User login
An FP’s guide to exercise counseling for older adults
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
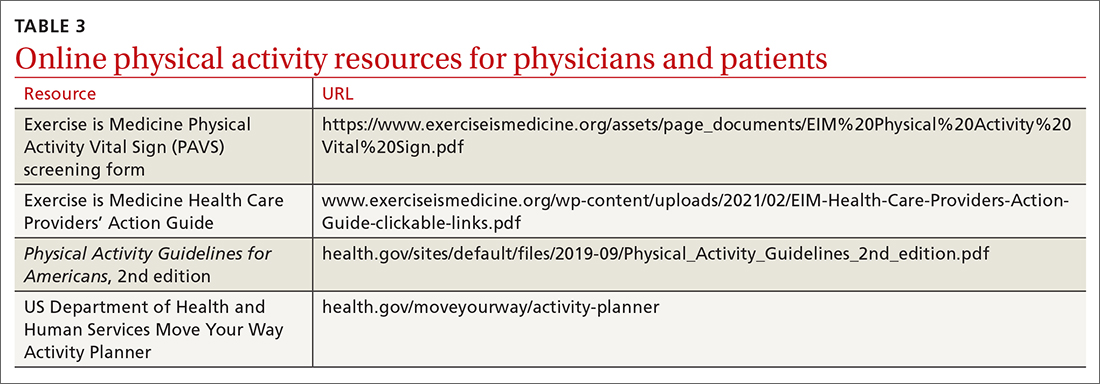
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)
Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
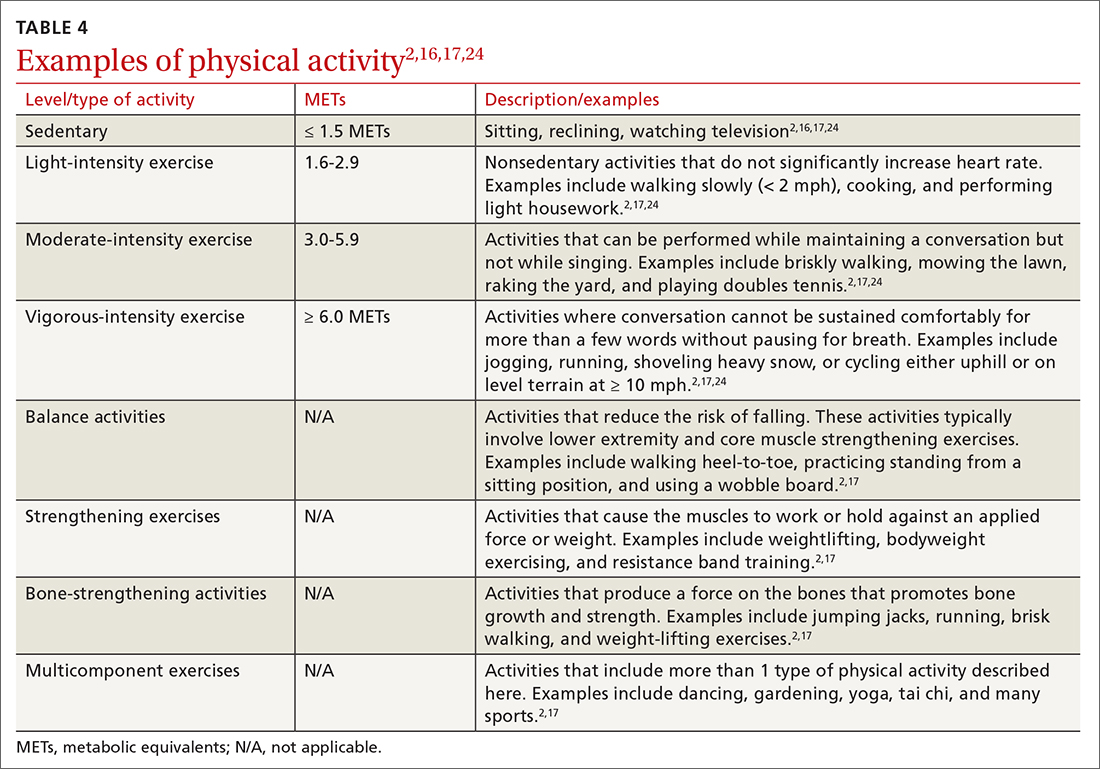
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2
The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
The health benefits of maintaining a physically active lifestyle are vast and irrefutable.1 Physical activity is an important modifiable behavior demonstrated to reduce the risk for many chronic diseases while improving physical function (TABLE 12).3 Physical inactivity increases with age, making older adults (ages ≥ 65 years) the least active age group and the group at greatest risk for inactivity-related health consequences.4-6 Engaging in a physically active lifestyle is especially important for older adults to maintain independence,7 quality of life,8 and the ability to perform activities of daily living.3,9

Prescribe physical activity for older adults
The 2018 Physical Activity Guidelines for Americans recommend that all healthy adults (including healthy older adults) ideally should perform muscle-strengthening activities of moderate or greater intensity that involve all major muscle groups on 2 or more days per week and either (a) 150 to 300 minutes per week of moderate-intensity aerobic physical activity, (b) 75 to 150 minutes per week of vigorous-intensity aerobic physical activity, or (c) an equivalent combination, if possible (TABLE 22).3 It is recommended that older adults specifically follow a multicomponent physical activity program that includes balance training, as well as aerobic and muscle-strengthening activities.3 Unfortunately, nearly 80% of older adults do not meet the recommended guidelines for aerobic or muscle-strengthening exercise.3

Identify barriers to exercise
Older adults report several barriers that limit physical activity. Some of the most commonly reported barriers include a lack of motivation, low self-efficacy for being active, physical limitations due to health conditions, inconvenient physical activity locations, boredom with physical activity, and lack of guidance from professionals.10-12 Physical activity programs designed for older adults should specifically target these barriers for maximum effectiveness.
Clinicians also face potential barriers for promoting physical activity among older adults. Screening patients for physical inactivity can be a challenge, given the robust number of clinical preventive services and conversations that are already recommended for older adults. Additionally, screening for physical activity is not a reimbursable service. In July, the US Preventive Services Task Force (USPSTF) reaffirmed its 2017 recommendation to individualize the decision to offer or refer adults without obesity, hypertension, dyslipidemia, or abnormal blood glucose levels or diabetes to behavioral counseling to promote a healthy diet and physical activity (Grade C rating).13
Treat physical activity as a vital sign
The Exercise is Medicine (EIM) model is based on the principle that physical activity should be treated as a vital sign and discussed during all health care visits. Health care professionals have a unique opportunity to promote physical activity, since more than 80% of US adults see a physician annually. Evidence also suggests clinician advice is associated with patients’ healthy lifestyle behaviors.14,15
EIM is a global health initiative that was established in 2007 and is managed by the American College of Sports Medicine (ACSM). The primary objective of the EIM model is to treat physical activity behavior as a vital sign and include physical activity promotion as a standard of clinical care. In order to achieve this objective, the EIM model recommends health care systems follow 3 simple rules: (1) treat physical activity as a vital sign by measuring physical activity of every patient at every visit, (2) prescribe exercise to those patients who report not meeting the physical activity guidelines, and/or (3) refer inactive patients to evidence-based physical activity resources to receive exercise counseling.16,17
Screen for physical activity using this 2-question self-report
Clinicians may employ multiple tactics to screen patients for their current levels of physical activity. Physical Activity Vital Sign (PAVS) is a 2-item self-report measure developed to briefly assess a patient’s level of physical activity; results can be entered into the patient’s electronic medical record and used to begin a process of referring inactive patients for behavioral counseling.17,18 The PAVS can be administered in less than 1 minute by a medical assistant and/or nursing staff during rooming or intake of patients. The PAVS questions include, “On average, how many days per week do you engage in moderate-to-vigorous physical activity?” and “On average, how many minutes do you engage in physical activity at this level?” The clinician can then multiply the 2 numbers to calculate the patient’s total minutes of moderate-to-vigorous physical activity per week to determine whether a patient is meeting the recommended physical activity guidelines.16 (For more on the PAVS and other resources, see TABLE 3.)

Continue to: The PAVS has been established...
The PAVS has been established as a valid instrument for detecting patients who may need counseling on physical activity for chronic disease recognition, management, and prevention.17 Furthermore, there is a strong association between PAVS, elevated body mass index, and chronic disease burden.19 Therefore, we recommend that primary care physicians screen their patients for physical activity levels. It has been demonstrated, however, that many primary care visits for older individuals include discussions of diet and physical activity but do not provide recommendations for lifestyle change.19 Thus, exploring ways to counsel patients on lifestyle change in an efficient manner is recommended. It has been demonstrated that counseling and referral from primary care centers can promote increased adherence to physical activity practices.20,21
Determine physical activity readiness
Prior to recommending a physical activity regimen, it is important to evaluate the patient’s readiness to make a change. Various questionnaires—such as the Physical Activity Readiness Questionnaire—have been developed to determine a patient’s level of readiness, evaluating both psychological and physical factors (www.nasm.org/docs/pdf/parqplus-2020.pdf?sfvrsn=401bf1af_24). Questionnaires also help you to determine whether further medical evaluation prior to beginning an exercise regimen is necessary. It’s important to note that, as is true with any office intervention, patients may be in a precontemplation or contemplation phase and may not be prepared to immediately make changes.
Evaluate risk level
Assess cardiovascular risk. Physicians and patients are often concerned about cardiovascular risk or injury risk during physical activity counseling, which may lead to fewer exercise prescriptions. As a physician, it is important to remember that for most adults, the benefits of exercise will outweigh any potential risks,3 and there is generally a low risk of cardiovascular events related to light to moderate–intensity exercise regimens.2 Additionally, it has been demonstrated that exercise and cardiovascular rehabilitation are highly beneficial for primary and secondary prevention of cardiovascular disease.22 Given that cardiovascular comorbidities are relatively common in older adults, some older adults will need to undergo risk stratification evaluation prior to initiating an exercise regimen.
Review preparticipation screening guidelines and recommendations
Guidelines can be contradictory regarding the ideal pre-exercise evaluation. In general, the USPSTF recommends against screening with resting or exercise electrocardiography (EKG) to prevent cardiovascular disease events in asymptomatic adults who are at low risk. It also finds insufficient evidence to assess the balance of benefits and harms of screening with resting or exercise EKG to prevent cardiovascular disease events in asymptomatic adults who are at intermediate or high risk.22
Similarly, the 2020 ACSM Guidelines for Exercise Testing and Prescription reflect that routine exercise testing is not recommended for all older adult patients prior to starting an exercise regimen.17 However, the ACSM does recommend all patients with signs or symptoms of a cardiovascular, renal, or metabolic disease consult with a clinician for medical risk stratification and potential subsequent testing prior to starting an exercise regimen. If an individual already exercises and is having new/worsening signs or symptoms of a cardiovascular, renal, or metabolic disease, that patient should cease exercise until medical evaluation is performed. Additionally, ACSM recommends that asymptomatic patients who do not exercise but who have known cardiovascular, renal, or metabolic disease receive medical evaluation prior to starting an exercise regimen.17
Continue to: Is there evidence of cardiovascular, renal, or metabolic disease?
Is there evidence of cardiovascular, renal, or metabolic disease?
Initial screening can be completed by obtaining the patient’s history and conducting a physical examination. Patients reporting chest pain or discomfort (or any anginal equivalent), dyspnea, syncope, orthopnea, lower extremity edema, signs of tachyarrhythmia/bradyarrhythmia, intermittent claudication, exertional fatigue, or new exertional symptoms should all be considered for cardiovascular stress testing. Patients with a diagnosis of renal disease or either type 1 or type 2 diabetes should also be considered for cardiovascular stress testing.
Ready to prescribe exercise? Cover these 4 points
When prescribing any exercise plan for older adults, it is important for clinicians to specify 4 key components: frequency, intensity, time, and type (this can be remembered using the acronym “FITT”).23 A sedentary adult should be encouraged to engage in moderate-intensity exercise, such as walking, for 15 minutes 3 times per week. The key with a sedentary adult is appropriate follow-up to monitor progression and modify activity to help ensure the patient can achieve the goal number of minutes per week. It can be helpful to share the “next step” with the patient, as well (eg, increase to 4 times per week after 2 weeks, or increase by 5 minutes every week). For the intermittent exerciser, a program of moderate exercise, such as using an elliptical, for 30 to 40 minutes 5 times per week is a recommended prescription. FITT components can be tailored to meet individual patient physical readiness.23
Frequency. While the 2018 Physical Activity Guidelines for Americans recommend a specific frequency of physical activity throughout the week, it is important to remember that some older adults will be unable to meet these recommendations, particularly in the setting of frailty and comorbidities (TABLE 22). In these cases, the guidelines simply recommend that older adults should be as physically active as their abilities and comorbidities allow. Some exercise is better than none, and generally moving more and sitting less will yield health benefits for older adult patients.
Intensity is a description of how hard an individual is working during physical activity. An older adult’s individual capacity for exercise intensity will depend on many factors, including their comorbidities. An activity’s intensity will be relative to a person’s unique level of fitness. Given this heterogeneity, exercise prescriptions should be tailored to the individual. Light-intensity exercise generally causes a slight increase in pulse and respiratory rate, moderate-intensity exercise causes a noticeable increase in pulse and respiratory rate, and vigorous-intensity exercise causes a significant increase in pulse and respiratory rate (TABLE 42,16,17,24).2

The “talk test” is a simple, practical, and validated test that can help one determine an individual’s capacity for moderate- or vigorous-intensity exercise.23 In general, a person performing vigorous-intensity exercise will be unable to talk comfortably during activity for more than a few words without pausing for breath. Similarly, a person will be able to talk but not sing comfortably during moderate-intensity exercise.3,23
Continue to: Time
Time. The 2018 Physical Activity Guidelines for Americans recommend a specific duration of physical activity throughout the week; however, as with frequency, it is important to remember that duration of exercise is individualized (TABLE 22). Older adults should be as physically active as their abilities and comorbidities allow, and in the setting of frailty, numerous comorbidities, and/or a sedentary lifestyle, it is reasonable to initiate exercise recommendations with shorter durations.
Type of exercise. As noted in the 2018 Physical Activity Guidelines for Americans, recommendations for older adults include multiple types of exercise. In addition to these general exercise recommendations, exercise prescriptions can be individualized to target specific comorbidities (TABLE 22). Weight-bearing, bone-strengthening exercises can benefit patients with disorders of low bone density and possibly those with osteoarthritis.3,23 Patients at increased risk for falls should focus on balance-training options that strengthen the muscles of the back, abdomen, and legs, such as tai chi.3,23 Patients with cardiovascular risk can benefit from moderate- to high-intensity aerobic exercise (although exercise should be performed below anginal threshold in patients with known cardiovascular disease). Patients with type 2 diabetes achieve improved glycemic control when engaging in combined moderate-intensity aerobic exercise and resistance training.7,23
Referral to a physical therapist or sport and exercise medicine specialist can always be considered, particularly for patients with significant neurologic disorders, disability secondary to traumatic injury, or health conditions.3
An improved quality of life. Incorporating physical activity into older adults’ lives can enhance their quality of life. Family physicians are well positioned to counsel older adults on the importance and benefits of exercise and to help them overcome the barriers or resistance to undertaking a change in behavior. Guidelines, recommendations, patient history, and resources provide the support needed to prescribe individualized exercise plans for this distinct population.
CORRESPONDENCE
Scott T. Larson, MD, 200 Hawkins Drive, Iowa City, IA, 52242; [email protected]
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
1.
2. US Department of Health and Human Services. Physical Activity Guidelines for Americans. 2nd ed. 2018. Accessed June 15, 2022. https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf
3. Piercy KL, Troiano RP, Ballard RM, et al. The Physical Activity Guidelines for Americans. JAMA. 2018;320:2020-2028. doi: 10.1001/jama.2018.14854
4. Harvey JA, Chastin SF, Skelton DA. How sedentary are older people? A systematic review of the amount of sedentary behavior. J Aging Phys Act. 2015;23:471-487. doi: 10.1123/japa.2014-0164
5. Yang L, Cao C, Kantor ED, et al. Trends in sedentary behavior among the US population, 2001-2016. JAMA. 2019;321:1587-1597. doi: 10.1001/jama.2019.3636
6. Watson KB, Carlson SA, Gunn JP, et al. Physical inactivity among adults aged 50 years and older—United States, 2014. MMWR Morb Mortal Wkly Rep. 2016;65:954-958. doi: 10.15585/mmwr.mm6536a3
7. Taylor D. Physical activity is medicine for older adults. Postgrad Med J. 2014;90:26-32. doi: 10.1136/postgradmedj-2012-131366
8. Marquez DX, Aguinaga S, Vasquez PM, et al. A systematic review of physical activity and quality of life and well-being. Transl Behav Med. 2020;10:1098-1109. doi: 10.1093/tbm/ibz198
9. Dionigi R. Resistance training and older adults’ beliefs about psychological benefits: the importance of self-efficacy and social interaction. J Sport Exerc Psychol. 2007;29:723-746. doi: 10.1123/jsep.29.6.723
10. Bethancourt HJ, Rosenberg DE, Beatty T, et al. Barriers to and facilitators of physical activity program use among older adults. Clin Med Res. 2014;12:10-20. doi: 10.3121/cmr.2013.1171
11. Strand KA, Francis SL, Margrett JA, et al. Community-based exergaming program increases physical activity and perceived wellness in older adults. J Aging Phys Act. 2014;22:364-371. doi: 10.1123/japa.2012-0302
12. Franco MR, Tong A, Howard K, et al. Older people’s perspectives on participation in physical activity: a systematic review and thematic synthesis of qualitative literature. Br J Sports Med. 2015;49:1268-1276. doi: 10.1136/bjsports-2014-094015
13. US Preventive Services Task Force. Behavioral Counseling Interventions to Promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. July 26, 2022. Accessed August 7, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/healthy-lifestyle-and-physical-activity-for-cvd-prevention-adults-without-known-risk-factors-behavioral-counseling#bootstrap-panel--7
14. Elley CR, Kerse N, Arroll B, et al. Effectiveness of counselling patients on physical activity in general practice: cluster randomised controlled trial. BMJ. 2003;326:793. doi: 10.1136/bmj.326.7393.793
15. Grandes G, Sanchez A, Sanchez-Pinella RO, et al. Effectiveness of physical activity advice and prescription by physicians in routine primary care: a cluster randomized trial. Arch Intern Med. 2009;169:694-701. doi: 10.1001/archinternmed.2009.23
16. Lobelo F, Young DR, Sallis R, et al. Routine assessment and promotion of physical activity in healthcare settings: a scientific statement from the American Heart Association. Circulation. 2018;137:e495-e522. doi: 10.1161/CIR.0000000000000559
