User login
Myasthenia Gravis Highlights From AANEM 2024
The latest data on myasthenia gravis (MG) research, reported at the American Association of Neuromuscular and Electrodiagnostic Medicine 2024 Annual Meeting, are presented by Dr Pushpa Narayanswami of Harvard Medical School in Boston, Massachusetts.
Dr Narayanswami begins with a safety, tolerability, and efficacy study for subcutaneous efgartigimod. Results showed that the mean change in MG activities of daily living (MG-ADL) was no different between the fixed-dose and cyclic regimens, demonstrating another dosage option for patients.
Next, Dr Narayanswami discusses two separate complement C5 inhibitor therapy trials. The first was a global registry study looking at ravulizumab. Patient cohorts consisted of those who started and remained on ravulizumab vs another that switched from initial eculizumab to ravulizumab. In both groups, MG-ADL was improved. The other study investigated zilucoplan in acetylcholine receptor autoantibody–positive generalized MG patient populations; similarly, researchers found favorable results.
She then details a study looking at the safety outcomes in pregnant patients treated with eculizumab. Because of limited disease-specific data in the registry, further investigation is recommended.
Finally, Dr Narayanaswami examines results for inebilizumab, a first-in-class anti-CD19 B cell–depleting agent. The drug demonstrated safety and beneficial efficacy compared with placebo in seropositive generalized MG patients.
--
Pushpa Narayanaswami, MD, Associate Professor, Department of Neurology, Harvard Medical School; Vice Chair of Clinical Operations, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Pushpa Narayanaswami, MD, has disclosed the following relevant financial relationships:
Serve(d) as an advisor or consultant for: Alexion; Argenx; Janssen; Dianthus; UCB; GSK
Received research grant from: Alexion; UCB; Dianthus; Janssen
The latest data on myasthenia gravis (MG) research, reported at the American Association of Neuromuscular and Electrodiagnostic Medicine 2024 Annual Meeting, are presented by Dr Pushpa Narayanswami of Harvard Medical School in Boston, Massachusetts.
Dr Narayanswami begins with a safety, tolerability, and efficacy study for subcutaneous efgartigimod. Results showed that the mean change in MG activities of daily living (MG-ADL) was no different between the fixed-dose and cyclic regimens, demonstrating another dosage option for patients.
Next, Dr Narayanswami discusses two separate complement C5 inhibitor therapy trials. The first was a global registry study looking at ravulizumab. Patient cohorts consisted of those who started and remained on ravulizumab vs another that switched from initial eculizumab to ravulizumab. In both groups, MG-ADL was improved. The other study investigated zilucoplan in acetylcholine receptor autoantibody–positive generalized MG patient populations; similarly, researchers found favorable results.
She then details a study looking at the safety outcomes in pregnant patients treated with eculizumab. Because of limited disease-specific data in the registry, further investigation is recommended.
Finally, Dr Narayanaswami examines results for inebilizumab, a first-in-class anti-CD19 B cell–depleting agent. The drug demonstrated safety and beneficial efficacy compared with placebo in seropositive generalized MG patients.
--
Pushpa Narayanaswami, MD, Associate Professor, Department of Neurology, Harvard Medical School; Vice Chair of Clinical Operations, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Pushpa Narayanaswami, MD, has disclosed the following relevant financial relationships:
Serve(d) as an advisor or consultant for: Alexion; Argenx; Janssen; Dianthus; UCB; GSK
Received research grant from: Alexion; UCB; Dianthus; Janssen
The latest data on myasthenia gravis (MG) research, reported at the American Association of Neuromuscular and Electrodiagnostic Medicine 2024 Annual Meeting, are presented by Dr Pushpa Narayanswami of Harvard Medical School in Boston, Massachusetts.
Dr Narayanswami begins with a safety, tolerability, and efficacy study for subcutaneous efgartigimod. Results showed that the mean change in MG activities of daily living (MG-ADL) was no different between the fixed-dose and cyclic regimens, demonstrating another dosage option for patients.
Next, Dr Narayanswami discusses two separate complement C5 inhibitor therapy trials. The first was a global registry study looking at ravulizumab. Patient cohorts consisted of those who started and remained on ravulizumab vs another that switched from initial eculizumab to ravulizumab. In both groups, MG-ADL was improved. The other study investigated zilucoplan in acetylcholine receptor autoantibody–positive generalized MG patient populations; similarly, researchers found favorable results.
She then details a study looking at the safety outcomes in pregnant patients treated with eculizumab. Because of limited disease-specific data in the registry, further investigation is recommended.
Finally, Dr Narayanaswami examines results for inebilizumab, a first-in-class anti-CD19 B cell–depleting agent. The drug demonstrated safety and beneficial efficacy compared with placebo in seropositive generalized MG patients.
--
Pushpa Narayanaswami, MD, Associate Professor, Department of Neurology, Harvard Medical School; Vice Chair of Clinical Operations, Department of Neurology, Beth Israel Deaconess Medical Center, Boston, Massachusetts
Pushpa Narayanaswami, MD, has disclosed the following relevant financial relationships:
Serve(d) as an advisor or consultant for: Alexion; Argenx; Janssen; Dianthus; UCB; GSK
Received research grant from: Alexion; UCB; Dianthus; Janssen

Evaluating Use of Empagliflozin for Diabetes Management in Veterans With Chronic Kidney Disease
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
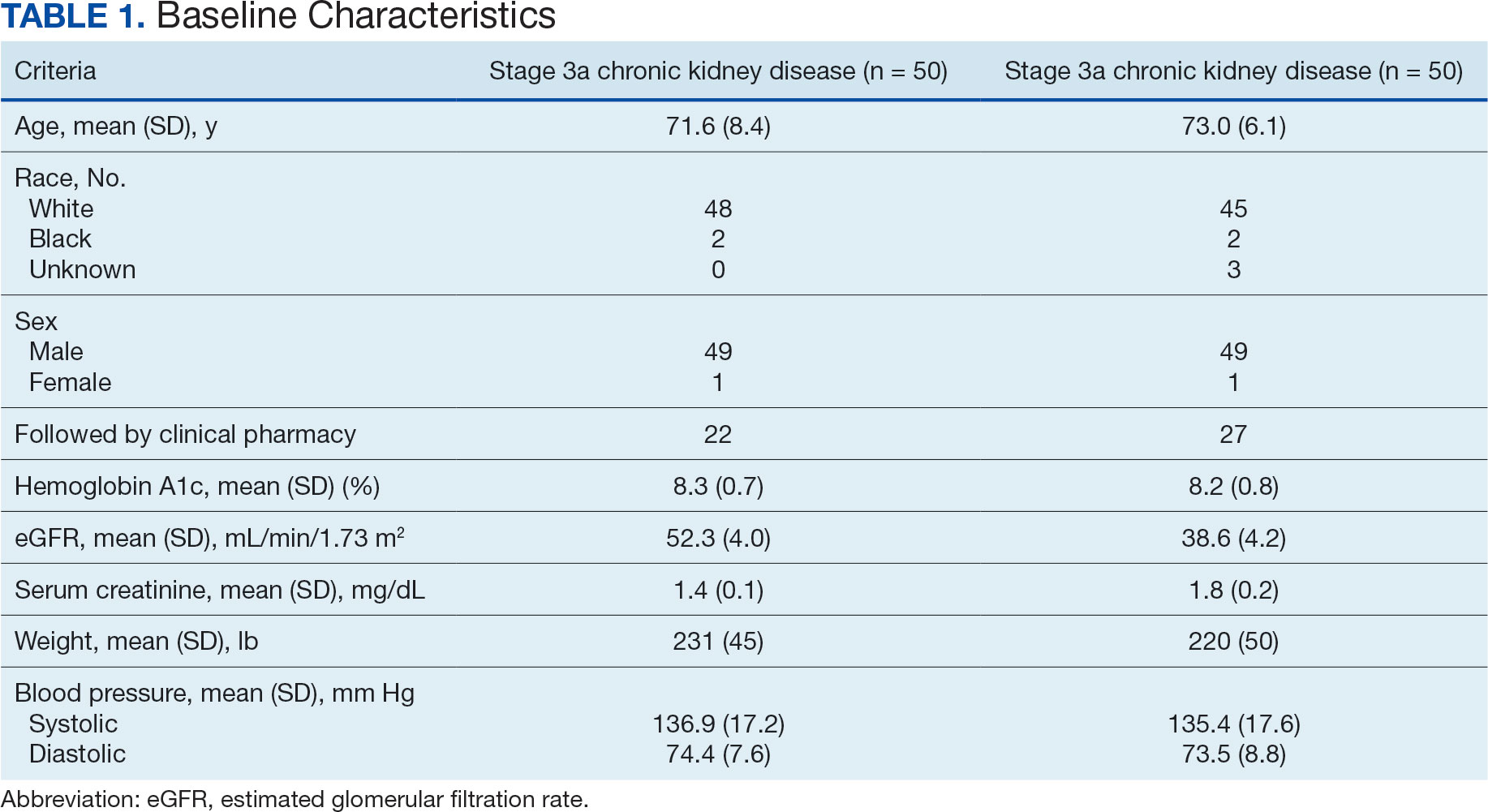
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.
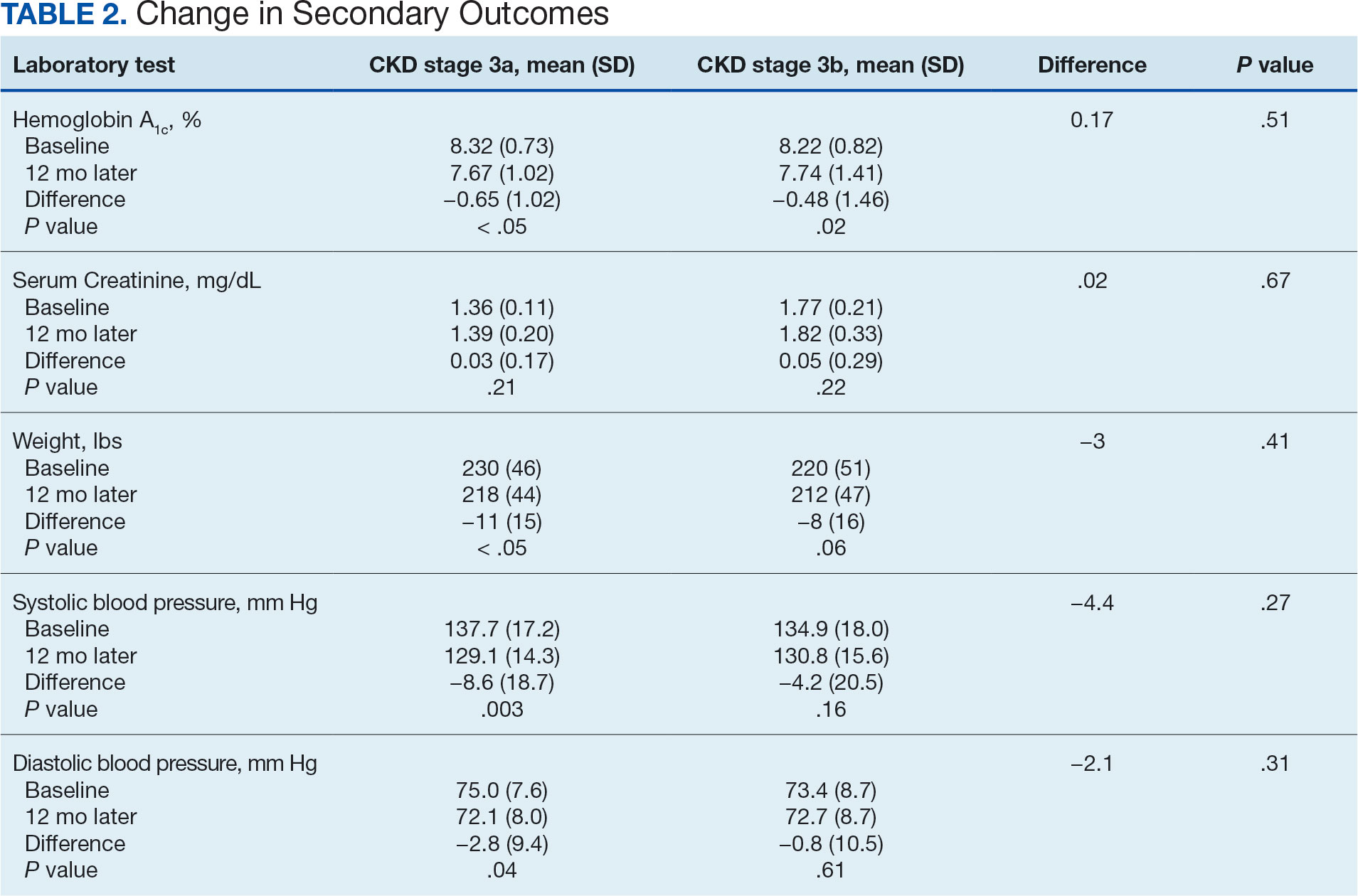
The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).
Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
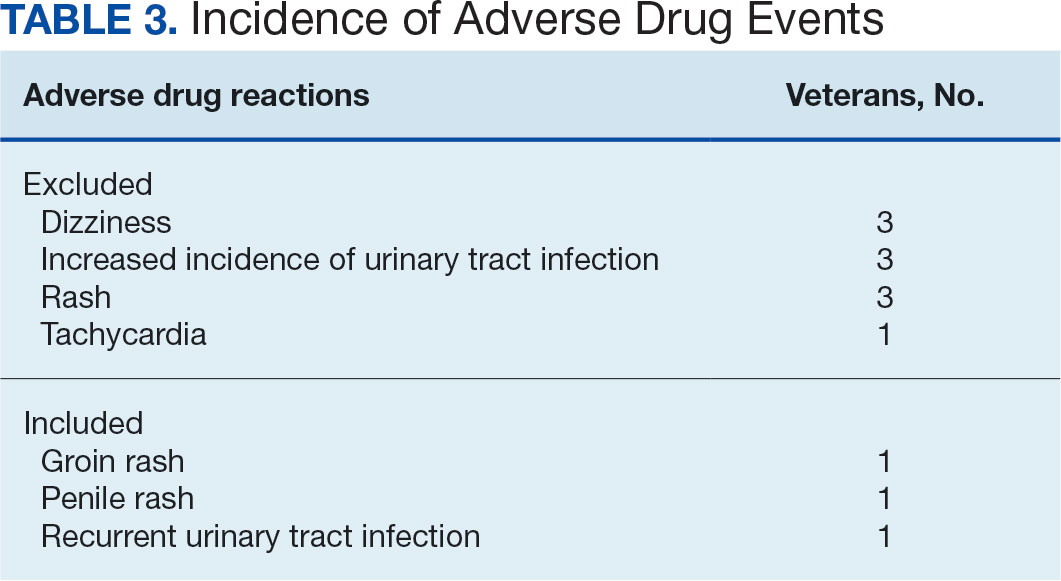
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).
Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.

The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).

Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).

Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
More than 37 million Americans have diabetes mellitus (DM), and approximately 90% have type 2 DM (T2DM), including about 25% of veterans.1,2 The current guidelines suggest that therapy depends on a patient's comorbidities, management needs, and patient-centered treatment factors.3 About 1 in 3 adults with DM have chronic kidney disease (CKD), defined as the presence of kidney damage or an estimated glomerular filtration rate (eGFR) < 60 mL/min per 1.73 m2, persisting for ≥ 3 months.4
Sodium-glucose cotransporter-2 (SGLT-2) inhibitors are a class of antihyperglycemic agents acting on the SGLT-2 proteins expressed in the renal proximal convoluted tubules. They exert their effects by preventing the reabsorption of filtered glucose from the tubular lumen. There are 4 SGLT-2 inhibitors approved by the US Food and Drug Administration: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin. Empagliflozin is currently the preferred SGLT-2 inhibitor on the US Department of Veterans Affairs (VA) formulary.
According to the American Diabetes Association guidelines, empagliflozin is considered when an individual has or is at risk for atherosclerotic cardiovascular disease, heart failure, and CKD.3 SGLT-2 inhibitors are a favorable option due to their low risk for hypoglycemia while also promoting weight loss. The EMPEROR-Reduced trial demonstrated that, in addition to benefits for patients with heart failure, empagliflozin also slowed the progressive decline in kidney function in those with and without DM.5 The purpose of this study was to evaluate the effectiveness of empagliflozin on hemoglobin A1c (HbA1c) levels in patients with CKD at the Hershel “Woody” Williams VA Medical Center (HWWVAMC) in Huntington, West Virginia, along with other laboratory test markers.
Methods
The Marshall University Institutional Review Board #1 (Medical) and the HWWVAMC institutional review board and research and development committee each reviewed and approved this study. A retrospective chart review was conducted on patients diagnosed with T2DM and stage 3 CKD who were prescribed empagliflozin for DM management between January 1, 2015, and October 1, 2022, yielding 1771 patients. Data were obtained through the VHA Corporate Data Warehouse (CDW) and stored on the VA Informatics and Computing Infrastructure (VINCI) research server.
Patients were included if they were aged 18 to 89 years, prescribed empagliflozin by a VA clinician for the treatment of T2DM, had an eGFR between 30 and 59 mL/min/1.73 m2, and had an initial HbA1c between 7% and 10%. Using further random sampling, patients were either excluded or divided into, those with stage 3a CKD and those with stage 3b CKD. The primary endpoint of this study was the change in HbA1c levels in patients with stage 3b CKD (eGFR 30-44 mL/min/1.73 m2) compared with stage 3a (eGFR 45-59 mL/min/1.73 m2) after 12 months. The secondary endpoints included effects on renal function, weight, blood pressure, incidence of adverse drug events, and cardiovascular events. Of the excluded, 38 had HbA1c < 7%, 30 had HbA1c ≥ 10%, 21 did not have data at 1-year mark, 15 had the medication discontinued due to decline in renal function, 14 discontinued their medication without documented reason, 10 discontinued their medication due to adverse drug reactions (ADRs), 12 had eGFR > 60 mL/ min/1.73 m2, 9 died within 1 year of initiation, 4 had eGFR < 30 mL/min/1.73 m2, 1 had no baseline eGFR, and 1 was the spouse of a veteran.
Statistical Analysis
All statistical analyses were performed using STATA v.15. We used t tests to examine changes within each group, along with paired t tests to compare the 2 groups. Two-sample t tests were used to analyze the continuous data at both the primary and secondary endpoints.
Results
Of the 1771 patients included in the initial data set, a randomized sample of 255 charts were reviewed, 155 were excluded, and 100 were included. Fifty patients, had stage 3a CKD and 50 had stage 3b CKD. Baseline demographics were similar between the stage 3a and 3b groups (Table 1). Both groups were predominantly White and male, with mean age > 70 years.

The primary endpoint was the differences in HbA1c levels over time and between groups for patients with stage 3a and stage 3b CKD 1 year after initiation of empagliflozin. The starting doses of empagliflozin were either 12.5 mg or 25.0 mg. For both groups, the changes in HbA1c levels were statistically significant (Table 2). HbA1c levels dropped 0.65% for the stage 3a group and 0.48% for the 3b group. When compared to one another, the results were not statistically significant (P = .51).

Secondary Endpoint
There was no statistically significant difference in serum creatinine levels within each group between baselines and 1 year later for the stage 3a (P = .21) and stage 3b (P = .22) groups, or when compared to each other (P = .67). There were statistically significant changes in weight for patients in the stage 3a group (P < .05), but not for stage 3b group (P = .06) or when compared to each other (P = .41). A statistically significant change in systolic blood pressure was observed for the stage 3a group (P = .003), but not the stage 3b group (P = .16) or when compared to each other (P = .27). There were statistically significant changes in diastolic blood pressure within the stage 3a group (P = .04), but not within the stage 3b group (P = .61) or when compared to each other (P = .31).
Ten patients discontinued empagliflozin before the 1-year mark due to ADRs, including dizziness, increased incidence of urinary tract infections, rash, and tachycardia (Table 3). Additionally, 3 ADRs resulted in the empagliflozin discontinuation after 1 year (Table 3).

Discussion
This study showed a statistically significant change in HbA1c levels for patients with stage 3a and stage 3b CKD. With eGFR levels in these 2 groups > 30 mL/min/1.73 m2, patients were able to achieve glycemic benefits. There were no significant changes to the serum creatinine levels. Both groups saw statistically significant changes in weight loss within their own group; however, there were no statistically significant changes when compared to each other. With both systolic and diastolic blood pressure, the stage 3a group had statistically significant changes.
The EMPA-REG BP study demonstrated that empagliflozin was associated with significant and clinically meaningful reductions in blood pressure and HbA1c levels compared with placebo and was well tolerated in patients with T2DM and hypertension.6,7,8
Limitations
This study had a retrospective study design, which resulted in missing information for many patients and higher rates of exclusion. The population was predominantly older, White, and male and may not reflect other populations. The starting doses of empagliflozin varied between the groups. The VA employs tablet splitting for some patients, and the available doses were either 10.0 mg, 12.5 mg, or 25.0 mg. Some prescribers start veterans at lower doses and gradually increase to the higher dose of 25.0 mg, adding to the variability in starting doses.
Patients with eGFR < 30 mL/min/1.73 m2 make it difficult to determine any potential benefit in this population. The EMPA-KIDNEY trial demonstrated that the benefits of empagliflozin treatment were consistent among patients with or without DM and regardless of eGFR at randomization.9 Furthermore, many veterans had an initial HbA1c levels outside the inclusion criteria range, which was a factor in the smaller sample size.
Conclusions
While the reduction in HbA1c levels was less in patients with stage 3b CKD compared to patients stage 3a CKD, all patients experienced a benefit. The overall incidence of ADRs was low in the study population, showing empagliflozin as a favorable choice for those with T2DM and CKD. Based on the findings of this study, empagliflozin is a potentially beneficial option for reducing HbA1c levels in patients with CKD.
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated May 25, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/about/about-type-2-diabetes.html?CDC_AAref_Val
- US Department of Veterans Affairs, VA research on diabetes. Updated September 2019. Accessed September 27, 2024. https://www.research.va.gov/pubs/docs/va_factsheets/Diabetes.pdf
- American Diabetes Association. Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin Diabetes. 2022;40(1):10-38. doi:10.2337/cd22-as01
- Centers for Disease Control and Prevention. Diabetes, chronic kidney disease. Updated May 15, 2024. Accessed September 27, 2024. https://www.cdc.gov/diabetes/diabetes-complications/diabetes-and-chronic-kidney-disease.html
- Packer M, Anker SD, Butler J, et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383(15):1413-1424. doi:10.1056/NEJMoa2022190
- Tikkanen I, Narko K, Zeller C, et al. Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care. 2015;38(3):420-428. doi:10.2337/dc14-1096
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117-2128. doi:10.1056/NEJMoa1504720
- Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab. 2015;17(12):1180-1193. doi:10.1111/dom.12572
- The EMPA-KIDNEY Collaborative Group, Herrington WG, Staplin N, et al. Empagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2023;388(2):117-127. doi:10.1056/NEJMoa2204233
Lichenoid Drug Eruption Secondary to Apalutamide Treatment
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
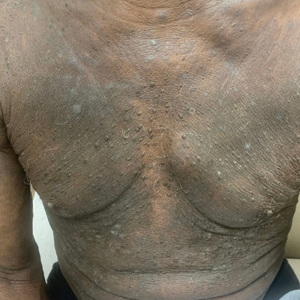
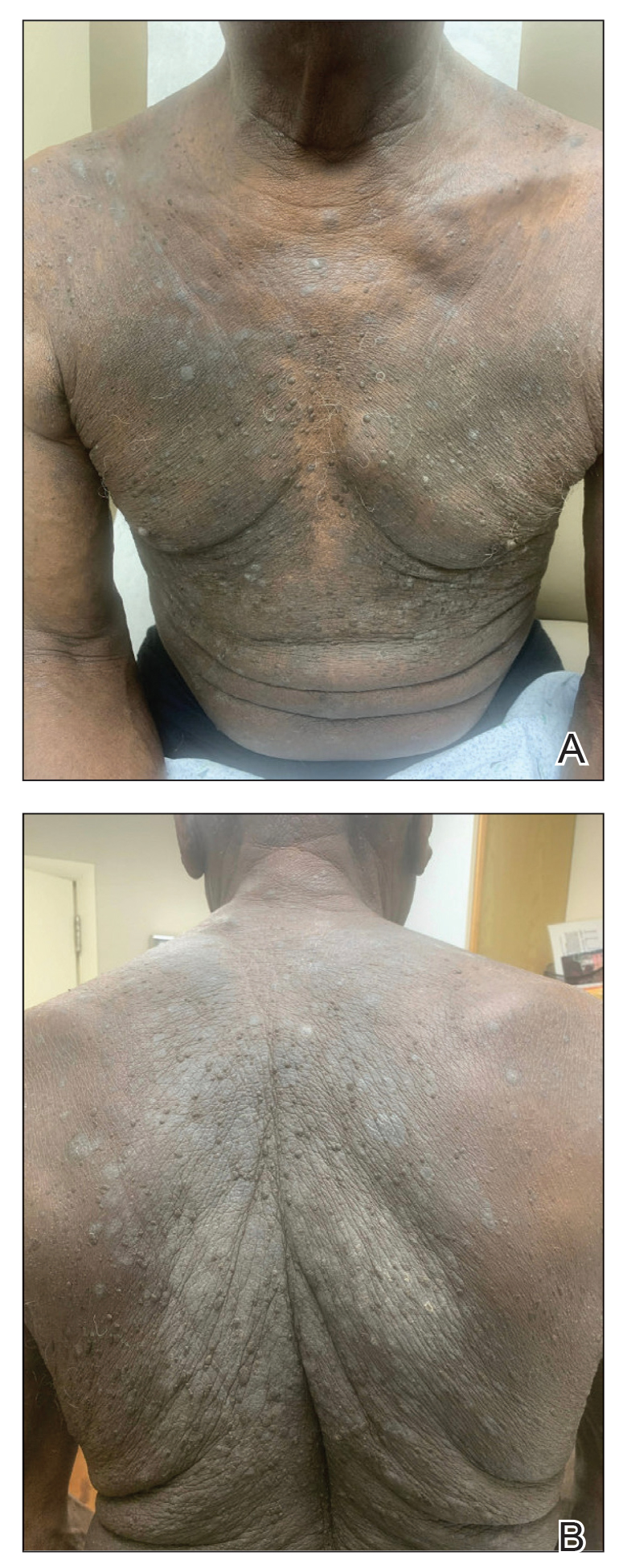
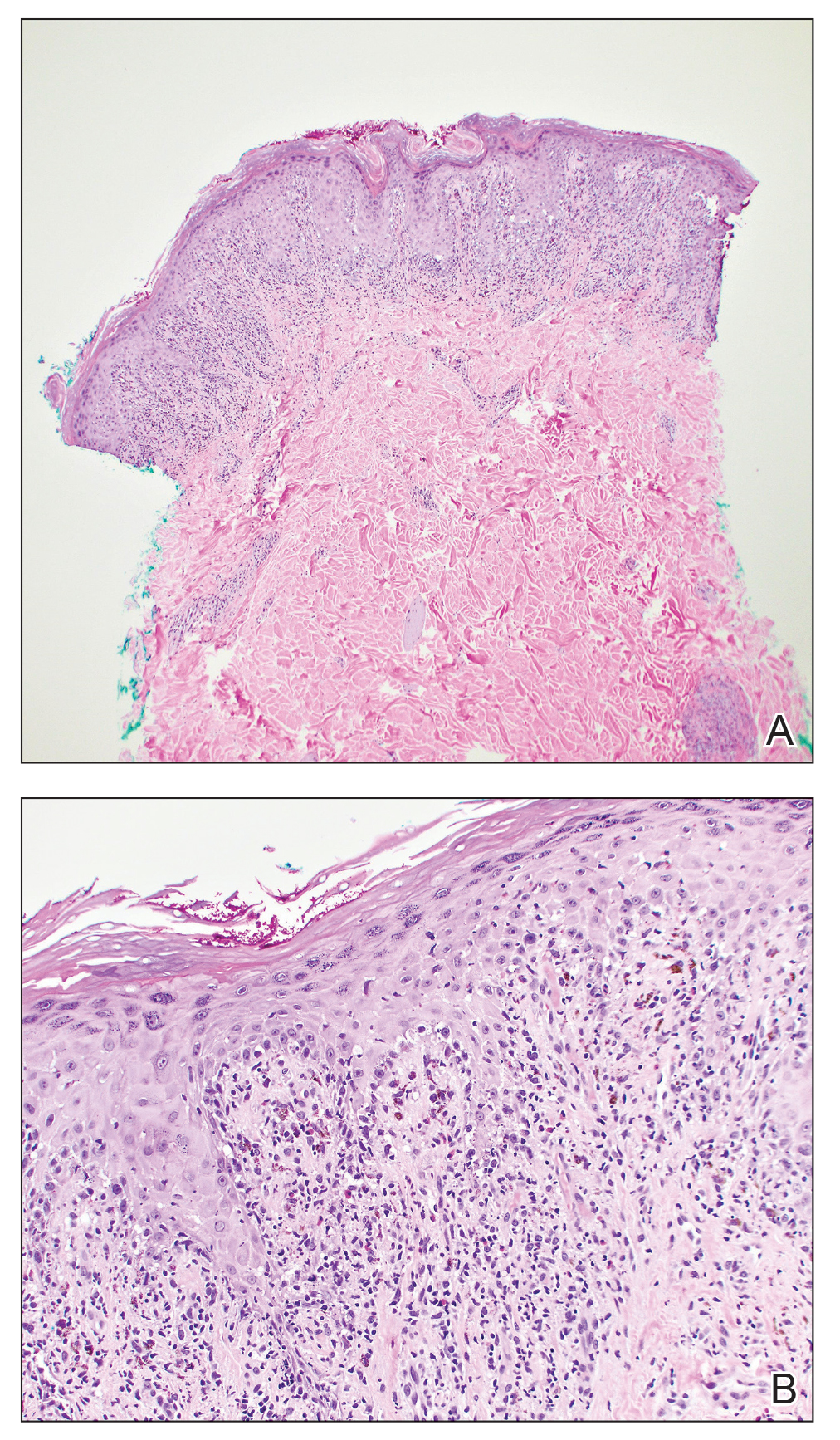
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.
Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.


Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.


Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
Practice Points
- Although it is rare, patients can develop lichenoid drug eruptions secondary to treatment with second-generation nonsteroidal androgen receptor antagonists such as apalutamide.
- If a patient develops a lichenoid drug eruption while taking a specific second-generation nonsteroidal androgen receptor antagonist, the entire class of medications should not be ruled out, as some patients can tolerate other drugs from that class.
Botulinum Toxin Injection for Treatment of Scleroderma-Related Anterior Neck Sclerosis
To the Editor:
Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body. On its own or in the setting of mixed connective tissue disease, scleroderma can result in systemic or localized symptoms that can limit patients’ functional capabilities, cause pain and discomfort, and reduce self-esteem—all negatively impacting patients’ quality of life.1,2 Neck sclerosis is a common manifestation of scleroderma. There is no curative treatment for scleroderma; thus, therapy is focused on slowing disease progression and improving quality of life. We present a case of neck sclerosis in a 44-year-old woman with scleroderma that was successfully treated with botulinum toxin (BTX) type A injection, resulting in improved skin laxity and appearance with high patient satisfaction. Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region.
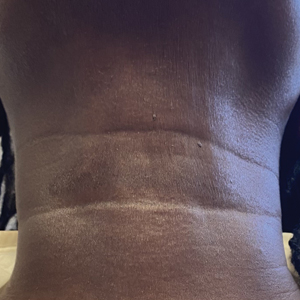
A 44-year-old woman presented to the dermatology clinic for treatment of thickened neck skin with stiffness and tightness that had been present for months to years. She had a history of mixed connective tissue disease (MCTD)(positive anti-ribonucleoprotein, anti–Sjögren syndrome–related antigen, and anti-Smith antibodies) with features of scleroderma and polyarthritis. The patient currently was taking sulfasalazine for the polyarthritis; she previously had taken hydroxychloroquine but discontinued treatment due to ineffectiveness. She was not taking any topical or systemic medications for scleroderma. On physical examination, the skin on the anterior neck appeared thickened with shiny patches (Figure 1). Pinching the skin in the affected area demonstrated sclerosis with high tension.
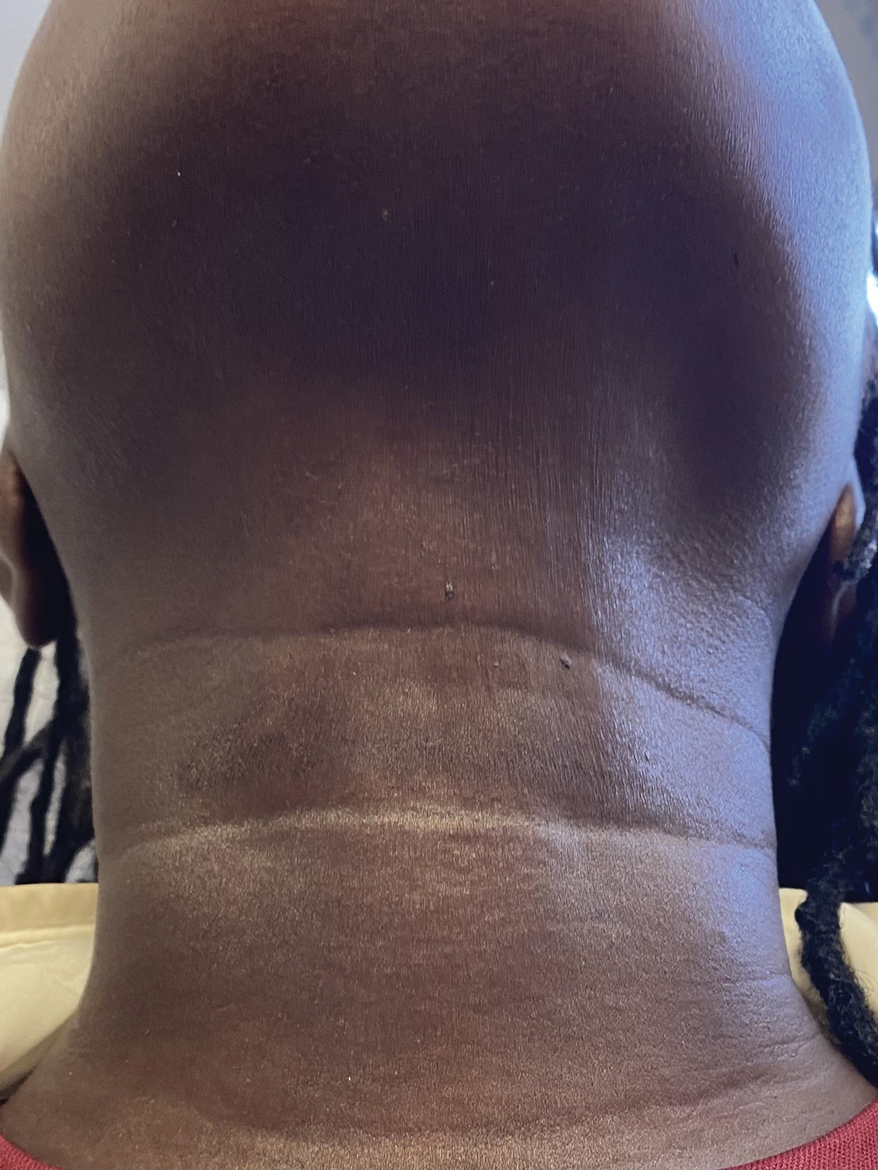
The dermatologist (J.J.) discussed potential treatment options to help relax the tension in the skin of the anterior neck, including BTX injections. After receiving counsel on adverse effects, alternative treatments, and postprocedural care, the patient decided to proceed with the procedure. The anterior neck was cleansed with an alcohol swab and 37 units (range, 25–50 units) of incobotulinumtoxinA (reconstituted using 2.5-mL bacteriostatic normal saline per 100 units) was injected transdermally using a 9-point injection technique, with each injection placed approximately 1 cm apart. The approximate treatment area included the space between the sternocleidomastoid anterior edges and below the hyoid bone up to the cricothyroid membrane (anatomic zone II).
When the patient returned for follow-up 3 weeks later, she reported considerable improvement in the stiffness and appearance of the skin on the anterior neck. On physical examination, the skin of the neck appeared softened, and improved laxity was seen on pinching the skin compared to the initial presentation (Figure 2). The patient expressed satisfaction with the results and denied any adverse events following the procedure.
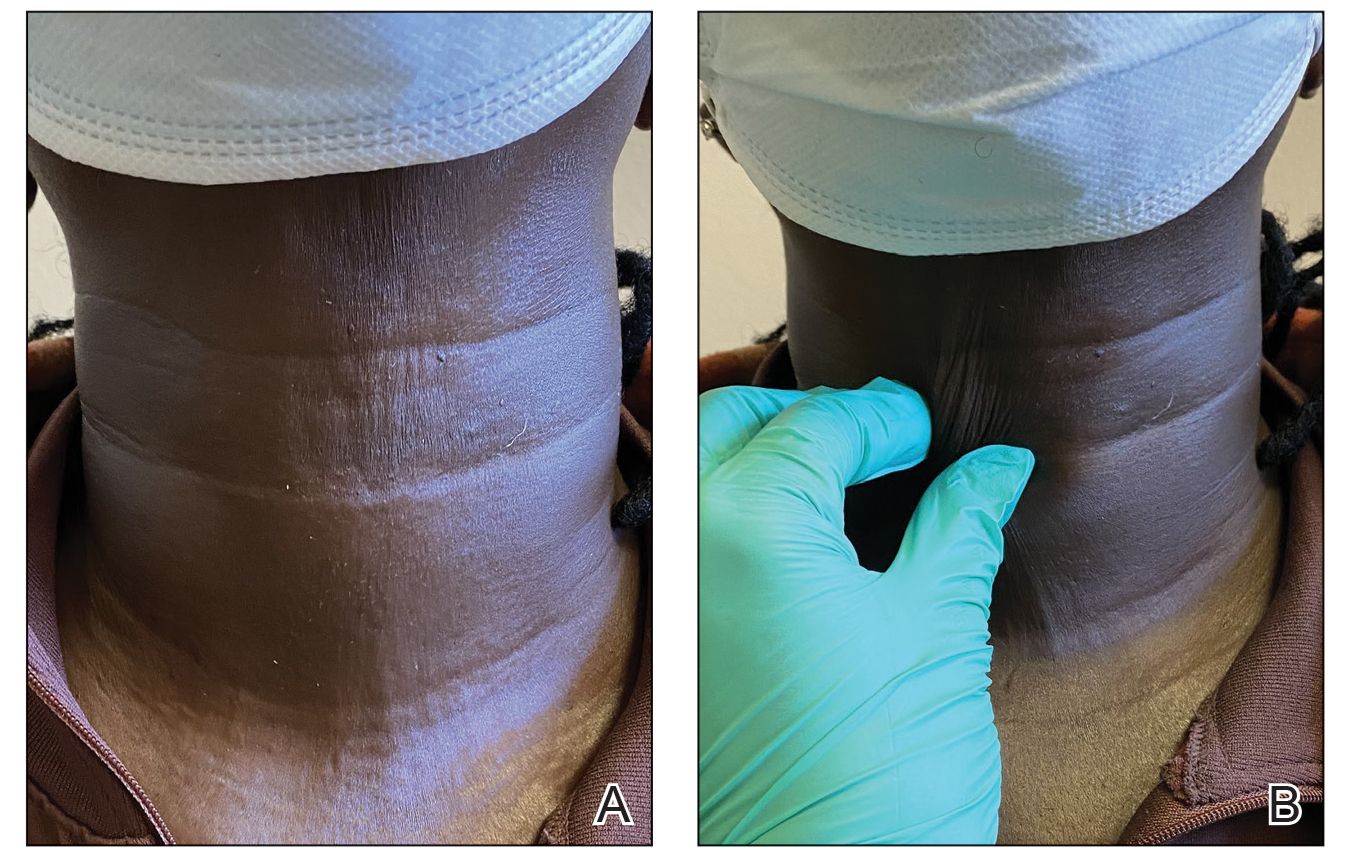
Mixed connective tissue disease manifests with a combination of features from various disorders—mainly lupus, scleroderma, polymyositis, and rheumatoid arthritis. It is most prevalent in females and often is diagnosed in the third decade of life.3 It is associated with positive antinuclear antibodies and human leukocyte antigen (HLA) II alleles (HLA-DR4, HLA-DR1, and HLA-DR2). Raynaud phenomenon (RP), one of the most common skin manifestations in both scleroderma and MCTD, is present in 75% to 90% of patients with MCTD.3
Scleroderma is a chronic connective tissue disorder that results in excessive collagen deposition in the skin and other organs throughout the body.4 Although the etiology is unknown, scleroderma develops when overactivation of the immune system leads to CD4+ T-lymphocyte infiltration in the skin, along with the release of profibrotic interleukins and growth factors, resulting in fibrosis.4 Subtypes include localized scleroderma (morphea), limited cutaneous systemic sclerosis (formerly known as CREST [calcinosis, RP, esophageal dysmotility, sclerodactyly, and telangiectasia] syndrome), diffuse cutaneous systemic sclerosis, and systemic sclerosis sine scleroderma.5 Scleroderma is associated with positive antinuclear antibodies and HLA II alleles (HLA-DR2 and HLA-DR5).
On its own or in the setting of MCTD, scleroderma can result in systemic or localized symptoms. Overall, the most common symptom is RP.5 Localized scleroderma and limited cutaneous systemic sclerosis manifest with symptoms of the skin and underlying tissues. Diffuse cutaneous systemic sclerosis involves cutaneous and visceral symptoms, including lung, esophageal, and vascular involvement.6 Similar to MCTD, scleroderma is most prevalent in middle-aged females,7 though it occurs at a higher rate and with a more severe disease course in Black patients.8
A highly sensitive and specific test for scleroderma that can aid in diagnosis is the neck sign—tightening of the skin of the neck when the head extends.9,10 In one study, the neck sign was positive in more than 90% of patients with scleroderma and negative for control patients and those with primary RP.9 Thus, neck sclerosis is a common manifestation of scleroderma for which patients may seek treatment.
While there is no curative treatment for scleroderma, skin manifestations can be treated with mycophenolate mofetil or methotrexate.5 Systemic treatments may be recommended if the patient has additional symptoms, such as azathioprine for myositis/arthritis and cyclophosphamide for interstitial lung disease.5 However, it is important to note that these medications are associated with risk for gastrointestinal upset, mouth sores, fatigue, or other complications.
Botulinum toxin is a bacterial protein toxin and neuromodulator that inhibits neurotransmitter release by cleaving SNARE proteins at peripheral nerve terminal junctions.11 It has been used in a variety of dermatologic and nondermatologic conditions, including migraines, hyperhidrosis, contractures, scars, and overactive bladder. It also has been used in aesthetics for facial rejuvenation and minimization of wrinkle appearance. Dermatologists and rheumatologists have successfully used BTX to treat primary and secondary RP—the most common symptom of scleroderma—due to its vasodilatation properties.12 Although our patient did not have RP, use of BTX to treat other features of scleroderma, including en coup de sabre, thoracic outlet syndrome, dyspareunia, gastroparesis, pterygium inversum unguis, and dysphagia has been documented.13-18 An in vivo mouse study that examined the possible mechanism for BTX as a treatment in scleroderma found that BTX injections significantly decreased dermal thickness and inflammation in fibrosis (P<.05). An analysis of oxidative stress and mRNA expression showed that BTX may treat fibrosis by suppressing oxidative stress and inflammatory cells, resulting in decreased apoptosis and oxidant-induced intracellular accumulation of reactive oxygen species.19 Another animal study demonstrated the positive effects of BTX treatment for fibrosis of the bladder in rats.20 In one case report, a female patient with scleroderma and facial fibrosis received perioral BTX injections for cosmetic purposes but also observed improvement in mouth constriction, demonstrating the potential efficacy of BTX for facial fibrosis.21
Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region. We recommend assessing the efficacy of the initial BTX treatment after 2 to 3 weeks, with additional injections as needed to achieve the patient’s desired level of comfort and appearance at approximately 3-month intervals (aligning with the expected duration of efficacy of BTX).22 Our patient experienced considerable relief and high satisfaction with BTX treatment. Given the limitations of sclerosis treatments and the unwanted adverse-effect profile of systemic treatments, BTX injections may be a preferrable treatment option for cutaneous manifestations of scleroderma among patients. Future studies with larger patient populations and a control group are warranted to further explore the use of BTX for the dermatologic treatment of scleroderma.
- Lis-S´wie¸ty A, Skrzypek-Salamon A, Ranosz-Janicka I, et al. Health-related quality of life and its influencing factors in adult patients with localized scleroderma—a cross-sectional study. Health Qual Life Outcomes. 2020;18:133. doi:10.1186/s12955-020-01386-0
- Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. 2015;14:1087-1096. doi:10.1016/j.autrev.2015.07.012
- Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2012;26:61-72. doi:10.1016/j.berh.2012.01.009
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018;153:208-215. doi:10.23736/S0392-0488.18.05922-9
- Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437. doi:10.1016/j.clindermatol.2013.01.010
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Calderon LM, Pope JE. Scleroderma epidemiology update. Curr Opin Rheumatol. 2021;33:122-127. doi:10.1097/BOR.0000000000000785
- Morgan ND, Gelber AC. African Americans and scleroderma: examining the root cause of the association. Arthritis Care Res (Hoboken). 2019;71:1151-1153. doi:10.1002/acr.23860
- Barnett AJ. The “neck sign” in scleroderma. Arthritis Rheum. 1989;32:209-211. doi:10.1002/anr.1780320215
- Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis). Postgrad Med J. 1988;64:121-125. doi:10.1136/pgmj.64.748.121
- Rossetto O, Pirazzini M, Fabris F, et al. Botulinum neurotoxins: mechanism of action. Handb Exp Pharmacol. 2021;263:35-47.doi:10.1007/164_2020_355
- Ennis D, Ahmad Z, Anderson MA, et al. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2021;35:101684. doi:10.1016/j.berh.2021.101684
- Turkmani MG, Alnomair N. Enhancement of the aesthetic outcome of scleroderma en coup de sabre with botulinum toxin injection. JAAD Case Rep. 2018;4:579-581. doi:10.1016/j.jdcr.2018.03.023
- Le EN, Freischlag JA, Christo PJ, et al. Thoracic outlet syndrome secondary to localized scleroderma treated with botulinum toxin injection. Arthritis Care Res (Hoboken). 2010;62:430-433. doi:10.1002/acr.20099
- Mousty E, Rathat G, Rouleau C, et al. Botulinum toxin type A for treatment of dyspareunia caused by localized scleroderma. Acta Obstet Gynecol Scand. 2011;90:926-927. doi:10.1111/j.1600-0412.2011.01183.x
- Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74-101. doi:10.1016/j.disamonth.2010.12.007
- Katschinski M. [Diagnosis and treatment of esophageal motility disorders]. Ther Umsch. 2001;58:128-133. doi:10.1024/0040-5930.58.3.128
- Kim DJ, Odell ID. Improvement of pterygium inversum unguis and Raynaud phenomenon with interdigital botulinum toxin injections. JAAD Case Rep. 2022;26:79-81. doi:10.1016/j.jdcr.2022.06.009
- Baral H, Sekiguchi A, Uchiyama A, et al. Inhibition of skin fibrosis in systemic sclerosis by botulinum toxin B via the suppression of oxidative stress. J Dermatol. 2021;48:1052-1061. doi:10.1111/1346-8138.15888
- Jia C, Xing T, Shang Z, et al. Botulinum toxin A improves neurogenic bladder fibrosis by suppressing transforming growth factor β1 expression in rats. Transl Androl Urol. 2021;10:2000-2007. doi:10.21037/tau-21-62
- Hoverson K, Love T, Lam TK, et al. A novel treatment for limited mouth opening due to facial fibrosis: a case series. J Am Acad Dermatol. 2018;78:190-192. doi:10.1016/j.jaad.2017.07.006
- Kollewe K, Mohammadi B, Köhler S, et al. Blepharospasm: long-term treatment with either Botox®, Xeomin® or Dysport®. J Neural Transm (Vienna). 2015;122:427-431. doi:10.1007/s00702-014-1278-z
To the Editor:
Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body. On its own or in the setting of mixed connective tissue disease, scleroderma can result in systemic or localized symptoms that can limit patients’ functional capabilities, cause pain and discomfort, and reduce self-esteem—all negatively impacting patients’ quality of life.1,2 Neck sclerosis is a common manifestation of scleroderma. There is no curative treatment for scleroderma; thus, therapy is focused on slowing disease progression and improving quality of life. We present a case of neck sclerosis in a 44-year-old woman with scleroderma that was successfully treated with botulinum toxin (BTX) type A injection, resulting in improved skin laxity and appearance with high patient satisfaction. Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region.
A 44-year-old woman presented to the dermatology clinic for treatment of thickened neck skin with stiffness and tightness that had been present for months to years. She had a history of mixed connective tissue disease (MCTD)(positive anti-ribonucleoprotein, anti–Sjögren syndrome–related antigen, and anti-Smith antibodies) with features of scleroderma and polyarthritis. The patient currently was taking sulfasalazine for the polyarthritis; she previously had taken hydroxychloroquine but discontinued treatment due to ineffectiveness. She was not taking any topical or systemic medications for scleroderma. On physical examination, the skin on the anterior neck appeared thickened with shiny patches (Figure 1). Pinching the skin in the affected area demonstrated sclerosis with high tension.

The dermatologist (J.J.) discussed potential treatment options to help relax the tension in the skin of the anterior neck, including BTX injections. After receiving counsel on adverse effects, alternative treatments, and postprocedural care, the patient decided to proceed with the procedure. The anterior neck was cleansed with an alcohol swab and 37 units (range, 25–50 units) of incobotulinumtoxinA (reconstituted using 2.5-mL bacteriostatic normal saline per 100 units) was injected transdermally using a 9-point injection technique, with each injection placed approximately 1 cm apart. The approximate treatment area included the space between the sternocleidomastoid anterior edges and below the hyoid bone up to the cricothyroid membrane (anatomic zone II).
When the patient returned for follow-up 3 weeks later, she reported considerable improvement in the stiffness and appearance of the skin on the anterior neck. On physical examination, the skin of the neck appeared softened, and improved laxity was seen on pinching the skin compared to the initial presentation (Figure 2). The patient expressed satisfaction with the results and denied any adverse events following the procedure.

