User login
Growing Periumbilical Plaque: A Case of Perforating Calcific Elastosis
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.
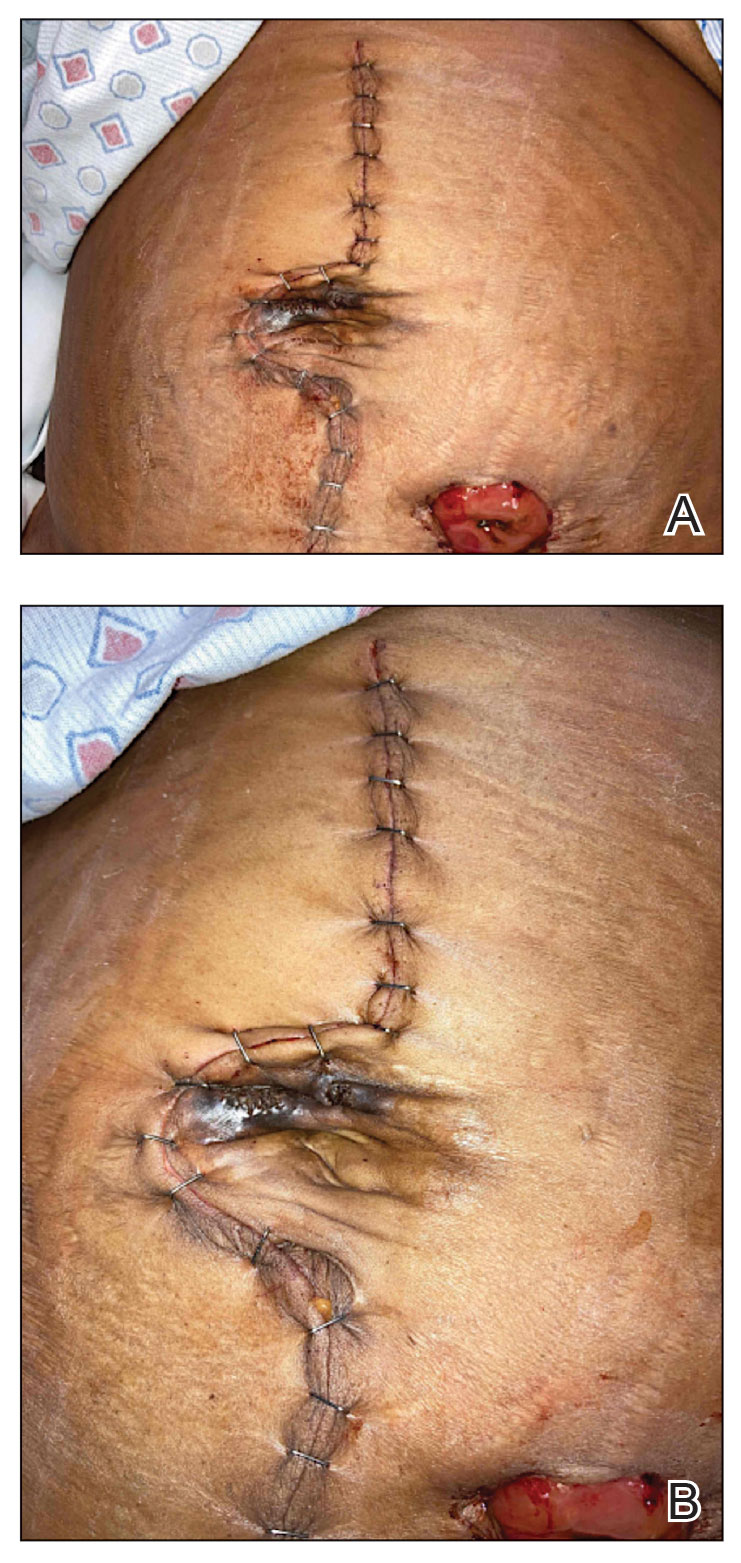
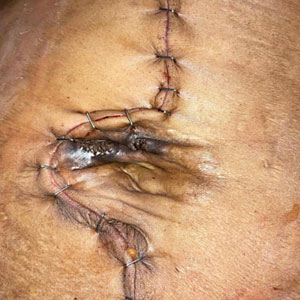
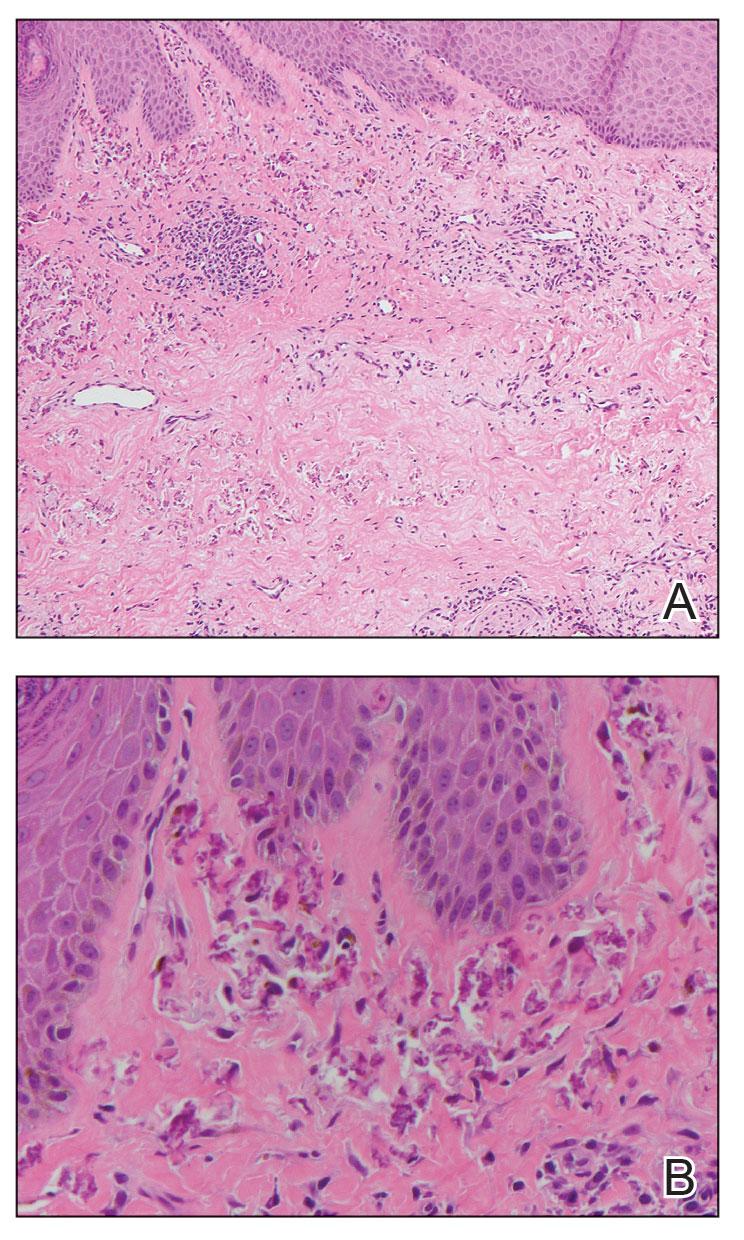
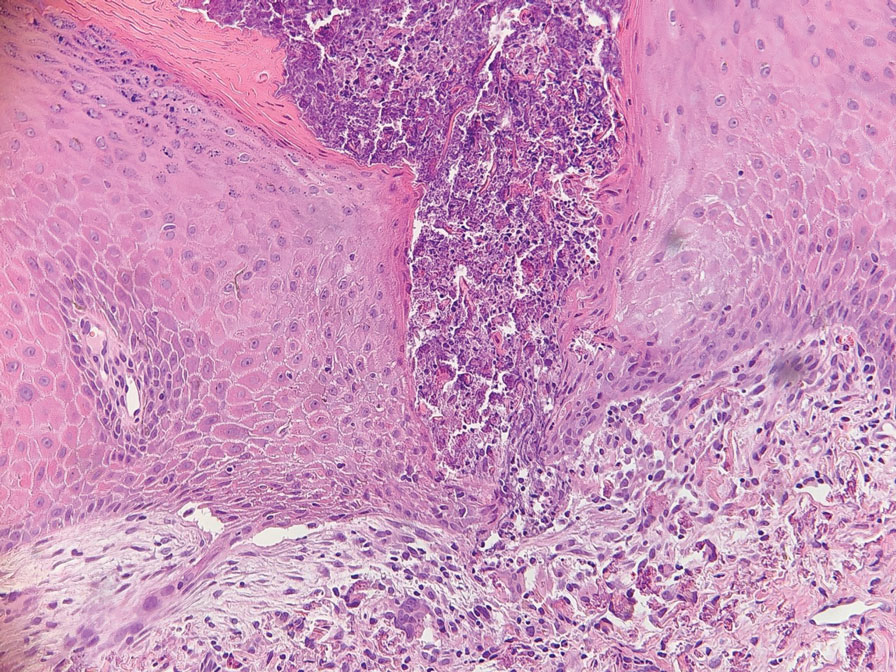
A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.
Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6
The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
To the Editor:
Pseudoxanthoma elasticum (PXE) is a genetic perforating dermatosis characterized by fragmentation and calcification of elastic fibers that most commonly manifests on the skin, eyes, gastrointestinal tract, or cardiovascular system.1 Classic skin findings include multiple symmetric yellowish papules favoring the flexural surfaces of the body and neck as well as the periumbilical and inguinal regions.1,2 Many life-threatening complications from this disease can occur due to calcification of elastic fibers in other parts of the body, such as the internal elastic lamina of arteries, which can cause gastrointestinal tract bleeding and accelerated cardiovascular disease including valvular disease.2,3 If PXE is localized to the skin only without systemic involvement or a family history, a diagnosis of perforating calcific elastosis (PCE) can be made. We report a case of PCE in a patient with a growing umbilical lesion.

A 49-year-old multiparous (gravida 3, para 3) woman presented for evaluation of an evolving periumbilical lesion of 4 months’ duration. She denied pain, bleeding, or drainage from the area, as well as any systemic symptoms. The patient had a surgical history of a laparoscopic hysterectomy 7 years prior to the current presentation due to uterine fibroids, which resulted in a periumbilical scar. At the current presentation, physical examination revealed 2 hyperpigmented to violaceous periumbilical papules coalescing into a plaque with overlying hyperkeratosis and crusting (Figure 1). A punch biopsy was performed and histopathology showed diffuse dermal collections of degenerated eosinophilic distorted elastic fibers with calcification (Figure 2). Further sections showed a transepidermal channel in which the elastic fibers extruded from the dermis through the epidermis (Figure 3). The diagnosis of acquired PCE was made based on the clinical presentation, relevant medical history, and lack of underlying medical conditions or family history of PXE. No further workup was needed, and the patient reported no further progression and rather some improvement (decrease in size) of the lesion at 3-month follow-up.

Perforating calcific elastosis (also known as periumbilical perforating PXE) is a rare acquired condition that is seen predominantly in multiparous middle-aged women.4-6 This diagnosis consists of degenerated calcified elastic fibers that may perforate the skin of the abdominal or periumbilical region. It clinically manifests as multiple painless hyperkeratotic papules surrounding the periumbilical region.4-6

The etiology and pathogenesis of PCE have not been defined but have been attributed to recurrent stressing of elastic fibers due to repeat traumas,1 which is proposed to lead to degeneration of elastic fibers and calcification of damaged tissue.4-7 As a result, PCE most commonly manifests in multiparous, obese, middle-aged women and patients with multiple abdominal surgeries or ascites.1 It also has been reported in patients with renal failure due to deposition of abnormal calcium phosphate products onto elastic fibers.4 In our patient, the development of PCE was related to both multiparity and trauma from prior surgery.
The histopathologic findings of PCE and PXE are similar, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history. Both show degenerated, fragmented, curly elastic fibers with calcium deposition throughout the dermis and a transepidermal channel extruding these elastic fibers.7,8 The biopsies stain positive for elastic fibers and calcium deposition. Calcium staining can help to differentiate these entities from elastosis perforans serpiginosa, which lacks the presence of calcium staining.7
There are no definitive treatments for PCE. A single case report of a patient with PCE and renal failure showed regression with hemodialysis.9 In a study evaluating patients with inherited PXE, notable improvement was seen in skin lesions treated with bisphosphonates, possibly suggesting that regulating serum calcium may contribute to improvement of the disease.3 Most cases spontaneously resolve with atrophic plaques. Our patient required no additional treatment with no further progression and reported improvement of the lesion with spontaneous decrease in size.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
- Jha AK, Zheeshan MD, Sinha BK, et al. Periumbilical perforating pseudoxanthoma elasticum: a rare case report. Dermatol Pract Concept. 2018;8:75-77. doi:10.5826/dpc.0802a02
- Ko JH, Shih YC, Huang YC, et al. Pseudoxanthoma elasticum. Lancet. 2013;381:565.
- Sherer DW, Singer G, Uribarri J, et al. Oral phosphate binders in the treatment of pseudoxanthoma elasticum. J Am Acad Dermatol. 2005;53:610-615.
- Lal NR, Bandyopadhyay D, Verma R, et al. Perforating calcific elastosis: revisiting a rare entity. Indian J Dermatol. 2018;63:186-188. doi:10.4103/ijd.IJD_111_17
- Kocatürk E, Kavala M, Zindanci I, et al. Periumbilical perforating pseudoxanthoma elasticum. Indian J Dermatol Venereol Leprol. 2009;75:329.
- Bressan AL, Vasconcelos BN, Silva RDS, et al. Periumbilical and periareolar perforating pseudoxanthoma elasticum. An Bras Dermatol. 2010;85:705-707. doi:10.1590/s0365-05962010000500018
- Hosen MJ, Lamoen A, De Paepe A, et al. Histopathology of pseudoxanthoma elasticum and related disorders: histological hallmarks and diagnostic clues. Scientifica (Cairo). 2012;2012:598262.
- Bathina M, Hedge SP, Shanavaz AA, et al. Pruritic periumbilical plaque as a presentation of rare perforating dermatosis. Indian Dermatol Online J. 2020;11:68-71. doi:10.4103/idoj.IDOJ_95_19
- Sapadin AN, Lebwohl MG, Teich SA, et al. Periumbilical pseudoxanthoma elasticum associated with chronic renal failure and angioid streaks—apparent regression with hemodialysis. J Am Acad Dermatol. 1998;39:338-344.
PRACTICE POINTS
- Perforating calcific elastosis (PCE) is a rare, localized, acquired variant of the inherited connective tissue disorder pseudoxanthoma elasticum (PXE).
- Histopathologic findings are identical for PCE and PXE, warranting differentiation via thorough clinical examination as well as further investigation of the patient’s medical and family history.
- Although there are no definitive treatments, most cases of PCE resolve spontaneously.
- Dermatologists should be aware of the importance of clinically differentiating PCE from PXE to prevent extensive workup, which can lead to unnecessary testing and increased morbidity in patients.
Early Treatment of Lyme Disease Prompted by Histopathologic Analysis of the Abdomen of an Engorged Tick
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.
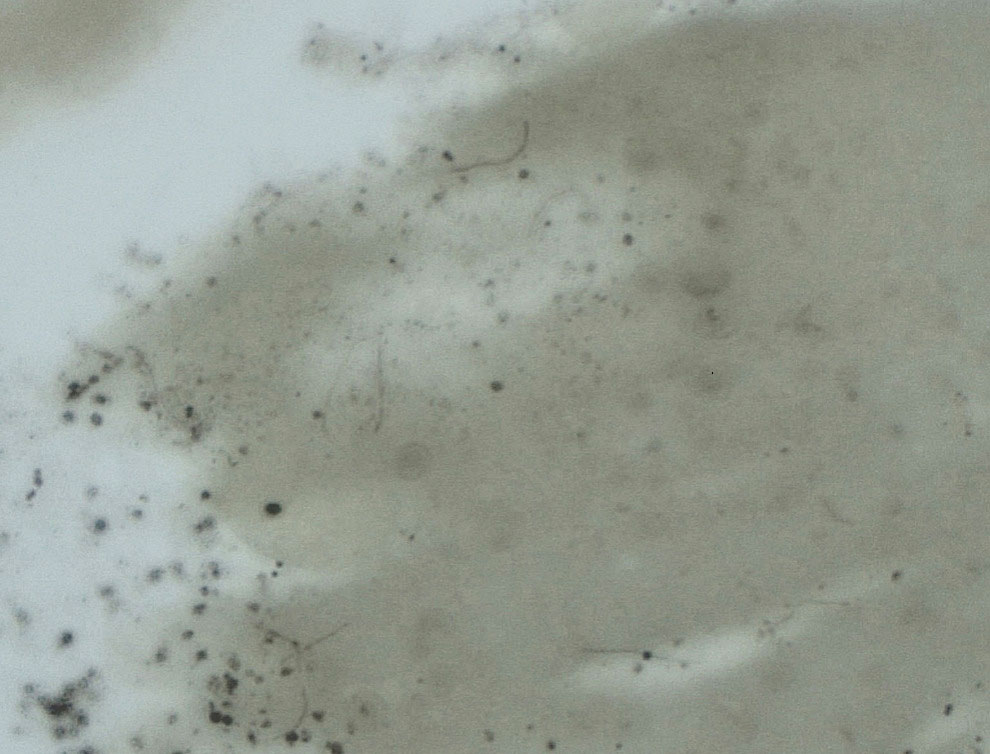
Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
To the Editor:
Lyme disease is caused by spirochetes of the Borrelia burgdorferi sensu lato species complex and transmitted to humans by the bite of the Ixodes scapularis tick. It was first classified as a nationally notifiable disease in 1991, and the incidence has risen remarkably since then.1 More than 63,000 cases are reported annually to the Centers for Disease Control and Prevention; however, this number reflects severe underreporting, as the true incidence of the disease is projected to be closer to 476,000 cases per year.2 Additionally, 95% of US cases occur in the Northeast and upper Midwest.3 Given the pervasiveness of Lyme disease, early and reliable diagnostic methodology is critical, especially in cases in which the timeline of inoculation is unclear. We present a case of Lyme disease that was discovered during a routine dermatologic visit.
A 77-year-old White man with no relevant medical history presented to a dermatology clinic in west-central Virginia for a routine skin check. Physical examination revealed a well-appearing patient without overt skin abnormalities. However, on closer evaluation, a 0.2×0.1-cm engorged black I scapularis tick was visualized on the left lateral upper back. There was a surrounding zone of erythema that measured less than the 5-cm-diameter criterion for erythema migrans.1
Upon questioning, the patient reported that he was unaware of the tick and could not provide a timeline for inoculation. To ensure proper treatment, the tick was removed in the office and a specimen was sent for histopathology. The arthropod was formalin fixed and paraffin embedded, and it was examined using hematoxylin and eosin and Warthin-Starry stains. Histopathology of the specimen revealed a blood-engorged arthropod. Warthin-Starry stain of the abdomen of the tick highlighted tiny strandlike spirochetes within the gut that were compatible with B burgdorferi (Figure). This finding prompted treatment with a 3-week course of doxycycline. Following treatment, erythema resolved. The patient experienced no sequelae.

Lyme disease can cause a range of serious complications if left untreated, including arthritis, neurologic deficits, and heart block. During the early stages of disease, the sensitivity and specificity of diagnostic methods such as serologic testing are limited.4 The gold standard for the diagnosis of Lyme disease comprises culture and subsequent confirmation by polymerase chain reaction.1 However, cultivation of B burgdorferi is challenging.5 The Centers for Disease Control and Prevention recommends 2-tiered serologic antibody analysis, which has 27% sensitivity during the first week of cutaneous symptoms, and involves an enzyme-linked immunoassay followed by reflexive immunoblotting for positive or indeterminate cases.2,6 The precision of this method is limited by several variables; for example, seroconversion fails to occur in approximately 40% of cases, even after proven exposure to the spirochete.7 Furthermore, the sensitivity of the test is particularly low during the first 4 to 6 weeks of infection—before the body mounts a proper immune response; fewer than 50% of patients exhibit a positive response to the test at initial presentation.3
Clinical diagnosis of Lyme disease is possible, though the pathognomonic erythema migrans rash can be delayed for as long as 30 days and remains absent in 20% to 30% of patients.1 Prophylactic treatment can be offered to individuals who reside in a hyperendemic area and have a rash or have had an engorged Ixodes tick attached for longer than 36 hours.8
More definitive techniques for early diagnosis are needed to enable selective and accurate treatment. The standard of care for Lyme disease includes a 10-day course of doxycycline or a 14-day course of cefuroxime axetil or amoxicillin.9 Many patients tolerate treatment and achieve resolution of disease, but antibiotics are not benign, as some patients experience drug-related adverse effects such as photosensitivity, urticaria, diarrhea, nausea, vomiting, esophagitis, hepatotoxicity, and the Jarisch-Herxheimer reaction (fever, chills, rigors, nausea and vomiting, headache, tachycardia, hypotension, hyperventilation, flushing, myalgia, and exacerbation of lesions).10,11 In a group of 123 patients with Lyme disease, 30% treated with cefuroxime axetil and 32% treated with doxycycline had 1 or more drug-related adverse events.10 Additionally, avoidable antibiotic use is associated with increasing antibiotic resistance.12 Improved diagnostic accuracy would prevent unnecessary treatment. Galan and colleagues7 reported that Warthin-Starry staining of prepared sections of the abdomen of a tick allowed for detection of B burgdorferi with a sensitivity of 71% and specificity of 83%. This technique did not delay the final biopsy report and may be a promising adjunct to the diagnosis of early Lyme disease.7
Anecdotally, many patients who present with an attached and engorged tick are unaware of the timeline of their exposure. Histologic analysis of a removed tick could aid in early clinical decision-making—ie, when the diagnosis is unclear and treatment guidelines vary by region and circumstance. Improved sensitivity and specificity of diagnosis can prevent unnecessary antibiotic treatment, which is associated with adverse effects and escalation of antibiotic resistance.
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
- Borchers AT, Keen CL, Huntley AC, et al. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82-115. doi:10.1016/j.jaut.2014.09.004
- Centers for Disease Control and Prevention. Lyme disease: data and surveillance. February 14, 2024. Accessed March 5, 2024. https://www.cdc.gov/lyme/datasurveillance/index.html
- Marques AR. Laboratory diagnosis of Lyme disease. Infect Dis Clin North Am. 2015;29:295-307. doi:10.1016/j.idc.2015.02.005
- Bratton RL, Whiteside JW, Hovan MJ, et al. Diagnosis and treatment of Lyme disease. Mayo Clin Proc. 2008;83:566-571. doi:10.4065/83.5.566
- Berger B, Johnson R, Kodner C. Cultivation of Borrelia burgdorferi from human tick bite sites: a guide to the risk of infection. J Am Acad Dermatol. 1995;32(2 pt 1):184-187. doi:10.1016/0190-9622(95)90123-x
- Branda JA, Linskey K, Kim YA, et al. Two-tiered antibody testing for Lyme disease with use of 2 enzyme immunoassays, a whole-cell sonicate enzyme immunoassay followed by a VlsE C6 peptide enzyme immunoassay. Clin Infect Dis. 2011;53:541-547. doi:10.1093/cid/cir464
- Galan A, Kupernik P, Cowper SE. Detection of Borrelia in Ixodes scapularis ticks by silver stain, immunohistochemical and direct immunofluorescent methods. J Cutan Pathol. 2018;45:473-477. doi:10.1111/cup.13143
- Nadelman RB, Nowakowski J, Fish D, et al; Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345:79-84. doi:10.1056/NEJM200107123450201
- Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis, and treatment of Lyme disease. Arthritis Rheumatol. 2021;73:12-20. doi:10.1002/art.41562
- Nadelman RB, Luger SW, Frank E, et al. Comparison of cefuroxime axetil and doxycycline in the treatment of early Lyme disease. Ann Intern Med. 1992;117:273-280. doi:10.7326/0003-4819-117-4-273
- Gresser U. Amoxicillin–clavulanic acid therapy may be associated with severe side effects—review of the literature. Eur J Med Res. 2001;6:139-149.
- Nathan C, Cars O. Antibiotic resistance—problems, progress, and prospects. N Engl J Med. 2014;371:1761-1763. doi:10.1056/NEJMp1408040
PRACTICE POINTS
- Lyme disease is increasingly common in the United States.
- Lyme disease can cause debilitating sequelae if left untreated, including arthritis, neurologic deficits, and heart block.
- Diagnostic methods for identifying early Lyme disease have limited sensitivity and specificity, necessitating alternative strategies for making an accurate diagnosis and initiating treatment.
Purpuric Eruption in a Patient With Hairy Cell Leukemia
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).
Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).

Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
The Diagnosis: Purpuric Drug Eruption
Histopathology revealed interface dermatitis, spongiosis, and a perivascular lymphocytic infiltrate with extravasated red blood cells consistent with a purpuric drug eruption. Our patient achieved remission of hairy cell leukemia after receiving only 2 of 5 expected doses of cladribine. The rash resolved completely in 3 weeks following a prednisone taper (Figure).