17. American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription. 11th ed. Wolters Kluwer; 2021.
18. Sallis R. Developing healthcare systems to support exercise: exercise as the fifth vital sign. Br J Sports Med. 2011;45:473-474. doi: 10.1136/bjsm.2010.083469
19. Bardach SH, Schoenberg NE. The content of diet and physical activity consultations with older adults in primary care. Patient Educ Couns. 2014;95:319-324. doi: 10.1016/j.pec.2014.03.020
20. Martín-Borràs C, Giné-Garriga M, Puig-Ribera A, et al. A new model of exercise referral scheme in primary care: is the effect on adherence to physical activity sustainable in the long term? A 15-month randomised controlled trial. BMJ Open. 2018;8:e017211. doi: 10.1136/bmjopen-2017-017211
21. Stoutenberg M, Shaya GE, Feldman DI, et al. Practical strategies for assessing patient physical activity levels in primary care. Mayo Clin Proc Innov Qual Outcomes. 2017;1:8-15. doi: 10.1016/j.mayocpiqo.2017.04.006
22. US Preventive Services Task Force. Cardiovascular disease risk: screening with electrocardiography. June 2018. Accessed July 19, 2022. www.uspreventiveservicestaskforce.org/uspstf/recommendation/cardiovascular-disease-risk-screening-with-electrocardiography
23. Reed JL, Pipe AL. Practical approaches to prescribing physical activity and monitoring exercise intensity. Can J Cardiol. 2016;32:514-522. doi: 10.1016/j.cjca.2015.12.024
24. Verschuren O, Mead G, Visser-Meily A. Sedentary behaviour and stroke: foundational knowledge is crucial. Transl Stroke Res. 2015;6:9-12. doi: 10.1007/s12975-014-0370
PRACTICE RECOMMENDATIONS
› Encourage older adults to engage in at least 150 minutes of moderate-intensity aerobic physical activity throughout the week, OR at least 75 minutes of vigorous-intensity aerobic physical activity throughout the week, OR an equivalent combination of moderate- and vigorous-intensity activity. A
› Recommend older adults perform muscle-strengthening activities involving major muscle groups on 2 or more days per week. A
› Encourage older adults to be as physically active as possible, even when their health conditions and abilities prevent them from reaching their minimum levels of physical activity. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Barriers to System Quality Improvement in Health Care
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Process improvement in any industry sector aims to increase the efficiency of resource utilization and delivery methods (cost) and the quality of the product (outcomes), with the goal of ultimately achieving continuous development.1 In the health care industry, variation in processes and outcomes along with inefficiency in resource use that result in changes in value (the product of outcomes/costs) are the general targets of quality improvement (QI) efforts employing various implementation methodologies.2 When the ultimate aim is to serve the patient (customer), best clinical practice includes both maintaining high quality (individual care delivery) and controlling costs (efficient care system delivery), leading to optimal delivery (value-based care). High-quality individual care and efficient care delivery are not competing concepts, but when working to improve both health care outcomes and cost, traditional and nontraditional barriers to system QI often arise.3
The possible scenarios after a QI intervention include backsliding (regression to the mean over time), steady-state (minimal fixed improvement that could sustain), and continuous improvement (tangible enhancement after completing the intervention with legacy effect).4 The scalability of results can be considered during the process measurement and the intervention design phases of all QI projects; however, the complex nature of barriers in the health care environment during each level of implementation should be accounted for to prevent failure in the scalability phase.5
The barriers to optimal QI outcomes leading to continuous improvement are multifactorial and are related to intrinsic and extrinsic factors.6 These factors include 3 fundamental levels: (1) individual level inertia/beliefs, prior personal knowledge, and team-related factors7,8; (2) intervention-related and process-specific barriers and clinical practice obstacles; and (3) organizational level challenges and macro-level and population-level barriers (Figure). The obstacles faced during the implementation phase will likely include 2 of these levels simultaneously, which could add complexity and hinder or prevent the implementation of a tangible successful QI process and eventually lead to backsliding or minimal fixed improvement rather than continuous improvement. Furthermore, a patient-centered approach to QI would contribute to further complexity in design and execution, given the importance of reaching sustainable, meaningful improvement by adding elements of patient’s preferences, caregiver engagement, and the shared decision-making processes.9
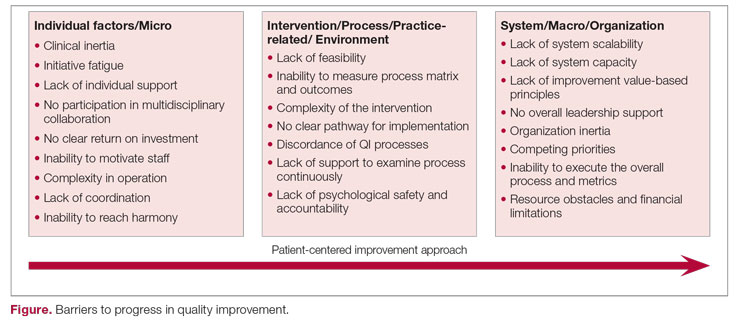
Overcoming these multidomain barriers and reaching resilience and sustainability requires thoughtful planning and execution through a multifaceted approach.10 A meaningful start could include addressing the clinical inertia for the individual and the team by promoting open innovation and allowing outside institutional collaborations and ideas through networks.11 On the individual level, encouraging participation and motivating health care workers in QI to reach a multidisciplinary operation approach will lead to harmony in collaboration. Concurrently, the organization should support the QI capability and scalability by removing competing priorities and establishing effective leadership that ensures resource allocation, communicates clear value-based principles, and engenders a psychological safety environment.
A continuous improvement state is the optimal QI target, a target that can be attained by removing obstacles and paving a clear pathway to implementation. Focusing on the 3 levels of barriers will position the organization for meaningful and successful QI phases to achieve continuous improvement.
1. Adesola S, Baines T. Developing and evaluating a methodology for business process improvement. Business Process Manage J. 2005;11(1):37-46. doi:10.1108/14637150510578719
2. Gershon M. Choosing which process improvement methodology to implement. J Appl Business & Economics. 2010;10(5):61-69.
3. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
4. Holweg M, Davies J, De Meyer A, Lawson B, Schmenner RW. Process Theory: The Principles of Operations Management. Oxford University Press; 2018.
5. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. doi:10.1111/1468-0009.00107
6. Solomons NM, Spross JA. Evidence‐based practice barriers and facilitators from a continuous quality improvement perspective: an integrative review. J Nurs Manage. 2011;19(1):109-120. doi:10.1111/j.1365-2834.2010.01144.x
7. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-34. doi:10.7326/0003-4819-135-9-200111060-00012
8. Stevenson K, Baker R, Farooqi A, Sorrie R, Khunti K. Features of primary health care teams associated with successful quality improvement of diabetes care: a qualitative study. Fam Pract. 2001;18(1):21-26. doi:10.1093/fampra/18.1.21
9. What is patient-centered care? NEJM Catalyst. January 1, 2017. Accessed August 31, 2022. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0559
10. Kilbourne AM, Beck K, Spaeth‐Rublee B, et al. Measuring and improving the quality of mental health care: a global perspective. World Psychiatry. 2018;17(1):30-8. doi:10.1002/wps.20482
11. Huang HC, Lai MC, Lin LH, Chen CT. Overcoming organizational inertia to strengthen business model innovation: An open innovation perspective. J Organizational Change Manage. 2013;26(6):977-1002. doi:10.1108/JOCM-04-2012-0047
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Process improvement in any industry sector aims to increase the efficiency of resource utilization and delivery methods (cost) and the quality of the product (outcomes), with the goal of ultimately achieving continuous development.1 In the health care industry, variation in processes and outcomes along with inefficiency in resource use that result in changes in value (the product of outcomes/costs) are the general targets of quality improvement (QI) efforts employing various implementation methodologies.2 When the ultimate aim is to serve the patient (customer), best clinical practice includes both maintaining high quality (individual care delivery) and controlling costs (efficient care system delivery), leading to optimal delivery (value-based care). High-quality individual care and efficient care delivery are not competing concepts, but when working to improve both health care outcomes and cost, traditional and nontraditional barriers to system QI often arise.3
The possible scenarios after a QI intervention include backsliding (regression to the mean over time), steady-state (minimal fixed improvement that could sustain), and continuous improvement (tangible enhancement after completing the intervention with legacy effect).4 The scalability of results can be considered during the process measurement and the intervention design phases of all QI projects; however, the complex nature of barriers in the health care environment during each level of implementation should be accounted for to prevent failure in the scalability phase.5
The barriers to optimal QI outcomes leading to continuous improvement are multifactorial and are related to intrinsic and extrinsic factors.6 These factors include 3 fundamental levels: (1) individual level inertia/beliefs, prior personal knowledge, and team-related factors7,8; (2) intervention-related and process-specific barriers and clinical practice obstacles; and (3) organizational level challenges and macro-level and population-level barriers (Figure). The obstacles faced during the implementation phase will likely include 2 of these levels simultaneously, which could add complexity and hinder or prevent the implementation of a tangible successful QI process and eventually lead to backsliding or minimal fixed improvement rather than continuous improvement. Furthermore, a patient-centered approach to QI would contribute to further complexity in design and execution, given the importance of reaching sustainable, meaningful improvement by adding elements of patient’s preferences, caregiver engagement, and the shared decision-making processes.9

Overcoming these multidomain barriers and reaching resilience and sustainability requires thoughtful planning and execution through a multifaceted approach.10 A meaningful start could include addressing the clinical inertia for the individual and the team by promoting open innovation and allowing outside institutional collaborations and ideas through networks.11 On the individual level, encouraging participation and motivating health care workers in QI to reach a multidisciplinary operation approach will lead to harmony in collaboration. Concurrently, the organization should support the QI capability and scalability by removing competing priorities and establishing effective leadership that ensures resource allocation, communicates clear value-based principles, and engenders a psychological safety environment.
A continuous improvement state is the optimal QI target, a target that can be attained by removing obstacles and paving a clear pathway to implementation. Focusing on the 3 levels of barriers will position the organization for meaningful and successful QI phases to achieve continuous improvement.
Corresponding author: Ebrahim Barkoudah, MD, MPH, Department of Medicine, Brigham and Women’s Hospital, Boston, MA; [email protected]
Process improvement in any industry sector aims to increase the efficiency of resource utilization and delivery methods (cost) and the quality of the product (outcomes), with the goal of ultimately achieving continuous development.1 In the health care industry, variation in processes and outcomes along with inefficiency in resource use that result in changes in value (the product of outcomes/costs) are the general targets of quality improvement (QI) efforts employing various implementation methodologies.2 When the ultimate aim is to serve the patient (customer), best clinical practice includes both maintaining high quality (individual care delivery) and controlling costs (efficient care system delivery), leading to optimal delivery (value-based care). High-quality individual care and efficient care delivery are not competing concepts, but when working to improve both health care outcomes and cost, traditional and nontraditional barriers to system QI often arise.3
The possible scenarios after a QI intervention include backsliding (regression to the mean over time), steady-state (minimal fixed improvement that could sustain), and continuous improvement (tangible enhancement after completing the intervention with legacy effect).4 The scalability of results can be considered during the process measurement and the intervention design phases of all QI projects; however, the complex nature of barriers in the health care environment during each level of implementation should be accounted for to prevent failure in the scalability phase.5
The barriers to optimal QI outcomes leading to continuous improvement are multifactorial and are related to intrinsic and extrinsic factors.6 These factors include 3 fundamental levels: (1) individual level inertia/beliefs, prior personal knowledge, and team-related factors7,8; (2) intervention-related and process-specific barriers and clinical practice obstacles; and (3) organizational level challenges and macro-level and population-level barriers (Figure). The obstacles faced during the implementation phase will likely include 2 of these levels simultaneously, which could add complexity and hinder or prevent the implementation of a tangible successful QI process and eventually lead to backsliding or minimal fixed improvement rather than continuous improvement. Furthermore, a patient-centered approach to QI would contribute to further complexity in design and execution, given the importance of reaching sustainable, meaningful improvement by adding elements of patient’s preferences, caregiver engagement, and the shared decision-making processes.9

Overcoming these multidomain barriers and reaching resilience and sustainability requires thoughtful planning and execution through a multifaceted approach.10 A meaningful start could include addressing the clinical inertia for the individual and the team by promoting open innovation and allowing outside institutional collaborations and ideas through networks.11 On the individual level, encouraging participation and motivating health care workers in QI to reach a multidisciplinary operation approach will lead to harmony in collaboration. Concurrently, the organization should support the QI capability and scalability by removing competing priorities and establishing effective leadership that ensures resource allocation, communicates clear value-based principles, and engenders a psychological safety environment.
A continuous improvement state is the optimal QI target, a target that can be attained by removing obstacles and paving a clear pathway to implementation. Focusing on the 3 levels of barriers will position the organization for meaningful and successful QI phases to achieve continuous improvement.
1. Adesola S, Baines T. Developing and evaluating a methodology for business process improvement. Business Process Manage J. 2005;11(1):37-46. doi:10.1108/14637150510578719
2. Gershon M. Choosing which process improvement methodology to implement. J Appl Business & Economics. 2010;10(5):61-69.
3. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
4. Holweg M, Davies J, De Meyer A, Lawson B, Schmenner RW. Process Theory: The Principles of Operations Management. Oxford University Press; 2018.
5. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. doi:10.1111/1468-0009.00107
6. Solomons NM, Spross JA. Evidence‐based practice barriers and facilitators from a continuous quality improvement perspective: an integrative review. J Nurs Manage. 2011;19(1):109-120. doi:10.1111/j.1365-2834.2010.01144.x
7. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-34. doi:10.7326/0003-4819-135-9-200111060-00012
8. Stevenson K, Baker R, Farooqi A, Sorrie R, Khunti K. Features of primary health care teams associated with successful quality improvement of diabetes care: a qualitative study. Fam Pract. 2001;18(1):21-26. doi:10.1093/fampra/18.1.21
9. What is patient-centered care? NEJM Catalyst. January 1, 2017. Accessed August 31, 2022. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0559
10. Kilbourne AM, Beck K, Spaeth‐Rublee B, et al. Measuring and improving the quality of mental health care: a global perspective. World Psychiatry. 2018;17(1):30-8. doi:10.1002/wps.20482
11. Huang HC, Lai MC, Lin LH, Chen CT. Overcoming organizational inertia to strengthen business model innovation: An open innovation perspective. J Organizational Change Manage. 2013;26(6):977-1002. doi:10.1108/JOCM-04-2012-0047
1. Adesola S, Baines T. Developing and evaluating a methodology for business process improvement. Business Process Manage J. 2005;11(1):37-46. doi:10.1108/14637150510578719
2. Gershon M. Choosing which process improvement methodology to implement. J Appl Business & Economics. 2010;10(5):61-69.
3. Porter ME, Teisberg EO. Redefining Health Care: Creating Value-Based Competition on Results. Harvard Business Press; 2006.
4. Holweg M, Davies J, De Meyer A, Lawson B, Schmenner RW. Process Theory: The Principles of Operations Management. Oxford University Press; 2018.
5. Shortell SM, Bennett CL, Byck GR. Assessing the impact of continuous quality improvement on clinical practice: what it will take to accelerate progress. Milbank Q. 1998;76(4):593-624. doi:10.1111/1468-0009.00107
6. Solomons NM, Spross JA. Evidence‐based practice barriers and facilitators from a continuous quality improvement perspective: an integrative review. J Nurs Manage. 2011;19(1):109-120. doi:10.1111/j.1365-2834.2010.01144.x
7. Phillips LS, Branch WT, Cook CB, et al. Clinical inertia. Ann Intern Med. 2001;135(9):825-34. doi:10.7326/0003-4819-135-9-200111060-00012
8. Stevenson K, Baker R, Farooqi A, Sorrie R, Khunti K. Features of primary health care teams associated with successful quality improvement of diabetes care: a qualitative study. Fam Pract. 2001;18(1):21-26. doi:10.1093/fampra/18.1.21
9. What is patient-centered care? NEJM Catalyst. January 1, 2017. Accessed August 31, 2022. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0559
10. Kilbourne AM, Beck K, Spaeth‐Rublee B, et al. Measuring and improving the quality of mental health care: a global perspective. World Psychiatry. 2018;17(1):30-8. doi:10.1002/wps.20482
11. Huang HC, Lai MC, Lin LH, Chen CT. Overcoming organizational inertia to strengthen business model innovation: An open innovation perspective. J Organizational Change Manage. 2013;26(6):977-1002. doi:10.1108/JOCM-04-2012-0047
Reporting Coronary Artery Calcium on Low-Dose Computed Tomography Impacts Statin Management in a Lung Cancer Screening Population
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
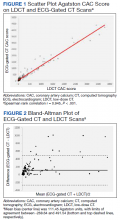
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
Cigarette smoking is an independent risk factor for lung cancer and atherosclerotic cardiovascular disease (ASCVD).1-3 The National Lung Screening Trial (NLST) demonstrated both lung cancer mortality reduction with the use of surveillance low-dose computed tomography (LDCT) and ASCVD as the most common cause of death among smokers.4,5 ASCVD remains the leading cause of death in the lung cancer screening (LCS) population.2,3 After publication of the NLST results, the US Preventive Services Task Force (USPSTF) established LCS eligibility among smokers and the Center for Medicare and Medicaid Services approved payment for annual LDCT in this group.1,6,7
Recently LDCT has been proposed as an adjunct diagnostic tool for detecting coronary artery calcium (CAC), which is independently associated with ASCVD and mortality.8-13 CAC scores have been recommended by the 2019 American College of Cardiology/American Heart Association cholesterol treatment guidelines and shown to be cost-effective in guiding statin therapy for patients with borderline to intermediate ASCVD risk.14-16 While CAC is conventionally quantified using electrocardiogram (ECG)-gated CT, these scans are not routinely performed in clinical practice because preventive CAC screening is neither recommended by the USPSTF nor covered by most insurance providers.17,18 LDCT, conversely, is reimbursable and a well-validated ASCVD risk predictor.18,19
In this study, we aimed to determine the validity of LDCT in identifying CAC among the military LCS population and whether it would impact statin recommendations based on 10-year ASCVD risk.
Methods
Participants were recruited from a retrospective cohort of 563 Military Health System (MHS) beneficiaries who received LCS with LDCT at Naval Medical Center Portsmouth (NMCP) in Virginia between January 1, 2019, and December 31, 2020. The 2013 USPSTF LCS guidelines were followed as the 2021 guidelines had not been published before the start of the study; thus, eligible participants included adults aged 55 to 80 years with at least a 30-pack-year smoking history and currently smoked or had quit within 15 years from the date of study consent.6,7
Between November 2020 and May 2021, study investigators screened 287 patient records and recruited 190 participants by telephone, starting with individuals who had the most recent LDCT and working backward until reaching the predetermined 170 subjects who had undergone in-office consents before ECG-gated CT scans. Since LDCT was not obtained simultaneously with the ECG-gated CT, participants were required to complete their gated CT within 24 months of their last LDCT. Of the 190 subjects initially recruited, those who were ineligible for LCS (n = 4), had a history of angioplasty, stent, or bypass revascularization procedure (n = 4), did not complete their ECG-gated CT within the specified time frame (n = 8), or withdrew from the study (n = 4) were excluded. While gated CT scans were scored for CAC in the present time, LDCT (previously only read for general lung pathology) was not scored until after participant consent. Patients were peripherally followed, via health record reviews, for 3 months after their gated CT to document any additional imaging ordered by their primary care practitioners. The study was approved by the NMCP Institutional Review Board.
