User login
56-year-old man • increased heart rate • weakness • intense sweating • horseradish consumption • Dx?
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
THE CASE
A 56-year-old physician (CUL) visited a local seafood restaurant, after having fasted since the prior evening. He had a history of hypertension that was well controlled with lisinopril/hydrochlorothiazide.
The physician and his party were seated outside, where the temperature was in the mid-70s. The group ordered oysters on the half shell accompanied by mignonette sauce, cocktail sauce, and horseradish. The physician ate an olive-size amount of horseradish with an oyster. He immediately complained of a sharp burning sensation in his stomach and remarked that the horseradish was significantly stronger than what he was accustomed to. Within 30 seconds, he noted an increased heart rate, weakness, and intense sweating. There was no increase in nasal secretions. Observers noted that he was very pale.
About 5 minutes after eating the horseradish, the physician leaned his head back and briefly lost consciousness. His wife, while supporting his head and checking his pulse, instructed other diners to call for emergency services, at which point the physician regained consciousness and the dispatcher was told that an ambulance was no longer necessary. Within a matter of minutes, all symptoms had abated, except for some mild weakness.
THE DIAGNOSIS
Ten minutes after the event, the physician identified his symptoms as a horseradish-induced vasovagal syncope (VVS), based on a case report published in JAMA in 1988, which his wife found after he asked her to do an Internet search of his symptoms.1
THE DISCUSSION
Horseradish’s active component is isothiocyanate. Horseradish-induced syncope is also called Seder syncope after the Jewish Passover holiday dinner at which observant Jews are required to eat “bitter herbs.”1,2 This type of syncope is thought to occur when horseradish vapors directly irritate the gastric or respiratory tract mucosa.
VVS commonly manifests for the first time at around age 13 years; however, the timing of that first occurrence can vary significantly among individuals (as in this case)
The loss of consciousness may be caused by an emotional trigger (eg, sight of blood, cast removal,8 blood or platelet donations9,10), a painful event (eg, an injection11), an orthostatic trigger12 (eg, prolonged standing), or visceral reflexes such as swallowing.13 In approximately 30% of cases, loss of consciousness is associated with memory loss.14 Loss of consciousness with VVS may be associated with injury in 33% of cases.15
Continue to: The recovery with awareness
The recovery with awareness of time, place, and person may be a feature of VVS, which would differentiate it from seizures and brainstem vascular events. Autonomic prodromal symptoms—including abdominal discomfort, pallor, sweating, and nausea—may precede the loss of consciousness.8
An evolutionary response?
VVS may have developed as a trait through evolution, although modern medicine treats it as a disease. Many potential explanations for VVS as a body defense mechanism have been proposed. Examples include fainting at the sight of blood, which developed during the Old Stone Age—a period with extreme human-to-human violence—or acting like a “possum playing dead” as a tactic designed to confuse an attacker.16
Another theory involves clot production and suggests that VVS-induced hypotension is a defense against bleeding by improving clot formation.17
A psychological defense theory maintains that the fainting and memory loss are designed to prevent a painful or overwhelming experience from being remembered. None of these theories, however, explain orthostatic VVS.18
The brain defense theory could explain all forms of VVS. It postulates that hypotension causes decreased cerebral perfusion, which leads to syncope resulting in the body returning to a more orthostatic position with increased cerebral profusion.19
Continue to: The patient
The patient in this case was able to leave the restaurant on his own volition 30 minutes after the event and resume normal activities. Ten days later, an electrocardiogram was performed, with negative results. In this case, the use of a potassium-wasting diuretic exacerbated the risk of a fluid-deprived state, hypokalemia, and hypotension, possibly contributing to the syncope. The patient has since “gotten back on the horseradish” without ill effect.
THE TAKEAWAY
Consumers and health care providers should be aware of the risks associated with consumption of fresh horseradish and should allow it to rest prior to ingestion to allow some evaporation of its active ingredient. An old case report saved the patient from an unnecessary (and costly) emergency department visit.
ACKNOWLEDGEMENTS
The authors would like to thank Terry J. Hannan, MBBS, FRACP, FACHI, FACMI for his critical review of the manuscript.
CORRESPONDENCE
Christoph U. Lehmann, MD, Clinical Informatics Center, 5323 Harry Hines Boulevard, Dallas, TX 75390; [email protected]
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
1. Rubin HR, Wu AW. The bitter herbs of Seder: more on horseradish horrors. JAMA. 1988;259:1943. doi: 10.1001/jama.259.13.1943b
2. Seder syncope. The Free Dictionary. Accessed July 20, 2022. https://medical-dictionary.thefreedictionary.com/Horseradish+Syncope
3. Sheldon RS, Sheldon AG, Connolly SJ, et al. Age of first faint in patients with vasovagal syncope. J Cardiovasc Electrophysiol. 2006;17:49-54. doi: 10.1111/j.1540-8167.2005.00267.x
4. Wallin BG, Sundlöf G. Sympathetic outflow to muscles during vasovagal syncope. J Auton Nerv Syst. 1982;6:287-291. doi: 10.1016/0165-1838(82)90001-7
5. Jardine DL, Melton IC, Crozier IG, et al. Decrease in cardiac output and muscle sympathetic activity during vasovagal syncope. Am J Physiol Heart Circ Physiol. 2002;282:H1804-H1809. doi: 10.1152/ajpheart.00640.2001
6. Waxman MB, Asta JA, Cameron DA. Localization of the reflex pathway responsible for the vasodepressor reaction induced by inferior vena caval occlusion and isoproterenol. Can J Physiol Pharmacol. 1992;70:882-889. doi: 10.1139/y92-118
7. Alboni P, Alboni M. Typical vasovagal syncope as a “defense mechanism” for the heart by contrasting sympathetic overactivity. Clin Auton Res. 2017;27:253-261. doi: 10.1007/s10286-017-0446-2
8. Moya A, Sutton R, Ammirati F, et al. Guidelines for the diagnosis and management of syncope (version 2009). Eur Heart J. 2009;30:2631-2671. doi: 10.1093/eurheartj/ehp298
9. Davies J, MacDonald L, Sivakumar B, et al. Prospective analysis of syncope/pre-syncope in a tertiary paediatric orthopaedic fracture outpatient clinic. ANZ J Surg. 2021;91:668-672. doi: 10.1111/ans.16664
10. Almutairi H, Salam M, Batarfi K, et al. Incidence and severity of adverse events among platelet donors: a three-year retrospective study. Medicine (Baltimore). 2020;99:e23648. doi: 10.1097/MD.0000000000023648
11. Coakley A, Bailey A, Tao J, et al. Video education to improve clinical skills in the prevention of and response to vasovagal syncopal episodes. Int J Womens Dermatol. 2020;6:186-190. doi: 10.1016/j.ijwd.2020.02.002
12. Thijs RD, Brignole M, Falup-Pecurariu C, et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton Neurosci. 2021;233:102792. doi: 10.1016/j.autneu.2021.102792
13. Nakagawa S, Hisanaga S, Kondoh H, et al. A case of swallow syncope induced by vagotonic visceral reflex resulting in atrioventricular node suppression. J Electrocardiol. 1987;20:65-69. doi: 10.1016/0022-0736(87)90010-0
14. O’Dwyer C, Bennett K, Langan Y, et al. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040-1045. doi: 10.1093/europace/eur069
15. Jorge JG, Raj SR, Teixeira PS, et al. Likelihood of injury due to vasovagal syncope: a systematic review and meta-analysis. Europace. 2021;23:1092-1099. doi: 10.1093/europace/euab041
16. Bracha HS, Bracha AS, Williams AE, et al. The human fear-circuitry and fear-induced fainting in healthy individuals—the paleolithic-threat hypothesis. Clin Auton Res. 2005;15:238-241. doi: 10.1007/s10286-005-0245-z
17. Diehl RR. Vasovagal syncope and Darwinian fitness. Clin Auton Res. 2005;15:126-129. doi: 10.1007/s10286-005-0244-0
18. Engel CL, Romano J. Studies of syncope; biologic interpretation of vasodepressor syncope. Psychosom Med. 1947;9:288-294. doi: 10.1097/00006842-194709000-00002
19. Blanc JJ, Benditt DG. Vasovagal syncope: hypothesis focusing on its being a clinical feature unique to humans. J Cardiovasc Electrophysiol. 2016;27:623-629. doi: 10.1111/jce.12945
Noncardiac inpatient has acute hypertension: Treat or not?
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
ILLUSTRATIVE CASE
A 48-year-old man is admitted to your family medicine service for cellulitis after failed outpatient therapy. He has presumed community-acquired methicillin-resistant Staphylococcus aureus infection of the left lower extremity and is receiving intravenous (IV) vancomycin. His BP this morning is 176/98 mm Hg, and the reading from the previous shift was 168/94 mm Hg. He is asymptomatic from this elevated BP. Based on protocol, his nurse is asking about treatment in response to the multiple elevated readings. How should you address the patient’s elevated BP, knowing that you will see him for a transition management appointment in 2 weeks?
Elevated BP is common in the adult inpatient setting. Prevalence estimates range from 25% to > 50%. Many factors can contribute to elevated BP in the acute illness setting, such as pain, anxiety, medication withdrawal, and volume status.2,3
Treatment of elevated BP in outpatients is well researched, with evidence-based guidelines for physicians. That is not the case for treatment of asymptomatic elevated BP in the inpatient setting. Most published guidance on inpatient management of acutely elevated BP recommends IV medications, such as hydralazine or labetalol, although there is limited evidence to support such recommendations. There is minimal evidence for outcomes-based benefit in treating acute elevations of inpatient BP, such as reduced myocardial injury or stroke; however, there is some evidence of adverse outcomes, such as hypotension and prolonged hospital stays.4-8
Although the possibility of intensifying antihypertensive therapy for those with known hypertension or those with presumed “new-onset” hypertension could theoretically lead to improved outcomes over the long term, there is little evidence to support this presumption. Rather, there is evidence that intensification of antihypertensive therapy at discharge is linked to short-term harms. This was demonstrated in a propensity-matched veteran cohort that included 4056 hospitalized older adults with hypertension (mean age, 77 years; 3961 men), equally split between those who received antihypertensive intensification at hospital discharge and those who did not. Within 30 days, patients receiving intensification had a higher risk of readmission (number needed to harm [NNH] = 27) and serious adverse events (NNH = 63).9
The current study aimed to put all these pieces together by quantifying the prevalence of hypertension in hospitalized patients, characterizing clinician response to patients’ acutely elevated BP, and comparing both short- and long-term outcomes in patients treated for acute BP elevations while hospitalized vs those who were not. The study also assessed the potential effects of antihypertensive intensification at discharge.
STUDY SUMMARY
Treatment of acute hypertension was associated with end-organ injury
This retrospective, propensity score–matched cohort study (N = 22,834) evaluated the electronic health records of all adult patients (age > 18 years) admitted to a medicine service with a noncardiovascular diagnosis over a 1-year period at 10 Cleveland Clinic hospitals, with 1 year of follow-up data.
Exclusion criteria included hospitalization for a cardiovascular diagnosis; admission for a cerebrovascular event or acute coronary syndrome within the previous 30 days; pregnancy; length of stay of less than 2 days or more than 14 days; and lack of outpatient medication data. Patients were propensity-score matched using BP, demographic features, comorbidities, hospital shift, and time since admission. Exposure was defined as administration of IV antihypertensive medication or a new class of oral antihypertensive medication.
Continue to: Outcomes were defined...
Outcomes were defined as a temporal association between acute hypertension treatment and subsequent end-organ damage, such as AKI (serum creatinine increase ≥ 0.3 mg/dL or 1.5 × initial value [Acute Kidney Injury Network definition]), myocardial injury (elevated troponin: > 0.029 ng/mL for troponin T; > 0.045 ng/mL for troponin I), and/or stroke (indicated by discharge diagnosis, with confirmation by chart review). Monitored outcomes included stroke and myocardial infarction (MI) within 30 days of discharge and BP control up to 1 year later.
The 22,834 patients had a mean (SD) age of 65.6 (17.9) years; 12,993 (56.9%) were women, and 15,963 (69.9%) were White. Of the 17,821 (78%) who had at least 1 inpatient hypertensive systolic BP (SBP) episode, defined as an SBP ≥ 140 mm Hg, 5904 (33.1%) received a new treatment. Of those receiving a new treatment, 4378 (74.2%) received only oral treatment, and 1516 (25.7%) received at least 1 dose of IV medication with or without oral dosing.
Using the propensity-matched sample (4520 treated for elevated BP matched to 4520 who were not treated), treated patients had higher rates of AKI (10.3% vs 7.9%; P < .001) and myocardial injury (1.2% vs 0.6%; P = .003). When assessed by SBP, nontreatment of BP was still superior up to an SBP of 199 mm Hg. At an SBP of ≥ 200 mm Hg, there was no difference in rates of AKI or MI between the treatment and nontreatment groups. There was no difference in stroke in either cohort, although the overall numbers were quite low.
Patients with and without antihypertensive intensification at discharge had similar rates of MI (0.1% vs 0.2%; P > .99) and stroke (0.5% vs 0.4%; P > .99) in a matched cohort at 30 days post discharge. At 1 year, BP control in the intensification vs no-intensification groups was nearly the same: maximum SBP was 157.2 mm Hg vs 157.8 mm Hg, respectively (P = .54) and maximum diastolic BP was 86.5 mm Hg vs 86.1 mm Hg, respectively (P = .49).
WHAT’S NEW
Previous research is confirmed in a more diverse population
Whereas previous research showed no benefit to intensification of treatment among hospitalized older male patients, this large, retrospective, propensity score–matched cohort study demonstrated the short- and long-term effects of treating acute, asymptomatic BP elevations in a younger, more generalizable population that included women. Regardless of treatment modality, there appeared to be more harm than good from treating these BP elevations.
In addition, the study appears to corroborate previous research showing that intensification of BP treatment at discharge did not lead to better outcomes.9 At the very least, the study makes a reasonable argument that treating acute BP elevations in noncardiac patients in the hospital setting is not beneficial.
CAVEATS
Impact of existing therapy could be underestimated
This study had several important limitations. First, 23% of treated participants were excluded from the propensity analysis without justification from the authors. Additionally, there was no reporting of missing data and how it was managed. The authors’ definition of treatment excluded dose intensification of existing antihypertensive therapy, which would undercount the number of treated patients. However, this could underestimate the actual harms of the acute antihypertensive therapy. The authors also included patients with atrial fibrillation and heart failure in the study population, even though they already may have been taking antihypertensive agents.
CHALLENGES TO IMPLEMENTATION
Potential delays in translating findings to patient care
Although several recent studies have shown the potential benefit of not treating asymptomatic acute BP elevations in inpatients, incorporating that information into electronic health record order sets or clinical decision support, and disseminating it to clinical end users, will take time. In the interim, despite these findings, patients may continue to receive IV or oral medications to treat acute, asymptomatic BP elevations while hospitalized for noncardiac diagnoses.
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
1. Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352. doi: 10.1001/jamainternmed.2020.7501
2. Jacobs ZG, Najafi N, Fang MC, et al. Reducing unnecessary treatment of asymptomatic elevated blood pressure with intravenous medications on the general internal medicine wards: a quality improvement initiative. J Hosp Med. 2019;14:144-150. doi: 10.12788/jhm.3087
3. Pasik SD, Chiu S, Yang J, et al. Assess before Rx: reducing the overtreatment of asymptomatic blood pressure elevation in the inpatient setting. J Hosp Med. 2019;14:151-156. doi: 10.12788/jhm.3190
4. Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5:473-477. doi: 10.1016/j.jash.2011.07.002
5. Gauer R. Severe asymptomatic hypertension: evaluation and treatment. Am Fam Physician. 2017;95:492-500.
6. Lipari M, Moser LR, Petrovitch EA, et al. As-needed intravenous antihypertensive therapy and blood pressure control. J Hosp Med. 2016;11:193-198. doi: 10.1002/jhm.2510
7. Gaynor MF, Wright GC, Vondracek S. Retrospective review of the use of as-needed hydralazine and labetalol for the treatment of acute hypertension in hospitalized medicine patients. Ther Adv Cardiovasc Dis. 2018;12:7-15. doi: 10.1177/1753944717746613
8. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12:29-33. doi: 10.1111/j.1751-7176.2009.00196.x
9. Anderson TS, Jing B, Auerbach A, et al. Clinical outcomes after intensifying antihypertensive medication regimens among older adults at hospital discharge. JAMA Intern Med. 2019;179:1528-1536. doi: 10.1001/jamainternmed.2019.3007
PRACTICE CHANGER
Manage blood pressure (BP) elevations conservatively in patients admitted for noncardiac diagnoses, as acute hypertension treatment may increase the risk for acute kidney injury (AKI) and myocardial injury.
STRENGTH OF RECOMMENDATION
C: Based on a single, large, retrospective cohort study.1
Rastogi R, Sheehan MM, Hu B, et al. Treatment and outcomes of inpatient hypertension among adults with noncardiac admissions. JAMA Intern Med. 2021;181:345-352.
When the public misplaces their trust
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
Not long ago, the grandmother of my son’s friend died of COVID-19 infection. She was elderly and unvaccinated. Her grandson had no regrets over her unvaccinated status. “Why would she inject poison into her body?” he said, and then expressed a strong opinion that she had died because the hospital physicians refused to give her ivermectin and hydroxychloroquine. My son, wisely, did not push the issue.
Soon thereafter, my personal family physician emailed a newsletter to his patients (me included) with 3 important messages: (1) COVID vaccines were available in the office; (2) He was not going to prescribe hydroxychloroquine, no matter how adamantly it was requested; and (3) He warned against threatening him or his staff with lawsuits or violence over refusal to prescribe any unproven medication.
How, as a country, have we come to this? A sizeable portion of the public trusts the advice of quacks, hacks, and political opportunists over that of the nation’s most expert scientists and physicians. The National Institutes of Health maintains a website with up-to-date recommendations on the use of treatments for COVID-19. They assess the existing evidence and make recommendations for or against a wide array of interventions. (They recommend against the use of both ivermectin and hydroxychloroquine.) The Centers for Disease Control and Prevention publishes extensively about the current knowledge on the safety and efficacy of vaccines. Neither agency is part of a “deep state” or conspiracy. They are comprised of some of the nation’s leading scientists, including physicians, trying to protect the public from disease and foster good health.
Sadly, some physicians have been a source of inaccurate vaccine information; some even prescribe ineffective treatments despite the evidence. These physicians are either letting their politics override their good sense or are improperly assessing the scientific literature, or both. Medical licensing agencies, and specialty certification boards, need to find ways to prevent this—ways that can survive judicial scrutiny and allow for legitimate scientific debate.
I have been tempted to just accept the current situation as the inevitable outcome of social media–fueled tribalism. But when we know that the COVID death rate among the unvaccinated is 9 times that of people who have received a booster dose,1 I can’t sit idly and watch the Internet pundits prevail. Instead, I continue to advise and teach my students to have confidence in trustworthy authorities and websites. Mistakes will be made; corrections will be issued. However, this is not evidence of malintent or incompetence, but rather, the scientific process in action.
I tell my students that one of the biggest challenges facing them and society is to figure out how to stop, or at least minimize the effects of, incorrect information, misleading statements, and outright lies in a society that values free speech. Physicians—young and old alike—must remain committed to communicating factual information to a not-always-receptive audience. And I wish my young colleagues luck; I hope that their passion for family medicine and their insights into social media may be just the combination that’s needed to redirect the public’s trust back to where it belongs during a health care crisis.
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
1. Fleming-Dutra KE. COVID-19 Epidemiology and Vaccination Rates in the United States. Presented to the Authorization Committee on Immunization Practices, July 19, 2022. Accessed August 9, 2022. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-07-19/02-COVID-Fleming-Dutra-508.pdf
COPD inhaler therapy: A path to success
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).
