User login
Orf Virus in Humans: Case Series and Clinical Review
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
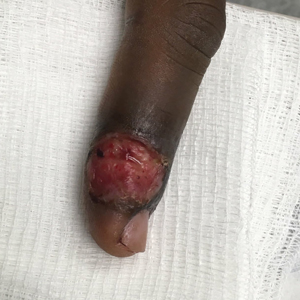
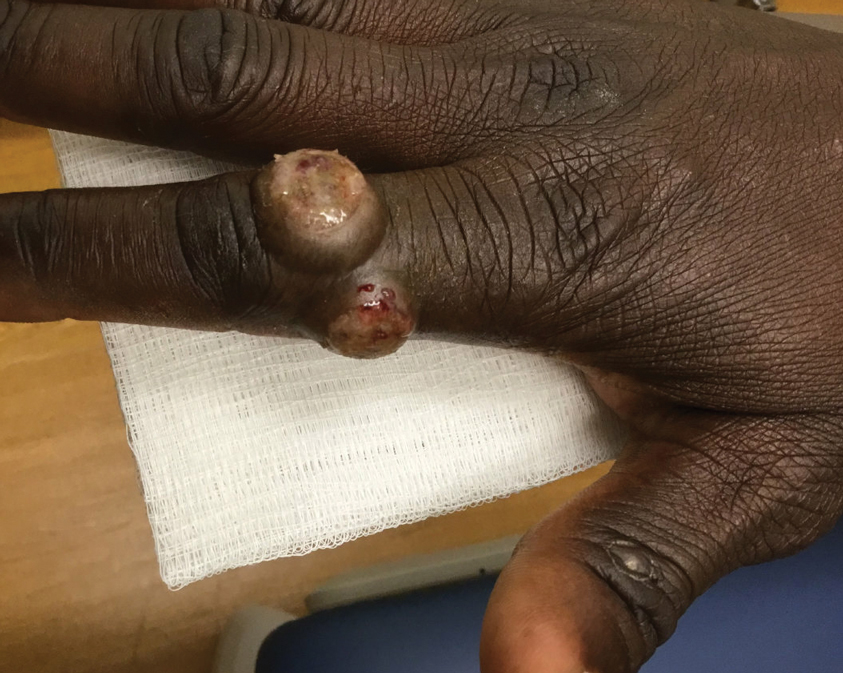
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.
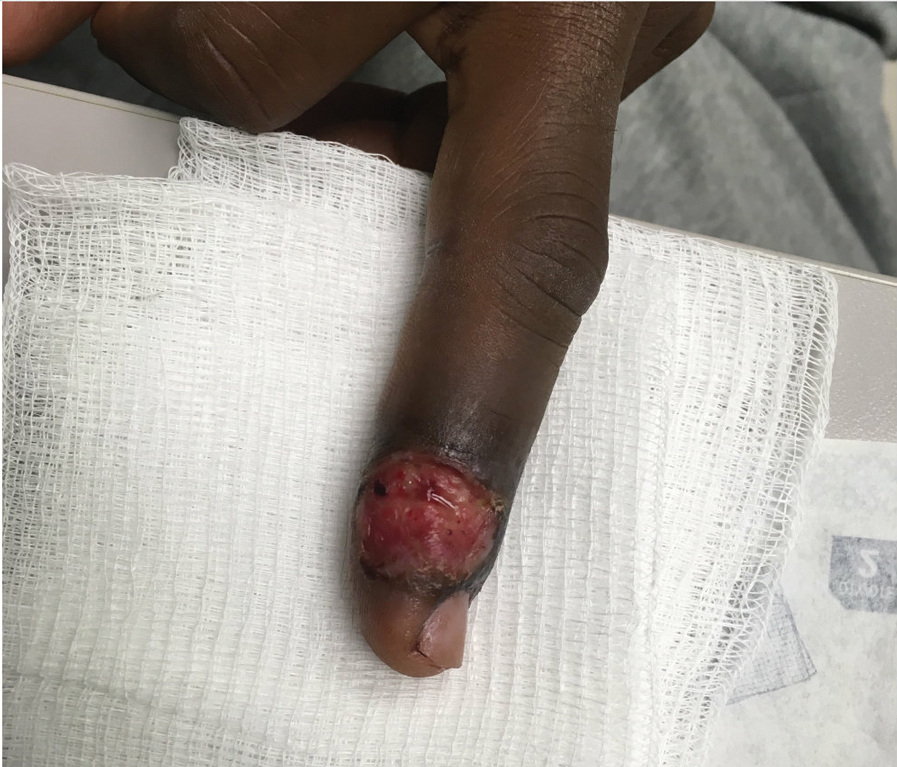
Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.
Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
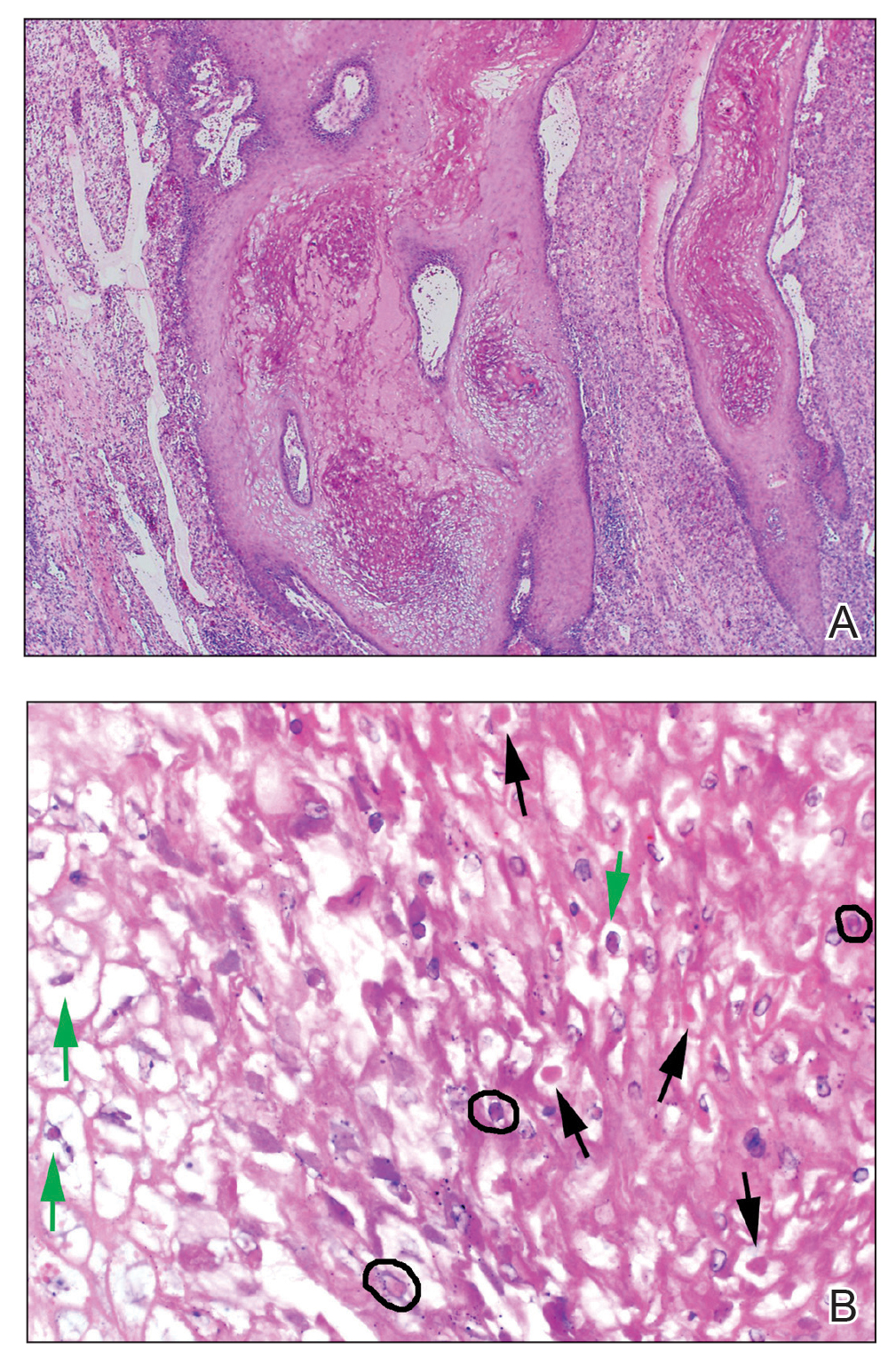
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8
Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24
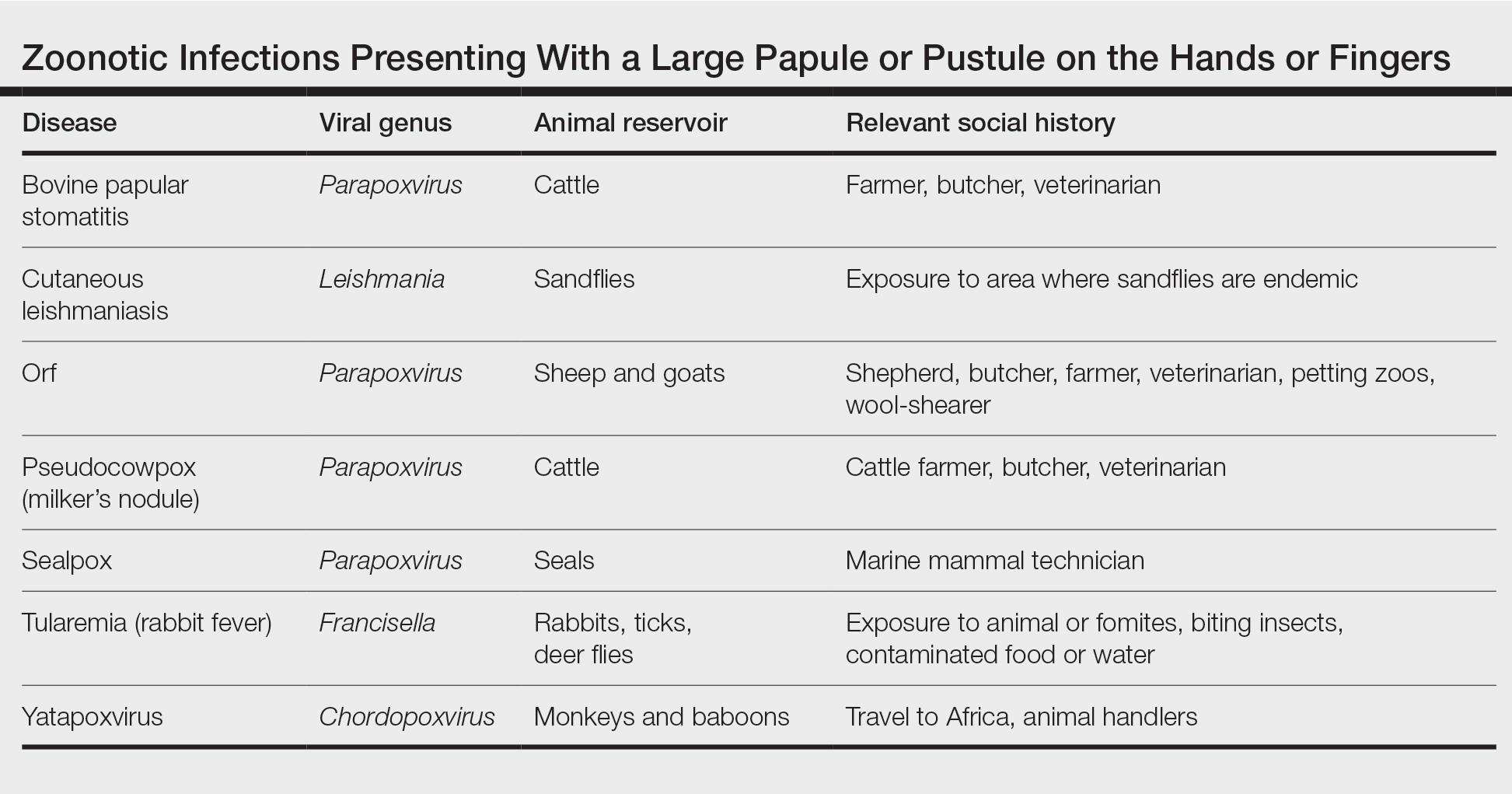
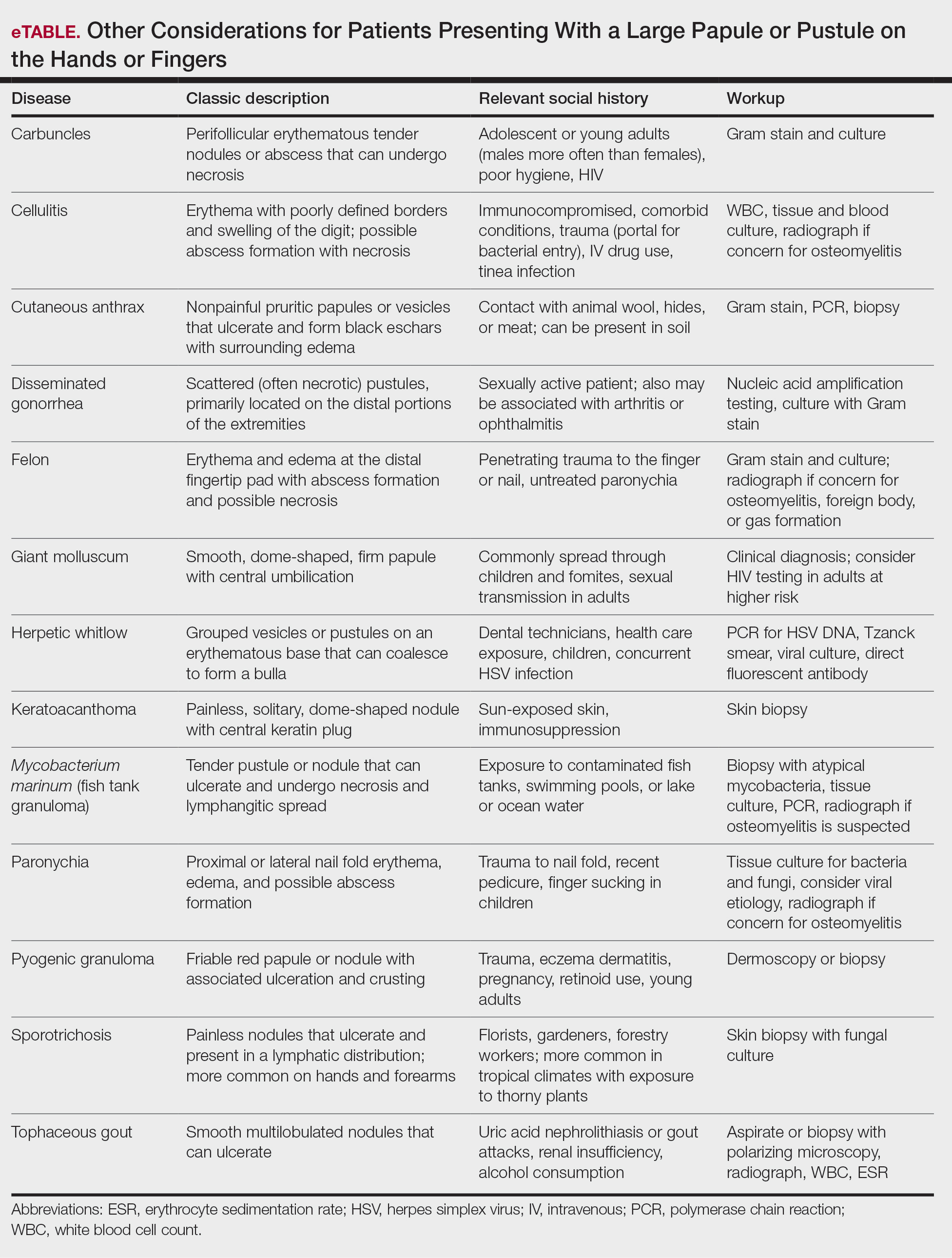
Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26
Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.

Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.

Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8

Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24

Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26

Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
A patient presenting with a hand pustule is a phenomenon encountered worldwide requiring careful history-taking. Some occupations, activities, and various religious practices (eg, Eid al-Adha, Passover, Easter) have been implicated worldwide in orf infection. In the United States, orf virus usually is spread from infected animal hosts to humans. Herein, we review the differential for a single hand pustule, which includes both infectious and noninfectious causes. Recognizing orf virus as the etiology of a cutaneous hand pustule in patients is important, as misdiagnosis can lead to unnecessary invasive testing and/or treatments with suboptimal clinical outcomes.
Case Series
When conducting a search for orf virus cases at our institution (University of Iowa Hospitals and Clinics, Iowa City, Iowa), 5 patient cases were identified.
Patient 1—A 27-year-old otherwise healthy woman presented to clinic with a tender red bump on the right ring finger that had been slowly growing over the course of 2 weeks and had recently started to bleed. A social history revealed that she owned several goats, which she frequently milked; 1 of the goats had a cyst on the mouth, which she popped approximately 1 to 2 weeks prior to the appearance of the lesion on the finger. She also endorsed that she owned several cattle and various other animals with which she had frequent contact. A biopsy was obtained with features consistent with orf virus.
Patient 2—A 33-year-old man presented to clinic with a lesion of concern on the left index finger. Several days prior to presentation, the patient had visited the emergency department for swelling and erythema of the same finger after cutting himself with a knife while preparing sheep meat. Radiographs were normal, and the patient was referred to dermatology. In clinic, there was a 0.5-cm fluctuant mass on the distal interphalangeal joint of the third finger. The patient declined a biopsy, and the lesion healed over 4 to 6 weeks without complication.
Patient 3—A 38-year-old man presented to clinic with 2 painless, large, round nodules on the right proximal index finger, with open friable centers noted on physical examination (Figure 1). The patient reported cutting the finger while preparing sheep meat several days prior. The nodules had been present for a few weeks and continued to grow. A punch biopsy revealed evidence of parapoxvirus infection consistent with a diagnosis of orf.

Patient 4—A 48-year-old man was referred to our dermatology clinic for evaluation of a bleeding lesion on the left middle finger. Physical examination revealed an exophytic, friable, ulcerated nodule on the dorsal aspect of the left middle finger (Figure 2). Upon further questioning, the patient mentioned that he handled raw lamb meat after cutting the finger. A punch biopsy was obtained and was consistent with orf virus infection.

Patient 5—A 43-year-old woman presented to clinic with a chronic wound on the mid lower back that was noted to drain and crust over. She thought the lesion was improving, but it had become painful over the last few weeks. A shave biopsy of the lesion was consistent with orf virus. At follow-up, the patient was unable to identify any recent contact with animals.
Comment
Transmission From Animals to Humans—Orf virus is a member of the Parapoxvirus genus of the Poxviridae family.1 This virus is highly contagious among animals and has been described around the globe. The resulting disease also is known as contagious pustular dermatitis,2 soremuzzle,3 ecthyma contagiosum of sheep,4 and scabby mouth.5 This virus most commonly infects young lambs and manifests as raw to crusty papules, pustules, or vesicles around the mouth and nose of the animal.4 Additional signs include excessive salivation and weight loss or starvation from the inability to suckle because of the lesions.5 Although ecthyma contagiosum infection of sheep and goats has been well known for centuries, human infection was first reported in the literature in 1934.6
Transmission of orf to humans can occur when direct contact with an infected animal exhibiting active lesions occurs.7 Orf virus also can be transmitted through fomites (eg, from knives, wool, buildings, equipment) that previously were in contact with infected animals, making it relevant to ask all farmers about any animals with pustules around the mouth, nose, udders, or other commonly affected areas. Although sanitation efforts are important for prevention, orf virus is hardy, and fomites can remain on surfaces for many months.8 Transmission among animals and from animals to humans frequently occurs; however, human-to-human transmission is less common.9 Ecthyma contagiosum is considered an occupational hazard, with the disease being most prevalent in shepherds, veterinarians, and butchers.1,8 Disease prevalence in these occupations has been reported to be as high as 50%.10 Infections also are seen in patients who attend petting zoos or who slaughter goats and sheep for cultural practices.8
Clinical Characteristics in Humans—The clinical diagnosis of orf is dependent on taking a thorough patient history that includes social, occupational, and religious activities. Development of a nodule or papule on a patient’s hand with recent exposure to fomites or direct contact with a goat or sheep up to 1 week prior is extremely suggestive of an orf virus infection.
Clinically, orf most often begins as an individual papule or nodule on the dorsal surface of the patient’s finger or hand and ranges from completely asymptomatic to pruritic or even painful.1,8 Depending on how the infection was inoculated, lesions can vary in size and number. Other sites that have been reported less frequently include the genitals, legs, axillae, and head.11,12 Lesions are roughly 1 cm in diameter but can vary in size. Ecthyma contagiosum is not a static disease but changes in appearance over the course of infection. Typically, lesions will appear 3 to 7 days after inoculation with the orf virus and will self-resolve 6 to 8 weeks later.
Orf lesions have been described to progress through 6 distinct phases before resolving: maculopapular (erythematous macule or papule forms), targetoid (formation of a necrotic center with red outer halo), acute (lesion begins to weep), regenerative (lesion becomes dry), papilloma (dry crust becomes papillomatous), and regression (skin returns to normal appearance).1,8,9 Each phase of ecthyma contagiosum is unique and will last up to 1 week before progressing. Because of this prolonged clinical course, patients can present at any stage.
Reports of systemic symptoms are uncommon but can include lymphadenopathy, fever, and malaise.13 Although the disease course in immunocompetent individuals is quite mild, immunocompromised patients may experience persistent orf lesions that are painful and can be much larger, with reports of several centimeters in diameter.14
Dermatopathology and Molecular Studies—When a clinical diagnosis is not possible, biopsy or molecular studies can be helpful.8 Histopathology can vary depending on the phase of the lesion. Early stages are characterized by spongiform degeneration of the epidermis with variable vesiculation of the superficial epidermis and eosinophilic cytoplasmic inclusion bodies of keratinocytes (Figure 3). Later stages demonstrate full-thickness necrosis with epidermal balloon degeneration and dense inflammation of the dermis with edema and extravasated erythrocytes from dilated blood vessels. Both early- and late-stage disease commonly show characteristic elongated thin rete ridges.8

Molecular studies are another reliable method for diagnosis, though these are not always readily available. Polymerase chain reaction can be used for sensitive and rapid diagnosis.15 Less commonly, electron microscopy, Western blot, or enzyme-linked immunosorbent assays are used.16 Laboratory studies, such as complete blood cell count with differential, erythrocyte sedimentation rate, and C-reactive protein, often are unnecessary but may be helpful in ruling out other infectious causes. Tissue culture can be considered if bacterial, fungal, or acid-fast bacilli are in the differential; however, no growth will be seen in the case of orf viral infection.
Differential Diagnosis—The differential diagnosis for patients presenting with a large pustule on the hand or fingers can depend on geographic location, as the potential etiology may vary widely around the world. Several zoonotic viral infections other than orf can present with pustular lesions on the hands (Table).17-24

Clinically, infection with these named viruses can be hard to distinguish; however, appropriate social history or polymerase chain reaction can be obtained to differentiate them. Other infectious entities include herpetic whitlow, giant molluscum, and anthrax (eTable).24-26 Biopsy of the lesion with bacterial tissue culture may lead to definitive diagnosis.26

Treatment—Because of the self-resolving nature of orf, treatment usually is not needed in immunocompetent patients with a solitary lesion. However, wound care is essential to prevent secondary infections of the lesion. If secondarily infected, topical or oral antibiotics may be prescribed. Immunocompromised individuals are at increased risk for developing large persistent lesions and sometimes require intervention for successful treatment. Several successful treatment methods have been described and include intralesional interferon injections, electrocautery, topical imiquimod, topical cidofovir, and cryotherapy.8,14,27-30 Infections that continue to be refractory to less-invasive treatment can be considered for wide local excision; however, recurrence is possible.8 Vaccinations are available for animals to prevent the spread of infection in the flock, but there are no formulations of vaccines for human use. Prevention of spread to humans can be done through animal vaccination, careful handling of animal products while wearing nonporous gloves, and proper sanitation techniques.
Complications—Orf has an excellent long-term prognosis in immunocompetent patients, as the virus is epitheliotropic, and inoculation does not lead to viremia.2 Although lesions typically are asymptomatic in most patients, complications can occur, especially in immunosuppressed individuals. These complications include systemic symptoms, giant persistent lesions prone to infection or scarring, erysipelas, lymphadenitis, and erythema multiforme.8,31 Common systemic symptoms of ecthyma contagiosum include fever, fatigue, and myalgia. Lymphadenitis can occur along with local swelling and lymphatic streaking. Although erythema multiforme is a rare complication occurring after initial ecthyma contagiosum infection, this hypersensitivity reaction is postulated to be in response to the immunologic clearing of the orf virus.32,33 Patients receiving systemic immunosuppressive medications are at an increased risk of developing complications from infection and may even be required to pause systemic treatment for complete resolution of orf lesions.34 Other cutaneous diseases that decrease the skin’s barrier protection, such as bullous pemphigoid or eczema, also can place patients at an increased risk for complications.35 Although human-to-human orf virus transmission is exceptionally rare, there is a case report of this phenomenon in immunosuppressed patients residing in a burn unit.36 Transplant recipients on immunosuppressive medications also can experience orf lesions with exaggerated presentations that continue to grow up to several centimeters in diameter.31 Long-term prognosis is still good in these patients with appropriate disease recognition and treatment. Reinfection is not uncommon with repeated exposure to the source, but lesions are less severe and resolve faster than with initial infection.1,8
Conclusion
The contagious hand pustule caused by orf virus is a distinct clinical entity that is prevalent worldwide and requires thorough evaluation of the clinical course of the lesion and the patient’s social history. Several zoonotic viral infections have been implicated in this presentation. Although biopsy and molecular studies can be helpful, the expert diagnostician can make a clinical diagnosis with careful attention to social history, geographic location, and cultural practices.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
- Haig DM, Mercer AA. Ovine diseases. orf. Vet Res. 1998;29:311-326.
- Glover RE. Contagious pustular dermatitis of the sheep. J Comp Pathol Ther. 1928;41:318-340.
- Hardy WT, Price DA. Soremuzzle of sheep.
J Am Vet Med Assoc. 1952;120:23-25. - Boughton IB, Hardy WT. Contagious ecthyma (sore mouth) of sheep and goats. J Am Vet Med Assoc. 1934;85:150-178.
- Gardiner MR, Craig VMD, Nairn ME. An unusual outbreak of contagious ecthyma (scabby mouth) in sheep. Aust Vet J. 1967;43:163-165.
- Newsome IE, Cross F. Sore mouth in sheep transmissible to man. J Am Vet Med Assoc. 1934;84:790-802.
- Demiraslan H, Dinc G, Doganay M. An overview of orf virus infection in humans and animals. Recent Pat Anti Infect Drug Discov. 2017;12:21-30.
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017;27:E1932.
- Duchateau NC, Aerts O, Lambert J. Autoinoculation with orf virus (ecthyma contagiosum). Int J Dermatol. 2014;53:E60-E62.
- Paiba GA, Thomas DR, Morgan KL, et al. Orf (contagious pustular dermatitis) in farmworkers: prevalence and risk factors in three areas of England. Vet Rec. 1999;145:7-11
- Kandemir H, Ciftcioglu MA, Yilmaz E. Genital orf. Eur J Dermatol. 2008;18:460-461.
- Weide B, Metzler G, Eigentler TK, et al. Inflammatory nodules around the axilla: an uncommon localization of orf virus infection. Clin Exp Dermatol. 2009;34:240-242.
- Wilkinson JD. Orf: a family with unusual complications. Br J Dermatol. 1977;97:447-450.
- Zaharia D, Kanitakis J, Pouteil-Noble C, et al. Rapidly growing orf in a renal transplant recipient: favourable outcome with reduction of immunosuppression and imiquimod. Transpl Int. 2010;23:E62-E64.
- Bora DP, Venkatesan G, Bhanuprakash V, et al. TaqMan real-time PCR assay based on DNA polymerase gene for rapid detection of orf infection. J Virol Methods. 2011;178:249-252.
- Töndury B, Kühne A, Kutzner H, et al. Molecular diagnostics of parapox virus infections. J Dtsch Dermatol Ges. 2010;8:681-684.
- Handler NS, Handler MZ, Rubins A, et al. Milker’s nodule: an occupational infection and threat to the immunocompromised. J Eur Acad Dermatol Venereol. 2018;32:537-541.
- Groves RW, Wilson-Jones E, MacDonald DM. Human orf and milkers’ nodule: a clinicopathologic study. J Am Acad Dermatol. 1991;25:706-711.
- Bowman KF, Barbery RT, Swango LJ, et al. Cutaneous form of bovine papular stomatitis in man. JAMA. 1981;246;1813-1818.
- Nagington J, Lauder IM, Smith JS. Bovine papular stomatitis, pseudocowpox and milker’s nodules. Vet Rec. 1967;79:306-313.
- Clark C, McIntyre PG, Evans A, et al. Human sealpox resulting from a seal bite: confirmation that sealpox virus is zoonotic. Br J Dermatol. 2005;152:791-793.
- Downie AW, Espana C. A comparative study of tanapox and yaba viruses. J Gen Virol. 1973;19:37-49.
- Zimmermann P, Thordsen I, Frangoulidis D, et al. Real-time PCR assay for the detection of tanapox virus and yaba-like disease virus. J Virol Methods. 2005;130:149-153.
- Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier Saunders; 2018.
- Wenner KA, Kenner JR. Anthrax. Dermatol Clin. 2004;22:247-256.
- Brachman P, Kaufmann A. Anthrax. In: Evans A, Brachman P, eds. Bacterial Infections of Humans: Epidemiology and Control. 3rd ed. Plenum Publishing; 1998:95.
- Ran M, Lee M, Gong J, et al. Oral acyclovir and intralesional interferon injections for treatment of giant pyogenic granuloma-like lesions in an immunocompromised patient with human orf. JAMA Dermatol. 2015;151:1032-1034.
- Degraeve C, De Coninck A, Senneseael J, et al. Recurrent contagious ecthyma (orf) in an immunocompromised host successfully treated with cryotherapy. Dermatology. 1999;198:162-163.
- Geerinck K, Lukito G, Snoeck R, et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J Med Virol. 2001;64:543-549.
- Ertekin S, Gurel M, Erdemir A, et al. Systemic interferon alfa injections for the treatment of a giant orf. Cutis. 2017;99:E19-E21.
- Hunskaar S. Giant orf in a patient with chronic lymphocytic leukaemia. Br J Dermatol. 1986;114:631-634.
- Ozturk P, Sayar H, Karakas T, et al. Erythema multiforme as a result of orf disease. Acta Dermatovenereol Alp Pannonica Adriat. 2012;21:45-46.
- Shahmoradi Z, Abtahi-Naeini B, Pourazizi M, et al. Orf disease following ‘eid ul-adha’: a rare cause of erythema multiforme. Int J Prev Med. 2014;5:912-914.
- Kostopoulos M, Gerodimos C, Batsila E, et al. Orf disease in a patient with rheumatoid arthritis. Mediterr J Rheumatol. 2018;29:89-91.
- Murphy JK, Ralphs IG. Bullous pemphigoid complicating human orf. Br J Dermatol. 1996;134:929-930.
- Midilli K, Erkiliç A, Kus¸kucu M, et al. Nosocomial outbreak of disseminated orf infection in a burn unit, Gaziantep, Turkey, October to December 2012. Euro Surveill. 2013;18:20425.
Practice Points
- Ecthyma contagiosum is a discrete clinical entity that occurs worldwide and demands careful attention to clinical course and social history.
- Ecthyma contagiosum is caused by orf virus, an epitheliotropic zoonotic infection that spreads from ruminants to humans.
- Early and rapid diagnosis of this classic condition is critical to prevent unnecessary biopsies or extensive testing, and determination of etiology can be important in preventing reinfection or spread to other humans by the same infected animal.
Surgical Specimens and Margins
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.
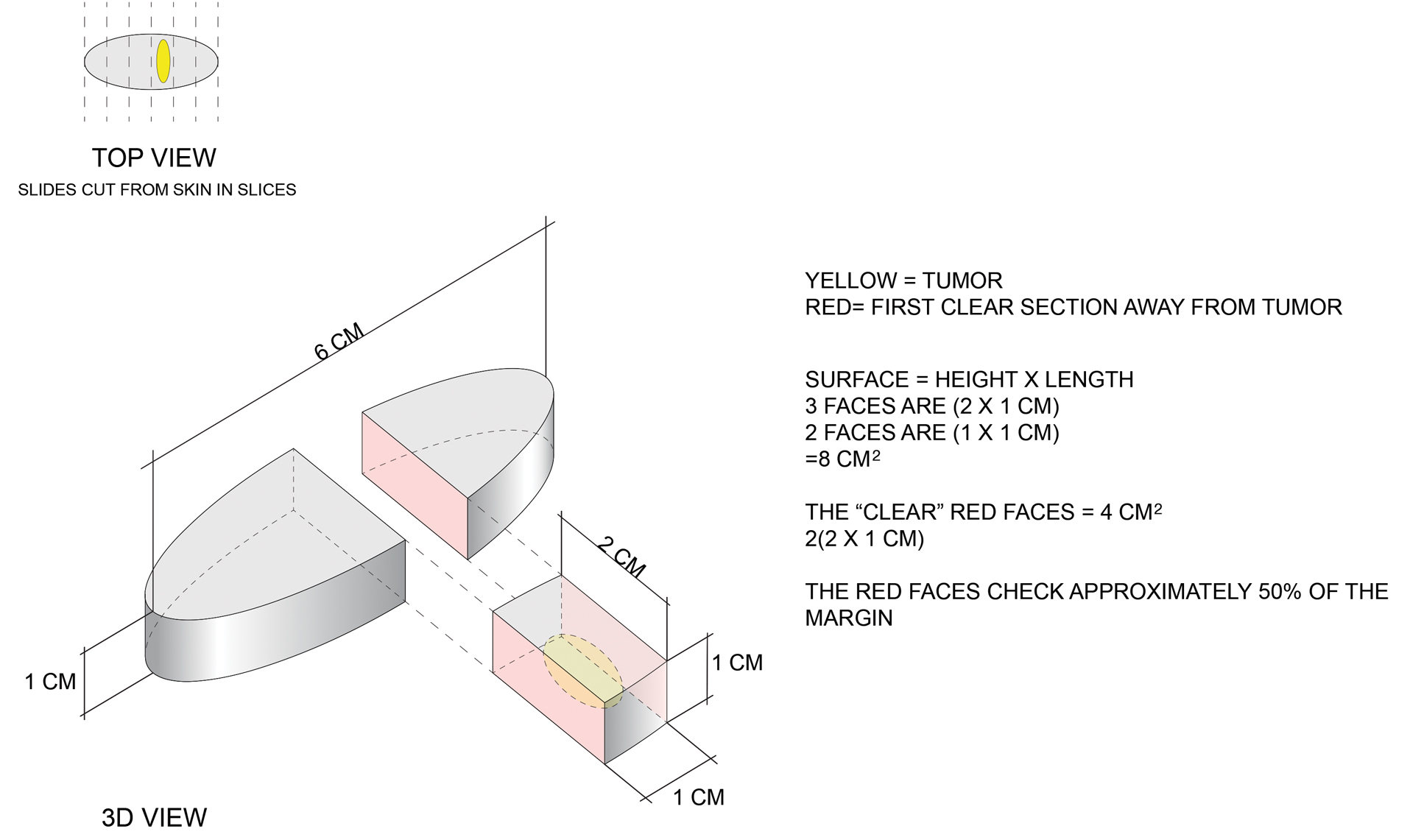
Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
We have attended grand rounds presentations at which students announce that Mohs micrographic surgery evaluates 100% of the surgical margin, whereas standard excision samples 1% to 2% of the margin; we have even fielded questions from neighbors who have come across this information on the internet.1-5 This statement describes a best-case scenario for Mohs surgery and a worst-case scenario for standard excision. We believe that it is important for clinicians to have a more nuanced understanding of how simple excisions are processed so that they can have pertinent discussions with patients, especially now that there is increasing access to personal health information along with increased agency in patient decision-making.
Margins for Mohs Surgery
Theoretically, Mohs surgery should sample all true surgical margins by complete circumferential, peripheral, and deep-margin assessment. Unfortunately, some sections are not cut full face—sections may not always sample a complete surface—when technicians make an error or lack expertise. Some sections may have small tissue folds or small gaps that prevent complete visualization. We estimate that the Mohs sections we review in consultation that are prepared by private practice Mohs surgeons in our communities visualize approximately 98% of surgical margins on average. Incomplete sections contribute to the rare tumor recurrences after Mohs surgery of approximately 2% to 3%.6
Standard Excision Margins
When we obtained the references cited in articles asserting that
Here is a simple example to show that more margin is accessed in some cases. Consider this hypothetical situation: If a tumor can be readily visualized grossly and housed entirely within an imaginary cuboid (rectangular) prism that is removed in an elliptical specimen with a length of 6 cm, a width of 2 cm, and a height of 1 cm (Figure), then standard sectioning assesses a greater margin.

