User login
Appropriate antibiotic selection for 12 common infections in obstetric patients
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
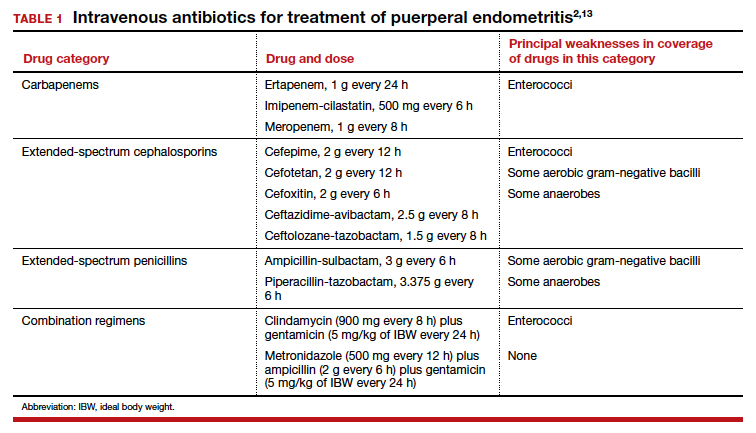
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13
7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17
Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17
11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13

7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17

Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17

11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
For the infections we most commonly encounter in obstetric practice, I review in this article the selection of specific antibiotics. I focus on the key pathogens that cause these infections, the most useful diagnostic tests, and the most cost-effective antibiotic therapy. Relative cost estimates (high vs low) for drugs are based on information published on the GoodRx website (https://www.goodrx.com/). Actual charges to patients, of course, may vary widely depending on contractual relationships between hospitals, insurance companies, and wholesale vendors. The infections are listed in alphabetical order, not in order of frequency or severity.
1. Bacterial vaginosis
Bacterial vaginosis (BV) is a polymicrobial infection that results from perturbation of the normal vaginal flora due to conditions such as pregnancy, hormonal therapy, and changes in the menstrual cycle. It is characterized by a decrease in the vaginal concentration of Lactobacillus crispatus, followed by an increase in Prevotella bivia, Gardnerella vaginalis, Mobiluncus species, Atopobium vaginae, and Megasphaera type 1.1,2
BV is characterized by a thin, white-gray malodorous (fishlike smell) discharge. The vaginal pH is >4.5. Clue cells are apparent on saline microscopy, and the whiff (amine) test is positive when potassium hydroxide is added to a drop of vaginal secretions. Diagnostic accuracy can be improved using one of the new vaginal panel assays such as BD MAX Vaginal Panel (Becton, Dickinson and Company).3
Antibiotic selection
Antibiotic treatment of BV is directed primarily at the anaerobic component of the infection. The preferred treatment is oral metronidazole 500 mg twice daily for 7 days. If the patient cannot tolerate metronidazole, oral clindamycin 300 mg twice daily for 7 days, can be used, although it is more expensive than metronidazole. Topical metronidazole vaginal gel (0.75%), 1 applicatorful daily for 5 days, is effective in treating the local vaginal infection, but it is not effective in preventing systemic complications such as preterm labor, chorioamnionitis, and puerperal endometritis.2 It also is significantly more expensive than the oral formulation of metronidazole. Topical clindamycin cream, 1 applicatorful daily for 5 days, is even more expensive.
Tinidazole 2 g orally daily for 2 days is an effective alternative to oral metronidazole. Single-dose therapy with oral secnidazole (2 g), a 5-nitroimidazole with a longer half-life than metronidazole, has been effective in small studies, but experience with this drug in the United States is limited. Secnidazole is also very expensive.4
2. Candidiasis
Vulvovaginal candidiasis usually is caused by Candida albicans. Other less common species include C tropicalis, C glabrata, C auris, C lusitaniae, and C krusei. The most common clinical findings are vulvovaginal pruritus in association with a curdlike white vaginal discharge. The diagnosis can be established by confirmation of a normal vaginal pH and identification of budding yeast and hyphae on a potassium hydroxide preparation. As noted above for BV, the vaginal panel assay improves the accuracy of clinical diagnosis.3 Culture usually is indicated only in patients with infections that are refractory to therapy.
Continue to: Antibiotic selection...
Antibiotic selection
In the first trimester of pregnancy, vulvovaginal candidiasis should be treated with a topical medication such as clotrimazole cream 1% (50 mg intravaginally daily for 7 days), miconazole cream 2% (100 mg intravaginally daily for 7 days), or terconazole cream 0.4% (50 g intravaginally daily for 7 days). Single-dose formulations or 3-day courses of treatment may not be quite as effective in pregnant patients, but they do offer a more convenient dosing schedule.2,5
Oral fluconazole should not be used in the first trimester of pregnancy because it has been associated with an increased risk for spontaneous abortion and with fetal cardiac septal defects. Beyond the first trimester, oral fluconazole offers an attractive option for treatment of vulvovaginal candidiasis. The appropriate dose is 150 mg initially, with a repeat dose in 3 days if symptoms persist.2,5
Ibrexafungerp (300 mg twice daily for 1 day) was recently approved by the US Food and Drug Administration (FDA) for oral treatment of vulvovaginal candidiasis. However, this drug is teratogenic and is contraindicated during pregnancy and lactation. It also is significantly more expensive than fluconazole.6
3. Cesarean delivery prophylaxis
All women having a cesarean delivery (CD) should receive antibiotic prophylaxis to reduce the risk of endometritis and wound infection.
Antibiotic selection
In my opinion, the preferred regimen is intravenous cefazolin 2 g plus azithromycin 500 mg administered preoperatively.7 Cefazolin can be administered in a rapid bolus; azithromycin should be administered over 1 hour.
In an exceptionally rigorous investigation called the C/SOAP trial (Cesarean Section Optimal Antibiotic Prophylaxis trial), Tita and colleagues showed that the combination of cefazolin plus azithromycin was superior to single-agent prophylaxis (usually with cefazolin) in preventing the composite of endometritis, wound infection, or other infection occurring within 6 weeks of surgery.8 The additive effect of azithromycin was particularly pronounced in patients having CD after labor and rupture of membranes. Harper and associates subsequently validated the cost-effectiveness of this combination regimen using a decision analytic model.9
If the patient has a serious allergy to β-lactam antibiotics, the best alternative regimen for prophylaxis is clindamycin plus gentamicin. The appropriate single intravenous dose of clindamycin is 900 mg; the single dose of gentamicin should be 5 mg/kg of ideal body weight (IBW).7
4. Chlamydia
Chlamydia trachomatis is an obligate intracellular bacterium. In pregnant women, it typically causes urethritis, endocervicitis, and inflammatory proctitis. Along with gonorrhea, it is the cause of an unusual infection/inflammation of the liver capsule, termed Fitz-Hugh-Curtis syndrome (perihepatitis). The diagnosis of chlamydia infection is best confirmed with a nucleic acid amplification test (NAAT). The NAAT simultaneously tests for chlamydia and gonorrhea in urine or in secretions obtained from the urethra, endocervix, and rectum.2
Antibiotic selection
The drug of choice for treating chlamydia in pregnancy is azithromycin 1,000 mg orally in a single dose. Erythromycin can be used as an alternative to azithromycin, but it usually is not well tolerated because of gastrointestinal adverse effects. In my practice, the preferred alternative for a patient who cannot tolerate azithromycin is amoxicillin 500 mg orally 3 times daily for 7 days.2,10
Continue to: 5. Chorioamnionitis...
5. Chorioamnionitis
Chorioamnionitis is a polymicrobial infection caused by anaerobes, aerobic gram-negative bacilli (predominantly Escherichia coli), and aerobic gram-positive cocci (primarily group B streptococci [GBS]). The diagnosis usually is made based on clinical examination: maternal fever, maternal and fetal tachycardia, and no other localizing sign of infection. The diagnosis can be confirmed by obtaining a sample of amniotic fluid via amniocentesis or via aspiration through the intrauterine pressure catheter and demonstrating a positive Gram stain, low glucose concentration (<20 mg/dL), positive nitrites, positive leukocyte esterase, and ultimately, a positive bacteriologic culture.2
Antibiotic selection
The initial treatment of chorioamnionitis specifically targets the 2 major organisms that cause neonatal pneumonia, meningitis, and sepsis: GBS and E coli. For many years, the drugs of choice have been intravenous ampicillin (2 g every 6 hours) plus intravenous gentamicin (5 mg/kg of IBW every 24 hours). Gentamicin also can be administered intravenously at a dose of 1.5 mg/kg every 8 hours. I prefer the once-daily dosing for 3 reasons:
- Gentamicin works by a concentration-dependent mechanism; the higher the initial serum concentration, the better the killing effect.
- Once-daily dosing preserves long periods with low trough levels, an effect that minimizes ototoxicity and nephrotoxicity.
- Once-daily dosing is more convenient.
In a patient who has a contraindication to use of an aminoglycoside, aztreonam (2 g intravenously every 8 hours) may be combined with ampicillin.2
If the patient delivers vaginally, 1 dose of each drug should be administered postpartum, and then the antibiotics should be discontinued. If the patient delivers by cesarean, a single dose of a medication with strong anaerobic coverage should be administered immediately after the infant’s umbilical cord is clamped. Options include clindamycin (900 mg intravenously) or metronidazole (500 mg intravenously).11
There are 2 key exceptions to the single postpartum dose rule, however. If the patient is obese (body mass index [BMI] >30 kg/m2) or if the membranes have been ruptured for more than 24 hours, antibiotics should be continued until she has been afebrile and asymptomatic for 24 hours.12
Two single agents are excellent alternatives to the combination ampicillin-gentamicin regimen. One is ampicillin-sulbactam, 3 g intravenously every 6 hours. The other is piperacillin-tazobactam, 3.375 g intravenously every 6 hours. These extended-spectrum penicillins provide exceptionally good coverage against the major pathogens that cause chorioamnionitis. Although more expensive than the combination regimen, they avoid the potential ototoxicity and nephrotoxicity associated with gentamicin.2
6. Endometritis
Puerperal endometritis is significantly more common after CD than after vaginal delivery. The infection is polymicrobial, and the principal pathogens are anaerobic gram-positive cocci, anaerobic gram-negative bacilli, aerobic gram-negative bacilli, and aerobic gram-positive cocci. The diagnosis usually is made almost exclusively based on clinical findings: fever within 24 to 36 hours of delivery, tachycardia, mild tachypnea, and lower abdominal/pelvic pain and tenderness in the absence of any other localizing sign of infection.13
Antibiotic selection
Effective treatment of endometritis requires administration of antibiotics that provide coverage against the broad range of pelvic pathogens. For many years, the gold standard of treatment has been the combination regimens of clindamycin plus gentamicin or metronidazole plus ampicillin plus gentamicin. These drugs are available in generic form and are relatively inexpensive. However, several broad-spectrum single agents are now available for treatment of endometritis. Although they are moderately more expensive than the generic combination regimens, they usually are very well tolerated, and they avoid the potential nephrotoxicity and ototoxicity associated with gentamicin. TABLE 1 summarizes the dosing regimens of these various agents and their potential weaknesses in coverage.2,13

7. Gonorrhea
Gonorrhea is caused by the gram-negative diplococcus, Neisseria gonorrhoeae. The organism has a propensity to infect columnar epithelium and uroepithelium, and, typically, it causes a localized infection of the urethra, endocervix, and rectum. The organism also can cause an oropharyngeal infection, a disseminated infection (most commonly manifested by dermatitis and arthritis), and perihepatitis.
The diagnosis is best confirmed by a NAAT that can simultaneously test for gonorrhea and chlamydia in urine or in secretions obtained from the urethra, endocervix, and rectum.2,10
Antibiotic selection
The drugs of choice for treating uncomplicated gonococcal infection in pregnancy are a single dose of ceftriaxone 500 mg intramuscularly, or cefixime 800 mg orally. If the patient is allergic to β-lactam antibiotics, the recommended treatment is gentamicin 240 mg intramuscularly in a single dose, combined with azithromycin 2,000 mg orally.14
8. Group B streptococci prophylaxis
The first-line agents for GBS prophylaxis are penicillin and ampicillin. Resistance of GBS to either of these antibiotics is extremely rare. The appropriate penicillin dose is 3 million U intravenously every 4 hours; the intravenous dose of ampicillin is 2 g initially, then 1 g every 4 hours. I prefer penicillin for prophylaxis because it has a narrower spectrum of activity and is less likely to cause antibiotic-associated diarrhea. The antibiotic should be continued until delivery of the neonate.2,15,16
If the patient has a mild allergy to penicillin, the drug of choice is cefazolin 2 g intravenously initially, then 1 g every 8 hours. If the patient’s allergy to β-lactam antibiotics is severe, the alternative agents are vancomycin (20 mg/kg intravenously every 8 hours infused over 1–2 hours; maximum single dose of 2 g) and clindamycin (900 mg intravenously every 8 hours). The latter drug should be used only if sensitivity testing has confirmed that the GBS strain is sensitive to clindamycin. Resistance to clindamycin usually ranges from 10% to 15%.2,15,16
9. Puerperal mastitis
The principal microorganisms that cause puerperal mastitis are the aerobic streptococci and staphylococci that form part of the normal skin flora. The diagnosis usually is made based on the characteristic clinical findings: erythema, tenderness, and warmth in an area of the breast accompanied by a purulent nipple discharge and fever and chills. The vast majority of cases can be treated with oral antibiotics on an outpatient basis. The key indications for hospitalization are severe illness, particularly in an immunocompromised patient, and suspicion of a breast abscess.2
Continue to: Antibiotic selection...
Antibiotic selection
The initial drug of choice for treatment of mastitis is dicloxacillin sodium 500 mg every 6 hours for 7 to 10 days. If the patient has a mild allergy to penicillin, the appropriate alternative is cephalexin 500 mg every 8 hours for 7 to 10 days. If the patient’s allergy to penicillin is severe, 2 alternatives are possible. One is clindamycin 300 mg twice daily for 7 to 10 days; the other is trimethoprim-sulfamethoxazole double strength (800 mg/160 mg), twice daily for 7 to 10 days. The latter 2 drugs are also of great value if the patient fails to respond to initial therapy and/or infection with methicillin-resistant Staphylococcus aureus (MRSA) is suspected.2 I prefer the latter agent because it is less expensive than clindamycin and is less likely to cause antibiotic-induced diarrhea.