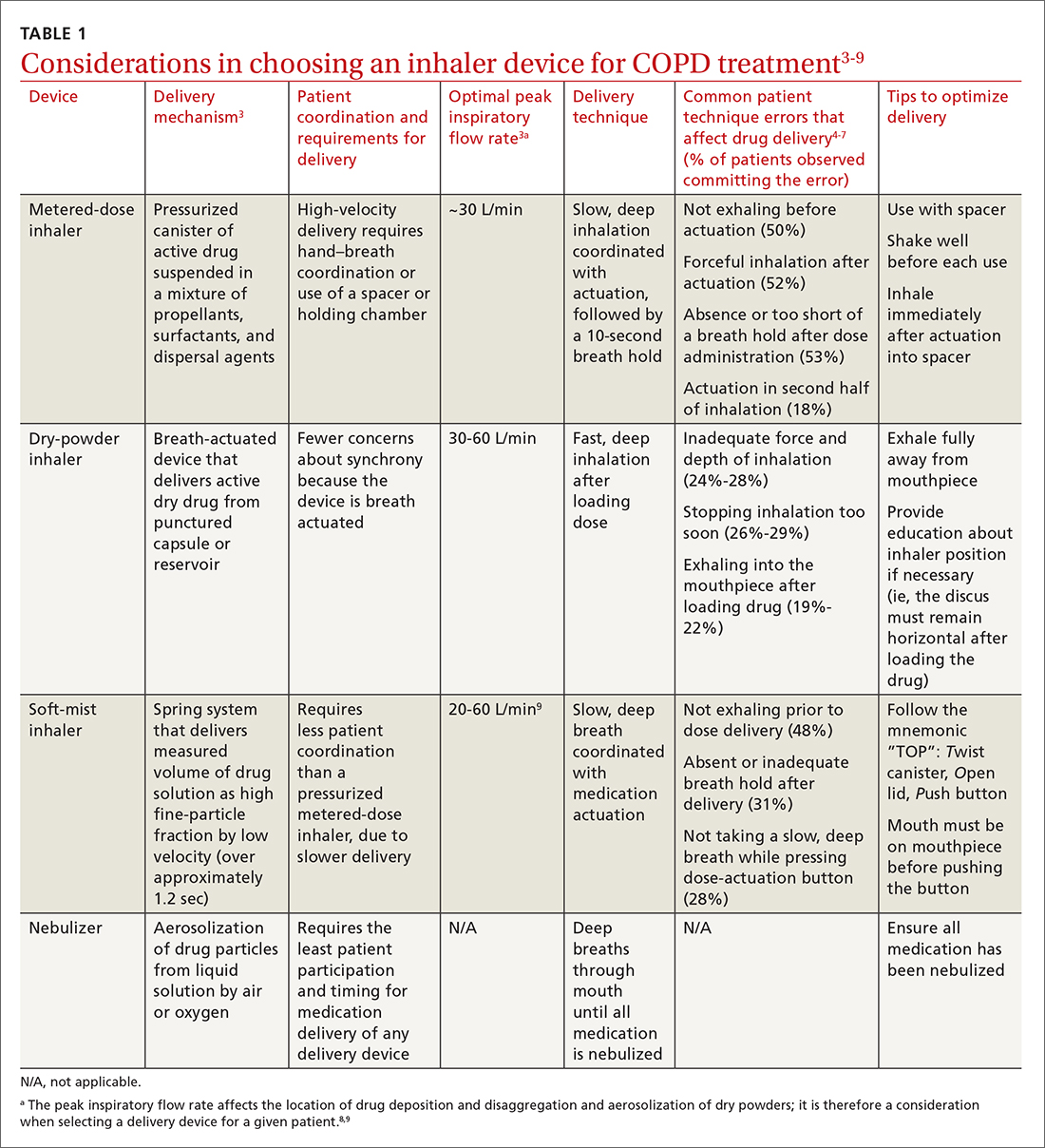
Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.
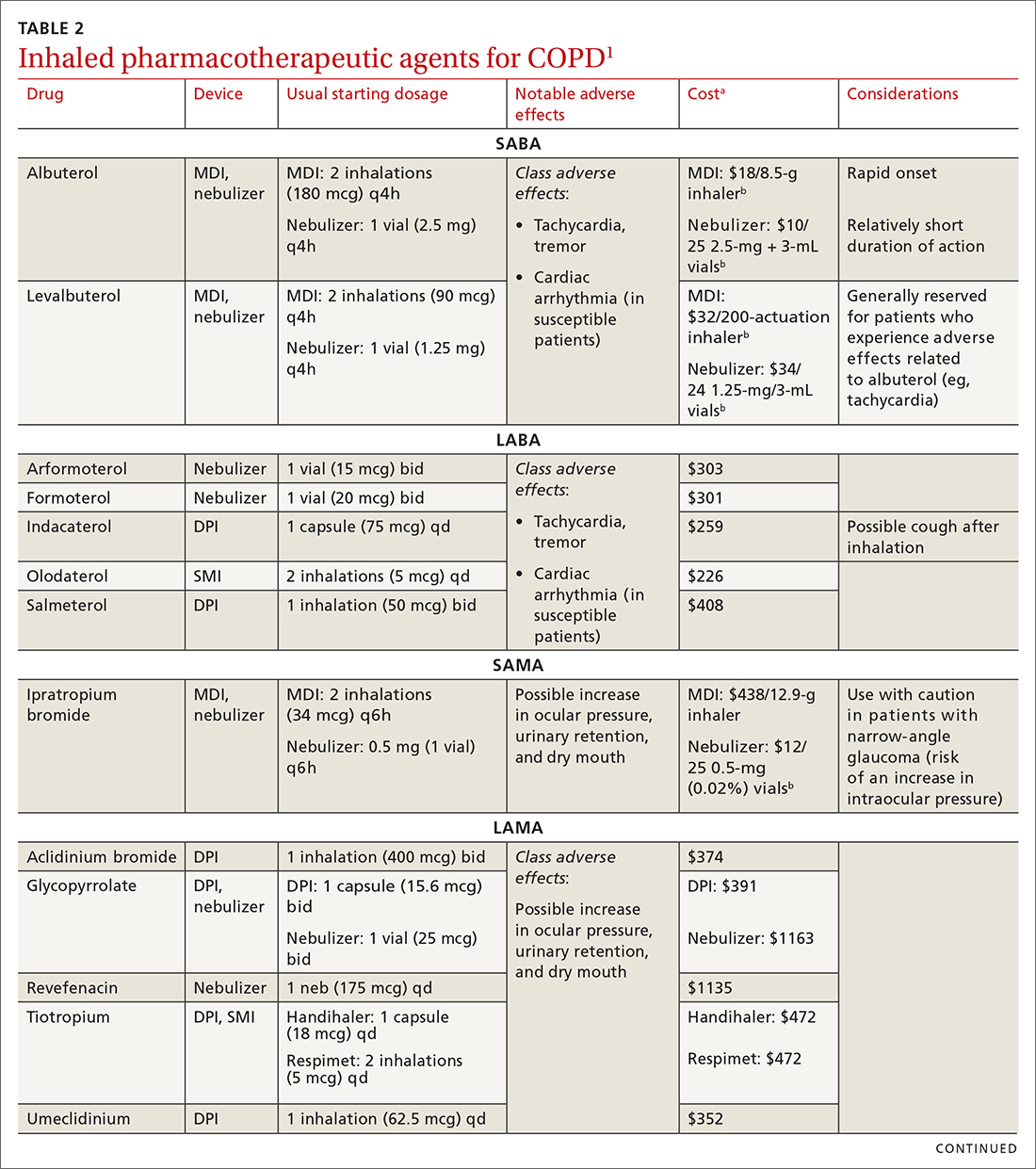
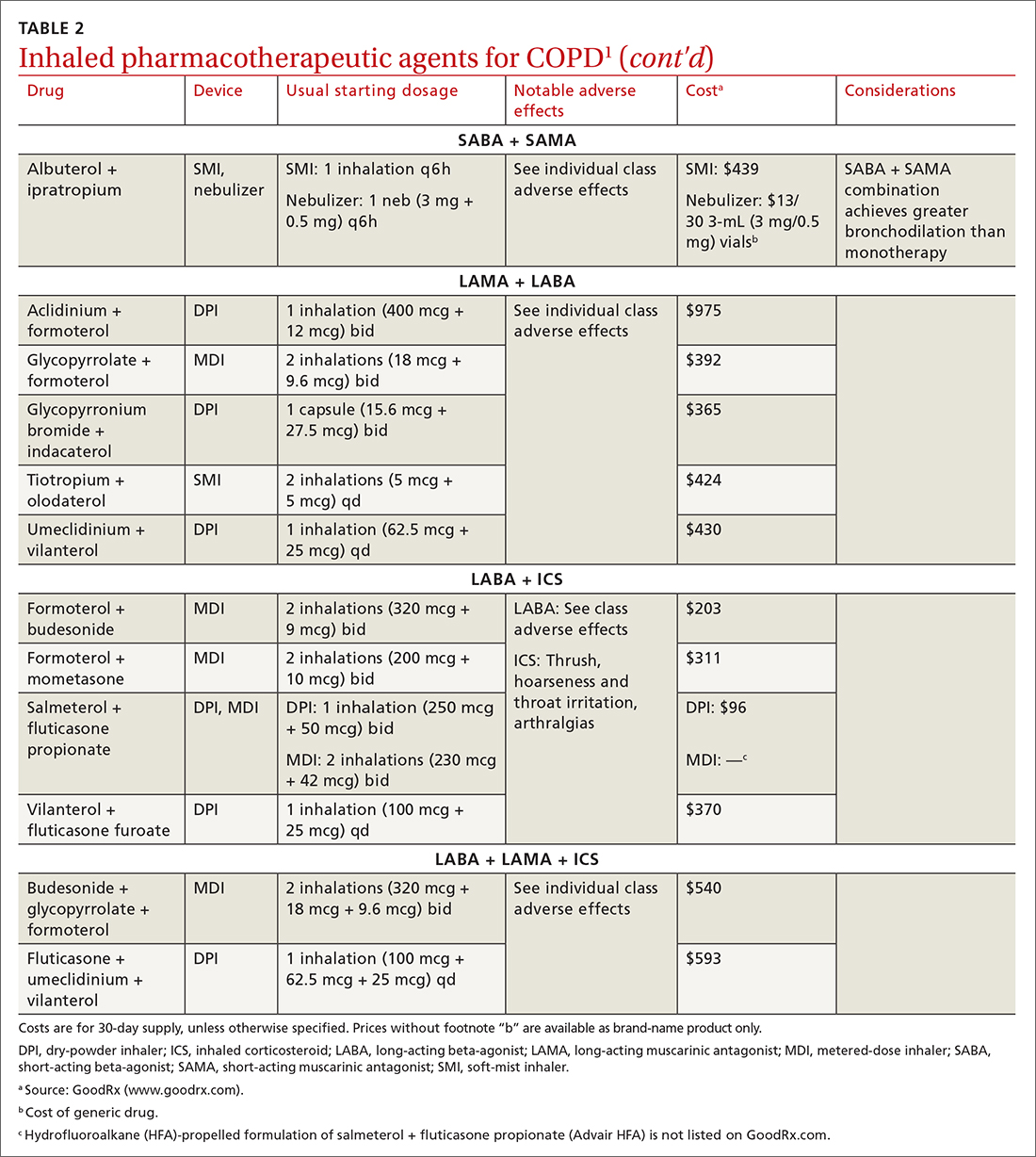
Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1
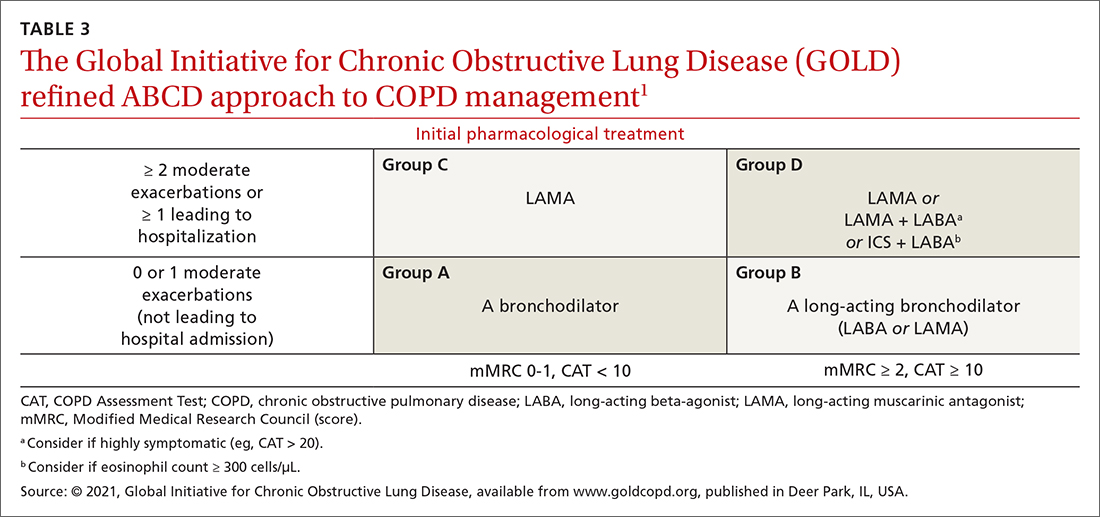
Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
PRACTICE RECOMMENDATIONS
› Follow guideline advice that (1) in general, short-acting beta-agonists (SABAs) are not for daily use in stable chronic obstructive pulmonary disease (COPD) but (2) agents in this class of drugs might have a role in relieving occasional COPD-associated dyspnea. C
› Prescribe albuterol over levalbuterol when a SABA is indicated because of the lower cost of albuterol, its comparative efficacy, and its lower incidence of tachycardia and palpitations, even in patients with cardiovascular disease. B
› Avoid the use of an inhaled corticosteroid, or consider withdrawing inhaled corticosteroid therapy, in patients with COPD whose blood eosinophil count is < 100 cells/μL or who have repeated bouts of pneumonia or a history of mycobacterial infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Hidradenitis Suppurativa
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).
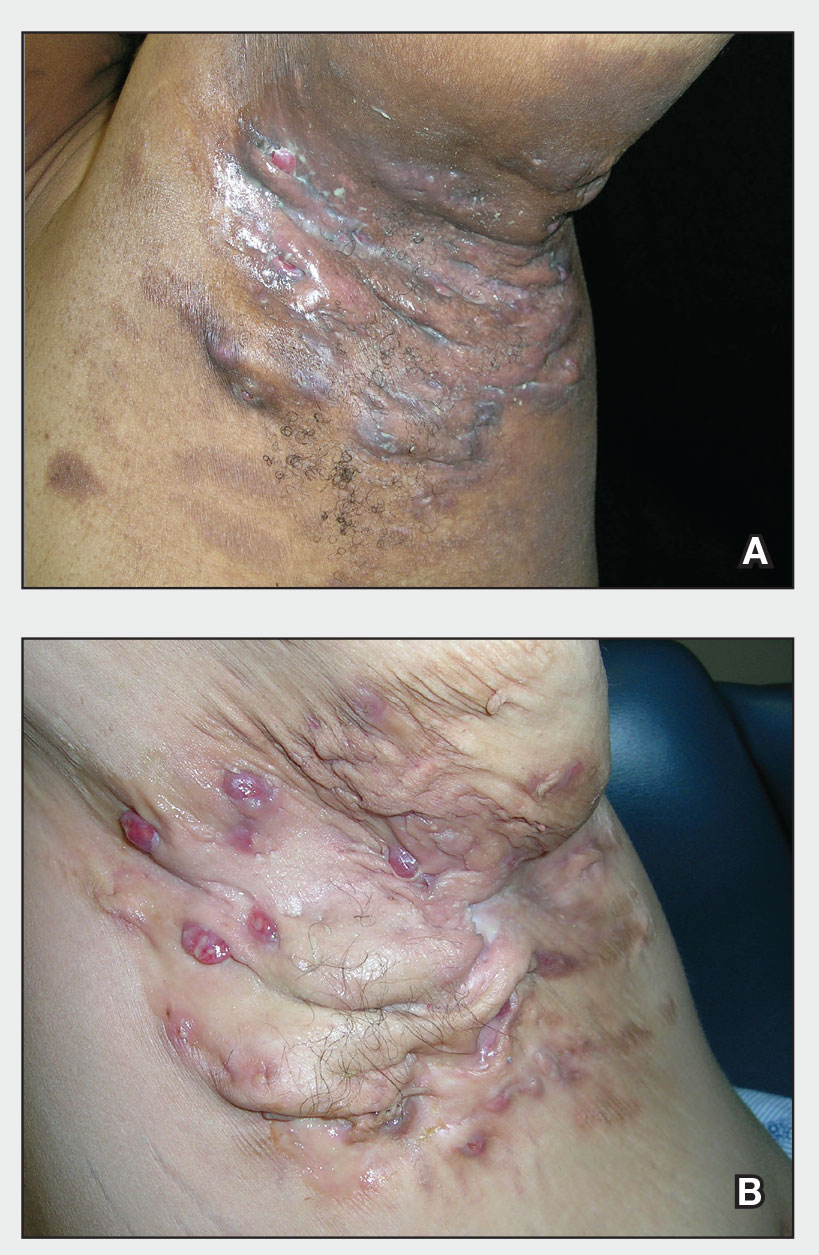
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE PRESENTATION
Severe long-standing hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of a 35-year-old Black man (A) and a 42-year-old Hispanic woman with a light skin tone (B).

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in HS patients:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts (tunnels) and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts (tunnels), abscesses, and/or scarring across the entire area.
Epidemiology
Hidradenitis suppurativa is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
Hidradenitis suppurativa is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to lack of HS knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
- Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa [published online November 11, 2020]. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
- Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
- Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
- Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
- Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73 (5 suppl 1):S4-S7.
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm [published online September 17, 2020]. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management [published online March 11, 2019]. J Am Acad Dermatol. 2019;81:91-101.
- Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
- Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations [published online January 23, 2021]. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j. jaad.2021.01.059
Erythematous Papule on the Nasal Ala
The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.
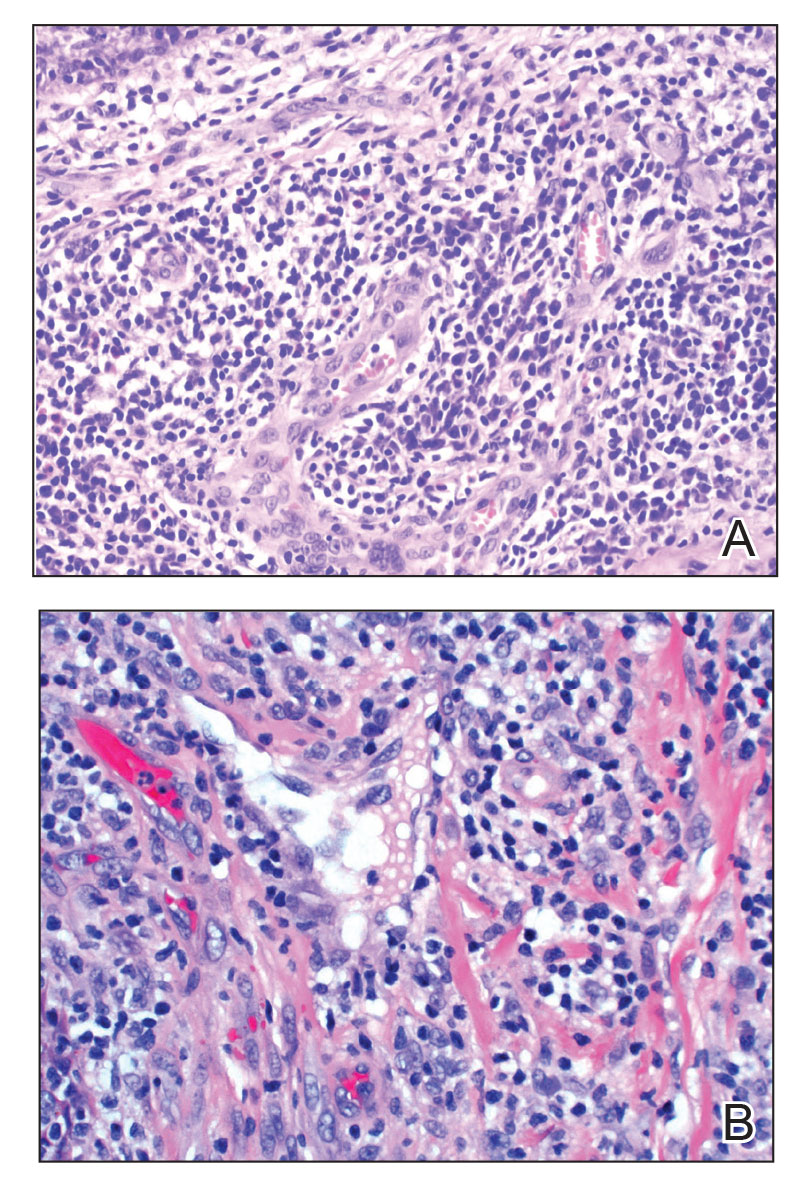
Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12
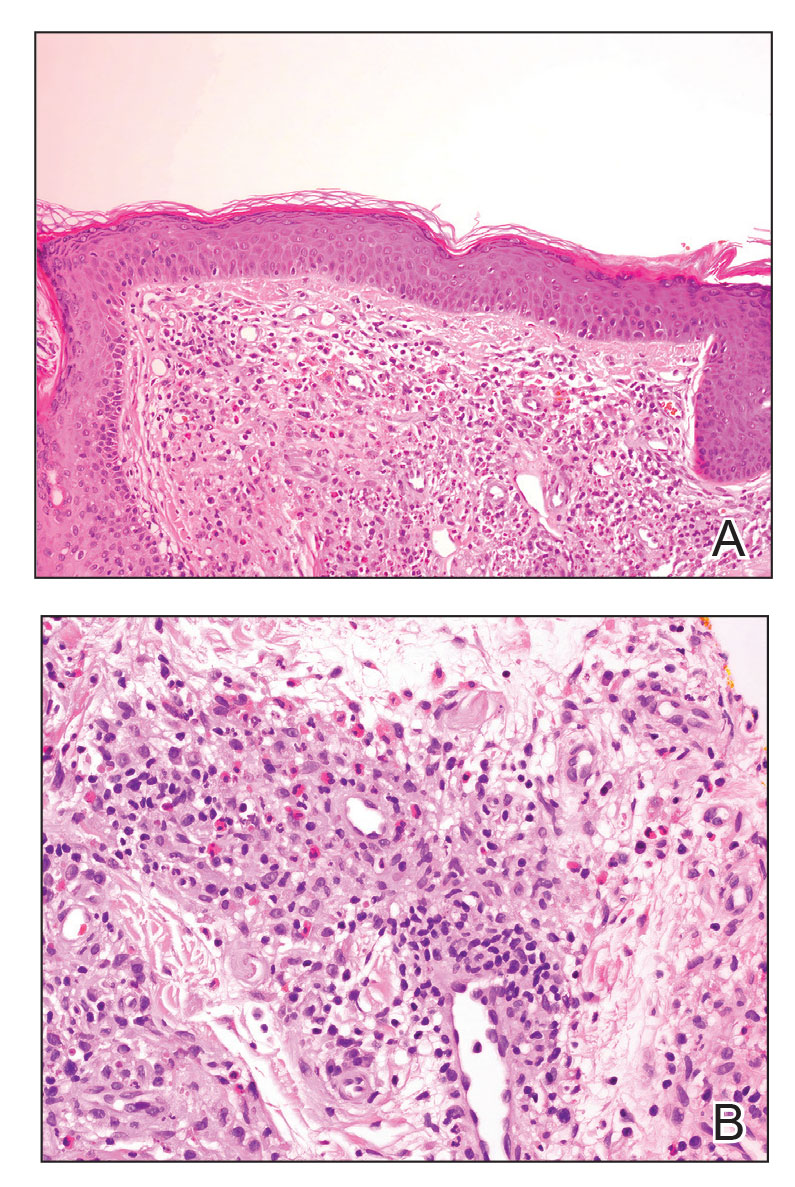
Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.