Bread-loaf sectioning would be expected to examine the complete surface of 2 sides (faces) of the cuboid. Assessing 2 of the 5 clinically relevant sides provides information for approximately 50% of the margins, as sections in the next parallel plane can be expected to be clear after the first clear section is identified. The clinically useful information is not limited to the sum of the widths of sections. Encountering a clear plane typically indicates that there will be no tumor in more distal parallel planes. Warne et al6 developed a formula that can accurately predict the percentage of the margin evaluated by proxy that considers the curvature of the ellipse.
Comparing Standard Excision and Mohs Surgery
Mohs surgery consistently results in the best outcomes, but standard excision is effective, too. Standard excision is relatively simple, requires less equipment, is less time consuming, and can provide good value when resources are finite. Data on recurrence of basal cell carcinoma after simple excision are limited, but the recurrence rate is reported to be approximately 3%.7,8 A meta-analysis found that the recurrence rate of basal cell carcinoma treated with standard excision was 0.4%, 1.6%, 2.6%, and 4% with 5-mm, 4-mm, 3-mm, and 2-mm surgical margins, respectively.9
Mohs surgery is the best, most effective, and most tissue-sparing technique for certain nonmelanoma skin cancers. This observation is reflected in guidelines worldwide.10 The adequacy of standard approaches to margin evaluation depends on the capabilities and focus of the laboratory team. Dermatopathologists often are called to the laboratory to decide which technique will be best for a particular case.11 Technicians are trained to take more sections in areas where abnormalities are seen, and some laboratories take photographs of specimens or provide sketches for correlation. Dermatopathologists also routinely request additional sections in areas where visible tumor extends close to surgical margins on microscopic examination.
It is not simply a matter of knowing how much of the margin is sampled but if the most pertinent areas are adequately sampled. Simple sectioning can work well and be cost effective. Many clinicians are unaware of how tissue processing can vary from laboratory to laboratory. There are no uniformly accepted standards for how tissue should be processed. Assiduous and thoughtful evaluation of specimens can affect results. As with any service, some laboratories provide more detailed and conscientious care while others focus more on immediate costs. Clinicians should understand how their specimens are processed by discussing margin evaluation with their dermatopathologist.
Final Thoughts
Used appropriately, Mohs surgery is an excellent technique that can provide outstanding results. Standard excision also has an important place in the dermatologist’s armamentarium and typically provides information about more than 1% to 2% of the margin. Understanding the techniques used to process specimens is critical to delivering the best possible care.
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
- Tolkachjov SN, Brodland DG, Coldiron BM, et al. Understanding Mohs micrographic surgery: a review and practical guide for the nondermatologist. Mayo Clin Proc. 2017;92:1261-1271. doi:10.1016/j.mayocp.2017.04.009
- Thomas RM, Amonette RA. Mohs micrographic surgery. Am Fam Physician. 1988;37:135-142.
- Buker JL, Amonette RA. Micrographic surgery. Clin Dermatol. 1992:10:309-315. doi:10.1016/0738-081x(92)90074-9
- Kauvar ANB. Mohs: the gold standard. The Skin Cancer Foundation website. Updated March 9, 2021. Accessed June 15, 2022. https://www.skincancer.org/treatment-resources/mohs-surgery/mohs-the-gold-standard/
- van Delft LCJ, Nelemans PJ, van Loo E, et al. The illusion of conventional histological resection margin control. Br J Dermatol. 2019;180:1240-1241. doi:10.1111/bjd.17510
- Warne MM, Klawonn MM, Brodell RT. Bread loaf sections provide useful information on more than 0.5% of surgical margins [published July 5, 2022]. Br J Dermatol. doi:10.1111/bjd.21740
- Mehrany K, Weenig RH, Pittelkow MR, et al. High recurrence rates of basal cell carcinoma after Mohs surgery in patients with chronic lymphocytic leukemia. Arch Dermatol. 2004;140:985-988. doi:10.1001/archderm.140.8.985
- Smeets NWJ, Krekels GAM, Ostertag JU, et al. Surgical excision vs Mohs’ micrographic surgery for basal-cell carcinoma of the face: randomised controlled trial. Lancet. 2004;364:1766-1772. doi:10.1016/S0140-6736(04)17399-6
- Gulleth Y, Goldberg N, Silverman RP, et al. What is the best surgical margin for a basal cell carcinoma: a meta-analysis of theliterature. Plast Reconstr Surg. 2010;126:1222-1231. doi:10.1097/PRS.0b013e3181ea450d
- Nahhas AF, Scarbrough CA, Trotter S. A review of the global guidelines on surgical margins for nonmelanoma skin cancers. J Clin Aesthet Dermatol. 2017;10:37-46.
- Rapini RP. Comparison of methods for checking surgical margins. J Am Acad Dermatol. 1990; 23:288-294. doi:10.1016/0190-9622(90)70212-z
Practice Points
- Margin analysis in simple excisions can provide useful information by proxy about more than the 1% of the margin often quoted in the literature.
- Simple excisions of uncomplicated keratinocytic carcinomas are associated with high cure rates.
Nail dystrophy and foot pain
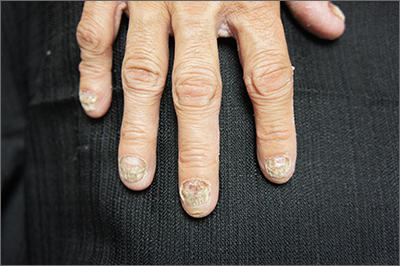
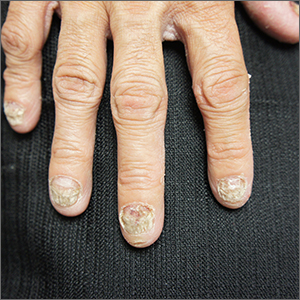
These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

These findings are consistent with a type of heritable keratoderma called pachyonychia congenita (also called twenty-nails dystrophy). It is easy to mistake this unusual cause of thickening nails with a more common cause: onychomycosis.
Pachyonychia congenita describes a set of disorders driven by heritable defects in 1 of 5 keratin genes. The disorder is often transmitted in an autosomal dominant fashion, although a third of patients are thought to have a spontaneous mutation.1 These gene changes can cause 1 or multiple dystrophic nails, thickened nail beds, natal teeth, thick plantar or palmar nodules or plaques, and hearing difficulties. Some patients may have symptoms at birth, while other patients do not develop symptoms until later in life.1
There is currently no cure for pachyonychia congenita. Patients with suspected heritable keratoderma benefit from referral to Medical Genetics and a dermatologist who is comfortable treating keratodermas. Patients can obtain free genetic testing, educational material, and additional resources through pachyonychia.org.
This patient was prescribed topical urea 40% cream that was to be applied to the feet nightly, until the nodules became less painful. He was also evaluated for pressure-offloading orthotics. Nails may be treated with topical urea lacquer nightly until patients are satisfied with the appearance, although this patient chose to forgo the lacquer.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
1. Smith FJD, Hansen CD, Hull PR, et al. Pachyonychia congenita. In: Adam MP, Mirzaa GM, Pagon RA, et al., eds. GeneReviews. Seattle (WA): University of Washington, Seattle; 2006. Updated November 30, 2017. Accessed June 27, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1280/
Evolving Treatment Options for Generalized Myasthenia Gravis
Myasthenia gravis (MG) is an autoimmune disease leading to muscle weakness and fatigue. Medical therapy for MG has traditionally focused on treatments to alleviate symptoms, but a range of new therapies are improving outcomes.
Dr Raghav Govindarajan, from HSHS Medical Group in O'Fallon, Illinois, reports on therapeutic advances for patients with MG presented at the American Academy of Neurology 2022 annual meeting.
First, Dr Govindarajan discusses interim results from the ADAPT+ study, an ongoing 3-year extension of ADAPT that evaluated the long-term safety, tolerability, and efficacy of efgartigimod.
Next, he highlights CHAMPION MG, an open-label extension trial that looked at the long-term efficacy and safety profile of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG.
Dr Govindarajan concludes by reviewing a phase 2 study on nipocalimab, a monoclonal antibody that targets the IgG binding site on FcRn with high affinity, therefore reducing serum levels of total IgG and pathogenic IgG autoantibodies — the underlying cause of MG. This study evaluated efficacy data including myasthenia gravis activities of daily living score evaluated efficacy data including myasthenia gravis activities of daily living.
--
Raghav Govindarajan, MD, Staff neurologist, Stroke Director, HSHS Medical Group-St Elizabeth, O'Fallon, Illinois
Serve(d) as a speaker or a member of a speakers bureau for: Alexion
Received research grant from: Alexion
Received income in an amount equal to or greater than $250 from: Alexion
Myasthenia gravis (MG) is an autoimmune disease leading to muscle weakness and fatigue. Medical therapy for MG has traditionally focused on treatments to alleviate symptoms, but a range of new therapies are improving outcomes.
Dr Raghav Govindarajan, from HSHS Medical Group in O'Fallon, Illinois, reports on therapeutic advances for patients with MG presented at the American Academy of Neurology 2022 annual meeting.
First, Dr Govindarajan discusses interim results from the ADAPT+ study, an ongoing 3-year extension of ADAPT that evaluated the long-term safety, tolerability, and efficacy of efgartigimod.
Next, he highlights CHAMPION MG, an open-label extension trial that looked at the long-term efficacy and safety profile of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG.
Dr Govindarajan concludes by reviewing a phase 2 study on nipocalimab, a monoclonal antibody that targets the IgG binding site on FcRn with high affinity, therefore reducing serum levels of total IgG and pathogenic IgG autoantibodies — the underlying cause of MG. This study evaluated efficacy data including myasthenia gravis activities of daily living score evaluated efficacy data including myasthenia gravis activities of daily living.
--
Raghav Govindarajan, MD, Staff neurologist, Stroke Director, HSHS Medical Group-St Elizabeth, O'Fallon, Illinois
Serve(d) as a speaker or a member of a speakers bureau for: Alexion
Received research grant from: Alexion
Received income in an amount equal to or greater than $250 from: Alexion
Myasthenia gravis (MG) is an autoimmune disease leading to muscle weakness and fatigue. Medical therapy for MG has traditionally focused on treatments to alleviate symptoms, but a range of new therapies are improving outcomes.
Dr Raghav Govindarajan, from HSHS Medical Group in O'Fallon, Illinois, reports on therapeutic advances for patients with MG presented at the American Academy of Neurology 2022 annual meeting.
First, Dr Govindarajan discusses interim results from the ADAPT+ study, an ongoing 3-year extension of ADAPT that evaluated the long-term safety, tolerability, and efficacy of efgartigimod.
Next, he highlights CHAMPION MG, an open-label extension trial that looked at the long-term efficacy and safety profile of ravulizumab in adults with anti-acetylcholine receptor antibody–positive generalized MG.
Dr Govindarajan concludes by reviewing a phase 2 study on nipocalimab, a monoclonal antibody that targets the IgG binding site on FcRn with high affinity, therefore reducing serum levels of total IgG and pathogenic IgG autoantibodies — the underlying cause of MG. This study evaluated efficacy data including myasthenia gravis activities of daily living score evaluated efficacy data including myasthenia gravis activities of daily living.
--
Raghav Govindarajan, MD, Staff neurologist, Stroke Director, HSHS Medical Group-St Elizabeth, O'Fallon, Illinois
Serve(d) as a speaker or a member of a speakers bureau for: Alexion
Received research grant from: Alexion
Received income in an amount equal to or greater than $250 from: Alexion

