User login
Lost keys, missed appointments: The struggle of treating executive dysfunction
Maybe you know some of these patients: They may come late or not show up at all. They may have little to say and minimize their difficulties, often because they are ashamed of how much effort it takes to meet ordinary obligations. They may struggle to complete assignments, fail classes, or lose jobs. And being in the right place at the right time can feel monumental to them: They forget appointments, double book themselves, or sometimes sleep through important events.
It’s not just appointments. They lose their keys and valuables, forget to pay bills, and may not answer calls, texts, or emails. Their voicemail may be full and people are often frustrated with them. These are all characteristics of executive dysfunction, which together can make the routine responsibilities of life very difficult.
Treatments include stimulants, and because of their potential for abuse, these medications are more strictly regulated when it comes to prescribing. The FDA does not allow them to be phoned into a pharmacy or refills to be added to prescriptions. Patients must wait until right before they are due to run out to get the next prescription, and this can present a problem if the patient travels or takes long vacations.
And although it is not the patient’s fault that stimulants can’t be ordered with refills, this adds to the burden of treating patients who take them. It’s hard to imagine that these restrictions on stimulants and opiates (but not on benzodiazepines) do much to deter abuse or diversion.
I trained at a time when ADD and ADHD were disorders of childhood, and as an adult psychiatrist, I was not exposed to patients on these medications. Occasionally, a stimulant was prescribed in a low dose to help activate a very depressed patient, but it was thought that children outgrow issues of attention and focus, and I have never felt fully confident in the more nuanced use of these medications with adults. Most of the patients I now treat with ADD have come to me on stable doses of the medications or at least with a history that directs care.
With others, the tip-off to look for the disorder is their disorganization in the absence of a substance use or active mood disorder. Medications help, sometimes remarkably, yet patients still struggle with organization and planning, and sometimes I find myself frustrated when patients forget their appointments or the issues around prescribing stimulants become time-consuming.
David W. Goodman, MD, director of the Adult Attention Deficit Center of Maryland, Lutherville, currently treats hundreds of patients with ADD and has written and spoken extensively about treating this disorder in adults.
“There are three things that make it difficult to manage patients with ADD,” Dr. Goodman noted, referring specifically to administrative issues. “You can’t write for refills, but with e-prescribing you can write a sequence of prescriptions with ‘fill-after’ dates. Or some patients are able to get a 90-day supply from mail-order pharmacies. Still, it’s a hassle if the patient moves around, as college students often do, and there are inventory shortages when some pharmacies can’t get the medications.”
“The second issue,” he adds, “is that it’s the nature of this disorder that patients struggle with organizational issues. Yelling at someone with ADD to pay attention is like yelling at a blind person not to run into furniture when they are in a new room. They go through life with people being impatient that they can’t do the things an ordinary person can do easily.”
Finally, Dr. Goodman noted that the clinicians who treat patients with ADD may have counter-transference issues.
“You have to understand that this is a disability and be sympathetic to it. They often have comorbid disorders, including personality disorders, and this can all bleed over to cause frustrations in their care. Psychiatrists who treat patients with ADD need to know they can deal with them compassionately.”
“I am occasionally contacted by patients who already have an ADHD diagnosis and are on stimulants, and who seem like they just want to get their prescriptions filled and aren’t interested in working on their issues,” says Douglas Beech, MD, a psychiatrist in private practice in Worthington, Ohio. “The doctor in this situation can feel like they are functioning as a sort of drug dealer. There are logistical matters that are structurally inherent in trying to assist these patients, from both a regulatory perspective and from a functional perspective. Dr. Beech feels that it’s helpful to acknowledge these issues when seeing patients with ADHD, so that he is prepared when problems do arise.
“It can almost feel cruel to charge a patient for a “no-show,” when difficulty keeping appointments may be a symptom of their illness, Dr. Beech adds. But he does believe it’s important to apply any fee policy equitably to all patients. “I don’t apply the ‘missed appointment’ policy differently to a person with an ADHD diagnosis versus any other diagnosis.” Though for their first missed appointment, he does give patients a “mulligan.”
“I don’t charge, but it puts both patient and doctor on notice,” he says.
And when his patients do miss an appointment, he offers to send a reminder for the next time, which is he says is effective. “With electronic messaging, this is a quick and easy way to prevent missed appointments and the complications that arise with prescriptions and rescheduling,” says Dr. Beech.
Dr. Goodman speaks about manging a large caseload of patients, many of whom have organizational issues.
“I have a full-time office manager who handles a lot of the logistics of scheduling and prescribing. Patients are sent multiple reminders, and I charge a nominal administrative fee if prescriptions need to be sent outside of appointments. This is not to make money, but to encourage patients to consider the administrative time.”
“I charge for appointments that are not canceled 48 hours in advance, and for patients who have missed appointments, a credit card is kept on file,” he says.
In a practice similar to Dr. Beech, Dr. Goodman notes that he shows some flexibility for new patients when they miss an appointment the first time. “By the second time, they know this is the policy. Having ADHD can be financially costly.”
He notes that about 10% of his patients, roughly one a day, cancel late or don’t show up for scheduled appointments: “We keep a waitlist, and if someone cancels before the appointment, we can often fill the time with another patient in need on our waitlist.”
Dr. Goodman noted repeatedly that the clinician needs to be able to empathize with the patient’s condition and how they suffer. “This is not something people choose to have. The trap is that people think that if you’re successful you can’t have ADHD, and that’s not true. Often people with this condition work harder, are brighter, and find ways to compensate.”
If a practice is set up to accommodate the needs of patients with attention and organizational issues, treating them can be very gratifying. In settings without administrative support, the psychiatrist needs to stay cognizant of this invisible disability and the frustration that may come with this disorder, not just for the patient, but also for the family, friends, and employers, and even for the psychiatrist.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, Baltimore. Dr. Miller has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maybe you know some of these patients: They may come late or not show up at all. They may have little to say and minimize their difficulties, often because they are ashamed of how much effort it takes to meet ordinary obligations. They may struggle to complete assignments, fail classes, or lose jobs. And being in the right place at the right time can feel monumental to them: They forget appointments, double book themselves, or sometimes sleep through important events.
It’s not just appointments. They lose their keys and valuables, forget to pay bills, and may not answer calls, texts, or emails. Their voicemail may be full and people are often frustrated with them. These are all characteristics of executive dysfunction, which together can make the routine responsibilities of life very difficult.
Treatments include stimulants, and because of their potential for abuse, these medications are more strictly regulated when it comes to prescribing. The FDA does not allow them to be phoned into a pharmacy or refills to be added to prescriptions. Patients must wait until right before they are due to run out to get the next prescription, and this can present a problem if the patient travels or takes long vacations.
And although it is not the patient’s fault that stimulants can’t be ordered with refills, this adds to the burden of treating patients who take them. It’s hard to imagine that these restrictions on stimulants and opiates (but not on benzodiazepines) do much to deter abuse or diversion.
I trained at a time when ADD and ADHD were disorders of childhood, and as an adult psychiatrist, I was not exposed to patients on these medications. Occasionally, a stimulant was prescribed in a low dose to help activate a very depressed patient, but it was thought that children outgrow issues of attention and focus, and I have never felt fully confident in the more nuanced use of these medications with adults. Most of the patients I now treat with ADD have come to me on stable doses of the medications or at least with a history that directs care.
With others, the tip-off to look for the disorder is their disorganization in the absence of a substance use or active mood disorder. Medications help, sometimes remarkably, yet patients still struggle with organization and planning, and sometimes I find myself frustrated when patients forget their appointments or the issues around prescribing stimulants become time-consuming.
David W. Goodman, MD, director of the Adult Attention Deficit Center of Maryland, Lutherville, currently treats hundreds of patients with ADD and has written and spoken extensively about treating this disorder in adults.
“There are three things that make it difficult to manage patients with ADD,” Dr. Goodman noted, referring specifically to administrative issues. “You can’t write for refills, but with e-prescribing you can write a sequence of prescriptions with ‘fill-after’ dates. Or some patients are able to get a 90-day supply from mail-order pharmacies. Still, it’s a hassle if the patient moves around, as college students often do, and there are inventory shortages when some pharmacies can’t get the medications.”
“The second issue,” he adds, “is that it’s the nature of this disorder that patients struggle with organizational issues. Yelling at someone with ADD to pay attention is like yelling at a blind person not to run into furniture when they are in a new room. They go through life with people being impatient that they can’t do the things an ordinary person can do easily.”
Finally, Dr. Goodman noted that the clinicians who treat patients with ADD may have counter-transference issues.
“You have to understand that this is a disability and be sympathetic to it. They often have comorbid disorders, including personality disorders, and this can all bleed over to cause frustrations in their care. Psychiatrists who treat patients with ADD need to know they can deal with them compassionately.”
“I am occasionally contacted by patients who already have an ADHD diagnosis and are on stimulants, and who seem like they just want to get their prescriptions filled and aren’t interested in working on their issues,” says Douglas Beech, MD, a psychiatrist in private practice in Worthington, Ohio. “The doctor in this situation can feel like they are functioning as a sort of drug dealer. There are logistical matters that are structurally inherent in trying to assist these patients, from both a regulatory perspective and from a functional perspective. Dr. Beech feels that it’s helpful to acknowledge these issues when seeing patients with ADHD, so that he is prepared when problems do arise.
“It can almost feel cruel to charge a patient for a “no-show,” when difficulty keeping appointments may be a symptom of their illness, Dr. Beech adds. But he does believe it’s important to apply any fee policy equitably to all patients. “I don’t apply the ‘missed appointment’ policy differently to a person with an ADHD diagnosis versus any other diagnosis.” Though for their first missed appointment, he does give patients a “mulligan.”
“I don’t charge, but it puts both patient and doctor on notice,” he says.
And when his patients do miss an appointment, he offers to send a reminder for the next time, which is he says is effective. “With electronic messaging, this is a quick and easy way to prevent missed appointments and the complications that arise with prescriptions and rescheduling,” says Dr. Beech.
Dr. Goodman speaks about manging a large caseload of patients, many of whom have organizational issues.
“I have a full-time office manager who handles a lot of the logistics of scheduling and prescribing. Patients are sent multiple reminders, and I charge a nominal administrative fee if prescriptions need to be sent outside of appointments. This is not to make money, but to encourage patients to consider the administrative time.”
“I charge for appointments that are not canceled 48 hours in advance, and for patients who have missed appointments, a credit card is kept on file,” he says.