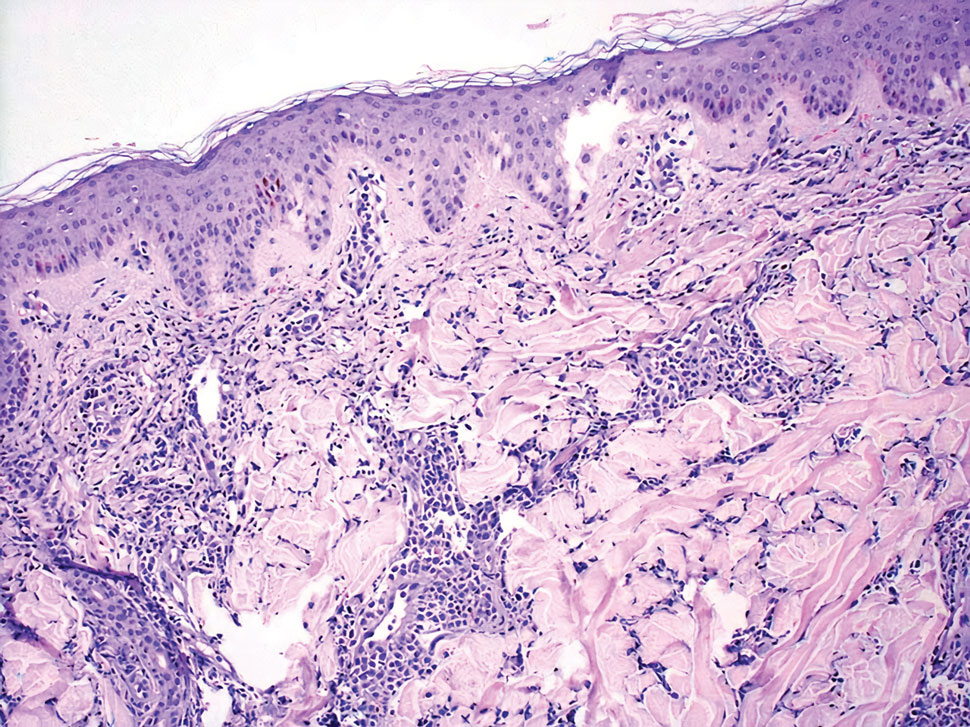
Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.
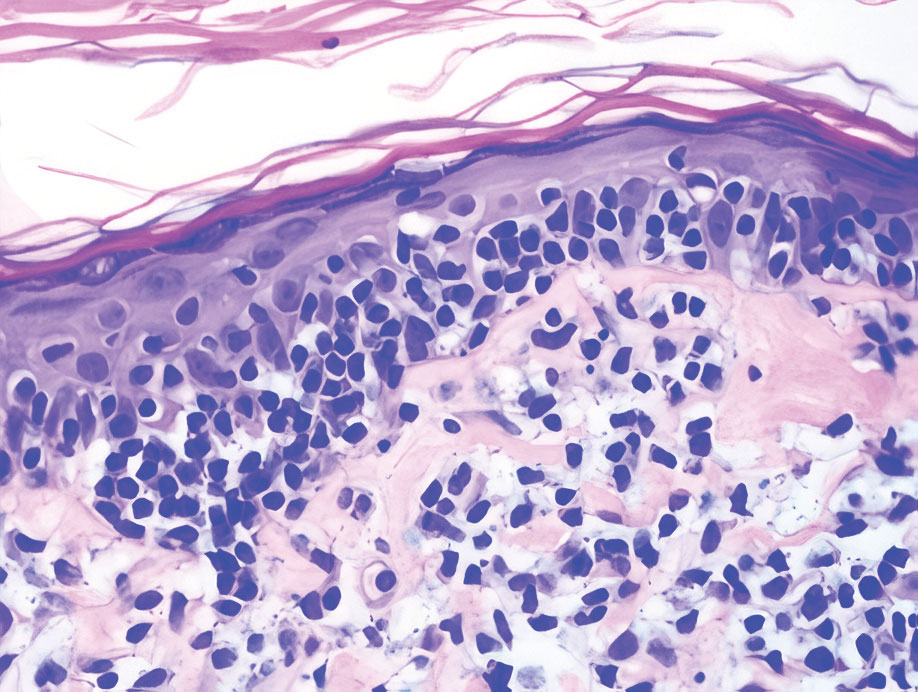
- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.

Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12

Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.

Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.

The Diagnosis: Cutaneous Lymphoid Hyperplasia
Cutaneous lymphoid hyperplasia (CLH)(also known as pseudolymphoma or lymphocytoma cutis) is a benign inflammatory condition that typically presents as a flesh-colored to erythematous or violaceous papule or nodule on the head or neck. Cutaneous lymphoid hyperplasia may arise in response to an antigenic stimulus, such as an insect bite, infectious agent (eg, Borrelia species), medication, or foreign body (eg, tattoos and piercings).1,2 Given the benign nature and potential for spontaneous resolution, treatment is conservative; however, high-potency topical steroids, cryosurgery, surgical excision, or local radiotherapy may lead to improvement.3 Our patient was started on clobetasol ointment 0.05% and topical tacrolimus 0.1%. After 3 months of use, she reported lesion improvement, but a new lesion appeared on the nose superior to the original. She was offered a steroid injection and liquid nitrogen freezing but was lost to follow-up.
The histopathologic features of CLH are variable and can resemble a cutaneous B- or T-cell lymphoma (quiz images). If there is B-cell predominance, histopathology typically shows a dense dermal infiltrate of lymphocytes admixed with sparse histiocytes, eosinophils, and plasma cells. Multiple germinal-center phenotype lymphoid follicles also may be seen.4 Histopathology of T-cell–predominant CLH commonly shows CD4+ T helper lymphocytes admixed with CD8+ T cells within the dermis with possible papillary dermal edema and red cell extravasation.5 Immunohistochemical stains for CD3, CD4, CD8, and CD20 usually are positive. Most lymphocytes are CD3+ T cells. Admixed clusters of CD20+ B cells may be present.
Angiolymphoid hyperplasia with eosinophilia is a vascular tumor of the skin composed of endothelial cells and inflammatory cells.6,7 The condition presents as single or multiple flesh-colored to purple papules most commonly on the face, scalp, and ears.8 Histologically, lesions appear as well-circumscribed collections of blood vessels composed of plump endothelial cells and an inflammatory infiltrate with lymphocytes and eosinophils (Figure 1A). Endothelial cells also may have an epithelioid appearance.7 Apparent fenestrations—holes within endothelial cells—may be present (Figure 1B). Surgical excision is the preferred treatment of angiolymphoid hyperplasia with eosinophilia. Success with laser and cryosurgery also has been reported.

Granuloma faciale typically presents as a solitary redbrown papule or plaque on the face. Linear arborizing vessels and dilated follicular openings with brown globules frequently are seen on dermoscopy.9 Although it may resemble CLH clinically, the histopathology of granuloma faciale is characterized by a perivascular and interstitial dermal infiltrate of numerous eosinophils admixed with lymphocytes, plasma cells, and neutrophils underneath a grenz zone (Figure 2).10 Leukocytoclastic vasculitis may be seen in early lesions, and lesions can show variable angiocentric fibrosis.11 Treatment options include intralesional triamcinolone, topical steroids or calcineurin inhibitors, topical psoralen plus UVA, surgical excision, and laser therapy, but outcomes are variable.12

Leukemia cutis is a malignant hematopoietic skin infiltration that presents as multiple pink to red-brown, firm, hemorrhagic papules most frequently involving the head, neck, and trunk.13 Rarely, lesions of leukemia cutis may present as ulcers or bullae. Most lesions occur at presentation of systemic leukemia or in the setting of established leukemia. The cutaneous involvement portends a poor prognosis, strongly correlating with additional extramedullary leukemic involvement.14 Histologic features vary based on the specific type of leukemia (eg, acute myelogenous leukemia). Generally, neoplastic infiltration of the dermis and subcutaneous tissue in a nodular, diffuse, perivascular, or interstitial pattern is seen (Figure 3).15 Leukemia cutis typically resolves after successful treatment of the underlying leukemia.

Mycosis fungoides (MF) is the most common type of cutaneous T-cell lymphoma. In its early stages, MF presents as erythematous, brown, scaly patches and plaques. With progression to the tumor stage of disease, clonal expansion of CD4+ T cells leads to the development of purple papules and nodules.16 Microscopic findings of MF are dependent on the stage of disease. Early patch lesions show superficial or lichenoid lymphocytic infiltration of the epidermal basal layer.17 In the plaque stage, dermal infiltrates and epidermotropism become more pronounced, with increased atypical lymphocytes with cerebriform nuclei and interspersed inflammatory cells (Figure 4). In the tumor stage, lymphocytic infiltrates may involve the entirety of the dermis or extend into the subcutaneous tissue, and malignant cells become larger in size.17 Mycosis fungoides lesions typically stain positive for helper T-cell markers with a minority staining positive for CD8.

- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
- Zhou LL, Mistry N. Cutaneous lymphoid hyperplasia (pseudolymphoma). CMAJ. 2018;190:E398.
- Lackey JN, Xia Y, Cho S, et al. Cutaneous lymphoid hyperplasia: a case report and brief review of the literature. Cutis. 2007;79:445-448.
- Albrecht J, Fine LA, Piette W. Drug-associated lymphoma and pseudolymphoma: recognition and management. Dermatol Clin. 2007;25:233-244, vii.
- Arai E, Shimizu M, Hirose T. A review of 55 cases of cutaneous lymphoid hyperplasia: reassessment of the histopathologic findings leading to reclassification of 4 lesions as cutaneous marginal zone lymphoma and 19 as pseudolymphomatous folliculitis. Hum Pathol. 2005;36:505-511.
- Bergman R, Khamaysi Z, Sahar D, et al. Cutaneous lymphoid hyperplasia presenting as a solitary facial nodule: clinical, histopathological, immunophenotypical, and molecular studies. Arch Dermatol. 2006;142:1561-1566.
- Wells GC, Whimster IW. Subcutaneous angiolymphoid hyperplasia with eosinophilia. Br J Dermatol. 1969;81:1-14.
- Guo R, Gavino AC. Angiolymphoid hyperplasia with eosinophilia. Arch Pathol Lab Med. 2015;139:683-686.
- Olsen TG, Helwig EB. Angiolymphoid hyperplasia with eosinophilia. a clinicopathologic study of 116 patients. J Am Acad Dermatol. 1985;12:781-796.
- Lallas A, Sidiropoulos T, Lefaki I, et al. Photo letter to the editor: dermoscopy of granuloma faciale. J Dermatol Case Rep. 2012;6:59-60.
- Oliveira CC, Ianhez PE, Marques SA, et al. Granuloma faciale: clinical, morphological and immunohistochemical aspects in a series of 10 patients. An Bras Dermatol. 2016;91:803-807.
- Marcoval J, Moreno A, Peyr J. Granuloma faciale: a clinicopathological study of 11 cases. J Am Acad Dermatol. 2004;51:269-273.
- Lindhaus C, Elsner P. Granuloma faciale treatment: a systematic review. Acta Derm Venereol. 2018;98:14-18.
- Haidari W, Strowd LC. Clinical characterization of leukemia cutis presentation. Cutis. 2019;104:326-330; E3.
- Rao AG, Danturty I. Leukemia cutis. Indian J Dermatol. 2012;57:504.
- Desch JK, Smoller BR. The spectrum of cutaneous disease in leukemias. J Cutan Pathol. 1993;20:407-410.
- Yamashita T, Abbade LP, Marques ME, et al. Mycosis fungoides and Sezary syndrome: clinical, histopathological and immunohistochemical review and update. An Bras Dermatol. 2012;87:817-828; quiz 829-830.
- Smoller BR, Bishop K, Glusac E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19:1423-1430.
A 35-year-old woman presented with a slowly growing, smooth, erythematous papule of 2 months’ duration on the left nasal ala surrounding a piercing (top, inset) that had been performed 4 years prior. A tangential biopsy was obtained for histopathologic evaluation.
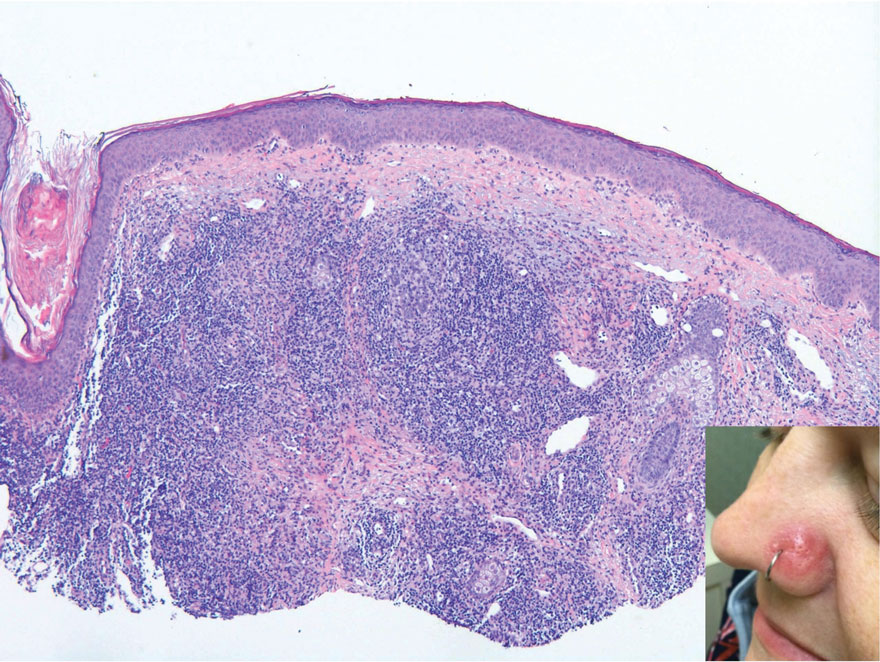
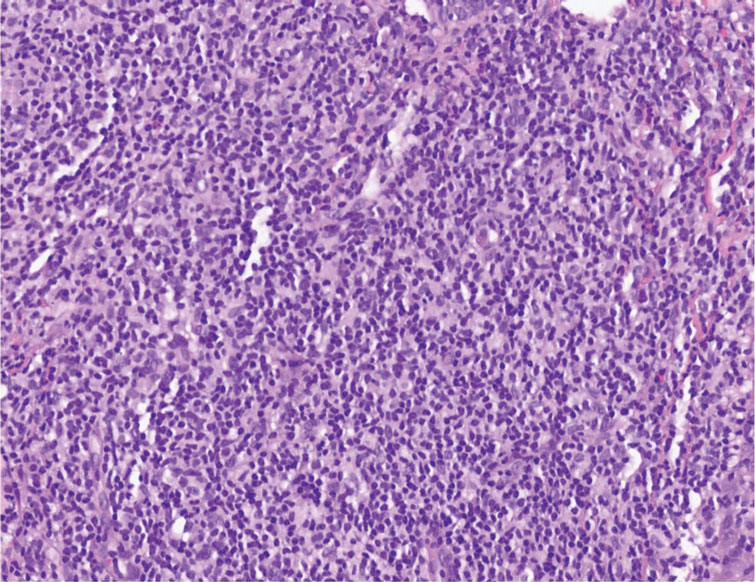
Can Atopic Dermatitis and Allergic Contact Dermatitis Coexist?
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are 2 common inflammatory skin conditions that may have similar clinical presentations. Historically, it was thought that these conditions could not be diagnosed simultaneously due to their differing immune mechanisms; however, this belief has been challenged by recent evidence suggesting a more nuanced relationship between the 2 disease processes. In this review, we examine the complex interplay between AD and ACD and explain how shifts in conventional understanding of the 2 conditions shaped our evolving recognition of their ability to coexist.
Epidemiology of AD and ACD
Atopic dermatitis is the most common inflammatory skin disease in children and adolescents, with an estimated prevalence reaching 21%.1 In 60% of cases, onset of AD will occur within the first year of life, and 90% of cases begin within the first 5 years.2 Resolution may occur by adulthood; however, AD may continue to impact up to 8% to 9% of adults, with an increased prevalence in those older than 75 years.1 This may represent an underestimation of the burden of adult AD; one systematic review of 17 studies found that the pooled proportion of adult-onset AD was greater than 25%.3
In contrast, ACD previously was assumed to be a disease that more commonly impacted adults and only rarely children, primarily due to an early misconception that children were not frequently exposed to contact allergens and their immune systems were too immature to react to them even if exposed.4,5 However, it is now known that children do have risk factors for development of ACD, including a thinner stratum corneum and potentially a more absorbent skin surface.4 In addition, a 2022 study by the North American Contact Dermatitis Group (NACDG) found similar rates of ACD in children (n=1871) and adults (n=41,699) referred for patch testing (55.2% and 57.3%, respectively) as well as similar rates of having at least 1 relevant positive patch test (49.2% and 52.2%).6
In opposition to traditional beliefs, these findings highlight that AD and ACD can occur across age groups.
Immune Mechanism
The pathogenesis of AD represents a multifactorial process involving the immune system, cutaneous flora, genetic predisposition, and surrounding environment. Immunologically, acute AD is driven by a predominantly TH2 helper T-cell response with high levels of IL-4, IL-5, and IL-137; TH22, TH17, and TH1 also have been implicated.8 Notably, TH17 is found in high levels during the acute eczema phase, while TH1 and TH22are associated with the chronic phase.7
The pathophysiology of ACD is not completely understood. The classic paradigm involves 2 phases: sensitization and elicitation. Sensitization involves antigen-presenting cells that take up allergens absorbed by the skin to present them in regional lymph nodes where antigen-specific T lymphocytes are generated. Elicitation occurs upon re-exposure to the allergen, at which time the primed T lymphocytes are recruited to the skin, causing inflammation.9 Allergic contact dermatitis initially was thought to be driven by TH1 cytokines and IL-17 but now is understood to be more complex.10 Studies have revealed immune polarization of contact allergens, demonstrating that nickel primarily induces a TH1/TH17 response, whereas fragrance and rubber accelerators skew to TH2; TH9 and TH22 also may be involved depending on the causative allergen.11,12
Of note, the immunologic differences between AD and ACD led early investigators to believe that patients with AD were relatively protected from ACD.13 However, as previously described, there are several overlapping cytokines between AD and ACD. Furthermore, research has revealed that risk of contact sensitization might be increased in the chronic eczema phase due to the shared TH1 pathway.14 Barrier-disrupted skin (such as that in AD) also may increase the cytokine response and the density of antigen-presenting cells, leading to a proallergic state.15 This suggests that the immunologic pathways of AD and ACD are more intertwined than was previously understood.