Commentary, Treatment of Refractory Migraine, June 2022
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
After an initial first dose of a CGRP mAb treatment, many patients ask whether a suboptimal response necessitates switching to another agent or whether a second (or third) dose should be given first. Eptinezumab is an intravenously administered mAb that is repeated every 12 weeks. Schim and colleagues present post hoc data for patients who initially had a minimally beneficial response to eptinezumab and received a second dose at week 13.
The authors define a suboptimal response as having less than a 50% decrease in monthly migraine days after 12 weeks. There were two pooled samples of patients—those who received 100 mg eptinezumab and those who received a 300 mg dose. Approximately 45% of patients in the pivotal trials of eptinezumab (PROMISE-1 and -2) were considered suboptimal responders, and 33%-37% of those suboptimal responders had a more than 50% decrease of their monthly migraine days after a second dose (week 24).
Further analysis determined predictive factors that favored a second dose response. The most prominent (and arguably most obvious) predictive factor was a favorable response after the first dose; the greater percent change in monthly migraine days after the first dose was proportional to the response after the second dose.Change in the Headache Impact Test (HIT-6) disability score after the first dose was also seen to be a strong predictive factor for improvement after the second dose.
When we discuss continuation of medications with our patients, especially when they have a suboptimal response, we should first keep in mind the degree of improvement that the patient initially had.There can be benefit from further treatment with the same medication; however, if the response truly was minimal, it may be better to consider another treatment option.
Practically every patient taking a preventive medication is taking at least one acute medication as well.Even the best preventive medication is not a guarantee that further exacerbations will not occur, and our patients will still need some acute treatment option even when their preventive medications are very effective. The study by Ambrosini and colleagues specifically shows how effective a preventive medication can be, specifically in allowing the patient to use fewer acute medications over time in a population of patients who have been resistant to two to four treatments.
Galcanezumab is a once-monthly mAb for the prevention of migraine.The authors of this study compared the acute use of medications for migraine in both the randomized and open-label stages of a study assessing treatment-refractory patients.A total of 462 patients were enrolled who were all resistant to two to four standard-of-care migraine-preventive medications that had been stopped either because of lack of efficacy or tolerance.The double-blind stage lasted 3 months; the open-label stage lasted another 3 months.
The treatment group was seen to use significantly fewer acute medications after just the first month and continued to improve through month 3.In the open-label phase, a similar improvement was noted in patients transitioning from placebo. In addition to acute medication use, emergency department use for migraine treatment was decreased significantly as well, by more than two thirds in month 3.
Migraine prevention will always remain the key ingredient for improvement for patients with higher frequencies of migraine, and adequate prevention will allow for the lower use of acute medications, and for less healthcare system use in general.
Most practitioners recommend migraine-specific medications for the acute treatment ofmigraine. Since the advent of sumatriptan, this has usually meant a triptan medication. However, a significant percentage of the population (up to 44% in one study) are either intolerant to, contraindicated for, or respond insufficiently to triptan medications. This can either be due to a strong triptan side effect (worsened nausea; tightness/soreness of the muscles of the chest, shoulders, and neck), having cardiovascular risk factors, or not responding adequately 2 hours after treatment.The study by Lipton and colleagues specifically assessed the efficacy of ubrogepant in this population.
Ubrogepant is a small-molecule CGRP antagonist for the acute treatment of migraine. Although somewhat controversial, most practitioners use ubrogepant in patients with some cardiovascular risk, a situation where they would be more likely to avoid the use of triptans.The study authors pooled post hoc data from the pivotal ubrogepant trials (ACHIEVE-1 and -2)to isolate patients with insufficient response to triptans, and their primary outcome was improvement in function 2 hours after medication dose.
Participants in the pivotal trials were separated into three groups: triptan responders, triptaninsufficient responders, and triptan-naive patients. Triptan response was defined as achieving pain freedom 2 hours after medication dose. Both those who had an insufficient response and those who no longer use the triptan owing to intolerance or contraindications were included in the group with insufficient triptan response. Function improvement was defined as the primary outcomeon the basis of a 4-point response scale (0 = no disability, 1 = mildly impaired, 2 = moderately impaired, 3 = severely impaired).In addition, patients were asked to report scores of satisfaction with the medication (yes or no) at 2 and 24 hours and their impression of overall change at 2 hours using a 7-point scale.
The population group of triptan insufficient responders (451 patients) had significant improvement in the primary outcome functional disability at 2, 4, and 7 hours after receipt of medications, but there was no statistical difference at 1 hour. This was similar when comparing those with intolerance to triptans, insufficient response to triptans, or contraindications for triptans. The secondary outcomes of satisfaction and global impression of change were also statistically improved in the insufficient-responders group. No additional tolerance issues or adverse events were noted in this group either.
It would certainly be worth considering the use of agepant acute medication, such as ubrogepant, in patients who are intolerant to or inadequately treated by triptan medications.There still is much to learn about cardiovascular risk and the use of CGRP antagonists, and although no adverse events were noted, more data may be necessary to widely prescribe this class in higher-risk patients.
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
After an initial first dose of a CGRP mAb treatment, many patients ask whether a suboptimal response necessitates switching to another agent or whether a second (or third) dose should be given first. Eptinezumab is an intravenously administered mAb that is repeated every 12 weeks. Schim and colleagues present post hoc data for patients who initially had a minimally beneficial response to eptinezumab and received a second dose at week 13.
The authors define a suboptimal response as having less than a 50% decrease in monthly migraine days after 12 weeks. There were two pooled samples of patients—those who received 100 mg eptinezumab and those who received a 300 mg dose. Approximately 45% of patients in the pivotal trials of eptinezumab (PROMISE-1 and -2) were considered suboptimal responders, and 33%-37% of those suboptimal responders had a more than 50% decrease of their monthly migraine days after a second dose (week 24).
Further analysis determined predictive factors that favored a second dose response. The most prominent (and arguably most obvious) predictive factor was a favorable response after the first dose; the greater percent change in monthly migraine days after the first dose was proportional to the response after the second dose.Change in the Headache Impact Test (HIT-6) disability score after the first dose was also seen to be a strong predictive factor for improvement after the second dose.
When we discuss continuation of medications with our patients, especially when they have a suboptimal response, we should first keep in mind the degree of improvement that the patient initially had.There can be benefit from further treatment with the same medication; however, if the response truly was minimal, it may be better to consider another treatment option.
Practically every patient taking a preventive medication is taking at least one acute medication as well.Even the best preventive medication is not a guarantee that further exacerbations will not occur, and our patients will still need some acute treatment option even when their preventive medications are very effective. The study by Ambrosini and colleagues specifically shows how effective a preventive medication can be, specifically in allowing the patient to use fewer acute medications over time in a population of patients who have been resistant to two to four treatments.
Galcanezumab is a once-monthly mAb for the prevention of migraine.The authors of this study compared the acute use of medications for migraine in both the randomized and open-label stages of a study assessing treatment-refractory patients.A total of 462 patients were enrolled who were all resistant to two to four standard-of-care migraine-preventive medications that had been stopped either because of lack of efficacy or tolerance.The double-blind stage lasted 3 months; the open-label stage lasted another 3 months.
The treatment group was seen to use significantly fewer acute medications after just the first month and continued to improve through month 3.In the open-label phase, a similar improvement was noted in patients transitioning from placebo. In addition to acute medication use, emergency department use for migraine treatment was decreased significantly as well, by more than two thirds in month 3.
Migraine prevention will always remain the key ingredient for improvement for patients with higher frequencies of migraine, and adequate prevention will allow for the lower use of acute medications, and for less healthcare system use in general.
Most practitioners recommend migraine-specific medications for the acute treatment ofmigraine. Since the advent of sumatriptan, this has usually meant a triptan medication. However, a significant percentage of the population (up to 44% in one study) are either intolerant to, contraindicated for, or respond insufficiently to triptan medications. This can either be due to a strong triptan side effect (worsened nausea; tightness/soreness of the muscles of the chest, shoulders, and neck), having cardiovascular risk factors, or not responding adequately 2 hours after treatment.The study by Lipton and colleagues specifically assessed the efficacy of ubrogepant in this population.
Ubrogepant is a small-molecule CGRP antagonist for the acute treatment of migraine. Although somewhat controversial, most practitioners use ubrogepant in patients with some cardiovascular risk, a situation where they would be more likely to avoid the use of triptans.The study authors pooled post hoc data from the pivotal ubrogepant trials (ACHIEVE-1 and -2)to isolate patients with insufficient response to triptans, and their primary outcome was improvement in function 2 hours after medication dose.
Participants in the pivotal trials were separated into three groups: triptan responders, triptaninsufficient responders, and triptan-naive patients. Triptan response was defined as achieving pain freedom 2 hours after medication dose. Both those who had an insufficient response and those who no longer use the triptan owing to intolerance or contraindications were included in the group with insufficient triptan response. Function improvement was defined as the primary outcomeon the basis of a 4-point response scale (0 = no disability, 1 = mildly impaired, 2 = moderately impaired, 3 = severely impaired).In addition, patients were asked to report scores of satisfaction with the medication (yes or no) at 2 and 24 hours and their impression of overall change at 2 hours using a 7-point scale.
The population group of triptan insufficient responders (451 patients) had significant improvement in the primary outcome functional disability at 2, 4, and 7 hours after receipt of medications, but there was no statistical difference at 1 hour. This was similar when comparing those with intolerance to triptans, insufficient response to triptans, or contraindications for triptans. The secondary outcomes of satisfaction and global impression of change were also statistically improved in the insufficient-responders group. No additional tolerance issues or adverse events were noted in this group either.
It would certainly be worth considering the use of agepant acute medication, such as ubrogepant, in patients who are intolerant to or inadequately treated by triptan medications.There still is much to learn about cardiovascular risk and the use of CGRP antagonists, and although no adverse events were noted, more data may be necessary to widely prescribe this class in higher-risk patients.
Many of our patients with refractory migraine do not respond to first-line acute or preventive treatments, and, almost by definition, first- and second-line treatments have failed in the majority of patients on calcitonin gene-related peptide (CGRP) antagonist medications. Three studies this month highlight the efficacy of CGRP monoclonal antibody (mAb) and small-molecule medications in this population specifically.
After an initial first dose of a CGRP mAb treatment, many patients ask whether a suboptimal response necessitates switching to another agent or whether a second (or third) dose should be given first. Eptinezumab is an intravenously administered mAb that is repeated every 12 weeks. Schim and colleagues present post hoc data for patients who initially had a minimally beneficial response to eptinezumab and received a second dose at week 13.
The authors define a suboptimal response as having less than a 50% decrease in monthly migraine days after 12 weeks. There were two pooled samples of patients—those who received 100 mg eptinezumab and those who received a 300 mg dose. Approximately 45% of patients in the pivotal trials of eptinezumab (PROMISE-1 and -2) were considered suboptimal responders, and 33%-37% of those suboptimal responders had a more than 50% decrease of their monthly migraine days after a second dose (week 24).
Further analysis determined predictive factors that favored a second dose response. The most prominent (and arguably most obvious) predictive factor was a favorable response after the first dose; the greater percent change in monthly migraine days after the first dose was proportional to the response after the second dose.Change in the Headache Impact Test (HIT-6) disability score after the first dose was also seen to be a strong predictive factor for improvement after the second dose.
When we discuss continuation of medications with our patients, especially when they have a suboptimal response, we should first keep in mind the degree of improvement that the patient initially had.There can be benefit from further treatment with the same medication; however, if the response truly was minimal, it may be better to consider another treatment option.
Practically every patient taking a preventive medication is taking at least one acute medication as well.Even the best preventive medication is not a guarantee that further exacerbations will not occur, and our patients will still need some acute treatment option even when their preventive medications are very effective. The study by Ambrosini and colleagues specifically shows how effective a preventive medication can be, specifically in allowing the patient to use fewer acute medications over time in a population of patients who have been resistant to two to four treatments.
Galcanezumab is a once-monthly mAb for the prevention of migraine.The authors of this study compared the acute use of medications for migraine in both the randomized and open-label stages of a study assessing treatment-refractory patients.A total of 462 patients were enrolled who were all resistant to two to four standard-of-care migraine-preventive medications that had been stopped either because of lack of efficacy or tolerance.The double-blind stage lasted 3 months; the open-label stage lasted another 3 months.
The treatment group was seen to use significantly fewer acute medications after just the first month and continued to improve through month 3.In the open-label phase, a similar improvement was noted in patients transitioning from placebo. In addition to acute medication use, emergency department use for migraine treatment was decreased significantly as well, by more than two thirds in month 3.
