User login
Older diabetes drugs linked to dementia risk -- one lower, one higher
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
a new observational study in patients with type 2 diabetes suggests.
The data, obtained from nationwide electronic medical records from the Department of Veterans Affairs, yielded a 22% lower risk of dementia with TZD monotherapy and a 12% elevated risk with sulfonylurea monotherapy, compared with metformin monotherapy. The apparent protective effects of TZDs were greater among individuals with overweight or obesity.
“Our findings provide additional information to aid clinicians’ selection of [glucose-lowering medications] for patients with mild or moderate type 2 diabetes and [who] are at high risk of dementia,” Xin Tang and colleagues wrote in their article, published online in BMJ Open Diabetes Research & Care.
The results “add substantially to the literature concerning the effects of [glucose-lowering medications] on dementia where previous findings have been inconsistent. Studies with a follow-up time of less than 3 years have mainly reported null associations, while studies with longer a follow-up time typically yielded protective findings. With a mean follow-up time of 6.8 years, we had a sufficient duration to detect treatment differences,” the investigators wrote.
“Supplementing [a] sulfonylurea with either metformin or [a] TZD may partially offset its prodementia effects. These findings may help inform medication selection for elderly patients with T2D at high risk of dementia,” they added.
Randomized trials needed to determine cause and effect
Ivan Koychev, PhD, a senior clinical researcher in the department of psychiatry at the University of Oxford (England), told the UK Science Media Centre: “This is a large, well-conducted real-world data study that highlights the importance of checking whether already prescribed medications may be useful for preventing dementia.”
The findings regarding TZDs, also known as glitazones, are in line with existing literature suggesting dementia protection with other drugs prescribed for type 2 diabetes that weren’t examined in the current study, such as newer agents like glucagonlike peptide–1 (GLP-1) agonists and sodium-glucose cotransporter 2 (SGLT2) inhibitors, Dr. Koychev said.
“The main limitations of this study is that following the initial 2-year period the authors were interested in, the participants may have been prescribed one of the other type 2 diabetes drugs [GLP-1 agonists or SGLT2 inhibitors] that have been found to reduce dementia risk, thus potentially making the direct glitazone [TZD] effect more difficult to discern,” Dr. Koychev noted.
And, he pointed out that the study design limits attribution of causality. “It is also important to note that people with type 2 diabetes do run a higher risk of both dementia and cognitive deficits and that these medications are only prescribed in these patients, so all this data is from this patient group rather than the general population.”
James Connell, PhD, head of translational science at Alzheimer’s Research UK, agreed. “While this observational study found that those with type 2 diabetes taking thiazolidinedione had a lower dementia risk than those on the most common medication for type 2 diabetes, it only shows an association between taking the drug and dementia risk and not a causal relationship.
“Double-blind and placebo-controlled clinical trials are needed to see whether the drug [TDZ] could help lower dementia risk in people with and without diabetes. Anyone with any questions about what treatments they are receiving should speak to their doctor,” he told the UK Science Media Centre.
Opposite effects of sulfonylureas, TZDs versus metformin
The study authors analyzed 559,106 VA patients with type 2 diabetes who initiated glucose-lowering medication during 2001-2017 and took it for at least a year. They were aged 60 years or older and did not have dementia at baseline. Most were White (76.8%) and male (96.9%), two-thirds (63.1%) had obesity, and mean hemoglobin A1c was 6.8%.
Overall, 31,125 developed all-cause dementia. The incidence rate was 8.2 cases per 1,000 person-years, ranging from 6.2 cases per 1,000 person-years among those taking metformin monotherapy to 13.4 cases per 1,000 person-years in those taking both sulfonylurea and a TZD.
Compared with metformin monotherapy, the hazard ratio for all-cause dementia for sulfonylurea monotherapy was a significant 1.12. The increased risk was also seen for vascular dementia, with an HR of 1.14.
In contrast, TZD monotherapy was associated with a significantly lower risk for all-cause dementia (HR, 0.78), as well as for Alzheimer’s disease (HR, 0.89) and vascular dementia (HR, 0.43), compared with metformin monotherapy.
The combination of metformin and TZD also lowered the risk of all-cause dementia, while regimens including sulfonylureas raised the risks for all-cause and vascular dementia.
Most of the results didn’t change significantly when the drug exposure window was extended to 2 years.
Effects more pronounced in those with obesity
The protective 1-year effects of TZD monotherapy and of metformin plus TZD, compared with metformin alone, were more significant among participants aged 75 or younger and with a body mass index above 25 kg/m2, compared with those who were older than 75 years and with normal BMIs, respectively.
On the other hand, the greater risk for dementia incurred with sulfonylureas was further increased among those with higher BMI.
This research was partially funded by grants from the National Human Genome Research Institute, the National Science Foundation, the National Institute of Diabetes and Digestive and Kidney Disease, and the National Heart, Lung, and Blood Institute. Dr. Koychev is chief investigator for a trial, sponsored by Oxford University and funded by Novo Nordisk, testing whether the GLP-1 agonist semaglutide reduces the risk for dementia in aging adults.
A version of this article first appeared on Medscape.com.
FROM BMJ OPEN DIABETES RESEARCH & CARE
Psychedelics and the Military: What a Long, Strange Trip It’s Been
In 2019 the Defense Advanced Research Projects Agency invested $27 million in the Focused Pharma program to develop new, more efficacious, rapid-acting drugs, including hallucinogens.1 While Focused Pharma does not include human studies, the Veterans Health Administration’s (VHA) newly launched psychedelics program research does include clinical trials.2 When I read of these ambitious projects, I recalled 2 prescient memories from my youth.
The first memory was of a dinner table conversation between my father, then chief of pediatrics at a military hospital, and one of my older brothers, a burgeoning hippie. My father mentioned that the military was doing research on lysergic acid diethylamide (LSD), and my brother asked whether he could bring some home for my brother to try. My father looked up from the dinner table with incredulity and in an ironic monotone replied, “No you would not qualify for the research, you are not in the Army.”
The second was about 10 years later, when I visited the state psychiatric hospital where my father directed the adolescent ward. I saw a group of young adults watching test patterns on an old-fashioned television set. When I asked my father what was wrong with them, he shook his head and said, “Too much LSD.”
Albert Hoffman was a Sandoz chemist when in 1938 he serendipitously developed LSD while working on a fungus that grew on grain. LSD’s psychoactive properties were not discovered until 1943. About a decade later, as the Cold War chilled international relations, the Central Intelligence Agency (CIA) began conducting experiments on military personnel in the MKUltra program using LSD, electroshock, hypnosis, and other techniques to develop a mind control program before its rivals did.3
Beginning in the 1950s, the US government collaborated with pharmaceutical companies and research universities to develop LSD as part of a campaign of psychological warfare. Though planned to be used against enemies, the program instead exploited US service members to develop hallucinogens as a form of chemical warfare that could render enemy troops mentally incapacitated. That psychiatrists, who then (as now) led much of this research, raised a host of ethical concerns about dual roles, disclosure, and duty.4
Government investigations and academic studies have shown that even soldiers who volunteered for the research were not given adequate information about the nature of the experiments and the potential adverse effects, such as persisting flashbacks. The military’s research on LSD ended in 1963, not because of the unethical aspects of the research, but because the effects of LSD were so unpredictable that the drug could not be effectively weaponized. Like Tuskegee and other research abuses of the time, when the MKUltra program was exposed, there were congressional investigations.5 Later studies found that many of the active-duty research subjects experienced a plethora of lasting and serious psychiatric symptoms. VHA practitioners had to put back together many of these broken service members. This program was rife with violations of research ethics and human rights, and those abuses tainted the field of hallucinogenic research in US Department of Defense (DoD) and VHA circles for decades.5 These research abuses, in part, have led to hallucinogens being categorized as Schedule I controlled substances, effectively blocking federal funding for research until recently.
LSD, Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), and 3,4-methylenedioxy-methamphetamine (MDMA), popularly known as psychedelics, are again receiving attention. However, the current investigations into psychedelics are vastly different—scientifically and ethically. The most important difference is that the context and leadership of these studies is not national security—it is health care.
The goal of this new wave of psychedelic research is not mind control or brain alteration, but liberation of the mind from cycles of rumination and trauma and empowerment to change patterns of self-destruction to affirmation of life. The impetus for this research is not international espionage but to find better treatments for chronic posttraumatic stress disorder, severe substance use disorders, and treatment-resistant depression that contribute to unquantifiable mental pain, psychosocial dysfunction, and an epidemic of suicide among military service members and veterans.6 Though we have some effective treatments for these often combat-inflicted maladies—primarily evidence-based psychotherapies—yet these treatments are not tolerable or safe, fast-acting, or long-lasting enough to succor each and every troubled soul. The success of ketamine, a dissociative drug, in relieving the most distressing service-connected psychiatric diagnoses has provided a proof of concept to reinvigorate the moribund hallucinogenic research idea.7
This dark chapter in US military research is a cautionary tale. The often quoted and more often ignored advice of the Spanish American philosopher George Santayana, “Those who cannot remember the past are condemned to repeat it,” should serve as the guiding principle of the new hallucinogenic research.8 Human subjects’ protections have exponentially improved since the days of the secret LSD project even for active-duty personnel. The Common Rule governs that all research participants are given adequate information that includes whatever is known about the risks and benefits of the research.10 Participants must provide full and free informed consent to enroll in these clinical trials, a consent that encompasses the right to withdraw from the research at any time without jeopardizing their careers, benefits, or ongoing health care.10
These rules, though, can be bent, broken, avoided, or worked around. Only the moral integrity of study personnel, administrators, oversight agencies, research compliance officers, and most important, principal investigators can assure that the rules are upheld and the rights they guarantee are respected.9 It would be a tragic shame if the promised hope for the relief of psychic pain went unrealized due to media hype, shared desperation of clinicians and patients, and conflicts of interests that today are more likely to come from profit-driven pharmaceutical companies than national security agencies. And for all of us in federal practice, remembering the sordid past forays with LSD can redeem the present research so future service members and veterans and the clinicians who care for them have better balms to heal the wounds of war.
1. US Department of Defense, Defense Advanced Research Projects Agency. Structure-guided drug design could yield fast-acting remedies for complex neuropsychiatric conditions. Accessed September 12, 2022. https://www.darpa.mil/news-events/2019-09-11#
2. Londono E. After six-decade hiatus, experimental psychedelic therapy returns to the VA. https://www.nytimes.com/2022/06/24/us/politics/psychedelic-therapy-veterans.html
3. Disbennett B. ‘This is the happy warrior, this is he:’ an analysis of CIA and military testing of LSD on non-consenting U.S. service-members and recovery through the VA disability system. Tennessee J Race, Gender, Social Justice. 2015;3(2):1-32. doi:10.2139/ssrn.2416478
4. Smith H. James Ketchum, who conducted mind-altering experiments on soldiers dies at 87. Accessed September 12, 2022. https://www.washingtonpost.com/local/obituaries/james-ketchum-who-conducted-mind-altering-experiments-on-soldiers-dies-at-87/2019/06/04/7b5ad322-86cc-11e9-a491-25df61c78dc4_story.html
5. Ross CA. LSD experiments by the United States Army. Hist Psychiatry. 2017;28(4):427-442. doi:10.1177/0957154X17717678
6. Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions of comorbid posttraumatic stress disorder and treatment resistant depression. Clin Psychiatry. 2018;79(3): 17m11634. doi:10.4088/JCP.17m11634
7. Shawler IC, Jordan CH, Jackson CA. Veteran and military mental health issues. Stat Pearls. Updated May 23, 2022. Accessed September 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK572092/#_NBK572092_pubdet_
8. Santayana G. The Life of Reason. 1905. Accessed September 12, 2022. https://www.gutenberg.org/files/15000/15000-h/15000-h.htm
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1200.05(2). Requirements for the protection of human subjects in research. Amended January 8, 2021. Accessed September 12, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8171
10. US Department of Defense, Military Health System. Research protections. Accessed September 12, 2022. https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Engineering/Research-Protections
In 2019 the Defense Advanced Research Projects Agency invested $27 million in the Focused Pharma program to develop new, more efficacious, rapid-acting drugs, including hallucinogens.1 While Focused Pharma does not include human studies, the Veterans Health Administration’s (VHA) newly launched psychedelics program research does include clinical trials.2 When I read of these ambitious projects, I recalled 2 prescient memories from my youth.
The first memory was of a dinner table conversation between my father, then chief of pediatrics at a military hospital, and one of my older brothers, a burgeoning hippie. My father mentioned that the military was doing research on lysergic acid diethylamide (LSD), and my brother asked whether he could bring some home for my brother to try. My father looked up from the dinner table with incredulity and in an ironic monotone replied, “No you would not qualify for the research, you are not in the Army.”
The second was about 10 years later, when I visited the state psychiatric hospital where my father directed the adolescent ward. I saw a group of young adults watching test patterns on an old-fashioned television set. When I asked my father what was wrong with them, he shook his head and said, “Too much LSD.”
Albert Hoffman was a Sandoz chemist when in 1938 he serendipitously developed LSD while working on a fungus that grew on grain. LSD’s psychoactive properties were not discovered until 1943. About a decade later, as the Cold War chilled international relations, the Central Intelligence Agency (CIA) began conducting experiments on military personnel in the MKUltra program using LSD, electroshock, hypnosis, and other techniques to develop a mind control program before its rivals did.3
Beginning in the 1950s, the US government collaborated with pharmaceutical companies and research universities to develop LSD as part of a campaign of psychological warfare. Though planned to be used against enemies, the program instead exploited US service members to develop hallucinogens as a form of chemical warfare that could render enemy troops mentally incapacitated. That psychiatrists, who then (as now) led much of this research, raised a host of ethical concerns about dual roles, disclosure, and duty.4
Government investigations and academic studies have shown that even soldiers who volunteered for the research were not given adequate information about the nature of the experiments and the potential adverse effects, such as persisting flashbacks. The military’s research on LSD ended in 1963, not because of the unethical aspects of the research, but because the effects of LSD were so unpredictable that the drug could not be effectively weaponized. Like Tuskegee and other research abuses of the time, when the MKUltra program was exposed, there were congressional investigations.5 Later studies found that many of the active-duty research subjects experienced a plethora of lasting and serious psychiatric symptoms. VHA practitioners had to put back together many of these broken service members. This program was rife with violations of research ethics and human rights, and those abuses tainted the field of hallucinogenic research in US Department of Defense (DoD) and VHA circles for decades.5 These research abuses, in part, have led to hallucinogens being categorized as Schedule I controlled substances, effectively blocking federal funding for research until recently.
LSD, Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), and 3,4-methylenedioxy-methamphetamine (MDMA), popularly known as psychedelics, are again receiving attention. However, the current investigations into psychedelics are vastly different—scientifically and ethically. The most important difference is that the context and leadership of these studies is not national security—it is health care.
The goal of this new wave of psychedelic research is not mind control or brain alteration, but liberation of the mind from cycles of rumination and trauma and empowerment to change patterns of self-destruction to affirmation of life. The impetus for this research is not international espionage but to find better treatments for chronic posttraumatic stress disorder, severe substance use disorders, and treatment-resistant depression that contribute to unquantifiable mental pain, psychosocial dysfunction, and an epidemic of suicide among military service members and veterans.6 Though we have some effective treatments for these often combat-inflicted maladies—primarily evidence-based psychotherapies—yet these treatments are not tolerable or safe, fast-acting, or long-lasting enough to succor each and every troubled soul. The success of ketamine, a dissociative drug, in relieving the most distressing service-connected psychiatric diagnoses has provided a proof of concept to reinvigorate the moribund hallucinogenic research idea.7
This dark chapter in US military research is a cautionary tale. The often quoted and more often ignored advice of the Spanish American philosopher George Santayana, “Those who cannot remember the past are condemned to repeat it,” should serve as the guiding principle of the new hallucinogenic research.8 Human subjects’ protections have exponentially improved since the days of the secret LSD project even for active-duty personnel. The Common Rule governs that all research participants are given adequate information that includes whatever is known about the risks and benefits of the research.10 Participants must provide full and free informed consent to enroll in these clinical trials, a consent that encompasses the right to withdraw from the research at any time without jeopardizing their careers, benefits, or ongoing health care.10
These rules, though, can be bent, broken, avoided, or worked around. Only the moral integrity of study personnel, administrators, oversight agencies, research compliance officers, and most important, principal investigators can assure that the rules are upheld and the rights they guarantee are respected.9 It would be a tragic shame if the promised hope for the relief of psychic pain went unrealized due to media hype, shared desperation of clinicians and patients, and conflicts of interests that today are more likely to come from profit-driven pharmaceutical companies than national security agencies. And for all of us in federal practice, remembering the sordid past forays with LSD can redeem the present research so future service members and veterans and the clinicians who care for them have better balms to heal the wounds of war.
In 2019 the Defense Advanced Research Projects Agency invested $27 million in the Focused Pharma program to develop new, more efficacious, rapid-acting drugs, including hallucinogens.1 While Focused Pharma does not include human studies, the Veterans Health Administration’s (VHA) newly launched psychedelics program research does include clinical trials.2 When I read of these ambitious projects, I recalled 2 prescient memories from my youth.
The first memory was of a dinner table conversation between my father, then chief of pediatrics at a military hospital, and one of my older brothers, a burgeoning hippie. My father mentioned that the military was doing research on lysergic acid diethylamide (LSD), and my brother asked whether he could bring some home for my brother to try. My father looked up from the dinner table with incredulity and in an ironic monotone replied, “No you would not qualify for the research, you are not in the Army.”
The second was about 10 years later, when I visited the state psychiatric hospital where my father directed the adolescent ward. I saw a group of young adults watching test patterns on an old-fashioned television set. When I asked my father what was wrong with them, he shook his head and said, “Too much LSD.”
Albert Hoffman was a Sandoz chemist when in 1938 he serendipitously developed LSD while working on a fungus that grew on grain. LSD’s psychoactive properties were not discovered until 1943. About a decade later, as the Cold War chilled international relations, the Central Intelligence Agency (CIA) began conducting experiments on military personnel in the MKUltra program using LSD, electroshock, hypnosis, and other techniques to develop a mind control program before its rivals did.3
Beginning in the 1950s, the US government collaborated with pharmaceutical companies and research universities to develop LSD as part of a campaign of psychological warfare. Though planned to be used against enemies, the program instead exploited US service members to develop hallucinogens as a form of chemical warfare that could render enemy troops mentally incapacitated. That psychiatrists, who then (as now) led much of this research, raised a host of ethical concerns about dual roles, disclosure, and duty.4
Government investigations and academic studies have shown that even soldiers who volunteered for the research were not given adequate information about the nature of the experiments and the potential adverse effects, such as persisting flashbacks. The military’s research on LSD ended in 1963, not because of the unethical aspects of the research, but because the effects of LSD were so unpredictable that the drug could not be effectively weaponized. Like Tuskegee and other research abuses of the time, when the MKUltra program was exposed, there were congressional investigations.5 Later studies found that many of the active-duty research subjects experienced a plethora of lasting and serious psychiatric symptoms. VHA practitioners had to put back together many of these broken service members. This program was rife with violations of research ethics and human rights, and those abuses tainted the field of hallucinogenic research in US Department of Defense (DoD) and VHA circles for decades.5 These research abuses, in part, have led to hallucinogens being categorized as Schedule I controlled substances, effectively blocking federal funding for research until recently.
LSD, Psilocybin (4-phosphoryloxy-N,N-dimethyltryptamine), and 3,4-methylenedioxy-methamphetamine (MDMA), popularly known as psychedelics, are again receiving attention. However, the current investigations into psychedelics are vastly different—scientifically and ethically. The most important difference is that the context and leadership of these studies is not national security—it is health care.
The goal of this new wave of psychedelic research is not mind control or brain alteration, but liberation of the mind from cycles of rumination and trauma and empowerment to change patterns of self-destruction to affirmation of life. The impetus for this research is not international espionage but to find better treatments for chronic posttraumatic stress disorder, severe substance use disorders, and treatment-resistant depression that contribute to unquantifiable mental pain, psychosocial dysfunction, and an epidemic of suicide among military service members and veterans.6 Though we have some effective treatments for these often combat-inflicted maladies—primarily evidence-based psychotherapies—yet these treatments are not tolerable or safe, fast-acting, or long-lasting enough to succor each and every troubled soul. The success of ketamine, a dissociative drug, in relieving the most distressing service-connected psychiatric diagnoses has provided a proof of concept to reinvigorate the moribund hallucinogenic research idea.7
This dark chapter in US military research is a cautionary tale. The often quoted and more often ignored advice of the Spanish American philosopher George Santayana, “Those who cannot remember the past are condemned to repeat it,” should serve as the guiding principle of the new hallucinogenic research.8 Human subjects’ protections have exponentially improved since the days of the secret LSD project even for active-duty personnel. The Common Rule governs that all research participants are given adequate information that includes whatever is known about the risks and benefits of the research.10 Participants must provide full and free informed consent to enroll in these clinical trials, a consent that encompasses the right to withdraw from the research at any time without jeopardizing their careers, benefits, or ongoing health care.10
These rules, though, can be bent, broken, avoided, or worked around. Only the moral integrity of study personnel, administrators, oversight agencies, research compliance officers, and most important, principal investigators can assure that the rules are upheld and the rights they guarantee are respected.9 It would be a tragic shame if the promised hope for the relief of psychic pain went unrealized due to media hype, shared desperation of clinicians and patients, and conflicts of interests that today are more likely to come from profit-driven pharmaceutical companies than national security agencies. And for all of us in federal practice, remembering the sordid past forays with LSD can redeem the present research so future service members and veterans and the clinicians who care for them have better balms to heal the wounds of war.
1. US Department of Defense, Defense Advanced Research Projects Agency. Structure-guided drug design could yield fast-acting remedies for complex neuropsychiatric conditions. Accessed September 12, 2022. https://www.darpa.mil/news-events/2019-09-11#
2. Londono E. After six-decade hiatus, experimental psychedelic therapy returns to the VA. https://www.nytimes.com/2022/06/24/us/politics/psychedelic-therapy-veterans.html
3. Disbennett B. ‘This is the happy warrior, this is he:’ an analysis of CIA and military testing of LSD on non-consenting U.S. service-members and recovery through the VA disability system. Tennessee J Race, Gender, Social Justice. 2015;3(2):1-32. doi:10.2139/ssrn.2416478
4. Smith H. James Ketchum, who conducted mind-altering experiments on soldiers dies at 87. Accessed September 12, 2022. https://www.washingtonpost.com/local/obituaries/james-ketchum-who-conducted-mind-altering-experiments-on-soldiers-dies-at-87/2019/06/04/7b5ad322-86cc-11e9-a491-25df61c78dc4_story.html
5. Ross CA. LSD experiments by the United States Army. Hist Psychiatry. 2017;28(4):427-442. doi:10.1177/0957154X17717678
6. Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions of comorbid posttraumatic stress disorder and treatment resistant depression. Clin Psychiatry. 2018;79(3): 17m11634. doi:10.4088/JCP.17m11634
7. Shawler IC, Jordan CH, Jackson CA. Veteran and military mental health issues. Stat Pearls. Updated May 23, 2022. Accessed September 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK572092/#_NBK572092_pubdet_
8. Santayana G. The Life of Reason. 1905. Accessed September 12, 2022. https://www.gutenberg.org/files/15000/15000-h/15000-h.htm
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1200.05(2). Requirements for the protection of human subjects in research. Amended January 8, 2021. Accessed September 12, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8171
10. US Department of Defense, Military Health System. Research protections. Accessed September 12, 2022. https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Engineering/Research-Protections
1. US Department of Defense, Defense Advanced Research Projects Agency. Structure-guided drug design could yield fast-acting remedies for complex neuropsychiatric conditions. Accessed September 12, 2022. https://www.darpa.mil/news-events/2019-09-11#
2. Londono E. After six-decade hiatus, experimental psychedelic therapy returns to the VA. https://www.nytimes.com/2022/06/24/us/politics/psychedelic-therapy-veterans.html
3. Disbennett B. ‘This is the happy warrior, this is he:’ an analysis of CIA and military testing of LSD on non-consenting U.S. service-members and recovery through the VA disability system. Tennessee J Race, Gender, Social Justice. 2015;3(2):1-32. doi:10.2139/ssrn.2416478
4. Smith H. James Ketchum, who conducted mind-altering experiments on soldiers dies at 87. Accessed September 12, 2022. https://www.washingtonpost.com/local/obituaries/james-ketchum-who-conducted-mind-altering-experiments-on-soldiers-dies-at-87/2019/06/04/7b5ad322-86cc-11e9-a491-25df61c78dc4_story.html
5. Ross CA. LSD experiments by the United States Army. Hist Psychiatry. 2017;28(4):427-442. doi:10.1177/0957154X17717678
6. Albott CS, Lim KO, Forbes MK, et al. Efficacy, safety, and durability of repeated ketamine infusions of comorbid posttraumatic stress disorder and treatment resistant depression. Clin Psychiatry. 2018;79(3): 17m11634. doi:10.4088/JCP.17m11634
7. Shawler IC, Jordan CH, Jackson CA. Veteran and military mental health issues. Stat Pearls. Updated May 23, 2022. Accessed September 12, 2022. https://www.ncbi.nlm.nih.gov/books/NBK572092/#_NBK572092_pubdet_
8. Santayana G. The Life of Reason. 1905. Accessed September 12, 2022. https://www.gutenberg.org/files/15000/15000-h/15000-h.htm
9. US Department of Veterans Affairs, Veterans Health Administration. VHA Directive 1200.05(2). Requirements for the protection of human subjects in research. Amended January 8, 2021. Accessed September 12, 2022. https://www.va.gov/vhapublications/ViewPublication.asp?pub_ID=8171
10. US Department of Defense, Military Health System. Research protections. Accessed September 12, 2022. https://www.health.mil/About-MHS/OASDHA/Defense-Health-Agency/Research-and-Engineering/Research-Protections
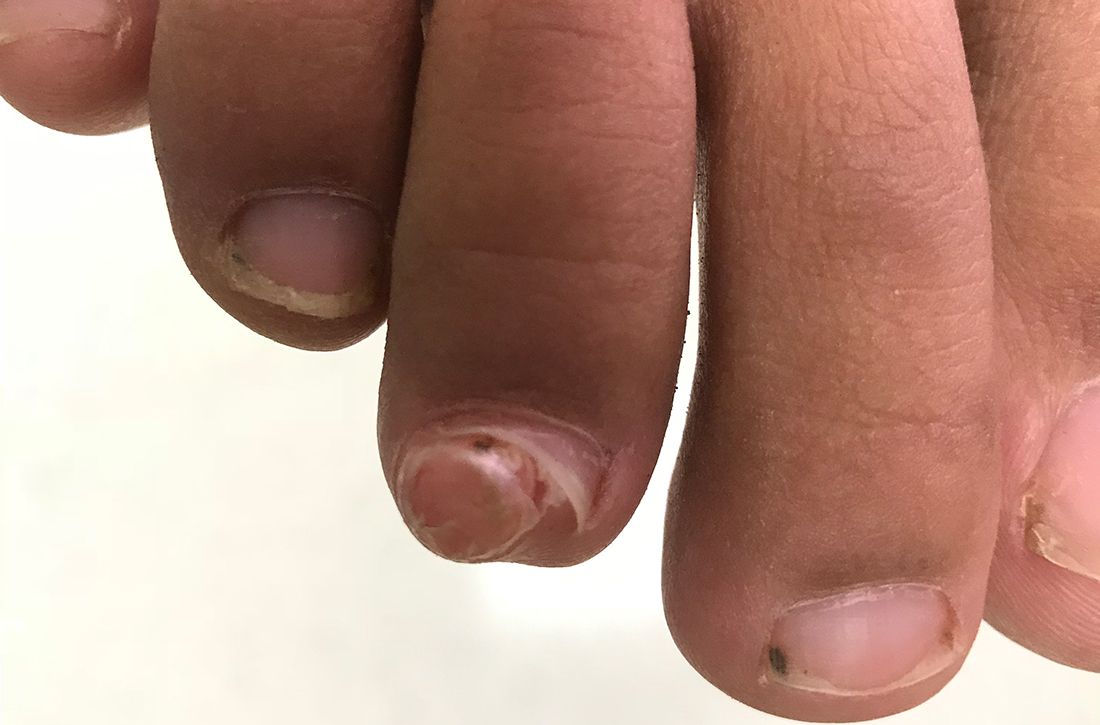
Soccer player with painful toe
A 13-YEAR-OLD GIRL presented to the clinic with a 1-year history of a slow-growing mass on the third toe of her right foot. As a soccer player, she experienced associated pain when kicking the ball or when wearing tight-fitting shoes. The lesion was otherwise asymptomatic. She denied any overt trauma to the area and indicated that the mass had enlarged over the previous year.
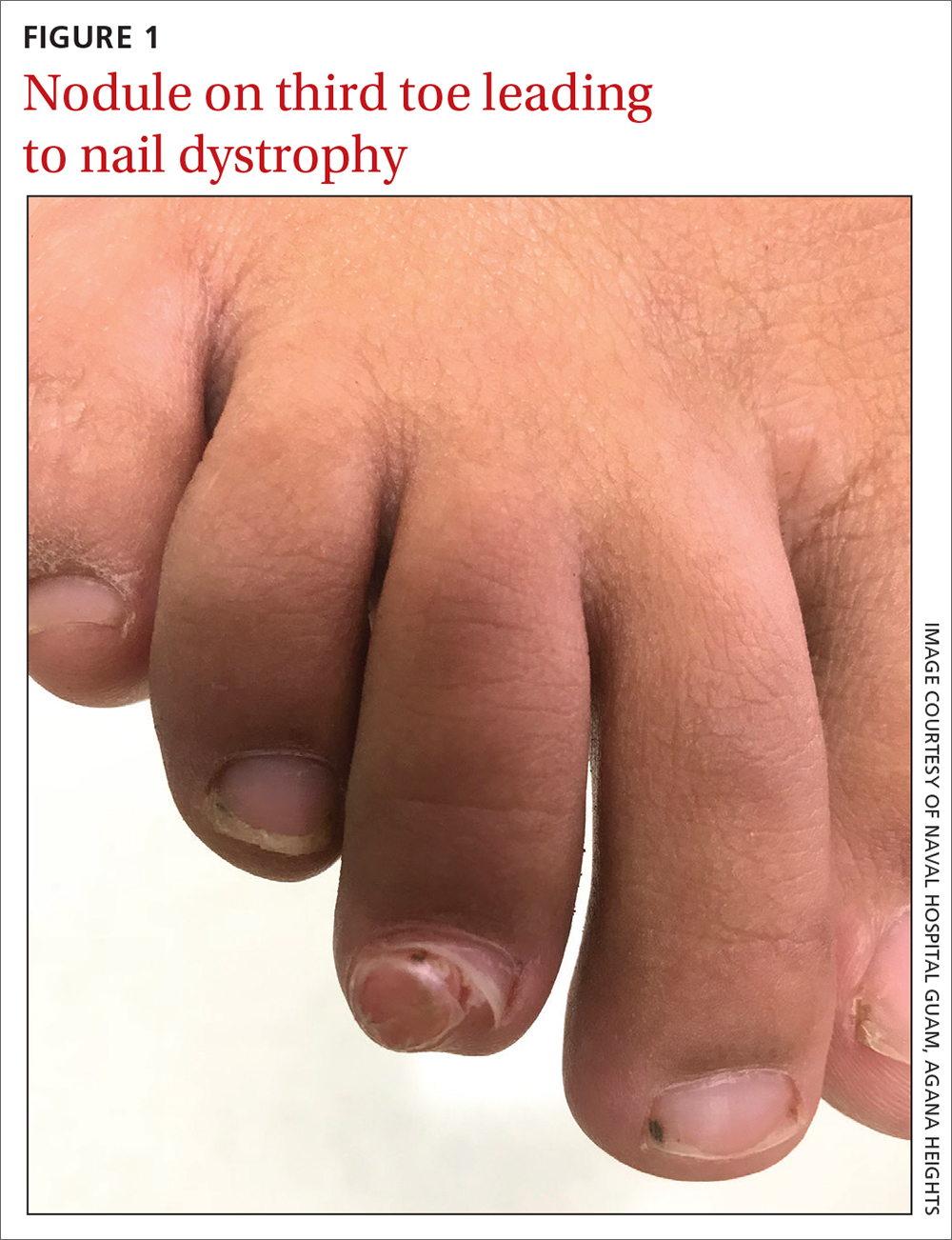
On exam, there was a nontender 8 × 8-mm firm nodule underneath the nail with associated nail dystrophy (FIGURE 1). The toe had full mobility, sensation was intact, and capillary refill time was < 2 seconds.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Subungual exostosis
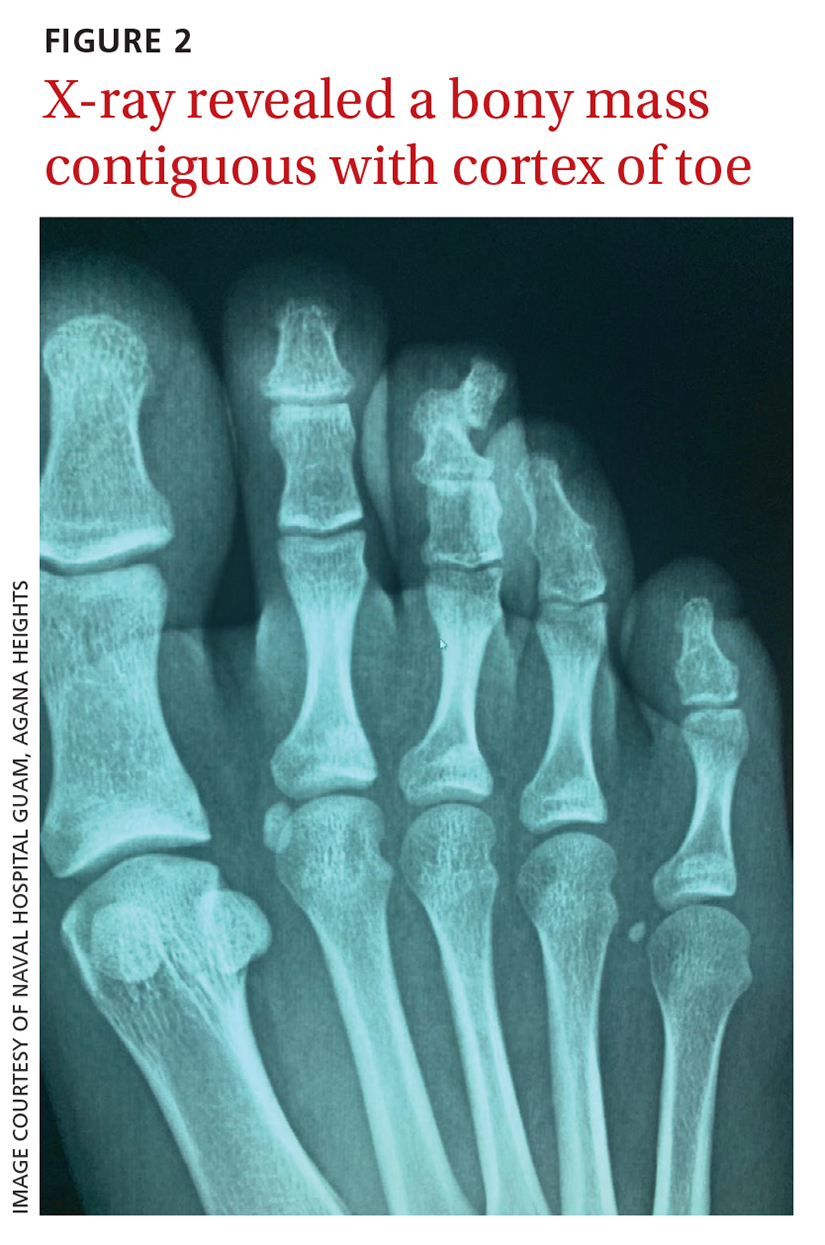
A plain radiograph of the patient’s foot showed continuity with the bony cortex and medullary space, confirming the diagnosis of subungual exostosis (FIGURE 2).1 An exostosis, or osteochondroma, is a form of benign bone tumor in which trabecular bone overgrows its normal border in a nodular pattern. When this occurs under the nail bed, it is called subungual exostosis.2 Exostosis represents 10% to 15% of all benign bone tumors, making it the most common benign bone tumor.3 Generally, the age of occurrence is 10 to 15 years.3
Repetitive trauma can be a culprit. Up to 8% of exostoses occur in the foot, with the most commonly affected area being the distal medial portion of the big toe.3,4 Repetitive trauma and infection are potential risk factors.3,4 The affected toe may be painful, but that is not always the case.4 Typically, lesions are solitary; however, multiple lesions can occur.4
Most pediatric foot lesions are benign and involve soft tissue
Benign soft-tissue masses make up the overwhelming majority of pediatric foot lesions, accounting for 61% to 87% of all foot lesions.3 Malignancies such as chondrosarcoma can occur and can be difficult to diagnose. Rapid growth, family history, size > 5 cm, heterogenous appearance on magnetic resonance imaging, and poorly defined margins are a few characteristics that should increase suspicion for possible malignancy.5
The differential diagnosis for a growth on the toe similar to the one our patient had would include pyogenic granuloma,
Pyogenic granulomas are benign vascular lesions that occur in patients of all ages. They tend to be dome-shaped and flesh-toned to violaceous red, and they are usually found on the head, neck, and extremities—especially fingers.6 They are associated with trauma and are classically tender with a propensity to bleed.6
Acral fibromyxoma is a benign, slow-growing, predominately painless, firm mass with an affinity for the great toe; the affected area includes the nail in 50% of cases.7 A radiograph may show bony erosion or scalloping due to mass effect; however, there will be no continuity with the bony matrix. (Such continuity would suggest exostosis.)
Periungual fibromas are benign soft-tissue masses, which are pink to red and firm, and emerge from underneath the nails, potentially resulting in dystrophy.8 They can bleed and cause pain, and are strongly associated with tuberous sclerosis.5
Continue to: Verruca vulgaris
Verruca vulgaris, the common wart, can also manifest in the subungual region as a firm, generally painless mass. It is the most common neoplasm of the hand and fingers.6 Tiny black dots that correspond to thrombosed capillaries are key to identifying this lesion.
Surgical excision when patient reaches maturity
The definitive treatment for subungual exostosis is surgical excision, preferably once the patient has reached skeletal maturity. Surgery at this point is associated with decreased recurrence rates.3,4 That said, excision may need to be performed sooner if the lesion is painful and leading to deformity.3
Our patient’s persistent pain prompted us to recommend surgical excision. She underwent a third digit exostectomy, which she tolerated without any issues. The patient was fitted with a postoperative shoe that she wore until her 2-week follow-up appointment, when her sutures were removed. The patient’s activity level progressed as tolerated. She regained full function and returned to playing soccer, without any pain, 3 months after her surgery.
1. Das PC, Hassan S, Kumar P. Subungual exostosis – clinical, radiological, and histological findings. Indian Dermatol Online J. 2019;10:202-203. doi: 10.4103/idoj.IDOJ_104_18
2. Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257. doi:10.12788/cutis.0380
3. Bouchard B, Bartlett M, Donnan L. Assessment of the pediatric foot mass. J Am Acad Orthop Surg. 2017;25:32-41. doi: 10.5435/JAAOS-D-15-00397
4. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi: 10.1007/s11999-013-3345-4
5. Shah SH, Callahan MJ. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol. 2013;43 suppl 1:S23-S40. doi: 10.1007/s00247-012-2590-0
6. Habif, Thomas P. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby/Elsevier, 2016.
7. Ramya C, Nayak C, Tambe S. Superficial acral fibromyxoma. Indian J Dermatol. 2016;61:457-459. doi: 10.4103/0019-5154.185734
8. Ma D, Darling T, Moss J, et al. Histologic variants of periungual fibromas in tuberous sclerosis complex. J Am Acad Dermatol. 2011;64:442-444. doi: 10.1016/j.jaad.2010.03.002
A 13-YEAR-OLD GIRL presented to the clinic with a 1-year history of a slow-growing mass on the third toe of her right foot. As a soccer player, she experienced associated pain when kicking the ball or when wearing tight-fitting shoes. The lesion was otherwise asymptomatic. She denied any overt trauma to the area and indicated that the mass had enlarged over the previous year.
On exam, there was a nontender 8 × 8-mm firm nodule underneath the nail with associated nail dystrophy (FIGURE 1). The toe had full mobility, sensation was intact, and capillary refill time was < 2 seconds.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Subungual exostosis
A plain radiograph of the patient’s foot showed continuity with the bony cortex and medullary space, confirming the diagnosis of subungual exostosis (FIGURE 2).1 An exostosis, or osteochondroma, is a form of benign bone tumor in which trabecular bone overgrows its normal border in a nodular pattern. When this occurs under the nail bed, it is called subungual exostosis.2 Exostosis represents 10% to 15% of all benign bone tumors, making it the most common benign bone tumor.3 Generally, the age of occurrence is 10 to 15 years.3