Underlying Risk Factors
Skin barrier dysfunction is a key step in the pathogenesis of AD. Patients with AD commonly have loss-of-function mutations in the filaggrin gene, a protein that is key to the function of the stratum corneum. Loss of this protein may not only impact the immune response as previously noted but also may lead to increased transepidermal water loss and bacterial colonization.16 Interestingly, a 2014 review examined how this mutation could lead to an increased risk of sensitization to bivalent metal ions via an impaired chelating ability of the skin.17 Furthermore, a 2016 study conducted in Dutch construction workers revealed an increased risk for contact dermatitis (irritant and allergic) for those with a loss-of-function filaggrin mutation.18
Importantly, this same mutation may explain why patients with AD tend to have increased skin colonization by Staphylococcus aureus. The abundance of S aureus and the relative decrease in the diversity of other microorganisms on the skin may be associated with increased AD severity.19 Likewise, S aureus may play a role in the pathogenesis of ACD via production of its exotoxin directed at the T-cell receptor V beta 17 region. In particular, this receptor has been associated with nickel sensitization.17
Another risk factor to consider is increased exposure to contact sensitizers when treating AD. For instance, management often includes use of over-the-counter emollients, natural or botanical remedies with purported benefits for AD, cleansers, and detergents. However, these products can contain some of the most prevalent contact allergens seen in those with AD, including methyl-isothiazolinone, formaldehyde releasers, and fragrance.20 Topical corticosteroids also are frequently used, and ACD to steroid molecules can occur, particularly to tixocortol-21-pivalate (a marker for class A corticosteroids) and budesonide (a marker for class B corticosteroids).21 Other allergens (eg, benzyl alcohol, propylene glycol) also may be found as inactive ingredients of topical corticosteroids.22 These exposures may place AD patients at risk for ACD.
The Coexistence of AD and ACD
Given the overlapping epidemiology, immunology, and potentially increased risk for the development of ACD in patients with AD, it would be reasonable to assume that the 2 diagnoses could coexist; however, is there clinical data to support this idea? Based on recent database reviews, the answer appears to be yes.20,23-26 An analysis from the Pediatric Contact Dermatitis Registry revealed that 30% of 1142 pediatric patch test cases analyzed were diagnosed as AD and ACD simultaneously.24 The NACDG found similar results in its 2021 review, as 29.5% of children (n=1648) and 20.7% of adults (n=36,834) had a concurrent diagnosis of AD and ACD.20 Notably, older results from these databases also demonstrated an association between the 2 conditions.23,25,26
It remains unclear whether the prevalence of ACD is higher in those with or without AD. A comprehensive systematic review conducted in 2017 examined this topic through analysis of 74 studies. The results demonstrated a similar prevalence of contact sensitization in individuals with and without AD.27 Another systematic review of 31 studies conducted in 2017 found a higher prevalence for ACD in children without AD; however, the authors noted that the included studies were too variable (eg, size, design, allergens tested) to draw definitive conclusions.28
Even though there is no clear overall increased risk for ACD in patients with AD, research has suggested that certain allergens may be more prevalent in the setting of AD. An NACDG study found that adults with AD had increased odds of reacting to 10 of the top 25 NACDG screening allergens compared to those without AD.20 Other studies have found that AD patients may be more likely to become sensitized to certain allergens, such as fragrance and lanolin.14
Considerations for Management
Diagnosis of ACD in patients with AD can be challenging because these conditions may present similarly with chronic, pruritic, inflammatory patches and plaques. Chronic ACD may be misdiagnosed as AD if patch testing is not performed.29 Given the prevalence of ACD in the setting of AD, there should be a low threshold to pursue patch testing, especially when dermatitis is recalcitrant to standard therapies or presents in an atypical distribution (ie, perioral, predominantly head/neck, hand and foot, isolated eyelid involvement, buttocks).4,30 Various allergen series are available for patch testing adults and children including the NACDG Standard Series, American Contact Dermatitis Society Core Allergen Series, or the Pediatric Baseline Series.31-33
If potentially relevant allergens are uncovered by patch testing, patients should be counseled on avoidance strategies. However, allergen avoidance may not always lead to complete symptom resolution, especially if AD is present concomitantly with ACD. Therefore, use of topical or systemic therapies still may be required. Topical corticosteroids can be used when dermatitis is acute and localized. Systemic corticosteroids are utilized for both diagnoses when cases are more severe or extensive, but their adverse-effect profile limits long-term use. Other systemic treatments, including conventional agents (ie, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil), biologics, and small molecule inhibitors also may be considered for severe cases.34,35 Dupilumab, a monoclonal antibody targeting IL-4/IL-13, is approved for use in moderate to severe AD in patients 6 months and older. Recent evidence has suggested that dupilumab also may be an effective off-label treatment choice for ACD when allergen avoidance alone is insufficient.36 Studies have been conducted on secukinumab, a monoclonal antibody against IL-17; however, it has not been shown to be effective in either AD or ACD.37,38 This indicates that targeted biologics may not always be successful in treating these diagnoses, likely due to their complex immune pathways. Finally, there is an emerging role for JAK inhibitors. Three are approved for AD: topical ruxolitinib, oral abrocitinib, and oral upadacitinib.39 Further investigation is needed to determine the efficacy of JAK inhibitors in ACD.
Final Interpretation
Evolving evidence shows that AD and ACD can occur at the same time despite the historical perspective that their immune pathways were too polarized for this to happen. Atopic dermatitis may be an important risk factor for subsequent development of ACD. Management should include a low threshold to perform patch testing, while pharmacotherapies utilized in the treatment of both conditions should be considered.
- Chan LN, Magyari A, Ye M, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One. 2021;16:E0258219. doi:10.1371/journal.pone.0258219
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis [published online November 27, 2013]. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis [published online June 2, 2018]. J Am Acad Dermatol. 2019;80:1526-1532.e7. doi:10.1016/j.jaad.2018.05.1241
- Borok J, Matiz C, Goldenberg A, et al. Contact dermatitis in atopic dermatitis children—past, present, and future. Clin Rev Allergy Immunol. 2019;56:86-98. doi:10.1007/s12016-018-8711-2
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668. doi:10.1016/j.jaip.2015.02.007
- Silverberg JI, Hou A, Warshaw EM, et al. Age-related differences in patch testing results among children: analysis of North American Contact Dermatitis Group data, 2001-2018 [published online July 24, 2021]. J Am Acad Dermatol. 2022;86:818-826. doi:10.1016/j.jaad.2021.07.030
- Tokura Y, Phadungsaksawasdi P, Ito T. Atopic dermatitis as Th2 disease revisited. J Cutan Immunol Allergy. 2018;1:158-164. doi:10.1002/cia2.12033
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(suppl 4):S65-S76. doi:10.1016/j.jaci.2017.01.011
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- He D, Wu L, Kim HK, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses [published online June 24, 2009]. J Immunol. 2009;183:1463-1470. doi:10.4049/jimmunol.0804108.
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response [published April 25, 2014]. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol. 1989;125:366-368.
- Yüksel YT, Nørreslet LB, Thyssen JP. Allergic contact dermatitis in patients with atopic dermatitis. Curr Derm Rep. 2021;10:67-76.
- Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis [published online August 28, 2012]. J Allergy Clin Immunol. 2013;131:300-313. doi:10.1016/j.jaci.2012.06.048
- Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease [published online October 14, 2019]. Ann Allergy Asthma Immunol. 2020;124:36-43. doi:10.1016/j.anai.2019.10.008
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affectingthe association between atopic dermatitis and contact sensitization [published online December 26, 2013]. Allergy. 2014;69:28-36. doi:10.1111/all.12358
- Timmerman JG, Heederik D, Spee T, et al. Contact dermatitis in the construction industry: the role of filaggrin loss-of-function mutations [published online December 12, 2015]. Br J Dermatol. 2016;174:348-355. doi:10.1111/bjd.14215
- Edslev SM, Agner T, Andersen PS. Skin microbiome in atopic dermatitis. Acta Derm Venereol. 2020;100:adv00164. doi:
10.2340/00015555-3514 - Silverberg JI, Hou A, Warshaw EM, et al. Prevalence and trend of allergen sensitization in adults and children with atopic dermatitis referred for patch testing, North American Contact Dermatitis Group data, 2001-2016 [published online March 27, 2021]. J Allergy Clin Immunol Pract. 2021;9:2853-2866.e14. doi:10.1016/j.jaip.2021.03.028
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Xiong M, Peterson MY, Hylwa S. Allergic contact dermatitis from benzyl alcohol in hydrocortisone cream [published online January 14, 2022]. Contact Dermatitis. 2022;86:424-425. doi:10.1111/cod.14042
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302. doi:10.1097/DER.0000000000000214
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol.2016.6136
- Zug KA, McGinley-Smith D, Warshaw EM, et al. Contact allergy in children referred for patch testing: North American Contact Dermatitis Group data, 2001-2004. Arch Dermatol. 2008;144:1329-1336. doi:10.1001/archderm.144.10.1329
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis [published online April 6, 2017]. J Am Acad Dermatol. 2017;77:70-78. doi:10.1016/j.jaad.2017.02.001
- Simonsen AB, Johansen JD, Deleuran M, et al. Contact allergy in children with atopic dermatitis: a systematic review [published online June 12, 2017]. Br J Dermatol. 2017;177:395-405. doi:10.1111/bjd.15628
- Chen R, Raffi J, Murase JE. Tocopherol allergic dermatitis masquerading as lifelong atopic dermatitis. Dermatitis. 2020;31:E3-E4. doi:10.1097/DER.0000000000000543
- Tam I, Yu J. Pediatric contact dermatitis: what’s new. Curr Opin Pediatr. 2020;32:524-530. doi:10.1097/MOP.0000000000000919
- Cohen DE, Rao S, Brancaccio RR. Use of the North American Contact Dermatitis Group Standard 65-allergen series alone in the evaluation of allergic contact dermatitis: a series of 794 patients. Dermatitis. 2008;19:137-141.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212. doi:10.1097/DER.0000000000000385
- Bußmann C, Novak N. Systemic therapy of atopic dermatitis. Allergol Select. 2017;1:1-8. doi:10.5414/ALX01285E
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Johnson H, Adler BL, Yu J. Dupilumab for allergic contact dermatitis: an overview of its use and impact on patch testing. Cutis. 2022;109:265-267, E4-E5. doi:10.12788/cutis.0519
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Ungar B, Pavel AB, Li R, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis [published online May 16, 2020]. J Allergy Clin Immunol. 2021;147:394-397. doi:10.1016/j.jaci.2020.04.055
- Perche PO, Cook MK, Feldman SR. Abrocitinib: a new FDA-approved drug for moderate-to-severe atopic dermatitis [published online May 19, 2022]. Ann Pharmacother. doi:10.1177/10600280221096713
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are 2 common inflammatory skin conditions that may have similar clinical presentations. Historically, it was thought that these conditions could not be diagnosed simultaneously due to their differing immune mechanisms; however, this belief has been challenged by recent evidence suggesting a more nuanced relationship between the 2 disease processes. In this review, we examine the complex interplay between AD and ACD and explain how shifts in conventional understanding of the 2 conditions shaped our evolving recognition of their ability to coexist.
Epidemiology of AD and ACD
Atopic dermatitis is the most common inflammatory skin disease in children and adolescents, with an estimated prevalence reaching 21%.1 In 60% of cases, onset of AD will occur within the first year of life, and 90% of cases begin within the first 5 years.2 Resolution may occur by adulthood; however, AD may continue to impact up to 8% to 9% of adults, with an increased prevalence in those older than 75 years.1 This may represent an underestimation of the burden of adult AD; one systematic review of 17 studies found that the pooled proportion of adult-onset AD was greater than 25%.3
In contrast, ACD previously was assumed to be a disease that more commonly impacted adults and only rarely children, primarily due to an early misconception that children were not frequently exposed to contact allergens and their immune systems were too immature to react to them even if exposed.4,5 However, it is now known that children do have risk factors for development of ACD, including a thinner stratum corneum and potentially a more absorbent skin surface.4 In addition, a 2022 study by the North American Contact Dermatitis Group (NACDG) found similar rates of ACD in children (n=1871) and adults (n=41,699) referred for patch testing (55.2% and 57.3%, respectively) as well as similar rates of having at least 1 relevant positive patch test (49.2% and 52.2%).6
In opposition to traditional beliefs, these findings highlight that AD and ACD can occur across age groups.
Immune Mechanism
The pathogenesis of AD represents a multifactorial process involving the immune system, cutaneous flora, genetic predisposition, and surrounding environment. Immunologically, acute AD is driven by a predominantly TH2 helper T-cell response with high levels of IL-4, IL-5, and IL-137; TH22, TH17, and TH1 also have been implicated.8 Notably, TH17 is found in high levels during the acute eczema phase, while TH1 and TH22are associated with the chronic phase.7
The pathophysiology of ACD is not completely understood. The classic paradigm involves 2 phases: sensitization and elicitation. Sensitization involves antigen-presenting cells that take up allergens absorbed by the skin to present them in regional lymph nodes where antigen-specific T lymphocytes are generated. Elicitation occurs upon re-exposure to the allergen, at which time the primed T lymphocytes are recruited to the skin, causing inflammation.9 Allergic contact dermatitis initially was thought to be driven by TH1 cytokines and IL-17 but now is understood to be more complex.10 Studies have revealed immune polarization of contact allergens, demonstrating that nickel primarily induces a TH1/TH17 response, whereas fragrance and rubber accelerators skew to TH2; TH9 and TH22 also may be involved depending on the causative allergen.11,12
Of note, the immunologic differences between AD and ACD led early investigators to believe that patients with AD were relatively protected from ACD.13 However, as previously described, there are several overlapping cytokines between AD and ACD. Furthermore, research has revealed that risk of contact sensitization might be increased in the chronic eczema phase due to the shared TH1 pathway.14 Barrier-disrupted skin (such as that in AD) also may increase the cytokine response and the density of antigen-presenting cells, leading to a proallergic state.15 This suggests that the immunologic pathways of AD and ACD are more intertwined than was previously understood.
Underlying Risk Factors
Skin barrier dysfunction is a key step in the pathogenesis of AD. Patients with AD commonly have loss-of-function mutations in the filaggrin gene, a protein that is key to the function of the stratum corneum. Loss of this protein may not only impact the immune response as previously noted but also may lead to increased transepidermal water loss and bacterial colonization.16 Interestingly, a 2014 review examined how this mutation could lead to an increased risk of sensitization to bivalent metal ions via an impaired chelating ability of the skin.17 Furthermore, a 2016 study conducted in Dutch construction workers revealed an increased risk for contact dermatitis (irritant and allergic) for those with a loss-of-function filaggrin mutation.18
Importantly, this same mutation may explain why patients with AD tend to have increased skin colonization by Staphylococcus aureus. The abundance of S aureus and the relative decrease in the diversity of other microorganisms on the skin may be associated with increased AD severity.19 Likewise, S aureus may play a role in the pathogenesis of ACD via production of its exotoxin directed at the T-cell receptor V beta 17 region. In particular, this receptor has been associated with nickel sensitization.17
Another risk factor to consider is increased exposure to contact sensitizers when treating AD. For instance, management often includes use of over-the-counter emollients, natural or botanical remedies with purported benefits for AD, cleansers, and detergents. However, these products can contain some of the most prevalent contact allergens seen in those with AD, including methyl-isothiazolinone, formaldehyde releasers, and fragrance.20 Topical corticosteroids also are frequently used, and ACD to steroid molecules can occur, particularly to tixocortol-21-pivalate (a marker for class A corticosteroids) and budesonide (a marker for class B corticosteroids).21 Other allergens (eg, benzyl alcohol, propylene glycol) also may be found as inactive ingredients of topical corticosteroids.22 These exposures may place AD patients at risk for ACD.
The Coexistence of AD and ACD
Given the overlapping epidemiology, immunology, and potentially increased risk for the development of ACD in patients with AD, it would be reasonable to assume that the 2 diagnoses could coexist; however, is there clinical data to support this idea? Based on recent database reviews, the answer appears to be yes.20,23-26 An analysis from the Pediatric Contact Dermatitis Registry revealed that 30% of 1142 pediatric patch test cases analyzed were diagnosed as AD and ACD simultaneously.24 The NACDG found similar results in its 2021 review, as 29.5% of children (n=1648) and 20.7% of adults (n=36,834) had a concurrent diagnosis of AD and ACD.20 Notably, older results from these databases also demonstrated an association between the 2 conditions.23,25,26
It remains unclear whether the prevalence of ACD is higher in those with or without AD. A comprehensive systematic review conducted in 2017 examined this topic through analysis of 74 studies. The results demonstrated a similar prevalence of contact sensitization in individuals with and without AD.27 Another systematic review of 31 studies conducted in 2017 found a higher prevalence for ACD in children without AD; however, the authors noted that the included studies were too variable (eg, size, design, allergens tested) to draw definitive conclusions.28
Even though there is no clear overall increased risk for ACD in patients with AD, research has suggested that certain allergens may be more prevalent in the setting of AD. An NACDG study found that adults with AD had increased odds of reacting to 10 of the top 25 NACDG screening allergens compared to those without AD.20 Other studies have found that AD patients may be more likely to become sensitized to certain allergens, such as fragrance and lanolin.14
Considerations for Management
Diagnosis of ACD in patients with AD can be challenging because these conditions may present similarly with chronic, pruritic, inflammatory patches and plaques. Chronic ACD may be misdiagnosed as AD if patch testing is not performed.29 Given the prevalence of ACD in the setting of AD, there should be a low threshold to pursue patch testing, especially when dermatitis is recalcitrant to standard therapies or presents in an atypical distribution (ie, perioral, predominantly head/neck, hand and foot, isolated eyelid involvement, buttocks).4,30 Various allergen series are available for patch testing adults and children including the NACDG Standard Series, American Contact Dermatitis Society Core Allergen Series, or the Pediatric Baseline Series.31-33
If potentially relevant allergens are uncovered by patch testing, patients should be counseled on avoidance strategies. However, allergen avoidance may not always lead to complete symptom resolution, especially if AD is present concomitantly with ACD. Therefore, use of topical or systemic therapies still may be required. Topical corticosteroids can be used when dermatitis is acute and localized. Systemic corticosteroids are utilized for both diagnoses when cases are more severe or extensive, but their adverse-effect profile limits long-term use. Other systemic treatments, including conventional agents (ie, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil), biologics, and small molecule inhibitors also may be considered for severe cases.34,35 Dupilumab, a monoclonal antibody targeting IL-4/IL-13, is approved for use in moderate to severe AD in patients 6 months and older. Recent evidence has suggested that dupilumab also may be an effective off-label treatment choice for ACD when allergen avoidance alone is insufficient.36 Studies have been conducted on secukinumab, a monoclonal antibody against IL-17; however, it has not been shown to be effective in either AD or ACD.37,38 This indicates that targeted biologics may not always be successful in treating these diagnoses, likely due to their complex immune pathways. Finally, there is an emerging role for JAK inhibitors. Three are approved for AD: topical ruxolitinib, oral abrocitinib, and oral upadacitinib.39 Further investigation is needed to determine the efficacy of JAK inhibitors in ACD.
Final Interpretation
Evolving evidence shows that AD and ACD can occur at the same time despite the historical perspective that their immune pathways were too polarized for this to happen. Atopic dermatitis may be an important risk factor for subsequent development of ACD. Management should include a low threshold to perform patch testing, while pharmacotherapies utilized in the treatment of both conditions should be considered.
Atopic dermatitis (AD) and allergic contact dermatitis (ACD) are 2 common inflammatory skin conditions that may have similar clinical presentations. Historically, it was thought that these conditions could not be diagnosed simultaneously due to their differing immune mechanisms; however, this belief has been challenged by recent evidence suggesting a more nuanced relationship between the 2 disease processes. In this review, we examine the complex interplay between AD and ACD and explain how shifts in conventional understanding of the 2 conditions shaped our evolving recognition of their ability to coexist.
Epidemiology of AD and ACD
Atopic dermatitis is the most common inflammatory skin disease in children and adolescents, with an estimated prevalence reaching 21%.1 In 60% of cases, onset of AD will occur within the first year of life, and 90% of cases begin within the first 5 years.2 Resolution may occur by adulthood; however, AD may continue to impact up to 8% to 9% of adults, with an increased prevalence in those older than 75 years.1 This may represent an underestimation of the burden of adult AD; one systematic review of 17 studies found that the pooled proportion of adult-onset AD was greater than 25%.3
In contrast, ACD previously was assumed to be a disease that more commonly impacted adults and only rarely children, primarily due to an early misconception that children were not frequently exposed to contact allergens and their immune systems were too immature to react to them even if exposed.4,5 However, it is now known that children do have risk factors for development of ACD, including a thinner stratum corneum and potentially a more absorbent skin surface.4 In addition, a 2022 study by the North American Contact Dermatitis Group (NACDG) found similar rates of ACD in children (n=1871) and adults (n=41,699) referred for patch testing (55.2% and 57.3%, respectively) as well as similar rates of having at least 1 relevant positive patch test (49.2% and 52.2%).6
In opposition to traditional beliefs, these findings highlight that AD and ACD can occur across age groups.
