User login
Adapting to Changes in Acne Management: Take One Step at a Time
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
After most dermatology residents graduate from their programs, they go out into practice and will often carry with them what they learned from their teachers, especially clinicians. Everyone else in their dermatology residency programs approaches disease management and the use of different therapies in the same way, right?
It does not take very long before these same dermatology residents realize that things are different in real-world clinical practice in many ways. Most clinicians develop a range of fairly predictable patterns in how they approach and treat common skin disorders such as acne, rosacea, psoriasis, atopic dermatitis/eczema, and seborrheic dermatitis. These patterns often include what testing is performed at baseline and at follow-up.
Recently, I have been giving thought to how clinicians—myself included—change their approaches to management of specific skin diseases over time, especially as new information and therapies emerge. Are we fast adopters, or are we slow adopters? How much evidence do we need to see before we consider adjusting our approach? Is the needle moving too fast or not fast enough?
I would like to use an example that relates to acne treatment, especially as this is one of the most common skin disorders encountered in outpatient dermatologic practice. Despite lack of US Food and Drug Administration (FDA) approval for use in acne, oral spironolactone commonly is used in females, especially adults, with acne vulgaris and has a long history as an acceptable approach in dermatology.1 Because spironolactone is a potassium-sparing diuretic, one question that commonly arises is: Do we monitor serum potassium levels at baseline and periodically during treatment with spironolactone? There has never been a definitive consensus on which approach to take. However, there has been evidence to suggest that such monitoring is not necessary in young healthy women due to a negligible risk for clinically relevant hyperkalemia.2,3
In fact, the suggestion that there is a very low risk for clinically significant hyperkalemia in healthy young women treated with spironolactone is accurate based on population-based studies. Nevertheless, the clinician is faced with confirming the patient is in fact healthy rather than assuming this is the case due to her “young” age. In addition, it is important to exclude potential drug-drug interactions that can increase the risk for hyperkalemia when coadministered with spironolactone and also to exclude an unknown underlying decrease in renal function.1 At the end of the day, I support the continued research that is being done to evaluate questions that can challenge the recycled dogma on how we manage patients, and I do not fault those who follow what they believe to be new cogent evidence. However, in the case of oral spironolactone use, I also could never fault a clinician for monitoring renal function and electrolytes including serum potassium levels in a female patient treated for acne, especially with a drug that has the known potential to cause hyperkalemia in certain clinical situations and is not FDA approved for the indication of acne (ie, the guidance that accompanies the level of investigation needed for such FDA approval is missing). The clinical judgment of the clinician who is responsible for the individual patient trumps the results from population-based studies completed thus far. Ultimately, it is the responsibility of that clinician to assure the safety of their patient in a manner that they are comfortable with.
It takes time to make changes in our approaches to patient management, and in the majority of cases, that is rightfully so. There are several potential limitations to how certain data are collected, and a reasonable verification of results over time is what tends to change behavior patterns. Ultimately, the common goal is to do what is in the best interest of our patients. No one can argue successfully against that.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
- Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
- Plovanich M, Weng QY, Arash Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
- Barbieri JS, Margolis DJ, Mostaghimi A. Temporal trends and clinician variability in potassium monitoring of healthy young women treated for acne with spironolactone. JAMA Dermatol. 2021;157:296-300.
Administrative Burden of iPLEDGE Deters Isotretinoin Prescriptions: Results From a Survey of Dermatologists
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.
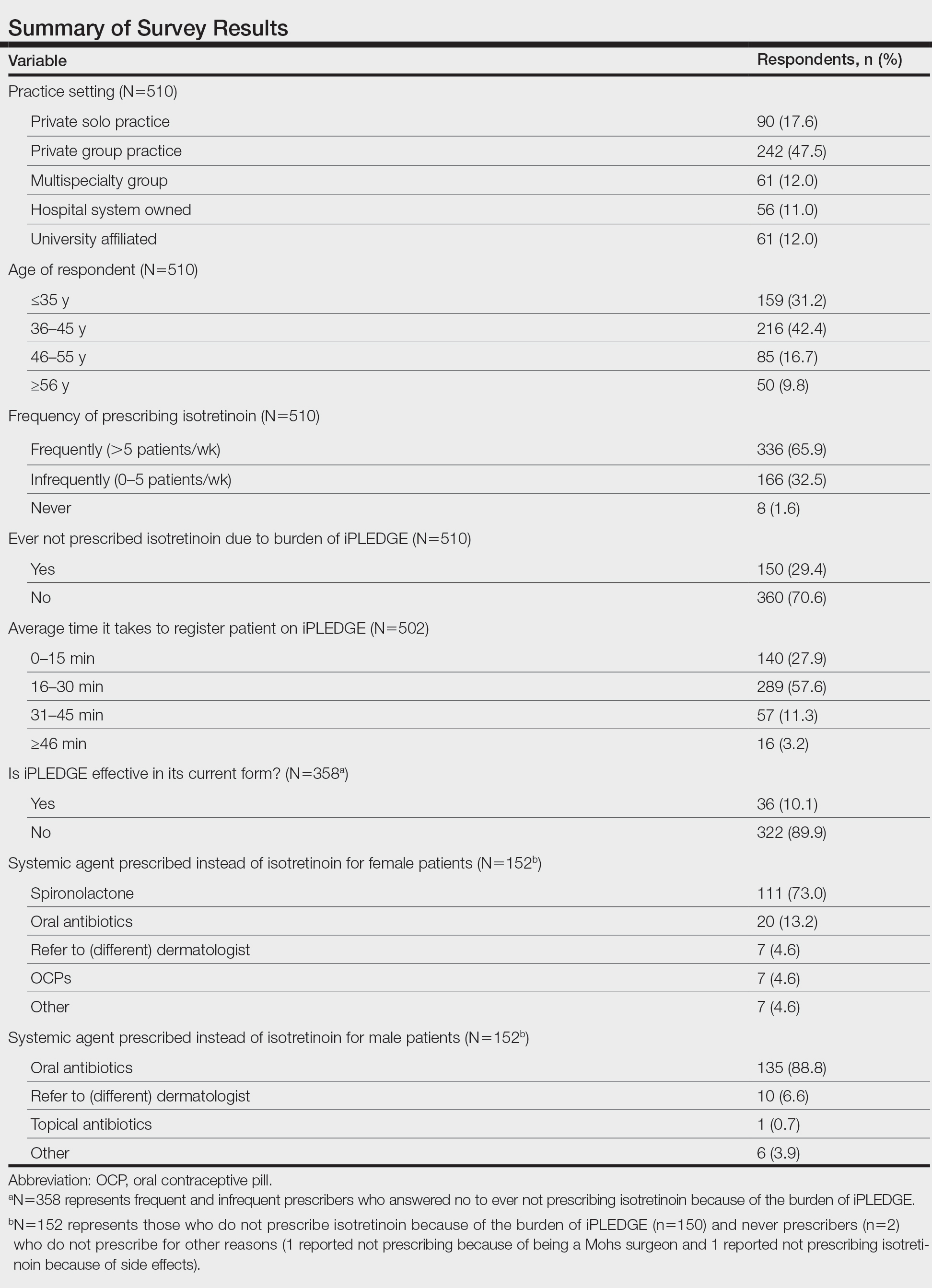
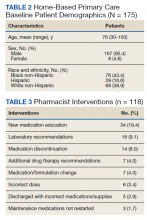
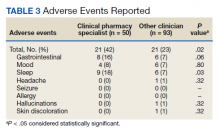
Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
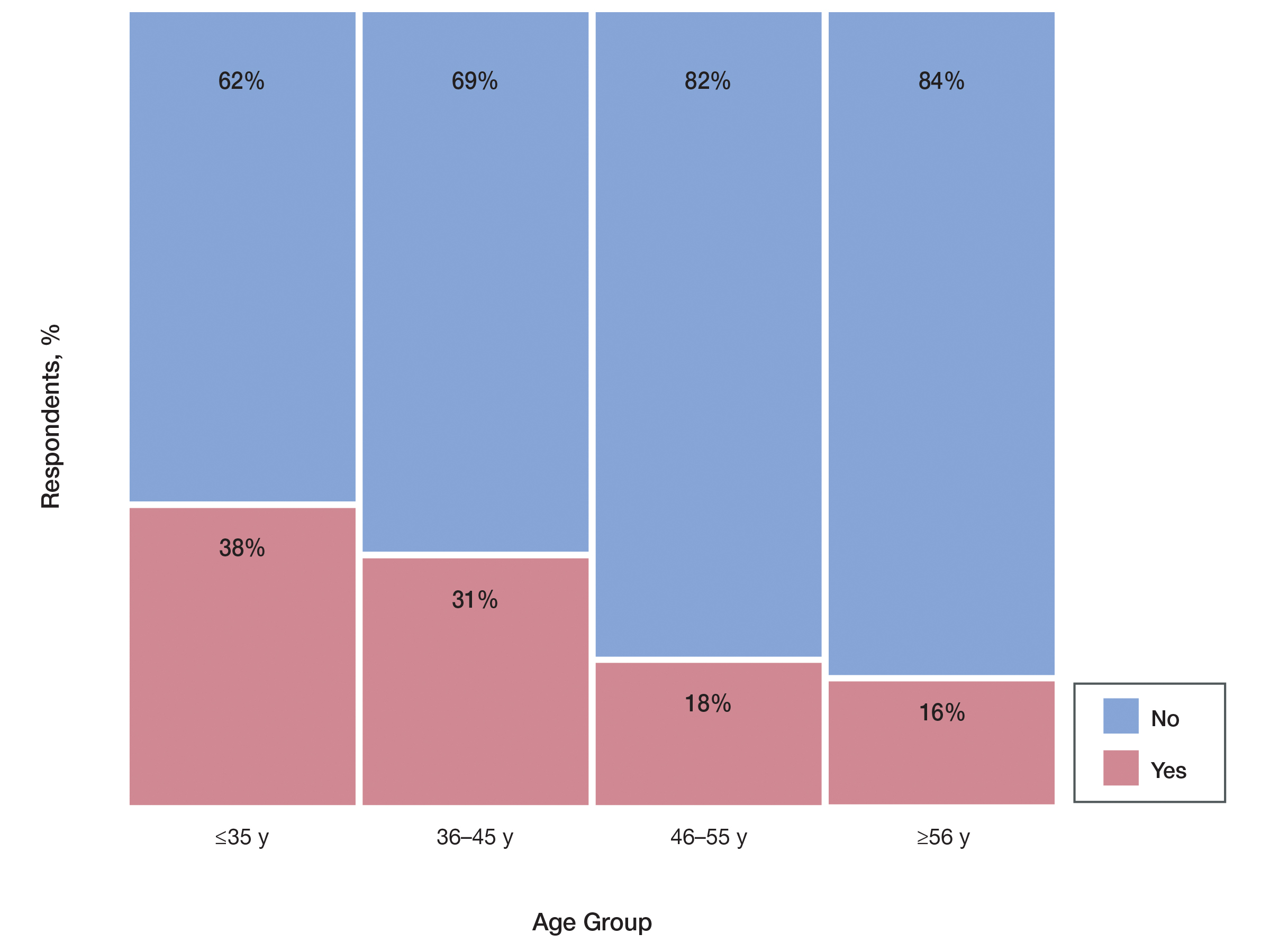
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.
Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.

Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.

Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
Isotretinoin is the most effective treatment of recalcitrant acne, but because of its teratogenicity and potential association with psychiatric adverse effects, it has been heavily regulated by the US Food and Drug Administration (FDA) through the iPLEDGE program since 2006.1,2 To manage the risk of teratogenicity associated with isotretinoin, various pregnancy prevention programs have been developed, but none of these programs have demonstrated a zero fetal exposure rate. The FDA reported 122 isotretinoin-exposed pregnancies during the first year iPLEDGE was implemented, which was a slight increase from the 120 pregnancies reported the year after the implementation of the System to Manage Accutane-Related Teratogenicity program, iPLEDGE’s predecessor.3 The iPLEDGE program requires registration of all wholesalers distributing isotretinoin, all health care providers prescribing isotretinoin, all pharmacies dispensing isotretinoin, and all female and male patients prescribed isotretinoin to create a verifiable link that only enables patients who have met all criteria to pick up their prescriptions. For patients of reproductive potential, there are additional qualification criteria and monthly requirements; before receiving their prescription every month, patients of reproductive potential must undergo a urine or serum pregnancy test with negative results, and patients must be counseled by prescribers regarding the risks of the drug and verify they are using 2 methods of contraception (or practicing abstinence) each month before completing online questions that test their understanding of the drug’s side effects and their chosen methods of contraception.4 These requirements place burdens on both patients and prescribers. Studies have shown that in the 2 years after the implementation of iPLEDGE, there was a 29% decrease in isotretinoin prescriptions.1-3
We conducted a survey study to see if clinicians chose not to prescribe isotretinoin to appropriate candidates specifically because of the administrative burden of iPLEDGE. Secondarily, we investigated the medications these clinicians would prescribe instead of isotretinoin.
