User login
Tinea Capitis
THE COMPARISON
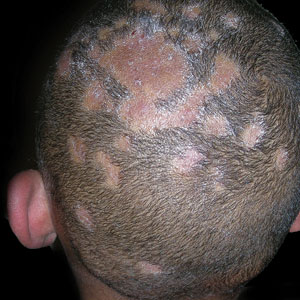
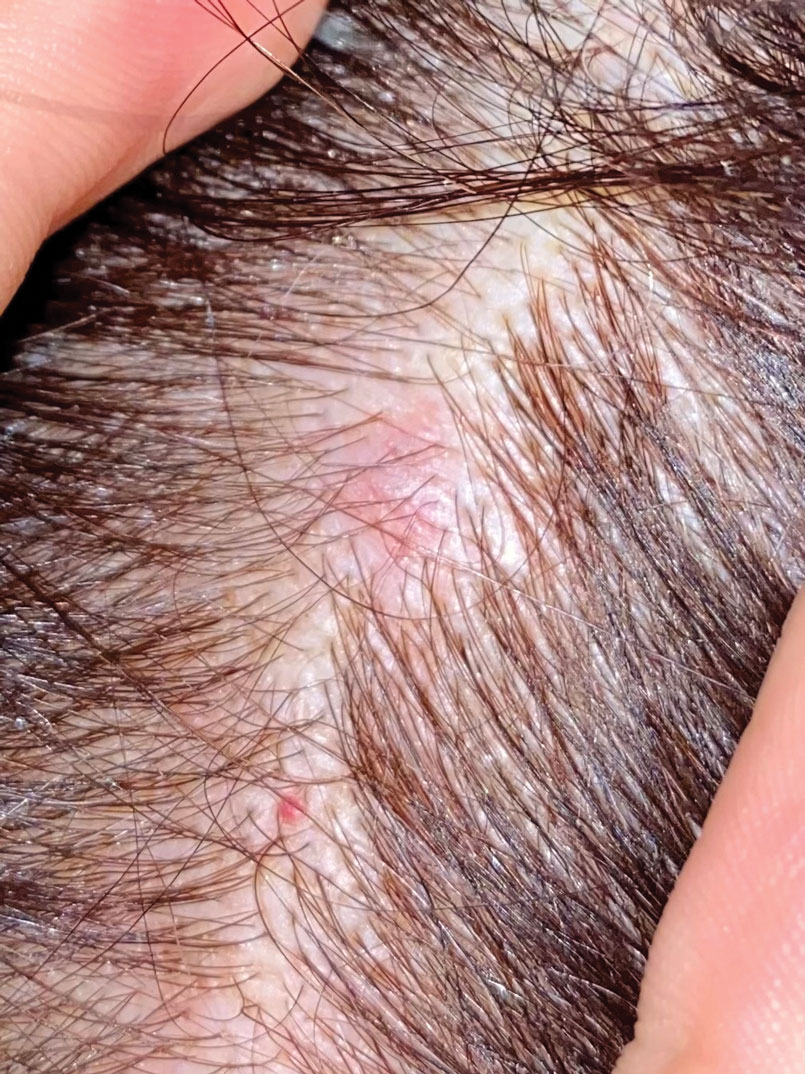
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.
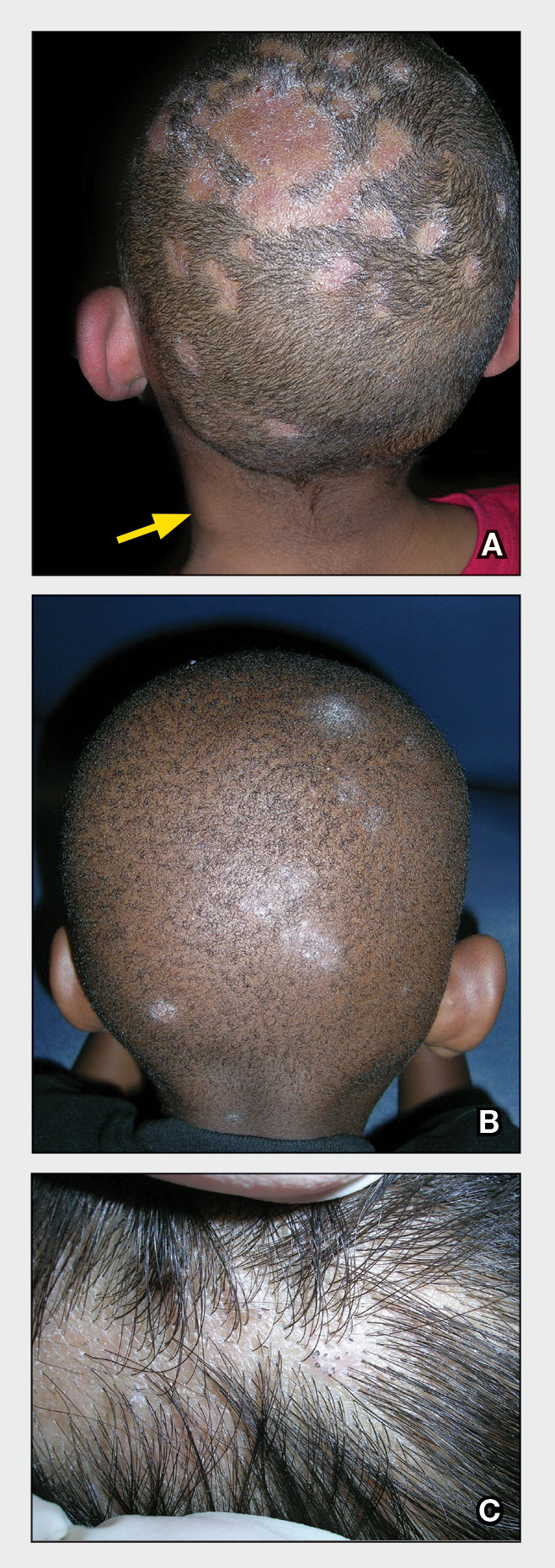
Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
THE COMPARISON
A Areas of alopecia with erythema and scale in a young Black boy with tinea capitis. He also had an enlarged posterior cervical lymph node (arrow) from this fungal infection.
B White patches of scale from tinea capitis in a young Black boy with no obvious hair loss; however, a potassium hydroxide preparation from the scale was positive for fungus.
C A subtle area of tinea capitis on the scalp of a Latina girl showed comma hairs.