Immune Mechanism
The pathogenesis of AD represents a multifactorial process involving the immune system, cutaneous flora, genetic predisposition, and surrounding environment. Immunologically, acute AD is driven by a predominantly TH2 helper T-cell response with high levels of IL-4, IL-5, and IL-137; TH22, TH17, and TH1 also have been implicated.8 Notably, TH17 is found in high levels during the acute eczema phase, while TH1 and TH22are associated with the chronic phase.7
The pathophysiology of ACD is not completely understood. The classic paradigm involves 2 phases: sensitization and elicitation. Sensitization involves antigen-presenting cells that take up allergens absorbed by the skin to present them in regional lymph nodes where antigen-specific T lymphocytes are generated. Elicitation occurs upon re-exposure to the allergen, at which time the primed T lymphocytes are recruited to the skin, causing inflammation.9 Allergic contact dermatitis initially was thought to be driven by TH1 cytokines and IL-17 but now is understood to be more complex.10 Studies have revealed immune polarization of contact allergens, demonstrating that nickel primarily induces a TH1/TH17 response, whereas fragrance and rubber accelerators skew to TH2; TH9 and TH22 also may be involved depending on the causative allergen.11,12
Of note, the immunologic differences between AD and ACD led early investigators to believe that patients with AD were relatively protected from ACD.13 However, as previously described, there are several overlapping cytokines between AD and ACD. Furthermore, research has revealed that risk of contact sensitization might be increased in the chronic eczema phase due to the shared TH1 pathway.14 Barrier-disrupted skin (such as that in AD) also may increase the cytokine response and the density of antigen-presenting cells, leading to a proallergic state.15 This suggests that the immunologic pathways of AD and ACD are more intertwined than was previously understood.
Underlying Risk Factors
Skin barrier dysfunction is a key step in the pathogenesis of AD. Patients with AD commonly have loss-of-function mutations in the filaggrin gene, a protein that is key to the function of the stratum corneum. Loss of this protein may not only impact the immune response as previously noted but also may lead to increased transepidermal water loss and bacterial colonization.16 Interestingly, a 2014 review examined how this mutation could lead to an increased risk of sensitization to bivalent metal ions via an impaired chelating ability of the skin.17 Furthermore, a 2016 study conducted in Dutch construction workers revealed an increased risk for contact dermatitis (irritant and allergic) for those with a loss-of-function filaggrin mutation.18
Importantly, this same mutation may explain why patients with AD tend to have increased skin colonization by Staphylococcus aureus. The abundance of S aureus and the relative decrease in the diversity of other microorganisms on the skin may be associated with increased AD severity.19 Likewise, S aureus may play a role in the pathogenesis of ACD via production of its exotoxin directed at the T-cell receptor V beta 17 region. In particular, this receptor has been associated with nickel sensitization.17
Another risk factor to consider is increased exposure to contact sensitizers when treating AD. For instance, management often includes use of over-the-counter emollients, natural or botanical remedies with purported benefits for AD, cleansers, and detergents. However, these products can contain some of the most prevalent contact allergens seen in those with AD, including methyl-isothiazolinone, formaldehyde releasers, and fragrance.20 Topical corticosteroids also are frequently used, and ACD to steroid molecules can occur, particularly to tixocortol-21-pivalate (a marker for class A corticosteroids) and budesonide (a marker for class B corticosteroids).21 Other allergens (eg, benzyl alcohol, propylene glycol) also may be found as inactive ingredients of topical corticosteroids.22 These exposures may place AD patients at risk for ACD.
The Coexistence of AD and ACD
Given the overlapping epidemiology, immunology, and potentially increased risk for the development of ACD in patients with AD, it would be reasonable to assume that the 2 diagnoses could coexist; however, is there clinical data to support this idea? Based on recent database reviews, the answer appears to be yes.20,23-26 An analysis from the Pediatric Contact Dermatitis Registry revealed that 30% of 1142 pediatric patch test cases analyzed were diagnosed as AD and ACD simultaneously.24 The NACDG found similar results in its 2021 review, as 29.5% of children (n=1648) and 20.7% of adults (n=36,834) had a concurrent diagnosis of AD and ACD.20 Notably, older results from these databases also demonstrated an association between the 2 conditions.23,25,26
It remains unclear whether the prevalence of ACD is higher in those with or without AD. A comprehensive systematic review conducted in 2017 examined this topic through analysis of 74 studies. The results demonstrated a similar prevalence of contact sensitization in individuals with and without AD.27 Another systematic review of 31 studies conducted in 2017 found a higher prevalence for ACD in children without AD; however, the authors noted that the included studies were too variable (eg, size, design, allergens tested) to draw definitive conclusions.28
Even though there is no clear overall increased risk for ACD in patients with AD, research has suggested that certain allergens may be more prevalent in the setting of AD. An NACDG study found that adults with AD had increased odds of reacting to 10 of the top 25 NACDG screening allergens compared to those without AD.20 Other studies have found that AD patients may be more likely to become sensitized to certain allergens, such as fragrance and lanolin.14
Considerations for Management
Diagnosis of ACD in patients with AD can be challenging because these conditions may present similarly with chronic, pruritic, inflammatory patches and plaques. Chronic ACD may be misdiagnosed as AD if patch testing is not performed.29 Given the prevalence of ACD in the setting of AD, there should be a low threshold to pursue patch testing, especially when dermatitis is recalcitrant to standard therapies or presents in an atypical distribution (ie, perioral, predominantly head/neck, hand and foot, isolated eyelid involvement, buttocks).4,30 Various allergen series are available for patch testing adults and children including the NACDG Standard Series, American Contact Dermatitis Society Core Allergen Series, or the Pediatric Baseline Series.31-33
If potentially relevant allergens are uncovered by patch testing, patients should be counseled on avoidance strategies. However, allergen avoidance may not always lead to complete symptom resolution, especially if AD is present concomitantly with ACD. Therefore, use of topical or systemic therapies still may be required. Topical corticosteroids can be used when dermatitis is acute and localized. Systemic corticosteroids are utilized for both diagnoses when cases are more severe or extensive, but their adverse-effect profile limits long-term use. Other systemic treatments, including conventional agents (ie, azathioprine, cyclosporine, methotrexate, mycophenolate mofetil), biologics, and small molecule inhibitors also may be considered for severe cases.34,35 Dupilumab, a monoclonal antibody targeting IL-4/IL-13, is approved for use in moderate to severe AD in patients 6 months and older. Recent evidence has suggested that dupilumab also may be an effective off-label treatment choice for ACD when allergen avoidance alone is insufficient.36 Studies have been conducted on secukinumab, a monoclonal antibody against IL-17; however, it has not been shown to be effective in either AD or ACD.37,38 This indicates that targeted biologics may not always be successful in treating these diagnoses, likely due to their complex immune pathways. Finally, there is an emerging role for JAK inhibitors. Three are approved for AD: topical ruxolitinib, oral abrocitinib, and oral upadacitinib.39 Further investigation is needed to determine the efficacy of JAK inhibitors in ACD.
Final Interpretation
Evolving evidence shows that AD and ACD can occur at the same time despite the historical perspective that their immune pathways were too polarized for this to happen. Atopic dermatitis may be an important risk factor for subsequent development of ACD. Management should include a low threshold to perform patch testing, while pharmacotherapies utilized in the treatment of both conditions should be considered.
- Chan LN, Magyari A, Ye M, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One. 2021;16:E0258219. doi:10.1371/journal.pone.0258219
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis [published online November 27, 2013]. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis [published online June 2, 2018]. J Am Acad Dermatol. 2019;80:1526-1532.e7. doi:10.1016/j.jaad.2018.05.1241
- Borok J, Matiz C, Goldenberg A, et al. Contact dermatitis in atopic dermatitis children—past, present, and future. Clin Rev Allergy Immunol. 2019;56:86-98. doi:10.1007/s12016-018-8711-2
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668. doi:10.1016/j.jaip.2015.02.007
- Silverberg JI, Hou A, Warshaw EM, et al. Age-related differences in patch testing results among children: analysis of North American Contact Dermatitis Group data, 2001-2018 [published online July 24, 2021]. J Am Acad Dermatol. 2022;86:818-826. doi:10.1016/j.jaad.2021.07.030
- Tokura Y, Phadungsaksawasdi P, Ito T. Atopic dermatitis as Th2 disease revisited. J Cutan Immunol Allergy. 2018;1:158-164. doi:10.1002/cia2.12033
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(suppl 4):S65-S76. doi:10.1016/j.jaci.2017.01.011
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- He D, Wu L, Kim HK, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses [published online June 24, 2009]. J Immunol. 2009;183:1463-1470. doi:10.4049/jimmunol.0804108.
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response [published April 25, 2014]. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol. 1989;125:366-368.
- Yüksel YT, Nørreslet LB, Thyssen JP. Allergic contact dermatitis in patients with atopic dermatitis. Curr Derm Rep. 2021;10:67-76.
- Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis [published online August 28, 2012]. J Allergy Clin Immunol. 2013;131:300-313. doi:10.1016/j.jaci.2012.06.048
- Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease [published online October 14, 2019]. Ann Allergy Asthma Immunol. 2020;124:36-43. doi:10.1016/j.anai.2019.10.008
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affectingthe association between atopic dermatitis and contact sensitization [published online December 26, 2013]. Allergy. 2014;69:28-36. doi:10.1111/all.12358
- Timmerman JG, Heederik D, Spee T, et al. Contact dermatitis in the construction industry: the role of filaggrin loss-of-function mutations [published online December 12, 2015]. Br J Dermatol. 2016;174:348-355. doi:10.1111/bjd.14215
- Edslev SM, Agner T, Andersen PS. Skin microbiome in atopic dermatitis. Acta Derm Venereol. 2020;100:adv00164. doi:
10.2340/00015555-3514 - Silverberg JI, Hou A, Warshaw EM, et al. Prevalence and trend of allergen sensitization in adults and children with atopic dermatitis referred for patch testing, North American Contact Dermatitis Group data, 2001-2016 [published online March 27, 2021]. J Allergy Clin Immunol Pract. 2021;9:2853-2866.e14. doi:10.1016/j.jaip.2021.03.028
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Xiong M, Peterson MY, Hylwa S. Allergic contact dermatitis from benzyl alcohol in hydrocortisone cream [published online January 14, 2022]. Contact Dermatitis. 2022;86:424-425. doi:10.1111/cod.14042
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302. doi:10.1097/DER.0000000000000214
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol.2016.6136
- Zug KA, McGinley-Smith D, Warshaw EM, et al. Contact allergy in children referred for patch testing: North American Contact Dermatitis Group data, 2001-2004. Arch Dermatol. 2008;144:1329-1336. doi:10.1001/archderm.144.10.1329
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis [published online April 6, 2017]. J Am Acad Dermatol. 2017;77:70-78. doi:10.1016/j.jaad.2017.02.001
- Simonsen AB, Johansen JD, Deleuran M, et al. Contact allergy in children with atopic dermatitis: a systematic review [published online June 12, 2017]. Br J Dermatol. 2017;177:395-405. doi:10.1111/bjd.15628
- Chen R, Raffi J, Murase JE. Tocopherol allergic dermatitis masquerading as lifelong atopic dermatitis. Dermatitis. 2020;31:E3-E4. doi:10.1097/DER.0000000000000543
- Tam I, Yu J. Pediatric contact dermatitis: what’s new. Curr Opin Pediatr. 2020;32:524-530. doi:10.1097/MOP.0000000000000919
- Cohen DE, Rao S, Brancaccio RR. Use of the North American Contact Dermatitis Group Standard 65-allergen series alone in the evaluation of allergic contact dermatitis: a series of 794 patients. Dermatitis. 2008;19:137-141.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212. doi:10.1097/DER.0000000000000385
- Bußmann C, Novak N. Systemic therapy of atopic dermatitis. Allergol Select. 2017;1:1-8. doi:10.5414/ALX01285E
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Johnson H, Adler BL, Yu J. Dupilumab for allergic contact dermatitis: an overview of its use and impact on patch testing. Cutis. 2022;109:265-267, E4-E5. doi:10.12788/cutis.0519
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Ungar B, Pavel AB, Li R, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis [published online May 16, 2020]. J Allergy Clin Immunol. 2021;147:394-397. doi:10.1016/j.jaci.2020.04.055
- Perche PO, Cook MK, Feldman SR. Abrocitinib: a new FDA-approved drug for moderate-to-severe atopic dermatitis [published online May 19, 2022]. Ann Pharmacother. doi:10.1177/10600280221096713
- Chan LN, Magyari A, Ye M, et al. The epidemiology of atopic dermatitis in older adults: a population-based study in the United Kingdom. PLoS One. 2021;16:E0258219. doi:10.1371/journal.pone.0258219
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis [published online November 27, 2013]. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Lee HH, Patel KR, Singam V, et al. A systematic review and meta-analysis of the prevalence and phenotype of adult-onset atopic dermatitis [published online June 2, 2018]. J Am Acad Dermatol. 2019;80:1526-1532.e7. doi:10.1016/j.jaad.2018.05.1241
- Borok J, Matiz C, Goldenberg A, et al. Contact dermatitis in atopic dermatitis children—past, present, and future. Clin Rev Allergy Immunol. 2019;56:86-98. doi:10.1007/s12016-018-8711-2
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668. doi:10.1016/j.jaip.2015.02.007
- Silverberg JI, Hou A, Warshaw EM, et al. Age-related differences in patch testing results among children: analysis of North American Contact Dermatitis Group data, 2001-2018 [published online July 24, 2021]. J Am Acad Dermatol. 2022;86:818-826. doi:10.1016/j.jaad.2021.07.030
- Tokura Y, Phadungsaksawasdi P, Ito T. Atopic dermatitis as Th2 disease revisited. J Cutan Immunol Allergy. 2018;1:158-164. doi:10.1002/cia2.12033
- Brunner PM, Guttman-Yassky E, Leung DY. The immunology of atopic dermatitis and its reversibility with broad-spectrum and targeted therapies. J Allergy Clin Immunol. 2017;139(suppl 4):S65-S76. doi:10.1016/j.jaci.2017.01.011
- Murphy PB, Atwater AR, Mueller M. Allergic Contact Dermatitis. StatPearls Publishing; 2021. https://www.ncbi.nlm.nih.gov/books/NBK532866/
- He D, Wu L, Kim HK, et al. IL-17 and IFN-gamma mediate the elicitation of contact hypersensitivity responses by different mechanisms and both are required for optimal responses [published online June 24, 2009]. J Immunol. 2009;183:1463-1470. doi:10.4049/jimmunol.0804108.
- Dhingra N, Shemer A, Correa da Rosa J, et al. Molecular profiling of contact dermatitis skin identifies allergen-dependent differences in immune response [published April 25, 2014]. J Allergy Clin Immunol. 2014;134:362-372. doi:10.1016/j.jaci.2014.03.009
- Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19:293-302. doi:10.1007/s40257-017-0340-7
- Uehara M, Sawai T. A longitudinal study of contact sensitivity in patients with atopic dermatitis. Arch Dermatol. 1989;125:366-368.
- Yüksel YT, Nørreslet LB, Thyssen JP. Allergic contact dermatitis in patients with atopic dermatitis. Curr Derm Rep. 2021;10:67-76.
- Gittler JK, Krueger JG, Guttman-Yassky E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: implications for contact dermatitis [published online August 28, 2012]. J Allergy Clin Immunol. 2013;131:300-313. doi:10.1016/j.jaci.2012.06.048
- Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease [published online October 14, 2019]. Ann Allergy Asthma Immunol. 2020;124:36-43. doi:10.1016/j.anai.2019.10.008
- Thyssen JP, McFadden JP, Kimber I. The multiple factors affectingthe association between atopic dermatitis and contact sensitization [published online December 26, 2013]. Allergy. 2014;69:28-36. doi:10.1111/all.12358
- Timmerman JG, Heederik D, Spee T, et al. Contact dermatitis in the construction industry: the role of filaggrin loss-of-function mutations [published online December 12, 2015]. Br J Dermatol. 2016;174:348-355. doi:10.1111/bjd.14215
- Edslev SM, Agner T, Andersen PS. Skin microbiome in atopic dermatitis. Acta Derm Venereol. 2020;100:adv00164. doi:
10.2340/00015555-3514 - Silverberg JI, Hou A, Warshaw EM, et al. Prevalence and trend of allergen sensitization in adults and children with atopic dermatitis referred for patch testing, North American Contact Dermatitis Group data, 2001-2016 [published online March 27, 2021]. J Allergy Clin Immunol Pract. 2021;9:2853-2866.e14. doi:10.1016/j.jaip.2021.03.028
- Pratt MD, Mufti A, Lipson J, et al. Patch test reactions to corticosteroids: retrospective analysis from the North American Contact Dermatitis Group 2007-2014. Dermatitis. 2017;28:58-63. doi:10.1097/DER.0000000000000251
- Xiong M, Peterson MY, Hylwa S. Allergic contact dermatitis from benzyl alcohol in hydrocortisone cream [published online January 14, 2022]. Contact Dermatitis. 2022;86:424-425. doi:10.1111/cod.14042
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302. doi:10.1097/DER.0000000000000214
- Jacob SE, McGowan M, Silverberg NB, et al. Pediatric Contact Dermatitis Registry data on contact allergy in children with atopic dermatitis. JAMA Dermatol. 2017;153:765-770. doi:10.1001/jamadermatol.2016.6136
- Zug KA, McGinley-Smith D, Warshaw EM, et al. Contact allergy in children referred for patch testing: North American Contact Dermatitis Group data, 2001-2004. Arch Dermatol. 2008;144:1329-1336. doi:10.1001/archderm.144.10.1329
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355. doi:10.1097/DER.0000000000000083
- Hamann CR, Hamann D, Egeberg A, et al. Association between atopic dermatitis and contact sensitization: a systematic review and meta-analysis [published online April 6, 2017]. J Am Acad Dermatol. 2017;77:70-78. doi:10.1016/j.jaad.2017.02.001
- Simonsen AB, Johansen JD, Deleuran M, et al. Contact allergy in children with atopic dermatitis: a systematic review [published online June 12, 2017]. Br J Dermatol. 2017;177:395-405. doi:10.1111/bjd.15628
- Chen R, Raffi J, Murase JE. Tocopherol allergic dermatitis masquerading as lifelong atopic dermatitis. Dermatitis. 2020;31:E3-E4. doi:10.1097/DER.0000000000000543
- Tam I, Yu J. Pediatric contact dermatitis: what’s new. Curr Opin Pediatr. 2020;32:524-530. doi:10.1097/MOP.0000000000000919
- Cohen DE, Rao S, Brancaccio RR. Use of the North American Contact Dermatitis Group Standard 65-allergen series alone in the evaluation of allergic contact dermatitis: a series of 794 patients. Dermatitis. 2008;19:137-141.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 update. Dermatitis. 2020;31:279-282. doi:10.1097/DER.0000000000000621
- Yu J, Atwater AR, Brod B, et al. Pediatric baseline patch test series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212. doi:10.1097/DER.0000000000000385
- Bußmann C, Novak N. Systemic therapy of atopic dermatitis. Allergol Select. 2017;1:1-8. doi:10.5414/ALX01285E
- Sung CT, McGowan MA, Machler BC, et al. Systemic treatments for allergic contact dermatitis. Dermatitis. 2019;30:46-53. doi:10.1097/DER.0000000000000435
- Johnson H, Adler BL, Yu J. Dupilumab for allergic contact dermatitis: an overview of its use and impact on patch testing. Cutis. 2022;109:265-267, E4-E5. doi:10.12788/cutis.0519
- Todberg T, Zachariae C, Krustrup D, et al. The effect of treatment with anti-interleukin-17 in patients with allergic contact dermatitis. Contact Dermatitis. 2018;78:431-432. doi:10.1111/cod.12988
- Ungar B, Pavel AB, Li R, et al. Phase 2 randomized, double-blind study of IL-17 targeting with secukinumab in atopic dermatitis [published online May 16, 2020]. J Allergy Clin Immunol. 2021;147:394-397. doi:10.1016/j.jaci.2020.04.055
- Perche PO, Cook MK, Feldman SR. Abrocitinib: a new FDA-approved drug for moderate-to-severe atopic dermatitis [published online May 19, 2022]. Ann Pharmacother. doi:10.1177/10600280221096713
Practice Points
- Although it previously was thought that atopic dermatitis (AD) and allergic contact dermatitis (ACD) could not coexist due to their polarized immune pathways, current evidence suggests otherwise.
- When both diagnoses are suspected, patch testing should be considered as well as therapeutic strategies that can treat both AD and ACD simultaneously.