Hairy cell leukemia is a rare indolent lymphoproliferative disorder of B cells that accounts for approximately 2% of adult leukemias in the United States. Cladribine, a purine nucleoside analog that impairs DNA synthesis and repair, has become the mainstay of therapy, demonstrating a 95% complete response rate.1 Although few reports have addressed the cutaneous reactions seen with cladribine therapy, they can occur in more than 50% of patients.1,2 The most common skin manifestation associated with cladribine therapy is a morbilliform rash, but Stevens-Johnson syndrome and toxic epidermal necrolysis (TEN) have been reported.1
Few cases of purpuric eruption secondary to cladribine treatment have been described, and nearly all reports involve concomitant medications such as allopurinol, which our patient was taking, and antibiotics including trimethoprim-sulfamethoxazole and penicillins.1,3,4 In a cohort of 35 patients receiving cladribine,1 only concomitant treatment with cladribine and allopurinol caused cutaneous reactions, further supporting the hypothesis of cladribine-induced drug sensitivity. Allopurinol often is prescribed during induction therapy for prophylaxis against tumor lysis syndrome; similarly, antibiotics frequently are given prophylactically and therapeutically for neutropenic fever. It is believed that T-cell imbalance and profound lymphopenia induced by cladribine increase susceptibility to drug hypersensitivity reactions.1,3
The typical purpuric eruption develops within 2 days of starting cladribine therapy. Diascopy will reveal petechiae, and biopsy should be performed to rule out other serious drug-induced reactions, such as erythema multiforme, Stevens-Johnson syndrome, and TEN. A cladribine-induced purpuric eruption typically is self-resolving and carries a favorable prognosis, though high-dose corticosteroids often are prescribed to hasten recovery. The rare reports of serious cutaneous reactions secondary to cladribine therapy have been with maculopapular, not purpuric eruptions.2 Based on limited available data, cladribine-induced purpura should not be a limitation to continued treatment in patients who need it.1 Careful consideration of concomitant drug use is necessary, as the current literature demonstrates resolution of rash with withdrawal of other therapies, namely allopurinol.2-4 Future studies are needed to examine the safety of withholding offending medications and to further elucidate the mechanisms contributing to drug hypersensitivity due to cladribine.
Widespread purpura and petechiae can pose a wide differential; the patient’s recent history of cladribine administration pointed to a classic purpuric eruption. Other diagnoses such as toxic erythema of chemotherapy (TEC) and TEN are not purpuric, though plaques can be violaceous. Lack of bullae, blisters, and facial or mucosal surface involvement suggest TEN.5 Thrombotic thrombocytopenic purpura and disseminated intravascular coagulation do manifest with petechiae and purpura, though such a robust eruption in the context of recent cladribine therapy is less likely. The classic retiform purpura and necrosis were not present to suggest purpura fulminans from disseminated intravascular coagulation.
Several of the proposed diagnoses as well as a purpuric drug eruption would demonstrate extravasated red blood cells on histopathology, but the presence of interface dermatitis narrows the differential to a purpuric drug eruption. Necrotic keratinocytes and full-thickness necrosis were not present on biopsy to support a diagnosis of TEN in our patient. Characteristic features of TEC—including eccrine squamous syringometaplasia, dermal edema, and keratinocyte atypia—were not present on biopsy.6 Finally, although TEN should resolve with steroid treatment, TEC is self-limited and thrombotic thrombocytopenic purpura and disseminated intravascular coagulation would not resolve with use of steroids alone.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
- Ganzel C, Gatt ME, Maly A, et al. High incidence of skin rash in patients with hairy cell leukemia treated with cladribine. Leuk Lymphoma. 2012;53:1169-1173. doi:10.3109/10428194.2011.635864
- Chubar Y, Bennett M. Cutaneous reactions in hairy cell leukaemia treated with 2-chlorodeoxyadenosine and allopurinol. Br J Haematol. 2003;122:768-770. doi:10.1046/j.1365-2141.2003.04506.x
- Espinosa Lara P, Quirós Redondo V, Aguado Lobo M, et al. Purpuric exanthema in a patient with hairy cell leukemia treated with cladribine and allopurinol. Ann Hematol. 2017;96:1209-1210. doi:10.1007 /s00277-017-2992-z
- Hendrick A. Purpuric rash following treatment with 2-chlorodeoxyadenosine. Clin Lab Haematol. 2001;23:67-68. doi:10.1046 /j.1365-2257.2001.0346b.x
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw-Hill Education; 2019.
- Bolognia JL, Cooper DL, Glusac EJ. Toxic erythema of chemotherapy: a useful clinical term. J Am Acad Dermatol. 2008;59:524-529.
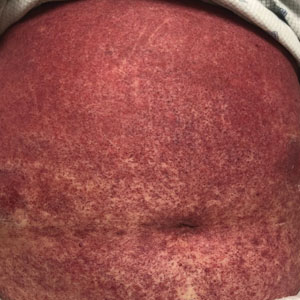
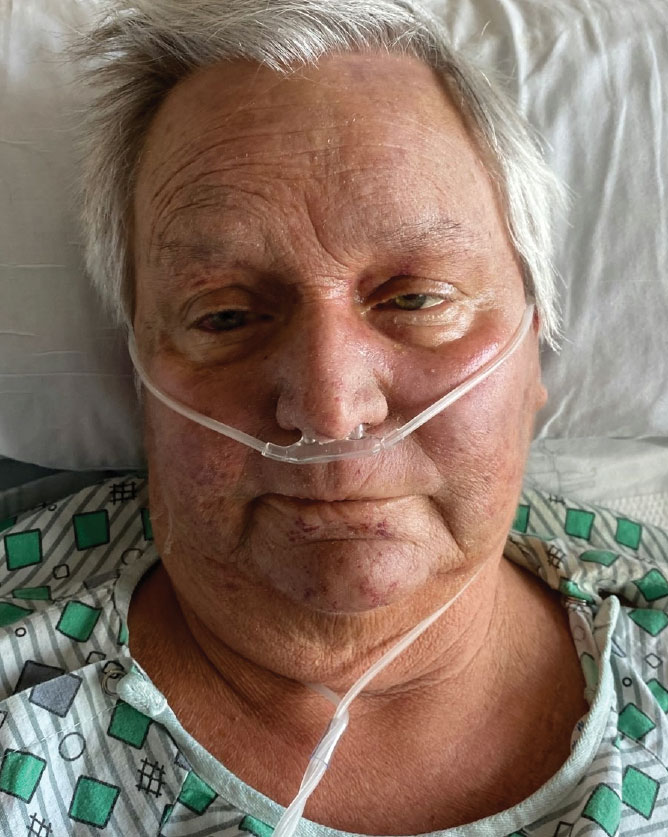
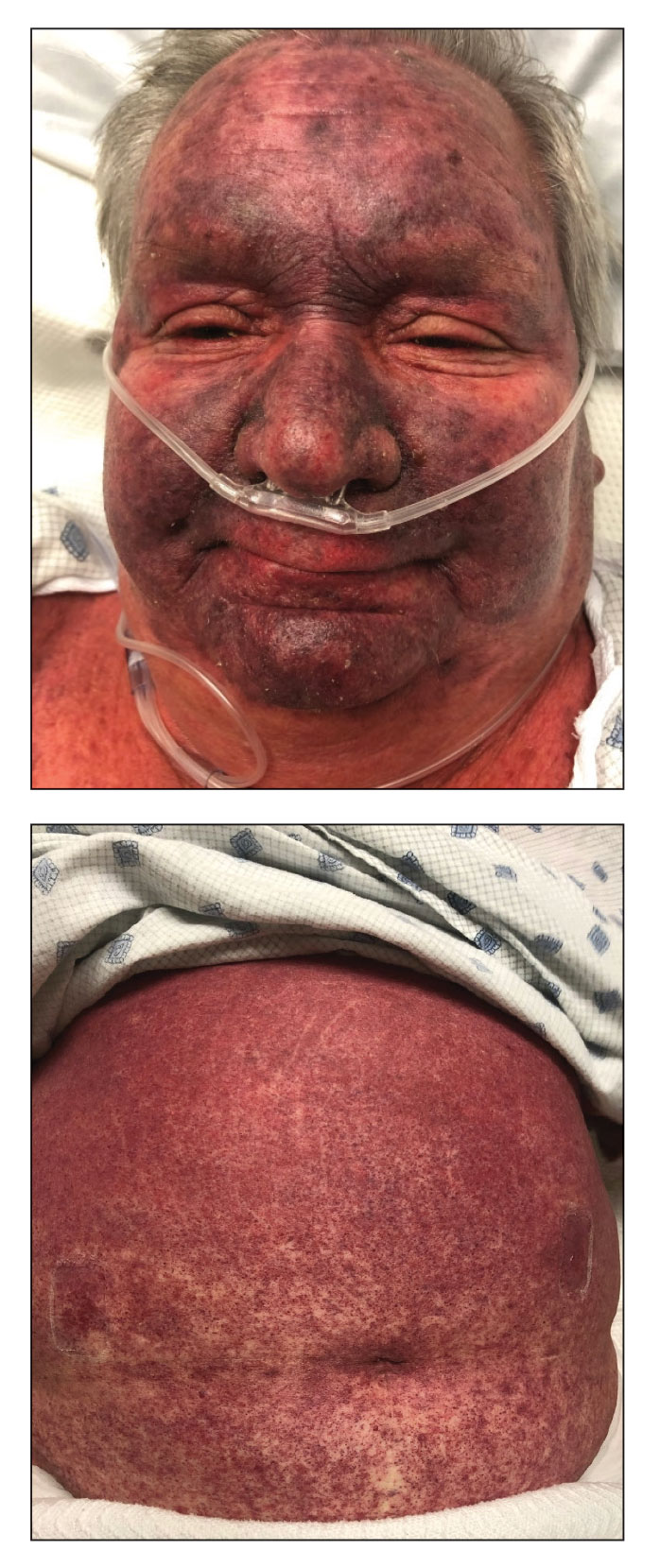
A 68-year-old woman presented to the emergency department with neutropenic fever and a rash over the body after receiving 2 doses of cladribine therapy for hairy cell leukemia. Physical examination demonstrated marked facial (top), lip, and tongue swelling, as well as a diffuse dusky nonpalpable purpuric rash on the abdomen (bottom) and back involving 90% of the body surface area. Bilateral ear edema was appreciated with accentuation of the earlobe crease. The patient exhibited subconjunctival hemorrhage, ectropion, and scleral injection. A punch biopsy of the thigh was performed.
Multiple Sclerosis Highlights From AAN 2024
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (RRMS) presented at the American Academy of Neurology (AAN) 2024 annual meeting is reported by Dr Pavan Bhargava from the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Bhargava first discusses a small study out of Germany exploring child development after exposure to monoclonal antibodies (mAbs) during breastfeeding. Currently, most mAbs are not approved for use during lactation. However, researchers found that infants studied for up to 36 months showed no evidence of adverse development or health effects compared with controls.
Next, Dr Bhargava discusses a trial examining pregnancy and infant outcomes in patients receiving ocrelizumab. They analyzed registry data of 3000 pregnancies and determined that in-utero exposure to ocrelizumab was not associated with an increased risk for adverse outcomes.
He then details a small, single-center cohort study evaluating the infection rates associated with anti-CD20 use in pediatric-onset RRMS. The study reported that approximately one third of participants experienced moderate to severe infections over 5 years of follow-up.
Finally, Dr Bhargava highlights the CHIMES trial, a 1-year analysis of efficacy and safety data from Black and Hispanic persons with RRMS who received ocrelizumab. Researchers found that the overall efficacy and safety results were similar to prior ocrelizumab clinical trials.
--
Pavan Bhargava, MD, Associate Professor, Staff Physician, Department of Neurology, Johns Hopkins Hospital, Baltimore, Maryland
Pavan Bhargava, MD, has disclosed no relevant financial relationships
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (RRMS) presented at the American Academy of Neurology (AAN) 2024 annual meeting is reported by Dr Pavan Bhargava from the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Bhargava first discusses a small study out of Germany exploring child development after exposure to monoclonal antibodies (mAbs) during breastfeeding. Currently, most mAbs are not approved for use during lactation. However, researchers found that infants studied for up to 36 months showed no evidence of adverse development or health effects compared with controls.
Next, Dr Bhargava discusses a trial examining pregnancy and infant outcomes in patients receiving ocrelizumab. They analyzed registry data of 3000 pregnancies and determined that in-utero exposure to ocrelizumab was not associated with an increased risk for adverse outcomes.
He then details a small, single-center cohort study evaluating the infection rates associated with anti-CD20 use in pediatric-onset RRMS. The study reported that approximately one third of participants experienced moderate to severe infections over 5 years of follow-up.
Finally, Dr Bhargava highlights the CHIMES trial, a 1-year analysis of efficacy and safety data from Black and Hispanic persons with RRMS who received ocrelizumab. Researchers found that the overall efficacy and safety results were similar to prior ocrelizumab clinical trials.
--
Pavan Bhargava, MD, Associate Professor, Staff Physician, Department of Neurology, Johns Hopkins Hospital, Baltimore, Maryland
Pavan Bhargava, MD, has disclosed no relevant financial relationships
The latest research on therapeutic management of patients with relapsing-remitting multiple sclerosis (RRMS) presented at the American Academy of Neurology (AAN) 2024 annual meeting is reported by Dr Pavan Bhargava from the Johns Hopkins University School of Medicine in Baltimore, Maryland.
Dr Bhargava first discusses a small study out of Germany exploring child development after exposure to monoclonal antibodies (mAbs) during breastfeeding. Currently, most mAbs are not approved for use during lactation. However, researchers found that infants studied for up to 36 months showed no evidence of adverse development or health effects compared with controls.
Next, Dr Bhargava discusses a trial examining pregnancy and infant outcomes in patients receiving ocrelizumab. They analyzed registry data of 3000 pregnancies and determined that in-utero exposure to ocrelizumab was not associated with an increased risk for adverse outcomes.
He then details a small, single-center cohort study evaluating the infection rates associated with anti-CD20 use in pediatric-onset RRMS. The study reported that approximately one third of participants experienced moderate to severe infections over 5 years of follow-up.
Finally, Dr Bhargava highlights the CHIMES trial, a 1-year analysis of efficacy and safety data from Black and Hispanic persons with RRMS who received ocrelizumab. Researchers found that the overall efficacy and safety results were similar to prior ocrelizumab clinical trials.
--
Pavan Bhargava, MD, Associate Professor, Staff Physician, Department of Neurology, Johns Hopkins Hospital, Baltimore, Maryland
Pavan Bhargava, MD, has disclosed no relevant financial relationships

Progressive Multiple Sclerosis Highlights From AAN 2024
Biomarkers indicating worsening of progressive multiple sclerosis (MS) can inform decisions about treatment, and two studies presented at the 2024 American Academy of Neurology meeting show promise in this area.
Dr Patricia Coyle of Stony Brook University Hospital in Stony Brook, New York, discusses a study showing that stool glial fibrillary acidic protein (GFAP) was markedly increased in patients with progressive MS vs those with relapsing-remitting disease or healthy controls.
A separate study using brain and cervical spine MRI showed that cervical spine gray matter atrophy, particularly at C2-3, strongly correlated with disability markers in patients with progressive MS.
Dr Coyle closes by outlining a small but important study showing that nasal foralumab dampened microglial activation and stabilized clinical progression in patients with progressive MS.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; NINDS; Sanofi Genzyme
Biomarkers indicating worsening of progressive multiple sclerosis (MS) can inform decisions about treatment, and two studies presented at the 2024 American Academy of Neurology meeting show promise in this area.
Dr Patricia Coyle of Stony Brook University Hospital in Stony Brook, New York, discusses a study showing that stool glial fibrillary acidic protein (GFAP) was markedly increased in patients with progressive MS vs those with relapsing-remitting disease or healthy controls.
A separate study using brain and cervical spine MRI showed that cervical spine gray matter atrophy, particularly at C2-3, strongly correlated with disability markers in patients with progressive MS.
Dr Coyle closes by outlining a small but important study showing that nasal foralumab dampened microglial activation and stabilized clinical progression in patients with progressive MS.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; NINDS; Sanofi Genzyme
Biomarkers indicating worsening of progressive multiple sclerosis (MS) can inform decisions about treatment, and two studies presented at the 2024 American Academy of Neurology meeting show promise in this area.
Dr Patricia Coyle of Stony Brook University Hospital in Stony Brook, New York, discusses a study showing that stool glial fibrillary acidic protein (GFAP) was markedly increased in patients with progressive MS vs those with relapsing-remitting disease or healthy controls.
A separate study using brain and cervical spine MRI showed that cervical spine gray matter atrophy, particularly at C2-3, strongly correlated with disability markers in patients with progressive MS.
Dr Coyle closes by outlining a small but important study showing that nasal foralumab dampened microglial activation and stabilized clinical progression in patients with progressive MS.
--
Patricia K. Coyle, MD, Professor and Interim Chair, Department of Neurology; Director, MS Comprehensive Care Center, Stony Brook University Hospital, Stony Brook, New York
Patricia K. Coyle, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: Accordant; Amgen; Biogen; Bristol Myers Squibb; Eli Lilly & Company; EMD Serono; GSK; Genentech; Horizon; LabCorp; Mylan; Novartis; Sanofi Genzyme; Viatris
Received research grant from: Celgene; CorEvitas LLC; Genentech/Roche; NINDS; Sanofi Genzyme

Myasthenia Gravis Highlights From AAN 2024
Highlights of the latest research on therapeutic management of patients with myasthenia gravis (MG) presented at the American Academy of Neurology (AAN) 2024 annual meeting are discussed by Dr Richard Nowak of Yale University, New Haven, Connecticut.
Dr Nowak first discusses LUMINESCE, a phase 3, randomized, double-blind study assessing the efficacy and safety of satralizumab, a humanized interleukin-6 receptor monoclonal recycling antibody. In this trial with 188 participants, satralizumab provided a statistically relevant, though modest, improvement in the Myasthenia Gravis Activities of Daily Living score.
Next, Dr Nowak details part A of ADAPT NXT, comparing a fixed- cycle dosing vs every-other-week dosing of intravenous efgartigimod. The researchers found that efgartigimod was well tolerated regardless of the regimen used, offering a way to individualize treatment for patients with MG.
He then discusses the CHAMPION MG open-label extension trial, which examined the long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG. The final analysis demonstrated the drug's durable efficacy through 164 weeks in this patient population.
Finally, Dr Nowak reports on a small trial using retrospective data determining the effectiveness of eculizumab treatment by start time. The study found that early eculizumab initiation in the first 2 years of diagnosis may offer greater clinical benefit compared with later initiation.
--
Richard J. Nowak, MD
Director, Yale Myasthenia Gravis Clinic, Associate Professor of Neurology; Division of Neuromuscular Medicine, Department of Neurology
Yale School of Medicine, New Haven, Connecticut
Richard J. Nowak, MD, has disclosed the following relevant financial relationships:
Serve(d) as a board of directors for: Myasthenia Gravis Foundation of America
Serve(d) as a consultant for: Alexion; argenx; Amgen; Janssen; Cour; UCB; Immunovant
Received research grant from: National Institutes of Health; Myasthenia Gravis Foundation of America; Alexion; argenx; Amgen; Janssen; Immunovant; UCB
Highlights of the latest research on therapeutic management of patients with myasthenia gravis (MG) presented at the American Academy of Neurology (AAN) 2024 annual meeting are discussed by Dr Richard Nowak of Yale University, New Haven, Connecticut.
Dr Nowak first discusses LUMINESCE, a phase 3, randomized, double-blind study assessing the efficacy and safety of satralizumab, a humanized interleukin-6 receptor monoclonal recycling antibody. In this trial with 188 participants, satralizumab provided a statistically relevant, though modest, improvement in the Myasthenia Gravis Activities of Daily Living score.
Next, Dr Nowak details part A of ADAPT NXT, comparing a fixed- cycle dosing vs every-other-week dosing of intravenous efgartigimod. The researchers found that efgartigimod was well tolerated regardless of the regimen used, offering a way to individualize treatment for patients with MG.
He then discusses the CHAMPION MG open-label extension trial, which examined the long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG. The final analysis demonstrated the drug's durable efficacy through 164 weeks in this patient population.
Finally, Dr Nowak reports on a small trial using retrospective data determining the effectiveness of eculizumab treatment by start time. The study found that early eculizumab initiation in the first 2 years of diagnosis may offer greater clinical benefit compared with later initiation.
--
Richard J. Nowak, MD
Director, Yale Myasthenia Gravis Clinic, Associate Professor of Neurology; Division of Neuromuscular Medicine, Department of Neurology
Yale School of Medicine, New Haven, Connecticut
Richard J. Nowak, MD, has disclosed the following relevant financial relationships:
Serve(d) as a board of directors for: Myasthenia Gravis Foundation of America
Serve(d) as a consultant for: Alexion; argenx; Amgen; Janssen; Cour; UCB; Immunovant
Received research grant from: National Institutes of Health; Myasthenia Gravis Foundation of America; Alexion; argenx; Amgen; Janssen; Immunovant; UCB
Highlights of the latest research on therapeutic management of patients with myasthenia gravis (MG) presented at the American Academy of Neurology (AAN) 2024 annual meeting are discussed by Dr Richard Nowak of Yale University, New Haven, Connecticut.
Dr Nowak first discusses LUMINESCE, a phase 3, randomized, double-blind study assessing the efficacy and safety of satralizumab, a humanized interleukin-6 receptor monoclonal recycling antibody. In this trial with 188 participants, satralizumab provided a statistically relevant, though modest, improvement in the Myasthenia Gravis Activities of Daily Living score.
Next, Dr Nowak details part A of ADAPT NXT, comparing a fixed- cycle dosing vs every-other-week dosing of intravenous efgartigimod. The researchers found that efgartigimod was well tolerated regardless of the regimen used, offering a way to individualize treatment for patients with MG.
He then discusses the CHAMPION MG open-label extension trial, which examined the long-term efficacy and safety of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG. The final analysis demonstrated the drug's durable efficacy through 164 weeks in this patient population.
Finally, Dr Nowak reports on a small trial using retrospective data determining the effectiveness of eculizumab treatment by start time. The study found that early eculizumab initiation in the first 2 years of diagnosis may offer greater clinical benefit compared with later initiation.
--
Richard J. Nowak, MD
Director, Yale Myasthenia Gravis Clinic, Associate Professor of Neurology; Division of Neuromuscular Medicine, Department of Neurology
Yale School of Medicine, New Haven, Connecticut
Richard J. Nowak, MD, has disclosed the following relevant financial relationships:
Serve(d) as a board of directors for: Myasthenia Gravis Foundation of America
Serve(d) as a consultant for: Alexion; argenx; Amgen; Janssen; Cour; UCB; Immunovant
Received research grant from: National Institutes of Health; Myasthenia Gravis Foundation of America; Alexion; argenx; Amgen; Janssen; Immunovant; UCB

Persistent Flu-Like Symptoms in a Patient With Glaucoma and Osteoporosis
A 62-year-old man presented to the emergency department (ED) with 3 days of chills, myalgias, and nausea. The patient’s oral temperature at home ranged from 99.9 to 100.1 °F. He came to the ED after multiple phone discussions with primary care nursing over 3 days. His medical history included posttraumatic stress disorder, enlarged prostate, osteoporosis, gastroesophageal reflux, glaucoma, and left eye central retinal vein occlusion. Medications included fluoxetine 20 mg twice daily, omeprazole 20 mg twice daily, rosuvastatin 10 mg once daily, tamsulosin 0.4 mg nightly, and zolpidem 10 mg nightly. The patient’s glaucoma had been treated with a dexamethasone intraocular implant about 90 days earlier. The patient started on intravenous (IV) zoledronic acid for osteoporosis, with the first infusion 5 days prior to presentation.
In the ED, the patient’s temperature was 98.2 °F, blood pressure was 156/76 mm Hg, pulse was 94 bpm, respiratory rate was 16 breaths per minute, and 98% oxygen saturation on room air. He was in no acute distress, with an unremarkable physical examination reporting no abnormal respiratory sounds, no arrhythmia, normal gait, and no focal neurologic deficits. A comprehensive metabolic panel was unremarkable, creatine phosphokinase was 155 U/L (reference range, 30-240 U/L), and the complete blood count was notable only for an elevated white blood count of 15.3 × 109/L (reference range, 4.0-11.0 × 109/L), with 73.4% neutrophils, 16.2% lymphocytes, 9.1% monocytes, 0.5% eosinophils, and 0.4% basophils. The patient’s urinalysis was unremarkable.
What is your diagnosis?
How would you treat this patient?
Discussion
The ED physician considered viral infection and tested for both influenza and COVID-19. Laboratory results eliminated urinary tract infection and rhabdomyolysis as possible diagnoses. An acute phase reaction to zoledronic acid was determined to be the most likely cause. The patient was treated with IV saline in the ED, and acetaminophen both in the ED and at home.