In a practice similar to Dr. Beech, Dr. Goodman notes that he shows some flexibility for new patients when they miss an appointment the first time. “By the second time, they know this is the policy. Having ADHD can be financially costly.”
He notes that about 10% of his patients, roughly one a day, cancel late or don’t show up for scheduled appointments: “We keep a waitlist, and if someone cancels before the appointment, we can often fill the time with another patient in need on our waitlist.”
Dr. Goodman noted repeatedly that the clinician needs to be able to empathize with the patient’s condition and how they suffer. “This is not something people choose to have. The trap is that people think that if you’re successful you can’t have ADHD, and that’s not true. Often people with this condition work harder, are brighter, and find ways to compensate.”
If a practice is set up to accommodate the needs of patients with attention and organizational issues, treating them can be very gratifying. In settings without administrative support, the psychiatrist needs to stay cognizant of this invisible disability and the frustration that may come with this disorder, not just for the patient, but also for the family, friends, and employers, and even for the psychiatrist.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, Baltimore. Dr. Miller has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Maybe you know some of these patients: They may come late or not show up at all. They may have little to say and minimize their difficulties, often because they are ashamed of how much effort it takes to meet ordinary obligations. They may struggle to complete assignments, fail classes, or lose jobs. And being in the right place at the right time can feel monumental to them: They forget appointments, double book themselves, or sometimes sleep through important events.
It’s not just appointments. They lose their keys and valuables, forget to pay bills, and may not answer calls, texts, or emails. Their voicemail may be full and people are often frustrated with them. These are all characteristics of executive dysfunction, which together can make the routine responsibilities of life very difficult.
Treatments include stimulants, and because of their potential for abuse, these medications are more strictly regulated when it comes to prescribing. The FDA does not allow them to be phoned into a pharmacy or refills to be added to prescriptions. Patients must wait until right before they are due to run out to get the next prescription, and this can present a problem if the patient travels or takes long vacations.
And although it is not the patient’s fault that stimulants can’t be ordered with refills, this adds to the burden of treating patients who take them. It’s hard to imagine that these restrictions on stimulants and opiates (but not on benzodiazepines) do much to deter abuse or diversion.
I trained at a time when ADD and ADHD were disorders of childhood, and as an adult psychiatrist, I was not exposed to patients on these medications. Occasionally, a stimulant was prescribed in a low dose to help activate a very depressed patient, but it was thought that children outgrow issues of attention and focus, and I have never felt fully confident in the more nuanced use of these medications with adults. Most of the patients I now treat with ADD have come to me on stable doses of the medications or at least with a history that directs care.
With others, the tip-off to look for the disorder is their disorganization in the absence of a substance use or active mood disorder. Medications help, sometimes remarkably, yet patients still struggle with organization and planning, and sometimes I find myself frustrated when patients forget their appointments or the issues around prescribing stimulants become time-consuming.
David W. Goodman, MD, director of the Adult Attention Deficit Center of Maryland, Lutherville, currently treats hundreds of patients with ADD and has written and spoken extensively about treating this disorder in adults.
“There are three things that make it difficult to manage patients with ADD,” Dr. Goodman noted, referring specifically to administrative issues. “You can’t write for refills, but with e-prescribing you can write a sequence of prescriptions with ‘fill-after’ dates. Or some patients are able to get a 90-day supply from mail-order pharmacies. Still, it’s a hassle if the patient moves around, as college students often do, and there are inventory shortages when some pharmacies can’t get the medications.”
“The second issue,” he adds, “is that it’s the nature of this disorder that patients struggle with organizational issues. Yelling at someone with ADD to pay attention is like yelling at a blind person not to run into furniture when they are in a new room. They go through life with people being impatient that they can’t do the things an ordinary person can do easily.”
Finally, Dr. Goodman noted that the clinicians who treat patients with ADD may have counter-transference issues.
“You have to understand that this is a disability and be sympathetic to it. They often have comorbid disorders, including personality disorders, and this can all bleed over to cause frustrations in their care. Psychiatrists who treat patients with ADD need to know they can deal with them compassionately.”
“I am occasionally contacted by patients who already have an ADHD diagnosis and are on stimulants, and who seem like they just want to get their prescriptions filled and aren’t interested in working on their issues,” says Douglas Beech, MD, a psychiatrist in private practice in Worthington, Ohio. “The doctor in this situation can feel like they are functioning as a sort of drug dealer. There are logistical matters that are structurally inherent in trying to assist these patients, from both a regulatory perspective and from a functional perspective. Dr. Beech feels that it’s helpful to acknowledge these issues when seeing patients with ADHD, so that he is prepared when problems do arise.
“It can almost feel cruel to charge a patient for a “no-show,” when difficulty keeping appointments may be a symptom of their illness, Dr. Beech adds. But he does believe it’s important to apply any fee policy equitably to all patients. “I don’t apply the ‘missed appointment’ policy differently to a person with an ADHD diagnosis versus any other diagnosis.” Though for their first missed appointment, he does give patients a “mulligan.”
“I don’t charge, but it puts both patient and doctor on notice,” he says.
And when his patients do miss an appointment, he offers to send a reminder for the next time, which is he says is effective. “With electronic messaging, this is a quick and easy way to prevent missed appointments and the complications that arise with prescriptions and rescheduling,” says Dr. Beech.
Dr. Goodman speaks about manging a large caseload of patients, many of whom have organizational issues.
“I have a full-time office manager who handles a lot of the logistics of scheduling and prescribing. Patients are sent multiple reminders, and I charge a nominal administrative fee if prescriptions need to be sent outside of appointments. This is not to make money, but to encourage patients to consider the administrative time.”
“I charge for appointments that are not canceled 48 hours in advance, and for patients who have missed appointments, a credit card is kept on file,” he says.
In a practice similar to Dr. Beech, Dr. Goodman notes that he shows some flexibility for new patients when they miss an appointment the first time. “By the second time, they know this is the policy. Having ADHD can be financially costly.”
He notes that about 10% of his patients, roughly one a day, cancel late or don’t show up for scheduled appointments: “We keep a waitlist, and if someone cancels before the appointment, we can often fill the time with another patient in need on our waitlist.”
Dr. Goodman noted repeatedly that the clinician needs to be able to empathize with the patient’s condition and how they suffer. “This is not something people choose to have. The trap is that people think that if you’re successful you can’t have ADHD, and that’s not true. Often people with this condition work harder, are brighter, and find ways to compensate.”
If a practice is set up to accommodate the needs of patients with attention and organizational issues, treating them can be very gratifying. In settings without administrative support, the psychiatrist needs to stay cognizant of this invisible disability and the frustration that may come with this disorder, not just for the patient, but also for the family, friends, and employers, and even for the psychiatrist.
Dr. Dinah Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is assistant professor of psychiatry and behavioral sciences at Johns Hopkins, Baltimore. Dr. Miller has no conflicts of interest.
A version of this article first appeared on Medscape.com.
Student loan forgiveness plans exclude physicians
In the run up to the midterm elections in November, President Biden has warmed to student loan forgiveness. However, before even being proposed,
What was the plan?
During the 2020 election, student loan forgiveness was a hot topic as the COVID epidemic raged. The CARES Act has placed all federal student loans in forbearance, with no payments made and the interest rate set to 0% to prevent further accrual. While this was tremendously useful to 45 million borrowers around the country (including the author), nothing material was done to deal with the loans.
The Biden Administration’s approach at that time was multi-tiered and chaotic. Plans were put forward that either expanded Public Service Loan Forgiveness (PSLF) or capped it. Plans were put forward that either extended free undergraduate or severely limited it through Pell Grants. Unfortunately, that duality continues today, with current plans not having a clear goal or a target group of beneficiaries.
Necessary CARES Act extensions
The Biden Administration has attempted repeatedly to turn the student loan apparatus back on, restarting payments en masse. However, each time, they are beset by challenges, ranging from repeat COVID spikes to servicer withdrawals or macroeconomic indicators of a recession.
At each step, the administration has had little choice but to extend the CARES Act forbearance, lest they suffer retribution for hastily resuming payments for 45 million borrowers without the apparatus to do so. Two years ago, the major federal servicers laid off hundreds, if not thousands, of staffers responsible for payment processing, accounting, customer care, and taxation. Hiring, training, and staffing these positions is nontrivial.
The administration has been out of step with servicers such that three of the largest have chosen not to renew their contracts: Navient, MyFedLoan, and Granite State Management and Resources. This has left 15 million borrowers in the lurch, not knowing who their servicer is – and, even worse, losing track of qualifying payments toward programs like PSLF.
Avenues of forgiveness
There are two major pathways to forgiveness. It is widely believed that the executive branch has the authority to broadly forgive student loans under executive order and managed through the U.S. Department of Education.
The alternative is through congressional action, voting on forgiveness as an economic stimulus plan. There is little appetite in Congress for forgiveness, and prominent congresspeople like Senator Warren and Senator Schumer have both pushed the executive branch for forgiveness in recognition of this.
What has been proposed?
First, it’s important to state that as headline-grabbing as it is to see that $50,000 of forgiveness has been proposed, the reality is that President Biden has repeatedly stated that he will not be in favor of that level of forgiveness. Instead, the number most commonly being discussed is $10,000. This would represent an unprecedented amount of support, alleviating 35% of borrowers of all student debt.
The impact of proposed forgiveness plans for physicians
For the medical community, sadly, this doesn’t represent a significant amount of forgiveness. At graduation, the average MD has $203,000 in debt, and the average DO has $258,000 in debt. These numbers grow during residency for years before any meaningful payments are made.
Further weakening forgiveness plans for physicians has been two caps proposed by the administration in recent days. The first is an income cap of $125,000. While this would maintain forgiveness for nearly all residents and fellows, this would exclude nearly every practicing physician. The alternative to an income cap is specific exclusion of certain careers seen to be high-earning: doctors and lawyers.
The bottom line
Physicians are unlikely to be included in any forgiveness plans being proposed recently by the Biden Administration. If they are considered, it will be for exclusion from any forgiveness offered.
For physicians no longer eligible for PSLF, this exclusion needs to be considered in managing the student loan debt associated with becoming a doctor.
Dr. Palmer is a part-time instructor, department of pediatrics, Harvard Medical School, Boston, and staff physician, department of medical critical care, Boston Children’s Hospital. He disclosed that he serves as director for Panacea Financial.
A version of this article first appeared on Medscape.com.