Mixed connective tissue disease manifests with a combination of features from various disorders—mainly lupus, scleroderma, polymyositis, and rheumatoid arthritis. It is most prevalent in females and often is diagnosed in the third decade of life.3 It is associated with positive antinuclear antibodies and human leukocyte antigen (HLA) II alleles (HLA-DR4, HLA-DR1, and HLA-DR2). Raynaud phenomenon (RP), one of the most common skin manifestations in both scleroderma and MCTD, is present in 75% to 90% of patients with MCTD.3
Scleroderma is a chronic connective tissue disorder that results in excessive collagen deposition in the skin and other organs throughout the body.4 Although the etiology is unknown, scleroderma develops when overactivation of the immune system leads to CD4+ T-lymphocyte infiltration in the skin, along with the release of profibrotic interleukins and growth factors, resulting in fibrosis.4 Subtypes include localized scleroderma (morphea), limited cutaneous systemic sclerosis (formerly known as CREST [calcinosis, RP, esophageal dysmotility, sclerodactyly, and telangiectasia] syndrome), diffuse cutaneous systemic sclerosis, and systemic sclerosis sine scleroderma.5 Scleroderma is associated with positive antinuclear antibodies and HLA II alleles (HLA-DR2 and HLA-DR5).
On its own or in the setting of MCTD, scleroderma can result in systemic or localized symptoms. Overall, the most common symptom is RP.5 Localized scleroderma and limited cutaneous systemic sclerosis manifest with symptoms of the skin and underlying tissues. Diffuse cutaneous systemic sclerosis involves cutaneous and visceral symptoms, including lung, esophageal, and vascular involvement.6 Similar to MCTD, scleroderma is most prevalent in middle-aged females,7 though it occurs at a higher rate and with a more severe disease course in Black patients.8
A highly sensitive and specific test for scleroderma that can aid in diagnosis is the neck sign—tightening of the skin of the neck when the head extends.9,10 In one study, the neck sign was positive in more than 90% of patients with scleroderma and negative for control patients and those with primary RP.9 Thus, neck sclerosis is a common manifestation of scleroderma for which patients may seek treatment.
While there is no curative treatment for scleroderma, skin manifestations can be treated with mycophenolate mofetil or methotrexate.5 Systemic treatments may be recommended if the patient has additional symptoms, such as azathioprine for myositis/arthritis and cyclophosphamide for interstitial lung disease.5 However, it is important to note that these medications are associated with risk for gastrointestinal upset, mouth sores, fatigue, or other complications.
Botulinum toxin is a bacterial protein toxin and neuromodulator that inhibits neurotransmitter release by cleaving SNARE proteins at peripheral nerve terminal junctions.11 It has been used in a variety of dermatologic and nondermatologic conditions, including migraines, hyperhidrosis, contractures, scars, and overactive bladder. It also has been used in aesthetics for facial rejuvenation and minimization of wrinkle appearance. Dermatologists and rheumatologists have successfully used BTX to treat primary and secondary RP—the most common symptom of scleroderma—due to its vasodilatation properties.12 Although our patient did not have RP, use of BTX to treat other features of scleroderma, including en coup de sabre, thoracic outlet syndrome, dyspareunia, gastroparesis, pterygium inversum unguis, and dysphagia has been documented.13-18 An in vivo mouse study that examined the possible mechanism for BTX as a treatment in scleroderma found that BTX injections significantly decreased dermal thickness and inflammation in fibrosis (P<.05). An analysis of oxidative stress and mRNA expression showed that BTX may treat fibrosis by suppressing oxidative stress and inflammatory cells, resulting in decreased apoptosis and oxidant-induced intracellular accumulation of reactive oxygen species.19 Another animal study demonstrated the positive effects of BTX treatment for fibrosis of the bladder in rats.20 In one case report, a female patient with scleroderma and facial fibrosis received perioral BTX injections for cosmetic purposes but also observed improvement in mouth constriction, demonstrating the potential efficacy of BTX for facial fibrosis.21
Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region. We recommend assessing the efficacy of the initial BTX treatment after 2 to 3 weeks, with additional injections as needed to achieve the patient’s desired level of comfort and appearance at approximately 3-month intervals (aligning with the expected duration of efficacy of BTX).22 Our patient experienced considerable relief and high satisfaction with BTX treatment. Given the limitations of sclerosis treatments and the unwanted adverse-effect profile of systemic treatments, BTX injections may be a preferrable treatment option for cutaneous manifestations of scleroderma among patients. Future studies with larger patient populations and a control group are warranted to further explore the use of BTX for the dermatologic treatment of scleroderma.
To the Editor:
Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body. On its own or in the setting of mixed connective tissue disease, scleroderma can result in systemic or localized symptoms that can limit patients’ functional capabilities, cause pain and discomfort, and reduce self-esteem—all negatively impacting patients’ quality of life.1,2 Neck sclerosis is a common manifestation of scleroderma. There is no curative treatment for scleroderma; thus, therapy is focused on slowing disease progression and improving quality of life. We present a case of neck sclerosis in a 44-year-old woman with scleroderma that was successfully treated with botulinum toxin (BTX) type A injection, resulting in improved skin laxity and appearance with high patient satisfaction. Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region.
A 44-year-old woman presented to the dermatology clinic for treatment of thickened neck skin with stiffness and tightness that had been present for months to years. She had a history of mixed connective tissue disease (MCTD)(positive anti-ribonucleoprotein, anti–Sjögren syndrome–related antigen, and anti-Smith antibodies) with features of scleroderma and polyarthritis. The patient currently was taking sulfasalazine for the polyarthritis; she previously had taken hydroxychloroquine but discontinued treatment due to ineffectiveness. She was not taking any topical or systemic medications for scleroderma. On physical examination, the skin on the anterior neck appeared thickened with shiny patches (Figure 1). Pinching the skin in the affected area demonstrated sclerosis with high tension.

The dermatologist (J.J.) discussed potential treatment options to help relax the tension in the skin of the anterior neck, including BTX injections. After receiving counsel on adverse effects, alternative treatments, and postprocedural care, the patient decided to proceed with the procedure. The anterior neck was cleansed with an alcohol swab and 37 units (range, 25–50 units) of incobotulinumtoxinA (reconstituted using 2.5-mL bacteriostatic normal saline per 100 units) was injected transdermally using a 9-point injection technique, with each injection placed approximately 1 cm apart. The approximate treatment area included the space between the sternocleidomastoid anterior edges and below the hyoid bone up to the cricothyroid membrane (anatomic zone II).
When the patient returned for follow-up 3 weeks later, she reported considerable improvement in the stiffness and appearance of the skin on the anterior neck. On physical examination, the skin of the neck appeared softened, and improved laxity was seen on pinching the skin compared to the initial presentation (Figure 2). The patient expressed satisfaction with the results and denied any adverse events following the procedure.

Mixed connective tissue disease manifests with a combination of features from various disorders—mainly lupus, scleroderma, polymyositis, and rheumatoid arthritis. It is most prevalent in females and often is diagnosed in the third decade of life.3 It is associated with positive antinuclear antibodies and human leukocyte antigen (HLA) II alleles (HLA-DR4, HLA-DR1, and HLA-DR2). Raynaud phenomenon (RP), one of the most common skin manifestations in both scleroderma and MCTD, is present in 75% to 90% of patients with MCTD.3
Scleroderma is a chronic connective tissue disorder that results in excessive collagen deposition in the skin and other organs throughout the body.4 Although the etiology is unknown, scleroderma develops when overactivation of the immune system leads to CD4+ T-lymphocyte infiltration in the skin, along with the release of profibrotic interleukins and growth factors, resulting in fibrosis.4 Subtypes include localized scleroderma (morphea), limited cutaneous systemic sclerosis (formerly known as CREST [calcinosis, RP, esophageal dysmotility, sclerodactyly, and telangiectasia] syndrome), diffuse cutaneous systemic sclerosis, and systemic sclerosis sine scleroderma.5 Scleroderma is associated with positive antinuclear antibodies and HLA II alleles (HLA-DR2 and HLA-DR5).
On its own or in the setting of MCTD, scleroderma can result in systemic or localized symptoms. Overall, the most common symptom is RP.5 Localized scleroderma and limited cutaneous systemic sclerosis manifest with symptoms of the skin and underlying tissues. Diffuse cutaneous systemic sclerosis involves cutaneous and visceral symptoms, including lung, esophageal, and vascular involvement.6 Similar to MCTD, scleroderma is most prevalent in middle-aged females,7 though it occurs at a higher rate and with a more severe disease course in Black patients.8
A highly sensitive and specific test for scleroderma that can aid in diagnosis is the neck sign—tightening of the skin of the neck when the head extends.9,10 In one study, the neck sign was positive in more than 90% of patients with scleroderma and negative for control patients and those with primary RP.9 Thus, neck sclerosis is a common manifestation of scleroderma for which patients may seek treatment.
While there is no curative treatment for scleroderma, skin manifestations can be treated with mycophenolate mofetil or methotrexate.5 Systemic treatments may be recommended if the patient has additional symptoms, such as azathioprine for myositis/arthritis and cyclophosphamide for interstitial lung disease.5 However, it is important to note that these medications are associated with risk for gastrointestinal upset, mouth sores, fatigue, or other complications.
Botulinum toxin is a bacterial protein toxin and neuromodulator that inhibits neurotransmitter release by cleaving SNARE proteins at peripheral nerve terminal junctions.11 It has been used in a variety of dermatologic and nondermatologic conditions, including migraines, hyperhidrosis, contractures, scars, and overactive bladder. It also has been used in aesthetics for facial rejuvenation and minimization of wrinkle appearance. Dermatologists and rheumatologists have successfully used BTX to treat primary and secondary RP—the most common symptom of scleroderma—due to its vasodilatation properties.12 Although our patient did not have RP, use of BTX to treat other features of scleroderma, including en coup de sabre, thoracic outlet syndrome, dyspareunia, gastroparesis, pterygium inversum unguis, and dysphagia has been documented.13-18 An in vivo mouse study that examined the possible mechanism for BTX as a treatment in scleroderma found that BTX injections significantly decreased dermal thickness and inflammation in fibrosis (P<.05). An analysis of oxidative stress and mRNA expression showed that BTX may treat fibrosis by suppressing oxidative stress and inflammatory cells, resulting in decreased apoptosis and oxidant-induced intracellular accumulation of reactive oxygen species.19 Another animal study demonstrated the positive effects of BTX treatment for fibrosis of the bladder in rats.20 In one case report, a female patient with scleroderma and facial fibrosis received perioral BTX injections for cosmetic purposes but also observed improvement in mouth constriction, demonstrating the potential efficacy of BTX for facial fibrosis.21
Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region. We recommend assessing the efficacy of the initial BTX treatment after 2 to 3 weeks, with additional injections as needed to achieve the patient’s desired level of comfort and appearance at approximately 3-month intervals (aligning with the expected duration of efficacy of BTX).22 Our patient experienced considerable relief and high satisfaction with BTX treatment. Given the limitations of sclerosis treatments and the unwanted adverse-effect profile of systemic treatments, BTX injections may be a preferrable treatment option for cutaneous manifestations of scleroderma among patients. Future studies with larger patient populations and a control group are warranted to further explore the use of BTX for the dermatologic treatment of scleroderma.
- Lis-S´wie¸ty A, Skrzypek-Salamon A, Ranosz-Janicka I, et al. Health-related quality of life and its influencing factors in adult patients with localized scleroderma—a cross-sectional study. Health Qual Life Outcomes. 2020;18:133. doi:10.1186/s12955-020-01386-0
- Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. 2015;14:1087-1096. doi:10.1016/j.autrev.2015.07.012
- Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2012;26:61-72. doi:10.1016/j.berh.2012.01.009
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018;153:208-215. doi:10.23736/S0392-0488.18.05922-9
- Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437. doi:10.1016/j.clindermatol.2013.01.010
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Calderon LM, Pope JE. Scleroderma epidemiology update. Curr Opin Rheumatol. 2021;33:122-127. doi:10.1097/BOR.0000000000000785
- Morgan ND, Gelber AC. African Americans and scleroderma: examining the root cause of the association. Arthritis Care Res (Hoboken). 2019;71:1151-1153. doi:10.1002/acr.23860
- Barnett AJ. The “neck sign” in scleroderma. Arthritis Rheum. 1989;32:209-211. doi:10.1002/anr.1780320215
- Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis). Postgrad Med J. 1988;64:121-125. doi:10.1136/pgmj.64.748.121
- Rossetto O, Pirazzini M, Fabris F, et al. Botulinum neurotoxins: mechanism of action. Handb Exp Pharmacol. 2021;263:35-47.doi:10.1007/164_2020_355
- Ennis D, Ahmad Z, Anderson MA, et al. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2021;35:101684. doi:10.1016/j.berh.2021.101684
- Turkmani MG, Alnomair N. Enhancement of the aesthetic outcome of scleroderma en coup de sabre with botulinum toxin injection. JAAD Case Rep. 2018;4:579-581. doi:10.1016/j.jdcr.2018.03.023
- Le EN, Freischlag JA, Christo PJ, et al. Thoracic outlet syndrome secondary to localized scleroderma treated with botulinum toxin injection. Arthritis Care Res (Hoboken). 2010;62:430-433. doi:10.1002/acr.20099
- Mousty E, Rathat G, Rouleau C, et al. Botulinum toxin type A for treatment of dyspareunia caused by localized scleroderma. Acta Obstet Gynecol Scand. 2011;90:926-927. doi:10.1111/j.1600-0412.2011.01183.x
- Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74-101. doi:10.1016/j.disamonth.2010.12.007
- Katschinski M. [Diagnosis and treatment of esophageal motility disorders]. Ther Umsch. 2001;58:128-133. doi:10.1024/0040-5930.58.3.128
- Kim DJ, Odell ID. Improvement of pterygium inversum unguis and Raynaud phenomenon with interdigital botulinum toxin injections. JAAD Case Rep. 2022;26:79-81. doi:10.1016/j.jdcr.2022.06.009
- Baral H, Sekiguchi A, Uchiyama A, et al. Inhibition of skin fibrosis in systemic sclerosis by botulinum toxin B via the suppression of oxidative stress. J Dermatol. 2021;48:1052-1061. doi:10.1111/1346-8138.15888
- Jia C, Xing T, Shang Z, et al. Botulinum toxin A improves neurogenic bladder fibrosis by suppressing transforming growth factor β1 expression in rats. Transl Androl Urol. 2021;10:2000-2007. doi:10.21037/tau-21-62
- Hoverson K, Love T, Lam TK, et al. A novel treatment for limited mouth opening due to facial fibrosis: a case series. J Am Acad Dermatol. 2018;78:190-192. doi:10.1016/j.jaad.2017.07.006
- Kollewe K, Mohammadi B, Köhler S, et al. Blepharospasm: long-term treatment with either Botox®, Xeomin® or Dysport®. J Neural Transm (Vienna). 2015;122:427-431. doi:10.1007/s00702-014-1278-z
- Lis-S´wie¸ty A, Skrzypek-Salamon A, Ranosz-Janicka I, et al. Health-related quality of life and its influencing factors in adult patients with localized scleroderma—a cross-sectional study. Health Qual Life Outcomes. 2020;18:133. doi:10.1186/s12955-020-01386-0
- Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. 2015;14:1087-1096. doi:10.1016/j.autrev.2015.07.012
- Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2012;26:61-72. doi:10.1016/j.berh.2012.01.009
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018;153:208-215. doi:10.23736/S0392-0488.18.05922-9
- Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437. doi:10.1016/j.clindermatol.2013.01.010
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Calderon LM, Pope JE. Scleroderma epidemiology update. Curr Opin Rheumatol. 2021;33:122-127. doi:10.1097/BOR.0000000000000785
- Morgan ND, Gelber AC. African Americans and scleroderma: examining the root cause of the association. Arthritis Care Res (Hoboken). 2019;71:1151-1153. doi:10.1002/acr.23860
- Barnett AJ. The “neck sign” in scleroderma. Arthritis Rheum. 1989;32:209-211. doi:10.1002/anr.1780320215
- Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis). Postgrad Med J. 1988;64:121-125. doi:10.1136/pgmj.64.748.121
- Rossetto O, Pirazzini M, Fabris F, et al. Botulinum neurotoxins: mechanism of action. Handb Exp Pharmacol. 2021;263:35-47.doi:10.1007/164_2020_355
- Ennis D, Ahmad Z, Anderson MA, et al. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2021;35:101684. doi:10.1016/j.berh.2021.101684
- Turkmani MG, Alnomair N. Enhancement of the aesthetic outcome of scleroderma en coup de sabre with botulinum toxin injection. JAAD Case Rep. 2018;4:579-581. doi:10.1016/j.jdcr.2018.03.023
- Le EN, Freischlag JA, Christo PJ, et al. Thoracic outlet syndrome secondary to localized scleroderma treated with botulinum toxin injection. Arthritis Care Res (Hoboken). 2010;62:430-433. doi:10.1002/acr.20099
- Mousty E, Rathat G, Rouleau C, et al. Botulinum toxin type A for treatment of dyspareunia caused by localized scleroderma. Acta Obstet Gynecol Scand. 2011;90:926-927. doi:10.1111/j.1600-0412.2011.01183.x
- Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74-101. doi:10.1016/j.disamonth.2010.12.007
- Katschinski M. [Diagnosis and treatment of esophageal motility disorders]. Ther Umsch. 2001;58:128-133. doi:10.1024/0040-5930.58.3.128
- Kim DJ, Odell ID. Improvement of pterygium inversum unguis and Raynaud phenomenon with interdigital botulinum toxin injections. JAAD Case Rep. 2022;26:79-81. doi:10.1016/j.jdcr.2022.06.009
- Baral H, Sekiguchi A, Uchiyama A, et al. Inhibition of skin fibrosis in systemic sclerosis by botulinum toxin B via the suppression of oxidative stress. J Dermatol. 2021;48:1052-1061. doi:10.1111/1346-8138.15888
- Jia C, Xing T, Shang Z, et al. Botulinum toxin A improves neurogenic bladder fibrosis by suppressing transforming growth factor β1 expression in rats. Transl Androl Urol. 2021;10:2000-2007. doi:10.21037/tau-21-62
- Hoverson K, Love T, Lam TK, et al. A novel treatment for limited mouth opening due to facial fibrosis: a case series. J Am Acad Dermatol. 2018;78:190-192. doi:10.1016/j.jaad.2017.07.006
- Kollewe K, Mohammadi B, Köhler S, et al. Blepharospasm: long-term treatment with either Botox®, Xeomin® or Dysport®. J Neural Transm (Vienna). 2015;122:427-431. doi:10.1007/s00702-014-1278-z
Practice Points
- Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body.
- Although there is no curative treatment for scleroderma, there are options to slow disease progression and improve quality of life.
- Botulinum toxin injection may be a preferred treatment option in patients with features of sclerosis or fibrosis related to scleroderma, particularly in the neck region.
Hyperkeratotic Papules and Black Macules on the Hands
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3
Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3

Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3

Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
An elderly woman with a long history of hyperkeratotic papules on the abdomen, forearms, dorsal hands, and skinfolds presented with new lesions on the dorsal hands that had developed over the preceding few months after a lapse in treatment with her previous dermatologist. Her medical history was otherwise unremarkable. Physical examination revealed hyperkeratotic papules, black hemorrhagic macules with jagged borders, and a thin hemorrhagic plaque on the dorsal hands. Nail findings were notable for alternating white and red longitudinal bands with nicking of the distal nail plates. She also had scattered leucodermic macules over the trunk, feet, arms, and legs, as well as numerous hyperkeratotic papules coalescing into plaques over the mons pubis and in the inguinal folds.
VHA Support for Home Health Agency Staff and Patients During Natural Disasters
As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12
Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).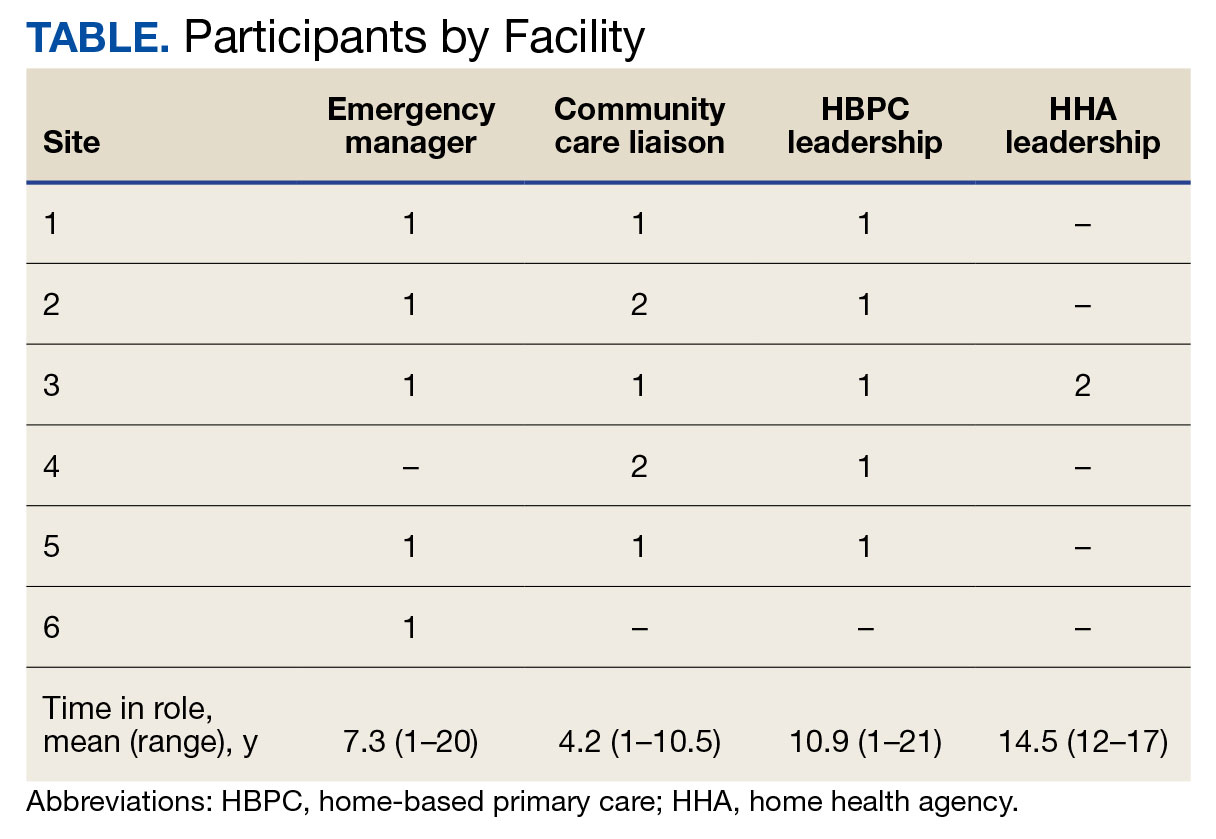
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular 
Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12
Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular 
Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