Coronary Artery Calcification Scoring
We performed CT scans using Siemens SOMATOM Flash, a second-generation dual-source scanner; and GE LightSpeed VCT, a single-source, 64-slice scanner. A step-and-shoot prospective trigger technique was used, and contiguous axial images were reconstructed at 2.5-mm or 3-mm intervals for CAC quantification using the Agatston method.20 ECG-gated CT scans were electrocardiographically triggered at mid-diastole (70% of the R-R interval). Radiation dose reduction techniques involved adjustments of the mA according to body mass index and iterative reconstruction. LDCT scans were performed without ECG gating. We reconstructed contiguous axial images at 1-mm intervals for evaluation of the lung parenchyma. Similar dose-reduction techniques were used, to limit radiation exposure for each LDCT scan to < 1.5 mSv, per established guidelines.21 CAC on LDCT was also scored using the Agatston method. CAC was scored on the 2 scan types by different blinded reviewers.
Covariates
We reviewed outpatient health records to obtain participants’ age, sex, medical history, statin use, smoking status (current or former), and pack-years. International Classification of Diseases, Tenth Revision codes within medical encounters were used to document prevalent hypertension, hyperlipidemia, and diabetes mellitus. Participants’ most recent low-density lipoprotein value (within 24 months of ECG-gated CT) was recorded and 10-year ASCVD risk scores were calculated using the pooled cohorts equation.
Statistical Analysis
A power analysis performed before study initiation determined that a prospective sample size of 170 would be sufficient to provide strength of correlation between CAC scores calculated from ECG-gated CT and LDCT and achieve a statistical power of at least 80%. The Wilcoxon rank sum and Fisher exact tests were used to evaluate differences in continuous and categorical CAC scores, respectively. Given skewed distributions, Spearman rank correlations and Kendall W coefficient of concordance were respectively used to evaluate correlation and concordance of CAC scores between the 2 scan types. κ statistics were used to rate agreement between categorical CAC scores. Bland-Altman analysis was performed to determine the bias and limits of agreement between ECG-gated CT and LDCT.22 For categorical CAC score analysis, participants were categorized into 5 groups according to standard Agatston score cut-off points. We defined the 5 categories of CAC for both scan types based on previous analysis from Rumberger and colleagues: CAC = 0 (absent), CAC = 1-10 (minimal), CAC = 11-100 (mild), CAC = 101-400 (moderate), CAC > 400 (severe).23 Of note, LDCT reports at NMCP include a visual CAC score using these qualitative descriptors that were available to LDCT reviewers. Analyses were conducted using SAS version 9.4 and Microsoft Excel; P values < .05 were considered statistically significant.
Results
The 170 participants had a mean (SD) age of 62.1 (4.6) years and were 70.6% male (Table 1). Hyperlipidemia was the most prevalent cardiac risk factor with almost 70% of participants on a statin. There was no incidence of ischemic ASCVD during follow-up, although 1 participant was later diagnosed with lung cancer after evaluation of suspicious pulmonary findings on ECG-gated CT. CAC was identified on both scan types in 126 participants; however, LDCT was discordant with gated CT in identifying CAC in 24 subjects (P < .001).
The correlation between CAC scores on ECG-gated CT and LDCT was 0.945 (P < .001) and the concordance was 0.643, indicating moderate agreement between CAC scores on the 2 different scans (Figure 1). Median CAC scores were significantly higher on ECG-gated CT when compared with LDCT (107.5 vs 48.1 Agatston units, respectively; P < .05). Table 2 shows the CAC score characteristics for both scan types. The κ statistic for agreement between categorical CAC scores on ECG-gated CT compared with LDCT was 0.49 (SEκ= 0.05; 95% CI, -0.73-1.71), and the weighted κ statistic was 0.71, indicating moderate to substantial agreement between the 2 scans using the specified cutoff points. The Bland-Altman analysis presented a mean bias of 111.45 Agatston units, with limits of agreement between -268.64 and 491.54, as shown in Figure 2, suggesting that CAC scores on ECG-gated CT were, on average, about 111 units higher than those on LDCT. Finally, there were 24 participants with CAC seen on ECG-gated CT but none identified on LDCT (P < .001); of this cohort 20 were already on a statin, and of the remaining 4 individuals, 1 met statin criteria based on a > 20% ASCVD risk score alone (regardless of CAC score), 1 with an intermediate risk score met statin criteria based on CAC score reporting, 1 did not meet criteria due to a low-risk score, and the last had no reportable ASCVD risk score.
In the study, there were 80 participants with reportable borderline to intermediate 10-year ASCVD risk scores (5% ≤ 10-year ASCVD risk < 20%), 49 of which were taking a statin. Of the remaining 31 participants not on a statin, 19 met statin criteria after CAC was identified on ECG-gated CT (of these 18 also had CAC identified on LDCT). Subsequently, the number of participants who met statin criteria after additional CAC reporting (on ECG-gated CT and LDCT) was statistically significant (P < .001 and P < .05, respectively). Of the 49 participants on a statin, only 1 individual no longer met statin criteria due to a CAC score < 1 on gated CT.
Discussion
In this study population of recruited MHS beneficiaries, there was a strong correlation and moderate to substantial agreement between CAC scores calculated from LDCT and conventional ECG-gated CT. The number of nonstatin participants who met statin criteria and would have benefited from additional CAC score reporting was statistically significant as compared to their statin counterparts who no longer met the criteria.
CAC screening using nongated CT has become an increasingly available and consistently reproducible means for stratifying ASCVD risk and guiding statin therapy in individuals with equivocal ASCVD risk scores.24-26 As has been demonstrated in previous studies, our study additionally highlights the effective use of LDCT in not only identifying CAC, but also in beneficially impacting statin decisions in the high-risk smoking population.24-26 Our results also showed LDCT missed CAC in participants, the majority of which were already on a statin, and only 1 nonstatin individual benefited from additional CAC reporting. CAC scoring on LDCT should be an adjunct, not a substitute, for ASCVD risk stratification to help guide statin management.25,27
Our results may provide cost considerate implications for preventive CAC screening. While TRICARE covers the cost of ECG-gated CT for MHS beneficiaries, the same is not true of most nonmilitary insurance providers. Concerns about cancer risk from radiation exposure may also lead to hesitation about receiving additional CTs in the smoking population. Since the LCS population already receives annual LDCT, these scans can also be used for CAC scoring to help primary care professionals risk stratify their patients, as has been previously shown.28-31 Clinicians should consider implementing CAC scoring with annual LDCT scans, which would curtail further risks and expenses from CAC-specified scans.
Although CAC is scored visually and routinely reported in the body of LDCT reports at our facility, this is not a universal practice and was performed in only 44% of subjects with known CAC by a previous study.32 In 2007, there were 600,000 CAC scoring scans and > 9 million routine chest CTs performed in the United States.33 Based on our results and the growing consensus in the existing literature, CAC scoring on nongated CT is not only valid and reliable, but also can estimate ASCVD risk and subsequent mortality.34-36 Routine chest CTs remain an available resource for providing additional ASCVD risk stratification.
As we demonstrated, median CAC scores on LDCT were on average significantly lower than those from gated CT. This could be due to slice thickness variability between the GE and Siemens scanners or CAC progression between the time of the retrospective LDCT and prospective ECG-gated CT. Aside from this potential limitation, LDCT has been shown to have a high level of agreement with gated CT in predicting CAC, both visually and by the Agatston technique.37-39 Our results further support previous recommendations of utilizing CAC score categories when determining ASCVD risk from LDCT and that establishing scoring cutoff points warrants further development for potential standardization.37-39 Readers should be mindful that LDCT may still be less sensitive and underestimate low CAC levels and that ECG-gated CT may occasionally be more optimal in determining ASCVD risk when considering the negative predictive value of CAC.40
Limitations
Our study cohort was composed of MHS beneficiaries. Compared with the general population, these individuals may have greater access to care and be more likely to receive statins after preventive screenings. Additional studies may be required to assess CAC-associated statin eligibility among the general population. As discussed previously LDCT was not performed concomitantly with the ECG-gated CT. Although there was moderate to substantial CAC agreement between the 2 scan types, the timing difference could have led to absolute differences in CAC scores across both scan types and impacted the ability to detect low-level CAC on LDCT. CAC values should be interpreted based on the respective scan type.
Conclusions
LDCT is a reliable diagnostic alternative to ECG-gated CT in predicting CAC. CAC scores from LDCT are highly correlated and concordant with those from gated CT and can help guide statin management in individuals with intermediate ASCVD risk. The proposed duality of LDCT to assess ASCVD risk in addition to lung cancer can reduce the need for unnecessary scans while optimizing preventive clinical care. While coronary calcium and elevated CAC scores can facilitate clinical decision making to initiate statin therapy for intermediate-risk patients, physicians must still determine whether additional cardiac testing is warranted to avoid unnecessary procedures and health care costs. Smokers undergoing annual LDCT may benefit from standardized CAC scoring to help further stratify ASCVD risk while limiting the expense and radiation of additional scans.
Acknowledgments
The authors thank Ms. Lorie Gower for her contributions to the study.
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
1. Leigh A, McEvoy JW, Garg P, et al. Coronary artery calcium scores and atherosclerotic cardiovascular disease risk stratification in smokers. JACC Cardiovasc Imaging. 2019;12(5):852-861. doi:10.1016/j.jcmg.2017.12.017
2. Lu MT, Onuma OK, Massaro JM, D’Agostino RB Sr, O’Donnell CJ, Hoffmann U. Lung cancer screening eligibility in the community: cardiovascular risk factors, coronary artery calcification, and cardiovascular events. Circulation. 2016;134(12):897-899. doi:10.1161/CIRCULATIONAHA.116.023957
3. Tailor TD, Chiles C, Yeboah J, et al. Cardiovascular risk in the lung cancer screening population: a multicenter study evaluating the association between coronary artery calcification and preventive statin prescription. J Am Coll Radiol. 2021;18(9):1258-1266. doi:10.1016/j.jacr.2021.01.015
4. National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013;368(21):1980-1991. doi:10.1056/NEJMoa1209120
5. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29-e322. doi:10.1161/CIR.0000000000000152
6. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330-338. doi:10.7326/M13-2771
7. US Preventive Services Task Force, Krist AH, Davidson KW, et al. Screening for lung cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
8. Arcadi T, Maffei E, Sverzellati N, et al. Coronary artery calcium score on low-dose computed tomography for lung cancer screening. World J Radiol. 2014;6(6):381-387. doi:10.4329/wjr.v6.i6.381
9. Kim SM, Chung MJ, Lee KS, Choe YH, Yi CA, Choe BK. Coronary calcium screening using low-dose lung cancer screening: effectiveness of MDCT with retrospective reconstruction. AJR Am J Roentgenol. 2008;190(4):917-922. doi:10.2214/AJR.07.2979
10. Ruparel M, Quaife SL, Dickson JL, et al. Evaluation of cardiovascular risk in a lung cancer screening cohort. Thorax. 2019;74(12):1140-1146. doi:10.1136/thoraxjnl-2018-212812
11. Jacobs PC, Gondrie MJ, van der Graaf Y, et al. Coronary artery calcium can predict all-cause mortality and cardiovascular events on low-dose CT screening for lung cancer. AJR Am J Roentgenol. 2012;198(3):505-511. doi:10.2214/AJR.10.5577
12. Fan L, Fan K. Lung cancer screening CT-based coronary artery calcification in predicting cardiovascular events: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97(20):e10461. doi:10.1097/MD.0000000000010461
13. Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol. 2018;72(4):434-447. doi:10.1016/j.jacc.2018.05.027
14. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e563-e595. doi:10.1161/CIR.0000000000000677
15. Pletcher MJ, Pignone M, Earnshaw S, et al. Using the coronary artery calcium score to guide statin therapy: a cost-effectiveness analysis. Circ Cardiovasc Qual Outcomes. 2014;7(2):276-284. doi:10.1161/CIRCOUTCOMES.113.000799
16. Hong JC, Blankstein R, Shaw LJ, et al. Implications of coronary artery calcium testing for treatment decisions among statin candidates according to the ACC/AHA Cholesterol Management Guidelines: a cost-effectiveness analysis. JACC Cardiovasc Imaging. 2017;10(8):938-952. doi:10.1016/j.jcmg.2017.04.014
17. US Preventive Services Task Force, Curry SJ, Krist AH, et al. Risk assessment for cardiovascular disease with nontraditional risk factors: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320(3):272-280. doi:10.1001/jama.2018.8359
18. Hughes-Austin JM, Dominguez A 3rd, Allison MA, et al. Relationship of coronary calcium on standard chest CT scans with mortality. JACC Cardiovasc Imaging. 2016;9(2):152-159. doi:10.1016/j.jcmg.2015.06.030
19. Haller C, Vandehei A, Fisher R, et al. Incidence and implication of coronary artery calcium on non-gated chest computed tomography scans: a large observational cohort. Cureus. 2019;11(11):e6218. Published 2019 Nov 22. doi:10.7759/cureus.6218
20. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827-832. doi:10.1016/0735-1097(90)90282-t
21. Aberle D, Berg C, Black W, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-53. doi:10.1148/radiol.10091808
22. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8(2):135-160. doi:10.1177/096228029900800204
23. Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: a review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74(3):243-252. doi:10.4065/74.3.243
24. Douthit NT, Wyatt N, Schwartz B. Clinical impact of reporting coronary artery calcium scores of non-gated chest computed tomography on statin management. Cureus. 2021;13(5):e14856. Published 2021 May 5. doi:10.7759/cureus.14856
25. Miedema MD, Dardari ZA, Kianoush S, et al. Statin eligibility, coronary artery calcium, and subsequent cardiovascular events according to the 2016 United States Preventive Services Task Force (USPSTF) Statin Guidelines: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7(12):e008920. Published 2018 Jun 13. doi:10.1161/JAHA.118.008920
26. Fisher R, Vandehei A, Haller C, et al. Reporting the presence of coronary artery calcium in the final impression of non-gated CT chest scans increases the appropriate utilization of statins. Cureus. 2020;12(9):e10579. Published 2020 Sep 21. doi:10.7759/cureus.10579
27. Blaha MJ, Budoff MJ, DeFilippis AP, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684-692. doi:10.1016/S0140-6736(11)60784-8
28. Waheed S, Pollack S, Roth M, Reichek N, Guerci A, Cao JJ. Collective impact of conventional cardiovascular risk factors and coronary calcium score on clinical outcomes with or without statin therapy: the St Francis Heart Study. Atherosclerosis. 2016;255:193-199. doi:10.1016/j.atherosclerosis.2016.09.060
29. Mahabadi AA, Möhlenkamp S, Lehmann N, et al. CAC score improves coronary and CV risk assessment above statin indication by ESC and AHA/ACC Primary Prevention Guidelines. JACC Cardiovasc Imaging. 2017;10(2):143-153. doi:10.1016/j.jcmg.2016.03.022
30. Blaha MJ, Cainzos-Achirica M, Greenland P, et al. Role of coronary artery calcium score of zero and other negative risk markers for cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2016;133(9):849-858. doi:10.1161/CIRCULATIONAHA.115.018524
31. Hoffmann U, Massaro JM, D’Agostino RB Sr, Kathiresan S, Fox CS, O’Donnell CJ. Cardiovascular event prediction and risk reclassification by coronary, aortic, and valvular calcification in the Framingham Heart Study. J Am Heart Assoc. 2016;5(2):e003144. Published 2016 Feb 22. doi:10.1161/JAHA.115.003144
32. Williams KA Sr, Kim JT, Holohan KM. Frequency of unrecognized, unreported, or underreported coronary artery and cardiovascular calcification on noncardiac chest CT. J Cardiovasc Comput Tomogr. 2013;7(3):167-172. doi:10.1016/j.jcct.2013.05.003

33. Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi:10.1001/archinternmed.2009.440
34. Azour L, Kadoch MA, Ward TJ, Eber CD, Jacobi AH. Estimation of cardiovascular risk on routine chest CT: Ordinal coronary artery calcium scoring as an accurate predictor of Agatston score ranges. J Cardiovasc Comput Tomogr. 2017;11(1):8-15. doi:10.1016/j.jcct.2016.10.001
35. Waltz J, Kocher M, Kahn J, Dirr M, Burt JR. The future of concurrent automated coronary artery calcium scoring on screening low-dose computed tomography. Cureus. 2020;12(6):e8574. Published 2020 Jun 12. doi:10.7759/cureus.8574
36. Huang YL, Wu FZ, Wang YC, et al. Reliable categorisation of visual scoring of coronary artery calcification on low-dose CT for lung cancer screening: validation with the standard Agatston score. Eur Radiol. 2013;23(5):1226-1233. doi:10.1007/s00330-012-2726-5
37. Kim YK, Sung YM, Cho SH, Park YN, Choi HY. Reliability analysis of visual ranking of coronary artery calcification on low-dose CT of the thorax for lung cancer screening: comparison with ECG-gated calcium scoring CT. Int J Cardiovasc Imaging. 2014;30 Suppl 2:81-87. doi:10.1007/s10554-014-0507-8
38. Xia C, Vonder M, Pelgrim GJ, et al. High-pitch dual-source CT for coronary artery calcium scoring: A head-to-head comparison of non-triggered chest versus triggered cardiac acquisition. J Cardiovasc Comput Tomogr. 2021;15(1):65-72. doi:10.1016/j.jcct.2020.04.013
39. Hutt A, Duhamel A, Deken V, et al. Coronary calcium screening with dual-source CT: reliability of ungated, high-pitch chest CT in comparison with dedicated calcium-scoring CT. Eur Radiol. 2016;26(6):1521-1528. doi:10.1007/s00330-015-3978-7
40. Blaha MJ, Budoff MJ, Tota-Maharaj R, et al. Improving the CAC score by addition of regional measures of calcium distribution: Multi-Ethnic Study of Atherosclerosis. JACC Cardiovasc Imaging. 2016;9(12):1407-1416. doi:10.1016/j.jcmg.2016.03.001
Engaging Veterans With Serious Mental Illness in Primary Care
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
People with serious mental illness (SMI) are at substantial risk for premature mortality, dying on average 10 to 20 years earlier than others.1 The reasons for this disparity are complex; however, the high prevalence of chronic disease and physical comorbidities in the SMI population have been identified as prominent factors.2 Engagement and reengagement in care, including primary care for medical comorbidities, can mitigate these mortality risks.2-4 Among veterans with SMI lost to follow-up care for more than 12 months, those not successfully reengaged in care were more likely to die compared with those reengaged in care.2,3
Given this evidence, health care systems, including the US Department of Veterans Affairs (VA), have looked to better engage these patients in care. These efforts have included mental health population health management, colocation of mental health with primary care, designation of primary care teams specializing in SMI, and integration of mental health and primary care services for patients experiencing homelessness.5-8
As part of a national approach to encourage locally driven quality improvement (QI), the VA compiles performance metrics for each facility, across a gamut of care settings, conditions, and veteran populations.9 Quarterly facility report cards, with longitudinal data and cross-facility comparisons, enable facilities to identify targets for QI and track improvement progress. One metric reports on the proportion of enrolled veterans with SMI who have primary care engagement, defined as having an assigned primary care practitioner (PCP) and a primary care visit in the prior 12 months.