Methods
In March 2020, we administered an anonymous online survey consisting of 12 multiple-choice questions to verified board-certified dermatologists in the United States using a social media group. The University of Rochester’s (Rochester, New York) institutional review board determined that our protocol met criteria for exemption (IRB STUDY00004693).
Statistical Analysis—Primary analyses used Pearson χ2 tests to identify significant differences among respondent groups, practice settings, age of respondents, and time spent registering patients for iPLEDGE.
Results
Survey results from 510 respondents are summarized in the Table.

Burden of iPLEDGE—Of the respondents, 336 (65.9%) were frequent prescribers of isotretinoin, 166 (32.5%) were infrequent prescribers, and 8 (1.6%) were never prescribers. Significantly more isotretinoin prescribers estimated that their offices spend 16 to 30 minutes registering a new isotretinoin patient with the iPLEDGE program (289 [57.6%]) compared with 0 to 15 minutes (140 [27.9%]), 31 to 45 minutes (57 [11.3%]), and morethan 45 minutes (16 [3.2%])(χ23=22.09, P<.0001). Furthermore, 150 dermatologists reported sometimes not prescribing, and 2 reported never prescribing isotretinoin because of the burden of iPLEDGE.
Systemic Agents Prescribed Instead of Isotretinoin—Of the respondents, 73.0% (n=111) prescribed spironolactone to female patients and 88.8% (n=135) prescribed oral antibiotics to male patients instead of isotretinoin. Spironolactone typically is not prescribed to male patients with acne because of its feminizing side effects, such as gynecomastia.5 According to the American Academy of Dermatology guidelines on acne, systemic antibiotic usage should be limited to the shortest possible duration (ie, less than 3–4 months) because of potential bacterial resistance and reported associations with inflammatory bowel disease, Clostridium difficile infection, and candidiasis.6,7
Prescriber Demographics—The frequency of not prescribing isotretinoin did not vary by practice setting (χ 24=6.44, P=.1689) but did vary by age of the dermatologist (χ23=15.57, P=.0014). Dermatologists younger than 46 years were more likely (Figure) to report not prescribing isotretinoin because of the administrative burden of iPLEDGE. We speculate that this is because younger dermatologists are less established in their practices and therefore may have less support to complete registration without interruption of clinic workflow.

Comment
The results of our survey suggest that the administrative burden of iPLEDGE may be compelling prescribers to refrain from prescribing isotretinoin therapy to appropriate candidates when it would otherwise be the drug of choice.
Recent Changes to iPLEDGE—The FDA recently approved a modification to the iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) program based on the advocacy efforts from the American Academy of Dermatology. Starting December 13, 2021, the 3 patient risk categories were consolidated into 2 gender-neutral categories: patients who can get pregnant and patients who cannot get pregnant.8 The iPLEDGE website was transitioned to a new system, and all iPLEDGE REMS users had to update their iPLEDGE accounts. After the implementation of the modified program, user access issues arose, leading to delayed treatment when patients, providers, and pharmacists were all locked out of the online system; users also experienced long hold times with the call center.8 This change highlights the ongoing critical need for a streamlined program that increases patient access to isotretinoin while maintaining safety.
Study Limitations—The main limitation of this study was the inability to calculate a true response rate to our survey. We distributed the survey via social media to maintain anonymity of the respondents. We could not track how many saw the link to compare with the number of respondents. Therefore, the only way we could calculate a response rate was with the total number of members in the group, which fluctuated around 4000 at the time we administered the survey. We calculated that we would need at least 351 responses to have a 5% margin of error at 95% confidence for our results to be generalizable and significant. We ultimately received 510 responses, which gave us a 4.05% margin of error at 95% confidence and an estimated 12.7% response rate. Since some members of the group are not active and did not see the survey link, our true response rate was likely higher. Therefore, we concluded that the survey was successful, and our significant responses were representative of US dermatologists.
Suggestions to Improve iPLEDGE Process—Our survey study should facilitate further discussions on the importance of simplifying iPLEDGE. One suggestion for improving iPLEDGE is to remove the initial registration month so care is not delayed. Currently, a patient who can get pregnant must be on 2 forms of contraception for 30 days after they register as a patient before they are eligible to fill their prescription.4 This process is unnecessarily long and arduous and could be eliminated as long as the patient has already been on an effective form of contraception and has a negative pregnancy test on the day of registration. The need to repeat contraception comprehension questions monthly is redundant and also could be removed. Another suggestion is to remove the category of patients who cannot become pregnant from the system entirely. Isotretinoin does not appear to be associated with adverse psychiatric effects as shown through the systematic review and meta-analysis of numerous studies.9 If anything, the treatment of acne with isotretinoin appears to mitigate depressive symptoms. The iPLEDGE program does not manage this largely debunked idea. Because the program’s sole goal is to manage the risk of isotretinoin’s teratogenicity, the category of those who cannot become pregnant should not be included.
Conclusion
This survey highlights the burdens of iPLEDGE for dermatologists and the need for a more streamlined risk management program. The burden was felt equally among all practice types but especially by younger dermatologists (<46 years). This time-consuming program is deterring some dermatologists from prescribing isotretinoin and ultimately limiting patient access to an effective medication.
Acknowledgment—The authors thank all of the responding clinicians who provided insight into the impact of iPLEDGE on their isotretinoin prescribing patterns.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
- Prevost N, English JC. Isotretinoin: update on controversial issues. J Pediatr Adolesc Gynecol. 2013;26:290-293.
- Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin. JAMA Dermatol. 2019;155:1175-1179.
- Shin J, Cheetham TC, Wong L, et al. The impact of the IPLEDGE program on isotretinoin fetal exposure in an integrated health care system. J Am Acad Dermatol. 2011;65:1117-1125.
- iPLEDGE Program. About iPLEDGE. Accessed June 13, 2022. https://ipledgeprogram.com/#Main/AboutiPledge
- Marson JW, Baldwin HE. An overview of acne therapy, part 2: hormonal therapy and isotretinoin. Dermatol Clin. 2019;37:195-203.
- Margolis DJ, Fanelli M, Hoffstad O, et al. Potential association between the oral tetracycline class of antimicrobials used to treat acne and inflammatory bowel disease. Am J Gastroenterol. 2010;105:2610-2616.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e33.
- iPLEDGE Risk Evaluation and Mitigation Strategy (REMS). Updated January 14, 2022. Accessed June 13, 2022. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
- Huang YC, Cheng YC. Isotretinoin treatment for acne and risk of depression: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;76:1068-1076.e9.
Practice Points
- Of clinicians who regularly prescribe isotretinoin, approximately 30% have at times chosen not to prescribe isotretinoin to patients with severe acne because of the burden of the iPLEDGE program.
- The US Food and Drug Administration should consider further streamlining the iPLEDGE program, as it is causing physician burden and therefore suboptimal treatment plans for patients.
Simple Intraoperative Technique to Improve Wound Edge Approximation for Residents
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
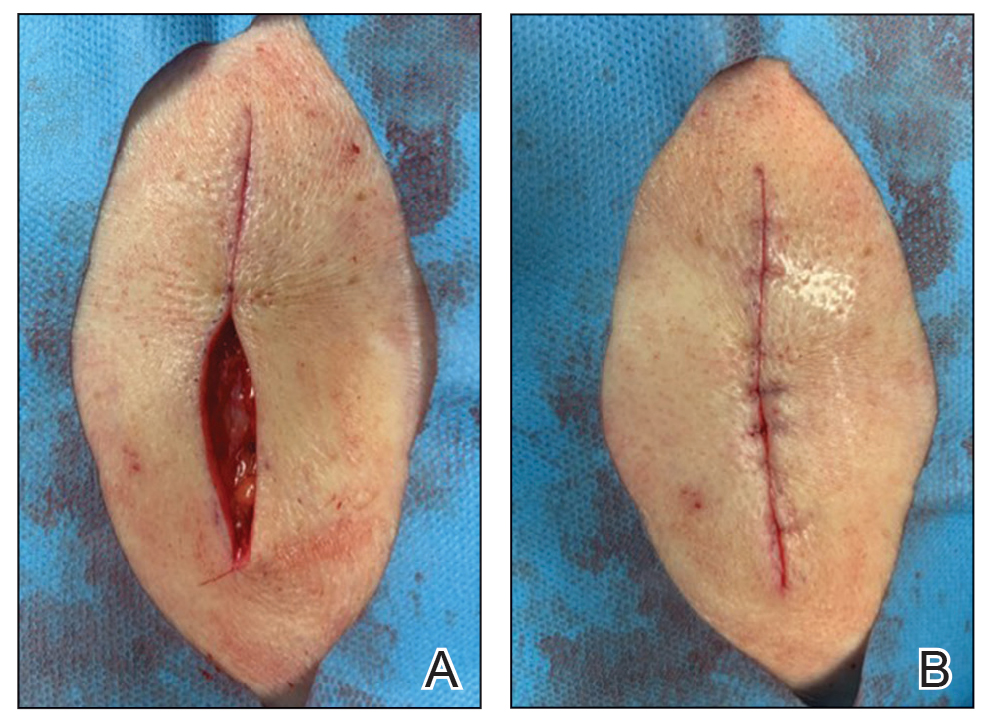
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.
Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.

Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
Practice Gap
Dermatology residents can struggle with surgical closure early in their training years. Although experienced dermatologic surgeons may intuitively be able to align edges for maximal cosmesis, doing so can prove challenging in the context of learning basic surgical techniques for early residents.
Furthermore, local anesthesia can distort cutaneous anatomy and surgical landmarks, requiring the surgeon to reexamine their closure technique. Patients may require position changes or may make involuntary movements, both of which require dynamic thinking and planning on the part of the dermatologic surgeon to achieve optimal outcomes.
The Technique
We propose the use of sutures to intraoperatively guide placement of the dermal needle. This technique can be used for various closure types; here, we demonstrate its use in a standard elliptical excision.
To begin, a standard length to width ellipse ratio of 3:1 is drawn with appropriate margins around a neoplasm.1 After excision and appropriate undermining of the ellipse, we typically use deep sutures to close the deep space. The first pass of the needle through tissue can be performed in a place of the surgeon’s preference but typically abides by the rule of halves or the zipper method (Figure 1A). To determine optimal placement of the second needle pass through tissue, we recommend applying gentle opposing traction forces to the wound apices to approximate the linear outcome of the wound edges. The surgeon can use a skin hook to guide placement of the needle to the contralateral wound edge in an unassisted method of this technique (Figure 1B). The surgeon’s assistant also can aid in applying cutaneous traction along the length of the excision if the surgeon wishes to free their hands (Figure 1C). Because the risk of needlestick injury at this step is small, it is prudent for the surgeon to advise the assistant to avoid needlestick injury by keeping their hands away from the needle path in the surgical site.
Although traction is being applied to the wound apices, the deep suture should extend across the wound with just enough pressure to leave a serosanguineous notched mark in the contralateral tissue edge (Figure 1D). After releasing traction on the wound edges, the surgeon can effortlessly visualize the target for needle placement and make a throw through the tissue accordingly.

This process can be continued until wound closure is complete (Figure 2). Top sutures or adhesive strips can be placed afterward for completing approximation of the wound edges superficially.

Practice Implications
By using this technique to align wound edges intraoperatively, the surgeon can have a functional guide for needle placement. The technique allows improvement of function and cosmesis of surgical wounds, while also accounting for topographical variations in the patient’s surgical site. Approximation of the wound edges is particularly important at the beginning of closure, as the wound edges align and approximate more with each subsequent stitch, with decreasing tension.2
In addition, when operating on a curvilinear or challenging topographical surface of the body, this technique can provide a clear template for guiding suture placement for approximating wound edges. Furthermore, local biodynamic anatomy might become distorted after excision of the tissue specimen due to release of centripetal tangential forces that were present in the pre-excised skin.1 Local change in biodynamic forces may be difficult to plan for preoperatively using other techniques.3
Although this technique can be utilized for all suture placements in closure, it is of greatest value when placing the first few sutures and when operating on nonplanar surfaces that might become distorted after excision. To ensure the best outcome, it is important to be certain that the area has been properly cleaned prior to surgery and a sterile technique is used.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
- Paul SP. Biodynamic excisional skin tension lines for excisional surgery of the lower limb and the technique of using parallel relaxing incisions to further reduce wound tension. Plast Reconstr Surg Glob Open. 2017;5:E1614. doi:10.1097/GOX.0000000000001614
- Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402. doi:10.1016/j.jaad.2014.08.006
- Parikh SA, Sloan B. Clinical pearl: a simple and effective technique for improving surgical closures for the early-learning resident. Cutis. 2017;100:338-339.
