User login
Generalized Pustular Psoriasis: A Review of the Pathophysiology, Clinical Manifestations, Diagnosis, and Treatment
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
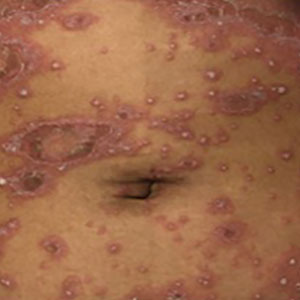
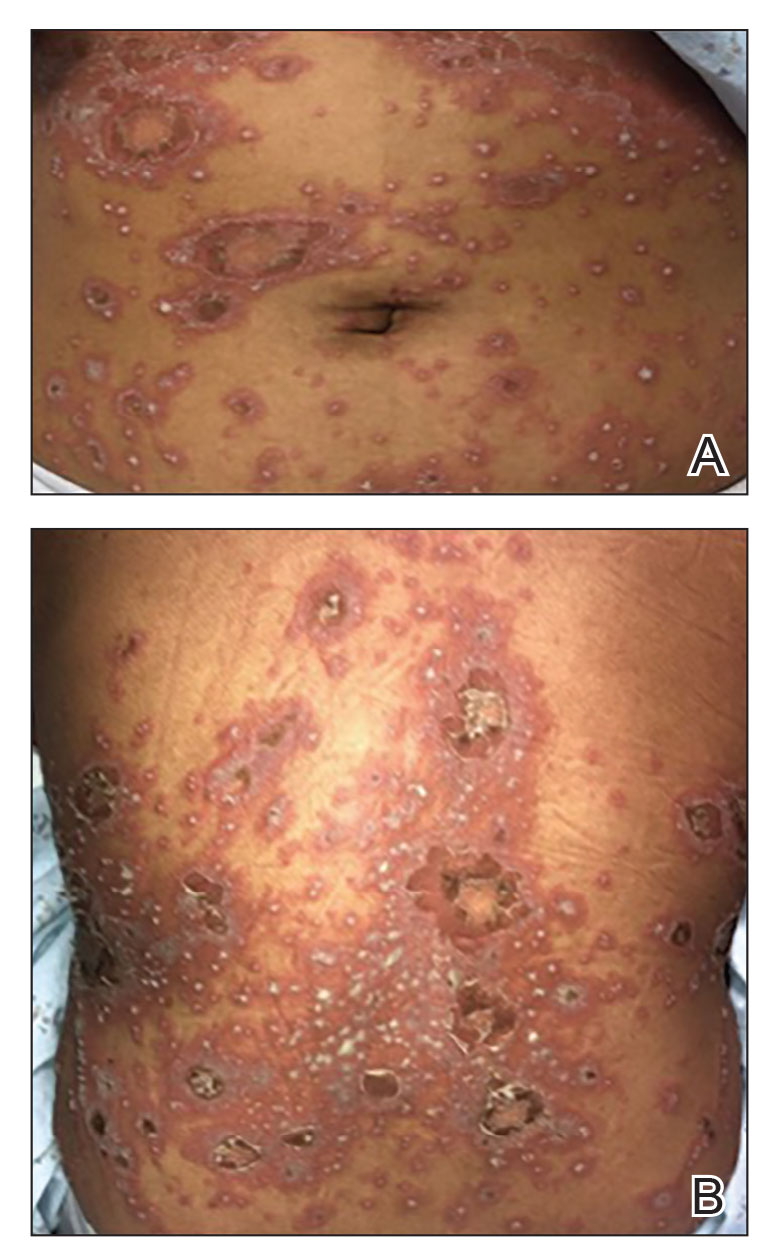
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4
The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25
Histologic Features
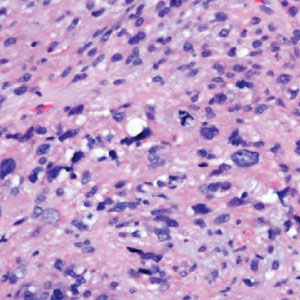
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
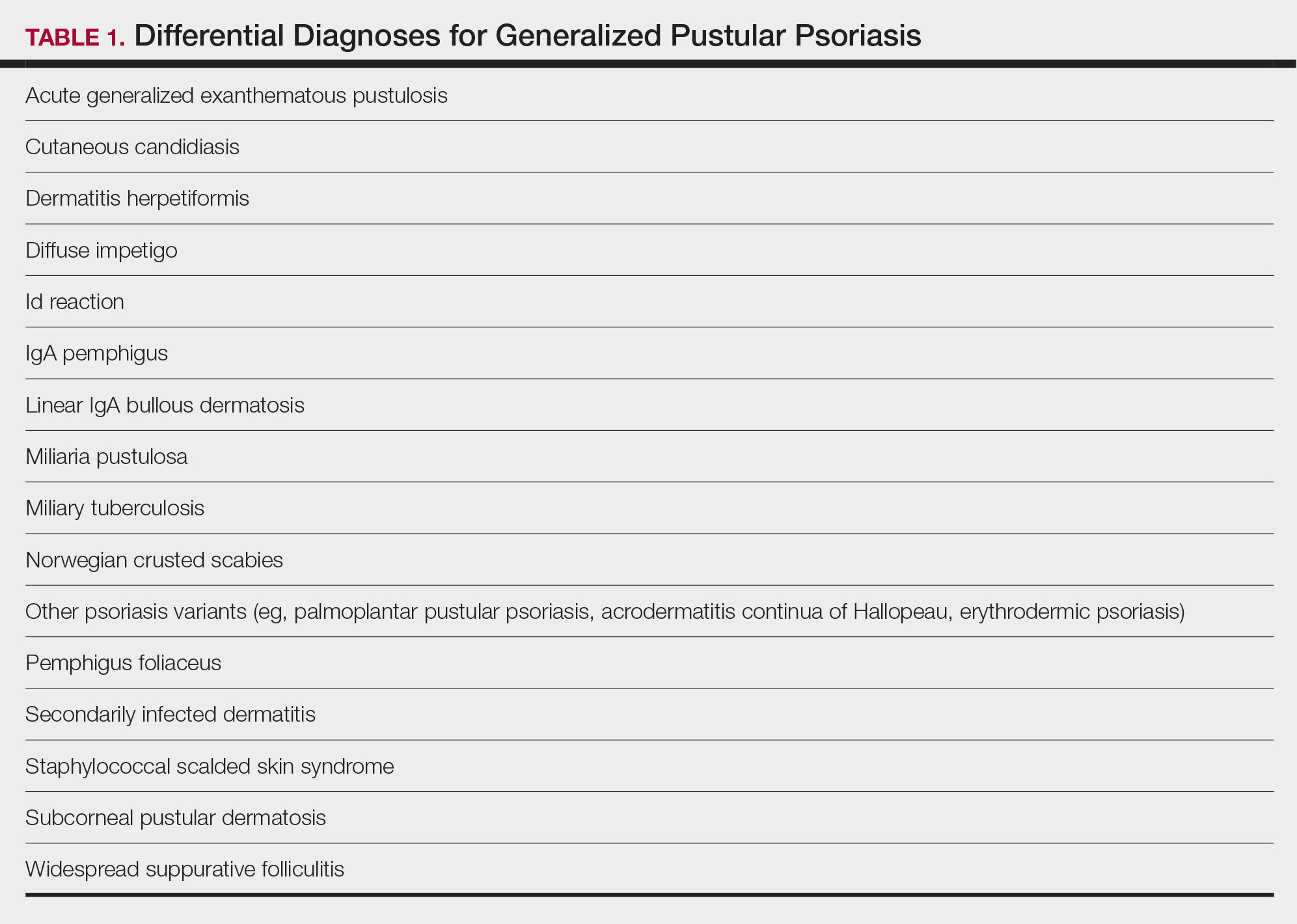
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4
Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
Acute generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis characterized by the sudden widespread eruption of sterile pustules.1,2 The cutaneous manifestations of GPP also may be accompanied by signs of systemic inflammation, including fever, malaise, and leukocytosis.2 Complications are common and may be life-threatening, especially in older patients with comorbid diseases.3 Generalized pustular psoriasis most commonly occurs in patients with a preceding history of psoriasis, but it also may occur de novo.4 Generalized pustular psoriasis is associated with notable morbidity and mortality, and relapses are common.3,4 Many triggers of GPP have been identified, including initiation and withdrawal of various medications, infections, pregnancy, and other conditions.5,6 Although GPP most often occurs in adults, it also may arise in children and infants.3 In pregnancy, GPP is referred to as impetigo herpetiformis, despite having no etiologic ties with either herpes simplex virus or staphylococcal or streptococcal infection. Impetigo herpetiformis is considered one of the most dangerous dermatoses of pregnancy because of high rates of associated maternal and fetal morbidity.6,7
Acute GPP has proven to be a challenging disease to treat due to the rarity and relapsing-remitting nature of the disease; additionally, there are relatively few randomized controlled trials investigating the efficacy and safety of treatments for GPP. This review summarizes the features of GPP, including the pathophysiology of the disease, clinical and histological manifestations, and recommendations for management based on a PubMed search of articles indexed for MEDLINE using MeSH terms pertaining to the disease, including generalized pustular psoriasis, impetigo herpetiformis, and von Zumbusch psoriasis.
Pathophysiology
The pathophysiology of GPP is only partially understood, but it is thought to have a distinct pattern of immune activation compared with plaque psoriasis.8 Although there is a considerable amount of overlap and cross-talk among cytokine pathways, GPP generally is driven by innate immunity and unrestrained IL-36 cytokine activity. In contrast, adaptive immune responses—namely the tumor necrosis factor (TNF) α, IL-23, IL-17, and IL-22 axes—underlie plaque psoriasis.8-10
Proinflammatory IL-36 cytokines α, β, and γ, which are all part of the IL-1 superfamily, bind to the IL-36 receptor (IL-36R) to recruit and activate immune cells via various mediators, including IL-1β; IL-8; and chemokines CXCL1, CXCL2, and CXCL8.3 The IL-36 receptor antagonist (IL-36ra) acts to inhibit this inflammatory cascade.3,8 Microarray analyses of skin biopsy samples have shown that overexpression of IL-17A, TNF-α, IL-1, and IL-36 are seen in both GPP and plaque psoriasis lesions, but GPP lesions had higher expression of IL-1β, IL-36α, and IL-36γ and elevated neutrophil chemokines—CXCL1, CXCL2, and CXCL8—compared with plaque psoriasis lesions.8
Gene Mutations Associated With GPP
There are 3 gene mutations that have been associated with pustular variants of psoriasis, though these mutations account for a minority of cases of GPP.4 Genetic screenings are not routinely indicated in patients with GPP, but they may be warranted in severe cases when a familial pattern of inheritance is suspected.4
IL36RN—The gene IL36RN codes the anti-inflammatory IL-36ra. Loss-of-function mutations in IL36RN lead to impairment of IL-36ra and consequently hyperactivity of the proinflammatory responses triggered by IL-36.3 Homozygous and heterozygous mutations in IL36RN have been observed in both familial and sporadic cases of GPP.11-13 Subsequent retrospective analyses have identified the presence of IL36RN mutations in patients with GPP with frequencies ranging from 23% to 37%.14-17IL36RN mutations are thought to be more common in patients without concomitant plaque psoriasis and have been associated with severe disease and early disease onset.15
CARD14—A gain-of-function mutation in CARD14 results in overactivation of the proinflammatory nuclear factor κB pathway and has been implicated in cases of GPP with concurrent psoriasis vulgaris. Interestingly, this may suggest distinct etiologies underlying GPP de novo and GPP in patients with a history of psoriasis.18,19
AP1S3—A loss-of-function mutation in AP1S3 results in abnormal endosomal trafficking and autophagy as well as increased expression of IL-36α.20,21
Clinical Presentation and Diagnosis Cutaneous Manifestations of GPP
Generalized pustular psoriasis is characterized by the onset of widespread 2- to 3-mm sterile pustules on erythematous skin or within psoriasiform plaques4 (Figure). In patients with skin of color, the erythema may appear less obvious or perhaps slightly violaceous compared to White skin. Pustules may coalesce to form “lakes” of pus.5 Cutaneous symptoms include pain, burning, and pruritus. Associated mucosal findings may include cheilitis, geographic tongue, conjunctivitis, and uveitis.4

The severity of symptoms can vary greatly among patients as well as between flares within the same patient.2,3 Four distinct patterns of GPP have been described. The von Zumbusch pattern is characterized by a rapid, generalized, painful, erythematous and pustular eruption accompanied by fever and asthenia. The pustules usually resolve after several days with extensive scaling. The annular pattern is characterized by annular, erythematous, scaly lesions with pustules present centrifugally. The lesions enlarge by centrifugal expansion over a period of hours to days, while healing occurs centrally. The exanthematic type is an acute eruption of small pustules that abruptly appear and disappear within a few days, usually from infection or medication initiation. Sometimes pustules appear within or at the edge of existing psoriatic plaques in a localized pattern—the fourth pattern—often following the exposure to irritants (eg, tars, anthralin).5
Impetigo Herpetiformis—Impetigo herpetiformis is a form of GPP associated with pregnancy. It generally presents early in the third trimester with symmetric erythematous plaques in flexural and intertriginous areas with pustules present at lesion margins. Lesions expand centrifugally, with pustulation present at the advancing edge.6,7 Patients often are acutely ill with fever, delirium, vomiting, and tetany. Mucous membranes, including the tongue, mouth, and esophagus, also may be involved. The eruption typically resolves after delivery, though it often recurs with subsequent pregnancies, with the morbidity risk rising with each successive pregnancy.7
Systemic and Extracutaneous Manifestations of GPP
Although the severity of GPP is highly variable, skin manifestations often are accompanied by systemic manifestations of inflammation, including fever and malaise. Common laboratory abnormalities include leukocytosis with peripheral neutrophilia, a high serum C-reactive protein level, hypocalcemia, and hypoalbuminemia.22 Abnormal liver enzymes often are present and result from neutrophilic cholangitis, with alternating strictures and dilations of biliary ducts observed on magnetic resonance imaging.23 Additional laboratory abnormalities are provided in Table 2. Other extracutaneous findings associated with GPP include arthralgia, edema, and characteristic psoriatic nail changes.4 Fatal complications include acute respiratory distress syndrome, renal dysfunction, cardiovascular shock, and sepsis.24,25

Histologic Features
Given the potential for the skin manifestations of GPP to mimic other disorders, a skin biopsy is warranted to confirm the diagnosis. Generalized pustular psoriasis is histologically characterized by the presence of subcorneal macropustules (ie, spongiform pustules of Kogoj) formed by neutrophil infiltration into the spongelike network of the epidermis.6 Otherwise, the architecture of the epithelium in GPP is similar to that seen with plaque psoriasis, with parakeratosis, acanthosis, rete-ridge elongation, diminished stratum granulosum, and thinning of the suprapapillary epidermis, though the inflammatory cell infiltrate and edema are markedly more severe in GPP than plaque psoriasis.3,4
Differential Diagnosis
There are many other cutaneous pustular diagnoses that must be ruled out when evaluating a patient with GPP (Table 1).26 Acute generalized exanthematous pustulosis (AGEP) is a common mimicker of GPP that is differentiated histologically by the presence of eosinophils and necrotic keratinocytes.4 In addition to its distinct histopathologic findings, AGEP is classically associated with recent initiation of certain medications, most commonly penicillins, macrolides, quinolones, sulfonamides, terbinafine, and diltiazem.27 In contrast, GPP more commonly is related to withdrawal of corticosteroids as well as initiation of some biologic medications, including anti-TNF agents.3 Generalized pustular psoriasis should be suspected over AGEP in patients with a personal or family history of psoriasis, though GPP may arise in patients with or without a history of psoriasis. Acute generalized exanthematous pustulosis usually is more abrupt in both onset and resolution compared with GPP, with clearance of pustules within a few days to weeks following cessation of the triggering factor.4

Other pustular variants of psoriasis (eg, palmoplantar pustular psoriasis, acrodermatitis continua of Hallopeau) are differentiated from GPP by their chronicity and localization to palmoplantar and/or ungual surfaces.5 Other differential diagnoses are listed in Table 1.
Diagnostic Criteria for GPP
Diagnostic criteria have been proposed for GPP (Table 2), including (1) the presence of sterile pustules, (2) systemic signs of inflammation, (3) laboratory abnormalities, (4) histopathologic confirmation of spongiform pustules of Kogoj, and (5) recurrence of symptoms.22 To definitively diagnose GPP, all 5 criteria must be met. To rule out mimickers, it may be worthwhile to perform Gram staining, potassium hydroxide preparation, in vitro cultures, and/or immunofluorescence testing.6
Treatment
Given the high potential for mortality associated with GPP, the most essential component of management is to ensure adequate supportive care. Any temperature, fluid, or electrolyte imbalances should be corrected as they arise. Secondary infections also must be identified and treated, if present, to reduce the risk for fatal complications, including systemic infection and sepsis. Precautions must be taken to ensure that serious end-organ damage, including hepatic, renal, and respiratory dysfunction, is avoided.