In support of a QI initiative at the VA Greater Los Angeles Healthcare System (VAGLAHS), we sought to describe promising practices being utilized by VA facilities with higher levels of primary care engagement among their veterans with SMI populations.
Methods
We conducted semistructured telephone interviews with a purposeful sample of key informants at VA facilities with high levels of engagement in primary care among veterans with SMI. All project components were conducted by an interdisciplinary team, which included a medical anthropologist (JM), a mental health physician (PR), an internal medicine physician (KC), and other health services researchers (JB, AG). Because the primary objective of the project was QI, this project was designated as nonresearch by the VAGLAHS Institutional Review Board.
The VA Facility Complexity Model classifies facilities into 5 tiers: 1a (most complex), 1b, 1c, 2, and 3 (least complex), based on patient care volume, patient risk, complexity of clinical programs, and size of research and teaching programs. We sampled informants at VA facilities with complexity ratings of 1a or 1b with better than median scores for primary care engagement of veterans with SMI based on report cards from January 2019 to March 2019. To increase the likelihood of identifying lessons that can generalize to the VAGLAHS with its large population of veterans experiencing homelessness, we selected facilities serving populations consisting of more than 1000 veterans experiencing homelessness.
At each selected facility, we first aimed to interview mental health leaders responsible for quality measurement and improvement identified from a national VA database. We then used snowball sampling to identify other informants at these VA facilities who were knowledgeable about relevant processes. Potential interviewees were contacted via email.
Interviews
The interview guide was developed by the interdisciplinary team and based on published literature about strategies for engaging patients with SMI in care. Interview guide questions focused on local practice arrangements, panel management, population health practices, and quality measurement and improvement efforts for engaging veterans with SMI in primary care (Appendix). Interviews were conducted by telephone, from May 2019 through July 2019, by experienced qualitative interviewers (JM, JB). Interviewees were assured confidentiality of their responses.
Interview audio recordings were used to generate detailed notes (AG). Structured summaries were prepared from these notes, using a template based on the interview guide. We organized these summaries into matrices for analysis, grouping summarized points by interview domains to facilitate comparison across interviews.10-11 Our team reviewed and discussed the matrices, and iteratively identified and defined themes to identify the common engagement approaches and the nature of the connections between mental health and primary care. To ensure rigor, findings were checked by the senior qualitative lead (JM).
Results
The median SMI engagement score—defined as the proportion of veterans with SMI who have had a primary care visit in the prior 12 months and who have an assigned PCP—was 75.6% across 1a and 1b VA facilities. We identified 16 VA facilities that had a median or higher score and more than 1000 enrolled veterans experiencing homelessness. From these16 facilities, we emailed 31 potential interviewees, 14 of whom were identified from a VA database and 17 referred by other interviewees. In total, we interviewed 18 key informants across 11 (69%) facilities, including chiefs of psychology and mental health services, PCPs with mental health expertise, QI specialists, a psychosocial rehabilitation leader, and a local recovery coordinator, who helps veterans with SMI access recovery-oriented services. Characteristics of the facilities and interviewees are shown in Table 1. Interviews lasted a mean 35 (range, 26-50) minutes.
Engagement Approaches
The strategies used to engage veterans with SMI were heterogenous, with no single strategy common across all facilities. However, we identified 2 categories of engagement approaches: targeted outreach and routine practices.
Targeted outreach strategies included deliberate, systematic approaches to reach veterans with SMI outside of regularly scheduled visits. These strategies were designed to be proactive, often prioritizing veterans at risk of disengaging from care. Designated VA care team members identified and reached out to veterans well before 12 months had passed since their prior visit (the VA definition of disengagement from care); visits included any care at VA, including, but not exclusively, primary care. Table 2 describes the key components of targeted outreach strategies: (1) identifying veterans’ last visit; (2) prioritizing which veterans to outreach to; and (3) assigning responsibility and reaching out. A key defining feature of targeted outreach is that veterans were identified and prioritized for outreach independent from any visits with mental health or other VA services.
In identifying veterans at risk for disengagement, a designated employee in mental health or primary care (eg, local recovery coordinator) reviewed a VA dashboard or locally developed report that identified veterans who have not engaged in care for several months. This process was repeated regularly. The designated employee either contacted those veterans directly or coordinated with other clinicians and support staff. When possible, a clinician or nurse with an existing relationship with the veteran would call them. If no such relationship existed, an administrative staff member made a cold call, sometimes accompanied by mailed outreach materials.
Routine practices were business-as-usual activities embedded in regular clinical workflows that facilitated engagement or reengagement of veterans with SMI in care. Of note, and in contrast to targeted outreach, these activities were tied to veteran visits with mental health practitioners. These practices were typically described as being at least as important as targeted outreach efforts. For example, during mental health visits, clinicians routinely checked the VA electronic health record to assess whether veterans had an assigned primary care team. If not, they would contact the primary care service to refer the patient for a primary care visit and assignment. If the patient already had a primary care team assigned, the mental health practitioner checked for recent primary care visits. If none were evident, the mental health practitioner might email the assigned PCP or contact them via instant message.
At some facilities, mental health support staff were able to directly schedule primary care appointments, which was identified as an important enabling factor in promoting mental health patient engagement in primary care. Some interviewees seemed to take for granted the idea that mental health practitioners would help engage patients in primary care—suggesting that these practices had perhaps become a cultural norm within their facility. However, some interviewees identified clear strategies for making these practices a consistent part of care—for example, by designing a protocol for initial mental health assessments to include a routine check for primary care engagement.
Mental Health/Primary Care Connections
Interviewees characterized the nature of the connections between mental health and primary care at their facilities. Nearly all interviewees described that their medical centers had extensive ties, formal and informal, between mental health and primary care.
Formal ties may include the reverse integration care model, in which primary care services are embedded in mental health settings. Interviewees at sites with programs based on this model noted that these programs enabled warm hand-offs from mental health to primary care and suggested that it can foster integration between primary care and mental health care for patients with SMI. However, the size, scope, and structure of these programs varied, sometimes serving a small proportion of a facility’s population of SMI patients. Other examples of formal ties included written agreements, establishing frequent, regular meetings between mental health and primary care leadership and front-line staff, and giving mental health clerks the ability to directly schedule primary care appointments.
Informal ties between mental health and primary care included communication and personal working relationships between mental health and PCPs, facilitated by mental health and primary care leaders working together in workgroups and other administrative activities. Some participants described a history of collaboration between mental health and primary care leaders yielding productive and trusting working relationships. Some interviewees described frequent direct communication between individual mental health practitioners and PCPs—either face-to-face or via secure messaging.
Discussion
VA facilities with high levels of primary care engagement among veterans with SMI used extensive engagement strategies, including a diverse array of targeted outreach and routine practices. In both approaches, intentional organizational structural and process decisions, as well as formal and informal ties between mental health and primary care, established and supported them. In addition, organizational cultural factors were especially relevant to routine practice strategies.
To enable targeted outreach, a bevy of organizational resources, both local and national were required. Large accountable care organizations and integrated delivery systems, like the VA, are often better able to create dashboards and other informational resources for population health management compared with smaller, less integrated health care systems. Though these resources are difficult to create in fragmented systems, comparable tools have been explored by multiple state health departments.12 Our findings suggest that these data tools, though resource intensive to develop, may enable facilities to be more methodical and reliable in conducting outreach to vulnerable patients.
In contrast to targeted outreach, routine practices depend less on population health management resources and more on cultural norms. Such norms are notoriously difficult to change, but intentional structural decisions like embedding primary care engagement in mental health protocols may signal that primary care engagement is an important and legitimate consideration for mental health care.13
We identified extensive and heterogenous connections between mental health and primary care in our sample of VA facilities with high engagement of patients with SMI in primary care. A growing body of literature on relational coordination studies the factors that contribute to organizational siloing and mechanisms for breaking down those silos so work can be coordinated across boundaries (eg, the organizational boundary between mental health and primary care).14 Coordinating care across these boundaries, through good relational coordination practices has been shown to improve outcomes in health care and other sectors. Notably, VA facilities in our sample had several of the defining characteristics of good relational coordination: relationships between mental health and primary care that include shared goals, shared knowledge, and mutual respect, all reinforced by frequent communication structured around problem solving.15 The relational coordination literature also offers a way to identify evidence-based interventions for facilitating relational coordination in places where it is lacking, for example, with information systems, boundary-spanning individuals, facility design, and formal conflict resolution.15 Future work might explore how relational coordination can be further used to optimize mental health and primary care connections to keep veterans with SMI engaged in care.
Our approach of interviewing informants in higher-performing facilities draws heavily on the idea of positive deviance, which holds that information on what works in health care is available from organizations that already are demonstrating “consistently exceptional performance.”16 This approach works best when high performance and organizational characteristics are observable for a large number of facilities, and when high-performing facilities are willing to share their strategies. These features allow investigators to identify promising practices and hypotheses that can then be empirically tested and compared. Such testing, including assessing for unintended consequences, is needed for the approaches we identified. Research is also needed to assess for factors that would promote the implementation of effective strategies.
Limitations
As a QI project seeking to identify promising practices, our interviews were limited to 18 key informants across 11 VA facilities with high engagement of care among veterans with SMI. No inferences can be made that these practices are directly related to this high level of engagement, nor the differential impact of different practices. Future work is needed to assess for these relationships. We also did not interview veterans to understand their perspectives on these strategies, which is an additional important topic for future work. In addition, these interviews were performed before the start of the COVID-19 pandemic. Further work is needed to understand how these strategies may have been modified in response to changes in practice. The shift to care from in-person to virtual services may have impacted both clinical interactions with veterans, as well as between clinicians.
Conclusions
Interviews with key informants demonstrate that while engaging and retaining veterans with SMI in primary care is vital, it also requires intentional and potentially resource-intensive practices, including targeted outreach and routine engagement strategies embedded into mental health visits. These promising practices can provide valuable insights for both VA and community health care systems providing care to patients with SMI.
Acknowledgments
We thank Gracielle J. Tan, MD for administrative assistance in preparing this manuscript.
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
1. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16(1):30-40. doi:10.1002/wps.20384
2. Bowersox NW, Kilbourne AM, Abraham KM, et al. Cause-specific mortality among veterans with serious mental illness lost to follow-up. Gen Hosp Psychiatry. 2012;34(6):651-653. doi:10.1016/j.genhosppsych.2012.05.014
3. Davis CL, Kilbourne AM, Blow FC, et al. Reduced mortality among Department of Veterans Affairs patients with schizophrenia or bipolar disorder lost to follow-up and engaged in active outreach to return for care. Am J Public Health. 2012;102(suppl 1):S74-S79. doi:10.2105/AJPH.2011.300502
4. Copeland LA, Zeber JE, Wang CP, et al. Patterns of primary care and mortality among patients with schizophrenia or diabetes: a cluster analysis approach to the retrospective study of healthcare utilization. BMC Health Serv Res. 2009;9:127. doi:10.1186/1472-6963-9-127
5. Abraham KM, Mach J, Visnic S, McCarthy JF. Enhancing treatment reengagement for veterans with serious mental illness: evaluating the effectiveness of SMI re-engage. Psychiatr Serv. 2018;69(8):887-895. doi:10.1176/appi.ps.201700407
6. Ward MC, Druss BG. Reverse integration initiatives for individuals with serious mental illness. Focus (Am Psychiatr Publ). 2017;15(3):271-278. doi:10.1176/appi.focus.20170011
7. Chang ET, Vinzon M, Cohen AN, Young AS. Effective models urgently needed to improve physical care for people with serious mental illnesses. Health Serv Insights. 2019;12:1178632919837628. Published 2019 Apr 2. doi:10.1177/1178632919837628
8. Gabrielian S, Gordon AJ, Gelberg L, et al. Primary care medical services for homeless veterans. Fed Pract. 2014;31(10):10-19.
9. Lemke S, Boden MT, Kearney LK, et al. Measurement-based management of mental health quality and access in VHA: SAIL mental health domain. Psychol Serv. 2017;14(1):1-12. doi:10.1037/ser0000097
10. Averill JB. Matrix analysis as a complementary analytic strategy in qualitative inquiry. Qual Health Res. 2002;12(6):855-866. doi:10.1177/104973230201200611
11. Zuchowski JL, Chrystal JG, Hamilton AB, et al. Coordinating care across health care systems for Veterans with gynecologic malignancies: a qualitative analysis. Med Care. 2017;55(suppl 1):S53-S60. doi:10.1097/MLR.0000000000000737
12. Daumit GL, Stone EM, Kennedy-Hendricks A, Choksy S, Marsteller JA, McGinty EE. Care coordination and population health management strategies and challenges in a behavioral health home model. Med Care. 2019;57(1):79-84. doi:10.1097/MLR.0000000000001023
13. Parmelli E, Flodgren G, Beyer F, et al. The effectiveness of strategies to change organisational culture to improve healthcare performance: a systematic review. Implement Sci. 2011;6(33):1-8. doi:10.1186/1748-5908-6-33
14. Bolton R, Logan C, Gittell JH. Revisiting relational coordination: a systematic review. J Appl Behav Sci. 2021;57(3):290-322. doi:10.1177/0021886321991597
15. Gittell JH, Godfrey M, Thistlethwaite J. Interprofessional collaborative practice and relational coordination: improving healthcare through relationships. J Interprof Care. 2013;27(3):210-13. doi:10.3109/13561820.2012.730564
16. Bradley EH, Curry LA, Ramanadhan S, Rowe L, Nembhard IM, Krumholz HM. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Published 2009 May 8. doi:10.1186/1748-5908-4-25
Catheter-Directed Retrieval of an Infected Fragment in a Vietnam War Veteran
Shrapnel injuries are commonly encountered in war zones.1 Shrapnel injuries can remain asymptomatic or become systemic, with health effects of the retained foreign body ranging from local to systemic toxicities depending on the patient’s reaction to the chemical composition and corrosiveness of the fragments in vivo.2 We present a case of a reactivating shrapnel injury in the form of a retroperitoneal infection and subsequent iliopsoas abscess. A collaborative procedure was performed between surgery and interventional radiology to snare and remove the infected fragment and drain the abscess.
Case Presentation
While serving in Vietnam, a soldier sustained a fragment injury to his left lower abdomen. He underwent a laparotomy, small bowel resection, and a temporary ileostomy at the time of the injury. Nearly 50 years later, the patient presented with chronic left lower quadrant pain and a low-grade fever. He was diagnosed clinically in the emergency department (ED) with diverticulitis and treated with antibiotics. The patient initially responded to treatment but returned 6 months later with similar symptoms, low-grade fever, and mild leukocytosis. A computed tomography (CT) scan during that encounter without IV contrast revealed a few scattered colonic diverticula without definite diverticulitis as well as a metallic fragment embedded in the left iliopsoas with increased soft tissue density.
The patient was diagnosed with a pelvic/abdominal wall hematoma and was discharged with pain medication. The patient reported recurrent attacks of left lower quadrant pain, fever, and changes in bowel habits, prompting gastrointestinal consultation and a colonoscopy that was unremarkable. Ten months later, the patient again presented to the ED, with recurrent symptoms, a fever of 102 °F, and leukocytosis with a white blood cell count of 11.7 × 109/L. CT scan with IV contrast revealed a large left iliopsoas abscess associated with an approximately 1-cm metallic fragment (Figure 1). A drainage catheter was placed under CT guidance and approximately 270 mL of purulent fluid was drained. Culture of the fluid was positive for Escherichia coli (E coli). Two days after drain placement, the fragment was removed as a joint procedure with interventional radiology and surgery. Using the drainage catheter tract as a point of entry, multiple attempts were made to retrieve the fragment with Olympus EndoJaw endoscopic forceps without success.
Ultimately a stiff directional sheath from a Cook Medical transjugular liver biopsy kit was used with a Merit Medical EnSnare to relocate the fragment to the left inguinal region for surgical excision (Figures 2, 3, and 4). The fragment was removed and swabbed for culture and sensitivity and a BLAKE drain was placed in the evacuated abscess cavity. The patient tolerated the procedure well and was discharged the following day. Three days later, culture and sensitivity grew E coli and Acinetobacter, thus confirming infection and a nidus for the surrounding abscess formation. On follow-up with general surgery 7 days later, the patient reported he was doing well, and the drain was removed without difficulty.
Discussion
Foreign body injuries can be benign or debilitating depending on the initial damage, anatomical location of the foreign body, composition of the foreign body, and the patient’s response to it. Retained shrapnel deep within the muscle tissue rarely causes complications. Although many times embedded objects can be asymptomatic and require no further management, migration of the foreign body or the formation of a fistula is possible, causing symptoms and requiring surgical intervention.1 One case involved the formation of a purulent fistula appearing a year after an explosive wound to the lumbosacral spine, which was treated with antimicrobials. Recurrence of the fistula several times after treatment led to surgical removal of the shrapnel along with antibiotic treatment of the osteomyelitis.3 Although uncommon, lead exposure that occurs due to retained foreign body fragments from gunshot or military-related injuries can cause systemic lead toxicity. Symptoms may range from abdominal pain, nausea, and constipation to jaundice and hepatitis.4 The severity has also been stated to correlate with the surface area of the lead exposed for dissolution.5 Migration of foreign bodies and shrapnel to other sites in the body, such as movement from soft tissues into distantly located body cavities, have been reported as well. Such a case involved the spontaneous onset of knee synovitis due to an intra-articular metallic object that was introduced via a blast injury to the upper third of the ipsilateral thigh.1
In this patient’s case, a large intramuscular abscess had formed nearly 50 years after the initial combat injury, requiring drainage of the abscess and removal of the fragment. By snaring the foreign body to a more superficial site, the surgical removal only required a minor incision, decreasing recovery time and the likelihood of postoperative complications that would have been associated with a large retroperitoneal dissection. While loop snare is often the first-line technique for the removal of intravascular foreign bodies, its use in soft tissue retained materials is scarcely reported.6 The more typical uses involve the removal of intraluminal materials, such as partially fractured venous catheters, guide wires, stents, and vena cava filters. The same report mentioned that in all 16 cases of percutaneous foreign body retrieval, no surgical intervention was required.7 In the case of most nonvascular foreign bodies, however, surgical retrieval is usually performed.8
Surgical removal of foreign bodies can be difficult in cases where a foreign body is anatomically located next to vital structures.9 An additional challenge with a sole surgical approach to foreign body retrieval is when it is small in size and lies deep within the soft tissue, as was the case for our patient. In such cases, the surgical procedure can be time consuming and lead to more trauma to the surrounding tissues.10 These factors alone necessitate consideration of postoperative morbidity and mortality.