Itchy Vesicular Rash
The Diagnosis: Tinea Corporis Bullosa
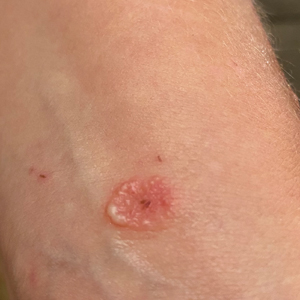
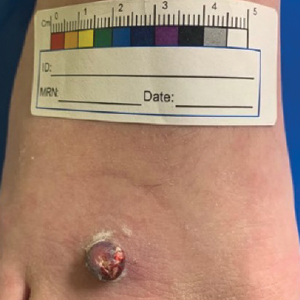
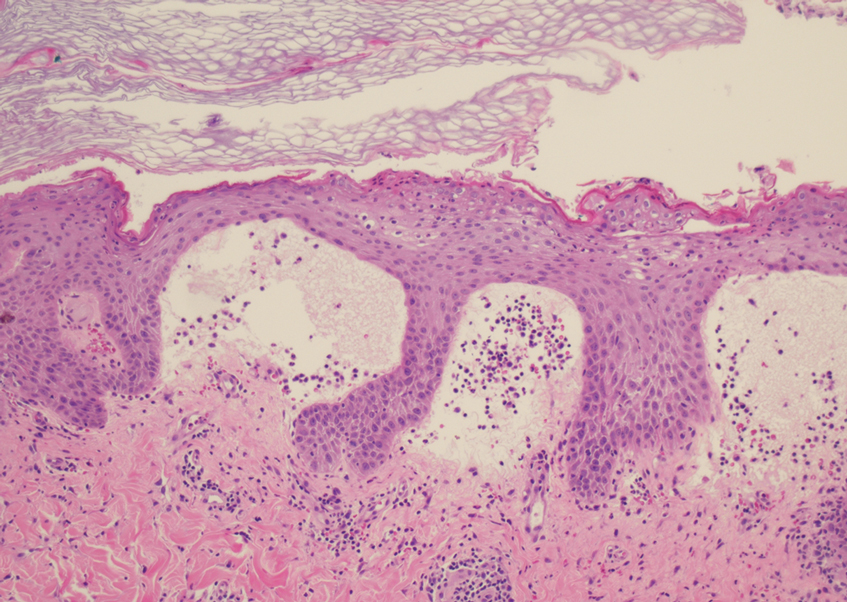
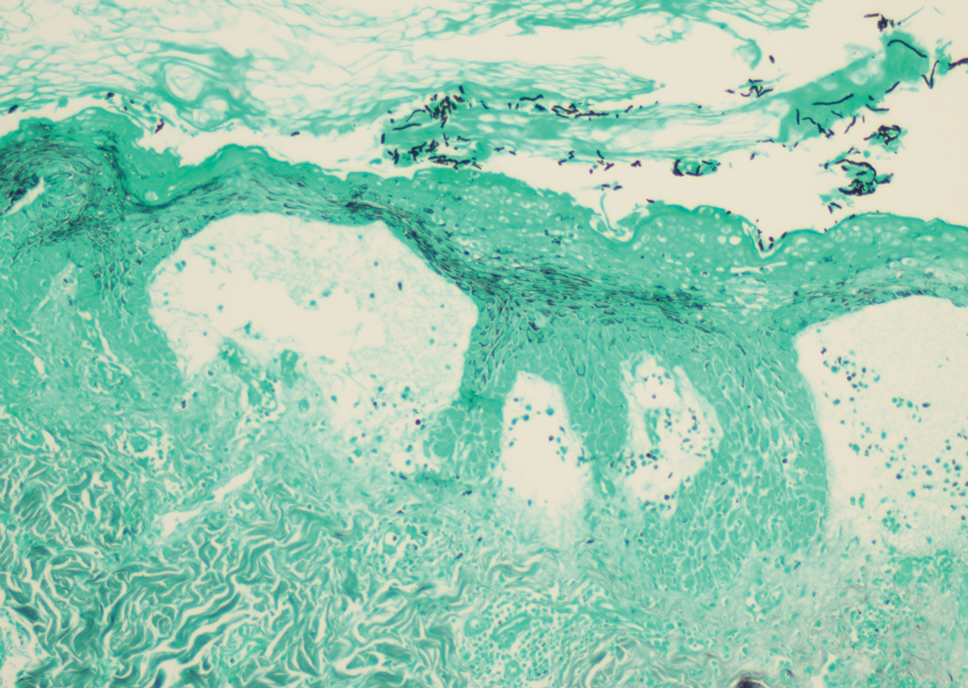
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.
Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1
Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
The Diagnosis: Tinea Corporis Bullosa
At the time of presentation, a potassium hydroxide (KOH) preparation, fungal culture, and punch biopsy of the right ventral wrist was performed. The KOH preparation was positive for fungal hyphae characteristic of dermatophyte infections. Histologically, the biopsy showed intraepidermal and subepidermal blisters with neutrophil- and lymphocyte-rich contents (Figure 1). Fungal hyphae and spores were present within the stratum corneum and superficial epidermis (Figure 2), and fungal cultures grew Microsporum canis. The extent of the rash (upper and lower extremities, chest, and back), positive fungal culture, and KOH preparation all supported the diagnosis of tinea corporis bullosa, which was confirmed with biopsy. Oral prednisone use was discouraged and triamcinolone ointment was discontinued given that inappropriate treatment with steroids in the setting of fungal infection suppresses an inflammatory response and alters clinical appearance, obviating the persistent underlying infection.

Tinea corporis bullosa is a rare superficial dermatophyte fungal infection that often is acquired by close personto- person contact or contact with domestic animals. The infection begins as a circular pruritic plaque, generally with raised borders, which may be erythematous or hyperpigmented. By definition, tinea corporis occurs in sites other than the face, feet, hands, or groin area. Bullae formation is thought to be secondary to a delayed hypersensitivity reaction provoked by the presence of a dermatophyte antigen.1

Linear IgA bullous dermatosis is an immunemediated disease characterized by IgA deposition at the dermoepidermal junction. Linear IgA bullous dermatosis classically presents as widespread tense vesicles in an arciform or annular pattern. Mucosal involvement is common and typically presents with erosions, ulcerations, and scarring.2 Given the absence of mucosal involvement in our patient and a positive KOH preparation, linear IgA bullous dermatosis was an unlikely diagnosis.
Benign inoculation lymphoreticulosis, more commonly known as cat scratch disease (CSD), is a Bartonella henselae infection that results from a cat scratch or bite. Cat scratch disease can present as localized cutaneous and nodal involvement (lymphadenopathy) near the site of inoculation, or it may present as disseminated disease. Cutaneous lesions generally progress through vesicular, erythematous, and papular phases. Regional lymphadenopathy proximal to the inoculation site is the hallmark of CSD.3 Given the absence of lymphadenopathy in our patient as well as the sporadic distribution of lesions, CSD was an unlikely diagnosis.
Dermatitis herpetiformis (DH) is an autoimmune disorder with cutaneous manifestations of gluten sensitivity. Dermatitis herpetiformis presents as extremely pruritic papules and vesicles arranged in groups on areas such as the elbows, dorsal aspects of the forearms, knees, scalp, back, and buttocks. Most patients with DH have celiac disease or small bowel disease related to gluten sensitivity.4 Given our patient’s acute presentation in adulthood and lack of gluten sensitivity, DH was an unlikely diagnosis.
Bullous fixed drug reaction is a cutaneous eruption that typically presents in the setting of exposure to an offending drug/agent. Drug reactions can have various cutaneous presentations, with the most common being pigmented macules that progress into plaques.5 Given the isolated nature of our patient’s episode and apparent lack of association with medication, bullous fixed drug reaction was an unlikely diagnosis.
Tinea corporis bullosa is a rare clinical variant of tinea corporis that has only been reported in patients with a history of contact with different animals. There are many causative organisms related to tinea corporis; Trichophyton rubrum is the most common etiology of tinea corporis, while tinea corporis due to close contact with domesticated animals often is caused by M canis.6 The immunoinhibitory properties of the mannans in the fungal cell wall allow the organisms to adhere to the skin prior to invasion. Cutaneous invasion into dead cornified layers of the skin is credited to the proteases, subtilisinlike proteases (subtilases), and keratinases produced by the fungus.1 There are many different clinical presentations of tinea corporis due to the variability of causative organisms. An annular (ring-shaped) lesion with a central plaque and advancing border is the most typical presentation. Tinea corporis bullosa is characterized by the presence of bullae or vesicles in the borders of the scaly plaque. Rupture of the bullae subsequently leads to erosions and crusts over the plaque.
The diagnosis of tinea corporis bullosa often is clinical if the lesion is typical and can be confirmed using KOH preparation and fungal culture. Once the diagnosis is confirmed, topical antifungals are the standard treatment approach for localized superficial tinea corporis. Systemic antifungal treatment can be initiated if the lesion is extensive, recurrent, chronic, or unresponsive to topical treatment.1 Given our patient’s characteristic presentation, she was managed with an over-the-counter topical antifungal (terbinafine). The patient’s lesions dramatically improved, rendering oral therapy unnecessary. At 1-month follow-up, the rash had nearly resolved.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
- Leung AK, Lam JM, Leong KF, et al. Tinea corporis: an updated review [published online July 20, 2020]. Drugs Context. doi:10.7573/dic.2020-5-6
- Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol. 2001;19:719-727.
- Lamps LW, Scott MA. Cat-scratch disease: historic, clinical, and pathologic perspectives. Pathology Patterns Reviews. 2004;121(suppl):S71-S80.
- Caproni M, Antiga E, Melani L, et al. Guidelines for the diagnosis and treatment of dermatitis herpetiformis. J Eur Acad Dermatol Venereol. 2009;23:633-638.
- Patel S, John AM, Handler MZ, et al. Fixed drug eruptions: an update, emphasizing the potentially lethal generalized bullous fixed drug eruption. Am J Clin Dermatol. 2020;21:393-399.
- Ziemer M, Seyfarth F, Elsner P, et al. Atypical manifestations of tinea corporis. Mycoses. 2007;50:31-35.
A 38-year-old woman presented with a rash of 5 days’ duration that initially appeared on the wrists after playing with her kitten, with subsequent involvement of the chest, back, abdomen, and upper and lower extremities. Physical examination revealed multiple annular plaques with raised erythematous borders, rare peripheral vesicles, and superficial central scaling. Extreme pruritus accompanied the plaques, both of which developed after playing with her kitten. The patient noted that all lesions on the upper extremities evolved in areas subject to deep puncture while more superficially excoriated areas were unaffected. She denied any other prior skin conditions and had received a 5-day course of azithromycin without improvement prior to presentation; triamcinolone ointment 0.1% had provided only temporary relief. Primary care providers prescribed a short course of oral prednisone; however, she did not start it prior to presentation.
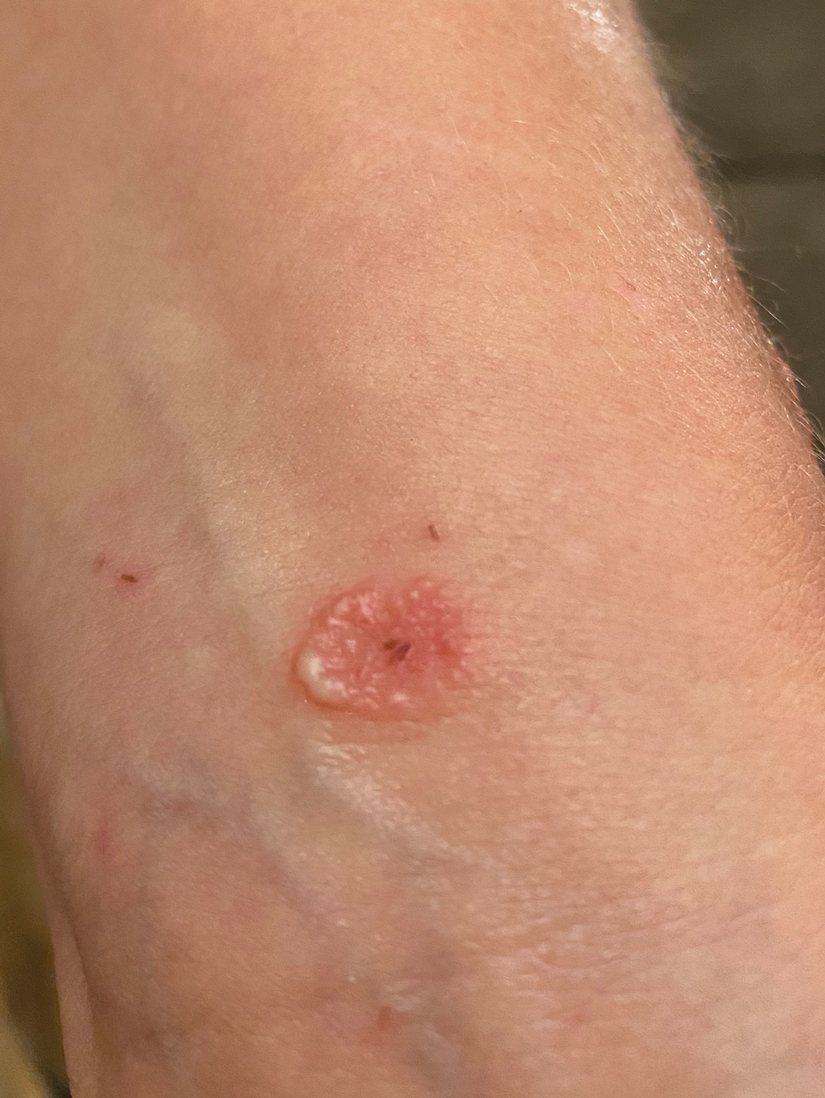
Monkeypox: What FPs need to know, now
The Centers for Disease Control and Prevention (CDC) and the World Health Organization are investigating an outbreak of monkeypox cases that have occurred around the world in countries that do not have endemic monkeypox virus.1,2 As of July 5, there have been 6924 cases documented in 52 countries, including 560 cases that have occurred in the United States.2 In the United States, as well as globally, a large proportion of cases have been in men who have sex with men.