Although initial nursing triage notes document consideration of acute phase reaction to zoledronic acid, the endocrinology service, which had recommended and arranged the zoledronic acid infusion, was not immediately notified of the reaction. It does not appear any treatment (eg, acetaminophen) was suggested, only that the patient was given advice this may resolve over 3 to 4 days. When he was seen 2 months later for an endocrinology follow-up appointment, he reported that all symptoms (chills, myalgias, and nausea) resolved gradually over 1 week. Since then, he has felt as well as he did before taking zoledronic acid. However, the patient was wary of further zoledronic acid, opting to defer deciding on a second dose until a future appointment.
Prior to starting zoledronic acid therapy, the patient was being treated for vitamin D deficiency. Four months prior to infusion, his 25-hydroxyvitamin D level was 12.0 ng/mL (reference range, 30 to 80 ng/mL). He then started taking cholecalciferol 100 mcg (4000 IU) daily. Eight days prior to infusion his 25-hydroxyvitamin D level was 29.5 ng/mL.
Federal health care practitioners, especially those working in the Veterans Health Administration (VHA), will commonly encounter patients similar to this case. Osteoporosisis is common in the United States with > 10 million diagnoses (including > 2 million men) and in VHA primary care populations.1,2 Zoledronic acid is a frequently prescribed treatment, appearing in guidelines for osteoporosis management.3-5
The acute phase reaction is a common adverse effect of both oral and IV bisphosphonates, although it’s substantially more common with IV bisphosphonates such as zoledronic acid. This reaction is characterized by flu-like symptoms of fever, myalgia, and arthralgia that occur within the first few days following bisphosphonate administration, and tends to be rated mild to moderate by patients.6 Clinical trial data from > 7000 women with postmenopausal osteoporosis found that 42% experienced ≥ 1 acute phase symptom following the first infusion (fever was most common, followed by musculoskeletal symptoms and gastrointestinal symptoms), compared with 12% for placebo. Incidence decreases with each subsequent infusion.7 Risk factors for reactions include low 25-hydroxyvitamin D levels,8,9 no prior bisphosphonate exposure,9 younger age (aged 64-67 years vs 78-89 years),7 lower body mass index,10 and higher lymphocyte levels at baseline.11 While most cases are mild and self-limited, severe consequences have been noted, such as precipitation of adrenal crisis.12,13 Additionally, more prolonged bone pain, sometimes quite severe, has been rarely reported with bisphosphonate use. However, it’s unclear whether this represents a separate adverse effect or a more severe acute phase reaction.6
The acute phase reaction is a transient inflammatory state marked by increases in proinflammatory cytokines such as C-reactive protein, interleukin-6, and tumor necrosis factor-α. Proposed mechanisms include: (1) inhibition of farnesyl pyrophosphate synthase, an enzyme of the mevalonate pathway, resulting inactivation of γϐ T cells and increased production of proinflammatory cytokines; (2) inhibition of the suppressor of cytokine signalling-3 in the macrophages, resulting in cessation of the suppression in cytokine signaling; or (3) negative regulation of γϐ T-cell expansion and interferon-c production by low serum 25-hydroxyvitamin D concentrations.11
Prevention
Can an acute phase reaction to zoledronic acid be prevented? Bourke and colleagues reported that baseline calcium and/or vitamin D intake do not appear to affect rates of acute phase reaction in data pooled from 2 trials of zoledronic acid in postmenopausal women.14 However, patients receiving zoledronic acid had 25-hydroxyvitamin D values > 20 ng/mL 86% of the time, and values > 30 ng/mL 36% of the time. Bourke and colleagues suggest that “coadministration of calcium and vitamin D with zoledronate may not be necessary for individuals not at risk of marked vitamin D deficiency.”14 However, they did not prospectively test this hypothesis.
In our patient, vitamin D deficiency had been identified and treated, nearly achieving 30 ng/mL. The 2020 guidelines for postmenopausal osteoporosis recommend maintaining serum 25-hydroxyvitamin D levels 30 to 50 ng/mL, advising to supplement with vitamin D3 as needed.5 The 2012 guidelines for osteoporosis in men from the Endocrine Society suggest that men with low vitamin D levels receive vitamin D supplements to raise the level > 30 ng/ml.4
Oral analgesics have been studied for the prevention of adverse effects related to zoledronic acid. Initiating 650 mg acetaminophen 45 minutes before zoledronic acid infusion and then every 6 hours over the next 3 days has been shown to significantly reduce symptoms.15 Acetaminophen or ibuprofen given every 6 hours for 3 days (starting 4 hours after zoledronic acid infusion) has been shown to reduce fever and other symptoms.16
Statins have been shown in vitro to prevent bisphosphonate-induced γϐ T cell activation.17 This has led to studies with various statins, although none have yet shown benefit in vivo. A double-blind, randomized, placebo-controlled trial of postmenopausal women for fluvastatin (single dose of 40 mg or 3 doses of 40 mg, each 24 hours apart) did not prevent acute phase reaction symptoms, nor did it prevent zoledronic acid-induced cytokine release.17 Rosuvastatin 10 mg daily starting 5 days before zoledronic acid treatment and taken for a total of 11 days did not show any difference in fever or pain.18 A protocol for pravastatin has been disseminated, but no study results have been published yet.19
Prophylactic dexamethasone has also been studied. A randomized double-blind, placebo-controlled trial of oral dexamethasone 4 mg at the time of first infusion of zoledronic acid found no significant difference in temperature change or symptom score over the following 3 days.20 Chen and colleagues compared the efficacy of acetaminophen alone vs acetaminophen plus dexamethasone over several days.21 Acetaminophen 500 mg was given on the day of infusion and 4 times daily for 3 to 7 days for both groups, while dexamethasone 4 mg was given for 3 to 7 days. The dexamethasone group reported substantially lower incidence of any acute phase reaction symptoms (34% vs 67%, P = .003). A more recent study by Murdoch and colleagues comparing dexamethasone (4 mg daily for 3 days with the first dose 90 minutes before zoledronic acid infusion) with placebo found that the dexamethasone group had a statistically significant lower mean temperature change and acute phase reaction symptom score.22
Adverse Effect Treatment
Treatment after development of acute phase reaction due to zoledronic acid infusion is generally limited to supportive care and/or nonsteroidal anti-inflammatory drugs (NSAIDs) acetaminophen or dexamethasone, largely based on extrapolation of the noted preventive trials and expert opinion.3,6 Experiencing an acute phase reaction may portend better fracture risk reduction from zoledronic acid, although there is a potential association between acute phase reaction and mortality risk.23,24
Our case was typical for acute phase reaction to zoledronic acid. The patient was already taking rosuvastatin 10 mg daily for hypercholesterolemia as prescribed by his primary care physician. Rosuvastatin was not shown to prevent symptoms, although it was not studied in patients on long-term statin therapy at the time of zoledronic acid infusion.18 The patient was also taking vitamin D3 supplementation and was nearly in the reference range.5 His ED treatment included IV fluids and acetaminophen. Pretreatment (prior to or at the time of zoledronic acid infusion) with acetaminophen or ibuprofen may have prevented his symptoms, or at least lessened them to the point that an ED visit would not have resulted. The endocrinologist who prescribed the zoledronic acid documented a detailed discussion of the adverse effects of zoledronic acid with the patient, and the initial nursing call documents consideration of acute phase reaction. It is unclear whether the persistence of symptoms or worsening of symptoms ultimately led to the ED visit. Because no treatment was offered, it is unknown whether earlier posttreatment with acetaminophen, ibuprofen, or dexamethasone might have prevented his ED visit.
Conclusions
Clinicians who treat patients with osteoporosis should be aware of several key points. First, acute phase reaction symptoms are common with bisphosphonates, especially zoledronic acid infusions. Second, the symptoms are nonspecific but should have a suggestive time course. Third, dexamethasone may be partially protective, but based on the various trials discussed, it likely needs to be given for multiple days (instead of a single dose on the day of infusion). Given that acetaminophen and NSAIDs also seem to be protective (when given for multiple days starting on the day of infusion), both have lower overall adverse effect profiles than dexamethasone, consideration may be given to using either of these prophylactically.6 Dexamethasone could then be prescribed if symptoms are severe or persistent despite the use of acetaminophen or NSAIDs.
1. Choksi P, Gay BL, Reyes-Gastelum D, Haymart MR, Papaleontiou M. Understanding osteoporosis screening practices in men: a nationwide physician survey. Endocr Pract. 2020;26(11):1237-1243. doi:10.4158/EP-2020-0123
2. Yu ZL, Fisher L, Hand J. Osteoporosis screening for male veterans in a resident based primary care clinic at Northport Veterans Affairs Medical Center. Am J Med Qual. 2023;38(5):272.doi:10.1097/JMQ.0000000000000134
3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595-1622. doi:10.1210/jc.2019-00221
4. Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822. doi:10.1210/jc.2011-3045
5. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update. Endocr Pract. 2020;26(suppl 1):1-46. doi:10.4158/GL-2020-0524SUPPL
6. Lim SY, Bolster MB. What can we do about musculoskeletal pain from bisphosphonates?. Cleve Clin J Med. 2018;85(9):675-678. doi:10.3949/ccjm.85a.18005
7. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380-4387. doi:10.1210/jc.2010-0597
8. Lu K, Shi Q, Gong YQ, Li C. Association between vitamin D and zoledronate-induced acute-phase response fever risk in osteoporotic patients. Front Endocrinol (Lausanne). 2022;13:991913. Published 2022 Oct 10. doi:10.3389/fendo.2022.991913
9. Popp AW, Senn R, Curkovic I, et al. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28(6):1995-2002. doi:10.1007/s00198-017-3992-5
10. Zheng X, Ye J, Zhan Q, et al. Prediction of musculoskeletal pain after the first intravenous zoledronic acid injection in patients with primary osteoporosis: development and evaluation of a new nomogram. BMC Musculoskelet Disord. 2023;24(1):841. Published 2023 Oct 25. doi:10.1186/s12891-023-06965-y
11. Anastasilakis AD, Polyzos SA, Delaroudis S, et al. The role of cytokines and adipocytokines in zoledronate-induced acute phase reaction in postmenopausal women with low bone mass. Clin Endocrinol (Oxf). 2012;77(6):816-822. doi:10.1111/j.1365-2265.2012.04459.x
12. Smrecnik M, Kavcic Trsinar Z, Kocjan T. Adrenal crisis after first infusion of zoledronic acid: a case report. Osteoporos Int. 2018;29(7):1675-1678. doi:10.1007/s00198-018-4508-7
13. Kuo B, Koransky A, Vaz Wicks CL. Adrenal crisis as an adverse reaction to zoledronic acid in a patient with primary adrenal insufficiency: a case report and literature review. AACE Clin Case Rep. 2022;9(2):32-34. Published 2022 Dec 17. doi:10.1016/j.aace.2022.12.003
14. Bourke S, Bolland MJ, Grey A, et al. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos Int. 2013;24(1):349-354. doi:10.1007/s00198-012-2117-4
15. Silverman SL, Kriegman A, and Goncalves J, et al. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int. 2011;22(8):2337-2345.
16. Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int. 2012;23(2):503-512. doi:10.1007/s00198-011-1563-8
17. Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone. 2011;49(1):140-145. doi:10.1016/j.bone.2010.10.177
18. Makras P, Anastasilakis AD, Polyzos SA, Bisbinas I, Sakellariou GT, Papapoulos SE. No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif Tissue Int. 2011;88(5):402-408. doi:10.1007/s00223-011-9468-2
19. Liu Q, Han G, Li R, et al. Reduction effect of oral pravastatin on the acute phase response to intravenous zoledronic acid: protocol for a real-world prospective, placebo-controlled trial. BMJ Open. 2022;12(7):e060703. Published 2022 Jul 13. doi:10.1136/bmjopen-2021-060703
20. Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int. 2017;28(6):1867-1874. doi:10.1007/s00198-017-3960-0
21. Chen FP, Fu TS, Lin YC, Lin YJ. Addition of dexamethasone to manage acute phase responses following initial zoledronic acid infusion. Osteoporos Int. 2021;32(4):663-670. doi:10.1007/s00198-020-05653-0
22. Murdoch R, Mellar A, Horne AM, et al. Effect of a three-day course of dexamethasone on acute phase response following treatment with zoledronate: a randomized controlled trial. J Bone Miner Res. 2023;38(5):631-638. doi:10.1002/jbmr.4802
23. Black DM, Reid IR, Napoli N, et al. The interaction of acute-phase reaction and efficacy for osteoporosis after zoledronic acid: HORIZON pivotal fracture trial. J Bone Miner Res. 2022;37(1):21-28. doi:10.1002/jbmr.4434
24. Lu K, Wu YM, Shi Q, Gong YQ, Zhang T, Li C. The impact of acute-phase reaction on mortality and re-fracture after zoledronic acid in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. 2023;34(9):1613-1623. doi:10.1007/s00198-023-06803-w
A 62-year-old man presented to the emergency department (ED) with 3 days of chills, myalgias, and nausea. The patient’s oral temperature at home ranged from 99.9 to 100.1 °F. He came to the ED after multiple phone discussions with primary care nursing over 3 days. His medical history included posttraumatic stress disorder, enlarged prostate, osteoporosis, gastroesophageal reflux, glaucoma, and left eye central retinal vein occlusion. Medications included fluoxetine 20 mg twice daily, omeprazole 20 mg twice daily, rosuvastatin 10 mg once daily, tamsulosin 0.4 mg nightly, and zolpidem 10 mg nightly. The patient’s glaucoma had been treated with a dexamethasone intraocular implant about 90 days earlier. The patient started on intravenous (IV) zoledronic acid for osteoporosis, with the first infusion 5 days prior to presentation.
In the ED, the patient’s temperature was 98.2 °F, blood pressure was 156/76 mm Hg, pulse was 94 bpm, respiratory rate was 16 breaths per minute, and 98% oxygen saturation on room air. He was in no acute distress, with an unremarkable physical examination reporting no abnormal respiratory sounds, no arrhythmia, normal gait, and no focal neurologic deficits. A comprehensive metabolic panel was unremarkable, creatine phosphokinase was 155 U/L (reference range, 30-240 U/L), and the complete blood count was notable only for an elevated white blood count of 15.3 × 109/L (reference range, 4.0-11.0 × 109/L), with 73.4% neutrophils, 16.2% lymphocytes, 9.1% monocytes, 0.5% eosinophils, and 0.4% basophils. The patient’s urinalysis was unremarkable.
What is your diagnosis?
How would you treat this patient?
Discussion
The ED physician considered viral infection and tested for both influenza and COVID-19. Laboratory results eliminated urinary tract infection and rhabdomyolysis as possible diagnoses. An acute phase reaction to zoledronic acid was determined to be the most likely cause. The patient was treated with IV saline in the ED, and acetaminophen both in the ED and at home.
Although initial nursing triage notes document consideration of acute phase reaction to zoledronic acid, the endocrinology service, which had recommended and arranged the zoledronic acid infusion, was not immediately notified of the reaction. It does not appear any treatment (eg, acetaminophen) was suggested, only that the patient was given advice this may resolve over 3 to 4 days. When he was seen 2 months later for an endocrinology follow-up appointment, he reported that all symptoms (chills, myalgias, and nausea) resolved gradually over 1 week. Since then, he has felt as well as he did before taking zoledronic acid. However, the patient was wary of further zoledronic acid, opting to defer deciding on a second dose until a future appointment.
Prior to starting zoledronic acid therapy, the patient was being treated for vitamin D deficiency. Four months prior to infusion, his 25-hydroxyvitamin D level was 12.0 ng/mL (reference range, 30 to 80 ng/mL). He then started taking cholecalciferol 100 mcg (4000 IU) daily. Eight days prior to infusion his 25-hydroxyvitamin D level was 29.5 ng/mL.
Federal health care practitioners, especially those working in the Veterans Health Administration (VHA), will commonly encounter patients similar to this case. Osteoporosisis is common in the United States with > 10 million diagnoses (including > 2 million men) and in VHA primary care populations.1,2 Zoledronic acid is a frequently prescribed treatment, appearing in guidelines for osteoporosis management.3-5
The acute phase reaction is a common adverse effect of both oral and IV bisphosphonates, although it’s substantially more common with IV bisphosphonates such as zoledronic acid. This reaction is characterized by flu-like symptoms of fever, myalgia, and arthralgia that occur within the first few days following bisphosphonate administration, and tends to be rated mild to moderate by patients.6 Clinical trial data from > 7000 women with postmenopausal osteoporosis found that 42% experienced ≥ 1 acute phase symptom following the first infusion (fever was most common, followed by musculoskeletal symptoms and gastrointestinal symptoms), compared with 12% for placebo. Incidence decreases with each subsequent infusion.7 Risk factors for reactions include low 25-hydroxyvitamin D levels,8,9 no prior bisphosphonate exposure,9 younger age (aged 64-67 years vs 78-89 years),7 lower body mass index,10 and higher lymphocyte levels at baseline.11 While most cases are mild and self-limited, severe consequences have been noted, such as precipitation of adrenal crisis.12,13 Additionally, more prolonged bone pain, sometimes quite severe, has been rarely reported with bisphosphonate use. However, it’s unclear whether this represents a separate adverse effect or a more severe acute phase reaction.6
The acute phase reaction is a transient inflammatory state marked by increases in proinflammatory cytokines such as C-reactive protein, interleukin-6, and tumor necrosis factor-α. Proposed mechanisms include: (1) inhibition of farnesyl pyrophosphate synthase, an enzyme of the mevalonate pathway, resulting inactivation of γϐ T cells and increased production of proinflammatory cytokines; (2) inhibition of the suppressor of cytokine signalling-3 in the macrophages, resulting in cessation of the suppression in cytokine signaling; or (3) negative regulation of γϐ T-cell expansion and interferon-c production by low serum 25-hydroxyvitamin D concentrations.11
Prevention
Can an acute phase reaction to zoledronic acid be prevented? Bourke and colleagues reported that baseline calcium and/or vitamin D intake do not appear to affect rates of acute phase reaction in data pooled from 2 trials of zoledronic acid in postmenopausal women.14 However, patients receiving zoledronic acid had 25-hydroxyvitamin D values > 20 ng/mL 86% of the time, and values > 30 ng/mL 36% of the time. Bourke and colleagues suggest that “coadministration of calcium and vitamin D with zoledronate may not be necessary for individuals not at risk of marked vitamin D deficiency.”14 However, they did not prospectively test this hypothesis.
In our patient, vitamin D deficiency had been identified and treated, nearly achieving 30 ng/mL. The 2020 guidelines for postmenopausal osteoporosis recommend maintaining serum 25-hydroxyvitamin D levels 30 to 50 ng/mL, advising to supplement with vitamin D3 as needed.5 The 2012 guidelines for osteoporosis in men from the Endocrine Society suggest that men with low vitamin D levels receive vitamin D supplements to raise the level > 30 ng/ml.4
Oral analgesics have been studied for the prevention of adverse effects related to zoledronic acid. Initiating 650 mg acetaminophen 45 minutes before zoledronic acid infusion and then every 6 hours over the next 3 days has been shown to significantly reduce symptoms.15 Acetaminophen or ibuprofen given every 6 hours for 3 days (starting 4 hours after zoledronic acid infusion) has been shown to reduce fever and other symptoms.16
Statins have been shown in vitro to prevent bisphosphonate-induced γϐ T cell activation.17 This has led to studies with various statins, although none have yet shown benefit in vivo. A double-blind, randomized, placebo-controlled trial of postmenopausal women for fluvastatin (single dose of 40 mg or 3 doses of 40 mg, each 24 hours apart) did not prevent acute phase reaction symptoms, nor did it prevent zoledronic acid-induced cytokine release.17 Rosuvastatin 10 mg daily starting 5 days before zoledronic acid treatment and taken for a total of 11 days did not show any difference in fever or pain.18 A protocol for pravastatin has been disseminated, but no study results have been published yet.19
Prophylactic dexamethasone has also been studied. A randomized double-blind, placebo-controlled trial of oral dexamethasone 4 mg at the time of first infusion of zoledronic acid found no significant difference in temperature change or symptom score over the following 3 days.20 Chen and colleagues compared the efficacy of acetaminophen alone vs acetaminophen plus dexamethasone over several days.21 Acetaminophen 500 mg was given on the day of infusion and 4 times daily for 3 to 7 days for both groups, while dexamethasone 4 mg was given for 3 to 7 days. The dexamethasone group reported substantially lower incidence of any acute phase reaction symptoms (34% vs 67%, P = .003). A more recent study by Murdoch and colleagues comparing dexamethasone (4 mg daily for 3 days with the first dose 90 minutes before zoledronic acid infusion) with placebo found that the dexamethasone group had a statistically significant lower mean temperature change and acute phase reaction symptom score.22
Adverse Effect Treatment
Treatment after development of acute phase reaction due to zoledronic acid infusion is generally limited to supportive care and/or nonsteroidal anti-inflammatory drugs (NSAIDs) acetaminophen or dexamethasone, largely based on extrapolation of the noted preventive trials and expert opinion.3,6 Experiencing an acute phase reaction may portend better fracture risk reduction from zoledronic acid, although there is a potential association between acute phase reaction and mortality risk.23,24
Our case was typical for acute phase reaction to zoledronic acid. The patient was already taking rosuvastatin 10 mg daily for hypercholesterolemia as prescribed by his primary care physician. Rosuvastatin was not shown to prevent symptoms, although it was not studied in patients on long-term statin therapy at the time of zoledronic acid infusion.18 The patient was also taking vitamin D3 supplementation and was nearly in the reference range.5 His ED treatment included IV fluids and acetaminophen. Pretreatment (prior to or at the time of zoledronic acid infusion) with acetaminophen or ibuprofen may have prevented his symptoms, or at least lessened them to the point that an ED visit would not have resulted. The endocrinologist who prescribed the zoledronic acid documented a detailed discussion of the adverse effects of zoledronic acid with the patient, and the initial nursing call documents consideration of acute phase reaction. It is unclear whether the persistence of symptoms or worsening of symptoms ultimately led to the ED visit. Because no treatment was offered, it is unknown whether earlier posttreatment with acetaminophen, ibuprofen, or dexamethasone might have prevented his ED visit.
Conclusions
Clinicians who treat patients with osteoporosis should be aware of several key points. First, acute phase reaction symptoms are common with bisphosphonates, especially zoledronic acid infusions. Second, the symptoms are nonspecific but should have a suggestive time course. Third, dexamethasone may be partially protective, but based on the various trials discussed, it likely needs to be given for multiple days (instead of a single dose on the day of infusion). Given that acetaminophen and NSAIDs also seem to be protective (when given for multiple days starting on the day of infusion), both have lower overall adverse effect profiles than dexamethasone, consideration may be given to using either of these prophylactically.6 Dexamethasone could then be prescribed if symptoms are severe or persistent despite the use of acetaminophen or NSAIDs.
A 62-year-old man presented to the emergency department (ED) with 3 days of chills, myalgias, and nausea. The patient’s oral temperature at home ranged from 99.9 to 100.1 °F. He came to the ED after multiple phone discussions with primary care nursing over 3 days. His medical history included posttraumatic stress disorder, enlarged prostate, osteoporosis, gastroesophageal reflux, glaucoma, and left eye central retinal vein occlusion. Medications included fluoxetine 20 mg twice daily, omeprazole 20 mg twice daily, rosuvastatin 10 mg once daily, tamsulosin 0.4 mg nightly, and zolpidem 10 mg nightly. The patient’s glaucoma had been treated with a dexamethasone intraocular implant about 90 days earlier. The patient started on intravenous (IV) zoledronic acid for osteoporosis, with the first infusion 5 days prior to presentation.
In the ED, the patient’s temperature was 98.2 °F, blood pressure was 156/76 mm Hg, pulse was 94 bpm, respiratory rate was 16 breaths per minute, and 98% oxygen saturation on room air. He was in no acute distress, with an unremarkable physical examination reporting no abnormal respiratory sounds, no arrhythmia, normal gait, and no focal neurologic deficits. A comprehensive metabolic panel was unremarkable, creatine phosphokinase was 155 U/L (reference range, 30-240 U/L), and the complete blood count was notable only for an elevated white blood count of 15.3 × 109/L (reference range, 4.0-11.0 × 109/L), with 73.4% neutrophils, 16.2% lymphocytes, 9.1% monocytes, 0.5% eosinophils, and 0.4% basophils. The patient’s urinalysis was unremarkable.
What is your diagnosis?
How would you treat this patient?
Discussion
The ED physician considered viral infection and tested for both influenza and COVID-19. Laboratory results eliminated urinary tract infection and rhabdomyolysis as possible diagnoses. An acute phase reaction to zoledronic acid was determined to be the most likely cause. The patient was treated with IV saline in the ED, and acetaminophen both in the ED and at home.
