality trumps selfishness: The neural valuation system underpins more optimal choices when learning to avoid harm to others than to oneself. J Neurosci. 2020 Sep 16;40(38):7286-99. doi: 10.1523/JNEUROSCI.0842-20.2020.
5. Meyer ML et al. Evidence that default network connectivity during rest consolidates social information. Cereb Cortex. 2019 May 1;29(5):1910-20. doi: 10.1093/cercor/bhy071.
6. Tienari P et al. The Finnish adoptive family study of schizophrenia. Implications for family research. Br J Psychiatry Suppl. 1994 Apr;(23):20-6.
7. Luhrmann, TM. Of two minds: The growing disorder in American psychiatry. New York, NY: Alfred A. Knopf, 2000.
8. Libbon R et al. Family skills for the resident toolbox: The 10-min. Genogram, Ecomap, and Prescribing Homework. Acad Psychiatry. 2019 Aug;43(4):435-439. doi: 10.1007/s40596-019-01054-6.
New law will awaken employers to health care’s ‘transparency gap’
It has become increasingly apparent that our health care system is suffering from a severe case of “transparency gap.” There is a lack of transparency at every level of care in the system. Whether it is the hidden rebate/fee kickbacks from drug manufacturers to pharmacy benefit managers (PBMs), or the variability in pricing of imaging and procedures based on site of care, the need for transparency has become acute. The health insurance sector seems to specialize in opaqueness. The vertical integration of the three largest insurance companies with the three largest PBMs seems to have trapped the flow of money and services in one big black box. It can be difficult to decide if this transparency gap is a case of missing information, misinformation, or a deliberate hiding of information … or maybe a combination of all three.
Recently, I testified before a Wisconsin State Senate committee about the consequences and potential harm to patients and physician offices caused by mandated “white bagging,” which refers to the process whereby provider-administered drugs are shipped to the provider by a specialty pharmacy, as opposed to the provider buying the drug and billing the insurance company. I was very surprised to hear the large employers testifying against our position.
As I listened to the employer groups, it was clear that their protestations were predominantly focused on hospital billing, where markups on the administered medications can be 500% and upward. It made sense that if a business has a “self-funded” health plan, where the employer pays for the cost of care of the employees, those very high markups on the hospital administered medications would eventually become unsustainable. In addition to paying for the care of employees, employers also pay the health insurance company/PBMs to administer the plan. It is obvious that self-funded businesses are being overwhelmed by all these rising costs. What is not so clear is how much information employers get from their plan administrators on their policies and pricing.
An Employee Benefit Research Institute (ERBI) study examined the difference in prices of health care procedures, labs, and imaging based on site of care. It clearly shows that physicians’ offices are the least expensive overall for infusion therapy, even when compared with home infusion in most cases.
Here is where the missing information and the white-bagging issue intersect. When insurance administrators tell employers that letting the provider “buy and bill” costs an outrageous amount, they fail to tell the employers that physicians’ office prices are comparable, or, in some cases, less than what the employer would pay with white bagging. In addition, the possible harm to patients and to the physicians’ practices are never mentioned to the employer. Here is a list of some of the problems associated with white bagging:
- Delays in patient care when dosages or treatment plans are modified during the patient visit.
- Significant waste of drugs when patients’ treatments change or appointments are rescheduled.
- Unnecessary administrative burden for both the patient and physician, including inventory nightmares.
We see the transparency gap again when formularies are created with higher-priced, branded drugs in place of lower-priced generics and alternatives. How can a PBM explain that a formulary that prefers a $10,000 prostate cancer drug but excludes the $400 generic of that drug actually saves money? If the employer doesn’t know about the generic, no explanation is needed.
When physician offices attempt to override some of these harmful policies, the PBM or insurance company often points the finger at the employer as the culprit responsible for the policy. Often, the employers have no idea of the ramifications of the contracts that they have signed. As health care costs continue to rise, it is important that employers are educated on how they can save money and improve patient care by directly contracting with independent physician practices.
In addition, the Consolidated Appropriation Act of 2020-21 (CAA) “seeks to enforce good value from providers and vendors, and forbids hidden contracting terms that disfavor employers and their employees.” This year and next, the employers will become responsible for transparency reporting and demonstrating cost effectiveness of therapies for their employees. In theory, this should uncover many of the hidden policies that favor only the health plans and not the patients or their employers. Many employers are unaware of the CAA, and vendors are in no hurry to inform them of it.
Not only will the CAA help to eliminate much of the transparency gap in the system, but it may encourage employers to work directly with independent physicians’ offices to provide more cost effective and transparent services for their patients. The Coalition of State Rheumatology Organizations is working on a framework to enable practicing rheumatologists to do exactly this.
In the meantime, we must continue educating employers on white bagging and other policies that harm both their patients and their “bottom line.” This education is just one of the steps needed to rid the health care system of the transparency gap that leads to higher prices and poorer care for all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
It has become increasingly apparent that our health care system is suffering from a severe case of “transparency gap.” There is a lack of transparency at every level of care in the system. Whether it is the hidden rebate/fee kickbacks from drug manufacturers to pharmacy benefit managers (PBMs), or the variability in pricing of imaging and procedures based on site of care, the need for transparency has become acute. The health insurance sector seems to specialize in opaqueness. The vertical integration of the three largest insurance companies with the three largest PBMs seems to have trapped the flow of money and services in one big black box. It can be difficult to decide if this transparency gap is a case of missing information, misinformation, or a deliberate hiding of information … or maybe a combination of all three.
Recently, I testified before a Wisconsin State Senate committee about the consequences and potential harm to patients and physician offices caused by mandated “white bagging,” which refers to the process whereby provider-administered drugs are shipped to the provider by a specialty pharmacy, as opposed to the provider buying the drug and billing the insurance company. I was very surprised to hear the large employers testifying against our position.
As I listened to the employer groups, it was clear that their protestations were predominantly focused on hospital billing, where markups on the administered medications can be 500% and upward. It made sense that if a business has a “self-funded” health plan, where the employer pays for the cost of care of the employees, those very high markups on the hospital administered medications would eventually become unsustainable. In addition to paying for the care of employees, employers also pay the health insurance company/PBMs to administer the plan. It is obvious that self-funded businesses are being overwhelmed by all these rising costs. What is not so clear is how much information employers get from their plan administrators on their policies and pricing.