Adjunctive topical intervention often is initiated with bland emollients, corticosteroids, calcineurin inhibitors, and/or vitamin D derivatives to help soothe skin symptoms, but treatment with systemic therapies usually is warranted to achieve symptom control.2,25 Importantly, there are no systemic or topical agents that have specifically been approved for the treatment of GPP in Europe or the United States.3 Given the absence of universally accepted treatment guidelines, therapeutic agents for GPP usually are selected based on clinical experience while also taking the extent of involvement and disease severity into consideration.3
Treatment Recommendations for Adults
Oral Systemic Agents—Treatment guidelines set forth by the National Psoriasis Foundation (NPF) in 2012 proposed that first-line therapies for GPP should be acitretin, cyclosporine, methotrexate, and infliximab.28 However, since those guidelines were established, many new biologic therapies have been approved for the treatment of psoriasis and often are considered in the treatment of psoriasis subtypes, including GPP.29 Although retinoids previously were considered to be a preferred first-line therapy, they are associated with a high incidence of adverse effects and must be used with caution in women of childbearing age.6 Oral acitretin at a dosage of 0.75 to 1.0 mg/kg/d has been shown to result in clinical improvement within 1 to 2 weeks, and a maintenance dosage of 0.125 to 0.25 mg/kg/d is required for several months to prevent recurrence.30 Methotrexate—5.0 to 15.0 mg/wk, or perhaps higher in patients with refractory disease, increased by 2.5-mg intervals until symptoms improve—is recommended by the NPF in patients who are unresponsive or cannot tolerate retinoids, though close monitoring for hematologic abnormalities is required. Cyclosporine 2.5 to 5.0 mg/kg/d is considered an alternative to methotrexate and retinoids; it has a faster onset of action, with improvement reported as early as 2 weeks after initiation of therapy.1,28 Although cyclosporine may be effective in the acute phase, especially in severe cases of GPP, long-term use of cyclosporine is not recommended because of the potential for renal dysfunction and hypertension.31
Biologic Agents—More recent evidence has accumulated supporting the efficacy of anti-TNF agents in the treatment of GPP, suggesting the positioning of these agents as first line. A number of case series have shown dramatic and rapid improvement of GPP with intravenous infliximab 3 to 5 mg/kg, with results observed hours to days after the first infusion.32-37 Thus, infliximab is recommended as first-line treatment in severe acute cases, though its efficacy as a maintenance therapy has not been sufficiently investigated.6 Case reports and case series document the safety and efficacy of adalimumab 40 to 80 mg every 1 to 2 weeks38,39 and etanercept 25 to 50 mg twice weekly40-42 in patients with recalcitrantGPP. Therefore, these anti-TNF agents may be considered in patients who are nonresponsive to treatment with infliximab.
Rarely, there have been reports of paradoxical induction of GPP with the use of some anti-TNF agents,43-45 which may be due to a cytokine imbalance characterized by unopposed IFN-α activation.6 In patients with a history of GPP after initiation of a biologic, treatment with agents from within the offending class should be avoided.
The IL-17A monoclonal antibodies secukinumab, ixekizumab, and brodalumab have been shown in open-label phase 3 studies to result in disease remission at 12 weeks.46-48 Treatment with guselkumab, an IL-23 monoclonal antibody, also has demonstrated efficacy in patients with GPP.49 Ustekinumab, an IL-12/23 inhibitor, in combination with acitretin also has been shown to be successful in achieving disease remission after a few weeks of treatment.50
More recent case reports have shown the efficacy of IL-1 inhibitors including gevokizumab, canakinumab, and anakinra in achieving GPP clearance, though more prospective studies are needed to evaluate their efficacy.51-53 Given the etiologic association between IL-1 disinhibition and GPP, future investigations of these therapies as well as those that target the IL-36 pathway may prove to be particularly interesting.
Phototherapy and Combination Therapies—Phototherapy may be considered as maintenance therapy after disease control is achieved, though it is not considered appropriate for acute cases.28 Combination therapies with a biologic plus a nonbiologic systemic agent or alternating among various biologics may allow physicians to maximize benefits and minimize adverse effects in the long term, though there is insufficient evidence to suggest any specific combination treatment algorithm for GPP.28
Treatment Recommendations for Pediatric Patients
Based on a small number of case series and case reports, the first-line treatment strategy for children with GPP is similar to adults. Given the notable adverse events of most oral systemic agents, biologic therapies may emerge as first-line therapy in the pediatric population as more evidence accumulates.28
Treatment Recommendations for Pregnant Patients
Systemic corticosteroids are widely considered to be the first-line treatments for the management of impetigo herpetiformis.7 Low-dose prednisone (15–30 mg/d) usually is effective, but severe cases may require increasing the dosage to 60 mg/d.6 Given the potential for rebound flares upon withdrawal of systemic corticosteroids, these agents must be gradually tapered after the resolution of symptoms.
Certolizumab pegol also is an attractive option in pregnant patients with impetigo herpetiformis because of its favorable safety profile and negligible mother-to-infant transfer through the placenta or breast milk. It has been shown to be effective in treating GPP and impetigo herpetiformis during pregnancy in recently published case reports.54,55 In refractory cases, other TNF-α inhibitors (eg, adalimumab, infliximab, etanercept) or cyclosporine may be considered. However, cautious medical monitoring is warranted, as little is known about the potential adverse effects of these agents to the mother and fetus.28,56 Data from transplant recipients along with several case reports indicate that cyclosporine is not associated with an increased risk for adverse effects during pregnancy at a dose of 2 to 3 mg/kg.57-59 Both methotrexate and retinoids are known teratogens and are therefore contraindicated in pregnant patients.56
If pustules do not resolve in the postpartum period, patients should be treated with standard GPP therapies. However, long-term and population studies are lacking regarding the potential for infant exposure to systemic agents in breast milk. Therefore, the NPF recommends avoiding breastfeeding while taking systemic medications, if possible.56
Limitations of Treatment Recommendations
The ability to generate an evidence-based treatment strategy for GPP is limited by a lack of high-quality studies investigating the efficacy and safety of treatments in patients with GPP due to the rarity and relapsing-remitting nature of the disease, which makes randomized controlled trials difficult to conduct. The quality of the available research is further limited by the lack of validated outcome measures to specifically assess improvements in patients with GPP, such that results are difficult to synthesize and compare among studies.31
Conclusion
Although limited, the available research suggests that treatment with various biologics, especially infliximab, is effective in achieving rapid clearance in patients with GPP. In general, biologics may be the most appropriate treatment option in patients with GPP given their relatively favorable safety profiles. Other oral systemic agents, including acitretin, cyclosporine, and methotrexate, have limited evidence to support their use in the acute phase, but their safety profiles often limit their utility in the long-term. Emerging evidence regarding the association of GPP with IL36RN mutations suggests a unique role for agents targeting the IL-36 or IL-1 pathways, though this has yet to be thoroughly investigated.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
- Benjegerdes KE, Hyde K, Kivelevitch D, et al. Pustular psoriasis: pathophysiology and current treatment perspectives. Psoriasis (Auckl). 2016;6:131‐144.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614‐618.
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907‐919.
- Ly K, Beck KM, Smith MP, et al. Diagnosis and screening of patients with generalized pustular psoriasis. Psoriasis (Auckl). 2019;9:37‐42.
- van de Kerkhof PCM, Nestle FO. Psoriasis. In: Bolognia JL, Jorizzo JJ, Schaffer JV, eds. Dermatology. 3rd ed. Elsevier; 2012:138-160.
- Hoegler KM, John AM, Handler MZ, et al. Generalized pustular psoriasis: a review and update on treatment. J Eur Acad Dermatol Venereol. 2018;32:1645‐1651.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101‐104.
- Johnston A, Xing X, Wolterink L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109-120.
- Furue K, Yamamura K, Tsuji G, et al. Highlighting interleukin-36 signalling in plaque psoriasis and pustular psoriasis. Acta Derm Venereol. 2018;98:5-13.
- Ogawa E, Sato Y, Minagawa A, et al. Pathogenesis of psoriasis and development of treatment. J Dermatol. 2018;45:264-272.
- Marrakchi S, Guigue P, Renshaw BR, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med. 2011;365:620-628.
- Onoufriadis A, Simpson MA, Pink AE, et al. Mutations in IL36RN/IL1F5 are associated with the severe episodic inflammatory skin disease known as generalized pustular psoriasis. Am J Hum Genet. 2011;89:432-437.
- Setta-Kaffetzi N, Navarini AA, Patel VM, et al. Rare pathogenic variants in IL36RN underlie a spectrum of psoriasis-associated pustular phenotypes. J Invest Dermatol. 2013;133:1366-1369.
- Sugiura K, Takemoto A, Yamaguchi M, et al. The majority of generalized pustular psoriasis without psoriasis vulgaris is caused by deficiency of interleukin-36 receptor antagonist. J Invest Dermatol. 2013;133:2514-2521.
- Hussain S, Berki DM, Choon SE, et al. IL36RN mutations define a severe autoinflammatory phenotype of generalized pustular psoriasis. J Allergy Clin Immunol. 2015;135:1067-1070.e9.
- Körber A, Mossner R, Renner R, et al. Mutations in IL36RN in patients with generalized pustular psoriasis. J Invest Dermatol. 2013;133:2634-2637.
- Twelves S, Mostafa A, Dand N, et al. Clinical and genetic differences between pustular psoriasis subtypes. J Allergy Clin Immunol. 2019;143:1021-1026.
- Sugiura K. The genetic background of generalized pustular psoriasis: IL36RN mutations and CARD14 gain-of-function variants. J Dermatol Sci. 2014;74:187-192
- Wang Y, Cheng R, Lu Z, et al. Clinical profiles of pediatric patients with GPP alone and with different IL36RN genotypes. J Dermatol Sci. 2017;85:235-240.
- Setta-Kaffetzi N, Simpson MA, Navarini AA, et al. AP1S3 mutations are associated with pustular psoriasis and impaired Toll-like receptor 3 trafficking. Am J Hum Genet. 2014;94:790-797.
- Mahil SK, Twelves S, Farkas K, et al. AP1S3 mutations cause skin autoinflammation by disrupting keratinocyte autophagy and upregulating IL-36 production. J Invest Dermatol. 2016;136:2251-2259.
- Umezawa Y, Ozawa A, Kawasima T, et al. Therapeutic guidelines for the treatment of generalized pustular psoriasis (GPP) based on a proposed classification of disease severity. Arch Dermatol Res. 2003;295(suppl 1):S43-S54.
- Viguier M, Allez M, Zagdanski AM, et al. High frequency of cholestasis in generalized pustular psoriasis: evidence for neutrophilic involvement of the biliary tract. Hepatology. 2004;40:452-458.
- Ryan TJ, Baker H. The prognosis of generalized pustular psoriasis. Br J Dermatol. 1971;85:407-411.
- Kalb RE. Pustular psoriasis: management. In: Ofori AO, Duffin KC, eds. UpToDate. UpToDate; 2014. Accessed July 20, 2022. https://www.uptodate.com/contents/pustular-psoriasis-management/print
- Naik HB, Cowen EW. Autoinflammatory pustular neutrophilic diseases. Dermatol Clin. 2013;31:405-425.
- Sidoroff A, Dunant A, Viboud C, et al. Risk factors for acute generalized exanthematous pustulosis (AGEP)—results of a multinational case-control study (EuroSCAR). Br J Dermatol. 2007;157:989-996.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279‐288.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Mengesha YM, Bennett ML. Pustular skin disorders: diagnosis and treatment. Am J Clin Dermatol 2002;3:389-400.
- Zhou LL, Georgakopoulos JR, Ighani A, et al. Systemic monotherapy treatments for generalized pustular psoriasis: a systematic review. J Cutan Med Surg. 2018;22:591‐601.
- Elewski BE. Infliximab for the treatment of severe pustular psoriasis. J Am Acad Dermatol. 2002;47:796-797.
- Kim HS, You HS, Cho HH, et al. Two cases of generalized pustular psoriasis: successful treatment with infliximab. Ann Dermatol. 2014;26:787-788.
- Trent JT, Kerdel FA. Successful treatment of Von Zumbusch pustular psoriasis with infliximab. J Cutan Med Surg. 2004;8:224-228.
- Poulalhon N, Begon E, Lebbé C, et al. A follow-up study in 28 patients treated with infliximab for severe recalcitrant psoriasis: evidence for efficacy and high incidence of biological autoimmunity. Br J Dermatol. 2007;156:329-336.
- Routhouska S, Sheth PB, Korman NJ. Long-term management of generalized pustular psoriasis with infliximab: case series. J Cutan Med Surg. 2008;12:184-188.
- Lisby S, Gniadecki R. Infliximab (Remicade) for acute, severe pustular and erythrodermic psoriasis. Acta Derm Venereol. 2004;84:247-248.
- Zangrilli A, Papoutsaki M, Talamonti M, et al. Long-term efficacy of adalimumab in generalized pustular psoriasis. J Dermatol Treat. 2008;19:185-187.
- Matsumoto A, Komine M, Karakawa M, et al. Adalimumab administration after infliximab therapy is a successful treatment strategy for generalized pustular psoriasis. J Dermatol. 2017;44:202-204.
- Kamarashev J, Lor P, Forster A, et al. Generalized pustular psoriasis induced by cyclosporin in a withdrawal responding to the tumour necrosis factor alpha inhibitor etanercept. Dermatology. 2002;205:213-216.
- Esposito M, Mazzotta A, Casciello C, et al. Etanercept at different dosages in the treatment of generalized pustular psoriasis: a case series. Dermatology. 2008;216:355-360.
- Lo Schiavo A, Brancaccio G, Puca RV, et al. Etanercept in the treatment of generalized annular pustular psoriasis. Ann Dermatol. 2012;24:233-234.
- Goiriz R, Daudén E, Pérez-Gala S, et al. Flare and change of psoriasis morphology during the course of treatment with tumor necrosis factor blockers. Clin Exp Dermatol. 2006;32:176-179.
- Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233-240.
- Almutairi D, Sheasgreen C, Weizman A, et al. Generalized pustular psoriasis induced by infliximab in a patient with inflammatory bowel disease. J Cutan Med Surg. 2018;1:507-510.
- Imafuku S, Honma M, Okubo Y, et al. Efficacy and safety of secukinumab in patients with generalized pustular psoriasis: a 52-week analysis from phase III open-label multicenter Japanese study. J Dermatol. 2016;43:1011-1017
- Saeki H, Nakagawa H, Ishii T, et al. Efficacy and safety of open-label ixekizumab treatment in Japanese patients with moderate-to-severe plaque psoriasis, erythrodermic psoriasis, and generalized pustular psoriasis. J Eur Acad Dermatol Venereol. 2015;29:1148-1155.
- Yamasaki K, Nakagawa H, Kubo Y, et al. Efficacy and safety of brodalumab in patients with generalized pustular psoriasis and psoriatic erythroderma: results from a 52-week, open-label study. Br J Dermatol. 2017;176:741-751.
- Sano S, Kubo H, Morishima H, et al. Guselkumab, a human interleukin-23 monoclonal antibody in Japanese patients with generalized pustular psoriasis and erythrodermic psoriasis: efficacy and safety analyses of a 52-week, phase 3, multicenter, open-label study. J Dermatol. 2018;45:529‐539.
- Arakawa A, Ruzicka T, Prinz JC. Therapeutic efficacy of interleukin 12/interleukin 23 blockade in generalized pustular psoriasis regardless of IL36RN mutation status. JAMA Dermatol. 2016;152:825-828.
- Mansouri B, Richards L, Menter A. Treatment of two patients with generalized pustular psoriasis with the interleukin-1beta inhibitor gevokizumab. Br J Dermatol. 2015;173:239-241.
- Skendros P, Papagoras C, Lefaki I, et al. Successful response in a case of severe pustular psoriasis after interleukin-1 beta inhibition. Br J Dermatol. 2017;176:212-215.
- Viguier M, Guigue P, Pagès C, et al. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist Anakinra: lack of correlation with IL1RN mutations. Ann Intern Med. 2010;153:66-67.
- Fukushima H, Iwata Y, Arima M, et al. Efficacy and safety of treatment with anti-tumor necrosis factor‐α drugs for severe impetigo herpetiformis. J Dermatol. 2021;48:207-210.
- Mizutani Y, Mizutani YH, Matsuyama K, et al. Generalized pustular psoriasis in pregnancy, successfully treated with certolizumab pegol. J Dermatol. 2021;47:e262-e263.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459‐477.
- Finch TM, Tan CY. Pustular psoriasis exacerbated by pregnancy and controlled by cyclosporin A. Br J Dermatol. 2000;142:582-584.
- Gaughan WJ, Moritz MJ, Radomski JS, et al. National Transplantation Pregnancy Registry: report on outcomes of cyclosporine-treated female kidney transplant recipients with an interval from transplantation to pregnancy of greater than five years. Am J Kidney Dis. 1996;28:266-269.
- Kura MM, Surjushe AU. Generalized pustular psoriasis of pregnancy treated with oral cyclosporin. Indian J Dermatol Venereol Leprol. 2006;72:458-459.
Practice Points
- Generalized pustular psoriasis (GPP) is a rare severe variant of psoriasis that is characterized by the abrupt widespread onset of small pustules.