Although initial nursing triage notes document consideration of acute phase reaction to zoledronic acid, the endocrinology service, which had recommended and arranged the zoledronic acid infusion, was not immediately notified of the reaction. It does not appear any treatment (eg, acetaminophen) was suggested, only that the patient was given advice this may resolve over 3 to 4 days. When he was seen 2 months later for an endocrinology follow-up appointment, he reported that all symptoms (chills, myalgias, and nausea) resolved gradually over 1 week. Since then, he has felt as well as he did before taking zoledronic acid. However, the patient was wary of further zoledronic acid, opting to defer deciding on a second dose until a future appointment.
Prior to starting zoledronic acid therapy, the patient was being treated for vitamin D deficiency. Four months prior to infusion, his 25-hydroxyvitamin D level was 12.0 ng/mL (reference range, 30 to 80 ng/mL). He then started taking cholecalciferol 100 mcg (4000 IU) daily. Eight days prior to infusion his 25-hydroxyvitamin D level was 29.5 ng/mL.
Federal health care practitioners, especially those working in the Veterans Health Administration (VHA), will commonly encounter patients similar to this case. Osteoporosisis is common in the United States with > 10 million diagnoses (including > 2 million men) and in VHA primary care populations.1,2 Zoledronic acid is a frequently prescribed treatment, appearing in guidelines for osteoporosis management.3-5
The acute phase reaction is a common adverse effect of both oral and IV bisphosphonates, although it’s substantially more common with IV bisphosphonates such as zoledronic acid. This reaction is characterized by flu-like symptoms of fever, myalgia, and arthralgia that occur within the first few days following bisphosphonate administration, and tends to be rated mild to moderate by patients.6 Clinical trial data from > 7000 women with postmenopausal osteoporosis found that 42% experienced ≥ 1 acute phase symptom following the first infusion (fever was most common, followed by musculoskeletal symptoms and gastrointestinal symptoms), compared with 12% for placebo. Incidence decreases with each subsequent infusion.7 Risk factors for reactions include low 25-hydroxyvitamin D levels,8,9 no prior bisphosphonate exposure,9 younger age (aged 64-67 years vs 78-89 years),7 lower body mass index,10 and higher lymphocyte levels at baseline.11 While most cases are mild and self-limited, severe consequences have been noted, such as precipitation of adrenal crisis.12,13 Additionally, more prolonged bone pain, sometimes quite severe, has been rarely reported with bisphosphonate use. However, it’s unclear whether this represents a separate adverse effect or a more severe acute phase reaction.6
The acute phase reaction is a transient inflammatory state marked by increases in proinflammatory cytokines such as C-reactive protein, interleukin-6, and tumor necrosis factor-α. Proposed mechanisms include: (1) inhibition of farnesyl pyrophosphate synthase, an enzyme of the mevalonate pathway, resulting inactivation of γϐ T cells and increased production of proinflammatory cytokines; (2) inhibition of the suppressor of cytokine signalling-3 in the macrophages, resulting in cessation of the suppression in cytokine signaling; or (3) negative regulation of γϐ T-cell expansion and interferon-c production by low serum 25-hydroxyvitamin D concentrations.11
Prevention
Can an acute phase reaction to zoledronic acid be prevented? Bourke and colleagues reported that baseline calcium and/or vitamin D intake do not appear to affect rates of acute phase reaction in data pooled from 2 trials of zoledronic acid in postmenopausal women.14 However, patients receiving zoledronic acid had 25-hydroxyvitamin D values > 20 ng/mL 86% of the time, and values > 30 ng/mL 36% of the time. Bourke and colleagues suggest that “coadministration of calcium and vitamin D with zoledronate may not be necessary for individuals not at risk of marked vitamin D deficiency.”14 However, they did not prospectively test this hypothesis.
In our patient, vitamin D deficiency had been identified and treated, nearly achieving 30 ng/mL. The 2020 guidelines for postmenopausal osteoporosis recommend maintaining serum 25-hydroxyvitamin D levels 30 to 50 ng/mL, advising to supplement with vitamin D3 as needed.5 The 2012 guidelines for osteoporosis in men from the Endocrine Society suggest that men with low vitamin D levels receive vitamin D supplements to raise the level > 30 ng/ml.4
Oral analgesics have been studied for the prevention of adverse effects related to zoledronic acid. Initiating 650 mg acetaminophen 45 minutes before zoledronic acid infusion and then every 6 hours over the next 3 days has been shown to significantly reduce symptoms.15 Acetaminophen or ibuprofen given every 6 hours for 3 days (starting 4 hours after zoledronic acid infusion) has been shown to reduce fever and other symptoms.16
Statins have been shown in vitro to prevent bisphosphonate-induced γϐ T cell activation.17 This has led to studies with various statins, although none have yet shown benefit in vivo. A double-blind, randomized, placebo-controlled trial of postmenopausal women for fluvastatin (single dose of 40 mg or 3 doses of 40 mg, each 24 hours apart) did not prevent acute phase reaction symptoms, nor did it prevent zoledronic acid-induced cytokine release.17 Rosuvastatin 10 mg daily starting 5 days before zoledronic acid treatment and taken for a total of 11 days did not show any difference in fever or pain.18 A protocol for pravastatin has been disseminated, but no study results have been published yet.19
Prophylactic dexamethasone has also been studied. A randomized double-blind, placebo-controlled trial of oral dexamethasone 4 mg at the time of first infusion of zoledronic acid found no significant difference in temperature change or symptom score over the following 3 days.20 Chen and colleagues compared the efficacy of acetaminophen alone vs acetaminophen plus dexamethasone over several days.21 Acetaminophen 500 mg was given on the day of infusion and 4 times daily for 3 to 7 days for both groups, while dexamethasone 4 mg was given for 3 to 7 days. The dexamethasone group reported substantially lower incidence of any acute phase reaction symptoms (34% vs 67%, P = .003). A more recent study by Murdoch and colleagues comparing dexamethasone (4 mg daily for 3 days with the first dose 90 minutes before zoledronic acid infusion) with placebo found that the dexamethasone group had a statistically significant lower mean temperature change and acute phase reaction symptom score.22
Adverse Effect Treatment
Treatment after development of acute phase reaction due to zoledronic acid infusion is generally limited to supportive care and/or nonsteroidal anti-inflammatory drugs (NSAIDs) acetaminophen or dexamethasone, largely based on extrapolation of the noted preventive trials and expert opinion.3,6 Experiencing an acute phase reaction may portend better fracture risk reduction from zoledronic acid, although there is a potential association between acute phase reaction and mortality risk.23,24
Our case was typical for acute phase reaction to zoledronic acid. The patient was already taking rosuvastatin 10 mg daily for hypercholesterolemia as prescribed by his primary care physician. Rosuvastatin was not shown to prevent symptoms, although it was not studied in patients on long-term statin therapy at the time of zoledronic acid infusion.18 The patient was also taking vitamin D3 supplementation and was nearly in the reference range.5 His ED treatment included IV fluids and acetaminophen. Pretreatment (prior to or at the time of zoledronic acid infusion) with acetaminophen or ibuprofen may have prevented his symptoms, or at least lessened them to the point that an ED visit would not have resulted. The endocrinologist who prescribed the zoledronic acid documented a detailed discussion of the adverse effects of zoledronic acid with the patient, and the initial nursing call documents consideration of acute phase reaction. It is unclear whether the persistence of symptoms or worsening of symptoms ultimately led to the ED visit. Because no treatment was offered, it is unknown whether earlier posttreatment with acetaminophen, ibuprofen, or dexamethasone might have prevented his ED visit.
Conclusions
Clinicians who treat patients with osteoporosis should be aware of several key points. First, acute phase reaction symptoms are common with bisphosphonates, especially zoledronic acid infusions. Second, the symptoms are nonspecific but should have a suggestive time course. Third, dexamethasone may be partially protective, but based on the various trials discussed, it likely needs to be given for multiple days (instead of a single dose on the day of infusion). Given that acetaminophen and NSAIDs also seem to be protective (when given for multiple days starting on the day of infusion), both have lower overall adverse effect profiles than dexamethasone, consideration may be given to using either of these prophylactically.6 Dexamethasone could then be prescribed if symptoms are severe or persistent despite the use of acetaminophen or NSAIDs.
1. Choksi P, Gay BL, Reyes-Gastelum D, Haymart MR, Papaleontiou M. Understanding osteoporosis screening practices in men: a nationwide physician survey. Endocr Pract. 2020;26(11):1237-1243. doi:10.4158/EP-2020-0123
2. Yu ZL, Fisher L, Hand J. Osteoporosis screening for male veterans in a resident based primary care clinic at Northport Veterans Affairs Medical Center. Am J Med Qual. 2023;38(5):272.doi:10.1097/JMQ.0000000000000134
3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595-1622. doi:10.1210/jc.2019-00221
4. Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822. doi:10.1210/jc.2011-3045
5. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update. Endocr Pract. 2020;26(suppl 1):1-46. doi:10.4158/GL-2020-0524SUPPL
6. Lim SY, Bolster MB. What can we do about musculoskeletal pain from bisphosphonates?. Cleve Clin J Med. 2018;85(9):675-678. doi:10.3949/ccjm.85a.18005
7. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380-4387. doi:10.1210/jc.2010-0597
8. Lu K, Shi Q, Gong YQ, Li C. Association between vitamin D and zoledronate-induced acute-phase response fever risk in osteoporotic patients. Front Endocrinol (Lausanne). 2022;13:991913. Published 2022 Oct 10. doi:10.3389/fendo.2022.991913
9. Popp AW, Senn R, Curkovic I, et al. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28(6):1995-2002. doi:10.1007/s00198-017-3992-5
10. Zheng X, Ye J, Zhan Q, et al. Prediction of musculoskeletal pain after the first intravenous zoledronic acid injection in patients with primary osteoporosis: development and evaluation of a new nomogram. BMC Musculoskelet Disord. 2023;24(1):841. Published 2023 Oct 25. doi:10.1186/s12891-023-06965-y
11. Anastasilakis AD, Polyzos SA, Delaroudis S, et al. The role of cytokines and adipocytokines in zoledronate-induced acute phase reaction in postmenopausal women with low bone mass. Clin Endocrinol (Oxf). 2012;77(6):816-822. doi:10.1111/j.1365-2265.2012.04459.x
12. Smrecnik M, Kavcic Trsinar Z, Kocjan T. Adrenal crisis after first infusion of zoledronic acid: a case report. Osteoporos Int. 2018;29(7):1675-1678. doi:10.1007/s00198-018-4508-7
13. Kuo B, Koransky A, Vaz Wicks CL. Adrenal crisis as an adverse reaction to zoledronic acid in a patient with primary adrenal insufficiency: a case report and literature review. AACE Clin Case Rep. 2022;9(2):32-34. Published 2022 Dec 17. doi:10.1016/j.aace.2022.12.003
14. Bourke S, Bolland MJ, Grey A, et al. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos Int. 2013;24(1):349-354. doi:10.1007/s00198-012-2117-4
15. Silverman SL, Kriegman A, and Goncalves J, et al. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int. 2011;22(8):2337-2345.
16. Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int. 2012;23(2):503-512. doi:10.1007/s00198-011-1563-8
17. Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone. 2011;49(1):140-145. doi:10.1016/j.bone.2010.10.177
18. Makras P, Anastasilakis AD, Polyzos SA, Bisbinas I, Sakellariou GT, Papapoulos SE. No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif Tissue Int. 2011;88(5):402-408. doi:10.1007/s00223-011-9468-2
19. Liu Q, Han G, Li R, et al. Reduction effect of oral pravastatin on the acute phase response to intravenous zoledronic acid: protocol for a real-world prospective, placebo-controlled trial. BMJ Open. 2022;12(7):e060703. Published 2022 Jul 13. doi:10.1136/bmjopen-2021-060703
20. Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int. 2017;28(6):1867-1874. doi:10.1007/s00198-017-3960-0
21. Chen FP, Fu TS, Lin YC, Lin YJ. Addition of dexamethasone to manage acute phase responses following initial zoledronic acid infusion. Osteoporos Int. 2021;32(4):663-670. doi:10.1007/s00198-020-05653-0
22. Murdoch R, Mellar A, Horne AM, et al. Effect of a three-day course of dexamethasone on acute phase response following treatment with zoledronate: a randomized controlled trial. J Bone Miner Res. 2023;38(5):631-638. doi:10.1002/jbmr.4802
23. Black DM, Reid IR, Napoli N, et al. The interaction of acute-phase reaction and efficacy for osteoporosis after zoledronic acid: HORIZON pivotal fracture trial. J Bone Miner Res. 2022;37(1):21-28. doi:10.1002/jbmr.4434
24. Lu K, Wu YM, Shi Q, Gong YQ, Zhang T, Li C. The impact of acute-phase reaction on mortality and re-fracture after zoledronic acid in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. 2023;34(9):1613-1623. doi:10.1007/s00198-023-06803-w
1. Choksi P, Gay BL, Reyes-Gastelum D, Haymart MR, Papaleontiou M. Understanding osteoporosis screening practices in men: a nationwide physician survey. Endocr Pract. 2020;26(11):1237-1243. doi:10.4158/EP-2020-0123
2. Yu ZL, Fisher L, Hand J. Osteoporosis screening for male veterans in a resident based primary care clinic at Northport Veterans Affairs Medical Center. Am J Med Qual. 2023;38(5):272.doi:10.1097/JMQ.0000000000000134
3. Eastell R, Rosen CJ, Black DM, Cheung AM, Murad MH, Shoback D. Pharmacological management of osteoporosis in postmenopausal women: an Endocrine Society* clinical practice guideline. J Clin Endocrinol Metab. 2019;104(5):1595-1622. doi:10.1210/jc.2019-00221
4. Watts NB, Adler RA, Bilezikian JP, et al. Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802-1822. doi:10.1210/jc.2011-3045
5. Camacho PM, Petak SM, Binkley N, et al. American Association of Clinical Endocrinologists/American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis – 2020 update. Endocr Pract. 2020;26(suppl 1):1-46. doi:10.4158/GL-2020-0524SUPPL
6. Lim SY, Bolster MB. What can we do about musculoskeletal pain from bisphosphonates?. Cleve Clin J Med. 2018;85(9):675-678. doi:10.3949/ccjm.85a.18005
7. Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM. Characterization of and risk factors for the acute-phase response after zoledronic acid. J Clin Endocrinol Metab. 2010;95(9):4380-4387. doi:10.1210/jc.2010-0597
8. Lu K, Shi Q, Gong YQ, Li C. Association between vitamin D and zoledronate-induced acute-phase response fever risk in osteoporotic patients. Front Endocrinol (Lausanne). 2022;13:991913. Published 2022 Oct 10. doi:10.3389/fendo.2022.991913
9. Popp AW, Senn R, Curkovic I, et al. Factors associated with acute-phase response of bisphosphonate-naïve or pretreated women with osteoporosis receiving an intravenous first dose of zoledronate or ibandronate. Osteoporos Int. 2017;28(6):1995-2002. doi:10.1007/s00198-017-3992-5
10. Zheng X, Ye J, Zhan Q, et al. Prediction of musculoskeletal pain after the first intravenous zoledronic acid injection in patients with primary osteoporosis: development and evaluation of a new nomogram. BMC Musculoskelet Disord. 2023;24(1):841. Published 2023 Oct 25. doi:10.1186/s12891-023-06965-y
11. Anastasilakis AD, Polyzos SA, Delaroudis S, et al. The role of cytokines and adipocytokines in zoledronate-induced acute phase reaction in postmenopausal women with low bone mass. Clin Endocrinol (Oxf). 2012;77(6):816-822. doi:10.1111/j.1365-2265.2012.04459.x
12. Smrecnik M, Kavcic Trsinar Z, Kocjan T. Adrenal crisis after first infusion of zoledronic acid: a case report. Osteoporos Int. 2018;29(7):1675-1678. doi:10.1007/s00198-018-4508-7
13. Kuo B, Koransky A, Vaz Wicks CL. Adrenal crisis as an adverse reaction to zoledronic acid in a patient with primary adrenal insufficiency: a case report and literature review. AACE Clin Case Rep. 2022;9(2):32-34. Published 2022 Dec 17. doi:10.1016/j.aace.2022.12.003
14. Bourke S, Bolland MJ, Grey A, et al. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos Int. 2013;24(1):349-354. doi:10.1007/s00198-012-2117-4
15. Silverman SL, Kriegman A, and Goncalves J, et al. Effect of acetaminophen and fluvastatin on post-dose symptoms following infusion of zoledronic acid. Osteoporos Int. 2011;22(8):2337-2345.
16. Wark JD, Bensen W, Recknor C, et al. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int. 2012;23(2):503-512. doi:10.1007/s00198-011-1563-8
17. Thompson K, Keech F, McLernon DJ, et al. Fluvastatin does not prevent the acute-phase response to intravenous zoledronic acid in post-menopausal women. Bone. 2011;49(1):140-145. doi:10.1016/j.bone.2010.10.177
18. Makras P, Anastasilakis AD, Polyzos SA, Bisbinas I, Sakellariou GT, Papapoulos SE. No effect of rosuvastatin in the zoledronate-induced acute-phase response. Calcif Tissue Int. 2011;88(5):402-408. doi:10.1007/s00223-011-9468-2
19. Liu Q, Han G, Li R, et al. Reduction effect of oral pravastatin on the acute phase response to intravenous zoledronic acid: protocol for a real-world prospective, placebo-controlled trial. BMJ Open. 2022;12(7):e060703. Published 2022 Jul 13. doi:10.1136/bmjopen-2021-060703
20. Billington EO, Horne A, Gamble GD, Maslowski K, House M, Reid IR. Effect of single-dose dexamethasone on acute phase response following zoledronic acid: a randomized controlled trial. Osteoporos Int. 2017;28(6):1867-1874. doi:10.1007/s00198-017-3960-0
21. Chen FP, Fu TS, Lin YC, Lin YJ. Addition of dexamethasone to manage acute phase responses following initial zoledronic acid infusion. Osteoporos Int. 2021;32(4):663-670. doi:10.1007/s00198-020-05653-0
22. Murdoch R, Mellar A, Horne AM, et al. Effect of a three-day course of dexamethasone on acute phase response following treatment with zoledronate: a randomized controlled trial. J Bone Miner Res. 2023;38(5):631-638. doi:10.1002/jbmr.4802
23. Black DM, Reid IR, Napoli N, et al. The interaction of acute-phase reaction and efficacy for osteoporosis after zoledronic acid: HORIZON pivotal fracture trial. J Bone Miner Res. 2022;37(1):21-28. doi:10.1002/jbmr.4434
24. Lu K, Wu YM, Shi Q, Gong YQ, Zhang T, Li C. The impact of acute-phase reaction on mortality and re-fracture after zoledronic acid in hospitalized elderly osteoporotic fracture patients. Osteoporos Int. 2023;34(9):1613-1623. doi:10.1007/s00198-023-06803-w
Evaluation of a Stress, Coping, and Resourcefulness Program for VA Nurses During the COVID-19 Pandemic
Nurses are recognized among the most trusted professions in the United States.1 Since the time of Florence Nightingale, nurses have been challenged to provide care to patients and soldiers with complex needs, including acute and chronic physical illness, as well as mental health issues. Nurses have traditionally met those challenges with perseverance and creativity but have also experienced stress and burnout.
A shortage of nurses has been linked to many interrelated factors including the retirement of bedside caregivers and educators, diverse care settings, expanding roles for nurses, and nurse burnout.2-4 Therefore, there is a critical need to better understand of how nurses can be supported while they care for patients, cope with stress, and maintain positive personal and professional outcomes. The objective of this pilot project was to assess US Department of Veterans Affairs (VA) nurses’ levels of burnout and test an intervention to enhance resourcefulness skills during the COVID-19 pandemic.
Background
Stress has many definitions. Hans Selye described it as a biological response of the body to any demand.5,6 Occupational stress is a process that occurs in which work environment stressors result in the development of psychological, behavioral, or physiological effects that can contribute to health.6 Occupational stress has been observed as prevalent among nurses.6 In 1960, Menzies identified sources of stress among nurses that include complex decision-making within a dynamic environment.7 Since the mid-1980s, nurses’ stress at work has increased because of legal, accreditation, ethical issues, fiscal pressures, staffing shortages, and the increasing integration of technology associated with clinical care.8
Sustained stress can lead to emotional exhaustion or burnout, which has been associated with nursing turnover, lower patient satisfaction, and patient safety risk.2,9 An American Nurses Foundation survey reported that 51% of US nurses feel exhausted, 43% overwhelmed, and 36% anxious; 28% express willingness to leave the profession.2 Burnout has been described as a response to physical or emotional stress leading to exhaustion, self-doubt, cynicism, and ineffectiveness.10 Employees with burnout are more likely to leave their jobs, take sick leave, and suffer from depression and relationship problems, and it affects nearly half of all US nurses, especially among critical care, pediatric, and oncology specialities.10,11 It has been well documented that unmitigated stress can lead to burnout and contribute to nurses leaving bedside care and the health care profession.2,3 Several studies on nursing stress and burnout have focused on its prevalence and negative outcomes.4,7,9 However, few studies have addressed building resiliency and resourcefulness for nurses.10,12,13
A 2021 National Academy of Medicine report advocated a multilevel approach to managing burnout and building resiliency among nurses.14 Taylor further identified specific interventions, ranging from primary prevention to treatment.15 Primary prevention could include educating nurses on self-awareness, coping strategies, and communication skills. Screening for burnout and providing resources for support would be a secondary level of intervention. For nurses who experienced severe burnout symptoms and left the workplace, strategies are sorely needed to provide healing and a return-to-work plan.15 This may include adjusting nurse schedules and nursing roles (such as admitting/discharge nurse or resource nurse).
RESILIENCY AND RESOURCEFULNESS
Rushton and colleagues describe resiliency as the “ability to face adverse situations, remain focused, and continue to be optimistic for the future.”4 For nurses in complex health care systems, resiliency is associated with reduced turnover and symptoms of burnout and improved mental health. Humans are thought to have an innate resiliency potential that evolves over time and fluctuates depending on the context (eg, societal conditions, moral/ethical values, commitments).4 It is believed that resiliency can contribute to the development of new neuropathways that can be used to manage and cope with stress, prevent burnout, and improve quality of life. However, it appears these adaptations are individualized and contingent on situations, available resources, and changing priorities.16 Consequently, resiliency may be an essential tool for nurses to combat burnout in today’s complex health care systems.17
Although resilience and resourcefulness are conceptually related, each has distinctive features.18 Celinski frames resilience as transcendence and resourcefulness as transformation.19 Thus, while resilience suggests transcendence in terms of rising above, going beyond, exceeding, or excelling; resourcefulness reflects transformation, such as making changes in thoughts, feelings, behaviors, actions, or reactions. Resourcefulness has been conceptualized as an indicator of resilience.18
Resourcefulness comprises 2 dimensions, including the use of self-help strategies (personal resourcefulness) and seeking help from others (social resourcefulness), to self-regulate one’s thoughts, feelings, and behaviors to cope with high levels of stress, anxiety, or depression.18,20,21 Personal resourcefulness skills include the use of cognitive reframing, positive thinking, problem-solving, priority-setting, and planning ahead. Social resourcefulness involves actively seeking help from others. Formal sources of help include, but are not limited to, nursing and medical care practitioners and community organizations such as hospitals and clinics. Informal sources of help include family members, friends, peers, and coworkers.
During the COVID-19 pandemic, nurses were especially challenged to provide support for each other because of limited nursing staff and treatment options, increased complex patient assignments, shortages of supplies, and reduced support services. Many nurses, however, were able to find innovative, peer-supported strategies for coping.13 Nurses’ use of resourcefulness skills is believed to be indicative of their resilience. This pilot project aimed to identify and evaluate some of these strategies and resourcefulness skills.
INTERVENTION
This pilot study among VA Northeast Ohio Health Care System (VANEOHS) nurses was designed to assess nursing burnout and resourcefulness during the pandemic. Those who agreed to participate completed a baseline survey on burnout and resourcefulness. Participants agreed to review a training video on resourcefulness skills (eg, relying on and exchanging ideas with others, and reframing and using ‘positive self-talk’). They were encouraged to document their experience with familiar and new resourcefulness skills. Weekly reminders (eg, emails and phone messages) reminded and coached participants in their journey.
The study identified and implemented an existing Resourcefulness Training (RT) intervention, which was developed for informal family caregivers and found to be effective.22 We measured burnout and resourcefulness preintervention and postintervention.23 This survey and educational intervention were reviewed by the VANEOHS institutional review board and ruled exempt. The survey also gathered information on nurses' contact with individuals infected with COVID-19.
Despite the many staffing and resource challenges during the COVID-19 pandemic, a convenience sample of 12 nurses was recruited from nursing committees that continued to have scheduled meetings. These meetings allowed time to answer questions and provide information about the study. The majority of nurses queried declined to participate, citing no time, interest, or burnout. Participants completed a baseline survey, reviewed a 30-minute RT video, and tracked their experience for 28 days. Participants completed postintervention surveys 6 weeks after the video. Details of the survey and measures can be found in previous studies.20,21
RT is an online cognitive-behavioral intervention that teaches and reinforces personal (self-help) and social (help-seeking) resourcefulness skills that have not yet been tested in nurses or other health care professionals.22,24 The training included social resourcefulness (eg, from family, friends, others, and professionals) and personal resourcefulness (eg, problem-solving, positive thinking, self-control, organization skills). Participants were encouraged to review the videos as often as they preferred during these 4 weeks.