An Employee Benefit Research Institute (ERBI) study examined the difference in prices of health care procedures, labs, and imaging based on site of care. It clearly shows that physicians’ offices are the least expensive overall for infusion therapy, even when compared with home infusion in most cases.
Here is where the missing information and the white-bagging issue intersect. When insurance administrators tell employers that letting the provider “buy and bill” costs an outrageous amount, they fail to tell the employers that physicians’ office prices are comparable, or, in some cases, less than what the employer would pay with white bagging. In addition, the possible harm to patients and to the physicians’ practices are never mentioned to the employer. Here is a list of some of the problems associated with white bagging:
- Delays in patient care when dosages or treatment plans are modified during the patient visit.
- Significant waste of drugs when patients’ treatments change or appointments are rescheduled.
- Unnecessary administrative burden for both the patient and physician, including inventory nightmares.
We see the transparency gap again when formularies are created with higher-priced, branded drugs in place of lower-priced generics and alternatives. How can a PBM explain that a formulary that prefers a $10,000 prostate cancer drug but excludes the $400 generic of that drug actually saves money? If the employer doesn’t know about the generic, no explanation is needed.
When physician offices attempt to override some of these harmful policies, the PBM or insurance company often points the finger at the employer as the culprit responsible for the policy. Often, the employers have no idea of the ramifications of the contracts that they have signed. As health care costs continue to rise, it is important that employers are educated on how they can save money and improve patient care by directly contracting with independent physician practices.
In addition, the Consolidated Appropriation Act of 2020-21 (CAA) “seeks to enforce good value from providers and vendors, and forbids hidden contracting terms that disfavor employers and their employees.” This year and next, the employers will become responsible for transparency reporting and demonstrating cost effectiveness of therapies for their employees. In theory, this should uncover many of the hidden policies that favor only the health plans and not the patients or their employers. Many employers are unaware of the CAA, and vendors are in no hurry to inform them of it.
Not only will the CAA help to eliminate much of the transparency gap in the system, but it may encourage employers to work directly with independent physicians’ offices to provide more cost effective and transparent services for their patients. The Coalition of State Rheumatology Organizations is working on a framework to enable practicing rheumatologists to do exactly this.
In the meantime, we must continue educating employers on white bagging and other policies that harm both their patients and their “bottom line.” This education is just one of the steps needed to rid the health care system of the transparency gap that leads to higher prices and poorer care for all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
It has become increasingly apparent that our health care system is suffering from a severe case of “transparency gap.” There is a lack of transparency at every level of care in the system. Whether it is the hidden rebate/fee kickbacks from drug manufacturers to pharmacy benefit managers (PBMs), or the variability in pricing of imaging and procedures based on site of care, the need for transparency has become acute. The health insurance sector seems to specialize in opaqueness. The vertical integration of the three largest insurance companies with the three largest PBMs seems to have trapped the flow of money and services in one big black box. It can be difficult to decide if this transparency gap is a case of missing information, misinformation, or a deliberate hiding of information … or maybe a combination of all three.
Recently, I testified before a Wisconsin State Senate committee about the consequences and potential harm to patients and physician offices caused by mandated “white bagging,” which refers to the process whereby provider-administered drugs are shipped to the provider by a specialty pharmacy, as opposed to the provider buying the drug and billing the insurance company. I was very surprised to hear the large employers testifying against our position.
As I listened to the employer groups, it was clear that their protestations were predominantly focused on hospital billing, where markups on the administered medications can be 500% and upward. It made sense that if a business has a “self-funded” health plan, where the employer pays for the cost of care of the employees, those very high markups on the hospital administered medications would eventually become unsustainable. In addition to paying for the care of employees, employers also pay the health insurance company/PBMs to administer the plan. It is obvious that self-funded businesses are being overwhelmed by all these rising costs. What is not so clear is how much information employers get from their plan administrators on their policies and pricing.
An Employee Benefit Research Institute (ERBI) study examined the difference in prices of health care procedures, labs, and imaging based on site of care. It clearly shows that physicians’ offices are the least expensive overall for infusion therapy, even when compared with home infusion in most cases.
Here is where the missing information and the white-bagging issue intersect. When insurance administrators tell employers that letting the provider “buy and bill” costs an outrageous amount, they fail to tell the employers that physicians’ office prices are comparable, or, in some cases, less than what the employer would pay with white bagging. In addition, the possible harm to patients and to the physicians’ practices are never mentioned to the employer. Here is a list of some of the problems associated with white bagging:
- Delays in patient care when dosages or treatment plans are modified during the patient visit.
- Significant waste of drugs when patients’ treatments change or appointments are rescheduled.
- Unnecessary administrative burden for both the patient and physician, including inventory nightmares.
We see the transparency gap again when formularies are created with higher-priced, branded drugs in place of lower-priced generics and alternatives. How can a PBM explain that a formulary that prefers a $10,000 prostate cancer drug but excludes the $400 generic of that drug actually saves money? If the employer doesn’t know about the generic, no explanation is needed.
When physician offices attempt to override some of these harmful policies, the PBM or insurance company often points the finger at the employer as the culprit responsible for the policy. Often, the employers have no idea of the ramifications of the contracts that they have signed. As health care costs continue to rise, it is important that employers are educated on how they can save money and improve patient care by directly contracting with independent physician practices.
In addition, the Consolidated Appropriation Act of 2020-21 (CAA) “seeks to enforce good value from providers and vendors, and forbids hidden contracting terms that disfavor employers and their employees.” This year and next, the employers will become responsible for transparency reporting and demonstrating cost effectiveness of therapies for their employees. In theory, this should uncover many of the hidden policies that favor only the health plans and not the patients or their employers. Many employers are unaware of the CAA, and vendors are in no hurry to inform them of it.
Not only will the CAA help to eliminate much of the transparency gap in the system, but it may encourage employers to work directly with independent physicians’ offices to provide more cost effective and transparent services for their patients. The Coalition of State Rheumatology Organizations is working on a framework to enable practicing rheumatologists to do exactly this.