Repetitive trauma can be a culprit. Up to 8% of exostoses occur in the foot, with the most commonly affected area being the distal medial portion of the big toe.3,4 Repetitive trauma and infection are potential risk factors.3,4 The affected toe may be painful, but that is not always the case.4 Typically, lesions are solitary; however, multiple lesions can occur.4
Most pediatric foot lesions are benign and involve soft tissue
Benign soft-tissue masses make up the overwhelming majority of pediatric foot lesions, accounting for 61% to 87% of all foot lesions.3 Malignancies such as chondrosarcoma can occur and can be difficult to diagnose. Rapid growth, family history, size > 5 cm, heterogenous appearance on magnetic resonance imaging, and poorly defined margins are a few characteristics that should increase suspicion for possible malignancy.5
The differential diagnosis for a growth on the toe similar to the one our patient had would include pyogenic granuloma,
Pyogenic granulomas are benign vascular lesions that occur in patients of all ages. They tend to be dome-shaped and flesh-toned to violaceous red, and they are usually found on the head, neck, and extremities—especially fingers.6 They are associated with trauma and are classically tender with a propensity to bleed.6
Acral fibromyxoma is a benign, slow-growing, predominately painless, firm mass with an affinity for the great toe; the affected area includes the nail in 50% of cases.7 A radiograph may show bony erosion or scalloping due to mass effect; however, there will be no continuity with the bony matrix. (Such continuity would suggest exostosis.)
Periungual fibromas are benign soft-tissue masses, which are pink to red and firm, and emerge from underneath the nails, potentially resulting in dystrophy.8 They can bleed and cause pain, and are strongly associated with tuberous sclerosis.5
Continue to: Verruca vulgaris
Verruca vulgaris, the common wart, can also manifest in the subungual region as a firm, generally painless mass. It is the most common neoplasm of the hand and fingers.6 Tiny black dots that correspond to thrombosed capillaries are key to identifying this lesion.
Surgical excision when patient reaches maturity
The definitive treatment for subungual exostosis is surgical excision, preferably once the patient has reached skeletal maturity. Surgery at this point is associated with decreased recurrence rates.3,4 That said, excision may need to be performed sooner if the lesion is painful and leading to deformity.3
Our patient’s persistent pain prompted us to recommend surgical excision. She underwent a third digit exostectomy, which she tolerated without any issues. The patient was fitted with a postoperative shoe that she wore until her 2-week follow-up appointment, when her sutures were removed. The patient’s activity level progressed as tolerated. She regained full function and returned to playing soccer, without any pain, 3 months after her surgery.
A 13-YEAR-OLD GIRL presented to the clinic with a 1-year history of a slow-growing mass on the third toe of her right foot. As a soccer player, she experienced associated pain when kicking the ball or when wearing tight-fitting shoes. The lesion was otherwise asymptomatic. She denied any overt trauma to the area and indicated that the mass had enlarged over the previous year.
On exam, there was a nontender 8 × 8-mm firm nodule underneath the nail with associated nail dystrophy (FIGURE 1). The toe had full mobility, sensation was intact, and capillary refill time was < 2 seconds.

WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Subungual exostosis
A plain radiograph of the patient’s foot showed continuity with the bony cortex and medullary space, confirming the diagnosis of subungual exostosis (FIGURE 2).1 An exostosis, or osteochondroma, is a form of benign bone tumor in which trabecular bone overgrows its normal border in a nodular pattern. When this occurs under the nail bed, it is called subungual exostosis.2 Exostosis represents 10% to 15% of all benign bone tumors, making it the most common benign bone tumor.3 Generally, the age of occurrence is 10 to 15 years.3

Repetitive trauma can be a culprit. Up to 8% of exostoses occur in the foot, with the most commonly affected area being the distal medial portion of the big toe.3,4 Repetitive trauma and infection are potential risk factors.3,4 The affected toe may be painful, but that is not always the case.4 Typically, lesions are solitary; however, multiple lesions can occur.4
Most pediatric foot lesions are benign and involve soft tissue
Benign soft-tissue masses make up the overwhelming majority of pediatric foot lesions, accounting for 61% to 87% of all foot lesions.3 Malignancies such as chondrosarcoma can occur and can be difficult to diagnose. Rapid growth, family history, size > 5 cm, heterogenous appearance on magnetic resonance imaging, and poorly defined margins are a few characteristics that should increase suspicion for possible malignancy.5
The differential diagnosis for a growth on the toe similar to the one our patient had would include pyogenic granuloma,
Pyogenic granulomas are benign vascular lesions that occur in patients of all ages. They tend to be dome-shaped and flesh-toned to violaceous red, and they are usually found on the head, neck, and extremities—especially fingers.6 They are associated with trauma and are classically tender with a propensity to bleed.6
Acral fibromyxoma is a benign, slow-growing, predominately painless, firm mass with an affinity for the great toe; the affected area includes the nail in 50% of cases.7 A radiograph may show bony erosion or scalloping due to mass effect; however, there will be no continuity with the bony matrix. (Such continuity would suggest exostosis.)
Periungual fibromas are benign soft-tissue masses, which are pink to red and firm, and emerge from underneath the nails, potentially resulting in dystrophy.8 They can bleed and cause pain, and are strongly associated with tuberous sclerosis.5
Continue to: Verruca vulgaris
Verruca vulgaris, the common wart, can also manifest in the subungual region as a firm, generally painless mass. It is the most common neoplasm of the hand and fingers.6 Tiny black dots that correspond to thrombosed capillaries are key to identifying this lesion.
Surgical excision when patient reaches maturity
The definitive treatment for subungual exostosis is surgical excision, preferably once the patient has reached skeletal maturity. Surgery at this point is associated with decreased recurrence rates.3,4 That said, excision may need to be performed sooner if the lesion is painful and leading to deformity.3
Our patient’s persistent pain prompted us to recommend surgical excision. She underwent a third digit exostectomy, which she tolerated without any issues. The patient was fitted with a postoperative shoe that she wore until her 2-week follow-up appointment, when her sutures were removed. The patient’s activity level progressed as tolerated. She regained full function and returned to playing soccer, without any pain, 3 months after her surgery.
1. Das PC, Hassan S, Kumar P. Subungual exostosis – clinical, radiological, and histological findings. Indian Dermatol Online J. 2019;10:202-203. doi: 10.4103/idoj.IDOJ_104_18
2. Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257. doi:10.12788/cutis.0380
3. Bouchard B, Bartlett M, Donnan L. Assessment of the pediatric foot mass. J Am Acad Orthop Surg. 2017;25:32-41. doi: 10.5435/JAAOS-D-15-00397
4. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi: 10.1007/s11999-013-3345-4
5. Shah SH, Callahan MJ. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol. 2013;43 suppl 1:S23-S40. doi: 10.1007/s00247-012-2590-0
6. Habif, Thomas P. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby/Elsevier, 2016.
7. Ramya C, Nayak C, Tambe S. Superficial acral fibromyxoma. Indian J Dermatol. 2016;61:457-459. doi: 10.4103/0019-5154.185734
8. Ma D, Darling T, Moss J, et al. Histologic variants of periungual fibromas in tuberous sclerosis complex. J Am Acad Dermatol. 2011;64:442-444. doi: 10.1016/j.jaad.2010.03.002
1. Das PC, Hassan S, Kumar P. Subungual exostosis – clinical, radiological, and histological findings. Indian Dermatol Online J. 2019;10:202-203. doi: 10.4103/idoj.IDOJ_104_18
2. Yousefian F, Davis B, Browning JC. Pediatric subungual exostosis. Cutis. 2021;108:256-257. doi:10.12788/cutis.0380
3. Bouchard B, Bartlett M, Donnan L. Assessment of the pediatric foot mass. J Am Acad Orthop Surg. 2017;25:32-41. doi: 10.5435/JAAOS-D-15-00397
4. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259. doi: 10.1007/s11999-013-3345-4
5. Shah SH, Callahan MJ. Ultrasound evaluation of superficial lumps and bumps of the extremities in children: a 5-year retrospective review. Pediatr Radiol. 2013;43 suppl 1:S23-S40. doi: 10.1007/s00247-012-2590-0
6. Habif, Thomas P. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. Mosby/Elsevier, 2016.
7. Ramya C, Nayak C, Tambe S. Superficial acral fibromyxoma. Indian J Dermatol. 2016;61:457-459. doi: 10.4103/0019-5154.185734
8. Ma D, Darling T, Moss J, et al. Histologic variants of periungual fibromas in tuberous sclerosis complex. J Am Acad Dermatol. 2011;64:442-444. doi: 10.1016/j.jaad.2010.03.002
Would your patient benefit from a monoclonal antibody?
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
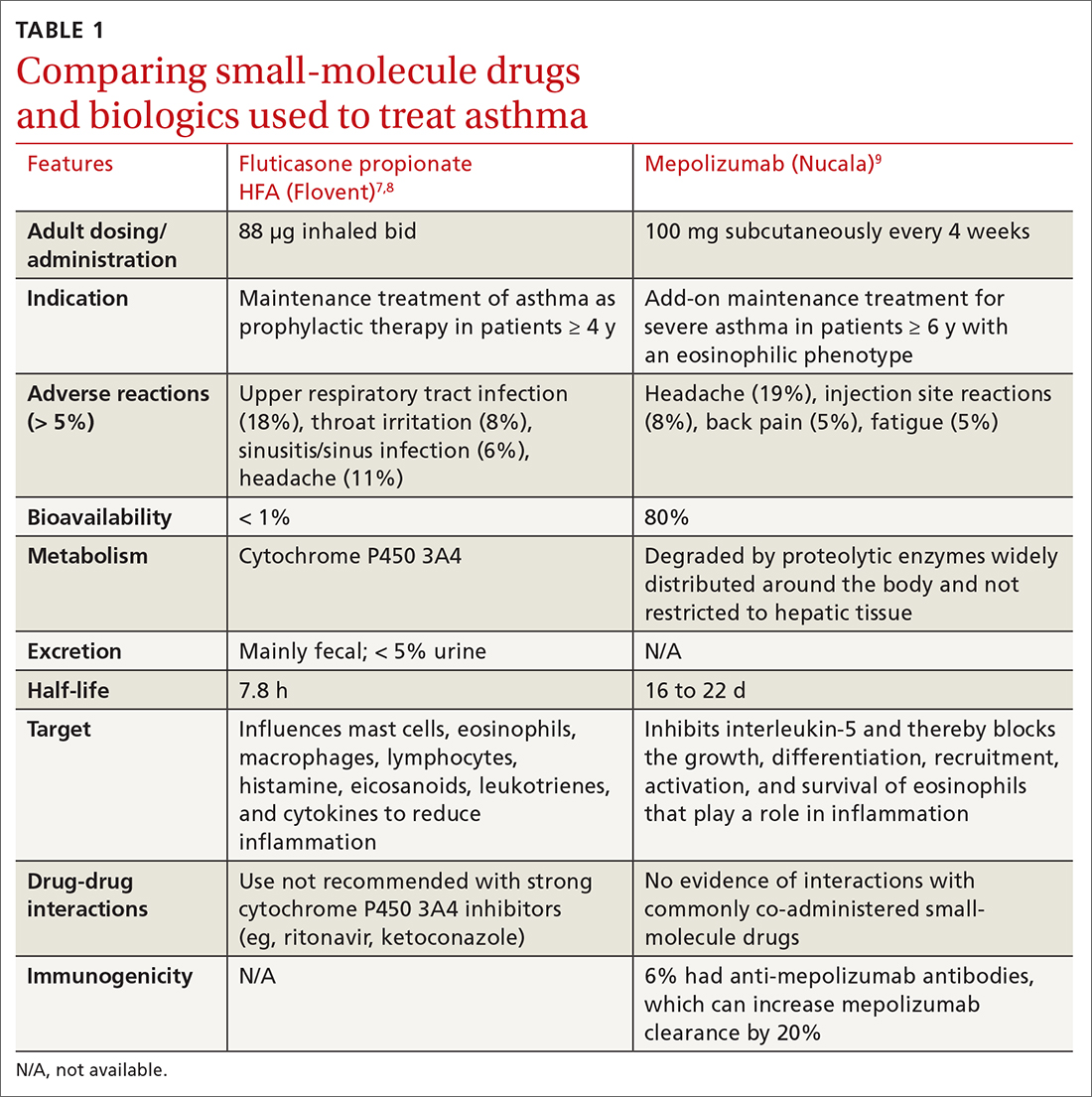
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).
MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46
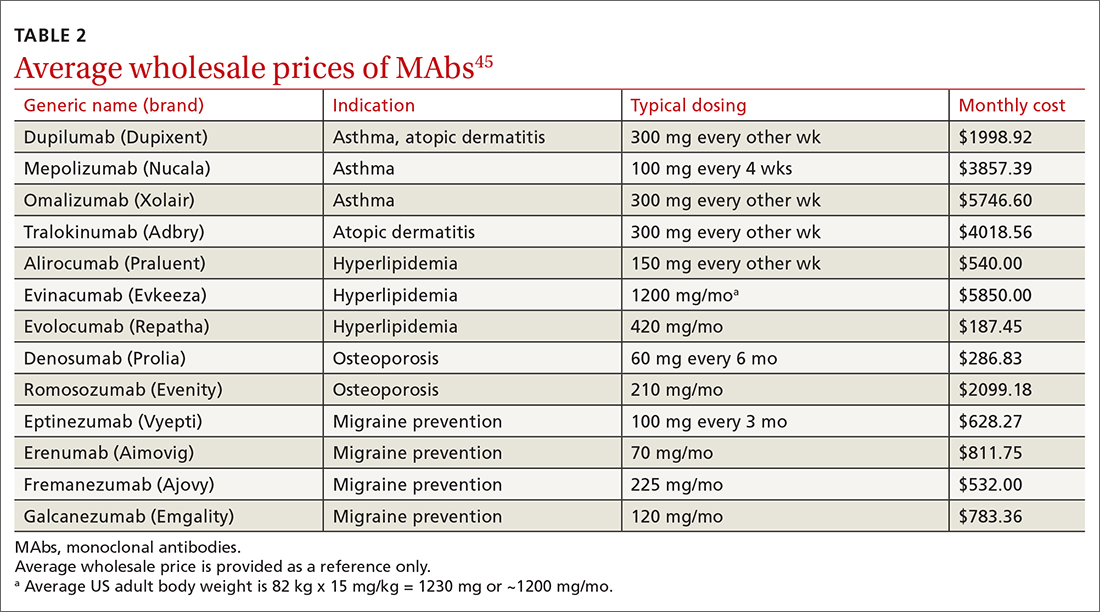
MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
Small-molecule drugs such as aspirin, albuterol, atorvastatin, and lisinopril are the backbone of disease management in family medicine.1 However, large-molecule biological drugs such as monoclonal antibodies (MAbs) are increasingly prescribed to treat common conditions. In the past decade, MAbs comprised 20% of all drug approvals by the US Food and Drug Administration (FDA), and today they represent more than half of drugs currently in development.2 Fifteen MAbs have been approved by the FDA over the past decade for asthma, atopic dermatitis (AD), hyperlipidemia, osteoporosis, and migraine prevention.3 This review details what makes MAbs unique and what you should know about them.
The uniqueness of monoclonal antibodies
MAbs are biologics, but not all biologics are MAbs—eg, adalimumab (Humira) is a MAb, but etanercept (Enbrel) is not. MAbs are therapeutic proteins made possible by hybridoma technology used to create an antibody with single specificity.4-6 Monoclonal antibodies differ from small-molecule drugs in structure, dosing, route of administration, manufacturing, metabolism, drug interactions, and elimination (TABLE 17-9).