As large-scale natural disasters become more common, health care coalitions and the engagement of health systems with local, state, and federal public health departments have effectively bolstered communities’ resilience via collective sharing and distribution of resources.1 These resources may include supplies and the dissemination of emergency information, education, and training.2 The COVID-19 pandemic demonstrated that larger health care systems including hospital networks and nursing homes are better connected to health care coalition resources than smaller, independent systems, such as community home health agencies.3 This leaves some organizations on their own to meet requirements that maintain continuity of care and support their patients and staff throughout a natural disaster.
Home health care workers play important roles in the care of older adults.4 Older adults experience high levels of disability and comorbidities that put them at risk during emergencies; they often require support from paid, family, and neighborhood caregivers to live independently.5 More than 9.3 million US adults receive paid care from 2.6 million home health care workers (eg, home health aides and personal care assistants).6 Many of these individuals are hired through small independent home health agencies (HHAs), while others may work directly for an individual. When neighborhood resources and family caregiving are disrupted during emergencies, the critical services these workers administer become even more essential to ensuring continued access to medical care and social services.
The importance of these services was underscored by the Centers for Medicare and Medicaid Services 2017 inclusion of HHAs in federal emergency preparedness guidelines.7,8 The fractured and decentralized nature of the home health care industry means many HHAs struggle to maintain continuous care during emergencies and protect their staff. HHAs, and health care workers in the home, are often isolated, under-resourced, and disconnected from broader emergency planning efforts. Additionally, home care jobs are largely part-time, unstable, and low paying, making the workers themselves vulnerable during emergencies.3,9-13
This is a significant issue for the Veterans Health Administration (VHA), which annually purchases 10.5 million home health care worker visits for 150,000 veterans from community-based HHAs to enable those individuals to live independently. Figure 1 illustrates the existing structure of directly provided and contracted VHA services for community-dwelling veterans, highlighting the circle of care around the veteran.8,9 Home health care workers anchored health care teams during the COVID-19 pandemic, observing and reporting on patients’ well-being to family caregivers, primary care practitioners, and HHAs. They also provided critical emotional support and companionship to patients isolated from family and friends.9 These workers also exposed themselves and their families to considerable risk and often lacked the protection afforded by personal protective equipment (PPE) in accordance with infection prevention guidance.3,12
Abbreviations: HBPC, home based primary care; HHA, home health agency; VHA, Veterans Health Administration.
aAdapted with permission from Wyte-Lake and Franzosa.8,9
Through a combination of its national and local health care networks, the VHA has a robust and well-positioned emergency infrastructure to supportcommunity-dwelling older adults during disasters.14 This network is supported by the VHA Office of Emergency Management, which shares resources and guidance with local emergency managers at each facility as well as individual programs such as the VHA Home Based Primary Care (HBPC) program, which provides 38,000 seriously ill veterans with home medical visits.15 Working closely with their local and national hospital networks and emergency managers, individual VHA HBPC programs were able to maintain the safety of staff and continuity of care for patients enrolled in HBPC by rapidly administering COVID-19 vaccines to patients, caregivers, and staff, and providing emergency assistance during the 2017 hurricane season.16,17 These efforts were successful because HBPC practitioners and their patients, had access to a level of emergency-related information, resources, and technology that are often out of reach for individual community-based health care practitioners (HCPs). The US Department of Veterans Affairs (VA) also supports local communities through its Fourth Mission, which provides emergency resources to non-VHA health care facilities (ie, hospitals and nursing homes) during national emergencies and natural disasters.17 Although there has been an expansion in the definition of shared resources, such as extending behavioral health support to local communities, the VHA has not historically provided these resources to HHAs.14
This study examines opportunities to leverage VHA emergency management resources to support contracted HHAs and inform other large health system emergency planning efforts. The findings from the exploratory phase are described in this article. We interviewed VHA emergency managers, HBPC and VA staff who coordinate home health care worker services, as well as administrators at contracted HHAs within a Veterans Integrated Services Network (VISN). These findings will inform the second (single-site pilot study) and third (feasibility study) phases. Our intent was to (1) better understand the relationships between VA medical centers (VAMCs) and their contracted HHAs; (2) identify existing VHA emergency protocols to support community-dwelling older adults; and (3) determine opportunities to build on existing infrastructure and relationships to better support contracted HHAs and their staff in emergencies.
Methods
The 18 VISNs act as regional systems of care that are loosely connected to better meet local health needs and maximize access to care. This study was conducted at 6 of 9 VAMCs within VISN 2, the New York/New Jersey VHA Health Care Network.18 VAMCs that serve urban, rural, and mixed urban/rural catchment areas were included.
Each VAMC has an emergency management program led by an emergency manager, an HBPC program led by a program director and medical director, and a community care or purchased care office that has a liaison who manages contracted home health care worker services. The studyfocused on HBPC programs because they are most likely to interact with veterans’ home health care workers in the home and care for community-dwelling veterans during emergencies. Each VHA also contracts with a series of local HHAs that generally have a dedicated staff member who interfaces with the VHA liaison. Our goal was to interview ≥ 1 emergency manager, ≥ 1 HBPC team member, ≥ 1 community care staff person, and ≥ 1 contracted home health agency administrator at each site to gain multiple perspectives from the range of HCPs serving veterans in the community.
Recruitment and Data Collection
The 6 sites were selected in consultation with VISN 2 leadership for their strong HBPC and emergency management programs. To recruit respondents, we contacted VISN and VAMC leads and used our professional networks to identify a sample of multidisciplinary individuals who represent both community care and HBPC programs who were contacted via email.
Since each VAMC is organized differently, we utilized a snowball sampling approach to identify the appropriate contacts.19 At the completion of each interview, we asked the participant to suggest additional contacts and introduce us to any remaining stakeholders (eg, the emergency manager) at that site or colleagues at other VISN facilities. Because roles vary among VAMCs, we contacted the person who most closely resembled the identified role and asked them to direct us to a more appropriate contact, if necessary. We asked community care managers to identify 1 to 2 agencies serving the highest volume of patients who are veterans at their site and requested interviews with those liaisons. This resulted in the recruitment of key stakeholders from 4 teams across the 6 sites (Table).
A semistructured interview guide was jointly developed based on constructs of interest, including relationships within VAMCs and between VAMCs and HHAs; existing emergency protocols and experience during disasters; and suggestions and opportunities for supporting agencies during emergencies and potential barriers. Two researchers (TWL and EF) who were trained in qualitative methods jointly conducted interviews using the interview guide, with 1 researcher leading and another taking notes and asking clarifying questions.
Interviews were conducted virtually via Microsoft Teams with respondents at their work locations between September 2022 and January 2023. Interviews were audio recorded and transcribed and 2 authors (TWL and ESO) reviewed transcripts for accuracy. Interviews averaged 47 minutes in length (range, 20-59).
The study was reviewed and determined to be exempt by institutional review boards at the James J. Peters VAMC and Greater Los Angeles VAMC. We asked participants for verbal consent to participate and preserved their confidentiality.
Analysis
Data were analyzed via an inductive approach, which involves drawing salient themes rather than imposing preconceived theories.20 Three researchers (TWL, EF, and ES) listened to and discussed 2 staff interviews and tagged text with specific codes (eg, communication between the VHA and HHA, internal communication, and barriers to case fulfillment) so the team could selectively return to the interview text for deeper analysis, allowing for the development of a final codebook. The project team synthesized the findings to identify higher-level themes, drawing comparisons across and within the respondent groups, including within and between health care systems. Throughout the analysis, we maintained analytic memos, documented discussions, and engaged in analyst triangulation to ensure trustworthiness.21,22 To ensure the analysis accurately reflected the participants’ understanding, we held 2 virtual member-checking sessions with participants to share preliminary findings and conclusions and solicit feedback. Analysis was conducted using ATLAS.ti version 20.
Results
VHA-based participants described internal emergency management systems that are deployed during a disaster to support patients and staff. Agency participants described their own internal emergency management protocols. Respondents discussed how and when the 2 intersected, as well as opportunities for future mutual support. The analysis identified several themes: (1) relationships between VAMC teams; (2) relationships between VHA and HHAs; (3) VHA and agencies responses during emergencies; (4) receptivity and opportunities for extending VHA resources into the community; and (5) barriers and facilitators to deeper engagement.
Relationships Within VHA (n = 17)
Staff at all VHA sites described close relationships between the internal emergency management and HBPC teams. HBPC teams identified patients who were most at risk during emergencies to triage those with the highest medical needs (eg, patients dependent on home infusion, oxygen, or electronic medical devices) and worked alongside emergency managers to develop plans to continue care during an emergency. HBPC representatives were part of their facilities’ local emergency response committees. Due to this close collaboration, VHA emergency managers were familiar with the needs of homebound veterans and caregivers. “I invite our [HBPC] program manager to attend [committee] meetings and … they’re part of the EOC [emergency operations center]," an emergency manager said. “We work together and I’m constantly in contact with that individual, especially during natural disasters and so forth, to ensure that everybody’s prepared in the community.”
On the other hand, community caremanagers—who described frequent interactions with HBPC teams, largely around coordinating and managing non-VHA home care services—were less likely to have direct relationships with their facility emergency managers. For example, when asked if they had a relationship with their emergency manager, a community care manager admitted, “I [only] know who he is.” They also did not report having structured protocols for veteran outreach during emergencies, “because all those veterans who are receiving [home health care worker] services also belong to a primary care team,” and considered the outreach to be the responsibility of the primary care team and HHA.
Relationships Between the VHA and HHAs (n = 17)
Communication between VAMCs and contracted agencies primarily went through community care managers, who described established long-term relationships with agency administrators. Communication was commonly restricted to operational activities, such as processing referrals and occasional troubleshooting. According to a community care manager most communication is “why haven’t you signed my orders?” There was a general sense from participants that communication was promptly answered, problems were addressed, and professional collegiality existed between the agencies as patients were referred and placed for services. One community care manager reported meeting with agencies regularly, noting, “I talk to them pretty much daily.”
If problems arose, community care managers described themselves as “the liaison” between agencies and VHA HCPs who ordered the referrals. This is particularly the case if the agency needed help finding a VHA clinician or addressing differences in care delivery protocols.
Responding During Emergencies (n = 19)
During emergencies, VHA and agency staff described following their own organization’s protocols and communicating with each other only on a case-by-case basis rather than through formal or systematic channels and had little knowledge of their counterpart’s emergency protocols. Beyond patient care, there was no evidence of information sharing between VHA and agency staff. Regarding sharing information with their local community, an HBPC Program Director said, “it’s almost like the VHA had become siloed” and operated on its own without engaging with community health systems or emergency managers.
Beyond the guidance provided by state departments of public health, HHAs described collaborating with other agencies in their network and relying on their informal professional network to manage the volume of information and updates they followed during emergencies like the COVID-19 pandemic. One agency administrator did not frequently communicate with VHA partners during the pandemic but explained that the local public health department helped work through challenges. However, “we realized pretty quickly they were overloaded and there was only so much they could do.” The agency administrator turned to a “sister agency” and local hospitals, noting, “Wherever you have connections in the field or in the industry, you know you’re going to reach out to people for guidance on policies and… protocol.”
Opportunities for Extending VHA Resources to the Community (n = 16)
All VHA emergency managers were receptive to extending support to community-based HCPS and, in some cases, felt strongly that they were an essential part of veterans’ care networks. Emergency managers offered examples for how they supportedcommunity-based HCPs, such as helping those in the VAMC medical foster home program develop and evaluate emergency plans. Many said they had not explicitly considered HHAs before (Appendix).
Emergency managers also described how supporting community-based HCPs could be considered within the scope of the VHA role and mission, specifically the Fourth Mission. “I think that we should be making our best effort to make sure that we’re also providing that same level [of protection] to the people taking care of the veteran [as our VHA staff],” an emergency manager said. “It’s our responsibility to provide the best for the staff that are going into those homes to take care of that patient.”
In many cases, emergency managers had already developed practical tools that could be easily shared outside the VHA, including weather alerts, trainings, emergency plan templates, and lists of community resources and shelters (Figure 2). A number of these examples built on existing communication channels. One emergency manager said that the extension of resources could be an opportunity to decrease the perceived isolation of home health care workers through regular 
Abbreviations: PPE, personal protective equipment; VA, US Department of Veterans Affairs.
On the agency side, participants noted that some HHAs could benefit more from support than others. While some agencies are well staffed and have good protocols and keep up to date, “There are smaller agencies, agencies that are starting up that may not have the resources to just disseminate all the information. Those are the agencies [that] could well benefit from the VHA,” an HBPC medical director explained. Agency administrators suggested several areas where they would welcome support, including a deeper understanding of available community resources and access to PPE for staff. Regarding informational resources, an administrator said, “Anytime we can get information, it’s good to have it come to you and not always have to go out searching for it.”
Barriers and Facilitators to Partnering With Community Agencies (n = 16)
A primary barrier regarding resource sharing was potential misalignment between each organization’s policies. HHAs followed state and federal public health guidelines, which sometimes differed from VHA policies. Given that agencies care for both VHA and non-VHA clients, questions also arose around how agencies would prioritize information from the VHA, if they were already receiving information from other sources. When asked about information sharing, both VHA staff and agencies agreed staff time to support any additional activities should be weighed against the value of the information gained.
Six participants also shared that education around emergency preparedness could be an opportunity to bridge gaps between VAMCs and their surrounding communities.
Two emergency managers noted the need to be sensitive in the way they engaged with partners, respecting and building on the work that agencies were already doing in this area to ensure VHA was seen as a trusted partner and resource rather than trying to impose new policies or rules on community-based HCPs. “I know that like all leadership in various organizations, there’s a little bit of bristling going on when other people try and tell them what to do,” an HBPC medical director said. “However, if it is established that as a sort of greater level like a state level or a federal level, that VHA can be a resource. I think that as long as that’s recognized by their own professional organizations within each state, then I think that that would be a tremendous advantage to many agencies.”
In terms of sharing physical resources, emergency managers raised concerns around potential liability, although they also acknowledged this issue was important enough to think about potential workarounds. As one emergency manager said, “I want to know that my PPE is not compromised in any way shape or form and that I am in charge of that PPE, so to rely upon going to a home and hoping that [the PPE] wasn’t compromised … would kind of make me a little uneasy.” This emergency manager suggested possible solutions, such as creating a sealed PPE package to give directly to an aide.
Discussion
As the prevalence of climate-related disasters increases, the need to ensure the safety and independence of older adults during emergencies grows more urgent. Health systems must think beyond the direct services they provide and consider the community resources upon which their patients rely. While relationships did not formally exist between VHA emergency managers and community home health HCPs in the sample analyzed in this article, there is precedent and interest in supporting contracted home health agencies caring for veterans in the community. Although not historically part of the VA Fourth Mission, creating a pipeline of support for contracted HHAs by leveraging existing relationships and resources can potentially strengthen its mission to protect older veterans in emergencies, help them age safely in place, and provide a model for health systems to collaborate with community-based HCPs around emergency planning and response (Figure 3).23

Existing research on the value of health care coalitions highlights the need for established and growing partnerships with a focus on ensuring they are value-added, which echoes concerns we heard in interviews.24 Investment in community partnerships not only includes sharing supplies but also relying on bidirectional support that can be a trusted form of timely information.1,25 The findings in this study exhibit strong communication practices within the VHA during periods of nonemergency and underscore the untapped value of the pre-existing relationship between VAMCs and their contracted HHAs as an area of potential growth for health care coalitions.
Sharing resources in a way that does not put new demands on partners contributes to the sustainability and value-added nature of coalitions. Examples include establishing new low-investment practices (ie, information sharing) that support capacity and compliance with existing requirements rather than create new responsibilities for either member of the coalition. The relationship between the VHA emergency managers and the VHA HBPC program can act as a guide. The emergency managers interviewed for this study are currently engaged with HBPC programs and therefore understand the needs of homebound older adults and their caregivers. Extending the information already available to the HBPC teams via existing channels strengthens workforce practices and increased security for the shared patient, even without direct relationships between emergency managers and agencies. It is important to understand the limitations of these practices, including concerns around conflicting federal and state mandates, legal concerns around the liability of sharing physical resources (such as PPE), and awareness that the objective is not for the VHA to increase burdens (eg, increasing compliance requirements) but rather to serve as a resource for a mutual population in a shared community.
Offering training and practical resources to HHA home health care workers can help them meet disaster preparedness requirements. This is particularly important considering the growing home care workforce shortages, a topic mentioned by all HBPC and community care participants interviewed for this study.26,27 Home health care workers report feeling underprepared and isolated while on the job in normal conditions, a sentiment exacerbated by the COVID-19 pandemic.3,10 Supporting these individuals may help them feel more prepared and connected to their work, improving stability and quality of care.
While these issues are priorities within the VHA, there is growing recognition at the state and federal level of the importance of including older adults and their HCPs in disaster preparedness and response.5,28 The US Department of Health and Human Services, for example, includes older adults and organizations that serve them on its National Advisory Committee on Seniors and Disasters. The Senate version of the 2023 reauthorization of the Pandemic and All-Hazards Preparedness and Response Act included specific provisions to support community-dwelling older adults and people with disabilities, incorporating funding for community organizations to support continuity of services and avoid institutionalization in an emergency.29 Other proposed legislation includes the Real Emergency Access for Aging and Disability Inclusion for Disasters Act, which would ensure the needs of older adults and people with disabilities are explicitly included in all phases of emergency planning and response.30
The VHA expansion of the its VEText program to include disaster response is an effort to more efficiently extend outreach to older and vulnerable patients who are veterans.31 Given these growing efforts, the VHA and other health systems have an opportunity to expand internal emergency preparedness efforts to ensure the health and safety of individuals living in the community.
Limitations
VISN 2 has been a target of terrorism and other disasters. In addition to the sites being initially recruited for their strong emergency management protocols, this context may have biased respondents who are favorable to extending their resources into the community. At the time of recruitment, contracted HHAs were still experiencing staff shortages due to the COVID-19 pandemic, which limited the ability of agency staff to participate in interviews. Additionally, while the comprehensive exploration of VISN 2 facilities allows for confidence of the organizational structures described, the qualitative research design and small study sample, the study findings cannot be immediately generalized to all VISNs.
Conclusions
Many older veterans increasingly rely on home health care workers to age safely. The VHA, as a large national health care system and leader in emergency preparedness, could play an important role in supporting home health care workers and ameliorating their sense of isolation during emergencies and natural disasters. Leveraging existing resources and relationships may be a low-cost, low-effort opportunity to build higher-level interventions that support the needs of patients. Future research and work in this field, including the authors’ ongoing work, will expand agency participation and engage agency staff in conceptualizing pilot projects to ensure they are viable and feasible for the field.

- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
- Barnett DJ, Knieser L, Errett NA, Rosenblum AJ, Seshamani M, Kirsch TD. Reexamining health-care coalitions in light of COVID-19. Disaster Med public Health Prep. 2022;16(3):859-863. doi:10.1017/dmp.2020.431
- Wulff K, Donato D, Lurie N. What is health resilience and how can we build it? Annu Rev Public Health. 2015;36:361-374. doi:10.1146/annurev-publhealth-031914-122829
- Franzosa E, Wyte-Lake T, Tsui EK, Reckrey JM, Sterling MR. Essential but excluded: building disaster preparedness capacity for home health care workers and home care agencies. J Am Med Dir Assoc. 2022;23(12):1990-1996. doi:10.1016/j.jamda.2022.09.012
- Miner S, Masci L, Chimenti C, Rin N, Mann A, Noonan B. An outreach phone call project: using home health to reach isolated community dwelling adults during the COVID 19 lockdown. J Community Health. 2022;47(2):266-272. doi:10.1007/s10900-021-01044-6
- National Institute on Aging. Protecting older adults from the effects of natural disasters and extreme weather. October 18, 2022. Accessed August 19, 2024. https://www.nia.nih.gov/news/protecting-older-adults-effects-natural-disasters-and-extreme-weather
- PHI. Direct Care Workers in the United States: Key Facts. September 7, 2021. Accessed August 19, 2024. https://www.phinational.org/resource/direct-care-workers-in-the-united-states-key-facts-2/
- Centers for Medicare & Medicaid Services. Emergency Preparedness Rule. September 8, 2016. Updated September 6, 2023. Accessed August 19, 2024. https://www.cms.gov/medicare/health-safety-standards/quality-safety-oversight-emergency-preparedness/emergency-preparedness-rule
- Wyte-Lake T, Claver M, Tubbesing S, Davis D, Dobalian A. Development of a home health patient assessment tool for disaster planning. Gerontology. 2019;65(4):353-361. doi:10.1159/000494971
- Franzosa E, Judon KM, Gottesman EM, et al. Home health aides’ increased role in supporting older veterans and primary healthcare teams during COVID-19: a qualitative analysis. J Gen Intern Med. 2022;37(8):1830-1837. doi:10.1007/s11606-021-07271-w
- Franzosa E, Tsui EK, Baron S. “Who’s caring for us?”: understanding and addressing the effects of emotional labor on home health aides’ well-being. Gerontologist. 2019;59(6):1055-1064. doi:10.1093/geront/gny099
- Osakwe ZT, Osborne JC, Samuel T, et al. All alone: a qualitative study of home health aides’ experiences during the COVID-19 pandemic in New York. Am J Infect Control. 2021;49(11):1362-1368. doi:10.1016/j.ajic.2021.08.004
- Feldman PH, Russell D, Onorato N, et al. Ensuring the safety of the home health aide workforce and the continuation of essential patient care through sustainable pandemic preparedness. July 2022. Accessed August 19, 2024. https://www.vnshealth.org/wp-content/uploads/2022/08/Pandemic_Preparedness_IB_07_21_22.pdf
- Sterling MR, Tseng E, Poon A, et al. Experiences of home health care workers in New York City during the coronavirus disease 2019 pandemic: a qualitative analysis. JAMA Internal Med. 2020;180(11):1453-1459. doi:10.1001/jamainternmed.2020.3930
- Wyte-Lake T, Schmitz S, Kornegay RJ, Acevedo F, Dobalian A. Three case studies of community behavioral health support from the US Department of Veterans Affairs after disasters. BMC Public Health. 2021;21(1):639. doi:10.1186/s12889-021-10650-x
- Beales JL, Edes T. Veteran’s affairs home based primary care. Clin Geriatr Med. 2009;25(1):149-ix. doi:10.1016/j.cger.2008.11.002
- Wyte-Lake T, Manheim C, Gillespie SM, Dobalian A, Haverhals LM. COVID-19 vaccination in VA home based primary care: experience of interdisciplinary team members. J Am Med Dir Assoc. 2022;23(6):917-922. doi:10.1016/j.jamda.2022.03.014
- Wyte-Lake T, Schmitz S, Cosme Torres-Sabater R, Dobalian A. Case study of VA Caribbean Healthcare System’s community response to Hurricane Maria. J Emerg Manag. 2022;19(8):189-199. doi:10.5055/jem.0536
- US Department of Veterans Affairs. New York/New Jersey VA Health Care Network, VISN 2 Locations. Updated January 3, 2024. Accessed August 19, 2024. https://www.visn2.va.gov/visn2/facilities.asp
- Noy C. Sampling knowledge: the hermeneutics of snowball sampling in qualitative research. Int J Soc Res Methodol. 2008;11(4):327-344. doi:10.1080/13645570701401305
- Ritchie J, Lewis J, Nicholls CM, Ormston R, eds. Qualitative Research Practice: A Guide for Social Science Students and Researchers. 2nd ed. Sage; 2013.
- Morrow SL. Quality and trustworthiness in qualitative research in counseling psychology. J Couns Psychol. 2005;52(2):250-260. doi:10.1037/0022-0167.52.2.250
- Rolfe G. Validity, trustworthiness and rigour: quality and the idea of qualitative research. J Adv Nurs. 2006;53(3):304-310. doi:10.1111/j.1365-2648.2006.03727.x
- Schmitz S, Wyte-Lake T, Dobalian A. Facilitators and barriers to preparedness partnerships: a veterans affairs medical center perspective. Disaster Med Public Health Prep. 2018;12(4):431-436. doi:10.1017/dmp.2017.92
- Koch AE, Bohn J, Corvin JA, Seaberg J. Maturing into high-functioning health-care coalitions: a qualitative Nationwide study of emergency preparedness and response leadership. Disaster Med Public Health Prep. 2022;17:e111. doi:10.1017/dmp.2022.13
- Lin JS, Webber EM, Bean SI, Martin AM, Davies MC. Rapid evidence review: policy actions for the integration of public health and health care in the United States. Front Public Health. 2023;11:1098431. doi:10.3389/fpubh.2023.1098431
- Watts MOM, Burns A, Ammula M. Ongoing impacts of the pandemic on medicaid home & community-based services (HCBS) programs: findings from a 50-state survey. November 28, 2022. Accessed August 19, 2024. https://www.kff.org/medicaid/issue-brief/ongoing-impacts-of-the-pandemic-on-medicaid-home-community-based-services-hcbs-programs-findings-from-a-50-state-survey/
- Kreider AR, Werner RM. The home care workforce has not kept pace with growth in home and community-based services. Health Aff (Millwood). 2023;42(5):650-657. doi:10.1377/hlthaff.2022.01351
- FEMA introduces disaster preparedness guide for older adults. News release. FEMA. September 20, 2023. Accessed August 19, 2024. https://www.fema.gov/press-release/20230920/fema-introduces-disaster-preparedness-guide-older-adults
- Pandemic and All-Hazards Preparedness and Response Act, S 2333, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/senate-bill/2333/text
- REAADI for Disasters Act, HR 2371, 118th Cong, 1st Sess (2023). https://www.congress.gov/bill/118th-congress/house-bill/2371
- Wyte-Lake T, Brewster P, Hubert T, Gin J, Davis D, Dobalian A. VA’s experience building capability to conduct outreach to vulnerable patients during emergencies. Innov Aging. 2023;7(suppl 1):209. doi:10.1093/geroni/igad104.0690
Open Clinical Trials for Patients With Diabetes
Actively Recruiting
→Continuous Glucose Monitoring Devices in Hospitalized Veterans With Diabetes
More than 25% of the patients admitted in the general wards have a history of diabetes mellitus. Up to 30% of the hospitalized diabetics develop hypoglycemia (low glucose values); a condition that is associated with seizures, cardiac arrhythmias, and even death. In veterans, the prevalence is disproportionally higher. It is estimated that 40% to 50% of hospitalized veterans are diabetics. In this clinical trial the investigators describe the development of a novel system, the Glucose Telemetry System, with which glucose values can be wirelessly transmitted from the patient’s bedside to a monitor device at the nursing station. The goal of this work is to develop a more effective glucose surveillance system at the general wards, which can decrease hypoglycemia in the hospital and improve clinical outcomes.
ID: NCT03508934
Sponsor; Investigator: VA Office of Research and Development; Ilias Spanakis, MD
Locations: Baltimore VA Medical Center, Maryland
→Optimizing Gait Rehabilitation for Veterans With Non-Traumatic Lower Limb Amputation (GEM)
The population of older veterans with nontraumatic lower limb amputation is growing. Following lower limb amputation, asymmetrical movements persist during walking and likely contribute to disabling sequelae including secondary pain conditions, poor gait efficiency, impaired physical function, and compromised skin integrity of the residual limb. This study seeks to address chronic gait asymmetry by evaluating the efficacy of two error-manipulation gait training programs to improve gait symmetry for veterans with nontraumatic lower limb amputation. Additionally, this study will evaluate the potential of error-manipulation training programs to improve secondary measures of disability and residual limb skin health. Ultimately, this study aims to improve conventional prosthetic rehabilitation for veterans with nontraumatic amputation through gait training programs based in motor learning principles, resulting in improved.
ID: NCT003995238
Sponsor; Investigator: VA Office of Research and Development; Cory L. Christiansen, PhD
Locations: Rocky Mountain Regional VA Medical Center, Aurora, Colorado; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
→Empowering Veterans to Actively Communicate and Engage in Shared Decision Making in Medical Visits, A Randomized Controlled Trial (ACTIVet-2)
Type 2 diabetes is a significant condition in VA affecting 20% of VA patients. Adherence to medication regimens and lifestyle factors is important to achieve care goals for these patients. Patients who use active participatory communication behaviors with their providers have better adherence to treatment and better biomedical outcomes, yet many patients are not prepared to engage in active communication with their providers. Existing coaching interventions have not been adopted in practice because of the cost of trained personnel. The investigators haveshown the efficacy of a low-cost video that did not require trained personnel. This proposal proposes to test implementation strategies to deliver that video in VA primary care clinics and to test the effectiveness of the video to improve outcomes in a hybrid type 2 effectiveness implementation trial using a cluster randomized stepped wedgedesign at eight sites. This proposal will test feasibility of implementing the video and if successful will generate the evidence to justify widespread dissemination of the video.
ID: NCT05169359
Sponsor; Investigator: VA Office of Research and Development; Howard S. Gordon, MD
Location: 8 locations, including Jesse Brown VA Medical Center, Chicago; Edward Hines Jr. VA Hopsital, Hines, Illinois
→The Diabetes Staging System in Patient Aligned Care Teams
The purpose of this study is to examine the feasibility/acceptability of the Diabetes Staging System in patient aligned care teams and its ability to increase sodium-glucosecotransporter-2 inhibitor and glucagon-like-1 peptide use in veteran patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease. A novel type 2 diabetes staging system patterned after Tumor Node Metastasis cancer staging that uses the number of macrovascular and microvascular complications and most recent hemoglobin A1c and glomerular filtration rate to determine Diabetes Staging System stage which reflects disease severity.
ID: NCT06142006
Sponsor; Collaborator: Durham VA Medical Center; Moahad Dar, MD
Location: Greenville VA Health Care Center, North Carolina
→Enhancing Mental and Physical Health of Women Veterans (EMPOWER)
Women veterans are the fastest growing segment of VA users. This dramatic growth has created challenges for VA to ensure that appropriate services are available to meet women veterans’ needs, and that they will want and be able to use those services. The EMPOWER QUERI 2.0 Program is a cluster randomized type 3 hybrid implementation effectiveness trial testing 2 strategies designed to support implementation and sustainment of evidence-based practices for women veterans in at least 20 VA facilities from 4 regions.
ID: NCT05050266
Sponsor; Investigator: VA Office of Research and Development; Alison B. Hamilton, PhD, MPH
Location: VA Greater Los Angeles Healthcare System
→Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA INTREPID)
The purpose of this research study is to determine if rifampin, an antibiotic (a medicine that treats infections), is effective in treating osteomyelitis (infection of the bone) of the foot in patients with diabetes. Despite use of powerful antibiotics prescribed over a long period of time, many diabetic patients remain at a high risk for needing an amputation of part of the foot or lower leg because the osteomyelitis is not cured. Some small research studies have shown that addition of rifampin to other antibiotics is effective in treating osteomyelitis. However, because few diabetics with osteomyelitis have been studied, there is no definite proof that it is better than the usual treatments for diabetic patients. If this study finds that adding rifampin to the usual antibiotics prescribed for osteomyelitis reduces the risk for amputations, doctors will be able to more effectively treat many veteran patients with this serious infection. Improving treatment outcomes is an important healthcare goal of the VA.
ID: NCT03012529
Sponsor; Investigator: VA Office of Research and Development; Paul A. Monach, MD, PhD
Location: 30 VA locations, including South Texas Health Care System, San Antonio; Cincinnati VA Medical Center, Ohio; VA Northern California Health Care System, Mather; Washington DC VA Medical Center; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
→Effectiveness of Remote Foot Temperature Monitoring (STOP)
Diabetic foot ulcers are common, debilitating, and costly complications of diabetes, disproportionately impacting Black and rural veterans. Forty percent of individuals have an ulcer recurrence within a year of ulcer healing and 65% within 5 years. Monitoring plantar foot temperatures is one of the few interventions that reduces the risk of ulcer recurrence. Despite the evidence, adoption has been poorbecause the original procedures, including the use of handheld thermometers, were burdensome and time-consuming. Podimetrics, a private company, has developed a temperature monitoring system involving a “smart” mat that can wirelessly transmit data and a remote monitoring team that works with VA providers to assist with triage and monitoring. This care model has incredible promise, but has been untested in VA. The investigators propose to conduct a randomized trial to evaluate effectiveness of remote temperature monitoring as well as costs. Additionally, the investigators will evaluate the implementation process, including barriers and facilitators to use among key stakeholders.
ID: NCT05728411
Sponsor; Investigator: VA Office of Research and Development; Rachel M. Thomas
Location: Edward Hines Jr. VA Hospital, Hines, Illinois; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia; VA Puget Sound Health Care System, Seattle, Washington
→Continuous Glucose Monitoring for Hyperglycemia in Critically Ill Patients
The investigators intend to conduct a single-center, prospective, randomized comparative trial of patients admitted to the intensive care unit who received continuous glucose monitoring vs point of care glucose monitoring. The study will examine relevant outcomes for patients in the intensive care unit with diabetes mellitus and/or hyperglycemia. The primary outcome of the study will be the proportion of time in target range (blood glucose 70-180 mg/dL).
ID: NCT05442853
Sponsor; Investigator: Malcom Randall VA Medical Center; Andrew J. Franck, PharmD
Location: Malcolm Randall VA Medical Center, Gainesville, Florida
→Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT)
CSP #2002 is a multicenter, prospective, randomized, double blind, secondary prevention trial to test the hypothesis that treatment with metformin, compared with placebo, reduces mortality and cardiovascular morbidity in veterans with pre-diabetes and established atherosclerotic cardiovascular disease. Qualifying patients have pre-diabetes defined by HbA 1c , fasting blood glucose, or oral glucose tolerance test criteria; clinically evident coronary, cerebrovascular, or peripheral arterial atherosclerotic cardiovascular disease; and estimated glomerular filtration rate of at least 45 mL/min/1.73 m 2 ; and do not fulfill any exclusion criteria.
ID: NCT04838392
Sponsor; Investigator: VA Office of Research and Development; Gregory G. Schwartz, PhD, MD
Locations: 40 locations
Actively Recruiting
→Continuous Glucose Monitoring Devices in Hospitalized Veterans With Diabetes
More than 25% of the patients admitted in the general wards have a history of diabetes mellitus. Up to 30% of the hospitalized diabetics develop hypoglycemia (low glucose values); a condition that is associated with seizures, cardiac arrhythmias, and even death. In veterans, the prevalence is disproportionally higher. It is estimated that 40% to 50% of hospitalized veterans are diabetics. In this clinical trial the investigators describe the development of a novel system, the Glucose Telemetry System, with which glucose values can be wirelessly transmitted from the patient’s bedside to a monitor device at the nursing station. The goal of this work is to develop a more effective glucose surveillance system at the general wards, which can decrease hypoglycemia in the hospital and improve clinical outcomes.
ID: NCT03508934
Sponsor; Investigator: VA Office of Research and Development; Ilias Spanakis, MD
Locations: Baltimore VA Medical Center, Maryland
→Optimizing Gait Rehabilitation for Veterans With Non-Traumatic Lower Limb Amputation (GEM)
The population of older veterans with nontraumatic lower limb amputation is growing. Following lower limb amputation, asymmetrical movements persist during walking and likely contribute to disabling sequelae including secondary pain conditions, poor gait efficiency, impaired physical function, and compromised skin integrity of the residual limb. This study seeks to address chronic gait asymmetry by evaluating the efficacy of two error-manipulation gait training programs to improve gait symmetry for veterans with nontraumatic lower limb amputation. Additionally, this study will evaluate the potential of error-manipulation training programs to improve secondary measures of disability and residual limb skin health. Ultimately, this study aims to improve conventional prosthetic rehabilitation for veterans with nontraumatic amputation through gait training programs based in motor learning principles, resulting in improved.
ID: NCT003995238
Sponsor; Investigator: VA Office of Research and Development; Cory L. Christiansen, PhD
Locations: Rocky Mountain Regional VA Medical Center, Aurora, Colorado; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
→Empowering Veterans to Actively Communicate and Engage in Shared Decision Making in Medical Visits, A Randomized Controlled Trial (ACTIVet-2)
Type 2 diabetes is a significant condition in VA affecting 20% of VA patients. Adherence to medication regimens and lifestyle factors is important to achieve care goals for these patients. Patients who use active participatory communication behaviors with their providers have better adherence to treatment and better biomedical outcomes, yet many patients are not prepared to engage in active communication with their providers. Existing coaching interventions have not been adopted in practice because of the cost of trained personnel. The investigators haveshown the efficacy of a low-cost video that did not require trained personnel. This proposal proposes to test implementation strategies to deliver that video in VA primary care clinics and to test the effectiveness of the video to improve outcomes in a hybrid type 2 effectiveness implementation trial using a cluster randomized stepped wedgedesign at eight sites. This proposal will test feasibility of implementing the video and if successful will generate the evidence to justify widespread dissemination of the video.
ID: NCT05169359
Sponsor; Investigator: VA Office of Research and Development; Howard S. Gordon, MD
Location: 8 locations, including Jesse Brown VA Medical Center, Chicago; Edward Hines Jr. VA Hopsital, Hines, Illinois
→The Diabetes Staging System in Patient Aligned Care Teams
The purpose of this study is to examine the feasibility/acceptability of the Diabetes Staging System in patient aligned care teams and its ability to increase sodium-glucosecotransporter-2 inhibitor and glucagon-like-1 peptide use in veteran patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease. A novel type 2 diabetes staging system patterned after Tumor Node Metastasis cancer staging that uses the number of macrovascular and microvascular complications and most recent hemoglobin A1c and glomerular filtration rate to determine Diabetes Staging System stage which reflects disease severity.
ID: NCT06142006
Sponsor; Collaborator: Durham VA Medical Center; Moahad Dar, MD
Location: Greenville VA Health Care Center, North Carolina
→Enhancing Mental and Physical Health of Women Veterans (EMPOWER)
Women veterans are the fastest growing segment of VA users. This dramatic growth has created challenges for VA to ensure that appropriate services are available to meet women veterans’ needs, and that they will want and be able to use those services. The EMPOWER QUERI 2.0 Program is a cluster randomized type 3 hybrid implementation effectiveness trial testing 2 strategies designed to support implementation and sustainment of evidence-based practices for women veterans in at least 20 VA facilities from 4 regions.
ID: NCT05050266
Sponsor; Investigator: VA Office of Research and Development; Alison B. Hamilton, PhD, MPH
Location: VA Greater Los Angeles Healthcare System
→Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA INTREPID)
The purpose of this research study is to determine if rifampin, an antibiotic (a medicine that treats infections), is effective in treating osteomyelitis (infection of the bone) of the foot in patients with diabetes. Despite use of powerful antibiotics prescribed over a long period of time, many diabetic patients remain at a high risk for needing an amputation of part of the foot or lower leg because the osteomyelitis is not cured. Some small research studies have shown that addition of rifampin to other antibiotics is effective in treating osteomyelitis. However, because few diabetics with osteomyelitis have been studied, there is no definite proof that it is better than the usual treatments for diabetic patients. If this study finds that adding rifampin to the usual antibiotics prescribed for osteomyelitis reduces the risk for amputations, doctors will be able to more effectively treat many veteran patients with this serious infection. Improving treatment outcomes is an important healthcare goal of the VA.
ID: NCT03012529
Sponsor; Investigator: VA Office of Research and Development; Paul A. Monach, MD, PhD
Location: 30 VA locations, including South Texas Health Care System, San Antonio; Cincinnati VA Medical Center, Ohio; VA Northern California Health Care System, Mather; Washington DC VA Medical Center; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
→Effectiveness of Remote Foot Temperature Monitoring (STOP)
Diabetic foot ulcers are common, debilitating, and costly complications of diabetes, disproportionately impacting Black and rural veterans. Forty percent of individuals have an ulcer recurrence within a year of ulcer healing and 65% within 5 years. Monitoring plantar foot temperatures is one of the few interventions that reduces the risk of ulcer recurrence. Despite the evidence, adoption has been poorbecause the original procedures, including the use of handheld thermometers, were burdensome and time-consuming. Podimetrics, a private company, has developed a temperature monitoring system involving a “smart” mat that can wirelessly transmit data and a remote monitoring team that works with VA providers to assist with triage and monitoring. This care model has incredible promise, but has been untested in VA. The investigators propose to conduct a randomized trial to evaluate effectiveness of remote temperature monitoring as well as costs. Additionally, the investigators will evaluate the implementation process, including barriers and facilitators to use among key stakeholders.
ID: NCT05728411
Sponsor; Investigator: VA Office of Research and Development; Rachel M. Thomas
Location: Edward Hines Jr. VA Hospital, Hines, Illinois; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia; VA Puget Sound Health Care System, Seattle, Washington
→Continuous Glucose Monitoring for Hyperglycemia in Critically Ill Patients
The investigators intend to conduct a single-center, prospective, randomized comparative trial of patients admitted to the intensive care unit who received continuous glucose monitoring vs point of care glucose monitoring. The study will examine relevant outcomes for patients in the intensive care unit with diabetes mellitus and/or hyperglycemia. The primary outcome of the study will be the proportion of time in target range (blood glucose 70-180 mg/dL).
ID: NCT05442853
Sponsor; Investigator: Malcom Randall VA Medical Center; Andrew J. Franck, PharmD
Location: Malcolm Randall VA Medical Center, Gainesville, Florida
→Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT)
CSP #2002 is a multicenter, prospective, randomized, double blind, secondary prevention trial to test the hypothesis that treatment with metformin, compared with placebo, reduces mortality and cardiovascular morbidity in veterans with pre-diabetes and established atherosclerotic cardiovascular disease. Qualifying patients have pre-diabetes defined by HbA 1c , fasting blood glucose, or oral glucose tolerance test criteria; clinically evident coronary, cerebrovascular, or peripheral arterial atherosclerotic cardiovascular disease; and estimated glomerular filtration rate of at least 45 mL/min/1.73 m 2 ; and do not fulfill any exclusion criteria.
ID: NCT04838392
Sponsor; Investigator: VA Office of Research and Development; Gregory G. Schwartz, PhD, MD
Locations: 40 locations
Actively Recruiting
→Continuous Glucose Monitoring Devices in Hospitalized Veterans With Diabetes
More than 25% of the patients admitted in the general wards have a history of diabetes mellitus. Up to 30% of the hospitalized diabetics develop hypoglycemia (low glucose values); a condition that is associated with seizures, cardiac arrhythmias, and even death. In veterans, the prevalence is disproportionally higher. It is estimated that 40% to 50% of hospitalized veterans are diabetics. In this clinical trial the investigators describe the development of a novel system, the Glucose Telemetry System, with which glucose values can be wirelessly transmitted from the patient’s bedside to a monitor device at the nursing station. The goal of this work is to develop a more effective glucose surveillance system at the general wards, which can decrease hypoglycemia in the hospital and improve clinical outcomes.
ID: NCT03508934
Sponsor; Investigator: VA Office of Research and Development; Ilias Spanakis, MD
Locations: Baltimore VA Medical Center, Maryland
→Optimizing Gait Rehabilitation for Veterans With Non-Traumatic Lower Limb Amputation (GEM)
The population of older veterans with nontraumatic lower limb amputation is growing. Following lower limb amputation, asymmetrical movements persist during walking and likely contribute to disabling sequelae including secondary pain conditions, poor gait efficiency, impaired physical function, and compromised skin integrity of the residual limb. This study seeks to address chronic gait asymmetry by evaluating the efficacy of two error-manipulation gait training programs to improve gait symmetry for veterans with nontraumatic lower limb amputation. Additionally, this study will evaluate the potential of error-manipulation training programs to improve secondary measures of disability and residual limb skin health. Ultimately, this study aims to improve conventional prosthetic rehabilitation for veterans with nontraumatic amputation through gait training programs based in motor learning principles, resulting in improved.
ID: NCT003995238
Sponsor; Investigator: VA Office of Research and Development; Cory L. Christiansen, PhD
Locations: Rocky Mountain Regional VA Medical Center, Aurora, Colorado; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia
→Empowering Veterans to Actively Communicate and Engage in Shared Decision Making in Medical Visits, A Randomized Controlled Trial (ACTIVet-2)
Type 2 diabetes is a significant condition in VA affecting 20% of VA patients. Adherence to medication regimens and lifestyle factors is important to achieve care goals for these patients. Patients who use active participatory communication behaviors with their providers have better adherence to treatment and better biomedical outcomes, yet many patients are not prepared to engage in active communication with their providers. Existing coaching interventions have not been adopted in practice because of the cost of trained personnel. The investigators haveshown the efficacy of a low-cost video that did not require trained personnel. This proposal proposes to test implementation strategies to deliver that video in VA primary care clinics and to test the effectiveness of the video to improve outcomes in a hybrid type 2 effectiveness implementation trial using a cluster randomized stepped wedgedesign at eight sites. This proposal will test feasibility of implementing the video and if successful will generate the evidence to justify widespread dissemination of the video.
ID: NCT05169359
Sponsor; Investigator: VA Office of Research and Development; Howard S. Gordon, MD
Location: 8 locations, including Jesse Brown VA Medical Center, Chicago; Edward Hines Jr. VA Hopsital, Hines, Illinois
→The Diabetes Staging System in Patient Aligned Care Teams
The purpose of this study is to examine the feasibility/acceptability of the Diabetes Staging System in patient aligned care teams and its ability to increase sodium-glucosecotransporter-2 inhibitor and glucagon-like-1 peptide use in veteran patients with type 2 diabetes and cardiovascular disease and/or chronic kidney disease. A novel type 2 diabetes staging system patterned after Tumor Node Metastasis cancer staging that uses the number of macrovascular and microvascular complications and most recent hemoglobin A1c and glomerular filtration rate to determine Diabetes Staging System stage which reflects disease severity.
ID: NCT06142006
Sponsor; Collaborator: Durham VA Medical Center; Moahad Dar, MD
Location: Greenville VA Health Care Center, North Carolina
→Enhancing Mental and Physical Health of Women Veterans (EMPOWER)
Women veterans are the fastest growing segment of VA users. This dramatic growth has created challenges for VA to ensure that appropriate services are available to meet women veterans’ needs, and that they will want and be able to use those services. The EMPOWER QUERI 2.0 Program is a cluster randomized type 3 hybrid implementation effectiveness trial testing 2 strategies designed to support implementation and sustainment of evidence-based practices for women veterans in at least 20 VA facilities from 4 regions.
ID: NCT05050266
Sponsor; Investigator: VA Office of Research and Development; Alison B. Hamilton, PhD, MPH
Location: VA Greater Los Angeles Healthcare System
→Investigation of Rifampin to Reduce Pedal Amputations for Osteomyelitis in Diabetics (VA INTREPID)
The purpose of this research study is to determine if rifampin, an antibiotic (a medicine that treats infections), is effective in treating osteomyelitis (infection of the bone) of the foot in patients with diabetes. Despite use of powerful antibiotics prescribed over a long period of time, many diabetic patients remain at a high risk for needing an amputation of part of the foot or lower leg because the osteomyelitis is not cured. Some small research studies have shown that addition of rifampin to other antibiotics is effective in treating osteomyelitis. However, because few diabetics with osteomyelitis have been studied, there is no definite proof that it is better than the usual treatments for diabetic patients. If this study finds that adding rifampin to the usual antibiotics prescribed for osteomyelitis reduces the risk for amputations, doctors will be able to more effectively treat many veteran patients with this serious infection. Improving treatment outcomes is an important healthcare goal of the VA.
ID: NCT03012529
Sponsor; Investigator: VA Office of Research and Development; Paul A. Monach, MD, PhD
Location: 30 VA locations, including South Texas Health Care System, San Antonio; Cincinnati VA Medical Center, Ohio; VA Northern California Health Care System, Mather; Washington DC VA Medical Center; William S. Middleton Memorial Veterans Hospital, Madison, Wisconsin
→Effectiveness of Remote Foot Temperature Monitoring (STOP)
Diabetic foot ulcers are common, debilitating, and costly complications of diabetes, disproportionately impacting Black and rural veterans. Forty percent of individuals have an ulcer recurrence within a year of ulcer healing and 65% within 5 years. Monitoring plantar foot temperatures is one of the few interventions that reduces the risk of ulcer recurrence. Despite the evidence, adoption has been poorbecause the original procedures, including the use of handheld thermometers, were burdensome and time-consuming. Podimetrics, a private company, has developed a temperature monitoring system involving a “smart” mat that can wirelessly transmit data and a remote monitoring team that works with VA providers to assist with triage and monitoring. This care model has incredible promise, but has been untested in VA. The investigators propose to conduct a randomized trial to evaluate effectiveness of remote temperature monitoring as well as costs. Additionally, the investigators will evaluate the implementation process, including barriers and facilitators to use among key stakeholders.
ID: NCT05728411
Sponsor; Investigator: VA Office of Research and Development; Rachel M. Thomas
Location: Edward Hines Jr. VA Hospital, Hines, Illinois; Hunter Holmes McGuire VA Medical Center, Richmond, Virginia; VA Puget Sound Health Care System, Seattle, Washington
→Continuous Glucose Monitoring for Hyperglycemia in Critically Ill Patients
The investigators intend to conduct a single-center, prospective, randomized comparative trial of patients admitted to the intensive care unit who received continuous glucose monitoring vs point of care glucose monitoring. The study will examine relevant outcomes for patients in the intensive care unit with diabetes mellitus and/or hyperglycemia. The primary outcome of the study will be the proportion of time in target range (blood glucose 70-180 mg/dL).
ID: NCT05442853
Sponsor; Investigator: Malcom Randall VA Medical Center; Andrew J. Franck, PharmD
Location: Malcolm Randall VA Medical Center, Gainesville, Florida
→Investigation of Metformin in Pre-Diabetes on Atherosclerotic Cardiovascular OuTcomes (VA-IMPACT)
CSP #2002 is a multicenter, prospective, randomized, double blind, secondary prevention trial to test the hypothesis that treatment with metformin, compared with placebo, reduces mortality and cardiovascular morbidity in veterans with pre-diabetes and established atherosclerotic cardiovascular disease. Qualifying patients have pre-diabetes defined by HbA 1c , fasting blood glucose, or oral glucose tolerance test criteria; clinically evident coronary, cerebrovascular, or peripheral arterial atherosclerotic cardiovascular disease; and estimated glomerular filtration rate of at least 45 mL/min/1.73 m 2 ; and do not fulfill any exclusion criteria.
ID: NCT04838392
Sponsor; Investigator: VA Office of Research and Development; Gregory G. Schwartz, PhD, MD
Locations: 40 locations
Commentary: Comparing Migraine Treatments, November 2024
With increasing options for migraine therapy, the right choice for each patient might not be clear. And many individual patients could experience relief from any of the different choices, meaning that there is often more than one “right” answer when it comes to selecting a migraine treatment approach for each patient. Triptans and nonsteroidal anti-inflammatory drugs (NSAIDs), which have been around for decades, have shown consistent success in treating migraine episodes. Newer therapies could be safer for patients who have contraindications to triptans or NSAIDs, and these newer medications could be more effective for some patients, but we are still trying to fully understand which types of patients. Studies aimed at reaching conclusions regarding comparisons between triptans, calcitonin gene-related peptide inhibitors (CGRPi), and other treatments can help us determine which of the different categories of treatments are most effective for certain migraine populations (age or migraine subtype) or indications (acute vs preventive therapy).
A review published in 2023 in Aging and Disease described several markers of aging that are associated with migraine, including epigenetic aging and oxidative stress.2 The review authors noted that markers of cellular senescence (ie, irreversible inhibition of cellular division) were increased in association with migraine. Additionally, endothelial progenitor cells, which reflect an increased ability for cell renewal, were decreased among migraine patients compared with the control group.
Telomeres, composed of nucleotides, are part of chromosome structures, serving to protect the molecular integrity of DNA. It has been established that shortened telomeres, often considered a reflection of aging and a marker of high potential for genetic and cellular damage, are a risk factor for physiologic changes that occur with the aging process. The 2024 Scientific Reports cross-sectional study included data from 6169 participants in the National Health and Nutrition Survey (NHANES) from 1999 to 2002.1 The researchers used statistical analysis to determine whether there was an age-influenced telomere length in relation to migraine. They found that “telomere length was inversely associated with migraine risk in those aged 20-50 years, while no relationship was observed in those aged > 50 years.” The significance of this association among the younger group, but not among the older group, is not clear.
The limited research regarding the links between migraine and physiologic markers of aging has not untangled cause-and-effect distinctions. And while there is no evidence that preventing or treating migraine could slow down these pro-aging molecular processes, we do know that the distress of migraine episodes contributes to pain, anxiety, stress, depression, and sleep disruption. Given that we can’t change a patient’s hereditary predisposition to migraines, we can make an effort to alleviate the impact of migraine by using the tools that we have.
An article published in September 2024 in the BMJ described the results of a meta-analysis that included “137 randomized controlled trials with 89,445 participants allocated to 1 of 17 active interventions or placebo.”3 Treatments included NSAIDs, paracetamol, triptans, and CGRPi. The authors observed that triptans “had the best profiles and were more efficacious” than other treatment categories, including CGRPi. Interestingly, they observed that eletriptan and ibuprofen performed better for sustained pain freedom. Efficacy and sustained relief are crucial for patients with migraine, and for those who experience relief with simple over-the-counter ibuprofen, it makes sense to avoid making changes. But for those who are not getting the relief they need with established migraine therapies, trying the newer medications, such as CGRPi, could provide a solution. It is also important to keep in mind that triptans are contraindicated for some patients, such as those with a high-risk cardiovascular profile. Additionally, some patients may have contraindications to NSAIDs.
Prevention is another important aspect of migraine care. A September 2024 article in Headache: The Journal of Head and Face Pain used a retrospective cohort analysis of Patient-Reported Outcomes Measurement Information System (PROMIS) data, which included 1245 patients using a variety of migraine preventive therapies: antidepressants, antiseizure medications, beta-blockers, and CGRPi.4 The researchers reported that patients taking “CGRPi had a statistically significant reduction in pain T-scores (60.4 [standard deviation (SD) 7.4] to 58.4 [SD 8.2], p = 0.003), especially those who switched from other preventative medications to CGRPi.” This, along with the BMJ meta-analysis,3 helps in assessing relative benefits for treatments of acute migraine episodes and for migraine prevention. However, the efficacy of various types of therapy highlights the value of considering all options for each patient.
Individual patient characteristics, particularly contraindications, also play an important role in guiding therapeutic selection. And trial and error remain part of migraine treatment, given that there are no pretesting determinants that can predict treatment success for individual patients. We need to emphasize to patients that effective migraine therapy is obtainable and important — for comfort, quality of life, and possibly overall healthy aging.
References
- Geng D, Liu H, Wang H, Wang H. Telomere length exhibits inverse association with migraine among Americans aged 20-50 years, without implications beyond age 50: a cross-sectional study. Sci Rep. 2024;14:22597. Source
- Fila M, Pawlowska E, Szczepanska J, Blasiak J. Different aspects of aging in migraine. Aging Dis. 2023;14:6. Source
- Karlsson WK, Ostinelli EG, Zhuang ZA, et al. Comparative effects of drug interventions for the acute management of migraine episodes in adults: Systematic review and network meta-analysis. BMJ. 2024;386:e080107. Source
- Peasah SK, Soh YH, Huang Y, Nguyen J, Hanmer J, Good C. Patient reported outcomes and real-world use of calcitonin gene-related peptide medications in migraine. Headache. Published online September 30, 202 Source
With increasing options for migraine therapy, the right choice for each patient might not be clear. And many individual patients could experience relief from any of the different choices, meaning that there is often more than one “right” answer when it comes to selecting a migraine treatment approach for each patient. Triptans and nonsteroidal anti-inflammatory drugs (NSAIDs), which have been around for decades, have shown consistent success in treating migraine episodes. Newer therapies could be safer for patients who have contraindications to triptans or NSAIDs, and these newer medications could be more effective for some patients, but we are still trying to fully understand which types of patients. Studies aimed at reaching conclusions regarding comparisons between triptans, calcitonin gene-related peptide inhibitors (CGRPi), and other treatments can help us determine which of the different categories of treatments are most effective for certain migraine populations (age or migraine subtype) or indications (acute vs preventive therapy).
A review published in 2023 in Aging and Disease described several markers of aging that are associated with migraine, including epigenetic aging and oxidative stress.2 The review authors noted that markers of cellular senescence (ie, irreversible inhibition of cellular division) were increased in association with migraine. Additionally, endothelial progenitor cells, which reflect an increased ability for cell renewal, were decreased among migraine patients compared with the control group.
Telomeres, composed of nucleotides, are part of chromosome structures, serving to protect the molecular integrity of DNA. It has been established that shortened telomeres, often considered a reflection of aging and a marker of high potential for genetic and cellular damage, are a risk factor for physiologic changes that occur with the aging process. The 2024 Scientific Reports cross-sectional study included data from 6169 participants in the National Health and Nutrition Survey (NHANES) from 1999 to 2002.1 The researchers used statistical analysis to determine whether there was an age-influenced telomere length in relation to migraine. They found that “telomere length was inversely associated with migraine risk in those aged 20-50 years, while no relationship was observed in those aged > 50 years.” The significance of this association among the younger group, but not among the older group, is not clear.
The limited research regarding the links between migraine and physiologic markers of aging has not untangled cause-and-effect distinctions. And while there is no evidence that preventing or treating migraine could slow down these pro-aging molecular processes, we do know that the distress of migraine episodes contributes to pain, anxiety, stress, depression, and sleep disruption. Given that we can’t change a patient’s hereditary predisposition to migraines, we can make an effort to alleviate the impact of migraine by using the tools that we have.
An article published in September 2024 in the BMJ described the results of a meta-analysis that included “137 randomized controlled trials with 89,445 participants allocated to 1 of 17 active interventions or placebo.”3 Treatments included NSAIDs, paracetamol, triptans, and CGRPi. The authors observed that triptans “had the best profiles and were more efficacious” than other treatment categories, including CGRPi. Interestingly, they observed that eletriptan and ibuprofen performed better for sustained pain freedom. Efficacy and sustained relief are crucial for patients with migraine, and for those who experience relief with simple over-the-counter ibuprofen, it makes sense to avoid making changes. But for those who are not getting the relief they need with established migraine therapies, trying the newer medications, such as CGRPi, could provide a solution. It is also important to keep in mind that triptans are contraindicated for some patients, such as those with a high-risk cardiovascular profile. Additionally, some patients may have contraindications to NSAIDs.
Prevention is another important aspect of migraine care. A September 2024 article in Headache: The Journal of Head and Face Pain used a retrospective cohort analysis of Patient-Reported Outcomes Measurement Information System (PROMIS) data, which included 1245 patients using a variety of migraine preventive therapies: antidepressants, antiseizure medications, beta-blockers, and CGRPi.4 The researchers reported that patients taking “CGRPi had a statistically significant reduction in pain T-scores (60.4 [standard deviation (SD) 7.4] to 58.4 [SD 8.2], p = 0.003), especially those who switched from other preventative medications to CGRPi.” This, along with the BMJ meta-analysis,3 helps in assessing relative benefits for treatments of acute migraine episodes and for migraine prevention. However, the efficacy of various types of therapy highlights the value of considering all options for each patient.
Individual patient characteristics, particularly contraindications, also play an important role in guiding therapeutic selection. And trial and error remain part of migraine treatment, given that there are no pretesting determinants that can predict treatment success for individual patients. We need to emphasize to patients that effective migraine therapy is obtainable and important — for comfort, quality of life, and possibly overall healthy aging.
References
- Geng D, Liu H, Wang H, Wang H. Telomere length exhibits inverse association with migraine among Americans aged 20-50 years, without implications beyond age 50: a cross-sectional study. Sci Rep. 2024;14:22597. Source
- Fila M, Pawlowska E, Szczepanska J, Blasiak J. Different aspects of aging in migraine. Aging Dis. 2023;14:6. Source
- Karlsson WK, Ostinelli EG, Zhuang ZA, et al. Comparative effects of drug interventions for the acute management of migraine episodes in adults: Systematic review and network meta-analysis. BMJ. 2024;386:e080107. Source
- Peasah SK, Soh YH, Huang Y, Nguyen J, Hanmer J, Good C. Patient reported outcomes and real-world use of calcitonin gene-related peptide medications in migraine. Headache. Published online September 30, 202 Source
With increasing options for migraine therapy, the right choice for each patient might not be clear. And many individual patients could experience relief from any of the different choices, meaning that there is often more than one “right” answer when it comes to selecting a migraine treatment approach for each patient. Triptans and nonsteroidal anti-inflammatory drugs (NSAIDs), which have been around for decades, have shown consistent success in treating migraine episodes. Newer therapies could be safer for patients who have contraindications to triptans or NSAIDs, and these newer medications could be more effective for some patients, but we are still trying to fully understand which types of patients. Studies aimed at reaching conclusions regarding comparisons between triptans, calcitonin gene-related peptide inhibitors (CGRPi), and other treatments can help us determine which of the different categories of treatments are most effective for certain migraine populations (age or migraine subtype) or indications (acute vs preventive therapy).
A review published in 2023 in Aging and Disease described several markers of aging that are associated with migraine, including epigenetic aging and oxidative stress.2 The review authors noted that markers of cellular senescence (ie, irreversible inhibition of cellular division) were increased in association with migraine. Additionally, endothelial progenitor cells, which reflect an increased ability for cell renewal, were decreased among migraine patients compared with the control group.
Telomeres, composed of nucleotides, are part of chromosome structures, serving to protect the molecular integrity of DNA. It has been established that shortened telomeres, often considered a reflection of aging and a marker of high potential for genetic and cellular damage, are a risk factor for physiologic changes that occur with the aging process. The 2024 Scientific Reports cross-sectional study included data from 6169 participants in the National Health and Nutrition Survey (NHANES) from 1999 to 2002.1 The researchers used statistical analysis to determine whether there was an age-influenced telomere length in relation to migraine. They found that “telomere length was inversely associated with migraine risk in those aged 20-50 years, while no relationship was observed in those aged > 50 years.” The significance of this association among the younger group, but not among the older group, is not clear.
The limited research regarding the links between migraine and physiologic markers of aging has not untangled cause-and-effect distinctions. And while there is no evidence that preventing or treating migraine could slow down these pro-aging molecular processes, we do know that the distress of migraine episodes contributes to pain, anxiety, stress, depression, and sleep disruption. Given that we can’t change a patient’s hereditary predisposition to migraines, we can make an effort to alleviate the impact of migraine by using the tools that we have.
An article published in September 2024 in the BMJ described the results of a meta-analysis that included “137 randomized controlled trials with 89,445 participants allocated to 1 of 17 active interventions or placebo.”3 Treatments included NSAIDs, paracetamol, triptans, and CGRPi. The authors observed that triptans “had the best profiles and were more efficacious” than other treatment categories, including CGRPi. Interestingly, they observed that eletriptan and ibuprofen performed better for sustained pain freedom. Efficacy and sustained relief are crucial for patients with migraine, and for those who experience relief with simple over-the-counter ibuprofen, it makes sense to avoid making changes. But for those who are not getting the relief they need with established migraine therapies, trying the newer medications, such as CGRPi, could provide a solution. It is also important to keep in mind that triptans are contraindicated for some patients, such as those with a high-risk cardiovascular profile. Additionally, some patients may have contraindications to NSAIDs.
Prevention is another important aspect of migraine care. A September 2024 article in Headache: The Journal of Head and Face Pain used a retrospective cohort analysis of Patient-Reported Outcomes Measurement Information System (PROMIS) data, which included 1245 patients using a variety of migraine preventive therapies: antidepressants, antiseizure medications, beta-blockers, and CGRPi.4 The researchers reported that patients taking “CGRPi had a statistically significant reduction in pain T-scores (60.4 [standard deviation (SD) 7.4] to 58.4 [SD 8.2], p = 0.003), especially those who switched from other preventative medications to CGRPi.” This, along with the BMJ meta-analysis,3 helps in assessing relative benefits for treatments of acute migraine episodes and for migraine prevention. However, the efficacy of various types of therapy highlights the value of considering all options for each patient.
Individual patient characteristics, particularly contraindications, also play an important role in guiding therapeutic selection. And trial and error remain part of migraine treatment, given that there are no pretesting determinants that can predict treatment success for individual patients. We need to emphasize to patients that effective migraine therapy is obtainable and important — for comfort, quality of life, and possibly overall healthy aging.
References
- Geng D, Liu H, Wang H, Wang H. Telomere length exhibits inverse association with migraine among Americans aged 20-50 years, without implications beyond age 50: a cross-sectional study. Sci Rep. 2024;14:22597. Source
- Fila M, Pawlowska E, Szczepanska J, Blasiak J. Different aspects of aging in migraine. Aging Dis. 2023;14:6. Source
- Karlsson WK, Ostinelli EG, Zhuang ZA, et al. Comparative effects of drug interventions for the acute management of migraine episodes in adults: Systematic review and network meta-analysis. BMJ. 2024;386:e080107. Source
- Peasah SK, Soh YH, Huang Y, Nguyen J, Hanmer J, Good C. Patient reported outcomes and real-world use of calcitonin gene-related peptide medications in migraine. Headache. Published online September 30, 202 Source
The Impact of a Metformin Recall on Patient Hemoglobin A1c Levels at a VA Network
About 1 in 10 Americans have diabetes mellitus (DM), of which about 90% to 95% are diagnosed with type 2 DM (T2DM) and veterans are disproportionately affected.1,2 About 25% enrolled in the Veterans Health Administration (VHA) have T2DM, which has been attributed to exposure to herbicides (eg, Agent Orange), decreased physical activity resulting from past physical strain, chronic pain, and other physical limitations resulting from military service.3-5
Pharmacologic management of DM is guided by the effectiveness of lifestyle interventions and comorbid diagnoses. Current DM management guidelines recommend patients with comorbid atherosclerotic cardiovascular disease, chronic kidney disease, or congestive heart failure receive first-line diabetes therapy with a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide-1 receptor (GLP-1) agonist.
Metformin remains a first-line pharmacologic option for the treatment of T2DM with the goal of achieving glycemic management when lifestyle interventions are insufficient.6,7 Newer antihyperglycemic therapies have been studied as adjunct therapy to metformin. However, there is limited literature comparing metformin directly to other medication classes for the treatment of T2DM.8-13 A systematic review of treatment-naive patients found HbA1c reductions were similar whether patients received metformin vs an SGLT-2 inhibitor, GLP-1 agonist, sulfonylurea, or thiazolidinedione monotherapy.10 The analysis found dipeptidyl-peptidase-4 (DPP-4) inhibitors had inferior HbA1c reduction compared to metformin.10 A Japanese systematic review compared metformin to thiazolidinediones, sulfonylureas, glinides, DPP-4 inhibitors, α-glucosidase inhibitors, or SGLT-2 inhibitors for ≥ 12 weeks but found no statistically significant differences in
On May 28, 2020, the US Food and Drug Administration (FDA) asked 5 pharmaceutical companies to voluntarily recall certain formulations of metformin. This action was taken when FDA testing revealed unacceptably high levels of N-Nitrosodimethylamine, a probable carcinogen.14 This FDA recall of metformin extended-release, referred to as metformin sustained-action (SA) within the VHA electronic medication file but the same type of formulation, prompted clinicians to revisit and revise the pharmacologic regimens of patients taking the drug. Because of the paucity of head-to-head trials comparing metformin with newer alternative antihyperglycemic therapies, the effect of treatment change was unknown. In response, we aimed to establish a data registry within Veterans Integrated Service Network (VISN) 6.
Registry Development
The VISN 6 registry was established to gather long-term, observational, head-to-head data that would allow review of HbA1c levels before and after the recall, as well as HbA1c levels broken down by the agent that patients were switched to after the recall. Another goal was to explore prescribing trends following the recall.
Data Access Request Tracker approval was obtained and a US Department of Veterans Affairs (VA) Information and Computing Infrastructure workspace was developed to host the registry data. The research cohort was established from this data, and the registry framework was finalized using Structured Query Language (SQL). The SQL coding allows for recurring data updates for all individuals within the cohort including date of birth, race, sex, ethnicity, VHA facility visited, weight, body mass index, HbA1c level, creatinine clearance, serum creatinine, antihyperglycemic medication prescriptions, adverse drug reactions, medication adherence (as defined by ≥ 80% refill history), and hospitalizations related to diabetes. For the purposes of this initial analysis, registry data included demographics, diabetes medications, and HbA1c results.
METHODS
This study was a concurrent, observational, multicenter, registry-based study conducted at the Western North Carolina VA Health Care System (WNCVAHCS). The study was approved by the WNCVAHCS institutional review board and research and development committees.
All patients aged ≥ 18 years with T2DM and receiving health care from VISN 6 facilities who had an active metformin SA prescription on, and 1 year prior to, June 1, 2020 (the initial date VHA began implementing the FDA metformin recall) were entered into the registry. Data from 1 year prior were collected to provide a baseline. Veterans were excluded if they received metformin SA for any indication other than T2DM, there was no pre- or postrecall HbA1c measurement, or death. We included 15,594 VISN 6 veterans.
Registry data were analyzed to determine whether a significant change in HbA1c level occurred after the metformin recall and in response to alternative agents being prescribed. Data from veterans who met all inclusion criteria were assessed during the year before and after June 1, 2020. Demographic data were analyzed using frequency and descriptive statistics. The Shapiro Wilkes test was performed, and data were found to be nonparametric; therefore the Wilcoxon signed-rank test was used to evaluate the hypothesis that HbA1c levels were not impacted by the recall.
Our sample size allowed us to create exact matched pairs of 9130 individuals and utilize rank-biserial correlation to establish effect size. Following this initial population-level test, we constructed 2 models. The first, a linear mixed-effects model, focused solely on the interaction effects between the pre- and postrecall periods and various medication classes on HbA1c levels. Second, we constructed a random-effects within-between model (REWB) to evaluate the impact ofmedication classes and demographic variables. Statistical significance was measured at P < .05 with conservative power at .90. The effect size was set to 1.0, reflecting a minimum clinically important difference. Literature establishes 0.5 as a modest level of HbA1c improvement and 1.0 as a clinically significant improvement.
RESULTS
Preliminary results included 15,594 veterans who received a metformin SA prescription as of June 1, 2020 from VISN 6 facilities; 15,392 veterans had a drug exposure end on June 1, 2020, indicating their standard therapy of metformin SA was discontinued following the FDA recall. Two hundred and two veterans were excluded from the registry because they continued to receive metformin SA from existing stock at a VISN6 facility.