In the meantime, we must continue educating employers on white bagging and other policies that harm both their patients and their “bottom line.” This education is just one of the steps needed to rid the health care system of the transparency gap that leads to higher prices and poorer care for all patients.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is president of the CSRO, past chair of the Alliance for Safe Biologic Medicines, and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Selling your practice
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
My previous column on practice valuation prompted a number of questions on the mechanics of selling a private practice. As usual, I cannot hope to cover this complex topic comprehensively in only 750 words, but here are the basics.
A generation ago, the sale of a medical practice was much like the sale of any other business: A retiring physician would sell his or her practice to a young doctor and the practice would continue on as before. Occasionally, that still happens, but changes in the business of medicine – most significantly the growth of managed care – have had a big impact on the way medical practices are bought and sold.
For one thing, there are far fewer solo practitioners these days, and polls indicate that most young physicians intend to continue that trend. .
For another, because the rules governing such sales have become so numbingly complex, the services of expert (and expensive) third parties are essential.
While these issues may complicate matters, there is still a market for the sale of medical practices. However, you must do everything possible to ensure you identify the best possible buyer and structure the best deal.
The first hurdle is the accurate valuation of your practice, which was covered in some detail in my last column. Briefly, for the protection of both parties, it is important that the appraisal be done by an experienced and neutral financial consultant, that all techniques used in the valuation be divulged and explained, and that documentation be supplied to support the conclusions reached.
Keep in mind that the valuation will not necessarily equal the purchase price; other factors may need to be considered before a final price can be agreed upon. Keep in mind, too, that there may be legal constraints on the purchase price. For example, if the buyer is a nonprofit corporation such as a hospital or HMO, by law it cannot pay in excess of fair market value for the practice – which may rule out any valuation of “good will.” In some states, the purchase of private practices by hospitals is prohibited altogether – so you might need to consider a long-term lease rather than a sale.
Once a value has been agreed upon, you must consider how the transaction will be structured. The most popular structures include purchase of assets, purchase of corporate stock, and merger.
Many buyers prefer to purchase assets, because it allows them to pick and choose only those items that have value to them. This can leave you with a bunch of “odd lot” assets to dispose of. But depending on the circumstances, an asset sale may still be to your advantage.
Sellers typically prefer to sell stock, because it allows them to sell their entire practice, which is often worth more than the sum of its parts, and often provides tax advantages.
The third option, merger, continues to grow in popularity and is a column subject in itself, and I will address it separately next month.
Tax issues must always be considered. Most private practices are corporations, and the sale of corporate stock will result in a long-term capital gain that will be taxed – currently at 15%-20%. As the saying goes, it’s not what you earn, it’s what you keep. So it may benefit you to accept a slightly lower price if the sale can be structured to provide significantly lower tax treatment. However, any gain that does not qualify as a long-term capital gain will be taxed as regular income – currently in the 32%-37% percent range – plus a Social Security tax of about 15%.
Payment in installments is a popular way to defer taxes, since they are incurred on each installment as it is paid; but such payments may be mistaken by the IRS for payments for referrals, which is illegal. And there is always the problem of making certain all payments are eventually made.
You may wish to continue working at the practice as an employee for an agreed-upon period of time, and this is often to the buyer’s advantage as well. Transitioning to new ownership in stages often maximizes the value of the business by improving patient retention, and allows patients to become accustomed to the transition. However, care must be taken, with the aid of good legal advice, to structure such an arrangement in a way that minimizes concerns of fraud and abuse.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The science of clean skin care and the clean beauty movement
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).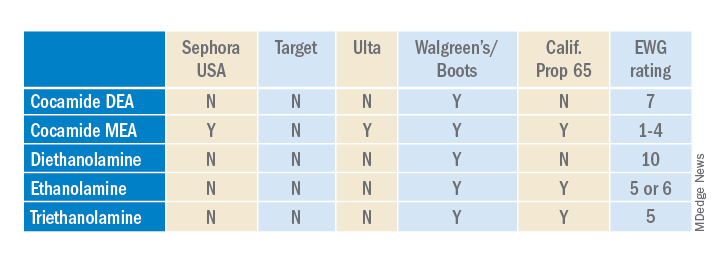
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
. I see numerous social media posts, blogs, and magazine articles about toxic skin care ingredients, while more patients are asking their dermatologists about clean beauty products. So, I decided it was time to dissect the issues and figure out what “clean” really means to me.
The problem is that no one agrees on a clean ingredient standard for beauty products. Many companies, like Target, Walgreens/Boots, Sephora, Neiman Marcus, Whole Foods, and Ulta, have their own varying clean standards. Even Allure magazine has a “Clean Best of Beauty” seal. California has Proposition 65, otherwise known as the Safe Drinking Water and Toxic Enforcement Act of 1986, which contains a list of banned chemicals “known to the state to cause cancer or reproductive toxicity.” In January 2021, Hawai‘i law prohibited the sale of oxybenzone and octinoxate in sunscreens in response to scientific studies showing that these ingredients “are toxic to corals and other marine life.” The Environmental Working Group (EWG) rates the safety of ingredients based on carcinogenicity, developmental and reproductive toxicity, allergenicity, and immunotoxicity. The Cosmetic Ingredient Review (CIR), funded by the Personal Care Products Council, consists of a seven-member steering committee that has at least one dermatologist representing the American Academy of Dermatology and a toxicologist representing the Society of Toxicology. The CIR publishes detailed reviews of ingredients that can be easily found on PubMed and Google Scholar and closely reviews animal and human data and reports on safety and contact dermatitis risk.
Which clean beauty standard is best?
I reviewed most of the various standards, clean seals, laws, and safety reports and found significant discrepancies resulting from misunderstandings of the science, lack of depth in the scientific evaluations, lumping of ingredients into a larger category, or lack of data. The most salient cause of misinformation and confusion seems to be hyperbolic claims by the media and clean beauty advocates who do not understand the basic science.
When I conducted a survey of cosmetic chemists on my LinkedIn account, most of the chemists stated that “ ‘Clean Beauty’ is a marketing term, more than a scientific term.” None of the chemists could give an exact definition of clean beauty. However, I thought I needed a good answer for my patients and for doctors who want to use and recommend “clean skin care brands.”