MAbs can be classified as naked, “without any drug or radioactive material attached to them,” or conjugated, “joined to a chemotherapy drug, radioactive isotope, or toxin.”10 MAbs work in several ways, including competitively inhibiting ligand-receptor binding, receptor blockade, or cell elimination from indirect immune system activities such as antibody-dependent cell-mediated cytotoxicity.11,12
Monoclonal antibody uses in family medicine
Asthma
Several MAbs have been approved for use in severe asthma, including but not limited to: omalizumab (Xolair),13 mepolizumab (Nucala),9,14 and dupilumab (Dupixent).15
Omalizumab is a humanized MAb that prevents IgE antibodies from binding to mast cells and basophils, thereby reducing inflammatory mediators.13 A systematic review found that, compared with placebo, omalizumab used in patients with inadequately controlled moderate-to-severe asthma led to significantly fewer asthma exacerbations (absolute risk reduction [ARR], 16% vs 26%; odds ratio [OR] = 0.55; 95% CI, 0.42-0.60; number needed to treat [NNT] = 10) and fewer hospitalizations (ARR, 0.5% vs 3%; OR = 0.16; 95% CI, 0.06-0.42; NNT = 40).13
Significantly more patients in the omalizumab group were able to withdraw from, or reduce, the dose of ICS. GINA recommends omalizumab for patients with positive skin sensitization, total serum IgE ≥ 30 IU/mL, weight within 30 kg to 150 kg, history of childhood asthma and recent exacerbations, and blood eosinophils ≥ 260/mcL.16 Omalizumab is also approved for use in chronic spontaneous urticaria and nasal polyps.
Mepolizumab
Continue to: Another trial found that...
Another trial found that mepolizumab reduced total OCS doses in patients with severe asthma by 50% without increasing exacerbations or worsening asthma control.18 All 3 anti-IL-5 drugs—including not only mepolizumab, but also benralizumab (Fasenra) and reslizumab (Cinqair)—appear to yield similar improvements. A 2017 systematic review found all anti-IL-5 treatments reduced rates of clinically significant asthma exacerbations (treatment with OCS for ≥ 3 days) by roughly 50% in patients with severe eosinophilic asthma and a history of ≥ 2 exacerbations in the past year.
Dupilumab is a humanized MAb that inhibits IL-4 and IL-13, which influence multiple cell types involved in inflammation (eg, mast cells, eosinophils) and inflammatory mediators (histamine, leukotrienes, cytokines).15 In a recent study of patients with uncontrolled asthma, dupilumab 200 mg every 2 weeks compared with placebo showed a modest reduction in the annualized rate of severe asthma exacerbations (0.46 exacerbations vs 0.87, respectively). Dupilumab was effective in patients with blood eosinophil counts ≥ 150/μL but was ineffective in patients with eosinophil counts < 150/μL.15
For patients ≥ 12 years old with severe eosinophilic asthma, GINA recommends using dupilumab as add-on therapy for an initial trial of 4 months at doses of 200 or 300 mg SC every 2 weeks, with preference for 300 mg SC every 2 weeks for OCS-dependent asthma. Dupilumab is approved for use in AD and chronic rhinosinusitis with nasal polyposis. If a biologic agent is not successful after a 4-month trial, consider a 6- to 12-month trial. If efficacy is still minimal, consider switching to an alternative biologic therapy approved for asthma.16
❯ Asthma: Test your skills
Subjective findings: A 19-year-old man presents to your clinic. He has a history of nasal polyps and allergic asthma. At age 18, he was given a diagnosis of severe persistent asthma. He has shortness of breath during waking hours 4 times per week, and treats each of these episodes with albuterol. He also wakes up about twice a week with shortness of breath and has some limitations in normal activities. He reports missing his prescribed fluticasone/salmeterol 500/50 μg, 1 inhalation bid, only once each month. In the last year, he has had 2 exacerbations requiring oral steroids.
Medications: Albuterol 90 μg, 1-2 inhalations, q6h prn; fluticasone/salmeterol 500/50 μg, 1 inhalation bid; tiotropium 1.25 μg, 2 puffs/d; montelukast 10 mg every morning; prednisone 10 mg/d.
Continue to: Objective data
Objective data: Patient is in no apparent distress and afebrile, and oxygen saturation on room air is 97%. Ht, 70 inches; wt, 75 kg. Labs: IgE, 15 IU/mL; serum eosinophils, 315/μL.
Which MAb would be appropriate for this patient? Given that the patient has a blood eosinophil level ≥ 300/μL and severe exacerbations, adult-onset asthma, nasal polyposis, and maintenance OCS at baseline, it would be reasonable to initiate mepolizumab 100 mg SC every 4 weeks, or dupilumab 600 mg once, then 300 mg SC every 2 weeks. Both agents can be self-administered.
Atopic dermatitis
Two MAbs—dupilumab and tralokinumab (Adbry; inhibits IL-13)—are approved for treatment of AD in adults that is uncontrolled with conventional therapy.15,19 Dupilumab is also approved for children ≥ 6 months old.20 Both MAbs are dosed at 600 mg SC, followed by 300 mg every 2 weeks. Dupilumab was compared with placebo in adult patients who had moderate-to-severe AD inadequately controlled on topical corticosteroids (TCSs), to determine the proportion of patients in each group achieving improvement of either 0 or 1 points or ≥ 2 points in the 5-point Investigator Global Assessment (IGA) score from baseline to 16 weeks.21 Thirty-seven percent of patients receiving dupilumab 300 mg SC weekly and 38% of patients receiving dupilumab 300 mg SC every 2 weeks achieved the primary outcome, compared with 10% of those receiving placebo (P < .001).21 Similar IGA scores were reported when dupilumab was combined with TCS, compared with placebo.22
It would be reasonable to consider dupilumab or tralokinumab in patients with: cutaneous atrophy or hypothalamic-pituitary-adrenal axis suppression with TCS, concerns of malignancy with topical calcineurin inhibitors, or problems with the alternative systemic therapies (cyclosporine-induced hypertension, nephrotoxicity, or immunosuppression; azathioprine-induced malignancy; or methotrexate-induced bone marrow suppression, renal impairment, hepatotoxicity, pneumonitis, or gastrointestinal toxicity).23
A distinct advantage of MAbs over other systemic agents in the management of AD is that MAbs do not require frequent monitoring of blood pressure, renal or liver function, complete blood count with differential, electrolytes, or uric acid. Additionally, MAbs have fewer black box warnings and adverse reactions when compared with other systemic agents.
Continue to: Hyperlipidemia
Hyperlipidemia
Three MAbs are approved for use in hyperlipidemia: the angiopoietin-like protein 3 (ANGPTL3) inhibitor evinacumab (Evkeeza)24 and 2 proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, evolocumab (Repatha)25 and alirocumab (Praluent).26
ANGPTL3 inhibitors block ANGPTL3 and reduce endothelial lipase and lipoprotein lipase activity, which in turn decreases low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride formation. PCSK9 inhibitors prevent PCSK9 from binding to LDL receptors, thereby maintaining the number of active LDL receptors and increasing LDL-C removal.
Evinacumab is indicated for homozygous familial hypercholesterolemia and is administered intravenously every 4 weeks. Evinacumab has not been evaluated for effects on cardiovascular morbidity and mortality.
Evolocumab 140 mg SC every 2 weeks or 420 mg SC monthly has been studied in patients on statin therapy with LDL-C ≥ 70 mg/dL. Patients on evolocumab experienced significantly less of the composite endpoint of cardiovascular death, myocardial infarction (MI), stroke, hospitalization for unstable angina, or coronary revascularization compared with placebo (9.8% vs 11.3%; hazard ratio [HR] = 0.85; 95% CI, 0.79-0.92; NNT = 67.27
Alirocumab 75 mg SC every 2 weeks has also been studied in patients receiving statin therapy with LDL-C ≥ 70 mg/dL. Patients taking alirocumab experienced significantly less of the composite endpoint of death from coronary heart disease, nonfatal MI, ischemic stroke, or hospitalization for unstable angina compared with placebo (9.5% vs 11.1%; HR = 0.85; 95% CI, 0.78-0.93; NNT = 63).
Continue to: According to the 2018...
According to the 2018 AHA Cholesterol Guidelines, PCSK9 inhibitors are indicated for patients receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe) with LDL-C ≥ 70 mg/dL, if they have had multiple atherosclerotic cardiovascular disease (ASCVD) events or 1 major ASCVD event with multiple high-risk conditions (eg, heterozygous familial hypercholesterolemia, history of coronary artery bypass grafting or percutaneous coronary intervention, hypertension, estimated glomerular filtration rate of 15 to 59 mL/min/1.73m2).29 For patients without prior ASCVD events or high-risk conditions who are receiving maximally tolerated LDL-C-lowering therapy (statin and ezetimibe), PCSK9 inhibitors are indicated if the LDL-C remains ≥ 100 mg/dL.
Osteoporosis
The 2 MAbs approved for use in osteoporosis are the receptor activator of nuclear factor kB ligand (RANKL) inhibitor denosumab (Prolia)30 and the sclerostin inhibitor romosozumab (Evenity).31
Denosumab prevents RANKL from binding to the RANK receptor, thereby inhibiting osteoclast formation and decreasing bone resorption. Denosumab is approved for use in women and men who are at high risk of osteoporotic fracture, including those taking OCSs, men receiving androgen deprivation therapy for prostate cancer, and women receiving adjuvant aromatase inhibitor therapy for breast cancer.
In a 3-year randomized trial, denosumab 60 mg SC every 6 months was compared with placebo in postmenopausal women with T-scores < –2.5, but not < –4.0 at the lumbar spine or total hip. Denosumab significantly reduced new radiographic vertebral fractures (2.3% vs 7.2%; risk ratio [RR] = 0.32; 95% CI, 0.26-0.41; NNT = 21), hip fracture (0.7% vs 1.2%), and nonvertebral fracture (6.5% vs 8.0%).32 Denosumab carries an increased risk of multiple vertebral fractures following discontinuation, skin infections, dermatologic reactions, and severe bone, joint, and muscle pain.
Romosozumab inhibits sclerostin, thereby increasing bone formation and, to a lesser degree, decreasing bone resorption. Romosozumab is approved for use in postmenopausal women at high risk for fracture (ie, those with a history of osteoporotic fracture or multiple risk factors for fracture) or in patients who have not benefited from or are intolerant of other therapies. In one study, postmenopausal women with a T-score of –2.5 to –3.5 at the total hip or femoral neck were randomly assigned to receive either romosozumab 210 mg SC or placebo for 12 months, then each group was switched to denosumab 60 mg SC for 12 months. After the first year, prior to initiating denosumab, patients taking romosozumab experienced significantly fewer new vertebral fractures than patients taking placebo (0.5% vs 1.8%; RR = 0.27; 95% CI, 0.16-0.47; NNT = 77); however, there was no significant difference between the 2 groups with nonvertebral fractures (HR = 0.75; 95% CI, 0.53-1.05).33
Continue to: In another study...
In another study, romosozumab 210 mg SC was compared with alendronate 70 mg weekly, followed by alendronate 70 mg weekly in both groups. Over the first 12 months, patients treated with romosozumab saw a significant reduction in the incidence of new vertebral fractures (4% vs 6.3%; RR = 0.63, P < .003; NNT = 44). Patients treated with romosozumab with alendronate added for another 12 months also saw a significant reduction in new incidence of vertebral fractures (6.2% vs 11.9%; RR = 0.52; P < .001; NNT = 18).34 There was a higher risk of cardiovascular events among patients receiving romosozumab compared with those treated with alendronate, so romosozumab should not be used in individuals who have had an MI or stroke within the previous year.34 Denosumab and romosozumab offer an advantage over some bisphosphonates in that they require less frequent dosing and can be used in patients with renal impairment (creatinine clearance < 35 mL/min, in which zoledronic acid is contraindicated and alendronate is not recommended; < 30 mL/min, in which risedronate and ibandronate are not recommended).
Migraine prevention
Four
Erenumab, fremanezumab, and galcanezumab are all available in subcutaneous autoinjectors (or syringe with fremanezumab). Eptinezumab is an intravenous (IV) infusion given every 3 months.
Erenumab is available in both 70-mg and 140-mg dosing options. Fremanezumab can be given as 225 mg monthly or 675 mg quarterly. Galcanezumab has an initial loading dose of 240 mg followed by 120 mg given monthly. Erenumab targets the CGRP receptor; the others target the CGRP ligand. Eptinezumab has 100% bioavailability and reaches maximum serum concentration sooner than the other antagonists (due to its route of administration), but it must be given in an infusion center. Few insurers approve the use of eptinezumab unless a trial of least 1 of the monthly injectables has failed.
There are no head-to-head studies of the medications in this class. Additionally, differing study designs, definitions, statistical analyses, endpoints, and responder-rate calculations make it challenging to compare them directly against one another. At the very least, all of the CGRP MAbs have efficacy comparable to conventional preventive migraine medications such as propranolol, amitriptyline, and topiramate.40
Continue to: The most commonly reported adverse...
The most commonly reported adverse effect for all 4 CGRPs is injection site reaction, which was highest with the quarterly fremanezumab dose (45%).37 Constipation was most notable with the 140-mg dose of erenumab (3%)35; with the other CGRP MAbs it is comparable to that seen with placebo (< 1%).
Erenumab-induced hypertension has been identified in 61 cases reported through the FDA Adverse Event Reporting System (FAERS) as of 2021.41 This was not reported during MAb development programs, nor was it noted during clinical trials. Blood pressure elevation was seen within 1 week of injection in nearly 50% of the cases, and nearly one-third had pre-existing hypertension.41 Due to these findings, the erenumab prescribing information was updated to include hypertension in its warnings and precautions. It is possible that hypertension could be a class effect, although trial data and posthoc studies have yet to bear that out. Since erenumab was the first CGRP antagonist brought to market (May 2018 vs September 2018 for fremanezumab and galcanezumab), it may have accumulated more FAERS reports. Nearly all studies exclude patients with older age, uncontrolled hypertension, and unstable cardiovascular disease, which could impact data.41
Overall, this class of medications is very well tolerated, easy to use (again, excluding eptinezumab), and maintains a low adverse effect profile, giving added value compared with conventional preventive migraine medications.
The American Headache Society recommends a preventive oral therapy for at least 3 months before trying an alternative medication. After treatment failure with at least 2 oral agents, CGRP MAbs are recommended.42 CGRP antagonists offer convenient dosing, bypass gastrointestinal metabolism (which is useful in patients with nausea/vomiting), and have fewer adverse effects than traditional oral medications.
Worth noting. Several newer oral agents have been recently approved for migraine prevention, including atogepant (Qulipta) and rimegepant (Nurtec), which are also CGRP antagonists. Rimegepant is approved for both acute migraine treatment and prevention.
Continue to: Migraine
❯ Migraine: Test your skills
Subjective findings: A 25-year-old woman presents to your clinic for management of episodic migraines with aura. Her baseline average migraine frequency is 9 headache days/month. Her migraines are becoming more frequent despite treatment. She fears IV medication use and avoids hospitals.
History: Hypertension, irritable bowel syndrome with constipation (IBS-C), and depression. The patient is not pregnant or trying to get pregnant.
Medications: Current medications (for previous 4 months) include propranolol 40 mg at bedtime, linaclotide 145 μg/d, citalopram 20 mg/d, and sumatriptan 50 mg prn. Past medications include venlafaxine 150 mg po bid for 5 months.
What would be appropriate for this patient? This patient meets the criteria for using a CGRP antagonist because she has tried 2 preventive treatments for more than 60 to 90 days. Erenumab is not the best option, given the patient’s history of hypertension and IBS-C. The patient fears hospitals and IV medications, making eptinezumab a less-than-ideal choice. Depending on her insurance, fremanezumab or galcanezumab would be good options at this time.
CGRP antagonists have not been studied or evaluated in pregnancy, but if this patient becomes pregnant, a first-line agent for prevention would be propranolol, and a second-line agent would be a tricyclic antidepressant, memantine, or verapamil. Avoid ergotamines and antiepileptics (topiramate or valproate) in pregnancy.43,44
Continue to: The challenges associated with MAbs
The challenges associated with MAbs
MAbs can be expensive (TABLE 2),45 some prohibitively so. On a population scale, biologics account for around 40% of prescription drug spending and may cost 22 times more than small-molecule drugs.46 Estimates in 2016 showed that MAbs comprise $90.2 billion (43%) of the biologic market.46