- Although no treatments have specifically been approved for GPP, various biologics, especially infliximab, may be effective in achieving rapid clearance in patients with GPP. Other oral systemic agents including acitretin, cyclosporine, and methotrexate also have been shown to be effective.
Racial Disparities in the Diagnosis of Psoriasis
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.
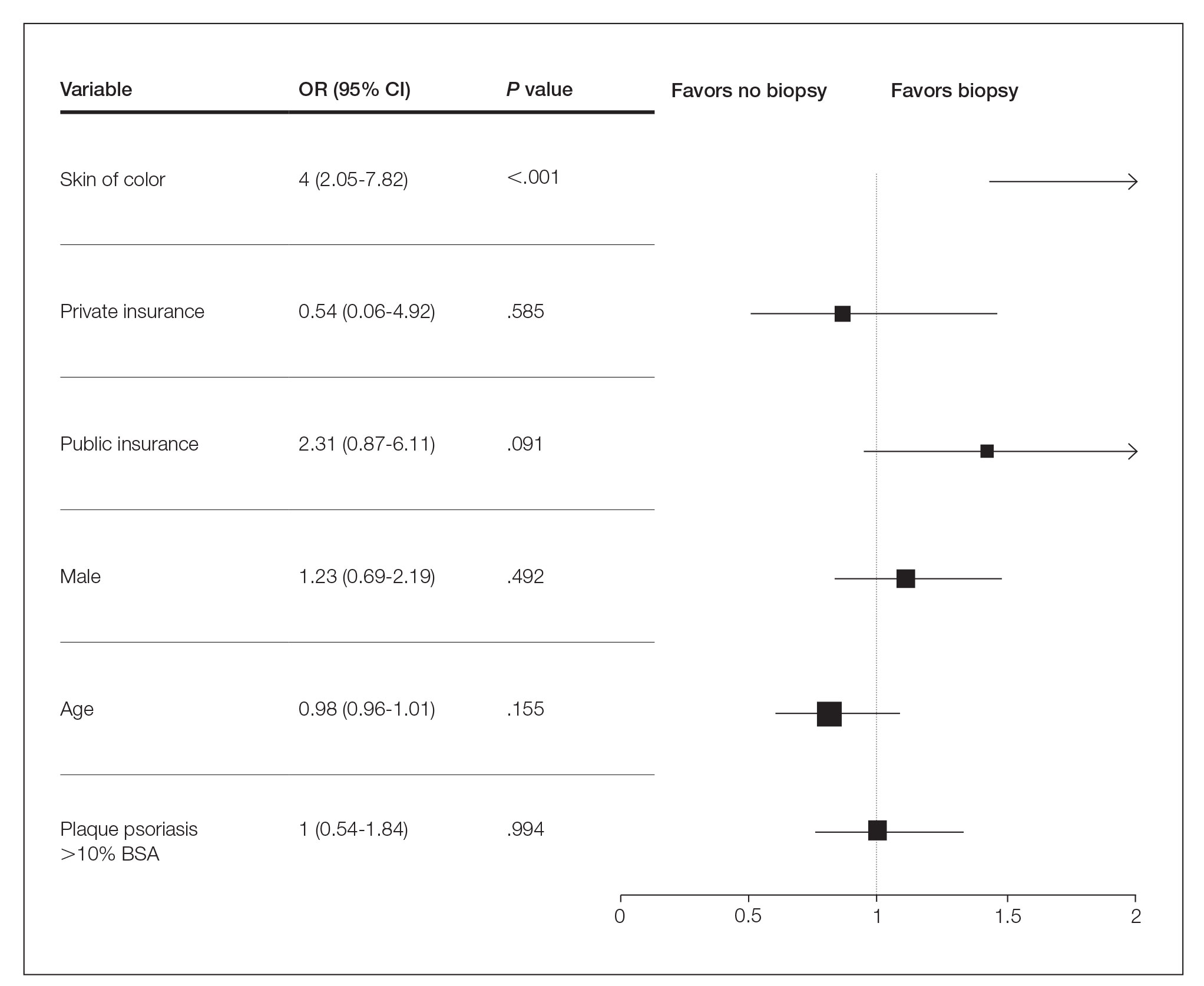
Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).
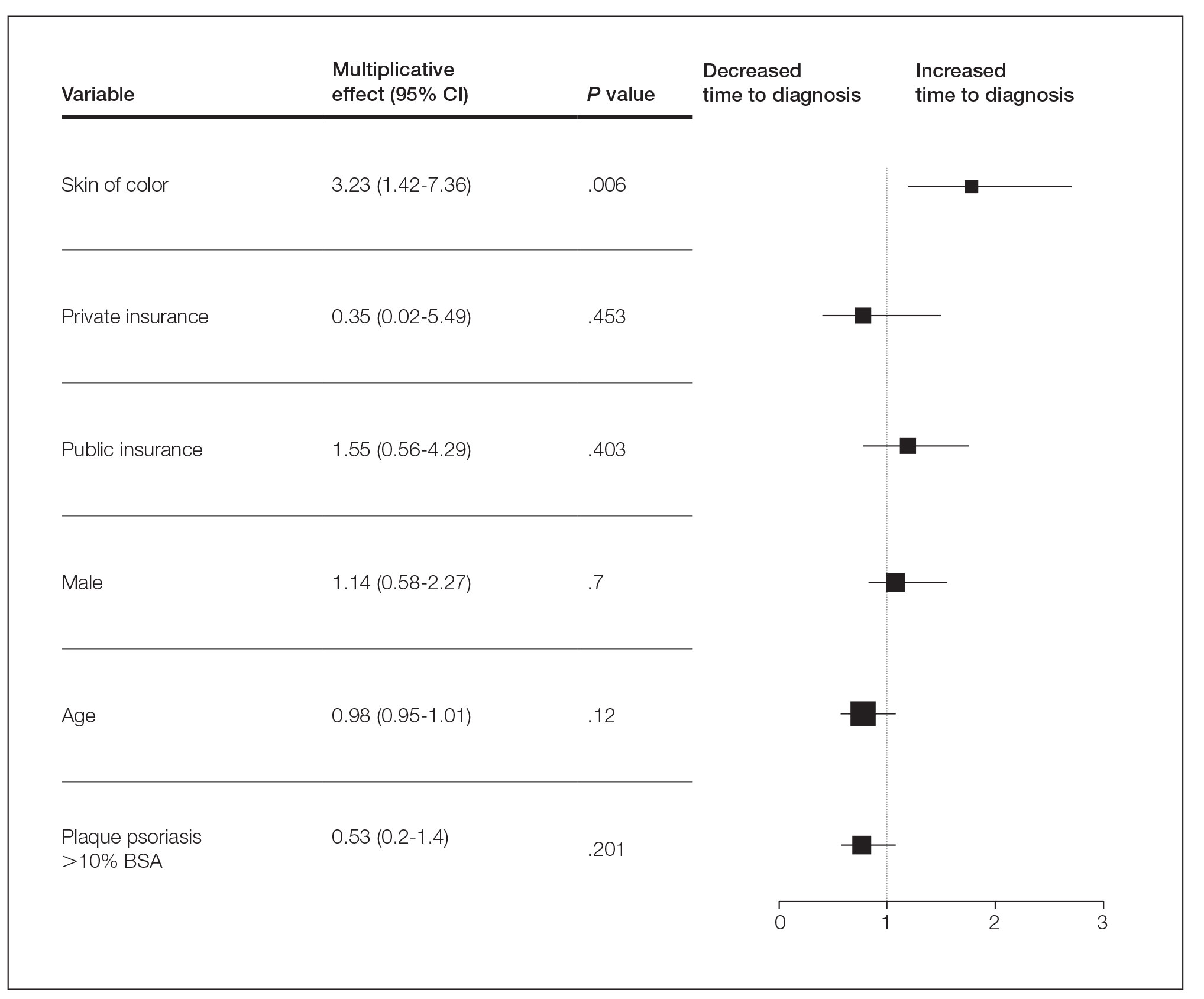
Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
To the Editor:
Psoriasis affects 2% to 3% of the US population and is one of the more commonly diagnosed dermatologic conditions.1-3 Experts agree that common cutaneous diseases such as psoriasis present differently in patients with skin of color (SOC) compared to non-SOC patients.3,4 Despite the prevalence of psoriasis, data on these morphologic differences are limited.3-5 We performed a retrospective chart review comparing characteristics of psoriasis in SOC and non-SOC patients.
Through a search of electronic health records, we identified patients with an International Classification of Diseases, 10th Revision, diagnosis of psoriasis who were 18 years or older and were evaluated in the dermatology department between August 2015 and June 2020 at University Medical Center, an academic institution in New Orleans, Louisiana. Photographs and descriptions of lesions from these patients were reviewed. Patient data collected included age, sex, psoriasis classification, insurance status, self-identified race and ethnicity, location of lesion(s), biopsy, final diagnosis, and average number of visits or days required for accurate diagnosis. Self-identified SOC race and ethnicity categories included Black or African American, Hispanic, Asian, American Indian and Alaskan Native, Native Hawaiian and Other Pacific Islander, and “other.”
All analyses were conducted using R-4.0.1 statistics software. Categorical variables were compared in SOC and non-SOC groups using Fisher exact tests. Continuous covariates were conducted using a Wilcoxon rank sum test.
In total, we reviewed 557 charts. Four patients who declined to identify their race or ethnicity were excluded, yielding 286 SOC and 267 non-SOC patients (N=553). A total of 276 patients (131 SOC; 145 non-SOC) with a prior diagnosis of psoriasis were excluded in the days to diagnosis analysis. Twenty patients (15, SOC; 5, non-SOC) were given a diagnosis of a disease other than psoriasis when evaluated in the dermatology department.
Distributions between racial groups differed for insurance status, sex, psoriasis classification, biopsy status, and days between first dermatology visit and diagnosis. Skin of color patients had significantly longer days between initial presentation to dermatology and final diagnosis vs non-SOC patients (180.11 and 60.27 days, respectively; P=.001). Skin of color patients had a higher rate of palmoplantar psoriasis and severe plaque psoriasis (ie, >10% body surface area involvement) at presentation.

Several multivariable regression analyses were performed. Skin of color patients had significantly higher odds of biopsy compared to non-SOC patients (adjusted odds ratio [95% CI]=4 [2.05-7.82]; P<.001)(Figure 1). There were no significant predictors for severe plaque psoriasis involving more than 10% body surface area. Skin of color patients had a significantly longer time to diagnosis than non-SOC patients (P=.006)(Figure 2). On average, patients with SOC waited 3.23 times longer for a diagnosis than their non-SOC counterparts (95% CI, 1.42-7.36).

Our data reveal striking racial disparities in psoriasis care. Worse outcomes for patients with SOC compared to non-SOC patients may result from physicians’ inadequate familiarity with diverse presentations of psoriasis, including more frequent involvement of special body sites in SOC. Other likely contributing factors that we did not evaluate include socioeconomic barriers to health care, lack of physician diversity, missed appointments, and a paucity of literature on the topic of differentiating morphologies of psoriasis in SOC and non-SOC patients. Our study did not examine the effects of sex, tobacco use, or prior or current therapy, and it excluded pediatric patients.
To improve dermatologic outcomes for our increasingly diverse patient population, more studies must be undertaken to elucidate and document disparities in care for SOC populations.
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
- Gelfand JM, Stern RS, Nijsten T, et al. The prevalence of psoriasis in African Americans: results from a population-based study. J Am Acad Dermatol. 2005;52:23-26. doi:10.1016/j.jaad.2004.07.045
- Stern RS, Nijsten T, Feldman SR, et al. Psoriasis is common, carries a substantial burden even when not extensive, and is associated with widespread treatment dissatisfaction. J Investig Dermatol Symp Proc. 2004;9:136-139. doi:10.1046/j.1087-0024.2003.09102.x
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Alexis AF, Blackcloud P. Psoriasis in skin of color: epidemiology, genetics, clinical presentation, and treatment nuances. J Clin Aesthet Dermatol. 2014;7:16-24.
- Kaufman BP, Alexis AF. Psoriasis in skin of color: insights into the epidemiology, clinical presentation, genetics, quality-of-life impact, and treatment of psoriasis in non-white racial/ethnic groups. Am J Clin Dermatol. 2018;19:405-423. doi:10.1007/s40257-017-0332-7
Practice Points
- Skin of color (SOC) patients can wait 3 times longer to receive a diagnosis of psoriasis than non-SOC patients.
- Patients with SOC more often present with severe forms of psoriasis and are more likely to have palmoplantar psoriasis.
- Skin of color patients can be 4 times as likely to require a biopsy to confirm psoriasis diagnosis compared to non-SOC patients.
Management of Psoriasis With Topicals: Applying the 2020 AAD-NPF Guidelines of Care to Clinical Practice
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
Psoriasis is a chronic inflammatory skin disease characterized by erythematous scaly plaques that can invoke substantial pain, pruritus, and quality-of-life disturbance in patients. Topical therapies are the most commonly used medications for treating psoriasis, with one study (N = 128,308) showing that more than 85% of patients with psoriasis were managed solely with topical medications. 1 For patients with mild to moderate psoriasis, topical agents alone may be able to control disease completely. For those with more severe disease, topical agents are used adjunctively with systemic or biologic agents to optimize disease control in localized areas.
The American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) published guidelines in 2020 for managing psoriasis with topical agents in adults.2 This review presents the most up-to-date clinical recommendations for topical agent use in adult patients with psoriasis and elaborates on each drug’s pharmacologic and safety profile. Specifically, evidence-based treatment recommendations for topical steroids, calcineurin inhibitors (CNIs), vitamin D analogues, retinoids (tazarotene), emollients, keratolytics (salicylic acid), anthracenes (anthralin), and keratoplastics (coal tar) will be addressed (Table 1). Recommendations for combination therapy with other treatment modalities including UVB light therapy, biologics, and systemic nonbiologic agents also will be discussed.


Selecting a Topical Agent Based on Disease Localization
When treating patients with psoriasis with topical therapies, clinicians should take into consideration drug potency, as it determines how effective a treatment will be in penetrating the skin barrier. Plaque characteristics, such as distribution (localized vs widespread), anatomical localization (flexural, scalp, palms/soles/nails), size (large vs small), and thickness (thick vs thin), not only influence treatment effectiveness but also the incidence of drug-related adverse events. Furthermore, preferred topical therapies are tailored to each patient based on disease characteristics and activity. Coal tar and anthralin have been used less frequently than other topical therapies for psoriasis because of their undesirable side-effect profiles (Table 1).3
Face and Intertriginous Regions—The face and intertriginous areas are sensitive because skin tends to be thin in these regions. Emollients are recommended for disease in these locations given their safety and flexibility in use for most areas. Conversely, anthralin should be avoided on the face, intertriginous areas, and even highly visible locations because of the potential for skin staining. Low-potency corticosteroids also have utility in psoriasis distributed on the face and intertriginous regions. Additionally, application of steroids around the eyes should be cautioned because topical steroids can induce ocular complications such as glaucoma and cataracts in rare circumstances.4
Off-label use of CNIs for psoriasis on the face and intertriginous areas also is effective. Currently, there is a level B recommendation for off-label use of 0.1% tacrolimus for up to 8 weeks for inverse psoriasis or psoriasis on the face. Off-label use of pimecrolimus for 4 to 8 weeks also can be considered for inverse psoriasis. Combination therapy consisting of hydrocortisone with calcipotriol ointment is another effective regimen.5 One study also suggested that use of crisaborole for 4 to 8 weeks in intertriginous psoriasis can be effective and well tolerated.6
Scalp—The vehicle of medication administration is especially important in hair-bearing areas such as the scalp, as these areas are challenging for medication application and patient adherence. Thus, patient preferences for the vehicle must be considered. Several studies have been conducted to assess preference for various vehicles in scalp psoriasis. A foam or solution may be preferable to ointments, gels, or creams.7 Gels may be preferred over ointments.8 There is a level A recommendation supporting the use of class 1 to 7 topical steroids for a minimum of 4 weeks as initial and maintenance treatment of scalp psoriasis. The highest level of evidence (level A) also supports the use of calcipotriol foam or combination therapy of calcipotriol–betamethasone dipropionate gel for 4 to 12 weeks as treatment of mild to moderate scalp psoriasis.
Nails—Several options for topical medications have been recommended for the treatment of nail psoriasis. Currently, there is a level B recommendation for the use of tazarotene for the treatment of nail psoriasis. Another effective regimen is combination therapy with vitamin D analogues and betamethasone dipropionate.9 Topical steroid use for nail psoriasis should be limited to 12 weeks because of the risk for bone atrophy with chronic steroid use.
Palmoplantar—The palms and soles have a thicker epidermal layer than other areas of the body. As a result, class 1 corticosteroids can be used for palmoplantar psoriasis for more than 4 weeks with vigilant monitoring for adverse effects such as skin atrophy, tachyphylaxis, or tinea infection. Tazarotene also has been shown to be helpful in treating palmoplantar psoriasis.