In our patient, the retained fragment was embedded in the wall of an abscess located retroperitoneally in his iliopsoas muscle. When considering the proximity of the iliopsoas muscle to the digestive tract, urinary tract, and iliac lymph nodes, it is reasonable for infectious material to come in contact with the foreign body from these nearby structures, resulting in secondary infection.11 Surgery was previously considered the first-line treatment for retroperitoneal abscesses until the advent of imaging-guided percutaneous drainage.12
In some instances, surgical drainage may still be attempted, such as if there are different disease processes requiring open surgery or if percutaneous catheter drainage is not technically possible due to the location of the abscess, thick exudate, loculation/septations, or phlegmon. In these cases, laparoscopic drainage as opposed to open surgical drainage can provide the benefits of an open procedure (ie, total drainage and resection of infected tissue) but is less invasive, requires a smaller incision, and heals faster.13 Percutaneous drainage is the current first-line treatment due to the lack of need for general anesthesia, lower cost, and better morbidity and mortality outcomes compared to surgical methods.12 While percutaneous drainage proved to be immediately therapeutic for our patient, the risk of abscess recurrence with the retained infected fragment necessitated coordination of procedures across specialties to provide the best outcome for the patient.
Conclusions
This case demonstrates a multidisciplinary approach to transforming an otherwise large retroperitoneal dissection to a minimally invasive and technically efficient abscess drainage and foreign body retrieval.
1. Schroeder JE, Lowe J, Chaimsky G, Liebergall M, Mosheiff R. Migrating shrapnel: a rare cause of knee synovitis. Mil Med. 2010;175(11):929-930. doi:10.7205/milmed-d-09-00254
2. Centeno JA, Rogers DA, van der Voet GB, et al. Embedded fragments from U.S. military personnel—chemical analysis and potential health implications. Int J Environ Res Public Health. 2014;11(2):1261-1278. Published 2014 Jan 23. doi:10.3390/ijerph110201261
3. Carija R, Busic Z, Bradaric N, Bulovic B, Borzic Z, Pavicic-Perkovic S. Surgical removal of metallic foreign body (shrapnel) from the lumbosacral spine and the treatment of chronic osteomyelitis: a case report. West Indian Med J. 2014;63(4):373-375. doi:10.7727/wimj.2012.290
4. Grasso I, Blattner M, Short T, Downs J. Severe systemic lead toxicity resulting from extra-articular retained shrapnel presenting as jaundice and hepatitis: a case report and review of the literature. Mil Med. 2017;182(3-4):e1843-e1848. doi:10.7205/MILMED-D-16-00231
5. Dillman RO, Crumb CK, Lidsky MJ. Lead poisoning from a gunshot wound: report of a case and review of the literature. Am J Med. 1979;66(3):509-514. doi:10.1016/0002-9343(79)91083-0
6. Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36(4):888-897. doi:10.1007/s00270-012-0488-8
7. Mallmann CV, Wolf KJ, Wacker FK. Retrieval of vascular foreign bodies using a self-made wire snare. Acta Radiol. 2008;49(10):1124-1128. doi:10.1080/02841850802454741
8. Nosher JL, Siegel R. Percutaneous retrieval of nonvascular foreign bodies. Radiology. 1993;187(3):649-651. doi:10.1148/radiology.187.3.8497610
9. Fu Y, Cui LG, Romagnoli C, Li ZQ, Lei YT. Ultrasound-guided removal of retained soft tissue foreign body with late presentation. Chin Med J (Engl). 2017;130(14):1753-1754. doi:10.4103/0366-6999.209910
10. Liang HD, Li H, Feng H, Zhao ZN, Song WJ, Yuan B. Application of intraoperative navigation and positioning system in the removal of deep foreign bodies in the limbs. Chin Med J (Engl). 2019;132(11):1375-1377. doi:10.1097/CM9.0000000000000253
11. Moriarty CM, Baker RJ. A pain in the psoas. Sports Health. 2016;8(6):568-572. doi:10.1177/1941738116665112
12. Akhan O, Durmaz H, Balcı S, Birgi E, Çiftçi T, Akıncı D. Percutaneous drainage of retroperitoneal abscesses: variables for success, failure, and recurrence. Diagn Interv Radiol. 2020;26(2):124-130. doi:10.5152/dir.2019.19199
13. Hong CH, Hong YC, Bae SH, et al. Laparoscopic drainage as a minimally invasive treatment for a psoas abscess: a single center case series and literature review. Medicine (Baltimore). 2020;99(14):e19640. doi:10.1097/MD.0000000000019640
Shrapnel injuries are commonly encountered in war zones.1 Shrapnel injuries can remain asymptomatic or become systemic, with health effects of the retained foreign body ranging from local to systemic toxicities depending on the patient’s reaction to the chemical composition and corrosiveness of the fragments in vivo.2 We present a case of a reactivating shrapnel injury in the form of a retroperitoneal infection and subsequent iliopsoas abscess. A collaborative procedure was performed between surgery and interventional radiology to snare and remove the infected fragment and drain the abscess.
Case Presentation
While serving in Vietnam, a soldier sustained a fragment injury to his left lower abdomen. He underwent a laparotomy, small bowel resection, and a temporary ileostomy at the time of the injury. Nearly 50 years later, the patient presented with chronic left lower quadrant pain and a low-grade fever. He was diagnosed clinically in the emergency department (ED) with diverticulitis and treated with antibiotics. The patient initially responded to treatment but returned 6 months later with similar symptoms, low-grade fever, and mild leukocytosis. A computed tomography (CT) scan during that encounter without IV contrast revealed a few scattered colonic diverticula without definite diverticulitis as well as a metallic fragment embedded in the left iliopsoas with increased soft tissue density.
The patient was diagnosed with a pelvic/abdominal wall hematoma and was discharged with pain medication. The patient reported recurrent attacks of left lower quadrant pain, fever, and changes in bowel habits, prompting gastrointestinal consultation and a colonoscopy that was unremarkable. Ten months later, the patient again presented to the ED, with recurrent symptoms, a fever of 102 °F, and leukocytosis with a white blood cell count of 11.7 × 109/L. CT scan with IV contrast revealed a large left iliopsoas abscess associated with an approximately 1-cm metallic fragment (Figure 1). A drainage catheter was placed under CT guidance and approximately 270 mL of purulent fluid was drained. Culture of the fluid was positive for Escherichia coli (E coli). Two days after drain placement, the fragment was removed as a joint procedure with interventional radiology and surgery. Using the drainage catheter tract as a point of entry, multiple attempts were made to retrieve the fragment with Olympus EndoJaw endoscopic forceps without success.
Ultimately a stiff directional sheath from a Cook Medical transjugular liver biopsy kit was used with a Merit Medical EnSnare to relocate the fragment to the left inguinal region for surgical excision (Figures 2, 3, and 4). The fragment was removed and swabbed for culture and sensitivity and a BLAKE drain was placed in the evacuated abscess cavity. The patient tolerated the procedure well and was discharged the following day. Three days later, culture and sensitivity grew E coli and Acinetobacter, thus confirming infection and a nidus for the surrounding abscess formation. On follow-up with general surgery 7 days later, the patient reported he was doing well, and the drain was removed without difficulty.
Discussion
Foreign body injuries can be benign or debilitating depending on the initial damage, anatomical location of the foreign body, composition of the foreign body, and the patient’s response to it. Retained shrapnel deep within the muscle tissue rarely causes complications. Although many times embedded objects can be asymptomatic and require no further management, migration of the foreign body or the formation of a fistula is possible, causing symptoms and requiring surgical intervention.1 One case involved the formation of a purulent fistula appearing a year after an explosive wound to the lumbosacral spine, which was treated with antimicrobials. Recurrence of the fistula several times after treatment led to surgical removal of the shrapnel along with antibiotic treatment of the osteomyelitis.3 Although uncommon, lead exposure that occurs due to retained foreign body fragments from gunshot or military-related injuries can cause systemic lead toxicity. Symptoms may range from abdominal pain, nausea, and constipation to jaundice and hepatitis.4 The severity has also been stated to correlate with the surface area of the lead exposed for dissolution.5 Migration of foreign bodies and shrapnel to other sites in the body, such as movement from soft tissues into distantly located body cavities, have been reported as well. Such a case involved the spontaneous onset of knee synovitis due to an intra-articular metallic object that was introduced via a blast injury to the upper third of the ipsilateral thigh.1
In this patient’s case, a large intramuscular abscess had formed nearly 50 years after the initial combat injury, requiring drainage of the abscess and removal of the fragment. By snaring the foreign body to a more superficial site, the surgical removal only required a minor incision, decreasing recovery time and the likelihood of postoperative complications that would have been associated with a large retroperitoneal dissection. While loop snare is often the first-line technique for the removal of intravascular foreign bodies, its use in soft tissue retained materials is scarcely reported.6 The more typical uses involve the removal of intraluminal materials, such as partially fractured venous catheters, guide wires, stents, and vena cava filters. The same report mentioned that in all 16 cases of percutaneous foreign body retrieval, no surgical intervention was required.7 In the case of most nonvascular foreign bodies, however, surgical retrieval is usually performed.8
Surgical removal of foreign bodies can be difficult in cases where a foreign body is anatomically located next to vital structures.9 An additional challenge with a sole surgical approach to foreign body retrieval is when it is small in size and lies deep within the soft tissue, as was the case for our patient. In such cases, the surgical procedure can be time consuming and lead to more trauma to the surrounding tissues.10 These factors alone necessitate consideration of postoperative morbidity and mortality.
In our patient, the retained fragment was embedded in the wall of an abscess located retroperitoneally in his iliopsoas muscle. When considering the proximity of the iliopsoas muscle to the digestive tract, urinary tract, and iliac lymph nodes, it is reasonable for infectious material to come in contact with the foreign body from these nearby structures, resulting in secondary infection.11 Surgery was previously considered the first-line treatment for retroperitoneal abscesses until the advent of imaging-guided percutaneous drainage.12
In some instances, surgical drainage may still be attempted, such as if there are different disease processes requiring open surgery or if percutaneous catheter drainage is not technically possible due to the location of the abscess, thick exudate, loculation/septations, or phlegmon. In these cases, laparoscopic drainage as opposed to open surgical drainage can provide the benefits of an open procedure (ie, total drainage and resection of infected tissue) but is less invasive, requires a smaller incision, and heals faster.13 Percutaneous drainage is the current first-line treatment due to the lack of need for general anesthesia, lower cost, and better morbidity and mortality outcomes compared to surgical methods.12 While percutaneous drainage proved to be immediately therapeutic for our patient, the risk of abscess recurrence with the retained infected fragment necessitated coordination of procedures across specialties to provide the best outcome for the patient.
Conclusions
This case demonstrates a multidisciplinary approach to transforming an otherwise large retroperitoneal dissection to a minimally invasive and technically efficient abscess drainage and foreign body retrieval.
Shrapnel injuries are commonly encountered in war zones.1 Shrapnel injuries can remain asymptomatic or become systemic, with health effects of the retained foreign body ranging from local to systemic toxicities depending on the patient’s reaction to the chemical composition and corrosiveness of the fragments in vivo.2 We present a case of a reactivating shrapnel injury in the form of a retroperitoneal infection and subsequent iliopsoas abscess. A collaborative procedure was performed between surgery and interventional radiology to snare and remove the infected fragment and drain the abscess.
Case Presentation
While serving in Vietnam, a soldier sustained a fragment injury to his left lower abdomen. He underwent a laparotomy, small bowel resection, and a temporary ileostomy at the time of the injury. Nearly 50 years later, the patient presented with chronic left lower quadrant pain and a low-grade fever. He was diagnosed clinically in the emergency department (ED) with diverticulitis and treated with antibiotics. The patient initially responded to treatment but returned 6 months later with similar symptoms, low-grade fever, and mild leukocytosis. A computed tomography (CT) scan during that encounter without IV contrast revealed a few scattered colonic diverticula without definite diverticulitis as well as a metallic fragment embedded in the left iliopsoas with increased soft tissue density.
The patient was diagnosed with a pelvic/abdominal wall hematoma and was discharged with pain medication. The patient reported recurrent attacks of left lower quadrant pain, fever, and changes in bowel habits, prompting gastrointestinal consultation and a colonoscopy that was unremarkable. Ten months later, the patient again presented to the ED, with recurrent symptoms, a fever of 102 °F, and leukocytosis with a white blood cell count of 11.7 × 109/L. CT scan with IV contrast revealed a large left iliopsoas abscess associated with an approximately 1-cm metallic fragment (Figure 1). A drainage catheter was placed under CT guidance and approximately 270 mL of purulent fluid was drained. Culture of the fluid was positive for Escherichia coli (E coli). Two days after drain placement, the fragment was removed as a joint procedure with interventional radiology and surgery. Using the drainage catheter tract as a point of entry, multiple attempts were made to retrieve the fragment with Olympus EndoJaw endoscopic forceps without success.
Ultimately a stiff directional sheath from a Cook Medical transjugular liver biopsy kit was used with a Merit Medical EnSnare to relocate the fragment to the left inguinal region for surgical excision (Figures 2, 3, and 4). The fragment was removed and swabbed for culture and sensitivity and a BLAKE drain was placed in the evacuated abscess cavity. The patient tolerated the procedure well and was discharged the following day. Three days later, culture and sensitivity grew E coli and Acinetobacter, thus confirming infection and a nidus for the surrounding abscess formation. On follow-up with general surgery 7 days later, the patient reported he was doing well, and the drain was removed without difficulty.
Discussion
Foreign body injuries can be benign or debilitating depending on the initial damage, anatomical location of the foreign body, composition of the foreign body, and the patient’s response to it. Retained shrapnel deep within the muscle tissue rarely causes complications. Although many times embedded objects can be asymptomatic and require no further management, migration of the foreign body or the formation of a fistula is possible, causing symptoms and requiring surgical intervention.1 One case involved the formation of a purulent fistula appearing a year after an explosive wound to the lumbosacral spine, which was treated with antimicrobials. Recurrence of the fistula several times after treatment led to surgical removal of the shrapnel along with antibiotic treatment of the osteomyelitis.3 Although uncommon, lead exposure that occurs due to retained foreign body fragments from gunshot or military-related injuries can cause systemic lead toxicity. Symptoms may range from abdominal pain, nausea, and constipation to jaundice and hepatitis.4 The severity has also been stated to correlate with the surface area of the lead exposed for dissolution.5 Migration of foreign bodies and shrapnel to other sites in the body, such as movement from soft tissues into distantly located body cavities, have been reported as well. Such a case involved the spontaneous onset of knee synovitis due to an intra-articular metallic object that was introduced via a blast injury to the upper third of the ipsilateral thigh.1
In this patient’s case, a large intramuscular abscess had formed nearly 50 years after the initial combat injury, requiring drainage of the abscess and removal of the fragment. By snaring the foreign body to a more superficial site, the surgical removal only required a minor incision, decreasing recovery time and the likelihood of postoperative complications that would have been associated with a large retroperitoneal dissection. While loop snare is often the first-line technique for the removal of intravascular foreign bodies, its use in soft tissue retained materials is scarcely reported.6 The more typical uses involve the removal of intraluminal materials, such as partially fractured venous catheters, guide wires, stents, and vena cava filters. The same report mentioned that in all 16 cases of percutaneous foreign body retrieval, no surgical intervention was required.7 In the case of most nonvascular foreign bodies, however, surgical retrieval is usually performed.8
Surgical removal of foreign bodies can be difficult in cases where a foreign body is anatomically located next to vital structures.9 An additional challenge with a sole surgical approach to foreign body retrieval is when it is small in size and lies deep within the soft tissue, as was the case for our patient. In such cases, the surgical procedure can be time consuming and lead to more trauma to the surrounding tissues.10 These factors alone necessitate consideration of postoperative morbidity and mortality.
In our patient, the retained fragment was embedded in the wall of an abscess located retroperitoneally in his iliopsoas muscle. When considering the proximity of the iliopsoas muscle to the digestive tract, urinary tract, and iliac lymph nodes, it is reasonable for infectious material to come in contact with the foreign body from these nearby structures, resulting in secondary infection.11 Surgery was previously considered the first-line treatment for retroperitoneal abscesses until the advent of imaging-guided percutaneous drainage.12
In some instances, surgical drainage may still be attempted, such as if there are different disease processes requiring open surgery or if percutaneous catheter drainage is not technically possible due to the location of the abscess, thick exudate, loculation/septations, or phlegmon. In these cases, laparoscopic drainage as opposed to open surgical drainage can provide the benefits of an open procedure (ie, total drainage and resection of infected tissue) but is less invasive, requires a smaller incision, and heals faster.13 Percutaneous drainage is the current first-line treatment due to the lack of need for general anesthesia, lower cost, and better morbidity and mortality outcomes compared to surgical methods.12 While percutaneous drainage proved to be immediately therapeutic for our patient, the risk of abscess recurrence with the retained infected fragment necessitated coordination of procedures across specialties to provide the best outcome for the patient.
Conclusions
This case demonstrates a multidisciplinary approach to transforming an otherwise large retroperitoneal dissection to a minimally invasive and technically efficient abscess drainage and foreign body retrieval.