Wilcoxon Signed-Rank Test
We created exact pairs by iterating the data and finding the closest measurements for each patient before and after the recall. This has the advantage over averaging a patient’s pre- and post-HbA1c levels, as it allows for a rank-biserial correlation. Using the nonparametric Wilcoxon signed-rank test, V was 20,100,707 (P < .001), indicating a significant effect. The –0.29 rank-biserial correlation, which was computed to assess the effect size of the recall, suggests that the median HbA1c level was lower postrecall vs prerecall. The magnitude of the correlation suggests a moderate effect size, and while the recall had a noticeable impact at a population level, it was not extreme (Table 2).
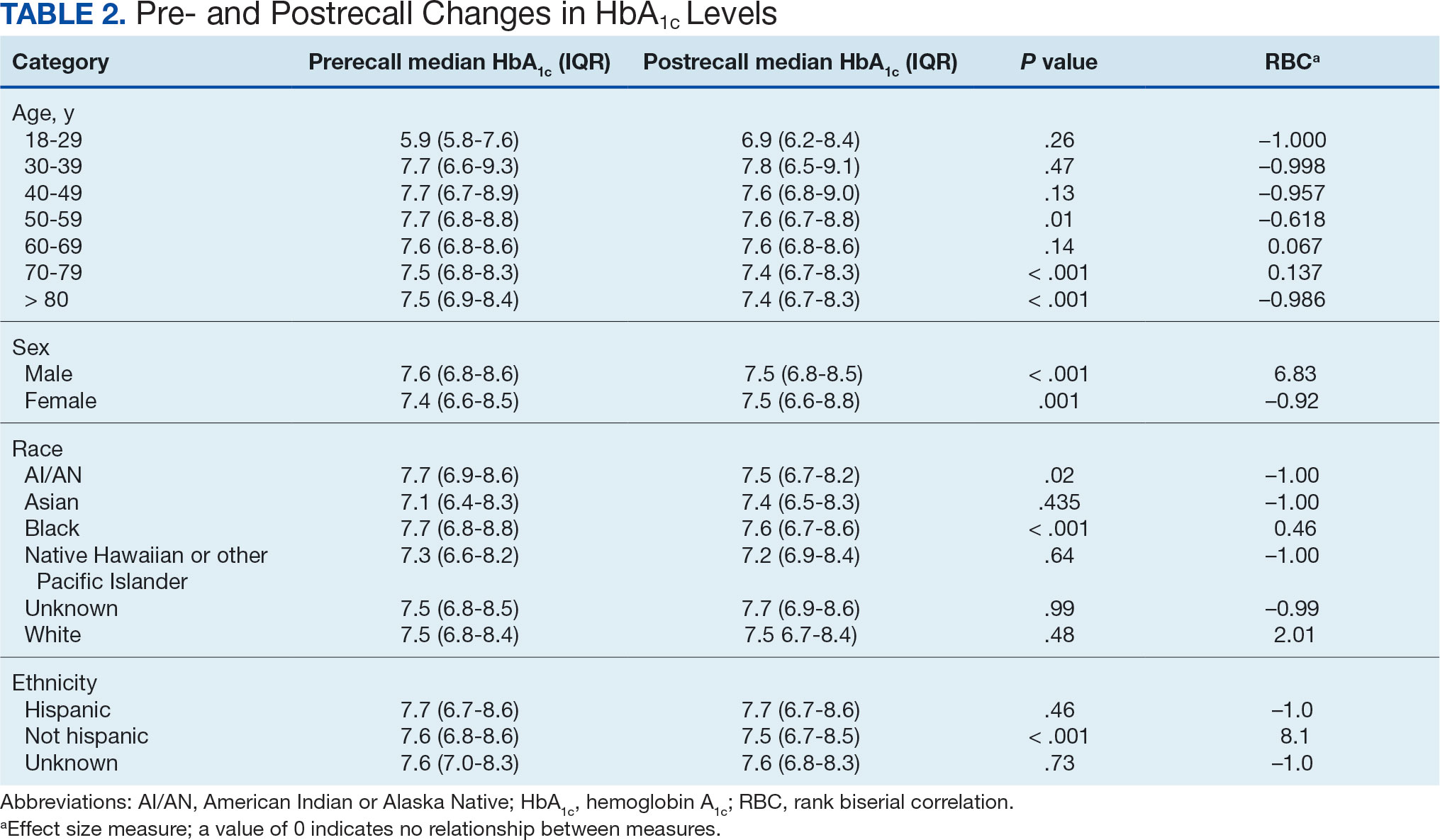
Linear Mixed-Effects Model
The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We employed a linear mixed-effects model to investigate the impact that switching from metformin SA to other T2DM medications had on HbA1c levels. The model was adjusted for patient-specific random effects and included interaction terms between the recall period (before and after) and the usage of different T2DM medications.
Model Fit and Random Effects
The model demonstrated a residual maximum likelihood criterion of 100,219.7, indicating its fit to the data. Notably, the random effects analysis revealed a substantial variability in baseline HbA1c levels across patients (SD, 0.94), highlighting the importance of individual differences in DM management. Medication classes with zero or near-zero exposure rate were removed. Due to demographic homogeneity, the model did not converge on demographic variables. Veterans were taking a mean of 1.8 T2DM medications and metformin SA was most common (Table 3).
During the postrecall period, metformin SA remained the most frequently prescribed medication class. This may be attributed to the existence of multiple manufacturers of metformin SA, some of which may not have been impacted by the recall. VISN 6 medical centers could have sought metformin SA outside of the usual procurement path following the recall.
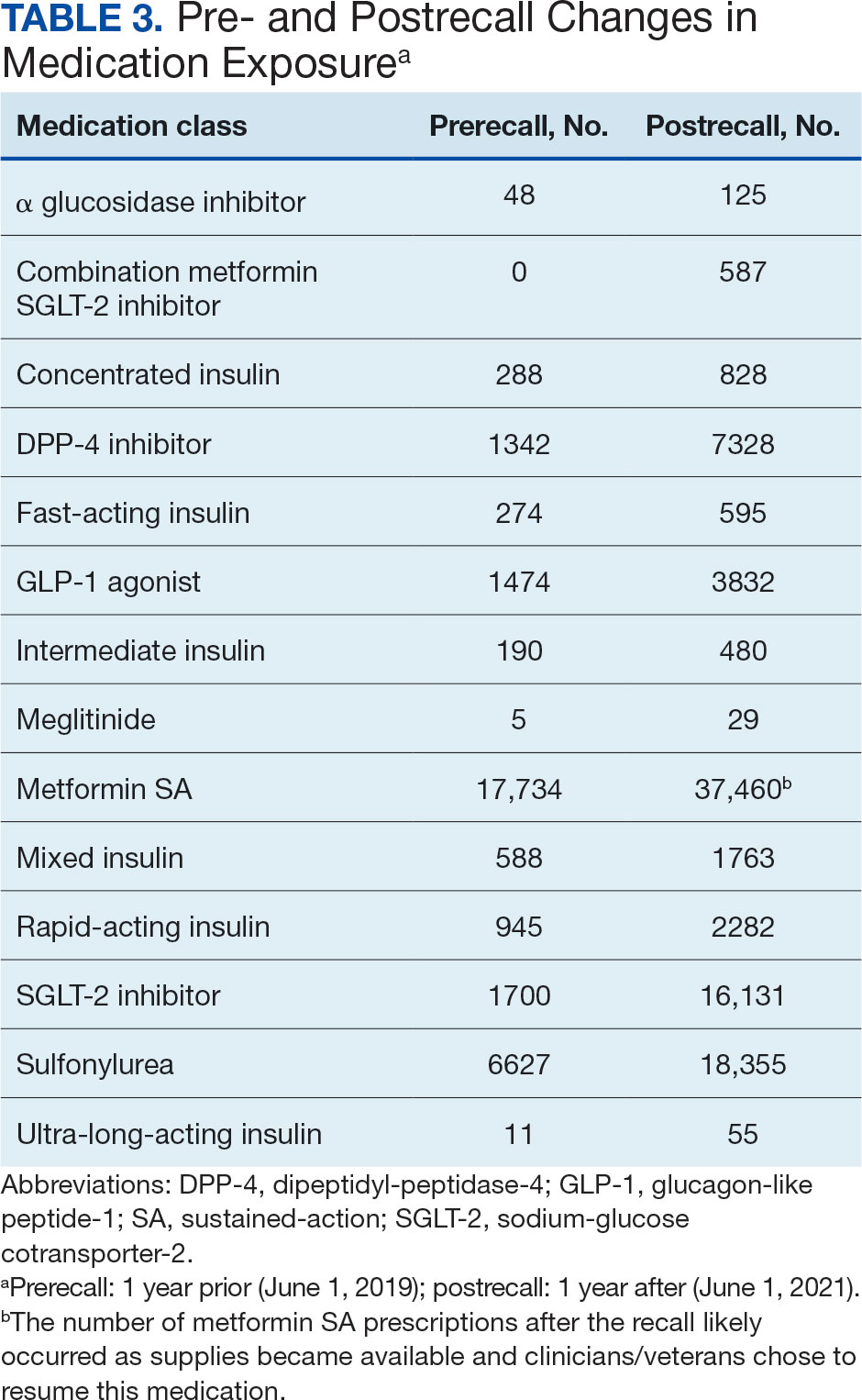
Complex Random Effects Model
We employed a complex REWB model that evaluated the impact of medication classes on HbA1c levels, accounting for both within and between subject effects of these medications, along with demographic variables (sex, race, and ethnicity) (eAppendix). This model accounts for individual-level changes over time (within-patient effects) and between groups of patients (between-patient effects). This is a more comprehensive model aimed at understanding the broader impact of medications on HbA1c levels across diverse patient groups.
Most demographic categories did not demonstrate significant effects in this model. Black individuals experienced a slight increase in HbA1c levels compared with other racial categories that was not statistically significant. However, this model confirms the findings from the linear mixed-effects model that GLP-1 agonists showed a substantial decrease in HbA1c levels within patients (coefficient –0.5; 95% CI, –0.56 to –0.44; P < .001) and a moderate increase between patients (coefficient, 0.21; 95% CI, 0.12-0.31; P < .001). Additionally, SGLT-2 inhibitors had a notable decrease within patients (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001).Another notable finding with our REWB model is insulin usage was associated with high HbA1c levels, but only between subjects. Long-acting insulin (coefficient, 0.96; 95% CI, 0.90-1.01; P <. 001) and mixed insulin (coefficient, 1.09; 95% CI, 0.94-1.24; P < .001) both displayed marked increases between patients, suggesting future analysis may benefit from stratifying across insulin users and nonusers.
Fixed Effect Analysis
The fixed effects analysis yielded several notable findings. The intercept, representing the mean baseline HbA1c level, was estimated at 7.8% (58 mmol/mol). The coefficient for the period (postrecall) was not statistically significant, indicating no overall change in HbA1c levels from before to after the recall when specific medication classes were not considered (Table 4). Among medication classes examined, several showed significant associations with HbA1c levels. DPP-4 inhibitors and GLP-1 agonists were associated with a decrease in HbA1c levels, with coefficients of −0.08 and −0.24, respectively. Long-acting insulin and metformin immediate-release (IR) were associated with an increase in HbA1c levels, as indicated by their positive coefficients of 0.38 and 0.16, respectively. Mixed insulin formulations and sulfonylureas showed an association with decreased HbA1c levels.
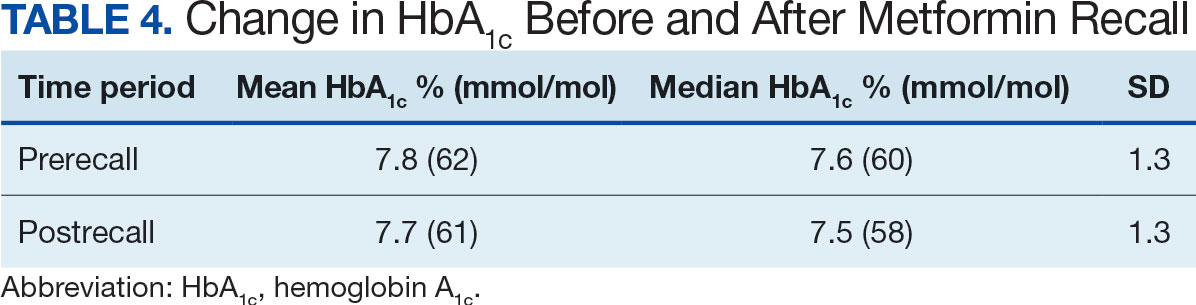
Interaction Effects
The interaction terms between the recall period and the medication classes provided insights into the differential impact of the medication switch postrecall. Notably, the interaction term for long-acting insulin (coefficient, −0.10) was significant, suggesting a differential effect on HbA1c levels postrecall. Other medications, like metformin IR, also exhibited significant interaction effects, indicating changes in the impact on HbA1c levels in the postrecall period. The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We did not address the potential for cross cluster heterogeneity due to different medication classes.
DISCUSSION
This study is an ongoing, concurrent, observational, multicenter, registry-based study consisting of VISN 6 veterans who have T2DM and were prescribed metformin SA on June 1, 2020. This initial aim was to evaluate change in HbA1c levels following the FDA metformin recall. While there was substantial variability in baseline HbA1c levels across the patients, the mean baseline HbA1c level at 7.5% (58 mmol/mol). Patients taking GLP-1 agonists showed substantial decrease in HbA1c levels (coefficient; –0.5; 95% CI, –0.56 to –0.44; P <. 001). Patients taking SGLT-2 inhibitors had a notable decrease in HbA1c (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001). Despite this, the coefficient for the postrecall period was not statistically significant, indicating no overall change in HbA1c levels from pre- to postrecall when specific medication classes were not considered.
Further analysis included assessment of prescribing trends postrecall. There was an increase in SGLT-2 inhibitor, GLP-1 agonist, and DPP-4 inhibitor prescribing. Considering the growing evidence of the cardiovascular and renal benefits of these medication classes, specifically the GLP-1 agonists and SGLT-2 inhibitors, this trend would be expected.
Limitations
This study cohort did not capture veterans with T2DM who transferred their health care to VISN 6 after June 1, 2020, and continued to receive metformin SA from the prior facility. Inclusion of these veterans would have increased the registry population. Additionally, the cohort did not identify veterans who continued to receive metformin SA through a source other than the VA. Without that information, the registry cohort may include veterans thought to have either transitioned to a different therapy or to no other T2DM therapy after the recall.
Given that DM can progress over time, it is possible the transition to a new medication after the recall was the result of suboptimal management, or in response to an adverse effect from a previous medication, and not solely due to the metformin SA recall. In addition, there are several factors that could impact HbA1c level over time that were not accounted for in this study, such as medication adherence and lifestyle modifications.
The notable level of metformin SA prescriptions, despite the recall, may be attributed to several factors. First, not all patients stopped metformin completely. Review of the prescription data indicated that some veterans were provided with limited refills at select VA medical centers that had supplies (medication lots not recalled). Access to a safe supply of metformin SA after the recall may have varied among VISN 6 facilities. It is also possible that as new supplies of metformin SA became available, veterans restarted metformin SA. This may have been resumed while continuing a new medication prescribed at the beginning of the recall. As the year progressed after the recall, an increase in metformin SA prescriptions likely occurred as supplies became available and clinicians/veterans chose to resume this medication therapy.
Conclusions
Results of this initial registry study found no difference in HbA1c levels across the study population after the metformin SA recall. However, there was clinical difference in the HbA1c within veterans prescribed SGLT-2 inhibitors and GLP-1 agonists. As expected, prescribing trends showed an increase in these agents after the recall. With the known benefits of these medications beyond glucose lowering, it is anticipated the cohort of veterans prescribed these medications will continue to grow.
The VISN 6 research registry allowed this study to gain an important snapshot in time following the metformin SA recall, and will serve as an important resource for future DM research endeavors. It will allow for ongoing evaluation of the impact of the transition to alternative T2DM medications after the metformin SA recall. Future exploration will include evaluation of adverse drug reactions, DM-related hospitalizations, emergency department visits related to T2DM, changes in renal function, and cardiovascular events among all diabetes medication classes.
Acknowledgments
The study team thanks the Veterans Affairs Informatics and Computing Infrastructure for their help and expertise throughout this project. The authors acknowledge the contributions of Philip Nelson, PharmD, and Brian Peek, PharmD.
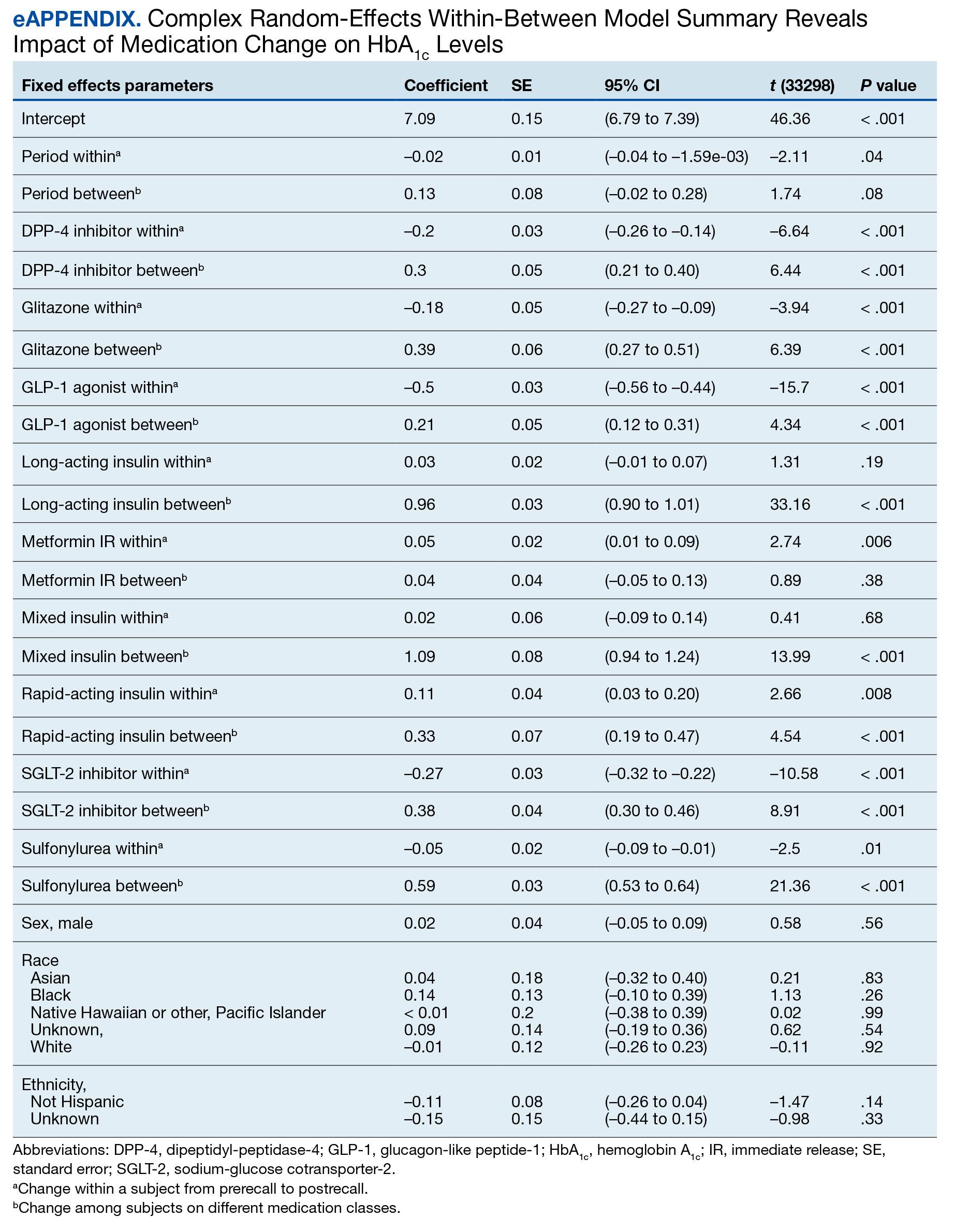
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated April 18, 2023. Accessed September 18, 2023. https://www.cdc.gov/diabetes/basics/type2.html
- ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. 2023;46(Supplement_1):S19-S40. doi:10.2337/dc23-S002
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005–2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
- Price LE, Gephart S, Shea K. The VA’s Corporate Data Warehouse: Uses and Implications for Nursing Research and Practice. Nurs Adm Q. 2015;39(4):311-318. doi:10.1097/NAQ.0000000000000118
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm - 2023 Update. Endocr Pract. 2023;29(5):305-340. doi:10.1016/j.eprac.2023.02.001
- Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602-613. doi:10.7326/0003-4819-154-9-201105030-00336
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386-399. doi:10.7326/0003-4819-147-6-200709180-00178
- Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173(4):278-286. doi:10.7326/M20-0864
- Nishimura R, Taniguchi M, Takeshima T, Iwasaki K. Efficacy and safety of metformin versus the other oral antidiabetic drugs in Japanese type 2 diabetes patients: a network meta-analysis. Adv Ther. 2022;39(1):632-654. doi:10.1007/s12325-021-01979-1
- Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252-258. doi:10.2337/dc11-1107
- Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168-2176. doi:10.2337/dc13-2759
- US Food and Drug Administration. FDA alerts patients and health care professionals to nitrosamine impurity findings in certain metformin extended-release products [press release]. May 28, 2020. Accessed October 16, 2024. https://www.fda.gov/news-events/press-announcements/fda-alerts-patients-and-health-care-professionals-nitrosamine-impurity-findings-certain-metformin
- Bell A, Jones K. Explaining fixed effects: random effects modeling of time-series cross-sectional and panel data. PSRM. 2015;3(1):133-153. doi:10.1017/psrm.2014.7
About 1 in 10 Americans have diabetes mellitus (DM), of which about 90% to 95% are diagnosed with type 2 DM (T2DM) and veterans are disproportionately affected.1,2 About 25% enrolled in the Veterans Health Administration (VHA) have T2DM, which has been attributed to exposure to herbicides (eg, Agent Orange), decreased physical activity resulting from past physical strain, chronic pain, and other physical limitations resulting from military service.3-5
Pharmacologic management of DM is guided by the effectiveness of lifestyle interventions and comorbid diagnoses. Current DM management guidelines recommend patients with comorbid atherosclerotic cardiovascular disease, chronic kidney disease, or congestive heart failure receive first-line diabetes therapy with a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide-1 receptor (GLP-1) agonist.
Metformin remains a first-line pharmacologic option for the treatment of T2DM with the goal of achieving glycemic management when lifestyle interventions are insufficient.6,7 Newer antihyperglycemic therapies have been studied as adjunct therapy to metformin. However, there is limited literature comparing metformin directly to other medication classes for the treatment of T2DM.8-13 A systematic review of treatment-naive patients found HbA1c reductions were similar whether patients received metformin vs an SGLT-2 inhibitor, GLP-1 agonist, sulfonylurea, or thiazolidinedione monotherapy.10 The analysis found dipeptidyl-peptidase-4 (DPP-4) inhibitors had inferior HbA1c reduction compared to metformin.10 A Japanese systematic review compared metformin to thiazolidinediones, sulfonylureas, glinides, DPP-4 inhibitors, α-glucosidase inhibitors, or SGLT-2 inhibitors for ≥ 12 weeks but found no statistically significant differences in
On May 28, 2020, the US Food and Drug Administration (FDA) asked 5 pharmaceutical companies to voluntarily recall certain formulations of metformin. This action was taken when FDA testing revealed unacceptably high levels of N-Nitrosodimethylamine, a probable carcinogen.14 This FDA recall of metformin extended-release, referred to as metformin sustained-action (SA) within the VHA electronic medication file but the same type of formulation, prompted clinicians to revisit and revise the pharmacologic regimens of patients taking the drug. Because of the paucity of head-to-head trials comparing metformin with newer alternative antihyperglycemic therapies, the effect of treatment change was unknown. In response, we aimed to establish a data registry within Veterans Integrated Service Network (VISN) 6.
Registry Development
The VISN 6 registry was established to gather long-term, observational, head-to-head data that would allow review of HbA1c levels before and after the recall, as well as HbA1c levels broken down by the agent that patients were switched to after the recall. Another goal was to explore prescribing trends following the recall.
Data Access Request Tracker approval was obtained and a US Department of Veterans Affairs (VA) Information and Computing Infrastructure workspace was developed to host the registry data. The research cohort was established from this data, and the registry framework was finalized using Structured Query Language (SQL). The SQL coding allows for recurring data updates for all individuals within the cohort including date of birth, race, sex, ethnicity, VHA facility visited, weight, body mass index, HbA1c level, creatinine clearance, serum creatinine, antihyperglycemic medication prescriptions, adverse drug reactions, medication adherence (as defined by ≥ 80% refill history), and hospitalizations related to diabetes. For the purposes of this initial analysis, registry data included demographics, diabetes medications, and HbA1c results.
METHODS
This study was a concurrent, observational, multicenter, registry-based study conducted at the Western North Carolina VA Health Care System (WNCVAHCS). The study was approved by the WNCVAHCS institutional review board and research and development committees.
All patients aged ≥ 18 years with T2DM and receiving health care from VISN 6 facilities who had an active metformin SA prescription on, and 1 year prior to, June 1, 2020 (the initial date VHA began implementing the FDA metformin recall) were entered into the registry. Data from 1 year prior were collected to provide a baseline. Veterans were excluded if they received metformin SA for any indication other than T2DM, there was no pre- or postrecall HbA1c measurement, or death. We included 15,594 VISN 6 veterans.
Registry data were analyzed to determine whether a significant change in HbA1c level occurred after the metformin recall and in response to alternative agents being prescribed. Data from veterans who met all inclusion criteria were assessed during the year before and after June 1, 2020. Demographic data were analyzed using frequency and descriptive statistics. The Shapiro Wilkes test was performed, and data were found to be nonparametric; therefore the Wilcoxon signed-rank test was used to evaluate the hypothesis that HbA1c levels were not impacted by the recall.
Our sample size allowed us to create exact matched pairs of 9130 individuals and utilize rank-biserial correlation to establish effect size. Following this initial population-level test, we constructed 2 models. The first, a linear mixed-effects model, focused solely on the interaction effects between the pre- and postrecall periods and various medication classes on HbA1c levels. Second, we constructed a random-effects within-between model (REWB) to evaluate the impact ofmedication classes and demographic variables. Statistical significance was measured at P < .05 with conservative power at .90. The effect size was set to 1.0, reflecting a minimum clinically important difference. Literature establishes 0.5 as a modest level of HbA1c improvement and 1.0 as a clinically significant improvement.
RESULTS
Preliminary results included 15,594 veterans who received a metformin SA prescription as of June 1, 2020 from VISN 6 facilities; 15,392 veterans had a drug exposure end on June 1, 2020, indicating their standard therapy of metformin SA was discontinued following the FDA recall. Two hundred and two veterans were excluded from the registry because they continued to receive metformin SA from existing stock at a VISN6 facility.