Vismodegib for Basal Cell Carcinoma and Beyond: What Dermatologists Need to Know
Basal cell carcinomas (BCCs) are considered the most common cutaneous cancers. Approximately 80% of nonmelanoma skin cancers are BCCs.1,2 Surgical management is the gold standard for early-stage and localized BCCs; it may include simple excision vs Mohs micrographic surgery.3,4 However, if left untreated, these lesions can progress to an advanced stage (locally advanced BCC) or infrequently may spread to distant sites (metastatic BCC). In the advanced stage, the lesions are no longer manageable by surgery or radiation therapy.5,6 Recently, inhibitors targeting the hedgehog (Hh) pathway have shown great promise for these patients. The first drug approved by the US Food and Drug Administration (FDA) for locally advanced and metastatic BCC is vismodegib.7 In this article, we provide a clinical review of vismodegib for the management of BCC, including a discussion of the Hh pathway in BCC, adverse effects of vismodegib, use of vismodegib in adnexal skin tumors, recommended doses for vismodegib therapy in BCC, and management of the side effects of treatment.
Hh Pathway in BCC
In embryonic development, the Hh signaling pathway is crucial across a broad spectrum of species, including humans. Various members of the Hh family have been recognized, all working as secreted regulatory proteins.8 The name of the Hh signaling pathway is derived from a polypeptide ligand called hedgehog found in some fruit flies. Mutations in the gene led to fruit fly larvae that had a spiky hairy pattern of denticles similar to hedgehogs, leading to the name of this molecule.9 The transmembrane protein smoothened (SMO) is the main component of the Hh signaling pathway and initiates a signaling cascade that in turn leads to an increased expression of target genes, such as GLI1. Patched (PTCH), also a transmembrane protein and a cell-surface receptor for the secreted Hh ligand, suppresses the signaling capacity of SMO. Upon binding of the Hh ligand to the PTCH receptor, the suppression of SMO is relieved and a signal is propagated, evoking a cellular response.10 Molecular and genetic studies have reported that genetic alterations in the Hh signaling pathway are almost universally present in all BCCs, leading to an aberrant activation of the pathway and an uncontrolled proliferation of the basal cells. Frequently, these alterations have been shown to cause loss of function of PTCH homologue 1, which usually acts to inhibit the SMO homologue signaling activity.11,12
Because of the potential importance of Hh signaling in other solid malignancies and the failure of topical inhibition of SMO,13 subsequent studies on the development of Hh pathway inhibitors have mostly focused on the systemic approach. A multitude of Hh pathway inhibitors have been developed thus far, such as SANT1-SANT4, GDC-0449, IPI-926, BMS-833923 (XL139), HhAntag-691, and MK-4101.14 Many of these inhibitors have been clinically investigated.13,15,16
Systemic SMO Inhibitor: Vismodegib
Vismodegib was the earliest systemic SMO inhibitor to fulfill early clinical evaluation15,16 and the first drug to receive FDA approval for the management of advanced or metastatic BCC. Vismodegib is a small-molecule SMO inhibitor used for the management of selected locally advanced BCC and metastatic BCC in adults.3,17 Although there is a possibility of recurrence following drug withdrawal, vismodegib constitutes a new therapeutic strategy presenting positive benefits to patients. It may provide superior improvement over sunitinib, which has shown efficacy in a few patients; however, the efficacy and tolerance of sunitinib have been shown to be limited.18,19
Adverse Effects of Vismodegib Therapy
Adverse events with vismodegib use have been reported in 98% of patients (N=491); most of these were mild to moderate.20 However, the frequency of adverse events could prove to be a therapeutic challenge for patients requiring extended treatment. The most frequently reported reversible side effects were muscle spasms (64%), alopecia (62%), weight loss (33%), fatigue (28%), decreased appetite (25%), diarrhea (17%), nausea (16%), dysgeusia (54%), and ageusia (22%).20 In clinical trials, amenorrhea was noticed in 30% (3/10) of females with reproductive potential.2 Apart from alopecia and possibly amenorrhea, these side effects are reversible.17 Alkeraye et al17 reported 3 clinical cases of persistent alopecia following the use of vismodegib. Amenorrhea is a possible side effect of unknown reversibility.7
Vismodegib is a pregnancy category D medication.4 Severe birth defects, including craniofacial abnormalities, retardations in normal growth, open perineum, and absence of digital fusion at a corresponding 20% of the recommended daily dose, were found in rat studies. Embryo-fetal death was noted when rats were exposedto concentrations comparable to the recommended human dose.4
Hepatic events with the use of vismodegib have been reported. The use of vismodegib in randomized controlled trials resulted in elevation of both alanine aminotransferase and aspartate aminotransferase levels compared with placebo.21 Moreover, severe hepatotoxicity with vismodegib has been reported.22-24 A study conducted by Edwards et al25 concluded that the use of vismodegib in patients with severe liver disease must include thorough risk-benefit assessment, with caution in using other concomitant hepatotoxic medications.
Rare adverse events also have been reported in the literature, including vismodegib-induced pancreatitis in a 79-year-old patient treated for locally advanced, recurrent BCC that was cleared following cessation of therapy.26 Additionally, atypical fibroxanthoma was observed in an 83-year-old patient after 30 days of treatment with vismodegib for multiple BCCs.27 The development of other secondary malignancies, such as squamous cell carcinoma, melanoma, keratoacanthomas, and pilomatricomas, during or after the long-term use of vismodegib also have been described.28-35
Use of Vismodegib for Adnexal Skin Tumors
The role of the sonic Hh–PTCH pathway in the pathogenesis of adnexal tumors varies in the literature. Some studies propose the involvement of this pathway in the formation of adnexal tumors such as trichoblastoma, trichoepithelioma, and cylindroma, as in BCC. Various lines of evidence support this involvement. Firstly, in mice, the spontaneous generation of numerous BCCs, trichoblastomas, trichoepitheliomas, and cylindromas has been observed following constitutive activation of the sonic Hh–PTCH pathway.36 Secondly, in trichoepitheliomas, there have been positive results in molecular research into the tumor suppressor gene PTCH homologue 1, PTCH1, whose mutations cause constitutive activation of the sonic Hh–PTCH pathway.37 Thirdly, GLI138 and SOX939 transcription factors associated with the signaling pathway of sonic Hh–PTCH appear to have increased levels in adnexal carcinomas.19 Lepesant et al19 reported a notable clinical response to vismodegib in trichoblastic carcinoma. Baur et al40 reported successful treatment of multiple familial trichoepitheliomas with vismodegib. Nonetheless, more studies are required to assess the efficacy and reliability of vismodegib in the management of adnexal tumors.
Recommended Dose of Vismodegib Therapy
The vismodegib dosage that is approved by the FDA is 150 mg/d until disease progression or the development of intolerable side effects.4 Higher dosing regimens were evaluated with 270 mg/d and 540 mg/d. No added therapeutic benefit was noted with the increase in the dose, and no dose-limiting toxic effects were observed.41
Management of Vismodegib Side Effects
Managing patient expectations is a crucial step in improving dysgeusia. The experience of dysgeusia varies among patients; thus, patients should be instructed to adjust their diets according to their level of dysgeusia, which can be achieved by changing ingredients or dressings used with their diet. This step has been proven to be effective in overcoming vismodegib-related dysgeusia. Also, fluid taste distortion may lead to dehydration and an increase in creatine level. Thus, patients should be encouraged to monitor fluid intake. Moreover, a treatment hiatus of 2 to 8 months results in near-complete improvement of taste distortion.
For muscle spasms, quinine, treatment break for 1 month, gentle exercise of affected areas, or muscle relaxants such as baclofen and temazepam all are effective methods. For vismodegib-related alopecia, managing patient expectations is key; patients should be aware that hair may take 6 to 12 months or even longer to regrow. In addition, shaving less frequently helps improve alopecia.
For gastrointestinal disorders, loperamide with or without codeine phosphate is effective in resolving diarrhea, and metoclopramide is mostly adequate in treating nausea. Another adverse event is weight loss; weight loss of 5% or more of total body weight prompts dietetic referral. If weight loss persists, a treatment break might be needed to regain weight.
Overall, treatment breaks are sufficient to resolve adverse events caused by vismodegib and do not compromise efficacy of treatment. The duration of a treatment break should be considered before initiation. In one clinical trial, a longer treatment break was associated with fewer adverse effects without affecting the efficacy of treatment.42
Conclusion
Vismodegib provides an effective alternative to surgical intervention in the management of BCC. However, patients must be monitored vigilantly, as adverse events are common (>90%).
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Cirrone F, Harris CS. Vismodegib and the hedgehog pathway: a new treatment for basal cell carcinoma. Clin Ther. 2012;34:2039-2050.
- Ruiz-Salas V, Alegre M, López-Ferrer A, et al. Vismodegib: a review [article in English, Spanish]. Actas Dermosifiliogr. 2014;105:744-751.
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Cusack CA, Nijhawan R, Miller B, et al. Vismodegib for locally advanced basal cell carcinoma in a heart transplant patient. JAMA Dermatol. 2015;151:70-72.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885-888.
- Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46:3-12.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103-115.
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434.
- Sekulic A, Mangold AR, Northfelt DW, et al. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218-223.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nature Rev Genet. 2006;7:841-850.
- Alkeraye S, Maire C, Desmedt E, et al. Persistent alopecia induced by vismodegib. Br J Dermatol. 2015;172:1671-1672.
- Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24:199-203.
- Lepesant P, Crinquette M, Alkeraye S, et al. Vismodegib induces significant clinical response in locally advanced trichoblastic carcinoma. Br J Dermatol. 2015;173:1059-1062.
- Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-plannedinterim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284-4292.
- Sanchez BE, Hajjafar L. Severe hepatotoxicity in a patient treated with hedgehog inhibitor: first case report. Gastroenterology. 2011;140:S974-S975.
- Ly P, Wolf K, Wilson J. A case of hepatotoxicity associated with vismodegib. JAAD Case Rep. 2018;5:57-59.
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70.
- Edwards BJ, Raisch DW, Saraykar SS, et al. Hepatotoxicity with vismodegib: an MD Anderson Cancer Center and Research on Adverse Drug Events and Reports Project. Drugs R D. 2017;17:211-218.
- Velter C, Blanc J, Robert C. Acute pancreatitis after vismodegib for basal cell carcinoma: a causal relation? Eur J Cancer. 2019;118:67-69.
- Giorgini C, Barbaccia V, Croci GA, et al. Rapid development of atypical fibroxanthoma during vismodegib treatment. Clin Exp Dermatol. 2019;44:86-88.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Zhu GA, Sundram U, Chang ALS. Two different scenarios of squamous cell carcinoma within advanced basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol. 2014;150:970-973.
- Poulalhon N, Dalle S, Balme B, et al. Fast-growing cutaneous squamous cell carcinoma in a patient treated with vismodegib. Dermatology. 2015;230:101-104.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:E33-E34.
- Giuffrida R, Kashofer K, Dika E, et al. Fast growing melanoma following treatment with vismodegib for locally advanced basal cell carcinomas: report of two cases. Eur J Cancer. 2018;91:177-179.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Magdaleno-Tapial J, Valenzuela-Oñate C, Ortiz-Salvador JM, et al. Pilomatricomas secondary to treatment with vismodegib. JAAD Case Rep. 2018;5:12-14.
- Nilsson M, Undèn AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438-3443.
- Vorechovský I, Undén AB, Sandstedt B, et al. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57:4677-4681.
- Hatta N, Hirano T, Kimura T, et al. Molecular diagnosis of basal cell carcinoma and other basaloid cell neoplasms of the skin by the quantification of Gli1 transcript levels. J Cutan Pathol. 2005;32:131-136.
- Vidal VP, Ortonne N, Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. 2008;35:373-379.
- Baur V, Papadopoulos T, Kazakov DV, et al. A case of multiple familial trichoepitheliomas responding to treatment with the hedgehog signaling pathway inhibitor vismodegib. Virchows Arch. 2018;473:241-246.
- LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502-2511.
- Fife K, Herd R, Lalondrelle S, et al. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017;13:175-184.
Basal cell carcinomas (BCCs) are considered the most common cutaneous cancers. Approximately 80% of nonmelanoma skin cancers are BCCs.1,2 Surgical management is the gold standard for early-stage and localized BCCs; it may include simple excision vs Mohs micrographic surgery.3,4 However, if left untreated, these lesions can progress to an advanced stage (locally advanced BCC) or infrequently may spread to distant sites (metastatic BCC). In the advanced stage, the lesions are no longer manageable by surgery or radiation therapy.5,6 Recently, inhibitors targeting the hedgehog (Hh) pathway have shown great promise for these patients. The first drug approved by the US Food and Drug Administration (FDA) for locally advanced and metastatic BCC is vismodegib.7 In this article, we provide a clinical review of vismodegib for the management of BCC, including a discussion of the Hh pathway in BCC, adverse effects of vismodegib, use of vismodegib in adnexal skin tumors, recommended doses for vismodegib therapy in BCC, and management of the side effects of treatment.
Hh Pathway in BCC
In embryonic development, the Hh signaling pathway is crucial across a broad spectrum of species, including humans. Various members of the Hh family have been recognized, all working as secreted regulatory proteins.8 The name of the Hh signaling pathway is derived from a polypeptide ligand called hedgehog found in some fruit flies. Mutations in the gene led to fruit fly larvae that had a spiky hairy pattern of denticles similar to hedgehogs, leading to the name of this molecule.9 The transmembrane protein smoothened (SMO) is the main component of the Hh signaling pathway and initiates a signaling cascade that in turn leads to an increased expression of target genes, such as GLI1. Patched (PTCH), also a transmembrane protein and a cell-surface receptor for the secreted Hh ligand, suppresses the signaling capacity of SMO. Upon binding of the Hh ligand to the PTCH receptor, the suppression of SMO is relieved and a signal is propagated, evoking a cellular response.10 Molecular and genetic studies have reported that genetic alterations in the Hh signaling pathway are almost universally present in all BCCs, leading to an aberrant activation of the pathway and an uncontrolled proliferation of the basal cells. Frequently, these alterations have been shown to cause loss of function of PTCH homologue 1, which usually acts to inhibit the SMO homologue signaling activity.11,12
Because of the potential importance of Hh signaling in other solid malignancies and the failure of topical inhibition of SMO,13 subsequent studies on the development of Hh pathway inhibitors have mostly focused on the systemic approach. A multitude of Hh pathway inhibitors have been developed thus far, such as SANT1-SANT4, GDC-0449, IPI-926, BMS-833923 (XL139), HhAntag-691, and MK-4101.14 Many of these inhibitors have been clinically investigated.13,15,16
Systemic SMO Inhibitor: Vismodegib
Vismodegib was the earliest systemic SMO inhibitor to fulfill early clinical evaluation15,16 and the first drug to receive FDA approval for the management of advanced or metastatic BCC. Vismodegib is a small-molecule SMO inhibitor used for the management of selected locally advanced BCC and metastatic BCC in adults.3,17 Although there is a possibility of recurrence following drug withdrawal, vismodegib constitutes a new therapeutic strategy presenting positive benefits to patients. It may provide superior improvement over sunitinib, which has shown efficacy in a few patients; however, the efficacy and tolerance of sunitinib have been shown to be limited.18,19
Adverse Effects of Vismodegib Therapy
Adverse events with vismodegib use have been reported in 98% of patients (N=491); most of these were mild to moderate.20 However, the frequency of adverse events could prove to be a therapeutic challenge for patients requiring extended treatment. The most frequently reported reversible side effects were muscle spasms (64%), alopecia (62%), weight loss (33%), fatigue (28%), decreased appetite (25%), diarrhea (17%), nausea (16%), dysgeusia (54%), and ageusia (22%).20 In clinical trials, amenorrhea was noticed in 30% (3/10) of females with reproductive potential.2 Apart from alopecia and possibly amenorrhea, these side effects are reversible.17 Alkeraye et al17 reported 3 clinical cases of persistent alopecia following the use of vismodegib. Amenorrhea is a possible side effect of unknown reversibility.7
Vismodegib is a pregnancy category D medication.4 Severe birth defects, including craniofacial abnormalities, retardations in normal growth, open perineum, and absence of digital fusion at a corresponding 20% of the recommended daily dose, were found in rat studies. Embryo-fetal death was noted when rats were exposedto concentrations comparable to the recommended human dose.4
Hepatic events with the use of vismodegib have been reported. The use of vismodegib in randomized controlled trials resulted in elevation of both alanine aminotransferase and aspartate aminotransferase levels compared with placebo.21 Moreover, severe hepatotoxicity with vismodegib has been reported.22-24 A study conducted by Edwards et al25 concluded that the use of vismodegib in patients with severe liver disease must include thorough risk-benefit assessment, with caution in using other concomitant hepatotoxic medications.
Rare adverse events also have been reported in the literature, including vismodegib-induced pancreatitis in a 79-year-old patient treated for locally advanced, recurrent BCC that was cleared following cessation of therapy.26 Additionally, atypical fibroxanthoma was observed in an 83-year-old patient after 30 days of treatment with vismodegib for multiple BCCs.27 The development of other secondary malignancies, such as squamous cell carcinoma, melanoma, keratoacanthomas, and pilomatricomas, during or after the long-term use of vismodegib also have been described.28-35
Use of Vismodegib for Adnexal Skin Tumors
The role of the sonic Hh–PTCH pathway in the pathogenesis of adnexal tumors varies in the literature. Some studies propose the involvement of this pathway in the formation of adnexal tumors such as trichoblastoma, trichoepithelioma, and cylindroma, as in BCC. Various lines of evidence support this involvement. Firstly, in mice, the spontaneous generation of numerous BCCs, trichoblastomas, trichoepitheliomas, and cylindromas has been observed following constitutive activation of the sonic Hh–PTCH pathway.36 Secondly, in trichoepitheliomas, there have been positive results in molecular research into the tumor suppressor gene PTCH homologue 1, PTCH1, whose mutations cause constitutive activation of the sonic Hh–PTCH pathway.37 Thirdly, GLI138 and SOX939 transcription factors associated with the signaling pathway of sonic Hh–PTCH appear to have increased levels in adnexal carcinomas.19 Lepesant et al19 reported a notable clinical response to vismodegib in trichoblastic carcinoma. Baur et al40 reported successful treatment of multiple familial trichoepitheliomas with vismodegib. Nonetheless, more studies are required to assess the efficacy and reliability of vismodegib in the management of adnexal tumors.
Recommended Dose of Vismodegib Therapy
The vismodegib dosage that is approved by the FDA is 150 mg/d until disease progression or the development of intolerable side effects.4 Higher dosing regimens were evaluated with 270 mg/d and 540 mg/d. No added therapeutic benefit was noted with the increase in the dose, and no dose-limiting toxic effects were observed.41
Management of Vismodegib Side Effects
Managing patient expectations is a crucial step in improving dysgeusia. The experience of dysgeusia varies among patients; thus, patients should be instructed to adjust their diets according to their level of dysgeusia, which can be achieved by changing ingredients or dressings used with their diet. This step has been proven to be effective in overcoming vismodegib-related dysgeusia. Also, fluid taste distortion may lead to dehydration and an increase in creatine level. Thus, patients should be encouraged to monitor fluid intake. Moreover, a treatment hiatus of 2 to 8 months results in near-complete improvement of taste distortion.
For muscle spasms, quinine, treatment break for 1 month, gentle exercise of affected areas, or muscle relaxants such as baclofen and temazepam all are effective methods. For vismodegib-related alopecia, managing patient expectations is key; patients should be aware that hair may take 6 to 12 months or even longer to regrow. In addition, shaving less frequently helps improve alopecia.
For gastrointestinal disorders, loperamide with or without codeine phosphate is effective in resolving diarrhea, and metoclopramide is mostly adequate in treating nausea. Another adverse event is weight loss; weight loss of 5% or more of total body weight prompts dietetic referral. If weight loss persists, a treatment break might be needed to regain weight.
Overall, treatment breaks are sufficient to resolve adverse events caused by vismodegib and do not compromise efficacy of treatment. The duration of a treatment break should be considered before initiation. In one clinical trial, a longer treatment break was associated with fewer adverse effects without affecting the efficacy of treatment.42
Conclusion
Vismodegib provides an effective alternative to surgical intervention in the management of BCC. However, patients must be monitored vigilantly, as adverse events are common (>90%).
Basal cell carcinomas (BCCs) are considered the most common cutaneous cancers. Approximately 80% of nonmelanoma skin cancers are BCCs.1,2 Surgical management is the gold standard for early-stage and localized BCCs; it may include simple excision vs Mohs micrographic surgery.3,4 However, if left untreated, these lesions can progress to an advanced stage (locally advanced BCC) or infrequently may spread to distant sites (metastatic BCC). In the advanced stage, the lesions are no longer manageable by surgery or radiation therapy.5,6 Recently, inhibitors targeting the hedgehog (Hh) pathway have shown great promise for these patients. The first drug approved by the US Food and Drug Administration (FDA) for locally advanced and metastatic BCC is vismodegib.7 In this article, we provide a clinical review of vismodegib for the management of BCC, including a discussion of the Hh pathway in BCC, adverse effects of vismodegib, use of vismodegib in adnexal skin tumors, recommended doses for vismodegib therapy in BCC, and management of the side effects of treatment.