If hospitalization is required, the drug of choice is intravenous vancomycin. The appropriate dosage is 20 mg/kg every 8 to 12 hours (maximum single dose of 2 g).2
10. Syphilis
Syphilis is caused by the spirochete bacterium, Treponema pallidum. The diagnosis can be made by clinical examination if the characteristic findings listed in TABLE 2 are present.2,17 However, most patients in our practice will have latent syphilis, and the diagnosis must be established based on serologic screening.17

Antibiotic selection
In pregnancy, the treatment of choice for syphilis is penicillin (TABLE 3).2,10,17 Only penicillin has been proven effective in treating both maternal and fetal infection. If the patient has a history of allergy to penicillin, she should undergo skin testing to determine if she is truly allergic. If hypersensitivity is confirmed, the patient should be desensitized and then treated with the appropriate regimen outlined in TABLE 3. Of interest, within a short period of time after treatment, the patient’s sensitivity to penicillin will be reestablished, and she should not be treated again with penicillin unless she undergoes another desensitization process.2,17

11. Trichomoniasis
Trichomoniasis is caused by the flagellated protozoan, Trichomonas vaginalis. The condition is characterized by a distinct yellowish-green vaginal discharge. The vaginal pH is >4.5, and motile flagellated organisms are easily visualized on saline microscopy. The vaginal panel assay also is a valuable diagnostic test.3
Antibiotic selection
The drug of choice for trichomoniasis is oral metronidazole 500 mg twice daily for 7 days. The patient’s sexual partner(s) should be treated concurrently to prevent reinfection. Most treatment failures are due to poor compliance with therapy on the part of either the patient or her partner(s); true drug resistance is uncommon. When antibiotic resistance is strongly suspected, the patient may be treated with a single 2-g oral dose of tinidazole.2
12. Urinary tract infections
Urethritis
Acute urethritis usually is caused by C trachomatis or N gonorrhoeae. The treatment of infections with these 2 organisms is discussed above.
Asymptomatic bacteriuria and acute cystitis
Bladder infections are caused primarily by E coli, Klebsiella pneumoniae, and Proteus species. Gram-positive cocci such as enterococci, Staphylococcus saprophyticus, and GBS are less common pathogens.18
The key diagnostic criterion for asymptomatic bacteriuria is a colony count greater than 100,000 organisms/mL of a single uropathogen on a clean-catch midstream urine specimen.18
The usual clinical manifestations of acute cystitis include frequency, urgency, hesitancy, suprapubic discomfort, and a low-grade fever. The diagnosis is most effectively confirmed by obtaining urine by catheterization and demonstrating a positive nitrite and positive leukocyte esterase reaction on dipstick examination. The finding of a urine pH of 8 or greater usually indicates an infection caused by Proteus species. When urine is obtained by catheterization, the criterion for defining a positive culture is greater than 100 colonies/mL.18
Antibiotic selection. In the first trimester, the preferred agents for treatment of a lower urinary tract infection are oral amoxicillin (875 mg twice daily) or cephalexin (500 mg every 8 hours). For an initial infection, a 3-day course of therapy usually is adequate. For a recurrent infection, a 7- to 10-day course is indicated.
Beyond the first trimester, nitrofurantoin monohydrate macrocrystals (100 mg orally twice daily) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily) are the preferred agents. Unless no other oral drug is likely to be effective, these 2 drugs should be avoided in the first trimester. The former has been associated with eye, heart, and cleft defects. The latter has been associated with neural tube defects, cardiac anomalies, choanal atresia, and diaphragmatic hernia.18
Acute pyelonephritis
Acute infections of the kidney usually are caused by the aerobic gram-negative bacilli: E coli, K pneumoniae, and Proteus species. Enterococci, S saprophyticus, and GBS are less likely to cause upper tract infection as opposed to bladder infection.
The typical clinical manifestations of acute pyelonephritis include high fever and chills in association with flank pain and tenderness. The diagnosis is best confirmed by obtaining urine by catheterization and documenting the presence of a positive nitrite and leukocyte esterase reaction. Again, an elevated urine pH is indicative of an infection secondary to Proteus species. The criterion for defining a positive culture from catheterized urine is greater than 100 colonies/mL.2,18
Antibiotic selection. Patients in the first half of pregnancy who are hemodynamically stable and who show no signs of preterm labor may be treated with oral antibiotics as outpatients. The 2 drugs of choice are amoxicillin-clavulanate (875 mg twice daily for 7 to 10 days) or trimethoprim-sulfamethoxazole double strength (800 mg/160 mg twice daily for 7 to 10 days).
For unstable patients in the first half of pregnancy and for essentially all patients in the second half of pregnancy, parenteral treatment should be administered on an inpatient basis. My preference for treatment is ceftriaxone, 2 g intravenously every 24 hours. The drug provides excellent coverage against almost all the uropathogens. It has a convenient dosing schedule, and it usually is very well tolerated. Parenteral therapy should be continued until the patient has been afebrile and asymptomatic for 24 to 48 hours. At this point, the patient can be transitioned to one of the oral regimens listed above and managed as an outpatient. If the patient is allergic to β-lactam antibiotics, an excellent alternative is aztreonam, 2 g intravenously every 8 hours.2,18 ●
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
- Reeder CF, Duff P. A case of BV during pregnancy: best management approach. OBG Manag. 2021;33(2):38-42.
- Duff P. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies, 8th ed. Elsevier; 2021:1124-1145.
- Broache M, Cammarata CL, Stonebraker E, et al. Performance of a vaginal panel assay compared with the clinical diagnosis of vaginitis. Obstet Gynecol. 2021;138:853-859.
- Hiller SL, Nyirjesy P, Waldbaum AS, et al. Secnidazole treatment of bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2017;130:379-386.
- Kirkpatrick K, Duff P. Candidiasis: the essentials of diagnosis and treatment. OBG Manag. 2020;32(8):27-29, 34.
- Ibrexafungerp (Brexafemme) for vulvovaginal candidiasis. Med Lett Drugs Ther. 2021;63:141-143.
- Duff P. Prevention of infection after cesarean delivery. Clin Obstet Gynecol. 2019;62:758-770.
- Tita AT, Szychowski JM, Boggess K, et al; for the C/SOAP Trial Consortium. Adjunctive azithromycin prophylaxis for cesarean delivery. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Morbid Mortal Wkly Rep. 2015;64(RR3):1-137.
- Edwards RK, Duff P. Single additional dose postpartum therapy for women with chorioamnionitis. Obstet Gynecol. 2003;102(5 pt 1):957-961.
- Black LP, Hinson L, Duff P. Limited course of antibiotic treatment for chorioamnionitis. Obstet Gynecol. 2012;119:1102-1105.
- Duff P. Fever following cesarean delivery: what are your steps for management? OBG Manag. 2021;33(12):26-30, 35.
- St Cyr S, Barbee L, Warkowski KA, et al. Update to CDC’s treatment guidelines for gonococcal infection, 2020. MMWR Morbid Mortal Wkly Rep. 2020;69:1911-1916.
- Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion summary, number 782. Obstet Gynecol. 2019;134:1.
- Duff P. Preventing early-onset group B streptococcal disease in newborns. OBG Manag. 2019;31(12):26, 28-31.
- Finley TA, Duff P. Syphilis: cutting risk through primary prevention and prenatal screening. OBG Manag. 2020;32(11):20, 22-27.
- Duff P. UTIs in pregnancy: managing urethritis, asymptomatic bacteriuria, cystitis, and pyelonephritis. OBG Manag. 2022;34(1):42-46.
Amniotic fluid embolism: Management using a checklist
CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●

CASE Part 1: CPR initiated during induction of labor
A 32-year-old gravida 4 para 3-0-0-3 is undergoing induction of labor with intravenous (IV) oxytocin at 39 weeks of gestation. She has no significant medical or obstetric history. Fifteen minutes after reaching complete cervical dilation, she says “I don’t feel right,” then suddenly loses consciousness. The nurse finds no detectable pulse, calls a “code blue,” and initiates cardiopulmonary resuscitation (CPR). The obstetrician is notified, appears promptly, assesses the situation, and delivers a 3.6-kg baby via vacuum extraction. Apgar score is 2/10 at 1 minute and 6/10 at 5 minutes. After delivery of the placenta, there is uterine atony and brisk hemorrhage with 2 L of blood loss.
Management of AFE: A rare complication
This case demonstrates a classic presentation of amniotic fluid embolism (AFE) syndrome—a patient in labor or within 30 minutes after delivery has sudden onset of cardiorespiratory collapse followed by disseminated intravascular coagulation (DIC). AFE is rare, affecting only about 2 to 6 per 100,000 births, but classic cases have a reported maternal mortality rate that exceeds 50%.1 It is thought to reflect a complex, systemic proinflammatory response to maternal intravasation of pregnancy material, such as trophoblast, thromboplastins, fetal cells, or amniotic fluid. Because the syndrome is not necessarily directly caused by emboli or by amniotic fluid per se,2 it has been proposed that AFE be called “anaphylactoid syndrome of pregnancy,” but this terminology has not yet been widely adopted.3
Guidelines from the Society for Maternal-Fetal Medicine (SMFM) recommend several time-critical steps for the initial stabilization and management of patients with AFE.4 However, because AFE is rare, most obstetric providers may not encounter a case for many years or even decades after they have received training, so it is unrealistic to expect that they will remember these guidelines when they are needed. For this reason, when AFE occurs, it is important to have a readily accessible cognitive aid, such as a checklist that summarizes the key management steps. The SMFM provides a checklist for initial management of AFE that can be used at your institution; it is presented in the FIGURE and provides the outline for this discussion.5

Provide CPR immediately
Most AFE cases are accompanied by cardiorespiratory arrest. If the patient has no pulse, call a “code” to mobilize additional help and immediately start CPR. Use a backboard to make cardiac compressions most effective and manually displace the uterus or tilt the patient to avoid supine hypotension. Designate a timekeeper to call out 1-minute intervals and record critical data, such as medication administration and laboratory orders/results.
Expedite delivery
Immediate delivery is needed if maternal cardiac activity is not restored within 4 minutes of starting CPR, with a target to have delivery completed within 5 minutes. Operative vaginal delivery may be an option if delivery is imminent, as in the case presented, but cesarean delivery (CD) will be needed in most cases. This was previously called “perimortem cesarean” delivery, but the term “resuscitative hysterotomy” has been proposed because the primary goal is to improve the effectiveness of CPR6 and prevent both maternal and perinatal death. CPR is less effective in pregnant women because the pregnant uterus takes a substantial fraction of the maternal cardiac output, as well as compresses the vena cava. Some experts suggest that, rather than waiting 4 minutes, CD should be started as soon as an obstetrician or other surgeon is present, unless there is an immediate response to electrical cardioversion.6,7
In most cases, immediate CD should be performed wherever the patient is located rather than using precious minutes to move the patient to an operating room. Antiseptic preparation is expedited by simply pouring povidone-iodine or chlorhexidine over the lower abdomen if readily available; if not available, skip this step. Enter the abdomen and uterus as rapidly as possible using only a scalpel to make generous midline incisions.
If CPR is not required, proceed with cesarean or operative vaginal delivery as soon as the mother has been stabilized. These procedures should be performed using standard safety precautions outlined in the SMFM patient safety checklists for cesarean or operative vaginal delivery.8,9
Continue to: Anticipate hemorrhage...
Anticipate hemorrhage
Be prepared for uterine atony, coagulopathy, and catastrophic hemorrhage. Initiate IV oxytocin prophylaxis as soon as the infant is delivered. Have a low threshold for giving other uterotonic agents such as methylergonovine, carboprost, or misoprostol. If hemorrhage or DIC occurs, give tranexamic acid. Have the anesthesiologist or trauma team (if available) insert an intraosseous line for fluid resuscitation if peripheral IV access is inadequate.
Massive transfusion is often needed to treat DIC, which occurs in most AFE cases. Anticipate—do not wait—for DIC to occur. We propose activating your hospital’s massive transfusion protocol (MTP) as soon as you diagnose AFE so that blood products will be available as soon as possible. A typical MTP provides several units of red blood cells, a pheresis pack of platelets, and fresh/frozen plasma (FFP). If clinically indicated, administer cryoprecipitate instead of FFP to minimize volume overload, which may occur with FFP.
CASE Part 2: MTP initiated to treat DIC
The MTP is initiated. Laboratory results immediately pre-transfusion include hemoglobin 11.3 g/dL, platelet count 46,000 per mm3, fibrinogen 87 mg/dL, and an elevated prothrombin time international normalized ratio.
Expect heart failure
The initial hemodynamic picture in AFE is right heart failure, which should optimally be managed by a specialist from anesthesiology, cardiology, or critical care as soon as they are available. An emergency department physician may manage the hemodynamics until a specialist arrives. Avoidance of fluid overload is one important principle. If fluid challenges are needed for hypovolemic shock, boluses should be restricted to 500 mL rather than the traditional 1000 mL.
Pharmacologic treatment may include vasopressors, inotropic agents, and pulmonary vasodilators. Example medications and dosages recommended by SMFM are summarized in the checklist (FIGURE).5
After the initial phase of recovery, the hemodynamic picture often changes from right heart failure to left heart failure. Management of left heart failure is not covered in the SMFM checklist because, by the time it appears, the patient will usually be in the intensive care unit, managed by the critical care team. Management of left heart failure generally includes diuresis as needed for cardiogenic pulmonary edema, optimization of cardiac preload, and inotropic agents or vasopressors if needed to maintain cardiac output or perfusion pressure.4
Debrief, learning opportunities
Complex emergencies such as AFE are rarely handled 100% perfectly, even those with a good outcome, so they present opportunities for team learning and improvement. The team should conduct a 10- to 15-minute debrief soon after the patient is stabilized. Make an explicit statement that the main goal of the debrief is to gather suggestions as to how systems and processes could be improved for next time, not to find fault or lay blame on individuals. Encourage all personnel involved in the initial management to attend and discuss what went well and what did not. Another goal is to provide support for individuals who may feel traumatized by the dramatic, frightening events surrounding an AFE and by the poor patient outcome or guarded prognosis that frequently follows. Another goal is to discuss the plan for providing support and disclosure to the patient and family.
The vast majority of AFE cases meet criteria to be designated as “sentinel events,” because of patient transfer to the intensive care unit, multi-unit blood transfusion, other severe maternal morbidities, or maternal death. Therefore, most AFE cases will trigger a root cause analysis (RCA) or other formal sentinel event analysis conducted by the hospital’s Safety or Quality Department. As with the immediate post-event debrief, the first goal of the RCA is to identify systems issues that may have resulted in suboptimal care and that can be modified to improve future care. Specific issues regarding the checklist should also be addressed:
- Was the checklist used?