Wilcoxon Signed-Rank Test
We created exact pairs by iterating the data and finding the closest measurements for each patient before and after the recall. This has the advantage over averaging a patient’s pre- and post-HbA1c levels, as it allows for a rank-biserial correlation. Using the nonparametric Wilcoxon signed-rank test, V was 20,100,707 (P < .001), indicating a significant effect. The –0.29 rank-biserial correlation, which was computed to assess the effect size of the recall, suggests that the median HbA1c level was lower postrecall vs prerecall. The magnitude of the correlation suggests a moderate effect size, and while the recall had a noticeable impact at a population level, it was not extreme (Table 2).

Linear Mixed-Effects Model
The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We employed a linear mixed-effects model to investigate the impact that switching from metformin SA to other T2DM medications had on HbA1c levels. The model was adjusted for patient-specific random effects and included interaction terms between the recall period (before and after) and the usage of different T2DM medications.
Model Fit and Random Effects
The model demonstrated a residual maximum likelihood criterion of 100,219.7, indicating its fit to the data. Notably, the random effects analysis revealed a substantial variability in baseline HbA1c levels across patients (SD, 0.94), highlighting the importance of individual differences in DM management. Medication classes with zero or near-zero exposure rate were removed. Due to demographic homogeneity, the model did not converge on demographic variables. Veterans were taking a mean of 1.8 T2DM medications and metformin SA was most common (Table 3).
During the postrecall period, metformin SA remained the most frequently prescribed medication class. This may be attributed to the existence of multiple manufacturers of metformin SA, some of which may not have been impacted by the recall. VISN 6 medical centers could have sought metformin SA outside of the usual procurement path following the recall.

Complex Random Effects Model
We employed a complex REWB model that evaluated the impact of medication classes on HbA1c levels, accounting for both within and between subject effects of these medications, along with demographic variables (sex, race, and ethnicity) (eAppendix). This model accounts for individual-level changes over time (within-patient effects) and between groups of patients (between-patient effects). This is a more comprehensive model aimed at understanding the broader impact of medications on HbA1c levels across diverse patient groups.
Most demographic categories did not demonstrate significant effects in this model. Black individuals experienced a slight increase in HbA1c levels compared with other racial categories that was not statistically significant. However, this model confirms the findings from the linear mixed-effects model that GLP-1 agonists showed a substantial decrease in HbA1c levels within patients (coefficient –0.5; 95% CI, –0.56 to –0.44; P < .001) and a moderate increase between patients (coefficient, 0.21; 95% CI, 0.12-0.31; P < .001). Additionally, SGLT-2 inhibitors had a notable decrease within patients (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001).Another notable finding with our REWB model is insulin usage was associated with high HbA1c levels, but only between subjects. Long-acting insulin (coefficient, 0.96; 95% CI, 0.90-1.01; P <. 001) and mixed insulin (coefficient, 1.09; 95% CI, 0.94-1.24; P < .001) both displayed marked increases between patients, suggesting future analysis may benefit from stratifying across insulin users and nonusers.
Fixed Effect Analysis
The fixed effects analysis yielded several notable findings. The intercept, representing the mean baseline HbA1c level, was estimated at 7.8% (58 mmol/mol). The coefficient for the period (postrecall) was not statistically significant, indicating no overall change in HbA1c levels from before to after the recall when specific medication classes were not considered (Table 4). Among medication classes examined, several showed significant associations with HbA1c levels. DPP-4 inhibitors and GLP-1 agonists were associated with a decrease in HbA1c levels, with coefficients of −0.08 and −0.24, respectively. Long-acting insulin and metformin immediate-release (IR) were associated with an increase in HbA1c levels, as indicated by their positive coefficients of 0.38 and 0.16, respectively. Mixed insulin formulations and sulfonylureas showed an association with decreased HbA1c levels.

Interaction Effects
The interaction terms between the recall period and the medication classes provided insights into the differential impact of the medication switch postrecall. Notably, the interaction term for long-acting insulin (coefficient, −0.10) was significant, suggesting a differential effect on HbA1c levels postrecall. Other medications, like metformin IR, also exhibited significant interaction effects, indicating changes in the impact on HbA1c levels in the postrecall period. The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We did not address the potential for cross cluster heterogeneity due to different medication classes.
DISCUSSION
This study is an ongoing, concurrent, observational, multicenter, registry-based study consisting of VISN 6 veterans who have T2DM and were prescribed metformin SA on June 1, 2020. This initial aim was to evaluate change in HbA1c levels following the FDA metformin recall. While there was substantial variability in baseline HbA1c levels across the patients, the mean baseline HbA1c level at 7.5% (58 mmol/mol). Patients taking GLP-1 agonists showed substantial decrease in HbA1c levels (coefficient; –0.5; 95% CI, –0.56 to –0.44; P <. 001). Patients taking SGLT-2 inhibitors had a notable decrease in HbA1c (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001). Despite this, the coefficient for the postrecall period was not statistically significant, indicating no overall change in HbA1c levels from pre- to postrecall when specific medication classes were not considered.
Further analysis included assessment of prescribing trends postrecall. There was an increase in SGLT-2 inhibitor, GLP-1 agonist, and DPP-4 inhibitor prescribing. Considering the growing evidence of the cardiovascular and renal benefits of these medication classes, specifically the GLP-1 agonists and SGLT-2 inhibitors, this trend would be expected.
Limitations
This study cohort did not capture veterans with T2DM who transferred their health care to VISN 6 after June 1, 2020, and continued to receive metformin SA from the prior facility. Inclusion of these veterans would have increased the registry population. Additionally, the cohort did not identify veterans who continued to receive metformin SA through a source other than the VA. Without that information, the registry cohort may include veterans thought to have either transitioned to a different therapy or to no other T2DM therapy after the recall.
Given that DM can progress over time, it is possible the transition to a new medication after the recall was the result of suboptimal management, or in response to an adverse effect from a previous medication, and not solely due to the metformin SA recall. In addition, there are several factors that could impact HbA1c level over time that were not accounted for in this study, such as medication adherence and lifestyle modifications.
The notable level of metformin SA prescriptions, despite the recall, may be attributed to several factors. First, not all patients stopped metformin completely. Review of the prescription data indicated that some veterans were provided with limited refills at select VA medical centers that had supplies (medication lots not recalled). Access to a safe supply of metformin SA after the recall may have varied among VISN 6 facilities. It is also possible that as new supplies of metformin SA became available, veterans restarted metformin SA. This may have been resumed while continuing a new medication prescribed at the beginning of the recall. As the year progressed after the recall, an increase in metformin SA prescriptions likely occurred as supplies became available and clinicians/veterans chose to resume this medication therapy.
Conclusions
Results of this initial registry study found no difference in HbA1c levels across the study population after the metformin SA recall. However, there was clinical difference in the HbA1c within veterans prescribed SGLT-2 inhibitors and GLP-1 agonists. As expected, prescribing trends showed an increase in these agents after the recall. With the known benefits of these medications beyond glucose lowering, it is anticipated the cohort of veterans prescribed these medications will continue to grow.
The VISN 6 research registry allowed this study to gain an important snapshot in time following the metformin SA recall, and will serve as an important resource for future DM research endeavors. It will allow for ongoing evaluation of the impact of the transition to alternative T2DM medications after the metformin SA recall. Future exploration will include evaluation of adverse drug reactions, DM-related hospitalizations, emergency department visits related to T2DM, changes in renal function, and cardiovascular events among all diabetes medication classes.
Acknowledgments
The study team thanks the Veterans Affairs Informatics and Computing Infrastructure for their help and expertise throughout this project. The authors acknowledge the contributions of Philip Nelson, PharmD, and Brian Peek, PharmD.