In the run up to the midterm elections in November, President Biden has warmed to student loan forgiveness. However, before even being proposed,
What was the plan?
During the 2020 election, student loan forgiveness was a hot topic as the COVID epidemic raged. The CARES Act has placed all federal student loans in forbearance, with no payments made and the interest rate set to 0% to prevent further accrual. While this was tremendously useful to 45 million borrowers around the country (including the author), nothing material was done to deal with the loans.
The Biden Administration’s approach at that time was multi-tiered and chaotic. Plans were put forward that either expanded Public Service Loan Forgiveness (PSLF) or capped it. Plans were put forward that either extended free undergraduate or severely limited it through Pell Grants. Unfortunately, that duality continues today, with current plans not having a clear goal or a target group of beneficiaries.
Necessary CARES Act extensions
The Biden Administration has attempted repeatedly to turn the student loan apparatus back on, restarting payments en masse. However, each time, they are beset by challenges, ranging from repeat COVID spikes to servicer withdrawals or macroeconomic indicators of a recession.
At each step, the administration has had little choice but to extend the CARES Act forbearance, lest they suffer retribution for hastily resuming payments for 45 million borrowers without the apparatus to do so. Two years ago, the major federal servicers laid off hundreds, if not thousands, of staffers responsible for payment processing, accounting, customer care, and taxation. Hiring, training, and staffing these positions is nontrivial.
The administration has been out of step with servicers such that three of the largest have chosen not to renew their contracts: Navient, MyFedLoan, and Granite State Management and Resources. This has left 15 million borrowers in the lurch, not knowing who their servicer is – and, even worse, losing track of qualifying payments toward programs like PSLF.
Avenues of forgiveness
There are two major pathways to forgiveness. It is widely believed that the executive branch has the authority to broadly forgive student loans under executive order and managed through the U.S. Department of Education.
The alternative is through congressional action, voting on forgiveness as an economic stimulus plan. There is little appetite in Congress for forgiveness, and prominent congresspeople like Senator Warren and Senator Schumer have both pushed the executive branch for forgiveness in recognition of this.
What has been proposed?
First, it’s important to state that as headline-grabbing as it is to see that $50,000 of forgiveness has been proposed, the reality is that President Biden has repeatedly stated that he will not be in favor of that level of forgiveness. Instead, the number most commonly being discussed is $10,000. This would represent an unprecedented amount of support, alleviating 35% of borrowers of all student debt.
The impact of proposed forgiveness plans for physicians
For the medical community, sadly, this doesn’t represent a significant amount of forgiveness. At graduation, the average MD has $203,000 in debt, and the average DO has $258,000 in debt. These numbers grow during residency for years before any meaningful payments are made.
Further weakening forgiveness plans for physicians has been two caps proposed by the administration in recent days. The first is an income cap of $125,000. While this would maintain forgiveness for nearly all residents and fellows, this would exclude nearly every practicing physician. The alternative to an income cap is specific exclusion of certain careers seen to be high-earning: doctors and lawyers.
The bottom line
Physicians are unlikely to be included in any forgiveness plans being proposed recently by the Biden Administration. If they are considered, it will be for exclusion from any forgiveness offered.
For physicians no longer eligible for PSLF, this exclusion needs to be considered in managing the student loan debt associated with becoming a doctor.
Dr. Palmer is a part-time instructor, department of pediatrics, Harvard Medical School, Boston, and staff physician, department of medical critical care, Boston Children’s Hospital. He disclosed that he serves as director for Panacea Financial.
A version of this article first appeared on Medscape.com.
In the run up to the midterm elections in November, President Biden has warmed to student loan forgiveness. However, before even being proposed,
What was the plan?
During the 2020 election, student loan forgiveness was a hot topic as the COVID epidemic raged. The CARES Act has placed all federal student loans in forbearance, with no payments made and the interest rate set to 0% to prevent further accrual. While this was tremendously useful to 45 million borrowers around the country (including the author), nothing material was done to deal with the loans.
The Biden Administration’s approach at that time was multi-tiered and chaotic. Plans were put forward that either expanded Public Service Loan Forgiveness (PSLF) or capped it. Plans were put forward that either extended free undergraduate or severely limited it through Pell Grants. Unfortunately, that duality continues today, with current plans not having a clear goal or a target group of beneficiaries.
Necessary CARES Act extensions
The Biden Administration has attempted repeatedly to turn the student loan apparatus back on, restarting payments en masse. However, each time, they are beset by challenges, ranging from repeat COVID spikes to servicer withdrawals or macroeconomic indicators of a recession.
At each step, the administration has had little choice but to extend the CARES Act forbearance, lest they suffer retribution for hastily resuming payments for 45 million borrowers without the apparatus to do so. Two years ago, the major federal servicers laid off hundreds, if not thousands, of staffers responsible for payment processing, accounting, customer care, and taxation. Hiring, training, and staffing these positions is nontrivial.
The administration has been out of step with servicers such that three of the largest have chosen not to renew their contracts: Navient, MyFedLoan, and Granite State Management and Resources. This has left 15 million borrowers in the lurch, not knowing who their servicer is – and, even worse, losing track of qualifying payments toward programs like PSLF.
Avenues of forgiveness
There are two major pathways to forgiveness. It is widely believed that the executive branch has the authority to broadly forgive student loans under executive order and managed through the U.S. Department of Education.
The alternative is through congressional action, voting on forgiveness as an economic stimulus plan. There is little appetite in Congress for forgiveness, and prominent congresspeople like Senator Warren and Senator Schumer have both pushed the executive branch for forgiveness in recognition of this.
What has been proposed?
First, it’s important to state that as headline-grabbing as it is to see that $50,000 of forgiveness has been proposed, the reality is that President Biden has repeatedly stated that he will not be in favor of that level of forgiveness. Instead, the number most commonly being discussed is $10,000. This would represent an unprecedented amount of support, alleviating 35% of borrowers of all student debt.
The impact of proposed forgiveness plans for physicians
For the medical community, sadly, this doesn’t represent a significant amount of forgiveness. At graduation, the average MD has $203,000 in debt, and the average DO has $258,000 in debt. These numbers grow during residency for years before any meaningful payments are made.
Further weakening forgiveness plans for physicians has been two caps proposed by the administration in recent days. The first is an income cap of $125,000. While this would maintain forgiveness for nearly all residents and fellows, this would exclude nearly every practicing physician. The alternative to an income cap is specific exclusion of certain careers seen to be high-earning: doctors and lawyers.
The bottom line
Physicians are unlikely to be included in any forgiveness plans being proposed recently by the Biden Administration. If they are considered, it will be for exclusion from any forgiveness offered.
For physicians no longer eligible for PSLF, this exclusion needs to be considered in managing the student loan debt associated with becoming a doctor.
Dr. Palmer is a part-time instructor, department of pediatrics, Harvard Medical School, Boston, and staff physician, department of medical critical care, Boston Children’s Hospital. He disclosed that he serves as director for Panacea Financial.
A version of this article first appeared on Medscape.com.
More practice merger options
The continuing than larger ones. While there are some smaller offices offering unique services that may be able to remain small, most small general practices will be forced to at least consider a larger alternative. Recently, I discussed one option – merging individual practices into a larger one – but others are available.
One alternate strategy is to form a cooperative group. If you look around your area of practice, you will likely find other small practices in similar situations that might be willing to collaborate with you for the purpose of pooling your billing and purchasing resources. This allows each participant to maintain independence, yet share office overhead expenses and employee salaries for mutual benefit. If that arrangement works, and remains satisfactory for all participants, you can consider expanding your sharing of expenditures, such as collective purchasing of supplies and equipment, and centralizing appointment scheduling. Such an arrangement might be particularly attractive to physicians in later stages of their careers who need to alleviate financial burdens but don’t wish to close up shop just yet.
After more time has passed, if everyone remains happy with the arrangement, an outright merger can be considered, allowing the group to negotiate higher insurance remunerations and even lower overhead costs. Obviously, projects of this size and scope require careful planning and implementation, and should not be undertaken without the help of competent legal counsel and an experienced business consultant.
Another option is to join an independent practice association (IPA), if one is operating in your area. IPAs are physician-directed legal entities, formed to provide the same advantages enjoyed by large group practices while allowing individual members to remain independent. IPAs have greater purchasing power, allowing members to cut costs on medical and office supplies. They can also negotiate more favorable contracts with insurance companies and other payers.
Before joining such an organization, examine its legal status carefully. Some IPAs have been charged with antitrust violations because their member practices are, in reality, competitors. Make certain that any IPA you consider joining abides by antitrust and price fixing laws. Look carefully at its financial solvency as well, as IPAs have also been known to fail, leaving former members to pick up the tab.
An alternative to the IPA is the accountable care organization (ACO), a relatively new entity created as part of the Affordable Care Act. Like an IPA, an ACO’s basic purpose is to limit unnecessary spending; but ACOs are typically limited to Medicare and Medicaid recipients, and involve a larger network of doctors and hospitals sharing financial and medical responsibility for patient care. Criteria for limits on spending are established by the Centers for Medicare & Medicaid Services (CMS).
ACOs offer financial incentives to cooperate, and to save money by avoiding unnecessary tests and procedures. A key component is the sharing of information. Providers who save money while also meeting quality targets are theoretically entitled to a portion of the savings. According to federal data, ACOs saved Medicare $4.1 billion in 2020). As of January 2022, 483 ACOs were participating in the Medicare Shared Savings Program. A similar entity designed for private-sector patients is the clinically integrated network (CIN), created by the Federal Trade Commission to serve the commercial or self-insured market, while ACOs treat Medicare and Medicaid patients. Like ACOs, the idea is to work together to improve care and reduce costs by sharing records and tracking data.
When joining any group, read the agreement carefully for any clauses that might infringe on your clinical judgment. In particular, be sure that there are no restrictions on patient treatment or physician referral options for your patients. You should also negotiate an escape clause, allowing you to opt out if you become unhappy with the arrangement.
Clearly, the price of remaining autonomous is significant, and many private practitioners are unwilling to pay it. In 2019, the American Medical Association reported that for the first time, there were fewer physician owners (45.9%) than employees (47.4%).
But as I have written many times, those of us who remain committed to independence will find ways to preserve it. In medicine, as in life, those most responsive to change will survive and flourish.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The continuing than larger ones. While there are some smaller offices offering unique services that may be able to remain small, most small general practices will be forced to at least consider a larger alternative. Recently, I discussed one option – merging individual practices into a larger one – but others are available.