Resistant Disease—Intralesional steroids are beneficial treatment options for recalcitrant psoriasis in glabrous areas, as well as for palmoplantar, nail, and scalp psoriasis. Up to 10 mg/mL of triamcinolone acetonide used every 3 to 4 weeks is an effective regimen.10Pregnancy/Breastfeeding—Women of childbearing potential have additional safety precautions that should be considered during medication selection. Emollients have been shown to be safe during pregnancy and lactation. Currently, there is little known about CNI use during pregnancy. During lactation, CNIs can be used by breastfeeding mothers in most areas, excluding the breasts. Evaluation of the safety of anthralin and vitamin D analogues during pregnancy and lactation have not been studied. For these agents, dermatologists need to use their clinical judgment to weigh the risks and benefits of medication, particularly in patients requiring occlusion, higher medication doses, or treatment over a large surface area. Salicylic acid should be used with caution in pregnant and breastfeeding mothers because it is a pregnancy category C drug. Lower-potency corticosteroids may be used with caution during pregnancy and breastfeeding. More potent corticosteroids and coal tar, however, should be avoided. Similarly, tazarotene use is contraindicated in pregnancy. According to the US Food and Drug Administration labels for all forms of topical tazarotene, a pregnancy test must be obtained 2 weeks prior to tazarotene treatment initiation in women of childbearing potential because of the risk for serious fetal malformations and toxicity.
Recommendations, Risks, and Benefits of Topical Therapy for the Management of Psoriasis
Topical Corticosteroids—Topical corticosteroids (TCs) are widely used for inflammatory skin conditions and are available in a variety of strengths (Table 2). They are thought to exert their action by regulating the gene transcription of proinflammatory mediators. For psoriasis, steroids are recommended for 2 to 4 weeks, depending on disease severity. Although potent and superpotent steroids are more effective than mild- to moderate-strength TCs, use of lower-potency TCs may be warranted depending on disease distribution and localization.11 For treatment of psoriasis with no involvement of the intertriginous areas, use of class 1 to 5 TCs for up to 4 weeks is recommended.

For moderate to severe psoriasis with 20% or less body surface area (BSA) affected, combination therapy consisting of mometasone and salicylic acid has been shown to be more effective than mometasone alone.12,13 There currently is a level A recommendation for the use of combination therapy with class 1 TCs and etanercept for 12 weeks in patients with moderate to severe psoriasis who require both systemic and topical therapies for disease control. Similarly, combination therapy with infliximab and high-potency TCs has a level B recommendation to enhance efficacy for the treatment of moderate to severe psoriasis.14 High-quality studies on the use of TCs with anti–IL-12/IL-23, anti–IL-23, and anti–IL-17 currently are unavailable, but the combination is not expected to be unsafe.14,15 Combination therapy of betamethasone dipropionate ointment and low-dose cyclosporine is an alternative regimen with a level B recommendation.
The most common adverse effects with use of TCs are skin thinning and atrophy, telangiectasia, and striae (Table 1). With clinical improvement of disease, it is recommended that clinicians taper TCs to prevent rebound effect. To decrease TC-related adverse effects, clinicians should use combination therapy with steroid-sparing agents for disease maintenance, transition to lower-potency corticosteroids, or use intermittent steroid therapy. Systemic effects of TC use include hypothalamic-pituitary-adrenal axis suppression, Cushing syndrome, and osteonecrosis of the femoral head.16-18 These systemic effects with TC use are rare unless treatment is for disease involving greater than 20% BSA or occlusion for more than 4 weeks.
Calcineurin Inhibitors—Calcineurin inhibitors inhibit calcineurin phosphorylation and T-cell activation, subsequently decreasing the expression of proinflammatory cytokines. Currently, they are not approved by the US Food and Drug Administration to treat psoriasis but have demonstrated efficacy in randomized control trials (RCTs) for facial and intertriginous psoriasis. In RCTs, 71% of patients using pimecrolimus cream 0.1% twice daily for 8 weeks achieved an investigator global assessment score of clear (0) or almost clear (1) compared with 21% of placebo-treated patients (N=57).19 Other trials have shown that 65% of patients receiving tacrolimus ointment 0.1% for 8 weeks achieved an investigator global assessment score of 0 or 1 compared with 31% of placebo-treated patients (N=167).20 Because of their efficacy in RCTs, CNIs commonly are used off label to treat psoriasis.
The most common adverse effects with CNI use are burning, pruritus, and flushing with alcohol ingestion (Table 1). Additionally, CNIs have a black box warning that use may increase the risk for malignancy, but this risk has not been demonstrated with topical use in humans.21Vitamin D Analogues—The class of vitamin D analogues—calcipotriol/calcipotriene and calcitriol—frequently are used to treat psoriasis. Vitamin D analogues exert their beneficial effects by inhibiting keratinocyte proliferation and enhancing keratinocyte differentiation. They also are ideal for long-term use (up to 52 weeks) in mild to moderate psoriasis and can be used in combination with class 2 and 3 TCs. There is a level A recommendation that supports the use of combination therapy with calcipotriol and TCs for the treatment of mild to moderate psoriasis.
For severe psoriasis, many studies have investigated the efficacy of combination therapy with vitamin D analogues and systemic treatments. Combination therapy with calcipotriol and methotrexate or calcipotriol and acitretin are effective treatment regimens with level A recommendations. Calcipotriol–betamethasone dipropionate ointment in combination with low-dose cyclosporine is an alternative option with a level B recommendation. Because vitamin D analogues are inactivated by UVA and UVB radiation, clinicians should advise their patients to use vitamin D analogues after receiving UVB phototherapy.22
Common adverse effects of vitamin D analogues include burning, pruritus, erythema, and dryness (Table 1). Hypercalcemia and parathyroid hormone suppression are extremely rare unless treatment occurs over a large surface area (>30% BSA) or the patient has concurrent renal disease or impairments in calcium metabolism.
Tazarotene—Tazarotene is a topical retinoid that acts by decreasing keratinocyte proliferation, facilitating keratinocyte differentiation, and inhibiting inflammation. Patients with mild to moderate psoriasis are recommended to receive tazarotene treatment for 8 to 12 weeks. In several RCTs, tazarotene gel 0.1% and tazarotene cream 0.1% and 0.05% achieved treatment success in treating plaque psoriasis.23,24
For increased efficacy, clinicians can recommend combination therapy with tazarotene and a TC. Combination therapy with tazarotene and a mid- or high-potency TC for 8 to 16 weeks has been shown to be more effective than treatment with tazarotene alone.25 Thus, there is a level A recommendation for use of this combination to treat mild to moderate psoriasis. Agents used in combination therapy work synergistically to decrease the length of treatment and increase the duration of remission. The frequency of adverse effects, such as irritation from tazarotene and skin atrophy from TCs, also are reduced.26 Combination therapy with tazarotene and narrowband UVB (NB-UVB) is another effective option that requires less UV radiation than NB-UVB alone because of the synergistic effects of both treatment modalities.27 Clinicians should counsel patients on the adverse effects of tazarotene, which include local irritation, burning, pruritus, and erythema (Table 1).
Emollients—Emollients are nonmedicated moisturizers that decrease the amount of transepidermal water loss. There is a level B recommendation for use of emollients and TCs in combination for 4 to 8 weeks to treat psoriasis. In fact, combination therapy with mometasone and emollients has demonstrated greater improvement in symptoms of palmoplantar psoriasis (ie, erythema, desquamation, infiltration, BSA involvement) than mometasone alone.28 Emollients are safe options that can be used on all areas of the body and during pregnancy and lactation. Although adverse effects of emollients are rare, clinicians should counsel patients on the risk for contact dermatitis if specific allergies to ingredients/fragrances exist (Table 1).
Salicylic Acid—Salicylic acid is a topical keratolytic that can be used to treat psoriatic plaques. Use of salicylic acid for 8 to 16 weeks has been shown to be effective for mild to moderate psoriasis. Combination therapy of salicylic acid and TCs in patients with 20% or less BSA affected is a safe and effective option with a level B recommendation. Combination therapy with salicylic acid and calcipotriene, however, should be avoided because calcipotriene is inactivated by salicylic acid. It also is recommended that salicylic acid application follow phototherapy when both treatment modalities are used in combination.29,30 Clinicians should be cautious about using salicylic acid in patients with renal or hepatic disease because of the increased risk for salicylate toxicity (Table 1).
Anthralin—Anthralin is a synthetic hydrocarbon derivative that has been shown to reduce inflammation and normalize keratinocyte proliferation through an unknown mechanism. It is recommended that patients with mild to moderate psoriasis receive anthralin treatment for 8 to 12 weeks, with a maximum application time of 2 hours per day. Combination therapy of excimer laser and anthralin has been shown to be more effective in treating psoriasis than anthralin alone.31 Therefore, clinicians have the option of including excimer laser therapy for additional disease control. Anthralin should be avoided on the face, flexural regions, and highly visible areas because of potential skin staining (Table 1). Other adverse effects include application-site burning and erythema.
Coal Tar—Coal tar is a heterogenous mixture of aromatic hydrocarbons that is an effective treatment of psoriasis because of its inherent anti-inflammatory and keratoplastic properties. There is high-quality evidence supporting a level A recommendation for coal tar use in mild to moderate psoriasis. Combination therapy with NB-UVB and coal tar (also known as Goeckerman therapy) is a recommended treatment option with a quicker onset of action and improved outcomes compared with NB-UVB therapy alone.32,33 Adverse events of coal tar include application-site irritation, folliculitis, contact dermatitis, phototoxicity, and skin pigmentation (Table 1).
Conclusion
Topical medications are versatile treatment options that can be utilized as monotherapy or adjunct therapy for mild to severe psoriasis. Benefits of topical agents include minimal required monitoring, few contraindications, and direct localized effect on plaques. Therefore, side effects with topical agent use rarely are systemic. Medication interactions are less of a concern with topical therapies; thus, they have better safety profiles compared with systemic therapies. This clinical review summarizes the recently published evidence-based guidelines from the AAD and NPF on the use of topical agents in psoriasis and may be a useful guiding framework for clinicians in their everyday practice.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
- Murage MJ, Kern DM, Chang L, et al. Treatment patterns among patients with psoriasis using a large national payer database in the United States: a retrospective study. J Med Econ. 2018:1-9.
- Elmets CA, Korman NJ, Prater EF, et al. Joint AAD-NPF Guidelines of care for the management and treatment of psoriasis with topical therapy and alternative medicine modalities for psoriasis severity measures. J Am Acad Dermatol. 2021;84:432-470.
- Svendsen MT, Jeyabalan J, Andersen KE, et al. Worldwide utilization of topical remedies in treatment of psoriasis: a systematic review. J Dermatolog Treat. 2017;28:374-383.
- Day A, Abramson AK, Patel M, et al. The spectrum of oculocutaneous disease: part II. neoplastic and drug-related causes of oculocutaneous disease. J Am Acad Dermatol. 2014;70:821.e821-819.
- Choi JW, Choi JW, Kwon IH, et al. High-concentration (20 μg g-¹) tacalcitol ointment in the treatment of facial psoriasis: an 8-week open-label clinical trial. Br J Dermatol. 2010;162:1359-1364.
- Hashim PW, Chima M, Kim HJ, et al. Crisaborole 2% ointment for the treatment of intertriginous, anogenital, and facial psoriasis: a double-blind, randomized, vehicle-controlled trial. J Am Acad Dermatol. 2020;82:360-365.
- Housman TS, Mellen BG, Rapp SR, et al. Patients with psoriasis prefer solution and foam vehicles: a quantitative assessment of vehicle preference. Cutis. 2002;70:327-332.
- Iversen L, Jakobsen HB. Patient preferences for topical psoriasis treatments are diverse and difficult to predict. Dermatol Ther. 2016;6:273-285.
- Clobex Package insert. Galderma Laboratories, LP; 2012.
- Kenalog-10 Injection. Package insert. Bristol-Myers Squibb Company; 2018.
- Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: a systematic review. Br J Dermatol. 2002;146:351-364.
- Koo J, Cuffie CA, Tanner DJ, et al. Mometasone furoate 0.1%-salicylic acid 5% ointment versus mometasone furoate 0.1% ointment in the treatment of moderate-to-severe psoriasis: a multicenter study. Clin Ther. 1998;20:283-291.
- Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905-912.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Strober BE, Bissonnette R, Fiorentino D, et al. Comparative effectiveness of biologic agents for the treatment of psoriasis in a real-world setting: results from a large, prospective, observational study (Psoriasis Longitudinal Assessment and Registry [PSOLAR]). J Am Acad Dermatol. 2016;74:851-861.e854.
- Castela E, Archier E, Devaux S, et al. Topical corticosteroids in plaque psoriasis: a systematic review of risk of adrenal axis suppression and skin atrophy. J Eur Acad Dermatol Venereol. 2012;26(suppl 3):47-51.
- Takahashi H, Tsuji H, Honma M, et al. Femoral head osteonecrosis after long-term topical corticosteroid treatment in a psoriasis patient. J Dermatol. 2012;39:887-888.
- el Maghraoui A, Tabache F, Bezza A, et al. Femoral head osteonecrosis after topical corticosteroid therapy. Clin Exp Rheumatol. 2001;19:233.
- Gribetz C, Ling M, Lebwohl M, et al. Pimecrolimus cream 1% in the treatment of intertriginous psoriasis: a double-blind, randomized study. J Am Acad Dermatol. 2004;51:731-738.
- Lebwohl M, Freeman AK, Chapman MS, et al. Tacrolimus ointment is effective for facial and intertriginous psoriasis. J Am Acad Dermatol. 2004;51:723-730.
- Paller AS, Fölster-Holst R, Chen SC, et al. No evidence of increased cancer incidence in children using topical tacrolimus for atopic dermatitis. J Am Acad Dermatol. 2020;83:375-381.
- Elmets CA, Lim HW, Stoff B, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81:775-804.
- Lebwohl M, Ast E, Callen JP, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38:705-711.
- Weinstein GD, Koo JY, Krueger GG, et al. Tazarotene cream in the treatment of psoriasis: two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760-767.
- Lebwohl M, Lombardi K, Tan MH. Duration of improvement in psoriasis after treatment with tazarotene 0.1% gel plus clobetasol propionate 0.05% ointment: comparison of maintenance treatments. Int J Dermatol. 2001;40:64-66.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Koo JY, Lowe NJ, Lew-Kaya DA, et al. Tazarotene plus UVB phototherapy in the treatment of psoriasis. J Am Acad Dermatol. 2000;43:821-828.
- Cassano N, Mantegazza R, Battaglini S, et al. Adjuvant role of a new emollient cream in patients with palmar and/or plantar psoriasis: a pilot randomized open-label study. G Ital Dermatol Venereol. 2010;145:789-792.
- Kristensen B, Kristensen O. Topical salicylic acid interferes with UVB therapy for psoriasis. Acta Derm Venereol. 1991;71:37-40.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis. section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Rogalski C, Grunewald S, Schetschorke M, et al. Treatment of plaque-type psoriasis with the 308 nm excimer laser in combination with dithranol or calcipotriol. Int J Hyperthermia. 2012;28:184-190.
- Bagel J. LCD plus NB-UVB reduces time to improvement of psoriasis vs. NB-UVB alone. J Drugs Dermatol. 2009;8:351-357.
- Abdallah MA, El-Khateeb EA, Abdel-Rahman SH. The influence of psoriatic plaques pretreatment with crude coal tar vs. petrolatum on the efficacy of narrow-band ultraviolet B: a half-vs.-half intra-individual double-blinded comparative study. Photodermatol Photoimmunol Photomed. 2011;27:226-230.
Practice Points
- Topical medications collectively represent the most common form of psoriasis treatment. Depending on disease severity and distribution, topical agents can be used as monotherapy or adjunct therapy, offering the benefit of localized treatment without systemic side effects.
- Dermatologists should base the selection of an appropriate topical medication on factors including adverse effects, potency, vehicle, and anatomic localization of disease.
Guidance From the National Psoriasis Foundation COVID-19 Task Force
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
When COVID-19 emerged in March 2020, physicians were forced to evaluate the potential impacts of the pandemic on our patients and the conditions that we treat. For dermatologists, psoriasis came into particular focus, as many patients were being treated with biologic therapies. The initial concern was that these biologics might render our patients more susceptible to both COVID-19 infection and/or a more severe disease course.
In early 2020, the National Psoriasis Foundation (NPF) presented its own recommendations for treating patients with psoriatic disease during the pandemic.1 Some highlights included the following1:
• At the time, it was stipulated that patients with COVID-19 infection should stop taking a biologic.
• Psoriasis patients in high-risk groups (eg, concomitant systemic disease) should discuss with their dermatologist if their therapeutic regimen should be continued or altered.
• Patients taking oral immunosuppressive therapy may be at greater risk for COVID-19 infection, though there is no strong COVID-19–related evidence to provide specific guidelines or risk level.
In May 2020, the NPF COVID-19 Task Force was formed. This group—chaired by dermatologist Joel M. Gelfand, MD, MSCE (Philadelphia, Pennsylvania), and rheumatologist Christopher T. Ritchlin, MD, MPH (Rochester, New York)—was comprised of members from both the NPF Medical Board and Scientific Advisory Committee in dermatology, rheumatology, infectious disease, and critical care. The NPF COVID-19 Task Force has been critical in keeping the dermatology community apprised of the latest scientific thinking related to COVID-19 and publishing guidance statements that are updated and amended on a regular basis as new data becomes available.2 Key recommendations most relevant to the daily care of patients with psoriatic disease included the following2:
• Patients with psoriasis and/or psoriatic arthritis have similar rates of SARS-CoV-2 infection and COVID-19 outcomes as the general population based on existing data, with some exceptions.