MAbs also require prior authorization forms to be submitted. Prior authorization criteria vary by state and by insurance plan. In my (ES) experience, submitting letters of medical necessity justifying the need for therapy or expertise in the disease states for which the MAb is being prescribed help your patient get the medication they need.
Expect to see additional MAbs approved in the future. If the costs come down, adoption of these agents into practice will likely increase.
CORRESPONDENCE
Evelyn Sbar, MD, Texas Tech University Health Sciences Center, 1400 South Coulter Street, Suite 5100, Amarillo, TX 79106; [email protected]
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
1. Rui P, Okeyode T. National Ambulatory Medical Care Survey: 2016 national summary tables. National Center for Health Statistics. Accessed June 15, 2022. www.cdc.gov/nchs/data/ahcd/namcs_summary/2016_namcs_web_tables.pdf
2. IDBS. The future of biologics drug development is today. June 27, 2018. Accessed June 15, 2022. www.idbs.com/blog/2018/06/the-future-of-biologics-drug-development-is-today/
3. Antibody therapeutics approved or in regulatory review in the EU or US. Antibody Society. Accessed June 15, 2022. www.antibodysociety.org/resources/approved-antibodies/
4. FDA. Code of Federal Regulations, Title 21, Chapter I, Subchapter F biologics. March 29, 2022. Accessed June 15, 2022. www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=600.3
5. Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495-497. doi: 10.1038/256495a0
6. Raejewsky K. The advent and rise of monoclonal antibodies. Nature. November 4, 2019. Accessed June 15, 2022. www.nature.com/articles/d41586-019-02840-w
7. Flovent. Prescribing information. GlaxoSmithKline; 2010. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2010/021433s015lbl.pdf
8. NLM. National Center for Biotechnology Information. PubChem. Method for the preparation of fluticasone and related 17beta-carbothioic esters using a novel carbothioic acid synthesis and novel purification methods. Accessed June 15, 2022. pubchem.ncbi.nlm.nih.gov/patent/WO-0162722-A2
9. Nucala. Prescribing information. GlaxoSmithKline; 2019. Accessed June 15, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761122s000lbl.pdf
10. Argyriou AA, Kalofonos HP. Recent advances relating to the clinical application of naked monoclonal antibodies in solid tumors. Mol Med. 2009;15:183-191. doi: 10.2119/molmed.2009.00007
11. Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548-558. doi: 10.1038/clpt.2008.170
12. Zahavi D, AlDeghaither D, O’Connell A, et al. Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy. Antib Ther. 2018;1:7-12. doi: 10.1093/abt/tby002
13. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014:CD003559. doi: 10.1002/14651858.CD003559.pub4
14. Farne HA, Wilson A, Powell C, et al. Anti-IL5 therapies for asthma. Cochrane Database Syst Rev. 2017;9:CD010834. doi: 10.1002/14651858.CD010834.pub3
15. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378:2486-2496. doi: 10.1056/NEJMoa1804092
16. GINA. Global strategy for asthma management and prevention. 2022 Difficult-to-treat and severe asthma guide—slide set. Accessed June 23, 2022. https://ginasthma.org/severeasthma/
17. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207. doi: 10.1056/NEJMoa1403290
18. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197. doi: 10.1056/NEJMoa1403291
19. Adbry. Prescribing information. Leo Pharma Inc; 2021. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/nda/2022/761180Orig1s000lbl.pdf
20. Dupixent. Prescribing information. Regeneron Pharmaceuticals; 2022. Accessed October 5, 2022. https://www.regeneron.com/downloads/dupixent_fpi.pdf
21. Simpson EL, Bieber T, Guttman-Yassky E, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016;375:2335-2348. doi: 10.1056/NEJMoa1610020
22. Blauvelt A, de Bruin-Weller M, Gooderham M, et al. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017;389:2287-2303. doi: 10.1016/s0140-6736(17)31191-1
23. Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi: 10.1016/j.jaad.2014.03.030
24. Evkeeza. Prescribing information. Regeneron Pharmaceuticals; 2021. Accessed June 24, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761181s000lbl.pdf
25. Repatha. Prescribing information. Amgen; 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125522s014lbl.pdf
26. Praluent. Prescribing information. Sanofi Aventis and Regeneron Pharmaceuticals. 2015. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2017/125559s002lbl.pdf
27. Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713-1722. doi: 10.1056/NEJMoa1615664
28. Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097-2107. doi:10.1056/NEJMoa1801174
29. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2019;73:e285-e350. doi: 10.1016/j.jacc.2018.11.003
30. Prolia. Prescribing information. Amgen; 2010. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2013/125320s094lbl.pdf
31. Evenity. Prescribing information. Amgen; 2019. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2019/761062s000lbl.pdf
32. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756-765. doi: 10.1056/NEJMoa0809493
33. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532-1543. doi: 10.1056/NEJMoa1607948
34. Saag KG, Petersen J, Brandi ML, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377:1417-1427. doi: 10.1056/NEJMoa1708322
35. Aimovig. Prescribing information. Amgen; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761077s000lbl.pdf
36. Vyepti. Prescribing information. Lundbeck Seattle BioPharmaceuticals; 2020. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2020/761119s000lbl.pdf
37. Ajovy. Prescribing information. Teva Pharmaceuticals; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf
38. Emgality. Prescribing information. Eli Lilly and Co.; 2018. Accessed June 24, 2022. www.accessdata.fda.gov/drugsatfda_docs/label/2018/761063s000lbl.pdf
39. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat Rev Neurol. 2018;14:338-350. doi: 10.1038/s41582-018-0003-1
40. Vandervorst F. Van Deun L, Van Dycke A, et al. CGRP monoclonal antibodies in migraine: an efficacy and tolerability comparison with standard prophylactic drugs. J Headache Pain. 2021;22:128. doi: 10.1186/s10194-021-01335-2
41. Saely S, Croteau D, Jawidzik L, et al. Hypertension: a new safety risk for patients treated with erenumab. Headache. 2021;61:202-208. doi: 10.1111/head.14051
42. American Headache Society. The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1-18. doi: 10.1111/head.13456
43. Burch R. Headache in pregnancy and the puerperium. Neurol Clin. 2019;37:31-51. doi: 10.1016/j.ncl.2018.09.004
44. Burch R. Epidemiology and treatment of menstrual migraine and migraine during pregnancy and lactation: a narrative review. Headache. 2020;60:200-216. doi: 10.1111/head.13665
45. Lexi-Comp. Lexi-drug database. Accessed April 4, 2022. https://online.lexi.com/lco/action/login
46. Walker N. Biologics: driving force in pharma. Pharma’s Almanac. June 5, 2017. Accessed June 15, 2020. www.pharmasalmanac.com/articles/biologics-driving-force-in-pharma
PRACTICE RECOMMENDATIONS
› Consider anti-immunoglobulin E, anti-interleukin 5, or anti-interleukin 4/interleukin 13 for patients with moderate-to-severe asthma and type 2 airway inflammation. B
› Consider dupilumab for patients with moderate-to-severe atopic dermatitis (with or without topical corticosteroids), or when traditional oral therapies are inadequate or contraindicated. B
› Consider proprotein convertase subtilisin/kexin type 9 inhibitors for patients with heterozygous familial hypercholesterolemia or clinical atherosclerotic cardiovascular disease when maximally tolerated statins or ezetimibe have not lowered low-density lipoprotein cholesterol levels far enough. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Going the distance with our patients
Many years ago, I had a patient I’ll call “Hannah,” who was well into her 80s and always came into the office with her daughter. She was a heavy smoker and had hypertension and type 2 diabetes.
At each visit, I would ask her if she still smoked and if she was interested in talking about quitting. At every visit, she would say that she was still smoking and didn’t want to quit. My response was always something along the lines of: “When you’re ready, we can talk more. But I think it is the most important thing you can do to improve your health.” From there, we would discuss any concerns she or her daughter had.
A few years shy of her 100th birthday, Hannah told me she had quit smoking. I was amazed and asked her why, after all these years, she’d done it.
“I quit,” she said, “because I was tired of you nagging me, sonny!” And we both had a good laugh about that.
Hannah’s story reminds me that, as family physicians, we often have an impact on our patients in ways we don’t see in the short term. It is our longitudinal relationships with patients that allow us to plant seeds and reap the benefits over time.
It is these relationships that we can draw upon when counseling our patients with type 2 diabetes to address lifestyle issues such as exercise and a healthy diet. In this issue, McMullan et al1 provide us with a rather hopeful review of the evidence in support of lifestyle changes. For our patients with type 2 diabetes, lifestyle changes can decrease A1C levels by 0.5% (with environmental changes related to diet)2 and 0.7% (with moderate aerobic exercise).3 This is comparable to what is reported for the starting doses of most medications.4 In fact, a meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients.5 (Caveat: The result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used.)
And yet, we often focus more on the various medications we can prescribe, with professional guidelines pointing the way.
Continue to: The National Institute for Health and Care Excellence
The National Institute for Health and Care Excellence,6 American Diabetes Association,7 American College of Physicians,8 and American Academy of Family Physicians8 have followed the accumulating evidence that various medications improve outcomes—especially in patients at high risk or with established atherosclerotic cardiovascular disease. They have endorsed a stepwise pharmacologic approach beginning with metformin and recommend assessing each patient’s comorbidities to guide whether to add a sodium glucose co-transporter 2 (SGLT2) inhibitor or another agent. Where the groups diverge is what that second agent should be (glucagon-like peptide 1 receptor agonist, SGLT2 inhibitor, or dipeptidyl peptidase-4 inhibitor).
But what about lifestyle? Each organization’s guidelines address lifestyle changes as a foundation for managing patients with type 2 diabetes. But is that call loud enough? Do we heed it well enough?
Implementing lifestyle changes in office practice can be time consuming. Many clinicians lack adequate training or experience to gain any traction with it. Also, there is skepticism about success and sustainability.
I believe change starts when we recognize that while we have a priority list for each patient encounter, so do our patients. But they may not share that list with us unless we open the door by asking questions, such as:
- Of all the things you have heard about caring for your diabetes, what would you like to work on?
- What are you currently doing and what prevents you from meeting your goals?
- How would you like me to help you?
From there, we can start small and build on successes over time. We can go the distance with our patients. In the case of Hannah, I had the honor of caring for her until she died at age 104.
1. McMullan S, Smith DK, Kimsey J. Maximizing lifestyle changes to manage type 2 diabetes. J Fam Pract. 2022;71;342-348. doi: 10.12788/jfp.0482
2. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
3. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. doi: 10.1186/s12933-017-0518-6
Many years ago, I had a patient I’ll call “Hannah,” who was well into her 80s and always came into the office with her daughter. She was a heavy smoker and had hypertension and type 2 diabetes.
At each visit, I would ask her if she still smoked and if she was interested in talking about quitting. At every visit, she would say that she was still smoking and didn’t want to quit. My response was always something along the lines of: “When you’re ready, we can talk more. But I think it is the most important thing you can do to improve your health.” From there, we would discuss any concerns she or her daughter had.
A few years shy of her 100th birthday, Hannah told me she had quit smoking. I was amazed and asked her why, after all these years, she’d done it.
“I quit,” she said, “because I was tired of you nagging me, sonny!” And we both had a good laugh about that.
Hannah’s story reminds me that, as family physicians, we often have an impact on our patients in ways we don’t see in the short term. It is our longitudinal relationships with patients that allow us to plant seeds and reap the benefits over time.
It is these relationships that we can draw upon when counseling our patients with type 2 diabetes to address lifestyle issues such as exercise and a healthy diet. In this issue, McMullan et al1 provide us with a rather hopeful review of the evidence in support of lifestyle changes. For our patients with type 2 diabetes, lifestyle changes can decrease A1C levels by 0.5% (with environmental changes related to diet)2 and 0.7% (with moderate aerobic exercise).3 This is comparable to what is reported for the starting doses of most medications.4 In fact, a meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients.5 (Caveat: The result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used.)
And yet, we often focus more on the various medications we can prescribe, with professional guidelines pointing the way.
Continue to: The National Institute for Health and Care Excellence
The National Institute for Health and Care Excellence,6 American Diabetes Association,7 American College of Physicians,8 and American Academy of Family Physicians8 have followed the accumulating evidence that various medications improve outcomes—especially in patients at high risk or with established atherosclerotic cardiovascular disease. They have endorsed a stepwise pharmacologic approach beginning with metformin and recommend assessing each patient’s comorbidities to guide whether to add a sodium glucose co-transporter 2 (SGLT2) inhibitor or another agent. Where the groups diverge is what that second agent should be (glucagon-like peptide 1 receptor agonist, SGLT2 inhibitor, or dipeptidyl peptidase-4 inhibitor).
But what about lifestyle? Each organization’s guidelines address lifestyle changes as a foundation for managing patients with type 2 diabetes. But is that call loud enough? Do we heed it well enough?
Implementing lifestyle changes in office practice can be time consuming. Many clinicians lack adequate training or experience to gain any traction with it. Also, there is skepticism about success and sustainability.
I believe change starts when we recognize that while we have a priority list for each patient encounter, so do our patients. But they may not share that list with us unless we open the door by asking questions, such as:
- Of all the things you have heard about caring for your diabetes, what would you like to work on?
- What are you currently doing and what prevents you from meeting your goals?
- How would you like me to help you?
From there, we can start small and build on successes over time. We can go the distance with our patients. In the case of Hannah, I had the honor of caring for her until she died at age 104.
Many years ago, I had a patient I’ll call “Hannah,” who was well into her 80s and always came into the office with her daughter. She was a heavy smoker and had hypertension and type 2 diabetes.
At each visit, I would ask her if she still smoked and if she was interested in talking about quitting. At every visit, she would say that she was still smoking and didn’t want to quit. My response was always something along the lines of: “When you’re ready, we can talk more. But I think it is the most important thing you can do to improve your health.” From there, we would discuss any concerns she or her daughter had.
A few years shy of her 100th birthday, Hannah told me she had quit smoking. I was amazed and asked her why, after all these years, she’d done it.
“I quit,” she said, “because I was tired of you nagging me, sonny!” And we both had a good laugh about that.
Hannah’s story reminds me that, as family physicians, we often have an impact on our patients in ways we don’t see in the short term. It is our longitudinal relationships with patients that allow us to plant seeds and reap the benefits over time.
It is these relationships that we can draw upon when counseling our patients with type 2 diabetes to address lifestyle issues such as exercise and a healthy diet. In this issue, McMullan et al1 provide us with a rather hopeful review of the evidence in support of lifestyle changes. For our patients with type 2 diabetes, lifestyle changes can decrease A1C levels by 0.5% (with environmental changes related to diet)2 and 0.7% (with moderate aerobic exercise).3 This is comparable to what is reported for the starting doses of most medications.4 In fact, a meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients.5 (Caveat: The result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used.)
And yet, we often focus more on the various medications we can prescribe, with professional guidelines pointing the way.
Continue to: The National Institute for Health and Care Excellence
The National Institute for Health and Care Excellence,6 American Diabetes Association,7 American College of Physicians,8 and American Academy of Family Physicians8 have followed the accumulating evidence that various medications improve outcomes—especially in patients at high risk or with established atherosclerotic cardiovascular disease. They have endorsed a stepwise pharmacologic approach beginning with metformin and recommend assessing each patient’s comorbidities to guide whether to add a sodium glucose co-transporter 2 (SGLT2) inhibitor or another agent. Where the groups diverge is what that second agent should be (glucagon-like peptide 1 receptor agonist, SGLT2 inhibitor, or dipeptidyl peptidase-4 inhibitor).
But what about lifestyle? Each organization’s guidelines address lifestyle changes as a foundation for managing patients with type 2 diabetes. But is that call loud enough? Do we heed it well enough?
Implementing lifestyle changes in office practice can be time consuming. Many clinicians lack adequate training or experience to gain any traction with it. Also, there is skepticism about success and sustainability.
I believe change starts when we recognize that while we have a priority list for each patient encounter, so do our patients. But they may not share that list with us unless we open the door by asking questions, such as:
- Of all the things you have heard about caring for your diabetes, what would you like to work on?
- What are you currently doing and what prevents you from meeting your goals?
- How would you like me to help you?
From there, we can start small and build on successes over time. We can go the distance with our patients. In the case of Hannah, I had the honor of caring for her until she died at age 104.
1. McMullan S, Smith DK, Kimsey J. Maximizing lifestyle changes to manage type 2 diabetes. J Fam Pract. 2022;71;342-348. doi: 10.12788/jfp.0482
2. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
3. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. doi: 10.1186/s12933-017-0518-6
1. McMullan S, Smith DK, Kimsey J. Maximizing lifestyle changes to manage type 2 diabetes. J Fam Pract. 2022;71;342-348. doi: 10.12788/jfp.0482
2. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
3. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. doi: 10.1186/s12933-017-0518-6
Vaccine update for the 2022-23 influenza season
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.
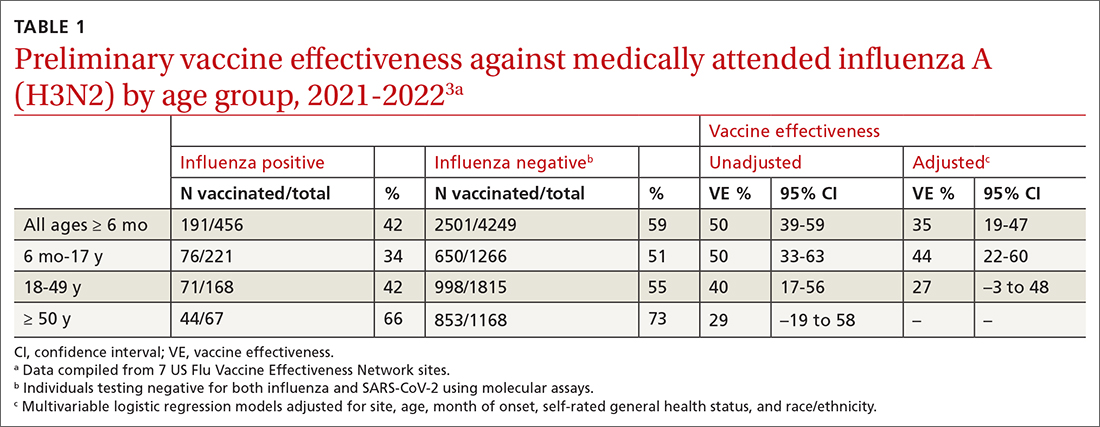
The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).
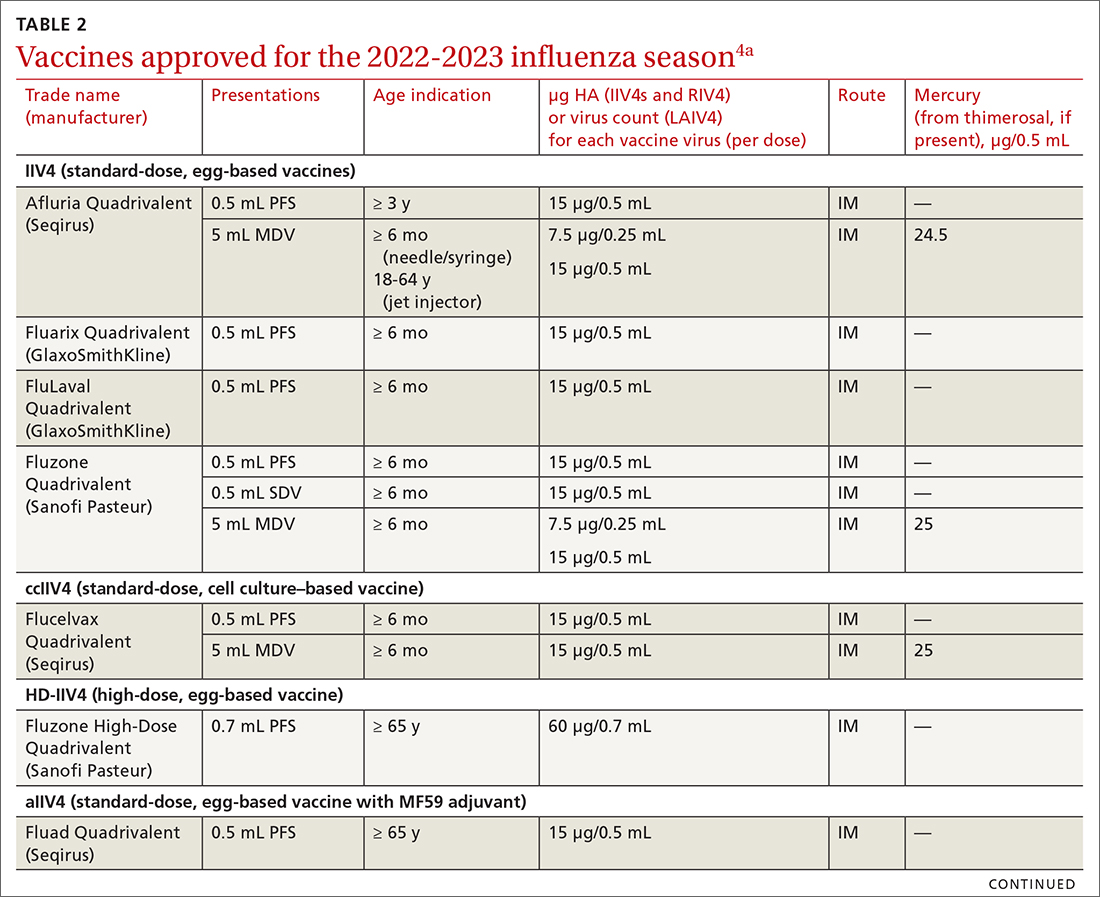
Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).
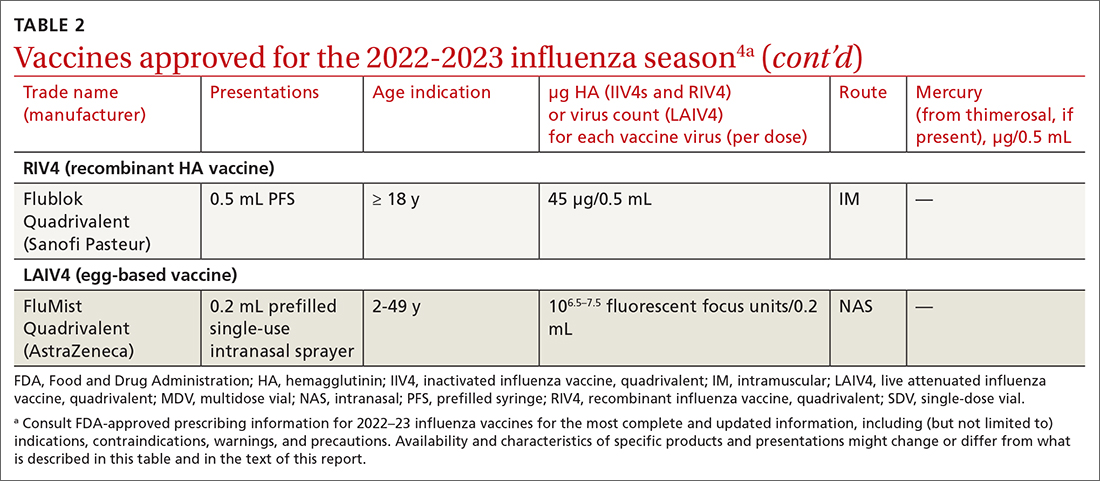
Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.
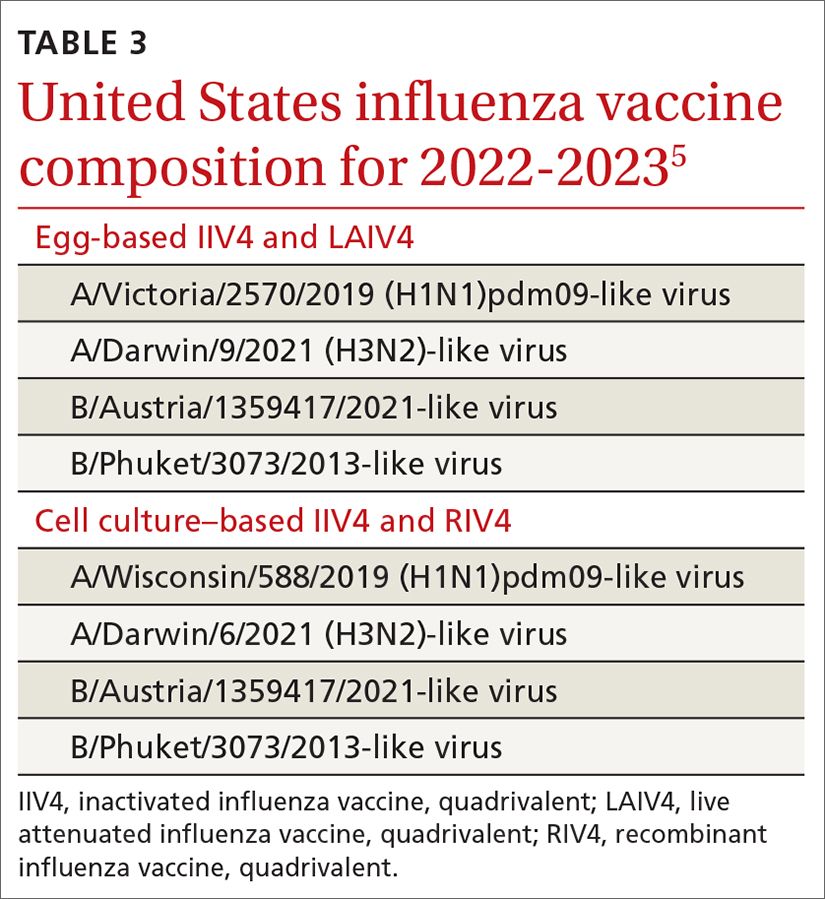
All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.
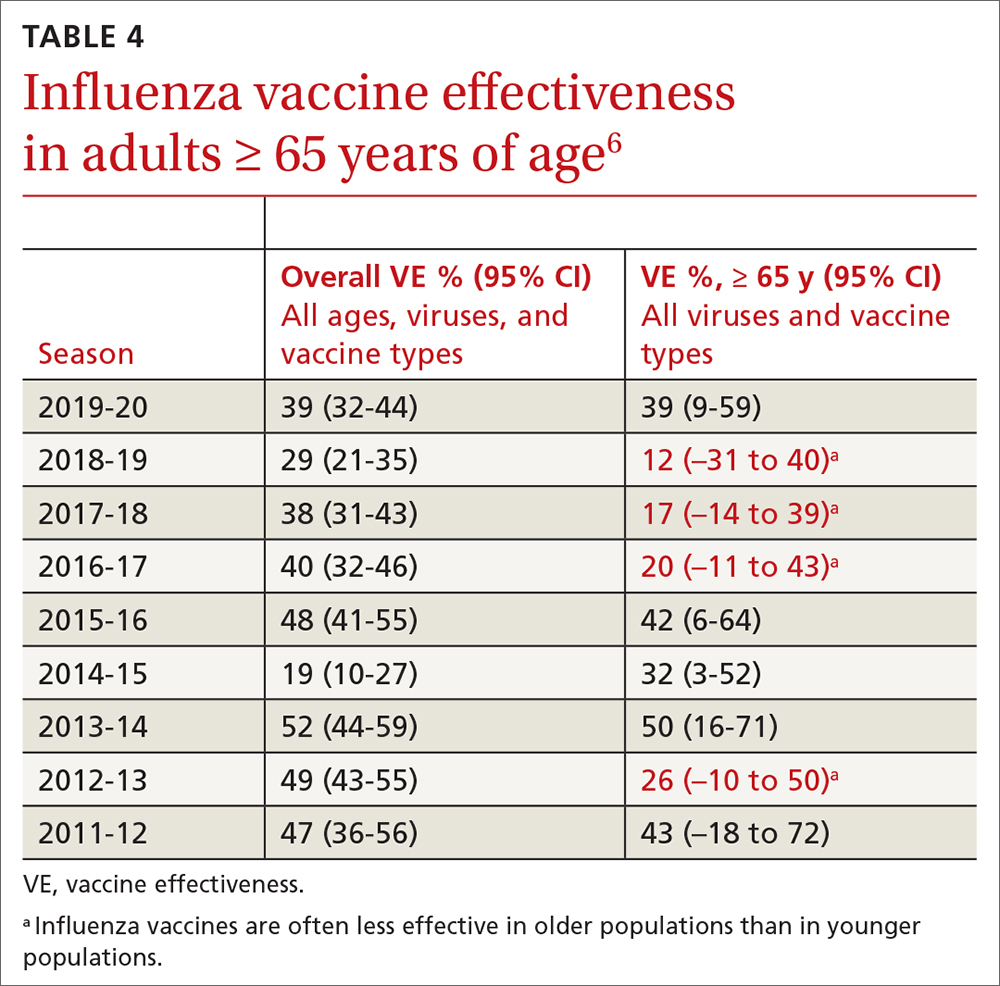
One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
In the 2020-2021 influenza season, there was practically no influenza circulating in the United States. This decline from seasonal expectations, described in a previous Practice Alert, was probably due to the interventions aimed at limiting the spread of COVID-19: masking, social distancing, working from home, and cancellation of large, crowded events.1 In 2021-2022 influenza returned, but only in moderation.