All 12 survey respondents were female and had received COVID-19 vaccinations according to the federal policy at the time of data collection. The number of patients cared for with COVID-19 infections varied widely (range, 1-1000). The baseline burnout score ranged from 1 (no burnout) to 3 (1 symptom of burnout, such as physical and emotional exhaustion), with a mean score of 2.2. In the follow-up survey, the mean score was 2.0. At baseline, participants reported a variety of activities to manage stress and burnout, including times with friends and family, engaging in hobbies, and prayer. Postintervention, some participants mentioned using skills learned from RT, including reframing the situation positively by refocusing and putting stressors in perspective (Table 1).
DISCUSSION
Recent American Nurses Association efforts to develop organizational and professional goals include the importance of nurses to recognize and manage stress to prevent burnout.25 The American Nurses Association Code of Ethics notes that nurses have the same duties to care for themselves as they do for others.25 Nurses have demonstrated the ability to adapt and remain resilient during stressful times. VA nurses are a resourceful group. Many used resourcefulness skills to manage stress and burnout even before the pandemic. For example, nurses identified using family/friends for support and validation, as well as prayer and meditation. Some of the new activities may have been influenced/inspired by RT, such as organizing schedules for problem-solving and distraction.
Relying on family and peers emerged as an essential resourcefulness skill. Support from peers—battle buddies—has been recognized as a key strategy among combat soldiers. A battle buddy is paired with a fellow soldier for support to keep each other informed about key instructions and information. This promotes cooperative problem-solving. Outcomes associated with battle buddies include increased morale and confidence, and decreased stress.25 Over time, it is hoped that these coaching/mentoring relationships will result in enhanced leadership skills. Battle buddy strategies are currently being adapted into health care environments.12,26 Such programs need to be further evaluated and information disseminated.
Findings from this pilot program support the use of interventions such as RT to decrease burnout among nurses. This study suggests that RT should be tested in a larger more representative sample to determine efficacy.
Limitations
This pilot study was limited by its small sample size, single facility, and female-only participants; the findings are not generalizable. Nurses were recruited from VA nursing committees and may not be representative of nurses in the general population. In addition, the RT intervention may require a longer time commitment to adequately determine efficacy. Another limitation was that personal or family exposure to COVID-19 was not measured, but may be a confounding variable. An additional limitation may have been the time interval. A baseline survey was completed prior to watching the teaching video. Daily logs were to be completed for 28 days. A post survey followed at 6 weeks. It is possible that there was insufficient time for the nurses to have the opportunity to use their resourcefulness skills within the short time frame of the study. While it supports the need for further studies, findings should be interpreted cautiously and not generalized. It may be premature based on these findings to conclude that the intervention will be effective for other populations. Further studies are needed to assess nurses’ preferences for healthy coping mechanisms, including RT.
Conclusions
As the nursing shortage continues, efforts to support diverse, innovative coping strategies remain a priority postpandemic. Nurses must be vigilant in appraising and managing their ability to cope and adapt to individual stress, while also being aware of the stress their colleagues are experiencing. Educational institutions, professional organizations, and health care facilities must strive to educate and support nurses to identify not only stress, but healthy coping mechanisms.
Acknowledgments
This work was supported by the US Department of Veterans Affairs Central Office rapid response COVID-19 funding initiative, the Veteran Affairs Northeast Ohio Health Care System, and Geriatric Research, Education, and Clinical Center (GRECC). The Resourcefulness Scale, Resourcefulness Skills Scale, and the Resourcefulness Training intervention are copyrighted and were used with permission of the copyright holder, Jaclene A. Zauszniewski, PhD, RN-BC.
1. Walker A. Nursing ranked as the most trusted profession for 22nd year in a row. January 23, 2024. Accessed January 31, 2024. https://nurse.org/articles/nursing-ranked-most-honest-profession
2. Mental health and wellness survey 1. American Nurses Foundation. August 2020. Accessed January 31, 2024. https://www.nursingworld.org/practice-policy/work-environment/health-safety/disaster-preparedness/coronavirus/what-you-need-to-know/mental-health-and-wellbeing-survey/
3. Healthy nurse, healthy nation. American Nurses Association. May 1, 2017. Accessed January 31, 2024. https://www.healthynursehealthynation.org/
4. Rushton CH, Batcheller J, Schroeder K, Donohue P. Burnout and resilience among nurses practicing in high-intensity settings. Am J Crit Care. 2015;24(5):412-420. doi:10.4037/ajcc2015291
5. Selye HA. History and general outline of the stress concept. Stress in Health and Disease. Butterworths; 1976:3-34.
6. Levy BS, Wegman DH, Baron SL, Sokas RK. Recognizing and preventing occupational and environmental disease and injury. Occupational and Environmental Health: Recognizing and Preventing Disease and Injury. 6th ed. Oxford University Press; 2011:59-77.
7. Menzies IEP. Nurses under stress. Int Nurs Rev. 1960;7:9-16.
8. Jennings BM. Turbulence. In: Hughes RG, ed. Advances in Patient Safety and Quality: An Evidence-Based Handbook for Nurses. 3rd ed. AHRQ Publication; 2007;2;193-202.
9. Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288(16):1987-1993. doi:10.1001/jama.288.16.1987
10. Magtibay DL, Chesak SS, Coughlin K, Sood A. Decreasing stress and burnout in nurses: efficacy of blended learning with stress management and resilience training program. J Nurs Adm. 2017;47(7-8):391-395. doi:10.1097/NNA.0000000000000501
11. Halbesleben JR, Wakefield BJ, Wakefield DS, Cooper LB. Nurse burnout and patient safety outcomes: nurse safety perception versus reporting behavior. West J Nurs Res. 2008;30(5):560-577. doi:10.1177/0193945907311322
12. Sherman RO. Creating a Battle Buddy program. September 2, 2021. Accessed September 27, 2022. https://www.emergingrnleader.com/creating-a-battle-buddy-program
13. Godfrey KM, Scott SD. At the heart of the pandemic: nursing peer support. Nurse Leader. 2021:19(2),188-193. doi:10.1016/j.mnl.2020.09.006
14. Wakefield M, Williams DR, Le Menestrel S, and Flaubert JL, Editors; Committee on the future of nursing 2020 2030; National Academy of Medicine; National Academies of Sciences, Engineering, and Medicine Institute of Medicine 2008. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: The National Academies Press. doi:10.17226/12089
15. Taylor RA. Contemporary issues: resilience training alone is an incomplete intervention. Nurs Educ Today. 2019;78:10-13. doi:10.1016/j.nedt.2019.03.014
16. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749. doi:10.1016/j.psc.2017.08.008
17. Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br J Psychiatry. 1985;147:598-611. doi:10.1192/bjp.147.6.598
18. Zauszniewski JA, Bekhet AK, Suresky MJ. Indicators of resilience in family members of persons with serious mental Illness. Psychiatr Clin North Am. 2015;38(1):131-146. doi:10.1016/j.psc.2014.11.009
19. Celinski MJ. Framing resilience as transcendence and resourcefulness as transformation. In: Celinski MJ, Gow KM, eds. Continuity Versus Creative Response to Challenge: The Primacy of Resilience and Resourcefulness in Life and Therapy. Nova Science Pub Inc; 2011:11-30.
20. Zauszniewski JA, Lai CY, Tithiphontumrong S. Development and testing of the Resourcefulness Scale for Older Adults. J Nurs Meas. 2006:14(1):57-68. doi:10.1891.jnum.14.1.57
21. Zauszniewski JA, Bekhet AK. Measuring use of resourcefulness skills: psychometric testing of a new scale. ISRN Nurs. 2011;2011:787363. doi:10.5402/2011/787363
22. Zauszniewski JA, Lekhak N, Burant CJ, Variath M, Morris DL. preliminary evidence for effectiveness of resourcefulness training for women dementia caregivers. J Fam Med. 2016:3(5):1069.
23. Dolan ED, Mohr D, Lempa M, et al. Using a single item to measure burnout in primary care staff: a psychometric evaluation. J Gen Intern Med. 2015;30(5):582-587. doi:10.1007/s11606-014-3112-6
24. Zauszniewski JA Resourcefulness. In: Fitzpatrick JJ, ed. Encyclopedia of Nursing Research. 4th ed. 2018:632-634.
25. Combating Stress. American Nurses Association. Accessed November 28, 2022. https://www.nursingworld.org/practice-policy/work-environment/health-safety/combating-stress/
26. Albott CS, Wozniak JR, McGlinch BP, Wall MH, Gold BS, Vinogradov S. Battle Buddies: Rapid deployment of a psychological resilience intervention for health care workers during the COVID-19 pandemic. Anesth Analg. 2020;131(1):43-54. doi:10.1213/ANE.0000000000004912
Nurses are recognized among the most trusted professions in the United States.1 Since the time of Florence Nightingale, nurses have been challenged to provide care to patients and soldiers with complex needs, including acute and chronic physical illness, as well as mental health issues. Nurses have traditionally met those challenges with perseverance and creativity but have also experienced stress and burnout.
A shortage of nurses has been linked to many interrelated factors including the retirement of bedside caregivers and educators, diverse care settings, expanding roles for nurses, and nurse burnout.2-4 Therefore, there is a critical need to better understand of how nurses can be supported while they care for patients, cope with stress, and maintain positive personal and professional outcomes. The objective of this pilot project was to assess US Department of Veterans Affairs (VA) nurses’ levels of burnout and test an intervention to enhance resourcefulness skills during the COVID-19 pandemic.
Background
Stress has many definitions. Hans Selye described it as a biological response of the body to any demand.5,6 Occupational stress is a process that occurs in which work environment stressors result in the development of psychological, behavioral, or physiological effects that can contribute to health.6 Occupational stress has been observed as prevalent among nurses.6 In 1960, Menzies identified sources of stress among nurses that include complex decision-making within a dynamic environment.7 Since the mid-1980s, nurses’ stress at work has increased because of legal, accreditation, ethical issues, fiscal pressures, staffing shortages, and the increasing integration of technology associated with clinical care.8
Sustained stress can lead to emotional exhaustion or burnout, which has been associated with nursing turnover, lower patient satisfaction, and patient safety risk.2,9 An American Nurses Foundation survey reported that 51% of US nurses feel exhausted, 43% overwhelmed, and 36% anxious; 28% express willingness to leave the profession.2 Burnout has been described as a response to physical or emotional stress leading to exhaustion, self-doubt, cynicism, and ineffectiveness.10 Employees with burnout are more likely to leave their jobs, take sick leave, and suffer from depression and relationship problems, and it affects nearly half of all US nurses, especially among critical care, pediatric, and oncology specialities.10,11 It has been well documented that unmitigated stress can lead to burnout and contribute to nurses leaving bedside care and the health care profession.2,3 Several studies on nursing stress and burnout have focused on its prevalence and negative outcomes.4,7,9 However, few studies have addressed building resiliency and resourcefulness for nurses.10,12,13
A 2021 National Academy of Medicine report advocated a multilevel approach to managing burnout and building resiliency among nurses.14 Taylor further identified specific interventions, ranging from primary prevention to treatment.15 Primary prevention could include educating nurses on self-awareness, coping strategies, and communication skills. Screening for burnout and providing resources for support would be a secondary level of intervention. For nurses who experienced severe burnout symptoms and left the workplace, strategies are sorely needed to provide healing and a return-to-work plan.15 This may include adjusting nurse schedules and nursing roles (such as admitting/discharge nurse or resource nurse).
RESILIENCY AND RESOURCEFULNESS
Rushton and colleagues describe resiliency as the “ability to face adverse situations, remain focused, and continue to be optimistic for the future.”4 For nurses in complex health care systems, resiliency is associated with reduced turnover and symptoms of burnout and improved mental health. Humans are thought to have an innate resiliency potential that evolves over time and fluctuates depending on the context (eg, societal conditions, moral/ethical values, commitments).4 It is believed that resiliency can contribute to the development of new neuropathways that can be used to manage and cope with stress, prevent burnout, and improve quality of life. However, it appears these adaptations are individualized and contingent on situations, available resources, and changing priorities.16 Consequently, resiliency may be an essential tool for nurses to combat burnout in today’s complex health care systems.17
Although resilience and resourcefulness are conceptually related, each has distinctive features.18 Celinski frames resilience as transcendence and resourcefulness as transformation.19 Thus, while resilience suggests transcendence in terms of rising above, going beyond, exceeding, or excelling; resourcefulness reflects transformation, such as making changes in thoughts, feelings, behaviors, actions, or reactions. Resourcefulness has been conceptualized as an indicator of resilience.18
Resourcefulness comprises 2 dimensions, including the use of self-help strategies (personal resourcefulness) and seeking help from others (social resourcefulness), to self-regulate one’s thoughts, feelings, and behaviors to cope with high levels of stress, anxiety, or depression.18,20,21 Personal resourcefulness skills include the use of cognitive reframing, positive thinking, problem-solving, priority-setting, and planning ahead. Social resourcefulness involves actively seeking help from others. Formal sources of help include, but are not limited to, nursing and medical care practitioners and community organizations such as hospitals and clinics. Informal sources of help include family members, friends, peers, and coworkers.
During the COVID-19 pandemic, nurses were especially challenged to provide support for each other because of limited nursing staff and treatment options, increased complex patient assignments, shortages of supplies, and reduced support services. Many nurses, however, were able to find innovative, peer-supported strategies for coping.13 Nurses’ use of resourcefulness skills is believed to be indicative of their resilience. This pilot project aimed to identify and evaluate some of these strategies and resourcefulness skills.
INTERVENTION
This pilot study among VA Northeast Ohio Health Care System (VANEOHS) nurses was designed to assess nursing burnout and resourcefulness during the pandemic. Those who agreed to participate completed a baseline survey on burnout and resourcefulness. Participants agreed to review a training video on resourcefulness skills (eg, relying on and exchanging ideas with others, and reframing and using ‘positive self-talk’). They were encouraged to document their experience with familiar and new resourcefulness skills. Weekly reminders (eg, emails and phone messages) reminded and coached participants in their journey.
The study identified and implemented an existing Resourcefulness Training (RT) intervention, which was developed for informal family caregivers and found to be effective.22 We measured burnout and resourcefulness preintervention and postintervention.23 This survey and educational intervention were reviewed by the VANEOHS institutional review board and ruled exempt. The survey also gathered information on nurses' contact with individuals infected with COVID-19.
Despite the many staffing and resource challenges during the COVID-19 pandemic, a convenience sample of 12 nurses was recruited from nursing committees that continued to have scheduled meetings. These meetings allowed time to answer questions and provide information about the study. The majority of nurses queried declined to participate, citing no time, interest, or burnout. Participants completed a baseline survey, reviewed a 30-minute RT video, and tracked their experience for 28 days. Participants completed postintervention surveys 6 weeks after the video. Details of the survey and measures can be found in previous studies.20,21
RT is an online cognitive-behavioral intervention that teaches and reinforces personal (self-help) and social (help-seeking) resourcefulness skills that have not yet been tested in nurses or other health care professionals.22,24 The training included social resourcefulness (eg, from family, friends, others, and professionals) and personal resourcefulness (eg, problem-solving, positive thinking, self-control, organization skills). Participants were encouraged to review the videos as often as they preferred during these 4 weeks.
All 12 survey respondents were female and had received COVID-19 vaccinations according to the federal policy at the time of data collection. The number of patients cared for with COVID-19 infections varied widely (range, 1-1000). The baseline burnout score ranged from 1 (no burnout) to 3 (1 symptom of burnout, such as physical and emotional exhaustion), with a mean score of 2.2. In the follow-up survey, the mean score was 2.0. At baseline, participants reported a variety of activities to manage stress and burnout, including times with friends and family, engaging in hobbies, and prayer. Postintervention, some participants mentioned using skills learned from RT, including reframing the situation positively by refocusing and putting stressors in perspective (Table 1).
DISCUSSION
Recent American Nurses Association efforts to develop organizational and professional goals include the importance of nurses to recognize and manage stress to prevent burnout.25 The American Nurses Association Code of Ethics notes that nurses have the same duties to care for themselves as they do for others.25 Nurses have demonstrated the ability to adapt and remain resilient during stressful times. VA nurses are a resourceful group. Many used resourcefulness skills to manage stress and burnout even before the pandemic. For example, nurses identified using family/friends for support and validation, as well as prayer and meditation. Some of the new activities may have been influenced/inspired by RT, such as organizing schedules for problem-solving and distraction.
Relying on family and peers emerged as an essential resourcefulness skill. Support from peers—battle buddies—has been recognized as a key strategy among combat soldiers. A battle buddy is paired with a fellow soldier for support to keep each other informed about key instructions and information. This promotes cooperative problem-solving. Outcomes associated with battle buddies include increased morale and confidence, and decreased stress.25 Over time, it is hoped that these coaching/mentoring relationships will result in enhanced leadership skills. Battle buddy strategies are currently being adapted into health care environments.12,26 Such programs need to be further evaluated and information disseminated.
Findings from this pilot program support the use of interventions such as RT to decrease burnout among nurses. This study suggests that RT should be tested in a larger more representative sample to determine efficacy.
Limitations
This pilot study was limited by its small sample size, single facility, and female-only participants; the findings are not generalizable. Nurses were recruited from VA nursing committees and may not be representative of nurses in the general population. In addition, the RT intervention may require a longer time commitment to adequately determine efficacy. Another limitation was that personal or family exposure to COVID-19 was not measured, but may be a confounding variable. An additional limitation may have been the time interval. A baseline survey was completed prior to watching the teaching video. Daily logs were to be completed for 28 days. A post survey followed at 6 weeks. It is possible that there was insufficient time for the nurses to have the opportunity to use their resourcefulness skills within the short time frame of the study. While it supports the need for further studies, findings should be interpreted cautiously and not generalized. It may be premature based on these findings to conclude that the intervention will be effective for other populations. Further studies are needed to assess nurses’ preferences for healthy coping mechanisms, including RT.
Conclusions
As the nursing shortage continues, efforts to support diverse, innovative coping strategies remain a priority postpandemic. Nurses must be vigilant in appraising and managing their ability to cope and adapt to individual stress, while also being aware of the stress their colleagues are experiencing. Educational institutions, professional organizations, and health care facilities must strive to educate and support nurses to identify not only stress, but healthy coping mechanisms.
Acknowledgments
This work was supported by the US Department of Veterans Affairs Central Office rapid response COVID-19 funding initiative, the Veteran Affairs Northeast Ohio Health Care System, and Geriatric Research, Education, and Clinical Center (GRECC). The Resourcefulness Scale, Resourcefulness Skills Scale, and the Resourcefulness Training intervention are copyrighted and were used with permission of the copyright holder, Jaclene A. Zauszniewski, PhD, RN-BC.
Nurses are recognized among the most trusted professions in the United States.1 Since the time of Florence Nightingale, nurses have been challenged to provide care to patients and soldiers with complex needs, including acute and chronic physical illness, as well as mental health issues. Nurses have traditionally met those challenges with perseverance and creativity but have also experienced stress and burnout.
A shortage of nurses has been linked to many interrelated factors including the retirement of bedside caregivers and educators, diverse care settings, expanding roles for nurses, and nurse burnout.2-4 Therefore, there is a critical need to better understand of how nurses can be supported while they care for patients, cope with stress, and maintain positive personal and professional outcomes. The objective of this pilot project was to assess US Department of Veterans Affairs (VA) nurses’ levels of burnout and test an intervention to enhance resourcefulness skills during the COVID-19 pandemic.
Background
Stress has many definitions. Hans Selye described it as a biological response of the body to any demand.5,6 Occupational stress is a process that occurs in which work environment stressors result in the development of psychological, behavioral, or physiological effects that can contribute to health.6 Occupational stress has been observed as prevalent among nurses.6 In 1960, Menzies identified sources of stress among nurses that include complex decision-making within a dynamic environment.7 Since the mid-1980s, nurses’ stress at work has increased because of legal, accreditation, ethical issues, fiscal pressures, staffing shortages, and the increasing integration of technology associated with clinical care.8
Sustained stress can lead to emotional exhaustion or burnout, which has been associated with nursing turnover, lower patient satisfaction, and patient safety risk.2,9 An American Nurses Foundation survey reported that 51% of US nurses feel exhausted, 43% overwhelmed, and 36% anxious; 28% express willingness to leave the profession.2 Burnout has been described as a response to physical or emotional stress leading to exhaustion, self-doubt, cynicism, and ineffectiveness.10 Employees with burnout are more likely to leave their jobs, take sick leave, and suffer from depression and relationship problems, and it affects nearly half of all US nurses, especially among critical care, pediatric, and oncology specialities.10,11 It has been well documented that unmitigated stress can lead to burnout and contribute to nurses leaving bedside care and the health care profession.2,3 Several studies on nursing stress and burnout have focused on its prevalence and negative outcomes.4,7,9 However, few studies have addressed building resiliency and resourcefulness for nurses.10,12,13
A 2021 National Academy of Medicine report advocated a multilevel approach to managing burnout and building resiliency among nurses.14 Taylor further identified specific interventions, ranging from primary prevention to treatment.15 Primary prevention could include educating nurses on self-awareness, coping strategies, and communication skills. Screening for burnout and providing resources for support would be a secondary level of intervention. For nurses who experienced severe burnout symptoms and left the workplace, strategies are sorely needed to provide healing and a return-to-work plan.15 This may include adjusting nurse schedules and nursing roles (such as admitting/discharge nurse or resource nurse).
RESILIENCY AND RESOURCEFULNESS
Rushton and colleagues describe resiliency as the “ability to face adverse situations, remain focused, and continue to be optimistic for the future.”4 For nurses in complex health care systems, resiliency is associated with reduced turnover and symptoms of burnout and improved mental health. Humans are thought to have an innate resiliency potential that evolves over time and fluctuates depending on the context (eg, societal conditions, moral/ethical values, commitments).4 It is believed that resiliency can contribute to the development of new neuropathways that can be used to manage and cope with stress, prevent burnout, and improve quality of life. However, it appears these adaptations are individualized and contingent on situations, available resources, and changing priorities.16 Consequently, resiliency may be an essential tool for nurses to combat burnout in today’s complex health care systems.17
Although resilience and resourcefulness are conceptually related, each has distinctive features.18 Celinski frames resilience as transcendence and resourcefulness as transformation.19 Thus, while resilience suggests transcendence in terms of rising above, going beyond, exceeding, or excelling; resourcefulness reflects transformation, such as making changes in thoughts, feelings, behaviors, actions, or reactions. Resourcefulness has been conceptualized as an indicator of resilience.18
Resourcefulness comprises 2 dimensions, including the use of self-help strategies (personal resourcefulness) and seeking help from others (social resourcefulness), to self-regulate one’s thoughts, feelings, and behaviors to cope with high levels of stress, anxiety, or depression.18,20,21 Personal resourcefulness skills include the use of cognitive reframing, positive thinking, problem-solving, priority-setting, and planning ahead. Social resourcefulness involves actively seeking help from others. Formal sources of help include, but are not limited to, nursing and medical care practitioners and community organizations such as hospitals and clinics. Informal sources of help include family members, friends, peers, and coworkers.
During the COVID-19 pandemic, nurses were especially challenged to provide support for each other because of limited nursing staff and treatment options, increased complex patient assignments, shortages of supplies, and reduced support services. Many nurses, however, were able to find innovative, peer-supported strategies for coping.13 Nurses’ use of resourcefulness skills is believed to be indicative of their resilience. This pilot project aimed to identify and evaluate some of these strategies and resourcefulness skills.
INTERVENTION
This pilot study among VA Northeast Ohio Health Care System (VANEOHS) nurses was designed to assess nursing burnout and resourcefulness during the pandemic. Those who agreed to participate completed a baseline survey on burnout and resourcefulness. Participants agreed to review a training video on resourcefulness skills (eg, relying on and exchanging ideas with others, and reframing and using ‘positive self-talk’). They were encouraged to document their experience with familiar and new resourcefulness skills. Weekly reminders (eg, emails and phone messages) reminded and coached participants in their journey.
The study identified and implemented an existing Resourcefulness Training (RT) intervention, which was developed for informal family caregivers and found to be effective.22 We measured burnout and resourcefulness preintervention and postintervention.23 This survey and educational intervention were reviewed by the VANEOHS institutional review board and ruled exempt. The survey also gathered information on nurses' contact with individuals infected with COVID-19.