• Therapies for psoriasis and/or psoriatic arthritis do not meaningfully alter the risk for acquiring SARS-CoV-2 infection or having worse COVID-19 outcomes.
• Patients should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases, unless they become infected with SARS-CoV-2.
• Chronic systemic steroid use for psoriatic disease in the setting of acute infection with COVID-19 may be associated with worse outcomes; however, steroids may improve outcomes for COVID-19 when initiated in hospitalized patients who require oxygen therapy.
• When local restrictions or pandemic conditions limit the ability for in-person visits, offer telemedicine to manage patients.
• Patients with psoriatic disease who do not have contraindications to vaccination should receive a messenger RNA (mRNA)–based COVID-19 vaccine and boosters, based on federal, state, and local guidance. Systemic medications for psoriasis or psoriatic arthritis are not a contraindication to the mRNA-based COVID-19 vaccine.
• Patients who are to receive an mRNA-based COVID-19 vaccine should continue their biologic or oral therapies for psoriasis and/or psoriatic arthritis in most cases.
• The use of hydroxychloroquine, chloroquine, and ivermectin is not suggested for the prevention or treatment of COVID-19 disease.
These guidelines have been critical in addressing some of the most pressing issues in psoriasis patient care, particularly the susceptibility to COVID-19, the role of psoriasis therapies in initial infection and health outcomes, and issues related to the administration of vaccines in those on systemic therapies. Based on these recommendations, we have been given a solid foundation that our current standard of care can (for the most part) continue with the continued presence of COVID-19 in our society. I encourage all providers to familiarize themselves with the NPF COVID-19 Task Force guidelines and keep abreast of updates as they become available (https://www.psoriasis.org/covid-19-task-force-guidance-statements/).
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
- Gelfand JM, Armstrong AW, Bell S, et al. National Psoriasis Foundation COVID-19 Task Force guidance for management of psoriatic disease during the pandemic: version 1. J Am Acad Dermatol. 2020;83:1704-1716.
- COVID-19 Task Force guidance statements. National Psoriasis Foundation website. Updated April 28, 2022. Accessed July 12, 2022. https://www.psoriasis.org/covid-19-task-force-guidance-statements/
Firm Exophytic Tumor on the Shin
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
A 62-year-old man presented with a firm, exophytic, 2.8×1.5-cm tumor on the left shin of 6 to 7 years’ duration. An excisional biopsy was obtained for histopathologic evaluation.
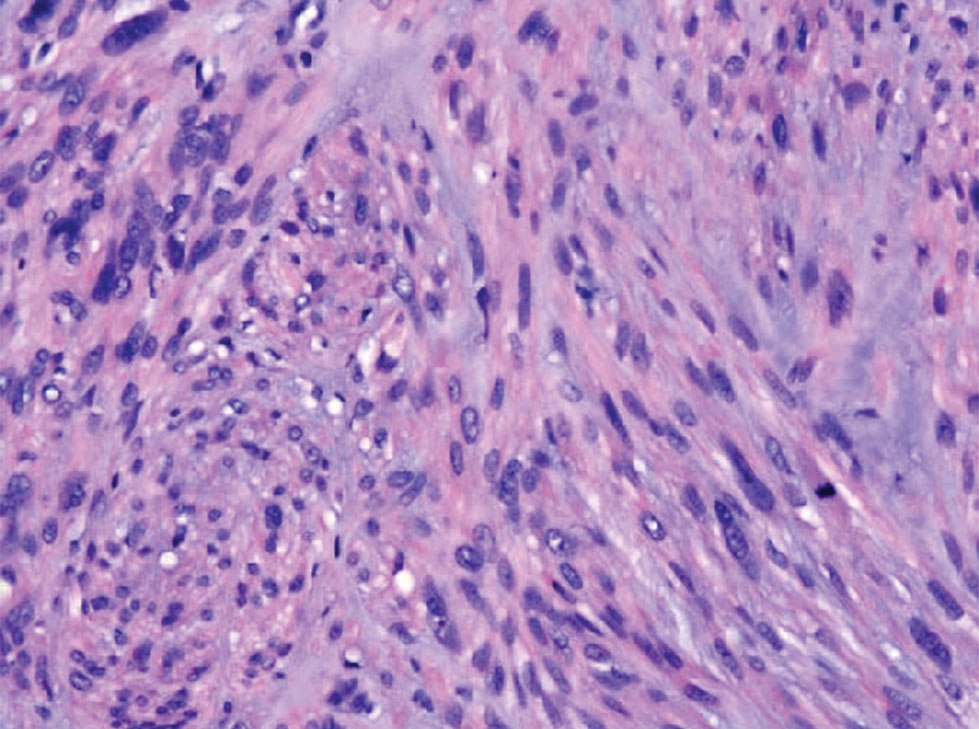
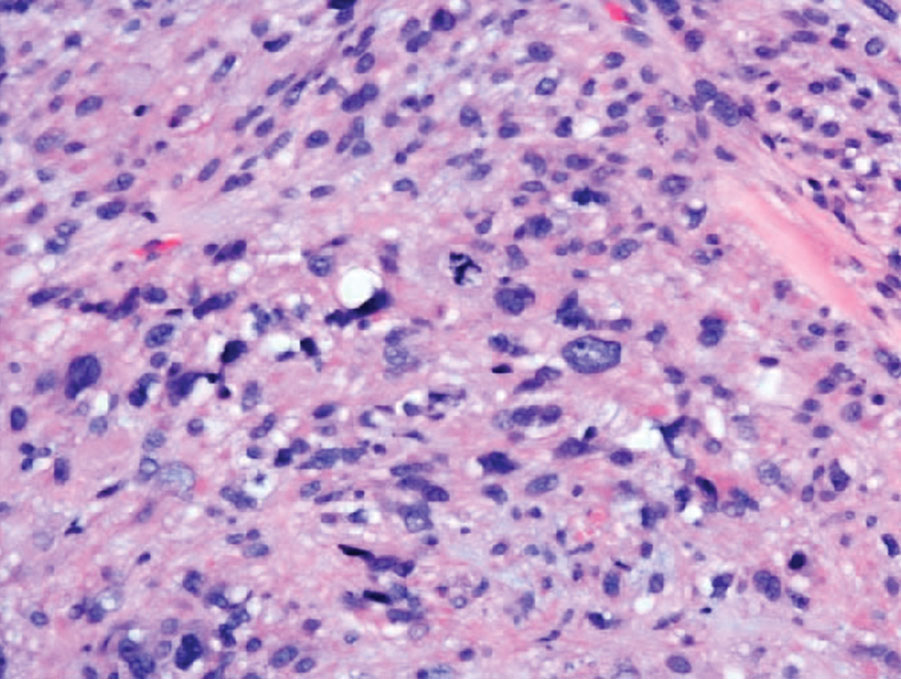
Unique Treatment for Alopecia Areata Combining Epinephrine With an Intralesional Steroid
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.
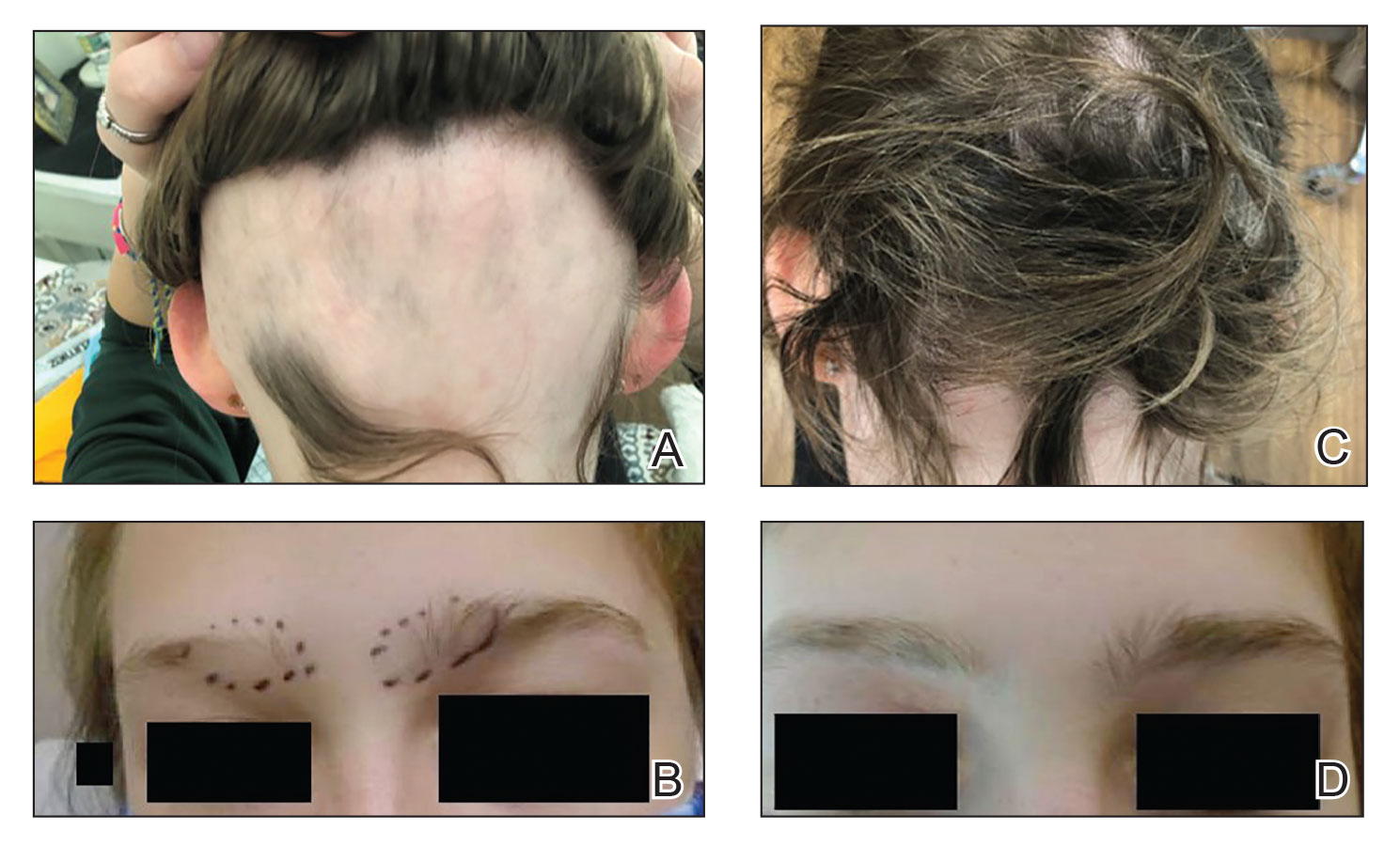
Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.
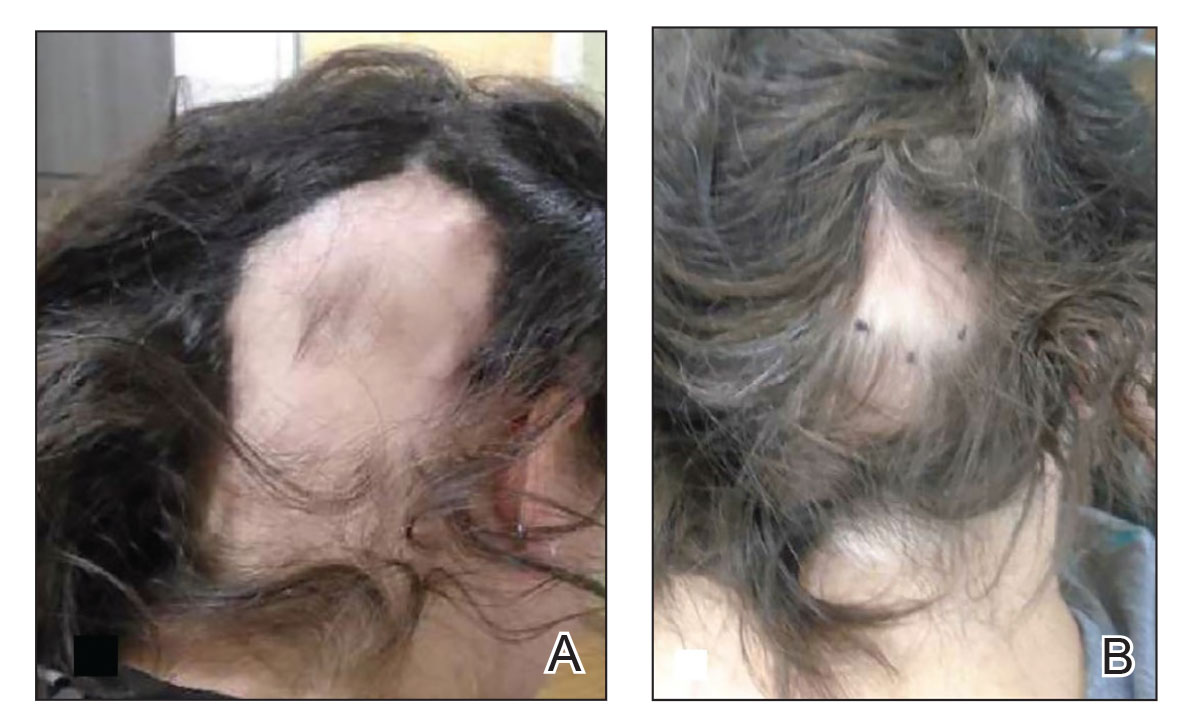
Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.
Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Practice Points
- Patients with alopecia areata that is refractory to first-line treatments may benefit from intralesional triamcinolone acetonide (ILTA) diluted to 2.5 mg/mL in 1% lidocaine and epinephrine 1:100,000 in place of normal saline.
- Local vasoconstriction due to epinephrine may potentiate ILTA effects and play an independent role.
2022 Update on female sexual health
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
Many authors have commented on the lack of research into female sexual dysfunction, especially when compared with the hundreds of research publications related to male sexual health and dysfunction. Not surprisingly, very little has been published in the past year on the subject of female sexual health.
Recently, the International Society for the Study of Women’s Sexual Health (ISSWSH) published 2 important papers: a guideline on the use of testosterone for hypoactive sexual desire disorder (HSDD) in women and a consensus document on the management of persistent genital arousal disorder (PGAD). The lack of funding and support for female sexual health leaves women’s health professionals with little education or guidance on how to identify and treat conditions that are likely as common in women as erectile dysfunction is in men. While we would like to rely on randomized trials to inform our clinical care, the very limited literature on female sexual health makes this difficult. Bringing together experienced clinicians who focus their practices on sexual health, ISSWSH has provided some much-needed recommendations for the management of difficult conditions.
ISSWSH provides clinical guidance on testosterone therapy for women with HSDD
Parish S, Simon J, Davis S, et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J Sex Med. 2021;18:849-867.
For development of the ISSWSH clinical practice guideline on testosterone therapy for women with HSDD, 16 international researchers and clinicians were convened. A modified Delphi method was used to establish consensus at the meeting on the recommended indications for testosterone treatment, formulations, and when measurement of testosterone levels is appropriate.
An extensive evidence-based literature review was performed, which included original research, meta-analyses, reviews, and clinical practice guidelines, to address the use of testosterone in women for management of HSDD. Notably, in 2019, representatives of 10 medical societies published a Global Consensus Position Statement on the Use of Testosterone Therapy for Women that reviewed the existing literature on testosterone’s effects on sexual dysfunction, mood, cognition, musculoskeletal, cardiovascular, and breast health as well as androgenic side effects and adverse events.1 Based on their review, the only evidence-based indication for testosterone use is for the treatment of HSDD.
Testosterone formulations, HSDD diagnosis, and sex steroid physiology
More than 10 years ago, the US Food and Drug Administration (FDA) reviewed an application for the use of a transdermal testosterone patch (Intrinsa) in women for the treatment of HSDD. Efficacy of treatment was clearly demonstrated, and no safety signals were found in the placebo-controlled trial. Based, however, on the opinions of regulators who were “concerned” about the potential for cardiovascular adverse outcomes and worry that the peripheral conversion of testosterone to estradiol might lead to an increase in breast cancer—worry generated from the findings of the Women’s Health Initiative (which did not demonstrate an increase in breast cancer risk with estrogen alone but only when estrogen was combined with medroxyprogesterone acetate)—the FDA declined to approve the testosterone patch for women.
The Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) defined HSDD as “persistent or recurrent deficiency or absence of sexual fantasies and desire for sexual activity with marked distress or interpersonal difficulty.” The guideline authors noted that although the DSM-5 edition merged female arousal disorder with desire disorder into a single diagnosis, they used the DSM-IV definition as it had been the basis for the studies and literature reviewed. HSDD is a prevalent condition worldwide that affects between 12% and 53% of peri- and postmenopausal women.
The consensus guideline authors extensively reviewed the physiology and mechanism of action of sex steroids in women, particularly their impact on sexual function and the biologic alterations that occur during peri- and postmenopause.