A dermatologist’s approach to develop a clean beauty standard
Many of the standards combine all of the following into the “clean” designation: nontoxic to the environment (both the production process and the resulting ingredient), nontoxic to marine life and coral, cruelty-free (not tested on animals), hypoallergenic, lacking in known health risks (carcinogenicity, reproductive toxicity), vegan, and gluten free. As a dermatologist, I am a splitter more than a lumper, so I prefer that “clean” be split into categories to make it easier to understand. With that in mind, I will focus on clean beauty ingredients only as they pertain to health: carcinogenicity, endocrine effects, nephrotoxicity, hepatotoxicity, immunotoxicity, etc. This discussion will not consider environmental effects, reproductive toxicity (some ingredients may decrease fertility, which is beyond the scope of this article), ingredient sources, and sustainability, animal testing, or human rights violations during production. Those issues are important, of course, but for clarity and simplicity, we will focus on the health risks of skin care ingredients.
In this month’s column, I will focus on a few ingredients and will continue the discussion in subsequent columns. Please note that commercial standards such as Target standards are based on the product type (e.g., cleansers, sunscreens, or moisturizers). So, when I mention an ingredient not allowed by certain company standards, note that it can vary by product type. My comments pertain mostly to facial moisturizers and facial serums to try and simplify the information. The Good Face Project has a complete list of standards by product type, which I recommend as a resource if you want more detailed information.
Are ethanolamines safe or toxic in cosmetics?
Ethanolamines are common ingredients in surfactants, fragrances, and emulsifying agents and include cocamide diethanolamine (DEA), cocamide monoethanolamine (MEA), and triethanolamine (TEA). Cocamide DEA, lauramide DEA, linoleamide DEA, and oleamide DEA are fatty acid diethanolamides that may contain 4% to 33% diethanolamine.1 A Google search of toxic ingredients in beauty products consistently identifies ethanolamines among such offending product constituents. Table 1 reveals that ethanolamines are excluded from some standards and included in others (N = not allowed or restricted by amount used and Y = allowed with no restrictions). As you can see, the standards don’t correspond to the EWG rating of the ingredients, which ranges from 1 (low hazard) to 10 (high hazard).
Why are ethanolamines sometimes considered safe and sometimes not?
Ethanolamines are reputed to be allergenic, but as we know as dermatologists, that does not mean that everyone will react to them. (In my opinion, allergenicity is a separate issue than the clean issue.) One study showed that TEA in 2.5% petrolatum had a 0.4% positive patch test rate in humans, which was thought to be related more to irritation than allergenicity.2 Cocamide DEA allergy is seen in those with hand dermatitis resulting from hand cleansers but is more commonly seen in metal workers.3 For this reason, these ethanolamines are usually found in rinse-off products to decrease exposure time. But there are many irritating ingredients not banned by Target, Sephora, and Ulta, so why does ethanolamine end up on toxic ingredient lists?
First, there is the issue of oral studies in animals. Oral forms of some ethanolamines have shown mild toxicity in rats, but topical forms have not been demonstrated to cause mutagenicity.1
For this reason, ethanolamines in their native form are considered safe.
The main issue with ethanolamines is that, when they are formulated with ingredients that break down into nitrogen, such as certain preservatives, the combination forms nitrosamines, such as N-nitrosodiethylamine (NDEA), which are carcinogenic.4 The European Commission prohibits DEA in cosmetics based on concerns about formation of these carcinogenic nitrosamines. Some standards limit ethanolamines to rinse-off products.5 The CIR panel concluded that diethanolamine and its 16 salts are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed and that TEA and TEA-related compounds are safe if they are not used in cosmetic products in which N-nitroso compounds can be formed.6,7 The FDA states that there is no reason for consumers to be alarmed based on the use of DEA in cosmetics.8
The safety issues surrounding the use of ethanolamines in a skin care routine illustrate an important point: Every single product in the skin care routine should be compatible with the other products in the regimen. Using ethanolamines in a rinse-off product is one solution, as is ensuring that no other products in the skin care routine contain N-nitroso compounds that can combine with ethanolamines to form nitrosamines.
Are natural products safer?
Natural products are not necessarily any safer than synthetic products. Considering ethanolamines as the example here, note that cocamide DEA is an ethanolamine derived from coconut. It is often found in “green” or “natural” skin care products.9 It can still combine with N-nitroso compounds to form carcinogenic nitrosamines.
What is the bottom line? Are ethanolamines safe in cosmetics?
For now, if a patient asks if ethanolamine is safe in skin care, my answer would be yes, so long as the following is true:
- It is in a rinse-off product.
- The patient is not allergic to it.
- They do not have hand dermatitis.
- Their skin care routine does not include nitrogen-containing compounds like N-nitrosodiethanolamine (NDELA) or NDEA.
Conclusion
This column uses ethanolamines as an example to show the disparity in clean standards in the cosmetic industry. As you can see, there are multiple factors to consider. I will begin including clean information in my cosmeceutical critique columns to address some of these issues.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. Cocamide DE. J Am Coll Toxicol. 1986;5(5).
2. Lessmann H et al. Contact Dermatitis. 2009 May;60(5):243-55.
3. Aalto-Korte K et al. 2014 Mar;70(3):169-74.
4. Kraeling ME et al. Food Chem Toxicol. 2004 Oct;42(10):1553-61.
5. Fiume MM et al. Int J Toxicol. 2015 Sep;34(2 Suppl):84S-98S.
6. Fiume MM.. Int J Toxicol. 2017 Sep/Oct;36(5_suppl2):89S-110S.
7. Fiume MM et al. Int J Toxicol. 2013 May-Jun;32(3 Suppl):59S-83S.
8. U.S. Food & Drug Administration. Diethanolamine. https://www.fda.gov/cosmetics/cosmetic-ingredients/diethanolamine. Accessed Feb. 12, 2022.
9. Aryanti N et al. IOP Conference Series: Materials Science and Engineering 2021 Feb 1 (Vol. 1053, No. 1, p. 012066). IOP Publishing.
When the EMR is MIA
“It’s in the computer.”
How many times a week do you hear that?
The advent of the modern EMR has created a new belief in many patients: That all medical office computers are connected, and information from one can be obtained from any of them. So when I ask patients if they had labs, or an MRI, or what their medications are, that’s what I sometimes hear back.