The Centers for Disease Control and Prevention (CDC) estimates there were between 82,000 to 170,000 hospitalizations and 5000 to 14,000 deaths attributed to influenza.2 In addition, US virologic surveillance indicates that 98.6% of specimens tested positive for influenza A.2 While the vaccine’s effectiveness in 2021-2022 was far below what was desired, it still prevented a great deal of flu morbidity and mortality and reduced acute respiratory illness due to influenza A(H3N2) virus by 35% (TABLE 1).3 All vaccines for the upcoming flu season are quadrivalent, containing 2 influenza A antigens and 2 influenza B antigens (TABLES 24 and 35).

Vaccine effectiveness in older adults (≥ 65 years) has been very low. TABLE 46 shows vaccine effectiveness in the elderly for 10 influenza seasons between 2011 and 2020.6 In nearly half of those seasons, the estimated vaccine effectiveness was possibly nil. All influenza vaccines licensed for use in the United States are approved for use in those ≥ 65 years of age, except live attenuated influenza vaccine (LAIV).

Three products were developed to address the issue of low vaccine effectiveness in the elderly. The Advisory Committee on Immunization Practices (ACIP) has not expressed a preference for any specific vaccine for this age group. The high-dose qudrivalent vaccine (HD-IIV4), Fluzone, contains 4 times the antigen level of the standard-dose vaccines (SD-IIV4)—60 μg vs 15 μg. Fluzone was initially approved in 2014 as a trivalent vaccine and was approved as a quadrivalent vaccine in 2019. The adjuvanted quadrivalent influenza vaccine (aIIV4), Fluad, was also inititally approved as a trivalent vaccine in 2015 and as quadrivalent in 2021. Both HD-IIV4 and aIIV4 are approved only for those ≥ 65 years of age. Recombinant quadrivalent influenza vaccine (RIV4), Flublok, is approved for ages ≥ 18 years and is produced by a process that does not involve eggs. It contains 3 times the antigen level as SD-IIV4 vaccines.

All 3 of these vaccines (HD-IIV4, aIIV4, and RIV4) have been compared with SD-IIV4 for effectiveness in the elderly and have yielded better outcomes. However, direct comparisons among the 3 vaccines have not shown robust evidence of superiority, and ACIP is unwilling to preferentially recommend one of them at this time. At its June 2022 meeting, ACIP voted to recommend any of these 3 options over the SD-IIV 4 options for those ≥ 65 years of age, with the caveat that if only an SD-IIV4 option is available it should be administered in preference to delaying vaccination.