- Was the checklist available?
- Are there items on the checklist that need to be modified, added, or deleted?
The RCA concludes with the development of a performance improvement plan.
Ultimately, we encourage all AFE cases be reported to the registry maintained by the Amniotic Fluid Embolism Foundation at https://www.afesupport.org/, regardless of whether the outcome was favorable for the mother and newborn. The registry includes over 130 AFE cases since 2013 from around the world. Researchers periodically report on the registry findings.10 If providers report cases with both good and bad outcomes, the registry may provide future insights regarding which adjunctive or empiric treatments may or may not be promising.
Continue to: Empiric treatments...
Empiric treatments
From time-to-time, new regimens for empiric treatment of AFE are reported. It is important to recognize that these reports are generally uncontrolled case reports of favorable outcomes and that, without a control group, it is impossible to determine to what extent the treatment contributed to the outcome or was merely incidental. Given the rarity of AFE, it seems unlikely that there will ever be a randomized clinical trial or even a controlled prospective study comparing treatment regimens.
The “A-OK” regimen is an empiric treatment that has garnered some interest after an initial case report.11 It consists of an anticholinergic agent (atropine 0.2 mg IV), a selective 5-HT3 receptor antagonist (ondansetron 8 mg IV), and a nonsteroidal anti-inflammatory drug (ketorolac 15 mg IV). We have some reservations about this regimen, however, because atropine is relatively contraindicated if the patient has tachycardia (which is common in patients with hemorrhage) and ketorolac may suppress platelet function, which might be harmful for patients with DIC or thrombocytopenia.
Another empiric treatment is the “50-50-500” regimen, which includes an H1 antihistamine (diphenhydramine 50 mg IV), an H2 antihistamine (famotidine 50 mg IV), and a corticosteroid (hydrocortisone 500 mg IV). This regimen aims to suppress histamine-mediated and cell-mediated inflammatory responses, based on the notion that proinflammatory responses likely mediate much of the underlying pathophysiology of the AFE syndrome.
We would emphasize that these empiric regimens are not clinically validated, US Food and Drug Administration approved for treatment of AFE, or considered standard of care. Future reports of these and other regimens will be needed to evaluate their efficacy, limitations, and risks. Again, we encourage providers to report all AFE cases to the AFE Foundation registry, regardless of whether the treatments are successful.
CASE Conclusion
The hemorrhage stops after administration of oxytocin, carboprost, 6 units of cryoprecipitate, and a 6-unit platelet pheresis pack. The patient is transferred to the intensive care unit where she eventually requires a total of 10 units of red cells, 8 more units of cryoprecipitate, and another platelet pheresis pack. She is discharged to home in stable condition on postpartum day 4.
Be prepared, have the checklist ready
Because AFE is rare, most members of the health care team will have no prior experience managing a real case. It may have been years or decades since they had any education on AFE or they last read a review article such as this one. It is even possible the anesthesiologist, cardiologist, or critical care specialist has never heard of AFE. Thus if they rely on memory alone, there is substantial risk of forgetting items, getting dosages wrong, or other errors. With this in mind, what is the best way to prepare the team to expeditiously employ the management steps outlined here?
Use of a checklist that summarizes these key steps for early management, such as the SMFM checklist in the FIGURE, will help ensure that all relevant steps are performed in every AFE case. It is designed to be printed on a single sheet of letter-sized paper, and we propose that every labor and delivery (L&D) unit keep laminated copies of this checklist in several places where they will be immediately available should an AFE occur. Copies can be kept on the anesthesia carts in the L&D operating rooms, in an emergency procedures binder on the unit, and on the “crash carts” and hemorrhage supply carts in the L&D unit. Effective implementation of an AFE checklist requires all personnel know where to readily find it and have some familiarity with its contents.
An interdisciplinary team comprising representatives from nursing, obstetrics, and anesthesia should meet to discuss whether the checklist needs to be modified to fit the local hospital formulary or other unique local circumstances. The team should develop an implementation plan that includes where to keep checklist copies, a process to periodically ensure that the copies are still present and readable, a roll-out plan to inform all personnel about the checklist process, and most importantly a training plan that includes incorporating AFE cases into the schedule of multidisciplinary simulations and drills for obstetric emergencies. Other implementation strategies are outlined in the SMFM document.5
Ultimately an organized, systematic approach is recommended for management of AFE. There is no single best treatment of AFE; it is supportive and directed toward the underlying pathophysiology, which may vary from patient to patient. Therefore, although a checklist, in conjunction with regular education and simulation activities, may help optimize care and improve outcomes, there is still a high risk of maternal morbidity and mortality from AFE. ●
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
- Clark SL. Amniotic fluid embolism. Obstet Gynecol. 2014;123(2 Pt 1):337-348. doi:10.1097/AOG.0000000000000107.
- Funk M, Damron A, Bandi V, et al. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism. Am J Obstet Gynecol. 2018;218:460-461. doi:10.1016/j.ajog.2017.12.225.
- Gilmore DA, Wakim J, Secrest J, et al. Anaphylactoid syndrome of pregnancy: a review of the literature with latest management and outcome data. AANA J. 2003;71:120-126.
- Society for Maternal-Fetal Medicine, Pacheco LD, Saade G, et al. Amniotic fluid embolism: diagnosis and management. Am J Obstet Gynecol. 2016;215:B16-24. doi:10.1016/j.ajog.2016.03.012.
- Patient Safety and Quality Committee, Society for Maternal-Fetal Medicine; Combs CA, Montgomery DM, et al. Society for Maternal-Fetal Medicine Special Statement: checklist for initial management of amniotic fluid embolism. Am J Obstet Gynecol. 2021;224:B29-B32. doi:10.1016/j.ajog.2021.01.001.
- Rose CH, Faksh A, Traynor KD, et al. Challenging the 4- to 5-minute rule: from perimortem cesarean to resuscitative hysterotomy. Am J Obstet Gynecol. 2015;213:653-6, 653.e1. doi:10.1016/j.ajog.2015.07.019.
- Pacheco LD, Clark SL, Klassen M, et al. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol. 2020;222:48-52. doi:10.1016/j.ajog.2019.07.036.
- Combs CA, Einerson BD, Toner LE, SMFM Patient Safety and Quality Committee. SMFM Special Statement: surgical safety checklists for cesarean delivery. Am J Obstet Gynecol. 2021;225:B43-B49. doi:10.1016/j.ajog.2021.07.011.
- SMFM Patient Safety and Quality Committee, Staat B, Combs CA. SMFM Special Statement: operative vaginal delivery: checklists for performance and documentation. Am J Obstet Gynecol. 2020;222:B15-B21. doi:10.1016/j.ajog.2020.02.011.
- Stafford IA, Moaddab A, Dildy GA, et al. Amniotic fluid embolism syndrome: analysis of the United States international registry. Am J Obstet Gynecol MFM. 2020;2:100083. doi:10.1016/j.ajogmf.2019.100083.
- Rezai S, Hughes AZC, Larsen TB, et al. Atypical amniotic f luid embolism managed with a novel therapeutic regimen. Case Rep Obstet Gynecol. 2017; 2017:8458375. doi:10.1155/2017/8458375.
Pain and photophobia
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
On the basis of the patient's medical history and presentation, this is probably a case of uveitis, a common extra-articular manifestation of psoriatic disease. In fact, the presence of uveitis can help distinguish PsA from osteoarthritis. Uveitis is characterized by inflammation of the uvea tract, with the retina, optic nerve, vitreous body, and sclera potentially becoming inflamed as well. Among patients with PsA, the prevalence of uveitis rises with ongoing disease duration, though the condition may also precede the development of PsA in patients with psoriasis, and is common among patients with severe psoriatic disease in Western and Asian populations. Overall, the prevalence of uveitis has been estimated to be 6%-9%. HLA-B27 genotype is strongly associated with uveitis in patients with concomitant PsA.
Symptoms of uveitis, as seen in the present case, include blurred vision, photophobia, pain, and ciliary flush. The condition is classified as anterior, intermediate, posterior, or panuveitis, with the majority of cases diagnosed as anterior. In anterior uveitis, the inflamed pupil may become constricted or take on an irregular shape caused by iris adhesions to the anterior lens capsule. Uveitis in PsA is bilateral and has a chronic relapsing course. Onset is typically insidious.
Workup for uveitis should comprise visual acuity testing, slit lamp biomicroscopy, measurement of intraocular pressures, and a dilated eye exam. Conditions in the differential which threaten a patient's sight include retinal vasculitis, vitritis, cystoid macular edema, Behçet disease, and tubulo-interstitial nephritis. Other autoimmune diseases which can cause uveitis with systemic manifestations (multiple sclerosis, sarcoidosis, lupus) should be investigated. Infectious causes must also be eliminated. However, considering this patient's history of psoriatic disease, uveitis should be highly suspected.
Uveitis demands urgent treatment to control ocular inflammation. Tumor necrosis factor (TNF) inhibitors are the recommended first-line and second-line treatment for PsA, including in patients with complications such as uveitis. However, etanercept should not be used as it is less effective than adalimumab or other TNF inhibitors for uveitis. Because uveitis may sometimes respond to MTX therapy, patients with severe PsA may use a biologic agent in combination with MTX if they have had a partial response to current MTX therapy, as recommended by the American College of Rheumatology.
Herbert S. Diamond, MD, Professor of Medicine (retired), Temple University School of Medicine, University of Pittsburgh; Chairman, Department of Medicine Emeritus, Western Pennsylvania Hospital, Pittsburgh, PA.
Herbert S. Diamond, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 48-year-old male patient presents with blurred vision, pain, and photophobia. He recently had a routine visit with an ophthalmologist, which was normal. The affected pupil appears irregular in shape. The anterior chamber appears foggy. Local ciliary flush is observed on slit lamp exam. The physical examination is also notable for axial arthropathy. The patient has an 11-year history of moderate to severe psoriatic arthritis (PsA) which he typically manages with methotrexate (MTX) therapy, to which he has had a partial response. He was initially diagnosed when he presented with worsening psoriasis and enthesitis on the insertion sites of the plantar fascia, as well as dactylitis.
Dry cough and dyspnea
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
Based on the patient's presentation and workup, the likely diagnosis is adenosquamous carcinoma of the lung, a relatively rare subtype of non–small cell lung cancer (NSCLC). Adenosquamous carcinoma displays qualities of both squamous cell carcinoma and adenocarcinoma; for definitive diagnosis, the cancer must contain 10% of each of these major NSCLC subtypes. Maeda and colleagues concluded that adenosquamous carcinoma occurs more frequently among men and that the age at the time of diagnosis is higher among such cancers compared with adenocarcinoma. Several studies have confirmed that adenosquamous carcinoma of the lung is also more prevalent among smokers.
Though a diagnosis of adenosquamous carcinoma may be suspected after small biopsies, cytology, or excisional biopsies, definitive diagnosis necessitates a resection specimen. If any adenocarcinoma component is observed in a biopsy specimen that is otherwise squamous, as in the present case, this finding is an indication for molecular testing. Epidermal growth factor receptor (EGFR) mutations may be present in adenosquamous carcinoma cancers, despite a majority of cancers with EGFR mutations being among nonsmokers or former light smokers with adenocarcinoma histology. In addition, even for patients diagnosed with squamous cell carcinoma, adenosquamous carcinoma should be considered if genetic testing suggests EGFR mutations.
Relative to adenocarcinoma and squamous cell carcinoma, adenosquamous carcinoma has higher grade malignancy, more advanced postoperative stage, and stronger lymph nodal invasiveness. In terms of treatment, surgical resection is the curative option for adenosquamous carcinoma of the lung, with lobectomy with lymphadenectomy considered for first-line treatment. Though the most beneficial chemotherapy regimen for patients with adenosquamous carcinoma of the lung remains the subject of investigation, platinum-based doublet chemotherapy is the current standard treatment option. EGFR tyrosine kinase inhibitors may be an effective option for EGFR-positive patients.
Karl J. D'Silva, MD, Clinical Assistant Professor, Department of Medicine, Tufts University School of Medicine, Boston; Medical Director, Department of Oncology and Hematology, Lahey Hospital and Medical Center, Peabody, Massachusetts.
Karl J. D'Silva, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 58-year-old man with a 20-year–pack history of smoking initially presented with a persistent dry cough and dyspnea. Clubbing was noted on physical examination and breath sounds in the right upper lung were weak. Other than hypertension, which the patient manages with angiotensin-converting enzyme (ACE) inhibitors, medical history is unremarkable. The patient notes that this medication has always made him cough, but dyspnea has only developed over the past 6 weeks. Respiratory symptoms prompted a chest radiograph which revealed a mass in the upper lobe of the right lung. Transbronchial lung biopsy of the right lung reveals components of adenocarcinoma; the specimen is otherwise squamous.
Do behavioral interventions improve nighttime sleep in children < 1 year old?
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
Most interventions resulted in at least modest improvements in sleep
A randomized controlled trial (RCT) of 279 newborn infants and their mothers evaluated developmentally appropriate sleep interventions.1 Mothers were given guidance on bedtime sleep routines, including starting the routine 30 to 45 minutes before bedtime, choosing age-appropriate calming bedtime activities, not using feeding as the last step before bedtime, and offering the child choices with their routine. Mothers were also given guidance on sleep location and behaviors, including recommendations on the best bedtime (between 7 and 8
These interventions were compared to a control group that received instructions on crib safety, sudden infant death syndrome prevention, and other sleep safety recommendations. Infant nocturnal sleep duration was determined by maternal report using the Brief Infant Sleep Questionnaire (BISQ). After 40 weeks, infants in the intervention group demonstrated longer sleep duration than did those in the control group (624.6 ± 67.6 minutes vs 602.9 ± 76.1 minutes; P = .01).1
An RCT of 82 infants (ages 2-4 months) and their mothers evaluated the effect of behavioral sleep interventions on maternal and infant sleep.2 Parents were offered either a 90-minute class and take-home booklet about behavioral sleep interventions or a 30-minute training on general infant safety with an accompanying pamphlet.