“It’s in the computer.”
True, but not MY computer.
Then they get perplexed, and irritated. Didn’t someone at the other office, or their friends, or something they read online, tell them I’d have access to it? Isn’t all medical info in a giant online database, somewhere, and all doctors can get into it?
Admittedly, I’m probably in better shape than other solo-practice docs. I have access codes to two local radiology places, two large labs, and the largest health care system in my corner of Phoenix. So although I’m not technically a part of them, I can still pull records when I need them for patient care. But if the patient is in my office right then, it takes a minute. My office wifi isn’t the fastest, I have to enter passwords, then do two-factor authentication.
I understand – very much – the importance of the added layers of security, but it adds time to the visit.
Some people, with perhaps more faith in technology than is justified, still don’t understand this. Another doctor sent them to see me, so why don’t I have the results of previous tests and labs? If they tell us what tests they had, and where, and when they make the appointment I can often be prepared for them. But this isn’t consistent.
On the surface some sort of large-scale medical database for everyone sounds good. It would be nice to not have to scramble to get past test results when people come in, and would probably save a lot of money on duplicated labs. But there are legitimate concerns about security and privacy, too.
Not only that, but “it’s in the computer” is also only as good as the people working them. Recently I got a call from an office in a local health care system asking for my notes on a patient. I’d sent them, but they insisted they hadn’t received them. From my desk I logged into their system and found my notes, neatly scanned in and labeled with my name and specialty. When I picked up the phone and told the young lady where to look, she was nice enough to apologize.
I get that. I often overlook things under my own nose, too. But no amount of technology will fix that issue for me or anyone else.
Unfortunately, this misplaced faith in technology doesn’t seem to be going away. People will still keep believing that it works much better than it really does.
That’s human nature, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“It’s in the computer.”
How many times a week do you hear that?
The advent of the modern EMR has created a new belief in many patients: That all medical office computers are connected, and information from one can be obtained from any of them. So when I ask patients if they had labs, or an MRI, or what their medications are, that’s what I sometimes hear back.
“It’s in the computer.”
True, but not MY computer.
Then they get perplexed, and irritated. Didn’t someone at the other office, or their friends, or something they read online, tell them I’d have access to it? Isn’t all medical info in a giant online database, somewhere, and all doctors can get into it?
Admittedly, I’m probably in better shape than other solo-practice docs. I have access codes to two local radiology places, two large labs, and the largest health care system in my corner of Phoenix. So although I’m not technically a part of them, I can still pull records when I need them for patient care. But if the patient is in my office right then, it takes a minute. My office wifi isn’t the fastest, I have to enter passwords, then do two-factor authentication.
I understand – very much – the importance of the added layers of security, but it adds time to the visit.
Some people, with perhaps more faith in technology than is justified, still don’t understand this. Another doctor sent them to see me, so why don’t I have the results of previous tests and labs? If they tell us what tests they had, and where, and when they make the appointment I can often be prepared for them. But this isn’t consistent.
On the surface some sort of large-scale medical database for everyone sounds good. It would be nice to not have to scramble to get past test results when people come in, and would probably save a lot of money on duplicated labs. But there are legitimate concerns about security and privacy, too.
Not only that, but “it’s in the computer” is also only as good as the people working them. Recently I got a call from an office in a local health care system asking for my notes on a patient. I’d sent them, but they insisted they hadn’t received them. From my desk I logged into their system and found my notes, neatly scanned in and labeled with my name and specialty. When I picked up the phone and told the young lady where to look, she was nice enough to apologize.
I get that. I often overlook things under my own nose, too. But no amount of technology will fix that issue for me or anyone else.
Unfortunately, this misplaced faith in technology doesn’t seem to be going away. People will still keep believing that it works much better than it really does.
That’s human nature, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“It’s in the computer.”
How many times a week do you hear that?
The advent of the modern EMR has created a new belief in many patients: That all medical office computers are connected, and information from one can be obtained from any of them. So when I ask patients if they had labs, or an MRI, or what their medications are, that’s what I sometimes hear back.
“It’s in the computer.”
True, but not MY computer.
Then they get perplexed, and irritated. Didn’t someone at the other office, or their friends, or something they read online, tell them I’d have access to it? Isn’t all medical info in a giant online database, somewhere, and all doctors can get into it?
Admittedly, I’m probably in better shape than other solo-practice docs. I have access codes to two local radiology places, two large labs, and the largest health care system in my corner of Phoenix. So although I’m not technically a part of them, I can still pull records when I need them for patient care. But if the patient is in my office right then, it takes a minute. My office wifi isn’t the fastest, I have to enter passwords, then do two-factor authentication.
I understand – very much – the importance of the added layers of security, but it adds time to the visit.
Some people, with perhaps more faith in technology than is justified, still don’t understand this. Another doctor sent them to see me, so why don’t I have the results of previous tests and labs? If they tell us what tests they had, and where, and when they make the appointment I can often be prepared for them. But this isn’t consistent.
On the surface some sort of large-scale medical database for everyone sounds good. It would be nice to not have to scramble to get past test results when people come in, and would probably save a lot of money on duplicated labs. But there are legitimate concerns about security and privacy, too.
Not only that, but “it’s in the computer” is also only as good as the people working them. Recently I got a call from an office in a local health care system asking for my notes on a patient. I’d sent them, but they insisted they hadn’t received them. From my desk I logged into their system and found my notes, neatly scanned in and labeled with my name and specialty. When I picked up the phone and told the young lady where to look, she was nice enough to apologize.
I get that. I often overlook things under my own nose, too. But no amount of technology will fix that issue for me or anyone else.
Unfortunately, this misplaced faith in technology doesn’t seem to be going away. People will still keep believing that it works much better than it really does.
That’s human nature, too.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Pharma should stop doing business in Russia, says ethicist
Should pharmaceutical companies continue to do business in Russia, running ongoing clinical trials, starting new ones, or continuing to sell their products there?
Some argue that medicine and science must not get enmeshed in politics, staying above the fray to protect their independence and credibility. Other defenders of business-as-usual say the pharmaceutical industry deals in health and aids the vulnerable. Humanitarianism requires continued interaction with Russia.
I think both arguments fail.