Tinea capitis is a common dermatophyte infection of the scalp in school-aged children. The infection is spread by close contact with infected people or with their personal items, including combs, brushes, pillowcases, and hats, as well as animals. It is uncommon in adults.
Epidemiology
Tinea capitis is the most common fungal infection among school-aged children worldwide.1 In a US-based study of more than 10,000 school-aged children, the prevalence of tinea capitis ranged from 0% to 19.4%, with Black children having the highest rates of infection at 12.9%.2 However, people of all races and ages may develop tinea capitis.3
Tinea capitis most commonly is caused by Trichophyton tonsurans and Microsporum canis. Dermatophyte scalp infections caused by T tonsurans produce fungal spores that may occur within the hair shaft (endothrix) or with fungal elements external to the hair shaft (exothrix) such as M canis. Microsporum canis usually fluoresces an apple green color on Wood lamp examination because of the location of the spores.
Key clinical features
Tinea capitis has a variety of clinical presentations: • broken hairs that appear as black dots on the scalp • diffuse scale mimicking seborrheic dermatitis • well-demarcated annular plaques • exudate and tenderness caused by inflammation • scalp pruritus • occipital scalp lymphadenopathy. Worth noting Tinea capitis impacts all patient groups, not just Black patients. In the United States, Black and Hispanic children are most commonly affected.4 Due to a tendency to have dry hair and hair breakage, those with more tightly coiled, textured hair may routinely apply oil and/or grease to the scalp; however, the application of heavy emollients, oils, and grease to camouflage scale contributes to falsenegative fungal cultures of the scalp if applied within 1 week of the fungal culture, which may delay diagnosis. If tinea capitis is suspected, occipital lymphadenopathy on physical examination should prompt treatment for tinea capitis, even without a fungal culture.5 Health disparity highlight A risk factor for tinea capitis is crowded living environments. Some families may live in crowded environments due to economic and housing disparities. This close contact increases the risk for conditions such as tinea capitis.6 Treatment delays may occur due to some cultural practices of applying oils and grease to the hair and scalp, camouflaging the clinical signs of tinea capitis.
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
- Gupta AK, Mays RR, Versteeg SG, et al. Tinea capitis in children: a systematic review of management [published online July 12, 2018]. J Eur Acad Dermatol Venereol. 2018;32:2264-2274. doi:10.1111/jdv.15088
- Abdel-Rahman SM, Farrand N, Schuenemann E, et al. The prevalence of infections with Trichophyton tonsurans in schoolchildren: the CAPITIS study [published online April 19, 2010]. Pediatrics. 2010;125:966-973. doi:10.1542/peds.2009-2522
- Silverberg NB, Weinberg JM, DeLeo VA. Tinea capitis: focus on African American women. J Am Acad Dermatol. 2002;46(2 suppl understanding):S120-S124. doi:10.1067/mjd.2002.120793
- Alvarez MS, Silverberg NB. Tinea capitis. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. McGraw Hill Medical; 2009:246-255.
- Nguyen CV, Collier S, Merten AH, et al. Tinea capitis: a singleinstitution retrospective review from 2010 to 2015 [published online January 20, 2020]. Pediatr Dermatol. 2020;37:305-310. doi:10.1111 /pde.14092
- Emele FE, Oyeka CA. Tinea capitis among primary school children in Anambra state of Nigeria [published online April 16, 2008]. Mycoses. 2008;51:536-541. doi:10.1111/j.1439-0507.2008.01507.x
Burnout Is Rampant, But Oncologists Can Turn the Tide
SAN DIEGO—Before the pandemic, an estimated one-third of oncologists worldwide suffered a high level of burnout. Cancer physicians face many of the same risk factors as their colleagues—high workloads, lack of autonomy, and no support—along with the added pressure of working in a medical field where patients often die. Then COVID-19 hit, and the burnout crisis got even worse.
This tide can be reversed with a focus on best practices and resilience, a mental health researcher told cancer professionals at the September 2022 annual meeting of the Association of VA Hematology/Oncology. Assessments, long-term interventions, and communication are all key, said Fay J. Hlubocky, PhD, MA, a clinical health psychologist and ethicist at the University of Chicago.
Even simple actions like taking time for “mindful moments” and checking in with a colleague can make a difference, she said. But institutions must act, she said. “Long-term tailored strategies are incredibly important to promote well-being.”
Hlubocky, who led an American Society of Clinical Oncology committee on burnout prior to the pandemic, noted that statistics about burnout in American medicine and oncology specifically, are grim. In 2017, a systematic review and meta-analysis found that significant numbers of oncologists suffered from high burnout (32%), high psychiatric morbidity (27%), depression (at least 12%), and alcohol misuse (as many as 30%).
The pandemic piled on more stressors. In the second half of 2020, researchers interviewed 25 American oncologists in focus groups and found that their “underlying oncologist burnout exacerbated stressors associated with disruptions in care, education, research, financial practice health, and telemedicine. Many feared delays in cancer screening, diagnosis, and treatment [and] strongly considered working part-time or taking early retirement.”
As one participant put it, “everyone is seeing a lot of death and heartache and social isolation and anger that they’re not used to encountering and in very new and different ways.”
Major contributors to oncologist burnout, Hlubocky said, include moral distress, moral injury, and compassion fatigue. “Moral distress occurs when that individual believes he or she knows the right thing to do, but institutional constraints make it really difficult to do what is right,” Hlubocky said. “The individual is aware of the moral problem, acknowledges and takes moral responsibility, makes some moral judgments, but yet—as a result of these constraints — participates in perceived moral wrongdoing.”
Moral injury refers to the damage that can be caused by moral distress or by witnessing acts that violate morals, such as during military service. Compassion fatigue, meanwhile, is defined by the American Stress Institute as “a low level, chronic clouding of caring and concern for others in your life.”
What can be done? Hlubocky highlighted multiple interventions, such as adjustment of work patterns, cognitive behavioral therapy, and training in mindfulness, relaxation, and communication. One strategy is to adopt multiple in-person interventions simultaneously.
But first it’s crucial for administrators to understand the problem in a specific workplace: “You have to know what’s going on in your organization to intervene on it,” she said. “There are multiple tools that have been validated in other health care fields and can be used on a regular basis over time to measure burnout, satisfaction, and engagement.”
For individuals, other strategies include daily check-ins with colleagues to catch signs of stress, she said, as Toronto oncologists started doing amid the pandemic. The check-ins can include simple questions like: How are you doing? How are you feeling? Are you sleeping, eating and exercising? Do you need help?
As for resilience, Hlubocky said it must grow at the individual level. “We can't rely so much on the organization. We need to develop our personal resilience in order for professional resilience to flourish again, and we have to do a lot to protect ourselves. It’s about focusing on the strength of the individual—that empowerment to rise above adversity, that vitality, that engagement, that self-efficacy. It supports health and enhances coping, and it is the key element of physician and clinician well-being.”
Research into resilience offers guidance about how to achieve it, she said. A 2013 German study of 200 physicians found that the most resilient physicians change their attitudes and behaviors, take time off, set boundaries, spend time with family and friends, and ask colleagues for help. And they gained resilience, the study found, by getting older and becoming more experienced.
Hlubocky pointed to several useful resources for burned-out medical professionals, including mindfulness, cognitive behavioral therapy and breathing apps: She highlighted Breathe2Relax, Headspace, MoodGYM, Stress Gym, and guided audio files from the University of California at San Diego. And she said ASCO has resources on combatting burnout and promoting well-being.
Hlubocky has no relevant disclosures.
SAN DIEGO—Before the pandemic, an estimated one-third of oncologists worldwide suffered a high level of burnout. Cancer physicians face many of the same risk factors as their colleagues—high workloads, lack of autonomy, and no support—along with the added pressure of working in a medical field where patients often die. Then COVID-19 hit, and the burnout crisis got even worse.
This tide can be reversed with a focus on best practices and resilience, a mental health researcher told cancer professionals at the September 2022 annual meeting of the Association of VA Hematology/Oncology. Assessments, long-term interventions, and communication are all key, said Fay J. Hlubocky, PhD, MA, a clinical health psychologist and ethicist at the University of Chicago.
Even simple actions like taking time for “mindful moments” and checking in with a colleague can make a difference, she said. But institutions must act, she said. “Long-term tailored strategies are incredibly important to promote well-being.”
Hlubocky, who led an American Society of Clinical Oncology committee on burnout prior to the pandemic, noted that statistics about burnout in American medicine and oncology specifically, are grim. In 2017, a systematic review and meta-analysis found that significant numbers of oncologists suffered from high burnout (32%), high psychiatric morbidity (27%), depression (at least 12%), and alcohol misuse (as many as 30%).
The pandemic piled on more stressors. In the second half of 2020, researchers interviewed 25 American oncologists in focus groups and found that their “underlying oncologist burnout exacerbated stressors associated with disruptions in care, education, research, financial practice health, and telemedicine. Many feared delays in cancer screening, diagnosis, and treatment [and] strongly considered working part-time or taking early retirement.”
As one participant put it, “everyone is seeing a lot of death and heartache and social isolation and anger that they’re not used to encountering and in very new and different ways.”
Major contributors to oncologist burnout, Hlubocky said, include moral distress, moral injury, and compassion fatigue. “Moral distress occurs when that individual believes he or she knows the right thing to do, but institutional constraints make it really difficult to do what is right,” Hlubocky said. “The individual is aware of the moral problem, acknowledges and takes moral responsibility, makes some moral judgments, but yet—as a result of these constraints — participates in perceived moral wrongdoing.”
Moral injury refers to the damage that can be caused by moral distress or by witnessing acts that violate morals, such as during military service. Compassion fatigue, meanwhile, is defined by the American Stress Institute as “a low level, chronic clouding of caring and concern for others in your life.”
What can be done? Hlubocky highlighted multiple interventions, such as adjustment of work patterns, cognitive behavioral therapy, and training in mindfulness, relaxation, and communication. One strategy is to adopt multiple in-person interventions simultaneously.
But first it’s crucial for administrators to understand the problem in a specific workplace: “You have to know what’s going on in your organization to intervene on it,” she said. “There are multiple tools that have been validated in other health care fields and can be used on a regular basis over time to measure burnout, satisfaction, and engagement.”
For individuals, other strategies include daily check-ins with colleagues to catch signs of stress, she said, as Toronto oncologists started doing amid the pandemic. The check-ins can include simple questions like: How are you doing? How are you feeling? Are you sleeping, eating and exercising? Do you need help?
As for resilience, Hlubocky said it must grow at the individual level. “We can't rely so much on the organization. We need to develop our personal resilience in order for professional resilience to flourish again, and we have to do a lot to protect ourselves. It’s about focusing on the strength of the individual—that empowerment to rise above adversity, that vitality, that engagement, that self-efficacy. It supports health and enhances coping, and it is the key element of physician and clinician well-being.”
Research into resilience offers guidance about how to achieve it, she said. A 2013 German study of 200 physicians found that the most resilient physicians change their attitudes and behaviors, take time off, set boundaries, spend time with family and friends, and ask colleagues for help. And they gained resilience, the study found, by getting older and becoming more experienced.
Hlubocky pointed to several useful resources for burned-out medical professionals, including mindfulness, cognitive behavioral therapy and breathing apps: She highlighted Breathe2Relax, Headspace, MoodGYM, Stress Gym, and guided audio files from the University of California at San Diego. And she said ASCO has resources on combatting burnout and promoting well-being.
Hlubocky has no relevant disclosures.
SAN DIEGO—Before the pandemic, an estimated one-third of oncologists worldwide suffered a high level of burnout. Cancer physicians face many of the same risk factors as their colleagues—high workloads, lack of autonomy, and no support—along with the added pressure of working in a medical field where patients often die. Then COVID-19 hit, and the burnout crisis got even worse.
This tide can be reversed with a focus on best practices and resilience, a mental health researcher told cancer professionals at the September 2022 annual meeting of the Association of VA Hematology/Oncology. Assessments, long-term interventions, and communication are all key, said Fay J. Hlubocky, PhD, MA, a clinical health psychologist and ethicist at the University of Chicago.
Even simple actions like taking time for “mindful moments” and checking in with a colleague can make a difference, she said. But institutions must act, she said. “Long-term tailored strategies are incredibly important to promote well-being.”
Hlubocky, who led an American Society of Clinical Oncology committee on burnout prior to the pandemic, noted that statistics about burnout in American medicine and oncology specifically, are grim. In 2017, a systematic review and meta-analysis found that significant numbers of oncologists suffered from high burnout (32%), high psychiatric morbidity (27%), depression (at least 12%), and alcohol misuse (as many as 30%).
The pandemic piled on more stressors. In the second half of 2020, researchers interviewed 25 American oncologists in focus groups and found that their “underlying oncologist burnout exacerbated stressors associated with disruptions in care, education, research, financial practice health, and telemedicine. Many feared delays in cancer screening, diagnosis, and treatment [and] strongly considered working part-time or taking early retirement.”
As one participant put it, “everyone is seeing a lot of death and heartache and social isolation and anger that they’re not used to encountering and in very new and different ways.”
Major contributors to oncologist burnout, Hlubocky said, include moral distress, moral injury, and compassion fatigue. “Moral distress occurs when that individual believes he or she knows the right thing to do, but institutional constraints make it really difficult to do what is right,” Hlubocky said. “The individual is aware of the moral problem, acknowledges and takes moral responsibility, makes some moral judgments, but yet—as a result of these constraints — participates in perceived moral wrongdoing.”
Moral injury refers to the damage that can be caused by moral distress or by witnessing acts that violate morals, such as during military service. Compassion fatigue, meanwhile, is defined by the American Stress Institute as “a low level, chronic clouding of caring and concern for others in your life.”
What can be done? Hlubocky highlighted multiple interventions, such as adjustment of work patterns, cognitive behavioral therapy, and training in mindfulness, relaxation, and communication. One strategy is to adopt multiple in-person interventions simultaneously.
But first it’s crucial for administrators to understand the problem in a specific workplace: “You have to know what’s going on in your organization to intervene on it,” she said. “There are multiple tools that have been validated in other health care fields and can be used on a regular basis over time to measure burnout, satisfaction, and engagement.”
For individuals, other strategies include daily check-ins with colleagues to catch signs of stress, she said, as Toronto oncologists started doing amid the pandemic. The check-ins can include simple questions like: How are you doing? How are you feeling? Are you sleeping, eating and exercising? Do you need help?
As for resilience, Hlubocky said it must grow at the individual level. “We can't rely so much on the organization. We need to develop our personal resilience in order for professional resilience to flourish again, and we have to do a lot to protect ourselves. It’s about focusing on the strength of the individual—that empowerment to rise above adversity, that vitality, that engagement, that self-efficacy. It supports health and enhances coping, and it is the key element of physician and clinician well-being.”
Research into resilience offers guidance about how to achieve it, she said. A 2013 German study of 200 physicians found that the most resilient physicians change their attitudes and behaviors, take time off, set boundaries, spend time with family and friends, and ask colleagues for help. And they gained resilience, the study found, by getting older and becoming more experienced.
Hlubocky pointed to several useful resources for burned-out medical professionals, including mindfulness, cognitive behavioral therapy and breathing apps: She highlighted Breathe2Relax, Headspace, MoodGYM, Stress Gym, and guided audio files from the University of California at San Diego. And she said ASCO has resources on combatting burnout and promoting well-being.
Hlubocky has no relevant disclosures.
Roselyn Tso confirmed to head Indian Health Service
It took 609 days, but the US Senate has finally (unanimously) confirmed President Biden’s choice to head the Indian Health Service (IHS: Roselyn Tso.)
President Biden nominated Tso in March 2022, and she was formally sworn in on September 27, 2022. The long-awaited confirmation filled a space that hadn’t had a permanent director since Michael Weahkee, a Pueblo of Zuni citizen, stepped down in 2021. In the interim, Elizabeth Fowler, of the Comanche Nation, served as acting director.
Tso’s resume includes almost 40 years of professional experience working at all levels of the IHS. Before taking over as IHS director, she led the IHS Navajo area, the largest IHS regional area, managing more than 4000 employees and a budget of nearly $1 billion.
She also brings “decades of lived experience as a member of the Navajo Nation,” she said in a 40-minute Senate hearing with the US Senate Committee on Indian Affairs in May.
The first Navajo Nation citizen to head the IHS (and only the second woman to do so), Tso introduced herself in Navajo: Deeschii’nii (Start of the Red Streak People) and born for Hashk’aa hadzohi (Yucca Fruit Strung Out). “This is a historic achievement for all of our Navajo people and tribal nations across the country,” Navajo Nation President Jonathan Nez said. “To have one of our own Navajo members in the highest position with IHS is remarkable.”
Tso spoke of having to “navigate the services provided by the Agency for myself, family, and friends.” Her personal and professional backgrounds, she said, help her understand how patients experience the system and how that can be improved. “The health care provided at IHS is critical for those we serve. I understand this not just because I work there,” she said. “My family relies on IHS. My friends rely on IHS. I rely on the IHS.”
The long lacuna in confirming a permanent IHS director left the Native peoples particularly vulnerable—when the COVID-19 pandemic essentially worsened the existing problems they faced, such as diabetes mellitus and cancer. Life expectancy for Native people fell by more than 6 years between 2019 and 2021, to 65 years, compared with the US average of 76 years.
Without a full-time IHS leader, the National Council of Urban Indian Health said in a statement, tribal nations and other Native health care providers struggled to raise and address the issues they were facing amid the pandemic. “Since the resignation of Rear Admiral Weahkee, there have been countless requests from Indian Country calling on Congress and the Administration to nominate a new IHS director to address the growing health disparities experienced by AI/ANs.”
Tso laid out her priorities in her May testimony: creating a more unified health care system using the latest technology to develop centralized systems; improving accountability, transparency, and patient safety; addressing workforce needs and challenges, improving recruitment and retention.
Meeting her goals, she noted, would take “strong partnerships and communication with our Tribal partners…. Each tribe has unique needs, and those needs cannot be met if you do not understand them.”
Last year, President Joseph R. Biden asked Congress to significantly increase IHS funding, but his proposal was cut to $400 million. “For years, IHS has been funded at a rate that is far below its level of need, and the results of this historical neglect can be seen in the disparities in health outcomes for AI/AN people,” William Smith, Valdez Native Tribe, Chairman of the National Indian Health Board (NIHB), wrote to the Senate Committee on Indian Affairs, on the topic of the next IHS director. “Perhaps one of the greatest challenges facing the [Indian, tribal and urban] system is the chronic and severe underfunding and budgetary instability for health care and public health services infrastructure and delivery. Since its creation in 1955, IHS has been chronically underfunded, with annual appropriations never exceeding 50% of demonstrated need. This underfunding has contributed to substandard investment in health delivery systems, some of the worst health disparities among any US population and a severe lack of public health infrastructure and services for our people. At the start of the COVID-19 pandemic these vulnerabilities were starkly exposed and while Congress moved decisively to invest into Tribal health and public health, the new Director must work to maintain these one-time investments.”
Stacy Bohlen, NIHB chief executive, told The Oklahoman that tribal leaders will look to Tso to press Congress for more money and to secure mandatory full funding for IHS—in contrast with the current annual appropriations, where Congress includes IHS in much larger budget bills. “When those bills stall, so does the money tribal clinics need to pay employees and suppliers,” making it hard to recruit and retain employees. “In the Indian Health System,” Bohlen says, “we simply can’t afford that kind of vulnerability.”
Securing advance appropriations and, ultimately, full mandatory funding for IHS, Smith wrote in his letter to the Senate committee, “fulfills the commitment made to our people generations ago and breaks down the systemic healthcare funding inequities the federal government tolerates for Tribes.”
Tso emphasized her intent to “improve the physical, mental, social, and spiritual health and well-being of all American Indians and Alaskan Natives served by the Agency.” Tso “understands the healthcare needs that many first people of this country deal with,” President Nez said. “Her work ethic, value system and approach to problem solving demonstrates the resilience of Indigenous peoples and the commitment to combat the systemic inequities that impact tribal nations.”
It took 609 days, but the US Senate has finally (unanimously) confirmed President Biden’s choice to head the Indian Health Service (IHS: Roselyn Tso.)
President Biden nominated Tso in March 2022, and she was formally sworn in on September 27, 2022. The long-awaited confirmation filled a space that hadn’t had a permanent director since Michael Weahkee, a Pueblo of Zuni citizen, stepped down in 2021. In the interim, Elizabeth Fowler, of the Comanche Nation, served as acting director.
Tso’s resume includes almost 40 years of professional experience working at all levels of the IHS. Before taking over as IHS director, she led the IHS Navajo area, the largest IHS regional area, managing more than 4000 employees and a budget of nearly $1 billion.
She also brings “decades of lived experience as a member of the Navajo Nation,” she said in a 40-minute Senate hearing with the US Senate Committee on Indian Affairs in May.
The first Navajo Nation citizen to head the IHS (and only the second woman to do so), Tso introduced herself in Navajo: Deeschii’nii (Start of the Red Streak People) and born for Hashk’aa hadzohi (Yucca Fruit Strung Out). “This is a historic achievement for all of our Navajo people and tribal nations across the country,” Navajo Nation President Jonathan Nez said. “To have one of our own Navajo members in the highest position with IHS is remarkable.”
Tso spoke of having to “navigate the services provided by the Agency for myself, family, and friends.” Her personal and professional backgrounds, she said, help her understand how patients experience the system and how that can be improved. “The health care provided at IHS is critical for those we serve. I understand this not just because I work there,” she said. “My family relies on IHS. My friends rely on IHS. I rely on the IHS.”
The long lacuna in confirming a permanent IHS director left the Native peoples particularly vulnerable—when the COVID-19 pandemic essentially worsened the existing problems they faced, such as diabetes mellitus and cancer. Life expectancy for Native people fell by more than 6 years between 2019 and 2021, to 65 years, compared with the US average of 76 years.
Without a full-time IHS leader, the National Council of Urban Indian Health said in a statement, tribal nations and other Native health care providers struggled to raise and address the issues they were facing amid the pandemic. “Since the resignation of Rear Admiral Weahkee, there have been countless requests from Indian Country calling on Congress and the Administration to nominate a new IHS director to address the growing health disparities experienced by AI/ANs.”
Tso laid out her priorities in her May testimony: creating a more unified health care system using the latest technology to develop centralized systems; improving accountability, transparency, and patient safety; addressing workforce needs and challenges, improving recruitment and retention.
Meeting her goals, she noted, would take “strong partnerships and communication with our Tribal partners…. Each tribe has unique needs, and those needs cannot be met if you do not understand them.”
Last year, President Joseph R. Biden asked Congress to significantly increase IHS funding, but his proposal was cut to $400 million. “For years, IHS has been funded at a rate that is far below its level of need, and the results of this historical neglect can be seen in the disparities in health outcomes for AI/AN people,” William Smith, Valdez Native Tribe, Chairman of the National Indian Health Board (NIHB), wrote to the Senate Committee on Indian Affairs, on the topic of the next IHS director. “Perhaps one of the greatest challenges facing the [Indian, tribal and urban] system is the chronic and severe underfunding and budgetary instability for health care and public health services infrastructure and delivery. Since its creation in 1955, IHS has been chronically underfunded, with annual appropriations never exceeding 50% of demonstrated need. This underfunding has contributed to substandard investment in health delivery systems, some of the worst health disparities among any US population and a severe lack of public health infrastructure and services for our people. At the start of the COVID-19 pandemic these vulnerabilities were starkly exposed and while Congress moved decisively to invest into Tribal health and public health, the new Director must work to maintain these one-time investments.”
Stacy Bohlen, NIHB chief executive, told The Oklahoman that tribal leaders will look to Tso to press Congress for more money and to secure mandatory full funding for IHS—in contrast with the current annual appropriations, where Congress includes IHS in much larger budget bills. “When those bills stall, so does the money tribal clinics need to pay employees and suppliers,” making it hard to recruit and retain employees. “In the Indian Health System,” Bohlen says, “we simply can’t afford that kind of vulnerability.”
Securing advance appropriations and, ultimately, full mandatory funding for IHS, Smith wrote in his letter to the Senate committee, “fulfills the commitment made to our people generations ago and breaks down the systemic healthcare funding inequities the federal government tolerates for Tribes.”
Tso emphasized her intent to “improve the physical, mental, social, and spiritual health and well-being of all American Indians and Alaskan Natives served by the Agency.” Tso “understands the healthcare needs that many first people of this country deal with,” President Nez said. “Her work ethic, value system and approach to problem solving demonstrates the resilience of Indigenous peoples and the commitment to combat the systemic inequities that impact tribal nations.”
It took 609 days, but the US Senate has finally (unanimously) confirmed President Biden’s choice to head the Indian Health Service (IHS: Roselyn Tso.)
President Biden nominated Tso in March 2022, and she was formally sworn in on September 27, 2022. The long-awaited confirmation filled a space that hadn’t had a permanent director since Michael Weahkee, a Pueblo of Zuni citizen, stepped down in 2021. In the interim, Elizabeth Fowler, of the Comanche Nation, served as acting director.
Tso’s resume includes almost 40 years of professional experience working at all levels of the IHS. Before taking over as IHS director, she led the IHS Navajo area, the largest IHS regional area, managing more than 4000 employees and a budget of nearly $1 billion.
She also brings “decades of lived experience as a member of the Navajo Nation,” she said in a 40-minute Senate hearing with the US Senate Committee on Indian Affairs in May.
The first Navajo Nation citizen to head the IHS (and only the second woman to do so), Tso introduced herself in Navajo: Deeschii’nii (Start of the Red Streak People) and born for Hashk’aa hadzohi (Yucca Fruit Strung Out). “This is a historic achievement for all of our Navajo people and tribal nations across the country,” Navajo Nation President Jonathan Nez said. “To have one of our own Navajo members in the highest position with IHS is remarkable.”
Tso spoke of having to “navigate the services provided by the Agency for myself, family, and friends.” Her personal and professional backgrounds, she said, help her understand how patients experience the system and how that can be improved. “The health care provided at IHS is critical for those we serve. I understand this not just because I work there,” she said. “My family relies on IHS. My friends rely on IHS. I rely on the IHS.”
The long lacuna in confirming a permanent IHS director left the Native peoples particularly vulnerable—when the COVID-19 pandemic essentially worsened the existing problems they faced, such as diabetes mellitus and cancer. Life expectancy for Native people fell by more than 6 years between 2019 and 2021, to 65 years, compared with the US average of 76 years.
Without a full-time IHS leader, the National Council of Urban Indian Health said in a statement, tribal nations and other Native health care providers struggled to raise and address the issues they were facing amid the pandemic. “Since the resignation of Rear Admiral Weahkee, there have been countless requests from Indian Country calling on Congress and the Administration to nominate a new IHS director to address the growing health disparities experienced by AI/ANs.”
Tso laid out her priorities in her May testimony: creating a more unified health care system using the latest technology to develop centralized systems; improving accountability, transparency, and patient safety; addressing workforce needs and challenges, improving recruitment and retention.
Meeting her goals, she noted, would take “strong partnerships and communication with our Tribal partners…. Each tribe has unique needs, and those needs cannot be met if you do not understand them.”
Last year, President Joseph R. Biden asked Congress to significantly increase IHS funding, but his proposal was cut to $400 million. “For years, IHS has been funded at a rate that is far below its level of need, and the results of this historical neglect can be seen in the disparities in health outcomes for AI/AN people,” William Smith, Valdez Native Tribe, Chairman of the National Indian Health Board (NIHB), wrote to the Senate Committee on Indian Affairs, on the topic of the next IHS director. “Perhaps one of the greatest challenges facing the [Indian, tribal and urban] system is the chronic and severe underfunding and budgetary instability for health care and public health services infrastructure and delivery. Since its creation in 1955, IHS has been chronically underfunded, with annual appropriations never exceeding 50% of demonstrated need. This underfunding has contributed to substandard investment in health delivery systems, some of the worst health disparities among any US population and a severe lack of public health infrastructure and services for our people. At the start of the COVID-19 pandemic these vulnerabilities were starkly exposed and while Congress moved decisively to invest into Tribal health and public health, the new Director must work to maintain these one-time investments.”
Stacy Bohlen, NIHB chief executive, told The Oklahoman that tribal leaders will look to Tso to press Congress for more money and to secure mandatory full funding for IHS—in contrast with the current annual appropriations, where Congress includes IHS in much larger budget bills. “When those bills stall, so does the money tribal clinics need to pay employees and suppliers,” making it hard to recruit and retain employees. “In the Indian Health System,” Bohlen says, “we simply can’t afford that kind of vulnerability.”
Securing advance appropriations and, ultimately, full mandatory funding for IHS, Smith wrote in his letter to the Senate committee, “fulfills the commitment made to our people generations ago and breaks down the systemic healthcare funding inequities the federal government tolerates for Tribes.”
Tso emphasized her intent to “improve the physical, mental, social, and spiritual health and well-being of all American Indians and Alaskan Natives served by the Agency.” Tso “understands the healthcare needs that many first people of this country deal with,” President Nez said. “Her work ethic, value system and approach to problem solving demonstrates the resilience of Indigenous peoples and the commitment to combat the systemic inequities that impact tribal nations.”
Firm Mobile Nodule on the Scalp
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
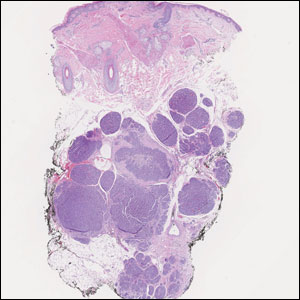
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12
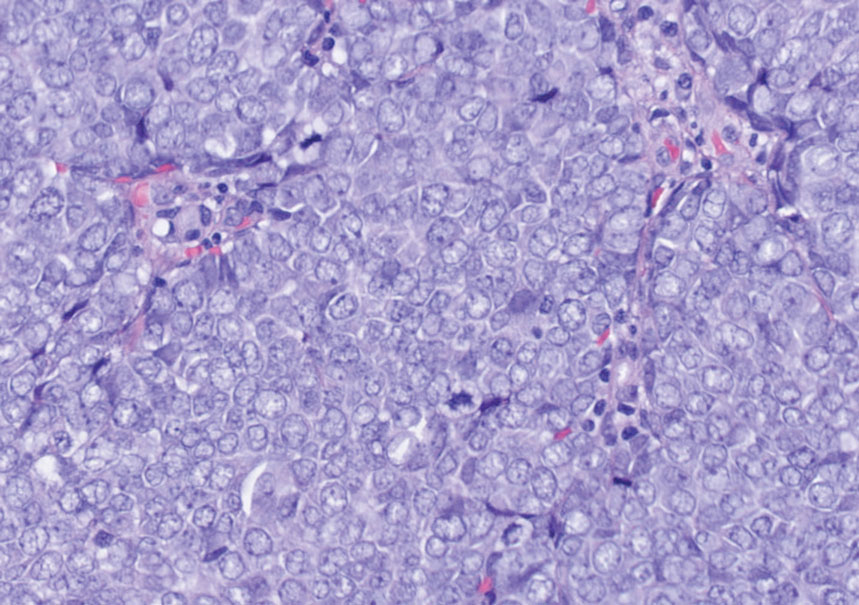
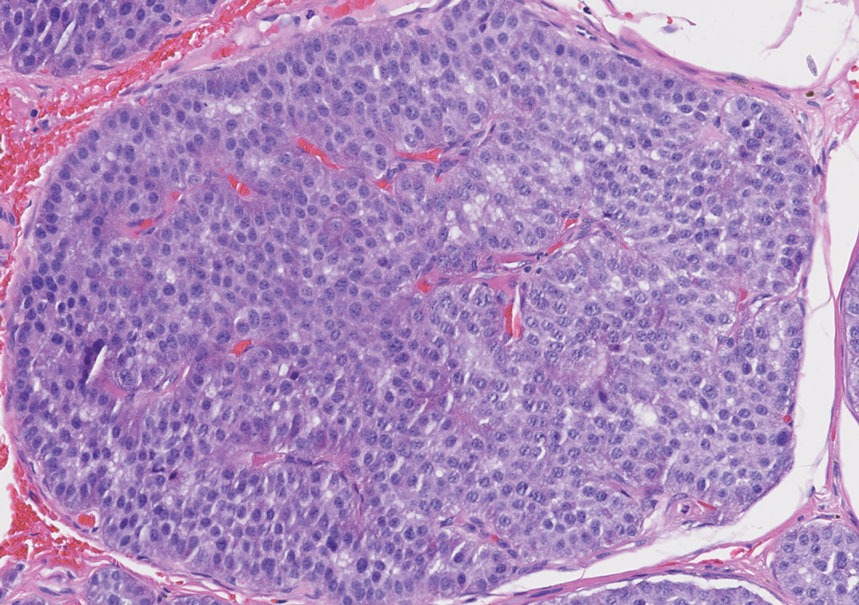
Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.
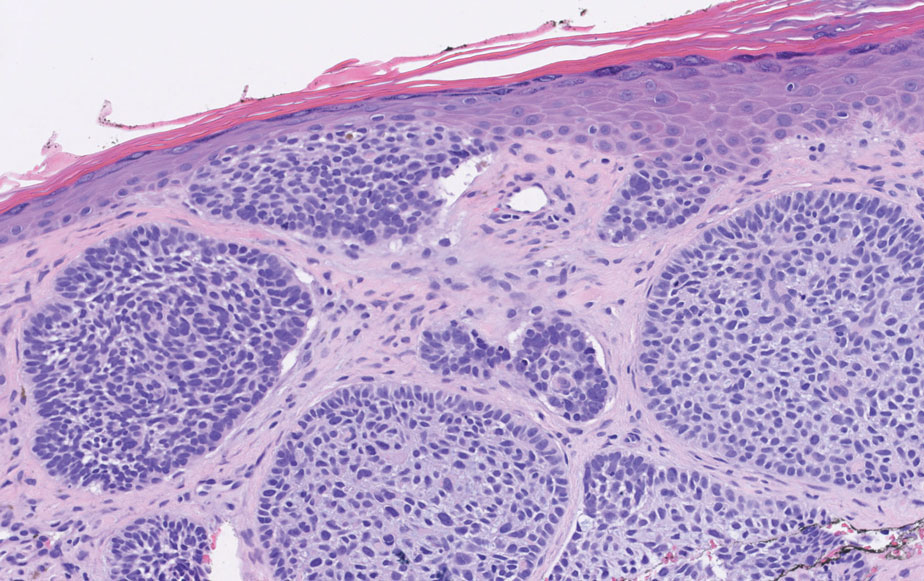
An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14
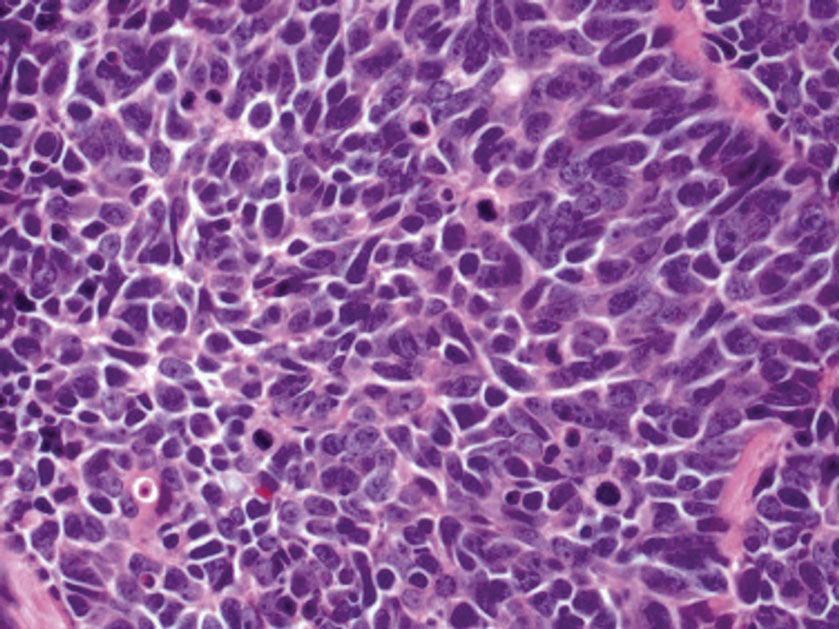
The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19
Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
The Diagnosis: Metastatic Carcinoid Tumor
Carcinoid tumors are derived from neuroendocrine cell compartments and generally arise in the gastrointestinal tract, with a quarter of carcinoids arising in the small bowel.1 Carcinoid tumors have an incidence of approximately 2 to 5 per 100,000 patients.2 Metastasis of carcinoids is approximately 31.2% to 46.7%.1 Metastasis to the skin is uncommon; we present a rare case of a carcinoid tumor of the terminal ileum with metastasis to the scalp.
Unlike our patient, most patients with carcinoid tumors have an indolent clinical course. The most common cutaneous symptom is flushing, which occurs in 75% of patients.3 Secreted vasoactive peptides such as serotonin may cause other symptoms such as tachycardia, diarrhea, and bronchospasm; together, these symptoms comprise carcinoid syndrome. Carcinoid syndrome requires metastasis of the tumor to the liver or a site outside of the gastrointestinal tract because the liver will metabolize the secreted serotonin. However, even in patients with liver metastasis, carcinoid syndrome only occurs in approximately 10% of patients.4 Common skin findings of carcinoid syndrome include pellagralike dermatitis, flushing, and sclerodermalike changes.5 Our patient experienced several episodes of presyncope with symptoms of dyspnea, lightheadedness, and flushing but did not have bronchospasm or recurrent diarrhea. Intramuscular octreotide improved some symptoms.
The scalp accounts for approximately 15% of cutaneous metastases, the most common being from the lung, renal, and breast cancers.6 Cutaneous metastases of carcinoid tumors are rare. A PubMed search of articles indexed for MEDLINE using the terms metastatic AND [carcinoid OR neuroendocrine] tumors AND [skin OR cutaneous] revealed 47 cases.7-11 Similar to other skin metastases, cutaneous metastases of carcinoid tumors commonly present as firm erythematous nodules of varying sizes that may be asymptomatic, tender, or pruritic (Figure 1). Cases of carcinoid tumors with cutaneous metastasis as the initial and only presenting sign are exceedingly rare.12

Histology of carcinoid tumors reveals a dermal neoplasm composed of loosely cohesive, mildly atypical, polygonal cells with salt-and-pepper chromatin and eosinophilic cytoplasm, which are similar findings to the primary tumor. The cells may grow in the typical trabecular or organoid neuroendocrine pattern or exhibit a pseudoglandular growth pattern with prominent vessels (quiz image, top).12 Positive chromogranin and synaptophysin immunostaining are the most common and reliable markers used for the diagnosis of carcinoid tumors.

An important histopathologic differential diagnosis is the aggressive Merkel cell carcinoma, which also demonstrates homogenous salt-and-pepper chromatin but exhibits a higher mitotic rate and positive cytokeratin 20 staining (Figure 2).13 Basal cell carcinoma (BCC) also may display similar features, including a blue tumor at scanning magnification and nodular or infiltrative growth patterns. The cell morphology of BCC is characterized by islands of basaloid cells with minimal cytoplasm and frequent apoptosis, connecting to the epidermis with peripheral palisading, retraction artifact, and a myxoid stroma; BCC lacks the salt-and-pepper chromatin commonly seen in carcinoid tumors (Figure 3). Basal cell carcinoma is characterized by positive BerEP4 (epithelial cell adhesion molecule immunostain), cytokeratin 5/6, and cytokeratin 14 uptake. Cytokeratin 20, often used to diagnose Merkel cell carcinoma, is negative in BCC. Chromogranin and synaptophysin occasionally may be positive in BCC.14

The superficial Ewing sarcoma family of tumors also may be included in the differential diagnosis of small round cell tumors of the skin, but they are very rare. These tumors possess strong positive membranous staining of cytokeratin 99 and also can stain positively for synaptophysin and chromogranin.15 Epithelial membrane antigen, which is negative in Ewing sarcomas, is positive in carcinoid tumors.16 Neuroendocrine tumors of all sites share similar basic morphologic patterns, and multiple primary tumors should be considered, including small cell lung carcinoma (Figure 4).17,18 Red granulations and true glandular lumina typically are not seen in the lungs but are common in gastrointestinal carcinoids.18 Regarding immunohistochemistry, TTF-1 is negative and CDX2 is positive in gastroenteropancreatic carcinoids, suggesting that these 2 markers can help distinguish carcinoids of unknown primary origin.19

Metastases in carcinoid tumors are common, with one study noting that the highest frequency of small intestinal metastases was from the ileal subset.20 At the time of diagnosis, 58% to 64% of patients with small intestine carcinoid tumors already had nonlocalized disease, with frequent sites being the lymph nodes (89.8%), liver (44.1%), lungs (13.6%), and peritoneum (13.6%). Regional and distant metastases are associated with substantially worse prognoses, with survival rates of 71.7% and 38.5%, respectively.1 Treatment of symptomatic unresectable disease focuses on symptomatic management with somatostatin analogs that also control tumor growth.21
We present a rare case of scalp metastasis of a carcinoid tumor of the terminal ileum. Distant metastasis is associated with poorer prognosis and should be considered in patients with a known history of a carcinoid tumor.
Acknowledgment—We would like to acknowledge the Research Histology and Tissue Imaging Core at University of Illinois Chicago Research Resources Center for the immunohistochemistry studies.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
- Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934-959.
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am. 2011;40:1-18, vii.
- Sabir S, James WD, Schuchter LM. Cutaneous manifestations of cancer. Curr Opin Oncol. 1999;11:139-144.
- Tomassetti P. Clinical aspects of carcinoid tumours. Italian J Gastroenterol Hepatol. 1999;31(suppl 2):S143-S146.
- Bell HK, Poston GJ, Vora J, et al. Cutaneous manifestations of the malignant carcinoid syndrome. Br J Dermatol. 2005;152:71-75.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Garcia A, Mays S, Silapunt S. Metastatic neuroendocrine carcinoma in the skin. Dermatol Online J. 2017;23:13030/qt9052w9x1.
- Ciliberti MP, Carbonara R, Grillo A, et al. Unexpected response to palliative radiotherapy for subcutaneous metastases of an advanced small cell pancreatic neuroendocrine carcinoma: a case report of two different radiation schedules. BMC Cancer. 2020;20:311.
- Devnani B, Kumar R, Pathy S, et al. Cutaneous metastases from neuroendocrine carcinoma of the cervix: an unusual metastatic lesion from an uncommon malignancy. Curr Probl Cancer. 2018; 42:527-533.
- Falto-Aizpurua L, Seyfer S, Krishnan B, et al. Cutaneous metastasis of a pulmonary carcinoid tumor. Cutis. 2017;99:E13-E15.
- Dhingra R, Tse JY, Saif MW. Cutaneous metastasis of gastroenteropancreatic neuroendocrine tumors (GEP-Nets)[published online September 8, 2018]. JOP. 2018;19.
- Jedrych J, Busam K, Klimstra DS, et al. Cutaneous metastases as an initial manifestation of visceral well-differentiated neuroendocrine tumor: a report of four cases and a review of literature. J Cutan Pathol. 2014;41:113-122.
- Lloyd RV. Practical markers used in the diagnosis of neuroendocrine tumors. Endocr Pathol. 2003;14:293-301.
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502.
- Machado I, Llombart B, Calabuig-Fariñas S, et al. Superficial Ewing’s sarcoma family of tumors: a clinicopathological study with differential diagnoses. J Cutan Pathol. 2011;38:636-643.
- D’Cruze L, Dutta R, Rao S, et al. The role of immunohistochemistry in the analysis of the spectrum of small round cell tumours at a tertiary care centre. J Clin Diagn Res. 2013;7:1377-1382.
- Chirila DN, Turdeanu NA, Constantea NA, et al. Multiple malignant tumors. Chirurgia (Bucur). 2013;108:498-502.
- Rekhtman N. Neuroendocrine tumors of the lung: an update. Arch Pathol Lab Med. 2010;134:1628-1638.
- Lin X, Saad RS, Luckasevic TM, et al. Diagnostic value of CDX-2 and TTF-1 expressions in separating metastatic neuroendocrine neoplasms of unknown origin. Appl Immunohistochem Mol Morphol. 2007;15:407-414.
- Olney JR, Urdaneta LF, Al-Jurf AS, et al. Carcinoid tumors of the gastrointestinal tract. Am Surg. 1985;51:37-41.
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas. 2017;46:707-714.
A 47-year-old woman was admitted to the hospital with abdominal pain and flushing. She had a history of a midgut carcinoid that originated in the ileum with metastasis to the colon, liver, and pancreas. Dermatologic examination revealed a firm, nontender, mobile, 7-mm scalp nodule with a pink-purple overlying epidermis. The lesion was associated with a slight decrease in hair density. A 4-mm punch biopsy was performed.