One alternate strategy is to form a cooperative group. If you look around your area of practice, you will likely find other small practices in similar situations that might be willing to collaborate with you for the purpose of pooling your billing and purchasing resources. This allows each participant to maintain independence, yet share office overhead expenses and employee salaries for mutual benefit. If that arrangement works, and remains satisfactory for all participants, you can consider expanding your sharing of expenditures, such as collective purchasing of supplies and equipment, and centralizing appointment scheduling. Such an arrangement might be particularly attractive to physicians in later stages of their careers who need to alleviate financial burdens but don’t wish to close up shop just yet.
After more time has passed, if everyone remains happy with the arrangement, an outright merger can be considered, allowing the group to negotiate higher insurance remunerations and even lower overhead costs. Obviously, projects of this size and scope require careful planning and implementation, and should not be undertaken without the help of competent legal counsel and an experienced business consultant.
Another option is to join an independent practice association (IPA), if one is operating in your area. IPAs are physician-directed legal entities, formed to provide the same advantages enjoyed by large group practices while allowing individual members to remain independent. IPAs have greater purchasing power, allowing members to cut costs on medical and office supplies. They can also negotiate more favorable contracts with insurance companies and other payers.
Before joining such an organization, examine its legal status carefully. Some IPAs have been charged with antitrust violations because their member practices are, in reality, competitors. Make certain that any IPA you consider joining abides by antitrust and price fixing laws. Look carefully at its financial solvency as well, as IPAs have also been known to fail, leaving former members to pick up the tab.
An alternative to the IPA is the accountable care organization (ACO), a relatively new entity created as part of the Affordable Care Act. Like an IPA, an ACO’s basic purpose is to limit unnecessary spending; but ACOs are typically limited to Medicare and Medicaid recipients, and involve a larger network of doctors and hospitals sharing financial and medical responsibility for patient care. Criteria for limits on spending are established by the Centers for Medicare & Medicaid Services (CMS).
ACOs offer financial incentives to cooperate, and to save money by avoiding unnecessary tests and procedures. A key component is the sharing of information. Providers who save money while also meeting quality targets are theoretically entitled to a portion of the savings. According to federal data, ACOs saved Medicare $4.1 billion in 2020). As of January 2022, 483 ACOs were participating in the Medicare Shared Savings Program. A similar entity designed for private-sector patients is the clinically integrated network (CIN), created by the Federal Trade Commission to serve the commercial or self-insured market, while ACOs treat Medicare and Medicaid patients. Like ACOs, the idea is to work together to improve care and reduce costs by sharing records and tracking data.
When joining any group, read the agreement carefully for any clauses that might infringe on your clinical judgment. In particular, be sure that there are no restrictions on patient treatment or physician referral options for your patients. You should also negotiate an escape clause, allowing you to opt out if you become unhappy with the arrangement.
Clearly, the price of remaining autonomous is significant, and many private practitioners are unwilling to pay it. In 2019, the American Medical Association reported that for the first time, there were fewer physician owners (45.9%) than employees (47.4%).
But as I have written many times, those of us who remain committed to independence will find ways to preserve it. In medicine, as in life, those most responsive to change will survive and flourish.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The continuing than larger ones. While there are some smaller offices offering unique services that may be able to remain small, most small general practices will be forced to at least consider a larger alternative. Recently, I discussed one option – merging individual practices into a larger one – but others are available.
One alternate strategy is to form a cooperative group. If you look around your area of practice, you will likely find other small practices in similar situations that might be willing to collaborate with you for the purpose of pooling your billing and purchasing resources. This allows each participant to maintain independence, yet share office overhead expenses and employee salaries for mutual benefit. If that arrangement works, and remains satisfactory for all participants, you can consider expanding your sharing of expenditures, such as collective purchasing of supplies and equipment, and centralizing appointment scheduling. Such an arrangement might be particularly attractive to physicians in later stages of their careers who need to alleviate financial burdens but don’t wish to close up shop just yet.
After more time has passed, if everyone remains happy with the arrangement, an outright merger can be considered, allowing the group to negotiate higher insurance remunerations and even lower overhead costs. Obviously, projects of this size and scope require careful planning and implementation, and should not be undertaken without the help of competent legal counsel and an experienced business consultant.
Another option is to join an independent practice association (IPA), if one is operating in your area. IPAs are physician-directed legal entities, formed to provide the same advantages enjoyed by large group practices while allowing individual members to remain independent. IPAs have greater purchasing power, allowing members to cut costs on medical and office supplies. They can also negotiate more favorable contracts with insurance companies and other payers.
Before joining such an organization, examine its legal status carefully. Some IPAs have been charged with antitrust violations because their member practices are, in reality, competitors. Make certain that any IPA you consider joining abides by antitrust and price fixing laws. Look carefully at its financial solvency as well, as IPAs have also been known to fail, leaving former members to pick up the tab.
An alternative to the IPA is the accountable care organization (ACO), a relatively new entity created as part of the Affordable Care Act. Like an IPA, an ACO’s basic purpose is to limit unnecessary spending; but ACOs are typically limited to Medicare and Medicaid recipients, and involve a larger network of doctors and hospitals sharing financial and medical responsibility for patient care. Criteria for limits on spending are established by the Centers for Medicare & Medicaid Services (CMS).
ACOs offer financial incentives to cooperate, and to save money by avoiding unnecessary tests and procedures. A key component is the sharing of information. Providers who save money while also meeting quality targets are theoretically entitled to a portion of the savings. According to federal data, ACOs saved Medicare $4.1 billion in 2020). As of January 2022, 483 ACOs were participating in the Medicare Shared Savings Program. A similar entity designed for private-sector patients is the clinically integrated network (CIN), created by the Federal Trade Commission to serve the commercial or self-insured market, while ACOs treat Medicare and Medicaid patients. Like ACOs, the idea is to work together to improve care and reduce costs by sharing records and tracking data.
When joining any group, read the agreement carefully for any clauses that might infringe on your clinical judgment. In particular, be sure that there are no restrictions on patient treatment or physician referral options for your patients. You should also negotiate an escape clause, allowing you to opt out if you become unhappy with the arrangement.
Clearly, the price of remaining autonomous is significant, and many private practitioners are unwilling to pay it. In 2019, the American Medical Association reported that for the first time, there were fewer physician owners (45.9%) than employees (47.4%).
But as I have written many times, those of us who remain committed to independence will find ways to preserve it. In medicine, as in life, those most responsive to change will survive and flourish.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Measles outbreaks: Protecting your patients during international travel
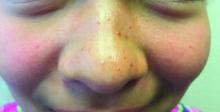
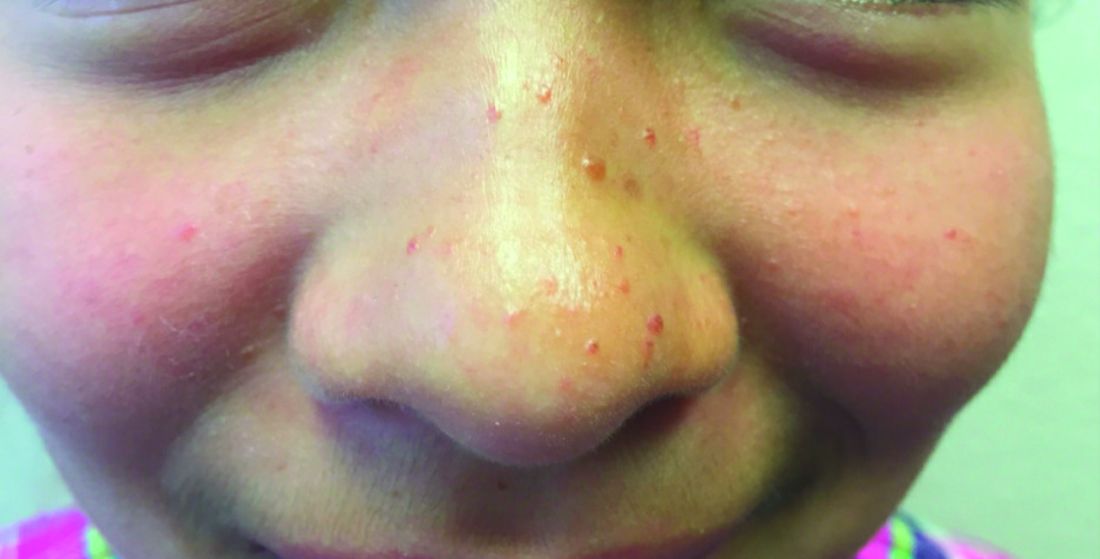
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3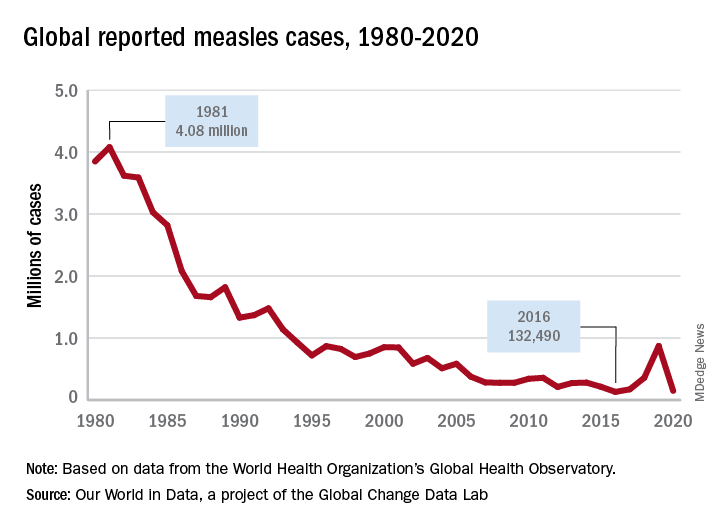
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
The U.S. immunization program is one of the best public health success stories. Physicians who provide care for children are familiar with the routine childhood immunization schedule and administer a measles-containing vaccine at age-appropriate times. Thanks to its rigorous implementation and acceptance, endemic measles (absence of continuous virus transmission for > 1 year) was eliminated in the U.S. in 2000. Loss of this status was in jeopardy in 2019 when 22 measles outbreaks occurred in 17 states (7 were multistate outbreaks). That year, 1,163 cases were reported.1 Most cases occurred in unvaccinated persons (89%) and 81 cases were imported of which 54 were in U.S. citizens returning from international travel. All outbreaks were linked to travel. Fortunately, the outbreaks were controlled prior to the elimination deadline, or the United States would have lost its measles elimination status. Restrictions on travel because of COVID-19 have relaxed significantly since the introduction of COVID-19 vaccines, resulting in increased regional and international travel. Multiple countries, including the United States noted a decline in routine immunizations rates during the last 2 years. Recent U.S. data for the 2020-2021 school year indicates that MMR immunizations rates (two doses) for kindergarteners declined to 93.9% (range 78.9% to > 98.9%), while the overall percentage of those students with an exemption remained low at 2.2%. Vaccine coverage greater than 95% was reported in only 16 states. Coverage of less than 90% was reported in seven states and the District of Columbia (Georgia, Idaho, Kentucky, Maryland, Minnesota, Ohio, and Wisconsin).2 Vaccine coverage should be 95% or higher to maintain herd immunity and control outbreaks.