Despite the many staffing and resource challenges during the COVID-19 pandemic, a convenience sample of 12 nurses was recruited from nursing committees that continued to have scheduled meetings. These meetings allowed time to answer questions and provide information about the study. The majority of nurses queried declined to participate, citing no time, interest, or burnout. Participants completed a baseline survey, reviewed a 30-minute RT video, and tracked their experience for 28 days. Participants completed postintervention surveys 6 weeks after the video. Details of the survey and measures can be found in previous studies.20,21
RT is an online cognitive-behavioral intervention that teaches and reinforces personal (self-help) and social (help-seeking) resourcefulness skills that have not yet been tested in nurses or other health care professionals.22,24 The training included social resourcefulness (eg, from family, friends, others, and professionals) and personal resourcefulness (eg, problem-solving, positive thinking, self-control, organization skills). Participants were encouraged to review the videos as often as they preferred during these 4 weeks.
All 12 survey respondents were female and had received COVID-19 vaccinations according to the federal policy at the time of data collection. The number of patients cared for with COVID-19 infections varied widely (range, 1-1000). The baseline burnout score ranged from 1 (no burnout) to 3 (1 symptom of burnout, such as physical and emotional exhaustion), with a mean score of 2.2. In the follow-up survey, the mean score was 2.0. At baseline, participants reported a variety of activities to manage stress and burnout, including times with friends and family, engaging in hobbies, and prayer. Postintervention, some participants mentioned using skills learned from RT, including reframing the situation positively by refocusing and putting stressors in perspective (Table 1).
DISCUSSION
Recent American Nurses Association efforts to develop organizational and professional goals include the importance of nurses to recognize and manage stress to prevent burnout.25 The American Nurses Association Code of Ethics notes that nurses have the same duties to care for themselves as they do for others.25 Nurses have demonstrated the ability to adapt and remain resilient during stressful times. VA nurses are a resourceful group. Many used resourcefulness skills to manage stress and burnout even before the pandemic. For example, nurses identified using family/friends for support and validation, as well as prayer and meditation. Some of the new activities may have been influenced/inspired by RT, such as organizing schedules for problem-solving and distraction.
Relying on family and peers emerged as an essential resourcefulness skill. Support from peers—battle buddies—has been recognized as a key strategy among combat soldiers. A battle buddy is paired with a fellow soldier for support to keep each other informed about key instructions and information. This promotes cooperative problem-solving. Outcomes associated with battle buddies include increased morale and confidence, and decreased stress.25 Over time, it is hoped that these coaching/mentoring relationships will result in enhanced leadership skills. Battle buddy strategies are currently being adapted into health care environments.12,26 Such programs need to be further evaluated and information disseminated.
Findings from this pilot program support the use of interventions such as RT to decrease burnout among nurses. This study suggests that RT should be tested in a larger more representative sample to determine efficacy.
Limitations
This pilot study was limited by its small sample size, single facility, and female-only participants; the findings are not generalizable. Nurses were recruited from VA nursing committees and may not be representative of nurses in the general population. In addition, the RT intervention may require a longer time commitment to adequately determine efficacy. Another limitation was that personal or family exposure to COVID-19 was not measured, but may be a confounding variable. An additional limitation may have been the time interval. A baseline survey was completed prior to watching the teaching video. Daily logs were to be completed for 28 days. A post survey followed at 6 weeks. It is possible that there was insufficient time for the nurses to have the opportunity to use their resourcefulness skills within the short time frame of the study. While it supports the need for further studies, findings should be interpreted cautiously and not generalized. It may be premature based on these findings to conclude that the intervention will be effective for other populations. Further studies are needed to assess nurses’ preferences for healthy coping mechanisms, including RT.
Conclusions
As the nursing shortage continues, efforts to support diverse, innovative coping strategies remain a priority postpandemic. Nurses must be vigilant in appraising and managing their ability to cope and adapt to individual stress, while also being aware of the stress their colleagues are experiencing. Educational institutions, professional organizations, and health care facilities must strive to educate and support nurses to identify not only stress, but healthy coping mechanisms.
Acknowledgments
This work was supported by the US Department of Veterans Affairs Central Office rapid response COVID-19 funding initiative, the Veteran Affairs Northeast Ohio Health Care System, and Geriatric Research, Education, and Clinical Center (GRECC). The Resourcefulness Scale, Resourcefulness Skills Scale, and the Resourcefulness Training intervention are copyrighted and were used with permission of the copyright holder, Jaclene A. Zauszniewski, PhD, RN-BC.
1. Walker A. Nursing ranked as the most trusted profession for 22nd year in a row. January 23, 2024. Accessed January 31, 2024. https://nurse.org/articles/nursing-ranked-most-honest-profession
2. Mental health and wellness survey 1. American Nurses Foundation. August 2020. Accessed January 31, 2024. https://www.nursingworld.org/practice-policy/work-environment/health-safety/disaster-preparedness/coronavirus/what-you-need-to-know/mental-health-and-wellbeing-survey/
3. Healthy nurse, healthy nation. American Nurses Association. May 1, 2017. Accessed January 31, 2024. https://www.healthynursehealthynation.org/
4. Rushton CH, Batcheller J, Schroeder K, Donohue P. Burnout and resilience among nurses practicing in high-intensity settings. Am J Crit Care. 2015;24(5):412-420. doi:10.4037/ajcc2015291
5. Selye HA. History and general outline of the stress concept. Stress in Health and Disease. Butterworths; 1976:3-34.
6. Levy BS, Wegman DH, Baron SL, Sokas RK. Recognizing and preventing occupational and environmental disease and injury. Occupational and Environmental Health: Recognizing and Preventing Disease and Injury. 6th ed. Oxford University Press; 2011:59-77.
7. Menzies IEP. Nurses under stress. Int Nurs Rev. 1960;7:9-16.
8. Jennings BM. Turbulence. In: Hughes RG, ed. Advances in Patient Safety and Quality: An Evidence-Based Handbook for Nurses. 3rd ed. AHRQ Publication; 2007;2;193-202.
9. Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288(16):1987-1993. doi:10.1001/jama.288.16.1987
10. Magtibay DL, Chesak SS, Coughlin K, Sood A. Decreasing stress and burnout in nurses: efficacy of blended learning with stress management and resilience training program. J Nurs Adm. 2017;47(7-8):391-395. doi:10.1097/NNA.0000000000000501
11. Halbesleben JR, Wakefield BJ, Wakefield DS, Cooper LB. Nurse burnout and patient safety outcomes: nurse safety perception versus reporting behavior. West J Nurs Res. 2008;30(5):560-577. doi:10.1177/0193945907311322
12. Sherman RO. Creating a Battle Buddy program. September 2, 2021. Accessed September 27, 2022. https://www.emergingrnleader.com/creating-a-battle-buddy-program
13. Godfrey KM, Scott SD. At the heart of the pandemic: nursing peer support. Nurse Leader. 2021:19(2),188-193. doi:10.1016/j.mnl.2020.09.006
14. Wakefield M, Williams DR, Le Menestrel S, and Flaubert JL, Editors; Committee on the future of nursing 2020 2030; National Academy of Medicine; National Academies of Sciences, Engineering, and Medicine Institute of Medicine 2008. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: The National Academies Press. doi:10.17226/12089
15. Taylor RA. Contemporary issues: resilience training alone is an incomplete intervention. Nurs Educ Today. 2019;78:10-13. doi:10.1016/j.nedt.2019.03.014
16. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749. doi:10.1016/j.psc.2017.08.008
17. Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br J Psychiatry. 1985;147:598-611. doi:10.1192/bjp.147.6.598
18. Zauszniewski JA, Bekhet AK, Suresky MJ. Indicators of resilience in family members of persons with serious mental Illness. Psychiatr Clin North Am. 2015;38(1):131-146. doi:10.1016/j.psc.2014.11.009
19. Celinski MJ. Framing resilience as transcendence and resourcefulness as transformation. In: Celinski MJ, Gow KM, eds. Continuity Versus Creative Response to Challenge: The Primacy of Resilience and Resourcefulness in Life and Therapy. Nova Science Pub Inc; 2011:11-30.
20. Zauszniewski JA, Lai CY, Tithiphontumrong S. Development and testing of the Resourcefulness Scale for Older Adults. J Nurs Meas. 2006:14(1):57-68. doi:10.1891.jnum.14.1.57
21. Zauszniewski JA, Bekhet AK. Measuring use of resourcefulness skills: psychometric testing of a new scale. ISRN Nurs. 2011;2011:787363. doi:10.5402/2011/787363
22. Zauszniewski JA, Lekhak N, Burant CJ, Variath M, Morris DL. preliminary evidence for effectiveness of resourcefulness training for women dementia caregivers. J Fam Med. 2016:3(5):1069.
23. Dolan ED, Mohr D, Lempa M, et al. Using a single item to measure burnout in primary care staff: a psychometric evaluation. J Gen Intern Med. 2015;30(5):582-587. doi:10.1007/s11606-014-3112-6
24. Zauszniewski JA Resourcefulness. In: Fitzpatrick JJ, ed. Encyclopedia of Nursing Research. 4th ed. 2018:632-634.
25. Combating Stress. American Nurses Association. Accessed November 28, 2022. https://www.nursingworld.org/practice-policy/work-environment/health-safety/combating-stress/
26. Albott CS, Wozniak JR, McGlinch BP, Wall MH, Gold BS, Vinogradov S. Battle Buddies: Rapid deployment of a psychological resilience intervention for health care workers during the COVID-19 pandemic. Anesth Analg. 2020;131(1):43-54. doi:10.1213/ANE.0000000000004912
1. Walker A. Nursing ranked as the most trusted profession for 22nd year in a row. January 23, 2024. Accessed January 31, 2024. https://nurse.org/articles/nursing-ranked-most-honest-profession
2. Mental health and wellness survey 1. American Nurses Foundation. August 2020. Accessed January 31, 2024. https://www.nursingworld.org/practice-policy/work-environment/health-safety/disaster-preparedness/coronavirus/what-you-need-to-know/mental-health-and-wellbeing-survey/
3. Healthy nurse, healthy nation. American Nurses Association. May 1, 2017. Accessed January 31, 2024. https://www.healthynursehealthynation.org/
4. Rushton CH, Batcheller J, Schroeder K, Donohue P. Burnout and resilience among nurses practicing in high-intensity settings. Am J Crit Care. 2015;24(5):412-420. doi:10.4037/ajcc2015291
5. Selye HA. History and general outline of the stress concept. Stress in Health and Disease. Butterworths; 1976:3-34.
6. Levy BS, Wegman DH, Baron SL, Sokas RK. Recognizing and preventing occupational and environmental disease and injury. Occupational and Environmental Health: Recognizing and Preventing Disease and Injury. 6th ed. Oxford University Press; 2011:59-77.
7. Menzies IEP. Nurses under stress. Int Nurs Rev. 1960;7:9-16.
8. Jennings BM. Turbulence. In: Hughes RG, ed. Advances in Patient Safety and Quality: An Evidence-Based Handbook for Nurses. 3rd ed. AHRQ Publication; 2007;2;193-202.
9. Aiken LH, Clarke SP, Sloane DM, Sochalski J, Silber JH. Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction. JAMA. 2002;288(16):1987-1993. doi:10.1001/jama.288.16.1987
10. Magtibay DL, Chesak SS, Coughlin K, Sood A. Decreasing stress and burnout in nurses: efficacy of blended learning with stress management and resilience training program. J Nurs Adm. 2017;47(7-8):391-395. doi:10.1097/NNA.0000000000000501
11. Halbesleben JR, Wakefield BJ, Wakefield DS, Cooper LB. Nurse burnout and patient safety outcomes: nurse safety perception versus reporting behavior. West J Nurs Res. 2008;30(5):560-577. doi:10.1177/0193945907311322
12. Sherman RO. Creating a Battle Buddy program. September 2, 2021. Accessed September 27, 2022. https://www.emergingrnleader.com/creating-a-battle-buddy-program
13. Godfrey KM, Scott SD. At the heart of the pandemic: nursing peer support. Nurse Leader. 2021:19(2),188-193. doi:10.1016/j.mnl.2020.09.006
14. Wakefield M, Williams DR, Le Menestrel S, and Flaubert JL, Editors; Committee on the future of nursing 2020 2030; National Academy of Medicine; National Academies of Sciences, Engineering, and Medicine Institute of Medicine 2008. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: The National Academies Press. doi:10.17226/12089
15. Taylor RA. Contemporary issues: resilience training alone is an incomplete intervention. Nurs Educ Today. 2019;78:10-13. doi:10.1016/j.nedt.2019.03.014
16. Hofmann SG, Gómez AF. Mindfulness-based interventions for anxiety and depression. Psychiatr Clin North Am. 2017;40(4):739-749. doi:10.1016/j.psc.2017.08.008
17. Rutter M. Resilience in the face of adversity. Protective factors and resistance to psychiatric disorder. Br J Psychiatry. 1985;147:598-611. doi:10.1192/bjp.147.6.598
18. Zauszniewski JA, Bekhet AK, Suresky MJ. Indicators of resilience in family members of persons with serious mental Illness. Psychiatr Clin North Am. 2015;38(1):131-146. doi:10.1016/j.psc.2014.11.009
19. Celinski MJ. Framing resilience as transcendence and resourcefulness as transformation. In: Celinski MJ, Gow KM, eds. Continuity Versus Creative Response to Challenge: The Primacy of Resilience and Resourcefulness in Life and Therapy. Nova Science Pub Inc; 2011:11-30.
20. Zauszniewski JA, Lai CY, Tithiphontumrong S. Development and testing of the Resourcefulness Scale for Older Adults. J Nurs Meas. 2006:14(1):57-68. doi:10.1891.jnum.14.1.57
21. Zauszniewski JA, Bekhet AK. Measuring use of resourcefulness skills: psychometric testing of a new scale. ISRN Nurs. 2011;2011:787363. doi:10.5402/2011/787363
22. Zauszniewski JA, Lekhak N, Burant CJ, Variath M, Morris DL. preliminary evidence for effectiveness of resourcefulness training for women dementia caregivers. J Fam Med. 2016:3(5):1069.
23. Dolan ED, Mohr D, Lempa M, et al. Using a single item to measure burnout in primary care staff: a psychometric evaluation. J Gen Intern Med. 2015;30(5):582-587. doi:10.1007/s11606-014-3112-6
24. Zauszniewski JA Resourcefulness. In: Fitzpatrick JJ, ed. Encyclopedia of Nursing Research. 4th ed. 2018:632-634.
25. Combating Stress. American Nurses Association. Accessed November 28, 2022. https://www.nursingworld.org/practice-policy/work-environment/health-safety/combating-stress/
26. Albott CS, Wozniak JR, McGlinch BP, Wall MH, Gold BS, Vinogradov S. Battle Buddies: Rapid deployment of a psychological resilience intervention for health care workers during the COVID-19 pandemic. Anesth Analg. 2020;131(1):43-54. doi:10.1213/ANE.0000000000004912
Robotic Pet Therapy in the Intensive Care Unit
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
Small Fiber Neuropathy in Veterans With Gulf War Illness
Following deployment to operations Desert Shield and Desert Storm (Gulf War) in 1990 and 1991, many Gulf War veterans (GWVs) developed chronic, complex symptoms, including pain, dyscognition, and fatigue, with gastrointestinal, skin, and respiratory manifestations. This Gulf War Illness (GWI) is reported to affect about 30% of those deployed. More than 30 years later, there is no consensus as to the etiology of GWI, although some deployment-related exposures have been implicated.1
Accepted research definitions for GWI include the Centers for Disease Control and Prevention and Kansas definitions.2 The US Department of Veterans Affairs (VA) uses the terminology chronic multisymptom illness (CMI), which is an overarching diagnosis under which GWI falls. Although there is no consensus case definition for CMI, there is overlap with conditions such as fibromyalgia, myalgic encephalomyelitis/chronic fatigue syndrome, and irritable bowel syndrome; the VA considers these as qualifying clinical diagnoses.3 The pathophysiology of GWI is also unknown, though a frequently reported unifying feature is that of autonomic nervous system (ANS) dysfunction. Studies have demonstrated differences between veterans with GWI and those without GWI in both the reporting of symptoms attributable to ANS dysfunction and in physiologic evaluations of the ANS.4-10
Small fiber neuropathy (SFN), a condition with damage to the A-δ and C small nerve fibers, has been proposed as a potential mechanism for the pain and ANS dysfunction experienced in GWI.11-13 Symptoms of SFN are similar to those of GWI, with pain and ANS symptoms commonly reported.14,15 There are multiple diagnostic criteria for SFN, the most commonly used requiring the presence of appropriate symptoms in the absence of large fiber neuropathy and a skin biopsy demonstrating reduced intraepidermal nerve fiber density.16-19 Several conditions reportedly cause SFN, most notably diabetes/prediabetes. Autoimmune disease, vitamin B12 deficiency, monoclonal gammopathies, celiac disease, paraneoplastic syndromes, and sodium channel gene mutations may also contribute to SFN.20 Hyperlipidemia has been identified as a contributor, although it has been variably reported.21,22
Idiopathic neuropathies, SFN included, may be secondary to neurotoxicant exposures. Agents whose exposure or consumption have been associated with SFN include alcohol most prominently, but also the organic solvent n-hexane, heavy metals, and excess vitamin B6.20,23-25 Agents associated with large fiber neuropathy may also have relevance for SFN, as small fibers have been likened to the “canary in the coal mine” in that they may be more susceptible to neurotoxicants and are affected earlier in the disease process.26 In this way, SFN may be the harbinger of large fiber neuropathy in some cases. Of specific relevance for GWVs, organophosphates and carbamates are known to produce a delayed onset large fiber neuropathy.27-30 Exposure to petrochemical solvents has also been associated with large fiber neuropathies.31,32
The War Related Illness and Injury Study Center (WRIISC) is a clinical, research, and education center established by Congress in 2001. Its primary focus is on military exposures and postdeployment health of veterans. It is located at 3 sites: East Orange, New Jersey; Washington, DC; and Palo Alto, California. The New Jersey WRIISC began a program to evaluate GWVs with characteristic symptoms for possible SFN with use of a skin biopsy.
We hypothesize that SFN may underly much of GWI symptomatology and may not be accounted for by the putative etiologies detailed in review of the medical literature. This retrospective review of clinical evaluations for SFN in GWVs who sought care at the New Jersey WRIISC explored and addressed the following questions: (1) how common is biopsy-confirmed SFN in veterans with GWI; (2) do veterans with GWI and SFN report more symptoms attributable to ANS dysfunction when compared with veterans with GWI and no SFN; and (3) can SFN in veterans with GWI and SFN be explained by conditions and substances commonly associated with SFN? Institutional review board approval and waiver of consent was obtained from the Veterans Affairs New Jersey Health Care Center for the study.
Methods
A retrospective chart review was conducted on veterans evaluated at the WRIISC from March 1, 2015, to January 31, 2019. Inclusion criteria were: deployment to operations Desert Shield and Desert Storm between August 2, 1990, and February 28, 1991, and skin biopsy conducted at the WRIISC. Skin biopsies were obtained at the discretion of an examining clinician based on clinical indications, including neuropathic pain, ANS symptoms, and/or a fibromyalgia/chronic pain–type presentation.
Electronic health record review explicitly abstracted GWI status, results of the skin biopsy, and ANS symptom burden as determined by the Composite Autonomic Symptom Scale 31 (COMPASS 31) completed at the time of the WRIISC evaluation.
COMPASS 31 assesses symptoms across 6 domains (orthostatic, vasomotor, secretomotor, gastrointestinal, bladder, andpupillomotor). Patients are asked about symptom frequency (rarely to almost always), severity (mild to severe), and improvement (much worse to completely gone). Individual domain scores and a total weighted score (0-100) have demonstrated good validity, reliability, and consistency in SFN.33,34
In veterans with GWI and documented SFN, a health record review was performed to identify potential etiologies for SFN (Appendix).
Statistical Analysis
Microsoft Excel and IBM SPSS 12.0.1 for Windows were used for data collection and statistical analysis. Fisher exact test was used for comparing the prevalence of SFN in veterans with GWI vs without GWI. The independent samples t test was used for comparing COMPASS 31 scores for veterans with GWI by SFN status. α < .05 was used for determining statistical significance. For those GWVs documented with SFN and GWI, potential explanations were documented in total and by condition.
Results
From March 1, 2015, to January 31, 2019, 141 GWVs received a comprehensive in person clinical evaluation at the WRIISC and 51 veterans (36%) received a skin biopsy and were included in this retrospective observational study (Figure). The mean age was 48.6 years, and the majority were male and served in the US Army. Skin biopsies met clinical criteria for GWI for 42 (82%) and 24 of 42 (57%) were determined to have SFN. Four of 9 (44%) veterans without GWI had positive SFN biopsies, though this difference was not statistically significant (Table 1). Veterans with SFN but no GWI were not included in the further analysis.
Thirty-five veterans with GWI—18 with SFN and 17 without SFN—completed the COMPASS 31 (Table 2). COMPASS 31 data were not analyzed for veterans without GWI. Individual domain scores and the difference in COMPASS 31 scores for veterans with GWI and SFN vs GWI and no SFN (38.3 vs 37.8, respectively) were not statistically significant.
Sixteen of 24 veterans with GWI and SFN (67%) had ≥ 1 conditions that could potentially be responsible for SFN (Table 3), including 11 veterans (46%) with prediabetes/diabetes. Hyperlipidemia is only variably reported as a cause of SFN; when included, 19 of 24 (79%) SFN cases were accounted for. We could not identify a medical explanation for SFN in 5 of 24 veterans (21%) with GWI, which were deemed to be idiopathic.
Discussion
Biopsy-confirmed SFN was present in more than half of our sample of veterans with GWI, which is broadly consistent with what has been reported in the literature.13,35-38 In this clinical observation study, SFN was similarly prevalent in veterans with and without GWI; although it should be noted that biopsies only were obtained when there was a strong clinical suspicion for SFN. Almost half of patients with GWI did not have SFN, so our study does not support SFN as the underlying explanation for all GWI. Although our data cannot provide clinical guidance as to when skin biopsy may be indicated in GWI, work done in fibromyalgia found symptoms of dysautonomia and paresthesias are more specific for SFN and may be useful to help guide medical decision making.39
Veterans with GWI in our clinical sample reported a high burden of clinical symptoms conceivably attributable to ANS dysfunction. This symptom reporting is consistent with that seen in other GWI studies, as well as in other studies of SFN.4,5,7-9,14,15,34,38,40 Our clinical sample of veterans with GWI found no differences in the ANS symptom reporting between those with and without SFN. Therefore, our study cannot support SFN alone as accounting for ANS symptom burden in patients with GWI.
Two-thirds of biopsy-confirmed SFN in our clinical sample of veterans with GWI could potentially be explained by established medical conditions. As in other studies of SFN, prediabetes and diabetes represented a plurality (46%). Even after considering hyperlipidemia as a potential explanation, about 21% of SFN cases in veterans with GWI still were deemed idiopathic.
Evidence supports certain environmental agents as causal factors for GWI. Neurotoxicants reportedly related to GWI include pesticides (particularly organophosphates and carbamates), pyridostigmine bromide (used during the Gulf War as a prophylactic agent against the use of chemical weapons), and low levels of the nerve agent sarin from environmental contamination due to chemical weapons detonations.1 Some of these agents have been implicated in neuropathy as well.1,28-30 It is biologically plausible that deployment-related exposures could trigger SFN, though the traditional consensus has been that remote exposure to neurotoxic substances is unlikely to produce neuropathy that presents many years after the exposure.41 In the WRIISC clinical experience, however, veterans often report that their neuropathic symptoms predate the diagnosis of the associated medical conditions, sometimes by decades. It is conceivable that remote exposures may trigger the condition that is then potentiated by ongoing exposures, metabolic factors, and/or other medical conditions. These may perpetuate neuropathic symptoms and the illness experience of affected veterans. Our clinical observation study cannot clarify the extent to which this may be the case. Despite these findings and arguments, an environmental contribution to SFN cannot be discounted, and further research is needed to explore a potential relationship.
Limitations
This study’s conclusions are limited by its observational/retrospective design in a relatively small clinical sample of veterans evaluated at a tertiary referral center for postdeployment exposure-related health concerns. The WRIISC clinical sample is not representative of all GWVs or even of all veterans with GWI, as there is inherent selection bias as to who gets referred to and evaluated at the WRIISC. As with studies based on retrospective chart review, data are reliant on clinical documentation andaccuracy/consistency of the reviewer. Evaluation for SFN with skin biopsy is an invasive procedure and was performed when a high index of clinical suspicion for this condition existed, possibly representing confirmation bias. Therefore, the relatively high prevalence ofbiopsy-confirmed SFN seen in our clinical sample cannot be generalized to GWVs as a whole or even to veterans with GWI.
Assessment of autonomic dysfunction was based on COMPASS 31 symptom reporting by an small subset of the clinical cohort. Symptom reporting may not be reflective of true abnormality in ANS function. Physiologic tests of the ANS were not performed; such studies could more objectively establish whether ANS dysfunction is more prevalent in GWI veterans with SFN.