Continue to: Consensus position and recommendations...
Consensus position and recommendations
The ISSWSH consensus guideline concluded that there is a moderate therapeutic benefit in adding testosterone therapy to achieve up to premenopausal levels in postmenopausal women with self-reported reduction in sexual desire that is causing distress as determined by a validated instrument.
The authors advise baseline hormone testing to rule out androgen excess and baseline renal, lipid, liver, and metabolic testing, even though transdermal testosterone therapy was not shown to alter these parameters in randomized trials of more than 3,000 women. Laboratory assays for both total and free testosterone are “highly unreliable” in the female range as they have been calibrated for male levels of hormone.
FDA-approved testosterone treatments for men with hypogonadism include transdermal gels, patches, intramuscular injection, and an oral formulation. Dosing for women is approximately one-tenth the dosage for treatment of men. Patients should be informed that this treatment is off-label and that long-term studies to establish safety are not available. The authors advised against the use of compounded formulations based on the National Academies of Science, Engineering, and Medicine guidelines, but they went on to say that if compounded products are used, the pharmacy should adhere to Good Manufacturing Practice and Active Pharmaceutical Ingredients standards.
Transdermal testosterone is beneficial for the treatment of HSDD in postmenopausal women after other causes of decreased desire, such as dyspareunia, relationship issues, and other general medical conditions, have been ruled out. There is no diagnostic laboratory test to confirm HSDD or to use as a therapeutic target in treatment (for total or free testosterone, as these are highly unreliable laboratory values). Although large trials have identified no safety signals, they were generally limited to 6 months in duration. Prescribing one-tenth the dose indicated for male hypogonadism results in premenopausal testosterone levels for most women. If there is no benefit after 6 months of treatment, testosterone should be discontinued.
Rare, complex sexual function disorder requires integrated biopsychosocial approach, says ISSWSH
Goldstein I, Komisaruk BR, Pukall CF, et al. International Society for the Study of Women’s Sexual Health (ISSWSH) review of epidemiology and pathophysiology, and a consensus nomenclature and process of care for the management of persistent genital arousal disorder/genito-pelvic dyesthesia (PGAD/GPD). J Sex Med. 2021;18:665-697.
Persistent genital arousal disorder is a poorly understood and relatively rare sexual dysfunction in women. The American College of Obstetricians and Gynecologists (ACOG) Practice Bulletin on Female Sexual Dysfunction does not mention this condition, leaving women’s health practitioners with little guidance as to diagnosis or management.2 Prevalence for the condition is estimated at 1% to 3%. The symptoms may be intermittent or continuous.
In a recent ISSWSH review, a consensus panel defined 5 criteria for this disorder: the perception of genital arousal that is involuntary, unrelated to sexual desire, without any identified cause, not relieved with orgasm, and distressing to the patient. The panel made a clear distinction between PGAD/ genito-pelvic dysesthesia (GPD) and Compulsive Sexual Behavior Disorder (defined by the International Classification of Diseases revision 11 as “a persistent pattern of failure to control intense, repetitive sexual impulses or urges). Because there is considerable overlap with syndromes of genital dysesthesia—itching, burning, tingling, or pain— the consensus panel elected to expand the nomenclature to describe both persistent genital arousal and genito-pelvic dysesthesia as a single syndrome, namely, PGAD/GPD.
Continue to: Negative impact of PGAD/GPD...
Negative impact of PGAD/GPD
The consensus panel identified several contributors to the overall morbidity of this complex disorder, including end organ pathology, peripheral nerve, spinal cord and central sensory processing malfunction, and significant psychological issues. PGAD/GPD also may be associated with spinal cysts, cauda equina pathology, and withdrawal from selective serotonin reuptake inhibitors (SSRIs). Functional magnetic resonance imaging has identified specific brain regions (for example, the paracentral lobule) that are active during clitoral stimulation and that also activate during patients’ experience of persistent genital arousal.
PGAD/GPD negatively impacts sexual function, mental health, and ability to function in daily life. Of major importance is that a large proportion of people with this disorder have significant mental health disorders; in a survey, 54% of patients with PGAD reported suicidal ideation, compared with 25% of participants in a control group.
Evaluation and management recommendations
Diagnosis and management of PGAD/GPD are directed at the 5 areas of evaluation:
- end organ
- pelvis and perineum (assess for pelvic floor tension myalgia, pudendal neuropathy, pelvic congestion syndrome, or pelvic arteriovenous malformation)
- cauda equina (evaluate for neurologic deficits related to cysts compressing S2-S3 nerve roots)
- spinal cord (serotonin and norepinephrine pathways modulate nociceptive sensory activity; either SSRI/serotonin and norepinephrine reuptake inhibitor (SNRI) withdrawal or treatment could impact PGAD/ GPD based on their actions in the spinal cord)
- brain.
The consensus panel recommends an integrated biopsychosocial model for evaluation and treatment of PGAD/GPD. Comorbid mental health conditions, such as depression and anxiety, are common. Small studies suggest that a history of sexual trauma may contribute to catastrophizing and the experience of distressing persistent genital sensations, either arousal or dyesthesia, with 46.7% to 52.6% of patients reporting childhood sexual abuse.3
PGAD/GPD is a poorly recognized source of major distress to a small but significant group of patients. Diagnosis and management require a multidisciplinary team to identify end organ, pharmacologic, neurologic, vascular, and emotional components that contribute to the syndrome. Treatment requires a biopsychosocial approach that addresses the various sources of aberrant sensory processing, including end organ disease, neuropathic signaling, spinal cord pathways, and brain signal processing. Recognizing the existence of, and approaches to, this disorder will help gynecologists understand the considerable distress and potential life-threatening consequences our patients with PGAD/GPD experience.
Future possibilities and current actualities for patient care
Research dollars and investment in female sexual dysfunction remain inadequate to address the considerable gaps that exist in evidence-based clinical guidelines. ISSWSH is working to help clinicians approach these evidence gaps with guidelines and consensus statements to help women’s health professionals identify and manage our patients with sexual concerns and symptoms. An expert consensus guideline on the assessment and management of female orgasmic disorder is currently under development (personal communication, Dr. Sheryl Kingsberg). In addition, a phase 2b trial is underway to assess the impact of topical sildenafil cream for the treatment of female arousal disorder. Stay tuned for the results of these studies.
For now, women’s health professionals have 2 FDA-approved treatment options for premenopausal women with arousal disorder, flibanserin (a daily oral medication that requires abstinence from alcohol) and bremelanotide (an injectable medication that can be used just prior to a sexual encounter). For postmenopausal women, there are no FDA-approved therapies; however, based on the ISSWSH guideline summarized above, transdermal testosterone may be offered to postmenopausal women with distressing loss of sexual desire in doses approximately one-tenth those used to treat men with androgen deficiency. These small doses are challenging to achieve consistently with the delivery systems available for FDA-approved products sold for men.
The National Academies of Science, Engineering, and Medicine advise against the use of compounded hormonal products due to the potential for inconsistency and lack of FDA oversight in the manufacturing/compounding process. I have found and used some compounding pharmacies that are dedicated to safety, quality control, and compliance; test their products; and provide consistent, reliable compounded drugs for my patients. Consideration of compounded testosterone should be discussed with patients, and they should be informed of the current professional association guidelines. Testosterone creams may be compounded to a 1% product—20 mg/mL. Researchers in Australia have demonstrated that 5 mg of transdermal testosterone cream (one-quarter of a mL) results in typical premenopausal testosterone levels.4 When prescribing testosterone for postmenopausal women, check in with them after 6 weeks of treatment to assess impact and check blood levels to ensure that levels are not too high.
Testosterone pellets and intramuscular testosterone are not recommended and in fact should be actively avoided. These methods of administration are associated with extreme variation in hormone levels over time. There are typically supraphysiologic and quite high levels immediately after implantation or injection, followed by fairly significant drop-offs and rapid return of symptoms over time. This may lead to more and more frequent dosing and markedly elevated serum levels.
Management of PGAD/GPD is difficult, but knowing it exists as a valid syndrome will help clinicians validate patients’ symptoms and begin to approach appropriate evaluation and workup targeted to the 5 domains suggested by the ISSWSH expert panel. It is useful to understand the possible relationship to initiation or withdrawal from SSRIs or SNRIs and how aberrant norepinephrine signaling along the sensory pathways may contribute to genital dysesthesia or chronic sensations of arousal. Nonpharmacologic therapies, such as cognitive-behavioral therapy and others, are essential components of the multifaceted approach to treatment. Finally, many complex problems, such as chronic pelvic pain, vestibulodynia, vulvodynia, and chronic fatigue syndrome, are associated with childhood adverse experiences and sexual trauma. Approaching these patients with trauma-informed care is important to create the trust and therapeutic environment they need for successful multidisciplinary care. ●
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
- Davis SR, Baber R, Panay N, et al. Global consensus position statement on the use of testosterone therapy for women. J Sex Med. 2019;16:1331-1337.
- American College of Obstetricians and Gynecologists’ Committee on Practice Bulletins—Gynecology. ACOG practice bulletin no. 213: Female sexual dysfunction: clinical management guidelines for obstetrician-gynecologists. Obstet Gynecol. 2019;134:e1-e18.
- Leiblum S, Seehuus M, Goldmeier D, et al. Psychological, medical, and pharmacological correlates of persistent genital arousal disorder. J Sex Med. 2007;4:1358-1366.
- Fooladi E, Reuter SE, Bell RJ, et al. Pharmacokinetics of a transdermal testosterone cream in healthy postmenopausal women. Menopause. 2015;22:44-49.
Should we rethink maternal monitoring of fetal movement through “kick counts”?

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●

It is time to reconsider the recommendation for practicing fetal kick counts. A meta-analysis demonstrated no decrease in the outcome of stillbirth, but instead an increased risk of iatrogenic delivery.1
CASE 1 8 vs 10 fetal movements in 2 hours
Ms. M is 38 weeks pregnant with an uncomplicated pregnancy. She calls your practice with concerns about fetal kick counts. During her prenatal care, she was counseled to ensure that the baby moved 10 times over a period of 2 hours. This morning, however, she only perceived 8 movements in 2 hours. She is scheduled for evaluation with a nonstress test (NST) on the labor and delivery unit. The NST reveals a reassuring, reactive tracing. Ultrasonography evaluation demonstrates a normal amniotic fluid index and normal fetal growth. The patient is reassured, returns home, and goes on to deliver a healthy baby at 39 weeks and 5 days.
Perception of decreased movement triggers evaluation and monitoring
Maternal perception of normal fetal movement has conceivably been used throughout history as a means of reassurance of fetal well-being; it is highly predictive of fetal viability.2,3 When fetal movement is lacking or decreased, it can be an alarm sign and may result in concerns by the mother that her baby is unwell. Maternal perception of decreased fetal movements affects 5% to 15% of all pregnancies.2,4 While decreased fetal movement can be associated with poor perinatal outcomes such as fetal growth restriction, oligohydramnios, and neuro-developmental disability, it also can be reflective of more benign issues such as anterior placenta, maternal activity, maternal caffeine or sugar consumption, or maternal position.4,5
However, the definition of decreased fetal movement is subject to significant variation, from a total absence of movement over an entire day or what has commonly become accepted as the definition of fetal kick counts with Pearson’s Cardiff chart (which was defined in the 1970s as 10 movements within 12 hours).6,7 Today, women in the United States are commonly recommended to monitor their baby over a 2-hour period and to look for 10 movements during that time.8 Anything less is considered reduced fetal movement and results in recommendations to undergo assessment of previously known high-risk conditions or any possible underlying conditions, such as hypertension, gestational diabetes, or fetal growth restriction. Further evaluation with more objective measures such as electronic fetal monitoring or ultrasonography with biophysical profile are often recommended concurrently.9
It is estimated that up to 15% of women present reporting decreased fetal movement in the third trimester and, as such, require additional monitoring and evaluation. This is not without cost of time and money to the health care system and pregnant patients.
It is uncertain that fetal kick counting prevents stillbirth
Intrauterine fetal demise is neither an uncommon nor completely preventable outcome, despite advances in antenatal care. Many cases occur without evidence of fetal abnormality or other risk factors, and 30% to 55% of women who experience intrauterine fetal demise experience decreased fetal movement in the preceding week.10 It makes physiologic sense that a fetus’ adaptive response to decreased oxygenation is reduced fetal movement, resulting from the prioritization of blood to the fetal brain and other organs over skeletal muscle.4,9,11 Results of a 1976 small study of 61 low-risk pregnancies seemed to confirm that a decrease in fetal movement preceded intrauterine death by 3 to 4 days. Conversely, they found that a normal fetal movement count was generally associated with a good neonatal outcome.6 Thus, experts have long extrapolated that decreased fetal movement can be an indicator for utero-placental insufficiency and, in turn, chronic or acute hypoxia.
However, in larger studies, the ability of fetal movement counting to predict fetal death and fetal compromise appears limited.8,10,11 A meta-analysis of studies, including 5 randomized controlled trials and 468,000 fetuses, compared the incidence of stillbirth in women receiving instructions for fetal movement counting versus women who did not. Rates of stillbirth were the same for each group, demonstrating no advantage to fetal kick counts to prevent a poor perinatal outcome, including stillbirth.1
CASE 2 Reported reduced fetal movement over 4 weeks
Ms. E is a 20-year-old nullipara at 36 weeks’ and 6 days gestation who has come in to triage weekly for the last 4 weeks with concerns about decreased fetal movement. She states that she goes for several hours each day without feeling 10 movements in 2 hours. Recent fetal growth recorded 3 weeks ago was in the 45th percentile, and the amniotic fluid index has been above 10 cm on each weekly ultrasound. Her weekly NSTs have been reactive, and she has been normotensive. However, because she has had several weeks of persistent decreased fetal movement, the labor and delivery team opts to keep her for induction as she is “close to term.”
Decreased kick count frequency may increase unnecessary interventions
Women with fewer kick counts are more likely to present with concerns about the well-being of their baby. In a survey of obstetricians and midwives, a large proportion of providers were more apt to recommend delivery or admission to the hospital for women presenting with decreased fetal movements.2 It stands to reason that recommendations for delivery or admission can lead to outcomes like preterm delivery or recommendations for cesarean delivery (CD). However, using fetal kick counts to portend stillbirth or other poor fetal and neonatal outcomes has been shown to be limited in its value with the AFFIRM trial.10 The results of this large study, which included more than 400,000 pregnancies from 37 hospitals, show the challenges of any study to address the use of management strategies for recent change in the frequency of fetal movements in the reduction of and cause of stillbirth. Additionally, the relatively low risk of stillbirth overall (4.06 stillbirths per 1,000 livebirths during the intervention period and 4.40 per 1,000 livebirths during the control period) but higher incidence of other outcomes, such as prolonged (>48 hours) antepartum admission (6.7% in the intervention period and 6.2% in the control period), induction of labor (40.7% in the intervention period and 35.9% in the control period), and CD (28.4% and 25.5%, respectively) may result in increased harm for many women rather than the intended benefit of preventing stillbirth.10,12
Mindfetalness may be a viable and valuable alternative to kick counts
Alternatives have been proposed as a measure of fetal movement without using kick counts specifically. Mindfetalness has been a method studied in Sweden; its purpose is to strengthen the mother’s awareness of her baby through developing an understanding of the fetal-movement pattern. It is practiced starting at 28 weeks’ gestation for 15 minutes a day, with the woman instructed to lie on her left side and discern the intensity and character of the movements, as well as frequency, without overtly counting the movements.12 In one small study, women felt more connected to their babies and felt less worried.12 In a much larger study of 13,000 women, the authors found no evidence of harm from generalized awareness of fetal movements in a population of pregnant women at or beyond 32 weeks; in fact, they did see significant reductions in iatrogenic outcomes such as CDs and labor inductions
The case for movement awareness over kick counts
Stillbirth risk does not appear to be modified by the use of methods to detect fetal movement.10,12 However, a perceived decrease in fetal kick counts has been shown to result in increased interventions and preterm deliveries. A more prudent approach appears to be educating mothers about general fetal movement, which appears to reduce potentially unnecessary visits and interventions without sacrificing the ability to reassure mothers about the well-being of their babies in utero. ●
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.
- Haezell AEP, Green M, Wright C, et al. Midwives’ and obstetricians’ knowledge and management of women presenting with decreased fetal movements. Acta Obstetricia et Gynecologica. 2008:87;331-339. doi: 10.1080/00016340801902034.
- Froen JF. A kick from within – fetal movement counting and the cancelled progress in antenatal care. J Perinat Med. 2004;32:13-24. doi: 10.1515/JPM.2004.003.
- Heazell AEP, Froen JF. Methods of fetal movement counting and the detection of fetal compromise. J Obstet Gynaecol. 2008;28:147-154. doi: 10.1080/01443610801912618.
- Froen JF, Heazell AEP, Holm Tveit JV, et al. Fetal movement assessment. Semin Perinatal. 2008;32:243-246. doi: 10.1053/j.semperi.2008.04.004
- Pearson JF, Weaver JB. Fetal activity and fetal wellbeing: an evaluation. British Med J. 1976;1:1305-1307. doi: 10.1136/bmj.1.6021.1305.