Hh Pathway in BCC
In embryonic development, the Hh signaling pathway is crucial across a broad spectrum of species, including humans. Various members of the Hh family have been recognized, all working as secreted regulatory proteins.8 The name of the Hh signaling pathway is derived from a polypeptide ligand called hedgehog found in some fruit flies. Mutations in the gene led to fruit fly larvae that had a spiky hairy pattern of denticles similar to hedgehogs, leading to the name of this molecule.9 The transmembrane protein smoothened (SMO) is the main component of the Hh signaling pathway and initiates a signaling cascade that in turn leads to an increased expression of target genes, such as GLI1. Patched (PTCH), also a transmembrane protein and a cell-surface receptor for the secreted Hh ligand, suppresses the signaling capacity of SMO. Upon binding of the Hh ligand to the PTCH receptor, the suppression of SMO is relieved and a signal is propagated, evoking a cellular response.10 Molecular and genetic studies have reported that genetic alterations in the Hh signaling pathway are almost universally present in all BCCs, leading to an aberrant activation of the pathway and an uncontrolled proliferation of the basal cells. Frequently, these alterations have been shown to cause loss of function of PTCH homologue 1, which usually acts to inhibit the SMO homologue signaling activity.11,12
Because of the potential importance of Hh signaling in other solid malignancies and the failure of topical inhibition of SMO,13 subsequent studies on the development of Hh pathway inhibitors have mostly focused on the systemic approach. A multitude of Hh pathway inhibitors have been developed thus far, such as SANT1-SANT4, GDC-0449, IPI-926, BMS-833923 (XL139), HhAntag-691, and MK-4101.14 Many of these inhibitors have been clinically investigated.13,15,16
Systemic SMO Inhibitor: Vismodegib
Vismodegib was the earliest systemic SMO inhibitor to fulfill early clinical evaluation15,16 and the first drug to receive FDA approval for the management of advanced or metastatic BCC. Vismodegib is a small-molecule SMO inhibitor used for the management of selected locally advanced BCC and metastatic BCC in adults.3,17 Although there is a possibility of recurrence following drug withdrawal, vismodegib constitutes a new therapeutic strategy presenting positive benefits to patients. It may provide superior improvement over sunitinib, which has shown efficacy in a few patients; however, the efficacy and tolerance of sunitinib have been shown to be limited.18,19
Adverse Effects of Vismodegib Therapy
Adverse events with vismodegib use have been reported in 98% of patients (N=491); most of these were mild to moderate.20 However, the frequency of adverse events could prove to be a therapeutic challenge for patients requiring extended treatment. The most frequently reported reversible side effects were muscle spasms (64%), alopecia (62%), weight loss (33%), fatigue (28%), decreased appetite (25%), diarrhea (17%), nausea (16%), dysgeusia (54%), and ageusia (22%).20 In clinical trials, amenorrhea was noticed in 30% (3/10) of females with reproductive potential.2 Apart from alopecia and possibly amenorrhea, these side effects are reversible.17 Alkeraye et al17 reported 3 clinical cases of persistent alopecia following the use of vismodegib. Amenorrhea is a possible side effect of unknown reversibility.7
Vismodegib is a pregnancy category D medication.4 Severe birth defects, including craniofacial abnormalities, retardations in normal growth, open perineum, and absence of digital fusion at a corresponding 20% of the recommended daily dose, were found in rat studies. Embryo-fetal death was noted when rats were exposedto concentrations comparable to the recommended human dose.4
Hepatic events with the use of vismodegib have been reported. The use of vismodegib in randomized controlled trials resulted in elevation of both alanine aminotransferase and aspartate aminotransferase levels compared with placebo.21 Moreover, severe hepatotoxicity with vismodegib has been reported.22-24 A study conducted by Edwards et al25 concluded that the use of vismodegib in patients with severe liver disease must include thorough risk-benefit assessment, with caution in using other concomitant hepatotoxic medications.
Rare adverse events also have been reported in the literature, including vismodegib-induced pancreatitis in a 79-year-old patient treated for locally advanced, recurrent BCC that was cleared following cessation of therapy.26 Additionally, atypical fibroxanthoma was observed in an 83-year-old patient after 30 days of treatment with vismodegib for multiple BCCs.27 The development of other secondary malignancies, such as squamous cell carcinoma, melanoma, keratoacanthomas, and pilomatricomas, during or after the long-term use of vismodegib also have been described.28-35
Use of Vismodegib for Adnexal Skin Tumors
The role of the sonic Hh–PTCH pathway in the pathogenesis of adnexal tumors varies in the literature. Some studies propose the involvement of this pathway in the formation of adnexal tumors such as trichoblastoma, trichoepithelioma, and cylindroma, as in BCC. Various lines of evidence support this involvement. Firstly, in mice, the spontaneous generation of numerous BCCs, trichoblastomas, trichoepitheliomas, and cylindromas has been observed following constitutive activation of the sonic Hh–PTCH pathway.36 Secondly, in trichoepitheliomas, there have been positive results in molecular research into the tumor suppressor gene PTCH homologue 1, PTCH1, whose mutations cause constitutive activation of the sonic Hh–PTCH pathway.37 Thirdly, GLI138 and SOX939 transcription factors associated with the signaling pathway of sonic Hh–PTCH appear to have increased levels in adnexal carcinomas.19 Lepesant et al19 reported a notable clinical response to vismodegib in trichoblastic carcinoma. Baur et al40 reported successful treatment of multiple familial trichoepitheliomas with vismodegib. Nonetheless, more studies are required to assess the efficacy and reliability of vismodegib in the management of adnexal tumors.
Recommended Dose of Vismodegib Therapy
The vismodegib dosage that is approved by the FDA is 150 mg/d until disease progression or the development of intolerable side effects.4 Higher dosing regimens were evaluated with 270 mg/d and 540 mg/d. No added therapeutic benefit was noted with the increase in the dose, and no dose-limiting toxic effects were observed.41
Management of Vismodegib Side Effects
Managing patient expectations is a crucial step in improving dysgeusia. The experience of dysgeusia varies among patients; thus, patients should be instructed to adjust their diets according to their level of dysgeusia, which can be achieved by changing ingredients or dressings used with their diet. This step has been proven to be effective in overcoming vismodegib-related dysgeusia. Also, fluid taste distortion may lead to dehydration and an increase in creatine level. Thus, patients should be encouraged to monitor fluid intake. Moreover, a treatment hiatus of 2 to 8 months results in near-complete improvement of taste distortion.
For muscle spasms, quinine, treatment break for 1 month, gentle exercise of affected areas, or muscle relaxants such as baclofen and temazepam all are effective methods. For vismodegib-related alopecia, managing patient expectations is key; patients should be aware that hair may take 6 to 12 months or even longer to regrow. In addition, shaving less frequently helps improve alopecia.
For gastrointestinal disorders, loperamide with or without codeine phosphate is effective in resolving diarrhea, and metoclopramide is mostly adequate in treating nausea. Another adverse event is weight loss; weight loss of 5% or more of total body weight prompts dietetic referral. If weight loss persists, a treatment break might be needed to regain weight.
Overall, treatment breaks are sufficient to resolve adverse events caused by vismodegib and do not compromise efficacy of treatment. The duration of a treatment break should be considered before initiation. In one clinical trial, a longer treatment break was associated with fewer adverse effects without affecting the efficacy of treatment.42
Conclusion
Vismodegib provides an effective alternative to surgical intervention in the management of BCC. However, patients must be monitored vigilantly, as adverse events are common (>90%).
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Cirrone F, Harris CS. Vismodegib and the hedgehog pathway: a new treatment for basal cell carcinoma. Clin Ther. 2012;34:2039-2050.
- Ruiz-Salas V, Alegre M, López-Ferrer A, et al. Vismodegib: a review [article in English, Spanish]. Actas Dermosifiliogr. 2014;105:744-751.
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Cusack CA, Nijhawan R, Miller B, et al. Vismodegib for locally advanced basal cell carcinoma in a heart transplant patient. JAMA Dermatol. 2015;151:70-72.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885-888.
- Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46:3-12.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103-115.
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434.
- Sekulic A, Mangold AR, Northfelt DW, et al. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218-223.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nature Rev Genet. 2006;7:841-850.
- Alkeraye S, Maire C, Desmedt E, et al. Persistent alopecia induced by vismodegib. Br J Dermatol. 2015;172:1671-1672.
- Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24:199-203.
- Lepesant P, Crinquette M, Alkeraye S, et al. Vismodegib induces significant clinical response in locally advanced trichoblastic carcinoma. Br J Dermatol. 2015;173:1059-1062.
- Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-plannedinterim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284-4292.
- Sanchez BE, Hajjafar L. Severe hepatotoxicity in a patient treated with hedgehog inhibitor: first case report. Gastroenterology. 2011;140:S974-S975.
- Ly P, Wolf K, Wilson J. A case of hepatotoxicity associated with vismodegib. JAAD Case Rep. 2018;5:57-59.
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70.
- Edwards BJ, Raisch DW, Saraykar SS, et al. Hepatotoxicity with vismodegib: an MD Anderson Cancer Center and Research on Adverse Drug Events and Reports Project. Drugs R D. 2017;17:211-218.
- Velter C, Blanc J, Robert C. Acute pancreatitis after vismodegib for basal cell carcinoma: a causal relation? Eur J Cancer. 2019;118:67-69.
- Giorgini C, Barbaccia V, Croci GA, et al. Rapid development of atypical fibroxanthoma during vismodegib treatment. Clin Exp Dermatol. 2019;44:86-88.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Zhu GA, Sundram U, Chang ALS. Two different scenarios of squamous cell carcinoma within advanced basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol. 2014;150:970-973.
- Poulalhon N, Dalle S, Balme B, et al. Fast-growing cutaneous squamous cell carcinoma in a patient treated with vismodegib. Dermatology. 2015;230:101-104.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:E33-E34.
- Giuffrida R, Kashofer K, Dika E, et al. Fast growing melanoma following treatment with vismodegib for locally advanced basal cell carcinomas: report of two cases. Eur J Cancer. 2018;91:177-179.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Magdaleno-Tapial J, Valenzuela-Oñate C, Ortiz-Salvador JM, et al. Pilomatricomas secondary to treatment with vismodegib. JAAD Case Rep. 2018;5:12-14.
- Nilsson M, Undèn AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438-3443.
- Vorechovský I, Undén AB, Sandstedt B, et al. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57:4677-4681.
- Hatta N, Hirano T, Kimura T, et al. Molecular diagnosis of basal cell carcinoma and other basaloid cell neoplasms of the skin by the quantification of Gli1 transcript levels. J Cutan Pathol. 2005;32:131-136.
- Vidal VP, Ortonne N, Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. 2008;35:373-379.
- Baur V, Papadopoulos T, Kazakov DV, et al. A case of multiple familial trichoepitheliomas responding to treatment with the hedgehog signaling pathway inhibitor vismodegib. Virchows Arch. 2018;473:241-246.
- LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502-2511.
- Fife K, Herd R, Lalondrelle S, et al. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017;13:175-184.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of nonmelanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283-287.
- Von Hoff DD, LoRusso PM, Rudin CM, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361:1164-1172.
- Cirrone F, Harris CS. Vismodegib and the hedgehog pathway: a new treatment for basal cell carcinoma. Clin Ther. 2012;34:2039-2050.
- Ruiz-Salas V, Alegre M, López-Ferrer A, et al. Vismodegib: a review [article in English, Spanish]. Actas Dermosifiliogr. 2014;105:744-751.
- Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Cusack CA, Nijhawan R, Miller B, et al. Vismodegib for locally advanced basal cell carcinoma in a heart transplant patient. JAMA Dermatol. 2015;151:70-72.
- Aszterbaum M, Rothman A, Johnson RL, et al. Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885-888.
- Abidi A. Hedgehog signaling pathway: a novel target for cancer therapy: vismodegib, a promising therapeutic option in treatment of basal cell carcinomas. Indian J Pharmacol. 2014;46:3-12.
- St-Jacques B, Dassule HR, Karavanova I, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058-1068.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Hall JM, Bell ML, Finger TE. Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol. 2003;255:263-277.
- Bai CB, Stephen D, Joyner AL. All mouse ventral spinal cord patterning by hedgehog is Gli dependent and involves an activator function of Gli3. Dev Cell. 2004;6:103-115.
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423-434.
- Sekulic A, Mangold AR, Northfelt DW, et al. Advanced basal cell carcinoma of the skin: targeting the hedgehog pathway. Curr Opin Oncol. 2013;25:218-223.
- Ingham PW, Placzek M. Orchestrating ontogenesis: variations on a theme by sonic hedgehog. Nature Rev Genet. 2006;7:841-850.
- Alkeraye S, Maire C, Desmedt E, et al. Persistent alopecia induced by vismodegib. Br J Dermatol. 2015;172:1671-1672.
- Battistella M, Mateus C, Lassau N, et al. Sunitinib efficacy in the treatment of metastatic skin adnexal carcinomas: report of two patients with hidradenocarcinoma and trichoblastic carcinoma. J Eur Acad Dermatol Venereol. 2010;24:199-203.
- Lepesant P, Crinquette M, Alkeraye S, et al. Vismodegib induces significant clinical response in locally advanced trichoblastic carcinoma. Br J Dermatol. 2015;173:1059-1062.
- Basset-Seguin N, Hauschild A, Grob JJ, et al. Vismodegib in patients with advanced basal cell carcinoma (STEVIE): a pre-plannedinterim analysis of an international, open-label trial. Lancet Oncol. 2015;16:729-736.
- Catenacci DV, Junttila MR, Karrison T, et al. Randomized phase Ib/II study of gemcitabine plus placebo or vismodegib, a hedgehog pathway inhibitor, in patients with metastatic pancreatic cancer. J Clin Oncol. 2015;33:4284-4292.
- Sanchez BE, Hajjafar L. Severe hepatotoxicity in a patient treated with hedgehog inhibitor: first case report. Gastroenterology. 2011;140:S974-S975.
- Ly P, Wolf K, Wilson J. A case of hepatotoxicity associated with vismodegib. JAAD Case Rep. 2018;5:57-59.
- Eiger-Moscovich M, Reich E, Tauber G, et al. Efficacy of vismodegib for the treatment of orbital and advanced periocular basal cell carcinoma. Am J Ophthalmol. 2019;207:62-70.
- Edwards BJ, Raisch DW, Saraykar SS, et al. Hepatotoxicity with vismodegib: an MD Anderson Cancer Center and Research on Adverse Drug Events and Reports Project. Drugs R D. 2017;17:211-218.
- Velter C, Blanc J, Robert C. Acute pancreatitis after vismodegib for basal cell carcinoma: a causal relation? Eur J Cancer. 2019;118:67-69.
- Giorgini C, Barbaccia V, Croci GA, et al. Rapid development of atypical fibroxanthoma during vismodegib treatment. Clin Exp Dermatol. 2019;44:86-88.
- Saintes C, Saint-Jean M, Brocard A, et al. Development of squamous cell carcinoma into basal cell carcinoma under treatment with vismodegib. J Eur Acad Dermatol Venereol. 2015;29:1006-1009.
- Zhu GA, Sundram U, Chang ALS. Two different scenarios of squamous cell carcinoma within advanced basal cell carcinomas: cases illustrating the importance of serial biopsy during vismodegib usage. JAMA Dermatol. 2014;150:970-973.
- Poulalhon N, Dalle S, Balme B, et al. Fast-growing cutaneous squamous cell carcinoma in a patient treated with vismodegib. Dermatology. 2015;230:101-104.
- Orouji A, Goerdt S, Utikal J, et al. Multiple highly and moderately differentiated squamous cell carcinomas of the skin during vismodegib treatment of inoperable basal cell carcinoma. Br J Dermatol. 2014;171:431-433.
- Iarrobino A, Messina JL, Kudchadkar R, et al. Emergence of a squamous cell carcinoma phenotype following treatment of metastatic basal cell carcinoma with vismodegib. J Am Acad Dermatol. 2013;69:E33-E34.
- Giuffrida R, Kashofer K, Dika E, et al. Fast growing melanoma following treatment with vismodegib for locally advanced basal cell carcinomas: report of two cases. Eur J Cancer. 2018;91:177-179.
- Aasi S, Silkiss R, Tang JY, et al. New onset of keratoacanthomas after vismodegib treatment for locally advanced basal cell carcinomas: a report of 2 cases. JAMA Dermatol. 2013;149:242-243.
- Magdaleno-Tapial J, Valenzuela-Oñate C, Ortiz-Salvador JM, et al. Pilomatricomas secondary to treatment with vismodegib. JAAD Case Rep. 2018;5:12-14.
- Nilsson M, Undèn AB, Krause D, et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438-3443.
- Vorechovský I, Undén AB, Sandstedt B, et al. Trichoepitheliomas contain somatic mutations in the overexpressed PTCH gene: support for a gatekeeper mechanism in skin tumorigenesis. Cancer Res. 1997;57:4677-4681.
- Hatta N, Hirano T, Kimura T, et al. Molecular diagnosis of basal cell carcinoma and other basaloid cell neoplasms of the skin by the quantification of Gli1 transcript levels. J Cutan Pathol. 2005;32:131-136.
- Vidal VP, Ortonne N, Schedl A. SOX9 expression is a general marker of basal cell carcinoma and adnexal-related neoplasms. J Cutan Pathol. 2008;35:373-379.
- Baur V, Papadopoulos T, Kazakov DV, et al. A case of multiple familial trichoepitheliomas responding to treatment with the hedgehog signaling pathway inhibitor vismodegib. Virchows Arch. 2018;473:241-246.
- LoRusso PM, Rudin CM, Reddy JC, et al. Phase I trial of hedgehog pathway inhibitor vismodegib (GDC-0449) in patients with refractory, locally advanced or metastatic solid tumors. Clin Cancer Res. 2011;17:2502-2511.
- Fife K, Herd R, Lalondrelle S, et al. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Future Oncol. 2017;13:175-184.
Practice Points
- The recommended dosage of vismodegib is 150 mg/d until unendurable side effects develop or disease progression occurs.
- The efficacy of vismodegib in the management of locally advanced basal cell carcinoma (BCC) and metastatic BCC is promising. Thus, it is now considered an effective substitute to surgical therapy.
- Patients using vismodegib must be closely monitored, as it is commonly associated with adverse events.
Surgical Deroofing for Hidradenitis Suppurativa
Practice Gap
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by inflammatory nodules, abscesses, sinus tracts, fistulae, and scarring, mainly in intertriginous areas. The extent of disease—classified using the Hurley staging system (stages I–III)—helps guide treatment, which includes medical management and surgical intervention in later stages.
First-line treatment of HS includes topical or systemic medications, or both. Surgical therapy typically is reserved for refractory HS in moderate to severe disease (Hurley stages II and III) and is combined with pharmacotherapy. Specifically, clinical management guidelines issued by an expert committee of the United States and Canadian Hidradenitis Suppurativa Foundations recommend excision or deroofing for recurrent nodules and tunnels.1
Surgical options for HS that are available to the outpatient dermatologist include incision and drainage, electrosurgery, CO2 laser evaporation, excision, and deroofing (also known as unroofing).2 Deroofing is a fairly novel therapy; many dermatologists are unfamiliar with the procedure. A PubMed search of articles indexed for MEDLINE related to HS prior to 2010 revealed only 1 article containing the word deroofing and only 4 articles containing unroofing.
The pathophysiology of HS has important implications for successful treatment. Inflammation of the follicular pilosebaceous unit along with follicular occlusion create challenges with treatment.3 It is postulated that a defect in the glassy membrane of the infra-infundibular wall predisposes the pilosebaceous follicle to lose its structural integrality as pressure builds from plugging of the duct,4 which can result in the clinical hallmarks of HS including tunneling tracts, bridging nodules, abscesses, and fistulae that form with lateral expansion of the plugged follicle.
Leaking of the contents of these plugged follicles into surrounding tissue produces an inflammatory response in characteristic HS lesions. Because debris within the lesions moves laterally instead of being able to burst to the surface, the lesions have difficulty fully healing. Unroofing the lesions and removing built-up debris allows them to heal more expediently and quiets the underlying immune response by removing the stimulus.4
Herein, we describe the benefits, risks, and surgical process of deroofing for HS.
Technique and Tools
Deroofing is performed under local anesthesia, stepwise as follows:
1. Identify sinus tracts and infiltrate the area with lidocaine (Figure, A).
2. Use a blunt probe to define the borders of the area to be unroofed and to evaluate for any communicating sinus tracts (Figure, B).
3. Remove the roof of underlying abscesses and tracts, using a probe as a guide (Figure, C).
4. Enter through the skin or sinus opening using electrocautery or with a scalpel or scissors; perform blunt dissection.
5. Reflect back the entirety of skin overlying the probed areas and remove the skin to expose the base of the lesion (Figure, D).
6. Explore the exposed base and walls of the lesion with the probe again to assess for hidden tracts; take care not to create false tracts.
7. Debride the surgical wound using curettage or rough gauze grattage to remove remaining inflammatory debris or biofilm. To achieve hemostasis, apply aluminum chloride or ferric chloride. Coat the wound with petroleum jelly and gauze and allow it to heal by secondary intention.