Evaluation for all potential etiologic/contributory conditions to SFN was not exhaustive. For example, sodium channel gene mutations have been documented to account for up to one-third of all cases of idiopathic SFN.42 For those cases in which no compelling etiology was identified, it is plausible that medical explanations for SFN may be found on further investigation.
Clinical assessments at the WRIISC were performed on GWVs ≥ 26 years after their deployment-related exposures. Other conditions/exposures may have occurred in the interim. What is not clear is whether the SFN predated the onset of any of these medical conditions or other putative contributors. This observational study is not able to tease out a temporal association to make a cause-and-effect assessment.
Conclusions
Retrospective analysis of clinical data of veterans evaluated at a specialized center for postdeployment health demonstrated that skin biopsy–confirmed SFN was prevalent, but not ubiquitous, in veterans with GWI. Symptom that may be attributed to ANS dysfunction in this clinical sample was consistent with literature on SFN and with GWI, but we could not definitively attribute ANS symptoms to SFN. Our study does not support the hypothesis that GWI symptoms are solely due to SFN, though it may still be relevant in a subset of veterans with GWI with strongly suggestive clinical features. We were able to identify a potential etiology for SFN in most veterans with GWI. Further investigations are recommended to explore any potential relationship between Gulf War exposures and SFN.
1. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. doi:10.1016/j.cortex.2015.08.022
2. Committee on the Development of a Consensus Case Definition for Chronic Multisymptom Illness in 1990-1991 Gulf War Veterans, Board on the Health of Select Populations, Institute of Medicine. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. National Academies Press; 2014.
3. Robbins R, Helmer D, Monahan P, et al. Management of chronic multisymptom illness: synopsis of the 2021 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guideline. Mayo Clin Proc. 2022;97(5):991-1002. doi:10.1016/j.mayocp.2022.01.031
4. Fox A, Helmer D, Tseng CL, Patrick-DeLuca L, Osinubi O. Report of autonomic symptoms in a clinical sample of veterans with Gulf War Illness. Mil Med. 2018;183(3-4):e179-e185. doi:10.1093/milmed/usx052
5. Fox A, Helmer D, Tseng CL, McCarron K, Satcher S, Osinubi O. Autonomic symptoms in Gulf War veterans evaluated at the War Related Illness and Injury Study Center. Mil Med. 2019;184(3-4):e191-e196. doi:10.1093/milmed/usy227
6. Reyes L, Falvo M, Blatt M, Ghobreal B, Acosta A, Serrador J. Autonomic dysfunction in veterans with Gulf War illness [abstract]. FASEB J. 2014;28(S1):1068.19. doi:10.1096/fasebj.28.1_supplement.1068.19
7. Haley RW, Charuvastra E, Shell WE, et al. Cholinergic autonomic dysfunction in veterans with Gulf War illness: confirmation in a population-based sample. JAMA Neurol. 2013;70(2):191-200. doi:10.1001/jamaneurol.2013.596
8. Haley RW, Vongpatanasin W, Wolfe GI, et al. Blunted circadian variation in autonomic regulation of sinus node function in veterans with Gulf War syndrome. Am J Med. 2004;117(7):469-478. doi:10.1016/j.amjmed.2004.03.041
9. Avery TJ, Mathersul DC, Schulz-Heik RJ, Mahoney L, Bayley PJ. Self-reported autonomic dysregulation in Gulf War Illness. Mil Med. Published online December 30, 2021. doi:10.1093/milmed/usab546
10. Verne ZT, Fields JZ, Zhang BB, Zhou Q. Autonomic dysfunction and gastroparesis in Gulf War veterans. J Investig Med. 2023;71(1):7-10. doi:10.1136/jim-2021-002291
11. Levine TD. Small fiber neuropathy: disease classification beyond pain and burning. J Cent Nerv Syst Dis. 2018;10:1179573518771703. doi:10.1177/1179573518771703
12. Novak P. Autonomic disorders. Am J Med. 2019;132(4):420-436. doi:10.1016/j.amjmed.2018.09.027
13. Oaklander AL, Klein MM. Undiagnosed small-fiber polyneuropathy: is it a component of Gulf War Illness? Defense Technical Information Center. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/ADA613891
 14. Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine. 2018;85(5):553-559. doi:10.1016/j.jbspin.2017.11.002
14. Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine. 2018;85(5):553-559. doi:10.1016/j.jbspin.2017.11.002
15. Novak V, Freimer ML, Kissel JT, et al. Autonomic impairment in painful neuropathy. Neurology. 2001;56(7):861-868. doi:10.1212/wnl.56.7.861
16. Myers MI, Peltier AC. Uses of skin biopsy for sensory and autonomic nerve assessment. Curr Neurol Neurosci Rep. 2013;13(1):323. doi:10.1007/s11910-012-0323-2
17. Haroutounian S, Todorovic MS, Leinders M, et al. Diagnostic criteria for idiopathic small fiber neuropathy: a systematic review. Muscle Nerve. 2021;63(2):170-177. doi:10.1002/mus.27070
18. Levine TD, Saperstein DS. Routine use of punch biopsy to diagnose small fiber neuropathy in fibromyalgia patients. Clin Rheumatol. 2015;34(3):413-417. doi:10.1007/s10067-014-2850-5
19. England JD, Gronseth G S, Franklin G, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2009;1(1):14-22. doi:10.1016/j.pmrj.2008.11.011
20. de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy - a large cohort study and review of the literature. Eur J Neurol. 2018;25(2):348-355. doi:10.1111/ene.13508
21. Morkavuk G, Leventoglu A. Small fiber neuropathy associated with hyperlipidemia: utility of cutaneous silent periods and autonomic tests. ISRN Neurol. 2014;2014:579242. doi:10.1155/2014/579242
22. Bednarik J, Vlckova-Moravcova E, Bursova S, Belobradkova J, Dusek L, Sommer C. Etiology of small-fiber neuropathy. J Peripher Nerv Syst. 2009;14(3):177-183. doi:10.1111/j.1529-8027.2009.00229.x
23. Kokotis P, Papantoniou M, Schmelz M, Buntziouka C, Tzavellas E, Paparrigopoulos T. Pure small fiber neuropathy in alcohol dependency detected by skin biopsy. Alcohol Fayettev N. 2023;111:67-73. doi:10.1016/j.alcohol.2023.05.006
24. Guimarães-Costa R, Schoindre Y, Metlaine A, et al. N-hexane exposure: a cause of small fiber neuropathy. J Peripher Nerv Syst. 2018;23(2):143-146. doi:10.1111/jns.12261
25. Koszewicz M, Markowska K, Waliszewska-Prosol M, et al. The impact of chronic co-exposure to different heavy metals on small fibers of peripheral nerves. A study of metal industry workers. J Occup Med Toxicol. 2021;16(1):12. doi:10.1186/s12995-021-00302-6
26. Johns Hopkins Medicine. Small nerve fibers defy neuropathy conventions. April 11, 2016. Accessed February 21, 2024. https://www.hopkinsmedicine.org/news/media/releases/small_nerve_fibers_defy_neuropathy_conventions
27. Jett DA. Neurotoxic pesticides and neurologic effects. Neurol Clin. 2011;29(3):667-677. doi:10.1016/j.ncl.2011.06.002
28. Berger AR, Schaumburg HH. Human toxic neuropathy caused by industrial agents. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed. Saunders; 2005:2505-2525. doi:10.1016/B978-0-7216-9491-7.50115-0
29. Herskovitz S, Schaumburg HH. Neuropathy caused by drugs. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed. Saunders; 2005:2553-2583.
30. Katona I, Weis J. Chapter 31 - Diseases of the peripheral nerves. Handb Clin Neurol. 2017;145:453-474. doi:10.1016/B978-0-12-802395-2.00031-6
31. Matikainen E, Juntunen J. Autonomic nervous system dysfunction in workers exposed to organic solvents. J Neurol Neurosurg Psychiatry. 1985;48(10):1021-1024. doi:10.1136/jnnp.48.10.1021
32. Murata K, Araki S, Yokoyama K, Maeda K. Autonomic and peripheral nervous system dysfunction in workers exposed to mixed organic solvents. Int Arch Occup Environ Health. 1991;63(5):335-340. doi:10.1007/BF00381584
33. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87(12):1196-1201. doi:10.1016/j.mayocp.2012.10.013
34. Treister R, O’Neil K, Downs HM, Oaklander AL. Validation of the Composite Autonomic Symptom Scale-31 (COMPASS-31) in patients with and without small-fiber polyneuropathy. Eur J Neurol. 2015;22(7):1124-1130. doi:10.1111/ene.12717
35. Joseph P, Arevalo C, Oliveira RKF, et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160(2):642-651. doi:10.1016/j.chest.2021.01.082
36. Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve. 2014;49(5):757-759. doi:10.1002/mus.24156
37. Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2310-2316. doi:10.1016/j.pain.2013.06.001
38. Serrador JM. Diagnosis of late-stage, early-onset, small-fiber polyneuropathy. Defense Technical Information Center. December 1, 2019. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/AD1094831
39. Lodahl M, Treister R, Oaklander AL. Specific symptoms may discriminate between fibromyalgia patients with vs without objective test evidence of small-fiber polyneuropathy. Pain Rep. 2018;3(1):e633. doi:10.1097/PR9.0000000000000633
40. Sastre A, Cook MR. Autonomic dysfunction in Gulf War veterans. Defense Technical Information Center. April 1, 2004. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/ADA429525
41. Little AA, Albers JW. Clinical description of toxic neuropathies. Handb Clin Neurol. 2015;131:253-296. doi:10.1016/B978-0-444-62627-1.00015-9
42. Faber CG, Hoeijmakers JGJ, Ahn HS, et al. Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71(1):26-39.
Following deployment to operations Desert Shield and Desert Storm (Gulf War) in 1990 and 1991, many Gulf War veterans (GWVs) developed chronic, complex symptoms, including pain, dyscognition, and fatigue, with gastrointestinal, skin, and respiratory manifestations. This Gulf War Illness (GWI) is reported to affect about 30% of those deployed. More than 30 years later, there is no consensus as to the etiology of GWI, although some deployment-related exposures have been implicated.1
Accepted research definitions for GWI include the Centers for Disease Control and Prevention and Kansas definitions.2 The US Department of Veterans Affairs (VA) uses the terminology chronic multisymptom illness (CMI), which is an overarching diagnosis under which GWI falls. Although there is no consensus case definition for CMI, there is overlap with conditions such as fibromyalgia, myalgic encephalomyelitis/chronic fatigue syndrome, and irritable bowel syndrome; the VA considers these as qualifying clinical diagnoses.3 The pathophysiology of GWI is also unknown, though a frequently reported unifying feature is that of autonomic nervous system (ANS) dysfunction. Studies have demonstrated differences between veterans with GWI and those without GWI in both the reporting of symptoms attributable to ANS dysfunction and in physiologic evaluations of the ANS.4-10
Small fiber neuropathy (SFN), a condition with damage to the A-δ and C small nerve fibers, has been proposed as a potential mechanism for the pain and ANS dysfunction experienced in GWI.11-13 Symptoms of SFN are similar to those of GWI, with pain and ANS symptoms commonly reported.14,15 There are multiple diagnostic criteria for SFN, the most commonly used requiring the presence of appropriate symptoms in the absence of large fiber neuropathy and a skin biopsy demonstrating reduced intraepidermal nerve fiber density.16-19 Several conditions reportedly cause SFN, most notably diabetes/prediabetes. Autoimmune disease, vitamin B12 deficiency, monoclonal gammopathies, celiac disease, paraneoplastic syndromes, and sodium channel gene mutations may also contribute to SFN.20 Hyperlipidemia has been identified as a contributor, although it has been variably reported.21,22
Idiopathic neuropathies, SFN included, may be secondary to neurotoxicant exposures. Agents whose exposure or consumption have been associated with SFN include alcohol most prominently, but also the organic solvent n-hexane, heavy metals, and excess vitamin B6.20,23-25 Agents associated with large fiber neuropathy may also have relevance for SFN, as small fibers have been likened to the “canary in the coal mine” in that they may be more susceptible to neurotoxicants and are affected earlier in the disease process.26 In this way, SFN may be the harbinger of large fiber neuropathy in some cases. Of specific relevance for GWVs, organophosphates and carbamates are known to produce a delayed onset large fiber neuropathy.27-30 Exposure to petrochemical solvents has also been associated with large fiber neuropathies.31,32
The War Related Illness and Injury Study Center (WRIISC) is a clinical, research, and education center established by Congress in 2001. Its primary focus is on military exposures and postdeployment health of veterans. It is located at 3 sites: East Orange, New Jersey; Washington, DC; and Palo Alto, California. The New Jersey WRIISC began a program to evaluate GWVs with characteristic symptoms for possible SFN with use of a skin biopsy.
We hypothesize that SFN may underly much of GWI symptomatology and may not be accounted for by the putative etiologies detailed in review of the medical literature. This retrospective review of clinical evaluations for SFN in GWVs who sought care at the New Jersey WRIISC explored and addressed the following questions: (1) how common is biopsy-confirmed SFN in veterans with GWI; (2) do veterans with GWI and SFN report more symptoms attributable to ANS dysfunction when compared with veterans with GWI and no SFN; and (3) can SFN in veterans with GWI and SFN be explained by conditions and substances commonly associated with SFN? Institutional review board approval and waiver of consent was obtained from the Veterans Affairs New Jersey Health Care Center for the study.
Methods
A retrospective chart review was conducted on veterans evaluated at the WRIISC from March 1, 2015, to January 31, 2019. Inclusion criteria were: deployment to operations Desert Shield and Desert Storm between August 2, 1990, and February 28, 1991, and skin biopsy conducted at the WRIISC. Skin biopsies were obtained at the discretion of an examining clinician based on clinical indications, including neuropathic pain, ANS symptoms, and/or a fibromyalgia/chronic pain–type presentation.
Electronic health record review explicitly abstracted GWI status, results of the skin biopsy, and ANS symptom burden as determined by the Composite Autonomic Symptom Scale 31 (COMPASS 31) completed at the time of the WRIISC evaluation.
COMPASS 31 assesses symptoms across 6 domains (orthostatic, vasomotor, secretomotor, gastrointestinal, bladder, andpupillomotor). Patients are asked about symptom frequency (rarely to almost always), severity (mild to severe), and improvement (much worse to completely gone). Individual domain scores and a total weighted score (0-100) have demonstrated good validity, reliability, and consistency in SFN.33,34
In veterans with GWI and documented SFN, a health record review was performed to identify potential etiologies for SFN (Appendix).
Statistical Analysis
Microsoft Excel and IBM SPSS 12.0.1 for Windows were used for data collection and statistical analysis. Fisher exact test was used for comparing the prevalence of SFN in veterans with GWI vs without GWI. The independent samples t test was used for comparing COMPASS 31 scores for veterans with GWI by SFN status. α < .05 was used for determining statistical significance. For those GWVs documented with SFN and GWI, potential explanations were documented in total and by condition.
Results
From March 1, 2015, to January 31, 2019, 141 GWVs received a comprehensive in person clinical evaluation at the WRIISC and 51 veterans (36%) received a skin biopsy and were included in this retrospective observational study (Figure). The mean age was 48.6 years, and the majority were male and served in the US Army. Skin biopsies met clinical criteria for GWI for 42 (82%) and 24 of 42 (57%) were determined to have SFN. Four of 9 (44%) veterans without GWI had positive SFN biopsies, though this difference was not statistically significant (Table 1). Veterans with SFN but no GWI were not included in the further analysis.
Thirty-five veterans with GWI—18 with SFN and 17 without SFN—completed the COMPASS 31 (Table 2). COMPASS 31 data were not analyzed for veterans without GWI. Individual domain scores and the difference in COMPASS 31 scores for veterans with GWI and SFN vs GWI and no SFN (38.3 vs 37.8, respectively) were not statistically significant.
Sixteen of 24 veterans with GWI and SFN (67%) had ≥ 1 conditions that could potentially be responsible for SFN (Table 3), including 11 veterans (46%) with prediabetes/diabetes. Hyperlipidemia is only variably reported as a cause of SFN; when included, 19 of 24 (79%) SFN cases were accounted for. We could not identify a medical explanation for SFN in 5 of 24 veterans (21%) with GWI, which were deemed to be idiopathic.
Discussion
Biopsy-confirmed SFN was present in more than half of our sample of veterans with GWI, which is broadly consistent with what has been reported in the literature.13,35-38 In this clinical observation study, SFN was similarly prevalent in veterans with and without GWI; although it should be noted that biopsies only were obtained when there was a strong clinical suspicion for SFN. Almost half of patients with GWI did not have SFN, so our study does not support SFN as the underlying explanation for all GWI. Although our data cannot provide clinical guidance as to when skin biopsy may be indicated in GWI, work done in fibromyalgia found symptoms of dysautonomia and paresthesias are more specific for SFN and may be useful to help guide medical decision making.39
Veterans with GWI in our clinical sample reported a high burden of clinical symptoms conceivably attributable to ANS dysfunction. This symptom reporting is consistent with that seen in other GWI studies, as well as in other studies of SFN.4,5,7-9,14,15,34,38,40 Our clinical sample of veterans with GWI found no differences in the ANS symptom reporting between those with and without SFN. Therefore, our study cannot support SFN alone as accounting for ANS symptom burden in patients with GWI.
Two-thirds of biopsy-confirmed SFN in our clinical sample of veterans with GWI could potentially be explained by established medical conditions. As in other studies of SFN, prediabetes and diabetes represented a plurality (46%). Even after considering hyperlipidemia as a potential explanation, about 21% of SFN cases in veterans with GWI still were deemed idiopathic.
Evidence supports certain environmental agents as causal factors for GWI. Neurotoxicants reportedly related to GWI include pesticides (particularly organophosphates and carbamates), pyridostigmine bromide (used during the Gulf War as a prophylactic agent against the use of chemical weapons), and low levels of the nerve agent sarin from environmental contamination due to chemical weapons detonations.1 Some of these agents have been implicated in neuropathy as well.1,28-30 It is biologically plausible that deployment-related exposures could trigger SFN, though the traditional consensus has been that remote exposure to neurotoxic substances is unlikely to produce neuropathy that presents many years after the exposure.41 In the WRIISC clinical experience, however, veterans often report that their neuropathic symptoms predate the diagnosis of the associated medical conditions, sometimes by decades. It is conceivable that remote exposures may trigger the condition that is then potentiated by ongoing exposures, metabolic factors, and/or other medical conditions. These may perpetuate neuropathic symptoms and the illness experience of affected veterans. Our clinical observation study cannot clarify the extent to which this may be the case. Despite these findings and arguments, an environmental contribution to SFN cannot be discounted, and further research is needed to explore a potential relationship.
Limitations
This study’s conclusions are limited by its observational/retrospective design in a relatively small clinical sample of veterans evaluated at a tertiary referral center for postdeployment exposure-related health concerns. The WRIISC clinical sample is not representative of all GWVs or even of all veterans with GWI, as there is inherent selection bias as to who gets referred to and evaluated at the WRIISC. As with studies based on retrospective chart review, data are reliant on clinical documentation andaccuracy/consistency of the reviewer. Evaluation for SFN with skin biopsy is an invasive procedure and was performed when a high index of clinical suspicion for this condition existed, possibly representing confirmation bias. Therefore, the relatively high prevalence ofbiopsy-confirmed SFN seen in our clinical sample cannot be generalized to GWVs as a whole or even to veterans with GWI.
Assessment of autonomic dysfunction was based on COMPASS 31 symptom reporting by an small subset of the clinical cohort. Symptom reporting may not be reflective of true abnormality in ANS function. Physiologic tests of the ANS were not performed; such studies could more objectively establish whether ANS dysfunction is more prevalent in GWI veterans with SFN.
Evaluation for all potential etiologic/contributory conditions to SFN was not exhaustive. For example, sodium channel gene mutations have been documented to account for up to one-third of all cases of idiopathic SFN.42 For those cases in which no compelling etiology was identified, it is plausible that medical explanations for SFN may be found on further investigation.
Clinical assessments at the WRIISC were performed on GWVs ≥ 26 years after their deployment-related exposures. Other conditions/exposures may have occurred in the interim. What is not clear is whether the SFN predated the onset of any of these medical conditions or other putative contributors. This observational study is not able to tease out a temporal association to make a cause-and-effect assessment.
Conclusions
Retrospective analysis of clinical data of veterans evaluated at a specialized center for postdeployment health demonstrated that skin biopsy–confirmed SFN was prevalent, but not ubiquitous, in veterans with GWI. Symptom that may be attributed to ANS dysfunction in this clinical sample was consistent with literature on SFN and with GWI, but we could not definitively attribute ANS symptoms to SFN. Our study does not support the hypothesis that GWI symptoms are solely due to SFN, though it may still be relevant in a subset of veterans with GWI with strongly suggestive clinical features. We were able to identify a potential etiology for SFN in most veterans with GWI. Further investigations are recommended to explore any potential relationship between Gulf War exposures and SFN.
Following deployment to operations Desert Shield and Desert Storm (Gulf War) in 1990 and 1991, many Gulf War veterans (GWVs) developed chronic, complex symptoms, including pain, dyscognition, and fatigue, with gastrointestinal, skin, and respiratory manifestations. This Gulf War Illness (GWI) is reported to affect about 30% of those deployed. More than 30 years later, there is no consensus as to the etiology of GWI, although some deployment-related exposures have been implicated.1
Accepted research definitions for GWI include the Centers for Disease Control and Prevention and Kansas definitions.2 The US Department of Veterans Affairs (VA) uses the terminology chronic multisymptom illness (CMI), which is an overarching diagnosis under which GWI falls. Although there is no consensus case definition for CMI, there is overlap with conditions such as fibromyalgia, myalgic encephalomyelitis/chronic fatigue syndrome, and irritable bowel syndrome; the VA considers these as qualifying clinical diagnoses.3 The pathophysiology of GWI is also unknown, though a frequently reported unifying feature is that of autonomic nervous system (ANS) dysfunction. Studies have demonstrated differences between veterans with GWI and those without GWI in both the reporting of symptoms attributable to ANS dysfunction and in physiologic evaluations of the ANS.4-10
Small fiber neuropathy (SFN), a condition with damage to the A-δ and C small nerve fibers, has been proposed as a potential mechanism for the pain and ANS dysfunction experienced in GWI.11-13 Symptoms of SFN are similar to those of GWI, with pain and ANS symptoms commonly reported.14,15 There are multiple diagnostic criteria for SFN, the most commonly used requiring the presence of appropriate symptoms in the absence of large fiber neuropathy and a skin biopsy demonstrating reduced intraepidermal nerve fiber density.16-19 Several conditions reportedly cause SFN, most notably diabetes/prediabetes. Autoimmune disease, vitamin B12 deficiency, monoclonal gammopathies, celiac disease, paraneoplastic syndromes, and sodium channel gene mutations may also contribute to SFN.20 Hyperlipidemia has been identified as a contributor, although it has been variably reported.21,22
Idiopathic neuropathies, SFN included, may be secondary to neurotoxicant exposures. Agents whose exposure or consumption have been associated with SFN include alcohol most prominently, but also the organic solvent n-hexane, heavy metals, and excess vitamin B6.20,23-25 Agents associated with large fiber neuropathy may also have relevance for SFN, as small fibers have been likened to the “canary in the coal mine” in that they may be more susceptible to neurotoxicants and are affected earlier in the disease process.26 In this way, SFN may be the harbinger of large fiber neuropathy in some cases. Of specific relevance for GWVs, organophosphates and carbamates are known to produce a delayed onset large fiber neuropathy.27-30 Exposure to petrochemical solvents has also been associated with large fiber neuropathies.31,32
The War Related Illness and Injury Study Center (WRIISC) is a clinical, research, and education center established by Congress in 2001. Its primary focus is on military exposures and postdeployment health of veterans. It is located at 3 sites: East Orange, New Jersey; Washington, DC; and Palo Alto, California. The New Jersey WRIISC began a program to evaluate GWVs with characteristic symptoms for possible SFN with use of a skin biopsy.
We hypothesize that SFN may underly much of GWI symptomatology and may not be accounted for by the putative etiologies detailed in review of the medical literature. This retrospective review of clinical evaluations for SFN in GWVs who sought care at the New Jersey WRIISC explored and addressed the following questions: (1) how common is biopsy-confirmed SFN in veterans with GWI; (2) do veterans with GWI and SFN report more symptoms attributable to ANS dysfunction when compared with veterans with GWI and no SFN; and (3) can SFN in veterans with GWI and SFN be explained by conditions and substances commonly associated with SFN? Institutional review board approval and waiver of consent was obtained from the Veterans Affairs New Jersey Health Care Center for the study.
Methods
A retrospective chart review was conducted on veterans evaluated at the WRIISC from March 1, 2015, to January 31, 2019. Inclusion criteria were: deployment to operations Desert Shield and Desert Storm between August 2, 1990, and February 28, 1991, and skin biopsy conducted at the WRIISC. Skin biopsies were obtained at the discretion of an examining clinician based on clinical indications, including neuropathic pain, ANS symptoms, and/or a fibromyalgia/chronic pain–type presentation.