1. Schroeder JE, Lowe J, Chaimsky G, Liebergall M, Mosheiff R. Migrating shrapnel: a rare cause of knee synovitis. Mil Med. 2010;175(11):929-930. doi:10.7205/milmed-d-09-00254
2. Centeno JA, Rogers DA, van der Voet GB, et al. Embedded fragments from U.S. military personnel—chemical analysis and potential health implications. Int J Environ Res Public Health. 2014;11(2):1261-1278. Published 2014 Jan 23. doi:10.3390/ijerph110201261
3. Carija R, Busic Z, Bradaric N, Bulovic B, Borzic Z, Pavicic-Perkovic S. Surgical removal of metallic foreign body (shrapnel) from the lumbosacral spine and the treatment of chronic osteomyelitis: a case report. West Indian Med J. 2014;63(4):373-375. doi:10.7727/wimj.2012.290
4. Grasso I, Blattner M, Short T, Downs J. Severe systemic lead toxicity resulting from extra-articular retained shrapnel presenting as jaundice and hepatitis: a case report and review of the literature. Mil Med. 2017;182(3-4):e1843-e1848. doi:10.7205/MILMED-D-16-00231
5. Dillman RO, Crumb CK, Lidsky MJ. Lead poisoning from a gunshot wound: report of a case and review of the literature. Am J Med. 1979;66(3):509-514. doi:10.1016/0002-9343(79)91083-0
6. Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36(4):888-897. doi:10.1007/s00270-012-0488-8
7. Mallmann CV, Wolf KJ, Wacker FK. Retrieval of vascular foreign bodies using a self-made wire snare. Acta Radiol. 2008;49(10):1124-1128. doi:10.1080/02841850802454741
8. Nosher JL, Siegel R. Percutaneous retrieval of nonvascular foreign bodies. Radiology. 1993;187(3):649-651. doi:10.1148/radiology.187.3.8497610
9. Fu Y, Cui LG, Romagnoli C, Li ZQ, Lei YT. Ultrasound-guided removal of retained soft tissue foreign body with late presentation. Chin Med J (Engl). 2017;130(14):1753-1754. doi:10.4103/0366-6999.209910
10. Liang HD, Li H, Feng H, Zhao ZN, Song WJ, Yuan B. Application of intraoperative navigation and positioning system in the removal of deep foreign bodies in the limbs. Chin Med J (Engl). 2019;132(11):1375-1377. doi:10.1097/CM9.0000000000000253
11. Moriarty CM, Baker RJ. A pain in the psoas. Sports Health. 2016;8(6):568-572. doi:10.1177/1941738116665112
12. Akhan O, Durmaz H, Balcı S, Birgi E, Çiftçi T, Akıncı D. Percutaneous drainage of retroperitoneal abscesses: variables for success, failure, and recurrence. Diagn Interv Radiol. 2020;26(2):124-130. doi:10.5152/dir.2019.19199
13. Hong CH, Hong YC, Bae SH, et al. Laparoscopic drainage as a minimally invasive treatment for a psoas abscess: a single center case series and literature review. Medicine (Baltimore). 2020;99(14):e19640. doi:10.1097/MD.0000000000019640
1. Schroeder JE, Lowe J, Chaimsky G, Liebergall M, Mosheiff R. Migrating shrapnel: a rare cause of knee synovitis. Mil Med. 2010;175(11):929-930. doi:10.7205/milmed-d-09-00254
2. Centeno JA, Rogers DA, van der Voet GB, et al. Embedded fragments from U.S. military personnel—chemical analysis and potential health implications. Int J Environ Res Public Health. 2014;11(2):1261-1278. Published 2014 Jan 23. doi:10.3390/ijerph110201261
3. Carija R, Busic Z, Bradaric N, Bulovic B, Borzic Z, Pavicic-Perkovic S. Surgical removal of metallic foreign body (shrapnel) from the lumbosacral spine and the treatment of chronic osteomyelitis: a case report. West Indian Med J. 2014;63(4):373-375. doi:10.7727/wimj.2012.290
4. Grasso I, Blattner M, Short T, Downs J. Severe systemic lead toxicity resulting from extra-articular retained shrapnel presenting as jaundice and hepatitis: a case report and review of the literature. Mil Med. 2017;182(3-4):e1843-e1848. doi:10.7205/MILMED-D-16-00231
5. Dillman RO, Crumb CK, Lidsky MJ. Lead poisoning from a gunshot wound: report of a case and review of the literature. Am J Med. 1979;66(3):509-514. doi:10.1016/0002-9343(79)91083-0
6. Woodhouse JB, Uberoi R. Techniques for intravascular foreign body retrieval. Cardiovasc Intervent Radiol. 2013;36(4):888-897. doi:10.1007/s00270-012-0488-8
7. Mallmann CV, Wolf KJ, Wacker FK. Retrieval of vascular foreign bodies using a self-made wire snare. Acta Radiol. 2008;49(10):1124-1128. doi:10.1080/02841850802454741
8. Nosher JL, Siegel R. Percutaneous retrieval of nonvascular foreign bodies. Radiology. 1993;187(3):649-651. doi:10.1148/radiology.187.3.8497610
9. Fu Y, Cui LG, Romagnoli C, Li ZQ, Lei YT. Ultrasound-guided removal of retained soft tissue foreign body with late presentation. Chin Med J (Engl). 2017;130(14):1753-1754. doi:10.4103/0366-6999.209910
10. Liang HD, Li H, Feng H, Zhao ZN, Song WJ, Yuan B. Application of intraoperative navigation and positioning system in the removal of deep foreign bodies in the limbs. Chin Med J (Engl). 2019;132(11):1375-1377. doi:10.1097/CM9.0000000000000253
11. Moriarty CM, Baker RJ. A pain in the psoas. Sports Health. 2016;8(6):568-572. doi:10.1177/1941738116665112
12. Akhan O, Durmaz H, Balcı S, Birgi E, Çiftçi T, Akıncı D. Percutaneous drainage of retroperitoneal abscesses: variables for success, failure, and recurrence. Diagn Interv Radiol. 2020;26(2):124-130. doi:10.5152/dir.2019.19199
13. Hong CH, Hong YC, Bae SH, et al. Laparoscopic drainage as a minimally invasive treatment for a psoas abscess: a single center case series and literature review. Medicine (Baltimore). 2020;99(14):e19640. doi:10.1097/MD.0000000000019640
Successful Use of Lanadelumab in an Older Patient With Type II Hereditary Angioedema
Hereditary angioedema (HAE) is a rare genetic disorder affecting about 1 in 67,000 individuals and may lead to increased morbidity and mortality.1,2 HAE is characterized by recurring episodes of subcutaneous and/or submucosal edema without urticaria due to an excess of bradykinin.2,3 Autosomal dominant inheritance is present in 75% of patients with HAE and is classified into 2 main types.2 Type I HAE is caused by deficiency of C1 esterase inhibitor, accounting for 85% of cases.2 Type II HAE is marked by normal to elevated levels of C1 esterase inhibitor but with reduced activity.2
Cutaneous and abdominal angioedema attacks are the most common presentation.1 However, any location may be affected, including the face, oropharynx, and larynx.1 Only 0.9% of all HAE attacks cause laryngeal edema, but 50% of HAE patients have experienced a laryngeal attack, which may be lethal.1 An angioedema attack can range in severity, depending on the location and degree of edema.3 In addition, patients with HAE often are diagnosed with anxiety and depression secondary to their poor quality of life.4 Thus, long-term prophylaxis of attacks is crucial to reduce the physical and psychological implications.
Previously, HAE was treated with antifibrinolytic agents and attenuated androgens for short- and long-term prophylaxis.1 These treatment modalities are now considered second-line since the development of novel medications with improved efficacy and limited adverse effects (AEs).1 For long-term prophylaxis, subcutaneous and IV C1 esterase inhibitor has been proven effective in both types I and II HAE.1 Another option, lanadelumab, a subcutaneously delivered monoclonal antibody inhibitor of plasma kallikrein, has been proven to decrease the frequency of HAE attacks without significant AEs.5 Lanadelumab works by binding to the active site of plasma kallikrein, which reduces its activity and slows the production of bradykinin.6 This results in decreasing vascular permeability and swelling episodes in patients with HAE.7 Data, however, are limited, specifically regarding patients with type II HAE and patients aged ≥ 65 years.5 This article reports on an older male with type II HAE successfully treated with lanadelumab.
Case Presentation
An 81-year-old male patient with hypertension, hypertriglyceridemia, and aortic aneurysm had recurrent, frequent episodes of severe abdominal pain with a remote history of extremity and scrotal swelling since adolescence. He was misdiagnosed for years and was eventually determined to have HAE at age 75 years after his niece was diagnosed, prompting him to be reevaluated for his frequent bouts of abdominal pain. His laboratory findings were consistent with HAE type II with low C4 (7.8 mg/dL), normal C1 esterase inhibitor levels (24 mg/dL), and low levels of C1 esterase inhibitor activity (28% of normal).
Initially, he described having weekly attacks of abdominal pain that could last 1 to several days. At worst, these attacks would last up to a month, causing a decrease in appetite and weight loss. At age 77 years, he began an on-demand treatment, icatibant, a bradykinin receptor blocker. After initiating icatibant during an acute attack, the pain would diminish within 1 to 2 hours, and within several hours, he would be pain free. Previously, pain relief would take several days to weeks. He continued to use icatibant on-demand, typically requiring treatment every 1 to 2 months for only the more severe attacks.
After an increasing frequency of abdominal pain attacks, prophylactic medication was recommended. Therefore, subcutaneous lanadelumab 300 mg every 2 weeks was initiated for long-term prophylaxis. The patient went from requiring on-demand treatment 2 to 3 times per month to once in 6 months after starting lanadelumab. In addition, he tolerated the medication well without any AEs.
Discussion
According to the international WAO/EAACI 2021 guidelines, HAE treatment goals are “to achieve complete control of the disease and to normalize patients’ lives.”8 On-demand treatment options include C1 esterase inhibitor, icatibant, or ecallantide (a kallikrein inhibitor).8 Long-term prophylaxis in HAE should be considered, accounting for disease activity, burden, control, and patient preference. Five medications have been used for long-term prophylaxis: antifibrinolytic agents (not recommended), attenuated androgens (considered second-line), C1 esterase inhibitor, berotralstat, and lanadelumab.8
Antifibrinolytics are no longer recommended for long-term prophylactic treatment as their efficacy is poor and was not considered for our patient. Attenuated androgens, such as danazol, have a history of prophylactic use in patients with HAE due to their good efficacy but are suboptimal due to their significant AE profile and many drug-drug interactions.8 In addition, androgens have many contraindications, including hypertension and hypertriglyceridemia, which were both present in our patient. Consequently, danazol was not an advised treatment for our patient. C1 esterase inhibitor is often used to prevent HAE attacks and can be given intravenously or subcutaneously, typically administered biweekly. A potential AE of C1 esterase inhibitor is thrombosis.Therefore, C1 esterase inhibitor was not a preferred choice in our older patient with a history of hypercoagulability. Berotralstat, a plasma kallikrein inhibitor, is an oral treatment option that also has shown efficacy in long-term prophylaxis. The most common AEs of berotralstat tend to be gastrointestinal symptoms, and the medication requires dose adjustment for patients with hepatic impairment.8 Berotralstat was not considered because it was not an approved treatment option at the time of this patient’s treatment. Lanadelumab is a human monoclonal antibody against plasma kallikrein, which decreases bradykinin production in patients with HAE, thus preventing angioedema attacks.5 Data regarding the use of lanadelumab in patients with type II HAE are limited, but because HAE with normal C1 esterase inhibitor levels involves the production of bradykinin via kallikrein, lanadelumab should still be effective.1 Lanadelumab was chosen for our patient because of its minimal AEs and is not known to increase the risk of thrombosis.
Lanadelumab is a novel medication, recently approved in 2018 by the US Food and Drug Administration for the treatment of type I and type 2 HAE in patients aged ≥ 12 years.7 The phase 3 Hereditary Angioedema Long-term Prophylaxis (HELP) study concluded that treatment with subcutaneous lanadelumab for 26 weeks significantly decreased the frequency of angioedema attacks compared with placebo.5 However, 113 (90.4%) of patients in the phase III HELP study had type I HAE.5 Of the 125 patients that completed this randomized, double-blind study, only 12 had type II HAE.5 In addition, this study only included 5 patients aged ≥ 65 years.5 Also, no patients aged ≥ 65 years were part of the treatment arms that included a lanadelumab dose of 300 mg.5 In a case series of 12 patients in Canada, treatment with lanadelumab decreased angioedema attacks by 72%.9 However, the series only included 1 patient with type II HAE who was aged 36 years.9 Therefore, our case demonstrates the efficacy of lanadelumab in a patient aged ≥ 65 years with type II HAE.
Conclusions
HAE is a rare and potentially fatal disease characterized by recurrent, unpredictable attacks of edema throughout the body. The disease burden adversely affects a patient’s quality of life. Therefore, long-term prophylaxis is critical to managing patients with HAE. Lanadelumab has been proven as an effective long-term prophylactic treatment option for HAE attacks. This case supports the use of lanadelumab in patients with type II HAE and patients aged ≥ 65 years.
Acknowledgments
The patient was initially written up based on his delayed diagnosis as a case report.3 An earlier version of this article was presented by Samuel Weiss, MD, and Derek Smith, MD, as a poster at the American Academy of Allergy, Asthma, and Immunology virtual conference February 26 to March 1, 2021.
1. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136-1148. doi:10.1056/NEJMra1808012
2. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14)(suppl):S292-S298.
3. Berger J, Carroll MP Jr, Champoux E, Coop CA. Extremely delayed diagnosis of type II hereditary angioedema: case report and review of the literature. Mil Med. 2018;183(11-12):e765-e767. doi:10.1093/milmed/usy031
4. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(4):371-375. doi:10.1016/j.anai.2013.05.028
5. Banerji A, Riedl MA, Bernstein JA, et al; HELP Investigators. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773
6. Busse PJ, Farkas H, Banerji A, et al. Lanadelumab for the prophylactic treatment of hereditary angioedema with C1 inhibitor deficiency: a review of preclinical and phase I studies. BioDrugs. 2019;33(1):33-43. doi:10.1007/s40259-018-0325-y
7. Riedl MA, Maurer M, Bernstein JA, et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75(11):2879-2887. doi:10.1111/all.14416
8. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214
9. Iaboni A, Kanani A, Lacuesta G, Song C, Kan M, Betschel SD. Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada. Allergy Asthma Clin Immunol. 2021;17(1):78. Published 2021 Jul 23. doi:10.1186/s13223-021-00579-6
Hereditary angioedema (HAE) is a rare genetic disorder affecting about 1 in 67,000 individuals and may lead to increased morbidity and mortality.1,2 HAE is characterized by recurring episodes of subcutaneous and/or submucosal edema without urticaria due to an excess of bradykinin.2,3 Autosomal dominant inheritance is present in 75% of patients with HAE and is classified into 2 main types.2 Type I HAE is caused by deficiency of C1 esterase inhibitor, accounting for 85% of cases.2 Type II HAE is marked by normal to elevated levels of C1 esterase inhibitor but with reduced activity.2
Cutaneous and abdominal angioedema attacks are the most common presentation.1 However, any location may be affected, including the face, oropharynx, and larynx.1 Only 0.9% of all HAE attacks cause laryngeal edema, but 50% of HAE patients have experienced a laryngeal attack, which may be lethal.1 An angioedema attack can range in severity, depending on the location and degree of edema.3 In addition, patients with HAE often are diagnosed with anxiety and depression secondary to their poor quality of life.4 Thus, long-term prophylaxis of attacks is crucial to reduce the physical and psychological implications.
Previously, HAE was treated with antifibrinolytic agents and attenuated androgens for short- and long-term prophylaxis.1 These treatment modalities are now considered second-line since the development of novel medications with improved efficacy and limited adverse effects (AEs).1 For long-term prophylaxis, subcutaneous and IV C1 esterase inhibitor has been proven effective in both types I and II HAE.1 Another option, lanadelumab, a subcutaneously delivered monoclonal antibody inhibitor of plasma kallikrein, has been proven to decrease the frequency of HAE attacks without significant AEs.5 Lanadelumab works by binding to the active site of plasma kallikrein, which reduces its activity and slows the production of bradykinin.6 This results in decreasing vascular permeability and swelling episodes in patients with HAE.7 Data, however, are limited, specifically regarding patients with type II HAE and patients aged ≥ 65 years.5 This article reports on an older male with type II HAE successfully treated with lanadelumab.
Case Presentation
An 81-year-old male patient with hypertension, hypertriglyceridemia, and aortic aneurysm had recurrent, frequent episodes of severe abdominal pain with a remote history of extremity and scrotal swelling since adolescence. He was misdiagnosed for years and was eventually determined to have HAE at age 75 years after his niece was diagnosed, prompting him to be reevaluated for his frequent bouts of abdominal pain. His laboratory findings were consistent with HAE type II with low C4 (7.8 mg/dL), normal C1 esterase inhibitor levels (24 mg/dL), and low levels of C1 esterase inhibitor activity (28% of normal).
Initially, he described having weekly attacks of abdominal pain that could last 1 to several days. At worst, these attacks would last up to a month, causing a decrease in appetite and weight loss. At age 77 years, he began an on-demand treatment, icatibant, a bradykinin receptor blocker. After initiating icatibant during an acute attack, the pain would diminish within 1 to 2 hours, and within several hours, he would be pain free. Previously, pain relief would take several days to weeks. He continued to use icatibant on-demand, typically requiring treatment every 1 to 2 months for only the more severe attacks.
After an increasing frequency of abdominal pain attacks, prophylactic medication was recommended. Therefore, subcutaneous lanadelumab 300 mg every 2 weeks was initiated for long-term prophylaxis. The patient went from requiring on-demand treatment 2 to 3 times per month to once in 6 months after starting lanadelumab. In addition, he tolerated the medication well without any AEs.
Discussion
According to the international WAO/EAACI 2021 guidelines, HAE treatment goals are “to achieve complete control of the disease and to normalize patients’ lives.”8 On-demand treatment options include C1 esterase inhibitor, icatibant, or ecallantide (a kallikrein inhibitor).8 Long-term prophylaxis in HAE should be considered, accounting for disease activity, burden, control, and patient preference. Five medications have been used for long-term prophylaxis: antifibrinolytic agents (not recommended), attenuated androgens (considered second-line), C1 esterase inhibitor, berotralstat, and lanadelumab.8
Antifibrinolytics are no longer recommended for long-term prophylactic treatment as their efficacy is poor and was not considered for our patient. Attenuated androgens, such as danazol, have a history of prophylactic use in patients with HAE due to their good efficacy but are suboptimal due to their significant AE profile and many drug-drug interactions.8 In addition, androgens have many contraindications, including hypertension and hypertriglyceridemia, which were both present in our patient. Consequently, danazol was not an advised treatment for our patient. C1 esterase inhibitor is often used to prevent HAE attacks and can be given intravenously or subcutaneously, typically administered biweekly. A potential AE of C1 esterase inhibitor is thrombosis.Therefore, C1 esterase inhibitor was not a preferred choice in our older patient with a history of hypercoagulability. Berotralstat, a plasma kallikrein inhibitor, is an oral treatment option that also has shown efficacy in long-term prophylaxis. The most common AEs of berotralstat tend to be gastrointestinal symptoms, and the medication requires dose adjustment for patients with hepatic impairment.8 Berotralstat was not considered because it was not an approved treatment option at the time of this patient’s treatment. Lanadelumab is a human monoclonal antibody against plasma kallikrein, which decreases bradykinin production in patients with HAE, thus preventing angioedema attacks.5 Data regarding the use of lanadelumab in patients with type II HAE are limited, but because HAE with normal C1 esterase inhibitor levels involves the production of bradykinin via kallikrein, lanadelumab should still be effective.1 Lanadelumab was chosen for our patient because of its minimal AEs and is not known to increase the risk of thrombosis.