8. Educate the patient on wound care—once-daily gentle cleansing with soap and water, followed by application of a moist dressing—which is similar to wound healing by secondary intention from other causes.2,4

Practice Implications
A deroofing procedure has many benefits compared to other surgical modalities for the treatment of HS. Deroofing requires only a probe, curette, and electrocautery device, making the procedure more cost-effective than excision, which requires a full tray of equipment and sutures. Furthermore, margins do not need to be taken with deroofing, and no undermining or closure is needed, which saves time during the operation and minimizes the risk for complications, including dehiscence and formation of new sinus tracts.4 No specialized equipment, such as a CO2 laser, is required, which makes deroofing accessible to every clinical dermatologist in any demographic or geographic setting.
Evidence of Benefit—Saylor and colleagues5 found that deroofing carries a 12.5% complication rate, which includes postoperative bleeding, hypergranulation tissue, and rarely wound infection. This rate is significantly lower than the 26% complication rate associated with local excision, which includes wound dehiscence, infection, and contracture (P<.001). Deroofing also was found to have an HS recurrence rate of 14.5%, which is significantly less than the 30% recurrence rate seen with local excision (P=.015). Saylor et al5 also concluded that incision and drainage was recommended only for immediate relief of HS because of its 100% recurrence rate.
van der Zee2 reported on 88 lesions from 44 patients that were treated by surgical deroofing, resulting in an average defect of 3.0 cm in length and a mean healing time of 14 days. The typical outcome was cosmetically acceptable scarring; this finding was supported by a postoperative survey (>1 year), to which 37 of 44 patients responded and assigned an average satisfaction score of 8 (of a possible 10) and a recommendation rate of 90%.2
Procedural Coding—Specific Current Procedural Terminology codes (11450-11471) from the International Classification of Diseases, Tenth Revision, exist for HS deroofing procedures; the applicable code for a given case depends on the final length of the surgical defect. Documentation to support these codes is similar to the note for an excision procedure, taking care to include location, depth, and length of the excision; healing by secondary intention; and the diagnosis of HS.
Final Thoughts
Deroofing is a surgical option that can be beneficial to patients with HS. It is a relatively simple procedure available to any dermatologist, regardless of setting. We encourage dermatologists to consider deroofing, even in patients with Hurley stage II lesions, because it can yield cosmetically acceptable and definitive results, given the variety of therapies available for HS. Deroofing also can be superior to standard excision, especially because of the potential complications with standard excision and quicker operative time with deroofing. As more providers become familiar with the deroofing procedure for HS, further studies can be undertaken to add to the paucity of data about deroofing and how it compares to other surgical treatments.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi:10.1016/j.jaad.2009.12.018
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Danby FW. Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010;63:481.e1-481.e3. doi:10.1016/j.jaad.2010.01.033
- Saylor DK, Brownstone ND, Naik HB. Office-based surgical intervention for hidradenitis suppurativa (HS): a focused review for dermatologists. Dermatol Ther (Heidelb). 2020;10:529-549. doi:10.1007/s13555-020-00391-x
Practice Gap
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by inflammatory nodules, abscesses, sinus tracts, fistulae, and scarring, mainly in intertriginous areas. The extent of disease—classified using the Hurley staging system (stages I–III)—helps guide treatment, which includes medical management and surgical intervention in later stages.
First-line treatment of HS includes topical or systemic medications, or both. Surgical therapy typically is reserved for refractory HS in moderate to severe disease (Hurley stages II and III) and is combined with pharmacotherapy. Specifically, clinical management guidelines issued by an expert committee of the United States and Canadian Hidradenitis Suppurativa Foundations recommend excision or deroofing for recurrent nodules and tunnels.1
Surgical options for HS that are available to the outpatient dermatologist include incision and drainage, electrosurgery, CO2 laser evaporation, excision, and deroofing (also known as unroofing).2 Deroofing is a fairly novel therapy; many dermatologists are unfamiliar with the procedure. A PubMed search of articles indexed for MEDLINE related to HS prior to 2010 revealed only 1 article containing the word deroofing and only 4 articles containing unroofing.
The pathophysiology of HS has important implications for successful treatment. Inflammation of the follicular pilosebaceous unit along with follicular occlusion create challenges with treatment.3 It is postulated that a defect in the glassy membrane of the infra-infundibular wall predisposes the pilosebaceous follicle to lose its structural integrality as pressure builds from plugging of the duct,4 which can result in the clinical hallmarks of HS including tunneling tracts, bridging nodules, abscesses, and fistulae that form with lateral expansion of the plugged follicle.
Leaking of the contents of these plugged follicles into surrounding tissue produces an inflammatory response in characteristic HS lesions. Because debris within the lesions moves laterally instead of being able to burst to the surface, the lesions have difficulty fully healing. Unroofing the lesions and removing built-up debris allows them to heal more expediently and quiets the underlying immune response by removing the stimulus.4
Herein, we describe the benefits, risks, and surgical process of deroofing for HS.
Technique and Tools
Deroofing is performed under local anesthesia, stepwise as follows:
1. Identify sinus tracts and infiltrate the area with lidocaine (Figure, A).
2. Use a blunt probe to define the borders of the area to be unroofed and to evaluate for any communicating sinus tracts (Figure, B).
3. Remove the roof of underlying abscesses and tracts, using a probe as a guide (Figure, C).
4. Enter through the skin or sinus opening using electrocautery or with a scalpel or scissors; perform blunt dissection.
5. Reflect back the entirety of skin overlying the probed areas and remove the skin to expose the base of the lesion (Figure, D).
6. Explore the exposed base and walls of the lesion with the probe again to assess for hidden tracts; take care not to create false tracts.
7. Debride the surgical wound using curettage or rough gauze grattage to remove remaining inflammatory debris or biofilm. To achieve hemostasis, apply aluminum chloride or ferric chloride. Coat the wound with petroleum jelly and gauze and allow it to heal by secondary intention.
8. Educate the patient on wound care—once-daily gentle cleansing with soap and water, followed by application of a moist dressing—which is similar to wound healing by secondary intention from other causes.2,4

Practice Implications
A deroofing procedure has many benefits compared to other surgical modalities for the treatment of HS. Deroofing requires only a probe, curette, and electrocautery device, making the procedure more cost-effective than excision, which requires a full tray of equipment and sutures. Furthermore, margins do not need to be taken with deroofing, and no undermining or closure is needed, which saves time during the operation and minimizes the risk for complications, including dehiscence and formation of new sinus tracts.4 No specialized equipment, such as a CO2 laser, is required, which makes deroofing accessible to every clinical dermatologist in any demographic or geographic setting.
Evidence of Benefit—Saylor and colleagues5 found that deroofing carries a 12.5% complication rate, which includes postoperative bleeding, hypergranulation tissue, and rarely wound infection. This rate is significantly lower than the 26% complication rate associated with local excision, which includes wound dehiscence, infection, and contracture (P<.001). Deroofing also was found to have an HS recurrence rate of 14.5%, which is significantly less than the 30% recurrence rate seen with local excision (P=.015). Saylor et al5 also concluded that incision and drainage was recommended only for immediate relief of HS because of its 100% recurrence rate.
van der Zee2 reported on 88 lesions from 44 patients that were treated by surgical deroofing, resulting in an average defect of 3.0 cm in length and a mean healing time of 14 days. The typical outcome was cosmetically acceptable scarring; this finding was supported by a postoperative survey (>1 year), to which 37 of 44 patients responded and assigned an average satisfaction score of 8 (of a possible 10) and a recommendation rate of 90%.2
Procedural Coding—Specific Current Procedural Terminology codes (11450-11471) from the International Classification of Diseases, Tenth Revision, exist for HS deroofing procedures; the applicable code for a given case depends on the final length of the surgical defect. Documentation to support these codes is similar to the note for an excision procedure, taking care to include location, depth, and length of the excision; healing by secondary intention; and the diagnosis of HS.
Final Thoughts
Deroofing is a surgical option that can be beneficial to patients with HS. It is a relatively simple procedure available to any dermatologist, regardless of setting. We encourage dermatologists to consider deroofing, even in patients with Hurley stage II lesions, because it can yield cosmetically acceptable and definitive results, given the variety of therapies available for HS. Deroofing also can be superior to standard excision, especially because of the potential complications with standard excision and quicker operative time with deroofing. As more providers become familiar with the deroofing procedure for HS, further studies can be undertaken to add to the paucity of data about deroofing and how it compares to other surgical treatments.
Practice Gap
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by inflammatory nodules, abscesses, sinus tracts, fistulae, and scarring, mainly in intertriginous areas. The extent of disease—classified using the Hurley staging system (stages I–III)—helps guide treatment, which includes medical management and surgical intervention in later stages.
First-line treatment of HS includes topical or systemic medications, or both. Surgical therapy typically is reserved for refractory HS in moderate to severe disease (Hurley stages II and III) and is combined with pharmacotherapy. Specifically, clinical management guidelines issued by an expert committee of the United States and Canadian Hidradenitis Suppurativa Foundations recommend excision or deroofing for recurrent nodules and tunnels.1
Surgical options for HS that are available to the outpatient dermatologist include incision and drainage, electrosurgery, CO2 laser evaporation, excision, and deroofing (also known as unroofing).2 Deroofing is a fairly novel therapy; many dermatologists are unfamiliar with the procedure. A PubMed search of articles indexed for MEDLINE related to HS prior to 2010 revealed only 1 article containing the word deroofing and only 4 articles containing unroofing.
The pathophysiology of HS has important implications for successful treatment. Inflammation of the follicular pilosebaceous unit along with follicular occlusion create challenges with treatment.3 It is postulated that a defect in the glassy membrane of the infra-infundibular wall predisposes the pilosebaceous follicle to lose its structural integrality as pressure builds from plugging of the duct,4 which can result in the clinical hallmarks of HS including tunneling tracts, bridging nodules, abscesses, and fistulae that form with lateral expansion of the plugged follicle.
Leaking of the contents of these plugged follicles into surrounding tissue produces an inflammatory response in characteristic HS lesions. Because debris within the lesions moves laterally instead of being able to burst to the surface, the lesions have difficulty fully healing. Unroofing the lesions and removing built-up debris allows them to heal more expediently and quiets the underlying immune response by removing the stimulus.4
Herein, we describe the benefits, risks, and surgical process of deroofing for HS.
Technique and Tools
Deroofing is performed under local anesthesia, stepwise as follows:
1. Identify sinus tracts and infiltrate the area with lidocaine (Figure, A).
2. Use a blunt probe to define the borders of the area to be unroofed and to evaluate for any communicating sinus tracts (Figure, B).
3. Remove the roof of underlying abscesses and tracts, using a probe as a guide (Figure, C).
4. Enter through the skin or sinus opening using electrocautery or with a scalpel or scissors; perform blunt dissection.
5. Reflect back the entirety of skin overlying the probed areas and remove the skin to expose the base of the lesion (Figure, D).
6. Explore the exposed base and walls of the lesion with the probe again to assess for hidden tracts; take care not to create false tracts.
7. Debride the surgical wound using curettage or rough gauze grattage to remove remaining inflammatory debris or biofilm. To achieve hemostasis, apply aluminum chloride or ferric chloride. Coat the wound with petroleum jelly and gauze and allow it to heal by secondary intention.
8. Educate the patient on wound care—once-daily gentle cleansing with soap and water, followed by application of a moist dressing—which is similar to wound healing by secondary intention from other causes.2,4

Practice Implications
A deroofing procedure has many benefits compared to other surgical modalities for the treatment of HS. Deroofing requires only a probe, curette, and electrocautery device, making the procedure more cost-effective than excision, which requires a full tray of equipment and sutures. Furthermore, margins do not need to be taken with deroofing, and no undermining or closure is needed, which saves time during the operation and minimizes the risk for complications, including dehiscence and formation of new sinus tracts.4 No specialized equipment, such as a CO2 laser, is required, which makes deroofing accessible to every clinical dermatologist in any demographic or geographic setting.
Evidence of Benefit—Saylor and colleagues5 found that deroofing carries a 12.5% complication rate, which includes postoperative bleeding, hypergranulation tissue, and rarely wound infection. This rate is significantly lower than the 26% complication rate associated with local excision, which includes wound dehiscence, infection, and contracture (P<.001). Deroofing also was found to have an HS recurrence rate of 14.5%, which is significantly less than the 30% recurrence rate seen with local excision (P=.015). Saylor et al5 also concluded that incision and drainage was recommended only for immediate relief of HS because of its 100% recurrence rate.
van der Zee2 reported on 88 lesions from 44 patients that were treated by surgical deroofing, resulting in an average defect of 3.0 cm in length and a mean healing time of 14 days. The typical outcome was cosmetically acceptable scarring; this finding was supported by a postoperative survey (>1 year), to which 37 of 44 patients responded and assigned an average satisfaction score of 8 (of a possible 10) and a recommendation rate of 90%.2
Procedural Coding—Specific Current Procedural Terminology codes (11450-11471) from the International Classification of Diseases, Tenth Revision, exist for HS deroofing procedures; the applicable code for a given case depends on the final length of the surgical defect. Documentation to support these codes is similar to the note for an excision procedure, taking care to include location, depth, and length of the excision; healing by secondary intention; and the diagnosis of HS.
Final Thoughts
Deroofing is a surgical option that can be beneficial to patients with HS. It is a relatively simple procedure available to any dermatologist, regardless of setting. We encourage dermatologists to consider deroofing, even in patients with Hurley stage II lesions, because it can yield cosmetically acceptable and definitive results, given the variety of therapies available for HS. Deroofing also can be superior to standard excision, especially because of the potential complications with standard excision and quicker operative time with deroofing. As more providers become familiar with the deroofing procedure for HS, further studies can be undertaken to add to the paucity of data about deroofing and how it compares to other surgical treatments.
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi:10.1016/j.jaad.2009.12.018
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Danby FW. Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010;63:481.e1-481.e3. doi:10.1016/j.jaad.2010.01.033
- Saylor DK, Brownstone ND, Naik HB. Office-based surgical intervention for hidradenitis suppurativa (HS): a focused review for dermatologists. Dermatol Ther (Heidelb). 2020;10:529-549. doi:10.1007/s13555-020-00391-x
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part I: diagnosis, evaluation, and the use of complementary and procedural management. J Am Acad Dermatol. 2019;81:76-90. doi:10.1016/j.jaad.2019.02.067
- van der Zee HH, Prens EP, Boer J. Deroofing: a tissue-saving surgical technique for the treatment of mild to moderate hidradenitis suppurativa lesions. J Am Acad Dermatol. 2010;63:475-480. doi:10.1016/j.jaad.2009.12.018
- Napolitano M, Megna M, Timoshchuk EA, et al. Hidradenitis suppurativa: from pathogenesis to diagnosis and treatment. Clin Cosmet Investig Dermatol. 2017;10:105-115. doi:10.2147/CCID.S111019
- Danby FW. Commentary: unroofing for hidradenitis suppurativa, why and how. J Am Acad Dermatol. 2010;63:481.e1-481.e3. doi:10.1016/j.jaad.2010.01.033
- Saylor DK, Brownstone ND, Naik HB. Office-based surgical intervention for hidradenitis suppurativa (HS): a focused review for dermatologists. Dermatol Ther (Heidelb). 2020;10:529-549. doi:10.1007/s13555-020-00391-x
Implementation of a Bone Marrow Biopsy Clinic: Effect on Wait Times for the Procedure, Diagnosis and Treatment Initiation
Clinical Situation
Bone marrow biopsies often need to be performed expeditiously in order to alleviate patient concerns and quickly determine a diagnosis and treatment plan. However, with increasing subspecialization there are fewer hematology/oncology providers available to perform this procedure.
Literature
Our VA previously addressed this issue by having all bone marrow biopsies performed through Interventional Radiology (IR). The average time from order to procedure, though, was 18.6 days (Arfons LM, AVAHO 2016).
Intervention
A weekly bone marrow biopsy clinic was formed, utilizing a small group (heme/onc physician, nurse practitioner and key nursing staff). In collaboration with pathology, interior design, pharmacy, facilities and environmental services, a standard operating procedure was developed, which included a staffing model, procedural checklist, documentation template, scheduling and ordering system.
Outcomes/Implications
Bone marrow biopsies performed before and after initiation of the bone marrow biopsy clinic were tracked for time from order placement to: procedure being done; diagnosis rendered; and for those whose biopsy result needed therapy, initiation of treatment. From 8/4/2020 to 8/12/2021 there were 140 bone marrow biopsies performed, all through IR. The average time from order to the procedure was 23.1 days; from order to diagnosis was 27.8 days and from order to treatment was 54.8 days. After implementation of the bone marrow biopsy clinic, from 9/8/2021 to 5/25/2022 there have been 61 bone marrow biopsies performed (those ordered through IR were excluded). The average time from order to the procedure was 6.8 days; from order to diagnosis was 11.4 days and from order to treatment was 27.3 days. The differences in the average wait times for all 3 measures (time to procedure, diagnosis and treatment) were highly statistically significant (P < .001 for each), in favor of shorter wait times for those performed in the bone marrow clinic as compared to those done through IR. Implementation of a dedicated weekly bone marrow biopsy clinic significantly reduced wait times for the procedure, diagnosis and treatment initiation. This should be considered at other VA centers to improve the care of our veterans.
Clinical Situation
Bone marrow biopsies often need to be performed expeditiously in order to alleviate patient concerns and quickly determine a diagnosis and treatment plan. However, with increasing subspecialization there are fewer hematology/oncology providers available to perform this procedure.
Literature
Our VA previously addressed this issue by having all bone marrow biopsies performed through Interventional Radiology (IR). The average time from order to procedure, though, was 18.6 days (Arfons LM, AVAHO 2016).
Intervention
A weekly bone marrow biopsy clinic was formed, utilizing a small group (heme/onc physician, nurse practitioner and key nursing staff). In collaboration with pathology, interior design, pharmacy, facilities and environmental services, a standard operating procedure was developed, which included a staffing model, procedural checklist, documentation template, scheduling and ordering system.
Outcomes/Implications
Bone marrow biopsies performed before and after initiation of the bone marrow biopsy clinic were tracked for time from order placement to: procedure being done; diagnosis rendered; and for those whose biopsy result needed therapy, initiation of treatment. From 8/4/2020 to 8/12/2021 there were 140 bone marrow biopsies performed, all through IR. The average time from order to the procedure was 23.1 days; from order to diagnosis was 27.8 days and from order to treatment was 54.8 days. After implementation of the bone marrow biopsy clinic, from 9/8/2021 to 5/25/2022 there have been 61 bone marrow biopsies performed (those ordered through IR were excluded). The average time from order to the procedure was 6.8 days; from order to diagnosis was 11.4 days and from order to treatment was 27.3 days. The differences in the average wait times for all 3 measures (time to procedure, diagnosis and treatment) were highly statistically significant (P < .001 for each), in favor of shorter wait times for those performed in the bone marrow clinic as compared to those done through IR. Implementation of a dedicated weekly bone marrow biopsy clinic significantly reduced wait times for the procedure, diagnosis and treatment initiation. This should be considered at other VA centers to improve the care of our veterans.
Clinical Situation
Bone marrow biopsies often need to be performed expeditiously in order to alleviate patient concerns and quickly determine a diagnosis and treatment plan. However, with increasing subspecialization there are fewer hematology/oncology providers available to perform this procedure.
Literature
Our VA previously addressed this issue by having all bone marrow biopsies performed through Interventional Radiology (IR). The average time from order to procedure, though, was 18.6 days (Arfons LM, AVAHO 2016).
Intervention
A weekly bone marrow biopsy clinic was formed, utilizing a small group (heme/onc physician, nurse practitioner and key nursing staff). In collaboration with pathology, interior design, pharmacy, facilities and environmental services, a standard operating procedure was developed, which included a staffing model, procedural checklist, documentation template, scheduling and ordering system.
Outcomes/Implications
Bone marrow biopsies performed before and after initiation of the bone marrow biopsy clinic were tracked for time from order placement to: procedure being done; diagnosis rendered; and for those whose biopsy result needed therapy, initiation of treatment. From 8/4/2020 to 8/12/2021 there were 140 bone marrow biopsies performed, all through IR. The average time from order to the procedure was 23.1 days; from order to diagnosis was 27.8 days and from order to treatment was 54.8 days. After implementation of the bone marrow biopsy clinic, from 9/8/2021 to 5/25/2022 there have been 61 bone marrow biopsies performed (those ordered through IR were excluded). The average time from order to the procedure was 6.8 days; from order to diagnosis was 11.4 days and from order to treatment was 27.3 days. The differences in the average wait times for all 3 measures (time to procedure, diagnosis and treatment) were highly statistically significant (P < .001 for each), in favor of shorter wait times for those performed in the bone marrow clinic as compared to those done through IR. Implementation of a dedicated weekly bone marrow biopsy clinic significantly reduced wait times for the procedure, diagnosis and treatment initiation. This should be considered at other VA centers to improve the care of our veterans.