One other vaccine change for the upcoming season involves the cell culture–based quadrivalent inactivated influenza vaccine (ccIIV4), Flucelvax, which is now approved for those ages ≥ 6 months. It previously was approved only for ages ≥ 2 years. All unadjuvanted SD-IIV4 vaccines as well as ccIIV4 are now approved for everyone ≥ 6 months of age. LAIV continues to be approved for ages 2 through 49 years. The only influenza vaccine products that contain thimerosal are those in multidose vials (TABLE 24).
Promote vaccination and infection-control practices. ACIP continues to recommend influenza vaccine for all those ages ≥ 6 months, with 2 doses for those < 9 years old not previously vaccinated with an influenza vaccine. In addition to encouraging and offering influenza vaccine to patients and staff, we can minimize the spread of influenza in the community by robust infection-control practices in the clinical setting: masking and isolation of patients with respiratory symptoms, encouraging those with symptoms to stay at home and mask when around family members, advising frequent hand washing and respiratory hygiene, and using pre- and post-exposure chemoprophylaxis as appropriate. All recommendations regarding influenza for 2022-2023 can be found on the CDC website.4
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
1. Campos-Outcalt D. Influenza vaccine update, 2021-2022. J Fam Pract. 2021;70:399-402. doi: 10.12788/jfp.0277
2. Merced-Morales A, Daly P, Abd Elal AI, et al. Influenza activity and composition of the 2022-23 influenza vaccine—United States, 2021-22 season. MMWR Morb Mortal Wkly Rep. 2022;71;913-919. doi: 10.15585/mmwr.mm7129a1
3. CDC. National Center for Immunization and Respiratory Diseases. Preliminary Estimates of 2021–22 Seasonal Influenza Vaccine Effectiveness against Medically Attended Influenza. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/02-influenza-chung-508.pdf
4. Grohskopf LA, Blanton LH, Ferdinands JM, et al. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices – United States, 2022-23 influenza season. MMWR Recomm Rep. 2022;71:1-28. doi: http://dx.doi.org/10.15585/mmwr.rr7101a1
5. FDA. Influenza vaccine for the 2022-2023 season. Accessed September 22, 2022. www.fda.gov/vaccines-blood-biologics/lot-release/influenza-vaccine-2022-2023-season
6. Grohskopf L. Influenza vaccines for persons aged ≥ 65 years: evidence to recommendation (EtR) framework. Presented to the ACIP June 22, 2022. Accessed September 22, 2022. www.cdc.gov/vaccines/acip/meetings/downloads/slides-2022-06-22-23/03-influenza-grohskopf-508.pdf
Maximizing lifestyle changes to manage type 2 diabetes
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11
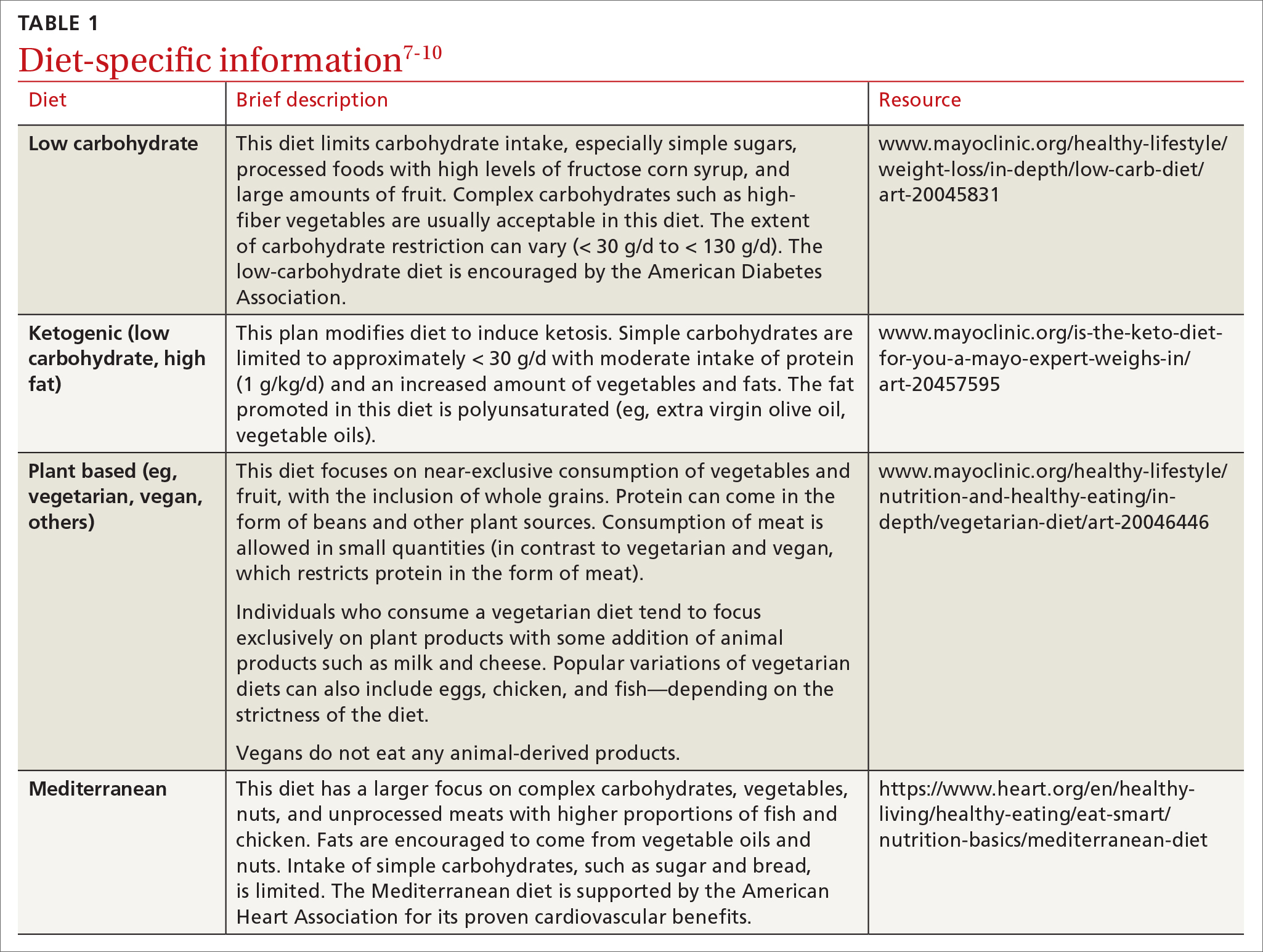
In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23
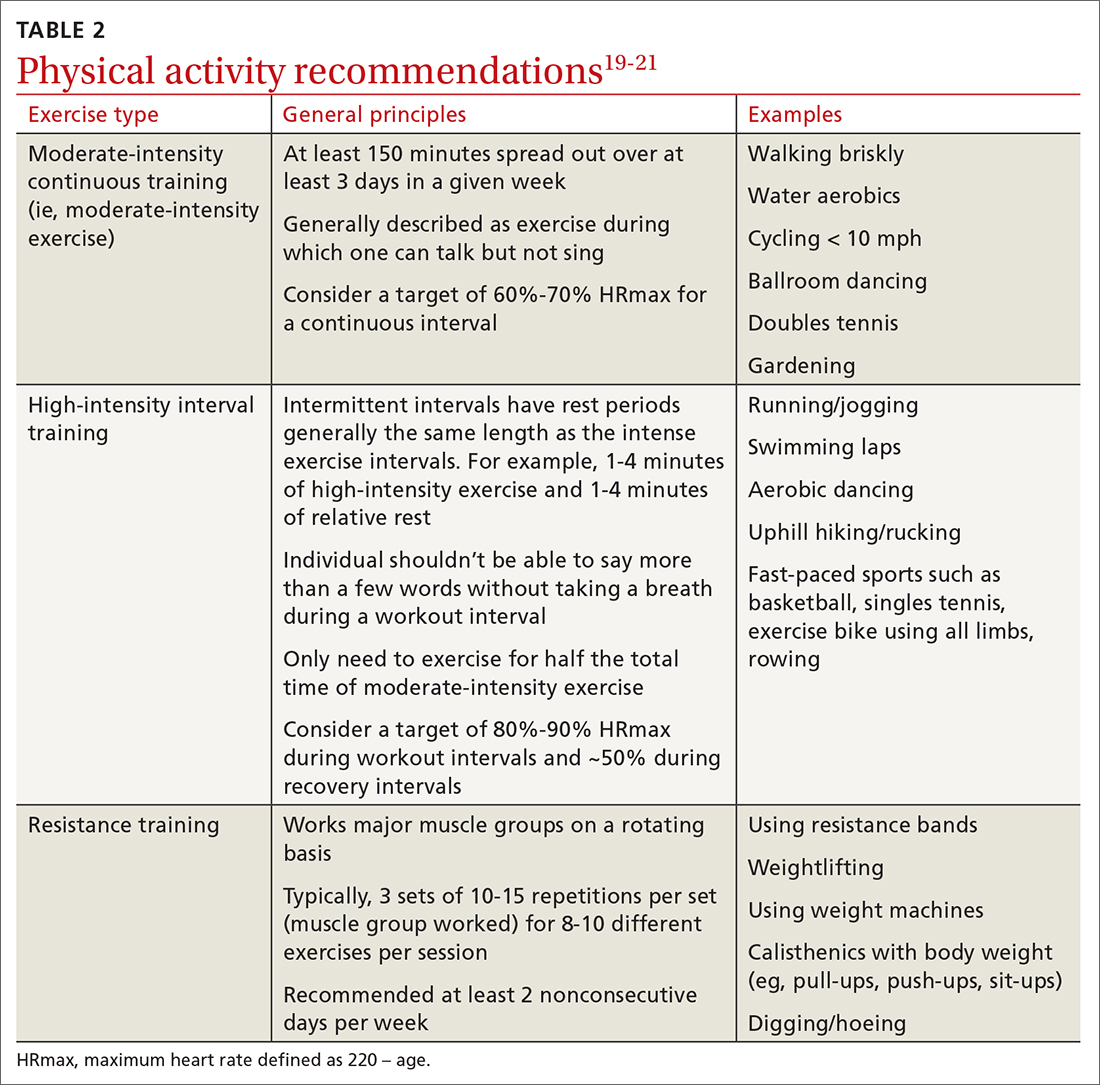
Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).
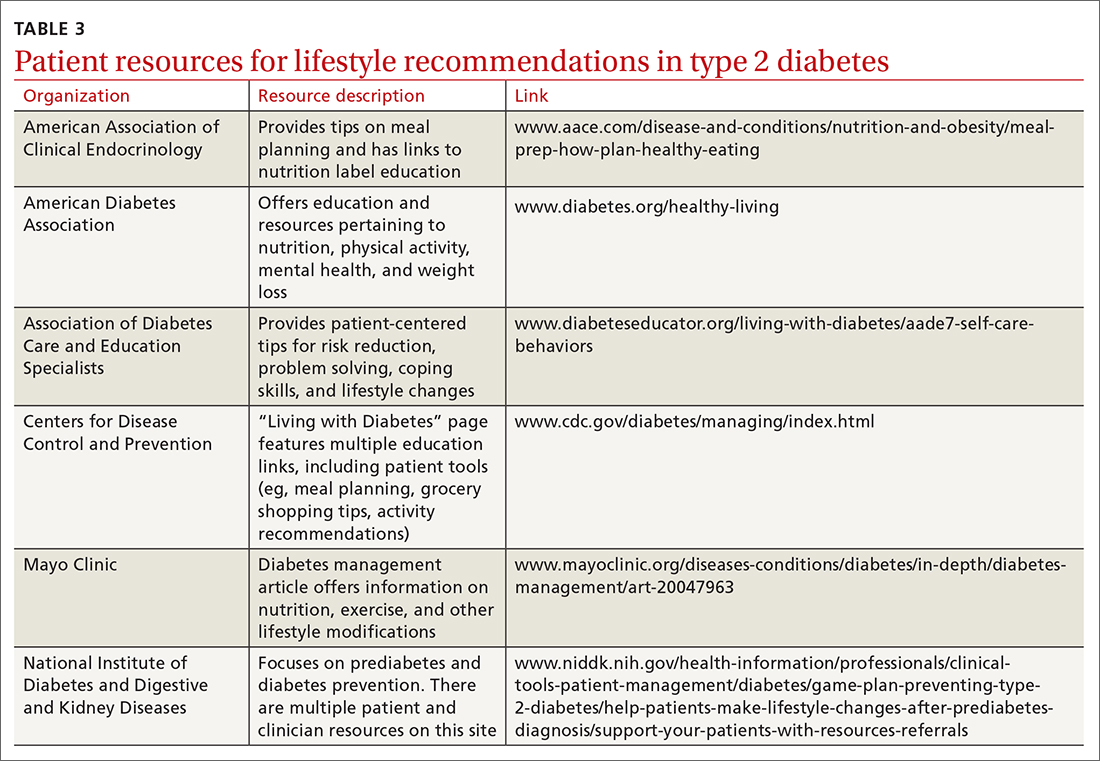
C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11

In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23

Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).

C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
Type 2 diabetes has been increasing in incidence and prevalence over the past 20 years, with worldwide prevalence estimated at 6.28%.1 The estimated cost of diagnosed diabetes in the United States was $327 billion in 2017; this included direct medical costs and reduced productivity.2 Type 2 diabetes can be prevented in most patients, given that it is a metabolic derangement caused by a complicated interaction between a patient’s genetic predisposition and lifestyle. A consensus statement by the American Academy of Clinical Endocrinologists (AACE) and American College of Endocrinology indicates that the recommended lifestyle modifications for diabetes include medical nutrition therapy with healthy eating patterns, regular physical activity, adequate sleep, behavioral support/counseling, and smoking cessation.3 Evidence shows that adherence to these lifestyle changes alone yields a relative reduction in type 2 diabetes mortality of 57%.4

In the discussion that follows, we review the current guideline recommendations for dietary modifications and physical activity and summarize their effectiveness in the treatment of type 2 diabetes. We also describe practical clinical strategies to promote change in patient behavior, and examine current literature supporting intensive lifestyle changes that, if achieved, may induce disease remission.5
Dietary strategies
Low, or very low, carbohydrate diet
Carbohydrates can affect blood glucose levels in varying degrees depending on their intrinsic properties such as fiber content, sugars, and starches . 6 According to the American Diabetes Association’s (ADA) 2019 consensus report, 6 the carbohydrate quality that generally should be recommended is high in fiber, vitamins, and minerals, and low in added sugars, fats, and sodium (processed carbohydrates) ( TABLE 1 7-10 ). A low-carbohydrate diet (LCD) typically has a carbohydrate content < 130 g/d or < 26% of a 2000 kcal/d diet. 11 A very low–carbohydrate diet (VLCD) is 20-50 g/d or < 10% of the 2000 kcal/day diet. 11

In a meta-analysis by Goldenberg et al11, the LCD was shown to reduce A1C by 0.47% at 6 months (95% CI, –0.6 to –0.34) and by 0.23% at 12 months when compared with control diets. A review of multiple meta-analyses also showed a significant reduction in A1C especially with VLCD patterns; however, the results waned at the 12-month follow-up.5 In addition, confounding factors were seen when comparing adherence between LCD and VLCD, with patients in the latter group having larger problems with adherence, which decreased the benefit seen in the overall group comparison.11
Very low–carbohydrate/high-fat (ketogenic) diet
Ketogenic diets generally follow a VLCD with the carbohydrate portion set at 5% to 10% of total caloric intake (generally < 30 g/d) and the rest of the calories taken up by protein (typically 1 g/kg/d) and fat (TABLE 17-10).12 The fat content recommended is primarily polyunsaturated fat such as olive oil, while saturated fats such as butter and lard (animal fat) should be limited.
A recent meta-analysis by Choi et al12 showed that in overweight or obese patients with type 2 diabetes, the average A1C reduction was 0.62% (95% CI, –0.89 to –0.35) in the ketogenic intervention group. Another meta-analysis showed an even more significant A1C reduction at 1.07% (95% CI, –1.37 to –0.78).13 Concerns have been raised about the ketogenic diet, particularly as it relates to lipid metabolism and cholesterol levels; however, in the 2 referenced meta-analyses, the total cholesterol and triglyceride levels actually declined in the ketogenic intervention groups with minimal effect on LDL-C.12,13 This may alleviate some of the concerns of lipid management with this diet.
Plant-based diet
Popularized by Dr. T. Colin Campbell, a plant-based diet refers to a low-fat, high-fiber, whole-foods diet (whole fruits, vegetables, and naturally occurring carbohydrates, as opposed to processed foods). Examples of this type of diet include the popular vegan diet, which restricts all animal-derived products, and the vegetarian diet, which is generally limited to foods in the plant category with some addition of animal products, such as milk and cheese. Other variations of these diets exist and include other sources of protein (eg, chicken, eggs, or fish) (TABLE 17-10).
Continue to: A review by...
A review by Salas-Salvadó et al14 showed that a vegan diet yields an average A1C reduction of 0.41% (95% CI, –0.58 to –0.23).Several meta-analyses report similar effects on A1C with vegetarian and vegan eating patterns.6,15,16 The ADA review notes that weight loss was more significant in the vegan group and concluded that this diet should be studied further while controlling for weight loss.6
Mediterranean diet
The Mediterranean diet emphasizes vegetables, whole grains, fruits, lean meats, nuts, and olive oil. The benefits of the Mediterranean diet are well known and, as a result, the diet is recommended by organizations including the American Heart Association as part of a strategy to reduce cardiovascular risk (TABLE 17-10).
Mediterranean diet interventions have generally shown mixed effects on A1C reduction, weight management, and lipid control in type 2 diabetes. 6 The PREDIMED trial is the largest and longest randomized controlled trial to date comparing the Mediterranean diet to a low-fat diet. 17 This trial has reliably shown a reduced risk for type 2 diabetes and a trend to reduced A1C. 17 A reduction in the need for glucose-lowering medications was demonstrated in a subgroup analysis of the intervention group (adjusted hazard ratio = 0.78; 95% CI, 0.62-0.98). 18 Also, the Mediterranean diet has shown a significant reduction in the incidence of cardiovascular disease in patients with type 2 diabetes. 6
Physical activity and exercise
What do current guidelines recommend?
For most adults with type 2 diabetes, current guidelines by the ADA and by the National Institute of Diabetes and Digestive and Kidney Diseases recommend at least 150 minutes of moderate-to-vigorous intensity exercise every week spread out over at least 3 days, with no more than 2 consecutive days without exercise; and resistance training at least 2 other days per week which should balance all major muscle groups (TABLE 219-21). The benefits of exercise for type 2 diabetes have been well reviewed: positive effects on glucose control, insulin sensitivity, cardiovascular disease, lipid profiles, skeletal muscle metabolism, and solid-organ functioning.19,22,23

Grace et al24 showed in a meta-analysis that moderate aerobic exercise reduced A1C by 0.69% (95% CI, –1.09 to –0.3) at 13 weeks, and a Cochrane review showed an average A1C reduction of 0.6% with moderate-intensity exercise.25 Borror et al26 demonstrated in a systematic review that postprandial moderate-intensity aerobic exercise starting 1 hour after meals results in a reduced 24-hour prevalence of hyperglycemia (33.5% reduction vs control). A meta-analysis in China showed an average A1C reduction of 0.68% for patients performing a Tai Chi physical activity intervention.27
Continue to: Consider high-intensity interval training
Consider high-intensity interval training
Multiple randomized controlled trials highlight the benefits of high-intensity interval training (HIIT) (TABLE 219-21) compared with moderate-intensity continuous training (MICT) on improving A1C. A meta-analysis showed a weighted mean difference in A1C of 0.23% (95% CI, –0.43 to –0.02%).28 Also, a patient could spend less time performing HIIT as opposed to MICT to achieve the same benefits. For example, a patient typically performing 30 minutes of MICT may only need to perform 15 minutes of HIIT,a time-saving option for patients.20,22
Interrupt sedentary behavior
Risk for incident type 2 diabetes increases when someone is sedentary for more than 6 to 8 hours daily or watches TV for 3 to 4 hours (relative risk [RR] = 1.12).29 Recommendations for interrupting a sedentary lifestyle include standing from a seated position at least every 30 minutes and engaging in a light activity during the break interval for at least 3 minutes.19 Most studies have reliably shown that interrupting sedentary behavior reduces postprandial and 24-hour average blood glucose levels.19 Interrupted sitting/sedentary behavior has also been shown to reduce resting blood pressure in patients with type 2 diabetes.30
O ther important lifestyle factors
Encourage 7 to 8 hours of sleep
There is a U-shaped association between glycemic control and sleep quantity based on a meta-analysis by Lee et al 31 that showed a 0.23% increase in A1C in patients with insufficient sleep (< 4.5-6 hours/night) and a 0.13% increase in patients with ≥ 8 hours of sleep per night. Patients should be encouraged to obtain 7 to 8 hours of sleep per night to help maximize their diabetes control.
Address stress reduction
Although evidence for stress reduction interventions on glycemic control is mixed, there does seem to be a benefit in diminishing emotional distress in patients with diabetes. A systematic review by Noordali et al32 demonstrated that patients who received mindfulness-based interventions had improvements in stress, anxiety, and depression symptoms which resulted in improved quality of life. These psychological benefits may subsequently lead to positive behavioral changes.
Assist patients with smoking cessation
A large meta-analysis showed that active smoking increases the risk of cardiovascular events in patients with type 2 diabetes (RR = 1.44; 95% CI, 1.34-1.54).33 Former smokers still have an increased risk (RR = 1.09; 95% CI, 1.05-1.13), but it is lower than that of current smokers, so patients should be encouraged to quit smoking.3,33
Continue to: How can I get my patient to change?
H ow can I get my patient to change?
The AACE recommends using motivational interviewing, behavioral therapy consultation, and wearable feedback devices (eg, accelerometers/pedometers) to stimulate behavioral change in patients.3 Motivational interviewing is the principal counseling strategy and is supported by multiple studies showing the benefits of using this technique in a clinical encounter to induce behavioral changes.34 In general, offer receptive patients intensive behavioral interventions and provide them with resources to accomplish their goals.35 For example, a 7-step yearly intensive behavioral counseling intervention over 3 years showed significant improvements in activity of any intensity, reduced sedentary time, and led to favorable metabolic outcomes.36 Wearable devices result in up to a 1 hour increase in physical activity per week for the wearers vs control, although there was no appreciable effect on A1C.37
One systematic review showed a 0.5% reduction in A1C (95% CI, –0.65 to –0.34) by focusing on environmental changes related to the diet, with the most effective intervention being full meal replacement for calorie control (ie, each meal was pre-made and provided to the patients based on macronutrient and caloric goals).38 Additionally, diabetes self-management education includes coping strategies, problem solving, self-advocacy, and health care system navigation, which have been shown to reduce A1C by an average of 0.6%.21 Patient resources are available for further assistance with lifestyle modifications (TABLE 3).