Electronic health record review explicitly abstracted GWI status, results of the skin biopsy, and ANS symptom burden as determined by the Composite Autonomic Symptom Scale 31 (COMPASS 31) completed at the time of the WRIISC evaluation.
COMPASS 31 assesses symptoms across 6 domains (orthostatic, vasomotor, secretomotor, gastrointestinal, bladder, andpupillomotor). Patients are asked about symptom frequency (rarely to almost always), severity (mild to severe), and improvement (much worse to completely gone). Individual domain scores and a total weighted score (0-100) have demonstrated good validity, reliability, and consistency in SFN.33,34
In veterans with GWI and documented SFN, a health record review was performed to identify potential etiologies for SFN (Appendix).
Statistical Analysis
Microsoft Excel and IBM SPSS 12.0.1 for Windows were used for data collection and statistical analysis. Fisher exact test was used for comparing the prevalence of SFN in veterans with GWI vs without GWI. The independent samples t test was used for comparing COMPASS 31 scores for veterans with GWI by SFN status. α < .05 was used for determining statistical significance. For those GWVs documented with SFN and GWI, potential explanations were documented in total and by condition.
Results
From March 1, 2015, to January 31, 2019, 141 GWVs received a comprehensive in person clinical evaluation at the WRIISC and 51 veterans (36%) received a skin biopsy and were included in this retrospective observational study (Figure). The mean age was 48.6 years, and the majority were male and served in the US Army. Skin biopsies met clinical criteria for GWI for 42 (82%) and 24 of 42 (57%) were determined to have SFN. Four of 9 (44%) veterans without GWI had positive SFN biopsies, though this difference was not statistically significant (Table 1). Veterans with SFN but no GWI were not included in the further analysis.
Thirty-five veterans with GWI—18 with SFN and 17 without SFN—completed the COMPASS 31 (Table 2). COMPASS 31 data were not analyzed for veterans without GWI. Individual domain scores and the difference in COMPASS 31 scores for veterans with GWI and SFN vs GWI and no SFN (38.3 vs 37.8, respectively) were not statistically significant.
Sixteen of 24 veterans with GWI and SFN (67%) had ≥ 1 conditions that could potentially be responsible for SFN (Table 3), including 11 veterans (46%) with prediabetes/diabetes. Hyperlipidemia is only variably reported as a cause of SFN; when included, 19 of 24 (79%) SFN cases were accounted for. We could not identify a medical explanation for SFN in 5 of 24 veterans (21%) with GWI, which were deemed to be idiopathic.
Discussion
Biopsy-confirmed SFN was present in more than half of our sample of veterans with GWI, which is broadly consistent with what has been reported in the literature.13,35-38 In this clinical observation study, SFN was similarly prevalent in veterans with and without GWI; although it should be noted that biopsies only were obtained when there was a strong clinical suspicion for SFN. Almost half of patients with GWI did not have SFN, so our study does not support SFN as the underlying explanation for all GWI. Although our data cannot provide clinical guidance as to when skin biopsy may be indicated in GWI, work done in fibromyalgia found symptoms of dysautonomia and paresthesias are more specific for SFN and may be useful to help guide medical decision making.39
Veterans with GWI in our clinical sample reported a high burden of clinical symptoms conceivably attributable to ANS dysfunction. This symptom reporting is consistent with that seen in other GWI studies, as well as in other studies of SFN.4,5,7-9,14,15,34,38,40 Our clinical sample of veterans with GWI found no differences in the ANS symptom reporting between those with and without SFN. Therefore, our study cannot support SFN alone as accounting for ANS symptom burden in patients with GWI.
Two-thirds of biopsy-confirmed SFN in our clinical sample of veterans with GWI could potentially be explained by established medical conditions. As in other studies of SFN, prediabetes and diabetes represented a plurality (46%). Even after considering hyperlipidemia as a potential explanation, about 21% of SFN cases in veterans with GWI still were deemed idiopathic.
Evidence supports certain environmental agents as causal factors for GWI. Neurotoxicants reportedly related to GWI include pesticides (particularly organophosphates and carbamates), pyridostigmine bromide (used during the Gulf War as a prophylactic agent against the use of chemical weapons), and low levels of the nerve agent sarin from environmental contamination due to chemical weapons detonations.1 Some of these agents have been implicated in neuropathy as well.1,28-30 It is biologically plausible that deployment-related exposures could trigger SFN, though the traditional consensus has been that remote exposure to neurotoxic substances is unlikely to produce neuropathy that presents many years after the exposure.41 In the WRIISC clinical experience, however, veterans often report that their neuropathic symptoms predate the diagnosis of the associated medical conditions, sometimes by decades. It is conceivable that remote exposures may trigger the condition that is then potentiated by ongoing exposures, metabolic factors, and/or other medical conditions. These may perpetuate neuropathic symptoms and the illness experience of affected veterans. Our clinical observation study cannot clarify the extent to which this may be the case. Despite these findings and arguments, an environmental contribution to SFN cannot be discounted, and further research is needed to explore a potential relationship.
Limitations
This study’s conclusions are limited by its observational/retrospective design in a relatively small clinical sample of veterans evaluated at a tertiary referral center for postdeployment exposure-related health concerns. The WRIISC clinical sample is not representative of all GWVs or even of all veterans with GWI, as there is inherent selection bias as to who gets referred to and evaluated at the WRIISC. As with studies based on retrospective chart review, data are reliant on clinical documentation andaccuracy/consistency of the reviewer. Evaluation for SFN with skin biopsy is an invasive procedure and was performed when a high index of clinical suspicion for this condition existed, possibly representing confirmation bias. Therefore, the relatively high prevalence ofbiopsy-confirmed SFN seen in our clinical sample cannot be generalized to GWVs as a whole or even to veterans with GWI.
Assessment of autonomic dysfunction was based on COMPASS 31 symptom reporting by an small subset of the clinical cohort. Symptom reporting may not be reflective of true abnormality in ANS function. Physiologic tests of the ANS were not performed; such studies could more objectively establish whether ANS dysfunction is more prevalent in GWI veterans with SFN.
Evaluation for all potential etiologic/contributory conditions to SFN was not exhaustive. For example, sodium channel gene mutations have been documented to account for up to one-third of all cases of idiopathic SFN.42 For those cases in which no compelling etiology was identified, it is plausible that medical explanations for SFN may be found on further investigation.
Clinical assessments at the WRIISC were performed on GWVs ≥ 26 years after their deployment-related exposures. Other conditions/exposures may have occurred in the interim. What is not clear is whether the SFN predated the onset of any of these medical conditions or other putative contributors. This observational study is not able to tease out a temporal association to make a cause-and-effect assessment.
Conclusions
Retrospective analysis of clinical data of veterans evaluated at a specialized center for postdeployment health demonstrated that skin biopsy–confirmed SFN was prevalent, but not ubiquitous, in veterans with GWI. Symptom that may be attributed to ANS dysfunction in this clinical sample was consistent with literature on SFN and with GWI, but we could not definitively attribute ANS symptoms to SFN. Our study does not support the hypothesis that GWI symptoms are solely due to SFN, though it may still be relevant in a subset of veterans with GWI with strongly suggestive clinical features. We were able to identify a potential etiology for SFN in most veterans with GWI. Further investigations are recommended to explore any potential relationship between Gulf War exposures and SFN.
1. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. doi:10.1016/j.cortex.2015.08.022
2. Committee on the Development of a Consensus Case Definition for Chronic Multisymptom Illness in 1990-1991 Gulf War Veterans, Board on the Health of Select Populations, Institute of Medicine. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. National Academies Press; 2014.
3. Robbins R, Helmer D, Monahan P, et al. Management of chronic multisymptom illness: synopsis of the 2021 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guideline. Mayo Clin Proc. 2022;97(5):991-1002. doi:10.1016/j.mayocp.2022.01.031
4. Fox A, Helmer D, Tseng CL, Patrick-DeLuca L, Osinubi O. Report of autonomic symptoms in a clinical sample of veterans with Gulf War Illness. Mil Med. 2018;183(3-4):e179-e185. doi:10.1093/milmed/usx052
5. Fox A, Helmer D, Tseng CL, McCarron K, Satcher S, Osinubi O. Autonomic symptoms in Gulf War veterans evaluated at the War Related Illness and Injury Study Center. Mil Med. 2019;184(3-4):e191-e196. doi:10.1093/milmed/usy227
6. Reyes L, Falvo M, Blatt M, Ghobreal B, Acosta A, Serrador J. Autonomic dysfunction in veterans with Gulf War illness [abstract]. FASEB J. 2014;28(S1):1068.19. doi:10.1096/fasebj.28.1_supplement.1068.19
7. Haley RW, Charuvastra E, Shell WE, et al. Cholinergic autonomic dysfunction in veterans with Gulf War illness: confirmation in a population-based sample. JAMA Neurol. 2013;70(2):191-200. doi:10.1001/jamaneurol.2013.596
8. Haley RW, Vongpatanasin W, Wolfe GI, et al. Blunted circadian variation in autonomic regulation of sinus node function in veterans with Gulf War syndrome. Am J Med. 2004;117(7):469-478. doi:10.1016/j.amjmed.2004.03.041
9. Avery TJ, Mathersul DC, Schulz-Heik RJ, Mahoney L, Bayley PJ. Self-reported autonomic dysregulation in Gulf War Illness. Mil Med. Published online December 30, 2021. doi:10.1093/milmed/usab546
10. Verne ZT, Fields JZ, Zhang BB, Zhou Q. Autonomic dysfunction and gastroparesis in Gulf War veterans. J Investig Med. 2023;71(1):7-10. doi:10.1136/jim-2021-002291
11. Levine TD. Small fiber neuropathy: disease classification beyond pain and burning. J Cent Nerv Syst Dis. 2018;10:1179573518771703. doi:10.1177/1179573518771703
12. Novak P. Autonomic disorders. Am J Med. 2019;132(4):420-436. doi:10.1016/j.amjmed.2018.09.027
13. Oaklander AL, Klein MM. Undiagnosed small-fiber polyneuropathy: is it a component of Gulf War Illness? Defense Technical Information Center. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/ADA613891
 14. Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine. 2018;85(5):553-559. doi:10.1016/j.jbspin.2017.11.002
14. Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine. 2018;85(5):553-559. doi:10.1016/j.jbspin.2017.11.002
15. Novak V, Freimer ML, Kissel JT, et al. Autonomic impairment in painful neuropathy. Neurology. 2001;56(7):861-868. doi:10.1212/wnl.56.7.861
16. Myers MI, Peltier AC. Uses of skin biopsy for sensory and autonomic nerve assessment. Curr Neurol Neurosci Rep. 2013;13(1):323. doi:10.1007/s11910-012-0323-2
17. Haroutounian S, Todorovic MS, Leinders M, et al. Diagnostic criteria for idiopathic small fiber neuropathy: a systematic review. Muscle Nerve. 2021;63(2):170-177. doi:10.1002/mus.27070
18. Levine TD, Saperstein DS. Routine use of punch biopsy to diagnose small fiber neuropathy in fibromyalgia patients. Clin Rheumatol. 2015;34(3):413-417. doi:10.1007/s10067-014-2850-5
19. England JD, Gronseth G S, Franklin G, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2009;1(1):14-22. doi:10.1016/j.pmrj.2008.11.011
20. de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy - a large cohort study and review of the literature. Eur J Neurol. 2018;25(2):348-355. doi:10.1111/ene.13508
21. Morkavuk G, Leventoglu A. Small fiber neuropathy associated with hyperlipidemia: utility of cutaneous silent periods and autonomic tests. ISRN Neurol. 2014;2014:579242. doi:10.1155/2014/579242
22. Bednarik J, Vlckova-Moravcova E, Bursova S, Belobradkova J, Dusek L, Sommer C. Etiology of small-fiber neuropathy. J Peripher Nerv Syst. 2009;14(3):177-183. doi:10.1111/j.1529-8027.2009.00229.x
23. Kokotis P, Papantoniou M, Schmelz M, Buntziouka C, Tzavellas E, Paparrigopoulos T. Pure small fiber neuropathy in alcohol dependency detected by skin biopsy. Alcohol Fayettev N. 2023;111:67-73. doi:10.1016/j.alcohol.2023.05.006
24. Guimarães-Costa R, Schoindre Y, Metlaine A, et al. N-hexane exposure: a cause of small fiber neuropathy. J Peripher Nerv Syst. 2018;23(2):143-146. doi:10.1111/jns.12261
25. Koszewicz M, Markowska K, Waliszewska-Prosol M, et al. The impact of chronic co-exposure to different heavy metals on small fibers of peripheral nerves. A study of metal industry workers. J Occup Med Toxicol. 2021;16(1):12. doi:10.1186/s12995-021-00302-6
26. Johns Hopkins Medicine. Small nerve fibers defy neuropathy conventions. April 11, 2016. Accessed February 21, 2024. https://www.hopkinsmedicine.org/news/media/releases/small_nerve_fibers_defy_neuropathy_conventions
27. Jett DA. Neurotoxic pesticides and neurologic effects. Neurol Clin. 2011;29(3):667-677. doi:10.1016/j.ncl.2011.06.002
28. Berger AR, Schaumburg HH. Human toxic neuropathy caused by industrial agents. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed. Saunders; 2005:2505-2525. doi:10.1016/B978-0-7216-9491-7.50115-0
29. Herskovitz S, Schaumburg HH. Neuropathy caused by drugs. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed. Saunders; 2005:2553-2583.
30. Katona I, Weis J. Chapter 31 - Diseases of the peripheral nerves. Handb Clin Neurol. 2017;145:453-474. doi:10.1016/B978-0-12-802395-2.00031-6
31. Matikainen E, Juntunen J. Autonomic nervous system dysfunction in workers exposed to organic solvents. J Neurol Neurosurg Psychiatry. 1985;48(10):1021-1024. doi:10.1136/jnnp.48.10.1021
32. Murata K, Araki S, Yokoyama K, Maeda K. Autonomic and peripheral nervous system dysfunction in workers exposed to mixed organic solvents. Int Arch Occup Environ Health. 1991;63(5):335-340. doi:10.1007/BF00381584
33. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87(12):1196-1201. doi:10.1016/j.mayocp.2012.10.013
34. Treister R, O’Neil K, Downs HM, Oaklander AL. Validation of the Composite Autonomic Symptom Scale-31 (COMPASS-31) in patients with and without small-fiber polyneuropathy. Eur J Neurol. 2015;22(7):1124-1130. doi:10.1111/ene.12717
35. Joseph P, Arevalo C, Oliveira RKF, et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160(2):642-651. doi:10.1016/j.chest.2021.01.082
36. Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve. 2014;49(5):757-759. doi:10.1002/mus.24156
37. Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2310-2316. doi:10.1016/j.pain.2013.06.001
38. Serrador JM. Diagnosis of late-stage, early-onset, small-fiber polyneuropathy. Defense Technical Information Center. December 1, 2019. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/AD1094831
39. Lodahl M, Treister R, Oaklander AL. Specific symptoms may discriminate between fibromyalgia patients with vs without objective test evidence of small-fiber polyneuropathy. Pain Rep. 2018;3(1):e633. doi:10.1097/PR9.0000000000000633
40. Sastre A, Cook MR. Autonomic dysfunction in Gulf War veterans. Defense Technical Information Center. April 1, 2004. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/ADA429525
41. Little AA, Albers JW. Clinical description of toxic neuropathies. Handb Clin Neurol. 2015;131:253-296. doi:10.1016/B978-0-444-62627-1.00015-9
42. Faber CG, Hoeijmakers JGJ, Ahn HS, et al. Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71(1):26-39.
1. White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: effects of toxicant exposures during deployment. Cortex. 2016;74:449-475. doi:10.1016/j.cortex.2015.08.022
2. Committee on the Development of a Consensus Case Definition for Chronic Multisymptom Illness in 1990-1991 Gulf War Veterans, Board on the Health of Select Populations, Institute of Medicine. Chronic Multisymptom Illness in Gulf War Veterans: Case Definitions Reexamined. National Academies Press; 2014.
3. Robbins R, Helmer D, Monahan P, et al. Management of chronic multisymptom illness: synopsis of the 2021 US Department of Veterans Affairs and US Department of Defense Clinical Practice Guideline. Mayo Clin Proc. 2022;97(5):991-1002. doi:10.1016/j.mayocp.2022.01.031
4. Fox A, Helmer D, Tseng CL, Patrick-DeLuca L, Osinubi O. Report of autonomic symptoms in a clinical sample of veterans with Gulf War Illness. Mil Med. 2018;183(3-4):e179-e185. doi:10.1093/milmed/usx052
5. Fox A, Helmer D, Tseng CL, McCarron K, Satcher S, Osinubi O. Autonomic symptoms in Gulf War veterans evaluated at the War Related Illness and Injury Study Center. Mil Med. 2019;184(3-4):e191-e196. doi:10.1093/milmed/usy227
6. Reyes L, Falvo M, Blatt M, Ghobreal B, Acosta A, Serrador J. Autonomic dysfunction in veterans with Gulf War illness [abstract]. FASEB J. 2014;28(S1):1068.19. doi:10.1096/fasebj.28.1_supplement.1068.19
7. Haley RW, Charuvastra E, Shell WE, et al. Cholinergic autonomic dysfunction in veterans with Gulf War illness: confirmation in a population-based sample. JAMA Neurol. 2013;70(2):191-200. doi:10.1001/jamaneurol.2013.596
8. Haley RW, Vongpatanasin W, Wolfe GI, et al. Blunted circadian variation in autonomic regulation of sinus node function in veterans with Gulf War syndrome. Am J Med. 2004;117(7):469-478. doi:10.1016/j.amjmed.2004.03.041
9. Avery TJ, Mathersul DC, Schulz-Heik RJ, Mahoney L, Bayley PJ. Self-reported autonomic dysregulation in Gulf War Illness. Mil Med. Published online December 30, 2021. doi:10.1093/milmed/usab546
10. Verne ZT, Fields JZ, Zhang BB, Zhou Q. Autonomic dysfunction and gastroparesis in Gulf War veterans. J Investig Med. 2023;71(1):7-10. doi:10.1136/jim-2021-002291
11. Levine TD. Small fiber neuropathy: disease classification beyond pain and burning. J Cent Nerv Syst Dis. 2018;10:1179573518771703. doi:10.1177/1179573518771703
12. Novak P. Autonomic disorders. Am J Med. 2019;132(4):420-436. doi:10.1016/j.amjmed.2018.09.027
13. Oaklander AL, Klein MM. Undiagnosed small-fiber polyneuropathy: is it a component of Gulf War Illness? Defense Technical Information Center. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/ADA613891
 14. Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine. 2018;85(5):553-559. doi:10.1016/j.jbspin.2017.11.002
14. Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine. 2018;85(5):553-559. doi:10.1016/j.jbspin.2017.11.002
15. Novak V, Freimer ML, Kissel JT, et al. Autonomic impairment in painful neuropathy. Neurology. 2001;56(7):861-868. doi:10.1212/wnl.56.7.861
16. Myers MI, Peltier AC. Uses of skin biopsy for sensory and autonomic nerve assessment. Curr Neurol Neurosci Rep. 2013;13(1):323. doi:10.1007/s11910-012-0323-2
17. Haroutounian S, Todorovic MS, Leinders M, et al. Diagnostic criteria for idiopathic small fiber neuropathy: a systematic review. Muscle Nerve. 2021;63(2):170-177. doi:10.1002/mus.27070
18. Levine TD, Saperstein DS. Routine use of punch biopsy to diagnose small fiber neuropathy in fibromyalgia patients. Clin Rheumatol. 2015;34(3):413-417. doi:10.1007/s10067-014-2850-5
19. England JD, Gronseth G S, Franklin G, et al. Practice parameter: the evaluation of distal symmetric polyneuropathy: the role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. PM R. 2009;1(1):14-22. doi:10.1016/j.pmrj.2008.11.011
20. de Greef BTA, Hoeijmakers JGJ, Gorissen-Brouwers CML, Geerts M, Faber CG, Merkies ISJ. Associated conditions in small fiber neuropathy - a large cohort study and review of the literature. Eur J Neurol. 2018;25(2):348-355. doi:10.1111/ene.13508
21. Morkavuk G, Leventoglu A. Small fiber neuropathy associated with hyperlipidemia: utility of cutaneous silent periods and autonomic tests. ISRN Neurol. 2014;2014:579242. doi:10.1155/2014/579242
22. Bednarik J, Vlckova-Moravcova E, Bursova S, Belobradkova J, Dusek L, Sommer C. Etiology of small-fiber neuropathy. J Peripher Nerv Syst. 2009;14(3):177-183. doi:10.1111/j.1529-8027.2009.00229.x
23. Kokotis P, Papantoniou M, Schmelz M, Buntziouka C, Tzavellas E, Paparrigopoulos T. Pure small fiber neuropathy in alcohol dependency detected by skin biopsy. Alcohol Fayettev N. 2023;111:67-73. doi:10.1016/j.alcohol.2023.05.006
24. Guimarães-Costa R, Schoindre Y, Metlaine A, et al. N-hexane exposure: a cause of small fiber neuropathy. J Peripher Nerv Syst. 2018;23(2):143-146. doi:10.1111/jns.12261
25. Koszewicz M, Markowska K, Waliszewska-Prosol M, et al. The impact of chronic co-exposure to different heavy metals on small fibers of peripheral nerves. A study of metal industry workers. J Occup Med Toxicol. 2021;16(1):12. doi:10.1186/s12995-021-00302-6
26. Johns Hopkins Medicine. Small nerve fibers defy neuropathy conventions. April 11, 2016. Accessed February 21, 2024. https://www.hopkinsmedicine.org/news/media/releases/small_nerve_fibers_defy_neuropathy_conventions
27. Jett DA. Neurotoxic pesticides and neurologic effects. Neurol Clin. 2011;29(3):667-677. doi:10.1016/j.ncl.2011.06.002
28. Berger AR, Schaumburg HH. Human toxic neuropathy caused by industrial agents. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed. Saunders; 2005:2505-2525. doi:10.1016/B978-0-7216-9491-7.50115-0
29. Herskovitz S, Schaumburg HH. Neuropathy caused by drugs. In: Dyck PJ, Thomas PK, eds. Peripheral Neuropathy. 4th ed. Saunders; 2005:2553-2583.
30. Katona I, Weis J. Chapter 31 - Diseases of the peripheral nerves. Handb Clin Neurol. 2017;145:453-474. doi:10.1016/B978-0-12-802395-2.00031-6
31. Matikainen E, Juntunen J. Autonomic nervous system dysfunction in workers exposed to organic solvents. J Neurol Neurosurg Psychiatry. 1985;48(10):1021-1024. doi:10.1136/jnnp.48.10.1021
32. Murata K, Araki S, Yokoyama K, Maeda K. Autonomic and peripheral nervous system dysfunction in workers exposed to mixed organic solvents. Int Arch Occup Environ Health. 1991;63(5):335-340. doi:10.1007/BF00381584
33. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc. 2012;87(12):1196-1201. doi:10.1016/j.mayocp.2012.10.013
34. Treister R, O’Neil K, Downs HM, Oaklander AL. Validation of the Composite Autonomic Symptom Scale-31 (COMPASS-31) in patients with and without small-fiber polyneuropathy. Eur J Neurol. 2015;22(7):1124-1130. doi:10.1111/ene.12717
35. Joseph P, Arevalo C, Oliveira RKF, et al. Insights from invasive cardiopulmonary exercise testing of patients with myalgic encephalomyelitis/chronic fatigue syndrome. Chest. 2021;160(2):642-651. doi:10.1016/j.chest.2021.01.082
36. Giannoccaro MP, Donadio V, Incensi A, Avoni P, Liguori R. Small nerve fiber involvement in patients referred for fibromyalgia. Muscle Nerve. 2014;49(5):757-759. doi:10.1002/mus.24156
37. Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2310-2316. doi:10.1016/j.pain.2013.06.001
38. Serrador JM. Diagnosis of late-stage, early-onset, small-fiber polyneuropathy. Defense Technical Information Center. December 1, 2019. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/AD1094831
39. Lodahl M, Treister R, Oaklander AL. Specific symptoms may discriminate between fibromyalgia patients with vs without objective test evidence of small-fiber polyneuropathy. Pain Rep. 2018;3(1):e633. doi:10.1097/PR9.0000000000000633
40. Sastre A, Cook MR. Autonomic dysfunction in Gulf War veterans. Defense Technical Information Center. April 1, 2004. Accessed February 21, 2024. https://apps.dtic.mil/sti/citations/ADA429525
41. Little AA, Albers JW. Clinical description of toxic neuropathies. Handb Clin Neurol. 2015;131:253-296. doi:10.1016/B978-0-444-62627-1.00015-9
42. Faber CG, Hoeijmakers JGJ, Ahn HS, et al. Gain of function NaV1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol. 2012;71(1):26-39.