First, what is monkeypox? Monkeypox is an orthopox virus that is closely related to variola (smallpox) and vaccinia (the virus used in the smallpox vaccine). It is endemic in western and central Africa and is contracted by contact with an infected mammal (including humans). Transmission can occur through direct contact with infected body fluids or lesions, via infectious fomites, or through respiratory secretions (although this usually requires prolonged exposure).
What is the disease course? The incubation period is 4 to 17 days. The initial symptoms include fever, malaise, headache, sore throat, and lymphadenopathy. A rash erupts 1 to 4 days after the prodrome and progresses synchronously from macules to papules to vesicles and then to pustules, which eventually scab over and fall off. In some cases reported in the United States, the rash started in the groin and genital area.
Don’t be fooled by other exanthems. Monkeypox can be confused with chickenpox and molluscum contagiosum (MC). However, the lesions in chickenpox appear asynchronously (all 4 stages present at the same time) and the papules of MC contain a central pit.
Can monkeypox be prevented? There are currently 2 vaccines against orthopox viruses: ACAM2000 and Jynneos. Currently, these vaccines are routinely recommended only for those at occupational risk of orthopox exposure.3
What you should know—and do. Be alert for any patient who presents with a suspicious rash; if there is a possibility of monkeypox, the local public health department should be contacted. They will investigate and collect samples for laboratory testing and will elicit contact names and locations. If monkeypox is confirmed, they may offer close contacts 1 of the 2 vaccines, which if administered within 4 days of exposure can prevent infection.
Advise all patients confirmed to have monkeypox to self-isolate until all skin lesions have healed. Good infection control practices in the clinical setting will prevent spread to staff and other patients.
More information about monkeypox, including images of typical lesions—as well as an update on the current investigation in the United States and worldwide—can be found on the CDC website.4
1. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:764-769. doi: http://dx.doi.org/10.15585/mmwr.mm7123e1
2. CDC. US monkeypox outbreak 2022: situation summary. Updated June 29, 2022. Accessed July 5, 2022.
3. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
4. CDC. 2022 monkeypox: information for healthcare professionals. Updated June 23, 2022. Accessed July 5, 2022.
The Centers for Disease Control and Prevention (CDC) and the World Health Organization are investigating an outbreak of monkeypox cases that have occurred around the world in countries that do not have endemic monkeypox virus.1,2 As of July 5, there have been 6924 cases documented in 52 countries, including 560 cases that have occurred in the United States.2 In the United States, as well as globally, a large proportion of cases have been in men who have sex with men.
First, what is monkeypox? Monkeypox is an orthopox virus that is closely related to variola (smallpox) and vaccinia (the virus used in the smallpox vaccine). It is endemic in western and central Africa and is contracted by contact with an infected mammal (including humans). Transmission can occur through direct contact with infected body fluids or lesions, via infectious fomites, or through respiratory secretions (although this usually requires prolonged exposure).
What is the disease course? The incubation period is 4 to 17 days. The initial symptoms include fever, malaise, headache, sore throat, and lymphadenopathy. A rash erupts 1 to 4 days after the prodrome and progresses synchronously from macules to papules to vesicles and then to pustules, which eventually scab over and fall off. In some cases reported in the United States, the rash started in the groin and genital area.
Don’t be fooled by other exanthems. Monkeypox can be confused with chickenpox and molluscum contagiosum (MC). However, the lesions in chickenpox appear asynchronously (all 4 stages present at the same time) and the papules of MC contain a central pit.
Can monkeypox be prevented? There are currently 2 vaccines against orthopox viruses: ACAM2000 and Jynneos. Currently, these vaccines are routinely recommended only for those at occupational risk of orthopox exposure.3
What you should know—and do. Be alert for any patient who presents with a suspicious rash; if there is a possibility of monkeypox, the local public health department should be contacted. They will investigate and collect samples for laboratory testing and will elicit contact names and locations. If monkeypox is confirmed, they may offer close contacts 1 of the 2 vaccines, which if administered within 4 days of exposure can prevent infection.
Advise all patients confirmed to have monkeypox to self-isolate until all skin lesions have healed. Good infection control practices in the clinical setting will prevent spread to staff and other patients.
More information about monkeypox, including images of typical lesions—as well as an update on the current investigation in the United States and worldwide—can be found on the CDC website.4
The Centers for Disease Control and Prevention (CDC) and the World Health Organization are investigating an outbreak of monkeypox cases that have occurred around the world in countries that do not have endemic monkeypox virus.1,2 As of July 5, there have been 6924 cases documented in 52 countries, including 560 cases that have occurred in the United States.2 In the United States, as well as globally, a large proportion of cases have been in men who have sex with men.
First, what is monkeypox? Monkeypox is an orthopox virus that is closely related to variola (smallpox) and vaccinia (the virus used in the smallpox vaccine). It is endemic in western and central Africa and is contracted by contact with an infected mammal (including humans). Transmission can occur through direct contact with infected body fluids or lesions, via infectious fomites, or through respiratory secretions (although this usually requires prolonged exposure).
What is the disease course? The incubation period is 4 to 17 days. The initial symptoms include fever, malaise, headache, sore throat, and lymphadenopathy. A rash erupts 1 to 4 days after the prodrome and progresses synchronously from macules to papules to vesicles and then to pustules, which eventually scab over and fall off. In some cases reported in the United States, the rash started in the groin and genital area.
Don’t be fooled by other exanthems. Monkeypox can be confused with chickenpox and molluscum contagiosum (MC). However, the lesions in chickenpox appear asynchronously (all 4 stages present at the same time) and the papules of MC contain a central pit.
Can monkeypox be prevented? There are currently 2 vaccines against orthopox viruses: ACAM2000 and Jynneos. Currently, these vaccines are routinely recommended only for those at occupational risk of orthopox exposure.3
What you should know—and do. Be alert for any patient who presents with a suspicious rash; if there is a possibility of monkeypox, the local public health department should be contacted. They will investigate and collect samples for laboratory testing and will elicit contact names and locations. If monkeypox is confirmed, they may offer close contacts 1 of the 2 vaccines, which if administered within 4 days of exposure can prevent infection.
Advise all patients confirmed to have monkeypox to self-isolate until all skin lesions have healed. Good infection control practices in the clinical setting will prevent spread to staff and other patients.
More information about monkeypox, including images of typical lesions—as well as an update on the current investigation in the United States and worldwide—can be found on the CDC website.4
1. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:764-769. doi: http://dx.doi.org/10.15585/mmwr.mm7123e1
2. CDC. US monkeypox outbreak 2022: situation summary. Updated June 29, 2022. Accessed July 5, 2022.
3. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
4. CDC. 2022 monkeypox: information for healthcare professionals. Updated June 23, 2022. Accessed July 5, 2022.
1. Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:764-769. doi: http://dx.doi.org/10.15585/mmwr.mm7123e1
2. CDC. US monkeypox outbreak 2022: situation summary. Updated June 29, 2022. Accessed July 5, 2022.
3. Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morbid Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
4. CDC. 2022 monkeypox: information for healthcare professionals. Updated June 23, 2022. Accessed July 5, 2022.
Erythematous Pedunculated Plaque on the Dorsal Aspect of the Foot
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).
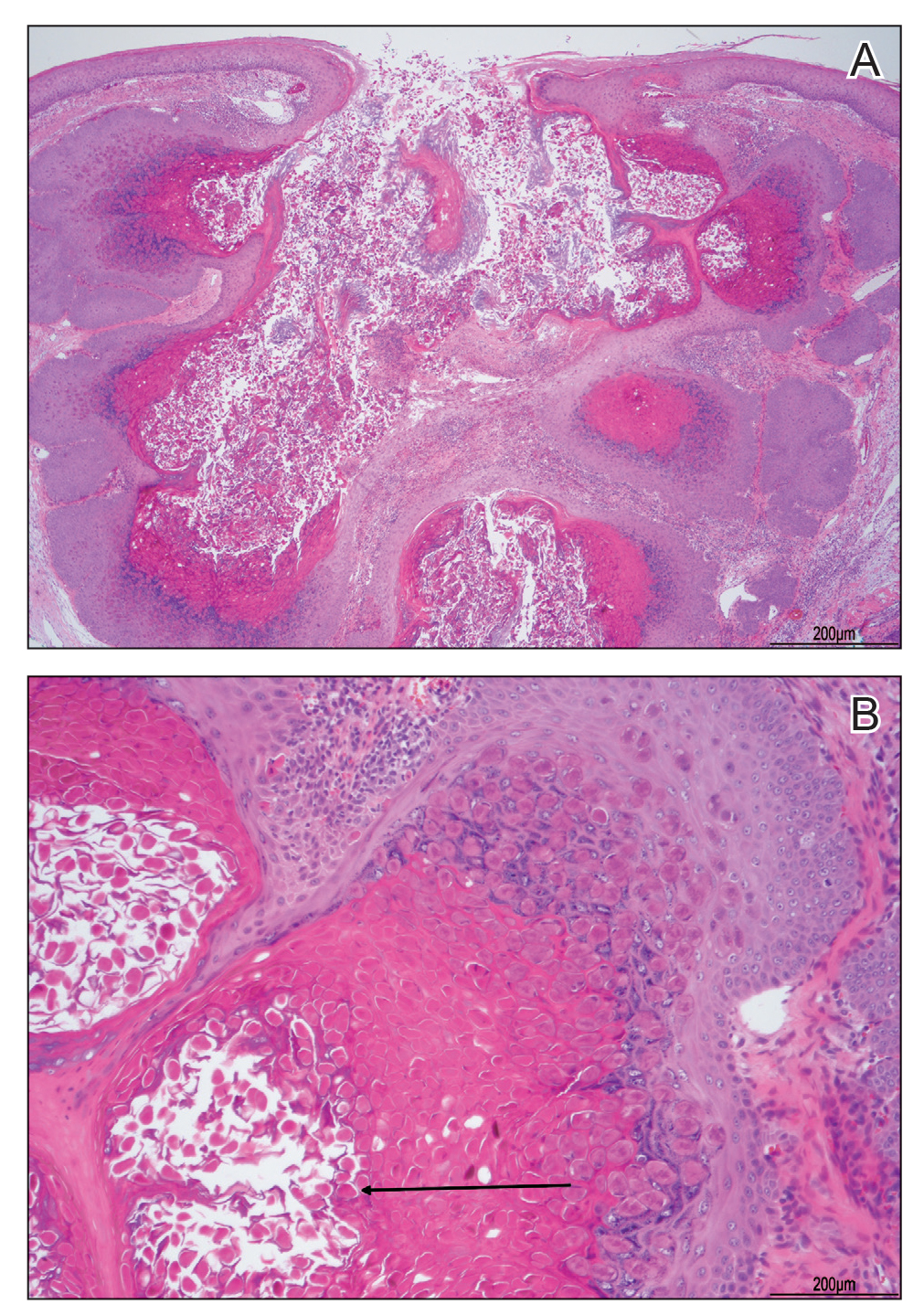
Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).

Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
The Diagnosis: Molluscum Contagiosum
A tangential shave removal with electrocautery was performed. Histopathology demonstrated numerous eosinophilic intracytoplasmic inclusion bodies (Figure), confirming a diagnosis of molluscum contagiosum (MC).