Migraine prevention will always remain the key ingredient for improvement for patients with higher frequencies of migraine, and adequate prevention will allow for the lower use of acute medications, and for less healthcare system use in general.
Most practitioners recommend migraine-specific medications for the acute treatment ofmigraine. Since the advent of sumatriptan, this has usually meant a triptan medication. However, a significant percentage of the population (up to 44% in one study) are either intolerant to, contraindicated for, or respond insufficiently to triptan medications. This can either be due to a strong triptan side effect (worsened nausea; tightness/soreness of the muscles of the chest, shoulders, and neck), having cardiovascular risk factors, or not responding adequately 2 hours after treatment.The study by Lipton and colleagues specifically assessed the efficacy of ubrogepant in this population.
Ubrogepant is a small-molecule CGRP antagonist for the acute treatment of migraine. Although somewhat controversial, most practitioners use ubrogepant in patients with some cardiovascular risk, a situation where they would be more likely to avoid the use of triptans.The study authors pooled post hoc data from the pivotal ubrogepant trials (ACHIEVE-1 and -2)to isolate patients with insufficient response to triptans, and their primary outcome was improvement in function 2 hours after medication dose.
Participants in the pivotal trials were separated into three groups: triptan responders, triptaninsufficient responders, and triptan-naive patients. Triptan response was defined as achieving pain freedom 2 hours after medication dose. Both those who had an insufficient response and those who no longer use the triptan owing to intolerance or contraindications were included in the group with insufficient triptan response. Function improvement was defined as the primary outcomeon the basis of a 4-point response scale (0 = no disability, 1 = mildly impaired, 2 = moderately impaired, 3 = severely impaired).In addition, patients were asked to report scores of satisfaction with the medication (yes or no) at 2 and 24 hours and their impression of overall change at 2 hours using a 7-point scale.
The population group of triptan insufficient responders (451 patients) had significant improvement in the primary outcome functional disability at 2, 4, and 7 hours after receipt of medications, but there was no statistical difference at 1 hour. This was similar when comparing those with intolerance to triptans, insufficient response to triptans, or contraindications for triptans. The secondary outcomes of satisfaction and global impression of change were also statistically improved in the insufficient-responders group. No additional tolerance issues or adverse events were noted in this group either.
It would certainly be worth considering the use of agepant acute medication, such as ubrogepant, in patients who are intolerant to or inadequately treated by triptan medications.There still is much to learn about cardiovascular risk and the use of CGRP antagonists, and although no adverse events were noted, more data may be necessary to widely prescribe this class in higher-risk patients.
Commentary: Treating ER+ Breast Cancer and Brain Metastases, July 2022
The clinical benefit of fulvestrant as a second-line treatment after tumor progression with CDK4/6i-based therapy has been discouraging, highlighting an unmet need for a better SERD in this space.1 Multiple oral SERD are currently in trials. The EMERALD trial is a phase 3 study that randomly assigned 477 patients with ER+/HER2- mBC in a 1:1 ratio to elacestrant, an oral SERD, vs standard of care (SOC) endocrine therapy (ET). Enrolled patients had received one to two prior ET and one or less chemotherapy treatments in the metastatic disease settings. All patients had prior treatment with a CDK4/6i. Fulvestrant-naive patients were required to have fulvestrant as the SOC ET. In contrast, patients previously treated with fulvestrant received an AI, the selection of which was based on prior AI therapy. Primary endpoints were progression-free survival (PFS) in all patients and in patients with detectable ESR1 mutation. The median PFS in the elacestrant arm was 2.8 months vs 1.9 months in the control arm [hazard ratio (HR) 0.70; 95% CI 0.55-0.88; P = .002]. The 6-month PFS was 34% vs 20% in all patients and 41% vs 19% in patients with detectable ESR1 mutation, favoring the elacestrant arm. In the subgroup analysis among patients who received fulvestrant in the control arm, the 6-month PFS was 34% vs 21% in all patients and 41% vs 19% in patients with ESR1 mutation. Grade 3 or 4 adverse events developed in 27% patients in the elacestrant arm compared with 20% in the SOC arm. More patients in the elacestrant arm developed nausea, vomiting, and liver function abnormalities compared with patients in the SOC arm.
This is the first phase 3 trial demonstrating statistically significant prolongation of PFS associated with an oral SERD compared with SOC ET in patients with ER+/HER2- mBC who had prior treatment with a CDK4/6i. A new drug application has been submitted to the US Food and Drug Administration based on this data. If approved, elacestrant may be favored over fulvestrant as the standard ET for ER+/HER2- mBC after progression on a CDK4/6i-based therapy.
Patients with breast cancer brain metastasis (BrM) have a poor prognosis. Systemic therapies with good central nervous system (CNS) permeability and strong activity against BrM are much needed. An exploratory subset of the phase 3 BEACON trial demonstrated improvement in overall survival (OS) with etirinotecan pegol, a long-acting polymer conjugate of irinotecan, compared with physicians' choice chemotherapy in patients with mBC with treated and stable BrMs.2
Based on this data, a large phase 3 study (the ATTAIN study) was conducted. Patients with mBC with treated and stable BrM (n = 178) were randomly assigned to receive etirinotecan pegol vs physicians' choice of chemotherapy (eribulin, ixabepilone, vinorelbine, gemcitabine, paclitaxel, docetaxel, or nab-paclitaxel). The primary endpoint of OS was similar in both groups (etirinotecan pegol 7.8 months; chemotherapy 7.5 months; HR 0.90; 95% CI 0.61-1.33; P = .60). Median PFS for mBC with CNS metastases (etirinotecan pegol vs chemotherapy) were 3.9 vs 3.3 months (HR 0.59; 95% CI 0.33-1.05; P = .07) and for non-CNS metastases were 2.8 vs 1.9 months (HR 0.72; 95% CI 0.45-1.16; P = .18). Adverse events were grade 3 or 4 in 57% patients receiving etirinotecan pegol compared with 64% receiving SOC chemotherapy.
This trial failed to meet its primary endpoint. The possible explanations proposed by the investigators are a protocol amendment that reduced the power of the trial to 80%, some key differences in the patient population in the ATTAIN trial compared with the BEACON trial, and the possibility of the BEACON trial exploratory analysis result being a false positive. The OS of around 7 months highlights the unmet need for better systemic therapy for patients with BrM breast cancer, especially those with HER2- breast cancer.
Additional References
- Lindeman GJ, Fernando TM, Bowen R, et al. VERONICA: Randomized phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors – Efficacy, safety, and biomarker results. Clin Cancer Res. 2022 (June 21). Doi: 10.1158/1078-0432.CCR-21-3811
- Cortés J, Rugo HS, Awada A, et al. Prolonged survival in patients with breast cancer and a history of brain metastases: Results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat. 2017;165:329-341. Doi: 10.1007/s10549-017-4304-7
The clinical benefit of fulvestrant as a second-line treatment after tumor progression with CDK4/6i-based therapy has been discouraging, highlighting an unmet need for a better SERD in this space.1 Multiple oral SERD are currently in trials. The EMERALD trial is a phase 3 study that randomly assigned 477 patients with ER+/HER2- mBC in a 1:1 ratio to elacestrant, an oral SERD, vs standard of care (SOC) endocrine therapy (ET). Enrolled patients had received one to two prior ET and one or less chemotherapy treatments in the metastatic disease settings. All patients had prior treatment with a CDK4/6i. Fulvestrant-naive patients were required to have fulvestrant as the SOC ET. In contrast, patients previously treated with fulvestrant received an AI, the selection of which was based on prior AI therapy. Primary endpoints were progression-free survival (PFS) in all patients and in patients with detectable ESR1 mutation. The median PFS in the elacestrant arm was 2.8 months vs 1.9 months in the control arm [hazard ratio (HR) 0.70; 95% CI 0.55-0.88; P = .002]. The 6-month PFS was 34% vs 20% in all patients and 41% vs 19% in patients with detectable ESR1 mutation, favoring the elacestrant arm. In the subgroup analysis among patients who received fulvestrant in the control arm, the 6-month PFS was 34% vs 21% in all patients and 41% vs 19% in patients with ESR1 mutation. Grade 3 or 4 adverse events developed in 27% patients in the elacestrant arm compared with 20% in the SOC arm. More patients in the elacestrant arm developed nausea, vomiting, and liver function abnormalities compared with patients in the SOC arm.
This is the first phase 3 trial demonstrating statistically significant prolongation of PFS associated with an oral SERD compared with SOC ET in patients with ER+/HER2- mBC who had prior treatment with a CDK4/6i. A new drug application has been submitted to the US Food and Drug Administration based on this data. If approved, elacestrant may be favored over fulvestrant as the standard ET for ER+/HER2- mBC after progression on a CDK4/6i-based therapy.
Patients with breast cancer brain metastasis (BrM) have a poor prognosis. Systemic therapies with good central nervous system (CNS) permeability and strong activity against BrM are much needed. An exploratory subset of the phase 3 BEACON trial demonstrated improvement in overall survival (OS) with etirinotecan pegol, a long-acting polymer conjugate of irinotecan, compared with physicians' choice chemotherapy in patients with mBC with treated and stable BrMs.2
Based on this data, a large phase 3 study (the ATTAIN study) was conducted. Patients with mBC with treated and stable BrM (n = 178) were randomly assigned to receive etirinotecan pegol vs physicians' choice of chemotherapy (eribulin, ixabepilone, vinorelbine, gemcitabine, paclitaxel, docetaxel, or nab-paclitaxel). The primary endpoint of OS was similar in both groups (etirinotecan pegol 7.8 months; chemotherapy 7.5 months; HR 0.90; 95% CI 0.61-1.33; P = .60). Median PFS for mBC with CNS metastases (etirinotecan pegol vs chemotherapy) were 3.9 vs 3.3 months (HR 0.59; 95% CI 0.33-1.05; P = .07) and for non-CNS metastases were 2.8 vs 1.9 months (HR 0.72; 95% CI 0.45-1.16; P = .18). Adverse events were grade 3 or 4 in 57% patients receiving etirinotecan pegol compared with 64% receiving SOC chemotherapy.
This trial failed to meet its primary endpoint. The possible explanations proposed by the investigators are a protocol amendment that reduced the power of the trial to 80%, some key differences in the patient population in the ATTAIN trial compared with the BEACON trial, and the possibility of the BEACON trial exploratory analysis result being a false positive. The OS of around 7 months highlights the unmet need for better systemic therapy for patients with BrM breast cancer, especially those with HER2- breast cancer.
Additional References
- Lindeman GJ, Fernando TM, Bowen R, et al. VERONICA: Randomized phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors – Efficacy, safety, and biomarker results. Clin Cancer Res. 2022 (June 21). Doi: 10.1158/1078-0432.CCR-21-3811
- Cortés J, Rugo HS, Awada A, et al. Prolonged survival in patients with breast cancer and a history of brain metastases: Results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat. 2017;165:329-341. Doi: 10.1007/s10549-017-4304-7
The clinical benefit of fulvestrant as a second-line treatment after tumor progression with CDK4/6i-based therapy has been discouraging, highlighting an unmet need for a better SERD in this space.1 Multiple oral SERD are currently in trials. The EMERALD trial is a phase 3 study that randomly assigned 477 patients with ER+/HER2- mBC in a 1:1 ratio to elacestrant, an oral SERD, vs standard of care (SOC) endocrine therapy (ET). Enrolled patients had received one to two prior ET and one or less chemotherapy treatments in the metastatic disease settings. All patients had prior treatment with a CDK4/6i. Fulvestrant-naive patients were required to have fulvestrant as the SOC ET. In contrast, patients previously treated with fulvestrant received an AI, the selection of which was based on prior AI therapy. Primary endpoints were progression-free survival (PFS) in all patients and in patients with detectable ESR1 mutation. The median PFS in the elacestrant arm was 2.8 months vs 1.9 months in the control arm [hazard ratio (HR) 0.70; 95% CI 0.55-0.88; P = .002]. The 6-month PFS was 34% vs 20% in all patients and 41% vs 19% in patients with detectable ESR1 mutation, favoring the elacestrant arm. In the subgroup analysis among patients who received fulvestrant in the control arm, the 6-month PFS was 34% vs 21% in all patients and 41% vs 19% in patients with ESR1 mutation. Grade 3 or 4 adverse events developed in 27% patients in the elacestrant arm compared with 20% in the SOC arm. More patients in the elacestrant arm developed nausea, vomiting, and liver function abnormalities compared with patients in the SOC arm.
This is the first phase 3 trial demonstrating statistically significant prolongation of PFS associated with an oral SERD compared with SOC ET in patients with ER+/HER2- mBC who had prior treatment with a CDK4/6i. A new drug application has been submitted to the US Food and Drug Administration based on this data. If approved, elacestrant may be favored over fulvestrant as the standard ET for ER+/HER2- mBC after progression on a CDK4/6i-based therapy.
Patients with breast cancer brain metastasis (BrM) have a poor prognosis. Systemic therapies with good central nervous system (CNS) permeability and strong activity against BrM are much needed. An exploratory subset of the phase 3 BEACON trial demonstrated improvement in overall survival (OS) with etirinotecan pegol, a long-acting polymer conjugate of irinotecan, compared with physicians' choice chemotherapy in patients with mBC with treated and stable BrMs.2
Based on this data, a large phase 3 study (the ATTAIN study) was conducted. Patients with mBC with treated and stable BrM (n = 178) were randomly assigned to receive etirinotecan pegol vs physicians' choice of chemotherapy (eribulin, ixabepilone, vinorelbine, gemcitabine, paclitaxel, docetaxel, or nab-paclitaxel). The primary endpoint of OS was similar in both groups (etirinotecan pegol 7.8 months; chemotherapy 7.5 months; HR 0.90; 95% CI 0.61-1.33; P = .60). Median PFS for mBC with CNS metastases (etirinotecan pegol vs chemotherapy) were 3.9 vs 3.3 months (HR 0.59; 95% CI 0.33-1.05; P = .07) and for non-CNS metastases were 2.8 vs 1.9 months (HR 0.72; 95% CI 0.45-1.16; P = .18). Adverse events were grade 3 or 4 in 57% patients receiving etirinotecan pegol compared with 64% receiving SOC chemotherapy.
This trial failed to meet its primary endpoint. The possible explanations proposed by the investigators are a protocol amendment that reduced the power of the trial to 80%, some key differences in the patient population in the ATTAIN trial compared with the BEACON trial, and the possibility of the BEACON trial exploratory analysis result being a false positive. The OS of around 7 months highlights the unmet need for better systemic therapy for patients with BrM breast cancer, especially those with HER2- breast cancer.
Additional References
- Lindeman GJ, Fernando TM, Bowen R, et al. VERONICA: Randomized phase II study of fulvestrant and venetoclax in ER-positive metastatic breast cancer post-CDK4/6 inhibitors – Efficacy, safety, and biomarker results. Clin Cancer Res. 2022 (June 21). Doi: 10.1158/1078-0432.CCR-21-3811
- Cortés J, Rugo HS, Awada A, et al. Prolonged survival in patients with breast cancer and a history of brain metastases: Results of a preplanned subgroup analysis from the randomized phase III BEACON trial. Breast Cancer Res Treat. 2017;165:329-341. Doi: 10.1007/s10549-017-4304-7
Acute Generalized Exanthematous Pustulosis Induced by the Second-Generation Antipsychotic Cariprazine
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.
A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.
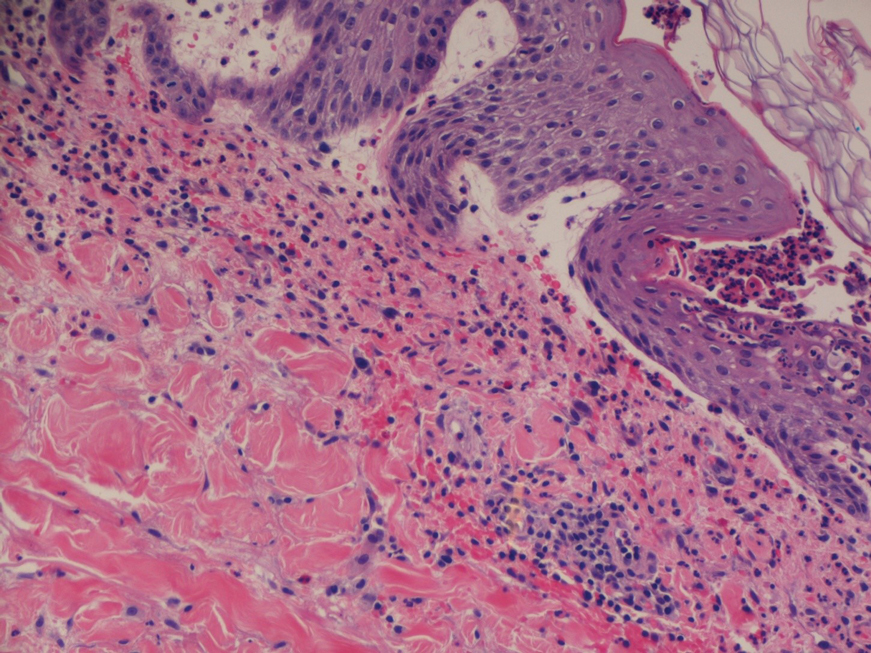
Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.