Lanadelumab is a novel medication, recently approved in 2018 by the US Food and Drug Administration for the treatment of type I and type 2 HAE in patients aged ≥ 12 years.7 The phase 3 Hereditary Angioedema Long-term Prophylaxis (HELP) study concluded that treatment with subcutaneous lanadelumab for 26 weeks significantly decreased the frequency of angioedema attacks compared with placebo.5 However, 113 (90.4%) of patients in the phase III HELP study had type I HAE.5 Of the 125 patients that completed this randomized, double-blind study, only 12 had type II HAE.5 In addition, this study only included 5 patients aged ≥ 65 years.5 Also, no patients aged ≥ 65 years were part of the treatment arms that included a lanadelumab dose of 300 mg.5 In a case series of 12 patients in Canada, treatment with lanadelumab decreased angioedema attacks by 72%.9 However, the series only included 1 patient with type II HAE who was aged 36 years.9 Therefore, our case demonstrates the efficacy of lanadelumab in a patient aged ≥ 65 years with type II HAE.
Conclusions
HAE is a rare and potentially fatal disease characterized by recurrent, unpredictable attacks of edema throughout the body. The disease burden adversely affects a patient’s quality of life. Therefore, long-term prophylaxis is critical to managing patients with HAE. Lanadelumab has been proven as an effective long-term prophylactic treatment option for HAE attacks. This case supports the use of lanadelumab in patients with type II HAE and patients aged ≥ 65 years.
Acknowledgments
The patient was initially written up based on his delayed diagnosis as a case report.3 An earlier version of this article was presented by Samuel Weiss, MD, and Derek Smith, MD, as a poster at the American Academy of Allergy, Asthma, and Immunology virtual conference February 26 to March 1, 2021.
Hereditary angioedema (HAE) is a rare genetic disorder affecting about 1 in 67,000 individuals and may lead to increased morbidity and mortality.1,2 HAE is characterized by recurring episodes of subcutaneous and/or submucosal edema without urticaria due to an excess of bradykinin.2,3 Autosomal dominant inheritance is present in 75% of patients with HAE and is classified into 2 main types.2 Type I HAE is caused by deficiency of C1 esterase inhibitor, accounting for 85% of cases.2 Type II HAE is marked by normal to elevated levels of C1 esterase inhibitor but with reduced activity.2
Cutaneous and abdominal angioedema attacks are the most common presentation.1 However, any location may be affected, including the face, oropharynx, and larynx.1 Only 0.9% of all HAE attacks cause laryngeal edema, but 50% of HAE patients have experienced a laryngeal attack, which may be lethal.1 An angioedema attack can range in severity, depending on the location and degree of edema.3 In addition, patients with HAE often are diagnosed with anxiety and depression secondary to their poor quality of life.4 Thus, long-term prophylaxis of attacks is crucial to reduce the physical and psychological implications.
Previously, HAE was treated with antifibrinolytic agents and attenuated androgens for short- and long-term prophylaxis.1 These treatment modalities are now considered second-line since the development of novel medications with improved efficacy and limited adverse effects (AEs).1 For long-term prophylaxis, subcutaneous and IV C1 esterase inhibitor has been proven effective in both types I and II HAE.1 Another option, lanadelumab, a subcutaneously delivered monoclonal antibody inhibitor of plasma kallikrein, has been proven to decrease the frequency of HAE attacks without significant AEs.5 Lanadelumab works by binding to the active site of plasma kallikrein, which reduces its activity and slows the production of bradykinin.6 This results in decreasing vascular permeability and swelling episodes in patients with HAE.7 Data, however, are limited, specifically regarding patients with type II HAE and patients aged ≥ 65 years.5 This article reports on an older male with type II HAE successfully treated with lanadelumab.
Case Presentation
An 81-year-old male patient with hypertension, hypertriglyceridemia, and aortic aneurysm had recurrent, frequent episodes of severe abdominal pain with a remote history of extremity and scrotal swelling since adolescence. He was misdiagnosed for years and was eventually determined to have HAE at age 75 years after his niece was diagnosed, prompting him to be reevaluated for his frequent bouts of abdominal pain. His laboratory findings were consistent with HAE type II with low C4 (7.8 mg/dL), normal C1 esterase inhibitor levels (24 mg/dL), and low levels of C1 esterase inhibitor activity (28% of normal).
Initially, he described having weekly attacks of abdominal pain that could last 1 to several days. At worst, these attacks would last up to a month, causing a decrease in appetite and weight loss. At age 77 years, he began an on-demand treatment, icatibant, a bradykinin receptor blocker. After initiating icatibant during an acute attack, the pain would diminish within 1 to 2 hours, and within several hours, he would be pain free. Previously, pain relief would take several days to weeks. He continued to use icatibant on-demand, typically requiring treatment every 1 to 2 months for only the more severe attacks.
After an increasing frequency of abdominal pain attacks, prophylactic medication was recommended. Therefore, subcutaneous lanadelumab 300 mg every 2 weeks was initiated for long-term prophylaxis. The patient went from requiring on-demand treatment 2 to 3 times per month to once in 6 months after starting lanadelumab. In addition, he tolerated the medication well without any AEs.
Discussion
According to the international WAO/EAACI 2021 guidelines, HAE treatment goals are “to achieve complete control of the disease and to normalize patients’ lives.”8 On-demand treatment options include C1 esterase inhibitor, icatibant, or ecallantide (a kallikrein inhibitor).8 Long-term prophylaxis in HAE should be considered, accounting for disease activity, burden, control, and patient preference. Five medications have been used for long-term prophylaxis: antifibrinolytic agents (not recommended), attenuated androgens (considered second-line), C1 esterase inhibitor, berotralstat, and lanadelumab.8
Antifibrinolytics are no longer recommended for long-term prophylactic treatment as their efficacy is poor and was not considered for our patient. Attenuated androgens, such as danazol, have a history of prophylactic use in patients with HAE due to their good efficacy but are suboptimal due to their significant AE profile and many drug-drug interactions.8 In addition, androgens have many contraindications, including hypertension and hypertriglyceridemia, which were both present in our patient. Consequently, danazol was not an advised treatment for our patient. C1 esterase inhibitor is often used to prevent HAE attacks and can be given intravenously or subcutaneously, typically administered biweekly. A potential AE of C1 esterase inhibitor is thrombosis.Therefore, C1 esterase inhibitor was not a preferred choice in our older patient with a history of hypercoagulability. Berotralstat, a plasma kallikrein inhibitor, is an oral treatment option that also has shown efficacy in long-term prophylaxis. The most common AEs of berotralstat tend to be gastrointestinal symptoms, and the medication requires dose adjustment for patients with hepatic impairment.8 Berotralstat was not considered because it was not an approved treatment option at the time of this patient’s treatment. Lanadelumab is a human monoclonal antibody against plasma kallikrein, which decreases bradykinin production in patients with HAE, thus preventing angioedema attacks.5 Data regarding the use of lanadelumab in patients with type II HAE are limited, but because HAE with normal C1 esterase inhibitor levels involves the production of bradykinin via kallikrein, lanadelumab should still be effective.1 Lanadelumab was chosen for our patient because of its minimal AEs and is not known to increase the risk of thrombosis.
Lanadelumab is a novel medication, recently approved in 2018 by the US Food and Drug Administration for the treatment of type I and type 2 HAE in patients aged ≥ 12 years.7 The phase 3 Hereditary Angioedema Long-term Prophylaxis (HELP) study concluded that treatment with subcutaneous lanadelumab for 26 weeks significantly decreased the frequency of angioedema attacks compared with placebo.5 However, 113 (90.4%) of patients in the phase III HELP study had type I HAE.5 Of the 125 patients that completed this randomized, double-blind study, only 12 had type II HAE.5 In addition, this study only included 5 patients aged ≥ 65 years.5 Also, no patients aged ≥ 65 years were part of the treatment arms that included a lanadelumab dose of 300 mg.5 In a case series of 12 patients in Canada, treatment with lanadelumab decreased angioedema attacks by 72%.9 However, the series only included 1 patient with type II HAE who was aged 36 years.9 Therefore, our case demonstrates the efficacy of lanadelumab in a patient aged ≥ 65 years with type II HAE.
Conclusions
HAE is a rare and potentially fatal disease characterized by recurrent, unpredictable attacks of edema throughout the body. The disease burden adversely affects a patient’s quality of life. Therefore, long-term prophylaxis is critical to managing patients with HAE. Lanadelumab has been proven as an effective long-term prophylactic treatment option for HAE attacks. This case supports the use of lanadelumab in patients with type II HAE and patients aged ≥ 65 years.
Acknowledgments
The patient was initially written up based on his delayed diagnosis as a case report.3 An earlier version of this article was presented by Samuel Weiss, MD, and Derek Smith, MD, as a poster at the American Academy of Allergy, Asthma, and Immunology virtual conference February 26 to March 1, 2021.
1. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136-1148. doi:10.1056/NEJMra1808012
2. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14)(suppl):S292-S298.
3. Berger J, Carroll MP Jr, Champoux E, Coop CA. Extremely delayed diagnosis of type II hereditary angioedema: case report and review of the literature. Mil Med. 2018;183(11-12):e765-e767. doi:10.1093/milmed/usy031
4. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(4):371-375. doi:10.1016/j.anai.2013.05.028
5. Banerji A, Riedl MA, Bernstein JA, et al; HELP Investigators. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773
6. Busse PJ, Farkas H, Banerji A, et al. Lanadelumab for the prophylactic treatment of hereditary angioedema with C1 inhibitor deficiency: a review of preclinical and phase I studies. BioDrugs. 2019;33(1):33-43. doi:10.1007/s40259-018-0325-y
7. Riedl MA, Maurer M, Bernstein JA, et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75(11):2879-2887. doi:10.1111/all.14416
8. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214
9. Iaboni A, Kanani A, Lacuesta G, Song C, Kan M, Betschel SD. Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada. Allergy Asthma Clin Immunol. 2021;17(1):78. Published 2021 Jul 23. doi:10.1186/s13223-021-00579-6
1. Busse PJ, Christiansen SC. Hereditary angioedema. N Engl J Med. 2020;382(12):1136-1148. doi:10.1056/NEJMra1808012
2. Bernstein JA. Severity of hereditary angioedema, prevalence, and diagnostic considerations. Am J Manag Care. 2018;24(14)(suppl):S292-S298.
3. Berger J, Carroll MP Jr, Champoux E, Coop CA. Extremely delayed diagnosis of type II hereditary angioedema: case report and review of the literature. Mil Med. 2018;183(11-12):e765-e767. doi:10.1093/milmed/usy031
4. Fouche AS, Saunders EF, Craig T. Depression and anxiety in patients with hereditary angioedema. Ann Allergy Asthma Immunol. 2014;112(4):371-375. doi:10.1016/j.anai.2013.05.028
5. Banerji A, Riedl MA, Bernstein JA, et al; HELP Investigators. Effect of lanadelumab compared with placebo on prevention of hereditary angioedema attacks: a randomized clinical trial. JAMA. 2018;320(20):2108-2121. doi:10.1001/jama.2018.16773
6. Busse PJ, Farkas H, Banerji A, et al. Lanadelumab for the prophylactic treatment of hereditary angioedema with C1 inhibitor deficiency: a review of preclinical and phase I studies. BioDrugs. 2019;33(1):33-43. doi:10.1007/s40259-018-0325-y
7. Riedl MA, Maurer M, Bernstein JA, et al. Lanadelumab demonstrates rapid and sustained prevention of hereditary angioedema attacks. Allergy. 2020;75(11):2879-2887. doi:10.1111/all.14416
8. Maurer M, Magerl M, Betschel S, et al. The international WAO/EAACI guideline for the management of hereditary angioedema—the 2021 revision and update. Allergy. 2022;77(7):1961-1990. doi:10.1111/all.15214
9. Iaboni A, Kanani A, Lacuesta G, Song C, Kan M, Betschel SD. Impact of lanadelumab in hereditary angioedema: a case series of 12 patients in Canada. Allergy Asthma Clin Immunol. 2021;17(1):78. Published 2021 Jul 23. doi:10.1186/s13223-021-00579-6
75 Years of the Historic Partnership Between the VA and Academic Medical Centers
The US government has a legacy of providing support for veterans. Pensions were offered to disabled veterans as early as 1776, and benefits were expanded to cover medical needs as the country grew and modernized.1,2 Enacted during the Civil War, the General Pension Act increased benefits for widows and dependents.2 Rehabilitation and vocational training assistance benefits were added after World War I, and the US Department of Veterans Affairs (VA) was created in 1930 to consolidate all benefits under one umbrella organization.2,3
Prior to World War II, the VA lacked the bed capacity for the 4 million veterans who were eligible for care. This shortage became more acute by the end of the war, when the number of eligible veterans increased by 15 million.4 Although the VA successfully built bed capacity through acquisition of military hospitals, VA hospitals struggled to recruit clinical staff.2 Physicians were hesitant to join the VA because civil service salaries were lower than comparable positions in the community, and the VA offered limited opportunities for research or continuing education. These limitations negatively impacted the overall reputation of the VA. The American Medical Association (AMA) was reluctant to directly admit VA physicians for membership because of a “lower” standard of care at VA hospitals.2 This review will describe how passage of 2 legislative actions, the Servicemen’s Readjustment Act and Public Law (PL)79-293, and a key policy memorandum set the foundation for the partnership between the VA and academic medical centers. This led to improved medical care for veterans and expansion of health professions education for VA and the nation.5,6
GI Bill of Rights
The passage of the Servicemen’s Readjustment Act of 1944, better known as the GI Bill of Rights, provided education assistance, guaranteed home loans, and unemployment payments to veterans.5 All medical officers serving during the war were eligible for this benefit, which effectively increased the number of potential physician trainees at the end of World War II by almost 60,000.7 Medical education at the time was simultaneously undergoing a transformation with more rigorous training and a push to standardize medical education across state lines. While prerequisite training was not required for admission to many medical schools and curricula varied in length based on state licensing requirements, more programs were adding premedical education requirements and transitioning to the 4-year curricula seen today. At this time, only 23 states required postgraduate internships for licensure, but this number was growing.8 The American Board of Medical Specialties was established several years prior to World War II in 1934 to elevate the quality of care; the desire for residency training and board certification continued to gain traction during the 1940s.9
Medical Training
In anticipation of an influx of medical trainees, the Committee on Postwar Medical Service conducted a comprehensive survey to understand the training needs of physician veterans returning from World War II.7 The survey collected data from medical officers on their desired length of training, interest in specialty board certification, time served, and type of medical practice prior to enlisting. Length of desired training was categorized as short (up to 6 months), which would serve as a refresher course and provide updates on recent advances in medicine and surgery, and long (> 6 months), which resembled a modern internship or residency. Nineteen percent did not want additional training, 22% wished to pursue short courses, and 51% were interested in longer courses. Most respondents also wished to obtain board certification.7 The AMA played a significant role in supporting the expansion of training opportunities, encouraging all accredited hospitals to assess their capacity to determine the number of additional residents they could accommodate. The AMA also awarded hospitals with existing internship programs temporary accreditation to allow them to add extended training through residency programs.7
Medical schools devised creative solutions to meet the needs of returning physician veterans and capitalize on the available educational benefits. Postgraduate refresher courses that varied in length from hours to months were developed focusing on an array of topics. In addition to basic medical principles, courses covered general topics, such as advances in medicine, to specialty topics, such as nutrition or ophthalmology.7 Although the courses could not be counted toward board certification, participation increased by almost 300% in the 1945/1946 academic year relative to the previous year.7 Increasing access to the longer training courses, including internships and residencies, was often achieved through experiences outside the clinical setting. Yale University modified its curriculum to reduce time devoted to lectures on published materials and encourage active learning and community outreach.10 Northwestern University assigned residents to spend 1 of their 3 years “out of residence” in basic science and clinical instruction provided by the medical school. Tuition assistance from the GI Bill supported the additional expenses incurred by the medical school to fund laboratory space, equipment, and the salaries of the basic science instructors and administrative staff.11
Public Law 79-293
Public Law 79-293 was passed on January 3, 1946, establishing the Department of Medicine and Surgery within the VA. The law, which became the basis for Title 38 chapters 73 and 74, allowed VA hospitals flexibility to hire doctors, dentists, and nurses without regard to the civil service regulations and salary restrictions associated with other federal positions.6
Concerns about quality of care had been mounting for years, and the release of several sensationalized and critical articles motivated VA leadership to make sweeping changes. One article described neglect at VA hospitals.12 Excessive paperwork and low economic benefits were identified as barriers to the recruitment of qualified clinicians at the VA.2 The VA Special Medical Advisory Group investigating the claims recommended that the VA encourage their hospitals to affiliate with medical schools to improve the quality of care. This group also recommended that new VA hospitals be constructed near academic medical centers to allow access to consultants.2 Three large veterans service organizations (American Legion, Veterans of Foreign Wars, and Disabled American Veterans) conducted their own investigations in response to the media reports. The organizations reported that the quality of care in most VA hospitals was already on par with the community but indicated that the VA would benefit from expansion of medical research and training, increased bed capacity, reduction in the administrative burden on clinicians, and increased salaries for clinical staff.2
Policy Memorandum No. 2
The relationship between VA and academic medical centers was solidified on January 30, 1946, with adoption of Policy Memorandum No. 2.13 This memorandum allowed for the establishment of relationships with academic medical centers to provide “the veteran a much higher standard of medical care than could be given him with a wholly full-time medical staff.” Shortly after this memorandum was signed, residents from Northwestern University and the University of Illinois at Chicago began clinical rotations at the Hines VA facility in Chicago, Illinois.2 By 1947, 62 medical schools had committed to an affiliation with local VA hospitals and 21 deans’ committees were in operation, which were responsible for the appointment of physician residents and consultants. The AMA extended direct membership privileges to VA physicians, and by 1947 the number of residency positions doubled nationally.14,15 The almost universal support of the relationship between VA and academic affiliates provided educational opportunities for returning veterans and raised standards for medical education nationally.
Current State
Since the passage of PL 79-293 and PM No. 2, the VA-academic health professions education partnership has grown to include 113,000 trainees rotating through 150 VA medical centers annually from more than 1400 colleges and universities.16 Most VA podiatrists, psychologists, optometrists, and physicians working in VA medical centers also trained at VA, and trainees are 37% more likely to consider a job at VA after completing their clinical rotations. This unique partnership began 76 years ago and continues to provide clinicians “for VA and the nation.”
1. Glasson WH. History of military pension legislation in the United States. Columbia University Press; 1900.
2. Lewis BJ. Veterans Administration medical program relationship with medical schools in the United States. Dissertation. The American University; 1969.
3. Kracke RR. The role of the medical college in the medical care of the veteran. J Med Assoc State Ala. 1950;19(8):225-230.
4. US Department of Veterans Affairs, Office of Public Affairs. VA History in Brief. VA Pamphlet 80-97-2. Washington, DC: United States Department of Veterans Affairs; 1997.