COMMENT & CONTROVERSY
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Misoprostol: Clinical pharmacology in obstetrics and gynecology
ROBERT L. BARBIERI, MD (JULY 2022)
Outcomes from my practice’s pilot study
In his recent editorial, Dr. Barbieri addressed the important topic of office-based cervical ripening prior to inpatient induction of labor. In order to decrease the length of labor and increase the success of vaginal delivery, the cervical factor is of prime importance. Patients with an unfavorable cervix (Bishop score of ≥6) are more likely to experience longer labor, risk of infection, fetal distress, etc, and may end up with an unwanted cesarean delivery. To prevent the above, numerous approaches (mechanical methods, double-balloon catheter, laminaria, misoprostol among others) have been discussed.
The inclusion criteria for office-based cervical ripening are low-risk patients, singleton pregnancies between 39 and 40 weeks of gestation, and cephalic presentation. The details of inclusion and exclusion criteria have to be determined by each practice individually. Our practice went a step further. We performed a small pilot study to assess the safety and efficacy of office cervical ripening in low-risk primigravid patients with low Bishop scores who were not scheduled for induction in anticipation of labor. Ten primigravid patients with poor Bishop scores (6 or less) were administered 50 µg misoprostol at 39+ weeks of pregnancy in the office setting. Bishop scores were taken twice per week until delivery. In 7 out of 10 patients, the Bishop score became favorable within a week of treatment, and in 3 patients the Bishop score remained the same. Three out of 10 patients experienced self-limited episodes of uterine contractility, and 2 of the patients went into labor within 3 days of using misoprostol. All patients were delivered within 2 weeks of treatment without an induction: 8 delivered vaginally, and 2 by cesarean delivery.2
Cesarean delivery was done for fetal distress (1 case) and prolonged second stage of labor (1 case). All neonates were born in satisfactory condition with Apgar scores between 7 and 10. Our preliminary results demonstrated marked improvement in cervical ripening judged by the Bishop score in 70% of patients.2
A prospective randomized study should be performed with the following agenda:
- Does late pregnancy medical cervical ripening in low-risk patients affect labor course and cesarean delivery rate?
- What is the optimal dose and route of administration of misoprostol?3,4
References
- Barbieri R. Office-based ambulatory cervical ripening prior to in patient induction of labor. OBG Manag. 2021;33:9-13.
- Petrikovsky B. Should cervical ripening become routine in primigravid low risk patients [In press]. Neonat Int Care. 2022:1, 4-6.
- Sharami SH, Milani F, Faraji R. Comparison of 25 µg sublingual and 50 µg intravaginal misoprostol for cervical ripening and labor: a randomized controlled equivalence trial. Arch Med. 2014:10:653-656.
- Barbieri R. Misoprostol: clinical pharmacology in obstetrics and gynecology. OBG Manag. 2022:34:7, 8-12.
B. Petrikovsky, MD, PhD
New Hyde Park, New York
Dr. Barbieri responds
I appreciate that Dr. Petrikovsky took time from a busy practice to provide our readers with his very innovative idea. I agree with him that a clinical trial is warranted to test the effects of late pregnancy medical cervical ripening in low-risk patients on labor course and birth outcome. Maybe one of our readers will take on the challenge to complete such a trial! ●
Options and outcomes for uterine preservation at the time of prolapse surgery
CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7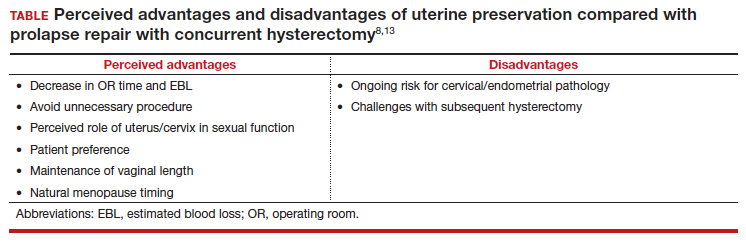
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●

CASE Patient desires prolapse repair
A 65-year-old postmenopausal patient (G3P3) presents to your office with symptoms of a vaginal bulge for more than 1 year. She has no urinary incontinence symptoms and no bowel dysfunction symptoms. On examination, you diagnose stage 2 uterovaginal prolapse with both anterior and apical defects. The patient declines expectant and pessary management and desires surgery, but she states that she feels her uterus “is important for me to keep, as my babies grew in there and it is part of me.” She denies any family or personal history of breast, endometrial, or ovarian cancer and has no history of abnormal cervical cancer screening or postmenopausal bleeding. What are the options for this patient?
Who is the appropriate hysteropexy patient, and how do we counsel her?
Uterine prolapse is the third leading cause of benign hysterectomy, with approximately 70,000 procedures performed each year in the United States. It has long been acknowledged that the uterus is a passive bystander to the prolapse process,1 but modern practice often involves a hysterectomy as part of addressing apical prolapse. However, more and more uterine-preserving surgeries are being performed, with one study showing an increase from 1.8% to 5% from 2002 and 2012.2
When presented with the option to keep or remove their uterus during the time of prolapse surgery, 36% of patients indicated that they would prefer to keep their uterus with similar outcomes while 21% would still prefer uterine preservation even if outcomes were inferior compared with hysterectomy.3 Another study showed that 60% of patients would decline concurrent hysterectomy if there were equal surgical outcomes,4 and popular platforms, such as Health magazine (www.health.com) and AARP magazine (www.aarp.org), have listed benign hysterectomy as a “top surgery to avoid.”
Patients desire uterine preservation for many reasons, including concerns about sexual function and pleasure, the uterus being important to their sense of identity or womanhood, and concerns around menopausal symptoms. Early patient counseling and discussion of surgical goals can help clinicians fully understand a patient’s thoughts toward uterine preservation. Women who identified their uterus as important to their sense of self had a 28.2-times chance of preferring uterine preservation.3 Frequently, concerns about menopausal symptoms are more directly related to hormones and ovary removal, not uterus removal, but clinicians should be careful to also counsel patients on the increased risk of menopause in the 5 years after hysterectomy, even with ovarian preservation.5
There are some patients for whom experts do not recommend uterine preservation.6 Patients with an increased risk of cervical or endometrial pathology should be counseled on the benefits of hysterectomy. Additionally, patients who have abnormal uterine bleeding from benign pathology should consider hysterectomy to treat these issues and avoid future workups (TABLE). For postmenopausal patients with recent postmenopausal bleeding, we encourage hysterectomy. A study of patients undergoing hysterectomy at the time of prolapse repair found a rate of 13% unanticipated endometrial pathology with postmenopausal bleeding and negative preoperative workup.7
At this time, a majority of clinicians consider the desire for future fertility to be a relative contraindication to surgical prolapse repair and advise conservative management with pessary until childbearing is complete. This is reasonable, given the paucity of safety data in subsequent pregnancies as well as the lack of prolapse outcomes after those pregnancies.8,9 Lastly, cervical elongation is considered a relative contraindication, as it represents a risk for surgical failure.10,11 This may be counteracted with trachelectomy at the time of hysteropexy or surgeries such as the Manchester repair, which involve a trachelectomy routinely,12 but currently there is no strong evidence for this as routine practice.
Continue to: Uterine preservation surgical techniques and outcomes...
Uterine preservation surgical techniques and outcomes
Le Fort colpocleisis
First described in 1840 by Neugebauer of Poland and later by Le Fort in Paris in 1877, the Le Fort colpocleisis repair technique remains the most reliable prolapse surgery to date.14 The uterus is left in place while the vagina is narrowed and shortened. It typically also is performed with a levator plication to reduce the genital hiatus.
This procedure is quick and effective, with a 90% to 95% success rate. If necessary, it can be performed under local or regional anesthesia, making it a good option for medically frail patients. It is not an option for everyone, however, as penetrative intercourse is no longer an option after surgery. Studies suggest an approximately 13% dissatisfaction rate after the procedure, with most of that coming from postoperative urinary symptoms, such as urgency or stress incontinence,15 and some studies show a dissatisfaction rate as low as 0% in a well-counseled patient population.16,17
Vaginal native tissue hysteropexy
Many patients who elect for uterine preservation at the time of prolapse surgery are “minimalists,” meaning that a vaginal native tissue procedure appeals to them due to the lack of abdominal incisions, decreased operating room time, and lack of permanent graft materials.
Of all the hysteropexy procedures, sacrospinous hysteropexy (SSHP) has the most robust data available. The approach to SSHP can be tailored to the patient’s anatomy and it is performed in a manner similar to posthysterectomy sacrospinous ligament fixation. The traditional posterior approach can be used with predominantly posterior prolapse, while an apical approach through a semilunar paracervical incision can be used for predominantly apical prolapse. Expert surgeons agree that one key to success is anchoring the suspension sutures through the cervical stroma, not just the vaginal epithelium.
Researchers in the Netherlands published the 5-year outcomes of a randomized trial that compared SSHP with vaginal hysterectomy with uterosacral ligament suspension.18 Their data showed no difference between groups in composite failure, reoperation rates, quality of life measures, and postoperative sexual function. Adverse events were very similar to those reported for posthysterectomy sacrospinous ligament fixation, including 15% transient buttock pain. Of note, the same authors explored risk factors for recurrence after SSHP and found that higher body mass index, smoking, and a large point Ba measurement were risk factors for prolapse recurrence.19
A randomized, controlled trial in the United Kingdom (the VUE trial) compared vaginal hysterectomy with apical suspension to uterine preservation with a variety of apical suspension techniques, mostly SSHP, and demonstrated no significant differences in outcomes.20 Overall, SSHP is an excellent option for many patients interested in uterine preservation.
Uterosacral ligament hysteropexy (USHP), when performed vaginally, is very similar to uterosacral ligament suspension at the time of vaginal hysterectomy, with entry into the peritoneal cavity through a posterior colpotomy. The uterosacral ligaments are grasped and delayed absorbable suture placed through the ligaments and anchored into the posterior cervical stroma. Given the maintenance of the normal axis of the vagina, USHP is a good technique for patients with isolated apical defects. Unfortunately, the least amount of quality data is available for USHP at this time. Currently, evidence suggests that complications are rare and that the procedure may offer acceptable anatomic and symptomatic outcomes.21 Some surgeons approach the uterosacral suspension laparoscopically, which also has mixed results in the literature, with failure rates between 8% and 27% and few robust studies.22–24
The Manchester-Fothergill operation, currently not common in the United States but popular in Europe, primarily is considered a treatment for cervical elongation when the uterosacral ligaments are intact. In this procedure, trachelectomy is performed and the uterosacral ligaments are plicated to the uterine body. Sturmdorf sutures are frequently placed to close off the endometrial canal, which can lead to hematometra and other complications of cervical stenosis. Previous unmatched studies have shown similar outcomes with the Manchester procedure compared with vaginal hysterectomy.25,26
The largest study currently available is a registry study from Denmark, with matched cohort populations, that compared the Manchester procedure, SSHP, and total vaginal hysterectomy with uterosacral ligament suspension.27 This study indicated less morbidity related to the Manchester procedure, decreased anterior recurrence compared with SSHP, and a 7% reoperation rate.27 The same authors also established better cost-effectiveness with the Manchester procedure as opposed to vaginal hysterectomy with uterosacral ligament suspension.28
Continue to: Vaginal mesh hysteropexy...
Vaginal mesh hysteropexy
Hysteropexy using vaginal mesh is limited in the United States given the removal of vaginal mesh kits from the market by the US Food and Drug Administration in 2019. However, a Pelvic Floor Disorders Network randomized trial compared vaginal mesh hysteropexy using the Uphold LITE transvaginal mesh support system (Boston Scientific) and vaginal hysterectomy with uterosacral ligament suspension.29 At 5 years, mesh hysteropexy had fewer failures than hysterectomy (37% vs 54%) and there was no difference in retreatment (9% vs 13%). The authors noted an 8% mesh exposure rate in the mesh hysteropexy group but 12% granulation tissue and 21% suture exposure rate in the hysterectomy group.29
While vaginal mesh hysteropexy was effective in the treatment of apical prolapse, the elevated mesh exposure rate and postoperative complications ultimately led to its removal from the market.
Sacrohysteropexy
Lastly, prolapse surgery with uterine preservation may be accomplished abdominally, most commonly laparoscopically with or without robotic assistance.
Sacrohysteropexy (SHP) involves the attachment of permanent synthetic mesh posteriorly to the posterior vagina and cervix with or without the additional placement of mesh to the anterior vagina and cervix. When the anterior mesh is placed, the arms are typically routed through the broad ligament bilaterally and joined with the posterior mesh for attachment to the anterior longitudinal ligament, overlying the sacrum.
Proponents of this technique endorse the use of mesh to augment already failing native tissues and propose similarities to the durability of sacrocolpopexy. While no randomized controlled trials have compared hysterectomy with sacrocolpopexy or supracervical hysterectomy with sacrocolpopexy to sacrohysteropexy, a meta-analysis suggests that sacrohysteropexy may have a decreased risk of mesh exposure but a higher reoperation rate with lower anatomic success.9 Randomized trials that compared abdominal sacrohysteropexy with vaginal hysterectomy and suspension indicate that apical support may be improved with sacrohysteropexy,30 but reoperations, postoperative pain and disability, and urinary dysfunction was higher with SHP.31,32
What further research is needed?
With the increasing patient and clinician interest in uterine preservation, more research is needed to improve patient counseling and surgical planning. Much of the current research compares hysteropexy outcomes with those of traditional prolapse repairs with hysterectomy, with only a few randomized trials. We are lacking robust, prospective comparison studies between hysteropexy methods, especially vaginal native tissue techniques, long-term follow-up on the prevalence of uterine or cervical pathology after hysteropexy, and pregnancy or postpartum outcomes following uterine preservation surgery.
Currently, work is underway to validate and test the effectiveness of a questionnaire to evaluate the uterus’s importance to the patient seeking prolapse surgery in order to optimize counseling. The VUE trial, which randomizes women to vaginal hysterectomy with suspension versus various prolapse surgeries with uterine preservation, is continuing its 6-year follow-up.20 In the Netherlands, an ongoing randomized, controlled trial (the SAM trial) is comparing the Manchester procedure with sacrospinous hysteropexy and will follow patients up to 24 months.33 Fortunately, both of these trials are rigorously assessing both objective and patient-centered outcomes.
CASE Counseling helps the patient weigh surgical options
After thorough review of her surgical options, the patient elects for a uterine-preserving prolapse repair. She would like to have the most minimally invasive procedure and does not want any permanent mesh used. You suggest, and she agrees to, a sacrospinous ligament hysteropexy, as it is the current technique with the most robust data. ●
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.
- DeLancey JO. Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol. 1992;166(6 pt 1):1717-1724; discussion 1724-1728. doi:10.1016/0002-9378(92)91562-o.
- Madsen AM, Raker C, Sung VW. Trends in hysteropexy and apical support for uterovaginal prolapse in the United States from 2002 to 2012. Female Pelvic Med Reconstr Surg. 2017;23:365-371. doi:10.1097/SPV.0000000000000426.
- Korbly NB, Kassis NC, Good MM, et al. Patient preferences for uterine preservation and hysterectomy in women with pelvic organ prolapse. Am J Obstet Gynecol. 2013;209:470.e16. doi:10.1016/j.ajog.2013.08.003.
- Frick AC, Barber MD, Paraiso MF, et al. Attitudes toward hysterectomy in women undergoing evaluation for uterovaginal prolapse. Female Pelvic Med Reconstr Surg. 2013;19:103-109. doi:10.1097/SPV.0b013e31827d8667.
- Farquhar CM, Sadler L, Harvey SA, et al. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112:956-962. doi:10.1111/j.1471-0528.2005.00696.x
- Gutman R, Maher C. Uterine-preserving POP surgery. Int Urogynecol J. 2013;24:1803-1813. doi:10.1007/s00192-0132171-2.
- Frick AC, Walters MD, Larkin KS, et al. Risk of unanticipated abnormal gynecologic pathology at the time of hysterectomy for uterovaginal prolapse. Am J Obstet Gynecol. 2010;202:507. e1-4. doi:10.1016/j.ajog.2010.01.077.
- Meriwether KV, Balk EM, Antosh DD, et al. Uterine-preserving surgeries for the repair of pelvic organ prolapse: a systematic review with meta-analysis and clinical practice guidelines. Int Urogynecol J. 2019;30:505-522. doi:10.1007/s00192-01903876-2.
- Meriwether KV, Antosh DD, Olivera CK, et al. Uterine preservation vs hysterectomy in pelvic organ prolapse surgery: a systematic review with meta-analysis and clinical practice guidelines. Am J Obstet Gynecol. 2018;219:129-146. e2. doi:10.1016/j.ajog.2018.01.018.
- Lin TY, Su TH, Wang YL, et al. Risk factors for failure of transvaginal sacrospinous uterine suspension in the treatment of uterovaginal prolapse. J Formos Med Assoc. 2005;104:249-253.
- Hyakutake MT, Cundiff GW, Geoffrion R. Cervical elongation following sacrospinous hysteropexy: a case series. Int Urogynecol J. 2014;25:851-854. doi:10.1007/s00192-013-2258-9.
- Thys SD, Coolen AL, Martens IR, et al. A comparison of long-term outcome between Manchester Fothergill and vaginal hysterectomy as treatment for uterine descent. Int Urogynecol J. 2011;22:1171-1178. doi:10.1007/s00192-011-1422-3.
- Ridgeway BM, Meriwether KV. Uterine preservation in pelvic organ prolapse surgery. In: Walters & Karram Urogynecology and Reconstructive Pelvic Surgery. 5th ed. Elsevier, Inc; 2022:358-373.
- FitzGerald MP, Richter HE, Siddique S, et al; for the Pelvic Floor Disorders Network. Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:261-271. doi:10.1007/s00192005-1339-9.
- Winkelman WD, Haviland MJ, Elkadry EA. Long-term pelvic f loor symptoms, recurrence, satisfaction, and regret following colpocleisis. Female Pelvic Med Reconstr Surg. 2020;26:558562. doi:10.1097/SPV.000000000000602.
- Lu M, Zeng W, Ju R, et al. Long-term clinical outcomes, recurrence, satisfaction, and regret after total colpocleisis with concomitant vaginal hysterectomy: a retrospective single-center study. Female Pelvic Med Reconstr Surg. 2021;27(4):e510-e515. doi:10.1097/SPV.0000000000000900.
- Wang X, Chen Y, Hua K. Pelvic symptoms, body image, and regret after LeFort colpocleisis: a long-term follow-up. J Minim Invasive Gynecol. 2017;24:415-419. doi:10.1016/j. jmig.2016.12.015.
- Schulten SFM, Detollenaere RJ, Stekelenburg J, et al. Sacrospinous hysteropexy versus vaginal hysterectomy with uterosacral ligament suspension in women with uterine prolapse stage 2 or higher: observational followup of a multicentre randomised trial. BMJ. 2019;366:I5149. doi:10.1136/bmj.l5149.
- Schulten SF, Detollenaere RJ, IntHout J, et al. Risk factors for pelvic organ prolapse recurrence after sacrospinous hysteropexy or vaginal hysterectomy with uterosacral ligament suspension. Am J Obstet Gynecol. 2022;227:252.e1252.e9. doi:10.1016/j.ajog.2022.04.017.
- Hemming C, Constable L, Goulao B, et al. Surgical interventions for uterine prolapse and for vault prolapse: the two VUE RCTs. Health Technol Assess. 2020;24:1-220. doi:10.3310/hta24130.
- Romanzi LJ, Tyagi R. Hysteropexy compared to hysterectomy for uterine prolapse surgery: does durability differ? Int Urogynecol J. 2012;23:625-631. doi:10.1007/s00192-011-1635-5.
- Rosen DM, Shukla A, Cario GM, et al. Is hysterectomy necessary for laparoscopic pelvic floor repair? A prospective study. J Minim Invasive Gynecol. 2008;15:729-734. doi:10.1016/j.jmig.2008.08.010.
- Bedford ND, Seman EI, O’Shea RT, et al. Effect of uterine preservation on outcome of laparoscopic uterosacral suspension. J Minim Invasive Gynecol. 2013;20(2):172-177. doi:10.1016/j.jmig.2012.10.014.
- Diwan A, Rardin CR, Strohsnitter WC, et al. Laparoscopic uterosacral ligament uterine suspension compared with vaginal hysterectomy with vaginal vault suspension for uterovaginal prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17:79-83. doi:10.1007/s00192-005-1346-x.
- de Boer TA, Milani AL, Kluivers KB, et al. The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:13131319. doi:10.1007/s00192-009-0945-3.
- Thomas AG, Brodman ML, Dottino PR, et al. Manchester procedure vs. vaginal hysterectomy for uterine prolapse. A comparison. J Reprod Med. 1995;40:299-304.
- Husby KR, Larsen MD, Lose G, et al. Surgical treatment of primary uterine prolapse: a comparison of vaginal native tissue surgical techniques. Int Urogynecol J. 2019;30:18871893. doi:10.1007/s00192-019-03950-9.
- Husby KR, Tolstrup CK, Lose G, et al. Manchester-Fothergill procedure versus vaginal hysterectomy with uterosacral ligament suspension: an activity-based costing analysis. Int Urogynecol J. 2018;29:1161-1171. doi:10.1007/s00192-0183575-9.
- Nager CW, Visco AG, Richter HE, et al; National Institute of Child Health and Human Development Pelvic Floor Disorders Network. Effect of sacrospinous hysteropexy with graft vs vaginal hysterectomy with uterosacral ligament suspension on treatment failure in women with uterovaginal prolapse: 5-year results of a randomized clinical trial. Am J Obstet Gynecol. 2021;225:153.e1-153.e31. doi:10.1016/j. ajog.2021.03.012.
- Rahmanou P, Price N, Jackson SR. Laparoscopic hysteropexy versus vaginal hysterectomy for the treatment of uterovaginal prolapse: a prospective randomized pilot study. Int Urogynecol J. 2015;26:1687-1694. doi:10.1007/s00192-0152761-2.
- Roovers JP, van der Vaart CH, van der Bom JG, et al. A randomised controlled trial comparing abdominal and vaginal prolapse surgery: effects on urogenital function. BJOG. 2004;111:50-56. doi:10.1111/j.1471-0528.2004.00001.x.
- Roovers JP, van der Bom JG, van der Vaart CH, et al. A randomized comparison of post-operative pain, quality of life, and physical performance during the first 6 weeks after abdominal or vaginal surgical correction of descensus uteri. Neurourol Urodyn. 2005;24:334-340. doi:10.1002/nau.20104.
- Schulten SFM, Enklaar RA, Kluivers KB, et al. Evaluation of two vaginal, uterus sparing operations for pelvic organ prolapse: modified Manchester operation (MM) and sacrospinous hysteropexy (SSH), a study protocol for a multicentre randomized non-inferiority trial (the SAM study). BMC Womens Health. 20192;19:49. doi:10.1186/ s12905-019-0749-7.
2022 Update on contraception
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.
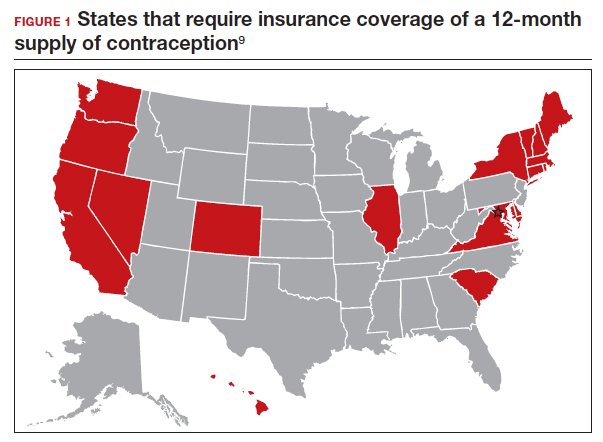
Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14
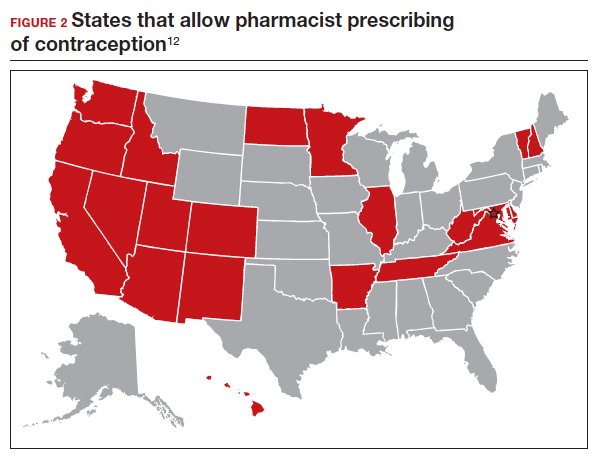
Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).
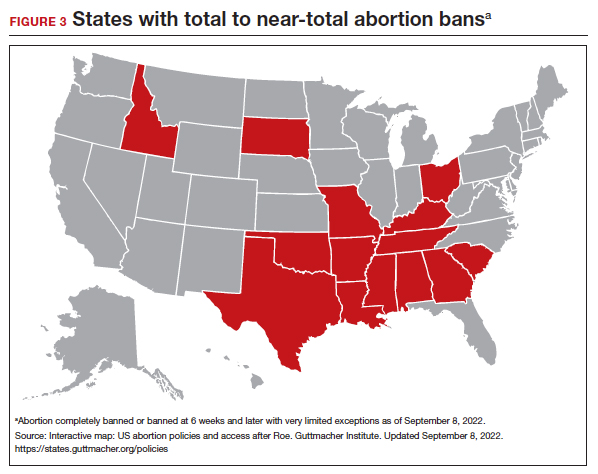
Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●
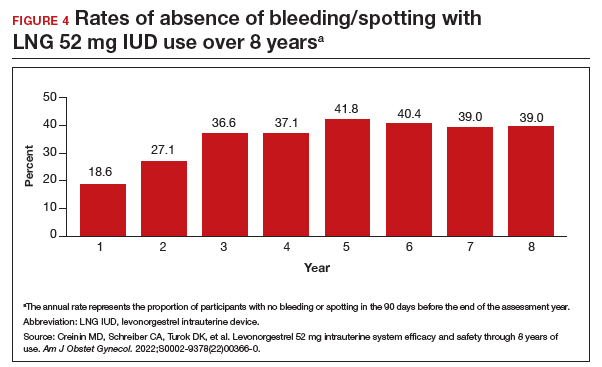
As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
On June 24, 2022, the US Supreme Court ruled in Dobbs v Jackson to overturn the landmark Roe v Wade decision, deeming that abortion is not protected by statutes that provide the right to privacy, liberty, or autonomy. With this historic ruling, other rights founded on the same principles, including the freedom to use contraception, may be called into question in the future. Clinics that provide abortion care typically play a vital role in providing contraception services. Due to abortion restriction across the country, many of these clinics are predicted to close and many have already closed. Within one month of the Dobbs decision, 43 clinics in 11 states had shut their doors to patients, reducing access to basic contraception services.1 It is more important now than ever that clinicians address barriers and lead the effort to improve and ensure that patients have access to contraceptive services.
In this Update, we review recent evidence that may help aid patients in obtaining contraception more easily and for longer periods of time. We review strategies demonstrated to improve contraceptive access, including how to increase prescribing rates of 1-year contraceptive supplies and pharmacist-prescribed contraception. We also review new data on extended use of the levonorgestrel 52 mg intrauterine device (LNG 52 mg IUD).
One-year prescribing of hormonal contraception decreases an access barrier
Uhm S, Chen MJ, Cutler ED, et al. Twelve-month prescribing of contraceptive pill, patch, and ring before and after a standardized electronic medical record order change. Contraception. 2021;103:60-63.
Providing a 1-year supply of self-administered contraception can lead to higher likelihood of continued use and is associated with reduced cost, unintended pregnancy, and abortion rates.2-4 Although some patients may not use a full year’s supply of pills, rings, or patches under such programs, the lower rates of unintended pregnancy result in significant cost savings as compared with the unused contraceptives.2,3 Accordingly, the Centers for Disease Control and Prevention (CDC) advises dispensing a 1-year supply of self-administered hormonal contraception.5 Insurance coverage and providers’ prescribing practices can be barriers to patients obtaining a year’s supply of hormonal contraception. Currently, 18 states and the District of Columbia legally require insurers to cover a 12-month supply of prescription contraceptives (FIGURE 1). Despite these laws and the CDC recommendation, studies show that most people continue to receive only a 1- to 3-month supply.6-8 One strategy to increase the number of 1-year supplies of self-administered contraception is institutional changes to default prescription orders.