Why is measles prevention so important? Many physicians practicing in the United States today have never seen a case or know its potential complications. I saw my first case as a resident in an immigrant child. It took our training director to point out the subtle signs and symptoms. It was the first time I saw Kolpik spots. Measles is transmitted person to person via large respiratory droplets and less often by airborne spread. It is highly contagious for susceptible individuals with an attack rate of 90%. In this case, a medical student on the team developed symptoms about 10 days later. Six years would pass before I diagnosed my next case of measles. An HIV patient acquired it after close contact with someone who was in the prodromal stage. He presented with the 3 C’s: Cough, coryza, and conjunctivitis, in addition to fever and an erythematous rash. He did not recover from complications of the disease.
Prior to the routine administration of a measles vaccine, 3-4 million cases with almost 500 deaths occurred annually in the United States. Worldwide, 35 million cases and more than 6 million deaths occurred each year. Here, most patients recover completely; however, complications including otitis media, pneumonia, croup, and encephalitis can develop. Complications commonly occur in immunocompromised individuals and young children. Groups with the highest fatality rates include children aged less than 5 years, immunocompromised persons, and pregnant women. Worldwide, fatality rates are dependent on the patients underlying nutritional and health status in addition to the quality of health care available.3
Measles vaccine was licensed in 1963 and cases began to decline (Figure 1). There was a resurgence in 1989 but it was not limited to the United States. The cause of the U.S. resurgence was multifactorial: Widespread viral transmission among unvaccinated preschool-age children residing in inner cities, outbreaks in vaccinated school-age children, outbreaks in students and personnel on college campuses, and primary vaccine failure (2%-5% of recipients failed to have an adequate response). In 1989, to help prevent future outbreaks, the United States recommended a two-dose schedule for measles and in 1993, the Vaccines for Children Program, a federally funded program, was established to improve access to vaccines for all children.
What is going on internationally?
Figure 2 lists the top 10 countries with current measles outbreaks.

Most countries on the list may not be typical travel destinations for tourists; however, they are common destinations for individuals visiting friends and relatives after immigrating to the United States. In contrast to the United States, most countries with limited resources and infrastructure have mass-vaccination campaigns to ensure vaccine administration to large segments of the population. They too have been affected by the COVID-19 pandemic. By report, at least 41 countries delayed implementation of their measles campaign in 2020 and 2021, thus, leading to the potential for even larger outbreaks.4
Progress toward the global elimination of measles is evidenced by the following: All 194 countries now include one dose of measles in their routine schedules; between 2000 and 2019 coverage of one dose of measles increased from 72% to 85% and countries with more than 90% coverage increased from 45% to 63%. Finally, the number of countries offering two doses of measles increased from 50% to 91% and vaccine coverage increased from 18% to 71% over the same time period.3
What can you do for your patients and their parents before they travel abroad?
- Inform all staff that the MMR vaccine can be administered to children as young as 6 months and at times other than those listed on the routine immunization schedule. This will help avoid parents seeking vaccine being denied an appointment.
- Children 6-11 months need 1 dose of MMR. Two additional doses will still need to be administered at the routine time.
- Children 12 months or older need 2 doses of MMR at least 4 weeks apart.
- If yellow fever vaccine is needed, coordinate administration with a travel medicine clinic since both are live vaccines and must be given on the same day.
- Any person born after 1956 should have 2 doses of MMR at least 4 weeks apart if they have no evidence of immunity.
- Encourage parents to always inform you and your staff of any international travel plans.
Moving forward, remember this increased global activity and the presence of inadequately vaccinated individuals/communities keeps the United States at continued risk for measles outbreaks. The source of the next outbreak may only be one plane ride away.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She said she had no relevant financial disclosures.
This article was updated 6/29/22.
References
1. Patel M et al. MMWR. 2019 Oct 11; 68(40):893-6.
2. Seither R et al. MMWR. 2022 Apr 22;71(16):561-8.
3. Gastañaduy PA et al. J Infect Dis. 2021 Sep 30;224(12 Suppl 2):S420-8. doi: 10.1093/infdis/jiaa793.
4. Centers for Disease Control and Prevention. Measles (Rubeola). http://www.CDC.gov/Measles.
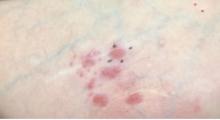
A 64-year-old woman presents with a history of asymptomatic erythematous grouped papules on the right breast
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
. Recurrences may occur. Rarely, lymph nodes, the gastrointestinal system, lung, bone and bone marrow may be involved as extracutaneous sites.
Primary cutaneous B-cell lymphomas account for approximately 25% of all cutaneous lymphomas. Clinically, patients present with either solitary or multiple papules or plaques, typically on the upper extremities or trunk.
Histopathology is vital for the correct diagnosis. In this patient, the histologic report was written as follows: “The findings are those of a well-differentiated but atypical diffuse mixed small lymphocytic infiltrate representing a mixture of T-cells and B-cells. The minor component of the infiltrate is of T-cell lineage, whereby the cells do not show any phenotypic abnormalities. The background cell population is interpreted as reactive. However, the dominant cell population is in fact of B-cell lineage. It is extensively highlighted by CD20. Only a minor component of the B cell infiltrate appeared to be in the context of representing germinal centers as characterized by small foci of centrocytic and centroblastic infiltration highlighted by BCL6 and CD10. The overwhelming B-cell component is a non–germinal center small B cell that does demonstrate BCL2 positivity and significant immunoreactivity for CD23. This small lymphocytic infiltrate obscures the germinal centers. There are only a few plasma cells; they do not show light chain restriction.”
The pathologist remarked that “this type of morphology of a diffuse small B-cell lymphocytic infiltrate that is without any evidence of light chain restriction amidst plasma cells, whereby the B cell component is dominant over the T-cell component would in fact be consistent with a unique variant of marginal zone lymphoma derived from a naive mantle zone.”
PCMZL has an excellent prognosis. When limited to the skin, local radiation or excision are effective treatments. Intravenous rituximab has been used to treat multifocal PCMZL. This patient was found to have no extracutaneous involvement and was treated with radiation.
This case and photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Virmani P et al. JAAD Case Rep. 2017 Jun 14;3(4):269-72.
Magro CM and Olson LC. Ann Diagn Pathol. 2018 Jun;34:116-21.
Move away from the screen ...
“Go outside and play!”
How often have you said that to your kids (or grandkids)? For that matter, how often did you hear it when you were a kid?
A lot, if memory serves me correctly. Some of it was just my mom wanting me out of the house, some of it an innate realization on her part that too much time spent planted in front of the TV was bad for you. (When I was a kid, Brady Bunch reruns kicked off my summer day at 8:00 a.m.).
The idea that too much time in front of a screen can be bad is nothing new. Regrettably, some of this ancient wisdom has been lost in the eons since I was a kid.
Does this surprise you?
Humans, like all primates, are a social species. We’ve benefited from the combined power of our minds to leave caves, harness nature, and build civilizations. But this has a cost, and perhaps the social media screen has been a tipping point for mental health.
I’m not knocking the basic idea. Share a joke with a friend, see pictures of the new baby, hear out about a new job. That’s fine. The trouble is that it’s gone beyond that. A lot of it is perfectly innocuous ... but a lot isn’t.
As it’s evolved, social media has also become the home of anger. Political and otherwise. It’s so much easier to post memes making fun of other people and their viewpoints than to speak to them in person. Trolls and bots lurk everywhere to get you riled up – things you wouldn’t be encountering if you were talking to your neighbor at the fence or a friend on the phone.
Recent trends on TikTok included students bragging about things they’d stolen from their high schools and people boasting of having “ripped off” Six Flags amusement parks with an annual membership loophole (the latter resulted in park management canceling the plan). How do such things benefit anyone (beyond those posting them getting clicks)?
I’m pretty sure they do nothing to make you feel good, or happy, or positive in any way. And that’s not even counting the political nastiness, cheap shots, and conspiracy theories that drown out rational thought.
Unfortunately, social media in today’s forms is addictive. Seeing one good thing from a friend gives you a dopamine boost, and this drives you to overlook all the bad things the screen does. Like the meth addict who lives for the high, and ignores all the negative aspects – loss of money, family, a home, teeth – that it brings.
So it’s not a surprise that walking away from it for a week made people happier and gave them time to do things that were more important than staring at a screen. Though I do wonder how many of the subjects ended up going back to it, forgetting about the benefits they’d just experienced.
When Frank Zappa released “I’m the Slime” in 1973, it was about television. But today the song is far closer to describing what social media has become than he could have ever imagined. (He died in 1993, never knowing how accurate he’d become).
We encourage our patients to exercise. The benefits of doing so are beyond question. But maybe it’s time to point out not only the good things that come from exercise, but also those that come from turning off the screen in order to do so.
As my mother said: “Go outside and play!”
It’s good for the body and sanity, and both are important.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“Go outside and play!”
How often have you said that to your kids (or grandkids)? For that matter, how often did you hear it when you were a kid?
A lot, if memory serves me correctly. Some of it was just my mom wanting me out of the house, some of it an innate realization on her part that too much time spent planted in front of the TV was bad for you. (When I was a kid, Brady Bunch reruns kicked off my summer day at 8:00 a.m.).
The idea that too much time in front of a screen can be bad is nothing new. Regrettably, some of this ancient wisdom has been lost in the eons since I was a kid.
Does this surprise you?
Humans, like all primates, are a social species. We’ve benefited from the combined power of our minds to leave caves, harness nature, and build civilizations. But this has a cost, and perhaps the social media screen has been a tipping point for mental health.