- Pearson JF. Fetal movements – a new approach to antenatal care. Nursing Mirror Midwives J. 1977;144:49-51.
- American College of Obstetricians and Gynecologists’ Committee on Obstetric Practice, Society for Maternal-Fetal Medicine. Indications for outpatient antenatal fetal surveillance: ACOG committee opinion no. 828. Obstet Gynecol. 2021;137:e177-197. doi: 10.1097/AOG.0000000000004407.
- Christensen FC, Rayburn WF. Fetal movement counts. Obstet Gynecol Clin North Am. 1999;26:4(607-621). doi: 10.1016/s0889-8545(05)70102-9.
- Norman JE, Heazell AEP, Rodriguez A, et al. Awareness of fetal movements and care package to reduce fetal mortality (AFFIRM): a stepped wedge cluster-randomized trial. Lancet. 2018;392:1629-1638. doi: 10.1016/S0140-6736(18)31543-5.
- Warrender LK, Batra G, Bernatavicius G, et al. Maternal perception of reduced fetal movement is associated with altered placental structure and function. PLoS One. 2012;7:4. doi: 10.1371/journal.pone.0034851.
- Bellussi F, Po’ G, Livi A, et al. Fetal movement counting and perinatal mortality. A systematic review and meta-analysis. Obstet Gynecol. 2020;135:453-462. doi: 10.1097/AOG.0000000000003645.
- Akselsson A, Georgsson S, Lindgren H, et al. Women’s attitudes, experiences and compliance concerning the use of mindfetalness – a method for systematic observation of fetal movements in late pregnancy. BMC Pregnancy Childbirth. 2017;17:1-7. doi: 10.1186/s12884-017-1548-5.
- Akselsson A, Lindgren H, Skokic V, et al. A decrease in cesarean sections and labor inductions among Swedish women by awareness of fetal movements with the Mindfetalness method. BMC Pregnancy Childbirth. 2020;20;577:1-10.
How should you advise your 54-year-old patient about the use of HT?
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16
Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
CASE Healthy woman with hot flashes inquires about HT
A 54-year-old healthy woman with a history of hypothyroidism taking thyroid replacement medication comes in for her annual visit. Her last menstrual period was over 2 years ago and she reports severe hot flashes. They have greatly affected her quality of life and she must take frequent breaks at work. She wakes up frequently at night due to night sweats, which is impacting her sleep and, subsequently, her energy level. She has noted increased vaginal dryness so has been abstaining from sexual intercourse due to the discomfort. She has an intact uterus. Her family history is significant for heart disease, diagnosed in her mother at age 75.
On physical examination, she is normotensive and well-appearing. Her body mass index (BMI) is 21 kg/m2. Labs obtained prior to her visit show normal renal and liver function. Her high-density lipid (HDL) level is 55 mg/dL, her low-density lipid (LDL) level is 80 mg/dL, and her triglyceride level is 100 mg/dL; HbA1c is 5.5 mmol/mol.
She is interested in learning more about menopausal hormone therapy (HT) and whether or not she would be a candidate.
What information do you need to know to counsel and manage this patient?
Menopausal HT prescribing practices have changed over the last few decades as a better understanding of the risks and benefits of treatment have emerged. Prior to 2002, HT was commonly used for treatment of symptoms associated with menopause and was thought to have beneficial effects for chronic disease prevention.1-4 After data from the Women’s Health Initiative (WHI) was released, concerns arose around the effect of HT on cardiovascular health and risk of breast cancer. As a result, HT prescriptions fell precipitously after around 2002.5 Since then, postintervention analysis and cumulative 18-year follow-up of WHI data, along with results from subsequent randomized controlled trials, including the Kronos Early Estrogen Prevention Study (KEEPS) and the Early Versus Late Intervention Trial with Estradiol (ELITE), have demonstrated a favorable safety profile for healthy women starting HT early in menopause (less than age 60, or within 10 years from their final menstrual period).5-11
There are many types, formulations, and routes of HT, and the effects and risks differ for each (TABLE). For example, oral estrogen therapy, such as conjugated equine estrogens, portend a higher risk of adverse effects compared with transdermal formulations. Topical and transdermal estrogens bypass first-pass hepatic metabolism and thus are associated with a lower risk of venous thromboembolism (VTE) compared with oral formulations.12-14 A progestogen such as micronized progesterone is used in postmenopausal women with a uterus to protect the endometrium from unopposed estrogen therapy (ET). While it comes in oral and transdermal forms, the oral formulation is most widely used and studied in the United States; transdermal forms do not provide adequate endometrial protection and should not be used in combination therapy.15,16

Risks and benefits
Cardiovascular risk
Over time, the benefits and risks of HT use in menopausal patients have been further elucidated and defined, although they remain complex and dependent on patient clinical characteristics. HT remains the most effective treatment for vasomotor symptoms (VMS) and the genitourinary syndrome of menopause.17,18 In 2002, concerns for increased cardiovascular disease (CVD) and breast cancer risk resulted in early cessation of the WHI trial. Since that time the risk of CVD in postmenopausal women taking HT has been found to be more nuanced. In fact, updates in the literature have shown that HT results in a reduction of coronary heart disease if started in healthy women younger than age 60 years within 10 years of menopause.7,9-11 With this updated information, the North American Menopause society (NAMS), American College of Obstetricians and Gynecologists and the Endocrine Society have published guidelines supporting the initiation of HT for symptomatic healthy women: under the age of 60, within 10 years of menopause, and without contraindications. After age 60 years and further from menopause, the benefits and risks become less known.18-20
Risk stratification allows for more comprehensive counseling in use of HT for treatment of bothersome VMS. From a cardiovascular health standpoint, calculating an atherosclerotic CVD (ASCVD) risk score helps to evaluate appropriateness of HT prescribing:
- For those with low 10-year CVD risk (<5%), either oral or transdermal HT is appropriate.
- For those with moderate 10-year CVD risk (5%-10%), transdermal HT is recommended over oral HT.
- For those with high 10-year CVD risk (>10%), HT is not recommended.19,21
Breast cancer risk
Follow up since the initial WHI publication have shown that breast cancer risk is largely dependent on the formulation and route of HT used. Oral estrogen combined with a progestogen has been shown to increase the risk of invasive breast cancer, though very rarely.22 To put it into context, the absolute risk of breast cancer based on follow-up studies from WHI showed less than 1 additional case per 1,000 person years of use; less risk than associated with drinking 2 glasses of wine per day and similar to that of obesity and/or sedentary lifestyle.23,24 Studies have shown estrogen treatment alone for postmenopausal women does not appear to increase the risk of breast cancer. In fact, follow-up data from WHI showed a nonsignificant reduction in breast cancer risk for those taking ET alone.25
Breast cancer risk stratification is helpful when determining appropriateness of HT in postmenopausal women. Generally, if using risk stratification models for breast cancer (ie, Gail Risk model or international breast cancer intervention study [IBIS] tool), a patient who is average to moderate risk, HT can be offered with appropriate counseling. By contrast, a patient who is high risk should have a more detailed discussion about their risk (surveillance and risk-reducing treatments), and they may consider nonhormonal options for treatment of VMS. Women with a history of breast cancer should not be prescribed systemic HT.
Continue to: Additional HT benefits...
Additional HT benefits
The benefits of HT in postmenopausal women include improved bone health and reduction of fractures; reduction of risk for type 2 diabetes mellitus (T2DM); improvement of insulin sensitivity; improvement of lipid profiles with increased HDL and decreased LDL levels; and reduction of colon cancer risk.25 For women aged younger than 60 years who start HT within 10 years of their last menstrual period, HT has been shown to cause a reduction in all-cause mortality. Important risks to counsel patients on when starting HT include the low risk of stroke and venous thromboembolism (VTE) when using oral formulations.26
CASE Resolved
Her ASCVD risk score, based on her history, estimates her 10-year CVD risk to be low (<5%). Thus, from a cardiovascular standpoint, either oral or transdermal HT would be an appropriate option. Her IBIS 10-year score is 1.5%, placing her in a low-risk category for breast cancer based on her personal and family history. Given that she is less than 60 years of age and within 10 years of menopause, along with her low-risk stratification for CVD and breast cancer, she would be an appropriate patient to begin combined HT with an estrogen plus an oral progesterone, such as an estradiol patch 0.0375 mg twice weekly, along with oral micronized progesterone 100 mg nightly. The dose could be increased over time based on symptoms and tolerability of the treatment.
ALTERNATE CASE 1 The patient has additional risk factors
Consider the patient case with the following additions to her history: the patient has a BMI of 34 kg/m2, a history of well-controlled hypertension while taking amlodipine 5 mg, and an ASCVD risk score of 7.5%. She reports severe VMS that are greatly impacting her quality of life. How would your recommendations or counseling change?
Focus on healthy lifestyle
Obesity and hypertension, both common chronic conditions, pose additional risks to be accounted for when counseling on and approaching HT prescribing. Her alternate ASCVD risk score places her at moderate risk for CVD within 10 years, based on guidelines as discussed above. It would still be appropriate to offer her combined HT after a shared decision-making discussion that includes a focus on healthy lifestyle habits.
Consider transdermal HT in obese women
Longitudinal studies have found that weight gain is more a consequence of aging, regardless of menopausal status. Fat distribution and body composition changes are a menopause-related phenomenon driven by estrogen deficiency. HT has been shown to preserve lean body mass and reduce visceral adiposity, resulting in favorable effects of body composition. Still, obesity results in increased risk of CVD, VTE, and certain hormone-sensitive cancers.27 When considering HT in obese patients, a transdermal estrogen route is preferred to reduce risks.
For women with hypertension, prescribe transdermal HT
Overall, studies have found that HT has a neutral effect on blood pressure.25 When considering formulation of HT, micronized progesterone, dydrogesterone, and drospirenone seem to be most neutral and possibly even beneficial on blood pressure compared with synthetic progestins.26 Oral estrogen is associated with increased vasoconstriction and/or increased sodium retention with resultant worsened regulation of blood pressure in women with hypertension, so transdermal estrogen is preferred for women with hypertension.26 Hypertension is a component of the ASCVD risk score; factoring this into a patient’s clinical picture is important when discussing appropriateness of HT prescribing. To minimize risks, the transdermal route of estrogen is preferred for those with hypertension.
Continue to: ALTERNATE CASE 1 Resolved...
ALTERNATE CASE 1 Resolved
She has a moderate ASCVD risk score, is obese, and has a history of hypertension. Through shared decision making, you ultimately start her on transdermal estrogen and micronized progesterone to treat her quality-of-life-impacting VMS, a formulation that is most likely to mitigate the possible risks in her clinical case. You see her back in the clinic every 3-6 months to monitor her blood pressure.
ALTERNATE CASE 2 The patient has a high risk for breast cancer
The patient reveals further her significant family history of breast cancer in her maternal grandmother and mother, both diagnosed in their 50s. You calculate her risk of breast cancer with a model that incorporates family history. Her Tyrer Cuzick-IBIS 10-year risk score is >5% and lifetime risk is >20%, putting her at high risk for breast cancer. Since she has a uterus and would need concomitant progesterone therapy, her risk for breast cancer is higher than if she was taking ET alone. Ultimately, together you and the patient decide to trial nonhormonal options for her VMS.
What are nonhormonal options for treatment of VMS?
While HT remains the most effective treatment for VMS, there are multiple nonhormonal treatments for women who are either at too high a risk for HT or who favor other options, which are outlined in the NAMS 2015 nonhormonal management position statement.27 Cognitive behavioral therapy (CBT) has been shown to decrease bother related to VMS but not frequency. Clinical hypnosis has been shown to reduce hot flash frequency and improve sleep. Paroxetine salt (7.5 mg/day) remains the only FDA nonhormonal-approved medication for treatment of moderate to severe vasomotor symptoms. Off label use of other selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors have been shown in studies to reduce VMS including paroxetine at slightly higher doses (10 mg/day–20 mg/day), citalopram (10 mg/day–20 mg/day), escitalopram (10 mg/day–20 mg/day), venlafaxine (37.5 mg/day–150 mg/day), and desvenlafaxine (50 mg/day–100 mg/day). Other treatments that could be considered include off-label use of gabapentin (900 mg/day–2,400 mg/day), oxybutynin (2.5–5 mg twice daily) or clonidine (0.1 mg/day–1 mg/day divided in doses) since they all have data demonstrating they are beneficial at reducing VMS.
Nonhormonal options that may be helpful but are recommended with caution due to lack of data include weight loss, mindfulness-based stress reduction, s-equol derivatives of soy isoflavones and a stellate ganglion block. Further evidence and studies are needed for the aforementioned options.27
ALTERNATE CASE 2 Resolved
She may consider any of the nonhormonal options discussed. If she meets with a medical breast specialist to discuss her elevated risk of breast cancer and considers starting risk-reducing medications, particularly tamoxifen, you will want to avoid medications that have significant CPY 2D6 inhibition, such as paroxetine and fluoxetine. Safer choices would include venlafaxine, escitalopram, or citalopram.
The bottom line
In summary, the benefits and risks of HT in the treatment of VMS remain nuanced. For healthy women younger than 60 years of age and within 10 years from their last menstrual period, the benefits of HT largely outweigh the risks. Shared decision making, along with individualized and appropriate risk stratification specific for women, can guide appropriateness of HT prescribing. For those women who cannot take HT or choose not to, there are many nonhormonal options that will help manage their bothersome VMS. ●
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
- Carr BR, Wilson JD. Disorders of the ovary and female reproductive tract. In: Isselbacher KJ, Braunwald E, Wilson JD, eds. Harrisons’ Principles of Internal Medicine, 13th ed. New York, NY: McGraw-Hill; 1994:2016-2017.
- Davidson MH, Maki KC, Marx P, et al. Effects of continuous estrogen and estrogen-progestin replacement regimens on cardiovascular risk markers in postmenopausel women. Arch Intern Med. 2000;160:3315-3325. doi: 10.1001/archinte.160.21.3315.
- Grodstein F, Manson JE, Colditz GA, et al. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Ann Intern Med. 2000;133:933-941. doi: 10.7326/0003-4819-133-12-200012190-00008.
- Grady D, Rubin SM, Petitti DB, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016-1037. doi: 10.7326/0003-4819-117-12-1016.
- Rossouw JE, Manson JE, Kaunitz AM, et al. Lessons learned from the Women’s Health Initiative trials of menopausal hormone therapy. Obstet Gynecol. 2013;121:172-176. doi: 10.1097/aog.0b013e31827a08c8.
- Manson JE, Hsia J, Johnson KC, et al. Estrogen plus progestin and the risk of coronary heart disease. NEJM. 2003;349:523-534. doi: 10.1056/NEJMoa030808.
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. JAMA. 2013;310:1353-1368. doi: 10.1001/jama.2013.278040.
- Santen RJ, Allred DC, Ardoin SP, et al. Postmenopausal hormone therapy: an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2010;95:S1-S66. doi: 10.1210/jc.2009-2509.
- Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality: the Women’s Health Initiative randomized trials. JAMA. 2017;318:927-938. doi: 10.1001/jama.2017.11217.
- Hodis HN, Mack WJ, Henderson VW, et al. Vacular effects of early versus late postmenopausal treatment with estradiol. NEJM. 2016;374:1221-1231. doi: 10.1056/NEJMoa1505241.
- Taylor HS, Tal A, Pal L, et al. Effects of oral vs transdermal estrogen therapy on sexual function in early postmenopause: ancillary study of the Kronos Early Estrogen Prevention Study (KEEPS). JAMA Intern Med. 2017;177:1471-1479. doi: 10.1001/jamainternmed.2017.3877.
- Liu JH, Pinkerton JV. Prescription therapies. In: CJ Crandall, ed. Menopause Practice: A Clinician’s Guide, 6th ed. Pepper Pike, OH: The North American Menopause Society; 2019:277-309.
- Files J, Kling JM. Transdermal delivery of bioidentical estrogen in menopausal hormone therapy: a clinical review. Expert Opin Drug Deliv. 2020;17:543-549. doi: 10.1080/17425247.2020.1700949.
- Canonico M, Carcaillon L, Plu-Bureau G, et al. Postmenopausal hormone therapy and risk of stroke: impact of the route of estrogen administration and type of progestogen. Stroke. 2016;47:1734-1741. doi: 10.1161/STROKEAHA.116.013052.
- Hitchcok CL, Prior JC. Oral micronized progesterone for vasomotor symptoms—a placebo-controlled randomized trial in healthy post-menopausal women. Menopause. 2001;8:10-16.
- Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) Trial. The writing Group for the PEPI Trial. JAMA. 1996;275:370-375. doi: 10.1001/jama.1996.03530290040035.
- Pinkerton JV. Hormone therapy for postmenopausal women. N Engl J Med. 2020;382:446-55. doi:10.1056/NEJMcp1714787.
- The 2022 hormone therapy position statement of The North American Menopause Society. Menopause. 2022;29:767-794. doi:10.1097/GME.00000000000000002028.
- Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:3975-4011. doi: 10.1210/jc.2015-2236.
- American College of Obstetricians and Gynecologists. Practice Bulletin No. 141: Management of Menopausal Symptoms. Obstet Gynecol. 2014;123:202-216. doi: 10.1097/01.AOG.0000441353.20693.78.