Molluscum contagiosum is a common poxvirus infection that is transmitted through fomites, contact, or self-inoculation.1 This infection most frequently occurs in school-aged children younger than 8 years1-3; peak incidence is 6 years of age.2,3 The worldwide estimated prevalence in children is 5.1% to 11.5%.1,3 In children cohabitating with others infected by MC, approximately 40% of households experienced a spread of infection; the risk of transmission is not associated with greater number of lesions.4 In adults, infection most commonly occurs in the setting of immunodeficiency or as a sexually transmitted infection in immunocompetent patients.3 Molluscum contagiosum infection classically presents as 1- to 3-mm, flesh- or white-colored, dome-shaped, smooth papules with central umbilication.1 Lesions often occur in clusters or lines, indicating local spread. The trunk, extremities, and face are areas that frequently are involved.2,3
Atypical presentations of MC infection can occur, as demonstrated by our case. Involvement of hair follicles by the infection can result in follicular induction.1,5 Secondary infection can mimic abscess formation.1 Inflamed MC lesions demonstrating the “beginning of the end” sign often are mistaken for primary infection, which is thought to be an inflammatory immune response to the virus.6 Lesions located on the eye or eyelid can present as unilateral conjunctivitis, conjunctival or corneal nodules, eyelid abscesses, or chalazions.1 Giant MC is a nodular variant of this infection measuring larger than 1 cm in size that can present similar to epidermoid cysts, condyloma acuminatum, or verruca vulgaris.1,7 Other reported mimicked conditions include basal cell carcinoma, trichoepithelioma, appendageal tumors, keratoacanthoma, foreign body granulomas, nevus sebaceous, or ecthyma.1,3 Molluscum contagiosum also has been reported to present as large ulcerative growths.8 In immunocompromised patients, deep fungal infection is another mimicker.1 Lesions on the plantar surfaces of the feet often are misdiagnosed as plantar verruca and present with pain during ambulation.9
The diagnosis of MC is clinical, with additional diagnostic tools reserved for more challenging situations.1 In cases with atypical presentations, dermoscopy may aid diagnosis through visualization of orifices and vascular patterns including crown, radial, and punctiform vessels.10 Biopsy or fine-needle aspiration also can be utilized as a diagnostic tool. Histopathology often reveals pathognomonic intracytoplasmic inclusions or Henderson-Paterson bodies.8,10 The appearance of MC can mimic other conditions that should be included in the differential diagnosis. Pyogenic granuloma often presents as a benign red papule that may grow rapidly and become pedunculated, sometimes with bleeding and crusting, though histology reveals groups of proliferating capillaries.11 More than half of amelanotic melanomas present in the papulonodular form as vascular or ulcerated nodules, and others may appear as erythematous macules. Diagnosis of amelanotic melanoma is made through histologic examination, which reveals atypical melanocytes in nests or cords, in conjunction with immunohistochemical stains such as S-100.12 Spitz nevi often appear as round, dome-shaped papules that most commonly are red, pink, or fleshcolored. They appear histologically similar to melanoma with nests of atypical melanocytes and nuclear atypia.13
A variety of treatment modalities can be used for MC including cantharidin, curettage, and cryotherapy.14 Imiquimod no longer is recommended due to a lack of demonstrated superiority over placebo in recent studies as well as its adverse effects.3 Topical retinoids have been recommended; however, their use frequently is limited by local irritation.3,14 Cantharidin is the most frequently utilized treatment by pediatric dermatologists. Most health care providers report subjective satisfaction with its results and efficacy, though some side effects may occur including discomfort and temporary changes in pigmentation. Treatment for MC is not required, as the condition is self-limiting.14 Therapy often is reserved for those with extensive disease, complications from lesions, cosmetic or psychological concerns, or genital involvement given the potential for sexual transmission.3 Time to resolution without treatment varies and is more prolonged in immunocompromised patients. Mean time to resolution in immunocompetent hosts has been reported as 13.3 months, but most infections are noted to clear within 2 to 4 years.1,4 Although resolution without treatment occurs, transmission to others and negative impact on quality of life (QOL) can occur and support the need for treatment. Greater impact on QOL was observed in females, those with more lesions, and patients with a longer duration of symptoms. Moderate impact on QOL was reported in 28% of patients (n=301), and severe effects were reported in 11%.4
In conclusion, MC is a common, benign, treatable cutaneous viral infection that often presents as small, flesh-colored papules in children. Its appearance can mimic a variety of other conditions. In cases with abnormal presentations, definitive diagnosis with pathology can be important to differentiate MC from more dangerous etiologies that may require further treatment.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
- Brown J, Janniger CK, Schwartz RA, et al. Childhood molluscum contagiosum. Int J Dermatol. 2006;45:93-99. doi:10.1111 /j.1365-4632.2006.02737.x
- Dohil MA, Lin P, Lee J, et al. The epidemiology of molluscum contagiosum in children. J Am Acad Dermatol. 2006;54:47-54. doi:10.1016/j.jaad.2005.08.035
- Robinson G, Townsend S, Jahnke MN. Molluscum contagiosum: review and update on clinical presentation, diagnosis, risk, prevention, and treatment. Curr Derm Rep. 2020;9:83-92.
- Olsen JR, Gallacher J, Finlay AY, et al. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15:190-195. doi:10.1016/S1473-3099(14)71053-9
- Davey J, Biswas A. Follicular induction in a case of molluscum contagiosum: possible link with secondary anetoderma-like changes? Am J Dermatopathol. 2014;36:E19-E21. doi:10.1097/DAD.0b013e31828bc7c7
- Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131:E1650-E1653. doi:10.1542/peds.2012-2933
- Uzuncakmak TK, Kuru BC, Zemheri EI, et al. Isolated giant molluscum contagiosum mimicking epidermoid cyst. Dermatol Pract Concept. 2016;6:71-73. doi:10.5826/dpc.0603a15
- Singh S, Swain M, Shukla S, et al. An unusual presentation of giant molluscum contagiosum diagnosed on cytology. Diagn Cytopathol. 2018;46:794-796. doi:10.1002/dc.23964
- Cohen PR, Tschen JA. Plantar molluscum contagiosum: a case report of molluscum contagiosum occurring on the sole of the foot and a review of the world literature. Cutis. 2012;90:35-41.
- Megalla M, Bronsnick T, Noor O, et al. Dermoscopic, confocal microscopic, and histologic characteristics of an atypical presentation of molluscum contagiosum. Ann Clin Pathol. 2014;2:1038.
- Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8:267-276. doi:10.1111/j.1525-1470.1991.tb00931.x
- Gong H-Z, Zheng H-Y, Li J. Amelanotic melanoma. Melanoma Res. 2019;29:221-230. doi:10.1097/CMR.0000000000000571
- Casso EM, Grin-Jorgensen CM, Grant-Kels JM. Spitz nevi. J Am Acad Dermatol. 1992;27(6 pt 1):901-913. doi:10.1016/0190-9622(92)70286-o
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26:405-408.
A 13-year-old adolescent girl presented for evaluation of a lesion on the dorsal aspect of the right foot of 1 week’s duration. She had a history of acne vulgaris and seasonal allergic rhinitis. She previously had noticed a persistent, small, flesh-colored bump of unknown chronicity in the same location, which had been diagnosed as a skin tag at an outside clinic. She denied any prior treatment in this area. Approximately a week prior to presentation, the lesion became painful, larger, and darkened in color before draining yellowish fluid. Due to concern for superinfection, the patient was prescribed cephalexin by her pediatrician. Dermatologic examination revealed a 1-cm, violaceous, pedunculated plaque with hemorrhagic crust on the dorsal aspect of the right foot with surrounding erythema and tenderness.
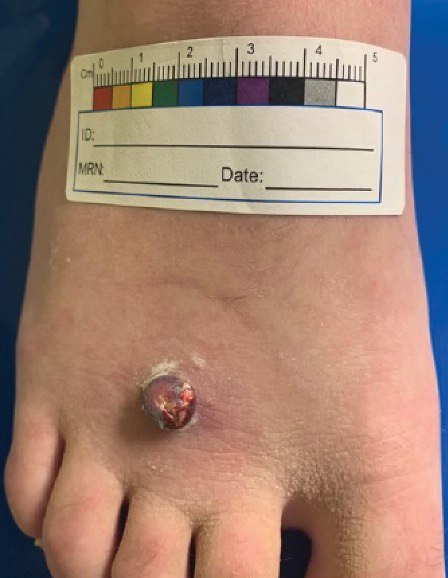
Innovations in Dermatology Spring Abstract Compendium
Effect of Pharmacist Interventions on Hospital Readmissions for Home-Based Primary Care Veterans
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
Following hospital discharge, patients are often in a vulnerable state due to new medical diagnoses, changes in medications, lack of understanding, and concerns for medical costs. In addition, the discharge process is complex and encompasses decisions regarding the postdischarge site of care, conveying patient instructions, and obtaining supplies and medications. There are several disciplines involved in the transitions of care process that are all essential for ensuring a successful transition and reducing the risk of hospital readmissions. Pharmacists play an integral role in the process.
When pharmacists are provided the opportunity to make therapeutic interventions, medication errors and hospital readmissions decrease and quality of life improves.1 Studies have shown that many older patients return home from the hospital with a limited understanding of their discharge instructions and oftentimes are unable to recall their discharge diagnoses and treatment plan, leaving opportunities for error when patients transition from one level of care to another.2,3 Additionally, high-quality transitional care is especially important for older adults with multiple comorbidities and complex therapeutic regimens as well as for their families and caregivers.4 To prevent hospital readmissions, pharmacists and other health care professionals (HCPs) should work diligently to prevent gaps in care as patients transition between settings. Common factors that lead to increased readmissions include premature discharge, inadequate follow-up, therapeutic errors, and medication-related problems. Furthermore, unintended hospital readmissions are common within the first 30 days following hospital discharge and lead to increased health care costs.2 For these reasons, many health care institutions have developed comprehensive models to improve the discharge process, decrease hospital readmissions, and reduce incidence of adverse events in general medical patients and high-risk populations.5
A study evaluating 693 hospital discharges found that 27.6% of patients were recommended for outpatient workups; however only 9% were actually completed.6 Due to lack of communication regarding discharge summaries, primary care practitioners (PCPs) were unaware of the need for outpatient workups; thus, these patients were lost to follow-up, and appropriate care was not received. Future studies should focus on interventions to improve the quality and dissemination of discharge information to PCPs.6 Fosnight and colleagues assessed a new transitions process focusing on the role of pharmacists. They evaluated medication reconciliations performed and discussed medication adherence barriers, medication recommendations, and time spent performing the interventions.7 After patients received a pharmacy intervention, Fosnight and colleagues reported that readmission rates decreased from 21.0% to 15.3% and mean length of stay decreased from 5.3 to 4.4 days. They also observed greater improvements in patients who received the full pharmacy intervention vs those receiving only parts of the intervention. This study concluded that adding a comprehensive pharmacy intervention to transitions of care resulted in an average of nearly 10 medication recommendations per patient, improved length of stay, and reduced readmission rates. After a review of similar studies, we concluded that a comprehensive discharge model is imperative to improve patient outcomes, along with HCP monitoring of the process to ensure appropriate follow-up.8
At Michael E. DeBakey Veteran Affairs Medical Center (MEDVAMC) in Houston, Texas, 30-day readmissions data were reviewed for veterans 6 months before and 12 months after enrollment in the Home-Based Primary Care (HBPC) service. HBPC is an in-home health care service provided to home-bound veterans with complex health care needs or when routine clinic-based care is not feasible. HBPC programs may differ among various US Department of Veterans Affairs (VA) medical centers. Currently, there are 9 HBPC teams at MEDVAMC and nearly 540 veterans are enrolled in the program. HBPC teams typically consist of PCPs, pharmacists, nurses, psychologists, occupational/physical therapists, social workers, medical support assistants, and dietitians.
Readmissions data are reviewed quarterly by fiscal year (FY) (Table 1). In FY 2019 quarter (Q) 2, the readmission rate before HBPC enrollment was 31% and decreased to 20% after enrollment. In FY 2019 Q3, the readmission rate was 29% before enrollment and decreased to 16% afterward. In FY 2019 Q4, the readmission rate before HBPC enrollment was 28% and decreased to 19% afterward. Although the readmission rates appeared to be decreasing overall, improvements were needed to decrease these rates further and to ensure readmissions were not rising as there was a slight increase in Q4. After reviewing these data, the HBPC service implemented a streamlined hospital discharge process to lower readmission rates and improve patient outcomes.
HBPC at MEDVAMC incorporates a team-based approach and the new streamlined discharge process implemented in 2019 highlights the role of each team member (Figure). Medical support assistants send daily emails of hospital discharges occurring in the last 7 days. Registered nurses are responsible for postdischarge calls within 2 days and home visits within 5 days. Pharmacists perform medication reconciliation within 14 days of discharge, review and/or educate on new medications, and change medications. The PCP is responsible for posthospital calls within 2 days and conducts a home visit within 5 days. Because HBPC programs vary among VA medical centers, the streamlined discharge process discussed may be applicable only to MEDVAMC. The primary objective of this quality improvement project was to identify specific pharmacist interventions to improve the HBPC discharge process and improve hospital readmission rates.