I’m not knocking the basic idea. Share a joke with a friend, see pictures of the new baby, hear out about a new job. That’s fine. The trouble is that it’s gone beyond that. A lot of it is perfectly innocuous ... but a lot isn’t.
As it’s evolved, social media has also become the home of anger. Political and otherwise. It’s so much easier to post memes making fun of other people and their viewpoints than to speak to them in person. Trolls and bots lurk everywhere to get you riled up – things you wouldn’t be encountering if you were talking to your neighbor at the fence or a friend on the phone.
Recent trends on TikTok included students bragging about things they’d stolen from their high schools and people boasting of having “ripped off” Six Flags amusement parks with an annual membership loophole (the latter resulted in park management canceling the plan). How do such things benefit anyone (beyond those posting them getting clicks)?
I’m pretty sure they do nothing to make you feel good, or happy, or positive in any way. And that’s not even counting the political nastiness, cheap shots, and conspiracy theories that drown out rational thought.
Unfortunately, social media in today’s forms is addictive. Seeing one good thing from a friend gives you a dopamine boost, and this drives you to overlook all the bad things the screen does. Like the meth addict who lives for the high, and ignores all the negative aspects – loss of money, family, a home, teeth – that it brings.
So it’s not a surprise that walking away from it for a week made people happier and gave them time to do things that were more important than staring at a screen. Though I do wonder how many of the subjects ended up going back to it, forgetting about the benefits they’d just experienced.
When Frank Zappa released “I’m the Slime” in 1973, it was about television. But today the song is far closer to describing what social media has become than he could have ever imagined. (He died in 1993, never knowing how accurate he’d become).
We encourage our patients to exercise. The benefits of doing so are beyond question. But maybe it’s time to point out not only the good things that come from exercise, but also those that come from turning off the screen in order to do so.
As my mother said: “Go outside and play!”
It’s good for the body and sanity, and both are important.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
“Go outside and play!”
How often have you said that to your kids (or grandkids)? For that matter, how often did you hear it when you were a kid?
A lot, if memory serves me correctly. Some of it was just my mom wanting me out of the house, some of it an innate realization on her part that too much time spent planted in front of the TV was bad for you. (When I was a kid, Brady Bunch reruns kicked off my summer day at 8:00 a.m.).
The idea that too much time in front of a screen can be bad is nothing new. Regrettably, some of this ancient wisdom has been lost in the eons since I was a kid.
Does this surprise you?
Humans, like all primates, are a social species. We’ve benefited from the combined power of our minds to leave caves, harness nature, and build civilizations. But this has a cost, and perhaps the social media screen has been a tipping point for mental health.
I’m not knocking the basic idea. Share a joke with a friend, see pictures of the new baby, hear out about a new job. That’s fine. The trouble is that it’s gone beyond that. A lot of it is perfectly innocuous ... but a lot isn’t.
As it’s evolved, social media has also become the home of anger. Political and otherwise. It’s so much easier to post memes making fun of other people and their viewpoints than to speak to them in person. Trolls and bots lurk everywhere to get you riled up – things you wouldn’t be encountering if you were talking to your neighbor at the fence or a friend on the phone.
Recent trends on TikTok included students bragging about things they’d stolen from their high schools and people boasting of having “ripped off” Six Flags amusement parks with an annual membership loophole (the latter resulted in park management canceling the plan). How do such things benefit anyone (beyond those posting them getting clicks)?
I’m pretty sure they do nothing to make you feel good, or happy, or positive in any way. And that’s not even counting the political nastiness, cheap shots, and conspiracy theories that drown out rational thought.
Unfortunately, social media in today’s forms is addictive. Seeing one good thing from a friend gives you a dopamine boost, and this drives you to overlook all the bad things the screen does. Like the meth addict who lives for the high, and ignores all the negative aspects – loss of money, family, a home, teeth – that it brings.
So it’s not a surprise that walking away from it for a week made people happier and gave them time to do things that were more important than staring at a screen. Though I do wonder how many of the subjects ended up going back to it, forgetting about the benefits they’d just experienced.
When Frank Zappa released “I’m the Slime” in 1973, it was about television. But today the song is far closer to describing what social media has become than he could have ever imagined. (He died in 1993, never knowing how accurate he’d become).
We encourage our patients to exercise. The benefits of doing so are beyond question. But maybe it’s time to point out not only the good things that come from exercise, but also those that come from turning off the screen in order to do so.
As my mother said: “Go outside and play!”
It’s good for the body and sanity, and both are important.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Nurses under fire: The stress of medical malpractice
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
Just because nurses are sued less often than doctors doesn’t mean that their actions aren’t a focus of a large number of medical malpractice lawsuits. A condition known as medical malpractice stress syndrome (MMSS) is increasingly being recognized as affecting medical professionals who are subjected to litigation.
According to a 2019 report by CRICO, the risk management arm of Harvard’s medical facilities, nursing was a “primary service” in 34% of cases with a high-severity injury and in 44% of cases that were closed with a payment. And even though nurses were named as defendants only 14% of the time, likely because many nurses don’t have their own personal malpractice coverage, their hospitals or facilities were sued in most of these cases – making the nurses important witnesses for the defense.
We have every reason to believe that things have gotten worse since the CRICO study was published. Chronic nursing shortages were exacerbated during the COVID pandemic, and we have seen a large number of nurses leave the workforce altogether. In a recent survey of nurses by Hospital IQ, 90% of respondents said they were considering leaving the nursing profession in the next year, with 71% of nurses who have more than 15 years of nursing experience thinking about leaving within the next few months.
Those remaining are faced with increased workloads and extra shifts – often mandated – and working with too little sleep. Their commitment to their mission is heroic, but they are only human; it’s hard to imagine the number of errors, the number of bad outcomes, and the number of lawsuits going anywhere but up.
And of course, the entire profession has been fixated on the recent case of the Tennessee nurse who was prosecuted criminally and convicted in connection with a fatal medication error.
These are all reasons to expect that an increasing number of nurses are going to be trying to cope with symptoms of MMSS. Too many of them will initially be viewed by lawyers or claims professionals as simply defensive, arrogant, or difficult to work with. In fact, it’s impossible to know how many cases are settled just to avoid the risk of such a “difficult client” being deposed.
These caring, hard-working, and committed individuals have had their lives shaken in ways that they never expected. Nurses with MMSS need support, but traditional psychotherapy, with a diffuse focus and long-time horizon, is not the most effective option. What’s necessary is practical support that is short term, goal oriented, and tailored to the specifics of the pending litigation process.
Most important, they need to know that they are not experiencing this alone, that MMSS is a common phenomenon, and that a productive coaching relationship can be highly effective.
When approached and supported effectively, nurses – and indeed all medical professionals – can regain their confidence and focus, continue having productive professional and personal lives, and reduce the likelihood of a downhill spiral. And it makes it more likely that they’ll remain in the profession rather than becoming just another statistic in the ever-worsening shortage of nurses in the United States.
Signs of MMSS in nurses
Mixed with their feelings of anxiety and depression, nurses with MMSS often have thoughts such as:
- Am I going to lose my license?
- Am I going to lose my job?
- Will my reputation be destroyed? Will I ever be able to work as a nurse again?
- What am I going to do for a living?
- If I lose everything, will my spouse divorce me? Will I lose my kids?
- I don’t think I did anything wrong, but what if I’m still found to be at fault?
- Did I miss something? Did I make a mistake? Was there something more that I should have done?
- What’s going to happen next? What else could go wrong?
- Are there more people out there who are going to sue me?
- Everything feels overwhelming and out of control.
- My entire identity is now in question.
- How do I get this case out of my head? I can’t focus on anything else.
- I’m developing medical problems of my own.
- I’m having difficulty focusing at work and relating to patients; how do I know who’s going to sue me next?
- I wish that I could escape it all; I feel like killing myself.
Gail Fiore is president of The Winning Focus, which works with physicians and other professionals involved in litigation who are having difficulty coping with stress, anxiety, and other emotional issues. A version of this article first appeared on Medscape.com.
How to make visits run more smoothly and be more productive
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
We all feel pressure from not having enough time to care for our patients the way we want to.
Organ recital
Some of our patients need to share an update on all their symptoms each visit, old and new, including those that are minor or possibly concerning. I have learned over the years that, for many patients, this allows them to release the worry about symptoms.
Some symptoms are so distressing and severe that symptomatic treatment is needed, but most aren’t.
I am very honest with patients when I have no idea what is causing their symptoms. I tell them, we will watch for other clues to see if the symptom needs a workup.
One thing I don’t do, and I strongly recommend against, is doing a review of systems. This leads a patient to believe you are concerned about exploring each possible symptom, ones that they didn’t even bring up! The yield is very low, and the time commitment is great.
The angry patient
Imagine a scenario when you are running 15 minutes behind and, when you step into the room, your patient is angry. You are already behind, and helping the patient navigate their anger will be part of your clinic visit.
In these situations, I always address the patient’s anger immediately. Problems with getting appointments with specialists, delays in diagnostic tests, or a broken entry to the parking garage have all been sources of my patients’ frustrations.
When we have limited time, using much of the clinic visit to process frustration leads to empty clinic visits. I listen and work to empathize with the patient, often agreeing that there are so many messed up aspects of the health care system. I do not like to use the corporate “I am sad you feel that way” response, because I feel it is not helpful. Instead, I tell them how much I want to help them today in any way I can at this visit.
The Internet sleuth
When our patients have new symptoms, some of them will go to the Internet to try to self-diagnose. Sometimes they make a correct diagnosis, but other times consider scary diagnoses we would not consider based on their symptoms and risk factors.
In these scenarios, I always ask the patient why they think their diagnosis is accurate. Their response to this question gives me insight into where their beliefs come from and helps me understand what information I need to provide.
McMullan said physicians can be defensive, collaborative, and informative when they interact with patients about information they have found on the Internet. In the first model, the physician is authoritative. The second involves working with the patient and obtaining and analyzing information. In the third model, the physician provides reputable internet sites to patients for obtaining information.
‘Oh, by the way’
Patients frequently bring up sensitive topics or complicated requests after the visit has wrapped up. Topics such as insomnia, erectile dysfunction, and anxiety are often brought up with the assumption that a quick prescription is the answer. For many years, I would add time to the appointment and try to address these issues as quickly as I could. But I invariably did a poor job at helping with these problems. Now, I offer to see the patient back soon to spend an entire visit discussing the newly brought up concern. I tell them that the problem is too important to not have my full attention and focus.