About 1 in 10 Americans have diabetes mellitus (DM), of which about 90% to 95% are diagnosed with type 2 DM (T2DM) and veterans are disproportionately affected.1,2 About 25% enrolled in the Veterans Health Administration (VHA) have T2DM, which has been attributed to exposure to herbicides (eg, Agent Orange), decreased physical activity resulting from past physical strain, chronic pain, and other physical limitations resulting from military service.3-5
Pharmacologic management of DM is guided by the effectiveness of lifestyle interventions and comorbid diagnoses. Current DM management guidelines recommend patients with comorbid atherosclerotic cardiovascular disease, chronic kidney disease, or congestive heart failure receive first-line diabetes therapy with a sodium-glucose cotransporter-2 (SGLT-2) inhibitor or glucagon-like peptide-1 receptor (GLP-1) agonist.
Metformin remains a first-line pharmacologic option for the treatment of T2DM with the goal of achieving glycemic management when lifestyle interventions are insufficient.6,7 Newer antihyperglycemic therapies have been studied as adjunct therapy to metformin. However, there is limited literature comparing metformin directly to other medication classes for the treatment of T2DM.8-13 A systematic review of treatment-naive patients found HbA1c reductions were similar whether patients received metformin vs an SGLT-2 inhibitor, GLP-1 agonist, sulfonylurea, or thiazolidinedione monotherapy.10 The analysis found dipeptidyl-peptidase-4 (DPP-4) inhibitors had inferior HbA1c reduction compared to metformin.10 A Japanese systematic review compared metformin to thiazolidinediones, sulfonylureas, glinides, DPP-4 inhibitors, α-glucosidase inhibitors, or SGLT-2 inhibitors for ≥ 12 weeks but found no statistically significant differences in
On May 28, 2020, the US Food and Drug Administration (FDA) asked 5 pharmaceutical companies to voluntarily recall certain formulations of metformin. This action was taken when FDA testing revealed unacceptably high levels of N-Nitrosodimethylamine, a probable carcinogen.14 This FDA recall of metformin extended-release, referred to as metformin sustained-action (SA) within the VHA electronic medication file but the same type of formulation, prompted clinicians to revisit and revise the pharmacologic regimens of patients taking the drug. Because of the paucity of head-to-head trials comparing metformin with newer alternative antihyperglycemic therapies, the effect of treatment change was unknown. In response, we aimed to establish a data registry within Veterans Integrated Service Network (VISN) 6.
Registry Development
The VISN 6 registry was established to gather long-term, observational, head-to-head data that would allow review of HbA1c levels before and after the recall, as well as HbA1c levels broken down by the agent that patients were switched to after the recall. Another goal was to explore prescribing trends following the recall.
Data Access Request Tracker approval was obtained and a US Department of Veterans Affairs (VA) Information and Computing Infrastructure workspace was developed to host the registry data. The research cohort was established from this data, and the registry framework was finalized using Structured Query Language (SQL). The SQL coding allows for recurring data updates for all individuals within the cohort including date of birth, race, sex, ethnicity, VHA facility visited, weight, body mass index, HbA1c level, creatinine clearance, serum creatinine, antihyperglycemic medication prescriptions, adverse drug reactions, medication adherence (as defined by ≥ 80% refill history), and hospitalizations related to diabetes. For the purposes of this initial analysis, registry data included demographics, diabetes medications, and HbA1c results.
METHODS
This study was a concurrent, observational, multicenter, registry-based study conducted at the Western North Carolina VA Health Care System (WNCVAHCS). The study was approved by the WNCVAHCS institutional review board and research and development committees.
All patients aged ≥ 18 years with T2DM and receiving health care from VISN 6 facilities who had an active metformin SA prescription on, and 1 year prior to, June 1, 2020 (the initial date VHA began implementing the FDA metformin recall) were entered into the registry. Data from 1 year prior were collected to provide a baseline. Veterans were excluded if they received metformin SA for any indication other than T2DM, there was no pre- or postrecall HbA1c measurement, or death. We included 15,594 VISN 6 veterans.
Registry data were analyzed to determine whether a significant change in HbA1c level occurred after the metformin recall and in response to alternative agents being prescribed. Data from veterans who met all inclusion criteria were assessed during the year before and after June 1, 2020. Demographic data were analyzed using frequency and descriptive statistics. The Shapiro Wilkes test was performed, and data were found to be nonparametric; therefore the Wilcoxon signed-rank test was used to evaluate the hypothesis that HbA1c levels were not impacted by the recall.
Our sample size allowed us to create exact matched pairs of 9130 individuals and utilize rank-biserial correlation to establish effect size. Following this initial population-level test, we constructed 2 models. The first, a linear mixed-effects model, focused solely on the interaction effects between the pre- and postrecall periods and various medication classes on HbA1c levels. Second, we constructed a random-effects within-between model (REWB) to evaluate the impact ofmedication classes and demographic variables. Statistical significance was measured at P < .05 with conservative power at .90. The effect size was set to 1.0, reflecting a minimum clinically important difference. Literature establishes 0.5 as a modest level of HbA1c improvement and 1.0 as a clinically significant improvement.
RESULTS
Preliminary results included 15,594 veterans who received a metformin SA prescription as of June 1, 2020 from VISN 6 facilities; 15,392 veterans had a drug exposure end on June 1, 2020, indicating their standard therapy of metformin SA was discontinued following the FDA recall. Two hundred and two veterans were excluded from the registry because they continued to receive metformin SA from existing stock at a VISN6 facility.

Wilcoxon Signed-Rank Test
We created exact pairs by iterating the data and finding the closest measurements for each patient before and after the recall. This has the advantage over averaging a patient’s pre- and post-HbA1c levels, as it allows for a rank-biserial correlation. Using the nonparametric Wilcoxon signed-rank test, V was 20,100,707 (P < .001), indicating a significant effect. The –0.29 rank-biserial correlation, which was computed to assess the effect size of the recall, suggests that the median HbA1c level was lower postrecall vs prerecall. The magnitude of the correlation suggests a moderate effect size, and while the recall had a noticeable impact at a population level, it was not extreme (Table 2).

Linear Mixed-Effects Model
The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We employed a linear mixed-effects model to investigate the impact that switching from metformin SA to other T2DM medications had on HbA1c levels. The model was adjusted for patient-specific random effects and included interaction terms between the recall period (before and after) and the usage of different T2DM medications.
Model Fit and Random Effects
The model demonstrated a residual maximum likelihood criterion of 100,219.7, indicating its fit to the data. Notably, the random effects analysis revealed a substantial variability in baseline HbA1c levels across patients (SD, 0.94), highlighting the importance of individual differences in DM management. Medication classes with zero or near-zero exposure rate were removed. Due to demographic homogeneity, the model did not converge on demographic variables. Veterans were taking a mean of 1.8 T2DM medications and metformin SA was most common (Table 3).
During the postrecall period, metformin SA remained the most frequently prescribed medication class. This may be attributed to the existence of multiple manufacturers of metformin SA, some of which may not have been impacted by the recall. VISN 6 medical centers could have sought metformin SA outside of the usual procurement path following the recall.

Complex Random Effects Model
We employed a complex REWB model that evaluated the impact of medication classes on HbA1c levels, accounting for both within and between subject effects of these medications, along with demographic variables (sex, race, and ethnicity) (eAppendix). This model accounts for individual-level changes over time (within-patient effects) and between groups of patients (between-patient effects). This is a more comprehensive model aimed at understanding the broader impact of medications on HbA1c levels across diverse patient groups.
Most demographic categories did not demonstrate significant effects in this model. Black individuals experienced a slight increase in HbA1c levels compared with other racial categories that was not statistically significant. However, this model confirms the findings from the linear mixed-effects model that GLP-1 agonists showed a substantial decrease in HbA1c levels within patients (coefficient –0.5; 95% CI, –0.56 to –0.44; P < .001) and a moderate increase between patients (coefficient, 0.21; 95% CI, 0.12-0.31; P < .001). Additionally, SGLT-2 inhibitors had a notable decrease within patients (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001).Another notable finding with our REWB model is insulin usage was associated with high HbA1c levels, but only between subjects. Long-acting insulin (coefficient, 0.96; 95% CI, 0.90-1.01; P <. 001) and mixed insulin (coefficient, 1.09; 95% CI, 0.94-1.24; P < .001) both displayed marked increases between patients, suggesting future analysis may benefit from stratifying across insulin users and nonusers.
Fixed Effect Analysis
The fixed effects analysis yielded several notable findings. The intercept, representing the mean baseline HbA1c level, was estimated at 7.8% (58 mmol/mol). The coefficient for the period (postrecall) was not statistically significant, indicating no overall change in HbA1c levels from before to after the recall when specific medication classes were not considered (Table 4). Among medication classes examined, several showed significant associations with HbA1c levels. DPP-4 inhibitors and GLP-1 agonists were associated with a decrease in HbA1c levels, with coefficients of −0.08 and −0.24, respectively. Long-acting insulin and metformin immediate-release (IR) were associated with an increase in HbA1c levels, as indicated by their positive coefficients of 0.38 and 0.16, respectively. Mixed insulin formulations and sulfonylureas showed an association with decreased HbA1c levels.

Interaction Effects
The interaction terms between the recall period and the medication classes provided insights into the differential impact of the medication switch postrecall. Notably, the interaction term for long-acting insulin (coefficient, −0.10) was significant, suggesting a differential effect on HbA1c levels postrecall. Other medications, like metformin IR, also exhibited significant interaction effects, indicating changes in the impact on HbA1c levels in the postrecall period. The binary variable for medication class exposure suggests the use of a logit link function for binary outcomes within the multilevel modeling framework.15 We did not address the potential for cross cluster heterogeneity due to different medication classes.
DISCUSSION
This study is an ongoing, concurrent, observational, multicenter, registry-based study consisting of VISN 6 veterans who have T2DM and were prescribed metformin SA on June 1, 2020. This initial aim was to evaluate change in HbA1c levels following the FDA metformin recall. While there was substantial variability in baseline HbA1c levels across the patients, the mean baseline HbA1c level at 7.5% (58 mmol/mol). Patients taking GLP-1 agonists showed substantial decrease in HbA1c levels (coefficient; –0.5; 95% CI, –0.56 to –0.44; P <. 001). Patients taking SGLT-2 inhibitors had a notable decrease in HbA1c (coefficient, –0.27; 95% CI, –0.32 to –0.22; P < .001). Despite this, the coefficient for the postrecall period was not statistically significant, indicating no overall change in HbA1c levels from pre- to postrecall when specific medication classes were not considered.
Further analysis included assessment of prescribing trends postrecall. There was an increase in SGLT-2 inhibitor, GLP-1 agonist, and DPP-4 inhibitor prescribing. Considering the growing evidence of the cardiovascular and renal benefits of these medication classes, specifically the GLP-1 agonists and SGLT-2 inhibitors, this trend would be expected.
Limitations
This study cohort did not capture veterans with T2DM who transferred their health care to VISN 6 after June 1, 2020, and continued to receive metformin SA from the prior facility. Inclusion of these veterans would have increased the registry population. Additionally, the cohort did not identify veterans who continued to receive metformin SA through a source other than the VA. Without that information, the registry cohort may include veterans thought to have either transitioned to a different therapy or to no other T2DM therapy after the recall.
Given that DM can progress over time, it is possible the transition to a new medication after the recall was the result of suboptimal management, or in response to an adverse effect from a previous medication, and not solely due to the metformin SA recall. In addition, there are several factors that could impact HbA1c level over time that were not accounted for in this study, such as medication adherence and lifestyle modifications.
The notable level of metformin SA prescriptions, despite the recall, may be attributed to several factors. First, not all patients stopped metformin completely. Review of the prescription data indicated that some veterans were provided with limited refills at select VA medical centers that had supplies (medication lots not recalled). Access to a safe supply of metformin SA after the recall may have varied among VISN 6 facilities. It is also possible that as new supplies of metformin SA became available, veterans restarted metformin SA. This may have been resumed while continuing a new medication prescribed at the beginning of the recall. As the year progressed after the recall, an increase in metformin SA prescriptions likely occurred as supplies became available and clinicians/veterans chose to resume this medication therapy.
Conclusions
Results of this initial registry study found no difference in HbA1c levels across the study population after the metformin SA recall. However, there was clinical difference in the HbA1c within veterans prescribed SGLT-2 inhibitors and GLP-1 agonists. As expected, prescribing trends showed an increase in these agents after the recall. With the known benefits of these medications beyond glucose lowering, it is anticipated the cohort of veterans prescribed these medications will continue to grow.
The VISN 6 research registry allowed this study to gain an important snapshot in time following the metformin SA recall, and will serve as an important resource for future DM research endeavors. It will allow for ongoing evaluation of the impact of the transition to alternative T2DM medications after the metformin SA recall. Future exploration will include evaluation of adverse drug reactions, DM-related hospitalizations, emergency department visits related to T2DM, changes in renal function, and cardiovascular events among all diabetes medication classes.
Acknowledgments
The study team thanks the Veterans Affairs Informatics and Computing Infrastructure for their help and expertise throughout this project. The authors acknowledge the contributions of Philip Nelson, PharmD, and Brian Peek, PharmD.

- Centers for Disease Control and Prevention. Type 2 diabetes. Updated April 18, 2023. Accessed September 18, 2023. https://www.cdc.gov/diabetes/basics/type2.html
- ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. 2023;46(Supplement_1):S19-S40. doi:10.2337/dc23-S002
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005–2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
- Price LE, Gephart S, Shea K. The VA’s Corporate Data Warehouse: Uses and Implications for Nursing Research and Practice. Nurs Adm Q. 2015;39(4):311-318. doi:10.1097/NAQ.0000000000000118
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm - 2023 Update. Endocr Pract. 2023;29(5):305-340. doi:10.1016/j.eprac.2023.02.001
- Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602-613. doi:10.7326/0003-4819-154-9-201105030-00336
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386-399. doi:10.7326/0003-4819-147-6-200709180-00178
- Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173(4):278-286. doi:10.7326/M20-0864
- Nishimura R, Taniguchi M, Takeshima T, Iwasaki K. Efficacy and safety of metformin versus the other oral antidiabetic drugs in Japanese type 2 diabetes patients: a network meta-analysis. Adv Ther. 2022;39(1):632-654. doi:10.1007/s12325-021-01979-1
- Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252-258. doi:10.2337/dc11-1107
- Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168-2176. doi:10.2337/dc13-2759
- US Food and Drug Administration. FDA alerts patients and health care professionals to nitrosamine impurity findings in certain metformin extended-release products [press release]. May 28, 2020. Accessed October 16, 2024. https://www.fda.gov/news-events/press-announcements/fda-alerts-patients-and-health-care-professionals-nitrosamine-impurity-findings-certain-metformin
- Bell A, Jones K. Explaining fixed effects: random effects modeling of time-series cross-sectional and panel data. PSRM. 2015;3(1):133-153. doi:10.1017/psrm.2014.7
- Centers for Disease Control and Prevention. Type 2 diabetes. Updated April 18, 2023. Accessed September 18, 2023. https://www.cdc.gov/diabetes/basics/type2.html
- ElSayed NA, Aleppo G, Aroda VR, et al. 2. Classification and diagnosis of diabetes: standards of care in diabetes—2023. Diabetes Care. 2023;46(Supplement_1):S19-S40. doi:10.2337/dc23-S002
- Liu Y, Sayam S, Shao X, et al. Prevalence of and trends in diabetes among veterans, United States, 2005–2014. Prev Chronic Dis. 2017;14:E135. doi:10.5888/pcd14.170230
- Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56-65. doi:10.1016/j.envres.2014.04.027
- Price LE, Gephart S, Shea K. The VA’s Corporate Data Warehouse: Uses and Implications for Nursing Research and Practice. Nurs Adm Q. 2015;39(4):311-318. doi:10.1097/NAQ.0000000000000118
- ElSayed NA, Aleppo G, Aroda VR, et al. 9. Pharmacologic approaches to glycemic treatment: standards of care in diabetes-2023. Diabetes Care. 2023;46(suppl 1):S140-S157. doi:10.2337/dc23-S009
- Samson SL, Vellanki P, Blonde L, et al. American Association of Clinical Endocrinology Consensus Statement: Comprehensive Type 2 Diabetes Management Algorithm - 2023 Update. Endocr Pract. 2023;29(5):305-340. doi:10.1016/j.eprac.2023.02.001
- Bennett WL, Maruthur NM, Singh S, et al. Comparative effectiveness and safety of medications for type 2 diabetes: an update including new drugs and 2-drug combinations. Ann Intern Med. 2011;154(9):602-613. doi:10.7326/0003-4819-154-9-201105030-00336
- Bolen S, Feldman L, Vassy J, et al. Systematic review: comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147(6):386-399. doi:10.7326/0003-4819-147-6-200709180-00178
- Tsapas A, Avgerinos I, Karagiannis T, et al. Comparative effectiveness of glucose-lowering drugs for type 2 diabetes: a systematic review and network meta-analysis. Ann Intern Med. 2020;173(4):278-286. doi:10.7326/M20-0864
- Nishimura R, Taniguchi M, Takeshima T, Iwasaki K. Efficacy and safety of metformin versus the other oral antidiabetic drugs in Japanese type 2 diabetes patients: a network meta-analysis. Adv Ther. 2022;39(1):632-654. doi:10.1007/s12325-021-01979-1
- Russell-Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug-naive patients with type 2 diabetes (DURATION-4): a 26-week double-blind study. Diabetes Care. 2012;35(2):252-258. doi:10.2337/dc11-1107
- Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD-3). Diabetes Care. 2014;37(8):2168-2176. doi:10.2337/dc13-2759
- US Food and Drug Administration. FDA alerts patients and health care professionals to nitrosamine impurity findings in certain metformin extended-release products [press release]. May 28, 2020. Accessed October 16, 2024. https://www.fda.gov/news-events/press-announcements/fda-alerts-patients-and-health-care-professionals-nitrosamine-impurity-findings-certain-metformin
- Bell A, Jones K. Explaining fixed effects: random effects modeling of time-series cross-sectional and panel data. PSRM. 2015;3(1):133-153. doi:10.1017/psrm.2014.7
Commentary: Factors Affecting PsA and Updated Therapy Efficacy Data, November 2024
Smoking is another important modifiable environmental factor. Smoking generally has an adverse impact on treatment. In a post hoc analysis of pooled data from phase 2 and 3 trials and a long-term extension study involving 914 patients with PsA and 372 patients with ankylosing spondylitis who received tofacitinib (a Janus kinase inhibitor) or placebo, Ogdie and coworkers assessed the impact of smoking on treatment efficacy and safety. The efficacy rates were generally similar in current/past smokers and never-smokers. The incidence rates of treatment-emergent adverse events were higher in current/past smokers compared with never-smokers. Thus, in contrast to tumor necrosis factor inhibitors, smoking status may not have an impact on tofacitinib efficacy. However, current/past smokers experienced increased rates of adverse events.
Secukinumab, an anti-interleukin (IL)-17A antibody, is an established treatment for PsA and is approved for use as fixed-dose (150/300 mg) subcutaneous injections. The efficacy and safety of weight-based intravenous (IV) therapy is unknown. Kivitz and colleagues recently reported the results of the phase 3 INVIGORATE-2 trial, in which 381 patients with active PsA and either plaque psoriasis or nail psoriasis were randomly assigned to receive IV secukinumab or placebo with crossover to IV secukinumab at week 16. They demonstrated that at week 16, IV secukinumab significantly improved the American College of Rheumatology 50 response rate (ACR50) compared with placebo (31.4% vs 6.3%; adjusted P < .0001). Improvements were observed as early as week 4 and were sustained through week 52. No new safety signals were reported. Thus, IV secukinumab is a safe and efficacious treatment for PsA. This mode of administration of secukinumab is a welcome addition to the PsA therapeutic armamentarium.
There are many targeted therapies available for PsA. However, data on comparative effectiveness is lacking. Kristensen and associates reported the results of an interim analysis of the PRO-SPIRIT real-world study that included 1192 patients with PsA across six countries who initiated or switched to a new biologic or targeted synthetic disease-modifying antirheumatic drug. They showed that at 3 months, ixekizumab significantly improved clinical disease activity in patients with PsA compared with IL-12/23 inhibitors and IL-23 inhibitors. The improvements in the joints were similar to those with TNF inhibitors and JAK inhibitors, but the improvement in psoriasis was higher. Thus, ixekizumab leads to rapid response to active skin and musculoskeletal disease activity in PsA. Comparative data on treatment persistence as well as adverse events are required.
Smoking is another important modifiable environmental factor. Smoking generally has an adverse impact on treatment. In a post hoc analysis of pooled data from phase 2 and 3 trials and a long-term extension study involving 914 patients with PsA and 372 patients with ankylosing spondylitis who received tofacitinib (a Janus kinase inhibitor) or placebo, Ogdie and coworkers assessed the impact of smoking on treatment efficacy and safety. The efficacy rates were generally similar in current/past smokers and never-smokers. The incidence rates of treatment-emergent adverse events were higher in current/past smokers compared with never-smokers. Thus, in contrast to tumor necrosis factor inhibitors, smoking status may not have an impact on tofacitinib efficacy. However, current/past smokers experienced increased rates of adverse events.
Secukinumab, an anti-interleukin (IL)-17A antibody, is an established treatment for PsA and is approved for use as fixed-dose (150/300 mg) subcutaneous injections. The efficacy and safety of weight-based intravenous (IV) therapy is unknown. Kivitz and colleagues recently reported the results of the phase 3 INVIGORATE-2 trial, in which 381 patients with active PsA and either plaque psoriasis or nail psoriasis were randomly assigned to receive IV secukinumab or placebo with crossover to IV secukinumab at week 16. They demonstrated that at week 16, IV secukinumab significantly improved the American College of Rheumatology 50 response rate (ACR50) compared with placebo (31.4% vs 6.3%; adjusted P < .0001). Improvements were observed as early as week 4 and were sustained through week 52. No new safety signals were reported. Thus, IV secukinumab is a safe and efficacious treatment for PsA. This mode of administration of secukinumab is a welcome addition to the PsA therapeutic armamentarium.
There are many targeted therapies available for PsA. However, data on comparative effectiveness is lacking. Kristensen and associates reported the results of an interim analysis of the PRO-SPIRIT real-world study that included 1192 patients with PsA across six countries who initiated or switched to a new biologic or targeted synthetic disease-modifying antirheumatic drug. They showed that at 3 months, ixekizumab significantly improved clinical disease activity in patients with PsA compared with IL-12/23 inhibitors and IL-23 inhibitors. The improvements in the joints were similar to those with TNF inhibitors and JAK inhibitors, but the improvement in psoriasis was higher. Thus, ixekizumab leads to rapid response to active skin and musculoskeletal disease activity in PsA. Comparative data on treatment persistence as well as adverse events are required.
Smoking is another important modifiable environmental factor. Smoking generally has an adverse impact on treatment. In a post hoc analysis of pooled data from phase 2 and 3 trials and a long-term extension study involving 914 patients with PsA and 372 patients with ankylosing spondylitis who received tofacitinib (a Janus kinase inhibitor) or placebo, Ogdie and coworkers assessed the impact of smoking on treatment efficacy and safety. The efficacy rates were generally similar in current/past smokers and never-smokers. The incidence rates of treatment-emergent adverse events were higher in current/past smokers compared with never-smokers. Thus, in contrast to tumor necrosis factor inhibitors, smoking status may not have an impact on tofacitinib efficacy. However, current/past smokers experienced increased rates of adverse events.
Secukinumab, an anti-interleukin (IL)-17A antibody, is an established treatment for PsA and is approved for use as fixed-dose (150/300 mg) subcutaneous injections. The efficacy and safety of weight-based intravenous (IV) therapy is unknown. Kivitz and colleagues recently reported the results of the phase 3 INVIGORATE-2 trial, in which 381 patients with active PsA and either plaque psoriasis or nail psoriasis were randomly assigned to receive IV secukinumab or placebo with crossover to IV secukinumab at week 16. They demonstrated that at week 16, IV secukinumab significantly improved the American College of Rheumatology 50 response rate (ACR50) compared with placebo (31.4% vs 6.3%; adjusted P < .0001). Improvements were observed as early as week 4 and were sustained through week 52. No new safety signals were reported. Thus, IV secukinumab is a safe and efficacious treatment for PsA. This mode of administration of secukinumab is a welcome addition to the PsA therapeutic armamentarium.
There are many targeted therapies available for PsA. However, data on comparative effectiveness is lacking. Kristensen and associates reported the results of an interim analysis of the PRO-SPIRIT real-world study that included 1192 patients with PsA across six countries who initiated or switched to a new biologic or targeted synthetic disease-modifying antirheumatic drug. They showed that at 3 months, ixekizumab significantly improved clinical disease activity in patients with PsA compared with IL-12/23 inhibitors and IL-23 inhibitors. The improvements in the joints were similar to those with TNF inhibitors and JAK inhibitors, but the improvement in psoriasis was higher. Thus, ixekizumab leads to rapid response to active skin and musculoskeletal disease activity in PsA. Comparative data on treatment persistence as well as adverse events are required.