Study design
In California, legislation enacted in January 2017 required commercial and medical assistance health plans to cover up to 12 months of US Food and Drug Administration (FDA)-approved self-administered hormonal contraceptives dispensed at 1 time as prescribed or requested. To better serve patients, a multidisciplinary team from the University of California Davis Health worked with the institution’s pharmacy to institute an electronic medical record (EMR) default order change from dispensing 1-month with refills to dispensing 12-month quantities for all combined and progestin-only pills, patches, and rings on formulary.
After this EMR order change in December 2019, Uhm and colleagues conducted a retrospective pre-post study using outpatient prescription data that included nearly 5,000 contraceptive pill, patch, and ring prescriptions over an 8-month period. They compared the frequency of 12-month prescriptions for each of these methods 4 months before and 4 months after the default order change. They compared the proportion of 12-month prescriptions by prescriber department affiliation and by clinic location. Department affiliation was categorized as obstetrics-gynecology or non–obstetrics-gynecology. Clinic location was categorized as medical center campus or community clinics.
Increase in 12-month prescriptions
The authors found an overall increase in 12-month prescriptions, from 11% to 27%, after the EMR order change. Prescribers at the medical center campus clinics more frequently ordered a 12-month supply compared with prescribers at community clinics both before (33% vs 4%, respectively) and after (53% vs 19%, respectively) the EMR change. The only group of providers without a significant increase in 12-month prescriptions was among obstetrics-gynecology providers at community clinics (4% before vs 6% after).
The system EMR change modified only the standard facility order settings and did not affect individual favorite orders, which may help explain the differences in prescribing practices. While this study found an increase in 12-month prescriptions, there were no data on the actual number of supplies a patient received or on reimbursement.
The study by Uhm and colleagues showed that making a relatively simple change to default EMR orders can increase 12-month contraception prescribing and lead to greater patient-centered care. Evidence shows that providers and pharmacists are not necessarily aware of laws that require 12-month supply coverage and routinely prescribe smaller supplies.6,7,9 For clinicians in states that have these laws (FIGURE 1), we urge you to provide as full a supply of contraceptives as possible as this approach is both evidence based and patient centered. Although this study shows the benefit of universal system change to the EMR, individual clinicians also must be sure to modify personal order preferences. In addition, pharmacists can play an important role by updating policies that comply with these laws and by increasing pharmacy stocks of contraception supplies.7 For those living in states that do not currently have these laws, we encourage you to reach out to your legislators to advocate for similar laws as the data show clear medical and cost benefits for patients and society.
Continue to: Pharmacist prescription of hormonal contraception is safe and promotes continuation...
Pharmacist prescription of hormonal contraception is safe and promotes continuation
Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
Patients often face difficulty obtaining both new and timely refills of self-administered contraception.10,11 To expand contraception access, Oregon became the first state (in 2016) to enact legislation to authorize direct pharmacist prescribing of hormonal contraceptives.12 Currently, 17 states and the District of Columbia have protocols for pharmacist prescribing privileges (FIGURE 2), and proposed legislation is pending in another 14 states.10,12 These protocols vary, but basic processes include screening, documentation, monitoring, and referrals when necessary. Typically, protocols require a pharmacist to review a patient’s medical history, pregnancy status, medication use, and blood pressure, followed by contraceptive counseling.10 Pharmacies are generally located in the community they serve, have extended hours, and usually do not require an appointment.8,13,14

Pharmacist prescribing increases the number of new contraceptive users, and pharmacists are more likely to prescribe a 6-month or longer supply of contraceptives compared with clinicians.8,13,15 Also, pharmacist prescribing is safe, with adherence rates to the CDC’s US Medical Eligibility Criteria for Contraceptive Use similar to those of prescriptions provided by a clinician.13
Authors of a recent multi-state study further assessed the impact of pharmacist prescribing by evaluating 12-month continuation and perfect use rates.
Study design
Rodriguez and colleagues evaluated the results of a 1-year prospective cohort study conducted in 2019 that included 388 participants who sought contraception in California, Colorado, Hawaii, and Oregon. All these states had laws permitting pharmacist prescribing and 12-month supply of hormonal contraception. Participants received prescriptions directly from a pharmacist at 1 of 139 pharmacies (n = 149) or filled a prescription provided by a clinician (n = 239). The primary outcomes were continuation of an effective method and perfect use of contraception across 12 months.
Participant demographics were similar between the 2 groups except for education and insurance status. Participants who received a prescription from a clinician reported higher levels of education. A greater proportion of uninsured participants received a prescription from a pharmacist (11%) compared with from a clinician (3%).
Contraceptive continuation rates
Participants were surveyed 3 times during the 12-month study about their current contraceptive method, if they had switched methods, or if they had any missed days of contraception.
Overall, 340 participants (88%) completed a full 12 months of follow-up. Continuation rates were similar between the 2 groups: 89% in the clinician-prescribed and 90% in the pharmacist-prescribed group (P=.86). Participants in the 2 groups also reported similar rates of perfect use (no missed days: 54% and 47%, respectively [P=.69]). Additionally, the authors reported that 29 participants changed from a tier 2 (pill, patch, ring, injection) to a tier 1 (intrauterine device or implant) method during follow-up, with no difference in switch rates for participants who received care from a clinician (10%) or a pharmacist (7%).

Patients have difficulties in obtaining both an initial contraceptive prescription and refills in time to avoid breaks in coverage.16 Pharmacist prescription of contraception is a proven strategy to increase access to contraception for new users or to promote continuation among current users. This practice is evidence based, decreases unintended pregnancy rates, and is safe.8,13,15,17
Promoting universal pharmacist prescribing is even more important given the overruling of Roe v Wade. With abortion restrictions, many family planning clinics that also play a vital role in providing contraception will close. Most states that are limiting abortion care (FIGURE 3) are the same states without pharmacist-prescribing provisions (FIGURE 2). As patient advocates, we need to continue to support this evidence-based practice in states where it is available and push legislators in states where it is not. Pharmacists should receive support to complete the training and certification needed to not only provide this service but also to receive appropriate reimbursements. Restrictions, such as requiring patients to be 18 years or older or to have prior consultation with a physician, should be limited as these are not necessary to provide self-administered contraception safely. Clinicians and pharmacists should inform patients, in states where this is available, that they can access initial or refill prescriptions at their local pharmacy if that is more convenient or their preference. Clinicians who live in states without these laws can advocate for their community by encouraging their legislators to pass laws that allow this evidence-based practice.
Continue to: LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use...
LNG 52 mg IUD demonstrates efficacy and safety through 8 years of use
Creinin MD, Schreiber CA, Turok DK, et al. Levonorgestrel 52 mg intrauterine system efficacy and safety through 8 years of use. Am J Obstet Gynecol. 2022;S00029378(22)00366-0.
Given the potential difficulty accessing contraceptive and abortion services due to state restrictions, patients may be more motivated to maintain long-acting reversible contraceptives for maximum periods of time. The LNG 52 mg IUD was first marketed as a 5-year product, but multiple studies suggested that it had potential longer duration of efficacy and safety.18,19 The most recent clinical trial report shows that the LNG 52 mg IUD has at least 8 years of efficacy and safety.
Evidence supports 8 years’ use
The ACCESS IUS (A Comprehensive Contraceptive Efficacy and Safety Study of an IUS) phase 3 trial was designed to assess the safety and efficacy of a LNG 52 mg IUD (Liletta) for up to 10 years of use. The recent publication by Creinin and colleagues extends the available data from this study from 6 to 8 years.
Five-hundred and sixty-nine participants started year 7; 478 completed year 7 and 343 completed year 8 by the time the study was discontinued. Two pregnancies occurred in year 7 and no pregnancies occurred in year 8. One of the pregnancies in year 7 was determined by ultrasound examination to have implantation on day 4 after LNG IUD removal. According to the FDA, any pregnancy that occurs within 7 days of discontinuation is included as on-treatment, whereas the European Medicines Agency (EMA) has a 2-day cutoff. Over 8 years, 11 pregnancies occurred. The cumulative life-table pregnancy rate in the primary efficacy population through year 8 was 1.32% (95% confidence interval [CI], 0.69–2.51) under FDA rules and 1.09% (95% CI, 0.56–2.13) according to EMA guidance.
Absence of bleeding/spotting rates and adverse events
Rates of absence of bleeding/spotting remained relatively stable in years 7 and 8 at around 40%, similar to the rates during years 3 to 8 (FIGURE 4). Overall, only 2.6% of participants discontinued LNG IUD use because of bleeding problems, with a total of 4 participants discontinuing for this reason in years 7 and 8. Expulsion rates remained low at a rate of approximately 0.5% in years 7 and 8. Vulvovaginal infections were the most common adverse effect during year 7–8 of use. These findings are consistent with those found at 6 years.20 ●

As abortion and contraception services become more difficult to access, patients may be more motivated to initiate or maintain an intrauterine device for longer. The ACCESS IUS trial provides contemporary data that are generalizable across the US population. Clinicians should educate patients about the efficacy, low incidence of new adverse events, and the steady rate at which patients experience absence of bleeding/spotting. The most recent data analysis supports continued use of LNG 52 mg IUD products for up to 8 years with an excellent extended safety profile. While some providers may express concern that patients may experience more bleeding with prolonged use, this study demonstrated low discontinuation rates due to bleeding in years 7 and 8. Perforations were diagnosed only during the first year, meaning that they most likely are related to the insertion process. Additionally, in this long-term study, expulsions occurred most frequently in the first year after placement. This study, which shows that the LNG IUD can continue to be used for longer than before, is important because it means that many patients will need fewer removals and reinsertions over their lifetime, reducing a patient’s risks and discomfort associated with these procedures. Sharing these data is important, as longer LNG IUD retention may reduce burdens faced by patients who desire long-acting reversible contraception.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
- Kirstein M, Jones RK, Philbin J. One month post-Roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. July 28, 2022. Accessed September 14, 2022. https://www.guttmacher.org /article/2022/07/one-month-post-roe-least-43-abortion-clinics-across -11-states-have-stopped-offering
- Foster DG, Hulett D, Bradsberry M, et al. Number of oral contraceptive pill packages dispensed and subsequent unintended pregnancies. Obstet Gynecol. 2011;117:566-572.
- Foster DG, Parvataneni R, de Bocanegra HT, et al. Number of oral contraceptive pill packages dispensed, method continuation, and costs. Obstet Gynecol. 2006;108:1107-114.
- Niu F, Cornelius J, Aboubechara N, et al. Real world outcomes related to providing an annual supply of short-acting hormonal contraceptives. Contraception. 2022;107:58-61.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. US selected practice recommendations for contraceptive use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Women’s sexual and reproductive health services: key findings from the 2017 Kaiser Women’s Health Survey. KFF: Kaiser Family Foundation. March 13, 2018. Accessed September 14, 2022. https://www.kff.org/womens-health-policy /issue-brief/womens-sexual-and-reproductive-health-services-key-findings -from-the-2017-kaiser-womens-health-survey/
- Nikpour G, Allen A, Rafie S, et al. Pharmacy implementation of a new law allowing year-long hormonal contraception supplies. Pharmacy (Basel). 2020;8:E165.
- Rodriguez MI, Edelman AB, Skye M, et al. Association of pharmacist prescription with dispensed duration of hormonal contraception. JAMA Netw Open. 2020;3:e205252.
- Insurance coverage of contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state-policy /explore/insurance-coverage-contraceptives
- Chim C, Sharma P. Pharmacists prescribing hormonal contraceptives: a status update. US Pharm. 2021;46:45-49.
- Rodriguez MI, Hersh A, Anderson LB, et al. Association of pharmacist prescription of hormonal contraception with unintended pregnancies and Medicaid costs. Obstet Gynecol. 2019;133:1238-1246.
- Pharmacist-prescribed contraceptives. Guttmacher Institute. Updated August 1, 2022. Accessed September 14, 2022. https://www.guttmacher.org/state -policy/explore/pharmacist-prescribed-contraceptives
- Anderson L, Hartung DM, Middleton L, et al. Pharmacist provision of hormonal contraception in the Oregon Medicaid population. Obstet Gynecol. 2019;133:1231-1237.
- Rodriguez MI, Edelman AB, Skye M, et al. Reasons for and experience in obtaining pharmacist prescribed contraception. Contraception. 2020;102:259-261.
- Rodriguez MI, Manibusan B, Kaufman M, et al. Association of pharmacist prescription of contraception with breaks in coverage. Obstet Gynecol. 2022;139:781-787.
- Pittman ME, Secura GM, Allsworth JE, et al. Understanding prescription adherence: pharmacy claims data from the Contraceptive CHOICE Project. Contraception. 2011;83:340-345.
- Rodriguez MI, Skye M, Edelman AB, et al. Association of pharmacist prescription and 12-month contraceptive continuation rates. Am J Obstet Gynecol. 2021;225:647.e1-647.e9.
- Secura GM, Allsworth JE, Madden T, et al. The Contraceptive CHOICE Project: reducing barriers to long-acting reversible contraception. Am J Obstet Gynecol. 2010;203:115.e1-7.
- Rowe P, Farley T, Peregoudov A, et al. Safety and efficacy in parous women of a 52-mg levonorgestrel-medicated intrauterine device: a 7-year randomized comparative study with the TCu380A. Contraception. 2016;93:498-506.
- Westhoff CL, Keder LM, Gangestad A, et al. Six-year contraceptive efficacy and continued safety of a levonorgestrel 52 mg intrauterine system. Contraception. 2020;101:159-161.
Disjointed states of America: The medical is political

Like many of you, I am an obstetrician-gynecologist who provides full-spectrum reproductive health care. Our jobs demand great intimacy—we are with patients as they meet their first born, learn of a miscarriage diagnosis, or decide to end their pregnancy. I have performed an uncomplicated, joyful vaginal delivery, then within an hour rushed a different patient’s gurney to the intensive care unit as she became acutely hypotensive and hypoxic, developing ARDS after a stillbirth. The care we provide is uniquely personal, and in that, has become deeply political. We have spent a long time here—news pundits, members of our family, even us—viewing abortion and reproductive health as something innately political. Although abortion is at the forefront of legislative interference and politicization, more than 1,300 abortion restrictions have been passed in the United States since Roe v Wade in 1973. It is not the only medical care affected by political interference.1 The United States ranks last in maternal mortality among industrialized nations, and Black women are more than twice as likely to die.2 As we grapple with the fallout of the Dobbs v Jackson Women’s Health Organization opinion and begin to recognize how fractured medical care has become—based on zip code—we should take stock of the way legislation and politics have already dictated reproductive health care. Abortion is the salient example, but state policy and legislation have unjustly been determining medical care available to women and other patients on a broader scale for decades. Here are just a few examples.
Postpartum care
The postpartum period is critical for maternal health; it is the time period in which many comorbidities emerge, including hypertensive disorders, postpartum thyroiditis, and mood disorders. Fifty percent of maternal deaths in the United States occur postpartum. Despite the importance of this care, Medicaid coverage for longer than 60 days postpartum varies greatly state to state. After the Affordable Care Act was implemented, it was assumed that all states would expand their Medicaid programs to include parents in their coverage plans beyond the guaranteed 60 days, negating the need for a specific postpartum coverage time period. However, the 2012 Supreme Court decision in National Federation of Independent Business v Sebelius allowed states to opt out of Medicaid expansion.3 In many states, postpartum patients lose their Medicaid insurance after 60 days if they do not meet the stringent income criteria.
The income level that makes patients ineligible for Medicaid coverage at day 61 postpartum varies widely. In Maryland, a patient can extend their Medicaid coverage for 12 months postpartum if their family of 4 earns less than $73,260 annually (264% of the federal poverty level). However, in Mississippi, an income of more than $6,936 per year for a family of 4 (approximately 25% of the federal poverty level) renders mothers who are 61 days postpartum ineligible for Medicaid coverage.4 Thus, many low-income postpartum patients (who are at twice the risk of maternal mortality as affluent patients) find themselves without access to this critical care depending on the decisions of their state legislatures.5 The American Rescue Plan Act of 2021 (known as the COVID-19 Stimulus Package) included a provision that allows states to expand their postpartum Medicaid coverage from 60 days to 12 months; currently, 10 additional states are planning to expand postpartum Medicaid for 12 months. While encouraging, 14 states still have not announced plans to utilize this provision or apply for a waiver to extend Medicaid coverage in the postpartum period.6
Treatment for substance use
Drug overdose is a leading cause of pregnancy-related death from unintentional causes.7 Overdose deaths in the general population climbed between 2020 and 2021, reaching historic highs of more than 100,000 deaths in a 12-month period.8 Given the impact of substance use and overdose on maternal mortality, health systems should be maximizing efforts to respond to this public health crisis by implementing effective screening and treatment interventions and establishing clinics and hospitals as safe places to seek care. However, many states have criminalized substance use in pregnant patients and mandate that clinicians report patients who use substances, creating an ethical dilemma for clinicians seeking to screen and treat patients for substance use disorder. Twenty-three states consider substance use in pregnancy to be child abuse, and 3 states consider substance use in pregnancy to be grounds for civil commitment. In Wisconsin, a patient can be detained against their will for the duration of the pregnancy. Twenty-five states require health care professionals to report suspected substance abuse in pregnancy to child protective services or a similar state office.9 Even when universal substance use screening is implemented, it has disparate impact on patients of color; Black women who screened positive for substance use in pregnancy were more likely to be reported to child protective services than their White counterparts.10 The criminalization of pregnant bodies does not lead to improvements in individual, community, or public health, it infringes on the ethical principle of bodily autonomy and puts clinicians at odds with what is best for their patients.
Gender-affirming care
Gender-affirming care is supported by major medical organizations and reduces the risk of depression and suicidality in transgender youth.11 Despite this evidence, several states have passed legislation restricting or banning this care, criminalizing the doctors who provide it. Idaho’s house of representatives passed House Bill 675,12 which would make providing gender-affirming care a felony, punishable by up to a life sentence. This would extend to parents trying to access care for their children as well as clinicians.
Although abortion is the medical care most conspicuously manipulated by politics and legislation, it is far from the only example. No area of medicine will be untouched by eliminating access to reproductive health care and by the regulation and criminalization of health care workers who provide it. This is a sea change, although state legislative interference and disparities in reproductive health care have been a tocsin of such change for years. We can no longer afford to believe there is a separation between politics and medicine; this directly interferes with our Hippocratic oath to do no harm. A politician in Ohio should not decide whether or not a 13-year-old patient should have to carry a pregnancy to term; the house of representatives in Idaho should not put someone’s transgender child at increased risk of depression and suicidality by making their medical care a felony. Colleagues in Texas should not be punishable by life in prison for providing abortion care.13 As a physician, I cannot stand by when, facing a maternal mortality crisis, state politicians decide whether a patient living below the poverty line should have access to postpartum care.
I am neither a politician nor a legal scholar. I am a physician who takes care of people in this intimate and powerful space of healing and support between doctor and patient. What should we do? We need to come together to find the answers. We need to vote if we haven’t before. And we need to vote differently if we have elected lawmakers who politicize and dangerously interfere with medicine, the well-being of our patients, and our ability to carry out our duty as physicians in our patients’ best interests. We need to tell our stories—to each other, to our newspapers, to our neighbors, and to our legislatures. If we are leading organizations, we can use the power held in our institutions to commit to providing care to the fullest extent possible, commit to protecting our clinicians providing evidence-based care, and encourage legislators who use medicine as a political bargaining chip to reverse course. Medicine is not an apolitical field, and we can no longer uphold that paradigm. Our patients lives, and our livelihood as healers and caretakers, depends on our collective action against it. ●
Acknowledgement
The author would like to thank Lauren Sobel, DO, MPH, for her contributions to a presentation on this subject.
- Nash E, Ephross P. State policy trends at midyear 2022: with Roe about to be overturned, some states double down on abortion restrictions. Guttmacher Institute. June 22, 2022. https://live. guttmacher.org/article/2022/06/state-policy -trends-midyear-2022-roe-about-be-overturnedsome-states-double-down. Accessed September 12, 2022.
- Declercq E, Zephyrin L. Maternal mortality in the United States: a primer. Commonwealth Fund; 2020. https://www.commonwealthfund .org/publications/issue-brief-report/2020 /dec/maternal-mortality-united-states-primer. Accessed September 12, 2022.
- Santa Clara Law Digital Communications website. Supreme Court of the United States. National Federation of Independent Business v Sebelius. (2012). Patient Protection and Affordable Care Act Litigation. 333. https://digitalcommons.law.scu.edu /aca/333. Accessed September 13, 2022.
- Ranji U, Salganicoff A, Gomez I. Postpartum coverage extension in the American Rescue Plan Act of 2021. San Francisco, CA: Kaiser Family Foundation; 2021.
- Singh GK, Lee H. Trends and racial/ethnic, socioeconomic, and geographic disparities in maternal mortality from indirect obstetric causes in the United States, 1999-2017. Int J MCH AIDS. 2021;10:43.
- Kaiser Family Foundation. Medicaid Postpartum Coverage Extension Tracker. https://www.kff. org/medicaid/issue-brief/medicaid-postpartum -coverage-extension-tracker/. Accessed September 7, 2022.
- Mehta PK, Bachhuber MA, Hoffman R, et al. Deaths from unintentional injury, homicide, and suicide during or within 1 year of pregnancy in Philadelphia. Am J Public Health. 2016;106: 2208-2210.
- O’Donnell J, Tanz LJ, Gladden RM, et al. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls— United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740.
- State laws and policies: substance use during pregnancy. Guttmacher Institute. https://www .guttmacher.org/state-policy/explore/substance -use-during-pregnancy. August 1, 2022. Accessed September 13, 2022.
- Roberts S, Nuru-Jeter A. Universal screening for alcohol and drug use and racial disparities in child protective services reporting. J Behav Health Serv Res. 2012;39;3-16.
- Tordoff DM, Wanta JW, Collin, et al. (2022). Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Network Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978.
- House Bill 675. Idaho Legislature web site. https:// legislature.idaho.gov/sessioninfo/2022/legislation/h0675/. Accessed September 9, 2022.
- Simon S. New Texas trigger law makes abortion a felony. NPR. August 27, 2022. https://www.npr. org/2022/08/27/1119795665/new-texas-trigger -law-makes-abortion-a-felony. Accessed September 13, 2022.