The behavioral interventions booklet included instructions on differentiating day and night routines for baby, avoiding digital devices and television in the evenings, playing more active games in the morning, dimming lights and reducing house noises in the afternoon, and having a consistent nighttime routine with consistent bedtime and sleep space. Participants completed an infant sleep diary prior to the intervention and repeated the sleep diary 8 weeks after the intervention. The infants whose mothers received the education on behavioral sleep interventions demonstrated an increase in nighttime sleep duration when compared to the control group (7.4 to 8.8 hours vs 7.3 to 7.5 hours; ANCOVA P < .001).
An RCT of 235 families with infants ages 6 to 8 months evaluated the effect of 45 minutes of nurse-provided education regarding normal infant sleep, effects of inadequate sleep, setting limits around infant sleep, importance of daytime routines, and negative sleep associations combined with a booklet and weekly phone follow-ups.3 This intervention was compared to routine infant education. At age 6 weeks, infants were monitored for 48 hours with actigraphy and the mothers completed a sleep diary to correlate activities. There was no difference in average nightly waking (2 nightly wakes; risk difference = –0.2%; 95% CI, –1.32 to 0.91).
An RCT of 268 families with infants (ages 2-3 weeks) evaluated the effect of 45 minutes of nurse-provided education on behavioral sleep interventions including the cyclical nature of infant sleep, environmental factors that influence sleep, and parent-independent sleep cues (eg, leaving a settling infant alone for 5 minutes before responding) combined with written information.4 This was compared to infants receiving standard care without parental sleep intervention education. Participants recorded sleep diaries for 7 days when their infant reached age 6 weeks and again at age 12 weeks. At both 6 weeks and 12 weeks, there was a significant increase in infant nocturnal sleep time in the intervention group vs the control group (mean difference [MD] at 6 weeks = 0.5 hours; 95% CI, 0.32 to 0.69 vs MD at 12 weeks = 0.64 hours; 95% CI, 0.19 to 0.89).
A nonrandomized controlled trial with 84 mothers and infants (ages 0-6 months) evaluated the effectiveness of a multifaceted intervention involving brief focused negotiation by pediatricians, motivational counseling by a health educator, and group parenting workshops, compared to mother–infant pairs receiving standard care.5 Parents completed the BISQ at 0 and 6 months to assess nocturnal sleep duration. At 6 months, the intervention group had a significantly higher increase in infant nocturnal sleep duration compared to the control group (mean increase = 1.9 vs 1.3 hours; P = .05).
In a prospective cohort study involving 79 infants (ages 3-24 months) with parent- or pediatrician-reported day and night sleep problems, parents were given education on the promotion of nighttime sleep by gradually reducing contact with the infant over several nights and only leaving the room after the infant fell asleep or allowing the child to self-soothe for 1-3 minutes.6 The intervention was performed over 3 weeks, with in-person follow-up performed on Day 15 and phone follow-up on Days 8 and 21. Infants in this study demonstrated an increase in the average hours of total night sleep from 10.2 to 10.5 hours (P < .001).
Editor’s takeaway
Providing behavioral recommendations to parents about infant sleep routines improves sleep duration. This increased sleep duration, and the supporting evidence, is modest, but the low cost and risk of these interventions make them worthwhile.
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
1. Paul IM, Savage JS, Anzman-Frasca S, et al. INSIGHT responsive parenting intervention and infant sleep. Pediatrics. 2016;138:e20160762. doi:10.1542/peds.2016-0762
2. Rouzafzoon M, Farnam F, Khakbazan Z. The effects of infant behavioural sleep interventions on maternal sleep and mood, and infant sleep: a randomised controlled trial. J Sleep Res. 2021;30:e13344. doi: 10.1111/jsr.13344
3. Hall WA, Hutton E, Brant RF, et al. A randomized controlled trial of an intervention for infants’ behavioral sleep problems. BMC Pediatr. 2015;15:181. doi:10.1186/s12887-015-0492-7
4. Symon BG, Marley JE, Martin AJ, et al. Effect of a consultation teaching behaviour modification on sleep performance in infants: a randomised controlled trial. Med J Aust. 2005;182:215-218. doi: 10.5694/j.1326-5377.2005.tb06669.x
5. Taveras EM, Blackburn K, Gillman MW, et al. First steps for mommy and me: a pilot intervention to improve nutrition and physical activity behaviors of postpartum mothers and their infants. Matern Child Health J. 2011;15:1217-1227. doi: 10.1007/s10995-010-0696-2
6. Skuladottir A, Thome M, Ramel A. Improving day and night sleep problems in infants by changing day time sleep rhythm: a single group before and after study. Int J Nurs Stud. 2005;42:843-850. doi: 10.1016/j.ijnurstu.2004.12.004
EVIDENCE-BASED ANSWER:
YES. Infants respond to behavioral interventions, although objective data are limited. Behavioral interventions include establishing regular daytime and sleep routines for the infant, reducing environmental noises or distractions, and allowing for self-soothing at bedtime (strength of recommendation: B, based on multiple randomized and nonrandomized studies).
Are antipsychotics effective adjunctive Tx for patients with moderate-to-severe depression?
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
Evidence summary
Depression symptoms improved with any of 4 antipsychotics
A 2021 systematic review of 16 RCTs (N = 3649) assessed data from trials that used an atypical antipsychotic—either aripiprazole, quetiapine, olanzapine, or risperidone—as augmentation therapy to an antidepressant vs placebo.1 Study participants included adults ages 18 to 65 who experienced an episode of depression and did not respond adequately to at least 1 optimally dosed antidepressant. In most studies, treatment-resistant depression (TRD) was defined as the failure of at least 1 major class of antidepressants. Trial lengths ranged from 4 to 12 weeks.
Six RCTs evaluated the effectiveness of augmentation with aripiprazole (2-20 mg/d) in patients with unipolar depression, with 5 trials demonstrating greater improvement in clinical symptoms with aripiprazole compared to placebo. Augmentation with quetiapine (150-300 mg/d) was evaluated in 5 trials, with all trials showing improvement in depression symptoms; however, in 1 trial the difference in remission rates was not significant, and in another trial significant improvement was seen only at a quetia-pine dose of 300 mg/d. Two trials examining olanzapine found that patients receiving fluoxetine plus olanzapine augmentation demonstrated greater improvement in depression symptoms than did those receiving either agent alone. Three trials examined augmentation with risperidone (0.5-3 mg/d); in all 3, risperidone demonstrated significant improvement in depression symptoms and remission rates compared to placebo.1
This systematic review was limited by small sample size and heterogeneity of antipsychotic dosages in the RCTs included, as well as the lack of a standardized and globally accepted definition of TRD.
Augmentation reduced symptom severity, but dropout rates were high
A 2019 Cochrane review of 10 RCTs (N = 2731) compared 5 strategies, including augmenting treatment with an antipsychotic vs continuing antidepressant monotherapy.2 Participants were adults ages 18 to 74 with unipolar depression who had not responded to a minimum of 4 weeks of antidepressant treatment at a recommended dose. The primary outcome was depressive symptom severity, as measured by the Montgomery-Asberg Depression Rating Scale (MADRS; range of 0-60) or the Hamilton Depression Rating Scale (HAM-D; range, 0-52).
Compared with continued antidepressant monotherapy, symptom severity was reduced when current treatment was augmented with cariprazine 1-4.5 mg/d (1 trial; N = 808; mean difference [MD] on MADRS = –1.5; 95% CI, –2.7 to –0.25; high-quality evidence); quetiapine 150-300 mg/d (3 trials; N = 977; standardized MD = –0.32; 95% CI, –0.46 to –0.18; high-quality evidence); ziprasidone 40-160 mg/d (2 trials; N = 199; MD on HAM-D = –2.7; 95% CI, –4.5 to –0.93; moderate-quality evidence); or olanzapine 5-20 mg/d (1 trial; N = 20; MD on MADRS = –12; 95% CI, –22 to –2.4; low-quality evidence). One trial did not show a significant difference on the HAM-D for olanzapine (1 trial; N = 20; MD = –7.9; 95% CI, –17 to 0.96; low-quality evidence).2
Dropout rates, which were most commonly secondary to adverse effects, ranged from 10% to 39% in the groups augmented with an antipsychotic and from 12% to 23% in the comparison groups.2 This systematic review was limited by the small number of studies included in the various comparisons.
Antipsychotic augmentation was effective but came with adverse effects
A 2017 RCT (N = 1522) examined the effectiveness of augmenting an antidepressant with aripiprazole in patients with TRD.3 Participants were adults (mean age, 54.4 years; 85% men) at 35 US Veterans Health Administration (VA) medical centers who had a diagnosis of nonpsychotic major depressive disorder that was unresponsive to at least 1 antidepressant course meeting minimal standards for treatment dose and duration.
Continue to: Patients were randomly...
Patients were randomly assigned to 1 of 3 different treatment groups, which included switching to a different antidepressant (bupropion sustained release 150-500 mg/d); augmenting current treatment with bupropion; or augmenting with an atypical antipsychotic (aripiprazole 2-15 mg/d) for 12 to 36 weeks. The primary outcome was remission rate at 12 weeks, which was defined as a score ≤ 5 on the Quick Inventory of Depressive Symptomatology–Clinician Rated (QIDS-C; range, 0-27) at 2 consecutive visits. The secondary outcome, symptom response to treatment, was defined as ≥ 50% reduction on QIDS-C score.
The augment-aripiprazole group (N = 146) exceeded the switch group (N = 114) in remission rate (absolute remission rates = 28.9% vs 22.3%; relative risk [RR] = 1.3; 95% CI, 1.1-1.6; number needed to treat [NNT] = 15), but had similar remission rates to the augment-bupropion group (N = 136; absolute remission rate = 26.9%; RR = 1.1; 95% CI, 0.88-1.3). Symptom response in the augment-aripiprazole group (74.3%) was higher than in either the switch group (62.4%; RR = 1.19; 95% CI, 1.09-1.29; NNT = 8) or the augment-bupropion group (65.6%; RR = 1.13; 95% CI, 1.0-1.2; NNT = 11). There was no difference noted in response rate between the switch group and the augment-bupropion group (RR = 1.05; 95% CI, 0.96-1.15).3
The adverse events that occurred more often in the augment-aripiprazole group than in the other groups included weight gain ≥ 7% (25% at 36 weeks) and extrapyramidal symptoms (19%).3 Limitations of the study included the evaluation of only 1 antipsychotic and 1 antidepressant, the dropout rate (only 75% of patients completed the 12-week follow-up), and the homogeneity of the patient population (older, male, veterans), all of which may limit the effect size.
Editor’s takeaway
Multiple trials show that adjunctive antipsychotic medications such as aripiprazole and quetiapine more effectively treat resistant depression than adding a placebo, increasing antidepressant dosage, switching to a different antidepressant, or adding another antidepressant. However, while primary care physicians should be comfortable with this option, the magnitude of difference between these options was modest, and adverse effects were common. All options can still be reasonably considered.
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
1. Cantù F, Ciappolino V, Enrico P, et al. Augmentation with atypical antipsychotics for treatment-resistant depression. J Affect Disord. 2021;280(pt A):45-53. doi: 10.1016/j.jad.2020.11.006
2. Davies P, Ijaz S, Williams CJ, et al. Pharmacological interventions for treatment-resistant depression in adults. Cochrane Database Syst Rev. 2019;12:CD010557. doi: 10.1002/14651858.CD010557.pub2
3. Mohamed S, Johnson GR, Chen P, et al. Effect of antidepressant switching vs augmentation on remission among patients with major depressive disorder unresponsive to antidepressant treatment: the VAST-D randomized clinical trial. JAMA. 2017;318:132-145. doi: 10.1001/jama.2017.8036
EVIDENCE-BASED ANSWER:
YES. Augmentation with second- generation antipsychotics, especially aripiprazole and quetiapine, appears to be effective in patients with moderate-to-severe depression who have had a suboptimal response to a selective serotonin reuptake inhibitor or a serotonin-norepinephrine reuptake inhibitor (strength of recommendation [SOR]: A, based on a systematic review of randomized controlled trials [RCTs] and an individual RCT). Augmenting antidepressant therapy with cariprazine, ziprasidone, or olanzapine also appears to improve depressive symptoms over the short term. All antipsychotics studied carried an increased likelihood of adverse effects that could lead to discontinuation (SOR: A, based on a systematic review of RCTs).
Insulin Injection-Site Acanthosis Nigricans: Skin Reactions and Clinical Implications
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
Insulin injection therapy is one of the most widely used health care interventions to manage both type 1 and type 2 diabetes mellitus (T1DM/T2DM). Globally, more than 150 to 200 million people inject insulin into their upper posterior arms, buttocks, anterior and lateral thighs, or abdomen.1,2 In an ideal world, every patient would be using the correct site and rotating their insulin injection sites in accordance with health care professional (HCP) recommendations—systematic injections in one general body location, at least 1 cm away from the previous injection.2 Unfortunately, same-site insulin injection (repeatedly in the same region within 1 cm of previous injections) is a common mistake made by patients with DM—in one study, 63% of participants either did not rotate sites correctly or failed to do so at all.
Insulin-resistant cutaneous complications may occur as a result of same-site insulin injections. The most common is lipohypertrophy, reported in some studies in nearly 50% of patients with DM on insulin therapy.4 Other common cutaneous complications include lipoatrophy and amyloidosis. Injection-site acanthosis nigricans, although uncommon, has been reported in 18 cases in the literature.
Most articles suggest that same-site insulin injections decrease local insulin sensitivity and result in tissue hypertrophy because of the anabolic properties of insulin and increase in insulin binding to insulin-like growth factor-1 (IGF-1) receptor.5-20 The hyperkeratotic growth and varying insulin absorption rates associated with these cutaneous complications increase chances of either hyper- or hypoglycemic episodes in patients.10,11,13 It is the responsibility of the DM care professional to provide proper insulin-injection technique education and perform routine inspection of injection sites to reduce cutaneous complications of insulin therapy. The purpose of this article is to (1) describe a case of acanthosis nigricans resulting from insulin injection at the same site; (2) review case reports
Case Presentation
A 75-year-old patient with an 8-year history of T2DM, as well as stable coronary artery disease, atrial fibrillation, hypertension, hyperlipidemia, chronic obstructive pulmonary disease, and stage 3 chronic kidney disease, presented with 2 discrete abdominal hyperpigmented plaques. At the time of the initial clinic visit, the patient was taking metformin 1000 mg twice daily and insulin glargine 40 units once daily. When insulin was initiated 7 years prior, the patient received
The patient reported 5 years of progressive, asymptomatic hyperpigmentation of the skin surrounding his insulin glargine injection sites and injecting in these same sites daily without rotation. He reported no additional skin changes or symptoms. He had noticed no skin changes while using NPH insulin during his first year of insulin therapy. On examination, the abdominal wall skin demonstrated 2 well-demarcated, nearly black, soft, velvety plaques, measuring 9 × 8 cm on the left side and 4 × 3.5 cm on the right, suggesting acanthosis nigricans (Figure 1A). The remainder of the skin examination, including the flexures, was normal. Of note, the patient received biweekly intramuscular testosterone injections in the gluteal region for secondary hypogonadism with no adverse dermatologic effects. A skin punch biopsy was performed and revealed epidermal papillomatosis and hyperkeratosis, confirming the clinical diagnosis of acanthosis nigricans (Figure 2).