Methods
We conducted a Plan-Do-Study-Act quality improvement project. The first step was to conduct a review of veterans enrolled in HBPC at MEDVAMC.9 Patients included were enrolled in HBPC at MEDVAMC from October 2019 to March 2020 (FY 2020 Q1 and Q2). The Computerized Patient Record System was used to access the patients’ electronic health records. Patient information collected included race, age, sex, admission diagnosis, date of discharge, HBPC pharmacist name, PCP notification on the discharge summary, and 30-day readmission rates. Unplanned return to the hospital within 30 days, which was counted as a readmission, was defined as any admission for acute clinical events that required urgent hospital management.10
Next, we identified specific pharmacist interventions, including medication reconciliation completed by an HBPC pharmacist postdischarge; mean time to contact patients postdischarge; correct medications and supplies on discharge; incorrect dose; incorrect medication frequency or route of administration; therapeutic duplications; discontinuation of medications; additional drug therapy recommendations; laboratory test recommendations; maintenance medications not restarted or omitted; new medication education; and medication or formulation changes.
In the third step, we reviewed discharge summaries and clinical pharmacy notes to collect pharmacist intervention data. These data were analyzed to develop a standardized discharge process. Descriptive statistics were used to represent the results of the study.
Results
Medication reconciliation was completed postdischarge by an HBPC pharmacist in 118 of 175 study patients (67.4%). The mean age of patients was 76 years, about 95% were male (Table 2). There was a wide variety of admission diagnoses but sepsis, chronic obstructive pulmonary disease, and chronic kidney disease were most common. The PCP was notified on the discharge note for 68 (38.9%) patients. The mean time for HBPC pharmacists to contact patients postdischarge was about 3 days, which was much less than the 14 days allowed in the streamlined discharge process.
Pharmacists made the following interventions during medication reconciliation: New medication education was provided for 34 (19.4%) patients and was the largest intervention completed by HBPC pharmacists. Laboratory tests were recommended for 16 (9.1%) patients, medications were discontinued in 14 (8.0%) patients, and additional drug therapy recommendations were made for 7 (4.0%) patients. Medication or formulation changes were completed in 7 (4.0%) patients, incorrect doses were identified in 6 (3.4%) patients, 5 (2.9%) patients were not discharged with the correct medications or supplies, maintenance medications were not restarted in 3 (1.7%) patients, and there were no therapeutic duplications identified. In total, there were 92 (77.9%) patients with interventions compared with the 118 medication reconciliations completed (Table 3).
Process Improvement
As this was a new streamlined discharge process, it was important to assess the progress of the pharmacist role over time. We evaluated the number of medication reconciliations completed by quarter to determine whether more interventions were completed as the streamlined discharge process was being fully implemented. In FY 2020 Q1, medication reconciliation was completed by an HBPC pharmacist at a rate of 35%, and in FY 2020 Q2, at a rate of 65%.
In addition to assessing interventions completed by an HBPC pharmacist, we noted how many medication reconciliations were completed by an inpatient pharmacist as this may have impacted the results of this study. Of the 175 patients in this study, 49 (28%) received a medication reconciliation by an inpatient clinical pharmacy specialist before discharge. Last, when reviewing the readmissions data for the study period, it was evident that the streamlined discharge process was improving. In FY 2020 Q1, the readmissions rate prior to HBPC enrollment was 30% and decreased to 15% after and in FY 2020 Q2 was 31% before and decreased to 13% after HBPC enrollment. Before the study period in FY 2019 Q4, the readmissions rate after HBPC enrollment was 19%. Therefore, the readmissions rate decreased from 19% before the study period to 13% by the end of the study period.
Discussion
A comparison of the readmissions data from FYs 2019, 2020, and 2021 revealed that the newly implemented discharge process at MEDVAMC had been more effective.
There were 92 interventions made during the study period, which is about 78% of all medication reconciliations completed. Medication doses were changed based on patients’ renal function. Additional laboratory tests were recommended after discharge to ensure safety of therapy. Medications were discontinued if inappropriate or if patients were no longer on them to simplify their medication list and limit polypharmacy. New medication education was provided, including drug name, dose, route of administration, time of administration, frequency, indication, mechanism of action, adverse effect profile, monitoring parameters, and more. The HBPC pharmacists were able to make suitable interventions in a timely fashion as the average time to contact patients postdischarge was 3 days.
Areas for Improvement
The PCP was notified on the discharge note only in 68 (38.9%) patients. This could lead to gaps in care if other mechanisms are not in place to notify the PCP of the patient’s discharge. For this reason, it is imperative not only to implement a streamlined discharge process, but to review it and determine methods for continued improvement.9 The streamlined discharge process implemented by the HBPC team highlights when each team member should contact the patient postdischarge. However, it may be beneficial for each team member to have a list of vital information that should be communicated to the patient postdischarge and to other HCPs. For pharmacists, a standardized discharge note template may aid in the consistency of the medication reconciliation process postdischarge and may also increase interventions from pharmacists. For example, only some HBPC pharmacists inserted a new medication template in their discharge follow-up note. In addition, 23 (13.1%) patients were unreachable, and although a complete medication reconciliation was not feasible, a standardized note to review inpatient and outpatient medications along with the discharge plan may still serve as an asset for HCPs.
As the HBPC team continues to improve the discharge process, it is also important to highlight roles of the inpatient team who may assist with a smoother transition. For example, discharge summaries should be clear, complete, and concise, incorporating key elements from the hospital visit. Methods of communication on discharge should be efficient and understood by both inpatient and outpatient teams. Patients’ health literacy status should be considered when providing discharge instructions. Finally, patients should have a clear understanding of who is included in their primary care team should any questions arise. The potential interventions for HCPs highlighted in this study are critical for preventing adverse outcomes, improving patients’ quality of life, and decreasing hospital readmissions. However, implementing the streamlined discharge process was only step 1. Areas of improvement still exist to provide exceptional patient care.
Our goal is to increase pharmacist-led medication reconciliation after discharge to ≥ 80%. This will be assessed monthly after providing education to the HBPC team regarding the study results. The second goal is to maintain hospital readmission rates to ≤ 10%, which will be assessed with each quarterly review.
Strengths and Limitations
This study was one of the first to evaluate the impact of pharmacist intervention on improving patient outcomes in HBPC veterans. Additionally, only 1 investigator conducted the data collection, which decreased the opportunity for errors.
A notable limitation of this study is that the discharge processes may not be able to be duplicated in other HBPC settings due to variability in programs. Additionally, as this was a new discharge process, there were a few aspects that needed to be worked out in the beginning as it was established. Furthermore, this study did not clarify whether a medication reconciliation was conducted by a physician or nurse after discharge; therefore, this study cannot conclude that the medication interventions were solely attributed to pharmacists. Also this study did not assess readmissions for recurrent events only, which may have impacted the results in a different way from the current results that assessed readmission rates for any hospitalization. Other limitations include the retrospective study design at a single center.
Conclusions
This study outlines several opportunities for interventions to improve patient outcomes and aid in decreasing hospital readmission rates. Using the results from this study, education has been provided for the HBPC Service and its readmission committee. Additionally, the safety concerns identified have been addressed with inpatient and outpatient pharmacy leadership to improve the practices in both settings, prevent delays in patient care, and avoid future adverse outcomes. This project highlights the advantages of having pharmacists involved in transitions of care and demonstrates the benefit of HBPC pharmacists’ role in the streamlined discharge process. This project will be reviewed biannually to further improve the discharge process and quality of care for our veterans.
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
1. Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383-391. doi:10.1177/1062860612472931
2. Hume AL, Kirwin J, Bieber HL, et al. Improving care transitions: current practice and future opportunities for pharmacists. Pharmacotherapy. 2012;32(11):e326-e337. doi:10.1002/phar.1215
3. Milfred-LaForest SK, Gee JA, Pugacz AM, et al. Heart failure transitions of care: a pharmacist-led post discharge pilot experience. Prog Cardiovasc Dis. 2017;60(2):249-258. doi:10.1016/j.pcad.2017.08.005
4. Naylor M, Keating SA. Transitional care: moving patients from one care setting to another. Am J Nurs. 2008;108(suppl 9):58-63. doi:10.1097/01.NAJ.0000336420.34946.3a
5. Rennke S, Nguyen OK, Shoeb MH, Magan Y, Wachter RM, Ranji SR. Hospital-initiated transitional care interventions as a patient safety strategy. Ann Intern Med. 2013;158(5, pt 2):433-440. doi:10.7326/0003-4819-158-5-201303051-00011
6. Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med. 2007;167:1305-1311. doi:10.1001/archinte.167.12.1305
7. Fosnight S, King P, Ewald J, et al. Effects of pharmacy interventions at transitions of care on patient outcomes. Am J Health Syst Pharm. 2020;77(12):943-949. doi:10.1093/ajhp/zxaa081
8. Shull MT, Braitman LE, Stites SD, DeLuca A, Hauser D. Effects of a pharmacist-driven intervention program on hospital readmissions. Am J Health Syst Pharm. 2018;75(9):e221-e230. doi:10.2146/ajhp170287
9. US Department of Health and Human Services, Agency for Healthcare Research and Quality. Plan-Do-Study-Act (PDSA) cycle. February 2015. Accessed June 2, 2022. https://www.ahrq.gov/health-literacy/improve/precautions/tool2b.html10. Horwitz L, Partovian C, Lin Z, et al. Yale New Haven Health Services Corporation/Center for Outcomes Research & Evaluation. Hospital-wide (all-condition) 30-day risk-standardized readmission measure. Updated August 20 2011. Accessed June 2, 2022. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.cms.gov/medicare/quality-initiatives-patient-assessment-instruments/mms/downloads/mmshospital-wideall-conditionreadmissionrate.pdf
A Learning Health System Approach to Long COVID Care
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
The Veterans Health Administration (VHA)—along with systems across the world—has spent the past 2 years continuously adapting to meet the emerging needs of persons infected with COVID-19. With the development of effective vaccines and global efforts to mitigate transmission, attention has now shifted to long COVID care as the need for further outpatient health care becomes increasingly apparent.1,2
Background
Multiple terms describe the lingering, multisystem sequelae of COVID-19 that last longer than 4 weeks: long COVID, postacute COVID-19 syndrome, post-COVID condition, postacute sequalae of COVID-19, and COVID long hauler.1,3 Common symptoms include fatigue, shortness of breath, cough, sleep disorders, brain fog or cognitive dysfunction, depression, anxiety, pain, and changes in taste or smell that impact a person’s functioning.4,5 The multisystem nature of the postacute course of COVID-19 necessitates an interdisciplinary approach to devise comprehensive and individualized care plans.6-9 Research is needed to better understand this postacute state (eg, prevalence, underlying effects, characteristics of those who experience long COVID) to establish and evaluate cost-effective treatment approaches.
Many patients who are experiencing symptoms beyond the acute course of COVID-19 have been referred to general outpatient clinics or home health, which may lack the capacity and knowledge of this novel disease to effectively manage complex long COVID cases.2,3 To address this growing need, clinicians and leadership across a variety of disciplines and settings in the VHA created a community of practice (CoP) to create a mechanism for cross-facility communication, identify gaps in long COVID care and research, and cocreate knowledge on best practices for care delivery.
In this spirit, we are embracing a learning health system (LHS) approach that uses rapid-cycle methods to integrate data and real-world experience to iteratively evaluate and adapt models of long COVID care.10 Our clinically identified and data-driven objective is to provide high value health care to patients with long COVID sequalae by creating a framework to learn about this novel condition and develop innovative care models. This article provides an overview of our emerging LHS approach to the study of long COVID care that is fostering innovation and adaptability within the VHA. We describe 3 aspects of our engagement approach central to LHS: the ongoing development of a long COVID CoP dedicated to iteratively informing the bidirectional cycle of data from practice to research, results of a broad environmental scan of VHA long COVID care, and results of a survey administered to CoP members to inform ongoing needs of the community and identify early successful outcomes from participation.
Learning Health System Approach
The VHA is one of the largest integrated health care systems in the United States serving more than 9 million veterans.11 Since 2017, the VHA has articulated a vision to become an LHS that informs and improves patient-centered care through practice-based and data-driven research (eAppendix).12 During the early COVID-19 pandemic, an LHS approach in the VHA was critical to rapidly establishing a data infrastructure for disease surveillance, coordinating data-driven solutions, leveraging use of technology, collaborating across the globe to identify best practices, and implementing systematic responses (eg, policies, workforce adjustments).