A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.

Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
To the Editor:
A 57-year-old woman presented to an outpatient clinic with severe pruritus and burning of the skin as well as subjective fevers and chills. She had been discharged from a psychiatric hospital for attempted suicide 1 day prior. There were no recent changes in the medication regimen, which consisted of linaclotide, fluoxetine, lorazepam, and gabapentin. While admitted, the patient was started on the atypical antipsychotic cariprazine. Within 24 hours of the first dose, she developed severe facial erythema that progressed to diffuse erythema over more than 60% of the body surface area. The attending psychiatrist promptly discontinued cariprazine. During the next 24 hours, there were no reports of fever, leukocytosis, or signs of systemic organ involvement. Given the patient’s mental and medical stability, she was discharged with instructions to follow up with the outpatient dermatology clinic.
At the current presentation, physical examination revealed innumerable 1- to 4-mm pustules coalescing to lakes of pus on an erythematous base over more than 60% of the body surface area (Figure 1). The mucous membranes were clear of lesions, the Nikolsky sign was negative, and the patient’s temperature was 99.6 °F in the office. Complete blood cell count and complete metabolic panel results were within reference range.

A 4-mm abdominal punch biopsy showed subcorneal neutrophilic pustules, papillary dermal edema, and superficial dermal lymphohistiocytic inflammation with numerous neutrophils, eosinophils, and extravasated red blood cells, consistent with acute generalized exanthematous pustulosis (AGEP)(Figure 2). The patient was started on wet wraps with triamcinolone cream 0.1%.

Two days later, physical examination revealed the erythema noted on initial examination had notably decreased, and the patient no longer reported burning or pruritus. One week after initial presentation to the clinic, the patient’s rash had resolved, and only a few small areas of desquamation remained.
Acute generalized exanthematous pustulosis is a severe cutaneous adverse reaction characterized by the development of numerous nonfollicular sterile pustules on an edematous and erythematous base. In almost 90% of reported cases, the cause is related to use of antibiotics, antifungals, antimalarials, or diltiazem (a calcium channel blocker). This rare cutaneous reaction occurs in 1 to 5 patients per million per year1; it carries a 1% to 2% mortality rate with proper supportive treatment.
The clinical symptoms of AGEP typically present 24 to 48 hours after drug initiation with the rapid development of dozens to thousands of 1- to 4-mm pustules, typically localized to the flexor surfaces and face. In the setting of AGEP, acute onset of fever and leukocytosis typically occur at the time of the cutaneous eruption. These features were absent in this patient. The eruption usually starts on the face and then migrates to the trunk and extremities, sparing the palms and soles. Systemic involvement most commonly presents as hepatic, renal, or pulmonary insufficiency, which has been seen in 20% of cases.2
The immunologic response associated with the reaction has been studied in vitro. Drug-specific CD8 T cells use perforin/granzyme B and Fas ligand mechanisms to induce apoptosis of the keratinocytes within the epidermis, leading to vesicle formation.3 During the very first stages of formation, vesicles mainly comprise CD8 T cells and keratinocytes. These cells then begin producing CXC-18, a potent neutrophil chemokine, leading to extensive chemotaxis of neutrophils into vesicles, which then rapidly transform to pustules.3 This rapid transformation leads to the lakes of pustules, a description often associated with AGEP.
Treatment of AGEP is mainly supportive and consists of discontinuing use of the causative agent. Topical corticosteroids can be used during the pustular phase for symptom management. There is no evidence that systemic steroids reduce the duration of the disease.2 Other supportive measures such as application of wet wraps can be used to provide comfort.
Cutaneous adverse drug reactions commonly are associated with psychiatric pharmacotherapy, but first-and second-generation antipsychotics rarely are associated with these types of reactions. In this patient, the causative agent of the AGEP was cariprazine, an atypical antipsychotic that had no reported association with AGEP or cutaneous adverse drug reactions prior to this presentation.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
- Fernando SL. Acute generalised exanthematous pustulosis. Australas J Dermatol. 2012;53:87-92.
- Feldmeyer L, Heidemeyer K, Yawalkar N. Acute generalized exanthematous pustulosis: pathogenesis, genetic background, clinical variants and therapy. Int J Mol Sci. 2016;17:1214.
- Szatkowski J, Schwartz RA. Acute generalized exanthematous pustulosis (AGEP): a review and update. J Am Acad Dermatol. 2015;73:843-848.
Practice Points
- The second-generation antipsychotic cariprazine has been shown to be a potential causative agent in acute generalized exanthematous pustulosis (AGEP).
- Treatment of AGEP is mainly supportive and consists of discontinuation of the causative agent as well as symptom control using cold compresses and topical corticosteroids.
Nevus Lipomatosis Deemed Suspicious by Airport Security
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.
Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.

Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
To the Editor:
A 47-year-old man presented at the dermatology clinic with a growing lesion on the left medial thigh.
Physical examination revealed a 5-cm, pedunculated, fatty nodule on the left medial thigh that was clinically consistent with nevus lipomatosis (NL)(Figure). Although benign, trouble traveling through airport security prompted the patient to request shave removal, which subsequently was performed. Histology showed a large pedunculated nodule with prominent adipose tissue, consistent with NL. At 3-month follow-up, the patient reported getting through airport security multiple times without incident.

Nevus lipomatosis is a benign fatty lesion most commonly found on the medial thighs or trunk of adults. The lesion usually is asymptomatic but can become irritated by rubbing or catching on clothing. Our patient had symptomatic NL that caused delays getting through airport security; he experienced full resolution after simple shave removal. In rare instances, both benign and malignant skin conditions have been seen on airport scanning devices since the introduction of increased security measures following September 11, 2001. In 2016, Heymann1 reported a man with a 1.5-cm epidermal inclusion cyst detected by airport security scanners, prompting the traveler to request and carry a medically explanatory letter used to get through security. In 2015 Mayer and Adams2 described a case of nodular melanoma that was detected 20 times over a period of 2 months by airport scanners, and in 2016, Caine et al3 reported a case of desmoplastic melanoma that was detected by airport security, but after its removal was not identified by security for the next 40 flights. Noncutaneous pathology also can be detected by airport scanners. In 2013, Naraynsingh et al4 reported a man with a large left reducible inguinal hernia who was stopped by airport security and subjected to an invasive physical examination of the area. These instances demonstrate the breadth of conditions that can be cumbersome when individuals are traveling by airplane in our current security climate.
Our patient had to go through the trouble of having the benign NL lesion removed to avoid the hassle of repeatedly being stopped by airport security. The patient had the lesion removed and is doing well, but the procedure could have been avoided if systems existed to help patients with dermatologic and medical conditions at airport security. Our patient likely will never be stopped again for the suspicious lump on the left inner thigh, but many others will be stopped for similar reasons.
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
- Heymann WR. A cyst misinterpreted on airport scan as security threat. JAMA Dermatol. 2016;152:1388. doi:10.1001/jamadermatol.2016.3329
- Mayer JE, Adams BB. Nodular melanoma serendipitously detected by airport full body scanners. Dermatology. 2015;230:16-17. doi:10.1159/000368045
- Caine P, Javed MU, Karoo ROS. A desmoplastic melanoma detected by an airport security scanner. J Plast Reconstr Aesthet Surg. 2016;69:874-876. doi:10.1016/j.bjps.2016.02.022
- Naraynsingh V, Cawich SO, Maharaj R, et al. Inguinal hernia and airport scanners: an emerging indication for repair? 2013;2013:952835. Case Rep Med. doi:10.1155/2013/952835
Practice Points
- Nevus lipomatosis is a benign fatty lesion that most commonly is found on the medial thighs or trunk of adults.
- Both benign and malignant skin conditions have been detected on airport scanning devices.
- At times, patients must go through the hassle of having the benign lesions removed to avoid repeated problems at airport security.
Telemedicine and Home Pregnancy Testing for iPLEDGE: A Survey of Clinician Perspectives
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).