We are fighting a war with Russia. It is a war of economic strangulation, social isolation, and pushing Russia as hard as we can to become a pariah state so that internal pressure on Putin will cause him to rethink his cruel, unjustified invasion or the Russian people to replace him. This pressure must be harsh and it must happen quickly. Why?
Having failed to rapidly defeat the Ukrainian army in the war’s first weeks, Russian commanders are now resorting to the horrible barbarism they used in previous wars in Chechnya and Syria: flattening cities, attacking civilians, killing children with massive and indiscriminate firepower.
To mention one recent horror among many, Russian shelling destroyed a maternity hospital in Mariupol. Ukraine’s president, Volodymyr Zelensky, in bemoaning the Russians for their continuing series of war crimes called on the world to act.
“Mariupol. Direct Strike of Russian troops at the maternity hospital,” he wrote in a Twitter post. “People, children are under the wreckage. Atrocity! How much longer will the world be an accomplice ignoring terror?”
The Russian government’s response: “It is not the first time we have seen pathetic outcries concerning the so-called atrocities,” said Minister of Foreign Affairs Sergei Lavrov, claiming the hospital was being used as a base by an “ultra-radical” Ukrainian battalion.
Health and its preservation are key parts of the aim of medicine and science. There is no way that medicine and science can ignore what war does to health, what attacks on hospitals do to the sick and those who serve them there, the psychological toll that intentional terrorism takes on civilians and their defenders, and what the destruction of infrastructure means for the long-term well-being of Ukrainians.
There can be no collusion with war criminals. There can be no denial of the inextricable link between medicine, science, and politics. Medicine and science are controlled by political forces; their use for good or evil is driven by political considerations, and each doctor, scientist, and scientific society must take a stand when politics corrodes the underlying aims of research and healing.
How far does noncooperation with Russia go? Very, very far. All research, both ongoing and new, must cease immediately. Whatever can be done to minimize harm to existing subjects in a short period of time ought to be done, but that is it.
Similarly, no sale of medicines or therapies ought to be occurring, be they life-saving or consumer products. Putin will see to it that such shipments go to the military or are sold on the black market for revenue, and there is nothing pharma companies can do to stop that.
The Russian people need to be pinched not only by the loss of cheeseburgers and boutique coffee but by products they use to maintain their well-being. War is cruel that way, but if you tolerate a government that is bombing and shelling a peaceful neighbor to oblivion, then pharma must ensure that efforts to make Putin and his kleptocratic goons feel the wrath of their fellow citizens.
Given the realities of nuclear Armageddon, the civilized world must fight obvious barbarity as best it can with sanctions, financial assaults, property seizures, and forgoing commerce, including important raw materials and health products. War, even in a fiscal form, is not without terrible costs; but achieving a rapid, just resolution against tyranny permits no exceptions for pharma or any other business if it is a war that must be fought.
Dr. Caplan is director of the division of medical ethics at New York University. He has consulted with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
Should pharmaceutical companies continue to do business in Russia, running ongoing clinical trials, starting new ones, or continuing to sell their products there?
Some argue that medicine and science must not get enmeshed in politics, staying above the fray to protect their independence and credibility. Other defenders of business-as-usual say the pharmaceutical industry deals in health and aids the vulnerable. Humanitarianism requires continued interaction with Russia.
I think both arguments fail.
We are fighting a war with Russia. It is a war of economic strangulation, social isolation, and pushing Russia as hard as we can to become a pariah state so that internal pressure on Putin will cause him to rethink his cruel, unjustified invasion or the Russian people to replace him. This pressure must be harsh and it must happen quickly. Why?
Having failed to rapidly defeat the Ukrainian army in the war’s first weeks, Russian commanders are now resorting to the horrible barbarism they used in previous wars in Chechnya and Syria: flattening cities, attacking civilians, killing children with massive and indiscriminate firepower.
To mention one recent horror among many, Russian shelling destroyed a maternity hospital in Mariupol. Ukraine’s president, Volodymyr Zelensky, in bemoaning the Russians for their continuing series of war crimes called on the world to act.
“Mariupol. Direct Strike of Russian troops at the maternity hospital,” he wrote in a Twitter post. “People, children are under the wreckage. Atrocity! How much longer will the world be an accomplice ignoring terror?”
The Russian government’s response: “It is not the first time we have seen pathetic outcries concerning the so-called atrocities,” said Minister of Foreign Affairs Sergei Lavrov, claiming the hospital was being used as a base by an “ultra-radical” Ukrainian battalion.
Health and its preservation are key parts of the aim of medicine and science. There is no way that medicine and science can ignore what war does to health, what attacks on hospitals do to the sick and those who serve them there, the psychological toll that intentional terrorism takes on civilians and their defenders, and what the destruction of infrastructure means for the long-term well-being of Ukrainians.
There can be no collusion with war criminals. There can be no denial of the inextricable link between medicine, science, and politics. Medicine and science are controlled by political forces; their use for good or evil is driven by political considerations, and each doctor, scientist, and scientific society must take a stand when politics corrodes the underlying aims of research and healing.
How far does noncooperation with Russia go? Very, very far. All research, both ongoing and new, must cease immediately. Whatever can be done to minimize harm to existing subjects in a short period of time ought to be done, but that is it.
Similarly, no sale of medicines or therapies ought to be occurring, be they life-saving or consumer products. Putin will see to it that such shipments go to the military or are sold on the black market for revenue, and there is nothing pharma companies can do to stop that.
The Russian people need to be pinched not only by the loss of cheeseburgers and boutique coffee but by products they use to maintain their well-being. War is cruel that way, but if you tolerate a government that is bombing and shelling a peaceful neighbor to oblivion, then pharma must ensure that efforts to make Putin and his kleptocratic goons feel the wrath of their fellow citizens.
Given the realities of nuclear Armageddon, the civilized world must fight obvious barbarity as best it can with sanctions, financial assaults, property seizures, and forgoing commerce, including important raw materials and health products. War, even in a fiscal form, is not without terrible costs; but achieving a rapid, just resolution against tyranny permits no exceptions for pharma or any other business if it is a war that must be fought.
Dr. Caplan is director of the division of medical ethics at New York University. He has consulted with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
Should pharmaceutical companies continue to do business in Russia, running ongoing clinical trials, starting new ones, or continuing to sell their products there?