Pearls
- Empathetically listen to descriptions of symptoms, but don’t focus on fixing them.
- Empathize with the angry patient, and move on to taking care of their medical problems.
- Avoid the urge to address newly raised problems at the end of the visit.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and serves as third-year medical student clerkship director at the University of Washington. He is a member of the editorial advisory board of Internal Medicine News. Dr. Paauw has no conflicts to disclose.
A 7-year-old with red bumps on her nose
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
The finding of individual, 1- to 4-mm firm, red papules depicted in the image are consistent with facial angiofibromas, which are most commonly seen in pediatric patients as a manifestation of tuberous sclerosis (TSC). Angiofibromas, previously called adenoma sebaceum, a misnomer, are seen in TSC as smooth papules, nodules, and occasionally plaques that typically involve the malar region of the face. These lesions usually develop in childhood and adolescence and can be misdiagnosed as lesions of acne. The number of lesions tend to increase with age, though there is no significant risk of malignant transformation. Ultraviolet-induced DNA damage is thought to play a role in the development of facial angiofibromas, so sun protection is called for.1 Patients may seek treatment to minimize deformity and the stigma of angiofibromas. Recently, the mammalian target of rapamycin inhibitor sirolimus (rapamycin) topical gel received Food and Drug Administration approval for the treatment of facial angiofibromas associated with TSC in patients age at least 6 years.2
The presence of angiofibromas should prompt consideration of TSC and as such, a thorough family history, medical history, and full-body skin examination. TSC is a rare autosomal-dominant genetic disorder, caused by a pathogenic variant in either the TSC1 or TSC2 gene. This neurocutaneous disorder is characterized by the development of multiple benign hamartomas across many organ systems including the brain, eyes, heart, lung, liver, kidney, and skin. The phenotypic expression of TSC is highly variable. Besides angiofibromas, some other characteristic dermatological findings in TSC include periungual fibromas, hypopigmented macules usually elliptical in shape (known as ash-leaf spots), and irregularly shaped elevated flesh-colored fibrous tissue most often found over the lower back (known as shagreen patches).3
What is on the differential?
Agminated spitz nevi refers to multiple spitz nevi in a localized area. Spitz nevi present as a well-circumscribed, dome-shaped, pink-red or brown papules, most commonly located on the face or lower extremities.4 The finding of agminated spitz nevi is very rare and less likely for this patient given the concomitant skin findings of dental pitting, renal cysts, and cortical tubers.
Juvenile xanthogranulomas are benign,proliferations of histiocytic cells that present as reddish or yellowish-to-brown papules, plaques, or nodules that typically develop in young children around the age of 1. With time, juvenile xanthogranulomas may flatten and become more yellow.
Basal cell carcinomas present as dome-shaped lesions with centralized erosions on sun-exposed areas of the skin. They are remarkably uncommon in children but are occasionally seen in basal cell nevus syndrome (also known as nevoid basal cell carcinoma syndrome or Gorlin syndrome). Affected patients may have other findings such as developmental anomalies, bifid ribs, palmar and plantar pitting, odontogenic keratocysts, and/or medulloblastomas.5
Flat warts commonly occur in children and occur by direct skin contact with human papillomavirus. Of the various types of warts, flat warts are smaller and tend to be smooth on top. The diagnosis of cutaneous warts is based on clinical appearance, showing thrombosed capillaries underneath the overlying hyperkeratotic debris. Our patient’s history of having a common wart on her hands raises suspicion for inoculation onto her face, but the morphology, distribution, and lack of response to tretinoin makes this diagnosis much less likely.
Disease workup and course
Our patient’s physical exam revealed dental pits but no evidence of hypopigmented macules, shagreen patches, or periungual lesions. Ultrasound of the kidney displayed renal cortical cysts and brain MRI showed cortical tubers, confirming extracutaneous TSC involvement. Over time, our patient developed angiofibromas on the forehead and was ultimately started on topical sirolimus, which led to marked improvement within months.
Ms. Kleinman is a pediatric dermatology research associate, division of pediatric and adolescent dermatology, University of California, San Diego, and Rady Children’s Hospital, also in San Diego. Dr. Eichenfield is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego, and Rady Children’s Hospital. They have no relevant financial disclosures.
References
1. Tyburczy ME et al. Hum Molec Genet. 2014;23(8):2023-9.
2. Food & Drug Administration. New drug application (NDA) approval for Hyftor (sirolimus topical gel). https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/213478Orig1s000ltr.pdf.
3. Webb DW et al. Br J Dermatol. 1996;135(1):1-5.
4. Ricci F et al. Eur J Dermatol. 2017;27(1):59-62.
5. Evans DG and Farndon PA. Nevoid basal cell carcinoma syndrome, in “GeneReviews®.” Seattle: University of Washington, 2002.
A 7-year-old female presented with a bump on the bridge of her nose that was present for 10 months, with subsequent development of multiple papules on the nose and cheeks.
A 7-year-old, previously healthy female presented with a bump on the bridge of her nose that was present for 10 months, with subsequent development of multiple papules on the nose and cheeks. She has no significant medical history aside from a wart on her hand that was recently frozen with liquid nitrogen and resolved. She denied pruritus, bumps, or skin changes elsewhere on the body. The patient was prescribed tretinoin 0.1% cream applied nightly for several months without response.
President’s report
There is little I enjoy more than an opportunity to get together with old friends.
I write this missive on the return trip from a week of CHEST leadership meetings held last month, and I find myself filled with joy, awe, and great appreciation for the hard work our volunteers contribute to making the American College of Chest Physicians an extraordinarily productive and successful organization. This year’s meetings meant more than any I can ever recall from the past, in the context of a return to in-person gatherings that let our members share laughs, stories, and even a game or two of laser tag in the context of celebrating good times and friendship. And while some great works were accomplished by our committees, some of which I will enumerate below, the highlight of the week was definitely the esprit de corps that was on broad display.
Our Membership Committee meeting was led by Vice-Chair Marie Budev, DO, FCCP. While this committee is tasked with the critical duty of reviewing applications for the prestigious FCCP designation, they are just as importantly tasked with promoting membership, to our domestic and international colleagues. This is a challenging task, because different members prioritize the variety of benefits from CHEST differently; some focus on access to our educational offerings, both throughout the year and at our annual meeting, while others find greater value in the chance to network with colleagues from around the world and to participate in leadership in an international society. Making sure that we are helping our members realize these benefits, while also identifying (and potentially enhancing) those opportunities in which members are most interested is a challenging task, and I very much enjoyed watching these folks brainstorm ways that we could further increase the value of joining CHEST for current and potential future members.
The Guidelines Oversight Committee, chaired by Lisa Moores, MD, FCCP, is responsible for the oversight of CHEST’s evidence-based guidelines. As our clinical guidelines are among the most highly regarded of all of the things we publish, the members of this committee take special care to ensure that the subjects selected for review as part of the guideline process meet strict criteria. They receive dozens of proposals for new guidelines each year and carefully examine each one to identify the potential public health impact, to ensure the availability of literature in the space worthy of review, and to provide the opportunity to illuminate areas where there are significant clinical uncertainties, often due to new treatments or diagnostic tests. Watching committee members meticulously debate the merits of the many good ideas received to finalize a short list of topics for guideline development in the coming year was incredibly informative and validated my longstanding perception that our members are some of the best clinical minds in the pulmonary, critical care, and sleep fields in the world.
The Professional Standards Committee (PSC), chaired by Scott Manaker, MD, PhD, FCCP, has the important duty of developing CHEST’s conflict of interest (COI) policy, as well as reviewing all potential COI among CHEST leaders and members of our guideline panels. While this may sound a little dry, the fascinating part of this meeting was the ongoing discussion of what constitutes a meaningful COI. As one would expect, many of the best medical experts in the world have relationships with pharmaceutical and medical device companies, who often seek the counsel and participation of high-performing, high-volume clinicians for research trials. CHEST has extremely strict rules with regard to COI among its many levels of leadership, but the question of what constitutes a potentially problematic COI for the large number of folks who volunteer their time and energy to teach at one of our many courses is an interesting (albeit possibly philosophical) question. Since PSC members cannot observe every CHEST faculty interaction, we rely on our members to let us know if they perceive any potential bias in faculty teaching (and we so very much appreciate those of you who bring the extremely rare concerns to our attention!), but this is an area that we continue to watch very closely, as we continue to ensure that all CHEST education is accurate, unbiased, and the best available throughout the world.
The reformulated Council of Networks met under the leadership of Angel Coz Yataco, MD, FCCP, and Cassie Kennedy, MD, FCCP, to discuss how to best engage our members in the new structure, which comprises seven Networks and 22 component Sections. The Council’s primary charges are to develop educational content, to review project applications from Sections, and to serve as expert consultants to the President in their specific clinical domains. While the new configuration provides a significant increase in leadership positions for our members, as well as more formal opportunities to cultivate relationships across different Sections, I have received a few emails from colleagues who were concerned about elimination of certain former Networks, or the placement of a specific Section under a specific Network. Some of these concerns were discussed at the April meeting. While there will be some growing pains, listening to the thoughtful discussion that ensued validated my belief that Drs. Coz and Kennedy are the right folks to be leading the Council as it matures into this new and stronger structure.
While I also had the opportunity to hear reports from the Training and Transitions Committee, the Education Committee, and the Council of Global Governors, I wanted to briefly mention the Scientific Program Committee and its Innovations Group. While we are looking forward to seeing everyone in Nashville this October, I cannot tell you how excited I am about some of the new things we have in store for our first in-person annual meeting in 3 years. (Literally ... I am absolutely sworn to secrecy!) But under the leadership of Program Chair Subani Chandra, MD, FCCP, and my two other “Chief Fun Officers” Aneesa Das, MD, FCCP, and William Kelly, MD, FCCP, I can say that attendees are going to be in for a heck of a lot of fun. Oh, and there’s going to be some education there, also.
In closing, I want to reiterate how much of a pleasure and privilege it has been to sit in the President’s chair over the first few months of 2022. If any of the committees I’ve described above sound interesting to you, please strongly consider throwing your hat into the ring when nominations open up in the coming months. Getting involved at CHEST has been one of the best experiences of my career, and I expect you’ll feel the same way after you join in the fun. As always, I remain available to you, either by emailing me at [email protected] or messaging me on Twitter @ChestPrez. And, please come find me in Nashville in October, either to say hello, or to challenge me to a game of laser tag. ... I’m not very hard to beat.