Like many of you, I am an obstetrician-gynecologist who provides full-spectrum reproductive health care. Our jobs demand great intimacy—we are with patients as they meet their first born, learn of a miscarriage diagnosis, or decide to end their pregnancy. I have performed an uncomplicated, joyful vaginal delivery, then within an hour rushed a different patient’s gurney to the intensive care unit as she became acutely hypotensive and hypoxic, developing ARDS after a stillbirth. The care we provide is uniquely personal, and in that, has become deeply political. We have spent a long time here—news pundits, members of our family, even us—viewing abortion and reproductive health as something innately political. Although abortion is at the forefront of legislative interference and politicization, more than 1,300 abortion restrictions have been passed in the United States since Roe v Wade in 1973. It is not the only medical care affected by political interference.1 The United States ranks last in maternal mortality among industrialized nations, and Black women are more than twice as likely to die.2 As we grapple with the fallout of the Dobbs v Jackson Women’s Health Organization opinion and begin to recognize how fractured medical care has become—based on zip code—we should take stock of the way legislation and politics have already dictated reproductive health care. Abortion is the salient example, but state policy and legislation have unjustly been determining medical care available to women and other patients on a broader scale for decades. Here are just a few examples.
Postpartum care
The postpartum period is critical for maternal health; it is the time period in which many comorbidities emerge, including hypertensive disorders, postpartum thyroiditis, and mood disorders. Fifty percent of maternal deaths in the United States occur postpartum. Despite the importance of this care, Medicaid coverage for longer than 60 days postpartum varies greatly state to state. After the Affordable Care Act was implemented, it was assumed that all states would expand their Medicaid programs to include parents in their coverage plans beyond the guaranteed 60 days, negating the need for a specific postpartum coverage time period. However, the 2012 Supreme Court decision in National Federation of Independent Business v Sebelius allowed states to opt out of Medicaid expansion.3 In many states, postpartum patients lose their Medicaid insurance after 60 days if they do not meet the stringent income criteria.
The income level that makes patients ineligible for Medicaid coverage at day 61 postpartum varies widely. In Maryland, a patient can extend their Medicaid coverage for 12 months postpartum if their family of 4 earns less than $73,260 annually (264% of the federal poverty level). However, in Mississippi, an income of more than $6,936 per year for a family of 4 (approximately 25% of the federal poverty level) renders mothers who are 61 days postpartum ineligible for Medicaid coverage.4 Thus, many low-income postpartum patients (who are at twice the risk of maternal mortality as affluent patients) find themselves without access to this critical care depending on the decisions of their state legislatures.5 The American Rescue Plan Act of 2021 (known as the COVID-19 Stimulus Package) included a provision that allows states to expand their postpartum Medicaid coverage from 60 days to 12 months; currently, 10 additional states are planning to expand postpartum Medicaid for 12 months. While encouraging, 14 states still have not announced plans to utilize this provision or apply for a waiver to extend Medicaid coverage in the postpartum period.6
Treatment for substance use
Drug overdose is a leading cause of pregnancy-related death from unintentional causes.7 Overdose deaths in the general population climbed between 2020 and 2021, reaching historic highs of more than 100,000 deaths in a 12-month period.8 Given the impact of substance use and overdose on maternal mortality, health systems should be maximizing efforts to respond to this public health crisis by implementing effective screening and treatment interventions and establishing clinics and hospitals as safe places to seek care. However, many states have criminalized substance use in pregnant patients and mandate that clinicians report patients who use substances, creating an ethical dilemma for clinicians seeking to screen and treat patients for substance use disorder. Twenty-three states consider substance use in pregnancy to be child abuse, and 3 states consider substance use in pregnancy to be grounds for civil commitment. In Wisconsin, a patient can be detained against their will for the duration of the pregnancy. Twenty-five states require health care professionals to report suspected substance abuse in pregnancy to child protective services or a similar state office.9 Even when universal substance use screening is implemented, it has disparate impact on patients of color; Black women who screened positive for substance use in pregnancy were more likely to be reported to child protective services than their White counterparts.10 The criminalization of pregnant bodies does not lead to improvements in individual, community, or public health, it infringes on the ethical principle of bodily autonomy and puts clinicians at odds with what is best for their patients.
Gender-affirming care
Gender-affirming care is supported by major medical organizations and reduces the risk of depression and suicidality in transgender youth.11 Despite this evidence, several states have passed legislation restricting or banning this care, criminalizing the doctors who provide it. Idaho’s house of representatives passed House Bill 675,12 which would make providing gender-affirming care a felony, punishable by up to a life sentence. This would extend to parents trying to access care for their children as well as clinicians.
Although abortion is the medical care most conspicuously manipulated by politics and legislation, it is far from the only example. No area of medicine will be untouched by eliminating access to reproductive health care and by the regulation and criminalization of health care workers who provide it. This is a sea change, although state legislative interference and disparities in reproductive health care have been a tocsin of such change for years. We can no longer afford to believe there is a separation between politics and medicine; this directly interferes with our Hippocratic oath to do no harm. A politician in Ohio should not decide whether or not a 13-year-old patient should have to carry a pregnancy to term; the house of representatives in Idaho should not put someone’s transgender child at increased risk of depression and suicidality by making their medical care a felony. Colleagues in Texas should not be punishable by life in prison for providing abortion care.13 As a physician, I cannot stand by when, facing a maternal mortality crisis, state politicians decide whether a patient living below the poverty line should have access to postpartum care.
I am neither a politician nor a legal scholar. I am a physician who takes care of people in this intimate and powerful space of healing and support between doctor and patient. What should we do? We need to come together to find the answers. We need to vote if we haven’t before. And we need to vote differently if we have elected lawmakers who politicize and dangerously interfere with medicine, the well-being of our patients, and our ability to carry out our duty as physicians in our patients’ best interests. We need to tell our stories—to each other, to our newspapers, to our neighbors, and to our legislatures. If we are leading organizations, we can use the power held in our institutions to commit to providing care to the fullest extent possible, commit to protecting our clinicians providing evidence-based care, and encourage legislators who use medicine as a political bargaining chip to reverse course. Medicine is not an apolitical field, and we can no longer uphold that paradigm. Our patients lives, and our livelihood as healers and caretakers, depends on our collective action against it. ●
Acknowledgement
The author would like to thank Lauren Sobel, DO, MPH, for her contributions to a presentation on this subject.

Like many of you, I am an obstetrician-gynecologist who provides full-spectrum reproductive health care. Our jobs demand great intimacy—we are with patients as they meet their first born, learn of a miscarriage diagnosis, or decide to end their pregnancy. I have performed an uncomplicated, joyful vaginal delivery, then within an hour rushed a different patient’s gurney to the intensive care unit as she became acutely hypotensive and hypoxic, developing ARDS after a stillbirth. The care we provide is uniquely personal, and in that, has become deeply political. We have spent a long time here—news pundits, members of our family, even us—viewing abortion and reproductive health as something innately political. Although abortion is at the forefront of legislative interference and politicization, more than 1,300 abortion restrictions have been passed in the United States since Roe v Wade in 1973. It is not the only medical care affected by political interference.1 The United States ranks last in maternal mortality among industrialized nations, and Black women are more than twice as likely to die.2 As we grapple with the fallout of the Dobbs v Jackson Women’s Health Organization opinion and begin to recognize how fractured medical care has become—based on zip code—we should take stock of the way legislation and politics have already dictated reproductive health care. Abortion is the salient example, but state policy and legislation have unjustly been determining medical care available to women and other patients on a broader scale for decades. Here are just a few examples.
Postpartum care
The postpartum period is critical for maternal health; it is the time period in which many comorbidities emerge, including hypertensive disorders, postpartum thyroiditis, and mood disorders. Fifty percent of maternal deaths in the United States occur postpartum. Despite the importance of this care, Medicaid coverage for longer than 60 days postpartum varies greatly state to state. After the Affordable Care Act was implemented, it was assumed that all states would expand their Medicaid programs to include parents in their coverage plans beyond the guaranteed 60 days, negating the need for a specific postpartum coverage time period. However, the 2012 Supreme Court decision in National Federation of Independent Business v Sebelius allowed states to opt out of Medicaid expansion.3 In many states, postpartum patients lose their Medicaid insurance after 60 days if they do not meet the stringent income criteria.
The income level that makes patients ineligible for Medicaid coverage at day 61 postpartum varies widely. In Maryland, a patient can extend their Medicaid coverage for 12 months postpartum if their family of 4 earns less than $73,260 annually (264% of the federal poverty level). However, in Mississippi, an income of more than $6,936 per year for a family of 4 (approximately 25% of the federal poverty level) renders mothers who are 61 days postpartum ineligible for Medicaid coverage.4 Thus, many low-income postpartum patients (who are at twice the risk of maternal mortality as affluent patients) find themselves without access to this critical care depending on the decisions of their state legislatures.5 The American Rescue Plan Act of 2021 (known as the COVID-19 Stimulus Package) included a provision that allows states to expand their postpartum Medicaid coverage from 60 days to 12 months; currently, 10 additional states are planning to expand postpartum Medicaid for 12 months. While encouraging, 14 states still have not announced plans to utilize this provision or apply for a waiver to extend Medicaid coverage in the postpartum period.6
Treatment for substance use
Drug overdose is a leading cause of pregnancy-related death from unintentional causes.7 Overdose deaths in the general population climbed between 2020 and 2021, reaching historic highs of more than 100,000 deaths in a 12-month period.8 Given the impact of substance use and overdose on maternal mortality, health systems should be maximizing efforts to respond to this public health crisis by implementing effective screening and treatment interventions and establishing clinics and hospitals as safe places to seek care. However, many states have criminalized substance use in pregnant patients and mandate that clinicians report patients who use substances, creating an ethical dilemma for clinicians seeking to screen and treat patients for substance use disorder. Twenty-three states consider substance use in pregnancy to be child abuse, and 3 states consider substance use in pregnancy to be grounds for civil commitment. In Wisconsin, a patient can be detained against their will for the duration of the pregnancy. Twenty-five states require health care professionals to report suspected substance abuse in pregnancy to child protective services or a similar state office.9 Even when universal substance use screening is implemented, it has disparate impact on patients of color; Black women who screened positive for substance use in pregnancy were more likely to be reported to child protective services than their White counterparts.10 The criminalization of pregnant bodies does not lead to improvements in individual, community, or public health, it infringes on the ethical principle of bodily autonomy and puts clinicians at odds with what is best for their patients.
Gender-affirming care
Gender-affirming care is supported by major medical organizations and reduces the risk of depression and suicidality in transgender youth.11 Despite this evidence, several states have passed legislation restricting or banning this care, criminalizing the doctors who provide it. Idaho’s house of representatives passed House Bill 675,12 which would make providing gender-affirming care a felony, punishable by up to a life sentence. This would extend to parents trying to access care for their children as well as clinicians.
Although abortion is the medical care most conspicuously manipulated by politics and legislation, it is far from the only example. No area of medicine will be untouched by eliminating access to reproductive health care and by the regulation and criminalization of health care workers who provide it. This is a sea change, although state legislative interference and disparities in reproductive health care have been a tocsin of such change for years. We can no longer afford to believe there is a separation between politics and medicine; this directly interferes with our Hippocratic oath to do no harm. A politician in Ohio should not decide whether or not a 13-year-old patient should have to carry a pregnancy to term; the house of representatives in Idaho should not put someone’s transgender child at increased risk of depression and suicidality by making their medical care a felony. Colleagues in Texas should not be punishable by life in prison for providing abortion care.13 As a physician, I cannot stand by when, facing a maternal mortality crisis, state politicians decide whether a patient living below the poverty line should have access to postpartum care.
I am neither a politician nor a legal scholar. I am a physician who takes care of people in this intimate and powerful space of healing and support between doctor and patient. What should we do? We need to come together to find the answers. We need to vote if we haven’t before. And we need to vote differently if we have elected lawmakers who politicize and dangerously interfere with medicine, the well-being of our patients, and our ability to carry out our duty as physicians in our patients’ best interests. We need to tell our stories—to each other, to our newspapers, to our neighbors, and to our legislatures. If we are leading organizations, we can use the power held in our institutions to commit to providing care to the fullest extent possible, commit to protecting our clinicians providing evidence-based care, and encourage legislators who use medicine as a political bargaining chip to reverse course. Medicine is not an apolitical field, and we can no longer uphold that paradigm. Our patients lives, and our livelihood as healers and caretakers, depends on our collective action against it. ●
Acknowledgement
The author would like to thank Lauren Sobel, DO, MPH, for her contributions to a presentation on this subject.
- Nash E, Ephross P. State policy trends at midyear 2022: with Roe about to be overturned, some states double down on abortion restrictions. Guttmacher Institute. June 22, 2022. https://live. guttmacher.org/article/2022/06/state-policy -trends-midyear-2022-roe-about-be-overturnedsome-states-double-down. Accessed September 12, 2022.
- Declercq E, Zephyrin L. Maternal mortality in the United States: a primer. Commonwealth Fund; 2020. https://www.commonwealthfund .org/publications/issue-brief-report/2020 /dec/maternal-mortality-united-states-primer. Accessed September 12, 2022.
- Santa Clara Law Digital Communications website. Supreme Court of the United States. National Federation of Independent Business v Sebelius. (2012). Patient Protection and Affordable Care Act Litigation. 333. https://digitalcommons.law.scu.edu /aca/333. Accessed September 13, 2022.
- Ranji U, Salganicoff A, Gomez I. Postpartum coverage extension in the American Rescue Plan Act of 2021. San Francisco, CA: Kaiser Family Foundation; 2021.
- Singh GK, Lee H. Trends and racial/ethnic, socioeconomic, and geographic disparities in maternal mortality from indirect obstetric causes in the United States, 1999-2017. Int J MCH AIDS. 2021;10:43.
- Kaiser Family Foundation. Medicaid Postpartum Coverage Extension Tracker. https://www.kff. org/medicaid/issue-brief/medicaid-postpartum -coverage-extension-tracker/. Accessed September 7, 2022.
- Mehta PK, Bachhuber MA, Hoffman R, et al. Deaths from unintentional injury, homicide, and suicide during or within 1 year of pregnancy in Philadelphia. Am J Public Health. 2016;106: 2208-2210.
- O’Donnell J, Tanz LJ, Gladden RM, et al. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls— United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740.
- State laws and policies: substance use during pregnancy. Guttmacher Institute. https://www .guttmacher.org/state-policy/explore/substance -use-during-pregnancy. August 1, 2022. Accessed September 13, 2022.
- Roberts S, Nuru-Jeter A. Universal screening for alcohol and drug use and racial disparities in child protective services reporting. J Behav Health Serv Res. 2012;39;3-16.
- Tordoff DM, Wanta JW, Collin, et al. (2022). Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Network Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978.
- House Bill 675. Idaho Legislature web site. https:// legislature.idaho.gov/sessioninfo/2022/legislation/h0675/. Accessed September 9, 2022.
- Simon S. New Texas trigger law makes abortion a felony. NPR. August 27, 2022. https://www.npr. org/2022/08/27/1119795665/new-texas-trigger -law-makes-abortion-a-felony. Accessed September 13, 2022.
- Nash E, Ephross P. State policy trends at midyear 2022: with Roe about to be overturned, some states double down on abortion restrictions. Guttmacher Institute. June 22, 2022. https://live. guttmacher.org/article/2022/06/state-policy -trends-midyear-2022-roe-about-be-overturnedsome-states-double-down. Accessed September 12, 2022.
- Declercq E, Zephyrin L. Maternal mortality in the United States: a primer. Commonwealth Fund; 2020. https://www.commonwealthfund .org/publications/issue-brief-report/2020 /dec/maternal-mortality-united-states-primer. Accessed September 12, 2022.
- Santa Clara Law Digital Communications website. Supreme Court of the United States. National Federation of Independent Business v Sebelius. (2012). Patient Protection and Affordable Care Act Litigation. 333. https://digitalcommons.law.scu.edu /aca/333. Accessed September 13, 2022.
- Ranji U, Salganicoff A, Gomez I. Postpartum coverage extension in the American Rescue Plan Act of 2021. San Francisco, CA: Kaiser Family Foundation; 2021.
- Singh GK, Lee H. Trends and racial/ethnic, socioeconomic, and geographic disparities in maternal mortality from indirect obstetric causes in the United States, 1999-2017. Int J MCH AIDS. 2021;10:43.
- Kaiser Family Foundation. Medicaid Postpartum Coverage Extension Tracker. https://www.kff. org/medicaid/issue-brief/medicaid-postpartum -coverage-extension-tracker/. Accessed September 7, 2022.
- Mehta PK, Bachhuber MA, Hoffman R, et al. Deaths from unintentional injury, homicide, and suicide during or within 1 year of pregnancy in Philadelphia. Am J Public Health. 2016;106: 2208-2210.
- O’Donnell J, Tanz LJ, Gladden RM, et al. Trends in and characteristics of drug overdose deaths involving illicitly manufactured fentanyls— United States, 2019–2020. MMWR Morb Mortal Wkly Rep. 2021;70:1740.
- State laws and policies: substance use during pregnancy. Guttmacher Institute. https://www .guttmacher.org/state-policy/explore/substance -use-during-pregnancy. August 1, 2022. Accessed September 13, 2022.
- Roberts S, Nuru-Jeter A. Universal screening for alcohol and drug use and racial disparities in child protective services reporting. J Behav Health Serv Res. 2012;39;3-16.
- Tordoff DM, Wanta JW, Collin, et al. (2022). Mental health outcomes in transgender and nonbinary youths receiving gender-affirming care. JAMA Network Open. 2022;5:e220978. doi: 10.1001/jamanetworkopen.2022.0978.
- House Bill 675. Idaho Legislature web site. https:// legislature.idaho.gov/sessioninfo/2022/legislation/h0675/. Accessed September 9, 2022.
- Simon S. New Texas trigger law makes abortion a felony. NPR. August 27, 2022. https://www.npr. org/2022/08/27/1119795665/new-texas-trigger -law-makes-abortion-a-felony. Accessed September 13, 2022.
Viral threats to the fetus and mother: Parvovirus and varicella
We review 2 important viral infections in this article. One, parvovirus, poses a major threat to the fetus. The second, varicella, poses less risk to the fetus but significantly greater risk to the mother. We focus on the epidemiology, clinical presentation, diagnosis, and management of each infection.
Parvovirus infection and its risks to the fetus
CASE #1 Pregnant teacher exposed to fifth disease
A 28-year-old primigravid woman at 16 weeks’ gestation works as an elementary school teacher. Over the past 3 weeks, she has been exposed to 4 children who had fifth disease. She now requests evaluation because she has malaise, arthralgias, myalgias, fever of 38.2°C, and a fine lacelike erythematous rash on her trunk, arms, and cheeks.
- What is the most likely diagnosis?
- What diagnostic tests are indicated?
- Is her fetus at risk?
Epidemiology of parvovirus
Parvovirus B19 is a small, single-stranded DNA virus. It is highly contagious and is transmitted primarily by respiratory droplets. Transmission also can occur via infected blood, for example, through a blood transfusion. The incubation period is 10 to 20 days. Among adults, the individuals at greatest risk for infection are those who have close contact with young children, such as parents, day-care workers, and elementary school teachers. With sustained exposure in the household or classroom, the risk of seroconversion approaches 50%.1 Approximately 50% to 60% of reproductive-aged women have evidence of prior infection, and immunity is usually lifelong.
Clinical manifestations
The classic presentation of parvovirus infection is erythema infectiosum, also called fifth disease. This condition is characterized by a “slapped cheek” facial rash, malaise, myalgias, arthralgias, and low-grade fever. A fine lacelike rash often develops over the torso. In adults, the characteristic rash may be absent, and the most common presentation is a flu-like illness with joint pains.1,2 In children and in adults with an underlying hemoglobinopathy, parvovirus can cause transient aplastic crisis, and patients present with signs of a severe anemia, such as dyspnea, pallor, and fatigue.
Although parvovirus infection usually poses no serious risk in otherwise healthy children and adults, it can cause major fetal injury when the pregnant woman is infected early in pregnancy. The principal manifestation of fetal infection is hydrops. Hydrops primarily results when the virus crosses the placenta and attaches to the P antigen on the surface of red cell progenitors in the fetal marrow, causing an aplastic anemia with resultant high-output congestive heart failure. The virus also may directly injure the fetal myocardium, thus exacerbating heart failure. Other manifestations of congenital parvovirus include thrombocytopenia and hepatitis.3
The severity of fetal injury is inversely proportional to the gestational age at the time of maternal infection. When primary maternal infection occurs in the first trimester, the frequency of fetal hydrops is 5% to 10%. If infection develops in weeks 13 to 20, the risk of hydrops decreases to 5% or less. If infection develops beyond week 20, the incidence of fetal hydrops is 1% or lower.2
Continue to: Diagnostic steps...
Diagnostic steps
Appropriate diagnostic evaluation for a pregnant woman with exposure to parvovirus or clinical manifestations suggestive of parvovirus infection is outlined in FIGURE 1.
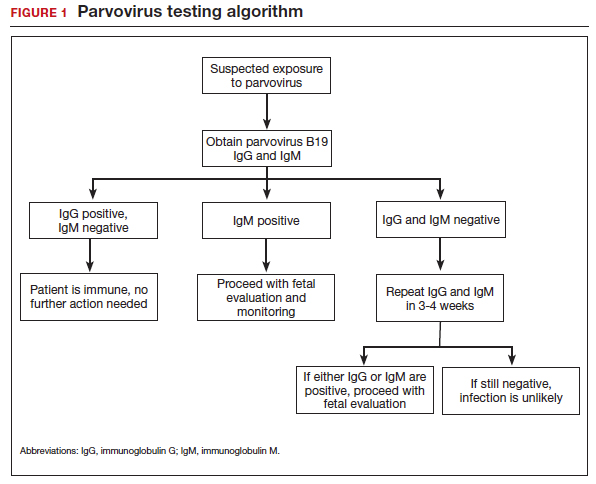
If infection is confirmed, serial ultrasound monitoring should be performed on a weekly to biweekly basis for 8 to 12 weeks, as delineated in FIGURE 2. Extended surveillance is necessary because the incubation period in the fetus is longer than that in the mother.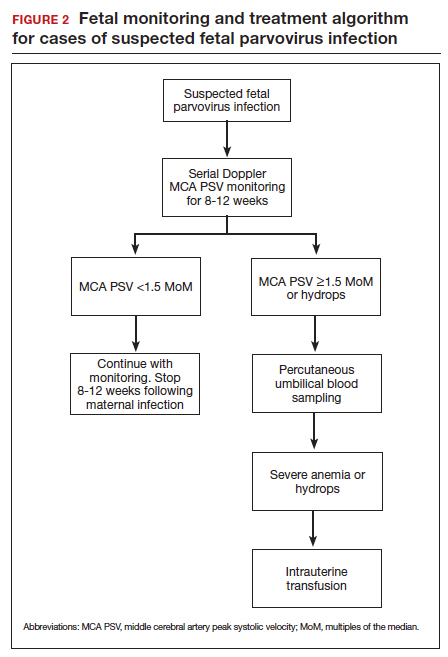
As the fetus develops anemia, peripheral tissues become hypoxic, leading to reflex peripheral vasoconstriction and increased cardiac output. At the same time, reduction in the number of fetal red blood cells decreases blood viscosity. The combination of these changes results in an increase in blood flow to the fetal brain, which can be detected by measuring the peak systolic velocity of flow in the middle cerebral artery (MCA PSV) with Doppler ultrasound imaging (FIGURE 3). The increase in MCA PSV parallels the decrease in fetal hematocrit and precedes the development of hydrops. In fact, signs of fetal hydrops do not usually develop until the fetal hematocrit falls to 15 to 20 vol%.
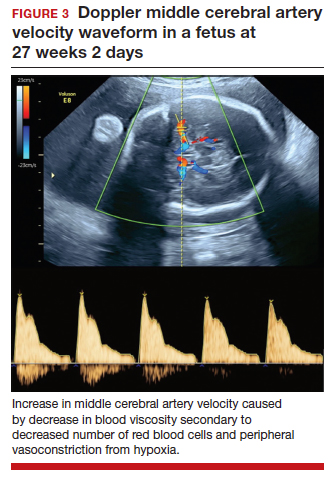
Management may necessitate intrauterine transfusion
Although some cases of fetal hydrops may resolve spontaneously, most authors agree that intrauterine transfusion is essential. In most instances, only a single intrauterine transfusion is necessary. In some fetuses, however, the infection is so prolonged and the anemia so severe that 2 to 3 transfusions may be required.
Infants who survive the intrauterine transfusion usually have an excellent long-term prognosis. However, isolated case reports have documented neurologic morbidity and prolonged transfusion-dependent anemia.4 In light of these reports, we recommend that a third trimester ultrasound exam be performed to assess fetal growth and evaluate the anatomy of the fetal brain. For the fetus with abnormal intracranial findings on ultrasonography, fetal magnetic resonance imaging is indicated.5
CASE #1 Diagnosis is probable parvovirus
The most likely diagnosis in this case is erythema infectiosum. This diagnosis can be confirmed by identifying positive immunogloblulin M (IgM) antibody and by detecting parvovirus in the maternal serum by polymerase chain reaction. Given the gestational age of 16 weeks, the risk of serious fetal injury should be less than 5%. Nevertheless, serial ultrasound examinations should be performed to assess for signs of fetal anemia.
Varicella exposure in pregnancy
CASE #2 Pregnant woman exposed to chickenpox has symptoms
Two weeks ago, a 32-year-old woman (G3P2002) at 24 weeks’ gestation was exposed to a neighbor’s child who had chickenpox. The patient has no history of natural infection or vaccination. She now has a fever of 38.6°C, malaise, headache, and a diffuse pruritic vesicular rash on her trunk and extremities. She also is experiencing a dry cough and mild dyspnea.
- What diagnostic tests are indicated?
- What treatment is indicated?
- What risk does this condition pose to the fetus?
Epidemiology of varicella
Varicella (chickenpox) is caused by the DNA varicella-zoster virus, an organism that is a member of the herpesvirus family. The disease occurs predominantly in children, and the infection is transmitted by respiratory droplets and by direct contact. Its incubation period is short (10–14 days), and it is highly contagious. More than 90% of susceptible close contacts will become infected after exposure to the index case. Like other herpesviruses, the varicella virus can establish a latent infection and then become manifest years later as herpes zoster (shingles).5,6
Continue to: Clinical manifestations...
Clinical manifestations
Patients with varicella usually have prodromal symptoms and signs that include malaise, fatigue, arthralgias, myalgias, and a low-grade fever. Varicella’s pathognomonic manifestation is a pruritic, macular rash that starts on the face and trunk and then spreads centripetally to the extremities. The lesions typically appear in “crops” and evolve through several distinct phases: macule, papule, vesicle, pustule, ulcer, and crust.5
In children, varicella is manifest almost entirely by mucocutaneous lesions. In adults, however, 2 serious and potentially life-threatening complications can occur. Approximately 1% of infected adults develop encephalitis and about 20% develop viral pneumonia, often accompanied by a severe superimposed bacterial pneumonia.5
When maternal infection develops in the first half of pregnancy, approximately 2% of fetuses will have evidence of congenital infection, usually manifested by circular, constricting scars on the extremities. These lesions typically occur in a dermatomal distribution. Spontaneous abortion and fetal death in utero also have been reported, but fortunately they are quite rare. When maternal infection occurs beyond 20 weeks of gestation, fetal injury is very uncommon.7
Interestingly, when maternal infection occurs at the time of delivery or shortly thereafter (from 5 days before until 2 days after delivery), neonatal varicella may develop. This infection may take 3 forms: disseminated mucocutaneous lesions, a deep-seated visceral infection, or severe pneumonia. In the era before the ready availability of antiviral agents, the case fatality rate from neonatal varicella was approximately 30%.5
Diagnosis is clinical
The diagnosis of varicella usually is established on the basis of clinical examination. It can be confirmed by identification of anti–varicella-zoster IgM.
Management includes assessing immunity
If a patient is seen for a preconception appointment, ask her whether she has ever had varicella or been vaccinated for this disease. If she is uncertain, a varicella-zoster immunoglobulin G (IgG) titer should be ordered. If the IgG titer is negative, denoting susceptibility to infection, the patient should be vaccinated before she tries to conceive (see below).8
If a patient has not had a preconception appointment and now presents for her first prenatal appointment, she should be asked about immunity to varicella. If she is uncertain, a varicella-zoster IgG assay should be obtained. Approximately 75% of patients who are uncertain about immunity will, in fact, be immune. Those who are not immune should be counseled to avoid exposure to individuals who may have varicella, and they should be targeted for vaccination immediately postpartum.5,9
If a susceptible pregnant patient has been exposed to an individual with varicella, she should receive 1 of 2 regimens within 72 to 96 hours to minimize the risk of maternal infection.5,9,10 One option is intramuscular varicella-zoster immune globulin (VariZIG), 125 U/10 kg body weight, with a maximum dose of 625 U (5 vials). The distributor of this agent is FFF Enterprises in Temucula, California (telephone: 800-843-7477). A company representative will assess the patient’s eligibility and deliver the drug within 24 hours if the patient is considered eligible. An alternative prophylactic regimen is oral acyclovir, 800 mg 5 times daily for 7 days, or oral valacyclovir, 1,000 mg 3 times daily for 7 days.
If, despite prophylaxis, the pregnant woman becomes infected, she should immediately be treated with 1 of the oral antiviral regimens described above. If she has evidence of encephalitis, pneumonia, or severe disseminated mucocutaneous infection, or if she is immunosuppressed, she should be hospitalized and treated with intravenous acyclovir, 10 mg/kg infused over 1 hour every 8 hours for 10 days.
Ultrasonography is the most valuable test to identify fetal infection. Key findings that suggest congenital varicella are fetal growth restriction, microcephaly, ventriculomegaly, echogenic foci in the liver, and limb abnormalities. There is no proven therapy for congenital varicella.
When a patient has varicella at the time of delivery, she should be isolated from her infant until all lesions have crusted over. In addition, the neonate should be treated with either VariZIG or an antiviral agent.5,9
Prevention with varicella vaccine
The varicella vaccine (Varivax) is a live-virus vaccine that is highly immunogenic. The vaccine is now part of the routine childhood immunization sequence. Children ages 1 to 12 years require only a single dose of the vaccine. Individuals older than 12 years of age require 2 doses, administered 4 to 6 weeks apart. The vaccine should not be administered during pregnancy. It also should not be administered to individuals who are severely immunocompromised, are receiving high-dose systemic steroids, have untreated tuberculosis, or have an allergy to neomycin, which is a component of the vaccine. The vaccine does not pose a risk to the breastfeeding infant.11
CASE #2 Hospitalization is recommended for this patient
The patient in this case developed acute varicella pneumonia as a result of her exposure to the neighbor’s child. The diagnosis can be confirmed by demonstrating a positive varicella-zoster IgM and by obtaining a chest x-ray that identifies the diffuse patchy infiltrates characteristic of viral pneumonia. Because this is such a potentially serious illness, the patient should be hospitalized and treated with intravenous acyclovir or valacyclovir. Antibiotics such as ceftriaxone and azithromycin may be indicated to treat superimposed bacterial pneumonia. Given the later gestational age, the fetus is at low risk for serious injury. ●
- Valeur-Jensen AK, Pedersen CB, Westergaard T, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA. 1999;281:1099-1105.
- Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
- Melamed N, Whittle W, Kelly EN, et al. Fetal thrombocytopenia in pregnancies with fetal human parvovirus-B19 infection. Am J Obstet Gynecol. 2015;212:793.e1-8.
- Nagel HTC, de Haan TR, Vandenbussche FPH, et al. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109:42-47.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al (eds). Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:911-912.
- Cohen JI. Herpes zoster. N Engl J Med. 2013;369:255-263.
- Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548-1551.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165.
- Marin M, Guris D, Chaves SS, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of varicella. MMWR Recommend Rep. 2007;56(RR-4):1-40.
- Swamy GK, Dotters-Katz SK. Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy. Am J Obstet Gynecol. 2019;221:655-656.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65.
We review 2 important viral infections in this article. One, parvovirus, poses a major threat to the fetus. The second, varicella, poses less risk to the fetus but significantly greater risk to the mother. We focus on the epidemiology, clinical presentation, diagnosis, and management of each infection.
Parvovirus infection and its risks to the fetus
CASE #1 Pregnant teacher exposed to fifth disease
A 28-year-old primigravid woman at 16 weeks’ gestation works as an elementary school teacher. Over the past 3 weeks, she has been exposed to 4 children who had fifth disease. She now requests evaluation because she has malaise, arthralgias, myalgias, fever of 38.2°C, and a fine lacelike erythematous rash on her trunk, arms, and cheeks.
- What is the most likely diagnosis?
- What diagnostic tests are indicated?
- Is her fetus at risk?
Epidemiology of parvovirus
Parvovirus B19 is a small, single-stranded DNA virus. It is highly contagious and is transmitted primarily by respiratory droplets. Transmission also can occur via infected blood, for example, through a blood transfusion. The incubation period is 10 to 20 days. Among adults, the individuals at greatest risk for infection are those who have close contact with young children, such as parents, day-care workers, and elementary school teachers. With sustained exposure in the household or classroom, the risk of seroconversion approaches 50%.1 Approximately 50% to 60% of reproductive-aged women have evidence of prior infection, and immunity is usually lifelong.
Clinical manifestations
The classic presentation of parvovirus infection is erythema infectiosum, also called fifth disease. This condition is characterized by a “slapped cheek” facial rash, malaise, myalgias, arthralgias, and low-grade fever. A fine lacelike rash often develops over the torso. In adults, the characteristic rash may be absent, and the most common presentation is a flu-like illness with joint pains.1,2 In children and in adults with an underlying hemoglobinopathy, parvovirus can cause transient aplastic crisis, and patients present with signs of a severe anemia, such as dyspnea, pallor, and fatigue.
Although parvovirus infection usually poses no serious risk in otherwise healthy children and adults, it can cause major fetal injury when the pregnant woman is infected early in pregnancy. The principal manifestation of fetal infection is hydrops. Hydrops primarily results when the virus crosses the placenta and attaches to the P antigen on the surface of red cell progenitors in the fetal marrow, causing an aplastic anemia with resultant high-output congestive heart failure. The virus also may directly injure the fetal myocardium, thus exacerbating heart failure. Other manifestations of congenital parvovirus include thrombocytopenia and hepatitis.3
The severity of fetal injury is inversely proportional to the gestational age at the time of maternal infection. When primary maternal infection occurs in the first trimester, the frequency of fetal hydrops is 5% to 10%. If infection develops in weeks 13 to 20, the risk of hydrops decreases to 5% or less. If infection develops beyond week 20, the incidence of fetal hydrops is 1% or lower.2
Continue to: Diagnostic steps...
Diagnostic steps
Appropriate diagnostic evaluation for a pregnant woman with exposure to parvovirus or clinical manifestations suggestive of parvovirus infection is outlined in FIGURE 1.