Some argue that medicine and science must not get enmeshed in politics, staying above the fray to protect their independence and credibility. Other defenders of business-as-usual say the pharmaceutical industry deals in health and aids the vulnerable. Humanitarianism requires continued interaction with Russia.
I think both arguments fail.
We are fighting a war with Russia. It is a war of economic strangulation, social isolation, and pushing Russia as hard as we can to become a pariah state so that internal pressure on Putin will cause him to rethink his cruel, unjustified invasion or the Russian people to replace him. This pressure must be harsh and it must happen quickly. Why?
Having failed to rapidly defeat the Ukrainian army in the war’s first weeks, Russian commanders are now resorting to the horrible barbarism they used in previous wars in Chechnya and Syria: flattening cities, attacking civilians, killing children with massive and indiscriminate firepower.
To mention one recent horror among many, Russian shelling destroyed a maternity hospital in Mariupol. Ukraine’s president, Volodymyr Zelensky, in bemoaning the Russians for their continuing series of war crimes called on the world to act.
“Mariupol. Direct Strike of Russian troops at the maternity hospital,” he wrote in a Twitter post. “People, children are under the wreckage. Atrocity! How much longer will the world be an accomplice ignoring terror?”
The Russian government’s response: “It is not the first time we have seen pathetic outcries concerning the so-called atrocities,” said Minister of Foreign Affairs Sergei Lavrov, claiming the hospital was being used as a base by an “ultra-radical” Ukrainian battalion.
Health and its preservation are key parts of the aim of medicine and science. There is no way that medicine and science can ignore what war does to health, what attacks on hospitals do to the sick and those who serve them there, the psychological toll that intentional terrorism takes on civilians and their defenders, and what the destruction of infrastructure means for the long-term well-being of Ukrainians.
There can be no collusion with war criminals. There can be no denial of the inextricable link between medicine, science, and politics. Medicine and science are controlled by political forces; their use for good or evil is driven by political considerations, and each doctor, scientist, and scientific society must take a stand when politics corrodes the underlying aims of research and healing.
How far does noncooperation with Russia go? Very, very far. All research, both ongoing and new, must cease immediately. Whatever can be done to minimize harm to existing subjects in a short period of time ought to be done, but that is it.
Similarly, no sale of medicines or therapies ought to be occurring, be they life-saving or consumer products. Putin will see to it that such shipments go to the military or are sold on the black market for revenue, and there is nothing pharma companies can do to stop that.
The Russian people need to be pinched not only by the loss of cheeseburgers and boutique coffee but by products they use to maintain their well-being. War is cruel that way, but if you tolerate a government that is bombing and shelling a peaceful neighbor to oblivion, then pharma must ensure that efforts to make Putin and his kleptocratic goons feel the wrath of their fellow citizens.
Given the realities of nuclear Armageddon, the civilized world must fight obvious barbarity as best it can with sanctions, financial assaults, property seizures, and forgoing commerce, including important raw materials and health products. War, even in a fiscal form, is not without terrible costs; but achieving a rapid, just resolution against tyranny permits no exceptions for pharma or any other business if it is a war that must be fought.
Dr. Caplan is director of the division of medical ethics at New York University. He has consulted with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
An 11-year-old female presented with skin discoloration on her back
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Becker’s nevus
The history and physical exam are most consistent with Becker’s nevus, also known as Becker’s melanosis. This is a benign cutaneous hamartoma, usually found in males, characterized by a large, irregularly shaped brown patch, often with hypertrichosis.1 Becker’s nevus can be congenital but is more commonly noticed in late childhood or early adolescence, with thickening, increased pigmentation, and hair growth. Becker’s nevus is considered an overgrowth of epidermal pigment cells and hair follicles and is thought to be attributable to postzygotic mutations (with ACTB mutations most reported).1 It is often located unilaterally on the upper trunk but is occasionally present elsewhere on the body. Acne may occasionally develop within the nevus, which is believed to be triggered by puberty-associated androgens.1 The lesion tends to persist indefinitely but has no propensity for malignant transformation.
Becker’s nevus is generally an isolated skin lesion without other anomalies. However, in rare instances, it may be associated with ipsilateral breast hypoplasia or hypoplastic defects of the muscle, skin, or skeleton, which is known as Becker’s nevus syndrome.2 Treatment is not medically warranted for an isolated Becker’s nevus but may be pursued for cosmetic reasons. Although treatment is generally discouraged because of variable success, laser hair removal and laser therapy may be pursued to address the hypertrichosis and hyperpigmentation, respectively.
What is on the differential?
A café-au-lait macule (CALM) is a light- to dark-brown, oval lesion that commonly presents at birth or in early childhood. CALMs vary widely in size from less than 1.5 cm to more than 20 cm in diameter. They are asymptomatic and grow in proportion to the individual over time.3 Becker’s nevus can be distinguished from CALMs by the development of hypertrichosis, typical location and course, and other skin changes within the nevus.
Postinflammatory hyperpigmentation (PIH) is characterized by asymptomatic, darkened macules or patches that are brown to blue-gray in color. It is one of the most common causes of hyperpigmentation, particularly in skin of color, and can take months to years to resolve. PIH is caused by increased melanin production in response to a cutaneous inflammatory process, such as a drug reaction, allergy, mechanical or thermal injury, infection, phototoxicity, or an underlying skin condition.3 Our patient’s history with the lack of an inciting inflammatory process is more consistent with Becker’s nevus.
Erythema ab igne is a cutaneous reaction to heat that presents as a hyperpigmented patch with a reticular or mottled configuration and superficial venular telangiectasia. The lesion is initially erythematous and progresses to a pale pink to purplish dark-brown color.4 Causes include long-term use of a heating pad, laptop, electric blanket, or a hot water bottle. The absence of prolonged heat exposure in our patient’s history does not favor erythema ab igne.
Pigmentary mosaicism is characterized by a distinctive pattern of hyperpigmentation that follows the lines of ectodermal embryologic development, known as the lines of Blaschko.5 This condition is also known as linear and whorled nevoid hypermelanosis because of its streaky or swirl-like pattern. Pigmentary mosaicism can be present at birth or appear within the first few weeks of life. It is caused by genetic heterogeneity in neuroectodermal cells, which results in skin with areas of varying colors. Pigmentary mosaicism is unlikely in this case as our patient’s lesion does not follow the lines of Blaschko.