5. Servicesmen’s Readjustment Act of 1944. 38 USC § 370 (1944).
6. To establish a Department of Medicine and Surgery in the Veterans’ Administration. 38 USC § 73-74 (1946). Accessed August 2, 2022.
7. Lueth HC. Postgraduate wishes of medical officers: final report on 21,029 questionnaires. J Am Med Assoc. 1945; 127(13):759-770.
8. Johnson V, Arestad FH, Tipner A. Medical education in the United States and Canada: forty-sixth annual report on medical education in the United States and Canada by the Council on Medical Education and Hospitals of the American Medical Association. J Am Med Assoc. 1946;131(16):1277-1310.
9. Chesney AM. Some impacts of the specialty board movement on medical education. J Assoc Am Med Coll. 1948;23(2):83-89.
10. Hiscock IV. New frontiers in health education. Can J Public Health. 1946;37(11):452-457.
11. Colwell AR. Principles of graduate medical instruction: with a specific plan of application in a medical school. J Am Med Assoc. 1945;127(13):741-746.
12. Maisel, AQ. The veteran betrayed. How long will the Veterans’ Administration continue to give third-rate medical care to first-rate men? Cosmopolitan. 1945(3):45.
13. US Veterans Administration. Policy Memorandum No. 2: Policy in association of veterans’ hospitals with medical schools. January 30, 1946.
14. American Medical Association. Digest of Official Actions: 1846-1958. JAMA. 1946;132:1094.
15. Wentz DK, Ford CV. A brief history of the internship. JAMA. 1984;252(24):3390-3394. doi:10.1001/jama.1984.03350240036035
16. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education academic year 2022-2021. Accessed August 8, 2022. https://www.va.gov/OAA/docs/OAA_Stats_AY_2020_2021_FINAL.pdf
The US government has a legacy of providing support for veterans. Pensions were offered to disabled veterans as early as 1776, and benefits were expanded to cover medical needs as the country grew and modernized.1,2 Enacted during the Civil War, the General Pension Act increased benefits for widows and dependents.2 Rehabilitation and vocational training assistance benefits were added after World War I, and the US Department of Veterans Affairs (VA) was created in 1930 to consolidate all benefits under one umbrella organization.2,3
Prior to World War II, the VA lacked the bed capacity for the 4 million veterans who were eligible for care. This shortage became more acute by the end of the war, when the number of eligible veterans increased by 15 million.4 Although the VA successfully built bed capacity through acquisition of military hospitals, VA hospitals struggled to recruit clinical staff.2 Physicians were hesitant to join the VA because civil service salaries were lower than comparable positions in the community, and the VA offered limited opportunities for research or continuing education. These limitations negatively impacted the overall reputation of the VA. The American Medical Association (AMA) was reluctant to directly admit VA physicians for membership because of a “lower” standard of care at VA hospitals.2 This review will describe how passage of 2 legislative actions, the Servicemen’s Readjustment Act and Public Law (PL)79-293, and a key policy memorandum set the foundation for the partnership between the VA and academic medical centers. This led to improved medical care for veterans and expansion of health professions education for VA and the nation.5,6
GI Bill of Rights
The passage of the Servicemen’s Readjustment Act of 1944, better known as the GI Bill of Rights, provided education assistance, guaranteed home loans, and unemployment payments to veterans.5 All medical officers serving during the war were eligible for this benefit, which effectively increased the number of potential physician trainees at the end of World War II by almost 60,000.7 Medical education at the time was simultaneously undergoing a transformation with more rigorous training and a push to standardize medical education across state lines. While prerequisite training was not required for admission to many medical schools and curricula varied in length based on state licensing requirements, more programs were adding premedical education requirements and transitioning to the 4-year curricula seen today. At this time, only 23 states required postgraduate internships for licensure, but this number was growing.8 The American Board of Medical Specialties was established several years prior to World War II in 1934 to elevate the quality of care; the desire for residency training and board certification continued to gain traction during the 1940s.9
Medical Training
In anticipation of an influx of medical trainees, the Committee on Postwar Medical Service conducted a comprehensive survey to understand the training needs of physician veterans returning from World War II.7 The survey collected data from medical officers on their desired length of training, interest in specialty board certification, time served, and type of medical practice prior to enlisting. Length of desired training was categorized as short (up to 6 months), which would serve as a refresher course and provide updates on recent advances in medicine and surgery, and long (> 6 months), which resembled a modern internship or residency. Nineteen percent did not want additional training, 22% wished to pursue short courses, and 51% were interested in longer courses. Most respondents also wished to obtain board certification.7 The AMA played a significant role in supporting the expansion of training opportunities, encouraging all accredited hospitals to assess their capacity to determine the number of additional residents they could accommodate. The AMA also awarded hospitals with existing internship programs temporary accreditation to allow them to add extended training through residency programs.7
Medical schools devised creative solutions to meet the needs of returning physician veterans and capitalize on the available educational benefits. Postgraduate refresher courses that varied in length from hours to months were developed focusing on an array of topics. In addition to basic medical principles, courses covered general topics, such as advances in medicine, to specialty topics, such as nutrition or ophthalmology.7 Although the courses could not be counted toward board certification, participation increased by almost 300% in the 1945/1946 academic year relative to the previous year.7 Increasing access to the longer training courses, including internships and residencies, was often achieved through experiences outside the clinical setting. Yale University modified its curriculum to reduce time devoted to lectures on published materials and encourage active learning and community outreach.10 Northwestern University assigned residents to spend 1 of their 3 years “out of residence” in basic science and clinical instruction provided by the medical school. Tuition assistance from the GI Bill supported the additional expenses incurred by the medical school to fund laboratory space, equipment, and the salaries of the basic science instructors and administrative staff.11
Public Law 79-293
Public Law 79-293 was passed on January 3, 1946, establishing the Department of Medicine and Surgery within the VA. The law, which became the basis for Title 38 chapters 73 and 74, allowed VA hospitals flexibility to hire doctors, dentists, and nurses without regard to the civil service regulations and salary restrictions associated with other federal positions.6
Concerns about quality of care had been mounting for years, and the release of several sensationalized and critical articles motivated VA leadership to make sweeping changes. One article described neglect at VA hospitals.12 Excessive paperwork and low economic benefits were identified as barriers to the recruitment of qualified clinicians at the VA.2 The VA Special Medical Advisory Group investigating the claims recommended that the VA encourage their hospitals to affiliate with medical schools to improve the quality of care. This group also recommended that new VA hospitals be constructed near academic medical centers to allow access to consultants.2 Three large veterans service organizations (American Legion, Veterans of Foreign Wars, and Disabled American Veterans) conducted their own investigations in response to the media reports. The organizations reported that the quality of care in most VA hospitals was already on par with the community but indicated that the VA would benefit from expansion of medical research and training, increased bed capacity, reduction in the administrative burden on clinicians, and increased salaries for clinical staff.2
Policy Memorandum No. 2
The relationship between VA and academic medical centers was solidified on January 30, 1946, with adoption of Policy Memorandum No. 2.13 This memorandum allowed for the establishment of relationships with academic medical centers to provide “the veteran a much higher standard of medical care than could be given him with a wholly full-time medical staff.” Shortly after this memorandum was signed, residents from Northwestern University and the University of Illinois at Chicago began clinical rotations at the Hines VA facility in Chicago, Illinois.2 By 1947, 62 medical schools had committed to an affiliation with local VA hospitals and 21 deans’ committees were in operation, which were responsible for the appointment of physician residents and consultants. The AMA extended direct membership privileges to VA physicians, and by 1947 the number of residency positions doubled nationally.14,15 The almost universal support of the relationship between VA and academic affiliates provided educational opportunities for returning veterans and raised standards for medical education nationally.
Current State
Since the passage of PL 79-293 and PM No. 2, the VA-academic health professions education partnership has grown to include 113,000 trainees rotating through 150 VA medical centers annually from more than 1400 colleges and universities.16 Most VA podiatrists, psychologists, optometrists, and physicians working in VA medical centers also trained at VA, and trainees are 37% more likely to consider a job at VA after completing their clinical rotations. This unique partnership began 76 years ago and continues to provide clinicians “for VA and the nation.”
The US government has a legacy of providing support for veterans. Pensions were offered to disabled veterans as early as 1776, and benefits were expanded to cover medical needs as the country grew and modernized.1,2 Enacted during the Civil War, the General Pension Act increased benefits for widows and dependents.2 Rehabilitation and vocational training assistance benefits were added after World War I, and the US Department of Veterans Affairs (VA) was created in 1930 to consolidate all benefits under one umbrella organization.2,3
Prior to World War II, the VA lacked the bed capacity for the 4 million veterans who were eligible for care. This shortage became more acute by the end of the war, when the number of eligible veterans increased by 15 million.4 Although the VA successfully built bed capacity through acquisition of military hospitals, VA hospitals struggled to recruit clinical staff.2 Physicians were hesitant to join the VA because civil service salaries were lower than comparable positions in the community, and the VA offered limited opportunities for research or continuing education. These limitations negatively impacted the overall reputation of the VA. The American Medical Association (AMA) was reluctant to directly admit VA physicians for membership because of a “lower” standard of care at VA hospitals.2 This review will describe how passage of 2 legislative actions, the Servicemen’s Readjustment Act and Public Law (PL)79-293, and a key policy memorandum set the foundation for the partnership between the VA and academic medical centers. This led to improved medical care for veterans and expansion of health professions education for VA and the nation.5,6
GI Bill of Rights
The passage of the Servicemen’s Readjustment Act of 1944, better known as the GI Bill of Rights, provided education assistance, guaranteed home loans, and unemployment payments to veterans.5 All medical officers serving during the war were eligible for this benefit, which effectively increased the number of potential physician trainees at the end of World War II by almost 60,000.7 Medical education at the time was simultaneously undergoing a transformation with more rigorous training and a push to standardize medical education across state lines. While prerequisite training was not required for admission to many medical schools and curricula varied in length based on state licensing requirements, more programs were adding premedical education requirements and transitioning to the 4-year curricula seen today. At this time, only 23 states required postgraduate internships for licensure, but this number was growing.8 The American Board of Medical Specialties was established several years prior to World War II in 1934 to elevate the quality of care; the desire for residency training and board certification continued to gain traction during the 1940s.9
Medical Training
In anticipation of an influx of medical trainees, the Committee on Postwar Medical Service conducted a comprehensive survey to understand the training needs of physician veterans returning from World War II.7 The survey collected data from medical officers on their desired length of training, interest in specialty board certification, time served, and type of medical practice prior to enlisting. Length of desired training was categorized as short (up to 6 months), which would serve as a refresher course and provide updates on recent advances in medicine and surgery, and long (> 6 months), which resembled a modern internship or residency. Nineteen percent did not want additional training, 22% wished to pursue short courses, and 51% were interested in longer courses. Most respondents also wished to obtain board certification.7 The AMA played a significant role in supporting the expansion of training opportunities, encouraging all accredited hospitals to assess their capacity to determine the number of additional residents they could accommodate. The AMA also awarded hospitals with existing internship programs temporary accreditation to allow them to add extended training through residency programs.7
Medical schools devised creative solutions to meet the needs of returning physician veterans and capitalize on the available educational benefits. Postgraduate refresher courses that varied in length from hours to months were developed focusing on an array of topics. In addition to basic medical principles, courses covered general topics, such as advances in medicine, to specialty topics, such as nutrition or ophthalmology.7 Although the courses could not be counted toward board certification, participation increased by almost 300% in the 1945/1946 academic year relative to the previous year.7 Increasing access to the longer training courses, including internships and residencies, was often achieved through experiences outside the clinical setting. Yale University modified its curriculum to reduce time devoted to lectures on published materials and encourage active learning and community outreach.10 Northwestern University assigned residents to spend 1 of their 3 years “out of residence” in basic science and clinical instruction provided by the medical school. Tuition assistance from the GI Bill supported the additional expenses incurred by the medical school to fund laboratory space, equipment, and the salaries of the basic science instructors and administrative staff.11
Public Law 79-293
Public Law 79-293 was passed on January 3, 1946, establishing the Department of Medicine and Surgery within the VA. The law, which became the basis for Title 38 chapters 73 and 74, allowed VA hospitals flexibility to hire doctors, dentists, and nurses without regard to the civil service regulations and salary restrictions associated with other federal positions.6
Concerns about quality of care had been mounting for years, and the release of several sensationalized and critical articles motivated VA leadership to make sweeping changes. One article described neglect at VA hospitals.12 Excessive paperwork and low economic benefits were identified as barriers to the recruitment of qualified clinicians at the VA.2 The VA Special Medical Advisory Group investigating the claims recommended that the VA encourage their hospitals to affiliate with medical schools to improve the quality of care. This group also recommended that new VA hospitals be constructed near academic medical centers to allow access to consultants.2 Three large veterans service organizations (American Legion, Veterans of Foreign Wars, and Disabled American Veterans) conducted their own investigations in response to the media reports. The organizations reported that the quality of care in most VA hospitals was already on par with the community but indicated that the VA would benefit from expansion of medical research and training, increased bed capacity, reduction in the administrative burden on clinicians, and increased salaries for clinical staff.2
Policy Memorandum No. 2
The relationship between VA and academic medical centers was solidified on January 30, 1946, with adoption of Policy Memorandum No. 2.13 This memorandum allowed for the establishment of relationships with academic medical centers to provide “the veteran a much higher standard of medical care than could be given him with a wholly full-time medical staff.” Shortly after this memorandum was signed, residents from Northwestern University and the University of Illinois at Chicago began clinical rotations at the Hines VA facility in Chicago, Illinois.2 By 1947, 62 medical schools had committed to an affiliation with local VA hospitals and 21 deans’ committees were in operation, which were responsible for the appointment of physician residents and consultants. The AMA extended direct membership privileges to VA physicians, and by 1947 the number of residency positions doubled nationally.14,15 The almost universal support of the relationship between VA and academic affiliates provided educational opportunities for returning veterans and raised standards for medical education nationally.
Current State
Since the passage of PL 79-293 and PM No. 2, the VA-academic health professions education partnership has grown to include 113,000 trainees rotating through 150 VA medical centers annually from more than 1400 colleges and universities.16 Most VA podiatrists, psychologists, optometrists, and physicians working in VA medical centers also trained at VA, and trainees are 37% more likely to consider a job at VA after completing their clinical rotations. This unique partnership began 76 years ago and continues to provide clinicians “for VA and the nation.”
1. Glasson WH. History of military pension legislation in the United States. Columbia University Press; 1900.
2. Lewis BJ. Veterans Administration medical program relationship with medical schools in the United States. Dissertation. The American University; 1969.
3. Kracke RR. The role of the medical college in the medical care of the veteran. J Med Assoc State Ala. 1950;19(8):225-230.
4. US Department of Veterans Affairs, Office of Public Affairs. VA History in Brief. VA Pamphlet 80-97-2. Washington, DC: United States Department of Veterans Affairs; 1997.
5. Servicesmen’s Readjustment Act of 1944. 38 USC § 370 (1944).
6. To establish a Department of Medicine and Surgery in the Veterans’ Administration. 38 USC § 73-74 (1946). Accessed August 2, 2022.
7. Lueth HC. Postgraduate wishes of medical officers: final report on 21,029 questionnaires. J Am Med Assoc. 1945; 127(13):759-770.
8. Johnson V, Arestad FH, Tipner A. Medical education in the United States and Canada: forty-sixth annual report on medical education in the United States and Canada by the Council on Medical Education and Hospitals of the American Medical Association. J Am Med Assoc. 1946;131(16):1277-1310.
9. Chesney AM. Some impacts of the specialty board movement on medical education. J Assoc Am Med Coll. 1948;23(2):83-89.
10. Hiscock IV. New frontiers in health education. Can J Public Health. 1946;37(11):452-457.
11. Colwell AR. Principles of graduate medical instruction: with a specific plan of application in a medical school. J Am Med Assoc. 1945;127(13):741-746.
12. Maisel, AQ. The veteran betrayed. How long will the Veterans’ Administration continue to give third-rate medical care to first-rate men? Cosmopolitan. 1945(3):45.
13. US Veterans Administration. Policy Memorandum No. 2: Policy in association of veterans’ hospitals with medical schools. January 30, 1946.
14. American Medical Association. Digest of Official Actions: 1846-1958. JAMA. 1946;132:1094.
15. Wentz DK, Ford CV. A brief history of the internship. JAMA. 1984;252(24):3390-3394. doi:10.1001/jama.1984.03350240036035
16. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education academic year 2022-2021. Accessed August 8, 2022. https://www.va.gov/OAA/docs/OAA_Stats_AY_2020_2021_FINAL.pdf
1. Glasson WH. History of military pension legislation in the United States. Columbia University Press; 1900.
2. Lewis BJ. Veterans Administration medical program relationship with medical schools in the United States. Dissertation. The American University; 1969.
3. Kracke RR. The role of the medical college in the medical care of the veteran. J Med Assoc State Ala. 1950;19(8):225-230.
4. US Department of Veterans Affairs, Office of Public Affairs. VA History in Brief. VA Pamphlet 80-97-2. Washington, DC: United States Department of Veterans Affairs; 1997.
5. Servicesmen’s Readjustment Act of 1944. 38 USC § 370 (1944).
6. To establish a Department of Medicine and Surgery in the Veterans’ Administration. 38 USC § 73-74 (1946). Accessed August 2, 2022.
7. Lueth HC. Postgraduate wishes of medical officers: final report on 21,029 questionnaires. J Am Med Assoc. 1945; 127(13):759-770.
8. Johnson V, Arestad FH, Tipner A. Medical education in the United States and Canada: forty-sixth annual report on medical education in the United States and Canada by the Council on Medical Education and Hospitals of the American Medical Association. J Am Med Assoc. 1946;131(16):1277-1310.
9. Chesney AM. Some impacts of the specialty board movement on medical education. J Assoc Am Med Coll. 1948;23(2):83-89.
10. Hiscock IV. New frontiers in health education. Can J Public Health. 1946;37(11):452-457.
11. Colwell AR. Principles of graduate medical instruction: with a specific plan of application in a medical school. J Am Med Assoc. 1945;127(13):741-746.
12. Maisel, AQ. The veteran betrayed. How long will the Veterans’ Administration continue to give third-rate medical care to first-rate men? Cosmopolitan. 1945(3):45.
13. US Veterans Administration. Policy Memorandum No. 2: Policy in association of veterans’ hospitals with medical schools. January 30, 1946.
14. American Medical Association. Digest of Official Actions: 1846-1958. JAMA. 1946;132:1094.
15. Wentz DK, Ford CV. A brief history of the internship. JAMA. 1984;252(24):3390-3394. doi:10.1001/jama.1984.03350240036035
16. US Department of Veterans Affairs, Veterans Health Administration, Office of Academic Affiliations. Health professions education academic year 2022-2021. Accessed August 8, 2022. https://www.va.gov/OAA/docs/OAA_Stats_AY_2020_2021_FINAL.pdf