If infection is confirmed, serial ultrasound monitoring should be performed on a weekly to biweekly basis for 8 to 12 weeks, as delineated in FIGURE 2. Extended surveillance is necessary because the incubation period in the fetus is longer than that in the mother.
As the fetus develops anemia, peripheral tissues become hypoxic, leading to reflex peripheral vasoconstriction and increased cardiac output. At the same time, reduction in the number of fetal red blood cells decreases blood viscosity. The combination of these changes results in an increase in blood flow to the fetal brain, which can be detected by measuring the peak systolic velocity of flow in the middle cerebral artery (MCA PSV) with Doppler ultrasound imaging (FIGURE 3). The increase in MCA PSV parallels the decrease in fetal hematocrit and precedes the development of hydrops. In fact, signs of fetal hydrops do not usually develop until the fetal hematocrit falls to 15 to 20 vol%.

Management may necessitate intrauterine transfusion
Although some cases of fetal hydrops may resolve spontaneously, most authors agree that intrauterine transfusion is essential. In most instances, only a single intrauterine transfusion is necessary. In some fetuses, however, the infection is so prolonged and the anemia so severe that 2 to 3 transfusions may be required.
Infants who survive the intrauterine transfusion usually have an excellent long-term prognosis. However, isolated case reports have documented neurologic morbidity and prolonged transfusion-dependent anemia.4 In light of these reports, we recommend that a third trimester ultrasound exam be performed to assess fetal growth and evaluate the anatomy of the fetal brain. For the fetus with abnormal intracranial findings on ultrasonography, fetal magnetic resonance imaging is indicated.5
CASE #1 Diagnosis is probable parvovirus
The most likely diagnosis in this case is erythema infectiosum. This diagnosis can be confirmed by identifying positive immunogloblulin M (IgM) antibody and by detecting parvovirus in the maternal serum by polymerase chain reaction. Given the gestational age of 16 weeks, the risk of serious fetal injury should be less than 5%. Nevertheless, serial ultrasound examinations should be performed to assess for signs of fetal anemia.
Varicella exposure in pregnancy
CASE #2 Pregnant woman exposed to chickenpox has symptoms
Two weeks ago, a 32-year-old woman (G3P2002) at 24 weeks’ gestation was exposed to a neighbor’s child who had chickenpox. The patient has no history of natural infection or vaccination. She now has a fever of 38.6°C, malaise, headache, and a diffuse pruritic vesicular rash on her trunk and extremities. She also is experiencing a dry cough and mild dyspnea.
- What diagnostic tests are indicated?
- What treatment is indicated?
- What risk does this condition pose to the fetus?
Epidemiology of varicella
Varicella (chickenpox) is caused by the DNA varicella-zoster virus, an organism that is a member of the herpesvirus family. The disease occurs predominantly in children, and the infection is transmitted by respiratory droplets and by direct contact. Its incubation period is short (10–14 days), and it is highly contagious. More than 90% of susceptible close contacts will become infected after exposure to the index case. Like other herpesviruses, the varicella virus can establish a latent infection and then become manifest years later as herpes zoster (shingles).5,6
Continue to: Clinical manifestations...
Clinical manifestations
Patients with varicella usually have prodromal symptoms and signs that include malaise, fatigue, arthralgias, myalgias, and a low-grade fever. Varicella’s pathognomonic manifestation is a pruritic, macular rash that starts on the face and trunk and then spreads centripetally to the extremities. The lesions typically appear in “crops” and evolve through several distinct phases: macule, papule, vesicle, pustule, ulcer, and crust.5
In children, varicella is manifest almost entirely by mucocutaneous lesions. In adults, however, 2 serious and potentially life-threatening complications can occur. Approximately 1% of infected adults develop encephalitis and about 20% develop viral pneumonia, often accompanied by a severe superimposed bacterial pneumonia.5
When maternal infection develops in the first half of pregnancy, approximately 2% of fetuses will have evidence of congenital infection, usually manifested by circular, constricting scars on the extremities. These lesions typically occur in a dermatomal distribution. Spontaneous abortion and fetal death in utero also have been reported, but fortunately they are quite rare. When maternal infection occurs beyond 20 weeks of gestation, fetal injury is very uncommon.7
Interestingly, when maternal infection occurs at the time of delivery or shortly thereafter (from 5 days before until 2 days after delivery), neonatal varicella may develop. This infection may take 3 forms: disseminated mucocutaneous lesions, a deep-seated visceral infection, or severe pneumonia. In the era before the ready availability of antiviral agents, the case fatality rate from neonatal varicella was approximately 30%.5
Diagnosis is clinical
The diagnosis of varicella usually is established on the basis of clinical examination. It can be confirmed by identification of anti–varicella-zoster IgM.
Management includes assessing immunity
If a patient is seen for a preconception appointment, ask her whether she has ever had varicella or been vaccinated for this disease. If she is uncertain, a varicella-zoster immunoglobulin G (IgG) titer should be ordered. If the IgG titer is negative, denoting susceptibility to infection, the patient should be vaccinated before she tries to conceive (see below).8
If a patient has not had a preconception appointment and now presents for her first prenatal appointment, she should be asked about immunity to varicella. If she is uncertain, a varicella-zoster IgG assay should be obtained. Approximately 75% of patients who are uncertain about immunity will, in fact, be immune. Those who are not immune should be counseled to avoid exposure to individuals who may have varicella, and they should be targeted for vaccination immediately postpartum.5,9
If a susceptible pregnant patient has been exposed to an individual with varicella, she should receive 1 of 2 regimens within 72 to 96 hours to minimize the risk of maternal infection.5,9,10 One option is intramuscular varicella-zoster immune globulin (VariZIG), 125 U/10 kg body weight, with a maximum dose of 625 U (5 vials). The distributor of this agent is FFF Enterprises in Temucula, California (telephone: 800-843-7477). A company representative will assess the patient’s eligibility and deliver the drug within 24 hours if the patient is considered eligible. An alternative prophylactic regimen is oral acyclovir, 800 mg 5 times daily for 7 days, or oral valacyclovir, 1,000 mg 3 times daily for 7 days.
If, despite prophylaxis, the pregnant woman becomes infected, she should immediately be treated with 1 of the oral antiviral regimens described above. If she has evidence of encephalitis, pneumonia, or severe disseminated mucocutaneous infection, or if she is immunosuppressed, she should be hospitalized and treated with intravenous acyclovir, 10 mg/kg infused over 1 hour every 8 hours for 10 days.
Ultrasonography is the most valuable test to identify fetal infection. Key findings that suggest congenital varicella are fetal growth restriction, microcephaly, ventriculomegaly, echogenic foci in the liver, and limb abnormalities. There is no proven therapy for congenital varicella.
When a patient has varicella at the time of delivery, she should be isolated from her infant until all lesions have crusted over. In addition, the neonate should be treated with either VariZIG or an antiviral agent.5,9
Prevention with varicella vaccine
The varicella vaccine (Varivax) is a live-virus vaccine that is highly immunogenic. The vaccine is now part of the routine childhood immunization sequence. Children ages 1 to 12 years require only a single dose of the vaccine. Individuals older than 12 years of age require 2 doses, administered 4 to 6 weeks apart. The vaccine should not be administered during pregnancy. It also should not be administered to individuals who are severely immunocompromised, are receiving high-dose systemic steroids, have untreated tuberculosis, or have an allergy to neomycin, which is a component of the vaccine. The vaccine does not pose a risk to the breastfeeding infant.11
CASE #2 Hospitalization is recommended for this patient
The patient in this case developed acute varicella pneumonia as a result of her exposure to the neighbor’s child. The diagnosis can be confirmed by demonstrating a positive varicella-zoster IgM and by obtaining a chest x-ray that identifies the diffuse patchy infiltrates characteristic of viral pneumonia. Because this is such a potentially serious illness, the patient should be hospitalized and treated with intravenous acyclovir or valacyclovir. Antibiotics such as ceftriaxone and azithromycin may be indicated to treat superimposed bacterial pneumonia. Given the later gestational age, the fetus is at low risk for serious injury. ●
We review 2 important viral infections in this article. One, parvovirus, poses a major threat to the fetus. The second, varicella, poses less risk to the fetus but significantly greater risk to the mother. We focus on the epidemiology, clinical presentation, diagnosis, and management of each infection.
Parvovirus infection and its risks to the fetus
CASE #1 Pregnant teacher exposed to fifth disease
A 28-year-old primigravid woman at 16 weeks’ gestation works as an elementary school teacher. Over the past 3 weeks, she has been exposed to 4 children who had fifth disease. She now requests evaluation because she has malaise, arthralgias, myalgias, fever of 38.2°C, and a fine lacelike erythematous rash on her trunk, arms, and cheeks.
- What is the most likely diagnosis?
- What diagnostic tests are indicated?
- Is her fetus at risk?
Epidemiology of parvovirus
Parvovirus B19 is a small, single-stranded DNA virus. It is highly contagious and is transmitted primarily by respiratory droplets. Transmission also can occur via infected blood, for example, through a blood transfusion. The incubation period is 10 to 20 days. Among adults, the individuals at greatest risk for infection are those who have close contact with young children, such as parents, day-care workers, and elementary school teachers. With sustained exposure in the household or classroom, the risk of seroconversion approaches 50%.1 Approximately 50% to 60% of reproductive-aged women have evidence of prior infection, and immunity is usually lifelong.
Clinical manifestations
The classic presentation of parvovirus infection is erythema infectiosum, also called fifth disease. This condition is characterized by a “slapped cheek” facial rash, malaise, myalgias, arthralgias, and low-grade fever. A fine lacelike rash often develops over the torso. In adults, the characteristic rash may be absent, and the most common presentation is a flu-like illness with joint pains.1,2 In children and in adults with an underlying hemoglobinopathy, parvovirus can cause transient aplastic crisis, and patients present with signs of a severe anemia, such as dyspnea, pallor, and fatigue.
Although parvovirus infection usually poses no serious risk in otherwise healthy children and adults, it can cause major fetal injury when the pregnant woman is infected early in pregnancy. The principal manifestation of fetal infection is hydrops. Hydrops primarily results when the virus crosses the placenta and attaches to the P antigen on the surface of red cell progenitors in the fetal marrow, causing an aplastic anemia with resultant high-output congestive heart failure. The virus also may directly injure the fetal myocardium, thus exacerbating heart failure. Other manifestations of congenital parvovirus include thrombocytopenia and hepatitis.3
The severity of fetal injury is inversely proportional to the gestational age at the time of maternal infection. When primary maternal infection occurs in the first trimester, the frequency of fetal hydrops is 5% to 10%. If infection develops in weeks 13 to 20, the risk of hydrops decreases to 5% or less. If infection develops beyond week 20, the incidence of fetal hydrops is 1% or lower.2
Continue to: Diagnostic steps...
Diagnostic steps
Appropriate diagnostic evaluation for a pregnant woman with exposure to parvovirus or clinical manifestations suggestive of parvovirus infection is outlined in FIGURE 1.

If infection is confirmed, serial ultrasound monitoring should be performed on a weekly to biweekly basis for 8 to 12 weeks, as delineated in FIGURE 2. Extended surveillance is necessary because the incubation period in the fetus is longer than that in the mother.
As the fetus develops anemia, peripheral tissues become hypoxic, leading to reflex peripheral vasoconstriction and increased cardiac output. At the same time, reduction in the number of fetal red blood cells decreases blood viscosity. The combination of these changes results in an increase in blood flow to the fetal brain, which can be detected by measuring the peak systolic velocity of flow in the middle cerebral artery (MCA PSV) with Doppler ultrasound imaging (FIGURE 3). The increase in MCA PSV parallels the decrease in fetal hematocrit and precedes the development of hydrops. In fact, signs of fetal hydrops do not usually develop until the fetal hematocrit falls to 15 to 20 vol%.