After a review of insulin-injection technique at his clinic visit, the patient started rotating insulin injection sites over his entire abdomen, and the acanthosis nigricans partially improved. A few months later, the patient stopped rotating the insulin injection site, and the acanthosis nigricans worsened again. Because of worsening glycemic control, the patient was then started on insulin aspart. He did not develop any skin changes at the insulin aspart injection site, although he was not rotating its site of injection.
Subsequently, with reeducation and proper injection-site rotation, the patient had resolution of his acanthosis nigricans (Figure 1b).
Discussion
A review of the literature revealed 18 reported cases of acanthosis nigricans at sites of repeated insulin injection (Table).5-20 Acanthosis nigricans at the site of insulin injection afflicts patients of any age, with cases observed in patients aged 14 to 75 years. Sixteen (84%) of 19 cases were male. Fourteen cases (73%) had T2DM; the rest of the patients had T1DM. The duration of insulin injection therapy prior to onset ranged from immediate to 13 years (median 4 years). Fourteen cases (73%) were reported on the abdomen; however, other sites, such as thighs and upper arm, also were reported. Lesions size varied from 12 to 360 cm2. Two cases had associated amyloidosis. The average HbA1c reported at presentation was 10%. Following insulin injection-site rotation, most of the cases reported improvement of both glycemic control and acanthosis nigricans appearance.
In the case described by Kudo and colleagues, a 59-year-old male patient with T2DM had been injecting insulin into the same spot on his abdomen for 10 years. He developed acanthosis nigricans and an amyloidoma so large and firm that it bent the needle when he injected insulin.11
Most of the cases we found in the literature were after 2005 and associated with the use of human or analog insulin. These cases may be related to a bias, as cases may be easier to find in digital archives in the later years, when human or analog insulins have been in common use. Also noteworthy, in cases that reported dosage, most were not very high, and the highest daily dose was 240 IU/d. Ten reports of injection-site acanthosis nigricans were in dermatology journals; only 5 reports were in endocrinology journals and 3 in general medical journals, indicating possible less awareness of this phenomenon in other HCPs who care for patients with DM.
Complications of Same-Site Injections
Acanthosis nigricans. Commonly found in the armpits, neck folds, and groin, acanthosis nigricans is known as one of the calling cards for insulin resistance, obesity, and hyperinsulinemia.21 Acanthosis nigricans can be seen in people with or without DM and is not limited to those on insulin therapy. However, same-site insulin injections for 4 to 6 years also may result in injection-site acanthosis nigricans–like lesions because of factors such as insulin exposure at the local tissue level.16
Acanthosis nigricans development is characterized by hyperpigmented, hyperkeratotic, velvety, and sometimes verrucous plaques.6 Acanthosis nigricans surrounding repeated injection sites is hypothesized to develop as a result of localized hyperinsulinemia secondary to insulin resistance, which increases the stimulation of IGF, thereby causing epidermal hypertrophy.5-20 If insulin injection therapy continues to be administered through the acanthosis nigricans lesion, it results in decreased insulin absorption, leading to poor glycemic control.13
Acanthosis nigricans associated with insulin injection is reversible. After rotation of injection sites, lesions either decrease in size or severity of appearance.5-8,11 Also, by avoiding injection into the hyperkeratotic plaques and using normal subcutaneous tissue for injection, patients’ response to insulin improves, as measured by HbA1c and by decreased daily insulin requirement.6-8,10,12,18-20
Lipohypertrophy. This is characterized by an increase in localized adipose tissue and is the most common cutaneous complication of insulin therapy.2 Lipohypertrophy presents as a firm, rubbery mass in the location of same-site insulin injections.22 Development of lipohypertrophy is suspected to be the result of either (1) anabolic effect of insulin on local adipocytes, promoting fat and protein synthesis; (2) an autoimmune response by immunoglobulin (Ig) G or IgE antibodies to insulin, immune response to insulin of different species, or to insulin injection techniques; or (3) repeated trauma to the injection site from repeated needle usage.4,23
In a study assessing the prevalence of lipohypertrophy and its relation to insulin technique, 49.1% of participants with
Primary prevention measures include injection site inspection and patient education about rotation and abstaining from needle reuse.22 If a patient already has signs of lipohypertrophy, data supports education and insulin injection technique practice as simple and effective means to reduce insulin action variability and increase glycemic control.24
Lipoatrophy. Lipoatrophy is described as a local loss of subcutaneous adipose tissue often in the face, buttocks, legs and arm regions and can be rooted in genetic, immune, or drug-associated etiologies.25 Insulin-induced lipoatrophy is suspected to be the result of tumor necrosis factor-α hyperproduction in reaction to insulin crystal presence at the injection site.26,27 Overall, lipoatrophy development has decreased since the use of recombinant human insulin and analog insulin therapy.28 The decrease is hypothesized to be due to increased subcutaneous tissue absorption rate of human insulin and its analog, decreasing overall adipocyte exposure to localized high insulin concentration.27 Treatments for same-site insulin-derived lipoatrophy include changing injection sites and preparation of insulin.26 When injection into the lipoatrophic site was avoided, glycemic control and lipoatrophy appearance improved.26
Amyloidosis. Amyloidosis indicates the presence of an extracellular bundle of insoluble polymeric protein fibrils in tissues and organs.29 Insulin-induced amyloidosis presents as a hard mass or ball near the injection site.29 Insulin is one of many hormones that can form amyloid fibrils, and there have been several dozen cases reported of amyloid formation at the site of insulin injection.29-31 Although insulin-derived amyloidosis is rare, it may be misdiagnosed as lipohypertrophy due to a lack of histopathologic testing or general awareness of the complication.29
In a case series of 7 patients with amyloidosis, all patients had a mean HbA1c of 9.3% (range, 8.5-10.2%) and averaged 1 IU/kg bodyweight before intervention.30 After the discovery of the mass, participants were instructed to avoid injection into the amyloidoma, and average insulin requirements decreased to 0.48 IU/kg body weight (P = .40).30 Patients with amyloidosis who rotated their injection sites experienced better glycemic control and decreased insulin requirements.30
Pathophysiology of Localized Insulin Resistance
Insulin regulates glucose homeostasis in skeletal muscle and adipose tissue, increases hepatic and adipocyte lipid synthesis, and decreases adipocyte fatty acid release.32 Generalized insulin resistance occurs when target tissues have decreased glucose uptake in response to circulating insulin.32 Insulin resistance increases the amount of free insulin in surrounding tissues. At high concentrations, insulin fosters tissue growth by binding to IGF-1 receptors, stimulating hypertrophy and reproduction of keratinocytes and fibroblasts.33 This pathophysiology helps explain the origin of localized acanthosis nigricans at same-site insulin injections.
Conclusions
Cutaneous complications are a local adverse effect of long-term failure to rotate insulin injection sites. Our case serves as a call to action for HCPs to improve education regarding insulin injection-site rotation, conduct routine injection-site inspection, and actively document cases as they occur to increase public awareness of these important complications.
If a patient with DM presents with unexplained poor glycemic control, consider questioning the patient about injection-site location and how often they are rotating the insulin injection site. Inspect the site for cutaneous complications. Of note, if a patient has a cutaneous complication due to insulin injection, adjust or decrease the insulin dosage when rotating sites to mitigate the risk of hypoglycemic episodes.
Improvement of glycemic control, cosmetic appearance of injection site, and insulin use all begin with skin inspection, injection technique education, and periodic review by a HCP.
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
1. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther. 2019;21(2):66-72. doi:10.1089/dia.2018.0384
2. Frid AH, Kreugel G, Grassi G, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91(9):1231-1255. doi:10.1016/j.mayocp.2016.06.010
3. Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39(5):445-453. doi:10.1016/j.diabet.2013.05.006
4. Johansson UB, Amsberg S, Hannerz L, et al. Impaired absorption of insulin aspart from lipohypertrophic injection sites. Diabetes Care. 2005;28(8):2025-2027. doi:10.2337/diacare.28.8.2025
5. Erickson L, Lipschutz DE, Wrigley W, Kearse WO. A peculiar cutaneous reaction to repeated injections of insulin. JAMA. 1969;209(6):934-935. doi:10.1001/jama.1969.03160190056019
6. Fleming MG, Simon SI. Cutaneous insulin reaction resembling acanthosis nigricans. Arch Dermatol. 1986;122(9):1054-1056. doi:10.1001/archderm.1986.01660210104028 7. Gannon D, Ross MW, Mahajan T. Acanthosis nigricans-like plaque and lipohypertrophy in type 1 diabetes. Pract Diabetes International. 2005;22(6).
8. Mailler-Savage EA, Adams BB. Exogenous insulin-derived acanthosis nigricans. Arch Dermatol. 2008;144(1):126-127. doi:10.1001/archdermatol.2007.27
9. Pachón Burgos A, Chan Aguilar MP. Visual vignette. Hyperpigmented hyperkeratotic cutaneous insulin reaction that resembles acanthosis nigricans with lipohypertrophy. Endocr Pract. 2008;14(4):514. doi:10.4158/EP.14.4.514
10. Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94(2):e34-e36. doi:10.1016/j.diabres.2011.07.023
11. Kudo-Watanuki S, Kurihara E, Yamamoto K, Mukai K, Chen KR. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2012;38(1):25-29. doi:10.1111/j.1365-2230.2012.04373.x
12. Brodell JD Jr, Cannella JD, Helms SE. Case report: acanthosis nigricans resulting from repetitive same-site insulin injections. J Drugs Dermatol. 2012;11(12):e85-e87.
13. Kanwar A, Sawatkar G, Dogra S, Bhadada S. Acanthosis nigricans—an uncommon cutaneous adverse effect of a common medication: report of two cases. Indian J Dermatol Venereol Leprol. 2013;79(4):553. doi:10.4103/0378-6323.113112
14. Dhingra M, Garg G, Gupta M, Khurana U, Thami GP. Exogenous insulin-derived acanthosis nigricans: could it be a cause of increased insulin requirement? Dermatol Online J. 2013;19(1):9. Published 2013 Jan 15.
15. Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57(1):127-129. doi:10.4103/0377-4929.130920
16. Yahagi E, Mabuchi T, Nuruki H, et al. Case of exogenous insulin-derived acanthosis nigricans caused by insulin injections. Tokai J Exp Clin Med. 2014;39(1):5-9.
17. Chapman SE, Bandino JP. A verrucous plaque on the abdomen: challenge. Am J Dermatopathol. 2017;39(12):e163. doi:10.1097/DAD.0000000000000659
18. Huang Y, Hessami-Booshehri M. Acanthosis nigricans at sites of insulin injection in a man with diabetes. CMAJ. 2018;190(47):E1390. doi:10.1503/cmaj.180705
19. Pal R, Bhattacharjee R, Chatterjee D, Bhadada SK, Bhansali A, Dutta P. Exogenous insulin-induced localized acanthosis nigricans: a rare injection site complication. Can J Diabetes. 2020;44(3):219-221. doi:10.1016/j.jcjd.2019.08.010
20. Bomar L, Lewallen R, Jorizzo J. Localized acanthosis nigricans at the site of repetitive insulin injections. Cutis. 2020;105(2);E20-E22.
21. Karadağ AS, You Y, Danarti R, Al-Khuzaei S, Chen W. Acanthosis nigricans and the metabolic syndrome. Clin Dermatol. 2018;36(1):48-53. doi:10.1016/j.clindermatol.2017.09.008
22. Kalra S, Kumar A, Gupta Y. Prevention of lipohypertrophy. J Pak Med Assoc. 2016;66(7):910-911.
23. Singha A, Bhattarcharjee R, Ghosh S, Chakrabarti SK, Baidya A, Chowdhury S. Concurrence of lipoatrophy and lipohypertrophy in children with type 1 diabetes using recombinant human insulin: two case reports. Clin Diabetes. 2016;34(1):51-53. doi:10.2337/diaclin.34.1.51
24. Famulla S, Hövelmann U, Fischer A, et al. Insulin injection into lipohypertrophic tissue: blunted and more variable insulin absorption and action and impaired postprandial glucose control. Diabetes Care. 2016;39(9):1486-1492. doi:10.2337/dc16-0610.
25. Reitman ML, Arioglu E, Gavrilova O, Taylor SI. Lipoatrophy revisited. Trends Endocrinol Metab. 2000;11(10):410-416. doi:10.1016/s1043-2760(00)00309-x
26. Kondo A, Nakamura A, Takeuchi J, Miyoshi H, Atsumi T. Insulin-Induced Distant Site Lipoatrophy. Diabetes Care. 2017;40(6):e67-e68. doi:10.2337/dc16-2385
27. Jermendy G, Nádas J, Sápi Z. “Lipoblastoma-like” lipoatrophy induced by human insulin: morphological evidence for local dedifferentiation of adipocytes?. Diabetologia. 2000;43(7):955-956. doi:10.1007/s001250051476
28. Mokta JK, Mokta KK, Panda P. Insulin lipodystrophy and lipohypertrophy. Indian J Endocrinol Metab. 2013;17(4):773-774. doi:10.4103/2230-8210.113788
29. Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19(1):174-177. doi:10.4103/2230-8210.146879
30. Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127(5):450-454. doi:10.1016/j.amjmed.2013.10.029
31. Swift B. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabet Med. 2002;19(10):881-882. doi:10.1046/j.1464-5491.2002.07581.x
32. Sesti G. Pathophysiology of insulin resistance. Best Pract Res Clin Endocrinol Metab. 2006;20(4):665-679. doi:10.1016/j.beem.2006.09.007
33. Phiske MM. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239-249. doi:10.4103/2229-5178.137765
Erythematous Papules on the Ears
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
The Diagnosis: Borrelial Lymphocytoma (Lymphocytoma Cutis)
A punch biopsy revealed an atypical lobular lymphoid infiltrate within the dermis and subcutaneous tissue with a mixed composition of CD3+ T cells and CD20+ B cells (quiz image, bottom). Immunohistochemical studies revealed a normal CD4:CD8 ratio with preservation of CD5 and CD7. CD30 was largely negative. CD21 failed to detect follicular dendritic cell networks, and κ/λ light chain staining confirmed a preserved ratio of polytypic plasma cells. There was limited staining with B-cell lymphoma (Bcl-2 and Bcl-6). Polymerase chain reaction studies for both T- and B-cell receptors were negative (polyclonal).