Our long COVID CoP was developed as clinical observations and ongoing conversations with stakeholders (eg, veterans, health care practitioners [HCPs], leadership) identified a need to effectively identify and treat the growing number of veterans with long COVID. This clinical issue is compounded by the limited but emerging evidence on the clinical presentation of prolonged COVID-19 symptoms, treatment, and subsequent care pathways. The VHA’s efforts and lessons learned within the lens of an LHS are applicable to other systems confronting the complex identification and management of patients with persistent and encumbering long COVID symptoms. The VHA is building upon the LHS approach to proactively prepare for and address future clinical or public health challenges that require cross-system and sector collaborations, expediency, inclusivity, and patient/family centeredness.11
Community of Practice
As of January 25, 2022, our workgroup consisted of 128 VHA employees representing 29 VHA medical centers. Members of the multidisciplinary workgroup have diverse backgrounds with HCPs from primary care (eg, physicians, nurse practitioners), rehabilitation (eg, physical therapists), specialty care (eg, pulmonologists, physiatrists), mental health (eg, psychologists), and complementary and integrated health/Whole Health services (eg, practitoners of services such as yoga, tai chi, mindfulness, acupuncture). Members also include clinical, operations, and research leadership at local, regional, and national VHA levels. Our first objective as a large, diverse group was to establish shared goals, which included: (1) determining efficient communication pathways; (2) identifying gaps in care or research; and (3) cocreating knowledge to provide solutions to identified gaps.
Communication Mechanisms
Our first goal was to create an efficient mechanism for cross-facility communication. The initial CoP was formed in April 2021 and the first virtual meeting focused on reaching a consensus regarding the best way to communicate and proceed. We agreed to convene weekly at a consistent time, created a standard agenda template, and elected a lead facilitator of meeting proceedings. In addition, a member of the CoP recorded and took extensive meeting notes, which were later distributed to the entire CoP to accommodate varying schedules and ability to attend live meetings. Approximately 20 to 30 participants attend the meetings in real-time.
To consolidate working documents, information, and resources in one location, we created a platform to communicate via a Microsoft Teams channel. All CoP members are given access to the folders and allowed to add to the growing library of resources. Resources include clinical assessment and note templates for electronic documentation of care, site-specific process maps, relevant literature on screening and interventions identified by practice members, and meeting notes along with the recordings. A chat feature alerts CoP members to questions posed by other members. Any resources or information shared on the chat discussion are curated by CoP leaders to disseminate to all members. Importantly, this platform allowed us to communicate efficiently within the VHA organization by creating a centralized space for documents and the ability to correspond with all or select members of the CoP. Additional VHA employees can easily be referred and request access.
To increase awareness of the CoP, expand reach, and diversify perspectives, every participant was encouraged to invite colleagues and stakeholders with interest or experience in long COVID care to join. While patients are not included in this CoP, we are working closely with the VHA user experience workgroup (many members overlap) that is gathering patient and caregiver perspectives on their COVID-19 experience and long COVID care. Concurrently, CoP members and leadership facilitate communication and set up formal collaborations with other non-VHA health care systems to create an intersystem network of collaboration for long COVID care. This approach further enhances the speed at which we can work together to share lessons learned and stay up-to-date on emerging evidence surrounding long COVID care.
Identifying Gaps in Care and Research
Our second goal was to identify gaps in care or knowledge to inform future research and quality improvement initiatives, while also creating a foundation to cocreate knowledge about safe, effective care management of the novel long COVID sequelae. To translate knowledge, we must first identify and understand the gaps between the current, best available evidence and current care practices or policies impacting that delivery.13 As such, the structured meeting agenda and facilitated meeting discussions focused on understanding current clinical decision making and the evidence base. We shared VHA evidence synthesis reports and living rapid reviews on complications following COVID-19 illness (ie, major organ damage and posthospitalization health care use) that provided an objective evidence base on common long COVID complications.14,15
Since long COVID is a novel condition, we drew from literature in similar patient populations and translated that information in the context of our current knowledge of this unique syndrome. For example, we discussed the predominant and persistent symptom of fatigue post-COVID.5 In particular, the CoP discussed challenges in identifying and treating post-COVID fatigue, which is often a vague symptom with multiple or interacting etiologies that require a comprehensive, interdisciplinary approach. As such, we reviewed, adapted, and translated identification and treatment strategies from the literature on chronic fatigue syndrome to patients with post-COVID syndrome.16,17 We continue to work collaboratively and engage the appropriate stakeholders to provide input on the gaps to prioritize targeting.
Cocreate Knowledge
Our third goal was to cocreate knowledge regarding the care of patients with long COVID. To accomplish this, our structured meetings and communication pathways invited members to share experiences on the who (delivers and receives care), what (type of care or HCPs), when (identification of post-COVID and access), and how (eg, telehealth) of care to patients post-COVID. As part of the workgroup, we identified and shared resources on standardized, facility-level practices to reduce variability across the VHA system. These resources included intake/assessment forms, care processes, and batteries of tests/measures used for screening and assessment. The knowledge obtained from outside the CoP and cocreated within is being used to inform data-driven tools to support and evaluate care for patients with long COVID. As such, members of the workgroup are in the formative stages of participating in quality improvement innovation pilots to test technologies and processes designed to improve and validate long COVID care pathways. These technologies include screening tools, clinical decision support tools, and population health management technologies. In addition, we are developing a formal collaboration with the VHA Office of Research and Development to create standardized intake forms across VHA long COVID clinics to facilitate both clinical monitoring and research.
Surveys
The US Department of Veterans Affairs Central Office collaborated with our workgroup to draft an initial set of survey questions designed to understand how each VHA facility defines, identifies, and provides care to veterans experiencing post-COVID sequalae. The 41-question survey was distributed through regional directors and chief medical officers at 139 VHA facilities in August 2021. One hundred nineteen responses (86%) were received. Sixteen facilities indicated they had established programs and 26 facilities were considering a program. Our CoP had representation from the 16 facilities with established programs indicating the deep and well-connected nature of our grassroots efforts to bring together stakeholders to learn as part of a CoP.
A separate, follow-up survey generated responses from 18 facilities and identified the need to capture evolving innovations and to develop smaller workstreams (eg, best practices, electronic documentation templates, pathway for referrals, veteran engagement, outcome measures). The survey not only exposed ongoing challenges to providing long COVID care, but importantly, outlined the ways in which CoP members were leveraging community knowledge and resources to inform innovations and processes of care changes at their specific sites. Fourteen of 18 facilities with long COVID programs in place explicitly identified the CoP as a resource they have found most beneficial when employing such innovations. Specific innovations reported included changes in care delivery, engagement in active outreach with veterans and local facility, and infrastructure development to sustain local long COVID clinics (Table).
Future Directions
Our CoP strives to contribute to an evidence base for long COVID care. At the system level, the CoP has the potential to impact access and continuity of care by identifying appropriate processes and ensuring that VHA patients receive outreach and an opportunity for post-COVID care. Comprehensive care requires input from HCP, clinical leadership, and operations levels. In this sense, our CoP provides an opportunity for diverse stakeholders to come together, discuss barriers to screening and delivering post-COVID care, and create an action plan to remove or lessen such barriers.18 Part of the process to remove barriers is to identify and support efficient resource allocation. Our CoP has worked to address issues in resource allocation (eg, space, personnel) for post-COVID care. For example, one facility is currently implementing interdisciplinary virtual post-COVID care. Another facility identified and restructured working assignments for psychologists who served in different capacities throughout the system to fill the need within the long COVID team.
At the HCP level, the CoP is currently developing workshops, media campaigns, written clinical resources, skills training, publications, and webinars/seminars with continuing medical education credits.19 The CoP may also provide learning and growth opportunities, such as clinical or VHA operational fellowships and research grants.
We are still in the formative stages of post-COVID care and future efforts will explore patient-centered outcomes. We are drawing on the Centers for Disease Control and Prevention’s guidance for evaluating patients with long COVID symptoms and examining the feasibility within VHA, as well as patient perspectives on post-COVID sequalae, to ensure we are selecting assessments that measure patient-centered constructs.18
Conclusions
A VHA-wide LHS approach is identifying issues related to the identification, delivery, and evaluation of long COVID care. This long COVID CoP has developed an infrastructure for communication, identified gaps in care, and cocreated knowledge related to best current practices for post-COVID care. This work is contributing to systemwide LHS efforts dedicated to creating a culture of quality care and innovation and is a process that is transferrable to other areas of care in the VHA, as well as other health care systems. The LHS approach continues to be highly relevant as we persist through the COVID-19 pandemic and reimagine a postpandemic world.
Acknowledg
We thank all the members of the Veterans Health Administration long COVID Community of Practice who participate in the meetings and contribute to the sharing and spread of knowledge.
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
1. Sivan M, Halpin S, Hollingworth L, Snook N, Hickman K, Clifton I. Development of an integrated rehabilitation pathway for individuals recovering from COVID-19 in the community. J Rehabil Med. 2020;52(8):jrm00089. doi:10.2340/16501977-2727
2. Understanding the long-term health effects of COVID-19. EClinicalMedicine. 2020;26:100586. doi:10.1016/j.eclinm.2020.100586
3. Greenhalgh T, Knight M, A’Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. Published online August 11, 2020:m3026. doi:10.1136/bmj.m3026
4. Iwua CJ, Iwu CD, Wiysonge CS. The occurrence of long COVID: a rapid review. Pan Afr Med J. 2021;38. doi:10.11604/pamj.2021.38.65.27366
5. Carfì A, Bernabei R, Landi F; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. doi:10.1001/jama.2020.12603
6. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. 2020;32(8):1613-1620. doi:10.1007/s40520-020-01616-x
7. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. doi:10.1038/s41591-022-01689-3
8. Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature. 2021;594:259-264. doi:10.1038/s41586-021-03553-9
9. Ayoubkhani D, Bermingham C, Pouwels KB, et al. Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ. 2022;377:e069676. doi:10.1136/bmj-2021-069676
10. Institute of Medicine (US) Roundtable on Evidence-Based Medicine, Olsen L, Aisner D, McGinnis JM, eds. The Learning Healthcare System: Workshop Summary. Washington (DC): National Academies Press (US); 2007. doi:10.17226/11903
11. Romanelli RJ, Azar KMJ, Sudat S, Hung D, Frosch DL, Pressman AR. Learning health system in crisis: lessons from the COVID-19 pandemic. Mayo Clin Proc Innov Qual Outcomes. 2021;5(1):171-176. doi:10.1016/j.mayocpiqo.2020.10.004
12. Atkins D, Kilbourne AM, Shulkin D. Moving from discovery to system-wide change: the role of research in a learning health care system: experience from three decades of health systems research in the Veterans Health Administration. Annu Rev Public Health. 2017;38:467-487. doi:10.1146/annurev-publhealth-031816-044255
13. Kitson A, Straus SE. The knowledge-to-action cycle: identifying the gaps. CMAJ. 2010;182(2):E73-77. doi:10.1503/cmaj.081231
14. Greer N, Bart B, Billington C, et al. COVID-19 post-acute care major organ damage: a living rapid review. Updated September 2021. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid-organ-damage.pdf
15. Sharpe JA, Burke C, Gordon AM, et al. COVID-19 post-hospitalization health care utilization: a living review. Updated February 2022. Accessed May 31, 2022. https://www.hsrd.research.va.gov/publications/esp/covid19-post-hosp.pdf
16. Bested AC, Marshall LM. Review of Myalgic Encephalomyelitis/chronic fatigue syndrome: an evidence-based approach to diagnosis and management by clinicians. Rev Environ Health. 2015;30(4):223-249. doi:10.1515/reveh-2015-0026