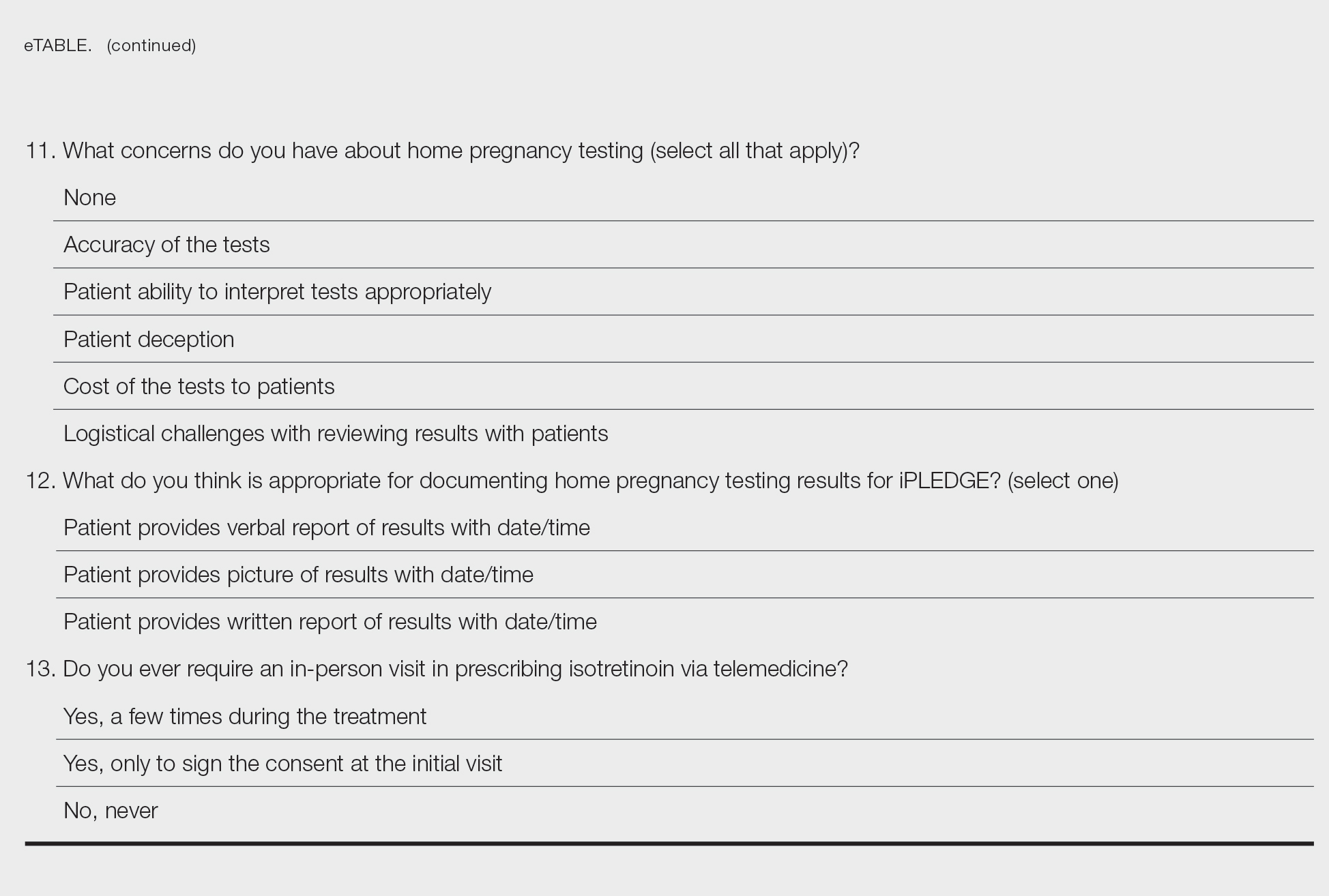
Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).
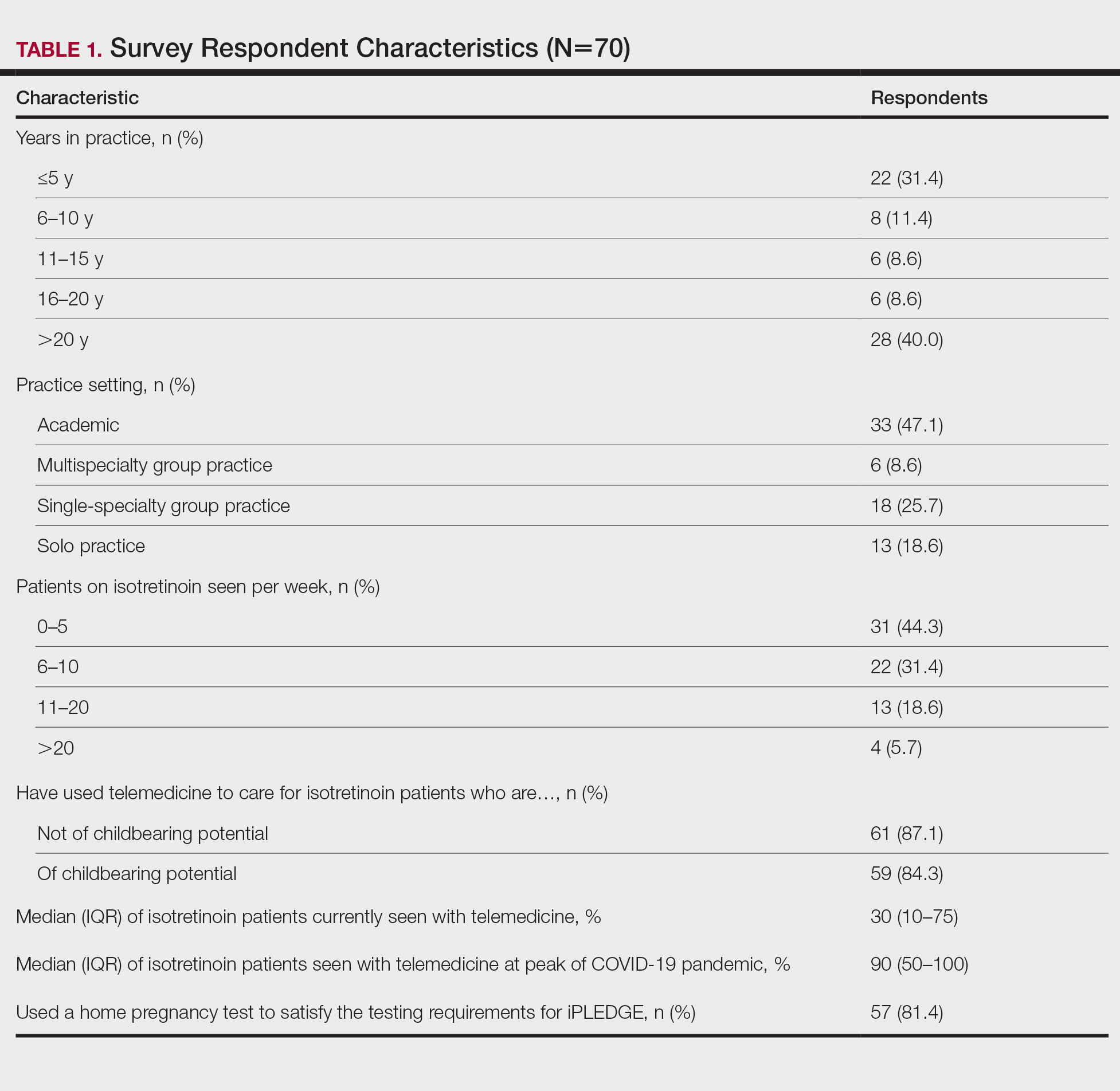
The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).
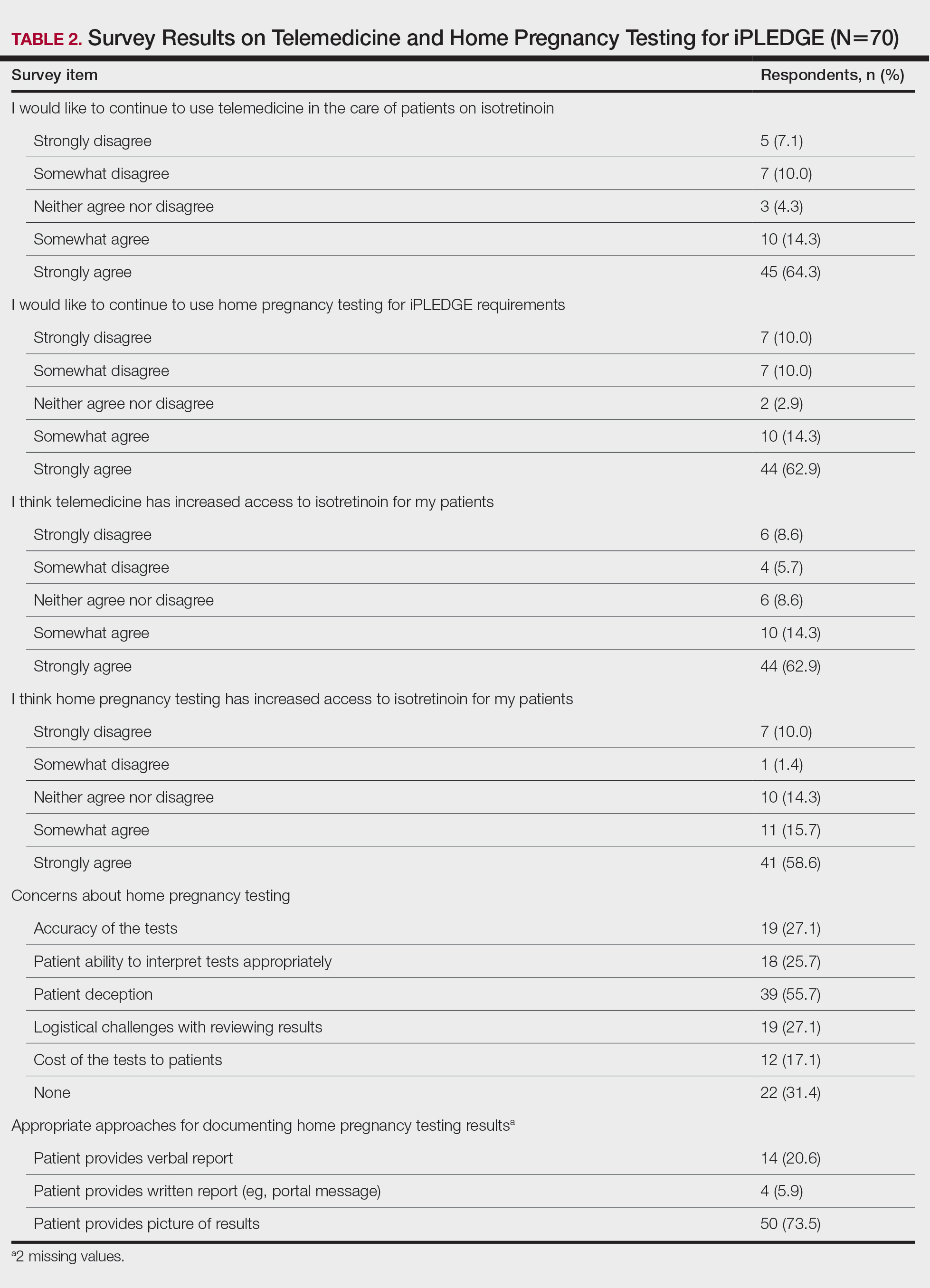
In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2
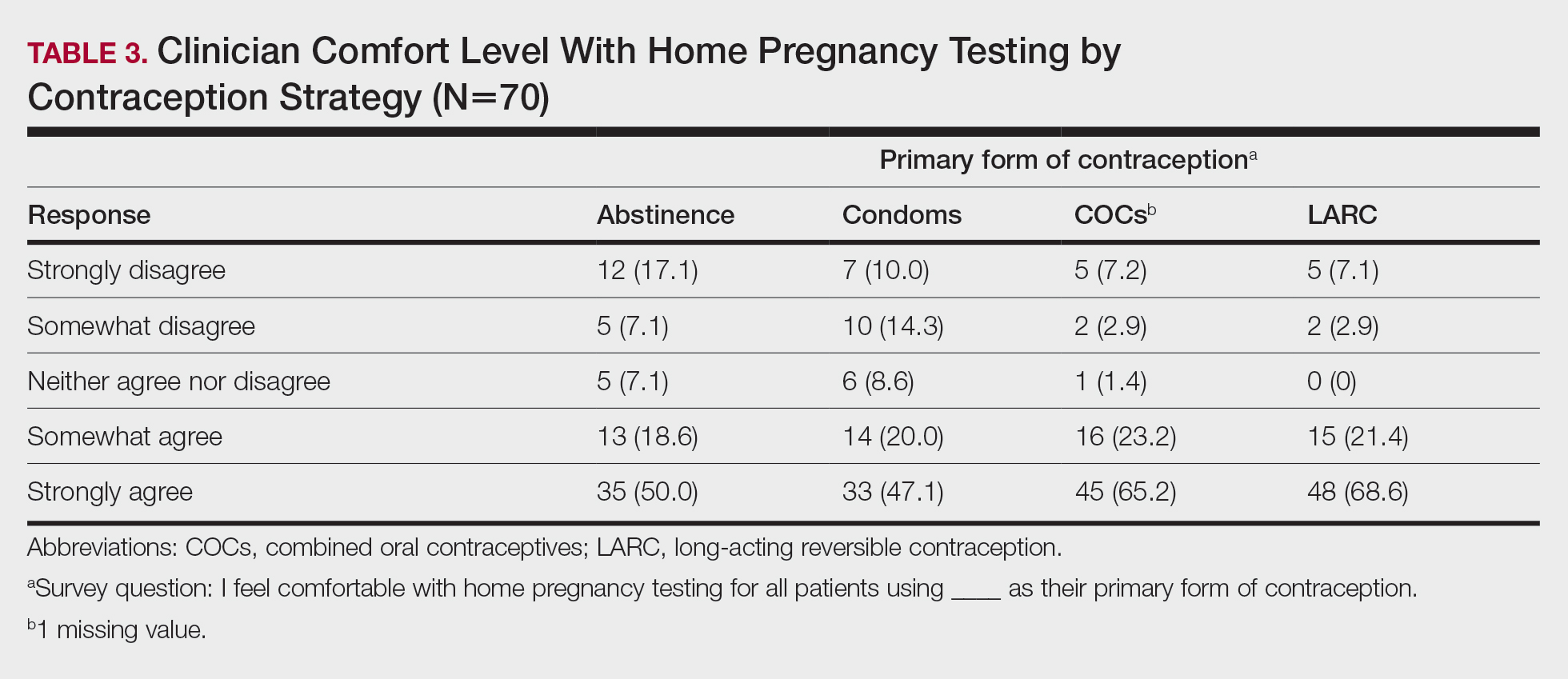
Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
To the Editor:
In response to the challenges of the COVID-19 pandemic, iPLEDGE announced that they would accept results from home pregnancy tests and explicitly permit telemedicine.1 Given the financial and logistical burdens associated with iPLEDGE, these changes have the potential to increase access.2 However, it is unclear whether these modifications will be allowed to continue. We sought to evaluate clinician perspectives on the role of telemedicine and home pregnancy testing for iPLEDGE.
After piloting among several clinicians, a 13-question survey was distributed using the Qualtrics platform to members of the American Acne & Rosacea Society between April 14, 2021, and June 14, 2021. This survey consisted of items addressing provider practices and perspectives on telemedicine and home pregnancy testing for patients taking isotretinoin (eTable). Respondents were asked whether they think telemedicine and home pregnancy testing have improved access to care and whether they would like to continue these practices going forward. In addition, participants were asked about their concerns with home pregnancy testing and how comfortable they feel with home pregnancy testing for various contraceptive strategies (abstinence, condoms, combined oral contraceptives, and long-acting reversible contraception). This study was deemed exempt (category 2) by the University of Pennsylvania (Philadelphia, Pennsylvania) institutional review board (Protocol #844549).


Among 70 clinicians who completed the survey (response rate, 6.4%), 33 (47.1%) practiced in an academic setting. At the peak of the COVID-19 pandemic, clinicians reported using telemedicine for a median of 90% (IQR=50%–100%) of their patients on isotretinoin, and 57 respondents (81.4%) reported having patients use a home pregnancy test for iPLEDGE (Table 1). More than 75% (55/70) agreed that they would like to continue to use telemedicine for patients on isotretinoin, and more than 75% (54/70) agreed that they would like to continue using home pregnancy testing for patients outside the setting of the COVID-19 pandemic. More than 75% (54/70) agreed that telemedicine has increased access for their patients, and more than 70% (52/70) agreed that home pregnancy testing has increased access (Table 2). Clinicians agreed that they would be comfortable using home pregnancy testing for patients choosing long-acting reversible contraception (63/70 [90.0%]), combined oral contraceptives (61/69 [88.4%]), condoms (47/70 [67.1%]), or abstinence (48/70 [68.6%])(Table 3).

The most common concerns about home pregnancy testing were patient deception (39/70 [55.7%]), logistical challenges with reviewing results (19/70 [27.1%]), accuracy of the tests (19/70 [27.1%]), and patient ability to interpret tests appropriately (18/70 [25.7%]). To document testing results, 50 respondents (73.5%) would require a picture of results, 4 (5.9%) would accept a written report from the patient, and 14 (20.6%) would accept a verbal report from the patient (Table 2).

In this survey, clinicians expressed interest in continuing to use telemedicine and home pregnancy testing to care for patients with acne treated with isotretinoin. More than 75% agreed that these changes have increased access, which is notable, as several studies have identified that female and minority patients may face iPLEDGE-associated access barriers.3,4 Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care.2