Management may necessitate intrauterine transfusion
Although some cases of fetal hydrops may resolve spontaneously, most authors agree that intrauterine transfusion is essential. In most instances, only a single intrauterine transfusion is necessary. In some fetuses, however, the infection is so prolonged and the anemia so severe that 2 to 3 transfusions may be required.
Infants who survive the intrauterine transfusion usually have an excellent long-term prognosis. However, isolated case reports have documented neurologic morbidity and prolonged transfusion-dependent anemia.4 In light of these reports, we recommend that a third trimester ultrasound exam be performed to assess fetal growth and evaluate the anatomy of the fetal brain. For the fetus with abnormal intracranial findings on ultrasonography, fetal magnetic resonance imaging is indicated.5
CASE #1 Diagnosis is probable parvovirus
The most likely diagnosis in this case is erythema infectiosum. This diagnosis can be confirmed by identifying positive immunogloblulin M (IgM) antibody and by detecting parvovirus in the maternal serum by polymerase chain reaction. Given the gestational age of 16 weeks, the risk of serious fetal injury should be less than 5%. Nevertheless, serial ultrasound examinations should be performed to assess for signs of fetal anemia.
Varicella exposure in pregnancy
CASE #2 Pregnant woman exposed to chickenpox has symptoms
Two weeks ago, a 32-year-old woman (G3P2002) at 24 weeks’ gestation was exposed to a neighbor’s child who had chickenpox. The patient has no history of natural infection or vaccination. She now has a fever of 38.6°C, malaise, headache, and a diffuse pruritic vesicular rash on her trunk and extremities. She also is experiencing a dry cough and mild dyspnea.
- What diagnostic tests are indicated?
- What treatment is indicated?
- What risk does this condition pose to the fetus?
Epidemiology of varicella
Varicella (chickenpox) is caused by the DNA varicella-zoster virus, an organism that is a member of the herpesvirus family. The disease occurs predominantly in children, and the infection is transmitted by respiratory droplets and by direct contact. Its incubation period is short (10–14 days), and it is highly contagious. More than 90% of susceptible close contacts will become infected after exposure to the index case. Like other herpesviruses, the varicella virus can establish a latent infection and then become manifest years later as herpes zoster (shingles).5,6
Continue to: Clinical manifestations...
Clinical manifestations
Patients with varicella usually have prodromal symptoms and signs that include malaise, fatigue, arthralgias, myalgias, and a low-grade fever. Varicella’s pathognomonic manifestation is a pruritic, macular rash that starts on the face and trunk and then spreads centripetally to the extremities. The lesions typically appear in “crops” and evolve through several distinct phases: macule, papule, vesicle, pustule, ulcer, and crust.5
In children, varicella is manifest almost entirely by mucocutaneous lesions. In adults, however, 2 serious and potentially life-threatening complications can occur. Approximately 1% of infected adults develop encephalitis and about 20% develop viral pneumonia, often accompanied by a severe superimposed bacterial pneumonia.5
When maternal infection develops in the first half of pregnancy, approximately 2% of fetuses will have evidence of congenital infection, usually manifested by circular, constricting scars on the extremities. These lesions typically occur in a dermatomal distribution. Spontaneous abortion and fetal death in utero also have been reported, but fortunately they are quite rare. When maternal infection occurs beyond 20 weeks of gestation, fetal injury is very uncommon.7
Interestingly, when maternal infection occurs at the time of delivery or shortly thereafter (from 5 days before until 2 days after delivery), neonatal varicella may develop. This infection may take 3 forms: disseminated mucocutaneous lesions, a deep-seated visceral infection, or severe pneumonia. In the era before the ready availability of antiviral agents, the case fatality rate from neonatal varicella was approximately 30%.5
Diagnosis is clinical
The diagnosis of varicella usually is established on the basis of clinical examination. It can be confirmed by identification of anti–varicella-zoster IgM.
Management includes assessing immunity
If a patient is seen for a preconception appointment, ask her whether she has ever had varicella or been vaccinated for this disease. If she is uncertain, a varicella-zoster immunoglobulin G (IgG) titer should be ordered. If the IgG titer is negative, denoting susceptibility to infection, the patient should be vaccinated before she tries to conceive (see below).8
If a patient has not had a preconception appointment and now presents for her first prenatal appointment, she should be asked about immunity to varicella. If she is uncertain, a varicella-zoster IgG assay should be obtained. Approximately 75% of patients who are uncertain about immunity will, in fact, be immune. Those who are not immune should be counseled to avoid exposure to individuals who may have varicella, and they should be targeted for vaccination immediately postpartum.5,9
If a susceptible pregnant patient has been exposed to an individual with varicella, she should receive 1 of 2 regimens within 72 to 96 hours to minimize the risk of maternal infection.5,9,10 One option is intramuscular varicella-zoster immune globulin (VariZIG), 125 U/10 kg body weight, with a maximum dose of 625 U (5 vials). The distributor of this agent is FFF Enterprises in Temucula, California (telephone: 800-843-7477). A company representative will assess the patient’s eligibility and deliver the drug within 24 hours if the patient is considered eligible. An alternative prophylactic regimen is oral acyclovir, 800 mg 5 times daily for 7 days, or oral valacyclovir, 1,000 mg 3 times daily for 7 days.
If, despite prophylaxis, the pregnant woman becomes infected, she should immediately be treated with 1 of the oral antiviral regimens described above. If she has evidence of encephalitis, pneumonia, or severe disseminated mucocutaneous infection, or if she is immunosuppressed, she should be hospitalized and treated with intravenous acyclovir, 10 mg/kg infused over 1 hour every 8 hours for 10 days.
Ultrasonography is the most valuable test to identify fetal infection. Key findings that suggest congenital varicella are fetal growth restriction, microcephaly, ventriculomegaly, echogenic foci in the liver, and limb abnormalities. There is no proven therapy for congenital varicella.
When a patient has varicella at the time of delivery, she should be isolated from her infant until all lesions have crusted over. In addition, the neonate should be treated with either VariZIG or an antiviral agent.5,9
Prevention with varicella vaccine
The varicella vaccine (Varivax) is a live-virus vaccine that is highly immunogenic. The vaccine is now part of the routine childhood immunization sequence. Children ages 1 to 12 years require only a single dose of the vaccine. Individuals older than 12 years of age require 2 doses, administered 4 to 6 weeks apart. The vaccine should not be administered during pregnancy. It also should not be administered to individuals who are severely immunocompromised, are receiving high-dose systemic steroids, have untreated tuberculosis, or have an allergy to neomycin, which is a component of the vaccine. The vaccine does not pose a risk to the breastfeeding infant.11
CASE #2 Hospitalization is recommended for this patient
The patient in this case developed acute varicella pneumonia as a result of her exposure to the neighbor’s child. The diagnosis can be confirmed by demonstrating a positive varicella-zoster IgM and by obtaining a chest x-ray that identifies the diffuse patchy infiltrates characteristic of viral pneumonia. Because this is such a potentially serious illness, the patient should be hospitalized and treated with intravenous acyclovir or valacyclovir. Antibiotics such as ceftriaxone and azithromycin may be indicated to treat superimposed bacterial pneumonia. Given the later gestational age, the fetus is at low risk for serious injury. ●
- Valeur-Jensen AK, Pedersen CB, Westergaard T, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA. 1999;281:1099-1105.
- Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
- Melamed N, Whittle W, Kelly EN, et al. Fetal thrombocytopenia in pregnancies with fetal human parvovirus-B19 infection. Am J Obstet Gynecol. 2015;212:793.e1-8.
- Nagel HTC, de Haan TR, Vandenbussche FPH, et al. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109:42-47.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al (eds). Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:911-912.
- Cohen JI. Herpes zoster. N Engl J Med. 2013;369:255-263.
- Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548-1551.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165.
- Marin M, Guris D, Chaves SS, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of varicella. MMWR Recommend Rep. 2007;56(RR-4):1-40.
- Swamy GK, Dotters-Katz SK. Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy. Am J Obstet Gynecol. 2019;221:655-656.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65.
- Valeur-Jensen AK, Pedersen CB, Westergaard T, et al. Risk factors for parvovirus B19 infection in pregnancy. JAMA. 1999;281:1099-1105.
- Harger JH, Adler SP, Koch WC, et al. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: risks and symptoms. Obstet Gynecol. 1998;91:413-420.
- Melamed N, Whittle W, Kelly EN, et al. Fetal thrombocytopenia in pregnancies with fetal human parvovirus-B19 infection. Am J Obstet Gynecol. 2015;212:793.e1-8.
- Nagel HTC, de Haan TR, Vandenbussche FPH, et al. Long-term outcome after fetal transfusion for hydrops associated with parvovirus B19 infection. Obstet Gynecol. 2007;109:42-47.
- Duff P. Maternal and fetal infections. In: Resnik R, Lockwood CJ, Moore TR, et al (eds). Creasy & Resnik’s Maternal-Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:911-912.
- Cohen JI. Herpes zoster. N Engl J Med. 2013;369:255-263.
- Enders G, Miller E, Cradock-Watson J, et al. Consequences of varicella and herpes zoster in pregnancy: prospective study of 1739 cases. Lancet. 1994;343:1548-1551.
- Duff P. Varicella in pregnancy: five priorities for clinicians. Infect Dis Obstet Gynecol. 1994;1:163-165.
- Marin M, Guris D, Chaves SS, et al; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention. Prevention of varicella. MMWR Recommend Rep. 2007;56(RR-4):1-40.
- Swamy GK, Dotters-Katz SK. Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy. Am J Obstet Gynecol. 2019;221:655-656.
- Duff P. Varicella vaccine. Infect Dis Obstet Gynecol. 1996;4:63-65.
Dietary sodium and potassium consumption and cardiovascular health
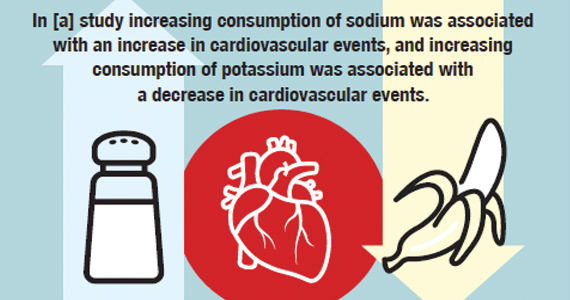
Hypertension is a prevalent medical problem among US women, with a higher prevalence among Black women, than among White, Hispanic, or Asian women (TABLE 1).1 Among US women aged 55 to 64 years, approximately 50% have hypertension or are taking a hypertension medicine.1 Hypertension is an important risk factor for cardiovascular disease, including stroke, coronary heart disease, heart failure, atrial fibrillation, and peripheral vascular disease.1,2 In a study of 1.3 million people, blood pressure (BP) ≥ 130/80 mm Hg was associated with an increased risk of a cardiovascular event, including myocardial infarction and stroke.2 Excessive sodium intake is an important risk factor for developing hypertension.3 In 2015–2016, 87% of US adults consumed >2,300 mg/d of sodium,4 an amount that is considered excessive.1 Less well known is the association between low potassium intake and hypertension. This editorial reviews the evidence that diets high in sodium and low in potassium contribute to the development of hypertension and cardiovascular disease.
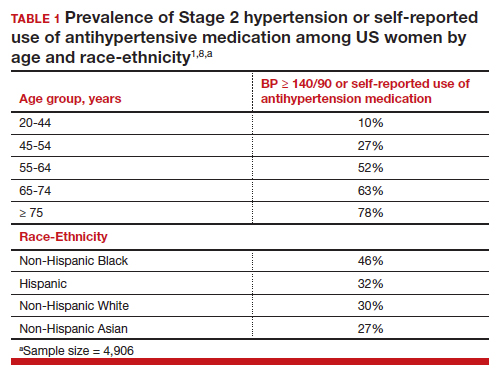
Sodium and potassium dueling cations
Many cohort studies report that diets high in sodium and low in potassium are associated with hypertension and an increased risk of cardiovascular disease. For example, in a cohort of 146,000 Chinese people, high sodium and low potassium intake was positively correlated with higher BP.5 In addition, the impact of increasing sodium intake or decreasing potassium intake was greater for people with a BMI ≥24 kg/m2, than people with a BMI <24 kg/m2. In a cohort of 11,095 US adults, high sodium and low potassium intake was associated with an increased risk of hypertension.6
In a study of 13,696 women, high potassium intake was associated with lower BP in participants with either a low or high sodium intake.7 In addition, over a 19-year follow up, higher potassium intake was associated with a lower risk of cardiovascular events.7 Comparing the highest (5,773 mg/d) vs lowest (2,783 mg/d) tertile of potassium intake, the decreased risk of a cardiovascular event was 0.89 (95% confidence interval [CI], 0.83–0.95).7
In a meta-analysis of data culled from 6 cohort studies, 10,709 adults with a mean age of 52 years, 54% of whom identified as women, were followed for a median of 8.8 years.8 Each adult contributed at least two 24-hour urine samples for measurement of sodium and potassium content. (Measurement of sodium and potassium in multiple 24-hour urine specimens from the same participant is thought to be the best way to assess sodium and potassium consumption.) The primary outcome was a cardiovascular event, including heart attack, stroke, or undergoing coronary revascularization procedures. In this study increasing consumption of sodium was associated with an increase in cardiovascular events, and increasing consumption of potassium was associated with a decrease in cardiovascular events. The hazard ratio for a cardiovascular event comparing high versus low consumption of sodium was 1.60 (95% CI, 1.19–2.14), and comparing high versus low consumption of potassium was 0.69 (95% CI, 0.51–0.91) (TABLE 2).8
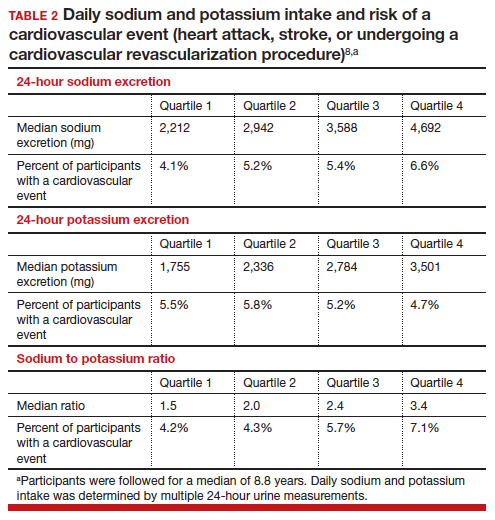
Continue to: Clinical trial data on decreasing Na and/or increasing K consumption on CV outcomes...
Clinical trial data on decreasing Na and/or increasing K consumption on CV outcomes
Building on the cohort studies reporting that diets high in sodium and low in potassium are associated with hypertension and cardiovascular disease, clinical trials report that decreasing dietary sodium intake reduces BP and the risk of a cardiovascular event. For example, in a meta-analysis of 85 clinical trials studying the link between sodium and BP, the investigators concluded that there was a linear relationship between sodium intake and BP, with larger reductions in sodium intake associated with greater reductions in BP, down to a daily sodium intake of 1,000 to 1,500 mg.9 The effect of sodium reduction on BP was greatest in study participants with higher BP at baseline.
In a cluster-randomized clinical trial in China, people living in 600 villages were assigned to a control group, continuing to use sodium chloride in their food preparation or an experimental intervention, replacing sodium chloride with a substitute product containing 75% sodium chloride and 25% potassium chloride by weight.10 The inclusion criteria included people ≥60 years of age with high BP or a history of stroke. The mean duration of follow-up was 4.7 years. Half of the participants were female. A total of 73% of the participants had a history of stroke and 88% had hypertension. In this study, the rate of death was lower in the group that used the salt substitute than in the group using sodium chloride (39 vs 45 deaths per 1,000 person-years; rate ratio (RR) 0.88; 95% CI, 0.82–0.95, P<.001). The rate of major cardiovascular events (nonfatal stroke, nonfatal acute coronary syndrome or death from vascular causes) was decreased in the group that used salt substitute compared with the group using sodium chloride (49 vs 56 events per 1,000 person-years, rate ratio (RR), 0.87; 95% CI, 0.80–0.94; P<.001). Similarly, the rate of stroke was decreased in the group that used salt substitute compared with the group using sodium chloride (29 vs 34 events per 1,000 person-years; rate ratio (RR), 0.86; 95% CI, 0.77–0.96; P = .006). This study shows that by decreasing sodium intake and increasing potassium, cardiovascular outcomes are improved in people at high risk for a cardiovascular event.10 People with kidney disease or taking medications that decrease renal excretion of potassium should consult with their health care provider before using potassium chloride containing salt substitutes.
What is your daily intake of sodium and potassium?
Almost all packaged prepared foods have labels indicating the amount of sodium in one serving. Many packaged foods also report the amount of potassium in one serving. Many processed foods contain high amounts of sodium and low amounts of potassium. Processed and ultra-processed foods are a major dietary source of sodium.11 In contrast to processed foods, fresh fruits, vegetables, and milk have high quantities of potassium and low amounts of sodium. As an example, a major brand of canned chicken broth has 750 mg of sodium and 40 mg of potassium per one-half cup, a ratio of sodium to potassium of 19:1. By contrast, canned red kidney beans have 135 mg of sodium and 425 mg of potassium in one-half cup, a ratio of sodium to potassium of 1:3. Patients can better understand their daily sodium and potassium intake by reading the food labels. Calculating a sodium to potassium ratio for a food may help people better understand their salt intake and identify foods associated with positive health outcomes.
The optimal target for daily consumption of sodium and potassium is controversial (TABLE 2). The mean daily intakes of sodium and potassium in the United States are approximately 3,380 mg and 2,499 mg,respectively.12 The American College of Cardiology (ACC) recommends that an optimal diet contains <1,500 mg/d of sodium, a stringent target.1 If that target is unattainable, people should at least aim for a 1,000 mg/d-reduction in their current sodium intake.1 The World Health Organization strongly recommends that adults consume <2,000 mg/d of sodium.13 The National Academy of Science recommends adults seeking to reduce the risk of cardiovascular disease consume <2,300 mg/d of sodium.14 The top dietary sources of sodium include deli meat, pizza, burritos and tacos, soups, savory snacks (chips, crackers, popcorn), fried poultry, burgers, and eggs.15
The optimal target for daily consumption of potassium is controversial. The ACC recommends that an optimal diet contains 3,500–5,000 mg/d of potassium.1 The World Health Organization recommends that adults consume >3,510 mg/d of potassium.16 The top dietary sources of potassium include milk, fruit, vegetables, coffee, savory snacks (chips, crackers, popcorn), fruit juice, white potatoes, deli meats, burritos, and tacos.15 The foods with the greatest amount of potassium include banana, avocado, acorn squash, spinach, sweet potatoes, salmon, apricots, grapefruit, broccoli, and white beans. People with kidney disease or those who are taking medications that interfere with renal excretion of potassium should consult with their health care provider before adding more potassium to their diet.
The ACC also recommends1:
- Maintaining an optimal weight (a 1-kg reduction in weight is associated with a 1-mm Hg reduction in BP).
- Eating a healthy diet rich in fruits, vegetables, whole grains, and low-fat dairy products with reduced saturated and total fat.
- Regular aerobic physical activity 90 to 150 min/wk.
- Moderation in alcohol consumption, with men limiting consumption ≤ 2 drinks/d and women limiting consumption to ≤ 1 drink/d.
- Smoking cessation.
Most adults in the US have too much sodium and too little potassium in their daily diet. Diets high in sodium and low in potassium increase the risk of hypertension. In turn, this increases the risk of cardiovascular disease, including myocardial infarction and stroke. Many personal choices and societal factors contribute to our current imbalanced and unhealthy diet, rich in sodium and deficient in potassium. Our best approach to improve health and reduce cardiovascular disease is to guide people to modify unhealthy lifestyle behaviors.17 For patients who are ready to change, a counseling intervention using the 5 A’s (including assess risk behaviors, advise change, agree on goals/action plan, assist with treatment, and arrange follow-up) has been shown to result in improved dietary choices, increased physical activity, and reduced use of tobacco products.18 ●
Two randomized clinical trials completed in the 1990s, comparing a low-sodium and a standard diet, showed no effect of reducing sodium intake by 32% and 57% on the risk of developing preeclampsia.1,2 Based on these 2 studies, a Cochrane review concluded that during pregnancy salt consumption should remain a matter of personal preference.3 Three recent observational studies report a relationship between sodium intake and the risk of developing pregnancy-associated hypertension.
In a study of 66,651 singleton pregnancies in the Danish Birth Cohort, participants with the greatest daily sodium intake, ranging from 3,520 to 7,520 mg/d had a 54% increased risk of developing gestational hypertension (95% confidence interval [CI], 16%–104%) and a 20% increased risk of developing preeclampsia (95% CI, 1%–42%).4 Another cohort study also reported that elevated sodium chloride intake was associated with an increased risk of developing preeclampsia.5 In one study, among patients with preeclampsia, those with lower urinary sodium to potassium ratio were less likely to develop severe preeclampsia.6 In a pregnant rat model, high salt intake is associated with a severe increase in blood pressure, the development of proteinuria, and an increase in circulating plasma soluble fmslike tyrosine-kinase 1 (sFlt-1)—changes also seen in preeclampsia.7 Pregnancy associated hypertension may not be as “salt sensitive” as chronic hypertension.
Future research could explore the effect of dietary sodium and potassium intake on the risk of developing severe hypertension during pregnancy in patients with chronic hypertension.
References
1. Knuist M, Bonsel GJ, Zondervan HA, et al. Low sodium diet and pregnancy-induced hypertension, a multicenter randomised controlled trial. Brit J Obstet Gynecol. 1998;105:430-434.
2. van der Maten GD, van Raaij JMA, Visman L, et al. Low-sodium in pregnancy: effects on blood pressure and maternal nutritional status. Brit J Nutr. 1997;77:703-720.
3. Duley L, Henderson-Smart DJ, Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database Syst Rev. 2005;CD005548.
4. Arvizu, M, Bjerregaard AA, Madsen MTB, et al. Sodium intake during pregnancy, but not other diet recommendations aimed at preventing cardiovascular disease, is positively related to risk of hypertensive disorders of pregnancy. J Nutr. 2020;150:159-166.
5. Birukov A, Andersen LB, Herse F, et al. Aldosterone, salt and potassium intakes as predictors of pregnancy outcome, including preeclampsia. Hypertension. 2019;74:391-398.
6. Yilmaz ZV, Akkas E, Turkmen GG, et al. Dietary sodium and potassium intake were associated with hypertension, kidney damage and adverse perinatal outcome in pregnant women with preeclampsia. Hypertension Preg. 2017;36:77-83.
7. Gillis EE, Williams JM, Garrett MR, et al. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309:R62-70.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/ AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ ASPC/NMA/PCNA guideline for the prevention, detection, evaluation and management of high blood pressure in adults: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426-e483.
- Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243-251.
- Aljuraiban G, Jose AP, Gupta P, et al. Sodium intake, health implications and the role of population-level strategies. Nutr Rev. 2021;79:351-359.
- Clarke LS, Overwyk K, Bates M, et al. Temporal trends in dietary sodium intake among adults aged ≥ 19 years--United States 2003-2016. MMWR. 2021;70:1478-1482.
- Guo X, Zhang M, Li C, et al. Association between urinary sodium and potassium excretion and blood pressure among non-hypertensive adults-China, 2018-2019. China CDC Wkly. 2022;4:522-526.
- Li M, Yan S, Li X, et al. Association between blood pressure and dietary intakes of sodium and potassium among US adults using quantile regression analysis NHANES 2007-2014. J Hum Hypertens. 2020;34:346-354.
- Wouda RD, Boekholdt SM, Khaw KT, et al. Sex-specific associations between potassium intake, blood pressure and cardiovascular outcomes: the EPIC-Norfolk study. Europ Heart J. 2022, Epub July 21.
- Ma Y, He, Sun Q, et al. 24-hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. 2022;386:252-263.
- Filippini T, Malavolti M, Whelton PK, et al. Blood pressure effects of sodium reduction: dose-response meta-analysis of experimental studies. Circulation. 2021;143:1542-1567.
- Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events. N Engl J Med. 2021;385:1067-1077.
- Monteiro CA, Cannon G, Moubarac JC, et al. The U.N. decade of nutrition: The NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;51:5-17.
- Nutrient intakes; From foods and beverages. Gender and Ag. WWEIA Data Tables. US Department of Health and Human Services, US Department of Agriculture. Web address Table 1. https://www .ars.usda.gov/ARSUserFiles/80400530/pdf /usual/Usual_Intake_gender_WWEIA_2015 _2018.pdf.
- WHO. Guideline: Sodium intake for adults and children. Geneva. World Health Organization (WHO), 2012. https://www.who.int /publications/i/item/9789241504836.
- National Academies of Sciences, Engineering and Medicine 2019. Dietary Reference Intakes for Sodium and Potassium. Washington DC: The National Academies Press. https://doi .org/10.17226/25353.
- Woodruff RC, Zhao L, Ahuja JKC, et al. Top food category contributors to sodium and potassium intake-United States 2015-2016. MMWR. 2020;69:1064-1069.
- WHO. Guideline: Potassium intake for adults and children. Geneva. World Health Organization (WHO), 2012. https://www.who.int /publications/i/item/9789241504829.
- Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138:345-355.
- US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. JAMA. 2022;328:367-374.

Hypertension is a prevalent medical problem among US women, with a higher prevalence among Black women, than among White, Hispanic, or Asian women (TABLE 1).1 Among US women aged 55 to 64 years, approximately 50% have hypertension or are taking a hypertension medicine.1 Hypertension is an important risk factor for cardiovascular disease, including stroke, coronary heart disease, heart failure, atrial fibrillation, and peripheral vascular disease.1,2 In a study of 1.3 million people, blood pressure (BP) ≥ 130/80 mm Hg was associated with an increased risk of a cardiovascular event, including myocardial infarction and stroke.2 Excessive sodium intake is an important risk factor for developing hypertension.3 In 2015–2016, 87% of US adults consumed >2,300 mg/d of sodium,4 an amount that is considered excessive.1 Less well known is the association between low potassium intake and hypertension. This editorial reviews the evidence that diets high in sodium and low in potassium contribute to the development of hypertension and cardiovascular disease.