17. Yancey JR, Thomas SM. Chronic fatigue syndrome: diagnosis and treatment. Am Fam Physician. 2012;86(8):741-746.
18. Kotter JP, Cohen DS. Change Leadership The Kotter Collection. Harvard Business Review Press; 2014.
19. Brownson RC, Eyler AA, Harris JK, Moore JB, Tabak RG. Getting the word out: new approaches for disseminating public health science. J Public Health Manag Pract. 2018;24(2):102-111. doi:10.1097/PHH.0000000000000673
Pharmacist-Assisted Varenicline Tobacco Cessation Treatment for Veterans
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
Tobacco smoking remains the leading cause of preventable disease and death in the United States, accounting for more than 480,000 deaths annually.1 An estimated 50.6 million US adults (20.8%) identify as tobacco users, with even higher rates among veterans (29.2%).2,3 Tobacco use is estimated to cost the US more than $300 billion annually in direct and indirect medical costs.4 According to a 2015 report, more than two-thirds of adult smokers reported a desire to quit, while only 7.5% reported successfully quitting in the past year.5 According to that same report, only 57.2% of smokers who had seen a health professional in the past year reported receiving advice to quit.5 This statistic is unfortunate, as interventions that combine behavioral and pharmacologic support can drastically increase tobacco cessation rates compared with self-help materials or no treatment.6
Currently, 7 first-line medications (5 nicotine, 2 nonnicotine) have been shown to increase long-term smoking abstinence rates. Varenicline was approved by the US Food and Drug Administration (FDA) in 2006 for use in adults as an aid to smoking cessation treatment. As a partial agonist of the α4β2 nicotinic acetylcholine receptor, varenicline’s mechanism of action is believed to involve reduction of nicotine’s rewarding capacity.7 Varenicline not only aids in complete tobacco cessation but also has been found to be effective for reducing cigarette consumption among smokers not yet willing or able to make a quit attempt.8 Furthermore, varenicline has demonstrated efficacy among users of smokeless tobacco in achieving continuous abstinence.9
Widespread adoption of varenicline into clinical practice was perhaps slowed by early concerns of psychiatric complications, prompting the FDA to issue a boxed warning for risk of serious neuropsychiatric events. This boxed warning was removed in 2016 in response to publication of the Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES). In this randomized controlled trial of more than 8000 participants, among whom 50.5% had a psychiatric disorder determined to be stable, varenicline significantly increased rates of continuous tobacco cessation compared with bupropion or the nicotine patch without an increased risk of neuropsychiatric events.10 This study underscored not only the safety of varenicline, but also its superiority over other first-line cessation products. The most recently published clinical practice guidelines recommend varenicline as a first-line agent for helping patients achieve long-term smoking cessation.11,12
Pharmacists are uniquely positioned to provide tobacco cessation interventions given their medication expertise and accessibility to the public. Indeed, multiple studies have demonstrated the effectiveness of pharmacist-led interventions on tobacco cessation.13-15 As of 2019, only 12 states had statutes or regulations addressing pharmacist prescribing of tobacco cessation aids without a collaborative practice agreement or local standing order.16 Until recently, most of these states limited pharmacists’ prescriptive authority to
Within the US Department of Veterans Affairs (VA), the clinical pharmacy specialist (CPS) is credentialed as an advanced practitioner with authority to independently manage patient medication therapy for a variety of diseases specified under a scope of practice. Although CPSs have provided tobacco cessation services for years, expansion of their scope to include varenicline did not occur until June 26, 2019, at the Southern Arizona VA Health Care System (SAVAHCS). All VA prescribers must follow the same criteria for prescribing varenicline. Unless previously trialed on varenicline, patients must have failed an appropriate trial of first-line agents (NRT, bupropion, or combination therapy) or have a contraindication to use of these first-line therapies before varenicline can be considered. Exclusions to therapy would include history of serious hypersensitivity to varenicline; suicidal intent, plan, or attempt within the past 12 months; current substance use disorder other than nicotine (unless varenicline recommended or prescribed by mental health professional); or unstable mental health disorder.18
The purpose of this study was to evaluate the efficacy and safety of CPS management of varenicline compared with other clinicians. We hope that this study provides insight regarding how the expansion of CPS scope to include prescriptive authority for varenicline has affected patient outcomes.
Methods
This retrospective chart review was conducted using SAVAHCS electronic health records. This study was granted approval by the institutional review board and the research and development committee at SAVAHCS. Data were obtained through the Computerized Patient Record System from the information provided by the pharmacist informatics department and was recorded electronically on a secure Microsoft Excel spreadsheet.
To be eligible for this study, patients must have been aged ≥ 18 years with a varenicline prescription between July 1, 2019, and July 31, 2020. Patients were excluded if tobacco cessation was managed by community-based (non-VA) clincians or if there was a lack of documentation of tobacco use at baseline and after at least 12 weeks of varenicline therapy. Sample size was not designed to achieve statistical power. Potential patients were queried by a pharmacist specializing in clinical informatics. All patients meeting initial inclusion criteria were then screened individually to evaluate for exclusion criteria.
Data collected included baseline age, sex, race, type of tobacco use (cigarettes, smokeless, both), mean daily tobacco use, prespecified comorbidities (depression, anxiety, or other psychiatric condition), and previous cessation medications prescribed (NRT, bupropion, and previous trials of varenicline).
The primary outcomes were reduction in tobacco use calculated as change at 12 weeks from baseline (and 24 weeks if available), continuous abstinence at 12 weeks (and 24 weeks if available), adherence to varenicline therapy measured by proportion of days covered (days covered by refills during the measurement period divided by days between the first fill and the end of the measurement period), and time to first follow-up in days. For safety evaluation, charts were reviewed for documented adverse events (AEs) in the health record. These AEs were categorized as follows: gastrointestinal, mood disturbance, sleep disturbance, headache, seizures, allergy, or other.
Statistical analyses regarding veteran baseline characteristics were descriptive in nature. χ2 test was used to analyze differences in complete cessation rates and AEs, whereas a Student t test was used to compare reductions of tobacco use, proportion of days covered (ie, adherence), and time to first follow-up. An α of .05 was used to determine significance.
Results
From the initial search, 255 charts met general inclusion criteria. After chart review, only 50 patients from the CPS group and 93 patients from the other clinician group met criteria to be included (Figure 1). The CPS group included pharmacists specializing in ambulatory care and outpatient mental health. The other clinician group was composed primarily of primary care practitioners, psychiatrists, and pulmonologists.
Overall, baseline characteristics were similar between the groups (Table 1). In the overall study population, the mean age was 57.5 years, 90% of patients were male, and 99% of patients were cigarette smokers. Baseline mean (SD) tobacco use was similar between the groups: 14.5 (10.8) vs 14.8 (8.6) cigarettes daily for the CPS and other clinician group, respectively.
While there was a significant reduction in daily cigarette use for both groups at 12 and 24 weeks (Figure 2), there was no mean (SD) between-group difference found among those patients prescribed varenicline by a CPS compared with other clinicians: -7.9 (10.4) vs -5.4 (9.8) cigarettes daily, respectively (P = .15) (Table 2). Change in tobacco use at 24 weeks and rates of complete tobacco abstinence were also not statistically significant between prescriber groups. Adherence (as evidenced by refill data) was higher in the CPS group than in the other clinician group (42% vs 31%, respectively; P = .01). There was also a significant difference in time to first follow-up; patients whose varenicline therapy was managed by a CPS had a mean (SD) follow-up time of 52 (66) vs 163 (110) days when patients were managed by other clinicians (P < .001). AEs were documented in 42% of patients in the CPS group compared with 23% of patients in the other clinician group (Table 3). The most reported AEs were gastrointestinal, as well as mood and sleep disturbances.
Discussion
The results of this single center study suggest that management of varenicline by CPSs is associated with similar reductions in tobacco use and abstinence rates compared with management by other clinicians. These results provide evidence that CPS management of varenicline may be as safe and effective as management by other clinicians.
Adherence rates (reported as proportion of days covered when assessing varenicline refill data) were higher on average among patients managed by a CPS compared with patients managed by other clinicians. However, this outcome may not be as reflective of adherence as initially intended, given delays in follow-up (see limitations section). Time to first follow-up was drastically different between the groups, with much sooner follow-up by CPSs compared with other clinicians. Despite similar tobacco cessation rates between groups, more frequent follow-up by CPSs helps to assess patient barriers to cessation, adherence to therapy, and AEs with varenicline. A higher percentage of AEs were documented within the CPS group that could be attributed to disparities in documentation rather than true rates of AEs. While rates of AEs were initially intended to serve as the primary safety outcome, they may instead reflect pharmacists’ diligence in monitoring and documenting tolerability of medication therapy.
Limitations
Several limitations to this study should be noted. First, the data collected were only as detailed as the extent to which prescribers documented tobacco use, previous cessation trials, and AEs; thus, various data points are likely missing within this study that could impact the results presented. In line with lack of documentation, delays in follow-up (ie, annual primary care visits) sorely undermined proportion of days covered, making these data less indicative of true medication adherence. Furthermore, this study did not account for concurrent therapies, such as combination varenicline and nicotine gum/lozenges, or behavioral treatment strategies like cessation classes.
Another limitation was that some primary care practitioners prescribed varenicline but then referred these patients to a CPS for tobacco cessation follow-up. Per the study’s protocol, these patients were included within the other clinician group, which could have brought results closer to the null. Finally, the timing of this chart review (July 1, 2019, to July 31, 2020) intersects with the start of the COVID-19 pandemic, presenting a possible confounding factor if patients’ quit attempts were hindered by the stress and isolation of the pandemic.19 All pharmacist visits during the pandemic were conducted by telephone, which may have affected results.
Conclusions
In this study of veterans receiving varenicline, management by CPSs resulted in similar reductions of tobacco use and rates of complete abstinence compared with management by other clinicians. Pharmacist management was associated with greater adherence and shorter time to first follow-up compared with other clinicians. Additional research is needed to fully characterize the impact of pharmacist management of varenicline, justify expansion of clinical pharmacist scope of practice, and ultimately enhance patient outcomes regarding tobacco cessation.
It would be interesting to see more studies outside of the VA system to determine the impact of pharmacist management of varenicline for a more heterogenous patient population. At some point, a prospective controlled trial should be conducted to overcome the various confounding factors that limit the results of retrospective chart reviews
Acknowledgments
This article was prepared, and research was conducted with resources and the use of facilities at the Southern Arizona Veterans Affairs Health Care System in Tucson.
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601
1. Centers for Disease Control and Prevention. Current cigarette smoking among adults in the United States. Updated March 17, 2022. Accessed May 31, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm 2. Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco product use among adults – United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(46):1736-1742. doi:10.15585/mmwr.mm6946a4
3. Odani S, Agaku IT, Graffunder CM, Tynan MA, Armour BS. Tobacco product use among military veterans – United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2018;67(1):7-12. doi:10.15585/mmwr.mm6701a2
4. Hall W, Doran C. How much can the USA reduce health care costs by reducing smoking? PLoS Med. 2016;13(5):e1002021. doi:10.1371/journal.pmed.1002021.
5. Centers for Disease Control and Prevention. Smoking cessation: fast facts. Updated March 21, 2022. Accessed June 1, 2022. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/cessation/smoking-cessation-fast-facts/index.html
6. US Public Health Service Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Chapter 6, Interventions for smoking cessation and treatments for nicotine dependence. In: Smoking Cessation: A Report of the Surgeon General [Internet]. Washington, DC: US Department of Health and Human Services; 2020. Accessed June 1, 2022. https://www.ncbi.nlm.nih.gov/books/NBK555596
7. Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the α4β2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985-994. doi:10.1016/j.neuropharm.2006.10.016
8. Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687-694. doi:10.1001/jama.2015.280
9. Fagerström K, Gilljam H, Metcalfe M, Tonstad S, Messig M. Stopping smokeless tobacco with varenicline: randomised double blind placebo controlled trial. BMJ. 2010;341:c6549. doi:10.1136/bmj.c6549
10. Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507-2520. doi:10.1016/S0140-6736(16)30272-0
11. Barua RS, Rigotti NA, Benowitz NL, et al. 2018 ACC expert consensus decision pathway on tobacco cessation treatment: a report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2018;72(25):3332-3365. doi:10.1016/j.jacc.2018.10.027
12. Leone FT, Zhang Y, Evers-Casey S, et al. Initiating pharmacologic treatment in tobacco-dependent adults. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. 2020;202(2):e5-e31. doi:10.1164/rccm.202005-1982ST
13. Saba M, Diep J, Saini B, Dhippayom T. Meta-analysis of the effectiveness of smoking cessation interventions in community pharmacy. J Clin Pharm Ther. 2014;39(3):240-247. doi:10.1111/jcpt.12131
14. Augustine JM, Taylor AM, Pelger M, Schiefer D, Warholak TL. Smoking quit rates among patients receiving pharmacist-provided pharmacotherapy and telephonic smoking cessation counseling. J Am Pharm Assoc. 2016;56(2):129-136. doi:10.1016/j.japh.2016.02.001
15. Dent LA, Harris KJ, Noonan CW. Tobacco interventions delivered by pharmacists: a summary and systematic review. Pharmacotherapy. 2007;27(7):1040-1051. doi:10.1592/phco.27.7.1040
16. National Alliance of State Pharmacy Associations. Pharmacist prescribing: tobacco cessation aids. February 10, 2021. Accessed June 1, 2022. https://naspa.us/resource/tobacco-cessation
17. Shen X, Bachyrycz A, Anderson JR, Tinker D, Raisch DW. Quitting patterns and predictors of success among participants in a tobacco cessation program provided by pharmacists in New Mexico. J Manag Care Spec Pharm. 2014;20(6):579-587. doi:10.18553/jmcp.2014.20.6.579
18. VA Center for Medication Safety, Tobacco Use Cessation Technical Advisory Group, Public Health Strategic Healthcare Group, VA Pharmacy Benefits Management Services, VISN Pharmacist Executives, and Medical Advisory Panel. Varenicline criteria for prescribing. 2008. Updated July 2011. Accessed June 9, 2022. https://www.healthquality.va.gov/tuc/VareniclineCriteriaforPrescribing.pdf
19. Jaklevic MC. COVID-19 and the “lost year” for smokers trying to quit. JAMA. 2021;325(19):1929-1930. doi:10.1001/jama.2021.5601