Lyme disease is the most frequently reported vectorborne infectious disease in the United States, and borrelial lymphocytoma (BL) is a rare clinical sequela. Borrelial lymphocytoma is a variant of lymphocytoma cutis (also known as benign reactive lymphoid hyperplasia), which is an inflammatory lesion that can mimic malignant lymphoma clinically and histologically. Lymphocytoma cutis is considered the prototypical example of cutaneous B-cell pseudolymphoma.1 Due to suspicion for lymphocytoma cutis based on the histologic findings and characteristic location of the lesions in our patient, Lyme serologies were ordered and were positive for IgM antibodies against p23, p39, and p41 antigens in high titers. Our patient was treated with doxycycline 100 mg twice daily for 3 weeks with complete resolution of the lesions at 3-month follow-up.
Clinically, BL appears as erythematous papules, plaques, or nodules commonly on the lobules of the ears (quiz image, top). Most cases of lymphocytoma cutis are idiopathic but may be triggered by identifiable associated etiologies including Borrelia burgdorferi, Leishmania donovani, molluscum contagiosum, herpes zoster virus, vaccinations, tattoos, insect bites, and drugs. The main differential diagnosis of lymphocytoma cutis is cutaneous B-cell lymphoma. Pseudolymphoma of the skin can mimic nearly all immunohistochemical staining patterns of true B-cell lymphomas.2
Primary cutaneous follicle center lymphoma frequently occurs on the head and neck. This true lymphoma of the skin can demonstrate prominent follicle centers with centrocytes and fragmented germinal centers (Figure 1) or show a diffuse pattern.3 Most cases show conspicuous Bcl-6 staining, and IgH gene rearrangements can detect a clonal B-cell population in more than 50% of cases.4
Diffuse large B-cell lymphoma can occur as a primary cutaneous malignancy or as a manifestation of systemic disease.4 When arising in the skin, lesions tend to affect the extremities, and the disease is classified as diffuse large B-cell lymphoma, leg type. Histologically, sheets of large atypical lymphocytes with numerous mitoses are seen (Figure 2). These cells stain positively with Bcl-2 and frequently demonstrate Bcl-6 and MUM-1, none of which were seen in our case.4 Lymphomatoid papulosis (LyP) tends to present with relapsing erythematous papules. Patients occasionally develop LyP in association with mycosis fungoides or other lymphomas. Both LyP and primary cutaneous anaplastic large cell lymphoma demonstrate conspicuous CD30+ large cells that can be multinucleated or resemble the Reed-Sternberg cells seen in Hodgkin lymphoma (Figure 3).4 Arthropod bite reactions are common but may be confused with lymphomas and pseudolymphomas. The perivascular lymphocytic infiltrate seen in arthropod bite reactions may be dense and usually is associated with numerous eosinophils (Figure 4). Occasional plasma cells also can be seen, and if the infiltrate closely adheres to vascular structures, a diagnosis of erythema chronicum migrans also can be considered. Patients with chronic lymphocytic leukemia/lymphoma may demonstrate exaggerated or persistent arthropod bite reactions, and atypical lymphocytes can be detected admixed with the otherwise reactive infiltrate.4
Borrelia burgdorferi is primarily endemic to North America and Europe. It is a spirochete bacterium spread by the Ixodes tick that was first recognized as the etiologic agent in 1975 in Old Lyme, Connecticut, where it received its name.5 Most reported cases of Lyme disease occur in the northeastern United States, which correlates with this case given our patient’s place of residence.6 Borrelial lymphocytoma cutis occurs in areas endemic for the Ixodes tick in Europe and North America.7 When describing the genotyping of Borrelia seen in BL, the strain B burgdorferi previously was grouped with Borrelia afzelii and Borrelia garinii.2 In the contemporary literature, however, B burgdorferi is referred to as sensu stricto when specifically talking about the strain B burgdorferi, and the term sensu lato is used when referencing the combination of strains (B burgdorferi, B afzelii, B garinii).
A 2016 study by Maraspin et al8 comprising 144 patients diagnosed with BL showed that the lesions mainly were located on the breast (106 patients [73.6%]) and the earlobe (27 patients [18.8%]), with the remaining cases occurring elsewhere on the body (11 patients [7.6%]). The Borrelia strains isolated from the BL lesions included B afzelii, Borrelia bissettii, and B garinii, with B afzelii being the most commonly identified (84.6% [11/13]).8
Borrelial lymphocytoma usually is categorized as a form of early disseminated Lyme disease and is treated as such. The treatment of choice for early disseminated Lyme disease is doxycycline 100 mg twice daily for 14 to 21 days. Ceftriaxone and azithromycin are reasonable treatment options for patients who have tetracycline allergies or who are pregnant.9
In conclusion, the presentation of red papules or nodules on the ears should prompt clinical suspicion of Lyme disease, particularly in endemic areas. Differentiating pseudolymphomas from true lymphomas and other reactive conditions can be challenging.
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
- Mitteldorf C, Kempf W. Cutaneous pseudolymphoma. Surg Pathol Clin. 2017;10:455-476. doi:10.1016/j.path.2017.01.002
- Colli C, Leinweber B, Müllegger R, et al. Borrelia burgdorferiassociated lymphocytoma cutis: clinicopathologic, immunophenotypic, and molecular study of 106 cases. J Cutan Pathol. 2004;31:232-240. doi:10.1111/j.0303-6987.2003.00167.x
- Wehbe AM, Neppalli V, Syrbu S, et al. Diffuse follicle centre lymphoma presents with high frequency of extranodal disease. J Clin Oncol. 2008;26(15 suppl):19511. doi:10.1200/jco.2008.26.15_suppl.19511
- Patterson JW, Hosler GA. Cutaneous infiltrates—lymphomatous and leukemic. In: Patterson JW, ed. Weedon’s Skin Pathology. 4th ed. Elsevier; 2016:1171-1217.
- Cardenas-de la Garza JA, De la Cruz-Valadez E, Ocampo -Candiani J, et al. Clinical spectrum of Lyme disease. Eur J Clin Microbiol Infect Dis. 2019;38:201-208. doi:10.1007/s10096-018-3417-1
- Shapiro ED, Gerber MA. Lyme disease. Clin Infect Dis. 2000;31:533-542. doi:10.1086/313982
- Kandhari R, Kandhari S, Jain S. Borrelial lymphocytoma cutis: a diagnostic dilemma. Indian J Dermatol. 2014;59:595-597. doi:10.4103/0019-5154.143530
- Maraspin V, Nahtigal Klevišar M, Ružic´-Sabljic´ E, et al. Borrelial lymphocytoma in adult patients. Clin Infect Dis. 2016;63:914-921. doi:10.1093/cid/ciw417
- Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006; 43:1089-1134. doi:10.1086/508667
A 53-year-old man with a history of atopic dermatitis presented with pain and redness of the lobules of both ears of 9 months’ duration. He had no known allergies and took no medications. He lived in suburban Virginia and had not recently traveled outside of the region. Physical examination revealed tender erythematous and edematous nodules on the lobules of both ears (top). There was no evidence of arthritis or neurologic deficits. A punch biopsy was performed (bottom).
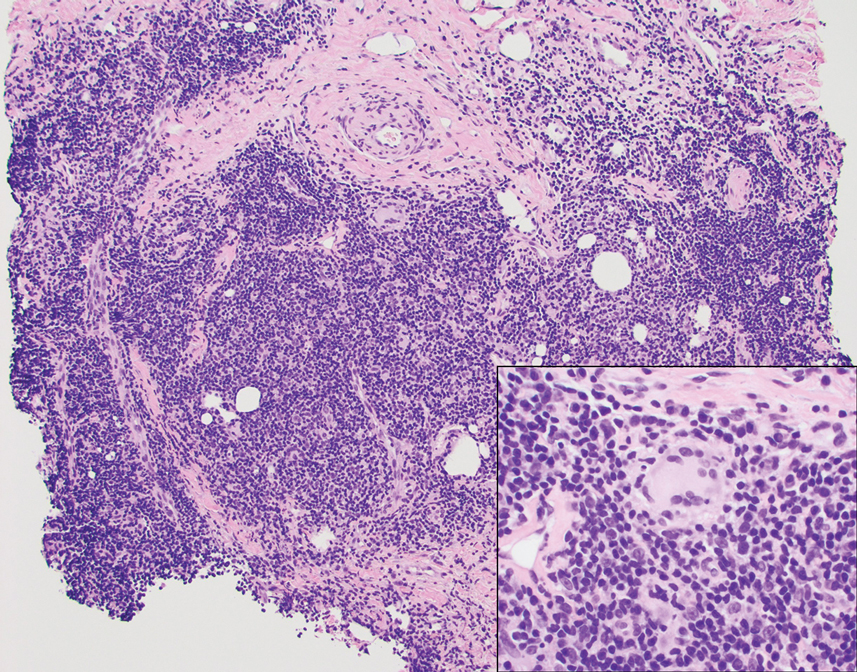
Botanical Briefs: Ginkgo (Ginkgo biloba)
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.
Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
An ancient tree of the Ginkgoaceae family, Ginkgo biloba is known as a living fossil because its genome has been identified in fossils older than 200 million years.1 An individual tree can live longer than 1000 years. Originating in China, G biloba (here, “ginkgo”) is cultivated worldwide for its attractive foliage (Figure 1). Ginkgo extract has long been used in traditional Chinese medicine; however, contact with the plant proper can provoke allergic contact dermatitis.

Dermatitis-Inducing Components
The allergenic component of the ginkgo tree is ginkgolic acid, which is structurally similar to urushiol and anacardic acid.2,3 This compound can cause a cross-reaction in a person previously sensitized by contact with other plants. Urushiol is found in poison ivy(Toxicodendron radicans); anacardic acid is found in the cashew tree (Anacardium occidentale). Both plants belong to the family Anacardiaceae, commonly known as the cashew family.
Members of Anacardiaceae are the most common causes of plant-induced allergic contact dermatitis and include the cashew tree, mango tree, poison ivy, poison oak, and poison sumac. These plants can cross-react to cause contact dermatitis (Table).3 Patch tests have revealed that some individuals who are sensitive to components of the ginkgo tree also demonstrate sensitivity to poison ivy and poison sumac4,5; countering this finding, Lepoittevin and colleagues6 demonstrated in animal studies that there was no cross-reactivity between ginkgo and urushiol, suggesting that patients with a reported cross-reaction might truly have been previously sensitized to both plants. In general, patients who have a history of a reaction to any Anacardiaceae plant should take precautions when handling them.

Therapeutic Benefit of Ginkgo
Ginkgo extract is sold as the herbal supplement EGB761, which acts as an antioxidant.7 In France, Germany, and China, it is a commonly prescribed herbal medicine.8 It is purported to support memory and attention; studies have shown improvement in cognition and in involvement with activities of daily living for patients with dementia.9,10 Ginkgo extract might lessen peripheral vascular disease and cerebral circulatory disease, having been shown in vitro and in animal models to prevent platelet aggregation induced by platelet-activating factor and to stimulate vasodilation by increasing production of nitric oxide.11,12
Furthermore, purified ginkgo extract might have beneficial effects on skin. A study in rats showed that when intraperitoneal ginkgo extract was given prior to radiation therapy, 100% of rats receiving placebo developed radiation dermatitis vs 13% of those that received ginkgo extract (P<.0001). An excisional skin biopsy showed a decrease in markers of oxidative stress in rats that received ginkgo extract prior to radiation.7
A randomized, double-blind clinical trial showed a significant reduction in disease progression in vitiligo patients assigned to receive ginkgo extract orally compared to placebo (P=.006).13 Research for many possible uses of ginkgo extract is ongoing.
Cutaneous Manifestations
Contact with the fruit of the ginkgo tree can induce allergic contact dermatitis,14 most often as erythematous papules, vesicles, and in some cases edema.5,15
Exposures While Picking Berries—In 1939, Bolus15 reported the case of a patient who presented with edema, erythema, and vesicular lesions involving the hands and face after picking berries from a ginkgo tree. Later, patch testing on this patient, using ginkgo fruit, resulted in burning and stinging that necessitated removal of the patch, suggesting an irritant reaction. This was followed by a vesicular reaction that then developed within 24 hours, which was more consistent with allergy. Similarly, in 1988, a case series of contact dermatitis was reported in 3 patients after gathering ginkgo fruit.5
Incidental Exposure While Walking—In 1965, dermatitis broke out in 35 high school students, mainly affecting exposed portions of the leg, after ginkgo fruit fell and its pulp was exposed on a path at their school.4 Subsequently, patch testing was performed on 29 volunteers—some who had been exposed to ginkgo on that path, others without prior exposure. It was established that testing with ginkgo pulp directly caused an irritant reaction in all students, regardless of prior ginkgo exposure, but all prior ginkgo-exposed students in this study reacted positively to an acetone extract of ginkgo pulp and either poison ivy extract or pentadecylcatechol.4
Systemic Contact After Eating Fruit—An illustrative case of dermatitis, stomatitis, and proctitis was reported in a man with history of poison oak contact dermatitis who had eaten fruit from a ginkgo tree, suggesting systemic contact dermatitis. Weeks after resolution of symptoms, he reacted positively to ginkgo fruit and poison ivy extracts on patch testing.16
Ginkgo dermatitis tends to resolve upon removal of the inciting agent and application of a topical steroid.8,17 Although many reported cases involve the fruit, allergic contact dermatitis can result from exposure to any part of the plant. In a reported case, a woman developed airborne contact dermatitis from working with sarcotesta of the ginkgo plant.18 Despite wearing rubber gloves, she broke out 1 week after exposure with erythema on the face and arms and severe facial edema.
Ginkgo leaves also can cause allergic contact dermatitis.19 Precautions should be taken when handling any component of the ginkgo tree.