Although clinicians felt comfortable with a variety of contraceptive strategies, particularly those with high reported effectiveness,5 there were concerns about deception and interpretation of test results. Future studies are needed to identify optimal workflows for home pregnancy testing and whether patients should be required to provide a photograph of the results.
This survey study is limited by the possibility of sampling and response bias due to the low response rate. Although the use of national listservs was employed to maximize the generalizability of the results, given the response rate, future studies are needed to evaluate whether these findings generalize to other settings. In addition, given iPLEDGE-associated access barriers, further research is needed to examine how changes such as telemedicine and home pregnancy testing influence both access to isotretinoin and pregnancy prevention.
Acknowledgments—We would like to thank Stacey Moore (Montclair, New Jersey) and the American Acne & Rosacea Society for their help distributing the survey.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
- Kane S, Admani S. COVID-19 pandemic leading to the accelerated development of a virtual health model for isotretinoin. J Dermatol Nurses Assoc. 2021;13:54-57.
- Barbieri JS, Frieden IJ, Nagler AR. Isotretinoin, patient safety, and patient-centered care-time to reform iPLEDGE. JAMA Dermatol. 2020;156:21-22.
- Barbieri JS, Shin DB, Wang S, et al. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156:312-319.
- Charrow A, Xia FD, Lu J, et al. Differences in isotretinoin start, interruption, and early termination across race and sex in the iPLEDGE era. PloS One. 2019;14:E0210445.
- Barbieri JS, Roe AH, Mostaghimi A. Simplifying contraception requirements for iPLEDGE: a decision analysis. J Am Acad Dermatol. 2020;83:104-108.
PRACTICE POINTS
- The majority of clinicians report that the use of telemedicine and home pregnancy testing for iPLEDGE has improved access to care and that they would like to continue these practices.
- Continuing to allow home pregnancy testing and explicitly permitting telemedicine can enable clinicians to provide patient-centered care for patients treated with isotretinoin.
What’s Diet Got to Do With It? Basic and Clinical Science Behind Diet and Acne
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
The current understanding of the pathogenesis of acne includes altered keratinization, follicular obstruction, overproduction of sebum, and microbial colonization ( Cutibacterium acnes ) of the pilosebaceous unit resulting in perifollicular inflammation. 1 A deeper dive into the hormonal and molecular drivers of acne have implicated insulin, insulinlike growth factor 1 (IGF-1), corticotropin-releasing hormone, the phosphoinositide 3 -kinase/Akt pathway, mitogen-activated protein kinase pathway, and the nuclear factor κ B pathway. 2-4 A Western diet comprised of high glycemic index foods, carbohydrates, and dairy enhances the production of insulin and IGF-1. A downstream effect of excess insulin and IGF-1 is overactivity of the mammalian target of rapamycin complex 1 (mTORC1), a major promoter of cellular growth and proliferation that primarily is regulated through nutrient availability. 5 This article will review our understanding of the impact of the Western diet on acne pathogenesis and highlight the existing evidence behind the contributions of the mTORC1 pathway in this process. Although quality randomized controlled trials analyzing these effects are limited, dermatologists should understand the existing evidence supporting the potential impacts of diet on acne.
The Western Diet
Glycemic Index—To assess the impact of a high glycemic index diet on acne, Kwon et al6 evaluated 32 patients with mild to moderate acne and placed them on a low or high glycemic index diet for 10 weeks. The low glycemic index diet group was found to have a 70% reduction in the mean number of inflammatory acne lesions from baseline (P<.05), while the high glycemic index diet group had no significant reduction. Noninflammatory lesion counts remained statistically unchanged.6 Smith et al7 studied 43 male patients with acne on either a low glycemic index diet or a self-directed high glycemic diet that was carbohydrate dense. The low glycemic index group showed greater improvement in lesion count as well as improved insulin sensitivity at 12 weeks. Specifically, the mean lesion count (SEM) decreased by 23.5 (3.9) in the low glycemic index group and by only 12.0 (3.5) in the control group (P=.03).7 Observational studies also have supported this hypothesis. After adjustment, an analysis of 24,452 participants in the NutriNet-Santé cohort found significant associations between current acne and the consumption of sugary beverages (adjusted OR, 1.18; 95% CI, 1.01-1.38) and the consumption of fatty and sugary products (adjusted OR, 1.54; 95% CI, 1.09-2.16).8 A Cochrane review that included only 2 studies (Kwon et al6 and Smith et al7) did not find evidence to suggest a low glycemic index diet for noninflammatory lesion count reduction but did note possible benefit for a reduction in inflammatory and total lesion counts; however, Kwon et al6 had incomplete data.9
Dairy—A large retrospective study including 47,355 nurses noted the frequency of milk intake was significantly associated with increased prevalence of acne in adolescence (prevalence ratio, 1.22; 95% CI, 1.03-1.44; P=.002).10 A 2019 meta-analysis further suggested a significant relationship between acne and milk in highest vs lowest intake groups (OR, 1.48; 95% CI, 1.31-1.66) with no significant heterogeneity between the studies (I2=23.6%, P=.24 for heterogeneity), as well as a positive relationship between the highest vs lowest intake of low-fat milk (OR, 1.25; 95% CI, 1.10-1.43) and skim milk (OR, 1.82; 95% CI, 1.34-2.47). In this meta-analysis, yogurt and cheese consumption were not significantly associated with acne (OR, 0.90; 95% CI, 0.73-1.11).11 One non–evidence-based explanation for this may be that fermented dairy products have different biological actions. Pasteurized milk allows microRNAs that directly activate mTORC1 to persist, whereas the bacteria present in the fermentation process may augment this.12 A separate meta-analysis from 2018 did find that yogurt consumption was positively associated with acne (OR, 1.36; 95% CI, 1.05-1.77; P=.022), highlighting the need for larger, more rigorous studies on this topic.13
Insulin and IGF-1—As reviewed above, acne has been considered a disease of Western society, with the Western diet at the center of this association.14 A typical Western diet consists of high glycemic index foods, carbohydrates, and dairy, all of which enhance the production of insulin and IGF-1. Insulin levels increase secondary to high blood glucose and to a lesser degree by protein intake.15 Insulinlike growth factor 1 production is most influenced by age and peaks during puberty; however, high protein diets also increase liver IGF-1 production and release.16 When present in excess, insulin can function as a growth factor. Insulin exerts its anabolic effects through the IGF-1 pathway; however, insulin and IGF-1 are produced in response to different signals.17 Endocrine production of IGF-1 represents 70% of blood levels, peaks at puberty, and rapidly declines in the third decade of life.18 Insulin is produced by the pancreas, and levels correspond to lifestyle and genetically induced insulin resistance.19
Adolescents have elevated levels of IGF-1 as a major driver of puberty-associated growth.20 Despite the natural decrease in IGF-1 following puberty, acne persists in many patients and can even develop for the first time in adulthood in a subset of patients. A study of 40 acne patients and 20 controls found that patients with acne who consumed a high glycemic–load diet was significantly higher than the number of controls consuming a similar diet (P=.008). Additionally, significantly higher levels of mean (SD) serum IGF-1 on quantitative sandwich enzyme-linked immunosorbent assay in acne patients vs controls (543.2 [174.7] ng/mL vs 316.9 [95.7] ng/mL; P<.001) was identified, and these levels correlated significantly with high glycemic–load diet consumption.21 In another study, Kartal et al22 found that basal and fasting insulin levels and homeostasis model assessment scores evaluating for insulin resistance were significantly higher in 36 women compared with 24 age/sex-matched controls (P<.05). This finding remained significant even after excluding women with hyperandrogenemia (P<.05).22
Highlighting the importance of IGF-1 in the pathogenesis of acne, patients with genetic disorders characterized by IGF-1 deficiency, such as Laron syndrome, do not develop acne despite having a functional androgen receptor. Treatment with IGF-1 in these patients induces acne, further supporting the role of IGF-1 in the pathogenesis of this condition.23
The mTORC1 Pathway
Comprised of mTOR in addition to other proteins, mTORC1 is a nutrient-sensitive regulator of cellular growth, proliferation, lipid synthesis, and protein translation.5 Increased activity of mTORC1 has been described in diabetes, neurodegenerative disease, and cancer,14,24 while decreased activity may promote longevity.25 Regulation of mTORC1 occurs through several mechanisms. Growth factors such as insulin and IGF-1 promote mTORC1 activation through the PI3K/Akt pathway. Several amino acids—specifically branched chain amino acids such as alanine, arginine, asparagine, glutamine, histidine, leucine, methionine, serine, threonine, and valine—also can activate mTORC1 independently.26 Excess glucose leads to decreased adenosine monophosphate–activated protein kinase and increased activity of mTORC1, which occurs separately from insulin or IGF-1.27 Starvation blocks mTORC1 via increased adenosine monophosphate–activated protein kinase and starvation-induced hypoxia.26,28 To activate mTORC1, both the IGF-1 or insulin signal and amino acid excess must be present.29 Although not studied in acne, altering the dietary protein content in obese mice has been shown to perturb the mTORC1 pathway, leading to pathologic changes in the mTORC1-autophagy signaling axis, increased amino acid release into the blood, and an acute elevation in mTORC1 signaling.30
Another major regulator of mTORC1 is Forkhead box protein O1 (FOXO1), which is a transcription factor that regulates mTORC1 through sestrin 3.31,32 Sestrin 3 is a stress-induced protein that helps regulate blood glucose and promote insulin sensitivity.33 When FOXO1 is translocated to the cell nucleus, it upregulates the expression of sestrin 3, resulting in mTORC1 inhibition.31,32 Insulin, IGF-1, and nutrient excess lead to FOXO1 translocation to the cell cytoplasm where it can no longer mitigate mTORC1 activity, while the fasted state leads to translocation to the nucleus.34 A single study evaluated the association between FOXO1, mTORC1, a high glycemic–load diet, and acne development. Immunohistochemical detection of mTORC1 assessed by digital image analysis revealed significantly greater expression in inflamed pilosebaceous units found in acne patients (P<.001). Immunohistochemical cytoplasmic expression of FOXO1 and mTOR (used as a proxy for mTORC1) was significantly higher in patients on a high glycemic–load diet (P=.021 and P=.009, respectively) as well as in patients with more severe forms of acne (P=.005 and P=.015, respectively) and elevated IGF-1 levels (P=.004 and P=.003, respectively).21
mTORC1 contributes to the proliferation of keratinocytes and excess sebum production, both independently and through androgen-mediated processes.35-40 Insulinlike growth factor 1 binding the IGF-1 receptor leads to proliferation of keratinocytes lining the sebaceous gland and hair follicle in vivo.35 In mice with epidermis-specific deletion of mTOR, keratinocyte proliferation was decreased and hair follicles were diminished both in number and development. Genetic loss of mTOR in the epidermis led to attenuated signaling pathways of mTORC1 and mTORC2.36
Androgen function is augmented by mTORC1, FOXO1, and IGF-1 through several mechanisms, which may partially explain the hormonal relationship to acne. Androgens increase IGF-1 within the hair follicle.37 In prostate cancer cells, IGF-1 then facilitates movement of FOXO1 to the cytoplasm, preventing it from blocking mTORC1. This effective inactivation of FOXO1 thus further augments the impact of androgens by both allowing unchecked mTORC1 pathway activity and increasing translocation of the androgen receptor (AR) to the nucleus where it exerts its effects.38 Interestingly, genetic polymorphisms of the AR have been shown to cause variable affinity of FOXO1 for the AR; specifically, shorter CAG (cytosine, adenine, guanine) repeat length may lead to decreased FOXO1 binding and is associated with an increased risk for acne.41-43 In addition to its effects on the hair follicle, IGF-1 stimulates production of testosterone and dehydroepiandrosterone as well as activates 5α-reductase, leading to higher dihydrotestosterone levels, which activate the AR with higher affinity than testosterone.44 In some tissues, androgens help regulate the mTORC1 pathway through positive feedback loops.45,46 At this time, we do not know if this occurs in the pathogenesis of acne.
Isotretinoin is the treatment of choice for refractory acne. It has been hypothesized that isotretinoin induces sebocyte apoptosis via the upregulation of FOXO transcription factors and p53.47 Elevated levels of nuclear FOXO1 have been found in the sebaceous glands of patients following initiation of treatment with isotretinoin and are hypothesized to play a major role in the drug’s effectiveness. Specifically, biopsies from 14 acne patients before and after 6 weeks of isotretinoin therapy were analyzed with immunohistochemical staining and found to have a significantly improved nuclear to cytoplasmic ratio of nonphosphorylated FOXO1 (P<.001).47
Practical Recommendations
Given the available evidence, it is important for dermatologists to address dietary recommendations in acne patients. Although large randomized controlled trials on diet and acne severity are challenging to conduct in this population, the existing literature suggests that patients should avoid high glycemic index simple sugars and processed grains, and patients should focus on eating more complex carbohydrates in the form of legumes, vegetables, fruits, and tubers.6-8 With regard to dairy, milk (especially skim) has been associated with increased risks for acne.11,13 Fermented dairy products may have less impact on acne severity and include cheese, yogurt (unsweetened to keep glycemic index low), and sour cream.12
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
- Zaenglein AL. Acne vulgaris. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. Elsevier; 2017:588-603.
- Ganceviciene R, Graziene V, Fimmel S, et al. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br J Dermatol. 2009;160:345-352.
- Kang S, Cho S, Chung JH, et al. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-kappaB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166:1691-1699.
- Cong TX, Hao D, Wen X, et al. From pathogenesis of acne vulgaris to anti-acne agents. Arch Dermatol Res. 2019;311:337-349.
- Pópulo H, Lopes JM, Soares P. The mTOR signalling pathway in human cancer. Int J Mol Sci. 2012;13:1886-1918.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Smith RN, Mann NJ, Braue A, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Penso L, Touvier M, Deschasaux M, et al. Association between adult acne and dietary behaviors: findings from the NutriNet-Santé prospective cohort study. JAMA Dermatol. 2020;156:854-862.
- Cao H, Yang G, Wang Y, et al. Complementary therapies for acne vulgaris. Cochrane Database Syst Rev. 2015;1:CD009436.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Melnik BC, Schmitz G. Pasteurized non-fermented cow’s milk but not fermented milk is a promoter of mTORC1-driven aging and increased mortality. Ageing Res Rev. 2021;67:101270.
- Juhl CR, Bergholdt HKM, Miller IM, et al. Dairy intake and acne vulgaris: a systematic review and meta-analysis of 78,529 children, adolescents, and young adults. Nutrients. 2018;10:1049. doi:10.3390/nu10081049
- Melnik BC. Linking diet to acne metabolomics, inflammation, and comedogenesis: an update. Clin Cosmet Investig Dermatol. 2015;8:371-388.
- Smart CEM, King BR, Lopez PE. Insulin dosing for fat and protein: is it time? Diabetes Care. 2020;43:13-15.
- Wan X, Wang S, Xu J, et al. Dietary protein-induced hepatic IGF-1 secretion mediated by PPARγ activation. PLoS One. 2017;12:E0173174.
- Bedinger DH, Adams SH. Metabolic, anabolic, and mitogenic insulin responses: a tissue-specific perspective for insulin receptor activators. Mol Cell Endocrinol. 2015;415:143-156.
- Gubbi S, Quipildor GF, Barzilai N, et al. 40 YEARS of IGF1: IGF1: the Jekyll and Hyde of the aging brain. J Mol Endocrinol. 2018;61:T171-T185.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. 2019;33:101265.
- Agamia NF, Abdallah DM, Sorour O, et al. Skin expression of mammalian target of rapamycin and forkhead box transcription factor O1, and serum insulin-like growth factor-1 in patients with acne vulgaris and their relationship with diet. Br J Dermatol. 2016;174:1299-1307.
- Kartal D, Yildiz H, Ertas R, et al. Association between isolated female acne and insulin resistance: a prospective study. G Ital Dermatol Venereol. 2016;151:353-357.
- Ben-Amitai D, Laron Z. Effect of insulin-like growth factor-1 deficiency or administration on the occurrence of acne. J Eur Acad Dermatol Venereol. 2011;25:950-954.
- Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36:2191-2201.
- Weichhart T. mTOR as regulator of lifespan, aging, and cellular senescence: a mini-review. Gerontology. 2018;64:127-134.
- Melick CH, Jewell JL. Regulation of mTORC1 by upstream stimuli. Genes. 2020;11:989. doi:10.3390/genes11090989
- Li M, Zhang CS, Feng JW, et al. Aldolase is a sensor for both low and high glucose, linking to AMPK and mTORC1. Cell Res. 2021;31:478-481.
- Yan T, Zhang J, Tang D, et al. Hypoxia regulates mTORC1-mediated keratinocyte motility and migration via the AMPK pathway. PLoS One. 2017;12:E0169155.
- Dennis MD, Baum JI, Kimball SR, et al. Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem. 2011;286:8287-8296.
- Choi BSY, Daniel N, Houde VP, et al. Feeding diversified protein sources exacerbates hepatic insulin resistance via increased gut microbial branched-chain fatty acids and mTORC1 signaling in obese mice. Nat Commun. 2021;12:3377.
- Chen CC, Jeon SM, Bhaskar PT, et al. FoxOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor. Dev Cell. 2010;18:592-604.
- Chen Y, Huang T, Yu Z, et al. The functions and roles of sestrins in regulating human diseases. Cell Mol Biol Lett. 2022;27:2.
- Tao R, Xiong X, Liangpunsakul S, et al. Sestrin 3 protein enhances hepatic insulin sensitivity by direct activation of the mTORC2-Akt signaling. Diabetes. 2015;64:1211-1223.
- Gross DN, Wan M, Birnbaum MJ. The role of FOXO in the regulation of metabolism. Curr Diab Rep. 2009;9:208-214.
- Gilhar A, Ish-Shalom S, Pillar T, et al. Effect of anti–insulin-like growth factor 1 on epidermal proliferation of human skin transplanted onto nude mice treated with growth hormone. Endocrinology. 1994;134:229-232.
- Ding X, Bloch W, Iden S, et al. mTORC1 and mTORC2 regulate skin morphogenesis and epidermal barrier formation. Nat Commun. 2016;7:13226.
- Inui S, Itami S. Androgen actions on the human hair follicle: perspectives. Exp Dermatol. 2013;22:168-171.
- Fan W, Yanase T, Morinaga H, et al. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem. 2007;282:7329-7338.
- Alestas T, Ganceviciene R, Fimmel S, et al. Enzymes involved in the biosynthesis of leukotriene B4 and prostaglandin E2 are active in sebaceous glands. J Mol Med. 2006;84:75-87.
- Smith TM, Gilliland K, Clawson GA, et al. IGF-1 induces SREBP-1 expression and lipogenesis in SEB-1 sebocytes via activation of the phosphoinositide 3-kinase/Akt pathway. J Invest Dermatol. 2008;128:1286-1293.
- Furtado GV, Yang J, Wu D, et al. FOXO1 controls protein synthesis and transcript abundance of mutant polyglutamine proteins, preventing protein aggregation. Hum Mol Genet. 2021;30:996-1005.
- Melnik BC. Isotretinoin and FoxO1: a scientific hypothesis. Dermatoendocrinol. 2011;3:141-165.
- Heng AHS, Say YH, Sio YY, et al. Gene variants associated with acne vulgaris presentation and severity: a systematic review and meta-analysis. BMC Med Genomics. 2021;14:103.
- Li J, Al-Azzawi F. Mechanism of androgen receptor action. Maturitas. 2009;63:142-148.
- Zhao Y, Tindall DJ, Huang H. Modulation of androgen receptor by FOXA1 and FOXO1 factors in prostate cancer. Int J Biol Sci. 2014;10:614-619.
- Hamdi MM, Mutungi G. Dihydrotestosterone stimulates amino acid uptake and the expression of LAT2 in mouse skeletal muscle fibres through an ERK1/2-dependent mechanism. J Physiol. 2011;589(pt 14):3623-3640.
- Agamia NF, Hussein OM, Abdelmaksoud RE, et al. Effect of oral isotretinoin on the nucleocytoplasmic distribution of FoxO1 and FoxO3 proteins in sebaceous glands of patients with acne vulgaris. Exp Dermatol. 2018;27:1344-1351.
- Kolovou GD, Watts GF, Mikhailidis DP, et al. Postprandial hypertriglyceridaemia revisited in the era of non-fasting lipid profile testing: a 2019 expert panel statement, main text. Curr Vasc Pharmacol. 2019;17:498-514.
- Svoboda SA, Shields BE. Cutaneous manifestations of nutritional excess: pathophysiologic effects of hyperglycemia and hyperinsulinemia on the skin. Cutis. 2021;107:74-78.
- González-González JG, Mancillas-Adame LG, Fernández-Reyes M, et al. Androgenetic alopecia and insulin resistance in young men. Clin Endocrinol . 2009;71:494-499.
- Livadas S, Anagnostis P, Bosdou JK, et al. Polycystic ovary syndrome and type 2 diabetes mellitus: a state-of-the-art review. World J Diabetes. 2022;13:5-26.
Practice Points
- Patients are frequently interested in the role that diet plays in acne, and dermatologists should be aware of the current evidence to answer these questions effectively.
- One of the primary pathways in acne pathogenesis, mTORC1 (mammalian target of rapamycin complex 1), is partially regulated by nutrient availability, insulin, and insulinlike growth factor 1.
- Dietary recommendations for acne based on available evidence may include a low glycemic index diet and avoidance of certain dairy products.
- Insulin resistance may underlie the pathogenesis of acne in a subset of patients, and assessing insulin resistance in acne patients should be considered.