- Manson JE. Current recommendations: what is the clinician to do? Fertil Steril. 2014;101:916. World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Alcoholic drinks and the risk of cancer. https://www.wcrf.org/sites/default/files/Alcoholic-Drinks.pdf. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, nutrition, physical activity and breast cancer. www.aicr.org/continuous-update-project/breast-cancer.html. 2018.
- Anderson GL, Chlebowski RT, Aragaki AK, et al. Conjugated equine oestrogen and breast cancer incidence and mortality in postmenopausal women with hysterectomy: Extended follow-up of the Women’s Health Initiative randomized placebo-controlled trial. Lancet Oncol. 2012;5:476-486. doi: 10.1016/S1470-2045(12)70075-X.
- Mehta J, Kling JM, Manson JE. Risks, benefits and treatment modalities of menopausal hormone therapy: current concepts. Front Endocrinol (Laussane). 2021;12:564781. doi: 10.3389/fendo.2021.564781.
- Kapoor E, Kling JM, Lobo AS, et al. Menopausal hormone therapy in women with chronic medical conditions. Best Pract Res Clin Endocrinol Metab. 2021:35;101578. doi: 10.1016/j.beem.2021.101578.
- NAMS position statement advisory panel. Nonhormonal management of menopause-associated vasomotor symptoms: 2015 position statement of The North American Menopause Society. Menopause. 2015:22:1155-72. doi: 10.1097/GME.0000000000000546.
Management considerations for women with von Willebrand disease
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
Von Willebrand disease (VWD) represents the most common inherited bleeding disorder, with a prevalence of approximately 1 in 1,000 people.
Bruising and mucocutaneous bleeding (epistaxis, gingival bleeding, and bleeding after dental extraction) are the most common presenting symptoms of VWD. Because VWD substantially increases the risk of heavy menstrual bleeding (HMB) and, to some extent, intrapartum bleeding complications, and postpartum hemorrhage, women experience a disproportionate burden from VWD. Thus, ObGyns are likely to be called on to make treatment recommendations in VWD patients with these concerns.1
In 2017, the American Society of Hematology, the International Society on Thrombosis and Haemostasis, the National Hemophilia Foundation, and the World Federation of Hemophilia determined that among clinical issues related to VWD, updating guidelines for women with VWD represented the highest priority.2 Accordingly, an international group of hematologists/coagulation specialists performed systematic literature reviews to address 3 questions faced by women with VWD and their clinicians:
- What are the most effective treatments for HMB?
- What is the safest approach for women desiring neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of tranexamic acid (TxA) on postpartum hemorrhage (PPH)?3
Evidence on management strategies for HMB in women with VWD
The prevalence of HMB in women with VWD ranges from 50% to 92%. Reports suggest that between 5% and 24% of women presenting with this symptom have VWD.3 However, the prevalence of VWD among women seeking care for HMB relates to referral patterns, with the prevalence of VWD substantially higher in patient populations who are referred to clinicians or centers that focus on care of patients with bleeding disorders.
The systematic review authors3 identified 2 comparative studies that assessed the treatment of HMB in women with VWD. One was a crossover trial that enrolled 116 VWD patients with HMB with a mean age of 36 years.4 All participants in this trial chose not to use combination oral contraceptives (COCs) as they had not experienced good results with prior COC use. Trial participants were randomly assigned to receive either intranasal desmopressin (DDAVP; a synthetic analog of the antidiuretic agent vasopressin, which stimulates the release of VWF from endothelial cells) or oral TxA therapy for 2 menstrual cycles. Participants then crossed over to the other drug for 2 additional cycles. Although both agents significantly reduced estimated menstrual blood loss, TxA was more effective in decreasing bleeding than intranasal DDAVP.4
In a retrospective cohort study, investigators compared COC use with intranasal DDAVP in 36 adolescents who had VWD and HMB.5 Participant follow-up ranged from 6 months to 4 years. The estimated efficacy of COCs and intranasal DDAVP was 86% and 77%, respectively, a difference that did not achieve statistical significance. Some of the adolescents who used intranasal DDAVP reported severe headaches and flushing.5
In addition, the systematic review authors3 identified 5 case series that described the use of the levonorgestrel (52 mg)-releasing intrauterine device (LNG 52 IUD) in women with VWD and HMB; 4 of these addressed the efficacy of progestin-releasing IUDs in reducing HMB in this patient population.6-9 Using different approaches to define HMB, the authors of these reports followed between 7 and 26 patients with bleeding disorders (most with confirmed VWD) and HMB for variable amounts of time after placement of an LNG 52 IUD. Many of the women described in these case series had tried other HMB treatments, including COCs, without success. Although these 4 reports assessed different outcomes, all reported that placement of the LNG 52 IUD substantially reduced menstrual blood loss, often resulting in amenorrhea. Several of these reports also noted important improvements in quality of life following LNG 52 IUD placement. One case series reported LNG 52 IUD placement in 13 adolescents with VWD and HMB. The mean time to achieve amenorrhea or occasional spotting was 94 days.6
The fifth report, which followed 20 women (median age, 31 years) with HMB associated with VWD or other bleeding disorders who underwent LNG 52 IUD placement, aimed to describe IUD expulsions and malpositioned IUDs in this population. In this small group of patients, 3 IUD expulsions and 2 malpositioned IUDs were observed. Furthermore, an additional 5 women had their device removed prematurely due to patient dissatisfaction. Accordingly, the IUD continuation rate in this case series was only 50%.10
Evidence on management of pregnancy, delivery, and the postpartum period
Heavy menstrual bleeding is not the only challenge for women with VWD. While pregnancy is accompanied by higher levels of VWF, potentially offsetting the risk of bleeding at the time of delivery, the levels do not achieve the same magnitude as they would in unaffected women.11 Women are at an increased risk of primary PPH12,13 and, importantly, since VWF levels fall exponentially after delivery when women are still experiencing lochia,11 they are at increased risk of secondary or delayed PPH.
Two questions arise frequently in the care of women with VWD at the time of delivery and during the postpartum period:
- What is the safest approach for women who desire neuraxial analgesia for intrapartum pain?
- What is the impact of postpartum administration of TxA on PPH?
The second systematic review the authors performed3 focused on VWF levels in women receiving neuraxial anesthesia during labor. After screening 27 studies, the authors included 5 case series, which did not describe outcomes based on VWF levels but rather described the outcomes of women with VWF levels of greater than 0.50 IU/mL (> 50% of normal compared with a normal standard).
Meta-analysis showed that the proportion of anesthesia complications was 6%, which sounds high, but the range of complications was what would be expected in any population (hypotension, accidental dural puncture, inadequate anesthesia, and bloody tap with no further complications). No spinal, subdural, or epidural hematomas were noted.3 Such hematomas are an extremely rare complication of neuraxial anesthesia, occurring in only 1 in 200,000 or 1 in 250,000 obstetric patients14,15; accordingly, an increase in the rate of hematomas among women with VWD could go undetected. The absence of hematomas among women with VWD as reported in the systematic review does not mean there is not an increase in the rate of hematomas in women with VWD. The relative risk is unknown and caution would be advised.
The third systematic review that the authors performed3 was on TxA treatment in the postpartum period. After screening 41 studies, the authors included 2 retrospective cohort studies.16,17 The majority of the participants had VWD. With very-low-certainty evidence, the authors found that TxA reduces the risk of:
- severe primary PPH (risk ratio [RR], 0.36; 95% confidence interval [CI], 0.05–2.59)
- primary PPH (RR, 0.25; 95% CI, 0.04–1.75)
- secondary PPH (RR, 0.42; 95% CI, 0.02–0.91—does not cross 1.0).
Note that the 95% confidence intervals for severe as well as primary PPH crossed 1.0 and therefore these reductions in risk did not achieve statistical significance. Additionally, there was very-low-certainty evidence on the effect of TxA on blood transfusions, vaginal hematomas, blood loss, and thrombotic complications.3
Continue to: Our recommendations for HMB management...
Our recommendations for HMB management
When first evaluating any woman with HMB, it is important to check a blood count and ferritin level, if not already done. If there is any suggestion of iron deficiency (with or without anemia), we recommend oral iron supplementation. This is best accomplished with slow-release iron supplement formulations (or less expensive generic or house brands that contain less than 65 mg of elemental iron per tablet) taken every other day. Such preparations may cause fewer gastrointestinal adverse effects than other oral iron formulations.18 Although it may appear counterintuitive, oral iron is better absorbed (and also may cause fewer gastrointestinal adverse effects) when taken every other day.19
Initial management of HMB, whether or not a bleeding disorder is present, often consists or oral hormonal management. If no contraindications are present, we recommend initiation of a COC with a short hormone-free interval (for example, a 24/4 formulation). If contraindications to contraceptive doses of estrogen are present, continuous use of norethindrone acetate 5-mg tablets or off-label use of combination tablets with 5 µg of ethinyl estradiol and 1 mg of norethindrone acetate (a formulation approved for the treatment of menopausal symptoms) is appropriate.20
Once a patient is established on oral hormonal management, placement of a levonorgestrel-releasing IUD should be considered. Given that expulsion rates may be higher in women with HMB, if feasible, consider using abdominal ultrasound guidance for IUD placement.
For women with VWD who fail first-line therapy (hormonal management) or are trying to become pregnant, TxA (two 650-mg tablets 3 times daily for up to 5 days during episodes of heavy flow) can reduce HMB.20,21
Our recommendations for management of pregnancy and delivery
The second and third systematic reviews discussed above provide very limited guidance on comprehensive management. The care of the pregnant patient with VWD starts with assessment of VWF levels and making an accurate diagnosis. This usually requires the input of a hematologist or other expert in hemostasis. If no recent VWF levels are available, the ObGyn can obtain a von Willebrand panel that includes VWF antigen, VWF activity (most commonly ristocetin cofactor), and factor VIII.
Levels should be reassessed around 36 weeks’ gestation in anticipation of delivery. VWF levels increase during pregnancy; accordingly, in mild, type 1 VWD, half the time treatment is not necessary.11 If VWF activity is less than 50 IU/dL (less than 50% of normal) at 36 weeks’ gestation, the patient should receive VWF concentrate (dosed in VWF units). This requires consultation with hematology and specialized pharmacy support.
For these reasons, the patient with a VWF level less than 50% should be delivered in a referral center with the necessary resources. Anesthesia should be aware of the patient. Unless
Due to the quantity of fluids administered during labor or at the time of delivery and the coexistent administration of oxytocin, desmopressin (synthetic vasopressin) should not be used without monitoring sodium levels, should not be dosed more than once, or should be avoided altogether due to the risk of water intoxication.
If the patient has sustained VWF and factor VIII levels greater than 50 IU/dL, she would be a candidate to deliver in her local hospital and receive neuraxial anesthesia.
Based on the best data we have for women with VWD, a patient with a VWF greater than 50 IU/dL is no more likely to experience PPH than other women.11 Intravenous TxA can be used for prevention or treatment of immediate postpartum bleeding per protocol (1 g after cord clamp and 1 g 30 minutes or more later).22 Oral TxA can be used for prevention or treatment of delayed postpartum bleeding as per HMB. Regardless of the outcome of any testing during pregnancy, nonsteroidal anti-inflammatory drugs should be avoided postpartum and the patient should be monitored closely for bleeding.
Neonatal care
As for the fetus/neonate, the parents should be aware that the infant has a 50% chance of inheriting VWD. If the baby’s father has no history of bleeding, it is unlikely that the infant would be any more affected than the patient herself. Nonetheless, cord blood (in one or more light blue top tubes) should be obtained at the time of delivery and sent for a von Willebrand panel. If the infant is male, a circumcision should be postponed until VWD is ruled out. In addition, fetal invasive procedures should be avoided during labor. Fetal scalp electrode placement should be avoided. Operative vaginal delivery also should be avoided. Cesarean delivery would be preferred to operative vaginal delivery, but if operative vaginal delivery is unavoidable, use of forceps is preferred to vacuum extraction. ●
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.
- ACOG committee opinion no. 451: Von Willebrand disease in women. Obstet Gynecol. 2009;114:1439-1443. doi: 10.1097 /AOG.0b013e3181c6f975.
- Kalot MA, Al-Khatib M, Connell NT, et al; VWD Working Group. An international survey to inform priorities for new guidance on von Willebrand disease. Hemophilia. 2020;26:106-116. doi: 10.1111/hae.13881.
- Brignardello-Petersen R, El Alayli A, Husainat N, et al. Gynecologic and obstetric management of women with von Willebrand disease: summary of 3 systematic reviews of the literature. Blood Adv. 2022;6:228-237. doi: 10.1182 /bloodadvances.2021005589.
- Kouides PA, Byams VR, Philipp CS, et al. Multisite management study of menorrhagia with abnormal laboratory haemostasis: a prospective crossover study of intranasal desmopressin and oral tranexamic acid. Br J Haematol. 2009;145:212-220. doi: 10.1111/j.1365-2141.2009.07610.x.
- Amesse LS, Pfaff-Amesse T, Leonardi R, et al. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27:357-363. doi: 10.1097/01.mph.0000173175.95152.95.
- Adeyemi-Fowode OA, Santos XM, Dietrich JE, et al. Levonorgestrel-releasing intrauterine device use in female adolescents with heavy menstrual bleeding and bleeding disorders: single institution review. J Pediatr Adolesc Gynecol. 2017;30:479-483. doi: 10.1016/j.jpag.2016.04.001.
- Chi C, Huq FY, Kadir RA. Levonorgestrel-releasing intrauterine system for the management of heavy menstrual bleeding in women with inherited bleeding disorders: long-term follow-up. Contraception. 2011;83:242-247. doi: 10.1016/j.contraception.2010.07.010.
- Kingman CE, Kadir RA, Lee CA, et al. The use of levonorgestrel-releasing intrauterine system for treatment of menorrhagia in women with inherited bleeding disorders. BJOG. 2004;111:1425-1428. doi: 10.1111/j.1471-0528.2004.00305.x.
- Lukes AS, Reardon B, Arepally G. Use of the levonorgestrel-releasing intrauterine system in women with hemostatic disorders. Fertil Steril. 2008;90:673-677. doi: 10.1016 /j.fertnstert.2007.07.1315.
- Rimmer E, Jamieson MA, James P. Malposition and expulsion of the levonorgestrel intrauterine system among women with inherited bleeding disorders. Haemophilia. 2013;19:933-938. doi: 10.1111/hae.12184.
- James AH, Konkle BA, Kouides P, et al. Postpartum von Willebrand factor levels in women with and without von Willebrand disease and implications for prophylaxis. Haemophilia. 2015;21:81-87. doi: 10.1111/hae.12568.
- James AH, Jamison MG. Bleeding events and other complications during pregnancy and childbirth in women with von Willebrand disease. J Thromb Haemost. 2007;5: 1165-1169. doi: 10.1111/j.1538-7836.2007.02563.x.
- Al-Zirqi I, Vangen S, Forsen L, et al. Prevalence and risk factors of severe obstetric haemorrhage. BJOG. 2008;115:1265-1272. doi: 10.1111/j.1471-0528.2008.01859.x.
- Moen V, Dahlgren N, Irestedt L. Severe neurological complications after central neuraxial blockades in Sweden 1990-1999. Anesthesiology. 2004;101:950-959. doi: 10.1097/00000542-200410000-00021.
- D’Angelo R, Smiley RM, Riley ET, et al. Serious complications related to obstetric anesthesia: the serious complication repository project of the Society for Obstetric Anesthesia and Perinatology. Anesthesiology. 2014;120:1505-1512. doi: 10.1097/ALN.000000000000253.
- Govorov I, Lofgren S, Chaireti R, et al. Postpartum hemorrhage in women with von Willebrand disease—a retrospective observational study. PLos One. 2016;11:e0164683. doi: 10.1371/journal.pone.0164683.
- Hawke L, Grabell J, Sim W, et al. Obstetric bleeding among women with inherited bleeding disorders: a retrospective study. Haemophilia. 2016;22:906-911. doi: 10.1111/hae.13067.
- James AH. Iron deficiency anemia in pregnancy. Obstet Gynecol. 2021;138:663-674. doi:10.1097/AOG .000000000000.4559.
- Stoffel NU, Cercamondi CI, Brittenham G, et al. Iron absorption from oral iron supplements given on consecutive versus alternate days and as single morning doses versus twice-daily split dosing in iron-depleted women: two open-label, randomised controlled trials. Lancet Haematol. 2017;4:e524-e533. doi: 10.1016/S2352-3026(17)30182-5.
- Kaunitz AM. Abnormal uterine bleeding in reproductiveage women. JAMA. 2019;321:2126-2127. doi: 10.1001 /jama.2019.5248.
- James AH, Kouides PA, Abdul-Kadir R, et al. Von Willebrand disease and other bleeding disorders in women: consensus on diagnosis and management from an international expert panel. Am J Obstet Gynecol. 2009;201:12.e1-8. doi: 10.1016 /j.ajog.2009.04.024.
- WOMAN Trial Collaborators. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017;389:2105-2116. doi: 10.1016/S0140-6736(17)30638-4.