Until next time,
David
There is little I enjoy more than an opportunity to get together with old friends.
I write this missive on the return trip from a week of CHEST leadership meetings held last month, and I find myself filled with joy, awe, and great appreciation for the hard work our volunteers contribute to making the American College of Chest Physicians an extraordinarily productive and successful organization. This year’s meetings meant more than any I can ever recall from the past, in the context of a return to in-person gatherings that let our members share laughs, stories, and even a game or two of laser tag in the context of celebrating good times and friendship. And while some great works were accomplished by our committees, some of which I will enumerate below, the highlight of the week was definitely the esprit de corps that was on broad display.
Our Membership Committee meeting was led by Vice-Chair Marie Budev, DO, FCCP. While this committee is tasked with the critical duty of reviewing applications for the prestigious FCCP designation, they are just as importantly tasked with promoting membership, to our domestic and international colleagues. This is a challenging task, because different members prioritize the variety of benefits from CHEST differently; some focus on access to our educational offerings, both throughout the year and at our annual meeting, while others find greater value in the chance to network with colleagues from around the world and to participate in leadership in an international society. Making sure that we are helping our members realize these benefits, while also identifying (and potentially enhancing) those opportunities in which members are most interested is a challenging task, and I very much enjoyed watching these folks brainstorm ways that we could further increase the value of joining CHEST for current and potential future members.
The Guidelines Oversight Committee, chaired by Lisa Moores, MD, FCCP, is responsible for the oversight of CHEST’s evidence-based guidelines. As our clinical guidelines are among the most highly regarded of all of the things we publish, the members of this committee take special care to ensure that the subjects selected for review as part of the guideline process meet strict criteria. They receive dozens of proposals for new guidelines each year and carefully examine each one to identify the potential public health impact, to ensure the availability of literature in the space worthy of review, and to provide the opportunity to illuminate areas where there are significant clinical uncertainties, often due to new treatments or diagnostic tests. Watching committee members meticulously debate the merits of the many good ideas received to finalize a short list of topics for guideline development in the coming year was incredibly informative and validated my longstanding perception that our members are some of the best clinical minds in the pulmonary, critical care, and sleep fields in the world.
The Professional Standards Committee (PSC), chaired by Scott Manaker, MD, PhD, FCCP, has the important duty of developing CHEST’s conflict of interest (COI) policy, as well as reviewing all potential COI among CHEST leaders and members of our guideline panels. While this may sound a little dry, the fascinating part of this meeting was the ongoing discussion of what constitutes a meaningful COI. As one would expect, many of the best medical experts in the world have relationships with pharmaceutical and medical device companies, who often seek the counsel and participation of high-performing, high-volume clinicians for research trials. CHEST has extremely strict rules with regard to COI among its many levels of leadership, but the question of what constitutes a potentially problematic COI for the large number of folks who volunteer their time and energy to teach at one of our many courses is an interesting (albeit possibly philosophical) question. Since PSC members cannot observe every CHEST faculty interaction, we rely on our members to let us know if they perceive any potential bias in faculty teaching (and we so very much appreciate those of you who bring the extremely rare concerns to our attention!), but this is an area that we continue to watch very closely, as we continue to ensure that all CHEST education is accurate, unbiased, and the best available throughout the world.
The reformulated Council of Networks met under the leadership of Angel Coz Yataco, MD, FCCP, and Cassie Kennedy, MD, FCCP, to discuss how to best engage our members in the new structure, which comprises seven Networks and 22 component Sections. The Council’s primary charges are to develop educational content, to review project applications from Sections, and to serve as expert consultants to the President in their specific clinical domains. While the new configuration provides a significant increase in leadership positions for our members, as well as more formal opportunities to cultivate relationships across different Sections, I have received a few emails from colleagues who were concerned about elimination of certain former Networks, or the placement of a specific Section under a specific Network. Some of these concerns were discussed at the April meeting. While there will be some growing pains, listening to the thoughtful discussion that ensued validated my belief that Drs. Coz and Kennedy are the right folks to be leading the Council as it matures into this new and stronger structure.
While I also had the opportunity to hear reports from the Training and Transitions Committee, the Education Committee, and the Council of Global Governors, I wanted to briefly mention the Scientific Program Committee and its Innovations Group. While we are looking forward to seeing everyone in Nashville this October, I cannot tell you how excited I am about some of the new things we have in store for our first in-person annual meeting in 3 years. (Literally ... I am absolutely sworn to secrecy!) But under the leadership of Program Chair Subani Chandra, MD, FCCP, and my two other “Chief Fun Officers” Aneesa Das, MD, FCCP, and William Kelly, MD, FCCP, I can say that attendees are going to be in for a heck of a lot of fun. Oh, and there’s going to be some education there, also.
In closing, I want to reiterate how much of a pleasure and privilege it has been to sit in the President’s chair over the first few months of 2022. If any of the committees I’ve described above sound interesting to you, please strongly consider throwing your hat into the ring when nominations open up in the coming months. Getting involved at CHEST has been one of the best experiences of my career, and I expect you’ll feel the same way after you join in the fun. As always, I remain available to you, either by emailing me at [email protected] or messaging me on Twitter @ChestPrez. And, please come find me in Nashville in October, either to say hello, or to challenge me to a game of laser tag. ... I’m not very hard to beat.
Until next time,
David
There is little I enjoy more than an opportunity to get together with old friends.
I write this missive on the return trip from a week of CHEST leadership meetings held last month, and I find myself filled with joy, awe, and great appreciation for the hard work our volunteers contribute to making the American College of Chest Physicians an extraordinarily productive and successful organization. This year’s meetings meant more than any I can ever recall from the past, in the context of a return to in-person gatherings that let our members share laughs, stories, and even a game or two of laser tag in the context of celebrating good times and friendship. And while some great works were accomplished by our committees, some of which I will enumerate below, the highlight of the week was definitely the esprit de corps that was on broad display.
Our Membership Committee meeting was led by Vice-Chair Marie Budev, DO, FCCP. While this committee is tasked with the critical duty of reviewing applications for the prestigious FCCP designation, they are just as importantly tasked with promoting membership, to our domestic and international colleagues. This is a challenging task, because different members prioritize the variety of benefits from CHEST differently; some focus on access to our educational offerings, both throughout the year and at our annual meeting, while others find greater value in the chance to network with colleagues from around the world and to participate in leadership in an international society. Making sure that we are helping our members realize these benefits, while also identifying (and potentially enhancing) those opportunities in which members are most interested is a challenging task, and I very much enjoyed watching these folks brainstorm ways that we could further increase the value of joining CHEST for current and potential future members.
The Guidelines Oversight Committee, chaired by Lisa Moores, MD, FCCP, is responsible for the oversight of CHEST’s evidence-based guidelines. As our clinical guidelines are among the most highly regarded of all of the things we publish, the members of this committee take special care to ensure that the subjects selected for review as part of the guideline process meet strict criteria. They receive dozens of proposals for new guidelines each year and carefully examine each one to identify the potential public health impact, to ensure the availability of literature in the space worthy of review, and to provide the opportunity to illuminate areas where there are significant clinical uncertainties, often due to new treatments or diagnostic tests. Watching committee members meticulously debate the merits of the many good ideas received to finalize a short list of topics for guideline development in the coming year was incredibly informative and validated my longstanding perception that our members are some of the best clinical minds in the pulmonary, critical care, and sleep fields in the world.
The Professional Standards Committee (PSC), chaired by Scott Manaker, MD, PhD, FCCP, has the important duty of developing CHEST’s conflict of interest (COI) policy, as well as reviewing all potential COI among CHEST leaders and members of our guideline panels. While this may sound a little dry, the fascinating part of this meeting was the ongoing discussion of what constitutes a meaningful COI. As one would expect, many of the best medical experts in the world have relationships with pharmaceutical and medical device companies, who often seek the counsel and participation of high-performing, high-volume clinicians for research trials. CHEST has extremely strict rules with regard to COI among its many levels of leadership, but the question of what constitutes a potentially problematic COI for the large number of folks who volunteer their time and energy to teach at one of our many courses is an interesting (albeit possibly philosophical) question. Since PSC members cannot observe every CHEST faculty interaction, we rely on our members to let us know if they perceive any potential bias in faculty teaching (and we so very much appreciate those of you who bring the extremely rare concerns to our attention!), but this is an area that we continue to watch very closely, as we continue to ensure that all CHEST education is accurate, unbiased, and the best available throughout the world.
The reformulated Council of Networks met under the leadership of Angel Coz Yataco, MD, FCCP, and Cassie Kennedy, MD, FCCP, to discuss how to best engage our members in the new structure, which comprises seven Networks and 22 component Sections. The Council’s primary charges are to develop educational content, to review project applications from Sections, and to serve as expert consultants to the President in their specific clinical domains. While the new configuration provides a significant increase in leadership positions for our members, as well as more formal opportunities to cultivate relationships across different Sections, I have received a few emails from colleagues who were concerned about elimination of certain former Networks, or the placement of a specific Section under a specific Network. Some of these concerns were discussed at the April meeting. While there will be some growing pains, listening to the thoughtful discussion that ensued validated my belief that Drs. Coz and Kennedy are the right folks to be leading the Council as it matures into this new and stronger structure.
While I also had the opportunity to hear reports from the Training and Transitions Committee, the Education Committee, and the Council of Global Governors, I wanted to briefly mention the Scientific Program Committee and its Innovations Group. While we are looking forward to seeing everyone in Nashville this October, I cannot tell you how excited I am about some of the new things we have in store for our first in-person annual meeting in 3 years. (Literally ... I am absolutely sworn to secrecy!) But under the leadership of Program Chair Subani Chandra, MD, FCCP, and my two other “Chief Fun Officers” Aneesa Das, MD, FCCP, and William Kelly, MD, FCCP, I can say that attendees are going to be in for a heck of a lot of fun. Oh, and there’s going to be some education there, also.
In closing, I want to reiterate how much of a pleasure and privilege it has been to sit in the President’s chair over the first few months of 2022. If any of the committees I’ve described above sound interesting to you, please strongly consider throwing your hat into the ring when nominations open up in the coming months. Getting involved at CHEST has been one of the best experiences of my career, and I expect you’ll feel the same way after you join in the fun. As always, I remain available to you, either by emailing me at [email protected] or messaging me on Twitter @ChestPrez. And, please come find me in Nashville in October, either to say hello, or to challenge me to a game of laser tag. ... I’m not very hard to beat.
Until next time,
David