Ms. Laborada is a pediatric dermatology research associate in the division of pediatric and adolescent dermatology at the University of California, San Diego, and Rady Children’s Hospital, San Diego. Dr. Eichenfield is the vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. Ms. Laborada and Dr. Eichenfield have no relevant financial disclosures.
References
1. Atzmony L et al. J Cutan Pathol. 2020;47(8):681-5.
2. Danarti R et al. J Am Acad Dermatol. 2004;51(6):965-9.
3. Paller A and Mancini AJ. “Hurwitz Clinical Pediatric Dermatology: A textbook of skin disorders of childhood and adolescence” 4th ed. Philadelphia: Elsevier Saunders, 2011.
4. Patel DP. JAMA Dermatol. 2017;153(7):685.
5. Kromann AB et al. Orphanet J Rare Dis. 2018;13(1):39.
Commentary: Norovirus vaccine candidates employ different approaches
Norovirus, as noted above, is now the most common cause of medically attended acute gastroenteritis (AGE) in the United States. Norovirus AGE resembles rotavirus AGE, but a bit heavier on the vomiting. What makes it scary is that it is a low-inoculum infection (as few as 16 virus particles can cause infection), and it survives for prolonged periods in food, 10% chlorinated water, and on environmental surfaces (J Med Virol 2008;80:1468-76); hence, the infamous outbreaks on cruise ships and daycare centers. So a vaccine would be very welcome. The two non-Chinese candidates GI.1/GII.4 vaccines are Takeda’s VLP vaccine and Vaxart’s oral adenovirus vector-based vaccine.
Takeda’s is injectable. If VLP sounds familiar, VLPs make up the FDA-approved HPV vaccine we use. Two doses of various formulations were tested in a recent phase 2 study of 1- to 3- and 4- to 8-year-olds in Finland, Panama, and Colombia with no safety issues identified. The 1- to 3-year-olds responded somewhat better than 4- to 8-year-olds, and titers remained elevated up to day 210 (Vaccine. 2022 Jun 9;40[26]:3588-96).
A recently as yet unpublished phase 1b trial of Vaxart’s vaccine in 55- to 80-year-olds (NCT04854746) showed a dose-dependent response. IgA mucosal cell responses were similar to those in younger adults. Adverse event profiles were similar between vaccinees and placebo recipients.
Progress continues for both vaccines, but we await efficacy trials. We are likely still years from a pediatric vaccine. My sense is that an oral vaccine would be more readily accepted into the pediatric schedule, but how to incorporate it and not cause issues with the rotavirus vaccine will need evaluation.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Norovirus, as noted above, is now the most common cause of medically attended acute gastroenteritis (AGE) in the United States. Norovirus AGE resembles rotavirus AGE, but a bit heavier on the vomiting. What makes it scary is that it is a low-inoculum infection (as few as 16 virus particles can cause infection), and it survives for prolonged periods in food, 10% chlorinated water, and on environmental surfaces (J Med Virol 2008;80:1468-76); hence, the infamous outbreaks on cruise ships and daycare centers. So a vaccine would be very welcome. The two non-Chinese candidates GI.1/GII.4 vaccines are Takeda’s VLP vaccine and Vaxart’s oral adenovirus vector-based vaccine.
Takeda’s is injectable. If VLP sounds familiar, VLPs make up the FDA-approved HPV vaccine we use. Two doses of various formulations were tested in a recent phase 2 study of 1- to 3- and 4- to 8-year-olds in Finland, Panama, and Colombia with no safety issues identified. The 1- to 3-year-olds responded somewhat better than 4- to 8-year-olds, and titers remained elevated up to day 210 (Vaccine. 2022 Jun 9;40[26]:3588-96).
A recently as yet unpublished phase 1b trial of Vaxart’s vaccine in 55- to 80-year-olds (NCT04854746) showed a dose-dependent response. IgA mucosal cell responses were similar to those in younger adults. Adverse event profiles were similar between vaccinees and placebo recipients.
Progress continues for both vaccines, but we await efficacy trials. We are likely still years from a pediatric vaccine. My sense is that an oral vaccine would be more readily accepted into the pediatric schedule, but how to incorporate it and not cause issues with the rotavirus vaccine will need evaluation.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.
Norovirus, as noted above, is now the most common cause of medically attended acute gastroenteritis (AGE) in the United States. Norovirus AGE resembles rotavirus AGE, but a bit heavier on the vomiting. What makes it scary is that it is a low-inoculum infection (as few as 16 virus particles can cause infection), and it survives for prolonged periods in food, 10% chlorinated water, and on environmental surfaces (J Med Virol 2008;80:1468-76); hence, the infamous outbreaks on cruise ships and daycare centers. So a vaccine would be very welcome. The two non-Chinese candidates GI.1/GII.4 vaccines are Takeda’s VLP vaccine and Vaxart’s oral adenovirus vector-based vaccine.
Takeda’s is injectable. If VLP sounds familiar, VLPs make up the FDA-approved HPV vaccine we use. Two doses of various formulations were tested in a recent phase 2 study of 1- to 3- and 4- to 8-year-olds in Finland, Panama, and Colombia with no safety issues identified. The 1- to 3-year-olds responded somewhat better than 4- to 8-year-olds, and titers remained elevated up to day 210 (Vaccine. 2022 Jun 9;40[26]:3588-96).
A recently as yet unpublished phase 1b trial of Vaxart’s vaccine in 55- to 80-year-olds (NCT04854746) showed a dose-dependent response. IgA mucosal cell responses were similar to those in younger adults. Adverse event profiles were similar between vaccinees and placebo recipients.
Progress continues for both vaccines, but we await efficacy trials. We are likely still years from a pediatric vaccine. My sense is that an oral vaccine would be more readily accepted into the pediatric schedule, but how to incorporate it and not cause issues with the rotavirus vaccine will need evaluation.
Christopher J. Harrison, MD, is professor, University of Missouri Kansas City School of Medicine, department of medicine, infectious diseases section, Kansas City. He has no financial conflicts of interest.