C an your patient achieve remission?
Emerging evidence suggests that patients may achieve remission from type 2 diabetes with intensive lifestyle interventions.39 This is supported by the American College of Lifestyle Medicine.5 Although there is no consensus definition for remission, in general it is reasonable to presume remission if a patient achieves normo-glycemia (A1C < 5.7%) for at least 1 year without any medication therapy.5 These intensive lifestyle interventions would include a mostly plant-based diet with moderate calorie restriction, appropriate and sustained physical activity, adequate sleep, and stress-reduction techniques.5 One study found that 46% of patients in a weight-management program across multiple primary care clinics achieved remission at 12 months.40 A meta-analysis showed that a low-carbohydrate diet induced remission at 6 months in 32% of patients (although the result was not controlled for weight loss as a possible confounding factor and an A1C cutoff of 6.5% was used).11 Thus far, most studies have focused on short-term follow-up intervals, but evidence is emerging that with intensive lifestyle interventions the effects are sustained at the 2-year mark.41
This evidence could reframe our understanding of type 2 diabetes therapy and could change the conversations we have with patients regarding their treatment. Instead of focusing on an A1C goal that is adequate for control of type 2 diabetes, we would instead focus on achieving remission.
CORRESPONDENCE
Stephen McMullan, MD, Mayo Clinic College of Medicine and Science, 4500 San Pablo Road, Jacksonville, FL 32224; [email protected]
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
1. Kahn MAB, Hashim MJ, King JK, et al. Epidemiology of type 2 diabetes – global burden of disease and forecasted trends. J Epidemiol Glob Health. 2020;10:107-111. doi: 10.2991/jegh.k.191028.001
2. American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917-928. doi:10.2337/dci18-0007
3. Garber AJ, Handelsman Y, Grunberger G, et al. Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm – 2020 Executive Summary. Endocr Pract. 2020;26:107-139. doi:10.4158/CS-2019-0472
4. Schlesinger S, Neuenschwander M, Ballon A, et al. Adherence to healthy lifestyles and incidence of diabetes and mortality among individuals with diabetes: a systematic review and meta-analysis of prospective studies. J Epidemiol Community Health. 2020;74:481-487. doi: 10.1136/jech-2019-213415
5. Kelly J, Karlsen M, Steinke G. Type 2 Diabetes Remission and Lifestyle Medicine: A Position Statement from the American College of Lifestyle Medicine. Am J Lifestyle Med. 2020;14:406-419. doi: 10.1177/1559827620930962
6. Evert AB, Dennison M, Gardner CD, et al. Nutrition Therapy for Adults with Diabetes or Prediabetes: A Consensus Report. Diabetes Care. 2019;42:731-754. doi: 10.2337/dci19-0014
7. Mayo Clinic. Low-carb diet: Can it help you lose weight? Accessed August 22, 2022. www.mayoclinic.org/healthylifestyle/weight-loss/in-depth/low-carb-diet/art-20045831
8. Mayo Clinic. Is the keto diet for You? A Mayo expert weighs in. Accessed September 16, 2022. www.mayoclinic.org/is-the-keto-diet-for-you-a-mayo-expert-weighs-in/art-20457595
9. Mayo Clinic. Vegetarian diet: How to get the best nutrition. Accessed August 22, 2022. www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/vegetarian-diet/art-20046446
10. AHA. What is the Mediterranean diet? Accessed September 16, 2022. www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/mediterranean-diet
11. Goldenberg JZ, Day A, Brinkworth GD, et al. Efficacy and safety of low and very low carbohydrate diets for type 2 diabetes remission: systematic review and meta-analysis of published and unpublished randomized trial data. BMJ. 2021;372:m4743. doi: 10.1136/bmj.m4743
12. Choi YJ, Jeon SM, Shin S. Impact of a ketogenic diet on metabolic parameters in patients with obesity or overweight and with or without type 2 diabetes: a meta-analysis of randomized controlled trials. Nutrients. 2020;12:2005. doi: 10.3390/nu12072005
13. Yuan X, Wang J, Yang S, et al. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: a systematic review and meta-analysis. Nutr Diabetes. 2020;10:38. doi: 10.1038/s41387-020-00142-z
14. Salas-Salvadó J, Becerra-Tomás N, Papandreou C, et al. Dietary patterns emphasizing the consumption of plant foods in the management of type 2 diabetes: a narrative review. Adv Nutr. 2019;10(suppl_4):S320-S331. doi: 10.1093/advances/nmy102
15. Viguiliouk E, Kendall CW, Kahleová H, et al. Effect of vegetarian dietary patterns on cardiometabolic risk factors in diabetes: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;38:1133-1145. doi: 10.1016/j.clnu.2018.05.032
16. Yokoyama Y, Barnard ND, Levin SM, et al. Vegetarian diets and glycemic control in diabetes: a systematic review and meta-analysis. Cardiovasc Diagn Ther. 2014;4:373-382. doi: 10.3978/j.issn.2223-3652.2014.10.04
17. Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:e34. doi: 10.1056/NEJMoa1800389
18. Basterra-Gortari FJ, Ruiz-Canela M, Martínez-González MA, et al. Effects of a Mediterranean eating plan on the need for glucose-lowering medications in participants with type 2 diabetes: a subgroup analysis of the PREDIMED trial. Diabetes Care. 2019;42:1390-1397. doi: 10.2337/dc18-2475
19. Colberg SR, Sigal RJ, Yardley JE, et al. Physical Activity/Exercise and Diabetes: A position Statement of the American Diabetes Association. Diabetes Care. 2016;39:2065-2079. doi:10.2337/dc16-1728
20. Hwang CL, Lim J, Yoo JK, et al. Effect of all-extremity high-intensity interval training vs. moderate-intensity continuous training on aerobic fitness in middle-aged and older adults with type 2 diabetes: a randomized controlled trial. Exp Gerontol. 2019;116:46-53. doi:10.1016/j.exger.2018.12.013
21. Zangeneh F, Boltri J, Dallas A, et al. National Institute of Diabetes and Digestive and Kidney Diseases. Guiding principles for the care of people with or at risk for diabetes. Accessed September 16, 2022. www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/diabetes/guiding-principles-care-people-risk-diabetes
22. Kirwan JP, Sacks J, Nieuwoudt S. The essential role of exercise in the management of type 2 diabetes. Cleve Clin J Med. 2017;84(7 suppl 1):S15-S21. doi: 10.3949/ccjm.84.s1.03
23. Zanuso S, Sacchetti M, Sundberg CJ, et al. Exercise in type 2 diabetes: genetic, metabolic and neuromuscular adaptations. a review of the evidence. Br J Sports Med. 2017;51:1533-1538. doi: 10.1136/bjsports-2016-096724
24. Grace A, Chan E, Giallauria F, et al. Clinical outcomes and glycaemic responses to different aerobic exercise training intensities in type II diabetes: a systematic review and meta-analysis. Cardiovasc Diabetol. 2017;16:37. Published 2017 Mar 14. doi: 10.1186/s12933-017-0518-6
25. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;(3):CD002968. doi: 10.1002/14651858.CD002968.pub2
26. Borror A, Zieff G, Battaglini C, et al. The effects of postprandial exercise on glucose control in individuals with type 2 diabetes: a systematic review. Sports Med. 2018;48:1479-1491. doi: 10.1007/s40279-018-0864-x
27. Xia TW, Yang Y, Li WH, et al. Different training durations and styles of tai chi for glucose control in patients with type 2 diabetes: a systematic review and meta-analysis of controlled trials. BMC Complement Altern Med. 2019;19:63. doi: 10.1186/s12906-019-2475-y
28. Liubaoerjijin Y, Terada T, Fletcher K, et al. Effect of aerobic exercise intensity on glycemic control in type 2 diabetes: a meta-analysis of head-to-head randomized trials. Acta Diabetol. 2016;53:769-781. doi: 10.1007/s00592-016-0870-0
29. Patterson R, McNamara E, Tainio M, et al. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol. 2018;33:811-829. doi: 10.1007/s10654-018-0380-1
30. Dempsey PC, Sacre JW, Larsen RN, et al. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens. 2016;34:2376-2382. doi: 10.1097/HJH.0000000000001101
31. Lee SWH, Ng KY, Chin WK. The impact of sleep amount and sleep quality on glycemic control in type 2 diabetes: a systematic review and meta-analysis. Sleep Med Rev. 2017;31:91-101. doi: 10.1016/j.smrv.2016.02.001.
32. Noordali F, Cumming J, Thompson JL. Effectiveness of mindfulness-based intervention on physiological and psychological complications in adults with diabetes: a systematic review. J Health Psychol. 2017;22:965-983. doi: 10.1177/1359105315620293
33. Pan A, Wang Y, Talaei M, et al. Relation of smoking with total mortality and cardiovascular events among patients with diabetes mellitus: a meta-analysis and systematic review. Circulation. 2015;132:1795-1804. doi:10.116/circulationaha.115.017926
34. VanBuskirk KA, Wetherell JL. Motivational interviewing with primary care populations: a systematic review and meta-analysis. J Behav Med. 2014;37:768-780. doi:10.1007/s10865-013-9527-4
35. Koenigsberg MR, Corliss J. Diabetes self-management: facilitating lifestyle change. Am Fam Physician. 2017;96:362-370.
36. Balducci S, D’Errico V, Haxhi J, et al. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care. 2017;40:1444-1452. doi: 10.2337/dc17-0594
37. Baskerville R, Ricci-Cabello I, Roberts N, et al. Impact of accelerometer and pedometer use on physical activity and glycaemic control in people with type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2017;34:612-620. doi:10.1111/dme.13331
38. Cradock KA, ÓLaighin G, Finucane FM, et al. Diet behavior change techniques in type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2017;40:1800-1810. doi: 10.2337/dc17-0462
39. Hallberg SJ, Gershuni VM, Hazbun TL, et al. Reversing type 2 diabetes: a narrative review of the evidence. Nutrients. 2019;11:766. doi: 10.3390/nu11040766
40. Lean MEJ, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet. 2018;391:541-551. doi: 10.1016/S0140-6736(17)33102-1
41. Sbroma Tomaro E, Pippi R, Reginato E, et al. Intensive lifestyle intervention is particularly advantageous in poorly controlled type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:688-694. doi:10.1016/j.numecd.2017.06.009
PRACTICE RECOMMENDATIONS
› Recommend a reduced-calorie diet that is generally plant based and low in carbohydrates as part of the treatment plan for type 2 diabetes. B
› Counsel all patients with type 2 diabetes to engage in physical activity for at least 150 minutes per week at moderate intensity and to add resistance training on at least 2 days to improve glycemic control. B
› Teach patients techniques to reduce stress and improve sleep quality. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Crohn Disease Medication Overview
Tinea capitis
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.
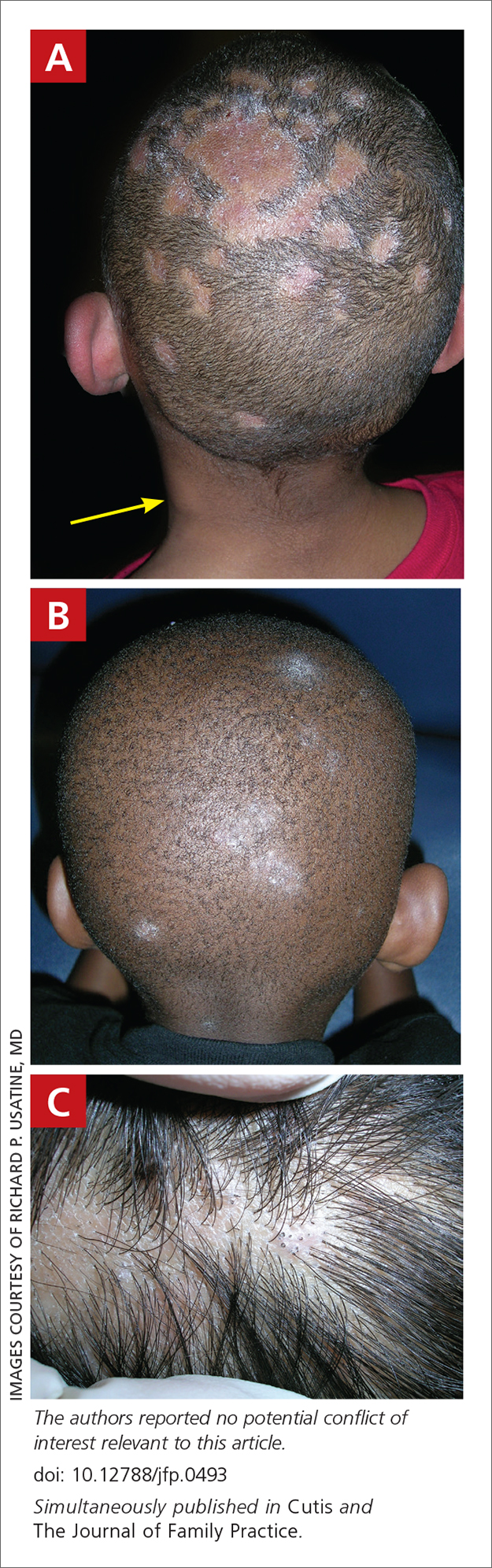
Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) caused by M canis. M canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations:
- broken hairs that appear as black dots on the scalp
- diffuse scale mimicking seborrheic dermatitis
- well-demarcated annular plaques
- exudate and tenderness caused by inflammation
- scalp pruritus
- occipital scalp lymphadenopathy.
Worth noting
Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp. However, the application of heavy emollients, oils, and grease to camouflage scale contributes to false-negative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5
Health disparity highlight
A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
1. Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi: 10.1111/jdv.15088
2. Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study. Pediatrics. 2010;125:966-973. doi: 10.1542/peds.2009-2522
3. Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi: 10.1067/mjd.2002.120793
4. Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
5. Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015. Pediatr Dermatol. 2020;37:305-310. doi: 10.1111/pde.14092
6. Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria. Mycoses. 2008;51:536-541. doi: 10.1111/j.1439-0507.2008.01507.x
Velvety brown lesion
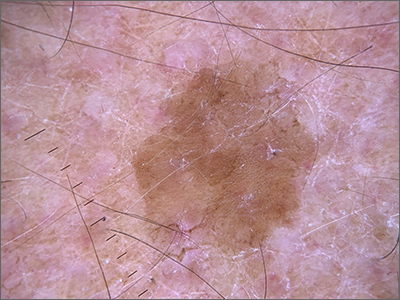
Dermoscopy revealed a uniform, sharply demarcated, slightly scaly lesion on a background of occasional scale and solar-damaged skin. This appearance, paired with the absence of abnormal blood vessels or suspicious, irregular pigmentation, pointed to a diagnosis of benign lichenoid keratosis also known as lichenoid keratosis (LK) or lichen planus-like keratosis. (It’s worth noting that in some cases, a dermoscopic evaluation will reveal blue-grey dots rather than the uniform, velvety brown pigmentation that was seen here.)
LK is a benign reactive inflammatory lesion that usually manifests as a solitary lesion in middle age. LKs can be found on the trunk or lower extremities. As the alternative name “lichen planus-like keratosis” implies, the lesions can be purple, polygonal, raised, and have stria. The etiology is unknown but thought to be a reaction to a lentigo or another lesion, resulting in an inflammatory infiltrate.1
If dermoscopic evaluation of the lesion is unclear, biopsy is warranted. Maor et al1 reported the pathology results of 263 consecutive patients with a histologic diagnosis of LK. Of those cases, 47% were clinically thought to be basal cell carcinoma (BCC) and 18% were submitted with a diagnosis of seborrheic keratosis.1 The high rate of concern for BCC and not listing a diagnosis of LK may have been the result of clinicians doing biopsies on the atypical lesions and clinically following the typical banal lesions.
At the patient’s request, he was given a written list of the diagnoses of his various skin lesions and advised that his LK was benign and did not require treatment. He was advised to continue coming in for serial skin examinations and report any concerning lesions in the interim.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo.
1. Maor D, Ondhia C, Yu LL, et al. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin Exp Dermatol. 2017;42:663-666. doi: 10.1111/ced.13178

Dermoscopy revealed a uniform, sharply demarcated, slightly scaly lesion on a background of occasional scale and solar-damaged skin. This appearance, paired with the absence of abnormal blood vessels or suspicious, irregular pigmentation, pointed to a diagnosis of benign lichenoid keratosis also known as lichenoid keratosis (LK) or lichen planus-like keratosis. (It’s worth noting that in some cases, a dermoscopic evaluation will reveal blue-grey dots rather than the uniform, velvety brown pigmentation that was seen here.)
LK is a benign reactive inflammatory lesion that usually manifests as a solitary lesion in middle age. LKs can be found on the trunk or lower extremities. As the alternative name “lichen planus-like keratosis” implies, the lesions can be purple, polygonal, raised, and have stria. The etiology is unknown but thought to be a reaction to a lentigo or another lesion, resulting in an inflammatory infiltrate.1
If dermoscopic evaluation of the lesion is unclear, biopsy is warranted. Maor et al1 reported the pathology results of 263 consecutive patients with a histologic diagnosis of LK. Of those cases, 47% were clinically thought to be basal cell carcinoma (BCC) and 18% were submitted with a diagnosis of seborrheic keratosis.1 The high rate of concern for BCC and not listing a diagnosis of LK may have been the result of clinicians doing biopsies on the atypical lesions and clinically following the typical banal lesions.
At the patient’s request, he was given a written list of the diagnoses of his various skin lesions and advised that his LK was benign and did not require treatment. He was advised to continue coming in for serial skin examinations and report any concerning lesions in the interim.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo.

Dermoscopy revealed a uniform, sharply demarcated, slightly scaly lesion on a background of occasional scale and solar-damaged skin. This appearance, paired with the absence of abnormal blood vessels or suspicious, irregular pigmentation, pointed to a diagnosis of benign lichenoid keratosis also known as lichenoid keratosis (LK) or lichen planus-like keratosis. (It’s worth noting that in some cases, a dermoscopic evaluation will reveal blue-grey dots rather than the uniform, velvety brown pigmentation that was seen here.)
LK is a benign reactive inflammatory lesion that usually manifests as a solitary lesion in middle age. LKs can be found on the trunk or lower extremities. As the alternative name “lichen planus-like keratosis” implies, the lesions can be purple, polygonal, raised, and have stria. The etiology is unknown but thought to be a reaction to a lentigo or another lesion, resulting in an inflammatory infiltrate.1
If dermoscopic evaluation of the lesion is unclear, biopsy is warranted. Maor et al1 reported the pathology results of 263 consecutive patients with a histologic diagnosis of LK. Of those cases, 47% were clinically thought to be basal cell carcinoma (BCC) and 18% were submitted with a diagnosis of seborrheic keratosis.1 The high rate of concern for BCC and not listing a diagnosis of LK may have been the result of clinicians doing biopsies on the atypical lesions and clinically following the typical banal lesions.
At the patient’s request, he was given a written list of the diagnoses of his various skin lesions and advised that his LK was benign and did not require treatment. He was advised to continue coming in for serial skin examinations and report any concerning lesions in the interim.
Image and text courtesy of Daniel Stulberg, MD, FAAFP, Professor and Chair, Department of Family and Community Medicine, Western Michigan University Homer Stryker MD School of Medicine, Kalamazoo.
1. Maor D, Ondhia C, Yu LL, et al. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin Exp Dermatol. 2017;42:663-666. doi: 10.1111/ced.13178
1. Maor D, Ondhia C, Yu LL, et al. Lichenoid keratosis is frequently misdiagnosed as basal cell carcinoma. Clin Exp Dermatol. 2017;42:663-666. doi: 10.1111/ced.13178