Sodium and potassium dueling cations
Many cohort studies report that diets high in sodium and low in potassium are associated with hypertension and an increased risk of cardiovascular disease. For example, in a cohort of 146,000 Chinese people, high sodium and low potassium intake was positively correlated with higher BP.5 In addition, the impact of increasing sodium intake or decreasing potassium intake was greater for people with a BMI ≥24 kg/m2, than people with a BMI <24 kg/m2. In a cohort of 11,095 US adults, high sodium and low potassium intake was associated with an increased risk of hypertension.6
In a study of 13,696 women, high potassium intake was associated with lower BP in participants with either a low or high sodium intake.7 In addition, over a 19-year follow up, higher potassium intake was associated with a lower risk of cardiovascular events.7 Comparing the highest (5,773 mg/d) vs lowest (2,783 mg/d) tertile of potassium intake, the decreased risk of a cardiovascular event was 0.89 (95% confidence interval [CI], 0.83–0.95).7
In a meta-analysis of data culled from 6 cohort studies, 10,709 adults with a mean age of 52 years, 54% of whom identified as women, were followed for a median of 8.8 years.8 Each adult contributed at least two 24-hour urine samples for measurement of sodium and potassium content. (Measurement of sodium and potassium in multiple 24-hour urine specimens from the same participant is thought to be the best way to assess sodium and potassium consumption.) The primary outcome was a cardiovascular event, including heart attack, stroke, or undergoing coronary revascularization procedures. In this study increasing consumption of sodium was associated with an increase in cardiovascular events, and increasing consumption of potassium was associated with a decrease in cardiovascular events. The hazard ratio for a cardiovascular event comparing high versus low consumption of sodium was 1.60 (95% CI, 1.19–2.14), and comparing high versus low consumption of potassium was 0.69 (95% CI, 0.51–0.91) (TABLE 2).8

Continue to: Clinical trial data on decreasing Na and/or increasing K consumption on CV outcomes...
Clinical trial data on decreasing Na and/or increasing K consumption on CV outcomes
Building on the cohort studies reporting that diets high in sodium and low in potassium are associated with hypertension and cardiovascular disease, clinical trials report that decreasing dietary sodium intake reduces BP and the risk of a cardiovascular event. For example, in a meta-analysis of 85 clinical trials studying the link between sodium and BP, the investigators concluded that there was a linear relationship between sodium intake and BP, with larger reductions in sodium intake associated with greater reductions in BP, down to a daily sodium intake of 1,000 to 1,500 mg.9 The effect of sodium reduction on BP was greatest in study participants with higher BP at baseline.
In a cluster-randomized clinical trial in China, people living in 600 villages were assigned to a control group, continuing to use sodium chloride in their food preparation or an experimental intervention, replacing sodium chloride with a substitute product containing 75% sodium chloride and 25% potassium chloride by weight.10 The inclusion criteria included people ≥60 years of age with high BP or a history of stroke. The mean duration of follow-up was 4.7 years. Half of the participants were female. A total of 73% of the participants had a history of stroke and 88% had hypertension. In this study, the rate of death was lower in the group that used the salt substitute than in the group using sodium chloride (39 vs 45 deaths per 1,000 person-years; rate ratio (RR) 0.88; 95% CI, 0.82–0.95, P<.001). The rate of major cardiovascular events (nonfatal stroke, nonfatal acute coronary syndrome or death from vascular causes) was decreased in the group that used salt substitute compared with the group using sodium chloride (49 vs 56 events per 1,000 person-years, rate ratio (RR), 0.87; 95% CI, 0.80–0.94; P<.001). Similarly, the rate of stroke was decreased in the group that used salt substitute compared with the group using sodium chloride (29 vs 34 events per 1,000 person-years; rate ratio (RR), 0.86; 95% CI, 0.77–0.96; P = .006). This study shows that by decreasing sodium intake and increasing potassium, cardiovascular outcomes are improved in people at high risk for a cardiovascular event.10 People with kidney disease or taking medications that decrease renal excretion of potassium should consult with their health care provider before using potassium chloride containing salt substitutes.
What is your daily intake of sodium and potassium?
Almost all packaged prepared foods have labels indicating the amount of sodium in one serving. Many packaged foods also report the amount of potassium in one serving. Many processed foods contain high amounts of sodium and low amounts of potassium. Processed and ultra-processed foods are a major dietary source of sodium.11 In contrast to processed foods, fresh fruits, vegetables, and milk have high quantities of potassium and low amounts of sodium. As an example, a major brand of canned chicken broth has 750 mg of sodium and 40 mg of potassium per one-half cup, a ratio of sodium to potassium of 19:1. By contrast, canned red kidney beans have 135 mg of sodium and 425 mg of potassium in one-half cup, a ratio of sodium to potassium of 1:3. Patients can better understand their daily sodium and potassium intake by reading the food labels. Calculating a sodium to potassium ratio for a food may help people better understand their salt intake and identify foods associated with positive health outcomes.
The optimal target for daily consumption of sodium and potassium is controversial (TABLE 2). The mean daily intakes of sodium and potassium in the United States are approximately 3,380 mg and 2,499 mg,respectively.12 The American College of Cardiology (ACC) recommends that an optimal diet contains <1,500 mg/d of sodium, a stringent target.1 If that target is unattainable, people should at least aim for a 1,000 mg/d-reduction in their current sodium intake.1 The World Health Organization strongly recommends that adults consume <2,000 mg/d of sodium.13 The National Academy of Science recommends adults seeking to reduce the risk of cardiovascular disease consume <2,300 mg/d of sodium.14 The top dietary sources of sodium include deli meat, pizza, burritos and tacos, soups, savory snacks (chips, crackers, popcorn), fried poultry, burgers, and eggs.15
The optimal target for daily consumption of potassium is controversial. The ACC recommends that an optimal diet contains 3,500–5,000 mg/d of potassium.1 The World Health Organization recommends that adults consume >3,510 mg/d of potassium.16 The top dietary sources of potassium include milk, fruit, vegetables, coffee, savory snacks (chips, crackers, popcorn), fruit juice, white potatoes, deli meats, burritos, and tacos.15 The foods with the greatest amount of potassium include banana, avocado, acorn squash, spinach, sweet potatoes, salmon, apricots, grapefruit, broccoli, and white beans. People with kidney disease or those who are taking medications that interfere with renal excretion of potassium should consult with their health care provider before adding more potassium to their diet.
The ACC also recommends1:
- Maintaining an optimal weight (a 1-kg reduction in weight is associated with a 1-mm Hg reduction in BP).
- Eating a healthy diet rich in fruits, vegetables, whole grains, and low-fat dairy products with reduced saturated and total fat.
- Regular aerobic physical activity 90 to 150 min/wk.
- Moderation in alcohol consumption, with men limiting consumption ≤ 2 drinks/d and women limiting consumption to ≤ 1 drink/d.
- Smoking cessation.
Most adults in the US have too much sodium and too little potassium in their daily diet. Diets high in sodium and low in potassium increase the risk of hypertension. In turn, this increases the risk of cardiovascular disease, including myocardial infarction and stroke. Many personal choices and societal factors contribute to our current imbalanced and unhealthy diet, rich in sodium and deficient in potassium. Our best approach to improve health and reduce cardiovascular disease is to guide people to modify unhealthy lifestyle behaviors.17 For patients who are ready to change, a counseling intervention using the 5 A’s (including assess risk behaviors, advise change, agree on goals/action plan, assist with treatment, and arrange follow-up) has been shown to result in improved dietary choices, increased physical activity, and reduced use of tobacco products.18 ●
Two randomized clinical trials completed in the 1990s, comparing a low-sodium and a standard diet, showed no effect of reducing sodium intake by 32% and 57% on the risk of developing preeclampsia.1,2 Based on these 2 studies, a Cochrane review concluded that during pregnancy salt consumption should remain a matter of personal preference.3 Three recent observational studies report a relationship between sodium intake and the risk of developing pregnancy-associated hypertension.
In a study of 66,651 singleton pregnancies in the Danish Birth Cohort, participants with the greatest daily sodium intake, ranging from 3,520 to 7,520 mg/d had a 54% increased risk of developing gestational hypertension (95% confidence interval [CI], 16%–104%) and a 20% increased risk of developing preeclampsia (95% CI, 1%–42%).4 Another cohort study also reported that elevated sodium chloride intake was associated with an increased risk of developing preeclampsia.5 In one study, among patients with preeclampsia, those with lower urinary sodium to potassium ratio were less likely to develop severe preeclampsia.6 In a pregnant rat model, high salt intake is associated with a severe increase in blood pressure, the development of proteinuria, and an increase in circulating plasma soluble fmslike tyrosine-kinase 1 (sFlt-1)—changes also seen in preeclampsia.7 Pregnancy associated hypertension may not be as “salt sensitive” as chronic hypertension.
Future research could explore the effect of dietary sodium and potassium intake on the risk of developing severe hypertension during pregnancy in patients with chronic hypertension.
References
1. Knuist M, Bonsel GJ, Zondervan HA, et al. Low sodium diet and pregnancy-induced hypertension, a multicenter randomised controlled trial. Brit J Obstet Gynecol. 1998;105:430-434.
2. van der Maten GD, van Raaij JMA, Visman L, et al. Low-sodium in pregnancy: effects on blood pressure and maternal nutritional status. Brit J Nutr. 1997;77:703-720.
3. Duley L, Henderson-Smart DJ, Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database Syst Rev. 2005;CD005548.
4. Arvizu, M, Bjerregaard AA, Madsen MTB, et al. Sodium intake during pregnancy, but not other diet recommendations aimed at preventing cardiovascular disease, is positively related to risk of hypertensive disorders of pregnancy. J Nutr. 2020;150:159-166.
5. Birukov A, Andersen LB, Herse F, et al. Aldosterone, salt and potassium intakes as predictors of pregnancy outcome, including preeclampsia. Hypertension. 2019;74:391-398.
6. Yilmaz ZV, Akkas E, Turkmen GG, et al. Dietary sodium and potassium intake were associated with hypertension, kidney damage and adverse perinatal outcome in pregnant women with preeclampsia. Hypertension Preg. 2017;36:77-83.
7. Gillis EE, Williams JM, Garrett MR, et al. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309:R62-70.

Hypertension is a prevalent medical problem among US women, with a higher prevalence among Black women, than among White, Hispanic, or Asian women (TABLE 1).1 Among US women aged 55 to 64 years, approximately 50% have hypertension or are taking a hypertension medicine.1 Hypertension is an important risk factor for cardiovascular disease, including stroke, coronary heart disease, heart failure, atrial fibrillation, and peripheral vascular disease.1,2 In a study of 1.3 million people, blood pressure (BP) ≥ 130/80 mm Hg was associated with an increased risk of a cardiovascular event, including myocardial infarction and stroke.2 Excessive sodium intake is an important risk factor for developing hypertension.3 In 2015–2016, 87% of US adults consumed >2,300 mg/d of sodium,4 an amount that is considered excessive.1 Less well known is the association between low potassium intake and hypertension. This editorial reviews the evidence that diets high in sodium and low in potassium contribute to the development of hypertension and cardiovascular disease.

Sodium and potassium dueling cations
Many cohort studies report that diets high in sodium and low in potassium are associated with hypertension and an increased risk of cardiovascular disease. For example, in a cohort of 146,000 Chinese people, high sodium and low potassium intake was positively correlated with higher BP.5 In addition, the impact of increasing sodium intake or decreasing potassium intake was greater for people with a BMI ≥24 kg/m2, than people with a BMI <24 kg/m2. In a cohort of 11,095 US adults, high sodium and low potassium intake was associated with an increased risk of hypertension.6
In a study of 13,696 women, high potassium intake was associated with lower BP in participants with either a low or high sodium intake.7 In addition, over a 19-year follow up, higher potassium intake was associated with a lower risk of cardiovascular events.7 Comparing the highest (5,773 mg/d) vs lowest (2,783 mg/d) tertile of potassium intake, the decreased risk of a cardiovascular event was 0.89 (95% confidence interval [CI], 0.83–0.95).7
In a meta-analysis of data culled from 6 cohort studies, 10,709 adults with a mean age of 52 years, 54% of whom identified as women, were followed for a median of 8.8 years.8 Each adult contributed at least two 24-hour urine samples for measurement of sodium and potassium content. (Measurement of sodium and potassium in multiple 24-hour urine specimens from the same participant is thought to be the best way to assess sodium and potassium consumption.) The primary outcome was a cardiovascular event, including heart attack, stroke, or undergoing coronary revascularization procedures. In this study increasing consumption of sodium was associated with an increase in cardiovascular events, and increasing consumption of potassium was associated with a decrease in cardiovascular events. The hazard ratio for a cardiovascular event comparing high versus low consumption of sodium was 1.60 (95% CI, 1.19–2.14), and comparing high versus low consumption of potassium was 0.69 (95% CI, 0.51–0.91) (TABLE 2).8

Continue to: Clinical trial data on decreasing Na and/or increasing K consumption on CV outcomes...
Clinical trial data on decreasing Na and/or increasing K consumption on CV outcomes
Building on the cohort studies reporting that diets high in sodium and low in potassium are associated with hypertension and cardiovascular disease, clinical trials report that decreasing dietary sodium intake reduces BP and the risk of a cardiovascular event. For example, in a meta-analysis of 85 clinical trials studying the link between sodium and BP, the investigators concluded that there was a linear relationship between sodium intake and BP, with larger reductions in sodium intake associated with greater reductions in BP, down to a daily sodium intake of 1,000 to 1,500 mg.9 The effect of sodium reduction on BP was greatest in study participants with higher BP at baseline.
In a cluster-randomized clinical trial in China, people living in 600 villages were assigned to a control group, continuing to use sodium chloride in their food preparation or an experimental intervention, replacing sodium chloride with a substitute product containing 75% sodium chloride and 25% potassium chloride by weight.10 The inclusion criteria included people ≥60 years of age with high BP or a history of stroke. The mean duration of follow-up was 4.7 years. Half of the participants were female. A total of 73% of the participants had a history of stroke and 88% had hypertension. In this study, the rate of death was lower in the group that used the salt substitute than in the group using sodium chloride (39 vs 45 deaths per 1,000 person-years; rate ratio (RR) 0.88; 95% CI, 0.82–0.95, P<.001). The rate of major cardiovascular events (nonfatal stroke, nonfatal acute coronary syndrome or death from vascular causes) was decreased in the group that used salt substitute compared with the group using sodium chloride (49 vs 56 events per 1,000 person-years, rate ratio (RR), 0.87; 95% CI, 0.80–0.94; P<.001). Similarly, the rate of stroke was decreased in the group that used salt substitute compared with the group using sodium chloride (29 vs 34 events per 1,000 person-years; rate ratio (RR), 0.86; 95% CI, 0.77–0.96; P = .006). This study shows that by decreasing sodium intake and increasing potassium, cardiovascular outcomes are improved in people at high risk for a cardiovascular event.10 People with kidney disease or taking medications that decrease renal excretion of potassium should consult with their health care provider before using potassium chloride containing salt substitutes.
What is your daily intake of sodium and potassium?
Almost all packaged prepared foods have labels indicating the amount of sodium in one serving. Many packaged foods also report the amount of potassium in one serving. Many processed foods contain high amounts of sodium and low amounts of potassium. Processed and ultra-processed foods are a major dietary source of sodium.11 In contrast to processed foods, fresh fruits, vegetables, and milk have high quantities of potassium and low amounts of sodium. As an example, a major brand of canned chicken broth has 750 mg of sodium and 40 mg of potassium per one-half cup, a ratio of sodium to potassium of 19:1. By contrast, canned red kidney beans have 135 mg of sodium and 425 mg of potassium in one-half cup, a ratio of sodium to potassium of 1:3. Patients can better understand their daily sodium and potassium intake by reading the food labels. Calculating a sodium to potassium ratio for a food may help people better understand their salt intake and identify foods associated with positive health outcomes.
The optimal target for daily consumption of sodium and potassium is controversial (TABLE 2). The mean daily intakes of sodium and potassium in the United States are approximately 3,380 mg and 2,499 mg,respectively.12 The American College of Cardiology (ACC) recommends that an optimal diet contains <1,500 mg/d of sodium, a stringent target.1 If that target is unattainable, people should at least aim for a 1,000 mg/d-reduction in their current sodium intake.1 The World Health Organization strongly recommends that adults consume <2,000 mg/d of sodium.13 The National Academy of Science recommends adults seeking to reduce the risk of cardiovascular disease consume <2,300 mg/d of sodium.14 The top dietary sources of sodium include deli meat, pizza, burritos and tacos, soups, savory snacks (chips, crackers, popcorn), fried poultry, burgers, and eggs.15
The optimal target for daily consumption of potassium is controversial. The ACC recommends that an optimal diet contains 3,500–5,000 mg/d of potassium.1 The World Health Organization recommends that adults consume >3,510 mg/d of potassium.16 The top dietary sources of potassium include milk, fruit, vegetables, coffee, savory snacks (chips, crackers, popcorn), fruit juice, white potatoes, deli meats, burritos, and tacos.15 The foods with the greatest amount of potassium include banana, avocado, acorn squash, spinach, sweet potatoes, salmon, apricots, grapefruit, broccoli, and white beans. People with kidney disease or those who are taking medications that interfere with renal excretion of potassium should consult with their health care provider before adding more potassium to their diet.
The ACC also recommends1:
- Maintaining an optimal weight (a 1-kg reduction in weight is associated with a 1-mm Hg reduction in BP).
- Eating a healthy diet rich in fruits, vegetables, whole grains, and low-fat dairy products with reduced saturated and total fat.
- Regular aerobic physical activity 90 to 150 min/wk.
- Moderation in alcohol consumption, with men limiting consumption ≤ 2 drinks/d and women limiting consumption to ≤ 1 drink/d.
- Smoking cessation.
Most adults in the US have too much sodium and too little potassium in their daily diet. Diets high in sodium and low in potassium increase the risk of hypertension. In turn, this increases the risk of cardiovascular disease, including myocardial infarction and stroke. Many personal choices and societal factors contribute to our current imbalanced and unhealthy diet, rich in sodium and deficient in potassium. Our best approach to improve health and reduce cardiovascular disease is to guide people to modify unhealthy lifestyle behaviors.17 For patients who are ready to change, a counseling intervention using the 5 A’s (including assess risk behaviors, advise change, agree on goals/action plan, assist with treatment, and arrange follow-up) has been shown to result in improved dietary choices, increased physical activity, and reduced use of tobacco products.18 ●
Two randomized clinical trials completed in the 1990s, comparing a low-sodium and a standard diet, showed no effect of reducing sodium intake by 32% and 57% on the risk of developing preeclampsia.1,2 Based on these 2 studies, a Cochrane review concluded that during pregnancy salt consumption should remain a matter of personal preference.3 Three recent observational studies report a relationship between sodium intake and the risk of developing pregnancy-associated hypertension.
In a study of 66,651 singleton pregnancies in the Danish Birth Cohort, participants with the greatest daily sodium intake, ranging from 3,520 to 7,520 mg/d had a 54% increased risk of developing gestational hypertension (95% confidence interval [CI], 16%–104%) and a 20% increased risk of developing preeclampsia (95% CI, 1%–42%).4 Another cohort study also reported that elevated sodium chloride intake was associated with an increased risk of developing preeclampsia.5 In one study, among patients with preeclampsia, those with lower urinary sodium to potassium ratio were less likely to develop severe preeclampsia.6 In a pregnant rat model, high salt intake is associated with a severe increase in blood pressure, the development of proteinuria, and an increase in circulating plasma soluble fmslike tyrosine-kinase 1 (sFlt-1)—changes also seen in preeclampsia.7 Pregnancy associated hypertension may not be as “salt sensitive” as chronic hypertension.
Future research could explore the effect of dietary sodium and potassium intake on the risk of developing severe hypertension during pregnancy in patients with chronic hypertension.
References
1. Knuist M, Bonsel GJ, Zondervan HA, et al. Low sodium diet and pregnancy-induced hypertension, a multicenter randomised controlled trial. Brit J Obstet Gynecol. 1998;105:430-434.
2. van der Maten GD, van Raaij JMA, Visman L, et al. Low-sodium in pregnancy: effects on blood pressure and maternal nutritional status. Brit J Nutr. 1997;77:703-720.
3. Duley L, Henderson-Smart DJ, Meher S. Altered dietary salt for preventing pre-eclampsia, and its complications. Cochrane Database Syst Rev. 2005;CD005548.
4. Arvizu, M, Bjerregaard AA, Madsen MTB, et al. Sodium intake during pregnancy, but not other diet recommendations aimed at preventing cardiovascular disease, is positively related to risk of hypertensive disorders of pregnancy. J Nutr. 2020;150:159-166.
5. Birukov A, Andersen LB, Herse F, et al. Aldosterone, salt and potassium intakes as predictors of pregnancy outcome, including preeclampsia. Hypertension. 2019;74:391-398.
6. Yilmaz ZV, Akkas E, Turkmen GG, et al. Dietary sodium and potassium intake were associated with hypertension, kidney damage and adverse perinatal outcome in pregnant women with preeclampsia. Hypertension Preg. 2017;36:77-83.
7. Gillis EE, Williams JM, Garrett MR, et al. The Dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015;309:R62-70.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/ AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ ASPC/NMA/PCNA guideline for the prevention, detection, evaluation and management of high blood pressure in adults: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426-e483.
- Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243-251.
- Aljuraiban G, Jose AP, Gupta P, et al. Sodium intake, health implications and the role of population-level strategies. Nutr Rev. 2021;79:351-359.
- Clarke LS, Overwyk K, Bates M, et al. Temporal trends in dietary sodium intake among adults aged ≥ 19 years--United States 2003-2016. MMWR. 2021;70:1478-1482.
- Guo X, Zhang M, Li C, et al. Association between urinary sodium and potassium excretion and blood pressure among non-hypertensive adults-China, 2018-2019. China CDC Wkly. 2022;4:522-526.
- Li M, Yan S, Li X, et al. Association between blood pressure and dietary intakes of sodium and potassium among US adults using quantile regression analysis NHANES 2007-2014. J Hum Hypertens. 2020;34:346-354.
- Wouda RD, Boekholdt SM, Khaw KT, et al. Sex-specific associations between potassium intake, blood pressure and cardiovascular outcomes: the EPIC-Norfolk study. Europ Heart J. 2022, Epub July 21.
- Ma Y, He, Sun Q, et al. 24-hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. 2022;386:252-263.
- Filippini T, Malavolti M, Whelton PK, et al. Blood pressure effects of sodium reduction: dose-response meta-analysis of experimental studies. Circulation. 2021;143:1542-1567.
- Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events. N Engl J Med. 2021;385:1067-1077.
- Monteiro CA, Cannon G, Moubarac JC, et al. The U.N. decade of nutrition: The NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;51:5-17.
- Nutrient intakes; From foods and beverages. Gender and Ag. WWEIA Data Tables. US Department of Health and Human Services, US Department of Agriculture. Web address Table 1. https://www .ars.usda.gov/ARSUserFiles/80400530/pdf /usual/Usual_Intake_gender_WWEIA_2015 _2018.pdf.
- WHO. Guideline: Sodium intake for adults and children. Geneva. World Health Organization (WHO), 2012. https://www.who.int /publications/i/item/9789241504836.
- National Academies of Sciences, Engineering and Medicine 2019. Dietary Reference Intakes for Sodium and Potassium. Washington DC: The National Academies Press. https://doi .org/10.17226/25353.
- Woodruff RC, Zhao L, Ahuja JKC, et al. Top food category contributors to sodium and potassium intake-United States 2015-2016. MMWR. 2020;69:1064-1069.
- WHO. Guideline: Potassium intake for adults and children. Geneva. World Health Organization (WHO), 2012. https://www.who.int /publications/i/item/9789241504829.
- Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138:345-355.
- US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. JAMA. 2022;328:367-374.
- Whelton PK, Carey RM, Aronow WS, et al. ACC/ AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ ASPC/NMA/PCNA guideline for the prevention, detection, evaluation and management of high blood pressure in adults: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138:e426-e483.
- Flint AC, Conell C, Ren X, et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. N Engl J Med. 2019;381:243-251.
- Aljuraiban G, Jose AP, Gupta P, et al. Sodium intake, health implications and the role of population-level strategies. Nutr Rev. 2021;79:351-359.
- Clarke LS, Overwyk K, Bates M, et al. Temporal trends in dietary sodium intake among adults aged ≥ 19 years--United States 2003-2016. MMWR. 2021;70:1478-1482.
- Guo X, Zhang M, Li C, et al. Association between urinary sodium and potassium excretion and blood pressure among non-hypertensive adults-China, 2018-2019. China CDC Wkly. 2022;4:522-526.
- Li M, Yan S, Li X, et al. Association between blood pressure and dietary intakes of sodium and potassium among US adults using quantile regression analysis NHANES 2007-2014. J Hum Hypertens. 2020;34:346-354.
- Wouda RD, Boekholdt SM, Khaw KT, et al. Sex-specific associations between potassium intake, blood pressure and cardiovascular outcomes: the EPIC-Norfolk study. Europ Heart J. 2022, Epub July 21.
- Ma Y, He, Sun Q, et al. 24-hour urinary sodium and potassium excretion and cardiovascular risk. N Engl J Med. 2022;386:252-263.
- Filippini T, Malavolti M, Whelton PK, et al. Blood pressure effects of sodium reduction: dose-response meta-analysis of experimental studies. Circulation. 2021;143:1542-1567.
- Neal B, Wu Y, Feng X, et al. Effect of salt substitution on cardiovascular events. N Engl J Med. 2021;385:1067-1077.
- Monteiro CA, Cannon G, Moubarac JC, et al. The U.N. decade of nutrition: The NOVA food classification and the trouble with ultra-processing. Public Health Nutr. 2018;51:5-17.
- Nutrient intakes; From foods and beverages. Gender and Ag. WWEIA Data Tables. US Department of Health and Human Services, US Department of Agriculture. Web address Table 1. https://www .ars.usda.gov/ARSUserFiles/80400530/pdf /usual/Usual_Intake_gender_WWEIA_2015 _2018.pdf.
- WHO. Guideline: Sodium intake for adults and children. Geneva. World Health Organization (WHO), 2012. https://www.who.int /publications/i/item/9789241504836.
- National Academies of Sciences, Engineering and Medicine 2019. Dietary Reference Intakes for Sodium and Potassium. Washington DC: The National Academies Press. https://doi .org/10.17226/25353.
- Woodruff RC, Zhao L, Ahuja JKC, et al. Top food category contributors to sodium and potassium intake-United States 2015-2016. MMWR. 2020;69:1064-1069.
- WHO. Guideline: Potassium intake for adults and children. Geneva. World Health Organization (WHO), 2012. https://www.who.int /publications/i/item/9789241504829.
- Li Y, Pan A, Wang DD, et al. Impact of healthy lifestyle factors on life expectancies in the US population. Circulation. 2018;138:345-355.
- US Preventive Services Task Force. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults without cardiovascular disease risk factors. JAMA. 2022;328:367-374.