Oral ginkgo supplementation has been implicated in a variety of other cutaneous reactions—from benign to life-threatening. When the ginkgo allergen concentration is too high within the supplement, as has been noted in some formulations, patients have presented with a diffuse morbilliform eruption within 1 or 2 weeks after taking ginkgo.20 One patient—who was not taking any other medication—experienced an episode of acute generalized exanthematous pustulosis 48 hours after taking ginkgo.21 Ingestion of ginkgo extract also has been associated with Stevens-Johnson syndrome.22-24
Other Adverse Reactions
The adverse effects of ginkgo supplement vary widely. In addition to dermatitis, ginkgo supplement can cause headaches, palpitations, tachycardia, vasculitis, nausea, and other symptoms.14
Metabolic Disturbance—One patient taking ginkgo who died after a seizure was found to have subtherapeutic levels of valproate and phenytoin,25 which could be due to ginkgo’s effect on cytochrome p450 enzyme CYP2C19.26 Ginkgo interactions with many cytochrome enzymes have been studied for potential drug interactions. Any other direct effects remain variable and controversial.27,28
Hemorrhage—Another serious effect associated with taking ginkgo supplements is hemorrhage, often in conjunction with warfarin14; however, a meta-analysis indicated that ginkgo generally does not increase the risk of bleeding.29 Other studies have shown that taking ginkgo with warfarin showed no difference in clotting status, and ginkgo with aspirin resulted in no clinically significant difference in bruising, bleeding, or platelet function in an analysis over a period of 1 month.30,31 These findings notwithstanding, pregnant women, surgical patients, and those taking a blood thinner are advised as a general precaution not to take ginkgo extract.
Carcinogenesis—Ginkgo extract has antioxidant properties, but there is evidence that it might act as a carcinogen. An animal study reported by the US National Toxicology Program found that ginkgo induced mutagenic activity in the liver, thyroid, and nose of mice and rats. Over time, rodent liver underwent changes consistent with hepatic enzyme induction.32 More research is needed to clarify the role of ginkgo in this process.
Toxicity by Ingestion—Ginkgo seeds can cause food poisoning due to the compound 4’-O-methylpyridoxine (also known as ginkgotoxin).33 Because methylpyridoxine can cause depletion of pyridoxal phosphate (a form of vitamin B6 necessary for the synthesis of γ-aminobutyric acid), overconsumption of ginkgo seeds, even when fully cooked, might result in convulsions and even death.33
Nomenclature and Distribution of Plants
Gingko biloba belongs to the Ginkgoaceae family (class Ginkgophytes). The tree originated in China but might no longer exist in a truly wild form. It is grown worldwide for its beauty and longevity. The female ginkgo tree is a gymnosperm, producing fruit with seeds that are not coated by an ovary wall15; male (nonfruiting) trees are preferentially planted because the fruit is surrounded by a pulp that, when dropped, emits a sour smell described variously as rancid butter, vomit, or excrement.5
Identifying Features and Plant Facts
The deciduous ginkgo tree has unique fan-shaped leaves and is cultivated for its beauty and resistance to disease (Figure 2).4,34 It is nicknamed the maidenhair tree because the leaves are similar to the pinnae of the maidenhair fern.34 Because G biloba is resistant to pollution, it often is planted along city streets.17 The leaf—5- to 8-cm wide and a symbol of the city of Tokyo, Japan34—grows in clusters (Figure 3)5 and is green but turns yellow before it falls in autumn.34 Leaf veins branch out into the blade without anastomosing.34

Male flowers grow in a catkinlike pattern; female flowers grow on long stems.5 The fruit is small, dark, and shriveled, with a hint of silver4; it typically is 2 to 2.5 cm in diameter and contains the ginkgo nut or seed. The kernel of the ginkgo nut is edible when roasted and is used in traditional Chinese and Japanese cuisine as a dish served on special occasions in autumn.33

Final Thoughts
Given that G biloba is a beautiful, commonly planted ornamental tree, gardeners and landscapers should be aware of the risk for allergic contact dermatitis and use proper protection. Dermatologists should be aware of its cross-reactivity with other common plants such as poison ivy and poison oak to help patients identify the cause of their reactions and avoid the inciting agent. Because ginkgo extract also can cause a cutaneous reaction or interact with other medications, providers should remember to take a thorough medication history that includes herbal medicines and supplements.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
- Lyu J. Ginkgo history told by genomes. Nat Plants. 2019;5:1029. doi:10.1038/s41477-019-0529-2
- ElSohly MA, Adawadkar PD, Benigni DA, et al. Analogues of poison ivy urushiol. Synthesis and biological activity of disubstituted n-alkylbenzenes. J Med Chem. 1986;29:606-611. doi:10.1021/jm00155a003
- He X, Bernart MW, Nolan GS, et al. High-performance liquid chromatography–electrospray ionization-mass spectrometry study of ginkgolic acid in the leaves and fruits of the ginkgo tree (Ginkgo biloba). J Chromatogr Sci. 2000;38:169-173. doi:10.1093/chromsci/38.4.169
- Sowers WF, Weary PE, Collins OD, et al. Ginkgo-tree dermatitis. Arch Dermatol. 1965;91:452-456. doi:10.1001/archderm.1965.01600110038009
- Tomb RR, Foussereau J, Sell Y. Mini-epidemic of contact dermatitis from ginkgo tree fruit (Ginkgo biloba L.). Contact Dermatitis. 1988;19:281-283. doi:10.1111/j.1600-0536.1988.tb02928.x
- Lepoittevin J-P, Benezra C, Asakawa Y. Allergic contact dermatitis to Ginkgo biloba L.: relationship with urushiol. Arch Dermatol Res. 1989;281:227-230. doi:10.1007/BF00431055
- Yirmibesoglu E, Karahacioglu E, Kilic D, et al. The protective effects of Ginkgo biloba extract (EGb-761) on radiation-induced dermatitis: an experimental study. Clin Exp Dermatol. 2012;37:387-394. doi:10.1111/j.1365-2230.2011.04253.x
- Jiang L, Su L, Cui H, et al. Ginkgo biloba extract for dementia: a systematic review. Shanghai Arch Psychiatry. 2013;25:10-21. doi:10.3969/j.issn.1002-0829.2013.01.005
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409-1415. doi:10.1001/archneur.55.11.1409
- Le Bars PL, Katz MM, Berman N, et al. A placebo-controlled, double-blind, randomized trial of an extract of Ginkgo biloba for dementia. North American EGb Study Group. JAMA. 1997;278:1327-1332. doi:10.1001/jama.278.16.1327
- Koltermann A, Hartkorn A, Koch E, et al. Ginkgo biloba extract EGb 761 increases endothelial nitric oxide production in vitro and in vivo. Cell Mol Life Sci. 2007;64:1715-1722. doi:10.1007/s00018-007-7085-z
- Touvay C, Vilain B, Taylor JE, et al. Proof of the involvement of platelet activating factor (paf-acether) in pulmonary complex immune systems using a specific paf-acether receptor antagonist: BN 52021. Prog Lipid Res. 1986;25:277-288. doi:10.1016/0163-7827(86)90057-3
- Parsad D, Pandhi R, Juneja A. Effectiveness of oral Ginkgo biloba in treating limited, slowly spreading vitiligo. Clin Exp Dermatol. 2003;28:285-287. doi:10.1046/j.1365-2230.2003.01207.x
- Jacobsson I, Jönsson AK, Gerdén B, et al. Spontaneously reported adverse reactions in association with complementary and alternative medicine substances in Sweden. Pharmacoepidemiol Drug Saf. 2009;18:1039-1047. doi:10.1002/pds.1818
- Bolus M. Dermatitis venenata due to Ginkgo berries. Arch Derm Syphilol. 1939;39:530.
- Becker LE, Skipworth GB. Ginkgo-tree dermatitis, stomatitis, and proctitis. JAMA. 1975;231:1162-1163.
- Nakamura T. Ginkgo tree dermatitis. Contact Dermatitis. 1985;12:281-282. doi:10.1111/j.1600-0536.1985.tb01138.x
- Jiang J, Ding Y, Qian G. Airborne contact dermatitis caused by the sarcotesta of Ginkgo biloba. Contact Dermatitis. 2016;75:384-385. doi:10.1111/cod.12646
- Hotta E, Tamagawa-Mineoka R, Katoh N. Allergic contact dermatitis due to ginkgo tree fruit and leaf. Eur J Dermatol. 2013;23:548-549. doi:10.1684/ejd.2013.2102
- Chiu AE, Lane AT, Kimball AB. Diffuse morbilliform eruption after consumption of Ginkgo biloba supplement. J Am Acad Dermatol. 2002;46:145-146. doi:10.1067/mjd.2001.118545
- Pennisi RS. Acute generalised exanthematous pustulosis induced by the herbal remedy Ginkgo biloba. Med J Aust. 2006;184:583-584. doi:10.5694/j.1326-5377.2006.tb00386.x
- Yuste M, Sánchez-Estella J, Santos JC, et al. Stevens-Johnson syndrome/toxic epidermal necrolysis treated with intravenous immunoglobulins. Actas Dermosifiliogr. 2005;96:589-592. doi:10.1016/s0001-7310(05)73141-0
- Jeyamani VP, Sabishruthi S, Kavitha S, et al. An illustrative case study on drug induced Steven-Johnson syndrome by Ginkgo biloba. J Clin Res. 2018;2:1-3.
- Davydov L, Stirling AL. Stevens-Johnson syndrome with Ginkgo biloba. J Herbal Pharmacother. 2001;1:65-69. doi:10.1080/J157v01n03_06
- Yin OQP, Tomlinson B, Waye MMY, et al. Pharmacogenetics and herb–drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841-850. doi:10.1097/00008571-200412000-00007
- Kupiec T, Raj V. Fatal seizures due to potential herb–drug interactions with Ginkgo biloba. J Anal Toxicol. 2005;29:755-758. doi:10.1093/jat/29.7.755
- Zadoyan G, Rokitta D, Klement S, et al. Effect of Ginkgo biloba special extract EGb 761® on human cytochrome P450 activity: a cocktail interaction study in healthy volunteers. Eur J Clin Pharmacol. 2012;68:553-560. doi:10.1007/s00228-011-1174-5
- Zhou S-F, Deng Y, Bi H-c, et al. Induction of cytochrome P450 3A by the Ginkgo biloba extract and bilobalides in human and rat primary hepatocytes. Drug Metab Lett. 2008;2:60-66. doi:10.2174/187231208783478489
- Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? a systematic review and meta-analysis. Pharmacotherapy. 2011;31:490-502. doi:10.1592/phco.31.5.490
- Gardner CD, Zehnder JL, Rigby AJ, et al. Effect of Ginkgo biloba (EGb 761) and aspirin on platelet aggregation and platelet function analysis among older adults at risk of cardiovascular disease: a randomized clinical trial. Blood Coagul Fibrinolysis. 2007;18:787-79. doi:10.1097/MBC.0b013e3282f102b1
- Jiang X, Williams KM, Liauw WS, et al. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol. 2005;59:425-432. doi:10.1111/j.1365-2125.2005.02322.x
- Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS No. 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser. 2013:1-183.
- Azuma F, Nokura K, Kako T, et al. An adult case of generalized convulsions caused by the ingestion of Ginkgo biloba seeds with alcohol. Intern Med. 2020;59:1555-1558. doi:10.2169/internalmedicine.4196-19
- Cohen PR. Fixed drug eruption to supplement containing Ginkgo biloba and vinpocetine: a case report and review of related cutaneous side effects. J Clin Aesthet Dermatol. 2017;10:44-47.
PRACTICE POINTS
- Contact with the Ginkgo biloba tree can cause allergic contact dermatitis; ingestion can cause systemic dermatitis in a previously sensitized patient.
- Ginkgo biloba can cross-react with plants of the family Anacardiaceae, such as poison ivy, poison oak, poison sumac, cashew tree, and mango.
- Ginkgo extract is widely considered safe for use; however, dermatologists should be aware that it can cause systemic dermatitis and serious adverse effects, including internal hemorrhage and convulsions.
Aluminum: The 2022 American Contact Dermatitis Society Allergen of the Year
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16
Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16

Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
No time of the year is more exciting than the unveiling of the American Contact Dermatitis Society Allergen of the Year. Sometimes the selected allergen represents a completely novel cause of allergic contact dermatitis (ACD) with an unpronounceable chemical name. Not this time! The 2022 Allergen of the Year is likely to be lurking in your kitchen drawer at this very moment, as this year aluminum was chosen for this most prestigious honor.1 But do not throw out your aluminum foil just yet—aluminum allergy tends to be confined to specific scenarios. In this article, we highlight the growing recognition of aluminum contact allergy, particularly in the pediatric population, focusing on distinct presentations of aluminum ACD, unique sources of exposure, and nuances of patch testing to this metal.
Aluminum Is All Around Us
As the third most common element in the Earth’s crust, aluminum can be found quite literally everywhere.1 However, aluminum rarely is found in its pure elemental form; instead, it reacts with other elements around it, most commonly oxygen, to form aluminum-containing compounds. Known for their stability and safety, aluminum and its salts are incorporated in myriad products ranging from electronic equipment to foods and their packaging, medications, cosmetics, orthopedic and dental implants, and even tattoos. Aluminum also is found in the air and water supply and may even be encountered in certain workplaces, such as aircraft and machine industries. As such, contact with aluminum is all but certain in modern life.
The use of aluminum in consumer products is widely accepted as safe by public health agencies in the United States.2 Although there has been public concern that aluminum could be linked to development of breast cancer or Alzheimer disease, there is no clear evidence that these conditions are associated with routine aluminum exposure through ingestion or consumer products.3-5
Aluminum Contact Allergy
In part because of its ubiquity and in part because of the stability of aluminum-containing compounds, it was long thought that aluminum was nonallergenic. Contact allergy to elemental aluminum is rare; on the other hand, aluminum salts (the forms we are likely to encounter in daily life) are now recognized in the field of contact dermatitis as allergens of significance, particularly in the pediatric population.1,6
First reported as a possible occupational allergen in 1944,7 aluminum allergy came to prominence in the 1990s in association with vaccines. Aluminum is included in some vaccines as an adjuvant that bolsters the immune response8; the eTable lists currently available aluminum-containing vaccines in the United States; of note, none of the COVID-19 vaccines approved in the United States or Europe contain aluminum.11 Although the use of aluminum in vaccines is considered to be safe by the US Food and Drug Administration and Centers for Disease Control and Prevention,12,13 a small number of children become sensitized to aluminum through vaccines and may develop persistent pruritic subcutaneous nodules (also known as vaccination granulomas) at the injection site; however, the incidence of this adverse effect was less than 1% in large studies including as many as 76,000 children, suggesting that it is relatively rare.14,15 Upon patch testing, aluminum allergy has been detected in 77% to 95% of such cases.14 There is wide variation in the onset of the nodules ranging from weeks to years following vaccination.15 Due to pruritus, the examination may reveal accompanying excoriations, hyperpigmentation, and sometimes hypertrichosis at the injection site. Aluminum allergy related to vaccination also can manifest with widespread eruptions representing systemic contact dermatitis.16

Along with vaccines, the second major source of aluminum sensitization is allergen-specific immunotherapies administered by allergists/immunologists, many of which contain aluminum hydroxide.17,18
On the consumer product front, antiperspirants are the most common source of cutaneous exposure to aluminum. Aluminum complexes react with electrolytes in sweat to form plugs in eccrine ducts, thereby preventing sweat excretion.6 Allergic contact dermatitis to these products presents with axillary-vault dermatitis. There also have been reports of ACD to aluminum in sunscreen and toothpaste, with the latter implicated in causing systemic ACD.19,20
Prevalence of Sensitization to Aluminum
There have been a few large-scale studies evaluating rates of sensitization to aluminum in general patch-test patient populations; additionally, because of the complexities of testing this metal, investigators have utilized differing formulations for patch testing. A recent Swedish study found that 0.9% of 5448 adults and 5.1% of 196 children showed positive reactions to aluminum chloride hexahydrate (ACH) 10% in petrolatum and/or aluminum lactate 12% in petrolatum.21 Notably, there was a significant association between aluminum allergy and history of atopy for both adults (P=.0056) and children (P=.046), which remains to be further explored. A systematic review and meta-analysis found comparable rates of aluminum allergy in 0.4% of adults and 5.6% of children without vaccine granulomas who were tested.22 With this evidence in mind, it has been recommended by contact dermatitis experts that aluminum be included in pediatric baseline patch test series and also investigated for potential inclusion in baseline series for adults.1
Differential Diagnosis of Aluminum ACD
The differential diagnosis for subcutaneous nodules following vaccination is broad and includes various forms of panniculitis, sarcoidosis, foreign body reactions, vascular malformations, infections, and malignancies.23-25 The diagnosis may be obscured in cases with delayed onset. Biopsy is not mandatory to establish the diagnosis; although variable histopathologic findings have been reported, a common feature is histiocytes with abundant granular cytoplasm.26 It may be possible to demonstrate the presence of aluminum particles in tissue using electron microscopy and X-ray microanalysis.
For those patients who present with axillary-vault dermatitis, the differential includes ACD to more common allergens in antiperspirants (eg, fragrance), as well as other axillary dermatoses including inverse psoriasis, erythrasma, Hailey-Hailey disease, and various forms of intertrigo. Dermatitis localized to the axillary rim suggests textile allergy.
Patch Testing to Aluminum
Due to its physicochemical properties, patch testing for aluminum allergy is complicated, and historically there has been a lack of consensus on the ideal test formulation.1,27,28 At this time, it appears that the most sensitive formulation for patch testing to aluminum is ACH 10% in petrolatum.1 Some contact dermatitis experts recommend that children younger than 8 years should be tested with ACH 2% in petrolatum to minimize the risk of extreme patch test reactions.29,30 In some patients sensitized to aluminum, the use of aluminum patch test chambers has been noted to produce false-positive reactions, taking the form of multiple ring-shaped reactions to the chambers themselves or reactions to certain allergens whose chemical properties cause corrosion of the aluminum within the chambers.31-33 Therefore, when testing for suspected aluminum allergy, plastic chambers should be used; given the higher prevalence of aluminum allergy in children, some clinics routinely use plastic chambers for all pediatric patch testing.34 Importantly, elemental aluminum, including empty aluminum test chambers or aluminum foil, alone is not sufficient for patch testing as it lacks sensitivity.1 Additionally, nearly 20% of positive tests will be missed if a day 7 reading is not performed, making delayed reading a must in cases with high suspicion for aluminum allergy.21
Management of Aluminum Allergy
The development of pruritic subcutaneous nodules is uncomfortable for children and their guardians alike and may be associated with prolonged symptoms that negatively impact quality of life35,36; nonetheless, expert authorities have determined that the preventive benefits of childhood vaccination far outweigh any risk posed by the presence of aluminum in vaccines.12,13,37 Because aluminum-free formulations may not be available for all vaccines, it is essential to educate patients and families who may be at risk for developing vaccine hesitancy or avoidance.35,36,38 Given the hypothesis that epidermal dendritic cells mediate aluminum sensitization, it has been proposed that vaccine administration via deep intramuscular rather than subcutaneous injection may mitigate the risk, but more evidence is needed to support this approach.39,40 The good news is that the nodules tend to fade with age, with a median time to resolution of 18 to 49 months.14 In addition, patients may experience loss of sensitization to aluminum over time41; in one study, 77% of 241 children lost patch test reactivity when retested 5 to 9 years later.42 The exact reason for this diminishment of reactivity is not well understood. Adjunctive treatments to relieve symptoms of vaccine granulomas include topical and intralesional corticosteroids and antihistamines.
For patients reacting to aluminum in antiperspirants, there are many aluminum-free formulations on the market as well as recipes for homemade antiperspirants.6 On a case-by-case basis, patients may need to avoid aluminum-containing medications, permanent tattoos, and orthopedic or dental implants. To the best of our knowledge, there is no evidence suggesting a need to avoid aluminum in foods and their containers in routine daily life; although some patients report exacerbations of their symptoms associated with food-related aluminum exposures (eg, canned food, dried fruit) and improvement with dietary modification, further investigation is needed to confirm the relevance of these sources of contact.36,38 For patients who require allergen-specific immunotherapy, aluminum-free allergen extracts are available.6
Final Interpretation
Exposure to aluminum is ubiquitous; although relatively uncommon, awareness of the potential for ACD to aluminum is increasingly important, particularly in children. Given the prevalence of aluminum contact allergy, it has been recommended by contact dermatitis experts for inclusion in baseline pediatric patch test series.1 Although it is a complex issue, the development of ACD in a small proportion of children exposed to aluminum in vaccines does not outweigh the benefit of vaccination for almost all children. When conducting patch testing to aluminum, studies support testing to ACH 10% in petrolatum for adults, and consider reducing the concentration to ACH 2% for children.
Acknowledgment—The authors thank Ian Fritz, MD (South Portland, Maine), for his critical input during preparation of this article.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
- Bruze M, Netterlid E, Siemund I. Aluminum—Allergen of the Year 2022. Dermatitis. 2022;33:10-15.
- Toxicological profile for aluminum. Agency for Toxic Substances and Disease Registry website. Accessed June 22, 2022. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=191&tid=34
- Klotz K, Weistenhöfer W, Neff F, et al. The health effects of aluminum exposure. Dtsch Arztebl Int. 2017;114:653-659.
- Liszewski W, Zaidi AJ, Fournier E, et al. Review of aluminum, paraben, and sulfate product disclaimers on personal care products [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j. jaad.2021.06.840
- Van Dyke N, Yenugadhati N, Birkett NJ, et al. Association between aluminum in drinking water and incident Alzheimer’s disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165.
- Kullberg SA, Ward JM, Liou YL, et al. Cutaneous reactions to aluminum. Dermatitis. 2020;31:335-349.
- Hall AF. Occupational contact dermatitis among aircraft workers. J Am Med Assoc. 1944;125:179-185.
- HogenEsch H. Mechanism of immunopotentiation and safety of aluminum adjuvants. Front Immunol. 2012;3:406.
- Vaccine exipient summary. Centers for Disease Control and Prevention website. Published November 2021. Accessed June 22, 2022. https://www.cdc.gov/vaccines/pubs/pinkbook/downloads/appendices/b/excipient-table-2.pdf
- Vaccines licensed for use in the United States. US Food and Drug Administration website. Updated January 31, 2022. Accessed June 22, 2022. https://www.fda.gov/vaccines-blood-biologics/vaccines/vaccines-licensed-use-united-states
- Swenson A. US and EU COVID vaccines don’t contain aluminum. AP News. Published March 16, 2021. Accessed June 22, 2022. https://apnews.com/article/fact-checking-afs:Content:9991020426
- Adjuvants and vaccines. Centers for Disease Control and Prevention website. Updated August 4, 2020. Accessed June 22, 2022. https://www.cdc.gov/vaccinesafety/concerns/adjuvants.html
- Common ingredients in U.S. licensed vaccines. US Food and Drug Administration website. Updated April 19, 2019. Accessed June 22, 2002. https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/common-ingredients-us-licensed-vaccines
- Bergfors E, Hermansson G, Nyström Kronander U, et al. How common are long-lasting, intensely itching vaccination granulomas and contact allergy to aluminium induced by currently used pediatric vaccines? a prospective cohort study. Eur J Pediatr. 2014;173:1297-1307.
- Bergfors E, Trollfors B, Inerot A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine. 2003;22:64-69.
- Mistry BD, DeKoven JG. Widespread cutaneous eruption after aluminum-containing vaccination: a case report and review of current literature. Pediatr Dermatol. 2021;38:872-874.
- Netterlid E, Hindsén M, Björk J, et al. There is an association between contact allergy to aluminium and persistent subcutaneous nodules in children undergoing hyposensitization therapy. Contact Dermatitis. 2009;60:41-49.
- Netterlid E, Hindsén M, Siemund I, et al. Does allergen-specific immunotherapy induce contact allergy to aluminium? Acta Derm Venereol. 2013;93:50-56.
- Hoffmann SS, Elberling J, Thyssen JP, et al. Does aluminium in sunscreens cause dermatitis in children with aluminium contact allergy: a repeated open application test study. Contact Dermatitis. 2022;86:9-14.
- Veien NK, Hattel T, Laurberg G. Systemically aggravated contact dermatitis caused by aluminium in toothpaste. Contact Dermatitis. 1993;28:199-200.
- Siemund I, Dahlin J, Hindsén M, et al. Contact allergy to two aluminum salts in consecutively patch-tested dermatitis patients. Dermatitis. 2022;33:31-35.
- Hoffmann SS, Wennervaldt M, Alinaghi F, et al. Aluminium contact allergy without vaccination granulomas: a systematic review and metaanalysis. Contact Dermatitis. 2021;85:129-135.
- Bergfors E, Lundmark K, Kronander UN. Case report: a child with a long-standing, intensely itching subcutaneous nodule on a thigh: an uncommon (?) reaction to commonly used vaccines [published online January 13, 2013]. BMJ Case Rep. doi:10.1136/bcr-2012-007779
- Mooser G, Gall H, Weber L, et al. Cold panniculitis—an unusual differential diagnosis from aluminium allergy in a patient hyposensitized with aluminium-precipitated antigen extract. Contact Dermatitis. 2001;44:366-375.
- Mulholland D, Joyce EA, Foran A, et al. The evaluation of palpable thigh nodularity in vaccination-age children—differentiating vaccination granulomas from other causes. J Med Ultrasound. 2021;29:129.
- Chong H, Brady K, Metze D, et al. Persistent nodules at injection sites (aluminium granuloma)—clinicopathological study of 14 cases with a diverse range of histological reaction patterns. Histopathology. 2006;48:182-188.
- Nikpour S, Hedberg YS. Using chemical speciation modelling to discuss variations in patch test reactions to different aluminium and chromium salts. Contact Dermatitis. 2021;85:415-420.
- Siemund I, Zimerson E, Hindsén M, et al. Establishing aluminium contact allergy. Contact Dermatitis. 2012;67:162-170.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Bruze M, Mowitz M, Netterlid E, et al. Patch testing with aluminum chloride hexahydrate in petrolatum. Contact Dermatitis. 2020;83:176-177.
- Hedberg YS, Wei Z, Matura M. Quantification of aluminium release from Finn Chambers under different in vitro test conditions of relevance for patch testing. Contact Dermatitis. 2020;83:380-386.
- King N, Moffitt D. Allergic contact dermatitis secondary to the use of aluminium Finn Chambers®. Contact Dermatitis. 2018;78:365-366.
- Rosholm Comstedt L, Dahlin J, Bruze M, et al. Patch testing with aluminium Finn Chambers could give false-positive reactions in patients with contact allergy to aluminium. Contact Dermatitis. 2021;85:407-414.
- Tran JM, Atwater AR, Reeder M. Patch testing in children: not just little adults. Cutis. 2019;104:288-290.
- Bergfors E, Trollfors B. Sixty-four children with persistent itching nodules and contact allergy to aluminium after vaccination with aluminium-adsorbed vaccines-prognosis and outcome after booster vaccination. Eur J Pediatr. 2013;172:171-177.
- Hoffmann SS, Thyssen JP, Elberling J, et al. Children with vaccination granulomas and aluminum contact allergy: evaluation of predispositions, avoidance behavior, and quality of life. Contact Dermatitis. 2020;83:99-107.
- Löffler P. Review: vaccine myth-buster-cleaning up with prejudices and dangerous misinformation [published online June 10, 2021]. Front Immunol. doi:10.3389/fimmu.2021.663280
- Salik E, Løvik I, Andersen KE, et al. Persistent skin reactions and aluminium hypersensitivity induced by childhood vaccines. Acta Derm Venereol. 2016;96:967-971.
- Beveridge MG, Polcari IC, Burns JL, et al. Local vaccine site reactions and contact allergy to aluminum. Pediatr Dermatol. 2012; 29:68-72.
- Frederiksen MS, Tofte H. Immunisation with aluminium-containing vaccine of a child with itching nodule following previous vaccination. Vaccine. 2004;23:1-2.
- Siemund I, Mowitz M, Zimerson E, et al. Variation in aluminium patch test reactivity over time. Contact Dermatitis. 2017;77:288-296.
- Lidholm AG, Bergfors E, Inerot A, et al. Unexpected loss of contact allergy to aluminium induced by vaccine. Contact Dermatitis. 2013;68:286.
Practice Points
- Aluminum is an allergen of significance relating to its use in vaccines, immunotherapies, and antiperspirants.
- There is a greater prevalence of aluminum contact allergy in children than in adults, affecting up to 5% of the pediatric patch-test population.
- The recommended patch test formulation is aluminum chloride hexahydrate 10% in petrolatum, with consideration of reducing the concentration to 2% in children younger than 8 years to avoid strong reactions.